User login
Cancer survivors with PCI die more often from cardiac causes
PARIS – Cancer survivors who subsequently undergo percutaneous coronary intervention are more likely to die of their cardiovascular disease than malignancy, Dr. Uri Landes reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective case-control study that included 969 patients who underwent PCI a mean of 3.6 years after being diagnosed with any form of cancer except nonmelanoma skin cancer and an equal number of PCI patients with no history of cancer. The two groups were matched for age, sex, left ventricular ejection fraction, estimated glomerular filtration rate, and diabetes and hypertension status.
In the patients with a history of cancer, 34% of in-hospital deaths were owing to cardiac causes, 25% to malignancy, and 24.5% to infection. In patients with no history of cancer, 42.5% of in-hospital deaths were cardiac, 5% were due to malignancy, and 33% were attributed to infection, according to Dr. Landes of Rabin Medical Center in Petah-Tikva, Israel.
During up to 10 years of follow-up, the all-cause mortality rate was 46% greater in patients with a prior cancer. Their risk of the composite endpoint of cardiac death or nonfatal MI was 40% greater than in controls without a history of cancer. Moreover, their risk of the composite outcome of cardiac death, MI, target vessel revascularization, and coronary artery bypass surgery was increased by 41% relative to that of PCI patients with no history of cancer.
The impetus for this study, Dr. Landes explained, is the dearth of information available on the long-term outcomes of PCI in patients with a history of cancer. The scarcity of information came about because cancer patients are typically excluded from participation in PCI clinical trials, and most PCI registries don’t include information as to whether or not a participant has a positive oncologic history.
Dr. Landes reported having no financial conflicts regarding this study, funded through Tel Aviv University.
PARIS – Cancer survivors who subsequently undergo percutaneous coronary intervention are more likely to die of their cardiovascular disease than malignancy, Dr. Uri Landes reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective case-control study that included 969 patients who underwent PCI a mean of 3.6 years after being diagnosed with any form of cancer except nonmelanoma skin cancer and an equal number of PCI patients with no history of cancer. The two groups were matched for age, sex, left ventricular ejection fraction, estimated glomerular filtration rate, and diabetes and hypertension status.
In the patients with a history of cancer, 34% of in-hospital deaths were owing to cardiac causes, 25% to malignancy, and 24.5% to infection. In patients with no history of cancer, 42.5% of in-hospital deaths were cardiac, 5% were due to malignancy, and 33% were attributed to infection, according to Dr. Landes of Rabin Medical Center in Petah-Tikva, Israel.
During up to 10 years of follow-up, the all-cause mortality rate was 46% greater in patients with a prior cancer. Their risk of the composite endpoint of cardiac death or nonfatal MI was 40% greater than in controls without a history of cancer. Moreover, their risk of the composite outcome of cardiac death, MI, target vessel revascularization, and coronary artery bypass surgery was increased by 41% relative to that of PCI patients with no history of cancer.
The impetus for this study, Dr. Landes explained, is the dearth of information available on the long-term outcomes of PCI in patients with a history of cancer. The scarcity of information came about because cancer patients are typically excluded from participation in PCI clinical trials, and most PCI registries don’t include information as to whether or not a participant has a positive oncologic history.
Dr. Landes reported having no financial conflicts regarding this study, funded through Tel Aviv University.
PARIS – Cancer survivors who subsequently undergo percutaneous coronary intervention are more likely to die of their cardiovascular disease than malignancy, Dr. Uri Landes reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective case-control study that included 969 patients who underwent PCI a mean of 3.6 years after being diagnosed with any form of cancer except nonmelanoma skin cancer and an equal number of PCI patients with no history of cancer. The two groups were matched for age, sex, left ventricular ejection fraction, estimated glomerular filtration rate, and diabetes and hypertension status.
In the patients with a history of cancer, 34% of in-hospital deaths were owing to cardiac causes, 25% to malignancy, and 24.5% to infection. In patients with no history of cancer, 42.5% of in-hospital deaths were cardiac, 5% were due to malignancy, and 33% were attributed to infection, according to Dr. Landes of Rabin Medical Center in Petah-Tikva, Israel.
During up to 10 years of follow-up, the all-cause mortality rate was 46% greater in patients with a prior cancer. Their risk of the composite endpoint of cardiac death or nonfatal MI was 40% greater than in controls without a history of cancer. Moreover, their risk of the composite outcome of cardiac death, MI, target vessel revascularization, and coronary artery bypass surgery was increased by 41% relative to that of PCI patients with no history of cancer.
The impetus for this study, Dr. Landes explained, is the dearth of information available on the long-term outcomes of PCI in patients with a history of cancer. The scarcity of information came about because cancer patients are typically excluded from participation in PCI clinical trials, and most PCI registries don’t include information as to whether or not a participant has a positive oncologic history.
Dr. Landes reported having no financial conflicts regarding this study, funded through Tel Aviv University.
AT EUROPCR 2016
Key clinical point: A malignancy background aggravates cardiovascular issues in percutaneous coronary intervention.
Major finding: Patients with a history of cancer who underwent PCI were 41% more likely to experience cardiac death, acute MI, target vessel revascularization, or CABG than were matched controls who had no history of cancer when they underwent PCI.
Data source: This was a matched-pairs retrospective study of 1,938 patients who underwent PCI, half of whom had prior cancer and were then followed for up to 10 years postprocedure.
Disclosures: The presenter reported having no financial conflicts regarding this study.
Low paravalvular leak shown in real-world registry of Sapien 3 recipients
PARIS – The initial report from a large, prospective registry documenting outcomes in recipients of the Edwards Sapien 3 transcatheter aortic valve in real-world clinical practice confirm that the excellent results seen earlier in the rarified, randomized clinical trial setting are routinely reproducible in everyday practice, Dr. Olaf Wendler said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The big thing with the Sapien 3 is the reduction in paravalvular leakage. Our rate of moderate or severe paravalvular leakage in the registry at 30 days is only 3.1%, with 73.6% of patients having no or only trace paravalvular leakage,” reported Dr. Wendler, professor of cardiothoracic surgery at King’s College Hospital, London.
The mean gradient improved from 43.8 mm Hg at baseline to 11.7 mm Hg at discharge, while the effective orifice area climbed from 0.72 to 1.67 cm2.
He presented 30-day outcomes from the SOURCE 3 registry for 1,947 recipients of the Edwards Sapien 3 valve during transcatheter aortic valve replacement at 80 European centers in 10 countries. Their average age was 81.6 years. Updates will continue during a planned 5 years of prospective follow-up.
“This will be a very rich dataset for subgroup analyses. We now have a number of procedural variables we can analyze over time. We will have good data to get to better outcomes in terms of how best to perform the procedure if you do it with a Sapien valve,” he explained.
Among the key findings: The lower profile of the Sapien 3 delivery system, compared with earlier iterations of the Sapien valve, enabled 87% of patients in the registry to undergo TAVR via transfemoral access. And, in these 1,695 patients, whose mean logistic EuroSCORE was 17.8, the 30-day rates of all-cause mortality and disabling stroke were just 1.9% and 0.5%, respectively.
“I think there are not many series that have shown better results than these,” Dr. Wendler commented.
The non–transfemoral-access group is a very different, higher surgical risk cohort with lots more comorbid conditions. Their mean logistic EuroSCORE was 21.8. Yet in this group, the 30-day all-cause mortality and disabling stroke rates were still only 4% and 0.8%.
The transfemoral access group had markedly lower rates of life-threatening bleeding (4%), new-onset atrial fibrillation (4.8%), extended ventilation (3.5%), stage 2-3 acute kidney injury (0.8%), plus a 2-day shorter mean hospital length of stay.
Sixty percent of transfemoral access patients had their TAVR done under conscious sedation. These 1,018 patients constitute the largest dataset ever treated using conscious sedation with one valve system. The conscious sedation group had significantly lower baseline rates of carotid artery disease, prior coronary artery bypass graft surgery, and heart failure than did patients who received general anesthesia. At 30 days post-TAVR, the conscious sedation group had significantly lower rates of extended ventilation and postdilatation, and they received less contrast volume than did the general anesthesia group. However, they had a significantly higher incidence of stroke: 1.7%, compared with 0.6% for the general anesthesia group.
“Why that is the case we don’t know at the moment. We need to look into this more in the future,” the surgeon said.
A particularly impressive finding was how well Sapien 3 valve recipients with a low left ventricular ejection fraction have done. For example, 30-day all-cause mortality in the 100 patients with a baseline LVEF below 30% was 3%, not significantly different from the 1.8% rate in the 1,198 subjects with an LVEF greater than 50% or the 2.6% in patients with an LVEF of 30%-50%.
“Three percent all-cause mortality with an ejection fraction below 30% is just remarkable from my point of view,” Dr. Wendler said.
The 778 who didn’t undergo balloon aortic valvuloplasty prior to Sapien 3 implantation did not fare any worse than did those who did in terms of 30-day all-cause mortality or all strokes, but they did have significantly higher rates of life-threatening bleeding and major vascular complications.
The SOURCE 3 registry is sponsored by Edwards Lifesciences. Dr. Wendler serves as a consultant to the company.
PARIS – The initial report from a large, prospective registry documenting outcomes in recipients of the Edwards Sapien 3 transcatheter aortic valve in real-world clinical practice confirm that the excellent results seen earlier in the rarified, randomized clinical trial setting are routinely reproducible in everyday practice, Dr. Olaf Wendler said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The big thing with the Sapien 3 is the reduction in paravalvular leakage. Our rate of moderate or severe paravalvular leakage in the registry at 30 days is only 3.1%, with 73.6% of patients having no or only trace paravalvular leakage,” reported Dr. Wendler, professor of cardiothoracic surgery at King’s College Hospital, London.
The mean gradient improved from 43.8 mm Hg at baseline to 11.7 mm Hg at discharge, while the effective orifice area climbed from 0.72 to 1.67 cm2.
He presented 30-day outcomes from the SOURCE 3 registry for 1,947 recipients of the Edwards Sapien 3 valve during transcatheter aortic valve replacement at 80 European centers in 10 countries. Their average age was 81.6 years. Updates will continue during a planned 5 years of prospective follow-up.
“This will be a very rich dataset for subgroup analyses. We now have a number of procedural variables we can analyze over time. We will have good data to get to better outcomes in terms of how best to perform the procedure if you do it with a Sapien valve,” he explained.
Among the key findings: The lower profile of the Sapien 3 delivery system, compared with earlier iterations of the Sapien valve, enabled 87% of patients in the registry to undergo TAVR via transfemoral access. And, in these 1,695 patients, whose mean logistic EuroSCORE was 17.8, the 30-day rates of all-cause mortality and disabling stroke were just 1.9% and 0.5%, respectively.
“I think there are not many series that have shown better results than these,” Dr. Wendler commented.
The non–transfemoral-access group is a very different, higher surgical risk cohort with lots more comorbid conditions. Their mean logistic EuroSCORE was 21.8. Yet in this group, the 30-day all-cause mortality and disabling stroke rates were still only 4% and 0.8%.
The transfemoral access group had markedly lower rates of life-threatening bleeding (4%), new-onset atrial fibrillation (4.8%), extended ventilation (3.5%), stage 2-3 acute kidney injury (0.8%), plus a 2-day shorter mean hospital length of stay.
Sixty percent of transfemoral access patients had their TAVR done under conscious sedation. These 1,018 patients constitute the largest dataset ever treated using conscious sedation with one valve system. The conscious sedation group had significantly lower baseline rates of carotid artery disease, prior coronary artery bypass graft surgery, and heart failure than did patients who received general anesthesia. At 30 days post-TAVR, the conscious sedation group had significantly lower rates of extended ventilation and postdilatation, and they received less contrast volume than did the general anesthesia group. However, they had a significantly higher incidence of stroke: 1.7%, compared with 0.6% for the general anesthesia group.
“Why that is the case we don’t know at the moment. We need to look into this more in the future,” the surgeon said.
A particularly impressive finding was how well Sapien 3 valve recipients with a low left ventricular ejection fraction have done. For example, 30-day all-cause mortality in the 100 patients with a baseline LVEF below 30% was 3%, not significantly different from the 1.8% rate in the 1,198 subjects with an LVEF greater than 50% or the 2.6% in patients with an LVEF of 30%-50%.
“Three percent all-cause mortality with an ejection fraction below 30% is just remarkable from my point of view,” Dr. Wendler said.
The 778 who didn’t undergo balloon aortic valvuloplasty prior to Sapien 3 implantation did not fare any worse than did those who did in terms of 30-day all-cause mortality or all strokes, but they did have significantly higher rates of life-threatening bleeding and major vascular complications.
The SOURCE 3 registry is sponsored by Edwards Lifesciences. Dr. Wendler serves as a consultant to the company.
PARIS – The initial report from a large, prospective registry documenting outcomes in recipients of the Edwards Sapien 3 transcatheter aortic valve in real-world clinical practice confirm that the excellent results seen earlier in the rarified, randomized clinical trial setting are routinely reproducible in everyday practice, Dr. Olaf Wendler said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The big thing with the Sapien 3 is the reduction in paravalvular leakage. Our rate of moderate or severe paravalvular leakage in the registry at 30 days is only 3.1%, with 73.6% of patients having no or only trace paravalvular leakage,” reported Dr. Wendler, professor of cardiothoracic surgery at King’s College Hospital, London.
The mean gradient improved from 43.8 mm Hg at baseline to 11.7 mm Hg at discharge, while the effective orifice area climbed from 0.72 to 1.67 cm2.
He presented 30-day outcomes from the SOURCE 3 registry for 1,947 recipients of the Edwards Sapien 3 valve during transcatheter aortic valve replacement at 80 European centers in 10 countries. Their average age was 81.6 years. Updates will continue during a planned 5 years of prospective follow-up.
“This will be a very rich dataset for subgroup analyses. We now have a number of procedural variables we can analyze over time. We will have good data to get to better outcomes in terms of how best to perform the procedure if you do it with a Sapien valve,” he explained.
Among the key findings: The lower profile of the Sapien 3 delivery system, compared with earlier iterations of the Sapien valve, enabled 87% of patients in the registry to undergo TAVR via transfemoral access. And, in these 1,695 patients, whose mean logistic EuroSCORE was 17.8, the 30-day rates of all-cause mortality and disabling stroke were just 1.9% and 0.5%, respectively.
“I think there are not many series that have shown better results than these,” Dr. Wendler commented.
The non–transfemoral-access group is a very different, higher surgical risk cohort with lots more comorbid conditions. Their mean logistic EuroSCORE was 21.8. Yet in this group, the 30-day all-cause mortality and disabling stroke rates were still only 4% and 0.8%.
The transfemoral access group had markedly lower rates of life-threatening bleeding (4%), new-onset atrial fibrillation (4.8%), extended ventilation (3.5%), stage 2-3 acute kidney injury (0.8%), plus a 2-day shorter mean hospital length of stay.
Sixty percent of transfemoral access patients had their TAVR done under conscious sedation. These 1,018 patients constitute the largest dataset ever treated using conscious sedation with one valve system. The conscious sedation group had significantly lower baseline rates of carotid artery disease, prior coronary artery bypass graft surgery, and heart failure than did patients who received general anesthesia. At 30 days post-TAVR, the conscious sedation group had significantly lower rates of extended ventilation and postdilatation, and they received less contrast volume than did the general anesthesia group. However, they had a significantly higher incidence of stroke: 1.7%, compared with 0.6% for the general anesthesia group.
“Why that is the case we don’t know at the moment. We need to look into this more in the future,” the surgeon said.
A particularly impressive finding was how well Sapien 3 valve recipients with a low left ventricular ejection fraction have done. For example, 30-day all-cause mortality in the 100 patients with a baseline LVEF below 30% was 3%, not significantly different from the 1.8% rate in the 1,198 subjects with an LVEF greater than 50% or the 2.6% in patients with an LVEF of 30%-50%.
“Three percent all-cause mortality with an ejection fraction below 30% is just remarkable from my point of view,” Dr. Wendler said.
The 778 who didn’t undergo balloon aortic valvuloplasty prior to Sapien 3 implantation did not fare any worse than did those who did in terms of 30-day all-cause mortality or all strokes, but they did have significantly higher rates of life-threatening bleeding and major vascular complications.
The SOURCE 3 registry is sponsored by Edwards Lifesciences. Dr. Wendler serves as a consultant to the company.
AT EUROPCR 2016
Key clinical point: Only 3.1% of patients had moderate or severe paravalvular leak at 30 days after TAVR with the Sapien 3.
Major finding: The 30-day, all-cause mortality and disabling stroke rates were 1.9% and 0.5% in Sapien 3 valve recipients whose transcatheter aortic valve replacement was done by the transfemoral route.
Data source: A prospective multicenter European registry which includes 1,947 patients who underwent transcatheter aortic valve replacement with the Edwards Sapien 3 valve in real-world commercial settings.
Disclosures: The SOURCE 3 registry is sponsored by Edwards Lifesciences. Dr. Wendler serves as a consultant to the company.
How to defeat radial artery spasm in transradial PCI
PARIS – The threat of radial artery spasm is the chief impediment to broader use of transradial access cardiac catheterization and percutaneous coronary intervention, but Dr. Julien Adjedj has a series of tips and tricks to defeat it.
At Cochin University Hospital in Paris, where he is chief of the interventional cardiology clinic, 95% of all PCIs are done transradially.
“With the tips and tricks we use, we have a transradial approach failure rate of only 1.5% at our center,” Dr. Adjedj said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He and his colleagues have conducted a series of prospective, randomized studies of various prophylactic vasodilator regimens in 1,950 patients undergoing transradial PCI.
The winning strategy? Place 2.5-5.0 mg of the calcium channel blocker verapamil in the arterial sheath as first-line preventive therapy.
In a multivariate analysis adjusted for potential confounders – for example, the investigators found that the incidence of radial artery spasm (RAS) is higher in women and younger patients – the use of prophylactic verapamil placed in the arterial sheath reduced the likelihood of RAS by 75% and 72%, respectively, compared with placebo.
Intra-arterial diltiazem at 5 mg, isosorbide dinitrate at 1 mg, and molsidomine at 1 mg were also more effective than placebo. However, diltiazem and isosorbide dinitrate were associated with an unacceptable increased risk of severe hypotension compared to placebo, and molsidomine is not widely available outside France.
In contrast, verapamil was not linked to severe hypotension.
Overall, RAS occurred in 22.2 % of patients on placebo, 7.1% of those on verapamil at 2.5 mg, 7.9% with verapamil at 5 mg, 6.5% with isosorbide dinitrate at 1 mg, 9.1% of those on diltiazem at 5 mg, 13.3% with molsidomine at 1 mg, and 4.8% with verapamil 2.5 mg plus molsidomine 1 mg.
When it proves difficult to advance the catheter during a transradial PCI despite prophylactic verapamil, the first thing to do is check whether the problem really is RAS or is instead a matter of having entered a remnant artery. This is accomplished by supplementing the verapamil with 1 mg of intra-arterial isosorbide dinitrate; if the catheter still won’t pass, seriously consider the possibility of a remnant artery.
Among Dr. Adjedj’s tips on how to successfully pass the catheter through a drug-refractory RAS: Use a hydrophilic 0.035-inch guide wire, switch from a 6 Fr to a smaller 5 or 4 Fr catheter, or use a long multipurpose 5 Fr catheter inside the 6 Fr guiding catheter.
“It’s like nested Russian dolls. It can pass through the spasm without any pain,” said Dr. Adjedj.
He reported having no financial conflicts regarding his presentation.
PARIS – The threat of radial artery spasm is the chief impediment to broader use of transradial access cardiac catheterization and percutaneous coronary intervention, but Dr. Julien Adjedj has a series of tips and tricks to defeat it.
At Cochin University Hospital in Paris, where he is chief of the interventional cardiology clinic, 95% of all PCIs are done transradially.
“With the tips and tricks we use, we have a transradial approach failure rate of only 1.5% at our center,” Dr. Adjedj said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He and his colleagues have conducted a series of prospective, randomized studies of various prophylactic vasodilator regimens in 1,950 patients undergoing transradial PCI.
The winning strategy? Place 2.5-5.0 mg of the calcium channel blocker verapamil in the arterial sheath as first-line preventive therapy.
In a multivariate analysis adjusted for potential confounders – for example, the investigators found that the incidence of radial artery spasm (RAS) is higher in women and younger patients – the use of prophylactic verapamil placed in the arterial sheath reduced the likelihood of RAS by 75% and 72%, respectively, compared with placebo.
Intra-arterial diltiazem at 5 mg, isosorbide dinitrate at 1 mg, and molsidomine at 1 mg were also more effective than placebo. However, diltiazem and isosorbide dinitrate were associated with an unacceptable increased risk of severe hypotension compared to placebo, and molsidomine is not widely available outside France.
In contrast, verapamil was not linked to severe hypotension.
Overall, RAS occurred in 22.2 % of patients on placebo, 7.1% of those on verapamil at 2.5 mg, 7.9% with verapamil at 5 mg, 6.5% with isosorbide dinitrate at 1 mg, 9.1% of those on diltiazem at 5 mg, 13.3% with molsidomine at 1 mg, and 4.8% with verapamil 2.5 mg plus molsidomine 1 mg.
When it proves difficult to advance the catheter during a transradial PCI despite prophylactic verapamil, the first thing to do is check whether the problem really is RAS or is instead a matter of having entered a remnant artery. This is accomplished by supplementing the verapamil with 1 mg of intra-arterial isosorbide dinitrate; if the catheter still won’t pass, seriously consider the possibility of a remnant artery.
Among Dr. Adjedj’s tips on how to successfully pass the catheter through a drug-refractory RAS: Use a hydrophilic 0.035-inch guide wire, switch from a 6 Fr to a smaller 5 or 4 Fr catheter, or use a long multipurpose 5 Fr catheter inside the 6 Fr guiding catheter.
“It’s like nested Russian dolls. It can pass through the spasm without any pain,” said Dr. Adjedj.
He reported having no financial conflicts regarding his presentation.
PARIS – The threat of radial artery spasm is the chief impediment to broader use of transradial access cardiac catheterization and percutaneous coronary intervention, but Dr. Julien Adjedj has a series of tips and tricks to defeat it.
At Cochin University Hospital in Paris, where he is chief of the interventional cardiology clinic, 95% of all PCIs are done transradially.
“With the tips and tricks we use, we have a transradial approach failure rate of only 1.5% at our center,” Dr. Adjedj said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He and his colleagues have conducted a series of prospective, randomized studies of various prophylactic vasodilator regimens in 1,950 patients undergoing transradial PCI.
The winning strategy? Place 2.5-5.0 mg of the calcium channel blocker verapamil in the arterial sheath as first-line preventive therapy.
In a multivariate analysis adjusted for potential confounders – for example, the investigators found that the incidence of radial artery spasm (RAS) is higher in women and younger patients – the use of prophylactic verapamil placed in the arterial sheath reduced the likelihood of RAS by 75% and 72%, respectively, compared with placebo.
Intra-arterial diltiazem at 5 mg, isosorbide dinitrate at 1 mg, and molsidomine at 1 mg were also more effective than placebo. However, diltiazem and isosorbide dinitrate were associated with an unacceptable increased risk of severe hypotension compared to placebo, and molsidomine is not widely available outside France.
In contrast, verapamil was not linked to severe hypotension.
Overall, RAS occurred in 22.2 % of patients on placebo, 7.1% of those on verapamil at 2.5 mg, 7.9% with verapamil at 5 mg, 6.5% with isosorbide dinitrate at 1 mg, 9.1% of those on diltiazem at 5 mg, 13.3% with molsidomine at 1 mg, and 4.8% with verapamil 2.5 mg plus molsidomine 1 mg.
When it proves difficult to advance the catheter during a transradial PCI despite prophylactic verapamil, the first thing to do is check whether the problem really is RAS or is instead a matter of having entered a remnant artery. This is accomplished by supplementing the verapamil with 1 mg of intra-arterial isosorbide dinitrate; if the catheter still won’t pass, seriously consider the possibility of a remnant artery.
Among Dr. Adjedj’s tips on how to successfully pass the catheter through a drug-refractory RAS: Use a hydrophilic 0.035-inch guide wire, switch from a 6 Fr to a smaller 5 or 4 Fr catheter, or use a long multipurpose 5 Fr catheter inside the 6 Fr guiding catheter.
“It’s like nested Russian dolls. It can pass through the spasm without any pain,” said Dr. Adjedj.
He reported having no financial conflicts regarding his presentation.
AT EUROPCR 2016
Key clinical point: Placing 2.5-5.0 mg of verapamil in the arterial sheath when performing transradial access PCI reduces the risk of radial artery spasm by three-quarters compared with placebo.
Major finding: The incidence of radial artery spasm during transradial access PCI was 7.1% when 2.5 mg of verapamil was introduced through the arterial sheath, compared with 22.2% with placebo.
Data source: This series of prospective randomized studies comprised 1,950 patients undergoing transradial access PCI by way of various intra-arterial vasodilators or placebo.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
LAA occlusion studied for stroke prevention in atrial fib with prior intracerebral hemorrhage
PARIS – Transcatheter left atrial appendage occlusion shows promise in providing a far better stroke prevention strategy than does standard medical management in patients with atrial fibrillation who have had a prior intracerebral hemorrhage, Dr. Jens E. Nielsen-Kudsk reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This is a difficult group of patients because there is no consensus on how to treat patients with atrial fibrillation after an intracerebral hemorrhage. They are very often left without any antithrombotic therapy because physicians and the patients themselves fear to resume anticoagulant therapy. Left atrial appendage occlusion is a nice nonpharmacologic way to obtain stroke prevention,” said Dr. Nielsen-Kudsk of Aarhus (Denmark) University.
He presented a propensity-matched follow-up study of 147 pairs of such patients at seven Nordic heart centers. Half received the percutaneously delivered St. Jude Medical Amplatzer Amulet device, which is approved in Europe for stroke prevention in patients with atrial fibrillation (AF). These 147 device recipients were matched for stroke risk by CHA2DS2-VASc score and for bleeding risk by HASBLED score with 147 patients with AF and a prior intracerebral hemorrhage who received standard medical therapy, ranging from warfarin or a novel oral anticoagulant in nearly half of patients to no antithrombotic therapy in 44%. The Amplatzer recipients were most often placed on aspirin for 6 months afterward unless they had an indication for continued aspirin, such as known coronary artery disease.
During a mean of 166 days of follow-up, the primary study endpoint – a composite of ischemic stroke, major bleeding, or death – occurred in the standard medical therapy group at a rate of 278.9 events per 1,000 patient-years, compared with 47.9 per 1,000 patient-years in patients with left atrial appendage occlusion (LAAO). This translates to an 81% relative risk reduction.
Moreover, the risk of ischemic stroke in the LAAO group was reduced by 65%, major bleeding was reduced by 61%, the rate of intracerebral hemorrhage was reduced by 71%, and mortality was decreased by 92%, the cardiologist continued.
On the basis of these encouraging results, a randomized, prospective clinical trial of LAAO using the Amplatzer device versus standard medical therapy in patients with AF and a prior intracerebral hemorrhage will get underway in the Nordic countries within the next several months. It will be called STROKECLOSE, he added.
Asked if the Watchman, the only LAAO device approved in the United States, might be similarly amenable for stroke prevention in this high-stroke-and-bleeding-risk population, Dr. Nielsen-Kudsk said in theory, yes, but the device’s labeling calls for an initial 45 days of anticoagulation therapy. That’s a daunting prospect in patients who’ve already had an intracerebral bleed, he observed.
Dr. Nicolo Piazza, who chaired a press conference where the Nordic study was highlighted, said the Watchman labeling needn’t be a deal breaker.
“Although the Watchman recommendations suggest an anticoagulant for the first 45 days afterward, real-world practice is not necessarily that. Many of those patients are put on dual-antiplatelet therapy or a single antiplatelet agent for a period of 45 days, 3 months, 6 months – it’s very heterogeneous out there,” said Dr. Piazza of McGill University in Montreal.
He added that STROKEBLED will be a very important trial in terms of providing guidance for daily clinical practice. A propensity matched-pairs analysis can’t be considered the final word because of the possibility of unrecognized and unmatched variables that could affect outcomes.
Dr. Nielsen-Kudsk reported receiving research grants from and serving as a consultant to St. Jude Medical.
PARIS – Transcatheter left atrial appendage occlusion shows promise in providing a far better stroke prevention strategy than does standard medical management in patients with atrial fibrillation who have had a prior intracerebral hemorrhage, Dr. Jens E. Nielsen-Kudsk reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This is a difficult group of patients because there is no consensus on how to treat patients with atrial fibrillation after an intracerebral hemorrhage. They are very often left without any antithrombotic therapy because physicians and the patients themselves fear to resume anticoagulant therapy. Left atrial appendage occlusion is a nice nonpharmacologic way to obtain stroke prevention,” said Dr. Nielsen-Kudsk of Aarhus (Denmark) University.
He presented a propensity-matched follow-up study of 147 pairs of such patients at seven Nordic heart centers. Half received the percutaneously delivered St. Jude Medical Amplatzer Amulet device, which is approved in Europe for stroke prevention in patients with atrial fibrillation (AF). These 147 device recipients were matched for stroke risk by CHA2DS2-VASc score and for bleeding risk by HASBLED score with 147 patients with AF and a prior intracerebral hemorrhage who received standard medical therapy, ranging from warfarin or a novel oral anticoagulant in nearly half of patients to no antithrombotic therapy in 44%. The Amplatzer recipients were most often placed on aspirin for 6 months afterward unless they had an indication for continued aspirin, such as known coronary artery disease.
During a mean of 166 days of follow-up, the primary study endpoint – a composite of ischemic stroke, major bleeding, or death – occurred in the standard medical therapy group at a rate of 278.9 events per 1,000 patient-years, compared with 47.9 per 1,000 patient-years in patients with left atrial appendage occlusion (LAAO). This translates to an 81% relative risk reduction.
Moreover, the risk of ischemic stroke in the LAAO group was reduced by 65%, major bleeding was reduced by 61%, the rate of intracerebral hemorrhage was reduced by 71%, and mortality was decreased by 92%, the cardiologist continued.
On the basis of these encouraging results, a randomized, prospective clinical trial of LAAO using the Amplatzer device versus standard medical therapy in patients with AF and a prior intracerebral hemorrhage will get underway in the Nordic countries within the next several months. It will be called STROKECLOSE, he added.
Asked if the Watchman, the only LAAO device approved in the United States, might be similarly amenable for stroke prevention in this high-stroke-and-bleeding-risk population, Dr. Nielsen-Kudsk said in theory, yes, but the device’s labeling calls for an initial 45 days of anticoagulation therapy. That’s a daunting prospect in patients who’ve already had an intracerebral bleed, he observed.
Dr. Nicolo Piazza, who chaired a press conference where the Nordic study was highlighted, said the Watchman labeling needn’t be a deal breaker.
“Although the Watchman recommendations suggest an anticoagulant for the first 45 days afterward, real-world practice is not necessarily that. Many of those patients are put on dual-antiplatelet therapy or a single antiplatelet agent for a period of 45 days, 3 months, 6 months – it’s very heterogeneous out there,” said Dr. Piazza of McGill University in Montreal.
He added that STROKEBLED will be a very important trial in terms of providing guidance for daily clinical practice. A propensity matched-pairs analysis can’t be considered the final word because of the possibility of unrecognized and unmatched variables that could affect outcomes.
Dr. Nielsen-Kudsk reported receiving research grants from and serving as a consultant to St. Jude Medical.
PARIS – Transcatheter left atrial appendage occlusion shows promise in providing a far better stroke prevention strategy than does standard medical management in patients with atrial fibrillation who have had a prior intracerebral hemorrhage, Dr. Jens E. Nielsen-Kudsk reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“This is a difficult group of patients because there is no consensus on how to treat patients with atrial fibrillation after an intracerebral hemorrhage. They are very often left without any antithrombotic therapy because physicians and the patients themselves fear to resume anticoagulant therapy. Left atrial appendage occlusion is a nice nonpharmacologic way to obtain stroke prevention,” said Dr. Nielsen-Kudsk of Aarhus (Denmark) University.
He presented a propensity-matched follow-up study of 147 pairs of such patients at seven Nordic heart centers. Half received the percutaneously delivered St. Jude Medical Amplatzer Amulet device, which is approved in Europe for stroke prevention in patients with atrial fibrillation (AF). These 147 device recipients were matched for stroke risk by CHA2DS2-VASc score and for bleeding risk by HASBLED score with 147 patients with AF and a prior intracerebral hemorrhage who received standard medical therapy, ranging from warfarin or a novel oral anticoagulant in nearly half of patients to no antithrombotic therapy in 44%. The Amplatzer recipients were most often placed on aspirin for 6 months afterward unless they had an indication for continued aspirin, such as known coronary artery disease.
During a mean of 166 days of follow-up, the primary study endpoint – a composite of ischemic stroke, major bleeding, or death – occurred in the standard medical therapy group at a rate of 278.9 events per 1,000 patient-years, compared with 47.9 per 1,000 patient-years in patients with left atrial appendage occlusion (LAAO). This translates to an 81% relative risk reduction.
Moreover, the risk of ischemic stroke in the LAAO group was reduced by 65%, major bleeding was reduced by 61%, the rate of intracerebral hemorrhage was reduced by 71%, and mortality was decreased by 92%, the cardiologist continued.
On the basis of these encouraging results, a randomized, prospective clinical trial of LAAO using the Amplatzer device versus standard medical therapy in patients with AF and a prior intracerebral hemorrhage will get underway in the Nordic countries within the next several months. It will be called STROKECLOSE, he added.
Asked if the Watchman, the only LAAO device approved in the United States, might be similarly amenable for stroke prevention in this high-stroke-and-bleeding-risk population, Dr. Nielsen-Kudsk said in theory, yes, but the device’s labeling calls for an initial 45 days of anticoagulation therapy. That’s a daunting prospect in patients who’ve already had an intracerebral bleed, he observed.
Dr. Nicolo Piazza, who chaired a press conference where the Nordic study was highlighted, said the Watchman labeling needn’t be a deal breaker.
“Although the Watchman recommendations suggest an anticoagulant for the first 45 days afterward, real-world practice is not necessarily that. Many of those patients are put on dual-antiplatelet therapy or a single antiplatelet agent for a period of 45 days, 3 months, 6 months – it’s very heterogeneous out there,” said Dr. Piazza of McGill University in Montreal.
He added that STROKEBLED will be a very important trial in terms of providing guidance for daily clinical practice. A propensity matched-pairs analysis can’t be considered the final word because of the possibility of unrecognized and unmatched variables that could affect outcomes.
Dr. Nielsen-Kudsk reported receiving research grants from and serving as a consultant to St. Jude Medical.
AT EUROPCR 2016
Key clinical point: Left atrial appendage occlusion appears to be better than medical management for stroke prevention in patients with AF and prior intracerebral hemorrhage.
Major finding: The risk of a combined endpoint of ischemic stroke, major bleeding, or death during follow-up was 81% lower in patients with atrial fibrillation and a prior intracerebral hemorrhage treated with a transcatheter left atrial appendage occlusion device than in controls on standard medical therapy.
Data source: This study included 147 patient pairs propensity-matched for stroke and bleeding risk.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to St. Jude Medical.
Optical coherence tomography for PCI gets boost in OPINION trial
PARIS – The first-ever head-to-head randomized trial comparing clinical outcomes of optical coherence tomography and intravascular ultrasound (IVUS) for guidance of percutaneous coronary intervention with a second-generation drug-eluting stent has ended in a draw.
“The clinical outcomes in both OCT-guided PCI and IVUS-guided PCI were excellent in the OPINION study,” Dr. Takashi Kubo reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The form of OCT used in this randomized trial is called optimal frequency domain imaging (OFDI). On the strength of the OPINION results, OFDI deserves to get an upgrade in the PCI treatment guidelines, said Dr. Kubo of Wakayama (Japan) University.
He noted that the 2014 European Society of Cardiology guidelines give IVUS a Class IIa recommendation in selected patients to optimize stent implantation, with a Level of Evidence of B (Eur Heart J. 2014 Oct 1;35:2541-619). The guidelines give OCT (optimal coherence tomography), the more recent and less-studied technology, a Class IIb, Level of Evidence C.
“Our results might influence the next ESC guidelines,” according to Dr. Kubo. “OCT use during PCI should have a Class IIa recommendation.”
The OPINION trial was a prospective, 42-site Japanese study in which 800 patients scheduled for PCI with the Terumo Nobori biolimus-eluting resorbable polymer stent were randomized to an OFDI- or IVUS-guided procedure. All participants underwent follow-up coronary angiography at 8 months and clinical assessment at 12 months.
The primary study endpoint was target vessel failure at 12 months post-PCI, a composite comprising cardiac death, target vessel–related MI, or clinically driven target vessel revascularization. The rate was 5.2% in the OFDI group and statistically similar at 4.9% in the IVUS arm. No cases of contrast-induced nephropathy occurred in either study arm, and stroke rates in both groups were similarly low.
Also noteworthy was the finding that the two intracoronary imaging technologies resulted in similar rates of procedural change: 38% of patients in the OFDI group had a procedural change as result of the imaging findings, as did 36% of the IVUS group. Examples of these procedural changes included upsizing the pre- or postdilatation balloon size or pressure, addition of an another stent, or the use of a distal protection device.
In Japan, where both OCT and IVUS during PCI are routinely reimbursed, roughly 80% of PCI patients undergo one of the two intracoronary imaging procedures. In the United States and Europe, the situation is reversed, Dr. Kubo observed.
Discussant Dr. Ron Waksman agreed with Dr. Kubo that the OPINION results warrant reconsideration of OCT’s Class IIb recommendation in the ESC PCI guidelines. But he thinks the study has a major limitation.
“In my view, this was a missed opportunity to include an angiographically guided PCI arm to establish the superiority of invasive imaging over angiographically guided PCI,” said Dr. Waksman of the MedStar Heart Institute in Washington. While he noted that a recent meta-analysis of 20 studies in more than 29,000 patients concluded that IVUS-guided implantation of drug-eluting stents was associated with a 38% reduction in the risk of mortality, a 23% decrease in major adverse cardiovascular events, and a 41% reduction in stent thrombosis, compared with angiographically guided PCI (BMC Cardiovasc Disord. 2015 Nov 17;15:153), given the inherent limitations of meta-analyses he’s not convinced that cardiologists really need imaging guidance.
“ILUMIEN III, to my view, is the right study design because it randomizes patients to OCT guidance, IVUS guidance, or angiographic guidance to see if there are important differences. We will have to wait for the ILUMIEN III study results to prove the superiority of invasive imaging over angiographically guided PCI,” according to Dr. Waksman.
It’s anticipated that the ILUMIEN III trial will be ready for presentation at EuroPCR 2017.
The OPINION trial was sponsored by Terumo. Dr. Kubo is a consultant to and recipient of an institutional research grant from the company.
PARIS – The first-ever head-to-head randomized trial comparing clinical outcomes of optical coherence tomography and intravascular ultrasound (IVUS) for guidance of percutaneous coronary intervention with a second-generation drug-eluting stent has ended in a draw.
“The clinical outcomes in both OCT-guided PCI and IVUS-guided PCI were excellent in the OPINION study,” Dr. Takashi Kubo reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The form of OCT used in this randomized trial is called optimal frequency domain imaging (OFDI). On the strength of the OPINION results, OFDI deserves to get an upgrade in the PCI treatment guidelines, said Dr. Kubo of Wakayama (Japan) University.
He noted that the 2014 European Society of Cardiology guidelines give IVUS a Class IIa recommendation in selected patients to optimize stent implantation, with a Level of Evidence of B (Eur Heart J. 2014 Oct 1;35:2541-619). The guidelines give OCT (optimal coherence tomography), the more recent and less-studied technology, a Class IIb, Level of Evidence C.
“Our results might influence the next ESC guidelines,” according to Dr. Kubo. “OCT use during PCI should have a Class IIa recommendation.”
The OPINION trial was a prospective, 42-site Japanese study in which 800 patients scheduled for PCI with the Terumo Nobori biolimus-eluting resorbable polymer stent were randomized to an OFDI- or IVUS-guided procedure. All participants underwent follow-up coronary angiography at 8 months and clinical assessment at 12 months.
The primary study endpoint was target vessel failure at 12 months post-PCI, a composite comprising cardiac death, target vessel–related MI, or clinically driven target vessel revascularization. The rate was 5.2% in the OFDI group and statistically similar at 4.9% in the IVUS arm. No cases of contrast-induced nephropathy occurred in either study arm, and stroke rates in both groups were similarly low.
Also noteworthy was the finding that the two intracoronary imaging technologies resulted in similar rates of procedural change: 38% of patients in the OFDI group had a procedural change as result of the imaging findings, as did 36% of the IVUS group. Examples of these procedural changes included upsizing the pre- or postdilatation balloon size or pressure, addition of an another stent, or the use of a distal protection device.
In Japan, where both OCT and IVUS during PCI are routinely reimbursed, roughly 80% of PCI patients undergo one of the two intracoronary imaging procedures. In the United States and Europe, the situation is reversed, Dr. Kubo observed.
Discussant Dr. Ron Waksman agreed with Dr. Kubo that the OPINION results warrant reconsideration of OCT’s Class IIb recommendation in the ESC PCI guidelines. But he thinks the study has a major limitation.
“In my view, this was a missed opportunity to include an angiographically guided PCI arm to establish the superiority of invasive imaging over angiographically guided PCI,” said Dr. Waksman of the MedStar Heart Institute in Washington. While he noted that a recent meta-analysis of 20 studies in more than 29,000 patients concluded that IVUS-guided implantation of drug-eluting stents was associated with a 38% reduction in the risk of mortality, a 23% decrease in major adverse cardiovascular events, and a 41% reduction in stent thrombosis, compared with angiographically guided PCI (BMC Cardiovasc Disord. 2015 Nov 17;15:153), given the inherent limitations of meta-analyses he’s not convinced that cardiologists really need imaging guidance.
“ILUMIEN III, to my view, is the right study design because it randomizes patients to OCT guidance, IVUS guidance, or angiographic guidance to see if there are important differences. We will have to wait for the ILUMIEN III study results to prove the superiority of invasive imaging over angiographically guided PCI,” according to Dr. Waksman.
It’s anticipated that the ILUMIEN III trial will be ready for presentation at EuroPCR 2017.
The OPINION trial was sponsored by Terumo. Dr. Kubo is a consultant to and recipient of an institutional research grant from the company.
PARIS – The first-ever head-to-head randomized trial comparing clinical outcomes of optical coherence tomography and intravascular ultrasound (IVUS) for guidance of percutaneous coronary intervention with a second-generation drug-eluting stent has ended in a draw.
“The clinical outcomes in both OCT-guided PCI and IVUS-guided PCI were excellent in the OPINION study,” Dr. Takashi Kubo reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The form of OCT used in this randomized trial is called optimal frequency domain imaging (OFDI). On the strength of the OPINION results, OFDI deserves to get an upgrade in the PCI treatment guidelines, said Dr. Kubo of Wakayama (Japan) University.
He noted that the 2014 European Society of Cardiology guidelines give IVUS a Class IIa recommendation in selected patients to optimize stent implantation, with a Level of Evidence of B (Eur Heart J. 2014 Oct 1;35:2541-619). The guidelines give OCT (optimal coherence tomography), the more recent and less-studied technology, a Class IIb, Level of Evidence C.
“Our results might influence the next ESC guidelines,” according to Dr. Kubo. “OCT use during PCI should have a Class IIa recommendation.”
The OPINION trial was a prospective, 42-site Japanese study in which 800 patients scheduled for PCI with the Terumo Nobori biolimus-eluting resorbable polymer stent were randomized to an OFDI- or IVUS-guided procedure. All participants underwent follow-up coronary angiography at 8 months and clinical assessment at 12 months.
The primary study endpoint was target vessel failure at 12 months post-PCI, a composite comprising cardiac death, target vessel–related MI, or clinically driven target vessel revascularization. The rate was 5.2% in the OFDI group and statistically similar at 4.9% in the IVUS arm. No cases of contrast-induced nephropathy occurred in either study arm, and stroke rates in both groups were similarly low.
Also noteworthy was the finding that the two intracoronary imaging technologies resulted in similar rates of procedural change: 38% of patients in the OFDI group had a procedural change as result of the imaging findings, as did 36% of the IVUS group. Examples of these procedural changes included upsizing the pre- or postdilatation balloon size or pressure, addition of an another stent, or the use of a distal protection device.
In Japan, where both OCT and IVUS during PCI are routinely reimbursed, roughly 80% of PCI patients undergo one of the two intracoronary imaging procedures. In the United States and Europe, the situation is reversed, Dr. Kubo observed.
Discussant Dr. Ron Waksman agreed with Dr. Kubo that the OPINION results warrant reconsideration of OCT’s Class IIb recommendation in the ESC PCI guidelines. But he thinks the study has a major limitation.
“In my view, this was a missed opportunity to include an angiographically guided PCI arm to establish the superiority of invasive imaging over angiographically guided PCI,” said Dr. Waksman of the MedStar Heart Institute in Washington. While he noted that a recent meta-analysis of 20 studies in more than 29,000 patients concluded that IVUS-guided implantation of drug-eluting stents was associated with a 38% reduction in the risk of mortality, a 23% decrease in major adverse cardiovascular events, and a 41% reduction in stent thrombosis, compared with angiographically guided PCI (BMC Cardiovasc Disord. 2015 Nov 17;15:153), given the inherent limitations of meta-analyses he’s not convinced that cardiologists really need imaging guidance.
“ILUMIEN III, to my view, is the right study design because it randomizes patients to OCT guidance, IVUS guidance, or angiographic guidance to see if there are important differences. We will have to wait for the ILUMIEN III study results to prove the superiority of invasive imaging over angiographically guided PCI,” according to Dr. Waksman.
It’s anticipated that the ILUMIEN III trial will be ready for presentation at EuroPCR 2017.
The OPINION trial was sponsored by Terumo. Dr. Kubo is a consultant to and recipient of an institutional research grant from the company.
AT EUROPCR 2016
Key clinical point: A large, randomized trial shows PCI clinical outcomes are equivalent with optical coherence tomography and intravascular ultrasound guidance.
Major finding: The composite rate of cardiac death, target vessel–related MI, or clinically driven target vessel revascularization within 12 months of PCI was 5.2% in the group whose procedure was guided by optical coherence tomography and statistically similar at 4.9% in patients whose PCI was guided by intravascular ultrasound.
Data source: This was a randomized, prospective, multicenter, 12-month follow-up trial of 800 Japanese patients scheduled for PCI under intracoronary imaging guidance provided by either IVUS or OCT.
Disclosures: The OPINION trial was sponsored by Terumo. The study presenter is a consultant to and recipient of an institutional research grant from the company.
TAVR cerebral protection device appears safe, effective
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
AT EUROPCR 2016
Key clinical point: The TriGuard neuroprotection device for use in TAVR effectively prevented strokes.
Major finding: The 30-day incidence of stroke in TAVR patients with the TriGard embolic protection device in place was 0, compared with 6% or 19% in controls, depending upon the stroke definition used.
Data source: A post hoc analysis of pooled data on 59 TriGuard recipients and 83 controls in three trials.
Disclosures: The presenter reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
Low hematocrit in elderly portends increased bleeding post PCI
PARIS – A low hematocrit in an elderly patient who’s going to undergo percutaneous coronary intervention signals a markedly increased risk of major bleeding within 30 days of the procedure, according to Dr. David Marti.
“Analysis of hematocrit in elderly patients can guide important procedural characteristics, such as access site and antithrombotic regimen,” he said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
For example, studies have established that transradial artery access percutaneous coronary intervention (PCI) results in significantly less bleeding than the transfemoral route, said Dr. Marti, an interventional cardiologist at the University of Alcalá in Madrid.
He presented a prospective study of 212 consecutive patients aged 75 or older who underwent PCI at a single university hospital. Their mean age was 81.4 years, and slightly over half of them presented with an acute coronary syndrome.
All patients received dual-antiplatelet therapy in accord with current guidelines. Stent type and procedural anticoagulant regimen were left to the discretion of the cardiologist; 80% of the subjects received bivalirudin-based anticoagulation.
The primary study outcome was the 30-day incidence of major bleeding, as defined by a Bleeding Academic Research Consortium (BARC) type 3-5 event. The overall rate in this elderly PCI population was 5.5%. However, the rate varied markedly by baseline hematocrit tertile, in accord with the investigators’ study hypothesis.
Major bleeding occurred in 2.9% of patients with an Hct greater than 42% and 3.1% in those with an Hct of 38%-52%, and jumped to 10.6% in the one-third of subjects whose baseline Hct was below 38%, Dr. Marti reported.
Thus, a preprocedural Hct below 38% was associated with a 4.1-fold increased risk of major bleeding within 30 days following PCI. An Hct in this range was a stronger predictor of BARC type 3-5 bleeding risk than were other factors better known as being important, including advanced age, greater body weight, female sex, or an elevated serum creatinine indicative of chronic kidney disease. Indeed, an Hct below 38% was the only statistically significant predictor of major bleeding in this elderly population.
The likely explanation for the observed results is that a low Hct level in elderly patients usually reflects subclinical blood loss that can be worsened by antithrombotic therapies, the cardiologist explained.
The presenter reported having no financial conflicts regarding this study, conducted without commercial support.
PARIS – A low hematocrit in an elderly patient who’s going to undergo percutaneous coronary intervention signals a markedly increased risk of major bleeding within 30 days of the procedure, according to Dr. David Marti.
“Analysis of hematocrit in elderly patients can guide important procedural characteristics, such as access site and antithrombotic regimen,” he said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
For example, studies have established that transradial artery access percutaneous coronary intervention (PCI) results in significantly less bleeding than the transfemoral route, said Dr. Marti, an interventional cardiologist at the University of Alcalá in Madrid.
He presented a prospective study of 212 consecutive patients aged 75 or older who underwent PCI at a single university hospital. Their mean age was 81.4 years, and slightly over half of them presented with an acute coronary syndrome.
All patients received dual-antiplatelet therapy in accord with current guidelines. Stent type and procedural anticoagulant regimen were left to the discretion of the cardiologist; 80% of the subjects received bivalirudin-based anticoagulation.
The primary study outcome was the 30-day incidence of major bleeding, as defined by a Bleeding Academic Research Consortium (BARC) type 3-5 event. The overall rate in this elderly PCI population was 5.5%. However, the rate varied markedly by baseline hematocrit tertile, in accord with the investigators’ study hypothesis.
Major bleeding occurred in 2.9% of patients with an Hct greater than 42% and 3.1% in those with an Hct of 38%-52%, and jumped to 10.6% in the one-third of subjects whose baseline Hct was below 38%, Dr. Marti reported.
Thus, a preprocedural Hct below 38% was associated with a 4.1-fold increased risk of major bleeding within 30 days following PCI. An Hct in this range was a stronger predictor of BARC type 3-5 bleeding risk than were other factors better known as being important, including advanced age, greater body weight, female sex, or an elevated serum creatinine indicative of chronic kidney disease. Indeed, an Hct below 38% was the only statistically significant predictor of major bleeding in this elderly population.
The likely explanation for the observed results is that a low Hct level in elderly patients usually reflects subclinical blood loss that can be worsened by antithrombotic therapies, the cardiologist explained.
The presenter reported having no financial conflicts regarding this study, conducted without commercial support.
PARIS – A low hematocrit in an elderly patient who’s going to undergo percutaneous coronary intervention signals a markedly increased risk of major bleeding within 30 days of the procedure, according to Dr. David Marti.
“Analysis of hematocrit in elderly patients can guide important procedural characteristics, such as access site and antithrombotic regimen,” he said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
For example, studies have established that transradial artery access percutaneous coronary intervention (PCI) results in significantly less bleeding than the transfemoral route, said Dr. Marti, an interventional cardiologist at the University of Alcalá in Madrid.
He presented a prospective study of 212 consecutive patients aged 75 or older who underwent PCI at a single university hospital. Their mean age was 81.4 years, and slightly over half of them presented with an acute coronary syndrome.
All patients received dual-antiplatelet therapy in accord with current guidelines. Stent type and procedural anticoagulant regimen were left to the discretion of the cardiologist; 80% of the subjects received bivalirudin-based anticoagulation.
The primary study outcome was the 30-day incidence of major bleeding, as defined by a Bleeding Academic Research Consortium (BARC) type 3-5 event. The overall rate in this elderly PCI population was 5.5%. However, the rate varied markedly by baseline hematocrit tertile, in accord with the investigators’ study hypothesis.
Major bleeding occurred in 2.9% of patients with an Hct greater than 42% and 3.1% in those with an Hct of 38%-52%, and jumped to 10.6% in the one-third of subjects whose baseline Hct was below 38%, Dr. Marti reported.
Thus, a preprocedural Hct below 38% was associated with a 4.1-fold increased risk of major bleeding within 30 days following PCI. An Hct in this range was a stronger predictor of BARC type 3-5 bleeding risk than were other factors better known as being important, including advanced age, greater body weight, female sex, or an elevated serum creatinine indicative of chronic kidney disease. Indeed, an Hct below 38% was the only statistically significant predictor of major bleeding in this elderly population.
The likely explanation for the observed results is that a low Hct level in elderly patients usually reflects subclinical blood loss that can be worsened by antithrombotic therapies, the cardiologist explained.
The presenter reported having no financial conflicts regarding this study, conducted without commercial support.
AT EUROPCR 2016
Key clinical point: Elderly patients scheduled for PCI have a fourfold greater risk of major bleeding within 30 days if their Hct is less than 38%.
Major finding: The 30-day incidence of BARC types 3-5 major bleeding was 10.9% in elderly patients with a pre-PCI Hct below 38%, compared with 2.9% in those in the top Hct tertile.
Data source: A prospective study of 212 consecutive patients aged 75 or older who underwent PCI at a single university hospital.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted without commercial support.
Obesity paradox extends to TAVR but not SAVR
PARIS – The obesity paradox appears to apply to patients undergoing transcatheter aortic valve replacement (TAVR) but doesn’t extend to those with surgical aortic valve replacement (SAVR), Dr. Marco Spaziano reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective single-center study of 3,340 consecutive patients who underwent either TAVR or SAVR, with all valve replacement procedures being performed by the same surgeons. Investigators divided the patients – 1,301 with TAVR, 2,039 SAVR – into quartiles on the basis of body mass index.
Rates of 30-day and 1-year mortality, major vascular complications, and major bleeding events were consistently lowest in TAVR patients in the top body mass index (BMI) quartile, defined as greater than 30.1 kg/m2, and highest in those in the bottom quartile, reserved for patients with a BMI below 24.3 kg/m2.
It’s worth noting that with a mean BMI of 21.8 kg/m2 in the bottom BMI quartile, most patients in that group were actually normal weight, not underweight, observed Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
Among the TAVR group, there were no significant differences between the BMI quartiles in TAVR devices, size, or procedural approach.
The TAVR patients’ BMI quartile had no impact on other outcomes, including rates of stroke, MI, permanent pacemaker implantation, acute kidney injury, or aortic regurgitation.
While being overweight or obese was protective in TAVR patients, BMI quartiles had no relationship with outcomes in the SAVR cohort. The SAVR patients were on average about 10 years younger than the TAVR group, and their logistic EuroSCORE – a tool for estimating mortality risk after cardiac surgery – was much lower as well.
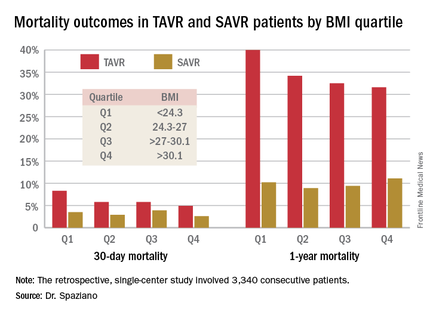
The obesity paradox remains an ongoing puzzle and source of intrigue for physicians in many different specialties. The paradox is this: Obesity is well established as a major risk factor for the development of cardiovascular diseases and diabetes, yet a higher BMI seems to be associated with lower mortality and better procedural outcomes for patients once they actually have a number of chronic diseases, including coronary artery disease, heart failure, peripheral arterial disease, hypertension, stroke, chronic obstructive pulmonary disease, renal disease, and acute venous thromboembolism.
The TAVR obesity paradox findings really got under the skin of at least one audience member.
“What’s the message?” he asked. “We’re telling people to lose weight, eat healthy, run thousands of miles and so forth, but then we’re supposed to tell patients undergoing a high-risk procedure that if you’re overweight you somehow survive better, live longer, feel better?”
Dr. Spaziano said the independent predictors of mortality in the lowest-BMI quartile of TAVR patients were a high serum creatinine, chronic obstructive pulmonary disease, and low BMI. Therein lies a likely explanation for the obesity paradox, at least in this particular study: The TAVR patients were generally sicker than the SAVR patients, which is why they weren’t undergoing surgery, and the lowest-BMI TAVR group was frailer than the others. Also, perhaps the heaviest patients benefited from having more energy reserves to draw upon.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
PARIS – The obesity paradox appears to apply to patients undergoing transcatheter aortic valve replacement (TAVR) but doesn’t extend to those with surgical aortic valve replacement (SAVR), Dr. Marco Spaziano reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective single-center study of 3,340 consecutive patients who underwent either TAVR or SAVR, with all valve replacement procedures being performed by the same surgeons. Investigators divided the patients – 1,301 with TAVR, 2,039 SAVR – into quartiles on the basis of body mass index.
Rates of 30-day and 1-year mortality, major vascular complications, and major bleeding events were consistently lowest in TAVR patients in the top body mass index (BMI) quartile, defined as greater than 30.1 kg/m2, and highest in those in the bottom quartile, reserved for patients with a BMI below 24.3 kg/m2.
It’s worth noting that with a mean BMI of 21.8 kg/m2 in the bottom BMI quartile, most patients in that group were actually normal weight, not underweight, observed Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
Among the TAVR group, there were no significant differences between the BMI quartiles in TAVR devices, size, or procedural approach.
The TAVR patients’ BMI quartile had no impact on other outcomes, including rates of stroke, MI, permanent pacemaker implantation, acute kidney injury, or aortic regurgitation.
While being overweight or obese was protective in TAVR patients, BMI quartiles had no relationship with outcomes in the SAVR cohort. The SAVR patients were on average about 10 years younger than the TAVR group, and their logistic EuroSCORE – a tool for estimating mortality risk after cardiac surgery – was much lower as well.

The obesity paradox remains an ongoing puzzle and source of intrigue for physicians in many different specialties. The paradox is this: Obesity is well established as a major risk factor for the development of cardiovascular diseases and diabetes, yet a higher BMI seems to be associated with lower mortality and better procedural outcomes for patients once they actually have a number of chronic diseases, including coronary artery disease, heart failure, peripheral arterial disease, hypertension, stroke, chronic obstructive pulmonary disease, renal disease, and acute venous thromboembolism.
The TAVR obesity paradox findings really got under the skin of at least one audience member.
“What’s the message?” he asked. “We’re telling people to lose weight, eat healthy, run thousands of miles and so forth, but then we’re supposed to tell patients undergoing a high-risk procedure that if you’re overweight you somehow survive better, live longer, feel better?”
Dr. Spaziano said the independent predictors of mortality in the lowest-BMI quartile of TAVR patients were a high serum creatinine, chronic obstructive pulmonary disease, and low BMI. Therein lies a likely explanation for the obesity paradox, at least in this particular study: The TAVR patients were generally sicker than the SAVR patients, which is why they weren’t undergoing surgery, and the lowest-BMI TAVR group was frailer than the others. Also, perhaps the heaviest patients benefited from having more energy reserves to draw upon.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
PARIS – The obesity paradox appears to apply to patients undergoing transcatheter aortic valve replacement (TAVR) but doesn’t extend to those with surgical aortic valve replacement (SAVR), Dr. Marco Spaziano reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented a retrospective single-center study of 3,340 consecutive patients who underwent either TAVR or SAVR, with all valve replacement procedures being performed by the same surgeons. Investigators divided the patients – 1,301 with TAVR, 2,039 SAVR – into quartiles on the basis of body mass index.
Rates of 30-day and 1-year mortality, major vascular complications, and major bleeding events were consistently lowest in TAVR patients in the top body mass index (BMI) quartile, defined as greater than 30.1 kg/m2, and highest in those in the bottom quartile, reserved for patients with a BMI below 24.3 kg/m2.
It’s worth noting that with a mean BMI of 21.8 kg/m2 in the bottom BMI quartile, most patients in that group were actually normal weight, not underweight, observed Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
Among the TAVR group, there were no significant differences between the BMI quartiles in TAVR devices, size, or procedural approach.
The TAVR patients’ BMI quartile had no impact on other outcomes, including rates of stroke, MI, permanent pacemaker implantation, acute kidney injury, or aortic regurgitation.
While being overweight or obese was protective in TAVR patients, BMI quartiles had no relationship with outcomes in the SAVR cohort. The SAVR patients were on average about 10 years younger than the TAVR group, and their logistic EuroSCORE – a tool for estimating mortality risk after cardiac surgery – was much lower as well.

The obesity paradox remains an ongoing puzzle and source of intrigue for physicians in many different specialties. The paradox is this: Obesity is well established as a major risk factor for the development of cardiovascular diseases and diabetes, yet a higher BMI seems to be associated with lower mortality and better procedural outcomes for patients once they actually have a number of chronic diseases, including coronary artery disease, heart failure, peripheral arterial disease, hypertension, stroke, chronic obstructive pulmonary disease, renal disease, and acute venous thromboembolism.
The TAVR obesity paradox findings really got under the skin of at least one audience member.
“What’s the message?” he asked. “We’re telling people to lose weight, eat healthy, run thousands of miles and so forth, but then we’re supposed to tell patients undergoing a high-risk procedure that if you’re overweight you somehow survive better, live longer, feel better?”
Dr. Spaziano said the independent predictors of mortality in the lowest-BMI quartile of TAVR patients were a high serum creatinine, chronic obstructive pulmonary disease, and low BMI. Therein lies a likely explanation for the obesity paradox, at least in this particular study: The TAVR patients were generally sicker than the SAVR patients, which is why they weren’t undergoing surgery, and the lowest-BMI TAVR group was frailer than the others. Also, perhaps the heaviest patients benefited from having more energy reserves to draw upon.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
AT EUROPCR 2016
Key clinical point: Being overweight or obese may have a protective effect in patients undergoing transcatheter aortic valve replacement, but not in those who have surgical replacement.
Major finding: Thirty-day mortality was 4.9% in TAVR patients in the top BMI quartile and climbed stepwise with lowering BMIs.
Data source: A single-center retrospective study of 3,340 consecutive patients who underwent TAVR or SAVR.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
TAVR degeneration estimated at 50% after 8 years
PARIS – The first-ever study of the long-term durability of transcatheter bioprosthetic aortic valves has documented a disturbing rise in the valve degeneration rate occurring 5-7 years post implant.
Prior consistently reassuring follow-up studies have been intermediate in length, with a maximum of 5 years. The PARTNER 2A trial, which generated enormous enthusiasm for moving TAVR to intermediate-risk patients on the basis of positive results presented at the 2016 meeting of the American College of Cardiology, reported 2-year results.
“We found, as have others, that there’s very little degeneration in the first 4 years: 94% freedom from degeneration. But at 6 years, it’s 82%, and we estimate that by 8 years, it’s about 50%,” Dr. Danny Dvir reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We need to be cautious: This is our first look at the data. But we have a signal for a problem,” said Dr. Dvir of St. Paul’s Hospital in Vancouver.
He presented a retrospective study of 378 patients who underwent TAVR 5-14 years ago at two pioneering centers for the procedure: St. Paul’s and Charles Nicolle Hospital in Rouen, France. Patients’ average age at the time of TAVR was 82.3 years, with an STS score of 8.3%. The study featured serial echocardiography conducted during house calls in this frail elderly population.
Thirty-five patients developed prosthetic valve degeneration, defined by at least moderate regurgitation and/or a mean gradient of at least 20 mm Hg in 23 cases and stenosis in 12. The risk of degeneration was unrelated to the use of warfarin, a finding that suggests the valve deterioration issue is unrelated to clotting. The strongest risk factor for transcatheter valve degeneration in this study was baseline renal failure at the time of TAVR.
Dr. Dvir’s presentation was the talk of the meeting, and it cast a pall over the proceedings. The red flag raised by the study regarding valve durability has major implications regarding the current enthusiasm among many interventional cardiologists to routinely extend TAVR to intermediate and even lower-risk patients. As one audience member later confessed, “I have felt sick since hearing that presentation.”
Discussant Dr. A. Pieter Kappetein observed that transcatheter heart valve durability was a hot topic of discussion about 4 years ago but subsequently faded below the radar as a concern – until Dr. Dvir’s study.
“This is a very important study that puts transcatheter heart valve implantation in a little bit different light,” said Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
He noted that the surgical aortic valve replacement (SAVR) literature shows that the rate of structural valve deterioration is age related. It’s higher in 75-year-olds than in 85-year-olds, and higher still in 65-year-olds.
“Valve degeneration didn’t play a major role when we were doing TAVR in 80- and 85-year-olds because of their limited life expectancy, but it will play a role in younger patients. So I think we have to be careful before we move toward lower-risk patients,” the surgeon continued.
Dr. Jean-Francois Obadia, who performs both SAVR and TAVR, noted that the median duration of freedom from valve degeneration for Edwards Lifescience’s Carpentier surgical aortic valve is a hefty 17.9 years.
“This should be the gold standard,” declared Dr. Obadia, head of the department of adult cardiovascular surgery and heart transplantation at the Louis Pradel Cardiothoracic Hospital of Claude Bernard University in Lyon, France.
“Dr. Dvir’s study is one of the key messages we all should take back home: a 50% rate of valve deterioration at 8 years. Valve deterioration is the Achilles’ heel of bioprostheses. There is a lot of improvement left to do for the TAVR,” he said.
Dr. Dvir and others were quick to note that his long-term study was of necessity restricted to early-generation, balloon-expandable devices: the Cribier Edwards, Edwards Sapien, and Sapien XT valves. Contemporary valves, patient selection methods, and procedural techniques are far advanced in comparison.
“The Sapien 3 has much less paravalvular leakage than earlier-generation valves. Maybe with less paravalvular leakage and better hemodynamics there will be a decreased rate of degeneration. It could be. We need to see. We have to wait a few more years to see if later-generation transcatheter heart valves are more durable,” Dr. Dvir said.
To gain a better understanding of the full dimensions of the valve degeneration issue, he and his coinvestigators have formed the VALID (VAlve Long-term Durability International Data) registry. Operators interested in contributing patients to what is hoped will be a very large and informative data base are encouraged to contact Dr. Dvir (ddvir@providencehealth.bc.ca).
In the meantime, he has reservations about extending TAVR to intermediate-risk patients outside of the rigorous clinical trial setting. He added that he’d feel far more comfortable in performing TAVR in intermediate-risk 70- to 75-year-olds if there was a tried and true valve-in-valve replacement method, something that doesn’t yet exist. The major limitation of current attempts at valve-in-valve replacement is underexpansion because the former valve doesn’t allow sufficient room for the new one to expand fully, resulting in residual stenosis.
“If you tell me that you can implant a platform that will enable a safe valve-in-valve procedure in 5, 7, 10 years – a less invasive bailout for a failed prosthetic valve – if you can do that safely and effectively I would be more keen to do TAVR even in a young patient,” the interventional cardiologist said.
He and others are working on this unmet need. Dr. Dvir’s novel valve, being developed with Edwards Lifesciences, has performed well in valve-in-valve procedures in cadavers and animals. The first clinical trials are being planned.
“We need to think always that a bioprosthetic valve is not a cure, it’s a palliation. We treat the patients, they feel better, but we leave them with some kind of a chronic disease that’s prone to thrombosis, prone to degeneration and failure, prone to many different things,” he reflected.
The study was conducted without commercial support. Dr. Dvir reported serving as a consultant to Edwards Lifesciences, Medtronic, and St. Jude Medical.
PARIS – The first-ever study of the long-term durability of transcatheter bioprosthetic aortic valves has documented a disturbing rise in the valve degeneration rate occurring 5-7 years post implant.
Prior consistently reassuring follow-up studies have been intermediate in length, with a maximum of 5 years. The PARTNER 2A trial, which generated enormous enthusiasm for moving TAVR to intermediate-risk patients on the basis of positive results presented at the 2016 meeting of the American College of Cardiology, reported 2-year results.
“We found, as have others, that there’s very little degeneration in the first 4 years: 94% freedom from degeneration. But at 6 years, it’s 82%, and we estimate that by 8 years, it’s about 50%,” Dr. Danny Dvir reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We need to be cautious: This is our first look at the data. But we have a signal for a problem,” said Dr. Dvir of St. Paul’s Hospital in Vancouver.
He presented a retrospective study of 378 patients who underwent TAVR 5-14 years ago at two pioneering centers for the procedure: St. Paul’s and Charles Nicolle Hospital in Rouen, France. Patients’ average age at the time of TAVR was 82.3 years, with an STS score of 8.3%. The study featured serial echocardiography conducted during house calls in this frail elderly population.
Thirty-five patients developed prosthetic valve degeneration, defined by at least moderate regurgitation and/or a mean gradient of at least 20 mm Hg in 23 cases and stenosis in 12. The risk of degeneration was unrelated to the use of warfarin, a finding that suggests the valve deterioration issue is unrelated to clotting. The strongest risk factor for transcatheter valve degeneration in this study was baseline renal failure at the time of TAVR.
Dr. Dvir’s presentation was the talk of the meeting, and it cast a pall over the proceedings. The red flag raised by the study regarding valve durability has major implications regarding the current enthusiasm among many interventional cardiologists to routinely extend TAVR to intermediate and even lower-risk patients. As one audience member later confessed, “I have felt sick since hearing that presentation.”
Discussant Dr. A. Pieter Kappetein observed that transcatheter heart valve durability was a hot topic of discussion about 4 years ago but subsequently faded below the radar as a concern – until Dr. Dvir’s study.
“This is a very important study that puts transcatheter heart valve implantation in a little bit different light,” said Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
He noted that the surgical aortic valve replacement (SAVR) literature shows that the rate of structural valve deterioration is age related. It’s higher in 75-year-olds than in 85-year-olds, and higher still in 65-year-olds.
“Valve degeneration didn’t play a major role when we were doing TAVR in 80- and 85-year-olds because of their limited life expectancy, but it will play a role in younger patients. So I think we have to be careful before we move toward lower-risk patients,” the surgeon continued.
Dr. Jean-Francois Obadia, who performs both SAVR and TAVR, noted that the median duration of freedom from valve degeneration for Edwards Lifescience’s Carpentier surgical aortic valve is a hefty 17.9 years.
“This should be the gold standard,” declared Dr. Obadia, head of the department of adult cardiovascular surgery and heart transplantation at the Louis Pradel Cardiothoracic Hospital of Claude Bernard University in Lyon, France.
“Dr. Dvir’s study is one of the key messages we all should take back home: a 50% rate of valve deterioration at 8 years. Valve deterioration is the Achilles’ heel of bioprostheses. There is a lot of improvement left to do for the TAVR,” he said.
Dr. Dvir and others were quick to note that his long-term study was of necessity restricted to early-generation, balloon-expandable devices: the Cribier Edwards, Edwards Sapien, and Sapien XT valves. Contemporary valves, patient selection methods, and procedural techniques are far advanced in comparison.
“The Sapien 3 has much less paravalvular leakage than earlier-generation valves. Maybe with less paravalvular leakage and better hemodynamics there will be a decreased rate of degeneration. It could be. We need to see. We have to wait a few more years to see if later-generation transcatheter heart valves are more durable,” Dr. Dvir said.
To gain a better understanding of the full dimensions of the valve degeneration issue, he and his coinvestigators have formed the VALID (VAlve Long-term Durability International Data) registry. Operators interested in contributing patients to what is hoped will be a very large and informative data base are encouraged to contact Dr. Dvir (ddvir@providencehealth.bc.ca).
In the meantime, he has reservations about extending TAVR to intermediate-risk patients outside of the rigorous clinical trial setting. He added that he’d feel far more comfortable in performing TAVR in intermediate-risk 70- to 75-year-olds if there was a tried and true valve-in-valve replacement method, something that doesn’t yet exist. The major limitation of current attempts at valve-in-valve replacement is underexpansion because the former valve doesn’t allow sufficient room for the new one to expand fully, resulting in residual stenosis.
“If you tell me that you can implant a platform that will enable a safe valve-in-valve procedure in 5, 7, 10 years – a less invasive bailout for a failed prosthetic valve – if you can do that safely and effectively I would be more keen to do TAVR even in a young patient,” the interventional cardiologist said.
He and others are working on this unmet need. Dr. Dvir’s novel valve, being developed with Edwards Lifesciences, has performed well in valve-in-valve procedures in cadavers and animals. The first clinical trials are being planned.
“We need to think always that a bioprosthetic valve is not a cure, it’s a palliation. We treat the patients, they feel better, but we leave them with some kind of a chronic disease that’s prone to thrombosis, prone to degeneration and failure, prone to many different things,” he reflected.
The study was conducted without commercial support. Dr. Dvir reported serving as a consultant to Edwards Lifesciences, Medtronic, and St. Jude Medical.
PARIS – The first-ever study of the long-term durability of transcatheter bioprosthetic aortic valves has documented a disturbing rise in the valve degeneration rate occurring 5-7 years post implant.
Prior consistently reassuring follow-up studies have been intermediate in length, with a maximum of 5 years. The PARTNER 2A trial, which generated enormous enthusiasm for moving TAVR to intermediate-risk patients on the basis of positive results presented at the 2016 meeting of the American College of Cardiology, reported 2-year results.
“We found, as have others, that there’s very little degeneration in the first 4 years: 94% freedom from degeneration. But at 6 years, it’s 82%, and we estimate that by 8 years, it’s about 50%,” Dr. Danny Dvir reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We need to be cautious: This is our first look at the data. But we have a signal for a problem,” said Dr. Dvir of St. Paul’s Hospital in Vancouver.
He presented a retrospective study of 378 patients who underwent TAVR 5-14 years ago at two pioneering centers for the procedure: St. Paul’s and Charles Nicolle Hospital in Rouen, France. Patients’ average age at the time of TAVR was 82.3 years, with an STS score of 8.3%. The study featured serial echocardiography conducted during house calls in this frail elderly population.
Thirty-five patients developed prosthetic valve degeneration, defined by at least moderate regurgitation and/or a mean gradient of at least 20 mm Hg in 23 cases and stenosis in 12. The risk of degeneration was unrelated to the use of warfarin, a finding that suggests the valve deterioration issue is unrelated to clotting. The strongest risk factor for transcatheter valve degeneration in this study was baseline renal failure at the time of TAVR.
Dr. Dvir’s presentation was the talk of the meeting, and it cast a pall over the proceedings. The red flag raised by the study regarding valve durability has major implications regarding the current enthusiasm among many interventional cardiologists to routinely extend TAVR to intermediate and even lower-risk patients. As one audience member later confessed, “I have felt sick since hearing that presentation.”
Discussant Dr. A. Pieter Kappetein observed that transcatheter heart valve durability was a hot topic of discussion about 4 years ago but subsequently faded below the radar as a concern – until Dr. Dvir’s study.
“This is a very important study that puts transcatheter heart valve implantation in a little bit different light,” said Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
He noted that the surgical aortic valve replacement (SAVR) literature shows that the rate of structural valve deterioration is age related. It’s higher in 75-year-olds than in 85-year-olds, and higher still in 65-year-olds.
“Valve degeneration didn’t play a major role when we were doing TAVR in 80- and 85-year-olds because of their limited life expectancy, but it will play a role in younger patients. So I think we have to be careful before we move toward lower-risk patients,” the surgeon continued.
Dr. Jean-Francois Obadia, who performs both SAVR and TAVR, noted that the median duration of freedom from valve degeneration for Edwards Lifescience’s Carpentier surgical aortic valve is a hefty 17.9 years.
“This should be the gold standard,” declared Dr. Obadia, head of the department of adult cardiovascular surgery and heart transplantation at the Louis Pradel Cardiothoracic Hospital of Claude Bernard University in Lyon, France.
“Dr. Dvir’s study is one of the key messages we all should take back home: a 50% rate of valve deterioration at 8 years. Valve deterioration is the Achilles’ heel of bioprostheses. There is a lot of improvement left to do for the TAVR,” he said.
Dr. Dvir and others were quick to note that his long-term study was of necessity restricted to early-generation, balloon-expandable devices: the Cribier Edwards, Edwards Sapien, and Sapien XT valves. Contemporary valves, patient selection methods, and procedural techniques are far advanced in comparison.
“The Sapien 3 has much less paravalvular leakage than earlier-generation valves. Maybe with less paravalvular leakage and better hemodynamics there will be a decreased rate of degeneration. It could be. We need to see. We have to wait a few more years to see if later-generation transcatheter heart valves are more durable,” Dr. Dvir said.
To gain a better understanding of the full dimensions of the valve degeneration issue, he and his coinvestigators have formed the VALID (VAlve Long-term Durability International Data) registry. Operators interested in contributing patients to what is hoped will be a very large and informative data base are encouraged to contact Dr. Dvir (ddvir@providencehealth.bc.ca).
In the meantime, he has reservations about extending TAVR to intermediate-risk patients outside of the rigorous clinical trial setting. He added that he’d feel far more comfortable in performing TAVR in intermediate-risk 70- to 75-year-olds if there was a tried and true valve-in-valve replacement method, something that doesn’t yet exist. The major limitation of current attempts at valve-in-valve replacement is underexpansion because the former valve doesn’t allow sufficient room for the new one to expand fully, resulting in residual stenosis.
“If you tell me that you can implant a platform that will enable a safe valve-in-valve procedure in 5, 7, 10 years – a less invasive bailout for a failed prosthetic valve – if you can do that safely and effectively I would be more keen to do TAVR even in a young patient,” the interventional cardiologist said.
He and others are working on this unmet need. Dr. Dvir’s novel valve, being developed with Edwards Lifesciences, has performed well in valve-in-valve procedures in cadavers and animals. The first clinical trials are being planned.
“We need to think always that a bioprosthetic valve is not a cure, it’s a palliation. We treat the patients, they feel better, but we leave them with some kind of a chronic disease that’s prone to thrombosis, prone to degeneration and failure, prone to many different things,” he reflected.
The study was conducted without commercial support. Dr. Dvir reported serving as a consultant to Edwards Lifesciences, Medtronic, and St. Jude Medical.
AT EUROPCR 2016
Key clinical point: The first study to examine transcatheter aortic bioprosthetic valve performance beyond 5 years has found a 50% rate of valve degeneration 8 years post TAVR.
Major finding: A sharp increase in the incidence of degeneration of these early-generation valves occurred 5-7 years post TAVR.
Data source: This retrospective study featured serial home echocardiography in 378 patients who underwent TAVR 5-14 years ago at two pioneering centers for the procedure.
Disclosures: The presenter of this study, conducted without commercial support, serves as a consultant to Edwards Lifesciences, Medtronic, and St. Jude Medical.
Prior pregnancy protects against TAVR complications
PARIS – Results of the first-ever all-female patient TAVR registry indicate that a history of pregnancy – albeit typically half a century or more ago – is strongly protective against stroke, major bleeding, and other serious adverse events within 30 days of undergoing transcatheter aortic valve replacement.
The explanation for this novel observation remains unclear and warrants further study. One plausible hypothesis: “We can postulate that there is a hormonal effect in pregnancy that is still present after 50 years,” Dr. Alaide Chieffo said in presenting the Women’s International Transcatheter Aortic Valve Implantation (WIN TAVI) registry findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
WIN TAVI is a prospective observational registry of women undergoing TAVR for symptomatic severe aortic stenosis. Dr. Chieffo reported on acute and 30-day outcomes in 1,019 women enrolled in the registry at 20 highly experienced TAVR centers in Europe and North America. The average age was 82.5 years and mean STS score was 8.3%, putting the women at intermediate to high risk. Nearly three-quarters of subjects were more older than 80 years. Most patients had at least three major procedural risk factors. Two-thirds of the women were deemed frail upon surgical assessment.
The rationale for creating the WIN TAVI registry lies in the fact that even though half of patients undergoing TAVR are women, their optimal treatment strategy remains undefined. This is an important issue, particularly in light of evidence to suggest that the superiority of transfemoral TAVR over surgical aortic valve replacement in the PARTNER 2A trial may have been driven by better outcomes in women than in men.
WIN TAVI is designed to evaluate the safety and performance of TAVR in women in real-world clinical practice. It’s also a unique opportunity to prospectively gather data on female sex-specific baseline characteristics that haven’t been collected in previous TAVR studies and to examine their influence on outcomes, explained Dr. Chieffo of San Raffaele Scientific Institute in Milan. The primary outcome was the 30-day standard Second Valve Academic Research Consortium (VARC 2) composite of all-cause mortality, stroke, life-threatening bleeding, major vascular complications, stage 2 or 3 acute kidney injury, coronary artery obstruction, or a repeat intervention for valve-related dysfunction. The incidence was 14.4%, including a 3.9% all-cause mortality rate, a 1.3% incidence of stroke, major vascular complications in 7.9% of women, and a 4.4% incidence of life-threatening bleeding.
“The findings of the current registry underscore the importance and safety of moving to a lower-risk population of women with TAVR,” Dr. Chieffo declared.
The VARC 2 rates seen in WIN TAVI are low in comparison to other reports in TAVR patients. Dr. Chieffo attributed this to several factors. Patients underwent current state-of-the-art TAVR procedures, with a percutaneous transfemoral approach used in 91% of cases. Only one-third of patients had general anesthesia; the rest underwent TAVR under conscious sedation or a local anesthetic. One-third of the procedures were carried out with sheath sizes of 16 French or less.
Moreover, 42% of patients received new-generation TAVR devices that are compatible with smaller sheath sizes, are completely or partially retrievable, and have a lower paravalvular leak rate than that of earlier devices. The predominantly female interventional cardiologists were all highly experienced, and more than three-quarters of patients were discharged on dual-antiplatelet therapy or an oral anticoagulant.
Seventy-two percent of women had a remote history of pregnancy. Their rate of the primary VARC 2 safety endpoint was 12.7%, compared to 18.9% in patients without a prior pregnancy. In a multivariate logistic regression analysis, prior pregnancy, regardless of whether it resulted in a live birth, was independently associated with a 43% reduction in the risk of a VARC 2 safety endpoint. The greater the number of a woman’s pregnancies, the larger the reduction in the rate of VARC 2 complications.
The other independent predictor of reduced risk was the use of a new-generation TAVR device, defined as any device commercially available in Europe other than the Edwards Sapien XT or Medtronic CoreValve. The newer devices were associated with a 41% relative risk reduction in the VARC 2 composite endpoint.
Risk factors for the primary safety endpoint were prior stroke, associated with an adjusted twofold increased risk; a left ventricular ejection fraction less than 30%, with a 2.6-fold increased risk; and advanced age.
Of note, while 17.5% of women had a history of osteoporosis, only one-fifth of them were on treatment for it. This may impose important limitations on their postprocedural functional recovery, according to Dr. Chieffo.
Further follow-up of WIN TAVI registry participants is planned at 12, 18, and 24 months post procedure.
The WIN TAVI registry is carried out with no external funding. All participating investigators worked gratis. Dr. Chieffo reported having no financial conflicts of interest.
PARIS – Results of the first-ever all-female patient TAVR registry indicate that a history of pregnancy – albeit typically half a century or more ago – is strongly protective against stroke, major bleeding, and other serious adverse events within 30 days of undergoing transcatheter aortic valve replacement.
The explanation for this novel observation remains unclear and warrants further study. One plausible hypothesis: “We can postulate that there is a hormonal effect in pregnancy that is still present after 50 years,” Dr. Alaide Chieffo said in presenting the Women’s International Transcatheter Aortic Valve Implantation (WIN TAVI) registry findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
WIN TAVI is a prospective observational registry of women undergoing TAVR for symptomatic severe aortic stenosis. Dr. Chieffo reported on acute and 30-day outcomes in 1,019 women enrolled in the registry at 20 highly experienced TAVR centers in Europe and North America. The average age was 82.5 years and mean STS score was 8.3%, putting the women at intermediate to high risk. Nearly three-quarters of subjects were more older than 80 years. Most patients had at least three major procedural risk factors. Two-thirds of the women were deemed frail upon surgical assessment.
The rationale for creating the WIN TAVI registry lies in the fact that even though half of patients undergoing TAVR are women, their optimal treatment strategy remains undefined. This is an important issue, particularly in light of evidence to suggest that the superiority of transfemoral TAVR over surgical aortic valve replacement in the PARTNER 2A trial may have been driven by better outcomes in women than in men.
WIN TAVI is designed to evaluate the safety and performance of TAVR in women in real-world clinical practice. It’s also a unique opportunity to prospectively gather data on female sex-specific baseline characteristics that haven’t been collected in previous TAVR studies and to examine their influence on outcomes, explained Dr. Chieffo of San Raffaele Scientific Institute in Milan. The primary outcome was the 30-day standard Second Valve Academic Research Consortium (VARC 2) composite of all-cause mortality, stroke, life-threatening bleeding, major vascular complications, stage 2 or 3 acute kidney injury, coronary artery obstruction, or a repeat intervention for valve-related dysfunction. The incidence was 14.4%, including a 3.9% all-cause mortality rate, a 1.3% incidence of stroke, major vascular complications in 7.9% of women, and a 4.4% incidence of life-threatening bleeding.
“The findings of the current registry underscore the importance and safety of moving to a lower-risk population of women with TAVR,” Dr. Chieffo declared.
The VARC 2 rates seen in WIN TAVI are low in comparison to other reports in TAVR patients. Dr. Chieffo attributed this to several factors. Patients underwent current state-of-the-art TAVR procedures, with a percutaneous transfemoral approach used in 91% of cases. Only one-third of patients had general anesthesia; the rest underwent TAVR under conscious sedation or a local anesthetic. One-third of the procedures were carried out with sheath sizes of 16 French or less.
Moreover, 42% of patients received new-generation TAVR devices that are compatible with smaller sheath sizes, are completely or partially retrievable, and have a lower paravalvular leak rate than that of earlier devices. The predominantly female interventional cardiologists were all highly experienced, and more than three-quarters of patients were discharged on dual-antiplatelet therapy or an oral anticoagulant.
Seventy-two percent of women had a remote history of pregnancy. Their rate of the primary VARC 2 safety endpoint was 12.7%, compared to 18.9% in patients without a prior pregnancy. In a multivariate logistic regression analysis, prior pregnancy, regardless of whether it resulted in a live birth, was independently associated with a 43% reduction in the risk of a VARC 2 safety endpoint. The greater the number of a woman’s pregnancies, the larger the reduction in the rate of VARC 2 complications.
The other independent predictor of reduced risk was the use of a new-generation TAVR device, defined as any device commercially available in Europe other than the Edwards Sapien XT or Medtronic CoreValve. The newer devices were associated with a 41% relative risk reduction in the VARC 2 composite endpoint.
Risk factors for the primary safety endpoint were prior stroke, associated with an adjusted twofold increased risk; a left ventricular ejection fraction less than 30%, with a 2.6-fold increased risk; and advanced age.
Of note, while 17.5% of women had a history of osteoporosis, only one-fifth of them were on treatment for it. This may impose important limitations on their postprocedural functional recovery, according to Dr. Chieffo.
Further follow-up of WIN TAVI registry participants is planned at 12, 18, and 24 months post procedure.
The WIN TAVI registry is carried out with no external funding. All participating investigators worked gratis. Dr. Chieffo reported having no financial conflicts of interest.
PARIS – Results of the first-ever all-female patient TAVR registry indicate that a history of pregnancy – albeit typically half a century or more ago – is strongly protective against stroke, major bleeding, and other serious adverse events within 30 days of undergoing transcatheter aortic valve replacement.
The explanation for this novel observation remains unclear and warrants further study. One plausible hypothesis: “We can postulate that there is a hormonal effect in pregnancy that is still present after 50 years,” Dr. Alaide Chieffo said in presenting the Women’s International Transcatheter Aortic Valve Implantation (WIN TAVI) registry findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
WIN TAVI is a prospective observational registry of women undergoing TAVR for symptomatic severe aortic stenosis. Dr. Chieffo reported on acute and 30-day outcomes in 1,019 women enrolled in the registry at 20 highly experienced TAVR centers in Europe and North America. The average age was 82.5 years and mean STS score was 8.3%, putting the women at intermediate to high risk. Nearly three-quarters of subjects were more older than 80 years. Most patients had at least three major procedural risk factors. Two-thirds of the women were deemed frail upon surgical assessment.
The rationale for creating the WIN TAVI registry lies in the fact that even though half of patients undergoing TAVR are women, their optimal treatment strategy remains undefined. This is an important issue, particularly in light of evidence to suggest that the superiority of transfemoral TAVR over surgical aortic valve replacement in the PARTNER 2A trial may have been driven by better outcomes in women than in men.
WIN TAVI is designed to evaluate the safety and performance of TAVR in women in real-world clinical practice. It’s also a unique opportunity to prospectively gather data on female sex-specific baseline characteristics that haven’t been collected in previous TAVR studies and to examine their influence on outcomes, explained Dr. Chieffo of San Raffaele Scientific Institute in Milan. The primary outcome was the 30-day standard Second Valve Academic Research Consortium (VARC 2) composite of all-cause mortality, stroke, life-threatening bleeding, major vascular complications, stage 2 or 3 acute kidney injury, coronary artery obstruction, or a repeat intervention for valve-related dysfunction. The incidence was 14.4%, including a 3.9% all-cause mortality rate, a 1.3% incidence of stroke, major vascular complications in 7.9% of women, and a 4.4% incidence of life-threatening bleeding.
“The findings of the current registry underscore the importance and safety of moving to a lower-risk population of women with TAVR,” Dr. Chieffo declared.
The VARC 2 rates seen in WIN TAVI are low in comparison to other reports in TAVR patients. Dr. Chieffo attributed this to several factors. Patients underwent current state-of-the-art TAVR procedures, with a percutaneous transfemoral approach used in 91% of cases. Only one-third of patients had general anesthesia; the rest underwent TAVR under conscious sedation or a local anesthetic. One-third of the procedures were carried out with sheath sizes of 16 French or less.
Moreover, 42% of patients received new-generation TAVR devices that are compatible with smaller sheath sizes, are completely or partially retrievable, and have a lower paravalvular leak rate than that of earlier devices. The predominantly female interventional cardiologists were all highly experienced, and more than three-quarters of patients were discharged on dual-antiplatelet therapy or an oral anticoagulant.
Seventy-two percent of women had a remote history of pregnancy. Their rate of the primary VARC 2 safety endpoint was 12.7%, compared to 18.9% in patients without a prior pregnancy. In a multivariate logistic regression analysis, prior pregnancy, regardless of whether it resulted in a live birth, was independently associated with a 43% reduction in the risk of a VARC 2 safety endpoint. The greater the number of a woman’s pregnancies, the larger the reduction in the rate of VARC 2 complications.
The other independent predictor of reduced risk was the use of a new-generation TAVR device, defined as any device commercially available in Europe other than the Edwards Sapien XT or Medtronic CoreValve. The newer devices were associated with a 41% relative risk reduction in the VARC 2 composite endpoint.
Risk factors for the primary safety endpoint were prior stroke, associated with an adjusted twofold increased risk; a left ventricular ejection fraction less than 30%, with a 2.6-fold increased risk; and advanced age.
Of note, while 17.5% of women had a history of osteoporosis, only one-fifth of them were on treatment for it. This may impose important limitations on their postprocedural functional recovery, according to Dr. Chieffo.
Further follow-up of WIN TAVI registry participants is planned at 12, 18, and 24 months post procedure.
The WIN TAVI registry is carried out with no external funding. All participating investigators worked gratis. Dr. Chieffo reported having no financial conflicts of interest.
AT EUROPCR 2016
Key clinical point: Initial results of the first all-female TAVR registry provide surprises.
Major finding: Women who underwent transcatheter aortic valve replacement were 43% less likely to experience a major adverse safety event within 30 days if they had a history of pregnancy.
Data source: WIN TAVI, a prospective observational registry including 1,019 women who underwent TAVR for symptomatic severe aortic stenosis at 20 European and North American centers.
Disclosures: The study is being conducted without external funding. The presenter reported having no financial conflicts of interest.





















