User login
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
THE DIAGNOSIS: Cutaneous Leishmaniasis
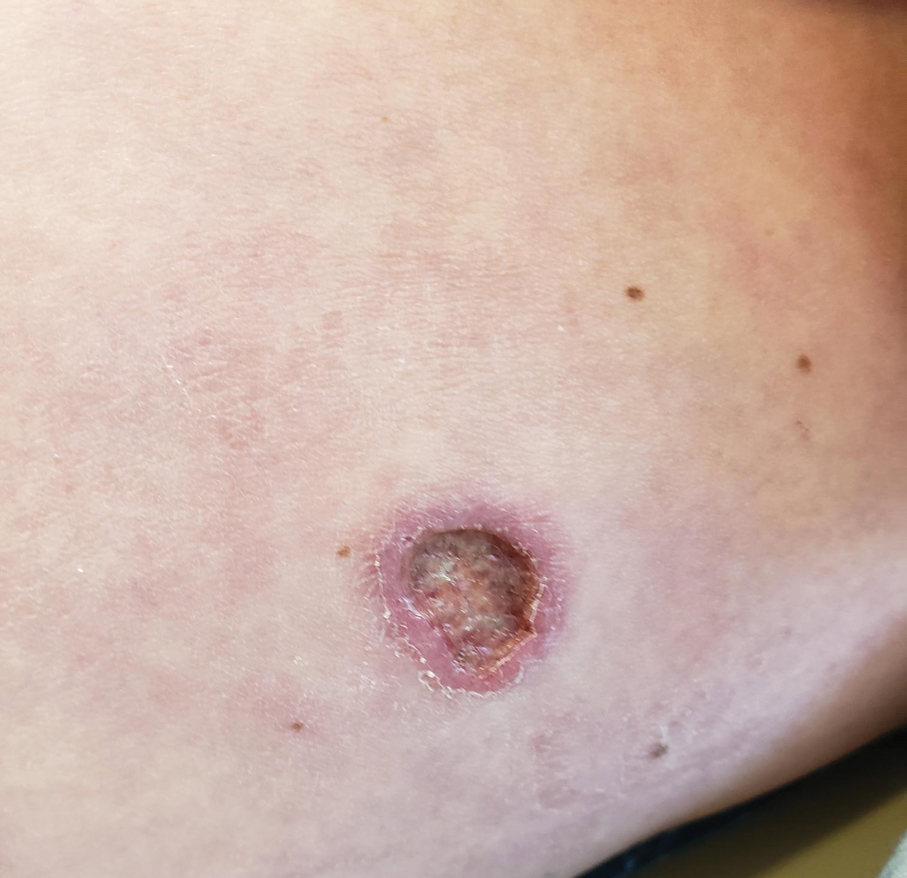
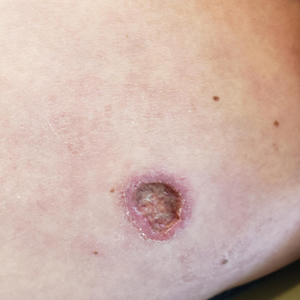
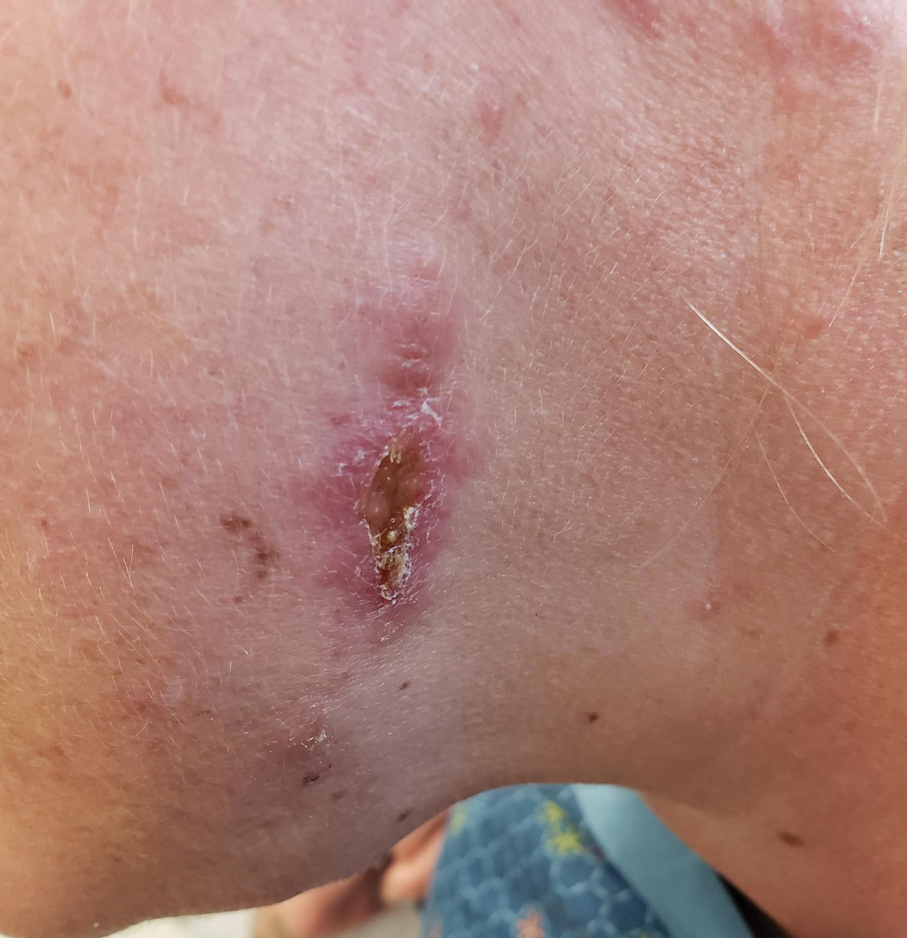
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.
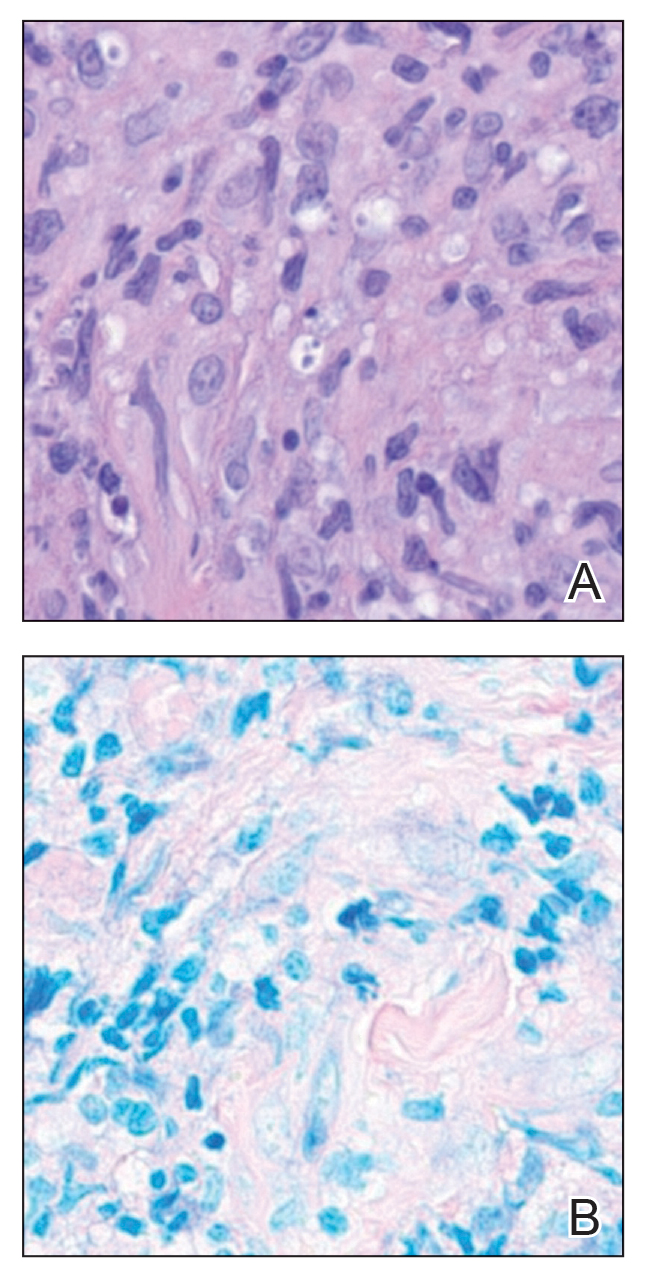
Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).
Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.

Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).

Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.

Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).

Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
A 43-year-old woman presented to the dermatology clinic with widespread scaly plaques and ulcerations of 2 months’ duration. Her medical history was otherwise unremarkable. The patient reported that the eruption began after returning from a vacation to Costa Rica, during which she spent time on the beach and white-water rafting. She noted that she had been exposed to numerous insects during her trip, and that her roommate, who had accompanied her, had similar exposure history and lesions. The plaques were refractory to multiple oral antibiotics previously prescribed by primary care. Physical examination revealed submental lymphadenopathy and painless ulcerations with indurated borders without purulent drainage alongside scattered scaly papules and plaques on the face, neck, arms, and legs. A biopsy was taken from an ulceration edge on the left thigh.