User login
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).
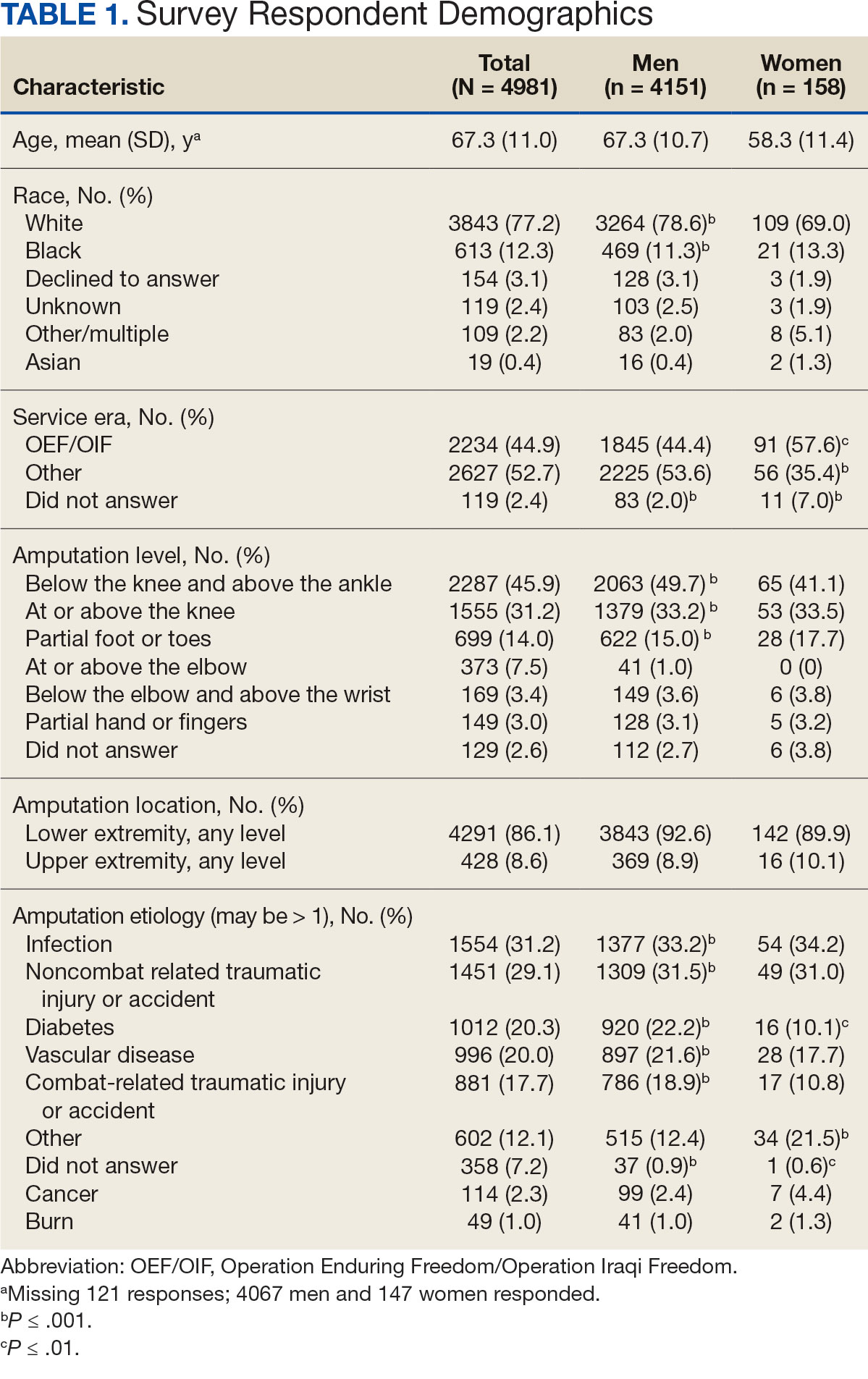
The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
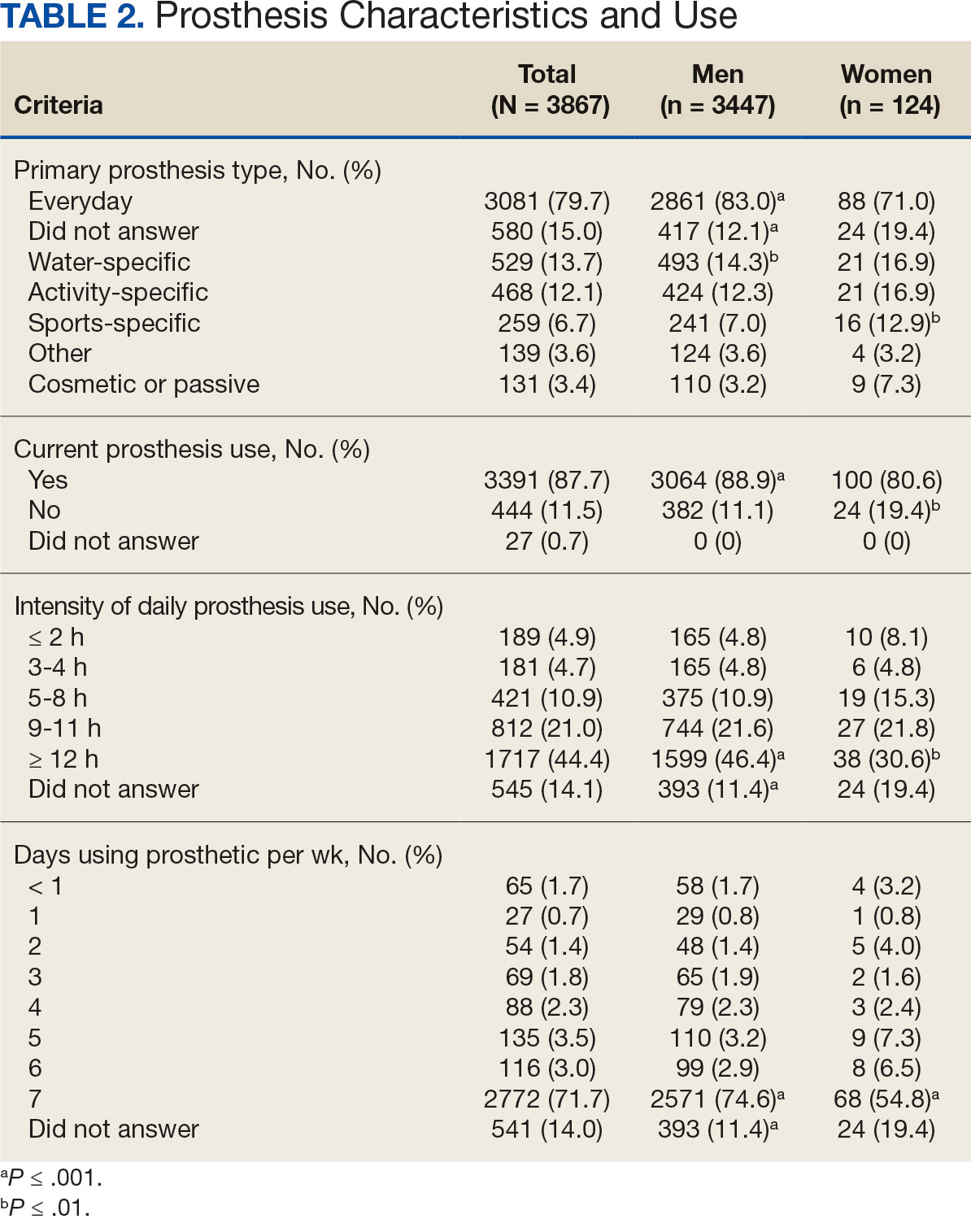
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).
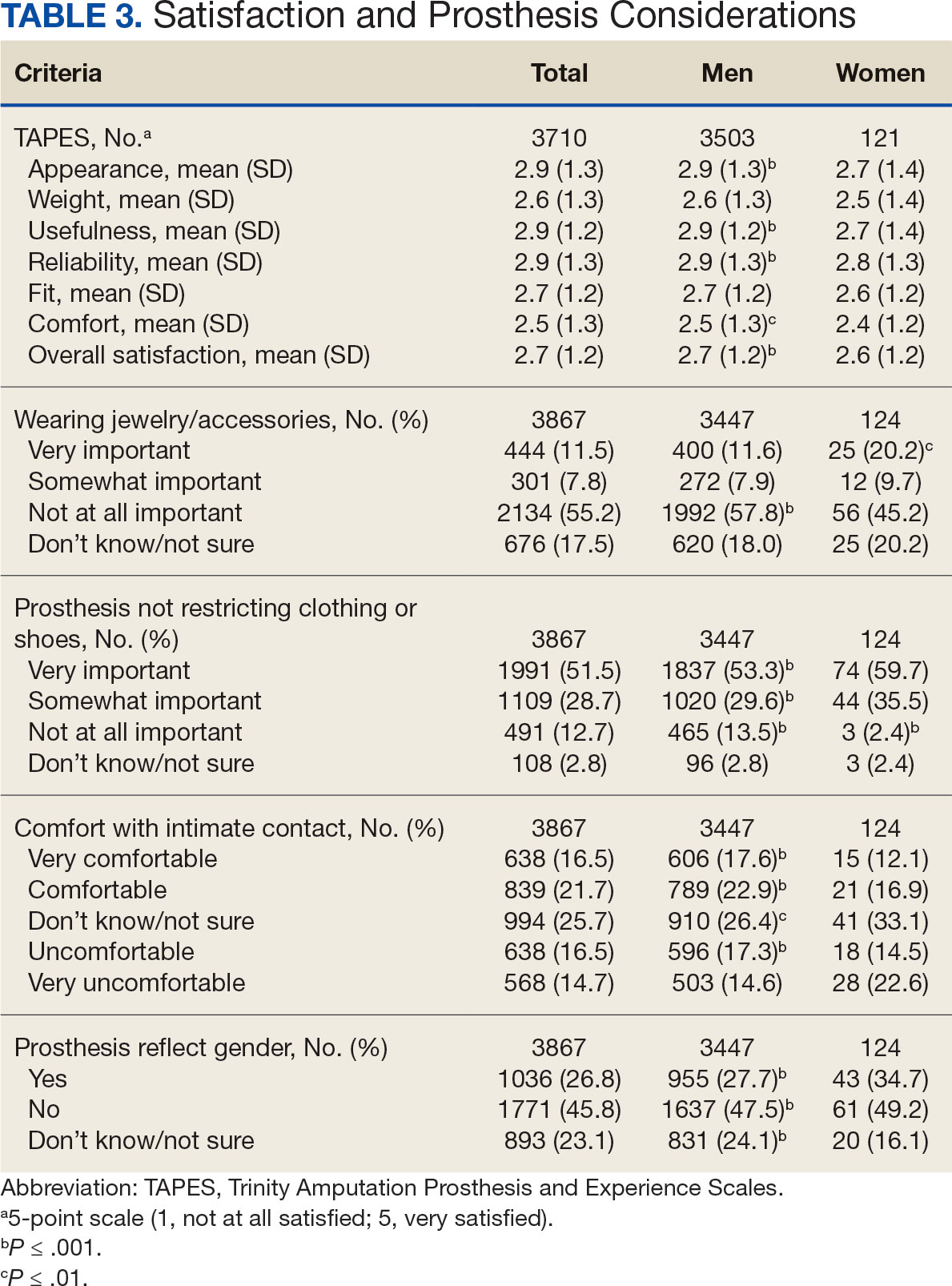
In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).
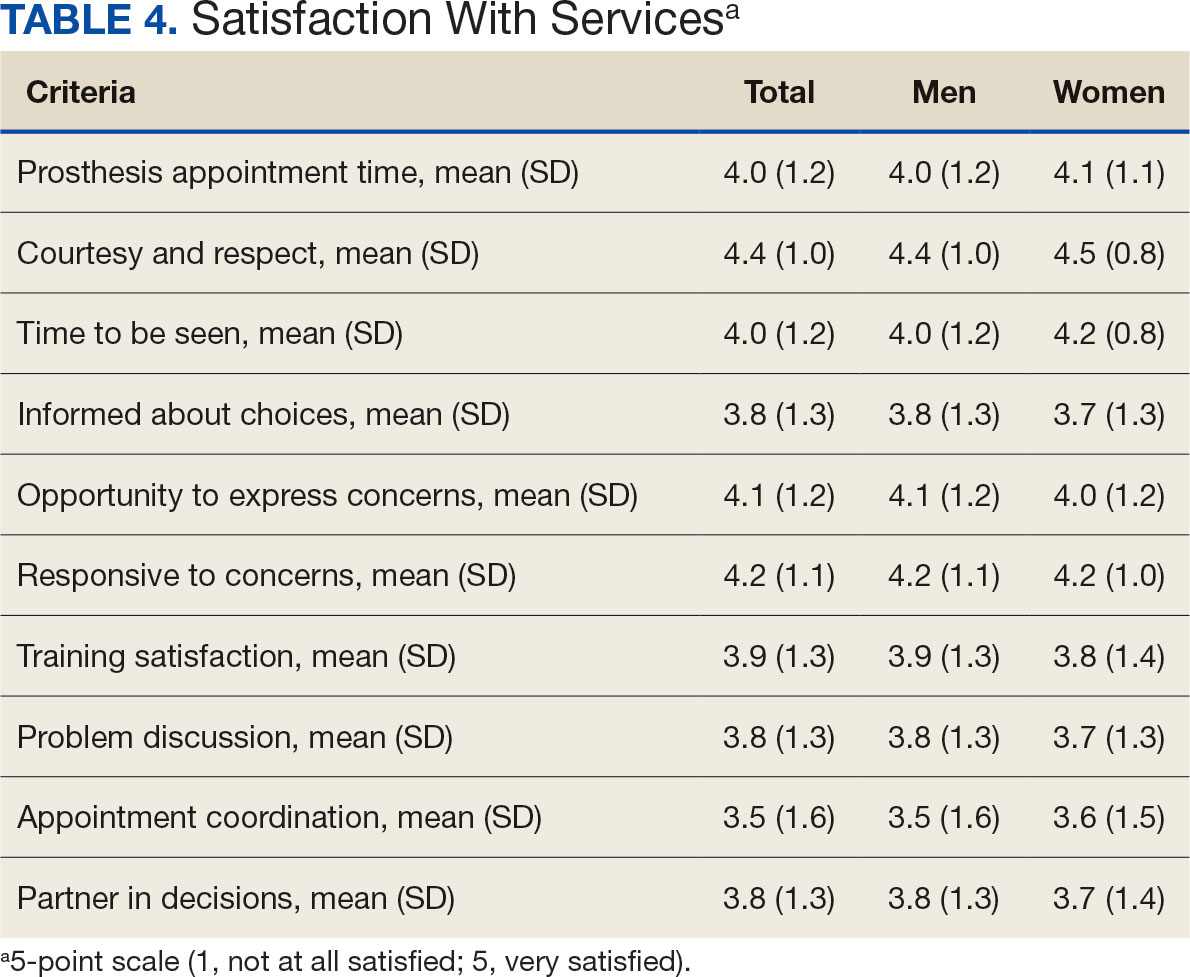
Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.
Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).

The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).

In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).

Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.

Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).

The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).

In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).

Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.

Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Heat Waves Pose Significant Health Risks for Dually Eligible Older Individuals
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
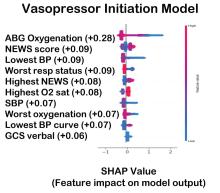
Digital Twin Model Predicts Sepsis Mortality
A “digital twin” model successfully predicted adverse outcomes in intensive care unit (ICU) patients treated for sepsis.
The digital twin could reduce the risk for some interventions, according to Amos Lal, MD, who presented the study at the CHEST Annual Meeting. That’s because the model can predict the outcome. “You don’t actually have to make an intervention to the patient, which might be risky. By doing that, you can actually prevent a lot of harm,” said Dr. Lal, assistant professor of medicine at Mayo Clinic in Rochester, Minnesota.
The researchers used a one-dimensional convolutional neural network (CNN), similar to two-dimensional CNNs that are used to classify images, substituting the color channels used in imaging with 38 time-dependent variables. They applied it to predicting outcomes in the ICU, focusing on data generated within the first 24 hours of admission. The team made the model dynamic by adding time-sensitive data like vitals, laboratory values, and interventions every 15 minutes. That contrasts with existing models that are usually static, relying on values at admission or at 24 hours, for example. It also takes into account time-insensitive data like age, gender, and comorbidities. “Combining these two and coming up with the prediction model in real time can give you a more informed decision about how these patients are going to perform over a period of 2 weeks or 4 weeks of their stay within the ICU. And of course, as we get more and more data within the first 24 hours, the performance of the model improves as well,” said Dr. Lal.
The researchers tested the model by creating a virtual model of the patient and then performing an intervention on the patient and a simulated intervention on the virtual patient. “Then we advance the clock and the patient either improved or deteriorated, and we compared how the digital twin performed, whether the changes were concordant or discordant [between the virtual and real-world patients],” said Dr. Lal.
The model was designed to predict which patients with sepsis would be at greater risk for death or ICU stays longer than 14 days. It was created using data from 28,617 patients with critical care sepsis at a single hospital who were treated between 2011 and 2018, with 70% used as a training set, 20% as a test set, and 10% as a validation set. The researchers conducted an external validation using MIMIC-IV data on 30,903 patients from the Beth Israel Deaconess Medical Center in Boston. The model included 31 time-independent variables and 38 time-dependent variables that were collected every 15 minutes at the Mayo Clinic and every 60 minutes at Beth Israel Deaconess. Surgical patients represented 24% of the Mayo dataset and 58% of the MIMIC-IV dataset, but otherwise the two groups were demographically similar.
At 24 hours, the area under the receiver operating characteristic curve for predicting 14-day mortality was −0.82 in the Mayo validation cohort and −0.78 in the MIMIC validation cohort. The model improved in accuracy over time as more data were accumulated.
The session’s co-moderators, Sandeep Jain, MD, and Casey Cable, MD, praised the work. Dr. Cable, associate professor of pulmonary care medicine at VCU Health, Richmond, Virginia, noted that the model used both surgical patients and medical patients with sepsis, and the two groups can present quite differently. Another variable was the COVID pandemic, where some patients presented at the hospital when they were quite sick. “I’m curious how different starting points would play into it,” she said.
She called for institutions to develop such models on their own rather than relying on companies that might develop software solutions. “I think that this needs to be clinician-led, from the ground up,” said Dr. Cable.
Dr. Jain, an associate professor of pulmonary care medicine at Broward Health, suggested that such models might need to be individualized for each institution, but “my fear is it could become too expensive, so I think a group like CHEST could come together and [create] an open source system to have their researchers jumpstart the research on this,” he said.
Dr. Lal, Dr. Jain, and Dr. Cable reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
A “digital twin” model successfully predicted adverse outcomes in intensive care unit (ICU) patients treated for sepsis.
The digital twin could reduce the risk for some interventions, according to Amos Lal, MD, who presented the study at the CHEST Annual Meeting. That’s because the model can predict the outcome. “You don’t actually have to make an intervention to the patient, which might be risky. By doing that, you can actually prevent a lot of harm,” said Dr. Lal, assistant professor of medicine at Mayo Clinic in Rochester, Minnesota.
The researchers used a one-dimensional convolutional neural network (CNN), similar to two-dimensional CNNs that are used to classify images, substituting the color channels used in imaging with 38 time-dependent variables. They applied it to predicting outcomes in the ICU, focusing on data generated within the first 24 hours of admission. The team made the model dynamic by adding time-sensitive data like vitals, laboratory values, and interventions every 15 minutes. That contrasts with existing models that are usually static, relying on values at admission or at 24 hours, for example. It also takes into account time-insensitive data like age, gender, and comorbidities. “Combining these two and coming up with the prediction model in real time can give you a more informed decision about how these patients are going to perform over a period of 2 weeks or 4 weeks of their stay within the ICU. And of course, as we get more and more data within the first 24 hours, the performance of the model improves as well,” said Dr. Lal.
The researchers tested the model by creating a virtual model of the patient and then performing an intervention on the patient and a simulated intervention on the virtual patient. “Then we advance the clock and the patient either improved or deteriorated, and we compared how the digital twin performed, whether the changes were concordant or discordant [between the virtual and real-world patients],” said Dr. Lal.
The model was designed to predict which patients with sepsis would be at greater risk for death or ICU stays longer than 14 days. It was created using data from 28,617 patients with critical care sepsis at a single hospital who were treated between 2011 and 2018, with 70% used as a training set, 20% as a test set, and 10% as a validation set. The researchers conducted an external validation using MIMIC-IV data on 30,903 patients from the Beth Israel Deaconess Medical Center in Boston. The model included 31 time-independent variables and 38 time-dependent variables that were collected every 15 minutes at the Mayo Clinic and every 60 minutes at Beth Israel Deaconess. Surgical patients represented 24% of the Mayo dataset and 58% of the MIMIC-IV dataset, but otherwise the two groups were demographically similar.
At 24 hours, the area under the receiver operating characteristic curve for predicting 14-day mortality was −0.82 in the Mayo validation cohort and −0.78 in the MIMIC validation cohort. The model improved in accuracy over time as more data were accumulated.
The session’s co-moderators, Sandeep Jain, MD, and Casey Cable, MD, praised the work. Dr. Cable, associate professor of pulmonary care medicine at VCU Health, Richmond, Virginia, noted that the model used both surgical patients and medical patients with sepsis, and the two groups can present quite differently. Another variable was the COVID pandemic, where some patients presented at the hospital when they were quite sick. “I’m curious how different starting points would play into it,” she said.
She called for institutions to develop such models on their own rather than relying on companies that might develop software solutions. “I think that this needs to be clinician-led, from the ground up,” said Dr. Cable.
Dr. Jain, an associate professor of pulmonary care medicine at Broward Health, suggested that such models might need to be individualized for each institution, but “my fear is it could become too expensive, so I think a group like CHEST could come together and [create] an open source system to have their researchers jumpstart the research on this,” he said.
Dr. Lal, Dr. Jain, and Dr. Cable reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
A “digital twin” model successfully predicted adverse outcomes in intensive care unit (ICU) patients treated for sepsis.
The digital twin could reduce the risk for some interventions, according to Amos Lal, MD, who presented the study at the CHEST Annual Meeting. That’s because the model can predict the outcome. “You don’t actually have to make an intervention to the patient, which might be risky. By doing that, you can actually prevent a lot of harm,” said Dr. Lal, assistant professor of medicine at Mayo Clinic in Rochester, Minnesota.
The researchers used a one-dimensional convolutional neural network (CNN), similar to two-dimensional CNNs that are used to classify images, substituting the color channels used in imaging with 38 time-dependent variables. They applied it to predicting outcomes in the ICU, focusing on data generated within the first 24 hours of admission. The team made the model dynamic by adding time-sensitive data like vitals, laboratory values, and interventions every 15 minutes. That contrasts with existing models that are usually static, relying on values at admission or at 24 hours, for example. It also takes into account time-insensitive data like age, gender, and comorbidities. “Combining these two and coming up with the prediction model in real time can give you a more informed decision about how these patients are going to perform over a period of 2 weeks or 4 weeks of their stay within the ICU. And of course, as we get more and more data within the first 24 hours, the performance of the model improves as well,” said Dr. Lal.
The researchers tested the model by creating a virtual model of the patient and then performing an intervention on the patient and a simulated intervention on the virtual patient. “Then we advance the clock and the patient either improved or deteriorated, and we compared how the digital twin performed, whether the changes were concordant or discordant [between the virtual and real-world patients],” said Dr. Lal.
The model was designed to predict which patients with sepsis would be at greater risk for death or ICU stays longer than 14 days. It was created using data from 28,617 patients with critical care sepsis at a single hospital who were treated between 2011 and 2018, with 70% used as a training set, 20% as a test set, and 10% as a validation set. The researchers conducted an external validation using MIMIC-IV data on 30,903 patients from the Beth Israel Deaconess Medical Center in Boston. The model included 31 time-independent variables and 38 time-dependent variables that were collected every 15 minutes at the Mayo Clinic and every 60 minutes at Beth Israel Deaconess. Surgical patients represented 24% of the Mayo dataset and 58% of the MIMIC-IV dataset, but otherwise the two groups were demographically similar.
At 24 hours, the area under the receiver operating characteristic curve for predicting 14-day mortality was −0.82 in the Mayo validation cohort and −0.78 in the MIMIC validation cohort. The model improved in accuracy over time as more data were accumulated.
The session’s co-moderators, Sandeep Jain, MD, and Casey Cable, MD, praised the work. Dr. Cable, associate professor of pulmonary care medicine at VCU Health, Richmond, Virginia, noted that the model used both surgical patients and medical patients with sepsis, and the two groups can present quite differently. Another variable was the COVID pandemic, where some patients presented at the hospital when they were quite sick. “I’m curious how different starting points would play into it,” she said.
She called for institutions to develop such models on their own rather than relying on companies that might develop software solutions. “I think that this needs to be clinician-led, from the ground up,” said Dr. Cable.
Dr. Jain, an associate professor of pulmonary care medicine at Broward Health, suggested that such models might need to be individualized for each institution, but “my fear is it could become too expensive, so I think a group like CHEST could come together and [create] an open source system to have their researchers jumpstart the research on this,” he said.
Dr. Lal, Dr. Jain, and Dr. Cable reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CHEST 2024
Biomarker use in ARDS resulting from COVID-19 infection
There is renewed interest in the use of immunomodulator therapies in patients with acute hypoxemic respiratory failure.
Beyond COVID-19, studies have also shown corticosteroid therapy improves clinical outcomes in patients with severe community-acquired pneumonia.3 However, the overwhelming majority of studies identifying plasma biomarkers that are associated with clinical outcomes in severe lung injury predate the routine use of corticosteroids.4 Two investigators at Massachusetts General Hospital, Jehan W. Alladina, MD, and George A. Alba, MD, performed a study to assess whether plasma biomarkers previously associated with clinical outcomes in ARDS maintained their predictive value in the setting of widespread immunomodulator therapy in the ICU. Drs. Alladina and Alba are physician-scientists and codirectors of the Program for Advancing Critical Care Translational Science at Massachusetts General Hospital in Boston.
In a study published in CHEST® Critical Care earlier this year, they prospectively enrolled patients with ARDS due to confirmed SARS-CoV-2 infection during the second wave of the COVID-19 pandemic from December 31, 2020, to March 31, 2021, at Massachusetts General Hospital.5 Plasma samples were collected within 24 hours of intubation for mechanical ventilation for protein analysis in 69 patients. Baseline demographics included a mean age of 62 plus or minus 15 years and a BMI of 31 plus or minus 8, and 45% were female. The median PaO2 to FiO2 ratio was 174 mm Hg, consistent with moderate ARDS, and the median duration of ventilation was 17 days. The patients had a median modified sequential organ failure assessment score of 8.5, and in-hospital mortality was 44% by 60 days. Notably, all patients in this cohort received steroids during their ICU stay.
Interestingly, the study investigators found no association between clinical outcomes and circulating proteins implicated in inflammation (eg, interleukin [IL]-6, IL-8), epithelial injury (eg, soluble receptor for advanced glycation end products, surfactant protein D), or coagulation (eg, D-dimer, tissue factor). However, four endothelial biomarkers—von Willebrand factor A2 domain; angiopoietin-2; syndecan-1; and neural precursor cell expressed, developmentally downregulated 9 (NEDD9)—were associated with 60-day mortality after adjusting for age, sex, and severity of illness. A sensitivity analysis, in which patients treated with the IL-6 inhibitor tocilizumab (n=4) were excluded, showed similar results.
Of the endothelial proteins, NEDD9 demonstrated the greatest effect size in its association with mortality in patients with ARDS due to COVID-19 who were treated with immunomodulators. NEDD9 is a scaffolding protein highly expressed in the pulmonary vascular endothelium, but its role in ARDS is not well known. In pulmonary vascular disease, plasma levels are associated with adverse pulmonary hemodynamics and clinical outcomes. Pulmonary artery endothelial NEDD9 is upregulated by cellular hypoxia and can mediate platelet-endothelial adhesion by interacting with P-selectin on the surface of activated platelets.6 Additionally, there is evidence of increased pulmonary endothelial NEDD9 expression and colocalization with fibrin within pulmonary arteries in lung tissue of patients who died from ARDS due to COVID-19.7 Thus, NEDD9 may be an important mediator of pulmonary vascular dysfunction observed in ARDS and could be a novel biomarker for patient subphenotyping and prognostication of clinical outcomes.
In summary, in a cohort of patients with COVID-19 ARDS uniformly treated with corticosteroids, plasma biomarkers of inflammation, coagulation, and epithelial injury were not associated with clinical outcomes, but endothelial biomarkers remained prognostic. It is biologically plausible that immunomodulators could attenuate the association between inflammatory biomarkers and patient outcomes. The findings of this study highlight the association of endothelial biomarkers with clinical outcomes in patients with COVID-19 ARDS treated with immunomodulators and warrant prospective validation, especially with the increasing evidence-based use of antiinflammatory therapy in acute lung injury. However, there are several important limitations to consider, including a small sample size from a single institution that precludes any definitive conclusions regarding any negative associations. Moreover, the single time point studied (the day of initiation of mechanical ventilation) and absence of a comparator group do not allow a comprehensive evaluation of the impact of antiinflammatory therapies across the trajectory of disease. Whether the findings are generalizable to all patients with ARDS treated with immunomodulators also remains unknown.
Overall, these data suggest that circulating signatures previously associated with ARDS, particularly those related to systemic inflammation, may have limited prognostic utility in the era of increasing immunomodulator use in critical illness. A deeper understanding of the pathobiology of ARDS, including the complex interplay with systemic immunomodulation, is needed to identify prognostic biomarkers and targeted therapies that improve patient outcomes.
Both authors work in the Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Harvard Medical School, in Boston.
References
1. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704.
2. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19. JAMA. 2020;324(13):1-11.
3. Dequin P-F, Meziani F, Quenot J-P, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941.
4. Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636-1643.
5. Alladina JW, Giacona FL, Haring AM, et al. Circulating biomarkers of endothelial dysfunction associated with ventilatory ratio and mortality in ARDS resulting from SARS-CoV-2 infection treated with antiinflammatory therapies. CHEST Crit Care. 2024;2(2):100054.
6. Alba GA, Samokhin AO, Wang R-S, et al. NEDD9 is a novel and modifiable mediator of platelet-endothelial adhesion in the pulmonary circulation. Am J Respir Crit Care Med. 2021;203(12):1533-1545.
7. Alba GA, Samokhin AO, Wang R-S, et al. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS‐CoV‐2 infection. Pulm Circ. 2022;12(2):e12071.
There is renewed interest in the use of immunomodulator therapies in patients with acute hypoxemic respiratory failure.
Beyond COVID-19, studies have also shown corticosteroid therapy improves clinical outcomes in patients with severe community-acquired pneumonia.3 However, the overwhelming majority of studies identifying plasma biomarkers that are associated with clinical outcomes in severe lung injury predate the routine use of corticosteroids.4 Two investigators at Massachusetts General Hospital, Jehan W. Alladina, MD, and George A. Alba, MD, performed a study to assess whether plasma biomarkers previously associated with clinical outcomes in ARDS maintained their predictive value in the setting of widespread immunomodulator therapy in the ICU. Drs. Alladina and Alba are physician-scientists and codirectors of the Program for Advancing Critical Care Translational Science at Massachusetts General Hospital in Boston.
In a study published in CHEST® Critical Care earlier this year, they prospectively enrolled patients with ARDS due to confirmed SARS-CoV-2 infection during the second wave of the COVID-19 pandemic from December 31, 2020, to March 31, 2021, at Massachusetts General Hospital.5 Plasma samples were collected within 24 hours of intubation for mechanical ventilation for protein analysis in 69 patients. Baseline demographics included a mean age of 62 plus or minus 15 years and a BMI of 31 plus or minus 8, and 45% were female. The median PaO2 to FiO2 ratio was 174 mm Hg, consistent with moderate ARDS, and the median duration of ventilation was 17 days. The patients had a median modified sequential organ failure assessment score of 8.5, and in-hospital mortality was 44% by 60 days. Notably, all patients in this cohort received steroids during their ICU stay.
Interestingly, the study investigators found no association between clinical outcomes and circulating proteins implicated in inflammation (eg, interleukin [IL]-6, IL-8), epithelial injury (eg, soluble receptor for advanced glycation end products, surfactant protein D), or coagulation (eg, D-dimer, tissue factor). However, four endothelial biomarkers—von Willebrand factor A2 domain; angiopoietin-2; syndecan-1; and neural precursor cell expressed, developmentally downregulated 9 (NEDD9)—were associated with 60-day mortality after adjusting for age, sex, and severity of illness. A sensitivity analysis, in which patients treated with the IL-6 inhibitor tocilizumab (n=4) were excluded, showed similar results.
Of the endothelial proteins, NEDD9 demonstrated the greatest effect size in its association with mortality in patients with ARDS due to COVID-19 who were treated with immunomodulators. NEDD9 is a scaffolding protein highly expressed in the pulmonary vascular endothelium, but its role in ARDS is not well known. In pulmonary vascular disease, plasma levels are associated with adverse pulmonary hemodynamics and clinical outcomes. Pulmonary artery endothelial NEDD9 is upregulated by cellular hypoxia and can mediate platelet-endothelial adhesion by interacting with P-selectin on the surface of activated platelets.6 Additionally, there is evidence of increased pulmonary endothelial NEDD9 expression and colocalization with fibrin within pulmonary arteries in lung tissue of patients who died from ARDS due to COVID-19.7 Thus, NEDD9 may be an important mediator of pulmonary vascular dysfunction observed in ARDS and could be a novel biomarker for patient subphenotyping and prognostication of clinical outcomes.
In summary, in a cohort of patients with COVID-19 ARDS uniformly treated with corticosteroids, plasma biomarkers of inflammation, coagulation, and epithelial injury were not associated with clinical outcomes, but endothelial biomarkers remained prognostic. It is biologically plausible that immunomodulators could attenuate the association between inflammatory biomarkers and patient outcomes. The findings of this study highlight the association of endothelial biomarkers with clinical outcomes in patients with COVID-19 ARDS treated with immunomodulators and warrant prospective validation, especially with the increasing evidence-based use of antiinflammatory therapy in acute lung injury. However, there are several important limitations to consider, including a small sample size from a single institution that precludes any definitive conclusions regarding any negative associations. Moreover, the single time point studied (the day of initiation of mechanical ventilation) and absence of a comparator group do not allow a comprehensive evaluation of the impact of antiinflammatory therapies across the trajectory of disease. Whether the findings are generalizable to all patients with ARDS treated with immunomodulators also remains unknown.
Overall, these data suggest that circulating signatures previously associated with ARDS, particularly those related to systemic inflammation, may have limited prognostic utility in the era of increasing immunomodulator use in critical illness. A deeper understanding of the pathobiology of ARDS, including the complex interplay with systemic immunomodulation, is needed to identify prognostic biomarkers and targeted therapies that improve patient outcomes.
Both authors work in the Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Harvard Medical School, in Boston.
References
1. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704.
2. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19. JAMA. 2020;324(13):1-11.
3. Dequin P-F, Meziani F, Quenot J-P, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941.
4. Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636-1643.
5. Alladina JW, Giacona FL, Haring AM, et al. Circulating biomarkers of endothelial dysfunction associated with ventilatory ratio and mortality in ARDS resulting from SARS-CoV-2 infection treated with antiinflammatory therapies. CHEST Crit Care. 2024;2(2):100054.
6. Alba GA, Samokhin AO, Wang R-S, et al. NEDD9 is a novel and modifiable mediator of platelet-endothelial adhesion in the pulmonary circulation. Am J Respir Crit Care Med. 2021;203(12):1533-1545.
7. Alba GA, Samokhin AO, Wang R-S, et al. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS‐CoV‐2 infection. Pulm Circ. 2022;12(2):e12071.
There is renewed interest in the use of immunomodulator therapies in patients with acute hypoxemic respiratory failure.
Beyond COVID-19, studies have also shown corticosteroid therapy improves clinical outcomes in patients with severe community-acquired pneumonia.3 However, the overwhelming majority of studies identifying plasma biomarkers that are associated with clinical outcomes in severe lung injury predate the routine use of corticosteroids.4 Two investigators at Massachusetts General Hospital, Jehan W. Alladina, MD, and George A. Alba, MD, performed a study to assess whether plasma biomarkers previously associated with clinical outcomes in ARDS maintained their predictive value in the setting of widespread immunomodulator therapy in the ICU. Drs. Alladina and Alba are physician-scientists and codirectors of the Program for Advancing Critical Care Translational Science at Massachusetts General Hospital in Boston.
In a study published in CHEST® Critical Care earlier this year, they prospectively enrolled patients with ARDS due to confirmed SARS-CoV-2 infection during the second wave of the COVID-19 pandemic from December 31, 2020, to March 31, 2021, at Massachusetts General Hospital.5 Plasma samples were collected within 24 hours of intubation for mechanical ventilation for protein analysis in 69 patients. Baseline demographics included a mean age of 62 plus or minus 15 years and a BMI of 31 plus or minus 8, and 45% were female. The median PaO2 to FiO2 ratio was 174 mm Hg, consistent with moderate ARDS, and the median duration of ventilation was 17 days. The patients had a median modified sequential organ failure assessment score of 8.5, and in-hospital mortality was 44% by 60 days. Notably, all patients in this cohort received steroids during their ICU stay.
Interestingly, the study investigators found no association between clinical outcomes and circulating proteins implicated in inflammation (eg, interleukin [IL]-6, IL-8), epithelial injury (eg, soluble receptor for advanced glycation end products, surfactant protein D), or coagulation (eg, D-dimer, tissue factor). However, four endothelial biomarkers—von Willebrand factor A2 domain; angiopoietin-2; syndecan-1; and neural precursor cell expressed, developmentally downregulated 9 (NEDD9)—were associated with 60-day mortality after adjusting for age, sex, and severity of illness. A sensitivity analysis, in which patients treated with the IL-6 inhibitor tocilizumab (n=4) were excluded, showed similar results.
Of the endothelial proteins, NEDD9 demonstrated the greatest effect size in its association with mortality in patients with ARDS due to COVID-19 who were treated with immunomodulators. NEDD9 is a scaffolding protein highly expressed in the pulmonary vascular endothelium, but its role in ARDS is not well known. In pulmonary vascular disease, plasma levels are associated with adverse pulmonary hemodynamics and clinical outcomes. Pulmonary artery endothelial NEDD9 is upregulated by cellular hypoxia and can mediate platelet-endothelial adhesion by interacting with P-selectin on the surface of activated platelets.6 Additionally, there is evidence of increased pulmonary endothelial NEDD9 expression and colocalization with fibrin within pulmonary arteries in lung tissue of patients who died from ARDS due to COVID-19.7 Thus, NEDD9 may be an important mediator of pulmonary vascular dysfunction observed in ARDS and could be a novel biomarker for patient subphenotyping and prognostication of clinical outcomes.
In summary, in a cohort of patients with COVID-19 ARDS uniformly treated with corticosteroids, plasma biomarkers of inflammation, coagulation, and epithelial injury were not associated with clinical outcomes, but endothelial biomarkers remained prognostic. It is biologically plausible that immunomodulators could attenuate the association between inflammatory biomarkers and patient outcomes. The findings of this study highlight the association of endothelial biomarkers with clinical outcomes in patients with COVID-19 ARDS treated with immunomodulators and warrant prospective validation, especially with the increasing evidence-based use of antiinflammatory therapy in acute lung injury. However, there are several important limitations to consider, including a small sample size from a single institution that precludes any definitive conclusions regarding any negative associations. Moreover, the single time point studied (the day of initiation of mechanical ventilation) and absence of a comparator group do not allow a comprehensive evaluation of the impact of antiinflammatory therapies across the trajectory of disease. Whether the findings are generalizable to all patients with ARDS treated with immunomodulators also remains unknown.
Overall, these data suggest that circulating signatures previously associated with ARDS, particularly those related to systemic inflammation, may have limited prognostic utility in the era of increasing immunomodulator use in critical illness. A deeper understanding of the pathobiology of ARDS, including the complex interplay with systemic immunomodulation, is needed to identify prognostic biomarkers and targeted therapies that improve patient outcomes.
Both authors work in the Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Harvard Medical School, in Boston.
References
1. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704.
2. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19. JAMA. 2020;324(13):1-11.
3. Dequin P-F, Meziani F, Quenot J-P, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941.
4. Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636-1643.
5. Alladina JW, Giacona FL, Haring AM, et al. Circulating biomarkers of endothelial dysfunction associated with ventilatory ratio and mortality in ARDS resulting from SARS-CoV-2 infection treated with antiinflammatory therapies. CHEST Crit Care. 2024;2(2):100054.
6. Alba GA, Samokhin AO, Wang R-S, et al. NEDD9 is a novel and modifiable mediator of platelet-endothelial adhesion in the pulmonary circulation. Am J Respir Crit Care Med. 2021;203(12):1533-1545.
7. Alba GA, Samokhin AO, Wang R-S, et al. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS‐CoV‐2 infection. Pulm Circ. 2022;12(2):e12071.
‘Door-to-Thrombectomy’ Time for Acute PE Linked to Better Outcomes
In the article "‘Door-to-thrombectomy ’ time linked to better acute PE outcomes" in the November 2024 issue of CHEST Physician, Dr. Patel was quoted as stating, "Mechanical thrombectomy in the FLASH registry showed mortality benefit," and, "There's a mortality benefit in any case whether the patient is high risk or intermediate-high." Dr. Patel was referring to the FLASH registry as a whole and not to the registry data study described in this article. CHEST Physician regrets the error and any confusion this may have caused.
BOSTON —
Among nearly 800 patients with acute PE whose data are recorded in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH), a prospective multicenter registry of individuals treated with mechanical thrombectomy using the FlowTriever system (Inari Medical), shorter time from admission to mechanical thrombectomy was associated with significantly greater reductions in intraprocedural mean and systolic pulmonary artery pressures (PAP), greater reductions in the right ventricular/left ventricular (RV/LV) ratio, and longer 6-minute walk times at 6 months, reported Krunal H. Patel, MD, a pulmonary and critical care fellow at the Lewis Katz School of Medicine at Temple University Hospital in Philadelphia.
“Mechanical thrombectomy in the FLASH registry showed a mortality benefit. I think as time progresses and mechanical thrombectomy becomes more popular, we’re just going to need to figure out what is the ideal time for intervention,” he said during an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
“There’s mortality benefit in any case whether the patient is high-risk or intermediate-high. This is a thought-provoking retrospective analysis that says that early intervention is probably better than doing it late, but regardless, the FLASH registry trial showed that early thrombectomy or thrombectomy in general shows positive mortality benefit,” Patel said in an interview.
He likened the challenge for pulmonary and critical care specialists to that of interventional cardiologists, who have determined that the ideal window for starting percutaneous coronary interventions is within 90 minutes of the patient’s arrival at the facility.
“I think we have to get our ‘door-to-balloon’ time for PE care,” he said.
Study Details
Patel and colleague Parth M. Rali, MD, FCCP, associate professor of thoracic medicine at Temple, conducted a retrospective review of data on 787 US patients in the FLASH registry for whom time to mechanical thrombectomy data were available. They stratified the patients into short and long time to mechanical thrombectomy groups, with “short” defined as ≤ 12 hours of presentation and “long” as > 12 hours.
They found that the median time to thrombectomy was 19.68 hours. In all, 242 patients (31%) were treated within the short window, and the remaining 545 patients (69%) were treated after at least 12 hours had passed.
Comparing clinical characteristics between the groups, the investigators noted that significantly more patients in the short time group vs long time group were categorized as high-risk (11.2% vs 6.2%; P = .0026). This difference is likely due to the need for greater urgency among high-risk patients, Patel said.
Patients in the short time group also had significantly higher baseline RV/LV ratios and lactate levels, but baseline dyspnea scores and pre-procedure median and systolic PAP were similar between the groups.
The mean time to thrombectomy was 6.08 hours in the short time group vs 34.04 hours in the long time group. Their respective median times were 6.01 and 24.73 hours.
The procedural time was similar between the groups, at 45 and 42 minutes, respectively.
The location of the treated thrombus was central only in 35.1% and 26.5% patients in the short and long time groups, respectively. Lobar-only thrombi were treated in 7.9% and 14.3%, respectively, and both central and lobar thrombi were treated in 57.0% and 59.2%, respectively.
Both 48-hour and 30-day all-cause mortality rates were similar between the groups (0.4%/0.2% and 0.5%/1.0%).
Patients in the short time group had slightly but significantly longer post-procedure hospital and intensive care unit stays, but 30-day readmission rates — whether for PE- or non-PE–related causes — were similar.
Where the differences between the groups really showed, however, were PAP reductions over baseline, with decline in median pressures of −8.7 mm Hg in the short group vs −7.2 mm Hg in the long group (P = .0008), and drops in systolic PAP of −14.4 vs −12.1 mm Hg, respectively (P = .0011).
In addition, reductions in RV/LV ratios from baseline were also significantly greater among patients whose thrombectomies had been expedited at the 48-hour, 30-day, and 6-month follow-up periods.
At 6 months, patients who had received mechanical thrombectomy within 12 hours also had significantly longer 6-minute walk distances (442.2 vs 390.5 m; P = .0032).
Low Thrombolysis Rate
Following his presentation, session co-moderator Galina Glazman-Kuczaj, MD, from the Division of Pulmonary and Critical Care Medicine at Albany Med Health System, Albany, New York, asked Patel what percentage of patients, if any, had received thrombolytic therapy before the thrombectomy procedure.
He noted that only 1% or 2% patients in the FLASH registry received thrombolysis.
In an interview, Glazman-Kuczaj said that “it was reassuring for [Patel] to report that it was only a small population of patients who got thrombolysis beforehand in either group because you would expect that maybe people in the group that took longer to have a thrombectomy got some thrombolysis beforehand and that perhaps they were more stable, but it seems like thrombectomy was the first-line treatment in both groups.”
The FLASH Registry is funded by Inari Medical. Patel and Glazman-Kuczaj reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In the article "‘Door-to-thrombectomy ’ time linked to better acute PE outcomes" in the November 2024 issue of CHEST Physician, Dr. Patel was quoted as stating, "Mechanical thrombectomy in the FLASH registry showed mortality benefit," and, "There's a mortality benefit in any case whether the patient is high risk or intermediate-high." Dr. Patel was referring to the FLASH registry as a whole and not to the registry data study described in this article. CHEST Physician regrets the error and any confusion this may have caused.
BOSTON —
Among nearly 800 patients with acute PE whose data are recorded in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH), a prospective multicenter registry of individuals treated with mechanical thrombectomy using the FlowTriever system (Inari Medical), shorter time from admission to mechanical thrombectomy was associated with significantly greater reductions in intraprocedural mean and systolic pulmonary artery pressures (PAP), greater reductions in the right ventricular/left ventricular (RV/LV) ratio, and longer 6-minute walk times at 6 months, reported Krunal H. Patel, MD, a pulmonary and critical care fellow at the Lewis Katz School of Medicine at Temple University Hospital in Philadelphia.
“Mechanical thrombectomy in the FLASH registry showed a mortality benefit. I think as time progresses and mechanical thrombectomy becomes more popular, we’re just going to need to figure out what is the ideal time for intervention,” he said during an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
“There’s mortality benefit in any case whether the patient is high-risk or intermediate-high. This is a thought-provoking retrospective analysis that says that early intervention is probably better than doing it late, but regardless, the FLASH registry trial showed that early thrombectomy or thrombectomy in general shows positive mortality benefit,” Patel said in an interview.
He likened the challenge for pulmonary and critical care specialists to that of interventional cardiologists, who have determined that the ideal window for starting percutaneous coronary interventions is within 90 minutes of the patient’s arrival at the facility.
“I think we have to get our ‘door-to-balloon’ time for PE care,” he said.
Study Details
Patel and colleague Parth M. Rali, MD, FCCP, associate professor of thoracic medicine at Temple, conducted a retrospective review of data on 787 US patients in the FLASH registry for whom time to mechanical thrombectomy data were available. They stratified the patients into short and long time to mechanical thrombectomy groups, with “short” defined as ≤ 12 hours of presentation and “long” as > 12 hours.
They found that the median time to thrombectomy was 19.68 hours. In all, 242 patients (31%) were treated within the short window, and the remaining 545 patients (69%) were treated after at least 12 hours had passed.
Comparing clinical characteristics between the groups, the investigators noted that significantly more patients in the short time group vs long time group were categorized as high-risk (11.2% vs 6.2%; P = .0026). This difference is likely due to the need for greater urgency among high-risk patients, Patel said.
Patients in the short time group also had significantly higher baseline RV/LV ratios and lactate levels, but baseline dyspnea scores and pre-procedure median and systolic PAP were similar between the groups.
The mean time to thrombectomy was 6.08 hours in the short time group vs 34.04 hours in the long time group. Their respective median times were 6.01 and 24.73 hours.
The procedural time was similar between the groups, at 45 and 42 minutes, respectively.
The location of the treated thrombus was central only in 35.1% and 26.5% patients in the short and long time groups, respectively. Lobar-only thrombi were treated in 7.9% and 14.3%, respectively, and both central and lobar thrombi were treated in 57.0% and 59.2%, respectively.
Both 48-hour and 30-day all-cause mortality rates were similar between the groups (0.4%/0.2% and 0.5%/1.0%).
Patients in the short time group had slightly but significantly longer post-procedure hospital and intensive care unit stays, but 30-day readmission rates — whether for PE- or non-PE–related causes — were similar.
Where the differences between the groups really showed, however, were PAP reductions over baseline, with decline in median pressures of −8.7 mm Hg in the short group vs −7.2 mm Hg in the long group (P = .0008), and drops in systolic PAP of −14.4 vs −12.1 mm Hg, respectively (P = .0011).
In addition, reductions in RV/LV ratios from baseline were also significantly greater among patients whose thrombectomies had been expedited at the 48-hour, 30-day, and 6-month follow-up periods.
At 6 months, patients who had received mechanical thrombectomy within 12 hours also had significantly longer 6-minute walk distances (442.2 vs 390.5 m; P = .0032).
Low Thrombolysis Rate
Following his presentation, session co-moderator Galina Glazman-Kuczaj, MD, from the Division of Pulmonary and Critical Care Medicine at Albany Med Health System, Albany, New York, asked Patel what percentage of patients, if any, had received thrombolytic therapy before the thrombectomy procedure.
He noted that only 1% or 2% patients in the FLASH registry received thrombolysis.
In an interview, Glazman-Kuczaj said that “it was reassuring for [Patel] to report that it was only a small population of patients who got thrombolysis beforehand in either group because you would expect that maybe people in the group that took longer to have a thrombectomy got some thrombolysis beforehand and that perhaps they were more stable, but it seems like thrombectomy was the first-line treatment in both groups.”
The FLASH Registry is funded by Inari Medical. Patel and Glazman-Kuczaj reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In the article "‘Door-to-thrombectomy ’ time linked to better acute PE outcomes" in the November 2024 issue of CHEST Physician, Dr. Patel was quoted as stating, "Mechanical thrombectomy in the FLASH registry showed mortality benefit," and, "There's a mortality benefit in any case whether the patient is high risk or intermediate-high." Dr. Patel was referring to the FLASH registry as a whole and not to the registry data study described in this article. CHEST Physician regrets the error and any confusion this may have caused.
BOSTON —
Among nearly 800 patients with acute PE whose data are recorded in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH), a prospective multicenter registry of individuals treated with mechanical thrombectomy using the FlowTriever system (Inari Medical), shorter time from admission to mechanical thrombectomy was associated with significantly greater reductions in intraprocedural mean and systolic pulmonary artery pressures (PAP), greater reductions in the right ventricular/left ventricular (RV/LV) ratio, and longer 6-minute walk times at 6 months, reported Krunal H. Patel, MD, a pulmonary and critical care fellow at the Lewis Katz School of Medicine at Temple University Hospital in Philadelphia.
“Mechanical thrombectomy in the FLASH registry showed a mortality benefit. I think as time progresses and mechanical thrombectomy becomes more popular, we’re just going to need to figure out what is the ideal time for intervention,” he said during an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
“There’s mortality benefit in any case whether the patient is high-risk or intermediate-high. This is a thought-provoking retrospective analysis that says that early intervention is probably better than doing it late, but regardless, the FLASH registry trial showed that early thrombectomy or thrombectomy in general shows positive mortality benefit,” Patel said in an interview.
He likened the challenge for pulmonary and critical care specialists to that of interventional cardiologists, who have determined that the ideal window for starting percutaneous coronary interventions is within 90 minutes of the patient’s arrival at the facility.
“I think we have to get our ‘door-to-balloon’ time for PE care,” he said.
Study Details
Patel and colleague Parth M. Rali, MD, FCCP, associate professor of thoracic medicine at Temple, conducted a retrospective review of data on 787 US patients in the FLASH registry for whom time to mechanical thrombectomy data were available. They stratified the patients into short and long time to mechanical thrombectomy groups, with “short” defined as ≤ 12 hours of presentation and “long” as > 12 hours.
They found that the median time to thrombectomy was 19.68 hours. In all, 242 patients (31%) were treated within the short window, and the remaining 545 patients (69%) were treated after at least 12 hours had passed.
Comparing clinical characteristics between the groups, the investigators noted that significantly more patients in the short time group vs long time group were categorized as high-risk (11.2% vs 6.2%; P = .0026). This difference is likely due to the need for greater urgency among high-risk patients, Patel said.
Patients in the short time group also had significantly higher baseline RV/LV ratios and lactate levels, but baseline dyspnea scores and pre-procedure median and systolic PAP were similar between the groups.
The mean time to thrombectomy was 6.08 hours in the short time group vs 34.04 hours in the long time group. Their respective median times were 6.01 and 24.73 hours.
The procedural time was similar between the groups, at 45 and 42 minutes, respectively.
The location of the treated thrombus was central only in 35.1% and 26.5% patients in the short and long time groups, respectively. Lobar-only thrombi were treated in 7.9% and 14.3%, respectively, and both central and lobar thrombi were treated in 57.0% and 59.2%, respectively.
Both 48-hour and 30-day all-cause mortality rates were similar between the groups (0.4%/0.2% and 0.5%/1.0%).
Patients in the short time group had slightly but significantly longer post-procedure hospital and intensive care unit stays, but 30-day readmission rates — whether for PE- or non-PE–related causes — were similar.
Where the differences between the groups really showed, however, were PAP reductions over baseline, with decline in median pressures of −8.7 mm Hg in the short group vs −7.2 mm Hg in the long group (P = .0008), and drops in systolic PAP of −14.4 vs −12.1 mm Hg, respectively (P = .0011).
In addition, reductions in RV/LV ratios from baseline were also significantly greater among patients whose thrombectomies had been expedited at the 48-hour, 30-day, and 6-month follow-up periods.
At 6 months, patients who had received mechanical thrombectomy within 12 hours also had significantly longer 6-minute walk distances (442.2 vs 390.5 m; P = .0032).
Low Thrombolysis Rate
Following his presentation, session co-moderator Galina Glazman-Kuczaj, MD, from the Division of Pulmonary and Critical Care Medicine at Albany Med Health System, Albany, New York, asked Patel what percentage of patients, if any, had received thrombolytic therapy before the thrombectomy procedure.
He noted that only 1% or 2% patients in the FLASH registry received thrombolysis.
In an interview, Glazman-Kuczaj said that “it was reassuring for [Patel] to report that it was only a small population of patients who got thrombolysis beforehand in either group because you would expect that maybe people in the group that took longer to have a thrombectomy got some thrombolysis beforehand and that perhaps they were more stable, but it seems like thrombectomy was the first-line treatment in both groups.”
The FLASH Registry is funded by Inari Medical. Patel and Glazman-Kuczaj reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CHEST 2024
ILD Linked to Poorer Outcomes in Pulmonary Embolism
BOSTON — Patients with pulmonary embolism (PE) who also present with interstitial lung disease (ILD) have worse outcomes with respect to in-hospital mortality, length of hospital stay, hospital cost, and all-cause readmission, according to results from a new retrospective analysis.
Unfortunately, there’s not a whole lot of evidence out there to really demonstrate it,” Leah Yuan, MD, said during a presentation of the results at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
The question is complicated by the nebulous nature of ILD, which includes a diverse set of diseases and etiologies, and different levels of inflammation and fibrosis. It has been employed in the Pulmonary Embolism Severity Index but counts for only 10 points out of 210. “If you look at ILD and PE outcomes, there’s nothing really out there [in the literature],” Yuan said in an interview. She is a resident physician at Cook County Health and Hospitals System.
The new study suggested that ILD could have an important influence and perhaps should have greater weight in risk stratification of patients with PE, she said. “We looked at all-cause readmissions and we looked at in-hospital mortality, [both] of which are significant for increased odds ratio. One thing that I’m very curious to see is whether there is increased PE readmissions [associated with ILD], which is something that we couldn’t find to be significant in our study,” said Yuan.
The researchers used data from hospitalizations for PE drawn from the Nationwide Readmissions Database in 2019, using International Classification of Diseases, Tenth Revision, codes to identify admissions. Among a total of 105,133 patients admitted for PE, 158 patients also had ILD. The mean age was 63.6 years for those without ILD (SD, 0.1) and 66.5 years for those with ILD (SD, 1.3).
Admission with ILD was associated with all-cause readmission (odds ratio [OR], 4.12; P < .01), in-hospital mortality (OR, 2.17; P = .01), a longer length of stay (+2.07 days; P < .01), and higher hospitalization charges (+$22,627; P < .01).
In the Q&A period after the presentation, Parth Rali, MD, professor of thoracic medicine and surgery at Temple University, Philadelphia, suggested phenotyping patients to better understand the location of the PE in relation to the ILD. “It may not fall into your classic PE classification. It may just depend on where the clot is in relationship to the interstitial lung disease. I think that’s where the field is going to evolve,” he later said in an interview.
“What is interesting is that patients with interstitial lung disease have a lot of fibrotic disease, and they do not need to have a large clot burden to make them sick. An example [is someone] who has undergone a lung transplant evaluation, and if their right lung is completely diseased from interstitial lung disease and if they get a big blood clot on the right side, it doesn’t affect them because the lung is already fibrotic, so the clot doesn’t matter. If they get a small clot [in the left lung], even though if you look at the standard PE classification they may qualify as a low-risk PE or even as an intermediate-low-risk PE, they are much sicker because that’s the functioning part of the lung,” said Rali.
He advised physicians to pay close attention to the location of PEs in relation to fibrotic tissue in patients with ILD. A PE in healthy lung tissue could have an outsized effect on hemodynamics, whereas a PE in fibrotic tissue may be clinically insignificant and not require treatment. “So it goes both ways: You don’t overtreat and you don’t undertreat,” Rali said.
Yuan and Rali disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
BOSTON — Patients with pulmonary embolism (PE) who also present with interstitial lung disease (ILD) have worse outcomes with respect to in-hospital mortality, length of hospital stay, hospital cost, and all-cause readmission, according to results from a new retrospective analysis.
Unfortunately, there’s not a whole lot of evidence out there to really demonstrate it,” Leah Yuan, MD, said during a presentation of the results at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
The question is complicated by the nebulous nature of ILD, which includes a diverse set of diseases and etiologies, and different levels of inflammation and fibrosis. It has been employed in the Pulmonary Embolism Severity Index but counts for only 10 points out of 210. “If you look at ILD and PE outcomes, there’s nothing really out there [in the literature],” Yuan said in an interview. She is a resident physician at Cook County Health and Hospitals System.
The new study suggested that ILD could have an important influence and perhaps should have greater weight in risk stratification of patients with PE, she said. “We looked at all-cause readmissions and we looked at in-hospital mortality, [both] of which are significant for increased odds ratio. One thing that I’m very curious to see is whether there is increased PE readmissions [associated with ILD], which is something that we couldn’t find to be significant in our study,” said Yuan.
The researchers used data from hospitalizations for PE drawn from the Nationwide Readmissions Database in 2019, using International Classification of Diseases, Tenth Revision, codes to identify admissions. Among a total of 105,133 patients admitted for PE, 158 patients also had ILD. The mean age was 63.6 years for those without ILD (SD, 0.1) and 66.5 years for those with ILD (SD, 1.3).
Admission with ILD was associated with all-cause readmission (odds ratio [OR], 4.12; P < .01), in-hospital mortality (OR, 2.17; P = .01), a longer length of stay (+2.07 days; P < .01), and higher hospitalization charges (+$22,627; P < .01).
In the Q&A period after the presentation, Parth Rali, MD, professor of thoracic medicine and surgery at Temple University, Philadelphia, suggested phenotyping patients to better understand the location of the PE in relation to the ILD. “It may not fall into your classic PE classification. It may just depend on where the clot is in relationship to the interstitial lung disease. I think that’s where the field is going to evolve,” he later said in an interview.
“What is interesting is that patients with interstitial lung disease have a lot of fibrotic disease, and they do not need to have a large clot burden to make them sick. An example [is someone] who has undergone a lung transplant evaluation, and if their right lung is completely diseased from interstitial lung disease and if they get a big blood clot on the right side, it doesn’t affect them because the lung is already fibrotic, so the clot doesn’t matter. If they get a small clot [in the left lung], even though if you look at the standard PE classification they may qualify as a low-risk PE or even as an intermediate-low-risk PE, they are much sicker because that’s the functioning part of the lung,” said Rali.
He advised physicians to pay close attention to the location of PEs in relation to fibrotic tissue in patients with ILD. A PE in healthy lung tissue could have an outsized effect on hemodynamics, whereas a PE in fibrotic tissue may be clinically insignificant and not require treatment. “So it goes both ways: You don’t overtreat and you don’t undertreat,” Rali said.
Yuan and Rali disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
BOSTON — Patients with pulmonary embolism (PE) who also present with interstitial lung disease (ILD) have worse outcomes with respect to in-hospital mortality, length of hospital stay, hospital cost, and all-cause readmission, according to results from a new retrospective analysis.
Unfortunately, there’s not a whole lot of evidence out there to really demonstrate it,” Leah Yuan, MD, said during a presentation of the results at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
The question is complicated by the nebulous nature of ILD, which includes a diverse set of diseases and etiologies, and different levels of inflammation and fibrosis. It has been employed in the Pulmonary Embolism Severity Index but counts for only 10 points out of 210. “If you look at ILD and PE outcomes, there’s nothing really out there [in the literature],” Yuan said in an interview. She is a resident physician at Cook County Health and Hospitals System.
The new study suggested that ILD could have an important influence and perhaps should have greater weight in risk stratification of patients with PE, she said. “We looked at all-cause readmissions and we looked at in-hospital mortality, [both] of which are significant for increased odds ratio. One thing that I’m very curious to see is whether there is increased PE readmissions [associated with ILD], which is something that we couldn’t find to be significant in our study,” said Yuan.
The researchers used data from hospitalizations for PE drawn from the Nationwide Readmissions Database in 2019, using International Classification of Diseases, Tenth Revision, codes to identify admissions. Among a total of 105,133 patients admitted for PE, 158 patients also had ILD. The mean age was 63.6 years for those without ILD (SD, 0.1) and 66.5 years for those with ILD (SD, 1.3).
Admission with ILD was associated with all-cause readmission (odds ratio [OR], 4.12; P < .01), in-hospital mortality (OR, 2.17; P = .01), a longer length of stay (+2.07 days; P < .01), and higher hospitalization charges (+$22,627; P < .01).
In the Q&A period after the presentation, Parth Rali, MD, professor of thoracic medicine and surgery at Temple University, Philadelphia, suggested phenotyping patients to better understand the location of the PE in relation to the ILD. “It may not fall into your classic PE classification. It may just depend on where the clot is in relationship to the interstitial lung disease. I think that’s where the field is going to evolve,” he later said in an interview.
“What is interesting is that patients with interstitial lung disease have a lot of fibrotic disease, and they do not need to have a large clot burden to make them sick. An example [is someone] who has undergone a lung transplant evaluation, and if their right lung is completely diseased from interstitial lung disease and if they get a big blood clot on the right side, it doesn’t affect them because the lung is already fibrotic, so the clot doesn’t matter. If they get a small clot [in the left lung], even though if you look at the standard PE classification they may qualify as a low-risk PE or even as an intermediate-low-risk PE, they are much sicker because that’s the functioning part of the lung,” said Rali.
He advised physicians to pay close attention to the location of PEs in relation to fibrotic tissue in patients with ILD. A PE in healthy lung tissue could have an outsized effect on hemodynamics, whereas a PE in fibrotic tissue may be clinically insignificant and not require treatment. “So it goes both ways: You don’t overtreat and you don’t undertreat,” Rali said.
Yuan and Rali disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
CHEST 2024
Locally Acquired Dengue Case Confirmed in California
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Controversy Surrounds Optimal Treatment for High-Risk Pulmonary Embolism
VIENNA — The optimal course of treatment when managing acute, high-risk pulmonary embolism (PE) remains a contentious topic among respiratory specialists.
Systemic thrombolysis, specifically using recombinant tissue plasminogen activator (rtPA), is the current gold standard treatment for high-risk PE. However, the real-world application is less straightforward due to patient complexities.
Here at the European Respiratory Society (ERS) 2024 Congress, respiratory specialists presented contrasting viewpoints and the latest evidence on each side of the issue to provide a comprehensive framework for navigating the complex decision-making process required for effective treatment.
“High-risk PE is a mechanical problem and thus needs a mechanical solution,” said Parth M. Rali, MD, an associate professor in thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia.
“The marketing on some of the mechanical techniques is very impressive,” said Olivier Sanchez, MD, a pulmonologist in the Department of Pneumology and Intensive Care at the Georges Pompidou European Hospital in France. “But what is the evidence of such treatment in the setting of pulmonary embolism?”
The Case Against rtPA as the Standard of Care
High-risk PE typically involves hemodynamically unstable patients presenting with conditions such as low blood pressure, cardiac arrest, or the need for mechanical circulatory support. There is a spectrum of severity within high-risk PE, making it a complex condition to manage, especially since many patients have comorbidities like anemia or active cancer, complicating treatment. “It’s a very dynamic and fluid condition, and we can’t take for granted that rtPA is a standard of care,” Dr. Rali said.
Alternative treatments such as catheter-directed therapies, extracorporeal membrane oxygenation (ECMO), and surgical embolectomy are emerging as promising options, especially for patients who do not respond to or cannot receive rtPA. Mechanical treatments offer benefits in reducing clot burden and stabilizing patients, but they come with their own challenges.
ECMO can stabilize patients who are in shock or cardiac arrest, buying time for the clot to resolve or for further interventions like surgery or catheter-based treatments, said Dr. Rali. However, it is an invasive procedure requiring cannulation of large blood vessels, often involving significant resources and expertise.
Catheter-directed thrombolysis is a minimally invasive technique where a catheter is inserted directly into the pulmonary artery to deliver thrombolytic drugs at lower doses. This method allows for more targeted treatment of the clot, reducing the risk for systemic bleeding that comes with higher doses of thrombolytic agents used in systemic therapy, Dr. Rali explained.
Dr. Rali reported results from the FLAME study, which investigated the effectiveness of FlowTriever mechanical thrombectomy compared with conventional therapies for high-risk PE. This prospective, multicenter observational study enrolled 53 patients in the FlowTriever arm and 61 in the context arm, which included patients treated with systemic thrombolysis or anticoagulation. The primary endpoint, a composite of adverse in-hospital outcomes, was reached in 17% of FlowTriever patients, significantly lower than the 32% performance goal and the 63.9% rate in the context arm. In-hospital mortality was dramatically lower in the FlowTriever arm (1.9%) compared to the context arm (29.5%).
When catheter-based treatment fails, surgical pulmonary embolectomy is a last-resort option. “Only a minority of the high-risk PE [patients] would qualify for rtPA without harmful side effects,” Dr. Rali concluded. “So think wise before you pull your trigger.”
rtPA Not a Matter of the Past
In high-risk PE, the therapeutic priority is rapid hemodynamic stabilization and restoration of pulmonary blood flow to prevent cardiovascular collapse. Systemic thrombolysis acts quickly, reducing pulmonary vascular resistance and obstruction within hours, said Dr. Sanchez.
Presenting at the ERS Congress, he reported numerous studies, including 15 randomized controlled trials that demonstrated its effectiveness in high-risk PE. The PEITHO trial, in particular, demonstrated the ability of systemic thrombolysis to reduce all-cause mortality and hemodynamic collapse within 7 days.
However, this benefit comes at the cost of increased bleeding risk, including a 10% rate of major bleeding and a 2% risk for intracranial hemorrhage. “These data come from old studies using invasive diagnostic procedures, and with current diagnostic procedures, the rate of bleeding is probably lower,” Dr. Sanchez said. The risk of bleeding is also related to the type of thrombolytic agent, with tenecteplase being strongly associated with a higher risk of bleeding, while alteplase shows no increase in the risk of major bleeding, he added. New strategies like reduced-dose thrombolysis offer comparable efficacy and improved safety, as demonstrated in ongoing trials like PEITHO-3, which aim to optimize the balance between efficacy and bleeding risk. Dr. Sanchez is the lead investigator of the PEITHO-3 study.
While rtPA might not be optimal for all patients, Dr. Sanchez thinks there is not enough evidence to replace it as a first-line treatment.
Existing studies on catheter-directed therapies often focus on surrogate endpoints, such as right-to-left ventricular ratio changes, rather than clinical outcomes like mortality, he said. Retrospective data suggest that catheter-directed therapies may reduce in-hospital mortality compared with systemic therapies, but they also increase the risk of intracranial bleeding, post-procedure complications, and device-related events.
Sanchez mentioned the same FLAME study described by Dr. Rali, which reported a 23% rate of device-related complications and 11% major bleeding in patients treated with catheter-directed therapies.
“Systemic thrombolysis remains the first treatment of choice,” Dr. Sanchez concluded. “The use of catheter-directed treatment should be discussed as an alternative in case of contraindications.”
The Debate Continues
Numerous ongoing clinical studies, such as the FLARE trial, will address gaps in evidence and refine treatment protocols, potentially reshaping the standard of care in high-risk PE in the near future by providing new data on the efficacy and safety of existing and emerging therapies.
“The coming data will make it clearer what the best option is,” said Thamer Al Khouzaie, MD, a pulmonary medicine consultant at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia. For now, he said, systemic thrombolysis remains the best option for most patients because it is widely available, easily administered with intravenous infusion, and at a limited cost. Catheter-directed treatment and surgical options are only available in specialized centers, require expertise and training, and are also very expensive.
Dr. Rali, Dr. Sanchez, and Dr. Khouzaie report no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — The optimal course of treatment when managing acute, high-risk pulmonary embolism (PE) remains a contentious topic among respiratory specialists.
Systemic thrombolysis, specifically using recombinant tissue plasminogen activator (rtPA), is the current gold standard treatment for high-risk PE. However, the real-world application is less straightforward due to patient complexities.
Here at the European Respiratory Society (ERS) 2024 Congress, respiratory specialists presented contrasting viewpoints and the latest evidence on each side of the issue to provide a comprehensive framework for navigating the complex decision-making process required for effective treatment.
“High-risk PE is a mechanical problem and thus needs a mechanical solution,” said Parth M. Rali, MD, an associate professor in thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia.
“The marketing on some of the mechanical techniques is very impressive,” said Olivier Sanchez, MD, a pulmonologist in the Department of Pneumology and Intensive Care at the Georges Pompidou European Hospital in France. “But what is the evidence of such treatment in the setting of pulmonary embolism?”
The Case Against rtPA as the Standard of Care
High-risk PE typically involves hemodynamically unstable patients presenting with conditions such as low blood pressure, cardiac arrest, or the need for mechanical circulatory support. There is a spectrum of severity within high-risk PE, making it a complex condition to manage, especially since many patients have comorbidities like anemia or active cancer, complicating treatment. “It’s a very dynamic and fluid condition, and we can’t take for granted that rtPA is a standard of care,” Dr. Rali said.
Alternative treatments such as catheter-directed therapies, extracorporeal membrane oxygenation (ECMO), and surgical embolectomy are emerging as promising options, especially for patients who do not respond to or cannot receive rtPA. Mechanical treatments offer benefits in reducing clot burden and stabilizing patients, but they come with their own challenges.
ECMO can stabilize patients who are in shock or cardiac arrest, buying time for the clot to resolve or for further interventions like surgery or catheter-based treatments, said Dr. Rali. However, it is an invasive procedure requiring cannulation of large blood vessels, often involving significant resources and expertise.
Catheter-directed thrombolysis is a minimally invasive technique where a catheter is inserted directly into the pulmonary artery to deliver thrombolytic drugs at lower doses. This method allows for more targeted treatment of the clot, reducing the risk for systemic bleeding that comes with higher doses of thrombolytic agents used in systemic therapy, Dr. Rali explained.
Dr. Rali reported results from the FLAME study, which investigated the effectiveness of FlowTriever mechanical thrombectomy compared with conventional therapies for high-risk PE. This prospective, multicenter observational study enrolled 53 patients in the FlowTriever arm and 61 in the context arm, which included patients treated with systemic thrombolysis or anticoagulation. The primary endpoint, a composite of adverse in-hospital outcomes, was reached in 17% of FlowTriever patients, significantly lower than the 32% performance goal and the 63.9% rate in the context arm. In-hospital mortality was dramatically lower in the FlowTriever arm (1.9%) compared to the context arm (29.5%).
When catheter-based treatment fails, surgical pulmonary embolectomy is a last-resort option. “Only a minority of the high-risk PE [patients] would qualify for rtPA without harmful side effects,” Dr. Rali concluded. “So think wise before you pull your trigger.”
rtPA Not a Matter of the Past
In high-risk PE, the therapeutic priority is rapid hemodynamic stabilization and restoration of pulmonary blood flow to prevent cardiovascular collapse. Systemic thrombolysis acts quickly, reducing pulmonary vascular resistance and obstruction within hours, said Dr. Sanchez.
Presenting at the ERS Congress, he reported numerous studies, including 15 randomized controlled trials that demonstrated its effectiveness in high-risk PE. The PEITHO trial, in particular, demonstrated the ability of systemic thrombolysis to reduce all-cause mortality and hemodynamic collapse within 7 days.
However, this benefit comes at the cost of increased bleeding risk, including a 10% rate of major bleeding and a 2% risk for intracranial hemorrhage. “These data come from old studies using invasive diagnostic procedures, and with current diagnostic procedures, the rate of bleeding is probably lower,” Dr. Sanchez said. The risk of bleeding is also related to the type of thrombolytic agent, with tenecteplase being strongly associated with a higher risk of bleeding, while alteplase shows no increase in the risk of major bleeding, he added. New strategies like reduced-dose thrombolysis offer comparable efficacy and improved safety, as demonstrated in ongoing trials like PEITHO-3, which aim to optimize the balance between efficacy and bleeding risk. Dr. Sanchez is the lead investigator of the PEITHO-3 study.
While rtPA might not be optimal for all patients, Dr. Sanchez thinks there is not enough evidence to replace it as a first-line treatment.
Existing studies on catheter-directed therapies often focus on surrogate endpoints, such as right-to-left ventricular ratio changes, rather than clinical outcomes like mortality, he said. Retrospective data suggest that catheter-directed therapies may reduce in-hospital mortality compared with systemic therapies, but they also increase the risk of intracranial bleeding, post-procedure complications, and device-related events.
Sanchez mentioned the same FLAME study described by Dr. Rali, which reported a 23% rate of device-related complications and 11% major bleeding in patients treated with catheter-directed therapies.
“Systemic thrombolysis remains the first treatment of choice,” Dr. Sanchez concluded. “The use of catheter-directed treatment should be discussed as an alternative in case of contraindications.”
The Debate Continues
Numerous ongoing clinical studies, such as the FLARE trial, will address gaps in evidence and refine treatment protocols, potentially reshaping the standard of care in high-risk PE in the near future by providing new data on the efficacy and safety of existing and emerging therapies.
“The coming data will make it clearer what the best option is,” said Thamer Al Khouzaie, MD, a pulmonary medicine consultant at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia. For now, he said, systemic thrombolysis remains the best option for most patients because it is widely available, easily administered with intravenous infusion, and at a limited cost. Catheter-directed treatment and surgical options are only available in specialized centers, require expertise and training, and are also very expensive.
Dr. Rali, Dr. Sanchez, and Dr. Khouzaie report no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — The optimal course of treatment when managing acute, high-risk pulmonary embolism (PE) remains a contentious topic among respiratory specialists.
Systemic thrombolysis, specifically using recombinant tissue plasminogen activator (rtPA), is the current gold standard treatment for high-risk PE. However, the real-world application is less straightforward due to patient complexities.
Here at the European Respiratory Society (ERS) 2024 Congress, respiratory specialists presented contrasting viewpoints and the latest evidence on each side of the issue to provide a comprehensive framework for navigating the complex decision-making process required for effective treatment.
“High-risk PE is a mechanical problem and thus needs a mechanical solution,” said Parth M. Rali, MD, an associate professor in thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia.
“The marketing on some of the mechanical techniques is very impressive,” said Olivier Sanchez, MD, a pulmonologist in the Department of Pneumology and Intensive Care at the Georges Pompidou European Hospital in France. “But what is the evidence of such treatment in the setting of pulmonary embolism?”
The Case Against rtPA as the Standard of Care
High-risk PE typically involves hemodynamically unstable patients presenting with conditions such as low blood pressure, cardiac arrest, or the need for mechanical circulatory support. There is a spectrum of severity within high-risk PE, making it a complex condition to manage, especially since many patients have comorbidities like anemia or active cancer, complicating treatment. “It’s a very dynamic and fluid condition, and we can’t take for granted that rtPA is a standard of care,” Dr. Rali said.
Alternative treatments such as catheter-directed therapies, extracorporeal membrane oxygenation (ECMO), and surgical embolectomy are emerging as promising options, especially for patients who do not respond to or cannot receive rtPA. Mechanical treatments offer benefits in reducing clot burden and stabilizing patients, but they come with their own challenges.
ECMO can stabilize patients who are in shock or cardiac arrest, buying time for the clot to resolve or for further interventions like surgery or catheter-based treatments, said Dr. Rali. However, it is an invasive procedure requiring cannulation of large blood vessels, often involving significant resources and expertise.
Catheter-directed thrombolysis is a minimally invasive technique where a catheter is inserted directly into the pulmonary artery to deliver thrombolytic drugs at lower doses. This method allows for more targeted treatment of the clot, reducing the risk for systemic bleeding that comes with higher doses of thrombolytic agents used in systemic therapy, Dr. Rali explained.
Dr. Rali reported results from the FLAME study, which investigated the effectiveness of FlowTriever mechanical thrombectomy compared with conventional therapies for high-risk PE. This prospective, multicenter observational study enrolled 53 patients in the FlowTriever arm and 61 in the context arm, which included patients treated with systemic thrombolysis or anticoagulation. The primary endpoint, a composite of adverse in-hospital outcomes, was reached in 17% of FlowTriever patients, significantly lower than the 32% performance goal and the 63.9% rate in the context arm. In-hospital mortality was dramatically lower in the FlowTriever arm (1.9%) compared to the context arm (29.5%).
When catheter-based treatment fails, surgical pulmonary embolectomy is a last-resort option. “Only a minority of the high-risk PE [patients] would qualify for rtPA without harmful side effects,” Dr. Rali concluded. “So think wise before you pull your trigger.”
rtPA Not a Matter of the Past
In high-risk PE, the therapeutic priority is rapid hemodynamic stabilization and restoration of pulmonary blood flow to prevent cardiovascular collapse. Systemic thrombolysis acts quickly, reducing pulmonary vascular resistance and obstruction within hours, said Dr. Sanchez.
Presenting at the ERS Congress, he reported numerous studies, including 15 randomized controlled trials that demonstrated its effectiveness in high-risk PE. The PEITHO trial, in particular, demonstrated the ability of systemic thrombolysis to reduce all-cause mortality and hemodynamic collapse within 7 days.
However, this benefit comes at the cost of increased bleeding risk, including a 10% rate of major bleeding and a 2% risk for intracranial hemorrhage. “These data come from old studies using invasive diagnostic procedures, and with current diagnostic procedures, the rate of bleeding is probably lower,” Dr. Sanchez said. The risk of bleeding is also related to the type of thrombolytic agent, with tenecteplase being strongly associated with a higher risk of bleeding, while alteplase shows no increase in the risk of major bleeding, he added. New strategies like reduced-dose thrombolysis offer comparable efficacy and improved safety, as demonstrated in ongoing trials like PEITHO-3, which aim to optimize the balance between efficacy and bleeding risk. Dr. Sanchez is the lead investigator of the PEITHO-3 study.
While rtPA might not be optimal for all patients, Dr. Sanchez thinks there is not enough evidence to replace it as a first-line treatment.
Existing studies on catheter-directed therapies often focus on surrogate endpoints, such as right-to-left ventricular ratio changes, rather than clinical outcomes like mortality, he said. Retrospective data suggest that catheter-directed therapies may reduce in-hospital mortality compared with systemic therapies, but they also increase the risk of intracranial bleeding, post-procedure complications, and device-related events.
Sanchez mentioned the same FLAME study described by Dr. Rali, which reported a 23% rate of device-related complications and 11% major bleeding in patients treated with catheter-directed therapies.
“Systemic thrombolysis remains the first treatment of choice,” Dr. Sanchez concluded. “The use of catheter-directed treatment should be discussed as an alternative in case of contraindications.”
The Debate Continues
Numerous ongoing clinical studies, such as the FLARE trial, will address gaps in evidence and refine treatment protocols, potentially reshaping the standard of care in high-risk PE in the near future by providing new data on the efficacy and safety of existing and emerging therapies.
“The coming data will make it clearer what the best option is,” said Thamer Al Khouzaie, MD, a pulmonary medicine consultant at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia. For now, he said, systemic thrombolysis remains the best option for most patients because it is widely available, easily administered with intravenous infusion, and at a limited cost. Catheter-directed treatment and surgical options are only available in specialized centers, require expertise and training, and are also very expensive.
Dr. Rali, Dr. Sanchez, and Dr. Khouzaie report no relevant financial relationships.
A version of this article appeared on Medscape.com.
The language of AI and its applications in health care
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
The Most Misinterpreted Study in Medicine: Don’t be TRICCed
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.