User login
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.
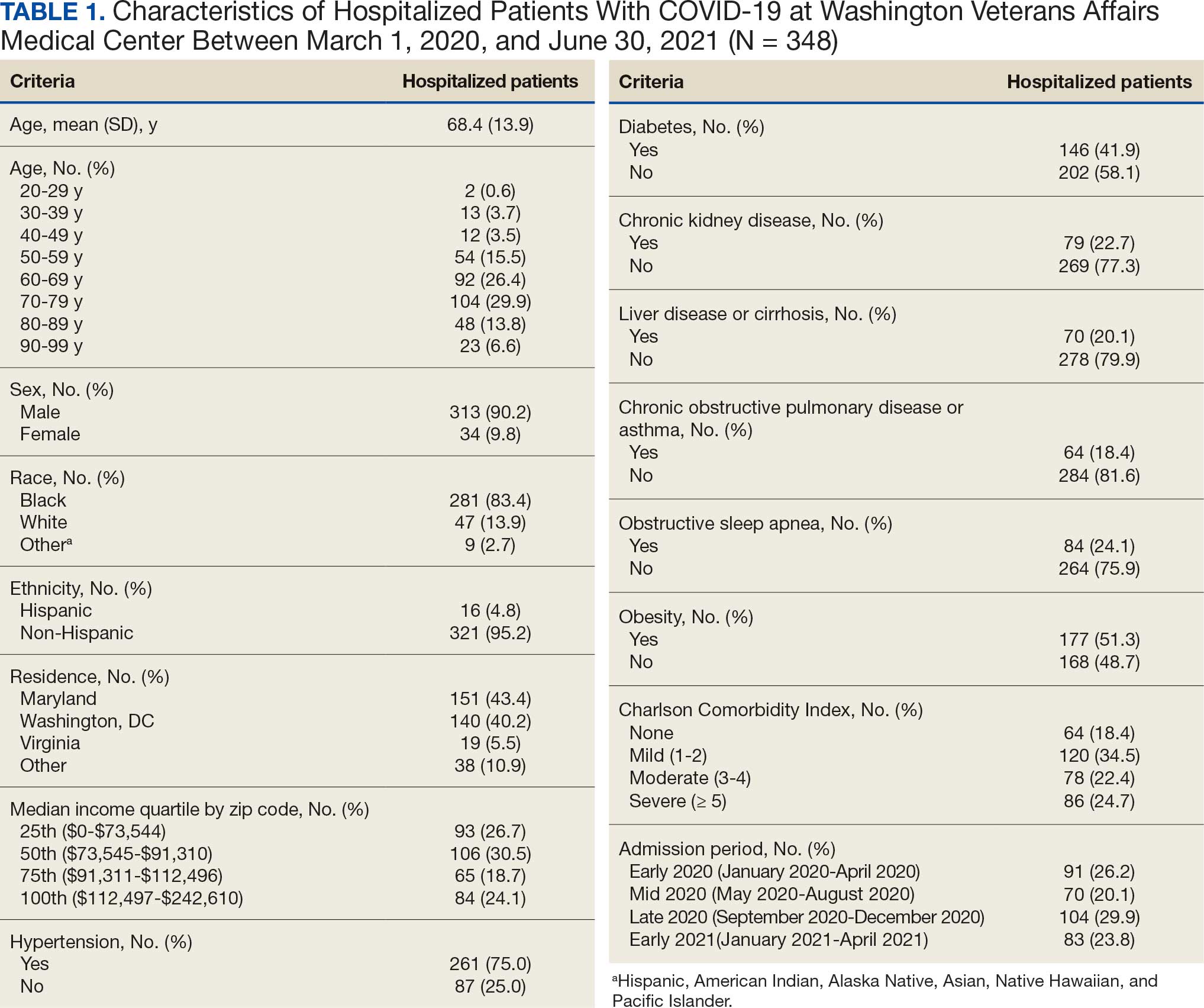
Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
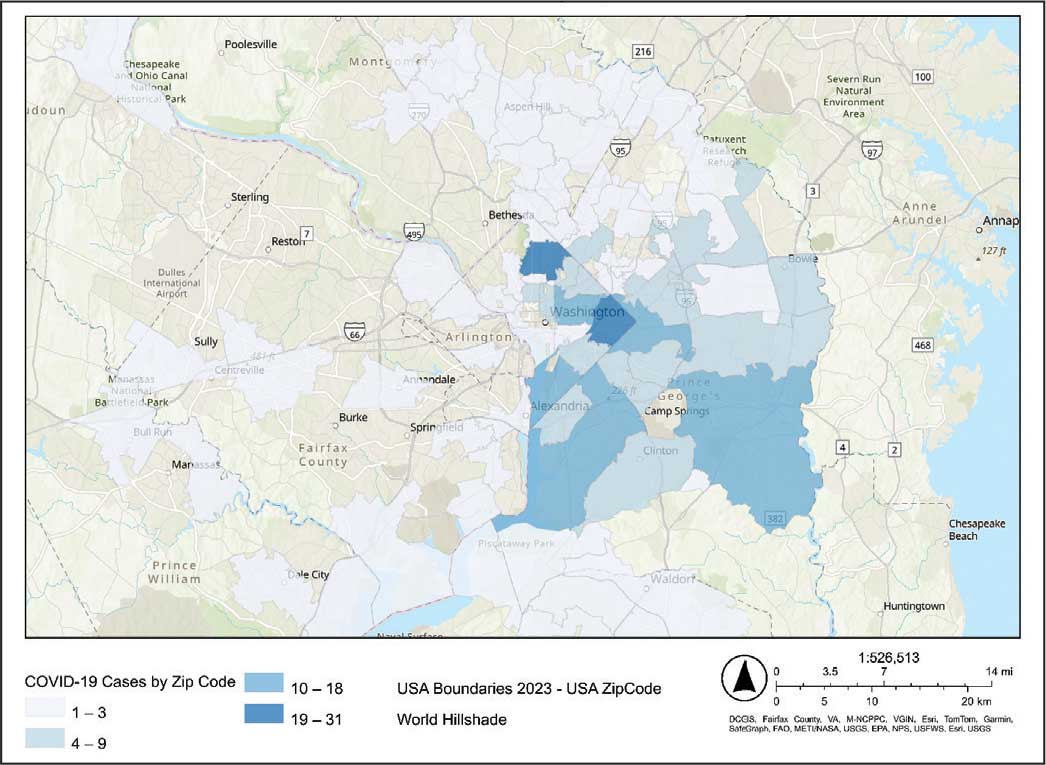
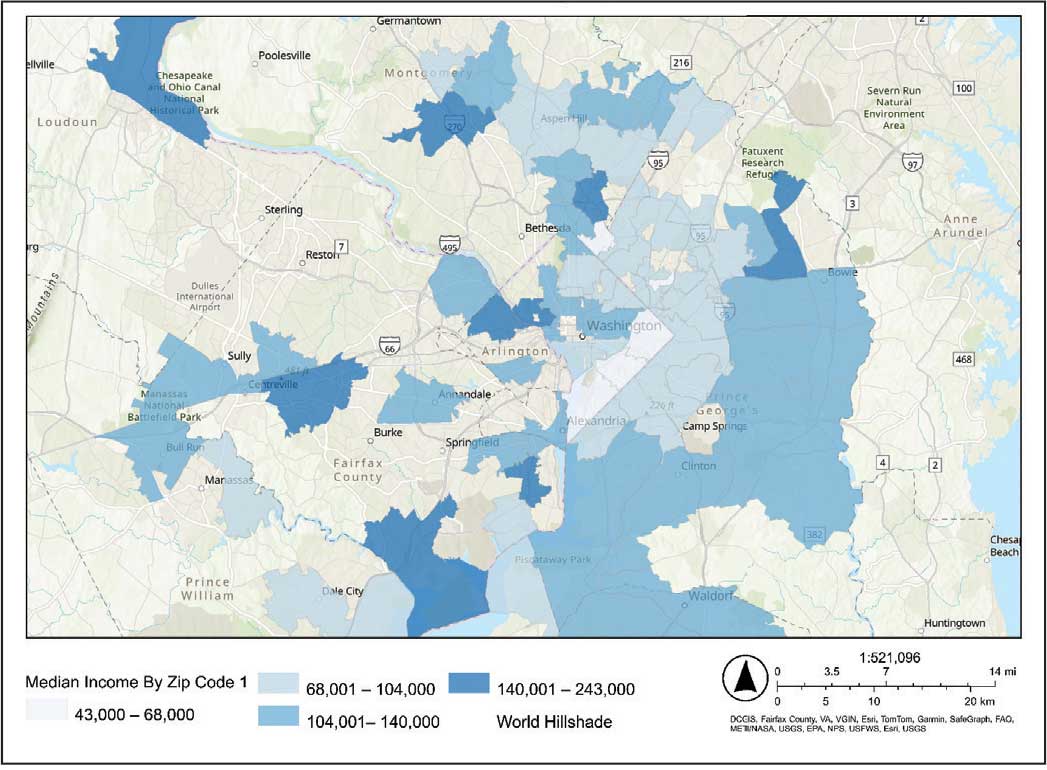
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).
Code
Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.
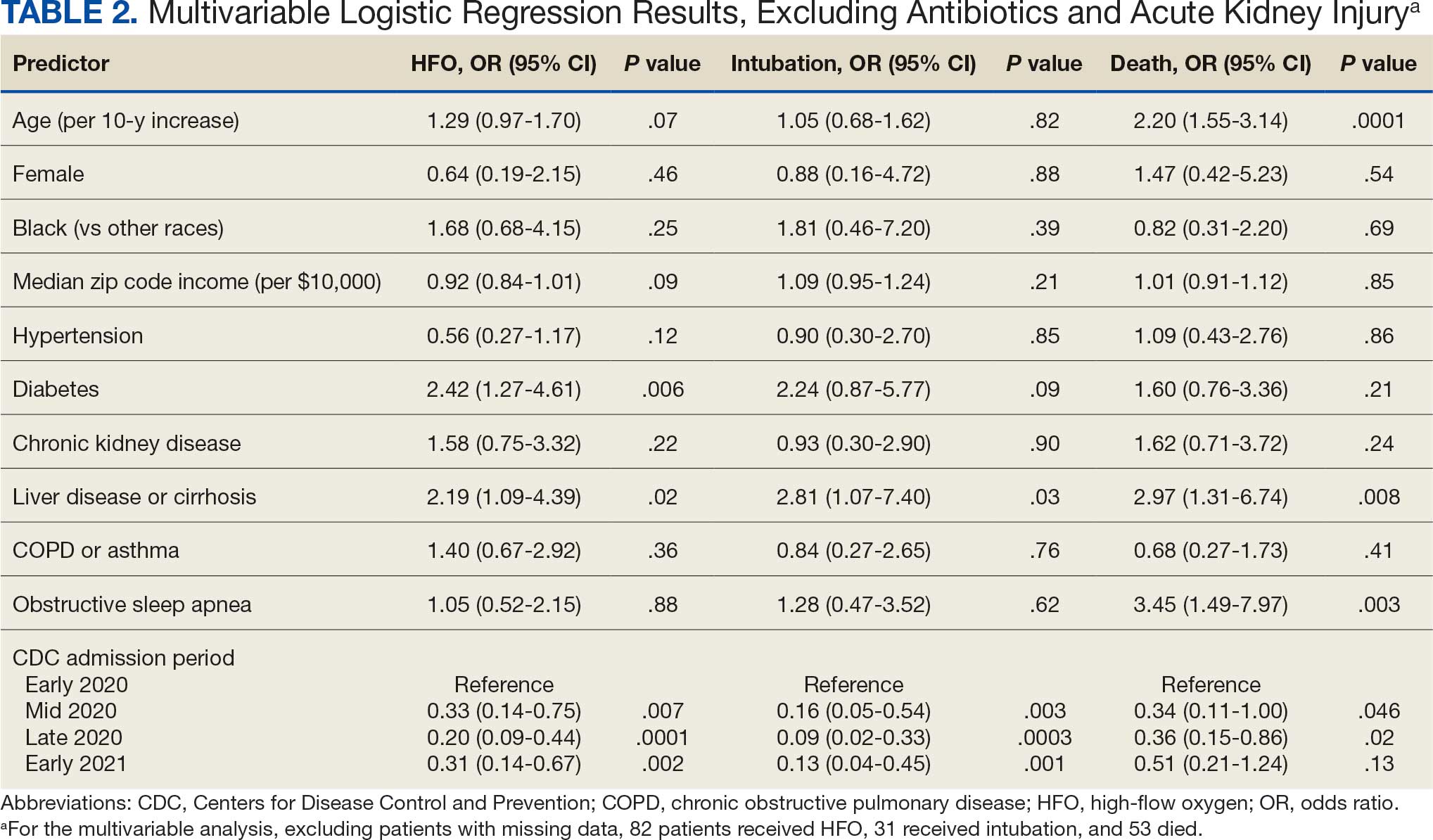
Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.

Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).

Code

Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.

Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.

Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).

Code

Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.

Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Weekends Off on Clinical Rotations? Examining Clinical Opportunity Trends on Weekdays vs Weekends During Internal Medicine Clerkship Rotations in Veterans Health Administration Inpatient Wards
Background
The Accreditation Council for Graduate Medical Education (ACGME) mandates an 80-hour weekly work limit for residents.1 In contrast, decisions regarding undergraduate medical education (UME) are strongly influenced locally, with individual institutions setting academic policy for students. These differences in oversight reflect fundamental differences in residents’ and students’ roles in patient care, power, and responsibility. Considering rotation schedules, internal medicine (IM) clerkship directors have discussed the relative value of weekend vs weekday duty during inpatient rotations, a scheduling topic of interest to students as well, though these conversations are limited by a lack of knowledge regarding admission patterns. Addressing this information gap would inform policy decisions.
The Veterans Health Administration (VHA) is uniquely positioned to address questions about UME clinical experiences nationwide: annually, over 118,000 students representing 97% of US medical schools train at VHA facilities.2,3 We aim to compare the number and variety of patient encounter opportunities presenting during inpatient VHA IM rotations on weekdays versus weekends to inform policy decisions for UME rotation schedules.
Innovation
The VHA Corporate Data Warehouse will be queried for all admissions, diagnoses, and length of stay on inpatient IM services at the 420 VHA hospitals affiliated with US medical schools from 2016-2026. We will aggregate case data for day of week, floor, hospital, and Veteran Integrated Service Network (VISN), and determine number of admissions by weekday (Monday-Friday) and weekend (Saturday-Sunday). Weekday vs. weekend admission data will be compared using generalized mixed effects models for clustered longitudinal data. Heterogeneity across hospitals and VISNs will be explored to examine unique regional trends.
Results
We have drafted strategies to query and curate relevant datasets, developed a preliminary analysis plan, and await data deployment from VHA data stewards.
Conclusions
We believe this will be the first VHA-wide evaluation of patient encounter trends on IM services to examine potential training experiences for medical students. This will increase understanding of the critical role VHA has in developing the nations’ healthcare workforce, and how patterns of opportunities for clinical education may be distributed over time, informing decisions about rotation schedules to maximize students’ abilities to interact with, learn from, and serve our nation’s veterans
- Dimitris KD, Taylor BC, Fankhauser RA. Resident work-week regulations: historical review and modern perspectives. J Surg Educ. 2008;65(4):290-296. doi:10.1016/j.jsurg.2008.05.011
- Health professions education statistics. Veterans Health Administration. Accessed March 19, 2025. https://www.va.gov/oaa/docs/OAACurrentStats.pdf
- Medical education at VA: It’s all about the Veterans. VA News. Updated August 16, 2021. Accessed March 19, 2025. https://news.va.gov/93370/medical-education-at-va-its-all-about-the-veterans/
Background
The Accreditation Council for Graduate Medical Education (ACGME) mandates an 80-hour weekly work limit for residents.1 In contrast, decisions regarding undergraduate medical education (UME) are strongly influenced locally, with individual institutions setting academic policy for students. These differences in oversight reflect fundamental differences in residents’ and students’ roles in patient care, power, and responsibility. Considering rotation schedules, internal medicine (IM) clerkship directors have discussed the relative value of weekend vs weekday duty during inpatient rotations, a scheduling topic of interest to students as well, though these conversations are limited by a lack of knowledge regarding admission patterns. Addressing this information gap would inform policy decisions.
The Veterans Health Administration (VHA) is uniquely positioned to address questions about UME clinical experiences nationwide: annually, over 118,000 students representing 97% of US medical schools train at VHA facilities.2,3 We aim to compare the number and variety of patient encounter opportunities presenting during inpatient VHA IM rotations on weekdays versus weekends to inform policy decisions for UME rotation schedules.
Innovation
The VHA Corporate Data Warehouse will be queried for all admissions, diagnoses, and length of stay on inpatient IM services at the 420 VHA hospitals affiliated with US medical schools from 2016-2026. We will aggregate case data for day of week, floor, hospital, and Veteran Integrated Service Network (VISN), and determine number of admissions by weekday (Monday-Friday) and weekend (Saturday-Sunday). Weekday vs. weekend admission data will be compared using generalized mixed effects models for clustered longitudinal data. Heterogeneity across hospitals and VISNs will be explored to examine unique regional trends.
Results
We have drafted strategies to query and curate relevant datasets, developed a preliminary analysis plan, and await data deployment from VHA data stewards.
Conclusions
We believe this will be the first VHA-wide evaluation of patient encounter trends on IM services to examine potential training experiences for medical students. This will increase understanding of the critical role VHA has in developing the nations’ healthcare workforce, and how patterns of opportunities for clinical education may be distributed over time, informing decisions about rotation schedules to maximize students’ abilities to interact with, learn from, and serve our nation’s veterans
Background
The Accreditation Council for Graduate Medical Education (ACGME) mandates an 80-hour weekly work limit for residents.1 In contrast, decisions regarding undergraduate medical education (UME) are strongly influenced locally, with individual institutions setting academic policy for students. These differences in oversight reflect fundamental differences in residents’ and students’ roles in patient care, power, and responsibility. Considering rotation schedules, internal medicine (IM) clerkship directors have discussed the relative value of weekend vs weekday duty during inpatient rotations, a scheduling topic of interest to students as well, though these conversations are limited by a lack of knowledge regarding admission patterns. Addressing this information gap would inform policy decisions.
The Veterans Health Administration (VHA) is uniquely positioned to address questions about UME clinical experiences nationwide: annually, over 118,000 students representing 97% of US medical schools train at VHA facilities.2,3 We aim to compare the number and variety of patient encounter opportunities presenting during inpatient VHA IM rotations on weekdays versus weekends to inform policy decisions for UME rotation schedules.
Innovation
The VHA Corporate Data Warehouse will be queried for all admissions, diagnoses, and length of stay on inpatient IM services at the 420 VHA hospitals affiliated with US medical schools from 2016-2026. We will aggregate case data for day of week, floor, hospital, and Veteran Integrated Service Network (VISN), and determine number of admissions by weekday (Monday-Friday) and weekend (Saturday-Sunday). Weekday vs. weekend admission data will be compared using generalized mixed effects models for clustered longitudinal data. Heterogeneity across hospitals and VISNs will be explored to examine unique regional trends.
Results
We have drafted strategies to query and curate relevant datasets, developed a preliminary analysis plan, and await data deployment from VHA data stewards.
Conclusions
We believe this will be the first VHA-wide evaluation of patient encounter trends on IM services to examine potential training experiences for medical students. This will increase understanding of the critical role VHA has in developing the nations’ healthcare workforce, and how patterns of opportunities for clinical education may be distributed over time, informing decisions about rotation schedules to maximize students’ abilities to interact with, learn from, and serve our nation’s veterans
- Dimitris KD, Taylor BC, Fankhauser RA. Resident work-week regulations: historical review and modern perspectives. J Surg Educ. 2008;65(4):290-296. doi:10.1016/j.jsurg.2008.05.011
- Health professions education statistics. Veterans Health Administration. Accessed March 19, 2025. https://www.va.gov/oaa/docs/OAACurrentStats.pdf
- Medical education at VA: It’s all about the Veterans. VA News. Updated August 16, 2021. Accessed March 19, 2025. https://news.va.gov/93370/medical-education-at-va-its-all-about-the-veterans/
- Dimitris KD, Taylor BC, Fankhauser RA. Resident work-week regulations: historical review and modern perspectives. J Surg Educ. 2008;65(4):290-296. doi:10.1016/j.jsurg.2008.05.011
- Health professions education statistics. Veterans Health Administration. Accessed March 19, 2025. https://www.va.gov/oaa/docs/OAACurrentStats.pdf
- Medical education at VA: It’s all about the Veterans. VA News. Updated August 16, 2021. Accessed March 19, 2025. https://news.va.gov/93370/medical-education-at-va-its-all-about-the-veterans/
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.
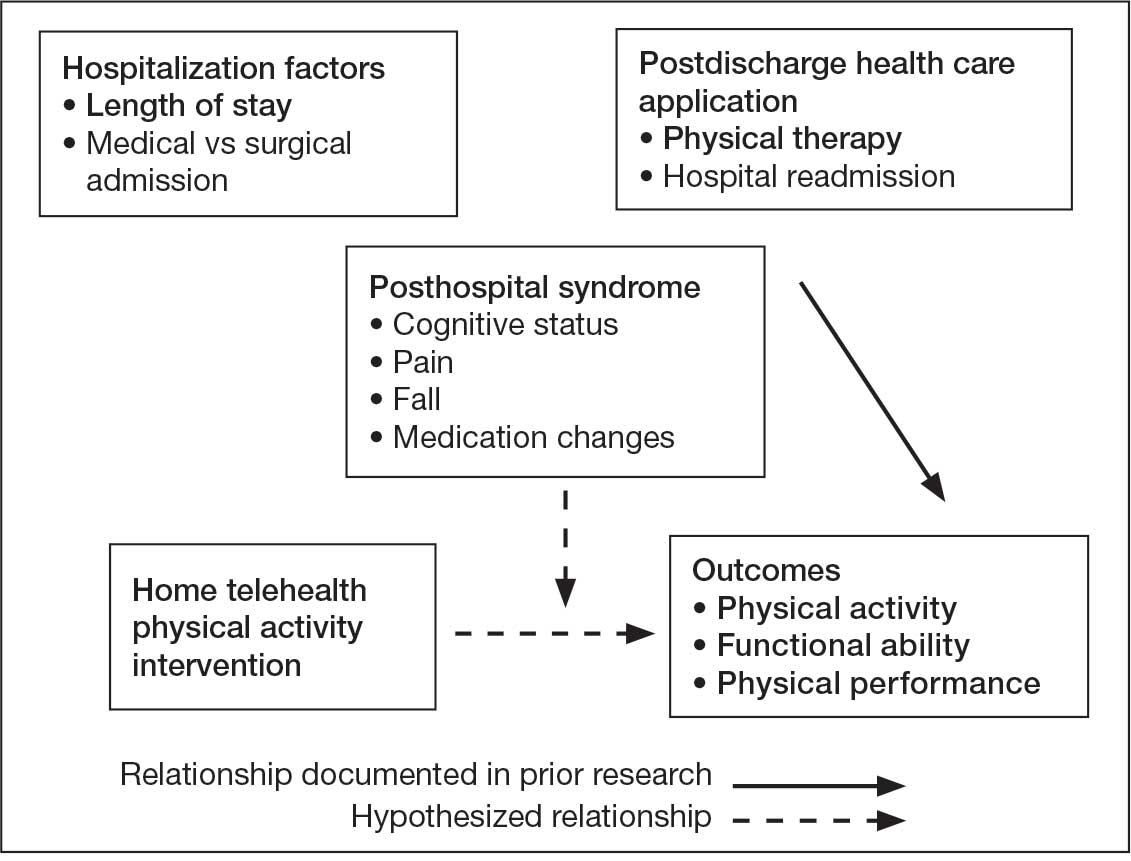
health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.
Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.
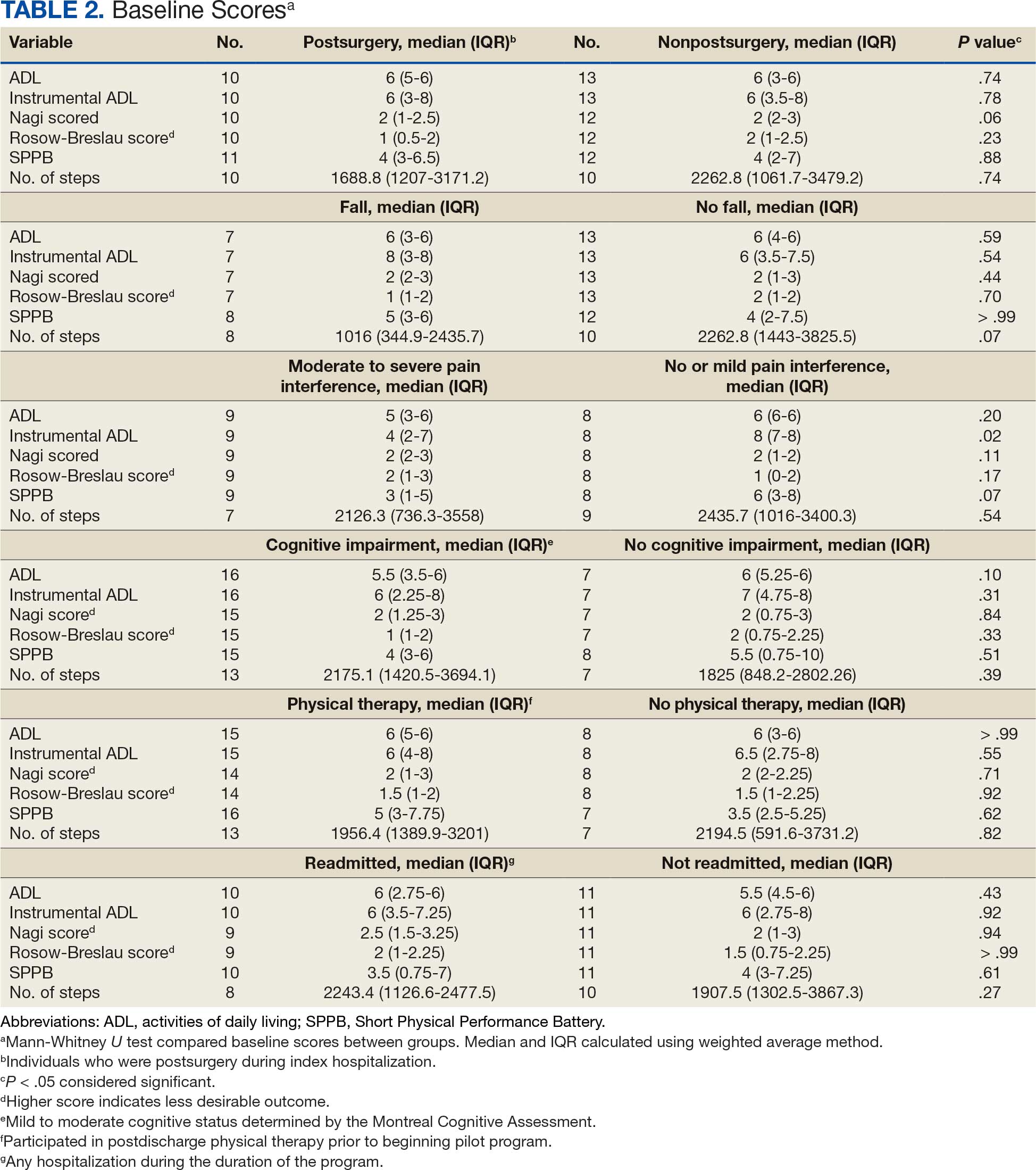
Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.
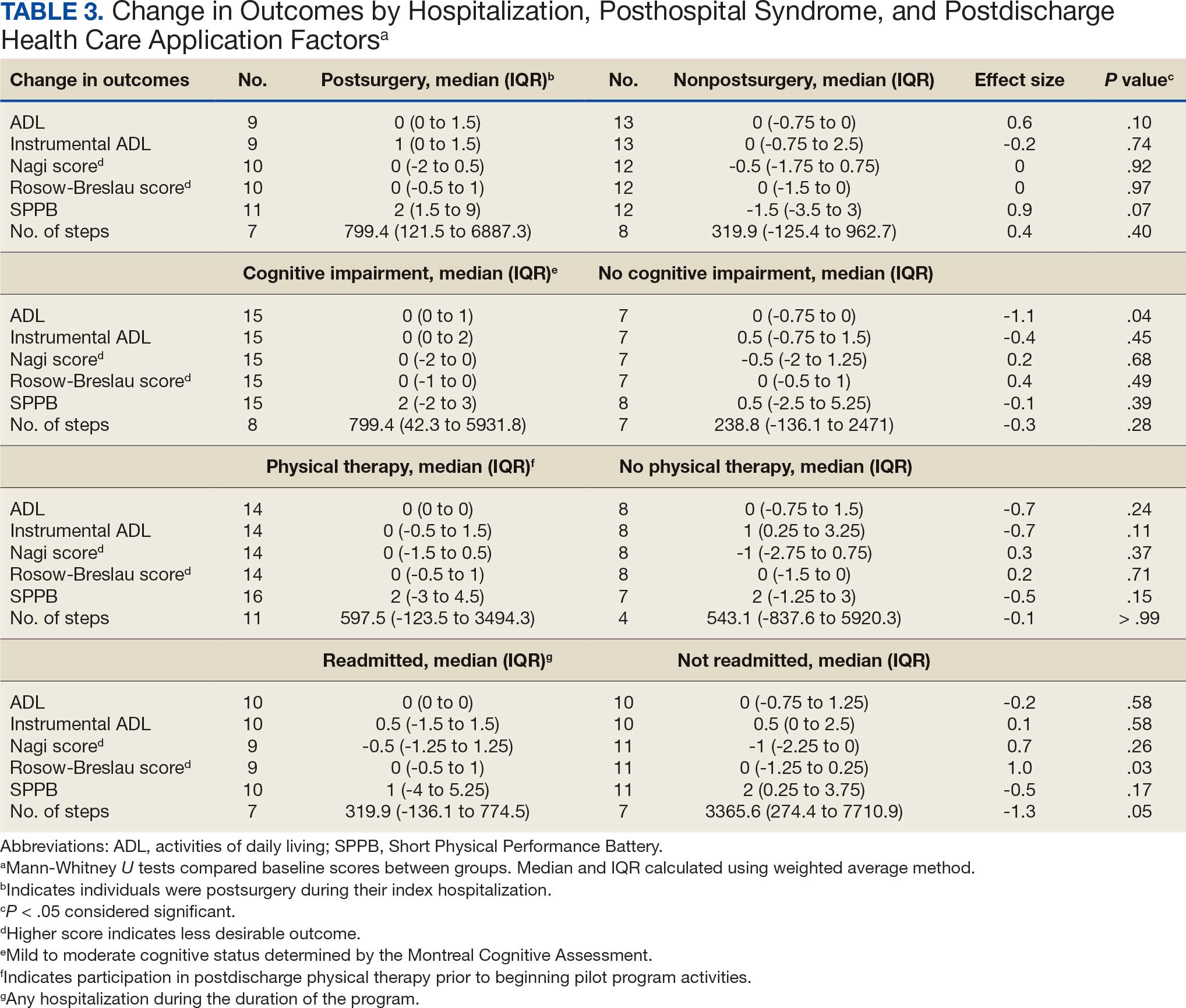
Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.

health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.

Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.

Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.

Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.

health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.

Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.

Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.

Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Hospitalists Must Encourage Mental Stimulation for Patients
As a hospitalist, you are in a unique position to notice changes in your hospitalized patients. This frontline perspective can be used to improve inpatient attention and care, and differs from primary care, where a clinician might only see a patient once or twice a year, and subtle, gradual changes may be missed, said George Cao, MD, MBA, a hospitalist at the University of Vermont Medical Center in Burlington and assistant professor at UVM’s Larner College of Medicine.
But in the hospital, Cao said even small shifts — like becoming less active, eating less, or changes in personality — can become much more obvious.
“As hospitalists…we see patients throughout the day, in different situations, and often end up spending more time with them over the course of a week than their primary care provider might in a year,” Cao explained. “This gives us a real advantage in picking up on subtle changes in mental awareness.”
These assessments can also be evaluated with the benefit of daily labs, frequent bedside interactions, and 24–hour observations.
With older adults, Cao said it’s important to go beyond just what’s in the chart.
“I always start by reviewing notes from the primary care provider and previous admissions, but some of the most valuable insights come from talking with family and close friends to get a true sense of the patient’s baseline — how they usually think, move, and interact,” he said.
Why to Watch for Declining Mental Awareness
Declining mental awareness in the inpatient setting is often a sign of an underlying problem — whether that’s a reversible medical condition, unrecognized dementia, or the development of delirium, Cao said.
“On the inpatient side, I pay close attention to more than just memory loss,” he said.
Changes in how patients function day–to–day, shifts in their behavior, or even something as simple as not wanting to get out of bed can be early signs of an aging mind or untreated psychiatric issues, he noted.
“Of course, we always rule out infections and medication side effects, but I also look for other reversible causes like thyroid problems, electrolyte imbalances, low oxygen, pain, urinary retention, constipation, and nutritional deficiencies,” Cao said.
Of note, delirium is the most common cause of sudden mental status changes in the hospital, and “it’s easy to miss if you’re not looking for it.”
He summarized that classic signs are an acute and fluctuating course with changes in alertness, but added there are other red flags too: disorientation, hallucinations, changes in sleep patterns, sporadic unsafe behaviors, mood swings, and changes in activity level, whether that’s agitation or just being unusually quiet.
By combining what he notices bedside and what is learned from the medical record (and from the people who know the patient best), Cao said he’s able to catch these changes early, identify the underlying cause, and work toward the best possible outcome.
“One of the main interventions is providing mental stimulation,” he said.
Why Mental Stimulation Is So Vital
Mental stimulation of the patient is critical to recovery and may prevent prolonged illness, said Meghana R. Medavaram, MD, associate director of consultation liaison and emergency psychiatry at Montefiore Medical Center’s Weiler Hospital in New York City. “Keeping a patient active both physically and mentally can help prevent deconditioning and risks of prolonged immobility,” she said.
It’s important to note that when patients are out of their familiar routines, away from their usual environment and people, and their sleep is fragmented, this can make them even more vulnerable. Keeping patients mentally stimulated during their hospital stay can help maintain their attention, orientation, and a healthy sleep-wake cycle — all things that are easily thrown off in the hospital, Cao said.
“These disruptions hit the pathways that control attention, wakefulness, and the sleep–wake cycle. That’s when you see attention drifting, orientation fading, and circadian rhythms unraveling, especially at night, which is why “sundowning” is so common, Cao said, referring to the syndrome where older adults or people with dementia experience behavioral changes in late afternoon or evening. “Mental stimulation is critical in the hospital because when the brain isn’t active and gets disoriented, it becomes an easy target for delirium.”
He said delirium often develops in older adults when acute stressors like inflammation, low oxygen, metabolic imbalances, or sedating medications disrupt the brain’s arousal systems and networks, especially in older adults.
Therefore, Cao said, encourage your patients to be more engaged during the day through conversation, activities, or regular reorientation. “This supports the brain networks that help prevent inattention and confusion, which are the hallmarks of delirium. Daytime stimulation also helps build up the natural drive for nighttime sleep, so patients are less likely to nap during the day and be awake and disoriented at night.”
To support this, it’s helpful to schedule medications during waking hours instead of around–the–clock dosing that interrupts sleep, and to cluster nighttime care activities to minimize disturbances, Cao explained. Ensuring patients have their glasses, hearing aids, and familiar routines, along with encouraging mobility and hydration, further protects against delirium and supports patients’ cognitive health during hospitalization. “These same principles are just as important in outpatient subacute rehab settings and at home, so it’s essential to take home these strategies after discharge,” he said.
A Family Member or Friend May Help
Hospitalists can suggest straightforward ways to encourage families and friends to keep patients engaged during a hospital stay. Visits and chats can go a long way as conversations are incredibly grounding, Cao said. Other methods could be bringing in favorite foods or snacks, a phone chat or video call, or even showing prerecorded video messages. “These can be effective. Patients respond well to seeing and hearing familiar faces and voices, even if it’s just on a screen,” Cao said.
Beyond that, he said, activities such as watching and discussing the news, reading aloud, using tablets for games, watching movies, doing crossword puzzles, knitting, reminiscing, and playing word games can also be mentally stimulating for patients.
In addition, safe exercises/activities that patients can do in bed — with advice from physical therapy and occupational therapy — are beneficial, Medavaram said. “These often include gentle range–of-motion activities,” she said.
Share Importance of Mental Stimulation With Patients and Caregivers
If a hospitalist wants to motivate patients to keep their minds active, the framing should be simple, positive, and tied directly to their goals of getting better and getting home, said Medavaram. She provided this script suggestion:
“One of the best ways to help your recovery isn’t just taking your medicine, it’s keeping your mind active. When you’re in the hospital, it’s easy to spend the day lying in bed and staring at the TV in your room, but that can make your brain slow down and even cause confusion. Simple things — like reading, talking with visitors, doing puzzles, listening to music you enjoy, or telling a nurse about your favorite memories — can keep your brain sharp. Staying mentally active helps your thinking stay clear and can even help you get home sooner. Think of it like physical therapy for your brain.”
A version of this article first appeared on Medscape.com.
As a hospitalist, you are in a unique position to notice changes in your hospitalized patients. This frontline perspective can be used to improve inpatient attention and care, and differs from primary care, where a clinician might only see a patient once or twice a year, and subtle, gradual changes may be missed, said George Cao, MD, MBA, a hospitalist at the University of Vermont Medical Center in Burlington and assistant professor at UVM’s Larner College of Medicine.
But in the hospital, Cao said even small shifts — like becoming less active, eating less, or changes in personality — can become much more obvious.
“As hospitalists…we see patients throughout the day, in different situations, and often end up spending more time with them over the course of a week than their primary care provider might in a year,” Cao explained. “This gives us a real advantage in picking up on subtle changes in mental awareness.”
These assessments can also be evaluated with the benefit of daily labs, frequent bedside interactions, and 24–hour observations.
With older adults, Cao said it’s important to go beyond just what’s in the chart.
“I always start by reviewing notes from the primary care provider and previous admissions, but some of the most valuable insights come from talking with family and close friends to get a true sense of the patient’s baseline — how they usually think, move, and interact,” he said.
Why to Watch for Declining Mental Awareness
Declining mental awareness in the inpatient setting is often a sign of an underlying problem — whether that’s a reversible medical condition, unrecognized dementia, or the development of delirium, Cao said.
“On the inpatient side, I pay close attention to more than just memory loss,” he said.
Changes in how patients function day–to–day, shifts in their behavior, or even something as simple as not wanting to get out of bed can be early signs of an aging mind or untreated psychiatric issues, he noted.
“Of course, we always rule out infections and medication side effects, but I also look for other reversible causes like thyroid problems, electrolyte imbalances, low oxygen, pain, urinary retention, constipation, and nutritional deficiencies,” Cao said.
Of note, delirium is the most common cause of sudden mental status changes in the hospital, and “it’s easy to miss if you’re not looking for it.”
He summarized that classic signs are an acute and fluctuating course with changes in alertness, but added there are other red flags too: disorientation, hallucinations, changes in sleep patterns, sporadic unsafe behaviors, mood swings, and changes in activity level, whether that’s agitation or just being unusually quiet.
By combining what he notices bedside and what is learned from the medical record (and from the people who know the patient best), Cao said he’s able to catch these changes early, identify the underlying cause, and work toward the best possible outcome.
“One of the main interventions is providing mental stimulation,” he said.
Why Mental Stimulation Is So Vital
Mental stimulation of the patient is critical to recovery and may prevent prolonged illness, said Meghana R. Medavaram, MD, associate director of consultation liaison and emergency psychiatry at Montefiore Medical Center’s Weiler Hospital in New York City. “Keeping a patient active both physically and mentally can help prevent deconditioning and risks of prolonged immobility,” she said.
It’s important to note that when patients are out of their familiar routines, away from their usual environment and people, and their sleep is fragmented, this can make them even more vulnerable. Keeping patients mentally stimulated during their hospital stay can help maintain their attention, orientation, and a healthy sleep-wake cycle — all things that are easily thrown off in the hospital, Cao said.
“These disruptions hit the pathways that control attention, wakefulness, and the sleep–wake cycle. That’s when you see attention drifting, orientation fading, and circadian rhythms unraveling, especially at night, which is why “sundowning” is so common, Cao said, referring to the syndrome where older adults or people with dementia experience behavioral changes in late afternoon or evening. “Mental stimulation is critical in the hospital because when the brain isn’t active and gets disoriented, it becomes an easy target for delirium.”
He said delirium often develops in older adults when acute stressors like inflammation, low oxygen, metabolic imbalances, or sedating medications disrupt the brain’s arousal systems and networks, especially in older adults.
Therefore, Cao said, encourage your patients to be more engaged during the day through conversation, activities, or regular reorientation. “This supports the brain networks that help prevent inattention and confusion, which are the hallmarks of delirium. Daytime stimulation also helps build up the natural drive for nighttime sleep, so patients are less likely to nap during the day and be awake and disoriented at night.”
To support this, it’s helpful to schedule medications during waking hours instead of around–the–clock dosing that interrupts sleep, and to cluster nighttime care activities to minimize disturbances, Cao explained. Ensuring patients have their glasses, hearing aids, and familiar routines, along with encouraging mobility and hydration, further protects against delirium and supports patients’ cognitive health during hospitalization. “These same principles are just as important in outpatient subacute rehab settings and at home, so it’s essential to take home these strategies after discharge,” he said.
A Family Member or Friend May Help
Hospitalists can suggest straightforward ways to encourage families and friends to keep patients engaged during a hospital stay. Visits and chats can go a long way as conversations are incredibly grounding, Cao said. Other methods could be bringing in favorite foods or snacks, a phone chat or video call, or even showing prerecorded video messages. “These can be effective. Patients respond well to seeing and hearing familiar faces and voices, even if it’s just on a screen,” Cao said.
Beyond that, he said, activities such as watching and discussing the news, reading aloud, using tablets for games, watching movies, doing crossword puzzles, knitting, reminiscing, and playing word games can also be mentally stimulating for patients.
In addition, safe exercises/activities that patients can do in bed — with advice from physical therapy and occupational therapy — are beneficial, Medavaram said. “These often include gentle range–of-motion activities,” she said.
Share Importance of Mental Stimulation With Patients and Caregivers
If a hospitalist wants to motivate patients to keep their minds active, the framing should be simple, positive, and tied directly to their goals of getting better and getting home, said Medavaram. She provided this script suggestion:
“One of the best ways to help your recovery isn’t just taking your medicine, it’s keeping your mind active. When you’re in the hospital, it’s easy to spend the day lying in bed and staring at the TV in your room, but that can make your brain slow down and even cause confusion. Simple things — like reading, talking with visitors, doing puzzles, listening to music you enjoy, or telling a nurse about your favorite memories — can keep your brain sharp. Staying mentally active helps your thinking stay clear and can even help you get home sooner. Think of it like physical therapy for your brain.”
A version of this article first appeared on Medscape.com.
As a hospitalist, you are in a unique position to notice changes in your hospitalized patients. This frontline perspective can be used to improve inpatient attention and care, and differs from primary care, where a clinician might only see a patient once or twice a year, and subtle, gradual changes may be missed, said George Cao, MD, MBA, a hospitalist at the University of Vermont Medical Center in Burlington and assistant professor at UVM’s Larner College of Medicine.
But in the hospital, Cao said even small shifts — like becoming less active, eating less, or changes in personality — can become much more obvious.
“As hospitalists…we see patients throughout the day, in different situations, and often end up spending more time with them over the course of a week than their primary care provider might in a year,” Cao explained. “This gives us a real advantage in picking up on subtle changes in mental awareness.”
These assessments can also be evaluated with the benefit of daily labs, frequent bedside interactions, and 24–hour observations.
With older adults, Cao said it’s important to go beyond just what’s in the chart.
“I always start by reviewing notes from the primary care provider and previous admissions, but some of the most valuable insights come from talking with family and close friends to get a true sense of the patient’s baseline — how they usually think, move, and interact,” he said.
Why to Watch for Declining Mental Awareness
Declining mental awareness in the inpatient setting is often a sign of an underlying problem — whether that’s a reversible medical condition, unrecognized dementia, or the development of delirium, Cao said.
“On the inpatient side, I pay close attention to more than just memory loss,” he said.
Changes in how patients function day–to–day, shifts in their behavior, or even something as simple as not wanting to get out of bed can be early signs of an aging mind or untreated psychiatric issues, he noted.
“Of course, we always rule out infections and medication side effects, but I also look for other reversible causes like thyroid problems, electrolyte imbalances, low oxygen, pain, urinary retention, constipation, and nutritional deficiencies,” Cao said.
Of note, delirium is the most common cause of sudden mental status changes in the hospital, and “it’s easy to miss if you’re not looking for it.”
He summarized that classic signs are an acute and fluctuating course with changes in alertness, but added there are other red flags too: disorientation, hallucinations, changes in sleep patterns, sporadic unsafe behaviors, mood swings, and changes in activity level, whether that’s agitation or just being unusually quiet.
By combining what he notices bedside and what is learned from the medical record (and from the people who know the patient best), Cao said he’s able to catch these changes early, identify the underlying cause, and work toward the best possible outcome.
“One of the main interventions is providing mental stimulation,” he said.
Why Mental Stimulation Is So Vital
Mental stimulation of the patient is critical to recovery and may prevent prolonged illness, said Meghana R. Medavaram, MD, associate director of consultation liaison and emergency psychiatry at Montefiore Medical Center’s Weiler Hospital in New York City. “Keeping a patient active both physically and mentally can help prevent deconditioning and risks of prolonged immobility,” she said.
It’s important to note that when patients are out of their familiar routines, away from their usual environment and people, and their sleep is fragmented, this can make them even more vulnerable. Keeping patients mentally stimulated during their hospital stay can help maintain their attention, orientation, and a healthy sleep-wake cycle — all things that are easily thrown off in the hospital, Cao said.
“These disruptions hit the pathways that control attention, wakefulness, and the sleep–wake cycle. That’s when you see attention drifting, orientation fading, and circadian rhythms unraveling, especially at night, which is why “sundowning” is so common, Cao said, referring to the syndrome where older adults or people with dementia experience behavioral changes in late afternoon or evening. “Mental stimulation is critical in the hospital because when the brain isn’t active and gets disoriented, it becomes an easy target for delirium.”
He said delirium often develops in older adults when acute stressors like inflammation, low oxygen, metabolic imbalances, or sedating medications disrupt the brain’s arousal systems and networks, especially in older adults.
Therefore, Cao said, encourage your patients to be more engaged during the day through conversation, activities, or regular reorientation. “This supports the brain networks that help prevent inattention and confusion, which are the hallmarks of delirium. Daytime stimulation also helps build up the natural drive for nighttime sleep, so patients are less likely to nap during the day and be awake and disoriented at night.”
To support this, it’s helpful to schedule medications during waking hours instead of around–the–clock dosing that interrupts sleep, and to cluster nighttime care activities to minimize disturbances, Cao explained. Ensuring patients have their glasses, hearing aids, and familiar routines, along with encouraging mobility and hydration, further protects against delirium and supports patients’ cognitive health during hospitalization. “These same principles are just as important in outpatient subacute rehab settings and at home, so it’s essential to take home these strategies after discharge,” he said.
A Family Member or Friend May Help
Hospitalists can suggest straightforward ways to encourage families and friends to keep patients engaged during a hospital stay. Visits and chats can go a long way as conversations are incredibly grounding, Cao said. Other methods could be bringing in favorite foods or snacks, a phone chat or video call, or even showing prerecorded video messages. “These can be effective. Patients respond well to seeing and hearing familiar faces and voices, even if it’s just on a screen,” Cao said.
Beyond that, he said, activities such as watching and discussing the news, reading aloud, using tablets for games, watching movies, doing crossword puzzles, knitting, reminiscing, and playing word games can also be mentally stimulating for patients.
In addition, safe exercises/activities that patients can do in bed — with advice from physical therapy and occupational therapy — are beneficial, Medavaram said. “These often include gentle range–of-motion activities,” she said.
Share Importance of Mental Stimulation With Patients and Caregivers
If a hospitalist wants to motivate patients to keep their minds active, the framing should be simple, positive, and tied directly to their goals of getting better and getting home, said Medavaram. She provided this script suggestion:
“One of the best ways to help your recovery isn’t just taking your medicine, it’s keeping your mind active. When you’re in the hospital, it’s easy to spend the day lying in bed and staring at the TV in your room, but that can make your brain slow down and even cause confusion. Simple things — like reading, talking with visitors, doing puzzles, listening to music you enjoy, or telling a nurse about your favorite memories — can keep your brain sharp. Staying mentally active helps your thinking stay clear and can even help you get home sooner. Think of it like physical therapy for your brain.”
A version of this article first appeared on Medscape.com.
VA Hospitals Score High in 2025 CMS Quality Survey
The number of US Department of Veterans Affairs (VA) hospitals receiving high scores in the Centers for Medicare & Medicaid Services (CMS) annual survey of quality measures is on the rise.
In 2023, VA hospitals became eligible to receive Overall Hospital Quality Star Ratings from the survey. In 2025, the survey covered 4609 hospitals (VA and non-VA). CMS analyzed 45 hospital quality measures across 5 different groups: mortality, safety of care, readmission, patient experience, and timely and effective care. The better the performance in these areas, the higher the star rating.
In the current ratings, 77% of surveyed VA hospitals earned 4- or 5-star ratings, a double digit increase over the previous 2 years (67% in 2023 and 58% in 2024). No VA hospitals received a 1-star rating, and > 90% of VA hospitals that received ratings maintained or improved on their 2024 mark.
“These ratings highlight the excellent care VA hospitals provide,” VA Secretary Doug Collins said. “Our job is to continue raising the bar for customer service and convenience throughout the department, so VA works better for the Veterans, families, caregivers and survivors we are charged with serving.”
According to a report from the Advisory Board, fewer hospitals are receiving 5-star ratings than ever, possibly due to the COVID-19 pandemic. According to CMS, of all the hospitals that received a rating, 291 earned 5 stars, 90 fewer than in 2024. At the same time, the number of hospitals with 1-star ratings dropped slightly, from 277 in 2024 to 233 in 2025.
The VA publishes its own data on its medical centers. VA Core Hospital Measures have been available from the Joint Commission since 2005. Additional performance measures, including safety, effectiveness, efficiency, timeliness, patient centeredness, and equity, have been published by the VA since 2008. In 2010, the VA began reporting on Hospital Compare, which has information about the quality of care at > 4000 Medicare-certified hospitals, including > 130 VA medical centers and > 50 military hospitals.
The number of US Department of Veterans Affairs (VA) hospitals receiving high scores in the Centers for Medicare & Medicaid Services (CMS) annual survey of quality measures is on the rise.
In 2023, VA hospitals became eligible to receive Overall Hospital Quality Star Ratings from the survey. In 2025, the survey covered 4609 hospitals (VA and non-VA). CMS analyzed 45 hospital quality measures across 5 different groups: mortality, safety of care, readmission, patient experience, and timely and effective care. The better the performance in these areas, the higher the star rating.
In the current ratings, 77% of surveyed VA hospitals earned 4- or 5-star ratings, a double digit increase over the previous 2 years (67% in 2023 and 58% in 2024). No VA hospitals received a 1-star rating, and > 90% of VA hospitals that received ratings maintained or improved on their 2024 mark.
“These ratings highlight the excellent care VA hospitals provide,” VA Secretary Doug Collins said. “Our job is to continue raising the bar for customer service and convenience throughout the department, so VA works better for the Veterans, families, caregivers and survivors we are charged with serving.”
According to a report from the Advisory Board, fewer hospitals are receiving 5-star ratings than ever, possibly due to the COVID-19 pandemic. According to CMS, of all the hospitals that received a rating, 291 earned 5 stars, 90 fewer than in 2024. At the same time, the number of hospitals with 1-star ratings dropped slightly, from 277 in 2024 to 233 in 2025.
The VA publishes its own data on its medical centers. VA Core Hospital Measures have been available from the Joint Commission since 2005. Additional performance measures, including safety, effectiveness, efficiency, timeliness, patient centeredness, and equity, have been published by the VA since 2008. In 2010, the VA began reporting on Hospital Compare, which has information about the quality of care at > 4000 Medicare-certified hospitals, including > 130 VA medical centers and > 50 military hospitals.
The number of US Department of Veterans Affairs (VA) hospitals receiving high scores in the Centers for Medicare & Medicaid Services (CMS) annual survey of quality measures is on the rise.
In 2023, VA hospitals became eligible to receive Overall Hospital Quality Star Ratings from the survey. In 2025, the survey covered 4609 hospitals (VA and non-VA). CMS analyzed 45 hospital quality measures across 5 different groups: mortality, safety of care, readmission, patient experience, and timely and effective care. The better the performance in these areas, the higher the star rating.
In the current ratings, 77% of surveyed VA hospitals earned 4- or 5-star ratings, a double digit increase over the previous 2 years (67% in 2023 and 58% in 2024). No VA hospitals received a 1-star rating, and > 90% of VA hospitals that received ratings maintained or improved on their 2024 mark.
“These ratings highlight the excellent care VA hospitals provide,” VA Secretary Doug Collins said. “Our job is to continue raising the bar for customer service and convenience throughout the department, so VA works better for the Veterans, families, caregivers and survivors we are charged with serving.”
According to a report from the Advisory Board, fewer hospitals are receiving 5-star ratings than ever, possibly due to the COVID-19 pandemic. According to CMS, of all the hospitals that received a rating, 291 earned 5 stars, 90 fewer than in 2024. At the same time, the number of hospitals with 1-star ratings dropped slightly, from 277 in 2024 to 233 in 2025.
The VA publishes its own data on its medical centers. VA Core Hospital Measures have been available from the Joint Commission since 2005. Additional performance measures, including safety, effectiveness, efficiency, timeliness, patient centeredness, and equity, have been published by the VA since 2008. In 2010, the VA began reporting on Hospital Compare, which has information about the quality of care at > 4000 Medicare-certified hospitals, including > 130 VA medical centers and > 50 military hospitals.
Enhancing Workforce Practices to Achieve Commission on Cancer Accreditation
Background
The American College of Surgeons’ Commission on Cancer (CoC) Accreditation requires establishment of a comprehensive cancer program, multi-disciplinary tumor boards, active cancer registry, quality improvement activities and cancer research.
Methods
In 2022, the Tibor Rubin VA Medical Center (TRVAMC) set out to obtain accreditation through enhancing workforce practices. Changes in workforce practices included (1) leadership engagement; (2) acquisition of staff; (3) enhancing staff efficiency and (4) inter-departmental collaboration, leading to CoC accreditation in August 2024. executive leadership team (ELT) buy-in was essential. ELT engagement included communicating the benefits of accreditation, alignment with organizational mission and values, protected time for Cancer Committee members, Chief of Staff presence in Cancer Committee, commitment to recruiting new staff, and membership in the Medical Executive Council to voice cancer program needs. New staff included a cancer program manager, cancer case conference RN care coordinator, certified oncology data specialist and survivorship nurse practitioner. Staff development included structured and focused training. Enhancing staff efficiency included developing standards of work with clear delineation of duties (delegation of specific CoC standards), decentralizing decision making, a shared governance council, and weekly Cancer Program meetings. These changes allowed staff members to be active, autonomous decision-making participants, and increased efficiency. Inter-departmental collaboration involved Hematology/Oncology, Surgery, Radiation Oncology, Pharmacy, Nutrition, Pathology, Palliative Care, Rehabilitation, Chaplaincy and Cancer Research, with key individuals serving as Cancer Committee members. Each department set performance goals and metrics. Each employee’s contribution was rated in annual performance reviews.
Results
TRVAMC thus elevated cancer care delivery standards through structured workforce practices within the framework of CoC standards required for accreditation. Additionally, the accreditation process achieved desirable and measurable outcomes, e.g. 100% growth in oncology dietitian referrals, 75% increase in early palliative care referrals (TRVAMC ranked in the top 5 in the US), and more than 200 patients enrolled in cancer clinical trials (TRVAMC was the highest enrolling VA in the US to NCI trials in 2024).
Conclusions
Our model demonstrates how strategic improvements in healthcare workforce practices at a VA can directly contribute to sustained improvements in quality and delivery of cancer care services.
Background
The American College of Surgeons’ Commission on Cancer (CoC) Accreditation requires establishment of a comprehensive cancer program, multi-disciplinary tumor boards, active cancer registry, quality improvement activities and cancer research.
Methods
In 2022, the Tibor Rubin VA Medical Center (TRVAMC) set out to obtain accreditation through enhancing workforce practices. Changes in workforce practices included (1) leadership engagement; (2) acquisition of staff; (3) enhancing staff efficiency and (4) inter-departmental collaboration, leading to CoC accreditation in August 2024. executive leadership team (ELT) buy-in was essential. ELT engagement included communicating the benefits of accreditation, alignment with organizational mission and values, protected time for Cancer Committee members, Chief of Staff presence in Cancer Committee, commitment to recruiting new staff, and membership in the Medical Executive Council to voice cancer program needs. New staff included a cancer program manager, cancer case conference RN care coordinator, certified oncology data specialist and survivorship nurse practitioner. Staff development included structured and focused training. Enhancing staff efficiency included developing standards of work with clear delineation of duties (delegation of specific CoC standards), decentralizing decision making, a shared governance council, and weekly Cancer Program meetings. These changes allowed staff members to be active, autonomous decision-making participants, and increased efficiency. Inter-departmental collaboration involved Hematology/Oncology, Surgery, Radiation Oncology, Pharmacy, Nutrition, Pathology, Palliative Care, Rehabilitation, Chaplaincy and Cancer Research, with key individuals serving as Cancer Committee members. Each department set performance goals and metrics. Each employee’s contribution was rated in annual performance reviews.
Results
TRVAMC thus elevated cancer care delivery standards through structured workforce practices within the framework of CoC standards required for accreditation. Additionally, the accreditation process achieved desirable and measurable outcomes, e.g. 100% growth in oncology dietitian referrals, 75% increase in early palliative care referrals (TRVAMC ranked in the top 5 in the US), and more than 200 patients enrolled in cancer clinical trials (TRVAMC was the highest enrolling VA in the US to NCI trials in 2024).
Conclusions
Our model demonstrates how strategic improvements in healthcare workforce practices at a VA can directly contribute to sustained improvements in quality and delivery of cancer care services.
Background
The American College of Surgeons’ Commission on Cancer (CoC) Accreditation requires establishment of a comprehensive cancer program, multi-disciplinary tumor boards, active cancer registry, quality improvement activities and cancer research.
Methods
In 2022, the Tibor Rubin VA Medical Center (TRVAMC) set out to obtain accreditation through enhancing workforce practices. Changes in workforce practices included (1) leadership engagement; (2) acquisition of staff; (3) enhancing staff efficiency and (4) inter-departmental collaboration, leading to CoC accreditation in August 2024. executive leadership team (ELT) buy-in was essential. ELT engagement included communicating the benefits of accreditation, alignment with organizational mission and values, protected time for Cancer Committee members, Chief of Staff presence in Cancer Committee, commitment to recruiting new staff, and membership in the Medical Executive Council to voice cancer program needs. New staff included a cancer program manager, cancer case conference RN care coordinator, certified oncology data specialist and survivorship nurse practitioner. Staff development included structured and focused training. Enhancing staff efficiency included developing standards of work with clear delineation of duties (delegation of specific CoC standards), decentralizing decision making, a shared governance council, and weekly Cancer Program meetings. These changes allowed staff members to be active, autonomous decision-making participants, and increased efficiency. Inter-departmental collaboration involved Hematology/Oncology, Surgery, Radiation Oncology, Pharmacy, Nutrition, Pathology, Palliative Care, Rehabilitation, Chaplaincy and Cancer Research, with key individuals serving as Cancer Committee members. Each department set performance goals and metrics. Each employee’s contribution was rated in annual performance reviews.
Results
TRVAMC thus elevated cancer care delivery standards through structured workforce practices within the framework of CoC standards required for accreditation. Additionally, the accreditation process achieved desirable and measurable outcomes, e.g. 100% growth in oncology dietitian referrals, 75% increase in early palliative care referrals (TRVAMC ranked in the top 5 in the US), and more than 200 patients enrolled in cancer clinical trials (TRVAMC was the highest enrolling VA in the US to NCI trials in 2024).
Conclusions
Our model demonstrates how strategic improvements in healthcare workforce practices at a VA can directly contribute to sustained improvements in quality and delivery of cancer care services.
Streamlining Health Care: Inpatient Dashboard as a User-Centric Solution in EHR Enhancement
Streamlining Health Care: Inpatient Dashboard as a User-Centric Solution in EHR Enhancement
Electronic health records (EHRs) are an integral part of modern health care. The 2009, Health Information Technology for Economic and Clinical Health Act established financial incentives for US hospitals to adopt EHRs. In 2009 only 12% of nonfederal acute care hospitals had adopted a certified EHR system, which increased to 96% by 2021.1
EHRs have transformed the way patient data are stored and accessed, streamlining the process of providing quality patient care with improvements in efficiency, effectiveness, patient satisfaction, and safety.2 Despite their widespread adoption and benefits, EHRs have generally been met with mixed physician satisfaction.3 Interactions with EHRs are linked to disproportionate time at the computer and physician burnout.4-6
The US Department of Veterans Affairs (VA) was at the forefront of EHR development, establishing the Veterans Health Information Systems and Technology Architecture (VistA) in the 1970s. The VA released the Computerized Patient Record System (CPRS) in 1997, the first clinical user interface for VistA. In May 2018, the VA signed a $10 billion contract with Cerner (now Oracle Health) to modernize its EHR.7 This was later revised to $16.1 billion, and the Institute for Defense Analyses estimates it will cost $49.8 billion.8 The transition to Oracle Health has been faced with significant challenges, including patient safety risks and workflow inefficiencies, leading to a pause in rollout.9
Due to the known challenges with EHRs and the aging CPRS system (without a scheduled replacement date), innovations that facilitate the synthesis and display of clinical information are needed. To address this gap, the VA Ann Arbor Healthcare System (VAAAHS) developed the Inpatient Dashboard, an online EHR companion tool. The Inpatient Dashboard was designed to draw data from VistA to reduce time spent at the computer by streamlining clinical information presentation, standardizing inpatient notes, improving safety measures, and enhancing overall clinician satisfaction. This study evaluated the adoption and user experience with the Inpatient Dashboard.
INPATIENT DASHBOARD
The Inpatient Dashboard consists of several modules created by a contractor for the VAAAHS that is housed on VA servers with access restricted to individuals with patient health data privileges. As the Inpatient Dashboard draws data from VistA, it can display laboratory information, studies, and notes from all VA sites.
The main dashboard is a snapshot summary of patient information, including patient location, code status, last vital sign readings, vital sign ranges over the previous 24 hours, intake/output, deep vein thrombosis (DVT) prophylaxis, the presence of telemetry orders, or use of Foley or central catheters (Figure). It also includes a customizable to-do list and contact information for the patient’s clinician and nurse. Significant events, such as abnormal vital signs or speciation/sensitivities for blood cultures, are automatically populated on the to-do list. From this main dashboard overview, clinicians can customize which patients are displayed, create and print a rounding list, print a sign-out sheet, or select individual patients to open a progress note module.
Notes can be written in the patient history and physical module, progress note module, and discharge summary module. The patient history and physical module has text blocks allocated to the traditional components of a history and physical note (ie, chief complaint, history of present illness, review of systems, past medical history, family history, social history, allergies, medications, physical examination, assessment, and plan) (eAppendix 1). Some elements, such as past medical history, family history, and social history are prepopulated if the patient was previously admitted. Vital signs, laboratory results, studies, microbiology/ pathology reports, and other CPRS notes are displayed in this module.
The progress note module contains text blocks allocated to the traditional components of a progress note, such as subjective/interval events, physical examination, assessment, and plan (eAppendix 2). Vital signs, laboratory results, studies, microbiology/ pathology reports, other CPRS notes, and the patient’s medication administration record are also displayed in this module. Lastly, the discharge summary module includes patient follow-up, patient instructions, hospitalization summary, medication reconciliation, laboratory results, and studies/procedures, ensuring a comprehensive discharge summary for patients and clinicians (eAppendix 3).
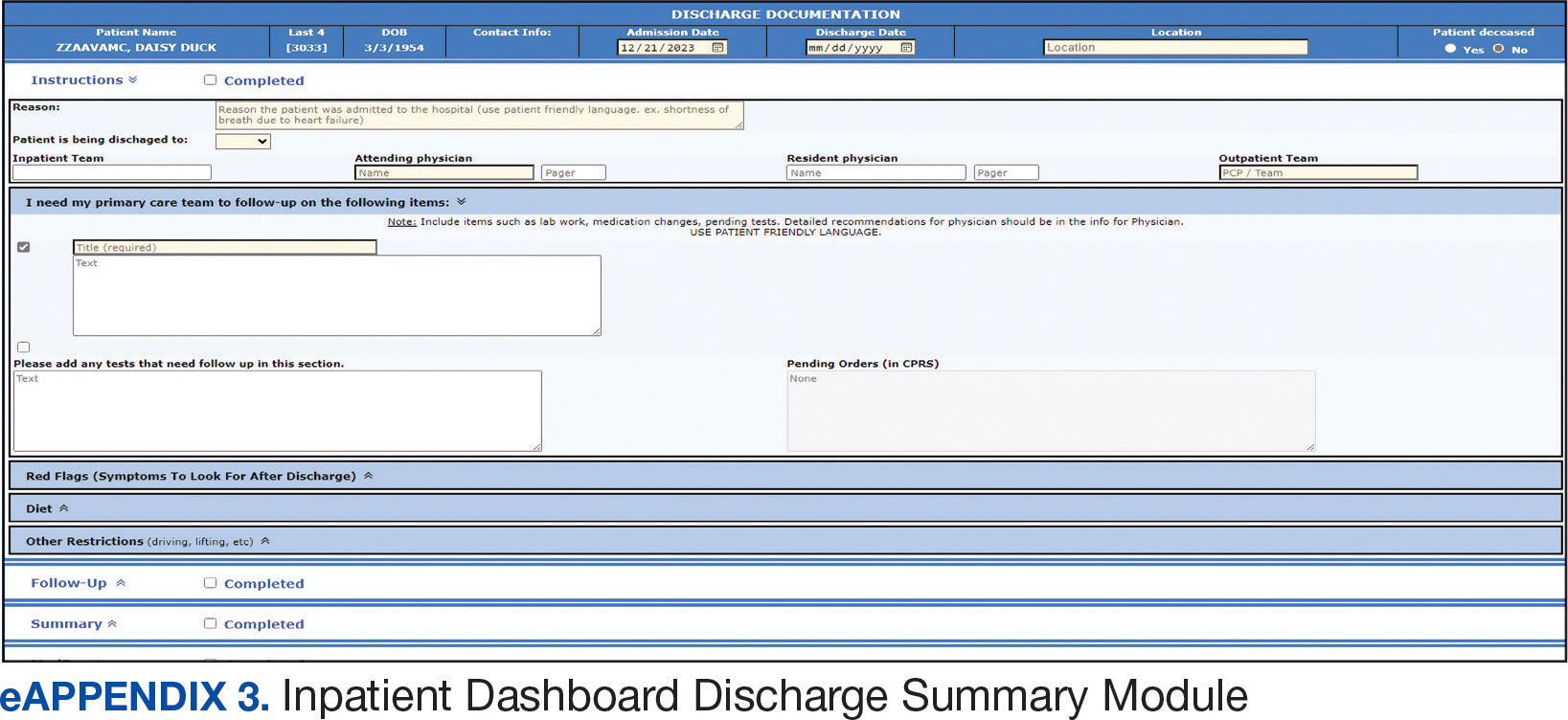
A medication reconciliation tool was embedded within the history and physical and discharge summary modules. This tool has been shown to reduce medication errors in patients admitted from the emergency department to the hospital (eAppendix 4).10 The handoff/sign-out tool (eAppendix 5) accessible through the main dashboard page is modeled on the I-PASS handoff framework.11,12 This includes the patient identifier, interval events, inpatient medications, specific sign-out guidance, sign-out tasks/to-dos, and any other pertinent information.
The Inpatient Dashboard is a team-based construct shared by the attending physicians, residents, and medical students. Each team (eg, general medicine, general surgery) is its own entity; only team members can change the content or add to the documentation. Each facility can have multiple teams caring for the same patient (eg, primary and consulting teams). Additional care members can also be incorporated (eg, pharmacists assist with medication reconciliation for admission and discharge at VAAAHS). The Inpatient Dashboard can export information directly to CPRS for clinicians to review and sign. It can also generate a note that can be pasted into CPRS.
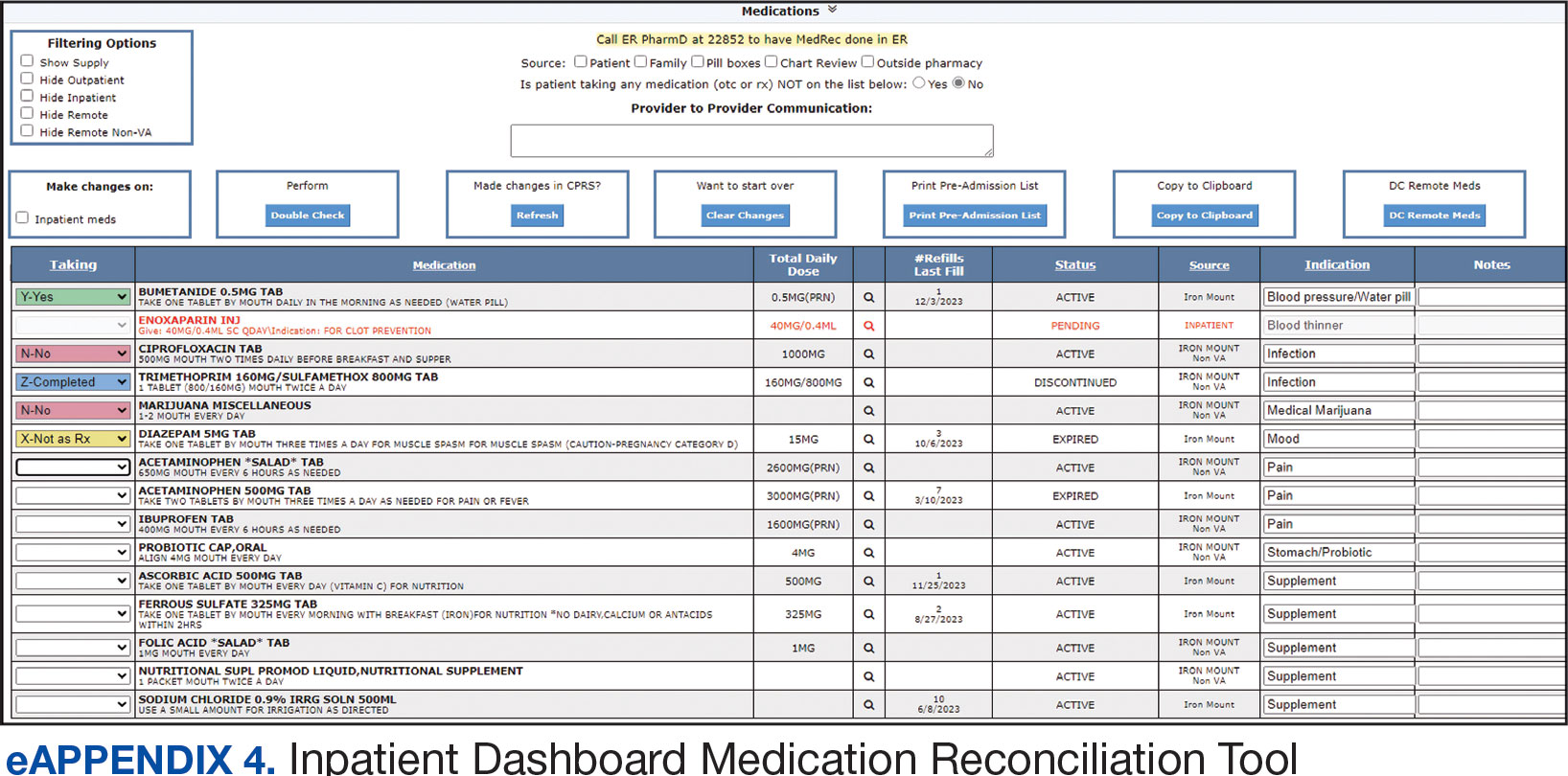
Clinician Feedback and Satisfaction
A survey was developed to evaluate clinician experiences with using the Inpatient Dashboard as an adjunct to the CPRS. The Inpatient Dashboard was made available to general medicine teams in November 2018. The survey was conducted from December 2018 to September 2019. The study was conducted at the VAAAHS and included 4 general medicine teams. Each team included an attending physician, a senior resident, 2 to 3 interns, and 3 to 4 medical students. Eligibility was extended to any team member who used both the CPRS and Inpatient Dashboard. Participation in the survey was voluntary. All respondents were informed of the study’s purpose and encouraged to provide candid feedback to ensure the reliability and validity of the findings.
Data were collected through a semistructured survey administered via the Qualtrics platform. The questionnaire was designed to capture multidimensional insights into clinician experience, with particular focus on satisfaction, efficiency, and perceived safety when using the tool as an adjunct to CPRS compared to using CPRS alone. The questionnaire primarily used a Likert scale for responses. Surveys were emailed at the completion of a team’s 1-month inpatient block. An answer was not required for every question, resulting in slightly different response numbers for some questions.
A question regarding the tool’s impact on workload stress was added halfway through the study period, which resulted in fewer responses. Adoption was assessed by counting the Inpatient Dashboard unique users. Descriptive statistics were used within individual survey responses to report the distribution of responses. Differences in response between levels of training were assessed using a X2 test of independence.
Survey Results
From September 2023 through November 2023, there were 1549 rounding printouts across 144 unique users (5 nurses, 40 medical students, 87 residents, and 12 attending physicians) and 1468 handoff printouts across 148 unique users (5 nurses, 10 medical students, 111 residents, and 22 attending physicians). The clinician survey received 68 responses from users at various levels of medical training: 23 medical students, 31 interns, 12 senior residents, and 2 attending physicians. All 68 participants confirmed they had used the Inpatient Dashboard.
User satisfaction and preference for the Inpatient Dashboard vs CPRS were assessed. Sixty-one respondents (90%) expressed overall satisfaction with the Inpatient Dashboard; 22 (32%) were extremely satisfied, and 39 (57%) were somewhat satisfied (Table 1). Three respondents (4%) were neutral, 2 (3%) were somewhat dissatisfied, and 2 (3%) were extremely dissatisfied with the Inpatient Dashboard. Responses differed by level of training (P = .03), with medical students trending towards higher satisfaction.
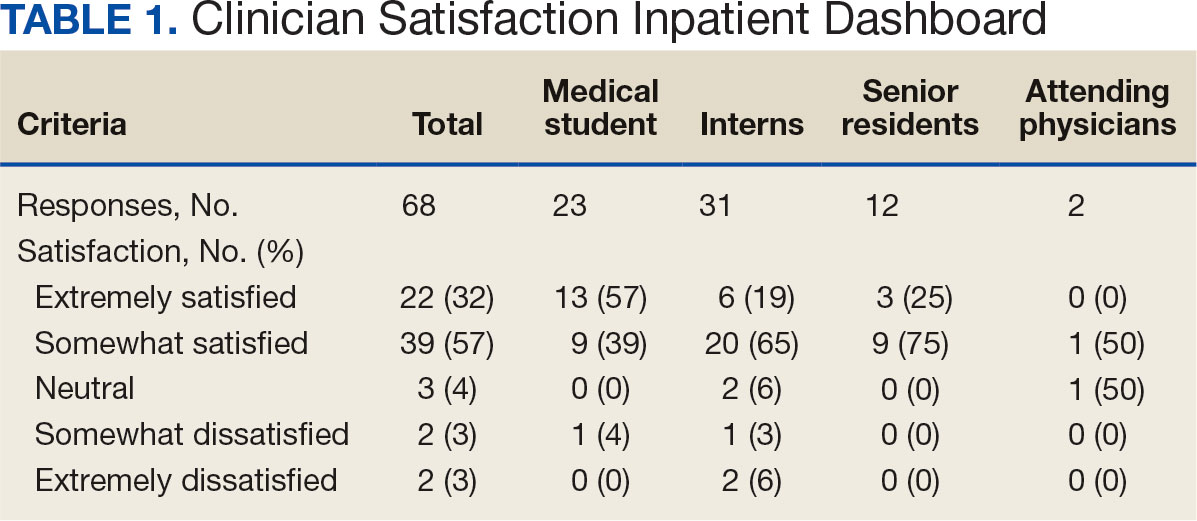
Respondents preferred the Inpatient Dashboard over CPRS for gathering information and writing progress notes; 42 (64%) respondents mostly favored the Inpatient Dashboard, 15 (23%) slightly favored the Inpatient Dashboard over CPRS, and 8 (12%) were neutral. One respondent (2%) slightly favored CPRS to the Inpatient Dashboard (Table 2).
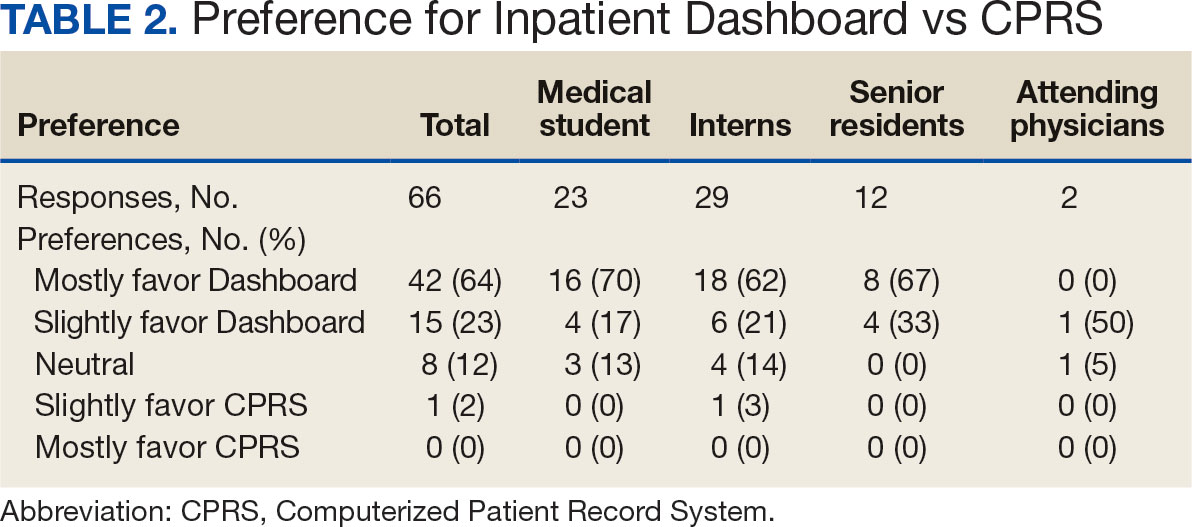
Sixty-five respondents (100%) found the Inpatient Dashboard’s ability to summarize patient information in a central place helpful (Table 3). Among them, 53 (82%) respondents reported it was very or extremely helpful, 10 (15%) respondents reported it was moderately helpful, and 2 (3%) respondents reported it was slightly helpful. This feature positively impacted users’ awareness of patients with DVT prophylaxis or a Foley catheter. Ten (15%) respondents reported being much more aware, and 29 (45%) respondents reporting they were slightly more aware. The remaining 26 (40%) respondents reported no change in awareness.
The Inpatient Dashboard was reported to save time preparing for physician rounds by 52 (80%) respondents, contributing to much greater efficiency for 29 (45%) respondents and slightly more efficiency for 23 (35%) respondents. However, 10 (15%) respondents reported no change in efficiency, and 3 (5%) respondents reduced efficiency, with 1 (2%) respondent reporting it slightly less efficient and 2 (3%) respondents reporting it much less efficient. Responses differed by level of training (P = .01), with medical students trending towards higher efficiency. Of the 23 respondents who reported on the Inpatient Dashboard’s impact on daily workload stress level, 22 (96%) indicated the tool had a stress-reducing effect, with 9 (39%) experiencing a major reduction in stress level, and 13 (57%) experiencing somewhat reduced stress level. Only 1 participant (4%) reported no change in stress. No participants reported an increase in stress.
DISCUSSION
The adoption of EHRs has transformed operational modalities in contemporary health care systems, heralding advancements in patient satisfaction, safety, and overall quality and efficiency of care.1,2 However, EHRs still present challenges, predominantly around clinician satisfaction, marked by instances of burnout and increased time spent on computers.2-6 In this context, the Inpatient Dashboard, an online companion to the CPRS, exemplifies how user-centered innovations in EHRs can address and mitigate associated challenges.
The Inpatient Dashboard has been well received with most respondents of the survey conducted in this study indicating they were both satisfied with the instrument and preferring it to CPRS. This high approval aligns with existing literature on the potential advantages of user-centered design in health care technology.13 The tool has gained widespread acceptance at the VAAAHS even in the absence of obligatory usage or institutional incentives. The appeal of the Inpatient Dashboard may stem from its increased efficiency, with most users affirming its timesaving nature. While CPRS can only display local notes, laboratory results, and studies, the Inpatient Dashboard can display data from across all VA sites. The VA Joint Longitudinal Viewer can similarly display data from across all sites, but the display is not streamlined as it is in the Inpatient Dashboard. The Inpatient Dashboard incorporates this clinical information into a single page to facilitate day-to-day workflow and dynamic documentation (ie, reviewing laboratory results, medications, writing notes, and signing out patients). This increased efficiency allows clinicians to counter 2 common barriers to EHR implementation: productivity loss and insufficient time.14
The association between EHRs and improved quality and safety in health care is well-documented.3 The Inpatient Dashboard fortifies this association by enhancing awareness around patient status, evidenced by a majority of respondents, and by integrating a medication reconciliation tool to decrease medication errors on transition from the emergency department to inpatient hospitalization.10
The Inpatient Dashboard’s impact on alleviating daily workload stress is noteworthy, with almost all respondents experiencing reduced stress levels and physician burnout, which has been linked to deteriorating well-being, compromised patient safety, and escalated health care costs.15,16 The heightened susceptibility of physicians to burnout compared to other professionals underscores the imperative for incorporating stress-mitigating interventions in the EHR.17,18
While responses to most questions did not significantly differ by training levels, overall satisfaction with the Inpatient Dashboard and its ability to save time preparing for rounds were rated higher by medical students. This may be attributable to a greater derived benefit from collating and presenting data to learners with less familiarity with the native EHR. It is also notable that the Inpatient Dashboard allows medical students to directly contribute to a patient’s note, which could be another driver in satisfaction. While most interns still felt the Inpatient Dashboard enabled them to save time preparing for rounds, there were a considerable number of ‘no change’ responses, which suggests some interns may not have modified their existing prerounding strategies. These associations are limited by the relatively small number of respondents by learner category, with senior medical residents and attending physicians being underrepresented.
While there are a multitude of dashboards available at the VA, most are made to track certain quality metrics and are used more by administrative and leadership staff. The Inpatient Dashboard was created specifically for frontline clinicians to facilitate their day-to-day workflow and dynamic documentation. This tool can additionally help with quality metrics, though its main purpose was and is to make clinician workflow easier and more efficient.
These results are especially timely because the VA is modernizing its EHR by transitioning to Oracle Health.7 Due to the numerous reports both from veterans and VA clinicians that the Oracle Health EHR is not meeting expectations, deployment at further sites has been halted while improving the experience of the 5 institutions using Oracle Health is prioritized.9 The Inpatient Dashboard, instead of being merely an enhancement to CPRS, could emerge as a potential bridge to Oracle Health if adapted to display data from Oracle Health as it does VistA. This would facilitate a smoother, more integrated transition for those health care institutions employing the Inpatient Dashboard.
Limitations
The reliance on self-reported data inherently carries the risk of bias, and the absence of objective measures, like time-tracking studies, limits the quantifiable assessment of the Inpatient Dashboard efficacy. The single-center nature of the study also may restrict the generalizability of the results.
CONCLUSIONS
Optimal integration of EHRs into health care delivery is critical to high-quality patient care and operational efficiency. The Inpatient Dashboard is an example of an innovative, user-centric solution that integrated and presented clinical information in a way that produced high satisfaction and adoption by users at a VA hospital.
- Office of the National Coordinator for Health Information Technology. National Trends in Hospital and Physician Adoption of Electronic Health Records. HealthIT.gov. Accessed February 5, 2025. https://www.healthit.gov/data/quickstats/national-trends-hospital-and-physician-adoption-electronic-health-records
- Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464-471. doi:10.1377/hlthaff.2011.0178
- Nguyen L, Bellucci E, Nguyen LT. Electronic health records implementation: an evaluation of information system impact and contingency factors. Int J Med Inf. 2014;83(11):779-796. doi:10.1016/j.ijmedinf.2014.06.011
- Alexander AG, Ballou KA. Work-life balance, burnout, and the electronic health record. Am J Med. 2018;131(8):857- 858. doi:10.1016/j.amjmed.2018.02.033
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165(11):753-760. doi:10.7326/M16-0961
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med. 2019;179(6):760-767. doi:10.1001/jamainternmed.2019.0095
- US Department of Veterans Affairs. Statement by Acting Secretary Robert Wilkie - VA signs contract with Cerner for an electronic health record system. New release. May 17, 2018. Accessed February 5, 2025. https://news.va.gov/press-room/statement-by-acting-secretary-robert-wilkie-va-signs-contract-with-cerner-for-an-electronic-health-record-system/
- US Government Publishing Office. VA’s Electronic health record modernization: an update on rollout, cost, and schedule. Subcommittee on Military Construction, Veterans Affairs, and Related Agencies, Committee on Appropriations, United States Senate. 117th Congress, 2nd Session. September 21, 2022. Accessed February 5, 2025. https://www.govinfo.gov/content/pkg/CHRG-117shrg52328/html/CHRG-117shrg52328.htm
- US Department of Veterans Affairs. VA announces reset of electronic health record project. Accessed December 21, 2023. https://news.va.gov/press-room/va-announces-reset-of-electronic-health-record-project/
- Grondin C, Gupta A, Houchens N, et al. Medication reconciliation tool reduces errors in patients admitted from the ED to hospital. Am J Med Qual. 2021;36(2):129. doi:10.1097/01.JMQ.0000741500.33781.eb
- Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi:10.1056/NEJMsa1405556
- Starmer AJ, O’Toole JK, Rosenbluth G, et al. Development, implementation, and dissemination of the I-PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876-884. doi:10.1097/ACM.0000000000000264
- Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
- Kruse CS, Kristof C, Jones B, Mitchell E, Martinez A. Barriers to electronic health record adoption: a systematic literature review. J Med Syst. 2016;40(12):252. doi:10.1007/s10916-016-0628-9
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516-529. doi:10.1111/joim.12752
- Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907- 912. doi:10.1016/j.amjsurg.2019.07.004
- Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385. doi:10.1001/archinternmed.2012.3199
- Budd J. Burnout related to electronic health record use in primary care. J Prim Care Community Health. 2023;14:21501319231166921. doi:10.1177/21501319231166921
Electronic health records (EHRs) are an integral part of modern health care. The 2009, Health Information Technology for Economic and Clinical Health Act established financial incentives for US hospitals to adopt EHRs. In 2009 only 12% of nonfederal acute care hospitals had adopted a certified EHR system, which increased to 96% by 2021.1
EHRs have transformed the way patient data are stored and accessed, streamlining the process of providing quality patient care with improvements in efficiency, effectiveness, patient satisfaction, and safety.2 Despite their widespread adoption and benefits, EHRs have generally been met with mixed physician satisfaction.3 Interactions with EHRs are linked to disproportionate time at the computer and physician burnout.4-6
The US Department of Veterans Affairs (VA) was at the forefront of EHR development, establishing the Veterans Health Information Systems and Technology Architecture (VistA) in the 1970s. The VA released the Computerized Patient Record System (CPRS) in 1997, the first clinical user interface for VistA. In May 2018, the VA signed a $10 billion contract with Cerner (now Oracle Health) to modernize its EHR.7 This was later revised to $16.1 billion, and the Institute for Defense Analyses estimates it will cost $49.8 billion.8 The transition to Oracle Health has been faced with significant challenges, including patient safety risks and workflow inefficiencies, leading to a pause in rollout.9
Due to the known challenges with EHRs and the aging CPRS system (without a scheduled replacement date), innovations that facilitate the synthesis and display of clinical information are needed. To address this gap, the VA Ann Arbor Healthcare System (VAAAHS) developed the Inpatient Dashboard, an online EHR companion tool. The Inpatient Dashboard was designed to draw data from VistA to reduce time spent at the computer by streamlining clinical information presentation, standardizing inpatient notes, improving safety measures, and enhancing overall clinician satisfaction. This study evaluated the adoption and user experience with the Inpatient Dashboard.
INPATIENT DASHBOARD
The Inpatient Dashboard consists of several modules created by a contractor for the VAAAHS that is housed on VA servers with access restricted to individuals with patient health data privileges. As the Inpatient Dashboard draws data from VistA, it can display laboratory information, studies, and notes from all VA sites.
The main dashboard is a snapshot summary of patient information, including patient location, code status, last vital sign readings, vital sign ranges over the previous 24 hours, intake/output, deep vein thrombosis (DVT) prophylaxis, the presence of telemetry orders, or use of Foley or central catheters (Figure). It also includes a customizable to-do list and contact information for the patient’s clinician and nurse. Significant events, such as abnormal vital signs or speciation/sensitivities for blood cultures, are automatically populated on the to-do list. From this main dashboard overview, clinicians can customize which patients are displayed, create and print a rounding list, print a sign-out sheet, or select individual patients to open a progress note module.

Notes can be written in the patient history and physical module, progress note module, and discharge summary module. The patient history and physical module has text blocks allocated to the traditional components of a history and physical note (ie, chief complaint, history of present illness, review of systems, past medical history, family history, social history, allergies, medications, physical examination, assessment, and plan) (eAppendix 1). Some elements, such as past medical history, family history, and social history are prepopulated if the patient was previously admitted. Vital signs, laboratory results, studies, microbiology/ pathology reports, and other CPRS notes are displayed in this module.

The progress note module contains text blocks allocated to the traditional components of a progress note, such as subjective/interval events, physical examination, assessment, and plan (eAppendix 2). Vital signs, laboratory results, studies, microbiology/ pathology reports, other CPRS notes, and the patient’s medication administration record are also displayed in this module. Lastly, the discharge summary module includes patient follow-up, patient instructions, hospitalization summary, medication reconciliation, laboratory results, and studies/procedures, ensuring a comprehensive discharge summary for patients and clinicians (eAppendix 3).


A medication reconciliation tool was embedded within the history and physical and discharge summary modules. This tool has been shown to reduce medication errors in patients admitted from the emergency department to the hospital (eAppendix 4).10 The handoff/sign-out tool (eAppendix 5) accessible through the main dashboard page is modeled on the I-PASS handoff framework.11,12 This includes the patient identifier, interval events, inpatient medications, specific sign-out guidance, sign-out tasks/to-dos, and any other pertinent information.
The Inpatient Dashboard is a team-based construct shared by the attending physicians, residents, and medical students. Each team (eg, general medicine, general surgery) is its own entity; only team members can change the content or add to the documentation. Each facility can have multiple teams caring for the same patient (eg, primary and consulting teams). Additional care members can also be incorporated (eg, pharmacists assist with medication reconciliation for admission and discharge at VAAAHS). The Inpatient Dashboard can export information directly to CPRS for clinicians to review and sign. It can also generate a note that can be pasted into CPRS.


Clinician Feedback and Satisfaction
A survey was developed to evaluate clinician experiences with using the Inpatient Dashboard as an adjunct to the CPRS. The Inpatient Dashboard was made available to general medicine teams in November 2018. The survey was conducted from December 2018 to September 2019. The study was conducted at the VAAAHS and included 4 general medicine teams. Each team included an attending physician, a senior resident, 2 to 3 interns, and 3 to 4 medical students. Eligibility was extended to any team member who used both the CPRS and Inpatient Dashboard. Participation in the survey was voluntary. All respondents were informed of the study’s purpose and encouraged to provide candid feedback to ensure the reliability and validity of the findings.
Data were collected through a semistructured survey administered via the Qualtrics platform. The questionnaire was designed to capture multidimensional insights into clinician experience, with particular focus on satisfaction, efficiency, and perceived safety when using the tool as an adjunct to CPRS compared to using CPRS alone. The questionnaire primarily used a Likert scale for responses. Surveys were emailed at the completion of a team’s 1-month inpatient block. An answer was not required for every question, resulting in slightly different response numbers for some questions.
A question regarding the tool’s impact on workload stress was added halfway through the study period, which resulted in fewer responses. Adoption was assessed by counting the Inpatient Dashboard unique users. Descriptive statistics were used within individual survey responses to report the distribution of responses. Differences in response between levels of training were assessed using a X2 test of independence.
Survey Results
From September 2023 through November 2023, there were 1549 rounding printouts across 144 unique users (5 nurses, 40 medical students, 87 residents, and 12 attending physicians) and 1468 handoff printouts across 148 unique users (5 nurses, 10 medical students, 111 residents, and 22 attending physicians). The clinician survey received 68 responses from users at various levels of medical training: 23 medical students, 31 interns, 12 senior residents, and 2 attending physicians. All 68 participants confirmed they had used the Inpatient Dashboard.
User satisfaction and preference for the Inpatient Dashboard vs CPRS were assessed. Sixty-one respondents (90%) expressed overall satisfaction with the Inpatient Dashboard; 22 (32%) were extremely satisfied, and 39 (57%) were somewhat satisfied (Table 1). Three respondents (4%) were neutral, 2 (3%) were somewhat dissatisfied, and 2 (3%) were extremely dissatisfied with the Inpatient Dashboard. Responses differed by level of training (P = .03), with medical students trending towards higher satisfaction.

Respondents preferred the Inpatient Dashboard over CPRS for gathering information and writing progress notes; 42 (64%) respondents mostly favored the Inpatient Dashboard, 15 (23%) slightly favored the Inpatient Dashboard over CPRS, and 8 (12%) were neutral. One respondent (2%) slightly favored CPRS to the Inpatient Dashboard (Table 2).

Sixty-five respondents (100%) found the Inpatient Dashboard’s ability to summarize patient information in a central place helpful (Table 3). Among them, 53 (82%) respondents reported it was very or extremely helpful, 10 (15%) respondents reported it was moderately helpful, and 2 (3%) respondents reported it was slightly helpful. This feature positively impacted users’ awareness of patients with DVT prophylaxis or a Foley catheter. Ten (15%) respondents reported being much more aware, and 29 (45%) respondents reporting they were slightly more aware. The remaining 26 (40%) respondents reported no change in awareness.

The Inpatient Dashboard was reported to save time preparing for physician rounds by 52 (80%) respondents, contributing to much greater efficiency for 29 (45%) respondents and slightly more efficiency for 23 (35%) respondents. However, 10 (15%) respondents reported no change in efficiency, and 3 (5%) respondents reduced efficiency, with 1 (2%) respondent reporting it slightly less efficient and 2 (3%) respondents reporting it much less efficient. Responses differed by level of training (P = .01), with medical students trending towards higher efficiency. Of the 23 respondents who reported on the Inpatient Dashboard’s impact on daily workload stress level, 22 (96%) indicated the tool had a stress-reducing effect, with 9 (39%) experiencing a major reduction in stress level, and 13 (57%) experiencing somewhat reduced stress level. Only 1 participant (4%) reported no change in stress. No participants reported an increase in stress.
DISCUSSION
The adoption of EHRs has transformed operational modalities in contemporary health care systems, heralding advancements in patient satisfaction, safety, and overall quality and efficiency of care.1,2 However, EHRs still present challenges, predominantly around clinician satisfaction, marked by instances of burnout and increased time spent on computers.2-6 In this context, the Inpatient Dashboard, an online companion to the CPRS, exemplifies how user-centered innovations in EHRs can address and mitigate associated challenges.
The Inpatient Dashboard has been well received with most respondents of the survey conducted in this study indicating they were both satisfied with the instrument and preferring it to CPRS. This high approval aligns with existing literature on the potential advantages of user-centered design in health care technology.13 The tool has gained widespread acceptance at the VAAAHS even in the absence of obligatory usage or institutional incentives. The appeal of the Inpatient Dashboard may stem from its increased efficiency, with most users affirming its timesaving nature. While CPRS can only display local notes, laboratory results, and studies, the Inpatient Dashboard can display data from across all VA sites. The VA Joint Longitudinal Viewer can similarly display data from across all sites, but the display is not streamlined as it is in the Inpatient Dashboard. The Inpatient Dashboard incorporates this clinical information into a single page to facilitate day-to-day workflow and dynamic documentation (ie, reviewing laboratory results, medications, writing notes, and signing out patients). This increased efficiency allows clinicians to counter 2 common barriers to EHR implementation: productivity loss and insufficient time.14
The association between EHRs and improved quality and safety in health care is well-documented.3 The Inpatient Dashboard fortifies this association by enhancing awareness around patient status, evidenced by a majority of respondents, and by integrating a medication reconciliation tool to decrease medication errors on transition from the emergency department to inpatient hospitalization.10
The Inpatient Dashboard’s impact on alleviating daily workload stress is noteworthy, with almost all respondents experiencing reduced stress levels and physician burnout, which has been linked to deteriorating well-being, compromised patient safety, and escalated health care costs.15,16 The heightened susceptibility of physicians to burnout compared to other professionals underscores the imperative for incorporating stress-mitigating interventions in the EHR.17,18
While responses to most questions did not significantly differ by training levels, overall satisfaction with the Inpatient Dashboard and its ability to save time preparing for rounds were rated higher by medical students. This may be attributable to a greater derived benefit from collating and presenting data to learners with less familiarity with the native EHR. It is also notable that the Inpatient Dashboard allows medical students to directly contribute to a patient’s note, which could be another driver in satisfaction. While most interns still felt the Inpatient Dashboard enabled them to save time preparing for rounds, there were a considerable number of ‘no change’ responses, which suggests some interns may not have modified their existing prerounding strategies. These associations are limited by the relatively small number of respondents by learner category, with senior medical residents and attending physicians being underrepresented.
While there are a multitude of dashboards available at the VA, most are made to track certain quality metrics and are used more by administrative and leadership staff. The Inpatient Dashboard was created specifically for frontline clinicians to facilitate their day-to-day workflow and dynamic documentation. This tool can additionally help with quality metrics, though its main purpose was and is to make clinician workflow easier and more efficient.
These results are especially timely because the VA is modernizing its EHR by transitioning to Oracle Health.7 Due to the numerous reports both from veterans and VA clinicians that the Oracle Health EHR is not meeting expectations, deployment at further sites has been halted while improving the experience of the 5 institutions using Oracle Health is prioritized.9 The Inpatient Dashboard, instead of being merely an enhancement to CPRS, could emerge as a potential bridge to Oracle Health if adapted to display data from Oracle Health as it does VistA. This would facilitate a smoother, more integrated transition for those health care institutions employing the Inpatient Dashboard.
Limitations
The reliance on self-reported data inherently carries the risk of bias, and the absence of objective measures, like time-tracking studies, limits the quantifiable assessment of the Inpatient Dashboard efficacy. The single-center nature of the study also may restrict the generalizability of the results.
CONCLUSIONS
Optimal integration of EHRs into health care delivery is critical to high-quality patient care and operational efficiency. The Inpatient Dashboard is an example of an innovative, user-centric solution that integrated and presented clinical information in a way that produced high satisfaction and adoption by users at a VA hospital.
Electronic health records (EHRs) are an integral part of modern health care. The 2009, Health Information Technology for Economic and Clinical Health Act established financial incentives for US hospitals to adopt EHRs. In 2009 only 12% of nonfederal acute care hospitals had adopted a certified EHR system, which increased to 96% by 2021.1
EHRs have transformed the way patient data are stored and accessed, streamlining the process of providing quality patient care with improvements in efficiency, effectiveness, patient satisfaction, and safety.2 Despite their widespread adoption and benefits, EHRs have generally been met with mixed physician satisfaction.3 Interactions with EHRs are linked to disproportionate time at the computer and physician burnout.4-6
The US Department of Veterans Affairs (VA) was at the forefront of EHR development, establishing the Veterans Health Information Systems and Technology Architecture (VistA) in the 1970s. The VA released the Computerized Patient Record System (CPRS) in 1997, the first clinical user interface for VistA. In May 2018, the VA signed a $10 billion contract with Cerner (now Oracle Health) to modernize its EHR.7 This was later revised to $16.1 billion, and the Institute for Defense Analyses estimates it will cost $49.8 billion.8 The transition to Oracle Health has been faced with significant challenges, including patient safety risks and workflow inefficiencies, leading to a pause in rollout.9
Due to the known challenges with EHRs and the aging CPRS system (without a scheduled replacement date), innovations that facilitate the synthesis and display of clinical information are needed. To address this gap, the VA Ann Arbor Healthcare System (VAAAHS) developed the Inpatient Dashboard, an online EHR companion tool. The Inpatient Dashboard was designed to draw data from VistA to reduce time spent at the computer by streamlining clinical information presentation, standardizing inpatient notes, improving safety measures, and enhancing overall clinician satisfaction. This study evaluated the adoption and user experience with the Inpatient Dashboard.
INPATIENT DASHBOARD
The Inpatient Dashboard consists of several modules created by a contractor for the VAAAHS that is housed on VA servers with access restricted to individuals with patient health data privileges. As the Inpatient Dashboard draws data from VistA, it can display laboratory information, studies, and notes from all VA sites.
The main dashboard is a snapshot summary of patient information, including patient location, code status, last vital sign readings, vital sign ranges over the previous 24 hours, intake/output, deep vein thrombosis (DVT) prophylaxis, the presence of telemetry orders, or use of Foley or central catheters (Figure). It also includes a customizable to-do list and contact information for the patient’s clinician and nurse. Significant events, such as abnormal vital signs or speciation/sensitivities for blood cultures, are automatically populated on the to-do list. From this main dashboard overview, clinicians can customize which patients are displayed, create and print a rounding list, print a sign-out sheet, or select individual patients to open a progress note module.

Notes can be written in the patient history and physical module, progress note module, and discharge summary module. The patient history and physical module has text blocks allocated to the traditional components of a history and physical note (ie, chief complaint, history of present illness, review of systems, past medical history, family history, social history, allergies, medications, physical examination, assessment, and plan) (eAppendix 1). Some elements, such as past medical history, family history, and social history are prepopulated if the patient was previously admitted. Vital signs, laboratory results, studies, microbiology/ pathology reports, and other CPRS notes are displayed in this module.

The progress note module contains text blocks allocated to the traditional components of a progress note, such as subjective/interval events, physical examination, assessment, and plan (eAppendix 2). Vital signs, laboratory results, studies, microbiology/ pathology reports, other CPRS notes, and the patient’s medication administration record are also displayed in this module. Lastly, the discharge summary module includes patient follow-up, patient instructions, hospitalization summary, medication reconciliation, laboratory results, and studies/procedures, ensuring a comprehensive discharge summary for patients and clinicians (eAppendix 3).


A medication reconciliation tool was embedded within the history and physical and discharge summary modules. This tool has been shown to reduce medication errors in patients admitted from the emergency department to the hospital (eAppendix 4).10 The handoff/sign-out tool (eAppendix 5) accessible through the main dashboard page is modeled on the I-PASS handoff framework.11,12 This includes the patient identifier, interval events, inpatient medications, specific sign-out guidance, sign-out tasks/to-dos, and any other pertinent information.
The Inpatient Dashboard is a team-based construct shared by the attending physicians, residents, and medical students. Each team (eg, general medicine, general surgery) is its own entity; only team members can change the content or add to the documentation. Each facility can have multiple teams caring for the same patient (eg, primary and consulting teams). Additional care members can also be incorporated (eg, pharmacists assist with medication reconciliation for admission and discharge at VAAAHS). The Inpatient Dashboard can export information directly to CPRS for clinicians to review and sign. It can also generate a note that can be pasted into CPRS.


Clinician Feedback and Satisfaction
A survey was developed to evaluate clinician experiences with using the Inpatient Dashboard as an adjunct to the CPRS. The Inpatient Dashboard was made available to general medicine teams in November 2018. The survey was conducted from December 2018 to September 2019. The study was conducted at the VAAAHS and included 4 general medicine teams. Each team included an attending physician, a senior resident, 2 to 3 interns, and 3 to 4 medical students. Eligibility was extended to any team member who used both the CPRS and Inpatient Dashboard. Participation in the survey was voluntary. All respondents were informed of the study’s purpose and encouraged to provide candid feedback to ensure the reliability and validity of the findings.
Data were collected through a semistructured survey administered via the Qualtrics platform. The questionnaire was designed to capture multidimensional insights into clinician experience, with particular focus on satisfaction, efficiency, and perceived safety when using the tool as an adjunct to CPRS compared to using CPRS alone. The questionnaire primarily used a Likert scale for responses. Surveys were emailed at the completion of a team’s 1-month inpatient block. An answer was not required for every question, resulting in slightly different response numbers for some questions.
A question regarding the tool’s impact on workload stress was added halfway through the study period, which resulted in fewer responses. Adoption was assessed by counting the Inpatient Dashboard unique users. Descriptive statistics were used within individual survey responses to report the distribution of responses. Differences in response between levels of training were assessed using a X2 test of independence.
Survey Results
From September 2023 through November 2023, there were 1549 rounding printouts across 144 unique users (5 nurses, 40 medical students, 87 residents, and 12 attending physicians) and 1468 handoff printouts across 148 unique users (5 nurses, 10 medical students, 111 residents, and 22 attending physicians). The clinician survey received 68 responses from users at various levels of medical training: 23 medical students, 31 interns, 12 senior residents, and 2 attending physicians. All 68 participants confirmed they had used the Inpatient Dashboard.
User satisfaction and preference for the Inpatient Dashboard vs CPRS were assessed. Sixty-one respondents (90%) expressed overall satisfaction with the Inpatient Dashboard; 22 (32%) were extremely satisfied, and 39 (57%) were somewhat satisfied (Table 1). Three respondents (4%) were neutral, 2 (3%) were somewhat dissatisfied, and 2 (3%) were extremely dissatisfied with the Inpatient Dashboard. Responses differed by level of training (P = .03), with medical students trending towards higher satisfaction.

Respondents preferred the Inpatient Dashboard over CPRS for gathering information and writing progress notes; 42 (64%) respondents mostly favored the Inpatient Dashboard, 15 (23%) slightly favored the Inpatient Dashboard over CPRS, and 8 (12%) were neutral. One respondent (2%) slightly favored CPRS to the Inpatient Dashboard (Table 2).

Sixty-five respondents (100%) found the Inpatient Dashboard’s ability to summarize patient information in a central place helpful (Table 3). Among them, 53 (82%) respondents reported it was very or extremely helpful, 10 (15%) respondents reported it was moderately helpful, and 2 (3%) respondents reported it was slightly helpful. This feature positively impacted users’ awareness of patients with DVT prophylaxis or a Foley catheter. Ten (15%) respondents reported being much more aware, and 29 (45%) respondents reporting they were slightly more aware. The remaining 26 (40%) respondents reported no change in awareness.

The Inpatient Dashboard was reported to save time preparing for physician rounds by 52 (80%) respondents, contributing to much greater efficiency for 29 (45%) respondents and slightly more efficiency for 23 (35%) respondents. However, 10 (15%) respondents reported no change in efficiency, and 3 (5%) respondents reduced efficiency, with 1 (2%) respondent reporting it slightly less efficient and 2 (3%) respondents reporting it much less efficient. Responses differed by level of training (P = .01), with medical students trending towards higher efficiency. Of the 23 respondents who reported on the Inpatient Dashboard’s impact on daily workload stress level, 22 (96%) indicated the tool had a stress-reducing effect, with 9 (39%) experiencing a major reduction in stress level, and 13 (57%) experiencing somewhat reduced stress level. Only 1 participant (4%) reported no change in stress. No participants reported an increase in stress.
DISCUSSION
The adoption of EHRs has transformed operational modalities in contemporary health care systems, heralding advancements in patient satisfaction, safety, and overall quality and efficiency of care.1,2 However, EHRs still present challenges, predominantly around clinician satisfaction, marked by instances of burnout and increased time spent on computers.2-6 In this context, the Inpatient Dashboard, an online companion to the CPRS, exemplifies how user-centered innovations in EHRs can address and mitigate associated challenges.
The Inpatient Dashboard has been well received with most respondents of the survey conducted in this study indicating they were both satisfied with the instrument and preferring it to CPRS. This high approval aligns with existing literature on the potential advantages of user-centered design in health care technology.13 The tool has gained widespread acceptance at the VAAAHS even in the absence of obligatory usage or institutional incentives. The appeal of the Inpatient Dashboard may stem from its increased efficiency, with most users affirming its timesaving nature. While CPRS can only display local notes, laboratory results, and studies, the Inpatient Dashboard can display data from across all VA sites. The VA Joint Longitudinal Viewer can similarly display data from across all sites, but the display is not streamlined as it is in the Inpatient Dashboard. The Inpatient Dashboard incorporates this clinical information into a single page to facilitate day-to-day workflow and dynamic documentation (ie, reviewing laboratory results, medications, writing notes, and signing out patients). This increased efficiency allows clinicians to counter 2 common barriers to EHR implementation: productivity loss and insufficient time.14
The association between EHRs and improved quality and safety in health care is well-documented.3 The Inpatient Dashboard fortifies this association by enhancing awareness around patient status, evidenced by a majority of respondents, and by integrating a medication reconciliation tool to decrease medication errors on transition from the emergency department to inpatient hospitalization.10
The Inpatient Dashboard’s impact on alleviating daily workload stress is noteworthy, with almost all respondents experiencing reduced stress levels and physician burnout, which has been linked to deteriorating well-being, compromised patient safety, and escalated health care costs.15,16 The heightened susceptibility of physicians to burnout compared to other professionals underscores the imperative for incorporating stress-mitigating interventions in the EHR.17,18
While responses to most questions did not significantly differ by training levels, overall satisfaction with the Inpatient Dashboard and its ability to save time preparing for rounds were rated higher by medical students. This may be attributable to a greater derived benefit from collating and presenting data to learners with less familiarity with the native EHR. It is also notable that the Inpatient Dashboard allows medical students to directly contribute to a patient’s note, which could be another driver in satisfaction. While most interns still felt the Inpatient Dashboard enabled them to save time preparing for rounds, there were a considerable number of ‘no change’ responses, which suggests some interns may not have modified their existing prerounding strategies. These associations are limited by the relatively small number of respondents by learner category, with senior medical residents and attending physicians being underrepresented.
While there are a multitude of dashboards available at the VA, most are made to track certain quality metrics and are used more by administrative and leadership staff. The Inpatient Dashboard was created specifically for frontline clinicians to facilitate their day-to-day workflow and dynamic documentation. This tool can additionally help with quality metrics, though its main purpose was and is to make clinician workflow easier and more efficient.
These results are especially timely because the VA is modernizing its EHR by transitioning to Oracle Health.7 Due to the numerous reports both from veterans and VA clinicians that the Oracle Health EHR is not meeting expectations, deployment at further sites has been halted while improving the experience of the 5 institutions using Oracle Health is prioritized.9 The Inpatient Dashboard, instead of being merely an enhancement to CPRS, could emerge as a potential bridge to Oracle Health if adapted to display data from Oracle Health as it does VistA. This would facilitate a smoother, more integrated transition for those health care institutions employing the Inpatient Dashboard.
Limitations
The reliance on self-reported data inherently carries the risk of bias, and the absence of objective measures, like time-tracking studies, limits the quantifiable assessment of the Inpatient Dashboard efficacy. The single-center nature of the study also may restrict the generalizability of the results.
CONCLUSIONS
Optimal integration of EHRs into health care delivery is critical to high-quality patient care and operational efficiency. The Inpatient Dashboard is an example of an innovative, user-centric solution that integrated and presented clinical information in a way that produced high satisfaction and adoption by users at a VA hospital.
- Office of the National Coordinator for Health Information Technology. National Trends in Hospital and Physician Adoption of Electronic Health Records. HealthIT.gov. Accessed February 5, 2025. https://www.healthit.gov/data/quickstats/national-trends-hospital-and-physician-adoption-electronic-health-records
- Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464-471. doi:10.1377/hlthaff.2011.0178
- Nguyen L, Bellucci E, Nguyen LT. Electronic health records implementation: an evaluation of information system impact and contingency factors. Int J Med Inf. 2014;83(11):779-796. doi:10.1016/j.ijmedinf.2014.06.011
- Alexander AG, Ballou KA. Work-life balance, burnout, and the electronic health record. Am J Med. 2018;131(8):857- 858. doi:10.1016/j.amjmed.2018.02.033
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165(11):753-760. doi:10.7326/M16-0961
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med. 2019;179(6):760-767. doi:10.1001/jamainternmed.2019.0095
- US Department of Veterans Affairs. Statement by Acting Secretary Robert Wilkie - VA signs contract with Cerner for an electronic health record system. New release. May 17, 2018. Accessed February 5, 2025. https://news.va.gov/press-room/statement-by-acting-secretary-robert-wilkie-va-signs-contract-with-cerner-for-an-electronic-health-record-system/
- US Government Publishing Office. VA’s Electronic health record modernization: an update on rollout, cost, and schedule. Subcommittee on Military Construction, Veterans Affairs, and Related Agencies, Committee on Appropriations, United States Senate. 117th Congress, 2nd Session. September 21, 2022. Accessed February 5, 2025. https://www.govinfo.gov/content/pkg/CHRG-117shrg52328/html/CHRG-117shrg52328.htm
- US Department of Veterans Affairs. VA announces reset of electronic health record project. Accessed December 21, 2023. https://news.va.gov/press-room/va-announces-reset-of-electronic-health-record-project/
- Grondin C, Gupta A, Houchens N, et al. Medication reconciliation tool reduces errors in patients admitted from the ED to hospital. Am J Med Qual. 2021;36(2):129. doi:10.1097/01.JMQ.0000741500.33781.eb
- Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi:10.1056/NEJMsa1405556
- Starmer AJ, O’Toole JK, Rosenbluth G, et al. Development, implementation, and dissemination of the I-PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876-884. doi:10.1097/ACM.0000000000000264
- Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
- Kruse CS, Kristof C, Jones B, Mitchell E, Martinez A. Barriers to electronic health record adoption: a systematic literature review. J Med Syst. 2016;40(12):252. doi:10.1007/s10916-016-0628-9
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516-529. doi:10.1111/joim.12752
- Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907- 912. doi:10.1016/j.amjsurg.2019.07.004
- Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385. doi:10.1001/archinternmed.2012.3199
- Budd J. Burnout related to electronic health record use in primary care. J Prim Care Community Health. 2023;14:21501319231166921. doi:10.1177/21501319231166921
- Office of the National Coordinator for Health Information Technology. National Trends in Hospital and Physician Adoption of Electronic Health Records. HealthIT.gov. Accessed February 5, 2025. https://www.healthit.gov/data/quickstats/national-trends-hospital-and-physician-adoption-electronic-health-records
- Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464-471. doi:10.1377/hlthaff.2011.0178
- Nguyen L, Bellucci E, Nguyen LT. Electronic health records implementation: an evaluation of information system impact and contingency factors. Int J Med Inf. 2014;83(11):779-796. doi:10.1016/j.ijmedinf.2014.06.011
- Alexander AG, Ballou KA. Work-life balance, burnout, and the electronic health record. Am J Med. 2018;131(8):857- 858. doi:10.1016/j.amjmed.2018.02.033
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165(11):753-760. doi:10.7326/M16-0961
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med. 2019;179(6):760-767. doi:10.1001/jamainternmed.2019.0095
- US Department of Veterans Affairs. Statement by Acting Secretary Robert Wilkie - VA signs contract with Cerner for an electronic health record system. New release. May 17, 2018. Accessed February 5, 2025. https://news.va.gov/press-room/statement-by-acting-secretary-robert-wilkie-va-signs-contract-with-cerner-for-an-electronic-health-record-system/
- US Government Publishing Office. VA’s Electronic health record modernization: an update on rollout, cost, and schedule. Subcommittee on Military Construction, Veterans Affairs, and Related Agencies, Committee on Appropriations, United States Senate. 117th Congress, 2nd Session. September 21, 2022. Accessed February 5, 2025. https://www.govinfo.gov/content/pkg/CHRG-117shrg52328/html/CHRG-117shrg52328.htm
- US Department of Veterans Affairs. VA announces reset of electronic health record project. Accessed December 21, 2023. https://news.va.gov/press-room/va-announces-reset-of-electronic-health-record-project/
- Grondin C, Gupta A, Houchens N, et al. Medication reconciliation tool reduces errors in patients admitted from the ED to hospital. Am J Med Qual. 2021;36(2):129. doi:10.1097/01.JMQ.0000741500.33781.eb
- Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi:10.1056/NEJMsa1405556
- Starmer AJ, O’Toole JK, Rosenbluth G, et al. Development, implementation, and dissemination of the I-PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876-884. doi:10.1097/ACM.0000000000000264
- Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
- Kruse CS, Kristof C, Jones B, Mitchell E, Martinez A. Barriers to electronic health record adoption: a systematic literature review. J Med Syst. 2016;40(12):252. doi:10.1007/s10916-016-0628-9
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516-529. doi:10.1111/joim.12752
- Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907- 912. doi:10.1016/j.amjsurg.2019.07.004
- Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385. doi:10.1001/archinternmed.2012.3199
- Budd J. Burnout related to electronic health record use in primary care. J Prim Care Community Health. 2023;14:21501319231166921. doi:10.1177/21501319231166921
Streamlining Health Care: Inpatient Dashboard as a User-Centric Solution in EHR Enhancement
Streamlining Health Care: Inpatient Dashboard as a User-Centric Solution in EHR Enhancement
Walter Reed National Military Medical Center Recovering After Flood
A burst sprinkler pipe and broken steam system caused significant infrastructure failures and wreaked havoc on patient care at Walter Reed National Military Medical Center in January.
An email sent to Walter Reed staff from the medical center’s director, Navy Capt. Melissa C. Austin, said 60,000 gallons of water, or enough “to fill a 25x50 foot swimming pool” flooded throughout the facility on Jan. 20 before it was contained, damaging 50 rooms and 6 elevators.
Frozen pipes burst due to extreme cold, and the issues were exacerbated by aging infrastructure and “deferred maintenance due to underfunding,” the Defense Health Agency (DHA), which oversees Walter Reed, said in a public statement.
The damage was severe enough to impact patient care. The facility had to evacuate the neonatal intensive care unit as well as several clinics. The steam system outages also meant operating rooms had fewer clean surgical tools available and had to send them to regional hospitals for sterilization, staffers told The Washington Post. Health care workers could not “flash sterilize” equipment in emergencies, further risking patient safety.
Rick McNamara, a spokesperson for the Defense Health Network National Capital Region, confirmed other hospitals are “sharing the burden” to sterilize equipment. McNamara said it could take 6 weeks to complete the immediate repairs, which will cost between $1 million and $2 million.
Patient appointments were delayed, and nonemergency procedures were canceled or delayed. Overall, 212 patients were “deferred or rescheduled,” and 56 other patients were sent to other hospitals to receive care.
Defense Secretary Pete Hegseth said on Jan. 31 the problem was “real and unacceptable” in response to a video circulating on social media that showed flooding.
Acknowledging that the water damage “temporarily impacted health care operations,” the Defense Department says DHA and Walter Reed staff were “working diligently around the clock” to find and implement solutions while minimizing disruptions to patient care: “High waters and loss of steam pressure impacted the capacity of services delivered, but the ability to deliver the hospital’s core capabilities of safe, quality care was never compromised,” the agency said.
In response to the flooding, the hospital moved quickly to provide the required urgent care: “We are utilizing all the hospitals and clinics in the National Capital Region Network from Malcom Grow at Joint Base Andrews to Kimbrough Ambulatory Care Center at Fort Meade to the Alexander T. Augusta Military Medical Center at Fort Belvoir,” Capt. Austin said.
DHA is also funding emergency work orders and contract modifications required to return Walter Reed to full operational capability. It is prioritizing resources for repairs and is collaborating with the Naval Installations Command and Naval Support Activity Bethesda to implement necessary repairs.
“This acute issue is being managed aggressively to ensure patient care continues to be delivered safely,” DHA said
A burst sprinkler pipe and broken steam system caused significant infrastructure failures and wreaked havoc on patient care at Walter Reed National Military Medical Center in January.
An email sent to Walter Reed staff from the medical center’s director, Navy Capt. Melissa C. Austin, said 60,000 gallons of water, or enough “to fill a 25x50 foot swimming pool” flooded throughout the facility on Jan. 20 before it was contained, damaging 50 rooms and 6 elevators.
Frozen pipes burst due to extreme cold, and the issues were exacerbated by aging infrastructure and “deferred maintenance due to underfunding,” the Defense Health Agency (DHA), which oversees Walter Reed, said in a public statement.
The damage was severe enough to impact patient care. The facility had to evacuate the neonatal intensive care unit as well as several clinics. The steam system outages also meant operating rooms had fewer clean surgical tools available and had to send them to regional hospitals for sterilization, staffers told The Washington Post. Health care workers could not “flash sterilize” equipment in emergencies, further risking patient safety.
Rick McNamara, a spokesperson for the Defense Health Network National Capital Region, confirmed other hospitals are “sharing the burden” to sterilize equipment. McNamara said it could take 6 weeks to complete the immediate repairs, which will cost between $1 million and $2 million.
Patient appointments were delayed, and nonemergency procedures were canceled or delayed. Overall, 212 patients were “deferred or rescheduled,” and 56 other patients were sent to other hospitals to receive care.
Defense Secretary Pete Hegseth said on Jan. 31 the problem was “real and unacceptable” in response to a video circulating on social media that showed flooding.
Acknowledging that the water damage “temporarily impacted health care operations,” the Defense Department says DHA and Walter Reed staff were “working diligently around the clock” to find and implement solutions while minimizing disruptions to patient care: “High waters and loss of steam pressure impacted the capacity of services delivered, but the ability to deliver the hospital’s core capabilities of safe, quality care was never compromised,” the agency said.
In response to the flooding, the hospital moved quickly to provide the required urgent care: “We are utilizing all the hospitals and clinics in the National Capital Region Network from Malcom Grow at Joint Base Andrews to Kimbrough Ambulatory Care Center at Fort Meade to the Alexander T. Augusta Military Medical Center at Fort Belvoir,” Capt. Austin said.
DHA is also funding emergency work orders and contract modifications required to return Walter Reed to full operational capability. It is prioritizing resources for repairs and is collaborating with the Naval Installations Command and Naval Support Activity Bethesda to implement necessary repairs.
“This acute issue is being managed aggressively to ensure patient care continues to be delivered safely,” DHA said
A burst sprinkler pipe and broken steam system caused significant infrastructure failures and wreaked havoc on patient care at Walter Reed National Military Medical Center in January.
An email sent to Walter Reed staff from the medical center’s director, Navy Capt. Melissa C. Austin, said 60,000 gallons of water, or enough “to fill a 25x50 foot swimming pool” flooded throughout the facility on Jan. 20 before it was contained, damaging 50 rooms and 6 elevators.
Frozen pipes burst due to extreme cold, and the issues were exacerbated by aging infrastructure and “deferred maintenance due to underfunding,” the Defense Health Agency (DHA), which oversees Walter Reed, said in a public statement.
The damage was severe enough to impact patient care. The facility had to evacuate the neonatal intensive care unit as well as several clinics. The steam system outages also meant operating rooms had fewer clean surgical tools available and had to send them to regional hospitals for sterilization, staffers told The Washington Post. Health care workers could not “flash sterilize” equipment in emergencies, further risking patient safety.
Rick McNamara, a spokesperson for the Defense Health Network National Capital Region, confirmed other hospitals are “sharing the burden” to sterilize equipment. McNamara said it could take 6 weeks to complete the immediate repairs, which will cost between $1 million and $2 million.
Patient appointments were delayed, and nonemergency procedures were canceled or delayed. Overall, 212 patients were “deferred or rescheduled,” and 56 other patients were sent to other hospitals to receive care.
Defense Secretary Pete Hegseth said on Jan. 31 the problem was “real and unacceptable” in response to a video circulating on social media that showed flooding.
Acknowledging that the water damage “temporarily impacted health care operations,” the Defense Department says DHA and Walter Reed staff were “working diligently around the clock” to find and implement solutions while minimizing disruptions to patient care: “High waters and loss of steam pressure impacted the capacity of services delivered, but the ability to deliver the hospital’s core capabilities of safe, quality care was never compromised,” the agency said.
In response to the flooding, the hospital moved quickly to provide the required urgent care: “We are utilizing all the hospitals and clinics in the National Capital Region Network from Malcom Grow at Joint Base Andrews to Kimbrough Ambulatory Care Center at Fort Meade to the Alexander T. Augusta Military Medical Center at Fort Belvoir,” Capt. Austin said.
DHA is also funding emergency work orders and contract modifications required to return Walter Reed to full operational capability. It is prioritizing resources for repairs and is collaborating with the Naval Installations Command and Naval Support Activity Bethesda to implement necessary repairs.
“This acute issue is being managed aggressively to ensure patient care continues to be delivered safely,” DHA said
VA Expanded Emergency Care Program Offers At-Home Clinical Evaluation
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.
VA Tele-Emergency Care Program Expanded Nationwide
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.