User login
New Classification of Lung Adenocarcinoma Calls for EGFR Testing
A joint effort by three medical groups has enabled a variety of specialists to join pathologists in revising the classification of lung adenocarcinoma, and they’ve made some major changes.
A new section addresses diagnosis and classification of non–small cell lung carcinoma (NSCLC) in small biopsies and cytology, including criteria to distinguish adenocarcinoma from squamous cell carcinoma.
The new classification also recommends epidermal growth factor receptor (EGFR) mutation testing in patients with advanced lung adenocarcinoma to help predict response to tyrosine kinase inhibitors.
And it dumps the term "bronchioalveolar carcinoma" while elsewhere adding some new terms (adenocarcinoma in situ and minimally invasive adenocarcinoma) in the document published in the February issue of the Journal of Thoracic Oncology (J. Thorac. Oncol. 2011;6:244-285).
The International Association for the Study of Lung Cancer convened the multidisciplinary panel of experts to revise the previous World Health Organization classification of lung adenocarcinoma, with support and scientific oversight from the American Thoracic Society and the European Respiratory Society. Pathologists, oncologists, pulmonologists, radiologists, thoracic surgeons, and molecular biologists joined the effort.
The revisions should make it easier to stratify patients and to individualize treatment, Dr. William D. Travis, chair of the expert panel, said in an interview. The changes also could significantly influence the next revision of the TNM (tumor, node, metastases) staging system, "not only for pathologic staging but also for clinical staging," said Dr. Travis, a thoracic pathologist at Memorial Sloan-Kettering Cancer Center, New York.
The new section on small biopsies and cytology specimens is especially important because 70% of lung cancers are diagnosed in samples like these, the consensus panel’s statement said. New criteria for diagnosing adenocarcinoma vs. squamous cell carcinoma include the use of special stains in difficult cases, and emphasize the importance of preserving tissue for molecular studies.
Dr. Travis outlined three important clinical reasons to distinguish adenocarcinomas from squamous cell carcinoma, especially in advanced disease.
• Patients with advanced lung adenocarcinoma or unspecified NSCLC who test positive for EGFR mutation are more likely to respond to treatment with tyrosine kinase inhibitors than are patients without mutation.
• Patients with adenocarcinoma or unspecific NSCLC are more likely to respond to pemetrexed (Alimta) than are patients with squamous cell carcinoma.
• Bevacizumab is contraindicated in patients with squamous cell carcinoma because it can lead to life-threatening hemorrhage, he said.
The statement attempts to banish the term bronchioloalveolar carcinoma from histopathology because it is used in ways that confuse five distinct categories: adenocarcinoma in situ; minimally invasive adenocarcinoma; lepidic predominant adenocarcinoma; adenocarcinoma that is predominantly invasive with some nonmucinous lepidic component; and invasive mucinous adenocarcinoma.
"Adenocarcinoma in situ" and "minimally invasive adenocarcinoma" appear in the classification for the first time for small solitary adenocarcinomas with either pure lepidic growth or predominant lepidic growth and no more than 5 mm invasion, because these terms identify patients who have nearly a sure shot at disease-free survival after complete resection.
The statement recommends a new approach for classification of resected invasive lung adenocarcinomas using comprehensive histologic subtyping and classification according to the predominant histologic subtype. "This allows for improved stratification of patients compared to the 2004 WHO classification, and allows for identification of subtypes that have prognostic significance and that can be correlated with molecular findings," Dr. Travis said.
Introducing the concept of in situ carcinoma raised the consideration that tumor size measured according to the size of the invasive component may be a better approach than measuring total tumor size in predicting survival for patients with small solitary adenocarcinomas with a lepidic component. This concept potentially could affect both pathologic and clinical staging in the next TNM, he said.
Using CT, prognosis may be better predicted by the size of the solid component in partly solid nodules rather than by total tumor size including the ground-glass component, Dr. Travis explained. "Hopefully, this will be investigated by lung cancer groups around the world in the next 5 years, so the TNM committee can address this issue in developing the 8th edition of TNM based on validated data," he said.
One of the consensus committee members, Dr. Giorgio Scagliotti, has received honoraria from Sanofi-Aventis, Roche, Eli Lilly, and AstraZeneca. Another committee member, Dr. David Yankelevitz, is a named inventor on some patents related to the evaluation of diseases; the patents are licensed to General Electric and may produce compensation if they are commercialized. The rest of the committee reported having no financial conflicts of interest.
A joint effort by three medical groups has enabled a variety of specialists to join pathologists in revising the classification of lung adenocarcinoma, and they’ve made some major changes.
A new section addresses diagnosis and classification of non–small cell lung carcinoma (NSCLC) in small biopsies and cytology, including criteria to distinguish adenocarcinoma from squamous cell carcinoma.
The new classification also recommends epidermal growth factor receptor (EGFR) mutation testing in patients with advanced lung adenocarcinoma to help predict response to tyrosine kinase inhibitors.
And it dumps the term "bronchioalveolar carcinoma" while elsewhere adding some new terms (adenocarcinoma in situ and minimally invasive adenocarcinoma) in the document published in the February issue of the Journal of Thoracic Oncology (J. Thorac. Oncol. 2011;6:244-285).
The International Association for the Study of Lung Cancer convened the multidisciplinary panel of experts to revise the previous World Health Organization classification of lung adenocarcinoma, with support and scientific oversight from the American Thoracic Society and the European Respiratory Society. Pathologists, oncologists, pulmonologists, radiologists, thoracic surgeons, and molecular biologists joined the effort.
The revisions should make it easier to stratify patients and to individualize treatment, Dr. William D. Travis, chair of the expert panel, said in an interview. The changes also could significantly influence the next revision of the TNM (tumor, node, metastases) staging system, "not only for pathologic staging but also for clinical staging," said Dr. Travis, a thoracic pathologist at Memorial Sloan-Kettering Cancer Center, New York.
The new section on small biopsies and cytology specimens is especially important because 70% of lung cancers are diagnosed in samples like these, the consensus panel’s statement said. New criteria for diagnosing adenocarcinoma vs. squamous cell carcinoma include the use of special stains in difficult cases, and emphasize the importance of preserving tissue for molecular studies.
Dr. Travis outlined three important clinical reasons to distinguish adenocarcinomas from squamous cell carcinoma, especially in advanced disease.
• Patients with advanced lung adenocarcinoma or unspecified NSCLC who test positive for EGFR mutation are more likely to respond to treatment with tyrosine kinase inhibitors than are patients without mutation.
• Patients with adenocarcinoma or unspecific NSCLC are more likely to respond to pemetrexed (Alimta) than are patients with squamous cell carcinoma.
• Bevacizumab is contraindicated in patients with squamous cell carcinoma because it can lead to life-threatening hemorrhage, he said.
The statement attempts to banish the term bronchioloalveolar carcinoma from histopathology because it is used in ways that confuse five distinct categories: adenocarcinoma in situ; minimally invasive adenocarcinoma; lepidic predominant adenocarcinoma; adenocarcinoma that is predominantly invasive with some nonmucinous lepidic component; and invasive mucinous adenocarcinoma.
"Adenocarcinoma in situ" and "minimally invasive adenocarcinoma" appear in the classification for the first time for small solitary adenocarcinomas with either pure lepidic growth or predominant lepidic growth and no more than 5 mm invasion, because these terms identify patients who have nearly a sure shot at disease-free survival after complete resection.
The statement recommends a new approach for classification of resected invasive lung adenocarcinomas using comprehensive histologic subtyping and classification according to the predominant histologic subtype. "This allows for improved stratification of patients compared to the 2004 WHO classification, and allows for identification of subtypes that have prognostic significance and that can be correlated with molecular findings," Dr. Travis said.
Introducing the concept of in situ carcinoma raised the consideration that tumor size measured according to the size of the invasive component may be a better approach than measuring total tumor size in predicting survival for patients with small solitary adenocarcinomas with a lepidic component. This concept potentially could affect both pathologic and clinical staging in the next TNM, he said.
Using CT, prognosis may be better predicted by the size of the solid component in partly solid nodules rather than by total tumor size including the ground-glass component, Dr. Travis explained. "Hopefully, this will be investigated by lung cancer groups around the world in the next 5 years, so the TNM committee can address this issue in developing the 8th edition of TNM based on validated data," he said.
One of the consensus committee members, Dr. Giorgio Scagliotti, has received honoraria from Sanofi-Aventis, Roche, Eli Lilly, and AstraZeneca. Another committee member, Dr. David Yankelevitz, is a named inventor on some patents related to the evaluation of diseases; the patents are licensed to General Electric and may produce compensation if they are commercialized. The rest of the committee reported having no financial conflicts of interest.
A joint effort by three medical groups has enabled a variety of specialists to join pathologists in revising the classification of lung adenocarcinoma, and they’ve made some major changes.
A new section addresses diagnosis and classification of non–small cell lung carcinoma (NSCLC) in small biopsies and cytology, including criteria to distinguish adenocarcinoma from squamous cell carcinoma.
The new classification also recommends epidermal growth factor receptor (EGFR) mutation testing in patients with advanced lung adenocarcinoma to help predict response to tyrosine kinase inhibitors.
And it dumps the term "bronchioalveolar carcinoma" while elsewhere adding some new terms (adenocarcinoma in situ and minimally invasive adenocarcinoma) in the document published in the February issue of the Journal of Thoracic Oncology (J. Thorac. Oncol. 2011;6:244-285).
The International Association for the Study of Lung Cancer convened the multidisciplinary panel of experts to revise the previous World Health Organization classification of lung adenocarcinoma, with support and scientific oversight from the American Thoracic Society and the European Respiratory Society. Pathologists, oncologists, pulmonologists, radiologists, thoracic surgeons, and molecular biologists joined the effort.
The revisions should make it easier to stratify patients and to individualize treatment, Dr. William D. Travis, chair of the expert panel, said in an interview. The changes also could significantly influence the next revision of the TNM (tumor, node, metastases) staging system, "not only for pathologic staging but also for clinical staging," said Dr. Travis, a thoracic pathologist at Memorial Sloan-Kettering Cancer Center, New York.
The new section on small biopsies and cytology specimens is especially important because 70% of lung cancers are diagnosed in samples like these, the consensus panel’s statement said. New criteria for diagnosing adenocarcinoma vs. squamous cell carcinoma include the use of special stains in difficult cases, and emphasize the importance of preserving tissue for molecular studies.
Dr. Travis outlined three important clinical reasons to distinguish adenocarcinomas from squamous cell carcinoma, especially in advanced disease.
• Patients with advanced lung adenocarcinoma or unspecified NSCLC who test positive for EGFR mutation are more likely to respond to treatment with tyrosine kinase inhibitors than are patients without mutation.
• Patients with adenocarcinoma or unspecific NSCLC are more likely to respond to pemetrexed (Alimta) than are patients with squamous cell carcinoma.
• Bevacizumab is contraindicated in patients with squamous cell carcinoma because it can lead to life-threatening hemorrhage, he said.
The statement attempts to banish the term bronchioloalveolar carcinoma from histopathology because it is used in ways that confuse five distinct categories: adenocarcinoma in situ; minimally invasive adenocarcinoma; lepidic predominant adenocarcinoma; adenocarcinoma that is predominantly invasive with some nonmucinous lepidic component; and invasive mucinous adenocarcinoma.
"Adenocarcinoma in situ" and "minimally invasive adenocarcinoma" appear in the classification for the first time for small solitary adenocarcinomas with either pure lepidic growth or predominant lepidic growth and no more than 5 mm invasion, because these terms identify patients who have nearly a sure shot at disease-free survival after complete resection.
The statement recommends a new approach for classification of resected invasive lung adenocarcinomas using comprehensive histologic subtyping and classification according to the predominant histologic subtype. "This allows for improved stratification of patients compared to the 2004 WHO classification, and allows for identification of subtypes that have prognostic significance and that can be correlated with molecular findings," Dr. Travis said.
Introducing the concept of in situ carcinoma raised the consideration that tumor size measured according to the size of the invasive component may be a better approach than measuring total tumor size in predicting survival for patients with small solitary adenocarcinomas with a lepidic component. This concept potentially could affect both pathologic and clinical staging in the next TNM, he said.
Using CT, prognosis may be better predicted by the size of the solid component in partly solid nodules rather than by total tumor size including the ground-glass component, Dr. Travis explained. "Hopefully, this will be investigated by lung cancer groups around the world in the next 5 years, so the TNM committee can address this issue in developing the 8th edition of TNM based on validated data," he said.
One of the consensus committee members, Dr. Giorgio Scagliotti, has received honoraria from Sanofi-Aventis, Roche, Eli Lilly, and AstraZeneca. Another committee member, Dr. David Yankelevitz, is a named inventor on some patents related to the evaluation of diseases; the patents are licensed to General Electric and may produce compensation if they are commercialized. The rest of the committee reported having no financial conflicts of interest.
FROM THE JOURNAL OF THORACIC ONCOLOGY
New Classification of Lung Adenocarcinoma Calls for EGFR Testing
A joint effort by three medical groups has enabled a variety of specialists to join pathologists in revising the classification of lung adenocarcinoma, and they’ve made some major changes.
A new section addresses diagnosis and classification of non–small cell lung carcinoma (NSCLC) in small biopsies and cytology, including criteria to distinguish adenocarcinoma from squamous cell carcinoma.
The new classification also recommends epidermal growth factor receptor (EGFR) mutation testing in patients with advanced lung adenocarcinoma to help predict response to tyrosine kinase inhibitors.
And it dumps the term "bronchioalveolar carcinoma" while elsewhere adding some new terms (adenocarcinoma in situ and minimally invasive adenocarcinoma) in the document published in the February issue of the Journal of Thoracic Oncology (J. Thorac. Oncol. 2011;6:244-285).
The International Association for the Study of Lung Cancer convened the multidisciplinary panel of experts to revise the previous World Health Organization classification of lung adenocarcinoma, with support and scientific oversight from the American Thoracic Society and the European Respiratory Society. Pathologists, oncologists, pulmonologists, radiologists, thoracic surgeons, and molecular biologists joined the effort.
The revisions should make it easier to stratify patients and to individualize treatment, Dr. William D. Travis, chair of the expert panel, said in an interview. The changes also could significantly influence the next revision of the TNM (tumor, node, metastases) staging system, "not only for pathologic staging but also for clinical staging," said Dr. Travis, a thoracic pathologist at Memorial Sloan-Kettering Cancer Center, New York.
The new section on small biopsies and cytology specimens is especially important because 70% of lung cancers are diagnosed in samples like these, the consensus panel’s statement said. New criteria for diagnosing adenocarcinoma vs. squamous cell carcinoma include the use of special stains in difficult cases, and emphasize the importance of preserving tissue for molecular studies.
Dr. Travis outlined three important clinical reasons to distinguish adenocarcinomas from squamous cell carcinoma, especially in advanced disease.
• Patients with advanced lung adenocarcinoma or unspecified NSCLC who test positive for EGFR mutation are more likely to respond to treatment with tyrosine kinase inhibitors than are patients without mutation.
• Patients with adenocarcinoma or unspecific NSCLC are more likely to respond to pemetrexed (Alimta) than are patients with squamous cell carcinoma.
• Bevacizumab is contraindicated in patients with squamous cell carcinoma because it can lead to life-threatening hemorrhage, he said.
The statement attempts to banish the term bronchioloalveolar carcinoma from histopathology because it is used in ways that confuse five distinct categories: adenocarcinoma in situ; minimally invasive adenocarcinoma; lepidic predominant adenocarcinoma; adenocarcinoma that is predominantly invasive with some nonmucinous lepidic component; and invasive mucinous adenocarcinoma.
"Adenocarcinoma in situ" and "minimally invasive adenocarcinoma" appear in the classification for the first time for small solitary adenocarcinomas with either pure lepidic growth or predominant lepidic growth and no more than 5 mm invasion, because these terms identify patients who have nearly a sure shot at disease-free survival after complete resection.
The statement recommends a new approach for classification of resected invasive lung adenocarcinomas using comprehensive histologic subtyping and classification according to the predominant histologic subtype. "This allows for improved stratification of patients compared to the 2004 WHO classification, and allows for identification of subtypes that have prognostic significance and that can be correlated with molecular findings," Dr. Travis said.
Introducing the concept of in situ carcinoma raised the consideration that tumor size measured according to the size of the invasive component may be a better approach than measuring total tumor size in predicting survival for patients with small solitary adenocarcinomas with a lepidic component. This concept potentially could affect both pathologic and clinical staging in the next TNM, he said.
Using CT, prognosis may be better predicted by the size of the solid component in partly solid nodules rather than by total tumor size including the ground-glass component, Dr. Travis explained. "Hopefully, this will be investigated by lung cancer groups around the world in the next 5 years, so the TNM committee can address this issue in developing the 8th edition of TNM based on validated data," he said.
One of the consensus committee members, Dr. Giorgio Scagliotti, has received honoraria from Sanofi-Aventis, Roche, Eli Lilly, and AstraZeneca. Another committee member, Dr. David Yankelevitz, is a named inventor on some patents related to the evaluation of diseases; the patents are licensed to General Electric and may produce compensation if they are commercialized. The rest of the committee reported having no financial conflicts of interest.
A joint effort by three medical groups has enabled a variety of specialists to join pathologists in revising the classification of lung adenocarcinoma, and they’ve made some major changes.
A new section addresses diagnosis and classification of non–small cell lung carcinoma (NSCLC) in small biopsies and cytology, including criteria to distinguish adenocarcinoma from squamous cell carcinoma.
The new classification also recommends epidermal growth factor receptor (EGFR) mutation testing in patients with advanced lung adenocarcinoma to help predict response to tyrosine kinase inhibitors.
And it dumps the term "bronchioalveolar carcinoma" while elsewhere adding some new terms (adenocarcinoma in situ and minimally invasive adenocarcinoma) in the document published in the February issue of the Journal of Thoracic Oncology (J. Thorac. Oncol. 2011;6:244-285).
The International Association for the Study of Lung Cancer convened the multidisciplinary panel of experts to revise the previous World Health Organization classification of lung adenocarcinoma, with support and scientific oversight from the American Thoracic Society and the European Respiratory Society. Pathologists, oncologists, pulmonologists, radiologists, thoracic surgeons, and molecular biologists joined the effort.
The revisions should make it easier to stratify patients and to individualize treatment, Dr. William D. Travis, chair of the expert panel, said in an interview. The changes also could significantly influence the next revision of the TNM (tumor, node, metastases) staging system, "not only for pathologic staging but also for clinical staging," said Dr. Travis, a thoracic pathologist at Memorial Sloan-Kettering Cancer Center, New York.
The new section on small biopsies and cytology specimens is especially important because 70% of lung cancers are diagnosed in samples like these, the consensus panel’s statement said. New criteria for diagnosing adenocarcinoma vs. squamous cell carcinoma include the use of special stains in difficult cases, and emphasize the importance of preserving tissue for molecular studies.
Dr. Travis outlined three important clinical reasons to distinguish adenocarcinomas from squamous cell carcinoma, especially in advanced disease.
• Patients with advanced lung adenocarcinoma or unspecified NSCLC who test positive for EGFR mutation are more likely to respond to treatment with tyrosine kinase inhibitors than are patients without mutation.
• Patients with adenocarcinoma or unspecific NSCLC are more likely to respond to pemetrexed (Alimta) than are patients with squamous cell carcinoma.
• Bevacizumab is contraindicated in patients with squamous cell carcinoma because it can lead to life-threatening hemorrhage, he said.
The statement attempts to banish the term bronchioloalveolar carcinoma from histopathology because it is used in ways that confuse five distinct categories: adenocarcinoma in situ; minimally invasive adenocarcinoma; lepidic predominant adenocarcinoma; adenocarcinoma that is predominantly invasive with some nonmucinous lepidic component; and invasive mucinous adenocarcinoma.
"Adenocarcinoma in situ" and "minimally invasive adenocarcinoma" appear in the classification for the first time for small solitary adenocarcinomas with either pure lepidic growth or predominant lepidic growth and no more than 5 mm invasion, because these terms identify patients who have nearly a sure shot at disease-free survival after complete resection.
The statement recommends a new approach for classification of resected invasive lung adenocarcinomas using comprehensive histologic subtyping and classification according to the predominant histologic subtype. "This allows for improved stratification of patients compared to the 2004 WHO classification, and allows for identification of subtypes that have prognostic significance and that can be correlated with molecular findings," Dr. Travis said.
Introducing the concept of in situ carcinoma raised the consideration that tumor size measured according to the size of the invasive component may be a better approach than measuring total tumor size in predicting survival for patients with small solitary adenocarcinomas with a lepidic component. This concept potentially could affect both pathologic and clinical staging in the next TNM, he said.
Using CT, prognosis may be better predicted by the size of the solid component in partly solid nodules rather than by total tumor size including the ground-glass component, Dr. Travis explained. "Hopefully, this will be investigated by lung cancer groups around the world in the next 5 years, so the TNM committee can address this issue in developing the 8th edition of TNM based on validated data," he said.
One of the consensus committee members, Dr. Giorgio Scagliotti, has received honoraria from Sanofi-Aventis, Roche, Eli Lilly, and AstraZeneca. Another committee member, Dr. David Yankelevitz, is a named inventor on some patents related to the evaluation of diseases; the patents are licensed to General Electric and may produce compensation if they are commercialized. The rest of the committee reported having no financial conflicts of interest.
A joint effort by three medical groups has enabled a variety of specialists to join pathologists in revising the classification of lung adenocarcinoma, and they’ve made some major changes.
A new section addresses diagnosis and classification of non–small cell lung carcinoma (NSCLC) in small biopsies and cytology, including criteria to distinguish adenocarcinoma from squamous cell carcinoma.
The new classification also recommends epidermal growth factor receptor (EGFR) mutation testing in patients with advanced lung adenocarcinoma to help predict response to tyrosine kinase inhibitors.
And it dumps the term "bronchioalveolar carcinoma" while elsewhere adding some new terms (adenocarcinoma in situ and minimally invasive adenocarcinoma) in the document published in the February issue of the Journal of Thoracic Oncology (J. Thorac. Oncol. 2011;6:244-285).
The International Association for the Study of Lung Cancer convened the multidisciplinary panel of experts to revise the previous World Health Organization classification of lung adenocarcinoma, with support and scientific oversight from the American Thoracic Society and the European Respiratory Society. Pathologists, oncologists, pulmonologists, radiologists, thoracic surgeons, and molecular biologists joined the effort.
The revisions should make it easier to stratify patients and to individualize treatment, Dr. William D. Travis, chair of the expert panel, said in an interview. The changes also could significantly influence the next revision of the TNM (tumor, node, metastases) staging system, "not only for pathologic staging but also for clinical staging," said Dr. Travis, a thoracic pathologist at Memorial Sloan-Kettering Cancer Center, New York.
The new section on small biopsies and cytology specimens is especially important because 70% of lung cancers are diagnosed in samples like these, the consensus panel’s statement said. New criteria for diagnosing adenocarcinoma vs. squamous cell carcinoma include the use of special stains in difficult cases, and emphasize the importance of preserving tissue for molecular studies.
Dr. Travis outlined three important clinical reasons to distinguish adenocarcinomas from squamous cell carcinoma, especially in advanced disease.
• Patients with advanced lung adenocarcinoma or unspecified NSCLC who test positive for EGFR mutation are more likely to respond to treatment with tyrosine kinase inhibitors than are patients without mutation.
• Patients with adenocarcinoma or unspecific NSCLC are more likely to respond to pemetrexed (Alimta) than are patients with squamous cell carcinoma.
• Bevacizumab is contraindicated in patients with squamous cell carcinoma because it can lead to life-threatening hemorrhage, he said.
The statement attempts to banish the term bronchioloalveolar carcinoma from histopathology because it is used in ways that confuse five distinct categories: adenocarcinoma in situ; minimally invasive adenocarcinoma; lepidic predominant adenocarcinoma; adenocarcinoma that is predominantly invasive with some nonmucinous lepidic component; and invasive mucinous adenocarcinoma.
"Adenocarcinoma in situ" and "minimally invasive adenocarcinoma" appear in the classification for the first time for small solitary adenocarcinomas with either pure lepidic growth or predominant lepidic growth and no more than 5 mm invasion, because these terms identify patients who have nearly a sure shot at disease-free survival after complete resection.
The statement recommends a new approach for classification of resected invasive lung adenocarcinomas using comprehensive histologic subtyping and classification according to the predominant histologic subtype. "This allows for improved stratification of patients compared to the 2004 WHO classification, and allows for identification of subtypes that have prognostic significance and that can be correlated with molecular findings," Dr. Travis said.
Introducing the concept of in situ carcinoma raised the consideration that tumor size measured according to the size of the invasive component may be a better approach than measuring total tumor size in predicting survival for patients with small solitary adenocarcinomas with a lepidic component. This concept potentially could affect both pathologic and clinical staging in the next TNM, he said.
Using CT, prognosis may be better predicted by the size of the solid component in partly solid nodules rather than by total tumor size including the ground-glass component, Dr. Travis explained. "Hopefully, this will be investigated by lung cancer groups around the world in the next 5 years, so the TNM committee can address this issue in developing the 8th edition of TNM based on validated data," he said.
One of the consensus committee members, Dr. Giorgio Scagliotti, has received honoraria from Sanofi-Aventis, Roche, Eli Lilly, and AstraZeneca. Another committee member, Dr. David Yankelevitz, is a named inventor on some patents related to the evaluation of diseases; the patents are licensed to General Electric and may produce compensation if they are commercialized. The rest of the committee reported having no financial conflicts of interest.
FROM THE JOURNAL OF THORACIC ONCOLOGY
Major Finding: Major revisions to the classification of lung adenocarcinoma by three of the world’s top lung associations update the previous World Health Organization classification from 2004.
Data Source: The International Association for the Study of Lung Cancer convened a multidisciplinary panel of 48 experts to update the classification, with support and scientific oversight from the American Thoracic Society and the European Respiratory Society.
Disclosures: One of the consensus committee members, Dr. Giorgio Scagliotti, has received honoraria from Sanofi-Aventis, Roche, Eli Lilly, and AstraZeneca. Another committee member, Dr. David Yankelevitz, is a named inventor on some patents related to the evaluation of diseases; the patents are licensed to General Electric and may produce compensation if they are commercialized. The rest of the committee reported having no financial conflicts of interest.
Explosion in Psoriasis Drug Development Promising
LAS VEGAS – There are so many psoriasis medications that have been approved or are in the experimental pipeline that it can be hard to track them all.
"I can't even keep them straight," Dr. Craig L. Leonardi said. "It's an amazing time right now."
He mapped out the major trends in psoriasis medications for physicians at a dermatology seminar sponsored by Skin Disease Education Foundation (SDEF).
The armamentarium started off with T-cell inhibitors, the main one being alefacept, said Dr. Leonardi of St. Louis University. "I'm not aware of any other T-cell inhibitors that are in development right now. This seems to be a strategy that has been pushed off to the side for now."
On the other hand, psoriasis drugs involving cytokines and cytokine inhibitors are booming. A few other anti-inflammatory strategies also are being tested, including activation of the sirtuin 1 (SIRT1) protein, an oral phospholipid analog, an oral phosphodiesterase inhibitor, and a drug that inhibits Janus kinase (JAK) 1 and 3.
Cytokines: "Cytokines and cytokine inhibitors are exploding," Dr. Leonardi said. The five approved tumor necrosis factor (TNF) antagonists – adalimumab, certolizumab, etanercept, infliximab, and golimumab – "have great utility," he said.
There are two drugs that block both interleukins (IL) 12 and 23. Ustekinumab is approved, and Abbott Laboratories in January 2011 withdrew its biologics license application for briakinumab to perform additional studies at the request of regulators. These two drugs seem to have a "class effect" associated with increased risk of major cardiovascular events, Dr. Leonardi said.
Two other experimental drugs inhibit just IL-23. "These drugs are not interacting with IL-12 at all. If the cardiovascular signal we have seen with briakinumab and with ustekinumab is real, maybe it has to do with IL-12 more than IL-23. Maybe we'll see an improved safety profile for targeting just IL-23," he said.
Trials are underway of three drugs that inhibit IL-17 and one that targets the IL-17 receptor.
"The IL-23 drugs, IL-17 drugs, and IL-17 receptor blocker drug have a lot of briakinumab/ustekinumab feel in terms of efficacy," Dr. Leonardi said. The studies are blinded, so he doesn't know which patients are getting drug or placebo, but he's noticed that in some patients "amazing things are happening to skin and they're happening fast. This is going to be a great moment for many psoriasis sufferers" if these drugs fulfill the promises he thinks he's glimpsed.
Another trial is studying an IL-22 blocking agent.
In other categories, the novel agents being studied are oral medications, "That's a good thing for those that like that approach, but when you use oral medication, you lose a lot of the specificity that we've come to really expect with our biologic agents," he said.
Resveratrol: This compound is believed to activate SIRT1, which may have anti-inflammatory properties. Preliminary trials are underway.
VB-201: The first drug in its class, VB-201 by Vascular Biogenics is an oral phospholipid analog that downregulates production of proinflammatory cytokines by mature dendritic cells. It inhibits the shared p40 subunit of IL-12 and IL-23, and is in preliminary trials for psoriasis.
Apremilast: An oral inhibitor of type 4 phosphodiesterase (PDE4), apremilast inhibits production of inflammatory cytokines. Its mechanism of action on psoriasis is unclear. "Is it a floor wax or is it a milk shake? I can't decide. How does this drug work?" Dr. Leonardi asked.
Reports from a phase II trial by Celgene, which is developing the drug, suggest that it has multiple effects including reducing TNF-alpha, IL-2, interferon-gamma, and several leukotrienes, he said. In the trial of 260 patients, 24% of patients on a 20-mg b.i.d. dosage achieved a 75% improvement in their Psoriasis Area and Severity Index (PASI 75) score at 12 weeks, compared with 10% of patients on either a 20-mg/day dosage or placebo.
Tasocitinib: Also known as CP-690,550, this is a relatively selective inhibitor of JAK1 and JAK3. In early trials for rheumatoid arthritis, the oral drug appears to decrease inflammatory cytokines and chemokines, and decrease the influx of inflammatory cells, "so that provides a rationale for using it in psoriasis," Dr. Leonardi said.
In a phase II trial, 67% of patients on 15 mg b.i.d. achieved a PASI 75 score at 12 weeks, compared with 41% on 5 mg b.i.d., 25% on 2 mg b.i.d., and 2% on placebo. There were signals of potential safety issues with the drug, however, with a total of five major adverse events, some decreases in hemoglobin levels, transient decreases in polymorphonuclear leukocytes, and dose-related increases in lipids.
"Whether or not these are going to be significant, we'll have to wait and see," Dr. Leonardi said. "At least it showed some promise."
SDEF and this news organization are owned by Elsevier.
Dr. Leonardi declared having potential conflicts of interest with Pfizer (which is developing tasocitinib), Celgene (apremilast), Abbott (briakinumab and adalimumab), Centocor (ustekinumab, infliximab, and golimumab), Amgen (etanercept), Abgenix, Allergan, Alza, Biogen-IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Connetics, Corixa, Fujisawa, Galderma, Genentech, Genzyme, GSK, Incyte, Isis, Lilly, MedImmune, Miravant, Schering Plough, Serono, Synta, Wyeth, and Xoma.
LAS VEGAS – There are so many psoriasis medications that have been approved or are in the experimental pipeline that it can be hard to track them all.
"I can't even keep them straight," Dr. Craig L. Leonardi said. "It's an amazing time right now."
He mapped out the major trends in psoriasis medications for physicians at a dermatology seminar sponsored by Skin Disease Education Foundation (SDEF).
The armamentarium started off with T-cell inhibitors, the main one being alefacept, said Dr. Leonardi of St. Louis University. "I'm not aware of any other T-cell inhibitors that are in development right now. This seems to be a strategy that has been pushed off to the side for now."
On the other hand, psoriasis drugs involving cytokines and cytokine inhibitors are booming. A few other anti-inflammatory strategies also are being tested, including activation of the sirtuin 1 (SIRT1) protein, an oral phospholipid analog, an oral phosphodiesterase inhibitor, and a drug that inhibits Janus kinase (JAK) 1 and 3.
Cytokines: "Cytokines and cytokine inhibitors are exploding," Dr. Leonardi said. The five approved tumor necrosis factor (TNF) antagonists – adalimumab, certolizumab, etanercept, infliximab, and golimumab – "have great utility," he said.
There are two drugs that block both interleukins (IL) 12 and 23. Ustekinumab is approved, and Abbott Laboratories in January 2011 withdrew its biologics license application for briakinumab to perform additional studies at the request of regulators. These two drugs seem to have a "class effect" associated with increased risk of major cardiovascular events, Dr. Leonardi said.
Two other experimental drugs inhibit just IL-23. "These drugs are not interacting with IL-12 at all. If the cardiovascular signal we have seen with briakinumab and with ustekinumab is real, maybe it has to do with IL-12 more than IL-23. Maybe we'll see an improved safety profile for targeting just IL-23," he said.
Trials are underway of three drugs that inhibit IL-17 and one that targets the IL-17 receptor.
"The IL-23 drugs, IL-17 drugs, and IL-17 receptor blocker drug have a lot of briakinumab/ustekinumab feel in terms of efficacy," Dr. Leonardi said. The studies are blinded, so he doesn't know which patients are getting drug or placebo, but he's noticed that in some patients "amazing things are happening to skin and they're happening fast. This is going to be a great moment for many psoriasis sufferers" if these drugs fulfill the promises he thinks he's glimpsed.
Another trial is studying an IL-22 blocking agent.
In other categories, the novel agents being studied are oral medications, "That's a good thing for those that like that approach, but when you use oral medication, you lose a lot of the specificity that we've come to really expect with our biologic agents," he said.
Resveratrol: This compound is believed to activate SIRT1, which may have anti-inflammatory properties. Preliminary trials are underway.
VB-201: The first drug in its class, VB-201 by Vascular Biogenics is an oral phospholipid analog that downregulates production of proinflammatory cytokines by mature dendritic cells. It inhibits the shared p40 subunit of IL-12 and IL-23, and is in preliminary trials for psoriasis.
Apremilast: An oral inhibitor of type 4 phosphodiesterase (PDE4), apremilast inhibits production of inflammatory cytokines. Its mechanism of action on psoriasis is unclear. "Is it a floor wax or is it a milk shake? I can't decide. How does this drug work?" Dr. Leonardi asked.
Reports from a phase II trial by Celgene, which is developing the drug, suggest that it has multiple effects including reducing TNF-alpha, IL-2, interferon-gamma, and several leukotrienes, he said. In the trial of 260 patients, 24% of patients on a 20-mg b.i.d. dosage achieved a 75% improvement in their Psoriasis Area and Severity Index (PASI 75) score at 12 weeks, compared with 10% of patients on either a 20-mg/day dosage or placebo.
Tasocitinib: Also known as CP-690,550, this is a relatively selective inhibitor of JAK1 and JAK3. In early trials for rheumatoid arthritis, the oral drug appears to decrease inflammatory cytokines and chemokines, and decrease the influx of inflammatory cells, "so that provides a rationale for using it in psoriasis," Dr. Leonardi said.
In a phase II trial, 67% of patients on 15 mg b.i.d. achieved a PASI 75 score at 12 weeks, compared with 41% on 5 mg b.i.d., 25% on 2 mg b.i.d., and 2% on placebo. There were signals of potential safety issues with the drug, however, with a total of five major adverse events, some decreases in hemoglobin levels, transient decreases in polymorphonuclear leukocytes, and dose-related increases in lipids.
"Whether or not these are going to be significant, we'll have to wait and see," Dr. Leonardi said. "At least it showed some promise."
SDEF and this news organization are owned by Elsevier.
Dr. Leonardi declared having potential conflicts of interest with Pfizer (which is developing tasocitinib), Celgene (apremilast), Abbott (briakinumab and adalimumab), Centocor (ustekinumab, infliximab, and golimumab), Amgen (etanercept), Abgenix, Allergan, Alza, Biogen-IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Connetics, Corixa, Fujisawa, Galderma, Genentech, Genzyme, GSK, Incyte, Isis, Lilly, MedImmune, Miravant, Schering Plough, Serono, Synta, Wyeth, and Xoma.
LAS VEGAS – There are so many psoriasis medications that have been approved or are in the experimental pipeline that it can be hard to track them all.
"I can't even keep them straight," Dr. Craig L. Leonardi said. "It's an amazing time right now."
He mapped out the major trends in psoriasis medications for physicians at a dermatology seminar sponsored by Skin Disease Education Foundation (SDEF).
The armamentarium started off with T-cell inhibitors, the main one being alefacept, said Dr. Leonardi of St. Louis University. "I'm not aware of any other T-cell inhibitors that are in development right now. This seems to be a strategy that has been pushed off to the side for now."
On the other hand, psoriasis drugs involving cytokines and cytokine inhibitors are booming. A few other anti-inflammatory strategies also are being tested, including activation of the sirtuin 1 (SIRT1) protein, an oral phospholipid analog, an oral phosphodiesterase inhibitor, and a drug that inhibits Janus kinase (JAK) 1 and 3.
Cytokines: "Cytokines and cytokine inhibitors are exploding," Dr. Leonardi said. The five approved tumor necrosis factor (TNF) antagonists – adalimumab, certolizumab, etanercept, infliximab, and golimumab – "have great utility," he said.
There are two drugs that block both interleukins (IL) 12 and 23. Ustekinumab is approved, and Abbott Laboratories in January 2011 withdrew its biologics license application for briakinumab to perform additional studies at the request of regulators. These two drugs seem to have a "class effect" associated with increased risk of major cardiovascular events, Dr. Leonardi said.
Two other experimental drugs inhibit just IL-23. "These drugs are not interacting with IL-12 at all. If the cardiovascular signal we have seen with briakinumab and with ustekinumab is real, maybe it has to do with IL-12 more than IL-23. Maybe we'll see an improved safety profile for targeting just IL-23," he said.
Trials are underway of three drugs that inhibit IL-17 and one that targets the IL-17 receptor.
"The IL-23 drugs, IL-17 drugs, and IL-17 receptor blocker drug have a lot of briakinumab/ustekinumab feel in terms of efficacy," Dr. Leonardi said. The studies are blinded, so he doesn't know which patients are getting drug or placebo, but he's noticed that in some patients "amazing things are happening to skin and they're happening fast. This is going to be a great moment for many psoriasis sufferers" if these drugs fulfill the promises he thinks he's glimpsed.
Another trial is studying an IL-22 blocking agent.
In other categories, the novel agents being studied are oral medications, "That's a good thing for those that like that approach, but when you use oral medication, you lose a lot of the specificity that we've come to really expect with our biologic agents," he said.
Resveratrol: This compound is believed to activate SIRT1, which may have anti-inflammatory properties. Preliminary trials are underway.
VB-201: The first drug in its class, VB-201 by Vascular Biogenics is an oral phospholipid analog that downregulates production of proinflammatory cytokines by mature dendritic cells. It inhibits the shared p40 subunit of IL-12 and IL-23, and is in preliminary trials for psoriasis.
Apremilast: An oral inhibitor of type 4 phosphodiesterase (PDE4), apremilast inhibits production of inflammatory cytokines. Its mechanism of action on psoriasis is unclear. "Is it a floor wax or is it a milk shake? I can't decide. How does this drug work?" Dr. Leonardi asked.
Reports from a phase II trial by Celgene, which is developing the drug, suggest that it has multiple effects including reducing TNF-alpha, IL-2, interferon-gamma, and several leukotrienes, he said. In the trial of 260 patients, 24% of patients on a 20-mg b.i.d. dosage achieved a 75% improvement in their Psoriasis Area and Severity Index (PASI 75) score at 12 weeks, compared with 10% of patients on either a 20-mg/day dosage or placebo.
Tasocitinib: Also known as CP-690,550, this is a relatively selective inhibitor of JAK1 and JAK3. In early trials for rheumatoid arthritis, the oral drug appears to decrease inflammatory cytokines and chemokines, and decrease the influx of inflammatory cells, "so that provides a rationale for using it in psoriasis," Dr. Leonardi said.
In a phase II trial, 67% of patients on 15 mg b.i.d. achieved a PASI 75 score at 12 weeks, compared with 41% on 5 mg b.i.d., 25% on 2 mg b.i.d., and 2% on placebo. There were signals of potential safety issues with the drug, however, with a total of five major adverse events, some decreases in hemoglobin levels, transient decreases in polymorphonuclear leukocytes, and dose-related increases in lipids.
"Whether or not these are going to be significant, we'll have to wait and see," Dr. Leonardi said. "At least it showed some promise."
SDEF and this news organization are owned by Elsevier.
Dr. Leonardi declared having potential conflicts of interest with Pfizer (which is developing tasocitinib), Celgene (apremilast), Abbott (briakinumab and adalimumab), Centocor (ustekinumab, infliximab, and golimumab), Amgen (etanercept), Abgenix, Allergan, Alza, Biogen-IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Connetics, Corixa, Fujisawa, Galderma, Genentech, Genzyme, GSK, Incyte, Isis, Lilly, MedImmune, Miravant, Schering Plough, Serono, Synta, Wyeth, and Xoma.
EXPERT ANALYSIS FROM THE LAS VEGAS DERMATOLOGY SEMINAR SPONSORED BY SDEF
Explosion in Psoriasis Drugs Development
LAS VEGAS – So many relatively new psoriasis medications have been approved or are in the experimental pipeline that it can be hard to track them all.
"I can’t even keep them straight," Dr. Craig L. Leonardi said. "It’s an amazing time right now."
He mapped out the major trends in psoriasis medications for physicians at a dermatology seminar sponsored by Skin Disease Education Foundation (SDEF).
The armamentarium started off with T-cell inhibitors, the main one being alefacept, said Dr. Leonardi of St. Louis University. "I’m not aware of any other T-cell inhibitors that are in development right now. This seems to be a strategy that has been pushed off to the side for now."
On the other hand, psoriasis drugs involving cytokines and cytokine inhibitors are booming. A few other anti-inflammatory strategies also are being tested, including activation of the sirtuin 1 (SIRT1) protein, an oral phospholipid analog, an oral phosphodiesterase inhibitor, and a drug that inhibits Janus kinase (JAK) 1 and 3.
Cytokines: "Cytokines and cytokine inhibitors are exploding," Dr. Leonardi said. The five approved tumor necrosis factor (TNF) antagonists – adalimumab, certolizumab, etanercept, infliximab, and golimumab – "have great utility," he said.
There are two drugs that block both interleukins (IL) 12 and 23. Ustekinumab is approved, and Abbott Laboratories in January 2011 withdrew its biologics license application for briakinumab to perform additional studies at the request of regulators. These two drugs seem to have a "class effect" associated with increased risk of major cardiovascular events, Dr. Leonardi said.
Two other experimental drugs inhibit just IL-23. "These drugs are not interacting with IL-12 at all. If the cardiovascular signal we have seen with briakinumab and with ustekinumab is real, maybe it has to do with IL-12 more than IL-23. Maybe we’ll see an improved safety profile for targeting just IL-23," he said.
Trials are underway of three drugs that inhibit IL-17 and one that targets the IL-17 receptor.
"The IL-23 drugs, IL-17 drugs, and IL-17 receptor blocker drug have a lot of briakinumab/ustekinumab feel in terms of efficacy," Dr. Leonardi said. The studies are blinded, so he doesn’t know which patients are getting drug or placebo, but he’s noticed that in some patients "amazing things are happening to skin and they’re happening fast. This is going to be a great moment for many psoriasis sufferers" if these drugs fulfill the promises he thinks he’s glimpsed.
Another trial is studying an IL-22 blocking agent.
In other categories, the novel agents being studied are oral medications, "That’s a good thing for those that like that approach, but when you use oral medication, you lose a lot of the specificity that we’ve come to really expect with our biologic agents," he said.
Resveratrol: This compound is believed to activate SIRT1, which may have anti-inflammatory properties. Preliminary trials are underway.
VB-201: The first drug in its class, VB-201 by Vascular Biogenics is an oral phospholipid analog that downregulates production of proinflammatory cytokines by mature dendritic cells. It inhibits the shared p40 subunit of IL-12 and IL-23, and is in preliminary trials for psoriasis.
Apremilast: An oral inhibitor of type 4 phosphodiesterase (PDE4), apremilast inhibits production of inflammatory cytokines. Its mechanism of action on psoriasis is unclear. "Is it a floor wax or is it a milk shake? I can’t decide. How does this drug work?" Dr. Leonardi asked.
Reports from a phase II trial by Celgene, which is developing the drug, suggest that it has multiple effects including reducing TNF-alpha, IL-2, interferon-gamma, and several leukotrienes, he said. In the trial of 260 patients, 24% of patients on a 20-mg b.i.d. dosage achieved a 75% improvement in their Psoriasis Area and Severity Index (PASI 75) score at 12 weeks, compared with 10% of patients on either a 20-mg/day dosage or placebo.
Tasocitinib: Also known as CP-690,550, this is a relatively selective inhibitor of JAK1 and JAK3. In early trials for rheumatoid arthritis, the oral drug appears to decrease inflammatory cytokines and chemokines, and decrease the influx of inflammatory cells, "so that provides a rationale for using it in psoriasis," Dr. Leonardi said.
In a phase II trial, 67% of patients on 15 mg b.i.d. achieved a PASI 75 score at 12 weeks, compared with 41% on 5 mg b.i.d., 25% on 2 mg b.i.d., and 2% on placebo. There were signals of potential safety issues with the drug, however, with a total of five major adverse events, some decreases in hemoglobin levels, transient decreases in polymorphonuclear leukocytes, and dose-related increases in lipids.
"Whether or not these are going to be significant, we’ll have to wait and see," Dr. Leonardi said. "At least it showed some promise."
SDEF and this news organization are owned by Elsevier.
Dr. Leonardi declared having potential conflicts of interest with Pfizer (which is developing tasocitinib), Celgene (apremilast), Abbott (briakinumab and adalimumab), Centocor (ustekinumab, infliximab, and golimumab), Amgen (etanercept), Abgenix, Allergan, Alza, Biogen-IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Connetics, Corixa, Fujisawa, Galderma, Genentech, Genzyme, GSK, Incyte, Isis, Lilly, MedImmune, Miravant, Schering Plough, Serono, Synta, Wyeth, and Xoma.
LAS VEGAS – So many relatively new psoriasis medications have been approved or are in the experimental pipeline that it can be hard to track them all.
"I can’t even keep them straight," Dr. Craig L. Leonardi said. "It’s an amazing time right now."
He mapped out the major trends in psoriasis medications for physicians at a dermatology seminar sponsored by Skin Disease Education Foundation (SDEF).
The armamentarium started off with T-cell inhibitors, the main one being alefacept, said Dr. Leonardi of St. Louis University. "I’m not aware of any other T-cell inhibitors that are in development right now. This seems to be a strategy that has been pushed off to the side for now."
On the other hand, psoriasis drugs involving cytokines and cytokine inhibitors are booming. A few other anti-inflammatory strategies also are being tested, including activation of the sirtuin 1 (SIRT1) protein, an oral phospholipid analog, an oral phosphodiesterase inhibitor, and a drug that inhibits Janus kinase (JAK) 1 and 3.
Cytokines: "Cytokines and cytokine inhibitors are exploding," Dr. Leonardi said. The five approved tumor necrosis factor (TNF) antagonists – adalimumab, certolizumab, etanercept, infliximab, and golimumab – "have great utility," he said.
There are two drugs that block both interleukins (IL) 12 and 23. Ustekinumab is approved, and Abbott Laboratories in January 2011 withdrew its biologics license application for briakinumab to perform additional studies at the request of regulators. These two drugs seem to have a "class effect" associated with increased risk of major cardiovascular events, Dr. Leonardi said.
Two other experimental drugs inhibit just IL-23. "These drugs are not interacting with IL-12 at all. If the cardiovascular signal we have seen with briakinumab and with ustekinumab is real, maybe it has to do with IL-12 more than IL-23. Maybe we’ll see an improved safety profile for targeting just IL-23," he said.
Trials are underway of three drugs that inhibit IL-17 and one that targets the IL-17 receptor.
"The IL-23 drugs, IL-17 drugs, and IL-17 receptor blocker drug have a lot of briakinumab/ustekinumab feel in terms of efficacy," Dr. Leonardi said. The studies are blinded, so he doesn’t know which patients are getting drug or placebo, but he’s noticed that in some patients "amazing things are happening to skin and they’re happening fast. This is going to be a great moment for many psoriasis sufferers" if these drugs fulfill the promises he thinks he’s glimpsed.
Another trial is studying an IL-22 blocking agent.
In other categories, the novel agents being studied are oral medications, "That’s a good thing for those that like that approach, but when you use oral medication, you lose a lot of the specificity that we’ve come to really expect with our biologic agents," he said.
Resveratrol: This compound is believed to activate SIRT1, which may have anti-inflammatory properties. Preliminary trials are underway.
VB-201: The first drug in its class, VB-201 by Vascular Biogenics is an oral phospholipid analog that downregulates production of proinflammatory cytokines by mature dendritic cells. It inhibits the shared p40 subunit of IL-12 and IL-23, and is in preliminary trials for psoriasis.
Apremilast: An oral inhibitor of type 4 phosphodiesterase (PDE4), apremilast inhibits production of inflammatory cytokines. Its mechanism of action on psoriasis is unclear. "Is it a floor wax or is it a milk shake? I can’t decide. How does this drug work?" Dr. Leonardi asked.
Reports from a phase II trial by Celgene, which is developing the drug, suggest that it has multiple effects including reducing TNF-alpha, IL-2, interferon-gamma, and several leukotrienes, he said. In the trial of 260 patients, 24% of patients on a 20-mg b.i.d. dosage achieved a 75% improvement in their Psoriasis Area and Severity Index (PASI 75) score at 12 weeks, compared with 10% of patients on either a 20-mg/day dosage or placebo.
Tasocitinib: Also known as CP-690,550, this is a relatively selective inhibitor of JAK1 and JAK3. In early trials for rheumatoid arthritis, the oral drug appears to decrease inflammatory cytokines and chemokines, and decrease the influx of inflammatory cells, "so that provides a rationale for using it in psoriasis," Dr. Leonardi said.
In a phase II trial, 67% of patients on 15 mg b.i.d. achieved a PASI 75 score at 12 weeks, compared with 41% on 5 mg b.i.d., 25% on 2 mg b.i.d., and 2% on placebo. There were signals of potential safety issues with the drug, however, with a total of five major adverse events, some decreases in hemoglobin levels, transient decreases in polymorphonuclear leukocytes, and dose-related increases in lipids.
"Whether or not these are going to be significant, we’ll have to wait and see," Dr. Leonardi said. "At least it showed some promise."
SDEF and this news organization are owned by Elsevier.
Dr. Leonardi declared having potential conflicts of interest with Pfizer (which is developing tasocitinib), Celgene (apremilast), Abbott (briakinumab and adalimumab), Centocor (ustekinumab, infliximab, and golimumab), Amgen (etanercept), Abgenix, Allergan, Alza, Biogen-IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Connetics, Corixa, Fujisawa, Galderma, Genentech, Genzyme, GSK, Incyte, Isis, Lilly, MedImmune, Miravant, Schering Plough, Serono, Synta, Wyeth, and Xoma.
LAS VEGAS – So many relatively new psoriasis medications have been approved or are in the experimental pipeline that it can be hard to track them all.
"I can’t even keep them straight," Dr. Craig L. Leonardi said. "It’s an amazing time right now."
He mapped out the major trends in psoriasis medications for physicians at a dermatology seminar sponsored by Skin Disease Education Foundation (SDEF).
The armamentarium started off with T-cell inhibitors, the main one being alefacept, said Dr. Leonardi of St. Louis University. "I’m not aware of any other T-cell inhibitors that are in development right now. This seems to be a strategy that has been pushed off to the side for now."
On the other hand, psoriasis drugs involving cytokines and cytokine inhibitors are booming. A few other anti-inflammatory strategies also are being tested, including activation of the sirtuin 1 (SIRT1) protein, an oral phospholipid analog, an oral phosphodiesterase inhibitor, and a drug that inhibits Janus kinase (JAK) 1 and 3.
Cytokines: "Cytokines and cytokine inhibitors are exploding," Dr. Leonardi said. The five approved tumor necrosis factor (TNF) antagonists – adalimumab, certolizumab, etanercept, infliximab, and golimumab – "have great utility," he said.
There are two drugs that block both interleukins (IL) 12 and 23. Ustekinumab is approved, and Abbott Laboratories in January 2011 withdrew its biologics license application for briakinumab to perform additional studies at the request of regulators. These two drugs seem to have a "class effect" associated with increased risk of major cardiovascular events, Dr. Leonardi said.
Two other experimental drugs inhibit just IL-23. "These drugs are not interacting with IL-12 at all. If the cardiovascular signal we have seen with briakinumab and with ustekinumab is real, maybe it has to do with IL-12 more than IL-23. Maybe we’ll see an improved safety profile for targeting just IL-23," he said.
Trials are underway of three drugs that inhibit IL-17 and one that targets the IL-17 receptor.
"The IL-23 drugs, IL-17 drugs, and IL-17 receptor blocker drug have a lot of briakinumab/ustekinumab feel in terms of efficacy," Dr. Leonardi said. The studies are blinded, so he doesn’t know which patients are getting drug or placebo, but he’s noticed that in some patients "amazing things are happening to skin and they’re happening fast. This is going to be a great moment for many psoriasis sufferers" if these drugs fulfill the promises he thinks he’s glimpsed.
Another trial is studying an IL-22 blocking agent.
In other categories, the novel agents being studied are oral medications, "That’s a good thing for those that like that approach, but when you use oral medication, you lose a lot of the specificity that we’ve come to really expect with our biologic agents," he said.
Resveratrol: This compound is believed to activate SIRT1, which may have anti-inflammatory properties. Preliminary trials are underway.
VB-201: The first drug in its class, VB-201 by Vascular Biogenics is an oral phospholipid analog that downregulates production of proinflammatory cytokines by mature dendritic cells. It inhibits the shared p40 subunit of IL-12 and IL-23, and is in preliminary trials for psoriasis.
Apremilast: An oral inhibitor of type 4 phosphodiesterase (PDE4), apremilast inhibits production of inflammatory cytokines. Its mechanism of action on psoriasis is unclear. "Is it a floor wax or is it a milk shake? I can’t decide. How does this drug work?" Dr. Leonardi asked.
Reports from a phase II trial by Celgene, which is developing the drug, suggest that it has multiple effects including reducing TNF-alpha, IL-2, interferon-gamma, and several leukotrienes, he said. In the trial of 260 patients, 24% of patients on a 20-mg b.i.d. dosage achieved a 75% improvement in their Psoriasis Area and Severity Index (PASI 75) score at 12 weeks, compared with 10% of patients on either a 20-mg/day dosage or placebo.
Tasocitinib: Also known as CP-690,550, this is a relatively selective inhibitor of JAK1 and JAK3. In early trials for rheumatoid arthritis, the oral drug appears to decrease inflammatory cytokines and chemokines, and decrease the influx of inflammatory cells, "so that provides a rationale for using it in psoriasis," Dr. Leonardi said.
In a phase II trial, 67% of patients on 15 mg b.i.d. achieved a PASI 75 score at 12 weeks, compared with 41% on 5 mg b.i.d., 25% on 2 mg b.i.d., and 2% on placebo. There were signals of potential safety issues with the drug, however, with a total of five major adverse events, some decreases in hemoglobin levels, transient decreases in polymorphonuclear leukocytes, and dose-related increases in lipids.
"Whether or not these are going to be significant, we’ll have to wait and see," Dr. Leonardi said. "At least it showed some promise."
SDEF and this news organization are owned by Elsevier.
Dr. Leonardi declared having potential conflicts of interest with Pfizer (which is developing tasocitinib), Celgene (apremilast), Abbott (briakinumab and adalimumab), Centocor (ustekinumab, infliximab, and golimumab), Amgen (etanercept), Abgenix, Allergan, Alza, Biogen-IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Connetics, Corixa, Fujisawa, Galderma, Genentech, Genzyme, GSK, Incyte, Isis, Lilly, MedImmune, Miravant, Schering Plough, Serono, Synta, Wyeth, and Xoma.
FROM A DERMATOLOGY SEMINAR SPONSORED BY SKIN DISEASE EDUCATION FOUNDATION
New Guidelines Raise Awareness of a Rare Stroke
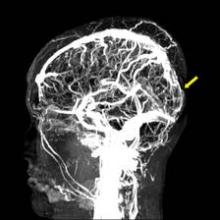
The American Heart Association for the first time released guidelines for clinicians to help detect and treat cerebral venous thrombosis, a rare stroke that disproportionately affects young people, especially women who are pregnant or on oral contraceptives, or who just gave birth.
The guidelines include an algorithm for diagnosing and managing cerebral venous thromboembolism (CVT), which is caused by a clot in the dural venous sinuses, veins that drain blood from the brain toward the heart. The guidelines were released Feb. 3 online in advance of publication in Stroke.
CVT is difficult to recognize because of its diverse risk factors and presentations. "The diagnosis and management of CVT requires a high level of suspicion," Dr. Gustavo Saposnik said in an e-mail interview. Dr. Saposnik, codirector of the stroke program at the University of Toronto, chaired the guidelines writing committee of nine experts from five countries, which reviewed the literature on CVT and rated the evidence behind their recommendations (Stroke 2011 Feb. 3 [doi:10.1161/STR.0b013e31820a8364]).
The guidelines have been endorsed by the American Academy of Neurology, the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, the Society of NeuroInterventional Surgery, and the Ibero-American Stroke Society.
Many kinds of physicians may be the first to encounter a patient with CVT because of its many causes and symptoms. The guidelines should be helpful not only to neurologists but also to emergency physicians, internists, family physicians, obstetricians, oncologists, pediatricians, hematologists, and more, he said.
Approximately five people per million develop CVT each year, accounting for 0.5%-1% of all strokes. In the largest cohort study of patients diagnosed with CVT, 54% were on oral contraceptives, 34% had an inherited or acquired prothrombotic condition, and 21% were pregnant or in the immediate postpartum period. Other predisposing conditions included infection in 12%, the presence of certain drugs in 8%, cancer in 7%, and other hematologic disorders in 12%. (Some patients had more than one predisposing condition.)
Patients may present with slowly progressive symptoms, and delays in diagnosis are common. Studies have reported a mean lapse of 4 days from onset of symptoms to hospital admission, and 7 days from onset of symptoms to diagnosis. Headache, the most common symptom, occurs in about 90% of cases. Seizures also are common.
Approximately 30%-40% of patients with CVT present with intracranial hemorrhage. To select the appropriate treatment, it is important to identify CVT as the cause of the hemorrhage, instead of a ruptured brain artery or other causes.
Women outnumber men with CVT at ages younger than 61 years. The largest cohort study of CVT reported that patients younger than 50 years accounted for 78% of cases. The incidence of CVT during pregnancy and post partum in Western countries ranges from one to four cases per 10,000 deliveries, with the greatest risk during the third trimester and in the first 4 weeks after delivery.
CVT is not a contraindication for future pregnancy, Dr. Saposnik said.
If a clinician suspects CVT, either MRI or magnetic resonance venography (MRV) is recommended to make the diagnosis by showing a thrombus obstructing the venous sinuses or cerebral veins. In emergency departments, either a CT scan or CT venography can be used if MRI is not available. "This allows different clinicians to initiate the appropriate work-up in the acute setting," Dr. Saposnik said.
Anticoagulation is the usual first-line therapy, with IV heparin or subcutaneous low-molecular-weight heparin in patients without contraindications. "There are several things that we still don’t know. For example, the anticoagulation regimen and duration of IV anticoagulation therapy is not clear," he said.
There is only limited, low-grade evidence for alternative treatments, such as endovascular therapy or decompressive hemicraniectomy. "These should be reserved for patients with progressive neurological deterioration despite anticoagulation therapy and the best medical treatment," Dr. Saposnik said.
A randomized trial is underway comparing anticoagulation therapy with endovascular thrombolysis to treat CVT.
One guidelines coauthor reported a financial relationship with Boehringer Ingelheim, and another reported being an adviser or consultant for Servier and Tecnifar. Another coauthor received less than $10,000 as an expert witness in a legal case concerning CVT. Disclosures of funding for the American Heart Association can be read online.
There has been a need for evaluation and management guidelines for this particular disease. Although there is not necessarily a controversy regarding the diagnosis of CVT, the general problem with detecting it is that it remains relatively uncommon in comparison with ischemic stroke, and it can present clinically with a very wide spectrum of symptoms, including headache, nausea, vomiting (likely from increased intracranial pressure), seizures, abrupt focal neurological deficits that mimic acute ischemic arterial stroke, and progressive mental status decline. These can occur in isolation or in any given combination.
Surveillance imaging such as CT or MR that does not include dedicated vascular imaging may reveal only subtle findings that can be overlooked. Identifying this problem early can be very challenging from both a clinical and imaging standpoint. One strength of this guideline statement will be to simply raise awareness of the disease and thus increase early detection.
The main controversy in CVT is focused on how best to treat it, particularly with regard to the safety and efficacy of anticoagulation with heparin and heparinoids (especially in patients with CVT-related intracranial hemorrhage) and the evolving use of endovascular techniques. The real strength of these guidelines is its clear recommendation that the first-line medical treatment for CVT is the use of intravenous heparin or subcutaneous heparinoids, including in patients with CVT-related intracranial hemorrhage.
Many physicians with limited experience in treating this disease (including neurologists) remain very hesitant to use heparin in CVT patients with intracranial hemorrhage secondary to long-standing fears of an increased risk of additional hemorrhage. The safety and efficacy of heparin in these patients seems pretty well established, and this strong statement will hopefully help ease some of these fears and increase the use of heparin in this patient population.
As a neurocritical care physician and neurointerventionalist, I also strongly agree with the statement that endovascular therapies should be reserved for patients who deteriorate despite the use of heparin. This population is rare. There has been a variety of case reports regarding endovascular therapies in CVT patients with intracranial hemorrhage – those felt to be at high risk for heparin therapy – and my hope is that such invasive procedures will no longer be offered in place of heparin therapy.
Joey D. English, M.D., is codirector of neurointerventional services at San Francisco General Hospital and the San Francisco Veterans Affairs Medical Center. He was not involved in the guidelines, and he has no relevant conflicts of interest.
There has been a need for evaluation and management guidelines for this particular disease. Although there is not necessarily a controversy regarding the diagnosis of CVT, the general problem with detecting it is that it remains relatively uncommon in comparison with ischemic stroke, and it can present clinically with a very wide spectrum of symptoms, including headache, nausea, vomiting (likely from increased intracranial pressure), seizures, abrupt focal neurological deficits that mimic acute ischemic arterial stroke, and progressive mental status decline. These can occur in isolation or in any given combination.
Surveillance imaging such as CT or MR that does not include dedicated vascular imaging may reveal only subtle findings that can be overlooked. Identifying this problem early can be very challenging from both a clinical and imaging standpoint. One strength of this guideline statement will be to simply raise awareness of the disease and thus increase early detection.
The main controversy in CVT is focused on how best to treat it, particularly with regard to the safety and efficacy of anticoagulation with heparin and heparinoids (especially in patients with CVT-related intracranial hemorrhage) and the evolving use of endovascular techniques. The real strength of these guidelines is its clear recommendation that the first-line medical treatment for CVT is the use of intravenous heparin or subcutaneous heparinoids, including in patients with CVT-related intracranial hemorrhage.
Many physicians with limited experience in treating this disease (including neurologists) remain very hesitant to use heparin in CVT patients with intracranial hemorrhage secondary to long-standing fears of an increased risk of additional hemorrhage. The safety and efficacy of heparin in these patients seems pretty well established, and this strong statement will hopefully help ease some of these fears and increase the use of heparin in this patient population.
As a neurocritical care physician and neurointerventionalist, I also strongly agree with the statement that endovascular therapies should be reserved for patients who deteriorate despite the use of heparin. This population is rare. There has been a variety of case reports regarding endovascular therapies in CVT patients with intracranial hemorrhage – those felt to be at high risk for heparin therapy – and my hope is that such invasive procedures will no longer be offered in place of heparin therapy.
Joey D. English, M.D., is codirector of neurointerventional services at San Francisco General Hospital and the San Francisco Veterans Affairs Medical Center. He was not involved in the guidelines, and he has no relevant conflicts of interest.
There has been a need for evaluation and management guidelines for this particular disease. Although there is not necessarily a controversy regarding the diagnosis of CVT, the general problem with detecting it is that it remains relatively uncommon in comparison with ischemic stroke, and it can present clinically with a very wide spectrum of symptoms, including headache, nausea, vomiting (likely from increased intracranial pressure), seizures, abrupt focal neurological deficits that mimic acute ischemic arterial stroke, and progressive mental status decline. These can occur in isolation or in any given combination.
Surveillance imaging such as CT or MR that does not include dedicated vascular imaging may reveal only subtle findings that can be overlooked. Identifying this problem early can be very challenging from both a clinical and imaging standpoint. One strength of this guideline statement will be to simply raise awareness of the disease and thus increase early detection.
The main controversy in CVT is focused on how best to treat it, particularly with regard to the safety and efficacy of anticoagulation with heparin and heparinoids (especially in patients with CVT-related intracranial hemorrhage) and the evolving use of endovascular techniques. The real strength of these guidelines is its clear recommendation that the first-line medical treatment for CVT is the use of intravenous heparin or subcutaneous heparinoids, including in patients with CVT-related intracranial hemorrhage.
Many physicians with limited experience in treating this disease (including neurologists) remain very hesitant to use heparin in CVT patients with intracranial hemorrhage secondary to long-standing fears of an increased risk of additional hemorrhage. The safety and efficacy of heparin in these patients seems pretty well established, and this strong statement will hopefully help ease some of these fears and increase the use of heparin in this patient population.
As a neurocritical care physician and neurointerventionalist, I also strongly agree with the statement that endovascular therapies should be reserved for patients who deteriorate despite the use of heparin. This population is rare. There has been a variety of case reports regarding endovascular therapies in CVT patients with intracranial hemorrhage – those felt to be at high risk for heparin therapy – and my hope is that such invasive procedures will no longer be offered in place of heparin therapy.
Joey D. English, M.D., is codirector of neurointerventional services at San Francisco General Hospital and the San Francisco Veterans Affairs Medical Center. He was not involved in the guidelines, and he has no relevant conflicts of interest.
The American Heart Association for the first time released guidelines for clinicians to help detect and treat cerebral venous thrombosis, a rare stroke that disproportionately affects young people, especially women who are pregnant or on oral contraceptives, or who just gave birth.
The guidelines include an algorithm for diagnosing and managing cerebral venous thromboembolism (CVT), which is caused by a clot in the dural venous sinuses, veins that drain blood from the brain toward the heart. The guidelines were released Feb. 3 online in advance of publication in Stroke.
CVT is difficult to recognize because of its diverse risk factors and presentations. "The diagnosis and management of CVT requires a high level of suspicion," Dr. Gustavo Saposnik said in an e-mail interview. Dr. Saposnik, codirector of the stroke program at the University of Toronto, chaired the guidelines writing committee of nine experts from five countries, which reviewed the literature on CVT and rated the evidence behind their recommendations (Stroke 2011 Feb. 3 [doi:10.1161/STR.0b013e31820a8364]).
The guidelines have been endorsed by the American Academy of Neurology, the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, the Society of NeuroInterventional Surgery, and the Ibero-American Stroke Society.
Many kinds of physicians may be the first to encounter a patient with CVT because of its many causes and symptoms. The guidelines should be helpful not only to neurologists but also to emergency physicians, internists, family physicians, obstetricians, oncologists, pediatricians, hematologists, and more, he said.
Approximately five people per million develop CVT each year, accounting for 0.5%-1% of all strokes. In the largest cohort study of patients diagnosed with CVT, 54% were on oral contraceptives, 34% had an inherited or acquired prothrombotic condition, and 21% were pregnant or in the immediate postpartum period. Other predisposing conditions included infection in 12%, the presence of certain drugs in 8%, cancer in 7%, and other hematologic disorders in 12%. (Some patients had more than one predisposing condition.)
Patients may present with slowly progressive symptoms, and delays in diagnosis are common. Studies have reported a mean lapse of 4 days from onset of symptoms to hospital admission, and 7 days from onset of symptoms to diagnosis. Headache, the most common symptom, occurs in about 90% of cases. Seizures also are common.
Approximately 30%-40% of patients with CVT present with intracranial hemorrhage. To select the appropriate treatment, it is important to identify CVT as the cause of the hemorrhage, instead of a ruptured brain artery or other causes.
Women outnumber men with CVT at ages younger than 61 years. The largest cohort study of CVT reported that patients younger than 50 years accounted for 78% of cases. The incidence of CVT during pregnancy and post partum in Western countries ranges from one to four cases per 10,000 deliveries, with the greatest risk during the third trimester and in the first 4 weeks after delivery.
CVT is not a contraindication for future pregnancy, Dr. Saposnik said.
If a clinician suspects CVT, either MRI or magnetic resonance venography (MRV) is recommended to make the diagnosis by showing a thrombus obstructing the venous sinuses or cerebral veins. In emergency departments, either a CT scan or CT venography can be used if MRI is not available. "This allows different clinicians to initiate the appropriate work-up in the acute setting," Dr. Saposnik said.
Anticoagulation is the usual first-line therapy, with IV heparin or subcutaneous low-molecular-weight heparin in patients without contraindications. "There are several things that we still don’t know. For example, the anticoagulation regimen and duration of IV anticoagulation therapy is not clear," he said.
There is only limited, low-grade evidence for alternative treatments, such as endovascular therapy or decompressive hemicraniectomy. "These should be reserved for patients with progressive neurological deterioration despite anticoagulation therapy and the best medical treatment," Dr. Saposnik said.
A randomized trial is underway comparing anticoagulation therapy with endovascular thrombolysis to treat CVT.
One guidelines coauthor reported a financial relationship with Boehringer Ingelheim, and another reported being an adviser or consultant for Servier and Tecnifar. Another coauthor received less than $10,000 as an expert witness in a legal case concerning CVT. Disclosures of funding for the American Heart Association can be read online.
The American Heart Association for the first time released guidelines for clinicians to help detect and treat cerebral venous thrombosis, a rare stroke that disproportionately affects young people, especially women who are pregnant or on oral contraceptives, or who just gave birth.
The guidelines include an algorithm for diagnosing and managing cerebral venous thromboembolism (CVT), which is caused by a clot in the dural venous sinuses, veins that drain blood from the brain toward the heart. The guidelines were released Feb. 3 online in advance of publication in Stroke.
CVT is difficult to recognize because of its diverse risk factors and presentations. "The diagnosis and management of CVT requires a high level of suspicion," Dr. Gustavo Saposnik said in an e-mail interview. Dr. Saposnik, codirector of the stroke program at the University of Toronto, chaired the guidelines writing committee of nine experts from five countries, which reviewed the literature on CVT and rated the evidence behind their recommendations (Stroke 2011 Feb. 3 [doi:10.1161/STR.0b013e31820a8364]).
The guidelines have been endorsed by the American Academy of Neurology, the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, the Society of NeuroInterventional Surgery, and the Ibero-American Stroke Society.
Many kinds of physicians may be the first to encounter a patient with CVT because of its many causes and symptoms. The guidelines should be helpful not only to neurologists but also to emergency physicians, internists, family physicians, obstetricians, oncologists, pediatricians, hematologists, and more, he said.
Approximately five people per million develop CVT each year, accounting for 0.5%-1% of all strokes. In the largest cohort study of patients diagnosed with CVT, 54% were on oral contraceptives, 34% had an inherited or acquired prothrombotic condition, and 21% were pregnant or in the immediate postpartum period. Other predisposing conditions included infection in 12%, the presence of certain drugs in 8%, cancer in 7%, and other hematologic disorders in 12%. (Some patients had more than one predisposing condition.)
Patients may present with slowly progressive symptoms, and delays in diagnosis are common. Studies have reported a mean lapse of 4 days from onset of symptoms to hospital admission, and 7 days from onset of symptoms to diagnosis. Headache, the most common symptom, occurs in about 90% of cases. Seizures also are common.
Approximately 30%-40% of patients with CVT present with intracranial hemorrhage. To select the appropriate treatment, it is important to identify CVT as the cause of the hemorrhage, instead of a ruptured brain artery or other causes.
Women outnumber men with CVT at ages younger than 61 years. The largest cohort study of CVT reported that patients younger than 50 years accounted for 78% of cases. The incidence of CVT during pregnancy and post partum in Western countries ranges from one to four cases per 10,000 deliveries, with the greatest risk during the third trimester and in the first 4 weeks after delivery.
CVT is not a contraindication for future pregnancy, Dr. Saposnik said.
If a clinician suspects CVT, either MRI or magnetic resonance venography (MRV) is recommended to make the diagnosis by showing a thrombus obstructing the venous sinuses or cerebral veins. In emergency departments, either a CT scan or CT venography can be used if MRI is not available. "This allows different clinicians to initiate the appropriate work-up in the acute setting," Dr. Saposnik said.
Anticoagulation is the usual first-line therapy, with IV heparin or subcutaneous low-molecular-weight heparin in patients without contraindications. "There are several things that we still don’t know. For example, the anticoagulation regimen and duration of IV anticoagulation therapy is not clear," he said.
There is only limited, low-grade evidence for alternative treatments, such as endovascular therapy or decompressive hemicraniectomy. "These should be reserved for patients with progressive neurological deterioration despite anticoagulation therapy and the best medical treatment," Dr. Saposnik said.
A randomized trial is underway comparing anticoagulation therapy with endovascular thrombolysis to treat CVT.
One guidelines coauthor reported a financial relationship with Boehringer Ingelheim, and another reported being an adviser or consultant for Servier and Tecnifar. Another coauthor received less than $10,000 as an expert witness in a legal case concerning CVT. Disclosures of funding for the American Heart Association can be read online.
Major Finding: The first guidelines to focus on CVT from the American Heart Association raise awareness of this rare stroke and provide an algorithm for diagnosis and treatment.
Data Source: A multidisciplinary group of experts from five countries reviewed the literature, rated the quality of the evidence, and drafted guidelines that were assessed and approved by the AHA’s Science Advisory and Coordinating Committee.
Disclosures: One coauthor reported a financial relationship with Boehringer Ingelheim, and another reported being an adviser or consultant for Servier and Tecnifar. Another coauthor received less than $10,000 as an expert witness in a legal case concerning CVT. Disclosures of funding for the AHA can be read at www.heart.org/corporatefunding.
New Guidance Aids Osteoporosis Screening
New federal recommendations on screening for osteoporosis provide more detail on when to screen women younger than age 65 years and – for the first time – point to a lack of data for screening decisions in men.
The U.S. Preventive Services Task Force updated its 2002 recommendations on osteoporosis screening to call for routine screening in all women aged 65 years or older and in any younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors (equivalent to a 9.3% or greater risk of fracture within 10 years). Previously, women younger than 65 would be screened if they were at least 60 years old with risk factors for fracture.
For the first time, the USPSTF evaluated the evidence for osteoporosis screening in men and found insufficient evidence to form any recommendation, Dr. Ned Colange, chair of the USPSTF, said in an interview. There's not enough evidence to recommend screening or treatment in men with no prior osteoporotic fractures, and “there's certainly not enough evidence to say, 'Don't' do it.”
“While there's not a call to action, that's an important call for research,” added Dr. Colange, president and CEO of the Colorado Trust Foundation, Denver.
In women, the recommendations do not say to stop osteoporosis screening at any specific age because the risk of fractures continues to increase with advancing age, and the minimal potential harms of treatment remain small. Clinicians who are considering treating older patients should consider data showing that the benefits of osteoporosis treatment emerge 18–24 months after starting treatment.
To predict an individual's risk for osteoporotic fracture, the USPSTF used the online FRAX tool, developed by the World Health Organization and the National Osteoporosis Foundation.
“The nice thing about the FRAX calculator is, the patient herself can determine that risk. It's available online. It uses measures that the woman should know,” Dr. Colange said.
The FRAX tool estimates 10-year fracture risk based on easily obtained information such as age, body mass index, parental fracture history, and tobacco or alcohol use. It asks about results of dual-energy x-ray absorptiometry scans but does not require this information to calculate fracture risk.
Younger women can reach the new threshold for screening because of various risk factors. For example, a white woman would qualify for screening if she is 50 years old, smokes, drinks alcohol daily, has a BMI less than 21, and has a parental history of fracture. A 55-year-old white woman would need only a parental fracture history to warrant osteoporosis screening. A 60-year-old white woman who smokes and drinks alcohol daily would fit the 10-year-risk profile for screening (Ann. Intern. Med. 2011 Jan. 18 [Epub ahead of print]).
White women are more likely than are nonwhite women to develop osteoporosis and fractures. Although there are fewer data on nonwhite women, the USPSTF recommended screening all women at age 65 years because the consequences of failing to identify and treat low bone-mineral density are considerable and the potential risks of treatment are small.
There aren't enough data to recommend when to rescreen women without osteoporosis on their first screen, the USPSTF stated, but an interval of at least 2 years would be needed to assess a change in bone density, and longer still for better prediction of fracture risk.
The recommendations are based on a 2010 review of studies published since 2002 by a team at the University of Oregon Health and Science University's Evidence-Based Practice Center in Portland.
In a new effort at transparency, the USPSTF first published a draft of the new recommendations online last summer and invited public comment. They received more than 50 comments from individuals, professional organizations, advocates, and drug companies, which led the USPSTF to clarify its approach to fracture risk assessment in the final version, Dr. Colange said.
He said he has no pertinent conflicts of interest.
View on the News
Online Access Will Help Screening Calculations
For clinicians, the biggest change in the new screening recommendations may be the need to calculate the 10-year fracture risk in women aged younger than 65, two experts suggested in interviews.
“They will need to know what tools are out there to be able to figure out whether a younger person is at equal to or greater risk than a 65-year-old woman with no addition risk factors,” Dr. Carolyn J. Crandall said.
The online FRAX calculator that was used by the USPSTF is a “really good tool” for this purpose, said Dr. Crandall. “Clinicians will have to access that tool in their clinics, which means they will either need Internet access at some point, or else they can download versions that are available for iPhone, or print versions that are available.”
Dr. Edward S. Leib also commended inclusion of the FRAX tool in the guidelines, but cautioned that it has some weaknesses that were discussed at a November 2010 “position development conference” conducted jointly by the International Osteoporosis Foundation and the International Society for Clinical Densitometry.
Some important risk factors that could affect the 10-year fracture risk would not necessarily be reflected in the FRAX calculation, he said. In addition, the FRAX tool is based on an international model, and although it included U.S. databases, the calculations may not reflect risks in regional populations.
“For example, in a retrospective review of our population of 15,000 postmenopausal women having bone density studies over the past 10 years, we did not find a correlation between history of fracturing and parental history of hip fractures,” he said.
Both Dr. Crandall and Dr. Leib also commended the USPSTF for acknowledging the need for more research in men, but Dr. Leib had hoped for more guidance. “It is known that the fracture risk in men who are age 75 is about equivalent to women who are age 65. I would have hoped that the USPSTF would have recommended screening at that age” despite the lack of primary prevention trials, he said.
DR. CRANDALL is professor of medicine at the University of California, Los Angeles. She said she has no pertinent conflicts of interest. DR. LEIB is professor of medicine at the University of Vermont, Burlington. He said he has no pertinent conflicts of interest.
New federal recommendations on screening for osteoporosis provide more detail on when to screen women younger than age 65 years and – for the first time – point to a lack of data for screening decisions in men.
The U.S. Preventive Services Task Force updated its 2002 recommendations on osteoporosis screening to call for routine screening in all women aged 65 years or older and in any younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors (equivalent to a 9.3% or greater risk of fracture within 10 years). Previously, women younger than 65 would be screened if they were at least 60 years old with risk factors for fracture.
For the first time, the USPSTF evaluated the evidence for osteoporosis screening in men and found insufficient evidence to form any recommendation, Dr. Ned Colange, chair of the USPSTF, said in an interview. There's not enough evidence to recommend screening or treatment in men with no prior osteoporotic fractures, and “there's certainly not enough evidence to say, 'Don't' do it.”
“While there's not a call to action, that's an important call for research,” added Dr. Colange, president and CEO of the Colorado Trust Foundation, Denver.
In women, the recommendations do not say to stop osteoporosis screening at any specific age because the risk of fractures continues to increase with advancing age, and the minimal potential harms of treatment remain small. Clinicians who are considering treating older patients should consider data showing that the benefits of osteoporosis treatment emerge 18–24 months after starting treatment.
To predict an individual's risk for osteoporotic fracture, the USPSTF used the online FRAX tool, developed by the World Health Organization and the National Osteoporosis Foundation.
“The nice thing about the FRAX calculator is, the patient herself can determine that risk. It's available online. It uses measures that the woman should know,” Dr. Colange said.
The FRAX tool estimates 10-year fracture risk based on easily obtained information such as age, body mass index, parental fracture history, and tobacco or alcohol use. It asks about results of dual-energy x-ray absorptiometry scans but does not require this information to calculate fracture risk.
Younger women can reach the new threshold for screening because of various risk factors. For example, a white woman would qualify for screening if she is 50 years old, smokes, drinks alcohol daily, has a BMI less than 21, and has a parental history of fracture. A 55-year-old white woman would need only a parental fracture history to warrant osteoporosis screening. A 60-year-old white woman who smokes and drinks alcohol daily would fit the 10-year-risk profile for screening (Ann. Intern. Med. 2011 Jan. 18 [Epub ahead of print]).
White women are more likely than are nonwhite women to develop osteoporosis and fractures. Although there are fewer data on nonwhite women, the USPSTF recommended screening all women at age 65 years because the consequences of failing to identify and treat low bone-mineral density are considerable and the potential risks of treatment are small.
There aren't enough data to recommend when to rescreen women without osteoporosis on their first screen, the USPSTF stated, but an interval of at least 2 years would be needed to assess a change in bone density, and longer still for better prediction of fracture risk.
The recommendations are based on a 2010 review of studies published since 2002 by a team at the University of Oregon Health and Science University's Evidence-Based Practice Center in Portland.
In a new effort at transparency, the USPSTF first published a draft of the new recommendations online last summer and invited public comment. They received more than 50 comments from individuals, professional organizations, advocates, and drug companies, which led the USPSTF to clarify its approach to fracture risk assessment in the final version, Dr. Colange said.
He said he has no pertinent conflicts of interest.
View on the News
Online Access Will Help Screening Calculations
For clinicians, the biggest change in the new screening recommendations may be the need to calculate the 10-year fracture risk in women aged younger than 65, two experts suggested in interviews.
“They will need to know what tools are out there to be able to figure out whether a younger person is at equal to or greater risk than a 65-year-old woman with no addition risk factors,” Dr. Carolyn J. Crandall said.
The online FRAX calculator that was used by the USPSTF is a “really good tool” for this purpose, said Dr. Crandall. “Clinicians will have to access that tool in their clinics, which means they will either need Internet access at some point, or else they can download versions that are available for iPhone, or print versions that are available.”
Dr. Edward S. Leib also commended inclusion of the FRAX tool in the guidelines, but cautioned that it has some weaknesses that were discussed at a November 2010 “position development conference” conducted jointly by the International Osteoporosis Foundation and the International Society for Clinical Densitometry.
Some important risk factors that could affect the 10-year fracture risk would not necessarily be reflected in the FRAX calculation, he said. In addition, the FRAX tool is based on an international model, and although it included U.S. databases, the calculations may not reflect risks in regional populations.
“For example, in a retrospective review of our population of 15,000 postmenopausal women having bone density studies over the past 10 years, we did not find a correlation between history of fracturing and parental history of hip fractures,” he said.
Both Dr. Crandall and Dr. Leib also commended the USPSTF for acknowledging the need for more research in men, but Dr. Leib had hoped for more guidance. “It is known that the fracture risk in men who are age 75 is about equivalent to women who are age 65. I would have hoped that the USPSTF would have recommended screening at that age” despite the lack of primary prevention trials, he said.
DR. CRANDALL is professor of medicine at the University of California, Los Angeles. She said she has no pertinent conflicts of interest. DR. LEIB is professor of medicine at the University of Vermont, Burlington. He said he has no pertinent conflicts of interest.
New federal recommendations on screening for osteoporosis provide more detail on when to screen women younger than age 65 years and – for the first time – point to a lack of data for screening decisions in men.
The U.S. Preventive Services Task Force updated its 2002 recommendations on osteoporosis screening to call for routine screening in all women aged 65 years or older and in any younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors (equivalent to a 9.3% or greater risk of fracture within 10 years). Previously, women younger than 65 would be screened if they were at least 60 years old with risk factors for fracture.
For the first time, the USPSTF evaluated the evidence for osteoporosis screening in men and found insufficient evidence to form any recommendation, Dr. Ned Colange, chair of the USPSTF, said in an interview. There's not enough evidence to recommend screening or treatment in men with no prior osteoporotic fractures, and “there's certainly not enough evidence to say, 'Don't' do it.”
“While there's not a call to action, that's an important call for research,” added Dr. Colange, president and CEO of the Colorado Trust Foundation, Denver.
In women, the recommendations do not say to stop osteoporosis screening at any specific age because the risk of fractures continues to increase with advancing age, and the minimal potential harms of treatment remain small. Clinicians who are considering treating older patients should consider data showing that the benefits of osteoporosis treatment emerge 18–24 months after starting treatment.
To predict an individual's risk for osteoporotic fracture, the USPSTF used the online FRAX tool, developed by the World Health Organization and the National Osteoporosis Foundation.
“The nice thing about the FRAX calculator is, the patient herself can determine that risk. It's available online. It uses measures that the woman should know,” Dr. Colange said.
The FRAX tool estimates 10-year fracture risk based on easily obtained information such as age, body mass index, parental fracture history, and tobacco or alcohol use. It asks about results of dual-energy x-ray absorptiometry scans but does not require this information to calculate fracture risk.
Younger women can reach the new threshold for screening because of various risk factors. For example, a white woman would qualify for screening if she is 50 years old, smokes, drinks alcohol daily, has a BMI less than 21, and has a parental history of fracture. A 55-year-old white woman would need only a parental fracture history to warrant osteoporosis screening. A 60-year-old white woman who smokes and drinks alcohol daily would fit the 10-year-risk profile for screening (Ann. Intern. Med. 2011 Jan. 18 [Epub ahead of print]).
White women are more likely than are nonwhite women to develop osteoporosis and fractures. Although there are fewer data on nonwhite women, the USPSTF recommended screening all women at age 65 years because the consequences of failing to identify and treat low bone-mineral density are considerable and the potential risks of treatment are small.
There aren't enough data to recommend when to rescreen women without osteoporosis on their first screen, the USPSTF stated, but an interval of at least 2 years would be needed to assess a change in bone density, and longer still for better prediction of fracture risk.
The recommendations are based on a 2010 review of studies published since 2002 by a team at the University of Oregon Health and Science University's Evidence-Based Practice Center in Portland.
In a new effort at transparency, the USPSTF first published a draft of the new recommendations online last summer and invited public comment. They received more than 50 comments from individuals, professional organizations, advocates, and drug companies, which led the USPSTF to clarify its approach to fracture risk assessment in the final version, Dr. Colange said.
He said he has no pertinent conflicts of interest.
View on the News
Online Access Will Help Screening Calculations
For clinicians, the biggest change in the new screening recommendations may be the need to calculate the 10-year fracture risk in women aged younger than 65, two experts suggested in interviews.
“They will need to know what tools are out there to be able to figure out whether a younger person is at equal to or greater risk than a 65-year-old woman with no addition risk factors,” Dr. Carolyn J. Crandall said.
The online FRAX calculator that was used by the USPSTF is a “really good tool” for this purpose, said Dr. Crandall. “Clinicians will have to access that tool in their clinics, which means they will either need Internet access at some point, or else they can download versions that are available for iPhone, or print versions that are available.”
Dr. Edward S. Leib also commended inclusion of the FRAX tool in the guidelines, but cautioned that it has some weaknesses that were discussed at a November 2010 “position development conference” conducted jointly by the International Osteoporosis Foundation and the International Society for Clinical Densitometry.
Some important risk factors that could affect the 10-year fracture risk would not necessarily be reflected in the FRAX calculation, he said. In addition, the FRAX tool is based on an international model, and although it included U.S. databases, the calculations may not reflect risks in regional populations.
“For example, in a retrospective review of our population of 15,000 postmenopausal women having bone density studies over the past 10 years, we did not find a correlation between history of fracturing and parental history of hip fractures,” he said.
Both Dr. Crandall and Dr. Leib also commended the USPSTF for acknowledging the need for more research in men, but Dr. Leib had hoped for more guidance. “It is known that the fracture risk in men who are age 75 is about equivalent to women who are age 65. I would have hoped that the USPSTF would have recommended screening at that age” despite the lack of primary prevention trials, he said.
DR. CRANDALL is professor of medicine at the University of California, Los Angeles. She said she has no pertinent conflicts of interest. DR. LEIB is professor of medicine at the University of Vermont, Burlington. He said he has no pertinent conflicts of interest.
From the Annals of Internal Medicine
Diabetes, Depression Raise Mortality in Women
The combination of type 2 diabetes and depression doubled the overall risk of death and nearly tripled the likelihood of dying of cardiovascular disease within 6 years, an analysis of data on 78,282 women found.
Previous studies have shown an association between depression or diabetes and increased risk of death from any cause or from CVD, but the combined effects of these diseases on mortality have not been well studied, especially in women. Earlier studies also tended to be smaller and to have shorter follow-up.
An Pan, Ph.D., of the Harvard School of Public Health, Boston, and his associates analyzed data on participants in the prospective Nurses' Health Study who were 54–79 years of age in 2000 and who were followed until 2006. There were 979 deaths from CVD and 4,654 deaths from any cause during that time.
Compared with the 80% of women who developed neither diabetes nor depression, the age-adjusted relative risk of death was 1.71 in the 5% of women with diabetes alone, 1.76 in the 14% with depression alone, and 3.11 in the 1% with both diseases. The relative risk of death from CVD was 1.67 in women with diabetes alone, 1.37 in women with depression alone, and 2.72 in those with both diabetes and depression (Arch. Gen. Psychiatry 2011;68:42-50).
The increased risks with either diabetes or depression were statistically significant, and the higher risks with both diseases were significant compared with either disease alone, even after adjustment for the effects of age, family history of diabetes and cancer, history of MI, current marital status, ethnicity, body mass index, and other confounders.
The highest risks were seen in women with depression combined with more severe diabetes, indicated by a longer duration of diabetes or treatment with oral medication or insulin. Death from CVD was three times more likely in depressed women who had had diabetes for more than 10 years, and four times more likely in depressed women who received insulin therapy for diabetes, compared with women who had neither depression nor diabetes.
The greater likelihood of death or of death from CVD in women with both diabetes and depression deserves greater attention, especially considering that 20%–25% of people with diabetes are depressed, the investigators suggested. An estimated 24 million U.S. adults have diabetes and 15 million U.S. adults are depressed. Adults with diabetes are twice as likely to be depressed, compared with those without diabetes.
In general, physicians don't do a great job of recognizing major depression, and the United States can claim a relatively high prevalence of untreated mental disorders, they added. Better strategies may be needed to provide adequate psychological management and support for people with diabetes. In addition, the coexistence of depression and diabetes should identify women who are at particularly high risk, the investigators concluded.
The mechanisms of the association between increased mortality and depression in women with diabetes are unknown.
The Nurses' Health Study, ongoing since 1976, has followed a large cohort of female nurses every 2 years with questionnaires and had better than a 94% follow-up rate through 2006. Deaths were identified by the next of kin, postal authorities, or National Death Index. The investigators obtained medical records and death certificates to determine the cause of death.
The current analysis excluded participants with a history of gestational diabetes, type 1 diabetes, secondary diabetes, or missing data regarding depression or diabetes.
The investigators reported having no conflicts of interest. The study was funded by the National Institutes of Health, the National Alliance for Research on Schizophrenia and Depression, and the Fonds de la Recherche en Santé du Québec.
View on the News
Don't Overlook Depression
The study shows that the risks of death in women with diabetes or depression are additive in those who have both diseases, but it doesn't establish a synergistic effect between the two diseases, Dr. Elizabeth Murphy said in an interview.
Given the increasing evidence for a link between diabetes and depression, “this article should provide an important reminder for clinicians to be vigilant about screening and treating depression in patients with diabetes,” said Dr. Murphy, who was not involved in the study.
Perhaps more interesting is a previous report by Dr. Pan and his associates that established a bidirectional association between type 2 diabetes and depression, Dr. Murphy added (Arch. Intern. Med. 2010;170: 1884-91). That report also drew from the Nurses' Health Study, analyzing data on 65,381 women who had neither diabetes nor depression in 1996. During 10 years of follow-up, 2,844 developed type 2 diabetes, and 7,415 developed clinical depression.
Depressed mood was associated with a 17% increased risk for developing diabetes, and use of antidepressants was associated with a 25% higher risk for diabetes, compared with women with the best depressive symptom scores. Women who developed diabetes were 24%–53% more likely to develop depression, depending on the severity of the diabetes.
DR. MURPHY is chief of endocrinology at San Francisco General Hospital.
The combination of type 2 diabetes and depression doubled the overall risk of death and nearly tripled the likelihood of dying of cardiovascular disease within 6 years, an analysis of data on 78,282 women found.
Previous studies have shown an association between depression or diabetes and increased risk of death from any cause or from CVD, but the combined effects of these diseases on mortality have not been well studied, especially in women. Earlier studies also tended to be smaller and to have shorter follow-up.
An Pan, Ph.D., of the Harvard School of Public Health, Boston, and his associates analyzed data on participants in the prospective Nurses' Health Study who were 54–79 years of age in 2000 and who were followed until 2006. There were 979 deaths from CVD and 4,654 deaths from any cause during that time.
Compared with the 80% of women who developed neither diabetes nor depression, the age-adjusted relative risk of death was 1.71 in the 5% of women with diabetes alone, 1.76 in the 14% with depression alone, and 3.11 in the 1% with both diseases. The relative risk of death from CVD was 1.67 in women with diabetes alone, 1.37 in women with depression alone, and 2.72 in those with both diabetes and depression (Arch. Gen. Psychiatry 2011;68:42-50).
The increased risks with either diabetes or depression were statistically significant, and the higher risks with both diseases were significant compared with either disease alone, even after adjustment for the effects of age, family history of diabetes and cancer, history of MI, current marital status, ethnicity, body mass index, and other confounders.
The highest risks were seen in women with depression combined with more severe diabetes, indicated by a longer duration of diabetes or treatment with oral medication or insulin. Death from CVD was three times more likely in depressed women who had had diabetes for more than 10 years, and four times more likely in depressed women who received insulin therapy for diabetes, compared with women who had neither depression nor diabetes.
The greater likelihood of death or of death from CVD in women with both diabetes and depression deserves greater attention, especially considering that 20%–25% of people with diabetes are depressed, the investigators suggested. An estimated 24 million U.S. adults have diabetes and 15 million U.S. adults are depressed. Adults with diabetes are twice as likely to be depressed, compared with those without diabetes.
In general, physicians don't do a great job of recognizing major depression, and the United States can claim a relatively high prevalence of untreated mental disorders, they added. Better strategies may be needed to provide adequate psychological management and support for people with diabetes. In addition, the coexistence of depression and diabetes should identify women who are at particularly high risk, the investigators concluded.
The mechanisms of the association between increased mortality and depression in women with diabetes are unknown.
The Nurses' Health Study, ongoing since 1976, has followed a large cohort of female nurses every 2 years with questionnaires and had better than a 94% follow-up rate through 2006. Deaths were identified by the next of kin, postal authorities, or National Death Index. The investigators obtained medical records and death certificates to determine the cause of death.
The current analysis excluded participants with a history of gestational diabetes, type 1 diabetes, secondary diabetes, or missing data regarding depression or diabetes.
The investigators reported having no conflicts of interest. The study was funded by the National Institutes of Health, the National Alliance for Research on Schizophrenia and Depression, and the Fonds de la Recherche en Santé du Québec.
View on the News
Don't Overlook Depression
The study shows that the risks of death in women with diabetes or depression are additive in those who have both diseases, but it doesn't establish a synergistic effect between the two diseases, Dr. Elizabeth Murphy said in an interview.
Given the increasing evidence for a link between diabetes and depression, “this article should provide an important reminder for clinicians to be vigilant about screening and treating depression in patients with diabetes,” said Dr. Murphy, who was not involved in the study.
Perhaps more interesting is a previous report by Dr. Pan and his associates that established a bidirectional association between type 2 diabetes and depression, Dr. Murphy added (Arch. Intern. Med. 2010;170: 1884-91). That report also drew from the Nurses' Health Study, analyzing data on 65,381 women who had neither diabetes nor depression in 1996. During 10 years of follow-up, 2,844 developed type 2 diabetes, and 7,415 developed clinical depression.
Depressed mood was associated with a 17% increased risk for developing diabetes, and use of antidepressants was associated with a 25% higher risk for diabetes, compared with women with the best depressive symptom scores. Women who developed diabetes were 24%–53% more likely to develop depression, depending on the severity of the diabetes.
DR. MURPHY is chief of endocrinology at San Francisco General Hospital.
The combination of type 2 diabetes and depression doubled the overall risk of death and nearly tripled the likelihood of dying of cardiovascular disease within 6 years, an analysis of data on 78,282 women found.
Previous studies have shown an association between depression or diabetes and increased risk of death from any cause or from CVD, but the combined effects of these diseases on mortality have not been well studied, especially in women. Earlier studies also tended to be smaller and to have shorter follow-up.
An Pan, Ph.D., of the Harvard School of Public Health, Boston, and his associates analyzed data on participants in the prospective Nurses' Health Study who were 54–79 years of age in 2000 and who were followed until 2006. There were 979 deaths from CVD and 4,654 deaths from any cause during that time.
Compared with the 80% of women who developed neither diabetes nor depression, the age-adjusted relative risk of death was 1.71 in the 5% of women with diabetes alone, 1.76 in the 14% with depression alone, and 3.11 in the 1% with both diseases. The relative risk of death from CVD was 1.67 in women with diabetes alone, 1.37 in women with depression alone, and 2.72 in those with both diabetes and depression (Arch. Gen. Psychiatry 2011;68:42-50).
The increased risks with either diabetes or depression were statistically significant, and the higher risks with both diseases were significant compared with either disease alone, even after adjustment for the effects of age, family history of diabetes and cancer, history of MI, current marital status, ethnicity, body mass index, and other confounders.
The highest risks were seen in women with depression combined with more severe diabetes, indicated by a longer duration of diabetes or treatment with oral medication or insulin. Death from CVD was three times more likely in depressed women who had had diabetes for more than 10 years, and four times more likely in depressed women who received insulin therapy for diabetes, compared with women who had neither depression nor diabetes.
The greater likelihood of death or of death from CVD in women with both diabetes and depression deserves greater attention, especially considering that 20%–25% of people with diabetes are depressed, the investigators suggested. An estimated 24 million U.S. adults have diabetes and 15 million U.S. adults are depressed. Adults with diabetes are twice as likely to be depressed, compared with those without diabetes.
In general, physicians don't do a great job of recognizing major depression, and the United States can claim a relatively high prevalence of untreated mental disorders, they added. Better strategies may be needed to provide adequate psychological management and support for people with diabetes. In addition, the coexistence of depression and diabetes should identify women who are at particularly high risk, the investigators concluded.
The mechanisms of the association between increased mortality and depression in women with diabetes are unknown.
The Nurses' Health Study, ongoing since 1976, has followed a large cohort of female nurses every 2 years with questionnaires and had better than a 94% follow-up rate through 2006. Deaths were identified by the next of kin, postal authorities, or National Death Index. The investigators obtained medical records and death certificates to determine the cause of death.
The current analysis excluded participants with a history of gestational diabetes, type 1 diabetes, secondary diabetes, or missing data regarding depression or diabetes.
The investigators reported having no conflicts of interest. The study was funded by the National Institutes of Health, the National Alliance for Research on Schizophrenia and Depression, and the Fonds de la Recherche en Santé du Québec.
View on the News
Don't Overlook Depression
The study shows that the risks of death in women with diabetes or depression are additive in those who have both diseases, but it doesn't establish a synergistic effect between the two diseases, Dr. Elizabeth Murphy said in an interview.
Given the increasing evidence for a link between diabetes and depression, “this article should provide an important reminder for clinicians to be vigilant about screening and treating depression in patients with diabetes,” said Dr. Murphy, who was not involved in the study.
Perhaps more interesting is a previous report by Dr. Pan and his associates that established a bidirectional association between type 2 diabetes and depression, Dr. Murphy added (Arch. Intern. Med. 2010;170: 1884-91). That report also drew from the Nurses' Health Study, analyzing data on 65,381 women who had neither diabetes nor depression in 1996. During 10 years of follow-up, 2,844 developed type 2 diabetes, and 7,415 developed clinical depression.
Depressed mood was associated with a 17% increased risk for developing diabetes, and use of antidepressants was associated with a 25% higher risk for diabetes, compared with women with the best depressive symptom scores. Women who developed diabetes were 24%–53% more likely to develop depression, depending on the severity of the diabetes.
DR. MURPHY is chief of endocrinology at San Francisco General Hospital.
From Archives of General Psychiatry
Statin Had No Effect on Atherosclerosis in Lupus Patients
Major Finding: Atorvastatin 40 mg/day did not affect subclinical measures of atherosclerosis or disease activity in patients with SLE, compared with placebo.
Data Source: Randomized, double-blind, placebo-controlled trial in 200 patients with SLE who were followed for 2 years.
Disclosures: Dr. Petri was formerly an adviser and speaker for Pfizer, which markets atorvastatin. None of her coauthors had conflicts of interest. The study was funded by the Alliance for Lupus Research, the Arthritis Foundation, the Hopkins Lupus Cohort, and the National Center for Research Resources.
Atorvastatin therapy did not improve subclinical measures of atherosclerosis or disease activity, compared with placebo, in a 2-year, randomized, double-blind study of 200 adults with systemic lupus erythematosus.
The results surprised the investigators because atorvastatin had seemed to be an ideal choice to interrupt the accelerated atherosclerosis seen in SLE, reported Dr. Michelle A. Petri.
Previous studies had shown some potential benefits from the anti-inflammatory effects of statins in other autoimmune diseases, including rheumatoid arthritis and multiple sclerosis. Findings from one small study of pravastatin in patients with SLE found that it reduced total cholesterol and LDL cholesterol levels but did not reduce C-reactive protein levels.
In the current study, there was no significant difference between the patients who were randomized to 40 mg/day atorvastatin and those receiving placebo in progression of coronary artery calcium, carotid intima media thickness, or carotid plaque.
There also were no significant differences between groups in measures of disease activity, inflammation, or endothelial cell activation, reported Dr. Petri, professor of medicine at Johns Hopkins University, Baltimore, and her associates (Ann. Rheum. Dis. 2011 Dec. 21 [doi: 10.1136/ard.2010.136762]).
The atorvastatin group, however, was more likely to develop elevations in liver function tests, even as late as 18 months into the study.
The lack of any evidence of an anti-inflammatory benefit from the statin suggests that something besides statins should be considered to manage the accelerated atherosclerosis of SLE, the investigators suggested.
Patients with SLE and hyperlipidemia should continue to be treated with statins and other lipid-lowering medications, they added, but with closer monitoring of liver function tests even if the statin dosage doesn't change, reported Dr. Petri, who is also the director of the lupus center at Johns Hopkins as well as the director of the Hopkins Lupus Cohort, and her associates.
At the start of the study, the groups were similar and none of the patients had taken statins for at least 3 months. They underwent helical CT scanning to assess coronary artery calcium and carotid duplex to assess carotid intima media thickness and carotid plaque. These tests were repeated at the 2-year follow-up.
Records of disease activity in the 2 years prior to starting the study were compared with quarterly measures of disease activity during the study.
In all, 96 patients on atorvastatin and 91 on placebo completed the study.
At baseline, 42% of patients in the atorvastatin group and 43% in the placebo group had coronary artery calcium. Carotid plaque was seen in 20% of the atorvastatin group and 15% of the placebo group, a difference that was not statistically significant.
Coronary artery calcium scores changed in 51% of those on atorvastatin and in 54% of those on placebo, with no significant differences between groups in the proportions whose scores increased or decreased, or by how much. All patients with carotid plaque at baseline also had it at follow-up. Among patients without carotid plaque at baseline, 25% in the atorvastatin group and 23% on placebo progressed to having plaque at follow-up, Dr. Petri and her associates said.
The mean carotid intima media thickness was 0.59 mm in the atorvastatin group and 0.57 mm in the placebo group at baseline, and 0.66 mm in both groups after 2 years. In a post hoc analysis of the proportions of patients in whom carotid intima media thickness improved, stayed the same, or got worse, results favored atorvastatin, the investigators noted.
Changes in the levels of total cholesterol and lipoprotein differed significantly between groups.
In the atorvastatin group, total cholesterol decreased by 31 mg/dL (or 17%), compared with an increase in the placebo group of 6 mg/dL (or 3%). In the atorvastatin group, lipoprotein levels increased by 8 mg/dL (or 12%), compared with a decrease in the placebo group of 7 mg/dL (or 10%).
Disease activity was measured using the Safety of Estrogens in Lupus: National Assessment revision of the SLE Disease Activity Index.
Major Finding: Atorvastatin 40 mg/day did not affect subclinical measures of atherosclerosis or disease activity in patients with SLE, compared with placebo.
Data Source: Randomized, double-blind, placebo-controlled trial in 200 patients with SLE who were followed for 2 years.
Disclosures: Dr. Petri was formerly an adviser and speaker for Pfizer, which markets atorvastatin. None of her coauthors had conflicts of interest. The study was funded by the Alliance for Lupus Research, the Arthritis Foundation, the Hopkins Lupus Cohort, and the National Center for Research Resources.
Atorvastatin therapy did not improve subclinical measures of atherosclerosis or disease activity, compared with placebo, in a 2-year, randomized, double-blind study of 200 adults with systemic lupus erythematosus.
The results surprised the investigators because atorvastatin had seemed to be an ideal choice to interrupt the accelerated atherosclerosis seen in SLE, reported Dr. Michelle A. Petri.
Previous studies had shown some potential benefits from the anti-inflammatory effects of statins in other autoimmune diseases, including rheumatoid arthritis and multiple sclerosis. Findings from one small study of pravastatin in patients with SLE found that it reduced total cholesterol and LDL cholesterol levels but did not reduce C-reactive protein levels.
In the current study, there was no significant difference between the patients who were randomized to 40 mg/day atorvastatin and those receiving placebo in progression of coronary artery calcium, carotid intima media thickness, or carotid plaque.
There also were no significant differences between groups in measures of disease activity, inflammation, or endothelial cell activation, reported Dr. Petri, professor of medicine at Johns Hopkins University, Baltimore, and her associates (Ann. Rheum. Dis. 2011 Dec. 21 [doi: 10.1136/ard.2010.136762]).
The atorvastatin group, however, was more likely to develop elevations in liver function tests, even as late as 18 months into the study.
The lack of any evidence of an anti-inflammatory benefit from the statin suggests that something besides statins should be considered to manage the accelerated atherosclerosis of SLE, the investigators suggested.
Patients with SLE and hyperlipidemia should continue to be treated with statins and other lipid-lowering medications, they added, but with closer monitoring of liver function tests even if the statin dosage doesn't change, reported Dr. Petri, who is also the director of the lupus center at Johns Hopkins as well as the director of the Hopkins Lupus Cohort, and her associates.
At the start of the study, the groups were similar and none of the patients had taken statins for at least 3 months. They underwent helical CT scanning to assess coronary artery calcium and carotid duplex to assess carotid intima media thickness and carotid plaque. These tests were repeated at the 2-year follow-up.
Records of disease activity in the 2 years prior to starting the study were compared with quarterly measures of disease activity during the study.
In all, 96 patients on atorvastatin and 91 on placebo completed the study.
At baseline, 42% of patients in the atorvastatin group and 43% in the placebo group had coronary artery calcium. Carotid plaque was seen in 20% of the atorvastatin group and 15% of the placebo group, a difference that was not statistically significant.
Coronary artery calcium scores changed in 51% of those on atorvastatin and in 54% of those on placebo, with no significant differences between groups in the proportions whose scores increased or decreased, or by how much. All patients with carotid plaque at baseline also had it at follow-up. Among patients without carotid plaque at baseline, 25% in the atorvastatin group and 23% on placebo progressed to having plaque at follow-up, Dr. Petri and her associates said.
The mean carotid intima media thickness was 0.59 mm in the atorvastatin group and 0.57 mm in the placebo group at baseline, and 0.66 mm in both groups after 2 years. In a post hoc analysis of the proportions of patients in whom carotid intima media thickness improved, stayed the same, or got worse, results favored atorvastatin, the investigators noted.
Changes in the levels of total cholesterol and lipoprotein differed significantly between groups.
In the atorvastatin group, total cholesterol decreased by 31 mg/dL (or 17%), compared with an increase in the placebo group of 6 mg/dL (or 3%). In the atorvastatin group, lipoprotein levels increased by 8 mg/dL (or 12%), compared with a decrease in the placebo group of 7 mg/dL (or 10%).
Disease activity was measured using the Safety of Estrogens in Lupus: National Assessment revision of the SLE Disease Activity Index.
Major Finding: Atorvastatin 40 mg/day did not affect subclinical measures of atherosclerosis or disease activity in patients with SLE, compared with placebo.
Data Source: Randomized, double-blind, placebo-controlled trial in 200 patients with SLE who were followed for 2 years.
Disclosures: Dr. Petri was formerly an adviser and speaker for Pfizer, which markets atorvastatin. None of her coauthors had conflicts of interest. The study was funded by the Alliance for Lupus Research, the Arthritis Foundation, the Hopkins Lupus Cohort, and the National Center for Research Resources.
Atorvastatin therapy did not improve subclinical measures of atherosclerosis or disease activity, compared with placebo, in a 2-year, randomized, double-blind study of 200 adults with systemic lupus erythematosus.
The results surprised the investigators because atorvastatin had seemed to be an ideal choice to interrupt the accelerated atherosclerosis seen in SLE, reported Dr. Michelle A. Petri.
Previous studies had shown some potential benefits from the anti-inflammatory effects of statins in other autoimmune diseases, including rheumatoid arthritis and multiple sclerosis. Findings from one small study of pravastatin in patients with SLE found that it reduced total cholesterol and LDL cholesterol levels but did not reduce C-reactive protein levels.
In the current study, there was no significant difference between the patients who were randomized to 40 mg/day atorvastatin and those receiving placebo in progression of coronary artery calcium, carotid intima media thickness, or carotid plaque.
There also were no significant differences between groups in measures of disease activity, inflammation, or endothelial cell activation, reported Dr. Petri, professor of medicine at Johns Hopkins University, Baltimore, and her associates (Ann. Rheum. Dis. 2011 Dec. 21 [doi: 10.1136/ard.2010.136762]).
The atorvastatin group, however, was more likely to develop elevations in liver function tests, even as late as 18 months into the study.
The lack of any evidence of an anti-inflammatory benefit from the statin suggests that something besides statins should be considered to manage the accelerated atherosclerosis of SLE, the investigators suggested.
Patients with SLE and hyperlipidemia should continue to be treated with statins and other lipid-lowering medications, they added, but with closer monitoring of liver function tests even if the statin dosage doesn't change, reported Dr. Petri, who is also the director of the lupus center at Johns Hopkins as well as the director of the Hopkins Lupus Cohort, and her associates.
At the start of the study, the groups were similar and none of the patients had taken statins for at least 3 months. They underwent helical CT scanning to assess coronary artery calcium and carotid duplex to assess carotid intima media thickness and carotid plaque. These tests were repeated at the 2-year follow-up.
Records of disease activity in the 2 years prior to starting the study were compared with quarterly measures of disease activity during the study.
In all, 96 patients on atorvastatin and 91 on placebo completed the study.
At baseline, 42% of patients in the atorvastatin group and 43% in the placebo group had coronary artery calcium. Carotid plaque was seen in 20% of the atorvastatin group and 15% of the placebo group, a difference that was not statistically significant.
Coronary artery calcium scores changed in 51% of those on atorvastatin and in 54% of those on placebo, with no significant differences between groups in the proportions whose scores increased or decreased, or by how much. All patients with carotid plaque at baseline also had it at follow-up. Among patients without carotid plaque at baseline, 25% in the atorvastatin group and 23% on placebo progressed to having plaque at follow-up, Dr. Petri and her associates said.
The mean carotid intima media thickness was 0.59 mm in the atorvastatin group and 0.57 mm in the placebo group at baseline, and 0.66 mm in both groups after 2 years. In a post hoc analysis of the proportions of patients in whom carotid intima media thickness improved, stayed the same, or got worse, results favored atorvastatin, the investigators noted.
Changes in the levels of total cholesterol and lipoprotein differed significantly between groups.
In the atorvastatin group, total cholesterol decreased by 31 mg/dL (or 17%), compared with an increase in the placebo group of 6 mg/dL (or 3%). In the atorvastatin group, lipoprotein levels increased by 8 mg/dL (or 12%), compared with a decrease in the placebo group of 7 mg/dL (or 10%).
Disease activity was measured using the Safety of Estrogens in Lupus: National Assessment revision of the SLE Disease Activity Index.
Osteoporosis Screening Not Supported for Men
New federal recommendations on screening for osteoporosis provide more detail on when to screen women younger than age 65 years and – for the first time – point to a lack of data to guide screening decisions in men.
The U.S. Preventive Services Task Force updated its 2002 recommendations on osteoporosis screening to call for routine screening in all women aged 65 years or older and in any younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors (equivalent to a 9.3% or greater risk of fracture within 10 years). Previously, women younger than 65 years would be screened if they were at least 60 years old with risk factors for fracture.
The new recommendations were posted on the USPSTF Web site (www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm
For the first time, the USPSTF evaluated the evidence for osteoporosis screening in men and found insufficient evidence to form any recommendation, Dr. Ned Colange, chair of the USPSTF, said in an interview. There's not enough evidence to recommend osteoporosis screening or treatment in men with no prior osteoporotic fractures, and “there's certainly not enough evidence to say, 'Don't' do it,” he said. “While there's not a call to action, that's an important call for research,” added Dr. Colange, who is president and CEO of the Colorado Trust Foundation, Denver.
In women, the recommendations do not say to stop osteoporosis screening at any specific age because the risk of fractures continues to increase with advancing age, and the minimal potential harms of treatment remain small. Clinicians who are considering treating older patients with significant morbidity should consider that the benefits of osteoporosis treatment eake 18-24 months af emerge.
To predict an individual's risk for osteoporotic fracture, the USPSTF used the online FRAX tool, developed by the World Health Organization and the National Osteoporosis Foundation. “The nice thing about the FRAX calculator is, the patient herself can determine that risk. It's available online. It uses measures that the woman should know,” Dr. Colange said.
The FRAX tool estimates 10-year fracture risk based on easily obtained information such as age, body mass index (BMI), parental fracture history, and tobacco or alcohol use. It asks about results of dual-energy x-ray absorptiometry scans but does not require this information.
Younger women can reach the new threshold for screening because of various risk factors. For example, a white woman would qualify for screening if she is 50 years old, smokes, drinks alcohol daily, has a BMI less than 21, and has a parental history of fracture. A 55-year-old white woman would need only a parental fracture history to warrant osteoporosis screening. A 60-year-old white woman who smokes and drinks alcohol daily would fit the 10-year-risk profile for screening.
White women are more likely than women of other races to develop osteoporosis and fractures. Although there are fewer data on nonwhite women, the USPSTF recommended screening all women at age 65 because the consequences of failing to identify and treat low bone-mineral density are considerable, and the potential risks of treatment are small,
There are not enough data to recommend when to rescreen women without osteoporosis on their initial screen, the USPSTF stated, but at least a 2-year interval would be needed to assess a change in bone density and perhaps longer for better prediction of fracture risk.
The new recommendations are based on a 2010 review of studies published since 2002; the review was done by a team at the University of Oregon Health and Science University's Evidence-Based Practice Center in Portland.
An estimated 12 million Americans aged 50 years or older will have osteoporosis in 2012. Among postmenopausal women, 15% will develop a hip fracture during their lifetime, 25% will develop a vertebral deformity, and osteoporotic fractures of any kind will affect 50%.
In a new effort at transparency, the USPSTF first published a draft of the new recommendations online in the summer of 2010 and invited public comment. They received more than 50 comments from individuals, professional organizations, advocates, and pharmaceutical companies, Dr. Colange said, which led the USPSTF to clarify its approach to fracture risk assessment in the final version.
Dr. Colange said he has no pertinent conflicts of interest.
Views on the News
Screening Calculations Now Needed
For clinicians, the biggest change in the new screening recommendations may be the need to calculate the 10-year fracture risk in women younger than 65, two experts suggested in interviews.
“They will need to know what tools are out there to be able to figure out whether a younger person is at equal to or greater risk than a 65-year-old woman with no additional risk factors,” Dr. Carolyn J. Crandall said.
The online FRAX calculator that was used by the USPSTF is a “really good tool” for this purpose, she said. “Clinicians will have to access that tool in their clinics, which means they will either need Internet access at some point, or else they can download versions that are available for iPhone, or print versions”
Dr. Edward S. Leib also commended inclusion of the FRAX tool in the guidelines, but cautioned that it has some weaknesses that were discussed at a November 2010 “position development conference” conducted jointly by the International Osteoporosis Foundation and the International Society for Clinical Densitometry.
Some important risk factors that could affect the 10-year fracture risk would not necessarily be reflected in the FRAX calculation, he said. In addition, the FRAX tool is based on an international model, and although it included U.S. databases, the calculations may not reflect risks in regional populations.
Both Dr. Crandall and Dr. Leib also commended the USPSTF for acknowledging the need for more research in men, but Dr. Leib had hoped for more guidance. “It is known that the fracture risk in men who are age 75 is about equivalent to women who are age 65. I would have hoped that the USPSTF would have recommended screening at that age” despite the lack of primary prevention trials, he said.
DR. CRANDALL is professor of medicine at the University of California, Los Angeles. DR. LEIB is professor of medicine at the University of Vermont, Burlington. Hoth said hey have no pertinent conflicts of interest.
New federal recommendations on screening for osteoporosis provide more detail on when to screen women younger than age 65 years and – for the first time – point to a lack of data to guide screening decisions in men.
The U.S. Preventive Services Task Force updated its 2002 recommendations on osteoporosis screening to call for routine screening in all women aged 65 years or older and in any younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors (equivalent to a 9.3% or greater risk of fracture within 10 years). Previously, women younger than 65 years would be screened if they were at least 60 years old with risk factors for fracture.
The new recommendations were posted on the USPSTF Web site (www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm
For the first time, the USPSTF evaluated the evidence for osteoporosis screening in men and found insufficient evidence to form any recommendation, Dr. Ned Colange, chair of the USPSTF, said in an interview. There's not enough evidence to recommend osteoporosis screening or treatment in men with no prior osteoporotic fractures, and “there's certainly not enough evidence to say, 'Don't' do it,” he said. “While there's not a call to action, that's an important call for research,” added Dr. Colange, who is president and CEO of the Colorado Trust Foundation, Denver.
In women, the recommendations do not say to stop osteoporosis screening at any specific age because the risk of fractures continues to increase with advancing age, and the minimal potential harms of treatment remain small. Clinicians who are considering treating older patients with significant morbidity should consider that the benefits of osteoporosis treatment eake 18-24 months af emerge.
To predict an individual's risk for osteoporotic fracture, the USPSTF used the online FRAX tool, developed by the World Health Organization and the National Osteoporosis Foundation. “The nice thing about the FRAX calculator is, the patient herself can determine that risk. It's available online. It uses measures that the woman should know,” Dr. Colange said.
The FRAX tool estimates 10-year fracture risk based on easily obtained information such as age, body mass index (BMI), parental fracture history, and tobacco or alcohol use. It asks about results of dual-energy x-ray absorptiometry scans but does not require this information.
Younger women can reach the new threshold for screening because of various risk factors. For example, a white woman would qualify for screening if she is 50 years old, smokes, drinks alcohol daily, has a BMI less than 21, and has a parental history of fracture. A 55-year-old white woman would need only a parental fracture history to warrant osteoporosis screening. A 60-year-old white woman who smokes and drinks alcohol daily would fit the 10-year-risk profile for screening.
White women are more likely than women of other races to develop osteoporosis and fractures. Although there are fewer data on nonwhite women, the USPSTF recommended screening all women at age 65 because the consequences of failing to identify and treat low bone-mineral density are considerable, and the potential risks of treatment are small,
There are not enough data to recommend when to rescreen women without osteoporosis on their initial screen, the USPSTF stated, but at least a 2-year interval would be needed to assess a change in bone density and perhaps longer for better prediction of fracture risk.
The new recommendations are based on a 2010 review of studies published since 2002; the review was done by a team at the University of Oregon Health and Science University's Evidence-Based Practice Center in Portland.
An estimated 12 million Americans aged 50 years or older will have osteoporosis in 2012. Among postmenopausal women, 15% will develop a hip fracture during their lifetime, 25% will develop a vertebral deformity, and osteoporotic fractures of any kind will affect 50%.
In a new effort at transparency, the USPSTF first published a draft of the new recommendations online in the summer of 2010 and invited public comment. They received more than 50 comments from individuals, professional organizations, advocates, and pharmaceutical companies, Dr. Colange said, which led the USPSTF to clarify its approach to fracture risk assessment in the final version.
Dr. Colange said he has no pertinent conflicts of interest.
Views on the News
Screening Calculations Now Needed
For clinicians, the biggest change in the new screening recommendations may be the need to calculate the 10-year fracture risk in women younger than 65, two experts suggested in interviews.
“They will need to know what tools are out there to be able to figure out whether a younger person is at equal to or greater risk than a 65-year-old woman with no additional risk factors,” Dr. Carolyn J. Crandall said.
The online FRAX calculator that was used by the USPSTF is a “really good tool” for this purpose, she said. “Clinicians will have to access that tool in their clinics, which means they will either need Internet access at some point, or else they can download versions that are available for iPhone, or print versions”
Dr. Edward S. Leib also commended inclusion of the FRAX tool in the guidelines, but cautioned that it has some weaknesses that were discussed at a November 2010 “position development conference” conducted jointly by the International Osteoporosis Foundation and the International Society for Clinical Densitometry.
Some important risk factors that could affect the 10-year fracture risk would not necessarily be reflected in the FRAX calculation, he said. In addition, the FRAX tool is based on an international model, and although it included U.S. databases, the calculations may not reflect risks in regional populations.
Both Dr. Crandall and Dr. Leib also commended the USPSTF for acknowledging the need for more research in men, but Dr. Leib had hoped for more guidance. “It is known that the fracture risk in men who are age 75 is about equivalent to women who are age 65. I would have hoped that the USPSTF would have recommended screening at that age” despite the lack of primary prevention trials, he said.
DR. CRANDALL is professor of medicine at the University of California, Los Angeles. DR. LEIB is professor of medicine at the University of Vermont, Burlington. Hoth said hey have no pertinent conflicts of interest.
New federal recommendations on screening for osteoporosis provide more detail on when to screen women younger than age 65 years and – for the first time – point to a lack of data to guide screening decisions in men.
The U.S. Preventive Services Task Force updated its 2002 recommendations on osteoporosis screening to call for routine screening in all women aged 65 years or older and in any younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors (equivalent to a 9.3% or greater risk of fracture within 10 years). Previously, women younger than 65 years would be screened if they were at least 60 years old with risk factors for fracture.
The new recommendations were posted on the USPSTF Web site (www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm
For the first time, the USPSTF evaluated the evidence for osteoporosis screening in men and found insufficient evidence to form any recommendation, Dr. Ned Colange, chair of the USPSTF, said in an interview. There's not enough evidence to recommend osteoporosis screening or treatment in men with no prior osteoporotic fractures, and “there's certainly not enough evidence to say, 'Don't' do it,” he said. “While there's not a call to action, that's an important call for research,” added Dr. Colange, who is president and CEO of the Colorado Trust Foundation, Denver.
In women, the recommendations do not say to stop osteoporosis screening at any specific age because the risk of fractures continues to increase with advancing age, and the minimal potential harms of treatment remain small. Clinicians who are considering treating older patients with significant morbidity should consider that the benefits of osteoporosis treatment eake 18-24 months af emerge.
To predict an individual's risk for osteoporotic fracture, the USPSTF used the online FRAX tool, developed by the World Health Organization and the National Osteoporosis Foundation. “The nice thing about the FRAX calculator is, the patient herself can determine that risk. It's available online. It uses measures that the woman should know,” Dr. Colange said.
The FRAX tool estimates 10-year fracture risk based on easily obtained information such as age, body mass index (BMI), parental fracture history, and tobacco or alcohol use. It asks about results of dual-energy x-ray absorptiometry scans but does not require this information.
Younger women can reach the new threshold for screening because of various risk factors. For example, a white woman would qualify for screening if she is 50 years old, smokes, drinks alcohol daily, has a BMI less than 21, and has a parental history of fracture. A 55-year-old white woman would need only a parental fracture history to warrant osteoporosis screening. A 60-year-old white woman who smokes and drinks alcohol daily would fit the 10-year-risk profile for screening.
White women are more likely than women of other races to develop osteoporosis and fractures. Although there are fewer data on nonwhite women, the USPSTF recommended screening all women at age 65 because the consequences of failing to identify and treat low bone-mineral density are considerable, and the potential risks of treatment are small,
There are not enough data to recommend when to rescreen women without osteoporosis on their initial screen, the USPSTF stated, but at least a 2-year interval would be needed to assess a change in bone density and perhaps longer for better prediction of fracture risk.
The new recommendations are based on a 2010 review of studies published since 2002; the review was done by a team at the University of Oregon Health and Science University's Evidence-Based Practice Center in Portland.
An estimated 12 million Americans aged 50 years or older will have osteoporosis in 2012. Among postmenopausal women, 15% will develop a hip fracture during their lifetime, 25% will develop a vertebral deformity, and osteoporotic fractures of any kind will affect 50%.
In a new effort at transparency, the USPSTF first published a draft of the new recommendations online in the summer of 2010 and invited public comment. They received more than 50 comments from individuals, professional organizations, advocates, and pharmaceutical companies, Dr. Colange said, which led the USPSTF to clarify its approach to fracture risk assessment in the final version.
Dr. Colange said he has no pertinent conflicts of interest.
Views on the News
Screening Calculations Now Needed
For clinicians, the biggest change in the new screening recommendations may be the need to calculate the 10-year fracture risk in women younger than 65, two experts suggested in interviews.
“They will need to know what tools are out there to be able to figure out whether a younger person is at equal to or greater risk than a 65-year-old woman with no additional risk factors,” Dr. Carolyn J. Crandall said.
The online FRAX calculator that was used by the USPSTF is a “really good tool” for this purpose, she said. “Clinicians will have to access that tool in their clinics, which means they will either need Internet access at some point, or else they can download versions that are available for iPhone, or print versions”
Dr. Edward S. Leib also commended inclusion of the FRAX tool in the guidelines, but cautioned that it has some weaknesses that were discussed at a November 2010 “position development conference” conducted jointly by the International Osteoporosis Foundation and the International Society for Clinical Densitometry.
Some important risk factors that could affect the 10-year fracture risk would not necessarily be reflected in the FRAX calculation, he said. In addition, the FRAX tool is based on an international model, and although it included U.S. databases, the calculations may not reflect risks in regional populations.
Both Dr. Crandall and Dr. Leib also commended the USPSTF for acknowledging the need for more research in men, but Dr. Leib had hoped for more guidance. “It is known that the fracture risk in men who are age 75 is about equivalent to women who are age 65. I would have hoped that the USPSTF would have recommended screening at that age” despite the lack of primary prevention trials, he said.
DR. CRANDALL is professor of medicine at the University of California, Los Angeles. DR. LEIB is professor of medicine at the University of Vermont, Burlington. Hoth said hey have no pertinent conflicts of interest.
Patient Representatives Are a Must in Research
New recommendations from the European League Against Rheumatism provide structure to the growing practice of including patient representatives in research projects.
The recommendations should be useful not only within the European League Against Rheumatism (EULAR) but also to other medical researchers, Maarten P. T. de Wit of Vrije Universiteit Medical Centre, Amsterdam, and his associates reported. EULAR convened a 16-member task force that crafted eight recommendations to promote inclusion of the patient perspective in EULAR-funded scientific research (Ann. Rheum. Dis. 2011[doi:10.1136/ard.2010.135129]).
Some previous reports suggest that the benefits of including patient representatives in research outweigh the drawbacks. Although including one or more patient representatives has become “usual practice” in EULAR scientific projects, the League's standardized procedures previously have not described how best to do this, the task force said.
They defined patient research partners as “persons with a relevant disease who operate as active research team members on an equal basis with professional researchers, adding the benefit of their experiential knowledge to any phase of the project.” Including patient research partners can help prevent mismatches between patient preferences and the focus of research, lead to more patient-oriented research agendas, empower patients, and build trust between patient organizations and medical institutions, data suggest.
The task force of three rheumatologists, one rheumatologist/epidemiologist, two allied health professionals, two research organization representatives, and eight patient research partners from six countries reviewed the literature and met twice to develop the recommendations, which were then evaluated by 28 patient representatives and 53 health professionals.
First, the task force urged that clinical researchers and groups work with patient research partners when developing guidelines or recommendations, and that other researchers consider patient participation. Patients' experiential knowledge is important even for laboratory-based research, and researchers who do not include patient partners should justify that decision, they said.
Second, patient participation should be considered for all phases of a research project and is essential in the early stages of research when critical decisions about the protocol and project are made. If this recommendation is not followed, investigators should explain why when publishing results.
Third, each project should include at least two patient research partners. There's no solid evidence to support this, but it adds to the team's diversity and supports the patient partners, and provides a substitute if one patient is absent due to rheumatic illness. This recommendation also drew more support from patients in the group of experts, winning agreement from 26 patients (93%) compared with 36 professionals (68%).
Fourth, give potential patient research partners a clear description of the minimum requirements for the position and clarify the roles of the patient partners and the principal investigator.
Fifth, take into account the patient's communication skills, motivation, and attitude when selecting patient research partners. Having a critical but constructive and proactive attitude is ideal. Academic training is not necessary and a medical background might even be undesirable, though some familiarity with medical terminology helps. “Thinking like an outsider is crucial to provide experiential knowledge,” the task force said.
Sixth, a good attitude, good communication, and good support from the principal investigator are crucial for the full participation of patient partners. These skills to create a safe and respectful environment for patient partners may not come naturally to researchers, who should learn these skills or get training.
Seventh, give patient partners the information and training they need to participate, including awareness of ethical issues such as confidentiality, privacy, and legislation.
Eighth, recognize the contributions of patient research partners, which is usually voluntary work.
The authors reported having no relevant conflicts of interest.
New recommendations from the European League Against Rheumatism provide structure to the growing practice of including patient representatives in research projects.
The recommendations should be useful not only within the European League Against Rheumatism (EULAR) but also to other medical researchers, Maarten P. T. de Wit of Vrije Universiteit Medical Centre, Amsterdam, and his associates reported. EULAR convened a 16-member task force that crafted eight recommendations to promote inclusion of the patient perspective in EULAR-funded scientific research (Ann. Rheum. Dis. 2011[doi:10.1136/ard.2010.135129]).
Some previous reports suggest that the benefits of including patient representatives in research outweigh the drawbacks. Although including one or more patient representatives has become “usual practice” in EULAR scientific projects, the League's standardized procedures previously have not described how best to do this, the task force said.
They defined patient research partners as “persons with a relevant disease who operate as active research team members on an equal basis with professional researchers, adding the benefit of their experiential knowledge to any phase of the project.” Including patient research partners can help prevent mismatches between patient preferences and the focus of research, lead to more patient-oriented research agendas, empower patients, and build trust between patient organizations and medical institutions, data suggest.
The task force of three rheumatologists, one rheumatologist/epidemiologist, two allied health professionals, two research organization representatives, and eight patient research partners from six countries reviewed the literature and met twice to develop the recommendations, which were then evaluated by 28 patient representatives and 53 health professionals.
First, the task force urged that clinical researchers and groups work with patient research partners when developing guidelines or recommendations, and that other researchers consider patient participation. Patients' experiential knowledge is important even for laboratory-based research, and researchers who do not include patient partners should justify that decision, they said.
Second, patient participation should be considered for all phases of a research project and is essential in the early stages of research when critical decisions about the protocol and project are made. If this recommendation is not followed, investigators should explain why when publishing results.
Third, each project should include at least two patient research partners. There's no solid evidence to support this, but it adds to the team's diversity and supports the patient partners, and provides a substitute if one patient is absent due to rheumatic illness. This recommendation also drew more support from patients in the group of experts, winning agreement from 26 patients (93%) compared with 36 professionals (68%).
Fourth, give potential patient research partners a clear description of the minimum requirements for the position and clarify the roles of the patient partners and the principal investigator.
Fifth, take into account the patient's communication skills, motivation, and attitude when selecting patient research partners. Having a critical but constructive and proactive attitude is ideal. Academic training is not necessary and a medical background might even be undesirable, though some familiarity with medical terminology helps. “Thinking like an outsider is crucial to provide experiential knowledge,” the task force said.
Sixth, a good attitude, good communication, and good support from the principal investigator are crucial for the full participation of patient partners. These skills to create a safe and respectful environment for patient partners may not come naturally to researchers, who should learn these skills or get training.
Seventh, give patient partners the information and training they need to participate, including awareness of ethical issues such as confidentiality, privacy, and legislation.
Eighth, recognize the contributions of patient research partners, which is usually voluntary work.
The authors reported having no relevant conflicts of interest.
New recommendations from the European League Against Rheumatism provide structure to the growing practice of including patient representatives in research projects.
The recommendations should be useful not only within the European League Against Rheumatism (EULAR) but also to other medical researchers, Maarten P. T. de Wit of Vrije Universiteit Medical Centre, Amsterdam, and his associates reported. EULAR convened a 16-member task force that crafted eight recommendations to promote inclusion of the patient perspective in EULAR-funded scientific research (Ann. Rheum. Dis. 2011[doi:10.1136/ard.2010.135129]).
Some previous reports suggest that the benefits of including patient representatives in research outweigh the drawbacks. Although including one or more patient representatives has become “usual practice” in EULAR scientific projects, the League's standardized procedures previously have not described how best to do this, the task force said.
They defined patient research partners as “persons with a relevant disease who operate as active research team members on an equal basis with professional researchers, adding the benefit of their experiential knowledge to any phase of the project.” Including patient research partners can help prevent mismatches between patient preferences and the focus of research, lead to more patient-oriented research agendas, empower patients, and build trust between patient organizations and medical institutions, data suggest.
The task force of three rheumatologists, one rheumatologist/epidemiologist, two allied health professionals, two research organization representatives, and eight patient research partners from six countries reviewed the literature and met twice to develop the recommendations, which were then evaluated by 28 patient representatives and 53 health professionals.
First, the task force urged that clinical researchers and groups work with patient research partners when developing guidelines or recommendations, and that other researchers consider patient participation. Patients' experiential knowledge is important even for laboratory-based research, and researchers who do not include patient partners should justify that decision, they said.
Second, patient participation should be considered for all phases of a research project and is essential in the early stages of research when critical decisions about the protocol and project are made. If this recommendation is not followed, investigators should explain why when publishing results.
Third, each project should include at least two patient research partners. There's no solid evidence to support this, but it adds to the team's diversity and supports the patient partners, and provides a substitute if one patient is absent due to rheumatic illness. This recommendation also drew more support from patients in the group of experts, winning agreement from 26 patients (93%) compared with 36 professionals (68%).
Fourth, give potential patient research partners a clear description of the minimum requirements for the position and clarify the roles of the patient partners and the principal investigator.
Fifth, take into account the patient's communication skills, motivation, and attitude when selecting patient research partners. Having a critical but constructive and proactive attitude is ideal. Academic training is not necessary and a medical background might even be undesirable, though some familiarity with medical terminology helps. “Thinking like an outsider is crucial to provide experiential knowledge,” the task force said.
Sixth, a good attitude, good communication, and good support from the principal investigator are crucial for the full participation of patient partners. These skills to create a safe and respectful environment for patient partners may not come naturally to researchers, who should learn these skills or get training.
Seventh, give patient partners the information and training they need to participate, including awareness of ethical issues such as confidentiality, privacy, and legislation.
Eighth, recognize the contributions of patient research partners, which is usually voluntary work.
The authors reported having no relevant conflicts of interest.