User login
Periprocedural Management of Chronically Anticoagulated Patients: A Practical Approach to Use of Novel Anticoagulants in Orthopedic Surgery
Chronic anticoagulation is a common preexisting condition in patients undergoing total joint arthroplasty (TJA). Atrial fibrillation (AF), the most common underlying disorder requiring chronic anticoagulation, affects more than 3 million patients in the United States—a number that is projected to increase to 16 million by 2050.1,2 Other common indications for anticoagulation are deep vein thrombosis (DVT) treatment, presence of a prosthetic heart valve, and venous thromboembolism (VTE) prevention after hip or knee arthroplasty. These patients face the additional risks of hemorrhage, persistent wound drainage, hematoma formation, transfusion requirements, periprosthetic joint infection, and longer hospital stay.1 Chronic anticoagulation traditionally has been managed with warfarin, which inhibits production of the vitamin K–dependent clotting factors II, VII, IX, and X. However, the new novel oral anticoagulants (NOACs), which target individual factors in the clotting cascade, are gaining favor as chronic anticoagulant agents because of their ease of use and improved efficacy and safety. These agents include the factor IIA inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
Management of patients at risk for thromboembolism and bleeding issues, particularly within the context of elective, urgent, or emergent orthopedic surgeries, is an evolving area. Understanding the pharmacokinetics, conventional laboratory tests, dosing, and reversal methods for NOACs is important, especially because clinical data are limited and the treatment itself can cause clinically significant harm.
In this article, we review the medical literature on these medications, their mechanism of action, and their reversal agents, and outline a practical approach for managing patients during the perioperative period.
Dabigatran
In October 2010, dabigatran became the first NOAC approved by the US Food and Drug Administration (FDA) for the prevention of arterial thromboembolic events in patients with nonvalvular AF, on the basis of the results of the RELY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Dabigatran is an oral factor IIA (thrombin) inhibitor. From time of ingestion, dabigatran takes 1.25 to 3 hours to reach peak plasma concentration. It has a half-life of 12 to 14 hours, is excreted predominantly by the kidneys (80%), and is renally dosed. The usual dose is 150 mg 2 times daily if creatinine clearance (CrCl) is >30 mL/minute, or 75 mg 2 times daily if CrCl is 15 to 30 mL/minute.3 Dabigatran is not recommended for patients with CrCl <15 mL/minute.
Dabigatran affects prothrombin time (PT), activated partial thromboplastin time (aPTT), ecarin clotting time, and thrombin time, with the latter 2 providing the most accurate means of monitoring appropriate drug levels.3,4 Of the tests commonly used to assess coagulation hemostasis in hospitals, normalization of thrombin time and aPTT provide the most accurate results (Table 1). The pharmacokinetics of dabigatran mandate consideration of dose, time of ingestion relative to time of blood sampling, and renal function in the assessment of coagulation hemostasis.
For elective surgeries, the periprocedure recommendation for patients being treated with dabigatran is to discontinue the medication 3 to 4 days before an operation if CrCl is ≥50 mL/minute, or 4 to 5 days beforehand if CrCl is <50 mL/minute.3 There is no antidote for dabigatran. In an in vitro model, activated charcoal reduced 99.9% of dabigatran absorption after recent ingestion.3 According to case reports, acute hemodialysis successfully removed 60% of the medication after 6 hours.5 In patients with end-stage renal disease, hemodialysis removed up to 68% of active dabigatran after 4 hours.3
Pernod and colleagues6 proposed that urgent surgeries can proceed if the concentration of dabigatran is ≤30 ng/mL—equivalent to normal aPTT. Their dictum was extrapolated from the data of patients who underwent elective surgeries while being treated with dabigatran, as recorded during the RELY trial. According to Pernod and colleagues,6 if aPTT is increased (probable drug level, ≥30 ng/mL), surgery should be postponed for up to 12 hours, with aPTT checked again and the process repeated if the concentration of dabigatran is still elevated and surgery can continue to be delayed. In patients who require urgent surgical interventions, we previously utilized nanofiltered activated prothrombin complex concentrate (aPCC; Feiba NF) 30 to 50 IU/kg over prothrombin complex concentrate (PCC; Kcentra or Bebulin) 25 to 50 IU/kg, as supported by in vitro and animal model studies and anecdotal case reports. However, neither aPCC nor PCC fully corrects the abnormalities evident on hemostasis tests.3,6 In October 2015, the FDA approved Idarucizumab (Praxbind), an injectable monoclonal antibody fragment that binds to dabigatran, as a reversing agent for use in urgent/emergent settings. Recommendation is to administer two 50-ml bolus infusions, each containing 2.5 g of idarucizumab, no more than 15 minutes apart.7 Additionally, hemodialysis could be discussed before surgery, with the understanding that it will take a long time to reach the threshold of 30 ng/mL in these patients (Table 2).
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor that was initially approved in November 2011 for the prevention of stroke and systemic embolism in patients with nonvalvular AF. Since then, clinical use of rivaroxaban has been expanded to include prevention of VTE after elective hip or knee arthroplasty as well as treatment of DVT and prevention of recurrent VTE after acute DVT. In the phase 3 ROCKET AF (Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) study, rivaroxaban 20 mg daily (CrCl, ≥50 mL/min) and rivaroxaban 15 mg daily (CrCl, 15-49 mL/min) were equally effective as warfarin. Compared with warfarin, rivaroxaban had a similar safety rate for bleeding and adverse events but fewer intracranial hemorrhage and fatal bleeding events.8 On the basis of the outcomes of the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) studies comparing rivaroxaban and enoxaparin sodium, rivaroxaban 10 mg daily was approved for the prevention of VTE and pulmonary embolism after elective hip or knee arthroplasty.8
The half-life of rivaroxaban is 5 to 9 hours in the young and 11 to 13 hours in the elderly.8 As rivaroxaban takes 2 to 4 hours after ingestion to reach peak plasma concentration, it is important to know the timing and the dose taken. Because of the short half-life and rapid onset of action of this medication, bridging with another anticoagulant is not required when rivaroxaban is discontinued before surgery or initiated after surgery.8 The recommendation is to withhold rivaroxaban for 24 to 48 hours before surgery and then to administer the first postoperative dose 6 to 10 hours after surgery, or when hemostasis is achieved (Table 1).
PT is recommended for rivaroxaban detection. Conventional assays are not sensitive at low concentrations, and degree of prolongation does not reliably predict amount of medication present.3,9 However, normal PT corresponds to a drug concentration of about 30 ng/mL and is considered safe for patients undergoing surgical intervention without increased risk for bleeding.6 This recommendation was extrapolated from data in the ROCKET AF study of patients who underwent elective surgeries while on rivaroxaban.6 Commercially available chromogenic anti–factor Xa assays, used with a rivaroxaban calibration curve, are sensitive and specific for rivaroxaban plasma concentrations.3,8 However, these assays are not widely available.
If a bleeding complication occurs in a patient who is being treated with rivaroxaban, the next rivaroxaban dose should be delayed, or treatment should be discontinued, as appropriate.8 Urgency of surgery should be weighed against risk for bleeding complications on a case-by-case basis. This decision is deferred to the clinical judgment of the surgeon. In the case of a patient with severe, life-threatening bleeding or a patient who requires emergent surgery, PCC 25-50 IU/kg is the recommended reversal agent.9 Recombinant factor VIIa and aPCC have been used in experimental settings, but there is concern about the greater prothrombotic potential of these agents compared with PCC8 (Table 2).
Apixaban
Apixaban is the second factor Xa inhibitor introduced in the United States and the first to show—in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) study—efficacy superior to that of warfarin for the prevention of stroke and systemic embolism, all-cause mortality, and major bleeding. Furthermore, in the AVERROES (Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) study, apixaban used in AF patients who were deemed not suitable for warfarin proved to be more effective than aspirin for stroke prevention, and had a similar rate of major bleeding.10 Apixaban is administered in a 5-mg dose 2 times daily. It has a half-life of 10 to 14 hours, is highly protein-bound, and has predominantly fecal excretion (27% is renal). Apixaban can prolong PT, but the correlation is nonlinear. Barrett and colleagues11 found that chromogenic anti–factor Xa assays provided the most accurate readings of apixaban plasma concentrations. Normal anti–factor Xa activity in patients being treated with apixaban suggests low drug levels and an intact hemostatic function, which are indicators of low bleeding risk with surgical intervention3 (Table 1).
Similar to other NOACs, apixaban has no antidote. In vitro testing showed that PCC improved thrombin generation when added to the blood of healthy donors who had received apixaban. Despite the lack of clinical experience, use of PCC 50 IU/kg may be reasonable for apixaban patients with severe or life-threatening bleeding3 (Table 2). Unlike dabigatran, apixaban cannot be eliminated with dialysis because of its high degree of protein binding. In nonemergent circumstances, delaying surgery 24 to 48 hours is considered effective in reducing the concentration of apixaban to a range that does not cause additional risk for bleeding.
Conclusion
Compared with warfarin, the NOACs dabigatran, rivaroxaban, and apixaban are efficacious and safe. Because of their steady pharmacokinetics, they do not require regular coagulation testing, as is the case with warfarin. These NOACs have been approved for the prevention of stroke and thromboembolic events in patients with nonvalvular AF; rivaroxaban has also been approved for VTE prevention after total hip or knee arthroplasty, for DVT treatment, and for prevention of recurrent VTE after acute DVT. Other options for VTE prophylaxis after hip and knee surgery are addressed in the guidelines issued by the American Academy of Orthopaedic Surgeons in 2011.12 As the incidence of chronic anticoagulation continues to increase among patients undergoing TJA, orthopedic surgeons need to be aware of the mechanism of action of these NOACs, as well as their pharmacokinetics and available reversal agents. Aggarwal and colleagues1 found that AF patients undergoing TJA had longer hospital stays, increased transfusion requirements, and increased risk for periprosthetic joint infection and unplanned hospital readmission.
The anticoagulation tests recommended for evaluation of hemostasis and drug reversal are normalization of aPTT for dabigatran; PT for rivaroxaban; and chromogenic anti–factor Xa activity for apixaban3 (Table 2). Although several research projects are being planned to develop an antidote for these medications, no antidote has been approved for human trials. The coagulation agents currently being used for reversal of NOACs are nonactivated PCC (Kcentra, Bebulin) and aPCC. Kcentra is a 4-factor PCC (II, VII, IX, X), and Bebulin is a 3-factor PCC (II, IX, X). Most authors recommend using 4-factor PCC 25 to 50 IU/kg. In vivo studies and animal studies have shown that nanofiltered aPCC (Feiba NF) at doses of 30 to 50 IU/kg can to some extent reverse anticoagulation in patients receiving NOACs. The current, limited data support use of reversal agent PCC for rivaroxaban and apixaban (no human studies for apixaban) and use of aPCC for dabigatran.3,6,8 Activated charcoal can be used for patients who have taken dabigatran <6 hours before presentation.3 Hemodialysis is another option for dabigatran removal. Hemodialysis, however, takes 4 to 6 hours or longer to remove about 60% of the medication (Table 2).3,5
In major orthopedic surgeries, such as TJA, bleeding is a critical concern. Using reversal agents to overcome the anticoagulation effect adds to the potential concern for thromboembolism secondary to these agents. Therefore, in cases in which surgery cannot be delayed any longer, the decision to use reversal agents should be made on a case-by-case basis. For most patients on rivaroxaban or apixaban, it is sufficient to delay for 24 to 48 hours before proceeding safely with surgery; for dabigatran, a delay of 3 to 4 days is recommended. Delay before surgery may need to be extended for the elderly and for patients with renal failure. The pharmacokinetics of these medications is summarized in Table 1.
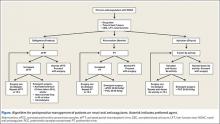
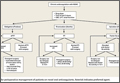
There are no guidelines for perioperative management of patients undergoing elective, urgent, or emergent surgeries while on NOACs. As discussed, Pernod and colleagues6 proposed better perioperative management of major bleeding risks in patients receiving rivaroxaban or dabigatran. Adapting their approach, and using the data available from the medical literature, we propose a perioperative algorithm that can guide practicing orthopedic surgeons performing urgent and emergent surgeries (Figure).
The population of patients receiving chronic anticoagulation therapy is growing, and anticoagulant and antiplatelet options are increasing in the United States and around the world. We propose a team approach for patient care, with orthopedic surgeon and cardiologist or vascular medicine specialist collaborating to ensure the safety and effectiveness of this treatment.
1. Aggarwal VK, Tischler EH, Post ZD, Kane I, Orozco FR, Ong A. Patients with atrial fibrillation undergoing total joint arthroplasty increase hospital burden. J Bone Joint Surg Am. 2013;95(17):1606-1611.
2. Curtis AB. Practice implications of the atrial fibrillation guidelines. Am J Cardiol. 2013;111(11):1660-1670.
3. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34(7):489-498b.
4. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116-1127.
5. Lillo-Le Louët A, Wolf M, Soufir L, et al. Life-threatening bleeding in four patients with an unusual excessive response to dabigatran: implications for emergency surgery and resuscitation. Thromb Haemost. 2012;108(3):583-585.
6. Pernod G, Albaladejo P, Godier A, et al; Working Group on Perioperative Haemostasis. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: proposals of the Working Group on Perioperative Haemostasis (GIHP) - March 2013. Arch Cardiovasc Dis. 2013;106(6-7):382-393.
7. Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511-520.
8. Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban: an oral, direct factor Xa inhibitor. Thromb Haemost. 2012;108(5):876-886.
9. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
10. Yates SW. Apixaban for stroke prevention in atrial fibrillation: a review of the clinical trial evidence. Hosp Pract. 2011;39(4):7-16.
11. Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104(6):1263-1271.
12. American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. Agency for Healthcare Research and Quality website. http://www.guideline.gov/content.aspx?id=35173. Released 2007. Revised 2011. Accessed March 21, 2016.
Chronic anticoagulation is a common preexisting condition in patients undergoing total joint arthroplasty (TJA). Atrial fibrillation (AF), the most common underlying disorder requiring chronic anticoagulation, affects more than 3 million patients in the United States—a number that is projected to increase to 16 million by 2050.1,2 Other common indications for anticoagulation are deep vein thrombosis (DVT) treatment, presence of a prosthetic heart valve, and venous thromboembolism (VTE) prevention after hip or knee arthroplasty. These patients face the additional risks of hemorrhage, persistent wound drainage, hematoma formation, transfusion requirements, periprosthetic joint infection, and longer hospital stay.1 Chronic anticoagulation traditionally has been managed with warfarin, which inhibits production of the vitamin K–dependent clotting factors II, VII, IX, and X. However, the new novel oral anticoagulants (NOACs), which target individual factors in the clotting cascade, are gaining favor as chronic anticoagulant agents because of their ease of use and improved efficacy and safety. These agents include the factor IIA inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
Management of patients at risk for thromboembolism and bleeding issues, particularly within the context of elective, urgent, or emergent orthopedic surgeries, is an evolving area. Understanding the pharmacokinetics, conventional laboratory tests, dosing, and reversal methods for NOACs is important, especially because clinical data are limited and the treatment itself can cause clinically significant harm.
In this article, we review the medical literature on these medications, their mechanism of action, and their reversal agents, and outline a practical approach for managing patients during the perioperative period.
Dabigatran
In October 2010, dabigatran became the first NOAC approved by the US Food and Drug Administration (FDA) for the prevention of arterial thromboembolic events in patients with nonvalvular AF, on the basis of the results of the RELY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Dabigatran is an oral factor IIA (thrombin) inhibitor. From time of ingestion, dabigatran takes 1.25 to 3 hours to reach peak plasma concentration. It has a half-life of 12 to 14 hours, is excreted predominantly by the kidneys (80%), and is renally dosed. The usual dose is 150 mg 2 times daily if creatinine clearance (CrCl) is >30 mL/minute, or 75 mg 2 times daily if CrCl is 15 to 30 mL/minute.3 Dabigatran is not recommended for patients with CrCl <15 mL/minute.
Dabigatran affects prothrombin time (PT), activated partial thromboplastin time (aPTT), ecarin clotting time, and thrombin time, with the latter 2 providing the most accurate means of monitoring appropriate drug levels.3,4 Of the tests commonly used to assess coagulation hemostasis in hospitals, normalization of thrombin time and aPTT provide the most accurate results (Table 1). The pharmacokinetics of dabigatran mandate consideration of dose, time of ingestion relative to time of blood sampling, and renal function in the assessment of coagulation hemostasis.
For elective surgeries, the periprocedure recommendation for patients being treated with dabigatran is to discontinue the medication 3 to 4 days before an operation if CrCl is ≥50 mL/minute, or 4 to 5 days beforehand if CrCl is <50 mL/minute.3 There is no antidote for dabigatran. In an in vitro model, activated charcoal reduced 99.9% of dabigatran absorption after recent ingestion.3 According to case reports, acute hemodialysis successfully removed 60% of the medication after 6 hours.5 In patients with end-stage renal disease, hemodialysis removed up to 68% of active dabigatran after 4 hours.3
Pernod and colleagues6 proposed that urgent surgeries can proceed if the concentration of dabigatran is ≤30 ng/mL—equivalent to normal aPTT. Their dictum was extrapolated from the data of patients who underwent elective surgeries while being treated with dabigatran, as recorded during the RELY trial. According to Pernod and colleagues,6 if aPTT is increased (probable drug level, ≥30 ng/mL), surgery should be postponed for up to 12 hours, with aPTT checked again and the process repeated if the concentration of dabigatran is still elevated and surgery can continue to be delayed. In patients who require urgent surgical interventions, we previously utilized nanofiltered activated prothrombin complex concentrate (aPCC; Feiba NF) 30 to 50 IU/kg over prothrombin complex concentrate (PCC; Kcentra or Bebulin) 25 to 50 IU/kg, as supported by in vitro and animal model studies and anecdotal case reports. However, neither aPCC nor PCC fully corrects the abnormalities evident on hemostasis tests.3,6 In October 2015, the FDA approved Idarucizumab (Praxbind), an injectable monoclonal antibody fragment that binds to dabigatran, as a reversing agent for use in urgent/emergent settings. Recommendation is to administer two 50-ml bolus infusions, each containing 2.5 g of idarucizumab, no more than 15 minutes apart.7 Additionally, hemodialysis could be discussed before surgery, with the understanding that it will take a long time to reach the threshold of 30 ng/mL in these patients (Table 2).
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor that was initially approved in November 2011 for the prevention of stroke and systemic embolism in patients with nonvalvular AF. Since then, clinical use of rivaroxaban has been expanded to include prevention of VTE after elective hip or knee arthroplasty as well as treatment of DVT and prevention of recurrent VTE after acute DVT. In the phase 3 ROCKET AF (Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) study, rivaroxaban 20 mg daily (CrCl, ≥50 mL/min) and rivaroxaban 15 mg daily (CrCl, 15-49 mL/min) were equally effective as warfarin. Compared with warfarin, rivaroxaban had a similar safety rate for bleeding and adverse events but fewer intracranial hemorrhage and fatal bleeding events.8 On the basis of the outcomes of the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) studies comparing rivaroxaban and enoxaparin sodium, rivaroxaban 10 mg daily was approved for the prevention of VTE and pulmonary embolism after elective hip or knee arthroplasty.8
The half-life of rivaroxaban is 5 to 9 hours in the young and 11 to 13 hours in the elderly.8 As rivaroxaban takes 2 to 4 hours after ingestion to reach peak plasma concentration, it is important to know the timing and the dose taken. Because of the short half-life and rapid onset of action of this medication, bridging with another anticoagulant is not required when rivaroxaban is discontinued before surgery or initiated after surgery.8 The recommendation is to withhold rivaroxaban for 24 to 48 hours before surgery and then to administer the first postoperative dose 6 to 10 hours after surgery, or when hemostasis is achieved (Table 1).
PT is recommended for rivaroxaban detection. Conventional assays are not sensitive at low concentrations, and degree of prolongation does not reliably predict amount of medication present.3,9 However, normal PT corresponds to a drug concentration of about 30 ng/mL and is considered safe for patients undergoing surgical intervention without increased risk for bleeding.6 This recommendation was extrapolated from data in the ROCKET AF study of patients who underwent elective surgeries while on rivaroxaban.6 Commercially available chromogenic anti–factor Xa assays, used with a rivaroxaban calibration curve, are sensitive and specific for rivaroxaban plasma concentrations.3,8 However, these assays are not widely available.
If a bleeding complication occurs in a patient who is being treated with rivaroxaban, the next rivaroxaban dose should be delayed, or treatment should be discontinued, as appropriate.8 Urgency of surgery should be weighed against risk for bleeding complications on a case-by-case basis. This decision is deferred to the clinical judgment of the surgeon. In the case of a patient with severe, life-threatening bleeding or a patient who requires emergent surgery, PCC 25-50 IU/kg is the recommended reversal agent.9 Recombinant factor VIIa and aPCC have been used in experimental settings, but there is concern about the greater prothrombotic potential of these agents compared with PCC8 (Table 2).
Apixaban
Apixaban is the second factor Xa inhibitor introduced in the United States and the first to show—in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) study—efficacy superior to that of warfarin for the prevention of stroke and systemic embolism, all-cause mortality, and major bleeding. Furthermore, in the AVERROES (Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) study, apixaban used in AF patients who were deemed not suitable for warfarin proved to be more effective than aspirin for stroke prevention, and had a similar rate of major bleeding.10 Apixaban is administered in a 5-mg dose 2 times daily. It has a half-life of 10 to 14 hours, is highly protein-bound, and has predominantly fecal excretion (27% is renal). Apixaban can prolong PT, but the correlation is nonlinear. Barrett and colleagues11 found that chromogenic anti–factor Xa assays provided the most accurate readings of apixaban plasma concentrations. Normal anti–factor Xa activity in patients being treated with apixaban suggests low drug levels and an intact hemostatic function, which are indicators of low bleeding risk with surgical intervention3 (Table 1).
Similar to other NOACs, apixaban has no antidote. In vitro testing showed that PCC improved thrombin generation when added to the blood of healthy donors who had received apixaban. Despite the lack of clinical experience, use of PCC 50 IU/kg may be reasonable for apixaban patients with severe or life-threatening bleeding3 (Table 2). Unlike dabigatran, apixaban cannot be eliminated with dialysis because of its high degree of protein binding. In nonemergent circumstances, delaying surgery 24 to 48 hours is considered effective in reducing the concentration of apixaban to a range that does not cause additional risk for bleeding.
Conclusion
Compared with warfarin, the NOACs dabigatran, rivaroxaban, and apixaban are efficacious and safe. Because of their steady pharmacokinetics, they do not require regular coagulation testing, as is the case with warfarin. These NOACs have been approved for the prevention of stroke and thromboembolic events in patients with nonvalvular AF; rivaroxaban has also been approved for VTE prevention after total hip or knee arthroplasty, for DVT treatment, and for prevention of recurrent VTE after acute DVT. Other options for VTE prophylaxis after hip and knee surgery are addressed in the guidelines issued by the American Academy of Orthopaedic Surgeons in 2011.12 As the incidence of chronic anticoagulation continues to increase among patients undergoing TJA, orthopedic surgeons need to be aware of the mechanism of action of these NOACs, as well as their pharmacokinetics and available reversal agents. Aggarwal and colleagues1 found that AF patients undergoing TJA had longer hospital stays, increased transfusion requirements, and increased risk for periprosthetic joint infection and unplanned hospital readmission.
The anticoagulation tests recommended for evaluation of hemostasis and drug reversal are normalization of aPTT for dabigatran; PT for rivaroxaban; and chromogenic anti–factor Xa activity for apixaban3 (Table 2). Although several research projects are being planned to develop an antidote for these medications, no antidote has been approved for human trials. The coagulation agents currently being used for reversal of NOACs are nonactivated PCC (Kcentra, Bebulin) and aPCC. Kcentra is a 4-factor PCC (II, VII, IX, X), and Bebulin is a 3-factor PCC (II, IX, X). Most authors recommend using 4-factor PCC 25 to 50 IU/kg. In vivo studies and animal studies have shown that nanofiltered aPCC (Feiba NF) at doses of 30 to 50 IU/kg can to some extent reverse anticoagulation in patients receiving NOACs. The current, limited data support use of reversal agent PCC for rivaroxaban and apixaban (no human studies for apixaban) and use of aPCC for dabigatran.3,6,8 Activated charcoal can be used for patients who have taken dabigatran <6 hours before presentation.3 Hemodialysis is another option for dabigatran removal. Hemodialysis, however, takes 4 to 6 hours or longer to remove about 60% of the medication (Table 2).3,5
In major orthopedic surgeries, such as TJA, bleeding is a critical concern. Using reversal agents to overcome the anticoagulation effect adds to the potential concern for thromboembolism secondary to these agents. Therefore, in cases in which surgery cannot be delayed any longer, the decision to use reversal agents should be made on a case-by-case basis. For most patients on rivaroxaban or apixaban, it is sufficient to delay for 24 to 48 hours before proceeding safely with surgery; for dabigatran, a delay of 3 to 4 days is recommended. Delay before surgery may need to be extended for the elderly and for patients with renal failure. The pharmacokinetics of these medications is summarized in Table 1.
There are no guidelines for perioperative management of patients undergoing elective, urgent, or emergent surgeries while on NOACs. As discussed, Pernod and colleagues6 proposed better perioperative management of major bleeding risks in patients receiving rivaroxaban or dabigatran. Adapting their approach, and using the data available from the medical literature, we propose a perioperative algorithm that can guide practicing orthopedic surgeons performing urgent and emergent surgeries (Figure).
The population of patients receiving chronic anticoagulation therapy is growing, and anticoagulant and antiplatelet options are increasing in the United States and around the world. We propose a team approach for patient care, with orthopedic surgeon and cardiologist or vascular medicine specialist collaborating to ensure the safety and effectiveness of this treatment.
Chronic anticoagulation is a common preexisting condition in patients undergoing total joint arthroplasty (TJA). Atrial fibrillation (AF), the most common underlying disorder requiring chronic anticoagulation, affects more than 3 million patients in the United States—a number that is projected to increase to 16 million by 2050.1,2 Other common indications for anticoagulation are deep vein thrombosis (DVT) treatment, presence of a prosthetic heart valve, and venous thromboembolism (VTE) prevention after hip or knee arthroplasty. These patients face the additional risks of hemorrhage, persistent wound drainage, hematoma formation, transfusion requirements, periprosthetic joint infection, and longer hospital stay.1 Chronic anticoagulation traditionally has been managed with warfarin, which inhibits production of the vitamin K–dependent clotting factors II, VII, IX, and X. However, the new novel oral anticoagulants (NOACs), which target individual factors in the clotting cascade, are gaining favor as chronic anticoagulant agents because of their ease of use and improved efficacy and safety. These agents include the factor IIA inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
Management of patients at risk for thromboembolism and bleeding issues, particularly within the context of elective, urgent, or emergent orthopedic surgeries, is an evolving area. Understanding the pharmacokinetics, conventional laboratory tests, dosing, and reversal methods for NOACs is important, especially because clinical data are limited and the treatment itself can cause clinically significant harm.
In this article, we review the medical literature on these medications, their mechanism of action, and their reversal agents, and outline a practical approach for managing patients during the perioperative period.
Dabigatran
In October 2010, dabigatran became the first NOAC approved by the US Food and Drug Administration (FDA) for the prevention of arterial thromboembolic events in patients with nonvalvular AF, on the basis of the results of the RELY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Dabigatran is an oral factor IIA (thrombin) inhibitor. From time of ingestion, dabigatran takes 1.25 to 3 hours to reach peak plasma concentration. It has a half-life of 12 to 14 hours, is excreted predominantly by the kidneys (80%), and is renally dosed. The usual dose is 150 mg 2 times daily if creatinine clearance (CrCl) is >30 mL/minute, or 75 mg 2 times daily if CrCl is 15 to 30 mL/minute.3 Dabigatran is not recommended for patients with CrCl <15 mL/minute.
Dabigatran affects prothrombin time (PT), activated partial thromboplastin time (aPTT), ecarin clotting time, and thrombin time, with the latter 2 providing the most accurate means of monitoring appropriate drug levels.3,4 Of the tests commonly used to assess coagulation hemostasis in hospitals, normalization of thrombin time and aPTT provide the most accurate results (Table 1). The pharmacokinetics of dabigatran mandate consideration of dose, time of ingestion relative to time of blood sampling, and renal function in the assessment of coagulation hemostasis.
For elective surgeries, the periprocedure recommendation for patients being treated with dabigatran is to discontinue the medication 3 to 4 days before an operation if CrCl is ≥50 mL/minute, or 4 to 5 days beforehand if CrCl is <50 mL/minute.3 There is no antidote for dabigatran. In an in vitro model, activated charcoal reduced 99.9% of dabigatran absorption after recent ingestion.3 According to case reports, acute hemodialysis successfully removed 60% of the medication after 6 hours.5 In patients with end-stage renal disease, hemodialysis removed up to 68% of active dabigatran after 4 hours.3
Pernod and colleagues6 proposed that urgent surgeries can proceed if the concentration of dabigatran is ≤30 ng/mL—equivalent to normal aPTT. Their dictum was extrapolated from the data of patients who underwent elective surgeries while being treated with dabigatran, as recorded during the RELY trial. According to Pernod and colleagues,6 if aPTT is increased (probable drug level, ≥30 ng/mL), surgery should be postponed for up to 12 hours, with aPTT checked again and the process repeated if the concentration of dabigatran is still elevated and surgery can continue to be delayed. In patients who require urgent surgical interventions, we previously utilized nanofiltered activated prothrombin complex concentrate (aPCC; Feiba NF) 30 to 50 IU/kg over prothrombin complex concentrate (PCC; Kcentra or Bebulin) 25 to 50 IU/kg, as supported by in vitro and animal model studies and anecdotal case reports. However, neither aPCC nor PCC fully corrects the abnormalities evident on hemostasis tests.3,6 In October 2015, the FDA approved Idarucizumab (Praxbind), an injectable monoclonal antibody fragment that binds to dabigatran, as a reversing agent for use in urgent/emergent settings. Recommendation is to administer two 50-ml bolus infusions, each containing 2.5 g of idarucizumab, no more than 15 minutes apart.7 Additionally, hemodialysis could be discussed before surgery, with the understanding that it will take a long time to reach the threshold of 30 ng/mL in these patients (Table 2).
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor that was initially approved in November 2011 for the prevention of stroke and systemic embolism in patients with nonvalvular AF. Since then, clinical use of rivaroxaban has been expanded to include prevention of VTE after elective hip or knee arthroplasty as well as treatment of DVT and prevention of recurrent VTE after acute DVT. In the phase 3 ROCKET AF (Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) study, rivaroxaban 20 mg daily (CrCl, ≥50 mL/min) and rivaroxaban 15 mg daily (CrCl, 15-49 mL/min) were equally effective as warfarin. Compared with warfarin, rivaroxaban had a similar safety rate for bleeding and adverse events but fewer intracranial hemorrhage and fatal bleeding events.8 On the basis of the outcomes of the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) studies comparing rivaroxaban and enoxaparin sodium, rivaroxaban 10 mg daily was approved for the prevention of VTE and pulmonary embolism after elective hip or knee arthroplasty.8
The half-life of rivaroxaban is 5 to 9 hours in the young and 11 to 13 hours in the elderly.8 As rivaroxaban takes 2 to 4 hours after ingestion to reach peak plasma concentration, it is important to know the timing and the dose taken. Because of the short half-life and rapid onset of action of this medication, bridging with another anticoagulant is not required when rivaroxaban is discontinued before surgery or initiated after surgery.8 The recommendation is to withhold rivaroxaban for 24 to 48 hours before surgery and then to administer the first postoperative dose 6 to 10 hours after surgery, or when hemostasis is achieved (Table 1).
PT is recommended for rivaroxaban detection. Conventional assays are not sensitive at low concentrations, and degree of prolongation does not reliably predict amount of medication present.3,9 However, normal PT corresponds to a drug concentration of about 30 ng/mL and is considered safe for patients undergoing surgical intervention without increased risk for bleeding.6 This recommendation was extrapolated from data in the ROCKET AF study of patients who underwent elective surgeries while on rivaroxaban.6 Commercially available chromogenic anti–factor Xa assays, used with a rivaroxaban calibration curve, are sensitive and specific for rivaroxaban plasma concentrations.3,8 However, these assays are not widely available.
If a bleeding complication occurs in a patient who is being treated with rivaroxaban, the next rivaroxaban dose should be delayed, or treatment should be discontinued, as appropriate.8 Urgency of surgery should be weighed against risk for bleeding complications on a case-by-case basis. This decision is deferred to the clinical judgment of the surgeon. In the case of a patient with severe, life-threatening bleeding or a patient who requires emergent surgery, PCC 25-50 IU/kg is the recommended reversal agent.9 Recombinant factor VIIa and aPCC have been used in experimental settings, but there is concern about the greater prothrombotic potential of these agents compared with PCC8 (Table 2).
Apixaban
Apixaban is the second factor Xa inhibitor introduced in the United States and the first to show—in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) study—efficacy superior to that of warfarin for the prevention of stroke and systemic embolism, all-cause mortality, and major bleeding. Furthermore, in the AVERROES (Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) study, apixaban used in AF patients who were deemed not suitable for warfarin proved to be more effective than aspirin for stroke prevention, and had a similar rate of major bleeding.10 Apixaban is administered in a 5-mg dose 2 times daily. It has a half-life of 10 to 14 hours, is highly protein-bound, and has predominantly fecal excretion (27% is renal). Apixaban can prolong PT, but the correlation is nonlinear. Barrett and colleagues11 found that chromogenic anti–factor Xa assays provided the most accurate readings of apixaban plasma concentrations. Normal anti–factor Xa activity in patients being treated with apixaban suggests low drug levels and an intact hemostatic function, which are indicators of low bleeding risk with surgical intervention3 (Table 1).
Similar to other NOACs, apixaban has no antidote. In vitro testing showed that PCC improved thrombin generation when added to the blood of healthy donors who had received apixaban. Despite the lack of clinical experience, use of PCC 50 IU/kg may be reasonable for apixaban patients with severe or life-threatening bleeding3 (Table 2). Unlike dabigatran, apixaban cannot be eliminated with dialysis because of its high degree of protein binding. In nonemergent circumstances, delaying surgery 24 to 48 hours is considered effective in reducing the concentration of apixaban to a range that does not cause additional risk for bleeding.
Conclusion
Compared with warfarin, the NOACs dabigatran, rivaroxaban, and apixaban are efficacious and safe. Because of their steady pharmacokinetics, they do not require regular coagulation testing, as is the case with warfarin. These NOACs have been approved for the prevention of stroke and thromboembolic events in patients with nonvalvular AF; rivaroxaban has also been approved for VTE prevention after total hip or knee arthroplasty, for DVT treatment, and for prevention of recurrent VTE after acute DVT. Other options for VTE prophylaxis after hip and knee surgery are addressed in the guidelines issued by the American Academy of Orthopaedic Surgeons in 2011.12 As the incidence of chronic anticoagulation continues to increase among patients undergoing TJA, orthopedic surgeons need to be aware of the mechanism of action of these NOACs, as well as their pharmacokinetics and available reversal agents. Aggarwal and colleagues1 found that AF patients undergoing TJA had longer hospital stays, increased transfusion requirements, and increased risk for periprosthetic joint infection and unplanned hospital readmission.
The anticoagulation tests recommended for evaluation of hemostasis and drug reversal are normalization of aPTT for dabigatran; PT for rivaroxaban; and chromogenic anti–factor Xa activity for apixaban3 (Table 2). Although several research projects are being planned to develop an antidote for these medications, no antidote has been approved for human trials. The coagulation agents currently being used for reversal of NOACs are nonactivated PCC (Kcentra, Bebulin) and aPCC. Kcentra is a 4-factor PCC (II, VII, IX, X), and Bebulin is a 3-factor PCC (II, IX, X). Most authors recommend using 4-factor PCC 25 to 50 IU/kg. In vivo studies and animal studies have shown that nanofiltered aPCC (Feiba NF) at doses of 30 to 50 IU/kg can to some extent reverse anticoagulation in patients receiving NOACs. The current, limited data support use of reversal agent PCC for rivaroxaban and apixaban (no human studies for apixaban) and use of aPCC for dabigatran.3,6,8 Activated charcoal can be used for patients who have taken dabigatran <6 hours before presentation.3 Hemodialysis is another option for dabigatran removal. Hemodialysis, however, takes 4 to 6 hours or longer to remove about 60% of the medication (Table 2).3,5
In major orthopedic surgeries, such as TJA, bleeding is a critical concern. Using reversal agents to overcome the anticoagulation effect adds to the potential concern for thromboembolism secondary to these agents. Therefore, in cases in which surgery cannot be delayed any longer, the decision to use reversal agents should be made on a case-by-case basis. For most patients on rivaroxaban or apixaban, it is sufficient to delay for 24 to 48 hours before proceeding safely with surgery; for dabigatran, a delay of 3 to 4 days is recommended. Delay before surgery may need to be extended for the elderly and for patients with renal failure. The pharmacokinetics of these medications is summarized in Table 1.
There are no guidelines for perioperative management of patients undergoing elective, urgent, or emergent surgeries while on NOACs. As discussed, Pernod and colleagues6 proposed better perioperative management of major bleeding risks in patients receiving rivaroxaban or dabigatran. Adapting their approach, and using the data available from the medical literature, we propose a perioperative algorithm that can guide practicing orthopedic surgeons performing urgent and emergent surgeries (Figure).
The population of patients receiving chronic anticoagulation therapy is growing, and anticoagulant and antiplatelet options are increasing in the United States and around the world. We propose a team approach for patient care, with orthopedic surgeon and cardiologist or vascular medicine specialist collaborating to ensure the safety and effectiveness of this treatment.
1. Aggarwal VK, Tischler EH, Post ZD, Kane I, Orozco FR, Ong A. Patients with atrial fibrillation undergoing total joint arthroplasty increase hospital burden. J Bone Joint Surg Am. 2013;95(17):1606-1611.
2. Curtis AB. Practice implications of the atrial fibrillation guidelines. Am J Cardiol. 2013;111(11):1660-1670.
3. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34(7):489-498b.
4. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116-1127.
5. Lillo-Le Louët A, Wolf M, Soufir L, et al. Life-threatening bleeding in four patients with an unusual excessive response to dabigatran: implications for emergency surgery and resuscitation. Thromb Haemost. 2012;108(3):583-585.
6. Pernod G, Albaladejo P, Godier A, et al; Working Group on Perioperative Haemostasis. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: proposals of the Working Group on Perioperative Haemostasis (GIHP) - March 2013. Arch Cardiovasc Dis. 2013;106(6-7):382-393.
7. Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511-520.
8. Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban: an oral, direct factor Xa inhibitor. Thromb Haemost. 2012;108(5):876-886.
9. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
10. Yates SW. Apixaban for stroke prevention in atrial fibrillation: a review of the clinical trial evidence. Hosp Pract. 2011;39(4):7-16.
11. Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104(6):1263-1271.
12. American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. Agency for Healthcare Research and Quality website. http://www.guideline.gov/content.aspx?id=35173. Released 2007. Revised 2011. Accessed March 21, 2016.
1. Aggarwal VK, Tischler EH, Post ZD, Kane I, Orozco FR, Ong A. Patients with atrial fibrillation undergoing total joint arthroplasty increase hospital burden. J Bone Joint Surg Am. 2013;95(17):1606-1611.
2. Curtis AB. Practice implications of the atrial fibrillation guidelines. Am J Cardiol. 2013;111(11):1660-1670.
3. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34(7):489-498b.
4. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116-1127.
5. Lillo-Le Louët A, Wolf M, Soufir L, et al. Life-threatening bleeding in four patients with an unusual excessive response to dabigatran: implications for emergency surgery and resuscitation. Thromb Haemost. 2012;108(3):583-585.
6. Pernod G, Albaladejo P, Godier A, et al; Working Group on Perioperative Haemostasis. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: proposals of the Working Group on Perioperative Haemostasis (GIHP) - March 2013. Arch Cardiovasc Dis. 2013;106(6-7):382-393.
7. Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511-520.
8. Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban: an oral, direct factor Xa inhibitor. Thromb Haemost. 2012;108(5):876-886.
9. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
10. Yates SW. Apixaban for stroke prevention in atrial fibrillation: a review of the clinical trial evidence. Hosp Pract. 2011;39(4):7-16.
11. Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104(6):1263-1271.
12. American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. Agency for Healthcare Research and Quality website. http://www.guideline.gov/content.aspx?id=35173. Released 2007. Revised 2011. Accessed March 21, 2016.