User login
2022 Update on abnormal uterine bleeding
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
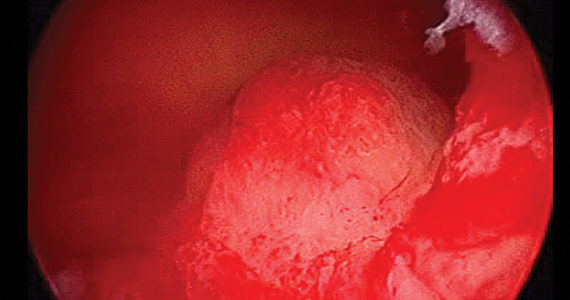
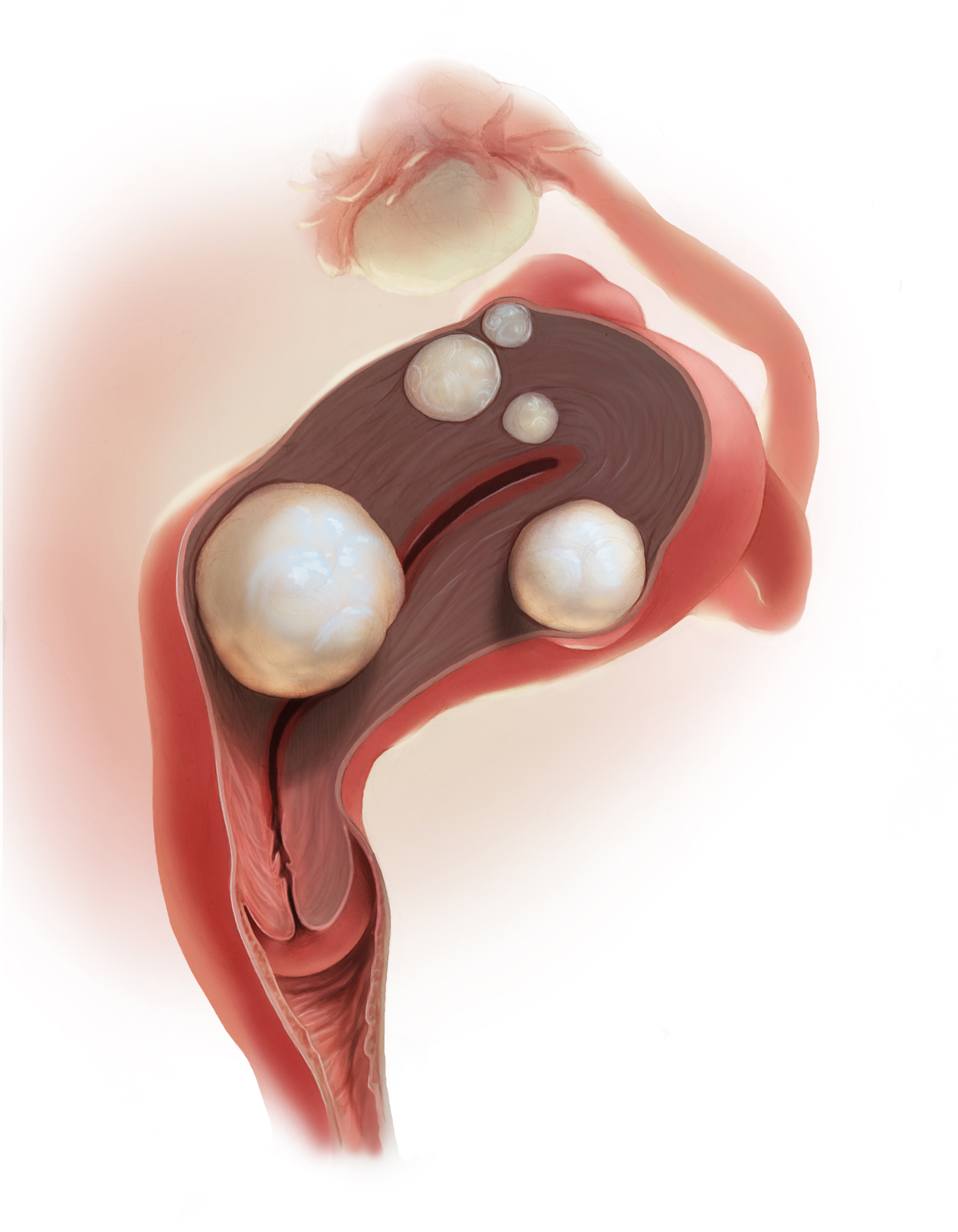
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
2021 Update on abnormal uterine bleeding
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
2019 Update on abnormal uterine bleeding
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
2018 Update on abnormal uterine bleeding
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.