User login
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
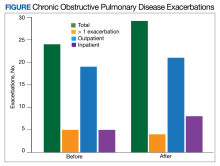
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4