User login
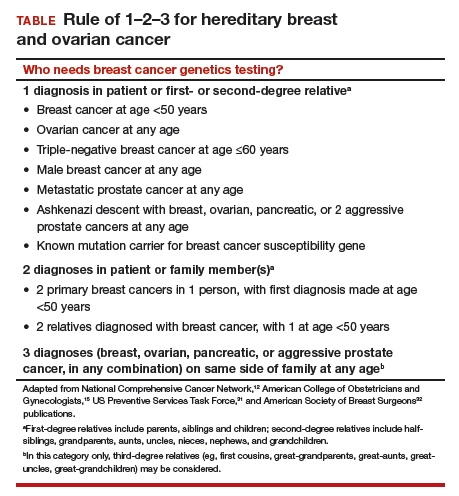
Who needs breast cancer genetics testing?
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Take-home points
- The best genetics test is a good family history, updated annually
- Each year, 35,000 breast cancers are attributable to hereditary risk
- It is crucial to identify families at risk for hereditary breast cancer early, as cancers may begin in a woman's 30s; screening begins at age 25
- Multigene panel testing is efficient and cost-effective
- For patients who have highly penetrant pathogenic variants and are of childbearing age, preimplantation genetics diagnosis is an option
Should breast cancer screening guidelines be tailored to a patient’s race and ethnicity?
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
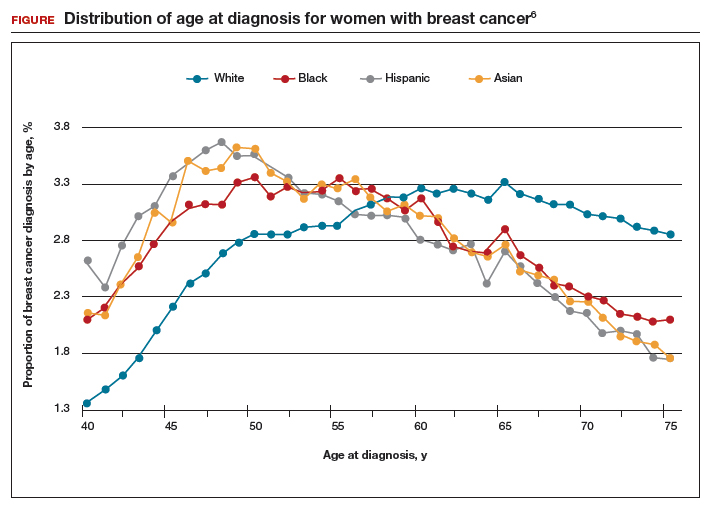
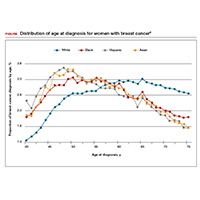
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.
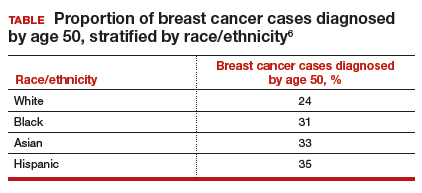
Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).
These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.
Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).
These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.
Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).
These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
Inside the complex, surprising world of MS comorbidities
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
EXPERT ANALYSIS FROM THE CMSC ANNUAL MEETING
iPad app puts cognitive screening in the hands of MS patients
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
REPORTING FROM THE CMSC ANNUAL MEETING
Dr Jame Abraham's top ASCO selections in breast cancer
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
CAR T therapy to enter early testing in multiple myeloma
Janssen Biotech is launching a phase 1b/2 trial of an .
The trial, which was cleared by the Food and Drug Administration to begin in the second half of 2018, will evaluate the safety and efficacy of LCAR-B38M (JNJ-68284528). The CAR T therapy targets B-cell Maturation Antigen and expresses a CAR protein that is identical to a product that was developed by Legend Biotech and evaluated in a first-in-human clinical study in China.
The drug is being developed as part of a collaboration between Legend Biotech and Janssen Biotech.
Janssen Biotech is launching a phase 1b/2 trial of an .
The trial, which was cleared by the Food and Drug Administration to begin in the second half of 2018, will evaluate the safety and efficacy of LCAR-B38M (JNJ-68284528). The CAR T therapy targets B-cell Maturation Antigen and expresses a CAR protein that is identical to a product that was developed by Legend Biotech and evaluated in a first-in-human clinical study in China.
The drug is being developed as part of a collaboration between Legend Biotech and Janssen Biotech.
Janssen Biotech is launching a phase 1b/2 trial of an .
The trial, which was cleared by the Food and Drug Administration to begin in the second half of 2018, will evaluate the safety and efficacy of LCAR-B38M (JNJ-68284528). The CAR T therapy targets B-cell Maturation Antigen and expresses a CAR protein that is identical to a product that was developed by Legend Biotech and evaluated in a first-in-human clinical study in China.
The drug is being developed as part of a collaboration between Legend Biotech and Janssen Biotech.
Does Age of Exposure to Tackle Football Affect CTE Severity?
Younger age of exposure to tackle football is not associated with chronic traumatic encephalopathy (CTE) pathologic severity, Alzheimer’s disease pathology, or Lewy body pathology, according to data published online ahead of print April 30 in Annals of Neurology. Younger age of exposure does appear to predict earlier neurobehavioral symptom onset, however, the authors said.
“These findings suggest that exposure to repetitive head impacts from tackle football as a youth may reduce resiliency to diseases, including, but not limited to, CTE, that affect the brain in later life,” said Michael L. Alosco, PhD, Assistant Professor of Neurology at the the Boston University Alzheimer’s Disease and CTE Center. “This study adds to growing research suggesting that incurring repeated head impacts through tackle football in earlier life can lead to both short-term and long-term effects on the brain.”
Repetitive Head Impacts and Neurodevelopment
Previous research has linked younger age of first exposure to tackle football with smaller thalamic volume in former National Football League players. A recent study of 214 former and amateur football players found that age of first exposure to tackle football—before age 12, in particular—predicted increased odds of self-reported neuropsychiatric and executive impairment.
“Youth exposure to repetitive head impacts may disrupt neurodevelopment to lower the threshold for later clinical dysfunction,” said the researchers.
To examine the effect of age of first exposure to tackle football on CTE pathologic severity and age of neurobehavioral symptom onset in tackle football players with neuropathologically confirmed CTE, Dr. Alosco and colleagues analyzed a sample of 246 amateur and professional tackle football players whose brains had been donated to the Veteran’s Affairs–Boston University–Concussion Legacy Foundation Brain Bank. The researchers interviewed informants to ascertain players’ age of first exposure and age of onset of cognitive, behavioral, or mood symptoms. A total of 211 football players were diagnosed with CTE; 35 did not have CTE. Of the 211 participants with CTE, 126 had CTE only, and the other participants had comorbid neurodegenerative diseases.
Onset of Cognitive, Behavioral, and Mood Symptoms
Of the 211 participants with CTE, 183 developed cognitive and behavioral or mood symptoms prior to death, eight had only cognitive symptoms, 12 had only behavioral or mood symptoms, and seven did not endorse any symptoms examined in the study. Clinical data for one participant were not available.
Among tackle football players with CTE, every one year younger that they began to play tackle football predicted earlier onset of cognitive symptoms by 2.44 years and of behavioral or mood symptoms by 2.50 years. Exposure before age 12 predicted earlier cognitive and behavioral or mood symptom onset by 13.39 years and 13.28 years, respectively.
Secondary subset analyses indicated that younger age of exposure to tackle football was associated with earlier onset of functional impairment in participants who were determined to have had dementia. Researchers observed nearly identical effects in participants with CTE only.
Study limitations include the lack of an appropriate control or comparison group, the researchers noted. In addition, the results may not be generalizable to a broader tackle football population.
“Given the growing public health concerns for participation in tackle football, prospective studies of former tackle football players that include objective clinical assessments are needed to better understand the relationship between youth tackle football exposure and long-term neurobehavioral outcomes,” said the researchers.
“More research on this topic is needed before any clinical recommendations, as well as recommendations on policy or rule changes, can be made,” said Dr. Alosco.
“Boston University and sites across the country are currently conducting longitudinal studies on former football players, which will allow us to begin to study cognition and behavior and mood functioning over time.”
—Erica Tricarico
Suggested Reading
Alosco ML, Mez J, Tripodis Y, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018 Apr 30 [Epub ahead of print].
Younger age of exposure to tackle football is not associated with chronic traumatic encephalopathy (CTE) pathologic severity, Alzheimer’s disease pathology, or Lewy body pathology, according to data published online ahead of print April 30 in Annals of Neurology. Younger age of exposure does appear to predict earlier neurobehavioral symptom onset, however, the authors said.
“These findings suggest that exposure to repetitive head impacts from tackle football as a youth may reduce resiliency to diseases, including, but not limited to, CTE, that affect the brain in later life,” said Michael L. Alosco, PhD, Assistant Professor of Neurology at the the Boston University Alzheimer’s Disease and CTE Center. “This study adds to growing research suggesting that incurring repeated head impacts through tackle football in earlier life can lead to both short-term and long-term effects on the brain.”
Repetitive Head Impacts and Neurodevelopment
Previous research has linked younger age of first exposure to tackle football with smaller thalamic volume in former National Football League players. A recent study of 214 former and amateur football players found that age of first exposure to tackle football—before age 12, in particular—predicted increased odds of self-reported neuropsychiatric and executive impairment.
“Youth exposure to repetitive head impacts may disrupt neurodevelopment to lower the threshold for later clinical dysfunction,” said the researchers.
To examine the effect of age of first exposure to tackle football on CTE pathologic severity and age of neurobehavioral symptom onset in tackle football players with neuropathologically confirmed CTE, Dr. Alosco and colleagues analyzed a sample of 246 amateur and professional tackle football players whose brains had been donated to the Veteran’s Affairs–Boston University–Concussion Legacy Foundation Brain Bank. The researchers interviewed informants to ascertain players’ age of first exposure and age of onset of cognitive, behavioral, or mood symptoms. A total of 211 football players were diagnosed with CTE; 35 did not have CTE. Of the 211 participants with CTE, 126 had CTE only, and the other participants had comorbid neurodegenerative diseases.
Onset of Cognitive, Behavioral, and Mood Symptoms
Of the 211 participants with CTE, 183 developed cognitive and behavioral or mood symptoms prior to death, eight had only cognitive symptoms, 12 had only behavioral or mood symptoms, and seven did not endorse any symptoms examined in the study. Clinical data for one participant were not available.
Among tackle football players with CTE, every one year younger that they began to play tackle football predicted earlier onset of cognitive symptoms by 2.44 years and of behavioral or mood symptoms by 2.50 years. Exposure before age 12 predicted earlier cognitive and behavioral or mood symptom onset by 13.39 years and 13.28 years, respectively.
Secondary subset analyses indicated that younger age of exposure to tackle football was associated with earlier onset of functional impairment in participants who were determined to have had dementia. Researchers observed nearly identical effects in participants with CTE only.
Study limitations include the lack of an appropriate control or comparison group, the researchers noted. In addition, the results may not be generalizable to a broader tackle football population.
“Given the growing public health concerns for participation in tackle football, prospective studies of former tackle football players that include objective clinical assessments are needed to better understand the relationship between youth tackle football exposure and long-term neurobehavioral outcomes,” said the researchers.
“More research on this topic is needed before any clinical recommendations, as well as recommendations on policy or rule changes, can be made,” said Dr. Alosco.
“Boston University and sites across the country are currently conducting longitudinal studies on former football players, which will allow us to begin to study cognition and behavior and mood functioning over time.”
—Erica Tricarico
Suggested Reading
Alosco ML, Mez J, Tripodis Y, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018 Apr 30 [Epub ahead of print].
Younger age of exposure to tackle football is not associated with chronic traumatic encephalopathy (CTE) pathologic severity, Alzheimer’s disease pathology, or Lewy body pathology, according to data published online ahead of print April 30 in Annals of Neurology. Younger age of exposure does appear to predict earlier neurobehavioral symptom onset, however, the authors said.
“These findings suggest that exposure to repetitive head impacts from tackle football as a youth may reduce resiliency to diseases, including, but not limited to, CTE, that affect the brain in later life,” said Michael L. Alosco, PhD, Assistant Professor of Neurology at the the Boston University Alzheimer’s Disease and CTE Center. “This study adds to growing research suggesting that incurring repeated head impacts through tackle football in earlier life can lead to both short-term and long-term effects on the brain.”
Repetitive Head Impacts and Neurodevelopment
Previous research has linked younger age of first exposure to tackle football with smaller thalamic volume in former National Football League players. A recent study of 214 former and amateur football players found that age of first exposure to tackle football—before age 12, in particular—predicted increased odds of self-reported neuropsychiatric and executive impairment.
“Youth exposure to repetitive head impacts may disrupt neurodevelopment to lower the threshold for later clinical dysfunction,” said the researchers.
To examine the effect of age of first exposure to tackle football on CTE pathologic severity and age of neurobehavioral symptom onset in tackle football players with neuropathologically confirmed CTE, Dr. Alosco and colleagues analyzed a sample of 246 amateur and professional tackle football players whose brains had been donated to the Veteran’s Affairs–Boston University–Concussion Legacy Foundation Brain Bank. The researchers interviewed informants to ascertain players’ age of first exposure and age of onset of cognitive, behavioral, or mood symptoms. A total of 211 football players were diagnosed with CTE; 35 did not have CTE. Of the 211 participants with CTE, 126 had CTE only, and the other participants had comorbid neurodegenerative diseases.
Onset of Cognitive, Behavioral, and Mood Symptoms
Of the 211 participants with CTE, 183 developed cognitive and behavioral or mood symptoms prior to death, eight had only cognitive symptoms, 12 had only behavioral or mood symptoms, and seven did not endorse any symptoms examined in the study. Clinical data for one participant were not available.
Among tackle football players with CTE, every one year younger that they began to play tackle football predicted earlier onset of cognitive symptoms by 2.44 years and of behavioral or mood symptoms by 2.50 years. Exposure before age 12 predicted earlier cognitive and behavioral or mood symptom onset by 13.39 years and 13.28 years, respectively.
Secondary subset analyses indicated that younger age of exposure to tackle football was associated with earlier onset of functional impairment in participants who were determined to have had dementia. Researchers observed nearly identical effects in participants with CTE only.
Study limitations include the lack of an appropriate control or comparison group, the researchers noted. In addition, the results may not be generalizable to a broader tackle football population.
“Given the growing public health concerns for participation in tackle football, prospective studies of former tackle football players that include objective clinical assessments are needed to better understand the relationship between youth tackle football exposure and long-term neurobehavioral outcomes,” said the researchers.
“More research on this topic is needed before any clinical recommendations, as well as recommendations on policy or rule changes, can be made,” said Dr. Alosco.
“Boston University and sites across the country are currently conducting longitudinal studies on former football players, which will allow us to begin to study cognition and behavior and mood functioning over time.”
—Erica Tricarico
Suggested Reading
Alosco ML, Mez J, Tripodis Y, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018 Apr 30 [Epub ahead of print].
Retinal Changes Indicate Parkinson’s Disease Pathology Severity
The accumulation of phosphorylated α-synuclein in the retina may serve as a biomarker of brain pathology severity and aid in diagnosis and monitoring of Parkinson’s disease, according to data published online ahead of print May 8 in Movement Disorders.
“These data suggest that phosphorylated α-synuclein accumulates in the retina in parallel with that in the brain, including in early stages preceding development of clinical signs of parkinsonism or dementia,” said Nicolás Cuenca, PhD, Assistant Professor of Physiology, Genetics, and Microbiology at the University of Alicante in Spain, and colleagues.
Parkinson’s disease pathology is mainly characterized by the accumulation of pathologic α-synuclein deposits in the brain, but little is known about how synucleinopathy affects the retina.
Dr. Cuenca and colleagues used immunohistochemistry to evaluate the presence of phosphorylated α-synuclein deposits in the retina of nine autopsied subjects with Parkinson’s disease, four with incidental Lewy body disease, and six controls. Eligible subjects had motor parkinsonism, Lewy body pathology, and pigmented neuron loss in the substantia nigra at autopsy. For each subject, the researchers compared the amount of retinal synucleinopathy with indicators of brain disease severity.
All subjects with Parkinson’s disease and three subjects with incidental Lewy body disease had phosphorylated α-synuclein deposits in ganglion cell perikarya, dendrites, and axons. Some of the deposits resembled brain Lewy bodies and Lewy neurites. Cells that contained phosphorylated α-synuclein had different morphologies, soma sizes (ie, from 15 µm to 30 µm), dendritic lengths (ie, from 570 µm to 1,620 µm), and receptive fields. Control subjects did not show any phosphorylated α-synuclein immunoreactivity in their retinas, however.
The Lewy-type synucleinopathy density in the retina significantly correlated with Lewy-type synucleinopathy density in the brain, with the Unified Parkinson’s disease pathology stage, and with the motor subscale of the Unifed Parkinson’s Disease Rating Scale. Confirmation of disease by autopsy partly compensated for the small number of subjects, according to the authors.
“Further investigations of the eye in Parkinson’s disease are desirable, given that ocular structures are involved in the pathology of several neurodegenerative diseases,” said Dr. Cuenca and colleagues.
—Erica Tricarico
Suggested Reading
Ortuño-Lizarán I, Beach TG, Serrano GE, et al. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018 May 8 [Epub ahead of print].
Ma LJ, Xu LL, Mao CJ, et al. Progressive changes in the retinal structure of patients with Parkinson’s disease. J Parkinsons Dis. 2018;8(1):85-92.
The accumulation of phosphorylated α-synuclein in the retina may serve as a biomarker of brain pathology severity and aid in diagnosis and monitoring of Parkinson’s disease, according to data published online ahead of print May 8 in Movement Disorders.
“These data suggest that phosphorylated α-synuclein accumulates in the retina in parallel with that in the brain, including in early stages preceding development of clinical signs of parkinsonism or dementia,” said Nicolás Cuenca, PhD, Assistant Professor of Physiology, Genetics, and Microbiology at the University of Alicante in Spain, and colleagues.
Parkinson’s disease pathology is mainly characterized by the accumulation of pathologic α-synuclein deposits in the brain, but little is known about how synucleinopathy affects the retina.
Dr. Cuenca and colleagues used immunohistochemistry to evaluate the presence of phosphorylated α-synuclein deposits in the retina of nine autopsied subjects with Parkinson’s disease, four with incidental Lewy body disease, and six controls. Eligible subjects had motor parkinsonism, Lewy body pathology, and pigmented neuron loss in the substantia nigra at autopsy. For each subject, the researchers compared the amount of retinal synucleinopathy with indicators of brain disease severity.
All subjects with Parkinson’s disease and three subjects with incidental Lewy body disease had phosphorylated α-synuclein deposits in ganglion cell perikarya, dendrites, and axons. Some of the deposits resembled brain Lewy bodies and Lewy neurites. Cells that contained phosphorylated α-synuclein had different morphologies, soma sizes (ie, from 15 µm to 30 µm), dendritic lengths (ie, from 570 µm to 1,620 µm), and receptive fields. Control subjects did not show any phosphorylated α-synuclein immunoreactivity in their retinas, however.
The Lewy-type synucleinopathy density in the retina significantly correlated with Lewy-type synucleinopathy density in the brain, with the Unified Parkinson’s disease pathology stage, and with the motor subscale of the Unifed Parkinson’s Disease Rating Scale. Confirmation of disease by autopsy partly compensated for the small number of subjects, according to the authors.
“Further investigations of the eye in Parkinson’s disease are desirable, given that ocular structures are involved in the pathology of several neurodegenerative diseases,” said Dr. Cuenca and colleagues.
—Erica Tricarico
Suggested Reading
Ortuño-Lizarán I, Beach TG, Serrano GE, et al. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018 May 8 [Epub ahead of print].
Ma LJ, Xu LL, Mao CJ, et al. Progressive changes in the retinal structure of patients with Parkinson’s disease. J Parkinsons Dis. 2018;8(1):85-92.
The accumulation of phosphorylated α-synuclein in the retina may serve as a biomarker of brain pathology severity and aid in diagnosis and monitoring of Parkinson’s disease, according to data published online ahead of print May 8 in Movement Disorders.
“These data suggest that phosphorylated α-synuclein accumulates in the retina in parallel with that in the brain, including in early stages preceding development of clinical signs of parkinsonism or dementia,” said Nicolás Cuenca, PhD, Assistant Professor of Physiology, Genetics, and Microbiology at the University of Alicante in Spain, and colleagues.
Parkinson’s disease pathology is mainly characterized by the accumulation of pathologic α-synuclein deposits in the brain, but little is known about how synucleinopathy affects the retina.
Dr. Cuenca and colleagues used immunohistochemistry to evaluate the presence of phosphorylated α-synuclein deposits in the retina of nine autopsied subjects with Parkinson’s disease, four with incidental Lewy body disease, and six controls. Eligible subjects had motor parkinsonism, Lewy body pathology, and pigmented neuron loss in the substantia nigra at autopsy. For each subject, the researchers compared the amount of retinal synucleinopathy with indicators of brain disease severity.
All subjects with Parkinson’s disease and three subjects with incidental Lewy body disease had phosphorylated α-synuclein deposits in ganglion cell perikarya, dendrites, and axons. Some of the deposits resembled brain Lewy bodies and Lewy neurites. Cells that contained phosphorylated α-synuclein had different morphologies, soma sizes (ie, from 15 µm to 30 µm), dendritic lengths (ie, from 570 µm to 1,620 µm), and receptive fields. Control subjects did not show any phosphorylated α-synuclein immunoreactivity in their retinas, however.
The Lewy-type synucleinopathy density in the retina significantly correlated with Lewy-type synucleinopathy density in the brain, with the Unified Parkinson’s disease pathology stage, and with the motor subscale of the Unifed Parkinson’s Disease Rating Scale. Confirmation of disease by autopsy partly compensated for the small number of subjects, according to the authors.
“Further investigations of the eye in Parkinson’s disease are desirable, given that ocular structures are involved in the pathology of several neurodegenerative diseases,” said Dr. Cuenca and colleagues.
—Erica Tricarico
Suggested Reading
Ortuño-Lizarán I, Beach TG, Serrano GE, et al. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018 May 8 [Epub ahead of print].
Ma LJ, Xu LL, Mao CJ, et al. Progressive changes in the retinal structure of patients with Parkinson’s disease. J Parkinsons Dis. 2018;8(1):85-92.
Mild TBI May Increase Risk of Parkinson’s Disease Among Military Veterans
Among military veterans, mild traumatic brain injury (TBI) is associated with a 56% increased risk of developing Parkinson’s disease over 12 years of follow-up, according to data published online ahead of print April 18 in Neurology. Prior TBI also is associated with a diagnosis of Parkinson’s disease two years earlier than among controls.
“Our findings highlight the critical importance of unraveling mechanisms subserving the association between TBI and Parkinson’s disease to inform treatment and prevention of post-TBI Parkinson’s disease,” said Raquel C. Gardner, MD, Assistant Professor of Neurology at the University of California, San Francisco.
A Longitudinal Cohort Study
Every year, mild TBI affects an estimated 42 million people worldwide. It is especially common among athletes and military personnel and is a growing epidemic among the elderly. In 2008, the Institute of Medicine found sufficient evidence to suggest an association between moderate to severe TBI and a clinical diagnosis of Parkinson’s disease, but limited evidence for an association between mild TBI with loss of consciousness and a clinical diagnosis of Parkinson’s disease. One small case–control study assessed the risk of Parkinson’s disease following mild TBI among military veterans, but the results were inconclusive, said the authors.
Dr. Gardner and colleagues conducted a longitudinal cohort study to evaluate the risk of Parkinson’s disease following TBI, including mild TBI, among patients in the Veterans Health Administration (VHA). They analyzed data from three nationwide VHA health care system databases and identified patients with a diagnosis of TBI from October 2002 to September 2014. Participants were age 18 or older without Parkinson’s disease or dementia at baseline and were age-matched 1:1 to a random sample of patients without TBI.
Researchers defined moderate to severe TBI as a loss of consciousness for more than 30 minutes, alteration of consciousness for more than 24 hours, or amnesia for more than 24 hours. They defined mild TBI as loss of consciousness for zero to 30 minutes, alteration of consciousness for a moment to 24 hours, or amnesia for zero to 24 hours.
TBI exposure and severity were determined via detailed clinical assessments or ICD-9 codes using Department of Defense and Defense and Veterans Brain Injury Center criteria. Baseline comorbidities and incident Parkinson’s disease at more than one year post TBI were identified using ICD-9 codes. In addition, investigators used Cox proportional hazard models adjusted for demographics and medical and psychiatric comorbidities to assess risk of Parkinson’s disease after TBI.
Prior TBI Was Associated With Minority Status
A total of 325,870 patients were included in the study with an average age of 47.9 and an average follow-up of 4.6 years. In all, 1,462 patients were diagnosed with Parkinson’s disease during follow-up. After adjusting for age, sex, race, education, and other health conditions, the researchers found that patients with any severity of TBI had a 71% increased risk of Parkinson’s disease; participants with moderate to severe TBI had an 83% increased risk.
Overall, patients with prior TBI were diagnosed with Parkinson’s disease at a significantly younger age, had significantly higher prevalence of non-Hispanic black and Hispanic race or ethnicity, and had significantly higher prevalence of all medical and psychiatric comorbidities, compared with those without prior TBI.
“Given the growing evidence for several potentially modifiable risk factors for Parkinson’s disease, an important area for future research will be to determine whether improved management of specific highly prevalent comorbidities among TBI-exposed veterans may reduce risk of subsequent Parkinson’s disease,” said the researchers.
Strengths of this study include the use of physicians’ diagnosis of TBI and Parkinson’s disease, a longitudinal cohort design, and a large sample size. One of the study’s limitations was the use of ICD-9 codes for the diagnosis of TBI and Parkinson’s disease, which may have overlooked some cases, such as TBI with polytrauma or mild
—Erica Tricarico
Suggested Reading
Gardner RC, Byers AL, Barnes DE, et al. Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology. 2018 Apr 18 [Epub ahead of print].
Among military veterans, mild traumatic brain injury (TBI) is associated with a 56% increased risk of developing Parkinson’s disease over 12 years of follow-up, according to data published online ahead of print April 18 in Neurology. Prior TBI also is associated with a diagnosis of Parkinson’s disease two years earlier than among controls.
“Our findings highlight the critical importance of unraveling mechanisms subserving the association between TBI and Parkinson’s disease to inform treatment and prevention of post-TBI Parkinson’s disease,” said Raquel C. Gardner, MD, Assistant Professor of Neurology at the University of California, San Francisco.
A Longitudinal Cohort Study
Every year, mild TBI affects an estimated 42 million people worldwide. It is especially common among athletes and military personnel and is a growing epidemic among the elderly. In 2008, the Institute of Medicine found sufficient evidence to suggest an association between moderate to severe TBI and a clinical diagnosis of Parkinson’s disease, but limited evidence for an association between mild TBI with loss of consciousness and a clinical diagnosis of Parkinson’s disease. One small case–control study assessed the risk of Parkinson’s disease following mild TBI among military veterans, but the results were inconclusive, said the authors.
Dr. Gardner and colleagues conducted a longitudinal cohort study to evaluate the risk of Parkinson’s disease following TBI, including mild TBI, among patients in the Veterans Health Administration (VHA). They analyzed data from three nationwide VHA health care system databases and identified patients with a diagnosis of TBI from October 2002 to September 2014. Participants were age 18 or older without Parkinson’s disease or dementia at baseline and were age-matched 1:1 to a random sample of patients without TBI.
Researchers defined moderate to severe TBI as a loss of consciousness for more than 30 minutes, alteration of consciousness for more than 24 hours, or amnesia for more than 24 hours. They defined mild TBI as loss of consciousness for zero to 30 minutes, alteration of consciousness for a moment to 24 hours, or amnesia for zero to 24 hours.
TBI exposure and severity were determined via detailed clinical assessments or ICD-9 codes using Department of Defense and Defense and Veterans Brain Injury Center criteria. Baseline comorbidities and incident Parkinson’s disease at more than one year post TBI were identified using ICD-9 codes. In addition, investigators used Cox proportional hazard models adjusted for demographics and medical and psychiatric comorbidities to assess risk of Parkinson’s disease after TBI.
Prior TBI Was Associated With Minority Status
A total of 325,870 patients were included in the study with an average age of 47.9 and an average follow-up of 4.6 years. In all, 1,462 patients were diagnosed with Parkinson’s disease during follow-up. After adjusting for age, sex, race, education, and other health conditions, the researchers found that patients with any severity of TBI had a 71% increased risk of Parkinson’s disease; participants with moderate to severe TBI had an 83% increased risk.
Overall, patients with prior TBI were diagnosed with Parkinson’s disease at a significantly younger age, had significantly higher prevalence of non-Hispanic black and Hispanic race or ethnicity, and had significantly higher prevalence of all medical and psychiatric comorbidities, compared with those without prior TBI.
“Given the growing evidence for several potentially modifiable risk factors for Parkinson’s disease, an important area for future research will be to determine whether improved management of specific highly prevalent comorbidities among TBI-exposed veterans may reduce risk of subsequent Parkinson’s disease,” said the researchers.
Strengths of this study include the use of physicians’ diagnosis of TBI and Parkinson’s disease, a longitudinal cohort design, and a large sample size. One of the study’s limitations was the use of ICD-9 codes for the diagnosis of TBI and Parkinson’s disease, which may have overlooked some cases, such as TBI with polytrauma or mild
—Erica Tricarico
Suggested Reading
Gardner RC, Byers AL, Barnes DE, et al. Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology. 2018 Apr 18 [Epub ahead of print].
Among military veterans, mild traumatic brain injury (TBI) is associated with a 56% increased risk of developing Parkinson’s disease over 12 years of follow-up, according to data published online ahead of print April 18 in Neurology. Prior TBI also is associated with a diagnosis of Parkinson’s disease two years earlier than among controls.
“Our findings highlight the critical importance of unraveling mechanisms subserving the association between TBI and Parkinson’s disease to inform treatment and prevention of post-TBI Parkinson’s disease,” said Raquel C. Gardner, MD, Assistant Professor of Neurology at the University of California, San Francisco.
A Longitudinal Cohort Study
Every year, mild TBI affects an estimated 42 million people worldwide. It is especially common among athletes and military personnel and is a growing epidemic among the elderly. In 2008, the Institute of Medicine found sufficient evidence to suggest an association between moderate to severe TBI and a clinical diagnosis of Parkinson’s disease, but limited evidence for an association between mild TBI with loss of consciousness and a clinical diagnosis of Parkinson’s disease. One small case–control study assessed the risk of Parkinson’s disease following mild TBI among military veterans, but the results were inconclusive, said the authors.
Dr. Gardner and colleagues conducted a longitudinal cohort study to evaluate the risk of Parkinson’s disease following TBI, including mild TBI, among patients in the Veterans Health Administration (VHA). They analyzed data from three nationwide VHA health care system databases and identified patients with a diagnosis of TBI from October 2002 to September 2014. Participants were age 18 or older without Parkinson’s disease or dementia at baseline and were age-matched 1:1 to a random sample of patients without TBI.
Researchers defined moderate to severe TBI as a loss of consciousness for more than 30 minutes, alteration of consciousness for more than 24 hours, or amnesia for more than 24 hours. They defined mild TBI as loss of consciousness for zero to 30 minutes, alteration of consciousness for a moment to 24 hours, or amnesia for zero to 24 hours.
TBI exposure and severity were determined via detailed clinical assessments or ICD-9 codes using Department of Defense and Defense and Veterans Brain Injury Center criteria. Baseline comorbidities and incident Parkinson’s disease at more than one year post TBI were identified using ICD-9 codes. In addition, investigators used Cox proportional hazard models adjusted for demographics and medical and psychiatric comorbidities to assess risk of Parkinson’s disease after TBI.
Prior TBI Was Associated With Minority Status
A total of 325,870 patients were included in the study with an average age of 47.9 and an average follow-up of 4.6 years. In all, 1,462 patients were diagnosed with Parkinson’s disease during follow-up. After adjusting for age, sex, race, education, and other health conditions, the researchers found that patients with any severity of TBI had a 71% increased risk of Parkinson’s disease; participants with moderate to severe TBI had an 83% increased risk.
Overall, patients with prior TBI were diagnosed with Parkinson’s disease at a significantly younger age, had significantly higher prevalence of non-Hispanic black and Hispanic race or ethnicity, and had significantly higher prevalence of all medical and psychiatric comorbidities, compared with those without prior TBI.
“Given the growing evidence for several potentially modifiable risk factors for Parkinson’s disease, an important area for future research will be to determine whether improved management of specific highly prevalent comorbidities among TBI-exposed veterans may reduce risk of subsequent Parkinson’s disease,” said the researchers.
Strengths of this study include the use of physicians’ diagnosis of TBI and Parkinson’s disease, a longitudinal cohort design, and a large sample size. One of the study’s limitations was the use of ICD-9 codes for the diagnosis of TBI and Parkinson’s disease, which may have overlooked some cases, such as TBI with polytrauma or mild
—Erica Tricarico
Suggested Reading
Gardner RC, Byers AL, Barnes DE, et al. Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology. 2018 Apr 18 [Epub ahead of print].
Ketorolac may reduce breast cancer recurrence risk, particularly in overweight patients
Ketorolac administered during primary tumor surgery may cut risk of distant recurrences in patients with breast cancer, results of a retrospective study show.
Overweight patients appeared most likely to benefit from interoperative treatment with this nonsteroidal anti-inflammatory drug, study investigators reported.
“This approach could be extremely appealing for parts of the globe where obesity has been strongly increasing during the last decade and where resources for cancer treatment are scarce,” they wrote. The report was published in the Journal of the National Cancer Institute.
Ketorolac inhibits enzymes upregulated by leptin, a hormone abnormally secreted in the setting of overweight or obesity, which might explain the concentration of benefit in high–body mass index individuals, noted Christine Desmedt, PhD, of the Breast Cancer Translational Research Laboratory, Institut Jules Bordet, Brussels, and her coauthors.
Indeed, the study also showed no benefit to intraoperative administration of another NSAID, diclofenac, which does not appear to have the same enzyme-inhibitory effects as ketorolac, the investigators said.
This recently published analysis by Dr. Desmedt and her colleagues was based on two retrospective series of patients: one evaluating intraoperative ketorolac in 529 patients versus 298 patients who received no ketorolac, and one evaluating intraoperative diclofenac in 787 patients, versus 220 who did not receive that NSAID.
The investigators found a significant association between ketorolac given during surgery and decreased incidence of distant metastasis (adjusted hazard ratio [aHR], 0.59, 95% confidence interval, 0.37-0.96, P = .03). Reduced recurrence was most evident in patients with high BMI (aHR, 0.55; 95% CI, 0.31-0.96; P = .04).
Further evaluation revealed that the benefit of ketorolac was “clearly associated” with a reduction in early metastases, both overall and in the high-BMI subgroup, the investigators said.
By contrast, intraoperative diclofenac was not associated with a decrease in distant recurrences, overall (adjusted HR, 1.04; 95% CI, 0.58-1.87, P = .88) or in BMI subgroup analysis, investigators said.
While some might be surprised that a single dose of ketorolac could reduce distant recurrence, it might be explained by the timing of NSAID delivery, they noted. In previous studies, primary tumor removal has been shown to disturb disease homeostasis, and thus might trigger early recurrences.
“Complex system dynamics are exquisitely sensitive on initial conditions, and, therefore, changes occurring in critical early times may be able to cause major changes in system evolution,” the investigators wrote in a discussion of the results.
The finding is also not without precedent. The authors cited one Scandinavian randomized trial in which a single course of perioperative cyclophosphamide significantly improved disease-free survival at more than 17 years of follow-up; by contrast, giving the treatment 2-4 weeks after mastectomy provided no such benefit.
In addition, ketorolac’s potential perioperative benefit has been shown in other tumor types, including improved disease-free survival in one institutional series of lung cancer patients, and reduced disease-specific mortality in a retrospective study of ovarian cancer patients.
The present breast cancer study is limited because it is retrospective, and does not address questions regarding optimal timing or duration of dose. However, “it suggests a potentially important repositioning of ketorolac in the intraoperative treatment of breast cancer patients with elevated BMI, and points to the need for a prospective confirmatory randomized trial,” the authors said.
Dr. Desmedt and her colleagues reported no conflicts of interest related to the study.
SOURCE: Desmedt C et al. J Natl Cancer Inst. 2018 Apr 30. doi: 10.1093/jnci/djy042.
Ketorolac administered during primary tumor surgery may cut risk of distant recurrences in patients with breast cancer, results of a retrospective study show.
Overweight patients appeared most likely to benefit from interoperative treatment with this nonsteroidal anti-inflammatory drug, study investigators reported.
“This approach could be extremely appealing for parts of the globe where obesity has been strongly increasing during the last decade and where resources for cancer treatment are scarce,” they wrote. The report was published in the Journal of the National Cancer Institute.
Ketorolac inhibits enzymes upregulated by leptin, a hormone abnormally secreted in the setting of overweight or obesity, which might explain the concentration of benefit in high–body mass index individuals, noted Christine Desmedt, PhD, of the Breast Cancer Translational Research Laboratory, Institut Jules Bordet, Brussels, and her coauthors.
Indeed, the study also showed no benefit to intraoperative administration of another NSAID, diclofenac, which does not appear to have the same enzyme-inhibitory effects as ketorolac, the investigators said.
This recently published analysis by Dr. Desmedt and her colleagues was based on two retrospective series of patients: one evaluating intraoperative ketorolac in 529 patients versus 298 patients who received no ketorolac, and one evaluating intraoperative diclofenac in 787 patients, versus 220 who did not receive that NSAID.
The investigators found a significant association between ketorolac given during surgery and decreased incidence of distant metastasis (adjusted hazard ratio [aHR], 0.59, 95% confidence interval, 0.37-0.96, P = .03). Reduced recurrence was most evident in patients with high BMI (aHR, 0.55; 95% CI, 0.31-0.96; P = .04).
Further evaluation revealed that the benefit of ketorolac was “clearly associated” with a reduction in early metastases, both overall and in the high-BMI subgroup, the investigators said.
By contrast, intraoperative diclofenac was not associated with a decrease in distant recurrences, overall (adjusted HR, 1.04; 95% CI, 0.58-1.87, P = .88) or in BMI subgroup analysis, investigators said.
While some might be surprised that a single dose of ketorolac could reduce distant recurrence, it might be explained by the timing of NSAID delivery, they noted. In previous studies, primary tumor removal has been shown to disturb disease homeostasis, and thus might trigger early recurrences.
“Complex system dynamics are exquisitely sensitive on initial conditions, and, therefore, changes occurring in critical early times may be able to cause major changes in system evolution,” the investigators wrote in a discussion of the results.
The finding is also not without precedent. The authors cited one Scandinavian randomized trial in which a single course of perioperative cyclophosphamide significantly improved disease-free survival at more than 17 years of follow-up; by contrast, giving the treatment 2-4 weeks after mastectomy provided no such benefit.
In addition, ketorolac’s potential perioperative benefit has been shown in other tumor types, including improved disease-free survival in one institutional series of lung cancer patients, and reduced disease-specific mortality in a retrospective study of ovarian cancer patients.
The present breast cancer study is limited because it is retrospective, and does not address questions regarding optimal timing or duration of dose. However, “it suggests a potentially important repositioning of ketorolac in the intraoperative treatment of breast cancer patients with elevated BMI, and points to the need for a prospective confirmatory randomized trial,” the authors said.
Dr. Desmedt and her colleagues reported no conflicts of interest related to the study.
SOURCE: Desmedt C et al. J Natl Cancer Inst. 2018 Apr 30. doi: 10.1093/jnci/djy042.
Ketorolac administered during primary tumor surgery may cut risk of distant recurrences in patients with breast cancer, results of a retrospective study show.
Overweight patients appeared most likely to benefit from interoperative treatment with this nonsteroidal anti-inflammatory drug, study investigators reported.
“This approach could be extremely appealing for parts of the globe where obesity has been strongly increasing during the last decade and where resources for cancer treatment are scarce,” they wrote. The report was published in the Journal of the National Cancer Institute.
Ketorolac inhibits enzymes upregulated by leptin, a hormone abnormally secreted in the setting of overweight or obesity, which might explain the concentration of benefit in high–body mass index individuals, noted Christine Desmedt, PhD, of the Breast Cancer Translational Research Laboratory, Institut Jules Bordet, Brussels, and her coauthors.
Indeed, the study also showed no benefit to intraoperative administration of another NSAID, diclofenac, which does not appear to have the same enzyme-inhibitory effects as ketorolac, the investigators said.
This recently published analysis by Dr. Desmedt and her colleagues was based on two retrospective series of patients: one evaluating intraoperative ketorolac in 529 patients versus 298 patients who received no ketorolac, and one evaluating intraoperative diclofenac in 787 patients, versus 220 who did not receive that NSAID.
The investigators found a significant association between ketorolac given during surgery and decreased incidence of distant metastasis (adjusted hazard ratio [aHR], 0.59, 95% confidence interval, 0.37-0.96, P = .03). Reduced recurrence was most evident in patients with high BMI (aHR, 0.55; 95% CI, 0.31-0.96; P = .04).
Further evaluation revealed that the benefit of ketorolac was “clearly associated” with a reduction in early metastases, both overall and in the high-BMI subgroup, the investigators said.
By contrast, intraoperative diclofenac was not associated with a decrease in distant recurrences, overall (adjusted HR, 1.04; 95% CI, 0.58-1.87, P = .88) or in BMI subgroup analysis, investigators said.
While some might be surprised that a single dose of ketorolac could reduce distant recurrence, it might be explained by the timing of NSAID delivery, they noted. In previous studies, primary tumor removal has been shown to disturb disease homeostasis, and thus might trigger early recurrences.
“Complex system dynamics are exquisitely sensitive on initial conditions, and, therefore, changes occurring in critical early times may be able to cause major changes in system evolution,” the investigators wrote in a discussion of the results.
The finding is also not without precedent. The authors cited one Scandinavian randomized trial in which a single course of perioperative cyclophosphamide significantly improved disease-free survival at more than 17 years of follow-up; by contrast, giving the treatment 2-4 weeks after mastectomy provided no such benefit.
In addition, ketorolac’s potential perioperative benefit has been shown in other tumor types, including improved disease-free survival in one institutional series of lung cancer patients, and reduced disease-specific mortality in a retrospective study of ovarian cancer patients.
The present breast cancer study is limited because it is retrospective, and does not address questions regarding optimal timing or duration of dose. However, “it suggests a potentially important repositioning of ketorolac in the intraoperative treatment of breast cancer patients with elevated BMI, and points to the need for a prospective confirmatory randomized trial,” the authors said.
Dr. Desmedt and her colleagues reported no conflicts of interest related to the study.
SOURCE: Desmedt C et al. J Natl Cancer Inst. 2018 Apr 30. doi: 10.1093/jnci/djy042.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: Administration of ketorolac during primary tumor surgery was associated with a reduction of distant recurrences, particularly in overweight patients.
Major finding: Reduced recurrence was most evident in patients with high BMI (adjusted hazard ratio, 0.55; 95% CI, 0.31-0.96; P = .04).
Study details: Analysis of two retrospective series, including a total of 1,834 patients with breast cancer, evaluating intraoperative administration of ketorolac or diclofenac.
Disclosures: The authors declared no conflicts of interest.
Source: Desmedt C et al. J Natl Cancer Inst. 2018 Apr 30. doi: 10.1093/jnci/djy042.