User login
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
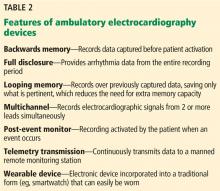
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
KEY POINTS
- Ambulatory ECG monitoring is commonly used to correlate symptoms with arrhythmia, confirm occult atrial fibrillation, and assess the efficacy of antiarrhythmic therapy.
- Devices have features such as access to the full monitoring time (“full disclosure”), extended monitoring, and telemetry, each with advantages and limitations.
- Consumer-oriented wearable devices are aimed at arrhythmia monitoring, which could lead to increased arrhythmia detection, but at the risk of more false-positive results and excessive use of healthcare resources.