User login
Establishing a strong and lasting mentor/mentee relationship
I. Finding a mentor
Case
You are a 27-year-old first-year resident who is seeking mentorship. You are halfway through the year and are thinking about your goals and future. You have a general interest in hematology/oncology but have limited experience and would like to gain more experience with clinically relevant scholarship. However, you do not know anyone in the field and are not sure who to ask for guidance.
Stage 1: Seeking the right mentor
Start first with your area of interest and then look broadly. In this case the resident is interested in heme/onc. The first place to look is on the heme/onc department website or in the faculty directory. It can be helpful to look at what the potential mentor has published recently and/or look at a version of their CV on the faculty directory or website. This can help determine how productive they are and help assess whether you share similar interests, and whether they have worked with many learners in the past.
It is also important to do some background work and ask around about potential mentors. Often resident colleagues and fellows have a good sense of current projects and which faculty work well with learners. Lastly, it is important to also look at non–heme/onc physicians as there may be internal medicine physicians or surgeons who are doing hematology or oncology research that more align with your interests.
After you have assessed whether you think this person would be a strong mentor for you, it is time to reach out. People are flattered to be asked and part of their promotion criteria is their ability to mentor. Do not assume that a potential mentor is too busy! Let him or her make that decision. Remember the worst a mentor can say is “no.” Even if they do not have time or the need for a mentee at the present time, they generally will offer some assistance or direction on who to ask.
Start with a straightforward, but pleasant email. Waiting up to 2 weeks for a response is reasonable. If after 2 weeks you have not received word, feel free to reach out again asking politely if he or she would be willing to work with you. Do not be afraid to ask bluntly for their guidance and mentorship and have a specific project or area of research that you would like their assistance with.
II. Optimizing the mentor/mentee relationship
Case continued
Success! Your email was received with interest by a hematologist who has done several projects, comes highly recommended by other residents, and worked with students and residents in the past. The project involves anticoagulation on the inpatient service. You are set to meet with her next month.
Stage 2: Establishing expectations and goals
Now comes the hard work in establishing an excellent mentor/mentee relationship. Before you meet with your mentor, brainstorm first. What do you want out of the relationship? A publication? Career advice? Attaining a fellowship position? You should feel empowered in knowing that you as the mentee are in the driver seat, but this relationship should be mutually beneficial. Consider basing the relationship and initial discussions on these key questions:
1. My goals
- What are my goals? It is okay not to know but be ready to communicate some information to your mentor.
- Remember to also ask your mentor what their goals are for you as well.
2. Outcome
- What type of outcome are both you and your mentor looking for from the relationship?
3. Expectations
- What mentorship expectations do you have?
- What are your mentor’s expectations of you?
Once you feel you have a sense of what you are looking for out of the relationship, it is important to communicate this with the mentor to establish congruent expectations of one another. For example, think about asking your mentor if the two of you can establish a mentor/mentee contract. This is a written document that can be found online and establishes a mutual agreement of roles, responsibilities, and expectations of one another for the relationship. It can further help to open a line for honest and consistent feedback. This can also give you a formalized endpoint and agreed upon scope for the mentoring relationship. Having a check-in preestablished in a contract reduces any potentially awkward conversations about redefining the relationship down the road. (For example, what if our case resident decides to pursue GI? It could happen.)
Stage 3: Establishing a common goal
After you have determined the goals and expectations of the relationship together (remember, this is a relationship), it is time to start exploring possible projects and establishing goals for those projects. Having a quality improvement or research project will determine a common goal to work towards and help establish and define the relationship.
Once you have delineated broadly what the project(s) should be, develop smaller SMART (specific, measurable, achievable, relevant, time-bound) goals to move the project forward. These goals determine stopping points for evaluation and feedback, which further establish the relationship and keep the project(s) progressing. For example, one goal could be to write the first draft of the proposal for your quality improvement project within 3 weeks.
Stage 4: Continued communication
With any project it is important to stay on the same page as your mentor and be clear to establish “who is doing what by when.” Do not expect accountability to be the mentor’s job. Remember that you are in the driver’s seat and that you should propose how often you need to meet and what those meetings look like by developing an agenda. You can have an open discussion and allow your mentor to help determine a reasonable timeline. Remember, the more you communicate your goals, the better your mentor will be able to address them.
One pro tip is to always exceed your mentor’s expectations – if you think you need 2 weeks to complete a task, ask for 3-4 weeks. This gives you extra padding in case of unforeseen circumstances and makes you look like a “rockstar” if you hit a deadline 1-2 weeks earlier than planned.
III. Ending and/or redefining the relationship
Case continued
You are now a senior resident who’s published multiple articles in the past year, and have completed an anticoagulation project for inpatients with pulmonary emboli. You look back on your experience and what stands out is the extent of your gratitude and appreciation for your incredible mentor. Not only do you feel that your mentor has guided you in your career and with your scholarship, but you feel that he or she has shaped your character and talent set. At this point your mentor is both a teacher and guide, but now also a friend. While you feel there is always more that you can learn from her, you are ready to explore new interests. How do you effectively end or redefine this relationship?
Stage 5: Redefining your mentoring relationship
First, go back to the expectations or contract established early in the relationship. The check-in is a key time in the relationship to reevaluate goals and priorities. At this point you may decide to amicably end the relationship or project, or move on to a new project with a change in your role. For example, the quality improvement project may change to research, or you as the mentee have a change in focus (e.g., change in specialty or scholarly focus).
In summary, the interaction between you and your mentor should be a relationship. And the keys to a great relationship are:
1. Establish clear expectations from the beginning. This clarifies the relationship and helps the mentee and mentor to become more successful.
2. Maintain clear and open communication throughout the relationship.3. Define your goals and discuss them with your mentor early. (Have we mentioned the importance of goals enough?) After all, your goal is the reason you started pursuing this relationship in the first place.
In clinical training having guidance can greatly enhance your experience and direct your future career in unexpected ways. We hope that using these tools will guide you towards forging a strong mentor/mentee relationship.
Dr. Zimmerberg-Helms is a resident physician at the University of New Mexico, Albuquerque. Dr. Rendon is an attending hospitalist at the University of New Mexico.
I. Finding a mentor
Case
You are a 27-year-old first-year resident who is seeking mentorship. You are halfway through the year and are thinking about your goals and future. You have a general interest in hematology/oncology but have limited experience and would like to gain more experience with clinically relevant scholarship. However, you do not know anyone in the field and are not sure who to ask for guidance.
Stage 1: Seeking the right mentor
Start first with your area of interest and then look broadly. In this case the resident is interested in heme/onc. The first place to look is on the heme/onc department website or in the faculty directory. It can be helpful to look at what the potential mentor has published recently and/or look at a version of their CV on the faculty directory or website. This can help determine how productive they are and help assess whether you share similar interests, and whether they have worked with many learners in the past.
It is also important to do some background work and ask around about potential mentors. Often resident colleagues and fellows have a good sense of current projects and which faculty work well with learners. Lastly, it is important to also look at non–heme/onc physicians as there may be internal medicine physicians or surgeons who are doing hematology or oncology research that more align with your interests.
After you have assessed whether you think this person would be a strong mentor for you, it is time to reach out. People are flattered to be asked and part of their promotion criteria is their ability to mentor. Do not assume that a potential mentor is too busy! Let him or her make that decision. Remember the worst a mentor can say is “no.” Even if they do not have time or the need for a mentee at the present time, they generally will offer some assistance or direction on who to ask.
Start with a straightforward, but pleasant email. Waiting up to 2 weeks for a response is reasonable. If after 2 weeks you have not received word, feel free to reach out again asking politely if he or she would be willing to work with you. Do not be afraid to ask bluntly for their guidance and mentorship and have a specific project or area of research that you would like their assistance with.
II. Optimizing the mentor/mentee relationship
Case continued
Success! Your email was received with interest by a hematologist who has done several projects, comes highly recommended by other residents, and worked with students and residents in the past. The project involves anticoagulation on the inpatient service. You are set to meet with her next month.
Stage 2: Establishing expectations and goals
Now comes the hard work in establishing an excellent mentor/mentee relationship. Before you meet with your mentor, brainstorm first. What do you want out of the relationship? A publication? Career advice? Attaining a fellowship position? You should feel empowered in knowing that you as the mentee are in the driver seat, but this relationship should be mutually beneficial. Consider basing the relationship and initial discussions on these key questions:
1. My goals
- What are my goals? It is okay not to know but be ready to communicate some information to your mentor.
- Remember to also ask your mentor what their goals are for you as well.
2. Outcome
- What type of outcome are both you and your mentor looking for from the relationship?
3. Expectations
- What mentorship expectations do you have?
- What are your mentor’s expectations of you?
Once you feel you have a sense of what you are looking for out of the relationship, it is important to communicate this with the mentor to establish congruent expectations of one another. For example, think about asking your mentor if the two of you can establish a mentor/mentee contract. This is a written document that can be found online and establishes a mutual agreement of roles, responsibilities, and expectations of one another for the relationship. It can further help to open a line for honest and consistent feedback. This can also give you a formalized endpoint and agreed upon scope for the mentoring relationship. Having a check-in preestablished in a contract reduces any potentially awkward conversations about redefining the relationship down the road. (For example, what if our case resident decides to pursue GI? It could happen.)
Stage 3: Establishing a common goal
After you have determined the goals and expectations of the relationship together (remember, this is a relationship), it is time to start exploring possible projects and establishing goals for those projects. Having a quality improvement or research project will determine a common goal to work towards and help establish and define the relationship.
Once you have delineated broadly what the project(s) should be, develop smaller SMART (specific, measurable, achievable, relevant, time-bound) goals to move the project forward. These goals determine stopping points for evaluation and feedback, which further establish the relationship and keep the project(s) progressing. For example, one goal could be to write the first draft of the proposal for your quality improvement project within 3 weeks.
Stage 4: Continued communication
With any project it is important to stay on the same page as your mentor and be clear to establish “who is doing what by when.” Do not expect accountability to be the mentor’s job. Remember that you are in the driver’s seat and that you should propose how often you need to meet and what those meetings look like by developing an agenda. You can have an open discussion and allow your mentor to help determine a reasonable timeline. Remember, the more you communicate your goals, the better your mentor will be able to address them.
One pro tip is to always exceed your mentor’s expectations – if you think you need 2 weeks to complete a task, ask for 3-4 weeks. This gives you extra padding in case of unforeseen circumstances and makes you look like a “rockstar” if you hit a deadline 1-2 weeks earlier than planned.
III. Ending and/or redefining the relationship
Case continued
You are now a senior resident who’s published multiple articles in the past year, and have completed an anticoagulation project for inpatients with pulmonary emboli. You look back on your experience and what stands out is the extent of your gratitude and appreciation for your incredible mentor. Not only do you feel that your mentor has guided you in your career and with your scholarship, but you feel that he or she has shaped your character and talent set. At this point your mentor is both a teacher and guide, but now also a friend. While you feel there is always more that you can learn from her, you are ready to explore new interests. How do you effectively end or redefine this relationship?
Stage 5: Redefining your mentoring relationship
First, go back to the expectations or contract established early in the relationship. The check-in is a key time in the relationship to reevaluate goals and priorities. At this point you may decide to amicably end the relationship or project, or move on to a new project with a change in your role. For example, the quality improvement project may change to research, or you as the mentee have a change in focus (e.g., change in specialty or scholarly focus).
In summary, the interaction between you and your mentor should be a relationship. And the keys to a great relationship are:
1. Establish clear expectations from the beginning. This clarifies the relationship and helps the mentee and mentor to become more successful.
2. Maintain clear and open communication throughout the relationship.3. Define your goals and discuss them with your mentor early. (Have we mentioned the importance of goals enough?) After all, your goal is the reason you started pursuing this relationship in the first place.
In clinical training having guidance can greatly enhance your experience and direct your future career in unexpected ways. We hope that using these tools will guide you towards forging a strong mentor/mentee relationship.
Dr. Zimmerberg-Helms is a resident physician at the University of New Mexico, Albuquerque. Dr. Rendon is an attending hospitalist at the University of New Mexico.
I. Finding a mentor
Case
You are a 27-year-old first-year resident who is seeking mentorship. You are halfway through the year and are thinking about your goals and future. You have a general interest in hematology/oncology but have limited experience and would like to gain more experience with clinically relevant scholarship. However, you do not know anyone in the field and are not sure who to ask for guidance.
Stage 1: Seeking the right mentor
Start first with your area of interest and then look broadly. In this case the resident is interested in heme/onc. The first place to look is on the heme/onc department website or in the faculty directory. It can be helpful to look at what the potential mentor has published recently and/or look at a version of their CV on the faculty directory or website. This can help determine how productive they are and help assess whether you share similar interests, and whether they have worked with many learners in the past.
It is also important to do some background work and ask around about potential mentors. Often resident colleagues and fellows have a good sense of current projects and which faculty work well with learners. Lastly, it is important to also look at non–heme/onc physicians as there may be internal medicine physicians or surgeons who are doing hematology or oncology research that more align with your interests.
After you have assessed whether you think this person would be a strong mentor for you, it is time to reach out. People are flattered to be asked and part of their promotion criteria is their ability to mentor. Do not assume that a potential mentor is too busy! Let him or her make that decision. Remember the worst a mentor can say is “no.” Even if they do not have time or the need for a mentee at the present time, they generally will offer some assistance or direction on who to ask.
Start with a straightforward, but pleasant email. Waiting up to 2 weeks for a response is reasonable. If after 2 weeks you have not received word, feel free to reach out again asking politely if he or she would be willing to work with you. Do not be afraid to ask bluntly for their guidance and mentorship and have a specific project or area of research that you would like their assistance with.
II. Optimizing the mentor/mentee relationship
Case continued
Success! Your email was received with interest by a hematologist who has done several projects, comes highly recommended by other residents, and worked with students and residents in the past. The project involves anticoagulation on the inpatient service. You are set to meet with her next month.
Stage 2: Establishing expectations and goals
Now comes the hard work in establishing an excellent mentor/mentee relationship. Before you meet with your mentor, brainstorm first. What do you want out of the relationship? A publication? Career advice? Attaining a fellowship position? You should feel empowered in knowing that you as the mentee are in the driver seat, but this relationship should be mutually beneficial. Consider basing the relationship and initial discussions on these key questions:
1. My goals
- What are my goals? It is okay not to know but be ready to communicate some information to your mentor.
- Remember to also ask your mentor what their goals are for you as well.
2. Outcome
- What type of outcome are both you and your mentor looking for from the relationship?
3. Expectations
- What mentorship expectations do you have?
- What are your mentor’s expectations of you?
Once you feel you have a sense of what you are looking for out of the relationship, it is important to communicate this with the mentor to establish congruent expectations of one another. For example, think about asking your mentor if the two of you can establish a mentor/mentee contract. This is a written document that can be found online and establishes a mutual agreement of roles, responsibilities, and expectations of one another for the relationship. It can further help to open a line for honest and consistent feedback. This can also give you a formalized endpoint and agreed upon scope for the mentoring relationship. Having a check-in preestablished in a contract reduces any potentially awkward conversations about redefining the relationship down the road. (For example, what if our case resident decides to pursue GI? It could happen.)
Stage 3: Establishing a common goal
After you have determined the goals and expectations of the relationship together (remember, this is a relationship), it is time to start exploring possible projects and establishing goals for those projects. Having a quality improvement or research project will determine a common goal to work towards and help establish and define the relationship.
Once you have delineated broadly what the project(s) should be, develop smaller SMART (specific, measurable, achievable, relevant, time-bound) goals to move the project forward. These goals determine stopping points for evaluation and feedback, which further establish the relationship and keep the project(s) progressing. For example, one goal could be to write the first draft of the proposal for your quality improvement project within 3 weeks.
Stage 4: Continued communication
With any project it is important to stay on the same page as your mentor and be clear to establish “who is doing what by when.” Do not expect accountability to be the mentor’s job. Remember that you are in the driver’s seat and that you should propose how often you need to meet and what those meetings look like by developing an agenda. You can have an open discussion and allow your mentor to help determine a reasonable timeline. Remember, the more you communicate your goals, the better your mentor will be able to address them.
One pro tip is to always exceed your mentor’s expectations – if you think you need 2 weeks to complete a task, ask for 3-4 weeks. This gives you extra padding in case of unforeseen circumstances and makes you look like a “rockstar” if you hit a deadline 1-2 weeks earlier than planned.
III. Ending and/or redefining the relationship
Case continued
You are now a senior resident who’s published multiple articles in the past year, and have completed an anticoagulation project for inpatients with pulmonary emboli. You look back on your experience and what stands out is the extent of your gratitude and appreciation for your incredible mentor. Not only do you feel that your mentor has guided you in your career and with your scholarship, but you feel that he or she has shaped your character and talent set. At this point your mentor is both a teacher and guide, but now also a friend. While you feel there is always more that you can learn from her, you are ready to explore new interests. How do you effectively end or redefine this relationship?
Stage 5: Redefining your mentoring relationship
First, go back to the expectations or contract established early in the relationship. The check-in is a key time in the relationship to reevaluate goals and priorities. At this point you may decide to amicably end the relationship or project, or move on to a new project with a change in your role. For example, the quality improvement project may change to research, or you as the mentee have a change in focus (e.g., change in specialty or scholarly focus).
In summary, the interaction between you and your mentor should be a relationship. And the keys to a great relationship are:
1. Establish clear expectations from the beginning. This clarifies the relationship and helps the mentee and mentor to become more successful.
2. Maintain clear and open communication throughout the relationship.3. Define your goals and discuss them with your mentor early. (Have we mentioned the importance of goals enough?) After all, your goal is the reason you started pursuing this relationship in the first place.
In clinical training having guidance can greatly enhance your experience and direct your future career in unexpected ways. We hope that using these tools will guide you towards forging a strong mentor/mentee relationship.
Dr. Zimmerberg-Helms is a resident physician at the University of New Mexico, Albuquerque. Dr. Rendon is an attending hospitalist at the University of New Mexico.
What Is Career Success for Academic Hospitalists? A Qualitative Analysis of Early-Career Faculty Perspectives
Academic hospital medicine is a young specialty, with most faculty at the rank of instructor or assistant professor.1 Traditional markers of academic success for clinical and translational investigators emphasize progressive, externally funded grants, achievements in basic science research, and prolific publication in the peer-reviewed literature.2 Promotion is often used as a proxy measure for academic success.
Conceptual models of career success derived from nonhealthcare industries and for physician-scientists include both extrinsic and intrinsic domains.3,4 Extrinsic domains of career success include financial rewards (compensation) and progression in hierarchical status (advancement).3,4 Intrinsic domains of career success include pleasure derived from daily work (job satisfaction) and satisfaction derived from aspects of the career over time (career satisfaction).3,4
Research is limited regarding hospitalist faculty beliefs about career success. A better understanding of hospitalist perspectives can inform program development to support junior faculty in academic hospital medicine. In this phenomenological, qualitative study, we explore the global concept of career success as perceived by early-career clinician-educator hospitalists.
METHODS
Study Design, Setting, and Participants
We conducted interviews with hospitalists from 3 academic medical centers between May 2016 and October 2016. Purposeful sampling was used.5 Leaders within each hospital medicine group identified early-career faculty with approximately 2 to 5 years in academic medicine with a rank of instructor or assistant professor at each institution likely to self-identify as clinician-educators for targeted solicitation to enroll. Additional subjects were recruited until thematic saturation had been achieved on the personal definition of career success. Participants received disclosure and consent documents prior to enrollment. No compensation was provided to participants. This study was approved by the Colorado Multiple Institutional Review Board.
Interview Guide Development and Content
The semistructured interview format was developed and validated through an iterative process. Proposed questions were developed by study investigators on the basis of review of the literature on career success in nonhealthcare industries and academic hospitalist promotion. The questions were assessed for content validity through a review of interview domains by an academic hospitalist program director (R. P.). Cognitive interviewing with 3 representative academic hospitalists who were not part of the study cohort was done as an additional face-validation step of the question probe structure. As a result of the cognitive interviews, 1 question was eliminated, and a framework for clarifications and answer probes was derived prior to the enrollment of the first study subject. No changes were made to the interview format during the study period.
Data Collection
The principal investigator (E.C.) performed all interviews by using the interview tool consisting of 7 demographic questions and 11 open-ended questions and exploring aspects of the concept of career success. The initial open-ended question, “How would you personally define career success as an academic hospitalist at this stage in your career?” represented the primary question of interest. Follow-up questions were used to better understand responses to the primary question. All interviews were audio recorded, deidentified, and transcribed by the principal investigator. Transcripts were randomly audited by a second investigator (E.Y.) for accuracy and completeness.
Sample Size Determination
Interviews were continued to thematic saturation. After the first 3 interviews were transcribed, 2 members of the research team (E.C. and P.K.) reviewed the transcripts and developed a preliminary thematic codebook for the primary question. Subsequent interviews were reviewed and analyzed against these themes. Interviews were continued to thematic saturation, which was defined as more than 3 sequential interviews with no new identified themes.6
Data Analysis
By using qualitative data analysis software (ATLAS.ti version 7; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany), transcriptions were analyzed with a team-based, mixed inductive-deductive approach. An inductive approach was utilized to allow basic theme codes to emerge from the raw text, and thus remaining open to unanticipated themes. Investigators assessed each distinct quote for new themes, confirmatory themes, and challenges to previously developed concepts. Basic themes were then discussed among research team members to determine prominent themes, with basic theme codes added, removed, or combined at this stage of the analysis. Responses to each follow-up question were subsequently assessed for new themes, confirmatory themes, or challenges to previously developed concepts related to the personal definition of career success. A deductive approach was then used to map our inductively generated themes back to the organizing themes of the existing conceptual framework.
RESULTS
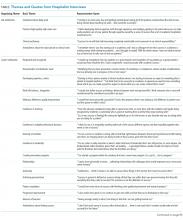
Thematic Mapping to Organizing Themes of the Conceptual Model (Table)
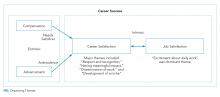
The single most dominant theme, “excitement about daily work” was connected to an intrinsic sense of job satisfaction. Career satisfaction emerged from interviews more frequently than extrinsic organizing themes, such as advancement or compensation. Advancement through promotion was infrequently referenced as part of success, and tenure was never raised despite being available for clinician-educators at 2 of the 3 institutions. Compensation was not referenced in any interviewee’s initial definition of career success, although in 1 interview, it came up in response to a follow-up question. The Figure visually represents the relative weighting (shown by the sizes of the boxes) of organizing themes to the early-career hospitalists’ self-concepts of career success. Relationships among organizing themes as they emerged from interviews are represented by arrows.
Intrinsic—Job Satisfaction
With regard to job satisfaction, early-career faculty often invoked words such as “excitement,” “enjoyment,” and “passionate” to describe an overall theme of “excitement about daily work.” A positive affective state created by the nature of daily work was described as integral to the personal sense of career success. It was also strongly associated with perception of sustainability in a hospitalist career.
“I think [career success] would be job satisfaction. …So, for me, that would be happiness with my job. I like coming to work. I like doing what I do and at the end of the day going home and saying that was a good day. I like to think that would be success at work…is how I would define it.”
This theme was also related to a negative aspect often referred to as burnout, which many identified as antithetical to career success. More often, they described success as a heightened state of enthusiasm for the daily work experience.
“I am staying engaged and excited. So, I am not just taking care of patients; I am not just teaching. Having enough excitement from my work to come home and talk about it at dinner. To enjoy my days off but at the same time being excited to get back to work.”
This description of passion toward the work of being a hospitalist was often linked to a sense of deeper purpose found through the delivery of clinical care and education of learners.
“I really feel that we have the opportunity to very meaningfully and powerfully impact people’s lives, and that to me is meaningful. …That’s value. ...That’s coming home at the end of the day and thinking that you have had a positive impact.”
The interviews reflected that core to meaningful work was a sense of personal efficacy as a clinician, which was reflected in the themes of clinical proficiency and practicing high-quality care.
“I think developing clinical expertise, both through experience and studying. Getting to the point to where you can take really excellent care of your patient through expertise would be a sense of success that a lot of academic hospitalists would strive for.”
Intrinsic—Career Satisfaction
Within career satisfaction, participants described that “being respected and recognized” and “dissemination of work” were important contributors to career success. Reputation was frequently referenced as a measure of career success. Reputation was defined by some in a local context of having the respect of learners, peers, and others as a national renown. As a prerequisite for developing a reputation beyond the local academic environment, dissemination of work was often referenced as an important component of satisfaction in the career. This dissemination extended beyond peer-reviewed publications and included other forms of scholarship, presentations at conferences, and sharing clinical innovations between hospitals.
“For me personally, I have less of an emphasis on research and some of the more, I don’t want to say ‘academic’ because I think education is academic, but maybe some of the more scholarly practice of medicine, doing research and the writing of papers and things like that, although I certainly view some of that as a part of career success.”
Within career satisfaction, participants also described a diverse set of themes, including progressive improvement in skills, developing a self-perception of excellence in 1 or more arenas of academic medicine, leadership, work–life integration, innovation, and relationships. The concept of developing a niche, or becoming an expert in a particular domain of hospital medicine, was frequently referenced.
“I think part of [success] is ‘Have they identified a niche?’ Because I think if you want to be in an academic center, as much as I value teaching and taking care of patients, I think 1 of the advantages is the opportunity to potentially identify an area of expertise.”
Participants frequently alluded to the idea that the most important aspects of career satisfaction are not static phenomena but rather values that could evolve over the course of a career. For instance, in the early-career, making a difference with individual learners or patients could have greater valence, but as the career progressed, finding a niche, disseminating work, and building a national reputation would gain importance to a personal sense of career satisfaction.
Extrinsic—Advancement
Promotion was typically referenced when discussing career success, but it was not uniformly valued by early career hospitalists. Some expressed significant ambivalence about its effect on their personal sense of career success. Academic hospitalists identified a number of organizations with definitions of success that influence them. Definitions of success for the university were more relevant to interviewees compared to those of the hospital or professional societies. Interviewees were able to describe a variety of criteria by which their universities define or recognize career success. These commonly included promotion, publications and/or scholarship, and research. The list of factors perceived as success by the hospital were often distinct from those of the university and included cost-effective care, patient safety, and clinical leadership roles.
Participants described a sense of internal conflict when external-stakeholder definitions of success diverged from internal motivators. This was particularly true when this divergence led academic hospitalists to engage in activities for advancement that they did not find personally fulfilling. Academic hospitalists recognized that advancement was central to the concept of career success for organizations even if this was not identified as being core to their personal definitions of success.
“I think that for me, the idea of being promoted and being a leader in the field is less important to me than...for the organization.”
Hospitalists expressed that objective markers, such as promotion and publications, were perceived as more important at higher levels of the academic organization, whereas more subjective aspects of success, aligned with intrinsic personal definitions, were more valued within the hospital medicine group.
Extrinsic—Compensation
Compensation was notable for its absence in participants’ discussion of career success. When asked about their definitions of career success, academic hospitalists did not spontaneously raise the topic of compensation. The only mention of compensation was in response to a question about how personal and external definitions of career success differ.
Unexpected Findings
While it was almost universally recognized by participants as important, ambivalence toward the “academic value of clinical work,” “scholarship,” and especially “promotion” represented an unexpected thematic family.
“I can’t quite get excited about a title attached to my name or the number of times my name pops up when I enter it into PubMed. My personal definition is more…where do I have something that I am interested [in] that someone else values. And that value is not shown as an associate professorship or an assistant professorship next to my name. …When you push me on it, you could call me clinical instructor forever, and I don’t think I would care too much.”
The interaction between work and personal activities as representing complementary aspects of a global sense of success was also unexpected and ran contrary to a simplistic conception of work and life in conflict. Academic hospitalists referenced that the ability to participate in aspects of life external to the workplace was important to their sense of career success. Participants frequently used phrases such as “work–life balance” to encompass a larger sense that work and nonwork life needed to merge to form a holistic sense of having a positive impact.
“Personal success is becoming what I have termed a ‘man of worth.’ I think [that is] someone who feels as though they make a positive impact in the world. Through both my career, but I guess the things that I do that are external to my career. Those would be defined by being a good husband, a good son, a philanthropist out in the community…sometimes, these are not things that can necessarily go on a [curriculum vitae].”
Conflict Among Organizing Themes
At times, academic hospitalists described a tension between day-to-day job satisfaction and what would be necessary to accomplish longer-term career success in the other organizing themes. This was reflected by a sense of trade-off. For instance, activities that lead to some aspects of career satisfaction or advancement would take time away from the direct exposure to learners and clinical care that currently drive job satisfaction.
“If the institution wanted me to be more productive from a research standpoint or…advocate that I receive funding so I could buy down clinical time and interactions I have with my students and my patients, then I can see my satisfaction going down.”
Many described a sense of engaging in activities they did not find personally fulfilling because of a sense of expectation that those activities were considered successful by others. Some described a state in which the drive toward advancement as an extrinsic incentive could come at the expense of the intrinsic rewards of being an academic hospitalist.
DISCUSSION
Career success has been defined as “the positive psychological or work-related outcomes or achievements one accumulates as a result of work experiences.”4,7,8 Academic career success for hospitalist faculty isn’t as well defined and has not been examined from the perspectives of early-career clinician-educator hospitalist faculty themselves.
The themes that emerged in this study describe a definition of success anchored in the daily work of striving to become an exceptional clinician and teacher. The major themes included (1) having excitement about daily work, (2) having meaningful impact, (3) development of a niche (4) a sense of respect within the sphere of academic medicine, and (5) disseminating work.
Success was very much internally defined as having a positive, meaningful impact on patients, learners, and the systems in which they practice. The faculty had a conception of what promotion committees value and often internalized aspects of this, such as developing a national reputation and giving talks at national meetings. Participants typically self-identified as clinician-educators, and yet dissemination of work remained an important component of personal success. While promotion was clearly identified as a marker of success, academic hospitalists often rejected the supposition of promotion itself as a professional goal. They expressed hope, and some skepticism, that external recognition of career success would follow the pursuit of internally meaningful goals.
While promotion and peer-reviewed publications represent easily measured markers often used as proxies for individual career and programmatic success, our research demonstrates that there is a deep well of externally imperceptible influences on an individual’s sense of success as an academic hospitalist. In our analysis, intrinsic elements of career success received far greater weight with early-career academic hospitalists. Our findings are supported by a prior survey of academic physicians that similarly found that faculty with >50% of their time devoted to clinical care placed greater career value in patient care, relationships with patients, and recognition by patients and residents compared to national reputation.9 Similar to our own findings, highly clinical faculty in that study were also less likely to value promotion and tenure as indicators of career success.9
The main focus of our questions was how early-career faculty define success at this point in their careers. When asked to extrapolate to a future state of career success, the concept of progression was repeatedly raised. This included successive promotions to higher academic ranks, increasing responsibility, titles, leadership, and achieving competitive roles or awards. It also included a progressively increasing impact of scholarship, growing national reputation, and becoming part of a network of accomplished academic hospitalists across the country. Looking forward, our early-career hospitalists felt that long-term career success would represent accomplishing these things and still being able to be focused on being excellent clinicians to patients, having a work–life balance, and keeping joy and excitement in daily activities.
Our work has limitations, including a focus on early-career clinician-educator hospitalists. The perception of career success may evolve over time, and future work to examine perceptions in more advanced academic hospitalists would be of interest. Our work used purposeful sampling to capture individuals who were likely to self-identify as academic clinician-educators, and results may not generalize to hospitalist physician-scientists or hospitalists in community practices.
Our analysis suggests that external organizations influence internal perceptions of career success. However, success is ultimately defined by the individual and not the institution. Efforts to measure and improve academic hospitalists’ attainment of career success should attend to intrinsic aspects of satisfaction in addition to objective measures, such as publications and promotion. This may provide a mechanism to address burnout and improve retention. As important as commonality in themes is the variation in self-definitions of career success among individuals. This suggests the value of inquiry by academic leadership in exploring and understanding what success is from the individual faculty perspective. This may enhance the alignment among personal definitions, organizational values, and, ultimately, sustainable, successful careers.
Disclosure: The authors have nothing to disclose.
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US Academic Hospitalist Leaders About Mentorship and Academic Activities in Hospitalist Groups. J Hosp Med. 2011;6(1):5-9. PubMed
2. Buddeberg-Fischer B, Stamm M, Buddeberg C, Klaghofer R. Career-Success Scale. A New Instrument to Assess Young Physicians Academic Career Steps. BMC Health Serv Res. 2008;8:120. PubMed
3. Rubio DM, Primack BA, Switzer GE, Bryce CL, Selzer DL, Kapoor WN. A Comprehensive Career-Success Model for Physician-Scientists. Acad Med. 2011;86(12):1571-1576. PubMed
4. Judge TA, Cable DM, Boudreau JW, Bretz RD. An empirical investigation of the predictors of executive career success (CAHRS Working Paper #94-08). Ithaca, NY: Cornell University, School of Industrial and Labor Relations, Center for Advanced Human Resource Studies. 1994. http://digitalcommons.ilr.cornell.edu/cahrswp/233. Accessed November 27, 2017.
5. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544. PubMed
6. Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25(10):1229-1245. PubMed
7. Abele AE, Spurk, D. The longitudinal impact of self-efficacy and career goals on objective and subjective career success. J Vocat Behav. 2009;74(1):53-62.
8. Seibert SE, Kraimer ML. The five-factor model of personality and career success. J Vocat Behav. 2011;58(1):1-21.
9. Buckley, LM, Sanders K, Shih M, Hampton CL. Attitudes of Clinical Faculty About Career Progress, Career Success, and Commitment to Academic Medicine: Results of a Survey. Arch Intern Med. 2000;160(17):2625-2629. PubMed
Academic hospital medicine is a young specialty, with most faculty at the rank of instructor or assistant professor.1 Traditional markers of academic success for clinical and translational investigators emphasize progressive, externally funded grants, achievements in basic science research, and prolific publication in the peer-reviewed literature.2 Promotion is often used as a proxy measure for academic success.
Conceptual models of career success derived from nonhealthcare industries and for physician-scientists include both extrinsic and intrinsic domains.3,4 Extrinsic domains of career success include financial rewards (compensation) and progression in hierarchical status (advancement).3,4 Intrinsic domains of career success include pleasure derived from daily work (job satisfaction) and satisfaction derived from aspects of the career over time (career satisfaction).3,4
Research is limited regarding hospitalist faculty beliefs about career success. A better understanding of hospitalist perspectives can inform program development to support junior faculty in academic hospital medicine. In this phenomenological, qualitative study, we explore the global concept of career success as perceived by early-career clinician-educator hospitalists.
METHODS
Study Design, Setting, and Participants
We conducted interviews with hospitalists from 3 academic medical centers between May 2016 and October 2016. Purposeful sampling was used.5 Leaders within each hospital medicine group identified early-career faculty with approximately 2 to 5 years in academic medicine with a rank of instructor or assistant professor at each institution likely to self-identify as clinician-educators for targeted solicitation to enroll. Additional subjects were recruited until thematic saturation had been achieved on the personal definition of career success. Participants received disclosure and consent documents prior to enrollment. No compensation was provided to participants. This study was approved by the Colorado Multiple Institutional Review Board.
Interview Guide Development and Content
The semistructured interview format was developed and validated through an iterative process. Proposed questions were developed by study investigators on the basis of review of the literature on career success in nonhealthcare industries and academic hospitalist promotion. The questions were assessed for content validity through a review of interview domains by an academic hospitalist program director (R. P.). Cognitive interviewing with 3 representative academic hospitalists who were not part of the study cohort was done as an additional face-validation step of the question probe structure. As a result of the cognitive interviews, 1 question was eliminated, and a framework for clarifications and answer probes was derived prior to the enrollment of the first study subject. No changes were made to the interview format during the study period.
Data Collection
The principal investigator (E.C.) performed all interviews by using the interview tool consisting of 7 demographic questions and 11 open-ended questions and exploring aspects of the concept of career success. The initial open-ended question, “How would you personally define career success as an academic hospitalist at this stage in your career?” represented the primary question of interest. Follow-up questions were used to better understand responses to the primary question. All interviews were audio recorded, deidentified, and transcribed by the principal investigator. Transcripts were randomly audited by a second investigator (E.Y.) for accuracy and completeness.
Sample Size Determination
Interviews were continued to thematic saturation. After the first 3 interviews were transcribed, 2 members of the research team (E.C. and P.K.) reviewed the transcripts and developed a preliminary thematic codebook for the primary question. Subsequent interviews were reviewed and analyzed against these themes. Interviews were continued to thematic saturation, which was defined as more than 3 sequential interviews with no new identified themes.6
Data Analysis
By using qualitative data analysis software (ATLAS.ti version 7; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany), transcriptions were analyzed with a team-based, mixed inductive-deductive approach. An inductive approach was utilized to allow basic theme codes to emerge from the raw text, and thus remaining open to unanticipated themes. Investigators assessed each distinct quote for new themes, confirmatory themes, and challenges to previously developed concepts. Basic themes were then discussed among research team members to determine prominent themes, with basic theme codes added, removed, or combined at this stage of the analysis. Responses to each follow-up question were subsequently assessed for new themes, confirmatory themes, or challenges to previously developed concepts related to the personal definition of career success. A deductive approach was then used to map our inductively generated themes back to the organizing themes of the existing conceptual framework.
RESULTS
Thematic Mapping to Organizing Themes of the Conceptual Model (Table)
The single most dominant theme, “excitement about daily work” was connected to an intrinsic sense of job satisfaction. Career satisfaction emerged from interviews more frequently than extrinsic organizing themes, such as advancement or compensation. Advancement through promotion was infrequently referenced as part of success, and tenure was never raised despite being available for clinician-educators at 2 of the 3 institutions. Compensation was not referenced in any interviewee’s initial definition of career success, although in 1 interview, it came up in response to a follow-up question. The Figure visually represents the relative weighting (shown by the sizes of the boxes) of organizing themes to the early-career hospitalists’ self-concepts of career success. Relationships among organizing themes as they emerged from interviews are represented by arrows.
Intrinsic—Job Satisfaction
With regard to job satisfaction, early-career faculty often invoked words such as “excitement,” “enjoyment,” and “passionate” to describe an overall theme of “excitement about daily work.” A positive affective state created by the nature of daily work was described as integral to the personal sense of career success. It was also strongly associated with perception of sustainability in a hospitalist career.
“I think [career success] would be job satisfaction. …So, for me, that would be happiness with my job. I like coming to work. I like doing what I do and at the end of the day going home and saying that was a good day. I like to think that would be success at work…is how I would define it.”
This theme was also related to a negative aspect often referred to as burnout, which many identified as antithetical to career success. More often, they described success as a heightened state of enthusiasm for the daily work experience.
“I am staying engaged and excited. So, I am not just taking care of patients; I am not just teaching. Having enough excitement from my work to come home and talk about it at dinner. To enjoy my days off but at the same time being excited to get back to work.”
This description of passion toward the work of being a hospitalist was often linked to a sense of deeper purpose found through the delivery of clinical care and education of learners.
“I really feel that we have the opportunity to very meaningfully and powerfully impact people’s lives, and that to me is meaningful. …That’s value. ...That’s coming home at the end of the day and thinking that you have had a positive impact.”
The interviews reflected that core to meaningful work was a sense of personal efficacy as a clinician, which was reflected in the themes of clinical proficiency and practicing high-quality care.
“I think developing clinical expertise, both through experience and studying. Getting to the point to where you can take really excellent care of your patient through expertise would be a sense of success that a lot of academic hospitalists would strive for.”
Intrinsic—Career Satisfaction
Within career satisfaction, participants described that “being respected and recognized” and “dissemination of work” were important contributors to career success. Reputation was frequently referenced as a measure of career success. Reputation was defined by some in a local context of having the respect of learners, peers, and others as a national renown. As a prerequisite for developing a reputation beyond the local academic environment, dissemination of work was often referenced as an important component of satisfaction in the career. This dissemination extended beyond peer-reviewed publications and included other forms of scholarship, presentations at conferences, and sharing clinical innovations between hospitals.
“For me personally, I have less of an emphasis on research and some of the more, I don’t want to say ‘academic’ because I think education is academic, but maybe some of the more scholarly practice of medicine, doing research and the writing of papers and things like that, although I certainly view some of that as a part of career success.”
Within career satisfaction, participants also described a diverse set of themes, including progressive improvement in skills, developing a self-perception of excellence in 1 or more arenas of academic medicine, leadership, work–life integration, innovation, and relationships. The concept of developing a niche, or becoming an expert in a particular domain of hospital medicine, was frequently referenced.
“I think part of [success] is ‘Have they identified a niche?’ Because I think if you want to be in an academic center, as much as I value teaching and taking care of patients, I think 1 of the advantages is the opportunity to potentially identify an area of expertise.”
Participants frequently alluded to the idea that the most important aspects of career satisfaction are not static phenomena but rather values that could evolve over the course of a career. For instance, in the early-career, making a difference with individual learners or patients could have greater valence, but as the career progressed, finding a niche, disseminating work, and building a national reputation would gain importance to a personal sense of career satisfaction.
Extrinsic—Advancement
Promotion was typically referenced when discussing career success, but it was not uniformly valued by early career hospitalists. Some expressed significant ambivalence about its effect on their personal sense of career success. Academic hospitalists identified a number of organizations with definitions of success that influence them. Definitions of success for the university were more relevant to interviewees compared to those of the hospital or professional societies. Interviewees were able to describe a variety of criteria by which their universities define or recognize career success. These commonly included promotion, publications and/or scholarship, and research. The list of factors perceived as success by the hospital were often distinct from those of the university and included cost-effective care, patient safety, and clinical leadership roles.
Participants described a sense of internal conflict when external-stakeholder definitions of success diverged from internal motivators. This was particularly true when this divergence led academic hospitalists to engage in activities for advancement that they did not find personally fulfilling. Academic hospitalists recognized that advancement was central to the concept of career success for organizations even if this was not identified as being core to their personal definitions of success.
“I think that for me, the idea of being promoted and being a leader in the field is less important to me than...for the organization.”
Hospitalists expressed that objective markers, such as promotion and publications, were perceived as more important at higher levels of the academic organization, whereas more subjective aspects of success, aligned with intrinsic personal definitions, were more valued within the hospital medicine group.
Extrinsic—Compensation
Compensation was notable for its absence in participants’ discussion of career success. When asked about their definitions of career success, academic hospitalists did not spontaneously raise the topic of compensation. The only mention of compensation was in response to a question about how personal and external definitions of career success differ.
Unexpected Findings
While it was almost universally recognized by participants as important, ambivalence toward the “academic value of clinical work,” “scholarship,” and especially “promotion” represented an unexpected thematic family.
“I can’t quite get excited about a title attached to my name or the number of times my name pops up when I enter it into PubMed. My personal definition is more…where do I have something that I am interested [in] that someone else values. And that value is not shown as an associate professorship or an assistant professorship next to my name. …When you push me on it, you could call me clinical instructor forever, and I don’t think I would care too much.”
The interaction between work and personal activities as representing complementary aspects of a global sense of success was also unexpected and ran contrary to a simplistic conception of work and life in conflict. Academic hospitalists referenced that the ability to participate in aspects of life external to the workplace was important to their sense of career success. Participants frequently used phrases such as “work–life balance” to encompass a larger sense that work and nonwork life needed to merge to form a holistic sense of having a positive impact.
“Personal success is becoming what I have termed a ‘man of worth.’ I think [that is] someone who feels as though they make a positive impact in the world. Through both my career, but I guess the things that I do that are external to my career. Those would be defined by being a good husband, a good son, a philanthropist out in the community…sometimes, these are not things that can necessarily go on a [curriculum vitae].”
Conflict Among Organizing Themes
At times, academic hospitalists described a tension between day-to-day job satisfaction and what would be necessary to accomplish longer-term career success in the other organizing themes. This was reflected by a sense of trade-off. For instance, activities that lead to some aspects of career satisfaction or advancement would take time away from the direct exposure to learners and clinical care that currently drive job satisfaction.
“If the institution wanted me to be more productive from a research standpoint or…advocate that I receive funding so I could buy down clinical time and interactions I have with my students and my patients, then I can see my satisfaction going down.”
Many described a sense of engaging in activities they did not find personally fulfilling because of a sense of expectation that those activities were considered successful by others. Some described a state in which the drive toward advancement as an extrinsic incentive could come at the expense of the intrinsic rewards of being an academic hospitalist.
DISCUSSION
Career success has been defined as “the positive psychological or work-related outcomes or achievements one accumulates as a result of work experiences.”4,7,8 Academic career success for hospitalist faculty isn’t as well defined and has not been examined from the perspectives of early-career clinician-educator hospitalist faculty themselves.
The themes that emerged in this study describe a definition of success anchored in the daily work of striving to become an exceptional clinician and teacher. The major themes included (1) having excitement about daily work, (2) having meaningful impact, (3) development of a niche (4) a sense of respect within the sphere of academic medicine, and (5) disseminating work.
Success was very much internally defined as having a positive, meaningful impact on patients, learners, and the systems in which they practice. The faculty had a conception of what promotion committees value and often internalized aspects of this, such as developing a national reputation and giving talks at national meetings. Participants typically self-identified as clinician-educators, and yet dissemination of work remained an important component of personal success. While promotion was clearly identified as a marker of success, academic hospitalists often rejected the supposition of promotion itself as a professional goal. They expressed hope, and some skepticism, that external recognition of career success would follow the pursuit of internally meaningful goals.
While promotion and peer-reviewed publications represent easily measured markers often used as proxies for individual career and programmatic success, our research demonstrates that there is a deep well of externally imperceptible influences on an individual’s sense of success as an academic hospitalist. In our analysis, intrinsic elements of career success received far greater weight with early-career academic hospitalists. Our findings are supported by a prior survey of academic physicians that similarly found that faculty with >50% of their time devoted to clinical care placed greater career value in patient care, relationships with patients, and recognition by patients and residents compared to national reputation.9 Similar to our own findings, highly clinical faculty in that study were also less likely to value promotion and tenure as indicators of career success.9
The main focus of our questions was how early-career faculty define success at this point in their careers. When asked to extrapolate to a future state of career success, the concept of progression was repeatedly raised. This included successive promotions to higher academic ranks, increasing responsibility, titles, leadership, and achieving competitive roles or awards. It also included a progressively increasing impact of scholarship, growing national reputation, and becoming part of a network of accomplished academic hospitalists across the country. Looking forward, our early-career hospitalists felt that long-term career success would represent accomplishing these things and still being able to be focused on being excellent clinicians to patients, having a work–life balance, and keeping joy and excitement in daily activities.
Our work has limitations, including a focus on early-career clinician-educator hospitalists. The perception of career success may evolve over time, and future work to examine perceptions in more advanced academic hospitalists would be of interest. Our work used purposeful sampling to capture individuals who were likely to self-identify as academic clinician-educators, and results may not generalize to hospitalist physician-scientists or hospitalists in community practices.
Our analysis suggests that external organizations influence internal perceptions of career success. However, success is ultimately defined by the individual and not the institution. Efforts to measure and improve academic hospitalists’ attainment of career success should attend to intrinsic aspects of satisfaction in addition to objective measures, such as publications and promotion. This may provide a mechanism to address burnout and improve retention. As important as commonality in themes is the variation in self-definitions of career success among individuals. This suggests the value of inquiry by academic leadership in exploring and understanding what success is from the individual faculty perspective. This may enhance the alignment among personal definitions, organizational values, and, ultimately, sustainable, successful careers.
Disclosure: The authors have nothing to disclose.
Academic hospital medicine is a young specialty, with most faculty at the rank of instructor or assistant professor.1 Traditional markers of academic success for clinical and translational investigators emphasize progressive, externally funded grants, achievements in basic science research, and prolific publication in the peer-reviewed literature.2 Promotion is often used as a proxy measure for academic success.
Conceptual models of career success derived from nonhealthcare industries and for physician-scientists include both extrinsic and intrinsic domains.3,4 Extrinsic domains of career success include financial rewards (compensation) and progression in hierarchical status (advancement).3,4 Intrinsic domains of career success include pleasure derived from daily work (job satisfaction) and satisfaction derived from aspects of the career over time (career satisfaction).3,4
Research is limited regarding hospitalist faculty beliefs about career success. A better understanding of hospitalist perspectives can inform program development to support junior faculty in academic hospital medicine. In this phenomenological, qualitative study, we explore the global concept of career success as perceived by early-career clinician-educator hospitalists.
METHODS
Study Design, Setting, and Participants
We conducted interviews with hospitalists from 3 academic medical centers between May 2016 and October 2016. Purposeful sampling was used.5 Leaders within each hospital medicine group identified early-career faculty with approximately 2 to 5 years in academic medicine with a rank of instructor or assistant professor at each institution likely to self-identify as clinician-educators for targeted solicitation to enroll. Additional subjects were recruited until thematic saturation had been achieved on the personal definition of career success. Participants received disclosure and consent documents prior to enrollment. No compensation was provided to participants. This study was approved by the Colorado Multiple Institutional Review Board.
Interview Guide Development and Content
The semistructured interview format was developed and validated through an iterative process. Proposed questions were developed by study investigators on the basis of review of the literature on career success in nonhealthcare industries and academic hospitalist promotion. The questions were assessed for content validity through a review of interview domains by an academic hospitalist program director (R. P.). Cognitive interviewing with 3 representative academic hospitalists who were not part of the study cohort was done as an additional face-validation step of the question probe structure. As a result of the cognitive interviews, 1 question was eliminated, and a framework for clarifications and answer probes was derived prior to the enrollment of the first study subject. No changes were made to the interview format during the study period.
Data Collection
The principal investigator (E.C.) performed all interviews by using the interview tool consisting of 7 demographic questions and 11 open-ended questions and exploring aspects of the concept of career success. The initial open-ended question, “How would you personally define career success as an academic hospitalist at this stage in your career?” represented the primary question of interest. Follow-up questions were used to better understand responses to the primary question. All interviews were audio recorded, deidentified, and transcribed by the principal investigator. Transcripts were randomly audited by a second investigator (E.Y.) for accuracy and completeness.
Sample Size Determination
Interviews were continued to thematic saturation. After the first 3 interviews were transcribed, 2 members of the research team (E.C. and P.K.) reviewed the transcripts and developed a preliminary thematic codebook for the primary question. Subsequent interviews were reviewed and analyzed against these themes. Interviews were continued to thematic saturation, which was defined as more than 3 sequential interviews with no new identified themes.6
Data Analysis
By using qualitative data analysis software (ATLAS.ti version 7; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany), transcriptions were analyzed with a team-based, mixed inductive-deductive approach. An inductive approach was utilized to allow basic theme codes to emerge from the raw text, and thus remaining open to unanticipated themes. Investigators assessed each distinct quote for new themes, confirmatory themes, and challenges to previously developed concepts. Basic themes were then discussed among research team members to determine prominent themes, with basic theme codes added, removed, or combined at this stage of the analysis. Responses to each follow-up question were subsequently assessed for new themes, confirmatory themes, or challenges to previously developed concepts related to the personal definition of career success. A deductive approach was then used to map our inductively generated themes back to the organizing themes of the existing conceptual framework.
RESULTS
Thematic Mapping to Organizing Themes of the Conceptual Model (Table)
The single most dominant theme, “excitement about daily work” was connected to an intrinsic sense of job satisfaction. Career satisfaction emerged from interviews more frequently than extrinsic organizing themes, such as advancement or compensation. Advancement through promotion was infrequently referenced as part of success, and tenure was never raised despite being available for clinician-educators at 2 of the 3 institutions. Compensation was not referenced in any interviewee’s initial definition of career success, although in 1 interview, it came up in response to a follow-up question. The Figure visually represents the relative weighting (shown by the sizes of the boxes) of organizing themes to the early-career hospitalists’ self-concepts of career success. Relationships among organizing themes as they emerged from interviews are represented by arrows.
Intrinsic—Job Satisfaction
With regard to job satisfaction, early-career faculty often invoked words such as “excitement,” “enjoyment,” and “passionate” to describe an overall theme of “excitement about daily work.” A positive affective state created by the nature of daily work was described as integral to the personal sense of career success. It was also strongly associated with perception of sustainability in a hospitalist career.
“I think [career success] would be job satisfaction. …So, for me, that would be happiness with my job. I like coming to work. I like doing what I do and at the end of the day going home and saying that was a good day. I like to think that would be success at work…is how I would define it.”
This theme was also related to a negative aspect often referred to as burnout, which many identified as antithetical to career success. More often, they described success as a heightened state of enthusiasm for the daily work experience.
“I am staying engaged and excited. So, I am not just taking care of patients; I am not just teaching. Having enough excitement from my work to come home and talk about it at dinner. To enjoy my days off but at the same time being excited to get back to work.”
This description of passion toward the work of being a hospitalist was often linked to a sense of deeper purpose found through the delivery of clinical care and education of learners.
“I really feel that we have the opportunity to very meaningfully and powerfully impact people’s lives, and that to me is meaningful. …That’s value. ...That’s coming home at the end of the day and thinking that you have had a positive impact.”
The interviews reflected that core to meaningful work was a sense of personal efficacy as a clinician, which was reflected in the themes of clinical proficiency and practicing high-quality care.
“I think developing clinical expertise, both through experience and studying. Getting to the point to where you can take really excellent care of your patient through expertise would be a sense of success that a lot of academic hospitalists would strive for.”
Intrinsic—Career Satisfaction
Within career satisfaction, participants described that “being respected and recognized” and “dissemination of work” were important contributors to career success. Reputation was frequently referenced as a measure of career success. Reputation was defined by some in a local context of having the respect of learners, peers, and others as a national renown. As a prerequisite for developing a reputation beyond the local academic environment, dissemination of work was often referenced as an important component of satisfaction in the career. This dissemination extended beyond peer-reviewed publications and included other forms of scholarship, presentations at conferences, and sharing clinical innovations between hospitals.
“For me personally, I have less of an emphasis on research and some of the more, I don’t want to say ‘academic’ because I think education is academic, but maybe some of the more scholarly practice of medicine, doing research and the writing of papers and things like that, although I certainly view some of that as a part of career success.”
Within career satisfaction, participants also described a diverse set of themes, including progressive improvement in skills, developing a self-perception of excellence in 1 or more arenas of academic medicine, leadership, work–life integration, innovation, and relationships. The concept of developing a niche, or becoming an expert in a particular domain of hospital medicine, was frequently referenced.
“I think part of [success] is ‘Have they identified a niche?’ Because I think if you want to be in an academic center, as much as I value teaching and taking care of patients, I think 1 of the advantages is the opportunity to potentially identify an area of expertise.”
Participants frequently alluded to the idea that the most important aspects of career satisfaction are not static phenomena but rather values that could evolve over the course of a career. For instance, in the early-career, making a difference with individual learners or patients could have greater valence, but as the career progressed, finding a niche, disseminating work, and building a national reputation would gain importance to a personal sense of career satisfaction.
Extrinsic—Advancement
Promotion was typically referenced when discussing career success, but it was not uniformly valued by early career hospitalists. Some expressed significant ambivalence about its effect on their personal sense of career success. Academic hospitalists identified a number of organizations with definitions of success that influence them. Definitions of success for the university were more relevant to interviewees compared to those of the hospital or professional societies. Interviewees were able to describe a variety of criteria by which their universities define or recognize career success. These commonly included promotion, publications and/or scholarship, and research. The list of factors perceived as success by the hospital were often distinct from those of the university and included cost-effective care, patient safety, and clinical leadership roles.
Participants described a sense of internal conflict when external-stakeholder definitions of success diverged from internal motivators. This was particularly true when this divergence led academic hospitalists to engage in activities for advancement that they did not find personally fulfilling. Academic hospitalists recognized that advancement was central to the concept of career success for organizations even if this was not identified as being core to their personal definitions of success.
“I think that for me, the idea of being promoted and being a leader in the field is less important to me than...for the organization.”
Hospitalists expressed that objective markers, such as promotion and publications, were perceived as more important at higher levels of the academic organization, whereas more subjective aspects of success, aligned with intrinsic personal definitions, were more valued within the hospital medicine group.
Extrinsic—Compensation
Compensation was notable for its absence in participants’ discussion of career success. When asked about their definitions of career success, academic hospitalists did not spontaneously raise the topic of compensation. The only mention of compensation was in response to a question about how personal and external definitions of career success differ.
Unexpected Findings
While it was almost universally recognized by participants as important, ambivalence toward the “academic value of clinical work,” “scholarship,” and especially “promotion” represented an unexpected thematic family.
“I can’t quite get excited about a title attached to my name or the number of times my name pops up when I enter it into PubMed. My personal definition is more…where do I have something that I am interested [in] that someone else values. And that value is not shown as an associate professorship or an assistant professorship next to my name. …When you push me on it, you could call me clinical instructor forever, and I don’t think I would care too much.”
The interaction between work and personal activities as representing complementary aspects of a global sense of success was also unexpected and ran contrary to a simplistic conception of work and life in conflict. Academic hospitalists referenced that the ability to participate in aspects of life external to the workplace was important to their sense of career success. Participants frequently used phrases such as “work–life balance” to encompass a larger sense that work and nonwork life needed to merge to form a holistic sense of having a positive impact.
“Personal success is becoming what I have termed a ‘man of worth.’ I think [that is] someone who feels as though they make a positive impact in the world. Through both my career, but I guess the things that I do that are external to my career. Those would be defined by being a good husband, a good son, a philanthropist out in the community…sometimes, these are not things that can necessarily go on a [curriculum vitae].”
Conflict Among Organizing Themes
At times, academic hospitalists described a tension between day-to-day job satisfaction and what would be necessary to accomplish longer-term career success in the other organizing themes. This was reflected by a sense of trade-off. For instance, activities that lead to some aspects of career satisfaction or advancement would take time away from the direct exposure to learners and clinical care that currently drive job satisfaction.
“If the institution wanted me to be more productive from a research standpoint or…advocate that I receive funding so I could buy down clinical time and interactions I have with my students and my patients, then I can see my satisfaction going down.”
Many described a sense of engaging in activities they did not find personally fulfilling because of a sense of expectation that those activities were considered successful by others. Some described a state in which the drive toward advancement as an extrinsic incentive could come at the expense of the intrinsic rewards of being an academic hospitalist.
DISCUSSION
Career success has been defined as “the positive psychological or work-related outcomes or achievements one accumulates as a result of work experiences.”4,7,8 Academic career success for hospitalist faculty isn’t as well defined and has not been examined from the perspectives of early-career clinician-educator hospitalist faculty themselves.
The themes that emerged in this study describe a definition of success anchored in the daily work of striving to become an exceptional clinician and teacher. The major themes included (1) having excitement about daily work, (2) having meaningful impact, (3) development of a niche (4) a sense of respect within the sphere of academic medicine, and (5) disseminating work.
Success was very much internally defined as having a positive, meaningful impact on patients, learners, and the systems in which they practice. The faculty had a conception of what promotion committees value and often internalized aspects of this, such as developing a national reputation and giving talks at national meetings. Participants typically self-identified as clinician-educators, and yet dissemination of work remained an important component of personal success. While promotion was clearly identified as a marker of success, academic hospitalists often rejected the supposition of promotion itself as a professional goal. They expressed hope, and some skepticism, that external recognition of career success would follow the pursuit of internally meaningful goals.
While promotion and peer-reviewed publications represent easily measured markers often used as proxies for individual career and programmatic success, our research demonstrates that there is a deep well of externally imperceptible influences on an individual’s sense of success as an academic hospitalist. In our analysis, intrinsic elements of career success received far greater weight with early-career academic hospitalists. Our findings are supported by a prior survey of academic physicians that similarly found that faculty with >50% of their time devoted to clinical care placed greater career value in patient care, relationships with patients, and recognition by patients and residents compared to national reputation.9 Similar to our own findings, highly clinical faculty in that study were also less likely to value promotion and tenure as indicators of career success.9
The main focus of our questions was how early-career faculty define success at this point in their careers. When asked to extrapolate to a future state of career success, the concept of progression was repeatedly raised. This included successive promotions to higher academic ranks, increasing responsibility, titles, leadership, and achieving competitive roles or awards. It also included a progressively increasing impact of scholarship, growing national reputation, and becoming part of a network of accomplished academic hospitalists across the country. Looking forward, our early-career hospitalists felt that long-term career success would represent accomplishing these things and still being able to be focused on being excellent clinicians to patients, having a work–life balance, and keeping joy and excitement in daily activities.
Our work has limitations, including a focus on early-career clinician-educator hospitalists. The perception of career success may evolve over time, and future work to examine perceptions in more advanced academic hospitalists would be of interest. Our work used purposeful sampling to capture individuals who were likely to self-identify as academic clinician-educators, and results may not generalize to hospitalist physician-scientists or hospitalists in community practices.
Our analysis suggests that external organizations influence internal perceptions of career success. However, success is ultimately defined by the individual and not the institution. Efforts to measure and improve academic hospitalists’ attainment of career success should attend to intrinsic aspects of satisfaction in addition to objective measures, such as publications and promotion. This may provide a mechanism to address burnout and improve retention. As important as commonality in themes is the variation in self-definitions of career success among individuals. This suggests the value of inquiry by academic leadership in exploring and understanding what success is from the individual faculty perspective. This may enhance the alignment among personal definitions, organizational values, and, ultimately, sustainable, successful careers.
Disclosure: The authors have nothing to disclose.
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US Academic Hospitalist Leaders About Mentorship and Academic Activities in Hospitalist Groups. J Hosp Med. 2011;6(1):5-9. PubMed
2. Buddeberg-Fischer B, Stamm M, Buddeberg C, Klaghofer R. Career-Success Scale. A New Instrument to Assess Young Physicians Academic Career Steps. BMC Health Serv Res. 2008;8:120. PubMed
3. Rubio DM, Primack BA, Switzer GE, Bryce CL, Selzer DL, Kapoor WN. A Comprehensive Career-Success Model for Physician-Scientists. Acad Med. 2011;86(12):1571-1576. PubMed
4. Judge TA, Cable DM, Boudreau JW, Bretz RD. An empirical investigation of the predictors of executive career success (CAHRS Working Paper #94-08). Ithaca, NY: Cornell University, School of Industrial and Labor Relations, Center for Advanced Human Resource Studies. 1994. http://digitalcommons.ilr.cornell.edu/cahrswp/233. Accessed November 27, 2017.
5. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544. PubMed
6. Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25(10):1229-1245. PubMed
7. Abele AE, Spurk, D. The longitudinal impact of self-efficacy and career goals on objective and subjective career success. J Vocat Behav. 2009;74(1):53-62.
8. Seibert SE, Kraimer ML. The five-factor model of personality and career success. J Vocat Behav. 2011;58(1):1-21.
9. Buckley, LM, Sanders K, Shih M, Hampton CL. Attitudes of Clinical Faculty About Career Progress, Career Success, and Commitment to Academic Medicine: Results of a Survey. Arch Intern Med. 2000;160(17):2625-2629. PubMed
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US Academic Hospitalist Leaders About Mentorship and Academic Activities in Hospitalist Groups. J Hosp Med. 2011;6(1):5-9. PubMed
2. Buddeberg-Fischer B, Stamm M, Buddeberg C, Klaghofer R. Career-Success Scale. A New Instrument to Assess Young Physicians Academic Career Steps. BMC Health Serv Res. 2008;8:120. PubMed
3. Rubio DM, Primack BA, Switzer GE, Bryce CL, Selzer DL, Kapoor WN. A Comprehensive Career-Success Model for Physician-Scientists. Acad Med. 2011;86(12):1571-1576. PubMed
4. Judge TA, Cable DM, Boudreau JW, Bretz RD. An empirical investigation of the predictors of executive career success (CAHRS Working Paper #94-08). Ithaca, NY: Cornell University, School of Industrial and Labor Relations, Center for Advanced Human Resource Studies. 1994. http://digitalcommons.ilr.cornell.edu/cahrswp/233. Accessed November 27, 2017.
5. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544. PubMed
6. Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25(10):1229-1245. PubMed
7. Abele AE, Spurk, D. The longitudinal impact of self-efficacy and career goals on objective and subjective career success. J Vocat Behav. 2009;74(1):53-62.
8. Seibert SE, Kraimer ML. The five-factor model of personality and career success. J Vocat Behav. 2011;58(1):1-21.
9. Buckley, LM, Sanders K, Shih M, Hampton CL. Attitudes of Clinical Faculty About Career Progress, Career Success, and Commitment to Academic Medicine: Results of a Survey. Arch Intern Med. 2000;160(17):2625-2629. PubMed
©2018 Society of Hospital Medicine
Optimizing diagnostic testing for venous thromboembolism
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
KEY POINTS
- A pretest clinical prediction tool such as the Wells score can help in deciding whether a patient with suspected venous thromboembolism warrants further workup.
- A clinical prediction tool should be used in concert with additional laboratory testing (eg, D-dimer) and imaging in patients at risk.
- In many cases, screening for thrombophilia to determine the cause of a venous thromboembolic event may be unwarranted.
- Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked.
Tips for Hospitalists on Improving Diagnostic Skills
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.
When Should Hospitalists Order Continuous Cardiac Monitoring?
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
What Is the Best Approach to a Cavitary Lung Lesion?
Case
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
Case
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
Case
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
Once-Weekly Antibiotic Might Be Effective for Treatment of Acute Bacterial Skin Infections
Background: Acute bacterial skin infections are common and often require hospitalization for intravenous antibiotic administration. Treatment covering gram-positive bacteria usually is indicated. Dalbavancin is effective against gram-positives, including MRSA. Its long half-life makes it an attractive alternative to other commonly used antibiotics, which require more frequent dosing.
Study design: Phase 3, double-blinded RCT.
Setting: Multiple international centers.
Synopsis: Researchers randomized 1,312 patients with acute bacterial skin and skin-structure infections with signs of systemic infection requiring intravenous antibiotics to receive dalbavancin on days one and eight, with placebo on other days, or several doses of vancomycin with an option to switch to oral linezolid. The primary endpoint was cessation of spread of erythema and temperature of =37.6°C at 48–72 hours. Secondary endpoints included a decrease in lesion area of =20% at 48–72 hours and clinical success at end of therapy (determined by clinical and historical features). Results of the primary endpoint were similar with dalbavancin and vancomycin-linezolid groups (79.7% and 79.8%, respectively) and were within 10 percentage points of noninferiority. The secondary endpoints were similar between both groups. Limitations of the study were the early primary endpoint, lack of noninferiority analysis of the secondary endpoints, and cost-effective analysis.
Bottom line: Once-weekly dalbavancin appears to be similarly efficacious to intravenous vancomycin in the treatment of acute bacterial skin infections in terms of outcomes within 48–72 hours of therapy and might provide an alternative to continued inpatient hospitalization for intravenous antibiotics in stable patients.
Citation: Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169-2179.
Background: Acute bacterial skin infections are common and often require hospitalization for intravenous antibiotic administration. Treatment covering gram-positive bacteria usually is indicated. Dalbavancin is effective against gram-positives, including MRSA. Its long half-life makes it an attractive alternative to other commonly used antibiotics, which require more frequent dosing.
Study design: Phase 3, double-blinded RCT.
Setting: Multiple international centers.
Synopsis: Researchers randomized 1,312 patients with acute bacterial skin and skin-structure infections with signs of systemic infection requiring intravenous antibiotics to receive dalbavancin on days one and eight, with placebo on other days, or several doses of vancomycin with an option to switch to oral linezolid. The primary endpoint was cessation of spread of erythema and temperature of =37.6°C at 48–72 hours. Secondary endpoints included a decrease in lesion area of =20% at 48–72 hours and clinical success at end of therapy (determined by clinical and historical features). Results of the primary endpoint were similar with dalbavancin and vancomycin-linezolid groups (79.7% and 79.8%, respectively) and were within 10 percentage points of noninferiority. The secondary endpoints were similar between both groups. Limitations of the study were the early primary endpoint, lack of noninferiority analysis of the secondary endpoints, and cost-effective analysis.
Bottom line: Once-weekly dalbavancin appears to be similarly efficacious to intravenous vancomycin in the treatment of acute bacterial skin infections in terms of outcomes within 48–72 hours of therapy and might provide an alternative to continued inpatient hospitalization for intravenous antibiotics in stable patients.
Citation: Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169-2179.
Background: Acute bacterial skin infections are common and often require hospitalization for intravenous antibiotic administration. Treatment covering gram-positive bacteria usually is indicated. Dalbavancin is effective against gram-positives, including MRSA. Its long half-life makes it an attractive alternative to other commonly used antibiotics, which require more frequent dosing.
Study design: Phase 3, double-blinded RCT.
Setting: Multiple international centers.
Synopsis: Researchers randomized 1,312 patients with acute bacterial skin and skin-structure infections with signs of systemic infection requiring intravenous antibiotics to receive dalbavancin on days one and eight, with placebo on other days, or several doses of vancomycin with an option to switch to oral linezolid. The primary endpoint was cessation of spread of erythema and temperature of =37.6°C at 48–72 hours. Secondary endpoints included a decrease in lesion area of =20% at 48–72 hours and clinical success at end of therapy (determined by clinical and historical features). Results of the primary endpoint were similar with dalbavancin and vancomycin-linezolid groups (79.7% and 79.8%, respectively) and were within 10 percentage points of noninferiority. The secondary endpoints were similar between both groups. Limitations of the study were the early primary endpoint, lack of noninferiority analysis of the secondary endpoints, and cost-effective analysis.
Bottom line: Once-weekly dalbavancin appears to be similarly efficacious to intravenous vancomycin in the treatment of acute bacterial skin infections in terms of outcomes within 48–72 hours of therapy and might provide an alternative to continued inpatient hospitalization for intravenous antibiotics in stable patients.
Citation: Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169-2179.
Once-Weekly Antibiotic Might Be Effective for Treatment of Acute Bacterial Skin Infections
Clinical question: Is once-weekly intravenous dalbavancin as effective as conventional therapy for the treatment of acute bacterial skin infections?
Background: Acute bacterial skin infections are common and often require hospitalization for intravenous antibiotic administration. Treatment covering gram-positive bacteria usually is indicated. Dalbavancin is effective against gram-positives, including MRSA. Its long half-life makes it an attractive alternative to other commonly used antibiotics, which require more frequent dosing.
Study design: Phase 3, double-blinded RCT.
Setting: Multiple international centers.
Synopsis: Researchers randomized 1,312 patients with acute bacterial skin and skin-structure infections with signs of systemic infection requiring intravenous antibiotics to receive dalbavancin on days one and eight, with placebo on other days, or several doses of vancomycin with an option to switch to oral linezolid. The primary endpoint was cessation of spread of erythema and temperature of ≤37.6°C at 48-72 hours. Secondary endpoints included a decrease in lesion area of ≥20% at 48-72 hours and clinical success at end of therapy (determined by clinical and historical features).
Results of the primary endpoint were similar with dalbavancin and vancomycin-linezolid groups (79.7% and 79.8%, respectively) and were within 10 percentage points of noninferiority. The secondary endpoints were similar between both groups.
Limitations of the study were the early primary endpoint, lack of noninferiority analysis of the secondary endpoints, and cost-effective analysis.
Bottom line: Once-weekly dalbavancin appears to be similarly efficacious to intravenous vancomycin in the treatment of acute bacterial skin infections in terms of outcomes within 48-72 hours of therapy and might provide an alternative to continued inpatient hospitalization for intravenous antibiotics in stable patients.
Citation: Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169-2179.
Clinical question: Is once-weekly intravenous dalbavancin as effective as conventional therapy for the treatment of acute bacterial skin infections?
Background: Acute bacterial skin infections are common and often require hospitalization for intravenous antibiotic administration. Treatment covering gram-positive bacteria usually is indicated. Dalbavancin is effective against gram-positives, including MRSA. Its long half-life makes it an attractive alternative to other commonly used antibiotics, which require more frequent dosing.
Study design: Phase 3, double-blinded RCT.
Setting: Multiple international centers.
Synopsis: Researchers randomized 1,312 patients with acute bacterial skin and skin-structure infections with signs of systemic infection requiring intravenous antibiotics to receive dalbavancin on days one and eight, with placebo on other days, or several doses of vancomycin with an option to switch to oral linezolid. The primary endpoint was cessation of spread of erythema and temperature of ≤37.6°C at 48-72 hours. Secondary endpoints included a decrease in lesion area of ≥20% at 48-72 hours and clinical success at end of therapy (determined by clinical and historical features).
Results of the primary endpoint were similar with dalbavancin and vancomycin-linezolid groups (79.7% and 79.8%, respectively) and were within 10 percentage points of noninferiority. The secondary endpoints were similar between both groups.
Limitations of the study were the early primary endpoint, lack of noninferiority analysis of the secondary endpoints, and cost-effective analysis.
Bottom line: Once-weekly dalbavancin appears to be similarly efficacious to intravenous vancomycin in the treatment of acute bacterial skin infections in terms of outcomes within 48-72 hours of therapy and might provide an alternative to continued inpatient hospitalization for intravenous antibiotics in stable patients.
Citation: Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169-2179.
Clinical question: Is once-weekly intravenous dalbavancin as effective as conventional therapy for the treatment of acute bacterial skin infections?
Background: Acute bacterial skin infections are common and often require hospitalization for intravenous antibiotic administration. Treatment covering gram-positive bacteria usually is indicated. Dalbavancin is effective against gram-positives, including MRSA. Its long half-life makes it an attractive alternative to other commonly used antibiotics, which require more frequent dosing.
Study design: Phase 3, double-blinded RCT.
Setting: Multiple international centers.
Synopsis: Researchers randomized 1,312 patients with acute bacterial skin and skin-structure infections with signs of systemic infection requiring intravenous antibiotics to receive dalbavancin on days one and eight, with placebo on other days, or several doses of vancomycin with an option to switch to oral linezolid. The primary endpoint was cessation of spread of erythema and temperature of ≤37.6°C at 48-72 hours. Secondary endpoints included a decrease in lesion area of ≥20% at 48-72 hours and clinical success at end of therapy (determined by clinical and historical features).
Results of the primary endpoint were similar with dalbavancin and vancomycin-linezolid groups (79.7% and 79.8%, respectively) and were within 10 percentage points of noninferiority. The secondary endpoints were similar between both groups.
Limitations of the study were the early primary endpoint, lack of noninferiority analysis of the secondary endpoints, and cost-effective analysis.
Bottom line: Once-weekly dalbavancin appears to be similarly efficacious to intravenous vancomycin in the treatment of acute bacterial skin infections in terms of outcomes within 48-72 hours of therapy and might provide an alternative to continued inpatient hospitalization for intravenous antibiotics in stable patients.
Citation: Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169-2179.
Continuous Positive Airway Pressure Outperforms Noctural Oxygen for Blood Pressure Reduction
Clinical question: What is the effect of continuous positive airway pressure (CPAP) or supplemental oxygen on ambulatory blood pressures and markers of cardiovascular risk when combined with sleep hygiene education in patients with obstructive sleep apnea (OSA) and coronary artery disease or cardiac risk factors?
Background: OSA is considered a risk factor for the development of hypertension. One meta-analysis showed reduction of mean arterial pressure (MAP) with CPAP therapy, but randomized controlled data on blood pressure reduction with treatment of OSA is lacking.
Study design: Randomized, parallel-group trial.
Setting: Four outpatient cardiology practices.
Synopsis: Patients ages 45-75 with OSA were randomized to receive nocturnal CPAP and healthy lifestyle and sleep education (HLSE), nocturnal oxygen therapy and HSLE, or HSLE alone. The primary outcome was 24-hour MAP. Secondary outcomes included fasting blood glucose, lipid panel, insulin level, erythrocyte sedimentation rate, C-reactive protein (CRP), and N-terminal pro-brain naturetic peptide.
Participants had high rates of diabetes, hypertension, and coronary artery disease. At 12 weeks, the CPAP arm experienced greater reductions in 24-hour MAP compared to both the nocturnal oxygen and HSLE arms (-2.8 mmHg [P=0.02] and -2.4 mmHg [P=0.04], respectively). No significant decrease in MAP was identified in the nocturnal oxygen arm when compared to the HSLE arm. The only significant difference in secondary outcomes was a decrease in CRP in the CPAP arm when compared to the HSLE arm, the clinical significance of which is unclear.
Bottom line: CPAP therapy with sleep hygiene education appears superior to nocturnal oxygen therapy with sleep hygiene education and sleep hygiene education alone in decreasing 24-hour MAP in patients with OSA and coronary artery disease or cardiac risk factors.
Citation: Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276-2285.
Clinical question: What is the effect of continuous positive airway pressure (CPAP) or supplemental oxygen on ambulatory blood pressures and markers of cardiovascular risk when combined with sleep hygiene education in patients with obstructive sleep apnea (OSA) and coronary artery disease or cardiac risk factors?
Background: OSA is considered a risk factor for the development of hypertension. One meta-analysis showed reduction of mean arterial pressure (MAP) with CPAP therapy, but randomized controlled data on blood pressure reduction with treatment of OSA is lacking.
Study design: Randomized, parallel-group trial.
Setting: Four outpatient cardiology practices.
Synopsis: Patients ages 45-75 with OSA were randomized to receive nocturnal CPAP and healthy lifestyle and sleep education (HLSE), nocturnal oxygen therapy and HSLE, or HSLE alone. The primary outcome was 24-hour MAP. Secondary outcomes included fasting blood glucose, lipid panel, insulin level, erythrocyte sedimentation rate, C-reactive protein (CRP), and N-terminal pro-brain naturetic peptide.
Participants had high rates of diabetes, hypertension, and coronary artery disease. At 12 weeks, the CPAP arm experienced greater reductions in 24-hour MAP compared to both the nocturnal oxygen and HSLE arms (-2.8 mmHg [P=0.02] and -2.4 mmHg [P=0.04], respectively). No significant decrease in MAP was identified in the nocturnal oxygen arm when compared to the HSLE arm. The only significant difference in secondary outcomes was a decrease in CRP in the CPAP arm when compared to the HSLE arm, the clinical significance of which is unclear.
Bottom line: CPAP therapy with sleep hygiene education appears superior to nocturnal oxygen therapy with sleep hygiene education and sleep hygiene education alone in decreasing 24-hour MAP in patients with OSA and coronary artery disease or cardiac risk factors.
Citation: Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276-2285.
Clinical question: What is the effect of continuous positive airway pressure (CPAP) or supplemental oxygen on ambulatory blood pressures and markers of cardiovascular risk when combined with sleep hygiene education in patients with obstructive sleep apnea (OSA) and coronary artery disease or cardiac risk factors?
Background: OSA is considered a risk factor for the development of hypertension. One meta-analysis showed reduction of mean arterial pressure (MAP) with CPAP therapy, but randomized controlled data on blood pressure reduction with treatment of OSA is lacking.
Study design: Randomized, parallel-group trial.
Setting: Four outpatient cardiology practices.
Synopsis: Patients ages 45-75 with OSA were randomized to receive nocturnal CPAP and healthy lifestyle and sleep education (HLSE), nocturnal oxygen therapy and HSLE, or HSLE alone. The primary outcome was 24-hour MAP. Secondary outcomes included fasting blood glucose, lipid panel, insulin level, erythrocyte sedimentation rate, C-reactive protein (CRP), and N-terminal pro-brain naturetic peptide.
Participants had high rates of diabetes, hypertension, and coronary artery disease. At 12 weeks, the CPAP arm experienced greater reductions in 24-hour MAP compared to both the nocturnal oxygen and HSLE arms (-2.8 mmHg [P=0.02] and -2.4 mmHg [P=0.04], respectively). No significant decrease in MAP was identified in the nocturnal oxygen arm when compared to the HSLE arm. The only significant difference in secondary outcomes was a decrease in CRP in the CPAP arm when compared to the HSLE arm, the clinical significance of which is unclear.
Bottom line: CPAP therapy with sleep hygiene education appears superior to nocturnal oxygen therapy with sleep hygiene education and sleep hygiene education alone in decreasing 24-hour MAP in patients with OSA and coronary artery disease or cardiac risk factors.
Citation: Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276-2285.
Lactate Clearance Portends Better Outcomes after Cardiac Arrest
Clinical question: Is greater lactate clearance following resuscitation from cardiac arrest associated with lower mortality and better neurologic outcomes?
Background: Recommendations from the International Liaison Committee on Resuscitation for monitoring serial lactate levels in post-resuscitation patients are based primarily on extrapolation from other conditions such as sepsis. Two single-retrospective analyses found effective lactate clearance was associated with decreased mortality. This association had not previously been validated in a multicenter, prospective study.
Study design: Multicenter, prospective, observational study.
Setting: Four urban, tertiary-care teaching hospitals.
Synopsis: Absolute lactate levels and the differences in the percent lactate change over 24 hours were compared in 100 patients who suffered out-of-hospital cardiac arrest. Ninety-seven percent received therapeutic hypothermia, and overall survival was 46%. Survivors and patients with a good neurologic outcome had lower lactate levels at zero hours (4.1 vs. 7.3), 12 hours (2.2 vs. 6.0), and 24 hours (1.6 vs. 4.4) compared with nonsurvivors and patients with bad neurologic outcomes.
The percent lactate decreased was greater in survivors and in those with good neurologic outcomes (odds ratio, 2.2; 95% confidence interval, 1.1–4.4).
Nonsurvivors or those with poor neurologic outcomes were less likely to have received bystander CPR, to have suffered a witnessed arrest, or to have had a shockable rhythm at presentation. Superior lactate clearance in survivors and those with good neurologic outcomes suggests a potential role in developing markers of effective resuscitation.
Bottom line: Lower lactate levels and more effective clearance of lactate in patients following cardiac arrest are associated with improved survival and good neurologic outcome.
Citation: Donnino MW, Andersen LW, Giberson T, et al. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med. 2014;42(8):1804-1811.
Clinical question: Is greater lactate clearance following resuscitation from cardiac arrest associated with lower mortality and better neurologic outcomes?
Background: Recommendations from the International Liaison Committee on Resuscitation for monitoring serial lactate levels in post-resuscitation patients are based primarily on extrapolation from other conditions such as sepsis. Two single-retrospective analyses found effective lactate clearance was associated with decreased mortality. This association had not previously been validated in a multicenter, prospective study.
Study design: Multicenter, prospective, observational study.
Setting: Four urban, tertiary-care teaching hospitals.
Synopsis: Absolute lactate levels and the differences in the percent lactate change over 24 hours were compared in 100 patients who suffered out-of-hospital cardiac arrest. Ninety-seven percent received therapeutic hypothermia, and overall survival was 46%. Survivors and patients with a good neurologic outcome had lower lactate levels at zero hours (4.1 vs. 7.3), 12 hours (2.2 vs. 6.0), and 24 hours (1.6 vs. 4.4) compared with nonsurvivors and patients with bad neurologic outcomes.
The percent lactate decreased was greater in survivors and in those with good neurologic outcomes (odds ratio, 2.2; 95% confidence interval, 1.1–4.4).
Nonsurvivors or those with poor neurologic outcomes were less likely to have received bystander CPR, to have suffered a witnessed arrest, or to have had a shockable rhythm at presentation. Superior lactate clearance in survivors and those with good neurologic outcomes suggests a potential role in developing markers of effective resuscitation.
Bottom line: Lower lactate levels and more effective clearance of lactate in patients following cardiac arrest are associated with improved survival and good neurologic outcome.
Citation: Donnino MW, Andersen LW, Giberson T, et al. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med. 2014;42(8):1804-1811.
Clinical question: Is greater lactate clearance following resuscitation from cardiac arrest associated with lower mortality and better neurologic outcomes?
Background: Recommendations from the International Liaison Committee on Resuscitation for monitoring serial lactate levels in post-resuscitation patients are based primarily on extrapolation from other conditions such as sepsis. Two single-retrospective analyses found effective lactate clearance was associated with decreased mortality. This association had not previously been validated in a multicenter, prospective study.
Study design: Multicenter, prospective, observational study.
Setting: Four urban, tertiary-care teaching hospitals.
Synopsis: Absolute lactate levels and the differences in the percent lactate change over 24 hours were compared in 100 patients who suffered out-of-hospital cardiac arrest. Ninety-seven percent received therapeutic hypothermia, and overall survival was 46%. Survivors and patients with a good neurologic outcome had lower lactate levels at zero hours (4.1 vs. 7.3), 12 hours (2.2 vs. 6.0), and 24 hours (1.6 vs. 4.4) compared with nonsurvivors and patients with bad neurologic outcomes.
The percent lactate decreased was greater in survivors and in those with good neurologic outcomes (odds ratio, 2.2; 95% confidence interval, 1.1–4.4).
Nonsurvivors or those with poor neurologic outcomes were less likely to have received bystander CPR, to have suffered a witnessed arrest, or to have had a shockable rhythm at presentation. Superior lactate clearance in survivors and those with good neurologic outcomes suggests a potential role in developing markers of effective resuscitation.
Bottom line: Lower lactate levels and more effective clearance of lactate in patients following cardiac arrest are associated with improved survival and good neurologic outcome.
Citation: Donnino MW, Andersen LW, Giberson T, et al. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med. 2014;42(8):1804-1811.