User login
Patients Get New Rights to Appeal Insurance Decisions
New federal regulations mandated by the Affordable Care Act will give patients new rights to appeal claims denials made by their health plans.
The rules will allow consumers in new health plans to appeal decisions both through their insurer's internal process and to an outside, independent entity. Most health plans already provide for an internal appeals process, but not all offer an external review of plan decisions, according to the U.S. Department of Health and Human Services. The types of appeals processes often depend on individual state laws.
HHS officials estimate that in 2011 about 31 million people in new employer plans and another 10 million people in new individual market plans will be able to take advantage of these new appeals opportunities. By 2013, that number is expected to grow to 88 million. The rules do not apply to grandfathered health plans.
Under the new rules, health plans that begin on or after Sept. 23, 2010, must have an internal appeals process that allows consumers to appeal whenever the plan denies a claim for a covered service or rescinds coverage. The internal appeals process must also offer consumers detailed information about the grounds for their denial and information on how to file an appeal.
The new rules aim to make internal appeals more objective by ensuring that the person considering the appeal does not have a conflict of interest. For example, the health plan is not allowed to offer financial incentives to employees based on the number of claims that are denied. Health plans will also have to provide an expedited appeals process, which would allow urgent cases to be reviewed within 24 hours.
The new federal appeals regulations also standardize rules for external appeals. Currently, 44 states require health plans to have some type of external appeal but those processes vary greatly, according to HHS. Under the federal rules, health plans must provide clear information about external appeals and expedited access to the process. The decisions made through external appeals are binding under the new federal rules.
New federal regulations mandated by the Affordable Care Act will give patients new rights to appeal claims denials made by their health plans.
The rules will allow consumers in new health plans to appeal decisions both through their insurer's internal process and to an outside, independent entity. Most health plans already provide for an internal appeals process, but not all offer an external review of plan decisions, according to the U.S. Department of Health and Human Services. The types of appeals processes often depend on individual state laws.
HHS officials estimate that in 2011 about 31 million people in new employer plans and another 10 million people in new individual market plans will be able to take advantage of these new appeals opportunities. By 2013, that number is expected to grow to 88 million. The rules do not apply to grandfathered health plans.
Under the new rules, health plans that begin on or after Sept. 23, 2010, must have an internal appeals process that allows consumers to appeal whenever the plan denies a claim for a covered service or rescinds coverage. The internal appeals process must also offer consumers detailed information about the grounds for their denial and information on how to file an appeal.
The new rules aim to make internal appeals more objective by ensuring that the person considering the appeal does not have a conflict of interest. For example, the health plan is not allowed to offer financial incentives to employees based on the number of claims that are denied. Health plans will also have to provide an expedited appeals process, which would allow urgent cases to be reviewed within 24 hours.
The new federal appeals regulations also standardize rules for external appeals. Currently, 44 states require health plans to have some type of external appeal but those processes vary greatly, according to HHS. Under the federal rules, health plans must provide clear information about external appeals and expedited access to the process. The decisions made through external appeals are binding under the new federal rules.
New federal regulations mandated by the Affordable Care Act will give patients new rights to appeal claims denials made by their health plans.
The rules will allow consumers in new health plans to appeal decisions both through their insurer's internal process and to an outside, independent entity. Most health plans already provide for an internal appeals process, but not all offer an external review of plan decisions, according to the U.S. Department of Health and Human Services. The types of appeals processes often depend on individual state laws.
HHS officials estimate that in 2011 about 31 million people in new employer plans and another 10 million people in new individual market plans will be able to take advantage of these new appeals opportunities. By 2013, that number is expected to grow to 88 million. The rules do not apply to grandfathered health plans.
Under the new rules, health plans that begin on or after Sept. 23, 2010, must have an internal appeals process that allows consumers to appeal whenever the plan denies a claim for a covered service or rescinds coverage. The internal appeals process must also offer consumers detailed information about the grounds for their denial and information on how to file an appeal.
The new rules aim to make internal appeals more objective by ensuring that the person considering the appeal does not have a conflict of interest. For example, the health plan is not allowed to offer financial incentives to employees based on the number of claims that are denied. Health plans will also have to provide an expedited appeals process, which would allow urgent cases to be reviewed within 24 hours.
The new federal appeals regulations also standardize rules for external appeals. Currently, 44 states require health plans to have some type of external appeal but those processes vary greatly, according to HHS. Under the federal rules, health plans must provide clear information about external appeals and expedited access to the process. The decisions made through external appeals are binding under the new federal rules.
Feds Release Final Meaningful Use Standards
The federal government has released the much-anticipated requirements for how physicians and hospitals can qualify for tens of thousands of dollars in incentive payments to adopt and use electronic health records.
The final rule on the meaningful use of electronic health records (EHRs) eases many of the requirements that officials in the Health and Human Services department had outlined in a proposal published in January. Physician organizations had objected to the initial proposal, saying that it asked doctors to do too much too quickly.
Physicians were also critical of the all or nothing framework of the proposal, which required them to meet all 25 objectives for meaningful use or lose out on incentive payments.
Federal officials aimed to address those concerns in the final rule by requiring physicians to first meet a core set of 15 requirements and then meet any 5 of 10 additional requirements. The core set includes requirements such as recording patient demographics and vital signs in the EHR and maintaining an up-to-date problem list and an active list of medications and allergies.
“We very much want well-intentioned providers to become meaningful users,” Dr. David Blumenthal, National Coordinator for Health Information Technology at HHS, said during a press briefing to announce the final rule.
HHS officials also relaxed some of the thresholds related to the requirements. For example, under the proposed rule, physicians would have had to generate and transmit 75% of their permissible prescriptions electronically to meet the e-prescribing requirement. Under the final rule, the threshold has been lowered to more than 40% of permissible prescriptions, Dr. Blumenthal said.
The final rule also creates an easier path for physicians to meet meaningful use requirements on electronic reporting of quality data. Under the final rule, physicians will need to report data on blood pressure, tobacco status, and adult weight screening, and follow-up in 2011 and 2012, in order to qualify.
The final rule outlines steps physicians must take in 2011 and 2012 to quality for the maximum incentive payments through Medicare and Medicaid. The incentives were mandated by the Health Information Technology for Economic and Clinical Health Act (HITECH), a part of 2009's American Recovery Act.
Starting in 2011, physicians who show meaningful use of certified EHRs can receive payments of up to $18,000 from Medicare. Those bonuses continue for 5 years, with physicians eligible to earn up to $44,000 in total incentives.
The federal government has released the much-anticipated requirements for how physicians and hospitals can qualify for tens of thousands of dollars in incentive payments to adopt and use electronic health records.
The final rule on the meaningful use of electronic health records (EHRs) eases many of the requirements that officials in the Health and Human Services department had outlined in a proposal published in January. Physician organizations had objected to the initial proposal, saying that it asked doctors to do too much too quickly.
Physicians were also critical of the all or nothing framework of the proposal, which required them to meet all 25 objectives for meaningful use or lose out on incentive payments.
Federal officials aimed to address those concerns in the final rule by requiring physicians to first meet a core set of 15 requirements and then meet any 5 of 10 additional requirements. The core set includes requirements such as recording patient demographics and vital signs in the EHR and maintaining an up-to-date problem list and an active list of medications and allergies.
“We very much want well-intentioned providers to become meaningful users,” Dr. David Blumenthal, National Coordinator for Health Information Technology at HHS, said during a press briefing to announce the final rule.
HHS officials also relaxed some of the thresholds related to the requirements. For example, under the proposed rule, physicians would have had to generate and transmit 75% of their permissible prescriptions electronically to meet the e-prescribing requirement. Under the final rule, the threshold has been lowered to more than 40% of permissible prescriptions, Dr. Blumenthal said.
The final rule also creates an easier path for physicians to meet meaningful use requirements on electronic reporting of quality data. Under the final rule, physicians will need to report data on blood pressure, tobacco status, and adult weight screening, and follow-up in 2011 and 2012, in order to qualify.
The final rule outlines steps physicians must take in 2011 and 2012 to quality for the maximum incentive payments through Medicare and Medicaid. The incentives were mandated by the Health Information Technology for Economic and Clinical Health Act (HITECH), a part of 2009's American Recovery Act.
Starting in 2011, physicians who show meaningful use of certified EHRs can receive payments of up to $18,000 from Medicare. Those bonuses continue for 5 years, with physicians eligible to earn up to $44,000 in total incentives.
The federal government has released the much-anticipated requirements for how physicians and hospitals can qualify for tens of thousands of dollars in incentive payments to adopt and use electronic health records.
The final rule on the meaningful use of electronic health records (EHRs) eases many of the requirements that officials in the Health and Human Services department had outlined in a proposal published in January. Physician organizations had objected to the initial proposal, saying that it asked doctors to do too much too quickly.
Physicians were also critical of the all or nothing framework of the proposal, which required them to meet all 25 objectives for meaningful use or lose out on incentive payments.
Federal officials aimed to address those concerns in the final rule by requiring physicians to first meet a core set of 15 requirements and then meet any 5 of 10 additional requirements. The core set includes requirements such as recording patient demographics and vital signs in the EHR and maintaining an up-to-date problem list and an active list of medications and allergies.
“We very much want well-intentioned providers to become meaningful users,” Dr. David Blumenthal, National Coordinator for Health Information Technology at HHS, said during a press briefing to announce the final rule.
HHS officials also relaxed some of the thresholds related to the requirements. For example, under the proposed rule, physicians would have had to generate and transmit 75% of their permissible prescriptions electronically to meet the e-prescribing requirement. Under the final rule, the threshold has been lowered to more than 40% of permissible prescriptions, Dr. Blumenthal said.
The final rule also creates an easier path for physicians to meet meaningful use requirements on electronic reporting of quality data. Under the final rule, physicians will need to report data on blood pressure, tobacco status, and adult weight screening, and follow-up in 2011 and 2012, in order to qualify.
The final rule outlines steps physicians must take in 2011 and 2012 to quality for the maximum incentive payments through Medicare and Medicaid. The incentives were mandated by the Health Information Technology for Economic and Clinical Health Act (HITECH), a part of 2009's American Recovery Act.
Starting in 2011, physicians who show meaningful use of certified EHRs can receive payments of up to $18,000 from Medicare. Those bonuses continue for 5 years, with physicians eligible to earn up to $44,000 in total incentives.
Proposal Tightens Privacy Protection
Patients could gain greater access to their health information and have more power to limit disclosures of certain personal information to health plans under a new proposal from the Health and Human Services department.
The new requirements are aimed at beefing up privacy and security, as the Obama administration pushes to get more physicians using electronic health records over the next few years.
“The benefits of health IT can only be fully realized if patients and providers are confident that electronic health information is kept private and secure at all times,” Georgina Verdugo, director of the HHS Office for Civil Rights, said in a statement. “This proposed rule … is an integral piece of the administration's efforts to broaden the use of health information technology in health care today.”
The proposal alters the Health Insurance Portability and Accountability Act (HIPAA) rules by setting new limits on the use of disclosure of protected health information for marketing and fundraising and by requiring business associates of HIPAA-covered entities to follow most of the same rules that covered entities follow. The proposal would also bar the sale of protected health information without explicit authorization from the patient.
The proposal also implements elements of the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act, which requires physicians and other covered entities to grant patient requests to restrict certain information from their health plans. For example, the proposed rule states that patients must be allowed to restrict protected health information if that information is related only to a service for which the patient paid in full and the information is not otherwise required by law to be reported.
Individuals can provide comments on the rule for 60 days, beginning on July 14.
Patients could gain greater access to their health information and have more power to limit disclosures of certain personal information to health plans under a new proposal from the Health and Human Services department.
The new requirements are aimed at beefing up privacy and security, as the Obama administration pushes to get more physicians using electronic health records over the next few years.
“The benefits of health IT can only be fully realized if patients and providers are confident that electronic health information is kept private and secure at all times,” Georgina Verdugo, director of the HHS Office for Civil Rights, said in a statement. “This proposed rule … is an integral piece of the administration's efforts to broaden the use of health information technology in health care today.”
The proposal alters the Health Insurance Portability and Accountability Act (HIPAA) rules by setting new limits on the use of disclosure of protected health information for marketing and fundraising and by requiring business associates of HIPAA-covered entities to follow most of the same rules that covered entities follow. The proposal would also bar the sale of protected health information without explicit authorization from the patient.
The proposal also implements elements of the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act, which requires physicians and other covered entities to grant patient requests to restrict certain information from their health plans. For example, the proposed rule states that patients must be allowed to restrict protected health information if that information is related only to a service for which the patient paid in full and the information is not otherwise required by law to be reported.
Individuals can provide comments on the rule for 60 days, beginning on July 14.
Patients could gain greater access to their health information and have more power to limit disclosures of certain personal information to health plans under a new proposal from the Health and Human Services department.
The new requirements are aimed at beefing up privacy and security, as the Obama administration pushes to get more physicians using electronic health records over the next few years.
“The benefits of health IT can only be fully realized if patients and providers are confident that electronic health information is kept private and secure at all times,” Georgina Verdugo, director of the HHS Office for Civil Rights, said in a statement. “This proposed rule … is an integral piece of the administration's efforts to broaden the use of health information technology in health care today.”
The proposal alters the Health Insurance Portability and Accountability Act (HIPAA) rules by setting new limits on the use of disclosure of protected health information for marketing and fundraising and by requiring business associates of HIPAA-covered entities to follow most of the same rules that covered entities follow. The proposal would also bar the sale of protected health information without explicit authorization from the patient.
The proposal also implements elements of the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act, which requires physicians and other covered entities to grant patient requests to restrict certain information from their health plans. For example, the proposed rule states that patients must be allowed to restrict protected health information if that information is related only to a service for which the patient paid in full and the information is not otherwise required by law to be reported.
Individuals can provide comments on the rule for 60 days, beginning on July 14.
Specialists Affected by Loss Of Consultation Billing
Medicare's decision to eliminate consultation codes has resulted in a loss of revenue for many physicians and forced some to cut back on appointments with Medicare beneficiaries, according to a survey commissioned by the American Medical Association and several other medical specialty societies.
In January, officials at the Centers for Medicare and Medicaid Services discontinued the use of inpatient and outpatient consultation codes when billing Medicare, except for telehealth codes. Physicians instead were asked to use new or established office visit codes, initial hospital care codes, or initial nursing facility care codes.
At the time of the policy change, CMS officials said they could no longer justify paying physicians more for a consultation when they had reduced so much of the documentation required to bill for a consultation. The agency also said that eliminating consultation codes would reduce the confusion around the differing definitions of consultations, transfers, and referrals.
But according to many specialists, the approach is flawed and is hurting both their bottom line and patient access to care.
In an online survey of approximately 5,500 physicians, about 72% said that not being able to bill for consultations had decreased their total revenues by more than 5%, with about 30% reporting that their revenues had fallen more than 15%.
The loss of revenue has in turn impacted physicians' practices. For example, 20% of respondents said they have already reduced the number of new Medicare patients seen in their practices. Additionally, 39% said they will hold off on purchasing new equipment or health information technology.
The policy change may also undermine efforts to improve care coordination. About 6% of responding physicians said they have stopped providing primary care physicians with written reports following consults with Medicare patients, and another 19% said they plan to do so.
“Patient health is best managed when physicians can work together across specialties to coordinate care,” Dr. J. James Rohack, AMA immediate past president, said in a statement.
“Twenty percent of patients over age 65 live with five or more chronic illnesses, and managing their care frequently requires primary care physicians to consult with a physician who specializes in the medical or surgical care of their conditions. CMS's new policy eliminating Medicare consultation codes fails to adequately recognize the additional time and effort involved in these consultations and limits physicians' ability to work together as a comprehensive health care team for their patients,” he said.
In a letter to CMS, officials from more than 30 medical specialty societies, including the American Academy of Dermatology Association, the American College of Gastroenterology, the American Gastroenterological Association, the American Geriatrics Society, the American Society of Clinical Oncology, the American Society for Gastrointestinal Endoscopy, and the American College of Physicians, urged the agency to revise the policy when they issue a final regulation on the 2011 Medicare Physician Fee Schedule this fall.
The organizations suggested that CMS consider paying consulting physicians for providing the referring physician with a comprehensive report. They also said CMS could ease some of the financial pressure on physicians by revising its guidelines for prolonged visits to allow for reimbursement for services provided outside of the face-to-face visit, such as reviewing charts and communicating with families and other health care providers.
Elsevier Global Medical News
Medicare's decision to eliminate consultation codes has resulted in a loss of revenue for many physicians and forced some to cut back on appointments with Medicare beneficiaries, according to a survey commissioned by the American Medical Association and several other medical specialty societies.
In January, officials at the Centers for Medicare and Medicaid Services discontinued the use of inpatient and outpatient consultation codes when billing Medicare, except for telehealth codes. Physicians instead were asked to use new or established office visit codes, initial hospital care codes, or initial nursing facility care codes.
At the time of the policy change, CMS officials said they could no longer justify paying physicians more for a consultation when they had reduced so much of the documentation required to bill for a consultation. The agency also said that eliminating consultation codes would reduce the confusion around the differing definitions of consultations, transfers, and referrals.
But according to many specialists, the approach is flawed and is hurting both their bottom line and patient access to care.
In an online survey of approximately 5,500 physicians, about 72% said that not being able to bill for consultations had decreased their total revenues by more than 5%, with about 30% reporting that their revenues had fallen more than 15%.
The loss of revenue has in turn impacted physicians' practices. For example, 20% of respondents said they have already reduced the number of new Medicare patients seen in their practices. Additionally, 39% said they will hold off on purchasing new equipment or health information technology.
The policy change may also undermine efforts to improve care coordination. About 6% of responding physicians said they have stopped providing primary care physicians with written reports following consults with Medicare patients, and another 19% said they plan to do so.
“Patient health is best managed when physicians can work together across specialties to coordinate care,” Dr. J. James Rohack, AMA immediate past president, said in a statement.
“Twenty percent of patients over age 65 live with five or more chronic illnesses, and managing their care frequently requires primary care physicians to consult with a physician who specializes in the medical or surgical care of their conditions. CMS's new policy eliminating Medicare consultation codes fails to adequately recognize the additional time and effort involved in these consultations and limits physicians' ability to work together as a comprehensive health care team for their patients,” he said.
In a letter to CMS, officials from more than 30 medical specialty societies, including the American Academy of Dermatology Association, the American College of Gastroenterology, the American Gastroenterological Association, the American Geriatrics Society, the American Society of Clinical Oncology, the American Society for Gastrointestinal Endoscopy, and the American College of Physicians, urged the agency to revise the policy when they issue a final regulation on the 2011 Medicare Physician Fee Schedule this fall.
The organizations suggested that CMS consider paying consulting physicians for providing the referring physician with a comprehensive report. They also said CMS could ease some of the financial pressure on physicians by revising its guidelines for prolonged visits to allow for reimbursement for services provided outside of the face-to-face visit, such as reviewing charts and communicating with families and other health care providers.
Elsevier Global Medical News
Medicare's decision to eliminate consultation codes has resulted in a loss of revenue for many physicians and forced some to cut back on appointments with Medicare beneficiaries, according to a survey commissioned by the American Medical Association and several other medical specialty societies.
In January, officials at the Centers for Medicare and Medicaid Services discontinued the use of inpatient and outpatient consultation codes when billing Medicare, except for telehealth codes. Physicians instead were asked to use new or established office visit codes, initial hospital care codes, or initial nursing facility care codes.
At the time of the policy change, CMS officials said they could no longer justify paying physicians more for a consultation when they had reduced so much of the documentation required to bill for a consultation. The agency also said that eliminating consultation codes would reduce the confusion around the differing definitions of consultations, transfers, and referrals.
But according to many specialists, the approach is flawed and is hurting both their bottom line and patient access to care.
In an online survey of approximately 5,500 physicians, about 72% said that not being able to bill for consultations had decreased their total revenues by more than 5%, with about 30% reporting that their revenues had fallen more than 15%.
The loss of revenue has in turn impacted physicians' practices. For example, 20% of respondents said they have already reduced the number of new Medicare patients seen in their practices. Additionally, 39% said they will hold off on purchasing new equipment or health information technology.
The policy change may also undermine efforts to improve care coordination. About 6% of responding physicians said they have stopped providing primary care physicians with written reports following consults with Medicare patients, and another 19% said they plan to do so.
“Patient health is best managed when physicians can work together across specialties to coordinate care,” Dr. J. James Rohack, AMA immediate past president, said in a statement.
“Twenty percent of patients over age 65 live with five or more chronic illnesses, and managing their care frequently requires primary care physicians to consult with a physician who specializes in the medical or surgical care of their conditions. CMS's new policy eliminating Medicare consultation codes fails to adequately recognize the additional time and effort involved in these consultations and limits physicians' ability to work together as a comprehensive health care team for their patients,” he said.
In a letter to CMS, officials from more than 30 medical specialty societies, including the American Academy of Dermatology Association, the American College of Gastroenterology, the American Gastroenterological Association, the American Geriatrics Society, the American Society of Clinical Oncology, the American Society for Gastrointestinal Endoscopy, and the American College of Physicians, urged the agency to revise the policy when they issue a final regulation on the 2011 Medicare Physician Fee Schedule this fall.
The organizations suggested that CMS consider paying consulting physicians for providing the referring physician with a comprehensive report. They also said CMS could ease some of the financial pressure on physicians by revising its guidelines for prolonged visits to allow for reimbursement for services provided outside of the face-to-face visit, such as reviewing charts and communicating with families and other health care providers.
Elsevier Global Medical News
Program Encourages Palliative Care Techniques in the ICU
Critical care and palliative care may seem like opposing concepts, but experts in both fields say bringing palliative care techniques into the intensive care unit can decrease costs and improve patient satisfaction.
A new project launched in partnership with the Center to Advance Palliative Care aims to jump-start the integration of palliative care techniques into ICU programs by providing a slew of online tools and resources.
The IPAL-ICU Project (www.capc.org/ipal-icu
The first step for anyone considering introducing palliative care into the ICU is to make the case to the multidisciplinary critical care team and to hospital leaders, said Dr. Judith E. Nelson, the project director for the IPAL-ICU Project and a professor of medicine at Mount Sinai School of Medicine in New York City. But it's not a difficult case to make, she said.
“There is absolutely no downside here,” Dr. Nelson said. “There is enhanced care and satisfaction for everyone involved, and efficiencies for the institution and the health care system as a whole. It's really a win across the system, and there aren't that many places or strategies in health care that we can say that about.”
Research shows that the use of palliative care consultation programs has resulted in cost savings throughout hospitals, including reductions in ICU costs (Arch. Intern. Med. 2008;168:1783-90). Those savings aren't achieved by increasing mortality, Dr. Nelson said. Instead, the better communication fostered by using palliative care strategies results in a reduced use of nonbeneficial ICU treatments and even a decreased length of stay. “It cuts back on delay and improves communication,” Dr. Nelson said.
The biggest barrier is convincing people to let go of the old model of sequential care, Dr. Nelson said. In that model, a patient receives aggressive care in the ICU and, when that is exhausted, moves to palliative care in a hospice setting.
There's a fear that the introduction of palliative care early on means that the intensive care will somehow be diminished, she noted. “That's not necessary, and it's not optimal,” Dr. Nelson explained. When done right, palliative care should support an aggressive care plan by making sure it is tailored to the patient's needs and desires. Palliative care can also help identify untreated pain and other symptoms.
Over the last decade, palliative care programs in general have spread across the country and increasingly been embraced by physicians. Dr. Nelson said she hopes that palliative care in the ICU setting will have similar success.
The concept of palliative care in the ICU is already catching on, noted Dr. J. Randall Curtis, professor of medicine at the University of Washington and head of the section of pulmonary and critical care medicine at Harborview Medical Center in Seattle. Just a few years ago, many people thought the very idea was crazy, he said—but he doesn't hear that anymore.
“I think people are still struggling with how to do it well, but I think there's a common acceptance that this is an important part of critical care,” said Dr. Curtis, who is a member of the IPAL-ICU advisory board.
Still, ICUs can be a difficult place to integrate palliative care, he cautioned.
For starters, critical care units are busy places. Physicians and nurses working there need to balance considerations such as providing supportive palliative care against the need to focus on reducing central line infections and using ventilators appropriately. In addition, palliative care isn't the primary goal in the ICU—so the clinicians there need training on how to provide both types of care.
Another potential pitfall can be a “clash of cultures” between the ICU team and palliative care consultants, Dr. Curtis said. Palliative care specialists need to learn about the culture of the ICU, or they risk coming in with the attitude that the critical care team is being overly aggressive in their approach to some patients. That can happen if they don't understand the outcomes of conditions commonly treated in the ICU.
Palliative and critical care teams need to operate on the same page, agreed Dr. Daniel E. Ray, director of the palliative medicine fellowship program at the Lehigh Valley Health Network in Allentown, Pa., and a member of the advisory board for the IPAL-ICU Project. Otherwise, it opens up the possibility that patients and families could receive conflicting recommendations from providers.
The IPAL-ICU Project resources should go a long way to helping institutions get started on the concept. However, he cautioned that the resources should be customized to the unique culture of each hospital, and that leaders need to work on getting buy-in from everyone on the team to ensure that the templates are actually used.
Critical care and palliative care may seem like opposing concepts, but experts in both fields say bringing palliative care techniques into the intensive care unit can decrease costs and improve patient satisfaction.
A new project launched in partnership with the Center to Advance Palliative Care aims to jump-start the integration of palliative care techniques into ICU programs by providing a slew of online tools and resources.
The IPAL-ICU Project (www.capc.org/ipal-icu
The first step for anyone considering introducing palliative care into the ICU is to make the case to the multidisciplinary critical care team and to hospital leaders, said Dr. Judith E. Nelson, the project director for the IPAL-ICU Project and a professor of medicine at Mount Sinai School of Medicine in New York City. But it's not a difficult case to make, she said.
“There is absolutely no downside here,” Dr. Nelson said. “There is enhanced care and satisfaction for everyone involved, and efficiencies for the institution and the health care system as a whole. It's really a win across the system, and there aren't that many places or strategies in health care that we can say that about.”
Research shows that the use of palliative care consultation programs has resulted in cost savings throughout hospitals, including reductions in ICU costs (Arch. Intern. Med. 2008;168:1783-90). Those savings aren't achieved by increasing mortality, Dr. Nelson said. Instead, the better communication fostered by using palliative care strategies results in a reduced use of nonbeneficial ICU treatments and even a decreased length of stay. “It cuts back on delay and improves communication,” Dr. Nelson said.
The biggest barrier is convincing people to let go of the old model of sequential care, Dr. Nelson said. In that model, a patient receives aggressive care in the ICU and, when that is exhausted, moves to palliative care in a hospice setting.
There's a fear that the introduction of palliative care early on means that the intensive care will somehow be diminished, she noted. “That's not necessary, and it's not optimal,” Dr. Nelson explained. When done right, palliative care should support an aggressive care plan by making sure it is tailored to the patient's needs and desires. Palliative care can also help identify untreated pain and other symptoms.
Over the last decade, palliative care programs in general have spread across the country and increasingly been embraced by physicians. Dr. Nelson said she hopes that palliative care in the ICU setting will have similar success.
The concept of palliative care in the ICU is already catching on, noted Dr. J. Randall Curtis, professor of medicine at the University of Washington and head of the section of pulmonary and critical care medicine at Harborview Medical Center in Seattle. Just a few years ago, many people thought the very idea was crazy, he said—but he doesn't hear that anymore.
“I think people are still struggling with how to do it well, but I think there's a common acceptance that this is an important part of critical care,” said Dr. Curtis, who is a member of the IPAL-ICU advisory board.
Still, ICUs can be a difficult place to integrate palliative care, he cautioned.
For starters, critical care units are busy places. Physicians and nurses working there need to balance considerations such as providing supportive palliative care against the need to focus on reducing central line infections and using ventilators appropriately. In addition, palliative care isn't the primary goal in the ICU—so the clinicians there need training on how to provide both types of care.
Another potential pitfall can be a “clash of cultures” between the ICU team and palliative care consultants, Dr. Curtis said. Palliative care specialists need to learn about the culture of the ICU, or they risk coming in with the attitude that the critical care team is being overly aggressive in their approach to some patients. That can happen if they don't understand the outcomes of conditions commonly treated in the ICU.
Palliative and critical care teams need to operate on the same page, agreed Dr. Daniel E. Ray, director of the palliative medicine fellowship program at the Lehigh Valley Health Network in Allentown, Pa., and a member of the advisory board for the IPAL-ICU Project. Otherwise, it opens up the possibility that patients and families could receive conflicting recommendations from providers.
The IPAL-ICU Project resources should go a long way to helping institutions get started on the concept. However, he cautioned that the resources should be customized to the unique culture of each hospital, and that leaders need to work on getting buy-in from everyone on the team to ensure that the templates are actually used.
Critical care and palliative care may seem like opposing concepts, but experts in both fields say bringing palliative care techniques into the intensive care unit can decrease costs and improve patient satisfaction.
A new project launched in partnership with the Center to Advance Palliative Care aims to jump-start the integration of palliative care techniques into ICU programs by providing a slew of online tools and resources.
The IPAL-ICU Project (www.capc.org/ipal-icu
The first step for anyone considering introducing palliative care into the ICU is to make the case to the multidisciplinary critical care team and to hospital leaders, said Dr. Judith E. Nelson, the project director for the IPAL-ICU Project and a professor of medicine at Mount Sinai School of Medicine in New York City. But it's not a difficult case to make, she said.
“There is absolutely no downside here,” Dr. Nelson said. “There is enhanced care and satisfaction for everyone involved, and efficiencies for the institution and the health care system as a whole. It's really a win across the system, and there aren't that many places or strategies in health care that we can say that about.”
Research shows that the use of palliative care consultation programs has resulted in cost savings throughout hospitals, including reductions in ICU costs (Arch. Intern. Med. 2008;168:1783-90). Those savings aren't achieved by increasing mortality, Dr. Nelson said. Instead, the better communication fostered by using palliative care strategies results in a reduced use of nonbeneficial ICU treatments and even a decreased length of stay. “It cuts back on delay and improves communication,” Dr. Nelson said.
The biggest barrier is convincing people to let go of the old model of sequential care, Dr. Nelson said. In that model, a patient receives aggressive care in the ICU and, when that is exhausted, moves to palliative care in a hospice setting.
There's a fear that the introduction of palliative care early on means that the intensive care will somehow be diminished, she noted. “That's not necessary, and it's not optimal,” Dr. Nelson explained. When done right, palliative care should support an aggressive care plan by making sure it is tailored to the patient's needs and desires. Palliative care can also help identify untreated pain and other symptoms.
Over the last decade, palliative care programs in general have spread across the country and increasingly been embraced by physicians. Dr. Nelson said she hopes that palliative care in the ICU setting will have similar success.
The concept of palliative care in the ICU is already catching on, noted Dr. J. Randall Curtis, professor of medicine at the University of Washington and head of the section of pulmonary and critical care medicine at Harborview Medical Center in Seattle. Just a few years ago, many people thought the very idea was crazy, he said—but he doesn't hear that anymore.
“I think people are still struggling with how to do it well, but I think there's a common acceptance that this is an important part of critical care,” said Dr. Curtis, who is a member of the IPAL-ICU advisory board.
Still, ICUs can be a difficult place to integrate palliative care, he cautioned.
For starters, critical care units are busy places. Physicians and nurses working there need to balance considerations such as providing supportive palliative care against the need to focus on reducing central line infections and using ventilators appropriately. In addition, palliative care isn't the primary goal in the ICU—so the clinicians there need training on how to provide both types of care.
Another potential pitfall can be a “clash of cultures” between the ICU team and palliative care consultants, Dr. Curtis said. Palliative care specialists need to learn about the culture of the ICU, or they risk coming in with the attitude that the critical care team is being overly aggressive in their approach to some patients. That can happen if they don't understand the outcomes of conditions commonly treated in the ICU.
Palliative and critical care teams need to operate on the same page, agreed Dr. Daniel E. Ray, director of the palliative medicine fellowship program at the Lehigh Valley Health Network in Allentown, Pa., and a member of the advisory board for the IPAL-ICU Project. Otherwise, it opens up the possibility that patients and families could receive conflicting recommendations from providers.
The IPAL-ICU Project resources should go a long way to helping institutions get started on the concept. However, he cautioned that the resources should be customized to the unique culture of each hospital, and that leaders need to work on getting buy-in from everyone on the team to ensure that the templates are actually used.
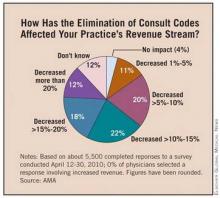
Survey Cites Impact of Consultation Code Elimination
Medicare's decision to eliminate consultation codes has resulted in a loss of revenue for many physicians and forced some to cut back on appointments with Medicare beneficiaries, according to a survey commissioned by the American Medical Association and several other medical specialty societies.
In January, officials at the Centers for Medicare and Medicaid Services discontinued the use of inpatient and outpatient consultation codes when billing Medicare, except for telehealth codes. Physicians instead were asked to use new or established office visit codes, initial hospital care codes, or initial nursing facility care codes. At the time of the policy change, CMS officials said they could no longer justify paying physicians more for a consultation when they had reduced so much of the documentation required to bill for a consultation. The agency also said that eliminating consultation codes would reduce the confusion around the differing definitions of consultations, transfers, and referrals.
In an online survey of about 5,500 physicians, about 72% said that not being able to bill for consultations had decreased their total revenues by more than 5%, with about 30% reporting their revenues had fallen more than 15%.
The loss of revenue has in turn impacted physicians' practices. For example, 20% of respondents said they have already reduced the number of new Medicare patients seen in their practices. Additionally, 39% said they will hold off on purchasing new equipment or health information technology.
The policy change may also undermine efforts to improve care coordination. About 6% of responding physicians said they have stopped providing primary care physicians with written reports following consults with Medicare patients, and another 19% said they plan to do so.
"Patient health is best managed when physicians can work together across specialties to coordinate care," Dr. J. James Rohack, AMA immediate past president, said in a statement. "Twenty percent of patients over age 65 live with five or more chronic illnesses, and managing their care frequently requires primary care physicians to consult with a physician who specializes in the medical or surgical care of their conditions. CMS’s new policy eliminating Medicare consultation codes fails to adequately recognize the additional time and effort involved in these consultations and limits physicians' ability to work together as a comprehensive health care team for their patients.
In a letter to CMS, officials from more than 30 medical specialty societies, including the American Academy of Dermatology, urged the agency to revise the policy when they issue a final regulation on the 2011 Medicare Physician Fee Schedule this fall.
The organizations suggested that CMS consider paying consulting physicians for providing the referring physician with a comprehensive report. They also said CMS could ease some of the financial pressure on physicians by revising its guidelines for prolonged visits to allow for reimbursement for services provided outside of the face-to-face visit, such as reviewing charts and communicating with families and other health care providers.
Medicare's decision to eliminate consultation codes has resulted in a loss of revenue for many physicians and forced some to cut back on appointments with Medicare beneficiaries, according to a survey commissioned by the American Medical Association and several other medical specialty societies.
In January, officials at the Centers for Medicare and Medicaid Services discontinued the use of inpatient and outpatient consultation codes when billing Medicare, except for telehealth codes. Physicians instead were asked to use new or established office visit codes, initial hospital care codes, or initial nursing facility care codes. At the time of the policy change, CMS officials said they could no longer justify paying physicians more for a consultation when they had reduced so much of the documentation required to bill for a consultation. The agency also said that eliminating consultation codes would reduce the confusion around the differing definitions of consultations, transfers, and referrals.
In an online survey of about 5,500 physicians, about 72% said that not being able to bill for consultations had decreased their total revenues by more than 5%, with about 30% reporting their revenues had fallen more than 15%.
The loss of revenue has in turn impacted physicians' practices. For example, 20% of respondents said they have already reduced the number of new Medicare patients seen in their practices. Additionally, 39% said they will hold off on purchasing new equipment or health information technology.
The policy change may also undermine efforts to improve care coordination. About 6% of responding physicians said they have stopped providing primary care physicians with written reports following consults with Medicare patients, and another 19% said they plan to do so.
"Patient health is best managed when physicians can work together across specialties to coordinate care," Dr. J. James Rohack, AMA immediate past president, said in a statement. "Twenty percent of patients over age 65 live with five or more chronic illnesses, and managing their care frequently requires primary care physicians to consult with a physician who specializes in the medical or surgical care of their conditions. CMS’s new policy eliminating Medicare consultation codes fails to adequately recognize the additional time and effort involved in these consultations and limits physicians' ability to work together as a comprehensive health care team for their patients.
In a letter to CMS, officials from more than 30 medical specialty societies, including the American Academy of Dermatology, urged the agency to revise the policy when they issue a final regulation on the 2011 Medicare Physician Fee Schedule this fall.
The organizations suggested that CMS consider paying consulting physicians for providing the referring physician with a comprehensive report. They also said CMS could ease some of the financial pressure on physicians by revising its guidelines for prolonged visits to allow for reimbursement for services provided outside of the face-to-face visit, such as reviewing charts and communicating with families and other health care providers.
Medicare's decision to eliminate consultation codes has resulted in a loss of revenue for many physicians and forced some to cut back on appointments with Medicare beneficiaries, according to a survey commissioned by the American Medical Association and several other medical specialty societies.
In January, officials at the Centers for Medicare and Medicaid Services discontinued the use of inpatient and outpatient consultation codes when billing Medicare, except for telehealth codes. Physicians instead were asked to use new or established office visit codes, initial hospital care codes, or initial nursing facility care codes. At the time of the policy change, CMS officials said they could no longer justify paying physicians more for a consultation when they had reduced so much of the documentation required to bill for a consultation. The agency also said that eliminating consultation codes would reduce the confusion around the differing definitions of consultations, transfers, and referrals.
In an online survey of about 5,500 physicians, about 72% said that not being able to bill for consultations had decreased their total revenues by more than 5%, with about 30% reporting their revenues had fallen more than 15%.
The loss of revenue has in turn impacted physicians' practices. For example, 20% of respondents said they have already reduced the number of new Medicare patients seen in their practices. Additionally, 39% said they will hold off on purchasing new equipment or health information technology.
The policy change may also undermine efforts to improve care coordination. About 6% of responding physicians said they have stopped providing primary care physicians with written reports following consults with Medicare patients, and another 19% said they plan to do so.
"Patient health is best managed when physicians can work together across specialties to coordinate care," Dr. J. James Rohack, AMA immediate past president, said in a statement. "Twenty percent of patients over age 65 live with five or more chronic illnesses, and managing their care frequently requires primary care physicians to consult with a physician who specializes in the medical or surgical care of their conditions. CMS’s new policy eliminating Medicare consultation codes fails to adequately recognize the additional time and effort involved in these consultations and limits physicians' ability to work together as a comprehensive health care team for their patients.
In a letter to CMS, officials from more than 30 medical specialty societies, including the American Academy of Dermatology, urged the agency to revise the policy when they issue a final regulation on the 2011 Medicare Physician Fee Schedule this fall.
The organizations suggested that CMS consider paying consulting physicians for providing the referring physician with a comprehensive report. They also said CMS could ease some of the financial pressure on physicians by revising its guidelines for prolonged visits to allow for reimbursement for services provided outside of the face-to-face visit, such as reviewing charts and communicating with families and other health care providers.
Patients Get New Rights to Appeal Insurance Decisions
New federal regulations mandated by the Affordable Care Act will give patients new rights to appeal claims denials made by their health plans.
The rules, which were announced on July 22, will allow consumers in new health plans to appeal decisions both through their insurer's internal process and to an outside, independent entity. While most health plans already provide for an internal appeals process, not all offer an external review of plan decisions, according to the U.S. Department of Health and Human Services. The types of appeals processes often depend on individual state laws.
HHS officials estimate that in 2011 there will be about 31 million people in new employer plans and another 10 million people in new individual market plans will be able to take advantage of these new appeals opportunities. By 2013, that number is expected to grow to 88 million people. The rules do not apply to grandfathered health plans.
Under the new rules, health plans that begin on or after Sept. 23, 2010 must have an internal appeals process that allows consumers to appeal whenever the plan denies a claim for a covered service or rescinds coverage. The internal appeals process must also offer consumers detailed information about the grounds for their denial and information on how to file an appeal.
The new rules aim to make internal appeals more objective by ensuring that the person considering the appeal does not have a conflict of interest. For example, the health plan is not allowed to offer financial incentives to employees based on the number of claims that are denied. Health plans will also have to provide an expedited appeals process, which would allow urgent cases to be reviewed within 24 hours.
The new federal appeals regulations also standardize rules for external appeals. Currently, 44 states require health plans to have some type of external appeal but those processes vary greatly, according to HHS. Under the federal rules, health plans must provide clear information about external appeals and expedited access to the process. The decisions made through external appeals are binding under the new federal rules.
New federal regulations mandated by the Affordable Care Act will give patients new rights to appeal claims denials made by their health plans.
The rules, which were announced on July 22, will allow consumers in new health plans to appeal decisions both through their insurer's internal process and to an outside, independent entity. While most health plans already provide for an internal appeals process, not all offer an external review of plan decisions, according to the U.S. Department of Health and Human Services. The types of appeals processes often depend on individual state laws.
HHS officials estimate that in 2011 there will be about 31 million people in new employer plans and another 10 million people in new individual market plans will be able to take advantage of these new appeals opportunities. By 2013, that number is expected to grow to 88 million people. The rules do not apply to grandfathered health plans.
Under the new rules, health plans that begin on or after Sept. 23, 2010 must have an internal appeals process that allows consumers to appeal whenever the plan denies a claim for a covered service or rescinds coverage. The internal appeals process must also offer consumers detailed information about the grounds for their denial and information on how to file an appeal.
The new rules aim to make internal appeals more objective by ensuring that the person considering the appeal does not have a conflict of interest. For example, the health plan is not allowed to offer financial incentives to employees based on the number of claims that are denied. Health plans will also have to provide an expedited appeals process, which would allow urgent cases to be reviewed within 24 hours.
The new federal appeals regulations also standardize rules for external appeals. Currently, 44 states require health plans to have some type of external appeal but those processes vary greatly, according to HHS. Under the federal rules, health plans must provide clear information about external appeals and expedited access to the process. The decisions made through external appeals are binding under the new federal rules.
New federal regulations mandated by the Affordable Care Act will give patients new rights to appeal claims denials made by their health plans.
The rules, which were announced on July 22, will allow consumers in new health plans to appeal decisions both through their insurer's internal process and to an outside, independent entity. While most health plans already provide for an internal appeals process, not all offer an external review of plan decisions, according to the U.S. Department of Health and Human Services. The types of appeals processes often depend on individual state laws.
HHS officials estimate that in 2011 there will be about 31 million people in new employer plans and another 10 million people in new individual market plans will be able to take advantage of these new appeals opportunities. By 2013, that number is expected to grow to 88 million people. The rules do not apply to grandfathered health plans.
Under the new rules, health plans that begin on or after Sept. 23, 2010 must have an internal appeals process that allows consumers to appeal whenever the plan denies a claim for a covered service or rescinds coverage. The internal appeals process must also offer consumers detailed information about the grounds for their denial and information on how to file an appeal.
The new rules aim to make internal appeals more objective by ensuring that the person considering the appeal does not have a conflict of interest. For example, the health plan is not allowed to offer financial incentives to employees based on the number of claims that are denied. Health plans will also have to provide an expedited appeals process, which would allow urgent cases to be reviewed within 24 hours.
The new federal appeals regulations also standardize rules for external appeals. Currently, 44 states require health plans to have some type of external appeal but those processes vary greatly, according to HHS. Under the federal rules, health plans must provide clear information about external appeals and expedited access to the process. The decisions made through external appeals are binding under the new federal rules.
HHS Proposes Tighter Health Privacy Requirements
Patients could gain greater access to their health information and have more power to limit disclosures of certain personal information to health plans under a new proposal from the Health and Human Services department.
The new requirements, which were announced recently, are aimed at beefing up privacy and security, as the Obama administration pushes to get more physicians using electronic health records over the next few years.
"The benefits of health IT can only be fully realized if patients and providers are confident that electronic health information is kept private and secure at all times," Georgina Verdugo, director of the HHS Office for Civil Rights, said in a statement. "This proposed rule strengthens the privacy and security of health information, and is an integral piece of the administration’s efforts to broaden the use of health information technology in health care today."
The proposal alters the Health Insurance Portability and Accountability Act (HIPAA) rules by setting new limits on the use of disclosure of protected health information for marketing and fundraising and by requiring business associates of HIPAA-covered entities to follow most of the same rules that covered entities follow. The proposal would also bar the sale of protected health information without explicit authorization from the patient.
The proposal also implements elements of the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act, which requires physicians and other covered entities to grant patient requests to restrict certain information from their health plans. For example, the proposed rule states that patients must be allowed to restrict protected health information if that information is related only to a service for which the patient paid in full and the information is not otherwise required by law to be reported.
Individuals can provide comments on the rule for 60 days; the comment period began July 14.
Along with the release of the proposed regulation, HHS has also launched a new Web site that provides consumers with information on their privacy rights under existing regulations.
Patients could gain greater access to their health information and have more power to limit disclosures of certain personal information to health plans under a new proposal from the Health and Human Services department.
The new requirements, which were announced recently, are aimed at beefing up privacy and security, as the Obama administration pushes to get more physicians using electronic health records over the next few years.
"The benefits of health IT can only be fully realized if patients and providers are confident that electronic health information is kept private and secure at all times," Georgina Verdugo, director of the HHS Office for Civil Rights, said in a statement. "This proposed rule strengthens the privacy and security of health information, and is an integral piece of the administration’s efforts to broaden the use of health information technology in health care today."
The proposal alters the Health Insurance Portability and Accountability Act (HIPAA) rules by setting new limits on the use of disclosure of protected health information for marketing and fundraising and by requiring business associates of HIPAA-covered entities to follow most of the same rules that covered entities follow. The proposal would also bar the sale of protected health information without explicit authorization from the patient.
The proposal also implements elements of the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act, which requires physicians and other covered entities to grant patient requests to restrict certain information from their health plans. For example, the proposed rule states that patients must be allowed to restrict protected health information if that information is related only to a service for which the patient paid in full and the information is not otherwise required by law to be reported.
Individuals can provide comments on the rule for 60 days; the comment period began July 14.
Along with the release of the proposed regulation, HHS has also launched a new Web site that provides consumers with information on their privacy rights under existing regulations.
Patients could gain greater access to their health information and have more power to limit disclosures of certain personal information to health plans under a new proposal from the Health and Human Services department.
The new requirements, which were announced recently, are aimed at beefing up privacy and security, as the Obama administration pushes to get more physicians using electronic health records over the next few years.
"The benefits of health IT can only be fully realized if patients and providers are confident that electronic health information is kept private and secure at all times," Georgina Verdugo, director of the HHS Office for Civil Rights, said in a statement. "This proposed rule strengthens the privacy and security of health information, and is an integral piece of the administration’s efforts to broaden the use of health information technology in health care today."
The proposal alters the Health Insurance Portability and Accountability Act (HIPAA) rules by setting new limits on the use of disclosure of protected health information for marketing and fundraising and by requiring business associates of HIPAA-covered entities to follow most of the same rules that covered entities follow. The proposal would also bar the sale of protected health information without explicit authorization from the patient.
The proposal also implements elements of the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act, which requires physicians and other covered entities to grant patient requests to restrict certain information from their health plans. For example, the proposed rule states that patients must be allowed to restrict protected health information if that information is related only to a service for which the patient paid in full and the information is not otherwise required by law to be reported.
Individuals can provide comments on the rule for 60 days; the comment period began July 14.
Along with the release of the proposed regulation, HHS has also launched a new Web site that provides consumers with information on their privacy rights under existing regulations.
New Regulations Outline Availability of Free Preventive Services
New health plans will soon be required to offer a range of recommended preventive health services to patients free of charge under the Affordable Care Act.
The requirements will affect new private health plans in the individual and group markets starting with plan years that begin on or after Sept. 23. The Health and Human Services department estimates that in 2011, the rules will impact about 30 million people in group health plans and another 10 million in individual market plans. The rules do not apply to grandfathered plans.
The administration released an interim final regulation detailing the new requirements on July 14.
Under the final rule, health plans may not collect copayments, coinsurance, or deductibles for a number of recommended preventive services. However, they may collect fees for the associated office visit if the preventive service wasn’t the primary purpose of the visit. Patients may also incur cost sharing if they go out of network for the recommended screenings.
The covered services include those given an evidence rating of “A” or “B” from the U.S. Preventive Services Task Force. Those services include breast and colon cancer screenings, diabetes screenings, blood pressure and cholesterol testing, and screening for vitamin deficiencies during pregnancy. Tobacco cessation counseling is also given a high evidence rating by the U.S. Preventive Services Task Force and would be covered under the new rule.
Health plans will have some extra time to begin covering newly recommended services. For recommendations that have been in effect for less than a year, plans will have 1 year to comply after the effective date, according to the interim final rule.
Health plans will also be required to cover the list of adult and childhood vaccines recommended by the Advisory Committee on Immunization Practices. For children, the rule also requires health plans to cover all preventive care recommended under the Bright Futures guidelines. The guidelines include screenings, developmental assessments, immunizations, and regular well-child visits from birth to age 21 years. These guidelines were developed jointly by the Health Resources and Services Administration and the American Academy of Pediatrics.
The rule also calls for coverage of additional preventive services for women, which will be developed by an independent group of experts. The recommendations from that group are expected by Aug. 1, 2011. There was no word from HHS on whether those recommendations are likely to include coverage for contraceptives, something many reproductive health advocates have been lobbying for in recent months.
HHS officials expect that the move to expand coverage and eliminate out-of-pocket costs for these services will decrease costs for many Americans, especially those at high risk for certain health conditions. At the same time, the change is expected to increase premiums for enrollees in nongrandfathered plans. The federal government estimates that premiums in the affected plans could increase about 1.5% on average.
A list of the recommended preventive services is available online.
New health plans will soon be required to offer a range of recommended preventive health services to patients free of charge under the Affordable Care Act.
The requirements will affect new private health plans in the individual and group markets starting with plan years that begin on or after Sept. 23. The Health and Human Services department estimates that in 2011, the rules will impact about 30 million people in group health plans and another 10 million in individual market plans. The rules do not apply to grandfathered plans.
The administration released an interim final regulation detailing the new requirements on July 14.
Under the final rule, health plans may not collect copayments, coinsurance, or deductibles for a number of recommended preventive services. However, they may collect fees for the associated office visit if the preventive service wasn’t the primary purpose of the visit. Patients may also incur cost sharing if they go out of network for the recommended screenings.
The covered services include those given an evidence rating of “A” or “B” from the U.S. Preventive Services Task Force. Those services include breast and colon cancer screenings, diabetes screenings, blood pressure and cholesterol testing, and screening for vitamin deficiencies during pregnancy. Tobacco cessation counseling is also given a high evidence rating by the U.S. Preventive Services Task Force and would be covered under the new rule.
Health plans will have some extra time to begin covering newly recommended services. For recommendations that have been in effect for less than a year, plans will have 1 year to comply after the effective date, according to the interim final rule.
Health plans will also be required to cover the list of adult and childhood vaccines recommended by the Advisory Committee on Immunization Practices. For children, the rule also requires health plans to cover all preventive care recommended under the Bright Futures guidelines. The guidelines include screenings, developmental assessments, immunizations, and regular well-child visits from birth to age 21 years. These guidelines were developed jointly by the Health Resources and Services Administration and the American Academy of Pediatrics.
The rule also calls for coverage of additional preventive services for women, which will be developed by an independent group of experts. The recommendations from that group are expected by Aug. 1, 2011. There was no word from HHS on whether those recommendations are likely to include coverage for contraceptives, something many reproductive health advocates have been lobbying for in recent months.
HHS officials expect that the move to expand coverage and eliminate out-of-pocket costs for these services will decrease costs for many Americans, especially those at high risk for certain health conditions. At the same time, the change is expected to increase premiums for enrollees in nongrandfathered plans. The federal government estimates that premiums in the affected plans could increase about 1.5% on average.
A list of the recommended preventive services is available online.
New health plans will soon be required to offer a range of recommended preventive health services to patients free of charge under the Affordable Care Act.
The requirements will affect new private health plans in the individual and group markets starting with plan years that begin on or after Sept. 23. The Health and Human Services department estimates that in 2011, the rules will impact about 30 million people in group health plans and another 10 million in individual market plans. The rules do not apply to grandfathered plans.
The administration released an interim final regulation detailing the new requirements on July 14.
Under the final rule, health plans may not collect copayments, coinsurance, or deductibles for a number of recommended preventive services. However, they may collect fees for the associated office visit if the preventive service wasn’t the primary purpose of the visit. Patients may also incur cost sharing if they go out of network for the recommended screenings.
The covered services include those given an evidence rating of “A” or “B” from the U.S. Preventive Services Task Force. Those services include breast and colon cancer screenings, diabetes screenings, blood pressure and cholesterol testing, and screening for vitamin deficiencies during pregnancy. Tobacco cessation counseling is also given a high evidence rating by the U.S. Preventive Services Task Force and would be covered under the new rule.
Health plans will have some extra time to begin covering newly recommended services. For recommendations that have been in effect for less than a year, plans will have 1 year to comply after the effective date, according to the interim final rule.
Health plans will also be required to cover the list of adult and childhood vaccines recommended by the Advisory Committee on Immunization Practices. For children, the rule also requires health plans to cover all preventive care recommended under the Bright Futures guidelines. The guidelines include screenings, developmental assessments, immunizations, and regular well-child visits from birth to age 21 years. These guidelines were developed jointly by the Health Resources and Services Administration and the American Academy of Pediatrics.
The rule also calls for coverage of additional preventive services for women, which will be developed by an independent group of experts. The recommendations from that group are expected by Aug. 1, 2011. There was no word from HHS on whether those recommendations are likely to include coverage for contraceptives, something many reproductive health advocates have been lobbying for in recent months.
HHS officials expect that the move to expand coverage and eliminate out-of-pocket costs for these services will decrease costs for many Americans, especially those at high risk for certain health conditions. At the same time, the change is expected to increase premiums for enrollees in nongrandfathered plans. The federal government estimates that premiums in the affected plans could increase about 1.5% on average.
A list of the recommended preventive services is available online.
Feds Release Final Meaningful Use Standards
The federal government on July 13 released the much-anticipated requirements for how physicians and hospitals can qualify for tens of thousands of dollars in incentive payments to adopt and use electronic health records.
The final rule on the meaningful use of electronic health records (EHRs) eases many of the requirements that officials in the Health and Human Services department had outlined in a proposal published in January. Physician organizations had objected to the initial proposal, saying that it asked doctors, especially those in small practices, to do too much too quickly. Physicians were also critical of the all or nothing framework of the proposal, which required them to meet all 25 objectives for meaningful use or lose out on incentive payments.
Federal officials aimed to address those concerns in the final rule by requiring physicians to first meet a core set of 15 requirements and then meet any 5 of 10 additional requirements. The core set includes requirements such as recording patient demographics and vital signs in the EHR, maintaining an up-to-date problem list and an active list of medications and allergies, and transmitting permissible prescriptions electronically.
"We very much want well-intentioned providers to become meaningful users," Dr. David Blumenthal, National Coordinator for Health Information Technology at HHS, said during a press briefing to announce the final rule.
HHS officials also relaxed some of the thresholds related to the requirements. For example, under the proposed rule, physicians would have had to generate and transmit 75% of their permissible prescriptions electronically to meet the e-prescribing requirement. Under the final rule, the threshold has been lowered to more than 40% of permissible prescriptions. The revision was made so that the requirement would be achievable by average practices in the early years of the program, Dr. Blumenthal said.
The final rule also creates an easier path for physicians to meet meaningful use requirements on electronic reporting of quality data. Under the final rule, physicians will need to report data on blood pressure, tobacco status, and adult weight screening, and follow-up in 2011 and 2012, in order to qualify. Alternatives are available if those measures do not apply to their practices. Physicians will also have to choose three other quality measures to report on through their EHRs.
The final rule outlines the steps physicians must take in 2011 and 2012 to quality for the maximum incentive payments through the Medicare and Medicaid programs. The incentives were mandated by the Health Information Technology for Economic and Clinical Health Act (HITECH), a part of 2009’s American Recovery Act.
Starting in 2011, physicians who demonstrate meaningful use of certified EHRs can receive payments of up to $18,000 from Medicare. Those bonuses continue for 5 years, with physicians eligible to earn up to $44,000 in total incentives. Physicians can still receive bonuses if they begin their meaningful use of the technology later, but they must start before 2013 to get all the available incentives.
A similar program is in place under the Medicaid program, with physicians eligible to receive up to $64,000 over 6 years for the adoption and use of certified EHRs.
Also on July 13, HHS released a final rule with new standards for the certification of EHR technology. The simultaneous release of these certification rules should assure physicians that any certified EHR they purchase will be capable of meeting meaningful use standards, Dr. Blumenthal said.
The American Medical Association was still reviewing the final rules at press time. However, the organization stressed that a key part of making EHRs affordable for physicians is to address Medicare payments in general, so that physicians aren’t facing double-digit cuts in their reimbursement every few months.
"Congress needs to repeal the flawed Medicare physician payment formula to help eliminate one major obstacle to physician adoption of new technologies," Dr. Steven J. Stack, an AMA board member said in a statement.
The federal government on July 13 released the much-anticipated requirements for how physicians and hospitals can qualify for tens of thousands of dollars in incentive payments to adopt and use electronic health records.
The final rule on the meaningful use of electronic health records (EHRs) eases many of the requirements that officials in the Health and Human Services department had outlined in a proposal published in January. Physician organizations had objected to the initial proposal, saying that it asked doctors, especially those in small practices, to do too much too quickly. Physicians were also critical of the all or nothing framework of the proposal, which required them to meet all 25 objectives for meaningful use or lose out on incentive payments.
Federal officials aimed to address those concerns in the final rule by requiring physicians to first meet a core set of 15 requirements and then meet any 5 of 10 additional requirements. The core set includes requirements such as recording patient demographics and vital signs in the EHR, maintaining an up-to-date problem list and an active list of medications and allergies, and transmitting permissible prescriptions electronically.
"We very much want well-intentioned providers to become meaningful users," Dr. David Blumenthal, National Coordinator for Health Information Technology at HHS, said during a press briefing to announce the final rule.
HHS officials also relaxed some of the thresholds related to the requirements. For example, under the proposed rule, physicians would have had to generate and transmit 75% of their permissible prescriptions electronically to meet the e-prescribing requirement. Under the final rule, the threshold has been lowered to more than 40% of permissible prescriptions. The revision was made so that the requirement would be achievable by average practices in the early years of the program, Dr. Blumenthal said.
The final rule also creates an easier path for physicians to meet meaningful use requirements on electronic reporting of quality data. Under the final rule, physicians will need to report data on blood pressure, tobacco status, and adult weight screening, and follow-up in 2011 and 2012, in order to qualify. Alternatives are available if those measures do not apply to their practices. Physicians will also have to choose three other quality measures to report on through their EHRs.
The final rule outlines the steps physicians must take in 2011 and 2012 to quality for the maximum incentive payments through the Medicare and Medicaid programs. The incentives were mandated by the Health Information Technology for Economic and Clinical Health Act (HITECH), a part of 2009’s American Recovery Act.
Starting in 2011, physicians who demonstrate meaningful use of certified EHRs can receive payments of up to $18,000 from Medicare. Those bonuses continue for 5 years, with physicians eligible to earn up to $44,000 in total incentives. Physicians can still receive bonuses if they begin their meaningful use of the technology later, but they must start before 2013 to get all the available incentives.
A similar program is in place under the Medicaid program, with physicians eligible to receive up to $64,000 over 6 years for the adoption and use of certified EHRs.
Also on July 13, HHS released a final rule with new standards for the certification of EHR technology. The simultaneous release of these certification rules should assure physicians that any certified EHR they purchase will be capable of meeting meaningful use standards, Dr. Blumenthal said.
The American Medical Association was still reviewing the final rules at press time. However, the organization stressed that a key part of making EHRs affordable for physicians is to address Medicare payments in general, so that physicians aren’t facing double-digit cuts in their reimbursement every few months.
"Congress needs to repeal the flawed Medicare physician payment formula to help eliminate one major obstacle to physician adoption of new technologies," Dr. Steven J. Stack, an AMA board member said in a statement.
The federal government on July 13 released the much-anticipated requirements for how physicians and hospitals can qualify for tens of thousands of dollars in incentive payments to adopt and use electronic health records.
The final rule on the meaningful use of electronic health records (EHRs) eases many of the requirements that officials in the Health and Human Services department had outlined in a proposal published in January. Physician organizations had objected to the initial proposal, saying that it asked doctors, especially those in small practices, to do too much too quickly. Physicians were also critical of the all or nothing framework of the proposal, which required them to meet all 25 objectives for meaningful use or lose out on incentive payments.
Federal officials aimed to address those concerns in the final rule by requiring physicians to first meet a core set of 15 requirements and then meet any 5 of 10 additional requirements. The core set includes requirements such as recording patient demographics and vital signs in the EHR, maintaining an up-to-date problem list and an active list of medications and allergies, and transmitting permissible prescriptions electronically.
"We very much want well-intentioned providers to become meaningful users," Dr. David Blumenthal, National Coordinator for Health Information Technology at HHS, said during a press briefing to announce the final rule.
HHS officials also relaxed some of the thresholds related to the requirements. For example, under the proposed rule, physicians would have had to generate and transmit 75% of their permissible prescriptions electronically to meet the e-prescribing requirement. Under the final rule, the threshold has been lowered to more than 40% of permissible prescriptions. The revision was made so that the requirement would be achievable by average practices in the early years of the program, Dr. Blumenthal said.
The final rule also creates an easier path for physicians to meet meaningful use requirements on electronic reporting of quality data. Under the final rule, physicians will need to report data on blood pressure, tobacco status, and adult weight screening, and follow-up in 2011 and 2012, in order to qualify. Alternatives are available if those measures do not apply to their practices. Physicians will also have to choose three other quality measures to report on through their EHRs.
The final rule outlines the steps physicians must take in 2011 and 2012 to quality for the maximum incentive payments through the Medicare and Medicaid programs. The incentives were mandated by the Health Information Technology for Economic and Clinical Health Act (HITECH), a part of 2009’s American Recovery Act.
Starting in 2011, physicians who demonstrate meaningful use of certified EHRs can receive payments of up to $18,000 from Medicare. Those bonuses continue for 5 years, with physicians eligible to earn up to $44,000 in total incentives. Physicians can still receive bonuses if they begin their meaningful use of the technology later, but they must start before 2013 to get all the available incentives.
A similar program is in place under the Medicaid program, with physicians eligible to receive up to $64,000 over 6 years for the adoption and use of certified EHRs.
Also on July 13, HHS released a final rule with new standards for the certification of EHR technology. The simultaneous release of these certification rules should assure physicians that any certified EHR they purchase will be capable of meeting meaningful use standards, Dr. Blumenthal said.
The American Medical Association was still reviewing the final rules at press time. However, the organization stressed that a key part of making EHRs affordable for physicians is to address Medicare payments in general, so that physicians aren’t facing double-digit cuts in their reimbursement every few months.
"Congress needs to repeal the flawed Medicare physician payment formula to help eliminate one major obstacle to physician adoption of new technologies," Dr. Steven J. Stack, an AMA board member said in a statement.