User login
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
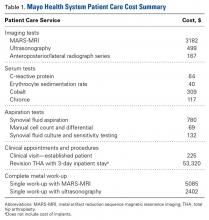
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
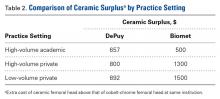
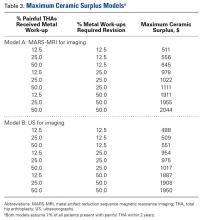
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.