User login
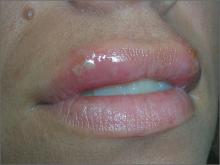
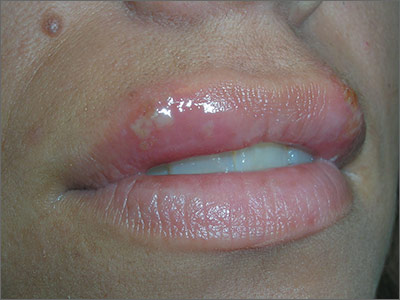
Ulcers on upper lip
The FP diagnosed recurrent orolabial herpes. Orolabial herpes typically takes the form of painful vesicles and ulcerative erosions on the tongue, palate, gingiva, buccal mucosa, and lips. Treatment for primary orolabial herpes includes oral acyclovir (200 mg 5 times daily for 5 days), which accelerates healing by one day and can reduce the mean duration of pain by 36%. Alternatively, valacyclovir can be given 2000 mg orally every 12 hours for one day and, like acyclovir, should be started as soon as possible after the onset of symptoms.
Docosanol cream is available without a prescription for oral herpes. One randomized controlled trial (RCT) of 743 patients with herpes labialis showed a faster healing time in patients treated with docosanol 10% cream when compared with placebo cream (4.1 vs 4.8 days), as well as reduced duration of pain symptoms (2.2 vs 2.7 days).1 More than 90% of patients in both groups healed completely within 10 days.1 Treatment with docosanol cream, when applied 5 times per day and within 12 hours of episode onset until symptoms are relieved, is safe and somewhat effective.
Suppression for patients with frequent recurrences may be provided with valacyclovir 500 mg daily or acyclovir 400 mg twice daily.
Suppression was not indicated for this patient since she had infrequent episodes. She was told that the best prevention would be to use a high-potency sunscreen on her lips and face when out in the sun and to use protective clothing such as a broad brim hat. To prevent skin cancers and recurrent oral labial herpes, she was told to avoid the midday sun whenever possible.
The FP also explained that it was too late now to start oral antiviral treatment, but that she might want to use topical petrolatum for symptom relief. He also offered her a prescription for an oral antiviral medicine that she could fill at the start of symptoms during a future outbreak. He explained that there is some benefit to the over-the-counter topical medicine, docosanol.
1. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed recurrent orolabial herpes. Orolabial herpes typically takes the form of painful vesicles and ulcerative erosions on the tongue, palate, gingiva, buccal mucosa, and lips. Treatment for primary orolabial herpes includes oral acyclovir (200 mg 5 times daily for 5 days), which accelerates healing by one day and can reduce the mean duration of pain by 36%. Alternatively, valacyclovir can be given 2000 mg orally every 12 hours for one day and, like acyclovir, should be started as soon as possible after the onset of symptoms.
Docosanol cream is available without a prescription for oral herpes. One randomized controlled trial (RCT) of 743 patients with herpes labialis showed a faster healing time in patients treated with docosanol 10% cream when compared with placebo cream (4.1 vs 4.8 days), as well as reduced duration of pain symptoms (2.2 vs 2.7 days).1 More than 90% of patients in both groups healed completely within 10 days.1 Treatment with docosanol cream, when applied 5 times per day and within 12 hours of episode onset until symptoms are relieved, is safe and somewhat effective.
Suppression for patients with frequent recurrences may be provided with valacyclovir 500 mg daily or acyclovir 400 mg twice daily.
Suppression was not indicated for this patient since she had infrequent episodes. She was told that the best prevention would be to use a high-potency sunscreen on her lips and face when out in the sun and to use protective clothing such as a broad brim hat. To prevent skin cancers and recurrent oral labial herpes, she was told to avoid the midday sun whenever possible.
The FP also explained that it was too late now to start oral antiviral treatment, but that she might want to use topical petrolatum for symptom relief. He also offered her a prescription for an oral antiviral medicine that she could fill at the start of symptoms during a future outbreak. He explained that there is some benefit to the over-the-counter topical medicine, docosanol.
1. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed recurrent orolabial herpes. Orolabial herpes typically takes the form of painful vesicles and ulcerative erosions on the tongue, palate, gingiva, buccal mucosa, and lips. Treatment for primary orolabial herpes includes oral acyclovir (200 mg 5 times daily for 5 days), which accelerates healing by one day and can reduce the mean duration of pain by 36%. Alternatively, valacyclovir can be given 2000 mg orally every 12 hours for one day and, like acyclovir, should be started as soon as possible after the onset of symptoms.
Docosanol cream is available without a prescription for oral herpes. One randomized controlled trial (RCT) of 743 patients with herpes labialis showed a faster healing time in patients treated with docosanol 10% cream when compared with placebo cream (4.1 vs 4.8 days), as well as reduced duration of pain symptoms (2.2 vs 2.7 days).1 More than 90% of patients in both groups healed completely within 10 days.1 Treatment with docosanol cream, when applied 5 times per day and within 12 hours of episode onset until symptoms are relieved, is safe and somewhat effective.
Suppression for patients with frequent recurrences may be provided with valacyclovir 500 mg daily or acyclovir 400 mg twice daily.
Suppression was not indicated for this patient since she had infrequent episodes. She was told that the best prevention would be to use a high-potency sunscreen on her lips and face when out in the sun and to use protective clothing such as a broad brim hat. To prevent skin cancers and recurrent oral labial herpes, she was told to avoid the midday sun whenever possible.
The FP also explained that it was too late now to start oral antiviral treatment, but that she might want to use topical petrolatum for symptom relief. He also offered her a prescription for an oral antiviral medicine that she could fill at the start of symptoms during a future outbreak. He explained that there is some benefit to the over-the-counter topical medicine, docosanol.
1. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
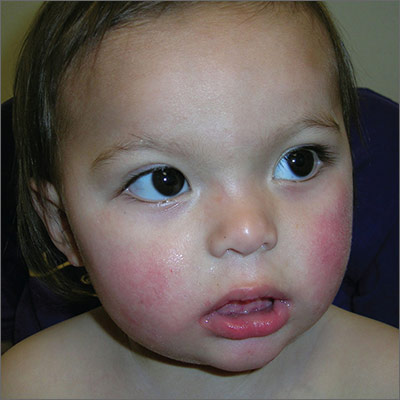
Child with malar rash
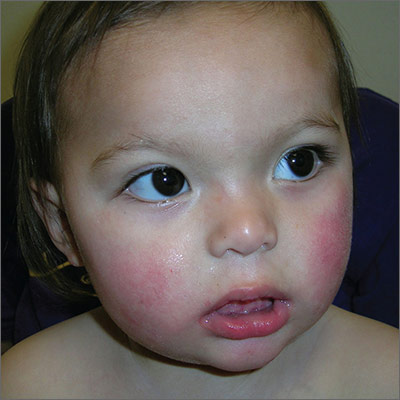
The patient’s “slapped-cheek” appearance led to the diagnosis: fifth disease (erythema infectiosum). The name of the diagnosis derives from the fact that it represents the fifth of 6 common childhood viral exanthems. Transmission of the causative parvovirus B19 occurs through respiratory secretions (possibly through fomites), parenterally via vertical transmission from mother to fetus, or by transfusion of blood or blood products.
Fifth disease is very contagious via the respiratory route and occurs more frequently between late winter and early summer. Up to 60% of the population is seropositive for antiparvovirus B19 immunoglobulin G (IgG) by age 20. Thirty to 40% of pregnant women lack measurable IgG to the infecting agent and are therefore presumed to be susceptible to infection. Infection during pregnancy can, in some cases, lead to fetal death.
Fifth disease is usually a biphasic illness, starting with upper respiratory tract symptoms that may include headache, fever, sore throat, pruritus, coryza, abdominal pain, diarrhea, and/or arthralgias. These constitutional symptoms coincide with the onset of viremia and usually resolve (for about a week) before the next stage begins. The second stage is characterized by a classic erythematous malar rash with relative circumoral pallor (the “slapped-cheek” appearance in children). This is followed by a “lace-like” erythematous rash on the trunk and extremities.
Pregnant women who are exposed to or have symptoms of parvovirus infection should have serologic testing. Fortunately in this case, the mother and the day care providers were not pregnant. The parents were reassured that this would go away on its own. They were told that their son should avoid excessive heat and sunlight, which can cause the rash to flare up. Children who present with the classic skin findings of fifth disease are past the infectious state and can attend school and day care. The child in this case returned to day care the next day with a note from the FP.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Fifth disease. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:728-731.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The patient’s “slapped-cheek” appearance led to the diagnosis: fifth disease (erythema infectiosum). The name of the diagnosis derives from the fact that it represents the fifth of 6 common childhood viral exanthems. Transmission of the causative parvovirus B19 occurs through respiratory secretions (possibly through fomites), parenterally via vertical transmission from mother to fetus, or by transfusion of blood or blood products.
Fifth disease is very contagious via the respiratory route and occurs more frequently between late winter and early summer. Up to 60% of the population is seropositive for antiparvovirus B19 immunoglobulin G (IgG) by age 20. Thirty to 40% of pregnant women lack measurable IgG to the infecting agent and are therefore presumed to be susceptible to infection. Infection during pregnancy can, in some cases, lead to fetal death.
Fifth disease is usually a biphasic illness, starting with upper respiratory tract symptoms that may include headache, fever, sore throat, pruritus, coryza, abdominal pain, diarrhea, and/or arthralgias. These constitutional symptoms coincide with the onset of viremia and usually resolve (for about a week) before the next stage begins. The second stage is characterized by a classic erythematous malar rash with relative circumoral pallor (the “slapped-cheek” appearance in children). This is followed by a “lace-like” erythematous rash on the trunk and extremities.
Pregnant women who are exposed to or have symptoms of parvovirus infection should have serologic testing. Fortunately in this case, the mother and the day care providers were not pregnant. The parents were reassured that this would go away on its own. They were told that their son should avoid excessive heat and sunlight, which can cause the rash to flare up. Children who present with the classic skin findings of fifth disease are past the infectious state and can attend school and day care. The child in this case returned to day care the next day with a note from the FP.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Fifth disease. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:728-731.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The patient’s “slapped-cheek” appearance led to the diagnosis: fifth disease (erythema infectiosum). The name of the diagnosis derives from the fact that it represents the fifth of 6 common childhood viral exanthems. Transmission of the causative parvovirus B19 occurs through respiratory secretions (possibly through fomites), parenterally via vertical transmission from mother to fetus, or by transfusion of blood or blood products.
Fifth disease is very contagious via the respiratory route and occurs more frequently between late winter and early summer. Up to 60% of the population is seropositive for antiparvovirus B19 immunoglobulin G (IgG) by age 20. Thirty to 40% of pregnant women lack measurable IgG to the infecting agent and are therefore presumed to be susceptible to infection. Infection during pregnancy can, in some cases, lead to fetal death.
Fifth disease is usually a biphasic illness, starting with upper respiratory tract symptoms that may include headache, fever, sore throat, pruritus, coryza, abdominal pain, diarrhea, and/or arthralgias. These constitutional symptoms coincide with the onset of viremia and usually resolve (for about a week) before the next stage begins. The second stage is characterized by a classic erythematous malar rash with relative circumoral pallor (the “slapped-cheek” appearance in children). This is followed by a “lace-like” erythematous rash on the trunk and extremities.
Pregnant women who are exposed to or have symptoms of parvovirus infection should have serologic testing. Fortunately in this case, the mother and the day care providers were not pregnant. The parents were reassured that this would go away on its own. They were told that their son should avoid excessive heat and sunlight, which can cause the rash to flare up. Children who present with the classic skin findings of fifth disease are past the infectious state and can attend school and day care. The child in this case returned to day care the next day with a note from the FP.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Fifth disease. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:728-731.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
From The Journal of Family Practice | 2016;65(5).
New Hospitalist Billing Code Should Benefit Hospitalists, Patients
The Centers for Medicare & Medicaid Services (CMS) recently announced that within the year hospitalists will be assigned their own specialty designation code.
Up to 85% of hospitalists are currently designated internal medicine, says Ron Greeno, MD, MHM, founding member of SHM and chair of SHM’s Public Policy Committee, but when it comes to quality metrics—and resulting penalties and bonuses—without a way to distinguish themselves from their clinic-based peers, hospitalists have been disadvantaged.
“It is almost impossible to look good when compared to a world of mostly outpatient physicians,” says Dr. Greeno, chief strategy officer at IPC Healthcare, based in North Hollywood, Calif., and SHM’s president-elect.
Today, hospitalists get lumped together with their office-based internal medicine or primary care counterparts, says Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians, based in Tacoma, Wash. Yet, he says, “The quality metrics should be different because it’s a different scope of practice.”
For example, with the Physician Quality Reporting System (PQRS) in recent years, hospitalists have been evaluated based on their patients’ HbA1c, a measure of their diabetic control over the three months prior to admission. But diabetic patients admitted to the hospital are there because they are sick and much less likely to have been well-managed.
“Hospitalists have had no control over their patients’ outpatient diabetes management during the time leading up to admissions, yet these admitted patients are compared to those in an outpatient setting, where their physicians do have control,” Dr. Sears says.
“[This] skews the data and real reporting patterns that are part of that specialty,” says Raemarie Jimenez, CPC, vice president of certifications and member development at AAPC, a professional organization for medical coders and more. “CMS wants the data it is using to be meaningful.”
Once the code is established, the choice to identify as a hospitalist will fall to individual physicians, hospitals, or hospitalist groups, Dr. Greeno says. The designation is noteworthy since hospital medicine does not have a board certification. Today, there are more than 48,000 hospitalists in the U.S., and the announcement comes as hospitalists celebrate 20 years as a specialty. SHM is calling 2016 the “Year of the Hospitalist.”
The decision to seek a hospitalist-specific billing code first arose at SHM several years ago, Dr. Greeno says, with discussions about the advantages, disadvantages, and possible unintended consequences of pursuing it. At the time, SHM chose to hold off, but that changed recently.
“A lot of thought was put into it, and two and a half years later, it’s very clear we made the right decision,” he says. “More and more depends on your data and a lot of different value-based measures. … The Public Policy Committee decided the benefits probably outweigh the potential risks.”
The billing code should make it easier to compare apples to apples, both for hospitalists and hospitals, and Dr. Sears says it should also enable patients to compare hospitalist performance to make better-informed healthcare decisions.
“When you have three or four hospitals in your community, you can compare inpatient hospitalist performance to determine who is providing the most consistent high-quality outcomes,” he says.
It may also enhance reimbursement, says Jimenez. Multiple providers often see patients in the hospital and handle their care. Two providers with the same designation may round on a patient on the same day and appear to CMS and private payors to deliver the same services.
“If a specialist is called in, or their family medicine provider is also seeing the patient, they will not be of the same designation, and that might help with some denials of payments that family or internal medicine physicians are getting,” she says.
Dr. Greeno also says the code may more effectively demonstrate to CMS that hospitalists do not have enough PQRS metrics to adequately qualify for value-based purchasing.
Yet challenges will remain that a specialty code cannot address. “A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears says. “I don’t think it’s an end-all, be-all, but it’s a place to start.”
SHM will continue to actively push CMS to implement the code, Dr. Greeno says, and it will develop strategies for educating members to help them make the decision that is right for them or their group.
Jimenez believes SHM will be capable of doing much more with the data that emerge through robust use of the code.
“Right now, in the industry, big data is it, and the more you can segregate or report on the specifics of data, the better you are at identifying trends,” she says. “We don’t even know yet about clinical outcomes: Are hospitalists’ patients seeing a better outcome of patient experience versus waiting all day to see a family physician? Are there shorter admission times? Trying to improve patient outcomes and reduce costs are two things CMS is desperately interested in.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
The Centers for Medicare & Medicaid Services (CMS) recently announced that within the year hospitalists will be assigned their own specialty designation code.
Up to 85% of hospitalists are currently designated internal medicine, says Ron Greeno, MD, MHM, founding member of SHM and chair of SHM’s Public Policy Committee, but when it comes to quality metrics—and resulting penalties and bonuses—without a way to distinguish themselves from their clinic-based peers, hospitalists have been disadvantaged.
“It is almost impossible to look good when compared to a world of mostly outpatient physicians,” says Dr. Greeno, chief strategy officer at IPC Healthcare, based in North Hollywood, Calif., and SHM’s president-elect.
Today, hospitalists get lumped together with their office-based internal medicine or primary care counterparts, says Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians, based in Tacoma, Wash. Yet, he says, “The quality metrics should be different because it’s a different scope of practice.”
For example, with the Physician Quality Reporting System (PQRS) in recent years, hospitalists have been evaluated based on their patients’ HbA1c, a measure of their diabetic control over the three months prior to admission. But diabetic patients admitted to the hospital are there because they are sick and much less likely to have been well-managed.
“Hospitalists have had no control over their patients’ outpatient diabetes management during the time leading up to admissions, yet these admitted patients are compared to those in an outpatient setting, where their physicians do have control,” Dr. Sears says.
“[This] skews the data and real reporting patterns that are part of that specialty,” says Raemarie Jimenez, CPC, vice president of certifications and member development at AAPC, a professional organization for medical coders and more. “CMS wants the data it is using to be meaningful.”
Once the code is established, the choice to identify as a hospitalist will fall to individual physicians, hospitals, or hospitalist groups, Dr. Greeno says. The designation is noteworthy since hospital medicine does not have a board certification. Today, there are more than 48,000 hospitalists in the U.S., and the announcement comes as hospitalists celebrate 20 years as a specialty. SHM is calling 2016 the “Year of the Hospitalist.”
The decision to seek a hospitalist-specific billing code first arose at SHM several years ago, Dr. Greeno says, with discussions about the advantages, disadvantages, and possible unintended consequences of pursuing it. At the time, SHM chose to hold off, but that changed recently.
“A lot of thought was put into it, and two and a half years later, it’s very clear we made the right decision,” he says. “More and more depends on your data and a lot of different value-based measures. … The Public Policy Committee decided the benefits probably outweigh the potential risks.”
The billing code should make it easier to compare apples to apples, both for hospitalists and hospitals, and Dr. Sears says it should also enable patients to compare hospitalist performance to make better-informed healthcare decisions.
“When you have three or four hospitals in your community, you can compare inpatient hospitalist performance to determine who is providing the most consistent high-quality outcomes,” he says.
It may also enhance reimbursement, says Jimenez. Multiple providers often see patients in the hospital and handle their care. Two providers with the same designation may round on a patient on the same day and appear to CMS and private payors to deliver the same services.
“If a specialist is called in, or their family medicine provider is also seeing the patient, they will not be of the same designation, and that might help with some denials of payments that family or internal medicine physicians are getting,” she says.
Dr. Greeno also says the code may more effectively demonstrate to CMS that hospitalists do not have enough PQRS metrics to adequately qualify for value-based purchasing.
Yet challenges will remain that a specialty code cannot address. “A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears says. “I don’t think it’s an end-all, be-all, but it’s a place to start.”
SHM will continue to actively push CMS to implement the code, Dr. Greeno says, and it will develop strategies for educating members to help them make the decision that is right for them or their group.
Jimenez believes SHM will be capable of doing much more with the data that emerge through robust use of the code.
“Right now, in the industry, big data is it, and the more you can segregate or report on the specifics of data, the better you are at identifying trends,” she says. “We don’t even know yet about clinical outcomes: Are hospitalists’ patients seeing a better outcome of patient experience versus waiting all day to see a family physician? Are there shorter admission times? Trying to improve patient outcomes and reduce costs are two things CMS is desperately interested in.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
The Centers for Medicare & Medicaid Services (CMS) recently announced that within the year hospitalists will be assigned their own specialty designation code.
Up to 85% of hospitalists are currently designated internal medicine, says Ron Greeno, MD, MHM, founding member of SHM and chair of SHM’s Public Policy Committee, but when it comes to quality metrics—and resulting penalties and bonuses—without a way to distinguish themselves from their clinic-based peers, hospitalists have been disadvantaged.
“It is almost impossible to look good when compared to a world of mostly outpatient physicians,” says Dr. Greeno, chief strategy officer at IPC Healthcare, based in North Hollywood, Calif., and SHM’s president-elect.
Today, hospitalists get lumped together with their office-based internal medicine or primary care counterparts, says Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians, based in Tacoma, Wash. Yet, he says, “The quality metrics should be different because it’s a different scope of practice.”
For example, with the Physician Quality Reporting System (PQRS) in recent years, hospitalists have been evaluated based on their patients’ HbA1c, a measure of their diabetic control over the three months prior to admission. But diabetic patients admitted to the hospital are there because they are sick and much less likely to have been well-managed.
“Hospitalists have had no control over their patients’ outpatient diabetes management during the time leading up to admissions, yet these admitted patients are compared to those in an outpatient setting, where their physicians do have control,” Dr. Sears says.
“[This] skews the data and real reporting patterns that are part of that specialty,” says Raemarie Jimenez, CPC, vice president of certifications and member development at AAPC, a professional organization for medical coders and more. “CMS wants the data it is using to be meaningful.”
Once the code is established, the choice to identify as a hospitalist will fall to individual physicians, hospitals, or hospitalist groups, Dr. Greeno says. The designation is noteworthy since hospital medicine does not have a board certification. Today, there are more than 48,000 hospitalists in the U.S., and the announcement comes as hospitalists celebrate 20 years as a specialty. SHM is calling 2016 the “Year of the Hospitalist.”
The decision to seek a hospitalist-specific billing code first arose at SHM several years ago, Dr. Greeno says, with discussions about the advantages, disadvantages, and possible unintended consequences of pursuing it. At the time, SHM chose to hold off, but that changed recently.
“A lot of thought was put into it, and two and a half years later, it’s very clear we made the right decision,” he says. “More and more depends on your data and a lot of different value-based measures. … The Public Policy Committee decided the benefits probably outweigh the potential risks.”
The billing code should make it easier to compare apples to apples, both for hospitalists and hospitals, and Dr. Sears says it should also enable patients to compare hospitalist performance to make better-informed healthcare decisions.
“When you have three or four hospitals in your community, you can compare inpatient hospitalist performance to determine who is providing the most consistent high-quality outcomes,” he says.
It may also enhance reimbursement, says Jimenez. Multiple providers often see patients in the hospital and handle their care. Two providers with the same designation may round on a patient on the same day and appear to CMS and private payors to deliver the same services.
“If a specialist is called in, or their family medicine provider is also seeing the patient, they will not be of the same designation, and that might help with some denials of payments that family or internal medicine physicians are getting,” she says.
Dr. Greeno also says the code may more effectively demonstrate to CMS that hospitalists do not have enough PQRS metrics to adequately qualify for value-based purchasing.
Yet challenges will remain that a specialty code cannot address. “A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears says. “I don’t think it’s an end-all, be-all, but it’s a place to start.”
SHM will continue to actively push CMS to implement the code, Dr. Greeno says, and it will develop strategies for educating members to help them make the decision that is right for them or their group.
Jimenez believes SHM will be capable of doing much more with the data that emerge through robust use of the code.
“Right now, in the industry, big data is it, and the more you can segregate or report on the specifics of data, the better you are at identifying trends,” she says. “We don’t even know yet about clinical outcomes: Are hospitalists’ patients seeing a better outcome of patient experience versus waiting all day to see a family physician? Are there shorter admission times? Trying to improve patient outcomes and reduce costs are two things CMS is desperately interested in.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
Free Webinars Aim to Help Reduce Admissions, Optimize Glycemic Control
Last month, SHM presented live webinars on two of its signature mentored implementation programs that continue to change the way hospitals manage readmissions and glycemic control.
Find out how Project BOOST can help your hospital reduce preventable readmissions, decrease average length of stay, and improve patient satisfaction with one year of individualized mentoring from a physician leader. Watch the webinar at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide; added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls, and more. View the free webinar to learn more at www.hospitalmedicine.org/gc.
Last month, SHM presented live webinars on two of its signature mentored implementation programs that continue to change the way hospitals manage readmissions and glycemic control.
Find out how Project BOOST can help your hospital reduce preventable readmissions, decrease average length of stay, and improve patient satisfaction with one year of individualized mentoring from a physician leader. Watch the webinar at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide; added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls, and more. View the free webinar to learn more at www.hospitalmedicine.org/gc.
Last month, SHM presented live webinars on two of its signature mentored implementation programs that continue to change the way hospitals manage readmissions and glycemic control.
Find out how Project BOOST can help your hospital reduce preventable readmissions, decrease average length of stay, and improve patient satisfaction with one year of individualized mentoring from a physician leader. Watch the webinar at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide; added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls, and more. View the free webinar to learn more at www.hospitalmedicine.org/gc.
Register for Pediatric Hospital Medicine 2016
Register now. Pediatric Hospital Medicine (PHM) 2016 is the premier educational conference for pediatric hospitalists and other clinicians involved in the care of hospitalized children. This year, PHM 2016 will be held at the Hyatt Regency Chicago in Illinois from July 28 to 31. For the latest information, visit www.phmmeeting.org.
Register now. Pediatric Hospital Medicine (PHM) 2016 is the premier educational conference for pediatric hospitalists and other clinicians involved in the care of hospitalized children. This year, PHM 2016 will be held at the Hyatt Regency Chicago in Illinois from July 28 to 31. For the latest information, visit www.phmmeeting.org.
Register now. Pediatric Hospital Medicine (PHM) 2016 is the premier educational conference for pediatric hospitalists and other clinicians involved in the care of hospitalized children. This year, PHM 2016 will be held at the Hyatt Regency Chicago in Illinois from July 28 to 31. For the latest information, visit www.phmmeeting.org.
Older Patients with Rosacea are Likely to be Diagnosed with Dementia
NEW YORK (Reuters Health) - Older patients with the inflammatory skin disorder rosacea appear significantly more likely to be diagnosed with dementia, according to Danish researchers.
As Dr. Alexander Egeberg told Reuters Health by email, "We found an increased risk of dementia, in particular Alzheimer's disease (AD), in patients with rosacea. The risk was only increased in patients older than 60 years, however."
"Emerging data," he added, "suggest a link between rosacea and neurological disorders. Yet, it is important for patients to remember that the absolute risk is still low."
In an April 28 online paper in Annals of Neurology, Dr. Egeberg, of the University of Copenhagen, and colleagues note that in rosacea there's upregulation of various inflammatory mediators, for example, cytokines, antimicrobial peptides (AMPs), chemokines, and matrix metalloproteinases (MMPs). Similar processes appear to be at work in certain neurodegenerative disorders.
To examine the possible relationship, the team studied data from 1997 to 2012 on almost 5.6 million Danes. Of these, 82,439 had rosacea at baseline. Over a maximum follow-up of 16 years, 99,040 developed dementia, of whom 29,193 were diagnosed with AD.
After adjustment, patients with rosacea were at significantly increased risk of dementia (hazard ratio 1.07) and of AD (HR 1.25). Women were at greater risk of AD (HR 1.28) than men (HR 1.16).
However, stratification by age at study entry showed that the risk of AD was significantly increased only in those enrolled at the age of 60 or more (HR 1.20). When analyses were limited to patients with a hospital dermatologist diagnosis of rosacea, the HR for dementia was 1.42 and for AD it was 1.92.
The current sum of evidence, conclude the investigators,"suggests that certain forms of dementia, in particular AD, have prominent inflammatory components, and MMPs and AMPs may provide mechanistic links for the observed association between rosacea and dementia."
"Increased focus on symptoms of cognitive dysfunction in patients with rosacea may be warranted," they say.
The authors reported no funding or disclosures.
NEW YORK (Reuters Health) - Older patients with the inflammatory skin disorder rosacea appear significantly more likely to be diagnosed with dementia, according to Danish researchers.
As Dr. Alexander Egeberg told Reuters Health by email, "We found an increased risk of dementia, in particular Alzheimer's disease (AD), in patients with rosacea. The risk was only increased in patients older than 60 years, however."
"Emerging data," he added, "suggest a link between rosacea and neurological disorders. Yet, it is important for patients to remember that the absolute risk is still low."
In an April 28 online paper in Annals of Neurology, Dr. Egeberg, of the University of Copenhagen, and colleagues note that in rosacea there's upregulation of various inflammatory mediators, for example, cytokines, antimicrobial peptides (AMPs), chemokines, and matrix metalloproteinases (MMPs). Similar processes appear to be at work in certain neurodegenerative disorders.
To examine the possible relationship, the team studied data from 1997 to 2012 on almost 5.6 million Danes. Of these, 82,439 had rosacea at baseline. Over a maximum follow-up of 16 years, 99,040 developed dementia, of whom 29,193 were diagnosed with AD.
After adjustment, patients with rosacea were at significantly increased risk of dementia (hazard ratio 1.07) and of AD (HR 1.25). Women were at greater risk of AD (HR 1.28) than men (HR 1.16).
However, stratification by age at study entry showed that the risk of AD was significantly increased only in those enrolled at the age of 60 or more (HR 1.20). When analyses were limited to patients with a hospital dermatologist diagnosis of rosacea, the HR for dementia was 1.42 and for AD it was 1.92.
The current sum of evidence, conclude the investigators,"suggests that certain forms of dementia, in particular AD, have prominent inflammatory components, and MMPs and AMPs may provide mechanistic links for the observed association between rosacea and dementia."
"Increased focus on symptoms of cognitive dysfunction in patients with rosacea may be warranted," they say.
The authors reported no funding or disclosures.
NEW YORK (Reuters Health) - Older patients with the inflammatory skin disorder rosacea appear significantly more likely to be diagnosed with dementia, according to Danish researchers.
As Dr. Alexander Egeberg told Reuters Health by email, "We found an increased risk of dementia, in particular Alzheimer's disease (AD), in patients with rosacea. The risk was only increased in patients older than 60 years, however."
"Emerging data," he added, "suggest a link between rosacea and neurological disorders. Yet, it is important for patients to remember that the absolute risk is still low."
In an April 28 online paper in Annals of Neurology, Dr. Egeberg, of the University of Copenhagen, and colleagues note that in rosacea there's upregulation of various inflammatory mediators, for example, cytokines, antimicrobial peptides (AMPs), chemokines, and matrix metalloproteinases (MMPs). Similar processes appear to be at work in certain neurodegenerative disorders.
To examine the possible relationship, the team studied data from 1997 to 2012 on almost 5.6 million Danes. Of these, 82,439 had rosacea at baseline. Over a maximum follow-up of 16 years, 99,040 developed dementia, of whom 29,193 were diagnosed with AD.
After adjustment, patients with rosacea were at significantly increased risk of dementia (hazard ratio 1.07) and of AD (HR 1.25). Women were at greater risk of AD (HR 1.28) than men (HR 1.16).
However, stratification by age at study entry showed that the risk of AD was significantly increased only in those enrolled at the age of 60 or more (HR 1.20). When analyses were limited to patients with a hospital dermatologist diagnosis of rosacea, the HR for dementia was 1.42 and for AD it was 1.92.
The current sum of evidence, conclude the investigators,"suggests that certain forms of dementia, in particular AD, have prominent inflammatory components, and MMPs and AMPs may provide mechanistic links for the observed association between rosacea and dementia."
"Increased focus on symptoms of cognitive dysfunction in patients with rosacea may be warranted," they say.
The authors reported no funding or disclosures.
Team creates bone marrow on a chip
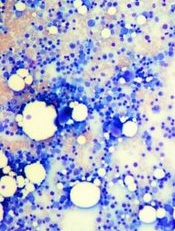
Image by Daniel E. Sabath
Engineered bone marrow grown in a microfluidic chip device mimics living bone marrow, according to research published in Tissue Engineering.
Experiments showed the engineered bone marrow responded in a way similar to living bone marrow when exposed to damaging radiation followed by treatment with compounds that aid in blood cell recovery.
The researchers said this new bone marrow-on-a-chip device holds promise for testing and developing improved radiation countermeasures.
Yu-suke Torisawa, PhD, of Kyoto University in Japan, and his colleagues conducted this research.
The team used a tissue engineering approach to induce formation of new marrow-containing bone in mice. They then surgically removed the bone, placed it in a microfluidic device, and continuously perfused it with medium in vitro.
Next, the researchers set out to determine if the device would keep the engineered bone marrow alive so they could perform tests on it.
To test the system, the team analyzed the dynamics of blood cell production and evaluated the radiation-protecting effects of granulocyte-colony stimulating factor (G-CSF) and bactericidal/permeability-increasing protein (BPI).
Experiments showed the microfluidic device could maintain hematopoietic stem and progenitor cells in normal proportions for at least 2 weeks in culture.
Over time, the researchers observed increases in the number of leukocytes and red blood cells in the microfluidic circulation. And they found that adding erythropoietin induced a significant increase in erythrocyte production.
When the researchers exposed the engineered bone marrow to gamma radiation, they saw reduced leukocyte production.
And when they treated the engineered bone marrow with G-CSF or BPI, the team saw significant increases in the number of hematopoietic stem cells and myeloid cells in the fluidic outflow.
On the other hand, BPI did not have such an effect on static bone marrow cultures. But the researchers pointed out that previous work has shown BPI can accelerate recovery from radiation-induced toxicity in vivo.
The team therefore concluded that, unlike static bone marrow cultures, engineered bone marrow grown in a microfluidic device effectively mimics the recovery response of bone marrow in the body. ![]()

Image by Daniel E. Sabath
Engineered bone marrow grown in a microfluidic chip device mimics living bone marrow, according to research published in Tissue Engineering.
Experiments showed the engineered bone marrow responded in a way similar to living bone marrow when exposed to damaging radiation followed by treatment with compounds that aid in blood cell recovery.
The researchers said this new bone marrow-on-a-chip device holds promise for testing and developing improved radiation countermeasures.
Yu-suke Torisawa, PhD, of Kyoto University in Japan, and his colleagues conducted this research.
The team used a tissue engineering approach to induce formation of new marrow-containing bone in mice. They then surgically removed the bone, placed it in a microfluidic device, and continuously perfused it with medium in vitro.
Next, the researchers set out to determine if the device would keep the engineered bone marrow alive so they could perform tests on it.
To test the system, the team analyzed the dynamics of blood cell production and evaluated the radiation-protecting effects of granulocyte-colony stimulating factor (G-CSF) and bactericidal/permeability-increasing protein (BPI).
Experiments showed the microfluidic device could maintain hematopoietic stem and progenitor cells in normal proportions for at least 2 weeks in culture.
Over time, the researchers observed increases in the number of leukocytes and red blood cells in the microfluidic circulation. And they found that adding erythropoietin induced a significant increase in erythrocyte production.
When the researchers exposed the engineered bone marrow to gamma radiation, they saw reduced leukocyte production.
And when they treated the engineered bone marrow with G-CSF or BPI, the team saw significant increases in the number of hematopoietic stem cells and myeloid cells in the fluidic outflow.
On the other hand, BPI did not have such an effect on static bone marrow cultures. But the researchers pointed out that previous work has shown BPI can accelerate recovery from radiation-induced toxicity in vivo.
The team therefore concluded that, unlike static bone marrow cultures, engineered bone marrow grown in a microfluidic device effectively mimics the recovery response of bone marrow in the body. ![]()

Image by Daniel E. Sabath
Engineered bone marrow grown in a microfluidic chip device mimics living bone marrow, according to research published in Tissue Engineering.
Experiments showed the engineered bone marrow responded in a way similar to living bone marrow when exposed to damaging radiation followed by treatment with compounds that aid in blood cell recovery.
The researchers said this new bone marrow-on-a-chip device holds promise for testing and developing improved radiation countermeasures.
Yu-suke Torisawa, PhD, of Kyoto University in Japan, and his colleagues conducted this research.
The team used a tissue engineering approach to induce formation of new marrow-containing bone in mice. They then surgically removed the bone, placed it in a microfluidic device, and continuously perfused it with medium in vitro.
Next, the researchers set out to determine if the device would keep the engineered bone marrow alive so they could perform tests on it.
To test the system, the team analyzed the dynamics of blood cell production and evaluated the radiation-protecting effects of granulocyte-colony stimulating factor (G-CSF) and bactericidal/permeability-increasing protein (BPI).
Experiments showed the microfluidic device could maintain hematopoietic stem and progenitor cells in normal proportions for at least 2 weeks in culture.
Over time, the researchers observed increases in the number of leukocytes and red blood cells in the microfluidic circulation. And they found that adding erythropoietin induced a significant increase in erythrocyte production.
When the researchers exposed the engineered bone marrow to gamma radiation, they saw reduced leukocyte production.
And when they treated the engineered bone marrow with G-CSF or BPI, the team saw significant increases in the number of hematopoietic stem cells and myeloid cells in the fluidic outflow.
On the other hand, BPI did not have such an effect on static bone marrow cultures. But the researchers pointed out that previous work has shown BPI can accelerate recovery from radiation-induced toxicity in vivo.
The team therefore concluded that, unlike static bone marrow cultures, engineered bone marrow grown in a microfluidic device effectively mimics the recovery response of bone marrow in the body. ![]()
Transparency doesn’t lower healthcare spending

Photo by Petr Kratochvil
Providing patients with a tool that enabled them to search for healthcare prices did not decrease their spending, according to a study published in JAMA.
Researchers studied the Truven Health Analytics Treatment Cost Calculator, an online price transparency tool that tells users how much they would pay out of pocket for services such as X-rays, lab tests, outpatient surgeries, or physician office visits at different sites.
The out-of-pocket cost estimates are based on the users’ health plan benefits and on how much they have already spent on healthcare during the year.
Two large national companies offered this tool to their employees in 2011 and 2012.
The researchers compared the healthcare spending patterns of employees (n=148,655) at these companies in the year before and after the tool was introduced with patterns among employees (n=295,983) of other companies that did not offer the tool.
Overall, having access to the tool was not associated with a reduction in outpatient spending, and subjects did not switch from more expensive outpatient hospital-based care to lower-cost settings.
The average outpatient spending among employees offered the tool was $2021 in the year before the tool was introduced and $2233 in the year after. Among control subjects, average outpatient spending increased from $1985 to $2138.
The average outpatient out-of-pocket spending among employees offered the tool was $507 in the year before it was introduced and $555 in the year after. In the control group, the average outpatient out-of-pocket spending increased from $490 to $520.
After the researchers adjusted for demographic and health characteristics, being offered the tool was associated with an average $59 increase in outpatient spending and an average $18 increase in out-of-pocket spending.
When the researchers looked only at patients with higher deductibles—who would be expected to have greater price-shopping incentives—they also found no evidence of reduction in spending.
“Despite large variation in healthcare prices, prevalence of high-deductible health plans, and widespread interest in price transparency, we did not find evidence that offering price transparency to employees generated savings,” said study author Sunita Desai, PhD, of Harvard Medical School in Boston, Massachusetts.
A possible explanation for this finding is that most patients did not actually use the tool. Only 10% of the employees who were offered the tool used it at least once in the first 12 months.
When patients did use the tool, more than half the searches were for relatively expensive services of over $1000.
“For expensive care that exceeds their deductible, patients may not see any reason to switch,” said study author Ateev Mehrotra, MD, also of Harvard Medical School. “They do not save by choosing a lower-cost provider, even if the health plan does.”
Still, the researchers said the tool does provide patients with valuable information, including their expected out-of-pocket costs, their deductible, and their health plan’s provider network.
“People might use the tools more—and focus more on choosing lower-priced care options—if they are combined with additional health plan benefit features that give greater incentive to price shop,” Dr Desai said. ![]()

Photo by Petr Kratochvil
Providing patients with a tool that enabled them to search for healthcare prices did not decrease their spending, according to a study published in JAMA.
Researchers studied the Truven Health Analytics Treatment Cost Calculator, an online price transparency tool that tells users how much they would pay out of pocket for services such as X-rays, lab tests, outpatient surgeries, or physician office visits at different sites.
The out-of-pocket cost estimates are based on the users’ health plan benefits and on how much they have already spent on healthcare during the year.
Two large national companies offered this tool to their employees in 2011 and 2012.
The researchers compared the healthcare spending patterns of employees (n=148,655) at these companies in the year before and after the tool was introduced with patterns among employees (n=295,983) of other companies that did not offer the tool.
Overall, having access to the tool was not associated with a reduction in outpatient spending, and subjects did not switch from more expensive outpatient hospital-based care to lower-cost settings.
The average outpatient spending among employees offered the tool was $2021 in the year before the tool was introduced and $2233 in the year after. Among control subjects, average outpatient spending increased from $1985 to $2138.
The average outpatient out-of-pocket spending among employees offered the tool was $507 in the year before it was introduced and $555 in the year after. In the control group, the average outpatient out-of-pocket spending increased from $490 to $520.
After the researchers adjusted for demographic and health characteristics, being offered the tool was associated with an average $59 increase in outpatient spending and an average $18 increase in out-of-pocket spending.
When the researchers looked only at patients with higher deductibles—who would be expected to have greater price-shopping incentives—they also found no evidence of reduction in spending.
“Despite large variation in healthcare prices, prevalence of high-deductible health plans, and widespread interest in price transparency, we did not find evidence that offering price transparency to employees generated savings,” said study author Sunita Desai, PhD, of Harvard Medical School in Boston, Massachusetts.
A possible explanation for this finding is that most patients did not actually use the tool. Only 10% of the employees who were offered the tool used it at least once in the first 12 months.
When patients did use the tool, more than half the searches were for relatively expensive services of over $1000.
“For expensive care that exceeds their deductible, patients may not see any reason to switch,” said study author Ateev Mehrotra, MD, also of Harvard Medical School. “They do not save by choosing a lower-cost provider, even if the health plan does.”
Still, the researchers said the tool does provide patients with valuable information, including their expected out-of-pocket costs, their deductible, and their health plan’s provider network.
“People might use the tools more—and focus more on choosing lower-priced care options—if they are combined with additional health plan benefit features that give greater incentive to price shop,” Dr Desai said. ![]()

Photo by Petr Kratochvil
Providing patients with a tool that enabled them to search for healthcare prices did not decrease their spending, according to a study published in JAMA.
Researchers studied the Truven Health Analytics Treatment Cost Calculator, an online price transparency tool that tells users how much they would pay out of pocket for services such as X-rays, lab tests, outpatient surgeries, or physician office visits at different sites.
The out-of-pocket cost estimates are based on the users’ health plan benefits and on how much they have already spent on healthcare during the year.
Two large national companies offered this tool to their employees in 2011 and 2012.
The researchers compared the healthcare spending patterns of employees (n=148,655) at these companies in the year before and after the tool was introduced with patterns among employees (n=295,983) of other companies that did not offer the tool.
Overall, having access to the tool was not associated with a reduction in outpatient spending, and subjects did not switch from more expensive outpatient hospital-based care to lower-cost settings.
The average outpatient spending among employees offered the tool was $2021 in the year before the tool was introduced and $2233 in the year after. Among control subjects, average outpatient spending increased from $1985 to $2138.
The average outpatient out-of-pocket spending among employees offered the tool was $507 in the year before it was introduced and $555 in the year after. In the control group, the average outpatient out-of-pocket spending increased from $490 to $520.
After the researchers adjusted for demographic and health characteristics, being offered the tool was associated with an average $59 increase in outpatient spending and an average $18 increase in out-of-pocket spending.
When the researchers looked only at patients with higher deductibles—who would be expected to have greater price-shopping incentives—they also found no evidence of reduction in spending.
“Despite large variation in healthcare prices, prevalence of high-deductible health plans, and widespread interest in price transparency, we did not find evidence that offering price transparency to employees generated savings,” said study author Sunita Desai, PhD, of Harvard Medical School in Boston, Massachusetts.
A possible explanation for this finding is that most patients did not actually use the tool. Only 10% of the employees who were offered the tool used it at least once in the first 12 months.
When patients did use the tool, more than half the searches were for relatively expensive services of over $1000.
“For expensive care that exceeds their deductible, patients may not see any reason to switch,” said study author Ateev Mehrotra, MD, also of Harvard Medical School. “They do not save by choosing a lower-cost provider, even if the health plan does.”
Still, the researchers said the tool does provide patients with valuable information, including their expected out-of-pocket costs, their deductible, and their health plan’s provider network.
“People might use the tools more—and focus more on choosing lower-priced care options—if they are combined with additional health plan benefit features that give greater incentive to price shop,” Dr Desai said. ![]()
FDA grants priority review for blinatumomab

and solution for infusion
Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application for blinatumomab (Blincyto) as a treatment for pediatric and adolescent patients with Philadelphia chromosome negative (Ph-) relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The Prescription Drug User Fee Act target action date for the supplemental biologics license application for blinatumomab is September 1, 2016.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab was previously granted breakthrough therapy and priority review designations by the FDA and is now approved in the US for the treatment of adults with Ph- relapsed or refractory B-cell precursor ALL.
This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials.
Blinatumomab is marketed by Amgen as Blincyto. The full US prescribing information is available at www.BLINCYTO.com.
‘205 trial
The supplemental biologics license application for blinatumomab in pediatric and adolescent patients is based on data from the phase 1/2 '205 trial.
In this study, researchers evaluated blinatumomab in patients younger than 18 years of age. The patients had B-cell precursor ALL that was refractory, had relapsed at least twice, or relapsed after an allogeneic hematopoietic stem cell transplant.
Treatment in this study has been completed, and subjects are being monitored for long-term efficacy. The data is being submitted for publication.
Preliminary data were presented at the 2014 ASH Annual Meeting (abstract 3703). ![]()

and solution for infusion
Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application for blinatumomab (Blincyto) as a treatment for pediatric and adolescent patients with Philadelphia chromosome negative (Ph-) relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The Prescription Drug User Fee Act target action date for the supplemental biologics license application for blinatumomab is September 1, 2016.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab was previously granted breakthrough therapy and priority review designations by the FDA and is now approved in the US for the treatment of adults with Ph- relapsed or refractory B-cell precursor ALL.
This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials.
Blinatumomab is marketed by Amgen as Blincyto. The full US prescribing information is available at www.BLINCYTO.com.
‘205 trial
The supplemental biologics license application for blinatumomab in pediatric and adolescent patients is based on data from the phase 1/2 '205 trial.
In this study, researchers evaluated blinatumomab in patients younger than 18 years of age. The patients had B-cell precursor ALL that was refractory, had relapsed at least twice, or relapsed after an allogeneic hematopoietic stem cell transplant.
Treatment in this study has been completed, and subjects are being monitored for long-term efficacy. The data is being submitted for publication.
Preliminary data were presented at the 2014 ASH Annual Meeting (abstract 3703). ![]()

and solution for infusion
Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application for blinatumomab (Blincyto) as a treatment for pediatric and adolescent patients with Philadelphia chromosome negative (Ph-) relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The Prescription Drug User Fee Act target action date for the supplemental biologics license application for blinatumomab is September 1, 2016.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab was previously granted breakthrough therapy and priority review designations by the FDA and is now approved in the US for the treatment of adults with Ph- relapsed or refractory B-cell precursor ALL.
This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials.
Blinatumomab is marketed by Amgen as Blincyto. The full US prescribing information is available at www.BLINCYTO.com.
‘205 trial
The supplemental biologics license application for blinatumomab in pediatric and adolescent patients is based on data from the phase 1/2 '205 trial.
In this study, researchers evaluated blinatumomab in patients younger than 18 years of age. The patients had B-cell precursor ALL that was refractory, had relapsed at least twice, or relapsed after an allogeneic hematopoietic stem cell transplant.
Treatment in this study has been completed, and subjects are being monitored for long-term efficacy. The data is being submitted for publication.
Preliminary data were presented at the 2014 ASH Annual Meeting (abstract 3703). ![]()
Low fitness levels linked to platelet activation in women

Women with poor physical fitness may have an increased risk of thrombotic events due to increased platelet activation, according to research published in Medicine & Science in Sports & Exercise.
In a study of 62 young women, those with poor cardiorespiratory fitness (CRF) had significantly higher platelet activation than women with average to very good CRF.
However, the study also showed that women with poor CRF were able to achieve normal platelet function by increasing their physical fitness.
The women achieved platelet normalization via endurance training—running for a maximum of 40 minutes—3 times a week over a 2-month period.
“Latently activated platelets release a number of mediators that can encourage the development of atherosclerotic vascular changes,” said study author Stefan Heber, of the Medical University of Vienna in Austria.
“If poor physical fitness is accompanied by a higher level of platelet activation, one can conclude that it also has an influence upon the early stages of this pathogenesis. The training effects we’ve found here are consistent with epidemiological data, according to which fit people have an approximately 40% lower risk of cardiovascular events than those who were physically inactive.”
To uncover their findings, Heber and his colleagues analyzed different aspects of platelet function in healthy, non-smoking women with low CRF, medium CRF, and high CRF.
The team assessed CRF using maximal oxygen consumption (VO2max), which was determined by an incremental treadmill exercise test. VO2max criteria were:
- less than 45 ml/min/kg bodyweight for the low CRF group
- 45 to 55 ml/min/kg bodyweight for the medium CRF group
- more than 55 ml/min/kg bodyweight for the high CRF group.
The researchers assessed platelet activation state and platelet reactivity in these groups of women by evaluating basal and agonist-induced surface expression of CD62P and CD40L, as well as the intraplatelet amount of reactive oxygen species.
The team observed higher basal platelet activation and agonist-induced platelet reactivity in the low CRF group, when compared to the medium and high CRF groups. However, platelet activation and reactivity were roughly the same in the medium and high CRF groups.
For the low CRF group, the researchers assessed platelet function again after the women completed a supervised endurance training program that lasted 2 menstrual cycles. The team found this program was able to normalize platelet function. ![]()

Women with poor physical fitness may have an increased risk of thrombotic events due to increased platelet activation, according to research published in Medicine & Science in Sports & Exercise.
In a study of 62 young women, those with poor cardiorespiratory fitness (CRF) had significantly higher platelet activation than women with average to very good CRF.
However, the study also showed that women with poor CRF were able to achieve normal platelet function by increasing their physical fitness.
The women achieved platelet normalization via endurance training—running for a maximum of 40 minutes—3 times a week over a 2-month period.
“Latently activated platelets release a number of mediators that can encourage the development of atherosclerotic vascular changes,” said study author Stefan Heber, of the Medical University of Vienna in Austria.
“If poor physical fitness is accompanied by a higher level of platelet activation, one can conclude that it also has an influence upon the early stages of this pathogenesis. The training effects we’ve found here are consistent with epidemiological data, according to which fit people have an approximately 40% lower risk of cardiovascular events than those who were physically inactive.”
To uncover their findings, Heber and his colleagues analyzed different aspects of platelet function in healthy, non-smoking women with low CRF, medium CRF, and high CRF.
The team assessed CRF using maximal oxygen consumption (VO2max), which was determined by an incremental treadmill exercise test. VO2max criteria were:
- less than 45 ml/min/kg bodyweight for the low CRF group
- 45 to 55 ml/min/kg bodyweight for the medium CRF group
- more than 55 ml/min/kg bodyweight for the high CRF group.
The researchers assessed platelet activation state and platelet reactivity in these groups of women by evaluating basal and agonist-induced surface expression of CD62P and CD40L, as well as the intraplatelet amount of reactive oxygen species.
The team observed higher basal platelet activation and agonist-induced platelet reactivity in the low CRF group, when compared to the medium and high CRF groups. However, platelet activation and reactivity were roughly the same in the medium and high CRF groups.
For the low CRF group, the researchers assessed platelet function again after the women completed a supervised endurance training program that lasted 2 menstrual cycles. The team found this program was able to normalize platelet function. ![]()

Women with poor physical fitness may have an increased risk of thrombotic events due to increased platelet activation, according to research published in Medicine & Science in Sports & Exercise.
In a study of 62 young women, those with poor cardiorespiratory fitness (CRF) had significantly higher platelet activation than women with average to very good CRF.
However, the study also showed that women with poor CRF were able to achieve normal platelet function by increasing their physical fitness.
The women achieved platelet normalization via endurance training—running for a maximum of 40 minutes—3 times a week over a 2-month period.
“Latently activated platelets release a number of mediators that can encourage the development of atherosclerotic vascular changes,” said study author Stefan Heber, of the Medical University of Vienna in Austria.
“If poor physical fitness is accompanied by a higher level of platelet activation, one can conclude that it also has an influence upon the early stages of this pathogenesis. The training effects we’ve found here are consistent with epidemiological data, according to which fit people have an approximately 40% lower risk of cardiovascular events than those who were physically inactive.”
To uncover their findings, Heber and his colleagues analyzed different aspects of platelet function in healthy, non-smoking women with low CRF, medium CRF, and high CRF.
The team assessed CRF using maximal oxygen consumption (VO2max), which was determined by an incremental treadmill exercise test. VO2max criteria were:
- less than 45 ml/min/kg bodyweight for the low CRF group
- 45 to 55 ml/min/kg bodyweight for the medium CRF group
- more than 55 ml/min/kg bodyweight for the high CRF group.
The researchers assessed platelet activation state and platelet reactivity in these groups of women by evaluating basal and agonist-induced surface expression of CD62P and CD40L, as well as the intraplatelet amount of reactive oxygen species.
The team observed higher basal platelet activation and agonist-induced platelet reactivity in the low CRF group, when compared to the medium and high CRF groups. However, platelet activation and reactivity were roughly the same in the medium and high CRF groups.
For the low CRF group, the researchers assessed platelet function again after the women completed a supervised endurance training program that lasted 2 menstrual cycles. The team found this program was able to normalize platelet function. ![]()