User login
Frailty in older adults: Implications for end-of-life care
As people get older, they have more things wrong with them. And the more things they have wrong with them, the more likely they are to die. But everyone accumulates deficits at a different rate, and not all people of the same age have the same short-term risk of dying. This variable susceptibility to death and other adverse outcomes in older people of the same age is called frailty.1
Frailty poses special challenges to how we organize and deliver health care. These challenges are sometimes seen most starkly when people are most frail, especially as they approach the end of life.
In this paper, we will review how frailty is conceptualized and defined, consider how frailty affects the care of people at the end of their lives, and suggest practices that can make end-of-life care better for frail older adults.
DEFINING FRAILTY
As with all complex systems, when frail people become acutely unwell their highest-order functions fail first. Thus, cognitive impairment, functional decline, impaired mobility, and social withdrawal are hallmark presentations of the further accumulation of deficits in vulnerable seniors.
Delirium and falls are important clues that a person’s resilience is becoming compromised and that the person is at risk of further insults in a downward spiral or acceleration of things going wrong.1,2 Frailty is associated with poor health outcomes, from disability to institutionalization and death.3
This idea of frailty as vulnerability arising from dysregulation of multiple physiologic systems is reasonably non-controversial. Even so, there are competing views on how to systematically quantify those who are at an increased risk of adverse sequelae.
Quantifying frailty is particularly important if it can tell us if a patient is at high risk of further decline and death. As frailty advances, it is appropriate to shift the focus of care to palliation, with the goal of optimizing quality of life and easing symptoms.4 Identifying someone as frail can aid decision-making in the setting of critical illness, where the system commonly defaults to an “always do everything” mode without considering the ramifications of such an approach. Furthermore, without a routine means of measuring frailty, it is often left to critical care units or rapid-response medical teams to initiate a discussion about whether an aggressive course of care is appropriate or desired.5,6
Frailty as a syndrome
Fried et al7 defined frailty as a syndrome arising from the “physiologic triad” of sarcopenia and immune and neuroendocrine dysregulation. Patients are considered frail if they have three or more of the following five criteria:
- Reduced activity
- Slowing of mobility
- Weight loss
- Diminished handgrip strength
- Exhaustion.
Someone who has only one or two of these items is said to be “pre-frail”; someone with none is said to be “robust.”
The frailty index
An alternative viewpoint is that frailty is a state arising from the accumulation of deficits, which can be counted in a frailty index.
The frailty index is based on the concept that frailty is a consequence of interacting physical, psychological, and social factors. As deficits accumulate, people become increasingly vulnerable to adverse outcomes.
The frailty index is calculated as the number of deficits the patient has, divided by the number of deficits considered. For example, in a frailty index based on a comprehensive geriatric assessment, an individual with impairments in 4 of 10 domains and with 10 of 24 possible comorbidities would have 14 of 34 possible deficits, for a frailty index of 0.41.8
A criticism of the frailty index is that it includes functional dependence as a deficit. The criticism stems from the view that frailty should be seen as occurring prior to disability. According to this view, including dependence in instrumental and basic activities of daily living as a deficit confuses disability with frailty.
Proponents of the frailty index counter that frailty is not “all or none” and needs to be graded. The frailty index can distinguish between people with and without disability by means of the number of deficits that they have, which is most important. For example, a person disabled by a paraplegic injury would have a lower frailty index score and therefore would be considered less frail than a person with advanced cancer affecting multiple body systems. (This is assuming the person who has suffered the injury resulting in paraplegia doesn’t have a concomitant condition such as renal failure or heart disease. In the absence of other health insults, such patients are less at risk of further morbidity or death than the patient with advanced cancer until they get another health insult or insults added to their frailty.)
In any case, functional capacity is fundamental in medical decision-making and when estimating prognoses. An example is the use of the Eastern Cooperative Oncology Group’s functional status measure.9,10
Sum of physical and psychological stressors
Consensus is growing for the concept that frail people are made more vulnerable by the combination of both physical and psychological stressors. This is particularly important to bear in mind for patients who may appear physically robust but whose total health burden makes them vulnerable to further insults.
For example, think of a relatively young overweight patient with hypertension, diabetes, dyslipidemia, and ischemic white matter changes (which can manifest as low mood and even mild vascular cognitive impairment). In such a patient, an acute illness could result in cognitive and functional decline that can be permanent.
Balance of assets and deficits
About 20 years ago, we used the metaphor of a balance beam to describe how frailty comes about in older adults. In this view, there is an interplay of physiological and functional health determinants. Assets such as health, resources, and caregivers are balanced against deficits such as illnesses, dependency on others, and support burden.8
For the most part, later concepts of frailty have focused on the individual, with social factors construed separately as social vulnerability.11
Tools for assessing frailty in people who are not yet disabled
Several tools exist to clinically assess frailty in people who are not yet disabled.
The FRAIL scale.12 The Geriatric Advisory Panel of the International Academy of Nutrition and Aging formulated a scale for measuring frailty as a “pre-disability state.” The FRAIL scale consists of five easily remembered items:
- Fatigue
- Resistance (inability to climb one flight of stairs)
- Ambulation (inability to walk one block)
- Illnesses (more than five)
- Loss of weight (> 5%).
Like the “reduced activity” criterion of the frailty syndrome mentioned above (in practical terms, described as the inability to do heavy household chores),13 the FRAIL scale seems to blur the distinction between disability (here, the inability to climb stairs or to walk a block) and “pre-disability,” to an uncertain end. It also seems to blend the notion of a state and a syndrome; these points will need to be clarified in due course.
The Tilburg Frailty Indicator14 was constructed around the multidimensional viewpoint of frailty, beyond disease or disability state, to identify frail community-dwelling older individuals. The first part of this two-part questionnaire consists of 10 questions on frailty determinants and medical comorbidities, while the second part contains physical, psychological, and social variables strongly associated with frailty, as well as information about disability in walking and balance. Interestingly, although it includes both social and physical factors, it does not include cognition.
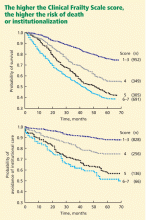
The Clinical Frailty Scale was developed as a practical approach to assess frailty using physical and functional indicators of health and illness burden. The descriptors for this 7-point scale guide clinicians in quantifying the degree of frailty present. It ranges from 1 (very fit) to 7 (severely frail).7 The higher the score, the higher the risks of death or institutionalization. Even mild frailty is associated with a 50% 5-year mortality rate in community-dwelling older adults (Figure 1).8
The Edmonton Frail Scale,15 like the Clinical Frailty Scale, was developed to be practical and usable at the bedside. It is based on the following domains: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and functional performance.
In a community-based sample, the Edmonton Frail Scale compared favorably with the clinical assessment of geriatric specialists who completed a comprehensive evaluation (Pearson’s correlation coefficient 0.64, P < .001).15
FRAILTY AS A PROGNOSTIC INDICATOR
Using frailty scales to aid in prognostication can be useful to clinicians. Survival prognostication is inherently challenging in individuals with multiple comorbidities and variable trajectories of decline, but it remains a vital clinical skill for all clinicians. Framing these difficult discussions in the context of degree of frailty provides a unifying concept, beyond a single-system construct, for care providers, patients, and their loved ones.
Patients nearing the end of their lives need this kind of clarity and support. Regardless of their diagnoses, patients typically want to know when they are at high risk of dying, as do their families and caregivers. People in general look for such information so that they can align medical decision-making congruently with predicted prognosis.16,17 They also use it to plan for the final chapter of their life and their death.
The frailty index is strongly correlated with risk of death
The frailty index is strongly correlated with the risk of death, with a correlation coefficient greater than 0.95. As such, an individual’s frailty index score is considered an estimate of biologic age, which has greater correlation with associated morbidity and death than does chronological age.18,19 In the general population, more than 99% of people have a frailty index value of less than 0.7. As people approach this value, the chance of survival is greatly diminished; indeed, one report suggested that of those who have a frailty index value of more than 0.5 (based on a comprehensive geriatric assessment), 100% are dead by about 20 months later.20,21
In short, there is a limit to which deficits can be added before the system fails. In this sense, the frailty index is akin to the concept of physiologic reserve. Reserve is finite, and as a system loses redundancy it can no longer survive new stresses.
What does this information mean for individual patients?
Even so, prognostication for individual patients remains probabilistic. Any patient has a chance to improve, stabilize, worsen, or die. However, a patient can reach an upper limit of frailty. At that point, instead of accumulating another deficit, death is much more likely. Similarly, although improvement can happen, the chance of improvement is low, and the improvement is typically modest.
Framing survival possibilities in terms of the number of things that people have wrong with them and the chance of death or of change (and the extent of change) makes sense to physicians, patients, and families. Being able to do so offers a much greater opportunity for realistic discussions of the likely outcomes of medical care than the foreseeable scenario of a junior doctor asking a senior citizen, “If your heart stops, do you want us to save your life?”
Understanding prognosis in the face of not just disease but also frailty can also help us focus not on disease but on health consequences of illness. Can the person think? Walk? Care for herself or himself? Interact with others? These questions need to be considered when end-of-life decisions are being discussed.22
Since making predictions about survival is most challenging when multiple comorbidities are present, using the concept of accumulating deficits to better define the slope of decline can be very helpful when discussing “the road ahead” with patients and their families. Visually mapping out the slope of decline and how it is accelerating as conditions progress and deficits accumulate can aid in medical decision-making. Looking individually at the deficits themselves and associated markers of progression can also help with prognostic discussions.
For example, a patient with chronic obstructive pulmonary disease could very well be unaware of the progression and ultimately terminal prognosis of this disease. The slope of clinical decline can be initially shallow, with saw-tooth fluctuations from acute exacerbations that seemingly “resolve to baseline” when antibiotic and steroid courses are completed. Talking with these patients and their families about heralding markers, such as more hospitalizations and cognitive decline with acute exacerbations, can clarify the steepening slope of decline and the way comorbidities interact.
FRAILTY AND END-OF-LIFE CARE
Frailty is progressive, and as it worsens, integrating a palliative approach is appropriate, with a focus on optimizing quality of life and relieving symptoms.4 This principle holds true regardless of the care setting, from acute care hospitals to hospice facilities and long-term care residences.
The principles of end-of-life care are applicable to frail individuals with progressive conditions from the time of diagnosis throughout the course of decline. As the population ages, more people suffer and die from progressive chronic conditions such as cerebrovascular disease, respiratory disease, and dementia.23 An interdisciplinary team approach can ensure all components of palliation are effectively delivered, such as easing symptoms, providing psychosocial and spiritual support, and improving quality of life.24
Pain management
Pain is widely underassessed and undertreated in older patients. Its management at the end of life is particularly challenging if the patient’s language is compromised, as in dementia.23,25,26
A recent cross-sectional analysis of self-reported pain in a longitudinal study of community-dwelling older adults showed an independent association between moderate or higher pain and frailty. The authors propose that persistent pain goes beyond physical discomfort in that it may contribute to homeostenosis (progressive diminishment of homeostatic reserve) and directly worsen frailty.27
Examine the medication list
In palliative care, medical interventions focus on optimizing quality of life.
This especially includes reexamining long medication lists that increase the chance of adverse drug effects.28 Many patients are on disease-modifying medications that may or may not help control symptoms—and might well exacerbate them. For example, betablockers for ischemic heart disease and angiotensin-converting enzyme inhibitors for diabetic nephropathy both can cause hypotension-induced lassitude or even falls due to orthostasis.
A sensible approach is to keep the drugs that may still contribute to quality of life, while discontinuing other drugs that may be causing side effects or that are unlikely to provide meaningful benefit in terms of prevention in patients who have limited life expectancy.29 Discontinuing ineffective, poorly tolerated, and duplicated medications also makes it easier to introduce new medications to manage symptoms—there will be fewer drug interactions, and fewer pills to take, an increasingly important issue in the setting of gastrointestinal symptoms such as dysphagia and gastroparesis or compliance issues as frequently encountered when cognitive impairment is present.29,30
In managing symptoms, start low and go slow—but get there
In managing symptoms in frail elderly patients we use the same classes of medications as in younger patients. The trick is to use appropriate doses.
The concept of “start low and go slow” is key, but so is “get there”—ie, reach the therapeutic goal. The principal drugs for symptom control, such as opioids for pain and dyspnea and anxiolytics for anxiety and restlessness, are associated with a higher rate of and more severe adverse effects in frail older adults.
Even so, most frail older adults appear to be undertreated in this regard, particularly if they are cognitively impaired.23 This fact, coupled with the reality that behavioral symptoms associated with advanced dementia can represent unmet care needs including undertreated pain, highlights the critical need to control symptoms optimally in frail seniors.
This is particularly relevant for those who can no longer verbally articulate their symptoms. Nonverbal pain scales and vigilant assessment of behavioral signs of pain are paramount skills for clinicians providing palliative care to patients with cognitive impairment. Caregivers and loved ones should be included in the assessment process.26,31
Adjunctive therapies for pain control
Maximizing adjunctive therapies can optimize pain control in this drug-sensitive population. Heat and cold packs to affected areas, acupuncture, massage therapy, and structured exercise regimens are some options that can improve quality of life. Cognitive behavioral therapy may offer coping strategies, provided the patient can participate in this process from a cognitive perspective.26,27 Topical preparations are often well tolerated and may include medicinal ingredients that are helpful without systemic effects, such as anti-inflammatory drugs or analgesics.
Optimal use of nonopioid drugs may help reduce the need for narcotics, particularly in the presence of musculoskeletal pain. An example is acetaminophen in regular doses—we would recommend no more than 3 g per day. Acetaminophen is preferred for older adults rather than nonsteroidal anti-inflammatory drugs, given the potential gastric and cardiovascular side effects of the latter medications.
Antidepressants and anticonvulsants such as gabapentin can also be considered as adjuncts for pain control, particularly in the setting of neuropathic pain, with careful monitoring for tolerance.25
When opioids are used, vigilance for constipation is essential.
Establishing goals of care
Goals of care need to be established incrementally along the course of clinical decline and as early as possible so that palliative support can be promptly implemented as symptoms worsen.32
End-of-life care can still include treatments with curative intent, depending on the overall prognosis and the state of the underlying terminal illness. On the other hand, frail older adults who are subjected to invasive treatments that are unlikely to provide cure, such as Whipple surgery, need special intervention postoperatively if they are not to suffer complications such as persisting delirium and functional decline.33
In this regard, geriatric palliative care is frequently about not “crossing a threshold.” Patients may still be receiving active management and be hospitalized for acute exacerbations of progressing chronic conditions, such as chronic obstructive pulmonary disease and heart failure, while palliative principles are introduced and increasingly become the focus of care.
To align goals of care with frailty burden, it is crucial to quantify frailty and to review the patient’s comorbidities. Particularly when dementia is present, lack of communication between the patient’s doctors or between the doctor and the family about disease burden can lead to inappropriately aggressive care.
Many family members and even clinicians do not recognize that advanced dementia is terminal.34,35 In light of this, a palliative approach to care may not even be considered as an appropriate plan when hallmark complications associated with progressing cognitive decline occur, such as aspiration pneumonia or dehydration. Education about dementia and other conditions with progressive organ failure should be done as soon as possible after diagnosis and readdressed at intervals throughout the patient’s clinical decline.
Earlier discussions also ensure that patients themselves can be involved in decision-making more often before cognitive impairment advances to the point where proxy discussions take over.16
BETTER PALLIATIVE CARE FOR ALL
Palliative care, developed initially to provide holistic and timely symptom-based care for patients with noncurable cancer, should also be available and offered to patients with nonmalignant, life-limiting diseases.23,36,37 Meeting this standard of geriatric care is not easy, given the burden of frailty in this population. Needed are multimodal palliative efforts across the spectrum of settings, from the home to the hospital and nursing home.23
To do this, we need to embrace the complexity posed by each person’s presentation and view care through the frailty lens. This will give us a common language in which to engage in a conversation with the same goal in mind: optimizing quality of life.
Furthermore, quantifying frailty can help minimize interventions that are futile or burdensome, that are not expected to ease symptoms, and that can worsen cognition and function. At the end of a patient’s life, we do not want to add to his or her frailty burden but rather minimize the morbidity associated with it.
The concept of frailty assessment is therefore essential for the timely delivery of holistic palliative care in geriatric patients who have progressive and ultimately terminal conditions.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Michel JP, Bonin-Guillame S, Gold G, Herrmann F. Cognition and frailty: possible interrelations. In:Carey JR, Robine JM, Michel JP, Christen Y, editors. Longevity and Frailty. Berlin Heidelberg: Springer; 2005:119–124. Research and Perspectives in Longevity.
- Espinoza S, Walston JD. Frailty in older adults: insights and interventions. Cleve Clin J Med 2005; 72:1105–1112.
- Boockvar KS, Meier DE. Palliative care for frail older adults: “there are things I can’t do anymore that I wish I could . . . “. JAMA 2006; 296:2245–2253.
- Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–286.
- McDermid RC, Bagshaw SM. ICU and critical care outreach for the elderly. Best Pract Res Clin Anaesthesiol 2011; 25:439–449.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ 1994; 150:489–495.
- Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 2011; 78:138–149.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS One 2008; 3:e2232.
- Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc 2008; 9:71–72.
- Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res 2011; 23:209–216.
- Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11:344–355.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care 2011; 15:301.
- Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc 2008; 56:898–903.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev 2006; 127:494–496.
- Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc 2010; 58:318–323.
- Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev 2006; 127:840–848.
- Davies E, Higginson IJ; WHO Europe. Better Palliative Care for Older People. Milan, Italy: Tipolitografia Trabella Sr; 2004.
- Raudonis BM, Daniel K. Frailty: an indication for palliative care. Geriatr Nurs 2010; 31:379–384.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1436.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6):S205–S224.
- Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc 2012; 60:113–117.
- Hanlon JT, Perera S, Sevick MA, Rodriguez KL, Jaffe EJ. Pain and its treatment in older nursing home hospice/palliative care residents. J Am Med Dir Assoc 2010; 11:579–583.
- Currow DC, Stevenson JP, Abernethy AP, Plummer J, Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc 2007; 55:590–595.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Kapo J, Morrison LJ, Liao S. Palliative care for the older adult. J Palliat Med 2007; 10:185–209.
- Gaertner J, Wolf J, Frechen S, et al. Recommending early integration of palliative care—does it work? Support Care Cancer 2012; 20:507–513.
- Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011; 213:245–252.
- Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009; 361:1529–1538.
- Arcand M, Monette J, Monette M, et al. Educating nursing home staff about the progression of dementia and the comfort care option: impact on family satisfaction with end-of-life care. J Am Med Dir Assoc 2009; 10:50–55.
- Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004; 164:321–326.
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008; 17:1144–1163.
As people get older, they have more things wrong with them. And the more things they have wrong with them, the more likely they are to die. But everyone accumulates deficits at a different rate, and not all people of the same age have the same short-term risk of dying. This variable susceptibility to death and other adverse outcomes in older people of the same age is called frailty.1
Frailty poses special challenges to how we organize and deliver health care. These challenges are sometimes seen most starkly when people are most frail, especially as they approach the end of life.
In this paper, we will review how frailty is conceptualized and defined, consider how frailty affects the care of people at the end of their lives, and suggest practices that can make end-of-life care better for frail older adults.
DEFINING FRAILTY
As with all complex systems, when frail people become acutely unwell their highest-order functions fail first. Thus, cognitive impairment, functional decline, impaired mobility, and social withdrawal are hallmark presentations of the further accumulation of deficits in vulnerable seniors.
Delirium and falls are important clues that a person’s resilience is becoming compromised and that the person is at risk of further insults in a downward spiral or acceleration of things going wrong.1,2 Frailty is associated with poor health outcomes, from disability to institutionalization and death.3
This idea of frailty as vulnerability arising from dysregulation of multiple physiologic systems is reasonably non-controversial. Even so, there are competing views on how to systematically quantify those who are at an increased risk of adverse sequelae.
Quantifying frailty is particularly important if it can tell us if a patient is at high risk of further decline and death. As frailty advances, it is appropriate to shift the focus of care to palliation, with the goal of optimizing quality of life and easing symptoms.4 Identifying someone as frail can aid decision-making in the setting of critical illness, where the system commonly defaults to an “always do everything” mode without considering the ramifications of such an approach. Furthermore, without a routine means of measuring frailty, it is often left to critical care units or rapid-response medical teams to initiate a discussion about whether an aggressive course of care is appropriate or desired.5,6
Frailty as a syndrome
Fried et al7 defined frailty as a syndrome arising from the “physiologic triad” of sarcopenia and immune and neuroendocrine dysregulation. Patients are considered frail if they have three or more of the following five criteria:
- Reduced activity
- Slowing of mobility
- Weight loss
- Diminished handgrip strength
- Exhaustion.
Someone who has only one or two of these items is said to be “pre-frail”; someone with none is said to be “robust.”
The frailty index
An alternative viewpoint is that frailty is a state arising from the accumulation of deficits, which can be counted in a frailty index.
The frailty index is based on the concept that frailty is a consequence of interacting physical, psychological, and social factors. As deficits accumulate, people become increasingly vulnerable to adverse outcomes.
The frailty index is calculated as the number of deficits the patient has, divided by the number of deficits considered. For example, in a frailty index based on a comprehensive geriatric assessment, an individual with impairments in 4 of 10 domains and with 10 of 24 possible comorbidities would have 14 of 34 possible deficits, for a frailty index of 0.41.8
A criticism of the frailty index is that it includes functional dependence as a deficit. The criticism stems from the view that frailty should be seen as occurring prior to disability. According to this view, including dependence in instrumental and basic activities of daily living as a deficit confuses disability with frailty.
Proponents of the frailty index counter that frailty is not “all or none” and needs to be graded. The frailty index can distinguish between people with and without disability by means of the number of deficits that they have, which is most important. For example, a person disabled by a paraplegic injury would have a lower frailty index score and therefore would be considered less frail than a person with advanced cancer affecting multiple body systems. (This is assuming the person who has suffered the injury resulting in paraplegia doesn’t have a concomitant condition such as renal failure or heart disease. In the absence of other health insults, such patients are less at risk of further morbidity or death than the patient with advanced cancer until they get another health insult or insults added to their frailty.)
In any case, functional capacity is fundamental in medical decision-making and when estimating prognoses. An example is the use of the Eastern Cooperative Oncology Group’s functional status measure.9,10
Sum of physical and psychological stressors
Consensus is growing for the concept that frail people are made more vulnerable by the combination of both physical and psychological stressors. This is particularly important to bear in mind for patients who may appear physically robust but whose total health burden makes them vulnerable to further insults.
For example, think of a relatively young overweight patient with hypertension, diabetes, dyslipidemia, and ischemic white matter changes (which can manifest as low mood and even mild vascular cognitive impairment). In such a patient, an acute illness could result in cognitive and functional decline that can be permanent.
Balance of assets and deficits
About 20 years ago, we used the metaphor of a balance beam to describe how frailty comes about in older adults. In this view, there is an interplay of physiological and functional health determinants. Assets such as health, resources, and caregivers are balanced against deficits such as illnesses, dependency on others, and support burden.8
For the most part, later concepts of frailty have focused on the individual, with social factors construed separately as social vulnerability.11
Tools for assessing frailty in people who are not yet disabled
Several tools exist to clinically assess frailty in people who are not yet disabled.
The FRAIL scale.12 The Geriatric Advisory Panel of the International Academy of Nutrition and Aging formulated a scale for measuring frailty as a “pre-disability state.” The FRAIL scale consists of five easily remembered items:
- Fatigue
- Resistance (inability to climb one flight of stairs)
- Ambulation (inability to walk one block)
- Illnesses (more than five)
- Loss of weight (> 5%).
Like the “reduced activity” criterion of the frailty syndrome mentioned above (in practical terms, described as the inability to do heavy household chores),13 the FRAIL scale seems to blur the distinction between disability (here, the inability to climb stairs or to walk a block) and “pre-disability,” to an uncertain end. It also seems to blend the notion of a state and a syndrome; these points will need to be clarified in due course.
The Tilburg Frailty Indicator14 was constructed around the multidimensional viewpoint of frailty, beyond disease or disability state, to identify frail community-dwelling older individuals. The first part of this two-part questionnaire consists of 10 questions on frailty determinants and medical comorbidities, while the second part contains physical, psychological, and social variables strongly associated with frailty, as well as information about disability in walking and balance. Interestingly, although it includes both social and physical factors, it does not include cognition.
The Clinical Frailty Scale was developed as a practical approach to assess frailty using physical and functional indicators of health and illness burden. The descriptors for this 7-point scale guide clinicians in quantifying the degree of frailty present. It ranges from 1 (very fit) to 7 (severely frail).7 The higher the score, the higher the risks of death or institutionalization. Even mild frailty is associated with a 50% 5-year mortality rate in community-dwelling older adults (Figure 1).8
The Edmonton Frail Scale,15 like the Clinical Frailty Scale, was developed to be practical and usable at the bedside. It is based on the following domains: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and functional performance.
In a community-based sample, the Edmonton Frail Scale compared favorably with the clinical assessment of geriatric specialists who completed a comprehensive evaluation (Pearson’s correlation coefficient 0.64, P < .001).15
FRAILTY AS A PROGNOSTIC INDICATOR
Using frailty scales to aid in prognostication can be useful to clinicians. Survival prognostication is inherently challenging in individuals with multiple comorbidities and variable trajectories of decline, but it remains a vital clinical skill for all clinicians. Framing these difficult discussions in the context of degree of frailty provides a unifying concept, beyond a single-system construct, for care providers, patients, and their loved ones.
Patients nearing the end of their lives need this kind of clarity and support. Regardless of their diagnoses, patients typically want to know when they are at high risk of dying, as do their families and caregivers. People in general look for such information so that they can align medical decision-making congruently with predicted prognosis.16,17 They also use it to plan for the final chapter of their life and their death.
The frailty index is strongly correlated with risk of death
The frailty index is strongly correlated with the risk of death, with a correlation coefficient greater than 0.95. As such, an individual’s frailty index score is considered an estimate of biologic age, which has greater correlation with associated morbidity and death than does chronological age.18,19 In the general population, more than 99% of people have a frailty index value of less than 0.7. As people approach this value, the chance of survival is greatly diminished; indeed, one report suggested that of those who have a frailty index value of more than 0.5 (based on a comprehensive geriatric assessment), 100% are dead by about 20 months later.20,21
In short, there is a limit to which deficits can be added before the system fails. In this sense, the frailty index is akin to the concept of physiologic reserve. Reserve is finite, and as a system loses redundancy it can no longer survive new stresses.
What does this information mean for individual patients?
Even so, prognostication for individual patients remains probabilistic. Any patient has a chance to improve, stabilize, worsen, or die. However, a patient can reach an upper limit of frailty. At that point, instead of accumulating another deficit, death is much more likely. Similarly, although improvement can happen, the chance of improvement is low, and the improvement is typically modest.
Framing survival possibilities in terms of the number of things that people have wrong with them and the chance of death or of change (and the extent of change) makes sense to physicians, patients, and families. Being able to do so offers a much greater opportunity for realistic discussions of the likely outcomes of medical care than the foreseeable scenario of a junior doctor asking a senior citizen, “If your heart stops, do you want us to save your life?”
Understanding prognosis in the face of not just disease but also frailty can also help us focus not on disease but on health consequences of illness. Can the person think? Walk? Care for herself or himself? Interact with others? These questions need to be considered when end-of-life decisions are being discussed.22
Since making predictions about survival is most challenging when multiple comorbidities are present, using the concept of accumulating deficits to better define the slope of decline can be very helpful when discussing “the road ahead” with patients and their families. Visually mapping out the slope of decline and how it is accelerating as conditions progress and deficits accumulate can aid in medical decision-making. Looking individually at the deficits themselves and associated markers of progression can also help with prognostic discussions.
For example, a patient with chronic obstructive pulmonary disease could very well be unaware of the progression and ultimately terminal prognosis of this disease. The slope of clinical decline can be initially shallow, with saw-tooth fluctuations from acute exacerbations that seemingly “resolve to baseline” when antibiotic and steroid courses are completed. Talking with these patients and their families about heralding markers, such as more hospitalizations and cognitive decline with acute exacerbations, can clarify the steepening slope of decline and the way comorbidities interact.
FRAILTY AND END-OF-LIFE CARE
Frailty is progressive, and as it worsens, integrating a palliative approach is appropriate, with a focus on optimizing quality of life and relieving symptoms.4 This principle holds true regardless of the care setting, from acute care hospitals to hospice facilities and long-term care residences.
The principles of end-of-life care are applicable to frail individuals with progressive conditions from the time of diagnosis throughout the course of decline. As the population ages, more people suffer and die from progressive chronic conditions such as cerebrovascular disease, respiratory disease, and dementia.23 An interdisciplinary team approach can ensure all components of palliation are effectively delivered, such as easing symptoms, providing psychosocial and spiritual support, and improving quality of life.24
Pain management
Pain is widely underassessed and undertreated in older patients. Its management at the end of life is particularly challenging if the patient’s language is compromised, as in dementia.23,25,26
A recent cross-sectional analysis of self-reported pain in a longitudinal study of community-dwelling older adults showed an independent association between moderate or higher pain and frailty. The authors propose that persistent pain goes beyond physical discomfort in that it may contribute to homeostenosis (progressive diminishment of homeostatic reserve) and directly worsen frailty.27
Examine the medication list
In palliative care, medical interventions focus on optimizing quality of life.
This especially includes reexamining long medication lists that increase the chance of adverse drug effects.28 Many patients are on disease-modifying medications that may or may not help control symptoms—and might well exacerbate them. For example, betablockers for ischemic heart disease and angiotensin-converting enzyme inhibitors for diabetic nephropathy both can cause hypotension-induced lassitude or even falls due to orthostasis.
A sensible approach is to keep the drugs that may still contribute to quality of life, while discontinuing other drugs that may be causing side effects or that are unlikely to provide meaningful benefit in terms of prevention in patients who have limited life expectancy.29 Discontinuing ineffective, poorly tolerated, and duplicated medications also makes it easier to introduce new medications to manage symptoms—there will be fewer drug interactions, and fewer pills to take, an increasingly important issue in the setting of gastrointestinal symptoms such as dysphagia and gastroparesis or compliance issues as frequently encountered when cognitive impairment is present.29,30
In managing symptoms, start low and go slow—but get there
In managing symptoms in frail elderly patients we use the same classes of medications as in younger patients. The trick is to use appropriate doses.
The concept of “start low and go slow” is key, but so is “get there”—ie, reach the therapeutic goal. The principal drugs for symptom control, such as opioids for pain and dyspnea and anxiolytics for anxiety and restlessness, are associated with a higher rate of and more severe adverse effects in frail older adults.
Even so, most frail older adults appear to be undertreated in this regard, particularly if they are cognitively impaired.23 This fact, coupled with the reality that behavioral symptoms associated with advanced dementia can represent unmet care needs including undertreated pain, highlights the critical need to control symptoms optimally in frail seniors.
This is particularly relevant for those who can no longer verbally articulate their symptoms. Nonverbal pain scales and vigilant assessment of behavioral signs of pain are paramount skills for clinicians providing palliative care to patients with cognitive impairment. Caregivers and loved ones should be included in the assessment process.26,31
Adjunctive therapies for pain control
Maximizing adjunctive therapies can optimize pain control in this drug-sensitive population. Heat and cold packs to affected areas, acupuncture, massage therapy, and structured exercise regimens are some options that can improve quality of life. Cognitive behavioral therapy may offer coping strategies, provided the patient can participate in this process from a cognitive perspective.26,27 Topical preparations are often well tolerated and may include medicinal ingredients that are helpful without systemic effects, such as anti-inflammatory drugs or analgesics.
Optimal use of nonopioid drugs may help reduce the need for narcotics, particularly in the presence of musculoskeletal pain. An example is acetaminophen in regular doses—we would recommend no more than 3 g per day. Acetaminophen is preferred for older adults rather than nonsteroidal anti-inflammatory drugs, given the potential gastric and cardiovascular side effects of the latter medications.
Antidepressants and anticonvulsants such as gabapentin can also be considered as adjuncts for pain control, particularly in the setting of neuropathic pain, with careful monitoring for tolerance.25
When opioids are used, vigilance for constipation is essential.
Establishing goals of care
Goals of care need to be established incrementally along the course of clinical decline and as early as possible so that palliative support can be promptly implemented as symptoms worsen.32
End-of-life care can still include treatments with curative intent, depending on the overall prognosis and the state of the underlying terminal illness. On the other hand, frail older adults who are subjected to invasive treatments that are unlikely to provide cure, such as Whipple surgery, need special intervention postoperatively if they are not to suffer complications such as persisting delirium and functional decline.33
In this regard, geriatric palliative care is frequently about not “crossing a threshold.” Patients may still be receiving active management and be hospitalized for acute exacerbations of progressing chronic conditions, such as chronic obstructive pulmonary disease and heart failure, while palliative principles are introduced and increasingly become the focus of care.
To align goals of care with frailty burden, it is crucial to quantify frailty and to review the patient’s comorbidities. Particularly when dementia is present, lack of communication between the patient’s doctors or between the doctor and the family about disease burden can lead to inappropriately aggressive care.
Many family members and even clinicians do not recognize that advanced dementia is terminal.34,35 In light of this, a palliative approach to care may not even be considered as an appropriate plan when hallmark complications associated with progressing cognitive decline occur, such as aspiration pneumonia or dehydration. Education about dementia and other conditions with progressive organ failure should be done as soon as possible after diagnosis and readdressed at intervals throughout the patient’s clinical decline.
Earlier discussions also ensure that patients themselves can be involved in decision-making more often before cognitive impairment advances to the point where proxy discussions take over.16
BETTER PALLIATIVE CARE FOR ALL
Palliative care, developed initially to provide holistic and timely symptom-based care for patients with noncurable cancer, should also be available and offered to patients with nonmalignant, life-limiting diseases.23,36,37 Meeting this standard of geriatric care is not easy, given the burden of frailty in this population. Needed are multimodal palliative efforts across the spectrum of settings, from the home to the hospital and nursing home.23
To do this, we need to embrace the complexity posed by each person’s presentation and view care through the frailty lens. This will give us a common language in which to engage in a conversation with the same goal in mind: optimizing quality of life.
Furthermore, quantifying frailty can help minimize interventions that are futile or burdensome, that are not expected to ease symptoms, and that can worsen cognition and function. At the end of a patient’s life, we do not want to add to his or her frailty burden but rather minimize the morbidity associated with it.
The concept of frailty assessment is therefore essential for the timely delivery of holistic palliative care in geriatric patients who have progressive and ultimately terminal conditions.
As people get older, they have more things wrong with them. And the more things they have wrong with them, the more likely they are to die. But everyone accumulates deficits at a different rate, and not all people of the same age have the same short-term risk of dying. This variable susceptibility to death and other adverse outcomes in older people of the same age is called frailty.1
Frailty poses special challenges to how we organize and deliver health care. These challenges are sometimes seen most starkly when people are most frail, especially as they approach the end of life.
In this paper, we will review how frailty is conceptualized and defined, consider how frailty affects the care of people at the end of their lives, and suggest practices that can make end-of-life care better for frail older adults.
DEFINING FRAILTY
As with all complex systems, when frail people become acutely unwell their highest-order functions fail first. Thus, cognitive impairment, functional decline, impaired mobility, and social withdrawal are hallmark presentations of the further accumulation of deficits in vulnerable seniors.
Delirium and falls are important clues that a person’s resilience is becoming compromised and that the person is at risk of further insults in a downward spiral or acceleration of things going wrong.1,2 Frailty is associated with poor health outcomes, from disability to institutionalization and death.3
This idea of frailty as vulnerability arising from dysregulation of multiple physiologic systems is reasonably non-controversial. Even so, there are competing views on how to systematically quantify those who are at an increased risk of adverse sequelae.
Quantifying frailty is particularly important if it can tell us if a patient is at high risk of further decline and death. As frailty advances, it is appropriate to shift the focus of care to palliation, with the goal of optimizing quality of life and easing symptoms.4 Identifying someone as frail can aid decision-making in the setting of critical illness, where the system commonly defaults to an “always do everything” mode without considering the ramifications of such an approach. Furthermore, without a routine means of measuring frailty, it is often left to critical care units or rapid-response medical teams to initiate a discussion about whether an aggressive course of care is appropriate or desired.5,6
Frailty as a syndrome
Fried et al7 defined frailty as a syndrome arising from the “physiologic triad” of sarcopenia and immune and neuroendocrine dysregulation. Patients are considered frail if they have three or more of the following five criteria:
- Reduced activity
- Slowing of mobility
- Weight loss
- Diminished handgrip strength
- Exhaustion.
Someone who has only one or two of these items is said to be “pre-frail”; someone with none is said to be “robust.”
The frailty index
An alternative viewpoint is that frailty is a state arising from the accumulation of deficits, which can be counted in a frailty index.
The frailty index is based on the concept that frailty is a consequence of interacting physical, psychological, and social factors. As deficits accumulate, people become increasingly vulnerable to adverse outcomes.
The frailty index is calculated as the number of deficits the patient has, divided by the number of deficits considered. For example, in a frailty index based on a comprehensive geriatric assessment, an individual with impairments in 4 of 10 domains and with 10 of 24 possible comorbidities would have 14 of 34 possible deficits, for a frailty index of 0.41.8
A criticism of the frailty index is that it includes functional dependence as a deficit. The criticism stems from the view that frailty should be seen as occurring prior to disability. According to this view, including dependence in instrumental and basic activities of daily living as a deficit confuses disability with frailty.
Proponents of the frailty index counter that frailty is not “all or none” and needs to be graded. The frailty index can distinguish between people with and without disability by means of the number of deficits that they have, which is most important. For example, a person disabled by a paraplegic injury would have a lower frailty index score and therefore would be considered less frail than a person with advanced cancer affecting multiple body systems. (This is assuming the person who has suffered the injury resulting in paraplegia doesn’t have a concomitant condition such as renal failure or heart disease. In the absence of other health insults, such patients are less at risk of further morbidity or death than the patient with advanced cancer until they get another health insult or insults added to their frailty.)
In any case, functional capacity is fundamental in medical decision-making and when estimating prognoses. An example is the use of the Eastern Cooperative Oncology Group’s functional status measure.9,10
Sum of physical and psychological stressors
Consensus is growing for the concept that frail people are made more vulnerable by the combination of both physical and psychological stressors. This is particularly important to bear in mind for patients who may appear physically robust but whose total health burden makes them vulnerable to further insults.
For example, think of a relatively young overweight patient with hypertension, diabetes, dyslipidemia, and ischemic white matter changes (which can manifest as low mood and even mild vascular cognitive impairment). In such a patient, an acute illness could result in cognitive and functional decline that can be permanent.
Balance of assets and deficits
About 20 years ago, we used the metaphor of a balance beam to describe how frailty comes about in older adults. In this view, there is an interplay of physiological and functional health determinants. Assets such as health, resources, and caregivers are balanced against deficits such as illnesses, dependency on others, and support burden.8
For the most part, later concepts of frailty have focused on the individual, with social factors construed separately as social vulnerability.11
Tools for assessing frailty in people who are not yet disabled
Several tools exist to clinically assess frailty in people who are not yet disabled.
The FRAIL scale.12 The Geriatric Advisory Panel of the International Academy of Nutrition and Aging formulated a scale for measuring frailty as a “pre-disability state.” The FRAIL scale consists of five easily remembered items:
- Fatigue
- Resistance (inability to climb one flight of stairs)
- Ambulation (inability to walk one block)
- Illnesses (more than five)
- Loss of weight (> 5%).
Like the “reduced activity” criterion of the frailty syndrome mentioned above (in practical terms, described as the inability to do heavy household chores),13 the FRAIL scale seems to blur the distinction between disability (here, the inability to climb stairs or to walk a block) and “pre-disability,” to an uncertain end. It also seems to blend the notion of a state and a syndrome; these points will need to be clarified in due course.
The Tilburg Frailty Indicator14 was constructed around the multidimensional viewpoint of frailty, beyond disease or disability state, to identify frail community-dwelling older individuals. The first part of this two-part questionnaire consists of 10 questions on frailty determinants and medical comorbidities, while the second part contains physical, psychological, and social variables strongly associated with frailty, as well as information about disability in walking and balance. Interestingly, although it includes both social and physical factors, it does not include cognition.
The Clinical Frailty Scale was developed as a practical approach to assess frailty using physical and functional indicators of health and illness burden. The descriptors for this 7-point scale guide clinicians in quantifying the degree of frailty present. It ranges from 1 (very fit) to 7 (severely frail).7 The higher the score, the higher the risks of death or institutionalization. Even mild frailty is associated with a 50% 5-year mortality rate in community-dwelling older adults (Figure 1).8
The Edmonton Frail Scale,15 like the Clinical Frailty Scale, was developed to be practical and usable at the bedside. It is based on the following domains: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and functional performance.
In a community-based sample, the Edmonton Frail Scale compared favorably with the clinical assessment of geriatric specialists who completed a comprehensive evaluation (Pearson’s correlation coefficient 0.64, P < .001).15
FRAILTY AS A PROGNOSTIC INDICATOR
Using frailty scales to aid in prognostication can be useful to clinicians. Survival prognostication is inherently challenging in individuals with multiple comorbidities and variable trajectories of decline, but it remains a vital clinical skill for all clinicians. Framing these difficult discussions in the context of degree of frailty provides a unifying concept, beyond a single-system construct, for care providers, patients, and their loved ones.
Patients nearing the end of their lives need this kind of clarity and support. Regardless of their diagnoses, patients typically want to know when they are at high risk of dying, as do their families and caregivers. People in general look for such information so that they can align medical decision-making congruently with predicted prognosis.16,17 They also use it to plan for the final chapter of their life and their death.
The frailty index is strongly correlated with risk of death
The frailty index is strongly correlated with the risk of death, with a correlation coefficient greater than 0.95. As such, an individual’s frailty index score is considered an estimate of biologic age, which has greater correlation with associated morbidity and death than does chronological age.18,19 In the general population, more than 99% of people have a frailty index value of less than 0.7. As people approach this value, the chance of survival is greatly diminished; indeed, one report suggested that of those who have a frailty index value of more than 0.5 (based on a comprehensive geriatric assessment), 100% are dead by about 20 months later.20,21
In short, there is a limit to which deficits can be added before the system fails. In this sense, the frailty index is akin to the concept of physiologic reserve. Reserve is finite, and as a system loses redundancy it can no longer survive new stresses.
What does this information mean for individual patients?
Even so, prognostication for individual patients remains probabilistic. Any patient has a chance to improve, stabilize, worsen, or die. However, a patient can reach an upper limit of frailty. At that point, instead of accumulating another deficit, death is much more likely. Similarly, although improvement can happen, the chance of improvement is low, and the improvement is typically modest.
Framing survival possibilities in terms of the number of things that people have wrong with them and the chance of death or of change (and the extent of change) makes sense to physicians, patients, and families. Being able to do so offers a much greater opportunity for realistic discussions of the likely outcomes of medical care than the foreseeable scenario of a junior doctor asking a senior citizen, “If your heart stops, do you want us to save your life?”
Understanding prognosis in the face of not just disease but also frailty can also help us focus not on disease but on health consequences of illness. Can the person think? Walk? Care for herself or himself? Interact with others? These questions need to be considered when end-of-life decisions are being discussed.22
Since making predictions about survival is most challenging when multiple comorbidities are present, using the concept of accumulating deficits to better define the slope of decline can be very helpful when discussing “the road ahead” with patients and their families. Visually mapping out the slope of decline and how it is accelerating as conditions progress and deficits accumulate can aid in medical decision-making. Looking individually at the deficits themselves and associated markers of progression can also help with prognostic discussions.
For example, a patient with chronic obstructive pulmonary disease could very well be unaware of the progression and ultimately terminal prognosis of this disease. The slope of clinical decline can be initially shallow, with saw-tooth fluctuations from acute exacerbations that seemingly “resolve to baseline” when antibiotic and steroid courses are completed. Talking with these patients and their families about heralding markers, such as more hospitalizations and cognitive decline with acute exacerbations, can clarify the steepening slope of decline and the way comorbidities interact.
FRAILTY AND END-OF-LIFE CARE
Frailty is progressive, and as it worsens, integrating a palliative approach is appropriate, with a focus on optimizing quality of life and relieving symptoms.4 This principle holds true regardless of the care setting, from acute care hospitals to hospice facilities and long-term care residences.
The principles of end-of-life care are applicable to frail individuals with progressive conditions from the time of diagnosis throughout the course of decline. As the population ages, more people suffer and die from progressive chronic conditions such as cerebrovascular disease, respiratory disease, and dementia.23 An interdisciplinary team approach can ensure all components of palliation are effectively delivered, such as easing symptoms, providing psychosocial and spiritual support, and improving quality of life.24
Pain management
Pain is widely underassessed and undertreated in older patients. Its management at the end of life is particularly challenging if the patient’s language is compromised, as in dementia.23,25,26
A recent cross-sectional analysis of self-reported pain in a longitudinal study of community-dwelling older adults showed an independent association between moderate or higher pain and frailty. The authors propose that persistent pain goes beyond physical discomfort in that it may contribute to homeostenosis (progressive diminishment of homeostatic reserve) and directly worsen frailty.27
Examine the medication list
In palliative care, medical interventions focus on optimizing quality of life.
This especially includes reexamining long medication lists that increase the chance of adverse drug effects.28 Many patients are on disease-modifying medications that may or may not help control symptoms—and might well exacerbate them. For example, betablockers for ischemic heart disease and angiotensin-converting enzyme inhibitors for diabetic nephropathy both can cause hypotension-induced lassitude or even falls due to orthostasis.
A sensible approach is to keep the drugs that may still contribute to quality of life, while discontinuing other drugs that may be causing side effects or that are unlikely to provide meaningful benefit in terms of prevention in patients who have limited life expectancy.29 Discontinuing ineffective, poorly tolerated, and duplicated medications also makes it easier to introduce new medications to manage symptoms—there will be fewer drug interactions, and fewer pills to take, an increasingly important issue in the setting of gastrointestinal symptoms such as dysphagia and gastroparesis or compliance issues as frequently encountered when cognitive impairment is present.29,30
In managing symptoms, start low and go slow—but get there
In managing symptoms in frail elderly patients we use the same classes of medications as in younger patients. The trick is to use appropriate doses.
The concept of “start low and go slow” is key, but so is “get there”—ie, reach the therapeutic goal. The principal drugs for symptom control, such as opioids for pain and dyspnea and anxiolytics for anxiety and restlessness, are associated with a higher rate of and more severe adverse effects in frail older adults.
Even so, most frail older adults appear to be undertreated in this regard, particularly if they are cognitively impaired.23 This fact, coupled with the reality that behavioral symptoms associated with advanced dementia can represent unmet care needs including undertreated pain, highlights the critical need to control symptoms optimally in frail seniors.
This is particularly relevant for those who can no longer verbally articulate their symptoms. Nonverbal pain scales and vigilant assessment of behavioral signs of pain are paramount skills for clinicians providing palliative care to patients with cognitive impairment. Caregivers and loved ones should be included in the assessment process.26,31
Adjunctive therapies for pain control
Maximizing adjunctive therapies can optimize pain control in this drug-sensitive population. Heat and cold packs to affected areas, acupuncture, massage therapy, and structured exercise regimens are some options that can improve quality of life. Cognitive behavioral therapy may offer coping strategies, provided the patient can participate in this process from a cognitive perspective.26,27 Topical preparations are often well tolerated and may include medicinal ingredients that are helpful without systemic effects, such as anti-inflammatory drugs or analgesics.
Optimal use of nonopioid drugs may help reduce the need for narcotics, particularly in the presence of musculoskeletal pain. An example is acetaminophen in regular doses—we would recommend no more than 3 g per day. Acetaminophen is preferred for older adults rather than nonsteroidal anti-inflammatory drugs, given the potential gastric and cardiovascular side effects of the latter medications.
Antidepressants and anticonvulsants such as gabapentin can also be considered as adjuncts for pain control, particularly in the setting of neuropathic pain, with careful monitoring for tolerance.25
When opioids are used, vigilance for constipation is essential.
Establishing goals of care
Goals of care need to be established incrementally along the course of clinical decline and as early as possible so that palliative support can be promptly implemented as symptoms worsen.32
End-of-life care can still include treatments with curative intent, depending on the overall prognosis and the state of the underlying terminal illness. On the other hand, frail older adults who are subjected to invasive treatments that are unlikely to provide cure, such as Whipple surgery, need special intervention postoperatively if they are not to suffer complications such as persisting delirium and functional decline.33
In this regard, geriatric palliative care is frequently about not “crossing a threshold.” Patients may still be receiving active management and be hospitalized for acute exacerbations of progressing chronic conditions, such as chronic obstructive pulmonary disease and heart failure, while palliative principles are introduced and increasingly become the focus of care.
To align goals of care with frailty burden, it is crucial to quantify frailty and to review the patient’s comorbidities. Particularly when dementia is present, lack of communication between the patient’s doctors or between the doctor and the family about disease burden can lead to inappropriately aggressive care.
Many family members and even clinicians do not recognize that advanced dementia is terminal.34,35 In light of this, a palliative approach to care may not even be considered as an appropriate plan when hallmark complications associated with progressing cognitive decline occur, such as aspiration pneumonia or dehydration. Education about dementia and other conditions with progressive organ failure should be done as soon as possible after diagnosis and readdressed at intervals throughout the patient’s clinical decline.
Earlier discussions also ensure that patients themselves can be involved in decision-making more often before cognitive impairment advances to the point where proxy discussions take over.16
BETTER PALLIATIVE CARE FOR ALL
Palliative care, developed initially to provide holistic and timely symptom-based care for patients with noncurable cancer, should also be available and offered to patients with nonmalignant, life-limiting diseases.23,36,37 Meeting this standard of geriatric care is not easy, given the burden of frailty in this population. Needed are multimodal palliative efforts across the spectrum of settings, from the home to the hospital and nursing home.23
To do this, we need to embrace the complexity posed by each person’s presentation and view care through the frailty lens. This will give us a common language in which to engage in a conversation with the same goal in mind: optimizing quality of life.
Furthermore, quantifying frailty can help minimize interventions that are futile or burdensome, that are not expected to ease symptoms, and that can worsen cognition and function. At the end of a patient’s life, we do not want to add to his or her frailty burden but rather minimize the morbidity associated with it.
The concept of frailty assessment is therefore essential for the timely delivery of holistic palliative care in geriatric patients who have progressive and ultimately terminal conditions.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Michel JP, Bonin-Guillame S, Gold G, Herrmann F. Cognition and frailty: possible interrelations. In:Carey JR, Robine JM, Michel JP, Christen Y, editors. Longevity and Frailty. Berlin Heidelberg: Springer; 2005:119–124. Research and Perspectives in Longevity.
- Espinoza S, Walston JD. Frailty in older adults: insights and interventions. Cleve Clin J Med 2005; 72:1105–1112.
- Boockvar KS, Meier DE. Palliative care for frail older adults: “there are things I can’t do anymore that I wish I could . . . “. JAMA 2006; 296:2245–2253.
- Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–286.
- McDermid RC, Bagshaw SM. ICU and critical care outreach for the elderly. Best Pract Res Clin Anaesthesiol 2011; 25:439–449.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ 1994; 150:489–495.
- Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 2011; 78:138–149.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS One 2008; 3:e2232.
- Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc 2008; 9:71–72.
- Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res 2011; 23:209–216.
- Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11:344–355.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care 2011; 15:301.
- Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc 2008; 56:898–903.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev 2006; 127:494–496.
- Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc 2010; 58:318–323.
- Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev 2006; 127:840–848.
- Davies E, Higginson IJ; WHO Europe. Better Palliative Care for Older People. Milan, Italy: Tipolitografia Trabella Sr; 2004.
- Raudonis BM, Daniel K. Frailty: an indication for palliative care. Geriatr Nurs 2010; 31:379–384.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1436.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6):S205–S224.
- Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc 2012; 60:113–117.
- Hanlon JT, Perera S, Sevick MA, Rodriguez KL, Jaffe EJ. Pain and its treatment in older nursing home hospice/palliative care residents. J Am Med Dir Assoc 2010; 11:579–583.
- Currow DC, Stevenson JP, Abernethy AP, Plummer J, Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc 2007; 55:590–595.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Kapo J, Morrison LJ, Liao S. Palliative care for the older adult. J Palliat Med 2007; 10:185–209.
- Gaertner J, Wolf J, Frechen S, et al. Recommending early integration of palliative care—does it work? Support Care Cancer 2012; 20:507–513.
- Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011; 213:245–252.
- Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009; 361:1529–1538.
- Arcand M, Monette J, Monette M, et al. Educating nursing home staff about the progression of dementia and the comfort care option: impact on family satisfaction with end-of-life care. J Am Med Dir Assoc 2009; 10:50–55.
- Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004; 164:321–326.
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008; 17:1144–1163.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Michel JP, Bonin-Guillame S, Gold G, Herrmann F. Cognition and frailty: possible interrelations. In:Carey JR, Robine JM, Michel JP, Christen Y, editors. Longevity and Frailty. Berlin Heidelberg: Springer; 2005:119–124. Research and Perspectives in Longevity.
- Espinoza S, Walston JD. Frailty in older adults: insights and interventions. Cleve Clin J Med 2005; 72:1105–1112.
- Boockvar KS, Meier DE. Palliative care for frail older adults: “there are things I can’t do anymore that I wish I could . . . “. JAMA 2006; 296:2245–2253.
- Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–286.
- McDermid RC, Bagshaw SM. ICU and critical care outreach for the elderly. Best Pract Res Clin Anaesthesiol 2011; 25:439–449.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ 1994; 150:489–495.
- Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 2011; 78:138–149.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS One 2008; 3:e2232.
- Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc 2008; 9:71–72.
- Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res 2011; 23:209–216.
- Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11:344–355.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care 2011; 15:301.
- Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc 2008; 56:898–903.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev 2006; 127:494–496.
- Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc 2010; 58:318–323.
- Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev 2006; 127:840–848.
- Davies E, Higginson IJ; WHO Europe. Better Palliative Care for Older People. Milan, Italy: Tipolitografia Trabella Sr; 2004.
- Raudonis BM, Daniel K. Frailty: an indication for palliative care. Geriatr Nurs 2010; 31:379–384.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1436.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6):S205–S224.
- Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc 2012; 60:113–117.
- Hanlon JT, Perera S, Sevick MA, Rodriguez KL, Jaffe EJ. Pain and its treatment in older nursing home hospice/palliative care residents. J Am Med Dir Assoc 2010; 11:579–583.
- Currow DC, Stevenson JP, Abernethy AP, Plummer J, Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc 2007; 55:590–595.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Kapo J, Morrison LJ, Liao S. Palliative care for the older adult. J Palliat Med 2007; 10:185–209.
- Gaertner J, Wolf J, Frechen S, et al. Recommending early integration of palliative care—does it work? Support Care Cancer 2012; 20:507–513.
- Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011; 213:245–252.
- Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009; 361:1529–1538.
- Arcand M, Monette J, Monette M, et al. Educating nursing home staff about the progression of dementia and the comfort care option: impact on family satisfaction with end-of-life care. J Am Med Dir Assoc 2009; 10:50–55.
- Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004; 164:321–326.
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008; 17:1144–1163.
KEY POINTS
- Frail older adults are more susceptible to delirium, functional decline, impaired mobility, falls, social withdrawal, and death.
- Evaluating the health care needs of people who are frail requires assessment of their cognition, function, mobility, balance, and social circumstances, in addition to understanding their medical problems.
- When people are so frail that they cannot withstand interventions that can cause significant injury, such as surgery or chemotherapy, then appropriate end-of-life care should focus on maintaining their highest-order functions.
- End-of-life care can include curative treatments of some episodes if they threaten cognition, mobility, or function or cause pain and suffering, even in the context of an overall palliative care plan.