User login
Laser-assisted PDT cleared majority of AKs in immunocompromised patients
BOSTON – Fractional laser-assisted photodynamic therapy can effectively treat actinic keratoses in organ transplant recipients, but at a cost of more intense postoperative skin reactions than with laser therapy alone, based on data from a randomized clinical trial.
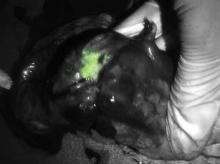
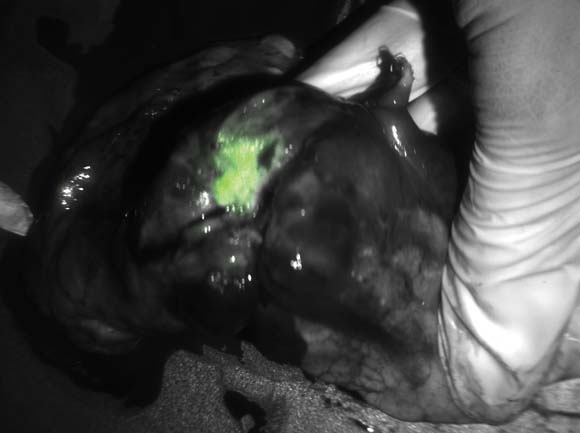
Intensified ablative fractional laser-assisted photodynamic therapy (AFXL-PDT) was significantly more effective at clearing actinic keratoses (AKs) and warty lesions on the dorsal aspect of the hands of organ transplant recipients than AFXL alone, reported Dr. Merete Haedersdal of the University of Copenhagen and a visiting scientist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
The treatment was painful, and 3 of 10 patients reported intense inflammation a week afterward, Dr. Haedersdal noted.
However, "this new treatment modality also works well for immunosuppressed patients, and I do think it works very well for immunosuppressed patients with multiple AKs," she said at the annual meeting of the American Society for Laser Medicine and Surgery.
High-risk population
Organ transplant recipients are at a 100-fold or greater risk for developing squamous cell carcinomas, which can arise from AKs, because of their chronic immunosuppressive treatment regimens. Cancers that develop in these patients tend to be quite aggressive, with a high rate of metastases and an attributable morality rate of 6%-8%, said Dr. Haedersdal.
"Therefore, there is a huge motivation to come up with intensified treatment regimens for these patients," she said.
PDT is a well-established therapy in transplant recipients, but it is less effective in these patients than in patients with intact immune responses. It is also less effective for thick lesions or lesions on the extremities.
Dr. Haedersdal and her colleagues previously demonstrated the efficacy of AFXL-PDT for treating AKs with a carbon dioxide laser in immunocompetent patients. In the current study, they compared the therapy with ablative fractional CO2 laser alone in 10 organ transplant recipients with a total of 680 AKs and 409 wartlike lesions on the dorsal hands, and a collective history of 21 previous squamous cell carcinomas.
In a randomized intrapatient trial, the participants first underwent targeted ablation of localized keratotic lesions, and then were randomly assigned to receive one field treatment with AFXL-PDT on one hand, and AFXL alone on the other.
For AFXL, energy was delivered with the laser set to 30 W, a 0.12-mm spot size, a 1.32- to 2.06-millisecond pulse duration, and 40-60 mJ with the target of 4.3%-5.2% coverage; settings were based on the severity of skin atrophy.
After laser exposure, lesions randomized to receive PDT were treated with methyl aminolevulinate applied under occlusion for 3 hours, and were then exposed to red diode light at 37 J/cm2.
‘Really impressive’ cure rate
The combined modality completely cleared 73% of all AKs, compared with 31% cleared by AFXL alone (P = .002), and 37% of all wartlike lesions, compared with 14% for AFXL (P = .02) at 4 months’ follow-up. The patients were clinically assessed by raters blinded to treatment type.
"Normally, when we deliver PDT for these patients, we have – from just a single treatment – cure rates on the acral lesions of about 30%-40%, so this was really impressive to get a complete cure rate for AKs at a level of 73%," Dr. Haedersdal said.
Overall, AKs treated with AFXL-PDT were rated as improved by a median of 83%, 15% as unchanged, and 3% as worsened, compared with AFXL-only rates of 52%, 47%, and 4%, respectively.
Thinner AKs responded better to treatment than thick lesions, with an odds ratio (OR) for grade 2 vs. grade 1 lesions of 0.34 (P = .001) and an OR for grade 3 vs. grade 1 of 0.21 (P = .001).
Safety data showed that AFXL treatment was generally not painful, with a mean visual analog scale (VAS) pain score of 1, and the PDT was more painful, with a mean VAS of 4.5 during LED illumination. Seven of 10 patients requested anesthesia during the procedure, Dr. Haedersdal noted.
In addition, at 1 week post treatment, 3 of 10 patients reported intense inflammation of the treated sites, 3 had pigment changes with AFXL-PDT, and 1 had a pigment change with AFXL alone.
The take-home message, Dr. Haedersdal said, is: "Do not deliver laser-assisted PDT to large areas; you have to deliver it to refined areas."
However, eight patients gave a favorable overall assessment to the combined treatments, and the remaining two rated the combined therapy and laser-only therapy as being equally effective.
The study was supported by a research grant to Dr. Haedersdal from Galderma. She also disclosed serving on a Galderma advisory board.
BOSTON – Fractional laser-assisted photodynamic therapy can effectively treat actinic keratoses in organ transplant recipients, but at a cost of more intense postoperative skin reactions than with laser therapy alone, based on data from a randomized clinical trial.
Intensified ablative fractional laser-assisted photodynamic therapy (AFXL-PDT) was significantly more effective at clearing actinic keratoses (AKs) and warty lesions on the dorsal aspect of the hands of organ transplant recipients than AFXL alone, reported Dr. Merete Haedersdal of the University of Copenhagen and a visiting scientist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
The treatment was painful, and 3 of 10 patients reported intense inflammation a week afterward, Dr. Haedersdal noted.
However, "this new treatment modality also works well for immunosuppressed patients, and I do think it works very well for immunosuppressed patients with multiple AKs," she said at the annual meeting of the American Society for Laser Medicine and Surgery.
High-risk population
Organ transplant recipients are at a 100-fold or greater risk for developing squamous cell carcinomas, which can arise from AKs, because of their chronic immunosuppressive treatment regimens. Cancers that develop in these patients tend to be quite aggressive, with a high rate of metastases and an attributable morality rate of 6%-8%, said Dr. Haedersdal.
"Therefore, there is a huge motivation to come up with intensified treatment regimens for these patients," she said.
PDT is a well-established therapy in transplant recipients, but it is less effective in these patients than in patients with intact immune responses. It is also less effective for thick lesions or lesions on the extremities.
Dr. Haedersdal and her colleagues previously demonstrated the efficacy of AFXL-PDT for treating AKs with a carbon dioxide laser in immunocompetent patients. In the current study, they compared the therapy with ablative fractional CO2 laser alone in 10 organ transplant recipients with a total of 680 AKs and 409 wartlike lesions on the dorsal hands, and a collective history of 21 previous squamous cell carcinomas.
In a randomized intrapatient trial, the participants first underwent targeted ablation of localized keratotic lesions, and then were randomly assigned to receive one field treatment with AFXL-PDT on one hand, and AFXL alone on the other.
For AFXL, energy was delivered with the laser set to 30 W, a 0.12-mm spot size, a 1.32- to 2.06-millisecond pulse duration, and 40-60 mJ with the target of 4.3%-5.2% coverage; settings were based on the severity of skin atrophy.
After laser exposure, lesions randomized to receive PDT were treated with methyl aminolevulinate applied under occlusion for 3 hours, and were then exposed to red diode light at 37 J/cm2.
‘Really impressive’ cure rate
The combined modality completely cleared 73% of all AKs, compared with 31% cleared by AFXL alone (P = .002), and 37% of all wartlike lesions, compared with 14% for AFXL (P = .02) at 4 months’ follow-up. The patients were clinically assessed by raters blinded to treatment type.
"Normally, when we deliver PDT for these patients, we have – from just a single treatment – cure rates on the acral lesions of about 30%-40%, so this was really impressive to get a complete cure rate for AKs at a level of 73%," Dr. Haedersdal said.
Overall, AKs treated with AFXL-PDT were rated as improved by a median of 83%, 15% as unchanged, and 3% as worsened, compared with AFXL-only rates of 52%, 47%, and 4%, respectively.
Thinner AKs responded better to treatment than thick lesions, with an odds ratio (OR) for grade 2 vs. grade 1 lesions of 0.34 (P = .001) and an OR for grade 3 vs. grade 1 of 0.21 (P = .001).
Safety data showed that AFXL treatment was generally not painful, with a mean visual analog scale (VAS) pain score of 1, and the PDT was more painful, with a mean VAS of 4.5 during LED illumination. Seven of 10 patients requested anesthesia during the procedure, Dr. Haedersdal noted.
In addition, at 1 week post treatment, 3 of 10 patients reported intense inflammation of the treated sites, 3 had pigment changes with AFXL-PDT, and 1 had a pigment change with AFXL alone.
The take-home message, Dr. Haedersdal said, is: "Do not deliver laser-assisted PDT to large areas; you have to deliver it to refined areas."
However, eight patients gave a favorable overall assessment to the combined treatments, and the remaining two rated the combined therapy and laser-only therapy as being equally effective.
The study was supported by a research grant to Dr. Haedersdal from Galderma. She also disclosed serving on a Galderma advisory board.
BOSTON – Fractional laser-assisted photodynamic therapy can effectively treat actinic keratoses in organ transplant recipients, but at a cost of more intense postoperative skin reactions than with laser therapy alone, based on data from a randomized clinical trial.
Intensified ablative fractional laser-assisted photodynamic therapy (AFXL-PDT) was significantly more effective at clearing actinic keratoses (AKs) and warty lesions on the dorsal aspect of the hands of organ transplant recipients than AFXL alone, reported Dr. Merete Haedersdal of the University of Copenhagen and a visiting scientist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
The treatment was painful, and 3 of 10 patients reported intense inflammation a week afterward, Dr. Haedersdal noted.
However, "this new treatment modality also works well for immunosuppressed patients, and I do think it works very well for immunosuppressed patients with multiple AKs," she said at the annual meeting of the American Society for Laser Medicine and Surgery.
High-risk population
Organ transplant recipients are at a 100-fold or greater risk for developing squamous cell carcinomas, which can arise from AKs, because of their chronic immunosuppressive treatment regimens. Cancers that develop in these patients tend to be quite aggressive, with a high rate of metastases and an attributable morality rate of 6%-8%, said Dr. Haedersdal.
"Therefore, there is a huge motivation to come up with intensified treatment regimens for these patients," she said.
PDT is a well-established therapy in transplant recipients, but it is less effective in these patients than in patients with intact immune responses. It is also less effective for thick lesions or lesions on the extremities.
Dr. Haedersdal and her colleagues previously demonstrated the efficacy of AFXL-PDT for treating AKs with a carbon dioxide laser in immunocompetent patients. In the current study, they compared the therapy with ablative fractional CO2 laser alone in 10 organ transplant recipients with a total of 680 AKs and 409 wartlike lesions on the dorsal hands, and a collective history of 21 previous squamous cell carcinomas.
In a randomized intrapatient trial, the participants first underwent targeted ablation of localized keratotic lesions, and then were randomly assigned to receive one field treatment with AFXL-PDT on one hand, and AFXL alone on the other.
For AFXL, energy was delivered with the laser set to 30 W, a 0.12-mm spot size, a 1.32- to 2.06-millisecond pulse duration, and 40-60 mJ with the target of 4.3%-5.2% coverage; settings were based on the severity of skin atrophy.
After laser exposure, lesions randomized to receive PDT were treated with methyl aminolevulinate applied under occlusion for 3 hours, and were then exposed to red diode light at 37 J/cm2.
‘Really impressive’ cure rate
The combined modality completely cleared 73% of all AKs, compared with 31% cleared by AFXL alone (P = .002), and 37% of all wartlike lesions, compared with 14% for AFXL (P = .02) at 4 months’ follow-up. The patients were clinically assessed by raters blinded to treatment type.
"Normally, when we deliver PDT for these patients, we have – from just a single treatment – cure rates on the acral lesions of about 30%-40%, so this was really impressive to get a complete cure rate for AKs at a level of 73%," Dr. Haedersdal said.
Overall, AKs treated with AFXL-PDT were rated as improved by a median of 83%, 15% as unchanged, and 3% as worsened, compared with AFXL-only rates of 52%, 47%, and 4%, respectively.
Thinner AKs responded better to treatment than thick lesions, with an odds ratio (OR) for grade 2 vs. grade 1 lesions of 0.34 (P = .001) and an OR for grade 3 vs. grade 1 of 0.21 (P = .001).
Safety data showed that AFXL treatment was generally not painful, with a mean visual analog scale (VAS) pain score of 1, and the PDT was more painful, with a mean VAS of 4.5 during LED illumination. Seven of 10 patients requested anesthesia during the procedure, Dr. Haedersdal noted.
In addition, at 1 week post treatment, 3 of 10 patients reported intense inflammation of the treated sites, 3 had pigment changes with AFXL-PDT, and 1 had a pigment change with AFXL alone.
The take-home message, Dr. Haedersdal said, is: "Do not deliver laser-assisted PDT to large areas; you have to deliver it to refined areas."
However, eight patients gave a favorable overall assessment to the combined treatments, and the remaining two rated the combined therapy and laser-only therapy as being equally effective.
The study was supported by a research grant to Dr. Haedersdal from Galderma. She also disclosed serving on a Galderma advisory board.
AT LASER 2013
Major finding: Laser-assisted photodynamic therapy completely cleared 73% of actinic keratoses on the hands of organ transplant recipients.
Data source: A randomized clinical trial comparing laser-assisted PDT with laser treatment alone in 10 patients.
Disclosures: The study was supported by a research grant to Dr. Haedersdal from Galderma. She also disclosed serving on a Galderma advisory board.
Risk factors for death in NAFLD patients remain elusive
ORLANDO – The risk factors for death in patients with nonalcoholic fatty liver disease include older age, male sex, truncal obesity, and a low HDL cholesterol level – in other words, the same factors that increase risk for death from cardiovascular disease and other causes, according to Dr. Naga P. Chalasani.
On the other hand, elevations in alanine aminotransferase (ALT) levels in patients with NAFLD are not associated with an increased risk for death or other poor outcomes, meaning that researchers may have to burrow more deeply through the available data to find risk predictors unique to NAFLD, said Dr Chalasani of Indiana University, Indianapolis.
"How do we identify someone with NAFLD who is at risk for poor outcomes? I think this is the first shot at risk mapping patients," Dr. Chalasani said at the annual Digestive Disease Week.
Dr. Keith D. Lindor, who moderated the session at which the data were presented, agreed.
"What we’re having trouble with, I think, is defining nonalcoholic fatty liver disease easily, particularly amongst the population," he said. "We saw data that ALT, which we commonly used to use, may not be telling, and there are questions about how well ultrasound detects [NAFLD], particularly given that the amount of steatosis in order to be detected by ultrasound has to be relatively dramatic."
It is still not known whether people with steatosis discovered during biopsy but not visible on ultrasound will have risk factors similar to those of people with more grossly evident steatosis, he said in an interview.
Although Dr. Chalasani and colleagues failed to find unique risk markers in this population, it was not for want of trying. The investigators pored over data from the third National Health and Nutrition Examination Survey (NHANES III) for baseline and follow-up information about patients with NAFLD.
The data were collected from 1988 through 1994, and included gallbladder ultrasound with liver images in 14,797 adults aged 20-74. The authors linked the data to the National Death Index in an attempt to determine which factors might be harbingers of early mortality in patients with NAFLD vs. controls.
They defined NAFLD by the presence of moderate to severe hepatic steatosis on ultrasonography, and by the absence of iron overload, hepatitis B or C infections, and excessive alcohol consumption. Controls were participants in the same data set who did not have underlying liver disease and had normal ultrasound and liver function tests.
There were a total of 2,441 people with NAFLD and 8,423 controls. During a median follow-up of 14.3 years, 14% of controls (1,193), and 21% of those with NAFLD (501) died, a difference that was significant in a univariate analysis (P = .0328).
But when they looked at overall mortality, cancer-related mortality, and cardiovascular mortality, they found that all three categories shared male sex, older age, and a low HDL level as independent predictors for death, with cardiovascular mortality having the added bonus of the metabolic syndrome as an additional risk factor.
The authors did not disclose a funding source. Dr. Chalasani and Dr. Lindor reported having no relevant financial disclosures.
ORLANDO – The risk factors for death in patients with nonalcoholic fatty liver disease include older age, male sex, truncal obesity, and a low HDL cholesterol level – in other words, the same factors that increase risk for death from cardiovascular disease and other causes, according to Dr. Naga P. Chalasani.
On the other hand, elevations in alanine aminotransferase (ALT) levels in patients with NAFLD are not associated with an increased risk for death or other poor outcomes, meaning that researchers may have to burrow more deeply through the available data to find risk predictors unique to NAFLD, said Dr Chalasani of Indiana University, Indianapolis.
"How do we identify someone with NAFLD who is at risk for poor outcomes? I think this is the first shot at risk mapping patients," Dr. Chalasani said at the annual Digestive Disease Week.
Dr. Keith D. Lindor, who moderated the session at which the data were presented, agreed.
"What we’re having trouble with, I think, is defining nonalcoholic fatty liver disease easily, particularly amongst the population," he said. "We saw data that ALT, which we commonly used to use, may not be telling, and there are questions about how well ultrasound detects [NAFLD], particularly given that the amount of steatosis in order to be detected by ultrasound has to be relatively dramatic."
It is still not known whether people with steatosis discovered during biopsy but not visible on ultrasound will have risk factors similar to those of people with more grossly evident steatosis, he said in an interview.
Although Dr. Chalasani and colleagues failed to find unique risk markers in this population, it was not for want of trying. The investigators pored over data from the third National Health and Nutrition Examination Survey (NHANES III) for baseline and follow-up information about patients with NAFLD.
The data were collected from 1988 through 1994, and included gallbladder ultrasound with liver images in 14,797 adults aged 20-74. The authors linked the data to the National Death Index in an attempt to determine which factors might be harbingers of early mortality in patients with NAFLD vs. controls.
They defined NAFLD by the presence of moderate to severe hepatic steatosis on ultrasonography, and by the absence of iron overload, hepatitis B or C infections, and excessive alcohol consumption. Controls were participants in the same data set who did not have underlying liver disease and had normal ultrasound and liver function tests.
There were a total of 2,441 people with NAFLD and 8,423 controls. During a median follow-up of 14.3 years, 14% of controls (1,193), and 21% of those with NAFLD (501) died, a difference that was significant in a univariate analysis (P = .0328).
But when they looked at overall mortality, cancer-related mortality, and cardiovascular mortality, they found that all three categories shared male sex, older age, and a low HDL level as independent predictors for death, with cardiovascular mortality having the added bonus of the metabolic syndrome as an additional risk factor.
The authors did not disclose a funding source. Dr. Chalasani and Dr. Lindor reported having no relevant financial disclosures.
ORLANDO – The risk factors for death in patients with nonalcoholic fatty liver disease include older age, male sex, truncal obesity, and a low HDL cholesterol level – in other words, the same factors that increase risk for death from cardiovascular disease and other causes, according to Dr. Naga P. Chalasani.
On the other hand, elevations in alanine aminotransferase (ALT) levels in patients with NAFLD are not associated with an increased risk for death or other poor outcomes, meaning that researchers may have to burrow more deeply through the available data to find risk predictors unique to NAFLD, said Dr Chalasani of Indiana University, Indianapolis.
"How do we identify someone with NAFLD who is at risk for poor outcomes? I think this is the first shot at risk mapping patients," Dr. Chalasani said at the annual Digestive Disease Week.
Dr. Keith D. Lindor, who moderated the session at which the data were presented, agreed.
"What we’re having trouble with, I think, is defining nonalcoholic fatty liver disease easily, particularly amongst the population," he said. "We saw data that ALT, which we commonly used to use, may not be telling, and there are questions about how well ultrasound detects [NAFLD], particularly given that the amount of steatosis in order to be detected by ultrasound has to be relatively dramatic."
It is still not known whether people with steatosis discovered during biopsy but not visible on ultrasound will have risk factors similar to those of people with more grossly evident steatosis, he said in an interview.
Although Dr. Chalasani and colleagues failed to find unique risk markers in this population, it was not for want of trying. The investigators pored over data from the third National Health and Nutrition Examination Survey (NHANES III) for baseline and follow-up information about patients with NAFLD.
The data were collected from 1988 through 1994, and included gallbladder ultrasound with liver images in 14,797 adults aged 20-74. The authors linked the data to the National Death Index in an attempt to determine which factors might be harbingers of early mortality in patients with NAFLD vs. controls.
They defined NAFLD by the presence of moderate to severe hepatic steatosis on ultrasonography, and by the absence of iron overload, hepatitis B or C infections, and excessive alcohol consumption. Controls were participants in the same data set who did not have underlying liver disease and had normal ultrasound and liver function tests.
There were a total of 2,441 people with NAFLD and 8,423 controls. During a median follow-up of 14.3 years, 14% of controls (1,193), and 21% of those with NAFLD (501) died, a difference that was significant in a univariate analysis (P = .0328).
But when they looked at overall mortality, cancer-related mortality, and cardiovascular mortality, they found that all three categories shared male sex, older age, and a low HDL level as independent predictors for death, with cardiovascular mortality having the added bonus of the metabolic syndrome as an additional risk factor.
The authors did not disclose a funding source. Dr. Chalasani and Dr. Lindor reported having no relevant financial disclosures.
AT DDW 2013
Major finding: Age, male sex, truncal obesity, and a low HDL level are risk factors for death in patients with NAFLD, but are common to other causes of death as well.
Data source: A review of data from the third National Health and Nutrition Examination Survey.
Disclosures: The authors did not disclose a funding source. Dr. Chalasani and Dr. Lindor reported having no relevant financial disclosures.
Serum cytokeratin fragments correlate with NASH histology
ORLANDO – Changes in serum levels of cytokeratin fragments appear to reflect changes in liver histology in patients with nonalcoholic steatohepatitis, Dr. Raj Vuppalanchi reported at the annual Digestive Disease Week.
Among 231 participants in the PIVENS (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis) trial, every 100-U/L decline in serum cytokeratin fragment (CK-18) level was significantly associated with overall histological improvement (P less than .001); resolution of NASH (P = .002); and improvement of at least 1 point in steatosis grade, hepatocellular ballooning, and nonalcoholic fatty liver disease (NAFLD) score (P less than .001 for all).
"We feel that serum CK-18 is a potentially useful surrogate marker for detection of improvement in clinical trials for NASH," said Dr. Vuppalanchi from Indiana University in Indianapolis.
Dr. Keith D. Lindor, executive vice provost for health at Arizona State University in Phoenix, said in an interview that CK-18 shows promise as a marker for disease activity in NASH but offers only limited information.
"It doesn’t hold the possibility of giving as much detailed information as a biopsy. We look at the amount of fat, amount of inflammation, amount of scarring – the biopsy lets us do that, but I don’t think a single serum assay will allow that," he said.
Dr. Lindor, who was not involved in the study, moderated the session at which the data were presented.
In previous cross-sectional studies, circulating CK-18 levels were shown to be associated with steatohepatitis in people with NAFLD, but it was unclear whether longitudinal changes in CK-18 would reflect changes in liver histology, Dr. Vuppalanchi said.
The investigators looked at CK-18 levels measured at baseline and at 16, 48, and 96 months among 231 of the 247 patients enrolled in the PIVENS trial, which compared vitamin E and/or pioglitazone against placebo in nondiabetic patients with NASH. The participants had liver biopsies at baseline and after 96 weeks of treatment.
The main trial results showed that vitamin E, but not pioglitazone, was significantly better than placebo at improvement of steatohepatitis.
In this substudy, the authors found that, compared with placebo, serum CK-18 levels were significantly lower in vitamin E–treated patients (P = .02 at 16 weeks, and P = .009 at 48 and 96 weeks). Among pioglitazone-treated patients, there was a similar pattern of lower CK-18 levels vs. placebo at all three time intervals (P = .001 for all).
Reductions in CK-18 correlated strongly with disease measures. For each 100-U/L decrease in CK-18 over 96 weeks, the odds ratios (ORs) were as follows: overall histological improvement (OR, 1.41; P less than .001); resolution of NASH (OR, 1.31; P = .002); and 1 point or more improvement in steatosis grade (OR, 1.45; P less than .001), hepatocellular ballooning (OR, 1.36; P less than .001), and NAFLD (OR, 1.41; P less than .001).
The study was supported by the National Institutes of Health with additional funding from Takeda Pharmaceuticals. Dr. Vuppalanchi and Dr. Lindor reported having no financial disclosures.
ORLANDO – Changes in serum levels of cytokeratin fragments appear to reflect changes in liver histology in patients with nonalcoholic steatohepatitis, Dr. Raj Vuppalanchi reported at the annual Digestive Disease Week.
Among 231 participants in the PIVENS (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis) trial, every 100-U/L decline in serum cytokeratin fragment (CK-18) level was significantly associated with overall histological improvement (P less than .001); resolution of NASH (P = .002); and improvement of at least 1 point in steatosis grade, hepatocellular ballooning, and nonalcoholic fatty liver disease (NAFLD) score (P less than .001 for all).
"We feel that serum CK-18 is a potentially useful surrogate marker for detection of improvement in clinical trials for NASH," said Dr. Vuppalanchi from Indiana University in Indianapolis.
Dr. Keith D. Lindor, executive vice provost for health at Arizona State University in Phoenix, said in an interview that CK-18 shows promise as a marker for disease activity in NASH but offers only limited information.
"It doesn’t hold the possibility of giving as much detailed information as a biopsy. We look at the amount of fat, amount of inflammation, amount of scarring – the biopsy lets us do that, but I don’t think a single serum assay will allow that," he said.
Dr. Lindor, who was not involved in the study, moderated the session at which the data were presented.
In previous cross-sectional studies, circulating CK-18 levels were shown to be associated with steatohepatitis in people with NAFLD, but it was unclear whether longitudinal changes in CK-18 would reflect changes in liver histology, Dr. Vuppalanchi said.
The investigators looked at CK-18 levels measured at baseline and at 16, 48, and 96 months among 231 of the 247 patients enrolled in the PIVENS trial, which compared vitamin E and/or pioglitazone against placebo in nondiabetic patients with NASH. The participants had liver biopsies at baseline and after 96 weeks of treatment.
The main trial results showed that vitamin E, but not pioglitazone, was significantly better than placebo at improvement of steatohepatitis.
In this substudy, the authors found that, compared with placebo, serum CK-18 levels were significantly lower in vitamin E–treated patients (P = .02 at 16 weeks, and P = .009 at 48 and 96 weeks). Among pioglitazone-treated patients, there was a similar pattern of lower CK-18 levels vs. placebo at all three time intervals (P = .001 for all).
Reductions in CK-18 correlated strongly with disease measures. For each 100-U/L decrease in CK-18 over 96 weeks, the odds ratios (ORs) were as follows: overall histological improvement (OR, 1.41; P less than .001); resolution of NASH (OR, 1.31; P = .002); and 1 point or more improvement in steatosis grade (OR, 1.45; P less than .001), hepatocellular ballooning (OR, 1.36; P less than .001), and NAFLD (OR, 1.41; P less than .001).
The study was supported by the National Institutes of Health with additional funding from Takeda Pharmaceuticals. Dr. Vuppalanchi and Dr. Lindor reported having no financial disclosures.
ORLANDO – Changes in serum levels of cytokeratin fragments appear to reflect changes in liver histology in patients with nonalcoholic steatohepatitis, Dr. Raj Vuppalanchi reported at the annual Digestive Disease Week.
Among 231 participants in the PIVENS (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis) trial, every 100-U/L decline in serum cytokeratin fragment (CK-18) level was significantly associated with overall histological improvement (P less than .001); resolution of NASH (P = .002); and improvement of at least 1 point in steatosis grade, hepatocellular ballooning, and nonalcoholic fatty liver disease (NAFLD) score (P less than .001 for all).
"We feel that serum CK-18 is a potentially useful surrogate marker for detection of improvement in clinical trials for NASH," said Dr. Vuppalanchi from Indiana University in Indianapolis.
Dr. Keith D. Lindor, executive vice provost for health at Arizona State University in Phoenix, said in an interview that CK-18 shows promise as a marker for disease activity in NASH but offers only limited information.
"It doesn’t hold the possibility of giving as much detailed information as a biopsy. We look at the amount of fat, amount of inflammation, amount of scarring – the biopsy lets us do that, but I don’t think a single serum assay will allow that," he said.
Dr. Lindor, who was not involved in the study, moderated the session at which the data were presented.
In previous cross-sectional studies, circulating CK-18 levels were shown to be associated with steatohepatitis in people with NAFLD, but it was unclear whether longitudinal changes in CK-18 would reflect changes in liver histology, Dr. Vuppalanchi said.
The investigators looked at CK-18 levels measured at baseline and at 16, 48, and 96 months among 231 of the 247 patients enrolled in the PIVENS trial, which compared vitamin E and/or pioglitazone against placebo in nondiabetic patients with NASH. The participants had liver biopsies at baseline and after 96 weeks of treatment.
The main trial results showed that vitamin E, but not pioglitazone, was significantly better than placebo at improvement of steatohepatitis.
In this substudy, the authors found that, compared with placebo, serum CK-18 levels were significantly lower in vitamin E–treated patients (P = .02 at 16 weeks, and P = .009 at 48 and 96 weeks). Among pioglitazone-treated patients, there was a similar pattern of lower CK-18 levels vs. placebo at all three time intervals (P = .001 for all).
Reductions in CK-18 correlated strongly with disease measures. For each 100-U/L decrease in CK-18 over 96 weeks, the odds ratios (ORs) were as follows: overall histological improvement (OR, 1.41; P less than .001); resolution of NASH (OR, 1.31; P = .002); and 1 point or more improvement in steatosis grade (OR, 1.45; P less than .001), hepatocellular ballooning (OR, 1.36; P less than .001), and NAFLD (OR, 1.41; P less than .001).
The study was supported by the National Institutes of Health with additional funding from Takeda Pharmaceuticals. Dr. Vuppalanchi and Dr. Lindor reported having no financial disclosures.
AT DDW 2013
Major finding: Every 100-U/L decline in serum cytokeratin fragment (CK-18) levels was significantly associated with overall histological improvement of NAFLD.
Data source: Subanalysis of data from the randomized controlled PIVENS trial.
Disclosures: The study was supported by the National Institutes of Health with additional funding from Takeda Pharmaceuticals. Dr. Vuppalanchi and Dr. Lindor reported having no financial disclosures.
Hint of prolonged response to vedoluzimab seen in Crohn's
ORLANDO – In patients with Crohn’s disease, a response to the investigational monoclonal antibody vedoluzimab within 6 weeks of initiating therapy was predictive of a continued response to the drug, even at lower doses.
Among patients in the GEMINI II trial with a documented response to vedoluzimab after 6 weeks, 32% of those who were then randomized to receive the drug once every 8 weeks for an additional 46 weeks had a corticosteroid-free clinical remission of Crohn’s disease (CD), as did 29% of those who continued to receive the same dose every 4 weeks and 16% of those on placebo, said Dr. William Sandborn at the annual Digestive Disease Week.
However, the rate of durable clinical remissions, defined as clinical remissions at 80% or more of study visits, was comparable for both dosing groups and the placebo group.
"Patients who had a clinical response to vedolizumab by week 6 then went on to have stable clinical remission rates throughout the maintenance phase and significantly higher clinical remission rates than placebo by week 52," said Dr. Sandborn of the University of California, San Diego.
Vedolizumab is an investigational, gut-selective monoclonal antibody targeting the alpha-4 beta-7 integrin. In GEMINI II, the drug was shown to be more effective than placebo for induction and maintenance therapy of CD.
For this analysis, the researchers dug deeper into the data from GEMINI II and looked at maintenance-phase outcomes for those patients who had a clinical response to the drug by week 6 of the trial.
Patients in the trial were adults 18-80 years old with a diagnosis of CD at least 3 months before study entry, moderate to severe CD as determined by a CD Activity Index (CDAI) score of 220-450 at screening, and either intolerance of or an inadequate response to purine antimetabolites or anti–tumor necrosis factor (anti-TNF) agents.
A clinical response to vedolizumab was defined as at least a 70-point decline in CDAI score from baseline value at week 6 following two induction doses of therapy. Patients were randomized on a 1:1:1 basis to receive vedolizumab via infusion every 8 weeks, the same dose every 4 weeks, or placebo until week 52.
Patients who did not have a clinical response by week 6 were treated with open-label vedolizumab at the 300-mg dose every 8 weeks until week 52, and were assessed with those patients who had been on placebo throughout the induction and maintenance phases.
CDAI scores among 153 patients on placebo stabilized at 26 weeks, but continued to decline through week 52 among patients on vedolizumab at both dosing frequencies (154 patients in each dosing group). Rates of clinical remission (CDAI score of 150 or lower) remained relatively stable among patients on vedolizumab, but declined among those on placebo.
A corticosteroid-free remission was seen at week 52 in 32% of patients on the 8-week schedule and in 16% of those on placebo (P = .015). The remission rate was 29% for those on the 4-week schedule (P vs. placebo = .045).
In addition, 21% of those on vedolizumab every 8 weeks had durable clinical remissions, as did 16% of those on the every-4-week dose and 14% of those on placebo. There were no statistically significant differences among the three groups.
In the question-and-answer session following presentation of the results, an attendee commented that "it’s a little disturbing that the more frequent dose seemed to be numerically inferior to the less-frequent dose at virtually every measured outcome."
Dr. Sandborn said that the investigators have extensively examined that question and determined that "there’s noise around the measurements, but you couldn’t draw any firm statistical conclusions."
The study was funded by Millennium/Takeda. Dr. Sandborn disclosed serving as a consultant and receiving grant and research support from the combined companies.
ORLANDO – In patients with Crohn’s disease, a response to the investigational monoclonal antibody vedoluzimab within 6 weeks of initiating therapy was predictive of a continued response to the drug, even at lower doses.
Among patients in the GEMINI II trial with a documented response to vedoluzimab after 6 weeks, 32% of those who were then randomized to receive the drug once every 8 weeks for an additional 46 weeks had a corticosteroid-free clinical remission of Crohn’s disease (CD), as did 29% of those who continued to receive the same dose every 4 weeks and 16% of those on placebo, said Dr. William Sandborn at the annual Digestive Disease Week.
However, the rate of durable clinical remissions, defined as clinical remissions at 80% or more of study visits, was comparable for both dosing groups and the placebo group.
"Patients who had a clinical response to vedolizumab by week 6 then went on to have stable clinical remission rates throughout the maintenance phase and significantly higher clinical remission rates than placebo by week 52," said Dr. Sandborn of the University of California, San Diego.
Vedolizumab is an investigational, gut-selective monoclonal antibody targeting the alpha-4 beta-7 integrin. In GEMINI II, the drug was shown to be more effective than placebo for induction and maintenance therapy of CD.
For this analysis, the researchers dug deeper into the data from GEMINI II and looked at maintenance-phase outcomes for those patients who had a clinical response to the drug by week 6 of the trial.
Patients in the trial were adults 18-80 years old with a diagnosis of CD at least 3 months before study entry, moderate to severe CD as determined by a CD Activity Index (CDAI) score of 220-450 at screening, and either intolerance of or an inadequate response to purine antimetabolites or anti–tumor necrosis factor (anti-TNF) agents.
A clinical response to vedolizumab was defined as at least a 70-point decline in CDAI score from baseline value at week 6 following two induction doses of therapy. Patients were randomized on a 1:1:1 basis to receive vedolizumab via infusion every 8 weeks, the same dose every 4 weeks, or placebo until week 52.
Patients who did not have a clinical response by week 6 were treated with open-label vedolizumab at the 300-mg dose every 8 weeks until week 52, and were assessed with those patients who had been on placebo throughout the induction and maintenance phases.
CDAI scores among 153 patients on placebo stabilized at 26 weeks, but continued to decline through week 52 among patients on vedolizumab at both dosing frequencies (154 patients in each dosing group). Rates of clinical remission (CDAI score of 150 or lower) remained relatively stable among patients on vedolizumab, but declined among those on placebo.
A corticosteroid-free remission was seen at week 52 in 32% of patients on the 8-week schedule and in 16% of those on placebo (P = .015). The remission rate was 29% for those on the 4-week schedule (P vs. placebo = .045).
In addition, 21% of those on vedolizumab every 8 weeks had durable clinical remissions, as did 16% of those on the every-4-week dose and 14% of those on placebo. There were no statistically significant differences among the three groups.
In the question-and-answer session following presentation of the results, an attendee commented that "it’s a little disturbing that the more frequent dose seemed to be numerically inferior to the less-frequent dose at virtually every measured outcome."
Dr. Sandborn said that the investigators have extensively examined that question and determined that "there’s noise around the measurements, but you couldn’t draw any firm statistical conclusions."
The study was funded by Millennium/Takeda. Dr. Sandborn disclosed serving as a consultant and receiving grant and research support from the combined companies.
ORLANDO – In patients with Crohn’s disease, a response to the investigational monoclonal antibody vedoluzimab within 6 weeks of initiating therapy was predictive of a continued response to the drug, even at lower doses.
Among patients in the GEMINI II trial with a documented response to vedoluzimab after 6 weeks, 32% of those who were then randomized to receive the drug once every 8 weeks for an additional 46 weeks had a corticosteroid-free clinical remission of Crohn’s disease (CD), as did 29% of those who continued to receive the same dose every 4 weeks and 16% of those on placebo, said Dr. William Sandborn at the annual Digestive Disease Week.
However, the rate of durable clinical remissions, defined as clinical remissions at 80% or more of study visits, was comparable for both dosing groups and the placebo group.
"Patients who had a clinical response to vedolizumab by week 6 then went on to have stable clinical remission rates throughout the maintenance phase and significantly higher clinical remission rates than placebo by week 52," said Dr. Sandborn of the University of California, San Diego.
Vedolizumab is an investigational, gut-selective monoclonal antibody targeting the alpha-4 beta-7 integrin. In GEMINI II, the drug was shown to be more effective than placebo for induction and maintenance therapy of CD.
For this analysis, the researchers dug deeper into the data from GEMINI II and looked at maintenance-phase outcomes for those patients who had a clinical response to the drug by week 6 of the trial.
Patients in the trial were adults 18-80 years old with a diagnosis of CD at least 3 months before study entry, moderate to severe CD as determined by a CD Activity Index (CDAI) score of 220-450 at screening, and either intolerance of or an inadequate response to purine antimetabolites or anti–tumor necrosis factor (anti-TNF) agents.
A clinical response to vedolizumab was defined as at least a 70-point decline in CDAI score from baseline value at week 6 following two induction doses of therapy. Patients were randomized on a 1:1:1 basis to receive vedolizumab via infusion every 8 weeks, the same dose every 4 weeks, or placebo until week 52.
Patients who did not have a clinical response by week 6 were treated with open-label vedolizumab at the 300-mg dose every 8 weeks until week 52, and were assessed with those patients who had been on placebo throughout the induction and maintenance phases.
CDAI scores among 153 patients on placebo stabilized at 26 weeks, but continued to decline through week 52 among patients on vedolizumab at both dosing frequencies (154 patients in each dosing group). Rates of clinical remission (CDAI score of 150 or lower) remained relatively stable among patients on vedolizumab, but declined among those on placebo.
A corticosteroid-free remission was seen at week 52 in 32% of patients on the 8-week schedule and in 16% of those on placebo (P = .015). The remission rate was 29% for those on the 4-week schedule (P vs. placebo = .045).
In addition, 21% of those on vedolizumab every 8 weeks had durable clinical remissions, as did 16% of those on the every-4-week dose and 14% of those on placebo. There were no statistically significant differences among the three groups.
In the question-and-answer session following presentation of the results, an attendee commented that "it’s a little disturbing that the more frequent dose seemed to be numerically inferior to the less-frequent dose at virtually every measured outcome."
Dr. Sandborn said that the investigators have extensively examined that question and determined that "there’s noise around the measurements, but you couldn’t draw any firm statistical conclusions."
The study was funded by Millennium/Takeda. Dr. Sandborn disclosed serving as a consultant and receiving grant and research support from the combined companies.
AT DDW 2013
Major finding: Among patients with a documented response to vedoluzimab after 6 weeks, 32% of those who received it every 8 weeks had a clinical remission at week 52, compared with 29% on an every-4-week dose and 16% of those on placebo.
Data source: Subanalysis of 461 patients in the maintenance phase of a randomized controlled trial.
Disclosures: The study was funded by Millennium/Takeda. Dr. Sandborn disclosed serving as a consultant and receiving grant and research support from the combined companies.
Skip the sphincterotomy before bile duct stent placement
ORLANDO – Patients with unresectable pancreatic cancer may be safely spared from endoscopic sphincterotomy prior to stent placement, investigators said at the annual Digestive Disease Week.
Among patients undergoing placement of a self-expanding, 10-mm-diameter stent into the bile duct*, there were no differences in the rates of early or late complications between patients randomized to receive endoscopic sphincterotomy (ES) before stent placement and those who underwent stent placement alone, reported Dr. Tsuyoshi Hayashi from Sapporo Medical University in Japan.
"ES had no effect on not only the incidence of complications but also SEMS [self-expanding metal stent] patency and patient survival. ES is an unnecessary pretreatment prior to SEMS placement for distal biliary obstruction due to pancreatic cancer," Dr. Hayashi said.
Although endoscopic sphincterotomy is intended to prevent potential pancreatitis from the expansion forces of the stent on the opening of the pancreatic duct, it adds significantly to operative time and carries the risk of bleeding or perforation, Dr. Hayashi said.
To see whether the endoscopist could safely forego sphincterotomy, investigators in 25 hospitals on Japan’s northern island of Hokkaido enrolled a total of 200 patients into a randomized trial and assigned them to stent placement with or without sphincterotomy. Four patients assigned to sphincterotomy and two assigned to stenting alone were unable to undergo biliary cannulation, leaving 96 and 98 patients, respectively, for the analysis.
The authors found that sphincterotomy added significantly to the procedural time, at a mean of 577 (plus or minus 310) seconds, compared with 388 (plus or minus 203) seconds for the no-sphincterotomy procedures.
But when they looked at early complications (within 30 days of the procedure), they found that there were no significant between-group differences in the rates of mild to severe pancreatitis, moderate bleeding, mild perforation, moderate hepatic abscess, pain, or vomiting.
In addition, there were no significant between-group differences in late complications, including either mild to severe cholecystitis, pancreatitis, bleeding, or duodenal ulcer. Similarly, there were no significant differences between the sphincterotomy group and the no-sphincterotomy group in either median time to stent dysfunction (170.5 vs. 148.0 days, respectively) or median overall survival (242 vs. 202 days).
Causes of stent dysfunction, which occurred in 25 patients in each group, included food impaction, sludge formation, intestinal obstruction, migration, and tumor ingrowth or overgrowth, but there were no significant between-group differences in the causes.
The study funding source was not disclosed. Dr. Hayashi and his colleagues reported having no financial disclosures.
*Correction 7/25/2013: An earlier version of this story missnamed this duct and stent.
For patients with unresectable pancreatic cancer, self-expandable metallic stents (SEMS) are preferred to smaller-caliber, plastic stents for the palliation of jaundice. SEMS have longer patency rates and reduce the overall costs of care by obviating the need for stent exchanges in the majority of cases. Further, SEMS have largely replaced surgical bypass of the bile duct because of reduced morbidity and acceptable durability since most patients with unresectable pancreatic cancer are not expected to survive more than 1 year.
The decision to place a SEMS with complete or partial coating, primarily designed to reduce the likelihood of tumor ingrowth within the interstices of the stent, is more complex. Coating reduces ingrowth and modestly prolongs patency, but increases the likelihood of stent migration and possibly cholecystitis (due to occlusion of the cystic duct). Presumably due to their larger diameter than plastic stents, previous studies suggested a higher rate of post-ERCP (post-endoscopic retrograde cholangiopancreatography) pancreatitis, irrespective of preexisting endoscopic sphincterotomy.
 |
| Dr. Gregory Cote |
Second, pancreatitis rates of 8%-9% are higher than expected for a low-risk indication such as malignant bile duct obstruction. The higher rates in this trial may be related to study design (patients in clinical trials are expected to have higher event rates due to meticulous follow-up compared with retrospective studies), use of SEMS in all patients, and specificity of the definition of post-ERCP pancreatitis in this population: Patients with pancreatic cancer are more likely to complain of pain following ERCP that is related to expansion of the metal stent, and a rise in serum pancreatic enzymes that may not necessarily connote true acute pancreatitis. This requires further investigation.
Third, bleeding and perforation are uncommon complications of ERCP with or without sphincterotomy when a high-volume physician performs the procedure. These data corroborate this - the argument that skipping the sphincterotomy will lower an already rare set of events is challenging. In fact, the only observed perforation occurred in a patient randomized to no sphincterotomy! Last, the higher rates (albeit not statistically significant) of hepatic abscess and cholecystitis among patients who did not undergo a sphincterotomy should not be ignored.
In some cases, sphincterotomy facilitates passage of the stent delivery catheter and tissue sampling devices, if needed. Nevertheless, endoscopists should be reassured by the equivalent rates of pancreatitis in this clinical trial. Based on these results, it seems appropriate to withhold sphincterotomy, at least in cases where bleeding may be high risk (e.g., with ongoing use of antiplatelet or anticoagulant medications).
Dr. Gregory A. Cote is an assistant professor of medicine in the department of medicine, division of gastroenterology and hepatology, Indiana University School of Medicine, Indianapolis. He is a consultant and member of the advisory board for Boston Scientific and a member of the advisory board for Olympus America.
For patients with unresectable pancreatic cancer, self-expandable metallic stents (SEMS) are preferred to smaller-caliber, plastic stents for the palliation of jaundice. SEMS have longer patency rates and reduce the overall costs of care by obviating the need for stent exchanges in the majority of cases. Further, SEMS have largely replaced surgical bypass of the bile duct because of reduced morbidity and acceptable durability since most patients with unresectable pancreatic cancer are not expected to survive more than 1 year.
The decision to place a SEMS with complete or partial coating, primarily designed to reduce the likelihood of tumor ingrowth within the interstices of the stent, is more complex. Coating reduces ingrowth and modestly prolongs patency, but increases the likelihood of stent migration and possibly cholecystitis (due to occlusion of the cystic duct). Presumably due to their larger diameter than plastic stents, previous studies suggested a higher rate of post-ERCP (post-endoscopic retrograde cholangiopancreatography) pancreatitis, irrespective of preexisting endoscopic sphincterotomy.
 |
| Dr. Gregory Cote |
Second, pancreatitis rates of 8%-9% are higher than expected for a low-risk indication such as malignant bile duct obstruction. The higher rates in this trial may be related to study design (patients in clinical trials are expected to have higher event rates due to meticulous follow-up compared with retrospective studies), use of SEMS in all patients, and specificity of the definition of post-ERCP pancreatitis in this population: Patients with pancreatic cancer are more likely to complain of pain following ERCP that is related to expansion of the metal stent, and a rise in serum pancreatic enzymes that may not necessarily connote true acute pancreatitis. This requires further investigation.
Third, bleeding and perforation are uncommon complications of ERCP with or without sphincterotomy when a high-volume physician performs the procedure. These data corroborate this - the argument that skipping the sphincterotomy will lower an already rare set of events is challenging. In fact, the only observed perforation occurred in a patient randomized to no sphincterotomy! Last, the higher rates (albeit not statistically significant) of hepatic abscess and cholecystitis among patients who did not undergo a sphincterotomy should not be ignored.
In some cases, sphincterotomy facilitates passage of the stent delivery catheter and tissue sampling devices, if needed. Nevertheless, endoscopists should be reassured by the equivalent rates of pancreatitis in this clinical trial. Based on these results, it seems appropriate to withhold sphincterotomy, at least in cases where bleeding may be high risk (e.g., with ongoing use of antiplatelet or anticoagulant medications).
Dr. Gregory A. Cote is an assistant professor of medicine in the department of medicine, division of gastroenterology and hepatology, Indiana University School of Medicine, Indianapolis. He is a consultant and member of the advisory board for Boston Scientific and a member of the advisory board for Olympus America.
For patients with unresectable pancreatic cancer, self-expandable metallic stents (SEMS) are preferred to smaller-caliber, plastic stents for the palliation of jaundice. SEMS have longer patency rates and reduce the overall costs of care by obviating the need for stent exchanges in the majority of cases. Further, SEMS have largely replaced surgical bypass of the bile duct because of reduced morbidity and acceptable durability since most patients with unresectable pancreatic cancer are not expected to survive more than 1 year.
The decision to place a SEMS with complete or partial coating, primarily designed to reduce the likelihood of tumor ingrowth within the interstices of the stent, is more complex. Coating reduces ingrowth and modestly prolongs patency, but increases the likelihood of stent migration and possibly cholecystitis (due to occlusion of the cystic duct). Presumably due to their larger diameter than plastic stents, previous studies suggested a higher rate of post-ERCP (post-endoscopic retrograde cholangiopancreatography) pancreatitis, irrespective of preexisting endoscopic sphincterotomy.
 |
| Dr. Gregory Cote |
Second, pancreatitis rates of 8%-9% are higher than expected for a low-risk indication such as malignant bile duct obstruction. The higher rates in this trial may be related to study design (patients in clinical trials are expected to have higher event rates due to meticulous follow-up compared with retrospective studies), use of SEMS in all patients, and specificity of the definition of post-ERCP pancreatitis in this population: Patients with pancreatic cancer are more likely to complain of pain following ERCP that is related to expansion of the metal stent, and a rise in serum pancreatic enzymes that may not necessarily connote true acute pancreatitis. This requires further investigation.
Third, bleeding and perforation are uncommon complications of ERCP with or without sphincterotomy when a high-volume physician performs the procedure. These data corroborate this - the argument that skipping the sphincterotomy will lower an already rare set of events is challenging. In fact, the only observed perforation occurred in a patient randomized to no sphincterotomy! Last, the higher rates (albeit not statistically significant) of hepatic abscess and cholecystitis among patients who did not undergo a sphincterotomy should not be ignored.
In some cases, sphincterotomy facilitates passage of the stent delivery catheter and tissue sampling devices, if needed. Nevertheless, endoscopists should be reassured by the equivalent rates of pancreatitis in this clinical trial. Based on these results, it seems appropriate to withhold sphincterotomy, at least in cases where bleeding may be high risk (e.g., with ongoing use of antiplatelet or anticoagulant medications).
Dr. Gregory A. Cote is an assistant professor of medicine in the department of medicine, division of gastroenterology and hepatology, Indiana University School of Medicine, Indianapolis. He is a consultant and member of the advisory board for Boston Scientific and a member of the advisory board for Olympus America.
ORLANDO – Patients with unresectable pancreatic cancer may be safely spared from endoscopic sphincterotomy prior to stent placement, investigators said at the annual Digestive Disease Week.
Among patients undergoing placement of a self-expanding, 10-mm-diameter stent into the bile duct*, there were no differences in the rates of early or late complications between patients randomized to receive endoscopic sphincterotomy (ES) before stent placement and those who underwent stent placement alone, reported Dr. Tsuyoshi Hayashi from Sapporo Medical University in Japan.
"ES had no effect on not only the incidence of complications but also SEMS [self-expanding metal stent] patency and patient survival. ES is an unnecessary pretreatment prior to SEMS placement for distal biliary obstruction due to pancreatic cancer," Dr. Hayashi said.
Although endoscopic sphincterotomy is intended to prevent potential pancreatitis from the expansion forces of the stent on the opening of the pancreatic duct, it adds significantly to operative time and carries the risk of bleeding or perforation, Dr. Hayashi said.
To see whether the endoscopist could safely forego sphincterotomy, investigators in 25 hospitals on Japan’s northern island of Hokkaido enrolled a total of 200 patients into a randomized trial and assigned them to stent placement with or without sphincterotomy. Four patients assigned to sphincterotomy and two assigned to stenting alone were unable to undergo biliary cannulation, leaving 96 and 98 patients, respectively, for the analysis.
The authors found that sphincterotomy added significantly to the procedural time, at a mean of 577 (plus or minus 310) seconds, compared with 388 (plus or minus 203) seconds for the no-sphincterotomy procedures.
But when they looked at early complications (within 30 days of the procedure), they found that there were no significant between-group differences in the rates of mild to severe pancreatitis, moderate bleeding, mild perforation, moderate hepatic abscess, pain, or vomiting.
In addition, there were no significant between-group differences in late complications, including either mild to severe cholecystitis, pancreatitis, bleeding, or duodenal ulcer. Similarly, there were no significant differences between the sphincterotomy group and the no-sphincterotomy group in either median time to stent dysfunction (170.5 vs. 148.0 days, respectively) or median overall survival (242 vs. 202 days).
Causes of stent dysfunction, which occurred in 25 patients in each group, included food impaction, sludge formation, intestinal obstruction, migration, and tumor ingrowth or overgrowth, but there were no significant between-group differences in the causes.
The study funding source was not disclosed. Dr. Hayashi and his colleagues reported having no financial disclosures.
*Correction 7/25/2013: An earlier version of this story missnamed this duct and stent.
ORLANDO – Patients with unresectable pancreatic cancer may be safely spared from endoscopic sphincterotomy prior to stent placement, investigators said at the annual Digestive Disease Week.
Among patients undergoing placement of a self-expanding, 10-mm-diameter stent into the bile duct*, there were no differences in the rates of early or late complications between patients randomized to receive endoscopic sphincterotomy (ES) before stent placement and those who underwent stent placement alone, reported Dr. Tsuyoshi Hayashi from Sapporo Medical University in Japan.
"ES had no effect on not only the incidence of complications but also SEMS [self-expanding metal stent] patency and patient survival. ES is an unnecessary pretreatment prior to SEMS placement for distal biliary obstruction due to pancreatic cancer," Dr. Hayashi said.
Although endoscopic sphincterotomy is intended to prevent potential pancreatitis from the expansion forces of the stent on the opening of the pancreatic duct, it adds significantly to operative time and carries the risk of bleeding or perforation, Dr. Hayashi said.
To see whether the endoscopist could safely forego sphincterotomy, investigators in 25 hospitals on Japan’s northern island of Hokkaido enrolled a total of 200 patients into a randomized trial and assigned them to stent placement with or without sphincterotomy. Four patients assigned to sphincterotomy and two assigned to stenting alone were unable to undergo biliary cannulation, leaving 96 and 98 patients, respectively, for the analysis.
The authors found that sphincterotomy added significantly to the procedural time, at a mean of 577 (plus or minus 310) seconds, compared with 388 (plus or minus 203) seconds for the no-sphincterotomy procedures.
But when they looked at early complications (within 30 days of the procedure), they found that there were no significant between-group differences in the rates of mild to severe pancreatitis, moderate bleeding, mild perforation, moderate hepatic abscess, pain, or vomiting.
In addition, there were no significant between-group differences in late complications, including either mild to severe cholecystitis, pancreatitis, bleeding, or duodenal ulcer. Similarly, there were no significant differences between the sphincterotomy group and the no-sphincterotomy group in either median time to stent dysfunction (170.5 vs. 148.0 days, respectively) or median overall survival (242 vs. 202 days).
Causes of stent dysfunction, which occurred in 25 patients in each group, included food impaction, sludge formation, intestinal obstruction, migration, and tumor ingrowth or overgrowth, but there were no significant between-group differences in the causes.
The study funding source was not disclosed. Dr. Hayashi and his colleagues reported having no financial disclosures.
*Correction 7/25/2013: An earlier version of this story missnamed this duct and stent.
AT DDW 2013
Major finding: There were no significant differences in early or late complications among patients with pancreatic cancer who underwent stent placement with or without sphincterotomy.
Data source: Randomized controlled trial in 200 patients from 25 treatment centers in Japan,
Disclosures: The study funding source was not disclosed. Dr. Hayashi and his colleagues reported having no financial disclosures.
Simultaneous resection reduced repeat intervention
ORLANDO – Combining surgical resection of primary carcinoid and neuroendocrine tumors with cytoreductive surgery of liver metastases is safe in select patients and can produce outcomes similar to those seen with surgery and adjuvant therapy, said investigators at the annual Digestive Disease Week.
Among 47 patients with carcinoid and neuroendocrine tumors (CNET), 22 of whom underwent simultaneous resection of primary tumors and surgical reduction of metastatic tumor burden with partial or near-total surgical cytoreduction (CR), and 25 of whom had resection of the primary with additional therapies, the 5-year survival rate was 82%, reported Dr. Nicholas N. Nissen of the Cedars-Sinai Medical Center in Los Angeles.
In addition, no patients who had near-total CR required a repeat hepatic intervention within 1 year of surgery, compared with 18 of 25 patients (72%) who had surgery followed by additional hepatic therapy, Dr. Nissen said.
"While most patients progress over time, overall survival is excellent, and we admit very freely that this reflects the growing and evolving multimodal therapies that are available to these patients," Dr. Nissen said.
"We would propose that simultaneous removal of the primary tumor and liver metastases may in fact be primarily beneficial as it consolidates procedures and allows some patients to delay or perhaps even avoid other locoregional hepatic therapies," he added.
The authors took a retrospective look at records of 51 patients, 47 of whom were available for the overall survival analysis. The patients all underwent resection of hepatic masses simultaneously with surgery to remove either small bowel carcinoid (32 patients) or pancreatic neuroendocrine tumor (15 patients). In all, 27 patients had partial surgical cytoreduction of metastases, and 22 had planned near-total surgical CR.
Of the 19 patients with carcinoid syndrome, all had what Dr. Nissen described as "dramatic improvement," and 11 had complete resolution of symptoms following surgery.
There were no deaths within 30 days of surgery. There were eight complications greater than Clavien grade 2, including two instances of bile leak requiring endoscopic retrograde cholangiopancreatography, and four cases requiring repeat laparotomy.
Overall survival for 47 patients with at least 5 years of data was 95% at 1 year, and 82% at years 3 and 5 of follow-up. Overall 5-year survival among those patients who had near-total CR was 88%, compared with 72% of those who had subtotal CR, but the difference was not statistically significant.
The respective progression-free survival rates were 77%, 37%, and 28%. Broken down by treatment type, 5-year PFS was 40% among patients who had near total CR, compared with 22% for those who had subtotal cytoreduction, but this difference was also not significant.
However, the seven patients who had no additional hepatic therapy had a more-than-threefold risk for progression, compared with either near total CR (hazard ratio [HR], 3.10; P = .044) or partial CR with additional liver treatment (HR, 3.37; P =.029).
No demographic or disease-related variables appeared to predict survival, but progression-free survival was significantly associated with poor differentiation (P = .022) and with large tumors (P = .044).
Dr. Carlos Corvera, a gastrointestinal surgeon at the University of California at San Francisco, who was not involved in the study, commented that the worse progression-free survival seen in the seven patients who did not receive adjuvant hepatic therapies suggested a benefit for additional cytoreduction, regardless of whether the patients are having symptoms.
"What do you tell your medical oncology colleagues who argue that disease-specific survival is really the most important thing, and that we end up predisposing these patients to possible [surgical] complications in situations where there’s really no significant survival benefit shown in asymptomatic patients?" he asked
Dr. Nissen replied that with the availability of new targeted systemic and locoregional therapies, in the near future the question may no longer when to operate and with what additional therapies, but "whether to operate."
The study sponsor was not disclosed. Dr. Nissen and Dr. Corvera reported having no relevant financial disclosures.
ORLANDO – Combining surgical resection of primary carcinoid and neuroendocrine tumors with cytoreductive surgery of liver metastases is safe in select patients and can produce outcomes similar to those seen with surgery and adjuvant therapy, said investigators at the annual Digestive Disease Week.
Among 47 patients with carcinoid and neuroendocrine tumors (CNET), 22 of whom underwent simultaneous resection of primary tumors and surgical reduction of metastatic tumor burden with partial or near-total surgical cytoreduction (CR), and 25 of whom had resection of the primary with additional therapies, the 5-year survival rate was 82%, reported Dr. Nicholas N. Nissen of the Cedars-Sinai Medical Center in Los Angeles.
In addition, no patients who had near-total CR required a repeat hepatic intervention within 1 year of surgery, compared with 18 of 25 patients (72%) who had surgery followed by additional hepatic therapy, Dr. Nissen said.
"While most patients progress over time, overall survival is excellent, and we admit very freely that this reflects the growing and evolving multimodal therapies that are available to these patients," Dr. Nissen said.
"We would propose that simultaneous removal of the primary tumor and liver metastases may in fact be primarily beneficial as it consolidates procedures and allows some patients to delay or perhaps even avoid other locoregional hepatic therapies," he added.
The authors took a retrospective look at records of 51 patients, 47 of whom were available for the overall survival analysis. The patients all underwent resection of hepatic masses simultaneously with surgery to remove either small bowel carcinoid (32 patients) or pancreatic neuroendocrine tumor (15 patients). In all, 27 patients had partial surgical cytoreduction of metastases, and 22 had planned near-total surgical CR.
Of the 19 patients with carcinoid syndrome, all had what Dr. Nissen described as "dramatic improvement," and 11 had complete resolution of symptoms following surgery.
There were no deaths within 30 days of surgery. There were eight complications greater than Clavien grade 2, including two instances of bile leak requiring endoscopic retrograde cholangiopancreatography, and four cases requiring repeat laparotomy.
Overall survival for 47 patients with at least 5 years of data was 95% at 1 year, and 82% at years 3 and 5 of follow-up. Overall 5-year survival among those patients who had near-total CR was 88%, compared with 72% of those who had subtotal CR, but the difference was not statistically significant.
The respective progression-free survival rates were 77%, 37%, and 28%. Broken down by treatment type, 5-year PFS was 40% among patients who had near total CR, compared with 22% for those who had subtotal cytoreduction, but this difference was also not significant.
However, the seven patients who had no additional hepatic therapy had a more-than-threefold risk for progression, compared with either near total CR (hazard ratio [HR], 3.10; P = .044) or partial CR with additional liver treatment (HR, 3.37; P =.029).
No demographic or disease-related variables appeared to predict survival, but progression-free survival was significantly associated with poor differentiation (P = .022) and with large tumors (P = .044).
Dr. Carlos Corvera, a gastrointestinal surgeon at the University of California at San Francisco, who was not involved in the study, commented that the worse progression-free survival seen in the seven patients who did not receive adjuvant hepatic therapies suggested a benefit for additional cytoreduction, regardless of whether the patients are having symptoms.
"What do you tell your medical oncology colleagues who argue that disease-specific survival is really the most important thing, and that we end up predisposing these patients to possible [surgical] complications in situations where there’s really no significant survival benefit shown in asymptomatic patients?" he asked
Dr. Nissen replied that with the availability of new targeted systemic and locoregional therapies, in the near future the question may no longer when to operate and with what additional therapies, but "whether to operate."
The study sponsor was not disclosed. Dr. Nissen and Dr. Corvera reported having no relevant financial disclosures.
ORLANDO – Combining surgical resection of primary carcinoid and neuroendocrine tumors with cytoreductive surgery of liver metastases is safe in select patients and can produce outcomes similar to those seen with surgery and adjuvant therapy, said investigators at the annual Digestive Disease Week.
Among 47 patients with carcinoid and neuroendocrine tumors (CNET), 22 of whom underwent simultaneous resection of primary tumors and surgical reduction of metastatic tumor burden with partial or near-total surgical cytoreduction (CR), and 25 of whom had resection of the primary with additional therapies, the 5-year survival rate was 82%, reported Dr. Nicholas N. Nissen of the Cedars-Sinai Medical Center in Los Angeles.
In addition, no patients who had near-total CR required a repeat hepatic intervention within 1 year of surgery, compared with 18 of 25 patients (72%) who had surgery followed by additional hepatic therapy, Dr. Nissen said.
"While most patients progress over time, overall survival is excellent, and we admit very freely that this reflects the growing and evolving multimodal therapies that are available to these patients," Dr. Nissen said.
"We would propose that simultaneous removal of the primary tumor and liver metastases may in fact be primarily beneficial as it consolidates procedures and allows some patients to delay or perhaps even avoid other locoregional hepatic therapies," he added.
The authors took a retrospective look at records of 51 patients, 47 of whom were available for the overall survival analysis. The patients all underwent resection of hepatic masses simultaneously with surgery to remove either small bowel carcinoid (32 patients) or pancreatic neuroendocrine tumor (15 patients). In all, 27 patients had partial surgical cytoreduction of metastases, and 22 had planned near-total surgical CR.
Of the 19 patients with carcinoid syndrome, all had what Dr. Nissen described as "dramatic improvement," and 11 had complete resolution of symptoms following surgery.
There were no deaths within 30 days of surgery. There were eight complications greater than Clavien grade 2, including two instances of bile leak requiring endoscopic retrograde cholangiopancreatography, and four cases requiring repeat laparotomy.
Overall survival for 47 patients with at least 5 years of data was 95% at 1 year, and 82% at years 3 and 5 of follow-up. Overall 5-year survival among those patients who had near-total CR was 88%, compared with 72% of those who had subtotal CR, but the difference was not statistically significant.
The respective progression-free survival rates were 77%, 37%, and 28%. Broken down by treatment type, 5-year PFS was 40% among patients who had near total CR, compared with 22% for those who had subtotal cytoreduction, but this difference was also not significant.
However, the seven patients who had no additional hepatic therapy had a more-than-threefold risk for progression, compared with either near total CR (hazard ratio [HR], 3.10; P = .044) or partial CR with additional liver treatment (HR, 3.37; P =.029).
No demographic or disease-related variables appeared to predict survival, but progression-free survival was significantly associated with poor differentiation (P = .022) and with large tumors (P = .044).
Dr. Carlos Corvera, a gastrointestinal surgeon at the University of California at San Francisco, who was not involved in the study, commented that the worse progression-free survival seen in the seven patients who did not receive adjuvant hepatic therapies suggested a benefit for additional cytoreduction, regardless of whether the patients are having symptoms.
"What do you tell your medical oncology colleagues who argue that disease-specific survival is really the most important thing, and that we end up predisposing these patients to possible [surgical] complications in situations where there’s really no significant survival benefit shown in asymptomatic patients?" he asked
Dr. Nissen replied that with the availability of new targeted systemic and locoregional therapies, in the near future the question may no longer when to operate and with what additional therapies, but "whether to operate."
The study sponsor was not disclosed. Dr. Nissen and Dr. Corvera reported having no relevant financial disclosures.
AT DDW 2013
Major finding: No patients who had surgical cytoreduction of liver metastases at the time or primary tumor resection required a repeat hepatic intervention within 1 year of surgery, compared with 18 of 25 patients (72%) who had surgery followed by additional hepatic therapy
Data source: Retrospective singe-institution case series.
Disclosures: The study sponsor was not disclosed. Dr. Nissen and Dr. Corvera reported having no relevant financial disclosures.
Nortriptyline no better than placebo for gastroparesis symptoms
ORLANDO – The tricyclic antidepressant nortriptyline is often prescribed for treatment of nausea, vomiting, and abdominal pain from gastroparesis, but results from a randomized clinical trial suggest that it doesn’t really work, investigators said at the annual Digestive Disease Week.
In a double-masked trial, 15 weeks of treatment with nortriptyline was no better than placebo for management of overall symptoms of idiopathic gastroparesis, reported Dr. Henry P. Parkman from Temple University in Philadelphia.
Although patients started on a 10-mg dose of nortriptyline had early improvement in nausea, this benefit disappeared as the dosage increased. In addition, 19 of the 65 patients assigned to the tricyclic antidepressant (TCA) opted to discontinue treatment early, primarily because of side effects, Dr. Parkman reported.
"Further studies will be needed to determine the role for TCAs and other neuromodulators in patients with idiopathic and also other etiologies of gastroparesis, and also to look at specific symptoms’ improvement associated with TCAs in gastroparesis," he said.
The investigators observed that the practice of treating idiopathic gastroparesis with TCAs is not well supported by clinical evidence, and took it on themselves to see whether they could gather sufficient evidence for or against the efficacy of these agents for this indication.
They chose nortriptyline because of its relatively low anticholinergic side effects, and used a dose-escalating scheme similar to that used in clinical practice.
They enrolled 130 patients into a placebo-controlled, parallel-group trial. Patients randomized to nortriptyline received it at a starting dose of 10 mg daily, which was escalated every 3 weeks to 25, 50, and finally 75 mg.
At the end of the 15 weeks, there were no significant differences between the groups in the primary endpoint, overall symptomatic improvement as measured by change in the 45-point, 9-symptom Gastroparesis Cardinal Symptom Index (GCSI). In all, 15 patients who received the TCA had at least a 50% decline from baseline GCSI score over two consecutive 3-week visits, compared with 14 patients on placebo.
Patients on nortriptyline did have a significant decline in both nausea (P = .04) and abdominal pain (P = .004) at the first visit, but this difference disappeared on subsequent visits.
At the end of the study, patients on the TCA also reported a greater improvement in appetite (P = .03), and showed a nonsignificant trend toward ability to finish a meal (P = .08). Patients on nortriptyline also had a slight gain in body mass index of 0.5 kg/m2, compared with a slight loss of –0.2 kg/m2 among patients on placebo (P = .03).
More than three times as many patients on nortriptyline stopped treatment early (19 vs. 6, P = .007), primarily because of side effects.
The investigators concluded that the role of TCAs and other neuromodulators for the treatment of idiopathic gastroparesis and other forms of gastrointestinal paralysis needs further exploration.
The study was funded by the National Institutes of Health. Dr. Parkman reported serving on advisory committees and review panels for Tranzyme, and consulting for Evoke, SmartPill, and other companies.
Gastroparesis (GP) is currently defined by a combination of symptoms (e.g., epigastric pain, nausea, vomiting, anorexia, bloating, and weight loss) and delayed gastric emptying in the absence of mechanical obstruction. The etiology of GP is diverse and includes diabetes, prior surgery, ischemia, connective tissue disorders, and vaccinations, although the largest group of GP patients is labeled as idiopathic.
Treatment of GP can be vexing for clinicians, as commonly used therapies (metoclopramide, erythromycin, and domperidone) are not uniformly effective, do not improve pain (one of the most common complaints in patients with GP), and are associated with side effects, some of which may be life-altering (i.e., tardive dyskinesia with metoclopramide). This has forced clinicians to search for alternative medications to treat GP symptoms.
Anecdotal reports have suggested that tricyclic antidepressants (TCAs) can improve symptoms in some patients with idiopathic GP. Theoretically, this appears to make sense, as TCAs improve visceral pain in patients with irritable bowel syndrome and functional abdominal pain. The prospective study by Parkman and colleagues was designed to evaluate the efficacy of nortriptyline in alleviating symptoms in patients with idiopathic GP. Frustratingly, nortriptyline was no better than placebo at improving global GP symptoms as measured by the Gastroparesis Cardinal Symptom Index.
These disappointing results may have occurred for a number of reasons, including the study being underpowered, the high dropout rate, too rapid dose escalation, use of a global scale, and not subtyping patients based on extent of pain or gastric emptying rates. These results should not dissuade clinicians from treating GP patients; rather, these results should serve as a stimulus to clinicians and researchers to identify an agent that can improve these chronic and debilitating symptoms.
Brian E. Lacy, M.D., Ph.D. is professor of medicine, section chief of gastroenterology and hepatology, and director of the GI Motility Laboratory at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H. He has no relevant financial disclosures.
Gastroparesis (GP) is currently defined by a combination of symptoms (e.g., epigastric pain, nausea, vomiting, anorexia, bloating, and weight loss) and delayed gastric emptying in the absence of mechanical obstruction. The etiology of GP is diverse and includes diabetes, prior surgery, ischemia, connective tissue disorders, and vaccinations, although the largest group of GP patients is labeled as idiopathic.
Treatment of GP can be vexing for clinicians, as commonly used therapies (metoclopramide, erythromycin, and domperidone) are not uniformly effective, do not improve pain (one of the most common complaints in patients with GP), and are associated with side effects, some of which may be life-altering (i.e., tardive dyskinesia with metoclopramide). This has forced clinicians to search for alternative medications to treat GP symptoms.
Anecdotal reports have suggested that tricyclic antidepressants (TCAs) can improve symptoms in some patients with idiopathic GP. Theoretically, this appears to make sense, as TCAs improve visceral pain in patients with irritable bowel syndrome and functional abdominal pain. The prospective study by Parkman and colleagues was designed to evaluate the efficacy of nortriptyline in alleviating symptoms in patients with idiopathic GP. Frustratingly, nortriptyline was no better than placebo at improving global GP symptoms as measured by the Gastroparesis Cardinal Symptom Index.
These disappointing results may have occurred for a number of reasons, including the study being underpowered, the high dropout rate, too rapid dose escalation, use of a global scale, and not subtyping patients based on extent of pain or gastric emptying rates. These results should not dissuade clinicians from treating GP patients; rather, these results should serve as a stimulus to clinicians and researchers to identify an agent that can improve these chronic and debilitating symptoms.
Brian E. Lacy, M.D., Ph.D. is professor of medicine, section chief of gastroenterology and hepatology, and director of the GI Motility Laboratory at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H. He has no relevant financial disclosures.
Gastroparesis (GP) is currently defined by a combination of symptoms (e.g., epigastric pain, nausea, vomiting, anorexia, bloating, and weight loss) and delayed gastric emptying in the absence of mechanical obstruction. The etiology of GP is diverse and includes diabetes, prior surgery, ischemia, connective tissue disorders, and vaccinations, although the largest group of GP patients is labeled as idiopathic.
Treatment of GP can be vexing for clinicians, as commonly used therapies (metoclopramide, erythromycin, and domperidone) are not uniformly effective, do not improve pain (one of the most common complaints in patients with GP), and are associated with side effects, some of which may be life-altering (i.e., tardive dyskinesia with metoclopramide). This has forced clinicians to search for alternative medications to treat GP symptoms.
Anecdotal reports have suggested that tricyclic antidepressants (TCAs) can improve symptoms in some patients with idiopathic GP. Theoretically, this appears to make sense, as TCAs improve visceral pain in patients with irritable bowel syndrome and functional abdominal pain. The prospective study by Parkman and colleagues was designed to evaluate the efficacy of nortriptyline in alleviating symptoms in patients with idiopathic GP. Frustratingly, nortriptyline was no better than placebo at improving global GP symptoms as measured by the Gastroparesis Cardinal Symptom Index.
These disappointing results may have occurred for a number of reasons, including the study being underpowered, the high dropout rate, too rapid dose escalation, use of a global scale, and not subtyping patients based on extent of pain or gastric emptying rates. These results should not dissuade clinicians from treating GP patients; rather, these results should serve as a stimulus to clinicians and researchers to identify an agent that can improve these chronic and debilitating symptoms.
Brian E. Lacy, M.D., Ph.D. is professor of medicine, section chief of gastroenterology and hepatology, and director of the GI Motility Laboratory at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H. He has no relevant financial disclosures.
ORLANDO – The tricyclic antidepressant nortriptyline is often prescribed for treatment of nausea, vomiting, and abdominal pain from gastroparesis, but results from a randomized clinical trial suggest that it doesn’t really work, investigators said at the annual Digestive Disease Week.
In a double-masked trial, 15 weeks of treatment with nortriptyline was no better than placebo for management of overall symptoms of idiopathic gastroparesis, reported Dr. Henry P. Parkman from Temple University in Philadelphia.
Although patients started on a 10-mg dose of nortriptyline had early improvement in nausea, this benefit disappeared as the dosage increased. In addition, 19 of the 65 patients assigned to the tricyclic antidepressant (TCA) opted to discontinue treatment early, primarily because of side effects, Dr. Parkman reported.
"Further studies will be needed to determine the role for TCAs and other neuromodulators in patients with idiopathic and also other etiologies of gastroparesis, and also to look at specific symptoms’ improvement associated with TCAs in gastroparesis," he said.
The investigators observed that the practice of treating idiopathic gastroparesis with TCAs is not well supported by clinical evidence, and took it on themselves to see whether they could gather sufficient evidence for or against the efficacy of these agents for this indication.
They chose nortriptyline because of its relatively low anticholinergic side effects, and used a dose-escalating scheme similar to that used in clinical practice.
They enrolled 130 patients into a placebo-controlled, parallel-group trial. Patients randomized to nortriptyline received it at a starting dose of 10 mg daily, which was escalated every 3 weeks to 25, 50, and finally 75 mg.
At the end of the 15 weeks, there were no significant differences between the groups in the primary endpoint, overall symptomatic improvement as measured by change in the 45-point, 9-symptom Gastroparesis Cardinal Symptom Index (GCSI). In all, 15 patients who received the TCA had at least a 50% decline from baseline GCSI score over two consecutive 3-week visits, compared with 14 patients on placebo.
Patients on nortriptyline did have a significant decline in both nausea (P = .04) and abdominal pain (P = .004) at the first visit, but this difference disappeared on subsequent visits.
At the end of the study, patients on the TCA also reported a greater improvement in appetite (P = .03), and showed a nonsignificant trend toward ability to finish a meal (P = .08). Patients on nortriptyline also had a slight gain in body mass index of 0.5 kg/m2, compared with a slight loss of –0.2 kg/m2 among patients on placebo (P = .03).
More than three times as many patients on nortriptyline stopped treatment early (19 vs. 6, P = .007), primarily because of side effects.
The investigators concluded that the role of TCAs and other neuromodulators for the treatment of idiopathic gastroparesis and other forms of gastrointestinal paralysis needs further exploration.
The study was funded by the National Institutes of Health. Dr. Parkman reported serving on advisory committees and review panels for Tranzyme, and consulting for Evoke, SmartPill, and other companies.
ORLANDO – The tricyclic antidepressant nortriptyline is often prescribed for treatment of nausea, vomiting, and abdominal pain from gastroparesis, but results from a randomized clinical trial suggest that it doesn’t really work, investigators said at the annual Digestive Disease Week.
In a double-masked trial, 15 weeks of treatment with nortriptyline was no better than placebo for management of overall symptoms of idiopathic gastroparesis, reported Dr. Henry P. Parkman from Temple University in Philadelphia.
Although patients started on a 10-mg dose of nortriptyline had early improvement in nausea, this benefit disappeared as the dosage increased. In addition, 19 of the 65 patients assigned to the tricyclic antidepressant (TCA) opted to discontinue treatment early, primarily because of side effects, Dr. Parkman reported.
"Further studies will be needed to determine the role for TCAs and other neuromodulators in patients with idiopathic and also other etiologies of gastroparesis, and also to look at specific symptoms’ improvement associated with TCAs in gastroparesis," he said.
The investigators observed that the practice of treating idiopathic gastroparesis with TCAs is not well supported by clinical evidence, and took it on themselves to see whether they could gather sufficient evidence for or against the efficacy of these agents for this indication.
They chose nortriptyline because of its relatively low anticholinergic side effects, and used a dose-escalating scheme similar to that used in clinical practice.
They enrolled 130 patients into a placebo-controlled, parallel-group trial. Patients randomized to nortriptyline received it at a starting dose of 10 mg daily, which was escalated every 3 weeks to 25, 50, and finally 75 mg.
At the end of the 15 weeks, there were no significant differences between the groups in the primary endpoint, overall symptomatic improvement as measured by change in the 45-point, 9-symptom Gastroparesis Cardinal Symptom Index (GCSI). In all, 15 patients who received the TCA had at least a 50% decline from baseline GCSI score over two consecutive 3-week visits, compared with 14 patients on placebo.
Patients on nortriptyline did have a significant decline in both nausea (P = .04) and abdominal pain (P = .004) at the first visit, but this difference disappeared on subsequent visits.
At the end of the study, patients on the TCA also reported a greater improvement in appetite (P = .03), and showed a nonsignificant trend toward ability to finish a meal (P = .08). Patients on nortriptyline also had a slight gain in body mass index of 0.5 kg/m2, compared with a slight loss of –0.2 kg/m2 among patients on placebo (P = .03).
More than three times as many patients on nortriptyline stopped treatment early (19 vs. 6, P = .007), primarily because of side effects.
The investigators concluded that the role of TCAs and other neuromodulators for the treatment of idiopathic gastroparesis and other forms of gastrointestinal paralysis needs further exploration.
The study was funded by the National Institutes of Health. Dr. Parkman reported serving on advisory committees and review panels for Tranzyme, and consulting for Evoke, SmartPill, and other companies.
AT DDW 2013
Major finding: There were no significant differences in improvement of gastroparesis symptom scores with nortriptyline vs. placebo.
Data source: Randomized, double-masked, placebo-controlled trial in 130 patients with gastroparesis.
Disclosures: The study was funded by the National Institutes of Health. Dr. Parkman reported serving on advisory committees and review panels for Tranzyme, and consulting for Evoke, SmartPill, and other companies.
Low vitamin D level may up risk for IBD flares
ORLANDO – Vitamin D may protect patients with inflammatory bowel disease from more serious disease flare-ups, investigators reported at the annual Digestive Disease Week.
Among 3,217 patients followed for a median of 8 years, those with Crohn’s disease who had the lowest levels of plasma 25-hydroxyvitamin D had a nearly twofold risk for surgery and double the risk for hospitalization related to IBD, compared with patients who had higher vitamin D levels.
A similar relationship was seen between vitamin D levels and risk of surgery and hospitalization among patients with ulcerative colitis (UC), reported Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston.
Tellingly, patients with Crohn’s disease (CD) who had initially low vitamin D levels that normalized during the study had significant reductions in their risk of surgery and hospitalization compared with patients whose vitamin D levels did not improve over time. In addition, patients with both CD and UC who normalized their vitamin D status during the study had significantly lower levels of the inflammatory marker C-reactive protein, the investigators found.
"There’s considerable evidence that supports a role for vitamin D in inflammatory bowel diseases," Dr. Ananthakrishnan said.
He noted that high vitamin D levels were associated with a reduced risk for CD in a prior study performed by his group (Gastroenterology 2012;142:482-9), and a second study showed that polymorphisms in the vitamin D receptor were associated with a risk of both CD and UC (Gut 2000;47:211-4).
Although vitamin D levels have been weakly associated with IBD exacerbations in retrospective studies, stronger evidence for the potential anti-inflammatory role of vitamin D has been hard to come by, partly because of researchers’ inability to determine vitamin D status before clinical outcomes such as surgery or hospitalization, Dr. Ananthakrishnan said.
He and colleagues prospectively followed all members of an IBD cohort treated at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston, who had a least one measured plasma 25(OH)D level before a first IBD-related surgery and/or hospitalization (the primary outcome; median C-reactive protein was a secondary outcome).
The researchers found that 16% of all patients had disease-related surgery, and 40% were hospitalized during the follow-up period.
A third of all patients (32%) were considered to be vitamin D deficient, defined as having a plasma 25(OH)D level below 20 ng/mL, and 28% were deemed to be vitamin D insufficient (20-30 ng/mL). The remaining 40% had sufficient vitamin D levels of 30 ng/mL and higher.
When the investigators controlled for age, sex, race, Charlson score (non-IBD comorbidity), disease-related complications, medication, vitamin D supplementation, and season of 25(OH)D measurement, analysis showed that patients with CD who had plasma vitamin D levels below 20 ng/mL had odds ratios of 1.76 for surgery and 2.07 for IBD-related hospitalization.
Similarly, for patients with UC and low vitamin D levels, the odds ratios for surgery and hospitalization were 1.70 and 2.26, respectively.
Overall, 76% of patients in the study with CD who had initial vitamin D levels below 30 ng/mL had subsequent normalization of their D values, as did 80% of those with ulcerative colitis.
In adjusted analysis, patients with CD had a nearly 50% reduction in the risk of surgery (odds ratio, 0.56) and a nearly 25% reduction in the risk of hospitalization (OR, 0.78), compared with patients whose vitamin D levels never corrected to the normal range. Patients with UC also had reductions in risk for both surgery and hospitalization, but these reductions were not significant.
In addition, patients with CD and UC who had vitamin D that normalized over the course of the study had significantly lower C-reactive protein levels than did those who remained vitamin D deficient (–5. 2 mg/L, P = .002).
The study was supported by grants and awards from the American Gastroenterological Association, the IBD Working Group, the Broad Foundation, and the National Institutes of Health. Dr. Ananthakrishnan reported having no financial disclosures.
ORLANDO – Vitamin D may protect patients with inflammatory bowel disease from more serious disease flare-ups, investigators reported at the annual Digestive Disease Week.
Among 3,217 patients followed for a median of 8 years, those with Crohn’s disease who had the lowest levels of plasma 25-hydroxyvitamin D had a nearly twofold risk for surgery and double the risk for hospitalization related to IBD, compared with patients who had higher vitamin D levels.
A similar relationship was seen between vitamin D levels and risk of surgery and hospitalization among patients with ulcerative colitis (UC), reported Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston.
Tellingly, patients with Crohn’s disease (CD) who had initially low vitamin D levels that normalized during the study had significant reductions in their risk of surgery and hospitalization compared with patients whose vitamin D levels did not improve over time. In addition, patients with both CD and UC who normalized their vitamin D status during the study had significantly lower levels of the inflammatory marker C-reactive protein, the investigators found.
"There’s considerable evidence that supports a role for vitamin D in inflammatory bowel diseases," Dr. Ananthakrishnan said.
He noted that high vitamin D levels were associated with a reduced risk for CD in a prior study performed by his group (Gastroenterology 2012;142:482-9), and a second study showed that polymorphisms in the vitamin D receptor were associated with a risk of both CD and UC (Gut 2000;47:211-4).
Although vitamin D levels have been weakly associated with IBD exacerbations in retrospective studies, stronger evidence for the potential anti-inflammatory role of vitamin D has been hard to come by, partly because of researchers’ inability to determine vitamin D status before clinical outcomes such as surgery or hospitalization, Dr. Ananthakrishnan said.
He and colleagues prospectively followed all members of an IBD cohort treated at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston, who had a least one measured plasma 25(OH)D level before a first IBD-related surgery and/or hospitalization (the primary outcome; median C-reactive protein was a secondary outcome).
The researchers found that 16% of all patients had disease-related surgery, and 40% were hospitalized during the follow-up period.
A third of all patients (32%) were considered to be vitamin D deficient, defined as having a plasma 25(OH)D level below 20 ng/mL, and 28% were deemed to be vitamin D insufficient (20-30 ng/mL). The remaining 40% had sufficient vitamin D levels of 30 ng/mL and higher.
When the investigators controlled for age, sex, race, Charlson score (non-IBD comorbidity), disease-related complications, medication, vitamin D supplementation, and season of 25(OH)D measurement, analysis showed that patients with CD who had plasma vitamin D levels below 20 ng/mL had odds ratios of 1.76 for surgery and 2.07 for IBD-related hospitalization.
Similarly, for patients with UC and low vitamin D levels, the odds ratios for surgery and hospitalization were 1.70 and 2.26, respectively.
Overall, 76% of patients in the study with CD who had initial vitamin D levels below 30 ng/mL had subsequent normalization of their D values, as did 80% of those with ulcerative colitis.
In adjusted analysis, patients with CD had a nearly 50% reduction in the risk of surgery (odds ratio, 0.56) and a nearly 25% reduction in the risk of hospitalization (OR, 0.78), compared with patients whose vitamin D levels never corrected to the normal range. Patients with UC also had reductions in risk for both surgery and hospitalization, but these reductions were not significant.
In addition, patients with CD and UC who had vitamin D that normalized over the course of the study had significantly lower C-reactive protein levels than did those who remained vitamin D deficient (–5. 2 mg/L, P = .002).
The study was supported by grants and awards from the American Gastroenterological Association, the IBD Working Group, the Broad Foundation, and the National Institutes of Health. Dr. Ananthakrishnan reported having no financial disclosures.
ORLANDO – Vitamin D may protect patients with inflammatory bowel disease from more serious disease flare-ups, investigators reported at the annual Digestive Disease Week.
Among 3,217 patients followed for a median of 8 years, those with Crohn’s disease who had the lowest levels of plasma 25-hydroxyvitamin D had a nearly twofold risk for surgery and double the risk for hospitalization related to IBD, compared with patients who had higher vitamin D levels.
A similar relationship was seen between vitamin D levels and risk of surgery and hospitalization among patients with ulcerative colitis (UC), reported Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston.
Tellingly, patients with Crohn’s disease (CD) who had initially low vitamin D levels that normalized during the study had significant reductions in their risk of surgery and hospitalization compared with patients whose vitamin D levels did not improve over time. In addition, patients with both CD and UC who normalized their vitamin D status during the study had significantly lower levels of the inflammatory marker C-reactive protein, the investigators found.
"There’s considerable evidence that supports a role for vitamin D in inflammatory bowel diseases," Dr. Ananthakrishnan said.
He noted that high vitamin D levels were associated with a reduced risk for CD in a prior study performed by his group (Gastroenterology 2012;142:482-9), and a second study showed that polymorphisms in the vitamin D receptor were associated with a risk of both CD and UC (Gut 2000;47:211-4).
Although vitamin D levels have been weakly associated with IBD exacerbations in retrospective studies, stronger evidence for the potential anti-inflammatory role of vitamin D has been hard to come by, partly because of researchers’ inability to determine vitamin D status before clinical outcomes such as surgery or hospitalization, Dr. Ananthakrishnan said.
He and colleagues prospectively followed all members of an IBD cohort treated at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston, who had a least one measured plasma 25(OH)D level before a first IBD-related surgery and/or hospitalization (the primary outcome; median C-reactive protein was a secondary outcome).
The researchers found that 16% of all patients had disease-related surgery, and 40% were hospitalized during the follow-up period.
A third of all patients (32%) were considered to be vitamin D deficient, defined as having a plasma 25(OH)D level below 20 ng/mL, and 28% were deemed to be vitamin D insufficient (20-30 ng/mL). The remaining 40% had sufficient vitamin D levels of 30 ng/mL and higher.
When the investigators controlled for age, sex, race, Charlson score (non-IBD comorbidity), disease-related complications, medication, vitamin D supplementation, and season of 25(OH)D measurement, analysis showed that patients with CD who had plasma vitamin D levels below 20 ng/mL had odds ratios of 1.76 for surgery and 2.07 for IBD-related hospitalization.
Similarly, for patients with UC and low vitamin D levels, the odds ratios for surgery and hospitalization were 1.70 and 2.26, respectively.
Overall, 76% of patients in the study with CD who had initial vitamin D levels below 30 ng/mL had subsequent normalization of their D values, as did 80% of those with ulcerative colitis.
In adjusted analysis, patients with CD had a nearly 50% reduction in the risk of surgery (odds ratio, 0.56) and a nearly 25% reduction in the risk of hospitalization (OR, 0.78), compared with patients whose vitamin D levels never corrected to the normal range. Patients with UC also had reductions in risk for both surgery and hospitalization, but these reductions were not significant.
In addition, patients with CD and UC who had vitamin D that normalized over the course of the study had significantly lower C-reactive protein levels than did those who remained vitamin D deficient (–5. 2 mg/L, P = .002).
The study was supported by grants and awards from the American Gastroenterological Association, the IBD Working Group, the Broad Foundation, and the National Institutes of Health. Dr. Ananthakrishnan reported having no financial disclosures.
AT DDW 2013
Major finding: In all, 32% of patients with Crohn’s disease who had plasma vitamin D below 20 ng/mL had IBD-related surgery, compared with 13% of those with normal vitamin D levels.
Data source: Prospective cohort study using electronic medical records of 3,217 patients with irritable bowel disease.
Disclosures: The study was supported by grants/awards from the American Gastroenterological Association, the IBD Working Group, the Broad Foundation, and the National Institutes of Health. Dr. Ananthakrishnan reported having no financial disclosures.
Barrett's recurrence more frequent in older, nonwhite patients
ORLANDO – Age, race, and extent of disease treated appear to predict which patients will have a recurrence of Barrett’s esophagus after apparently successful radiofrequency ablation, investigators reported at the annual Digestive Disease Week.
A review of registry data from 148 centers in the United States shows that patients who experience a recurrence of Barrett’s after radiofrequency ablation (RFA) are more likely to be older, to be nonwhite, and to have had larger areas of involvement treated and fewer treatment sessions than patients with no recurrence, said Dr. William J. Bulsiewicz, of the University of North Carolina in Chapel Hill.
"These risk factors may point out biological differences between the populations [with Barrett’s] and help us to better understand the process of regeneration of squamous epithelium," he said.
There were recurrences in 28% of 1,602 patients who had complete eradication of intestinal metaplasia with RFA for Barrett’s and had at least 2 years of follow-up during which at least two biopsies were performed. The median time to recurrence was 1.4 years. Additionally, 33 patients were retreated for a suspected recurrence despite a lack of histological confirmation.
Based on a bivariate analysis of characteristics, patients with recurrent disease were more likely to have a higher mean age (63 vs. 61 years, P less than .01), to be male (77% vs. 71%, P = .03), to have longer esophageal segments with disease involvement (mean 4.3 vs. 3.7 cm, P less than .01), and to have low-grade dysplasia or worse prior to treatment (54% vs. 45%, P less than. 01).
The researchers used these and other patient and treatment characteristics in a logistic regression model to see which factors, if any, could independently predict recurrence.
Significant independent predictors of recurrence included nonwhite race (odds ratio, 2.47), length of the tubular esophagus with Barrett’s esophagus (OR, 1.09 per centimeter), age (OR, 1.02 per year), and number of RFA sessions required to treat initial disease (OR, 0.90 per treatment session).
Risk of recurrence was not associated with sex, pretreatment dysplasias, previous endoscopic mucosal resection, or treatment setting (academic medical center vs. community setting), Dr. Bulsiewicz said.
The investigators suggested that future surveillance protocols include age, race, esophageal length of Barrett’s involvement, and number of treatment sessions.
The study was funded by Covidien, GI Solutions, and the National Institutes of Health. Dr. Bulsiewicz reported having no financial disclosures.
ORLANDO – Age, race, and extent of disease treated appear to predict which patients will have a recurrence of Barrett’s esophagus after apparently successful radiofrequency ablation, investigators reported at the annual Digestive Disease Week.
A review of registry data from 148 centers in the United States shows that patients who experience a recurrence of Barrett’s after radiofrequency ablation (RFA) are more likely to be older, to be nonwhite, and to have had larger areas of involvement treated and fewer treatment sessions than patients with no recurrence, said Dr. William J. Bulsiewicz, of the University of North Carolina in Chapel Hill.
"These risk factors may point out biological differences between the populations [with Barrett’s] and help us to better understand the process of regeneration of squamous epithelium," he said.
There were recurrences in 28% of 1,602 patients who had complete eradication of intestinal metaplasia with RFA for Barrett’s and had at least 2 years of follow-up during which at least two biopsies were performed. The median time to recurrence was 1.4 years. Additionally, 33 patients were retreated for a suspected recurrence despite a lack of histological confirmation.
Based on a bivariate analysis of characteristics, patients with recurrent disease were more likely to have a higher mean age (63 vs. 61 years, P less than .01), to be male (77% vs. 71%, P = .03), to have longer esophageal segments with disease involvement (mean 4.3 vs. 3.7 cm, P less than .01), and to have low-grade dysplasia or worse prior to treatment (54% vs. 45%, P less than. 01).
The researchers used these and other patient and treatment characteristics in a logistic regression model to see which factors, if any, could independently predict recurrence.
Significant independent predictors of recurrence included nonwhite race (odds ratio, 2.47), length of the tubular esophagus with Barrett’s esophagus (OR, 1.09 per centimeter), age (OR, 1.02 per year), and number of RFA sessions required to treat initial disease (OR, 0.90 per treatment session).
Risk of recurrence was not associated with sex, pretreatment dysplasias, previous endoscopic mucosal resection, or treatment setting (academic medical center vs. community setting), Dr. Bulsiewicz said.
The investigators suggested that future surveillance protocols include age, race, esophageal length of Barrett’s involvement, and number of treatment sessions.
The study was funded by Covidien, GI Solutions, and the National Institutes of Health. Dr. Bulsiewicz reported having no financial disclosures.
ORLANDO – Age, race, and extent of disease treated appear to predict which patients will have a recurrence of Barrett’s esophagus after apparently successful radiofrequency ablation, investigators reported at the annual Digestive Disease Week.
A review of registry data from 148 centers in the United States shows that patients who experience a recurrence of Barrett’s after radiofrequency ablation (RFA) are more likely to be older, to be nonwhite, and to have had larger areas of involvement treated and fewer treatment sessions than patients with no recurrence, said Dr. William J. Bulsiewicz, of the University of North Carolina in Chapel Hill.
"These risk factors may point out biological differences between the populations [with Barrett’s] and help us to better understand the process of regeneration of squamous epithelium," he said.
There were recurrences in 28% of 1,602 patients who had complete eradication of intestinal metaplasia with RFA for Barrett’s and had at least 2 years of follow-up during which at least two biopsies were performed. The median time to recurrence was 1.4 years. Additionally, 33 patients were retreated for a suspected recurrence despite a lack of histological confirmation.
Based on a bivariate analysis of characteristics, patients with recurrent disease were more likely to have a higher mean age (63 vs. 61 years, P less than .01), to be male (77% vs. 71%, P = .03), to have longer esophageal segments with disease involvement (mean 4.3 vs. 3.7 cm, P less than .01), and to have low-grade dysplasia or worse prior to treatment (54% vs. 45%, P less than. 01).
The researchers used these and other patient and treatment characteristics in a logistic regression model to see which factors, if any, could independently predict recurrence.
Significant independent predictors of recurrence included nonwhite race (odds ratio, 2.47), length of the tubular esophagus with Barrett’s esophagus (OR, 1.09 per centimeter), age (OR, 1.02 per year), and number of RFA sessions required to treat initial disease (OR, 0.90 per treatment session).
Risk of recurrence was not associated with sex, pretreatment dysplasias, previous endoscopic mucosal resection, or treatment setting (academic medical center vs. community setting), Dr. Bulsiewicz said.
The investigators suggested that future surveillance protocols include age, race, esophageal length of Barrett’s involvement, and number of treatment sessions.
The study was funded by Covidien, GI Solutions, and the National Institutes of Health. Dr. Bulsiewicz reported having no financial disclosures.
AT DDW 2013
Major finding: Significant independent predictors of recurrence included nonwhite race (OR, 2.47), length of the tubular esophagus with Barrett’s esophagus (OR, 1.09 per centimeter), age (OR, 1.02 per year), and number of RFA sessions required to treat initial disease (OR, 0.90 per treatment session).
Data source: Review of data on 1,602 patients who underwent radiofrequency ablation with complete eradication of intestinal metaplasia.
Disclosures: The study was funded by Covidien, GI Solutions, and the National Institutes of Health. Dr. Bulsiewicz reported having no financial disclosures.
Green glow locates tumors during surgery
NATIONAL HARBOR, MD. - Seeing is believing, especially when enhanced visualization of tumors during surgery helps improve chances for complete resection, investigators said at the annual Society of Surgical Oncology Cancer Symposium.
With near-infrared (NIR) fluorescence imaging and a portable camera that can be used in an operating room, surgeons can eliminate some of the guesswork involved in identifying involved surgical margins or lymph nodes, researchers from the United States and the Netherlands reported in oral and poster sessions.
In early human trials, a small, portable infrared camera has been successful at identifying dye-impregnated tumors -- including noncontiguous pockets of malignancy -- during surgery to resect squamous cell carcinomas and adenocarcinomas of the lung, reported Dr. Sunil Singhal, an ACS Fellow of the department of surgery at the University of Pennsylvania, Philadelphia.
"Even in this day and age, surgeons leave behind disease in 40% of the cases, and in about a quarter of those cases the tumor was within 2 centimeters of where the surgeon was working," Dr. Singhal said.
To improve the odds, he and his colleagues have been investigating optical contrast agents that can be delivered safely to tumors and cause them to fluoresce under light in the NIR portion of the spectrum. In preclinical studies with dogs, they found that indocyanine green had the right combination of toxicity, photostability, pharmacokinetics, and cost. The dye, currently used in retinal angiography, has an emission profile that makes it easy for observers to discriminate between the fluorescing dye and blood or tissues, Dr. Singhal said.
They also developed an intraoperative device, dubbed the "FloCam" which consists of a light source and NIR camera that sits above the patient and sends images to a computer monitor showing the operation in NIR.
In animal studies, the system found evidence of residual disease that was not visible to the naked eye or on x-ray microtomography. On pathologic examination, they saw that the dye was "remarkably precise in delineating margins from normal surrounding tissues," particularly in tumors with neovascular features.
Dr. Singhal said that the imaging technique has been effective at identifying tumor sites during surgery in 36 of 38 patients in early human trials, failing only for 1 patient with melanoma, and for 1 with a sarcoma.
One patient was a 64-year-old nonsmoking man who presented with a cough and was found to have a 2.5-cm right upper lobe lung tumor. Evaluation of the mediastinum with imaging and pathology samples was negative for malignancy, but during surgery, the dye highlighted previously undetected tumor hotspots in the right lower lobe.
Green hybrid
In a separate study, investigators in the Netherlands reported on improved intraoperative sentinel node identification and harvesting using a novel hybrid radiopharmaceutical tracer combining indocyanine green with technetium-99m in a nanocolloid suspension.
They found that in 96 patients with malignant melanomas of the head and neck, trunk, or extremities, the hybrid tracer, facilitated both preoperative SPECT/CT imaging and intraoperative radio- and fluorescence-guide sentinel node biopsy in all patients.
"The hybrid tracer was found to be particularly useful for the detection of sentinel nodes in the neck, and for sentinel nodes that failed to accumulate patent blue dye," wrote Dr. Oscar R. Brouwer from the division of nuclear medicine at the Netherlands Cancer Institute in Amsterdam, and colleagues in a scientific poster.
Dr. Singhal and Dr. Brouwer both reported that they had no disclosures.
NATIONAL HARBOR, MD. - Seeing is believing, especially when enhanced visualization of tumors during surgery helps improve chances for complete resection, investigators said at the annual Society of Surgical Oncology Cancer Symposium.
With near-infrared (NIR) fluorescence imaging and a portable camera that can be used in an operating room, surgeons can eliminate some of the guesswork involved in identifying involved surgical margins or lymph nodes, researchers from the United States and the Netherlands reported in oral and poster sessions.
In early human trials, a small, portable infrared camera has been successful at identifying dye-impregnated tumors -- including noncontiguous pockets of malignancy -- during surgery to resect squamous cell carcinomas and adenocarcinomas of the lung, reported Dr. Sunil Singhal, an ACS Fellow of the department of surgery at the University of Pennsylvania, Philadelphia.
"Even in this day and age, surgeons leave behind disease in 40% of the cases, and in about a quarter of those cases the tumor was within 2 centimeters of where the surgeon was working," Dr. Singhal said.
To improve the odds, he and his colleagues have been investigating optical contrast agents that can be delivered safely to tumors and cause them to fluoresce under light in the NIR portion of the spectrum. In preclinical studies with dogs, they found that indocyanine green had the right combination of toxicity, photostability, pharmacokinetics, and cost. The dye, currently used in retinal angiography, has an emission profile that makes it easy for observers to discriminate between the fluorescing dye and blood or tissues, Dr. Singhal said.
They also developed an intraoperative device, dubbed the "FloCam" which consists of a light source and NIR camera that sits above the patient and sends images to a computer monitor showing the operation in NIR.
In animal studies, the system found evidence of residual disease that was not visible to the naked eye or on x-ray microtomography. On pathologic examination, they saw that the dye was "remarkably precise in delineating margins from normal surrounding tissues," particularly in tumors with neovascular features.
Dr. Singhal said that the imaging technique has been effective at identifying tumor sites during surgery in 36 of 38 patients in early human trials, failing only for 1 patient with melanoma, and for 1 with a sarcoma.
One patient was a 64-year-old nonsmoking man who presented with a cough and was found to have a 2.5-cm right upper lobe lung tumor. Evaluation of the mediastinum with imaging and pathology samples was negative for malignancy, but during surgery, the dye highlighted previously undetected tumor hotspots in the right lower lobe.
Green hybrid
In a separate study, investigators in the Netherlands reported on improved intraoperative sentinel node identification and harvesting using a novel hybrid radiopharmaceutical tracer combining indocyanine green with technetium-99m in a nanocolloid suspension.
They found that in 96 patients with malignant melanomas of the head and neck, trunk, or extremities, the hybrid tracer, facilitated both preoperative SPECT/CT imaging and intraoperative radio- and fluorescence-guide sentinel node biopsy in all patients.
"The hybrid tracer was found to be particularly useful for the detection of sentinel nodes in the neck, and for sentinel nodes that failed to accumulate patent blue dye," wrote Dr. Oscar R. Brouwer from the division of nuclear medicine at the Netherlands Cancer Institute in Amsterdam, and colleagues in a scientific poster.
Dr. Singhal and Dr. Brouwer both reported that they had no disclosures.
NATIONAL HARBOR, MD. - Seeing is believing, especially when enhanced visualization of tumors during surgery helps improve chances for complete resection, investigators said at the annual Society of Surgical Oncology Cancer Symposium.
With near-infrared (NIR) fluorescence imaging and a portable camera that can be used in an operating room, surgeons can eliminate some of the guesswork involved in identifying involved surgical margins or lymph nodes, researchers from the United States and the Netherlands reported in oral and poster sessions.
In early human trials, a small, portable infrared camera has been successful at identifying dye-impregnated tumors -- including noncontiguous pockets of malignancy -- during surgery to resect squamous cell carcinomas and adenocarcinomas of the lung, reported Dr. Sunil Singhal, an ACS Fellow of the department of surgery at the University of Pennsylvania, Philadelphia.
"Even in this day and age, surgeons leave behind disease in 40% of the cases, and in about a quarter of those cases the tumor was within 2 centimeters of where the surgeon was working," Dr. Singhal said.
To improve the odds, he and his colleagues have been investigating optical contrast agents that can be delivered safely to tumors and cause them to fluoresce under light in the NIR portion of the spectrum. In preclinical studies with dogs, they found that indocyanine green had the right combination of toxicity, photostability, pharmacokinetics, and cost. The dye, currently used in retinal angiography, has an emission profile that makes it easy for observers to discriminate between the fluorescing dye and blood or tissues, Dr. Singhal said.
They also developed an intraoperative device, dubbed the "FloCam" which consists of a light source and NIR camera that sits above the patient and sends images to a computer monitor showing the operation in NIR.
In animal studies, the system found evidence of residual disease that was not visible to the naked eye or on x-ray microtomography. On pathologic examination, they saw that the dye was "remarkably precise in delineating margins from normal surrounding tissues," particularly in tumors with neovascular features.
Dr. Singhal said that the imaging technique has been effective at identifying tumor sites during surgery in 36 of 38 patients in early human trials, failing only for 1 patient with melanoma, and for 1 with a sarcoma.
One patient was a 64-year-old nonsmoking man who presented with a cough and was found to have a 2.5-cm right upper lobe lung tumor. Evaluation of the mediastinum with imaging and pathology samples was negative for malignancy, but during surgery, the dye highlighted previously undetected tumor hotspots in the right lower lobe.
Green hybrid
In a separate study, investigators in the Netherlands reported on improved intraoperative sentinel node identification and harvesting using a novel hybrid radiopharmaceutical tracer combining indocyanine green with technetium-99m in a nanocolloid suspension.
They found that in 96 patients with malignant melanomas of the head and neck, trunk, or extremities, the hybrid tracer, facilitated both preoperative SPECT/CT imaging and intraoperative radio- and fluorescence-guide sentinel node biopsy in all patients.
"The hybrid tracer was found to be particularly useful for the detection of sentinel nodes in the neck, and for sentinel nodes that failed to accumulate patent blue dye," wrote Dr. Oscar R. Brouwer from the division of nuclear medicine at the Netherlands Cancer Institute in Amsterdam, and colleagues in a scientific poster.
Dr. Singhal and Dr. Brouwer both reported that they had no disclosures.