User login
Team creates faster method for diagnosing sepsis

for Staphylococcus infection
Credit: Bill Branson
A new technique could cut hours off the time it takes to diagnose blood infections, according to a report published in mBio.
The
method combines a selective lysis step in which blood cells are destroyed, a centrifugation step to collect any bacteria or
fungi, and a fluorescence step that reveals the “fingerprint” of any
pathogens present in the blood sample.
In tests, this method identified
the species of bacteria or fungi in 96.5% of positive blood culture
samples.
“The primary benefit of getting a rapid identification is making sure the patient is on the right [antibiotic] therapy and to quickly make any needed adjustments to the initial therapy,” said study author John Walsh, of bioMérieux, Inc. in Durham, North Carolina.
Walsh added that the current standard approach to diagnosing bloodstream infections—Gram staining and overnight sub-culture followed by phenotypic ID tests—has limitations that can prevent timely treatment.
Gram staining provides early, low-level information about the type of microorganism present, but it sometimes takes hours to deliver a result, and technicians can make mistakes in the process that provide misleading results.
Other, more specific identification methods are also available for diagnosis, but they can take at least a day or two to produce results, and many require expensive equipment.
So Walsh and his colleagues developed a new technique. With this method, a sample of positive blood culture is treated with lysis buffer, to “pop” the blood cells, then transferred to a specialized optical tube.
Next, the tube is centrifuged, which drives bacteria or fungi down through a liquid density cushion to form a pellet at the bottom of the tube.
Then comes the intrinsic fluorescence spectroscopy. The microbial pellet is irradiated with light ranging from the deep ultraviolet to infrared. This excites certain organic molecules in the microorganisms—tryptophan, NADH, FAD, porphyrins, and others—and causes them to fluoresce in a characteristic way depending on the identity of the microbe.
The exact pattern of fluorescence is then compared with a database of 37 common pathogens to identify the organism present.
“We’re using intrinsic fluorescence to identify the microorganisms,” Walsh noted. “It’s an innate property of most living organisms. Because it’s intrinsic, no reagents are needed for the identification step.”
This removes many of the opportunities for mistakes and lowers test costs, he added.
In tests, this method correctly identified the species in 96.5% of all samples. In the 2.7% of samples for which no species identity was provided, the system was able to correctly identify 67% to the family level, which is often enough information to select an effective therapy.
More than 1000 samples were tested, and the method never gave an incorrect result as to the family level or the Gram type.
Walsh and his colleagues are now working on automating this system with robotics to make it a fully hands-off process. He noted that blood cultures grow in their own time, often producing a positive result at an inconvenient time of the day for clinical labs, so automation could speed up diagnosis significantly.
“Our vision is to have a system that will automatically identify the blood culture isolate within 15 minutes of the culture being called positive,” Walsh said.
If a culture is positive at 2 am, automating this method could make it possible to identify the organism by 2:15 am and send an electronic report to a patient’s physician.
The researchers hope to begin testing and evaluating the feasibility of an automated form of the system in a clinical environment within months. ![]()

for Staphylococcus infection
Credit: Bill Branson
A new technique could cut hours off the time it takes to diagnose blood infections, according to a report published in mBio.
The
method combines a selective lysis step in which blood cells are destroyed, a centrifugation step to collect any bacteria or
fungi, and a fluorescence step that reveals the “fingerprint” of any
pathogens present in the blood sample.
In tests, this method identified
the species of bacteria or fungi in 96.5% of positive blood culture
samples.
“The primary benefit of getting a rapid identification is making sure the patient is on the right [antibiotic] therapy and to quickly make any needed adjustments to the initial therapy,” said study author John Walsh, of bioMérieux, Inc. in Durham, North Carolina.
Walsh added that the current standard approach to diagnosing bloodstream infections—Gram staining and overnight sub-culture followed by phenotypic ID tests—has limitations that can prevent timely treatment.
Gram staining provides early, low-level information about the type of microorganism present, but it sometimes takes hours to deliver a result, and technicians can make mistakes in the process that provide misleading results.
Other, more specific identification methods are also available for diagnosis, but they can take at least a day or two to produce results, and many require expensive equipment.
So Walsh and his colleagues developed a new technique. With this method, a sample of positive blood culture is treated with lysis buffer, to “pop” the blood cells, then transferred to a specialized optical tube.
Next, the tube is centrifuged, which drives bacteria or fungi down through a liquid density cushion to form a pellet at the bottom of the tube.
Then comes the intrinsic fluorescence spectroscopy. The microbial pellet is irradiated with light ranging from the deep ultraviolet to infrared. This excites certain organic molecules in the microorganisms—tryptophan, NADH, FAD, porphyrins, and others—and causes them to fluoresce in a characteristic way depending on the identity of the microbe.
The exact pattern of fluorescence is then compared with a database of 37 common pathogens to identify the organism present.
“We’re using intrinsic fluorescence to identify the microorganisms,” Walsh noted. “It’s an innate property of most living organisms. Because it’s intrinsic, no reagents are needed for the identification step.”
This removes many of the opportunities for mistakes and lowers test costs, he added.
In tests, this method correctly identified the species in 96.5% of all samples. In the 2.7% of samples for which no species identity was provided, the system was able to correctly identify 67% to the family level, which is often enough information to select an effective therapy.
More than 1000 samples were tested, and the method never gave an incorrect result as to the family level or the Gram type.
Walsh and his colleagues are now working on automating this system with robotics to make it a fully hands-off process. He noted that blood cultures grow in their own time, often producing a positive result at an inconvenient time of the day for clinical labs, so automation could speed up diagnosis significantly.
“Our vision is to have a system that will automatically identify the blood culture isolate within 15 minutes of the culture being called positive,” Walsh said.
If a culture is positive at 2 am, automating this method could make it possible to identify the organism by 2:15 am and send an electronic report to a patient’s physician.
The researchers hope to begin testing and evaluating the feasibility of an automated form of the system in a clinical environment within months. ![]()

for Staphylococcus infection
Credit: Bill Branson
A new technique could cut hours off the time it takes to diagnose blood infections, according to a report published in mBio.
The
method combines a selective lysis step in which blood cells are destroyed, a centrifugation step to collect any bacteria or
fungi, and a fluorescence step that reveals the “fingerprint” of any
pathogens present in the blood sample.
In tests, this method identified
the species of bacteria or fungi in 96.5% of positive blood culture
samples.
“The primary benefit of getting a rapid identification is making sure the patient is on the right [antibiotic] therapy and to quickly make any needed adjustments to the initial therapy,” said study author John Walsh, of bioMérieux, Inc. in Durham, North Carolina.
Walsh added that the current standard approach to diagnosing bloodstream infections—Gram staining and overnight sub-culture followed by phenotypic ID tests—has limitations that can prevent timely treatment.
Gram staining provides early, low-level information about the type of microorganism present, but it sometimes takes hours to deliver a result, and technicians can make mistakes in the process that provide misleading results.
Other, more specific identification methods are also available for diagnosis, but they can take at least a day or two to produce results, and many require expensive equipment.
So Walsh and his colleagues developed a new technique. With this method, a sample of positive blood culture is treated with lysis buffer, to “pop” the blood cells, then transferred to a specialized optical tube.
Next, the tube is centrifuged, which drives bacteria or fungi down through a liquid density cushion to form a pellet at the bottom of the tube.
Then comes the intrinsic fluorescence spectroscopy. The microbial pellet is irradiated with light ranging from the deep ultraviolet to infrared. This excites certain organic molecules in the microorganisms—tryptophan, NADH, FAD, porphyrins, and others—and causes them to fluoresce in a characteristic way depending on the identity of the microbe.
The exact pattern of fluorescence is then compared with a database of 37 common pathogens to identify the organism present.
“We’re using intrinsic fluorescence to identify the microorganisms,” Walsh noted. “It’s an innate property of most living organisms. Because it’s intrinsic, no reagents are needed for the identification step.”
This removes many of the opportunities for mistakes and lowers test costs, he added.
In tests, this method correctly identified the species in 96.5% of all samples. In the 2.7% of samples for which no species identity was provided, the system was able to correctly identify 67% to the family level, which is often enough information to select an effective therapy.
More than 1000 samples were tested, and the method never gave an incorrect result as to the family level or the Gram type.
Walsh and his colleagues are now working on automating this system with robotics to make it a fully hands-off process. He noted that blood cultures grow in their own time, often producing a positive result at an inconvenient time of the day for clinical labs, so automation could speed up diagnosis significantly.
“Our vision is to have a system that will automatically identify the blood culture isolate within 15 minutes of the culture being called positive,” Walsh said.
If a culture is positive at 2 am, automating this method could make it possible to identify the organism by 2:15 am and send an electronic report to a patient’s physician.
The researchers hope to begin testing and evaluating the feasibility of an automated form of the system in a clinical environment within months. ![]()
Point-Counterpoint: Type II Endoleaks - To treat or not to treat
The management of Type II endoleaks remains controversial. At the Charing Cross meeting this year Jean- Pierre Becquemin and Hence Vehagen debated whether Type II endoleaks should be treated. Dr. Verhagen suggested that results of endoleak treatment are so poor and the natural history of Type II endoleaks so benign that perhaps we shouldn’t even try to treat them. Dr. Becquemin felt that an attempt at treatment for some endoleaks was still necessary. This prompted our editors to suggest a U.S. version of the debate. As you will see both Dr. Geraghty and Dr. Kwolek feel that there is merit in pursuing treatment but with certain caveats. We appreciate their insight into this important subject.
– Dr. Russell Samson is the medical editor of Vascular Specialist.
A slow start can be salvaged
Our group recently published the midterm outcomes of our series of percutaneous glue and /or coil embolizations to treat type II endoleaks (T2EL) accompanied by continued aneurysm sac growth.1 Painstaking retrospective data collection, augmented by blinded review of all follow-up CT scans, demonstrated that at 23 months post intervention, T2EL had persisted or recurred in 72% of patients. Furthermore, the rate of aneurysm sac growth was not altered by the percutaneous intervention.
Other institutions have also reported significant rates of T2EL persistence/recurrence following embolization, and in the Cleveland Clinic experience, continued sac growth in excess of 5 mm in diameter was documented in 56% of patients who had undergone an attempt at T2EL ablation.2-4 In light of this data, one might logically question why I continue to support intervention in this setting.
The midterm reappraisals of T2EL interventions are certainly sobering. We can no longer accept apparent procedural success as a guarantee that these endoleaks will remain clinically and radiographically dormant. Yet these studies were not without their bright spots. In our series, diagnostic angiography (via transfemoral and translumbar approaches) detected occult type I or type III endoleaks in 21% of patients, prompting definitive repair by additional endograft placement. Similar angiographic elucidation of type I and III endoleaks was confirmed by the University of Chicago experience, with a 16% detection rate.2 The diagnosis and treatment of these highly pressurized leaks likely contributed to the low incidence of aneurysm rupture and aneurysm-related mortality seen in most of these series.
Bright spots aside, current midterm results for T2EL intervention leave much to be desired. I would contend, however, that these interventions should be refined, not abandoned. We now appreciate that T2EL mimic the behavior of complex arteriovenous malformations in several ways: the presence of multiple feeding and draining branches, the finding that solitary branch vessel embolization often leads to inflow recruitment from less prominent branches, and the presence of a nidus whose ablation is critical. In retrospect, then, it is not surprising that treatment approaches consisting of single-vessel coiling, or simple embolization of the nidus without concomitant treatment of all feeding branches, failed to produce the desired result. The aggressiveness of early T2EL embolization procedures may have been tempered by the potential for visceral and spinal cord complications.5,6 However, current midterm outcomes data suggest that predictable and durable success in T2EL treatment will require complete nidus ablation and occlusion of all arterial branches of the sac.
How can we pursue this more aggressive goal while maintaining an appropriate safety profile? In several instances, we have pursued this objective via staged interventions: initial angiographic coil/glue deployment, followed by laparoscopic clipping of persistent feeding vessels. The use of Onyx® (EV3-Covidien), as championed by Abularrage and Bosiers,7,8 offers the potential to achieve full treatment with a single intervention.
Advantages of Onyx include predictable polymerization times and lack of adhesion to the delivery catheter. These features permit deliberate and complete obliteration of the nidus, with intentional extension of the polymerizing mixture into the feeding vessels.
T2EL intervention has thus completed its first iteration. The value of routine angiographic examination is clear, particularly as regards the detection of occult type I and III endoleaks. Failure analysis of first-generation T2EL interventions suggests that incomplete obliteration of the endoleak complex (nidus and all feeding branches) sets the stage for vessel recruitment and reestablishment of sac flow. Promising alternative therapies have been identified, and we await their thorough evaluation.
Dr. Geraghty is an associate professor, surgery and radiology, in the division of general surgery, vascular surgery section at Washington University School of Medicine, St. Louis, Mo., and co-director of the Washington University Limb Preservation Center.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55.
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013.
A call for a prospective randomized trial
While I agree with Dr. Geraghty’s comments concerning the initial poor results reported for the percutaneous treatment of Type II endoleaks,1 I disagree with comments made in April 2013 at the Charing Cross meeting by Dr. Hence Verhagen that there is "no need to worry about Type II endoleaks since they are low pressure."2
Early on in our experience we began measuring sac pressures after trans- lumbar catheterization and have found that some aneurysm sacs may in fact have systolic arterial pressures of 8-90 mm HG from large lumbar or IMA collaterals. Furthermore, we and others have described the treatment of patients with ruptured AAA after EVAR who upon open exploration where found to have large patent lumbar arteries as the sole etiology of the ruptured AAA.
Dr. Geraghty and Dr. Sarac at Cleveland Clinic have also described the important observation that these Type II endoleaks may be associated with a Type I or III endoleaks and thus put patients at even higher risk of rupture.3 The extent of these leaks may not be completely elucidated until the time of angiography, again emphasizing the importance of a more aggressive approach to diagnosis and treatment in patients with an enlarging aneurysm sac.
These authors have also appropriately pointed out that overall treatment success rates are low with persistence of type II endoleaks on long–term follow-up. While we initially described an overall success rate of 30%, our approach has changed over time to include direct access to the endoleak nidus with the injection of a flow directed glue (Onyx) to obliterate the flow channel and feeding vessels.
We have found this approach to be more successful than attempting to cannulate each of the feeding lumbar vessels or coil embolization of the aneurysm sac, and can be performed via either a translumbar or transarterial approach using SMA to IMA collaterals. While our success rate using this technique has improved to nearly 80%, several words of caution should be noted. 1) Onyx glue is approved for use in the treatment of intracerebral vascular malformations and does not have a peripheral indication at this time. 2) It is critically important that the interventionalist obtains access into the nidus/flow channel to allow the embolic agent to flow. Otherwise you are injecting glue directly into the sac thrombus and wasting time and an expensive embolic agent. 3) There are often multiple levels of lumbar vessels involved and we have found that we often have to go back several times at different levels to treat extensive Type II endoleaks. All of these observations emphasize the need to further study the treatment of Type II endoleaks and the need for a prospective randomized trial to better identify the best treatment options.
Dr. Kwolek is the director of the vascular and endovascular training program at Massachusetts General Hospital, Boston, and the chief of vascular surgery at Newton Wellesley Hospital.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. Management of Type II endoleaks divides the experts at CX 35. Vascular News International,58, May 2012, BIBA publishing.
3. J Vasc Surg, 2012. 55(1): p. 33-40.
4. J Vasc Surg, 2012. 56(3): p. 630-6.
The management of Type II endoleaks remains controversial. At the Charing Cross meeting this year Jean- Pierre Becquemin and Hence Vehagen debated whether Type II endoleaks should be treated. Dr. Verhagen suggested that results of endoleak treatment are so poor and the natural history of Type II endoleaks so benign that perhaps we shouldn’t even try to treat them. Dr. Becquemin felt that an attempt at treatment for some endoleaks was still necessary. This prompted our editors to suggest a U.S. version of the debate. As you will see both Dr. Geraghty and Dr. Kwolek feel that there is merit in pursuing treatment but with certain caveats. We appreciate their insight into this important subject.
– Dr. Russell Samson is the medical editor of Vascular Specialist.
A slow start can be salvaged
Our group recently published the midterm outcomes of our series of percutaneous glue and /or coil embolizations to treat type II endoleaks (T2EL) accompanied by continued aneurysm sac growth.1 Painstaking retrospective data collection, augmented by blinded review of all follow-up CT scans, demonstrated that at 23 months post intervention, T2EL had persisted or recurred in 72% of patients. Furthermore, the rate of aneurysm sac growth was not altered by the percutaneous intervention.
Other institutions have also reported significant rates of T2EL persistence/recurrence following embolization, and in the Cleveland Clinic experience, continued sac growth in excess of 5 mm in diameter was documented in 56% of patients who had undergone an attempt at T2EL ablation.2-4 In light of this data, one might logically question why I continue to support intervention in this setting.
The midterm reappraisals of T2EL interventions are certainly sobering. We can no longer accept apparent procedural success as a guarantee that these endoleaks will remain clinically and radiographically dormant. Yet these studies were not without their bright spots. In our series, diagnostic angiography (via transfemoral and translumbar approaches) detected occult type I or type III endoleaks in 21% of patients, prompting definitive repair by additional endograft placement. Similar angiographic elucidation of type I and III endoleaks was confirmed by the University of Chicago experience, with a 16% detection rate.2 The diagnosis and treatment of these highly pressurized leaks likely contributed to the low incidence of aneurysm rupture and aneurysm-related mortality seen in most of these series.
Bright spots aside, current midterm results for T2EL intervention leave much to be desired. I would contend, however, that these interventions should be refined, not abandoned. We now appreciate that T2EL mimic the behavior of complex arteriovenous malformations in several ways: the presence of multiple feeding and draining branches, the finding that solitary branch vessel embolization often leads to inflow recruitment from less prominent branches, and the presence of a nidus whose ablation is critical. In retrospect, then, it is not surprising that treatment approaches consisting of single-vessel coiling, or simple embolization of the nidus without concomitant treatment of all feeding branches, failed to produce the desired result. The aggressiveness of early T2EL embolization procedures may have been tempered by the potential for visceral and spinal cord complications.5,6 However, current midterm outcomes data suggest that predictable and durable success in T2EL treatment will require complete nidus ablation and occlusion of all arterial branches of the sac.
How can we pursue this more aggressive goal while maintaining an appropriate safety profile? In several instances, we have pursued this objective via staged interventions: initial angiographic coil/glue deployment, followed by laparoscopic clipping of persistent feeding vessels. The use of Onyx® (EV3-Covidien), as championed by Abularrage and Bosiers,7,8 offers the potential to achieve full treatment with a single intervention.
Advantages of Onyx include predictable polymerization times and lack of adhesion to the delivery catheter. These features permit deliberate and complete obliteration of the nidus, with intentional extension of the polymerizing mixture into the feeding vessels.
T2EL intervention has thus completed its first iteration. The value of routine angiographic examination is clear, particularly as regards the detection of occult type I and III endoleaks. Failure analysis of first-generation T2EL interventions suggests that incomplete obliteration of the endoleak complex (nidus and all feeding branches) sets the stage for vessel recruitment and reestablishment of sac flow. Promising alternative therapies have been identified, and we await their thorough evaluation.
Dr. Geraghty is an associate professor, surgery and radiology, in the division of general surgery, vascular surgery section at Washington University School of Medicine, St. Louis, Mo., and co-director of the Washington University Limb Preservation Center.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55.
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013.
A call for a prospective randomized trial
While I agree with Dr. Geraghty’s comments concerning the initial poor results reported for the percutaneous treatment of Type II endoleaks,1 I disagree with comments made in April 2013 at the Charing Cross meeting by Dr. Hence Verhagen that there is "no need to worry about Type II endoleaks since they are low pressure."2
Early on in our experience we began measuring sac pressures after trans- lumbar catheterization and have found that some aneurysm sacs may in fact have systolic arterial pressures of 8-90 mm HG from large lumbar or IMA collaterals. Furthermore, we and others have described the treatment of patients with ruptured AAA after EVAR who upon open exploration where found to have large patent lumbar arteries as the sole etiology of the ruptured AAA.
Dr. Geraghty and Dr. Sarac at Cleveland Clinic have also described the important observation that these Type II endoleaks may be associated with a Type I or III endoleaks and thus put patients at even higher risk of rupture.3 The extent of these leaks may not be completely elucidated until the time of angiography, again emphasizing the importance of a more aggressive approach to diagnosis and treatment in patients with an enlarging aneurysm sac.
These authors have also appropriately pointed out that overall treatment success rates are low with persistence of type II endoleaks on long–term follow-up. While we initially described an overall success rate of 30%, our approach has changed over time to include direct access to the endoleak nidus with the injection of a flow directed glue (Onyx) to obliterate the flow channel and feeding vessels.
We have found this approach to be more successful than attempting to cannulate each of the feeding lumbar vessels or coil embolization of the aneurysm sac, and can be performed via either a translumbar or transarterial approach using SMA to IMA collaterals. While our success rate using this technique has improved to nearly 80%, several words of caution should be noted. 1) Onyx glue is approved for use in the treatment of intracerebral vascular malformations and does not have a peripheral indication at this time. 2) It is critically important that the interventionalist obtains access into the nidus/flow channel to allow the embolic agent to flow. Otherwise you are injecting glue directly into the sac thrombus and wasting time and an expensive embolic agent. 3) There are often multiple levels of lumbar vessels involved and we have found that we often have to go back several times at different levels to treat extensive Type II endoleaks. All of these observations emphasize the need to further study the treatment of Type II endoleaks and the need for a prospective randomized trial to better identify the best treatment options.
Dr. Kwolek is the director of the vascular and endovascular training program at Massachusetts General Hospital, Boston, and the chief of vascular surgery at Newton Wellesley Hospital.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. Management of Type II endoleaks divides the experts at CX 35. Vascular News International,58, May 2012, BIBA publishing.
3. J Vasc Surg, 2012. 55(1): p. 33-40.
4. J Vasc Surg, 2012. 56(3): p. 630-6.
The management of Type II endoleaks remains controversial. At the Charing Cross meeting this year Jean- Pierre Becquemin and Hence Vehagen debated whether Type II endoleaks should be treated. Dr. Verhagen suggested that results of endoleak treatment are so poor and the natural history of Type II endoleaks so benign that perhaps we shouldn’t even try to treat them. Dr. Becquemin felt that an attempt at treatment for some endoleaks was still necessary. This prompted our editors to suggest a U.S. version of the debate. As you will see both Dr. Geraghty and Dr. Kwolek feel that there is merit in pursuing treatment but with certain caveats. We appreciate their insight into this important subject.
– Dr. Russell Samson is the medical editor of Vascular Specialist.
A slow start can be salvaged
Our group recently published the midterm outcomes of our series of percutaneous glue and /or coil embolizations to treat type II endoleaks (T2EL) accompanied by continued aneurysm sac growth.1 Painstaking retrospective data collection, augmented by blinded review of all follow-up CT scans, demonstrated that at 23 months post intervention, T2EL had persisted or recurred in 72% of patients. Furthermore, the rate of aneurysm sac growth was not altered by the percutaneous intervention.
Other institutions have also reported significant rates of T2EL persistence/recurrence following embolization, and in the Cleveland Clinic experience, continued sac growth in excess of 5 mm in diameter was documented in 56% of patients who had undergone an attempt at T2EL ablation.2-4 In light of this data, one might logically question why I continue to support intervention in this setting.
The midterm reappraisals of T2EL interventions are certainly sobering. We can no longer accept apparent procedural success as a guarantee that these endoleaks will remain clinically and radiographically dormant. Yet these studies were not without their bright spots. In our series, diagnostic angiography (via transfemoral and translumbar approaches) detected occult type I or type III endoleaks in 21% of patients, prompting definitive repair by additional endograft placement. Similar angiographic elucidation of type I and III endoleaks was confirmed by the University of Chicago experience, with a 16% detection rate.2 The diagnosis and treatment of these highly pressurized leaks likely contributed to the low incidence of aneurysm rupture and aneurysm-related mortality seen in most of these series.
Bright spots aside, current midterm results for T2EL intervention leave much to be desired. I would contend, however, that these interventions should be refined, not abandoned. We now appreciate that T2EL mimic the behavior of complex arteriovenous malformations in several ways: the presence of multiple feeding and draining branches, the finding that solitary branch vessel embolization often leads to inflow recruitment from less prominent branches, and the presence of a nidus whose ablation is critical. In retrospect, then, it is not surprising that treatment approaches consisting of single-vessel coiling, or simple embolization of the nidus without concomitant treatment of all feeding branches, failed to produce the desired result. The aggressiveness of early T2EL embolization procedures may have been tempered by the potential for visceral and spinal cord complications.5,6 However, current midterm outcomes data suggest that predictable and durable success in T2EL treatment will require complete nidus ablation and occlusion of all arterial branches of the sac.
How can we pursue this more aggressive goal while maintaining an appropriate safety profile? In several instances, we have pursued this objective via staged interventions: initial angiographic coil/glue deployment, followed by laparoscopic clipping of persistent feeding vessels. The use of Onyx® (EV3-Covidien), as championed by Abularrage and Bosiers,7,8 offers the potential to achieve full treatment with a single intervention.
Advantages of Onyx include predictable polymerization times and lack of adhesion to the delivery catheter. These features permit deliberate and complete obliteration of the nidus, with intentional extension of the polymerizing mixture into the feeding vessels.
T2EL intervention has thus completed its first iteration. The value of routine angiographic examination is clear, particularly as regards the detection of occult type I and III endoleaks. Failure analysis of first-generation T2EL interventions suggests that incomplete obliteration of the endoleak complex (nidus and all feeding branches) sets the stage for vessel recruitment and reestablishment of sac flow. Promising alternative therapies have been identified, and we await their thorough evaluation.
Dr. Geraghty is an associate professor, surgery and radiology, in the division of general surgery, vascular surgery section at Washington University School of Medicine, St. Louis, Mo., and co-director of the Washington University Limb Preservation Center.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55.
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013.
A call for a prospective randomized trial
While I agree with Dr. Geraghty’s comments concerning the initial poor results reported for the percutaneous treatment of Type II endoleaks,1 I disagree with comments made in April 2013 at the Charing Cross meeting by Dr. Hence Verhagen that there is "no need to worry about Type II endoleaks since they are low pressure."2
Early on in our experience we began measuring sac pressures after trans- lumbar catheterization and have found that some aneurysm sacs may in fact have systolic arterial pressures of 8-90 mm HG from large lumbar or IMA collaterals. Furthermore, we and others have described the treatment of patients with ruptured AAA after EVAR who upon open exploration where found to have large patent lumbar arteries as the sole etiology of the ruptured AAA.
Dr. Geraghty and Dr. Sarac at Cleveland Clinic have also described the important observation that these Type II endoleaks may be associated with a Type I or III endoleaks and thus put patients at even higher risk of rupture.3 The extent of these leaks may not be completely elucidated until the time of angiography, again emphasizing the importance of a more aggressive approach to diagnosis and treatment in patients with an enlarging aneurysm sac.
These authors have also appropriately pointed out that overall treatment success rates are low with persistence of type II endoleaks on long–term follow-up. While we initially described an overall success rate of 30%, our approach has changed over time to include direct access to the endoleak nidus with the injection of a flow directed glue (Onyx) to obliterate the flow channel and feeding vessels.
We have found this approach to be more successful than attempting to cannulate each of the feeding lumbar vessels or coil embolization of the aneurysm sac, and can be performed via either a translumbar or transarterial approach using SMA to IMA collaterals. While our success rate using this technique has improved to nearly 80%, several words of caution should be noted. 1) Onyx glue is approved for use in the treatment of intracerebral vascular malformations and does not have a peripheral indication at this time. 2) It is critically important that the interventionalist obtains access into the nidus/flow channel to allow the embolic agent to flow. Otherwise you are injecting glue directly into the sac thrombus and wasting time and an expensive embolic agent. 3) There are often multiple levels of lumbar vessels involved and we have found that we often have to go back several times at different levels to treat extensive Type II endoleaks. All of these observations emphasize the need to further study the treatment of Type II endoleaks and the need for a prospective randomized trial to better identify the best treatment options.
Dr. Kwolek is the director of the vascular and endovascular training program at Massachusetts General Hospital, Boston, and the chief of vascular surgery at Newton Wellesley Hospital.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. Management of Type II endoleaks divides the experts at CX 35. Vascular News International,58, May 2012, BIBA publishing.
3. J Vasc Surg, 2012. 55(1): p. 33-40.
4. J Vasc Surg, 2012. 56(3): p. 630-6.
How To Maximize a Minimal Incision
Mini-incision carotid surgery was first reported (not necessarily performed) by Ascher et al. in 2005. In light of this report and an ever-growing patient demand for minimally invasive procedures, it is surprising that this procedure has not been widely adopted by the vascular surgical community. The reasons for this are probably multifactorial and may include a concern that cranial nerve injuries are more likely to occur as well as an added difficulty in placing a shunt or sewing in a patch. These concerns are mitigated by a thorough knowledge of the usual and variant anatomy of this area and a broad experience in carotid surgery. If performed properly, mini-incision is not synonymous with mini exposure carotid surgery.
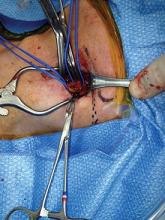
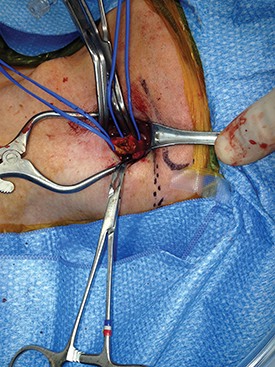
It is important to identify the carotid bifurcation with duplex ultrasound prior to making the skin incision. It is my preference to make a vertical skin incision which extends from approximately 2 cm above to below the carotid bifurcation at a slight outside to inside angle to the anterior border of the sternocleidomastoid muscle (some surgeons prefer a transverse skin incision). The platysma muscle is identified and divided for a short distance between forceps.
The edges of the platysma are grasped with DeBakey forceps while the skin is retracted as far inferiorly as possible.
The platysma is then divided to this point following which this is repeated at the superior aspect of the wound. The extended division of the platysma is what allows for an exposure equal to that of a much larger incision. Now adequate visualization of the common and internal carotid arteries is achieved by use of a small retractor applied alternately to the inferior and superior ends of the wound respectively.
Using this technique, I have easily been able to perform both standard and eversion endarterectomies (as in photo), place a shunt when necessary, and/or sew on a carotid patch.
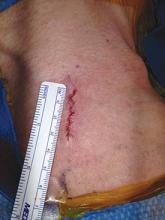
Once completed the platysma is closed with an absorbable suture and skin is closed with an absorbable monofilament suture.
In my experience, patients have less postoperative pain than those with a larger neck incision. They are also exceptionally pleased with the small, barely visible, neck scar. Finally, I have used the mini-incision for my carotid surgeries for more than 10 years during which time no patient has suffered a permanent cranial nerve injury.
Dr. Dietzek is the chief of the vascular and endovascular surgery section and the Linda and Stephen R. Cohen chair in vascular surgery at Danbury Hospital, Danbury Conn. He is also a clinical associate professor of surgery at the University of Vermont College of Medicine in Burlington.
Editor’s Note: If you would like to submit a similarly useful Tips and Tricks, contact us at vascularspecialist@frontlinemedcom.com.
Mini-incision carotid surgery was first reported (not necessarily performed) by Ascher et al. in 2005. In light of this report and an ever-growing patient demand for minimally invasive procedures, it is surprising that this procedure has not been widely adopted by the vascular surgical community. The reasons for this are probably multifactorial and may include a concern that cranial nerve injuries are more likely to occur as well as an added difficulty in placing a shunt or sewing in a patch. These concerns are mitigated by a thorough knowledge of the usual and variant anatomy of this area and a broad experience in carotid surgery. If performed properly, mini-incision is not synonymous with mini exposure carotid surgery.
It is important to identify the carotid bifurcation with duplex ultrasound prior to making the skin incision. It is my preference to make a vertical skin incision which extends from approximately 2 cm above to below the carotid bifurcation at a slight outside to inside angle to the anterior border of the sternocleidomastoid muscle (some surgeons prefer a transverse skin incision). The platysma muscle is identified and divided for a short distance between forceps.
The edges of the platysma are grasped with DeBakey forceps while the skin is retracted as far inferiorly as possible.
The platysma is then divided to this point following which this is repeated at the superior aspect of the wound. The extended division of the platysma is what allows for an exposure equal to that of a much larger incision. Now adequate visualization of the common and internal carotid arteries is achieved by use of a small retractor applied alternately to the inferior and superior ends of the wound respectively.
Using this technique, I have easily been able to perform both standard and eversion endarterectomies (as in photo), place a shunt when necessary, and/or sew on a carotid patch.
Once completed the platysma is closed with an absorbable suture and skin is closed with an absorbable monofilament suture.
In my experience, patients have less postoperative pain than those with a larger neck incision. They are also exceptionally pleased with the small, barely visible, neck scar. Finally, I have used the mini-incision for my carotid surgeries for more than 10 years during which time no patient has suffered a permanent cranial nerve injury.
Dr. Dietzek is the chief of the vascular and endovascular surgery section and the Linda and Stephen R. Cohen chair in vascular surgery at Danbury Hospital, Danbury Conn. He is also a clinical associate professor of surgery at the University of Vermont College of Medicine in Burlington.
Editor’s Note: If you would like to submit a similarly useful Tips and Tricks, contact us at vascularspecialist@frontlinemedcom.com.
Mini-incision carotid surgery was first reported (not necessarily performed) by Ascher et al. in 2005. In light of this report and an ever-growing patient demand for minimally invasive procedures, it is surprising that this procedure has not been widely adopted by the vascular surgical community. The reasons for this are probably multifactorial and may include a concern that cranial nerve injuries are more likely to occur as well as an added difficulty in placing a shunt or sewing in a patch. These concerns are mitigated by a thorough knowledge of the usual and variant anatomy of this area and a broad experience in carotid surgery. If performed properly, mini-incision is not synonymous with mini exposure carotid surgery.
It is important to identify the carotid bifurcation with duplex ultrasound prior to making the skin incision. It is my preference to make a vertical skin incision which extends from approximately 2 cm above to below the carotid bifurcation at a slight outside to inside angle to the anterior border of the sternocleidomastoid muscle (some surgeons prefer a transverse skin incision). The platysma muscle is identified and divided for a short distance between forceps.
The edges of the platysma are grasped with DeBakey forceps while the skin is retracted as far inferiorly as possible.
The platysma is then divided to this point following which this is repeated at the superior aspect of the wound. The extended division of the platysma is what allows for an exposure equal to that of a much larger incision. Now adequate visualization of the common and internal carotid arteries is achieved by use of a small retractor applied alternately to the inferior and superior ends of the wound respectively.
Using this technique, I have easily been able to perform both standard and eversion endarterectomies (as in photo), place a shunt when necessary, and/or sew on a carotid patch.
Once completed the platysma is closed with an absorbable suture and skin is closed with an absorbable monofilament suture.
In my experience, patients have less postoperative pain than those with a larger neck incision. They are also exceptionally pleased with the small, barely visible, neck scar. Finally, I have used the mini-incision for my carotid surgeries for more than 10 years during which time no patient has suffered a permanent cranial nerve injury.
Dr. Dietzek is the chief of the vascular and endovascular surgery section and the Linda and Stephen R. Cohen chair in vascular surgery at Danbury Hospital, Danbury Conn. He is also a clinical associate professor of surgery at the University of Vermont College of Medicine in Burlington.
Editor’s Note: If you would like to submit a similarly useful Tips and Tricks, contact us at vascularspecialist@frontlinemedcom.com.
Welcome Our New Resident Editor
We are pleased to have Dr. Sapan S. Desai come on board as our Resident/Fellow Editor for the next year. Dr. Desai was selected from an excellent candidate pool who submitted their credentials as well as samples of their writings.
Dr. Desai is currently a vascular fellow at University of Texas at Houston/Memorial Hermann Hospital Houston, Tex. He did his surgical residency at Duke University where he still holds the rank of adjunct assistant professor of surgery.
He also has a PhD from the University of Illinois at Chicago, College of Medicine and an MBA in Health Care Management from Western Governors University, Salt Lake City. He is the founder and Executive Editor of the Journal of Surgical Radiology.
We look forward to his contributions and insight into issues that pertain to residents and fellows.
Dr. Russell Samson, Medical Editor, Vascular Specialist
We are pleased to have Dr. Sapan S. Desai come on board as our Resident/Fellow Editor for the next year. Dr. Desai was selected from an excellent candidate pool who submitted their credentials as well as samples of their writings.
Dr. Desai is currently a vascular fellow at University of Texas at Houston/Memorial Hermann Hospital Houston, Tex. He did his surgical residency at Duke University where he still holds the rank of adjunct assistant professor of surgery.
He also has a PhD from the University of Illinois at Chicago, College of Medicine and an MBA in Health Care Management from Western Governors University, Salt Lake City. He is the founder and Executive Editor of the Journal of Surgical Radiology.
We look forward to his contributions and insight into issues that pertain to residents and fellows.
Dr. Russell Samson, Medical Editor, Vascular Specialist
We are pleased to have Dr. Sapan S. Desai come on board as our Resident/Fellow Editor for the next year. Dr. Desai was selected from an excellent candidate pool who submitted their credentials as well as samples of their writings.
Dr. Desai is currently a vascular fellow at University of Texas at Houston/Memorial Hermann Hospital Houston, Tex. He did his surgical residency at Duke University where he still holds the rank of adjunct assistant professor of surgery.
He also has a PhD from the University of Illinois at Chicago, College of Medicine and an MBA in Health Care Management from Western Governors University, Salt Lake City. He is the founder and Executive Editor of the Journal of Surgical Radiology.
We look forward to his contributions and insight into issues that pertain to residents and fellows.
Dr. Russell Samson, Medical Editor, Vascular Specialist
No sex differences in carotid revascularization outcomes?
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
From the Vascular Community
In November, Dr. George Andros was honored with the MedStar Georgetown 2013 Distinguished Achievement Award in Diabetic Limb Salvage at this year’s Diabetic Limb Salvage Conference in Washington, D.C. Dr. Andros is pictured with his award between DLS Conference co-chairs Dr. Christopher E. Attinger (left) and Dr. John S. Steinberg (right).
Dr. Andros is a founding partner of Los Angeles Vascular Specialists and is founder and medical director of the Amputation Prevention Center at Valley Presbyterian Hospital in Van Nuys, Calif.
For the last 14 years, Dr. Andros has been co-chair of the Diabetic Foot Global Conference (DFCon), held in Los Angeles. He is also the emeritus medical editor of Vascular Specialist .
In November, Dr. George Andros was honored with the MedStar Georgetown 2013 Distinguished Achievement Award in Diabetic Limb Salvage at this year’s Diabetic Limb Salvage Conference in Washington, D.C. Dr. Andros is pictured with his award between DLS Conference co-chairs Dr. Christopher E. Attinger (left) and Dr. John S. Steinberg (right).
Dr. Andros is a founding partner of Los Angeles Vascular Specialists and is founder and medical director of the Amputation Prevention Center at Valley Presbyterian Hospital in Van Nuys, Calif.
For the last 14 years, Dr. Andros has been co-chair of the Diabetic Foot Global Conference (DFCon), held in Los Angeles. He is also the emeritus medical editor of Vascular Specialist .
In November, Dr. George Andros was honored with the MedStar Georgetown 2013 Distinguished Achievement Award in Diabetic Limb Salvage at this year’s Diabetic Limb Salvage Conference in Washington, D.C. Dr. Andros is pictured with his award between DLS Conference co-chairs Dr. Christopher E. Attinger (left) and Dr. John S. Steinberg (right).
Dr. Andros is a founding partner of Los Angeles Vascular Specialists and is founder and medical director of the Amputation Prevention Center at Valley Presbyterian Hospital in Van Nuys, Calif.
For the last 14 years, Dr. Andros has been co-chair of the Diabetic Foot Global Conference (DFCon), held in Los Angeles. He is also the emeritus medical editor of Vascular Specialist .
From the Vascular Community
In November, Dr. George Andros was honored with the MedStar Georgetown 2013 Distinguished Achievement Award in Diabetic Limb Salvage at this year’s Diabetic Limb Salvage Conference in Washington, D.C. Dr. Andros is pictured with his award between DLS Conference co-chairs Dr. Christopher E. Attinger (left) and Dr. John S. Steinberg (right).
Dr. Andros is a founding partner of Los Angeles Vascular Specialists and is founder and medical director of the Amputation Prevention Center at Valley Presbyterian Hospital in Van Nuys, Calif.
For the last 14 years, Dr. Andros has been co-chair of the Diabetic Foot Global Conference (DFCon), held in Los Angeles. He is also the emeritus medical editor of Vascular Specialist .
In November, Dr. George Andros was honored with the MedStar Georgetown 2013 Distinguished Achievement Award in Diabetic Limb Salvage at this year’s Diabetic Limb Salvage Conference in Washington, D.C. Dr. Andros is pictured with his award between DLS Conference co-chairs Dr. Christopher E. Attinger (left) and Dr. John S. Steinberg (right).
Dr. Andros is a founding partner of Los Angeles Vascular Specialists and is founder and medical director of the Amputation Prevention Center at Valley Presbyterian Hospital in Van Nuys, Calif.
For the last 14 years, Dr. Andros has been co-chair of the Diabetic Foot Global Conference (DFCon), held in Los Angeles. He is also the emeritus medical editor of Vascular Specialist .
In November, Dr. George Andros was honored with the MedStar Georgetown 2013 Distinguished Achievement Award in Diabetic Limb Salvage at this year’s Diabetic Limb Salvage Conference in Washington, D.C. Dr. Andros is pictured with his award between DLS Conference co-chairs Dr. Christopher E. Attinger (left) and Dr. John S. Steinberg (right).
Dr. Andros is a founding partner of Los Angeles Vascular Specialists and is founder and medical director of the Amputation Prevention Center at Valley Presbyterian Hospital in Van Nuys, Calif.
For the last 14 years, Dr. Andros has been co-chair of the Diabetic Foot Global Conference (DFCon), held in Los Angeles. He is also the emeritus medical editor of Vascular Specialist .
No Benefit to Addition of Pentoxifylline to Steroids for Treatment of Severe Alcoholic Hepatitis
Clinical question
Does the combination of prednisolone and pentoxifylline improve survival in patients with severe alcoholic hepatitis?
Bottom line
Although pentoxifylline and prednisolone have both been demonstrated to be effective individual treatments for alcoholic hepatitis, the combination of both did not improve survival more than prednisolone alone. (LOE = 1b)
Reference
Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: A randomized clinical trial. JAMA 2013;310(10):1033-1041.
Study design
Randomized controlled trial (double-blinded)
Funding source
Government
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
Current guidelines recommend the use of either prednisolone or pentoxifylline for the treatment of severe alcoholic hepatitis. The benefit of the combination of these 2 medications in this setting is unclear. These investigators enrolled patients who were current heavy alcohol users and who had biopsy-proven alcoholic hepatitis with a Maddrey score of 32 or more. Patients were randomized, using concealed allocation, to receive prednisolone 40 mg daily plus pentoxifylline 400 mg 3 times daily (n = 133) or prednisolone 40 mg daily plus placebo (n = 137). Treatment lasted for 28 days and follow-up was 100% at 6 months. The patients had a mean age of 51 years and an average Maddrey score in the 50s (indicating severe disease). No significant difference was detected in 6-month survival between the 2 groups in either the intention-to-treat or per-protocol analyses. Overall, there were 82 deaths in the cohort at 6 months -- 40 in the pentoxifylline-prednisolone group and 42 in the placebo-prednisolone group. The risk of hepatorenal syndrome was decreased at 1 month in the combination therapy group as compared with the placebo group (3.1% vs 11.7%; P = .007), but this difference did not remain significant at 6 months. It is important to note, however, that this study was not powered to detect a difference in the incidence of hepatorenal syndrome.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does the combination of prednisolone and pentoxifylline improve survival in patients with severe alcoholic hepatitis?
Bottom line
Although pentoxifylline and prednisolone have both been demonstrated to be effective individual treatments for alcoholic hepatitis, the combination of both did not improve survival more than prednisolone alone. (LOE = 1b)
Reference
Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: A randomized clinical trial. JAMA 2013;310(10):1033-1041.
Study design
Randomized controlled trial (double-blinded)
Funding source
Government
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
Current guidelines recommend the use of either prednisolone or pentoxifylline for the treatment of severe alcoholic hepatitis. The benefit of the combination of these 2 medications in this setting is unclear. These investigators enrolled patients who were current heavy alcohol users and who had biopsy-proven alcoholic hepatitis with a Maddrey score of 32 or more. Patients were randomized, using concealed allocation, to receive prednisolone 40 mg daily plus pentoxifylline 400 mg 3 times daily (n = 133) or prednisolone 40 mg daily plus placebo (n = 137). Treatment lasted for 28 days and follow-up was 100% at 6 months. The patients had a mean age of 51 years and an average Maddrey score in the 50s (indicating severe disease). No significant difference was detected in 6-month survival between the 2 groups in either the intention-to-treat or per-protocol analyses. Overall, there were 82 deaths in the cohort at 6 months -- 40 in the pentoxifylline-prednisolone group and 42 in the placebo-prednisolone group. The risk of hepatorenal syndrome was decreased at 1 month in the combination therapy group as compared with the placebo group (3.1% vs 11.7%; P = .007), but this difference did not remain significant at 6 months. It is important to note, however, that this study was not powered to detect a difference in the incidence of hepatorenal syndrome.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does the combination of prednisolone and pentoxifylline improve survival in patients with severe alcoholic hepatitis?
Bottom line
Although pentoxifylline and prednisolone have both been demonstrated to be effective individual treatments for alcoholic hepatitis, the combination of both did not improve survival more than prednisolone alone. (LOE = 1b)
Reference
Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: A randomized clinical trial. JAMA 2013;310(10):1033-1041.
Study design
Randomized controlled trial (double-blinded)
Funding source
Government
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
Current guidelines recommend the use of either prednisolone or pentoxifylline for the treatment of severe alcoholic hepatitis. The benefit of the combination of these 2 medications in this setting is unclear. These investigators enrolled patients who were current heavy alcohol users and who had biopsy-proven alcoholic hepatitis with a Maddrey score of 32 or more. Patients were randomized, using concealed allocation, to receive prednisolone 40 mg daily plus pentoxifylline 400 mg 3 times daily (n = 133) or prednisolone 40 mg daily plus placebo (n = 137). Treatment lasted for 28 days and follow-up was 100% at 6 months. The patients had a mean age of 51 years and an average Maddrey score in the 50s (indicating severe disease). No significant difference was detected in 6-month survival between the 2 groups in either the intention-to-treat or per-protocol analyses. Overall, there were 82 deaths in the cohort at 6 months -- 40 in the pentoxifylline-prednisolone group and 42 in the placebo-prednisolone group. The risk of hepatorenal syndrome was decreased at 1 month in the combination therapy group as compared with the placebo group (3.1% vs 11.7%; P = .007), but this difference did not remain significant at 6 months. It is important to note, however, that this study was not powered to detect a difference in the incidence of hepatorenal syndrome.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Preventive PCI During Treatment for Acute STEMI Reduces Future Cardiovascular Events (PRAMI)
Clinical question
Does preventive percutaneous coronary intervention of noninfarct but stenosed arteries improve outcomes in patients with acute ST-segment elevation myocardial infarction?
Bottom line
Preventive percutaneous coronary intervention (PCI) of noninfarct, stenosed arteries duing treatment for acute ST-segment elevation myocardial infarction (STEMI) is effective in decreasing long-term cardiovascular events. You would need to treat 7 patients with preventive PCI to avoid one such event. (LOE = 1b)
Reference
Wald DS, Morris JK, Wald NJ, et al, for the PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369(12):1115-1123.
Study design
Randomized controlled trial (single-blinded)
Funding source
Foundation
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
This study enrolled 465 patients with acute STEMI who received successful PCI to the infarct artery and were noted to have stenosis greater than 50% in other noninfarct coronary arteries during angiography. Patients with history of, or indications for, coronary artery bypass grafting and those in cardiogenic shock were excluded. Using concealed allocation, the investigators randomized patients to receive no further PCI or immediate preventive PCI to noninfarct arteries. The 2 groups had similar comorbidities and a mean age of 62 years. The use of drug-eluting stents and medical therapy after discharge were also similar between groups. The primary outcome was the composite of death from cardiac causes, nonfatal myocardial infarction, or refractory angina. Mean follow-up was 2 years and analysis was by intention to treat. The trial was stopped early because of highly significant results favoring preventive PCI. Overall, there was a 9% event rate in the preventive PCI group as compared with 23% in the other group (hazard ratio = 0.35; 95% CI, 0.21-0.58; P < .001). The individual components of the primary outcome showed similar results, although the reduction in cardiac death was not statistically significant (P = .07). As expected, the procedure time and contrast volume used were higher in the preventive PCI group, but the complication rates, including contrast-induced nephropathy, did not differ between groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does preventive percutaneous coronary intervention of noninfarct but stenosed arteries improve outcomes in patients with acute ST-segment elevation myocardial infarction?
Bottom line
Preventive percutaneous coronary intervention (PCI) of noninfarct, stenosed arteries duing treatment for acute ST-segment elevation myocardial infarction (STEMI) is effective in decreasing long-term cardiovascular events. You would need to treat 7 patients with preventive PCI to avoid one such event. (LOE = 1b)
Reference
Wald DS, Morris JK, Wald NJ, et al, for the PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369(12):1115-1123.
Study design
Randomized controlled trial (single-blinded)
Funding source
Foundation
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
This study enrolled 465 patients with acute STEMI who received successful PCI to the infarct artery and were noted to have stenosis greater than 50% in other noninfarct coronary arteries during angiography. Patients with history of, or indications for, coronary artery bypass grafting and those in cardiogenic shock were excluded. Using concealed allocation, the investigators randomized patients to receive no further PCI or immediate preventive PCI to noninfarct arteries. The 2 groups had similar comorbidities and a mean age of 62 years. The use of drug-eluting stents and medical therapy after discharge were also similar between groups. The primary outcome was the composite of death from cardiac causes, nonfatal myocardial infarction, or refractory angina. Mean follow-up was 2 years and analysis was by intention to treat. The trial was stopped early because of highly significant results favoring preventive PCI. Overall, there was a 9% event rate in the preventive PCI group as compared with 23% in the other group (hazard ratio = 0.35; 95% CI, 0.21-0.58; P < .001). The individual components of the primary outcome showed similar results, although the reduction in cardiac death was not statistically significant (P = .07). As expected, the procedure time and contrast volume used were higher in the preventive PCI group, but the complication rates, including contrast-induced nephropathy, did not differ between groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does preventive percutaneous coronary intervention of noninfarct but stenosed arteries improve outcomes in patients with acute ST-segment elevation myocardial infarction?
Bottom line
Preventive percutaneous coronary intervention (PCI) of noninfarct, stenosed arteries duing treatment for acute ST-segment elevation myocardial infarction (STEMI) is effective in decreasing long-term cardiovascular events. You would need to treat 7 patients with preventive PCI to avoid one such event. (LOE = 1b)
Reference
Wald DS, Morris JK, Wald NJ, et al, for the PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369(12):1115-1123.
Study design
Randomized controlled trial (single-blinded)
Funding source
Foundation
Allocation
Concealed
Setting
Inpatient (any location) with outpatient follow-up
Synopsis
This study enrolled 465 patients with acute STEMI who received successful PCI to the infarct artery and were noted to have stenosis greater than 50% in other noninfarct coronary arteries during angiography. Patients with history of, or indications for, coronary artery bypass grafting and those in cardiogenic shock were excluded. Using concealed allocation, the investigators randomized patients to receive no further PCI or immediate preventive PCI to noninfarct arteries. The 2 groups had similar comorbidities and a mean age of 62 years. The use of drug-eluting stents and medical therapy after discharge were also similar between groups. The primary outcome was the composite of death from cardiac causes, nonfatal myocardial infarction, or refractory angina. Mean follow-up was 2 years and analysis was by intention to treat. The trial was stopped early because of highly significant results favoring preventive PCI. Overall, there was a 9% event rate in the preventive PCI group as compared with 23% in the other group (hazard ratio = 0.35; 95% CI, 0.21-0.58; P < .001). The individual components of the primary outcome showed similar results, although the reduction in cardiac death was not statistically significant (P = .07). As expected, the procedure time and contrast volume used were higher in the preventive PCI group, but the complication rates, including contrast-induced nephropathy, did not differ between groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
A one-size-fits-all fenestrated graft for iliac aneurysms?
CHICAGO – A novel bifurcated covered stent graft limb that uses an off-the-shelf graft can treat large common iliac aneurysms, while preserving good pelvic blood flow.
The alternative endovascular approach has been performed on 15 patients since April 2011, with a success rate of 100%. Bilateral stent grafts were placed in four patients.
The all-male cohort has been able to maintain appropriate exercise tolerance and remains free from erectile dysfunction, pelvic ischemia, buttock claudication, and paralysis.
"These people do well,extremely well," Dr. Patrick Kelly said at the annual meeting of the Midwestern Vascular Surgical Society.
Several iliac branch grafts are currently under investigation, including the Cook Zenith Branch iliac endovascular graft. They promise to preserve flow to the internal iliac artery and thus reduce the potential for ischemic sequelae resulting from iliac embolization. Depending on patient anatomy, however, the internal iliac may become jailed upon deployment of the main body graft, said Dr. Kelly of Sanford Health, Sioux Falls, S.D. The fenestrated systems are also limited by bridging stent technology and the relatively short bridging stent.
His alternative modified bifurcated limb divides the common iliac flow into the internal and external iliac arteries, while excluding the common iliac artery aneurysm.
"The pros are that it uses an off-the-shelf [graft], should be able to handle virtually any anatomy, can be used to treat either existing EVAR or previous open repairs, and has multiple off-ramps, so you don’t jail yourself," he said. "The cons: It requires arm access – although I’m not sure that’s a con – and it requires three stents."
Operative details
The bifurcated limb is created by sewing an 8-mm and 10-mm covered stent graft to the distal end of a standard 16 x 20 x 82-mm stent graft limb. The distal ends of both the 8-mm and 10-mm grafts are left free, allowing flexibility and easier selection of the internal iliac artery, he said.
Once the graft is resheathed using a spiral wire technique, a traditional infrarenal abdominal aneurysm repair is performed. In order to exclude the common iliac aneurysm, the graft is oriented with the 8-mm limb toward the internal iliac and with the distal end of the 8-mm limb being deployed 2-3 cm above the origin of the internal iliac artery. The internal iliac artery is selected from an arm approach, through the 8-mm limb of the bifurcated stent graft limb.
Angiograms are performed and a 3-cm covered, self-expanding bridge stent graft is deployed. The 10-mm limb is used to extend the graft into the external iliac, thus completing exclusion of the common iliac aneurysm, while preserving both the internal and external iliac arteries, Dr. Kelly said.
Thus far, occlusion of the external iliac artery has been reported in one patient, and there were no recurring endoleaks. There was a type 3 endoleak between the main body and bridging stent that was visible on diagnostic angiography, but it resolved after being reballooned and patent flow was established upon completion angiography, Dr. Kelly explained. There was also a retrograde fill that was fixed 1 year postoperatively by extending the limb to obtain a healthy seal.
The average patient age was 65.4 years (range, 46-87 years); fluoroscopy time, 46 minutes (range, 29-91 minutes); and average length of stay 3.1 days (range, 1-9 days).
This compares with an average hospital stay of 4-7 days for the tried-and-true method of open aneurysm repair, which has bleeding rates of 30% or more, colonic ischemia in 20%-30%, and paraplegia in 2%-3%, Dr. Kelly noted.
Audience reaction
Dr. Rebecca Kelso of the Cleveland Clinic, who co-moderated the session, was enthusiastic about the novel approach.
"The potential for it is quite significant, because the other main competitive device he mentioned that’s on the market still has anatomic limitations for use," she said in an interview. "So if he has something that can be used in any patient, no matter what the circumstances, that has significant implications for being available commercially for everyone."
Fellow moderator Dr. Patrick J. Geraghty of Washington University, St. Louis, remarked that while the approach uses a standardized graft, it is somewhat tailored since the length and the diameter of the grafts extending into the external and internal iliac arteries can be chosen separately. That said, the one-size-fits-all approach is particularly appealing because it could simplify treatment planning and reduce treatment delays.
"If you have a patient who is symptomatic and you have an off-the-shelf component, you could potentially treat them within the next 24 hours," he said in an interview. "The current turnaround time for the fenestrated system is about a month or so, so it would shorten treatment delays and might lead to a broader application of the technology."
A potentially shorter hospital length of stay could also reduce hospital costs, Dr. Kelso noted.
While the audience appeared equally enthusiastic about the results, some members questioned whether results on a physician-modified graft without an Investigational Device Exemption (IDE) should be presented at the meeting in light of recent warnings by the U.S. Food and Drug Administration that such interventions involve the use of significant-risk devices and need to be conducted under an IDE. Dr. Kelly responded that he is currently working with the FDA to obtain an IDE.
Earlier this year, the Society of Thoracic Surgeons and the American College of Cardiology became the first medical societies to receive an IDE to study alternative access for transcatheter aortic valve replacement using the STS/ACC TVT Registry.
Dr. Kelly and Dr. Kelso reported having no financial disclosures. Dr. Geraghty disclosed relationships with Cook Medical and Bard/Lutonix.
 | |
Dr. John F. Eidt |
Vascular surgeons are like cobblers – we try to make the perfect pair of shoes for each customer. While all aneurysm operations are similar in principle, in reality each successful operation depends on a unique blend of surgical skill and experience applied to the individual anatomy of each patient. By combining endovascular and open surgical skills, vascular surgeons are enticed to develop ever more innovative solutions for complex problems. Dr. Kelly should be congratulated for thinking outside the box and applying his imagination and skill to the solution of this common clinical scenario. While I am intrigued by the technique, I will reserve my enthusiastic endorsement because of the relatively high cost and the fact the common iliac artery must be sufficiently large to accommodate the physician-modified bifurcated graft. Others have described the use of a "stacked" Gore Excluder device to achieve a similar result. And the Cook Iliac Branched Device is nearing approval. In the long run, the FDA needs to adopt policies that encourage rather than discourage innovation in the develop- ment of novel surgical treatments. The current onerous IDE process is excessively complex and expensive. Physician-modified endografts fill an important gap in our ability to deliver quality, customized care for every patient. And there is no evidence that innovation in vascular surgery has been harmful to patients. After all, every operation is "physician modified."
Dr. John F. Eidt is a vascular surgeon at the Greenville (South Carolina) Health System, and an associate medical editor of Vascular Specialist.
 | |
Dr. John F. Eidt |
Vascular surgeons are like cobblers – we try to make the perfect pair of shoes for each customer. While all aneurysm operations are similar in principle, in reality each successful operation depends on a unique blend of surgical skill and experience applied to the individual anatomy of each patient. By combining endovascular and open surgical skills, vascular surgeons are enticed to develop ever more innovative solutions for complex problems. Dr. Kelly should be congratulated for thinking outside the box and applying his imagination and skill to the solution of this common clinical scenario. While I am intrigued by the technique, I will reserve my enthusiastic endorsement because of the relatively high cost and the fact the common iliac artery must be sufficiently large to accommodate the physician-modified bifurcated graft. Others have described the use of a "stacked" Gore Excluder device to achieve a similar result. And the Cook Iliac Branched Device is nearing approval. In the long run, the FDA needs to adopt policies that encourage rather than discourage innovation in the develop- ment of novel surgical treatments. The current onerous IDE process is excessively complex and expensive. Physician-modified endografts fill an important gap in our ability to deliver quality, customized care for every patient. And there is no evidence that innovation in vascular surgery has been harmful to patients. After all, every operation is "physician modified."
Dr. John F. Eidt is a vascular surgeon at the Greenville (South Carolina) Health System, and an associate medical editor of Vascular Specialist.
 | |
Dr. John F. Eidt |
Vascular surgeons are like cobblers – we try to make the perfect pair of shoes for each customer. While all aneurysm operations are similar in principle, in reality each successful operation depends on a unique blend of surgical skill and experience applied to the individual anatomy of each patient. By combining endovascular and open surgical skills, vascular surgeons are enticed to develop ever more innovative solutions for complex problems. Dr. Kelly should be congratulated for thinking outside the box and applying his imagination and skill to the solution of this common clinical scenario. While I am intrigued by the technique, I will reserve my enthusiastic endorsement because of the relatively high cost and the fact the common iliac artery must be sufficiently large to accommodate the physician-modified bifurcated graft. Others have described the use of a "stacked" Gore Excluder device to achieve a similar result. And the Cook Iliac Branched Device is nearing approval. In the long run, the FDA needs to adopt policies that encourage rather than discourage innovation in the develop- ment of novel surgical treatments. The current onerous IDE process is excessively complex and expensive. Physician-modified endografts fill an important gap in our ability to deliver quality, customized care for every patient. And there is no evidence that innovation in vascular surgery has been harmful to patients. After all, every operation is "physician modified."
Dr. John F. Eidt is a vascular surgeon at the Greenville (South Carolina) Health System, and an associate medical editor of Vascular Specialist.
CHICAGO – A novel bifurcated covered stent graft limb that uses an off-the-shelf graft can treat large common iliac aneurysms, while preserving good pelvic blood flow.
The alternative endovascular approach has been performed on 15 patients since April 2011, with a success rate of 100%. Bilateral stent grafts were placed in four patients.
The all-male cohort has been able to maintain appropriate exercise tolerance and remains free from erectile dysfunction, pelvic ischemia, buttock claudication, and paralysis.
"These people do well,extremely well," Dr. Patrick Kelly said at the annual meeting of the Midwestern Vascular Surgical Society.
Several iliac branch grafts are currently under investigation, including the Cook Zenith Branch iliac endovascular graft. They promise to preserve flow to the internal iliac artery and thus reduce the potential for ischemic sequelae resulting from iliac embolization. Depending on patient anatomy, however, the internal iliac may become jailed upon deployment of the main body graft, said Dr. Kelly of Sanford Health, Sioux Falls, S.D. The fenestrated systems are also limited by bridging stent technology and the relatively short bridging stent.
His alternative modified bifurcated limb divides the common iliac flow into the internal and external iliac arteries, while excluding the common iliac artery aneurysm.
"The pros are that it uses an off-the-shelf [graft], should be able to handle virtually any anatomy, can be used to treat either existing EVAR or previous open repairs, and has multiple off-ramps, so you don’t jail yourself," he said. "The cons: It requires arm access – although I’m not sure that’s a con – and it requires three stents."
Operative details
The bifurcated limb is created by sewing an 8-mm and 10-mm covered stent graft to the distal end of a standard 16 x 20 x 82-mm stent graft limb. The distal ends of both the 8-mm and 10-mm grafts are left free, allowing flexibility and easier selection of the internal iliac artery, he said.
Once the graft is resheathed using a spiral wire technique, a traditional infrarenal abdominal aneurysm repair is performed. In order to exclude the common iliac aneurysm, the graft is oriented with the 8-mm limb toward the internal iliac and with the distal end of the 8-mm limb being deployed 2-3 cm above the origin of the internal iliac artery. The internal iliac artery is selected from an arm approach, through the 8-mm limb of the bifurcated stent graft limb.
Angiograms are performed and a 3-cm covered, self-expanding bridge stent graft is deployed. The 10-mm limb is used to extend the graft into the external iliac, thus completing exclusion of the common iliac aneurysm, while preserving both the internal and external iliac arteries, Dr. Kelly said.
Thus far, occlusion of the external iliac artery has been reported in one patient, and there were no recurring endoleaks. There was a type 3 endoleak between the main body and bridging stent that was visible on diagnostic angiography, but it resolved after being reballooned and patent flow was established upon completion angiography, Dr. Kelly explained. There was also a retrograde fill that was fixed 1 year postoperatively by extending the limb to obtain a healthy seal.
The average patient age was 65.4 years (range, 46-87 years); fluoroscopy time, 46 minutes (range, 29-91 minutes); and average length of stay 3.1 days (range, 1-9 days).
This compares with an average hospital stay of 4-7 days for the tried-and-true method of open aneurysm repair, which has bleeding rates of 30% or more, colonic ischemia in 20%-30%, and paraplegia in 2%-3%, Dr. Kelly noted.
Audience reaction
Dr. Rebecca Kelso of the Cleveland Clinic, who co-moderated the session, was enthusiastic about the novel approach.
"The potential for it is quite significant, because the other main competitive device he mentioned that’s on the market still has anatomic limitations for use," she said in an interview. "So if he has something that can be used in any patient, no matter what the circumstances, that has significant implications for being available commercially for everyone."
Fellow moderator Dr. Patrick J. Geraghty of Washington University, St. Louis, remarked that while the approach uses a standardized graft, it is somewhat tailored since the length and the diameter of the grafts extending into the external and internal iliac arteries can be chosen separately. That said, the one-size-fits-all approach is particularly appealing because it could simplify treatment planning and reduce treatment delays.
"If you have a patient who is symptomatic and you have an off-the-shelf component, you could potentially treat them within the next 24 hours," he said in an interview. "The current turnaround time for the fenestrated system is about a month or so, so it would shorten treatment delays and might lead to a broader application of the technology."
A potentially shorter hospital length of stay could also reduce hospital costs, Dr. Kelso noted.
While the audience appeared equally enthusiastic about the results, some members questioned whether results on a physician-modified graft without an Investigational Device Exemption (IDE) should be presented at the meeting in light of recent warnings by the U.S. Food and Drug Administration that such interventions involve the use of significant-risk devices and need to be conducted under an IDE. Dr. Kelly responded that he is currently working with the FDA to obtain an IDE.
Earlier this year, the Society of Thoracic Surgeons and the American College of Cardiology became the first medical societies to receive an IDE to study alternative access for transcatheter aortic valve replacement using the STS/ACC TVT Registry.
Dr. Kelly and Dr. Kelso reported having no financial disclosures. Dr. Geraghty disclosed relationships with Cook Medical and Bard/Lutonix.
CHICAGO – A novel bifurcated covered stent graft limb that uses an off-the-shelf graft can treat large common iliac aneurysms, while preserving good pelvic blood flow.
The alternative endovascular approach has been performed on 15 patients since April 2011, with a success rate of 100%. Bilateral stent grafts were placed in four patients.
The all-male cohort has been able to maintain appropriate exercise tolerance and remains free from erectile dysfunction, pelvic ischemia, buttock claudication, and paralysis.
"These people do well,extremely well," Dr. Patrick Kelly said at the annual meeting of the Midwestern Vascular Surgical Society.
Several iliac branch grafts are currently under investigation, including the Cook Zenith Branch iliac endovascular graft. They promise to preserve flow to the internal iliac artery and thus reduce the potential for ischemic sequelae resulting from iliac embolization. Depending on patient anatomy, however, the internal iliac may become jailed upon deployment of the main body graft, said Dr. Kelly of Sanford Health, Sioux Falls, S.D. The fenestrated systems are also limited by bridging stent technology and the relatively short bridging stent.
His alternative modified bifurcated limb divides the common iliac flow into the internal and external iliac arteries, while excluding the common iliac artery aneurysm.
"The pros are that it uses an off-the-shelf [graft], should be able to handle virtually any anatomy, can be used to treat either existing EVAR or previous open repairs, and has multiple off-ramps, so you don’t jail yourself," he said. "The cons: It requires arm access – although I’m not sure that’s a con – and it requires three stents."
Operative details
The bifurcated limb is created by sewing an 8-mm and 10-mm covered stent graft to the distal end of a standard 16 x 20 x 82-mm stent graft limb. The distal ends of both the 8-mm and 10-mm grafts are left free, allowing flexibility and easier selection of the internal iliac artery, he said.
Once the graft is resheathed using a spiral wire technique, a traditional infrarenal abdominal aneurysm repair is performed. In order to exclude the common iliac aneurysm, the graft is oriented with the 8-mm limb toward the internal iliac and with the distal end of the 8-mm limb being deployed 2-3 cm above the origin of the internal iliac artery. The internal iliac artery is selected from an arm approach, through the 8-mm limb of the bifurcated stent graft limb.
Angiograms are performed and a 3-cm covered, self-expanding bridge stent graft is deployed. The 10-mm limb is used to extend the graft into the external iliac, thus completing exclusion of the common iliac aneurysm, while preserving both the internal and external iliac arteries, Dr. Kelly said.
Thus far, occlusion of the external iliac artery has been reported in one patient, and there were no recurring endoleaks. There was a type 3 endoleak between the main body and bridging stent that was visible on diagnostic angiography, but it resolved after being reballooned and patent flow was established upon completion angiography, Dr. Kelly explained. There was also a retrograde fill that was fixed 1 year postoperatively by extending the limb to obtain a healthy seal.
The average patient age was 65.4 years (range, 46-87 years); fluoroscopy time, 46 minutes (range, 29-91 minutes); and average length of stay 3.1 days (range, 1-9 days).
This compares with an average hospital stay of 4-7 days for the tried-and-true method of open aneurysm repair, which has bleeding rates of 30% or more, colonic ischemia in 20%-30%, and paraplegia in 2%-3%, Dr. Kelly noted.
Audience reaction
Dr. Rebecca Kelso of the Cleveland Clinic, who co-moderated the session, was enthusiastic about the novel approach.
"The potential for it is quite significant, because the other main competitive device he mentioned that’s on the market still has anatomic limitations for use," she said in an interview. "So if he has something that can be used in any patient, no matter what the circumstances, that has significant implications for being available commercially for everyone."
Fellow moderator Dr. Patrick J. Geraghty of Washington University, St. Louis, remarked that while the approach uses a standardized graft, it is somewhat tailored since the length and the diameter of the grafts extending into the external and internal iliac arteries can be chosen separately. That said, the one-size-fits-all approach is particularly appealing because it could simplify treatment planning and reduce treatment delays.
"If you have a patient who is symptomatic and you have an off-the-shelf component, you could potentially treat them within the next 24 hours," he said in an interview. "The current turnaround time for the fenestrated system is about a month or so, so it would shorten treatment delays and might lead to a broader application of the technology."
A potentially shorter hospital length of stay could also reduce hospital costs, Dr. Kelso noted.
While the audience appeared equally enthusiastic about the results, some members questioned whether results on a physician-modified graft without an Investigational Device Exemption (IDE) should be presented at the meeting in light of recent warnings by the U.S. Food and Drug Administration that such interventions involve the use of significant-risk devices and need to be conducted under an IDE. Dr. Kelly responded that he is currently working with the FDA to obtain an IDE.
Earlier this year, the Society of Thoracic Surgeons and the American College of Cardiology became the first medical societies to receive an IDE to study alternative access for transcatheter aortic valve replacement using the STS/ACC TVT Registry.
Dr. Kelly and Dr. Kelso reported having no financial disclosures. Dr. Geraghty disclosed relationships with Cook Medical and Bard/Lutonix.