User login
From the University of Arizona College of Medicine, Tucson, AZ (Dr. Beatty), and the Baylor College of Medicine, Houston, TX (Dr. Mohajer).
Abstract
- Objective: To review management issues regarding health care–associated urinary tract infections (UTIs) commonly encountered by practicing clinicians.
- Methods: Review of the literature.
- Results: Because urinary catheter (UC) placement plays a major role in the development of catheter-associated UTIs (CA-UTI), clinicians should be aware of the appropriate and inappropriate uses of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key to preventing CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. Percutaneous nephrostomy and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected. Candiduria rarely leads to symptoms unless it is related to an ascending process. Proper urine collection is crucial in determining whether contamination, colonization, or infection is present. Fluconazole is recommended in most cases of Candida UTI, while intravenous amphotericin B is recommended for fluconazole-resistant Candida species.
- Conclusion: Continued use of evidence-based strategies for preventing and managing health care–associated UTI should lead to further improvements in patient outcomes and overall decreased rates of infection.
Keywords: bacteriuria; catheter-associated UTI; catheterization; percutaneous nephrostomy; candiduria.
Health care–associated urinary tract infections (UTIs) are estimated to be the most common adverse infectious event in U.S. hospitals, occurring in 1 of 10 admitted patients.1-3 Approximately 32% of all health care–associated infections are UTIs.1 Furthermore, urinary catheters (UCs) are associated with 8% to 21% of health care–associated infections that occur in the intensive care unit.4 The most important predisposing factor for nosocomial UTI is urinary catheterization.5 Genitourinary manipulation and/or implementation also play a major role in the development of nosocomial UTIs.
In 2008, the U.S. Centers for Medicare & Medicaid Services instituted a new policy that reduced reimbursement rates for hospitalizations linked to health care–associated infections.6 Indwelling UCs are among the most overused health care devices in the hospital setting. They are placed in an estimated 15% to 25% of all hospitalized patients,7,8 and are often inserted in the emergency department (ED) without a physician order or appropriate indication.9 Intermittent straight catheterization, male or female condom catheterization, and/or placement of an indwelling UC are the most common causes of catheter-associated asymptomatic bacteriuria (CA-ASB) and catheter-associated UTIs (CA-UTI).5 Prevention and management of CA-ASB and CA-UTI can be challenging and require an evidence-based approach. Furthermore, guidelines for the management of UTIs in the setting of active percutaneous nephrostomy (PCN) drainage and/or ureteral stenting are not established.5 This may leave clinicians with little mainstream data to aid in management decisions.
In 2009 the Centers for Disease Control and Prevention provided a guideline for the appropriate and inappropriate use of indwelling UCs to help promote their proper use.10 In the time since the guideline’s initiatives were instituted around the United States, published data have shown some improvement in the use of UCs,11,12 but other recent reports indicate that rates of UC use have remained unchanged.13 This review discusses management issues regarding health care–associated UTIs that are commonly encountered by practicing clinicians, with a focus on current guidelines and evidence.
Catheter-Associated UTI
CA-UTI is defined as the presence of signs or symptoms of UTI with no other explainable infectious source along with ≥ 1000 colony-forming units (cfu) of ≥ 1 bacterial species per milliliter in a urine specimen from a catheter that has been changed within 48 hours of collection of the urine specimen.5 Signs and symptoms of CA-UTI include, but are not limited to: new-onset or worsening fever, chills, altered sensorium from baseline, lethargy, malaise, flank pain, pelvic pain, costovertebral angle tenderness, and acute hematuria.5 New-onset “foul-smelling” (odorous)urine and “cloudy” urine are neither sensitive nor specific when assessing for CA-UTI, and do not have significant clinical relevance when found alone.14,15 Patients who have removed or exchanged the UC during this event and then experience dysuria, increased frequency, urgency, or suprapubic pain are likely having symptoms of CA-UTI.5
What is the recommended method for collecting urine samples when CA-UTI is suspected?
In a patient with an indwelling catheter that has been in place for more than 2 weeks at the onset of a suspected CA-UTI, the catheter should be replaced (if still indicated) or removed to accelerate resolution of symptoms and to reduce the risk of subsequent catheter-associated bacteriuria and CA-UTI. The urine culture should be acquired from the freshly placed UC.5
When should a patient be empirically treated?
A patient presenting with evidence of sepsis should be empirically treated with antimicrobials. Empiric coverage should be based on risk factors for multidrug-resistant organisms and data pertaining to local antimicrobial resistance patterns. A urine specimen for urinalysis and possible culture should be sent prior to administering empiric antibiotics (if possible) in a symptomatic patient.5
What bacteria are commonly associated with CA-UTI?
The bacteria most commonly associated with CA-UTI are found in or around the gastrointestinal and genitourinary tracts and also are part of the normal skin flora. The introduction and/or facilitated ascension of these microorganisms is believed to occur during UC insertion.16,17 Two-thirds of all isolated uropathogens in those with indwelling UCs are extraluminally acquired (via ascension along the catheter-urethral mucosa interface), and one-third are believed to be intraluminally acquired.18
The most commonly isolated bacteria in CA-UTI are Enterobacteriaceae, which include Escherichia coli (most common), Klebsiella species (K. oxytoca, K. pneumoniae), Serratia species (S. marcescens), Citrobacter species (C. koseri), Enterobacter species (E. cloacae), and Proteus species; non-Enterobacteriaceae such as Pseudomonas species; and gram-positive cocci, which include coagulase-negative staphylococci (S. saprophyticus), Staphylococcus aureus, group B streptococci, and Enterococcus species (E. faecalis, E. faecium).19-21 Coagulase-negative staphylococci and Enterococcus species can lead to CA-UTI but are usually avirulent and more commonly isolated from asymptomatic individuals.19 Also, coagulase-negative staphylococci such as S. epidermidis and S. lugdunensis are usually the manifestation of contamination during the collection process and their presence should prompt a repeat sample collection under sterile techniques. Monomicrobial infection is usually seen in those with short-term catheter use and CA-UTI. In contrast, polymicrobial infection is more common in those with long-term indwelling UCs and CA-UTI.19 Providencia stuartii, Proteus mirabilis, S. aureus, and Morganella morganii have all been associated with CA-UTI in those with long-term indwelling UCs.
Growth of S. aureus in the urine should prompt further investigation with blood cultures to explore the possibility of hematogenous dissemination to the urinary tract. Organisms leading to bacteremia due to CA-UTI are most commonly gram-negative bacilli (E. coli, Klebsiella species, Pseudomonas aeruginosa) and E. faecalis.21
What is the difference between CA-ASB and CA-UTI?
CA-ASB is defined as the presence of ≥ 1 bacteria species growing on urine culture at ≥ 100,000 cfu/mL in a patient with a history of urinary catheterization and/or indwelling UC who lacks signs or symptoms of UTI. In a man with a condom catheter, CA-ASB is defined using the same criteria, but the urine sample is collected after a fresh condom catheter is applied.5 The difference between CA-ASB and CA-UTI is simply the presence or absence of signs and symptoms related to UTI. Currently, there is no standard definition for significant bacteriuria in a catheterized patient.5 Pyuria found on urinalysis is indicative of genitourinary inflammation and can be present in both CA-ASB and CA-UTI. The absence, presence, and/or degree of pyuria in catheterized patients does not accurately differentiate between CA-ASB and CA-UTI.5,22,23 On the other hand, the absence of pyuria in a symptomatic catheterized patient suggests an etiology other than CA-UTI.5
How can CA-UTI be prevented in patients with a short-term indwelling urinary catheter?
If a short-term UC is essential, the most important approach to preventing CA-UTI is limiting the duration of time it will be used. Strategies such as computer-based order entry and care maps with automated discontinuation of UCs have been shown to decrease catheter usage.19 Using closed-systems for UC collection with ports in the distal catheter for needle aspiration of urine has also been shown to decrease the incidence of CA-UTI.5 Securing the UC to avoid urethral trauma, aseptic techniques for insertion and repositioning, and placement of the tubing and collection bag below the level of the bladder to prevent reflux will likely also prevent CA-UTI, but these strategies have not been evaluated thoroughly.19
When should you screen for and treat CA-ASB?
The 2009 Infectious Diseases Society of America (IDSA) guidelines recommend that the only patients who should be screened and treated for CA-ASB are pregnant women and those who will undergo a potentially traumatic urologic procedure for which mucosal breaching may occur, causing bleeding. Routinely screening or treating patients for CA-ASB in not recommended in any other group of patients and will lead to unnecessary antibiotic use and antibiotic resistance.5
UTI Associated with Percutaneous Nephrostomy and Ureteral Stenting
Similar systemic symptoms of infection (fever, rigors, malaise, shock) are present in patients with and without percutaneous nephrostomy (PCN) and/or ureteral stent placement. Dysuria is not commonly present in those with PCN. The first signs of CA-UTI may be decreased urine output and pericatheter leakage due to an obstructive process resulting from the encrustation.24-27 The most common complaint among patients with either acute or chronic ureteral stenting is discomfort, which has been described as “urinary symptoms” and “body pain.”28 This discomfort can be related to ureteral hyperperistalsis after placement of the stent and is usually self-limiting. Ureteral stent migration, usually at the distal end, can also lead to discomfort, but is easily rectified with cystoscopy.25 Body pain and/or urinary symptoms in the setting of ureteral stenting are not indicative of infection alone.
What is urinary catheter and/or ureteral stent encrustation?
Encrustation is the formation of a conditioning film that develops on the surface of the UC or ureteral stent. The exact mechanism is not well understood, but it is believed to involve electrostatic interactions of urinary proteins that stimulate binding onto the stent or UC surface.25 Encrustation increases exponentially with the dwell time. Among patients with ureteral stents placed due to urolithiasis, encrustation occurred in 9.2% of stents removed prior to 6 weeks, 47.5% of stents removed at 6 weeks, and 76.3% of stents removed at 12 weeks.26 Encrustation is most common at the proximal and distal ends (pigtails) of the ureteral stent and usually spares or presents last within the lumen.29 Attempts have been made to prevent ureteral stent encrustation through the development of biodegradable, drug-eluting, and tissue-engineered substrates. These developments are promising, but currently there is limited observational data from large randomized trials to suggest that these new modalities decrease rates of encrustation.25 Encrustation is highly associated with certain microorganisms, especially those that create biofilms.30 Urease-producing bacteria, most commonly P. mirabilis, play a role in encrustation formation.31 Bacteria most commonly associated with encrustation include Proteus species (P. mirabilis is most common), P. aeruginosa, K. pneumoniae, Providencia species (P. stuartii is most common), and M. morganii.
Does ureteral stent bacterial colonization correlate with UTI?
Ureteral stent colonization with bacteria increases with dwell time and is found in 40% to 98.5% of stents placed.32-35 If UTI is suspected in a patient with an active indwelling ureteral stent, a sample of urine should be cultured while the stent is in place.25 Typically, genitourinary and normal skin flora pathogens are found when the ureteral stent is cultured. The top 3 organisms cultured from ureteral stents are S. aureus, P. aeruginosa, and E. faecalis.34 Urine culture usually does not correlate with stent culture results, which has brought up the debate of how bacterial colonization occurs. It has been postulated that colonization is actually a manifestation of contamination during the insertion procedure, but this has yet to be validated.25 In patients with symptoms of UTI in the setting of an indwelling ureteral stent, a positive culture has low sensitivity, with estimates between 21% and 40%.35 Therefore, a negative urine culture does not rule out UTI alone in a symptomatic patient. Multiple studies have suggested that colonization of the ureteral stent does not correlate strongly with developing a UTI.25,32-34
How can UTI be prevented in those receiving PCN or ureteral stent placement?
Antibiotic prophylaxis has been recommended to prevent UTI in patients who will undergo PCN or ureteral stent placement. The American Urological Association recommends empiric treatment even in the absence of signs and symptoms of UTI,36 but substantial evidence is lacking that this approach prevents infection.37 Ciprofloxacin or trimethoprim/sulfamethoxazole has been recommended by some for empiric coverage for enteric gram-negative bacilli and enterococcus in those undergoing genitourinary manipulation or instrumentation.32,38 Most patients who develop CA-UTI and pyelonephritis do so within the first 2 to 6 weeks after placement.37,39 Bacteriuria, candiduria, and/or pyuria are present in all patients approximately within 9 weeks even when sterile urine is confirmed prior to PCN placement.39 Data on the effectiveness of antibiotic prophylaxis to prevent CA-UTI in those with PCN or ureteral stenting is limited. Currently, there are no recommendations from the IDSA on how to prevent infection in these situations.5 Early or frequent stent removal or exchanges has been proven to reduce UTI in those with ureteral stenting.33 Patients with diabetes mellitus and chronic renal failure are at high-risk for UTI when ureteral stents are in place. This population should undergo close monitoring for UTI development and may warrant more frequent stent exchanges.27,40
What is the treatment of CA-UTI associated with PCN and/or ureteral stenting?
The IDSA guidelines do not apply to patients with PCN and/or ureteral stenting.5 There is no treatment protocol for UTI related to these processes. Generally speaking, they are considered “complicated UTI” by most experts. Broad-spectrum, empiric antibiotic administration along with prompt removal of the PCN and/or ureteral stent is the gold standard of therapy.27 The recommended duration of targeted antibiotic therapy is generally between 5 and 14 days.19 Most clinicians will treat this complicated UTI for at least 10 to 14 days. Antibiotic administration should be continued even after removal of the catheter and/or stent to complete the full course. Repeat urinalysis and culture is not indicated at the end of therapy if the patient is clinically improving or has remission of symptoms.
What is the exchange rate for those who require chronic PCN and/or ureteral stent use?
On average a PCN or ureteral stent should be exchanged every 2 to 3 months in patients who require chronic usage.24,27 Some patients with persistent complications may require more frequent exchanges (< 10 weeks).27 Encrustation and bacterial colonization become more prevalent the longer the devices are in place. This process is estimated to begin within the first 2 weeks after placement.27,33,34 A “forgotten stent” is one that has been left in place after the patient is lost to follow-up. This unfortunate event can lead to massive encrustation, UTI, stent fracturing, and complete ureteral obstruction.24 As noted, patients with diabetes mellitus, chronic renal failure, and frequent UTI may warrant more frequent exchanges, but this should be determined on a case-by-case basis.
Catheter-Associated UTI in Patients with Spinal Cord Injury
Spinal cord injury (SCI) at any level can cause neurogenic bladder. This process ultimately leads to urinary stasis and colonization of the bladder with bacteria. According to the IDSA, the acceptable indications for UC insertion are: clinically significant urinary retention (if medical therapy is not effective), urinary incontinence, accurate urine output monitoring required, and patient is unable and/or unwilling to collect urine (Table 1).5 Recently, further guidelines were published regarding appropriate and inappropriate indwelling UC placement in hospitalized medical patients (Table 2 and Table 3), expanding upon the earlier acceptable criteria provided by the IDSA.41 According to the IDSA and Ann Arbor Criteria for Appropriate Urinary Catheter Use, patients with SCI and subsequent neurogenic bladder without obstruction where intermittent bladder straight catheterization for the drainage of urine is not feasible will likely need an indwelling UC.41 SCI patients often experience decubitus ulcers, and an indwelling UC can be used if needed to help with wound healing if other urinary management alternatives have been attempted. Other such situations in which an indwelling UC can used before attempting alternative approaches would be in a patient who is actively dying and is pursuing comfort care and/or hospice.41
Which indwelling UCs should be used in patients with SCI?
Efforts to reduce the likelihood of infection in patients with SCI have led to several advances in the design and manufacturing of UCs. UCs are made of either latex, plastic, silicone, or polytetrafluoroethylene (Teflon). None of these substrates is free of complications, but of them latex UCs have been studied the most in regards to their associated complications. Aside from being allergenic in nature, latex UCs have an increased propensity to allow bacteria to adhere to their surface due to microscopic planes of unevenness.42 Silicone UCs are less frequently associated with infection but are more rigid, leading to increased discomfort.43,44 Hydrophilic and silver-hydrogel coatings are innovative methods that have been developed to increase comfort and reduce the likelihood of infection. Hydrophilic-coated UCs are associated with reduced microbial adherence, decreased encrustation, and better patient satisfaction.45,46 In SCI patients, these UCs have demonstrated lower complication rates, including UTI; fewer episodes of post-, intra-, and inter-catheterization bleeding; and decreased rates of antibiotic-resistant bacteria.45,46 Silver-hydrogel-coated UCs are less well studied but have also demonstrated reduced UTI rates in SCI patients; however, their efficacy over the long term has yet to be determined. Antibiotic-impregnated UCs are not currently recommended for either short- or long-term indwelling UC use.5
How can CA-UTI be prevented in a patient who will require a long-term indwelling catheter?
At this time, the data is insufficient to make a recommendation on routine UC exchange (eg, every 2 to 4 weeks) in patients who require long-term indwelling urethral or suprapubic catheters in an attempt to reduce the risk of CA-ASB or CA-UTI. This is also true for those who experience even repeated early catheter blockage from encrustation.5 Thus, the rate at which these exchanges occur can be controversial, but typically around every 4 weeks is a common approach. Some would argue that if the patient has repeated CA-UTI, an exchange rate of every 2 weeks might be needed, but data is currently lacking to support this practice.5 If intermittent urinary catheterization is feasible, it should be done at least every 6 hours and before bedtime. In general, when the volume of urine in the bladder reaches approximately 400 mL, the patient should undergo bladder catheterization to prevent stasis and infection.47 A closed drainage system is recommended in all patients who require long-term indwelling UC use.48,49 Placement of the collection bag above the catheter or above the level of the bladder and a breach in the closed drainage system have been shown to result in higher rates of catheter-associated bacteriuria.48,50 Proper hand hygiene and sterile and/or clean techniques should be used when placing or exchanging a UC. However, in one study there was no difference in bacteremia or UTIs when using sterile versus clean techniques.51
Asymptomatic bacteriuria should not be treated in patients with long-term indwelling UCs, and prophylactic antibiotics have led to the emergence of resistance.52 At this time it is not clear whether prophylactic weekly oral cyclic antibiotic administration can effectively reduce the frequency of CA-UTI in patients with SCI and chronic indwelling UC use.
What are the signs and symptoms of CA-UTI in a patient with SCI?
Subjective and objective findings may be limited when a patient with SCI presents with CA-UTI. These patients often lack the usual symptoms of UTI (dysuria, suprapubic discomfort, urgency, increased frequency) and pyelonephritis (flank pain, costovertebral angle tenderness). Caregivers and health care providers should be aware that nonspecific symptoms such as foul-smelling urine, pyuria, increased residual volume of urine in the bladder, change in voiding habits, worsening detrusor spasticity, and aggravation of autonomic dysreflexia can be the only initial presenting symptoms.53
What is the duration of therapy for CA-UTI in SCI, and how can antibiotic stewardship principles be applied in this patient population?
Antibiotic therapy is indicated for a duration of 7 days if there is prompt resolution of symptoms, or for a total of 10 to 14 days if the response is delayed, regardless of whether the patient remains with a UC.5 Antibiotic stewardship is very important to reduce the risk for developing drug resistance in this high-risk population. Methods such as prescriber education practices, institution protocols, guideline implementation, auditing and feedback, restriction and reauthorization practices, computer-assisted programs, de-escalation or streamlining, and antibiotic cycling or dosage optimization have all been shown to assist in antibiotic stewardship in UTIs.54
Candida UTI
What is the initial evaluation for a patient with candiduria?
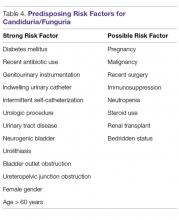
The workup for candiduria hinges on determining whether the candiduria likely represents contamination, colonization, or infection; certain predisposing risk factors are associated with Candida UTI (Table 4).55-57 The most important aspect to candiduria is the patient’s clinical status and comorbid conditions.58 Among funguria, Candida species are the most common and represent 95% of organisms isolated on urine cultures (Table 5).59 Candiduria is usually present in those with significant comorbidities and rarely is associated with healthy individuals.59,60 Candiduria is increasing in prevalence among hospitalized patients, representing 22% to 40% of all nosocomial UTIs.59,60 Markers in the urine (leukocyte esterase, colony count of culture growth, presence or absence of Candida casts and pseudohyphae) cannot alone differentiate colonization from infection.55 When candiduria is discovered in a patient with symptoms related to UTI in the setting of predisposing risk factors, it should be considered a real infection until proven otherwise.
In a situation where an asymptomatic patient without an indwelling UC has Candida species isolated from a urine culture, a repeat culture (clean-catch midstream sample) should be performed to assess for a likely contaminated collection.55 If the patient has an indwelling UC then it should be exchanged and urine collected from the fresh catheter.61 When candiduria is found in healthy asymptomatic adults, it is most commonly associated with poor collection techniques or postcollection contamination.59 If candiduria persists in an asymptomatic patient, the patient should be assessed for predisposing factors. This includes checking hemoglobin A1C for developing diabetes and renal ultrasound looking for urolithiasis, renal abscess, hydronephrosis, and fungus ball. Postvoid residual urinary retention should also be ruled out with bladder ultrasound. Treatment of predisposing factors can lead to resolution of candiduria without antifungal treatment, and a urine culture should be repeated (1 to 2 weeks later).61 Asymptomatic patients lacking any predisposing factors can be observed with repeat urine cultures in 1 to 3 months.61
Candiduria may actually represent candidemia in those patients who have a predisposing risk factor for disseminated candidiasis. These risk factors include central venous catheters, administration of total parental nutrition, antibiotic use (especially broad spectrum), critical illness, recent surgical intervention (especially intra-abdominal), acute renal failure, nasogastric tube use, and active gastric acid suppression (ie, proton pump inhibitors).62,63 The hematogenous spread of Candida can lead to the detection of candiduria in 46% to 80% of persons who are experiencing candidemia.59 If the patient is at risk for candidemia, then blood fungal cultures should be drawn. It is not unreasonable to also order a serum-D-glucan assay if suspicions are high. A thorough skin assessment should be completed and ophthalmology consulted for a detailed eye exam in the event that the patient has candidemia. Candiduria is highly prevalent among those who are candidemic, but overall candidemia is encountered in less than 5% of patients in most intensive care units.59 Thus, most patients with candiduria do not have disseminated candidiasis.
Candiduria rarely leads to symptoms of UTI,58 unless the pathogenesis is related to an ascending process.56 Symptoms of Candida UTI are no different from those experienced from a bacterial etiology. Some patients may complain of pneumaturia and/or endorse seeing particulate matter in their urine.55 Patients showing signs of sepsis (fever, chills, flank pain) should be investigated for possible Candida pyelonephritis in the setting of candiduria.64
When should asymptomatic candiduria be treated?
In adult patients with asymptomatic candiduria, there are 2 situations in which antifungal therapy is recommended. A patient undergoing a traumatic urologic procedure would be treated to avoid the risk for candidemia caused by the procedure. Also, in neutropenic patients empiric antifungal therapy should be administered because there is a high likelihood that this candiduria may actually represent hematogenous spread from candidemia.61,65
What is the treatment for symptomatic Candida cystitis?
Empiric treatment with oral fluconazole 200 to 400 mg daily for a total of 2 weeks is recommended in patients with persisting candiduria and symptoms of cystitis.65 Identifying the species is a crucial step in the treatment of Candida UTI. Several species (C. glabrata, C. krusei) are known to be resistant to fluconazole. Species identification and antifungal sensitivities should be done and therapy directed after obtaining these results.55
What is the recommended treatment for Candida pyelonephritis?
Treatment for pyelonephritis caused by fluconazole-susceptible Candida species is oral fluconazole 200 to 400 mg (3-6 mg/kg) for a total of 2 weeks.65 A fluconazole-resistant organism should be suspected when a non-albicans Candida species is isolated, such as C. krusei or C. glabrata. In this circumstance, in vitro antifungal susceptibility testing should be done. Echinocandins are not a good option in this situation because they do not reach adequate urine concentration and treatment failure is well documented.66-68 Amphotericin B deoxycholate (AmB) 0.3 to 0.6 mg/kg daily for 1 to 7 days, with or without oral flucytosine (25 mg/kg) 4 times daily, is recommended by the IDSA for the treatment of fluconazole-resistant isolates of C. glabrata and C. krusei.65 Further imaging with ultrasound, CT, or magnetic resonance should be done to rule out urinary tract obstruction and/or “fungus ball” formation. Emphysematous pyelonephritis and necrosis can occur and usually require nephrectomy. Perinephric abscess will need drainage, which can be accomplished through interventional radiological techniques.55
If a “fungus ball” is suspected in the kidney, how does the management change in a patient with Candida pyelonephritis?
A fungus ball must be treated with both antifungals and surgical intervention. Antifungal therapy should be continued during the surgical removal process to avoid fungemia. Interventional radiology should be consulted and is usually the best option for removal. Fungus ball(s) can and often do cause urinary obstruction. Temporary nephrostomy tube placement may be warranted in these situations to relieve the obstruction.55,65 AmB can be infused through the nephrostomy tube to increase local concentrations. This route of administration is not known to be nephrotoxic.55 Fluconazole infusion through a nephrostomy tube has also been used in the successful treatment of a fungus ball.69
Summary
Health care–associated UTIs are the most common nosocomial infection in the United States. UC placement and genitourinary manipulation or instrumentation play a major role in the development of CA-UTI. Clinicians should be aware of the appropriate and inappropriate use of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key in prevention of CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. A multidisciplinary approach is essential when managing chronic indwelling UCs in SCI. PCN and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected.
Candiduria is an emerging nosocomial source of UTI but rarely leads to symptoms unless related to an ascending process. Proper urine collection is crucial in determining whether you are dealing with contamination, colonization, or infection. If candiduria persists in an asymptomatic individual, then further investigations should be done in regards to possible predisposing risks factors. Fluconazole is recommended for treatment of most patients with Candida UTI, while intravenous AmB is the treatment of choice for fluconazole-resistant Candida species. As we continue to take an evidence-based approach to the prevention and management of health care-associated UTI, we will likely see continued improvement in patient outcomes and overall decreased rates of infection.
Corresponding author: Norman Beatty, MD, 1501 N. Campbell Ave., Tucson, AZ 85724; nbeatty@email.arizona.edu.
Financial disclosures: None.
1. Klevens RM, Edwards JR, Richards CL Jr. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
2. Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003;348:651-656.
3. Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Division of Healthcare Quality Promotion; National Center for Preparedness Detection and Control of Infectious Diseases; Coordinating Center for Infectious Diseases; Centers for Disease Control and Prevention. March 2009. Contract No.: CS200891-A. www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
4. Eriksen HM, Iverson BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40-45.
5. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
6. Mattie AS, Webster BL. Centers for Medicare and Medicaid Services’ “never events”: an analysis and recommendations to hospitals. Health Care Manag. 2008;27:338-349.
7. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents. 2001;17:299-303.
8. Weinstein JW, Mazon D, Pantelick E, et al. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol. 1999;20:543-548.
9. Fakih MG, Pena ME, Shemes S, et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337-340.
10. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
11. Fakih MG, Rey JE, Pena ME, et al. Sustained reductions in urinary catheter use over 5 years: bedside nurses view themselves responsible for evaluation of catheter necessity. Am J Infect Control. 2013;41:236-239.
12. Meddings J, Rogers MA, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infections: an integrative review. BMJ Qual Saf. 2014;23:277-289.
13. Gould C. Catheter-associated urinary tract infection: the national perspective. In: Essential Hospitals Engagement Network. Patient Harm Series II: new tools to prevent CAUTI webinar. April 16, 2014. http://bit.ly/1UWndRA.
14. Nicolle LE. Consequences of asymptomatic bacteriuria in the elderly. Int J Antimicrob Agents. 1994;4:107-111.
15. Nicolle LE. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22:167-175
16. Cohen A. A microbiological comparison of a povidone-iodine lubricating gel and a control as catheter lubricants. J Hosp Infect. 1985;6(Suppl A):155-161.
17. Daifuku R, Stamm WE. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986;314:1208-1213.
18. Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131-136.
19. Nicolle LE. Catheter-related urinary tract infection. Drugs Aging. 2005;22:627-639.
20. Redder JD, Leth RA, Møller JK. Incidence rates of hospital-acquired urinary tract and bloodstream infections generated by automated compilation of electronically available healthcare data. J Hosp Infect. 2015;91:231-236.
21. Ortega M, Marco F, Soriano A, et al. Epidemiology and prognostic determinants of bacteraemic catheter-acquired urinary tract infection in a single institution from 1991 to 2010. J Infect. 2013;67:282-287.
22. Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160:673-677.
23. Musher DM, Thorsteinsson SB, Airola VM, II. Quantitative urinalysis:diagnosing urinary tract infection in men. JAMA. 1976;236:2069-2072.
24. Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16:2016-2030.
25. Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications--where we are and where we are going. Nat Rev Urol. 2015;12:17-25.
26. el-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487-1491.
27. Adamo R, Saad WE, Brown DB. Management of nephrostomy drains and ureteral stents. Tech Vasc Interv Radiol. 2009;12:193-204.
28. Joshi HB, Newns N, Stainthorpe A, et al. Ureteral Stent Symptom Questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060-1064.
29. Singh I, Gupta NP, Hemal AK, et al. Severely encrusted polyurethane ureteral stents: management and analysis of potential risk factors. Urology. 2001;58:526-531.
30. Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am. 2003;17:411-432.
31. Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216-2217.
32. Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891-896.
33. Paick SH, Park HK, Oh SJ, Kim HH. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology. 2003;62:214-217.
34. Klis R, Korczak-Kozakiewicz E, Denys A, et al. Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol. 2009;23:1015-1019.
35. Farsi HM, Mosli HA, Al-Zemaity M, et al. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol. 1995;9:469-472.
36. Wolfe JS Jr, Bennet CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
37. Bahu R, Chaftari AM, Hachem RY, et al. Nephrostomy tube related pyelonephritis in patients with cancer: epidemiology, infection rate and risk factors. J Urol. 2013;189:130-135.
38. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14:73-156.
39. Cronan JJ, Horn DL, Marcello A, et al: Antibiotics and nephrostomy tube care: preliminary observations. Part II. Bacteremia. Radiology. 1989;172(3 Pt 2):1043-1045.
40. Akay AF, Aflay U, Gedik A, et al. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95-98.
41. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/ UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-34.
42. Stickler D, Young R, Jones G, et al. Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res. 2003;31:306-311.
43. Denstedt J, Wollin T, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493-500.
44. Morris N, Stickler D, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol. 1997;80:58-63.
45. Cardenas D, Moore K, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PMR. 2011;3:408-417.
46. Spinu A, Onose G, Daia C, et al. Intermittent catheterization in the management of post spinal cord injury (SCI) neurogenic bladder using new hydrophilic, with lubrication in close circuit devices-our own preliminary results. J Med Life. 2012;5:21-28.
47. Shekelle P, Morton S, Clark K, et al. Systematic review of risk factors for urinary tract infection in adults with spinal cord dysfunction. J Spinal Cord Med. 1998;22:258-272.
48. Siddiq D, Darouiche R. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9:305-314.
49. Gould C, Umscheid C, Agarwal R, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
50. Maki D, Tambyah P. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342-347.
51. Munasinghe R, Yazdani H, Siddique M, et al. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol. 2001;22:647-649.
52. Nicolle L, Bradley S, Colgan R, et al; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40: 643-654.
53. Linsenmeyer T, Oakley A. Accuracy of individuals with spinal cord injury at predicting urinary tract infections based on their symptoms. J Spinal Cord Med. 2002;26:352-357.
54. Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3:174-192.
55. Kauffman CA. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am. 2014;28:61-74.
56. Alvarez-Lerma F, Nolla-Salas J, Leon C, et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003;29:1069-1076.
57. Colodner R, Nuri Y, Chazan B, et al. Community-acquired and hospital-acquired candiduria: comparison of prevalence and clinical characteristics. Eur J Clin Microbiol Infect Dis. 2008;27:301-305.
58. Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14-18.
59. Sobel JD, Fisher JF, Kauffman CA, et al. Candida urinary tract infections—epidemiology. Clin Infect Dis. 2011;52(suppl 6): S433-436.
60. Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510-515.
61. Fisher JF, Sobel JD, Kauffman CA, et al. Candida urinary tract infections—treatment. Clin Infect Dis. 2011;52(suppl 6):S457-466.
62. Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis. 2001;33:177-186.
63. Puzniak LP, Teutsch S, Powderly W, et al. Has the epidemiology of nosocomial candidemia changed? Infect Control Hosp Epidemiol. 2004;25:628-633.
64. Siddique MS, Gayed N, McGuire N, et al. Salient features of Candida pyelonephritis in adults. Infect Dis Clin Pract. 1992;1:239-245
65. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-e50.
66. Sobel JD, Bradshaw SK, Lipka CJ, et al. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis. 2007;44:e46-9.
67. Malani AN. Failure of caspofungin for treatment of Candida glabrata candiduria. Case report and review of the literature. Infect Dis Clin Pract. 2010;18:271-272.
68. Schelenz S, Ross CN. Limitations of caspofugin in the treatment of obstructive pyelonephrosis due to Candida glabrata infection. BMC Infect Dis. 2006;56:126-130.
69. Chung BH, Chang SY, Kim SI, et al. Successfully treated renal fungal ball with continuous irrigation of fluconazole. J Urol. 2001;166:1835-1836.
From the University of Arizona College of Medicine, Tucson, AZ (Dr. Beatty), and the Baylor College of Medicine, Houston, TX (Dr. Mohajer).
Abstract
- Objective: To review management issues regarding health care–associated urinary tract infections (UTIs) commonly encountered by practicing clinicians.
- Methods: Review of the literature.
- Results: Because urinary catheter (UC) placement plays a major role in the development of catheter-associated UTIs (CA-UTI), clinicians should be aware of the appropriate and inappropriate uses of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key to preventing CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. Percutaneous nephrostomy and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected. Candiduria rarely leads to symptoms unless it is related to an ascending process. Proper urine collection is crucial in determining whether contamination, colonization, or infection is present. Fluconazole is recommended in most cases of Candida UTI, while intravenous amphotericin B is recommended for fluconazole-resistant Candida species.
- Conclusion: Continued use of evidence-based strategies for preventing and managing health care–associated UTI should lead to further improvements in patient outcomes and overall decreased rates of infection.
Keywords: bacteriuria; catheter-associated UTI; catheterization; percutaneous nephrostomy; candiduria.
Health care–associated urinary tract infections (UTIs) are estimated to be the most common adverse infectious event in U.S. hospitals, occurring in 1 of 10 admitted patients.1-3 Approximately 32% of all health care–associated infections are UTIs.1 Furthermore, urinary catheters (UCs) are associated with 8% to 21% of health care–associated infections that occur in the intensive care unit.4 The most important predisposing factor for nosocomial UTI is urinary catheterization.5 Genitourinary manipulation and/or implementation also play a major role in the development of nosocomial UTIs.
In 2008, the U.S. Centers for Medicare & Medicaid Services instituted a new policy that reduced reimbursement rates for hospitalizations linked to health care–associated infections.6 Indwelling UCs are among the most overused health care devices in the hospital setting. They are placed in an estimated 15% to 25% of all hospitalized patients,7,8 and are often inserted in the emergency department (ED) without a physician order or appropriate indication.9 Intermittent straight catheterization, male or female condom catheterization, and/or placement of an indwelling UC are the most common causes of catheter-associated asymptomatic bacteriuria (CA-ASB) and catheter-associated UTIs (CA-UTI).5 Prevention and management of CA-ASB and CA-UTI can be challenging and require an evidence-based approach. Furthermore, guidelines for the management of UTIs in the setting of active percutaneous nephrostomy (PCN) drainage and/or ureteral stenting are not established.5 This may leave clinicians with little mainstream data to aid in management decisions.
In 2009 the Centers for Disease Control and Prevention provided a guideline for the appropriate and inappropriate use of indwelling UCs to help promote their proper use.10 In the time since the guideline’s initiatives were instituted around the United States, published data have shown some improvement in the use of UCs,11,12 but other recent reports indicate that rates of UC use have remained unchanged.13 This review discusses management issues regarding health care–associated UTIs that are commonly encountered by practicing clinicians, with a focus on current guidelines and evidence.
Catheter-Associated UTI
CA-UTI is defined as the presence of signs or symptoms of UTI with no other explainable infectious source along with ≥ 1000 colony-forming units (cfu) of ≥ 1 bacterial species per milliliter in a urine specimen from a catheter that has been changed within 48 hours of collection of the urine specimen.5 Signs and symptoms of CA-UTI include, but are not limited to: new-onset or worsening fever, chills, altered sensorium from baseline, lethargy, malaise, flank pain, pelvic pain, costovertebral angle tenderness, and acute hematuria.5 New-onset “foul-smelling” (odorous)urine and “cloudy” urine are neither sensitive nor specific when assessing for CA-UTI, and do not have significant clinical relevance when found alone.14,15 Patients who have removed or exchanged the UC during this event and then experience dysuria, increased frequency, urgency, or suprapubic pain are likely having symptoms of CA-UTI.5
What is the recommended method for collecting urine samples when CA-UTI is suspected?
In a patient with an indwelling catheter that has been in place for more than 2 weeks at the onset of a suspected CA-UTI, the catheter should be replaced (if still indicated) or removed to accelerate resolution of symptoms and to reduce the risk of subsequent catheter-associated bacteriuria and CA-UTI. The urine culture should be acquired from the freshly placed UC.5
When should a patient be empirically treated?
A patient presenting with evidence of sepsis should be empirically treated with antimicrobials. Empiric coverage should be based on risk factors for multidrug-resistant organisms and data pertaining to local antimicrobial resistance patterns. A urine specimen for urinalysis and possible culture should be sent prior to administering empiric antibiotics (if possible) in a symptomatic patient.5
What bacteria are commonly associated with CA-UTI?
The bacteria most commonly associated with CA-UTI are found in or around the gastrointestinal and genitourinary tracts and also are part of the normal skin flora. The introduction and/or facilitated ascension of these microorganisms is believed to occur during UC insertion.16,17 Two-thirds of all isolated uropathogens in those with indwelling UCs are extraluminally acquired (via ascension along the catheter-urethral mucosa interface), and one-third are believed to be intraluminally acquired.18
The most commonly isolated bacteria in CA-UTI are Enterobacteriaceae, which include Escherichia coli (most common), Klebsiella species (K. oxytoca, K. pneumoniae), Serratia species (S. marcescens), Citrobacter species (C. koseri), Enterobacter species (E. cloacae), and Proteus species; non-Enterobacteriaceae such as Pseudomonas species; and gram-positive cocci, which include coagulase-negative staphylococci (S. saprophyticus), Staphylococcus aureus, group B streptococci, and Enterococcus species (E. faecalis, E. faecium).19-21 Coagulase-negative staphylococci and Enterococcus species can lead to CA-UTI but are usually avirulent and more commonly isolated from asymptomatic individuals.19 Also, coagulase-negative staphylococci such as S. epidermidis and S. lugdunensis are usually the manifestation of contamination during the collection process and their presence should prompt a repeat sample collection under sterile techniques. Monomicrobial infection is usually seen in those with short-term catheter use and CA-UTI. In contrast, polymicrobial infection is more common in those with long-term indwelling UCs and CA-UTI.19 Providencia stuartii, Proteus mirabilis, S. aureus, and Morganella morganii have all been associated with CA-UTI in those with long-term indwelling UCs.
Growth of S. aureus in the urine should prompt further investigation with blood cultures to explore the possibility of hematogenous dissemination to the urinary tract. Organisms leading to bacteremia due to CA-UTI are most commonly gram-negative bacilli (E. coli, Klebsiella species, Pseudomonas aeruginosa) and E. faecalis.21
What is the difference between CA-ASB and CA-UTI?
CA-ASB is defined as the presence of ≥ 1 bacteria species growing on urine culture at ≥ 100,000 cfu/mL in a patient with a history of urinary catheterization and/or indwelling UC who lacks signs or symptoms of UTI. In a man with a condom catheter, CA-ASB is defined using the same criteria, but the urine sample is collected after a fresh condom catheter is applied.5 The difference between CA-ASB and CA-UTI is simply the presence or absence of signs and symptoms related to UTI. Currently, there is no standard definition for significant bacteriuria in a catheterized patient.5 Pyuria found on urinalysis is indicative of genitourinary inflammation and can be present in both CA-ASB and CA-UTI. The absence, presence, and/or degree of pyuria in catheterized patients does not accurately differentiate between CA-ASB and CA-UTI.5,22,23 On the other hand, the absence of pyuria in a symptomatic catheterized patient suggests an etiology other than CA-UTI.5
How can CA-UTI be prevented in patients with a short-term indwelling urinary catheter?
If a short-term UC is essential, the most important approach to preventing CA-UTI is limiting the duration of time it will be used. Strategies such as computer-based order entry and care maps with automated discontinuation of UCs have been shown to decrease catheter usage.19 Using closed-systems for UC collection with ports in the distal catheter for needle aspiration of urine has also been shown to decrease the incidence of CA-UTI.5 Securing the UC to avoid urethral trauma, aseptic techniques for insertion and repositioning, and placement of the tubing and collection bag below the level of the bladder to prevent reflux will likely also prevent CA-UTI, but these strategies have not been evaluated thoroughly.19
When should you screen for and treat CA-ASB?
The 2009 Infectious Diseases Society of America (IDSA) guidelines recommend that the only patients who should be screened and treated for CA-ASB are pregnant women and those who will undergo a potentially traumatic urologic procedure for which mucosal breaching may occur, causing bleeding. Routinely screening or treating patients for CA-ASB in not recommended in any other group of patients and will lead to unnecessary antibiotic use and antibiotic resistance.5
UTI Associated with Percutaneous Nephrostomy and Ureteral Stenting
Similar systemic symptoms of infection (fever, rigors, malaise, shock) are present in patients with and without percutaneous nephrostomy (PCN) and/or ureteral stent placement. Dysuria is not commonly present in those with PCN. The first signs of CA-UTI may be decreased urine output and pericatheter leakage due to an obstructive process resulting from the encrustation.24-27 The most common complaint among patients with either acute or chronic ureteral stenting is discomfort, which has been described as “urinary symptoms” and “body pain.”28 This discomfort can be related to ureteral hyperperistalsis after placement of the stent and is usually self-limiting. Ureteral stent migration, usually at the distal end, can also lead to discomfort, but is easily rectified with cystoscopy.25 Body pain and/or urinary symptoms in the setting of ureteral stenting are not indicative of infection alone.
What is urinary catheter and/or ureteral stent encrustation?
Encrustation is the formation of a conditioning film that develops on the surface of the UC or ureteral stent. The exact mechanism is not well understood, but it is believed to involve electrostatic interactions of urinary proteins that stimulate binding onto the stent or UC surface.25 Encrustation increases exponentially with the dwell time. Among patients with ureteral stents placed due to urolithiasis, encrustation occurred in 9.2% of stents removed prior to 6 weeks, 47.5% of stents removed at 6 weeks, and 76.3% of stents removed at 12 weeks.26 Encrustation is most common at the proximal and distal ends (pigtails) of the ureteral stent and usually spares or presents last within the lumen.29 Attempts have been made to prevent ureteral stent encrustation through the development of biodegradable, drug-eluting, and tissue-engineered substrates. These developments are promising, but currently there is limited observational data from large randomized trials to suggest that these new modalities decrease rates of encrustation.25 Encrustation is highly associated with certain microorganisms, especially those that create biofilms.30 Urease-producing bacteria, most commonly P. mirabilis, play a role in encrustation formation.31 Bacteria most commonly associated with encrustation include Proteus species (P. mirabilis is most common), P. aeruginosa, K. pneumoniae, Providencia species (P. stuartii is most common), and M. morganii.
Does ureteral stent bacterial colonization correlate with UTI?
Ureteral stent colonization with bacteria increases with dwell time and is found in 40% to 98.5% of stents placed.32-35 If UTI is suspected in a patient with an active indwelling ureteral stent, a sample of urine should be cultured while the stent is in place.25 Typically, genitourinary and normal skin flora pathogens are found when the ureteral stent is cultured. The top 3 organisms cultured from ureteral stents are S. aureus, P. aeruginosa, and E. faecalis.34 Urine culture usually does not correlate with stent culture results, which has brought up the debate of how bacterial colonization occurs. It has been postulated that colonization is actually a manifestation of contamination during the insertion procedure, but this has yet to be validated.25 In patients with symptoms of UTI in the setting of an indwelling ureteral stent, a positive culture has low sensitivity, with estimates between 21% and 40%.35 Therefore, a negative urine culture does not rule out UTI alone in a symptomatic patient. Multiple studies have suggested that colonization of the ureteral stent does not correlate strongly with developing a UTI.25,32-34
How can UTI be prevented in those receiving PCN or ureteral stent placement?
Antibiotic prophylaxis has been recommended to prevent UTI in patients who will undergo PCN or ureteral stent placement. The American Urological Association recommends empiric treatment even in the absence of signs and symptoms of UTI,36 but substantial evidence is lacking that this approach prevents infection.37 Ciprofloxacin or trimethoprim/sulfamethoxazole has been recommended by some for empiric coverage for enteric gram-negative bacilli and enterococcus in those undergoing genitourinary manipulation or instrumentation.32,38 Most patients who develop CA-UTI and pyelonephritis do so within the first 2 to 6 weeks after placement.37,39 Bacteriuria, candiduria, and/or pyuria are present in all patients approximately within 9 weeks even when sterile urine is confirmed prior to PCN placement.39 Data on the effectiveness of antibiotic prophylaxis to prevent CA-UTI in those with PCN or ureteral stenting is limited. Currently, there are no recommendations from the IDSA on how to prevent infection in these situations.5 Early or frequent stent removal or exchanges has been proven to reduce UTI in those with ureteral stenting.33 Patients with diabetes mellitus and chronic renal failure are at high-risk for UTI when ureteral stents are in place. This population should undergo close monitoring for UTI development and may warrant more frequent stent exchanges.27,40
What is the treatment of CA-UTI associated with PCN and/or ureteral stenting?
The IDSA guidelines do not apply to patients with PCN and/or ureteral stenting.5 There is no treatment protocol for UTI related to these processes. Generally speaking, they are considered “complicated UTI” by most experts. Broad-spectrum, empiric antibiotic administration along with prompt removal of the PCN and/or ureteral stent is the gold standard of therapy.27 The recommended duration of targeted antibiotic therapy is generally between 5 and 14 days.19 Most clinicians will treat this complicated UTI for at least 10 to 14 days. Antibiotic administration should be continued even after removal of the catheter and/or stent to complete the full course. Repeat urinalysis and culture is not indicated at the end of therapy if the patient is clinically improving or has remission of symptoms.
What is the exchange rate for those who require chronic PCN and/or ureteral stent use?
On average a PCN or ureteral stent should be exchanged every 2 to 3 months in patients who require chronic usage.24,27 Some patients with persistent complications may require more frequent exchanges (< 10 weeks).27 Encrustation and bacterial colonization become more prevalent the longer the devices are in place. This process is estimated to begin within the first 2 weeks after placement.27,33,34 A “forgotten stent” is one that has been left in place after the patient is lost to follow-up. This unfortunate event can lead to massive encrustation, UTI, stent fracturing, and complete ureteral obstruction.24 As noted, patients with diabetes mellitus, chronic renal failure, and frequent UTI may warrant more frequent exchanges, but this should be determined on a case-by-case basis.
Catheter-Associated UTI in Patients with Spinal Cord Injury
Spinal cord injury (SCI) at any level can cause neurogenic bladder. This process ultimately leads to urinary stasis and colonization of the bladder with bacteria. According to the IDSA, the acceptable indications for UC insertion are: clinically significant urinary retention (if medical therapy is not effective), urinary incontinence, accurate urine output monitoring required, and patient is unable and/or unwilling to collect urine (Table 1).5 Recently, further guidelines were published regarding appropriate and inappropriate indwelling UC placement in hospitalized medical patients (Table 2 and Table 3), expanding upon the earlier acceptable criteria provided by the IDSA.41 According to the IDSA and Ann Arbor Criteria for Appropriate Urinary Catheter Use, patients with SCI and subsequent neurogenic bladder without obstruction where intermittent bladder straight catheterization for the drainage of urine is not feasible will likely need an indwelling UC.41 SCI patients often experience decubitus ulcers, and an indwelling UC can be used if needed to help with wound healing if other urinary management alternatives have been attempted. Other such situations in which an indwelling UC can used before attempting alternative approaches would be in a patient who is actively dying and is pursuing comfort care and/or hospice.41
Which indwelling UCs should be used in patients with SCI?
Efforts to reduce the likelihood of infection in patients with SCI have led to several advances in the design and manufacturing of UCs. UCs are made of either latex, plastic, silicone, or polytetrafluoroethylene (Teflon). None of these substrates is free of complications, but of them latex UCs have been studied the most in regards to their associated complications. Aside from being allergenic in nature, latex UCs have an increased propensity to allow bacteria to adhere to their surface due to microscopic planes of unevenness.42 Silicone UCs are less frequently associated with infection but are more rigid, leading to increased discomfort.43,44 Hydrophilic and silver-hydrogel coatings are innovative methods that have been developed to increase comfort and reduce the likelihood of infection. Hydrophilic-coated UCs are associated with reduced microbial adherence, decreased encrustation, and better patient satisfaction.45,46 In SCI patients, these UCs have demonstrated lower complication rates, including UTI; fewer episodes of post-, intra-, and inter-catheterization bleeding; and decreased rates of antibiotic-resistant bacteria.45,46 Silver-hydrogel-coated UCs are less well studied but have also demonstrated reduced UTI rates in SCI patients; however, their efficacy over the long term has yet to be determined. Antibiotic-impregnated UCs are not currently recommended for either short- or long-term indwelling UC use.5
How can CA-UTI be prevented in a patient who will require a long-term indwelling catheter?
At this time, the data is insufficient to make a recommendation on routine UC exchange (eg, every 2 to 4 weeks) in patients who require long-term indwelling urethral or suprapubic catheters in an attempt to reduce the risk of CA-ASB or CA-UTI. This is also true for those who experience even repeated early catheter blockage from encrustation.5 Thus, the rate at which these exchanges occur can be controversial, but typically around every 4 weeks is a common approach. Some would argue that if the patient has repeated CA-UTI, an exchange rate of every 2 weeks might be needed, but data is currently lacking to support this practice.5 If intermittent urinary catheterization is feasible, it should be done at least every 6 hours and before bedtime. In general, when the volume of urine in the bladder reaches approximately 400 mL, the patient should undergo bladder catheterization to prevent stasis and infection.47 A closed drainage system is recommended in all patients who require long-term indwelling UC use.48,49 Placement of the collection bag above the catheter or above the level of the bladder and a breach in the closed drainage system have been shown to result in higher rates of catheter-associated bacteriuria.48,50 Proper hand hygiene and sterile and/or clean techniques should be used when placing or exchanging a UC. However, in one study there was no difference in bacteremia or UTIs when using sterile versus clean techniques.51
Asymptomatic bacteriuria should not be treated in patients with long-term indwelling UCs, and prophylactic antibiotics have led to the emergence of resistance.52 At this time it is not clear whether prophylactic weekly oral cyclic antibiotic administration can effectively reduce the frequency of CA-UTI in patients with SCI and chronic indwelling UC use.
What are the signs and symptoms of CA-UTI in a patient with SCI?
Subjective and objective findings may be limited when a patient with SCI presents with CA-UTI. These patients often lack the usual symptoms of UTI (dysuria, suprapubic discomfort, urgency, increased frequency) and pyelonephritis (flank pain, costovertebral angle tenderness). Caregivers and health care providers should be aware that nonspecific symptoms such as foul-smelling urine, pyuria, increased residual volume of urine in the bladder, change in voiding habits, worsening detrusor spasticity, and aggravation of autonomic dysreflexia can be the only initial presenting symptoms.53
What is the duration of therapy for CA-UTI in SCI, and how can antibiotic stewardship principles be applied in this patient population?
Antibiotic therapy is indicated for a duration of 7 days if there is prompt resolution of symptoms, or for a total of 10 to 14 days if the response is delayed, regardless of whether the patient remains with a UC.5 Antibiotic stewardship is very important to reduce the risk for developing drug resistance in this high-risk population. Methods such as prescriber education practices, institution protocols, guideline implementation, auditing and feedback, restriction and reauthorization practices, computer-assisted programs, de-escalation or streamlining, and antibiotic cycling or dosage optimization have all been shown to assist in antibiotic stewardship in UTIs.54
Candida UTI
What is the initial evaluation for a patient with candiduria?
The workup for candiduria hinges on determining whether the candiduria likely represents contamination, colonization, or infection; certain predisposing risk factors are associated with Candida UTI (Table 4).55-57 The most important aspect to candiduria is the patient’s clinical status and comorbid conditions.58 Among funguria, Candida species are the most common and represent 95% of organisms isolated on urine cultures (Table 5).59 Candiduria is usually present in those with significant comorbidities and rarely is associated with healthy individuals.59,60 Candiduria is increasing in prevalence among hospitalized patients, representing 22% to 40% of all nosocomial UTIs.59,60 Markers in the urine (leukocyte esterase, colony count of culture growth, presence or absence of Candida casts and pseudohyphae) cannot alone differentiate colonization from infection.55 When candiduria is discovered in a patient with symptoms related to UTI in the setting of predisposing risk factors, it should be considered a real infection until proven otherwise.
In a situation where an asymptomatic patient without an indwelling UC has Candida species isolated from a urine culture, a repeat culture (clean-catch midstream sample) should be performed to assess for a likely contaminated collection.55 If the patient has an indwelling UC then it should be exchanged and urine collected from the fresh catheter.61 When candiduria is found in healthy asymptomatic adults, it is most commonly associated with poor collection techniques or postcollection contamination.59 If candiduria persists in an asymptomatic patient, the patient should be assessed for predisposing factors. This includes checking hemoglobin A1C for developing diabetes and renal ultrasound looking for urolithiasis, renal abscess, hydronephrosis, and fungus ball. Postvoid residual urinary retention should also be ruled out with bladder ultrasound. Treatment of predisposing factors can lead to resolution of candiduria without antifungal treatment, and a urine culture should be repeated (1 to 2 weeks later).61 Asymptomatic patients lacking any predisposing factors can be observed with repeat urine cultures in 1 to 3 months.61
Candiduria may actually represent candidemia in those patients who have a predisposing risk factor for disseminated candidiasis. These risk factors include central venous catheters, administration of total parental nutrition, antibiotic use (especially broad spectrum), critical illness, recent surgical intervention (especially intra-abdominal), acute renal failure, nasogastric tube use, and active gastric acid suppression (ie, proton pump inhibitors).62,63 The hematogenous spread of Candida can lead to the detection of candiduria in 46% to 80% of persons who are experiencing candidemia.59 If the patient is at risk for candidemia, then blood fungal cultures should be drawn. It is not unreasonable to also order a serum-D-glucan assay if suspicions are high. A thorough skin assessment should be completed and ophthalmology consulted for a detailed eye exam in the event that the patient has candidemia. Candiduria is highly prevalent among those who are candidemic, but overall candidemia is encountered in less than 5% of patients in most intensive care units.59 Thus, most patients with candiduria do not have disseminated candidiasis.
Candiduria rarely leads to symptoms of UTI,58 unless the pathogenesis is related to an ascending process.56 Symptoms of Candida UTI are no different from those experienced from a bacterial etiology. Some patients may complain of pneumaturia and/or endorse seeing particulate matter in their urine.55 Patients showing signs of sepsis (fever, chills, flank pain) should be investigated for possible Candida pyelonephritis in the setting of candiduria.64
When should asymptomatic candiduria be treated?
In adult patients with asymptomatic candiduria, there are 2 situations in which antifungal therapy is recommended. A patient undergoing a traumatic urologic procedure would be treated to avoid the risk for candidemia caused by the procedure. Also, in neutropenic patients empiric antifungal therapy should be administered because there is a high likelihood that this candiduria may actually represent hematogenous spread from candidemia.61,65
What is the treatment for symptomatic Candida cystitis?
Empiric treatment with oral fluconazole 200 to 400 mg daily for a total of 2 weeks is recommended in patients with persisting candiduria and symptoms of cystitis.65 Identifying the species is a crucial step in the treatment of Candida UTI. Several species (C. glabrata, C. krusei) are known to be resistant to fluconazole. Species identification and antifungal sensitivities should be done and therapy directed after obtaining these results.55
What is the recommended treatment for Candida pyelonephritis?
Treatment for pyelonephritis caused by fluconazole-susceptible Candida species is oral fluconazole 200 to 400 mg (3-6 mg/kg) for a total of 2 weeks.65 A fluconazole-resistant organism should be suspected when a non-albicans Candida species is isolated, such as C. krusei or C. glabrata. In this circumstance, in vitro antifungal susceptibility testing should be done. Echinocandins are not a good option in this situation because they do not reach adequate urine concentration and treatment failure is well documented.66-68 Amphotericin B deoxycholate (AmB) 0.3 to 0.6 mg/kg daily for 1 to 7 days, with or without oral flucytosine (25 mg/kg) 4 times daily, is recommended by the IDSA for the treatment of fluconazole-resistant isolates of C. glabrata and C. krusei.65 Further imaging with ultrasound, CT, or magnetic resonance should be done to rule out urinary tract obstruction and/or “fungus ball” formation. Emphysematous pyelonephritis and necrosis can occur and usually require nephrectomy. Perinephric abscess will need drainage, which can be accomplished through interventional radiological techniques.55
If a “fungus ball” is suspected in the kidney, how does the management change in a patient with Candida pyelonephritis?
A fungus ball must be treated with both antifungals and surgical intervention. Antifungal therapy should be continued during the surgical removal process to avoid fungemia. Interventional radiology should be consulted and is usually the best option for removal. Fungus ball(s) can and often do cause urinary obstruction. Temporary nephrostomy tube placement may be warranted in these situations to relieve the obstruction.55,65 AmB can be infused through the nephrostomy tube to increase local concentrations. This route of administration is not known to be nephrotoxic.55 Fluconazole infusion through a nephrostomy tube has also been used in the successful treatment of a fungus ball.69
Summary
Health care–associated UTIs are the most common nosocomial infection in the United States. UC placement and genitourinary manipulation or instrumentation play a major role in the development of CA-UTI. Clinicians should be aware of the appropriate and inappropriate use of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key in prevention of CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. A multidisciplinary approach is essential when managing chronic indwelling UCs in SCI. PCN and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected.
Candiduria is an emerging nosocomial source of UTI but rarely leads to symptoms unless related to an ascending process. Proper urine collection is crucial in determining whether you are dealing with contamination, colonization, or infection. If candiduria persists in an asymptomatic individual, then further investigations should be done in regards to possible predisposing risks factors. Fluconazole is recommended for treatment of most patients with Candida UTI, while intravenous AmB is the treatment of choice for fluconazole-resistant Candida species. As we continue to take an evidence-based approach to the prevention and management of health care-associated UTI, we will likely see continued improvement in patient outcomes and overall decreased rates of infection.
Corresponding author: Norman Beatty, MD, 1501 N. Campbell Ave., Tucson, AZ 85724; nbeatty@email.arizona.edu.
Financial disclosures: None.
From the University of Arizona College of Medicine, Tucson, AZ (Dr. Beatty), and the Baylor College of Medicine, Houston, TX (Dr. Mohajer).
Abstract
- Objective: To review management issues regarding health care–associated urinary tract infections (UTIs) commonly encountered by practicing clinicians.
- Methods: Review of the literature.
- Results: Because urinary catheter (UC) placement plays a major role in the development of catheter-associated UTIs (CA-UTI), clinicians should be aware of the appropriate and inappropriate uses of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key to preventing CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. Percutaneous nephrostomy and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected. Candiduria rarely leads to symptoms unless it is related to an ascending process. Proper urine collection is crucial in determining whether contamination, colonization, or infection is present. Fluconazole is recommended in most cases of Candida UTI, while intravenous amphotericin B is recommended for fluconazole-resistant Candida species.
- Conclusion: Continued use of evidence-based strategies for preventing and managing health care–associated UTI should lead to further improvements in patient outcomes and overall decreased rates of infection.
Keywords: bacteriuria; catheter-associated UTI; catheterization; percutaneous nephrostomy; candiduria.
Health care–associated urinary tract infections (UTIs) are estimated to be the most common adverse infectious event in U.S. hospitals, occurring in 1 of 10 admitted patients.1-3 Approximately 32% of all health care–associated infections are UTIs.1 Furthermore, urinary catheters (UCs) are associated with 8% to 21% of health care–associated infections that occur in the intensive care unit.4 The most important predisposing factor for nosocomial UTI is urinary catheterization.5 Genitourinary manipulation and/or implementation also play a major role in the development of nosocomial UTIs.
In 2008, the U.S. Centers for Medicare & Medicaid Services instituted a new policy that reduced reimbursement rates for hospitalizations linked to health care–associated infections.6 Indwelling UCs are among the most overused health care devices in the hospital setting. They are placed in an estimated 15% to 25% of all hospitalized patients,7,8 and are often inserted in the emergency department (ED) without a physician order or appropriate indication.9 Intermittent straight catheterization, male or female condom catheterization, and/or placement of an indwelling UC are the most common causes of catheter-associated asymptomatic bacteriuria (CA-ASB) and catheter-associated UTIs (CA-UTI).5 Prevention and management of CA-ASB and CA-UTI can be challenging and require an evidence-based approach. Furthermore, guidelines for the management of UTIs in the setting of active percutaneous nephrostomy (PCN) drainage and/or ureteral stenting are not established.5 This may leave clinicians with little mainstream data to aid in management decisions.
In 2009 the Centers for Disease Control and Prevention provided a guideline for the appropriate and inappropriate use of indwelling UCs to help promote their proper use.10 In the time since the guideline’s initiatives were instituted around the United States, published data have shown some improvement in the use of UCs,11,12 but other recent reports indicate that rates of UC use have remained unchanged.13 This review discusses management issues regarding health care–associated UTIs that are commonly encountered by practicing clinicians, with a focus on current guidelines and evidence.
Catheter-Associated UTI
CA-UTI is defined as the presence of signs or symptoms of UTI with no other explainable infectious source along with ≥ 1000 colony-forming units (cfu) of ≥ 1 bacterial species per milliliter in a urine specimen from a catheter that has been changed within 48 hours of collection of the urine specimen.5 Signs and symptoms of CA-UTI include, but are not limited to: new-onset or worsening fever, chills, altered sensorium from baseline, lethargy, malaise, flank pain, pelvic pain, costovertebral angle tenderness, and acute hematuria.5 New-onset “foul-smelling” (odorous)urine and “cloudy” urine are neither sensitive nor specific when assessing for CA-UTI, and do not have significant clinical relevance when found alone.14,15 Patients who have removed or exchanged the UC during this event and then experience dysuria, increased frequency, urgency, or suprapubic pain are likely having symptoms of CA-UTI.5
What is the recommended method for collecting urine samples when CA-UTI is suspected?
In a patient with an indwelling catheter that has been in place for more than 2 weeks at the onset of a suspected CA-UTI, the catheter should be replaced (if still indicated) or removed to accelerate resolution of symptoms and to reduce the risk of subsequent catheter-associated bacteriuria and CA-UTI. The urine culture should be acquired from the freshly placed UC.5
When should a patient be empirically treated?
A patient presenting with evidence of sepsis should be empirically treated with antimicrobials. Empiric coverage should be based on risk factors for multidrug-resistant organisms and data pertaining to local antimicrobial resistance patterns. A urine specimen for urinalysis and possible culture should be sent prior to administering empiric antibiotics (if possible) in a symptomatic patient.5
What bacteria are commonly associated with CA-UTI?
The bacteria most commonly associated with CA-UTI are found in or around the gastrointestinal and genitourinary tracts and also are part of the normal skin flora. The introduction and/or facilitated ascension of these microorganisms is believed to occur during UC insertion.16,17 Two-thirds of all isolated uropathogens in those with indwelling UCs are extraluminally acquired (via ascension along the catheter-urethral mucosa interface), and one-third are believed to be intraluminally acquired.18
The most commonly isolated bacteria in CA-UTI are Enterobacteriaceae, which include Escherichia coli (most common), Klebsiella species (K. oxytoca, K. pneumoniae), Serratia species (S. marcescens), Citrobacter species (C. koseri), Enterobacter species (E. cloacae), and Proteus species; non-Enterobacteriaceae such as Pseudomonas species; and gram-positive cocci, which include coagulase-negative staphylococci (S. saprophyticus), Staphylococcus aureus, group B streptococci, and Enterococcus species (E. faecalis, E. faecium).19-21 Coagulase-negative staphylococci and Enterococcus species can lead to CA-UTI but are usually avirulent and more commonly isolated from asymptomatic individuals.19 Also, coagulase-negative staphylococci such as S. epidermidis and S. lugdunensis are usually the manifestation of contamination during the collection process and their presence should prompt a repeat sample collection under sterile techniques. Monomicrobial infection is usually seen in those with short-term catheter use and CA-UTI. In contrast, polymicrobial infection is more common in those with long-term indwelling UCs and CA-UTI.19 Providencia stuartii, Proteus mirabilis, S. aureus, and Morganella morganii have all been associated with CA-UTI in those with long-term indwelling UCs.
Growth of S. aureus in the urine should prompt further investigation with blood cultures to explore the possibility of hematogenous dissemination to the urinary tract. Organisms leading to bacteremia due to CA-UTI are most commonly gram-negative bacilli (E. coli, Klebsiella species, Pseudomonas aeruginosa) and E. faecalis.21
What is the difference between CA-ASB and CA-UTI?
CA-ASB is defined as the presence of ≥ 1 bacteria species growing on urine culture at ≥ 100,000 cfu/mL in a patient with a history of urinary catheterization and/or indwelling UC who lacks signs or symptoms of UTI. In a man with a condom catheter, CA-ASB is defined using the same criteria, but the urine sample is collected after a fresh condom catheter is applied.5 The difference between CA-ASB and CA-UTI is simply the presence or absence of signs and symptoms related to UTI. Currently, there is no standard definition for significant bacteriuria in a catheterized patient.5 Pyuria found on urinalysis is indicative of genitourinary inflammation and can be present in both CA-ASB and CA-UTI. The absence, presence, and/or degree of pyuria in catheterized patients does not accurately differentiate between CA-ASB and CA-UTI.5,22,23 On the other hand, the absence of pyuria in a symptomatic catheterized patient suggests an etiology other than CA-UTI.5
How can CA-UTI be prevented in patients with a short-term indwelling urinary catheter?
If a short-term UC is essential, the most important approach to preventing CA-UTI is limiting the duration of time it will be used. Strategies such as computer-based order entry and care maps with automated discontinuation of UCs have been shown to decrease catheter usage.19 Using closed-systems for UC collection with ports in the distal catheter for needle aspiration of urine has also been shown to decrease the incidence of CA-UTI.5 Securing the UC to avoid urethral trauma, aseptic techniques for insertion and repositioning, and placement of the tubing and collection bag below the level of the bladder to prevent reflux will likely also prevent CA-UTI, but these strategies have not been evaluated thoroughly.19
When should you screen for and treat CA-ASB?
The 2009 Infectious Diseases Society of America (IDSA) guidelines recommend that the only patients who should be screened and treated for CA-ASB are pregnant women and those who will undergo a potentially traumatic urologic procedure for which mucosal breaching may occur, causing bleeding. Routinely screening or treating patients for CA-ASB in not recommended in any other group of patients and will lead to unnecessary antibiotic use and antibiotic resistance.5
UTI Associated with Percutaneous Nephrostomy and Ureteral Stenting
Similar systemic symptoms of infection (fever, rigors, malaise, shock) are present in patients with and without percutaneous nephrostomy (PCN) and/or ureteral stent placement. Dysuria is not commonly present in those with PCN. The first signs of CA-UTI may be decreased urine output and pericatheter leakage due to an obstructive process resulting from the encrustation.24-27 The most common complaint among patients with either acute or chronic ureteral stenting is discomfort, which has been described as “urinary symptoms” and “body pain.”28 This discomfort can be related to ureteral hyperperistalsis after placement of the stent and is usually self-limiting. Ureteral stent migration, usually at the distal end, can also lead to discomfort, but is easily rectified with cystoscopy.25 Body pain and/or urinary symptoms in the setting of ureteral stenting are not indicative of infection alone.
What is urinary catheter and/or ureteral stent encrustation?
Encrustation is the formation of a conditioning film that develops on the surface of the UC or ureteral stent. The exact mechanism is not well understood, but it is believed to involve electrostatic interactions of urinary proteins that stimulate binding onto the stent or UC surface.25 Encrustation increases exponentially with the dwell time. Among patients with ureteral stents placed due to urolithiasis, encrustation occurred in 9.2% of stents removed prior to 6 weeks, 47.5% of stents removed at 6 weeks, and 76.3% of stents removed at 12 weeks.26 Encrustation is most common at the proximal and distal ends (pigtails) of the ureteral stent and usually spares or presents last within the lumen.29 Attempts have been made to prevent ureteral stent encrustation through the development of biodegradable, drug-eluting, and tissue-engineered substrates. These developments are promising, but currently there is limited observational data from large randomized trials to suggest that these new modalities decrease rates of encrustation.25 Encrustation is highly associated with certain microorganisms, especially those that create biofilms.30 Urease-producing bacteria, most commonly P. mirabilis, play a role in encrustation formation.31 Bacteria most commonly associated with encrustation include Proteus species (P. mirabilis is most common), P. aeruginosa, K. pneumoniae, Providencia species (P. stuartii is most common), and M. morganii.
Does ureteral stent bacterial colonization correlate with UTI?
Ureteral stent colonization with bacteria increases with dwell time and is found in 40% to 98.5% of stents placed.32-35 If UTI is suspected in a patient with an active indwelling ureteral stent, a sample of urine should be cultured while the stent is in place.25 Typically, genitourinary and normal skin flora pathogens are found when the ureteral stent is cultured. The top 3 organisms cultured from ureteral stents are S. aureus, P. aeruginosa, and E. faecalis.34 Urine culture usually does not correlate with stent culture results, which has brought up the debate of how bacterial colonization occurs. It has been postulated that colonization is actually a manifestation of contamination during the insertion procedure, but this has yet to be validated.25 In patients with symptoms of UTI in the setting of an indwelling ureteral stent, a positive culture has low sensitivity, with estimates between 21% and 40%.35 Therefore, a negative urine culture does not rule out UTI alone in a symptomatic patient. Multiple studies have suggested that colonization of the ureteral stent does not correlate strongly with developing a UTI.25,32-34
How can UTI be prevented in those receiving PCN or ureteral stent placement?
Antibiotic prophylaxis has been recommended to prevent UTI in patients who will undergo PCN or ureteral stent placement. The American Urological Association recommends empiric treatment even in the absence of signs and symptoms of UTI,36 but substantial evidence is lacking that this approach prevents infection.37 Ciprofloxacin or trimethoprim/sulfamethoxazole has been recommended by some for empiric coverage for enteric gram-negative bacilli and enterococcus in those undergoing genitourinary manipulation or instrumentation.32,38 Most patients who develop CA-UTI and pyelonephritis do so within the first 2 to 6 weeks after placement.37,39 Bacteriuria, candiduria, and/or pyuria are present in all patients approximately within 9 weeks even when sterile urine is confirmed prior to PCN placement.39 Data on the effectiveness of antibiotic prophylaxis to prevent CA-UTI in those with PCN or ureteral stenting is limited. Currently, there are no recommendations from the IDSA on how to prevent infection in these situations.5 Early or frequent stent removal or exchanges has been proven to reduce UTI in those with ureteral stenting.33 Patients with diabetes mellitus and chronic renal failure are at high-risk for UTI when ureteral stents are in place. This population should undergo close monitoring for UTI development and may warrant more frequent stent exchanges.27,40
What is the treatment of CA-UTI associated with PCN and/or ureteral stenting?
The IDSA guidelines do not apply to patients with PCN and/or ureteral stenting.5 There is no treatment protocol for UTI related to these processes. Generally speaking, they are considered “complicated UTI” by most experts. Broad-spectrum, empiric antibiotic administration along with prompt removal of the PCN and/or ureteral stent is the gold standard of therapy.27 The recommended duration of targeted antibiotic therapy is generally between 5 and 14 days.19 Most clinicians will treat this complicated UTI for at least 10 to 14 days. Antibiotic administration should be continued even after removal of the catheter and/or stent to complete the full course. Repeat urinalysis and culture is not indicated at the end of therapy if the patient is clinically improving or has remission of symptoms.
What is the exchange rate for those who require chronic PCN and/or ureteral stent use?
On average a PCN or ureteral stent should be exchanged every 2 to 3 months in patients who require chronic usage.24,27 Some patients with persistent complications may require more frequent exchanges (< 10 weeks).27 Encrustation and bacterial colonization become more prevalent the longer the devices are in place. This process is estimated to begin within the first 2 weeks after placement.27,33,34 A “forgotten stent” is one that has been left in place after the patient is lost to follow-up. This unfortunate event can lead to massive encrustation, UTI, stent fracturing, and complete ureteral obstruction.24 As noted, patients with diabetes mellitus, chronic renal failure, and frequent UTI may warrant more frequent exchanges, but this should be determined on a case-by-case basis.
Catheter-Associated UTI in Patients with Spinal Cord Injury
Spinal cord injury (SCI) at any level can cause neurogenic bladder. This process ultimately leads to urinary stasis and colonization of the bladder with bacteria. According to the IDSA, the acceptable indications for UC insertion are: clinically significant urinary retention (if medical therapy is not effective), urinary incontinence, accurate urine output monitoring required, and patient is unable and/or unwilling to collect urine (Table 1).5 Recently, further guidelines were published regarding appropriate and inappropriate indwelling UC placement in hospitalized medical patients (Table 2 and Table 3), expanding upon the earlier acceptable criteria provided by the IDSA.41 According to the IDSA and Ann Arbor Criteria for Appropriate Urinary Catheter Use, patients with SCI and subsequent neurogenic bladder without obstruction where intermittent bladder straight catheterization for the drainage of urine is not feasible will likely need an indwelling UC.41 SCI patients often experience decubitus ulcers, and an indwelling UC can be used if needed to help with wound healing if other urinary management alternatives have been attempted. Other such situations in which an indwelling UC can used before attempting alternative approaches would be in a patient who is actively dying and is pursuing comfort care and/or hospice.41
Which indwelling UCs should be used in patients with SCI?
Efforts to reduce the likelihood of infection in patients with SCI have led to several advances in the design and manufacturing of UCs. UCs are made of either latex, plastic, silicone, or polytetrafluoroethylene (Teflon). None of these substrates is free of complications, but of them latex UCs have been studied the most in regards to their associated complications. Aside from being allergenic in nature, latex UCs have an increased propensity to allow bacteria to adhere to their surface due to microscopic planes of unevenness.42 Silicone UCs are less frequently associated with infection but are more rigid, leading to increased discomfort.43,44 Hydrophilic and silver-hydrogel coatings are innovative methods that have been developed to increase comfort and reduce the likelihood of infection. Hydrophilic-coated UCs are associated with reduced microbial adherence, decreased encrustation, and better patient satisfaction.45,46 In SCI patients, these UCs have demonstrated lower complication rates, including UTI; fewer episodes of post-, intra-, and inter-catheterization bleeding; and decreased rates of antibiotic-resistant bacteria.45,46 Silver-hydrogel-coated UCs are less well studied but have also demonstrated reduced UTI rates in SCI patients; however, their efficacy over the long term has yet to be determined. Antibiotic-impregnated UCs are not currently recommended for either short- or long-term indwelling UC use.5
How can CA-UTI be prevented in a patient who will require a long-term indwelling catheter?
At this time, the data is insufficient to make a recommendation on routine UC exchange (eg, every 2 to 4 weeks) in patients who require long-term indwelling urethral or suprapubic catheters in an attempt to reduce the risk of CA-ASB or CA-UTI. This is also true for those who experience even repeated early catheter blockage from encrustation.5 Thus, the rate at which these exchanges occur can be controversial, but typically around every 4 weeks is a common approach. Some would argue that if the patient has repeated CA-UTI, an exchange rate of every 2 weeks might be needed, but data is currently lacking to support this practice.5 If intermittent urinary catheterization is feasible, it should be done at least every 6 hours and before bedtime. In general, when the volume of urine in the bladder reaches approximately 400 mL, the patient should undergo bladder catheterization to prevent stasis and infection.47 A closed drainage system is recommended in all patients who require long-term indwelling UC use.48,49 Placement of the collection bag above the catheter or above the level of the bladder and a breach in the closed drainage system have been shown to result in higher rates of catheter-associated bacteriuria.48,50 Proper hand hygiene and sterile and/or clean techniques should be used when placing or exchanging a UC. However, in one study there was no difference in bacteremia or UTIs when using sterile versus clean techniques.51
Asymptomatic bacteriuria should not be treated in patients with long-term indwelling UCs, and prophylactic antibiotics have led to the emergence of resistance.52 At this time it is not clear whether prophylactic weekly oral cyclic antibiotic administration can effectively reduce the frequency of CA-UTI in patients with SCI and chronic indwelling UC use.
What are the signs and symptoms of CA-UTI in a patient with SCI?
Subjective and objective findings may be limited when a patient with SCI presents with CA-UTI. These patients often lack the usual symptoms of UTI (dysuria, suprapubic discomfort, urgency, increased frequency) and pyelonephritis (flank pain, costovertebral angle tenderness). Caregivers and health care providers should be aware that nonspecific symptoms such as foul-smelling urine, pyuria, increased residual volume of urine in the bladder, change in voiding habits, worsening detrusor spasticity, and aggravation of autonomic dysreflexia can be the only initial presenting symptoms.53
What is the duration of therapy for CA-UTI in SCI, and how can antibiotic stewardship principles be applied in this patient population?
Antibiotic therapy is indicated for a duration of 7 days if there is prompt resolution of symptoms, or for a total of 10 to 14 days if the response is delayed, regardless of whether the patient remains with a UC.5 Antibiotic stewardship is very important to reduce the risk for developing drug resistance in this high-risk population. Methods such as prescriber education practices, institution protocols, guideline implementation, auditing and feedback, restriction and reauthorization practices, computer-assisted programs, de-escalation or streamlining, and antibiotic cycling or dosage optimization have all been shown to assist in antibiotic stewardship in UTIs.54
Candida UTI
What is the initial evaluation for a patient with candiduria?
The workup for candiduria hinges on determining whether the candiduria likely represents contamination, colonization, or infection; certain predisposing risk factors are associated with Candida UTI (Table 4).55-57 The most important aspect to candiduria is the patient’s clinical status and comorbid conditions.58 Among funguria, Candida species are the most common and represent 95% of organisms isolated on urine cultures (Table 5).59 Candiduria is usually present in those with significant comorbidities and rarely is associated with healthy individuals.59,60 Candiduria is increasing in prevalence among hospitalized patients, representing 22% to 40% of all nosocomial UTIs.59,60 Markers in the urine (leukocyte esterase, colony count of culture growth, presence or absence of Candida casts and pseudohyphae) cannot alone differentiate colonization from infection.55 When candiduria is discovered in a patient with symptoms related to UTI in the setting of predisposing risk factors, it should be considered a real infection until proven otherwise.
In a situation where an asymptomatic patient without an indwelling UC has Candida species isolated from a urine culture, a repeat culture (clean-catch midstream sample) should be performed to assess for a likely contaminated collection.55 If the patient has an indwelling UC then it should be exchanged and urine collected from the fresh catheter.61 When candiduria is found in healthy asymptomatic adults, it is most commonly associated with poor collection techniques or postcollection contamination.59 If candiduria persists in an asymptomatic patient, the patient should be assessed for predisposing factors. This includes checking hemoglobin A1C for developing diabetes and renal ultrasound looking for urolithiasis, renal abscess, hydronephrosis, and fungus ball. Postvoid residual urinary retention should also be ruled out with bladder ultrasound. Treatment of predisposing factors can lead to resolution of candiduria without antifungal treatment, and a urine culture should be repeated (1 to 2 weeks later).61 Asymptomatic patients lacking any predisposing factors can be observed with repeat urine cultures in 1 to 3 months.61
Candiduria may actually represent candidemia in those patients who have a predisposing risk factor for disseminated candidiasis. These risk factors include central venous catheters, administration of total parental nutrition, antibiotic use (especially broad spectrum), critical illness, recent surgical intervention (especially intra-abdominal), acute renal failure, nasogastric tube use, and active gastric acid suppression (ie, proton pump inhibitors).62,63 The hematogenous spread of Candida can lead to the detection of candiduria in 46% to 80% of persons who are experiencing candidemia.59 If the patient is at risk for candidemia, then blood fungal cultures should be drawn. It is not unreasonable to also order a serum-D-glucan assay if suspicions are high. A thorough skin assessment should be completed and ophthalmology consulted for a detailed eye exam in the event that the patient has candidemia. Candiduria is highly prevalent among those who are candidemic, but overall candidemia is encountered in less than 5% of patients in most intensive care units.59 Thus, most patients with candiduria do not have disseminated candidiasis.
Candiduria rarely leads to symptoms of UTI,58 unless the pathogenesis is related to an ascending process.56 Symptoms of Candida UTI are no different from those experienced from a bacterial etiology. Some patients may complain of pneumaturia and/or endorse seeing particulate matter in their urine.55 Patients showing signs of sepsis (fever, chills, flank pain) should be investigated for possible Candida pyelonephritis in the setting of candiduria.64
When should asymptomatic candiduria be treated?
In adult patients with asymptomatic candiduria, there are 2 situations in which antifungal therapy is recommended. A patient undergoing a traumatic urologic procedure would be treated to avoid the risk for candidemia caused by the procedure. Also, in neutropenic patients empiric antifungal therapy should be administered because there is a high likelihood that this candiduria may actually represent hematogenous spread from candidemia.61,65
What is the treatment for symptomatic Candida cystitis?
Empiric treatment with oral fluconazole 200 to 400 mg daily for a total of 2 weeks is recommended in patients with persisting candiduria and symptoms of cystitis.65 Identifying the species is a crucial step in the treatment of Candida UTI. Several species (C. glabrata, C. krusei) are known to be resistant to fluconazole. Species identification and antifungal sensitivities should be done and therapy directed after obtaining these results.55
What is the recommended treatment for Candida pyelonephritis?
Treatment for pyelonephritis caused by fluconazole-susceptible Candida species is oral fluconazole 200 to 400 mg (3-6 mg/kg) for a total of 2 weeks.65 A fluconazole-resistant organism should be suspected when a non-albicans Candida species is isolated, such as C. krusei or C. glabrata. In this circumstance, in vitro antifungal susceptibility testing should be done. Echinocandins are not a good option in this situation because they do not reach adequate urine concentration and treatment failure is well documented.66-68 Amphotericin B deoxycholate (AmB) 0.3 to 0.6 mg/kg daily for 1 to 7 days, with or without oral flucytosine (25 mg/kg) 4 times daily, is recommended by the IDSA for the treatment of fluconazole-resistant isolates of C. glabrata and C. krusei.65 Further imaging with ultrasound, CT, or magnetic resonance should be done to rule out urinary tract obstruction and/or “fungus ball” formation. Emphysematous pyelonephritis and necrosis can occur and usually require nephrectomy. Perinephric abscess will need drainage, which can be accomplished through interventional radiological techniques.55
If a “fungus ball” is suspected in the kidney, how does the management change in a patient with Candida pyelonephritis?
A fungus ball must be treated with both antifungals and surgical intervention. Antifungal therapy should be continued during the surgical removal process to avoid fungemia. Interventional radiology should be consulted and is usually the best option for removal. Fungus ball(s) can and often do cause urinary obstruction. Temporary nephrostomy tube placement may be warranted in these situations to relieve the obstruction.55,65 AmB can be infused through the nephrostomy tube to increase local concentrations. This route of administration is not known to be nephrotoxic.55 Fluconazole infusion through a nephrostomy tube has also been used in the successful treatment of a fungus ball.69
Summary
Health care–associated UTIs are the most common nosocomial infection in the United States. UC placement and genitourinary manipulation or instrumentation play a major role in the development of CA-UTI. Clinicians should be aware of the appropriate and inappropriate use of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key in prevention of CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. A multidisciplinary approach is essential when managing chronic indwelling UCs in SCI. PCN and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected.
Candiduria is an emerging nosocomial source of UTI but rarely leads to symptoms unless related to an ascending process. Proper urine collection is crucial in determining whether you are dealing with contamination, colonization, or infection. If candiduria persists in an asymptomatic individual, then further investigations should be done in regards to possible predisposing risks factors. Fluconazole is recommended for treatment of most patients with Candida UTI, while intravenous AmB is the treatment of choice for fluconazole-resistant Candida species. As we continue to take an evidence-based approach to the prevention and management of health care-associated UTI, we will likely see continued improvement in patient outcomes and overall decreased rates of infection.
Corresponding author: Norman Beatty, MD, 1501 N. Campbell Ave., Tucson, AZ 85724; nbeatty@email.arizona.edu.
Financial disclosures: None.
1. Klevens RM, Edwards JR, Richards CL Jr. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
2. Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003;348:651-656.
3. Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Division of Healthcare Quality Promotion; National Center for Preparedness Detection and Control of Infectious Diseases; Coordinating Center for Infectious Diseases; Centers for Disease Control and Prevention. March 2009. Contract No.: CS200891-A. www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
4. Eriksen HM, Iverson BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40-45.
5. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
6. Mattie AS, Webster BL. Centers for Medicare and Medicaid Services’ “never events”: an analysis and recommendations to hospitals. Health Care Manag. 2008;27:338-349.
7. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents. 2001;17:299-303.
8. Weinstein JW, Mazon D, Pantelick E, et al. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol. 1999;20:543-548.
9. Fakih MG, Pena ME, Shemes S, et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337-340.
10. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
11. Fakih MG, Rey JE, Pena ME, et al. Sustained reductions in urinary catheter use over 5 years: bedside nurses view themselves responsible for evaluation of catheter necessity. Am J Infect Control. 2013;41:236-239.
12. Meddings J, Rogers MA, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infections: an integrative review. BMJ Qual Saf. 2014;23:277-289.
13. Gould C. Catheter-associated urinary tract infection: the national perspective. In: Essential Hospitals Engagement Network. Patient Harm Series II: new tools to prevent CAUTI webinar. April 16, 2014. http://bit.ly/1UWndRA.
14. Nicolle LE. Consequences of asymptomatic bacteriuria in the elderly. Int J Antimicrob Agents. 1994;4:107-111.
15. Nicolle LE. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22:167-175
16. Cohen A. A microbiological comparison of a povidone-iodine lubricating gel and a control as catheter lubricants. J Hosp Infect. 1985;6(Suppl A):155-161.
17. Daifuku R, Stamm WE. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986;314:1208-1213.
18. Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131-136.
19. Nicolle LE. Catheter-related urinary tract infection. Drugs Aging. 2005;22:627-639.
20. Redder JD, Leth RA, Møller JK. Incidence rates of hospital-acquired urinary tract and bloodstream infections generated by automated compilation of electronically available healthcare data. J Hosp Infect. 2015;91:231-236.
21. Ortega M, Marco F, Soriano A, et al. Epidemiology and prognostic determinants of bacteraemic catheter-acquired urinary tract infection in a single institution from 1991 to 2010. J Infect. 2013;67:282-287.
22. Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160:673-677.
23. Musher DM, Thorsteinsson SB, Airola VM, II. Quantitative urinalysis:diagnosing urinary tract infection in men. JAMA. 1976;236:2069-2072.
24. Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16:2016-2030.
25. Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications--where we are and where we are going. Nat Rev Urol. 2015;12:17-25.
26. el-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487-1491.
27. Adamo R, Saad WE, Brown DB. Management of nephrostomy drains and ureteral stents. Tech Vasc Interv Radiol. 2009;12:193-204.
28. Joshi HB, Newns N, Stainthorpe A, et al. Ureteral Stent Symptom Questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060-1064.
29. Singh I, Gupta NP, Hemal AK, et al. Severely encrusted polyurethane ureteral stents: management and analysis of potential risk factors. Urology. 2001;58:526-531.
30. Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am. 2003;17:411-432.
31. Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216-2217.
32. Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891-896.
33. Paick SH, Park HK, Oh SJ, Kim HH. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology. 2003;62:214-217.
34. Klis R, Korczak-Kozakiewicz E, Denys A, et al. Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol. 2009;23:1015-1019.
35. Farsi HM, Mosli HA, Al-Zemaity M, et al. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol. 1995;9:469-472.
36. Wolfe JS Jr, Bennet CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
37. Bahu R, Chaftari AM, Hachem RY, et al. Nephrostomy tube related pyelonephritis in patients with cancer: epidemiology, infection rate and risk factors. J Urol. 2013;189:130-135.
38. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14:73-156.
39. Cronan JJ, Horn DL, Marcello A, et al: Antibiotics and nephrostomy tube care: preliminary observations. Part II. Bacteremia. Radiology. 1989;172(3 Pt 2):1043-1045.
40. Akay AF, Aflay U, Gedik A, et al. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95-98.
41. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/ UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-34.
42. Stickler D, Young R, Jones G, et al. Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res. 2003;31:306-311.
43. Denstedt J, Wollin T, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493-500.
44. Morris N, Stickler D, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol. 1997;80:58-63.
45. Cardenas D, Moore K, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PMR. 2011;3:408-417.
46. Spinu A, Onose G, Daia C, et al. Intermittent catheterization in the management of post spinal cord injury (SCI) neurogenic bladder using new hydrophilic, with lubrication in close circuit devices-our own preliminary results. J Med Life. 2012;5:21-28.
47. Shekelle P, Morton S, Clark K, et al. Systematic review of risk factors for urinary tract infection in adults with spinal cord dysfunction. J Spinal Cord Med. 1998;22:258-272.
48. Siddiq D, Darouiche R. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9:305-314.
49. Gould C, Umscheid C, Agarwal R, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
50. Maki D, Tambyah P. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342-347.
51. Munasinghe R, Yazdani H, Siddique M, et al. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol. 2001;22:647-649.
52. Nicolle L, Bradley S, Colgan R, et al; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40: 643-654.
53. Linsenmeyer T, Oakley A. Accuracy of individuals with spinal cord injury at predicting urinary tract infections based on their symptoms. J Spinal Cord Med. 2002;26:352-357.
54. Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3:174-192.
55. Kauffman CA. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am. 2014;28:61-74.
56. Alvarez-Lerma F, Nolla-Salas J, Leon C, et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003;29:1069-1076.
57. Colodner R, Nuri Y, Chazan B, et al. Community-acquired and hospital-acquired candiduria: comparison of prevalence and clinical characteristics. Eur J Clin Microbiol Infect Dis. 2008;27:301-305.
58. Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14-18.
59. Sobel JD, Fisher JF, Kauffman CA, et al. Candida urinary tract infections—epidemiology. Clin Infect Dis. 2011;52(suppl 6): S433-436.
60. Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510-515.
61. Fisher JF, Sobel JD, Kauffman CA, et al. Candida urinary tract infections—treatment. Clin Infect Dis. 2011;52(suppl 6):S457-466.
62. Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis. 2001;33:177-186.
63. Puzniak LP, Teutsch S, Powderly W, et al. Has the epidemiology of nosocomial candidemia changed? Infect Control Hosp Epidemiol. 2004;25:628-633.
64. Siddique MS, Gayed N, McGuire N, et al. Salient features of Candida pyelonephritis in adults. Infect Dis Clin Pract. 1992;1:239-245
65. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-e50.
66. Sobel JD, Bradshaw SK, Lipka CJ, et al. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis. 2007;44:e46-9.
67. Malani AN. Failure of caspofungin for treatment of Candida glabrata candiduria. Case report and review of the literature. Infect Dis Clin Pract. 2010;18:271-272.
68. Schelenz S, Ross CN. Limitations of caspofugin in the treatment of obstructive pyelonephrosis due to Candida glabrata infection. BMC Infect Dis. 2006;56:126-130.
69. Chung BH, Chang SY, Kim SI, et al. Successfully treated renal fungal ball with continuous irrigation of fluconazole. J Urol. 2001;166:1835-1836.
1. Klevens RM, Edwards JR, Richards CL Jr. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
2. Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003;348:651-656.
3. Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Division of Healthcare Quality Promotion; National Center for Preparedness Detection and Control of Infectious Diseases; Coordinating Center for Infectious Diseases; Centers for Disease Control and Prevention. March 2009. Contract No.: CS200891-A. www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
4. Eriksen HM, Iverson BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40-45.
5. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
6. Mattie AS, Webster BL. Centers for Medicare and Medicaid Services’ “never events”: an analysis and recommendations to hospitals. Health Care Manag. 2008;27:338-349.
7. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents. 2001;17:299-303.
8. Weinstein JW, Mazon D, Pantelick E, et al. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol. 1999;20:543-548.
9. Fakih MG, Pena ME, Shemes S, et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337-340.
10. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
11. Fakih MG, Rey JE, Pena ME, et al. Sustained reductions in urinary catheter use over 5 years: bedside nurses view themselves responsible for evaluation of catheter necessity. Am J Infect Control. 2013;41:236-239.
12. Meddings J, Rogers MA, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infections: an integrative review. BMJ Qual Saf. 2014;23:277-289.
13. Gould C. Catheter-associated urinary tract infection: the national perspective. In: Essential Hospitals Engagement Network. Patient Harm Series II: new tools to prevent CAUTI webinar. April 16, 2014. http://bit.ly/1UWndRA.
14. Nicolle LE. Consequences of asymptomatic bacteriuria in the elderly. Int J Antimicrob Agents. 1994;4:107-111.
15. Nicolle LE. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22:167-175
16. Cohen A. A microbiological comparison of a povidone-iodine lubricating gel and a control as catheter lubricants. J Hosp Infect. 1985;6(Suppl A):155-161.
17. Daifuku R, Stamm WE. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986;314:1208-1213.
18. Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131-136.
19. Nicolle LE. Catheter-related urinary tract infection. Drugs Aging. 2005;22:627-639.
20. Redder JD, Leth RA, Møller JK. Incidence rates of hospital-acquired urinary tract and bloodstream infections generated by automated compilation of electronically available healthcare data. J Hosp Infect. 2015;91:231-236.
21. Ortega M, Marco F, Soriano A, et al. Epidemiology and prognostic determinants of bacteraemic catheter-acquired urinary tract infection in a single institution from 1991 to 2010. J Infect. 2013;67:282-287.
22. Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160:673-677.
23. Musher DM, Thorsteinsson SB, Airola VM, II. Quantitative urinalysis:diagnosing urinary tract infection in men. JAMA. 1976;236:2069-2072.
24. Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16:2016-2030.
25. Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications--where we are and where we are going. Nat Rev Urol. 2015;12:17-25.
26. el-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487-1491.
27. Adamo R, Saad WE, Brown DB. Management of nephrostomy drains and ureteral stents. Tech Vasc Interv Radiol. 2009;12:193-204.
28. Joshi HB, Newns N, Stainthorpe A, et al. Ureteral Stent Symptom Questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060-1064.
29. Singh I, Gupta NP, Hemal AK, et al. Severely encrusted polyurethane ureteral stents: management and analysis of potential risk factors. Urology. 2001;58:526-531.
30. Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am. 2003;17:411-432.
31. Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216-2217.
32. Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891-896.
33. Paick SH, Park HK, Oh SJ, Kim HH. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology. 2003;62:214-217.
34. Klis R, Korczak-Kozakiewicz E, Denys A, et al. Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol. 2009;23:1015-1019.
35. Farsi HM, Mosli HA, Al-Zemaity M, et al. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol. 1995;9:469-472.
36. Wolfe JS Jr, Bennet CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
37. Bahu R, Chaftari AM, Hachem RY, et al. Nephrostomy tube related pyelonephritis in patients with cancer: epidemiology, infection rate and risk factors. J Urol. 2013;189:130-135.
38. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14:73-156.
39. Cronan JJ, Horn DL, Marcello A, et al: Antibiotics and nephrostomy tube care: preliminary observations. Part II. Bacteremia. Radiology. 1989;172(3 Pt 2):1043-1045.
40. Akay AF, Aflay U, Gedik A, et al. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95-98.
41. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/ UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-34.
42. Stickler D, Young R, Jones G, et al. Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res. 2003;31:306-311.
43. Denstedt J, Wollin T, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493-500.
44. Morris N, Stickler D, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol. 1997;80:58-63.
45. Cardenas D, Moore K, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PMR. 2011;3:408-417.
46. Spinu A, Onose G, Daia C, et al. Intermittent catheterization in the management of post spinal cord injury (SCI) neurogenic bladder using new hydrophilic, with lubrication in close circuit devices-our own preliminary results. J Med Life. 2012;5:21-28.
47. Shekelle P, Morton S, Clark K, et al. Systematic review of risk factors for urinary tract infection in adults with spinal cord dysfunction. J Spinal Cord Med. 1998;22:258-272.
48. Siddiq D, Darouiche R. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9:305-314.
49. Gould C, Umscheid C, Agarwal R, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
50. Maki D, Tambyah P. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342-347.
51. Munasinghe R, Yazdani H, Siddique M, et al. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol. 2001;22:647-649.
52. Nicolle L, Bradley S, Colgan R, et al; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40: 643-654.
53. Linsenmeyer T, Oakley A. Accuracy of individuals with spinal cord injury at predicting urinary tract infections based on their symptoms. J Spinal Cord Med. 2002;26:352-357.
54. Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3:174-192.
55. Kauffman CA. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am. 2014;28:61-74.
56. Alvarez-Lerma F, Nolla-Salas J, Leon C, et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003;29:1069-1076.
57. Colodner R, Nuri Y, Chazan B, et al. Community-acquired and hospital-acquired candiduria: comparison of prevalence and clinical characteristics. Eur J Clin Microbiol Infect Dis. 2008;27:301-305.
58. Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14-18.
59. Sobel JD, Fisher JF, Kauffman CA, et al. Candida urinary tract infections—epidemiology. Clin Infect Dis. 2011;52(suppl 6): S433-436.
60. Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510-515.
61. Fisher JF, Sobel JD, Kauffman CA, et al. Candida urinary tract infections—treatment. Clin Infect Dis. 2011;52(suppl 6):S457-466.
62. Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis. 2001;33:177-186.
63. Puzniak LP, Teutsch S, Powderly W, et al. Has the epidemiology of nosocomial candidemia changed? Infect Control Hosp Epidemiol. 2004;25:628-633.
64. Siddique MS, Gayed N, McGuire N, et al. Salient features of Candida pyelonephritis in adults. Infect Dis Clin Pract. 1992;1:239-245
65. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-e50.
66. Sobel JD, Bradshaw SK, Lipka CJ, et al. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis. 2007;44:e46-9.
67. Malani AN. Failure of caspofungin for treatment of Candida glabrata candiduria. Case report and review of the literature. Infect Dis Clin Pract. 2010;18:271-272.
68. Schelenz S, Ross CN. Limitations of caspofugin in the treatment of obstructive pyelonephrosis due to Candida glabrata infection. BMC Infect Dis. 2006;56:126-130.
69. Chung BH, Chang SY, Kim SI, et al. Successfully treated renal fungal ball with continuous irrigation of fluconazole. J Urol. 2001;166:1835-1836.