User login
Clinical Challenges - May 2016: Pancreaticobiliary maljunction with bifid pancreatic ducts presenting as recurrent pancreatitis and concurrent gallbladder adenocarcinoma
What's Your Diagnosis?
The diagnosis
Figure A shows marked intrahepatic and extrahepatic biliary ductal dilation and an irregular enhancing mass along the lateral wall of the gallbladder (long arrow). Figure B shows an abnormal pancreaticobiliary junction, with the common bile duct inserting into a distal pancreatic duct to form a cystically dilated common channel (arrowhead), as well as a bifid main pancreatic duct (long arrow). Figure C shows a bifid pancreatic duct and no evidence of a pancreatic mass. Other endoscopic ultrasound images visualized an irregular gallbladder mass. Figure D shows an irregular mass in the gallbladder wall, with final pathology revealing an invasive, well-differentiated adenocarcinoma of the gallbladder (long arrow) with negative margins and no evidence of lymph node involvement (T1N0Mx). The final diagnosis was pancreaticobiliary maljunction (PBM) with bifid pancreatic ducts presenting as recurrent pancreatitis and concurrent gallbladder adenocarcinoma.
It is well established that PBM, an anomalous junction of the pancreaticobiliary ductal system, is frequently associated with carcinomas of the biliary tract. First described in 1916 by Kozumi and Kodama, PBM is a rare congenital malformation most prevalent in Asia that is defined as an anomalous junction of the pancreatic and biliary ducts located outside of the duodenal wall.1PBM often manifests clinically as intermittent abdominal pain, obstructive jaundice, and/or acute pancreatitis, although patients may be asymptomatic. The most concerning problem, however, is the close relationship of biliary tract carcinogenesis to PBM, with gallbladder carcinoma and bile duct cancers arising in 14.8% and 4.9% of patients with PBM, respectively.2 The anomalous junction is thought to preclude normal sphincter of Oddi function, thus facilitating the reciprocal reflux of bile and pancreatic juice and ultimately leading to biliary carcinogenesis. Tumor markers, such as CA 19-9 and carcinoembryonic antigen, may be of some diagnostic value in PBM and biliary tract neoplasms, although they lack sensitivity and specificity because of significant overlap with benign disease, such as pancreatitis. This particular case had the added novelty of a bifid pancreatic duct. The clinical significance of a bifid pancreatic duct is unclear, and no relationship has been demonstrated between this ductal anomaly and pancreaticobiliary disease. In this case, a pancreaticoduodenectomy with en bloc resection of the gallbladder was performed to resect the gallbladder mass with clear margins and eliminate the risk for further biliary tract carcinogenesis while simultaneously excising the anomalous junction thought to be causing the recurrent pancreatitis.
References
1. Todani T., Arima E., Eto T., et al. Diagnostic criteria of pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1994;1:219-21.
2. Funabiki T., Matsubara T., Miyakawa S., et al. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009;39:149-69.
The diagnosis
Figure A shows marked intrahepatic and extrahepatic biliary ductal dilation and an irregular enhancing mass along the lateral wall of the gallbladder (long arrow). Figure B shows an abnormal pancreaticobiliary junction, with the common bile duct inserting into a distal pancreatic duct to form a cystically dilated common channel (arrowhead), as well as a bifid main pancreatic duct (long arrow). Figure C shows a bifid pancreatic duct and no evidence of a pancreatic mass. Other endoscopic ultrasound images visualized an irregular gallbladder mass. Figure D shows an irregular mass in the gallbladder wall, with final pathology revealing an invasive, well-differentiated adenocarcinoma of the gallbladder (long arrow) with negative margins and no evidence of lymph node involvement (T1N0Mx). The final diagnosis was pancreaticobiliary maljunction (PBM) with bifid pancreatic ducts presenting as recurrent pancreatitis and concurrent gallbladder adenocarcinoma.
It is well established that PBM, an anomalous junction of the pancreaticobiliary ductal system, is frequently associated with carcinomas of the biliary tract. First described in 1916 by Kozumi and Kodama, PBM is a rare congenital malformation most prevalent in Asia that is defined as an anomalous junction of the pancreatic and biliary ducts located outside of the duodenal wall.1PBM often manifests clinically as intermittent abdominal pain, obstructive jaundice, and/or acute pancreatitis, although patients may be asymptomatic. The most concerning problem, however, is the close relationship of biliary tract carcinogenesis to PBM, with gallbladder carcinoma and bile duct cancers arising in 14.8% and 4.9% of patients with PBM, respectively.2 The anomalous junction is thought to preclude normal sphincter of Oddi function, thus facilitating the reciprocal reflux of bile and pancreatic juice and ultimately leading to biliary carcinogenesis. Tumor markers, such as CA 19-9 and carcinoembryonic antigen, may be of some diagnostic value in PBM and biliary tract neoplasms, although they lack sensitivity and specificity because of significant overlap with benign disease, such as pancreatitis. This particular case had the added novelty of a bifid pancreatic duct. The clinical significance of a bifid pancreatic duct is unclear, and no relationship has been demonstrated between this ductal anomaly and pancreaticobiliary disease. In this case, a pancreaticoduodenectomy with en bloc resection of the gallbladder was performed to resect the gallbladder mass with clear margins and eliminate the risk for further biliary tract carcinogenesis while simultaneously excising the anomalous junction thought to be causing the recurrent pancreatitis.
References
1. Todani T., Arima E., Eto T., et al. Diagnostic criteria of pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1994;1:219-21.
2. Funabiki T., Matsubara T., Miyakawa S., et al. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009;39:149-69.
The diagnosis
Figure A shows marked intrahepatic and extrahepatic biliary ductal dilation and an irregular enhancing mass along the lateral wall of the gallbladder (long arrow). Figure B shows an abnormal pancreaticobiliary junction, with the common bile duct inserting into a distal pancreatic duct to form a cystically dilated common channel (arrowhead), as well as a bifid main pancreatic duct (long arrow). Figure C shows a bifid pancreatic duct and no evidence of a pancreatic mass. Other endoscopic ultrasound images visualized an irregular gallbladder mass. Figure D shows an irregular mass in the gallbladder wall, with final pathology revealing an invasive, well-differentiated adenocarcinoma of the gallbladder (long arrow) with negative margins and no evidence of lymph node involvement (T1N0Mx). The final diagnosis was pancreaticobiliary maljunction (PBM) with bifid pancreatic ducts presenting as recurrent pancreatitis and concurrent gallbladder adenocarcinoma.
It is well established that PBM, an anomalous junction of the pancreaticobiliary ductal system, is frequently associated with carcinomas of the biliary tract. First described in 1916 by Kozumi and Kodama, PBM is a rare congenital malformation most prevalent in Asia that is defined as an anomalous junction of the pancreatic and biliary ducts located outside of the duodenal wall.1PBM often manifests clinically as intermittent abdominal pain, obstructive jaundice, and/or acute pancreatitis, although patients may be asymptomatic. The most concerning problem, however, is the close relationship of biliary tract carcinogenesis to PBM, with gallbladder carcinoma and bile duct cancers arising in 14.8% and 4.9% of patients with PBM, respectively.2 The anomalous junction is thought to preclude normal sphincter of Oddi function, thus facilitating the reciprocal reflux of bile and pancreatic juice and ultimately leading to biliary carcinogenesis. Tumor markers, such as CA 19-9 and carcinoembryonic antigen, may be of some diagnostic value in PBM and biliary tract neoplasms, although they lack sensitivity and specificity because of significant overlap with benign disease, such as pancreatitis. This particular case had the added novelty of a bifid pancreatic duct. The clinical significance of a bifid pancreatic duct is unclear, and no relationship has been demonstrated between this ductal anomaly and pancreaticobiliary disease. In this case, a pancreaticoduodenectomy with en bloc resection of the gallbladder was performed to resect the gallbladder mass with clear margins and eliminate the risk for further biliary tract carcinogenesis while simultaneously excising the anomalous junction thought to be causing the recurrent pancreatitis.
References
1. Todani T., Arima E., Eto T., et al. Diagnostic criteria of pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1994;1:219-21.
2. Funabiki T., Matsubara T., Miyakawa S., et al. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009;39:149-69.
What's Your Diagnosis?
What's Your Diagnosis?
By Dr. Katherine Albutt, Dr. Laurence Bailen, and Dr. Carlos Fernandez-del Castillo. Published previously in Gastroenterology (2012;143:896, 1121-2).
A 63-year-old African American woman with a history of recurrent pancreatitis was admitted with severe right upper quadrant pain radiating to the back. Her medical history was notable for three prior episodes of pancreatitis that required hospitalization in 2000, 2006, and 2007.
On the day of admission, physical examination revealed a soft abdomen that was tender to palpation in the right upper quadrant with a positive Murphy’s sign. Laboratory data were notable for an amylase level of 1,203 U/L and a lipase level of 2,091 U/L in the setting of normal liver function tests (LFTs) and a normal leukocyte count. The cancer antigen (CA) 19-9 level at this time was 299 U/mL.
The patient then underwent abdominal sonography and computed tomography (Figure A). Magnetic resonance cholangiopancreatography was also performed (Figure B). Because of rising LFTs, endoscopic retrograde cholangiopancreatography was performed, and a biliary stent was placed. An endoscopic ultrasound was performed at the time of endoscopic retrograde cholangiopancreatography (Figure C).
Once the acute pancreatitis resolved and the patient was tolerating a regular diet, she was discharged home. At this time, the level of CA 19-9 was 35 U/mL. She was subsequently taken to the operating room for a planned pancreaticoduodenectomy, cholecystectomy, and dissection of periportal lymph nodes. The Whipple specimen was removed en bloc with the gallbladder (Figure D).
The patient’s postoperative course was uneventful, and she was discharged home on postoperative day 7. Postoperative laboratory values were notable for a CA 19-9 of 23 U/mL and carcinoembryonic antigen of 3.3 ng/mL. What is the diagnosis?
Register for Pediatric Hospital Medicine 2016
PHM16 will provide in-depth review and challenge participants in various areas, including clinical practice, medical education, quality improvement, and professional development. Time will also be dedicated to networking and meeting with leaders in the field.
Register, book your hotel, and see the full course schedule at www.phmmeeting.org.
Brett Radler is SHM’s communications coordinator.
PHM16 will provide in-depth review and challenge participants in various areas, including clinical practice, medical education, quality improvement, and professional development. Time will also be dedicated to networking and meeting with leaders in the field.
Register, book your hotel, and see the full course schedule at www.phmmeeting.org.
Brett Radler is SHM’s communications coordinator.
PHM16 will provide in-depth review and challenge participants in various areas, including clinical practice, medical education, quality improvement, and professional development. Time will also be dedicated to networking and meeting with leaders in the field.
Register, book your hotel, and see the full course schedule at www.phmmeeting.org.
Brett Radler is SHM’s communications coordinator.
New SHM Members – June 2016
B. Abdalsm, MD, MPH, Alabama
A. Aboutalib, Alabama
D. Adams, MD, Arkansas
A. Afzal, MD, FACP, Arizona
J. Aheam, MD, California
S. Ahluwalia, MBBS, California
A. Alhusseini, MD, FAACP, MBchB, California
L. Anderson, MD, California
K. Arunachalam, MD, California
B. Asalone, California
L. Atkins, MD, California
T. Aultman, MD, California
T. Ayangade, MD, California
A. Azizi, MD, California
F. Azizi, MD, California
S. Balu, MD, California
K. Basra, APRN, FNP, California
N. Bassi, California
C. Batchelor, MD, California
K. Beinlich, MD, California
S. Bhat, MD, MBBS, California
H. Bilal, MBBS, California
G. Bismack, MD, California
M. Bokhari, MD, California
M. Brandenbug, MD, Colorado
H. Briggs, MD, PhD, Colorado
E. Burgh, MD, Colorado
M. Cabrera, BC, Delaware
J. Camden, BA, Delaware
P. Chandra Mohan, MD, Delaware
D. Chau, MD, Delaware
M. Chen, Florida
V. Chennamaneni, Florida
L. Cler, Florida
D. Cooks, Florida
S. Crenshaw, Florida
K. Cunningham, MD, FACP, Florida
V. De Guzman, APRN, MSN, NP, Florida
M. Del Rosario, MD, Florida
D. DeVere, MD, Florida
S. Dharmapuri, Florida
P. Dodson, MD, Florida
A. Domaoal, Georgia
J. Duncan, MD, Georgia
B. Dyck, BSC, MD, PhD, Georgia
J. Dzundza, MD, Georgia
A. Ellis, FNP, Georgia
R. Erickson, Idaho
A. Faraj, Idaho
S. Fernandez, MD, Illinois
G. Ferrari, MD, Illinois
W. Folad, MD, Illinois
L. Fowler, ACNP, APRN, MBA, Illinois
J. Golderberg, MD, Illinois
G. Goldman, MD, Illinois
L. Gonzales, MD, Indiana
A. Gonzalez, Kansas
W. Griffo, MD, Kansas
R. Guzman, Kansas
L. Guzman Vinasco, MD, Kansas
K. Hageman, DO, Kentucky
M. Haggerty, PA-C, Kentucky
B. Hammond, Louisiana
G. Harris, MD, Louisiana
J. Hasan-Jones, RN, FACHE, Louisiana
J. Herring, Louisiana
L. Hsu, MD, Massachusetts
A. C. Hunag, DO, Massachusetts
M. Huq, Massachusetts
M. Jandrin, PA-C, Massachusetts
C. Janish, MD, Maryland
J. Jarin, MD, Maine
A. Jenkins, Maine
S. Jindal, MD, Michigan
M. Johl, Michigan
T. John, Michigan
N. Kapadia, MD, Michigan
L. Katona, Michigan
K. Kaye, Michigan
M. Keating, Michigan
L. Keeton, MD, Michigan
L. Kendall, Michigan
M. Kerlin, Michigan
A. Kia, MD, Minnesota
R. Klett, Minnesota
L. Knapp, DO, Minnesota
K. Knox, Missouri
M. Kraynak, MD, Missouri
P. Kuppireddy, MBBS, Missouri
W. Landrum, MD, Missouri
C. Larion, ACNP, Missouri
E. Latcheva, MD, Mississippi
D. Leforce, North Carolina
V. Leigh, DO, North Carolina
C. Leon, North Carolina
T. Li, MD, North Dakota
X. Li, MD, Nebraska
Y. Li, New Hampshire
J. Liu, New Hampshire
L. Lu, DO, New Jersey
S. Mathapathi, New Jersey
L. McGann, New Jersey
S. Melkaveri, MD, Nevada
R. Mercado Garcia, New York
S. Merry, MD, New York
P. Meyer, DO, New York
J. Mikulca, PharmD, New York
Z. Moyenda, MD, MBA, New York
K. Murphy, DO, MPH, New York
J. Musenze, New York
P. Mutungi, New York
G. Nanna, USA, New York
I. Nasir, New York
U. Nazario-Vidah, MD, New York
D. Nguyen, New York
C. Ojha, MBBS, New York
K. Olson, MD, Ohio
V. Paulson, MD, Ohio
R. Pearson, DO, PhD, Ohio
A. Peel, MD, Ohio
S. Pettis, PA-C, Ohio
E. Picloglou, MD, Ohio
H. Pokhrel, MD, Ohio
H. Bush, Oklahoma
R. Porter, PA, Oklahoma
P. Prabhakar, Oklahoma
U. Qamar, Oklahoma
R. Quansah, MD, Oklahoma
M. Rahman, Oklahoma
R. Rajeshwar, Oregon
E. Randal, Oregon
A. Ray, Oregon
V. Reddy, Oregon
J. Reed, MD, RN, Oregon
R. Regidor, Oregon
A. Reitsma-Mathias, MD, Oregon
R. Reyes, MD, Oregon
T. Richardson, NP, Oregon
T. Ringer, Oregon
L. Rivera-Crespo, Pennsylvania
T. Rothwell, PA, Pennsylvania
E. Sacolick, MD, Pennsylvania
E. Saluke, MD, Pennsylvania
M. Santinelli, NP, Pennsylvania
M. Sapon-Amoah, FNP, Pennsylvania
D. Scarine, NP, Pennsylvania
K. Seger, Pennsylvania
A. Shah, Pennsylvania
K. Shah, MD, Pennsylvania
S. Shah, MD, Pennsylvania
G. Sharma, MD, Pennsylvania
K. Shaukat, MD, Pennsylvania
E. Sheindler, Rhode Island
D. Sheps, South Carolina
J. Shipe-Spotloe, South Carolina
S. Sim, South Carolina
M. Simon, MD, MMM, CPE, South Carolina
A. Singh, South Carolina
S. Singh-Patel, PO, South Dakota
M. Snyder, South Dakota
A. Srikanth, MBBS, Tennessee
B. Staats, Tennessee
C. Standley, Texas
R. Stanhiser, Texas
M. Stevens, Texas
K. Stuart, Texas
A. Summers, Texas
E. Taylo, Texas
L. Taylor, PA-C, Texas
L. Theaker, Texas
M. Thieman, Texas
J. Tong, Texas
N. Trivedi, MD, Texas
L. Tuazon, MD, FACP, Texas
S. Tummalapalli, Texas
A. Ufferman, MD, Virginia
R. Urrea, MD, Virginia
N. Van Groningen, Virginia
D. Vaughn, MD, Vermont
R. Vento, MD, MPH, Washington
Y. Villaran, MD, Washington
L. Viscome, DO, Washington
K. Vo, Washington
H. Vu, MD, Washington
T. Washko, MD, Washington
T. Waters, DO, Wisconsin
L. Weisberger, USA, Wisconsin
A. Whitehead, Wisconsin
A. Workman, West Virginia
F. Yasin, MD, West Virginia
A. Yoon, MD, West Virginia
M. Yu, West Virginia
A. Yuen, DO, West Virginia
K. Zwieg, West Virginia
B. Abdalsm, MD, MPH, Alabama
A. Aboutalib, Alabama
D. Adams, MD, Arkansas
A. Afzal, MD, FACP, Arizona
J. Aheam, MD, California
S. Ahluwalia, MBBS, California
A. Alhusseini, MD, FAACP, MBchB, California
L. Anderson, MD, California
K. Arunachalam, MD, California
B. Asalone, California
L. Atkins, MD, California
T. Aultman, MD, California
T. Ayangade, MD, California
A. Azizi, MD, California
F. Azizi, MD, California
S. Balu, MD, California
K. Basra, APRN, FNP, California
N. Bassi, California
C. Batchelor, MD, California
K. Beinlich, MD, California
S. Bhat, MD, MBBS, California
H. Bilal, MBBS, California
G. Bismack, MD, California
M. Bokhari, MD, California
M. Brandenbug, MD, Colorado
H. Briggs, MD, PhD, Colorado
E. Burgh, MD, Colorado
M. Cabrera, BC, Delaware
J. Camden, BA, Delaware
P. Chandra Mohan, MD, Delaware
D. Chau, MD, Delaware
M. Chen, Florida
V. Chennamaneni, Florida
L. Cler, Florida
D. Cooks, Florida
S. Crenshaw, Florida
K. Cunningham, MD, FACP, Florida
V. De Guzman, APRN, MSN, NP, Florida
M. Del Rosario, MD, Florida
D. DeVere, MD, Florida
S. Dharmapuri, Florida
P. Dodson, MD, Florida
A. Domaoal, Georgia
J. Duncan, MD, Georgia
B. Dyck, BSC, MD, PhD, Georgia
J. Dzundza, MD, Georgia
A. Ellis, FNP, Georgia
R. Erickson, Idaho
A. Faraj, Idaho
S. Fernandez, MD, Illinois
G. Ferrari, MD, Illinois
W. Folad, MD, Illinois
L. Fowler, ACNP, APRN, MBA, Illinois
J. Golderberg, MD, Illinois
G. Goldman, MD, Illinois
L. Gonzales, MD, Indiana
A. Gonzalez, Kansas
W. Griffo, MD, Kansas
R. Guzman, Kansas
L. Guzman Vinasco, MD, Kansas
K. Hageman, DO, Kentucky
M. Haggerty, PA-C, Kentucky
B. Hammond, Louisiana
G. Harris, MD, Louisiana
J. Hasan-Jones, RN, FACHE, Louisiana
J. Herring, Louisiana
L. Hsu, MD, Massachusetts
A. C. Hunag, DO, Massachusetts
M. Huq, Massachusetts
M. Jandrin, PA-C, Massachusetts
C. Janish, MD, Maryland
J. Jarin, MD, Maine
A. Jenkins, Maine
S. Jindal, MD, Michigan
M. Johl, Michigan
T. John, Michigan
N. Kapadia, MD, Michigan
L. Katona, Michigan
K. Kaye, Michigan
M. Keating, Michigan
L. Keeton, MD, Michigan
L. Kendall, Michigan
M. Kerlin, Michigan
A. Kia, MD, Minnesota
R. Klett, Minnesota
L. Knapp, DO, Minnesota
K. Knox, Missouri
M. Kraynak, MD, Missouri
P. Kuppireddy, MBBS, Missouri
W. Landrum, MD, Missouri
C. Larion, ACNP, Missouri
E. Latcheva, MD, Mississippi
D. Leforce, North Carolina
V. Leigh, DO, North Carolina
C. Leon, North Carolina
T. Li, MD, North Dakota
X. Li, MD, Nebraska
Y. Li, New Hampshire
J. Liu, New Hampshire
L. Lu, DO, New Jersey
S. Mathapathi, New Jersey
L. McGann, New Jersey
S. Melkaveri, MD, Nevada
R. Mercado Garcia, New York
S. Merry, MD, New York
P. Meyer, DO, New York
J. Mikulca, PharmD, New York
Z. Moyenda, MD, MBA, New York
K. Murphy, DO, MPH, New York
J. Musenze, New York
P. Mutungi, New York
G. Nanna, USA, New York
I. Nasir, New York
U. Nazario-Vidah, MD, New York
D. Nguyen, New York
C. Ojha, MBBS, New York
K. Olson, MD, Ohio
V. Paulson, MD, Ohio
R. Pearson, DO, PhD, Ohio
A. Peel, MD, Ohio
S. Pettis, PA-C, Ohio
E. Picloglou, MD, Ohio
H. Pokhrel, MD, Ohio
H. Bush, Oklahoma
R. Porter, PA, Oklahoma
P. Prabhakar, Oklahoma
U. Qamar, Oklahoma
R. Quansah, MD, Oklahoma
M. Rahman, Oklahoma
R. Rajeshwar, Oregon
E. Randal, Oregon
A. Ray, Oregon
V. Reddy, Oregon
J. Reed, MD, RN, Oregon
R. Regidor, Oregon
A. Reitsma-Mathias, MD, Oregon
R. Reyes, MD, Oregon
T. Richardson, NP, Oregon
T. Ringer, Oregon
L. Rivera-Crespo, Pennsylvania
T. Rothwell, PA, Pennsylvania
E. Sacolick, MD, Pennsylvania
E. Saluke, MD, Pennsylvania
M. Santinelli, NP, Pennsylvania
M. Sapon-Amoah, FNP, Pennsylvania
D. Scarine, NP, Pennsylvania
K. Seger, Pennsylvania
A. Shah, Pennsylvania
K. Shah, MD, Pennsylvania
S. Shah, MD, Pennsylvania
G. Sharma, MD, Pennsylvania
K. Shaukat, MD, Pennsylvania
E. Sheindler, Rhode Island
D. Sheps, South Carolina
J. Shipe-Spotloe, South Carolina
S. Sim, South Carolina
M. Simon, MD, MMM, CPE, South Carolina
A. Singh, South Carolina
S. Singh-Patel, PO, South Dakota
M. Snyder, South Dakota
A. Srikanth, MBBS, Tennessee
B. Staats, Tennessee
C. Standley, Texas
R. Stanhiser, Texas
M. Stevens, Texas
K. Stuart, Texas
A. Summers, Texas
E. Taylo, Texas
L. Taylor, PA-C, Texas
L. Theaker, Texas
M. Thieman, Texas
J. Tong, Texas
N. Trivedi, MD, Texas
L. Tuazon, MD, FACP, Texas
S. Tummalapalli, Texas
A. Ufferman, MD, Virginia
R. Urrea, MD, Virginia
N. Van Groningen, Virginia
D. Vaughn, MD, Vermont
R. Vento, MD, MPH, Washington
Y. Villaran, MD, Washington
L. Viscome, DO, Washington
K. Vo, Washington
H. Vu, MD, Washington
T. Washko, MD, Washington
T. Waters, DO, Wisconsin
L. Weisberger, USA, Wisconsin
A. Whitehead, Wisconsin
A. Workman, West Virginia
F. Yasin, MD, West Virginia
A. Yoon, MD, West Virginia
M. Yu, West Virginia
A. Yuen, DO, West Virginia
K. Zwieg, West Virginia
B. Abdalsm, MD, MPH, Alabama
A. Aboutalib, Alabama
D. Adams, MD, Arkansas
A. Afzal, MD, FACP, Arizona
J. Aheam, MD, California
S. Ahluwalia, MBBS, California
A. Alhusseini, MD, FAACP, MBchB, California
L. Anderson, MD, California
K. Arunachalam, MD, California
B. Asalone, California
L. Atkins, MD, California
T. Aultman, MD, California
T. Ayangade, MD, California
A. Azizi, MD, California
F. Azizi, MD, California
S. Balu, MD, California
K. Basra, APRN, FNP, California
N. Bassi, California
C. Batchelor, MD, California
K. Beinlich, MD, California
S. Bhat, MD, MBBS, California
H. Bilal, MBBS, California
G. Bismack, MD, California
M. Bokhari, MD, California
M. Brandenbug, MD, Colorado
H. Briggs, MD, PhD, Colorado
E. Burgh, MD, Colorado
M. Cabrera, BC, Delaware
J. Camden, BA, Delaware
P. Chandra Mohan, MD, Delaware
D. Chau, MD, Delaware
M. Chen, Florida
V. Chennamaneni, Florida
L. Cler, Florida
D. Cooks, Florida
S. Crenshaw, Florida
K. Cunningham, MD, FACP, Florida
V. De Guzman, APRN, MSN, NP, Florida
M. Del Rosario, MD, Florida
D. DeVere, MD, Florida
S. Dharmapuri, Florida
P. Dodson, MD, Florida
A. Domaoal, Georgia
J. Duncan, MD, Georgia
B. Dyck, BSC, MD, PhD, Georgia
J. Dzundza, MD, Georgia
A. Ellis, FNP, Georgia
R. Erickson, Idaho
A. Faraj, Idaho
S. Fernandez, MD, Illinois
G. Ferrari, MD, Illinois
W. Folad, MD, Illinois
L. Fowler, ACNP, APRN, MBA, Illinois
J. Golderberg, MD, Illinois
G. Goldman, MD, Illinois
L. Gonzales, MD, Indiana
A. Gonzalez, Kansas
W. Griffo, MD, Kansas
R. Guzman, Kansas
L. Guzman Vinasco, MD, Kansas
K. Hageman, DO, Kentucky
M. Haggerty, PA-C, Kentucky
B. Hammond, Louisiana
G. Harris, MD, Louisiana
J. Hasan-Jones, RN, FACHE, Louisiana
J. Herring, Louisiana
L. Hsu, MD, Massachusetts
A. C. Hunag, DO, Massachusetts
M. Huq, Massachusetts
M. Jandrin, PA-C, Massachusetts
C. Janish, MD, Maryland
J. Jarin, MD, Maine
A. Jenkins, Maine
S. Jindal, MD, Michigan
M. Johl, Michigan
T. John, Michigan
N. Kapadia, MD, Michigan
L. Katona, Michigan
K. Kaye, Michigan
M. Keating, Michigan
L. Keeton, MD, Michigan
L. Kendall, Michigan
M. Kerlin, Michigan
A. Kia, MD, Minnesota
R. Klett, Minnesota
L. Knapp, DO, Minnesota
K. Knox, Missouri
M. Kraynak, MD, Missouri
P. Kuppireddy, MBBS, Missouri
W. Landrum, MD, Missouri
C. Larion, ACNP, Missouri
E. Latcheva, MD, Mississippi
D. Leforce, North Carolina
V. Leigh, DO, North Carolina
C. Leon, North Carolina
T. Li, MD, North Dakota
X. Li, MD, Nebraska
Y. Li, New Hampshire
J. Liu, New Hampshire
L. Lu, DO, New Jersey
S. Mathapathi, New Jersey
L. McGann, New Jersey
S. Melkaveri, MD, Nevada
R. Mercado Garcia, New York
S. Merry, MD, New York
P. Meyer, DO, New York
J. Mikulca, PharmD, New York
Z. Moyenda, MD, MBA, New York
K. Murphy, DO, MPH, New York
J. Musenze, New York
P. Mutungi, New York
G. Nanna, USA, New York
I. Nasir, New York
U. Nazario-Vidah, MD, New York
D. Nguyen, New York
C. Ojha, MBBS, New York
K. Olson, MD, Ohio
V. Paulson, MD, Ohio
R. Pearson, DO, PhD, Ohio
A. Peel, MD, Ohio
S. Pettis, PA-C, Ohio
E. Picloglou, MD, Ohio
H. Pokhrel, MD, Ohio
H. Bush, Oklahoma
R. Porter, PA, Oklahoma
P. Prabhakar, Oklahoma
U. Qamar, Oklahoma
R. Quansah, MD, Oklahoma
M. Rahman, Oklahoma
R. Rajeshwar, Oregon
E. Randal, Oregon
A. Ray, Oregon
V. Reddy, Oregon
J. Reed, MD, RN, Oregon
R. Regidor, Oregon
A. Reitsma-Mathias, MD, Oregon
R. Reyes, MD, Oregon
T. Richardson, NP, Oregon
T. Ringer, Oregon
L. Rivera-Crespo, Pennsylvania
T. Rothwell, PA, Pennsylvania
E. Sacolick, MD, Pennsylvania
E. Saluke, MD, Pennsylvania
M. Santinelli, NP, Pennsylvania
M. Sapon-Amoah, FNP, Pennsylvania
D. Scarine, NP, Pennsylvania
K. Seger, Pennsylvania
A. Shah, Pennsylvania
K. Shah, MD, Pennsylvania
S. Shah, MD, Pennsylvania
G. Sharma, MD, Pennsylvania
K. Shaukat, MD, Pennsylvania
E. Sheindler, Rhode Island
D. Sheps, South Carolina
J. Shipe-Spotloe, South Carolina
S. Sim, South Carolina
M. Simon, MD, MMM, CPE, South Carolina
A. Singh, South Carolina
S. Singh-Patel, PO, South Dakota
M. Snyder, South Dakota
A. Srikanth, MBBS, Tennessee
B. Staats, Tennessee
C. Standley, Texas
R. Stanhiser, Texas
M. Stevens, Texas
K. Stuart, Texas
A. Summers, Texas
E. Taylo, Texas
L. Taylor, PA-C, Texas
L. Theaker, Texas
M. Thieman, Texas
J. Tong, Texas
N. Trivedi, MD, Texas
L. Tuazon, MD, FACP, Texas
S. Tummalapalli, Texas
A. Ufferman, MD, Virginia
R. Urrea, MD, Virginia
N. Van Groningen, Virginia
D. Vaughn, MD, Vermont
R. Vento, MD, MPH, Washington
Y. Villaran, MD, Washington
L. Viscome, DO, Washington
K. Vo, Washington
H. Vu, MD, Washington
T. Washko, MD, Washington
T. Waters, DO, Wisconsin
L. Weisberger, USA, Wisconsin
A. Whitehead, Wisconsin
A. Workman, West Virginia
F. Yasin, MD, West Virginia
A. Yoon, MD, West Virginia
M. Yu, West Virginia
A. Yuen, DO, West Virginia
K. Zwieg, West Virginia
CHMP recommends extending brentuximab approval

Photo from Business Wire
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended extending the current conditional approval of brentuximab vedotin (Adcetris) to include the treatment of adults with CD30+ Hodgkin lymphoma (HL) at increased risk of relapse or progression following autologous stem cell transplant (ASCT).
The CHMP’s recommendation will now be reviewed by the European Commission (EC).
If the recommendation is formally adopted by the EC, brentuximab vedotin will be approved for the aforementioned indication in the 28 member states of the European Union as well as Norway, Liechtenstein, and Iceland.
Brentuximab vedotin already has conditional marketing authorization from the EC for 2 indications:
- To treat adults with relapsed or refractory CD30+ HL after ASCT or following at least 2 prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- To treat adults with relapsed or refractory systemic anaplastic large-cell lymphoma (sALCL).
In January 2016, the EC approved a Type II variation to include data on the retreatment of adult patients with HL or sALCL who previously responded to brentuximab vedotin and later relapsed.
Brentuximab vedotin is under joint development by Seattle Genetics and Takeda Pharmaceutical Company Limited.
AETHERA trial
The CHMP’s recommendation to extend the approval of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015 and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()

Photo from Business Wire
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended extending the current conditional approval of brentuximab vedotin (Adcetris) to include the treatment of adults with CD30+ Hodgkin lymphoma (HL) at increased risk of relapse or progression following autologous stem cell transplant (ASCT).
The CHMP’s recommendation will now be reviewed by the European Commission (EC).
If the recommendation is formally adopted by the EC, brentuximab vedotin will be approved for the aforementioned indication in the 28 member states of the European Union as well as Norway, Liechtenstein, and Iceland.
Brentuximab vedotin already has conditional marketing authorization from the EC for 2 indications:
- To treat adults with relapsed or refractory CD30+ HL after ASCT or following at least 2 prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- To treat adults with relapsed or refractory systemic anaplastic large-cell lymphoma (sALCL).
In January 2016, the EC approved a Type II variation to include data on the retreatment of adult patients with HL or sALCL who previously responded to brentuximab vedotin and later relapsed.
Brentuximab vedotin is under joint development by Seattle Genetics and Takeda Pharmaceutical Company Limited.
AETHERA trial
The CHMP’s recommendation to extend the approval of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015 and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()

Photo from Business Wire
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended extending the current conditional approval of brentuximab vedotin (Adcetris) to include the treatment of adults with CD30+ Hodgkin lymphoma (HL) at increased risk of relapse or progression following autologous stem cell transplant (ASCT).
The CHMP’s recommendation will now be reviewed by the European Commission (EC).
If the recommendation is formally adopted by the EC, brentuximab vedotin will be approved for the aforementioned indication in the 28 member states of the European Union as well as Norway, Liechtenstein, and Iceland.
Brentuximab vedotin already has conditional marketing authorization from the EC for 2 indications:
- To treat adults with relapsed or refractory CD30+ HL after ASCT or following at least 2 prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- To treat adults with relapsed or refractory systemic anaplastic large-cell lymphoma (sALCL).
In January 2016, the EC approved a Type II variation to include data on the retreatment of adult patients with HL or sALCL who previously responded to brentuximab vedotin and later relapsed.
Brentuximab vedotin is under joint development by Seattle Genetics and Takeda Pharmaceutical Company Limited.
AETHERA trial
The CHMP’s recommendation to extend the approval of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015 and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()
Monitor Alarms in a Children's Hospital
Physiologic monitor alarms are an inescapable part of the soundtrack for hospitals. Data from primarily adult hospitals have shown that alarms occur at high rates, and most alarms are not actionable.[1] Small studies have suggested that high alarm rates can lead to alarm fatigue.[2, 3] To prioritize alarm types to target in future intervention studies, in this study we aimed to investigate the alarm rates on all inpatient units and the most common causes of alarms at a children's hospital.
METHODS
This was a cross‐sectional study of audible physiologic monitor alarms at Cincinnati Children's Hospital Medical Center (CCHMC) over 7 consecutive days during August 2014. CCHMC is a 522‐bed free‐standing children's hospital. Inpatient beds are equipped with GE Healthcare (Little Chalfont, United Kingdom) bedside monitors (models Dash 3000, 4000, and 5000, and Solar 8000). Age‐specific vital sign parameters were employed for monitors on all units.
We obtained date, time, and type of alarm from bedside physiologic monitors using Connexall middleware (GlobeStar Systems, Toronto, Ontario, Canada).
We determined unit census using the electronic health records for the time period concurrent with the alarm data collection. Given previously described variation in hospital census over the day,[4] we used 4 daily census measurements (6:00 am, 12:00 pm, 6:00 pm, and 11:00 pm) rather than 1 single measurement to more accurately reflect the hospital census.
The CCHMC Institutional Review Board determined this work to be not human subjects research.
Statistical Analysis
For each unit and each census time interval, we generated a rate based on the number of occupied beds (alarms per patient‐day) resulting in a total of 28 rates (4 census measurement periods per/day 7 days) for each unit over the study period. We used descriptive statistics to summarize alarms per patient‐day by unit. Analysis of variance was used to compare alarm rates between units. For significant main effects, we used Tukey's multiple comparisons tests for all pairwise comparisons to control the type I experiment‐wise error rate. Alarms were then classified by alarm cause (eg, high heart rate). We summarized the cause for all alarms using counts and percentages.
RESULTS
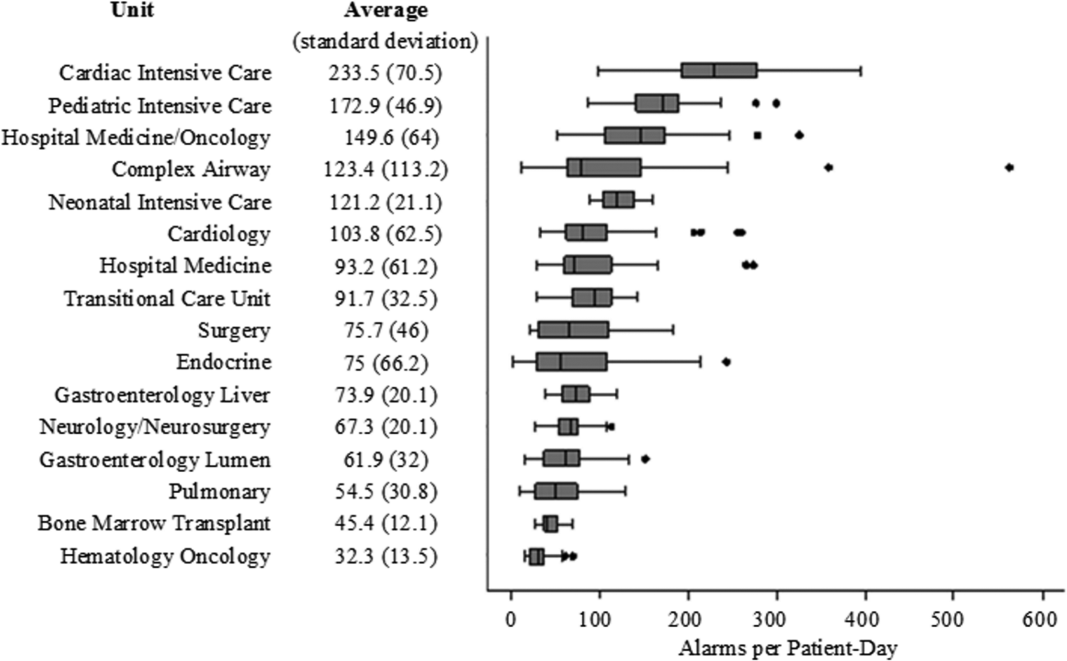
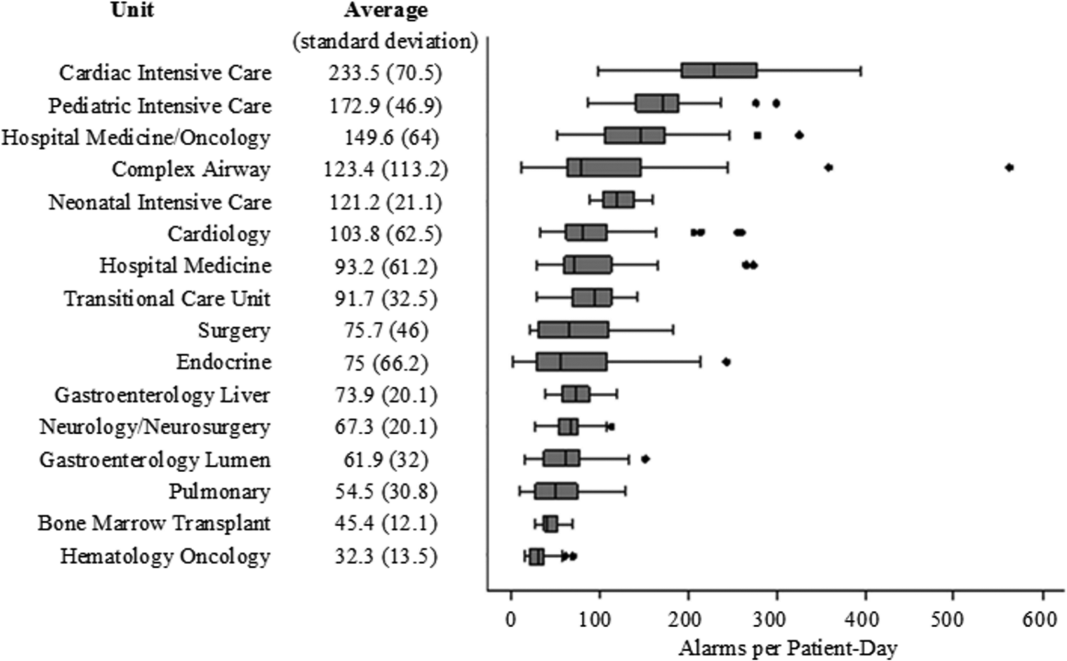
There were a total of 220,813 audible alarms over 1 week. Median alarm rate per patient‐day by unit ranged from 30.4 to 228.5; the highest alarm rates occurred in the cardiac intensive care unit, with a median of 228.5 (interquartile range [IQR], 193275) followed by the pediatric intensive care unit (172.4; IQR, 141188) (Figure 1). The average alarm rate was significantly different among the units (P < 0.01).
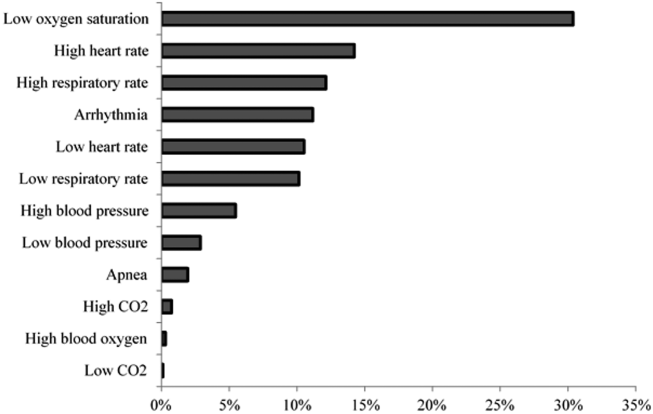
Technical alarms (eg, alarms for artifact, lead failure), comprised 33% of the total number of alarms. The remaining 67% of alarms were for clinical conditions, the most common of which was low oxygen saturation (30% of clinical alarms) (Figure 2).
DISCUSSION
We described alarm rates and causes over multiple units at a large children's hospital. To our knowledge, this is the first description of alarm rates across multiple pediatric inpatient units. Alarm counts were high even for the general units, indicating that a nurse taking care of 4 monitored patients would need to process a physiologic monitor alarm every 4 minutes on average, in addition to other sources of alarms such as infusion pumps.
Alarm rates were highest in the intensive care unit areas, which may be attributable to both higher rates of monitoring and sicker patients. Importantly, however, alarms were quite high and variable on the acute care units. This suggests that factors other than patient acuity may have substantial influence on alarm rates.
Technical alarms, alarms that do not indicate a change in patient condition, accounted for the largest percentage of alarms during the study period. This is consistent with prior literature that has suggested that regular electrode replacement, which decreases technical alarms, can be effective in reducing alarm rates.[5, 6] The most common vital sign change to cause alarms was low oxygen saturation, followed by elevated heart rate and elevated respiratory rate. Whereas in most healthy patients, certain low oxygen levels would prompt initiation of supplemental oxygen, there are many conditions in which elevated heart rate and respiratory rate may not require titration of any particular therapy. These may be potential intervention targets for hospitals trying to improve alarm rates.
Limitations
There are several limitations to our study. First, our results are not necessarily generalizable to other types of hospitals or those utilizing monitors from other vendors. Second, we were unable to include other sources of alarms such as infusion pumps and ventilators. However, given the high alarm rates from physiologic monitors alone, these data add urgency to the need for further investigation in the pediatric setting.
CONCLUSION
Alarm rates at a single children's hospital varied depending on the unit. Strategies targeted at reducing technical alarms and reducing nonactionable clinical alarms for low oxygen saturation, high heart rate, and high respiratory rate may offer the greatest opportunity to reduce alarm rates.
Acknowledgements
The authors acknowledge Melinda Egan for her assistance in obtaining data for this study and Ting Sa for her assistance with data management.
Disclosures: Dr. Bonafide is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. Dr. Bonafide also holds a Young Investigator Award grant from the Academic Pediatric Association evaluating the impact of a data‐driven monitor alarm reduction strategy implemented in safety huddles. Dr. Brady is supported by the Agency for Healthcare Research and Quality under award number K08HS23827. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality. This study was funded by the Arnold W. Strauss Fellow Grant, Cincinnati Children's Hospital Medical Center. The authors have no conflicts of interest to disclose.
- , , , et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136–144.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . Traditional measures of hospital utilization may not accurately reflect dynamic patient demand: findings from a children's hospital. Hosp Pediatr. 2012;2(1):10–18.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- , , , . Daily electrode change and effect on cardiac monitor alarms: an evidence‐based practice approach. J Nurs Care Qual. 2013;28(3):265–271.
Physiologic monitor alarms are an inescapable part of the soundtrack for hospitals. Data from primarily adult hospitals have shown that alarms occur at high rates, and most alarms are not actionable.[1] Small studies have suggested that high alarm rates can lead to alarm fatigue.[2, 3] To prioritize alarm types to target in future intervention studies, in this study we aimed to investigate the alarm rates on all inpatient units and the most common causes of alarms at a children's hospital.
METHODS
This was a cross‐sectional study of audible physiologic monitor alarms at Cincinnati Children's Hospital Medical Center (CCHMC) over 7 consecutive days during August 2014. CCHMC is a 522‐bed free‐standing children's hospital. Inpatient beds are equipped with GE Healthcare (Little Chalfont, United Kingdom) bedside monitors (models Dash 3000, 4000, and 5000, and Solar 8000). Age‐specific vital sign parameters were employed for monitors on all units.
We obtained date, time, and type of alarm from bedside physiologic monitors using Connexall middleware (GlobeStar Systems, Toronto, Ontario, Canada).
We determined unit census using the electronic health records for the time period concurrent with the alarm data collection. Given previously described variation in hospital census over the day,[4] we used 4 daily census measurements (6:00 am, 12:00 pm, 6:00 pm, and 11:00 pm) rather than 1 single measurement to more accurately reflect the hospital census.
The CCHMC Institutional Review Board determined this work to be not human subjects research.
Statistical Analysis
For each unit and each census time interval, we generated a rate based on the number of occupied beds (alarms per patient‐day) resulting in a total of 28 rates (4 census measurement periods per/day 7 days) for each unit over the study period. We used descriptive statistics to summarize alarms per patient‐day by unit. Analysis of variance was used to compare alarm rates between units. For significant main effects, we used Tukey's multiple comparisons tests for all pairwise comparisons to control the type I experiment‐wise error rate. Alarms were then classified by alarm cause (eg, high heart rate). We summarized the cause for all alarms using counts and percentages.
RESULTS
There were a total of 220,813 audible alarms over 1 week. Median alarm rate per patient‐day by unit ranged from 30.4 to 228.5; the highest alarm rates occurred in the cardiac intensive care unit, with a median of 228.5 (interquartile range [IQR], 193275) followed by the pediatric intensive care unit (172.4; IQR, 141188) (Figure 1). The average alarm rate was significantly different among the units (P < 0.01).

Technical alarms (eg, alarms for artifact, lead failure), comprised 33% of the total number of alarms. The remaining 67% of alarms were for clinical conditions, the most common of which was low oxygen saturation (30% of clinical alarms) (Figure 2).

DISCUSSION
We described alarm rates and causes over multiple units at a large children's hospital. To our knowledge, this is the first description of alarm rates across multiple pediatric inpatient units. Alarm counts were high even for the general units, indicating that a nurse taking care of 4 monitored patients would need to process a physiologic monitor alarm every 4 minutes on average, in addition to other sources of alarms such as infusion pumps.
Alarm rates were highest in the intensive care unit areas, which may be attributable to both higher rates of monitoring and sicker patients. Importantly, however, alarms were quite high and variable on the acute care units. This suggests that factors other than patient acuity may have substantial influence on alarm rates.
Technical alarms, alarms that do not indicate a change in patient condition, accounted for the largest percentage of alarms during the study period. This is consistent with prior literature that has suggested that regular electrode replacement, which decreases technical alarms, can be effective in reducing alarm rates.[5, 6] The most common vital sign change to cause alarms was low oxygen saturation, followed by elevated heart rate and elevated respiratory rate. Whereas in most healthy patients, certain low oxygen levels would prompt initiation of supplemental oxygen, there are many conditions in which elevated heart rate and respiratory rate may not require titration of any particular therapy. These may be potential intervention targets for hospitals trying to improve alarm rates.
Limitations
There are several limitations to our study. First, our results are not necessarily generalizable to other types of hospitals or those utilizing monitors from other vendors. Second, we were unable to include other sources of alarms such as infusion pumps and ventilators. However, given the high alarm rates from physiologic monitors alone, these data add urgency to the need for further investigation in the pediatric setting.
CONCLUSION
Alarm rates at a single children's hospital varied depending on the unit. Strategies targeted at reducing technical alarms and reducing nonactionable clinical alarms for low oxygen saturation, high heart rate, and high respiratory rate may offer the greatest opportunity to reduce alarm rates.
Acknowledgements
The authors acknowledge Melinda Egan for her assistance in obtaining data for this study and Ting Sa for her assistance with data management.
Disclosures: Dr. Bonafide is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. Dr. Bonafide also holds a Young Investigator Award grant from the Academic Pediatric Association evaluating the impact of a data‐driven monitor alarm reduction strategy implemented in safety huddles. Dr. Brady is supported by the Agency for Healthcare Research and Quality under award number K08HS23827. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality. This study was funded by the Arnold W. Strauss Fellow Grant, Cincinnati Children's Hospital Medical Center. The authors have no conflicts of interest to disclose.
Physiologic monitor alarms are an inescapable part of the soundtrack for hospitals. Data from primarily adult hospitals have shown that alarms occur at high rates, and most alarms are not actionable.[1] Small studies have suggested that high alarm rates can lead to alarm fatigue.[2, 3] To prioritize alarm types to target in future intervention studies, in this study we aimed to investigate the alarm rates on all inpatient units and the most common causes of alarms at a children's hospital.
METHODS
This was a cross‐sectional study of audible physiologic monitor alarms at Cincinnati Children's Hospital Medical Center (CCHMC) over 7 consecutive days during August 2014. CCHMC is a 522‐bed free‐standing children's hospital. Inpatient beds are equipped with GE Healthcare (Little Chalfont, United Kingdom) bedside monitors (models Dash 3000, 4000, and 5000, and Solar 8000). Age‐specific vital sign parameters were employed for monitors on all units.
We obtained date, time, and type of alarm from bedside physiologic monitors using Connexall middleware (GlobeStar Systems, Toronto, Ontario, Canada).
We determined unit census using the electronic health records for the time period concurrent with the alarm data collection. Given previously described variation in hospital census over the day,[4] we used 4 daily census measurements (6:00 am, 12:00 pm, 6:00 pm, and 11:00 pm) rather than 1 single measurement to more accurately reflect the hospital census.
The CCHMC Institutional Review Board determined this work to be not human subjects research.
Statistical Analysis
For each unit and each census time interval, we generated a rate based on the number of occupied beds (alarms per patient‐day) resulting in a total of 28 rates (4 census measurement periods per/day 7 days) for each unit over the study period. We used descriptive statistics to summarize alarms per patient‐day by unit. Analysis of variance was used to compare alarm rates between units. For significant main effects, we used Tukey's multiple comparisons tests for all pairwise comparisons to control the type I experiment‐wise error rate. Alarms were then classified by alarm cause (eg, high heart rate). We summarized the cause for all alarms using counts and percentages.
RESULTS
There were a total of 220,813 audible alarms over 1 week. Median alarm rate per patient‐day by unit ranged from 30.4 to 228.5; the highest alarm rates occurred in the cardiac intensive care unit, with a median of 228.5 (interquartile range [IQR], 193275) followed by the pediatric intensive care unit (172.4; IQR, 141188) (Figure 1). The average alarm rate was significantly different among the units (P < 0.01).

Technical alarms (eg, alarms for artifact, lead failure), comprised 33% of the total number of alarms. The remaining 67% of alarms were for clinical conditions, the most common of which was low oxygen saturation (30% of clinical alarms) (Figure 2).

DISCUSSION
We described alarm rates and causes over multiple units at a large children's hospital. To our knowledge, this is the first description of alarm rates across multiple pediatric inpatient units. Alarm counts were high even for the general units, indicating that a nurse taking care of 4 monitored patients would need to process a physiologic monitor alarm every 4 minutes on average, in addition to other sources of alarms such as infusion pumps.
Alarm rates were highest in the intensive care unit areas, which may be attributable to both higher rates of monitoring and sicker patients. Importantly, however, alarms were quite high and variable on the acute care units. This suggests that factors other than patient acuity may have substantial influence on alarm rates.
Technical alarms, alarms that do not indicate a change in patient condition, accounted for the largest percentage of alarms during the study period. This is consistent with prior literature that has suggested that regular electrode replacement, which decreases technical alarms, can be effective in reducing alarm rates.[5, 6] The most common vital sign change to cause alarms was low oxygen saturation, followed by elevated heart rate and elevated respiratory rate. Whereas in most healthy patients, certain low oxygen levels would prompt initiation of supplemental oxygen, there are many conditions in which elevated heart rate and respiratory rate may not require titration of any particular therapy. These may be potential intervention targets for hospitals trying to improve alarm rates.
Limitations
There are several limitations to our study. First, our results are not necessarily generalizable to other types of hospitals or those utilizing monitors from other vendors. Second, we were unable to include other sources of alarms such as infusion pumps and ventilators. However, given the high alarm rates from physiologic monitors alone, these data add urgency to the need for further investigation in the pediatric setting.
CONCLUSION
Alarm rates at a single children's hospital varied depending on the unit. Strategies targeted at reducing technical alarms and reducing nonactionable clinical alarms for low oxygen saturation, high heart rate, and high respiratory rate may offer the greatest opportunity to reduce alarm rates.
Acknowledgements
The authors acknowledge Melinda Egan for her assistance in obtaining data for this study and Ting Sa for her assistance with data management.
Disclosures: Dr. Bonafide is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. Dr. Bonafide also holds a Young Investigator Award grant from the Academic Pediatric Association evaluating the impact of a data‐driven monitor alarm reduction strategy implemented in safety huddles. Dr. Brady is supported by the Agency for Healthcare Research and Quality under award number K08HS23827. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality. This study was funded by the Arnold W. Strauss Fellow Grant, Cincinnati Children's Hospital Medical Center. The authors have no conflicts of interest to disclose.
- , , , et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136–144.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . Traditional measures of hospital utilization may not accurately reflect dynamic patient demand: findings from a children's hospital. Hosp Pediatr. 2012;2(1):10–18.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- , , , . Daily electrode change and effect on cardiac monitor alarms: an evidence‐based practice approach. J Nurs Care Qual. 2013;28(3):265–271.
- , , , et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136–144.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . Traditional measures of hospital utilization may not accurately reflect dynamic patient demand: findings from a children's hospital. Hosp Pediatr. 2012;2(1):10–18.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- , , , . Daily electrode change and effect on cardiac monitor alarms: an evidence‐based practice approach. J Nurs Care Qual. 2013;28(3):265–271.
Cystoscopy after hysterectomy: Consider more frequent use
WASHINGTON – Universal cystoscopy at the time of hysterectomy – or at least more frequent use of the procedure – is worth considering since delayed diagnosis of urinary tract injury causes increased morbidity for patients, and in all likelihood increases litigation, Dr. Jay Goldberg and Dr. Cheung Kim suggested at the annual meeting of the American College of Obstetricians and Gynecologists.
“We’re often hesitant to do cystoscopy because we don’t want to add time,” said Dr. Kim. “But I always feel that no matter how much time it takes, I’ll be happier in the end if I do it. [And] if you have [experience], a routine, and readily available equipment, it can take as little as 10 minutes.”
Universal cystoscopy to confirm ureteral patency is “fairly straightforward, low risk, and more likely to detect most injuries [than visual inspection alone], particularly ureteral injuries,” Dr. Kim said. On the other hand, it adds to operating time and increases procedure cost, and there is some research suggesting it may be relatively “low yield” and lead to some false positives.
Dr. Kim and Dr. Goldberg both practice at the Einstein Healthcare Network in Philadelphia. Here are some of the findings they shared, and advice they gave, on the use of cystoscopy – universal or selective – after hysterectomy.
Conflicting findings
There is conflicting opinion as to whether universal or selective cystoscopy after hysterectomy is best, and “there’s data on both sides,” said Dr. Goldberg, vice chairman of ob.gyn. and director of the Philadelphia Fibroid Center at Einstein.
A prospective study done at Louisiana State University, New Orleans, to evaluate the impact of a universal approach, for instance, showed an incidence of urinary tract injury of 4.3% (2.9% bladder injury, 1.8% ureteral injury, plus cases of simultaneous injury) in 839 hysterectomies for benign disease. The injury detection rate using intraoperative cystoscopy was 97.4%, and the majority of injuries – 76% – were not suspected prior to cystoscopy being performed (Obstet Gynecol. 2009 Jan;113[1]:6-10).
But researchers in Boston who looked retrospectively at 1,982 hysterectomies performed for any gynecologic indication found a much lower incidence of complications, and reported that cystoscopy did not detect any of the bladder injuries (0.71%) or ureteral injuries (0.25%) incurred in the group. Cystoscopy was performed selectively, however, in 250 of the patients, and was either normal or omitted in the patients who had complications (Obstet Gynecol. 2012 Dec;120[6]:1363-70).
Cystoscopy failed to detect any of the bladder injuries, but “all five of the ureteral injuries occurred in patients who had not undergone cystoscopy,” said Dr. Kim, chairman of ob.gyn. at Einstein Medical Center Montgomery in East Norriton, Pa.
Possible false-positives
Cystoscopy may lead on occasion to an incorrect presumption of a ureteral injury in patients with a pre-existing nonfunctional kidney, Dr. Goldberg noted.
He relayed the case of a 42-year-old patient who underwent a total abdominal hysterectomy without apparent complication. Cystoscopy was then performed with indigo carmine. An efflux of dye was seen from the left ureteral orifice but not from the right orifice.
Urology was consulted and investigated the presumed ureteral injury with additional surgical exploration. An intraoperative intravenous pyelogram (IVP) was eventually performed and was unable to identify the right kidney. A CT then showed an atrophic right kidney with compensatory hypertrophy of the left kidney, probably due to congenital right multicystic dysplastic kidney.
An estimated 0.2% of the population – 1 in 500 – will have a unilateral nonfunctional kidney, the majority of which have not been previously diagnosed. Etiologies include multicystic dysplastic kidney, congenital unilateral renal agenesis, and vascular events. “As we do more and more cystoscopies, this scenario is going to come up every so often,” said Dr. Goldberg, who reported on two such cases last year (Obstet Gynecol. 2015 Sep;126[3]:635-7).
It is also possible, Dr. Kim noted, that a weak urine jet observed on cystoscopy may not necessarily reflect injury. In the LSU study evaluating a universal approach, there was no injury detected on further evaluation in each of the 21 cases of low, subnormal dye efflux from the ureteral orifices. “So it’s not a benign process to undergo cystoscopy in terms of what the ramifications might be,” Dr. Kim said.
Increasing use
Ob.gyn. residents are required by the Accreditation Council for Graduate Medical Education to have completed 15 cystoscopies by the time they graduate, and according to recent survey findings, residents are more likely to utilize universal cystoscopy at the time of hysterectomy than currently practicing gynecologic surgeons.
The survey of ob.gyn residents (n = 56) shows universal cystoscopy (defined as greater than 90%) was performed in only a minority of cases during residency: 27% of total laparoscopic hysterectomies (TLH), 14% of laparoscopically assisted vaginal hysterectomies (LAVH), 12% of vaginal hysterectomies (VH), 2% of total abdominal hysterectomies (TAH), and 0% of supracervical hysterectomies (SCH), for instance.
Yet for every hysterectomy type, residents planned to perform universal cystoscopy post-residency more often than they had during their training (49% TLH, 34% LAVH, 34% VH, 15% TAH, 12% SCH), and “residents familiar with the literature on cystoscopy were statistically more likely to plan to perform universal cystoscopy,” said Dr. Goldberg, the senior author of the paper (Womens Health (Lond Engl). 2015 Nov;11[6]:825-31).
Litigation possible
Failure to detect a urinary tract injury at the time of hysterectomy may result in the need for future additional surgeries. Litigation in the Philadelphia market suggests that “if you have an injury that’s missed, there’s a chance that litigation may result,” Dr. Kim said.
Plaintiff’s attorneys have argued that not recognizing ureteral injury during surgery is a deviation from acceptable practice, while defense attorneys have contended that unavoidable complications occur and that no evaluation is required, or supported by the medical literature, when injury is not intraoperatively suspected. Currently, as cystoscopy is performed less than 25% of the time for all types of hysterectomy, the standard of care does not require the procedure intraoperatively if no injury is suspected, Dr. Goldberg said.
Primary prevention of urinary tract injury is most important, both physicians emphasized. The best way to accomplish this is to meticulously identify the anatomy and know the path of the ureter, and to document that the ureter has been identified and viewed as outside of the operative area, they said.
Dr. Kim and Dr. Goldberg reported having no relevant financial disclosures.
WASHINGTON – Universal cystoscopy at the time of hysterectomy – or at least more frequent use of the procedure – is worth considering since delayed diagnosis of urinary tract injury causes increased morbidity for patients, and in all likelihood increases litigation, Dr. Jay Goldberg and Dr. Cheung Kim suggested at the annual meeting of the American College of Obstetricians and Gynecologists.
“We’re often hesitant to do cystoscopy because we don’t want to add time,” said Dr. Kim. “But I always feel that no matter how much time it takes, I’ll be happier in the end if I do it. [And] if you have [experience], a routine, and readily available equipment, it can take as little as 10 minutes.”
Universal cystoscopy to confirm ureteral patency is “fairly straightforward, low risk, and more likely to detect most injuries [than visual inspection alone], particularly ureteral injuries,” Dr. Kim said. On the other hand, it adds to operating time and increases procedure cost, and there is some research suggesting it may be relatively “low yield” and lead to some false positives.
Dr. Kim and Dr. Goldberg both practice at the Einstein Healthcare Network in Philadelphia. Here are some of the findings they shared, and advice they gave, on the use of cystoscopy – universal or selective – after hysterectomy.
Conflicting findings
There is conflicting opinion as to whether universal or selective cystoscopy after hysterectomy is best, and “there’s data on both sides,” said Dr. Goldberg, vice chairman of ob.gyn. and director of the Philadelphia Fibroid Center at Einstein.
A prospective study done at Louisiana State University, New Orleans, to evaluate the impact of a universal approach, for instance, showed an incidence of urinary tract injury of 4.3% (2.9% bladder injury, 1.8% ureteral injury, plus cases of simultaneous injury) in 839 hysterectomies for benign disease. The injury detection rate using intraoperative cystoscopy was 97.4%, and the majority of injuries – 76% – were not suspected prior to cystoscopy being performed (Obstet Gynecol. 2009 Jan;113[1]:6-10).
But researchers in Boston who looked retrospectively at 1,982 hysterectomies performed for any gynecologic indication found a much lower incidence of complications, and reported that cystoscopy did not detect any of the bladder injuries (0.71%) or ureteral injuries (0.25%) incurred in the group. Cystoscopy was performed selectively, however, in 250 of the patients, and was either normal or omitted in the patients who had complications (Obstet Gynecol. 2012 Dec;120[6]:1363-70).
Cystoscopy failed to detect any of the bladder injuries, but “all five of the ureteral injuries occurred in patients who had not undergone cystoscopy,” said Dr. Kim, chairman of ob.gyn. at Einstein Medical Center Montgomery in East Norriton, Pa.
Possible false-positives
Cystoscopy may lead on occasion to an incorrect presumption of a ureteral injury in patients with a pre-existing nonfunctional kidney, Dr. Goldberg noted.
He relayed the case of a 42-year-old patient who underwent a total abdominal hysterectomy without apparent complication. Cystoscopy was then performed with indigo carmine. An efflux of dye was seen from the left ureteral orifice but not from the right orifice.
Urology was consulted and investigated the presumed ureteral injury with additional surgical exploration. An intraoperative intravenous pyelogram (IVP) was eventually performed and was unable to identify the right kidney. A CT then showed an atrophic right kidney with compensatory hypertrophy of the left kidney, probably due to congenital right multicystic dysplastic kidney.
An estimated 0.2% of the population – 1 in 500 – will have a unilateral nonfunctional kidney, the majority of which have not been previously diagnosed. Etiologies include multicystic dysplastic kidney, congenital unilateral renal agenesis, and vascular events. “As we do more and more cystoscopies, this scenario is going to come up every so often,” said Dr. Goldberg, who reported on two such cases last year (Obstet Gynecol. 2015 Sep;126[3]:635-7).
It is also possible, Dr. Kim noted, that a weak urine jet observed on cystoscopy may not necessarily reflect injury. In the LSU study evaluating a universal approach, there was no injury detected on further evaluation in each of the 21 cases of low, subnormal dye efflux from the ureteral orifices. “So it’s not a benign process to undergo cystoscopy in terms of what the ramifications might be,” Dr. Kim said.
Increasing use
Ob.gyn. residents are required by the Accreditation Council for Graduate Medical Education to have completed 15 cystoscopies by the time they graduate, and according to recent survey findings, residents are more likely to utilize universal cystoscopy at the time of hysterectomy than currently practicing gynecologic surgeons.
The survey of ob.gyn residents (n = 56) shows universal cystoscopy (defined as greater than 90%) was performed in only a minority of cases during residency: 27% of total laparoscopic hysterectomies (TLH), 14% of laparoscopically assisted vaginal hysterectomies (LAVH), 12% of vaginal hysterectomies (VH), 2% of total abdominal hysterectomies (TAH), and 0% of supracervical hysterectomies (SCH), for instance.
Yet for every hysterectomy type, residents planned to perform universal cystoscopy post-residency more often than they had during their training (49% TLH, 34% LAVH, 34% VH, 15% TAH, 12% SCH), and “residents familiar with the literature on cystoscopy were statistically more likely to plan to perform universal cystoscopy,” said Dr. Goldberg, the senior author of the paper (Womens Health (Lond Engl). 2015 Nov;11[6]:825-31).
Litigation possible
Failure to detect a urinary tract injury at the time of hysterectomy may result in the need for future additional surgeries. Litigation in the Philadelphia market suggests that “if you have an injury that’s missed, there’s a chance that litigation may result,” Dr. Kim said.
Plaintiff’s attorneys have argued that not recognizing ureteral injury during surgery is a deviation from acceptable practice, while defense attorneys have contended that unavoidable complications occur and that no evaluation is required, or supported by the medical literature, when injury is not intraoperatively suspected. Currently, as cystoscopy is performed less than 25% of the time for all types of hysterectomy, the standard of care does not require the procedure intraoperatively if no injury is suspected, Dr. Goldberg said.
Primary prevention of urinary tract injury is most important, both physicians emphasized. The best way to accomplish this is to meticulously identify the anatomy and know the path of the ureter, and to document that the ureter has been identified and viewed as outside of the operative area, they said.
Dr. Kim and Dr. Goldberg reported having no relevant financial disclosures.
WASHINGTON – Universal cystoscopy at the time of hysterectomy – or at least more frequent use of the procedure – is worth considering since delayed diagnosis of urinary tract injury causes increased morbidity for patients, and in all likelihood increases litigation, Dr. Jay Goldberg and Dr. Cheung Kim suggested at the annual meeting of the American College of Obstetricians and Gynecologists.
“We’re often hesitant to do cystoscopy because we don’t want to add time,” said Dr. Kim. “But I always feel that no matter how much time it takes, I’ll be happier in the end if I do it. [And] if you have [experience], a routine, and readily available equipment, it can take as little as 10 minutes.”
Universal cystoscopy to confirm ureteral patency is “fairly straightforward, low risk, and more likely to detect most injuries [than visual inspection alone], particularly ureteral injuries,” Dr. Kim said. On the other hand, it adds to operating time and increases procedure cost, and there is some research suggesting it may be relatively “low yield” and lead to some false positives.
Dr. Kim and Dr. Goldberg both practice at the Einstein Healthcare Network in Philadelphia. Here are some of the findings they shared, and advice they gave, on the use of cystoscopy – universal or selective – after hysterectomy.
Conflicting findings
There is conflicting opinion as to whether universal or selective cystoscopy after hysterectomy is best, and “there’s data on both sides,” said Dr. Goldberg, vice chairman of ob.gyn. and director of the Philadelphia Fibroid Center at Einstein.
A prospective study done at Louisiana State University, New Orleans, to evaluate the impact of a universal approach, for instance, showed an incidence of urinary tract injury of 4.3% (2.9% bladder injury, 1.8% ureteral injury, plus cases of simultaneous injury) in 839 hysterectomies for benign disease. The injury detection rate using intraoperative cystoscopy was 97.4%, and the majority of injuries – 76% – were not suspected prior to cystoscopy being performed (Obstet Gynecol. 2009 Jan;113[1]:6-10).
But researchers in Boston who looked retrospectively at 1,982 hysterectomies performed for any gynecologic indication found a much lower incidence of complications, and reported that cystoscopy did not detect any of the bladder injuries (0.71%) or ureteral injuries (0.25%) incurred in the group. Cystoscopy was performed selectively, however, in 250 of the patients, and was either normal or omitted in the patients who had complications (Obstet Gynecol. 2012 Dec;120[6]:1363-70).
Cystoscopy failed to detect any of the bladder injuries, but “all five of the ureteral injuries occurred in patients who had not undergone cystoscopy,” said Dr. Kim, chairman of ob.gyn. at Einstein Medical Center Montgomery in East Norriton, Pa.
Possible false-positives
Cystoscopy may lead on occasion to an incorrect presumption of a ureteral injury in patients with a pre-existing nonfunctional kidney, Dr. Goldberg noted.
He relayed the case of a 42-year-old patient who underwent a total abdominal hysterectomy without apparent complication. Cystoscopy was then performed with indigo carmine. An efflux of dye was seen from the left ureteral orifice but not from the right orifice.
Urology was consulted and investigated the presumed ureteral injury with additional surgical exploration. An intraoperative intravenous pyelogram (IVP) was eventually performed and was unable to identify the right kidney. A CT then showed an atrophic right kidney with compensatory hypertrophy of the left kidney, probably due to congenital right multicystic dysplastic kidney.
An estimated 0.2% of the population – 1 in 500 – will have a unilateral nonfunctional kidney, the majority of which have not been previously diagnosed. Etiologies include multicystic dysplastic kidney, congenital unilateral renal agenesis, and vascular events. “As we do more and more cystoscopies, this scenario is going to come up every so often,” said Dr. Goldberg, who reported on two such cases last year (Obstet Gynecol. 2015 Sep;126[3]:635-7).
It is also possible, Dr. Kim noted, that a weak urine jet observed on cystoscopy may not necessarily reflect injury. In the LSU study evaluating a universal approach, there was no injury detected on further evaluation in each of the 21 cases of low, subnormal dye efflux from the ureteral orifices. “So it’s not a benign process to undergo cystoscopy in terms of what the ramifications might be,” Dr. Kim said.
Increasing use
Ob.gyn. residents are required by the Accreditation Council for Graduate Medical Education to have completed 15 cystoscopies by the time they graduate, and according to recent survey findings, residents are more likely to utilize universal cystoscopy at the time of hysterectomy than currently practicing gynecologic surgeons.
The survey of ob.gyn residents (n = 56) shows universal cystoscopy (defined as greater than 90%) was performed in only a minority of cases during residency: 27% of total laparoscopic hysterectomies (TLH), 14% of laparoscopically assisted vaginal hysterectomies (LAVH), 12% of vaginal hysterectomies (VH), 2% of total abdominal hysterectomies (TAH), and 0% of supracervical hysterectomies (SCH), for instance.
Yet for every hysterectomy type, residents planned to perform universal cystoscopy post-residency more often than they had during their training (49% TLH, 34% LAVH, 34% VH, 15% TAH, 12% SCH), and “residents familiar with the literature on cystoscopy were statistically more likely to plan to perform universal cystoscopy,” said Dr. Goldberg, the senior author of the paper (Womens Health (Lond Engl). 2015 Nov;11[6]:825-31).
Litigation possible
Failure to detect a urinary tract injury at the time of hysterectomy may result in the need for future additional surgeries. Litigation in the Philadelphia market suggests that “if you have an injury that’s missed, there’s a chance that litigation may result,” Dr. Kim said.
Plaintiff’s attorneys have argued that not recognizing ureteral injury during surgery is a deviation from acceptable practice, while defense attorneys have contended that unavoidable complications occur and that no evaluation is required, or supported by the medical literature, when injury is not intraoperatively suspected. Currently, as cystoscopy is performed less than 25% of the time for all types of hysterectomy, the standard of care does not require the procedure intraoperatively if no injury is suspected, Dr. Goldberg said.
Primary prevention of urinary tract injury is most important, both physicians emphasized. The best way to accomplish this is to meticulously identify the anatomy and know the path of the ureter, and to document that the ureter has been identified and viewed as outside of the operative area, they said.
Dr. Kim and Dr. Goldberg reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2016
Emotional Abuse in Childhood May Be Linked to Migraine in Adulthood
VANCOUVER—Childhood emotional abuse may be associated with migraine in adulthood, according to data presented at the 68th Annual Meeting of the American Academy of Neurology. The research indicates that emotional abuse during childhood has a more significant effect on migraine, compared with physical and sexual abuse.
Childhood maltreatment, which includes neglect and abuse (ie, emotional, physical, and sexual), is confirmed in 12.5% of children by the age of 18, according to a report published in 2014 in JAMA Pediatrics. Previous studies have linked childhood abuse to headache, but there has been limited assessment of other major types of abuse. “In 2015, there were six different meta-analyses from all over the world linking childhood emotional abuse in particular with adult psychiatric disease,” said Gretchen Tietjen, MD, Professor and Chair of Neurology at the University of Toledo in Ohio.
Gretchen Tietjen, MD
Dr. Tietjen and her colleagues conducted a study to better understand the association between emotional abuse and migraine, independent of depression and anxiety. The study’s objectives were to examine the correlation between migraine and the number of types of abuse and frequency of abuse, and to examine the influence of sex and race on migraine. They also strove to determine the temporal relationship between age of onset of abuse, depression, and anxiety and age of onset of migraine.
There were 14,484 participants aged 24 to 32 in the study. About 14% of participants self-reported that they had been diagnosed with migraine. Fifteen percent of the population reported a diagnosis of depression (35% in the migraine sample vs 12% of controls), and 12% of the sample reported a diagnosis of anxiety (25% in the migraine sample vs 10% of controls). The mean age in the study was 29.5. Fifty-three percent of participants were women and 36% of the population was nonwhite.
All participants filled out a “Mistreatment by Adults” questionnaire about experiences before age 18. Emotional abuse was assessed by asking participants how often a parent or other adult caregiver had said things that hurt their feelings or made them feel unwanted or unloved. Physical abuse was assessed by asking whether a parent or caregiver had hit the participant with a fist, kicked him or her, or threw him or her to the floor, into a wall, or down stairs. And finally, sexual abuse was assessed by asking whether a parent or adult caregiver had touched the participant in a sexual way, forced him or her to touch him or her in a sexual way, or forced him or her to have sexual relations. The dependent variable in the study was self-reported doctor diagnosis of migraine. Sociodemographic characteristics recorded included age, sex, household income, and race. Self-reported physician diagnoses of depression and anxiety were examined as additional confounders.
Abuse was recalled by 60.5% of participants with migraine and 49% of the nonmigraine sample. Abuse increased the chance of migraine diagnosis by 55%. Emotional abuse had the strongest link to migraine, and the link was stronger in males than in females. When the researchers controlled the data for depression and anxiety, the effect of child abuse on migraine was attenuated, but remained significant. In addition, the effect of emotional abuse on migraine decreased, but remained significant when the investigators controlled for depression and anxiety. The researchers found no association between physical and sexual abuse when they adjusted for other abuse types, and there was no association with any abuse type and migraine in the non-Caucasian cohort.
Strengths of this study included its nationally representative population-based sample, its population’s relatively young mean age of 30 (which was 20–30 years younger than populations in other studies), and the inclusion of questions on three major types of abuse. Limitations of the study included its reliance on self-reported, retrospective data on abuse and on physician-diagnosed conditions; its broad definition of emotional abuse; and its potential for recall bias and reporting bias.
The next goal for this investigation is to determine the neurophysiologic mechanisms of the relationship between migraine and emotional abuse, said Dr. Tietjen. The relationship itself needs to be confirmed. “We’re currently looking at the database for gene–environment interactions that may link abuse to migraine for persons with a specific genotype. There’s also an opportunity for investigation of other different kinds of modifications that we know occur with abuse and whether these play a role in determining migraine,” she concluded.
—Erica Robinson
Suggested Reading
Tietjen G. Childhood maltreatment and headache disorders. Curr Pain Headache Rep. 2016; 20(4):26.
Wildeman C. Emanuel N, Leventhal J, et al. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr. 2014;168(8):706-713.
VANCOUVER—Childhood emotional abuse may be associated with migraine in adulthood, according to data presented at the 68th Annual Meeting of the American Academy of Neurology. The research indicates that emotional abuse during childhood has a more significant effect on migraine, compared with physical and sexual abuse.
Childhood maltreatment, which includes neglect and abuse (ie, emotional, physical, and sexual), is confirmed in 12.5% of children by the age of 18, according to a report published in 2014 in JAMA Pediatrics. Previous studies have linked childhood abuse to headache, but there has been limited assessment of other major types of abuse. “In 2015, there were six different meta-analyses from all over the world linking childhood emotional abuse in particular with adult psychiatric disease,” said Gretchen Tietjen, MD, Professor and Chair of Neurology at the University of Toledo in Ohio.
Gretchen Tietjen, MD
Dr. Tietjen and her colleagues conducted a study to better understand the association between emotional abuse and migraine, independent of depression and anxiety. The study’s objectives were to examine the correlation between migraine and the number of types of abuse and frequency of abuse, and to examine the influence of sex and race on migraine. They also strove to determine the temporal relationship between age of onset of abuse, depression, and anxiety and age of onset of migraine.
There were 14,484 participants aged 24 to 32 in the study. About 14% of participants self-reported that they had been diagnosed with migraine. Fifteen percent of the population reported a diagnosis of depression (35% in the migraine sample vs 12% of controls), and 12% of the sample reported a diagnosis of anxiety (25% in the migraine sample vs 10% of controls). The mean age in the study was 29.5. Fifty-three percent of participants were women and 36% of the population was nonwhite.
All participants filled out a “Mistreatment by Adults” questionnaire about experiences before age 18. Emotional abuse was assessed by asking participants how often a parent or other adult caregiver had said things that hurt their feelings or made them feel unwanted or unloved. Physical abuse was assessed by asking whether a parent or caregiver had hit the participant with a fist, kicked him or her, or threw him or her to the floor, into a wall, or down stairs. And finally, sexual abuse was assessed by asking whether a parent or adult caregiver had touched the participant in a sexual way, forced him or her to touch him or her in a sexual way, or forced him or her to have sexual relations. The dependent variable in the study was self-reported doctor diagnosis of migraine. Sociodemographic characteristics recorded included age, sex, household income, and race. Self-reported physician diagnoses of depression and anxiety were examined as additional confounders.
Abuse was recalled by 60.5% of participants with migraine and 49% of the nonmigraine sample. Abuse increased the chance of migraine diagnosis by 55%. Emotional abuse had the strongest link to migraine, and the link was stronger in males than in females. When the researchers controlled the data for depression and anxiety, the effect of child abuse on migraine was attenuated, but remained significant. In addition, the effect of emotional abuse on migraine decreased, but remained significant when the investigators controlled for depression and anxiety. The researchers found no association between physical and sexual abuse when they adjusted for other abuse types, and there was no association with any abuse type and migraine in the non-Caucasian cohort.
Strengths of this study included its nationally representative population-based sample, its population’s relatively young mean age of 30 (which was 20–30 years younger than populations in other studies), and the inclusion of questions on three major types of abuse. Limitations of the study included its reliance on self-reported, retrospective data on abuse and on physician-diagnosed conditions; its broad definition of emotional abuse; and its potential for recall bias and reporting bias.
The next goal for this investigation is to determine the neurophysiologic mechanisms of the relationship between migraine and emotional abuse, said Dr. Tietjen. The relationship itself needs to be confirmed. “We’re currently looking at the database for gene–environment interactions that may link abuse to migraine for persons with a specific genotype. There’s also an opportunity for investigation of other different kinds of modifications that we know occur with abuse and whether these play a role in determining migraine,” she concluded.
—Erica Robinson
VANCOUVER—Childhood emotional abuse may be associated with migraine in adulthood, according to data presented at the 68th Annual Meeting of the American Academy of Neurology. The research indicates that emotional abuse during childhood has a more significant effect on migraine, compared with physical and sexual abuse.
Childhood maltreatment, which includes neglect and abuse (ie, emotional, physical, and sexual), is confirmed in 12.5% of children by the age of 18, according to a report published in 2014 in JAMA Pediatrics. Previous studies have linked childhood abuse to headache, but there has been limited assessment of other major types of abuse. “In 2015, there were six different meta-analyses from all over the world linking childhood emotional abuse in particular with adult psychiatric disease,” said Gretchen Tietjen, MD, Professor and Chair of Neurology at the University of Toledo in Ohio.
Gretchen Tietjen, MD
Dr. Tietjen and her colleagues conducted a study to better understand the association between emotional abuse and migraine, independent of depression and anxiety. The study’s objectives were to examine the correlation between migraine and the number of types of abuse and frequency of abuse, and to examine the influence of sex and race on migraine. They also strove to determine the temporal relationship between age of onset of abuse, depression, and anxiety and age of onset of migraine.
There were 14,484 participants aged 24 to 32 in the study. About 14% of participants self-reported that they had been diagnosed with migraine. Fifteen percent of the population reported a diagnosis of depression (35% in the migraine sample vs 12% of controls), and 12% of the sample reported a diagnosis of anxiety (25% in the migraine sample vs 10% of controls). The mean age in the study was 29.5. Fifty-three percent of participants were women and 36% of the population was nonwhite.
All participants filled out a “Mistreatment by Adults” questionnaire about experiences before age 18. Emotional abuse was assessed by asking participants how often a parent or other adult caregiver had said things that hurt their feelings or made them feel unwanted or unloved. Physical abuse was assessed by asking whether a parent or caregiver had hit the participant with a fist, kicked him or her, or threw him or her to the floor, into a wall, or down stairs. And finally, sexual abuse was assessed by asking whether a parent or adult caregiver had touched the participant in a sexual way, forced him or her to touch him or her in a sexual way, or forced him or her to have sexual relations. The dependent variable in the study was self-reported doctor diagnosis of migraine. Sociodemographic characteristics recorded included age, sex, household income, and race. Self-reported physician diagnoses of depression and anxiety were examined as additional confounders.
Abuse was recalled by 60.5% of participants with migraine and 49% of the nonmigraine sample. Abuse increased the chance of migraine diagnosis by 55%. Emotional abuse had the strongest link to migraine, and the link was stronger in males than in females. When the researchers controlled the data for depression and anxiety, the effect of child abuse on migraine was attenuated, but remained significant. In addition, the effect of emotional abuse on migraine decreased, but remained significant when the investigators controlled for depression and anxiety. The researchers found no association between physical and sexual abuse when they adjusted for other abuse types, and there was no association with any abuse type and migraine in the non-Caucasian cohort.
Strengths of this study included its nationally representative population-based sample, its population’s relatively young mean age of 30 (which was 20–30 years younger than populations in other studies), and the inclusion of questions on three major types of abuse. Limitations of the study included its reliance on self-reported, retrospective data on abuse and on physician-diagnosed conditions; its broad definition of emotional abuse; and its potential for recall bias and reporting bias.
The next goal for this investigation is to determine the neurophysiologic mechanisms of the relationship between migraine and emotional abuse, said Dr. Tietjen. The relationship itself needs to be confirmed. “We’re currently looking at the database for gene–environment interactions that may link abuse to migraine for persons with a specific genotype. There’s also an opportunity for investigation of other different kinds of modifications that we know occur with abuse and whether these play a role in determining migraine,” she concluded.
—Erica Robinson
Suggested Reading
Tietjen G. Childhood maltreatment and headache disorders. Curr Pain Headache Rep. 2016; 20(4):26.
Wildeman C. Emanuel N, Leventhal J, et al. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr. 2014;168(8):706-713.
Suggested Reading
Tietjen G. Childhood maltreatment and headache disorders. Curr Pain Headache Rep. 2016; 20(4):26.
Wildeman C. Emanuel N, Leventhal J, et al. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr. 2014;168(8):706-713.
Anti-TNF certolizumab pegol effective in early RA
Anti-TNF drug certolizumab pegol in combination with methotrexate achieves significantly better outcomes in early active rheumatoid arthritis than does methotrexate alone, according to researchers.
They conducted a randomized, double-blind, placebo-controlled phase III trial of certolizumab pegol (CZP) in combination with dose-optimized methotrexate (MTX) versus dose-optimized methotrexate and placebo (PBO) in 879 treatment-naive patients with moderate to severe, active, progressive RA, and with poor prognostic factors.
After 1 year of treatment, 28.9% of the 660 patients who received the CZP and MTX combination had achieved sustained remission, compared with 15% of the 219 patients in the PBO+MTX group (P less than .001) (Ann Rheum Dis. 2016 May 10. doi: 10.1136/annrheumdis-2015-209057).
The study also found that 43.8% of patients in the treatment arm achieved sustained low disease activity, compared with 28.6% of the control arm (P less than .001). Adjustment for withdrawals in each arm did not significantly bias the results.
CZP with MTX has shown efficacy both in patients with established RA who have shown an insufficient response to MTX alone, and in MTX-naive individuals with early RA, which the authors said justified further study in recently-diagnosed individuals.
“These data demonstrate that CZP+MTX combination therapy results in a significantly higher proportion of patients achieving sREM [sustained remission] than those treated with PBO+MTX, even when using a ‘treat-to-tolerance’ dosing strategy for MTX,” wrote Dr. Paul Emery of Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds, England, and coauthors.
“Consequently, for patients with poor prognostic factors for severe disease progression, treating early and aggressively may represent a unique opportunity to achieve maximal clinical benefit.”
Significantly more patients treated with CZP and MTX than with PBO achieved an ACR50 response (61.8% vs. 52.6%, P = .023), signifying at least a 50% improvement in the number of tender and swollen joints, and in the patient assessments of disease status, pain, and function.
Patients in the active group also showed greater improvements in physical function, as measured by the Health Assessment Questionnaire–Disability Index, and significantly less radiographic progression. Researchers noted that patients in the active arm of the study showed less joint erosion and less joint space narrowing compared with those in the placebo arm.
“The analysis of radiographic data in C-EARLY demonstrates that CZP+MTX therapy can inhibit structural damage significantly more than MTX alone – the percentage of patients with radiographic nonprogression was significantly higher in the CZP+MTX group compared with the PBO+MTX group,” the authors reported.
The incidence of adverse events was similar in both groups, with nausea, upper respiratory tract infection, urinary tract infection, nasopharyngitis, headache, and increased levels of alanine aminotransferase the most commonly reported events in the active arm.
One death in a patient taking CZP and MTX was caused by disseminated, noncharacterized, mycobacterium infection, which the investigators considered to be related to the study medication. However, the overall rates of serious adverse events and withdrawals due to adverse events were similar between both arms of the study.
Patients given CZP began on a dose of 400 mg at baseline, week 2 and week 4, then 200 mg every 2 weeks until week 52, while the dose of oral MTX used in the study was titrated up from an initial dose of 10 mg/week by 5 mg every 2 weeks, to a maximum of 25 mg/week by week 8.
The authors suggested that the methotrexate titration was an important part of the treatment because it ensured each patient received the maximum-tolerated dose within 8 weeks.
“To our knowledge, there are no previous studies in MTX-naive or DMARD-naive patients with RA where MTX doses have been optimized per-protocol to the levels achieved in C-EARLY,” they wrote. “This optimization may have been responsible, in part, for the response observed for the PBO+MTX and CZP+MTX arms.”
The study and manuscript development was sponsored by UCB Pharma, which also signed off on the manuscript after a review. The authors declared consultancy, speaker’s fees, grants and other funding from a range of pharmaceutical companies including UCB Pharma, and five authors were employees of UCB Pharma.
Anti-TNF drug certolizumab pegol in combination with methotrexate achieves significantly better outcomes in early active rheumatoid arthritis than does methotrexate alone, according to researchers.
They conducted a randomized, double-blind, placebo-controlled phase III trial of certolizumab pegol (CZP) in combination with dose-optimized methotrexate (MTX) versus dose-optimized methotrexate and placebo (PBO) in 879 treatment-naive patients with moderate to severe, active, progressive RA, and with poor prognostic factors.
After 1 year of treatment, 28.9% of the 660 patients who received the CZP and MTX combination had achieved sustained remission, compared with 15% of the 219 patients in the PBO+MTX group (P less than .001) (Ann Rheum Dis. 2016 May 10. doi: 10.1136/annrheumdis-2015-209057).
The study also found that 43.8% of patients in the treatment arm achieved sustained low disease activity, compared with 28.6% of the control arm (P less than .001). Adjustment for withdrawals in each arm did not significantly bias the results.
CZP with MTX has shown efficacy both in patients with established RA who have shown an insufficient response to MTX alone, and in MTX-naive individuals with early RA, which the authors said justified further study in recently-diagnosed individuals.
“These data demonstrate that CZP+MTX combination therapy results in a significantly higher proportion of patients achieving sREM [sustained remission] than those treated with PBO+MTX, even when using a ‘treat-to-tolerance’ dosing strategy for MTX,” wrote Dr. Paul Emery of Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds, England, and coauthors.
“Consequently, for patients with poor prognostic factors for severe disease progression, treating early and aggressively may represent a unique opportunity to achieve maximal clinical benefit.”
Significantly more patients treated with CZP and MTX than with PBO achieved an ACR50 response (61.8% vs. 52.6%, P = .023), signifying at least a 50% improvement in the number of tender and swollen joints, and in the patient assessments of disease status, pain, and function.
Patients in the active group also showed greater improvements in physical function, as measured by the Health Assessment Questionnaire–Disability Index, and significantly less radiographic progression. Researchers noted that patients in the active arm of the study showed less joint erosion and less joint space narrowing compared with those in the placebo arm.
“The analysis of radiographic data in C-EARLY demonstrates that CZP+MTX therapy can inhibit structural damage significantly more than MTX alone – the percentage of patients with radiographic nonprogression was significantly higher in the CZP+MTX group compared with the PBO+MTX group,” the authors reported.
The incidence of adverse events was similar in both groups, with nausea, upper respiratory tract infection, urinary tract infection, nasopharyngitis, headache, and increased levels of alanine aminotransferase the most commonly reported events in the active arm.
One death in a patient taking CZP and MTX was caused by disseminated, noncharacterized, mycobacterium infection, which the investigators considered to be related to the study medication. However, the overall rates of serious adverse events and withdrawals due to adverse events were similar between both arms of the study.
Patients given CZP began on a dose of 400 mg at baseline, week 2 and week 4, then 200 mg every 2 weeks until week 52, while the dose of oral MTX used in the study was titrated up from an initial dose of 10 mg/week by 5 mg every 2 weeks, to a maximum of 25 mg/week by week 8.
The authors suggested that the methotrexate titration was an important part of the treatment because it ensured each patient received the maximum-tolerated dose within 8 weeks.
“To our knowledge, there are no previous studies in MTX-naive or DMARD-naive patients with RA where MTX doses have been optimized per-protocol to the levels achieved in C-EARLY,” they wrote. “This optimization may have been responsible, in part, for the response observed for the PBO+MTX and CZP+MTX arms.”
The study and manuscript development was sponsored by UCB Pharma, which also signed off on the manuscript after a review. The authors declared consultancy, speaker’s fees, grants and other funding from a range of pharmaceutical companies including UCB Pharma, and five authors were employees of UCB Pharma.
Anti-TNF drug certolizumab pegol in combination with methotrexate achieves significantly better outcomes in early active rheumatoid arthritis than does methotrexate alone, according to researchers.
They conducted a randomized, double-blind, placebo-controlled phase III trial of certolizumab pegol (CZP) in combination with dose-optimized methotrexate (MTX) versus dose-optimized methotrexate and placebo (PBO) in 879 treatment-naive patients with moderate to severe, active, progressive RA, and with poor prognostic factors.
After 1 year of treatment, 28.9% of the 660 patients who received the CZP and MTX combination had achieved sustained remission, compared with 15% of the 219 patients in the PBO+MTX group (P less than .001) (Ann Rheum Dis. 2016 May 10. doi: 10.1136/annrheumdis-2015-209057).
The study also found that 43.8% of patients in the treatment arm achieved sustained low disease activity, compared with 28.6% of the control arm (P less than .001). Adjustment for withdrawals in each arm did not significantly bias the results.
CZP with MTX has shown efficacy both in patients with established RA who have shown an insufficient response to MTX alone, and in MTX-naive individuals with early RA, which the authors said justified further study in recently-diagnosed individuals.
“These data demonstrate that CZP+MTX combination therapy results in a significantly higher proportion of patients achieving sREM [sustained remission] than those treated with PBO+MTX, even when using a ‘treat-to-tolerance’ dosing strategy for MTX,” wrote Dr. Paul Emery of Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds, England, and coauthors.
“Consequently, for patients with poor prognostic factors for severe disease progression, treating early and aggressively may represent a unique opportunity to achieve maximal clinical benefit.”
Significantly more patients treated with CZP and MTX than with PBO achieved an ACR50 response (61.8% vs. 52.6%, P = .023), signifying at least a 50% improvement in the number of tender and swollen joints, and in the patient assessments of disease status, pain, and function.
Patients in the active group also showed greater improvements in physical function, as measured by the Health Assessment Questionnaire–Disability Index, and significantly less radiographic progression. Researchers noted that patients in the active arm of the study showed less joint erosion and less joint space narrowing compared with those in the placebo arm.
“The analysis of radiographic data in C-EARLY demonstrates that CZP+MTX therapy can inhibit structural damage significantly more than MTX alone – the percentage of patients with radiographic nonprogression was significantly higher in the CZP+MTX group compared with the PBO+MTX group,” the authors reported.
The incidence of adverse events was similar in both groups, with nausea, upper respiratory tract infection, urinary tract infection, nasopharyngitis, headache, and increased levels of alanine aminotransferase the most commonly reported events in the active arm.
One death in a patient taking CZP and MTX was caused by disseminated, noncharacterized, mycobacterium infection, which the investigators considered to be related to the study medication. However, the overall rates of serious adverse events and withdrawals due to adverse events were similar between both arms of the study.
Patients given CZP began on a dose of 400 mg at baseline, week 2 and week 4, then 200 mg every 2 weeks until week 52, while the dose of oral MTX used in the study was titrated up from an initial dose of 10 mg/week by 5 mg every 2 weeks, to a maximum of 25 mg/week by week 8.
The authors suggested that the methotrexate titration was an important part of the treatment because it ensured each patient received the maximum-tolerated dose within 8 weeks.
“To our knowledge, there are no previous studies in MTX-naive or DMARD-naive patients with RA where MTX doses have been optimized per-protocol to the levels achieved in C-EARLY,” they wrote. “This optimization may have been responsible, in part, for the response observed for the PBO+MTX and CZP+MTX arms.”
The study and manuscript development was sponsored by UCB Pharma, which also signed off on the manuscript after a review. The authors declared consultancy, speaker’s fees, grants and other funding from a range of pharmaceutical companies including UCB Pharma, and five authors were employees of UCB Pharma.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Certolizumab pegol and methotrexate is more efficacious than methotrexate alone in treatment-naive individuals with early active rheumatoid arthritis.
Major finding: After 1 year of treatment, 28.9% of the 660 patients who received the certolizumab pegol and methotrexate combination had achieved sustained remission, compared with 15% of the 219 patients in the methotrexate and placebo group.
Data source: A randomized, double-blind, placebo-controlled phase III trial of 879 treatment-naive patients with moderate to severe, active, progressive rheumatoid arthritis.
Disclosures: The study and manuscript development was sponsored by UCB Pharma, which also signed off on the manuscript after a review. The authors declared consultancy, speaker fees, grants, and other funding from a range of pharmaceutical companies including UCB Pharma, and five authors were employees of UCB Pharma.
Thank you notes: Helpful on bad days
I get the occasional heartfelt thank you note from a patient. I also get hate mail, but fortunately the thank yous predominate.
I still have all of them, going back to residency, in an old Nike box. They sit in a closet at home, taken out here and there. On bad days.
You know what I mean. The days where you screwed up, or had an angry patient get on your nerves and/or in your face. Where the schedule was accidentally double-booked and you were running behind from the start. When you question your abilities and wonder why you are still doing this to yourself.
At the end of those days, I go home, dig out the box, and quietly read a few of the notes. Their neatly folded pages of gratitude remind me why I’m here, why I chose this path, why I need to be clear and ready for the patients depending on me the next day. They help me to realize that there’s more good than bad in this job, and that an unhappy, albeit vocal, few don’t represent most patients. That I really do know what I’m doing, regardless of what Mr. I’m-going-to-complain-about-you-on-Yelp says.
Of course, there are other reminders of what you have be thankfu for, like families and dogs. But sometimes you need a reminder directly from the people for whom you make a difference every day, to let you know that this isn’t just a job. It’s why you once volunteered at a hospital, fought through lorganic chemistry, wrote out 20 (or more) drafts of a personal statement, and studied for the MCAT. Because, once upon a time, this job was just a dream.
I don’t spend a lot of time with the notes – maybe 10 minutes reading a randomly pulled handful, but it’s enough to get me out of a funk. Then the old shoe box is carefully returned to my closet. Until I need it again.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I get the occasional heartfelt thank you note from a patient. I also get hate mail, but fortunately the thank yous predominate.
I still have all of them, going back to residency, in an old Nike box. They sit in a closet at home, taken out here and there. On bad days.
You know what I mean. The days where you screwed up, or had an angry patient get on your nerves and/or in your face. Where the schedule was accidentally double-booked and you were running behind from the start. When you question your abilities and wonder why you are still doing this to yourself.
At the end of those days, I go home, dig out the box, and quietly read a few of the notes. Their neatly folded pages of gratitude remind me why I’m here, why I chose this path, why I need to be clear and ready for the patients depending on me the next day. They help me to realize that there’s more good than bad in this job, and that an unhappy, albeit vocal, few don’t represent most patients. That I really do know what I’m doing, regardless of what Mr. I’m-going-to-complain-about-you-on-Yelp says.
Of course, there are other reminders of what you have be thankfu for, like families and dogs. But sometimes you need a reminder directly from the people for whom you make a difference every day, to let you know that this isn’t just a job. It’s why you once volunteered at a hospital, fought through lorganic chemistry, wrote out 20 (or more) drafts of a personal statement, and studied for the MCAT. Because, once upon a time, this job was just a dream.
I don’t spend a lot of time with the notes – maybe 10 minutes reading a randomly pulled handful, but it’s enough to get me out of a funk. Then the old shoe box is carefully returned to my closet. Until I need it again.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I get the occasional heartfelt thank you note from a patient. I also get hate mail, but fortunately the thank yous predominate.
I still have all of them, going back to residency, in an old Nike box. They sit in a closet at home, taken out here and there. On bad days.
You know what I mean. The days where you screwed up, or had an angry patient get on your nerves and/or in your face. Where the schedule was accidentally double-booked and you were running behind from the start. When you question your abilities and wonder why you are still doing this to yourself.
At the end of those days, I go home, dig out the box, and quietly read a few of the notes. Their neatly folded pages of gratitude remind me why I’m here, why I chose this path, why I need to be clear and ready for the patients depending on me the next day. They help me to realize that there’s more good than bad in this job, and that an unhappy, albeit vocal, few don’t represent most patients. That I really do know what I’m doing, regardless of what Mr. I’m-going-to-complain-about-you-on-Yelp says.
Of course, there are other reminders of what you have be thankfu for, like families and dogs. But sometimes you need a reminder directly from the people for whom you make a difference every day, to let you know that this isn’t just a job. It’s why you once volunteered at a hospital, fought through lorganic chemistry, wrote out 20 (or more) drafts of a personal statement, and studied for the MCAT. Because, once upon a time, this job was just a dream.
I don’t spend a lot of time with the notes – maybe 10 minutes reading a randomly pulled handful, but it’s enough to get me out of a funk. Then the old shoe box is carefully returned to my closet. Until I need it again.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What’s happening in the AGA Community this month?
Your year-round, members-only online networking platform opened to all AGA members last month, right in time for Digestive Disease Week® (DDW) 2016. AGA Community has opened the floor for conversations that build connections and collaborations.
In its first month, AGA Community had 42 new discussion threads, 326 public replies, 77 private replies, and 42 “recommends” (which are equivalent to a “like” on Facebook).
Here are the top discussions happening in the forum, many of which contain topical content from DDW:
• Recertification Board Exam
• Interested in Obesity Management?
• Ask the Expert: Legal Implications of Clinical Practice Guidelines
• Propofol for Colonoscopy
• When Would You Perform the Next Colonoscopy?
Have something to say? Join in or start your own discussion on topics that matter to you. Visit http://community.gastro.org/and sign in to start your AGA Community experience.
Your year-round, members-only online networking platform opened to all AGA members last month, right in time for Digestive Disease Week® (DDW) 2016. AGA Community has opened the floor for conversations that build connections and collaborations.
In its first month, AGA Community had 42 new discussion threads, 326 public replies, 77 private replies, and 42 “recommends” (which are equivalent to a “like” on Facebook).
Here are the top discussions happening in the forum, many of which contain topical content from DDW:
• Recertification Board Exam
• Interested in Obesity Management?
• Ask the Expert: Legal Implications of Clinical Practice Guidelines
• Propofol for Colonoscopy
• When Would You Perform the Next Colonoscopy?
Have something to say? Join in or start your own discussion on topics that matter to you. Visit http://community.gastro.org/and sign in to start your AGA Community experience.
Your year-round, members-only online networking platform opened to all AGA members last month, right in time for Digestive Disease Week® (DDW) 2016. AGA Community has opened the floor for conversations that build connections and collaborations.
In its first month, AGA Community had 42 new discussion threads, 326 public replies, 77 private replies, and 42 “recommends” (which are equivalent to a “like” on Facebook).
Here are the top discussions happening in the forum, many of which contain topical content from DDW:
• Recertification Board Exam
• Interested in Obesity Management?
• Ask the Expert: Legal Implications of Clinical Practice Guidelines
• Propofol for Colonoscopy
• When Would You Perform the Next Colonoscopy?
Have something to say? Join in or start your own discussion on topics that matter to you. Visit http://community.gastro.org/and sign in to start your AGA Community experience.