User login
- The treatment goal is to reduce blood pressure to a safe level to prevent maternal cerebral complications.This goal must be weighed against the risks of fetal exposure to antihypertensive drugs and the effects on uteroplacental blood flow.
- Gravidas with uncomplicated mild hypertension are at low risk; however, those with severe hypertension or associated complicating factors are at high risk of complications and adverse outcomes.
- Antihypertensive medications should not be used routinely in low-risk patients.
- Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.
The decision to use antihypertensive drug therapy in pregnant women is a tricky one—especially considering the ever-evolving nature of treatment. For instance, we now know that in some hypertensive gravidas, medical interventions may actually be deleterious.
With the aging of the obstetric population in the United States, hypertension in pregnancy—which currently affects 7% of gestations—will remain a major issue in preconception and prenatal care. Its reported risks, which include stroke, pulmonary edema, and death, underscore the importance of careful management (TABLE 1).
This article describes the indications for antihypertensive therapy in pregnancy, focusing on 2 basic categories—high-risk and lowrisk patients—and offers guidance in choosing the optimal agent for each patient.
TABLE 1
Maternal risks of severe hypertension in pregnancy
| Stroke |
| Cerebral hemorrhage |
| Hypertensive encephalopathy |
| Congestive heart failure/pulmonary edema |
| Acute renal dysfunction/acute renal failure |
| Abruptio placentae |
| Disseminated intravascular coagulopathy |
| Death |
Correct classification helps direct management
First, identify chronic hypertension. Chronic hypertension is defined as an elevation in blood pressure (BP) that exists prior to pregnancy. Unfortunately, because the pregestational BP is not always known, the diagnosis in many cases must be made on the basis of specific levels: systolic BP of at least 140 mm Hg or diastolic BP of at least 90 mm Hg on at least 2 occasions at least 4 hours apart prior to 20 weeks’ gestation.1
Even with these guidelines, however, diagnosis may be difficult, since early manifestations of preeclampsia can include hypertension prior to 20 weeks’ gestation.2,3 In addition, the physiologic decrease in BP during the first and second trimesters—seen in many patients with chronic hypertension—may obscure the condition early in gestation and lead to the erroneous diagnosis of gestational hypertension or preeclampsia later in pregnancy.4-6
Once a diagnosis of chronic hypertension is made, an accurate classification of the disease will help guide management and initiation of antihypertensive medication.
Mild versus severe hypertension. In pregnancy, chronic hypertension is classified as mild or severe. Mild hypertension has traditionally been defined as systolic BP less than 160 mm Hg and diastolic blood pressure less than 110 mm Hg.1,7 However, the American College of Obstetricians and Gynecologists recently changed its definition of mild hypertension to systolic BP less than 180 mm Hg.8,9 Most women with chronic hypertension in pregnancy have the mild form of the disease.
Low-risk hypertension. Patients with uncomplicated chronic mild hypertension are at low risk.
High-risk hypertension. Patients at high risk have either chronic severe hypertension or chronic mild hypertension in association with any of the complicating factors listed in TABLE 2.
History and laboratory studies. To properly classify the disease when first evaluating a patient with chronic hypertension, a thorough history is essential. Ask about related medical illnesses as well as target organ damage. Pay special attention to cardiac, renal, thyroid, and cerebrovascular disease, as well as diabetes. The outcomes of prior pregnancies also are important, especially complications such as abruptio placentae, preeclampsia, preterm delivery, growth restriction, fetal death, and neonatal complications.
Overall, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension.
Finally, laboratory evaluation should include urine analysis, urine culture, 24-hour urine protein, electrolytes, complete blood count, and glucose tolerance testing.
Other key examinations. In women with long-standing disease, ophthalmologic evaluation, electrocardiography, echocardiography, and assessment of creatinine clearance may be indicated.
TABLE 2
Criteria for low- versus high-risk chronic hypertension
| LOW-RISK CHRONIC HYPERTENSION |
| Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in the absence of complicating factors |
| HIGH-RISK CHRONIC HYPERTENSION |
| Either: |
| Chronic severe hypertension (systolic blood pressure ≥180 mm Hg and diastolic blood pressure ≥110 mm Hg) |
| or |
Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in association with anyof the following:
|
Objective of treatment: Prevent complications
In the nonpregnant state, the aim of hypertensive management is to prevent longterm vascular complications such as stroke and cardiovascular disease.10 A reasonable treatment goal for patients with mild to moderate hypertension may be benefits that are apparent after 5 years of therapy10—an acceptable time frame due to the long-term nature of the disease.
In pregnant women, however, the duration of the condition (pregnant and hypertensive) is finite and relatively short; as a result, maternal health benefits may not be clear. For that reason, the objective is to reduce BP to a safe level to prevent maternal cerebral complications (systolic BP below 160 mm Hg and diastolic BP below 110 mm Hg).Of course, these short-term maternal benefits must be weighed against the potential risks of fetal exposure (TABLE 3).11,12
TABLE 3
Rates of adverse pregnancy outcomes among patients with mild and severe chronic hypertension
| OUTCOME | MILD HYPERTENSION (%) | SEVERE HYPERTENSION (%) |
|---|---|---|
| Preeclampsia | 10-25 | 25-50 |
| Abruptio placentae | 0.7-1.5 | 2-5 |
| Fetal growth restriction | 8.0-15.5 | 10-20 |
| Preterm birth | 12-34.4 | 25-30 |
Low-risk disease: Avoid routine antihypertensive therapy
A limited number of randomized trials have studied the effectiveness of antihypertensive treatment in preventing adverse maternal outcomes such as superimposed preeclampsia and abruptio placentae. Here are 2 key findings:
- No demonstrable maternal benefit. Overall, there appears to be no clear benefit of antihypertensive treatment in women with mild hypertension. Indeed, the 2 largest studies had contradictory findings regarding preeclampsia,6,13 and there was no demonstrable benefit in regard to abruptio placentae.
- Antihypertensive drugs may adversely affect fetal growth. A recent meta-analysis examining antihypertensive medications in patients with mild to moderate hypertension investigated the relationship between a fall in mean arterial blood pressure and the delivery of small-for-gestational-age (SGA) infants.14 The authors concluded that antihypertensive medications induce BP drops that may adversely affect fetal growth (see FIGURE). Prior to this observation, prospective studies had shown no association between antihypertensive medications and SGA infants. (The only exception was atenolol; 3 separate studies found a relationship between treatment with atenolol and low birth weight.15-17)
Overall, maternal and perinatal data indicate that, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension. In fact, the indiscriminate use of such medications may have deleterious effects. Consequently, antihypertensive medications should not be used routinely in low-risk patients.18,19
Clinical care. When a woman with low-risk hypertension presents for prenatal care, it is our policy to discontinue antihypertensive medications at the first prenatal visit. Although many women will not require antihypertensive treatment during the pregnancy, careful management remains essential, as such patients can become high-risk at any time. Therapy should be initiated if her condition changes to severe hypertension (systolic BP of 180 mm Hg or more, or a diastolic blood pressure of 110 mm Hg or more).8
Low-risk women should be monitored closely for evidence of preeclampsia and fetal growth restriction. Thus, they should have a baseline ultrasound at 16 to 20 weeks’ gestation, with serial monthly ultrasounds beginning at 30 to 32 weeks to follow fetal growth. Nonstress testing or biophysical profiles are indicated in the presence of severe hypertension, preeclampsia, or abnormal fetal growth.
Patients with uncomplicated lowrisk hypertension may continue pregnancy until 40 weeks’ gestation. However, beyond 37 weeks, the presence of complications such as severe hypertension, documented growth restriction, and superimposed preeclampsia are indications for hospitalization and delivery.
FIGURE Placental effects of hypertension
In hypertensive gravidas, placental blood flow is reduced—particularly in cases of preeclampsia. Antihypertensive therapy in low-risk women may induce blood pressure drops that further compromise fetal growth.
High-risk disease: Initiate medical therapy
Randomized, controlled trials do not exist for gravidas with high-risk hypertension—that is, women with severe hypertension or complicating factors—due to concerns about the potential adverse consequences of uncontrolled disease, such as cerebrovascular accident, congestive heart failure, and renal failure.20 It is interesting to note, however, that although controlling hypertension in such patients may help prolong pregnancy, there is no evidence that it reduces the rates of preeclampsia or abruptio placentae.20,21
Labetalol provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation.
When to start treatment. For women with high-risk hypertension, hospitalization at the time of the first prenatal visit facilitates complete cardiovascular and renal evaluation, and is therefore often beneficial. If a woman has target organ damage, treatment should be initiated at a systolic BP of 140 mm Hg or a diastolicBP of 90 mm Hg. Indeed, many such women are already receiving treatment for their hypertension, in which case antihypertensive medications should be continued, though physicians should consider altering the regimen to optimize fetal safety.
Choosing the best agent. Before choosing an antihypertensive drug, review the patient’s history. If her disease was well controlled on a particular medication, that agent is probably a reasonable first choice, provided there is adequate published literature establishing the safety of her medication during pregnancy. Obviously, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II antagonists, and atenolol should be avoided because of the potential adverse effects on the fetus, including renal failure.The most commonly used medications for control of hypertension during pregnancy are listed in TABLE 4.
- Labetalol. We consider labetalol a first-line agent for controlling hypertension in pregnancy. This beta-blocking drug provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation—not seen with traditional beta-blockers. Overall, labetalol has an excellent record of safety in pregnancy.
In a randomized, controlled trial involving 86 mildly hypertensive patients who initiated labetalol therapy between 6 and 13 weeks’ gestation, no major congenital malformations were identified.6 Although there have been reports of an increased risk for SGA infants in patients treated with labetalol for mild pregnancy-induced hypertension during the second and third trimesters, this association has not been documented in women with chronic hypertension.6
- Thiazide diuretics. If labetalol fails to control blood pressure, we typically add either the calcium-channel blocker nifedipine or a thiazide diuretic. Use of the latter has been well documented in pregnancy. Indeed, thiazide diuretics can be given in the first trimester and throughout gestation without associated risks of major fetal malformations or adverse fetal-neonatal complications.
- Calcium-channel blockers. Calcium-channel–blocking agents also have an excellent safety profile in pregnancy. They have been studied both as antihypertensive medications (primarily in the second and third trimesters) and as tocolytic agents. In a prospective, multicenter, cohort study in which 78 women were exposed to calcium-channel blockers (mainly nifedipine and verapamil) during the first trimester, there was no increase in the rate of birth defects.22
A separate prospective, randomized trial evaluated the benefit of nifedipine in pregnancy. A total of 283 women—47% of whom had chronic hypertension—were enrolled between 12 and 34 weeks’ gestation (mean: 24 weeks). Researchers found patients on nifedipine therapy experienced no improvement in maternal or neonatal outcomes compared to subjects assigned to no treatment.23 Follow-up at 18 months of 94 of the infants exposed to nifedipine in utero showed no adverse effects on development.24
- Methyldopa. For many obstetricians, methyldopa remains a first-line agent for the treatment of chronic hypertension in pregnancy.1 It has a well-documented safety record in both short-term25 and long-term follow-up of children exposed in utero.26 Indeed, many studies have evaluated use of this medication to manage mild to moderate hypertension, with no evidence of adverse maternal or fetal outcome. However, it is now rarely used in the nonpregnant population, and the safety of other medications, such as labetalol and nifedipine, has prompted us to stop giving it.
- Other considerations. Finally, when choosing an antihypertensive drug, the physician must consider the benefits and response of specific agents in particular risk groups (TABLE 5).
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy, since ACE inhibitors, which also offer this benefit, must be avoided beyond 16 weeks’ gestation because of the potential adverse fetal effects.
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy.
Young African-American women frequently have low-renin, salt-sensitive hypertension, and therefore thiazide diuretics or nifedipine may be better first-line agents in this population.
TABLE 4
Agents for treating chronic hypertension in pregnancy
| DRUG | STARTING DOSE | MAXIMUM DAILY DOSE | COMMON SIDE EFFECTS |
|---|---|---|---|
| Labetalol | 100 mg every 8 h | 1,200–2,400 mg | Headache Tremulousness |
| Thiazide diuretic | 12.5 mg twice daily | 50 mg | Hypokalemia |
| Nifedipine | Hypotension | ||
| Short acting | 10 mg every 8 h | 120 mg | Headache |
| Long acting | 30 mg/d | 240 mg | Tachycardia |
| Alpha methyldopa | 250 mg twice daily | 4 g | Thirst Drowsiness Elevation of liver enzymes |
TABLE 5
Medical factors guiding selection of antihypertensive medication
| If the patient has… | It’s generally best to start with… |
|---|---|
| Diabetes | Calcium-channel blocker |
| Vascular disease | |
| Salt-wasting hypertension* | Thiazide diuretic |
| Left ventricular systolic dysfunction | |
| Mitral stenosis | |
| *Mostly African-American women | |
Severe gestational hypertension and preeclampsia
Women who develop severe gestational hypertension (systolic BP of 160 mm Hg or more or diastolic BP of 110 mm Hg or more) and/or preeclampsia require antihypertensive treatment during management remote from term. In this case, the aim of antihypertensive drug treatment is to keep systolic BP between 150 and 159 mm Hg and diastolic BP between 100 and 109 mm Hg in order to not compromise uteroplacental blood flow.
Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent for gravidas with hypertensive encephalopathy.
The drugs to use are oral labetalol and/or oral nifedipine. If maternal BP is not adequately controlled with maximum doses of labetalol plus nifedipine, the patient should be delivered.
Severe hypertension and encephalopathy
Hypertensive encephalopathy is a medical emergency. This rare complication of hypertension in pregnancy27 is marked by severely elevated BP, with the diastolic level frequently exceeding 130 mm Hg. Associated findings include headache, visual disturbances, nausea, vomiting, seizures, confusion, stupor, and coma. Also possible are retinal hemorrhage, exudates, papilledema, and evidence of renal or cardiac disease. Transient focal neurologic findings may be present as well, but more often suggest vascular disease, hemorrhage, embolism, or thrombosis.
Pathophysiology. In hypertensive encephalopathy, loss of autoregulation leads to generalized cerebral vasodilation. Under normal conditions, when the mean arterial pressure is between 60 and 130 mm Hg, patients maintain constant cerebral blood flow. In hypertensive patients, however, autoregulation occurs between mean arterial pressures of 110 and 180 mm Hg as a result of arteriolar thickening. When BP exceeds the ability of the vessels to autoregulate, blood flow hyperperfuses the brain, causing fluid to leak into the perivascular tissue and resulting in vasogenic cerebral edema. Altered vascular reactivity to normally circulating pressor agents, deficient levels of vasodilating prostaglandins, endothelial dysfunction, and activation of the coagulation cascade may further exacerbate this condition.28
Treatment options. Clinically, it may be impossible to differentiate hypertensive encephalopathy from eclampsia, and magnesium sulfate should be considered for seizure prophylaxis. The most frequently used antihypertensive medications for this syndrome are shown in TABLE 6. Nitroprusside lowers BP most predictably, but because of the associated risks of fetal cyanide toxicity, other medications may be more desirable first-line agents in the pregnant woman.
Importantly, because sudden drops in BP may impair cerebral perfusion, we recommend that the mean arterial pressure be lowered no more than 25% from baseline (TABLE 7). If pulmonary edema develops, oxygen and furosemide should be administered, and consultation with subspecialists considered. (We suggest such consultation for cases of renal dysfunction and cerebral complications, as well.) Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent in this situation.
TABLE 6
Medications for treating acute severe hypertension
| DRUG | DOSE | ONSET OF ACTION | DURATION OF ACTION | SIDE EFFECTS |
|---|---|---|---|---|
| Hydralazine | 5-10 mg IV every 20 min | 10-20 min | 3-6 h | Tachycardia Headache Flushing Angina |
| Labetalol | 20-80 mg IV every 10 min | 5-10 min | 3-6 h | Scalp tingling Vomiting Heart block |
| Sodium nitroprusside* | 0.25-5 mcg/kg/min | Immediate | 1-2 min | Nausea Vomiting Muscle twitching Thiocyanate and cyanide intoxication |
| Nicardipine* | 5-15 mg/h IV | 5-10 min | 1-4 h | Tachycardia Headache Phlebitis |
| *Drugs to use in the presence of hypertensive encephalopathy. | ||||
TABLE 7
Principles of management for severe hypertension and encephalopathy
Acute severe hypertension
|
Hypertensive encephalopathy
|
| *Lower mean arterial pressure no more than 25% from baseline. |
Postpartum management
Monitor BP for at least 48 hours. Women with high-risk chronic hypertension are more likely to suffer postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure than normotensive patients.8 This risk is even higher when these women also have target organ involvement, superimposed preeclampsia, abruptio placentae, morbid obesity, or longstanding hypertension.
In these patients, BP must be closely monitored and controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be administered for acute elevations of BP29; diuretics should also be used for women with circulatory congestion and pulmonary edema.30
Methyldopa remains the first-line agent for breastfeeding patients without compelling indications for another drug.
Oral antihypertensive therapy may be needed to maintain BP control. In choosing the appropriate agent, it is important to consider whether factors compel the choice of one medication over another. For example, for patients with a history of myocardial infarction, beta blockers and ACE inhibitors are excellent choices to decrease mortality.31 In patients with diabetes mellitus, as mentioned earlier, ACE inhibitors offer a renoprotective effect.10
Consider drug concentrations in breast milk. Another significant consideration in the postpartum period is whether the mother wishes to breastfeed her infant. All antihypertensive medications are found in breast milk to varying degrees,32 and the long-term effects of these medications on breastfeeding infants has not been specifically studied.
Because concentrations of methyldopa in milk are low and considered safe, it remains the first-line agent for patients without compelling indications for another antihypertensive drug. Concentrations of labetalol and propranolol also are low in breast milk; therefore, these may be better choices than atenolol and metoprolol, which are more highly concentrated in breast milk.12
Although diuretic agents have low concentrations in breast milk, they may decrease milk production.32
Little information exists regarding the excretion of calcium-channel blockers in breast milk, but no untoward effects are apparent.33 ACE inhibitors and angiotensin II receptor antagonists should be avoided because of potential deleterious effects on neonatal renal function, even though their concentrations in breast milk appear to be low. If ACE inhibitors are indicated for the breastfeeding mother, current data suggest that captopril and enalapril are safe.34
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. Sibai BM, Akl S, Fairlie F, Moretti M. A protocol for managing severe preeclampsia in the second trimester. Am J Obstet Gynecol. 1990;163:733-738.
3. Hermida RC, Ayala DA, Mojon A, et al. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000;36:149-158.
4. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
5. Benedetto C, Zonca M, Marozio L, Dolci C, Carandente F, Massobrio M. Blood pressure patterns in normal pregnancy and in pregnancy-induced hypertension, preeclampsia, and chronic hypertension. Obstet Gynecol. 1996;88:503-510.
6. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
7. Sibai BM. Diagnosis and management of chronic hypertension in pregnancy. Obstet Gynecol. 1991;78:451-461.
8. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: Chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
9. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
10. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413-2446.
11. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96:849-860.
12. Umans JG, Lindheimer MD. Antihypertensive treatment. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn: Appleton and Lange; 1998;581-604.
13. Steyn DW, Odendaal HJ. Randomized controlled trial of ketanserin and aspirin in prevention of pre-eclampsia. Lancet. 1997;350:1267-1271.
14. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355:87-92.
15. Lip GY, Beevers M, Churchill D, Shaffer LM, Beevers DG. Effect of atenolol on birth weight. Am J Cardiol. 1997;79:1436-1438.
16. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725-733.
17. Lydakis C, Lip GY, Beevers M, Beevers G. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12:541-547.
18. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335:257-265.
19. Magee LA, Ornstein MP, von Dadelszen P. Management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
20. Sibai BM, Anderson GD. Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstet Gynecol. 1986;67:517-522.
21. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
22. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174:823-828.
23. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722.
24. Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months. Br J Obstet Gynaecol. 2000;107:792-794.
25. Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1993;168:152-156.
26. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1:647-649.
27. Witlin AG, Friedman SA, Egerman RS, et al. Cerebrovascular disorders complicating pregnancy—beyond eclampsia. Am J Obstet Gynecol. 1997;176:1139-1148.
28. Cotton DB, Janusz CA, Berman RF. Anticonvulsant effects of magnesium sulfate on hippocampal seizures: therapeutic implication in preeclampsia-eclampsia. Am J Obstet Gynecol. 1992;166:1127-1136.
29. Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol. 1987;70:328-333.
30. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women: a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
31. Pfeffer MA, Braunwald E, Moye LA. for the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trials. N Engl J Med. 1992;327:669-677.
32. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
33. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore, Md: Williams and Wilkins; 1998.
34. Committee on Drugs, American Academy of Pediatrics. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137-150.
- The treatment goal is to reduce blood pressure to a safe level to prevent maternal cerebral complications.This goal must be weighed against the risks of fetal exposure to antihypertensive drugs and the effects on uteroplacental blood flow.
- Gravidas with uncomplicated mild hypertension are at low risk; however, those with severe hypertension or associated complicating factors are at high risk of complications and adverse outcomes.
- Antihypertensive medications should not be used routinely in low-risk patients.
- Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.
The decision to use antihypertensive drug therapy in pregnant women is a tricky one—especially considering the ever-evolving nature of treatment. For instance, we now know that in some hypertensive gravidas, medical interventions may actually be deleterious.
With the aging of the obstetric population in the United States, hypertension in pregnancy—which currently affects 7% of gestations—will remain a major issue in preconception and prenatal care. Its reported risks, which include stroke, pulmonary edema, and death, underscore the importance of careful management (TABLE 1).
This article describes the indications for antihypertensive therapy in pregnancy, focusing on 2 basic categories—high-risk and lowrisk patients—and offers guidance in choosing the optimal agent for each patient.
TABLE 1
Maternal risks of severe hypertension in pregnancy
| Stroke |
| Cerebral hemorrhage |
| Hypertensive encephalopathy |
| Congestive heart failure/pulmonary edema |
| Acute renal dysfunction/acute renal failure |
| Abruptio placentae |
| Disseminated intravascular coagulopathy |
| Death |
Correct classification helps direct management
First, identify chronic hypertension. Chronic hypertension is defined as an elevation in blood pressure (BP) that exists prior to pregnancy. Unfortunately, because the pregestational BP is not always known, the diagnosis in many cases must be made on the basis of specific levels: systolic BP of at least 140 mm Hg or diastolic BP of at least 90 mm Hg on at least 2 occasions at least 4 hours apart prior to 20 weeks’ gestation.1
Even with these guidelines, however, diagnosis may be difficult, since early manifestations of preeclampsia can include hypertension prior to 20 weeks’ gestation.2,3 In addition, the physiologic decrease in BP during the first and second trimesters—seen in many patients with chronic hypertension—may obscure the condition early in gestation and lead to the erroneous diagnosis of gestational hypertension or preeclampsia later in pregnancy.4-6
Once a diagnosis of chronic hypertension is made, an accurate classification of the disease will help guide management and initiation of antihypertensive medication.
Mild versus severe hypertension. In pregnancy, chronic hypertension is classified as mild or severe. Mild hypertension has traditionally been defined as systolic BP less than 160 mm Hg and diastolic blood pressure less than 110 mm Hg.1,7 However, the American College of Obstetricians and Gynecologists recently changed its definition of mild hypertension to systolic BP less than 180 mm Hg.8,9 Most women with chronic hypertension in pregnancy have the mild form of the disease.
Low-risk hypertension. Patients with uncomplicated chronic mild hypertension are at low risk.
High-risk hypertension. Patients at high risk have either chronic severe hypertension or chronic mild hypertension in association with any of the complicating factors listed in TABLE 2.
History and laboratory studies. To properly classify the disease when first evaluating a patient with chronic hypertension, a thorough history is essential. Ask about related medical illnesses as well as target organ damage. Pay special attention to cardiac, renal, thyroid, and cerebrovascular disease, as well as diabetes. The outcomes of prior pregnancies also are important, especially complications such as abruptio placentae, preeclampsia, preterm delivery, growth restriction, fetal death, and neonatal complications.
Overall, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension.
Finally, laboratory evaluation should include urine analysis, urine culture, 24-hour urine protein, electrolytes, complete blood count, and glucose tolerance testing.
Other key examinations. In women with long-standing disease, ophthalmologic evaluation, electrocardiography, echocardiography, and assessment of creatinine clearance may be indicated.
TABLE 2
Criteria for low- versus high-risk chronic hypertension
| LOW-RISK CHRONIC HYPERTENSION |
| Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in the absence of complicating factors |
| HIGH-RISK CHRONIC HYPERTENSION |
| Either: |
| Chronic severe hypertension (systolic blood pressure ≥180 mm Hg and diastolic blood pressure ≥110 mm Hg) |
| or |
Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in association with anyof the following:
|
Objective of treatment: Prevent complications
In the nonpregnant state, the aim of hypertensive management is to prevent longterm vascular complications such as stroke and cardiovascular disease.10 A reasonable treatment goal for patients with mild to moderate hypertension may be benefits that are apparent after 5 years of therapy10—an acceptable time frame due to the long-term nature of the disease.
In pregnant women, however, the duration of the condition (pregnant and hypertensive) is finite and relatively short; as a result, maternal health benefits may not be clear. For that reason, the objective is to reduce BP to a safe level to prevent maternal cerebral complications (systolic BP below 160 mm Hg and diastolic BP below 110 mm Hg).Of course, these short-term maternal benefits must be weighed against the potential risks of fetal exposure (TABLE 3).11,12
TABLE 3
Rates of adverse pregnancy outcomes among patients with mild and severe chronic hypertension
| OUTCOME | MILD HYPERTENSION (%) | SEVERE HYPERTENSION (%) |
|---|---|---|
| Preeclampsia | 10-25 | 25-50 |
| Abruptio placentae | 0.7-1.5 | 2-5 |
| Fetal growth restriction | 8.0-15.5 | 10-20 |
| Preterm birth | 12-34.4 | 25-30 |
Low-risk disease: Avoid routine antihypertensive therapy
A limited number of randomized trials have studied the effectiveness of antihypertensive treatment in preventing adverse maternal outcomes such as superimposed preeclampsia and abruptio placentae. Here are 2 key findings:
- No demonstrable maternal benefit. Overall, there appears to be no clear benefit of antihypertensive treatment in women with mild hypertension. Indeed, the 2 largest studies had contradictory findings regarding preeclampsia,6,13 and there was no demonstrable benefit in regard to abruptio placentae.
- Antihypertensive drugs may adversely affect fetal growth. A recent meta-analysis examining antihypertensive medications in patients with mild to moderate hypertension investigated the relationship between a fall in mean arterial blood pressure and the delivery of small-for-gestational-age (SGA) infants.14 The authors concluded that antihypertensive medications induce BP drops that may adversely affect fetal growth (see FIGURE). Prior to this observation, prospective studies had shown no association between antihypertensive medications and SGA infants. (The only exception was atenolol; 3 separate studies found a relationship between treatment with atenolol and low birth weight.15-17)
Overall, maternal and perinatal data indicate that, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension. In fact, the indiscriminate use of such medications may have deleterious effects. Consequently, antihypertensive medications should not be used routinely in low-risk patients.18,19
Clinical care. When a woman with low-risk hypertension presents for prenatal care, it is our policy to discontinue antihypertensive medications at the first prenatal visit. Although many women will not require antihypertensive treatment during the pregnancy, careful management remains essential, as such patients can become high-risk at any time. Therapy should be initiated if her condition changes to severe hypertension (systolic BP of 180 mm Hg or more, or a diastolic blood pressure of 110 mm Hg or more).8
Low-risk women should be monitored closely for evidence of preeclampsia and fetal growth restriction. Thus, they should have a baseline ultrasound at 16 to 20 weeks’ gestation, with serial monthly ultrasounds beginning at 30 to 32 weeks to follow fetal growth. Nonstress testing or biophysical profiles are indicated in the presence of severe hypertension, preeclampsia, or abnormal fetal growth.
Patients with uncomplicated lowrisk hypertension may continue pregnancy until 40 weeks’ gestation. However, beyond 37 weeks, the presence of complications such as severe hypertension, documented growth restriction, and superimposed preeclampsia are indications for hospitalization and delivery.
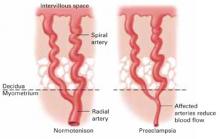
FIGURE Placental effects of hypertension
In hypertensive gravidas, placental blood flow is reduced—particularly in cases of preeclampsia. Antihypertensive therapy in low-risk women may induce blood pressure drops that further compromise fetal growth.
High-risk disease: Initiate medical therapy
Randomized, controlled trials do not exist for gravidas with high-risk hypertension—that is, women with severe hypertension or complicating factors—due to concerns about the potential adverse consequences of uncontrolled disease, such as cerebrovascular accident, congestive heart failure, and renal failure.20 It is interesting to note, however, that although controlling hypertension in such patients may help prolong pregnancy, there is no evidence that it reduces the rates of preeclampsia or abruptio placentae.20,21
Labetalol provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation.
When to start treatment. For women with high-risk hypertension, hospitalization at the time of the first prenatal visit facilitates complete cardiovascular and renal evaluation, and is therefore often beneficial. If a woman has target organ damage, treatment should be initiated at a systolic BP of 140 mm Hg or a diastolicBP of 90 mm Hg. Indeed, many such women are already receiving treatment for their hypertension, in which case antihypertensive medications should be continued, though physicians should consider altering the regimen to optimize fetal safety.
Choosing the best agent. Before choosing an antihypertensive drug, review the patient’s history. If her disease was well controlled on a particular medication, that agent is probably a reasonable first choice, provided there is adequate published literature establishing the safety of her medication during pregnancy. Obviously, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II antagonists, and atenolol should be avoided because of the potential adverse effects on the fetus, including renal failure.The most commonly used medications for control of hypertension during pregnancy are listed in TABLE 4.
- Labetalol. We consider labetalol a first-line agent for controlling hypertension in pregnancy. This beta-blocking drug provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation—not seen with traditional beta-blockers. Overall, labetalol has an excellent record of safety in pregnancy.
In a randomized, controlled trial involving 86 mildly hypertensive patients who initiated labetalol therapy between 6 and 13 weeks’ gestation, no major congenital malformations were identified.6 Although there have been reports of an increased risk for SGA infants in patients treated with labetalol for mild pregnancy-induced hypertension during the second and third trimesters, this association has not been documented in women with chronic hypertension.6
- Thiazide diuretics. If labetalol fails to control blood pressure, we typically add either the calcium-channel blocker nifedipine or a thiazide diuretic. Use of the latter has been well documented in pregnancy. Indeed, thiazide diuretics can be given in the first trimester and throughout gestation without associated risks of major fetal malformations or adverse fetal-neonatal complications.
- Calcium-channel blockers. Calcium-channel–blocking agents also have an excellent safety profile in pregnancy. They have been studied both as antihypertensive medications (primarily in the second and third trimesters) and as tocolytic agents. In a prospective, multicenter, cohort study in which 78 women were exposed to calcium-channel blockers (mainly nifedipine and verapamil) during the first trimester, there was no increase in the rate of birth defects.22
A separate prospective, randomized trial evaluated the benefit of nifedipine in pregnancy. A total of 283 women—47% of whom had chronic hypertension—were enrolled between 12 and 34 weeks’ gestation (mean: 24 weeks). Researchers found patients on nifedipine therapy experienced no improvement in maternal or neonatal outcomes compared to subjects assigned to no treatment.23 Follow-up at 18 months of 94 of the infants exposed to nifedipine in utero showed no adverse effects on development.24
- Methyldopa. For many obstetricians, methyldopa remains a first-line agent for the treatment of chronic hypertension in pregnancy.1 It has a well-documented safety record in both short-term25 and long-term follow-up of children exposed in utero.26 Indeed, many studies have evaluated use of this medication to manage mild to moderate hypertension, with no evidence of adverse maternal or fetal outcome. However, it is now rarely used in the nonpregnant population, and the safety of other medications, such as labetalol and nifedipine, has prompted us to stop giving it.
- Other considerations. Finally, when choosing an antihypertensive drug, the physician must consider the benefits and response of specific agents in particular risk groups (TABLE 5).
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy, since ACE inhibitors, which also offer this benefit, must be avoided beyond 16 weeks’ gestation because of the potential adverse fetal effects.
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy.
Young African-American women frequently have low-renin, salt-sensitive hypertension, and therefore thiazide diuretics or nifedipine may be better first-line agents in this population.
TABLE 4
Agents for treating chronic hypertension in pregnancy
| DRUG | STARTING DOSE | MAXIMUM DAILY DOSE | COMMON SIDE EFFECTS |
|---|---|---|---|
| Labetalol | 100 mg every 8 h | 1,200–2,400 mg | Headache Tremulousness |
| Thiazide diuretic | 12.5 mg twice daily | 50 mg | Hypokalemia |
| Nifedipine | Hypotension | ||
| Short acting | 10 mg every 8 h | 120 mg | Headache |
| Long acting | 30 mg/d | 240 mg | Tachycardia |
| Alpha methyldopa | 250 mg twice daily | 4 g | Thirst Drowsiness Elevation of liver enzymes |
TABLE 5
Medical factors guiding selection of antihypertensive medication
| If the patient has… | It’s generally best to start with… |
|---|---|
| Diabetes | Calcium-channel blocker |
| Vascular disease | |
| Salt-wasting hypertension* | Thiazide diuretic |
| Left ventricular systolic dysfunction | |
| Mitral stenosis | |
| *Mostly African-American women | |
Severe gestational hypertension and preeclampsia
Women who develop severe gestational hypertension (systolic BP of 160 mm Hg or more or diastolic BP of 110 mm Hg or more) and/or preeclampsia require antihypertensive treatment during management remote from term. In this case, the aim of antihypertensive drug treatment is to keep systolic BP between 150 and 159 mm Hg and diastolic BP between 100 and 109 mm Hg in order to not compromise uteroplacental blood flow.
Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent for gravidas with hypertensive encephalopathy.
The drugs to use are oral labetalol and/or oral nifedipine. If maternal BP is not adequately controlled with maximum doses of labetalol plus nifedipine, the patient should be delivered.
Severe hypertension and encephalopathy
Hypertensive encephalopathy is a medical emergency. This rare complication of hypertension in pregnancy27 is marked by severely elevated BP, with the diastolic level frequently exceeding 130 mm Hg. Associated findings include headache, visual disturbances, nausea, vomiting, seizures, confusion, stupor, and coma. Also possible are retinal hemorrhage, exudates, papilledema, and evidence of renal or cardiac disease. Transient focal neurologic findings may be present as well, but more often suggest vascular disease, hemorrhage, embolism, or thrombosis.
Pathophysiology. In hypertensive encephalopathy, loss of autoregulation leads to generalized cerebral vasodilation. Under normal conditions, when the mean arterial pressure is between 60 and 130 mm Hg, patients maintain constant cerebral blood flow. In hypertensive patients, however, autoregulation occurs between mean arterial pressures of 110 and 180 mm Hg as a result of arteriolar thickening. When BP exceeds the ability of the vessels to autoregulate, blood flow hyperperfuses the brain, causing fluid to leak into the perivascular tissue and resulting in vasogenic cerebral edema. Altered vascular reactivity to normally circulating pressor agents, deficient levels of vasodilating prostaglandins, endothelial dysfunction, and activation of the coagulation cascade may further exacerbate this condition.28
Treatment options. Clinically, it may be impossible to differentiate hypertensive encephalopathy from eclampsia, and magnesium sulfate should be considered for seizure prophylaxis. The most frequently used antihypertensive medications for this syndrome are shown in TABLE 6. Nitroprusside lowers BP most predictably, but because of the associated risks of fetal cyanide toxicity, other medications may be more desirable first-line agents in the pregnant woman.
Importantly, because sudden drops in BP may impair cerebral perfusion, we recommend that the mean arterial pressure be lowered no more than 25% from baseline (TABLE 7). If pulmonary edema develops, oxygen and furosemide should be administered, and consultation with subspecialists considered. (We suggest such consultation for cases of renal dysfunction and cerebral complications, as well.) Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent in this situation.
TABLE 6
Medications for treating acute severe hypertension
| DRUG | DOSE | ONSET OF ACTION | DURATION OF ACTION | SIDE EFFECTS |
|---|---|---|---|---|
| Hydralazine | 5-10 mg IV every 20 min | 10-20 min | 3-6 h | Tachycardia Headache Flushing Angina |
| Labetalol | 20-80 mg IV every 10 min | 5-10 min | 3-6 h | Scalp tingling Vomiting Heart block |
| Sodium nitroprusside* | 0.25-5 mcg/kg/min | Immediate | 1-2 min | Nausea Vomiting Muscle twitching Thiocyanate and cyanide intoxication |
| Nicardipine* | 5-15 mg/h IV | 5-10 min | 1-4 h | Tachycardia Headache Phlebitis |
| *Drugs to use in the presence of hypertensive encephalopathy. | ||||
TABLE 7
Principles of management for severe hypertension and encephalopathy
Acute severe hypertension
|
Hypertensive encephalopathy
|
| *Lower mean arterial pressure no more than 25% from baseline. |
Postpartum management
Monitor BP for at least 48 hours. Women with high-risk chronic hypertension are more likely to suffer postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure than normotensive patients.8 This risk is even higher when these women also have target organ involvement, superimposed preeclampsia, abruptio placentae, morbid obesity, or longstanding hypertension.
In these patients, BP must be closely monitored and controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be administered for acute elevations of BP29; diuretics should also be used for women with circulatory congestion and pulmonary edema.30
Methyldopa remains the first-line agent for breastfeeding patients without compelling indications for another drug.
Oral antihypertensive therapy may be needed to maintain BP control. In choosing the appropriate agent, it is important to consider whether factors compel the choice of one medication over another. For example, for patients with a history of myocardial infarction, beta blockers and ACE inhibitors are excellent choices to decrease mortality.31 In patients with diabetes mellitus, as mentioned earlier, ACE inhibitors offer a renoprotective effect.10
Consider drug concentrations in breast milk. Another significant consideration in the postpartum period is whether the mother wishes to breastfeed her infant. All antihypertensive medications are found in breast milk to varying degrees,32 and the long-term effects of these medications on breastfeeding infants has not been specifically studied.
Because concentrations of methyldopa in milk are low and considered safe, it remains the first-line agent for patients without compelling indications for another antihypertensive drug. Concentrations of labetalol and propranolol also are low in breast milk; therefore, these may be better choices than atenolol and metoprolol, which are more highly concentrated in breast milk.12
Although diuretic agents have low concentrations in breast milk, they may decrease milk production.32
Little information exists regarding the excretion of calcium-channel blockers in breast milk, but no untoward effects are apparent.33 ACE inhibitors and angiotensin II receptor antagonists should be avoided because of potential deleterious effects on neonatal renal function, even though their concentrations in breast milk appear to be low. If ACE inhibitors are indicated for the breastfeeding mother, current data suggest that captopril and enalapril are safe.34
The authors report no financial relationship with any companies whose products are mentioned in this article.
- The treatment goal is to reduce blood pressure to a safe level to prevent maternal cerebral complications.This goal must be weighed against the risks of fetal exposure to antihypertensive drugs and the effects on uteroplacental blood flow.
- Gravidas with uncomplicated mild hypertension are at low risk; however, those with severe hypertension or associated complicating factors are at high risk of complications and adverse outcomes.
- Antihypertensive medications should not be used routinely in low-risk patients.
- Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.
The decision to use antihypertensive drug therapy in pregnant women is a tricky one—especially considering the ever-evolving nature of treatment. For instance, we now know that in some hypertensive gravidas, medical interventions may actually be deleterious.
With the aging of the obstetric population in the United States, hypertension in pregnancy—which currently affects 7% of gestations—will remain a major issue in preconception and prenatal care. Its reported risks, which include stroke, pulmonary edema, and death, underscore the importance of careful management (TABLE 1).
This article describes the indications for antihypertensive therapy in pregnancy, focusing on 2 basic categories—high-risk and lowrisk patients—and offers guidance in choosing the optimal agent for each patient.
TABLE 1
Maternal risks of severe hypertension in pregnancy
| Stroke |
| Cerebral hemorrhage |
| Hypertensive encephalopathy |
| Congestive heart failure/pulmonary edema |
| Acute renal dysfunction/acute renal failure |
| Abruptio placentae |
| Disseminated intravascular coagulopathy |
| Death |
Correct classification helps direct management
First, identify chronic hypertension. Chronic hypertension is defined as an elevation in blood pressure (BP) that exists prior to pregnancy. Unfortunately, because the pregestational BP is not always known, the diagnosis in many cases must be made on the basis of specific levels: systolic BP of at least 140 mm Hg or diastolic BP of at least 90 mm Hg on at least 2 occasions at least 4 hours apart prior to 20 weeks’ gestation.1
Even with these guidelines, however, diagnosis may be difficult, since early manifestations of preeclampsia can include hypertension prior to 20 weeks’ gestation.2,3 In addition, the physiologic decrease in BP during the first and second trimesters—seen in many patients with chronic hypertension—may obscure the condition early in gestation and lead to the erroneous diagnosis of gestational hypertension or preeclampsia later in pregnancy.4-6
Once a diagnosis of chronic hypertension is made, an accurate classification of the disease will help guide management and initiation of antihypertensive medication.
Mild versus severe hypertension. In pregnancy, chronic hypertension is classified as mild or severe. Mild hypertension has traditionally been defined as systolic BP less than 160 mm Hg and diastolic blood pressure less than 110 mm Hg.1,7 However, the American College of Obstetricians and Gynecologists recently changed its definition of mild hypertension to systolic BP less than 180 mm Hg.8,9 Most women with chronic hypertension in pregnancy have the mild form of the disease.
Low-risk hypertension. Patients with uncomplicated chronic mild hypertension are at low risk.
High-risk hypertension. Patients at high risk have either chronic severe hypertension or chronic mild hypertension in association with any of the complicating factors listed in TABLE 2.
History and laboratory studies. To properly classify the disease when first evaluating a patient with chronic hypertension, a thorough history is essential. Ask about related medical illnesses as well as target organ damage. Pay special attention to cardiac, renal, thyroid, and cerebrovascular disease, as well as diabetes. The outcomes of prior pregnancies also are important, especially complications such as abruptio placentae, preeclampsia, preterm delivery, growth restriction, fetal death, and neonatal complications.
Overall, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension.
Finally, laboratory evaluation should include urine analysis, urine culture, 24-hour urine protein, electrolytes, complete blood count, and glucose tolerance testing.
Other key examinations. In women with long-standing disease, ophthalmologic evaluation, electrocardiography, echocardiography, and assessment of creatinine clearance may be indicated.
TABLE 2
Criteria for low- versus high-risk chronic hypertension
| LOW-RISK CHRONIC HYPERTENSION |
| Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in the absence of complicating factors |
| HIGH-RISK CHRONIC HYPERTENSION |
| Either: |
| Chronic severe hypertension (systolic blood pressure ≥180 mm Hg and diastolic blood pressure ≥110 mm Hg) |
| or |
Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in association with anyof the following:
|
Objective of treatment: Prevent complications
In the nonpregnant state, the aim of hypertensive management is to prevent longterm vascular complications such as stroke and cardiovascular disease.10 A reasonable treatment goal for patients with mild to moderate hypertension may be benefits that are apparent after 5 years of therapy10—an acceptable time frame due to the long-term nature of the disease.
In pregnant women, however, the duration of the condition (pregnant and hypertensive) is finite and relatively short; as a result, maternal health benefits may not be clear. For that reason, the objective is to reduce BP to a safe level to prevent maternal cerebral complications (systolic BP below 160 mm Hg and diastolic BP below 110 mm Hg).Of course, these short-term maternal benefits must be weighed against the potential risks of fetal exposure (TABLE 3).11,12
TABLE 3
Rates of adverse pregnancy outcomes among patients with mild and severe chronic hypertension
| OUTCOME | MILD HYPERTENSION (%) | SEVERE HYPERTENSION (%) |
|---|---|---|
| Preeclampsia | 10-25 | 25-50 |
| Abruptio placentae | 0.7-1.5 | 2-5 |
| Fetal growth restriction | 8.0-15.5 | 10-20 |
| Preterm birth | 12-34.4 | 25-30 |
Low-risk disease: Avoid routine antihypertensive therapy
A limited number of randomized trials have studied the effectiveness of antihypertensive treatment in preventing adverse maternal outcomes such as superimposed preeclampsia and abruptio placentae. Here are 2 key findings:
- No demonstrable maternal benefit. Overall, there appears to be no clear benefit of antihypertensive treatment in women with mild hypertension. Indeed, the 2 largest studies had contradictory findings regarding preeclampsia,6,13 and there was no demonstrable benefit in regard to abruptio placentae.
- Antihypertensive drugs may adversely affect fetal growth. A recent meta-analysis examining antihypertensive medications in patients with mild to moderate hypertension investigated the relationship between a fall in mean arterial blood pressure and the delivery of small-for-gestational-age (SGA) infants.14 The authors concluded that antihypertensive medications induce BP drops that may adversely affect fetal growth (see FIGURE). Prior to this observation, prospective studies had shown no association between antihypertensive medications and SGA infants. (The only exception was atenolol; 3 separate studies found a relationship between treatment with atenolol and low birth weight.15-17)
Overall, maternal and perinatal data indicate that, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension. In fact, the indiscriminate use of such medications may have deleterious effects. Consequently, antihypertensive medications should not be used routinely in low-risk patients.18,19
Clinical care. When a woman with low-risk hypertension presents for prenatal care, it is our policy to discontinue antihypertensive medications at the first prenatal visit. Although many women will not require antihypertensive treatment during the pregnancy, careful management remains essential, as such patients can become high-risk at any time. Therapy should be initiated if her condition changes to severe hypertension (systolic BP of 180 mm Hg or more, or a diastolic blood pressure of 110 mm Hg or more).8
Low-risk women should be monitored closely for evidence of preeclampsia and fetal growth restriction. Thus, they should have a baseline ultrasound at 16 to 20 weeks’ gestation, with serial monthly ultrasounds beginning at 30 to 32 weeks to follow fetal growth. Nonstress testing or biophysical profiles are indicated in the presence of severe hypertension, preeclampsia, or abnormal fetal growth.
Patients with uncomplicated lowrisk hypertension may continue pregnancy until 40 weeks’ gestation. However, beyond 37 weeks, the presence of complications such as severe hypertension, documented growth restriction, and superimposed preeclampsia are indications for hospitalization and delivery.
FIGURE Placental effects of hypertension
In hypertensive gravidas, placental blood flow is reduced—particularly in cases of preeclampsia. Antihypertensive therapy in low-risk women may induce blood pressure drops that further compromise fetal growth.
High-risk disease: Initiate medical therapy
Randomized, controlled trials do not exist for gravidas with high-risk hypertension—that is, women with severe hypertension or complicating factors—due to concerns about the potential adverse consequences of uncontrolled disease, such as cerebrovascular accident, congestive heart failure, and renal failure.20 It is interesting to note, however, that although controlling hypertension in such patients may help prolong pregnancy, there is no evidence that it reduces the rates of preeclampsia or abruptio placentae.20,21
Labetalol provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation.
When to start treatment. For women with high-risk hypertension, hospitalization at the time of the first prenatal visit facilitates complete cardiovascular and renal evaluation, and is therefore often beneficial. If a woman has target organ damage, treatment should be initiated at a systolic BP of 140 mm Hg or a diastolicBP of 90 mm Hg. Indeed, many such women are already receiving treatment for their hypertension, in which case antihypertensive medications should be continued, though physicians should consider altering the regimen to optimize fetal safety.
Choosing the best agent. Before choosing an antihypertensive drug, review the patient’s history. If her disease was well controlled on a particular medication, that agent is probably a reasonable first choice, provided there is adequate published literature establishing the safety of her medication during pregnancy. Obviously, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II antagonists, and atenolol should be avoided because of the potential adverse effects on the fetus, including renal failure.The most commonly used medications for control of hypertension during pregnancy are listed in TABLE 4.
- Labetalol. We consider labetalol a first-line agent for controlling hypertension in pregnancy. This beta-blocking drug provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation—not seen with traditional beta-blockers. Overall, labetalol has an excellent record of safety in pregnancy.
In a randomized, controlled trial involving 86 mildly hypertensive patients who initiated labetalol therapy between 6 and 13 weeks’ gestation, no major congenital malformations were identified.6 Although there have been reports of an increased risk for SGA infants in patients treated with labetalol for mild pregnancy-induced hypertension during the second and third trimesters, this association has not been documented in women with chronic hypertension.6
- Thiazide diuretics. If labetalol fails to control blood pressure, we typically add either the calcium-channel blocker nifedipine or a thiazide diuretic. Use of the latter has been well documented in pregnancy. Indeed, thiazide diuretics can be given in the first trimester and throughout gestation without associated risks of major fetal malformations or adverse fetal-neonatal complications.
- Calcium-channel blockers. Calcium-channel–blocking agents also have an excellent safety profile in pregnancy. They have been studied both as antihypertensive medications (primarily in the second and third trimesters) and as tocolytic agents. In a prospective, multicenter, cohort study in which 78 women were exposed to calcium-channel blockers (mainly nifedipine and verapamil) during the first trimester, there was no increase in the rate of birth defects.22
A separate prospective, randomized trial evaluated the benefit of nifedipine in pregnancy. A total of 283 women—47% of whom had chronic hypertension—were enrolled between 12 and 34 weeks’ gestation (mean: 24 weeks). Researchers found patients on nifedipine therapy experienced no improvement in maternal or neonatal outcomes compared to subjects assigned to no treatment.23 Follow-up at 18 months of 94 of the infants exposed to nifedipine in utero showed no adverse effects on development.24
- Methyldopa. For many obstetricians, methyldopa remains a first-line agent for the treatment of chronic hypertension in pregnancy.1 It has a well-documented safety record in both short-term25 and long-term follow-up of children exposed in utero.26 Indeed, many studies have evaluated use of this medication to manage mild to moderate hypertension, with no evidence of adverse maternal or fetal outcome. However, it is now rarely used in the nonpregnant population, and the safety of other medications, such as labetalol and nifedipine, has prompted us to stop giving it.
- Other considerations. Finally, when choosing an antihypertensive drug, the physician must consider the benefits and response of specific agents in particular risk groups (TABLE 5).
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy, since ACE inhibitors, which also offer this benefit, must be avoided beyond 16 weeks’ gestation because of the potential adverse fetal effects.
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy.
Young African-American women frequently have low-renin, salt-sensitive hypertension, and therefore thiazide diuretics or nifedipine may be better first-line agents in this population.
TABLE 4
Agents for treating chronic hypertension in pregnancy
| DRUG | STARTING DOSE | MAXIMUM DAILY DOSE | COMMON SIDE EFFECTS |
|---|---|---|---|
| Labetalol | 100 mg every 8 h | 1,200–2,400 mg | Headache Tremulousness |
| Thiazide diuretic | 12.5 mg twice daily | 50 mg | Hypokalemia |
| Nifedipine | Hypotension | ||
| Short acting | 10 mg every 8 h | 120 mg | Headache |
| Long acting | 30 mg/d | 240 mg | Tachycardia |
| Alpha methyldopa | 250 mg twice daily | 4 g | Thirst Drowsiness Elevation of liver enzymes |
TABLE 5
Medical factors guiding selection of antihypertensive medication
| If the patient has… | It’s generally best to start with… |
|---|---|
| Diabetes | Calcium-channel blocker |
| Vascular disease | |
| Salt-wasting hypertension* | Thiazide diuretic |
| Left ventricular systolic dysfunction | |
| Mitral stenosis | |
| *Mostly African-American women | |
Severe gestational hypertension and preeclampsia
Women who develop severe gestational hypertension (systolic BP of 160 mm Hg or more or diastolic BP of 110 mm Hg or more) and/or preeclampsia require antihypertensive treatment during management remote from term. In this case, the aim of antihypertensive drug treatment is to keep systolic BP between 150 and 159 mm Hg and diastolic BP between 100 and 109 mm Hg in order to not compromise uteroplacental blood flow.
Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent for gravidas with hypertensive encephalopathy.
The drugs to use are oral labetalol and/or oral nifedipine. If maternal BP is not adequately controlled with maximum doses of labetalol plus nifedipine, the patient should be delivered.
Severe hypertension and encephalopathy
Hypertensive encephalopathy is a medical emergency. This rare complication of hypertension in pregnancy27 is marked by severely elevated BP, with the diastolic level frequently exceeding 130 mm Hg. Associated findings include headache, visual disturbances, nausea, vomiting, seizures, confusion, stupor, and coma. Also possible are retinal hemorrhage, exudates, papilledema, and evidence of renal or cardiac disease. Transient focal neurologic findings may be present as well, but more often suggest vascular disease, hemorrhage, embolism, or thrombosis.
Pathophysiology. In hypertensive encephalopathy, loss of autoregulation leads to generalized cerebral vasodilation. Under normal conditions, when the mean arterial pressure is between 60 and 130 mm Hg, patients maintain constant cerebral blood flow. In hypertensive patients, however, autoregulation occurs between mean arterial pressures of 110 and 180 mm Hg as a result of arteriolar thickening. When BP exceeds the ability of the vessels to autoregulate, blood flow hyperperfuses the brain, causing fluid to leak into the perivascular tissue and resulting in vasogenic cerebral edema. Altered vascular reactivity to normally circulating pressor agents, deficient levels of vasodilating prostaglandins, endothelial dysfunction, and activation of the coagulation cascade may further exacerbate this condition.28
Treatment options. Clinically, it may be impossible to differentiate hypertensive encephalopathy from eclampsia, and magnesium sulfate should be considered for seizure prophylaxis. The most frequently used antihypertensive medications for this syndrome are shown in TABLE 6. Nitroprusside lowers BP most predictably, but because of the associated risks of fetal cyanide toxicity, other medications may be more desirable first-line agents in the pregnant woman.
Importantly, because sudden drops in BP may impair cerebral perfusion, we recommend that the mean arterial pressure be lowered no more than 25% from baseline (TABLE 7). If pulmonary edema develops, oxygen and furosemide should be administered, and consultation with subspecialists considered. (We suggest such consultation for cases of renal dysfunction and cerebral complications, as well.) Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent in this situation.
TABLE 6
Medications for treating acute severe hypertension
| DRUG | DOSE | ONSET OF ACTION | DURATION OF ACTION | SIDE EFFECTS |
|---|---|---|---|---|
| Hydralazine | 5-10 mg IV every 20 min | 10-20 min | 3-6 h | Tachycardia Headache Flushing Angina |
| Labetalol | 20-80 mg IV every 10 min | 5-10 min | 3-6 h | Scalp tingling Vomiting Heart block |
| Sodium nitroprusside* | 0.25-5 mcg/kg/min | Immediate | 1-2 min | Nausea Vomiting Muscle twitching Thiocyanate and cyanide intoxication |
| Nicardipine* | 5-15 mg/h IV | 5-10 min | 1-4 h | Tachycardia Headache Phlebitis |
| *Drugs to use in the presence of hypertensive encephalopathy. | ||||
TABLE 7
Principles of management for severe hypertension and encephalopathy
Acute severe hypertension
|
Hypertensive encephalopathy
|
| *Lower mean arterial pressure no more than 25% from baseline. |
Postpartum management
Monitor BP for at least 48 hours. Women with high-risk chronic hypertension are more likely to suffer postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure than normotensive patients.8 This risk is even higher when these women also have target organ involvement, superimposed preeclampsia, abruptio placentae, morbid obesity, or longstanding hypertension.
In these patients, BP must be closely monitored and controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be administered for acute elevations of BP29; diuretics should also be used for women with circulatory congestion and pulmonary edema.30
Methyldopa remains the first-line agent for breastfeeding patients without compelling indications for another drug.
Oral antihypertensive therapy may be needed to maintain BP control. In choosing the appropriate agent, it is important to consider whether factors compel the choice of one medication over another. For example, for patients with a history of myocardial infarction, beta blockers and ACE inhibitors are excellent choices to decrease mortality.31 In patients with diabetes mellitus, as mentioned earlier, ACE inhibitors offer a renoprotective effect.10
Consider drug concentrations in breast milk. Another significant consideration in the postpartum period is whether the mother wishes to breastfeed her infant. All antihypertensive medications are found in breast milk to varying degrees,32 and the long-term effects of these medications on breastfeeding infants has not been specifically studied.
Because concentrations of methyldopa in milk are low and considered safe, it remains the first-line agent for patients without compelling indications for another antihypertensive drug. Concentrations of labetalol and propranolol also are low in breast milk; therefore, these may be better choices than atenolol and metoprolol, which are more highly concentrated in breast milk.12
Although diuretic agents have low concentrations in breast milk, they may decrease milk production.32
Little information exists regarding the excretion of calcium-channel blockers in breast milk, but no untoward effects are apparent.33 ACE inhibitors and angiotensin II receptor antagonists should be avoided because of potential deleterious effects on neonatal renal function, even though their concentrations in breast milk appear to be low. If ACE inhibitors are indicated for the breastfeeding mother, current data suggest that captopril and enalapril are safe.34
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. Sibai BM, Akl S, Fairlie F, Moretti M. A protocol for managing severe preeclampsia in the second trimester. Am J Obstet Gynecol. 1990;163:733-738.
3. Hermida RC, Ayala DA, Mojon A, et al. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000;36:149-158.
4. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
5. Benedetto C, Zonca M, Marozio L, Dolci C, Carandente F, Massobrio M. Blood pressure patterns in normal pregnancy and in pregnancy-induced hypertension, preeclampsia, and chronic hypertension. Obstet Gynecol. 1996;88:503-510.
6. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
7. Sibai BM. Diagnosis and management of chronic hypertension in pregnancy. Obstet Gynecol. 1991;78:451-461.
8. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: Chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
9. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
10. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413-2446.
11. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96:849-860.
12. Umans JG, Lindheimer MD. Antihypertensive treatment. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn: Appleton and Lange; 1998;581-604.
13. Steyn DW, Odendaal HJ. Randomized controlled trial of ketanserin and aspirin in prevention of pre-eclampsia. Lancet. 1997;350:1267-1271.
14. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355:87-92.
15. Lip GY, Beevers M, Churchill D, Shaffer LM, Beevers DG. Effect of atenolol on birth weight. Am J Cardiol. 1997;79:1436-1438.
16. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725-733.
17. Lydakis C, Lip GY, Beevers M, Beevers G. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12:541-547.
18. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335:257-265.
19. Magee LA, Ornstein MP, von Dadelszen P. Management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
20. Sibai BM, Anderson GD. Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstet Gynecol. 1986;67:517-522.
21. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
22. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174:823-828.
23. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722.
24. Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months. Br J Obstet Gynaecol. 2000;107:792-794.
25. Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1993;168:152-156.
26. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1:647-649.
27. Witlin AG, Friedman SA, Egerman RS, et al. Cerebrovascular disorders complicating pregnancy—beyond eclampsia. Am J Obstet Gynecol. 1997;176:1139-1148.
28. Cotton DB, Janusz CA, Berman RF. Anticonvulsant effects of magnesium sulfate on hippocampal seizures: therapeutic implication in preeclampsia-eclampsia. Am J Obstet Gynecol. 1992;166:1127-1136.
29. Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol. 1987;70:328-333.
30. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women: a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
31. Pfeffer MA, Braunwald E, Moye LA. for the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trials. N Engl J Med. 1992;327:669-677.
32. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
33. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore, Md: Williams and Wilkins; 1998.
34. Committee on Drugs, American Academy of Pediatrics. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137-150.
1. National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. Sibai BM, Akl S, Fairlie F, Moretti M. A protocol for managing severe preeclampsia in the second trimester. Am J Obstet Gynecol. 1990;163:733-738.
3. Hermida RC, Ayala DA, Mojon A, et al. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000;36:149-158.
4. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
5. Benedetto C, Zonca M, Marozio L, Dolci C, Carandente F, Massobrio M. Blood pressure patterns in normal pregnancy and in pregnancy-induced hypertension, preeclampsia, and chronic hypertension. Obstet Gynecol. 1996;88:503-510.
6. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
7. Sibai BM. Diagnosis and management of chronic hypertension in pregnancy. Obstet Gynecol. 1991;78:451-461.
8. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: Chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
9. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
10. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413-2446.
11. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96:849-860.
12. Umans JG, Lindheimer MD. Antihypertensive treatment. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn: Appleton and Lange; 1998;581-604.
13. Steyn DW, Odendaal HJ. Randomized controlled trial of ketanserin and aspirin in prevention of pre-eclampsia. Lancet. 1997;350:1267-1271.
14. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355:87-92.
15. Lip GY, Beevers M, Churchill D, Shaffer LM, Beevers DG. Effect of atenolol on birth weight. Am J Cardiol. 1997;79:1436-1438.
16. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725-733.
17. Lydakis C, Lip GY, Beevers M, Beevers G. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12:541-547.
18. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335:257-265.
19. Magee LA, Ornstein MP, von Dadelszen P. Management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
20. Sibai BM, Anderson GD. Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstet Gynecol. 1986;67:517-522.
21. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
22. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174:823-828.
23. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722.
24. Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months. Br J Obstet Gynaecol. 2000;107:792-794.
25. Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1993;168:152-156.
26. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1:647-649.
27. Witlin AG, Friedman SA, Egerman RS, et al. Cerebrovascular disorders complicating pregnancy—beyond eclampsia. Am J Obstet Gynecol. 1997;176:1139-1148.
28. Cotton DB, Janusz CA, Berman RF. Anticonvulsant effects of magnesium sulfate on hippocampal seizures: therapeutic implication in preeclampsia-eclampsia. Am J Obstet Gynecol. 1992;166:1127-1136.
29. Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol. 1987;70:328-333.
30. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women: a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
31. Pfeffer MA, Braunwald E, Moye LA. for the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trials. N Engl J Med. 1992;327:669-677.
32. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
33. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore, Md: Williams and Wilkins; 1998.
34. Committee on Drugs, American Academy of Pediatrics. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137-150.