User login
Comanagement Following CRC Surgery
Colorectal cancer (CRC) is the second most common malignancy in the United States. In 2013, an estimated 142,820 men and women will be newly diagnosed, and 50,830 patients will die from colon or rectal cancer.[1] The majority of patients are aged 65 years or older at diagnosis, and with a growing elderly population, the CRC burden assumes increasing importance in this patient population.[1] Surgery remains the most important treatment option; however, surgical management of older CRC patients is often complicated because of the attendant comorbidities.[2, 3]
Recently, comanagement of surgical patients during their hospital stay has increased substantially in an effort to provide care to complex patients.[4, 5] Comanagement includes daily assessment of acute issues and comorbidities, and communication with surgeons by physicians including hospitalists and internists.[6] The presumed benefits of this comanaged approach to patient care include increased prescribing of evidence‐based treatments,[7] reduced time to surgery,[8] fewer transfers to an intensive care unit,[9] fewer postoperative complications,[9, 10, 11] shortened length of hospital stay,[12, 13] and lower readmission rates.[7] Information regarding detailed characteristics of patients receiving comanagement during hospitalization, specifically for CRC surgery, is lacking. This is an important consideration because comanagement may be particularly beneficial for CRC patients, who tend to be older at diagnosis and may have multiple comorbidities.[5] Hospitalists may be especially important for postoperative management of CRC patients, depending upon the complexity of the surgery and also the need for close medical and surgical monitoring in the perioperative setting, particularly among older CRC patients. Many CRC patients develop complications following surgery,[14] and it is possible that these patients may be rescued by comanagement with hospitalist physicians. Our aim was to assess the use of and characteristics associated with comanagement of patients undergoing surgical intervention for CRC.
METHODS
We obtained data from an existing linkage of 2000 to 2005 National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program data with 1999 to 2005 Medicare claim files from the Centers for Medicare and Medicaid Services (CMS).[15] In this study, data from 16 tumor registries and 1242 hospitals were included, covering approximately 28% of the US population. We selected patients aged 66 years or older with a first primary stage I to III colon or rectal cancer diagnosis between 2000 and 2005. Patients were eligible for inclusion when they had both Medicare parts A and B coverage and underwent surgical treatment for CRC. We excluded patients identified from autopsy or death certificate only, patients for whom the month of diagnosis was not available, and patients who were members of a Health Maintenance Organization and thereby lacking Medicare claims data. We excluded patients who were only eligible for Medicare because they were disabled or had end‐stage renal disease (ie, patients younger than 65 years of age). The age restriction of 66 years of age or older allowed for 1 year of Medicare claims data prior to CRC diagnosis to determine preexisting comorbidity. We used 1999 Medicare data to obtain information about comorbidity for CRC patients who were diagnosed in 2000. Definitive surgery for CRC was measured by searching inpatient, outpatient, and carrier claims using previously identified Healthcare Common Procedure Coding System and/or International Classification of Diseases, 9th Revision (ICD‐9). In the event of multiple surgical interventions in 1 patient record, the date on which the most invasive surgery was performed was used.[16]
Comanagement Use
The outcome of interest was whether or not CRC patients received comanaged care during hospitalization following surgical treatment (comanagement prior to surgery was not included). Comanagement was defined as having a relevant physician (ie, internal medicine hospitalist/generalist) submit a claim for evaluation and management services on 70% or more of the days of hospitalization of the patient, including partial days of admission and discharge. To identify inpatient physician claims, we used the Common Procedure TerminologyEvaluation and Management codes 99221 to 99223, 99251 to 99255, and 99231 to 99233.[5, 17]
Covariates
Characteristics potentially associated with comanagement use included patient, tumor, treatment, and hospital characteristics based on previous work.[4, 5] Patient sociodemographic characteristics included sex, age, race/ethnicity, comorbidity, dual eligibility for Medicare and Medicaid, and year of CRC diagnosis. Comorbidity was measured by searching inpatient or carrier claims for multiple chronic diseases occurring 1 to 12 months prior to diagnosis. We categorized comorbidity as a score of 0, 1, or 2 or more and computed an index score[18] to examine nonlinear trends. Dual MedicareMedicaid participation was present if a patient had Medicaid coverage for at least 1 month during the year before diagnosis. We used census‐tract poverty rate as a measure of neighborhood economic condition, Which was defined as the percentage of the population living in poverty in the census tract of the patient's residence at the time of diagnosis and was derived from the 2000 census. Tumor characteristics included American Joint Commission on Cancer (AJCC) tumor stage, grade, location, and histology. Treatment characteristics included mode of presentation, type of surgery, length of hospital stay, and presence of complications during hospitalization.[19, 20] Complications (eg, postoperative pneumonia, surgical‐site infection, deep vein thrombosis) during hospitalization but following CRC surgery were identified from Medicare data using a previously developed algorithm.[19] The complications algorithm was developed by clinical experts and consists of ICD‐9 diagnosis and procedure codes representing CRC‐resection complications and their treatment, including additional operations. Surgical interventions after emergency admission were defined as emergency surgery; all other surgical treatments were classified as elective. Additionally, characteristics of the hospital where the patient's surgery took place included number of hospital beds, obtained from the Healthcare Cost Report and the Provider of Service files from CMS, and surgeon caseload, based on the number of CRC surgeries performed during the study period. The hospital's surgery volume was calculated using the number of CRC surgeries performed during the study period and categorized into quartiles.
Statistical Analysis
Proportions of comanaged patients were calculated for each covariate. Univariable logistic regression analyses were performed to assess the association between comanagement use and each covariate separately. All analyses were adjusted for nesting of CRC patients within hospitals, using a 2‐level model with a random intercept. Next, we fitted a multivariable model that included covariates associated with comanagement in univariable analysis based on the likelihood ratio test (P < 0.05). Inclusion of covariates in the final multivariable model was based on statistically significant associations with comanagement. Adjusted odds ratios (ORs) were calculated with 95% confidence intervals. Variability across hospitals was calculated using the intraclass coefficient (ICC) for logistic regression.[21] The ICC ranges from 0.0 (no variability across hospitals) to 1.0 (extreme variability across hospitals). In addition, we calculated adjusted values for comanagement to describe the hospital variability. These adjusted values were computed based on the multivariable model by averaging the patient‐level probabilities for all patients who resided in that hospital. We conducted a series of analyses to challenge the robustness of our results. To investigate the potential effect of a different definition of comanagement, we defined comanagement as both (1) having a relevant physician submit a claim for evaluation and management services on 50% or more of the days of the patient's hospitalization, and (2) having a relevant hospitalist or generalist physician submit a claim for evaluation and management services on 100% of the days of hospitalization. All analyses were performed using Stata (version 12.0; StataCorp, College Station, TX).
RESULTS
There were 47,828 patients aged 66 years or older who were diagnosed with a first primary CRC between 2000 and 2005, had both Medicare parts A and B, and were not members of a Health Maintenance Organization during the study period. We excluded patients for whom we were unable to calculate complications (n = 2649), surgery or hospital volume (n = 6415), or who had missing data (n = 238). Finally, we excluded CRC patients with in situ, stage IV, or missing stage information, resulting in 37,065 patients in the study population. Patients in the study population were typically aged 75 to 84 years, were female, had 1 or more comorbid condition, lived in areas with the population poverty rate <10%, were diagnosed with AJCC stage II, had elective surgery, and were treated with partial colectomy (Table 1). During 2000 to 2005, 10,230 (27.6%) of 37,065 patients were comanaged during hospitalization for CRC surgery. In a model with no covariates, variability of comanagement across hospitals was significant (ICC = 0.382). All patient and hospital characteristics except for sex and race/ethnicity were significantly associated with comanagement use, when adjusted for clustering within hospitals. The most common characteristics associated with comanagement were emergency surgery (40.4%), complications (39.7%), and having 2 or more comorbid conditions (37.1%). Comanagement was less common among those with no comorbidity (21.0%), unknown tumor grade (21.9%), and other surgery (18.6%). Furthermore, comanagement increased from 24.8% in 2000 to 30.1% in 2005 (P for trend < 0.001; Table 1).
| Covariates | Total, N = 37,065 | Comanaged, n (%), n = 10,230 (27.6%) |
|---|---|---|
| ||
| Sociodemographics | ||
| Age, y | ||
| 6674 | 13,197 | 3,014 (22.8) |
| 7584 | 6,958 | 4,736 (27.9) |
| 85+ | 6,910 | 2,480 (35.9) |
| Sex | ||
| Male | 15,744 | 4,345 (27.6) |
| Female | 21,321 | 5,885 (27.6) |
| Racea | ||
| White | 31,958 | 8,579 (26.8) |
| African American | 2,641 | 745 (28.2) |
| Other | 2,466 | 906 (36.7) |
| Comorbiditya | ||
| 0 | 15,905 | 3,342 (21.0) |
| 1 | 10,860 | 3,068 (28.3) |
| 2+ | 10,300 | 3,820 (37.1) |
| Medicaida | ||
| No | 31,099 | 8,000 (25.7) |
| Yes | 5,966 | 2,230 (37.4) |
| Year of diagnosisa | ||
| 2000 | 6,041 | 1,497 (24.8) |
| 2001 | 6,193 | 1,624 (26.2) |
| 2002 | 6,289 | 1,692 (26.9) |
| 2003 | 6,489 | 1,829 (28.2) |
| 2004 | 6,219 | 1,832 (29.5) |
| 2005 | 5,834 | 1,756 (30.1) |
| Neighborhood characteristic | ||
| Poverty ratea | ||
| <10% | 21,772 | 5,698(26.2) |
| 10%19% | 9,458 | 2,722(28.8) |
| 20% | 5,835 | 1,810(31.0) |
| Tumor characteristics | ||
| AJCC stagea | ||
| I | 9,996 | 2,402 (24.0) |
| II | 14,662 | 4,287 (29.2) |
| III | 12,407 | 3,541 (28.5) |
| Tumor grade/differentiationa | ||
| Well | 3,278 | 799 (24.4) |
| Moderate | 25,129 | 6,905 (27.5) |
| Poor | 6,898 | 2,107 (30.6) |
| Undifferentiated | 350 | 110 (31.4) |
| Unknown | 1,410 | 309 (21.9) |
| Tumor locationa | ||
| Rectum | 6,977 | 1,616 (23.2) |
| Proximal colon | 14,811 | 4,252 (28.7) |
| Transverse colon | 5,650 | 1,679 (27.9) |
| Distal colon | 9,627 | 2,683 (23.2) |
| Tumor histologya | ||
| Mucinous adenocarcinoma | 32,109 | 8,766 (27.3) |
| Other adenocarcinoma | 4,761 | 1,407 (29.6) |
| Nonadenocarcinoma | 195 | 57 (29.2) |
| Treatment characteristics | ||
| Mode of presentationa | ||
| Elective | 28,673 | 6,836 (23.8) |
| Emergency | 8,392 | 3,394 (40.4) |
| Type of surgerya | ||
| Total (procto)colectomy | 1,475 | 372 (25.2) |
| Subtotal (hemi)colectomy | 18,840 | 5,367 (28.5) |
| Partial colectomyb | 16,017 | 4,298 (26.8) |
| Colectomy NOS | 647 | 177 (27.4) |
| Other surgery | 86 | 16 (18.6) |
| Length of stay, daysa, c | 12.8 (8.3) | |
| Complications during hospitalization | ||
| None | 28,580 | 6,863 (24.0) |
| Yes | 8,485 | 3,367 (39.7) |
| Hospital characteristics | ||
| Hospital volume (no. of beds)a | ||
| 1199 | 9,326 | 2,382 (25.5) |
| 200349 | 10,446 | 3,153 (30.2) |
| 350499 | 8,963 | 2,590 (28.9) |
| 500+ | 8,330 | 2,105 (25.3) |
| Hospital surgery volumea | ||
| 120 | 8,429 | 2,445 (29.0) |
| 2138 | 8,294 | 2,389 (28.8) |
| 3965 | 8,115 | 2,257 (27.8) |
| 66+ | 8,453 | 2,013 (23.8) |
| Unknown | 3,774 | 1,126 (29.8) |
Table 2 shows the adjusted ORs of the covariates associated with comanagement. This model includes only variables independently associated with comanagement. Increasing age was associated with increased odds of receiving comanagement, with OR = 1.22 for patients aged between 75 and 84 years, and OR = 1.52 for patients aged 85 years and older. Comorbidity scores of 1 or 2 or more were associated with increased odds of comanagement use following CRC surgery compared to patients without comorbidities (OR = 1.39 and OR = 1.92, respectively). Patients who received Medicaid were more likely to receive comanagement (OR = 1.11) compared with patients without Medicaid insurance. Higher AJCC stage was associated with increased use of comanagement, as was poor tumor differentiation. Patients undergoing surgery for colon versus rectal cancer were more likely to be comanaged during their hospital stay (OR = 1.23). Surgery after emergency admission was associated with an increased use of comanagement (OR = 1.95). The odds of comanagement increased slightly with increasing length of hospital stay (OR = 1.03), but were higher for patients who developed 1 or more complications during hospitalization compared with patients without complications (OR = 1.38). Compared with CRC patients treated in small hospitals (<200 beds), patients treated in hospitals with 200 to 349 beds were more likely to receive comanaged care (OR = 1.51), whereas patients treated in high‐volume hospitals with 500 beds were less likely to receive comanaged care during hospitalization (OR = 0.65). While census‐tract poverty rate was associated with comanagement in univariable analysis, no significant association was observed when adjusting for other covariates in the multivariable model. In a model with all covariates listed (Table 2), variability of comanagement across hospitals was significant (ICC = 0.376), suggesting that extensive variability across hospitals remained. The adjusted value of CRC patients receiving comanaged care varied widely, from 1.9% to 83.2% across hospitals. Our sensitivity analysis showed that odds ratios and confidence intervals were generally unchanged when using different definitions of comanagement.
| Covariates | OR | 95% CI |
|---|---|---|
| ||
| Age, y | ||
| 6674 | 1.00 | |
| 7584 | 1.22 | 1.151.30 |
| 85+ | 1.52 | 1.421.64 |
| Comorbidity | ||
| 0 | 1.00 | |
| 1 | 1.39 | 1.301.48 |
| 2+ | 1.92 | 1.802.05 |
| Medicaid | ||
| No | 1.00 | |
| Yes | 1.11 | 1.031.20 |
| Year of diagnosis, per year | 1.07 | 1.061.09 |
| AJCC stage | ||
| I | 1.00 | |
| II | 1.11 | 1.041.19 |
| III | 1.10 | 1.021.18 |
| Tumor grade/differentiation | ||
| Well | 1.00 | |
| Moderate | 1.08 | 0.981.19 |
| Poor | 1.17 | 1.041.31 |
| Undifferentiated | 1.14 | 0.851.52 |
| Unknown | 0.89 | 0.751.06 |
| Tumor location | ||
| Rectal | 1.00 | |
| Colon | 1.23 | 1.151.32 |
| Mode of presentation | ||
| Elective | 1.00 | |
| Emergency | 1.95 | 1.842.08 |
| Length of stay, per day | 1.03 | 1.021.03 |
| Complications during stay | ||
| No | 1.00 | |
| Yes | 1.38 | 1.291.48 |
| Hospital volume (no. of beds) | ||
| 1199 | 1.00 | |
| 200349 | 1.51 | 1.221.86 |
| 350499 | 1.26 | 0.981.62 |
| 500+ | 0.65 | 0.490.86 |
DISCUSSION
In all, 27.6% of CRC patients were comanaged during hospitalization, but large disparities existed across patient, hospital, and geographic characteristics. Our finding that more complex patients are more likely to receive comanaged care (eg, increasing age and comorbidity, higher tumor stage and grade, emergency presentation, and complications) are in line with other studies.[5] Importantly, there was a wide range in hospital use of comanagement; the variability across hospitals accounted for 37.6% of the total variability in comanagement use. In some hospitals, almost none of the patients received comanaged care, whereas in other hospitals 83.2% of patients received such care. One reason for the large variability across hospitals may be the lack of evidence about the effectiveness of comanagement. Although comanaged care may benefit orthopedic patients,[7, 8, 9, 10, 11] to our knowledge, no studies have shown benefit to CRC patients. Typically, procedures for which there is more ambiguity about its effectiveness show greater variability across hospitals and geographic areas.[22] Similar to a previous study,[5] we found that patients treated in midsize hospitals were more likely to receive comanagement, and patients treated at high‐volume hospitals were less likely to receive comanagement compared to smaller hospitals. However, because the variability was similar between the model without any variables and the full multivariable model, other variables not available in SEERMedicare data likely played a role in explaining this large interhospital variability. Quality of cancer care may be improved by reducing variation in underuse of effective and necessary care, variation that indicates misuse of preference‐sensitive care (ie, care that offers equivalent options to be chosen by the patient), and variation that indicates overuse of supply‐sensitive care (ie, care influenced by medical capacity).[23] Examining and reducing variability in medical care has been an important policy consideration for almost 30 years.[24, 25] Future studies should examine additional reasons for variability in comanagement across hospitals, including variables at the level of the patient, provider, and hospital. Cost‐effective interventions should subsequently target modifiable factors to reduce use of unnecessary care among patients unlikely to benefit from comanagement.
Our study benefits from using a population‐based SEERMedicare cohort of older CRC patients, representing the diversity of geographic areas and hospitals across the United States. Furthermore, we were able to include a variety of covariates available in the SEERMedicare data, including patient, hospital, and area characteristics. Whereas most studies examining the use or effectiveness of comanagement include single institutions or a variety of surgical patients,[5, 8, 11, 17] we examined the use of and characteristics associated with comanagement throughout the United States for a specific patient population.
We recognize several limitations in this study. Our study specifically assessed comanagement among hospitalized CRC patients aged 65 years and older without managed care insurance. Generalizabilty of our findings to other surgical patients, to younger CRC patients, and those with managed care insurance may therefore be limited. With regard to the definition of comanagement, we used a cutoff point of submitting a claim for management and evaluation on 70% or more of the days of hospitalization for CRC. This cutoff is somewhat arbitrary, but different cutoffs did not influence the results. However, in a prior study, changes in the cutoff used in this definition only affected the proportion of comanaged patients and did not change the observation of increasing trends in the use of comanagement.[5] In addition, we examined a CRC population diagnosed between 2000 and 2005. Because an increasing trend in use of comanagement was observed, it is possible that comanagement use has increased further in more recent years. Our findings of lower use of comanaged care in larger hospitals may be related to the increased use of hospitalists as consultants, whose care may not be accurately or completely captured in claims data. We do not report data regarding the use of physician extenders (nurse practitioners and physician assistants). Although their use is increasing in some contexts, in our data, if we counted patients comanaged by physician extenders, we would only add 79 additional CRC patients (0.6%) to our analyses, which is unlikely to influence our findings.
In conclusion, more complex patients are more likely to receive comanaged care following CRC surgery. Extensive variability existed across patients and hospitals likely due to the lack of evidence about the clinical effectiveness of comanagement for patients undergoing CRC surgery. Future studies should examine additional reasons for variability in comanagement across hospitals, including variables at the level of the patient, provider, and hospital.
Acknowledgements
The authors gratefully acknowledge James Struthers for his data management and programming services, provided through the Health Behavior, Communication, and Outreach Core of the Alvin J. Siteman Cancer Center at Barnes‐Jewish Hospital and Washington University School of Medicine. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; SEER Program tumor registries in the creation of the SEERMedicare database. The authors thank Dr. S. Hendren at the University of Michigan for the use of the SAS programming codes to identify complications.
Disclosures: This study used the linked SEERMedicare database. The interpretation and reporting of these data are the sole responsibility of the authors. This work was supported by grants from the National Institutes of Health National Cancer Institute (R01 CA137750 and P30 CA091842). Dr. Davidson was supported in part through National Institutes of Health grants HL‐38180, DK‐56260, and Digestive Disease Research Core Center DK‐52574.
- , , . Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- , , , , , . Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer. 2007;43(15):2179–2193.
- , , , et al. Which comorbid conditions predict complications after surgery for colorectal cancer? World J Surg. 2007;31(1):192–199.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , . Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394–397.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , . Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program. Clin Orthop Relat Res. 1992;(274):213–225.
- , , , . Impact of a comanaged Geriatric Fracture Center on short‐term hip fracture outcomes. Arch Intern Med. 2009;169(18):1712–1717.
- , , , et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28–38.
- , . Effect of hospitalists on length of stay in the Medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58(9):1649–1657.
- , , , et al. The impact of hospitalists on length of stay and costs: systematic review and meta‐analysis. Am J Manag Care. 2012;18(1):e23–e30.
- , , , et al. Hospital and geographic variability in two colorectal cancer surgery outcomes: complications and mortality after complications. Ann Surg Onc. In press.
- , , , , . Overview of the SEER‐Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 (8 suppl):IV–3–18.
- , , , , , . Use of SEER‐Medicare data for measuring cancer surgery. Med Care. 2002;40(8 suppl):IV–43–48.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
- , , , . Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267.
- , , , , , . Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53(12):1587–1593.
- , , , , , . Major postoperative complications and survival for colon cancer elderly patients. BMC Surgery. 2012;12(suppl 1):S20.
- , . Multilevel Analysis. An Introduction to Basic and Advanced Multilevel Modeling. London, UK: Sage Publications; 1999.
- , , , , , . The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298.
- . Tracking Medicine: A Researcher's Quest to Understand Health Care. Oxford, UK: Oxford University Press; 2010.
- . Understanding geographic variations in health care delivery. N Engl J Med. 1999;340:52–53.
- . Reducing variation in health care: the rhetorical politics of a policy idea. J Health Polit Policy Law. 2013;38(1):5–26.
Colorectal cancer (CRC) is the second most common malignancy in the United States. In 2013, an estimated 142,820 men and women will be newly diagnosed, and 50,830 patients will die from colon or rectal cancer.[1] The majority of patients are aged 65 years or older at diagnosis, and with a growing elderly population, the CRC burden assumes increasing importance in this patient population.[1] Surgery remains the most important treatment option; however, surgical management of older CRC patients is often complicated because of the attendant comorbidities.[2, 3]
Recently, comanagement of surgical patients during their hospital stay has increased substantially in an effort to provide care to complex patients.[4, 5] Comanagement includes daily assessment of acute issues and comorbidities, and communication with surgeons by physicians including hospitalists and internists.[6] The presumed benefits of this comanaged approach to patient care include increased prescribing of evidence‐based treatments,[7] reduced time to surgery,[8] fewer transfers to an intensive care unit,[9] fewer postoperative complications,[9, 10, 11] shortened length of hospital stay,[12, 13] and lower readmission rates.[7] Information regarding detailed characteristics of patients receiving comanagement during hospitalization, specifically for CRC surgery, is lacking. This is an important consideration because comanagement may be particularly beneficial for CRC patients, who tend to be older at diagnosis and may have multiple comorbidities.[5] Hospitalists may be especially important for postoperative management of CRC patients, depending upon the complexity of the surgery and also the need for close medical and surgical monitoring in the perioperative setting, particularly among older CRC patients. Many CRC patients develop complications following surgery,[14] and it is possible that these patients may be rescued by comanagement with hospitalist physicians. Our aim was to assess the use of and characteristics associated with comanagement of patients undergoing surgical intervention for CRC.
METHODS
We obtained data from an existing linkage of 2000 to 2005 National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program data with 1999 to 2005 Medicare claim files from the Centers for Medicare and Medicaid Services (CMS).[15] In this study, data from 16 tumor registries and 1242 hospitals were included, covering approximately 28% of the US population. We selected patients aged 66 years or older with a first primary stage I to III colon or rectal cancer diagnosis between 2000 and 2005. Patients were eligible for inclusion when they had both Medicare parts A and B coverage and underwent surgical treatment for CRC. We excluded patients identified from autopsy or death certificate only, patients for whom the month of diagnosis was not available, and patients who were members of a Health Maintenance Organization and thereby lacking Medicare claims data. We excluded patients who were only eligible for Medicare because they were disabled or had end‐stage renal disease (ie, patients younger than 65 years of age). The age restriction of 66 years of age or older allowed for 1 year of Medicare claims data prior to CRC diagnosis to determine preexisting comorbidity. We used 1999 Medicare data to obtain information about comorbidity for CRC patients who were diagnosed in 2000. Definitive surgery for CRC was measured by searching inpatient, outpatient, and carrier claims using previously identified Healthcare Common Procedure Coding System and/or International Classification of Diseases, 9th Revision (ICD‐9). In the event of multiple surgical interventions in 1 patient record, the date on which the most invasive surgery was performed was used.[16]
Comanagement Use
The outcome of interest was whether or not CRC patients received comanaged care during hospitalization following surgical treatment (comanagement prior to surgery was not included). Comanagement was defined as having a relevant physician (ie, internal medicine hospitalist/generalist) submit a claim for evaluation and management services on 70% or more of the days of hospitalization of the patient, including partial days of admission and discharge. To identify inpatient physician claims, we used the Common Procedure TerminologyEvaluation and Management codes 99221 to 99223, 99251 to 99255, and 99231 to 99233.[5, 17]
Covariates
Characteristics potentially associated with comanagement use included patient, tumor, treatment, and hospital characteristics based on previous work.[4, 5] Patient sociodemographic characteristics included sex, age, race/ethnicity, comorbidity, dual eligibility for Medicare and Medicaid, and year of CRC diagnosis. Comorbidity was measured by searching inpatient or carrier claims for multiple chronic diseases occurring 1 to 12 months prior to diagnosis. We categorized comorbidity as a score of 0, 1, or 2 or more and computed an index score[18] to examine nonlinear trends. Dual MedicareMedicaid participation was present if a patient had Medicaid coverage for at least 1 month during the year before diagnosis. We used census‐tract poverty rate as a measure of neighborhood economic condition, Which was defined as the percentage of the population living in poverty in the census tract of the patient's residence at the time of diagnosis and was derived from the 2000 census. Tumor characteristics included American Joint Commission on Cancer (AJCC) tumor stage, grade, location, and histology. Treatment characteristics included mode of presentation, type of surgery, length of hospital stay, and presence of complications during hospitalization.[19, 20] Complications (eg, postoperative pneumonia, surgical‐site infection, deep vein thrombosis) during hospitalization but following CRC surgery were identified from Medicare data using a previously developed algorithm.[19] The complications algorithm was developed by clinical experts and consists of ICD‐9 diagnosis and procedure codes representing CRC‐resection complications and their treatment, including additional operations. Surgical interventions after emergency admission were defined as emergency surgery; all other surgical treatments were classified as elective. Additionally, characteristics of the hospital where the patient's surgery took place included number of hospital beds, obtained from the Healthcare Cost Report and the Provider of Service files from CMS, and surgeon caseload, based on the number of CRC surgeries performed during the study period. The hospital's surgery volume was calculated using the number of CRC surgeries performed during the study period and categorized into quartiles.
Statistical Analysis
Proportions of comanaged patients were calculated for each covariate. Univariable logistic regression analyses were performed to assess the association between comanagement use and each covariate separately. All analyses were adjusted for nesting of CRC patients within hospitals, using a 2‐level model with a random intercept. Next, we fitted a multivariable model that included covariates associated with comanagement in univariable analysis based on the likelihood ratio test (P < 0.05). Inclusion of covariates in the final multivariable model was based on statistically significant associations with comanagement. Adjusted odds ratios (ORs) were calculated with 95% confidence intervals. Variability across hospitals was calculated using the intraclass coefficient (ICC) for logistic regression.[21] The ICC ranges from 0.0 (no variability across hospitals) to 1.0 (extreme variability across hospitals). In addition, we calculated adjusted values for comanagement to describe the hospital variability. These adjusted values were computed based on the multivariable model by averaging the patient‐level probabilities for all patients who resided in that hospital. We conducted a series of analyses to challenge the robustness of our results. To investigate the potential effect of a different definition of comanagement, we defined comanagement as both (1) having a relevant physician submit a claim for evaluation and management services on 50% or more of the days of the patient's hospitalization, and (2) having a relevant hospitalist or generalist physician submit a claim for evaluation and management services on 100% of the days of hospitalization. All analyses were performed using Stata (version 12.0; StataCorp, College Station, TX).
RESULTS
There were 47,828 patients aged 66 years or older who were diagnosed with a first primary CRC between 2000 and 2005, had both Medicare parts A and B, and were not members of a Health Maintenance Organization during the study period. We excluded patients for whom we were unable to calculate complications (n = 2649), surgery or hospital volume (n = 6415), or who had missing data (n = 238). Finally, we excluded CRC patients with in situ, stage IV, or missing stage information, resulting in 37,065 patients in the study population. Patients in the study population were typically aged 75 to 84 years, were female, had 1 or more comorbid condition, lived in areas with the population poverty rate <10%, were diagnosed with AJCC stage II, had elective surgery, and were treated with partial colectomy (Table 1). During 2000 to 2005, 10,230 (27.6%) of 37,065 patients were comanaged during hospitalization for CRC surgery. In a model with no covariates, variability of comanagement across hospitals was significant (ICC = 0.382). All patient and hospital characteristics except for sex and race/ethnicity were significantly associated with comanagement use, when adjusted for clustering within hospitals. The most common characteristics associated with comanagement were emergency surgery (40.4%), complications (39.7%), and having 2 or more comorbid conditions (37.1%). Comanagement was less common among those with no comorbidity (21.0%), unknown tumor grade (21.9%), and other surgery (18.6%). Furthermore, comanagement increased from 24.8% in 2000 to 30.1% in 2005 (P for trend < 0.001; Table 1).
| Covariates | Total, N = 37,065 | Comanaged, n (%), n = 10,230 (27.6%) |
|---|---|---|
| ||
| Sociodemographics | ||
| Age, y | ||
| 6674 | 13,197 | 3,014 (22.8) |
| 7584 | 6,958 | 4,736 (27.9) |
| 85+ | 6,910 | 2,480 (35.9) |
| Sex | ||
| Male | 15,744 | 4,345 (27.6) |
| Female | 21,321 | 5,885 (27.6) |
| Racea | ||
| White | 31,958 | 8,579 (26.8) |
| African American | 2,641 | 745 (28.2) |
| Other | 2,466 | 906 (36.7) |
| Comorbiditya | ||
| 0 | 15,905 | 3,342 (21.0) |
| 1 | 10,860 | 3,068 (28.3) |
| 2+ | 10,300 | 3,820 (37.1) |
| Medicaida | ||
| No | 31,099 | 8,000 (25.7) |
| Yes | 5,966 | 2,230 (37.4) |
| Year of diagnosisa | ||
| 2000 | 6,041 | 1,497 (24.8) |
| 2001 | 6,193 | 1,624 (26.2) |
| 2002 | 6,289 | 1,692 (26.9) |
| 2003 | 6,489 | 1,829 (28.2) |
| 2004 | 6,219 | 1,832 (29.5) |
| 2005 | 5,834 | 1,756 (30.1) |
| Neighborhood characteristic | ||
| Poverty ratea | ||
| <10% | 21,772 | 5,698(26.2) |
| 10%19% | 9,458 | 2,722(28.8) |
| 20% | 5,835 | 1,810(31.0) |
| Tumor characteristics | ||
| AJCC stagea | ||
| I | 9,996 | 2,402 (24.0) |
| II | 14,662 | 4,287 (29.2) |
| III | 12,407 | 3,541 (28.5) |
| Tumor grade/differentiationa | ||
| Well | 3,278 | 799 (24.4) |
| Moderate | 25,129 | 6,905 (27.5) |
| Poor | 6,898 | 2,107 (30.6) |
| Undifferentiated | 350 | 110 (31.4) |
| Unknown | 1,410 | 309 (21.9) |
| Tumor locationa | ||
| Rectum | 6,977 | 1,616 (23.2) |
| Proximal colon | 14,811 | 4,252 (28.7) |
| Transverse colon | 5,650 | 1,679 (27.9) |
| Distal colon | 9,627 | 2,683 (23.2) |
| Tumor histologya | ||
| Mucinous adenocarcinoma | 32,109 | 8,766 (27.3) |
| Other adenocarcinoma | 4,761 | 1,407 (29.6) |
| Nonadenocarcinoma | 195 | 57 (29.2) |
| Treatment characteristics | ||
| Mode of presentationa | ||
| Elective | 28,673 | 6,836 (23.8) |
| Emergency | 8,392 | 3,394 (40.4) |
| Type of surgerya | ||
| Total (procto)colectomy | 1,475 | 372 (25.2) |
| Subtotal (hemi)colectomy | 18,840 | 5,367 (28.5) |
| Partial colectomyb | 16,017 | 4,298 (26.8) |
| Colectomy NOS | 647 | 177 (27.4) |
| Other surgery | 86 | 16 (18.6) |
| Length of stay, daysa, c | 12.8 (8.3) | |
| Complications during hospitalization | ||
| None | 28,580 | 6,863 (24.0) |
| Yes | 8,485 | 3,367 (39.7) |
| Hospital characteristics | ||
| Hospital volume (no. of beds)a | ||
| 1199 | 9,326 | 2,382 (25.5) |
| 200349 | 10,446 | 3,153 (30.2) |
| 350499 | 8,963 | 2,590 (28.9) |
| 500+ | 8,330 | 2,105 (25.3) |
| Hospital surgery volumea | ||
| 120 | 8,429 | 2,445 (29.0) |
| 2138 | 8,294 | 2,389 (28.8) |
| 3965 | 8,115 | 2,257 (27.8) |
| 66+ | 8,453 | 2,013 (23.8) |
| Unknown | 3,774 | 1,126 (29.8) |
Table 2 shows the adjusted ORs of the covariates associated with comanagement. This model includes only variables independently associated with comanagement. Increasing age was associated with increased odds of receiving comanagement, with OR = 1.22 for patients aged between 75 and 84 years, and OR = 1.52 for patients aged 85 years and older. Comorbidity scores of 1 or 2 or more were associated with increased odds of comanagement use following CRC surgery compared to patients without comorbidities (OR = 1.39 and OR = 1.92, respectively). Patients who received Medicaid were more likely to receive comanagement (OR = 1.11) compared with patients without Medicaid insurance. Higher AJCC stage was associated with increased use of comanagement, as was poor tumor differentiation. Patients undergoing surgery for colon versus rectal cancer were more likely to be comanaged during their hospital stay (OR = 1.23). Surgery after emergency admission was associated with an increased use of comanagement (OR = 1.95). The odds of comanagement increased slightly with increasing length of hospital stay (OR = 1.03), but were higher for patients who developed 1 or more complications during hospitalization compared with patients without complications (OR = 1.38). Compared with CRC patients treated in small hospitals (<200 beds), patients treated in hospitals with 200 to 349 beds were more likely to receive comanaged care (OR = 1.51), whereas patients treated in high‐volume hospitals with 500 beds were less likely to receive comanaged care during hospitalization (OR = 0.65). While census‐tract poverty rate was associated with comanagement in univariable analysis, no significant association was observed when adjusting for other covariates in the multivariable model. In a model with all covariates listed (Table 2), variability of comanagement across hospitals was significant (ICC = 0.376), suggesting that extensive variability across hospitals remained. The adjusted value of CRC patients receiving comanaged care varied widely, from 1.9% to 83.2% across hospitals. Our sensitivity analysis showed that odds ratios and confidence intervals were generally unchanged when using different definitions of comanagement.
| Covariates | OR | 95% CI |
|---|---|---|
| ||
| Age, y | ||
| 6674 | 1.00 | |
| 7584 | 1.22 | 1.151.30 |
| 85+ | 1.52 | 1.421.64 |
| Comorbidity | ||
| 0 | 1.00 | |
| 1 | 1.39 | 1.301.48 |
| 2+ | 1.92 | 1.802.05 |
| Medicaid | ||
| No | 1.00 | |
| Yes | 1.11 | 1.031.20 |
| Year of diagnosis, per year | 1.07 | 1.061.09 |
| AJCC stage | ||
| I | 1.00 | |
| II | 1.11 | 1.041.19 |
| III | 1.10 | 1.021.18 |
| Tumor grade/differentiation | ||
| Well | 1.00 | |
| Moderate | 1.08 | 0.981.19 |
| Poor | 1.17 | 1.041.31 |
| Undifferentiated | 1.14 | 0.851.52 |
| Unknown | 0.89 | 0.751.06 |
| Tumor location | ||
| Rectal | 1.00 | |
| Colon | 1.23 | 1.151.32 |
| Mode of presentation | ||
| Elective | 1.00 | |
| Emergency | 1.95 | 1.842.08 |
| Length of stay, per day | 1.03 | 1.021.03 |
| Complications during stay | ||
| No | 1.00 | |
| Yes | 1.38 | 1.291.48 |
| Hospital volume (no. of beds) | ||
| 1199 | 1.00 | |
| 200349 | 1.51 | 1.221.86 |
| 350499 | 1.26 | 0.981.62 |
| 500+ | 0.65 | 0.490.86 |
DISCUSSION
In all, 27.6% of CRC patients were comanaged during hospitalization, but large disparities existed across patient, hospital, and geographic characteristics. Our finding that more complex patients are more likely to receive comanaged care (eg, increasing age and comorbidity, higher tumor stage and grade, emergency presentation, and complications) are in line with other studies.[5] Importantly, there was a wide range in hospital use of comanagement; the variability across hospitals accounted for 37.6% of the total variability in comanagement use. In some hospitals, almost none of the patients received comanaged care, whereas in other hospitals 83.2% of patients received such care. One reason for the large variability across hospitals may be the lack of evidence about the effectiveness of comanagement. Although comanaged care may benefit orthopedic patients,[7, 8, 9, 10, 11] to our knowledge, no studies have shown benefit to CRC patients. Typically, procedures for which there is more ambiguity about its effectiveness show greater variability across hospitals and geographic areas.[22] Similar to a previous study,[5] we found that patients treated in midsize hospitals were more likely to receive comanagement, and patients treated at high‐volume hospitals were less likely to receive comanagement compared to smaller hospitals. However, because the variability was similar between the model without any variables and the full multivariable model, other variables not available in SEERMedicare data likely played a role in explaining this large interhospital variability. Quality of cancer care may be improved by reducing variation in underuse of effective and necessary care, variation that indicates misuse of preference‐sensitive care (ie, care that offers equivalent options to be chosen by the patient), and variation that indicates overuse of supply‐sensitive care (ie, care influenced by medical capacity).[23] Examining and reducing variability in medical care has been an important policy consideration for almost 30 years.[24, 25] Future studies should examine additional reasons for variability in comanagement across hospitals, including variables at the level of the patient, provider, and hospital. Cost‐effective interventions should subsequently target modifiable factors to reduce use of unnecessary care among patients unlikely to benefit from comanagement.
Our study benefits from using a population‐based SEERMedicare cohort of older CRC patients, representing the diversity of geographic areas and hospitals across the United States. Furthermore, we were able to include a variety of covariates available in the SEERMedicare data, including patient, hospital, and area characteristics. Whereas most studies examining the use or effectiveness of comanagement include single institutions or a variety of surgical patients,[5, 8, 11, 17] we examined the use of and characteristics associated with comanagement throughout the United States for a specific patient population.
We recognize several limitations in this study. Our study specifically assessed comanagement among hospitalized CRC patients aged 65 years and older without managed care insurance. Generalizabilty of our findings to other surgical patients, to younger CRC patients, and those with managed care insurance may therefore be limited. With regard to the definition of comanagement, we used a cutoff point of submitting a claim for management and evaluation on 70% or more of the days of hospitalization for CRC. This cutoff is somewhat arbitrary, but different cutoffs did not influence the results. However, in a prior study, changes in the cutoff used in this definition only affected the proportion of comanaged patients and did not change the observation of increasing trends in the use of comanagement.[5] In addition, we examined a CRC population diagnosed between 2000 and 2005. Because an increasing trend in use of comanagement was observed, it is possible that comanagement use has increased further in more recent years. Our findings of lower use of comanaged care in larger hospitals may be related to the increased use of hospitalists as consultants, whose care may not be accurately or completely captured in claims data. We do not report data regarding the use of physician extenders (nurse practitioners and physician assistants). Although their use is increasing in some contexts, in our data, if we counted patients comanaged by physician extenders, we would only add 79 additional CRC patients (0.6%) to our analyses, which is unlikely to influence our findings.
In conclusion, more complex patients are more likely to receive comanaged care following CRC surgery. Extensive variability existed across patients and hospitals likely due to the lack of evidence about the clinical effectiveness of comanagement for patients undergoing CRC surgery. Future studies should examine additional reasons for variability in comanagement across hospitals, including variables at the level of the patient, provider, and hospital.
Acknowledgements
The authors gratefully acknowledge James Struthers for his data management and programming services, provided through the Health Behavior, Communication, and Outreach Core of the Alvin J. Siteman Cancer Center at Barnes‐Jewish Hospital and Washington University School of Medicine. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; SEER Program tumor registries in the creation of the SEERMedicare database. The authors thank Dr. S. Hendren at the University of Michigan for the use of the SAS programming codes to identify complications.
Disclosures: This study used the linked SEERMedicare database. The interpretation and reporting of these data are the sole responsibility of the authors. This work was supported by grants from the National Institutes of Health National Cancer Institute (R01 CA137750 and P30 CA091842). Dr. Davidson was supported in part through National Institutes of Health grants HL‐38180, DK‐56260, and Digestive Disease Research Core Center DK‐52574.
Colorectal cancer (CRC) is the second most common malignancy in the United States. In 2013, an estimated 142,820 men and women will be newly diagnosed, and 50,830 patients will die from colon or rectal cancer.[1] The majority of patients are aged 65 years or older at diagnosis, and with a growing elderly population, the CRC burden assumes increasing importance in this patient population.[1] Surgery remains the most important treatment option; however, surgical management of older CRC patients is often complicated because of the attendant comorbidities.[2, 3]
Recently, comanagement of surgical patients during their hospital stay has increased substantially in an effort to provide care to complex patients.[4, 5] Comanagement includes daily assessment of acute issues and comorbidities, and communication with surgeons by physicians including hospitalists and internists.[6] The presumed benefits of this comanaged approach to patient care include increased prescribing of evidence‐based treatments,[7] reduced time to surgery,[8] fewer transfers to an intensive care unit,[9] fewer postoperative complications,[9, 10, 11] shortened length of hospital stay,[12, 13] and lower readmission rates.[7] Information regarding detailed characteristics of patients receiving comanagement during hospitalization, specifically for CRC surgery, is lacking. This is an important consideration because comanagement may be particularly beneficial for CRC patients, who tend to be older at diagnosis and may have multiple comorbidities.[5] Hospitalists may be especially important for postoperative management of CRC patients, depending upon the complexity of the surgery and also the need for close medical and surgical monitoring in the perioperative setting, particularly among older CRC patients. Many CRC patients develop complications following surgery,[14] and it is possible that these patients may be rescued by comanagement with hospitalist physicians. Our aim was to assess the use of and characteristics associated with comanagement of patients undergoing surgical intervention for CRC.
METHODS
We obtained data from an existing linkage of 2000 to 2005 National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program data with 1999 to 2005 Medicare claim files from the Centers for Medicare and Medicaid Services (CMS).[15] In this study, data from 16 tumor registries and 1242 hospitals were included, covering approximately 28% of the US population. We selected patients aged 66 years or older with a first primary stage I to III colon or rectal cancer diagnosis between 2000 and 2005. Patients were eligible for inclusion when they had both Medicare parts A and B coverage and underwent surgical treatment for CRC. We excluded patients identified from autopsy or death certificate only, patients for whom the month of diagnosis was not available, and patients who were members of a Health Maintenance Organization and thereby lacking Medicare claims data. We excluded patients who were only eligible for Medicare because they were disabled or had end‐stage renal disease (ie, patients younger than 65 years of age). The age restriction of 66 years of age or older allowed for 1 year of Medicare claims data prior to CRC diagnosis to determine preexisting comorbidity. We used 1999 Medicare data to obtain information about comorbidity for CRC patients who were diagnosed in 2000. Definitive surgery for CRC was measured by searching inpatient, outpatient, and carrier claims using previously identified Healthcare Common Procedure Coding System and/or International Classification of Diseases, 9th Revision (ICD‐9). In the event of multiple surgical interventions in 1 patient record, the date on which the most invasive surgery was performed was used.[16]
Comanagement Use
The outcome of interest was whether or not CRC patients received comanaged care during hospitalization following surgical treatment (comanagement prior to surgery was not included). Comanagement was defined as having a relevant physician (ie, internal medicine hospitalist/generalist) submit a claim for evaluation and management services on 70% or more of the days of hospitalization of the patient, including partial days of admission and discharge. To identify inpatient physician claims, we used the Common Procedure TerminologyEvaluation and Management codes 99221 to 99223, 99251 to 99255, and 99231 to 99233.[5, 17]
Covariates
Characteristics potentially associated with comanagement use included patient, tumor, treatment, and hospital characteristics based on previous work.[4, 5] Patient sociodemographic characteristics included sex, age, race/ethnicity, comorbidity, dual eligibility for Medicare and Medicaid, and year of CRC diagnosis. Comorbidity was measured by searching inpatient or carrier claims for multiple chronic diseases occurring 1 to 12 months prior to diagnosis. We categorized comorbidity as a score of 0, 1, or 2 or more and computed an index score[18] to examine nonlinear trends. Dual MedicareMedicaid participation was present if a patient had Medicaid coverage for at least 1 month during the year before diagnosis. We used census‐tract poverty rate as a measure of neighborhood economic condition, Which was defined as the percentage of the population living in poverty in the census tract of the patient's residence at the time of diagnosis and was derived from the 2000 census. Tumor characteristics included American Joint Commission on Cancer (AJCC) tumor stage, grade, location, and histology. Treatment characteristics included mode of presentation, type of surgery, length of hospital stay, and presence of complications during hospitalization.[19, 20] Complications (eg, postoperative pneumonia, surgical‐site infection, deep vein thrombosis) during hospitalization but following CRC surgery were identified from Medicare data using a previously developed algorithm.[19] The complications algorithm was developed by clinical experts and consists of ICD‐9 diagnosis and procedure codes representing CRC‐resection complications and their treatment, including additional operations. Surgical interventions after emergency admission were defined as emergency surgery; all other surgical treatments were classified as elective. Additionally, characteristics of the hospital where the patient's surgery took place included number of hospital beds, obtained from the Healthcare Cost Report and the Provider of Service files from CMS, and surgeon caseload, based on the number of CRC surgeries performed during the study period. The hospital's surgery volume was calculated using the number of CRC surgeries performed during the study period and categorized into quartiles.
Statistical Analysis
Proportions of comanaged patients were calculated for each covariate. Univariable logistic regression analyses were performed to assess the association between comanagement use and each covariate separately. All analyses were adjusted for nesting of CRC patients within hospitals, using a 2‐level model with a random intercept. Next, we fitted a multivariable model that included covariates associated with comanagement in univariable analysis based on the likelihood ratio test (P < 0.05). Inclusion of covariates in the final multivariable model was based on statistically significant associations with comanagement. Adjusted odds ratios (ORs) were calculated with 95% confidence intervals. Variability across hospitals was calculated using the intraclass coefficient (ICC) for logistic regression.[21] The ICC ranges from 0.0 (no variability across hospitals) to 1.0 (extreme variability across hospitals). In addition, we calculated adjusted values for comanagement to describe the hospital variability. These adjusted values were computed based on the multivariable model by averaging the patient‐level probabilities for all patients who resided in that hospital. We conducted a series of analyses to challenge the robustness of our results. To investigate the potential effect of a different definition of comanagement, we defined comanagement as both (1) having a relevant physician submit a claim for evaluation and management services on 50% or more of the days of the patient's hospitalization, and (2) having a relevant hospitalist or generalist physician submit a claim for evaluation and management services on 100% of the days of hospitalization. All analyses were performed using Stata (version 12.0; StataCorp, College Station, TX).
RESULTS
There were 47,828 patients aged 66 years or older who were diagnosed with a first primary CRC between 2000 and 2005, had both Medicare parts A and B, and were not members of a Health Maintenance Organization during the study period. We excluded patients for whom we were unable to calculate complications (n = 2649), surgery or hospital volume (n = 6415), or who had missing data (n = 238). Finally, we excluded CRC patients with in situ, stage IV, or missing stage information, resulting in 37,065 patients in the study population. Patients in the study population were typically aged 75 to 84 years, were female, had 1 or more comorbid condition, lived in areas with the population poverty rate <10%, were diagnosed with AJCC stage II, had elective surgery, and were treated with partial colectomy (Table 1). During 2000 to 2005, 10,230 (27.6%) of 37,065 patients were comanaged during hospitalization for CRC surgery. In a model with no covariates, variability of comanagement across hospitals was significant (ICC = 0.382). All patient and hospital characteristics except for sex and race/ethnicity were significantly associated with comanagement use, when adjusted for clustering within hospitals. The most common characteristics associated with comanagement were emergency surgery (40.4%), complications (39.7%), and having 2 or more comorbid conditions (37.1%). Comanagement was less common among those with no comorbidity (21.0%), unknown tumor grade (21.9%), and other surgery (18.6%). Furthermore, comanagement increased from 24.8% in 2000 to 30.1% in 2005 (P for trend < 0.001; Table 1).
| Covariates | Total, N = 37,065 | Comanaged, n (%), n = 10,230 (27.6%) |
|---|---|---|
| ||
| Sociodemographics | ||
| Age, y | ||
| 6674 | 13,197 | 3,014 (22.8) |
| 7584 | 6,958 | 4,736 (27.9) |
| 85+ | 6,910 | 2,480 (35.9) |
| Sex | ||
| Male | 15,744 | 4,345 (27.6) |
| Female | 21,321 | 5,885 (27.6) |
| Racea | ||
| White | 31,958 | 8,579 (26.8) |
| African American | 2,641 | 745 (28.2) |
| Other | 2,466 | 906 (36.7) |
| Comorbiditya | ||
| 0 | 15,905 | 3,342 (21.0) |
| 1 | 10,860 | 3,068 (28.3) |
| 2+ | 10,300 | 3,820 (37.1) |
| Medicaida | ||
| No | 31,099 | 8,000 (25.7) |
| Yes | 5,966 | 2,230 (37.4) |
| Year of diagnosisa | ||
| 2000 | 6,041 | 1,497 (24.8) |
| 2001 | 6,193 | 1,624 (26.2) |
| 2002 | 6,289 | 1,692 (26.9) |
| 2003 | 6,489 | 1,829 (28.2) |
| 2004 | 6,219 | 1,832 (29.5) |
| 2005 | 5,834 | 1,756 (30.1) |
| Neighborhood characteristic | ||
| Poverty ratea | ||
| <10% | 21,772 | 5,698(26.2) |
| 10%19% | 9,458 | 2,722(28.8) |
| 20% | 5,835 | 1,810(31.0) |
| Tumor characteristics | ||
| AJCC stagea | ||
| I | 9,996 | 2,402 (24.0) |
| II | 14,662 | 4,287 (29.2) |
| III | 12,407 | 3,541 (28.5) |
| Tumor grade/differentiationa | ||
| Well | 3,278 | 799 (24.4) |
| Moderate | 25,129 | 6,905 (27.5) |
| Poor | 6,898 | 2,107 (30.6) |
| Undifferentiated | 350 | 110 (31.4) |
| Unknown | 1,410 | 309 (21.9) |
| Tumor locationa | ||
| Rectum | 6,977 | 1,616 (23.2) |
| Proximal colon | 14,811 | 4,252 (28.7) |
| Transverse colon | 5,650 | 1,679 (27.9) |
| Distal colon | 9,627 | 2,683 (23.2) |
| Tumor histologya | ||
| Mucinous adenocarcinoma | 32,109 | 8,766 (27.3) |
| Other adenocarcinoma | 4,761 | 1,407 (29.6) |
| Nonadenocarcinoma | 195 | 57 (29.2) |
| Treatment characteristics | ||
| Mode of presentationa | ||
| Elective | 28,673 | 6,836 (23.8) |
| Emergency | 8,392 | 3,394 (40.4) |
| Type of surgerya | ||
| Total (procto)colectomy | 1,475 | 372 (25.2) |
| Subtotal (hemi)colectomy | 18,840 | 5,367 (28.5) |
| Partial colectomyb | 16,017 | 4,298 (26.8) |
| Colectomy NOS | 647 | 177 (27.4) |
| Other surgery | 86 | 16 (18.6) |
| Length of stay, daysa, c | 12.8 (8.3) | |
| Complications during hospitalization | ||
| None | 28,580 | 6,863 (24.0) |
| Yes | 8,485 | 3,367 (39.7) |
| Hospital characteristics | ||
| Hospital volume (no. of beds)a | ||
| 1199 | 9,326 | 2,382 (25.5) |
| 200349 | 10,446 | 3,153 (30.2) |
| 350499 | 8,963 | 2,590 (28.9) |
| 500+ | 8,330 | 2,105 (25.3) |
| Hospital surgery volumea | ||
| 120 | 8,429 | 2,445 (29.0) |
| 2138 | 8,294 | 2,389 (28.8) |
| 3965 | 8,115 | 2,257 (27.8) |
| 66+ | 8,453 | 2,013 (23.8) |
| Unknown | 3,774 | 1,126 (29.8) |
Table 2 shows the adjusted ORs of the covariates associated with comanagement. This model includes only variables independently associated with comanagement. Increasing age was associated with increased odds of receiving comanagement, with OR = 1.22 for patients aged between 75 and 84 years, and OR = 1.52 for patients aged 85 years and older. Comorbidity scores of 1 or 2 or more were associated with increased odds of comanagement use following CRC surgery compared to patients without comorbidities (OR = 1.39 and OR = 1.92, respectively). Patients who received Medicaid were more likely to receive comanagement (OR = 1.11) compared with patients without Medicaid insurance. Higher AJCC stage was associated with increased use of comanagement, as was poor tumor differentiation. Patients undergoing surgery for colon versus rectal cancer were more likely to be comanaged during their hospital stay (OR = 1.23). Surgery after emergency admission was associated with an increased use of comanagement (OR = 1.95). The odds of comanagement increased slightly with increasing length of hospital stay (OR = 1.03), but were higher for patients who developed 1 or more complications during hospitalization compared with patients without complications (OR = 1.38). Compared with CRC patients treated in small hospitals (<200 beds), patients treated in hospitals with 200 to 349 beds were more likely to receive comanaged care (OR = 1.51), whereas patients treated in high‐volume hospitals with 500 beds were less likely to receive comanaged care during hospitalization (OR = 0.65). While census‐tract poverty rate was associated with comanagement in univariable analysis, no significant association was observed when adjusting for other covariates in the multivariable model. In a model with all covariates listed (Table 2), variability of comanagement across hospitals was significant (ICC = 0.376), suggesting that extensive variability across hospitals remained. The adjusted value of CRC patients receiving comanaged care varied widely, from 1.9% to 83.2% across hospitals. Our sensitivity analysis showed that odds ratios and confidence intervals were generally unchanged when using different definitions of comanagement.
| Covariates | OR | 95% CI |
|---|---|---|
| ||
| Age, y | ||
| 6674 | 1.00 | |
| 7584 | 1.22 | 1.151.30 |
| 85+ | 1.52 | 1.421.64 |
| Comorbidity | ||
| 0 | 1.00 | |
| 1 | 1.39 | 1.301.48 |
| 2+ | 1.92 | 1.802.05 |
| Medicaid | ||
| No | 1.00 | |
| Yes | 1.11 | 1.031.20 |
| Year of diagnosis, per year | 1.07 | 1.061.09 |
| AJCC stage | ||
| I | 1.00 | |
| II | 1.11 | 1.041.19 |
| III | 1.10 | 1.021.18 |
| Tumor grade/differentiation | ||
| Well | 1.00 | |
| Moderate | 1.08 | 0.981.19 |
| Poor | 1.17 | 1.041.31 |
| Undifferentiated | 1.14 | 0.851.52 |
| Unknown | 0.89 | 0.751.06 |
| Tumor location | ||
| Rectal | 1.00 | |
| Colon | 1.23 | 1.151.32 |
| Mode of presentation | ||
| Elective | 1.00 | |
| Emergency | 1.95 | 1.842.08 |
| Length of stay, per day | 1.03 | 1.021.03 |
| Complications during stay | ||
| No | 1.00 | |
| Yes | 1.38 | 1.291.48 |
| Hospital volume (no. of beds) | ||
| 1199 | 1.00 | |
| 200349 | 1.51 | 1.221.86 |
| 350499 | 1.26 | 0.981.62 |
| 500+ | 0.65 | 0.490.86 |
DISCUSSION
In all, 27.6% of CRC patients were comanaged during hospitalization, but large disparities existed across patient, hospital, and geographic characteristics. Our finding that more complex patients are more likely to receive comanaged care (eg, increasing age and comorbidity, higher tumor stage and grade, emergency presentation, and complications) are in line with other studies.[5] Importantly, there was a wide range in hospital use of comanagement; the variability across hospitals accounted for 37.6% of the total variability in comanagement use. In some hospitals, almost none of the patients received comanaged care, whereas in other hospitals 83.2% of patients received such care. One reason for the large variability across hospitals may be the lack of evidence about the effectiveness of comanagement. Although comanaged care may benefit orthopedic patients,[7, 8, 9, 10, 11] to our knowledge, no studies have shown benefit to CRC patients. Typically, procedures for which there is more ambiguity about its effectiveness show greater variability across hospitals and geographic areas.[22] Similar to a previous study,[5] we found that patients treated in midsize hospitals were more likely to receive comanagement, and patients treated at high‐volume hospitals were less likely to receive comanagement compared to smaller hospitals. However, because the variability was similar between the model without any variables and the full multivariable model, other variables not available in SEERMedicare data likely played a role in explaining this large interhospital variability. Quality of cancer care may be improved by reducing variation in underuse of effective and necessary care, variation that indicates misuse of preference‐sensitive care (ie, care that offers equivalent options to be chosen by the patient), and variation that indicates overuse of supply‐sensitive care (ie, care influenced by medical capacity).[23] Examining and reducing variability in medical care has been an important policy consideration for almost 30 years.[24, 25] Future studies should examine additional reasons for variability in comanagement across hospitals, including variables at the level of the patient, provider, and hospital. Cost‐effective interventions should subsequently target modifiable factors to reduce use of unnecessary care among patients unlikely to benefit from comanagement.
Our study benefits from using a population‐based SEERMedicare cohort of older CRC patients, representing the diversity of geographic areas and hospitals across the United States. Furthermore, we were able to include a variety of covariates available in the SEERMedicare data, including patient, hospital, and area characteristics. Whereas most studies examining the use or effectiveness of comanagement include single institutions or a variety of surgical patients,[5, 8, 11, 17] we examined the use of and characteristics associated with comanagement throughout the United States for a specific patient population.
We recognize several limitations in this study. Our study specifically assessed comanagement among hospitalized CRC patients aged 65 years and older without managed care insurance. Generalizabilty of our findings to other surgical patients, to younger CRC patients, and those with managed care insurance may therefore be limited. With regard to the definition of comanagement, we used a cutoff point of submitting a claim for management and evaluation on 70% or more of the days of hospitalization for CRC. This cutoff is somewhat arbitrary, but different cutoffs did not influence the results. However, in a prior study, changes in the cutoff used in this definition only affected the proportion of comanaged patients and did not change the observation of increasing trends in the use of comanagement.[5] In addition, we examined a CRC population diagnosed between 2000 and 2005. Because an increasing trend in use of comanagement was observed, it is possible that comanagement use has increased further in more recent years. Our findings of lower use of comanaged care in larger hospitals may be related to the increased use of hospitalists as consultants, whose care may not be accurately or completely captured in claims data. We do not report data regarding the use of physician extenders (nurse practitioners and physician assistants). Although their use is increasing in some contexts, in our data, if we counted patients comanaged by physician extenders, we would only add 79 additional CRC patients (0.6%) to our analyses, which is unlikely to influence our findings.
In conclusion, more complex patients are more likely to receive comanaged care following CRC surgery. Extensive variability existed across patients and hospitals likely due to the lack of evidence about the clinical effectiveness of comanagement for patients undergoing CRC surgery. Future studies should examine additional reasons for variability in comanagement across hospitals, including variables at the level of the patient, provider, and hospital.
Acknowledgements
The authors gratefully acknowledge James Struthers for his data management and programming services, provided through the Health Behavior, Communication, and Outreach Core of the Alvin J. Siteman Cancer Center at Barnes‐Jewish Hospital and Washington University School of Medicine. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; SEER Program tumor registries in the creation of the SEERMedicare database. The authors thank Dr. S. Hendren at the University of Michigan for the use of the SAS programming codes to identify complications.
Disclosures: This study used the linked SEERMedicare database. The interpretation and reporting of these data are the sole responsibility of the authors. This work was supported by grants from the National Institutes of Health National Cancer Institute (R01 CA137750 and P30 CA091842). Dr. Davidson was supported in part through National Institutes of Health grants HL‐38180, DK‐56260, and Digestive Disease Research Core Center DK‐52574.
- , , . Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- , , , , , . Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer. 2007;43(15):2179–2193.
- , , , et al. Which comorbid conditions predict complications after surgery for colorectal cancer? World J Surg. 2007;31(1):192–199.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , . Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394–397.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , . Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program. Clin Orthop Relat Res. 1992;(274):213–225.
- , , , . Impact of a comanaged Geriatric Fracture Center on short‐term hip fracture outcomes. Arch Intern Med. 2009;169(18):1712–1717.
- , , , et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28–38.
- , . Effect of hospitalists on length of stay in the Medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58(9):1649–1657.
- , , , et al. The impact of hospitalists on length of stay and costs: systematic review and meta‐analysis. Am J Manag Care. 2012;18(1):e23–e30.
- , , , et al. Hospital and geographic variability in two colorectal cancer surgery outcomes: complications and mortality after complications. Ann Surg Onc. In press.
- , , , , . Overview of the SEER‐Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 (8 suppl):IV–3–18.
- , , , , , . Use of SEER‐Medicare data for measuring cancer surgery. Med Care. 2002;40(8 suppl):IV–43–48.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
- , , , . Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267.
- , , , , , . Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53(12):1587–1593.
- , , , , , . Major postoperative complications and survival for colon cancer elderly patients. BMC Surgery. 2012;12(suppl 1):S20.
- , . Multilevel Analysis. An Introduction to Basic and Advanced Multilevel Modeling. London, UK: Sage Publications; 1999.
- , , , , , . The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298.
- . Tracking Medicine: A Researcher's Quest to Understand Health Care. Oxford, UK: Oxford University Press; 2010.
- . Understanding geographic variations in health care delivery. N Engl J Med. 1999;340:52–53.
- . Reducing variation in health care: the rhetorical politics of a policy idea. J Health Polit Policy Law. 2013;38(1):5–26.
- , , . Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- , , , , , . Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer. 2007;43(15):2179–2193.
- , , , et al. Which comorbid conditions predict complications after surgery for colorectal cancer? World J Surg. 2007;31(1):192–199.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , . Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394–397.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , . Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program. Clin Orthop Relat Res. 1992;(274):213–225.
- , , , . Impact of a comanaged Geriatric Fracture Center on short‐term hip fracture outcomes. Arch Intern Med. 2009;169(18):1712–1717.
- , , , et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28–38.
- , . Effect of hospitalists on length of stay in the Medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58(9):1649–1657.
- , , , et al. The impact of hospitalists on length of stay and costs: systematic review and meta‐analysis. Am J Manag Care. 2012;18(1):e23–e30.
- , , , et al. Hospital and geographic variability in two colorectal cancer surgery outcomes: complications and mortality after complications. Ann Surg Onc. In press.
- , , , , . Overview of the SEER‐Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 (8 suppl):IV–3–18.
- , , , , , . Use of SEER‐Medicare data for measuring cancer surgery. Med Care. 2002;40(8 suppl):IV–43–48.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
- , , , . Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267.
- , , , , , . Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53(12):1587–1593.
- , , , , , . Major postoperative complications and survival for colon cancer elderly patients. BMC Surgery. 2012;12(suppl 1):S20.
- , . Multilevel Analysis. An Introduction to Basic and Advanced Multilevel Modeling. London, UK: Sage Publications; 1999.
- , , , , , . The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298.
- . Tracking Medicine: A Researcher's Quest to Understand Health Care. Oxford, UK: Oxford University Press; 2010.
- . Understanding geographic variations in health care delivery. N Engl J Med. 1999;340:52–53.
- . Reducing variation in health care: the rhetorical politics of a policy idea. J Health Polit Policy Law. 2013;38(1):5–26.
© 2014 Society of Hospital Medicine
Rounding Practices and AGME Competencies
In 1999, the Accreditation Council for Graduate Medical Education (ACGME) established the requirement for residency programs to assess trainees' competencies in 6 core domains: patient care, medical knowledge, practice‐based learning, systems‐based practice, interpersonal and communication skills, and professionalism.[1] With the rollout of the Next Accreditation System (NAS), and a focus of graduate medical education turning to the assessment of milestones within the ACGME core competencies, it is essential for clinician educators to reflect on how current educational activities meet the needs of our learners and enable compliance with the new recommendations.[1]
On internal medicine services in the inpatient setting, clinician educators routinely supervise and teach trainees during attending rounds. The long‐standing practice of rounding innately offers a forum for making patient care decisions and for sharing medical knowledge.[2] However, the rounding process may also afford clinician educators opportunities to teach material relevant to the other 4 ACGME core competencies.[3] Despite the ubiquitous presence of rounds on internal medicine services, rounding practices vary markedly among and within institutions.[4, 5] Furthermore, there is no consensus with respect to best practices for rounds in general, or more specifically as they pertain to graduate medical education and teaching within the 6 core competencies.
We have conducted a multicenter survey study of internal medicine rounding practices at academic institutions from all US regions. As part of a larger investigation of rounding practices, we surveyed attending physicians regarding the frequency with which they participated in different rounding models (card‐flipping rounds [CFR], hallway rounds [HR], or bedside rounds [BR]), and the perceived capacity of each of these models to promote teaching of material relevant to the 6 ACGME core competencies.
METHODS
Sites and Subjects
We disseminated a survey using internal medicine educational leadership and hospital medicine clinical leadership electronic mailing lists (eg, the Society of General Internal Medicine [SGIM] and the Society of Hospital Medicine [SHM]). These listservs gave us access to leaders in the field at institutions affiliated with residency programs. Our initial survey distribution included attending physicians from 58 institutions. We asked these leaders for their assistance in distributing the survey within their respective institutions to physicians who attend on inpatient medicine teaching services.
Survey Development and Domains
The survey was composed of 24 multiple‐choice questions and 1 open‐ended question, and was adapted with permission from a survey created by Mittal and colleagues.[6] We initially piloted the survey with attending physicians in the Division of Hospital Medicine at the University of California, San Francisco. We defined the following 3 models for attending rounds based on our review of the literature, as well as interviews with inpatient clinician educators and internal medicine residency leadership at 3 different institutions: (1) BR, where the discussion of the patient and care plan occur in the presence of the patient with his or her active participation; (2) HR, where the discussion of the patient and care plan occurs partially outside the patient's room and partially at the patient's bedside in the presence of the patient; and (3) CFR, where the discussion of the patient and care plan occurs entirely outside of the patient's room and the team does not see the patient together. The survey asked respondents for their perceptions about how well each model promotes teaching content relevant to the 6 ACGME core competencies (options: very poorly, poorly, neutral, well, very well).
Survey Process
The survey was administered electronically using SurveyMonkey (SurveyMonkey, Menlo Park, CA). We sent an initial survey request to 58 institutional contacts. These contacts were designated clinical and educational leaders in the SHM and SGIM, and were an invited working group. Those leaders were asked to reach out to physicians within their institutions who attended on teaching services. We left the survey open for accrual for a total period of 80 days. Participants received 2 reminder emails asking for their assistance in distributing the survey. The study received approval by our institutional review board.
Data Analysis
We employed means and standard deviations to classify rounding model preference and prevalence. We used Pearson's [2] test to assess the association among the 3 rounding models and the perceptions of how well they worked for teaching material relevant to the ACGME competencies. We dichotomized measures with well and very well, forming the well category. All analysis was conducted using Stata 11.0 (StateCorp, College Station, TX).
RESULTS
Attending Characteristics
We received 153 completed surveys from attending physicians representing 34 unique institutions. All respondents were internal medicine physicians who attend on inpatient medicine teaching services. Institutions spanned all regions of the United States. The characteristics of the surveyed population are described in Table 1.
| Variable | Category | Percent |
|---|---|---|
| ||
| Age, y | 40 | 62% |
| 4150 | 21% | |
| 5160 | 13% | |
| >60 | 3% | |
| Sex | Female | 46% |
| Male | 54% | |
| Job description | Hospitalist | 61% |
| Outpatient internist | 10% | |
| Mixed internist* | 14% | |
| Specialist | 15% | |
| Experience, y | 2 | 21% |
| 3+ | 79% | |
| Months teaching/year | 3 | 50% |
| 3+ | 50% | |
| Decisions requiring attending input | 30% | 40% |
| >30% | 60% | |
| Team cap | <20 | 47% |
| 20 | 53% | |
| Average daily census | 10 | 51% |
| >10 | 49% | |
| Region | Midwest | 8% |
| Northeast | 19% | |
| South | 28% | |
| West | 44% | |
| Hospital type | University | 82% |
| Community | 8% | |
| Hospital size, beds | <300 | 23% |
| 300500 | 32% | |
| >500 | 55% | |
Rounding Characteristics
HR proved to be the model employed most frequently for both new and established patients (61% and 43%, respectively) (Table 2). The next most frequently utilized rounding models were CFR for established patients (36%) and BR for new patients (22%). Of attending physicians, 53% never used BR for established patients, and 46% never used them at all. When asked about barriers to bedside rounding, respondents cited time constraints, patient psychosocial complexities, and patient privacy as the most significant barriers to performing BR (64%, 39%, and 38%, respectively). Only 6% felt that patient preference was a barrier to bedside rounding.
| New Patients | Old Patients | |
|---|---|---|
| Card‐flipping rounds | 17% (12%22%) | 36% (31%42%) |
| Hallway rounds | 61% (55%68%) | 43% (37%48%) |
| Bedside rounds | 22% (16%27%) | 21% (15%26%) |
Rounding Models and Core Competencies
Most attending physicians surveyed perceived CFR to perform well or very well for teaching medical knowledge (78%), practice‐based learning (59%), and systems‐based practice (53%). Conversely, a minority thought CFR performed well or very well with respect to teaching patient care (21%), professionalism (27%), or interpersonal skills and communication (16%).
The majority of respondents perceived HR to perform well or very well across all ACGME domains, including teaching patient care (91%), medical knowledge (87%), practice‐based learning (74%), systems‐based practice (69%), professionalism (87%), and interpersonal skills and communication (90%).
Most attending physicians surveyed felt BR performed well or very well with respect to teaching patient care (88%), medical knowledge (58%), professionalism (92%), and interpersonal skills and communication (95%). A minority of participants perceived BR to perform well or very well in teaching practice‐based learning (47%) or systems‐based practice (47%).
Compared with CFR, both HR and BR were perceived to be significantly more effective in teaching patient care, professionalism, and interpersonal skills and communication (Figure 1). Respondents rated BR as significantly inferior to both CFR and HR in teaching medical knowledge. In addition, BR was perceived to be inferior to HR with respect to teaching systems‐based practice and practice‐based learning.
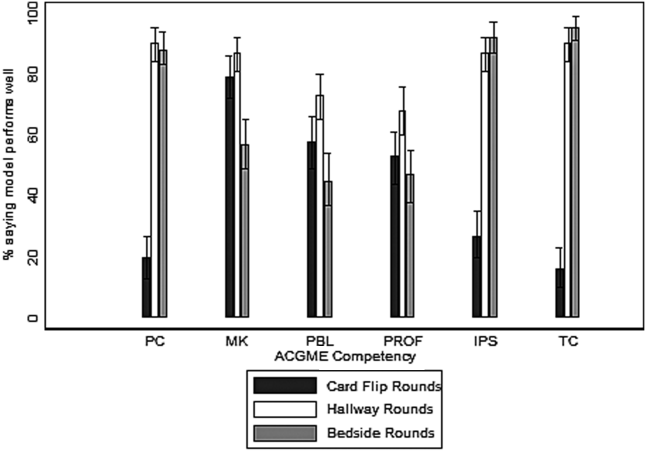
DISCUSSION
In the inpatient setting, attending rounds may offer a primary means for attending physician teaching of trainees. Although all trainees are assessed by their knowledge and skills within the 6 ACGME core competencies, little attention has been paid as to how various rounding models support resident education across these domains.[4] To our knowledge, this is the first cross‐national, multicenter survey study that examines how well the 3 most commonly employed internal medicine rounding practices promote teaching of material relevant to the 6 ACGME core competencies. We found that significant heterogeneity exists in current rounding practices, and different models are perceived to perform variably in their promotion of teaching content within the educational competencies.
In general, with respect to teaching across ACGME domains, CFR were perceived to be less effective compared with HR or BR, and significantly less so in the teaching of patient care, professionalism, and interpersonal skills and communication. Yet, CFR remain widely employed and are used by 17% of attending physicians for new patients and more than 36% for old patients. The reason for their ongoing use was not assessed by our survey; however, this practice does not appear to be driven by educational objectives. The prevalence of CFR may be related to a perception of improved efficiency and a frequent preference among trainees and attending physicians to do this model of rounding. There may be other perceived benefits, including physical comfort of providers or access to the electronic health record, but these qualities were not captured in this study. To our knowledge, there are no prior studies specifically examining CFR as a rounding model.
HR was the most commonly utilized rounding method for the majority of respondents. Attending physicians considered HR particularly effective in teaching patient care, medical knowledge, professionalism, and interpersonal skills and communication. The perceived value of HR may be related to the bimodal nature of the encounter. The discussion of the patient and the care plan outside of the room may include, but is not limited to, formulating the care plan through the formal oral case presentation, focusing on the patient management component of patient care, and sharing medical knowledge without fear of provoking patient anxiety or causing confusion. Subsequently, the time spent in the room may allow for observation and instruction of the physical examination, observation and modeling of professionalism in the patient interaction, and observation and modeling of effective communication with the patient and family members.
We found it interesting that attending physicians also considered HR superior to BR in their capacity to teach practice‐based learning and systems‐based practice. This requires further exploration, as it would seem that increased patient involvement in care plans could offer advantages for the teaching of both of these competencies. Despite the popularity of this rounding structure, there is little prior evidence examining the pros and cons of HR.
Conversely, there is significant literature exploring BR as a rounding model. Prior research has elucidated the multiple benefits of bedside rounding including, but not limited to, teaching history taking, physical examination skills, and clinical ethics; modeling humanism and professionalism; and promoting effective communication.[3, 7, 8, 9, 10, 11] Furthermore, the majority of patients may prefer bedside presentations.[11, 12, 13] Despite their apparent merits, roughly half of attendings surveyed never conduct BR. This may reflect the trend reported in the literature of diminishing bedside teaching, and more specifically, reports that in the United States, less than 5% of time is spent on observing learners' clinical skills and correcting faulty exam techniques.[2, 14] The perception that HR and CFR were superior to BR in teaching medical knowledge suggests that attending physicians value the teaching that occurs away from the patient's bedside. Prior studies suggest that, of the core clinical skills taught on the wards, trainees may find teaching of differential diagnosis to be most challenged by BR, and residents may not appreciate the educational benefits of BR in general.[11, 13] Although time constraints were cited as a significant barrier to BR, recent studies have suggested that BR do not necessarily take more time overall.[15] The notion that patient psychosocial complexities may limit BR has been reflected in the literature,[16, 17, 18, 19, 20] but these situations may also afford unique bedside teaching opportunities.[21] Finally, faculty, and in particular more junior attendings, may be uncomfortable teaching in the presence of the patient.[13] This barrier may be overcome through faculty development efforts.[15]
As internal medicine training transitions to the NAS and a milestone‐based assessment framework,[1] residency programs will need to consider how rounding can be structured to help trainees achieve the required milestones, and to help programs meaningfully assess trainee performance. Our survey indicates that HR may be effective across all of the competencies and the potential for this should be further explored. Yet, HR may allow for a limited ability to observe learners with patients, as there may only be cursory data gathering from the patient, a brief physical exam, and limited communication with patients and/or family members. Furthermore, the patient‐centeredness of HR may be called into question, given restricted emphasis on shared decision making. Finally, as efficiency remains crucial in the wake of duty‐hour reform, HR may also prove to be more time consuming than BR, given that it often requires information shared outside of the patient's room to be repeated in the patient's presence. Ultimately, there may not be a 1 size fits all solution, and institutions should ensure the organization and structure of their rounding models are optimally designed to enable the achievement and assessment of ACGME milestones.
Our study has several limitations. Due to our employment of snowball sampling, we could not calculate a response rate. We also recruited a self‐selected sample of internal medicine attending physicians, raising the possibility of selection bias. However, we captured a wide range of experience and opinion, and do not have reason to believe that any particular viewpoints are over‐ or under‐represented. Further, our study may have been influenced by sampling bias, reaching primarily attending physicians at university‐affiliated medical centers, calling into question the generalizability of our results and making any comparisons between academic and community health centers less meaningful. Nonetheless, we received responses from both large and small medical centers, as well as quaternary care and community‐based hospitals. The reported benefits and barriers were respondents' personal perceptions, rather than measured outcomes. Moreover, we focused primarily on the effectiveness of teaching of the ACGME competencies and did not explore other outcomes that could be impacted by rounding structure (eg, patient satisfaction, trainee satisfaction, length of stay, time of discharge). Our study also did not address the variety of complex factors that influence the location and methods of attending rounds. For example, the various institutions surveyed have a variety of team sizes and compositions, admitting schedules, geographic layouts, and time allotted for attending rounds, all of which can influence choices for rounding practices. Finally, we did not assess resident perceptions, an area of future study that would allow us to corroborate the findings of our survey.
In conclusion, in this cross‐national, multicenter survey study of the 3 most prevalent internal medicine rounding practices, respondents utilized HR most commonly and believed this model was effective in teaching across the 6 ACGME core competencies. Those surveyed identified the benefits and barriers to BR, and a substantial number continue to use CFR despite recognizing its educational limitations. Future studies should explore factors that promote various rounding models and assess the relationship between rounding structure and educational outcomes for trainees.
Disclosure: Nothing to report.
- , , , . The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366(11):1051–1056.
- , . Teaching the resident in internal medicine. Present practices and suggestions for the future. JAMA. 1986;256(6):725–729.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , , , . Relationships of the location and content of rounds to specialty, institution, patient‐census, and team size. PLoS One. 2010;5(6):e11246.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- . Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217–2225.
- , , , , . Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661–667.
- . Teaching approaches that reflect and promote professionalism. Acad Med. 2003;78(7):709–713.
- . Bedside rounds revisited. N Engl J Med. 1997;336(16):1174–1175.
- , . Sounding boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230–1233.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , . Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341–346.
- , , , , . An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646–648.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- , , . The attending physician as teacher. N Engl J Med. 1983;308(19):1129–1132.
- , , , , . Bedside case presentations: why patients like them but learners don't. J Gen Intern Med. 1989;4(4):284–287.
- . On bedside teaching. Ann Intern Med. 1997;126(3):217–220.
- , . Teaching at the bedside: a new model. Med Teach. 2003;25(2):127–130.
- . Twelve tips to improve bedside teaching. Med Teach. 2003;25(2):112–115.
- Wiese J, ed. Teaching in the Hospital. Philadelphia, PA: American College of Physicians; 2010.
In 1999, the Accreditation Council for Graduate Medical Education (ACGME) established the requirement for residency programs to assess trainees' competencies in 6 core domains: patient care, medical knowledge, practice‐based learning, systems‐based practice, interpersonal and communication skills, and professionalism.[1] With the rollout of the Next Accreditation System (NAS), and a focus of graduate medical education turning to the assessment of milestones within the ACGME core competencies, it is essential for clinician educators to reflect on how current educational activities meet the needs of our learners and enable compliance with the new recommendations.[1]
On internal medicine services in the inpatient setting, clinician educators routinely supervise and teach trainees during attending rounds. The long‐standing practice of rounding innately offers a forum for making patient care decisions and for sharing medical knowledge.[2] However, the rounding process may also afford clinician educators opportunities to teach material relevant to the other 4 ACGME core competencies.[3] Despite the ubiquitous presence of rounds on internal medicine services, rounding practices vary markedly among and within institutions.[4, 5] Furthermore, there is no consensus with respect to best practices for rounds in general, or more specifically as they pertain to graduate medical education and teaching within the 6 core competencies.
We have conducted a multicenter survey study of internal medicine rounding practices at academic institutions from all US regions. As part of a larger investigation of rounding practices, we surveyed attending physicians regarding the frequency with which they participated in different rounding models (card‐flipping rounds [CFR], hallway rounds [HR], or bedside rounds [BR]), and the perceived capacity of each of these models to promote teaching of material relevant to the 6 ACGME core competencies.
METHODS
Sites and Subjects
We disseminated a survey using internal medicine educational leadership and hospital medicine clinical leadership electronic mailing lists (eg, the Society of General Internal Medicine [SGIM] and the Society of Hospital Medicine [SHM]). These listservs gave us access to leaders in the field at institutions affiliated with residency programs. Our initial survey distribution included attending physicians from 58 institutions. We asked these leaders for their assistance in distributing the survey within their respective institutions to physicians who attend on inpatient medicine teaching services.
Survey Development and Domains
The survey was composed of 24 multiple‐choice questions and 1 open‐ended question, and was adapted with permission from a survey created by Mittal and colleagues.[6] We initially piloted the survey with attending physicians in the Division of Hospital Medicine at the University of California, San Francisco. We defined the following 3 models for attending rounds based on our review of the literature, as well as interviews with inpatient clinician educators and internal medicine residency leadership at 3 different institutions: (1) BR, where the discussion of the patient and care plan occur in the presence of the patient with his or her active participation; (2) HR, where the discussion of the patient and care plan occurs partially outside the patient's room and partially at the patient's bedside in the presence of the patient; and (3) CFR, where the discussion of the patient and care plan occurs entirely outside of the patient's room and the team does not see the patient together. The survey asked respondents for their perceptions about how well each model promotes teaching content relevant to the 6 ACGME core competencies (options: very poorly, poorly, neutral, well, very well).
Survey Process
The survey was administered electronically using SurveyMonkey (SurveyMonkey, Menlo Park, CA). We sent an initial survey request to 58 institutional contacts. These contacts were designated clinical and educational leaders in the SHM and SGIM, and were an invited working group. Those leaders were asked to reach out to physicians within their institutions who attended on teaching services. We left the survey open for accrual for a total period of 80 days. Participants received 2 reminder emails asking for their assistance in distributing the survey. The study received approval by our institutional review board.
Data Analysis
We employed means and standard deviations to classify rounding model preference and prevalence. We used Pearson's [2] test to assess the association among the 3 rounding models and the perceptions of how well they worked for teaching material relevant to the ACGME competencies. We dichotomized measures with well and very well, forming the well category. All analysis was conducted using Stata 11.0 (StateCorp, College Station, TX).
RESULTS
Attending Characteristics
We received 153 completed surveys from attending physicians representing 34 unique institutions. All respondents were internal medicine physicians who attend on inpatient medicine teaching services. Institutions spanned all regions of the United States. The characteristics of the surveyed population are described in Table 1.
| Variable | Category | Percent |
|---|---|---|
| ||
| Age, y | 40 | 62% |
| 4150 | 21% | |
| 5160 | 13% | |
| >60 | 3% | |
| Sex | Female | 46% |
| Male | 54% | |
| Job description | Hospitalist | 61% |
| Outpatient internist | 10% | |
| Mixed internist* | 14% | |
| Specialist | 15% | |
| Experience, y | 2 | 21% |
| 3+ | 79% | |
| Months teaching/year | 3 | 50% |
| 3+ | 50% | |
| Decisions requiring attending input | 30% | 40% |
| >30% | 60% | |
| Team cap | <20 | 47% |
| 20 | 53% | |
| Average daily census | 10 | 51% |
| >10 | 49% | |
| Region | Midwest | 8% |
| Northeast | 19% | |
| South | 28% | |
| West | 44% | |
| Hospital type | University | 82% |
| Community | 8% | |
| Hospital size, beds | <300 | 23% |
| 300500 | 32% | |
| >500 | 55% | |
Rounding Characteristics
HR proved to be the model employed most frequently for both new and established patients (61% and 43%, respectively) (Table 2). The next most frequently utilized rounding models were CFR for established patients (36%) and BR for new patients (22%). Of attending physicians, 53% never used BR for established patients, and 46% never used them at all. When asked about barriers to bedside rounding, respondents cited time constraints, patient psychosocial complexities, and patient privacy as the most significant barriers to performing BR (64%, 39%, and 38%, respectively). Only 6% felt that patient preference was a barrier to bedside rounding.
| New Patients | Old Patients | |
|---|---|---|
| Card‐flipping rounds | 17% (12%22%) | 36% (31%42%) |
| Hallway rounds | 61% (55%68%) | 43% (37%48%) |
| Bedside rounds | 22% (16%27%) | 21% (15%26%) |
Rounding Models and Core Competencies
Most attending physicians surveyed perceived CFR to perform well or very well for teaching medical knowledge (78%), practice‐based learning (59%), and systems‐based practice (53%). Conversely, a minority thought CFR performed well or very well with respect to teaching patient care (21%), professionalism (27%), or interpersonal skills and communication (16%).
The majority of respondents perceived HR to perform well or very well across all ACGME domains, including teaching patient care (91%), medical knowledge (87%), practice‐based learning (74%), systems‐based practice (69%), professionalism (87%), and interpersonal skills and communication (90%).
Most attending physicians surveyed felt BR performed well or very well with respect to teaching patient care (88%), medical knowledge (58%), professionalism (92%), and interpersonal skills and communication (95%). A minority of participants perceived BR to perform well or very well in teaching practice‐based learning (47%) or systems‐based practice (47%).
Compared with CFR, both HR and BR were perceived to be significantly more effective in teaching patient care, professionalism, and interpersonal skills and communication (Figure 1). Respondents rated BR as significantly inferior to both CFR and HR in teaching medical knowledge. In addition, BR was perceived to be inferior to HR with respect to teaching systems‐based practice and practice‐based learning.

DISCUSSION
In the inpatient setting, attending rounds may offer a primary means for attending physician teaching of trainees. Although all trainees are assessed by their knowledge and skills within the 6 ACGME core competencies, little attention has been paid as to how various rounding models support resident education across these domains.[4] To our knowledge, this is the first cross‐national, multicenter survey study that examines how well the 3 most commonly employed internal medicine rounding practices promote teaching of material relevant to the 6 ACGME core competencies. We found that significant heterogeneity exists in current rounding practices, and different models are perceived to perform variably in their promotion of teaching content within the educational competencies.
In general, with respect to teaching across ACGME domains, CFR were perceived to be less effective compared with HR or BR, and significantly less so in the teaching of patient care, professionalism, and interpersonal skills and communication. Yet, CFR remain widely employed and are used by 17% of attending physicians for new patients and more than 36% for old patients. The reason for their ongoing use was not assessed by our survey; however, this practice does not appear to be driven by educational objectives. The prevalence of CFR may be related to a perception of improved efficiency and a frequent preference among trainees and attending physicians to do this model of rounding. There may be other perceived benefits, including physical comfort of providers or access to the electronic health record, but these qualities were not captured in this study. To our knowledge, there are no prior studies specifically examining CFR as a rounding model.
HR was the most commonly utilized rounding method for the majority of respondents. Attending physicians considered HR particularly effective in teaching patient care, medical knowledge, professionalism, and interpersonal skills and communication. The perceived value of HR may be related to the bimodal nature of the encounter. The discussion of the patient and the care plan outside of the room may include, but is not limited to, formulating the care plan through the formal oral case presentation, focusing on the patient management component of patient care, and sharing medical knowledge without fear of provoking patient anxiety or causing confusion. Subsequently, the time spent in the room may allow for observation and instruction of the physical examination, observation and modeling of professionalism in the patient interaction, and observation and modeling of effective communication with the patient and family members.
We found it interesting that attending physicians also considered HR superior to BR in their capacity to teach practice‐based learning and systems‐based practice. This requires further exploration, as it would seem that increased patient involvement in care plans could offer advantages for the teaching of both of these competencies. Despite the popularity of this rounding structure, there is little prior evidence examining the pros and cons of HR.
Conversely, there is significant literature exploring BR as a rounding model. Prior research has elucidated the multiple benefits of bedside rounding including, but not limited to, teaching history taking, physical examination skills, and clinical ethics; modeling humanism and professionalism; and promoting effective communication.[3, 7, 8, 9, 10, 11] Furthermore, the majority of patients may prefer bedside presentations.[11, 12, 13] Despite their apparent merits, roughly half of attendings surveyed never conduct BR. This may reflect the trend reported in the literature of diminishing bedside teaching, and more specifically, reports that in the United States, less than 5% of time is spent on observing learners' clinical skills and correcting faulty exam techniques.[2, 14] The perception that HR and CFR were superior to BR in teaching medical knowledge suggests that attending physicians value the teaching that occurs away from the patient's bedside. Prior studies suggest that, of the core clinical skills taught on the wards, trainees may find teaching of differential diagnosis to be most challenged by BR, and residents may not appreciate the educational benefits of BR in general.[11, 13] Although time constraints were cited as a significant barrier to BR, recent studies have suggested that BR do not necessarily take more time overall.[15] The notion that patient psychosocial complexities may limit BR has been reflected in the literature,[16, 17, 18, 19, 20] but these situations may also afford unique bedside teaching opportunities.[21] Finally, faculty, and in particular more junior attendings, may be uncomfortable teaching in the presence of the patient.[13] This barrier may be overcome through faculty development efforts.[15]
As internal medicine training transitions to the NAS and a milestone‐based assessment framework,[1] residency programs will need to consider how rounding can be structured to help trainees achieve the required milestones, and to help programs meaningfully assess trainee performance. Our survey indicates that HR may be effective across all of the competencies and the potential for this should be further explored. Yet, HR may allow for a limited ability to observe learners with patients, as there may only be cursory data gathering from the patient, a brief physical exam, and limited communication with patients and/or family members. Furthermore, the patient‐centeredness of HR may be called into question, given restricted emphasis on shared decision making. Finally, as efficiency remains crucial in the wake of duty‐hour reform, HR may also prove to be more time consuming than BR, given that it often requires information shared outside of the patient's room to be repeated in the patient's presence. Ultimately, there may not be a 1 size fits all solution, and institutions should ensure the organization and structure of their rounding models are optimally designed to enable the achievement and assessment of ACGME milestones.
Our study has several limitations. Due to our employment of snowball sampling, we could not calculate a response rate. We also recruited a self‐selected sample of internal medicine attending physicians, raising the possibility of selection bias. However, we captured a wide range of experience and opinion, and do not have reason to believe that any particular viewpoints are over‐ or under‐represented. Further, our study may have been influenced by sampling bias, reaching primarily attending physicians at university‐affiliated medical centers, calling into question the generalizability of our results and making any comparisons between academic and community health centers less meaningful. Nonetheless, we received responses from both large and small medical centers, as well as quaternary care and community‐based hospitals. The reported benefits and barriers were respondents' personal perceptions, rather than measured outcomes. Moreover, we focused primarily on the effectiveness of teaching of the ACGME competencies and did not explore other outcomes that could be impacted by rounding structure (eg, patient satisfaction, trainee satisfaction, length of stay, time of discharge). Our study also did not address the variety of complex factors that influence the location and methods of attending rounds. For example, the various institutions surveyed have a variety of team sizes and compositions, admitting schedules, geographic layouts, and time allotted for attending rounds, all of which can influence choices for rounding practices. Finally, we did not assess resident perceptions, an area of future study that would allow us to corroborate the findings of our survey.
In conclusion, in this cross‐national, multicenter survey study of the 3 most prevalent internal medicine rounding practices, respondents utilized HR most commonly and believed this model was effective in teaching across the 6 ACGME core competencies. Those surveyed identified the benefits and barriers to BR, and a substantial number continue to use CFR despite recognizing its educational limitations. Future studies should explore factors that promote various rounding models and assess the relationship between rounding structure and educational outcomes for trainees.
Disclosure: Nothing to report.
In 1999, the Accreditation Council for Graduate Medical Education (ACGME) established the requirement for residency programs to assess trainees' competencies in 6 core domains: patient care, medical knowledge, practice‐based learning, systems‐based practice, interpersonal and communication skills, and professionalism.[1] With the rollout of the Next Accreditation System (NAS), and a focus of graduate medical education turning to the assessment of milestones within the ACGME core competencies, it is essential for clinician educators to reflect on how current educational activities meet the needs of our learners and enable compliance with the new recommendations.[1]
On internal medicine services in the inpatient setting, clinician educators routinely supervise and teach trainees during attending rounds. The long‐standing practice of rounding innately offers a forum for making patient care decisions and for sharing medical knowledge.[2] However, the rounding process may also afford clinician educators opportunities to teach material relevant to the other 4 ACGME core competencies.[3] Despite the ubiquitous presence of rounds on internal medicine services, rounding practices vary markedly among and within institutions.[4, 5] Furthermore, there is no consensus with respect to best practices for rounds in general, or more specifically as they pertain to graduate medical education and teaching within the 6 core competencies.
We have conducted a multicenter survey study of internal medicine rounding practices at academic institutions from all US regions. As part of a larger investigation of rounding practices, we surveyed attending physicians regarding the frequency with which they participated in different rounding models (card‐flipping rounds [CFR], hallway rounds [HR], or bedside rounds [BR]), and the perceived capacity of each of these models to promote teaching of material relevant to the 6 ACGME core competencies.
METHODS
Sites and Subjects
We disseminated a survey using internal medicine educational leadership and hospital medicine clinical leadership electronic mailing lists (eg, the Society of General Internal Medicine [SGIM] and the Society of Hospital Medicine [SHM]). These listservs gave us access to leaders in the field at institutions affiliated with residency programs. Our initial survey distribution included attending physicians from 58 institutions. We asked these leaders for their assistance in distributing the survey within their respective institutions to physicians who attend on inpatient medicine teaching services.
Survey Development and Domains
The survey was composed of 24 multiple‐choice questions and 1 open‐ended question, and was adapted with permission from a survey created by Mittal and colleagues.[6] We initially piloted the survey with attending physicians in the Division of Hospital Medicine at the University of California, San Francisco. We defined the following 3 models for attending rounds based on our review of the literature, as well as interviews with inpatient clinician educators and internal medicine residency leadership at 3 different institutions: (1) BR, where the discussion of the patient and care plan occur in the presence of the patient with his or her active participation; (2) HR, where the discussion of the patient and care plan occurs partially outside the patient's room and partially at the patient's bedside in the presence of the patient; and (3) CFR, where the discussion of the patient and care plan occurs entirely outside of the patient's room and the team does not see the patient together. The survey asked respondents for their perceptions about how well each model promotes teaching content relevant to the 6 ACGME core competencies (options: very poorly, poorly, neutral, well, very well).
Survey Process
The survey was administered electronically using SurveyMonkey (SurveyMonkey, Menlo Park, CA). We sent an initial survey request to 58 institutional contacts. These contacts were designated clinical and educational leaders in the SHM and SGIM, and were an invited working group. Those leaders were asked to reach out to physicians within their institutions who attended on teaching services. We left the survey open for accrual for a total period of 80 days. Participants received 2 reminder emails asking for their assistance in distributing the survey. The study received approval by our institutional review board.
Data Analysis
We employed means and standard deviations to classify rounding model preference and prevalence. We used Pearson's [2] test to assess the association among the 3 rounding models and the perceptions of how well they worked for teaching material relevant to the ACGME competencies. We dichotomized measures with well and very well, forming the well category. All analysis was conducted using Stata 11.0 (StateCorp, College Station, TX).
RESULTS
Attending Characteristics
We received 153 completed surveys from attending physicians representing 34 unique institutions. All respondents were internal medicine physicians who attend on inpatient medicine teaching services. Institutions spanned all regions of the United States. The characteristics of the surveyed population are described in Table 1.
| Variable | Category | Percent |
|---|---|---|
| ||
| Age, y | 40 | 62% |
| 4150 | 21% | |
| 5160 | 13% | |
| >60 | 3% | |
| Sex | Female | 46% |
| Male | 54% | |
| Job description | Hospitalist | 61% |
| Outpatient internist | 10% | |
| Mixed internist* | 14% | |
| Specialist | 15% | |
| Experience, y | 2 | 21% |
| 3+ | 79% | |
| Months teaching/year | 3 | 50% |
| 3+ | 50% | |
| Decisions requiring attending input | 30% | 40% |
| >30% | 60% | |
| Team cap | <20 | 47% |
| 20 | 53% | |
| Average daily census | 10 | 51% |
| >10 | 49% | |
| Region | Midwest | 8% |
| Northeast | 19% | |
| South | 28% | |
| West | 44% | |
| Hospital type | University | 82% |
| Community | 8% | |
| Hospital size, beds | <300 | 23% |
| 300500 | 32% | |
| >500 | 55% | |
Rounding Characteristics
HR proved to be the model employed most frequently for both new and established patients (61% and 43%, respectively) (Table 2). The next most frequently utilized rounding models were CFR for established patients (36%) and BR for new patients (22%). Of attending physicians, 53% never used BR for established patients, and 46% never used them at all. When asked about barriers to bedside rounding, respondents cited time constraints, patient psychosocial complexities, and patient privacy as the most significant barriers to performing BR (64%, 39%, and 38%, respectively). Only 6% felt that patient preference was a barrier to bedside rounding.
| New Patients | Old Patients | |
|---|---|---|
| Card‐flipping rounds | 17% (12%22%) | 36% (31%42%) |
| Hallway rounds | 61% (55%68%) | 43% (37%48%) |
| Bedside rounds | 22% (16%27%) | 21% (15%26%) |
Rounding Models and Core Competencies
Most attending physicians surveyed perceived CFR to perform well or very well for teaching medical knowledge (78%), practice‐based learning (59%), and systems‐based practice (53%). Conversely, a minority thought CFR performed well or very well with respect to teaching patient care (21%), professionalism (27%), or interpersonal skills and communication (16%).
The majority of respondents perceived HR to perform well or very well across all ACGME domains, including teaching patient care (91%), medical knowledge (87%), practice‐based learning (74%), systems‐based practice (69%), professionalism (87%), and interpersonal skills and communication (90%).
Most attending physicians surveyed felt BR performed well or very well with respect to teaching patient care (88%), medical knowledge (58%), professionalism (92%), and interpersonal skills and communication (95%). A minority of participants perceived BR to perform well or very well in teaching practice‐based learning (47%) or systems‐based practice (47%).
Compared with CFR, both HR and BR were perceived to be significantly more effective in teaching patient care, professionalism, and interpersonal skills and communication (Figure 1). Respondents rated BR as significantly inferior to both CFR and HR in teaching medical knowledge. In addition, BR was perceived to be inferior to HR with respect to teaching systems‐based practice and practice‐based learning.

DISCUSSION
In the inpatient setting, attending rounds may offer a primary means for attending physician teaching of trainees. Although all trainees are assessed by their knowledge and skills within the 6 ACGME core competencies, little attention has been paid as to how various rounding models support resident education across these domains.[4] To our knowledge, this is the first cross‐national, multicenter survey study that examines how well the 3 most commonly employed internal medicine rounding practices promote teaching of material relevant to the 6 ACGME core competencies. We found that significant heterogeneity exists in current rounding practices, and different models are perceived to perform variably in their promotion of teaching content within the educational competencies.
In general, with respect to teaching across ACGME domains, CFR were perceived to be less effective compared with HR or BR, and significantly less so in the teaching of patient care, professionalism, and interpersonal skills and communication. Yet, CFR remain widely employed and are used by 17% of attending physicians for new patients and more than 36% for old patients. The reason for their ongoing use was not assessed by our survey; however, this practice does not appear to be driven by educational objectives. The prevalence of CFR may be related to a perception of improved efficiency and a frequent preference among trainees and attending physicians to do this model of rounding. There may be other perceived benefits, including physical comfort of providers or access to the electronic health record, but these qualities were not captured in this study. To our knowledge, there are no prior studies specifically examining CFR as a rounding model.
HR was the most commonly utilized rounding method for the majority of respondents. Attending physicians considered HR particularly effective in teaching patient care, medical knowledge, professionalism, and interpersonal skills and communication. The perceived value of HR may be related to the bimodal nature of the encounter. The discussion of the patient and the care plan outside of the room may include, but is not limited to, formulating the care plan through the formal oral case presentation, focusing on the patient management component of patient care, and sharing medical knowledge without fear of provoking patient anxiety or causing confusion. Subsequently, the time spent in the room may allow for observation and instruction of the physical examination, observation and modeling of professionalism in the patient interaction, and observation and modeling of effective communication with the patient and family members.
We found it interesting that attending physicians also considered HR superior to BR in their capacity to teach practice‐based learning and systems‐based practice. This requires further exploration, as it would seem that increased patient involvement in care plans could offer advantages for the teaching of both of these competencies. Despite the popularity of this rounding structure, there is little prior evidence examining the pros and cons of HR.
Conversely, there is significant literature exploring BR as a rounding model. Prior research has elucidated the multiple benefits of bedside rounding including, but not limited to, teaching history taking, physical examination skills, and clinical ethics; modeling humanism and professionalism; and promoting effective communication.[3, 7, 8, 9, 10, 11] Furthermore, the majority of patients may prefer bedside presentations.[11, 12, 13] Despite their apparent merits, roughly half of attendings surveyed never conduct BR. This may reflect the trend reported in the literature of diminishing bedside teaching, and more specifically, reports that in the United States, less than 5% of time is spent on observing learners' clinical skills and correcting faulty exam techniques.[2, 14] The perception that HR and CFR were superior to BR in teaching medical knowledge suggests that attending physicians value the teaching that occurs away from the patient's bedside. Prior studies suggest that, of the core clinical skills taught on the wards, trainees may find teaching of differential diagnosis to be most challenged by BR, and residents may not appreciate the educational benefits of BR in general.[11, 13] Although time constraints were cited as a significant barrier to BR, recent studies have suggested that BR do not necessarily take more time overall.[15] The notion that patient psychosocial complexities may limit BR has been reflected in the literature,[16, 17, 18, 19, 20] but these situations may also afford unique bedside teaching opportunities.[21] Finally, faculty, and in particular more junior attendings, may be uncomfortable teaching in the presence of the patient.[13] This barrier may be overcome through faculty development efforts.[15]
As internal medicine training transitions to the NAS and a milestone‐based assessment framework,[1] residency programs will need to consider how rounding can be structured to help trainees achieve the required milestones, and to help programs meaningfully assess trainee performance. Our survey indicates that HR may be effective across all of the competencies and the potential for this should be further explored. Yet, HR may allow for a limited ability to observe learners with patients, as there may only be cursory data gathering from the patient, a brief physical exam, and limited communication with patients and/or family members. Furthermore, the patient‐centeredness of HR may be called into question, given restricted emphasis on shared decision making. Finally, as efficiency remains crucial in the wake of duty‐hour reform, HR may also prove to be more time consuming than BR, given that it often requires information shared outside of the patient's room to be repeated in the patient's presence. Ultimately, there may not be a 1 size fits all solution, and institutions should ensure the organization and structure of their rounding models are optimally designed to enable the achievement and assessment of ACGME milestones.
Our study has several limitations. Due to our employment of snowball sampling, we could not calculate a response rate. We also recruited a self‐selected sample of internal medicine attending physicians, raising the possibility of selection bias. However, we captured a wide range of experience and opinion, and do not have reason to believe that any particular viewpoints are over‐ or under‐represented. Further, our study may have been influenced by sampling bias, reaching primarily attending physicians at university‐affiliated medical centers, calling into question the generalizability of our results and making any comparisons between academic and community health centers less meaningful. Nonetheless, we received responses from both large and small medical centers, as well as quaternary care and community‐based hospitals. The reported benefits and barriers were respondents' personal perceptions, rather than measured outcomes. Moreover, we focused primarily on the effectiveness of teaching of the ACGME competencies and did not explore other outcomes that could be impacted by rounding structure (eg, patient satisfaction, trainee satisfaction, length of stay, time of discharge). Our study also did not address the variety of complex factors that influence the location and methods of attending rounds. For example, the various institutions surveyed have a variety of team sizes and compositions, admitting schedules, geographic layouts, and time allotted for attending rounds, all of which can influence choices for rounding practices. Finally, we did not assess resident perceptions, an area of future study that would allow us to corroborate the findings of our survey.
In conclusion, in this cross‐national, multicenter survey study of the 3 most prevalent internal medicine rounding practices, respondents utilized HR most commonly and believed this model was effective in teaching across the 6 ACGME core competencies. Those surveyed identified the benefits and barriers to BR, and a substantial number continue to use CFR despite recognizing its educational limitations. Future studies should explore factors that promote various rounding models and assess the relationship between rounding structure and educational outcomes for trainees.
Disclosure: Nothing to report.
- , , , . The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366(11):1051–1056.
- , . Teaching the resident in internal medicine. Present practices and suggestions for the future. JAMA. 1986;256(6):725–729.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , , , . Relationships of the location and content of rounds to specialty, institution, patient‐census, and team size. PLoS One. 2010;5(6):e11246.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- . Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217–2225.
- , , , , . Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661–667.
- . Teaching approaches that reflect and promote professionalism. Acad Med. 2003;78(7):709–713.
- . Bedside rounds revisited. N Engl J Med. 1997;336(16):1174–1175.
- , . Sounding boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230–1233.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , . Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341–346.
- , , , , . An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646–648.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- , , . The attending physician as teacher. N Engl J Med. 1983;308(19):1129–1132.
- , , , , . Bedside case presentations: why patients like them but learners don't. J Gen Intern Med. 1989;4(4):284–287.
- . On bedside teaching. Ann Intern Med. 1997;126(3):217–220.
- , . Teaching at the bedside: a new model. Med Teach. 2003;25(2):127–130.
- . Twelve tips to improve bedside teaching. Med Teach. 2003;25(2):112–115.
- Wiese J, ed. Teaching in the Hospital. Philadelphia, PA: American College of Physicians; 2010.
- , , , . The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366(11):1051–1056.
- , . Teaching the resident in internal medicine. Present practices and suggestions for the future. JAMA. 1986;256(6):725–729.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , , , . Relationships of the location and content of rounds to specialty, institution, patient‐census, and team size. PLoS One. 2010;5(6):e11246.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- . Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217–2225.
- , , , , . Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661–667.
- . Teaching approaches that reflect and promote professionalism. Acad Med. 2003;78(7):709–713.
- . Bedside rounds revisited. N Engl J Med. 1997;336(16):1174–1175.
- , . Sounding boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230–1233.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , . Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341–346.
- , , , , . An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646–648.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- , , . The attending physician as teacher. N Engl J Med. 1983;308(19):1129–1132.
- , , , , . Bedside case presentations: why patients like them but learners don't. J Gen Intern Med. 1989;4(4):284–287.
- . On bedside teaching. Ann Intern Med. 1997;126(3):217–220.
- , . Teaching at the bedside: a new model. Med Teach. 2003;25(2):127–130.
- . Twelve tips to improve bedside teaching. Med Teach. 2003;25(2):112–115.
- Wiese J, ed. Teaching in the Hospital. Philadelphia, PA: American College of Physicians; 2010.
© 2014 Society of Hospital Medicine
Specialties Performing Paracentesis
Cirrhosis affects up to 3% of the population and is 1 of the 10 most common causes of death in the United States.[1, 2, 3, 4] Paracentesis procedures are frequently performed in patients with liver disease and ascites for diagnostic and/or therapeutic purposes. These procedures can be performed safely by trained clinicians at the bedside or referred to interventional radiology (IR).[2, 3, 4]
National practice patterns show that paracentesis procedures are increasingly referred to IR rather than performed at the bedside by internal medicine or gastroenterology clinicians.[5, 6, 7] In fact, a recent study of Medicare beneficiaries showed that inpatient and outpatient paracentesis procedures performed by radiologists increased by 964% from 1993 to 2008.[7] Reasons for the decline in bedside procedures include the increased availability of IR, lack of sufficient reimbursement, and the time required to perform paracentesis procedures.[5, 6, 7, 8] Surveys of internal medicine and family medicine residents and gastroenterology fellows show trainees often lack the confidence and experience needed to perform the procedure safely.[9, 10, 11] Additionally, many clinicians do not have expertise with ultrasound use and may not have access to necessary equipment.
Inconsistent certification requirements may also impact the competence and experience of physicians to perform paracentesis procedures. Internal medicine residents are no longer required by the American Board of Internal Medicine (ABIM) to demonstrate competency in procedures such as paracentesis for certification.[12] However, the Accreditation Council for Graduate Medical Education (ACGME) requirements state that internal medicine programs must offer residents the opportunity to demonstrate competence in the performance of procedures such as paracentesis, thoracentesis, and central venous catheter insertion.[13] The American Board of Family Medicine (ABFM) does not outline specific procedural competence for initial certification.[14] The ACGME states that family medicine residents must receive training to perform those clinical procedures required for their future practices but allows each program to determine which procedures to require.[15] Due to this uncertainty, practicing hospitalists are likely to have variable training and competence in bedside procedures such as paracentesis.
We previously showed that internal medicine residents rotating on the hepatology service of an academic medical center performed 59% of paracentesis procedures at the bedside.[16] These findings are in contrast to national data showing that 74% of paracentesis procedures performed on Medicare beneficiaries were performed by radiologists.[7] Practice patterns at university hospitals may not be reflected in this data because the study was limited to Medicare beneficiaries and included ambulatory patients.[7] In addition to uncertainty about who is performing this procedure in inpatient settings, little is known about the effect of specialty on postparacentesis clinical outcomes.[16, 17]
The current study had 3 aims: (1) evaluate which clinical specialties perform paracentesis procedures at university hospitals; (2) model patient characteristics associated with procedures performed at the bedside versus those referred to IR; and (3) among patients with a similar likelihood of IR referral, evaluate length of stay (LOS) and hospital costs of patients undergoing procedures performed by different specialties.
METHODS
We performed an observational administrative database review of patients who underwent paracentesis procedures in hospitals participating in the University HealthSystem Consortium (UHC) Clinical Database from January 2010 through December 2012. UHC is an alliance of 120 nonprofit academic medical centers and their 290 affiliated hospitals. UHC maintains databases containing clinical, operational, financial, and patient safety data from affiliated hospitals. Using the UHC database, we described the characteristics of all patients who underwent paracentesis procedures by clinical specialty performing the procedure. We then modeled the effects of patient characteristics on decision‐making about IR referral. Finally, among patients with a homogeneous predicted probability of IR referral, we compared LOS and direct costs by specialty performing the procedure. The Northwestern University institutional review board approved this study.
Procedure
We queried the UHC database for all patients over the age of 18 years who underwent paracentesis procedures (International Classification of Disease Revision 9 [ICD‐9] procedure code 54.91) and had at least 1 diagnosis code of liver disease (571.x). We excluded patients admitted to obstetrics. The query included patient and clinical characteristics such as admission, discharge, and procedure date; age, gender, procedure provider specialty, and intensive care unit (ICU) stay. We also obtained all ICD‐9 codes associated with the admission including obesity, severe liver disease, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before or during the admission, awaiting liver transplant, and complications of liver transplant. We used ICD‐9 codes to calculate patients' Charlson score[18, 19] to assess severity of illness on admission.
LOS and total direct hospital costs were compared among patients with a paracentesis performed by a single clinical group and among patients with a similar predicted probability of IR referral. UHC generates direct cost estimates by applying Medicare Cost Report ratios of cost to charges with the labor cost further adjusted by the respective area wage index. Hospital costs were not available from 8.3% of UHC hospitals. We therefore based cost estimates on nonmissing data.
Paracentesis provider specialties were divided into 6 general categories: (1) IR (interventional and diagnostic radiology); (2) medicine (family medicine, general medicine, and hospital medicine); (3) subspecialty medicine (infectious disease, cardiology, nephrology, hematology/oncology, endocrinology, pulmonary, and geriatrics); (4) gastroenterology/hepatology (gastroenterology, hepatology, and transplant medicine); (5) general surgery (general surgery and transplant surgery); and (6) all other (included unclassified specialties). We present patient characteristics categorized by these specialty groups and for admissions in which multiple specialties performed procedures.
Study Design
To analyze an individual patient's likelihood of IR referral, we needed to restrict our sample to discharges where only 1 clinical specialty performed a paracentesis. Therefore, we excluded hybrid discharges with procedures performed by more than 1 specialty in a single admission as well as discharges with procedures performed by all other specialties. To compare LOS and direct cost outcomes, and to minimize selection bias among exclusively IR‐treated patients, we excluded hospitals without procedures done by both IR and medicine.
We modeled referral to IR as a function of patients' demographic and clinical variables, which we believed would affect the probability of referral. We then examined the IR referral model predicted probabilities (propensity score).[20] Finally, we examined mean differences in LOS and direct costs among discharges with a single clinical specialty group, while using the predicted probability of referral as a filter to compare these outcomes by specialty. We further tested specialty differences in LOS and direct costs controlling for demographic and clinical variables.
Statistical Analysis
To test the significance of differences between demographic and clinical characteristics of patients across specialties, we used 2 tests for categorical variables and analysis of variance or the Kruskal‐Wallis rank test for continuous variables. Random effects logistic regression, which adjusts standard errors for clustering by hospital, was used to model the likelihood of referral to IR. Independent variables included patient age, gender, obesity, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before hospitalization, liver transplant during hospitalization, awaiting transplant, complications of liver transplant, ICU stay, Charlson score, and number of paracentesis procedures performed during the admission. Predicted probabilities derived from this IR referral model were used to investigate selection bias in our subsequent analyses of LOS and costs.[20]
We used random effects multiple linear regression to test the association of procedure specialty with hospital LOS and total direct costs, controlling for the same independent variables listed above. Analyses were conducted using both actual LOS in days and Medicare costs. We also performed a log transformation of LOS and costs to account for rightward skew. We only present actual LOS and cost results because results were virtually identical. We used SAS version 9 (SAS Institute Inc., Cary, NC) to extract data from the UHC Clinical Database. We performed all statistical analyses using Stata version 12 (StataCorp LP, College Station, TX).
RESULTS
Procedure and Discharge Level Results
There were 97,577 paracentesis procedures performed during 70,862 hospital admissions in 204 UHC hospitals during the study period. Table 1 shows specific specialty groups for each procedure. The all other category consisted of 17,558 subspecialty groups including 9,434 with specialty unknown. Twenty‐nine percent of procedures were performed in IR versus 27% by medicine, 11% by gastroenterology/hepatology, and 11% by subspecialty medicine.
| Specialty Group | No. | % |
|---|---|---|
| Interventional radiology | 28,414 | 29.1 |
| Medicine | 26,031 | 26.7 |
| Family medicine | 1,026 | 1.1 |
| General medicine | 21,787 | 22.3 |
| Hospitalist | 3,218 | 3.3 |
| Subspecialty medicine | 10,558 | 10.8 |
| Infectious disease | 848 | 0.9 |
| Nephrology | 615 | 0.6 |
| Cardiology | 991 | 1.0 |
| Hematology oncology | 795 | 0.8 |
| Endocrinology | 359 | 0.4 |
| Pulmonology | 6,605 | 6.8 |
| Geriatrics | 345 | 0.4 |
| Gastroenterology/hepatology | 11,143 | 11.4 |
| Transplant medicine | 99 | 0.1 |
| Hepatology | 874 | 0.9 |
| Gastroenterology | 10,170 | 10.4 |
| General surgery | 3,873 | 4.0 |
| Transplant surgery | 2,146 | 2.2 |
| General surgery | 1,727 | 1.8 |
| All other | 17,558 | 18.0 |
| Specialty unknown | 9,434 | 9.7 |
Table 2 presents patient characteristics for 70,862 hospital discharges with paracentesis procedures grouped by whether single or multiple specialties performed procedures. Patient characteristics were significantly different across specialty groups. Medicine, subspecialty medicine, and gastroenterology/hepatology patients were younger, more likely to be male, and more likely to have severe liver disease, coagulation disorders, hypotension, and hyponatremia than IR patients.
| All Discharges, N=70,862 | Interventional Radiology, n=9,348 | Medicine, n=13,789 | Subspecialty Medicine, n=5,085 | Gastroenterology/Hepatology, n=6,664 | General Surgery, n=1,891 | All Other, n=7,912 | Discharges With Multiple Specialties, n=26,173 | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age group, y (%) | ||||||||
| 1849 | 25.4 | 22.5 | 27.6 | 24.9 | 23.5 | 20.8 | 25.5 | 26.1 |
| 5059 | 39.8 | 39.8 | 40.9 | 39.4 | 41.5 | 40.3 | 40.0 | 38.7 |
| 6069 | 24.7 | 24.9 | 21.6 | 24.7 | 26.5 | 30.0 | 23.6 | 25.8 |
| 70+ | 10.1 | 12.9 | 9.9 | 11.1 | 8.4 | 8.9 | 11.0 | 9.4 |
| Male (%) | 65.5 | 64.2 | 67.6 | 67.5 | 65.7 | 66.6 | 65.7 | 64.2 |
| Severe liver disease (%)a | 73.7 | 65.3 | 67.8 | 71.0 | 75.3 | 66.6 | 67.6 | 82.1 |
| Obesity (BMI 40+) (%) | 6.3 | 6.1 | 5.3 | 5.7 | 5.1 | 5.8 | 5.2 | 7.6 |
| Any intensive care unit stay (%) | 31.0 | 10.9 | 16.8 | 50.5 | 16.9 | 36.7 | 22.3 | 47.8 |
| Coagulation disorders (%) | 24.3 | 14.8 | 20.2 | 29.9 | 16.1 | 19.0 | 17.8 | 33.1 |
| Blood loss anemia (%) | 3.4 | 1.3 | 2.8 | 2.7 | 2.7 | 1.9 | 2.1 | 5.2 |
| Hyponatremia (%) | 29.9 | 27.1 | 29.2 | 28.9 | 28.0 | 26.6 | 27.3 | 33.1 |
| Hypotension (%) | 9.8 | 7.0 | 8.0 | 11.0 | 7.7 | 10.5 | 8.1 | 12.4 |
| Thrombocytopenia (%) | 29.6 | 24.6 | 28.3 | 32.5 | 22.1 | 21.5 | 24.0 | 35.8 |
| Complication of transplant (%) | 3.3 | 2.1 | 1.1 | 2.4 | 4.0 | 10.3 | 2.7 | 4.7 |
| Awaiting liver transplant (%) | 7.6 | 6.4 | 4.0 | 5.4 | 12.8 | 16.0 | 7.8 | 8.2 |
| Prior liver transplant (%) | 0.5 | 0.8 | 0.3 | 0.3 | 0.7 | 0.7 | 0.4 | 0.6 |
| Liver transplant procedure (%) | 2.7 | 0.0 | 0.0 | 0.3 | 0.4 | 15.6 | 1.6 | 5.6 |
| Mean Charlson score (SD) | 4.51 (2.17) | 4.28 (2.26) | 4.16 (2.17) | 4.72 (2.30) | 4.30 (1.98) | 4.26 (2.22) | 4.36 (2.30) | 4.84 (2.07) |
| Mean paracentesis procedures per discharge (SD) | 1.38 (0.88) | 1.21 (0.56) | 1.26 (0.66) | 1.30 (0.76) | 1.31 (0.70) | 1.28 (0.78) | 1.22 (0.61) | 1.58 (1.13) |
IR Referral Model
We first excluded 6030/70,862 discharges (8.5%) from 59 hospitals without both IR and medicine procedures. We then further excluded 24,986/70,862 (35.3%) discharges with procedures performed by multiple specialties during the same admission. Finally, we excluded 5555/70,862 (7.8%) of discharges with procedure specialty coded as all other. Therefore, 34,291 (48.4%) discharges (43,337/97,577; 44.4% procedures) from 145 UHC hospitals with paracentesis procedures performed by a single clinical specialty group remained for the IR referral analysis sample. Among admissions with multiple specialty paracentesis performed within the same admission, 3128/26,606 admissions with any IR procedure (11.8%) had a different specialty ascribed to the first, second, or third paracentesis with a subsequent IR procedure.
Model results (Table 3) indicate that patients who were obese (odds ratio [OR]: 1.25; 95% confidence interval [CI]: 1.10‐1.43) or had a liver transplant on a prior admission (OR: 2.03; 95% CI: 1.40‐2.95) were more likely to be referred to IR. However, male patients (OR: 0.89; 95% CI: 0.83‐0.95), or patients who required an ICU stay (OR: 0.39; 95% CI: 0.36‐0.43) were less likely to have IR procedures. Other patient factors reducing the likelihood of IR referral included characteristics associated with higher severity of illness (coagulation disorders, hyponatremia, hypotension, and thrombocytopenia).
| Odds Ratio | 95% CI | ||
|---|---|---|---|
| Lower | Upper | ||
| |||
| Age group, y | |||
| 1849 | Reference | ||
| 5059 | 1.05 | 0.97 | 1.14 |
| 6069 | 1.12 | 1.02 | 1.22 |
| 70+ | 1.11 | 0.99 | 1.24 |
| Male | 0.89 | 0.83 | 0.95 |
| Obesity, BMI 40+ | 1.25 | 1.10 | 1.43 |
| ICU care | 0.39 | 0.36 | 0.43 |
| Coagulation disorders | 0.68 | 0.63 | 0.75 |
| Blood loss anemia | 0.52 | 0.41 | 0.66 |
| Hyponatremia | 0.85 | 0.80 | 0.92 |
| Hypotension | 0.83 | 0.74 | 0.93 |
| Thrombocytopenia | 0.94 | 0.87 | 1.01 |
| Prior liver transplant | 0.08 | 0.03 | 0.23 |
| Awaiting liver transplant | 0.86 | 0.76 | 0.98 |
| Complication of liver transplant | 1.07 | 0.88 | 1.31 |
| Liver transplant procedure | 2.03 | 1.40 | 2.95 |
| Charlson score | 1.00 | 0.99 | 1.01 |
| Number of paracentesis procedures | 0.90 | 0.85 | 0.95 |
Predicted Probabilities of IR Referral
Figure 1 presents the distribution of predicted probabilities for IR referral. Predicted probabilities were low overall, with very few patients having an equal chance of referralthe standard often used in comparative effectiveness analyses from observational data. Figure 1 indicates that IR referral probabilities were clustered in an unusual bimodal distribution. The cluster on the left, which centers around a 15% predicted probability of IR referral, consists of discharges with patient characteristics that were associated with a very low chance of an IR paracentesis. We therefore used this distribution to conduct comparative analyses of admission outcomes between clinical specialty groups, choosing to examine patients with a 20% or greater chance of IR referral.
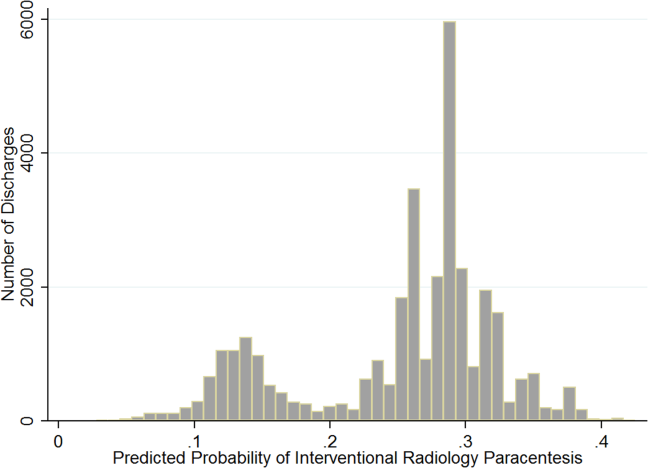
Post hoc analysis revealed that the biggest factor driving low predicted probability of IR referral was whether patients experienced an ICU stay at any time during hospitalization. Among the discharges with a predicted probability 0.2 (n=26,615 discharges), there were only 87 discharges with ICU stays (0.3%). For the discharges with predicted probability <0.2 (n=7676), 91.9% (n=7055) had an ICU admission. We therefore used a threshold of 0.2 or greater to present the most comparable LOS and direct cost differences.
LOS and Cost Comparisons by Specialty
Mean LOS and hospital direct costs by specialty for our final analysis sample can be found in Table 4; differences between specialties were significant (P<0.0001). Patients undergoing IR procedures had equivalent LOS and costs to medicine patients, but lower LOS and costs than other clinical specialty groups. Random effects linear regression showed that neither medicine nor gastroenterology/hepatology patients had significantly different LOS from IR patients, but subspecialty medicine was associated with 0.89 additional days and general surgery with 1.47 additional days (both P<0.0001; R2=0.10). In the direct cost regression model, medicine patients were associated with $1308 lower costs and gastroenterology/hepatology patients with $803 lower costs than IR patients (both P=0.0001), whereas subspecialty medicine and general surgery had higher direct costs per discharge of $1886 and $3039, respectively (both P<0.0001, R2=0.19). Older age, obesity, coagulopathy, hyponatremia, hypotension, thrombocytopenia, liver transplant status, ICU care, higher Charlson score, and higher number of paracentesis procedures performed were all significantly associated with higher LOS and hospital costs in these linear models.
| All Admissions n=26,615 | Interventional Radiology n=7,677 | Medicine n=10,413 | Medicine Subspecialties n=2,210 | Gastroenterology/ Hepatology n=5,182 | General Surgery n=1,133 | |
|---|---|---|---|---|---|---|
| All Admissions n=24,408 | Interventional Radiology n =7,265 | Medicine n=8,965, | Medicine Subspecialties n=2,064 | Gastroenterology/Hepatology n=5,031 | General Surgery n=1,083 | |
| ||||||
| Mean length of stay, d (SD) | 5.57 (5.63) | 5.20 (4.72) | 5.59 (5.85) | 6.28 (6.47) | 5.54 (5.31) | 6.67 (8.16) |
| Mean total direct cost, $ (SD)a | 11,447 (12,247) | 10,975 (9,723) | 10,517 (10,895) | 13,705 (16,591) | 12,000 (11,712) | 15,448 (23,807) |
DISCUSSION
This study showed that internal medicine‐ and family medicine‐trained clinicians perform approximately half of the inpatient paracentesis procedures at university hospitals and their affiliates. This confirms findings from our earlier single‐institution study[16] but contrasts with previously published reports involving Medicare data. The earlier report, using Medicare claims and including ambulatory procedures, revealed that primary care physicians and gastroenterologists only performed approximately 10% of US paracentesis procedures in 2008.[7] Our findings suggest that practices are different at university hospitals, where patients with severe liver disease often seek care. Because we used the UHC database, it was not possible to determine if the clinicians who performed paracentesis procedures in this study were internal medicine or family medicine residents, fellows, or attending physicians. However, findings from our own institution show that the vast majority of bedside paracentesis procedures are performed by internal medicine residents.[16]
Our findings have implications for certification of internal medicine and family medicine trainees. In 2008, the ABIM removed the requirement that internal medicine residents demonstrate competency in paracentesis.[12] This decision was informed by a lack of standardized methods to determine procedural competency and published surveys showing that internal medicine and hospitalist physicians rarely performed bedside procedures.[5, 6] Despite this policy change, our findings show that current clinical practice at university hospitals does not reflect national practice patterns or certification requirements, because many internal medicine‐ and family medicine‐trained clinicians still perform paracentesis procedures. This is concerning because internal medicine and family medicine trainees report variable confidence, experience, expertise, and supervision regarding performance of invasive procedures.[9, 10, 21, 22, 23, 24] Furthermore, earlier research also demonstrates that graduating residents and fellows are not able to competently perform common bedside procedures such as thoracentesis, temporary hemodialysis catheter insertion, and lumbar puncture.[25, 26, 27]
The American Association for the Study of Liver Diseases (AASLD) recommends that trained clinicians perform paracentesis procedures.[3, 4] However, the AASLD provides no definition for how training should occur. Because competency in this procedure is not specifically required by the ABIM, ABFM, or ACGME, a paradoxical situation occurs in which internal medicine and family medicine residents, and internal medicine‐trained fellows and faculty continue to perform paracentesis procedures on highly complex patients, but are no longer required to be competent to do so.
In earlier research we showed that simulation‐based mastery learning (SBML) was an effective method to boost internal medicine residents' paracentesis skills.[28] In SBML, all trainees must meet or exceed a minimum passing score on a simulated procedure before performing one on an actual patient.[29] This approach improves clinical care and outcomes in procedures such as central venous catheter insertion[30, 31] and advanced cardiac life support.[32] SBML‐trained residents also performed safe paracentesis procedures with shorter hospital LOS, fewer ICU transfers, and fewer blood product transfusions than IR procedures.[16] Based on the results of this study, AASLD guidelines regarding training, and our experience with SBML, we recommend that all clinicians complete paracentesis SBML training before performing procedures on patients.
Using our propensity model we identified patient characteristics that were associated with IR referral. Patients with a liver transplant were more likely to be cared for in IR. This may be due to a belief that postoperative procedures are anatomically more complex or because surgical trainees do not commonly perform this procedure. The current study confirms findings from earlier work that obese and female patients are more likely to be referred to IR.[16] IR referral of obese patients is likely to occur because paracentesis procedures are technically more difficult. We have no explanation why female patients were more likely to be referred to IR, because most decisions appear to be discretionary. Prospective studies are needed to determine evidence‐based recommendations regarding paracentesis procedure location. Patients with more comorbidities (eg, ICU stay, awaiting liver transplant, coagulation disorders) were more likely to undergo bedside procedures. The complexity of patients undergoing bedside paracentesis procedures reinforces the need for rigorous skill assessment for clinicians who perform them because complications such as intraperitoneal bleeding can be fatal.
Finally, we showed that LOS was similar but hospital direct costs were $800 to $1300 lower for patients whose paracentesis procedure was performed by medicine or gastroenterology/hepatology compared to IR. Medical subspecialties and surgery procedures were more expensive than IR, consistent with the higher LOS seen in these groups. IR procedures add costs due to facility charges for space, personnel, and equipment.[33] At our institution, the hospital cost of an IR paracentesis in 2012 was $361. If we use this figure, and assume costs are similar across university hospitals, the resultant cost savings would be $10,257,454 (for the procedure alone) if all procedures referred to IR in this 2‐year study were instead performed at the bedside. This estimate is approximate because it does not consider factors such as cost of clinician staffing models, which may differ across UHC hospitals. As hospitals look to reduce costs, potential savings due to appropriate use of bedside and IR procedures should be considered. This is especially important because there is no evidence that the extra expense of IR procedures is justified due to improved patient outcomes.
This study has several limitations. First, this was an observational study. Although the database was large, we were limited by coding accuracy and could not control for all potential confounding factors such as Model for End‐Stage Liver Disease score,[34, 35] other specific laboratory values, amount of ascites fluid removed, or bedside procedure failures later referred to IR. However, we do know that only a small number of second, third, or fourth procedures were subsequently referred to IR after earlier ones were performed at the bedside. Additionally the UHC database does not include patient‐specific data, and therefore we could not adjust for multiple visits or procedures by the same patient. Second, we were unable to determine the level of teaching involvement at each UHC affiliated hospital. Community hospitals where attendings managed most of the patients without trainees could not be differentiated from university hospitals where trainees were involved in most patients' care. Third, we did not have specialty information for 9434 (9.7%) procedures and had to exclude these cases. We also excluded a large number of paracentesis procedures in our final outcomes analysis. However, this was necessary because we needed to perform a patient‐level analysis to ensure the propensity and outcomes models were accurate. Finally, we did not evaluate inpatient mortality or 30‐day hospital readmission rates. Mortality and readmission from complications of a paracentesis procedure are rare events.[3, 4, 36] However, mortality and hospital readmission among patients with liver disease are relatively common.[37, 38] It was impossible to link these outcomes to a paracentesis procedure without the ability to perform medical records review.
In conclusion, paracentesis procedures are performed frequently by internal medicine‐ and family medicine‐trained clinicians in university hospitals. Because of these findings regarding current practice patterns, we believe the ACGME, ABIM, and ABFM should clarify their policies to require that residents are competent to perform paracentesis procedures before performing them on patients. This may improve supervision and training for paracentesis procedures that are already occurring and possibly encourage performance of additional, less costly bedside procedures.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Mark Williams for their support and encouragement of this work.
Disclosure: Nothing to report.
- . A primer on detecting cirrhosis and caring for these patients without causing harm. Int J Hepatol. 2011:801983.
- , , . Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787–799.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–2107.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology. 2009;49:2087–2107. Available at: http://www.aasld.org/practiceguidelines/Documents/ascitesupdate2013.pdf. Accessed October 21, 2013.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , . What procedures should internists do? Ann Intern Med. 2007;146(5):392–393.
- , , , , , . Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures. Am J Med. 2006;119(1)71.e17–e24.
- , , . Perception of competency to perform procedures and future practice intent: a national survey of family practice residents. Acad Med. 2003;78(9):926–932.
- , , , . Utilization of and adherence to the gastroenterology core curriculum on hepatology training during a gastrointestinal fellowship. Clin Gastroenterol Hepatol. 2008;6(6):682–688.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed December 21, 2013.
- A CGME program requirements for graduate medical education in internal medicine. Available at: http://acgme.org/acgmeweb/portals/0/PFassets/2013‐PR‐FAQ‐PIF/140_internal_medicine_07012013.pdf. Accessed December 17, 2013.
- American Board of Family Medicine residency requirements. Available at: https://www.theabfm.org/cert/guidelines.aspx. Accessed December 17, 2013.
- ACGME program requirements for graduate medical education in family medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/120pr07012007.pdf. Accessed December 17, 2013.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484–488.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- . The role of known effects in observational studies. Biometrics. 1998;45(2):557–569.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , , , et al. Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial. J Hosp Med. 2007;2(3):143–149.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , . Performance of procedures by nephrologists and nephrology fellows at U.S. nephrology training programs. Clin J Am Soc Nephrol. 2008;3(4):941–947.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3:49–54.
- , , , , . Mastery learning of temporary dialysis catheter insertion skills by nephrology fellows using simulation technology and deliberate practice. Am J Kidney Dis. 2009;54:70–76.
- , , , , , . Simulation‐based education with mastery learning improves residents' lumbar puncture skills. Neurology. 2012;79:132–137.
- , , , , , . Simulation‐based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23–27.
- , , , , . Medical education featuring mastery learning with deliberate practice can lead to better health for individuals and populations. Acad Med. 2011; 86(11):e8–e9.
- , , , , . Simulation‐based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697–2701.
- , , , , . Use of simulation‐based education to reduce catheter‐related bloodstream infections. Arch Intern Med. 2009;169(15):1420–1423.
- , , , et al. Progress toward improving the quality of cardiac arrest medical team responses at an academic teaching hospital. J Grad Med Educ. 2011;3(2):211–216.
- , , , et al. Technical cost of radiologic examinations: analysis across imaging modalities. Radiology. 2000;216(1):269–272.
- , , , et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33(2):464–470.
- , , , et al. Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- , , , . Videos in clinical medicine. Paracentesis. N Engl J Med. 2006;355(19):e21.
- Center for Disease Control and Prevention. Chronic liver disease or cirrhosis. National Hospital Discharge Survey: 2010 detailed diagnosis and procedure tables, number of first‐listed diagnoses (see ICD9‐CM code 571). Available at: http://www.cdc.gov/nchs/fastats/liverdis.htm. Accessed October 19, 2013.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254–259.
Cirrhosis affects up to 3% of the population and is 1 of the 10 most common causes of death in the United States.[1, 2, 3, 4] Paracentesis procedures are frequently performed in patients with liver disease and ascites for diagnostic and/or therapeutic purposes. These procedures can be performed safely by trained clinicians at the bedside or referred to interventional radiology (IR).[2, 3, 4]
National practice patterns show that paracentesis procedures are increasingly referred to IR rather than performed at the bedside by internal medicine or gastroenterology clinicians.[5, 6, 7] In fact, a recent study of Medicare beneficiaries showed that inpatient and outpatient paracentesis procedures performed by radiologists increased by 964% from 1993 to 2008.[7] Reasons for the decline in bedside procedures include the increased availability of IR, lack of sufficient reimbursement, and the time required to perform paracentesis procedures.[5, 6, 7, 8] Surveys of internal medicine and family medicine residents and gastroenterology fellows show trainees often lack the confidence and experience needed to perform the procedure safely.[9, 10, 11] Additionally, many clinicians do not have expertise with ultrasound use and may not have access to necessary equipment.
Inconsistent certification requirements may also impact the competence and experience of physicians to perform paracentesis procedures. Internal medicine residents are no longer required by the American Board of Internal Medicine (ABIM) to demonstrate competency in procedures such as paracentesis for certification.[12] However, the Accreditation Council for Graduate Medical Education (ACGME) requirements state that internal medicine programs must offer residents the opportunity to demonstrate competence in the performance of procedures such as paracentesis, thoracentesis, and central venous catheter insertion.[13] The American Board of Family Medicine (ABFM) does not outline specific procedural competence for initial certification.[14] The ACGME states that family medicine residents must receive training to perform those clinical procedures required for their future practices but allows each program to determine which procedures to require.[15] Due to this uncertainty, practicing hospitalists are likely to have variable training and competence in bedside procedures such as paracentesis.
We previously showed that internal medicine residents rotating on the hepatology service of an academic medical center performed 59% of paracentesis procedures at the bedside.[16] These findings are in contrast to national data showing that 74% of paracentesis procedures performed on Medicare beneficiaries were performed by radiologists.[7] Practice patterns at university hospitals may not be reflected in this data because the study was limited to Medicare beneficiaries and included ambulatory patients.[7] In addition to uncertainty about who is performing this procedure in inpatient settings, little is known about the effect of specialty on postparacentesis clinical outcomes.[16, 17]
The current study had 3 aims: (1) evaluate which clinical specialties perform paracentesis procedures at university hospitals; (2) model patient characteristics associated with procedures performed at the bedside versus those referred to IR; and (3) among patients with a similar likelihood of IR referral, evaluate length of stay (LOS) and hospital costs of patients undergoing procedures performed by different specialties.
METHODS
We performed an observational administrative database review of patients who underwent paracentesis procedures in hospitals participating in the University HealthSystem Consortium (UHC) Clinical Database from January 2010 through December 2012. UHC is an alliance of 120 nonprofit academic medical centers and their 290 affiliated hospitals. UHC maintains databases containing clinical, operational, financial, and patient safety data from affiliated hospitals. Using the UHC database, we described the characteristics of all patients who underwent paracentesis procedures by clinical specialty performing the procedure. We then modeled the effects of patient characteristics on decision‐making about IR referral. Finally, among patients with a homogeneous predicted probability of IR referral, we compared LOS and direct costs by specialty performing the procedure. The Northwestern University institutional review board approved this study.
Procedure
We queried the UHC database for all patients over the age of 18 years who underwent paracentesis procedures (International Classification of Disease Revision 9 [ICD‐9] procedure code 54.91) and had at least 1 diagnosis code of liver disease (571.x). We excluded patients admitted to obstetrics. The query included patient and clinical characteristics such as admission, discharge, and procedure date; age, gender, procedure provider specialty, and intensive care unit (ICU) stay. We also obtained all ICD‐9 codes associated with the admission including obesity, severe liver disease, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before or during the admission, awaiting liver transplant, and complications of liver transplant. We used ICD‐9 codes to calculate patients' Charlson score[18, 19] to assess severity of illness on admission.
LOS and total direct hospital costs were compared among patients with a paracentesis performed by a single clinical group and among patients with a similar predicted probability of IR referral. UHC generates direct cost estimates by applying Medicare Cost Report ratios of cost to charges with the labor cost further adjusted by the respective area wage index. Hospital costs were not available from 8.3% of UHC hospitals. We therefore based cost estimates on nonmissing data.
Paracentesis provider specialties were divided into 6 general categories: (1) IR (interventional and diagnostic radiology); (2) medicine (family medicine, general medicine, and hospital medicine); (3) subspecialty medicine (infectious disease, cardiology, nephrology, hematology/oncology, endocrinology, pulmonary, and geriatrics); (4) gastroenterology/hepatology (gastroenterology, hepatology, and transplant medicine); (5) general surgery (general surgery and transplant surgery); and (6) all other (included unclassified specialties). We present patient characteristics categorized by these specialty groups and for admissions in which multiple specialties performed procedures.
Study Design
To analyze an individual patient's likelihood of IR referral, we needed to restrict our sample to discharges where only 1 clinical specialty performed a paracentesis. Therefore, we excluded hybrid discharges with procedures performed by more than 1 specialty in a single admission as well as discharges with procedures performed by all other specialties. To compare LOS and direct cost outcomes, and to minimize selection bias among exclusively IR‐treated patients, we excluded hospitals without procedures done by both IR and medicine.
We modeled referral to IR as a function of patients' demographic and clinical variables, which we believed would affect the probability of referral. We then examined the IR referral model predicted probabilities (propensity score).[20] Finally, we examined mean differences in LOS and direct costs among discharges with a single clinical specialty group, while using the predicted probability of referral as a filter to compare these outcomes by specialty. We further tested specialty differences in LOS and direct costs controlling for demographic and clinical variables.
Statistical Analysis
To test the significance of differences between demographic and clinical characteristics of patients across specialties, we used 2 tests for categorical variables and analysis of variance or the Kruskal‐Wallis rank test for continuous variables. Random effects logistic regression, which adjusts standard errors for clustering by hospital, was used to model the likelihood of referral to IR. Independent variables included patient age, gender, obesity, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before hospitalization, liver transplant during hospitalization, awaiting transplant, complications of liver transplant, ICU stay, Charlson score, and number of paracentesis procedures performed during the admission. Predicted probabilities derived from this IR referral model were used to investigate selection bias in our subsequent analyses of LOS and costs.[20]
We used random effects multiple linear regression to test the association of procedure specialty with hospital LOS and total direct costs, controlling for the same independent variables listed above. Analyses were conducted using both actual LOS in days and Medicare costs. We also performed a log transformation of LOS and costs to account for rightward skew. We only present actual LOS and cost results because results were virtually identical. We used SAS version 9 (SAS Institute Inc., Cary, NC) to extract data from the UHC Clinical Database. We performed all statistical analyses using Stata version 12 (StataCorp LP, College Station, TX).
RESULTS
Procedure and Discharge Level Results
There were 97,577 paracentesis procedures performed during 70,862 hospital admissions in 204 UHC hospitals during the study period. Table 1 shows specific specialty groups for each procedure. The all other category consisted of 17,558 subspecialty groups including 9,434 with specialty unknown. Twenty‐nine percent of procedures were performed in IR versus 27% by medicine, 11% by gastroenterology/hepatology, and 11% by subspecialty medicine.
| Specialty Group | No. | % |
|---|---|---|
| Interventional radiology | 28,414 | 29.1 |
| Medicine | 26,031 | 26.7 |
| Family medicine | 1,026 | 1.1 |
| General medicine | 21,787 | 22.3 |
| Hospitalist | 3,218 | 3.3 |
| Subspecialty medicine | 10,558 | 10.8 |
| Infectious disease | 848 | 0.9 |
| Nephrology | 615 | 0.6 |
| Cardiology | 991 | 1.0 |
| Hematology oncology | 795 | 0.8 |
| Endocrinology | 359 | 0.4 |
| Pulmonology | 6,605 | 6.8 |
| Geriatrics | 345 | 0.4 |
| Gastroenterology/hepatology | 11,143 | 11.4 |
| Transplant medicine | 99 | 0.1 |
| Hepatology | 874 | 0.9 |
| Gastroenterology | 10,170 | 10.4 |
| General surgery | 3,873 | 4.0 |
| Transplant surgery | 2,146 | 2.2 |
| General surgery | 1,727 | 1.8 |
| All other | 17,558 | 18.0 |
| Specialty unknown | 9,434 | 9.7 |
Table 2 presents patient characteristics for 70,862 hospital discharges with paracentesis procedures grouped by whether single or multiple specialties performed procedures. Patient characteristics were significantly different across specialty groups. Medicine, subspecialty medicine, and gastroenterology/hepatology patients were younger, more likely to be male, and more likely to have severe liver disease, coagulation disorders, hypotension, and hyponatremia than IR patients.
| All Discharges, N=70,862 | Interventional Radiology, n=9,348 | Medicine, n=13,789 | Subspecialty Medicine, n=5,085 | Gastroenterology/Hepatology, n=6,664 | General Surgery, n=1,891 | All Other, n=7,912 | Discharges With Multiple Specialties, n=26,173 | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age group, y (%) | ||||||||
| 1849 | 25.4 | 22.5 | 27.6 | 24.9 | 23.5 | 20.8 | 25.5 | 26.1 |
| 5059 | 39.8 | 39.8 | 40.9 | 39.4 | 41.5 | 40.3 | 40.0 | 38.7 |
| 6069 | 24.7 | 24.9 | 21.6 | 24.7 | 26.5 | 30.0 | 23.6 | 25.8 |
| 70+ | 10.1 | 12.9 | 9.9 | 11.1 | 8.4 | 8.9 | 11.0 | 9.4 |
| Male (%) | 65.5 | 64.2 | 67.6 | 67.5 | 65.7 | 66.6 | 65.7 | 64.2 |
| Severe liver disease (%)a | 73.7 | 65.3 | 67.8 | 71.0 | 75.3 | 66.6 | 67.6 | 82.1 |
| Obesity (BMI 40+) (%) | 6.3 | 6.1 | 5.3 | 5.7 | 5.1 | 5.8 | 5.2 | 7.6 |
| Any intensive care unit stay (%) | 31.0 | 10.9 | 16.8 | 50.5 | 16.9 | 36.7 | 22.3 | 47.8 |
| Coagulation disorders (%) | 24.3 | 14.8 | 20.2 | 29.9 | 16.1 | 19.0 | 17.8 | 33.1 |
| Blood loss anemia (%) | 3.4 | 1.3 | 2.8 | 2.7 | 2.7 | 1.9 | 2.1 | 5.2 |
| Hyponatremia (%) | 29.9 | 27.1 | 29.2 | 28.9 | 28.0 | 26.6 | 27.3 | 33.1 |
| Hypotension (%) | 9.8 | 7.0 | 8.0 | 11.0 | 7.7 | 10.5 | 8.1 | 12.4 |
| Thrombocytopenia (%) | 29.6 | 24.6 | 28.3 | 32.5 | 22.1 | 21.5 | 24.0 | 35.8 |
| Complication of transplant (%) | 3.3 | 2.1 | 1.1 | 2.4 | 4.0 | 10.3 | 2.7 | 4.7 |
| Awaiting liver transplant (%) | 7.6 | 6.4 | 4.0 | 5.4 | 12.8 | 16.0 | 7.8 | 8.2 |
| Prior liver transplant (%) | 0.5 | 0.8 | 0.3 | 0.3 | 0.7 | 0.7 | 0.4 | 0.6 |
| Liver transplant procedure (%) | 2.7 | 0.0 | 0.0 | 0.3 | 0.4 | 15.6 | 1.6 | 5.6 |
| Mean Charlson score (SD) | 4.51 (2.17) | 4.28 (2.26) | 4.16 (2.17) | 4.72 (2.30) | 4.30 (1.98) | 4.26 (2.22) | 4.36 (2.30) | 4.84 (2.07) |
| Mean paracentesis procedures per discharge (SD) | 1.38 (0.88) | 1.21 (0.56) | 1.26 (0.66) | 1.30 (0.76) | 1.31 (0.70) | 1.28 (0.78) | 1.22 (0.61) | 1.58 (1.13) |
IR Referral Model
We first excluded 6030/70,862 discharges (8.5%) from 59 hospitals without both IR and medicine procedures. We then further excluded 24,986/70,862 (35.3%) discharges with procedures performed by multiple specialties during the same admission. Finally, we excluded 5555/70,862 (7.8%) of discharges with procedure specialty coded as all other. Therefore, 34,291 (48.4%) discharges (43,337/97,577; 44.4% procedures) from 145 UHC hospitals with paracentesis procedures performed by a single clinical specialty group remained for the IR referral analysis sample. Among admissions with multiple specialty paracentesis performed within the same admission, 3128/26,606 admissions with any IR procedure (11.8%) had a different specialty ascribed to the first, second, or third paracentesis with a subsequent IR procedure.
Model results (Table 3) indicate that patients who were obese (odds ratio [OR]: 1.25; 95% confidence interval [CI]: 1.10‐1.43) or had a liver transplant on a prior admission (OR: 2.03; 95% CI: 1.40‐2.95) were more likely to be referred to IR. However, male patients (OR: 0.89; 95% CI: 0.83‐0.95), or patients who required an ICU stay (OR: 0.39; 95% CI: 0.36‐0.43) were less likely to have IR procedures. Other patient factors reducing the likelihood of IR referral included characteristics associated with higher severity of illness (coagulation disorders, hyponatremia, hypotension, and thrombocytopenia).
| Odds Ratio | 95% CI | ||
|---|---|---|---|
| Lower | Upper | ||
| |||
| Age group, y | |||
| 1849 | Reference | ||
| 5059 | 1.05 | 0.97 | 1.14 |
| 6069 | 1.12 | 1.02 | 1.22 |
| 70+ | 1.11 | 0.99 | 1.24 |
| Male | 0.89 | 0.83 | 0.95 |
| Obesity, BMI 40+ | 1.25 | 1.10 | 1.43 |
| ICU care | 0.39 | 0.36 | 0.43 |
| Coagulation disorders | 0.68 | 0.63 | 0.75 |
| Blood loss anemia | 0.52 | 0.41 | 0.66 |
| Hyponatremia | 0.85 | 0.80 | 0.92 |
| Hypotension | 0.83 | 0.74 | 0.93 |
| Thrombocytopenia | 0.94 | 0.87 | 1.01 |
| Prior liver transplant | 0.08 | 0.03 | 0.23 |
| Awaiting liver transplant | 0.86 | 0.76 | 0.98 |
| Complication of liver transplant | 1.07 | 0.88 | 1.31 |
| Liver transplant procedure | 2.03 | 1.40 | 2.95 |
| Charlson score | 1.00 | 0.99 | 1.01 |
| Number of paracentesis procedures | 0.90 | 0.85 | 0.95 |
Predicted Probabilities of IR Referral
Figure 1 presents the distribution of predicted probabilities for IR referral. Predicted probabilities were low overall, with very few patients having an equal chance of referralthe standard often used in comparative effectiveness analyses from observational data. Figure 1 indicates that IR referral probabilities were clustered in an unusual bimodal distribution. The cluster on the left, which centers around a 15% predicted probability of IR referral, consists of discharges with patient characteristics that were associated with a very low chance of an IR paracentesis. We therefore used this distribution to conduct comparative analyses of admission outcomes between clinical specialty groups, choosing to examine patients with a 20% or greater chance of IR referral.

Post hoc analysis revealed that the biggest factor driving low predicted probability of IR referral was whether patients experienced an ICU stay at any time during hospitalization. Among the discharges with a predicted probability 0.2 (n=26,615 discharges), there were only 87 discharges with ICU stays (0.3%). For the discharges with predicted probability <0.2 (n=7676), 91.9% (n=7055) had an ICU admission. We therefore used a threshold of 0.2 or greater to present the most comparable LOS and direct cost differences.
LOS and Cost Comparisons by Specialty
Mean LOS and hospital direct costs by specialty for our final analysis sample can be found in Table 4; differences between specialties were significant (P<0.0001). Patients undergoing IR procedures had equivalent LOS and costs to medicine patients, but lower LOS and costs than other clinical specialty groups. Random effects linear regression showed that neither medicine nor gastroenterology/hepatology patients had significantly different LOS from IR patients, but subspecialty medicine was associated with 0.89 additional days and general surgery with 1.47 additional days (both P<0.0001; R2=0.10). In the direct cost regression model, medicine patients were associated with $1308 lower costs and gastroenterology/hepatology patients with $803 lower costs than IR patients (both P=0.0001), whereas subspecialty medicine and general surgery had higher direct costs per discharge of $1886 and $3039, respectively (both P<0.0001, R2=0.19). Older age, obesity, coagulopathy, hyponatremia, hypotension, thrombocytopenia, liver transplant status, ICU care, higher Charlson score, and higher number of paracentesis procedures performed were all significantly associated with higher LOS and hospital costs in these linear models.
| All Admissions n=26,615 | Interventional Radiology n=7,677 | Medicine n=10,413 | Medicine Subspecialties n=2,210 | Gastroenterology/ Hepatology n=5,182 | General Surgery n=1,133 | |
|---|---|---|---|---|---|---|
| All Admissions n=24,408 | Interventional Radiology n =7,265 | Medicine n=8,965, | Medicine Subspecialties n=2,064 | Gastroenterology/Hepatology n=5,031 | General Surgery n=1,083 | |
| ||||||
| Mean length of stay, d (SD) | 5.57 (5.63) | 5.20 (4.72) | 5.59 (5.85) | 6.28 (6.47) | 5.54 (5.31) | 6.67 (8.16) |
| Mean total direct cost, $ (SD)a | 11,447 (12,247) | 10,975 (9,723) | 10,517 (10,895) | 13,705 (16,591) | 12,000 (11,712) | 15,448 (23,807) |
DISCUSSION
This study showed that internal medicine‐ and family medicine‐trained clinicians perform approximately half of the inpatient paracentesis procedures at university hospitals and their affiliates. This confirms findings from our earlier single‐institution study[16] but contrasts with previously published reports involving Medicare data. The earlier report, using Medicare claims and including ambulatory procedures, revealed that primary care physicians and gastroenterologists only performed approximately 10% of US paracentesis procedures in 2008.[7] Our findings suggest that practices are different at university hospitals, where patients with severe liver disease often seek care. Because we used the UHC database, it was not possible to determine if the clinicians who performed paracentesis procedures in this study were internal medicine or family medicine residents, fellows, or attending physicians. However, findings from our own institution show that the vast majority of bedside paracentesis procedures are performed by internal medicine residents.[16]
Our findings have implications for certification of internal medicine and family medicine trainees. In 2008, the ABIM removed the requirement that internal medicine residents demonstrate competency in paracentesis.[12] This decision was informed by a lack of standardized methods to determine procedural competency and published surveys showing that internal medicine and hospitalist physicians rarely performed bedside procedures.[5, 6] Despite this policy change, our findings show that current clinical practice at university hospitals does not reflect national practice patterns or certification requirements, because many internal medicine‐ and family medicine‐trained clinicians still perform paracentesis procedures. This is concerning because internal medicine and family medicine trainees report variable confidence, experience, expertise, and supervision regarding performance of invasive procedures.[9, 10, 21, 22, 23, 24] Furthermore, earlier research also demonstrates that graduating residents and fellows are not able to competently perform common bedside procedures such as thoracentesis, temporary hemodialysis catheter insertion, and lumbar puncture.[25, 26, 27]
The American Association for the Study of Liver Diseases (AASLD) recommends that trained clinicians perform paracentesis procedures.[3, 4] However, the AASLD provides no definition for how training should occur. Because competency in this procedure is not specifically required by the ABIM, ABFM, or ACGME, a paradoxical situation occurs in which internal medicine and family medicine residents, and internal medicine‐trained fellows and faculty continue to perform paracentesis procedures on highly complex patients, but are no longer required to be competent to do so.
In earlier research we showed that simulation‐based mastery learning (SBML) was an effective method to boost internal medicine residents' paracentesis skills.[28] In SBML, all trainees must meet or exceed a minimum passing score on a simulated procedure before performing one on an actual patient.[29] This approach improves clinical care and outcomes in procedures such as central venous catheter insertion[30, 31] and advanced cardiac life support.[32] SBML‐trained residents also performed safe paracentesis procedures with shorter hospital LOS, fewer ICU transfers, and fewer blood product transfusions than IR procedures.[16] Based on the results of this study, AASLD guidelines regarding training, and our experience with SBML, we recommend that all clinicians complete paracentesis SBML training before performing procedures on patients.
Using our propensity model we identified patient characteristics that were associated with IR referral. Patients with a liver transplant were more likely to be cared for in IR. This may be due to a belief that postoperative procedures are anatomically more complex or because surgical trainees do not commonly perform this procedure. The current study confirms findings from earlier work that obese and female patients are more likely to be referred to IR.[16] IR referral of obese patients is likely to occur because paracentesis procedures are technically more difficult. We have no explanation why female patients were more likely to be referred to IR, because most decisions appear to be discretionary. Prospective studies are needed to determine evidence‐based recommendations regarding paracentesis procedure location. Patients with more comorbidities (eg, ICU stay, awaiting liver transplant, coagulation disorders) were more likely to undergo bedside procedures. The complexity of patients undergoing bedside paracentesis procedures reinforces the need for rigorous skill assessment for clinicians who perform them because complications such as intraperitoneal bleeding can be fatal.
Finally, we showed that LOS was similar but hospital direct costs were $800 to $1300 lower for patients whose paracentesis procedure was performed by medicine or gastroenterology/hepatology compared to IR. Medical subspecialties and surgery procedures were more expensive than IR, consistent with the higher LOS seen in these groups. IR procedures add costs due to facility charges for space, personnel, and equipment.[33] At our institution, the hospital cost of an IR paracentesis in 2012 was $361. If we use this figure, and assume costs are similar across university hospitals, the resultant cost savings would be $10,257,454 (for the procedure alone) if all procedures referred to IR in this 2‐year study were instead performed at the bedside. This estimate is approximate because it does not consider factors such as cost of clinician staffing models, which may differ across UHC hospitals. As hospitals look to reduce costs, potential savings due to appropriate use of bedside and IR procedures should be considered. This is especially important because there is no evidence that the extra expense of IR procedures is justified due to improved patient outcomes.
This study has several limitations. First, this was an observational study. Although the database was large, we were limited by coding accuracy and could not control for all potential confounding factors such as Model for End‐Stage Liver Disease score,[34, 35] other specific laboratory values, amount of ascites fluid removed, or bedside procedure failures later referred to IR. However, we do know that only a small number of second, third, or fourth procedures were subsequently referred to IR after earlier ones were performed at the bedside. Additionally the UHC database does not include patient‐specific data, and therefore we could not adjust for multiple visits or procedures by the same patient. Second, we were unable to determine the level of teaching involvement at each UHC affiliated hospital. Community hospitals where attendings managed most of the patients without trainees could not be differentiated from university hospitals where trainees were involved in most patients' care. Third, we did not have specialty information for 9434 (9.7%) procedures and had to exclude these cases. We also excluded a large number of paracentesis procedures in our final outcomes analysis. However, this was necessary because we needed to perform a patient‐level analysis to ensure the propensity and outcomes models were accurate. Finally, we did not evaluate inpatient mortality or 30‐day hospital readmission rates. Mortality and readmission from complications of a paracentesis procedure are rare events.[3, 4, 36] However, mortality and hospital readmission among patients with liver disease are relatively common.[37, 38] It was impossible to link these outcomes to a paracentesis procedure without the ability to perform medical records review.
In conclusion, paracentesis procedures are performed frequently by internal medicine‐ and family medicine‐trained clinicians in university hospitals. Because of these findings regarding current practice patterns, we believe the ACGME, ABIM, and ABFM should clarify their policies to require that residents are competent to perform paracentesis procedures before performing them on patients. This may improve supervision and training for paracentesis procedures that are already occurring and possibly encourage performance of additional, less costly bedside procedures.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Mark Williams for their support and encouragement of this work.
Disclosure: Nothing to report.
Cirrhosis affects up to 3% of the population and is 1 of the 10 most common causes of death in the United States.[1, 2, 3, 4] Paracentesis procedures are frequently performed in patients with liver disease and ascites for diagnostic and/or therapeutic purposes. These procedures can be performed safely by trained clinicians at the bedside or referred to interventional radiology (IR).[2, 3, 4]
National practice patterns show that paracentesis procedures are increasingly referred to IR rather than performed at the bedside by internal medicine or gastroenterology clinicians.[5, 6, 7] In fact, a recent study of Medicare beneficiaries showed that inpatient and outpatient paracentesis procedures performed by radiologists increased by 964% from 1993 to 2008.[7] Reasons for the decline in bedside procedures include the increased availability of IR, lack of sufficient reimbursement, and the time required to perform paracentesis procedures.[5, 6, 7, 8] Surveys of internal medicine and family medicine residents and gastroenterology fellows show trainees often lack the confidence and experience needed to perform the procedure safely.[9, 10, 11] Additionally, many clinicians do not have expertise with ultrasound use and may not have access to necessary equipment.
Inconsistent certification requirements may also impact the competence and experience of physicians to perform paracentesis procedures. Internal medicine residents are no longer required by the American Board of Internal Medicine (ABIM) to demonstrate competency in procedures such as paracentesis for certification.[12] However, the Accreditation Council for Graduate Medical Education (ACGME) requirements state that internal medicine programs must offer residents the opportunity to demonstrate competence in the performance of procedures such as paracentesis, thoracentesis, and central venous catheter insertion.[13] The American Board of Family Medicine (ABFM) does not outline specific procedural competence for initial certification.[14] The ACGME states that family medicine residents must receive training to perform those clinical procedures required for their future practices but allows each program to determine which procedures to require.[15] Due to this uncertainty, practicing hospitalists are likely to have variable training and competence in bedside procedures such as paracentesis.
We previously showed that internal medicine residents rotating on the hepatology service of an academic medical center performed 59% of paracentesis procedures at the bedside.[16] These findings are in contrast to national data showing that 74% of paracentesis procedures performed on Medicare beneficiaries were performed by radiologists.[7] Practice patterns at university hospitals may not be reflected in this data because the study was limited to Medicare beneficiaries and included ambulatory patients.[7] In addition to uncertainty about who is performing this procedure in inpatient settings, little is known about the effect of specialty on postparacentesis clinical outcomes.[16, 17]
The current study had 3 aims: (1) evaluate which clinical specialties perform paracentesis procedures at university hospitals; (2) model patient characteristics associated with procedures performed at the bedside versus those referred to IR; and (3) among patients with a similar likelihood of IR referral, evaluate length of stay (LOS) and hospital costs of patients undergoing procedures performed by different specialties.
METHODS
We performed an observational administrative database review of patients who underwent paracentesis procedures in hospitals participating in the University HealthSystem Consortium (UHC) Clinical Database from January 2010 through December 2012. UHC is an alliance of 120 nonprofit academic medical centers and their 290 affiliated hospitals. UHC maintains databases containing clinical, operational, financial, and patient safety data from affiliated hospitals. Using the UHC database, we described the characteristics of all patients who underwent paracentesis procedures by clinical specialty performing the procedure. We then modeled the effects of patient characteristics on decision‐making about IR referral. Finally, among patients with a homogeneous predicted probability of IR referral, we compared LOS and direct costs by specialty performing the procedure. The Northwestern University institutional review board approved this study.
Procedure
We queried the UHC database for all patients over the age of 18 years who underwent paracentesis procedures (International Classification of Disease Revision 9 [ICD‐9] procedure code 54.91) and had at least 1 diagnosis code of liver disease (571.x). We excluded patients admitted to obstetrics. The query included patient and clinical characteristics such as admission, discharge, and procedure date; age, gender, procedure provider specialty, and intensive care unit (ICU) stay. We also obtained all ICD‐9 codes associated with the admission including obesity, severe liver disease, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before or during the admission, awaiting liver transplant, and complications of liver transplant. We used ICD‐9 codes to calculate patients' Charlson score[18, 19] to assess severity of illness on admission.
LOS and total direct hospital costs were compared among patients with a paracentesis performed by a single clinical group and among patients with a similar predicted probability of IR referral. UHC generates direct cost estimates by applying Medicare Cost Report ratios of cost to charges with the labor cost further adjusted by the respective area wage index. Hospital costs were not available from 8.3% of UHC hospitals. We therefore based cost estimates on nonmissing data.
Paracentesis provider specialties were divided into 6 general categories: (1) IR (interventional and diagnostic radiology); (2) medicine (family medicine, general medicine, and hospital medicine); (3) subspecialty medicine (infectious disease, cardiology, nephrology, hematology/oncology, endocrinology, pulmonary, and geriatrics); (4) gastroenterology/hepatology (gastroenterology, hepatology, and transplant medicine); (5) general surgery (general surgery and transplant surgery); and (6) all other (included unclassified specialties). We present patient characteristics categorized by these specialty groups and for admissions in which multiple specialties performed procedures.
Study Design
To analyze an individual patient's likelihood of IR referral, we needed to restrict our sample to discharges where only 1 clinical specialty performed a paracentesis. Therefore, we excluded hybrid discharges with procedures performed by more than 1 specialty in a single admission as well as discharges with procedures performed by all other specialties. To compare LOS and direct cost outcomes, and to minimize selection bias among exclusively IR‐treated patients, we excluded hospitals without procedures done by both IR and medicine.
We modeled referral to IR as a function of patients' demographic and clinical variables, which we believed would affect the probability of referral. We then examined the IR referral model predicted probabilities (propensity score).[20] Finally, we examined mean differences in LOS and direct costs among discharges with a single clinical specialty group, while using the predicted probability of referral as a filter to compare these outcomes by specialty. We further tested specialty differences in LOS and direct costs controlling for demographic and clinical variables.
Statistical Analysis
To test the significance of differences between demographic and clinical characteristics of patients across specialties, we used 2 tests for categorical variables and analysis of variance or the Kruskal‐Wallis rank test for continuous variables. Random effects logistic regression, which adjusts standard errors for clustering by hospital, was used to model the likelihood of referral to IR. Independent variables included patient age, gender, obesity, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before hospitalization, liver transplant during hospitalization, awaiting transplant, complications of liver transplant, ICU stay, Charlson score, and number of paracentesis procedures performed during the admission. Predicted probabilities derived from this IR referral model were used to investigate selection bias in our subsequent analyses of LOS and costs.[20]
We used random effects multiple linear regression to test the association of procedure specialty with hospital LOS and total direct costs, controlling for the same independent variables listed above. Analyses were conducted using both actual LOS in days and Medicare costs. We also performed a log transformation of LOS and costs to account for rightward skew. We only present actual LOS and cost results because results were virtually identical. We used SAS version 9 (SAS Institute Inc., Cary, NC) to extract data from the UHC Clinical Database. We performed all statistical analyses using Stata version 12 (StataCorp LP, College Station, TX).
RESULTS
Procedure and Discharge Level Results
There were 97,577 paracentesis procedures performed during 70,862 hospital admissions in 204 UHC hospitals during the study period. Table 1 shows specific specialty groups for each procedure. The all other category consisted of 17,558 subspecialty groups including 9,434 with specialty unknown. Twenty‐nine percent of procedures were performed in IR versus 27% by medicine, 11% by gastroenterology/hepatology, and 11% by subspecialty medicine.
| Specialty Group | No. | % |
|---|---|---|
| Interventional radiology | 28,414 | 29.1 |
| Medicine | 26,031 | 26.7 |
| Family medicine | 1,026 | 1.1 |
| General medicine | 21,787 | 22.3 |
| Hospitalist | 3,218 | 3.3 |
| Subspecialty medicine | 10,558 | 10.8 |
| Infectious disease | 848 | 0.9 |
| Nephrology | 615 | 0.6 |
| Cardiology | 991 | 1.0 |
| Hematology oncology | 795 | 0.8 |
| Endocrinology | 359 | 0.4 |
| Pulmonology | 6,605 | 6.8 |
| Geriatrics | 345 | 0.4 |
| Gastroenterology/hepatology | 11,143 | 11.4 |
| Transplant medicine | 99 | 0.1 |
| Hepatology | 874 | 0.9 |
| Gastroenterology | 10,170 | 10.4 |
| General surgery | 3,873 | 4.0 |
| Transplant surgery | 2,146 | 2.2 |
| General surgery | 1,727 | 1.8 |
| All other | 17,558 | 18.0 |
| Specialty unknown | 9,434 | 9.7 |
Table 2 presents patient characteristics for 70,862 hospital discharges with paracentesis procedures grouped by whether single or multiple specialties performed procedures. Patient characteristics were significantly different across specialty groups. Medicine, subspecialty medicine, and gastroenterology/hepatology patients were younger, more likely to be male, and more likely to have severe liver disease, coagulation disorders, hypotension, and hyponatremia than IR patients.
| All Discharges, N=70,862 | Interventional Radiology, n=9,348 | Medicine, n=13,789 | Subspecialty Medicine, n=5,085 | Gastroenterology/Hepatology, n=6,664 | General Surgery, n=1,891 | All Other, n=7,912 | Discharges With Multiple Specialties, n=26,173 | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age group, y (%) | ||||||||
| 1849 | 25.4 | 22.5 | 27.6 | 24.9 | 23.5 | 20.8 | 25.5 | 26.1 |
| 5059 | 39.8 | 39.8 | 40.9 | 39.4 | 41.5 | 40.3 | 40.0 | 38.7 |
| 6069 | 24.7 | 24.9 | 21.6 | 24.7 | 26.5 | 30.0 | 23.6 | 25.8 |
| 70+ | 10.1 | 12.9 | 9.9 | 11.1 | 8.4 | 8.9 | 11.0 | 9.4 |
| Male (%) | 65.5 | 64.2 | 67.6 | 67.5 | 65.7 | 66.6 | 65.7 | 64.2 |
| Severe liver disease (%)a | 73.7 | 65.3 | 67.8 | 71.0 | 75.3 | 66.6 | 67.6 | 82.1 |
| Obesity (BMI 40+) (%) | 6.3 | 6.1 | 5.3 | 5.7 | 5.1 | 5.8 | 5.2 | 7.6 |
| Any intensive care unit stay (%) | 31.0 | 10.9 | 16.8 | 50.5 | 16.9 | 36.7 | 22.3 | 47.8 |
| Coagulation disorders (%) | 24.3 | 14.8 | 20.2 | 29.9 | 16.1 | 19.0 | 17.8 | 33.1 |
| Blood loss anemia (%) | 3.4 | 1.3 | 2.8 | 2.7 | 2.7 | 1.9 | 2.1 | 5.2 |
| Hyponatremia (%) | 29.9 | 27.1 | 29.2 | 28.9 | 28.0 | 26.6 | 27.3 | 33.1 |
| Hypotension (%) | 9.8 | 7.0 | 8.0 | 11.0 | 7.7 | 10.5 | 8.1 | 12.4 |
| Thrombocytopenia (%) | 29.6 | 24.6 | 28.3 | 32.5 | 22.1 | 21.5 | 24.0 | 35.8 |
| Complication of transplant (%) | 3.3 | 2.1 | 1.1 | 2.4 | 4.0 | 10.3 | 2.7 | 4.7 |
| Awaiting liver transplant (%) | 7.6 | 6.4 | 4.0 | 5.4 | 12.8 | 16.0 | 7.8 | 8.2 |
| Prior liver transplant (%) | 0.5 | 0.8 | 0.3 | 0.3 | 0.7 | 0.7 | 0.4 | 0.6 |
| Liver transplant procedure (%) | 2.7 | 0.0 | 0.0 | 0.3 | 0.4 | 15.6 | 1.6 | 5.6 |
| Mean Charlson score (SD) | 4.51 (2.17) | 4.28 (2.26) | 4.16 (2.17) | 4.72 (2.30) | 4.30 (1.98) | 4.26 (2.22) | 4.36 (2.30) | 4.84 (2.07) |
| Mean paracentesis procedures per discharge (SD) | 1.38 (0.88) | 1.21 (0.56) | 1.26 (0.66) | 1.30 (0.76) | 1.31 (0.70) | 1.28 (0.78) | 1.22 (0.61) | 1.58 (1.13) |
IR Referral Model
We first excluded 6030/70,862 discharges (8.5%) from 59 hospitals without both IR and medicine procedures. We then further excluded 24,986/70,862 (35.3%) discharges with procedures performed by multiple specialties during the same admission. Finally, we excluded 5555/70,862 (7.8%) of discharges with procedure specialty coded as all other. Therefore, 34,291 (48.4%) discharges (43,337/97,577; 44.4% procedures) from 145 UHC hospitals with paracentesis procedures performed by a single clinical specialty group remained for the IR referral analysis sample. Among admissions with multiple specialty paracentesis performed within the same admission, 3128/26,606 admissions with any IR procedure (11.8%) had a different specialty ascribed to the first, second, or third paracentesis with a subsequent IR procedure.
Model results (Table 3) indicate that patients who were obese (odds ratio [OR]: 1.25; 95% confidence interval [CI]: 1.10‐1.43) or had a liver transplant on a prior admission (OR: 2.03; 95% CI: 1.40‐2.95) were more likely to be referred to IR. However, male patients (OR: 0.89; 95% CI: 0.83‐0.95), or patients who required an ICU stay (OR: 0.39; 95% CI: 0.36‐0.43) were less likely to have IR procedures. Other patient factors reducing the likelihood of IR referral included characteristics associated with higher severity of illness (coagulation disorders, hyponatremia, hypotension, and thrombocytopenia).
| Odds Ratio | 95% CI | ||
|---|---|---|---|
| Lower | Upper | ||
| |||
| Age group, y | |||
| 1849 | Reference | ||
| 5059 | 1.05 | 0.97 | 1.14 |
| 6069 | 1.12 | 1.02 | 1.22 |
| 70+ | 1.11 | 0.99 | 1.24 |
| Male | 0.89 | 0.83 | 0.95 |
| Obesity, BMI 40+ | 1.25 | 1.10 | 1.43 |
| ICU care | 0.39 | 0.36 | 0.43 |
| Coagulation disorders | 0.68 | 0.63 | 0.75 |
| Blood loss anemia | 0.52 | 0.41 | 0.66 |
| Hyponatremia | 0.85 | 0.80 | 0.92 |
| Hypotension | 0.83 | 0.74 | 0.93 |
| Thrombocytopenia | 0.94 | 0.87 | 1.01 |
| Prior liver transplant | 0.08 | 0.03 | 0.23 |
| Awaiting liver transplant | 0.86 | 0.76 | 0.98 |
| Complication of liver transplant | 1.07 | 0.88 | 1.31 |
| Liver transplant procedure | 2.03 | 1.40 | 2.95 |
| Charlson score | 1.00 | 0.99 | 1.01 |
| Number of paracentesis procedures | 0.90 | 0.85 | 0.95 |
Predicted Probabilities of IR Referral
Figure 1 presents the distribution of predicted probabilities for IR referral. Predicted probabilities were low overall, with very few patients having an equal chance of referralthe standard often used in comparative effectiveness analyses from observational data. Figure 1 indicates that IR referral probabilities were clustered in an unusual bimodal distribution. The cluster on the left, which centers around a 15% predicted probability of IR referral, consists of discharges with patient characteristics that were associated with a very low chance of an IR paracentesis. We therefore used this distribution to conduct comparative analyses of admission outcomes between clinical specialty groups, choosing to examine patients with a 20% or greater chance of IR referral.

Post hoc analysis revealed that the biggest factor driving low predicted probability of IR referral was whether patients experienced an ICU stay at any time during hospitalization. Among the discharges with a predicted probability 0.2 (n=26,615 discharges), there were only 87 discharges with ICU stays (0.3%). For the discharges with predicted probability <0.2 (n=7676), 91.9% (n=7055) had an ICU admission. We therefore used a threshold of 0.2 or greater to present the most comparable LOS and direct cost differences.
LOS and Cost Comparisons by Specialty
Mean LOS and hospital direct costs by specialty for our final analysis sample can be found in Table 4; differences between specialties were significant (P<0.0001). Patients undergoing IR procedures had equivalent LOS and costs to medicine patients, but lower LOS and costs than other clinical specialty groups. Random effects linear regression showed that neither medicine nor gastroenterology/hepatology patients had significantly different LOS from IR patients, but subspecialty medicine was associated with 0.89 additional days and general surgery with 1.47 additional days (both P<0.0001; R2=0.10). In the direct cost regression model, medicine patients were associated with $1308 lower costs and gastroenterology/hepatology patients with $803 lower costs than IR patients (both P=0.0001), whereas subspecialty medicine and general surgery had higher direct costs per discharge of $1886 and $3039, respectively (both P<0.0001, R2=0.19). Older age, obesity, coagulopathy, hyponatremia, hypotension, thrombocytopenia, liver transplant status, ICU care, higher Charlson score, and higher number of paracentesis procedures performed were all significantly associated with higher LOS and hospital costs in these linear models.
| All Admissions n=26,615 | Interventional Radiology n=7,677 | Medicine n=10,413 | Medicine Subspecialties n=2,210 | Gastroenterology/ Hepatology n=5,182 | General Surgery n=1,133 | |
|---|---|---|---|---|---|---|
| All Admissions n=24,408 | Interventional Radiology n =7,265 | Medicine n=8,965, | Medicine Subspecialties n=2,064 | Gastroenterology/Hepatology n=5,031 | General Surgery n=1,083 | |
| ||||||
| Mean length of stay, d (SD) | 5.57 (5.63) | 5.20 (4.72) | 5.59 (5.85) | 6.28 (6.47) | 5.54 (5.31) | 6.67 (8.16) |
| Mean total direct cost, $ (SD)a | 11,447 (12,247) | 10,975 (9,723) | 10,517 (10,895) | 13,705 (16,591) | 12,000 (11,712) | 15,448 (23,807) |
DISCUSSION
This study showed that internal medicine‐ and family medicine‐trained clinicians perform approximately half of the inpatient paracentesis procedures at university hospitals and their affiliates. This confirms findings from our earlier single‐institution study[16] but contrasts with previously published reports involving Medicare data. The earlier report, using Medicare claims and including ambulatory procedures, revealed that primary care physicians and gastroenterologists only performed approximately 10% of US paracentesis procedures in 2008.[7] Our findings suggest that practices are different at university hospitals, where patients with severe liver disease often seek care. Because we used the UHC database, it was not possible to determine if the clinicians who performed paracentesis procedures in this study were internal medicine or family medicine residents, fellows, or attending physicians. However, findings from our own institution show that the vast majority of bedside paracentesis procedures are performed by internal medicine residents.[16]
Our findings have implications for certification of internal medicine and family medicine trainees. In 2008, the ABIM removed the requirement that internal medicine residents demonstrate competency in paracentesis.[12] This decision was informed by a lack of standardized methods to determine procedural competency and published surveys showing that internal medicine and hospitalist physicians rarely performed bedside procedures.[5, 6] Despite this policy change, our findings show that current clinical practice at university hospitals does not reflect national practice patterns or certification requirements, because many internal medicine‐ and family medicine‐trained clinicians still perform paracentesis procedures. This is concerning because internal medicine and family medicine trainees report variable confidence, experience, expertise, and supervision regarding performance of invasive procedures.[9, 10, 21, 22, 23, 24] Furthermore, earlier research also demonstrates that graduating residents and fellows are not able to competently perform common bedside procedures such as thoracentesis, temporary hemodialysis catheter insertion, and lumbar puncture.[25, 26, 27]
The American Association for the Study of Liver Diseases (AASLD) recommends that trained clinicians perform paracentesis procedures.[3, 4] However, the AASLD provides no definition for how training should occur. Because competency in this procedure is not specifically required by the ABIM, ABFM, or ACGME, a paradoxical situation occurs in which internal medicine and family medicine residents, and internal medicine‐trained fellows and faculty continue to perform paracentesis procedures on highly complex patients, but are no longer required to be competent to do so.
In earlier research we showed that simulation‐based mastery learning (SBML) was an effective method to boost internal medicine residents' paracentesis skills.[28] In SBML, all trainees must meet or exceed a minimum passing score on a simulated procedure before performing one on an actual patient.[29] This approach improves clinical care and outcomes in procedures such as central venous catheter insertion[30, 31] and advanced cardiac life support.[32] SBML‐trained residents also performed safe paracentesis procedures with shorter hospital LOS, fewer ICU transfers, and fewer blood product transfusions than IR procedures.[16] Based on the results of this study, AASLD guidelines regarding training, and our experience with SBML, we recommend that all clinicians complete paracentesis SBML training before performing procedures on patients.
Using our propensity model we identified patient characteristics that were associated with IR referral. Patients with a liver transplant were more likely to be cared for in IR. This may be due to a belief that postoperative procedures are anatomically more complex or because surgical trainees do not commonly perform this procedure. The current study confirms findings from earlier work that obese and female patients are more likely to be referred to IR.[16] IR referral of obese patients is likely to occur because paracentesis procedures are technically more difficult. We have no explanation why female patients were more likely to be referred to IR, because most decisions appear to be discretionary. Prospective studies are needed to determine evidence‐based recommendations regarding paracentesis procedure location. Patients with more comorbidities (eg, ICU stay, awaiting liver transplant, coagulation disorders) were more likely to undergo bedside procedures. The complexity of patients undergoing bedside paracentesis procedures reinforces the need for rigorous skill assessment for clinicians who perform them because complications such as intraperitoneal bleeding can be fatal.
Finally, we showed that LOS was similar but hospital direct costs were $800 to $1300 lower for patients whose paracentesis procedure was performed by medicine or gastroenterology/hepatology compared to IR. Medical subspecialties and surgery procedures were more expensive than IR, consistent with the higher LOS seen in these groups. IR procedures add costs due to facility charges for space, personnel, and equipment.[33] At our institution, the hospital cost of an IR paracentesis in 2012 was $361. If we use this figure, and assume costs are similar across university hospitals, the resultant cost savings would be $10,257,454 (for the procedure alone) if all procedures referred to IR in this 2‐year study were instead performed at the bedside. This estimate is approximate because it does not consider factors such as cost of clinician staffing models, which may differ across UHC hospitals. As hospitals look to reduce costs, potential savings due to appropriate use of bedside and IR procedures should be considered. This is especially important because there is no evidence that the extra expense of IR procedures is justified due to improved patient outcomes.
This study has several limitations. First, this was an observational study. Although the database was large, we were limited by coding accuracy and could not control for all potential confounding factors such as Model for End‐Stage Liver Disease score,[34, 35] other specific laboratory values, amount of ascites fluid removed, or bedside procedure failures later referred to IR. However, we do know that only a small number of second, third, or fourth procedures were subsequently referred to IR after earlier ones were performed at the bedside. Additionally the UHC database does not include patient‐specific data, and therefore we could not adjust for multiple visits or procedures by the same patient. Second, we were unable to determine the level of teaching involvement at each UHC affiliated hospital. Community hospitals where attendings managed most of the patients without trainees could not be differentiated from university hospitals where trainees were involved in most patients' care. Third, we did not have specialty information for 9434 (9.7%) procedures and had to exclude these cases. We also excluded a large number of paracentesis procedures in our final outcomes analysis. However, this was necessary because we needed to perform a patient‐level analysis to ensure the propensity and outcomes models were accurate. Finally, we did not evaluate inpatient mortality or 30‐day hospital readmission rates. Mortality and readmission from complications of a paracentesis procedure are rare events.[3, 4, 36] However, mortality and hospital readmission among patients with liver disease are relatively common.[37, 38] It was impossible to link these outcomes to a paracentesis procedure without the ability to perform medical records review.
In conclusion, paracentesis procedures are performed frequently by internal medicine‐ and family medicine‐trained clinicians in university hospitals. Because of these findings regarding current practice patterns, we believe the ACGME, ABIM, and ABFM should clarify their policies to require that residents are competent to perform paracentesis procedures before performing them on patients. This may improve supervision and training for paracentesis procedures that are already occurring and possibly encourage performance of additional, less costly bedside procedures.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Mark Williams for their support and encouragement of this work.
Disclosure: Nothing to report.
- . A primer on detecting cirrhosis and caring for these patients without causing harm. Int J Hepatol. 2011:801983.
- , , . Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787–799.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–2107.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology. 2009;49:2087–2107. Available at: http://www.aasld.org/practiceguidelines/Documents/ascitesupdate2013.pdf. Accessed October 21, 2013.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , . What procedures should internists do? Ann Intern Med. 2007;146(5):392–393.
- , , , , , . Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures. Am J Med. 2006;119(1)71.e17–e24.
- , , . Perception of competency to perform procedures and future practice intent: a national survey of family practice residents. Acad Med. 2003;78(9):926–932.
- , , , . Utilization of and adherence to the gastroenterology core curriculum on hepatology training during a gastrointestinal fellowship. Clin Gastroenterol Hepatol. 2008;6(6):682–688.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed December 21, 2013.
- A CGME program requirements for graduate medical education in internal medicine. Available at: http://acgme.org/acgmeweb/portals/0/PFassets/2013‐PR‐FAQ‐PIF/140_internal_medicine_07012013.pdf. Accessed December 17, 2013.
- American Board of Family Medicine residency requirements. Available at: https://www.theabfm.org/cert/guidelines.aspx. Accessed December 17, 2013.
- ACGME program requirements for graduate medical education in family medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/120pr07012007.pdf. Accessed December 17, 2013.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484–488.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- . The role of known effects in observational studies. Biometrics. 1998;45(2):557–569.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , , , et al. Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial. J Hosp Med. 2007;2(3):143–149.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , . Performance of procedures by nephrologists and nephrology fellows at U.S. nephrology training programs. Clin J Am Soc Nephrol. 2008;3(4):941–947.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3:49–54.
- , , , , . Mastery learning of temporary dialysis catheter insertion skills by nephrology fellows using simulation technology and deliberate practice. Am J Kidney Dis. 2009;54:70–76.
- , , , , , . Simulation‐based education with mastery learning improves residents' lumbar puncture skills. Neurology. 2012;79:132–137.
- , , , , , . Simulation‐based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23–27.
- , , , , . Medical education featuring mastery learning with deliberate practice can lead to better health for individuals and populations. Acad Med. 2011; 86(11):e8–e9.
- , , , , . Simulation‐based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697–2701.
- , , , , . Use of simulation‐based education to reduce catheter‐related bloodstream infections. Arch Intern Med. 2009;169(15):1420–1423.
- , , , et al. Progress toward improving the quality of cardiac arrest medical team responses at an academic teaching hospital. J Grad Med Educ. 2011;3(2):211–216.
- , , , et al. Technical cost of radiologic examinations: analysis across imaging modalities. Radiology. 2000;216(1):269–272.
- , , , et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33(2):464–470.
- , , , et al. Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- , , , . Videos in clinical medicine. Paracentesis. N Engl J Med. 2006;355(19):e21.
- Center for Disease Control and Prevention. Chronic liver disease or cirrhosis. National Hospital Discharge Survey: 2010 detailed diagnosis and procedure tables, number of first‐listed diagnoses (see ICD9‐CM code 571). Available at: http://www.cdc.gov/nchs/fastats/liverdis.htm. Accessed October 19, 2013.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254–259.
- . A primer on detecting cirrhosis and caring for these patients without causing harm. Int J Hepatol. 2011:801983.
- , , . Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787–799.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–2107.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology. 2009;49:2087–2107. Available at: http://www.aasld.org/practiceguidelines/Documents/ascitesupdate2013.pdf. Accessed October 21, 2013.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , . What procedures should internists do? Ann Intern Med. 2007;146(5):392–393.
- , , , , , . Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures. Am J Med. 2006;119(1)71.e17–e24.
- , , . Perception of competency to perform procedures and future practice intent: a national survey of family practice residents. Acad Med. 2003;78(9):926–932.
- , , , . Utilization of and adherence to the gastroenterology core curriculum on hepatology training during a gastrointestinal fellowship. Clin Gastroenterol Hepatol. 2008;6(6):682–688.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed December 21, 2013.
- A CGME program requirements for graduate medical education in internal medicine. Available at: http://acgme.org/acgmeweb/portals/0/PFassets/2013‐PR‐FAQ‐PIF/140_internal_medicine_07012013.pdf. Accessed December 17, 2013.
- American Board of Family Medicine residency requirements. Available at: https://www.theabfm.org/cert/guidelines.aspx. Accessed December 17, 2013.
- ACGME program requirements for graduate medical education in family medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/120pr07012007.pdf. Accessed December 17, 2013.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484–488.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- . The role of known effects in observational studies. Biometrics. 1998;45(2):557–569.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , , , et al. Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial. J Hosp Med. 2007;2(3):143–149.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , . Performance of procedures by nephrologists and nephrology fellows at U.S. nephrology training programs. Clin J Am Soc Nephrol. 2008;3(4):941–947.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3:49–54.
- , , , , . Mastery learning of temporary dialysis catheter insertion skills by nephrology fellows using simulation technology and deliberate practice. Am J Kidney Dis. 2009;54:70–76.
- , , , , , . Simulation‐based education with mastery learning improves residents' lumbar puncture skills. Neurology. 2012;79:132–137.
- , , , , , . Simulation‐based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23–27.
- , , , , . Medical education featuring mastery learning with deliberate practice can lead to better health for individuals and populations. Acad Med. 2011; 86(11):e8–e9.
- , , , , . Simulation‐based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697–2701.
- , , , , . Use of simulation‐based education to reduce catheter‐related bloodstream infections. Arch Intern Med. 2009;169(15):1420–1423.
- , , , et al. Progress toward improving the quality of cardiac arrest medical team responses at an academic teaching hospital. J Grad Med Educ. 2011;3(2):211–216.
- , , , et al. Technical cost of radiologic examinations: analysis across imaging modalities. Radiology. 2000;216(1):269–272.
- , , , et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33(2):464–470.
- , , , et al. Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- , , , . Videos in clinical medicine. Paracentesis. N Engl J Med. 2006;355(19):e21.
- Center for Disease Control and Prevention. Chronic liver disease or cirrhosis. National Hospital Discharge Survey: 2010 detailed diagnosis and procedure tables, number of first‐listed diagnoses (see ICD9‐CM code 571). Available at: http://www.cdc.gov/nchs/fastats/liverdis.htm. Accessed October 19, 2013.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254–259.
© 2014 Society of Hospital Medicine
Electronic Order Set for AMI
Although the prevalence of coronary heart disease and death from acute myocardial infarction (AMI) have declined steadily, about 935,000 heart attacks still occur annually in the United States, with approximately one‐third of these being fatal.[1, 2, 3] Studies have demonstrated decreased 30‐day and longer‐term mortality in AMI patients who receive evidence‐based treatment, including aspirin, ‐blockers, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), anticoagulation therapy, and statins.[4, 5, 6, 7] Despite clinical practice guidelines (CPGs) outlining evidence‐based care and considerable efforts to implement processes that improve patient outcomes, delivery of effective therapy remains suboptimal.[8] For example, the Hospital Quality Alliance Program[9] found that in AMI patients, use of aspirin on admission was only 81% to 92%, ‐blocker on admission 75% to 85%, and ACE inhibitors for left ventricular dysfunction 71% to 74%.
Efforts to increase adherence to CPGs and improve patient outcomes in AMI have resulted in variable degrees of success. They include promotion of CPGs,[4, 5, 6, 7] physician education with feedback, report cards, care paths, registries,[10] Joint Commission standardized measures,[11] and paper checklists or order sets (OS).[12, 13]
In this report, we describe the association between use of an evidence‐based, electronic OS for AMI (AMI‐OS) and better adherence to CPGs. This AMI‐OS was implemented in the inpatient electronic medical records (EMRs) of a large integrated healthcare delivery system, Kaiser Permanente Northern California (KPNC). The purpose of our investigation was to determine (1) whether use of the AMI‐OS was associated with improved AMI processes and patient outcomes, and (2) whether these associations persisted after risk adjustment using a comprehensive severity of illness scoring system.
MATERIALS AND METHODS
This project was approved by the KPNC institutional review board.
Under a mutual exclusivity arrangement, salaried physicians of The Permanente Medical Group, Inc., care for 3.4 million Kaiser Foundation Health Plan, Inc. members at facilities owned by Kaiser Foundation Hospitals, Inc. All KPNC facilities employ the same information systems with a common medical record number and can track care covered by the plan but delivered elsewhere.[14] Our setting consisted of 21 KPNC hospitals described in previous reports,[15, 16, 17, 18] using the same commercially available EMR system that includes computerized physician order entry (CPOE). Deployment of the customized inpatient Epic EMR (
In this EMR's CPOE, physicians have options to select individual orders (a la carte) or they can utilize an OS, which is a collection of the most appropriate orders associated with specific diagnoses, procedures, or treatments. The evidence‐based AMI‐OS studied in this project was developed by a multidisciplinary team (for detailed components see Supporting Appendix 1Appendix 5 in the online version of this article).
Our study focused on the first set of hospital admission orders for patients with AMI. The study sample consisted of patients meeting these criteria: (1) age 18 years at admission; (2) admitted to a KPNC hospital for an overnight stay between September 28, 2008 and December 31, 2010; (3) principal diagnosis was AMI (International Classification of Diseases, 9th Revision [ICD‐9][19] codes 410.00, 01, 10, 11, 20, 21, 30, 31, 40, 41, 50, 51, 60, 61, 70, 71, 80, 90, and 91); and (4) KPHC had been operational at the hospital for at least 3 months to be included (for assembly descriptions see Supporting Appendices 15 in the online version of this article). At the study hospitals, troponin I was measured using the Beckman Access AccuTnI assay (Beckman Coulter, Inc., Brea, CA), whose upper reference limit (99th percentile) is 0.04 ng/mL. We excluded patients initially hospitalized for AMI at a non‐KPNC site and transferred into a study hospital.
The data processing methods we employed have been detailed elsewhere.[14, 15, 17, 20, 21, 22] The dependent outcome variables were total hospital length of stay, inpatient mortality, 30‐day mortality, and all‐cause rehospitalization within 30 days of discharge. Linked state mortality data were unavailable for the entire study period, so we ascertained 30‐day mortality based on the combination of KPNC patient demographic data and publicly available Social Security Administration decedent files. We ascertained rehospitalization by scanning KPNC hospitalization databases, which also track out‐of‐plan use.
The dependent process variables were use of aspirin within 24 hours of admission, ‐blockers, anticoagulation, ACE inhibitors or ARBs, and statins. The primary independent variable of interest was whether or not the admitting physician employed the AMI‐OS when admission orders were entered. Consequently, this variable is dichotomous (AMI‐OS vs a la carte).
We controlled for acute illness severity and chronic illness burden using a recent modification[22] of an externally validated risk‐adjustment system applicable to all hospitalized patients.[15, 16, 23, 24, 25] Our methodology included vital signs, neurological status checks, and laboratory test results obtained in the 72 hours preceding hospital admission; comorbidities were captured longitudinally using data from the year preceding hospitalization (for comparison purposes, we also assigned a Charlson Comorbidity Index score[26]).
End‐of‐life care directives are mandatory on admission at KPNC hospitals. Physicians have 4 options: full code, partial code, do not resuscitate, and comfort care only. Because of small numbers in some categories, we collapsed these 4 categories into full code and not full code. Because patients' care directives may change, we elected to capture the care directive in effect when a patient first entered a hospital unit other than the emergency department (ED).
Two authors (M.B., P.C.L.), one of whom is a board‐certified cardiologist, reviewed all admission electrocardiograms and made a consensus determination as to whether or not criteria for ST‐segment elevation myocardial infarction (STEMI) were present (ie, new ST‐segment elevation or left bundle branch block); we also reviewed the records of all patients with missing troponin I data to confirm the AMI diagnosis.
Statistical Methods
We performed unadjusted comparisons between AMI‐OS and nonAMI‐OS patients using the t test or the [2] test, as appropriate.
We hypothesized that the AMI‐OS plays a mediating role on patient outcomes through its effect on adherence to recommended treatment. We evaluated this hypothesis for inpatient mortality by first fitting a multivariable logistic regression model for inpatient mortality as the outcome and either the 5 evidence‐based therapies or the total number of evidence‐based therapies used (ranging from 02, 3, 4, or 5) as the dependent variable controlling for age, gender, presence of STEMI, troponin I, comorbidities, illness severity, ED length of stay (LOS), care directive status, and timing of cardiac catheterization referral as covariates to confirm the protective effect of these therapies on mortality. We then used the same model to estimate the effect of AMI‐OS on inpatient mortality, substituting the therapies with AMI‐OS as the dependent variable and using the same covariates. Last, we included both the therapies and the AMI‐OS in the model to evaluate their combined effects.[27]
We used 2 different methods to estimate the effects of AMI‐OS and number of therapies provided on the outcomes while adjusting for observed baseline differences between the 2 groups of patients: propensity risk score matching, which estimates the average treatment effect for the treated,[28, 29] and inverse probability of treatment weighting, which is used to estimate the average treatment effect.[30, 31, 32] The propensity score was defined as the probability of receiving the intervention for a patient with specific predictive factors.[33, 34] We computed a propensity score for each patient by using logistic regression, with the dependent variable being receipt of AMI‐OS and the independent variables being the covariates used for the multivariate logistic regression as well as ICD‐9 code for final diagnosis. We calculated the Mahalanobis distance between patients who received AMI‐OS (cases) and patients who did not received AMI‐OS (controls) using the same set of covariates. We matched each case to a single control within the same facility based on the nearest available Mahalanobis metric matching within calipers defied as the maximum width of 0.2 standard deviations of the logit of the estimated propensity score.[29, 35] We estimated the odds ratios for the binary dependent variables based on a conditional logistic regression model to account for the matched pairs design.[28] We used a generalized linear model with the log‐transformed LOS as the outcome to estimate the ratio of the LOS geometric mean of the cases to the controls. We calculated the relative risk for patients receiving AMI‐OS via the inverse probability weighting method by first defining a weight for each patient. [We assigned a weight of 1/psi to patients who received the AMI‐OS and a weight of 1/(1psi) to patients who did not receive the AMI‐OS, where psi denotes the propensity score for patient i]. We used a logistic regression model for the binary dependent variables with the same set of covariates described above to estimate the adjusted odds ratios while weighting each observation by its corresponding weight. Last, we used a weighted generalized linear model to estimate the AMI‐OS effect on the log‐transformed LOS.
RESULTS
Table 1 summarizes the characteristics of the 5879 patients. It shows that AMI‐OS patients were more likely to receive evidence‐based therapies for AMI (aspirin, ‐blockers, ACE inhibitors or ARBs, anticoagulation, and statins) and had a 46% lower mortality rate in hospital (3.51 % vs 6.52%) and 33% lower rate at 30 days (5.66% vs 8.48%). AMI‐OS patients were also found to be at lower risk for an adverse outcome than nonAMI‐OS patients. The AMI‐OS patients had lower peak troponin I values, severity of illness (lower Laboratory‐Based Acute Physiology Score, version 2 [LAPS2] scores), comorbidity burdens (lower Comorbidity Point Score, version 2 [COPS2] and Charlson scores), and global predicted mortality risk. AMI‐OS patients were also less likely to have required intensive care. AMI‐OS patients were at higher risk of death than nonAMI‐OS patients with respect to only 1 variable (being full code at the time of admission), but although this difference was statistically significant, it was of minor clinical impact (86% vs 88%).
| Patients Initially Managed Using | P Valuea | ||
|---|---|---|---|
| AMI Order Set, N=3,531b | A La Carte Orders, N=2,348b | ||
| |||
| Age, y, median (meanSD) | 70 (69.413.8) | 70 (69.213.8) | 0.5603 |
| Age (% >65 years) | 2,134 (60.4%) | 1,415 (60.3%) | 0.8949 |
| Sex (% male) | 2,202 (62.4%) | 1,451 (61.8%) | 0.6620 |
| STEMI (% with)c | 166 (4.7%) | 369 (15.7%) | <0.0001 |
| Troponin I (% missing) | 111 (3.1%) | 151 (6.4%) | <0.0001 |
| Troponin I median (meanSD) | 0.57 (3.08.2) | 0.27 (2.58.9) | 0.0651 |
| Charlson score median (meanSD)d | 2.0 (2.51.5) | 2.0 (2.71.6) | <0.0001 |
| COPS2, median (meanSD)e | 14.0 (29.831.7) | 17.0 (34.334.4) | <0.0001 |
| LAPS2, median (meanSD)e | 0.0 (35.643.5) | 27.0 (40.948.1) | <0.0001 |
| Length of stay in ED, h, median (meanSD) | 5.7 (5.93.0) | 5.7 (5.43.1) | <0.0001 |
| Patients receiving aspirin within 24 hoursf | 3,470 (98.3%) | 2,202 (93.8%) | <0.0001 |
| Patients receiving anticoagulation therapyf | 2,886 (81.7%) | 1,846 (78.6%) | 0.0032 |
| Patients receiving ‐blockersf | 3,196 (90.5%) | 1,926 (82.0%) | <0.0001 |
| Patients receiving ACE inhibitors or ARBsf | 2,395 (67.8%) | 1,244 (53.0%) | <0.0001 |
| Patients receiving statinsf | 3,337 (94.5%) | 1,975 (84.1%) | <0.0001 |
| Patient received 1 or more therapies | 3,531 (100.0%) | 2,330 (99.2%) | <0.0001 |
| Patient received 2 or more therapies | 3,521 (99.7%) | 2,266 (96.5%) | <0.0001 |
| Patient received 3 or more therapies | 3,440 (97.4%) | 2,085 (88.8%) | <0.0001 |
| Patient received 4 or more therapies | 3,015 (85.4%) | 1,646 (70.1%) | <0.0001 |
| Patient received all 5 therapies | 1,777 (50.3%) | 866 (35.9%) | <0.0001 |
| Predicted mortality risk, %, median, (meanSD)f | 0.86 (3.27.4) | 1.19 (4.810.8) | <0.0001 |
| Full code at time of hospital entry (%)g | 3,041 (86.1%) | 2,066 (88.0%) | 0.0379 |
| Admitted to ICU (%)i | |||
| Direct admit | 826 (23.4%) | 567 (24.2%) | 0.5047 |
| Unplanned transfer | 222 (6.3%) | 133 (5.7%) | 0.3262 |
| Ever | 1,283 (36.3%) | 1,169 (49.8%) | <0.0001 |
| Length of stay, h, median (meanSD) | 68.3 (109.4140.9) | 68.9 (113.8154.3) | 0.2615 |
| Inpatient mortality (%) | 124 (3.5%) | 153 (6.5%) | <0.0001 |
| 30‐day mortality (%) | 200 (5.7%) | 199 (8.5%) | <0.0001 |
| All‐cause rehospitalization within 30 days (%) | 576 (16.3%) | 401 (17.1%) | 0.4398 |
| Cardiac catheterization procedure referral timing | |||
| 1 day preadmission to discharge | 2,018 (57.2%) | 1,348 (57.4%) | 0.1638 |
| 2 days preadmission or earlier | 97 (2.8%) | 87 (3.7%) | |
| After discharge | 149 (4.2%) | 104 (4.4%) | |
| No referral | 1,267 (35.9%) | 809 (34.5%) | |
Table 2 shows the result of a logistic regression model in which the dependent variable was inpatient mortality and either the 5 evidence‐based therapies or the total number of evidence‐based therapies are the dependent variables. ‐blocker, statin, and ACE inhibitor or ARB therapies all had a protective effect on mortality, with odds ratios ranging from 0.48 (95% confidence interval [CI]: 0.36‐0.64), 0.63 (95% CI: 0.45‐0.89), and 0.40 (95% CI: 0.30‐0.53), respectively. An increased number of therapies also had a beneficial effect on inpatient mortality, with patients having 3 or more of the evidence‐based therapies showing an adjusted odds ratio (AOR) of 0.49 (95% CI: 0.33‐0.73), 4 or more therapies an AOR of 0.29 (95% CI: 0.20‐0.42), and 0.17 (95% CI: 0.11‐0.25) for 5 or more therapies.
| Multiple Therapies Effect | Individual Therapies Effect | |||
|---|---|---|---|---|
| Outcome | Death | Death | ||
| Number of outcomes | 277 | 277 | ||
| AORa | 95% CIb | AORa | 95% CIb | |
| ||||
| Age in years | ||||
| 1839 | Ref | Ref | ||
| 4064 | 1.02 | (0.147.73) | 1.01 | (0.137.66) |
| 6584 | 4.05 | (0.5529.72) | 3.89 | (0.5328.66) |
| 85+ | 4.99 | (0.6737.13) | 4.80 | (0.6435.84) |
| Sex | ||||
| Female | Ref | |||
| Male | 1.05 | (0.811.37) | 1.07 | (0.821.39) |
| STEMIc | ||||
| Absent | Ref | Ref | ||
| Present | 4.00 | (2.755.81) | 3.86 | (2.645.63) |
| Troponin I | ||||
| 0.1 ng/ml | Ref | Ref | ||
| >0.1 ng/ml | 1.01 | (0.721.42) | 1.02 | (0.731.43) |
| COPS2d (AOR per 10 points) | 1.05 | (1.011.08) | 1.04 | (1.011.08) |
| LAPS2d (AOR per 10 points) | 1.09 | (1.061.11) | 1.09 | (1.061.11) |
| ED LOSe (hours) | ||||
| <6 | Ref | Ref | ||
| 67 | 0.74 | (0.531.03) | 0.76 | (0.541.06) |
| >=12 | 0.82 | (0.391.74) | 0.83 | (0.391.78) |
| Code Statusf | ||||
| Full Code | Ref | |||
| Not Full Code | 1.08 | (0.781.49) | 1.09 | (0.791.51) |
| Cardiac procedure referral | ||||
| None during stay | Ref | |||
| 1 day pre adm until discharge | 0.40 | (0.290.54) | 0.39 | (0.280.53) |
| Number of therapies received | ||||
| 2 or less | Ref | |||
| 3 | 0.49 | (0.330.73) | ||
| 4 | 0.29 | (0.200.42) | ||
| 5 | 0.17 | (0.110.25) | ||
| Aspirin therapy | 0.80 | (0.491.32) | ||
| Anticoagulation therapy | 0.86 | (0.641.16) | ||
| Beta Blocker therapy | 0.48 | (0.360.64) | ||
| Statin therapy | 0.63 | (0.450.89) | ||
| ACE inhibitors or ARBs | 0.40 | (0.300.53) | ||
| C Statistic | 0.814 | 0.822 | ||
| Hosmer‐Lemeshow p value | 0.509 | 0.934 | ||
Table 3 shows that the use of the AMI‐OS is protective, with an AOR of 0.59 and a 95% CI of 0.45‐0.76. Table 3 also shows that the most potent predictors were comorbidity burden (AOR: 1.07, 95% CI: 1.03‐1.10 per 10 COPS2 points), severity of illness (AOR: 1.09, 95% CI: 1.07‐1.12 per 10 LAPS2 points), STEMI (AOR: 3.86, 95% CI: 2.68‐5.58), and timing of cardiac catheterization referral occurring immediately prior to or during the admission (AOR: 0.37, 95% CI: 0.27‐0.51). The statistical significance of the AMI‐OS effect disappears when both AMI‐OS and the individual therapies are included in the same model (see Supporting Information, Appendices 15, in the online version of this article).
| Outcome | Death | |
|---|---|---|
| Number of outcomes | 277 | |
| AORa | 95% CIb | |
| ||
| Age in years | ||
| 1839 | Ref | |
| 4064 | 1.16 | (0.158.78) |
| 6584 | 4.67 | (0.6334.46) |
| 85+ | 5.45 | (0.7340.86) |
| Sex | ||
| Female | Ref | |
| Male | 1.05 | (0.811.36) |
| STEMIc | ||
| Absent | Ref | |
| Present | 3.86 | (2.685.58) |
| Troponin I | ||
| 0.1 ng/ml | Ref | |
| >0.1 ng/ml | 1.16 | (0.831.62) |
| COPS2d (AOR per 10 points) | 1.07 | (1.031.10) |
| LAPS2d (AOR per 10 points) | 1.09 | (1.071.12) |
| ED LOSe (hours) | ||
| <6 | Ref | |
| 67 | 0.72 | (0.521.00) |
| >=12 | 0.70 | (0.331.48) |
| Code statusf | ||
| Full code | Ref | |
| Not full code | 1.22 | (0.891.68) |
| Cardiac procedure referral | ||
| None during stay | Ref | |
| 1 day pre adm until discharge | 0.37 | (0.270.51) |
| Order set employedg | ||
| No | Ref | |
| Yes | 0.59 | (0.450.76) |
| C Statistic | 0.792 | |
| Hosmer‐Lemeshow p value | 0.273 | |
Table 4 shows separately the average treatment effect (ATE) and average treatment effect for the treated (ATT) of AMI‐OS and of increasing number of therapies on other outcomes (30‐day mortality, LOS, and readmission). Both the ATE and ATT show that the use of the AMI‐OS was significantly protective with respect to mortality and total hospital LOS but not significant with respect to readmission. The effect of the number of therapies on mortality is significantly higher with increasing number of therapies. For example, patients who received 5 therapies had an average treatment effect on 30‐day inpatient mortality of 0.23 (95% CI: 0.15‐0.35) compared to 0.64 (95% CI: 0.43‐0.96) for 3 therapies, almost a 3‐fold difference. The effects of increasing number of therapies were not significant for LOS or readmission. A sensitivity analysis in which the 535 STEMI patients were removed showed essentially the same results, so it is not reported here.
| Outcome | Order Seta | 3 Therapiesb | 4 Therapiesb | 5 Therapiesb |
|---|---|---|---|---|
| ||||
| Average treatment effectc | ||||
| Inpatient mortality | 0.67 (0.520.86) | 0.64 (0.430.96) | 0.37 (0.250.54) | 0.23 (0.150.35) |
| 30‐day mortality | 0.77 (0.620.96) | 0.68 (0.480.98) | 0.34 (0.240.48) | 0.26 (0.180.37) |
| Readmission | 1.03 (0.901.19) | 1.20 (0.871.66) | 1.19 (0.881.60) | 1.30 (0.961.76) |
| LOS, ratio of the geometric means | 0.91 (0.870.95) | 1.16 (1.031.30) | 1.17 (1.051.30) | 1.12 (1.001.24) |
| Average treatment effect on the treatedd | ||||
| Inpatient mortality | 0.69 (0.520.92) | 0.35 (0.130.93) | 0.17 (0.070.43) | 0.08 (0.030.20) |
| 30‐day mortality | 0.84 (0.661.06) | 0.35 (0.150.79) | 0.17 (0.070.37) | 0.09 (0.040.20) |
| Readmission | 1.02 (0.871.20) | 1.39 (0.852.26) | 1.36 (0.882.12) | 1.23 (0.801.89) |
| LOS, ratio of the geometric meanse | 0.92 (0.870.97) | 1.18 (1.021.37) | 1.16 (1.011.33) | 1.04 (0.911.19) |
To further elucidate possible reasons why physicians did not use the AMI‐OS, the lead author reviewed 105 randomly selected records where the AMI‐OS was not used, 5 records from each of the 21 study hospitals. This review found that in 36% of patients, the AMI‐OS was not used because emergent catheterization or transfer to a facility with percutaneous coronary intervention capability occurred. Presence of other significant medical conditions, including critical illness, was the reason in 17% of these cases, patient or family refusal of treatments in 8%, issues around end‐of‐life care in 3%, and specific medical contraindications in 1%. In the remaining 34%, no reason for not using the AMI‐OS could be identified.
DISCUSSION
We evaluated the use of an evidence‐based electronic AMI‐OS embedded in a comprehensive EMR and found that it was beneficial. Its use was associated with increased adherence to evidence‐based therapies, which in turn were associated with improved outcomes. Using data from a large cohort of hospitalized AMI patients in 21 community hospitals, we were able to use risk adjustment that included physiologic illness severity to adjust for baseline mortality risk. Patients in whom the AMI‐OS was employed tended to be at lower risk; nonetheless, after controlling for confounding variables and adjusting for bias using propensity scores, the AMI‐OS was associated with increased use of evidence‐based therapies and decreased mortality. Most importantly, it appears that the benefits of the OS were not just due to increased receipt of individual recommended therapies, but to increased concurrent receipt of multiple recommended therapies.
Modern EMRs have great potential for significant improvements in the quality, efficiency, and safety of care provided,[36] and our study highlights this potential. However, a number of important limitations to our study must be considered. Although we had access to a very rich dataset, we could not control for all possible confounders, and our risk adjustment cannot match the level of information available to clinicians. In particular, the measurements available to us with respect to cardiac risk are limited. Thus, we have to recognize that the strength of our findings does not approximate that of a randomized trial, and one would expect that the magnitude of the beneficial association would fall under more controlled conditions. Resource limitations also did not permit us to gather more time course data (eg, sequential measurements of patient instability, cardiac damage, or use of recommended therapies), which could provide a better delineation of differences in both processes and outcomes.
Limitations also exist to the generalizability of the use of order sets in other settings that go beyond the availability of a comprehensive EMR. Our study population was cared for in a setting with an unusually high level of integration.[1] For example, KPNC has an elaborate administrative infrastructure for training in the use of the EMR as well as ensuring that order sets are not just evidence‐based, but that they are perceived by clinicians to be of significant value. This infrastructure, established to ensure physician buy‐in, may not be easy to replicate in smaller or less‐integrated settings. Thus, it is conceivable that factors other than the degree of support during the EMR deployments can affect rates of order set use.
Although our use of counterfactual methods included illness severity (LAPS2) and longitudinal comorbidity burden (COPS2), which are not yet available outside highly integrated delivery services employing comprehensive EMRs, it is possible they are insufficient. We cannot exclude the possibility that other biases or patient characteristics were present that led clinicians to preferentially employ the electronic order set in some patients but not in others. One could also argue that future studies should consider using overall adherence to recommended AMI treatment guidelines as a risk adjustment tool that would permit one to analyze what other factors may be playing a role in residual differences in patient outcomes. Last, one could object to our inclusion of STEMI patients; however, this was not a study on optimum treatment strategies for STEMI patients. Rather, it was a study on the impact on AMI outcomes of a specific component of computerized order entry outside the research setting.
Despite these limitations, we believe that our findings provide strong support for the continued use of electronic evidence‐based order sets in the inpatient medical setting. Once the initial implementation of a comprehensive EMR has occurred, deployment of these electronic order sets is a relatively inexpensive but effective method to foster compliance with evidence‐based care.
Future research in healthcare information technology can take a number of directions. One important area, of course, revolves around ways to promote enhanced physician adoption of EMRs. Our audit of records where the AMI‐OS was not used found that specific reasons for not using the order set (eg, treatment refusals, emergent intervention) were present in two‐thirds of the cases. This suggests that future analyses of adherence involving EMRs and CPOE implementation should take a more nuanced look at how order entry is actually enabled. It may be that understanding how order sets affect care enhances clinician acceptance and thus could serve as an incentive to EMR adoption. However, once an EMR is adopted, a need exists to continue evaluations such as this because, ultimately, the gold standard should be improved patient care processes and better outcomes for patients.
Acknowledgement
The authors give special thanks to Dr. Brian Hoberman for sponsoring this work, Dr. Alan S. Go for providing assistance with obtaining copies of electrocardiograms for review, Drs. Tracy Lieu and Vincent Liu for reviewing the manuscript, and Ms. Rachel Lesser for formatting the manuscript.
Disclosures: This work was supported by The Permanente Medical Group, Inc. and Kaiser Foundation Hospitals, Inc. The algorithms used to extract data and perform risk adjustment were developed with funding from the Sidney Garfield Memorial Fund (Early Detection of Impending Physiologic Deterioration in Hospitalized Patients, 1159518), the Agency for Healthcare Quality and Research (Rapid Clinical Snapshots From the EMR Among Pneumonia Patients, 1R01HS018480‐01), and the Gordon and Betty Moore Foundation (Early Detection of Impending Physiologic Deterioration: Electronic Early Warning System).
- , , , , , . Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165.
- , , , et al. Twenty‐two‐year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125(15):1848–1857.
- , , , et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220.
- , , , et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157.
- , , , et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51(2):210–247.
- , , , , , . Association between adoption of evidence‐based treatment and survival for patients with ST‐elevation myocardial infarction. JAMA. 2011;305(16):1677–1684.
- , , , et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST‐elevation myocardial infarction. JAMA. 2012;308(10):998–1006.
- , , , et al. Changes in myocardial infarction guideline adherence as a function of patient risk: an end to paradoxical care? J Am Coll Cardiol. 2011;58(17):1760–1765.
- , , , . Care in U.S. hospitals—the Hospital Quality Alliance program. N Engl J Med. 2005;353(3):265–274.
- , , et al. Challenges in the treatment of NSTEMI patients at high risk for both ischemic and bleeding events: insights from the ACTION Registry‐GWTG. J Am Coll Cardiol. 2011;57:E913–E913.
- , , , , . Quality of care in U.S. hospitals as reflected by standardized measures, 2002–2004. N Engl J Med. 2005;353(3):255–264.
- , , . Guideline‐based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction. J Am Coll Cardiol. 2005;46(7):1242–1248.
- , , , et al. Impact of a standardized heart failure order set on mortality, readmission, and quality and costs of care. Int J Qual Health Care. 2010;22(6):437–444.
- . Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127(8 pt 2):719–724.
- , , , , , . Risk adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , . Length of stay predictions: improvements through the use of automated laboratory and comorbidity variables. Med Care. 2010;48(8):739–744.
- , , , , , . Intra‐hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74–80.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- International Classification of Diseases, 9th Revision‐Clinical Modification. 4th ed. 3 Vols. Los Angeles, CA: Practice Management Information Corporation; 2006.
- , , , et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685–2692.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , , . Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated healthcare delivery system. Med Care. 2013;51(5):446–453.
- , , . Effect of choice of estimation method on inter‐hospital mortality rate comparisons. Med Care. 2010;48(5):456–485.
- , , , . The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798–803.
- , , , , . Derivation and validation of a model to predict daily risk of death in hospital. Med Care. 2011;49(8):734–743.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- . Introduction to Statistical Mediation Analysis. New York, NY: Lawrence Erlbaum Associates; 2008.
- . Nonparametric estimation of average treatment effects under exogenity: a review. Rev Econ Stat. 2004;86:25.
- . Design of Observational Studies. New York, NY: Springer Science+Business Media; 2010.
- . Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:24.
- , , . Estimation of regression coefficients when some regressors are not always observed. J Am Stat Assoc. 1994(89):846–866.
- , . Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960.
- . Discussing hidden bias in observational studies. Ann Intern Med. 1991;115(11):901–905.
- . Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17(19):2265–2281.
- , , . A method/macro based on propensity score and Mahalanobis distance to reduce bias in treatment comparison in observational study, 2005. www.lexjansen.com/pharmasug/2006/publichealthresearch/pr05.pdf. Accessed on September 14, 2013.
- . Using health information technology to improve health care. Arch Intern Med. 2012;172(22):1728–1730.
Although the prevalence of coronary heart disease and death from acute myocardial infarction (AMI) have declined steadily, about 935,000 heart attacks still occur annually in the United States, with approximately one‐third of these being fatal.[1, 2, 3] Studies have demonstrated decreased 30‐day and longer‐term mortality in AMI patients who receive evidence‐based treatment, including aspirin, ‐blockers, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), anticoagulation therapy, and statins.[4, 5, 6, 7] Despite clinical practice guidelines (CPGs) outlining evidence‐based care and considerable efforts to implement processes that improve patient outcomes, delivery of effective therapy remains suboptimal.[8] For example, the Hospital Quality Alliance Program[9] found that in AMI patients, use of aspirin on admission was only 81% to 92%, ‐blocker on admission 75% to 85%, and ACE inhibitors for left ventricular dysfunction 71% to 74%.
Efforts to increase adherence to CPGs and improve patient outcomes in AMI have resulted in variable degrees of success. They include promotion of CPGs,[4, 5, 6, 7] physician education with feedback, report cards, care paths, registries,[10] Joint Commission standardized measures,[11] and paper checklists or order sets (OS).[12, 13]
In this report, we describe the association between use of an evidence‐based, electronic OS for AMI (AMI‐OS) and better adherence to CPGs. This AMI‐OS was implemented in the inpatient electronic medical records (EMRs) of a large integrated healthcare delivery system, Kaiser Permanente Northern California (KPNC). The purpose of our investigation was to determine (1) whether use of the AMI‐OS was associated with improved AMI processes and patient outcomes, and (2) whether these associations persisted after risk adjustment using a comprehensive severity of illness scoring system.
MATERIALS AND METHODS
This project was approved by the KPNC institutional review board.
Under a mutual exclusivity arrangement, salaried physicians of The Permanente Medical Group, Inc., care for 3.4 million Kaiser Foundation Health Plan, Inc. members at facilities owned by Kaiser Foundation Hospitals, Inc. All KPNC facilities employ the same information systems with a common medical record number and can track care covered by the plan but delivered elsewhere.[14] Our setting consisted of 21 KPNC hospitals described in previous reports,[15, 16, 17, 18] using the same commercially available EMR system that includes computerized physician order entry (CPOE). Deployment of the customized inpatient Epic EMR (
In this EMR's CPOE, physicians have options to select individual orders (a la carte) or they can utilize an OS, which is a collection of the most appropriate orders associated with specific diagnoses, procedures, or treatments. The evidence‐based AMI‐OS studied in this project was developed by a multidisciplinary team (for detailed components see Supporting Appendix 1Appendix 5 in the online version of this article).
Our study focused on the first set of hospital admission orders for patients with AMI. The study sample consisted of patients meeting these criteria: (1) age 18 years at admission; (2) admitted to a KPNC hospital for an overnight stay between September 28, 2008 and December 31, 2010; (3) principal diagnosis was AMI (International Classification of Diseases, 9th Revision [ICD‐9][19] codes 410.00, 01, 10, 11, 20, 21, 30, 31, 40, 41, 50, 51, 60, 61, 70, 71, 80, 90, and 91); and (4) KPHC had been operational at the hospital for at least 3 months to be included (for assembly descriptions see Supporting Appendices 15 in the online version of this article). At the study hospitals, troponin I was measured using the Beckman Access AccuTnI assay (Beckman Coulter, Inc., Brea, CA), whose upper reference limit (99th percentile) is 0.04 ng/mL. We excluded patients initially hospitalized for AMI at a non‐KPNC site and transferred into a study hospital.
The data processing methods we employed have been detailed elsewhere.[14, 15, 17, 20, 21, 22] The dependent outcome variables were total hospital length of stay, inpatient mortality, 30‐day mortality, and all‐cause rehospitalization within 30 days of discharge. Linked state mortality data were unavailable for the entire study period, so we ascertained 30‐day mortality based on the combination of KPNC patient demographic data and publicly available Social Security Administration decedent files. We ascertained rehospitalization by scanning KPNC hospitalization databases, which also track out‐of‐plan use.
The dependent process variables were use of aspirin within 24 hours of admission, ‐blockers, anticoagulation, ACE inhibitors or ARBs, and statins. The primary independent variable of interest was whether or not the admitting physician employed the AMI‐OS when admission orders were entered. Consequently, this variable is dichotomous (AMI‐OS vs a la carte).
We controlled for acute illness severity and chronic illness burden using a recent modification[22] of an externally validated risk‐adjustment system applicable to all hospitalized patients.[15, 16, 23, 24, 25] Our methodology included vital signs, neurological status checks, and laboratory test results obtained in the 72 hours preceding hospital admission; comorbidities were captured longitudinally using data from the year preceding hospitalization (for comparison purposes, we also assigned a Charlson Comorbidity Index score[26]).
End‐of‐life care directives are mandatory on admission at KPNC hospitals. Physicians have 4 options: full code, partial code, do not resuscitate, and comfort care only. Because of small numbers in some categories, we collapsed these 4 categories into full code and not full code. Because patients' care directives may change, we elected to capture the care directive in effect when a patient first entered a hospital unit other than the emergency department (ED).
Two authors (M.B., P.C.L.), one of whom is a board‐certified cardiologist, reviewed all admission electrocardiograms and made a consensus determination as to whether or not criteria for ST‐segment elevation myocardial infarction (STEMI) were present (ie, new ST‐segment elevation or left bundle branch block); we also reviewed the records of all patients with missing troponin I data to confirm the AMI diagnosis.
Statistical Methods
We performed unadjusted comparisons between AMI‐OS and nonAMI‐OS patients using the t test or the [2] test, as appropriate.
We hypothesized that the AMI‐OS plays a mediating role on patient outcomes through its effect on adherence to recommended treatment. We evaluated this hypothesis for inpatient mortality by first fitting a multivariable logistic regression model for inpatient mortality as the outcome and either the 5 evidence‐based therapies or the total number of evidence‐based therapies used (ranging from 02, 3, 4, or 5) as the dependent variable controlling for age, gender, presence of STEMI, troponin I, comorbidities, illness severity, ED length of stay (LOS), care directive status, and timing of cardiac catheterization referral as covariates to confirm the protective effect of these therapies on mortality. We then used the same model to estimate the effect of AMI‐OS on inpatient mortality, substituting the therapies with AMI‐OS as the dependent variable and using the same covariates. Last, we included both the therapies and the AMI‐OS in the model to evaluate their combined effects.[27]
We used 2 different methods to estimate the effects of AMI‐OS and number of therapies provided on the outcomes while adjusting for observed baseline differences between the 2 groups of patients: propensity risk score matching, which estimates the average treatment effect for the treated,[28, 29] and inverse probability of treatment weighting, which is used to estimate the average treatment effect.[30, 31, 32] The propensity score was defined as the probability of receiving the intervention for a patient with specific predictive factors.[33, 34] We computed a propensity score for each patient by using logistic regression, with the dependent variable being receipt of AMI‐OS and the independent variables being the covariates used for the multivariate logistic regression as well as ICD‐9 code for final diagnosis. We calculated the Mahalanobis distance between patients who received AMI‐OS (cases) and patients who did not received AMI‐OS (controls) using the same set of covariates. We matched each case to a single control within the same facility based on the nearest available Mahalanobis metric matching within calipers defied as the maximum width of 0.2 standard deviations of the logit of the estimated propensity score.[29, 35] We estimated the odds ratios for the binary dependent variables based on a conditional logistic regression model to account for the matched pairs design.[28] We used a generalized linear model with the log‐transformed LOS as the outcome to estimate the ratio of the LOS geometric mean of the cases to the controls. We calculated the relative risk for patients receiving AMI‐OS via the inverse probability weighting method by first defining a weight for each patient. [We assigned a weight of 1/psi to patients who received the AMI‐OS and a weight of 1/(1psi) to patients who did not receive the AMI‐OS, where psi denotes the propensity score for patient i]. We used a logistic regression model for the binary dependent variables with the same set of covariates described above to estimate the adjusted odds ratios while weighting each observation by its corresponding weight. Last, we used a weighted generalized linear model to estimate the AMI‐OS effect on the log‐transformed LOS.
RESULTS
Table 1 summarizes the characteristics of the 5879 patients. It shows that AMI‐OS patients were more likely to receive evidence‐based therapies for AMI (aspirin, ‐blockers, ACE inhibitors or ARBs, anticoagulation, and statins) and had a 46% lower mortality rate in hospital (3.51 % vs 6.52%) and 33% lower rate at 30 days (5.66% vs 8.48%). AMI‐OS patients were also found to be at lower risk for an adverse outcome than nonAMI‐OS patients. The AMI‐OS patients had lower peak troponin I values, severity of illness (lower Laboratory‐Based Acute Physiology Score, version 2 [LAPS2] scores), comorbidity burdens (lower Comorbidity Point Score, version 2 [COPS2] and Charlson scores), and global predicted mortality risk. AMI‐OS patients were also less likely to have required intensive care. AMI‐OS patients were at higher risk of death than nonAMI‐OS patients with respect to only 1 variable (being full code at the time of admission), but although this difference was statistically significant, it was of minor clinical impact (86% vs 88%).
| Patients Initially Managed Using | P Valuea | ||
|---|---|---|---|
| AMI Order Set, N=3,531b | A La Carte Orders, N=2,348b | ||
| |||
| Age, y, median (meanSD) | 70 (69.413.8) | 70 (69.213.8) | 0.5603 |
| Age (% >65 years) | 2,134 (60.4%) | 1,415 (60.3%) | 0.8949 |
| Sex (% male) | 2,202 (62.4%) | 1,451 (61.8%) | 0.6620 |
| STEMI (% with)c | 166 (4.7%) | 369 (15.7%) | <0.0001 |
| Troponin I (% missing) | 111 (3.1%) | 151 (6.4%) | <0.0001 |
| Troponin I median (meanSD) | 0.57 (3.08.2) | 0.27 (2.58.9) | 0.0651 |
| Charlson score median (meanSD)d | 2.0 (2.51.5) | 2.0 (2.71.6) | <0.0001 |
| COPS2, median (meanSD)e | 14.0 (29.831.7) | 17.0 (34.334.4) | <0.0001 |
| LAPS2, median (meanSD)e | 0.0 (35.643.5) | 27.0 (40.948.1) | <0.0001 |
| Length of stay in ED, h, median (meanSD) | 5.7 (5.93.0) | 5.7 (5.43.1) | <0.0001 |
| Patients receiving aspirin within 24 hoursf | 3,470 (98.3%) | 2,202 (93.8%) | <0.0001 |
| Patients receiving anticoagulation therapyf | 2,886 (81.7%) | 1,846 (78.6%) | 0.0032 |
| Patients receiving ‐blockersf | 3,196 (90.5%) | 1,926 (82.0%) | <0.0001 |
| Patients receiving ACE inhibitors or ARBsf | 2,395 (67.8%) | 1,244 (53.0%) | <0.0001 |
| Patients receiving statinsf | 3,337 (94.5%) | 1,975 (84.1%) | <0.0001 |
| Patient received 1 or more therapies | 3,531 (100.0%) | 2,330 (99.2%) | <0.0001 |
| Patient received 2 or more therapies | 3,521 (99.7%) | 2,266 (96.5%) | <0.0001 |
| Patient received 3 or more therapies | 3,440 (97.4%) | 2,085 (88.8%) | <0.0001 |
| Patient received 4 or more therapies | 3,015 (85.4%) | 1,646 (70.1%) | <0.0001 |
| Patient received all 5 therapies | 1,777 (50.3%) | 866 (35.9%) | <0.0001 |
| Predicted mortality risk, %, median, (meanSD)f | 0.86 (3.27.4) | 1.19 (4.810.8) | <0.0001 |
| Full code at time of hospital entry (%)g | 3,041 (86.1%) | 2,066 (88.0%) | 0.0379 |
| Admitted to ICU (%)i | |||
| Direct admit | 826 (23.4%) | 567 (24.2%) | 0.5047 |
| Unplanned transfer | 222 (6.3%) | 133 (5.7%) | 0.3262 |
| Ever | 1,283 (36.3%) | 1,169 (49.8%) | <0.0001 |
| Length of stay, h, median (meanSD) | 68.3 (109.4140.9) | 68.9 (113.8154.3) | 0.2615 |
| Inpatient mortality (%) | 124 (3.5%) | 153 (6.5%) | <0.0001 |
| 30‐day mortality (%) | 200 (5.7%) | 199 (8.5%) | <0.0001 |
| All‐cause rehospitalization within 30 days (%) | 576 (16.3%) | 401 (17.1%) | 0.4398 |
| Cardiac catheterization procedure referral timing | |||
| 1 day preadmission to discharge | 2,018 (57.2%) | 1,348 (57.4%) | 0.1638 |
| 2 days preadmission or earlier | 97 (2.8%) | 87 (3.7%) | |
| After discharge | 149 (4.2%) | 104 (4.4%) | |
| No referral | 1,267 (35.9%) | 809 (34.5%) | |
Table 2 shows the result of a logistic regression model in which the dependent variable was inpatient mortality and either the 5 evidence‐based therapies or the total number of evidence‐based therapies are the dependent variables. ‐blocker, statin, and ACE inhibitor or ARB therapies all had a protective effect on mortality, with odds ratios ranging from 0.48 (95% confidence interval [CI]: 0.36‐0.64), 0.63 (95% CI: 0.45‐0.89), and 0.40 (95% CI: 0.30‐0.53), respectively. An increased number of therapies also had a beneficial effect on inpatient mortality, with patients having 3 or more of the evidence‐based therapies showing an adjusted odds ratio (AOR) of 0.49 (95% CI: 0.33‐0.73), 4 or more therapies an AOR of 0.29 (95% CI: 0.20‐0.42), and 0.17 (95% CI: 0.11‐0.25) for 5 or more therapies.
| Multiple Therapies Effect | Individual Therapies Effect | |||
|---|---|---|---|---|
| Outcome | Death | Death | ||
| Number of outcomes | 277 | 277 | ||
| AORa | 95% CIb | AORa | 95% CIb | |
| ||||
| Age in years | ||||
| 1839 | Ref | Ref | ||
| 4064 | 1.02 | (0.147.73) | 1.01 | (0.137.66) |
| 6584 | 4.05 | (0.5529.72) | 3.89 | (0.5328.66) |
| 85+ | 4.99 | (0.6737.13) | 4.80 | (0.6435.84) |
| Sex | ||||
| Female | Ref | |||
| Male | 1.05 | (0.811.37) | 1.07 | (0.821.39) |
| STEMIc | ||||
| Absent | Ref | Ref | ||
| Present | 4.00 | (2.755.81) | 3.86 | (2.645.63) |
| Troponin I | ||||
| 0.1 ng/ml | Ref | Ref | ||
| >0.1 ng/ml | 1.01 | (0.721.42) | 1.02 | (0.731.43) |
| COPS2d (AOR per 10 points) | 1.05 | (1.011.08) | 1.04 | (1.011.08) |
| LAPS2d (AOR per 10 points) | 1.09 | (1.061.11) | 1.09 | (1.061.11) |
| ED LOSe (hours) | ||||
| <6 | Ref | Ref | ||
| 67 | 0.74 | (0.531.03) | 0.76 | (0.541.06) |
| >=12 | 0.82 | (0.391.74) | 0.83 | (0.391.78) |
| Code Statusf | ||||
| Full Code | Ref | |||
| Not Full Code | 1.08 | (0.781.49) | 1.09 | (0.791.51) |
| Cardiac procedure referral | ||||
| None during stay | Ref | |||
| 1 day pre adm until discharge | 0.40 | (0.290.54) | 0.39 | (0.280.53) |
| Number of therapies received | ||||
| 2 or less | Ref | |||
| 3 | 0.49 | (0.330.73) | ||
| 4 | 0.29 | (0.200.42) | ||
| 5 | 0.17 | (0.110.25) | ||
| Aspirin therapy | 0.80 | (0.491.32) | ||
| Anticoagulation therapy | 0.86 | (0.641.16) | ||
| Beta Blocker therapy | 0.48 | (0.360.64) | ||
| Statin therapy | 0.63 | (0.450.89) | ||
| ACE inhibitors or ARBs | 0.40 | (0.300.53) | ||
| C Statistic | 0.814 | 0.822 | ||
| Hosmer‐Lemeshow p value | 0.509 | 0.934 | ||
Table 3 shows that the use of the AMI‐OS is protective, with an AOR of 0.59 and a 95% CI of 0.45‐0.76. Table 3 also shows that the most potent predictors were comorbidity burden (AOR: 1.07, 95% CI: 1.03‐1.10 per 10 COPS2 points), severity of illness (AOR: 1.09, 95% CI: 1.07‐1.12 per 10 LAPS2 points), STEMI (AOR: 3.86, 95% CI: 2.68‐5.58), and timing of cardiac catheterization referral occurring immediately prior to or during the admission (AOR: 0.37, 95% CI: 0.27‐0.51). The statistical significance of the AMI‐OS effect disappears when both AMI‐OS and the individual therapies are included in the same model (see Supporting Information, Appendices 15, in the online version of this article).
| Outcome | Death | |
|---|---|---|
| Number of outcomes | 277 | |
| AORa | 95% CIb | |
| ||
| Age in years | ||
| 1839 | Ref | |
| 4064 | 1.16 | (0.158.78) |
| 6584 | 4.67 | (0.6334.46) |
| 85+ | 5.45 | (0.7340.86) |
| Sex | ||
| Female | Ref | |
| Male | 1.05 | (0.811.36) |
| STEMIc | ||
| Absent | Ref | |
| Present | 3.86 | (2.685.58) |
| Troponin I | ||
| 0.1 ng/ml | Ref | |
| >0.1 ng/ml | 1.16 | (0.831.62) |
| COPS2d (AOR per 10 points) | 1.07 | (1.031.10) |
| LAPS2d (AOR per 10 points) | 1.09 | (1.071.12) |
| ED LOSe (hours) | ||
| <6 | Ref | |
| 67 | 0.72 | (0.521.00) |
| >=12 | 0.70 | (0.331.48) |
| Code statusf | ||
| Full code | Ref | |
| Not full code | 1.22 | (0.891.68) |
| Cardiac procedure referral | ||
| None during stay | Ref | |
| 1 day pre adm until discharge | 0.37 | (0.270.51) |
| Order set employedg | ||
| No | Ref | |
| Yes | 0.59 | (0.450.76) |
| C Statistic | 0.792 | |
| Hosmer‐Lemeshow p value | 0.273 | |
Table 4 shows separately the average treatment effect (ATE) and average treatment effect for the treated (ATT) of AMI‐OS and of increasing number of therapies on other outcomes (30‐day mortality, LOS, and readmission). Both the ATE and ATT show that the use of the AMI‐OS was significantly protective with respect to mortality and total hospital LOS but not significant with respect to readmission. The effect of the number of therapies on mortality is significantly higher with increasing number of therapies. For example, patients who received 5 therapies had an average treatment effect on 30‐day inpatient mortality of 0.23 (95% CI: 0.15‐0.35) compared to 0.64 (95% CI: 0.43‐0.96) for 3 therapies, almost a 3‐fold difference. The effects of increasing number of therapies were not significant for LOS or readmission. A sensitivity analysis in which the 535 STEMI patients were removed showed essentially the same results, so it is not reported here.
| Outcome | Order Seta | 3 Therapiesb | 4 Therapiesb | 5 Therapiesb |
|---|---|---|---|---|
| ||||
| Average treatment effectc | ||||
| Inpatient mortality | 0.67 (0.520.86) | 0.64 (0.430.96) | 0.37 (0.250.54) | 0.23 (0.150.35) |
| 30‐day mortality | 0.77 (0.620.96) | 0.68 (0.480.98) | 0.34 (0.240.48) | 0.26 (0.180.37) |
| Readmission | 1.03 (0.901.19) | 1.20 (0.871.66) | 1.19 (0.881.60) | 1.30 (0.961.76) |
| LOS, ratio of the geometric means | 0.91 (0.870.95) | 1.16 (1.031.30) | 1.17 (1.051.30) | 1.12 (1.001.24) |
| Average treatment effect on the treatedd | ||||
| Inpatient mortality | 0.69 (0.520.92) | 0.35 (0.130.93) | 0.17 (0.070.43) | 0.08 (0.030.20) |
| 30‐day mortality | 0.84 (0.661.06) | 0.35 (0.150.79) | 0.17 (0.070.37) | 0.09 (0.040.20) |
| Readmission | 1.02 (0.871.20) | 1.39 (0.852.26) | 1.36 (0.882.12) | 1.23 (0.801.89) |
| LOS, ratio of the geometric meanse | 0.92 (0.870.97) | 1.18 (1.021.37) | 1.16 (1.011.33) | 1.04 (0.911.19) |
To further elucidate possible reasons why physicians did not use the AMI‐OS, the lead author reviewed 105 randomly selected records where the AMI‐OS was not used, 5 records from each of the 21 study hospitals. This review found that in 36% of patients, the AMI‐OS was not used because emergent catheterization or transfer to a facility with percutaneous coronary intervention capability occurred. Presence of other significant medical conditions, including critical illness, was the reason in 17% of these cases, patient or family refusal of treatments in 8%, issues around end‐of‐life care in 3%, and specific medical contraindications in 1%. In the remaining 34%, no reason for not using the AMI‐OS could be identified.
DISCUSSION
We evaluated the use of an evidence‐based electronic AMI‐OS embedded in a comprehensive EMR and found that it was beneficial. Its use was associated with increased adherence to evidence‐based therapies, which in turn were associated with improved outcomes. Using data from a large cohort of hospitalized AMI patients in 21 community hospitals, we were able to use risk adjustment that included physiologic illness severity to adjust for baseline mortality risk. Patients in whom the AMI‐OS was employed tended to be at lower risk; nonetheless, after controlling for confounding variables and adjusting for bias using propensity scores, the AMI‐OS was associated with increased use of evidence‐based therapies and decreased mortality. Most importantly, it appears that the benefits of the OS were not just due to increased receipt of individual recommended therapies, but to increased concurrent receipt of multiple recommended therapies.
Modern EMRs have great potential for significant improvements in the quality, efficiency, and safety of care provided,[36] and our study highlights this potential. However, a number of important limitations to our study must be considered. Although we had access to a very rich dataset, we could not control for all possible confounders, and our risk adjustment cannot match the level of information available to clinicians. In particular, the measurements available to us with respect to cardiac risk are limited. Thus, we have to recognize that the strength of our findings does not approximate that of a randomized trial, and one would expect that the magnitude of the beneficial association would fall under more controlled conditions. Resource limitations also did not permit us to gather more time course data (eg, sequential measurements of patient instability, cardiac damage, or use of recommended therapies), which could provide a better delineation of differences in both processes and outcomes.
Limitations also exist to the generalizability of the use of order sets in other settings that go beyond the availability of a comprehensive EMR. Our study population was cared for in a setting with an unusually high level of integration.[1] For example, KPNC has an elaborate administrative infrastructure for training in the use of the EMR as well as ensuring that order sets are not just evidence‐based, but that they are perceived by clinicians to be of significant value. This infrastructure, established to ensure physician buy‐in, may not be easy to replicate in smaller or less‐integrated settings. Thus, it is conceivable that factors other than the degree of support during the EMR deployments can affect rates of order set use.
Although our use of counterfactual methods included illness severity (LAPS2) and longitudinal comorbidity burden (COPS2), which are not yet available outside highly integrated delivery services employing comprehensive EMRs, it is possible they are insufficient. We cannot exclude the possibility that other biases or patient characteristics were present that led clinicians to preferentially employ the electronic order set in some patients but not in others. One could also argue that future studies should consider using overall adherence to recommended AMI treatment guidelines as a risk adjustment tool that would permit one to analyze what other factors may be playing a role in residual differences in patient outcomes. Last, one could object to our inclusion of STEMI patients; however, this was not a study on optimum treatment strategies for STEMI patients. Rather, it was a study on the impact on AMI outcomes of a specific component of computerized order entry outside the research setting.
Despite these limitations, we believe that our findings provide strong support for the continued use of electronic evidence‐based order sets in the inpatient medical setting. Once the initial implementation of a comprehensive EMR has occurred, deployment of these electronic order sets is a relatively inexpensive but effective method to foster compliance with evidence‐based care.
Future research in healthcare information technology can take a number of directions. One important area, of course, revolves around ways to promote enhanced physician adoption of EMRs. Our audit of records where the AMI‐OS was not used found that specific reasons for not using the order set (eg, treatment refusals, emergent intervention) were present in two‐thirds of the cases. This suggests that future analyses of adherence involving EMRs and CPOE implementation should take a more nuanced look at how order entry is actually enabled. It may be that understanding how order sets affect care enhances clinician acceptance and thus could serve as an incentive to EMR adoption. However, once an EMR is adopted, a need exists to continue evaluations such as this because, ultimately, the gold standard should be improved patient care processes and better outcomes for patients.
Acknowledgement
The authors give special thanks to Dr. Brian Hoberman for sponsoring this work, Dr. Alan S. Go for providing assistance with obtaining copies of electrocardiograms for review, Drs. Tracy Lieu and Vincent Liu for reviewing the manuscript, and Ms. Rachel Lesser for formatting the manuscript.
Disclosures: This work was supported by The Permanente Medical Group, Inc. and Kaiser Foundation Hospitals, Inc. The algorithms used to extract data and perform risk adjustment were developed with funding from the Sidney Garfield Memorial Fund (Early Detection of Impending Physiologic Deterioration in Hospitalized Patients, 1159518), the Agency for Healthcare Quality and Research (Rapid Clinical Snapshots From the EMR Among Pneumonia Patients, 1R01HS018480‐01), and the Gordon and Betty Moore Foundation (Early Detection of Impending Physiologic Deterioration: Electronic Early Warning System).
Although the prevalence of coronary heart disease and death from acute myocardial infarction (AMI) have declined steadily, about 935,000 heart attacks still occur annually in the United States, with approximately one‐third of these being fatal.[1, 2, 3] Studies have demonstrated decreased 30‐day and longer‐term mortality in AMI patients who receive evidence‐based treatment, including aspirin, ‐blockers, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), anticoagulation therapy, and statins.[4, 5, 6, 7] Despite clinical practice guidelines (CPGs) outlining evidence‐based care and considerable efforts to implement processes that improve patient outcomes, delivery of effective therapy remains suboptimal.[8] For example, the Hospital Quality Alliance Program[9] found that in AMI patients, use of aspirin on admission was only 81% to 92%, ‐blocker on admission 75% to 85%, and ACE inhibitors for left ventricular dysfunction 71% to 74%.
Efforts to increase adherence to CPGs and improve patient outcomes in AMI have resulted in variable degrees of success. They include promotion of CPGs,[4, 5, 6, 7] physician education with feedback, report cards, care paths, registries,[10] Joint Commission standardized measures,[11] and paper checklists or order sets (OS).[12, 13]
In this report, we describe the association between use of an evidence‐based, electronic OS for AMI (AMI‐OS) and better adherence to CPGs. This AMI‐OS was implemented in the inpatient electronic medical records (EMRs) of a large integrated healthcare delivery system, Kaiser Permanente Northern California (KPNC). The purpose of our investigation was to determine (1) whether use of the AMI‐OS was associated with improved AMI processes and patient outcomes, and (2) whether these associations persisted after risk adjustment using a comprehensive severity of illness scoring system.
MATERIALS AND METHODS
This project was approved by the KPNC institutional review board.
Under a mutual exclusivity arrangement, salaried physicians of The Permanente Medical Group, Inc., care for 3.4 million Kaiser Foundation Health Plan, Inc. members at facilities owned by Kaiser Foundation Hospitals, Inc. All KPNC facilities employ the same information systems with a common medical record number and can track care covered by the plan but delivered elsewhere.[14] Our setting consisted of 21 KPNC hospitals described in previous reports,[15, 16, 17, 18] using the same commercially available EMR system that includes computerized physician order entry (CPOE). Deployment of the customized inpatient Epic EMR (
In this EMR's CPOE, physicians have options to select individual orders (a la carte) or they can utilize an OS, which is a collection of the most appropriate orders associated with specific diagnoses, procedures, or treatments. The evidence‐based AMI‐OS studied in this project was developed by a multidisciplinary team (for detailed components see Supporting Appendix 1Appendix 5 in the online version of this article).
Our study focused on the first set of hospital admission orders for patients with AMI. The study sample consisted of patients meeting these criteria: (1) age 18 years at admission; (2) admitted to a KPNC hospital for an overnight stay between September 28, 2008 and December 31, 2010; (3) principal diagnosis was AMI (International Classification of Diseases, 9th Revision [ICD‐9][19] codes 410.00, 01, 10, 11, 20, 21, 30, 31, 40, 41, 50, 51, 60, 61, 70, 71, 80, 90, and 91); and (4) KPHC had been operational at the hospital for at least 3 months to be included (for assembly descriptions see Supporting Appendices 15 in the online version of this article). At the study hospitals, troponin I was measured using the Beckman Access AccuTnI assay (Beckman Coulter, Inc., Brea, CA), whose upper reference limit (99th percentile) is 0.04 ng/mL. We excluded patients initially hospitalized for AMI at a non‐KPNC site and transferred into a study hospital.
The data processing methods we employed have been detailed elsewhere.[14, 15, 17, 20, 21, 22] The dependent outcome variables were total hospital length of stay, inpatient mortality, 30‐day mortality, and all‐cause rehospitalization within 30 days of discharge. Linked state mortality data were unavailable for the entire study period, so we ascertained 30‐day mortality based on the combination of KPNC patient demographic data and publicly available Social Security Administration decedent files. We ascertained rehospitalization by scanning KPNC hospitalization databases, which also track out‐of‐plan use.
The dependent process variables were use of aspirin within 24 hours of admission, ‐blockers, anticoagulation, ACE inhibitors or ARBs, and statins. The primary independent variable of interest was whether or not the admitting physician employed the AMI‐OS when admission orders were entered. Consequently, this variable is dichotomous (AMI‐OS vs a la carte).
We controlled for acute illness severity and chronic illness burden using a recent modification[22] of an externally validated risk‐adjustment system applicable to all hospitalized patients.[15, 16, 23, 24, 25] Our methodology included vital signs, neurological status checks, and laboratory test results obtained in the 72 hours preceding hospital admission; comorbidities were captured longitudinally using data from the year preceding hospitalization (for comparison purposes, we also assigned a Charlson Comorbidity Index score[26]).
End‐of‐life care directives are mandatory on admission at KPNC hospitals. Physicians have 4 options: full code, partial code, do not resuscitate, and comfort care only. Because of small numbers in some categories, we collapsed these 4 categories into full code and not full code. Because patients' care directives may change, we elected to capture the care directive in effect when a patient first entered a hospital unit other than the emergency department (ED).
Two authors (M.B., P.C.L.), one of whom is a board‐certified cardiologist, reviewed all admission electrocardiograms and made a consensus determination as to whether or not criteria for ST‐segment elevation myocardial infarction (STEMI) were present (ie, new ST‐segment elevation or left bundle branch block); we also reviewed the records of all patients with missing troponin I data to confirm the AMI diagnosis.
Statistical Methods
We performed unadjusted comparisons between AMI‐OS and nonAMI‐OS patients using the t test or the [2] test, as appropriate.
We hypothesized that the AMI‐OS plays a mediating role on patient outcomes through its effect on adherence to recommended treatment. We evaluated this hypothesis for inpatient mortality by first fitting a multivariable logistic regression model for inpatient mortality as the outcome and either the 5 evidence‐based therapies or the total number of evidence‐based therapies used (ranging from 02, 3, 4, or 5) as the dependent variable controlling for age, gender, presence of STEMI, troponin I, comorbidities, illness severity, ED length of stay (LOS), care directive status, and timing of cardiac catheterization referral as covariates to confirm the protective effect of these therapies on mortality. We then used the same model to estimate the effect of AMI‐OS on inpatient mortality, substituting the therapies with AMI‐OS as the dependent variable and using the same covariates. Last, we included both the therapies and the AMI‐OS in the model to evaluate their combined effects.[27]
We used 2 different methods to estimate the effects of AMI‐OS and number of therapies provided on the outcomes while adjusting for observed baseline differences between the 2 groups of patients: propensity risk score matching, which estimates the average treatment effect for the treated,[28, 29] and inverse probability of treatment weighting, which is used to estimate the average treatment effect.[30, 31, 32] The propensity score was defined as the probability of receiving the intervention for a patient with specific predictive factors.[33, 34] We computed a propensity score for each patient by using logistic regression, with the dependent variable being receipt of AMI‐OS and the independent variables being the covariates used for the multivariate logistic regression as well as ICD‐9 code for final diagnosis. We calculated the Mahalanobis distance between patients who received AMI‐OS (cases) and patients who did not received AMI‐OS (controls) using the same set of covariates. We matched each case to a single control within the same facility based on the nearest available Mahalanobis metric matching within calipers defied as the maximum width of 0.2 standard deviations of the logit of the estimated propensity score.[29, 35] We estimated the odds ratios for the binary dependent variables based on a conditional logistic regression model to account for the matched pairs design.[28] We used a generalized linear model with the log‐transformed LOS as the outcome to estimate the ratio of the LOS geometric mean of the cases to the controls. We calculated the relative risk for patients receiving AMI‐OS via the inverse probability weighting method by first defining a weight for each patient. [We assigned a weight of 1/psi to patients who received the AMI‐OS and a weight of 1/(1psi) to patients who did not receive the AMI‐OS, where psi denotes the propensity score for patient i]. We used a logistic regression model for the binary dependent variables with the same set of covariates described above to estimate the adjusted odds ratios while weighting each observation by its corresponding weight. Last, we used a weighted generalized linear model to estimate the AMI‐OS effect on the log‐transformed LOS.
RESULTS
Table 1 summarizes the characteristics of the 5879 patients. It shows that AMI‐OS patients were more likely to receive evidence‐based therapies for AMI (aspirin, ‐blockers, ACE inhibitors or ARBs, anticoagulation, and statins) and had a 46% lower mortality rate in hospital (3.51 % vs 6.52%) and 33% lower rate at 30 days (5.66% vs 8.48%). AMI‐OS patients were also found to be at lower risk for an adverse outcome than nonAMI‐OS patients. The AMI‐OS patients had lower peak troponin I values, severity of illness (lower Laboratory‐Based Acute Physiology Score, version 2 [LAPS2] scores), comorbidity burdens (lower Comorbidity Point Score, version 2 [COPS2] and Charlson scores), and global predicted mortality risk. AMI‐OS patients were also less likely to have required intensive care. AMI‐OS patients were at higher risk of death than nonAMI‐OS patients with respect to only 1 variable (being full code at the time of admission), but although this difference was statistically significant, it was of minor clinical impact (86% vs 88%).
| Patients Initially Managed Using | P Valuea | ||
|---|---|---|---|
| AMI Order Set, N=3,531b | A La Carte Orders, N=2,348b | ||
| |||
| Age, y, median (meanSD) | 70 (69.413.8) | 70 (69.213.8) | 0.5603 |
| Age (% >65 years) | 2,134 (60.4%) | 1,415 (60.3%) | 0.8949 |
| Sex (% male) | 2,202 (62.4%) | 1,451 (61.8%) | 0.6620 |
| STEMI (% with)c | 166 (4.7%) | 369 (15.7%) | <0.0001 |
| Troponin I (% missing) | 111 (3.1%) | 151 (6.4%) | <0.0001 |
| Troponin I median (meanSD) | 0.57 (3.08.2) | 0.27 (2.58.9) | 0.0651 |
| Charlson score median (meanSD)d | 2.0 (2.51.5) | 2.0 (2.71.6) | <0.0001 |
| COPS2, median (meanSD)e | 14.0 (29.831.7) | 17.0 (34.334.4) | <0.0001 |
| LAPS2, median (meanSD)e | 0.0 (35.643.5) | 27.0 (40.948.1) | <0.0001 |
| Length of stay in ED, h, median (meanSD) | 5.7 (5.93.0) | 5.7 (5.43.1) | <0.0001 |
| Patients receiving aspirin within 24 hoursf | 3,470 (98.3%) | 2,202 (93.8%) | <0.0001 |
| Patients receiving anticoagulation therapyf | 2,886 (81.7%) | 1,846 (78.6%) | 0.0032 |
| Patients receiving ‐blockersf | 3,196 (90.5%) | 1,926 (82.0%) | <0.0001 |
| Patients receiving ACE inhibitors or ARBsf | 2,395 (67.8%) | 1,244 (53.0%) | <0.0001 |
| Patients receiving statinsf | 3,337 (94.5%) | 1,975 (84.1%) | <0.0001 |
| Patient received 1 or more therapies | 3,531 (100.0%) | 2,330 (99.2%) | <0.0001 |
| Patient received 2 or more therapies | 3,521 (99.7%) | 2,266 (96.5%) | <0.0001 |
| Patient received 3 or more therapies | 3,440 (97.4%) | 2,085 (88.8%) | <0.0001 |
| Patient received 4 or more therapies | 3,015 (85.4%) | 1,646 (70.1%) | <0.0001 |
| Patient received all 5 therapies | 1,777 (50.3%) | 866 (35.9%) | <0.0001 |
| Predicted mortality risk, %, median, (meanSD)f | 0.86 (3.27.4) | 1.19 (4.810.8) | <0.0001 |
| Full code at time of hospital entry (%)g | 3,041 (86.1%) | 2,066 (88.0%) | 0.0379 |
| Admitted to ICU (%)i | |||
| Direct admit | 826 (23.4%) | 567 (24.2%) | 0.5047 |
| Unplanned transfer | 222 (6.3%) | 133 (5.7%) | 0.3262 |
| Ever | 1,283 (36.3%) | 1,169 (49.8%) | <0.0001 |
| Length of stay, h, median (meanSD) | 68.3 (109.4140.9) | 68.9 (113.8154.3) | 0.2615 |
| Inpatient mortality (%) | 124 (3.5%) | 153 (6.5%) | <0.0001 |
| 30‐day mortality (%) | 200 (5.7%) | 199 (8.5%) | <0.0001 |
| All‐cause rehospitalization within 30 days (%) | 576 (16.3%) | 401 (17.1%) | 0.4398 |
| Cardiac catheterization procedure referral timing | |||
| 1 day preadmission to discharge | 2,018 (57.2%) | 1,348 (57.4%) | 0.1638 |
| 2 days preadmission or earlier | 97 (2.8%) | 87 (3.7%) | |
| After discharge | 149 (4.2%) | 104 (4.4%) | |
| No referral | 1,267 (35.9%) | 809 (34.5%) | |
Table 2 shows the result of a logistic regression model in which the dependent variable was inpatient mortality and either the 5 evidence‐based therapies or the total number of evidence‐based therapies are the dependent variables. ‐blocker, statin, and ACE inhibitor or ARB therapies all had a protective effect on mortality, with odds ratios ranging from 0.48 (95% confidence interval [CI]: 0.36‐0.64), 0.63 (95% CI: 0.45‐0.89), and 0.40 (95% CI: 0.30‐0.53), respectively. An increased number of therapies also had a beneficial effect on inpatient mortality, with patients having 3 or more of the evidence‐based therapies showing an adjusted odds ratio (AOR) of 0.49 (95% CI: 0.33‐0.73), 4 or more therapies an AOR of 0.29 (95% CI: 0.20‐0.42), and 0.17 (95% CI: 0.11‐0.25) for 5 or more therapies.
| Multiple Therapies Effect | Individual Therapies Effect | |||
|---|---|---|---|---|
| Outcome | Death | Death | ||
| Number of outcomes | 277 | 277 | ||
| AORa | 95% CIb | AORa | 95% CIb | |
| ||||
| Age in years | ||||
| 1839 | Ref | Ref | ||
| 4064 | 1.02 | (0.147.73) | 1.01 | (0.137.66) |
| 6584 | 4.05 | (0.5529.72) | 3.89 | (0.5328.66) |
| 85+ | 4.99 | (0.6737.13) | 4.80 | (0.6435.84) |
| Sex | ||||
| Female | Ref | |||
| Male | 1.05 | (0.811.37) | 1.07 | (0.821.39) |
| STEMIc | ||||
| Absent | Ref | Ref | ||
| Present | 4.00 | (2.755.81) | 3.86 | (2.645.63) |
| Troponin I | ||||
| 0.1 ng/ml | Ref | Ref | ||
| >0.1 ng/ml | 1.01 | (0.721.42) | 1.02 | (0.731.43) |
| COPS2d (AOR per 10 points) | 1.05 | (1.011.08) | 1.04 | (1.011.08) |
| LAPS2d (AOR per 10 points) | 1.09 | (1.061.11) | 1.09 | (1.061.11) |
| ED LOSe (hours) | ||||
| <6 | Ref | Ref | ||
| 67 | 0.74 | (0.531.03) | 0.76 | (0.541.06) |
| >=12 | 0.82 | (0.391.74) | 0.83 | (0.391.78) |
| Code Statusf | ||||
| Full Code | Ref | |||
| Not Full Code | 1.08 | (0.781.49) | 1.09 | (0.791.51) |
| Cardiac procedure referral | ||||
| None during stay | Ref | |||
| 1 day pre adm until discharge | 0.40 | (0.290.54) | 0.39 | (0.280.53) |
| Number of therapies received | ||||
| 2 or less | Ref | |||
| 3 | 0.49 | (0.330.73) | ||
| 4 | 0.29 | (0.200.42) | ||
| 5 | 0.17 | (0.110.25) | ||
| Aspirin therapy | 0.80 | (0.491.32) | ||
| Anticoagulation therapy | 0.86 | (0.641.16) | ||
| Beta Blocker therapy | 0.48 | (0.360.64) | ||
| Statin therapy | 0.63 | (0.450.89) | ||
| ACE inhibitors or ARBs | 0.40 | (0.300.53) | ||
| C Statistic | 0.814 | 0.822 | ||
| Hosmer‐Lemeshow p value | 0.509 | 0.934 | ||
Table 3 shows that the use of the AMI‐OS is protective, with an AOR of 0.59 and a 95% CI of 0.45‐0.76. Table 3 also shows that the most potent predictors were comorbidity burden (AOR: 1.07, 95% CI: 1.03‐1.10 per 10 COPS2 points), severity of illness (AOR: 1.09, 95% CI: 1.07‐1.12 per 10 LAPS2 points), STEMI (AOR: 3.86, 95% CI: 2.68‐5.58), and timing of cardiac catheterization referral occurring immediately prior to or during the admission (AOR: 0.37, 95% CI: 0.27‐0.51). The statistical significance of the AMI‐OS effect disappears when both AMI‐OS and the individual therapies are included in the same model (see Supporting Information, Appendices 15, in the online version of this article).
| Outcome | Death | |
|---|---|---|
| Number of outcomes | 277 | |
| AORa | 95% CIb | |
| ||
| Age in years | ||
| 1839 | Ref | |
| 4064 | 1.16 | (0.158.78) |
| 6584 | 4.67 | (0.6334.46) |
| 85+ | 5.45 | (0.7340.86) |
| Sex | ||
| Female | Ref | |
| Male | 1.05 | (0.811.36) |
| STEMIc | ||
| Absent | Ref | |
| Present | 3.86 | (2.685.58) |
| Troponin I | ||
| 0.1 ng/ml | Ref | |
| >0.1 ng/ml | 1.16 | (0.831.62) |
| COPS2d (AOR per 10 points) | 1.07 | (1.031.10) |
| LAPS2d (AOR per 10 points) | 1.09 | (1.071.12) |
| ED LOSe (hours) | ||
| <6 | Ref | |
| 67 | 0.72 | (0.521.00) |
| >=12 | 0.70 | (0.331.48) |
| Code statusf | ||
| Full code | Ref | |
| Not full code | 1.22 | (0.891.68) |
| Cardiac procedure referral | ||
| None during stay | Ref | |
| 1 day pre adm until discharge | 0.37 | (0.270.51) |
| Order set employedg | ||
| No | Ref | |
| Yes | 0.59 | (0.450.76) |
| C Statistic | 0.792 | |
| Hosmer‐Lemeshow p value | 0.273 | |
Table 4 shows separately the average treatment effect (ATE) and average treatment effect for the treated (ATT) of AMI‐OS and of increasing number of therapies on other outcomes (30‐day mortality, LOS, and readmission). Both the ATE and ATT show that the use of the AMI‐OS was significantly protective with respect to mortality and total hospital LOS but not significant with respect to readmission. The effect of the number of therapies on mortality is significantly higher with increasing number of therapies. For example, patients who received 5 therapies had an average treatment effect on 30‐day inpatient mortality of 0.23 (95% CI: 0.15‐0.35) compared to 0.64 (95% CI: 0.43‐0.96) for 3 therapies, almost a 3‐fold difference. The effects of increasing number of therapies were not significant for LOS or readmission. A sensitivity analysis in which the 535 STEMI patients were removed showed essentially the same results, so it is not reported here.
| Outcome | Order Seta | 3 Therapiesb | 4 Therapiesb | 5 Therapiesb |
|---|---|---|---|---|
| ||||
| Average treatment effectc | ||||
| Inpatient mortality | 0.67 (0.520.86) | 0.64 (0.430.96) | 0.37 (0.250.54) | 0.23 (0.150.35) |
| 30‐day mortality | 0.77 (0.620.96) | 0.68 (0.480.98) | 0.34 (0.240.48) | 0.26 (0.180.37) |
| Readmission | 1.03 (0.901.19) | 1.20 (0.871.66) | 1.19 (0.881.60) | 1.30 (0.961.76) |
| LOS, ratio of the geometric means | 0.91 (0.870.95) | 1.16 (1.031.30) | 1.17 (1.051.30) | 1.12 (1.001.24) |
| Average treatment effect on the treatedd | ||||
| Inpatient mortality | 0.69 (0.520.92) | 0.35 (0.130.93) | 0.17 (0.070.43) | 0.08 (0.030.20) |
| 30‐day mortality | 0.84 (0.661.06) | 0.35 (0.150.79) | 0.17 (0.070.37) | 0.09 (0.040.20) |
| Readmission | 1.02 (0.871.20) | 1.39 (0.852.26) | 1.36 (0.882.12) | 1.23 (0.801.89) |
| LOS, ratio of the geometric meanse | 0.92 (0.870.97) | 1.18 (1.021.37) | 1.16 (1.011.33) | 1.04 (0.911.19) |
To further elucidate possible reasons why physicians did not use the AMI‐OS, the lead author reviewed 105 randomly selected records where the AMI‐OS was not used, 5 records from each of the 21 study hospitals. This review found that in 36% of patients, the AMI‐OS was not used because emergent catheterization or transfer to a facility with percutaneous coronary intervention capability occurred. Presence of other significant medical conditions, including critical illness, was the reason in 17% of these cases, patient or family refusal of treatments in 8%, issues around end‐of‐life care in 3%, and specific medical contraindications in 1%. In the remaining 34%, no reason for not using the AMI‐OS could be identified.
DISCUSSION
We evaluated the use of an evidence‐based electronic AMI‐OS embedded in a comprehensive EMR and found that it was beneficial. Its use was associated with increased adherence to evidence‐based therapies, which in turn were associated with improved outcomes. Using data from a large cohort of hospitalized AMI patients in 21 community hospitals, we were able to use risk adjustment that included physiologic illness severity to adjust for baseline mortality risk. Patients in whom the AMI‐OS was employed tended to be at lower risk; nonetheless, after controlling for confounding variables and adjusting for bias using propensity scores, the AMI‐OS was associated with increased use of evidence‐based therapies and decreased mortality. Most importantly, it appears that the benefits of the OS were not just due to increased receipt of individual recommended therapies, but to increased concurrent receipt of multiple recommended therapies.
Modern EMRs have great potential for significant improvements in the quality, efficiency, and safety of care provided,[36] and our study highlights this potential. However, a number of important limitations to our study must be considered. Although we had access to a very rich dataset, we could not control for all possible confounders, and our risk adjustment cannot match the level of information available to clinicians. In particular, the measurements available to us with respect to cardiac risk are limited. Thus, we have to recognize that the strength of our findings does not approximate that of a randomized trial, and one would expect that the magnitude of the beneficial association would fall under more controlled conditions. Resource limitations also did not permit us to gather more time course data (eg, sequential measurements of patient instability, cardiac damage, or use of recommended therapies), which could provide a better delineation of differences in both processes and outcomes.
Limitations also exist to the generalizability of the use of order sets in other settings that go beyond the availability of a comprehensive EMR. Our study population was cared for in a setting with an unusually high level of integration.[1] For example, KPNC has an elaborate administrative infrastructure for training in the use of the EMR as well as ensuring that order sets are not just evidence‐based, but that they are perceived by clinicians to be of significant value. This infrastructure, established to ensure physician buy‐in, may not be easy to replicate in smaller or less‐integrated settings. Thus, it is conceivable that factors other than the degree of support during the EMR deployments can affect rates of order set use.
Although our use of counterfactual methods included illness severity (LAPS2) and longitudinal comorbidity burden (COPS2), which are not yet available outside highly integrated delivery services employing comprehensive EMRs, it is possible they are insufficient. We cannot exclude the possibility that other biases or patient characteristics were present that led clinicians to preferentially employ the electronic order set in some patients but not in others. One could also argue that future studies should consider using overall adherence to recommended AMI treatment guidelines as a risk adjustment tool that would permit one to analyze what other factors may be playing a role in residual differences in patient outcomes. Last, one could object to our inclusion of STEMI patients; however, this was not a study on optimum treatment strategies for STEMI patients. Rather, it was a study on the impact on AMI outcomes of a specific component of computerized order entry outside the research setting.
Despite these limitations, we believe that our findings provide strong support for the continued use of electronic evidence‐based order sets in the inpatient medical setting. Once the initial implementation of a comprehensive EMR has occurred, deployment of these electronic order sets is a relatively inexpensive but effective method to foster compliance with evidence‐based care.
Future research in healthcare information technology can take a number of directions. One important area, of course, revolves around ways to promote enhanced physician adoption of EMRs. Our audit of records where the AMI‐OS was not used found that specific reasons for not using the order set (eg, treatment refusals, emergent intervention) were present in two‐thirds of the cases. This suggests that future analyses of adherence involving EMRs and CPOE implementation should take a more nuanced look at how order entry is actually enabled. It may be that understanding how order sets affect care enhances clinician acceptance and thus could serve as an incentive to EMR adoption. However, once an EMR is adopted, a need exists to continue evaluations such as this because, ultimately, the gold standard should be improved patient care processes and better outcomes for patients.
Acknowledgement
The authors give special thanks to Dr. Brian Hoberman for sponsoring this work, Dr. Alan S. Go for providing assistance with obtaining copies of electrocardiograms for review, Drs. Tracy Lieu and Vincent Liu for reviewing the manuscript, and Ms. Rachel Lesser for formatting the manuscript.
Disclosures: This work was supported by The Permanente Medical Group, Inc. and Kaiser Foundation Hospitals, Inc. The algorithms used to extract data and perform risk adjustment were developed with funding from the Sidney Garfield Memorial Fund (Early Detection of Impending Physiologic Deterioration in Hospitalized Patients, 1159518), the Agency for Healthcare Quality and Research (Rapid Clinical Snapshots From the EMR Among Pneumonia Patients, 1R01HS018480‐01), and the Gordon and Betty Moore Foundation (Early Detection of Impending Physiologic Deterioration: Electronic Early Warning System).
- , , , , , . Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165.
- , , , et al. Twenty‐two‐year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125(15):1848–1857.
- , , , et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220.
- , , , et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157.
- , , , et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51(2):210–247.
- , , , , , . Association between adoption of evidence‐based treatment and survival for patients with ST‐elevation myocardial infarction. JAMA. 2011;305(16):1677–1684.
- , , , et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST‐elevation myocardial infarction. JAMA. 2012;308(10):998–1006.
- , , , et al. Changes in myocardial infarction guideline adherence as a function of patient risk: an end to paradoxical care? J Am Coll Cardiol. 2011;58(17):1760–1765.
- , , , . Care in U.S. hospitals—the Hospital Quality Alliance program. N Engl J Med. 2005;353(3):265–274.
- , , et al. Challenges in the treatment of NSTEMI patients at high risk for both ischemic and bleeding events: insights from the ACTION Registry‐GWTG. J Am Coll Cardiol. 2011;57:E913–E913.
- , , , , . Quality of care in U.S. hospitals as reflected by standardized measures, 2002–2004. N Engl J Med. 2005;353(3):255–264.
- , , . Guideline‐based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction. J Am Coll Cardiol. 2005;46(7):1242–1248.
- , , , et al. Impact of a standardized heart failure order set on mortality, readmission, and quality and costs of care. Int J Qual Health Care. 2010;22(6):437–444.
- . Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127(8 pt 2):719–724.
- , , , , , . Risk adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , . Length of stay predictions: improvements through the use of automated laboratory and comorbidity variables. Med Care. 2010;48(8):739–744.
- , , , , , . Intra‐hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74–80.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- International Classification of Diseases, 9th Revision‐Clinical Modification. 4th ed. 3 Vols. Los Angeles, CA: Practice Management Information Corporation; 2006.
- , , , et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685–2692.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , , . Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated healthcare delivery system. Med Care. 2013;51(5):446–453.
- , , . Effect of choice of estimation method on inter‐hospital mortality rate comparisons. Med Care. 2010;48(5):456–485.
- , , , . The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798–803.
- , , , , . Derivation and validation of a model to predict daily risk of death in hospital. Med Care. 2011;49(8):734–743.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- . Introduction to Statistical Mediation Analysis. New York, NY: Lawrence Erlbaum Associates; 2008.
- . Nonparametric estimation of average treatment effects under exogenity: a review. Rev Econ Stat. 2004;86:25.
- . Design of Observational Studies. New York, NY: Springer Science+Business Media; 2010.
- . Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:24.
- , , . Estimation of regression coefficients when some regressors are not always observed. J Am Stat Assoc. 1994(89):846–866.
- , . Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960.
- . Discussing hidden bias in observational studies. Ann Intern Med. 1991;115(11):901–905.
- . Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17(19):2265–2281.
- , , . A method/macro based on propensity score and Mahalanobis distance to reduce bias in treatment comparison in observational study, 2005. www.lexjansen.com/pharmasug/2006/publichealthresearch/pr05.pdf. Accessed on September 14, 2013.
- . Using health information technology to improve health care. Arch Intern Med. 2012;172(22):1728–1730.
- , , , , , . Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165.
- , , , et al. Twenty‐two‐year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125(15):1848–1857.
- , , , et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220.
- , , , et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157.
- , , , et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51(2):210–247.
- , , , , , . Association between adoption of evidence‐based treatment and survival for patients with ST‐elevation myocardial infarction. JAMA. 2011;305(16):1677–1684.
- , , , et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST‐elevation myocardial infarction. JAMA. 2012;308(10):998–1006.
- , , , et al. Changes in myocardial infarction guideline adherence as a function of patient risk: an end to paradoxical care? J Am Coll Cardiol. 2011;58(17):1760–1765.
- , , , . Care in U.S. hospitals—the Hospital Quality Alliance program. N Engl J Med. 2005;353(3):265–274.
- , , et al. Challenges in the treatment of NSTEMI patients at high risk for both ischemic and bleeding events: insights from the ACTION Registry‐GWTG. J Am Coll Cardiol. 2011;57:E913–E913.
- , , , , . Quality of care in U.S. hospitals as reflected by standardized measures, 2002–2004. N Engl J Med. 2005;353(3):255–264.
- , , . Guideline‐based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction. J Am Coll Cardiol. 2005;46(7):1242–1248.
- , , , et al. Impact of a standardized heart failure order set on mortality, readmission, and quality and costs of care. Int J Qual Health Care. 2010;22(6):437–444.
- . Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127(8 pt 2):719–724.
- , , , , , . Risk adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , . Length of stay predictions: improvements through the use of automated laboratory and comorbidity variables. Med Care. 2010;48(8):739–744.
- , , , , , . Intra‐hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74–80.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- International Classification of Diseases, 9th Revision‐Clinical Modification. 4th ed. 3 Vols. Los Angeles, CA: Practice Management Information Corporation; 2006.
- , , , et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685–2692.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , , . Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated healthcare delivery system. Med Care. 2013;51(5):446–453.
- , , . Effect of choice of estimation method on inter‐hospital mortality rate comparisons. Med Care. 2010;48(5):456–485.
- , , , . The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798–803.
- , , , , . Derivation and validation of a model to predict daily risk of death in hospital. Med Care. 2011;49(8):734–743.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- . Introduction to Statistical Mediation Analysis. New York, NY: Lawrence Erlbaum Associates; 2008.
- . Nonparametric estimation of average treatment effects under exogenity: a review. Rev Econ Stat. 2004;86:25.
- . Design of Observational Studies. New York, NY: Springer Science+Business Media; 2010.
- . Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:24.
- , , . Estimation of regression coefficients when some regressors are not always observed. J Am Stat Assoc. 1994(89):846–866.
- , . Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960.
- . Discussing hidden bias in observational studies. Ann Intern Med. 1991;115(11):901–905.
- . Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17(19):2265–2281.
- , , . A method/macro based on propensity score and Mahalanobis distance to reduce bias in treatment comparison in observational study, 2005. www.lexjansen.com/pharmasug/2006/publichealthresearch/pr05.pdf. Accessed on September 14, 2013.
- . Using health information technology to improve health care. Arch Intern Med. 2012;172(22):1728–1730.
© 2014 Society of Hospital Medicine
Group ‘rewrites rules’ on pluripotency
Credit: Haruko Obokata
Researchers say they have developed a novel technique for inducing pluripotency in somatic cells.
Unlike methods for creating induced pluripotent stem cells (iPSCs), the new process—called stimulus-triggered acquisition of pluripotency (STAP)—does not require the introduction of genetic material.
Instead, adult cells must only be injured in order to revert to a pluripotent state.
The investigators tested STAP in preclinical models and reported the results in both a letter and an article published in Nature.
Inspired by plants
Haruko Obokata, PhD, of the RIKEN Center for Developmental Biology in Japan, and her colleagues said this research was inspired by the ability of a plant callus—a node of plant cells created by injuring an existing plant—to grow into a new plant.
The researchers thought this phenomenon suggested that any somatic cell could be de-differentiated through injury.
To find out, they tested cells derived from mice. The team chose hematopoietic cells positive for CD45 because these are lineage-committed, somatic cells that do not express pluripotency-related markers unless they are reprogrammed.
The investigators stressed the cells almost to the point of death by exposing them to various stimuli in vitro, including trauma, a low-oxygen environment, and a low-pH environment.
Within a few days, the cells had recovered from the stressful stimuli by naturally reverting to a pluripotent state. These stem cells were then able to re-differentiate and mature into any type of cell and grow into any type of tissue, depending on the environment into which they were placed.
“It was really surprising to see that such a remarkable transformation could be triggered simply by stimuli from outside of the cell,” Dr Obokata said.
She and her colleagues found that the low-pH environment was most effective for inducing pluripotency.
“Once again, Japanese scientists have unexpectedly rewritten the rules on making pluripotent cells from adult cells,” said Chris Mason, MD, PhD, of the University College London in the UK, who was not involved in this research.
“In 2006, [Shinya] Yamanaka used 4 genes [to create iPSCs]. And now, the far simpler and quicker route discovered by Obokata . . . requires only transient exposure of adult cells to an acidic solution.”
Growth in mice
To examine the cells’ growth potential in vivo, Dr Obokata and her colleagues used CD45+ cells from GFP+ mice. The team exposed the cells to a low-pH environment and found that, in the days following the stress, the cells reverted back to a pluripotent state.
These stem cells then began growing in spherical clusters, similar to a plant callus. The researchers introduced the cell clusters into the developing embryo of a non-GFP mouse and found the clusters could create GFP+ tissues in all organs tested, thereby confirming that the cells are pluripotent.
The investigators think these findings raise the possibility that unknown cellular functions activated through external stress may set somatic cells free from their current commitment and permit them to revert to their naïve state.
“Our findings suggest that, somehow, through part of a natural repair process, mature cells turn off some of the epigenetic controls that inhibit expression of certain nuclear genes that result in differentiation,” said study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston.
If this process can be replicated in human cells, researchers might one day be able to use a skin biopsy or blood sample to create stem cells specific to each individual. And this could have implications for treating cancers and other diseases.
The investigators are now testing the STAP technique in human cells. ![]()

Credit: Haruko Obokata
Researchers say they have developed a novel technique for inducing pluripotency in somatic cells.
Unlike methods for creating induced pluripotent stem cells (iPSCs), the new process—called stimulus-triggered acquisition of pluripotency (STAP)—does not require the introduction of genetic material.
Instead, adult cells must only be injured in order to revert to a pluripotent state.
The investigators tested STAP in preclinical models and reported the results in both a letter and an article published in Nature.
Inspired by plants
Haruko Obokata, PhD, of the RIKEN Center for Developmental Biology in Japan, and her colleagues said this research was inspired by the ability of a plant callus—a node of plant cells created by injuring an existing plant—to grow into a new plant.
The researchers thought this phenomenon suggested that any somatic cell could be de-differentiated through injury.
To find out, they tested cells derived from mice. The team chose hematopoietic cells positive for CD45 because these are lineage-committed, somatic cells that do not express pluripotency-related markers unless they are reprogrammed.
The investigators stressed the cells almost to the point of death by exposing them to various stimuli in vitro, including trauma, a low-oxygen environment, and a low-pH environment.
Within a few days, the cells had recovered from the stressful stimuli by naturally reverting to a pluripotent state. These stem cells were then able to re-differentiate and mature into any type of cell and grow into any type of tissue, depending on the environment into which they were placed.
“It was really surprising to see that such a remarkable transformation could be triggered simply by stimuli from outside of the cell,” Dr Obokata said.
She and her colleagues found that the low-pH environment was most effective for inducing pluripotency.
“Once again, Japanese scientists have unexpectedly rewritten the rules on making pluripotent cells from adult cells,” said Chris Mason, MD, PhD, of the University College London in the UK, who was not involved in this research.
“In 2006, [Shinya] Yamanaka used 4 genes [to create iPSCs]. And now, the far simpler and quicker route discovered by Obokata . . . requires only transient exposure of adult cells to an acidic solution.”
Growth in mice
To examine the cells’ growth potential in vivo, Dr Obokata and her colleagues used CD45+ cells from GFP+ mice. The team exposed the cells to a low-pH environment and found that, in the days following the stress, the cells reverted back to a pluripotent state.
These stem cells then began growing in spherical clusters, similar to a plant callus. The researchers introduced the cell clusters into the developing embryo of a non-GFP mouse and found the clusters could create GFP+ tissues in all organs tested, thereby confirming that the cells are pluripotent.
The investigators think these findings raise the possibility that unknown cellular functions activated through external stress may set somatic cells free from their current commitment and permit them to revert to their naïve state.
“Our findings suggest that, somehow, through part of a natural repair process, mature cells turn off some of the epigenetic controls that inhibit expression of certain nuclear genes that result in differentiation,” said study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston.
If this process can be replicated in human cells, researchers might one day be able to use a skin biopsy or blood sample to create stem cells specific to each individual. And this could have implications for treating cancers and other diseases.
The investigators are now testing the STAP technique in human cells. ![]()

Credit: Haruko Obokata
Researchers say they have developed a novel technique for inducing pluripotency in somatic cells.
Unlike methods for creating induced pluripotent stem cells (iPSCs), the new process—called stimulus-triggered acquisition of pluripotency (STAP)—does not require the introduction of genetic material.
Instead, adult cells must only be injured in order to revert to a pluripotent state.
The investigators tested STAP in preclinical models and reported the results in both a letter and an article published in Nature.
Inspired by plants
Haruko Obokata, PhD, of the RIKEN Center for Developmental Biology in Japan, and her colleagues said this research was inspired by the ability of a plant callus—a node of plant cells created by injuring an existing plant—to grow into a new plant.
The researchers thought this phenomenon suggested that any somatic cell could be de-differentiated through injury.
To find out, they tested cells derived from mice. The team chose hematopoietic cells positive for CD45 because these are lineage-committed, somatic cells that do not express pluripotency-related markers unless they are reprogrammed.
The investigators stressed the cells almost to the point of death by exposing them to various stimuli in vitro, including trauma, a low-oxygen environment, and a low-pH environment.
Within a few days, the cells had recovered from the stressful stimuli by naturally reverting to a pluripotent state. These stem cells were then able to re-differentiate and mature into any type of cell and grow into any type of tissue, depending on the environment into which they were placed.
“It was really surprising to see that such a remarkable transformation could be triggered simply by stimuli from outside of the cell,” Dr Obokata said.
She and her colleagues found that the low-pH environment was most effective for inducing pluripotency.
“Once again, Japanese scientists have unexpectedly rewritten the rules on making pluripotent cells from adult cells,” said Chris Mason, MD, PhD, of the University College London in the UK, who was not involved in this research.
“In 2006, [Shinya] Yamanaka used 4 genes [to create iPSCs]. And now, the far simpler and quicker route discovered by Obokata . . . requires only transient exposure of adult cells to an acidic solution.”
Growth in mice
To examine the cells’ growth potential in vivo, Dr Obokata and her colleagues used CD45+ cells from GFP+ mice. The team exposed the cells to a low-pH environment and found that, in the days following the stress, the cells reverted back to a pluripotent state.
These stem cells then began growing in spherical clusters, similar to a plant callus. The researchers introduced the cell clusters into the developing embryo of a non-GFP mouse and found the clusters could create GFP+ tissues in all organs tested, thereby confirming that the cells are pluripotent.
The investigators think these findings raise the possibility that unknown cellular functions activated through external stress may set somatic cells free from their current commitment and permit them to revert to their naïve state.
“Our findings suggest that, somehow, through part of a natural repair process, mature cells turn off some of the epigenetic controls that inhibit expression of certain nuclear genes that result in differentiation,” said study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston.
If this process can be replicated in human cells, researchers might one day be able to use a skin biopsy or blood sample to create stem cells specific to each individual. And this could have implications for treating cancers and other diseases.
The investigators are now testing the STAP technique in human cells. ![]()
Listen Now! Dining and Nightlife Recommendations for HM14
Listen Up! What Hospitalists Need to Know About Healthcare Post-Obamacare
Click here to listen to more of our interview with Dr. Morrison
Click here to listen to more of our interview with Dr. Morrison
Click here to listen to more of our interview with Dr. Morrison
Likelihood for Readmission of Hospitalized Medicare Patients with Multiple Chronic Conditions Up 600%
600%
The increased likelihood of 30-day hospital readmission for hospitalized Medicare patients who have 10 or more chronic conditions, compared with those who have only one to four chronic conditions.4 These patients with multiple chronic conditions represent only 8.9% of Medicare beneficiaries but account for 50% of all rehospitalizations. The numbers are drawn from a 5% sample of Medicare fee-for-service beneficiaries during the first nine months of 2008. Those with five to nine chronic conditions had 2.5 times the odds for being readmitted.
Larry Beresford is a freelance writer in Alameda, Calif.
- Shieh L, Pummer E, Tsui J, et al. Septris: improving sepsis recognition and management through a mobile educational game [abstract]. J Hosp Med. 2013;8(Suppl 1):1053.
- Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30-day post-discharge hospital utilization. J Gen Intern Med. 2014;29(2):349-355.
- Daniels KR, Lee GC, Frei CR. Trends in catheter-associated urinary tract infections among a national cohort of hospitalized adults, 2001-2010. Am J Infect Control. 2014;42(1):17-22.
- Berkowitz SA. Anderson GF. Medicare beneficiaries most likely to be readmitted. J Hosp Med. 2013;8(11):639-641.
600%
The increased likelihood of 30-day hospital readmission for hospitalized Medicare patients who have 10 or more chronic conditions, compared with those who have only one to four chronic conditions.4 These patients with multiple chronic conditions represent only 8.9% of Medicare beneficiaries but account for 50% of all rehospitalizations. The numbers are drawn from a 5% sample of Medicare fee-for-service beneficiaries during the first nine months of 2008. Those with five to nine chronic conditions had 2.5 times the odds for being readmitted.
Larry Beresford is a freelance writer in Alameda, Calif.
- Shieh L, Pummer E, Tsui J, et al. Septris: improving sepsis recognition and management through a mobile educational game [abstract]. J Hosp Med. 2013;8(Suppl 1):1053.
- Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30-day post-discharge hospital utilization. J Gen Intern Med. 2014;29(2):349-355.
- Daniels KR, Lee GC, Frei CR. Trends in catheter-associated urinary tract infections among a national cohort of hospitalized adults, 2001-2010. Am J Infect Control. 2014;42(1):17-22.
- Berkowitz SA. Anderson GF. Medicare beneficiaries most likely to be readmitted. J Hosp Med. 2013;8(11):639-641.
600%
The increased likelihood of 30-day hospital readmission for hospitalized Medicare patients who have 10 or more chronic conditions, compared with those who have only one to four chronic conditions.4 These patients with multiple chronic conditions represent only 8.9% of Medicare beneficiaries but account for 50% of all rehospitalizations. The numbers are drawn from a 5% sample of Medicare fee-for-service beneficiaries during the first nine months of 2008. Those with five to nine chronic conditions had 2.5 times the odds for being readmitted.
Larry Beresford is a freelance writer in Alameda, Calif.
- Shieh L, Pummer E, Tsui J, et al. Septris: improving sepsis recognition and management through a mobile educational game [abstract]. J Hosp Med. 2013;8(Suppl 1):1053.
- Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30-day post-discharge hospital utilization. J Gen Intern Med. 2014;29(2):349-355.
- Daniels KR, Lee GC, Frei CR. Trends in catheter-associated urinary tract infections among a national cohort of hospitalized adults, 2001-2010. Am J Infect Control. 2014;42(1):17-22.
- Berkowitz SA. Anderson GF. Medicare beneficiaries most likely to be readmitted. J Hosp Med. 2013;8(11):639-641.
Shift from Productivity to Value-Based Compensation Gains Momentum
At the 2011 SHM annual meeting in Dallas, I served on an expert panel that reviewed the latest hospitalist survey data. Included in this review were the latest compensation and productivity figures. As the session concluded, I was satisfied that the panel had discussed important information in an accessible way; however, the keynote speaker who followed us to address an entirely different topic began his talk by pointing out that the data we had reviewed, including things like wRVUs, would very soon have little to do with compensation for any physician, regardless of specialty. He implied, quite persuasively, that we were pretty old school to be talking about wRVUs and compensation based on productivity; everyone should be prepared for and embrace compensation based on value, not production.
I hear a similar sentiment reasonably often. And I agree, but I think many make the mistake of oversimplifying the issue.
Physician Value-Based Payment
Measurement of physician performance using costs, quality, and outcomes has already begun and will influence Medicare payments to doctors beginning in 2015 for large groups (>100 providers with any mix of specialties billing under the same tax ID number) and in 2017 for smaller groups.
If Medicare is moving away from payment based on wRVUs, likely followed soon by other payors, then hospitalist compensation should do the same. But I don’t think that changes the potential role of compensation based on productivity.
Compensation Should Include Performance and Productivity Metrics
Survey data show a move from an essentially fixed annual compensation early in our field to an inclusion of components tied to performance several years before the introduction of the Physician Value-Based Payment Modifier program. Data from SHM’s 2010, 2011, and 2012 State of Hospital Medicine reports (www.hospitalmedicine.org/survey) show that a small, but probably increasing, part of compensation has been tied to performance on things like patient satisfaction and core measures (see “Distribution of Total Hospitalist Compensation,” below). Note that the percentages in the chart refer to the fraction of total compensation dollars allocated to each domain and not the portion of hospitalists who have compensation tied to each domain.
Over the same three years, the percentage of compensation tied to productivity has been decreasing overall, while “private groups are more likely to pay a higher proportion of compensation based on productivity, and hospital-employed groups are more likely to pay a higher proportion of compensation based on performance.”
Matching Performance Compensation to Medicare’s Value-Based Modifier
It makes sense for physician compensation to generally mirror Medicare and other payor professional fee reimbursement formulas. But, in that regard, hospitalists are ahead of the market already, because the portion of dollars allocated to performance (value) in hospitalist compensation plans already exceeds the 2% or less portion of Medicare reimbursement that is influenced by performance.
Medicare will steadily increase the portion of reimbursement allocated to performance (value) and decrease the part tied solely to wRVUs. So it makes sense that hospitalist compensation plans should do the same. Who knows, within the next 5-10 years, hospitalists, and potentially doctors in all specialties, might see 20% to 50% of their compensation tied to performance. I think that might be a good thing, as long as we can come up with effective measures of performance and value—not an easy thing to do in any business or industry.
Future Role of Productivity Compensation
I don’t think all the talk about value-based reimbursement means we should abandon the idea of connecting a portion of compensation to productivity. The first two practice management columns I wrote for The Hospitalist appeared in May 2006 (www.the-hospitalist.org/details/article/252413/The_Sweet_Spot.html) and June 2006 (www.the-hospitalist.org/details/article/246297.html) and recommended tying a meaningful portion of compensation to individual hospitalist productivity, and I think it still makes sense to do so.
Source: 2012 State of Hospital Medicine report
In any business or industry, financial performance is connected to the amount of product produced and its value. In the future, both metrics will determine reimbursement for even the highest performing healthcare providers. The new emphasis on value won’t ever make it unnecessary to produce at a reasonable level.
Unquestionably, there are many high-performing hospitalist practices with little or no productivity component in the compensation formula. So it isn’t an absolute sine qua non for success. But I think many practices dismiss it as a viable option when it might solve problems and liberate individuals in the group to exercise some autonomy in finding their own sweet spot between workload and compensation.
It will be interesting to see if future surveys show that the portion of dollars tied to hospitalist productivity continues to decrease, despite what I see as its potential benefits.

At the 2011 SHM annual meeting in Dallas, I served on an expert panel that reviewed the latest hospitalist survey data. Included in this review were the latest compensation and productivity figures. As the session concluded, I was satisfied that the panel had discussed important information in an accessible way; however, the keynote speaker who followed us to address an entirely different topic began his talk by pointing out that the data we had reviewed, including things like wRVUs, would very soon have little to do with compensation for any physician, regardless of specialty. He implied, quite persuasively, that we were pretty old school to be talking about wRVUs and compensation based on productivity; everyone should be prepared for and embrace compensation based on value, not production.
I hear a similar sentiment reasonably often. And I agree, but I think many make the mistake of oversimplifying the issue.
Physician Value-Based Payment
Measurement of physician performance using costs, quality, and outcomes has already begun and will influence Medicare payments to doctors beginning in 2015 for large groups (>100 providers with any mix of specialties billing under the same tax ID number) and in 2017 for smaller groups.
If Medicare is moving away from payment based on wRVUs, likely followed soon by other payors, then hospitalist compensation should do the same. But I don’t think that changes the potential role of compensation based on productivity.
Compensation Should Include Performance and Productivity Metrics
Survey data show a move from an essentially fixed annual compensation early in our field to an inclusion of components tied to performance several years before the introduction of the Physician Value-Based Payment Modifier program. Data from SHM’s 2010, 2011, and 2012 State of Hospital Medicine reports (www.hospitalmedicine.org/survey) show that a small, but probably increasing, part of compensation has been tied to performance on things like patient satisfaction and core measures (see “Distribution of Total Hospitalist Compensation,” below). Note that the percentages in the chart refer to the fraction of total compensation dollars allocated to each domain and not the portion of hospitalists who have compensation tied to each domain.
Over the same three years, the percentage of compensation tied to productivity has been decreasing overall, while “private groups are more likely to pay a higher proportion of compensation based on productivity, and hospital-employed groups are more likely to pay a higher proportion of compensation based on performance.”
Matching Performance Compensation to Medicare’s Value-Based Modifier
It makes sense for physician compensation to generally mirror Medicare and other payor professional fee reimbursement formulas. But, in that regard, hospitalists are ahead of the market already, because the portion of dollars allocated to performance (value) in hospitalist compensation plans already exceeds the 2% or less portion of Medicare reimbursement that is influenced by performance.
Medicare will steadily increase the portion of reimbursement allocated to performance (value) and decrease the part tied solely to wRVUs. So it makes sense that hospitalist compensation plans should do the same. Who knows, within the next 5-10 years, hospitalists, and potentially doctors in all specialties, might see 20% to 50% of their compensation tied to performance. I think that might be a good thing, as long as we can come up with effective measures of performance and value—not an easy thing to do in any business or industry.
Future Role of Productivity Compensation
I don’t think all the talk about value-based reimbursement means we should abandon the idea of connecting a portion of compensation to productivity. The first two practice management columns I wrote for The Hospitalist appeared in May 2006 (www.the-hospitalist.org/details/article/252413/The_Sweet_Spot.html) and June 2006 (www.the-hospitalist.org/details/article/246297.html) and recommended tying a meaningful portion of compensation to individual hospitalist productivity, and I think it still makes sense to do so.
Source: 2012 State of Hospital Medicine report
In any business or industry, financial performance is connected to the amount of product produced and its value. In the future, both metrics will determine reimbursement for even the highest performing healthcare providers. The new emphasis on value won’t ever make it unnecessary to produce at a reasonable level.
Unquestionably, there are many high-performing hospitalist practices with little or no productivity component in the compensation formula. So it isn’t an absolute sine qua non for success. But I think many practices dismiss it as a viable option when it might solve problems and liberate individuals in the group to exercise some autonomy in finding their own sweet spot between workload and compensation.
It will be interesting to see if future surveys show that the portion of dollars tied to hospitalist productivity continues to decrease, despite what I see as its potential benefits.

At the 2011 SHM annual meeting in Dallas, I served on an expert panel that reviewed the latest hospitalist survey data. Included in this review were the latest compensation and productivity figures. As the session concluded, I was satisfied that the panel had discussed important information in an accessible way; however, the keynote speaker who followed us to address an entirely different topic began his talk by pointing out that the data we had reviewed, including things like wRVUs, would very soon have little to do with compensation for any physician, regardless of specialty. He implied, quite persuasively, that we were pretty old school to be talking about wRVUs and compensation based on productivity; everyone should be prepared for and embrace compensation based on value, not production.
I hear a similar sentiment reasonably often. And I agree, but I think many make the mistake of oversimplifying the issue.
Physician Value-Based Payment
Measurement of physician performance using costs, quality, and outcomes has already begun and will influence Medicare payments to doctors beginning in 2015 for large groups (>100 providers with any mix of specialties billing under the same tax ID number) and in 2017 for smaller groups.
If Medicare is moving away from payment based on wRVUs, likely followed soon by other payors, then hospitalist compensation should do the same. But I don’t think that changes the potential role of compensation based on productivity.
Compensation Should Include Performance and Productivity Metrics
Survey data show a move from an essentially fixed annual compensation early in our field to an inclusion of components tied to performance several years before the introduction of the Physician Value-Based Payment Modifier program. Data from SHM’s 2010, 2011, and 2012 State of Hospital Medicine reports (www.hospitalmedicine.org/survey) show that a small, but probably increasing, part of compensation has been tied to performance on things like patient satisfaction and core measures (see “Distribution of Total Hospitalist Compensation,” below). Note that the percentages in the chart refer to the fraction of total compensation dollars allocated to each domain and not the portion of hospitalists who have compensation tied to each domain.
Over the same three years, the percentage of compensation tied to productivity has been decreasing overall, while “private groups are more likely to pay a higher proportion of compensation based on productivity, and hospital-employed groups are more likely to pay a higher proportion of compensation based on performance.”
Matching Performance Compensation to Medicare’s Value-Based Modifier
It makes sense for physician compensation to generally mirror Medicare and other payor professional fee reimbursement formulas. But, in that regard, hospitalists are ahead of the market already, because the portion of dollars allocated to performance (value) in hospitalist compensation plans already exceeds the 2% or less portion of Medicare reimbursement that is influenced by performance.
Medicare will steadily increase the portion of reimbursement allocated to performance (value) and decrease the part tied solely to wRVUs. So it makes sense that hospitalist compensation plans should do the same. Who knows, within the next 5-10 years, hospitalists, and potentially doctors in all specialties, might see 20% to 50% of their compensation tied to performance. I think that might be a good thing, as long as we can come up with effective measures of performance and value—not an easy thing to do in any business or industry.
Future Role of Productivity Compensation
I don’t think all the talk about value-based reimbursement means we should abandon the idea of connecting a portion of compensation to productivity. The first two practice management columns I wrote for The Hospitalist appeared in May 2006 (www.the-hospitalist.org/details/article/252413/The_Sweet_Spot.html) and June 2006 (www.the-hospitalist.org/details/article/246297.html) and recommended tying a meaningful portion of compensation to individual hospitalist productivity, and I think it still makes sense to do so.
Source: 2012 State of Hospital Medicine report
In any business or industry, financial performance is connected to the amount of product produced and its value. In the future, both metrics will determine reimbursement for even the highest performing healthcare providers. The new emphasis on value won’t ever make it unnecessary to produce at a reasonable level.
Unquestionably, there are many high-performing hospitalist practices with little or no productivity component in the compensation formula. So it isn’t an absolute sine qua non for success. But I think many practices dismiss it as a viable option when it might solve problems and liberate individuals in the group to exercise some autonomy in finding their own sweet spot between workload and compensation.
It will be interesting to see if future surveys show that the portion of dollars tied to hospitalist productivity continues to decrease, despite what I see as its potential benefits.

Mid-Flight Medical Emergencies Benefit from Hospitalist on Board
We were still climbing from the airport tarmac, and the movie on my iPad, “Star Trek II: The Wrath of Khan,” was at an exciting point where Klingons are attacking the USS Enterprise when it came: “Is there a doctor on the plane?”
If you talk to your physician and healthcare colleagues who fly, you’ll hear about this scenario enough to know that it is not a rare event. Healthcare providers who fly routinely are more likely to tend to a sick airline passenger than they are to diagnose pheochromocytoma in their day jobs. Pheo is a two-in-a-million disease, but getting ill on a plane happens to one to two people in every 20,000. In fact, the sick airline passenger is relatively common, with an FAA study estimating 13 events per day in the 1990s (Anesthesiology. 2008;108(4):749-755). There have been a number of interesting articles written about the doctor-on-the-plane scenario. Our own Bob Wachter, MD, MHM, blogged about it in his usual humorous and insightful way a few years ago here, (http://community.the-hospitalist.org/2010/08/22/if-there-s-a-doctor-on-board-please-ring-your-call-button), and The New England Journal of Medicine published a perspective on it at www.nejm.org/doi/full/10.1056/NEJMp1006331?query=TOC (NEJM; 2010;363(21):1988-1989).
My most recent experience happened on a flight just before the New Year, and because many of us will be flying to and from the annual meeting in Las Vegas and it seems to fit naturally (in many cases) with what we do as hospitalists, I thought I’d put pen to paper regarding the sick airline passenger in flight.
Fasten Your Seatbelt
As I was walked up to the first row, the flight attendant said a passenger had almost passed out. A doctor was tending to the sick woman already, as were two very concerned flight attendants. I have been through this before, so I knew I couldn’t go back to my seat just yet. I asked the physician if everything was OK and if he needed help. In my previous experiences, the initial doctor was often a specialist, or retired, or both. They often were relieved to see a hospitalist and happily handed over the care of the airline patient once they heard I’m a hospitalist. Sound familiar from your day job?
This episode was no different: Although pleasant and concerned, the initial doctor was retired, and he made it clear this was outside of his area of expertise. He didn’t exactly sprint back to his seat, but you get the picture.
The patient was pale, looked ill, and was semi-conscious. She was about 70 (later confirmed at 73) and was sitting with her son, who worriedly showed me the auto-blood pressure cuff they had brought with her; it read 81/60. She denied chest pain or shortness of breath. Her pulse was 65, and her breathing was not labored.
For a hospitalist, attending to the ill airline passenger can be quite rewarding. Most diagnoses are those we see every day: syncope/pre-syncope, respiratory, and GI complaints make up more than half of the calls. Death is rare (0.3%), and other “big” decisions, like whether to force the plane to land early (landing a plane still full of fuel or at a smaller airport is not to be taken lightly), are uncommon (7.3%). Still, the illnesses can be real, and more than a quarter of aircraft patients are transported to a hospital upon landing (N Engl J Med. 2013;368(22):2075-2083). Our skills at diagnosis are undoubtedly valuable in the air.
Also, as Dr. Wachter said in his blog on the subject, tending to the ill airline passenger is “one of the purest expressions of our Hippocratic oath, and our professionalism. We have no obligation to respond, and no contractual relationship. It’s just you, armed with your wits and experience, a sick and scared patient and family member, and about 200 interested observers.”
We broke open the aircraft medical kit, which was surprisingly well supplied, complete with a manual BP cuff and medications any registered respiratory therapist or code responder would find familiar. Bronchodilators, epinephrine and lidocaine, the usual aspirin, even IV tubing and needles. The one thing I was shocked to find was that there is limited supplemental oxygen: only enough to supply a nasal cannula at 4L max, and that for only a few hours.
As with the vast majority of medical cases, a thorough history of my 73-year-old air traveler proved invaluable. She felt light-headed but never lost consciousness. She had no other symptoms. Her past medical history was significant for hypertension but no heart disease. Was there anything else? She had been discharged from a hospital three days before for severe hypertension. Her ACE inhibitor and beta-blocker doses had been doubled and HCTZ added (her hospitalist had done an excellent job educating her on her disease, her medication changes, and possible side effects).
Anything else? She had been traveling more than 12 hours with little to drink, but she had taken all of her meds just before boarding the flight. After some oral rehydration, leaning back, and elevating her feet, her blood pressure increased to 125/71. I checked on her frequently for the rest of the flight, and she was talking happily to neighbors and her son long before we deplaned. They were en route to Boston, where she was moving and had no doctor, but she had an appointment scheduled with a new one soon. I gave her my card and my cell number and instructed them to call me if there were any problems. She and her son were thankful (and her neighbors were too!), and I was glad to have helped.
The Aftermath
The only thing left was the administrative paperwork for the airline. Would I please sign here? What was my license number (they were confused as to whether to take my NPI, my state license number, or DEA number, so I gave them all three), and where was I employed?
After getting home and recovering from my jet lag, I did some research on this topic. Colleagues of mine expressed concern over the legal liability of providing assistance in flight, but, compared to our day jobs, that concern seems to be unwarranted. The Aviation Medical Assistance Act of 1998 (www.gpo.gov) protects healthcare providers who render care in good faith.
As of the 2008 article by Ruskin, no physician providing care for an airline patient had been successfully sued. I learned that the medical kits are fairly well stocked and are set up for the physician/medical professional. I also learned that supplemental oxygen, so ubiquitous in the hospital, is more limited on an airplane. And, I found out that, while airlines contract with ground-based medical services, half of all emergencies are cared for by Good Samaritan doctors, licensed providers, nurses, and EMTs.
So, before my next flight, in addition to packing my iPad and thumb drive, boarding pass, and ID, I plan to pack those reference articles by Ruskin and Peterson.

We were still climbing from the airport tarmac, and the movie on my iPad, “Star Trek II: The Wrath of Khan,” was at an exciting point where Klingons are attacking the USS Enterprise when it came: “Is there a doctor on the plane?”
If you talk to your physician and healthcare colleagues who fly, you’ll hear about this scenario enough to know that it is not a rare event. Healthcare providers who fly routinely are more likely to tend to a sick airline passenger than they are to diagnose pheochromocytoma in their day jobs. Pheo is a two-in-a-million disease, but getting ill on a plane happens to one to two people in every 20,000. In fact, the sick airline passenger is relatively common, with an FAA study estimating 13 events per day in the 1990s (Anesthesiology. 2008;108(4):749-755). There have been a number of interesting articles written about the doctor-on-the-plane scenario. Our own Bob Wachter, MD, MHM, blogged about it in his usual humorous and insightful way a few years ago here, (http://community.the-hospitalist.org/2010/08/22/if-there-s-a-doctor-on-board-please-ring-your-call-button), and The New England Journal of Medicine published a perspective on it at www.nejm.org/doi/full/10.1056/NEJMp1006331?query=TOC (NEJM; 2010;363(21):1988-1989).
My most recent experience happened on a flight just before the New Year, and because many of us will be flying to and from the annual meeting in Las Vegas and it seems to fit naturally (in many cases) with what we do as hospitalists, I thought I’d put pen to paper regarding the sick airline passenger in flight.
Fasten Your Seatbelt
As I was walked up to the first row, the flight attendant said a passenger had almost passed out. A doctor was tending to the sick woman already, as were two very concerned flight attendants. I have been through this before, so I knew I couldn’t go back to my seat just yet. I asked the physician if everything was OK and if he needed help. In my previous experiences, the initial doctor was often a specialist, or retired, or both. They often were relieved to see a hospitalist and happily handed over the care of the airline patient once they heard I’m a hospitalist. Sound familiar from your day job?
This episode was no different: Although pleasant and concerned, the initial doctor was retired, and he made it clear this was outside of his area of expertise. He didn’t exactly sprint back to his seat, but you get the picture.
The patient was pale, looked ill, and was semi-conscious. She was about 70 (later confirmed at 73) and was sitting with her son, who worriedly showed me the auto-blood pressure cuff they had brought with her; it read 81/60. She denied chest pain or shortness of breath. Her pulse was 65, and her breathing was not labored.
For a hospitalist, attending to the ill airline passenger can be quite rewarding. Most diagnoses are those we see every day: syncope/pre-syncope, respiratory, and GI complaints make up more than half of the calls. Death is rare (0.3%), and other “big” decisions, like whether to force the plane to land early (landing a plane still full of fuel or at a smaller airport is not to be taken lightly), are uncommon (7.3%). Still, the illnesses can be real, and more than a quarter of aircraft patients are transported to a hospital upon landing (N Engl J Med. 2013;368(22):2075-2083). Our skills at diagnosis are undoubtedly valuable in the air.
Also, as Dr. Wachter said in his blog on the subject, tending to the ill airline passenger is “one of the purest expressions of our Hippocratic oath, and our professionalism. We have no obligation to respond, and no contractual relationship. It’s just you, armed with your wits and experience, a sick and scared patient and family member, and about 200 interested observers.”
We broke open the aircraft medical kit, which was surprisingly well supplied, complete with a manual BP cuff and medications any registered respiratory therapist or code responder would find familiar. Bronchodilators, epinephrine and lidocaine, the usual aspirin, even IV tubing and needles. The one thing I was shocked to find was that there is limited supplemental oxygen: only enough to supply a nasal cannula at 4L max, and that for only a few hours.
As with the vast majority of medical cases, a thorough history of my 73-year-old air traveler proved invaluable. She felt light-headed but never lost consciousness. She had no other symptoms. Her past medical history was significant for hypertension but no heart disease. Was there anything else? She had been discharged from a hospital three days before for severe hypertension. Her ACE inhibitor and beta-blocker doses had been doubled and HCTZ added (her hospitalist had done an excellent job educating her on her disease, her medication changes, and possible side effects).
Anything else? She had been traveling more than 12 hours with little to drink, but she had taken all of her meds just before boarding the flight. After some oral rehydration, leaning back, and elevating her feet, her blood pressure increased to 125/71. I checked on her frequently for the rest of the flight, and she was talking happily to neighbors and her son long before we deplaned. They were en route to Boston, where she was moving and had no doctor, but she had an appointment scheduled with a new one soon. I gave her my card and my cell number and instructed them to call me if there were any problems. She and her son were thankful (and her neighbors were too!), and I was glad to have helped.
The Aftermath
The only thing left was the administrative paperwork for the airline. Would I please sign here? What was my license number (they were confused as to whether to take my NPI, my state license number, or DEA number, so I gave them all three), and where was I employed?
After getting home and recovering from my jet lag, I did some research on this topic. Colleagues of mine expressed concern over the legal liability of providing assistance in flight, but, compared to our day jobs, that concern seems to be unwarranted. The Aviation Medical Assistance Act of 1998 (www.gpo.gov) protects healthcare providers who render care in good faith.
As of the 2008 article by Ruskin, no physician providing care for an airline patient had been successfully sued. I learned that the medical kits are fairly well stocked and are set up for the physician/medical professional. I also learned that supplemental oxygen, so ubiquitous in the hospital, is more limited on an airplane. And, I found out that, while airlines contract with ground-based medical services, half of all emergencies are cared for by Good Samaritan doctors, licensed providers, nurses, and EMTs.
So, before my next flight, in addition to packing my iPad and thumb drive, boarding pass, and ID, I plan to pack those reference articles by Ruskin and Peterson.

We were still climbing from the airport tarmac, and the movie on my iPad, “Star Trek II: The Wrath of Khan,” was at an exciting point where Klingons are attacking the USS Enterprise when it came: “Is there a doctor on the plane?”
If you talk to your physician and healthcare colleagues who fly, you’ll hear about this scenario enough to know that it is not a rare event. Healthcare providers who fly routinely are more likely to tend to a sick airline passenger than they are to diagnose pheochromocytoma in their day jobs. Pheo is a two-in-a-million disease, but getting ill on a plane happens to one to two people in every 20,000. In fact, the sick airline passenger is relatively common, with an FAA study estimating 13 events per day in the 1990s (Anesthesiology. 2008;108(4):749-755). There have been a number of interesting articles written about the doctor-on-the-plane scenario. Our own Bob Wachter, MD, MHM, blogged about it in his usual humorous and insightful way a few years ago here, (http://community.the-hospitalist.org/2010/08/22/if-there-s-a-doctor-on-board-please-ring-your-call-button), and The New England Journal of Medicine published a perspective on it at www.nejm.org/doi/full/10.1056/NEJMp1006331?query=TOC (NEJM; 2010;363(21):1988-1989).
My most recent experience happened on a flight just before the New Year, and because many of us will be flying to and from the annual meeting in Las Vegas and it seems to fit naturally (in many cases) with what we do as hospitalists, I thought I’d put pen to paper regarding the sick airline passenger in flight.
Fasten Your Seatbelt
As I was walked up to the first row, the flight attendant said a passenger had almost passed out. A doctor was tending to the sick woman already, as were two very concerned flight attendants. I have been through this before, so I knew I couldn’t go back to my seat just yet. I asked the physician if everything was OK and if he needed help. In my previous experiences, the initial doctor was often a specialist, or retired, or both. They often were relieved to see a hospitalist and happily handed over the care of the airline patient once they heard I’m a hospitalist. Sound familiar from your day job?
This episode was no different: Although pleasant and concerned, the initial doctor was retired, and he made it clear this was outside of his area of expertise. He didn’t exactly sprint back to his seat, but you get the picture.
The patient was pale, looked ill, and was semi-conscious. She was about 70 (later confirmed at 73) and was sitting with her son, who worriedly showed me the auto-blood pressure cuff they had brought with her; it read 81/60. She denied chest pain or shortness of breath. Her pulse was 65, and her breathing was not labored.
For a hospitalist, attending to the ill airline passenger can be quite rewarding. Most diagnoses are those we see every day: syncope/pre-syncope, respiratory, and GI complaints make up more than half of the calls. Death is rare (0.3%), and other “big” decisions, like whether to force the plane to land early (landing a plane still full of fuel or at a smaller airport is not to be taken lightly), are uncommon (7.3%). Still, the illnesses can be real, and more than a quarter of aircraft patients are transported to a hospital upon landing (N Engl J Med. 2013;368(22):2075-2083). Our skills at diagnosis are undoubtedly valuable in the air.
Also, as Dr. Wachter said in his blog on the subject, tending to the ill airline passenger is “one of the purest expressions of our Hippocratic oath, and our professionalism. We have no obligation to respond, and no contractual relationship. It’s just you, armed with your wits and experience, a sick and scared patient and family member, and about 200 interested observers.”
We broke open the aircraft medical kit, which was surprisingly well supplied, complete with a manual BP cuff and medications any registered respiratory therapist or code responder would find familiar. Bronchodilators, epinephrine and lidocaine, the usual aspirin, even IV tubing and needles. The one thing I was shocked to find was that there is limited supplemental oxygen: only enough to supply a nasal cannula at 4L max, and that for only a few hours.
As with the vast majority of medical cases, a thorough history of my 73-year-old air traveler proved invaluable. She felt light-headed but never lost consciousness. She had no other symptoms. Her past medical history was significant for hypertension but no heart disease. Was there anything else? She had been discharged from a hospital three days before for severe hypertension. Her ACE inhibitor and beta-blocker doses had been doubled and HCTZ added (her hospitalist had done an excellent job educating her on her disease, her medication changes, and possible side effects).
Anything else? She had been traveling more than 12 hours with little to drink, but she had taken all of her meds just before boarding the flight. After some oral rehydration, leaning back, and elevating her feet, her blood pressure increased to 125/71. I checked on her frequently for the rest of the flight, and she was talking happily to neighbors and her son long before we deplaned. They were en route to Boston, where she was moving and had no doctor, but she had an appointment scheduled with a new one soon. I gave her my card and my cell number and instructed them to call me if there were any problems. She and her son were thankful (and her neighbors were too!), and I was glad to have helped.
The Aftermath
The only thing left was the administrative paperwork for the airline. Would I please sign here? What was my license number (they were confused as to whether to take my NPI, my state license number, or DEA number, so I gave them all three), and where was I employed?
After getting home and recovering from my jet lag, I did some research on this topic. Colleagues of mine expressed concern over the legal liability of providing assistance in flight, but, compared to our day jobs, that concern seems to be unwarranted. The Aviation Medical Assistance Act of 1998 (www.gpo.gov) protects healthcare providers who render care in good faith.
As of the 2008 article by Ruskin, no physician providing care for an airline patient had been successfully sued. I learned that the medical kits are fairly well stocked and are set up for the physician/medical professional. I also learned that supplemental oxygen, so ubiquitous in the hospital, is more limited on an airplane. And, I found out that, while airlines contract with ground-based medical services, half of all emergencies are cared for by Good Samaritan doctors, licensed providers, nurses, and EMTs.
So, before my next flight, in addition to packing my iPad and thumb drive, boarding pass, and ID, I plan to pack those reference articles by Ruskin and Peterson.