User login
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
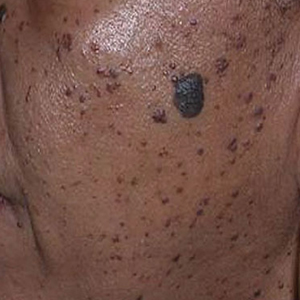
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3
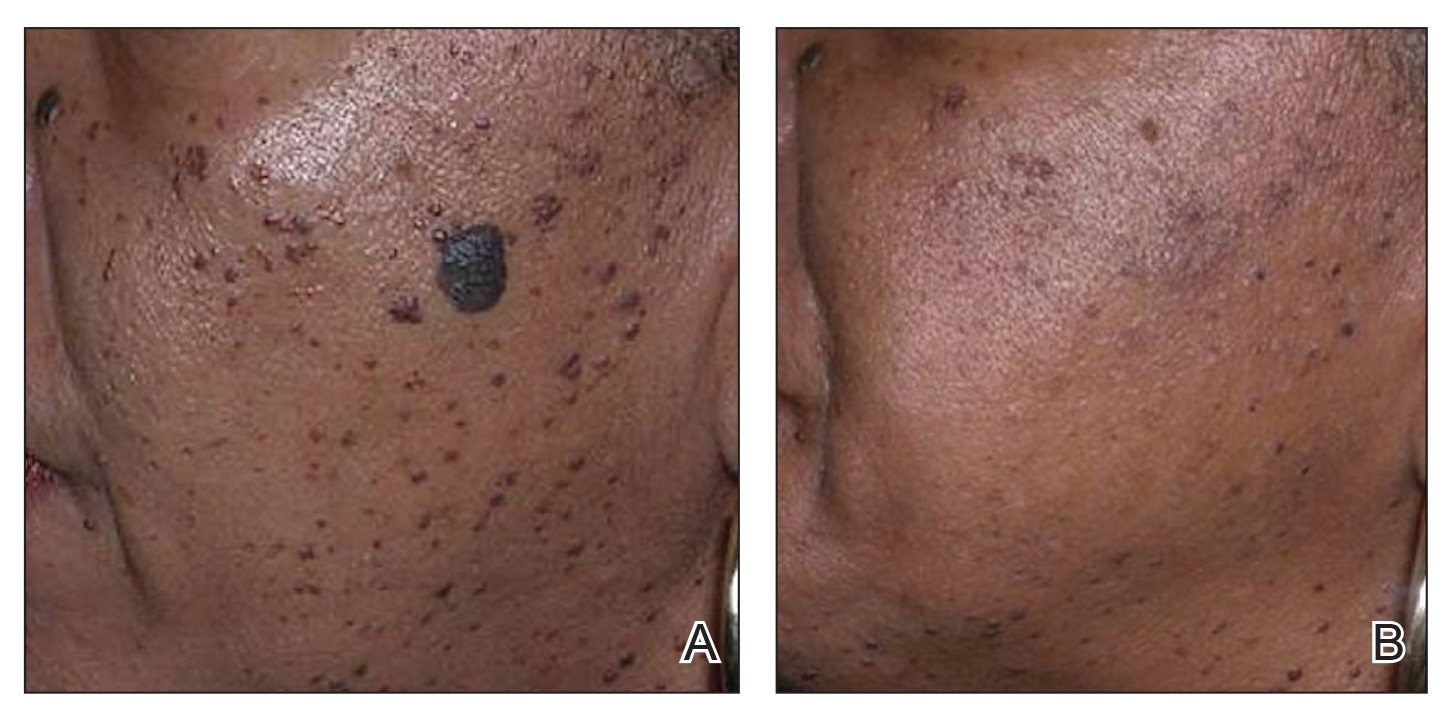
Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
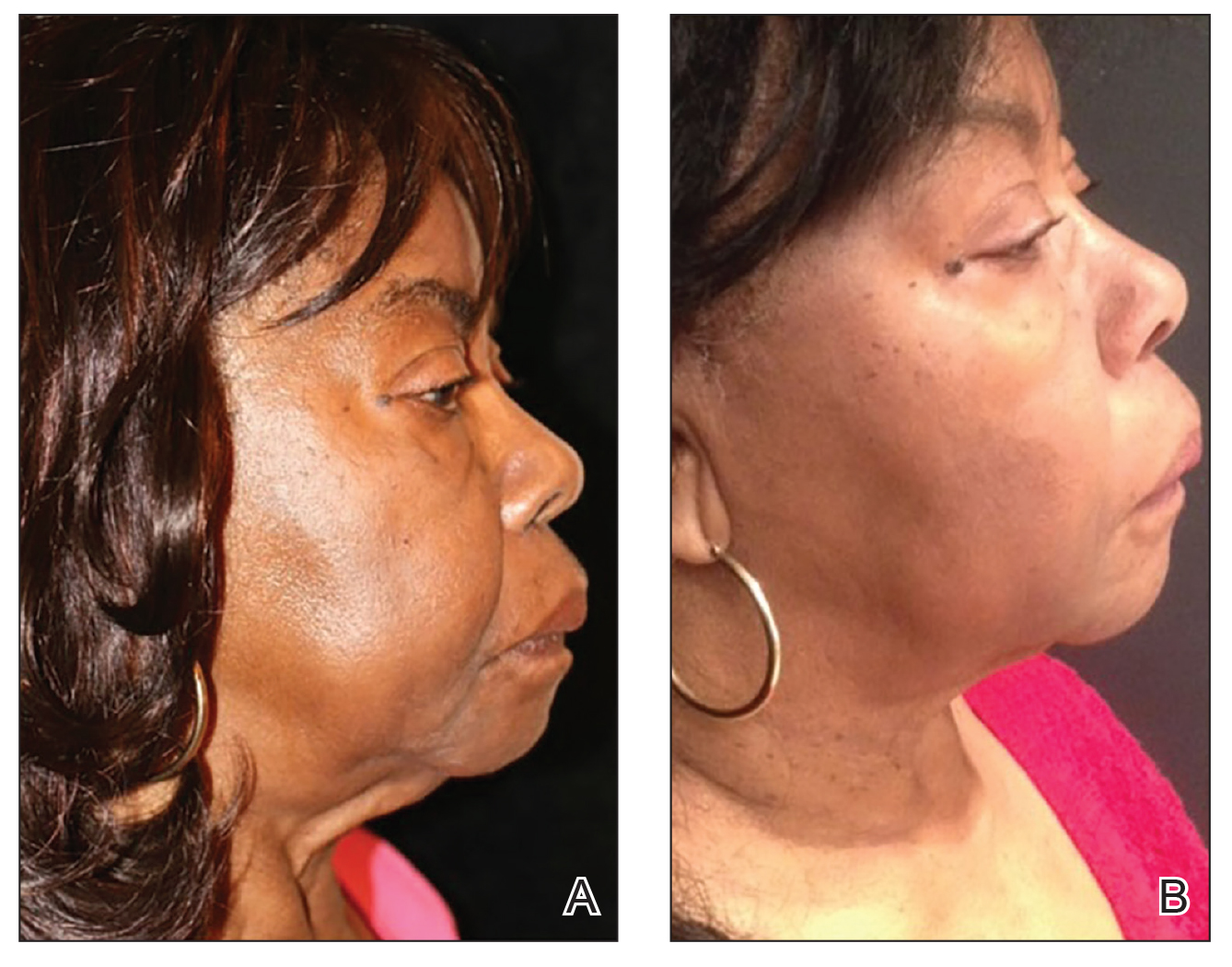
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.
Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3

Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.

Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3

Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.

Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
- Mazzoni D, Lin MJ, Dubin DP, et al. Review of non-invasive body contouring devices for fat reduction, skin tightening and muscle definition. Australas J Dermatol. 2019;60:278-283. doi:10.1111/ajd.13090
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083. doi:10.1111/j.1524-4725.2009.01186.x&
- Schweiger ES, Kwasniak L, Aires DJ. Treatment of dermatosis papulosa nigra with a 1064 nm Nd:YAG laser: report of two cases. J Cosmet Laser Ther. 2008;10:120-122. doi:10.1080/14764170801950070
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438. doi:10.1002/lsm.20048
- Alajlan AM, Alsuwaidan SN. Acne scars in ethnic skin treated with both non-ablative fractional 1,550 nm and ablative fractional CO2 lasers: comparative retrospective analysis with recommended guidelines. Lasers Surg Med. 2011;43effi:787-791. doi:10.1002/lsm.21092
- Ke R, Cai B, Ni X, et al. Efficacy and safety of non-ablative vs. ablative lasers for acne scarring: a meta-analysis. J Deutschen Dermatologischen Gesellschaft. Published online March 11, 2025. doi: 10.1111/ddg.15651
- Goel A, Krupashankar DS, Aurangabadkar S, et al. Fractional lasers in dermatology—current status and recommendations. Indian J Dermatol Venereol Leprol. 2011;77:369. doi:10.4103/0378-6323.79732
- Lee HS, Lee JH, Ahn GY, et al. Fractional photothermolysis for the treatment of acne scars: a report of 27 Korean patients. J Dermatolog Treat. 2008;19:45-49. doi:10.1080/09546630701691244
- Zhang AD, Clovie J, Lazar M, et al. Treatment of benign pigmented lesions using lasers: a scoping review. J Clin Med. 2025;14li:3985. doi:10.3390/jcm14113985
- Lipper GM, Perez M. Nonablative acne scar reduction after a series of treatments with a short-pulsed 1,064-nm neodymium:YAG laser. Dermatol Surg. 2006;32:998-1006. doi:10.1111/j.1524-4725.2006.32222.x
- Mar K, Khalid B, Maazi M, et al. Treatment of post-inflammatory hyperpigmentation in skin of colour: a systematic review. J Cutan Med Surg. 2024;28:473-480. doi:10.1177/12034754241265716
- Kono T, Chan HH, Groff WF, et al. Prospective direct comparison study of fractional resurfacing using different fluences and densities for skin rejuvenation in Asians. Lasers Surg Med. 2007;39:311-314. doi:10.1002/lsm.20484
- Sharkey JR, Sharf BF, St John JA. “Una persona derechita (staying right in the mind)”: perceptions of Spanish-speaking Mexican American older adults in South Texas colonias. Gerontologist. 2009;49 suppl 1:S79-85. doi:10.1093/geront/gnp086
- Wu X, Cen Q, Jin J, et al. An effective and safe laser treatment strategy of fractional carbon dioxide laser for Chinese populations with periorbital wrinkles: a randomized split-face trial. Dermatol Therapy. 2025;15:1307-1317.
- Milante RR, Doria-Ruiz MJ, Beloso MB, et al. Split-face comparison of grid fractional radiofrequency vs 1064-nm Nd-YAG laser treatment of periorbital rhytides among Filipino patients. Dermatol Ther. 2020;33:e14031. doi:10.1111/dth.14031
- Alexis AF, Andriessen A, Beach RA, et al. Periprocedural skincare for nonenergy and nonablative energy-based aesthetic procedures in patients with skin of color. J Cosmet Dermatol. 2025;24:E16712. doi:10.1111/jocd.16712
- Marmon S, Shek SYN, Yeung CK, et al. Evaluating the safety and efficacy of the 1,440-nm laser in the treatment of photodamage in Asian skin. Lasers Surg Med. 2014;46:375-379. doi:10.1002/lsm.22242
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118. doi:10.1016/j.jaad.2012.08.041
- Jih MH, Goldberg LH, Kimyai-Asadi A. Fractional photothermolysis for photoaging of hands. Dermatol Surg. 2008;34:73-78. doi:10.1111/j.1524-4725.2007.34011.x
- Prohaska J, Hohman MH. Laser complications. StatPearls. Updated August 28, 2023. Accessed July 23, 2025. http://www.ncbi.nlm.nih.gov/books/NBK532248/
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20. doi:10.1016/j.ijwd.2017.01.004
- Brauer JA, Kazlouskaya V, Alabdulrazzaq H, et al. Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol. 2015;151:278-284. doi:10.1001/jamadermatol.2014.3045
- Greywal T, Ortiz A. Treating melasma with the 1064 nm Nd:YAG laser with a 650-microsecond pulse duration: a clinical evaluation. J Cosmet Dermatol. 2021;20:3889-3892. doi:10.1111/jocd.14558
- Weaver SM, Sagaral EC. Treatment of pseudofolliculitis barbae using the long-pulse Nd:YAG laser on skin types V and VI. Dermatol Surg. 2003;29:1187-1191. doi:10.1111/j.1524-4725.2003.29387.x
- Negishi K, Tanaka S, Tobita S. Prospective, randomized, evaluator-blinded study of the long pulse 532-nm KTP laser alone or in combination with the long pulse 1064-nm Nd:YAG laser on facial rejuvenation in Asian skin. Lasers Surg Med. 2016;48:844-851. doi:10.1002/lsm.22582
- Kaushik S, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthetic Dermatol. 2017;10:51-67.
- Garg S, Vashisht KR, Garg D, et al. Advancements in laser therapies for dermal hyperpigmentation in skin of color: a comprehensive literature review and experience of sequential laser treatments in a cohort of 122 Indian patients. J Clin Med. 2024;13:2116. doi:10.3390/jcm13072116
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:e36727. doi:10.5812/ijem.36727
- Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79-93. doi:10.1111/j.1467-2494.2006.00302.x
- El-Domyati M, El-Ammawi TS, Medhat W, et al. Radiofrequency facial rejuvenation: Evidence-based effect. J Am Acad Dermatol. 2011;64:524-535. doi:10.1016/j.jaad.2010.06.045
- US Food and Drug Administration. Non-invasive body contouring technologies. Published December 7, 2022. Accessed July 23, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/non-invasive-body-contouring-technologies
- Robinson DM, Kaminer MS, Baumann L, et al. High-intensity focused ultrasound for the reduction of subcutaneous adipose tissue using multiple treatment techniques. Dermatol Surg. 2014;40:641-651. doi:10.1111/dsu.0000000000000022
- Biskanaki F, Tertipi N, Sfyri E, et al. Complications and risks of high-intensity focused ultrasound (HIFU) in esthetic procedures: a review. Applied Sciences. 2025;15:4958. doi:10.3390/app15094958
- Lu PH, Yang CH, Chang YC. Quantitative analysis of face and neck skin tightening by microfocused ultrasound with visualization in Asians. Dermatol Surg. 2017;43:1332-1338. doi:10.1097/DSS.0000000000001181
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708. doi:10.1002/lsm.20864
- Nishikawa A, Aikawa Y. Quantitative assessment of the cryolipolysis method for body contouring in Asian patients. Clin Cosmet Investig Dermatol. 2021;14:1773-1781. doi:10.2147/CCID.S337487
- Bass LS, Doherty ST. Safety and efficacy of a non-invasive 1060 nm diode laser for fat reduction of the abdomen. J Drugs Dermatol. 2018;17:106-112
- Shome D, Khare S, Kapoor R. The use of deoxycholic acid for the clinical reduction of excess submental fat in Indian patients. J Drugs Dermatol. 2019;18:266-272.
- Goodman GJ, Ho WWS, Chang KJ, et al. Efficacy of a novel injection lipolysis to induce targeted adipocyte apoptosis: a randomized, phase IIa study of CBL-514 injection on abdominal subcutaneous fat reduction. Aesthetic Surg J. 2022;42:NP662-NP674. doi:10.1093/asj/sjac162
- McDaniel D, Lozanova P. Human adipocyte apoptosis immediately following high frequency focused field radio frequency: case study.J Drugs Dermatol. 2015;14:622-623.
- Fritz K, Samková P, Salavastru C, et al. A novel selective RF applicator for reducing thigh circumference: a clinical evaluation. Dermatol Ther. 2016;29:92-95. doi:10.1111/dth.12304
- Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg Med. 2019;51:40-46. doi:10.1002/lsm.23024
- Negosanti F, Cannarozzo G, Zingoni T, et al. Is it possible to reshape the body and tone it at the same time? Schwarzy: the new technology for body sculpting. Bioengineering (Basel). 2022;9:284. doi:10.3390/bioengineering9070284
PRACTICE POINTS
- Nonablative fractional lasers are preferred for acne scars in skin of color (SOC), minimizing hyperpigmentation risk.
- The 1064-nm Nd:YAG and picosecond lasers are safe and effective when used with SOC-appropriate settings.
- Photoprotection and topical lightening agents reduce postprocedure pigmentation risks.
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Keloid Pathogenesis and Therapeutics
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
Central Centrifugal Cicatricial Alopecia in Males: Analysis of Time to Diagnosis and Disease Severity
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.
Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.


Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.


Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
Practice Points
- Most males with central centrifugal cicatricial alopecia (CCCA) experience considerable diagnostic delays and typically present to dermatology with late-stage disease.
- Dermatologists should consider CCCA in the differential diagnosis for adult Black males with alopecia.
- More research is needed to explore advanced CCCA in males, including factors limiting timely diagnosis and the impact on quality of life in this population.
Telemedicine Alopecia Assessment: Highlighting Patients With Skin of Color
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.

Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.

Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6

• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.

• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.

Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6

• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.

• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Increasing Skin of Color Publications in the Dermatology Literature: A Call to Action
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
Practice Points
- Submitting more articles related to skin of color for peer review and publication will increase educational opportunities.
- Journals that publish skin of color articles play a critical role in reducing educational gaps and ultimately help improve patient care for those with skin of color.
Multiethnic Training in Residency: A Survey of Dermatology Residents
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Practice Points
- To treat the ever-changing demographics of patients in the United States, dermatologists must receive adequate exposure and education regarding dermatologic conditions in patients from various ethnic backgrounds.
- Dermatology residents from less diverse regions are more likely to agree that dedicated clinics and rotations are important to gain competence compared to those from more diverse regions.
- In areas with less diversity, dedicated multiethnic skin clinics and faculty may be more important for assuring an adequate residency experience.
Hair Care Products Used by Women of African Descent: Review of Ingredients
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.
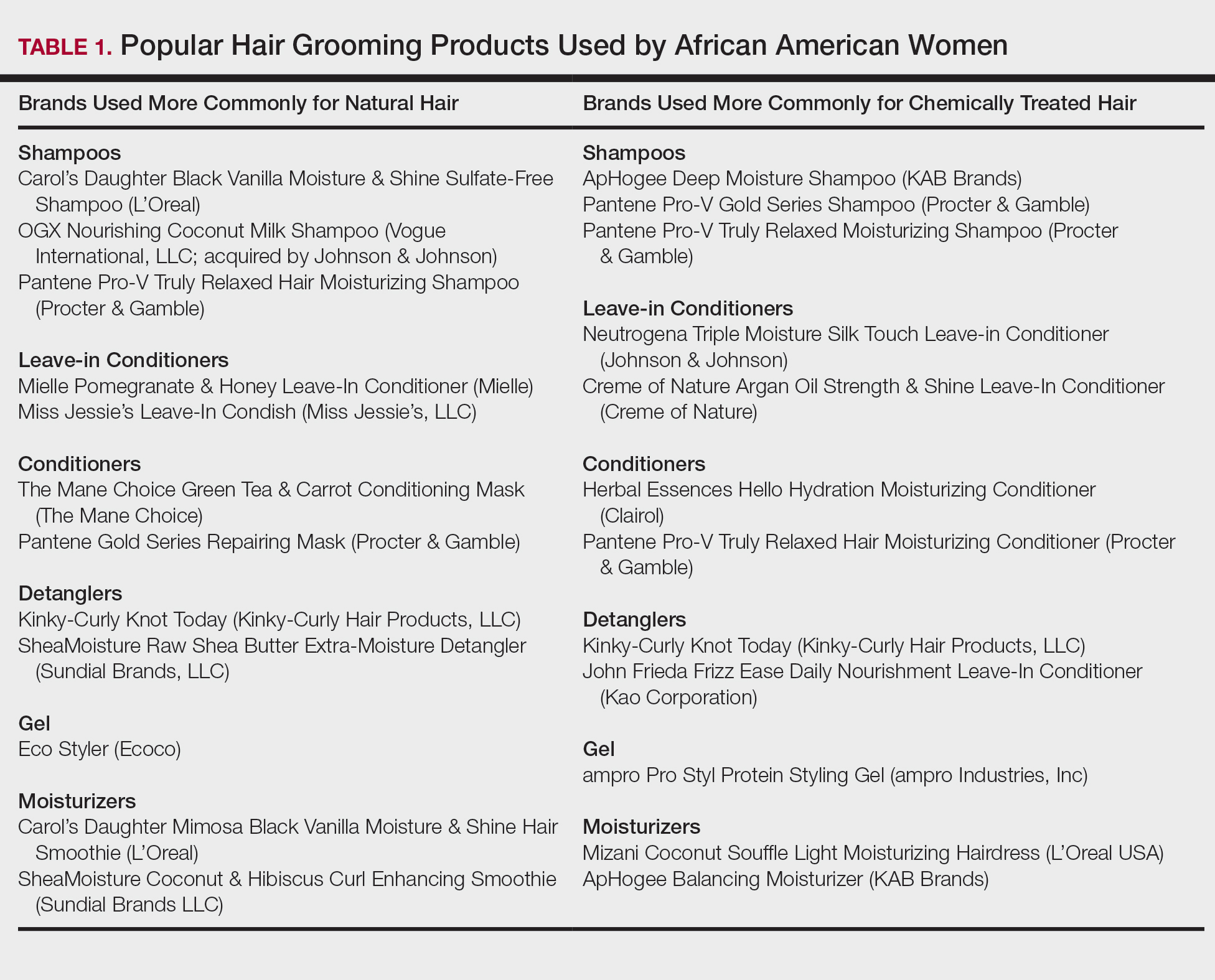
Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.

Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.

Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
Practice Points
- Dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent patient interactions and treatment.
- Common ingredients in popular hair care products used by African Americans include sulfates, cationic surfactants and polymers, silicone, oils, and parabens.
Racial Limitations of Fitzpatrick Skin Type
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20
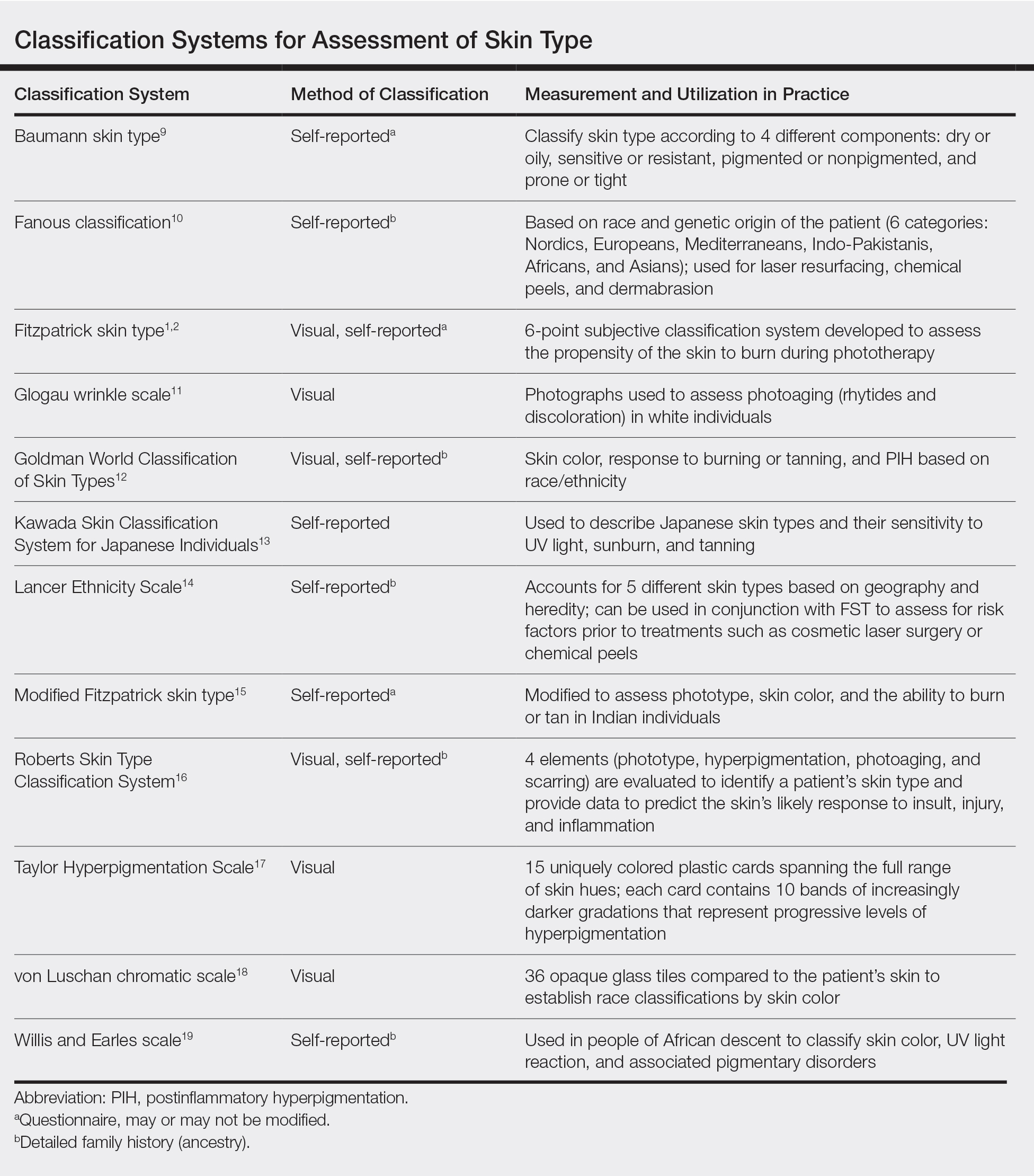
Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Practice Points
- Medical providers should be cognizant of conflating race and ethnicity with Fitzpatrick skin type (FST).
- Misuse of FST may occur more frequently among physicians who do not identify as having skin of color.
- Although alternative skin type classification systems have been proposed, more clinically relevant methods for describing skin of color need to be developed.