User login
Fat Embolism Syndrome With Cerebral Fat Embolism Associated With Long-Bone Fracture
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
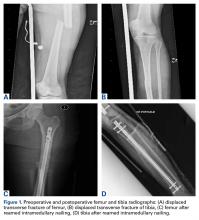
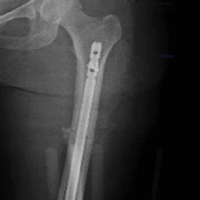
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
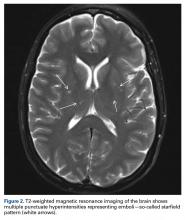
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
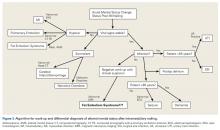
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
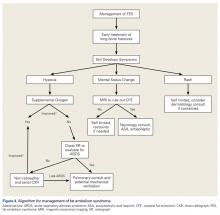
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.