User login
We must counsel against heat-not-burn cigarettes
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.
A skeptic’s view of bariatric surgery
Like JFP’s Editor-in-Chief, Dr. John Hickner, I have been skeptical about bariatric surgery (A [former] skeptic’s view of bariatric surgery. J Fam Pract. 2018;67:600), but I will recommend it for a select few patients who are unable or unwilling to undergo significant lifestyle changes. My experience in clinic has done nothing to change this skeptical view. I have many patients who opted for bariatric surgery, but did not change their lifestyle habits. These patients often regain weight and accumulate chronic diseases 2 to 7 years postop. In the end, if a patient does not change their lifestyle, bariatric surgery can push the consequences of obesity out 5 to 10 years, but at a very significant risk.
The most significant problem I see is that many primary care providers do not feel qualified to impart meaningful lifestyle recommendations to patients, which often leads to guidance that is inadequate and, in some cases, inaccurate. Furthermore, assuming patients have received evidence-based instructions, they often lack the support and means to apply these lifestyle changes. I would be very hesitant to recommend bariatric surgery before addressing all of these concerns.
An interesting study done by Lingvay et al1 showed that postsurgical starvation (600 kcal/d) without the bariatric surgery had better short-term outcomes than surgery with calorie restriction, which suggests that a period of starvation is better than surgery.
In general, the results of evidence-based lifestyle changes far surpass any medical or surgical treatment for obesity and its associated chronic diseases. The evidence for this is overwhelming. (See books by Drs. Joel Fuhrman, Michael Greger, Neal Barnard, Dean Ornish, and Garth Davis, as well as the hundreds of peer-reviewed studies cited in these books.) Yet most patients under-going bariatric surgery never receive proper instructions or attempt any meaningful lifestyle changes.
I think it is far more prudent to refer potential surgical candidates to someone who understands good nutrition and lifestyle changes, such as a doctor certified by the American College of Lifestyle Medicine (lifestylemedicine.org). Surgery, in my opinion, is a very poor and dangerous second choice.
John Reed, MD
Fishersville, Va
1. Lingvay I, GuthE, Eslam A, et al. Rapid improvement in diabetes after gastric bypass surgery: Is it the diet or surgery? Diabetes Care. 2013;36:2741-2747.
Like JFP’s Editor-in-Chief, Dr. John Hickner, I have been skeptical about bariatric surgery (A [former] skeptic’s view of bariatric surgery. J Fam Pract. 2018;67:600), but I will recommend it for a select few patients who are unable or unwilling to undergo significant lifestyle changes. My experience in clinic has done nothing to change this skeptical view. I have many patients who opted for bariatric surgery, but did not change their lifestyle habits. These patients often regain weight and accumulate chronic diseases 2 to 7 years postop. In the end, if a patient does not change their lifestyle, bariatric surgery can push the consequences of obesity out 5 to 10 years, but at a very significant risk.
The most significant problem I see is that many primary care providers do not feel qualified to impart meaningful lifestyle recommendations to patients, which often leads to guidance that is inadequate and, in some cases, inaccurate. Furthermore, assuming patients have received evidence-based instructions, they often lack the support and means to apply these lifestyle changes. I would be very hesitant to recommend bariatric surgery before addressing all of these concerns.
An interesting study done by Lingvay et al1 showed that postsurgical starvation (600 kcal/d) without the bariatric surgery had better short-term outcomes than surgery with calorie restriction, which suggests that a period of starvation is better than surgery.
In general, the results of evidence-based lifestyle changes far surpass any medical or surgical treatment for obesity and its associated chronic diseases. The evidence for this is overwhelming. (See books by Drs. Joel Fuhrman, Michael Greger, Neal Barnard, Dean Ornish, and Garth Davis, as well as the hundreds of peer-reviewed studies cited in these books.) Yet most patients under-going bariatric surgery never receive proper instructions or attempt any meaningful lifestyle changes.
I think it is far more prudent to refer potential surgical candidates to someone who understands good nutrition and lifestyle changes, such as a doctor certified by the American College of Lifestyle Medicine (lifestylemedicine.org). Surgery, in my opinion, is a very poor and dangerous second choice.
John Reed, MD
Fishersville, Va
Like JFP’s Editor-in-Chief, Dr. John Hickner, I have been skeptical about bariatric surgery (A [former] skeptic’s view of bariatric surgery. J Fam Pract. 2018;67:600), but I will recommend it for a select few patients who are unable or unwilling to undergo significant lifestyle changes. My experience in clinic has done nothing to change this skeptical view. I have many patients who opted for bariatric surgery, but did not change their lifestyle habits. These patients often regain weight and accumulate chronic diseases 2 to 7 years postop. In the end, if a patient does not change their lifestyle, bariatric surgery can push the consequences of obesity out 5 to 10 years, but at a very significant risk.
The most significant problem I see is that many primary care providers do not feel qualified to impart meaningful lifestyle recommendations to patients, which often leads to guidance that is inadequate and, in some cases, inaccurate. Furthermore, assuming patients have received evidence-based instructions, they often lack the support and means to apply these lifestyle changes. I would be very hesitant to recommend bariatric surgery before addressing all of these concerns.
An interesting study done by Lingvay et al1 showed that postsurgical starvation (600 kcal/d) without the bariatric surgery had better short-term outcomes than surgery with calorie restriction, which suggests that a period of starvation is better than surgery.
In general, the results of evidence-based lifestyle changes far surpass any medical or surgical treatment for obesity and its associated chronic diseases. The evidence for this is overwhelming. (See books by Drs. Joel Fuhrman, Michael Greger, Neal Barnard, Dean Ornish, and Garth Davis, as well as the hundreds of peer-reviewed studies cited in these books.) Yet most patients under-going bariatric surgery never receive proper instructions or attempt any meaningful lifestyle changes.
I think it is far more prudent to refer potential surgical candidates to someone who understands good nutrition and lifestyle changes, such as a doctor certified by the American College of Lifestyle Medicine (lifestylemedicine.org). Surgery, in my opinion, is a very poor and dangerous second choice.
John Reed, MD
Fishersville, Va
1. Lingvay I, GuthE, Eslam A, et al. Rapid improvement in diabetes after gastric bypass surgery: Is it the diet or surgery? Diabetes Care. 2013;36:2741-2747.
1. Lingvay I, GuthE, Eslam A, et al. Rapid improvement in diabetes after gastric bypass surgery: Is it the diet or surgery? Diabetes Care. 2013;36:2741-2747.
Another look at overdiagnosis/remission of asthma
I appreciated the PURL, “Should you reassess your patient’s asthma diagnosis?” (J Fam Pract. 2018;67:704-707) that reminded clinicians to taper asthma controller medications in asymptomatic patients. The articles cited1,2 by Drs. Stevermer and Hayes documented that one-third of the adults enrolled in the respective study with physician-diagnosed asthma did not have objective evidence for asthma and were either over-diagnosed or had remitted. These articles also contained evidence that: 1) over-diagnosis was likely much more common than remission,1 and 2) there was a significant temporal trend towards increasing over-diagnosis/remission during the last several decades. The authors of the cited article1 suggested that the temporal trend could be explained by increased public awareness of respiratory symptoms, more aggressive marketing of asthma medications, and a lack of objective measurement of reversible airway obstruction in primary care. These assertions deserve careful consideration as we strive to diagnose asthma appropriately.
Over-diagnosis/remission is almost certainly not as prevalent (33%) as the authors of the cited articles1,2 reported. The reason is simple selection bias: 1) the cited study2 excluded asthma patients who smoked >10 pack-years (it enrolled 701 asthma patients and excluded 812 asthma patients with a >10 pack-year smoking history), and 2) this study likely did not include asthma patients with the asthma-COPD overlap syndrome, which is treated as asthma and comprises an additional 30% of our patients with chronic airflow limitation (the asthma-COPD spectrum).3 Asthma patients who smoke and/or have the overlap syndrome are prone to severe asthma that is refractory to inhaled corticosteroids.3,4
In addition to making the correct diagnosis, it is equally important to be aware of efficacious therapies for severe refractory asthma that primary care clinicians can easily use. There is now good evidence that azithromycin is efficacious for severe refractory asthma5 and should be considered prior to referral for immunomodulatory asthma therapies.6
David L. Hahn, MD, MS
Madison, Wis
1. Aaron SD, Vandemheen KL, Boulet LP, et al; Canadian Respiratory Clinical Research Consortium. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al; Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
3. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728-735.
4. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24;313-322.
5. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017:390659-668.
6. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycin-Asthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
Continue to: Authors' response...
Authors’ response:
We appreciate Dr. Hahn’s observations about the PURL1 on overdiagnosis of asthma. This article focused on the results of a prospective, multicenter cohort study2 that evaluated the feasibility of tapering, and in many patients, stopping asthma medications. We agree that if the study had included people diagnosed with asthma who also had smoked at least 10 pack-years or who also had COPD, the proportion of those who would eventually no longer meet diagnostic criteria for asthma would be lower than in this study. We are uncertain of the relative proportion of cases that were overdiagnosis, when compared with true remission of disease, as only 43% of those no longer meeting the diagnostic criteria for asthma had evidence of prior lung function testing, whether by formal spirometry, serial peak function testing, or bronchial challenge testing.
We agree that using efficacious therapies for severe refractory asthma is essential, but the selection of those therapies was outside the scope of this PURL.
James J. Stevermer, MD, MSPH; Alisa Hayes, MD
Columbia, Mo
1. Stevermer JJ, Hayes A. Should you reassess your patient’s asthma diagnosis? J Fam Pract. 2018;67:704-707.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al; Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
I appreciated the PURL, “Should you reassess your patient’s asthma diagnosis?” (J Fam Pract. 2018;67:704-707) that reminded clinicians to taper asthma controller medications in asymptomatic patients. The articles cited1,2 by Drs. Stevermer and Hayes documented that one-third of the adults enrolled in the respective study with physician-diagnosed asthma did not have objective evidence for asthma and were either over-diagnosed or had remitted. These articles also contained evidence that: 1) over-diagnosis was likely much more common than remission,1 and 2) there was a significant temporal trend towards increasing over-diagnosis/remission during the last several decades. The authors of the cited article1 suggested that the temporal trend could be explained by increased public awareness of respiratory symptoms, more aggressive marketing of asthma medications, and a lack of objective measurement of reversible airway obstruction in primary care. These assertions deserve careful consideration as we strive to diagnose asthma appropriately.
Over-diagnosis/remission is almost certainly not as prevalent (33%) as the authors of the cited articles1,2 reported. The reason is simple selection bias: 1) the cited study2 excluded asthma patients who smoked >10 pack-years (it enrolled 701 asthma patients and excluded 812 asthma patients with a >10 pack-year smoking history), and 2) this study likely did not include asthma patients with the asthma-COPD overlap syndrome, which is treated as asthma and comprises an additional 30% of our patients with chronic airflow limitation (the asthma-COPD spectrum).3 Asthma patients who smoke and/or have the overlap syndrome are prone to severe asthma that is refractory to inhaled corticosteroids.3,4
In addition to making the correct diagnosis, it is equally important to be aware of efficacious therapies for severe refractory asthma that primary care clinicians can easily use. There is now good evidence that azithromycin is efficacious for severe refractory asthma5 and should be considered prior to referral for immunomodulatory asthma therapies.6
David L. Hahn, MD, MS
Madison, Wis
1. Aaron SD, Vandemheen KL, Boulet LP, et al; Canadian Respiratory Clinical Research Consortium. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al; Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
3. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728-735.
4. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24;313-322.
5. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017:390659-668.
6. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycin-Asthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
Continue to: Authors' response...
Authors’ response:
We appreciate Dr. Hahn’s observations about the PURL1 on overdiagnosis of asthma. This article focused on the results of a prospective, multicenter cohort study2 that evaluated the feasibility of tapering, and in many patients, stopping asthma medications. We agree that if the study had included people diagnosed with asthma who also had smoked at least 10 pack-years or who also had COPD, the proportion of those who would eventually no longer meet diagnostic criteria for asthma would be lower than in this study. We are uncertain of the relative proportion of cases that were overdiagnosis, when compared with true remission of disease, as only 43% of those no longer meeting the diagnostic criteria for asthma had evidence of prior lung function testing, whether by formal spirometry, serial peak function testing, or bronchial challenge testing.
We agree that using efficacious therapies for severe refractory asthma is essential, but the selection of those therapies was outside the scope of this PURL.
James J. Stevermer, MD, MSPH; Alisa Hayes, MD
Columbia, Mo
1. Stevermer JJ, Hayes A. Should you reassess your patient’s asthma diagnosis? J Fam Pract. 2018;67:704-707.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al; Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
I appreciated the PURL, “Should you reassess your patient’s asthma diagnosis?” (J Fam Pract. 2018;67:704-707) that reminded clinicians to taper asthma controller medications in asymptomatic patients. The articles cited1,2 by Drs. Stevermer and Hayes documented that one-third of the adults enrolled in the respective study with physician-diagnosed asthma did not have objective evidence for asthma and were either over-diagnosed or had remitted. These articles also contained evidence that: 1) over-diagnosis was likely much more common than remission,1 and 2) there was a significant temporal trend towards increasing over-diagnosis/remission during the last several decades. The authors of the cited article1 suggested that the temporal trend could be explained by increased public awareness of respiratory symptoms, more aggressive marketing of asthma medications, and a lack of objective measurement of reversible airway obstruction in primary care. These assertions deserve careful consideration as we strive to diagnose asthma appropriately.
Over-diagnosis/remission is almost certainly not as prevalent (33%) as the authors of the cited articles1,2 reported. The reason is simple selection bias: 1) the cited study2 excluded asthma patients who smoked >10 pack-years (it enrolled 701 asthma patients and excluded 812 asthma patients with a >10 pack-year smoking history), and 2) this study likely did not include asthma patients with the asthma-COPD overlap syndrome, which is treated as asthma and comprises an additional 30% of our patients with chronic airflow limitation (the asthma-COPD spectrum).3 Asthma patients who smoke and/or have the overlap syndrome are prone to severe asthma that is refractory to inhaled corticosteroids.3,4
In addition to making the correct diagnosis, it is equally important to be aware of efficacious therapies for severe refractory asthma that primary care clinicians can easily use. There is now good evidence that azithromycin is efficacious for severe refractory asthma5 and should be considered prior to referral for immunomodulatory asthma therapies.6
David L. Hahn, MD, MS
Madison, Wis
1. Aaron SD, Vandemheen KL, Boulet LP, et al; Canadian Respiratory Clinical Research Consortium. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al; Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
3. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728-735.
4. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24;313-322.
5. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017:390659-668.
6. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycin-Asthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
Continue to: Authors' response...
Authors’ response:
We appreciate Dr. Hahn’s observations about the PURL1 on overdiagnosis of asthma. This article focused on the results of a prospective, multicenter cohort study2 that evaluated the feasibility of tapering, and in many patients, stopping asthma medications. We agree that if the study had included people diagnosed with asthma who also had smoked at least 10 pack-years or who also had COPD, the proportion of those who would eventually no longer meet diagnostic criteria for asthma would be lower than in this study. We are uncertain of the relative proportion of cases that were overdiagnosis, when compared with true remission of disease, as only 43% of those no longer meeting the diagnostic criteria for asthma had evidence of prior lung function testing, whether by formal spirometry, serial peak function testing, or bronchial challenge testing.
We agree that using efficacious therapies for severe refractory asthma is essential, but the selection of those therapies was outside the scope of this PURL.
James J. Stevermer, MD, MSPH; Alisa Hayes, MD
Columbia, Mo
1. Stevermer JJ, Hayes A. Should you reassess your patient’s asthma diagnosis? J Fam Pract. 2018;67:704-707.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al; Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
Does left atrial appendage closure reduce stroke rates as well as oral anticoagulants and antiplatelet meds in A-fib patients?
EVIDENCE SUMMARY
A 2017 network meta-analysis included 19 RCTs and 87,831 patients receiving anticoagulation, antiplatelet therapy, or LAAC for NVAF.1 LAAC was superior to antiplatelet therapy (hazard ratio [HR]=0.44; 95% confidence interval [CI], 0.23-0.86; P<.05) and similar to NOACs (HR=1.01; 95% CI, 0.53-1.92; P=.969) for reducing risk of stroke.
LAAC and NOACs found “most effective”
A network meta-analysis of 21 RCTs, which included data from 96,017 patients, examined the effectiveness of 7 interventions to prevent stroke in patients with NVAF: 4 NOACs, VKA, aspirin, and LAAC; the analysis compared VKA with the other interventions.2 The 2 trials that investigated LAAC accounted for only 1114 patients.
When the 7 interventions were ranked simultaneously on 2 efficacy outcomes (stroke/systemic embolism and all-cause mortality), all 4 NOACs and LAAC clustered together as “the most effective and lifesaving.”
Fewer hemorrhagic strokes with LAAC than VKA
A 2016 meta-analysis of 6 RCTs compared risk of stroke for adults with NVAF who received LAAC, VKA, or NOACs.3 No significant differences were found between NOACs and VKA or LAAC and VKA. The LAAC group had a significantly smaller number of patients.
A 2015 meta-analysis of 2406 patients with NVAF found that patients who received LAAC had significantly fewer hemorrhagic strokes (HR=0.22; P<.05) than patients who received VKA.4 No differences in all-cause stroke were found between the 2 groups during an average follow-up of 2.69 years.
LAAC found superior to warfarin for stroke prevention in one trial
A 2014 multicenter, randomized study (PROTECT-AF) of 707 patients with NVAF plus 1 additional stroke risk factor compared LAAC with VKA (warfarin).5 LAAC met criteria at 3.8 years for both noninferiority and superiority in preventing stroke, based on 2.3 events per 100 patient-years compared with 3.8 events per 100 patient-years for VKA. The number needed to treat with LAAC was 67 to result in 1 less event per patient-year.
A 2014 RCT (PREVAIL) evaluated patients with NVAF plus 1 additional stroke risk factor. LAAC was noninferior to warfarin for ischemic stroke prevention.6
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Cardiology (ACC) recommends LAAC for patients with NVAF who are not candidates for long-term anticoagulation.7 Similarly, the 2016 European Society of Cardiology guidelines issued a Class IIb recommendation for LAAC for stroke prevention in those with contraindications for long-term anticoagulation.8 Lastly, in a 2014 guideline, the American Heart Association, ACC, and the Heart Rhythm Society issued a Class IIb recommendation for surgical excision of the left atrial appendage in patients with atrial fibrillation undergoing cardiac surgery, but did not provide recommendations regarding LAAC.9
1. Sahay S, Nombela-Franco L, Rodes-Cabau J, et al. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: a network meta-analysis from randomised trials. Heart. 2017;103:139-147.
2. Tereshchenko LG, Henrikson CA, Cigarroa, J, et al. Comparative effectiveness of interventions for stroke prevention in atrial fibrillation: a network meta-analysis. J Am Heart Assoc. 2016; 5:e003206.
3. Bajaj NS, Kalra R, Patel N, et al. Comparison of approaches for stroke prophylaxis in patients with non-valvular atrial fibrillation: network meta-analyses of randomized clinical trials. PLoS One. 2016;11:e0163608.
4. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614-2623.
5. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
6. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12.
7. Panaich S, Holmes DR. Left atrial appendage occlusion: Expert analysis. http://www.acc.org/latest-in-cardiology/articles/2017/ 01/31/13/08/left-atrial-appendage-occlusion. Accessed April 5, 2018.
8. Kirchof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678.
9. January CT, Wann LS, Alpert LS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. JACC. 2014;64:2246-2280.
EVIDENCE SUMMARY
A 2017 network meta-analysis included 19 RCTs and 87,831 patients receiving anticoagulation, antiplatelet therapy, or LAAC for NVAF.1 LAAC was superior to antiplatelet therapy (hazard ratio [HR]=0.44; 95% confidence interval [CI], 0.23-0.86; P<.05) and similar to NOACs (HR=1.01; 95% CI, 0.53-1.92; P=.969) for reducing risk of stroke.
LAAC and NOACs found “most effective”
A network meta-analysis of 21 RCTs, which included data from 96,017 patients, examined the effectiveness of 7 interventions to prevent stroke in patients with NVAF: 4 NOACs, VKA, aspirin, and LAAC; the analysis compared VKA with the other interventions.2 The 2 trials that investigated LAAC accounted for only 1114 patients.
When the 7 interventions were ranked simultaneously on 2 efficacy outcomes (stroke/systemic embolism and all-cause mortality), all 4 NOACs and LAAC clustered together as “the most effective and lifesaving.”
Fewer hemorrhagic strokes with LAAC than VKA
A 2016 meta-analysis of 6 RCTs compared risk of stroke for adults with NVAF who received LAAC, VKA, or NOACs.3 No significant differences were found between NOACs and VKA or LAAC and VKA. The LAAC group had a significantly smaller number of patients.
A 2015 meta-analysis of 2406 patients with NVAF found that patients who received LAAC had significantly fewer hemorrhagic strokes (HR=0.22; P<.05) than patients who received VKA.4 No differences in all-cause stroke were found between the 2 groups during an average follow-up of 2.69 years.
LAAC found superior to warfarin for stroke prevention in one trial
A 2014 multicenter, randomized study (PROTECT-AF) of 707 patients with NVAF plus 1 additional stroke risk factor compared LAAC with VKA (warfarin).5 LAAC met criteria at 3.8 years for both noninferiority and superiority in preventing stroke, based on 2.3 events per 100 patient-years compared with 3.8 events per 100 patient-years for VKA. The number needed to treat with LAAC was 67 to result in 1 less event per patient-year.
A 2014 RCT (PREVAIL) evaluated patients with NVAF plus 1 additional stroke risk factor. LAAC was noninferior to warfarin for ischemic stroke prevention.6
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Cardiology (ACC) recommends LAAC for patients with NVAF who are not candidates for long-term anticoagulation.7 Similarly, the 2016 European Society of Cardiology guidelines issued a Class IIb recommendation for LAAC for stroke prevention in those with contraindications for long-term anticoagulation.8 Lastly, in a 2014 guideline, the American Heart Association, ACC, and the Heart Rhythm Society issued a Class IIb recommendation for surgical excision of the left atrial appendage in patients with atrial fibrillation undergoing cardiac surgery, but did not provide recommendations regarding LAAC.9
EVIDENCE SUMMARY
A 2017 network meta-analysis included 19 RCTs and 87,831 patients receiving anticoagulation, antiplatelet therapy, or LAAC for NVAF.1 LAAC was superior to antiplatelet therapy (hazard ratio [HR]=0.44; 95% confidence interval [CI], 0.23-0.86; P<.05) and similar to NOACs (HR=1.01; 95% CI, 0.53-1.92; P=.969) for reducing risk of stroke.
LAAC and NOACs found “most effective”
A network meta-analysis of 21 RCTs, which included data from 96,017 patients, examined the effectiveness of 7 interventions to prevent stroke in patients with NVAF: 4 NOACs, VKA, aspirin, and LAAC; the analysis compared VKA with the other interventions.2 The 2 trials that investigated LAAC accounted for only 1114 patients.
When the 7 interventions were ranked simultaneously on 2 efficacy outcomes (stroke/systemic embolism and all-cause mortality), all 4 NOACs and LAAC clustered together as “the most effective and lifesaving.”
Fewer hemorrhagic strokes with LAAC than VKA
A 2016 meta-analysis of 6 RCTs compared risk of stroke for adults with NVAF who received LAAC, VKA, or NOACs.3 No significant differences were found between NOACs and VKA or LAAC and VKA. The LAAC group had a significantly smaller number of patients.
A 2015 meta-analysis of 2406 patients with NVAF found that patients who received LAAC had significantly fewer hemorrhagic strokes (HR=0.22; P<.05) than patients who received VKA.4 No differences in all-cause stroke were found between the 2 groups during an average follow-up of 2.69 years.
LAAC found superior to warfarin for stroke prevention in one trial
A 2014 multicenter, randomized study (PROTECT-AF) of 707 patients with NVAF plus 1 additional stroke risk factor compared LAAC with VKA (warfarin).5 LAAC met criteria at 3.8 years for both noninferiority and superiority in preventing stroke, based on 2.3 events per 100 patient-years compared with 3.8 events per 100 patient-years for VKA. The number needed to treat with LAAC was 67 to result in 1 less event per patient-year.
A 2014 RCT (PREVAIL) evaluated patients with NVAF plus 1 additional stroke risk factor. LAAC was noninferior to warfarin for ischemic stroke prevention.6
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Cardiology (ACC) recommends LAAC for patients with NVAF who are not candidates for long-term anticoagulation.7 Similarly, the 2016 European Society of Cardiology guidelines issued a Class IIb recommendation for LAAC for stroke prevention in those with contraindications for long-term anticoagulation.8 Lastly, in a 2014 guideline, the American Heart Association, ACC, and the Heart Rhythm Society issued a Class IIb recommendation for surgical excision of the left atrial appendage in patients with atrial fibrillation undergoing cardiac surgery, but did not provide recommendations regarding LAAC.9
1. Sahay S, Nombela-Franco L, Rodes-Cabau J, et al. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: a network meta-analysis from randomised trials. Heart. 2017;103:139-147.
2. Tereshchenko LG, Henrikson CA, Cigarroa, J, et al. Comparative effectiveness of interventions for stroke prevention in atrial fibrillation: a network meta-analysis. J Am Heart Assoc. 2016; 5:e003206.
3. Bajaj NS, Kalra R, Patel N, et al. Comparison of approaches for stroke prophylaxis in patients with non-valvular atrial fibrillation: network meta-analyses of randomized clinical trials. PLoS One. 2016;11:e0163608.
4. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614-2623.
5. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
6. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12.
7. Panaich S, Holmes DR. Left atrial appendage occlusion: Expert analysis. http://www.acc.org/latest-in-cardiology/articles/2017/ 01/31/13/08/left-atrial-appendage-occlusion. Accessed April 5, 2018.
8. Kirchof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678.
9. January CT, Wann LS, Alpert LS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. JACC. 2014;64:2246-2280.
1. Sahay S, Nombela-Franco L, Rodes-Cabau J, et al. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: a network meta-analysis from randomised trials. Heart. 2017;103:139-147.
2. Tereshchenko LG, Henrikson CA, Cigarroa, J, et al. Comparative effectiveness of interventions for stroke prevention in atrial fibrillation: a network meta-analysis. J Am Heart Assoc. 2016; 5:e003206.
3. Bajaj NS, Kalra R, Patel N, et al. Comparison of approaches for stroke prophylaxis in patients with non-valvular atrial fibrillation: network meta-analyses of randomized clinical trials. PLoS One. 2016;11:e0163608.
4. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614-2623.
5. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
6. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12.
7. Panaich S, Holmes DR. Left atrial appendage occlusion: Expert analysis. http://www.acc.org/latest-in-cardiology/articles/2017/ 01/31/13/08/left-atrial-appendage-occlusion. Accessed April 5, 2018.
8. Kirchof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678.
9. January CT, Wann LS, Alpert LS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. JACC. 2014;64:2246-2280.
EVIDENCE-BASED ANSWER:
Yes. Left atrial appendage closure (LAAC) with the Watchman device is noninferior to vitamin K antagonists (VKAs) and non-VKA oral anticoagulants (NOACs) for adults with nonvalvular atrial fibrillation (NVAF) and 1 additional stroke risk factor (strength of recommendation [SOR]: A, multiple meta-analyses).
LAAC has consistently been shown to be superior to antiplatelet therapy (SOR: A, single meta-analysis). One randomized controlled trial (RCT) demonstrated superiority of LAAC to VKA (SOR: B, single RCT).
To refer—or not?
When I was training to become a family physician, my mentor often told me that a competent family physician should be able to manage about 80% of patients’ office visits without consultation. I am not sure where that figure came from, but my 40 years of experience in family medicine supports that prediction. Of course, the flip-side of that coin is having the wisdom to make those referrals for patients who really need a specialist’s diagnostic or treatment skills. The “rub,” of course, is that when I do need a specialist’s help, the wait for an appointment is often unacceptably long—both for me and my patients.
One way to help alleviate the logjam of referrals is to manage more medical problems ourselves. Now I don’t mean holding on to patients who definitely need a referral. But I do think we should avoid being too quick to hand off a patient. Let me explain.
When I was Chair of Family Medicine at Cleveland Clinic, I asked my specialty colleagues what percentage of the referred patients they saw in their offices could be managed competently by a well-trained family physician. The usual answer—from a variety of specialists—was “about 30%.” If we took care of that 30% of patients ourselves, it would go a long way toward freeing up specialists’ schedules to see the patients who truly require their expertise.
Some public health systems, such as the University of California San Francisco Medical Center,1 have implemented successful triage systems to alleviate the referral backlog. Patients are triaged by a specialist and assigned to 1 of 3 categories: 1) urgent—the patient will be seen right away, 2) non-urgent—the patient will be seen as soon as possible (usually within 2 weeks), or 3) phone/email consultation—the specialist provides diagnostic and management advice electronically, or by phone, but does not see the patient.
Continue to: The issue of referral comes to mind...
The issue of referral comes to mind this month in light of our cover story on migraine headache management. Migraine is one of those conditions that is often referred for specialist care, but can, in many cases, be competently managed by family physicians. The diagnosis of migraine is made almost entirely by history and physical exam, and there are many treatments for acute attacks and prevention that are effective and can be prescribed by family physicians and other primary health care professionals.
Yes, patients with more severe migraine may need a specialist consultation. But let’s remain cognizant of the fact that a good percentage of our patients will be best served staying right where they are—in the office of their family physician.
1. Chen AH, Murphy EJ, Yee HF. eReferral—a new model for integrated care. N Engl J Med. 2013;368:2450-2453.
When I was training to become a family physician, my mentor often told me that a competent family physician should be able to manage about 80% of patients’ office visits without consultation. I am not sure where that figure came from, but my 40 years of experience in family medicine supports that prediction. Of course, the flip-side of that coin is having the wisdom to make those referrals for patients who really need a specialist’s diagnostic or treatment skills. The “rub,” of course, is that when I do need a specialist’s help, the wait for an appointment is often unacceptably long—both for me and my patients.
One way to help alleviate the logjam of referrals is to manage more medical problems ourselves. Now I don’t mean holding on to patients who definitely need a referral. But I do think we should avoid being too quick to hand off a patient. Let me explain.
When I was Chair of Family Medicine at Cleveland Clinic, I asked my specialty colleagues what percentage of the referred patients they saw in their offices could be managed competently by a well-trained family physician. The usual answer—from a variety of specialists—was “about 30%.” If we took care of that 30% of patients ourselves, it would go a long way toward freeing up specialists’ schedules to see the patients who truly require their expertise.
Some public health systems, such as the University of California San Francisco Medical Center,1 have implemented successful triage systems to alleviate the referral backlog. Patients are triaged by a specialist and assigned to 1 of 3 categories: 1) urgent—the patient will be seen right away, 2) non-urgent—the patient will be seen as soon as possible (usually within 2 weeks), or 3) phone/email consultation—the specialist provides diagnostic and management advice electronically, or by phone, but does not see the patient.
Continue to: The issue of referral comes to mind...
The issue of referral comes to mind this month in light of our cover story on migraine headache management. Migraine is one of those conditions that is often referred for specialist care, but can, in many cases, be competently managed by family physicians. The diagnosis of migraine is made almost entirely by history and physical exam, and there are many treatments for acute attacks and prevention that are effective and can be prescribed by family physicians and other primary health care professionals.
Yes, patients with more severe migraine may need a specialist consultation. But let’s remain cognizant of the fact that a good percentage of our patients will be best served staying right where they are—in the office of their family physician.
When I was training to become a family physician, my mentor often told me that a competent family physician should be able to manage about 80% of patients’ office visits without consultation. I am not sure where that figure came from, but my 40 years of experience in family medicine supports that prediction. Of course, the flip-side of that coin is having the wisdom to make those referrals for patients who really need a specialist’s diagnostic or treatment skills. The “rub,” of course, is that when I do need a specialist’s help, the wait for an appointment is often unacceptably long—both for me and my patients.
One way to help alleviate the logjam of referrals is to manage more medical problems ourselves. Now I don’t mean holding on to patients who definitely need a referral. But I do think we should avoid being too quick to hand off a patient. Let me explain.
When I was Chair of Family Medicine at Cleveland Clinic, I asked my specialty colleagues what percentage of the referred patients they saw in their offices could be managed competently by a well-trained family physician. The usual answer—from a variety of specialists—was “about 30%.” If we took care of that 30% of patients ourselves, it would go a long way toward freeing up specialists’ schedules to see the patients who truly require their expertise.
Some public health systems, such as the University of California San Francisco Medical Center,1 have implemented successful triage systems to alleviate the referral backlog. Patients are triaged by a specialist and assigned to 1 of 3 categories: 1) urgent—the patient will be seen right away, 2) non-urgent—the patient will be seen as soon as possible (usually within 2 weeks), or 3) phone/email consultation—the specialist provides diagnostic and management advice electronically, or by phone, but does not see the patient.
Continue to: The issue of referral comes to mind...
The issue of referral comes to mind this month in light of our cover story on migraine headache management. Migraine is one of those conditions that is often referred for specialist care, but can, in many cases, be competently managed by family physicians. The diagnosis of migraine is made almost entirely by history and physical exam, and there are many treatments for acute attacks and prevention that are effective and can be prescribed by family physicians and other primary health care professionals.
Yes, patients with more severe migraine may need a specialist consultation. But let’s remain cognizant of the fact that a good percentage of our patients will be best served staying right where they are—in the office of their family physician.
1. Chen AH, Murphy EJ, Yee HF. eReferral—a new model for integrated care. N Engl J Med. 2013;368:2450-2453.
1. Chen AH, Murphy EJ, Yee HF. eReferral—a new model for integrated care. N Engl J Med. 2013;368:2450-2453.
Persistent facial hyperpigmentation
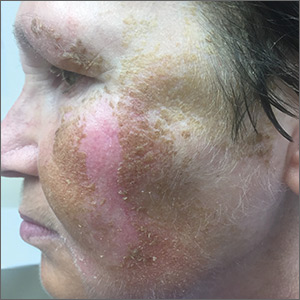
A 59-year-old woman presented to a dermatology clinic with an asymptomatic brown facial hyperpigmentation that had developed several years earlier, and had persisted, despite regular face washing. Physicians who previously treated this patient interpreted this as melasma and advised her to wear sunscreen. The condition was not aggravated by sun exposure. The patient reported that she was otherwise healthy.
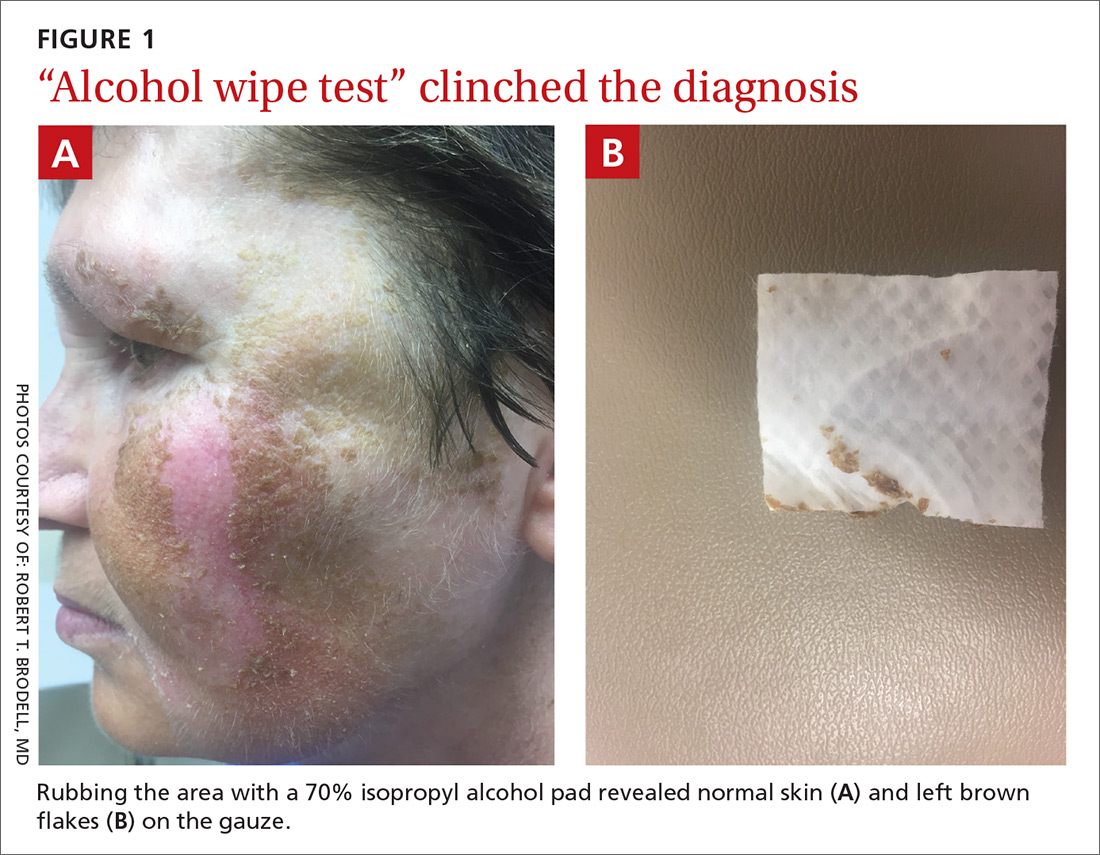
Physical examination revealed a brown discoloration with a slightly rough texture. Upon rubbing the affected area with a 70% isopropyl alcohol pad, normal skin was revealed (FIGURE 1A) and brown flakes were apparent on the gauze (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Terra firma-forme dermatosis
The physician diagnosed terra firma-forme dermatosis (TFFD) in this patient, noting the “dirty brown coloration” and distribution that did not suggest post-inflammatory hyperpigmentation or melasma. TFFD is a rare and benign form of acquired hyperpigmentation characterized by “velvety, pigmented patches or plaques.”1 A simple bedside test, known as an “alcohol wipe test,” both confirms and treats TFFD; it involves rubbing the affected area with a 70% isopropyl alcohol pad.1
TFFD typically affects the face, neck, trunk, or ankles, but the scalp, axilla, back, and pubis also can be affected.1 Histopathology will show negligible amounts of dermal inflammation, hyperkeratosis with mild acanthosis, and hyperkeratosis and papillomatosis.1 Most patients diagnosed with TFFD report that the hyperpigmentation does not improve despite washing with soap and water.2
Hygiene is not a factor
In 2015, Greywal and Cohen followed the case presentations of 10 Caucasian patients with TFFD who presented with “brown and/or black plaques or papules or both.”2 Many of the individuals followed in this case series reported “[practicing] good hygiene and showered a minimum of every other day or daily.”2 The same was reported by the patient in this case. This suggests that TFFD is not a consequence of poor hygiene but perhaps a result of “sticky” sebum that produces a buildup of keratin debris, sebum, and bacteria on the skin.3 This produces the hyperpigmentation seen clinically.
Differential includes post-inflammatory hyperpigmentation
Several other hyperpigmentation disorders were considered on the initial differential diagnosis for this case, including melasma and post-inflammatory hyperpigmentation. However, these 2 conditions are macular, whereas this hyperpigmented condition had a rough, mildly papular texture. Additionally, melasma flares up in the summer with UV exposure, and post-inflammatory hyperpigmentation presents with pruritus and/or a pre-existing rash.4 This patient reported that the condition did not itch nor change with increased sunlight, thus making melasma and post-inflammatory hyperpigmentation unlikely diagnoses.
Acanthosis nigricans also was considered because it presents with a velvety brown pigmentation similar to what was seen with this patient. Acanthosis nigricans, however, primarily affects flexural areas, not the face, making it improbable.
Continue to: Our patient
Our patient. A “wipe test” was performed on the patient. This removed the brown flaky scaling and revealed the underlying normal skin. We instructed the patient to wash daily with a soapy wash cloth and scrub with 70% isopropyl alcohol should the hyperpigmentation recur. The patient did not return.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; rbrodell@umc.edu
1. Lunge S, Supraja C. Terra firma-forme dermatosis—a dirty dermatosis: report of two cases. Our Dermatol Online. 2016;7:338-340.
2. Greywal T, Cohen PR. Terra firma-forme dermatosis: A report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
3. Alonso-Usero V, Gavrilova M, et al. Dermatosis neglecta or terra firma-forme dermatosis. Actas Dermosifiliogr. 2012;103:932-934.
4. Lucas J, Brodell RT, Feldman SR. Dermatosis neglecta: a series of case reports and review of other dirty-appearing dermatoses. Dermatol Online J. 2006;12:5.
A 59-year-old woman presented to a dermatology clinic with an asymptomatic brown facial hyperpigmentation that had developed several years earlier, and had persisted, despite regular face washing. Physicians who previously treated this patient interpreted this as melasma and advised her to wear sunscreen. The condition was not aggravated by sun exposure. The patient reported that she was otherwise healthy.
Physical examination revealed a brown discoloration with a slightly rough texture. Upon rubbing the affected area with a 70% isopropyl alcohol pad, normal skin was revealed (FIGURE 1A) and brown flakes were apparent on the gauze (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Terra firma-forme dermatosis
The physician diagnosed terra firma-forme dermatosis (TFFD) in this patient, noting the “dirty brown coloration” and distribution that did not suggest post-inflammatory hyperpigmentation or melasma. TFFD is a rare and benign form of acquired hyperpigmentation characterized by “velvety, pigmented patches or plaques.”1 A simple bedside test, known as an “alcohol wipe test,” both confirms and treats TFFD; it involves rubbing the affected area with a 70% isopropyl alcohol pad.1
TFFD typically affects the face, neck, trunk, or ankles, but the scalp, axilla, back, and pubis also can be affected.1 Histopathology will show negligible amounts of dermal inflammation, hyperkeratosis with mild acanthosis, and hyperkeratosis and papillomatosis.1 Most patients diagnosed with TFFD report that the hyperpigmentation does not improve despite washing with soap and water.2
Hygiene is not a factor
In 2015, Greywal and Cohen followed the case presentations of 10 Caucasian patients with TFFD who presented with “brown and/or black plaques or papules or both.”2 Many of the individuals followed in this case series reported “[practicing] good hygiene and showered a minimum of every other day or daily.”2 The same was reported by the patient in this case. This suggests that TFFD is not a consequence of poor hygiene but perhaps a result of “sticky” sebum that produces a buildup of keratin debris, sebum, and bacteria on the skin.3 This produces the hyperpigmentation seen clinically.
Differential includes post-inflammatory hyperpigmentation
Several other hyperpigmentation disorders were considered on the initial differential diagnosis for this case, including melasma and post-inflammatory hyperpigmentation. However, these 2 conditions are macular, whereas this hyperpigmented condition had a rough, mildly papular texture. Additionally, melasma flares up in the summer with UV exposure, and post-inflammatory hyperpigmentation presents with pruritus and/or a pre-existing rash.4 This patient reported that the condition did not itch nor change with increased sunlight, thus making melasma and post-inflammatory hyperpigmentation unlikely diagnoses.
Acanthosis nigricans also was considered because it presents with a velvety brown pigmentation similar to what was seen with this patient. Acanthosis nigricans, however, primarily affects flexural areas, not the face, making it improbable.
Continue to: Our patient
Our patient. A “wipe test” was performed on the patient. This removed the brown flaky scaling and revealed the underlying normal skin. We instructed the patient to wash daily with a soapy wash cloth and scrub with 70% isopropyl alcohol should the hyperpigmentation recur. The patient did not return.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; rbrodell@umc.edu
A 59-year-old woman presented to a dermatology clinic with an asymptomatic brown facial hyperpigmentation that had developed several years earlier, and had persisted, despite regular face washing. Physicians who previously treated this patient interpreted this as melasma and advised her to wear sunscreen. The condition was not aggravated by sun exposure. The patient reported that she was otherwise healthy.
Physical examination revealed a brown discoloration with a slightly rough texture. Upon rubbing the affected area with a 70% isopropyl alcohol pad, normal skin was revealed (FIGURE 1A) and brown flakes were apparent on the gauze (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Terra firma-forme dermatosis
The physician diagnosed terra firma-forme dermatosis (TFFD) in this patient, noting the “dirty brown coloration” and distribution that did not suggest post-inflammatory hyperpigmentation or melasma. TFFD is a rare and benign form of acquired hyperpigmentation characterized by “velvety, pigmented patches or plaques.”1 A simple bedside test, known as an “alcohol wipe test,” both confirms and treats TFFD; it involves rubbing the affected area with a 70% isopropyl alcohol pad.1
TFFD typically affects the face, neck, trunk, or ankles, but the scalp, axilla, back, and pubis also can be affected.1 Histopathology will show negligible amounts of dermal inflammation, hyperkeratosis with mild acanthosis, and hyperkeratosis and papillomatosis.1 Most patients diagnosed with TFFD report that the hyperpigmentation does not improve despite washing with soap and water.2
Hygiene is not a factor
In 2015, Greywal and Cohen followed the case presentations of 10 Caucasian patients with TFFD who presented with “brown and/or black plaques or papules or both.”2 Many of the individuals followed in this case series reported “[practicing] good hygiene and showered a minimum of every other day or daily.”2 The same was reported by the patient in this case. This suggests that TFFD is not a consequence of poor hygiene but perhaps a result of “sticky” sebum that produces a buildup of keratin debris, sebum, and bacteria on the skin.3 This produces the hyperpigmentation seen clinically.
Differential includes post-inflammatory hyperpigmentation
Several other hyperpigmentation disorders were considered on the initial differential diagnosis for this case, including melasma and post-inflammatory hyperpigmentation. However, these 2 conditions are macular, whereas this hyperpigmented condition had a rough, mildly papular texture. Additionally, melasma flares up in the summer with UV exposure, and post-inflammatory hyperpigmentation presents with pruritus and/or a pre-existing rash.4 This patient reported that the condition did not itch nor change with increased sunlight, thus making melasma and post-inflammatory hyperpigmentation unlikely diagnoses.
Acanthosis nigricans also was considered because it presents with a velvety brown pigmentation similar to what was seen with this patient. Acanthosis nigricans, however, primarily affects flexural areas, not the face, making it improbable.
Continue to: Our patient
Our patient. A “wipe test” was performed on the patient. This removed the brown flaky scaling and revealed the underlying normal skin. We instructed the patient to wash daily with a soapy wash cloth and scrub with 70% isopropyl alcohol should the hyperpigmentation recur. The patient did not return.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; rbrodell@umc.edu
1. Lunge S, Supraja C. Terra firma-forme dermatosis—a dirty dermatosis: report of two cases. Our Dermatol Online. 2016;7:338-340.
2. Greywal T, Cohen PR. Terra firma-forme dermatosis: A report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
3. Alonso-Usero V, Gavrilova M, et al. Dermatosis neglecta or terra firma-forme dermatosis. Actas Dermosifiliogr. 2012;103:932-934.
4. Lucas J, Brodell RT, Feldman SR. Dermatosis neglecta: a series of case reports and review of other dirty-appearing dermatoses. Dermatol Online J. 2006;12:5.
1. Lunge S, Supraja C. Terra firma-forme dermatosis—a dirty dermatosis: report of two cases. Our Dermatol Online. 2016;7:338-340.
2. Greywal T, Cohen PR. Terra firma-forme dermatosis: A report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
3. Alonso-Usero V, Gavrilova M, et al. Dermatosis neglecta or terra firma-forme dermatosis. Actas Dermosifiliogr. 2012;103:932-934.
4. Lucas J, Brodell RT, Feldman SR. Dermatosis neglecta: a series of case reports and review of other dirty-appearing dermatoses. Dermatol Online J. 2006;12:5.
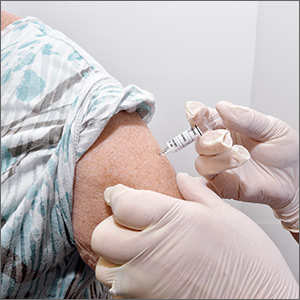
Pain in right shoulder • recent influenza vaccination • history of hypertension and myocardial infarction • Dx?
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
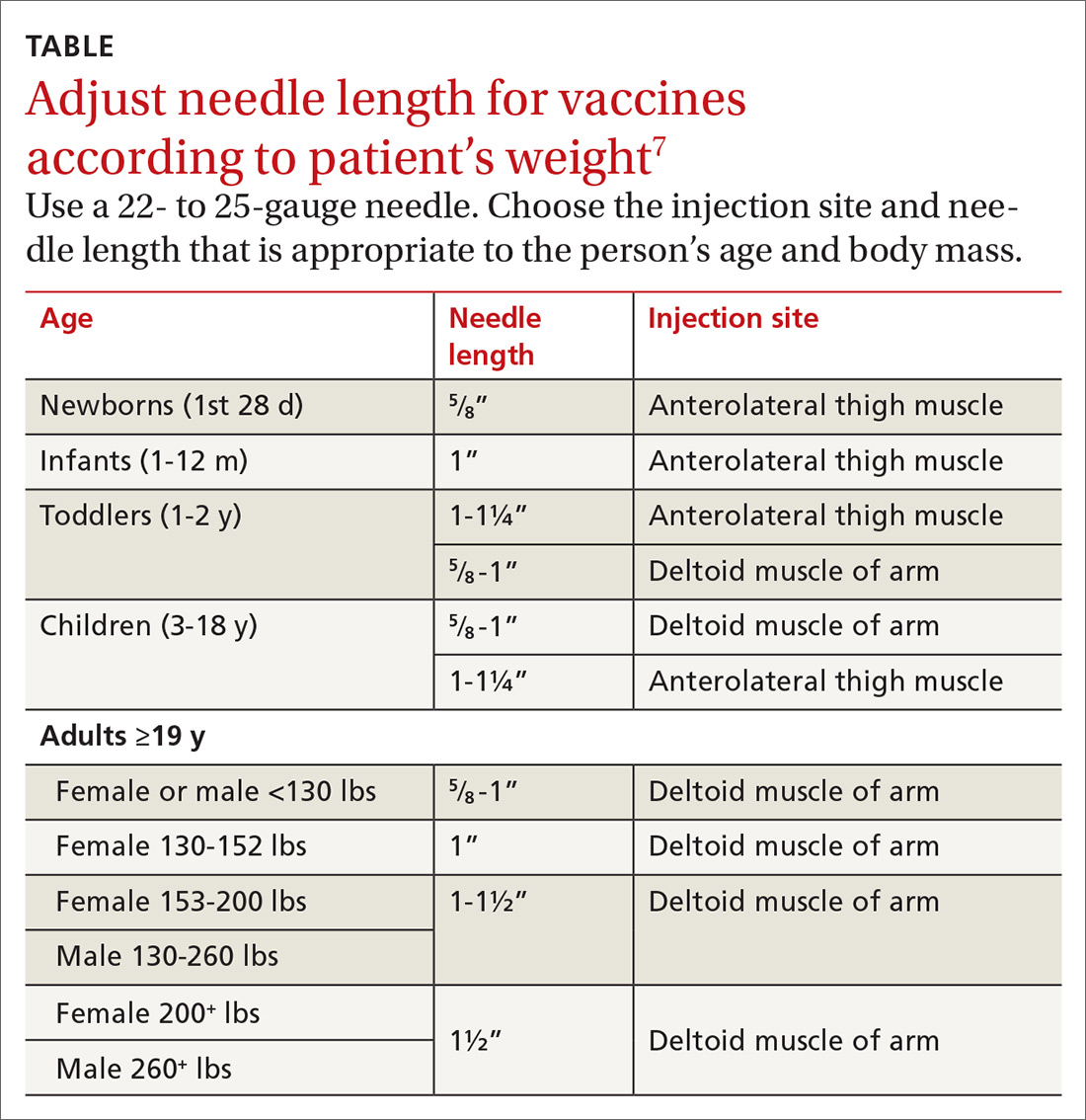
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; dustymarienarducci@gmail.com
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; dustymarienarducci@gmail.com
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; dustymarienarducci@gmail.com
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
Less is more when it comes to ketorolac for pain
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
PRACTICE CHANGER
Use a low dose (10 mg) of intravenous ketorolac for moderate to severe acute pain in adults because it is as effective as higher doses (15-30 mg).1
STRENGTH OF RECOMMENDATION
B: Based on a single, good-quality randomized controlled trial.
Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
Alcohol use disorder: How best to screen and intervene
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
The role of home BP monitoring: Answers to 10 common questions
National Health and Nutrition Examination Survey (NHANES) data from 2011 to 2014 revealed that 29% of adults in the United States have hypertension.1 Prevalence increases with age, so that 7% of adults ages 18 to 39 years, 32% of adults ages 40 to 59, and 65% of adults ages ≥60 years have the disease.1 This national survey data also showed that 53% of those given the diagnosis had uncontrolled hypertension, and that control of hypertension did not change significantly from 2009 to 2014.1
Elevated blood pressure (BP) has been the leading risk factor for death related to cardiovascular disease globally for the last 3 decades.2 Yet in 2 nationally representative samples, only 1 in 6 patients with documented BP ≥140/90 mm Hg received treatment intensification with new medication during primary care visits.3 Uncertainty about the representativeness of any single clinic BP measurement is a prominent reason for health care providers not to intensify therapy.4
Confirming the Dx outside the office. The 2015 US Preventive Services Task Force (USPSTF) guidelines on screening for hypertension state that, for most patients, a diagnosis of hypertension should be confirmed with out-of-office BP monitoring before initiating treatment.5 The USPSTF states that ambulatory BP monitoring (ABPM) is accurate for hypertension diagnosis and monitoring, and that home BP monitoring (HBPM) is an acceptable alternative, based on good quality evidence.
Access to ABPM, however, is often limited. In a 2015 survey of primary care clinics, only 25% of the 123 clinics that completed the questionnaire reported having access to it.6 Conversely, HBPM is widely available and acceptable to most patients. A recent NHANES survey showed that 43.5% of patients who were aware of their hypertension diagnosis engaged in HBPM.7
So what, exactly, should the role of HBPM be in the management of patients with hypertension? The evidence-based answers to the 10 questions that follow provide useful insights.
[polldaddy:10224678]
1. Can HBPM be used to confirm a Dx of hypertension?
Yes (Strength of recommendation [SOR] C).
In reviewing the diagnostic accuracy of various methods to confirm the diagnosis of hypertension, the USPSTF identified ABPM as the most accurate, followed by HBPM, with clinic BP measurements bringing up the rear.5 In adults ≥18 years of age, the USPSTF recommends obtaining BP measurements outside of the clinical setting for diagnostic confirmation before starting treatment unless the patient’s BP is ≥180/110 mm Hg, there is evidence of end-organ damage, or the patient has a diagnosis of secondary hypertension.5 The USPSTF recommends HBPM as an acceptable alternative to ABPM based on 6 studies including a total of 1253 participants.8 The percentage of patients with elevated office BP confirmed by HBPM to have hypertension was 45% to 84% across these 6 studies.
Sixteen studies from another systematic review evaluated the diagnostic accuracy of HBPM while using ABPM as a reference.9 This review found that HBPM had high specificity and negative predictive value, but low sensitivity and positive predictive value. There was moderate diagnostic agreement between HBPM and ABPM, with kappa statistic values of 0.37 to 0.73 across all studies.9
Continue to: In yet another study...
In yet another study, home BP and ambulatory BP measurements were identical when the same dual-mode device was used to measure both ambulatory and home BP.10
2. What are the diagnostic and treatment targets for home BP monitoring?
Treat patients if home BP is ≥130/80 mm Hg and categorize patients as normotensive if home BP is <125/76 mm Hg (SOR C). Monitor patients who are in between.
A 2017 joint statement from the American College of Cardiology/American Heart Association (ACC/AHA) Task Force states that the target BP for HBPM should be <130/80 mm Hg.11 The Joint National Commission (JNC) 8 issued BP goals of <140/90 mm Hg for adults <60 years of age and those with diabetes and/or chronic kidney disease, and a goal of <150/90 mm Hg for adults ≥60 years of age with no diabetes or chronic kidney disease,12 but much debate has recently surrounded these guidelines. JNC 8 does not provide a separate BP goal for HBPM.
Although based solely on evidence (and not patient-oriented outcomes), a home BP threshold of ≥135/85 mm Hg for the diagnosis and treatment of hypertension has been supported by the European Society of Hypertension consensus guidelines,13 results of a longitudinal study,14 meta-analyses of published studies, and a meta-analysis using individual subject data.15
Support for a home BP measurement of <125/76 mm Hg as normal is limited to a single cross-sectional study of 48 patients with 2 elevated office BP readings where the threshold of 125/76 mm Hg on home BP was shown to exclude 80% of patients diagnosed with hypertension by ambulatory readings.16 If home BP measurements are >125/76 mm Hgbut <135/85 mm Hg, 24-hour ambulatory BP monitoring is recommended to assess hypertension control.17
3. Does home BP monitoring improve hypertension control?
Yes, in the short term, but not in the long term (SOR C).
A meta-analysis of 13 comparative studies looking at HBPM alone vs usual care showed a small, but statistically significant, benefit of achieving target BP at 6 months with a relative risk ratio (RRR) of 1.3 (95% confidence interval [CI], 1.00-1.68; I2=77%).18 However, the pooled effects from 3 studies that measured the benefit of achieving a predefined BP target at the 12-month follow-up mark were not significant in this review (RRR=1.18; 95% CI, 0.95-1.46, I2=86%).18 The pooled effect from 19 studies from the same review showed that there was a statistically significant weighted mean difference of -3.9 mm Hg in systolic BP and a weighted mean difference of -2.4 mm Hg in diastolic BP at 6 months; however, the changes were no longer significant at the 12-month follow-up mark.18
Continue to: More than half of the studies included...
More than half of the studies included in the meta-analysis were of low quality, and none of the studies recruited patients based on differences in clinic BP and home BP patterns, but rather on controlled or uncontrolled hypertension status. The studies included in this meta-analysis measured final BP outcomes by measuring ambulatory BP or clinic BP.
Another systematic review of 19 studies and 7100 participants looking at how HBPM compared with ABPM as a measurement standard for BP control and patient outcomes found insufficient data to determine the benefit of using HBPM as a measurement standard for BP control.19
HBPM + added support. There was high-quality evidence from the meta-analysis that HBPM plus additional support vs usual care led to a reduction in BP and a higher proportion of patients achieving target BP.18 However, the additional support interventions in the studies were heterogeneous.
4. Should HBPM be used to detect a change in BP associated with medication alterations?
Yes (SOR B).
A 2008 meta-analysis20 and several other studies21,22 showed that HBPM has greater accuracy than office BP for identifying drug-induced BP changes. The 2008 meta-analysis looked at changes in office and home BP measurements produced by various antihypertensive drugs. In 7 studies that compared office BP measurements with home and ambulatory BP measurements, the 24-hour ambulatory BP measurements and home BP measurements showed less dramatic BP reductions with medications than clinic BP measurements.20 This meta-analysis included 30 studies with 6794 participants and showed that home BP readings fell 20% less than office BP readings; the difference was statistically significant. These findings suggest that treatment-attributable changes in home BP and clinic BP measurements are linearly related, with the treatment effect on home BP measurements being around 80% of the effect on clinic BP measurements.
5. Do home BP measurements correlate with clinical outcomes?
Yes, and better than office BP measurements do; however, most studies comparing home BP measurements with usual care while looking at clinical outcomes are observational or quasi-experimental (SOR B).
For example, a 2015 systematic review looking at associations between BP measurement type (office, home, and ambulatory) and patient mortality found 5 observational studies that showed that adding home or ambulatory BP information improved cardiovascular risk prediction models. Moreover, all-cause mortality was associated with home BP and ambulatory BP levels only and not with office BP levels.19 The number of participants in these 5 studies varied between 210 and 2051 with study duration between 2.4 and 12.3 years. Of note, every study had a distinct population, affecting the generalizability of the results.
Continue to: One quasi-experimental study...
One quasi-experimental study with 450 participants showed that home BP measurements were at least as good as ambulatory BP measurements at predicting end organ damage related to hypertension when organ damage was measured by cardiac echocardiography, detection of microalbuminuria, and carotid echocardiography.23 Similarly, a systematic review of 14 studies and 2485 participants comparing home, ambulatory, and office BP readings showed that home BP measurements’ association with left ventricular mass index is as good as that of ambulatory BP measurements, and superior to clinic BP readings.24
6. Does HBPM help improve medication adherence?
The jury is still out on this one (SOR B).
A 2006 systematic review of randomized controlled trials (RCTs) incorporating HBPM and evaluating medication adherence outcomes found that in 6 of the 11 studies identified, there was some improvement in medication adherence with HBPM.25 However, only 1 of the 6 studies in this review involved HBPM as the sole intervention; the remaining 5 studies employed additional adherence-enhancing strategies.
Another systematic review looking at HBPM vs usual care included 8 studies (3 of moderate quality and 5 of low quality) that measured medication adherence (using varying measures of adherence) of which only 3 studies showed some improvement in medication adherence with HBPM.18
7. Does HBPM reduce therapeutic inertia?
Yes (SOR B).
A meta-analysis of 15 studies showed that therapeutic inertia was less common with HBPM than with office BP monitoring alone; the relative risk for unchanged medication was 0.82 (95% CI, 0.68 to 0.99) with HBPM.26 However, 10 of the 15 studies were of low quality with a Jadad score ≤3.
8. Does HBPM, along with titration of treatment, improve BP outcomes?
Yes (SOR B).
Two RCTs that looked at self-monitoring of BP and self-titration of hypertensive medications showed significant reductions in BP levels.27,28 In a cluster RCT of home BP telemonitoring, in which the pharmacist adjusted antihypertensives based on transmitted BP measurements, hypertension control was significantly better in the intervention group than in the usual care group (57.2% vs 30%).29
Continue to: What are the recommended techniques for HBPM?
9. What are the recommended techniques for HBP
Patients should use a device that is validated, fully automated, and has an upper arm cuff (not a wrist monitor), according to a joint statement from the AHA, the American Society of Hypertension, and the Preventive Cardiovascular Nurses Association.17 (SOR C). (See validated BP monitor list at http://www.dableducational.org/sphygmomanometers/devices_2_sbpm.html.)
Patients should measure their BP in their nondominant arm after 5 minutes of rest with the arm at heart level, back supported, and feet flat on the ground. Patient technique and the accuracy of the home BP monitor should be checked annually. It is also recommended that patients check their BP 2 to 3 times every morning and evening. An average of 12 morning and evening measurements should be used for monitoring and treatment changes. An AHA informational sheet that shows how to measure BP properly can be found on their Web si
Several studies examining the accuracy of measuring BP over clothing did not find significant differences in BP measurements performed on a bare arm vs over a sleeve.30-33
10. What are the predictors of differences between home and office BP measurements?
Gender is one of the biggest predictors (SOR B).
A 2016 meta-analysis reported a total of 60 different hypothesized predictors of differences between home and clinic BP measurements (eg, gender, age, body mass index, systolic BP, diastolic BP). Masked hypertension was defined as a normal clinic BP reading and an elevated home BP reading. White coat hypertension was defined as an elevated clinic BP measurement with an acceptable home BP measurement. The researchers extracted odds ratios (ORs) for each study describing the association between patient characteristics and white coat or masked hypertension.34 Studies of masked hypertension diagnosed from HBPM showed male gender as the most significant predictor of home-clinic BP differences (OR=1.47, 95% CI, 1.18-1.75). In contrast, female gender was the only significant predictor of white coat hypertension (OR=3.38; 95% CI, 1.64-6.96) when comparing home BP with clinic BP measurements
Literature limitations and barriers to greater implementation
Most studies looking at HBPM outcomes have measured outcomes using ABPM or office BP measurements. The authors of studies using office BP as the outcome measure usually performed multiple BP measurements at often multiple office or clinic visits to calculate the true BP—a procedure that primary care practices rarely follow.35 Additionally, there are significant methodologic differences in HBPM and ABPM; home BP is measured at rest, while ambulatory BP is measured while the patient is mobile and functioning. There are insufficient prospective studies looking at HBPM effects on clinical and patient-oriented outcomes.
The evidence clearly supports using HBPM in the diagnosis of hypertension and suggests its benefit in hypertension management. However, there are significant barriers to incorporating HBPM into practice—barriers that are largely unaddressed in the literature.
For HBPM to be successful, patients need affordable validated home BP monitors covered by insurance that can translate home BP readings into usable information. Additional administrative and/or nursing assistance is required for patient education and support. Uploaded data need to be summarized in a way that is actionable and linked to the electronic health record.
Continue to: As the volume of patient-generated home date increases...
As the volume of patient-generated home data increases, there is a risk of information overload. Thus, meaningful summarization of the data is required to enable the physician, patient, and/or pharmacist to take prompt and effective action.
CORRESPONDENCE
Sonal J. Patil, MD, MSPH, Curtis W. and Ann H. Long Department of Family and Community Medicine, University of Missouri, MA306 Medical Sciences Building, DC032.00 Columbia, MO 65212; patilso@health.missouri.edu.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. David R. Mehr for his valuable expertise in editing and proofreading this manuscript.
1. Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011-2014. NCHS Data Brief. 2015;(220):1-8.
2. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardio-metabolic risk factors between 1980 and 2010: comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634-647.
3. Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:e004188.
4. Kerr EA, Zikmund-Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717-727.
5. U.S. Preventive Services Task Force. Final recommendation statement: High blood pressure in adults: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed July 19, 2017.
6. Woolsey S, Brown B, Ralls B, et al. Diagnosing hypertension in primary care clinics according to current guidelines. J Am Board Fam Med. 2017;30:170-177.
7. Ostchega Y, Zhang G, Kit BK, et al. Factors associated with home blood pressure monitoring among US adults: National Health and Nutrition Examination Survey, 2011-2014. Am J Hypertens. 2017;30:1126-1132.
8. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
9. Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011;24:123-134.
10. Stergiou GS, Tzamouranis D, Nasothimiou EG, et al. Are there really differences between home and daytime ambulatory blood pressure? Comparison using a novel dual-mode ambulatory and home monitor. J Hum Hypertens. 2009;24:207-212.
11. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:e13-e115.
12. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. Erratum in JAMA. 2014;311:1809.
13. O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821-848.
14. Tsunoda S, Kawano Y, Horio T, et al. Relationship between home blood pressure and longitudinal changes in target organ damage in treated hypertensive patients. Hypertens Res. 2002;25:167-173.
15. Staessen JA, Thijs L. Development of diagnostic thresholds for automated self-measurement of blood pressure in adults. First International Consensus Conference on Blood Pressure Self-Measurement. Blood Press Monit. 2000;5:101-109.
16. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17(11 Pt 1):1017-1022.
17. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. J Cardiovasc Nurs. 2008;23:299-323.
18. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185-194.
19. Breaux-Shropshire TL, Judd E, Vucovich LA, et al. Does home blood pressure monitoring improve patient outcomes? A systematic review comparing home and ambulatory blood pressure monitoring on blood pressure control and patient outcomes. Integr Blood Press Control. 2015;8:43-49.
20. Ishikawa J, Carroll DJ, Kuruvilla S, et al. Changes in home versus clinic blood pressure with antihypertensive treatments: a meta-analysis. Hypertension. 2008;52:856-864.
21. Imai Y, Ohkubo T, Hozawa A, et al. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19:179-185.
22. Vaur L, Dubroca I, Dutrey-Dupagne C, et al. Superiority of home blood pressure measurements over office measurements for testing antihypertensive drugs. Blood Press Monit. 1998;3:107-114.
23. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
24. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299.
25. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich). 2006;8:174-180.
26. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38.
27. McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799-808.
28. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163-172.
29. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: The HyperLink Cluster Randomized Trial. JAMA. 2013;310:46-56.
30. Ma G, Sabin N, Dawes M. A comparison of blood pressure measurement over a sleeved arm versus a bare arm. CMAJ. 2008;178:585-589.
31. Kahan E, Yaphe J, Knaani-Levinz H, et al. Comparison of blood pressure measurements on the bare arm, below a rolled-up sleeve, or over a sleeve. Fam Pract. 2003;20:730-732.
32. Liebl M, Holzgreve H, Schulz M, et al. The effect of clothes on sphygmomanometric and oscillometric blood pressure measurement. Blood Press. 2004;13:279-282.
33. Holleman DR Jr., Westman EC, McCrory DC, et al. The effect of sleeved arms on oscillometric blood pressure measurement. J Gen Intern Med. 1993;8:325-326.
34. Sheppard JP, Fletcher B, Gill P, et al. Predictors of the home-clinic blood pressure difference: a systematic review and meta-analysis. Am J Hypertens. 2016;29:614-625.
35. Stergiou GS, Skeva II, Baibas NM, et al. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18:1745-1751.
National Health and Nutrition Examination Survey (NHANES) data from 2011 to 2014 revealed that 29% of adults in the United States have hypertension.1 Prevalence increases with age, so that 7% of adults ages 18 to 39 years, 32% of adults ages 40 to 59, and 65% of adults ages ≥60 years have the disease.1 This national survey data also showed that 53% of those given the diagnosis had uncontrolled hypertension, and that control of hypertension did not change significantly from 2009 to 2014.1
Elevated blood pressure (BP) has been the leading risk factor for death related to cardiovascular disease globally for the last 3 decades.2 Yet in 2 nationally representative samples, only 1 in 6 patients with documented BP ≥140/90 mm Hg received treatment intensification with new medication during primary care visits.3 Uncertainty about the representativeness of any single clinic BP measurement is a prominent reason for health care providers not to intensify therapy.4
Confirming the Dx outside the office. The 2015 US Preventive Services Task Force (USPSTF) guidelines on screening for hypertension state that, for most patients, a diagnosis of hypertension should be confirmed with out-of-office BP monitoring before initiating treatment.5 The USPSTF states that ambulatory BP monitoring (ABPM) is accurate for hypertension diagnosis and monitoring, and that home BP monitoring (HBPM) is an acceptable alternative, based on good quality evidence.
Access to ABPM, however, is often limited. In a 2015 survey of primary care clinics, only 25% of the 123 clinics that completed the questionnaire reported having access to it.6 Conversely, HBPM is widely available and acceptable to most patients. A recent NHANES survey showed that 43.5% of patients who were aware of their hypertension diagnosis engaged in HBPM.7
So what, exactly, should the role of HBPM be in the management of patients with hypertension? The evidence-based answers to the 10 questions that follow provide useful insights.
[polldaddy:10224678]
1. Can HBPM be used to confirm a Dx of hypertension?
Yes (Strength of recommendation [SOR] C).
In reviewing the diagnostic accuracy of various methods to confirm the diagnosis of hypertension, the USPSTF identified ABPM as the most accurate, followed by HBPM, with clinic BP measurements bringing up the rear.5 In adults ≥18 years of age, the USPSTF recommends obtaining BP measurements outside of the clinical setting for diagnostic confirmation before starting treatment unless the patient’s BP is ≥180/110 mm Hg, there is evidence of end-organ damage, or the patient has a diagnosis of secondary hypertension.5 The USPSTF recommends HBPM as an acceptable alternative to ABPM based on 6 studies including a total of 1253 participants.8 The percentage of patients with elevated office BP confirmed by HBPM to have hypertension was 45% to 84% across these 6 studies.
Sixteen studies from another systematic review evaluated the diagnostic accuracy of HBPM while using ABPM as a reference.9 This review found that HBPM had high specificity and negative predictive value, but low sensitivity and positive predictive value. There was moderate diagnostic agreement between HBPM and ABPM, with kappa statistic values of 0.37 to 0.73 across all studies.9
Continue to: In yet another study...
In yet another study, home BP and ambulatory BP measurements were identical when the same dual-mode device was used to measure both ambulatory and home BP.10
2. What are the diagnostic and treatment targets for home BP monitoring?
Treat patients if home BP is ≥130/80 mm Hg and categorize patients as normotensive if home BP is <125/76 mm Hg (SOR C). Monitor patients who are in between.
A 2017 joint statement from the American College of Cardiology/American Heart Association (ACC/AHA) Task Force states that the target BP for HBPM should be <130/80 mm Hg.11 The Joint National Commission (JNC) 8 issued BP goals of <140/90 mm Hg for adults <60 years of age and those with diabetes and/or chronic kidney disease, and a goal of <150/90 mm Hg for adults ≥60 years of age with no diabetes or chronic kidney disease,12 but much debate has recently surrounded these guidelines. JNC 8 does not provide a separate BP goal for HBPM.
Although based solely on evidence (and not patient-oriented outcomes), a home BP threshold of ≥135/85 mm Hg for the diagnosis and treatment of hypertension has been supported by the European Society of Hypertension consensus guidelines,13 results of a longitudinal study,14 meta-analyses of published studies, and a meta-analysis using individual subject data.15
Support for a home BP measurement of <125/76 mm Hg as normal is limited to a single cross-sectional study of 48 patients with 2 elevated office BP readings where the threshold of 125/76 mm Hg on home BP was shown to exclude 80% of patients diagnosed with hypertension by ambulatory readings.16 If home BP measurements are >125/76 mm Hgbut <135/85 mm Hg, 24-hour ambulatory BP monitoring is recommended to assess hypertension control.17
3. Does home BP monitoring improve hypertension control?
Yes, in the short term, but not in the long term (SOR C).
A meta-analysis of 13 comparative studies looking at HBPM alone vs usual care showed a small, but statistically significant, benefit of achieving target BP at 6 months with a relative risk ratio (RRR) of 1.3 (95% confidence interval [CI], 1.00-1.68; I2=77%).18 However, the pooled effects from 3 studies that measured the benefit of achieving a predefined BP target at the 12-month follow-up mark were not significant in this review (RRR=1.18; 95% CI, 0.95-1.46, I2=86%).18 The pooled effect from 19 studies from the same review showed that there was a statistically significant weighted mean difference of -3.9 mm Hg in systolic BP and a weighted mean difference of -2.4 mm Hg in diastolic BP at 6 months; however, the changes were no longer significant at the 12-month follow-up mark.18
Continue to: More than half of the studies included...
More than half of the studies included in the meta-analysis were of low quality, and none of the studies recruited patients based on differences in clinic BP and home BP patterns, but rather on controlled or uncontrolled hypertension status. The studies included in this meta-analysis measured final BP outcomes by measuring ambulatory BP or clinic BP.
Another systematic review of 19 studies and 7100 participants looking at how HBPM compared with ABPM as a measurement standard for BP control and patient outcomes found insufficient data to determine the benefit of using HBPM as a measurement standard for BP control.19
HBPM + added support. There was high-quality evidence from the meta-analysis that HBPM plus additional support vs usual care led to a reduction in BP and a higher proportion of patients achieving target BP.18 However, the additional support interventions in the studies were heterogeneous.
4. Should HBPM be used to detect a change in BP associated with medication alterations?
Yes (SOR B).
A 2008 meta-analysis20 and several other studies21,22 showed that HBPM has greater accuracy than office BP for identifying drug-induced BP changes. The 2008 meta-analysis looked at changes in office and home BP measurements produced by various antihypertensive drugs. In 7 studies that compared office BP measurements with home and ambulatory BP measurements, the 24-hour ambulatory BP measurements and home BP measurements showed less dramatic BP reductions with medications than clinic BP measurements.20 This meta-analysis included 30 studies with 6794 participants and showed that home BP readings fell 20% less than office BP readings; the difference was statistically significant. These findings suggest that treatment-attributable changes in home BP and clinic BP measurements are linearly related, with the treatment effect on home BP measurements being around 80% of the effect on clinic BP measurements.
5. Do home BP measurements correlate with clinical outcomes?
Yes, and better than office BP measurements do; however, most studies comparing home BP measurements with usual care while looking at clinical outcomes are observational or quasi-experimental (SOR B).
For example, a 2015 systematic review looking at associations between BP measurement type (office, home, and ambulatory) and patient mortality found 5 observational studies that showed that adding home or ambulatory BP information improved cardiovascular risk prediction models. Moreover, all-cause mortality was associated with home BP and ambulatory BP levels only and not with office BP levels.19 The number of participants in these 5 studies varied between 210 and 2051 with study duration between 2.4 and 12.3 years. Of note, every study had a distinct population, affecting the generalizability of the results.
Continue to: One quasi-experimental study...
One quasi-experimental study with 450 participants showed that home BP measurements were at least as good as ambulatory BP measurements at predicting end organ damage related to hypertension when organ damage was measured by cardiac echocardiography, detection of microalbuminuria, and carotid echocardiography.23 Similarly, a systematic review of 14 studies and 2485 participants comparing home, ambulatory, and office BP readings showed that home BP measurements’ association with left ventricular mass index is as good as that of ambulatory BP measurements, and superior to clinic BP readings.24
6. Does HBPM help improve medication adherence?
The jury is still out on this one (SOR B).
A 2006 systematic review of randomized controlled trials (RCTs) incorporating HBPM and evaluating medication adherence outcomes found that in 6 of the 11 studies identified, there was some improvement in medication adherence with HBPM.25 However, only 1 of the 6 studies in this review involved HBPM as the sole intervention; the remaining 5 studies employed additional adherence-enhancing strategies.
Another systematic review looking at HBPM vs usual care included 8 studies (3 of moderate quality and 5 of low quality) that measured medication adherence (using varying measures of adherence) of which only 3 studies showed some improvement in medication adherence with HBPM.18
7. Does HBPM reduce therapeutic inertia?
Yes (SOR B).
A meta-analysis of 15 studies showed that therapeutic inertia was less common with HBPM than with office BP monitoring alone; the relative risk for unchanged medication was 0.82 (95% CI, 0.68 to 0.99) with HBPM.26 However, 10 of the 15 studies were of low quality with a Jadad score ≤3.
8. Does HBPM, along with titration of treatment, improve BP outcomes?
Yes (SOR B).
Two RCTs that looked at self-monitoring of BP and self-titration of hypertensive medications showed significant reductions in BP levels.27,28 In a cluster RCT of home BP telemonitoring, in which the pharmacist adjusted antihypertensives based on transmitted BP measurements, hypertension control was significantly better in the intervention group than in the usual care group (57.2% vs 30%).29
Continue to: What are the recommended techniques for HBPM?
9. What are the recommended techniques for HBP
Patients should use a device that is validated, fully automated, and has an upper arm cuff (not a wrist monitor), according to a joint statement from the AHA, the American Society of Hypertension, and the Preventive Cardiovascular Nurses Association.17 (SOR C). (See validated BP monitor list at http://www.dableducational.org/sphygmomanometers/devices_2_sbpm.html.)
Patients should measure their BP in their nondominant arm after 5 minutes of rest with the arm at heart level, back supported, and feet flat on the ground. Patient technique and the accuracy of the home BP monitor should be checked annually. It is also recommended that patients check their BP 2 to 3 times every morning and evening. An average of 12 morning and evening measurements should be used for monitoring and treatment changes. An AHA informational sheet that shows how to measure BP properly can be found on their Web si
Several studies examining the accuracy of measuring BP over clothing did not find significant differences in BP measurements performed on a bare arm vs over a sleeve.30-33
10. What are the predictors of differences between home and office BP measurements?
Gender is one of the biggest predictors (SOR B).
A 2016 meta-analysis reported a total of 60 different hypothesized predictors of differences between home and clinic BP measurements (eg, gender, age, body mass index, systolic BP, diastolic BP). Masked hypertension was defined as a normal clinic BP reading and an elevated home BP reading. White coat hypertension was defined as an elevated clinic BP measurement with an acceptable home BP measurement. The researchers extracted odds ratios (ORs) for each study describing the association between patient characteristics and white coat or masked hypertension.34 Studies of masked hypertension diagnosed from HBPM showed male gender as the most significant predictor of home-clinic BP differences (OR=1.47, 95% CI, 1.18-1.75). In contrast, female gender was the only significant predictor of white coat hypertension (OR=3.38; 95% CI, 1.64-6.96) when comparing home BP with clinic BP measurements
Literature limitations and barriers to greater implementation
Most studies looking at HBPM outcomes have measured outcomes using ABPM or office BP measurements. The authors of studies using office BP as the outcome measure usually performed multiple BP measurements at often multiple office or clinic visits to calculate the true BP—a procedure that primary care practices rarely follow.35 Additionally, there are significant methodologic differences in HBPM and ABPM; home BP is measured at rest, while ambulatory BP is measured while the patient is mobile and functioning. There are insufficient prospective studies looking at HBPM effects on clinical and patient-oriented outcomes.
The evidence clearly supports using HBPM in the diagnosis of hypertension and suggests its benefit in hypertension management. However, there are significant barriers to incorporating HBPM into practice—barriers that are largely unaddressed in the literature.
For HBPM to be successful, patients need affordable validated home BP monitors covered by insurance that can translate home BP readings into usable information. Additional administrative and/or nursing assistance is required for patient education and support. Uploaded data need to be summarized in a way that is actionable and linked to the electronic health record.
Continue to: As the volume of patient-generated home date increases...
As the volume of patient-generated home data increases, there is a risk of information overload. Thus, meaningful summarization of the data is required to enable the physician, patient, and/or pharmacist to take prompt and effective action.
CORRESPONDENCE
Sonal J. Patil, MD, MSPH, Curtis W. and Ann H. Long Department of Family and Community Medicine, University of Missouri, MA306 Medical Sciences Building, DC032.00 Columbia, MO 65212; patilso@health.missouri.edu.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. David R. Mehr for his valuable expertise in editing and proofreading this manuscript.
National Health and Nutrition Examination Survey (NHANES) data from 2011 to 2014 revealed that 29% of adults in the United States have hypertension.1 Prevalence increases with age, so that 7% of adults ages 18 to 39 years, 32% of adults ages 40 to 59, and 65% of adults ages ≥60 years have the disease.1 This national survey data also showed that 53% of those given the diagnosis had uncontrolled hypertension, and that control of hypertension did not change significantly from 2009 to 2014.1
Elevated blood pressure (BP) has been the leading risk factor for death related to cardiovascular disease globally for the last 3 decades.2 Yet in 2 nationally representative samples, only 1 in 6 patients with documented BP ≥140/90 mm Hg received treatment intensification with new medication during primary care visits.3 Uncertainty about the representativeness of any single clinic BP measurement is a prominent reason for health care providers not to intensify therapy.4
Confirming the Dx outside the office. The 2015 US Preventive Services Task Force (USPSTF) guidelines on screening for hypertension state that, for most patients, a diagnosis of hypertension should be confirmed with out-of-office BP monitoring before initiating treatment.5 The USPSTF states that ambulatory BP monitoring (ABPM) is accurate for hypertension diagnosis and monitoring, and that home BP monitoring (HBPM) is an acceptable alternative, based on good quality evidence.
Access to ABPM, however, is often limited. In a 2015 survey of primary care clinics, only 25% of the 123 clinics that completed the questionnaire reported having access to it.6 Conversely, HBPM is widely available and acceptable to most patients. A recent NHANES survey showed that 43.5% of patients who were aware of their hypertension diagnosis engaged in HBPM.7
So what, exactly, should the role of HBPM be in the management of patients with hypertension? The evidence-based answers to the 10 questions that follow provide useful insights.
[polldaddy:10224678]
1. Can HBPM be used to confirm a Dx of hypertension?
Yes (Strength of recommendation [SOR] C).
In reviewing the diagnostic accuracy of various methods to confirm the diagnosis of hypertension, the USPSTF identified ABPM as the most accurate, followed by HBPM, with clinic BP measurements bringing up the rear.5 In adults ≥18 years of age, the USPSTF recommends obtaining BP measurements outside of the clinical setting for diagnostic confirmation before starting treatment unless the patient’s BP is ≥180/110 mm Hg, there is evidence of end-organ damage, or the patient has a diagnosis of secondary hypertension.5 The USPSTF recommends HBPM as an acceptable alternative to ABPM based on 6 studies including a total of 1253 participants.8 The percentage of patients with elevated office BP confirmed by HBPM to have hypertension was 45% to 84% across these 6 studies.
Sixteen studies from another systematic review evaluated the diagnostic accuracy of HBPM while using ABPM as a reference.9 This review found that HBPM had high specificity and negative predictive value, but low sensitivity and positive predictive value. There was moderate diagnostic agreement between HBPM and ABPM, with kappa statistic values of 0.37 to 0.73 across all studies.9
Continue to: In yet another study...
In yet another study, home BP and ambulatory BP measurements were identical when the same dual-mode device was used to measure both ambulatory and home BP.10
2. What are the diagnostic and treatment targets for home BP monitoring?
Treat patients if home BP is ≥130/80 mm Hg and categorize patients as normotensive if home BP is <125/76 mm Hg (SOR C). Monitor patients who are in between.
A 2017 joint statement from the American College of Cardiology/American Heart Association (ACC/AHA) Task Force states that the target BP for HBPM should be <130/80 mm Hg.11 The Joint National Commission (JNC) 8 issued BP goals of <140/90 mm Hg for adults <60 years of age and those with diabetes and/or chronic kidney disease, and a goal of <150/90 mm Hg for adults ≥60 years of age with no diabetes or chronic kidney disease,12 but much debate has recently surrounded these guidelines. JNC 8 does not provide a separate BP goal for HBPM.
Although based solely on evidence (and not patient-oriented outcomes), a home BP threshold of ≥135/85 mm Hg for the diagnosis and treatment of hypertension has been supported by the European Society of Hypertension consensus guidelines,13 results of a longitudinal study,14 meta-analyses of published studies, and a meta-analysis using individual subject data.15
Support for a home BP measurement of <125/76 mm Hg as normal is limited to a single cross-sectional study of 48 patients with 2 elevated office BP readings where the threshold of 125/76 mm Hg on home BP was shown to exclude 80% of patients diagnosed with hypertension by ambulatory readings.16 If home BP measurements are >125/76 mm Hgbut <135/85 mm Hg, 24-hour ambulatory BP monitoring is recommended to assess hypertension control.17
3. Does home BP monitoring improve hypertension control?
Yes, in the short term, but not in the long term (SOR C).
A meta-analysis of 13 comparative studies looking at HBPM alone vs usual care showed a small, but statistically significant, benefit of achieving target BP at 6 months with a relative risk ratio (RRR) of 1.3 (95% confidence interval [CI], 1.00-1.68; I2=77%).18 However, the pooled effects from 3 studies that measured the benefit of achieving a predefined BP target at the 12-month follow-up mark were not significant in this review (RRR=1.18; 95% CI, 0.95-1.46, I2=86%).18 The pooled effect from 19 studies from the same review showed that there was a statistically significant weighted mean difference of -3.9 mm Hg in systolic BP and a weighted mean difference of -2.4 mm Hg in diastolic BP at 6 months; however, the changes were no longer significant at the 12-month follow-up mark.18
Continue to: More than half of the studies included...
More than half of the studies included in the meta-analysis were of low quality, and none of the studies recruited patients based on differences in clinic BP and home BP patterns, but rather on controlled or uncontrolled hypertension status. The studies included in this meta-analysis measured final BP outcomes by measuring ambulatory BP or clinic BP.
Another systematic review of 19 studies and 7100 participants looking at how HBPM compared with ABPM as a measurement standard for BP control and patient outcomes found insufficient data to determine the benefit of using HBPM as a measurement standard for BP control.19
HBPM + added support. There was high-quality evidence from the meta-analysis that HBPM plus additional support vs usual care led to a reduction in BP and a higher proportion of patients achieving target BP.18 However, the additional support interventions in the studies were heterogeneous.
4. Should HBPM be used to detect a change in BP associated with medication alterations?
Yes (SOR B).
A 2008 meta-analysis20 and several other studies21,22 showed that HBPM has greater accuracy than office BP for identifying drug-induced BP changes. The 2008 meta-analysis looked at changes in office and home BP measurements produced by various antihypertensive drugs. In 7 studies that compared office BP measurements with home and ambulatory BP measurements, the 24-hour ambulatory BP measurements and home BP measurements showed less dramatic BP reductions with medications than clinic BP measurements.20 This meta-analysis included 30 studies with 6794 participants and showed that home BP readings fell 20% less than office BP readings; the difference was statistically significant. These findings suggest that treatment-attributable changes in home BP and clinic BP measurements are linearly related, with the treatment effect on home BP measurements being around 80% of the effect on clinic BP measurements.
5. Do home BP measurements correlate with clinical outcomes?
Yes, and better than office BP measurements do; however, most studies comparing home BP measurements with usual care while looking at clinical outcomes are observational or quasi-experimental (SOR B).
For example, a 2015 systematic review looking at associations between BP measurement type (office, home, and ambulatory) and patient mortality found 5 observational studies that showed that adding home or ambulatory BP information improved cardiovascular risk prediction models. Moreover, all-cause mortality was associated with home BP and ambulatory BP levels only and not with office BP levels.19 The number of participants in these 5 studies varied between 210 and 2051 with study duration between 2.4 and 12.3 years. Of note, every study had a distinct population, affecting the generalizability of the results.
Continue to: One quasi-experimental study...
One quasi-experimental study with 450 participants showed that home BP measurements were at least as good as ambulatory BP measurements at predicting end organ damage related to hypertension when organ damage was measured by cardiac echocardiography, detection of microalbuminuria, and carotid echocardiography.23 Similarly, a systematic review of 14 studies and 2485 participants comparing home, ambulatory, and office BP readings showed that home BP measurements’ association with left ventricular mass index is as good as that of ambulatory BP measurements, and superior to clinic BP readings.24
6. Does HBPM help improve medication adherence?
The jury is still out on this one (SOR B).
A 2006 systematic review of randomized controlled trials (RCTs) incorporating HBPM and evaluating medication adherence outcomes found that in 6 of the 11 studies identified, there was some improvement in medication adherence with HBPM.25 However, only 1 of the 6 studies in this review involved HBPM as the sole intervention; the remaining 5 studies employed additional adherence-enhancing strategies.
Another systematic review looking at HBPM vs usual care included 8 studies (3 of moderate quality and 5 of low quality) that measured medication adherence (using varying measures of adherence) of which only 3 studies showed some improvement in medication adherence with HBPM.18
7. Does HBPM reduce therapeutic inertia?
Yes (SOR B).
A meta-analysis of 15 studies showed that therapeutic inertia was less common with HBPM than with office BP monitoring alone; the relative risk for unchanged medication was 0.82 (95% CI, 0.68 to 0.99) with HBPM.26 However, 10 of the 15 studies were of low quality with a Jadad score ≤3.
8. Does HBPM, along with titration of treatment, improve BP outcomes?
Yes (SOR B).
Two RCTs that looked at self-monitoring of BP and self-titration of hypertensive medications showed significant reductions in BP levels.27,28 In a cluster RCT of home BP telemonitoring, in which the pharmacist adjusted antihypertensives based on transmitted BP measurements, hypertension control was significantly better in the intervention group than in the usual care group (57.2% vs 30%).29
Continue to: What are the recommended techniques for HBPM?
9. What are the recommended techniques for HBP
Patients should use a device that is validated, fully automated, and has an upper arm cuff (not a wrist monitor), according to a joint statement from the AHA, the American Society of Hypertension, and the Preventive Cardiovascular Nurses Association.17 (SOR C). (See validated BP monitor list at http://www.dableducational.org/sphygmomanometers/devices_2_sbpm.html.)
Patients should measure their BP in their nondominant arm after 5 minutes of rest with the arm at heart level, back supported, and feet flat on the ground. Patient technique and the accuracy of the home BP monitor should be checked annually. It is also recommended that patients check their BP 2 to 3 times every morning and evening. An average of 12 morning and evening measurements should be used for monitoring and treatment changes. An AHA informational sheet that shows how to measure BP properly can be found on their Web si
Several studies examining the accuracy of measuring BP over clothing did not find significant differences in BP measurements performed on a bare arm vs over a sleeve.30-33
10. What are the predictors of differences between home and office BP measurements?
Gender is one of the biggest predictors (SOR B).
A 2016 meta-analysis reported a total of 60 different hypothesized predictors of differences between home and clinic BP measurements (eg, gender, age, body mass index, systolic BP, diastolic BP). Masked hypertension was defined as a normal clinic BP reading and an elevated home BP reading. White coat hypertension was defined as an elevated clinic BP measurement with an acceptable home BP measurement. The researchers extracted odds ratios (ORs) for each study describing the association between patient characteristics and white coat or masked hypertension.34 Studies of masked hypertension diagnosed from HBPM showed male gender as the most significant predictor of home-clinic BP differences (OR=1.47, 95% CI, 1.18-1.75). In contrast, female gender was the only significant predictor of white coat hypertension (OR=3.38; 95% CI, 1.64-6.96) when comparing home BP with clinic BP measurements
Literature limitations and barriers to greater implementation
Most studies looking at HBPM outcomes have measured outcomes using ABPM or office BP measurements. The authors of studies using office BP as the outcome measure usually performed multiple BP measurements at often multiple office or clinic visits to calculate the true BP—a procedure that primary care practices rarely follow.35 Additionally, there are significant methodologic differences in HBPM and ABPM; home BP is measured at rest, while ambulatory BP is measured while the patient is mobile and functioning. There are insufficient prospective studies looking at HBPM effects on clinical and patient-oriented outcomes.
The evidence clearly supports using HBPM in the diagnosis of hypertension and suggests its benefit in hypertension management. However, there are significant barriers to incorporating HBPM into practice—barriers that are largely unaddressed in the literature.
For HBPM to be successful, patients need affordable validated home BP monitors covered by insurance that can translate home BP readings into usable information. Additional administrative and/or nursing assistance is required for patient education and support. Uploaded data need to be summarized in a way that is actionable and linked to the electronic health record.
Continue to: As the volume of patient-generated home date increases...
As the volume of patient-generated home data increases, there is a risk of information overload. Thus, meaningful summarization of the data is required to enable the physician, patient, and/or pharmacist to take prompt and effective action.
CORRESPONDENCE
Sonal J. Patil, MD, MSPH, Curtis W. and Ann H. Long Department of Family and Community Medicine, University of Missouri, MA306 Medical Sciences Building, DC032.00 Columbia, MO 65212; patilso@health.missouri.edu.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. David R. Mehr for his valuable expertise in editing and proofreading this manuscript.
1. Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011-2014. NCHS Data Brief. 2015;(220):1-8.
2. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardio-metabolic risk factors between 1980 and 2010: comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634-647.
3. Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:e004188.
4. Kerr EA, Zikmund-Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717-727.
5. U.S. Preventive Services Task Force. Final recommendation statement: High blood pressure in adults: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed July 19, 2017.
6. Woolsey S, Brown B, Ralls B, et al. Diagnosing hypertension in primary care clinics according to current guidelines. J Am Board Fam Med. 2017;30:170-177.
7. Ostchega Y, Zhang G, Kit BK, et al. Factors associated with home blood pressure monitoring among US adults: National Health and Nutrition Examination Survey, 2011-2014. Am J Hypertens. 2017;30:1126-1132.
8. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
9. Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011;24:123-134.
10. Stergiou GS, Tzamouranis D, Nasothimiou EG, et al. Are there really differences between home and daytime ambulatory blood pressure? Comparison using a novel dual-mode ambulatory and home monitor. J Hum Hypertens. 2009;24:207-212.
11. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:e13-e115.
12. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. Erratum in JAMA. 2014;311:1809.
13. O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821-848.
14. Tsunoda S, Kawano Y, Horio T, et al. Relationship between home blood pressure and longitudinal changes in target organ damage in treated hypertensive patients. Hypertens Res. 2002;25:167-173.
15. Staessen JA, Thijs L. Development of diagnostic thresholds for automated self-measurement of blood pressure in adults. First International Consensus Conference on Blood Pressure Self-Measurement. Blood Press Monit. 2000;5:101-109.
16. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17(11 Pt 1):1017-1022.
17. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. J Cardiovasc Nurs. 2008;23:299-323.
18. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185-194.
19. Breaux-Shropshire TL, Judd E, Vucovich LA, et al. Does home blood pressure monitoring improve patient outcomes? A systematic review comparing home and ambulatory blood pressure monitoring on blood pressure control and patient outcomes. Integr Blood Press Control. 2015;8:43-49.
20. Ishikawa J, Carroll DJ, Kuruvilla S, et al. Changes in home versus clinic blood pressure with antihypertensive treatments: a meta-analysis. Hypertension. 2008;52:856-864.
21. Imai Y, Ohkubo T, Hozawa A, et al. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19:179-185.
22. Vaur L, Dubroca I, Dutrey-Dupagne C, et al. Superiority of home blood pressure measurements over office measurements for testing antihypertensive drugs. Blood Press Monit. 1998;3:107-114.
23. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
24. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299.
25. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich). 2006;8:174-180.
26. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38.
27. McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799-808.
28. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163-172.
29. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: The HyperLink Cluster Randomized Trial. JAMA. 2013;310:46-56.
30. Ma G, Sabin N, Dawes M. A comparison of blood pressure measurement over a sleeved arm versus a bare arm. CMAJ. 2008;178:585-589.
31. Kahan E, Yaphe J, Knaani-Levinz H, et al. Comparison of blood pressure measurements on the bare arm, below a rolled-up sleeve, or over a sleeve. Fam Pract. 2003;20:730-732.
32. Liebl M, Holzgreve H, Schulz M, et al. The effect of clothes on sphygmomanometric and oscillometric blood pressure measurement. Blood Press. 2004;13:279-282.
33. Holleman DR Jr., Westman EC, McCrory DC, et al. The effect of sleeved arms on oscillometric blood pressure measurement. J Gen Intern Med. 1993;8:325-326.
34. Sheppard JP, Fletcher B, Gill P, et al. Predictors of the home-clinic blood pressure difference: a systematic review and meta-analysis. Am J Hypertens. 2016;29:614-625.
35. Stergiou GS, Skeva II, Baibas NM, et al. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18:1745-1751.
1. Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011-2014. NCHS Data Brief. 2015;(220):1-8.
2. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardio-metabolic risk factors between 1980 and 2010: comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634-647.
3. Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:e004188.
4. Kerr EA, Zikmund-Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717-727.
5. U.S. Preventive Services Task Force. Final recommendation statement: High blood pressure in adults: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed July 19, 2017.
6. Woolsey S, Brown B, Ralls B, et al. Diagnosing hypertension in primary care clinics according to current guidelines. J Am Board Fam Med. 2017;30:170-177.
7. Ostchega Y, Zhang G, Kit BK, et al. Factors associated with home blood pressure monitoring among US adults: National Health and Nutrition Examination Survey, 2011-2014. Am J Hypertens. 2017;30:1126-1132.
8. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
9. Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011;24:123-134.
10. Stergiou GS, Tzamouranis D, Nasothimiou EG, et al. Are there really differences between home and daytime ambulatory blood pressure? Comparison using a novel dual-mode ambulatory and home monitor. J Hum Hypertens. 2009;24:207-212.
11. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:e13-e115.
12. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. Erratum in JAMA. 2014;311:1809.
13. O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821-848.
14. Tsunoda S, Kawano Y, Horio T, et al. Relationship between home blood pressure and longitudinal changes in target organ damage in treated hypertensive patients. Hypertens Res. 2002;25:167-173.
15. Staessen JA, Thijs L. Development of diagnostic thresholds for automated self-measurement of blood pressure in adults. First International Consensus Conference on Blood Pressure Self-Measurement. Blood Press Monit. 2000;5:101-109.
16. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17(11 Pt 1):1017-1022.
17. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. J Cardiovasc Nurs. 2008;23:299-323.
18. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185-194.
19. Breaux-Shropshire TL, Judd E, Vucovich LA, et al. Does home blood pressure monitoring improve patient outcomes? A systematic review comparing home and ambulatory blood pressure monitoring on blood pressure control and patient outcomes. Integr Blood Press Control. 2015;8:43-49.
20. Ishikawa J, Carroll DJ, Kuruvilla S, et al. Changes in home versus clinic blood pressure with antihypertensive treatments: a meta-analysis. Hypertension. 2008;52:856-864.
21. Imai Y, Ohkubo T, Hozawa A, et al. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19:179-185.
22. Vaur L, Dubroca I, Dutrey-Dupagne C, et al. Superiority of home blood pressure measurements over office measurements for testing antihypertensive drugs. Blood Press Monit. 1998;3:107-114.
23. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
24. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299.
25. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich). 2006;8:174-180.
26. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38.
27. McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799-808.
28. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163-172.
29. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: The HyperLink Cluster Randomized Trial. JAMA. 2013;310:46-56.
30. Ma G, Sabin N, Dawes M. A comparison of blood pressure measurement over a sleeved arm versus a bare arm. CMAJ. 2008;178:585-589.
31. Kahan E, Yaphe J, Knaani-Levinz H, et al. Comparison of blood pressure measurements on the bare arm, below a rolled-up sleeve, or over a sleeve. Fam Pract. 2003;20:730-732.
32. Liebl M, Holzgreve H, Schulz M, et al. The effect of clothes on sphygmomanometric and oscillometric blood pressure measurement. Blood Press. 2004;13:279-282.
33. Holleman DR Jr., Westman EC, McCrory DC, et al. The effect of sleeved arms on oscillometric blood pressure measurement. J Gen Intern Med. 1993;8:325-326.
34. Sheppard JP, Fletcher B, Gill P, et al. Predictors of the home-clinic blood pressure difference: a systematic review and meta-analysis. Am J Hypertens. 2016;29:614-625.
35. Stergiou GS, Skeva II, Baibas NM, et al. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18:1745-1751.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series