User login
Skin manifestations of diabetes
MANIFESTATIONS ASSOCIATED WITH TYPE 1 DIABETES
Periungual telangiectasia
The lesions of periungual telangiectasia, appearing as red, dilated, capillary veins, are easily visible with the naked eye and are the result of a loss of capillary loops and dilation of the remaining capillaries. A prevalence up to 49% has been described in all diabetic patients.1 Connective tissue diseases may also involve periungual telangiectases, although these lesions are morphologically different. In diabetes, periungual telangiectasia is often associated with nail fold erythema, accompanied by fingertip tenderness and “ragged” cuticles.2
Necrobiosis lipoidica
Metabolic control has no proven effect on the course of this condition,6 although Cohen et al7 reported that tight glucose control reduced the incidence in diabetic patients. Treatment includes application of a topical steroid with or without occlusion; intralesional steroids at the active border; or, in the rare severe or extensive case, systemic steroids.6,7 In some resistant cases, aspirin, chloroquine (Aralen), and cyclosporine (Sandimmune, Neoral) have been used with some success.3,8,9
Bullosis diabeticorum
Bullosis diabeticorum develops in approximately 0.5% of diabetic patients, but more often in those with type 1 diabetes, and more often in men and in patients with long-standing diabetes with peripheral neuropathy. It presents as asymptomatic bullae containing sterile fluid on a noninflamed base, usually arising spontaneously on the dorsa and sides of the lower legs and feet, sometimes on the hands or the forearms. The cause is unknown, and it is a diagnosis of exclusion. The differential diagnosis includes epidermolysis bullosa acquisita, porphyria cutanea tarda, bullous pemphigoid, bullous impetigo, coma blisters, and erythema multiforme.
Treatment is symptomatic and conservative. In case of discomfort, the bullae can be aspirated (leaving the blister roof intact), or compresses can be used. Topical antibiotics may be required to prevent secondary infection.3 Most lesions resolve in 2 to 3 weeks without residual scarring.5,6
Vitiligo
Vitiligo vulgaris, or skin depigmentation, occurs more often in type 1 diabetic patients. From 1% to 7% of all diabetic patients have vitiligo vs 0.2% to 1% of the general population. The mechanism behind the association has not been elucidated, although some have suggested polyglandular autoimmune syndrome (PAS), a rare immune endocrinopathy characterized by the coexistence of at least two endocrine gland insufficiencies that are based on autoimmune mechanisms. PAS type 2 is more common (estimated prevalence of 1:20,000), occurs mainly in the third or fourth decade, and is characterized by adrenal failure, autoimmune thyroid disease, or type 1 diabetes. Adrenal failure may precede other endocrinopathies. Vitiligo and gonadal failure occur more frequently in PAS type 1 than in PAS type 2, whereas immunogastritis, pernicious anemia, and alopecia areata are the main features of PAS type 2. In contrast to PAS type 1, family members of PAS type 2 patients are often affected as well. PAS type 2 is believed to be polygenic, with an autosomal dominant pattern of inheritance.10
Treatment of vitiligo is unsatisfactory in general. Patients should be advised to avoid the sun and to use broad-spectrum sunscreens. For localized vitiligo, topical corticosteroids are preferred, whereas for generalized vitiligo ultraviolet B light treatment is most effective. Cosmetic treatment is an option for improved well-being.11
Oral lichen planus
Clinically, lichen planus presents as polygonal erythematous flat lesions. Most often affected are the wrists, the dorsa of the feet, and the lower legs. Oral lichen planus presents as white stripes in a reticular pattern.
Clinical and histopathologic differentiation of these lesions from lichenoid reactions to drugs (eg, nonsteroidal anti-inflammatory drugs, antihypertensive drugs) may be difficult, although numerous eosinophils, parakeratosis, and perivascular inflammation around the mid and deep dermal plexuses, are seen in lichenoid drug reactions, but generally not in lichen planus.13
Treatment consists of topical corticosteroids or topical cyclosporine, or both.6
SKIN MANIFESTATIONS ASSOCIATED WITH TYPE 2 DIABETES
Yellow nails
Elderly type 2 diabetic patients tend to have yellow nails. A prevalence of 40% to 50% in patients with type 2 diabetes has been reported,14 but occasionally yellow nails are also found in normal elderly people and in patients with onychomycosis. The yellow discoloration in diabetes is most evident on the distal end of the hallux nail. It probably represents end-products of glycosylation, similar to the yellow color in diabetic skin, although this has not yet been confirmed.15
Diabetic thick skin
Diabetes mellitus is generally associated with a thickening of the skin,2 measurable via ultrasonography,16 and this thickening may increase with age in all diabetic patients, unlike normally aging skin.
Diabetic thick skin occurs in three forms. First is the general asymptomatic but measurable thickening. Second is a clinically apparent thickening of the skin involving the fingers and hands. Third is diabetic scleredema, an infrequent syndrome in which the dermis of the upper back becomes markedly thickened.2,6
Thickening of the skin on the dorsum of the hands occurs in 20% to 30% of all diabetic patients, regardless of the type of diabetes.17 Manifestations range from pebbled knuckles to diabetic hand syndrome.2 Pebbled knuckles (or Huntley papules) are multiple minute papules, grouped on the extensor side of the fingers, on the knuckles, or on the periungual surface.18 The prevalence of diabetic hand syndrome varies from 8% to 50%.19 It begins with stiffness of the metacarpophalangeal and proximal interphalangeal joints and progresses to limit joint mobility.20,21 Dupuytren contracture (or palmar fascial thickening) may further complicate diabetic hand syndrome.5,22
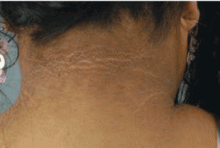
Scleredema diabeticorum is characterized by remarkable thickening of the skin of the posterior neck and upper back, occasionally extending to the deltoid and lumbar regions. A peau d’orange appearance of the skin can occur, often with decreased sensitivity to pain and touch.
Scleredema occurs in 2.5% to 14% of people with diabetes6 and is sometimes confused with scleredema of Buschke, a rare disorder in which areas of dermal thickening occur, mostly on the face, arms, and hands, often after an upper respiratory infection. It clears spontaneously in months or years. Women are affected more often than men. These characteristics differentiate scleredema of Buschke from scleredema diabeticorum, which almost exclusively occurs in long-standing diabetes, is usually permanent, is not related to previous infection, and is limited to the posterior neck and upper back. No effective treatment is known for scleredema diabeticorum.23
Skin tags or acrochordons
Skin tags are small, pedunculated, soft, often pigmented lesions occurring on the eyelids, the neck, and the axillae. A few studies have reported an association between multiple skin tags and diabetes, and between skin tags and insulin resistance.24–27 Crook28 found that skin tags were associated with the typical atherogenic lipid profile seen in insulin-resistant states: elevated triglycerides and low levels of high-density-lipoprotein cholesterol. In a large study of patients with skin tags,24 over 25% had diabetes and 8% had impaired glucose tolerance.24
Treatment is not necessary, but skin tags can be removed with grade 1 scissors, cryotherapy, or electrodessication.28 Skin tags may be regarded as a sign of impaired glucose tolerance, diabetes, and increased cardiovascular risk.28,29
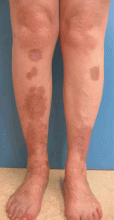
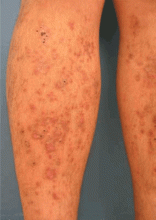
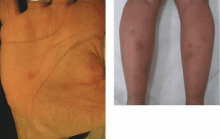
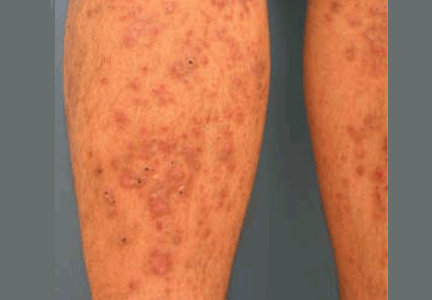
Diabetic dermopathy
Diabetic dermopathy (ie, shin spots and pigmented pretibial papules) affects 7% to 70% of all diabetic patients. It is not specific for diabetes: 20% of nondiabetic people show similar lesions. Men are affected more often than women, and the mean age is 50 years.
Shin spots present as multiple, bilateral, asymmetrical, annular or irregular red papules or plaques on the extensor surface of the lower legs and may precede abnormal glucose metabolism. The clinician usually sees only the end result: atrophic, scarred, hyperpigmented, finely scaled macules. Lesions may also be found on the forearms, thighs, and lateral malleoli. Several studies found severe microvascular complications in patients with diabetic dermopathy, indicating a close association with a high risk of accelerated diabetes complications.
Treatment is not very effective; however, some lesions resolve spontaneously.6,30
Acanthosis nigricans
The pathogenesis is most likely related to high levels of circulating insulin, which binds to insulin-like growth factor receptors to stimulate the growth of keratinocytes and dermal fibroblasts.
Although the lesions are generally asymptomatic, they can be painful, malodorous, or macerated.3 The most effective treatment is lifestyle alteration. Weight reduction and exercise can reduce insulin resistance. Acanthosis nigricans is reversible with weight reduction if it is seen as a complication of obesity. If the lesions are asymptomatic, they need no treatment. Ointments containing salicylic or retinoic acid can be used to reduce thicker lesions in areas of maceration in order to decrease odor and promote comfort. Systemic isotretinoin (Accutane) improves acanthosis nigricans, but it recurs when the drug is discontinued.3,5,6,32
Acquired perforating dermatosis
Acquired perforating dermatosis is seen in patients with kidney failure, type 2 diabetes, or type 1 diabetes. A prevalence of up to 10% has been reported in dialysis patients.33,34
The characteristic lesions are 2- to 10-mm, pruritic, dome-shaped papules and nodules with a hyperkeratotic plug. They occur mainly on the limbs, the trunk, and the dorsal surface of the hands, and to a lesser extent on the face. The Koebner phenomenon (also called isomorphic effect) may also occur.
Histologic study shows a hyperplastic epidermis with marked spongiosis directly over the plug. The contents of the plug itself are collagen, elastic fibers, nuclear debris, and polymorphonuclear leukocytes. These leukocytes have been implicated in the pathogenesis of acquired perforating dermatosis.6,17
The lesions are chronic but may heal after months if trauma and scratching are avoided. Further treatments include topical keratolytics, psoralen-ultraviolet A light, ultraviolet B light, topical and systemic retinoids, topical and intralesional steroids, oral antihistamines, and cryotherapy.6
Calciphylaxis
Calciphylaxis is a small-vessel vasculopathy accompanied by mural calcification with intimal proliferation, fibrosis, and thrombosis. It occurs mostly in patients with renal failure and causes a spectrum of end-organ damage due to ischemia. The reported prevalence is 1% to 4% in the dialysis population.
Damage is seen in the epidermal and the subcutaneous tissues. First, redness and tenderness evolve in a small area, which may be surrounded by ecchymosis or pallor, and eventually ischemia leads to the development of subcutaneous nodules and poor-healing, necrotizing skin ulcers. These ulcers serve as a port of entry for infectious agents.
Calciphylaxis has a predilection for vascular regions with thicker subcutaneous adipose tissue, such as the breasts, abdomen, and thighs. In renal failure patients, those who are women, white, obese, or diabetic (especially those with type 2 diabetes) are considered at risk.
Histologic features are medial wall calcification and fibrous expansion in capillaries, venules, arterioles, and small arteries of dermis and subcutaneous fat. Calciphylaxis should not be considered a small-vessel variant of Mönckeberg calcification, which is a medial wall calcification of medium and large vessels. Mönckeberg calcification has been described in patients with diabetes, renal failure, or vitamin D intoxification.
The outcome of calciphylaxis is poor because of impaired wound-healing and infection of the skin with progression to sepsis. Extremely aggressive treatment with analgesics is required for ischemic pain. Furthermore, weight reduction and aggressive control of blood sugar levels seem prudent.35
Eruptive xanthoma
Eruptive xanthoma presents as crops of small (1- to 2-mm) yellow papules with an erythematous halo; these papules may be pruritic and tender. They occur in less than 0.1% of diabetic patients.36 Areas of predilection are extensor surfaces and the buttocks.20
The key histologic feature is the formation of foam cells in the superficial dermis that are mixed with a lymphocytic and neutrophilic infiltrate.
Eruptive xanthomas appear in association with elevated levels of triglyceride-rich lipoproteins. The lipid changes appear in association with familial hypertriglyceridemia and diabetes mellitus, resulting in hypertriglyceridemia from a lack of lipoprotein lipase activity and impaired clearance of chylomicrons and very-low-density lipoproteins. These eruptive xanthomas tend to resolve with control of carbohydrate and lipid metabolism.5,6,17
Granuloma annulare: Not linked to diabetes
Although many have tried to prove an association between localized granuloma annulare and diabetes, no association has been clearly established, and the association between generalized (disseminated) granuloma annulare and diabetes is controversial.21
The cause is not known. The lesions are oval or ring-shaped, with a raised border of skin-colored or erythematous papules. The size varies from millimeters to centimeters. The dorsa of the hands and arms are the areas usually affected. Histologically, the epidermis usually appears normal, whereas the upper and mid dermis show focal degeneration of collagen, palisaded histiocytes around collagen bundles, and abundant mucin.
Localized lesions often resolve spontaneously, whereas the generalized form has a more protracted course which, in rare cases, resolves spontaneously. Sporadic therapeutic success has been reported with topical, systemic, and intralesional steroids; isotretinoin; chlorambucil (Leukeran); cryotherapy; chlorpropamide (Diabinese); chloroquine; potassium iodide/nicotinamide; dapsone; antimalarials; and psoralen-ultraviolet A light.5,6,20
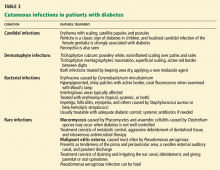
CUTANEOUS INFECTIONS
Candidal infection
A candidal infection (moniliasis) can be an early sign of undiagnosed diabetes. Perlèche is a classic sign of diabetes in children, and localized candidal infection of the female genitalia has a strong association with diabetes. This infection appears as erythema with scaling and typical satellite papules and pustules. Paronychia is another sign.
It is important to remember that in men, Candida balanitis, balanoposthitis, and intertrigo can be presenting signs of diabetes.
Candidal infections improve with adequate metabolic control and treatment with topical midazoles or nystatin (Mycostatin).5,37
Infections with dermatophytes
Common superficial infections are caused by Trichophyton rubrum, T mentagrophytes, and Epidermophyton floccosum. In diabetic patients, onychomycosis or tinea pedis needs to be monitored for and treated, as it can be a port of entry for infection. This is especially true for patients with neurovascular complications and intertrigo.
Signs of T rubrum infection are noninflamed, white, powdery scaling or skin creases on the palms and soles, often with nail involvement. T mentagrophytes-associated intertrigo or interdigital infection presents as maceration and superficial scaling with an active red border. Treatments of choice are drying the local area and applying one of the newer topical imidazole antifungal agents.5,37
Bacterial infections
Pyodermic infections such as impetigo, folliculitis, carbuncles, furunculosis, ecthyma, and erysipelas can be more severe and widespread in diabetic patients. Therapy consists of adequate diabetic control and, if necessary, adequate systemic antibiotic therapy; deeper infections require intravenous antibiotics.
Erythrasma, caused by Corynebacterium minutissimum, occurs with increased frequency in obese diabetic patients, but it is often missed. Intertriginous areas are the main affected site. Sweat, friction, and maceration play a role in the development. Erythrasma presents as shiny, hyperpigmented patches with an active border. With the Wood’s lamp, a characteristic coral fluorescence is seen. Treatment consists of topical or systemic erythromycin, or both. Prevention of sweating, friction, and maceration can limit the chances of developing this infection.5,6,37
Rare infections
Poor metabolic control and ketoacidosis may set the stage for severe infections by otherwise nonpathogenic microorganisms, such as mucormycosis by Phycomycetes and anaerobic cellulitis by Clostridium species. Treatment consists of metabolic control, aggressive debride ment of devitalized tissue, and intravenous antimicrobial therapy.37
In older diabetic patients, malignant otitis externa, often caused by Pseudomonas aeruginosa, can be fatal. This invasive infection may spread from the external auditory canal to the base of the skull, the meninges, and the brain itself. Treatment consists of irrigation and drainage of the ear canal, antibiotics, and sometimes debridement. A cure rate of more than 90% can be achieved using parenteral or oral quinolones.3
CUTANEOUS REACTIONS TO INSULIN
Impurities in insulin preparations, the presence of cow or pig proteins, the insulin molecule itself, preservatives, or additives cause allergic reactions. The use of human recombinant insulin has decreased the incidence of insulin allergy, so that now it is reported in fewer than 1% of diabetic patients treated with insulin.6
Allergic reactions to insulin can be classified as immediate-local, generalized, delayed, or biphasic.
Immediate-local reactions reach maximum intensity in 15 to 30 minutes and usually subside within 1 hour. Clinically, one finds erythema, which may become urticarial. This reaction probably is mediated by immunoglobulin E (IgE).
Generalized reactions. Immediate reactions may progress to generalized erythema and urticaria. Anaphylaxis is unusual.
Delayed hypersensitivity reactions are the most common. They usually appear about 2 weeks after the start of insulin therapy as an itchy nodule at the site of injection, 4 to 24 hours after injection.
Biphasic, or dual, reactions are rare events and consist of an immediate and a delayed local reaction, often with a generalized illness resembling serum sickness. They are considered Arthus-immune complex reactions.6
Other complications of insulin injections
Other local cutaneous complications include keloids, hyperkeratotic papules, purpura, and localized pigmentation.
The treatment of choice for localized immediate allergic reactions is a change of insulin to a more purified product.17 Other tools to manage allergic reactions are antihistamines, the addition of glucocorticoids to insulin, discontinuation of therapy, desensitization therapy, or a change in the insulin delivery system.5,6
The most important immunologic problem is IgE-mediated anaphylaxis, which can be managed by temporary reduction in dose or by insulin desensitization. Serum sickness responds to corticosteroid therapy.38
Insulin therapy may also cause lipoatrophy and lipohypertrophy that can coexist in the same patient. Lipoatrophy presents as circumscribed, depressed areas of skin at the insulin injection site 6 to 24 months after the start of therapy. Children and obese women are affected most often. It may be caused by lipolytic components in the insulin preparation or by an inflammatory process mediated by the immune complex. Other theories involve cryotrauma from refrigerated insulin, mechanical trauma due to the angle of injection, surface alcohol contamination, or local hyperproduction of tumor necrosis factor alpha from macrophages induced by injected insulin. Since the introduction of purified recombinant human insulin, lipoatrophy has become rare.37,39 Duration of the presence of an insulin depot has been implicated as well. That is why Murao et al40 suggested substituting rapid-acting insulin.
Lipohypertrophy clinically resembles lipoma and presents as soft dermal nodules at the site of frequent injections. Lipohypertrophy is regarded as a local response to the lipogenic action of insulin and can be prevented by rotation of the injection site.5,17,37
SKIN EFFECTS OF INSULIN ANALOGUES
Cutaneous side effects are not often described in insulin analogues, but there have been case reports. A case of IgE-mediated anaphylaxis41 and one case of vitiligo42 were described with insulin lispro. One case of allergy was described with insulin glargine.43 Although insulin detemir is well tolerated in general, several cases of local injection site reactions have been reported.44,45 Treatment depends on the extent of the reaction and can include desensitization, changing the type of insulin, rotating the injection site, or a combination of these.41–45
SKIN EFFECTS OF ORAL HYPOGLYCEMIC AGENTS
First-generation sulfonylureas
In 10% to 30% of patients using chlorpropamide, an alcohol flush is induced, consisting of redness and warmth, headache, tachycardia, and occasionally dyspnea, starting about 15 minutes after alcohol consumption. Usually, the symptoms disappear after an hour. This reaction pattern seems to be inherited in an autosomal-dominant pattern.6,37
Second-generation sulfonylureas
Second-generation sulfonylureas such as glipizide (Glucotrol) and glimepiride (Amaryl) have also been associated with cutaneous reactions. The most frequent reactions associated with glipizide are photosensitivity, rash, urticaria, and pruritus. These are reported less often with glimepiride. Deerochanawong46 reported patients with skin rash after the use of glimepiride. A case of lichenoid drug eruption was described by Noakes.47
Other oral hypoglycemic drugs
Metformin (Glucophage), a biguanide-derivative antihyperglycemic drug, is the first-choice oral drug in type 2 diabetic patients. Dermal side effects reported include psoriatiform drug eruption,48 erythema exsudativum multiforme,49 and leukocytoclastic vasculitis.50,51Litt’s Drug Eruption Manual gives the risk of photosensitivity reaction to metformin as 1% to 10%52 but cites no reference for this statement. Erythema, exanthema, pruritus, and urticaria have also been reported as side effects of metformin.52
Acarbose (Precose) is minimally absorbed from the gut: only about 1% of a dose reaches the bloodstream,53 and thus it seldom causes adverse effects. Kono et al54 reported a case of acarbose-induced generalized erythema multiforme confirmed by a challenge test. The drug-induced lymphocyte stimulation test and patch test for acarbose were negative. Ahr et al55 reported that acarbose labeled with carbon 14 was poorly absorbed when given orally, but that up to 35% of this formulation of acarbose was absorbed after degradation by digestive enzymes, intestinal microorganisms, or both. Because the drug-induced lymphocyte stimulation test and the patch test were negative in the patient described by Kono et al,54 it is possible that the degradation products of acarbose induced the allergic reaction after absorption. Poszepczynska-Guigné et al56 described the first case of acute generalized exanthematous pustulosis induced after administration of acarbose.
Thiazolidinediones. Edema has been reported as an adverse cutaneous effect of rosiglitazone (Avandia) and pioglitazone (Actos).52
- Landau J, Davis E. The small blood-vessels of the conjunctiva and nail bed in diabetes mellitus. Lancet 1960; 2:731
- Huntley A. Diabetes mellitus: review. Dermatology Online Journal 1995; vol 1(2). http://dermatology.cdlib.org/DOJvol1num2/diabetes/dmreview.html. Accessed July 30, 2008.
- Ahmed I, Goldstein B. Diabetes mellitus. Clin Dermatol 2006; 24:237–246.
- Petzelbauer P, Wolff K, Tappeiner G. Necrobiosis lipoidica: treatment with systemic corticoids. Br J Dermatol 1992; 126:542.
- Sibbald RG, Schachter RK. The skin and diabetes mellitus. Int J Dermatol 1984; 23:567–584.
- Ferringer T, Miller F. Cutaneous manifestations of diabetes mellitus. Dermatol Clin 2002; 20:483–492.
- Cohen O, Yaniv R, Karasik A, Trau H. Necrobiosis lipoidica and diabetic control revisited. Med Hypotheses 1996; 46:348–350.
- Nguyen K, Washenik K, Shupak J. Necrobiosis lipoidica diabeticorum treated with chloroquine. J Am Acad Dermatol 2002; 46 suppl 2:34–36.
- Stanway A, Rademaker M, Newman P. Healing of severe ulcerative necrobiosis lipoidica with cyclosporine. Australas J Dermatol 2004; 45:119–122.
- Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab 2003; 88:2983–2992.
- Forschner T, Buchholtz S, Stockfleth E. Current state of vitiligo therapy-evidence-based analysis of the literature. J Dtsch Dermatol Ges 2007; 5:467–475.
- Petrou-Amerikanou C, Markopoulos AK, Belazi M, Karamitsos D, Papanayotou P. Prevalence of oral lichen planus in diabetes mellitus according to the type of diabetes. Oral Dis 1998; 4:37–40.
- Mobini N, Toussaint S, Kamino H. Noninfectious, erythematous, papular, and squamous diseases. In:Elder DE, editor. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2005:179–214.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK. Characteristic changes of skin and its accessories in type 2 diabetes mellitus. Georgian Med News 2006; 131:43–46.
- Lithner F, Hietala S-O. Skeletal lesions of the feet in diabetics and their relationship to cutaneous erythema with or without necrosis of the feet. Acta Med Scand 1976; 200:155–161.
- Collier A, Matthews DM, Kellett HA, Clarke BF, Hunter JA. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1986; 292:936.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol 1994; 30:519–530.
- Libecco JF. Finger pebbles and diabetes: a case with broad involvement of the dorsal fingers and hands. Arch Dermatol 2001; 137:510–511.
- Brik R, Berant M, Vardi P. The scleroderma-like syndrome of insulin-dependent diabetes mellitus. Diabetes Metab Rev 1991; 7:121–128.
- Jelinek JE. Cutaneous manifestations of diabetes mellitus. Int J Dermatol 1994; 33:605–617.
- Huntley AC. The cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol 1989; 7:427–455.
- Jennings AM, Milner PC, Ward JD. Hand abnormalities are associated with the complications of diabetes in type 2 diabetes. Diabet Med 1989; 6:43–47.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care 1983; 6:189–192.
- Kahana M, Grossman E, Feinstein A, Ronnen M, Cohen M, Millet MS. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol 1987; 67:175–177.
- Margolis J, Margolis LS. Skin tags—a frequent sign of diabetes mellitus [letter]. N Engl J Med 1976; 294:1184.
- Garcia Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol 2002; 3:497–506.
- Scheinfeld NS. Obesity and dermatology. Clin Dermatol 2004; 22:303–309.
- Crook MA. Skin tags and the atherogenic lipid profile. J Clin Pathol 2000; 53:873–874.
- Tompkins RR. Skin tags and diabetes. Arch Dermatol 1977; 113:1463.
- Sibbald RG, Landolt SJ, Toth D. Skin and diabetes. Endocrinol Metab Clin North Am 1996; 25:463–472.
- Hermanns-Le T, Scheen A, Pierard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol 2004; 5:199–203.
- Katz RA. Treatment of acanthosis nigricans with oral isotretinoin. Arch Dermatol 1980; 116:110.
- Morton CA, Henderson IS, Jones MC, Lowe JG. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol 1996; 135:671–677.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol 2006; 20:679–688.
- Wilmer WA, Magro CM. Calciphylaxis:emerging concepts in prevention, diagnosis, and treatment. Dialysis 2002; 15:172–186.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc 1966; 41:689.
- Meurer M, Stumvoll M, Szeimies RM. Hautveränderungen bei Diabetes mellitus. Hautartz 2004; 55:428–435.
- Grammer L. Insulin allergy. Clin Rev Allergy 1986; 4:189–200.
- Richardson T, Kerr D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol 2003; 4:661–667.
- Murao S, Hirata K, Ishida T, Takahara J. Lipoatrophy induced by recombinant human insulin injection. Intern Med 1998; 37:1031–1033.
- Barranco R, Herrero T, Tornero P, et al. Systemic allergic reaction by a human insulin analog. Allergy 2003; 58:536–537.
- Burge MR, Carey JD. Vitiligo associated with subcutaneous insulin lispro infusion in type 1 diabetes. Diabetes Care 2004; 27:275–276.
- Durand-Gonzalez KN, Guillausseau N, Pecquet C, Gayno JP. Glargine insulin is not an alternative in insulin allergy. Diabetes Care 2003; 26:2216.
- Blumer IR. Severe injection site reaction to insulin detemir. Diabetes Care 2006; 29:946.
- Darmon P, Castera V, Koeppel MC, Petitjean C, Dutour A. Type III allergy to insulin detemir. Diabetes Care 2005; 28:2980.
- Deerochanawong C, Chandraprasert S. Glimepiride in type 2 diabetes mellitus Thai patients. J Med Assoc Thai 2001; 84:1221–1228.
- Noakes R. Lichenoid drug eruption as a result of the recently released sulfonylurea glimepiride. Australas J Dermatol 2003; 44:302–303.
- Koca R, Altinyazar HC, Yenidünya S, Tekin NS. Psoriasiform [sic] drug eruption associated with metformin hydrochloride: a case report. Dermatol Online J 2003; 9:11. http://dermatology.cdlib.org/93/case_reports/metformin/koca.html. Accessed July 30, 2008.
- Burger DE, Goyal S. Erythema multiforme from metformin. Ann Pharmacother 2004; 38:1537.
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. BMJ (Clin Res Ed) 1986; 293:483.
- Ben Salem C, Hmouda H, Slim R, Denguezli M, Belajouza C, Bouraoui K. Rare case of metformin-induced leucocytoclastic vasculitis. Ann Pharmacother 2006; 40:1685–1687.
- Litt JZ. Litt’s Drug Eruption Reference Manual. London: Taylor and Francis, 2001.
- Balfour JA, McTavish D. Acarbose: an update of its pharmacology and therapeutic use in diabetes mellitus. Drugs 1993; 46:1024–1054. Erratum in: Drugs 1994; 48:929.
- Kono T, Hayami M, Kobayashi H, Ishii M, Taniguchi S. Acarbose-induced generalized erythema multiforme. Lancet 1999; 354:396–397.
- Ahr HJ, Boberg M, Krause HP, et al. Pharmacokinetics of acarbose. Part 1: Absorption, concentration in plasma, metabolism, and excretion after single administration of 14C acarbose to rats, dogs and man. Arzneimittelforschung 1989; 39:1254–1260.
- Poszepczynska-Guigné E, Viguier M, Assier H, Pinquier L, Hochedez P, Dubertret L. Acute generalized exanthematous pustulosis induced by drugs with low-digestive absorption: acarbose and nystatin. Ann Dermatol Venereol 2003; 130:439–442.
MANIFESTATIONS ASSOCIATED WITH TYPE 1 DIABETES
Periungual telangiectasia
The lesions of periungual telangiectasia, appearing as red, dilated, capillary veins, are easily visible with the naked eye and are the result of a loss of capillary loops and dilation of the remaining capillaries. A prevalence up to 49% has been described in all diabetic patients.1 Connective tissue diseases may also involve periungual telangiectases, although these lesions are morphologically different. In diabetes, periungual telangiectasia is often associated with nail fold erythema, accompanied by fingertip tenderness and “ragged” cuticles.2
Necrobiosis lipoidica
Metabolic control has no proven effect on the course of this condition,6 although Cohen et al7 reported that tight glucose control reduced the incidence in diabetic patients. Treatment includes application of a topical steroid with or without occlusion; intralesional steroids at the active border; or, in the rare severe or extensive case, systemic steroids.6,7 In some resistant cases, aspirin, chloroquine (Aralen), and cyclosporine (Sandimmune, Neoral) have been used with some success.3,8,9
Bullosis diabeticorum
Bullosis diabeticorum develops in approximately 0.5% of diabetic patients, but more often in those with type 1 diabetes, and more often in men and in patients with long-standing diabetes with peripheral neuropathy. It presents as asymptomatic bullae containing sterile fluid on a noninflamed base, usually arising spontaneously on the dorsa and sides of the lower legs and feet, sometimes on the hands or the forearms. The cause is unknown, and it is a diagnosis of exclusion. The differential diagnosis includes epidermolysis bullosa acquisita, porphyria cutanea tarda, bullous pemphigoid, bullous impetigo, coma blisters, and erythema multiforme.
Treatment is symptomatic and conservative. In case of discomfort, the bullae can be aspirated (leaving the blister roof intact), or compresses can be used. Topical antibiotics may be required to prevent secondary infection.3 Most lesions resolve in 2 to 3 weeks without residual scarring.5,6
Vitiligo
Vitiligo vulgaris, or skin depigmentation, occurs more often in type 1 diabetic patients. From 1% to 7% of all diabetic patients have vitiligo vs 0.2% to 1% of the general population. The mechanism behind the association has not been elucidated, although some have suggested polyglandular autoimmune syndrome (PAS), a rare immune endocrinopathy characterized by the coexistence of at least two endocrine gland insufficiencies that are based on autoimmune mechanisms. PAS type 2 is more common (estimated prevalence of 1:20,000), occurs mainly in the third or fourth decade, and is characterized by adrenal failure, autoimmune thyroid disease, or type 1 diabetes. Adrenal failure may precede other endocrinopathies. Vitiligo and gonadal failure occur more frequently in PAS type 1 than in PAS type 2, whereas immunogastritis, pernicious anemia, and alopecia areata are the main features of PAS type 2. In contrast to PAS type 1, family members of PAS type 2 patients are often affected as well. PAS type 2 is believed to be polygenic, with an autosomal dominant pattern of inheritance.10
Treatment of vitiligo is unsatisfactory in general. Patients should be advised to avoid the sun and to use broad-spectrum sunscreens. For localized vitiligo, topical corticosteroids are preferred, whereas for generalized vitiligo ultraviolet B light treatment is most effective. Cosmetic treatment is an option for improved well-being.11
Oral lichen planus
Clinically, lichen planus presents as polygonal erythematous flat lesions. Most often affected are the wrists, the dorsa of the feet, and the lower legs. Oral lichen planus presents as white stripes in a reticular pattern.
Clinical and histopathologic differentiation of these lesions from lichenoid reactions to drugs (eg, nonsteroidal anti-inflammatory drugs, antihypertensive drugs) may be difficult, although numerous eosinophils, parakeratosis, and perivascular inflammation around the mid and deep dermal plexuses, are seen in lichenoid drug reactions, but generally not in lichen planus.13
Treatment consists of topical corticosteroids or topical cyclosporine, or both.6
SKIN MANIFESTATIONS ASSOCIATED WITH TYPE 2 DIABETES
Yellow nails
Elderly type 2 diabetic patients tend to have yellow nails. A prevalence of 40% to 50% in patients with type 2 diabetes has been reported,14 but occasionally yellow nails are also found in normal elderly people and in patients with onychomycosis. The yellow discoloration in diabetes is most evident on the distal end of the hallux nail. It probably represents end-products of glycosylation, similar to the yellow color in diabetic skin, although this has not yet been confirmed.15
Diabetic thick skin
Diabetes mellitus is generally associated with a thickening of the skin,2 measurable via ultrasonography,16 and this thickening may increase with age in all diabetic patients, unlike normally aging skin.
Diabetic thick skin occurs in three forms. First is the general asymptomatic but measurable thickening. Second is a clinically apparent thickening of the skin involving the fingers and hands. Third is diabetic scleredema, an infrequent syndrome in which the dermis of the upper back becomes markedly thickened.2,6
Thickening of the skin on the dorsum of the hands occurs in 20% to 30% of all diabetic patients, regardless of the type of diabetes.17 Manifestations range from pebbled knuckles to diabetic hand syndrome.2 Pebbled knuckles (or Huntley papules) are multiple minute papules, grouped on the extensor side of the fingers, on the knuckles, or on the periungual surface.18 The prevalence of diabetic hand syndrome varies from 8% to 50%.19 It begins with stiffness of the metacarpophalangeal and proximal interphalangeal joints and progresses to limit joint mobility.20,21 Dupuytren contracture (or palmar fascial thickening) may further complicate diabetic hand syndrome.5,22
Scleredema diabeticorum is characterized by remarkable thickening of the skin of the posterior neck and upper back, occasionally extending to the deltoid and lumbar regions. A peau d’orange appearance of the skin can occur, often with decreased sensitivity to pain and touch.
Scleredema occurs in 2.5% to 14% of people with diabetes6 and is sometimes confused with scleredema of Buschke, a rare disorder in which areas of dermal thickening occur, mostly on the face, arms, and hands, often after an upper respiratory infection. It clears spontaneously in months or years. Women are affected more often than men. These characteristics differentiate scleredema of Buschke from scleredema diabeticorum, which almost exclusively occurs in long-standing diabetes, is usually permanent, is not related to previous infection, and is limited to the posterior neck and upper back. No effective treatment is known for scleredema diabeticorum.23
Skin tags or acrochordons
Skin tags are small, pedunculated, soft, often pigmented lesions occurring on the eyelids, the neck, and the axillae. A few studies have reported an association between multiple skin tags and diabetes, and between skin tags and insulin resistance.24–27 Crook28 found that skin tags were associated with the typical atherogenic lipid profile seen in insulin-resistant states: elevated triglycerides and low levels of high-density-lipoprotein cholesterol. In a large study of patients with skin tags,24 over 25% had diabetes and 8% had impaired glucose tolerance.24
Treatment is not necessary, but skin tags can be removed with grade 1 scissors, cryotherapy, or electrodessication.28 Skin tags may be regarded as a sign of impaired glucose tolerance, diabetes, and increased cardiovascular risk.28,29
Diabetic dermopathy
Diabetic dermopathy (ie, shin spots and pigmented pretibial papules) affects 7% to 70% of all diabetic patients. It is not specific for diabetes: 20% of nondiabetic people show similar lesions. Men are affected more often than women, and the mean age is 50 years.
Shin spots present as multiple, bilateral, asymmetrical, annular or irregular red papules or plaques on the extensor surface of the lower legs and may precede abnormal glucose metabolism. The clinician usually sees only the end result: atrophic, scarred, hyperpigmented, finely scaled macules. Lesions may also be found on the forearms, thighs, and lateral malleoli. Several studies found severe microvascular complications in patients with diabetic dermopathy, indicating a close association with a high risk of accelerated diabetes complications.
Treatment is not very effective; however, some lesions resolve spontaneously.6,30
Acanthosis nigricans
The pathogenesis is most likely related to high levels of circulating insulin, which binds to insulin-like growth factor receptors to stimulate the growth of keratinocytes and dermal fibroblasts.
Although the lesions are generally asymptomatic, they can be painful, malodorous, or macerated.3 The most effective treatment is lifestyle alteration. Weight reduction and exercise can reduce insulin resistance. Acanthosis nigricans is reversible with weight reduction if it is seen as a complication of obesity. If the lesions are asymptomatic, they need no treatment. Ointments containing salicylic or retinoic acid can be used to reduce thicker lesions in areas of maceration in order to decrease odor and promote comfort. Systemic isotretinoin (Accutane) improves acanthosis nigricans, but it recurs when the drug is discontinued.3,5,6,32
Acquired perforating dermatosis
Acquired perforating dermatosis is seen in patients with kidney failure, type 2 diabetes, or type 1 diabetes. A prevalence of up to 10% has been reported in dialysis patients.33,34
The characteristic lesions are 2- to 10-mm, pruritic, dome-shaped papules and nodules with a hyperkeratotic plug. They occur mainly on the limbs, the trunk, and the dorsal surface of the hands, and to a lesser extent on the face. The Koebner phenomenon (also called isomorphic effect) may also occur.
Histologic study shows a hyperplastic epidermis with marked spongiosis directly over the plug. The contents of the plug itself are collagen, elastic fibers, nuclear debris, and polymorphonuclear leukocytes. These leukocytes have been implicated in the pathogenesis of acquired perforating dermatosis.6,17
The lesions are chronic but may heal after months if trauma and scratching are avoided. Further treatments include topical keratolytics, psoralen-ultraviolet A light, ultraviolet B light, topical and systemic retinoids, topical and intralesional steroids, oral antihistamines, and cryotherapy.6
Calciphylaxis
Calciphylaxis is a small-vessel vasculopathy accompanied by mural calcification with intimal proliferation, fibrosis, and thrombosis. It occurs mostly in patients with renal failure and causes a spectrum of end-organ damage due to ischemia. The reported prevalence is 1% to 4% in the dialysis population.
Damage is seen in the epidermal and the subcutaneous tissues. First, redness and tenderness evolve in a small area, which may be surrounded by ecchymosis or pallor, and eventually ischemia leads to the development of subcutaneous nodules and poor-healing, necrotizing skin ulcers. These ulcers serve as a port of entry for infectious agents.
Calciphylaxis has a predilection for vascular regions with thicker subcutaneous adipose tissue, such as the breasts, abdomen, and thighs. In renal failure patients, those who are women, white, obese, or diabetic (especially those with type 2 diabetes) are considered at risk.
Histologic features are medial wall calcification and fibrous expansion in capillaries, venules, arterioles, and small arteries of dermis and subcutaneous fat. Calciphylaxis should not be considered a small-vessel variant of Mönckeberg calcification, which is a medial wall calcification of medium and large vessels. Mönckeberg calcification has been described in patients with diabetes, renal failure, or vitamin D intoxification.
The outcome of calciphylaxis is poor because of impaired wound-healing and infection of the skin with progression to sepsis. Extremely aggressive treatment with analgesics is required for ischemic pain. Furthermore, weight reduction and aggressive control of blood sugar levels seem prudent.35
Eruptive xanthoma
Eruptive xanthoma presents as crops of small (1- to 2-mm) yellow papules with an erythematous halo; these papules may be pruritic and tender. They occur in less than 0.1% of diabetic patients.36 Areas of predilection are extensor surfaces and the buttocks.20
The key histologic feature is the formation of foam cells in the superficial dermis that are mixed with a lymphocytic and neutrophilic infiltrate.
Eruptive xanthomas appear in association with elevated levels of triglyceride-rich lipoproteins. The lipid changes appear in association with familial hypertriglyceridemia and diabetes mellitus, resulting in hypertriglyceridemia from a lack of lipoprotein lipase activity and impaired clearance of chylomicrons and very-low-density lipoproteins. These eruptive xanthomas tend to resolve with control of carbohydrate and lipid metabolism.5,6,17
Granuloma annulare: Not linked to diabetes
Although many have tried to prove an association between localized granuloma annulare and diabetes, no association has been clearly established, and the association between generalized (disseminated) granuloma annulare and diabetes is controversial.21
The cause is not known. The lesions are oval or ring-shaped, with a raised border of skin-colored or erythematous papules. The size varies from millimeters to centimeters. The dorsa of the hands and arms are the areas usually affected. Histologically, the epidermis usually appears normal, whereas the upper and mid dermis show focal degeneration of collagen, palisaded histiocytes around collagen bundles, and abundant mucin.
Localized lesions often resolve spontaneously, whereas the generalized form has a more protracted course which, in rare cases, resolves spontaneously. Sporadic therapeutic success has been reported with topical, systemic, and intralesional steroids; isotretinoin; chlorambucil (Leukeran); cryotherapy; chlorpropamide (Diabinese); chloroquine; potassium iodide/nicotinamide; dapsone; antimalarials; and psoralen-ultraviolet A light.5,6,20
CUTANEOUS INFECTIONS
Candidal infection
A candidal infection (moniliasis) can be an early sign of undiagnosed diabetes. Perlèche is a classic sign of diabetes in children, and localized candidal infection of the female genitalia has a strong association with diabetes. This infection appears as erythema with scaling and typical satellite papules and pustules. Paronychia is another sign.
It is important to remember that in men, Candida balanitis, balanoposthitis, and intertrigo can be presenting signs of diabetes.
Candidal infections improve with adequate metabolic control and treatment with topical midazoles or nystatin (Mycostatin).5,37
Infections with dermatophytes
Common superficial infections are caused by Trichophyton rubrum, T mentagrophytes, and Epidermophyton floccosum. In diabetic patients, onychomycosis or tinea pedis needs to be monitored for and treated, as it can be a port of entry for infection. This is especially true for patients with neurovascular complications and intertrigo.
Signs of T rubrum infection are noninflamed, white, powdery scaling or skin creases on the palms and soles, often with nail involvement. T mentagrophytes-associated intertrigo or interdigital infection presents as maceration and superficial scaling with an active red border. Treatments of choice are drying the local area and applying one of the newer topical imidazole antifungal agents.5,37
Bacterial infections
Pyodermic infections such as impetigo, folliculitis, carbuncles, furunculosis, ecthyma, and erysipelas can be more severe and widespread in diabetic patients. Therapy consists of adequate diabetic control and, if necessary, adequate systemic antibiotic therapy; deeper infections require intravenous antibiotics.
Erythrasma, caused by Corynebacterium minutissimum, occurs with increased frequency in obese diabetic patients, but it is often missed. Intertriginous areas are the main affected site. Sweat, friction, and maceration play a role in the development. Erythrasma presents as shiny, hyperpigmented patches with an active border. With the Wood’s lamp, a characteristic coral fluorescence is seen. Treatment consists of topical or systemic erythromycin, or both. Prevention of sweating, friction, and maceration can limit the chances of developing this infection.5,6,37
Rare infections
Poor metabolic control and ketoacidosis may set the stage for severe infections by otherwise nonpathogenic microorganisms, such as mucormycosis by Phycomycetes and anaerobic cellulitis by Clostridium species. Treatment consists of metabolic control, aggressive debride ment of devitalized tissue, and intravenous antimicrobial therapy.37
In older diabetic patients, malignant otitis externa, often caused by Pseudomonas aeruginosa, can be fatal. This invasive infection may spread from the external auditory canal to the base of the skull, the meninges, and the brain itself. Treatment consists of irrigation and drainage of the ear canal, antibiotics, and sometimes debridement. A cure rate of more than 90% can be achieved using parenteral or oral quinolones.3
CUTANEOUS REACTIONS TO INSULIN
Impurities in insulin preparations, the presence of cow or pig proteins, the insulin molecule itself, preservatives, or additives cause allergic reactions. The use of human recombinant insulin has decreased the incidence of insulin allergy, so that now it is reported in fewer than 1% of diabetic patients treated with insulin.6
Allergic reactions to insulin can be classified as immediate-local, generalized, delayed, or biphasic.
Immediate-local reactions reach maximum intensity in 15 to 30 minutes and usually subside within 1 hour. Clinically, one finds erythema, which may become urticarial. This reaction probably is mediated by immunoglobulin E (IgE).
Generalized reactions. Immediate reactions may progress to generalized erythema and urticaria. Anaphylaxis is unusual.
Delayed hypersensitivity reactions are the most common. They usually appear about 2 weeks after the start of insulin therapy as an itchy nodule at the site of injection, 4 to 24 hours after injection.
Biphasic, or dual, reactions are rare events and consist of an immediate and a delayed local reaction, often with a generalized illness resembling serum sickness. They are considered Arthus-immune complex reactions.6
Other complications of insulin injections
Other local cutaneous complications include keloids, hyperkeratotic papules, purpura, and localized pigmentation.
The treatment of choice for localized immediate allergic reactions is a change of insulin to a more purified product.17 Other tools to manage allergic reactions are antihistamines, the addition of glucocorticoids to insulin, discontinuation of therapy, desensitization therapy, or a change in the insulin delivery system.5,6
The most important immunologic problem is IgE-mediated anaphylaxis, which can be managed by temporary reduction in dose or by insulin desensitization. Serum sickness responds to corticosteroid therapy.38
Insulin therapy may also cause lipoatrophy and lipohypertrophy that can coexist in the same patient. Lipoatrophy presents as circumscribed, depressed areas of skin at the insulin injection site 6 to 24 months after the start of therapy. Children and obese women are affected most often. It may be caused by lipolytic components in the insulin preparation or by an inflammatory process mediated by the immune complex. Other theories involve cryotrauma from refrigerated insulin, mechanical trauma due to the angle of injection, surface alcohol contamination, or local hyperproduction of tumor necrosis factor alpha from macrophages induced by injected insulin. Since the introduction of purified recombinant human insulin, lipoatrophy has become rare.37,39 Duration of the presence of an insulin depot has been implicated as well. That is why Murao et al40 suggested substituting rapid-acting insulin.
Lipohypertrophy clinically resembles lipoma and presents as soft dermal nodules at the site of frequent injections. Lipohypertrophy is regarded as a local response to the lipogenic action of insulin and can be prevented by rotation of the injection site.5,17,37
SKIN EFFECTS OF INSULIN ANALOGUES
Cutaneous side effects are not often described in insulin analogues, but there have been case reports. A case of IgE-mediated anaphylaxis41 and one case of vitiligo42 were described with insulin lispro. One case of allergy was described with insulin glargine.43 Although insulin detemir is well tolerated in general, several cases of local injection site reactions have been reported.44,45 Treatment depends on the extent of the reaction and can include desensitization, changing the type of insulin, rotating the injection site, or a combination of these.41–45
SKIN EFFECTS OF ORAL HYPOGLYCEMIC AGENTS
First-generation sulfonylureas
In 10% to 30% of patients using chlorpropamide, an alcohol flush is induced, consisting of redness and warmth, headache, tachycardia, and occasionally dyspnea, starting about 15 minutes after alcohol consumption. Usually, the symptoms disappear after an hour. This reaction pattern seems to be inherited in an autosomal-dominant pattern.6,37
Second-generation sulfonylureas
Second-generation sulfonylureas such as glipizide (Glucotrol) and glimepiride (Amaryl) have also been associated with cutaneous reactions. The most frequent reactions associated with glipizide are photosensitivity, rash, urticaria, and pruritus. These are reported less often with glimepiride. Deerochanawong46 reported patients with skin rash after the use of glimepiride. A case of lichenoid drug eruption was described by Noakes.47
Other oral hypoglycemic drugs
Metformin (Glucophage), a biguanide-derivative antihyperglycemic drug, is the first-choice oral drug in type 2 diabetic patients. Dermal side effects reported include psoriatiform drug eruption,48 erythema exsudativum multiforme,49 and leukocytoclastic vasculitis.50,51Litt’s Drug Eruption Manual gives the risk of photosensitivity reaction to metformin as 1% to 10%52 but cites no reference for this statement. Erythema, exanthema, pruritus, and urticaria have also been reported as side effects of metformin.52
Acarbose (Precose) is minimally absorbed from the gut: only about 1% of a dose reaches the bloodstream,53 and thus it seldom causes adverse effects. Kono et al54 reported a case of acarbose-induced generalized erythema multiforme confirmed by a challenge test. The drug-induced lymphocyte stimulation test and patch test for acarbose were negative. Ahr et al55 reported that acarbose labeled with carbon 14 was poorly absorbed when given orally, but that up to 35% of this formulation of acarbose was absorbed after degradation by digestive enzymes, intestinal microorganisms, or both. Because the drug-induced lymphocyte stimulation test and the patch test were negative in the patient described by Kono et al,54 it is possible that the degradation products of acarbose induced the allergic reaction after absorption. Poszepczynska-Guigné et al56 described the first case of acute generalized exanthematous pustulosis induced after administration of acarbose.
Thiazolidinediones. Edema has been reported as an adverse cutaneous effect of rosiglitazone (Avandia) and pioglitazone (Actos).52
MANIFESTATIONS ASSOCIATED WITH TYPE 1 DIABETES
Periungual telangiectasia
The lesions of periungual telangiectasia, appearing as red, dilated, capillary veins, are easily visible with the naked eye and are the result of a loss of capillary loops and dilation of the remaining capillaries. A prevalence up to 49% has been described in all diabetic patients.1 Connective tissue diseases may also involve periungual telangiectases, although these lesions are morphologically different. In diabetes, periungual telangiectasia is often associated with nail fold erythema, accompanied by fingertip tenderness and “ragged” cuticles.2
Necrobiosis lipoidica
Metabolic control has no proven effect on the course of this condition,6 although Cohen et al7 reported that tight glucose control reduced the incidence in diabetic patients. Treatment includes application of a topical steroid with or without occlusion; intralesional steroids at the active border; or, in the rare severe or extensive case, systemic steroids.6,7 In some resistant cases, aspirin, chloroquine (Aralen), and cyclosporine (Sandimmune, Neoral) have been used with some success.3,8,9
Bullosis diabeticorum
Bullosis diabeticorum develops in approximately 0.5% of diabetic patients, but more often in those with type 1 diabetes, and more often in men and in patients with long-standing diabetes with peripheral neuropathy. It presents as asymptomatic bullae containing sterile fluid on a noninflamed base, usually arising spontaneously on the dorsa and sides of the lower legs and feet, sometimes on the hands or the forearms. The cause is unknown, and it is a diagnosis of exclusion. The differential diagnosis includes epidermolysis bullosa acquisita, porphyria cutanea tarda, bullous pemphigoid, bullous impetigo, coma blisters, and erythema multiforme.
Treatment is symptomatic and conservative. In case of discomfort, the bullae can be aspirated (leaving the blister roof intact), or compresses can be used. Topical antibiotics may be required to prevent secondary infection.3 Most lesions resolve in 2 to 3 weeks without residual scarring.5,6
Vitiligo
Vitiligo vulgaris, or skin depigmentation, occurs more often in type 1 diabetic patients. From 1% to 7% of all diabetic patients have vitiligo vs 0.2% to 1% of the general population. The mechanism behind the association has not been elucidated, although some have suggested polyglandular autoimmune syndrome (PAS), a rare immune endocrinopathy characterized by the coexistence of at least two endocrine gland insufficiencies that are based on autoimmune mechanisms. PAS type 2 is more common (estimated prevalence of 1:20,000), occurs mainly in the third or fourth decade, and is characterized by adrenal failure, autoimmune thyroid disease, or type 1 diabetes. Adrenal failure may precede other endocrinopathies. Vitiligo and gonadal failure occur more frequently in PAS type 1 than in PAS type 2, whereas immunogastritis, pernicious anemia, and alopecia areata are the main features of PAS type 2. In contrast to PAS type 1, family members of PAS type 2 patients are often affected as well. PAS type 2 is believed to be polygenic, with an autosomal dominant pattern of inheritance.10
Treatment of vitiligo is unsatisfactory in general. Patients should be advised to avoid the sun and to use broad-spectrum sunscreens. For localized vitiligo, topical corticosteroids are preferred, whereas for generalized vitiligo ultraviolet B light treatment is most effective. Cosmetic treatment is an option for improved well-being.11
Oral lichen planus
Clinically, lichen planus presents as polygonal erythematous flat lesions. Most often affected are the wrists, the dorsa of the feet, and the lower legs. Oral lichen planus presents as white stripes in a reticular pattern.
Clinical and histopathologic differentiation of these lesions from lichenoid reactions to drugs (eg, nonsteroidal anti-inflammatory drugs, antihypertensive drugs) may be difficult, although numerous eosinophils, parakeratosis, and perivascular inflammation around the mid and deep dermal plexuses, are seen in lichenoid drug reactions, but generally not in lichen planus.13
Treatment consists of topical corticosteroids or topical cyclosporine, or both.6
SKIN MANIFESTATIONS ASSOCIATED WITH TYPE 2 DIABETES
Yellow nails
Elderly type 2 diabetic patients tend to have yellow nails. A prevalence of 40% to 50% in patients with type 2 diabetes has been reported,14 but occasionally yellow nails are also found in normal elderly people and in patients with onychomycosis. The yellow discoloration in diabetes is most evident on the distal end of the hallux nail. It probably represents end-products of glycosylation, similar to the yellow color in diabetic skin, although this has not yet been confirmed.15
Diabetic thick skin
Diabetes mellitus is generally associated with a thickening of the skin,2 measurable via ultrasonography,16 and this thickening may increase with age in all diabetic patients, unlike normally aging skin.
Diabetic thick skin occurs in three forms. First is the general asymptomatic but measurable thickening. Second is a clinically apparent thickening of the skin involving the fingers and hands. Third is diabetic scleredema, an infrequent syndrome in which the dermis of the upper back becomes markedly thickened.2,6
Thickening of the skin on the dorsum of the hands occurs in 20% to 30% of all diabetic patients, regardless of the type of diabetes.17 Manifestations range from pebbled knuckles to diabetic hand syndrome.2 Pebbled knuckles (or Huntley papules) are multiple minute papules, grouped on the extensor side of the fingers, on the knuckles, or on the periungual surface.18 The prevalence of diabetic hand syndrome varies from 8% to 50%.19 It begins with stiffness of the metacarpophalangeal and proximal interphalangeal joints and progresses to limit joint mobility.20,21 Dupuytren contracture (or palmar fascial thickening) may further complicate diabetic hand syndrome.5,22
Scleredema diabeticorum is characterized by remarkable thickening of the skin of the posterior neck and upper back, occasionally extending to the deltoid and lumbar regions. A peau d’orange appearance of the skin can occur, often with decreased sensitivity to pain and touch.
Scleredema occurs in 2.5% to 14% of people with diabetes6 and is sometimes confused with scleredema of Buschke, a rare disorder in which areas of dermal thickening occur, mostly on the face, arms, and hands, often after an upper respiratory infection. It clears spontaneously in months or years. Women are affected more often than men. These characteristics differentiate scleredema of Buschke from scleredema diabeticorum, which almost exclusively occurs in long-standing diabetes, is usually permanent, is not related to previous infection, and is limited to the posterior neck and upper back. No effective treatment is known for scleredema diabeticorum.23
Skin tags or acrochordons
Skin tags are small, pedunculated, soft, often pigmented lesions occurring on the eyelids, the neck, and the axillae. A few studies have reported an association between multiple skin tags and diabetes, and between skin tags and insulin resistance.24–27 Crook28 found that skin tags were associated with the typical atherogenic lipid profile seen in insulin-resistant states: elevated triglycerides and low levels of high-density-lipoprotein cholesterol. In a large study of patients with skin tags,24 over 25% had diabetes and 8% had impaired glucose tolerance.24
Treatment is not necessary, but skin tags can be removed with grade 1 scissors, cryotherapy, or electrodessication.28 Skin tags may be regarded as a sign of impaired glucose tolerance, diabetes, and increased cardiovascular risk.28,29
Diabetic dermopathy
Diabetic dermopathy (ie, shin spots and pigmented pretibial papules) affects 7% to 70% of all diabetic patients. It is not specific for diabetes: 20% of nondiabetic people show similar lesions. Men are affected more often than women, and the mean age is 50 years.
Shin spots present as multiple, bilateral, asymmetrical, annular or irregular red papules or plaques on the extensor surface of the lower legs and may precede abnormal glucose metabolism. The clinician usually sees only the end result: atrophic, scarred, hyperpigmented, finely scaled macules. Lesions may also be found on the forearms, thighs, and lateral malleoli. Several studies found severe microvascular complications in patients with diabetic dermopathy, indicating a close association with a high risk of accelerated diabetes complications.
Treatment is not very effective; however, some lesions resolve spontaneously.6,30
Acanthosis nigricans
The pathogenesis is most likely related to high levels of circulating insulin, which binds to insulin-like growth factor receptors to stimulate the growth of keratinocytes and dermal fibroblasts.
Although the lesions are generally asymptomatic, they can be painful, malodorous, or macerated.3 The most effective treatment is lifestyle alteration. Weight reduction and exercise can reduce insulin resistance. Acanthosis nigricans is reversible with weight reduction if it is seen as a complication of obesity. If the lesions are asymptomatic, they need no treatment. Ointments containing salicylic or retinoic acid can be used to reduce thicker lesions in areas of maceration in order to decrease odor and promote comfort. Systemic isotretinoin (Accutane) improves acanthosis nigricans, but it recurs when the drug is discontinued.3,5,6,32
Acquired perforating dermatosis
Acquired perforating dermatosis is seen in patients with kidney failure, type 2 diabetes, or type 1 diabetes. A prevalence of up to 10% has been reported in dialysis patients.33,34
The characteristic lesions are 2- to 10-mm, pruritic, dome-shaped papules and nodules with a hyperkeratotic plug. They occur mainly on the limbs, the trunk, and the dorsal surface of the hands, and to a lesser extent on the face. The Koebner phenomenon (also called isomorphic effect) may also occur.
Histologic study shows a hyperplastic epidermis with marked spongiosis directly over the plug. The contents of the plug itself are collagen, elastic fibers, nuclear debris, and polymorphonuclear leukocytes. These leukocytes have been implicated in the pathogenesis of acquired perforating dermatosis.6,17
The lesions are chronic but may heal after months if trauma and scratching are avoided. Further treatments include topical keratolytics, psoralen-ultraviolet A light, ultraviolet B light, topical and systemic retinoids, topical and intralesional steroids, oral antihistamines, and cryotherapy.6
Calciphylaxis
Calciphylaxis is a small-vessel vasculopathy accompanied by mural calcification with intimal proliferation, fibrosis, and thrombosis. It occurs mostly in patients with renal failure and causes a spectrum of end-organ damage due to ischemia. The reported prevalence is 1% to 4% in the dialysis population.
Damage is seen in the epidermal and the subcutaneous tissues. First, redness and tenderness evolve in a small area, which may be surrounded by ecchymosis or pallor, and eventually ischemia leads to the development of subcutaneous nodules and poor-healing, necrotizing skin ulcers. These ulcers serve as a port of entry for infectious agents.
Calciphylaxis has a predilection for vascular regions with thicker subcutaneous adipose tissue, such as the breasts, abdomen, and thighs. In renal failure patients, those who are women, white, obese, or diabetic (especially those with type 2 diabetes) are considered at risk.
Histologic features are medial wall calcification and fibrous expansion in capillaries, venules, arterioles, and small arteries of dermis and subcutaneous fat. Calciphylaxis should not be considered a small-vessel variant of Mönckeberg calcification, which is a medial wall calcification of medium and large vessels. Mönckeberg calcification has been described in patients with diabetes, renal failure, or vitamin D intoxification.
The outcome of calciphylaxis is poor because of impaired wound-healing and infection of the skin with progression to sepsis. Extremely aggressive treatment with analgesics is required for ischemic pain. Furthermore, weight reduction and aggressive control of blood sugar levels seem prudent.35
Eruptive xanthoma
Eruptive xanthoma presents as crops of small (1- to 2-mm) yellow papules with an erythematous halo; these papules may be pruritic and tender. They occur in less than 0.1% of diabetic patients.36 Areas of predilection are extensor surfaces and the buttocks.20
The key histologic feature is the formation of foam cells in the superficial dermis that are mixed with a lymphocytic and neutrophilic infiltrate.
Eruptive xanthomas appear in association with elevated levels of triglyceride-rich lipoproteins. The lipid changes appear in association with familial hypertriglyceridemia and diabetes mellitus, resulting in hypertriglyceridemia from a lack of lipoprotein lipase activity and impaired clearance of chylomicrons and very-low-density lipoproteins. These eruptive xanthomas tend to resolve with control of carbohydrate and lipid metabolism.5,6,17
Granuloma annulare: Not linked to diabetes
Although many have tried to prove an association between localized granuloma annulare and diabetes, no association has been clearly established, and the association between generalized (disseminated) granuloma annulare and diabetes is controversial.21
The cause is not known. The lesions are oval or ring-shaped, with a raised border of skin-colored or erythematous papules. The size varies from millimeters to centimeters. The dorsa of the hands and arms are the areas usually affected. Histologically, the epidermis usually appears normal, whereas the upper and mid dermis show focal degeneration of collagen, palisaded histiocytes around collagen bundles, and abundant mucin.
Localized lesions often resolve spontaneously, whereas the generalized form has a more protracted course which, in rare cases, resolves spontaneously. Sporadic therapeutic success has been reported with topical, systemic, and intralesional steroids; isotretinoin; chlorambucil (Leukeran); cryotherapy; chlorpropamide (Diabinese); chloroquine; potassium iodide/nicotinamide; dapsone; antimalarials; and psoralen-ultraviolet A light.5,6,20
CUTANEOUS INFECTIONS
Candidal infection
A candidal infection (moniliasis) can be an early sign of undiagnosed diabetes. Perlèche is a classic sign of diabetes in children, and localized candidal infection of the female genitalia has a strong association with diabetes. This infection appears as erythema with scaling and typical satellite papules and pustules. Paronychia is another sign.
It is important to remember that in men, Candida balanitis, balanoposthitis, and intertrigo can be presenting signs of diabetes.
Candidal infections improve with adequate metabolic control and treatment with topical midazoles or nystatin (Mycostatin).5,37
Infections with dermatophytes
Common superficial infections are caused by Trichophyton rubrum, T mentagrophytes, and Epidermophyton floccosum. In diabetic patients, onychomycosis or tinea pedis needs to be monitored for and treated, as it can be a port of entry for infection. This is especially true for patients with neurovascular complications and intertrigo.
Signs of T rubrum infection are noninflamed, white, powdery scaling or skin creases on the palms and soles, often with nail involvement. T mentagrophytes-associated intertrigo or interdigital infection presents as maceration and superficial scaling with an active red border. Treatments of choice are drying the local area and applying one of the newer topical imidazole antifungal agents.5,37
Bacterial infections
Pyodermic infections such as impetigo, folliculitis, carbuncles, furunculosis, ecthyma, and erysipelas can be more severe and widespread in diabetic patients. Therapy consists of adequate diabetic control and, if necessary, adequate systemic antibiotic therapy; deeper infections require intravenous antibiotics.
Erythrasma, caused by Corynebacterium minutissimum, occurs with increased frequency in obese diabetic patients, but it is often missed. Intertriginous areas are the main affected site. Sweat, friction, and maceration play a role in the development. Erythrasma presents as shiny, hyperpigmented patches with an active border. With the Wood’s lamp, a characteristic coral fluorescence is seen. Treatment consists of topical or systemic erythromycin, or both. Prevention of sweating, friction, and maceration can limit the chances of developing this infection.5,6,37
Rare infections
Poor metabolic control and ketoacidosis may set the stage for severe infections by otherwise nonpathogenic microorganisms, such as mucormycosis by Phycomycetes and anaerobic cellulitis by Clostridium species. Treatment consists of metabolic control, aggressive debride ment of devitalized tissue, and intravenous antimicrobial therapy.37
In older diabetic patients, malignant otitis externa, often caused by Pseudomonas aeruginosa, can be fatal. This invasive infection may spread from the external auditory canal to the base of the skull, the meninges, and the brain itself. Treatment consists of irrigation and drainage of the ear canal, antibiotics, and sometimes debridement. A cure rate of more than 90% can be achieved using parenteral or oral quinolones.3
CUTANEOUS REACTIONS TO INSULIN
Impurities in insulin preparations, the presence of cow or pig proteins, the insulin molecule itself, preservatives, or additives cause allergic reactions. The use of human recombinant insulin has decreased the incidence of insulin allergy, so that now it is reported in fewer than 1% of diabetic patients treated with insulin.6
Allergic reactions to insulin can be classified as immediate-local, generalized, delayed, or biphasic.
Immediate-local reactions reach maximum intensity in 15 to 30 minutes and usually subside within 1 hour. Clinically, one finds erythema, which may become urticarial. This reaction probably is mediated by immunoglobulin E (IgE).
Generalized reactions. Immediate reactions may progress to generalized erythema and urticaria. Anaphylaxis is unusual.
Delayed hypersensitivity reactions are the most common. They usually appear about 2 weeks after the start of insulin therapy as an itchy nodule at the site of injection, 4 to 24 hours after injection.
Biphasic, or dual, reactions are rare events and consist of an immediate and a delayed local reaction, often with a generalized illness resembling serum sickness. They are considered Arthus-immune complex reactions.6
Other complications of insulin injections
Other local cutaneous complications include keloids, hyperkeratotic papules, purpura, and localized pigmentation.
The treatment of choice for localized immediate allergic reactions is a change of insulin to a more purified product.17 Other tools to manage allergic reactions are antihistamines, the addition of glucocorticoids to insulin, discontinuation of therapy, desensitization therapy, or a change in the insulin delivery system.5,6
The most important immunologic problem is IgE-mediated anaphylaxis, which can be managed by temporary reduction in dose or by insulin desensitization. Serum sickness responds to corticosteroid therapy.38
Insulin therapy may also cause lipoatrophy and lipohypertrophy that can coexist in the same patient. Lipoatrophy presents as circumscribed, depressed areas of skin at the insulin injection site 6 to 24 months after the start of therapy. Children and obese women are affected most often. It may be caused by lipolytic components in the insulin preparation or by an inflammatory process mediated by the immune complex. Other theories involve cryotrauma from refrigerated insulin, mechanical trauma due to the angle of injection, surface alcohol contamination, or local hyperproduction of tumor necrosis factor alpha from macrophages induced by injected insulin. Since the introduction of purified recombinant human insulin, lipoatrophy has become rare.37,39 Duration of the presence of an insulin depot has been implicated as well. That is why Murao et al40 suggested substituting rapid-acting insulin.
Lipohypertrophy clinically resembles lipoma and presents as soft dermal nodules at the site of frequent injections. Lipohypertrophy is regarded as a local response to the lipogenic action of insulin and can be prevented by rotation of the injection site.5,17,37
SKIN EFFECTS OF INSULIN ANALOGUES
Cutaneous side effects are not often described in insulin analogues, but there have been case reports. A case of IgE-mediated anaphylaxis41 and one case of vitiligo42 were described with insulin lispro. One case of allergy was described with insulin glargine.43 Although insulin detemir is well tolerated in general, several cases of local injection site reactions have been reported.44,45 Treatment depends on the extent of the reaction and can include desensitization, changing the type of insulin, rotating the injection site, or a combination of these.41–45
SKIN EFFECTS OF ORAL HYPOGLYCEMIC AGENTS
First-generation sulfonylureas
In 10% to 30% of patients using chlorpropamide, an alcohol flush is induced, consisting of redness and warmth, headache, tachycardia, and occasionally dyspnea, starting about 15 minutes after alcohol consumption. Usually, the symptoms disappear after an hour. This reaction pattern seems to be inherited in an autosomal-dominant pattern.6,37
Second-generation sulfonylureas
Second-generation sulfonylureas such as glipizide (Glucotrol) and glimepiride (Amaryl) have also been associated with cutaneous reactions. The most frequent reactions associated with glipizide are photosensitivity, rash, urticaria, and pruritus. These are reported less often with glimepiride. Deerochanawong46 reported patients with skin rash after the use of glimepiride. A case of lichenoid drug eruption was described by Noakes.47
Other oral hypoglycemic drugs
Metformin (Glucophage), a biguanide-derivative antihyperglycemic drug, is the first-choice oral drug in type 2 diabetic patients. Dermal side effects reported include psoriatiform drug eruption,48 erythema exsudativum multiforme,49 and leukocytoclastic vasculitis.50,51Litt’s Drug Eruption Manual gives the risk of photosensitivity reaction to metformin as 1% to 10%52 but cites no reference for this statement. Erythema, exanthema, pruritus, and urticaria have also been reported as side effects of metformin.52
Acarbose (Precose) is minimally absorbed from the gut: only about 1% of a dose reaches the bloodstream,53 and thus it seldom causes adverse effects. Kono et al54 reported a case of acarbose-induced generalized erythema multiforme confirmed by a challenge test. The drug-induced lymphocyte stimulation test and patch test for acarbose were negative. Ahr et al55 reported that acarbose labeled with carbon 14 was poorly absorbed when given orally, but that up to 35% of this formulation of acarbose was absorbed after degradation by digestive enzymes, intestinal microorganisms, or both. Because the drug-induced lymphocyte stimulation test and the patch test were negative in the patient described by Kono et al,54 it is possible that the degradation products of acarbose induced the allergic reaction after absorption. Poszepczynska-Guigné et al56 described the first case of acute generalized exanthematous pustulosis induced after administration of acarbose.
Thiazolidinediones. Edema has been reported as an adverse cutaneous effect of rosiglitazone (Avandia) and pioglitazone (Actos).52
- Landau J, Davis E. The small blood-vessels of the conjunctiva and nail bed in diabetes mellitus. Lancet 1960; 2:731
- Huntley A. Diabetes mellitus: review. Dermatology Online Journal 1995; vol 1(2). http://dermatology.cdlib.org/DOJvol1num2/diabetes/dmreview.html. Accessed July 30, 2008.
- Ahmed I, Goldstein B. Diabetes mellitus. Clin Dermatol 2006; 24:237–246.
- Petzelbauer P, Wolff K, Tappeiner G. Necrobiosis lipoidica: treatment with systemic corticoids. Br J Dermatol 1992; 126:542.
- Sibbald RG, Schachter RK. The skin and diabetes mellitus. Int J Dermatol 1984; 23:567–584.
- Ferringer T, Miller F. Cutaneous manifestations of diabetes mellitus. Dermatol Clin 2002; 20:483–492.
- Cohen O, Yaniv R, Karasik A, Trau H. Necrobiosis lipoidica and diabetic control revisited. Med Hypotheses 1996; 46:348–350.
- Nguyen K, Washenik K, Shupak J. Necrobiosis lipoidica diabeticorum treated with chloroquine. J Am Acad Dermatol 2002; 46 suppl 2:34–36.
- Stanway A, Rademaker M, Newman P. Healing of severe ulcerative necrobiosis lipoidica with cyclosporine. Australas J Dermatol 2004; 45:119–122.
- Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab 2003; 88:2983–2992.
- Forschner T, Buchholtz S, Stockfleth E. Current state of vitiligo therapy-evidence-based analysis of the literature. J Dtsch Dermatol Ges 2007; 5:467–475.
- Petrou-Amerikanou C, Markopoulos AK, Belazi M, Karamitsos D, Papanayotou P. Prevalence of oral lichen planus in diabetes mellitus according to the type of diabetes. Oral Dis 1998; 4:37–40.
- Mobini N, Toussaint S, Kamino H. Noninfectious, erythematous, papular, and squamous diseases. In:Elder DE, editor. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2005:179–214.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK. Characteristic changes of skin and its accessories in type 2 diabetes mellitus. Georgian Med News 2006; 131:43–46.
- Lithner F, Hietala S-O. Skeletal lesions of the feet in diabetics and their relationship to cutaneous erythema with or without necrosis of the feet. Acta Med Scand 1976; 200:155–161.
- Collier A, Matthews DM, Kellett HA, Clarke BF, Hunter JA. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1986; 292:936.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol 1994; 30:519–530.
- Libecco JF. Finger pebbles and diabetes: a case with broad involvement of the dorsal fingers and hands. Arch Dermatol 2001; 137:510–511.
- Brik R, Berant M, Vardi P. The scleroderma-like syndrome of insulin-dependent diabetes mellitus. Diabetes Metab Rev 1991; 7:121–128.
- Jelinek JE. Cutaneous manifestations of diabetes mellitus. Int J Dermatol 1994; 33:605–617.
- Huntley AC. The cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol 1989; 7:427–455.
- Jennings AM, Milner PC, Ward JD. Hand abnormalities are associated with the complications of diabetes in type 2 diabetes. Diabet Med 1989; 6:43–47.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care 1983; 6:189–192.
- Kahana M, Grossman E, Feinstein A, Ronnen M, Cohen M, Millet MS. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol 1987; 67:175–177.
- Margolis J, Margolis LS. Skin tags—a frequent sign of diabetes mellitus [letter]. N Engl J Med 1976; 294:1184.
- Garcia Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol 2002; 3:497–506.
- Scheinfeld NS. Obesity and dermatology. Clin Dermatol 2004; 22:303–309.
- Crook MA. Skin tags and the atherogenic lipid profile. J Clin Pathol 2000; 53:873–874.
- Tompkins RR. Skin tags and diabetes. Arch Dermatol 1977; 113:1463.
- Sibbald RG, Landolt SJ, Toth D. Skin and diabetes. Endocrinol Metab Clin North Am 1996; 25:463–472.
- Hermanns-Le T, Scheen A, Pierard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol 2004; 5:199–203.
- Katz RA. Treatment of acanthosis nigricans with oral isotretinoin. Arch Dermatol 1980; 116:110.
- Morton CA, Henderson IS, Jones MC, Lowe JG. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol 1996; 135:671–677.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol 2006; 20:679–688.
- Wilmer WA, Magro CM. Calciphylaxis:emerging concepts in prevention, diagnosis, and treatment. Dialysis 2002; 15:172–186.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc 1966; 41:689.
- Meurer M, Stumvoll M, Szeimies RM. Hautveränderungen bei Diabetes mellitus. Hautartz 2004; 55:428–435.
- Grammer L. Insulin allergy. Clin Rev Allergy 1986; 4:189–200.
- Richardson T, Kerr D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol 2003; 4:661–667.
- Murao S, Hirata K, Ishida T, Takahara J. Lipoatrophy induced by recombinant human insulin injection. Intern Med 1998; 37:1031–1033.
- Barranco R, Herrero T, Tornero P, et al. Systemic allergic reaction by a human insulin analog. Allergy 2003; 58:536–537.
- Burge MR, Carey JD. Vitiligo associated with subcutaneous insulin lispro infusion in type 1 diabetes. Diabetes Care 2004; 27:275–276.
- Durand-Gonzalez KN, Guillausseau N, Pecquet C, Gayno JP. Glargine insulin is not an alternative in insulin allergy. Diabetes Care 2003; 26:2216.
- Blumer IR. Severe injection site reaction to insulin detemir. Diabetes Care 2006; 29:946.
- Darmon P, Castera V, Koeppel MC, Petitjean C, Dutour A. Type III allergy to insulin detemir. Diabetes Care 2005; 28:2980.
- Deerochanawong C, Chandraprasert S. Glimepiride in type 2 diabetes mellitus Thai patients. J Med Assoc Thai 2001; 84:1221–1228.
- Noakes R. Lichenoid drug eruption as a result of the recently released sulfonylurea glimepiride. Australas J Dermatol 2003; 44:302–303.
- Koca R, Altinyazar HC, Yenidünya S, Tekin NS. Psoriasiform [sic] drug eruption associated with metformin hydrochloride: a case report. Dermatol Online J 2003; 9:11. http://dermatology.cdlib.org/93/case_reports/metformin/koca.html. Accessed July 30, 2008.
- Burger DE, Goyal S. Erythema multiforme from metformin. Ann Pharmacother 2004; 38:1537.
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. BMJ (Clin Res Ed) 1986; 293:483.
- Ben Salem C, Hmouda H, Slim R, Denguezli M, Belajouza C, Bouraoui K. Rare case of metformin-induced leucocytoclastic vasculitis. Ann Pharmacother 2006; 40:1685–1687.
- Litt JZ. Litt’s Drug Eruption Reference Manual. London: Taylor and Francis, 2001.
- Balfour JA, McTavish D. Acarbose: an update of its pharmacology and therapeutic use in diabetes mellitus. Drugs 1993; 46:1024–1054. Erratum in: Drugs 1994; 48:929.
- Kono T, Hayami M, Kobayashi H, Ishii M, Taniguchi S. Acarbose-induced generalized erythema multiforme. Lancet 1999; 354:396–397.
- Ahr HJ, Boberg M, Krause HP, et al. Pharmacokinetics of acarbose. Part 1: Absorption, concentration in plasma, metabolism, and excretion after single administration of 14C acarbose to rats, dogs and man. Arzneimittelforschung 1989; 39:1254–1260.
- Poszepczynska-Guigné E, Viguier M, Assier H, Pinquier L, Hochedez P, Dubertret L. Acute generalized exanthematous pustulosis induced by drugs with low-digestive absorption: acarbose and nystatin. Ann Dermatol Venereol 2003; 130:439–442.
- Landau J, Davis E. The small blood-vessels of the conjunctiva and nail bed in diabetes mellitus. Lancet 1960; 2:731
- Huntley A. Diabetes mellitus: review. Dermatology Online Journal 1995; vol 1(2). http://dermatology.cdlib.org/DOJvol1num2/diabetes/dmreview.html. Accessed July 30, 2008.
- Ahmed I, Goldstein B. Diabetes mellitus. Clin Dermatol 2006; 24:237–246.
- Petzelbauer P, Wolff K, Tappeiner G. Necrobiosis lipoidica: treatment with systemic corticoids. Br J Dermatol 1992; 126:542.
- Sibbald RG, Schachter RK. The skin and diabetes mellitus. Int J Dermatol 1984; 23:567–584.
- Ferringer T, Miller F. Cutaneous manifestations of diabetes mellitus. Dermatol Clin 2002; 20:483–492.
- Cohen O, Yaniv R, Karasik A, Trau H. Necrobiosis lipoidica and diabetic control revisited. Med Hypotheses 1996; 46:348–350.
- Nguyen K, Washenik K, Shupak J. Necrobiosis lipoidica diabeticorum treated with chloroquine. J Am Acad Dermatol 2002; 46 suppl 2:34–36.
- Stanway A, Rademaker M, Newman P. Healing of severe ulcerative necrobiosis lipoidica with cyclosporine. Australas J Dermatol 2004; 45:119–122.
- Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab 2003; 88:2983–2992.
- Forschner T, Buchholtz S, Stockfleth E. Current state of vitiligo therapy-evidence-based analysis of the literature. J Dtsch Dermatol Ges 2007; 5:467–475.
- Petrou-Amerikanou C, Markopoulos AK, Belazi M, Karamitsos D, Papanayotou P. Prevalence of oral lichen planus in diabetes mellitus according to the type of diabetes. Oral Dis 1998; 4:37–40.
- Mobini N, Toussaint S, Kamino H. Noninfectious, erythematous, papular, and squamous diseases. In:Elder DE, editor. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2005:179–214.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK. Characteristic changes of skin and its accessories in type 2 diabetes mellitus. Georgian Med News 2006; 131:43–46.
- Lithner F, Hietala S-O. Skeletal lesions of the feet in diabetics and their relationship to cutaneous erythema with or without necrosis of the feet. Acta Med Scand 1976; 200:155–161.
- Collier A, Matthews DM, Kellett HA, Clarke BF, Hunter JA. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1986; 292:936.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol 1994; 30:519–530.
- Libecco JF. Finger pebbles and diabetes: a case with broad involvement of the dorsal fingers and hands. Arch Dermatol 2001; 137:510–511.
- Brik R, Berant M, Vardi P. The scleroderma-like syndrome of insulin-dependent diabetes mellitus. Diabetes Metab Rev 1991; 7:121–128.
- Jelinek JE. Cutaneous manifestations of diabetes mellitus. Int J Dermatol 1994; 33:605–617.
- Huntley AC. The cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol 1989; 7:427–455.
- Jennings AM, Milner PC, Ward JD. Hand abnormalities are associated with the complications of diabetes in type 2 diabetes. Diabet Med 1989; 6:43–47.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care 1983; 6:189–192.
- Kahana M, Grossman E, Feinstein A, Ronnen M, Cohen M, Millet MS. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol 1987; 67:175–177.
- Margolis J, Margolis LS. Skin tags—a frequent sign of diabetes mellitus [letter]. N Engl J Med 1976; 294:1184.
- Garcia Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol 2002; 3:497–506.
- Scheinfeld NS. Obesity and dermatology. Clin Dermatol 2004; 22:303–309.
- Crook MA. Skin tags and the atherogenic lipid profile. J Clin Pathol 2000; 53:873–874.
- Tompkins RR. Skin tags and diabetes. Arch Dermatol 1977; 113:1463.
- Sibbald RG, Landolt SJ, Toth D. Skin and diabetes. Endocrinol Metab Clin North Am 1996; 25:463–472.
- Hermanns-Le T, Scheen A, Pierard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol 2004; 5:199–203.
- Katz RA. Treatment of acanthosis nigricans with oral isotretinoin. Arch Dermatol 1980; 116:110.
- Morton CA, Henderson IS, Jones MC, Lowe JG. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol 1996; 135:671–677.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol 2006; 20:679–688.
- Wilmer WA, Magro CM. Calciphylaxis:emerging concepts in prevention, diagnosis, and treatment. Dialysis 2002; 15:172–186.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc 1966; 41:689.
- Meurer M, Stumvoll M, Szeimies RM. Hautveränderungen bei Diabetes mellitus. Hautartz 2004; 55:428–435.
- Grammer L. Insulin allergy. Clin Rev Allergy 1986; 4:189–200.
- Richardson T, Kerr D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol 2003; 4:661–667.
- Murao S, Hirata K, Ishida T, Takahara J. Lipoatrophy induced by recombinant human insulin injection. Intern Med 1998; 37:1031–1033.
- Barranco R, Herrero T, Tornero P, et al. Systemic allergic reaction by a human insulin analog. Allergy 2003; 58:536–537.
- Burge MR, Carey JD. Vitiligo associated with subcutaneous insulin lispro infusion in type 1 diabetes. Diabetes Care 2004; 27:275–276.
- Durand-Gonzalez KN, Guillausseau N, Pecquet C, Gayno JP. Glargine insulin is not an alternative in insulin allergy. Diabetes Care 2003; 26:2216.
- Blumer IR. Severe injection site reaction to insulin detemir. Diabetes Care 2006; 29:946.
- Darmon P, Castera V, Koeppel MC, Petitjean C, Dutour A. Type III allergy to insulin detemir. Diabetes Care 2005; 28:2980.
- Deerochanawong C, Chandraprasert S. Glimepiride in type 2 diabetes mellitus Thai patients. J Med Assoc Thai 2001; 84:1221–1228.
- Noakes R. Lichenoid drug eruption as a result of the recently released sulfonylurea glimepiride. Australas J Dermatol 2003; 44:302–303.
- Koca R, Altinyazar HC, Yenidünya S, Tekin NS. Psoriasiform [sic] drug eruption associated with metformin hydrochloride: a case report. Dermatol Online J 2003; 9:11. http://dermatology.cdlib.org/93/case_reports/metformin/koca.html. Accessed July 30, 2008.
- Burger DE, Goyal S. Erythema multiforme from metformin. Ann Pharmacother 2004; 38:1537.
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. BMJ (Clin Res Ed) 1986; 293:483.
- Ben Salem C, Hmouda H, Slim R, Denguezli M, Belajouza C, Bouraoui K. Rare case of metformin-induced leucocytoclastic vasculitis. Ann Pharmacother 2006; 40:1685–1687.
- Litt JZ. Litt’s Drug Eruption Reference Manual. London: Taylor and Francis, 2001.
- Balfour JA, McTavish D. Acarbose: an update of its pharmacology and therapeutic use in diabetes mellitus. Drugs 1993; 46:1024–1054. Erratum in: Drugs 1994; 48:929.
- Kono T, Hayami M, Kobayashi H, Ishii M, Taniguchi S. Acarbose-induced generalized erythema multiforme. Lancet 1999; 354:396–397.
- Ahr HJ, Boberg M, Krause HP, et al. Pharmacokinetics of acarbose. Part 1: Absorption, concentration in plasma, metabolism, and excretion after single administration of 14C acarbose to rats, dogs and man. Arzneimittelforschung 1989; 39:1254–1260.
- Poszepczynska-Guigné E, Viguier M, Assier H, Pinquier L, Hochedez P, Dubertret L. Acute generalized exanthematous pustulosis induced by drugs with low-digestive absorption: acarbose and nystatin. Ann Dermatol Venereol 2003; 130:439–442.
KEY POINTS
- Patients with type 2 diabetes more often develop skin infections, whereas those with type 1 more often have autoimmune-related lesions.
- Insulin signaling supports normal skin proliferation, differentiation, and maintenance, and a lack of insulin may lead to impaired wound healing, which may affect insulin resorption.
- Skin manifestations of diabetes may also serve as ports of entry for secondary infection.
- A candidal infection (moniliasis) can be an early sign of undiagnosed diabetes.
- Watch for dermal side effects of insulin injections and oral hypoglycemic drugs.