User login
American Diabetes Association (ADA): Annual Scientific Sessions
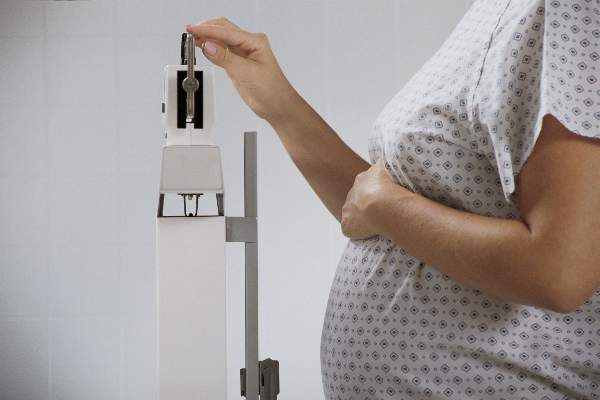
Pregnancy weight changes infant metabolic profiles
BOSTON – Maternal obesity and increased weight gain during pregnancy are associated with changes to newborns’ cardiometabolic markers, and these associations are largely independent of infant adiposity, according to new research findings.
Maternal obesity and weight gain during pregnancy are established risk factors for childhood obesity, but the reasons for this remain little understood.
In utero programming of infant metabolism may be occurring, providing reasons beyond fetal growth and fat accretion for the differences in lipid and hormonal profiles among infants, judging from the findings reported at the annual scientific sessions of the American Diabetes Association.
Presenting data from a cohort of 753 mother-infant pairs, Dominick J. Lemas, Ph.D., of the University of Colorado, Denver, said that samples of cord blood revealed “striking” associations.
Dr. Lemas and his colleagues found higher levels of cord blood glucose (P = .03), and leptin (P < .001) in newborns among mothers who gained more weight during pregnancy. Infants of women with higher body mass index when they became pregnant saw higher levels of leptin (P < .001) in cord blood and lower levels of HDL cholesterol (P = .05), even after adjusting for infant adiposity.
The leptin finding was particularly surprising, Dr. Lemas said. Leptin, a hormone that regulates food intake, energy output, and other functions, is synthesized by fatty tissues and therefore expected to be found in higher levels among infants with fatter body composition.
In this study, however, “the association actually became stronger when we adjusted for the adiposity of the infant,” he said.
The investigators looked carefully at cord blood lipids, including total and high-density lipoprotein (HDL) cholesterol, triglycerides, and free fatty acids. The finding that prepregnancy BMI was negatively associated with HDL cholesterol “was something very interesting for our group,” Dr. Lemas said at the conference. “In adults, abnormal lipid patterns are a very strong predictor of cardiovascular disease.”
The findings raise two main concerns, Dr. Lemas said. First, “what factors beyond maternal BMI and gestational weight gain contribute to the changes to these cardiometabolic biomarkers at delivery?” And second, he said, it remains to be seen if these markers persist or result in clinically meaningful differences: “It’s possible cord-blood biomarkers don’t predict the metabolic risk of these individuals a few years down the road.”
The investigators used data from Healthy Start, a cohort study funded by the National Institute of Diabetes and Digestive and Kidney Diseases. They disclosed no conflicts of interest, and the full paper on their findings will be published in the International Journal of Obesity.
BOSTON – Maternal obesity and increased weight gain during pregnancy are associated with changes to newborns’ cardiometabolic markers, and these associations are largely independent of infant adiposity, according to new research findings.
Maternal obesity and weight gain during pregnancy are established risk factors for childhood obesity, but the reasons for this remain little understood.
In utero programming of infant metabolism may be occurring, providing reasons beyond fetal growth and fat accretion for the differences in lipid and hormonal profiles among infants, judging from the findings reported at the annual scientific sessions of the American Diabetes Association.
Presenting data from a cohort of 753 mother-infant pairs, Dominick J. Lemas, Ph.D., of the University of Colorado, Denver, said that samples of cord blood revealed “striking” associations.
Dr. Lemas and his colleagues found higher levels of cord blood glucose (P = .03), and leptin (P < .001) in newborns among mothers who gained more weight during pregnancy. Infants of women with higher body mass index when they became pregnant saw higher levels of leptin (P < .001) in cord blood and lower levels of HDL cholesterol (P = .05), even after adjusting for infant adiposity.
The leptin finding was particularly surprising, Dr. Lemas said. Leptin, a hormone that regulates food intake, energy output, and other functions, is synthesized by fatty tissues and therefore expected to be found in higher levels among infants with fatter body composition.
In this study, however, “the association actually became stronger when we adjusted for the adiposity of the infant,” he said.
The investigators looked carefully at cord blood lipids, including total and high-density lipoprotein (HDL) cholesterol, triglycerides, and free fatty acids. The finding that prepregnancy BMI was negatively associated with HDL cholesterol “was something very interesting for our group,” Dr. Lemas said at the conference. “In adults, abnormal lipid patterns are a very strong predictor of cardiovascular disease.”
The findings raise two main concerns, Dr. Lemas said. First, “what factors beyond maternal BMI and gestational weight gain contribute to the changes to these cardiometabolic biomarkers at delivery?” And second, he said, it remains to be seen if these markers persist or result in clinically meaningful differences: “It’s possible cord-blood biomarkers don’t predict the metabolic risk of these individuals a few years down the road.”
The investigators used data from Healthy Start, a cohort study funded by the National Institute of Diabetes and Digestive and Kidney Diseases. They disclosed no conflicts of interest, and the full paper on their findings will be published in the International Journal of Obesity.
BOSTON – Maternal obesity and increased weight gain during pregnancy are associated with changes to newborns’ cardiometabolic markers, and these associations are largely independent of infant adiposity, according to new research findings.
Maternal obesity and weight gain during pregnancy are established risk factors for childhood obesity, but the reasons for this remain little understood.
In utero programming of infant metabolism may be occurring, providing reasons beyond fetal growth and fat accretion for the differences in lipid and hormonal profiles among infants, judging from the findings reported at the annual scientific sessions of the American Diabetes Association.
Presenting data from a cohort of 753 mother-infant pairs, Dominick J. Lemas, Ph.D., of the University of Colorado, Denver, said that samples of cord blood revealed “striking” associations.
Dr. Lemas and his colleagues found higher levels of cord blood glucose (P = .03), and leptin (P < .001) in newborns among mothers who gained more weight during pregnancy. Infants of women with higher body mass index when they became pregnant saw higher levels of leptin (P < .001) in cord blood and lower levels of HDL cholesterol (P = .05), even after adjusting for infant adiposity.
The leptin finding was particularly surprising, Dr. Lemas said. Leptin, a hormone that regulates food intake, energy output, and other functions, is synthesized by fatty tissues and therefore expected to be found in higher levels among infants with fatter body composition.
In this study, however, “the association actually became stronger when we adjusted for the adiposity of the infant,” he said.
The investigators looked carefully at cord blood lipids, including total and high-density lipoprotein (HDL) cholesterol, triglycerides, and free fatty acids. The finding that prepregnancy BMI was negatively associated with HDL cholesterol “was something very interesting for our group,” Dr. Lemas said at the conference. “In adults, abnormal lipid patterns are a very strong predictor of cardiovascular disease.”
The findings raise two main concerns, Dr. Lemas said. First, “what factors beyond maternal BMI and gestational weight gain contribute to the changes to these cardiometabolic biomarkers at delivery?” And second, he said, it remains to be seen if these markers persist or result in clinically meaningful differences: “It’s possible cord-blood biomarkers don’t predict the metabolic risk of these individuals a few years down the road.”
The investigators used data from Healthy Start, a cohort study funded by the National Institute of Diabetes and Digestive and Kidney Diseases. They disclosed no conflicts of interest, and the full paper on their findings will be published in the International Journal of Obesity.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Mothers’ prepregnancy BMI and gestational weight gain are both associated with changes in neonatal cardiometabolic biomarkers largely independent of neonatal adiposity.
Major finding: Gestational weight gain was associated with significant increases in cord blood glucose and leptin in newborns even after adjusting for newborn adiposity. Prepregnancy BMI was associated with higher cord blood leptin and inversely associated with cord blood HDL cholesterol after adjusting for adiposity.
Data source: A longitudinal, prebirth cohort of 753 ethnically diverse pregnant women 16 years of age and older and their newborns recruited from university hospital obstetric clinics in Colorado from 2009 to 2014, with prepregnancy weight extracted from medical records or self reported, and subsequent gains recorded. Cord blood samples and adiposity measurements were taken from newborns.
Disclosures: Study funded with grants from the National Institute of Diabetes and Digestive and Kidney Diseases. The investigators disclosed no conflicts of interest.
ADA: Why WAIT Program Promotes Long-term Weight Loss Maintenance
BOSTON – Significant weight reduction in obese patients with diabetes can be maintained for 5 years in a “real world” clinical practice setting, but such long-term maintenance is most likely in those who maintain at least 7% weight loss at 1 year, according to findings from the Weight Achievement and Intensive Treatment (Why WAIT) program.
Sustained weight loss in 129 program participants who were included in the longitudinal study was associated with lower hemoglobin A1c for 5 years, and with lower blood pressure for the first 18 months; HbA1c and triglycerides, however, were the first risk factors to deteriorate with weight regain, Dr. Osama Hamdy reported at the annual scientific sessions of the American Diabetes Association.
The study subjects had completed the Why WAIT program – a 12-week intensive lifestyle intervention model designed for clinical practice – and were grouped according to their percentage weight loss: Group A included 61 patients who failed to maintain at least 7% weight loss, and group B included 68 patients who maintained at least 7% weight loss.
Overall, the total cohort lost an average of 23.8 pounds (–9.7%) and maintained an average of 16.2 pounds lost (–6.4%). Group A maintained an average of 8.4 pounds lost (–3.5%) and group B maintained an average of 23.1 pounds lost (–9.0%) at 5 years.
HbA1c decreased from 7.5% to 6.7% at 12 weeks in the group A patients, but increased to 7.7% at 1 year, and to 8.0% at 5 years. HbA1c decreased from 7.4% to 6.4% at 12 weeks in the group B patients, but increased to 6.8% at 1 year, and to 7.3% at 5 years, said Dr. Hamdy, medical director of the obesity clinical program, and director of the inpatient program at Joslin Diabetes Center, Harvard Medical School, Boston.
Despite the weight regain, group A subjects maintained significant improvement in both low- and high-density lipoprotein cholesterol levels. They had no change in blood pressure, but had worsening of serum triglycerides, noted Dr. Hamdy, whose abstract received the 2015 ADA Michaela Modan Memorial Award for top abstracts in the areas of human studies on the epidemiology, complications, and prevention of diabetes.
Group B subjects experienced similar improvement in lipid profile, but had lower blood pressure for the first 18 months.
Weight loss through intensive lifestyle interventions are typically followed by either weight regain or sustained weight loss after the first year, but the impact of sustained weight loss vs. weight regain on cardiovascular risk factors was unknown, Dr. Hamdy said.
Thus, he and his colleagues evaluated the impact in Why WAIT participants. Why WAIT is a multidisciplinary approach to weight loss that involves structured dietary interventions and modified macronutrient composition, gradual balanced and individualized physical activity (including flexibility, aerobic, and strength training), adjustment of medications that affect body weight (including diabetes medications and antidepressants), cognitive behavioral modification, and group diabetes education.
“Our study demonstrated that sustained weight loss is associated with improved diabetes control for 5 years, and improved blood pressure for the first 18 months,” he said, noting that the ability to maintain at least 7% weight loss at 1 year predicts long-term wight loss.
The study “provides further understanding that regaining the weight does eliminate some of the benefits associated with the initial weight loss,” he added.
The findings change the misconception that people who lose weight with nonsurgical interventions will fail to maintain their weight loss beyond 6 months, he said.
“Our patients maintained 6.4% weight loss after 5 years, and approximately 53% of them achieved an average of 9% weight loss.”
The Why WAIT study is supported by Joslin Diabetes Center. Dr Hamdy reported that he has received research support and is an advisory panel member and author for Metagenics, that he is a consultant and author for Abbott Nutrition and Merck & Co., and that he has received research support and is an author for Neurometrix.
* This article was updated on 6/8/2015
BOSTON – Significant weight reduction in obese patients with diabetes can be maintained for 5 years in a “real world” clinical practice setting, but such long-term maintenance is most likely in those who maintain at least 7% weight loss at 1 year, according to findings from the Weight Achievement and Intensive Treatment (Why WAIT) program.
Sustained weight loss in 129 program participants who were included in the longitudinal study was associated with lower hemoglobin A1c for 5 years, and with lower blood pressure for the first 18 months; HbA1c and triglycerides, however, were the first risk factors to deteriorate with weight regain, Dr. Osama Hamdy reported at the annual scientific sessions of the American Diabetes Association.
The study subjects had completed the Why WAIT program – a 12-week intensive lifestyle intervention model designed for clinical practice – and were grouped according to their percentage weight loss: Group A included 61 patients who failed to maintain at least 7% weight loss, and group B included 68 patients who maintained at least 7% weight loss.
Overall, the total cohort lost an average of 23.8 pounds (–9.7%) and maintained an average of 16.2 pounds lost (–6.4%). Group A maintained an average of 8.4 pounds lost (–3.5%) and group B maintained an average of 23.1 pounds lost (–9.0%) at 5 years.
HbA1c decreased from 7.5% to 6.7% at 12 weeks in the group A patients, but increased to 7.7% at 1 year, and to 8.0% at 5 years. HbA1c decreased from 7.4% to 6.4% at 12 weeks in the group B patients, but increased to 6.8% at 1 year, and to 7.3% at 5 years, said Dr. Hamdy, medical director of the obesity clinical program, and director of the inpatient program at Joslin Diabetes Center, Harvard Medical School, Boston.
Despite the weight regain, group A subjects maintained significant improvement in both low- and high-density lipoprotein cholesterol levels. They had no change in blood pressure, but had worsening of serum triglycerides, noted Dr. Hamdy, whose abstract received the 2015 ADA Michaela Modan Memorial Award for top abstracts in the areas of human studies on the epidemiology, complications, and prevention of diabetes.
Group B subjects experienced similar improvement in lipid profile, but had lower blood pressure for the first 18 months.
Weight loss through intensive lifestyle interventions are typically followed by either weight regain or sustained weight loss after the first year, but the impact of sustained weight loss vs. weight regain on cardiovascular risk factors was unknown, Dr. Hamdy said.
Thus, he and his colleagues evaluated the impact in Why WAIT participants. Why WAIT is a multidisciplinary approach to weight loss that involves structured dietary interventions and modified macronutrient composition, gradual balanced and individualized physical activity (including flexibility, aerobic, and strength training), adjustment of medications that affect body weight (including diabetes medications and antidepressants), cognitive behavioral modification, and group diabetes education.
“Our study demonstrated that sustained weight loss is associated with improved diabetes control for 5 years, and improved blood pressure for the first 18 months,” he said, noting that the ability to maintain at least 7% weight loss at 1 year predicts long-term wight loss.
The study “provides further understanding that regaining the weight does eliminate some of the benefits associated with the initial weight loss,” he added.
The findings change the misconception that people who lose weight with nonsurgical interventions will fail to maintain their weight loss beyond 6 months, he said.
“Our patients maintained 6.4% weight loss after 5 years, and approximately 53% of them achieved an average of 9% weight loss.”
The Why WAIT study is supported by Joslin Diabetes Center. Dr Hamdy reported that he has received research support and is an advisory panel member and author for Metagenics, that he is a consultant and author for Abbott Nutrition and Merck & Co., and that he has received research support and is an author for Neurometrix.
* This article was updated on 6/8/2015
BOSTON – Significant weight reduction in obese patients with diabetes can be maintained for 5 years in a “real world” clinical practice setting, but such long-term maintenance is most likely in those who maintain at least 7% weight loss at 1 year, according to findings from the Weight Achievement and Intensive Treatment (Why WAIT) program.
Sustained weight loss in 129 program participants who were included in the longitudinal study was associated with lower hemoglobin A1c for 5 years, and with lower blood pressure for the first 18 months; HbA1c and triglycerides, however, were the first risk factors to deteriorate with weight regain, Dr. Osama Hamdy reported at the annual scientific sessions of the American Diabetes Association.
The study subjects had completed the Why WAIT program – a 12-week intensive lifestyle intervention model designed for clinical practice – and were grouped according to their percentage weight loss: Group A included 61 patients who failed to maintain at least 7% weight loss, and group B included 68 patients who maintained at least 7% weight loss.
Overall, the total cohort lost an average of 23.8 pounds (–9.7%) and maintained an average of 16.2 pounds lost (–6.4%). Group A maintained an average of 8.4 pounds lost (–3.5%) and group B maintained an average of 23.1 pounds lost (–9.0%) at 5 years.
HbA1c decreased from 7.5% to 6.7% at 12 weeks in the group A patients, but increased to 7.7% at 1 year, and to 8.0% at 5 years. HbA1c decreased from 7.4% to 6.4% at 12 weeks in the group B patients, but increased to 6.8% at 1 year, and to 7.3% at 5 years, said Dr. Hamdy, medical director of the obesity clinical program, and director of the inpatient program at Joslin Diabetes Center, Harvard Medical School, Boston.
Despite the weight regain, group A subjects maintained significant improvement in both low- and high-density lipoprotein cholesterol levels. They had no change in blood pressure, but had worsening of serum triglycerides, noted Dr. Hamdy, whose abstract received the 2015 ADA Michaela Modan Memorial Award for top abstracts in the areas of human studies on the epidemiology, complications, and prevention of diabetes.
Group B subjects experienced similar improvement in lipid profile, but had lower blood pressure for the first 18 months.
Weight loss through intensive lifestyle interventions are typically followed by either weight regain or sustained weight loss after the first year, but the impact of sustained weight loss vs. weight regain on cardiovascular risk factors was unknown, Dr. Hamdy said.
Thus, he and his colleagues evaluated the impact in Why WAIT participants. Why WAIT is a multidisciplinary approach to weight loss that involves structured dietary interventions and modified macronutrient composition, gradual balanced and individualized physical activity (including flexibility, aerobic, and strength training), adjustment of medications that affect body weight (including diabetes medications and antidepressants), cognitive behavioral modification, and group diabetes education.
“Our study demonstrated that sustained weight loss is associated with improved diabetes control for 5 years, and improved blood pressure for the first 18 months,” he said, noting that the ability to maintain at least 7% weight loss at 1 year predicts long-term wight loss.
The study “provides further understanding that regaining the weight does eliminate some of the benefits associated with the initial weight loss,” he added.
The findings change the misconception that people who lose weight with nonsurgical interventions will fail to maintain their weight loss beyond 6 months, he said.
“Our patients maintained 6.4% weight loss after 5 years, and approximately 53% of them achieved an average of 9% weight loss.”
The Why WAIT study is supported by Joslin Diabetes Center. Dr Hamdy reported that he has received research support and is an advisory panel member and author for Metagenics, that he is a consultant and author for Abbott Nutrition and Merck & Co., and that he has received research support and is an author for Neurometrix.
* This article was updated on 6/8/2015
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
ADA: Why WAIT program promotes long-term weight loss maintenance
BOSTON – Significant weight reduction in obese patients with diabetes can be maintained for 5 years in a “real world” clinical practice setting, but such long-term maintenance is most likely in those who maintain at least 7% weight loss at 1 year, according to findings from the Weight Achievement and Intensive Treatment (Why WAIT) program.
Sustained weight loss in 129 program participants who were included in the longitudinal study was associated with lower hemoglobin A1c for 5 years, and with lower blood pressure for the first 18 months; HbA1c and triglycerides, however, were the first risk factors to deteriorate with weight regain, Dr. Osama Hamdy reported at the annual scientific sessions of the American Diabetes Association.
The study subjects had completed the Why WAIT program – a 12-week intensive lifestyle intervention model designed for clinical practice – and were grouped according to their percentage weight loss: Group A included 61 patients who failed to maintain at least 7% weight loss, and group B included 68 patients who maintained at least 7% weight loss.
Overall, the total cohort lost an average of 23.8 pounds (–9.7%) and maintained an average of 16.2 pounds lost (–6.4%). Group A maintained an average of 8.4 pounds lost (–3.5%) and group B maintained an average of 23.1 pounds lost (–9.0%) at 5 years.
HbA1c decreased from 7.5% to 6.7% at 12 weeks in the group A patients, but increased to 7.7% at 1 year, and to 8.0% at 5 years. HbA1c decreased from 7.4% to 6.4% at 12 weeks in the group B patients, but increased to 6.8% at 1 year, and to 7.3% at 5 years, said Dr. Hamdy, medical director of the obesity clinical program, and director of the inpatient program at Joslin Diabetes Center, Harvard Medical School, Boston.
Despite the weight regain, group A subjects maintained significant improvement in both low- and high-density lipoprotein cholesterol levels. They had no change in blood pressure, but had worsening of serum triglycerides, noted Dr. Hamdy, whose abstract received the 2015 ADA Michaela Modan Memorial Award for top abstracts in the areas of human studies on the epidemiology, complications, and prevention of diabetes.
Group B subjects experienced similar improvement in lipid profile, but had lower blood pressure for the first 18 months.
Weight loss through intensive lifestyle interventions are typically followed by either weight regain or sustained weight loss after the first year, but the impact of sustained weight loss vs. weight regain on cardiovascular risk factors was unknown, Dr. Hamdy said.
Thus, he and his colleagues evaluated the impact in Why WAIT participants. Why WAIT is a multidisciplinary approach to weight loss that involves structured dietary interventions and modified macronutrient composition, gradual balanced and individualized physical activity (including flexibility, aerobic, and strength training), adjustment of medications that affect body weight (including diabetes medications and antidepressants), cognitive behavioral modification, and group diabetes education.
“Our study demonstrated that sustained weight loss is associated with improved diabetes control for 5 years, and improved blood pressure for the first 18 months,” he said, noting that the ability to maintain at least 7% weight loss at 1 year predicts long-term wight loss.
The study “provides further understanding that regaining the weight does eliminate some of the benefits associated with the initial weight loss,” he added.
The findings change the misconception that people who lose weight with nonsurgical interventions will fail to maintain their weight loss beyond 6 months, he said.
“Our patients maintained 6.4% weight loss after 5 years, and approximately 53% of them achieved an average of 9% weight loss.”
The Why WAIT study is supported by Joslin Diabetes Center. Dr Hamdy reported that he has received research support and is an advisory panel member and author for Metagenics, that he is a consultant and author for Abbott Nutrition and Merck & Co., and that he has received research support and is an author for Neurometrix.
sworcester@frontlinemedcom.com
* This article was updated on 6/8/2015
BOSTON – Significant weight reduction in obese patients with diabetes can be maintained for 5 years in a “real world” clinical practice setting, but such long-term maintenance is most likely in those who maintain at least 7% weight loss at 1 year, according to findings from the Weight Achievement and Intensive Treatment (Why WAIT) program.
Sustained weight loss in 129 program participants who were included in the longitudinal study was associated with lower hemoglobin A1c for 5 years, and with lower blood pressure for the first 18 months; HbA1c and triglycerides, however, were the first risk factors to deteriorate with weight regain, Dr. Osama Hamdy reported at the annual scientific sessions of the American Diabetes Association.
The study subjects had completed the Why WAIT program – a 12-week intensive lifestyle intervention model designed for clinical practice – and were grouped according to their percentage weight loss: Group A included 61 patients who failed to maintain at least 7% weight loss, and group B included 68 patients who maintained at least 7% weight loss.
Overall, the total cohort lost an average of 23.8 pounds (–9.7%) and maintained an average of 16.2 pounds lost (–6.4%). Group A maintained an average of 8.4 pounds lost (–3.5%) and group B maintained an average of 23.1 pounds lost (–9.0%) at 5 years.
HbA1c decreased from 7.5% to 6.7% at 12 weeks in the group A patients, but increased to 7.7% at 1 year, and to 8.0% at 5 years. HbA1c decreased from 7.4% to 6.4% at 12 weeks in the group B patients, but increased to 6.8% at 1 year, and to 7.3% at 5 years, said Dr. Hamdy, medical director of the obesity clinical program, and director of the inpatient program at Joslin Diabetes Center, Harvard Medical School, Boston.
Despite the weight regain, group A subjects maintained significant improvement in both low- and high-density lipoprotein cholesterol levels. They had no change in blood pressure, but had worsening of serum triglycerides, noted Dr. Hamdy, whose abstract received the 2015 ADA Michaela Modan Memorial Award for top abstracts in the areas of human studies on the epidemiology, complications, and prevention of diabetes.
Group B subjects experienced similar improvement in lipid profile, but had lower blood pressure for the first 18 months.
Weight loss through intensive lifestyle interventions are typically followed by either weight regain or sustained weight loss after the first year, but the impact of sustained weight loss vs. weight regain on cardiovascular risk factors was unknown, Dr. Hamdy said.
Thus, he and his colleagues evaluated the impact in Why WAIT participants. Why WAIT is a multidisciplinary approach to weight loss that involves structured dietary interventions and modified macronutrient composition, gradual balanced and individualized physical activity (including flexibility, aerobic, and strength training), adjustment of medications that affect body weight (including diabetes medications and antidepressants), cognitive behavioral modification, and group diabetes education.
“Our study demonstrated that sustained weight loss is associated with improved diabetes control for 5 years, and improved blood pressure for the first 18 months,” he said, noting that the ability to maintain at least 7% weight loss at 1 year predicts long-term wight loss.
The study “provides further understanding that regaining the weight does eliminate some of the benefits associated with the initial weight loss,” he added.
The findings change the misconception that people who lose weight with nonsurgical interventions will fail to maintain their weight loss beyond 6 months, he said.
“Our patients maintained 6.4% weight loss after 5 years, and approximately 53% of them achieved an average of 9% weight loss.”
The Why WAIT study is supported by Joslin Diabetes Center. Dr Hamdy reported that he has received research support and is an advisory panel member and author for Metagenics, that he is a consultant and author for Abbott Nutrition and Merck & Co., and that he has received research support and is an author for Neurometrix.
sworcester@frontlinemedcom.com
* This article was updated on 6/8/2015
BOSTON – Significant weight reduction in obese patients with diabetes can be maintained for 5 years in a “real world” clinical practice setting, but such long-term maintenance is most likely in those who maintain at least 7% weight loss at 1 year, according to findings from the Weight Achievement and Intensive Treatment (Why WAIT) program.
Sustained weight loss in 129 program participants who were included in the longitudinal study was associated with lower hemoglobin A1c for 5 years, and with lower blood pressure for the first 18 months; HbA1c and triglycerides, however, were the first risk factors to deteriorate with weight regain, Dr. Osama Hamdy reported at the annual scientific sessions of the American Diabetes Association.
The study subjects had completed the Why WAIT program – a 12-week intensive lifestyle intervention model designed for clinical practice – and were grouped according to their percentage weight loss: Group A included 61 patients who failed to maintain at least 7% weight loss, and group B included 68 patients who maintained at least 7% weight loss.
Overall, the total cohort lost an average of 23.8 pounds (–9.7%) and maintained an average of 16.2 pounds lost (–6.4%). Group A maintained an average of 8.4 pounds lost (–3.5%) and group B maintained an average of 23.1 pounds lost (–9.0%) at 5 years.
HbA1c decreased from 7.5% to 6.7% at 12 weeks in the group A patients, but increased to 7.7% at 1 year, and to 8.0% at 5 years. HbA1c decreased from 7.4% to 6.4% at 12 weeks in the group B patients, but increased to 6.8% at 1 year, and to 7.3% at 5 years, said Dr. Hamdy, medical director of the obesity clinical program, and director of the inpatient program at Joslin Diabetes Center, Harvard Medical School, Boston.
Despite the weight regain, group A subjects maintained significant improvement in both low- and high-density lipoprotein cholesterol levels. They had no change in blood pressure, but had worsening of serum triglycerides, noted Dr. Hamdy, whose abstract received the 2015 ADA Michaela Modan Memorial Award for top abstracts in the areas of human studies on the epidemiology, complications, and prevention of diabetes.
Group B subjects experienced similar improvement in lipid profile, but had lower blood pressure for the first 18 months.
Weight loss through intensive lifestyle interventions are typically followed by either weight regain or sustained weight loss after the first year, but the impact of sustained weight loss vs. weight regain on cardiovascular risk factors was unknown, Dr. Hamdy said.
Thus, he and his colleagues evaluated the impact in Why WAIT participants. Why WAIT is a multidisciplinary approach to weight loss that involves structured dietary interventions and modified macronutrient composition, gradual balanced and individualized physical activity (including flexibility, aerobic, and strength training), adjustment of medications that affect body weight (including diabetes medications and antidepressants), cognitive behavioral modification, and group diabetes education.
“Our study demonstrated that sustained weight loss is associated with improved diabetes control for 5 years, and improved blood pressure for the first 18 months,” he said, noting that the ability to maintain at least 7% weight loss at 1 year predicts long-term wight loss.
The study “provides further understanding that regaining the weight does eliminate some of the benefits associated with the initial weight loss,” he added.
The findings change the misconception that people who lose weight with nonsurgical interventions will fail to maintain their weight loss beyond 6 months, he said.
“Our patients maintained 6.4% weight loss after 5 years, and approximately 53% of them achieved an average of 9% weight loss.”
The Why WAIT study is supported by Joslin Diabetes Center. Dr Hamdy reported that he has received research support and is an advisory panel member and author for Metagenics, that he is a consultant and author for Abbott Nutrition and Merck & Co., and that he has received research support and is an author for Neurometrix.
sworcester@frontlinemedcom.com
* This article was updated on 6/8/2015
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Significant weight reduction in obese patients with diabetes can be maintained for 5 years in a “real world” clinical practice setting, but such long-term maintenance is most likely in those who maintain at least 7% weight loss at 1 year.
Major finding: Sustained weight loss was associated with lower hemoglobin A1c for 5 years, and with lower blood pressure for the first 18 months.
Data source: A longitudinal study of 129 participants in the Why WAIT program.
Disclosures: The Why WAIT study is supported by Joslin Diabetes Center. Dr Hamdy reported that he has received research support and is an advisory panel member and author for Metagenics, that he is a consultant and author for Abbott Nutrition and Merck & Co., and that he has received research support and is an author for Neurometrix.
ADA: Address obesity risk early among overweight children
BOSTON – Efforts to address obesity with a goal of reducing the risk of adult diabetes should involve overweight children, and should begin as early as age 2 years, particularly in those from racial or ethnic minorities and from low income families, findings from the Early Childhood Longitudinal Study–Birth Cohort of 2001 suggest.
Of 3,400 children from the nationally representative cohort, 15.9% were obese at age 2 years, and the risk of obesity during the preschool years, from age 2 years to 5 years, was 12.1%, Shivani A. Patel, Ph.D., reported in a poster at the annual scientific sessions of the American Diabetes Association.
Those who were overweight vs. normal weight at age 2 years were more likely to be obese at age 5 years (odds ratio, 2.7), said Dr Patel of Emory University, Atlanta.
Incident obesity at age 5 years was higher among Hispanic children (OR, 2.3) and black children (OR, 2.1), compared with white children, and among those from low vs. high income families (OR, 2.1).
Those with birth weight greater than 2,500 g vs. those with low birth weight also were more likely to be obese at age 5 years (OR, 6.6,) as were those whose mothers were obese vs. non-obese before their pregnancy (OR, 2.0), according to Dr. Patel.
“Of overweight 2-years-olds, more than 1 in 4 who were Hispanic, non-Hispanic black, low-income, or who were born to obese mothers became obese by age 5,” she noted.
The Early Childhood Longitudinal Study involves three nationally representative cohorts, including the birth cohort addressed in the current study (children born in 2001 and followed through kindergarten entry), a cohort of children in kindergarten during 1998-1999 who were followed through the eighth grade, and a cohort of children in kindergarten in 2010-2011, who will be followed through the fifth grade. The study is examining child development, school readiness, and early childhood experiences. Normal weight, overweight, and obesity were defined using age-appropriate Centers for Disease Control and Prevention body mass index cut points.
The findings provide important information about incident obesity – a potential risk factor for adult diabetes – in preschool age children; few prior studies have addressed incident obesity in this age group, Dr. Patel said.
She reported having no relevant financial disclosures.
BOSTON – Efforts to address obesity with a goal of reducing the risk of adult diabetes should involve overweight children, and should begin as early as age 2 years, particularly in those from racial or ethnic minorities and from low income families, findings from the Early Childhood Longitudinal Study–Birth Cohort of 2001 suggest.
Of 3,400 children from the nationally representative cohort, 15.9% were obese at age 2 years, and the risk of obesity during the preschool years, from age 2 years to 5 years, was 12.1%, Shivani A. Patel, Ph.D., reported in a poster at the annual scientific sessions of the American Diabetes Association.
Those who were overweight vs. normal weight at age 2 years were more likely to be obese at age 5 years (odds ratio, 2.7), said Dr Patel of Emory University, Atlanta.
Incident obesity at age 5 years was higher among Hispanic children (OR, 2.3) and black children (OR, 2.1), compared with white children, and among those from low vs. high income families (OR, 2.1).
Those with birth weight greater than 2,500 g vs. those with low birth weight also were more likely to be obese at age 5 years (OR, 6.6,) as were those whose mothers were obese vs. non-obese before their pregnancy (OR, 2.0), according to Dr. Patel.
“Of overweight 2-years-olds, more than 1 in 4 who were Hispanic, non-Hispanic black, low-income, or who were born to obese mothers became obese by age 5,” she noted.
The Early Childhood Longitudinal Study involves three nationally representative cohorts, including the birth cohort addressed in the current study (children born in 2001 and followed through kindergarten entry), a cohort of children in kindergarten during 1998-1999 who were followed through the eighth grade, and a cohort of children in kindergarten in 2010-2011, who will be followed through the fifth grade. The study is examining child development, school readiness, and early childhood experiences. Normal weight, overweight, and obesity were defined using age-appropriate Centers for Disease Control and Prevention body mass index cut points.
The findings provide important information about incident obesity – a potential risk factor for adult diabetes – in preschool age children; few prior studies have addressed incident obesity in this age group, Dr. Patel said.
She reported having no relevant financial disclosures.
BOSTON – Efforts to address obesity with a goal of reducing the risk of adult diabetes should involve overweight children, and should begin as early as age 2 years, particularly in those from racial or ethnic minorities and from low income families, findings from the Early Childhood Longitudinal Study–Birth Cohort of 2001 suggest.
Of 3,400 children from the nationally representative cohort, 15.9% were obese at age 2 years, and the risk of obesity during the preschool years, from age 2 years to 5 years, was 12.1%, Shivani A. Patel, Ph.D., reported in a poster at the annual scientific sessions of the American Diabetes Association.
Those who were overweight vs. normal weight at age 2 years were more likely to be obese at age 5 years (odds ratio, 2.7), said Dr Patel of Emory University, Atlanta.
Incident obesity at age 5 years was higher among Hispanic children (OR, 2.3) and black children (OR, 2.1), compared with white children, and among those from low vs. high income families (OR, 2.1).
Those with birth weight greater than 2,500 g vs. those with low birth weight also were more likely to be obese at age 5 years (OR, 6.6,) as were those whose mothers were obese vs. non-obese before their pregnancy (OR, 2.0), according to Dr. Patel.
“Of overweight 2-years-olds, more than 1 in 4 who were Hispanic, non-Hispanic black, low-income, or who were born to obese mothers became obese by age 5,” she noted.
The Early Childhood Longitudinal Study involves three nationally representative cohorts, including the birth cohort addressed in the current study (children born in 2001 and followed through kindergarten entry), a cohort of children in kindergarten during 1998-1999 who were followed through the eighth grade, and a cohort of children in kindergarten in 2010-2011, who will be followed through the fifth grade. The study is examining child development, school readiness, and early childhood experiences. Normal weight, overweight, and obesity were defined using age-appropriate Centers for Disease Control and Prevention body mass index cut points.
The findings provide important information about incident obesity – a potential risk factor for adult diabetes – in preschool age children; few prior studies have addressed incident obesity in this age group, Dr. Patel said.
She reported having no relevant financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Efforts to address obesity with a goal of reducing the risk of adult diabetes should involve overweight children, and should begin as early as age 2 years, particularly in those from racial or ethnic minorities and from low income families.
Major finding: Children who were overweight vs. normal weight at age 2 years were more likely to be obese at age 5 years (odds ratio, 2.7).
Data source: A longitudinal cohort of 3,400 children.
Disclosures: Dr. Patel reported having no relevant financial disclosures.
ADA: Nighttime system reduces hypoglycemia events in children with type 1 diabetes
BOSTON – A system to detect dropping blood glucose and suspend insulin delivery during sleep is safe and effective in children with type 1 diabetes as young as 4 years, according to results of a randomized controlled trial.
In a presentation to the annual scientific sessions of the American Diabetes Association, Dr. Bruce A. Buckingham of Stanford (Calif.) University showed that the system, which combines glucose monitoring with insulin pump connected wirelessly to a computer by the patient’s bedside, worked nearly as well in children as it had in a previous study enrolling adults (Diabetes Care 2014;37:1885-91).
Dr. Buckingham and his colleagues enrolled 36 children aged 4-10 years and 45 children aged 11-14 years. All the children had type 1 diabetes for a year or more and had been using an insulin pump at least 6 months. The youngsters had hemoglobin A1c levels of 8% or lower, and the investigators confirmed the presence of nighttime hypoglycemia during 2 weeks of pretrial monitoring. The children were then randomized to the active or inactive system for 42 nights, of which 21 were active. Patients were not aware whether the system was active on any given night.
Insulin delivery was suspended when the computer predicted glucose would fall below 80 mg/dL within 30 minutes, to anticipate and prevent further drops to levels of 60 mg/dL or lower that could, if prolonged, trigger a seizure.
Unlike other systems for continuous glucose monitoring that alert patients of dropping glucose, this one used alarms only when levels dropped below 60 mg/dL. More often than not, Dr. Buckingham said, “people do not awaken to alarms.” The idea “is for the system to work in the background – a good night’s sleep without a seizure.”
Dr. Buckingham and his colleagues found that among about 1,900 nights recorded in the 11- to 14-year-old subgroup, the active system reduced the number of nights children’s glucose fell below 60 mg/dL for 2 hours or more from 8% in the control group to 3% in the active system (P < .001). Among about 1,600 nights monitored in the 4- to 10-year-olds, prolonged hypoglycemia was reduced from 5% of nights in the control group to 1% in the active group (P < .001).
Although younger children have a higher risk of fasting ketonemia that can be increased with insulin pump shut-offs, no episodes of ketosis occurred during the study, and there were no seizures.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Buckingham disclosed advisory or consulting relationships with Medtronic, Sanofi, Novo Nordisk, Tandem, and ConvaTec.
BOSTON – A system to detect dropping blood glucose and suspend insulin delivery during sleep is safe and effective in children with type 1 diabetes as young as 4 years, according to results of a randomized controlled trial.
In a presentation to the annual scientific sessions of the American Diabetes Association, Dr. Bruce A. Buckingham of Stanford (Calif.) University showed that the system, which combines glucose monitoring with insulin pump connected wirelessly to a computer by the patient’s bedside, worked nearly as well in children as it had in a previous study enrolling adults (Diabetes Care 2014;37:1885-91).
Dr. Buckingham and his colleagues enrolled 36 children aged 4-10 years and 45 children aged 11-14 years. All the children had type 1 diabetes for a year or more and had been using an insulin pump at least 6 months. The youngsters had hemoglobin A1c levels of 8% or lower, and the investigators confirmed the presence of nighttime hypoglycemia during 2 weeks of pretrial monitoring. The children were then randomized to the active or inactive system for 42 nights, of which 21 were active. Patients were not aware whether the system was active on any given night.
Insulin delivery was suspended when the computer predicted glucose would fall below 80 mg/dL within 30 minutes, to anticipate and prevent further drops to levels of 60 mg/dL or lower that could, if prolonged, trigger a seizure.
Unlike other systems for continuous glucose monitoring that alert patients of dropping glucose, this one used alarms only when levels dropped below 60 mg/dL. More often than not, Dr. Buckingham said, “people do not awaken to alarms.” The idea “is for the system to work in the background – a good night’s sleep without a seizure.”
Dr. Buckingham and his colleagues found that among about 1,900 nights recorded in the 11- to 14-year-old subgroup, the active system reduced the number of nights children’s glucose fell below 60 mg/dL for 2 hours or more from 8% in the control group to 3% in the active system (P < .001). Among about 1,600 nights monitored in the 4- to 10-year-olds, prolonged hypoglycemia was reduced from 5% of nights in the control group to 1% in the active group (P < .001).
Although younger children have a higher risk of fasting ketonemia that can be increased with insulin pump shut-offs, no episodes of ketosis occurred during the study, and there were no seizures.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Buckingham disclosed advisory or consulting relationships with Medtronic, Sanofi, Novo Nordisk, Tandem, and ConvaTec.
BOSTON – A system to detect dropping blood glucose and suspend insulin delivery during sleep is safe and effective in children with type 1 diabetes as young as 4 years, according to results of a randomized controlled trial.
In a presentation to the annual scientific sessions of the American Diabetes Association, Dr. Bruce A. Buckingham of Stanford (Calif.) University showed that the system, which combines glucose monitoring with insulin pump connected wirelessly to a computer by the patient’s bedside, worked nearly as well in children as it had in a previous study enrolling adults (Diabetes Care 2014;37:1885-91).
Dr. Buckingham and his colleagues enrolled 36 children aged 4-10 years and 45 children aged 11-14 years. All the children had type 1 diabetes for a year or more and had been using an insulin pump at least 6 months. The youngsters had hemoglobin A1c levels of 8% or lower, and the investigators confirmed the presence of nighttime hypoglycemia during 2 weeks of pretrial monitoring. The children were then randomized to the active or inactive system for 42 nights, of which 21 were active. Patients were not aware whether the system was active on any given night.
Insulin delivery was suspended when the computer predicted glucose would fall below 80 mg/dL within 30 minutes, to anticipate and prevent further drops to levels of 60 mg/dL or lower that could, if prolonged, trigger a seizure.
Unlike other systems for continuous glucose monitoring that alert patients of dropping glucose, this one used alarms only when levels dropped below 60 mg/dL. More often than not, Dr. Buckingham said, “people do not awaken to alarms.” The idea “is for the system to work in the background – a good night’s sleep without a seizure.”
Dr. Buckingham and his colleagues found that among about 1,900 nights recorded in the 11- to 14-year-old subgroup, the active system reduced the number of nights children’s glucose fell below 60 mg/dL for 2 hours or more from 8% in the control group to 3% in the active system (P < .001). Among about 1,600 nights monitored in the 4- to 10-year-olds, prolonged hypoglycemia was reduced from 5% of nights in the control group to 1% in the active group (P < .001).
Although younger children have a higher risk of fasting ketonemia that can be increased with insulin pump shut-offs, no episodes of ketosis occurred during the study, and there were no seizures.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Buckingham disclosed advisory or consulting relationships with Medtronic, Sanofi, Novo Nordisk, Tandem, and ConvaTec.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Dangerous episodes of low blood sugar during sleep can be reduced with an computer-based glucose monitoring system that suspends insulin delivery automatically in children with type 1 diabetes.
Major finding: Nocturnal hypoglycemia events lasting more than 120 minutes were reduced from 5% of nights in controls to 1% in children on active therapy in the 4- to 10-year-old age group, and from 8% of nights in controls to 3% in children on active therapy in the 11- to 14-year-old age group.
Data source: Thirty-six subjects aged 4-10 years with type 1 diabetes, and 45 subjects aged 11-14 years with type 1 diabetes 1 year or longer with nocturnal hypoglycemia who were randomized nightly to the active or inactive system for 42 nights.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Buckingham disclosed advisory relationships with Medtronic, Sanofi, Novo Nordisk, Tandem, and ConvaTec.