User login
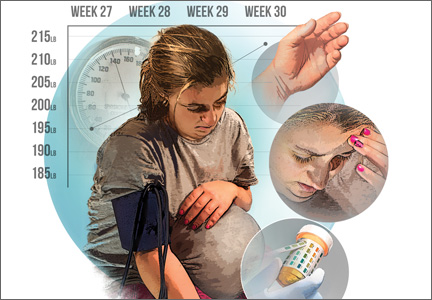
Recognition, evaluation, and management of postpartum hypertension
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.
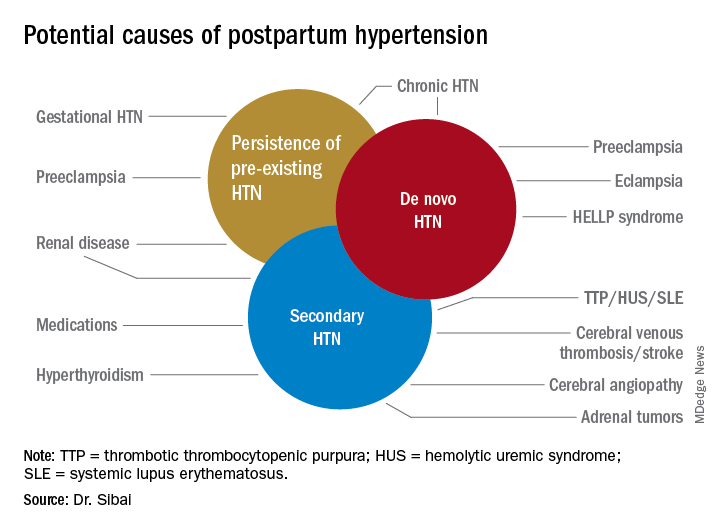
Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.

Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.

Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Management of hypertensive disorders in pregnancy
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 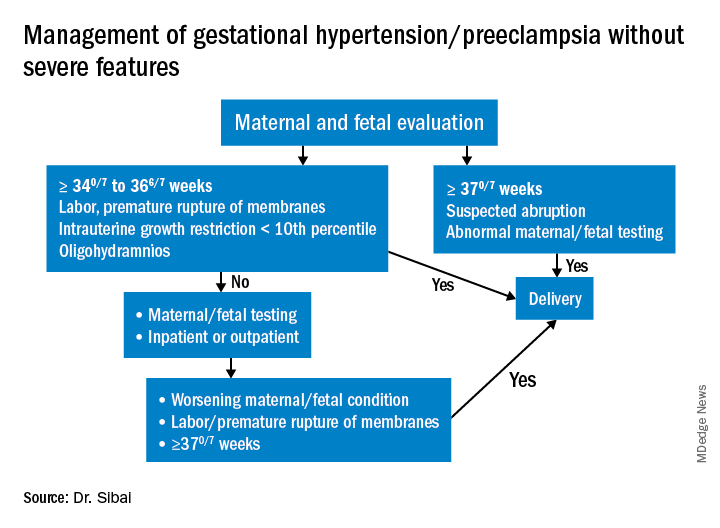
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 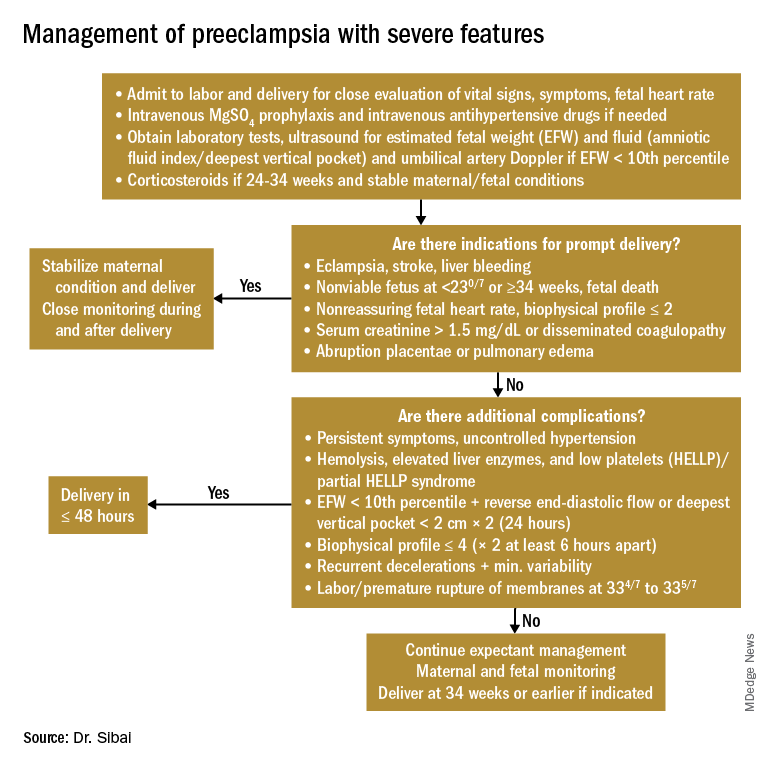
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
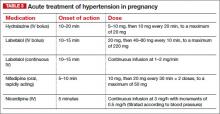
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.
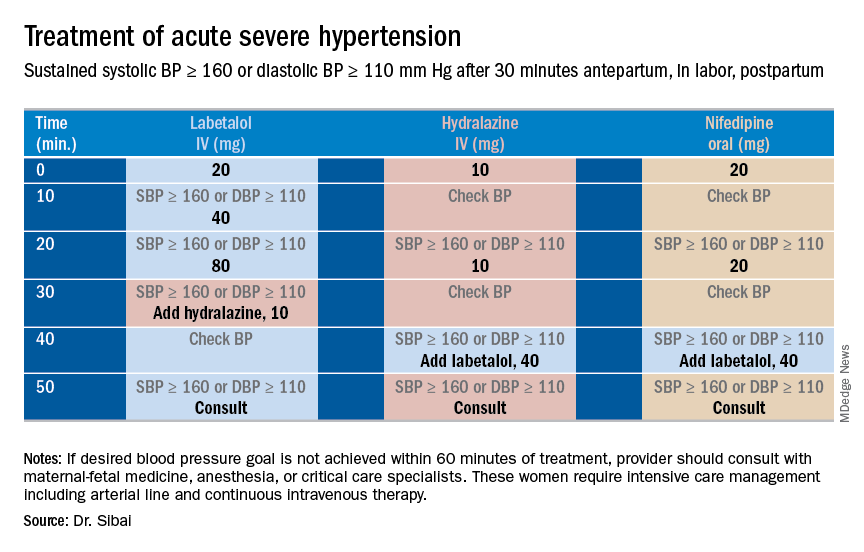
Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.

Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.

Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Clarifying the categories of hypertensive disorders in pregnancy
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Optimal obstetric care for women aged 40 and older
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
A stepwise approach to managing eclampsia and other hypertensive emergencies
CASE: MISSED PREECLAMPSIA
At her first prenatal visit at 14 weeks’ gestation, a 41-year-old woman (G2P1) presents with a dichorionic twin gestation, blood pressure (BP) of 105/68 mm Hg, and a body mass index (BMI) of 40 kg/m2. The pregnancy was achieved through in vitro fertilization. Ten years earlier, the patient’s first pregnancy was complicated by preeclampsia, requiring preterm delivery at 33 weeks’ gestation.
By 28 weeks’ gestation, the patient has gained 26 lb. Her BP is 120/70 mm Hg, with no proteinuria detected by urine dipstick. By 30 weeks, she has gained an additional 8 lb, her BP is 142/84 mm Hg, and no proteinuria is detected. At 32 weeks, her BP is 140/92 mm Hg, she has gained another 8 lb, and no proteinuria is present. She also reports new-onset headaches that do not respond to over-the-counter analgesics. She is sent to the obstetric triage area for BP monitoring, blood testing for preeclampsia and nonstress test fetal monitoring.
During the 2-hour observation period, the patient continues to report headaches, and swelling of her face and hands is present. Her systolic BP values range from 132 to 152 mm Hg, and diastolic values range from 80 to 96 mm Hg. No proteinuria is detected, blood testing results for preeclampsia (complete blood count, liver enzymes, serum creatinine, and uric acid) are normal, and the nonstress tests are reactive in both fetuses.
The patient is given a diagnosis of gestational hypertension, along with a prescription for oral labetalol 200 mg daily and two tablets of acetaminophen with codeine for the headaches (to be taken every 6 hours as needed). She is sent home with instructions to return to her physician’s office in 1 week.
Two days later, she wakes in the middle of the night with a severe headache, blurred vision, and vomiting. Her husband calls the obstetrician’s answering service and is instructed to call 911 immediately. While waiting for an ambulance, the patient experiences a grand mal eclamptic convulsion. A second convulsion occurs during her transfer to the ED.
This scenario could have been avoided.
The obstetrician in this case was negligent for failing to recognize preeclampsia in a patient who had two clear risk factors for it: multifetal gestation and a history of early-onset (<37 weeks) preeclampsia in an earlier pregnancy (other risk factors are listed in TABLE 1).
As a result, the patient developed eclampsia, a serious condition that can lead to grave maternal complications (TABLE 2), including death. It also can cause fetal complications, including growth restriction, hypoxia, acidosis, preterm birth, long-term developmental deficits, and death.1,2
The obstetrician in this case also overlooked published evidence indicating that, in the setting of hypertension and headaches, as many as 20% to 30% of pregnant women whose tests for proteinuria show a negative or trace result via dipstick will develop eclampsia.3 Instead of initiating outpatient administration of oral antihypertensive agents, the obstetrician should have hospitalized this patient for at least 48 hours, with steroid administration, to determine whether outpatient management was feasible.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia Baha Sibai, MD (November 2011)
Defining eclampsia
Eclampsia is marked by the onset of convulsions (during pregnancy or postpartum) in association with gestational hypertension alone, proteinuria, preeclampsia, or superimposed preeclampsia. Although it is rare, eclampsia is potentially life-threatening. For that reason, obstetricians, anesthesiologists, ED physicians, neurologists, and critical-care physicians should be well versed in its diagnosis and management. In this article, I focus on management.
A few preliminary points
Eclampsia can develop any time during the antenatal period (>16 weeks’ gestation), during labor and delivery, and as long as 6 weeks after delivery. Therefore, we should be vigilant for preeclampsia whenever a pregnant patient visits our office, as well as when she makes unscheduled visits to the ED or obstetric triage area or is hospitalized.
Early recognition of women at high risk for preeclampsia and eclampsia may allow for prompt intervention, including early hospitalization for close observation prior to delivery and postpartum.1,2,4–10
Hospitalization of high-risk women allows for use of antihypertensive agents to treat severe BP, administration of magnesium sulfate to prevent convulsions, and timely delivery of the infant. It also allows for intensive maternal support during and after an eclamptic seizure.
Hospitalization is essential for women who exhibit features that suggest severe disease. More specifically, the presence of gestational hypertension with any of the following features is an indication for immediate hospitalization for evaluation and management:
- persistent severe hypertension (systolic
BP ≥160 mm Hg or diastolic BP ≥110 mm Hg) for at least 1 hour - gestational hypertension requiring oral antihypertensive therapy
- progressive and excessive weight gain (≥20 lb prior to 28 weeks’ gestation)
- generalized swelling (edema of hands or face)
- new-onset or persistent headaches despite analgesics
- persistent visual changes (blurred vision, scotomata, photophobia, double vision)
- shortness of breath, dyspnea, orthopnea, or tightness in the chest
- persistent retrosternal chest pain, severe epigastric or right upper quadrant pain
- persistent nausea, vomiting, malaise
- altered mental state, confusion, numbness, tingling, or motor weakness
- platelet count below 100 3 103 µL
- aspartate aminotransferase (AST), alanine aminotransferase (ALT), or lactic acid dehydrogenase (LDH) levels more than twice the upper limit of normal
- serum creatinine level >1.1 mg/dL
- suspected abruptio placentae.
A stepwise approach to eclampsia
Eclampsia is an obstetric emergency. Inadequate preparation for it or an inappropriate response to maternal and fetal conditions during and after an eclamptic convulsion can be detrimental to the mother and fetus. All obstetric units should have up-to-date protocols in place and should conduct mandatory drills to prepare nursing staff, obstetric providers, and anesthesia staff working in these units to manage eclampsia.
Step 1: Let the seizure run its course
During a seizure, resist the impulse to administer anticonvulsive drugs, including intravenous (IV) magnesium sulfate, because most eclamptic convulsions are self-limiting. Also abstain from administering medications such as IV phenytoin, diazepam, or midazolam, as these drugs are less effective than magnesium sulfate, and some can suppress the laryngeal reflex, increasing the risk of aspiration.
If the patient develops status epilepticus, initiate muscle paralysis and intubate her.
Step 2: Support the maternal condition
It is vital to support maternal respiratory and cardiovascular functions to prevent hypoxia, acidosis, and cardiorespiratory arrest.
Begin by establishing airway patency and maternal oxygenation during and after the convulsion. Administer oxygen via a face mask, with or without a reservoir, at a rate of 8 to 10 L/min.
During the apneic period (see “Profile of an eclamptic seizure” on page 46), the patient will develop hypoxia. Use pulse oximetry to monitor oxygen saturation, with the goal of keeping it above 94%. Arterial blood gas analysis is required if oxygen saturation remains below 92% or if pulmonary edema or aspiration is suspected.
If the patient develops recurrent seizures, status epilepticus, florid alveolar pulmonary edema, or respiratory arrest, intubate her immediately.
Step 3: Prevent maternal injury and aspiration
Secure the side rails of the patient’s bed by elevating them to prevent a fall, and make sure they are padded to prevent trauma during convulsions and afterward, when some women become combative and agitated. Position the patient in a lateral decubitus position to minimize aspiration of oral secretions. If any secretions or vomitus are present, remove them via suction.
Step 4: After the convulsion, give magnesium sulfate
Magnesium is the drug of choice for seizure prophylaxis in women with preeclampsia and severe symptoms, and to prevent recurrent seizures in women with eclampsia.
In the latter group, once the eclamptic convulsion has ended, give a loading dose of IV magnesium (6 g/100 mL over 20 minutes), followed by a continuous infusion of 2 g/h for at least 24 hours. If the patient develops a second seizure during the maintenance infusion, administer another bolus of magnesium (2 g/100 mL over 3–5 minutes).
Step 5: Treat severe hypertension
If severe hypertension persists for 60 minutes or longer, it can lead to injury of the brain, heart, and kidneys. To avoid these complications, it is essential to reduce BP to a safe range and maintain that level without compromising cerebral perfusion pressure and uteroplacental blood flow (which already may be reduced in some patients).
The goal of antihypertensive therapy is to keep systolic BP between 140 and 155 mm Hg and diastolic values between 90 and 105 mm Hg.9 Several agents are available for the treatment of severe hypertension during pregnancy and postpartum. The most commonly used IV medications for this purpose are labetalol and hydralazine. Another option is oral, rapidly acting
nifedipine.
Several randomized trials have compared efficacy and side effects between IV bolus injections of hydralazine; IV labetalol; and oral, rapidly acting nifedipine. In general, the findings of these studies suggest that either IV hydralazine or labetalol or oral nifedipine can be used to treat severe hypertension in pregnancy, as long as the provider is familiar with the dose to be used, the expected onset of action, and potential side effects (TABLE 3).
Women who develop generalized swelling or hemoconcentration (hematocrit ≥40%), or both, usually experience markedly reduced plasma volume. For this reason, these women will benefit from treatment with labetalol. If this is ineffective, then add IV hydralazine. However, delay administration of a rapidly acting vasodilator such as hydralazine to prevent an excessive hypotensive response and a secondary reduction in tissue perfusion and uteroplacental blood flow. Rather, administer a bolus infusion of 250 to 500 mL of isotonic saline before giving a vasodilator.
Additional details about the use of antihypertensive drugs are given in the section on other hypertensive emergencies below.
Step 6: Evaluate the patient for complications
Pulmonary edema can develop in patients with eclampsia or another hypertensive emergency. Suspect it if the patient has respiratory symptoms in association with tachypnea, tachycardia, or sustained oxygen saturation values below 93%, as well as when the patient exhibits basal rales during auscultation of the lungs. Treatment involves the administration of oxygen and IV furosemide (20–40 mg push), repeated as needed.
Some women with eclampsia may develop severe cerebral edema, hemorrhage, or both. The edema can be vasogenic or cytotoxic, leading to increased intracerebral pressure. Suspect edema or hemorrhage if the patient remains unresponsive, continues to experience convulsions despite therapy, or exhibits sensory or motor neurologic deficits. In such cases, neuroimaging is indicated, and the patient should be managed in consultation with neurology or neurosurgery.
Step 7: Begin the process of induction and delivery
Once the patient has been stabilized—and not before—initiate the induction process. Be aware that during and after the convulsion, changes in fetal heart rate (FHR) and uterine monitoring will usually be evident:
- prolonged deceleration or bradycardia (3–10 minutes)
- compensatory tachycardia, decreased beat-to-beat variability
- transient recurrent decelerations
- increased uterine tone and greater frequency of uterine activity.
These changes in FHR and uterine activity usually last 3 to 15 minutes. For this reason, it is important to avoid rushing the patient for cesarean delivery, as FHR and uterine activity are likely to return to normal after maternal resuscitation and stabilization. If not, consider other causes, such as abruptio placentae.
Eclampsia itself is not an indication for cesarean delivery. The selection of mode of delivery should be based on the presence or absence of labor, the cervical Bishop score, fetal gestational age, fetal presentation, and overall fetal condition.
Choosing an anesthetic
Regional analgesia/anesthesia is the method of choice for most women with eclampsia. However, regional anesthesia is to be avoided in the presence of disseminated intravascular coagulation or thrombocytopenia (the threshold platelet count is usually less than 75 x 103 µL. In such a case, IV analgesia can be used during labor, and general anesthesia may be appropriate for cesarean delivery. Both spinal and epidural analgesia and anesthesia are appropriate for women with eclampsia.
How to manage other hypertensive emergencies
A hypertensive emergency during pregnancy or postpartum involves acute-onset, persistent (>15 minutes), severe systolic BP (≥160 mm Hg) or severe diastolic BP (≥110 mm Hg), or both. The first step in such an emergency is to ensure the accurate measurement of BP using standard techniques.
Patients with acute-onset, persistent, severe BP should be hospitalized promptly for evaluation and treatment to prevent organ damage. Once such a patient is hospitalized, BP should be recorded every 15 minutes, with continuous FHR monitoring to ensure fetal viability.
Related article: Failure to diagnose preeclampsia and more (Medical Verdicts, February 2013)
The timing of initiation of antihypertensive medications, as well as determination of the type of medication best suited for the patient, should be based on:
- systolic and diastolic BP levels
- maternal clinical and laboratory findings
- presence of associated symptoms
- preexisting medical comorbidities
- whether the patient is antepartum or postpartum.
For example, a sustained BP level of 200/120 mm Hg requires therapy after 15 minutes, whereas observation may be suitable for as long as 60 minutes for a sustained BP of 160/72 mm Hg during labor.
Rapid reduction of systolic BP can lead to marked reductions in uteroplacental blood flow and a nonreassuring FHR tracing. Moreover, a rapid reduction of severe systolic BP in patients who have constricted plasma volume can reduce perfusion to the kidney, brain, and placenta. However, sustained BP of 165/100 mm Hg in association with central nervous system signs or symptoms, congestive heart failure, thrombocytopenia, or postpartum status requires therapy within 1 hour.
In general, it is difficult to obtain accurate BP recordings using noninvasive electronic instruments during labor because of the effects of labor on systolic BP and the lack of standardized methods for positioning of the arm cuff and the patient.
For these reasons, the decision about when to start acute antihypertensive therapy, based on systolic or diastolic BP, or both, should be individualized. And the choice of antihypertensive agent should be based on maternal clinical findings.
Choosing an antihypertensive agent
Because both hydralazine and nifedipine are associated with tachycardia, avoid them in patients with a heart rate above 110 bpm, using labetalol instead.10
In patients with bradycardia (heart rate <60 bpm), asthma, or congestive heart failure, however, labetalol should be avoided. In these populations, hydralazine or nifedipine is the drug of choice. Nifedipine is associated with improved renal blood flow and a resultant increase in urine output, making it preferable for patients with decreased urine output or severe postpartum hypertension.10
One theoretical concern is that the combined use of nifedipine and magnesium sulfate can cause excessive hypotension and neuromuscular blockage. As a result, some experts recommend that nifedipine be avoided in patients receiving magnesium sulfate. However, a recent review of this subject concluded that combined use of these drugs does not increase the risks of excessive hypotension and neuromuscular blockage in patients with severe hypertension or preeclampsia.
The initial dose of labetalol, when it is your chosen agent, is 20 mg IV, with BP measured 10 minutes later. If the target BP threshold is not achieved, administer 40 mg, 80 mg, and 80 mg at 10-minute intervals, as needed, again measuring BP 10 minutes after every dose. If, after a maximum dose of 240 mg, the desired BP threshold still has not been reached, give 5 to 10 mg IV hydralazine and measure BP 20 minutes later. If the target BP threshold still has not been achieved, it is essential to obtain consultation on the need for continuous infusion of labetalol, nicardipine, or sodium nitroprusside.
The initial dose of hydralazine, when it is your chosen agent, is 5 to 10 mg IV, with BP measured 20 minutes later. If needed, give another 10 mg and measure BP after another 20-minute interval. After a maximum dose of hydralazine 20 mg, switch to IV labetalol, using the regimen described above for labetalol, if the BP threshold still has not been achieved.
Nitroglycerin may be helpful in carefully selected patients
This drug is an arterial—but mostly venous—dilator. It is administered via IV infusion at an initial rate of 5 µg/min, with the rate gradually increased every 3 to 5 minutes (titrated to BP) to a maximum dose of 100 µg/min. It is the drug of choice in any hypertensive emergency associated with pulmonary edema and for control of hypertension associated with tracheal manipulation during intubation and extubation with general anesthesia.
Nitroglycerin is contraindicated in hypertensive encephalopathy because it increases cerebral blood flow and intracranial pressure. This drug should be administered only under the supervision of an experienced obstetric intensivist.
Sodium nitroprusside: Only in an ICU
This agent causes arterial and venous relaxation by interfering with the influx and intracellular activation of calcium. It is the drug of choice in hypertensive encephalopathy because it controls both afterload (vascular resistance) and preload (fluid status). It should be used only in the setting of intensive care.
The recommended dose is IV infusion at a rate of 0.25 to 5.00 µg/kg/min. Sodium nitroprusside has an immediate onset of action and may continue to exert an effect 3 to 5 minutes after discontinuation. Any hypotension caused by the drug should subside within minutes after discontinuation of the drip, due to the drug’s short half-life.
Nitroprusside is metabolized into thiocyanate and excreted in the urine. Cyanide can accumulate with large doses (>10 µg/kg/min) or prolonged administration (>48 hours), or if the patient has renal insufficiency or decreased hepatic metabolism. Signs of toxicity include anorexia, disorientation, headache, fatigue, restlessness, tinnitus, delirium, hallucinations, nausea, vomiting, and metabolic acidosis. When infused at a rate of less than 2 µg/kg/min, however, cyanide toxicity is unlikely.
As is the case with nitroglycerin, this drug should be administered only under the supervision of an experienced obstetric intensivist.
Case: Resolved
Upon arrival at the ED, the patient exhibits shallow, rapid breathing and foaming from the mouth. She is placed in a lateral decubitus position, an oral airway is established, and all secretions are suctioned. Oxygen is administered via face mask at a rate of 8 L/min. Her initial oxygen saturation level is 92%. IV access is secured, and a loading dose of magnesium sulfate 6 g is given over 20 minutes. Oxygen saturation increases to 94% to 96%. Auscultation of both lungs is normal.
The patient remains in a postictal state for about 15 minutes, but then orients to name, place, and time. FHR monitoring of both fetuses reveals a normal baseline with moderate variability, as well as variable decelerations in the presenting twin.
A maintenance dose of magnesium sulfate is initiated at a rate of 2 g/h, with the BP level recorded every 15 minutes. Systolic values remain between 170 and 180 mm Hg, and diastolic values between 108 and 112 mm Hg for 60 minutes. The obstetrician administers IV labetalol (20 mg) over 2 minutes. About 15 minutes later, the BP level is 154/100 mm Hg, with values remaining in the range of 150 to 156 mm Hg systolic and 92 to 104 mm Hg diastolic.
Ultrasonography reveals that the presenting twin is in a breech position, with estimated fetal weight below the 10th percentile and oligohydramnios. As a result, the obstetrician elects to proceed to cesarean delivery. The twins are delivered by cesarean section using spinal anesthesia. Although the infants are premature, there are no complications.Profile of an eclamptic seizure
Witnessing an eclamptic convulsion can be a frightening experience for nurses and medical providers. The convulsion usually lasts 60 to 90 seconds and occurs in two phases:
- Phase 1 (15–25 seconds) involves facial twitching, rolling of the eyes, and stiffening of the body, with generalized muscular contractions.
- Phase 2 (20–50 seconds) involves alternate contraction and relaxation of the muscles of the body in rapid succession, starting in the face and spreading throughout the body. Foaming at the mouth also occurs, and the patient may bite her tongue if it isn’t protected.
Apnea develops during and immediately after the convulsion, lasting about 120 seconds. A period of hyperventilation follows to compensate for the respiratory acidosis during the apneic period.
A postictal state follows the convulsion, and the patient usually remembers nothing of the episode. Some patients also become restless, combative, and agitated, requiring sedation. Aspiration is possible during or after the convulsion.
We want to hear from you! Tell us what you think.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
- Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):182–192.
- Meyer NL, Mercer BM, Friedman SA, Sibai BM. Urinary dipstick protein: a poor predictor of absent or severe proteinuria. Am J Obstet Gynecol. 1994;170(1 Pt 1):137–141.
- Knight M; UK Obstetric Surveillance System (UKOSS). Eclampsia in the United Kingdom 2005. BJOG. 2007;114(9):1072–1078.
- ACOG Practice Bulletin #33: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167.
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia–eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e1–e7.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–475.
- ACOG Committee Opinion #514: Emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. Obstet Gynecol. 2011;118:1465–1468.
- Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987–994.
- Raheem IA, Saaid R, Omar Sz, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomized trial. BJOG. 2012;119(1):78–85.
CASE: MISSED PREECLAMPSIA
At her first prenatal visit at 14 weeks’ gestation, a 41-year-old woman (G2P1) presents with a dichorionic twin gestation, blood pressure (BP) of 105/68 mm Hg, and a body mass index (BMI) of 40 kg/m2. The pregnancy was achieved through in vitro fertilization. Ten years earlier, the patient’s first pregnancy was complicated by preeclampsia, requiring preterm delivery at 33 weeks’ gestation.
By 28 weeks’ gestation, the patient has gained 26 lb. Her BP is 120/70 mm Hg, with no proteinuria detected by urine dipstick. By 30 weeks, she has gained an additional 8 lb, her BP is 142/84 mm Hg, and no proteinuria is detected. At 32 weeks, her BP is 140/92 mm Hg, she has gained another 8 lb, and no proteinuria is present. She also reports new-onset headaches that do not respond to over-the-counter analgesics. She is sent to the obstetric triage area for BP monitoring, blood testing for preeclampsia and nonstress test fetal monitoring.
During the 2-hour observation period, the patient continues to report headaches, and swelling of her face and hands is present. Her systolic BP values range from 132 to 152 mm Hg, and diastolic values range from 80 to 96 mm Hg. No proteinuria is detected, blood testing results for preeclampsia (complete blood count, liver enzymes, serum creatinine, and uric acid) are normal, and the nonstress tests are reactive in both fetuses.
The patient is given a diagnosis of gestational hypertension, along with a prescription for oral labetalol 200 mg daily and two tablets of acetaminophen with codeine for the headaches (to be taken every 6 hours as needed). She is sent home with instructions to return to her physician’s office in 1 week.
Two days later, she wakes in the middle of the night with a severe headache, blurred vision, and vomiting. Her husband calls the obstetrician’s answering service and is instructed to call 911 immediately. While waiting for an ambulance, the patient experiences a grand mal eclamptic convulsion. A second convulsion occurs during her transfer to the ED.
This scenario could have been avoided.
The obstetrician in this case was negligent for failing to recognize preeclampsia in a patient who had two clear risk factors for it: multifetal gestation and a history of early-onset (<37 weeks) preeclampsia in an earlier pregnancy (other risk factors are listed in TABLE 1).
As a result, the patient developed eclampsia, a serious condition that can lead to grave maternal complications (TABLE 2), including death. It also can cause fetal complications, including growth restriction, hypoxia, acidosis, preterm birth, long-term developmental deficits, and death.1,2
The obstetrician in this case also overlooked published evidence indicating that, in the setting of hypertension and headaches, as many as 20% to 30% of pregnant women whose tests for proteinuria show a negative or trace result via dipstick will develop eclampsia.3 Instead of initiating outpatient administration of oral antihypertensive agents, the obstetrician should have hospitalized this patient for at least 48 hours, with steroid administration, to determine whether outpatient management was feasible.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia Baha Sibai, MD (November 2011)
Defining eclampsia
Eclampsia is marked by the onset of convulsions (during pregnancy or postpartum) in association with gestational hypertension alone, proteinuria, preeclampsia, or superimposed preeclampsia. Although it is rare, eclampsia is potentially life-threatening. For that reason, obstetricians, anesthesiologists, ED physicians, neurologists, and critical-care physicians should be well versed in its diagnosis and management. In this article, I focus on management.
A few preliminary points
Eclampsia can develop any time during the antenatal period (>16 weeks’ gestation), during labor and delivery, and as long as 6 weeks after delivery. Therefore, we should be vigilant for preeclampsia whenever a pregnant patient visits our office, as well as when she makes unscheduled visits to the ED or obstetric triage area or is hospitalized.
Early recognition of women at high risk for preeclampsia and eclampsia may allow for prompt intervention, including early hospitalization for close observation prior to delivery and postpartum.1,2,4–10
Hospitalization of high-risk women allows for use of antihypertensive agents to treat severe BP, administration of magnesium sulfate to prevent convulsions, and timely delivery of the infant. It also allows for intensive maternal support during and after an eclamptic seizure.
Hospitalization is essential for women who exhibit features that suggest severe disease. More specifically, the presence of gestational hypertension with any of the following features is an indication for immediate hospitalization for evaluation and management:
- persistent severe hypertension (systolic
BP ≥160 mm Hg or diastolic BP ≥110 mm Hg) for at least 1 hour - gestational hypertension requiring oral antihypertensive therapy
- progressive and excessive weight gain (≥20 lb prior to 28 weeks’ gestation)
- generalized swelling (edema of hands or face)
- new-onset or persistent headaches despite analgesics
- persistent visual changes (blurred vision, scotomata, photophobia, double vision)
- shortness of breath, dyspnea, orthopnea, or tightness in the chest
- persistent retrosternal chest pain, severe epigastric or right upper quadrant pain
- persistent nausea, vomiting, malaise
- altered mental state, confusion, numbness, tingling, or motor weakness
- platelet count below 100 3 103 µL
- aspartate aminotransferase (AST), alanine aminotransferase (ALT), or lactic acid dehydrogenase (LDH) levels more than twice the upper limit of normal
- serum creatinine level >1.1 mg/dL
- suspected abruptio placentae.
A stepwise approach to eclampsia
Eclampsia is an obstetric emergency. Inadequate preparation for it or an inappropriate response to maternal and fetal conditions during and after an eclamptic convulsion can be detrimental to the mother and fetus. All obstetric units should have up-to-date protocols in place and should conduct mandatory drills to prepare nursing staff, obstetric providers, and anesthesia staff working in these units to manage eclampsia.
Step 1: Let the seizure run its course
During a seizure, resist the impulse to administer anticonvulsive drugs, including intravenous (IV) magnesium sulfate, because most eclamptic convulsions are self-limiting. Also abstain from administering medications such as IV phenytoin, diazepam, or midazolam, as these drugs are less effective than magnesium sulfate, and some can suppress the laryngeal reflex, increasing the risk of aspiration.
If the patient develops status epilepticus, initiate muscle paralysis and intubate her.
Step 2: Support the maternal condition
It is vital to support maternal respiratory and cardiovascular functions to prevent hypoxia, acidosis, and cardiorespiratory arrest.
Begin by establishing airway patency and maternal oxygenation during and after the convulsion. Administer oxygen via a face mask, with or without a reservoir, at a rate of 8 to 10 L/min.
During the apneic period (see “Profile of an eclamptic seizure” on page 46), the patient will develop hypoxia. Use pulse oximetry to monitor oxygen saturation, with the goal of keeping it above 94%. Arterial blood gas analysis is required if oxygen saturation remains below 92% or if pulmonary edema or aspiration is suspected.
If the patient develops recurrent seizures, status epilepticus, florid alveolar pulmonary edema, or respiratory arrest, intubate her immediately.
Step 3: Prevent maternal injury and aspiration
Secure the side rails of the patient’s bed by elevating them to prevent a fall, and make sure they are padded to prevent trauma during convulsions and afterward, when some women become combative and agitated. Position the patient in a lateral decubitus position to minimize aspiration of oral secretions. If any secretions or vomitus are present, remove them via suction.
Step 4: After the convulsion, give magnesium sulfate
Magnesium is the drug of choice for seizure prophylaxis in women with preeclampsia and severe symptoms, and to prevent recurrent seizures in women with eclampsia.
In the latter group, once the eclamptic convulsion has ended, give a loading dose of IV magnesium (6 g/100 mL over 20 minutes), followed by a continuous infusion of 2 g/h for at least 24 hours. If the patient develops a second seizure during the maintenance infusion, administer another bolus of magnesium (2 g/100 mL over 3–5 minutes).
Step 5: Treat severe hypertension
If severe hypertension persists for 60 minutes or longer, it can lead to injury of the brain, heart, and kidneys. To avoid these complications, it is essential to reduce BP to a safe range and maintain that level without compromising cerebral perfusion pressure and uteroplacental blood flow (which already may be reduced in some patients).
The goal of antihypertensive therapy is to keep systolic BP between 140 and 155 mm Hg and diastolic values between 90 and 105 mm Hg.9 Several agents are available for the treatment of severe hypertension during pregnancy and postpartum. The most commonly used IV medications for this purpose are labetalol and hydralazine. Another option is oral, rapidly acting
nifedipine.
Several randomized trials have compared efficacy and side effects between IV bolus injections of hydralazine; IV labetalol; and oral, rapidly acting nifedipine. In general, the findings of these studies suggest that either IV hydralazine or labetalol or oral nifedipine can be used to treat severe hypertension in pregnancy, as long as the provider is familiar with the dose to be used, the expected onset of action, and potential side effects (TABLE 3).
Women who develop generalized swelling or hemoconcentration (hematocrit ≥40%), or both, usually experience markedly reduced plasma volume. For this reason, these women will benefit from treatment with labetalol. If this is ineffective, then add IV hydralazine. However, delay administration of a rapidly acting vasodilator such as hydralazine to prevent an excessive hypotensive response and a secondary reduction in tissue perfusion and uteroplacental blood flow. Rather, administer a bolus infusion of 250 to 500 mL of isotonic saline before giving a vasodilator.
Additional details about the use of antihypertensive drugs are given in the section on other hypertensive emergencies below.
Step 6: Evaluate the patient for complications
Pulmonary edema can develop in patients with eclampsia or another hypertensive emergency. Suspect it if the patient has respiratory symptoms in association with tachypnea, tachycardia, or sustained oxygen saturation values below 93%, as well as when the patient exhibits basal rales during auscultation of the lungs. Treatment involves the administration of oxygen and IV furosemide (20–40 mg push), repeated as needed.
Some women with eclampsia may develop severe cerebral edema, hemorrhage, or both. The edema can be vasogenic or cytotoxic, leading to increased intracerebral pressure. Suspect edema or hemorrhage if the patient remains unresponsive, continues to experience convulsions despite therapy, or exhibits sensory or motor neurologic deficits. In such cases, neuroimaging is indicated, and the patient should be managed in consultation with neurology or neurosurgery.
Step 7: Begin the process of induction and delivery
Once the patient has been stabilized—and not before—initiate the induction process. Be aware that during and after the convulsion, changes in fetal heart rate (FHR) and uterine monitoring will usually be evident:
- prolonged deceleration or bradycardia (3–10 minutes)
- compensatory tachycardia, decreased beat-to-beat variability
- transient recurrent decelerations
- increased uterine tone and greater frequency of uterine activity.
These changes in FHR and uterine activity usually last 3 to 15 minutes. For this reason, it is important to avoid rushing the patient for cesarean delivery, as FHR and uterine activity are likely to return to normal after maternal resuscitation and stabilization. If not, consider other causes, such as abruptio placentae.
Eclampsia itself is not an indication for cesarean delivery. The selection of mode of delivery should be based on the presence or absence of labor, the cervical Bishop score, fetal gestational age, fetal presentation, and overall fetal condition.
Choosing an anesthetic
Regional analgesia/anesthesia is the method of choice for most women with eclampsia. However, regional anesthesia is to be avoided in the presence of disseminated intravascular coagulation or thrombocytopenia (the threshold platelet count is usually less than 75 x 103 µL. In such a case, IV analgesia can be used during labor, and general anesthesia may be appropriate for cesarean delivery. Both spinal and epidural analgesia and anesthesia are appropriate for women with eclampsia.
How to manage other hypertensive emergencies
A hypertensive emergency during pregnancy or postpartum involves acute-onset, persistent (>15 minutes), severe systolic BP (≥160 mm Hg) or severe diastolic BP (≥110 mm Hg), or both. The first step in such an emergency is to ensure the accurate measurement of BP using standard techniques.
Patients with acute-onset, persistent, severe BP should be hospitalized promptly for evaluation and treatment to prevent organ damage. Once such a patient is hospitalized, BP should be recorded every 15 minutes, with continuous FHR monitoring to ensure fetal viability.
Related article: Failure to diagnose preeclampsia and more (Medical Verdicts, February 2013)
The timing of initiation of antihypertensive medications, as well as determination of the type of medication best suited for the patient, should be based on:
- systolic and diastolic BP levels
- maternal clinical and laboratory findings
- presence of associated symptoms
- preexisting medical comorbidities
- whether the patient is antepartum or postpartum.
For example, a sustained BP level of 200/120 mm Hg requires therapy after 15 minutes, whereas observation may be suitable for as long as 60 minutes for a sustained BP of 160/72 mm Hg during labor.
Rapid reduction of systolic BP can lead to marked reductions in uteroplacental blood flow and a nonreassuring FHR tracing. Moreover, a rapid reduction of severe systolic BP in patients who have constricted plasma volume can reduce perfusion to the kidney, brain, and placenta. However, sustained BP of 165/100 mm Hg in association with central nervous system signs or symptoms, congestive heart failure, thrombocytopenia, or postpartum status requires therapy within 1 hour.
In general, it is difficult to obtain accurate BP recordings using noninvasive electronic instruments during labor because of the effects of labor on systolic BP and the lack of standardized methods for positioning of the arm cuff and the patient.
For these reasons, the decision about when to start acute antihypertensive therapy, based on systolic or diastolic BP, or both, should be individualized. And the choice of antihypertensive agent should be based on maternal clinical findings.
Choosing an antihypertensive agent
Because both hydralazine and nifedipine are associated with tachycardia, avoid them in patients with a heart rate above 110 bpm, using labetalol instead.10
In patients with bradycardia (heart rate <60 bpm), asthma, or congestive heart failure, however, labetalol should be avoided. In these populations, hydralazine or nifedipine is the drug of choice. Nifedipine is associated with improved renal blood flow and a resultant increase in urine output, making it preferable for patients with decreased urine output or severe postpartum hypertension.10
One theoretical concern is that the combined use of nifedipine and magnesium sulfate can cause excessive hypotension and neuromuscular blockage. As a result, some experts recommend that nifedipine be avoided in patients receiving magnesium sulfate. However, a recent review of this subject concluded that combined use of these drugs does not increase the risks of excessive hypotension and neuromuscular blockage in patients with severe hypertension or preeclampsia.
The initial dose of labetalol, when it is your chosen agent, is 20 mg IV, with BP measured 10 minutes later. If the target BP threshold is not achieved, administer 40 mg, 80 mg, and 80 mg at 10-minute intervals, as needed, again measuring BP 10 minutes after every dose. If, after a maximum dose of 240 mg, the desired BP threshold still has not been reached, give 5 to 10 mg IV hydralazine and measure BP 20 minutes later. If the target BP threshold still has not been achieved, it is essential to obtain consultation on the need for continuous infusion of labetalol, nicardipine, or sodium nitroprusside.
The initial dose of hydralazine, when it is your chosen agent, is 5 to 10 mg IV, with BP measured 20 minutes later. If needed, give another 10 mg and measure BP after another 20-minute interval. After a maximum dose of hydralazine 20 mg, switch to IV labetalol, using the regimen described above for labetalol, if the BP threshold still has not been achieved.
Nitroglycerin may be helpful in carefully selected patients
This drug is an arterial—but mostly venous—dilator. It is administered via IV infusion at an initial rate of 5 µg/min, with the rate gradually increased every 3 to 5 minutes (titrated to BP) to a maximum dose of 100 µg/min. It is the drug of choice in any hypertensive emergency associated with pulmonary edema and for control of hypertension associated with tracheal manipulation during intubation and extubation with general anesthesia.
Nitroglycerin is contraindicated in hypertensive encephalopathy because it increases cerebral blood flow and intracranial pressure. This drug should be administered only under the supervision of an experienced obstetric intensivist.
Sodium nitroprusside: Only in an ICU
This agent causes arterial and venous relaxation by interfering with the influx and intracellular activation of calcium. It is the drug of choice in hypertensive encephalopathy because it controls both afterload (vascular resistance) and preload (fluid status). It should be used only in the setting of intensive care.
The recommended dose is IV infusion at a rate of 0.25 to 5.00 µg/kg/min. Sodium nitroprusside has an immediate onset of action and may continue to exert an effect 3 to 5 minutes after discontinuation. Any hypotension caused by the drug should subside within minutes after discontinuation of the drip, due to the drug’s short half-life.
Nitroprusside is metabolized into thiocyanate and excreted in the urine. Cyanide can accumulate with large doses (>10 µg/kg/min) or prolonged administration (>48 hours), or if the patient has renal insufficiency or decreased hepatic metabolism. Signs of toxicity include anorexia, disorientation, headache, fatigue, restlessness, tinnitus, delirium, hallucinations, nausea, vomiting, and metabolic acidosis. When infused at a rate of less than 2 µg/kg/min, however, cyanide toxicity is unlikely.
As is the case with nitroglycerin, this drug should be administered only under the supervision of an experienced obstetric intensivist.
Case: Resolved
Upon arrival at the ED, the patient exhibits shallow, rapid breathing and foaming from the mouth. She is placed in a lateral decubitus position, an oral airway is established, and all secretions are suctioned. Oxygen is administered via face mask at a rate of 8 L/min. Her initial oxygen saturation level is 92%. IV access is secured, and a loading dose of magnesium sulfate 6 g is given over 20 minutes. Oxygen saturation increases to 94% to 96%. Auscultation of both lungs is normal.
The patient remains in a postictal state for about 15 minutes, but then orients to name, place, and time. FHR monitoring of both fetuses reveals a normal baseline with moderate variability, as well as variable decelerations in the presenting twin.
A maintenance dose of magnesium sulfate is initiated at a rate of 2 g/h, with the BP level recorded every 15 minutes. Systolic values remain between 170 and 180 mm Hg, and diastolic values between 108 and 112 mm Hg for 60 minutes. The obstetrician administers IV labetalol (20 mg) over 2 minutes. About 15 minutes later, the BP level is 154/100 mm Hg, with values remaining in the range of 150 to 156 mm Hg systolic and 92 to 104 mm Hg diastolic.
Ultrasonography reveals that the presenting twin is in a breech position, with estimated fetal weight below the 10th percentile and oligohydramnios. As a result, the obstetrician elects to proceed to cesarean delivery. The twins are delivered by cesarean section using spinal anesthesia. Although the infants are premature, there are no complications.Profile of an eclamptic seizure
Witnessing an eclamptic convulsion can be a frightening experience for nurses and medical providers. The convulsion usually lasts 60 to 90 seconds and occurs in two phases:
- Phase 1 (15–25 seconds) involves facial twitching, rolling of the eyes, and stiffening of the body, with generalized muscular contractions.
- Phase 2 (20–50 seconds) involves alternate contraction and relaxation of the muscles of the body in rapid succession, starting in the face and spreading throughout the body. Foaming at the mouth also occurs, and the patient may bite her tongue if it isn’t protected.
Apnea develops during and immediately after the convulsion, lasting about 120 seconds. A period of hyperventilation follows to compensate for the respiratory acidosis during the apneic period.
A postictal state follows the convulsion, and the patient usually remembers nothing of the episode. Some patients also become restless, combative, and agitated, requiring sedation. Aspiration is possible during or after the convulsion.
We want to hear from you! Tell us what you think.
CASE: MISSED PREECLAMPSIA
At her first prenatal visit at 14 weeks’ gestation, a 41-year-old woman (G2P1) presents with a dichorionic twin gestation, blood pressure (BP) of 105/68 mm Hg, and a body mass index (BMI) of 40 kg/m2. The pregnancy was achieved through in vitro fertilization. Ten years earlier, the patient’s first pregnancy was complicated by preeclampsia, requiring preterm delivery at 33 weeks’ gestation.
By 28 weeks’ gestation, the patient has gained 26 lb. Her BP is 120/70 mm Hg, with no proteinuria detected by urine dipstick. By 30 weeks, she has gained an additional 8 lb, her BP is 142/84 mm Hg, and no proteinuria is detected. At 32 weeks, her BP is 140/92 mm Hg, she has gained another 8 lb, and no proteinuria is present. She also reports new-onset headaches that do not respond to over-the-counter analgesics. She is sent to the obstetric triage area for BP monitoring, blood testing for preeclampsia and nonstress test fetal monitoring.
During the 2-hour observation period, the patient continues to report headaches, and swelling of her face and hands is present. Her systolic BP values range from 132 to 152 mm Hg, and diastolic values range from 80 to 96 mm Hg. No proteinuria is detected, blood testing results for preeclampsia (complete blood count, liver enzymes, serum creatinine, and uric acid) are normal, and the nonstress tests are reactive in both fetuses.
The patient is given a diagnosis of gestational hypertension, along with a prescription for oral labetalol 200 mg daily and two tablets of acetaminophen with codeine for the headaches (to be taken every 6 hours as needed). She is sent home with instructions to return to her physician’s office in 1 week.
Two days later, she wakes in the middle of the night with a severe headache, blurred vision, and vomiting. Her husband calls the obstetrician’s answering service and is instructed to call 911 immediately. While waiting for an ambulance, the patient experiences a grand mal eclamptic convulsion. A second convulsion occurs during her transfer to the ED.
This scenario could have been avoided.
The obstetrician in this case was negligent for failing to recognize preeclampsia in a patient who had two clear risk factors for it: multifetal gestation and a history of early-onset (<37 weeks) preeclampsia in an earlier pregnancy (other risk factors are listed in TABLE 1).
As a result, the patient developed eclampsia, a serious condition that can lead to grave maternal complications (TABLE 2), including death. It also can cause fetal complications, including growth restriction, hypoxia, acidosis, preterm birth, long-term developmental deficits, and death.1,2
The obstetrician in this case also overlooked published evidence indicating that, in the setting of hypertension and headaches, as many as 20% to 30% of pregnant women whose tests for proteinuria show a negative or trace result via dipstick will develop eclampsia.3 Instead of initiating outpatient administration of oral antihypertensive agents, the obstetrician should have hospitalized this patient for at least 48 hours, with steroid administration, to determine whether outpatient management was feasible.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia Baha Sibai, MD (November 2011)
Defining eclampsia
Eclampsia is marked by the onset of convulsions (during pregnancy or postpartum) in association with gestational hypertension alone, proteinuria, preeclampsia, or superimposed preeclampsia. Although it is rare, eclampsia is potentially life-threatening. For that reason, obstetricians, anesthesiologists, ED physicians, neurologists, and critical-care physicians should be well versed in its diagnosis and management. In this article, I focus on management.
A few preliminary points
Eclampsia can develop any time during the antenatal period (>16 weeks’ gestation), during labor and delivery, and as long as 6 weeks after delivery. Therefore, we should be vigilant for preeclampsia whenever a pregnant patient visits our office, as well as when she makes unscheduled visits to the ED or obstetric triage area or is hospitalized.
Early recognition of women at high risk for preeclampsia and eclampsia may allow for prompt intervention, including early hospitalization for close observation prior to delivery and postpartum.1,2,4–10
Hospitalization of high-risk women allows for use of antihypertensive agents to treat severe BP, administration of magnesium sulfate to prevent convulsions, and timely delivery of the infant. It also allows for intensive maternal support during and after an eclamptic seizure.
Hospitalization is essential for women who exhibit features that suggest severe disease. More specifically, the presence of gestational hypertension with any of the following features is an indication for immediate hospitalization for evaluation and management:
- persistent severe hypertension (systolic
BP ≥160 mm Hg or diastolic BP ≥110 mm Hg) for at least 1 hour - gestational hypertension requiring oral antihypertensive therapy
- progressive and excessive weight gain (≥20 lb prior to 28 weeks’ gestation)
- generalized swelling (edema of hands or face)
- new-onset or persistent headaches despite analgesics
- persistent visual changes (blurred vision, scotomata, photophobia, double vision)
- shortness of breath, dyspnea, orthopnea, or tightness in the chest
- persistent retrosternal chest pain, severe epigastric or right upper quadrant pain
- persistent nausea, vomiting, malaise
- altered mental state, confusion, numbness, tingling, or motor weakness
- platelet count below 100 3 103 µL
- aspartate aminotransferase (AST), alanine aminotransferase (ALT), or lactic acid dehydrogenase (LDH) levels more than twice the upper limit of normal
- serum creatinine level >1.1 mg/dL
- suspected abruptio placentae.
A stepwise approach to eclampsia
Eclampsia is an obstetric emergency. Inadequate preparation for it or an inappropriate response to maternal and fetal conditions during and after an eclamptic convulsion can be detrimental to the mother and fetus. All obstetric units should have up-to-date protocols in place and should conduct mandatory drills to prepare nursing staff, obstetric providers, and anesthesia staff working in these units to manage eclampsia.
Step 1: Let the seizure run its course
During a seizure, resist the impulse to administer anticonvulsive drugs, including intravenous (IV) magnesium sulfate, because most eclamptic convulsions are self-limiting. Also abstain from administering medications such as IV phenytoin, diazepam, or midazolam, as these drugs are less effective than magnesium sulfate, and some can suppress the laryngeal reflex, increasing the risk of aspiration.
If the patient develops status epilepticus, initiate muscle paralysis and intubate her.
Step 2: Support the maternal condition
It is vital to support maternal respiratory and cardiovascular functions to prevent hypoxia, acidosis, and cardiorespiratory arrest.
Begin by establishing airway patency and maternal oxygenation during and after the convulsion. Administer oxygen via a face mask, with or without a reservoir, at a rate of 8 to 10 L/min.
During the apneic period (see “Profile of an eclamptic seizure” on page 46), the patient will develop hypoxia. Use pulse oximetry to monitor oxygen saturation, with the goal of keeping it above 94%. Arterial blood gas analysis is required if oxygen saturation remains below 92% or if pulmonary edema or aspiration is suspected.
If the patient develops recurrent seizures, status epilepticus, florid alveolar pulmonary edema, or respiratory arrest, intubate her immediately.
Step 3: Prevent maternal injury and aspiration
Secure the side rails of the patient’s bed by elevating them to prevent a fall, and make sure they are padded to prevent trauma during convulsions and afterward, when some women become combative and agitated. Position the patient in a lateral decubitus position to minimize aspiration of oral secretions. If any secretions or vomitus are present, remove them via suction.
Step 4: After the convulsion, give magnesium sulfate
Magnesium is the drug of choice for seizure prophylaxis in women with preeclampsia and severe symptoms, and to prevent recurrent seizures in women with eclampsia.
In the latter group, once the eclamptic convulsion has ended, give a loading dose of IV magnesium (6 g/100 mL over 20 minutes), followed by a continuous infusion of 2 g/h for at least 24 hours. If the patient develops a second seizure during the maintenance infusion, administer another bolus of magnesium (2 g/100 mL over 3–5 minutes).
Step 5: Treat severe hypertension
If severe hypertension persists for 60 minutes or longer, it can lead to injury of the brain, heart, and kidneys. To avoid these complications, it is essential to reduce BP to a safe range and maintain that level without compromising cerebral perfusion pressure and uteroplacental blood flow (which already may be reduced in some patients).
The goal of antihypertensive therapy is to keep systolic BP between 140 and 155 mm Hg and diastolic values between 90 and 105 mm Hg.9 Several agents are available for the treatment of severe hypertension during pregnancy and postpartum. The most commonly used IV medications for this purpose are labetalol and hydralazine. Another option is oral, rapidly acting
nifedipine.
Several randomized trials have compared efficacy and side effects between IV bolus injections of hydralazine; IV labetalol; and oral, rapidly acting nifedipine. In general, the findings of these studies suggest that either IV hydralazine or labetalol or oral nifedipine can be used to treat severe hypertension in pregnancy, as long as the provider is familiar with the dose to be used, the expected onset of action, and potential side effects (TABLE 3).
Women who develop generalized swelling or hemoconcentration (hematocrit ≥40%), or both, usually experience markedly reduced plasma volume. For this reason, these women will benefit from treatment with labetalol. If this is ineffective, then add IV hydralazine. However, delay administration of a rapidly acting vasodilator such as hydralazine to prevent an excessive hypotensive response and a secondary reduction in tissue perfusion and uteroplacental blood flow. Rather, administer a bolus infusion of 250 to 500 mL of isotonic saline before giving a vasodilator.
Additional details about the use of antihypertensive drugs are given in the section on other hypertensive emergencies below.
Step 6: Evaluate the patient for complications
Pulmonary edema can develop in patients with eclampsia or another hypertensive emergency. Suspect it if the patient has respiratory symptoms in association with tachypnea, tachycardia, or sustained oxygen saturation values below 93%, as well as when the patient exhibits basal rales during auscultation of the lungs. Treatment involves the administration of oxygen and IV furosemide (20–40 mg push), repeated as needed.
Some women with eclampsia may develop severe cerebral edema, hemorrhage, or both. The edema can be vasogenic or cytotoxic, leading to increased intracerebral pressure. Suspect edema or hemorrhage if the patient remains unresponsive, continues to experience convulsions despite therapy, or exhibits sensory or motor neurologic deficits. In such cases, neuroimaging is indicated, and the patient should be managed in consultation with neurology or neurosurgery.
Step 7: Begin the process of induction and delivery
Once the patient has been stabilized—and not before—initiate the induction process. Be aware that during and after the convulsion, changes in fetal heart rate (FHR) and uterine monitoring will usually be evident:
- prolonged deceleration or bradycardia (3–10 minutes)
- compensatory tachycardia, decreased beat-to-beat variability
- transient recurrent decelerations
- increased uterine tone and greater frequency of uterine activity.
These changes in FHR and uterine activity usually last 3 to 15 minutes. For this reason, it is important to avoid rushing the patient for cesarean delivery, as FHR and uterine activity are likely to return to normal after maternal resuscitation and stabilization. If not, consider other causes, such as abruptio placentae.
Eclampsia itself is not an indication for cesarean delivery. The selection of mode of delivery should be based on the presence or absence of labor, the cervical Bishop score, fetal gestational age, fetal presentation, and overall fetal condition.
Choosing an anesthetic
Regional analgesia/anesthesia is the method of choice for most women with eclampsia. However, regional anesthesia is to be avoided in the presence of disseminated intravascular coagulation or thrombocytopenia (the threshold platelet count is usually less than 75 x 103 µL. In such a case, IV analgesia can be used during labor, and general anesthesia may be appropriate for cesarean delivery. Both spinal and epidural analgesia and anesthesia are appropriate for women with eclampsia.
How to manage other hypertensive emergencies
A hypertensive emergency during pregnancy or postpartum involves acute-onset, persistent (>15 minutes), severe systolic BP (≥160 mm Hg) or severe diastolic BP (≥110 mm Hg), or both. The first step in such an emergency is to ensure the accurate measurement of BP using standard techniques.
Patients with acute-onset, persistent, severe BP should be hospitalized promptly for evaluation and treatment to prevent organ damage. Once such a patient is hospitalized, BP should be recorded every 15 minutes, with continuous FHR monitoring to ensure fetal viability.
Related article: Failure to diagnose preeclampsia and more (Medical Verdicts, February 2013)
The timing of initiation of antihypertensive medications, as well as determination of the type of medication best suited for the patient, should be based on:
- systolic and diastolic BP levels
- maternal clinical and laboratory findings
- presence of associated symptoms
- preexisting medical comorbidities
- whether the patient is antepartum or postpartum.
For example, a sustained BP level of 200/120 mm Hg requires therapy after 15 minutes, whereas observation may be suitable for as long as 60 minutes for a sustained BP of 160/72 mm Hg during labor.
Rapid reduction of systolic BP can lead to marked reductions in uteroplacental blood flow and a nonreassuring FHR tracing. Moreover, a rapid reduction of severe systolic BP in patients who have constricted plasma volume can reduce perfusion to the kidney, brain, and placenta. However, sustained BP of 165/100 mm Hg in association with central nervous system signs or symptoms, congestive heart failure, thrombocytopenia, or postpartum status requires therapy within 1 hour.
In general, it is difficult to obtain accurate BP recordings using noninvasive electronic instruments during labor because of the effects of labor on systolic BP and the lack of standardized methods for positioning of the arm cuff and the patient.
For these reasons, the decision about when to start acute antihypertensive therapy, based on systolic or diastolic BP, or both, should be individualized. And the choice of antihypertensive agent should be based on maternal clinical findings.
Choosing an antihypertensive agent
Because both hydralazine and nifedipine are associated with tachycardia, avoid them in patients with a heart rate above 110 bpm, using labetalol instead.10
In patients with bradycardia (heart rate <60 bpm), asthma, or congestive heart failure, however, labetalol should be avoided. In these populations, hydralazine or nifedipine is the drug of choice. Nifedipine is associated with improved renal blood flow and a resultant increase in urine output, making it preferable for patients with decreased urine output or severe postpartum hypertension.10
One theoretical concern is that the combined use of nifedipine and magnesium sulfate can cause excessive hypotension and neuromuscular blockage. As a result, some experts recommend that nifedipine be avoided in patients receiving magnesium sulfate. However, a recent review of this subject concluded that combined use of these drugs does not increase the risks of excessive hypotension and neuromuscular blockage in patients with severe hypertension or preeclampsia.
The initial dose of labetalol, when it is your chosen agent, is 20 mg IV, with BP measured 10 minutes later. If the target BP threshold is not achieved, administer 40 mg, 80 mg, and 80 mg at 10-minute intervals, as needed, again measuring BP 10 minutes after every dose. If, after a maximum dose of 240 mg, the desired BP threshold still has not been reached, give 5 to 10 mg IV hydralazine and measure BP 20 minutes later. If the target BP threshold still has not been achieved, it is essential to obtain consultation on the need for continuous infusion of labetalol, nicardipine, or sodium nitroprusside.
The initial dose of hydralazine, when it is your chosen agent, is 5 to 10 mg IV, with BP measured 20 minutes later. If needed, give another 10 mg and measure BP after another 20-minute interval. After a maximum dose of hydralazine 20 mg, switch to IV labetalol, using the regimen described above for labetalol, if the BP threshold still has not been achieved.
Nitroglycerin may be helpful in carefully selected patients
This drug is an arterial—but mostly venous—dilator. It is administered via IV infusion at an initial rate of 5 µg/min, with the rate gradually increased every 3 to 5 minutes (titrated to BP) to a maximum dose of 100 µg/min. It is the drug of choice in any hypertensive emergency associated with pulmonary edema and for control of hypertension associated with tracheal manipulation during intubation and extubation with general anesthesia.
Nitroglycerin is contraindicated in hypertensive encephalopathy because it increases cerebral blood flow and intracranial pressure. This drug should be administered only under the supervision of an experienced obstetric intensivist.
Sodium nitroprusside: Only in an ICU
This agent causes arterial and venous relaxation by interfering with the influx and intracellular activation of calcium. It is the drug of choice in hypertensive encephalopathy because it controls both afterload (vascular resistance) and preload (fluid status). It should be used only in the setting of intensive care.
The recommended dose is IV infusion at a rate of 0.25 to 5.00 µg/kg/min. Sodium nitroprusside has an immediate onset of action and may continue to exert an effect 3 to 5 minutes after discontinuation. Any hypotension caused by the drug should subside within minutes after discontinuation of the drip, due to the drug’s short half-life.
Nitroprusside is metabolized into thiocyanate and excreted in the urine. Cyanide can accumulate with large doses (>10 µg/kg/min) or prolonged administration (>48 hours), or if the patient has renal insufficiency or decreased hepatic metabolism. Signs of toxicity include anorexia, disorientation, headache, fatigue, restlessness, tinnitus, delirium, hallucinations, nausea, vomiting, and metabolic acidosis. When infused at a rate of less than 2 µg/kg/min, however, cyanide toxicity is unlikely.
As is the case with nitroglycerin, this drug should be administered only under the supervision of an experienced obstetric intensivist.
Case: Resolved
Upon arrival at the ED, the patient exhibits shallow, rapid breathing and foaming from the mouth. She is placed in a lateral decubitus position, an oral airway is established, and all secretions are suctioned. Oxygen is administered via face mask at a rate of 8 L/min. Her initial oxygen saturation level is 92%. IV access is secured, and a loading dose of magnesium sulfate 6 g is given over 20 minutes. Oxygen saturation increases to 94% to 96%. Auscultation of both lungs is normal.
The patient remains in a postictal state for about 15 minutes, but then orients to name, place, and time. FHR monitoring of both fetuses reveals a normal baseline with moderate variability, as well as variable decelerations in the presenting twin.
A maintenance dose of magnesium sulfate is initiated at a rate of 2 g/h, with the BP level recorded every 15 minutes. Systolic values remain between 170 and 180 mm Hg, and diastolic values between 108 and 112 mm Hg for 60 minutes. The obstetrician administers IV labetalol (20 mg) over 2 minutes. About 15 minutes later, the BP level is 154/100 mm Hg, with values remaining in the range of 150 to 156 mm Hg systolic and 92 to 104 mm Hg diastolic.
Ultrasonography reveals that the presenting twin is in a breech position, with estimated fetal weight below the 10th percentile and oligohydramnios. As a result, the obstetrician elects to proceed to cesarean delivery. The twins are delivered by cesarean section using spinal anesthesia. Although the infants are premature, there are no complications.Profile of an eclamptic seizure
Witnessing an eclamptic convulsion can be a frightening experience for nurses and medical providers. The convulsion usually lasts 60 to 90 seconds and occurs in two phases:
- Phase 1 (15–25 seconds) involves facial twitching, rolling of the eyes, and stiffening of the body, with generalized muscular contractions.
- Phase 2 (20–50 seconds) involves alternate contraction and relaxation of the muscles of the body in rapid succession, starting in the face and spreading throughout the body. Foaming at the mouth also occurs, and the patient may bite her tongue if it isn’t protected.
Apnea develops during and immediately after the convulsion, lasting about 120 seconds. A period of hyperventilation follows to compensate for the respiratory acidosis during the apneic period.
A postictal state follows the convulsion, and the patient usually remembers nothing of the episode. Some patients also become restless, combative, and agitated, requiring sedation. Aspiration is possible during or after the convulsion.
We want to hear from you! Tell us what you think.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
- Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):182–192.
- Meyer NL, Mercer BM, Friedman SA, Sibai BM. Urinary dipstick protein: a poor predictor of absent or severe proteinuria. Am J Obstet Gynecol. 1994;170(1 Pt 1):137–141.
- Knight M; UK Obstetric Surveillance System (UKOSS). Eclampsia in the United Kingdom 2005. BJOG. 2007;114(9):1072–1078.
- ACOG Practice Bulletin #33: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167.
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia–eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e1–e7.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–475.
- ACOG Committee Opinion #514: Emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. Obstet Gynecol. 2011;118:1465–1468.
- Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987–994.
- Raheem IA, Saaid R, Omar Sz, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomized trial. BJOG. 2012;119(1):78–85.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
- Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):182–192.
- Meyer NL, Mercer BM, Friedman SA, Sibai BM. Urinary dipstick protein: a poor predictor of absent or severe proteinuria. Am J Obstet Gynecol. 1994;170(1 Pt 1):137–141.
- Knight M; UK Obstetric Surveillance System (UKOSS). Eclampsia in the United Kingdom 2005. BJOG. 2007;114(9):1072–1078.
- ACOG Practice Bulletin #33: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167.
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia–eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e1–e7.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–475.
- ACOG Committee Opinion #514: Emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. Obstet Gynecol. 2011;118:1465–1468.
- Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987–994.
- Raheem IA, Saaid R, Omar Sz, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomized trial. BJOG. 2012;119(1):78–85.
Does the addition of the Foley bulb to vaginal misoprostol for cervical ripening and labor induction reduce the time to delivery?
In this trial, women who had a singleton pregnancy at 35 weeks’ gestation or later were randomly allocated to the Foley bulb plus vaginal misoprostol (n = 56) or to vaginal misoprostol alone (n = 61) for cervical ripening and labor induction. All women had a vertex presentation, intact membranes, and an unfavorable cervix.
Women assigned to the combination group received vaginal misoprostol 25 μg every 4 hours and a Foley bulb inserted into the internal cervical os and filled with 60 mL of normal saline. Women assigned to vaginal misoprostol alone were given 25 μg every 4 hours. Intravenous oxytocin was given in each group when indicated, according to the discretion of the managing physician, at a rate of 2 mU/min, increasing by 2 mU every 20 min until regular uterine contractions occurred.
The primary outcome of the trial was the time from induction to delivery. Secondary outcomes were mode of delivery, tachysystole, and postpartum hemorrhage.
The median Bishop score was 3 in each group (range, 3–6).
Strengths and limitations of the trial
The strength of this study is its randomized design.
Among the limitations are its small sample size, which was inadequate to evaluate serious maternal and neonatal morbidities, and its lack of blinding, which may introduce bias among the managing physicians.
The primary outcome of induction-to-delivery time is not clinically important, particularly when multiparous women and those with a Bishop score above 5 are included, as they were in this study. Moreover, only women with a gestational age of at least 35 weeks were included, so the results do not apply to those with lower gestational ages.
Goal of induction is to achieve vaginal delivery within 24 hours
In the United States, at least one in every four pregnant women undergo induction of labor for any of a variety of obstetric, medical, and social indications.1 In nulliparous women who have an unfavorable cervix, induction of labor is associated with increased rates of prolonged labor, cesarean delivery, chorioamnionitis, and postpartum hemorrhage. The goal of induction of labor should be to achieve vaginal delivery within 24 hours and reduce the rate of cesarean delivery without increasing adverse maternal and neonatal outcomes.
Click here to access other Examining the Evidence articles on obstetrics.
Several methods are used for induction of labor with or without cervical ripening. They include the administration of oxytocin, oral or vaginal misoprostol in various doses, different preparations of prostaglandins, use of a Foley balloon filled with 30 to 100 mL of saline, or a combination of these methods. To date, none of these approaches has been shown to reduce the rate of cesarean delivery.
A similarly designed study produced very different findings. Data from multiple studies are mixed in regard to the induction-to-delivery time, rate of delivery within 24 and 48 hours, and side effects.1-6 These studies vary in inclusion criteria, method of induction or cervical ripening, dose of induction agent, Bishop score at randomization, primary outcomes, and sample size. A study from Brazil with a design similar to that of the study by Carbone and colleagues found a shorter mean time from induction to vaginal delivery in the vaginal misoprostol group, compared with the group allocated to the Foley bulb plus oxytocin. There were also more vaginal deliveries in the misoprostol group at 12 hours and 18 hours.6
Given the contradictory findings of Carbone and colleagues and an earlier trial of similar design,6 it is very unlikely that this trial is robust enough to change current clinical practice, which is highly variable. Among the methods for cervical ripening and labor induction in current use are oxytocin administration, oral or vaginal misoprostol, prostaglandin administration, the Foley bulb, vaginal dinoprostone, or a combination of methods.
We want to hear from you! Tell us what you think.
1. Hill JB, Thigpen BD, Bofill JA, et al. A randomized clinical trial comparing misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33-38.
2. Kehl S, Ehard A, Berlit S, et al. Combination of misoprostol and mechanical dilation for induction of labor: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):315-319.
3. Kashanian M, Akbarian AR, Fekrat M. Cervical ripening and induction of labor with intravaginal misoprostol and Foley catheter cervical traction. Int J Gynaecol Obstet. 2006;92(1):79-80.
4. Chung JH, Huang WH, Rumney PJ, et al. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189(4):1031-1035.
5. Rust OA, Greybush M, Atlas RO, et al. Preinduction cervical ripening: a randomized trial of intravaginal misoprostol alone vs a combination of trancervical Foley balloon and intravaginal misoprostol. J Reprod Med. 2001;46(10):899-904.
6. Moraes Filho OB, Albuquerque RM, Cecatti JG. A randomized controlled trial comparing vaginal misoprostol versus Foley catheter plus oxytocin for labor induction. Acta Obstet Gynecol. 2010;89(8):1045-1052.
In this trial, women who had a singleton pregnancy at 35 weeks’ gestation or later were randomly allocated to the Foley bulb plus vaginal misoprostol (n = 56) or to vaginal misoprostol alone (n = 61) for cervical ripening and labor induction. All women had a vertex presentation, intact membranes, and an unfavorable cervix.
Women assigned to the combination group received vaginal misoprostol 25 μg every 4 hours and a Foley bulb inserted into the internal cervical os and filled with 60 mL of normal saline. Women assigned to vaginal misoprostol alone were given 25 μg every 4 hours. Intravenous oxytocin was given in each group when indicated, according to the discretion of the managing physician, at a rate of 2 mU/min, increasing by 2 mU every 20 min until regular uterine contractions occurred.
The primary outcome of the trial was the time from induction to delivery. Secondary outcomes were mode of delivery, tachysystole, and postpartum hemorrhage.
The median Bishop score was 3 in each group (range, 3–6).
Strengths and limitations of the trial
The strength of this study is its randomized design.
Among the limitations are its small sample size, which was inadequate to evaluate serious maternal and neonatal morbidities, and its lack of blinding, which may introduce bias among the managing physicians.
The primary outcome of induction-to-delivery time is not clinically important, particularly when multiparous women and those with a Bishop score above 5 are included, as they were in this study. Moreover, only women with a gestational age of at least 35 weeks were included, so the results do not apply to those with lower gestational ages.
Goal of induction is to achieve vaginal delivery within 24 hours
In the United States, at least one in every four pregnant women undergo induction of labor for any of a variety of obstetric, medical, and social indications.1 In nulliparous women who have an unfavorable cervix, induction of labor is associated with increased rates of prolonged labor, cesarean delivery, chorioamnionitis, and postpartum hemorrhage. The goal of induction of labor should be to achieve vaginal delivery within 24 hours and reduce the rate of cesarean delivery without increasing adverse maternal and neonatal outcomes.
Click here to access other Examining the Evidence articles on obstetrics.
Several methods are used for induction of labor with or without cervical ripening. They include the administration of oxytocin, oral or vaginal misoprostol in various doses, different preparations of prostaglandins, use of a Foley balloon filled with 30 to 100 mL of saline, or a combination of these methods. To date, none of these approaches has been shown to reduce the rate of cesarean delivery.
A similarly designed study produced very different findings. Data from multiple studies are mixed in regard to the induction-to-delivery time, rate of delivery within 24 and 48 hours, and side effects.1-6 These studies vary in inclusion criteria, method of induction or cervical ripening, dose of induction agent, Bishop score at randomization, primary outcomes, and sample size. A study from Brazil with a design similar to that of the study by Carbone and colleagues found a shorter mean time from induction to vaginal delivery in the vaginal misoprostol group, compared with the group allocated to the Foley bulb plus oxytocin. There were also more vaginal deliveries in the misoprostol group at 12 hours and 18 hours.6
Given the contradictory findings of Carbone and colleagues and an earlier trial of similar design,6 it is very unlikely that this trial is robust enough to change current clinical practice, which is highly variable. Among the methods for cervical ripening and labor induction in current use are oxytocin administration, oral or vaginal misoprostol, prostaglandin administration, the Foley bulb, vaginal dinoprostone, or a combination of methods.
We want to hear from you! Tell us what you think.
In this trial, women who had a singleton pregnancy at 35 weeks’ gestation or later were randomly allocated to the Foley bulb plus vaginal misoprostol (n = 56) or to vaginal misoprostol alone (n = 61) for cervical ripening and labor induction. All women had a vertex presentation, intact membranes, and an unfavorable cervix.
Women assigned to the combination group received vaginal misoprostol 25 μg every 4 hours and a Foley bulb inserted into the internal cervical os and filled with 60 mL of normal saline. Women assigned to vaginal misoprostol alone were given 25 μg every 4 hours. Intravenous oxytocin was given in each group when indicated, according to the discretion of the managing physician, at a rate of 2 mU/min, increasing by 2 mU every 20 min until regular uterine contractions occurred.
The primary outcome of the trial was the time from induction to delivery. Secondary outcomes were mode of delivery, tachysystole, and postpartum hemorrhage.
The median Bishop score was 3 in each group (range, 3–6).
Strengths and limitations of the trial
The strength of this study is its randomized design.
Among the limitations are its small sample size, which was inadequate to evaluate serious maternal and neonatal morbidities, and its lack of blinding, which may introduce bias among the managing physicians.
The primary outcome of induction-to-delivery time is not clinically important, particularly when multiparous women and those with a Bishop score above 5 are included, as they were in this study. Moreover, only women with a gestational age of at least 35 weeks were included, so the results do not apply to those with lower gestational ages.
Goal of induction is to achieve vaginal delivery within 24 hours
In the United States, at least one in every four pregnant women undergo induction of labor for any of a variety of obstetric, medical, and social indications.1 In nulliparous women who have an unfavorable cervix, induction of labor is associated with increased rates of prolonged labor, cesarean delivery, chorioamnionitis, and postpartum hemorrhage. The goal of induction of labor should be to achieve vaginal delivery within 24 hours and reduce the rate of cesarean delivery without increasing adverse maternal and neonatal outcomes.
Click here to access other Examining the Evidence articles on obstetrics.
Several methods are used for induction of labor with or without cervical ripening. They include the administration of oxytocin, oral or vaginal misoprostol in various doses, different preparations of prostaglandins, use of a Foley balloon filled with 30 to 100 mL of saline, or a combination of these methods. To date, none of these approaches has been shown to reduce the rate of cesarean delivery.
A similarly designed study produced very different findings. Data from multiple studies are mixed in regard to the induction-to-delivery time, rate of delivery within 24 and 48 hours, and side effects.1-6 These studies vary in inclusion criteria, method of induction or cervical ripening, dose of induction agent, Bishop score at randomization, primary outcomes, and sample size. A study from Brazil with a design similar to that of the study by Carbone and colleagues found a shorter mean time from induction to vaginal delivery in the vaginal misoprostol group, compared with the group allocated to the Foley bulb plus oxytocin. There were also more vaginal deliveries in the misoprostol group at 12 hours and 18 hours.6
Given the contradictory findings of Carbone and colleagues and an earlier trial of similar design,6 it is very unlikely that this trial is robust enough to change current clinical practice, which is highly variable. Among the methods for cervical ripening and labor induction in current use are oxytocin administration, oral or vaginal misoprostol, prostaglandin administration, the Foley bulb, vaginal dinoprostone, or a combination of methods.
We want to hear from you! Tell us what you think.
1. Hill JB, Thigpen BD, Bofill JA, et al. A randomized clinical trial comparing misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33-38.
2. Kehl S, Ehard A, Berlit S, et al. Combination of misoprostol and mechanical dilation for induction of labor: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):315-319.
3. Kashanian M, Akbarian AR, Fekrat M. Cervical ripening and induction of labor with intravaginal misoprostol and Foley catheter cervical traction. Int J Gynaecol Obstet. 2006;92(1):79-80.
4. Chung JH, Huang WH, Rumney PJ, et al. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189(4):1031-1035.
5. Rust OA, Greybush M, Atlas RO, et al. Preinduction cervical ripening: a randomized trial of intravaginal misoprostol alone vs a combination of trancervical Foley balloon and intravaginal misoprostol. J Reprod Med. 2001;46(10):899-904.
6. Moraes Filho OB, Albuquerque RM, Cecatti JG. A randomized controlled trial comparing vaginal misoprostol versus Foley catheter plus oxytocin for labor induction. Acta Obstet Gynecol. 2010;89(8):1045-1052.
1. Hill JB, Thigpen BD, Bofill JA, et al. A randomized clinical trial comparing misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33-38.
2. Kehl S, Ehard A, Berlit S, et al. Combination of misoprostol and mechanical dilation for induction of labor: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):315-319.
3. Kashanian M, Akbarian AR, Fekrat M. Cervical ripening and induction of labor with intravaginal misoprostol and Foley catheter cervical traction. Int J Gynaecol Obstet. 2006;92(1):79-80.
4. Chung JH, Huang WH, Rumney PJ, et al. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189(4):1031-1035.
5. Rust OA, Greybush M, Atlas RO, et al. Preinduction cervical ripening: a randomized trial of intravaginal misoprostol alone vs a combination of trancervical Foley balloon and intravaginal misoprostol. J Reprod Med. 2001;46(10):899-904.
6. Moraes Filho OB, Albuquerque RM, Cecatti JG. A randomized controlled trial comparing vaginal misoprostol versus Foley catheter plus oxytocin for labor induction. Acta Obstet Gynecol. 2010;89(8):1045-1052.
Does elimination of the bladder flap from cesarean delivery increase the risk of complications?
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Cesarean delivery is the most common major surgical procedure performed during pregnancy. In the United States, the rate of cesarean delivery approaches 30%. As this rate rises, it is likely to be accompanied by an increase in the rate of surgical complications, such as pelvic hematoma, infection, and bladder injury, and in the rate of long-term complications, such as adhesion formation.
Several studies have assessed technical aspects of cesarean delivery, but debate continues over whether a bladder flap is a necessary part of the standard procedure.
The bladder flap is developed by incising the peritoneal lining and dissecting the urinary bladder away from the lower uterine segment. Suggested benefits of the bladder flap are easy access to the lower uterine segment and avoidance of bladder injury—but these claims have not been confirmed in retrospective or randomized trials.1,2 On the contrary, some studies suggest that creation of a bladder flap prolongs the duration of surgery and may increase the risk of postoperative infection and adhesion formation, as well as bladder injury at the time of repeat cesarean.3
Details of the trial
This study by Tuuli and colleagues is a single-center, unblinded, randomized, controlled trial designed to explore the risks and benefits of creating a bladder flap versus those of omitting the flap at the time of cesarean delivery. Of the 258 women enrolled in the trial, 131 were allocated to creation of a bladder flap and 127 to omission of the flap.
The primary outcome was total operative time. Secondary outcomes were:
- bladder injury
- incision-to-delivery time
- incision-to-fascial closure time
- estimated blood loss
- postoperative pain
- hospital stay
- endometritis
- urinary tract infection.
Unlike an earlier trial that included only women undergoing primary cesarean, this study included both primary and repeat cesarean deliveries. Sample size for each group was calculated assuming a 5-minute difference in total operating time.
Of the 131 women allocated to the bladder-flap creation group, only 108 (82%) actually had a bladder flap; 23 (18%) did not. Conversely, among the 127 women allocated to the no-flap group, 14 (11%) had a bladder flap created, most commonly because of the presence of scar tissue (n = 9).
Neither group had any bladder injuries nor were there statistical differences in any of the other secondary outcomes studied.
The authors concluded that omission of the bladder flap from primary and repeat cesarean delivery does not increase intraoperative or postoperative complications.
Strengths and limitations
As I mentioned, the rationale for creating a bladder flap is to reduce the rate of bladder injury. Therefore, bladder injury should have been the primary outcome of this trial. However, because the expected rate of bladder injury during cesarean delivery is so low (0.14%–0.35%), a sample size of 40,000 women would have been needed to address this outcome.
Among women who do not have a bladder flap created during cesarean delivery, bladder injury may be more likely when the second stage of labor is prolonged (i.e., when the vertex is wedged low in the pelvis) and when the woman has a history of multiple cesarean deliveries. This study did not include information about the number of women meeting these criteria.
Another limitation of this trial: Adherence to the protocol was inadequate, as 18% of the women assigned to receive a bladder flap did not have one, and 11% of those assigned to receive no flap had a flap created. This failure to adhere to the protocol may explain the lack of significant differences in total delivery time between the two groups, as well as the clinically insignificant difference in the incision-to-delivery interval between groups.
The rationale for omitting a bladder flap is to shorten total operating and incision-to-delivery time and/or to reduce the rate of future adhesions. Regrettably, this trial provided no conclusive evidence regarding any of these benefits. We still need a randomized trial of adequate sample size to address some of the questions raised by this trial.
I agree with the authors of this trial that their findings—along with those of other studies—argue against routine creation of a bladder flap at cesarean delivery.
Consider clinical findings at the time of surgery when deciding whether or not to create a bladder flap. For example, a flap may ease delivery of the fetal head when pushing has been prolonged during the second stage of labor or when operative vaginal delivery has failed. A flap also may help the surgeon avoid injury to the bladder in cases involving accidental extension of the lower-segment incision.
Among women who have a history of cesarean delivery and in whom the bladder flap is attached high above the lower segment, the bladder should be dissected carefully away from the uterus to avoid injury during delivery.
Baha M. Sibai, MD
We want to hear from you! Tell us what you think.
1. Malvasi A, Tinelli A, Gustapane S, et al. Surgical technique to avoid bladder flap formation during cesarean section. G Chir. 2011;32(11–12):498-403.
2. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2011;98(6):1089-1092.
3. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in cesarean section on repeat cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):300-304.
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Cesarean delivery is the most common major surgical procedure performed during pregnancy. In the United States, the rate of cesarean delivery approaches 30%. As this rate rises, it is likely to be accompanied by an increase in the rate of surgical complications, such as pelvic hematoma, infection, and bladder injury, and in the rate of long-term complications, such as adhesion formation.
Several studies have assessed technical aspects of cesarean delivery, but debate continues over whether a bladder flap is a necessary part of the standard procedure.
The bladder flap is developed by incising the peritoneal lining and dissecting the urinary bladder away from the lower uterine segment. Suggested benefits of the bladder flap are easy access to the lower uterine segment and avoidance of bladder injury—but these claims have not been confirmed in retrospective or randomized trials.1,2 On the contrary, some studies suggest that creation of a bladder flap prolongs the duration of surgery and may increase the risk of postoperative infection and adhesion formation, as well as bladder injury at the time of repeat cesarean.3
Details of the trial
This study by Tuuli and colleagues is a single-center, unblinded, randomized, controlled trial designed to explore the risks and benefits of creating a bladder flap versus those of omitting the flap at the time of cesarean delivery. Of the 258 women enrolled in the trial, 131 were allocated to creation of a bladder flap and 127 to omission of the flap.
The primary outcome was total operative time. Secondary outcomes were:
- bladder injury
- incision-to-delivery time
- incision-to-fascial closure time
- estimated blood loss
- postoperative pain
- hospital stay
- endometritis
- urinary tract infection.
Unlike an earlier trial that included only women undergoing primary cesarean, this study included both primary and repeat cesarean deliveries. Sample size for each group was calculated assuming a 5-minute difference in total operating time.
Of the 131 women allocated to the bladder-flap creation group, only 108 (82%) actually had a bladder flap; 23 (18%) did not. Conversely, among the 127 women allocated to the no-flap group, 14 (11%) had a bladder flap created, most commonly because of the presence of scar tissue (n = 9).
Neither group had any bladder injuries nor were there statistical differences in any of the other secondary outcomes studied.
The authors concluded that omission of the bladder flap from primary and repeat cesarean delivery does not increase intraoperative or postoperative complications.
Strengths and limitations
As I mentioned, the rationale for creating a bladder flap is to reduce the rate of bladder injury. Therefore, bladder injury should have been the primary outcome of this trial. However, because the expected rate of bladder injury during cesarean delivery is so low (0.14%–0.35%), a sample size of 40,000 women would have been needed to address this outcome.
Among women who do not have a bladder flap created during cesarean delivery, bladder injury may be more likely when the second stage of labor is prolonged (i.e., when the vertex is wedged low in the pelvis) and when the woman has a history of multiple cesarean deliveries. This study did not include information about the number of women meeting these criteria.
Another limitation of this trial: Adherence to the protocol was inadequate, as 18% of the women assigned to receive a bladder flap did not have one, and 11% of those assigned to receive no flap had a flap created. This failure to adhere to the protocol may explain the lack of significant differences in total delivery time between the two groups, as well as the clinically insignificant difference in the incision-to-delivery interval between groups.
The rationale for omitting a bladder flap is to shorten total operating and incision-to-delivery time and/or to reduce the rate of future adhesions. Regrettably, this trial provided no conclusive evidence regarding any of these benefits. We still need a randomized trial of adequate sample size to address some of the questions raised by this trial.
I agree with the authors of this trial that their findings—along with those of other studies—argue against routine creation of a bladder flap at cesarean delivery.
Consider clinical findings at the time of surgery when deciding whether or not to create a bladder flap. For example, a flap may ease delivery of the fetal head when pushing has been prolonged during the second stage of labor or when operative vaginal delivery has failed. A flap also may help the surgeon avoid injury to the bladder in cases involving accidental extension of the lower-segment incision.
Among women who have a history of cesarean delivery and in whom the bladder flap is attached high above the lower segment, the bladder should be dissected carefully away from the uterus to avoid injury during delivery.
Baha M. Sibai, MD
We want to hear from you! Tell us what you think.
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Cesarean delivery is the most common major surgical procedure performed during pregnancy. In the United States, the rate of cesarean delivery approaches 30%. As this rate rises, it is likely to be accompanied by an increase in the rate of surgical complications, such as pelvic hematoma, infection, and bladder injury, and in the rate of long-term complications, such as adhesion formation.
Several studies have assessed technical aspects of cesarean delivery, but debate continues over whether a bladder flap is a necessary part of the standard procedure.
The bladder flap is developed by incising the peritoneal lining and dissecting the urinary bladder away from the lower uterine segment. Suggested benefits of the bladder flap are easy access to the lower uterine segment and avoidance of bladder injury—but these claims have not been confirmed in retrospective or randomized trials.1,2 On the contrary, some studies suggest that creation of a bladder flap prolongs the duration of surgery and may increase the risk of postoperative infection and adhesion formation, as well as bladder injury at the time of repeat cesarean.3
Details of the trial
This study by Tuuli and colleagues is a single-center, unblinded, randomized, controlled trial designed to explore the risks and benefits of creating a bladder flap versus those of omitting the flap at the time of cesarean delivery. Of the 258 women enrolled in the trial, 131 were allocated to creation of a bladder flap and 127 to omission of the flap.
The primary outcome was total operative time. Secondary outcomes were:
- bladder injury
- incision-to-delivery time
- incision-to-fascial closure time
- estimated blood loss
- postoperative pain
- hospital stay
- endometritis
- urinary tract infection.
Unlike an earlier trial that included only women undergoing primary cesarean, this study included both primary and repeat cesarean deliveries. Sample size for each group was calculated assuming a 5-minute difference in total operating time.
Of the 131 women allocated to the bladder-flap creation group, only 108 (82%) actually had a bladder flap; 23 (18%) did not. Conversely, among the 127 women allocated to the no-flap group, 14 (11%) had a bladder flap created, most commonly because of the presence of scar tissue (n = 9).
Neither group had any bladder injuries nor were there statistical differences in any of the other secondary outcomes studied.
The authors concluded that omission of the bladder flap from primary and repeat cesarean delivery does not increase intraoperative or postoperative complications.
Strengths and limitations
As I mentioned, the rationale for creating a bladder flap is to reduce the rate of bladder injury. Therefore, bladder injury should have been the primary outcome of this trial. However, because the expected rate of bladder injury during cesarean delivery is so low (0.14%–0.35%), a sample size of 40,000 women would have been needed to address this outcome.
Among women who do not have a bladder flap created during cesarean delivery, bladder injury may be more likely when the second stage of labor is prolonged (i.e., when the vertex is wedged low in the pelvis) and when the woman has a history of multiple cesarean deliveries. This study did not include information about the number of women meeting these criteria.
Another limitation of this trial: Adherence to the protocol was inadequate, as 18% of the women assigned to receive a bladder flap did not have one, and 11% of those assigned to receive no flap had a flap created. This failure to adhere to the protocol may explain the lack of significant differences in total delivery time between the two groups, as well as the clinically insignificant difference in the incision-to-delivery interval between groups.
The rationale for omitting a bladder flap is to shorten total operating and incision-to-delivery time and/or to reduce the rate of future adhesions. Regrettably, this trial provided no conclusive evidence regarding any of these benefits. We still need a randomized trial of adequate sample size to address some of the questions raised by this trial.
I agree with the authors of this trial that their findings—along with those of other studies—argue against routine creation of a bladder flap at cesarean delivery.
Consider clinical findings at the time of surgery when deciding whether or not to create a bladder flap. For example, a flap may ease delivery of the fetal head when pushing has been prolonged during the second stage of labor or when operative vaginal delivery has failed. A flap also may help the surgeon avoid injury to the bladder in cases involving accidental extension of the lower-segment incision.
Among women who have a history of cesarean delivery and in whom the bladder flap is attached high above the lower segment, the bladder should be dissected carefully away from the uterus to avoid injury during delivery.
Baha M. Sibai, MD
We want to hear from you! Tell us what you think.
1. Malvasi A, Tinelli A, Gustapane S, et al. Surgical technique to avoid bladder flap formation during cesarean section. G Chir. 2011;32(11–12):498-403.
2. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2011;98(6):1089-1092.
3. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in cesarean section on repeat cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):300-304.
1. Malvasi A, Tinelli A, Gustapane S, et al. Surgical technique to avoid bladder flap formation during cesarean section. G Chir. 2011;32(11–12):498-403.
2. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2011;98(6):1089-1092.
3. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in cesarean section on repeat cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):300-304.
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)
10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia
Because eclampsia occurs but rarely during pregnancy and the postpartum period, most health-care providers have little to no personal experience with management of this life-threatening obstetric emergency. Knowledge about maternal resuscitation during and after an eclamptic seizure is critical for improving maternal and perinatal outcomes.
In this round-up, I present 10 practical recommendations for prompt diagnosis and management of women who have eclampsia. Immediate implementation of these recommendations can lead to improved maternal and perinatal outcomes (both acute and long-term).
1. Practice. Practice again.
Implement regular monthly simulation training sessions
Fisher N, Bernstein PS, Satin A, et al. Resident training for eclampsia and magnesium toxicity management: simulation or traditional lecture? Am J Obstet Gynecol. 2010;203(4):379.e1–5.
Eclampsia is unpredictable and can develop rapidly at home, in labor and delivery, on the antepartum/postpartum ward, and in the emergency room. Therefore, it is prudent that all health-care providers who treat pregnant or postpartum women on a daily basis be trained and knowledgeable about early detection and management of eclampsia. This goal can be achieved by developing drills for rehearsal and by testing the response and skills of all providers.
2. Preventive: Magnesium sulfate
Do not attempt to arrest the seizure. Use MgSO4 to prevent recurrent convulsions.
Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulfate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;(12):CD000127.
Most eclamptic seizures are self-limiting. Therefore, there is no need to administer bolus drugs such as diazepam or midazolam. These drugs are usually used in the emergency room, but they inhibit maternal laryngeal reflexes and may lead to aspiration. They also suppress the central nervous system respiratory centers and can cause apnea, requiring intubation.
When used in the management of eclampsia, magnesium sulfate is associated with a lower rate of recurrent seizures and maternal death than is diazepam.
3. FHR changes? Be patient.
Do not rush the patient to emergent cesarean section because of an abnormal FHR tracing
Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
During an eclamptic convulsion, there is usually prolonged fetal heart rate (FHR) deceleration or even bradycardia—with or without an increase in both frequency and uterine tone. After the convulsion, as a result of maternal hypoxia and hypercarbia, the FHR tracing can show tachycardia, reduced beat-to-beat variability, and transient recurrent decelerations. When this happens, concern about fetal status can distract the obstetric provider from resuscitation of the mother. However, these FHR changes usually return to normal after maternal resuscitation. If the FHR changes persist for longer than 15 minutes, consider abruptio placentae and move to delivery.
4. Target: Lower BP
Reduce maternal blood pressure to a safe level to prevent stroke, but without compromising uteroplacental perfusion
Zwart JJ, Richters A, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Eclampsia in the Netherlands. Obstet Gynecol. 2008;112(4):820–827.
In this nationwide review of complications from eclampsia in the Netherlands, the authors found that failure to treat persistent severe hypertension was associated with hypertensive encephalopathy, cerebral infarction, bleeding, or congestive heart failure. They also found that 35.2% of women had systolic or diastolic blood pressure at or above 170/110 mm Hg at admission, but fewer than half were given antihypertensive drugs at that time. Among the cases deemed to have received substandard care, one third involved inadequate treatment of hypertension.
5. Know your antihypertensives
Learn which agents are best to control severe hypertension in eclampsia
Sibai BM. Hypertensive Emergencies. In: Foley MR, Strong TH, Garite TJ, eds. Obstetric Intensive Care Manual. 3rd ed. New York, NY: The McGraw-Hill Companies; 2010.
It is critical to familiarize oneself with the mechanism of action, dose, and potential side effects of agents used to control hypertension. For example, neither hydralazine nor nifedipine should be used in patients who have severe headache and persistent tachycardia (pulse, >100 bpm). Labetalol should be avoided in women who have persistent bradycardia (pulse, <60 bpm), asthma, or congestive heart failure.
For women who have persistent headache and tachycardia, I suggest intravenous (IV) labetalol, starting at a dose of 20 mg, 40 mg, or 80 mg every 10 minutes as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg. The maximum dose of labetalol should not exceed 300 mg in 1 hour.
For patients who have bradycardia and severe asthma, I suggest oral, rapid-acting nifedipine, starting at 10 mg to 20 mg, to be repeated in 20 to 30 minutes as needed, up to a maximum of 50 mg to 60 mg in 1 hour. Oral nifedipine can be used with magnesium sulfate. An alternative is an IV bolus injection of hydralazine, starting at a dose of 5 mg to 10 mg, to be repeated every 15 minutes, up to a maximum dose of 25 mg.
6. Avoid general anesthesia
Use neuraxial anesthesia for labor and delivery in eclampsia
Turner JA. Severe preeclampsia: anesthetic implications of the disease and its management. Am J Ther. 2009;16(4):284–248.
Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Cesarean delivery: a population-based study. Br J Anaesth. 2010;105(6):818–826.
Epidural, spinal, or combined anesthesia is safe in the absence of coagulopathy or severe thrombocytopenia. General anesthesia increases the risk of aspiration, failed intubation due to pharyngolaryngeal edema, and stroke secondary to the increase in systemic and intracerebral pressures during intubation and extubation.
Eclampsia is not an indication for cesarean delivery
Repke JT, Sibai BM. Preeclampsia and eclampsia. OBG Manage. 2009;21(4):44–55.
Once the mother has been resuscitated and stabilized, the provider should choose a mode of delivery that is based on fetal condition, gestational age, presence or absence of labor, and the cervical Bishop score. Vaginal delivery can be achieved in most patients who have a gestational age of 34 weeks or greater.
8. Late presentation happens
Be aware that eclampsia can develop for the first time as long as 28 days postpartum
Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e31–37.
Atypical eclampsia is any eclampsia that develops beyond 48 hours postpartum. A history of diagnosed predelivery preeclampsia is not necessary for development of late postpartum eclampsia. In general, more than 50% of patients who develop late postpartum eclampsia have no evidence of preeclampsia prior to delivery.
Be aware that the clinical and neuro-imaging features of eclampsia overlap with those of reversible cerebral vasoconstriction syndrome (angiopathy)
Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ. Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care. 2009;11(2):199–209.
Women who have reversible cerebral vasoconstriction syndrome have clinical findings (acute onset of recurrent headaches, visual changes, seizures, and hypertension) and cerebral magnetic resonance imaging (MRI) findings (posterior reversible encephalopathy syndrome) that are similar to those of women who have late postpartum eclampsia (FIGURE). However, in women who have postpartum cerebral angiopathy, cerebral angiography will show the presence of bead-like vasoconstriction—which is usually absent in eclampsia.
Posterior reversible encephalopathy syndrome
Green arrows point to vasogenic edema in the occipital lobes and, partially, the parietal lobes. The edema is gone on repeat magnetic resonance imaging (see Recommendation #9).
10. Act today, see a better outcome tomorrow
Avoid long-term maternal neurologic injury by managing eclampsia properly
Zeeman GG. Neurologic complications of preeclampsia. Semin Perinatol. 2009;33(3):166–172.
Residual neurologic damage is rare in the majority of women who have eclampsia. However, long-term cerebral white-matter injury (cytotoxic edema, infarction) on MRI imaging and impaired memory and cognitive function may develop in some women who have multiple seizures and who have inadequately controlled persistent severe hypertension.
We want to hear from you! Tell us what you think.
Because eclampsia occurs but rarely during pregnancy and the postpartum period, most health-care providers have little to no personal experience with management of this life-threatening obstetric emergency. Knowledge about maternal resuscitation during and after an eclamptic seizure is critical for improving maternal and perinatal outcomes.
In this round-up, I present 10 practical recommendations for prompt diagnosis and management of women who have eclampsia. Immediate implementation of these recommendations can lead to improved maternal and perinatal outcomes (both acute and long-term).
1. Practice. Practice again.
Implement regular monthly simulation training sessions
Fisher N, Bernstein PS, Satin A, et al. Resident training for eclampsia and magnesium toxicity management: simulation or traditional lecture? Am J Obstet Gynecol. 2010;203(4):379.e1–5.
Eclampsia is unpredictable and can develop rapidly at home, in labor and delivery, on the antepartum/postpartum ward, and in the emergency room. Therefore, it is prudent that all health-care providers who treat pregnant or postpartum women on a daily basis be trained and knowledgeable about early detection and management of eclampsia. This goal can be achieved by developing drills for rehearsal and by testing the response and skills of all providers.
2. Preventive: Magnesium sulfate
Do not attempt to arrest the seizure. Use MgSO4 to prevent recurrent convulsions.
Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulfate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;(12):CD000127.
Most eclamptic seizures are self-limiting. Therefore, there is no need to administer bolus drugs such as diazepam or midazolam. These drugs are usually used in the emergency room, but they inhibit maternal laryngeal reflexes and may lead to aspiration. They also suppress the central nervous system respiratory centers and can cause apnea, requiring intubation.
When used in the management of eclampsia, magnesium sulfate is associated with a lower rate of recurrent seizures and maternal death than is diazepam.
3. FHR changes? Be patient.
Do not rush the patient to emergent cesarean section because of an abnormal FHR tracing
Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
During an eclamptic convulsion, there is usually prolonged fetal heart rate (FHR) deceleration or even bradycardia—with or without an increase in both frequency and uterine tone. After the convulsion, as a result of maternal hypoxia and hypercarbia, the FHR tracing can show tachycardia, reduced beat-to-beat variability, and transient recurrent decelerations. When this happens, concern about fetal status can distract the obstetric provider from resuscitation of the mother. However, these FHR changes usually return to normal after maternal resuscitation. If the FHR changes persist for longer than 15 minutes, consider abruptio placentae and move to delivery.
4. Target: Lower BP
Reduce maternal blood pressure to a safe level to prevent stroke, but without compromising uteroplacental perfusion
Zwart JJ, Richters A, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Eclampsia in the Netherlands. Obstet Gynecol. 2008;112(4):820–827.
In this nationwide review of complications from eclampsia in the Netherlands, the authors found that failure to treat persistent severe hypertension was associated with hypertensive encephalopathy, cerebral infarction, bleeding, or congestive heart failure. They also found that 35.2% of women had systolic or diastolic blood pressure at or above 170/110 mm Hg at admission, but fewer than half were given antihypertensive drugs at that time. Among the cases deemed to have received substandard care, one third involved inadequate treatment of hypertension.
5. Know your antihypertensives
Learn which agents are best to control severe hypertension in eclampsia
Sibai BM. Hypertensive Emergencies. In: Foley MR, Strong TH, Garite TJ, eds. Obstetric Intensive Care Manual. 3rd ed. New York, NY: The McGraw-Hill Companies; 2010.
It is critical to familiarize oneself with the mechanism of action, dose, and potential side effects of agents used to control hypertension. For example, neither hydralazine nor nifedipine should be used in patients who have severe headache and persistent tachycardia (pulse, >100 bpm). Labetalol should be avoided in women who have persistent bradycardia (pulse, <60 bpm), asthma, or congestive heart failure.
For women who have persistent headache and tachycardia, I suggest intravenous (IV) labetalol, starting at a dose of 20 mg, 40 mg, or 80 mg every 10 minutes as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg. The maximum dose of labetalol should not exceed 300 mg in 1 hour.
For patients who have bradycardia and severe asthma, I suggest oral, rapid-acting nifedipine, starting at 10 mg to 20 mg, to be repeated in 20 to 30 minutes as needed, up to a maximum of 50 mg to 60 mg in 1 hour. Oral nifedipine can be used with magnesium sulfate. An alternative is an IV bolus injection of hydralazine, starting at a dose of 5 mg to 10 mg, to be repeated every 15 minutes, up to a maximum dose of 25 mg.
6. Avoid general anesthesia
Use neuraxial anesthesia for labor and delivery in eclampsia
Turner JA. Severe preeclampsia: anesthetic implications of the disease and its management. Am J Ther. 2009;16(4):284–248.
Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Cesarean delivery: a population-based study. Br J Anaesth. 2010;105(6):818–826.
Epidural, spinal, or combined anesthesia is safe in the absence of coagulopathy or severe thrombocytopenia. General anesthesia increases the risk of aspiration, failed intubation due to pharyngolaryngeal edema, and stroke secondary to the increase in systemic and intracerebral pressures during intubation and extubation.
Eclampsia is not an indication for cesarean delivery
Repke JT, Sibai BM. Preeclampsia and eclampsia. OBG Manage. 2009;21(4):44–55.
Once the mother has been resuscitated and stabilized, the provider should choose a mode of delivery that is based on fetal condition, gestational age, presence or absence of labor, and the cervical Bishop score. Vaginal delivery can be achieved in most patients who have a gestational age of 34 weeks or greater.
8. Late presentation happens
Be aware that eclampsia can develop for the first time as long as 28 days postpartum
Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e31–37.
Atypical eclampsia is any eclampsia that develops beyond 48 hours postpartum. A history of diagnosed predelivery preeclampsia is not necessary for development of late postpartum eclampsia. In general, more than 50% of patients who develop late postpartum eclampsia have no evidence of preeclampsia prior to delivery.
Be aware that the clinical and neuro-imaging features of eclampsia overlap with those of reversible cerebral vasoconstriction syndrome (angiopathy)
Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ. Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care. 2009;11(2):199–209.
Women who have reversible cerebral vasoconstriction syndrome have clinical findings (acute onset of recurrent headaches, visual changes, seizures, and hypertension) and cerebral magnetic resonance imaging (MRI) findings (posterior reversible encephalopathy syndrome) that are similar to those of women who have late postpartum eclampsia (FIGURE). However, in women who have postpartum cerebral angiopathy, cerebral angiography will show the presence of bead-like vasoconstriction—which is usually absent in eclampsia.
Posterior reversible encephalopathy syndrome
Green arrows point to vasogenic edema in the occipital lobes and, partially, the parietal lobes. The edema is gone on repeat magnetic resonance imaging (see Recommendation #9).
10. Act today, see a better outcome tomorrow
Avoid long-term maternal neurologic injury by managing eclampsia properly
Zeeman GG. Neurologic complications of preeclampsia. Semin Perinatol. 2009;33(3):166–172.
Residual neurologic damage is rare in the majority of women who have eclampsia. However, long-term cerebral white-matter injury (cytotoxic edema, infarction) on MRI imaging and impaired memory and cognitive function may develop in some women who have multiple seizures and who have inadequately controlled persistent severe hypertension.
We want to hear from you! Tell us what you think.
Because eclampsia occurs but rarely during pregnancy and the postpartum period, most health-care providers have little to no personal experience with management of this life-threatening obstetric emergency. Knowledge about maternal resuscitation during and after an eclamptic seizure is critical for improving maternal and perinatal outcomes.
In this round-up, I present 10 practical recommendations for prompt diagnosis and management of women who have eclampsia. Immediate implementation of these recommendations can lead to improved maternal and perinatal outcomes (both acute and long-term).
1. Practice. Practice again.
Implement regular monthly simulation training sessions
Fisher N, Bernstein PS, Satin A, et al. Resident training for eclampsia and magnesium toxicity management: simulation or traditional lecture? Am J Obstet Gynecol. 2010;203(4):379.e1–5.
Eclampsia is unpredictable and can develop rapidly at home, in labor and delivery, on the antepartum/postpartum ward, and in the emergency room. Therefore, it is prudent that all health-care providers who treat pregnant or postpartum women on a daily basis be trained and knowledgeable about early detection and management of eclampsia. This goal can be achieved by developing drills for rehearsal and by testing the response and skills of all providers.
2. Preventive: Magnesium sulfate
Do not attempt to arrest the seizure. Use MgSO4 to prevent recurrent convulsions.
Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulfate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;(12):CD000127.
Most eclamptic seizures are self-limiting. Therefore, there is no need to administer bolus drugs such as diazepam or midazolam. These drugs are usually used in the emergency room, but they inhibit maternal laryngeal reflexes and may lead to aspiration. They also suppress the central nervous system respiratory centers and can cause apnea, requiring intubation.
When used in the management of eclampsia, magnesium sulfate is associated with a lower rate of recurrent seizures and maternal death than is diazepam.
3. FHR changes? Be patient.
Do not rush the patient to emergent cesarean section because of an abnormal FHR tracing
Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
During an eclamptic convulsion, there is usually prolonged fetal heart rate (FHR) deceleration or even bradycardia—with or without an increase in both frequency and uterine tone. After the convulsion, as a result of maternal hypoxia and hypercarbia, the FHR tracing can show tachycardia, reduced beat-to-beat variability, and transient recurrent decelerations. When this happens, concern about fetal status can distract the obstetric provider from resuscitation of the mother. However, these FHR changes usually return to normal after maternal resuscitation. If the FHR changes persist for longer than 15 minutes, consider abruptio placentae and move to delivery.
4. Target: Lower BP
Reduce maternal blood pressure to a safe level to prevent stroke, but without compromising uteroplacental perfusion
Zwart JJ, Richters A, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Eclampsia in the Netherlands. Obstet Gynecol. 2008;112(4):820–827.
In this nationwide review of complications from eclampsia in the Netherlands, the authors found that failure to treat persistent severe hypertension was associated with hypertensive encephalopathy, cerebral infarction, bleeding, or congestive heart failure. They also found that 35.2% of women had systolic or diastolic blood pressure at or above 170/110 mm Hg at admission, but fewer than half were given antihypertensive drugs at that time. Among the cases deemed to have received substandard care, one third involved inadequate treatment of hypertension.
5. Know your antihypertensives
Learn which agents are best to control severe hypertension in eclampsia
Sibai BM. Hypertensive Emergencies. In: Foley MR, Strong TH, Garite TJ, eds. Obstetric Intensive Care Manual. 3rd ed. New York, NY: The McGraw-Hill Companies; 2010.
It is critical to familiarize oneself with the mechanism of action, dose, and potential side effects of agents used to control hypertension. For example, neither hydralazine nor nifedipine should be used in patients who have severe headache and persistent tachycardia (pulse, >100 bpm). Labetalol should be avoided in women who have persistent bradycardia (pulse, <60 bpm), asthma, or congestive heart failure.
For women who have persistent headache and tachycardia, I suggest intravenous (IV) labetalol, starting at a dose of 20 mg, 40 mg, or 80 mg every 10 minutes as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg. The maximum dose of labetalol should not exceed 300 mg in 1 hour.
For patients who have bradycardia and severe asthma, I suggest oral, rapid-acting nifedipine, starting at 10 mg to 20 mg, to be repeated in 20 to 30 minutes as needed, up to a maximum of 50 mg to 60 mg in 1 hour. Oral nifedipine can be used with magnesium sulfate. An alternative is an IV bolus injection of hydralazine, starting at a dose of 5 mg to 10 mg, to be repeated every 15 minutes, up to a maximum dose of 25 mg.
6. Avoid general anesthesia
Use neuraxial anesthesia for labor and delivery in eclampsia
Turner JA. Severe preeclampsia: anesthetic implications of the disease and its management. Am J Ther. 2009;16(4):284–248.
Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Cesarean delivery: a population-based study. Br J Anaesth. 2010;105(6):818–826.
Epidural, spinal, or combined anesthesia is safe in the absence of coagulopathy or severe thrombocytopenia. General anesthesia increases the risk of aspiration, failed intubation due to pharyngolaryngeal edema, and stroke secondary to the increase in systemic and intracerebral pressures during intubation and extubation.
Eclampsia is not an indication for cesarean delivery
Repke JT, Sibai BM. Preeclampsia and eclampsia. OBG Manage. 2009;21(4):44–55.
Once the mother has been resuscitated and stabilized, the provider should choose a mode of delivery that is based on fetal condition, gestational age, presence or absence of labor, and the cervical Bishop score. Vaginal delivery can be achieved in most patients who have a gestational age of 34 weeks or greater.
8. Late presentation happens
Be aware that eclampsia can develop for the first time as long as 28 days postpartum
Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e31–37.
Atypical eclampsia is any eclampsia that develops beyond 48 hours postpartum. A history of diagnosed predelivery preeclampsia is not necessary for development of late postpartum eclampsia. In general, more than 50% of patients who develop late postpartum eclampsia have no evidence of preeclampsia prior to delivery.
Be aware that the clinical and neuro-imaging features of eclampsia overlap with those of reversible cerebral vasoconstriction syndrome (angiopathy)
Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ. Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care. 2009;11(2):199–209.
Women who have reversible cerebral vasoconstriction syndrome have clinical findings (acute onset of recurrent headaches, visual changes, seizures, and hypertension) and cerebral magnetic resonance imaging (MRI) findings (posterior reversible encephalopathy syndrome) that are similar to those of women who have late postpartum eclampsia (FIGURE). However, in women who have postpartum cerebral angiopathy, cerebral angiography will show the presence of bead-like vasoconstriction—which is usually absent in eclampsia.
Posterior reversible encephalopathy syndrome
Green arrows point to vasogenic edema in the occipital lobes and, partially, the parietal lobes. The edema is gone on repeat magnetic resonance imaging (see Recommendation #9).
10. Act today, see a better outcome tomorrow
Avoid long-term maternal neurologic injury by managing eclampsia properly
Zeeman GG. Neurologic complications of preeclampsia. Semin Perinatol. 2009;33(3):166–172.
Residual neurologic damage is rare in the majority of women who have eclampsia. However, long-term cerebral white-matter injury (cytotoxic edema, infarction) on MRI imaging and impaired memory and cognitive function may develop in some women who have multiple seizures and who have inadequately controlled persistent severe hypertension.
We want to hear from you! Tell us what you think.
10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Dr. Sibai reports no financial relationships relevant to this article.
By the time a pregnancy reaches term, approximately 500 to 800 mL of blood are circulating through the uterus and placenta every minute, thanks to the intricate network of blood vessels permeating these organs. So it is not surprising that postpartum hemorrhage complicates as many as one in every 20 deliveries, both vaginal and cesarean. Usually, hemorrhage is the result of uterine atony, but other entities may also cause or contribute to acute bleeding.
Severe postpartum hemorrhage, defined as a loss of more than 1,500 mL of blood, complicates approximately 1% of all deliveries and is a leading cause of maternal death. Severe PPH poses serious and often unpredictable challenges to obstetric providers, from the need to make an early diagnosis and establish and treat the cause to the ability to manage hemorrhagic shock.
In this article, I sort through data on the management of this potentially catastrophic event and summarize 10 evidence-based recommendations that can help reduce acute and long-term maternal complications.
1. Plan and rehearse a step-by-step approach
Wise A, Clark V. Challenges of major obstetric haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2010;24(3):353–365.
It is important to anticipate and prepare for the possibility of PPH so that you can respond quickly and effectively when it occurs. Evaluation and management should be simultaneous and should not be hindered by confusion or chaos. Successful management requires early recognition; identification of the cause; the securing of help; continuous monitoring of vital signs and blood loss; prompt resuscitation with fluids, blood, and blood products; and medical or surgical treatment.
2. Know the signs and symptoms of severe hemorrhage
Moore J, Chandraharn E. Management of massive postpartum haemorrhage and coagulopathy. Obstet Gynaecol Reprod Med. 2010;20(6):174–180.
Persistent vaginal bleeding is the first sign of PPH. The bleeding may be continuous oozing or it may be profuse. In addition to bleeding, the patient will exhibit several of the signs and symptoms listed in the TABLE.
Signs and symptoms of postpartum hemorrhage
| Signs | Symptoms |
|---|---|
| Systolic pressure, ≤90 mm Hg | Anxiety |
| Restlessness | |
| Pulse, ≥110 beats per minute | Tachypnea |
| Narrow pulse pressure | Dizziness |
| Hunger for air | |
| Coldness and clamminess | Confusion |
| Pale appearance | |
| Oliguria or anuria |
3. Call for help within 10 minutes after making
the diagnosis of PPH
Driessen M, Bouvier-Colle MH, Dupont C, et al. Postpartum hemorrhage resulting from uterine atony after vaginal delivery. Factors associated with severity. Obstet Gynecol. 2011;117(1):21–31.
In the early stages of uterine atony, delaying care beyond 10 minutes increases the risk of severe PPH.
4. Identify patients at very high risk of hysterectomy
and end-organ dysfunction
O’Brien D, Babiker E, O’Sullivan O, et al. Prediction of peripartum hysterectomy and end organ dysfunction in major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2010;153(2):165–169.
Rossi AC, Lee RH, Chmait RH. Emergency postpartum hysterectomy for uncontrolled postpartum bleeding: a systematic review. Obstet Gynecol. 2010;115(3):637–644.
In a study of 117 cases of severe obstetric hemorrhage, several independent risk factors for peripartum hysterectomy and end-organ dysfunction were identified:
- number of previous cesarean deliveries (odds ratio [OR], 3.28; 95% confidence interval [CI], 1.95–5.5)
- placenta previa (OR, 13.5; 95% CI, 7.7–184)
- placenta accreta (OR, 37.7; 95% CI, 7.7–184)
- uterine rupture (OR, 7.25; 95% CI, 1.25–42)
- number of units of red blood cells (RBCs) transfused (OR, 1.31; 95% CI, 1.13–1.5).
5. Perform uterine-compression sutures within 1 hour
after delivery
Kayem G, Kurinczuik JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M; UK Obstetric Surveillance System (UKOSS). Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. 2011;117(1):14–20.
Balloon tamponade of the uterine cavity and uterine-compression sutures are crucial in the management of PPH. In a series of 211 women who were treated with a uterine-compression suture to control PPH, the rate of hysterectomy was 16% if the procedure was performed within an hour of delivery, but it rose to 42% with a delay of 2 to 6 hours.
6. When you suspect placenta previa or placenta accreta,
plan delivery by a multidisciplinary team
Eller AG, Bennett MA, Sharshiner M, et al. Maternal morbidity in cases of placenta accreta managed by multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011;117(2 Pt 1):331–337.
Placenta previa and placenta accreta are frequently associated with severe intrapartum and postpartum hemorrhage. In a retrospective cohort study of 141 cases of placenta accreta that were managed by a multidisciplinary care team (n=79) or received standard obstetric care (n=62), women managed by the multidisciplinary team were less likely (43% vs 61%) to require a large volume of transfusion. They were also less likely to require reoperation within 7 days of delivery for bleeding complications (3% vs 36%) and less likely to experience composite maternal morbidity (47% vs 75%).
Sentilhes L, Ambroselli C, Kayem G, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010;115(3):526–534.
Extirpative surgery in the form of hysterectomy—with or without partial bladder resection—is usually considered the treatment of choice for these conditions. A retrospective multicenter study reported maternal outcomes after conservative treatment of 167 women who had placenta accreta or percreta (18% had percreta). Conservative management included one or more of the following:
- stepwise uterine devascularization
- pelvic vessel ligation or embolization
- uterine-compression sutures
- administration of methotrexate and antibiotics.
Conservative treatment was successful in 131 (78.5%) cases. Eighteen women underwent primary hysterectomy, and 18 women underwent delayed hysterectomy. One woman died after intraumbilical methotrexate administration, and 10 women (6%) experienced severe morbidity.
Conservative management should be offered only in centers that have adequate equipment and resources for patients who are properly counseled and who are motivated and agree to close follow-up. Planned cesarean hysterectomy remains the treatment of choice for multiparous women, as well as for women who have multiple cesarean deliveries with accreta, and those who do not accept the risks or who are not motivated to undergo close and prolonged follow-up.
8. Beware of von Willebrand disease
Pacheco LD, Costantine M, Saade GR, et al. von Willebrand disease and pregnancy: a practical approach for the diagnosis and treatment. Am J Obstet Gynecol. 2010;203(3):194–200.
This disease can cause immediate and delayed postpartum hemorrhage and has a prevalence of approximately 1% in the general population. Sixteen percent to 29% of women who have von Willebrand disease will experience PPH within 24 hours after delivery, and 20% to 29% will experience delayed postpartum bleeding.
Patients who have this disease should be managed in consultation with a hematologist and blood bank personnel. It entails use of desmopressin, plasma concentrates that contain von Willebrand factor (Humate-P), or cryoprecipitate.
9. Have fibrinogen concentrate on hand
Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. Int J Obstet Anaesth. 2010;19(2):218–223.
Rahe-Mayer N, Sørensen B. Fibrinogen concentrate for management of bleeding. J Thromb Haemost. 2011;9(1):1–5.
This product can correct hypofibrinogenemia very rapidly. In women who have severe PPH, hypofibrinogenemia may develop as a result of dilutional coagulopathy or hypofibrinogemia in conditions such as abruptio placentae with fetal demise, acute fatty liver of pregnancy, or amniotic fluid embolism. Treatment requires a high volume of fresh frozen plasma or cryoprecipitate. Fibrinogen concentrate is stored at room temperature, requires no cross-matching, and can be prepared and infused within 3 minutes.
10. Implement a protocol for massive transfusion
Sibai BM. Evaluation and management of postpartum hemorrhage. In: Management of Acute Obstetric Emergencies. New York, NY: Elsevier; 2011:41–70.
A delay in the treatment of hypovolemic shock can cause ischemic injury to the kidneys, liver, myocardium, and brain and can lead to diffuse intravascular coagulation (DIC), adult respiratory distress syndrome, and death. The objectives for having a protocol for massive transfusion include:
- administration of adequate blood and blood products
- maintenance of tissue perfusion
- ensuring adequate oxygen delivery
- correction of DIC.
These objectives are vital while the team is working to control the source of bleeding.
BY EXPERT AUTHORS
- “Postpartum hemorrhage: 11 critical questions, answered by an expert”
Q&A with Haywood L. Brown, MD(January 2011) - “What you can do to optimize blood conservation in ObGyn practice”
Eric J. Bieber, MD; Linda Scott, RN; Corinna Muller, DO; Nancy Nuss, RN; and Edie L. Derian, MD(February 2010) - “Planning reduces the risk of maternal death. This tool helps.”
Robert L. Barbieri, MD (Editorial; August 2009) - “You should add the Bakri balloon to your treatments for OB bleeds”
Robert L. Barbieri, MD (Editorial; February 2009) - “Consider retroperitoneal packing for postpartum hemorrhage”
Maj. William R. Fulton, DO (July 2008) - “Give a uterotonic routinely during the third stage of labor”
Robert L. Barbieri, MD (Editorial; May 2007)
For a related malpractice case, read Medical Verdicts.
We want to hear from you! Tell us what you think.
Dr. Sibai reports no financial relationships relevant to this article.
By the time a pregnancy reaches term, approximately 500 to 800 mL of blood are circulating through the uterus and placenta every minute, thanks to the intricate network of blood vessels permeating these organs. So it is not surprising that postpartum hemorrhage complicates as many as one in every 20 deliveries, both vaginal and cesarean. Usually, hemorrhage is the result of uterine atony, but other entities may also cause or contribute to acute bleeding.
Severe postpartum hemorrhage, defined as a loss of more than 1,500 mL of blood, complicates approximately 1% of all deliveries and is a leading cause of maternal death. Severe PPH poses serious and often unpredictable challenges to obstetric providers, from the need to make an early diagnosis and establish and treat the cause to the ability to manage hemorrhagic shock.
In this article, I sort through data on the management of this potentially catastrophic event and summarize 10 evidence-based recommendations that can help reduce acute and long-term maternal complications.
1. Plan and rehearse a step-by-step approach
Wise A, Clark V. Challenges of major obstetric haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2010;24(3):353–365.
It is important to anticipate and prepare for the possibility of PPH so that you can respond quickly and effectively when it occurs. Evaluation and management should be simultaneous and should not be hindered by confusion or chaos. Successful management requires early recognition; identification of the cause; the securing of help; continuous monitoring of vital signs and blood loss; prompt resuscitation with fluids, blood, and blood products; and medical or surgical treatment.
2. Know the signs and symptoms of severe hemorrhage
Moore J, Chandraharn E. Management of massive postpartum haemorrhage and coagulopathy. Obstet Gynaecol Reprod Med. 2010;20(6):174–180.
Persistent vaginal bleeding is the first sign of PPH. The bleeding may be continuous oozing or it may be profuse. In addition to bleeding, the patient will exhibit several of the signs and symptoms listed in the TABLE.
Signs and symptoms of postpartum hemorrhage
| Signs | Symptoms |
|---|---|
| Systolic pressure, ≤90 mm Hg | Anxiety |
| Restlessness | |
| Pulse, ≥110 beats per minute | Tachypnea |
| Narrow pulse pressure | Dizziness |
| Hunger for air | |
| Coldness and clamminess | Confusion |
| Pale appearance | |
| Oliguria or anuria |
3. Call for help within 10 minutes after making
the diagnosis of PPH
Driessen M, Bouvier-Colle MH, Dupont C, et al. Postpartum hemorrhage resulting from uterine atony after vaginal delivery. Factors associated with severity. Obstet Gynecol. 2011;117(1):21–31.
In the early stages of uterine atony, delaying care beyond 10 minutes increases the risk of severe PPH.
4. Identify patients at very high risk of hysterectomy
and end-organ dysfunction
O’Brien D, Babiker E, O’Sullivan O, et al. Prediction of peripartum hysterectomy and end organ dysfunction in major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2010;153(2):165–169.
Rossi AC, Lee RH, Chmait RH. Emergency postpartum hysterectomy for uncontrolled postpartum bleeding: a systematic review. Obstet Gynecol. 2010;115(3):637–644.
In a study of 117 cases of severe obstetric hemorrhage, several independent risk factors for peripartum hysterectomy and end-organ dysfunction were identified:
- number of previous cesarean deliveries (odds ratio [OR], 3.28; 95% confidence interval [CI], 1.95–5.5)
- placenta previa (OR, 13.5; 95% CI, 7.7–184)
- placenta accreta (OR, 37.7; 95% CI, 7.7–184)
- uterine rupture (OR, 7.25; 95% CI, 1.25–42)
- number of units of red blood cells (RBCs) transfused (OR, 1.31; 95% CI, 1.13–1.5).
5. Perform uterine-compression sutures within 1 hour
after delivery
Kayem G, Kurinczuik JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M; UK Obstetric Surveillance System (UKOSS). Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. 2011;117(1):14–20.
Balloon tamponade of the uterine cavity and uterine-compression sutures are crucial in the management of PPH. In a series of 211 women who were treated with a uterine-compression suture to control PPH, the rate of hysterectomy was 16% if the procedure was performed within an hour of delivery, but it rose to 42% with a delay of 2 to 6 hours.
6. When you suspect placenta previa or placenta accreta,
plan delivery by a multidisciplinary team
Eller AG, Bennett MA, Sharshiner M, et al. Maternal morbidity in cases of placenta accreta managed by multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011;117(2 Pt 1):331–337.
Placenta previa and placenta accreta are frequently associated with severe intrapartum and postpartum hemorrhage. In a retrospective cohort study of 141 cases of placenta accreta that were managed by a multidisciplinary care team (n=79) or received standard obstetric care (n=62), women managed by the multidisciplinary team were less likely (43% vs 61%) to require a large volume of transfusion. They were also less likely to require reoperation within 7 days of delivery for bleeding complications (3% vs 36%) and less likely to experience composite maternal morbidity (47% vs 75%).
Sentilhes L, Ambroselli C, Kayem G, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010;115(3):526–534.
Extirpative surgery in the form of hysterectomy—with or without partial bladder resection—is usually considered the treatment of choice for these conditions. A retrospective multicenter study reported maternal outcomes after conservative treatment of 167 women who had placenta accreta or percreta (18% had percreta). Conservative management included one or more of the following:
- stepwise uterine devascularization
- pelvic vessel ligation or embolization
- uterine-compression sutures
- administration of methotrexate and antibiotics.
Conservative treatment was successful in 131 (78.5%) cases. Eighteen women underwent primary hysterectomy, and 18 women underwent delayed hysterectomy. One woman died after intraumbilical methotrexate administration, and 10 women (6%) experienced severe morbidity.
Conservative management should be offered only in centers that have adequate equipment and resources for patients who are properly counseled and who are motivated and agree to close follow-up. Planned cesarean hysterectomy remains the treatment of choice for multiparous women, as well as for women who have multiple cesarean deliveries with accreta, and those who do not accept the risks or who are not motivated to undergo close and prolonged follow-up.
8. Beware of von Willebrand disease
Pacheco LD, Costantine M, Saade GR, et al. von Willebrand disease and pregnancy: a practical approach for the diagnosis and treatment. Am J Obstet Gynecol. 2010;203(3):194–200.
This disease can cause immediate and delayed postpartum hemorrhage and has a prevalence of approximately 1% in the general population. Sixteen percent to 29% of women who have von Willebrand disease will experience PPH within 24 hours after delivery, and 20% to 29% will experience delayed postpartum bleeding.
Patients who have this disease should be managed in consultation with a hematologist and blood bank personnel. It entails use of desmopressin, plasma concentrates that contain von Willebrand factor (Humate-P), or cryoprecipitate.
9. Have fibrinogen concentrate on hand
Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. Int J Obstet Anaesth. 2010;19(2):218–223.
Rahe-Mayer N, Sørensen B. Fibrinogen concentrate for management of bleeding. J Thromb Haemost. 2011;9(1):1–5.
This product can correct hypofibrinogenemia very rapidly. In women who have severe PPH, hypofibrinogenemia may develop as a result of dilutional coagulopathy or hypofibrinogemia in conditions such as abruptio placentae with fetal demise, acute fatty liver of pregnancy, or amniotic fluid embolism. Treatment requires a high volume of fresh frozen plasma or cryoprecipitate. Fibrinogen concentrate is stored at room temperature, requires no cross-matching, and can be prepared and infused within 3 minutes.
10. Implement a protocol for massive transfusion
Sibai BM. Evaluation and management of postpartum hemorrhage. In: Management of Acute Obstetric Emergencies. New York, NY: Elsevier; 2011:41–70.
A delay in the treatment of hypovolemic shock can cause ischemic injury to the kidneys, liver, myocardium, and brain and can lead to diffuse intravascular coagulation (DIC), adult respiratory distress syndrome, and death. The objectives for having a protocol for massive transfusion include:
- administration of adequate blood and blood products
- maintenance of tissue perfusion
- ensuring adequate oxygen delivery
- correction of DIC.
These objectives are vital while the team is working to control the source of bleeding.
BY EXPERT AUTHORS
- “Postpartum hemorrhage: 11 critical questions, answered by an expert”
Q&A with Haywood L. Brown, MD(January 2011) - “What you can do to optimize blood conservation in ObGyn practice”
Eric J. Bieber, MD; Linda Scott, RN; Corinna Muller, DO; Nancy Nuss, RN; and Edie L. Derian, MD(February 2010) - “Planning reduces the risk of maternal death. This tool helps.”
Robert L. Barbieri, MD (Editorial; August 2009) - “You should add the Bakri balloon to your treatments for OB bleeds”
Robert L. Barbieri, MD (Editorial; February 2009) - “Consider retroperitoneal packing for postpartum hemorrhage”
Maj. William R. Fulton, DO (July 2008) - “Give a uterotonic routinely during the third stage of labor”
Robert L. Barbieri, MD (Editorial; May 2007)
For a related malpractice case, read Medical Verdicts.
We want to hear from you! Tell us what you think.
Dr. Sibai reports no financial relationships relevant to this article.
By the time a pregnancy reaches term, approximately 500 to 800 mL of blood are circulating through the uterus and placenta every minute, thanks to the intricate network of blood vessels permeating these organs. So it is not surprising that postpartum hemorrhage complicates as many as one in every 20 deliveries, both vaginal and cesarean. Usually, hemorrhage is the result of uterine atony, but other entities may also cause or contribute to acute bleeding.
Severe postpartum hemorrhage, defined as a loss of more than 1,500 mL of blood, complicates approximately 1% of all deliveries and is a leading cause of maternal death. Severe PPH poses serious and often unpredictable challenges to obstetric providers, from the need to make an early diagnosis and establish and treat the cause to the ability to manage hemorrhagic shock.
In this article, I sort through data on the management of this potentially catastrophic event and summarize 10 evidence-based recommendations that can help reduce acute and long-term maternal complications.
1. Plan and rehearse a step-by-step approach
Wise A, Clark V. Challenges of major obstetric haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2010;24(3):353–365.
It is important to anticipate and prepare for the possibility of PPH so that you can respond quickly and effectively when it occurs. Evaluation and management should be simultaneous and should not be hindered by confusion or chaos. Successful management requires early recognition; identification of the cause; the securing of help; continuous monitoring of vital signs and blood loss; prompt resuscitation with fluids, blood, and blood products; and medical or surgical treatment.
2. Know the signs and symptoms of severe hemorrhage
Moore J, Chandraharn E. Management of massive postpartum haemorrhage and coagulopathy. Obstet Gynaecol Reprod Med. 2010;20(6):174–180.
Persistent vaginal bleeding is the first sign of PPH. The bleeding may be continuous oozing or it may be profuse. In addition to bleeding, the patient will exhibit several of the signs and symptoms listed in the TABLE.
Signs and symptoms of postpartum hemorrhage
| Signs | Symptoms |
|---|---|
| Systolic pressure, ≤90 mm Hg | Anxiety |
| Restlessness | |
| Pulse, ≥110 beats per minute | Tachypnea |
| Narrow pulse pressure | Dizziness |
| Hunger for air | |
| Coldness and clamminess | Confusion |
| Pale appearance | |
| Oliguria or anuria |
3. Call for help within 10 minutes after making
the diagnosis of PPH
Driessen M, Bouvier-Colle MH, Dupont C, et al. Postpartum hemorrhage resulting from uterine atony after vaginal delivery. Factors associated with severity. Obstet Gynecol. 2011;117(1):21–31.
In the early stages of uterine atony, delaying care beyond 10 minutes increases the risk of severe PPH.
4. Identify patients at very high risk of hysterectomy
and end-organ dysfunction
O’Brien D, Babiker E, O’Sullivan O, et al. Prediction of peripartum hysterectomy and end organ dysfunction in major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2010;153(2):165–169.
Rossi AC, Lee RH, Chmait RH. Emergency postpartum hysterectomy for uncontrolled postpartum bleeding: a systematic review. Obstet Gynecol. 2010;115(3):637–644.
In a study of 117 cases of severe obstetric hemorrhage, several independent risk factors for peripartum hysterectomy and end-organ dysfunction were identified:
- number of previous cesarean deliveries (odds ratio [OR], 3.28; 95% confidence interval [CI], 1.95–5.5)
- placenta previa (OR, 13.5; 95% CI, 7.7–184)
- placenta accreta (OR, 37.7; 95% CI, 7.7–184)
- uterine rupture (OR, 7.25; 95% CI, 1.25–42)
- number of units of red blood cells (RBCs) transfused (OR, 1.31; 95% CI, 1.13–1.5).
5. Perform uterine-compression sutures within 1 hour
after delivery
Kayem G, Kurinczuik JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M; UK Obstetric Surveillance System (UKOSS). Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. 2011;117(1):14–20.
Balloon tamponade of the uterine cavity and uterine-compression sutures are crucial in the management of PPH. In a series of 211 women who were treated with a uterine-compression suture to control PPH, the rate of hysterectomy was 16% if the procedure was performed within an hour of delivery, but it rose to 42% with a delay of 2 to 6 hours.
6. When you suspect placenta previa or placenta accreta,
plan delivery by a multidisciplinary team
Eller AG, Bennett MA, Sharshiner M, et al. Maternal morbidity in cases of placenta accreta managed by multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011;117(2 Pt 1):331–337.
Placenta previa and placenta accreta are frequently associated with severe intrapartum and postpartum hemorrhage. In a retrospective cohort study of 141 cases of placenta accreta that were managed by a multidisciplinary care team (n=79) or received standard obstetric care (n=62), women managed by the multidisciplinary team were less likely (43% vs 61%) to require a large volume of transfusion. They were also less likely to require reoperation within 7 days of delivery for bleeding complications (3% vs 36%) and less likely to experience composite maternal morbidity (47% vs 75%).
Sentilhes L, Ambroselli C, Kayem G, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010;115(3):526–534.
Extirpative surgery in the form of hysterectomy—with or without partial bladder resection—is usually considered the treatment of choice for these conditions. A retrospective multicenter study reported maternal outcomes after conservative treatment of 167 women who had placenta accreta or percreta (18% had percreta). Conservative management included one or more of the following:
- stepwise uterine devascularization
- pelvic vessel ligation or embolization
- uterine-compression sutures
- administration of methotrexate and antibiotics.
Conservative treatment was successful in 131 (78.5%) cases. Eighteen women underwent primary hysterectomy, and 18 women underwent delayed hysterectomy. One woman died after intraumbilical methotrexate administration, and 10 women (6%) experienced severe morbidity.
Conservative management should be offered only in centers that have adequate equipment and resources for patients who are properly counseled and who are motivated and agree to close follow-up. Planned cesarean hysterectomy remains the treatment of choice for multiparous women, as well as for women who have multiple cesarean deliveries with accreta, and those who do not accept the risks or who are not motivated to undergo close and prolonged follow-up.
8. Beware of von Willebrand disease
Pacheco LD, Costantine M, Saade GR, et al. von Willebrand disease and pregnancy: a practical approach for the diagnosis and treatment. Am J Obstet Gynecol. 2010;203(3):194–200.
This disease can cause immediate and delayed postpartum hemorrhage and has a prevalence of approximately 1% in the general population. Sixteen percent to 29% of women who have von Willebrand disease will experience PPH within 24 hours after delivery, and 20% to 29% will experience delayed postpartum bleeding.
Patients who have this disease should be managed in consultation with a hematologist and blood bank personnel. It entails use of desmopressin, plasma concentrates that contain von Willebrand factor (Humate-P), or cryoprecipitate.
9. Have fibrinogen concentrate on hand
Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. Int J Obstet Anaesth. 2010;19(2):218–223.
Rahe-Mayer N, Sørensen B. Fibrinogen concentrate for management of bleeding. J Thromb Haemost. 2011;9(1):1–5.
This product can correct hypofibrinogenemia very rapidly. In women who have severe PPH, hypofibrinogenemia may develop as a result of dilutional coagulopathy or hypofibrinogemia in conditions such as abruptio placentae with fetal demise, acute fatty liver of pregnancy, or amniotic fluid embolism. Treatment requires a high volume of fresh frozen plasma or cryoprecipitate. Fibrinogen concentrate is stored at room temperature, requires no cross-matching, and can be prepared and infused within 3 minutes.
10. Implement a protocol for massive transfusion
Sibai BM. Evaluation and management of postpartum hemorrhage. In: Management of Acute Obstetric Emergencies. New York, NY: Elsevier; 2011:41–70.
A delay in the treatment of hypovolemic shock can cause ischemic injury to the kidneys, liver, myocardium, and brain and can lead to diffuse intravascular coagulation (DIC), adult respiratory distress syndrome, and death. The objectives for having a protocol for massive transfusion include:
- administration of adequate blood and blood products
- maintenance of tissue perfusion
- ensuring adequate oxygen delivery
- correction of DIC.
These objectives are vital while the team is working to control the source of bleeding.
BY EXPERT AUTHORS
- “Postpartum hemorrhage: 11 critical questions, answered by an expert”
Q&A with Haywood L. Brown, MD(January 2011) - “What you can do to optimize blood conservation in ObGyn practice”
Eric J. Bieber, MD; Linda Scott, RN; Corinna Muller, DO; Nancy Nuss, RN; and Edie L. Derian, MD(February 2010) - “Planning reduces the risk of maternal death. This tool helps.”
Robert L. Barbieri, MD (Editorial; August 2009) - “You should add the Bakri balloon to your treatments for OB bleeds”
Robert L. Barbieri, MD (Editorial; February 2009) - “Consider retroperitoneal packing for postpartum hemorrhage”
Maj. William R. Fulton, DO (July 2008) - “Give a uterotonic routinely during the third stage of labor”
Robert L. Barbieri, MD (Editorial; May 2007)
For a related malpractice case, read Medical Verdicts.
We want to hear from you! Tell us what you think.