User login
SAN FRANCISCO – The largest-ever randomized trial of niacin for cardiovascular protection has not only failed to show clinical benefit, it spotlighted a hitherto underrecognized and disturbingly high range of serious harms in users of the HDL-raising drug.
The HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) trial found a rate of serious adverse events warranting hospitalization of 30 cases per 1,000 treated patients. Although many of these adverse events were already known to be associated with niacin therapy, two types of serious adverse events not previously recognized as niacin related were also identified: infections and bleeding, Dr. Jane Armitage reported at the annual meeting of the American College of Cardiology.
"The role of extended-release niacin for the treatment and prevention of cardiovascular disease needs to be reconsidered in light of these findings," said Dr. Armitage, professor of clinical trials and epidemiology at the University of Oxford (England).
That’s exactly what is now happening, as the European Medicine Agency has announced it is reviewing niacin’s status and considering its possible withdrawal from the marketplace.
The HPS2-THRIVE study involved 25,673 high-cardiovascular-risk patients in six countries, all of whom were placed on simvastatin 40 mg/day, with ezetimibe added if necessary in order to achieve a target total cholesterol level below 135 mg/dL. On that regimen, their mean LDL was an impressively favorable 63 mg/dL, with an HDL of 44 mg/dL and triglycerides of 125 mg/dL. This was a high-risk population. Roughly 80% of participants had a history of coronary artery disease, one-third had a history of cerebrovascular disease, and one-third had diabetes at baseline. After the investigators established during a run-in period that all participants could tolerate full-dose niacin, they were randomized to 2 g/day of extended-release niacin plus 40 mg of laropiprant to mitigate flushing or to placebo.
The active treatment arm had an average further 10-mg/dL reduction in LDL, a 6-mg/dL rise in HDL, and a 33-mg/dL drop in triglycerides. Extrapolating from earlier studies, Dr. Armitage and his coworkers anticipated that these lipid improvements would lead to an estimated 10%-15% reduction in the primary study endpoint, a composite of cardiac death, MI, stroke, or revascularization. But that’s not what transpired.
Indeed, during a median follow-up of 3.9 years, the primary endpoint occurred in 14.5% of patients on niacin/laropiprant and 15% on placebo, a nonsignificant difference.
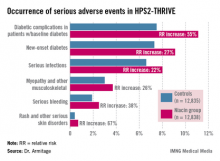
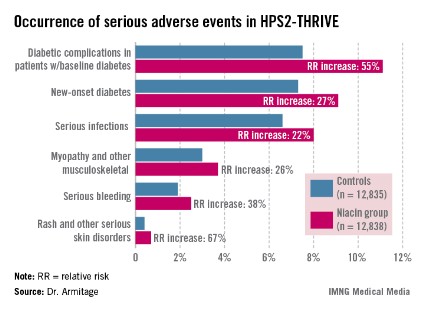
Diabetic complications among the more than 8,200 patients with diabetes at baseline occurred at an absolute 3.7% greater rate and 55% increased relative rate in the niacin group, compared with controls. Most of these diabetic complications led to multiday hospitalizations. Leading the way were major hyperglycemic episodes, which were 3.1-fold more common in the active treatment arm.
Among the other serious events that occurred at a significantly higher rate in the niacin group were serious infections in a variety of different organ systems, with a 22% increased rate; serious bleeding into the brain or gut, with a 38% increase; new-onset diabetes, with a 27% increase; myopathy and other serious musculoskeletal problems, with a 26% rise; and serious rash and other skin issues, which increased by 67%.
On the basis of prior studies, the most plausible culprit in the increased rates of serious adverse events is niacin rather than laropiprant, according to Dr. Armitage.
Discussant Donna Arnett, Ph.D., called HPS2-THRIVE "an exceptionally well-conducted study that definitively tells us the story of this drug combination in the setting of very well-controlled LDL."
"The results call into question the concept that increasing a low HDL in the context of a low LDL is really an important clinical problem," added Dr. Arnett, president of the American Heart Association and professor and chair of the department of epidemiology at the University of Alabama at Birmingham.
But panelist Dr. Christie M. Ballantyne indicated he has a big issue with the study: "If your LDL is 60, how important is your HDL, anyway? If I saw a patient who came to me with an LDL of 63, an HDL of 44, and triglycerides of 125, like the subjects in this trial, I would never even think of adding niacin because I know the drug has some risk and there is in my mind very little likelihood of seeing any benefit. But the majority of people in the U.S. who get niacin have LDLs greater than 70. I have high-risk patients who can only get their LDL down to 110 mg/dL with maximum statin therapy, and they can lower it by a further 30-40 mg/dL with niacin. What do I tell those patients now?
"Niacin has always been known as a drug that’s not easy to use, one with lots of side effects. It’s never been a first- or second-line therapy. It’s always been the drug where, when you can’t get there any other way, then you use it," observed Dr. Ballantyne, professor of medicine and chief of cardiology at Baylor College of Medicine, Houston.
Dr. Spencer B. King III called HPS2-THRIVE "another nail in the coffin for niacin," coming after the negative results of the National Institutes of Health–sponsored AIM-HIGH (Niacin Plus Statin to Prevent Vascular Events) study.
"I think the practice-changing part of this study is that many of us have been piling on a lot of niacin in people with low HDL. What you’ve shown is going to change attitudes among many physicians," predicted Dr. King, president of the Saint Joseph’s Heart and Vascular Institute, Atlanta.
When he asked Dr. Armitage whether any room remains now for individualized niacin therapy in selected patients, she replied that the investigators couldn’t identify any patient subgroup in whom the benefit outweighed the greater risk.
Dr. Rory Collins, chair of the HPS2-THRIVE trial, chimed in, adding: "Is niacin dead? I think in the light of these findings, it’s not healthy. The default position should change. It should be, Is there a good reason for using this therapy in any particular patient when there are additional ways, besides statins, of lowering LDL cholesterol, and where we don’t as yet have evidence that raising HDL cholesterol produces benefit?"
The HPS2-THRIVE trial was funded by Merck. Dr. Armitage, Dr. Collins, Dr. Arnett, and Dr. King reported having no relevant financial conflicts. Dr. Ballantyne serves as a consultant to numerous pharmaceutical companies, including Merck.
SAN FRANCISCO – The largest-ever randomized trial of niacin for cardiovascular protection has not only failed to show clinical benefit, it spotlighted a hitherto underrecognized and disturbingly high range of serious harms in users of the HDL-raising drug.
The HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) trial found a rate of serious adverse events warranting hospitalization of 30 cases per 1,000 treated patients. Although many of these adverse events were already known to be associated with niacin therapy, two types of serious adverse events not previously recognized as niacin related were also identified: infections and bleeding, Dr. Jane Armitage reported at the annual meeting of the American College of Cardiology.
"The role of extended-release niacin for the treatment and prevention of cardiovascular disease needs to be reconsidered in light of these findings," said Dr. Armitage, professor of clinical trials and epidemiology at the University of Oxford (England).
That’s exactly what is now happening, as the European Medicine Agency has announced it is reviewing niacin’s status and considering its possible withdrawal from the marketplace.
The HPS2-THRIVE study involved 25,673 high-cardiovascular-risk patients in six countries, all of whom were placed on simvastatin 40 mg/day, with ezetimibe added if necessary in order to achieve a target total cholesterol level below 135 mg/dL. On that regimen, their mean LDL was an impressively favorable 63 mg/dL, with an HDL of 44 mg/dL and triglycerides of 125 mg/dL. This was a high-risk population. Roughly 80% of participants had a history of coronary artery disease, one-third had a history of cerebrovascular disease, and one-third had diabetes at baseline. After the investigators established during a run-in period that all participants could tolerate full-dose niacin, they were randomized to 2 g/day of extended-release niacin plus 40 mg of laropiprant to mitigate flushing or to placebo.
The active treatment arm had an average further 10-mg/dL reduction in LDL, a 6-mg/dL rise in HDL, and a 33-mg/dL drop in triglycerides. Extrapolating from earlier studies, Dr. Armitage and his coworkers anticipated that these lipid improvements would lead to an estimated 10%-15% reduction in the primary study endpoint, a composite of cardiac death, MI, stroke, or revascularization. But that’s not what transpired.
Indeed, during a median follow-up of 3.9 years, the primary endpoint occurred in 14.5% of patients on niacin/laropiprant and 15% on placebo, a nonsignificant difference.
Diabetic complications among the more than 8,200 patients with diabetes at baseline occurred at an absolute 3.7% greater rate and 55% increased relative rate in the niacin group, compared with controls. Most of these diabetic complications led to multiday hospitalizations. Leading the way were major hyperglycemic episodes, which were 3.1-fold more common in the active treatment arm.
Among the other serious events that occurred at a significantly higher rate in the niacin group were serious infections in a variety of different organ systems, with a 22% increased rate; serious bleeding into the brain or gut, with a 38% increase; new-onset diabetes, with a 27% increase; myopathy and other serious musculoskeletal problems, with a 26% rise; and serious rash and other skin issues, which increased by 67%.
On the basis of prior studies, the most plausible culprit in the increased rates of serious adverse events is niacin rather than laropiprant, according to Dr. Armitage.
Discussant Donna Arnett, Ph.D., called HPS2-THRIVE "an exceptionally well-conducted study that definitively tells us the story of this drug combination in the setting of very well-controlled LDL."
"The results call into question the concept that increasing a low HDL in the context of a low LDL is really an important clinical problem," added Dr. Arnett, president of the American Heart Association and professor and chair of the department of epidemiology at the University of Alabama at Birmingham.
But panelist Dr. Christie M. Ballantyne indicated he has a big issue with the study: "If your LDL is 60, how important is your HDL, anyway? If I saw a patient who came to me with an LDL of 63, an HDL of 44, and triglycerides of 125, like the subjects in this trial, I would never even think of adding niacin because I know the drug has some risk and there is in my mind very little likelihood of seeing any benefit. But the majority of people in the U.S. who get niacin have LDLs greater than 70. I have high-risk patients who can only get their LDL down to 110 mg/dL with maximum statin therapy, and they can lower it by a further 30-40 mg/dL with niacin. What do I tell those patients now?
"Niacin has always been known as a drug that’s not easy to use, one with lots of side effects. It’s never been a first- or second-line therapy. It’s always been the drug where, when you can’t get there any other way, then you use it," observed Dr. Ballantyne, professor of medicine and chief of cardiology at Baylor College of Medicine, Houston.
Dr. Spencer B. King III called HPS2-THRIVE "another nail in the coffin for niacin," coming after the negative results of the National Institutes of Health–sponsored AIM-HIGH (Niacin Plus Statin to Prevent Vascular Events) study.
"I think the practice-changing part of this study is that many of us have been piling on a lot of niacin in people with low HDL. What you’ve shown is going to change attitudes among many physicians," predicted Dr. King, president of the Saint Joseph’s Heart and Vascular Institute, Atlanta.
When he asked Dr. Armitage whether any room remains now for individualized niacin therapy in selected patients, she replied that the investigators couldn’t identify any patient subgroup in whom the benefit outweighed the greater risk.
Dr. Rory Collins, chair of the HPS2-THRIVE trial, chimed in, adding: "Is niacin dead? I think in the light of these findings, it’s not healthy. The default position should change. It should be, Is there a good reason for using this therapy in any particular patient when there are additional ways, besides statins, of lowering LDL cholesterol, and where we don’t as yet have evidence that raising HDL cholesterol produces benefit?"
The HPS2-THRIVE trial was funded by Merck. Dr. Armitage, Dr. Collins, Dr. Arnett, and Dr. King reported having no relevant financial conflicts. Dr. Ballantyne serves as a consultant to numerous pharmaceutical companies, including Merck.
SAN FRANCISCO – The largest-ever randomized trial of niacin for cardiovascular protection has not only failed to show clinical benefit, it spotlighted a hitherto underrecognized and disturbingly high range of serious harms in users of the HDL-raising drug.
The HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) trial found a rate of serious adverse events warranting hospitalization of 30 cases per 1,000 treated patients. Although many of these adverse events were already known to be associated with niacin therapy, two types of serious adverse events not previously recognized as niacin related were also identified: infections and bleeding, Dr. Jane Armitage reported at the annual meeting of the American College of Cardiology.
"The role of extended-release niacin for the treatment and prevention of cardiovascular disease needs to be reconsidered in light of these findings," said Dr. Armitage, professor of clinical trials and epidemiology at the University of Oxford (England).
That’s exactly what is now happening, as the European Medicine Agency has announced it is reviewing niacin’s status and considering its possible withdrawal from the marketplace.
The HPS2-THRIVE study involved 25,673 high-cardiovascular-risk patients in six countries, all of whom were placed on simvastatin 40 mg/day, with ezetimibe added if necessary in order to achieve a target total cholesterol level below 135 mg/dL. On that regimen, their mean LDL was an impressively favorable 63 mg/dL, with an HDL of 44 mg/dL and triglycerides of 125 mg/dL. This was a high-risk population. Roughly 80% of participants had a history of coronary artery disease, one-third had a history of cerebrovascular disease, and one-third had diabetes at baseline. After the investigators established during a run-in period that all participants could tolerate full-dose niacin, they were randomized to 2 g/day of extended-release niacin plus 40 mg of laropiprant to mitigate flushing or to placebo.
The active treatment arm had an average further 10-mg/dL reduction in LDL, a 6-mg/dL rise in HDL, and a 33-mg/dL drop in triglycerides. Extrapolating from earlier studies, Dr. Armitage and his coworkers anticipated that these lipid improvements would lead to an estimated 10%-15% reduction in the primary study endpoint, a composite of cardiac death, MI, stroke, or revascularization. But that’s not what transpired.
Indeed, during a median follow-up of 3.9 years, the primary endpoint occurred in 14.5% of patients on niacin/laropiprant and 15% on placebo, a nonsignificant difference.
Diabetic complications among the more than 8,200 patients with diabetes at baseline occurred at an absolute 3.7% greater rate and 55% increased relative rate in the niacin group, compared with controls. Most of these diabetic complications led to multiday hospitalizations. Leading the way were major hyperglycemic episodes, which were 3.1-fold more common in the active treatment arm.
Among the other serious events that occurred at a significantly higher rate in the niacin group were serious infections in a variety of different organ systems, with a 22% increased rate; serious bleeding into the brain or gut, with a 38% increase; new-onset diabetes, with a 27% increase; myopathy and other serious musculoskeletal problems, with a 26% rise; and serious rash and other skin issues, which increased by 67%.
On the basis of prior studies, the most plausible culprit in the increased rates of serious adverse events is niacin rather than laropiprant, according to Dr. Armitage.
Discussant Donna Arnett, Ph.D., called HPS2-THRIVE "an exceptionally well-conducted study that definitively tells us the story of this drug combination in the setting of very well-controlled LDL."
"The results call into question the concept that increasing a low HDL in the context of a low LDL is really an important clinical problem," added Dr. Arnett, president of the American Heart Association and professor and chair of the department of epidemiology at the University of Alabama at Birmingham.
But panelist Dr. Christie M. Ballantyne indicated he has a big issue with the study: "If your LDL is 60, how important is your HDL, anyway? If I saw a patient who came to me with an LDL of 63, an HDL of 44, and triglycerides of 125, like the subjects in this trial, I would never even think of adding niacin because I know the drug has some risk and there is in my mind very little likelihood of seeing any benefit. But the majority of people in the U.S. who get niacin have LDLs greater than 70. I have high-risk patients who can only get their LDL down to 110 mg/dL with maximum statin therapy, and they can lower it by a further 30-40 mg/dL with niacin. What do I tell those patients now?
"Niacin has always been known as a drug that’s not easy to use, one with lots of side effects. It’s never been a first- or second-line therapy. It’s always been the drug where, when you can’t get there any other way, then you use it," observed Dr. Ballantyne, professor of medicine and chief of cardiology at Baylor College of Medicine, Houston.
Dr. Spencer B. King III called HPS2-THRIVE "another nail in the coffin for niacin," coming after the negative results of the National Institutes of Health–sponsored AIM-HIGH (Niacin Plus Statin to Prevent Vascular Events) study.
"I think the practice-changing part of this study is that many of us have been piling on a lot of niacin in people with low HDL. What you’ve shown is going to change attitudes among many physicians," predicted Dr. King, president of the Saint Joseph’s Heart and Vascular Institute, Atlanta.
When he asked Dr. Armitage whether any room remains now for individualized niacin therapy in selected patients, she replied that the investigators couldn’t identify any patient subgroup in whom the benefit outweighed the greater risk.
Dr. Rory Collins, chair of the HPS2-THRIVE trial, chimed in, adding: "Is niacin dead? I think in the light of these findings, it’s not healthy. The default position should change. It should be, Is there a good reason for using this therapy in any particular patient when there are additional ways, besides statins, of lowering LDL cholesterol, and where we don’t as yet have evidence that raising HDL cholesterol produces benefit?"
The HPS2-THRIVE trial was funded by Merck. Dr. Armitage, Dr. Collins, Dr. Arnett, and Dr. King reported having no relevant financial conflicts. Dr. Ballantyne serves as a consultant to numerous pharmaceutical companies, including Merck.
AT ACC 13
Major Finding: During an average 3.9-year prospective follow-up, the rate of the combined endpoint of coronary death, nonfatal MI, stroke, or revascularization occurred in 14.5% of patients on 2 g of extended-release niacin plus 40 mg of laropiprant per day and 15% of placebo-treated controls, a nonsignificant difference. Serious adverse events warranting hospitalization occurred in the niacin group at a rate of 30/1,000 treated patients.
Data Source: HPS2-THRIVE, which randomized 25,673 high-cardiovascular-risk patients with low baseline LDL levels on simvastatin with or without ezetimibe to niacin/laropiprant or placebo.
Disclosures: The study was sponsored by Merck. The presenter reported having no relevant financial conflicts.