User login
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
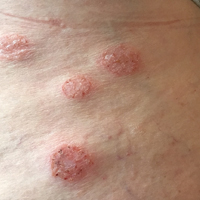
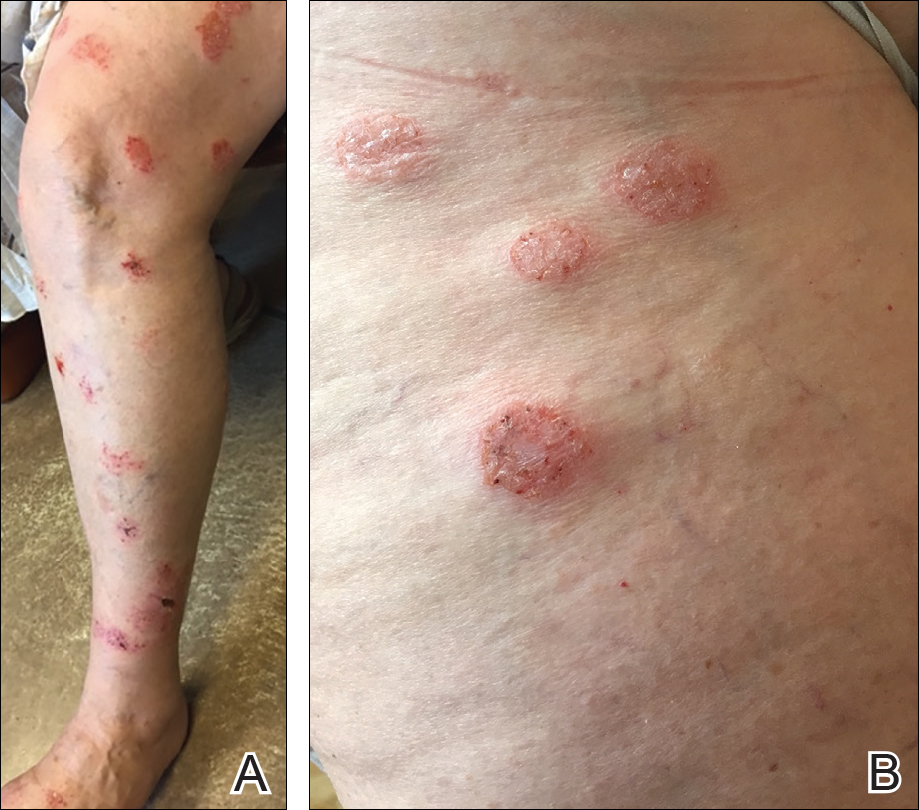
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.
A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.
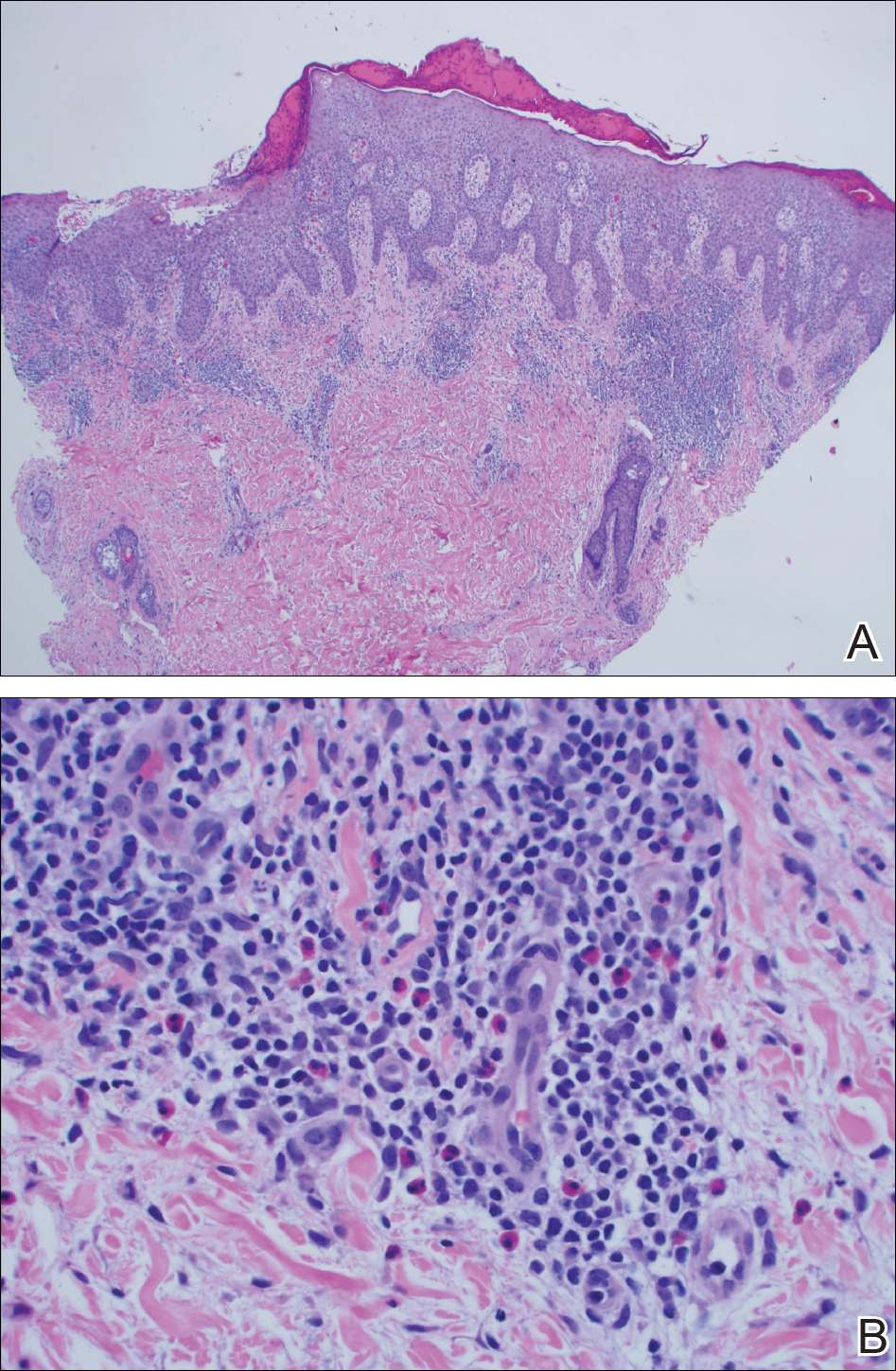
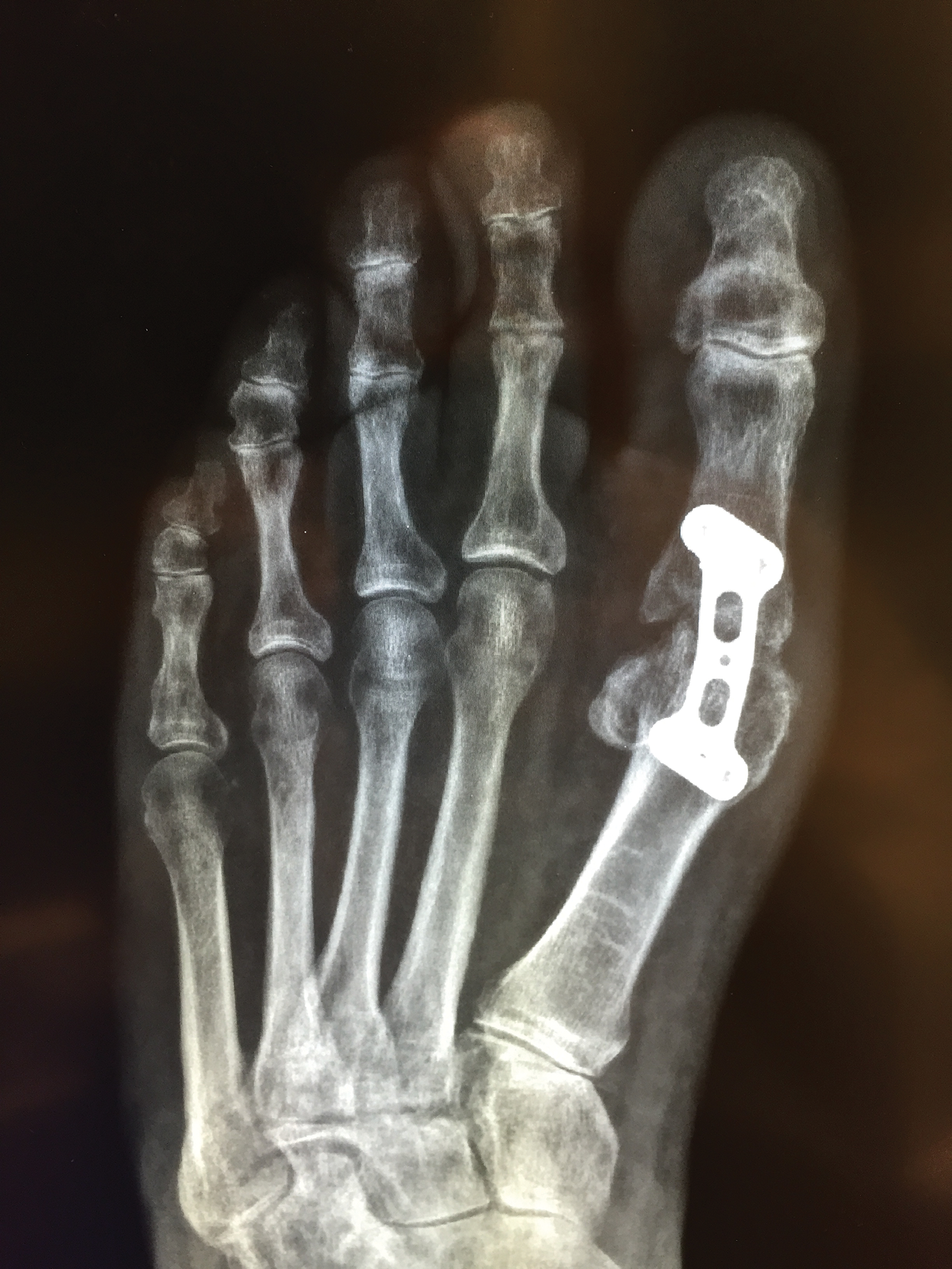
The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.

After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
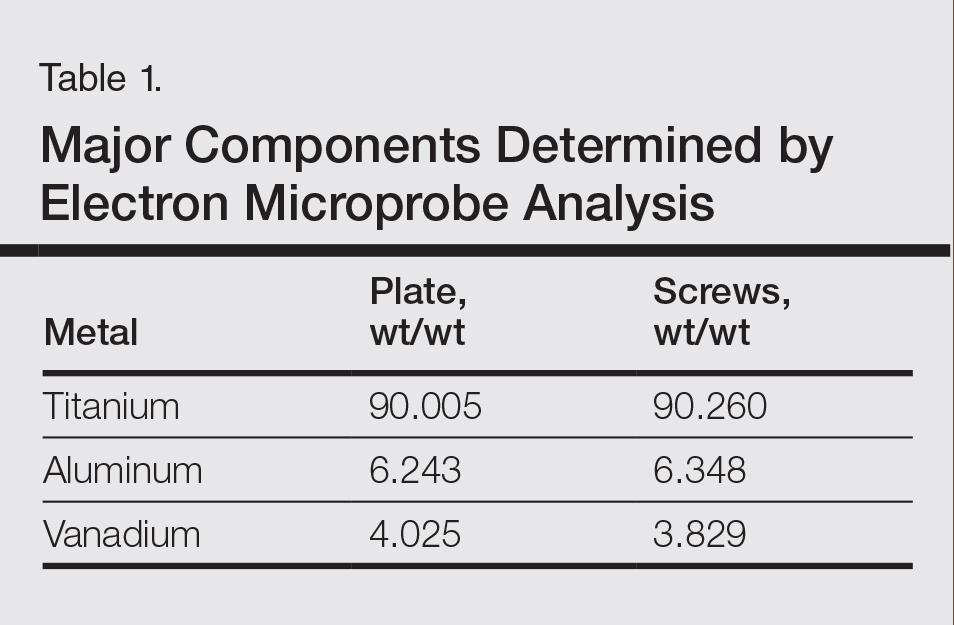
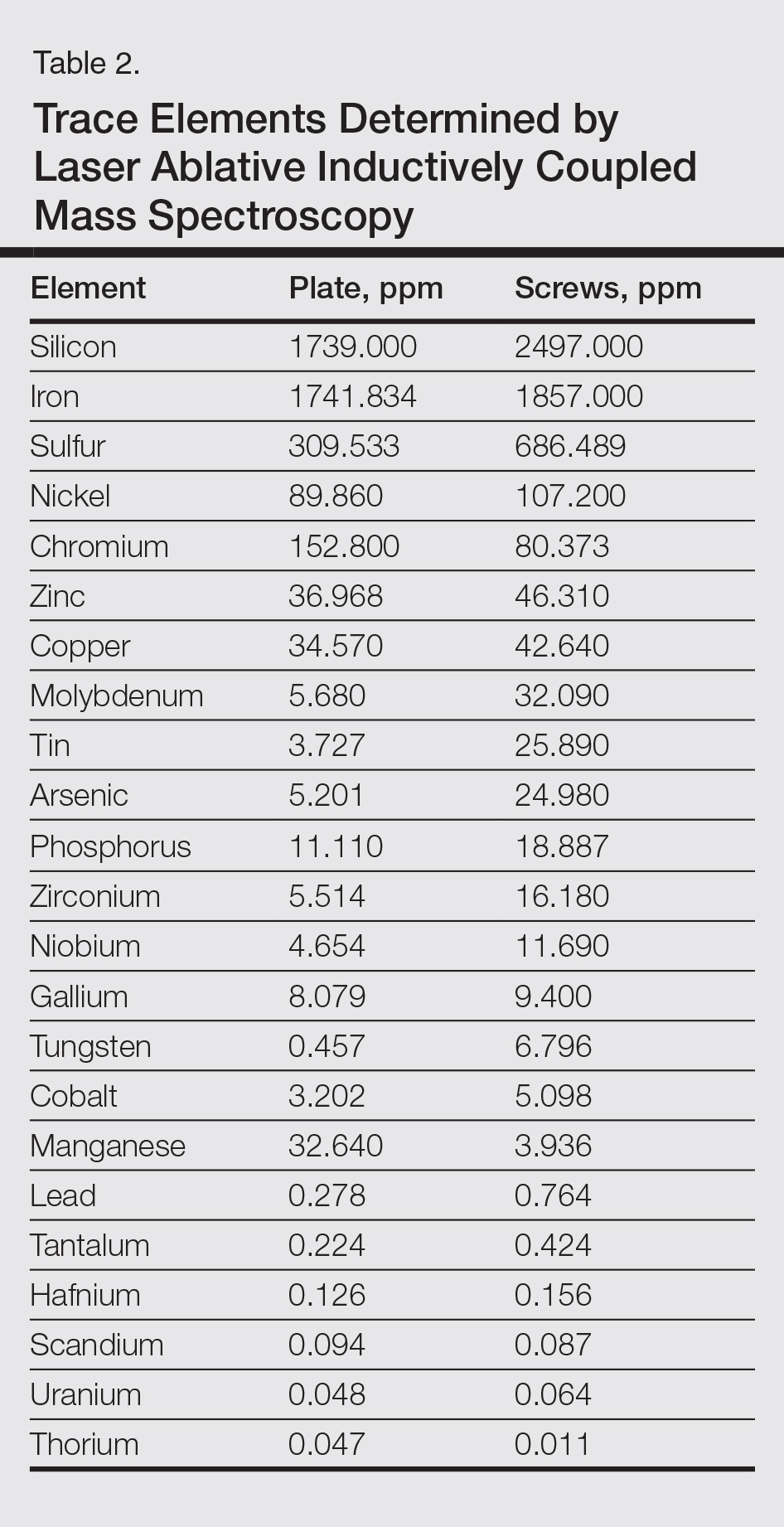
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Practice Points
- Vanadium may be an underrecognized allergen in patients with metal implants.
- Consider vanadium allergy in those with surgical implants and signs of hypersensitivity reaction.
- Test for allergy with vanadium trichloride.
- Niobium is an alternative for implants in vanadium-allergic patients.