User login
New Physician Editor of The Hospitalist Offers Broad Experience and an Eye for QI

—Danielle Scheurer, MD, MSCR, SFHM, physician editor, The Hospitalist
There has always been a journalist dwelling in Danielle Scheurer, MD, MSCR, SFHM. As an undergrad at Emory University, she saw TV reporting in her future.
“I was on the Katie Couric kick for a decade,” she says.
Her course eventually shifted—dramatically. But since she became a hospitalist, Dr. Scheurer, now the chief quality officer at the Medical University of South Carolina (MUSC) in Charleston, has stayed involved with a long slate of editorial projects.
Her latest: She is the new physician editor of The Hospitalist. With the appointment, the magazine gains a high-energy physician with a broad spectrum of knowledge. Colleagues say she has a knack for seeing the big picture and taking a bolus of information and conveying its relevance to hospitalists and other medical professionals.
Dr. Scheurer says that one of her aims will be to make the publication’s website—www.the-hospitalist.org—more interactive, allowing for more direct participation from readers, such as with polls and forums on topics covered.
“A lot of us in the hospital sort of struggle with the exact same things,” she says. “I think there’s some value in connecting, even if it’s in just short little snippets.”
She also would like to increase the website’s use of audio files so that doctors have more options in how they get their information.
But above all, she says, she wants to keep The Hospitalist “one of the most practical publications available to hospitalists,” a publication that is specifically tailored to deliver useful messages.
“I feel like it’s a very high-yield publication for really busy hospitalists,” she says.
Career Shuffle = Diverse Experience
Dr. Scheurer brings experience from a variety of settings, such as the small community hospital Trident Medical Center in Charleston to the large, urban medical centers that are Brigham and Women’s Hospital in Boston and MUSC.
She said some of her career moves came with some apprehension, including those that came about when her husband got a position that required her to move, too. But she says she has benefited from those experiences. Trident “gave me a window into hospital medicine that I never otherwise would have had,” she notes.
She never anticipated moving to Boston, and she admits it felt outside of her “comfort zone.” But in 2005, she found herself at Brigham. She had just earned a master’s degree in clinical research and thought she’d end up being a researcher. In Boston, she got a glimpse of what it meant to be a “hard-core, NIH-funded researcher” and decided it wasn’t for her.
While there, she took training courses in leadership and quality improvement. And QI stuck.
In 2010, she returned to MUSC and now leads QI for the whole hospital, a medium-size setting that she says is just right for promoting change.
“This is definitely my sweet spot,” she says. “If you’re going to change people’s minds, it’s a lot easer to change 200 people’s minds than 450 people’s minds.”
Chris Roy, MD, medical director of the hospitalist service at Brigham, says Dr. Scheurer was “one of the most-hard working people that I knew” and a strong leader with an “uncanny, almost photographic memory of all the hospital medicine literature.”
“Even though she was very forceful as a leader, she never irritated anyone,” he adds. “She was very skillful in managing people.”
Chris Rees, the director of quality and patient safety at MUSC, says Dr. Scheurer is adept at taking issues that evolve from the hospital and collaborating on them with other university departments. She is good at putting herself in other groups’ shoes and delivering messages succinctly, he says.
“She’s definitely not seen as just one of those white coats,” Rees says.
Highly Recommended
On top of her QI projects, Dr. Scheurer is involved as an advisor, contributor, or reviewer at 11 other publications or online venues. The Hospitalist will make it an even dozen.
“She’s just a dynamo,” Rees says. “She walks around with her MacAir book and she’s constantly writing stuff on it and sending out emails.”
Patrick Cawley, MD, MBA, MHM, the chief medical officer at MUSC who hired Dr. Scheurer when she first worked there in 2003, has seen her move from small projects to systemwide efforts.
“She did a great job and is very collaborative, very knowledgeable, [and] brings an evidence-based approach to problems,” says Dr. Cawley, a past president of SHM and recent inductee as a Master in Hospital Medicine (MHM).
She is quick to notice trends and patterns, he points out. “She’s very knowledgeable about what’s going on in the hospitalist arena,” he says, adding he anticipates she’ll be interested in “data-driven” coverage, along with QI topics.
Dr. Scheurer’s interest in disseminating information shouldn’t be a surprise—it’s a fundamental part of QI and instrumental in systemwide change. She finds it “appealing to work on a project and know that it’s going to affect the next 20,000 patients.”
“There’s no one single person that can ensure that the patient gets all of their needs met,” she says. “There has to be a system approach.”
At The Hospitalist, she will try to keep pace with all the change that hospitals are constantly trying to navigate.
“I don’t think there will ever be a deficiency of content to cover,” she says. “Something’s always brand-new.”
Tom Collins is a freelance writer in Florida.

—Danielle Scheurer, MD, MSCR, SFHM, physician editor, The Hospitalist
There has always been a journalist dwelling in Danielle Scheurer, MD, MSCR, SFHM. As an undergrad at Emory University, she saw TV reporting in her future.
“I was on the Katie Couric kick for a decade,” she says.
Her course eventually shifted—dramatically. But since she became a hospitalist, Dr. Scheurer, now the chief quality officer at the Medical University of South Carolina (MUSC) in Charleston, has stayed involved with a long slate of editorial projects.
Her latest: She is the new physician editor of The Hospitalist. With the appointment, the magazine gains a high-energy physician with a broad spectrum of knowledge. Colleagues say she has a knack for seeing the big picture and taking a bolus of information and conveying its relevance to hospitalists and other medical professionals.
Dr. Scheurer says that one of her aims will be to make the publication’s website—www.the-hospitalist.org—more interactive, allowing for more direct participation from readers, such as with polls and forums on topics covered.
“A lot of us in the hospital sort of struggle with the exact same things,” she says. “I think there’s some value in connecting, even if it’s in just short little snippets.”
She also would like to increase the website’s use of audio files so that doctors have more options in how they get their information.
But above all, she says, she wants to keep The Hospitalist “one of the most practical publications available to hospitalists,” a publication that is specifically tailored to deliver useful messages.
“I feel like it’s a very high-yield publication for really busy hospitalists,” she says.
Career Shuffle = Diverse Experience
Dr. Scheurer brings experience from a variety of settings, such as the small community hospital Trident Medical Center in Charleston to the large, urban medical centers that are Brigham and Women’s Hospital in Boston and MUSC.
She said some of her career moves came with some apprehension, including those that came about when her husband got a position that required her to move, too. But she says she has benefited from those experiences. Trident “gave me a window into hospital medicine that I never otherwise would have had,” she notes.
She never anticipated moving to Boston, and she admits it felt outside of her “comfort zone.” But in 2005, she found herself at Brigham. She had just earned a master’s degree in clinical research and thought she’d end up being a researcher. In Boston, she got a glimpse of what it meant to be a “hard-core, NIH-funded researcher” and decided it wasn’t for her.
While there, she took training courses in leadership and quality improvement. And QI stuck.
In 2010, she returned to MUSC and now leads QI for the whole hospital, a medium-size setting that she says is just right for promoting change.
“This is definitely my sweet spot,” she says. “If you’re going to change people’s minds, it’s a lot easer to change 200 people’s minds than 450 people’s minds.”
Chris Roy, MD, medical director of the hospitalist service at Brigham, says Dr. Scheurer was “one of the most-hard working people that I knew” and a strong leader with an “uncanny, almost photographic memory of all the hospital medicine literature.”
“Even though she was very forceful as a leader, she never irritated anyone,” he adds. “She was very skillful in managing people.”
Chris Rees, the director of quality and patient safety at MUSC, says Dr. Scheurer is adept at taking issues that evolve from the hospital and collaborating on them with other university departments. She is good at putting herself in other groups’ shoes and delivering messages succinctly, he says.
“She’s definitely not seen as just one of those white coats,” Rees says.
Highly Recommended
On top of her QI projects, Dr. Scheurer is involved as an advisor, contributor, or reviewer at 11 other publications or online venues. The Hospitalist will make it an even dozen.
“She’s just a dynamo,” Rees says. “She walks around with her MacAir book and she’s constantly writing stuff on it and sending out emails.”
Patrick Cawley, MD, MBA, MHM, the chief medical officer at MUSC who hired Dr. Scheurer when she first worked there in 2003, has seen her move from small projects to systemwide efforts.
“She did a great job and is very collaborative, very knowledgeable, [and] brings an evidence-based approach to problems,” says Dr. Cawley, a past president of SHM and recent inductee as a Master in Hospital Medicine (MHM).
She is quick to notice trends and patterns, he points out. “She’s very knowledgeable about what’s going on in the hospitalist arena,” he says, adding he anticipates she’ll be interested in “data-driven” coverage, along with QI topics.
Dr. Scheurer’s interest in disseminating information shouldn’t be a surprise—it’s a fundamental part of QI and instrumental in systemwide change. She finds it “appealing to work on a project and know that it’s going to affect the next 20,000 patients.”
“There’s no one single person that can ensure that the patient gets all of their needs met,” she says. “There has to be a system approach.”
At The Hospitalist, she will try to keep pace with all the change that hospitals are constantly trying to navigate.
“I don’t think there will ever be a deficiency of content to cover,” she says. “Something’s always brand-new.”
Tom Collins is a freelance writer in Florida.

—Danielle Scheurer, MD, MSCR, SFHM, physician editor, The Hospitalist
There has always been a journalist dwelling in Danielle Scheurer, MD, MSCR, SFHM. As an undergrad at Emory University, she saw TV reporting in her future.
“I was on the Katie Couric kick for a decade,” she says.
Her course eventually shifted—dramatically. But since she became a hospitalist, Dr. Scheurer, now the chief quality officer at the Medical University of South Carolina (MUSC) in Charleston, has stayed involved with a long slate of editorial projects.
Her latest: She is the new physician editor of The Hospitalist. With the appointment, the magazine gains a high-energy physician with a broad spectrum of knowledge. Colleagues say she has a knack for seeing the big picture and taking a bolus of information and conveying its relevance to hospitalists and other medical professionals.
Dr. Scheurer says that one of her aims will be to make the publication’s website—www.the-hospitalist.org—more interactive, allowing for more direct participation from readers, such as with polls and forums on topics covered.
“A lot of us in the hospital sort of struggle with the exact same things,” she says. “I think there’s some value in connecting, even if it’s in just short little snippets.”
She also would like to increase the website’s use of audio files so that doctors have more options in how they get their information.
But above all, she says, she wants to keep The Hospitalist “one of the most practical publications available to hospitalists,” a publication that is specifically tailored to deliver useful messages.
“I feel like it’s a very high-yield publication for really busy hospitalists,” she says.
Career Shuffle = Diverse Experience
Dr. Scheurer brings experience from a variety of settings, such as the small community hospital Trident Medical Center in Charleston to the large, urban medical centers that are Brigham and Women’s Hospital in Boston and MUSC.
She said some of her career moves came with some apprehension, including those that came about when her husband got a position that required her to move, too. But she says she has benefited from those experiences. Trident “gave me a window into hospital medicine that I never otherwise would have had,” she notes.
She never anticipated moving to Boston, and she admits it felt outside of her “comfort zone.” But in 2005, she found herself at Brigham. She had just earned a master’s degree in clinical research and thought she’d end up being a researcher. In Boston, she got a glimpse of what it meant to be a “hard-core, NIH-funded researcher” and decided it wasn’t for her.
While there, she took training courses in leadership and quality improvement. And QI stuck.
In 2010, she returned to MUSC and now leads QI for the whole hospital, a medium-size setting that she says is just right for promoting change.
“This is definitely my sweet spot,” she says. “If you’re going to change people’s minds, it’s a lot easer to change 200 people’s minds than 450 people’s minds.”
Chris Roy, MD, medical director of the hospitalist service at Brigham, says Dr. Scheurer was “one of the most-hard working people that I knew” and a strong leader with an “uncanny, almost photographic memory of all the hospital medicine literature.”
“Even though she was very forceful as a leader, she never irritated anyone,” he adds. “She was very skillful in managing people.”
Chris Rees, the director of quality and patient safety at MUSC, says Dr. Scheurer is adept at taking issues that evolve from the hospital and collaborating on them with other university departments. She is good at putting herself in other groups’ shoes and delivering messages succinctly, he says.
“She’s definitely not seen as just one of those white coats,” Rees says.
Highly Recommended
On top of her QI projects, Dr. Scheurer is involved as an advisor, contributor, or reviewer at 11 other publications or online venues. The Hospitalist will make it an even dozen.
“She’s just a dynamo,” Rees says. “She walks around with her MacAir book and she’s constantly writing stuff on it and sending out emails.”
Patrick Cawley, MD, MBA, MHM, the chief medical officer at MUSC who hired Dr. Scheurer when she first worked there in 2003, has seen her move from small projects to systemwide efforts.
“She did a great job and is very collaborative, very knowledgeable, [and] brings an evidence-based approach to problems,” says Dr. Cawley, a past president of SHM and recent inductee as a Master in Hospital Medicine (MHM).
She is quick to notice trends and patterns, he points out. “She’s very knowledgeable about what’s going on in the hospitalist arena,” he says, adding he anticipates she’ll be interested in “data-driven” coverage, along with QI topics.
Dr. Scheurer’s interest in disseminating information shouldn’t be a surprise—it’s a fundamental part of QI and instrumental in systemwide change. She finds it “appealing to work on a project and know that it’s going to affect the next 20,000 patients.”
“There’s no one single person that can ensure that the patient gets all of their needs met,” she says. “There has to be a system approach.”
At The Hospitalist, she will try to keep pace with all the change that hospitals are constantly trying to navigate.
“I don’t think there will ever be a deficiency of content to cover,” she says. “Something’s always brand-new.”
Tom Collins is a freelance writer in Florida.
In the Literature: Physician Reviews of HM-Related Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- IDSA/ATS guidelines for community-acquired pneumonia
- Improved asthma with IL-13 antibody
- Rivaroxaban vs. warfarin for stroke prevention in atrial fibrillation
- Apixaban vs. warfarin for stroke prevention in atrial fibrillation
- Ultrasonography more sensitive than chest radiograph for pneumothorax
- Current readmission risk models inadequate
- Optimal fluid volume for acute pancreatitis
- Low mortality in saddle pulmonary embolism
Triage Decisions for Patients with Severe Community-Acquired Pneumonia Should Be Based on IDSA/ATS Guidelines, Not Inflammatory Biomarkers
Clinical question: Can C-reactive protein levels (CRP), procalcitonin, TNF-alpha, and cytokine levels predict the need for intensive-care admission more accurately than IDSA/ATS guidelines in patients with severe community-acquired pneumonia (CAP)?
Background: Inflammatory biomarkers, such as CRP and procalcitonin, have diagnostic and prognostic utility in patients with CAP. Whether these inflammatory biomarkers can help triage patients to the appropriate level of care is unknown.
Study design: Prospective case control study.
Setting: Two university hospitals in Spain.
Synopsis: The study included 685 patients with severe CAP who did not require mechanical ventilation or vasopressor support. Serum levels of CRP, procalcitonin, TNF-alpha, IL-1, IL-6, IL-8, and IL-10, as well as Infectious Diseases Society of American/American Thoracic Society (IDSA/ATS) minor severity criteria data, were collected on admission. After controlling for age, comorbidities, and PSI risk class, serum levels of CRP and procalcitonin were found to be significantly higher in ICU patients compared with non-ICU patients. Despite this, these inflammatory biomarkers did not augment the IDSA/ATS guidelines, suggesting that patients who have three or more minor criteria be considered for ICU admission.
The study did suggest that patients with severe CAP and low levels of IL-6 and procalcitonin could potentially be managed safely outside of the ICU. However, hospitalists should be wary of applying the study results due to the small number of ICU patients in this study and the lack of real-time availability of these biomarkers at most institutions.
Bottom line: More studies of inflammatory biomarkers are needed before using them to determine the level of care required for patients with CAP. Until these data are available, physicians should use the IDSA/ATS guidelines to triage patients to the appropriate level of care.
Citation: Ramirez P, Ferrer M, Torres A, et al. Inflammatory biomarkers and prediction for intensive care unit admission pneumonia. Crit Care Med. 2011;39:2211-2217.
IL-13 Antibody Lebrikizumab Shows Promise as a New Therapy for Adults with Uncontrolled Asthma
Clinical question: Can lebrikizumab, an IL-13 antibody, improve asthma control in patients with uncontrolled asthma?
Background: Asthma is a complex disease, with varied patient response to treatment. Some patients have uncontrolled asthma despite inhaled glucocorticoids. It is postulated that IL-13 may account for this variability and that some patients with uncontrolled asthma are poorly controlled due to glucocorticoid resistance mediated by IL-13. Lebrikizumab is an IgG4 monoclonal antibody that binds to and inhibits the function of IL-13. This study was performed to see if this antibody would be effective in patients with uncontrolled asthma despite inhaled glucocorticoid therapy.
Study design: Randomized double-blinded placebo-controlled trial.
Setting: Multiple centers.
Synopsis: The study randomized 219 adult asthma patients who were inadequately controlled despite inhaled corticosteroids to a placebo or lebrikizumab. The primary outcome was improvement in prebronchodilator FEV1 from baseline. Secondary outcomes were exacerbations, use of rescue medications, and symptom scores. Patients were also stratified and analyzed based on surrogate markers for IL-13, which included serum IGE levels, eosinophil counts, and periostin levels.
In patients who were randomized to the lebrikizumab treatment, there was a statistically significant improvement in FEV1 of 5.5%, which occurred almost immediately and was sustained for the entire 32 weeks of the study. The improvement was more significant in patients who had high surrogate markers for IL-13. Despite this improvement in FEV1, there were no differences in secondary outcomes except in patients who had surrogate markers for high IL-13 levels.
Bottom line: In adults with asthma who remained uncontrolled despite inhaled corticosteroid therapy, IL-13 antagonism with lebrikizumab improved FEV1. However, the clinical relevance of these modest improvements remains unclear.
Citation: Corren J, Lemanske R, Matthews J, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088-1098.
Rivaroxaban Is Noninferior to Warfarin for Stroke Prevention in Atrial Fibrillation
Clinical question: How does rivaroxaban compare with warfarin in the prevention of stroke or systemic embolism in patients with nonvalvular atrial fibrillation?
Background: Warfarin is effective for the prevention of stroke in atrial fibrillation, but it requires close monitoring and adjustment. Rivaroxaban, an oral Xa inhibitor, may be safer, easier, and more effective than warfarin.
Study design: Multicenter, randomized, double-blind, double-dummy trial.
Setting: 1,178 sites in 45 countries.
Synopsis: The study included 14,264 patients with nonvalvular atrial fibrillation who were randomized to either fixed-dose rivaroxaban (20 mg daily or 15 mg daily for CrCl 30-49 mL/min) plus placebo or adjusted-dose warfarin (target INR 2.0 to 3.0) plus placebo. The mean CHADS2 score was 3.5. The primary endpoint (stroke or systemic embolism) occurred in 1.7% of patients per year in the rivaroxaban group and 2.2% per year in the warfarin group (hazard ratio for rivaroxaban 0.79; 95% CI: 0.66 to 0.96, P<0.001 for noninferiority). There was no difference in major or nonmajor clinically significant bleeding between the two groups (14.9% rivaroxaban vs. 14.5% warfarin, hazard ratio=1.03, 95% CI: 0.96 to 1.11, P=0.44). There were fewer intracranial hemorrhages (0.5% vs. 0.7%, P=0.02) and fatal bleeding (0.2% vs. 0.5%, P=0.003) in the rivaroxaban group.
Bottom line: In patients with atrial fibrillation, rivaroxaban was noninferior to warfarin for the prevention of stroke or systemic embolization, with a similar risk of major bleeding and a lower risk of intracranial hemorrhage or fatal bleeding.
Citation: Patel MR, Mahaffey K, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
Apixaban More Effective and Safer than Warfarin for Stroke Prevention in Atrial Fibrillation
Clinical question: How does the effectiveness and safety of apixaban compare with warfarin for stroke prevention in atrial fibrillation?
Background: Until recently, warfarin has been the only available oral anticoagulant for stroke prevention in patients with atrial fibrillation (AF). The oral factor Xa inhibitors have shown similar efficacy and safety, without the monitoring requirement and drug interactions associated with warfarin.
Study design: Prospective randomized double-blind controlled trial.
Setting: More than 1,000 clinical sites in 39 countries.
Synopsis: This study randomized 18,201 patients with atrial fibrillation or flutter and at least one CHADS2 risk factor for stroke to receive oral apixaban or warfarin therapy. Exclusion criteria were prosthetic valves and severe kidney disease. The median duration of follow-up was 1.8 years, and the major endpoints were incidence of stroke, systemic embolism, bleeding complications, and mortality.
Compared with warfarin, apixaban reduced the annual incidence of stroke and systemic embolism from 1.6% to 1.3% (HR 0.79, 95%: CI 0.66 to 0.95, P=0.01 for superiority), and reduced mortality (HR: 0.89, 95% CI: 0.80 to 0.998). For the combined endpoint of stroke, systemic embolism, MI, or death, the annual rate was reduced from 5.5% to 4.9% (HR: 0.88, 95% CI: 0.80 to 0.97). All measures of bleeding were less frequent with apixaban: major 2.1% vs. 3.1% (HR: 0.69, 95% CI: 0.60 to 0.80), and combined major and minor bleeding 4.1% vs. 6.0% (HR: 0.68, 95% CI: 0.61 to 0.75). The annual rate for the net outcome of stroke, embolism, or major bleeding was 3.2% with apixaban and 4.1% with warfarin (HR: 0.77, 95% CI: 0.69 to 0.86).
Bottom line: Compared with warfarin therapy, apixaban is more effective and safer for stroke prevention in patients with atrial fibrillation.
Citation: Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
Ultrasonography Is Useful in Diagnosis of Pneumothorax
Clinical question: Is transthoracic ultrasonography a useful tool to diagnose pneumothorax?
Background: CT is the diagnostic gold standard for pneumothorax, but it is associated with radiation exposure and requires patient transport. Chest radiograph is easy to perform but may be too insensitive for adequate diagnosis. Ultrasonography’s diagnostic performance for detecting pneumothorax needs further evaluation.
Study design: Systematic review and meta-analysis.
Setting: Critically ill, trauma, or post-biopsy patients were identified in each of the studies.
Synopsis: The meta-analysis of 20 eligible studies found a pooled sensitivity of ultrasound for the detection of pneumothorax of 0.88 (95% CI: 0.85 to 0.91) and specificity of 0.99 (0.98 to 0.99) compared with sensitivity of 0.52 (0.49 to 0.55) and specificity of 1.00 (1.00 to 1.00) for chest radiograph. Although the overall ROC curve was not significantly different between these modalities, the accuracy of ultrasonography was highly dependent on the skill of the operator.
Bottom line: When performed by a skilled operator, transthoracic ultrasonography is as specific, and more sensitive, than chest radiograph in diagnosing pneumothorax.
Citation: Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140:859-866.
Risk Prediction for Hospital Readmission Remains Challenging
Clinical question: Can readmission risk assessment be used to identify which patients would benefit most from care-transition interventions, or to risk-adjust readmission rates for hospital comparison?
Background: Multiple models to predict hospital readmission have been described and validated. Identifying patients at high risk for readmission could allow for customized care-transition interventions, or could be used to risk-adjust readmission rates to compare publicly reported rates by hospital.
Study design: Systematic review with qualitative synthesis of results.
Setting: Thirty studies (23 from the U.S.) tested 26 unique readmission models.
Synopsis: Each model had been tested in both a derivation and validation cohort. Fourteen models (nine from the U.S.), using retrospective administrative data to compare risk-adjusted rates between hospitals, had poor discriminative capacity (c statistic range: 0.55 to 0.65). Seven models could be used to identify high-risk patients early in the hospitalization (c statistic range: 0.56 to 0.72) and five could be used to identify high-risk patients at discharge (c statistic range: 0.68 to 0.83), but these also had poor to moderate discriminative capacity. Multiple variables were considered in each of the models; most incorporated medical comorbidities and prior use of healthcare services.
Bottom line: Current readmission risk prediction models do not perform adequately for comparative or clinical purposes.
Citation: Kansagara D, Englander H, Salanitro A, et. al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
Intravenous Fluids for Acute Pancreatitis: More May Be Less
Clinical question: What is the optimal volume of fluid administration for treatment of acute pancreatitis?
Background: Current guidelines for management of acute pancreatitis emphasize vigorous administration of intravenous fluids to reduce the risk of pancreatic necrosis and organ failure. This recommendation is based upon animal studies, and has not been subjected to clinical evaluation in humans.
Study design: Prospective observational cohort.
Setting: University-affiliated tertiary-care public hospital in Spain.
Synopsis: This study enrolled 247 patients admitted with acute pancreatitis to determine the association between the volume of fluid administered during the first 24 hours and the development of persistent organ failure, pancreatic fluid collection or necrosis, and mortality. The volume and rate of fluid administered were determined by the treating physician. Patients were classified into three groups: those receiving a volume <3.1 L, 3.1 to 4.1 L, or >4.1 L.
After multivariate adjustment, those receiving <3.1 L had no increased risk of necrosis or any other adverse outcome, compared with those who received the middle range of fluid volume.
Patients receiving >4.1 L had a higher risk of persistent organ failure (OR: 7.7, 95% CI: 1.5 to 38.7), particularly renal and respiratory insufficiency, and fluid collection development (OR: 1.9, 95% CI: 1 to 3.7) independent of disease severity. Pancreatic necrosis and mortality were similar in the three groups.
Bottom line: Administration of large-volume intravenous fluids (>4.1 L) in
the first 24 hours was associated with worse outcomes, although residual confounding cannot be excluded in this nonrandomized study.
Citation: de-Madaria E, Soler-Sala G, Sanchez-Paya J, et al. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;106:1843-1850.
Clinical Outcomes in Saddle Pulmonary Embolism
Clinical question: What are the treatments used and outcomes associated with saddle pulmonary embolism?
Background: Saddle pulmonary embolism is a risk for right ventricular dysfunction and sudden hemodynamic collapse. There are limited data on the clinical presentation and outcomes in these patients.
Study design: Retrospective case review.
Setting: Single academic medical center.
Synopsis: In this retrospective review of 680 patients diagnosed with pulmonary embolism on CT at a single academic medical center from 2004 to 2009, 5.4% (37 patients) had a saddle pulmonary embolism.
Most patients with saddle pulmonary embolism were hemodynamically stable and responded to standard therapy with unfractionated heparin. The mean length of stay was nine days, 46% received an inferior vena cava filter, 41% were treated in an ICU, and 5.4% (two patients) died in the hospital. Thrombolytics were used in only 11% of patients, most of which had sustained hypotension and/or were mechanically ventilated.
Bottom line: Most patients with saddle pulmonary embolus in this single institution study did not receive thrombolytics and had overall low mortality.
Citation: Sardi A, Gluskin J, Guttentag A, Kotler MN, Braitman LE, Lippmann M. Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience. Crit Care Med. 2011;39:2413-2418.
In This Edition
Literature At A Glance
A guide to this month’s studies
- IDSA/ATS guidelines for community-acquired pneumonia
- Improved asthma with IL-13 antibody
- Rivaroxaban vs. warfarin for stroke prevention in atrial fibrillation
- Apixaban vs. warfarin for stroke prevention in atrial fibrillation
- Ultrasonography more sensitive than chest radiograph for pneumothorax
- Current readmission risk models inadequate
- Optimal fluid volume for acute pancreatitis
- Low mortality in saddle pulmonary embolism
Triage Decisions for Patients with Severe Community-Acquired Pneumonia Should Be Based on IDSA/ATS Guidelines, Not Inflammatory Biomarkers
Clinical question: Can C-reactive protein levels (CRP), procalcitonin, TNF-alpha, and cytokine levels predict the need for intensive-care admission more accurately than IDSA/ATS guidelines in patients with severe community-acquired pneumonia (CAP)?
Background: Inflammatory biomarkers, such as CRP and procalcitonin, have diagnostic and prognostic utility in patients with CAP. Whether these inflammatory biomarkers can help triage patients to the appropriate level of care is unknown.
Study design: Prospective case control study.
Setting: Two university hospitals in Spain.
Synopsis: The study included 685 patients with severe CAP who did not require mechanical ventilation or vasopressor support. Serum levels of CRP, procalcitonin, TNF-alpha, IL-1, IL-6, IL-8, and IL-10, as well as Infectious Diseases Society of American/American Thoracic Society (IDSA/ATS) minor severity criteria data, were collected on admission. After controlling for age, comorbidities, and PSI risk class, serum levels of CRP and procalcitonin were found to be significantly higher in ICU patients compared with non-ICU patients. Despite this, these inflammatory biomarkers did not augment the IDSA/ATS guidelines, suggesting that patients who have three or more minor criteria be considered for ICU admission.
The study did suggest that patients with severe CAP and low levels of IL-6 and procalcitonin could potentially be managed safely outside of the ICU. However, hospitalists should be wary of applying the study results due to the small number of ICU patients in this study and the lack of real-time availability of these biomarkers at most institutions.
Bottom line: More studies of inflammatory biomarkers are needed before using them to determine the level of care required for patients with CAP. Until these data are available, physicians should use the IDSA/ATS guidelines to triage patients to the appropriate level of care.
Citation: Ramirez P, Ferrer M, Torres A, et al. Inflammatory biomarkers and prediction for intensive care unit admission pneumonia. Crit Care Med. 2011;39:2211-2217.
IL-13 Antibody Lebrikizumab Shows Promise as a New Therapy for Adults with Uncontrolled Asthma
Clinical question: Can lebrikizumab, an IL-13 antibody, improve asthma control in patients with uncontrolled asthma?
Background: Asthma is a complex disease, with varied patient response to treatment. Some patients have uncontrolled asthma despite inhaled glucocorticoids. It is postulated that IL-13 may account for this variability and that some patients with uncontrolled asthma are poorly controlled due to glucocorticoid resistance mediated by IL-13. Lebrikizumab is an IgG4 monoclonal antibody that binds to and inhibits the function of IL-13. This study was performed to see if this antibody would be effective in patients with uncontrolled asthma despite inhaled glucocorticoid therapy.
Study design: Randomized double-blinded placebo-controlled trial.
Setting: Multiple centers.
Synopsis: The study randomized 219 adult asthma patients who were inadequately controlled despite inhaled corticosteroids to a placebo or lebrikizumab. The primary outcome was improvement in prebronchodilator FEV1 from baseline. Secondary outcomes were exacerbations, use of rescue medications, and symptom scores. Patients were also stratified and analyzed based on surrogate markers for IL-13, which included serum IGE levels, eosinophil counts, and periostin levels.
In patients who were randomized to the lebrikizumab treatment, there was a statistically significant improvement in FEV1 of 5.5%, which occurred almost immediately and was sustained for the entire 32 weeks of the study. The improvement was more significant in patients who had high surrogate markers for IL-13. Despite this improvement in FEV1, there were no differences in secondary outcomes except in patients who had surrogate markers for high IL-13 levels.
Bottom line: In adults with asthma who remained uncontrolled despite inhaled corticosteroid therapy, IL-13 antagonism with lebrikizumab improved FEV1. However, the clinical relevance of these modest improvements remains unclear.
Citation: Corren J, Lemanske R, Matthews J, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088-1098.
Rivaroxaban Is Noninferior to Warfarin for Stroke Prevention in Atrial Fibrillation
Clinical question: How does rivaroxaban compare with warfarin in the prevention of stroke or systemic embolism in patients with nonvalvular atrial fibrillation?
Background: Warfarin is effective for the prevention of stroke in atrial fibrillation, but it requires close monitoring and adjustment. Rivaroxaban, an oral Xa inhibitor, may be safer, easier, and more effective than warfarin.
Study design: Multicenter, randomized, double-blind, double-dummy trial.
Setting: 1,178 sites in 45 countries.
Synopsis: The study included 14,264 patients with nonvalvular atrial fibrillation who were randomized to either fixed-dose rivaroxaban (20 mg daily or 15 mg daily for CrCl 30-49 mL/min) plus placebo or adjusted-dose warfarin (target INR 2.0 to 3.0) plus placebo. The mean CHADS2 score was 3.5. The primary endpoint (stroke or systemic embolism) occurred in 1.7% of patients per year in the rivaroxaban group and 2.2% per year in the warfarin group (hazard ratio for rivaroxaban 0.79; 95% CI: 0.66 to 0.96, P<0.001 for noninferiority). There was no difference in major or nonmajor clinically significant bleeding between the two groups (14.9% rivaroxaban vs. 14.5% warfarin, hazard ratio=1.03, 95% CI: 0.96 to 1.11, P=0.44). There were fewer intracranial hemorrhages (0.5% vs. 0.7%, P=0.02) and fatal bleeding (0.2% vs. 0.5%, P=0.003) in the rivaroxaban group.
Bottom line: In patients with atrial fibrillation, rivaroxaban was noninferior to warfarin for the prevention of stroke or systemic embolization, with a similar risk of major bleeding and a lower risk of intracranial hemorrhage or fatal bleeding.
Citation: Patel MR, Mahaffey K, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
Apixaban More Effective and Safer than Warfarin for Stroke Prevention in Atrial Fibrillation
Clinical question: How does the effectiveness and safety of apixaban compare with warfarin for stroke prevention in atrial fibrillation?
Background: Until recently, warfarin has been the only available oral anticoagulant for stroke prevention in patients with atrial fibrillation (AF). The oral factor Xa inhibitors have shown similar efficacy and safety, without the monitoring requirement and drug interactions associated with warfarin.
Study design: Prospective randomized double-blind controlled trial.
Setting: More than 1,000 clinical sites in 39 countries.
Synopsis: This study randomized 18,201 patients with atrial fibrillation or flutter and at least one CHADS2 risk factor for stroke to receive oral apixaban or warfarin therapy. Exclusion criteria were prosthetic valves and severe kidney disease. The median duration of follow-up was 1.8 years, and the major endpoints were incidence of stroke, systemic embolism, bleeding complications, and mortality.
Compared with warfarin, apixaban reduced the annual incidence of stroke and systemic embolism from 1.6% to 1.3% (HR 0.79, 95%: CI 0.66 to 0.95, P=0.01 for superiority), and reduced mortality (HR: 0.89, 95% CI: 0.80 to 0.998). For the combined endpoint of stroke, systemic embolism, MI, or death, the annual rate was reduced from 5.5% to 4.9% (HR: 0.88, 95% CI: 0.80 to 0.97). All measures of bleeding were less frequent with apixaban: major 2.1% vs. 3.1% (HR: 0.69, 95% CI: 0.60 to 0.80), and combined major and minor bleeding 4.1% vs. 6.0% (HR: 0.68, 95% CI: 0.61 to 0.75). The annual rate for the net outcome of stroke, embolism, or major bleeding was 3.2% with apixaban and 4.1% with warfarin (HR: 0.77, 95% CI: 0.69 to 0.86).
Bottom line: Compared with warfarin therapy, apixaban is more effective and safer for stroke prevention in patients with atrial fibrillation.
Citation: Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
Ultrasonography Is Useful in Diagnosis of Pneumothorax
Clinical question: Is transthoracic ultrasonography a useful tool to diagnose pneumothorax?
Background: CT is the diagnostic gold standard for pneumothorax, but it is associated with radiation exposure and requires patient transport. Chest radiograph is easy to perform but may be too insensitive for adequate diagnosis. Ultrasonography’s diagnostic performance for detecting pneumothorax needs further evaluation.
Study design: Systematic review and meta-analysis.
Setting: Critically ill, trauma, or post-biopsy patients were identified in each of the studies.
Synopsis: The meta-analysis of 20 eligible studies found a pooled sensitivity of ultrasound for the detection of pneumothorax of 0.88 (95% CI: 0.85 to 0.91) and specificity of 0.99 (0.98 to 0.99) compared with sensitivity of 0.52 (0.49 to 0.55) and specificity of 1.00 (1.00 to 1.00) for chest radiograph. Although the overall ROC curve was not significantly different between these modalities, the accuracy of ultrasonography was highly dependent on the skill of the operator.
Bottom line: When performed by a skilled operator, transthoracic ultrasonography is as specific, and more sensitive, than chest radiograph in diagnosing pneumothorax.
Citation: Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140:859-866.
Risk Prediction for Hospital Readmission Remains Challenging
Clinical question: Can readmission risk assessment be used to identify which patients would benefit most from care-transition interventions, or to risk-adjust readmission rates for hospital comparison?
Background: Multiple models to predict hospital readmission have been described and validated. Identifying patients at high risk for readmission could allow for customized care-transition interventions, or could be used to risk-adjust readmission rates to compare publicly reported rates by hospital.
Study design: Systematic review with qualitative synthesis of results.
Setting: Thirty studies (23 from the U.S.) tested 26 unique readmission models.
Synopsis: Each model had been tested in both a derivation and validation cohort. Fourteen models (nine from the U.S.), using retrospective administrative data to compare risk-adjusted rates between hospitals, had poor discriminative capacity (c statistic range: 0.55 to 0.65). Seven models could be used to identify high-risk patients early in the hospitalization (c statistic range: 0.56 to 0.72) and five could be used to identify high-risk patients at discharge (c statistic range: 0.68 to 0.83), but these also had poor to moderate discriminative capacity. Multiple variables were considered in each of the models; most incorporated medical comorbidities and prior use of healthcare services.
Bottom line: Current readmission risk prediction models do not perform adequately for comparative or clinical purposes.
Citation: Kansagara D, Englander H, Salanitro A, et. al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
Intravenous Fluids for Acute Pancreatitis: More May Be Less
Clinical question: What is the optimal volume of fluid administration for treatment of acute pancreatitis?
Background: Current guidelines for management of acute pancreatitis emphasize vigorous administration of intravenous fluids to reduce the risk of pancreatic necrosis and organ failure. This recommendation is based upon animal studies, and has not been subjected to clinical evaluation in humans.
Study design: Prospective observational cohort.
Setting: University-affiliated tertiary-care public hospital in Spain.
Synopsis: This study enrolled 247 patients admitted with acute pancreatitis to determine the association between the volume of fluid administered during the first 24 hours and the development of persistent organ failure, pancreatic fluid collection or necrosis, and mortality. The volume and rate of fluid administered were determined by the treating physician. Patients were classified into three groups: those receiving a volume <3.1 L, 3.1 to 4.1 L, or >4.1 L.
After multivariate adjustment, those receiving <3.1 L had no increased risk of necrosis or any other adverse outcome, compared with those who received the middle range of fluid volume.
Patients receiving >4.1 L had a higher risk of persistent organ failure (OR: 7.7, 95% CI: 1.5 to 38.7), particularly renal and respiratory insufficiency, and fluid collection development (OR: 1.9, 95% CI: 1 to 3.7) independent of disease severity. Pancreatic necrosis and mortality were similar in the three groups.
Bottom line: Administration of large-volume intravenous fluids (>4.1 L) in
the first 24 hours was associated with worse outcomes, although residual confounding cannot be excluded in this nonrandomized study.
Citation: de-Madaria E, Soler-Sala G, Sanchez-Paya J, et al. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;106:1843-1850.
Clinical Outcomes in Saddle Pulmonary Embolism
Clinical question: What are the treatments used and outcomes associated with saddle pulmonary embolism?
Background: Saddle pulmonary embolism is a risk for right ventricular dysfunction and sudden hemodynamic collapse. There are limited data on the clinical presentation and outcomes in these patients.
Study design: Retrospective case review.
Setting: Single academic medical center.
Synopsis: In this retrospective review of 680 patients diagnosed with pulmonary embolism on CT at a single academic medical center from 2004 to 2009, 5.4% (37 patients) had a saddle pulmonary embolism.
Most patients with saddle pulmonary embolism were hemodynamically stable and responded to standard therapy with unfractionated heparin. The mean length of stay was nine days, 46% received an inferior vena cava filter, 41% were treated in an ICU, and 5.4% (two patients) died in the hospital. Thrombolytics were used in only 11% of patients, most of which had sustained hypotension and/or were mechanically ventilated.
Bottom line: Most patients with saddle pulmonary embolus in this single institution study did not receive thrombolytics and had overall low mortality.
Citation: Sardi A, Gluskin J, Guttentag A, Kotler MN, Braitman LE, Lippmann M. Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience. Crit Care Med. 2011;39:2413-2418.
In This Edition
Literature At A Glance
A guide to this month’s studies
- IDSA/ATS guidelines for community-acquired pneumonia
- Improved asthma with IL-13 antibody
- Rivaroxaban vs. warfarin for stroke prevention in atrial fibrillation
- Apixaban vs. warfarin for stroke prevention in atrial fibrillation
- Ultrasonography more sensitive than chest radiograph for pneumothorax
- Current readmission risk models inadequate
- Optimal fluid volume for acute pancreatitis
- Low mortality in saddle pulmonary embolism
Triage Decisions for Patients with Severe Community-Acquired Pneumonia Should Be Based on IDSA/ATS Guidelines, Not Inflammatory Biomarkers
Clinical question: Can C-reactive protein levels (CRP), procalcitonin, TNF-alpha, and cytokine levels predict the need for intensive-care admission more accurately than IDSA/ATS guidelines in patients with severe community-acquired pneumonia (CAP)?
Background: Inflammatory biomarkers, such as CRP and procalcitonin, have diagnostic and prognostic utility in patients with CAP. Whether these inflammatory biomarkers can help triage patients to the appropriate level of care is unknown.
Study design: Prospective case control study.
Setting: Two university hospitals in Spain.
Synopsis: The study included 685 patients with severe CAP who did not require mechanical ventilation or vasopressor support. Serum levels of CRP, procalcitonin, TNF-alpha, IL-1, IL-6, IL-8, and IL-10, as well as Infectious Diseases Society of American/American Thoracic Society (IDSA/ATS) minor severity criteria data, were collected on admission. After controlling for age, comorbidities, and PSI risk class, serum levels of CRP and procalcitonin were found to be significantly higher in ICU patients compared with non-ICU patients. Despite this, these inflammatory biomarkers did not augment the IDSA/ATS guidelines, suggesting that patients who have three or more minor criteria be considered for ICU admission.
The study did suggest that patients with severe CAP and low levels of IL-6 and procalcitonin could potentially be managed safely outside of the ICU. However, hospitalists should be wary of applying the study results due to the small number of ICU patients in this study and the lack of real-time availability of these biomarkers at most institutions.
Bottom line: More studies of inflammatory biomarkers are needed before using them to determine the level of care required for patients with CAP. Until these data are available, physicians should use the IDSA/ATS guidelines to triage patients to the appropriate level of care.
Citation: Ramirez P, Ferrer M, Torres A, et al. Inflammatory biomarkers and prediction for intensive care unit admission pneumonia. Crit Care Med. 2011;39:2211-2217.
IL-13 Antibody Lebrikizumab Shows Promise as a New Therapy for Adults with Uncontrolled Asthma
Clinical question: Can lebrikizumab, an IL-13 antibody, improve asthma control in patients with uncontrolled asthma?
Background: Asthma is a complex disease, with varied patient response to treatment. Some patients have uncontrolled asthma despite inhaled glucocorticoids. It is postulated that IL-13 may account for this variability and that some patients with uncontrolled asthma are poorly controlled due to glucocorticoid resistance mediated by IL-13. Lebrikizumab is an IgG4 monoclonal antibody that binds to and inhibits the function of IL-13. This study was performed to see if this antibody would be effective in patients with uncontrolled asthma despite inhaled glucocorticoid therapy.
Study design: Randomized double-blinded placebo-controlled trial.
Setting: Multiple centers.
Synopsis: The study randomized 219 adult asthma patients who were inadequately controlled despite inhaled corticosteroids to a placebo or lebrikizumab. The primary outcome was improvement in prebronchodilator FEV1 from baseline. Secondary outcomes were exacerbations, use of rescue medications, and symptom scores. Patients were also stratified and analyzed based on surrogate markers for IL-13, which included serum IGE levels, eosinophil counts, and periostin levels.
In patients who were randomized to the lebrikizumab treatment, there was a statistically significant improvement in FEV1 of 5.5%, which occurred almost immediately and was sustained for the entire 32 weeks of the study. The improvement was more significant in patients who had high surrogate markers for IL-13. Despite this improvement in FEV1, there were no differences in secondary outcomes except in patients who had surrogate markers for high IL-13 levels.
Bottom line: In adults with asthma who remained uncontrolled despite inhaled corticosteroid therapy, IL-13 antagonism with lebrikizumab improved FEV1. However, the clinical relevance of these modest improvements remains unclear.
Citation: Corren J, Lemanske R, Matthews J, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088-1098.
Rivaroxaban Is Noninferior to Warfarin for Stroke Prevention in Atrial Fibrillation
Clinical question: How does rivaroxaban compare with warfarin in the prevention of stroke or systemic embolism in patients with nonvalvular atrial fibrillation?
Background: Warfarin is effective for the prevention of stroke in atrial fibrillation, but it requires close monitoring and adjustment. Rivaroxaban, an oral Xa inhibitor, may be safer, easier, and more effective than warfarin.
Study design: Multicenter, randomized, double-blind, double-dummy trial.
Setting: 1,178 sites in 45 countries.
Synopsis: The study included 14,264 patients with nonvalvular atrial fibrillation who were randomized to either fixed-dose rivaroxaban (20 mg daily or 15 mg daily for CrCl 30-49 mL/min) plus placebo or adjusted-dose warfarin (target INR 2.0 to 3.0) plus placebo. The mean CHADS2 score was 3.5. The primary endpoint (stroke or systemic embolism) occurred in 1.7% of patients per year in the rivaroxaban group and 2.2% per year in the warfarin group (hazard ratio for rivaroxaban 0.79; 95% CI: 0.66 to 0.96, P<0.001 for noninferiority). There was no difference in major or nonmajor clinically significant bleeding between the two groups (14.9% rivaroxaban vs. 14.5% warfarin, hazard ratio=1.03, 95% CI: 0.96 to 1.11, P=0.44). There were fewer intracranial hemorrhages (0.5% vs. 0.7%, P=0.02) and fatal bleeding (0.2% vs. 0.5%, P=0.003) in the rivaroxaban group.
Bottom line: In patients with atrial fibrillation, rivaroxaban was noninferior to warfarin for the prevention of stroke or systemic embolization, with a similar risk of major bleeding and a lower risk of intracranial hemorrhage or fatal bleeding.
Citation: Patel MR, Mahaffey K, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
Apixaban More Effective and Safer than Warfarin for Stroke Prevention in Atrial Fibrillation
Clinical question: How does the effectiveness and safety of apixaban compare with warfarin for stroke prevention in atrial fibrillation?
Background: Until recently, warfarin has been the only available oral anticoagulant for stroke prevention in patients with atrial fibrillation (AF). The oral factor Xa inhibitors have shown similar efficacy and safety, without the monitoring requirement and drug interactions associated with warfarin.
Study design: Prospective randomized double-blind controlled trial.
Setting: More than 1,000 clinical sites in 39 countries.
Synopsis: This study randomized 18,201 patients with atrial fibrillation or flutter and at least one CHADS2 risk factor for stroke to receive oral apixaban or warfarin therapy. Exclusion criteria were prosthetic valves and severe kidney disease. The median duration of follow-up was 1.8 years, and the major endpoints were incidence of stroke, systemic embolism, bleeding complications, and mortality.
Compared with warfarin, apixaban reduced the annual incidence of stroke and systemic embolism from 1.6% to 1.3% (HR 0.79, 95%: CI 0.66 to 0.95, P=0.01 for superiority), and reduced mortality (HR: 0.89, 95% CI: 0.80 to 0.998). For the combined endpoint of stroke, systemic embolism, MI, or death, the annual rate was reduced from 5.5% to 4.9% (HR: 0.88, 95% CI: 0.80 to 0.97). All measures of bleeding were less frequent with apixaban: major 2.1% vs. 3.1% (HR: 0.69, 95% CI: 0.60 to 0.80), and combined major and minor bleeding 4.1% vs. 6.0% (HR: 0.68, 95% CI: 0.61 to 0.75). The annual rate for the net outcome of stroke, embolism, or major bleeding was 3.2% with apixaban and 4.1% with warfarin (HR: 0.77, 95% CI: 0.69 to 0.86).
Bottom line: Compared with warfarin therapy, apixaban is more effective and safer for stroke prevention in patients with atrial fibrillation.
Citation: Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
Ultrasonography Is Useful in Diagnosis of Pneumothorax
Clinical question: Is transthoracic ultrasonography a useful tool to diagnose pneumothorax?
Background: CT is the diagnostic gold standard for pneumothorax, but it is associated with radiation exposure and requires patient transport. Chest radiograph is easy to perform but may be too insensitive for adequate diagnosis. Ultrasonography’s diagnostic performance for detecting pneumothorax needs further evaluation.
Study design: Systematic review and meta-analysis.
Setting: Critically ill, trauma, or post-biopsy patients were identified in each of the studies.
Synopsis: The meta-analysis of 20 eligible studies found a pooled sensitivity of ultrasound for the detection of pneumothorax of 0.88 (95% CI: 0.85 to 0.91) and specificity of 0.99 (0.98 to 0.99) compared with sensitivity of 0.52 (0.49 to 0.55) and specificity of 1.00 (1.00 to 1.00) for chest radiograph. Although the overall ROC curve was not significantly different between these modalities, the accuracy of ultrasonography was highly dependent on the skill of the operator.
Bottom line: When performed by a skilled operator, transthoracic ultrasonography is as specific, and more sensitive, than chest radiograph in diagnosing pneumothorax.
Citation: Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140:859-866.
Risk Prediction for Hospital Readmission Remains Challenging
Clinical question: Can readmission risk assessment be used to identify which patients would benefit most from care-transition interventions, or to risk-adjust readmission rates for hospital comparison?
Background: Multiple models to predict hospital readmission have been described and validated. Identifying patients at high risk for readmission could allow for customized care-transition interventions, or could be used to risk-adjust readmission rates to compare publicly reported rates by hospital.
Study design: Systematic review with qualitative synthesis of results.
Setting: Thirty studies (23 from the U.S.) tested 26 unique readmission models.
Synopsis: Each model had been tested in both a derivation and validation cohort. Fourteen models (nine from the U.S.), using retrospective administrative data to compare risk-adjusted rates between hospitals, had poor discriminative capacity (c statistic range: 0.55 to 0.65). Seven models could be used to identify high-risk patients early in the hospitalization (c statistic range: 0.56 to 0.72) and five could be used to identify high-risk patients at discharge (c statistic range: 0.68 to 0.83), but these also had poor to moderate discriminative capacity. Multiple variables were considered in each of the models; most incorporated medical comorbidities and prior use of healthcare services.
Bottom line: Current readmission risk prediction models do not perform adequately for comparative or clinical purposes.
Citation: Kansagara D, Englander H, Salanitro A, et. al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
Intravenous Fluids for Acute Pancreatitis: More May Be Less
Clinical question: What is the optimal volume of fluid administration for treatment of acute pancreatitis?
Background: Current guidelines for management of acute pancreatitis emphasize vigorous administration of intravenous fluids to reduce the risk of pancreatic necrosis and organ failure. This recommendation is based upon animal studies, and has not been subjected to clinical evaluation in humans.
Study design: Prospective observational cohort.
Setting: University-affiliated tertiary-care public hospital in Spain.
Synopsis: This study enrolled 247 patients admitted with acute pancreatitis to determine the association between the volume of fluid administered during the first 24 hours and the development of persistent organ failure, pancreatic fluid collection or necrosis, and mortality. The volume and rate of fluid administered were determined by the treating physician. Patients were classified into three groups: those receiving a volume <3.1 L, 3.1 to 4.1 L, or >4.1 L.
After multivariate adjustment, those receiving <3.1 L had no increased risk of necrosis or any other adverse outcome, compared with those who received the middle range of fluid volume.
Patients receiving >4.1 L had a higher risk of persistent organ failure (OR: 7.7, 95% CI: 1.5 to 38.7), particularly renal and respiratory insufficiency, and fluid collection development (OR: 1.9, 95% CI: 1 to 3.7) independent of disease severity. Pancreatic necrosis and mortality were similar in the three groups.
Bottom line: Administration of large-volume intravenous fluids (>4.1 L) in
the first 24 hours was associated with worse outcomes, although residual confounding cannot be excluded in this nonrandomized study.
Citation: de-Madaria E, Soler-Sala G, Sanchez-Paya J, et al. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;106:1843-1850.
Clinical Outcomes in Saddle Pulmonary Embolism
Clinical question: What are the treatments used and outcomes associated with saddle pulmonary embolism?
Background: Saddle pulmonary embolism is a risk for right ventricular dysfunction and sudden hemodynamic collapse. There are limited data on the clinical presentation and outcomes in these patients.
Study design: Retrospective case review.
Setting: Single academic medical center.
Synopsis: In this retrospective review of 680 patients diagnosed with pulmonary embolism on CT at a single academic medical center from 2004 to 2009, 5.4% (37 patients) had a saddle pulmonary embolism.
Most patients with saddle pulmonary embolism were hemodynamically stable and responded to standard therapy with unfractionated heparin. The mean length of stay was nine days, 46% received an inferior vena cava filter, 41% were treated in an ICU, and 5.4% (two patients) died in the hospital. Thrombolytics were used in only 11% of patients, most of which had sustained hypotension and/or were mechanically ventilated.
Bottom line: Most patients with saddle pulmonary embolus in this single institution study did not receive thrombolytics and had overall low mortality.
Citation: Sardi A, Gluskin J, Guttentag A, Kotler MN, Braitman LE, Lippmann M. Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience. Crit Care Med. 2011;39:2413-2418.
Early Fluids Might Decrease Renal Morbidity in Hemolytic Uremic Syndrome
Clinical question: Does intravenous volume expansion during diarrheal illness mitigate the nephrotoxic effects of hemolytic uremic syndrome (HUS)?
Background: HUS often results in significant morbidity, particularly when oligoanuria is also present. Shiga-toxin-producing bacteria, notoriously Escherichia coli O157:H7 in the context of a diarrheal illness, are the most common cause, and worldwide outbreaks have been increasingly described. One prior report suggests that early IV fluid administration results in improved outcomes.
Study design: Prospective cohort study.
Setting: Eleven pediatric hospitals in the U.S. and Scotland.
Synopsis: Fifty children with diarrhea-associated HUS were enrolled and received clinical care at the discretion of the local provider, independent of the study. A family questionnaire (to define initial illness) and chart review were subsequently performed. Oligoanuria, defined as a urine output of less than 0.5 mL/kg/hr for at least one calendar day after HUS onset, was present in 34 (68%) patients. Oligoanuric and nonoligoanuric patients were similar at baseline; however, there was a significant association between less fluid administration in the first four days of illness and oligoanuria. Specifically, lack of IV fluids portended a 1.6 times higher likelihood of oligoanuria (95% confidence interval, 1.1-2.4; P=0.02).
The authors also suggest a dose-response relationship to their findings, which potentially strengthens their findings. However, the practical applicability of these findings appears limited. Many of the patients who did not receive IV fluids early on were also not admitted to a hospital, likely signifying mild illness without notable dehydration. Replicability of the benefits of early volume expansion in HUS will depend on the ability to accurately identify patients with Shiga-toxin-producing diarrheal illnesses at presentation. If this is feasible, it would be interesting to examine the details of oral hydration as well, particularly in those who are not dehydrated enough to require hospitalization.
Bottom line: Early IV fluids might be nephroprotective in diarrhea-associated HUS.
Citation: Hickey CA, Beattie J, Cowieson J, et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch Pediatr Adolesc Med. 2011;165:884-889.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: Does intravenous volume expansion during diarrheal illness mitigate the nephrotoxic effects of hemolytic uremic syndrome (HUS)?
Background: HUS often results in significant morbidity, particularly when oligoanuria is also present. Shiga-toxin-producing bacteria, notoriously Escherichia coli O157:H7 in the context of a diarrheal illness, are the most common cause, and worldwide outbreaks have been increasingly described. One prior report suggests that early IV fluid administration results in improved outcomes.
Study design: Prospective cohort study.
Setting: Eleven pediatric hospitals in the U.S. and Scotland.
Synopsis: Fifty children with diarrhea-associated HUS were enrolled and received clinical care at the discretion of the local provider, independent of the study. A family questionnaire (to define initial illness) and chart review were subsequently performed. Oligoanuria, defined as a urine output of less than 0.5 mL/kg/hr for at least one calendar day after HUS onset, was present in 34 (68%) patients. Oligoanuric and nonoligoanuric patients were similar at baseline; however, there was a significant association between less fluid administration in the first four days of illness and oligoanuria. Specifically, lack of IV fluids portended a 1.6 times higher likelihood of oligoanuria (95% confidence interval, 1.1-2.4; P=0.02).
The authors also suggest a dose-response relationship to their findings, which potentially strengthens their findings. However, the practical applicability of these findings appears limited. Many of the patients who did not receive IV fluids early on were also not admitted to a hospital, likely signifying mild illness without notable dehydration. Replicability of the benefits of early volume expansion in HUS will depend on the ability to accurately identify patients with Shiga-toxin-producing diarrheal illnesses at presentation. If this is feasible, it would be interesting to examine the details of oral hydration as well, particularly in those who are not dehydrated enough to require hospitalization.
Bottom line: Early IV fluids might be nephroprotective in diarrhea-associated HUS.
Citation: Hickey CA, Beattie J, Cowieson J, et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch Pediatr Adolesc Med. 2011;165:884-889.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: Does intravenous volume expansion during diarrheal illness mitigate the nephrotoxic effects of hemolytic uremic syndrome (HUS)?
Background: HUS often results in significant morbidity, particularly when oligoanuria is also present. Shiga-toxin-producing bacteria, notoriously Escherichia coli O157:H7 in the context of a diarrheal illness, are the most common cause, and worldwide outbreaks have been increasingly described. One prior report suggests that early IV fluid administration results in improved outcomes.
Study design: Prospective cohort study.
Setting: Eleven pediatric hospitals in the U.S. and Scotland.
Synopsis: Fifty children with diarrhea-associated HUS were enrolled and received clinical care at the discretion of the local provider, independent of the study. A family questionnaire (to define initial illness) and chart review were subsequently performed. Oligoanuria, defined as a urine output of less than 0.5 mL/kg/hr for at least one calendar day after HUS onset, was present in 34 (68%) patients. Oligoanuric and nonoligoanuric patients were similar at baseline; however, there was a significant association between less fluid administration in the first four days of illness and oligoanuria. Specifically, lack of IV fluids portended a 1.6 times higher likelihood of oligoanuria (95% confidence interval, 1.1-2.4; P=0.02).
The authors also suggest a dose-response relationship to their findings, which potentially strengthens their findings. However, the practical applicability of these findings appears limited. Many of the patients who did not receive IV fluids early on were also not admitted to a hospital, likely signifying mild illness without notable dehydration. Replicability of the benefits of early volume expansion in HUS will depend on the ability to accurately identify patients with Shiga-toxin-producing diarrheal illnesses at presentation. If this is feasible, it would be interesting to examine the details of oral hydration as well, particularly in those who are not dehydrated enough to require hospitalization.
Bottom line: Early IV fluids might be nephroprotective in diarrhea-associated HUS.
Citation: Hickey CA, Beattie J, Cowieson J, et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch Pediatr Adolesc Med. 2011;165:884-889.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
What Are Common Causes of Glomerular Disease in Adults?
The Case
A 52-year-old man presents with abdominal pain. His temperature is 100.8°F, his blood pressure is 170/90 mm/Hg, and his pulse is 110 beats per minute. On exam, he has 2+ lower extremity edema, periorbital edema, and left-sided flank tenderness. His BUN is 42 mg/dL, his creatinine is 2.5 mg/dL, and his albumin is 1.4 g/dL. Urinalysis shows 2+ protein, large blood, and red blood cells (RBCs). What are the next steps in his diagnosis?
Overview
Glomerular diseases involve a wide spectrum of disease processes. They can result from an acute illness, such as an upper respiratory infection that self-resolves, or from chronic disease states, such as HIV. In some instances, such illnesses as systemic lupus erythematosus (SLE) can cause rapidly progressive renal failure, requiring prompt intervention. While glomerular diseases can be daunting, it is essential for hospitalists to be familiar with fundamental concepts and key features unique to each syndrome.
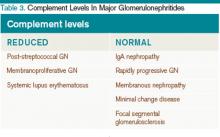
The approach to glomerulonephritis (GN) can be simplified by summarizing various types into the two broad categories of nephrotic and nephritic syndromes, and identifying the key clinical findings (see Table 1, p. below).
The major subtypes of nephrotic syndrome are minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), and membranoproliferative glomerulonephritis (MPGN). The clinical manifestations of nephrotic syndrome are edema, hyperlipidemia, lipiduria, and hypoalbuminemia.1 The urinalysis is significant for >3.5 g/day of proteinuria showing fatty casts or oval fat bodies.2 The loss of other proteins, such as anti-thrombin III, may put patients at higher risk for developing venous thromboses.1
The major subtypes of nephritic syndrome are post-streptococcal glomerulonephritis (PSGS), IgA nephropathy, Henoch-Schonlein Purpura (HSP), and rapidly progressive GN (RPGN types I, II, and III). The clinical manifestations of nephritic syndrome are hypertension (HTN) and hematuria.1 Nephritic syndromes may present with more rapidly progressive renal failure when compared with nephrotic syndrome.1 The urinalysis is significant for hematuria with RBC casts, and variable levels of proteinuria (typically, less than 3.5 g/day is seen in nephritic syndrome).1
Review of the Data
Nephrotic Syndromes
Minimal change disease. MCD is more common in children than adults, and only accounts for 10% to 15% of glomerular disease cases in adults.3 It is associated with Hodgkin’s lymphoma, NSAID use, and allergic conditions. There usually is an absence of hypertension (HTN). There are no glomerular basement membrane abnormalities seen on light microscopy. Electron microscopy shows effacement of podocytes. On urinalysis, oval fat bodies are seen, which are characteristic of heavy proteinuria. Complement levels are normal. Steroids are first-line treatment, but in adults with relapses or steroid resistance, immunosuppressive agents have also been used.2
Focal segment glomerlosclerosis.
FSGS is the most common primary glomerular disorder in the United States and is the most common cause of nephrotic syndrome among blacks.4,5 It is associated with HIV (collapsing variant), parvovirus B19, heroin use, sickle-cell disease, obesity, chronic vesicoureteral reflux, and HTN.4,6 Sclerosis of segmental glomeruli is seen on light microscopy.
Electron microscopy shows effacement of podocytes. Complement levels are normal. Treatment of primary idiopathic FSGS includes use of renin-angiotensin inhibitors and steroids.4 Immunosuppressives are reserved for relapses. Treatment of secondary FSGS involves identifying the underlying cause.
Membranous nephropathy. MN is twice as common in males as in females and is the most common cause of adult-onset idiopathic nephrotic syndrome, with the average presentation in the fifth or sixth decade of life.7,8 Aside from its idiopathic form, up to 25% of MN cases have an underlying disease process, such as solid organ tumors or hepatitis B.7,9 While nephrotic syndrome overall can increase the risk of thromboembolic complications, MN is the most common nephrotic disorder predisposing the development of renal vein thrombosis.7 Diffuse capillary wall thickening is seen on light microscopy, and electron microscopy shows sub-epithelial immune deposits. Complement levels are normal. Steroids and immunosuppressive agents are used for treatment.10
Membranoproliferative glomerulonephritis. MPGN is a nephrotic syndrome that is more common in children and young adults and can present with features of nephritic syndrome.1,11 It is associated with hepatitis C, SLE, and cryoglobulinemia.11 Light microscopy shows mesangial and endocapillary proliferation, as well as glomerular basement membrane thickening and splitting (“tram track” appearance). Electron microscopy shows subendothelial and dense deposits. It presents with reduced complement levels (C3 and C4).11 Treatment depends on the associated disease.
Nephritic Syndromes
Post-streptococcal glomerulonephritis. PSGN is seen in children and young adults and is associated with skin (impetigo) and throat infections (pharyngitis).12 Hematuria usually presents two to three weeks after a streptococcal infection. The urine is classically dark and smoky-colored. Levels of C3 and CH50 are low, but C4 levels are normal.1 In addition, there are positive antibody titers for ASO and anti-DNase. Light microscopy shows hypercellularity of glomeruli. Electron microscopy shows dome-shaped sub-endothelial deposits. Treatment is usually supportive.
IgA nephropathy. IgA nephropathy is the most common form of glomerular disease worldwide and the most common form of glomerular-related microscopic hematuria in all age groups.2,13 It occurs in all ages but more frequently in males.14 It occurs during or immediately after an upper respiratory infection. Light microscopy shows mesangial cell proliferation and crescentic GN. Electron microscopy shows immune deposits in the mesangium. Complement levels are normal. There has been no proven therapy, but ACE inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), fish oil, steroids, and tonsillectomy have been used with some success.14 The clinical course of IgA nephropathy can be highly variable, with the potential for a benign course to rapidly progressive renal failure, with 15% to 40% of patients developing end-stage renal disease.14
Henoch-Schonlein purpura. HSP affects children more than adults and is the systemic form of IgA nephropathy.14 Most cases are idiopathic. Clinical
manifestations include: HTN; purpuric palpable rash on buttocks, ankles, and legs; bloody diarrhea with abdominal cramps; and pain in wrist, ankle, and knee joints.15 Light microscopy shows mesangial cell proliferation. Immune deposits in the mesangium are seen on electron microscopy. Complement levels are normal. Treatment is supportive.
Rapidly progressive glomerulonephritis types I, II, and III. RPGN represents a wide variety of disease states in which rapid progression to renal failure is seen within days to weeks.16 They are categorized into three sub-categories: I, II, and III.
Type I is an anti-GBM disease, an example being Goodpasture’s syndrome. This condition presents with hemoptysis, pulmonary infiltrates, and hematuria with RBC casts. Anti-GBM antibodies are classically found.1 Complement levels are normal and a linear immunofluorescence pattern is seen. Treatment is steroids, immunosuppressive agents, and plasmapheresis.17
Type II is an immune complex deposition disease, such as HSP, SLE, or post-streptococcal GN, in which granular complex deposits are seen. Treatment is directed toward treating the underlying cause.
Type III is pauci-immune (no immune deposits), showing necrotizing crescentic GN on biopsy, and is associated with a positive ANCA.1,18 They are associated with systemic small-vessel vasculitis, such as granulomatosis with polyangiitis (formerly known as Wegener’s granulomatosis), microscopic polyangiitis, and Churg-Strauss syndrome, or can be limited to renal involvement.1,18 Complement levels are normal. Treatment is steroids and immunosuppressive agents, such as cyclophosphamide.
A summary of the findings found in the glomerulonephritides and how complement levels are affected are found in Table 2 and Table 3, respectively.
Secondary Causes of Nephrotic Diseases
Diabetic nephropathy. Diabetic nephropathy is the single most common cause of progressive renal failure in the United States.3 Up to 50% of patients with diabetes present with diabetic nephropathy.19 Current recommendations are to screen yearly for microalbuminuria at the time of diagnosis.3 Treatment involves use of ACE-Is or ARBs to reduce proteinuria and slow the progression of renal disease.
HIV-associated nephropathy. HIV-associated nephropathy commonly presents as the collapsing variant of FSGS. However, it can present as other forms of glomerulopathy, such as MPGN or IgA nephropathy, as well as an immune complex GN with “lupus-like” features without evidence of SLE.19,20 Therefore, HIV nephropathy has now been categorized as a separate entity.3 ACE-Is, HAART therapy, and corticosteroids are the mainstays of treatment.
Amyloidosis. Renal involvement is seen in both primary (AL) and secondary (AA) amyloidosis. Eighty percent of patients with AL have renal disease, and 25% of these patients have nephrotic syndrome.16 Diagnosis is made with Congo Red stain, which shows fibrillary amyloid deposits within the mesangium and capillary walls. Treatment is directed at the underlying process.
Systemic lupus erythematosus. SLE is divided into six classes (I-VI) based on the involvement and severity of renal disease, and steroids and immunosuppressive agents are used for treatment, also based on the severity of the disease.21
Back to the Case
Our patient presented to the hospital with abdominal pain, low-grade fever, HTN, edema, hypoalbuminemia, and new-onset renal failure with gross hematuria and proteinuria. The presence of proteinuria and hypoalbuminemia, combined with peripheral and periorbital edema, suggests glomerular loss of albumin, such as in nephrotic syndrome. His renal failure in the setting of the sudden development of gross hematuria with flank pain is concerning for a renal vein thrombosis, and an abdominal magnetic resonance venography did in fact visualize a renal vein thrombosis.
He was admitted to the hospital and was started on therapeutic intravenous heparin, and bridged to warfarin. Subsequent renal biopsy confirmed the findings of membranous nephropathy, which was suspected due to his renal vein thrombosis. Therapy was initiated with corticosteroids after the biopsy, and he responded well. Because of his risk factors for further thromboembolic events, lifelong anticoagulation therapy was recommended.
Bottom Line
For patients with glomerular disease, differentiating between nephrotic and nephritic syndromes and understanding key clinical and laboratory differences can lead to easier identification and treatment.
Drs. Khan and Smith are assistant professors of medicine, and Dr. Ansari is associate division director, in the Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
References
- Donegio RGB, Salant DJ. Nephrology: Glomerular Diseases. In: ACP Medicine. Dale D, Federman D, eds. Available at: http://www.acpmedicine.com/acpmedicine/institutional/instHtmlReader.action?readerFlag=chapt&chapId=part10_ch05. Accessed Feb. 15, 2012.
- Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338:1202-1211.
- Lewis JB, Neilson EG. Chapter 283. Glomerular Diseases. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2012.
- D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398-23411.
- Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815-825.
- Balow JE. Nephropathy in the context of HIV infection. Kidney Int. 2005;67:1632-1633.
- Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23:324-332.
- Nickolas TL, Radhakrishnan J, Appel GB. Hyperlipidemia and thrombotic complications in patients with membranous nephropathy. Semin Nephrol. 2003;23:406-411.
- Burstein DM, Korbet SM, Schwartz MM. Membranous glomerulonephritis and malignancy. Am J Kidney Dis. 1993;22:5-10.
- Hofstra JM, Wetzels JF. Management of patients with membranous nephropathy. Nephrol Dial Transplant. 2012;27:6-9.
- Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr Nephrol. 2010;25:1409-1418.
- Eison TM, Ault BH, Jones DP, Chesney RW, Wyatt RJ. Post-streptococcal acute glomerulonephritis in children: clinical features and pathogenesis. Pediatr Nephrol. 2011;26:165-180.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med. 2003;348:2330-2338.
- Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738-748.
- McCarthy HJ, Tizard EJ. Clinical practice: Diagnosis and management of henoch-schonlein purpura. Eur J Pediatr. 2010169:643-650.
- Appel GB, Radhakrishnan J. Cecil Medicine: Volume 1: Chapter 123: Glomerular Disorders and Nephrotic Syndromes. MD Consult Preview website. Available at: http://www.mdconsult.com/books/page.do?eid=4-u1.0-B978-1-4377-1604-7..00123-8&isbn=978-1-4377-1604-7&uniqId=313771243-2#4-u1.0-B978-1-4377-1604-7..00123-8. Accessed Feb. 16, 2012.
- Walters G, Willis NS, Craig JC. Interventions for renal vasculitis in adults. Cochrane Database Syst Rev. 2008;(3)(3):CD003232.
- Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164-1177.
- Falk, RJ: Medical Knowledge Self Assessment Program 14. Nephrology: 2006.
- Haas M, Kaul S, Eustace JA. HIV-associated immune complex glomerulonephritis with “lupus-like” features: a clinicopathologic study of 14 cases. Kidney Int. 2005;67:1381.
- Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black americans. glomerular disease collaborative network. Kidney Int. 1997;51:1188-1195.
The Case
A 52-year-old man presents with abdominal pain. His temperature is 100.8°F, his blood pressure is 170/90 mm/Hg, and his pulse is 110 beats per minute. On exam, he has 2+ lower extremity edema, periorbital edema, and left-sided flank tenderness. His BUN is 42 mg/dL, his creatinine is 2.5 mg/dL, and his albumin is 1.4 g/dL. Urinalysis shows 2+ protein, large blood, and red blood cells (RBCs). What are the next steps in his diagnosis?
Overview
Glomerular diseases involve a wide spectrum of disease processes. They can result from an acute illness, such as an upper respiratory infection that self-resolves, or from chronic disease states, such as HIV. In some instances, such illnesses as systemic lupus erythematosus (SLE) can cause rapidly progressive renal failure, requiring prompt intervention. While glomerular diseases can be daunting, it is essential for hospitalists to be familiar with fundamental concepts and key features unique to each syndrome.
The approach to glomerulonephritis (GN) can be simplified by summarizing various types into the two broad categories of nephrotic and nephritic syndromes, and identifying the key clinical findings (see Table 1, p. below).
The major subtypes of nephrotic syndrome are minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), and membranoproliferative glomerulonephritis (MPGN). The clinical manifestations of nephrotic syndrome are edema, hyperlipidemia, lipiduria, and hypoalbuminemia.1 The urinalysis is significant for >3.5 g/day of proteinuria showing fatty casts or oval fat bodies.2 The loss of other proteins, such as anti-thrombin III, may put patients at higher risk for developing venous thromboses.1
The major subtypes of nephritic syndrome are post-streptococcal glomerulonephritis (PSGS), IgA nephropathy, Henoch-Schonlein Purpura (HSP), and rapidly progressive GN (RPGN types I, II, and III). The clinical manifestations of nephritic syndrome are hypertension (HTN) and hematuria.1 Nephritic syndromes may present with more rapidly progressive renal failure when compared with nephrotic syndrome.1 The urinalysis is significant for hematuria with RBC casts, and variable levels of proteinuria (typically, less than 3.5 g/day is seen in nephritic syndrome).1
Review of the Data
Nephrotic Syndromes
Minimal change disease. MCD is more common in children than adults, and only accounts for 10% to 15% of glomerular disease cases in adults.3 It is associated with Hodgkin’s lymphoma, NSAID use, and allergic conditions. There usually is an absence of hypertension (HTN). There are no glomerular basement membrane abnormalities seen on light microscopy. Electron microscopy shows effacement of podocytes. On urinalysis, oval fat bodies are seen, which are characteristic of heavy proteinuria. Complement levels are normal. Steroids are first-line treatment, but in adults with relapses or steroid resistance, immunosuppressive agents have also been used.2
Focal segment glomerlosclerosis.
FSGS is the most common primary glomerular disorder in the United States and is the most common cause of nephrotic syndrome among blacks.4,5 It is associated with HIV (collapsing variant), parvovirus B19, heroin use, sickle-cell disease, obesity, chronic vesicoureteral reflux, and HTN.4,6 Sclerosis of segmental glomeruli is seen on light microscopy.
Electron microscopy shows effacement of podocytes. Complement levels are normal. Treatment of primary idiopathic FSGS includes use of renin-angiotensin inhibitors and steroids.4 Immunosuppressives are reserved for relapses. Treatment of secondary FSGS involves identifying the underlying cause.
Membranous nephropathy. MN is twice as common in males as in females and is the most common cause of adult-onset idiopathic nephrotic syndrome, with the average presentation in the fifth or sixth decade of life.7,8 Aside from its idiopathic form, up to 25% of MN cases have an underlying disease process, such as solid organ tumors or hepatitis B.7,9 While nephrotic syndrome overall can increase the risk of thromboembolic complications, MN is the most common nephrotic disorder predisposing the development of renal vein thrombosis.7 Diffuse capillary wall thickening is seen on light microscopy, and electron microscopy shows sub-epithelial immune deposits. Complement levels are normal. Steroids and immunosuppressive agents are used for treatment.10
Membranoproliferative glomerulonephritis. MPGN is a nephrotic syndrome that is more common in children and young adults and can present with features of nephritic syndrome.1,11 It is associated with hepatitis C, SLE, and cryoglobulinemia.11 Light microscopy shows mesangial and endocapillary proliferation, as well as glomerular basement membrane thickening and splitting (“tram track” appearance). Electron microscopy shows subendothelial and dense deposits. It presents with reduced complement levels (C3 and C4).11 Treatment depends on the associated disease.
Nephritic Syndromes
Post-streptococcal glomerulonephritis. PSGN is seen in children and young adults and is associated with skin (impetigo) and throat infections (pharyngitis).12 Hematuria usually presents two to three weeks after a streptococcal infection. The urine is classically dark and smoky-colored. Levels of C3 and CH50 are low, but C4 levels are normal.1 In addition, there are positive antibody titers for ASO and anti-DNase. Light microscopy shows hypercellularity of glomeruli. Electron microscopy shows dome-shaped sub-endothelial deposits. Treatment is usually supportive.
IgA nephropathy. IgA nephropathy is the most common form of glomerular disease worldwide and the most common form of glomerular-related microscopic hematuria in all age groups.2,13 It occurs in all ages but more frequently in males.14 It occurs during or immediately after an upper respiratory infection. Light microscopy shows mesangial cell proliferation and crescentic GN. Electron microscopy shows immune deposits in the mesangium. Complement levels are normal. There has been no proven therapy, but ACE inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), fish oil, steroids, and tonsillectomy have been used with some success.14 The clinical course of IgA nephropathy can be highly variable, with the potential for a benign course to rapidly progressive renal failure, with 15% to 40% of patients developing end-stage renal disease.14
Henoch-Schonlein purpura. HSP affects children more than adults and is the systemic form of IgA nephropathy.14 Most cases are idiopathic. Clinical
manifestations include: HTN; purpuric palpable rash on buttocks, ankles, and legs; bloody diarrhea with abdominal cramps; and pain in wrist, ankle, and knee joints.15 Light microscopy shows mesangial cell proliferation. Immune deposits in the mesangium are seen on electron microscopy. Complement levels are normal. Treatment is supportive.
Rapidly progressive glomerulonephritis types I, II, and III. RPGN represents a wide variety of disease states in which rapid progression to renal failure is seen within days to weeks.16 They are categorized into three sub-categories: I, II, and III.
Type I is an anti-GBM disease, an example being Goodpasture’s syndrome. This condition presents with hemoptysis, pulmonary infiltrates, and hematuria with RBC casts. Anti-GBM antibodies are classically found.1 Complement levels are normal and a linear immunofluorescence pattern is seen. Treatment is steroids, immunosuppressive agents, and plasmapheresis.17
Type II is an immune complex deposition disease, such as HSP, SLE, or post-streptococcal GN, in which granular complex deposits are seen. Treatment is directed toward treating the underlying cause.
Type III is pauci-immune (no immune deposits), showing necrotizing crescentic GN on biopsy, and is associated with a positive ANCA.1,18 They are associated with systemic small-vessel vasculitis, such as granulomatosis with polyangiitis (formerly known as Wegener’s granulomatosis), microscopic polyangiitis, and Churg-Strauss syndrome, or can be limited to renal involvement.1,18 Complement levels are normal. Treatment is steroids and immunosuppressive agents, such as cyclophosphamide.
A summary of the findings found in the glomerulonephritides and how complement levels are affected are found in Table 2 and Table 3, respectively.
Secondary Causes of Nephrotic Diseases
Diabetic nephropathy. Diabetic nephropathy is the single most common cause of progressive renal failure in the United States.3 Up to 50% of patients with diabetes present with diabetic nephropathy.19 Current recommendations are to screen yearly for microalbuminuria at the time of diagnosis.3 Treatment involves use of ACE-Is or ARBs to reduce proteinuria and slow the progression of renal disease.
HIV-associated nephropathy. HIV-associated nephropathy commonly presents as the collapsing variant of FSGS. However, it can present as other forms of glomerulopathy, such as MPGN or IgA nephropathy, as well as an immune complex GN with “lupus-like” features without evidence of SLE.19,20 Therefore, HIV nephropathy has now been categorized as a separate entity.3 ACE-Is, HAART therapy, and corticosteroids are the mainstays of treatment.
Amyloidosis. Renal involvement is seen in both primary (AL) and secondary (AA) amyloidosis. Eighty percent of patients with AL have renal disease, and 25% of these patients have nephrotic syndrome.16 Diagnosis is made with Congo Red stain, which shows fibrillary amyloid deposits within the mesangium and capillary walls. Treatment is directed at the underlying process.
Systemic lupus erythematosus. SLE is divided into six classes (I-VI) based on the involvement and severity of renal disease, and steroids and immunosuppressive agents are used for treatment, also based on the severity of the disease.21
Back to the Case
Our patient presented to the hospital with abdominal pain, low-grade fever, HTN, edema, hypoalbuminemia, and new-onset renal failure with gross hematuria and proteinuria. The presence of proteinuria and hypoalbuminemia, combined with peripheral and periorbital edema, suggests glomerular loss of albumin, such as in nephrotic syndrome. His renal failure in the setting of the sudden development of gross hematuria with flank pain is concerning for a renal vein thrombosis, and an abdominal magnetic resonance venography did in fact visualize a renal vein thrombosis.
He was admitted to the hospital and was started on therapeutic intravenous heparin, and bridged to warfarin. Subsequent renal biopsy confirmed the findings of membranous nephropathy, which was suspected due to his renal vein thrombosis. Therapy was initiated with corticosteroids after the biopsy, and he responded well. Because of his risk factors for further thromboembolic events, lifelong anticoagulation therapy was recommended.
Bottom Line
For patients with glomerular disease, differentiating between nephrotic and nephritic syndromes and understanding key clinical and laboratory differences can lead to easier identification and treatment.
Drs. Khan and Smith are assistant professors of medicine, and Dr. Ansari is associate division director, in the Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
References
- Donegio RGB, Salant DJ. Nephrology: Glomerular Diseases. In: ACP Medicine. Dale D, Federman D, eds. Available at: http://www.acpmedicine.com/acpmedicine/institutional/instHtmlReader.action?readerFlag=chapt&chapId=part10_ch05. Accessed Feb. 15, 2012.
- Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338:1202-1211.
- Lewis JB, Neilson EG. Chapter 283. Glomerular Diseases. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2012.
- D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398-23411.
- Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815-825.
- Balow JE. Nephropathy in the context of HIV infection. Kidney Int. 2005;67:1632-1633.
- Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23:324-332.
- Nickolas TL, Radhakrishnan J, Appel GB. Hyperlipidemia and thrombotic complications in patients with membranous nephropathy. Semin Nephrol. 2003;23:406-411.
- Burstein DM, Korbet SM, Schwartz MM. Membranous glomerulonephritis and malignancy. Am J Kidney Dis. 1993;22:5-10.
- Hofstra JM, Wetzels JF. Management of patients with membranous nephropathy. Nephrol Dial Transplant. 2012;27:6-9.
- Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr Nephrol. 2010;25:1409-1418.
- Eison TM, Ault BH, Jones DP, Chesney RW, Wyatt RJ. Post-streptococcal acute glomerulonephritis in children: clinical features and pathogenesis. Pediatr Nephrol. 2011;26:165-180.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med. 2003;348:2330-2338.
- Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738-748.
- McCarthy HJ, Tizard EJ. Clinical practice: Diagnosis and management of henoch-schonlein purpura. Eur J Pediatr. 2010169:643-650.
- Appel GB, Radhakrishnan J. Cecil Medicine: Volume 1: Chapter 123: Glomerular Disorders and Nephrotic Syndromes. MD Consult Preview website. Available at: http://www.mdconsult.com/books/page.do?eid=4-u1.0-B978-1-4377-1604-7..00123-8&isbn=978-1-4377-1604-7&uniqId=313771243-2#4-u1.0-B978-1-4377-1604-7..00123-8. Accessed Feb. 16, 2012.
- Walters G, Willis NS, Craig JC. Interventions for renal vasculitis in adults. Cochrane Database Syst Rev. 2008;(3)(3):CD003232.
- Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164-1177.
- Falk, RJ: Medical Knowledge Self Assessment Program 14. Nephrology: 2006.
- Haas M, Kaul S, Eustace JA. HIV-associated immune complex glomerulonephritis with “lupus-like” features: a clinicopathologic study of 14 cases. Kidney Int. 2005;67:1381.
- Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black americans. glomerular disease collaborative network. Kidney Int. 1997;51:1188-1195.
The Case
A 52-year-old man presents with abdominal pain. His temperature is 100.8°F, his blood pressure is 170/90 mm/Hg, and his pulse is 110 beats per minute. On exam, he has 2+ lower extremity edema, periorbital edema, and left-sided flank tenderness. His BUN is 42 mg/dL, his creatinine is 2.5 mg/dL, and his albumin is 1.4 g/dL. Urinalysis shows 2+ protein, large blood, and red blood cells (RBCs). What are the next steps in his diagnosis?
Overview
Glomerular diseases involve a wide spectrum of disease processes. They can result from an acute illness, such as an upper respiratory infection that self-resolves, or from chronic disease states, such as HIV. In some instances, such illnesses as systemic lupus erythematosus (SLE) can cause rapidly progressive renal failure, requiring prompt intervention. While glomerular diseases can be daunting, it is essential for hospitalists to be familiar with fundamental concepts and key features unique to each syndrome.
The approach to glomerulonephritis (GN) can be simplified by summarizing various types into the two broad categories of nephrotic and nephritic syndromes, and identifying the key clinical findings (see Table 1, p. below).
The major subtypes of nephrotic syndrome are minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), and membranoproliferative glomerulonephritis (MPGN). The clinical manifestations of nephrotic syndrome are edema, hyperlipidemia, lipiduria, and hypoalbuminemia.1 The urinalysis is significant for >3.5 g/day of proteinuria showing fatty casts or oval fat bodies.2 The loss of other proteins, such as anti-thrombin III, may put patients at higher risk for developing venous thromboses.1
The major subtypes of nephritic syndrome are post-streptococcal glomerulonephritis (PSGS), IgA nephropathy, Henoch-Schonlein Purpura (HSP), and rapidly progressive GN (RPGN types I, II, and III). The clinical manifestations of nephritic syndrome are hypertension (HTN) and hematuria.1 Nephritic syndromes may present with more rapidly progressive renal failure when compared with nephrotic syndrome.1 The urinalysis is significant for hematuria with RBC casts, and variable levels of proteinuria (typically, less than 3.5 g/day is seen in nephritic syndrome).1
Review of the Data
Nephrotic Syndromes
Minimal change disease. MCD is more common in children than adults, and only accounts for 10% to 15% of glomerular disease cases in adults.3 It is associated with Hodgkin’s lymphoma, NSAID use, and allergic conditions. There usually is an absence of hypertension (HTN). There are no glomerular basement membrane abnormalities seen on light microscopy. Electron microscopy shows effacement of podocytes. On urinalysis, oval fat bodies are seen, which are characteristic of heavy proteinuria. Complement levels are normal. Steroids are first-line treatment, but in adults with relapses or steroid resistance, immunosuppressive agents have also been used.2
Focal segment glomerlosclerosis.
FSGS is the most common primary glomerular disorder in the United States and is the most common cause of nephrotic syndrome among blacks.4,5 It is associated with HIV (collapsing variant), parvovirus B19, heroin use, sickle-cell disease, obesity, chronic vesicoureteral reflux, and HTN.4,6 Sclerosis of segmental glomeruli is seen on light microscopy.
Electron microscopy shows effacement of podocytes. Complement levels are normal. Treatment of primary idiopathic FSGS includes use of renin-angiotensin inhibitors and steroids.4 Immunosuppressives are reserved for relapses. Treatment of secondary FSGS involves identifying the underlying cause.
Membranous nephropathy. MN is twice as common in males as in females and is the most common cause of adult-onset idiopathic nephrotic syndrome, with the average presentation in the fifth or sixth decade of life.7,8 Aside from its idiopathic form, up to 25% of MN cases have an underlying disease process, such as solid organ tumors or hepatitis B.7,9 While nephrotic syndrome overall can increase the risk of thromboembolic complications, MN is the most common nephrotic disorder predisposing the development of renal vein thrombosis.7 Diffuse capillary wall thickening is seen on light microscopy, and electron microscopy shows sub-epithelial immune deposits. Complement levels are normal. Steroids and immunosuppressive agents are used for treatment.10
Membranoproliferative glomerulonephritis. MPGN is a nephrotic syndrome that is more common in children and young adults and can present with features of nephritic syndrome.1,11 It is associated with hepatitis C, SLE, and cryoglobulinemia.11 Light microscopy shows mesangial and endocapillary proliferation, as well as glomerular basement membrane thickening and splitting (“tram track” appearance). Electron microscopy shows subendothelial and dense deposits. It presents with reduced complement levels (C3 and C4).11 Treatment depends on the associated disease.
Nephritic Syndromes
Post-streptococcal glomerulonephritis. PSGN is seen in children and young adults and is associated with skin (impetigo) and throat infections (pharyngitis).12 Hematuria usually presents two to three weeks after a streptococcal infection. The urine is classically dark and smoky-colored. Levels of C3 and CH50 are low, but C4 levels are normal.1 In addition, there are positive antibody titers for ASO and anti-DNase. Light microscopy shows hypercellularity of glomeruli. Electron microscopy shows dome-shaped sub-endothelial deposits. Treatment is usually supportive.
IgA nephropathy. IgA nephropathy is the most common form of glomerular disease worldwide and the most common form of glomerular-related microscopic hematuria in all age groups.2,13 It occurs in all ages but more frequently in males.14 It occurs during or immediately after an upper respiratory infection. Light microscopy shows mesangial cell proliferation and crescentic GN. Electron microscopy shows immune deposits in the mesangium. Complement levels are normal. There has been no proven therapy, but ACE inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), fish oil, steroids, and tonsillectomy have been used with some success.14 The clinical course of IgA nephropathy can be highly variable, with the potential for a benign course to rapidly progressive renal failure, with 15% to 40% of patients developing end-stage renal disease.14
Henoch-Schonlein purpura. HSP affects children more than adults and is the systemic form of IgA nephropathy.14 Most cases are idiopathic. Clinical
manifestations include: HTN; purpuric palpable rash on buttocks, ankles, and legs; bloody diarrhea with abdominal cramps; and pain in wrist, ankle, and knee joints.15 Light microscopy shows mesangial cell proliferation. Immune deposits in the mesangium are seen on electron microscopy. Complement levels are normal. Treatment is supportive.
Rapidly progressive glomerulonephritis types I, II, and III. RPGN represents a wide variety of disease states in which rapid progression to renal failure is seen within days to weeks.16 They are categorized into three sub-categories: I, II, and III.
Type I is an anti-GBM disease, an example being Goodpasture’s syndrome. This condition presents with hemoptysis, pulmonary infiltrates, and hematuria with RBC casts. Anti-GBM antibodies are classically found.1 Complement levels are normal and a linear immunofluorescence pattern is seen. Treatment is steroids, immunosuppressive agents, and plasmapheresis.17
Type II is an immune complex deposition disease, such as HSP, SLE, or post-streptococcal GN, in which granular complex deposits are seen. Treatment is directed toward treating the underlying cause.
Type III is pauci-immune (no immune deposits), showing necrotizing crescentic GN on biopsy, and is associated with a positive ANCA.1,18 They are associated with systemic small-vessel vasculitis, such as granulomatosis with polyangiitis (formerly known as Wegener’s granulomatosis), microscopic polyangiitis, and Churg-Strauss syndrome, or can be limited to renal involvement.1,18 Complement levels are normal. Treatment is steroids and immunosuppressive agents, such as cyclophosphamide.
A summary of the findings found in the glomerulonephritides and how complement levels are affected are found in Table 2 and Table 3, respectively.
Secondary Causes of Nephrotic Diseases
Diabetic nephropathy. Diabetic nephropathy is the single most common cause of progressive renal failure in the United States.3 Up to 50% of patients with diabetes present with diabetic nephropathy.19 Current recommendations are to screen yearly for microalbuminuria at the time of diagnosis.3 Treatment involves use of ACE-Is or ARBs to reduce proteinuria and slow the progression of renal disease.
HIV-associated nephropathy. HIV-associated nephropathy commonly presents as the collapsing variant of FSGS. However, it can present as other forms of glomerulopathy, such as MPGN or IgA nephropathy, as well as an immune complex GN with “lupus-like” features without evidence of SLE.19,20 Therefore, HIV nephropathy has now been categorized as a separate entity.3 ACE-Is, HAART therapy, and corticosteroids are the mainstays of treatment.
Amyloidosis. Renal involvement is seen in both primary (AL) and secondary (AA) amyloidosis. Eighty percent of patients with AL have renal disease, and 25% of these patients have nephrotic syndrome.16 Diagnosis is made with Congo Red stain, which shows fibrillary amyloid deposits within the mesangium and capillary walls. Treatment is directed at the underlying process.
Systemic lupus erythematosus. SLE is divided into six classes (I-VI) based on the involvement and severity of renal disease, and steroids and immunosuppressive agents are used for treatment, also based on the severity of the disease.21
Back to the Case
Our patient presented to the hospital with abdominal pain, low-grade fever, HTN, edema, hypoalbuminemia, and new-onset renal failure with gross hematuria and proteinuria. The presence of proteinuria and hypoalbuminemia, combined with peripheral and periorbital edema, suggests glomerular loss of albumin, such as in nephrotic syndrome. His renal failure in the setting of the sudden development of gross hematuria with flank pain is concerning for a renal vein thrombosis, and an abdominal magnetic resonance venography did in fact visualize a renal vein thrombosis.
He was admitted to the hospital and was started on therapeutic intravenous heparin, and bridged to warfarin. Subsequent renal biopsy confirmed the findings of membranous nephropathy, which was suspected due to his renal vein thrombosis. Therapy was initiated with corticosteroids after the biopsy, and he responded well. Because of his risk factors for further thromboembolic events, lifelong anticoagulation therapy was recommended.
Bottom Line
For patients with glomerular disease, differentiating between nephrotic and nephritic syndromes and understanding key clinical and laboratory differences can lead to easier identification and treatment.
Drs. Khan and Smith are assistant professors of medicine, and Dr. Ansari is associate division director, in the Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
References
- Donegio RGB, Salant DJ. Nephrology: Glomerular Diseases. In: ACP Medicine. Dale D, Federman D, eds. Available at: http://www.acpmedicine.com/acpmedicine/institutional/instHtmlReader.action?readerFlag=chapt&chapId=part10_ch05. Accessed Feb. 15, 2012.
- Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338:1202-1211.
- Lewis JB, Neilson EG. Chapter 283. Glomerular Diseases. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2012.
- D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398-23411.
- Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815-825.
- Balow JE. Nephropathy in the context of HIV infection. Kidney Int. 2005;67:1632-1633.
- Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23:324-332.
- Nickolas TL, Radhakrishnan J, Appel GB. Hyperlipidemia and thrombotic complications in patients with membranous nephropathy. Semin Nephrol. 2003;23:406-411.
- Burstein DM, Korbet SM, Schwartz MM. Membranous glomerulonephritis and malignancy. Am J Kidney Dis. 1993;22:5-10.
- Hofstra JM, Wetzels JF. Management of patients with membranous nephropathy. Nephrol Dial Transplant. 2012;27:6-9.
- Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr Nephrol. 2010;25:1409-1418.
- Eison TM, Ault BH, Jones DP, Chesney RW, Wyatt RJ. Post-streptococcal acute glomerulonephritis in children: clinical features and pathogenesis. Pediatr Nephrol. 2011;26:165-180.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med. 2003;348:2330-2338.
- Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738-748.
- McCarthy HJ, Tizard EJ. Clinical practice: Diagnosis and management of henoch-schonlein purpura. Eur J Pediatr. 2010169:643-650.
- Appel GB, Radhakrishnan J. Cecil Medicine: Volume 1: Chapter 123: Glomerular Disorders and Nephrotic Syndromes. MD Consult Preview website. Available at: http://www.mdconsult.com/books/page.do?eid=4-u1.0-B978-1-4377-1604-7..00123-8&isbn=978-1-4377-1604-7&uniqId=313771243-2#4-u1.0-B978-1-4377-1604-7..00123-8. Accessed Feb. 16, 2012.
- Walters G, Willis NS, Craig JC. Interventions for renal vasculitis in adults. Cochrane Database Syst Rev. 2008;(3)(3):CD003232.
- Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164-1177.
- Falk, RJ: Medical Knowledge Self Assessment Program 14. Nephrology: 2006.
- Haas M, Kaul S, Eustace JA. HIV-associated immune complex glomerulonephritis with “lupus-like” features: a clinicopathologic study of 14 cases. Kidney Int. 2005;67:1381.
- Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black americans. glomerular disease collaborative network. Kidney Int. 1997;51:1188-1195.
Three Earn Master of Hospital Medicine Designation

—Joseph Ming-Wah Li, MD, SFHM, SHM president
If the SHM Award of Excellence winners are the MVPs of HM, then the specialty’s new Masters in Hospital Medicine are this year’s Hall of Famers.
SHM is proud to announce that Patrick J. Cawley, MD, MBA, MHM, Peter K. Lindenauer, MD, MSc, FACP, MHM, and Mark V. Williams, MD, FACP, MHM, have earned the Master in Hospital Medicine designation. They are the eighth, ninth, and 10th SHM members to receive the exclusive designation. In addition to being honored at HM12, they have earned the right to use the “MHM” credential.
“Hospitalists of all stripes owe our new Masters in Hospital Medicine a great debt for their commitment to the specialty, to their patients, and to healthcare,” says Joseph Ming Wah Li, MD, SFHM, SHM president. “Their innovation, leadership, and vision have helped grow the influence and credibility of the hospital medicine specialty.”
Dr. Cawley is a charter member and past president of SHM who has served on the Ethics and Public Policy committees. He is a frequent speaker at the society’s annual meetings. Dr. Cawley received his medical degree from Georgetown University School of Medicine and trained as a resident at Duke University Medical Center, where he also served as an assistant chief resident. He has been a contributing writer to The Hospitalist and several other journals and books.
Dr. Lindenauer is a board-certified internist and founding SHM board member. His research focuses on measuring the quality and outcomes of hospital care for patients with common medical conditions. He is supported by grants from the Agency for Healthcare Research and Quality and the NIH’s National Heart Lung and Blood Institute. His research has appeared in the New England Journal of Medicine, JAMA, Annals of Internal Medicine, Health Affairs, Medical Care, and other general internal medicine and subspecialty journals.
Dr. Williams is professor and chief of the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago. He is the principal investigator for SHM’s Project BOOST and is a former SHM president and board member. He has served in numerous leadership roles since the society’s establishment in 1996. In 1998, Dr. Williams established the first hospitalist program at a public hospital, Grady Memorial Hospital in Atlanta.
Drs. Cawley, Lindenauer, and Williams represent the third year of the Masters program. This marks the first year that nominations could be submitted to the SHM Masters Selection committee.
Joining the new masters in earning a new designation are 120 new Fellows in Hospital Medicine (FHM) and 52 Senior Fellows in Hospital Medicine (SFHM). The new members push the fellows program totals to 947 FHMs, 243 SFHMs, and 10 MHMs.
Brendon Shank is SHM’s associate vice president of communications.

—Joseph Ming-Wah Li, MD, SFHM, SHM president
If the SHM Award of Excellence winners are the MVPs of HM, then the specialty’s new Masters in Hospital Medicine are this year’s Hall of Famers.
SHM is proud to announce that Patrick J. Cawley, MD, MBA, MHM, Peter K. Lindenauer, MD, MSc, FACP, MHM, and Mark V. Williams, MD, FACP, MHM, have earned the Master in Hospital Medicine designation. They are the eighth, ninth, and 10th SHM members to receive the exclusive designation. In addition to being honored at HM12, they have earned the right to use the “MHM” credential.
“Hospitalists of all stripes owe our new Masters in Hospital Medicine a great debt for their commitment to the specialty, to their patients, and to healthcare,” says Joseph Ming Wah Li, MD, SFHM, SHM president. “Their innovation, leadership, and vision have helped grow the influence and credibility of the hospital medicine specialty.”
Dr. Cawley is a charter member and past president of SHM who has served on the Ethics and Public Policy committees. He is a frequent speaker at the society’s annual meetings. Dr. Cawley received his medical degree from Georgetown University School of Medicine and trained as a resident at Duke University Medical Center, where he also served as an assistant chief resident. He has been a contributing writer to The Hospitalist and several other journals and books.
Dr. Lindenauer is a board-certified internist and founding SHM board member. His research focuses on measuring the quality and outcomes of hospital care for patients with common medical conditions. He is supported by grants from the Agency for Healthcare Research and Quality and the NIH’s National Heart Lung and Blood Institute. His research has appeared in the New England Journal of Medicine, JAMA, Annals of Internal Medicine, Health Affairs, Medical Care, and other general internal medicine and subspecialty journals.
Dr. Williams is professor and chief of the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago. He is the principal investigator for SHM’s Project BOOST and is a former SHM president and board member. He has served in numerous leadership roles since the society’s establishment in 1996. In 1998, Dr. Williams established the first hospitalist program at a public hospital, Grady Memorial Hospital in Atlanta.
Drs. Cawley, Lindenauer, and Williams represent the third year of the Masters program. This marks the first year that nominations could be submitted to the SHM Masters Selection committee.
Joining the new masters in earning a new designation are 120 new Fellows in Hospital Medicine (FHM) and 52 Senior Fellows in Hospital Medicine (SFHM). The new members push the fellows program totals to 947 FHMs, 243 SFHMs, and 10 MHMs.
Brendon Shank is SHM’s associate vice president of communications.

—Joseph Ming-Wah Li, MD, SFHM, SHM president
If the SHM Award of Excellence winners are the MVPs of HM, then the specialty’s new Masters in Hospital Medicine are this year’s Hall of Famers.
SHM is proud to announce that Patrick J. Cawley, MD, MBA, MHM, Peter K. Lindenauer, MD, MSc, FACP, MHM, and Mark V. Williams, MD, FACP, MHM, have earned the Master in Hospital Medicine designation. They are the eighth, ninth, and 10th SHM members to receive the exclusive designation. In addition to being honored at HM12, they have earned the right to use the “MHM” credential.
“Hospitalists of all stripes owe our new Masters in Hospital Medicine a great debt for their commitment to the specialty, to their patients, and to healthcare,” says Joseph Ming Wah Li, MD, SFHM, SHM president. “Their innovation, leadership, and vision have helped grow the influence and credibility of the hospital medicine specialty.”
Dr. Cawley is a charter member and past president of SHM who has served on the Ethics and Public Policy committees. He is a frequent speaker at the society’s annual meetings. Dr. Cawley received his medical degree from Georgetown University School of Medicine and trained as a resident at Duke University Medical Center, where he also served as an assistant chief resident. He has been a contributing writer to The Hospitalist and several other journals and books.
Dr. Lindenauer is a board-certified internist and founding SHM board member. His research focuses on measuring the quality and outcomes of hospital care for patients with common medical conditions. He is supported by grants from the Agency for Healthcare Research and Quality and the NIH’s National Heart Lung and Blood Institute. His research has appeared in the New England Journal of Medicine, JAMA, Annals of Internal Medicine, Health Affairs, Medical Care, and other general internal medicine and subspecialty journals.
Dr. Williams is professor and chief of the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago. He is the principal investigator for SHM’s Project BOOST and is a former SHM president and board member. He has served in numerous leadership roles since the society’s establishment in 1996. In 1998, Dr. Williams established the first hospitalist program at a public hospital, Grady Memorial Hospital in Atlanta.
Drs. Cawley, Lindenauer, and Williams represent the third year of the Masters program. This marks the first year that nominations could be submitted to the SHM Masters Selection committee.
Joining the new masters in earning a new designation are 120 new Fellows in Hospital Medicine (FHM) and 52 Senior Fellows in Hospital Medicine (SFHM). The new members push the fellows program totals to 947 FHMs, 243 SFHMs, and 10 MHMs.
Brendon Shank is SHM’s associate vice president of communications.
Society of Hospital Medicine's Award of Excellence Winners Lead the Way

—Joseph Ming-Wah Li, MD, SFHM, SHM president
Hospitalists across the country are taking the lead—leading their HM groups, leading care teams, and leading quality-improvement (QI) efforts.
With this year’s annual SHM Awards of Excellence, thousands of hospitalists at HM12 saluted the leaders in the specialty. Five individuals and one team were recognized at the San Diego Convention Center for their efforts to transform healthcare and revolutionize patient care.
In addition to being recognized at HM12, the honorees were the subjects of a video presented at HM12 and posted on SHM’s YouTube channel.
Brendon Shank is associate vice president of communications at SHM
The 2012 SHM Award of Excellence winners are:
Excellence in Teamwork and Quality Improvement
The In-Hospital Stroke QI Team at the University of Colorado Hospital, led by Ethan Cumbler, MD, FACP, associate professor of medicine and hospitalist at University of Colorado Hospital, was recognized for exemplary quality improvement initiatives in hospital medicine that engage the full patient care team.
Excellence in Hospital Medicine for Non-Physicians
Jina Saltzman, physician assistant, University of Chicago.
Outstanding Service in Hospital Medicine
William D. Atchley Jr., MD, FACP, SFHM, regional senior medical director, Eagle Hospital Physicians.
Excellence in Teaching
Jeff Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern University Feinberg School of Medicine, Chicago.
Clinical Excellence
Douglas W. Carlson, MD, SFHM, professor of pediatrics and director of the Division of Pediatric Hospital Medicine at Washington University and St. Louis Children’s Hospital.
Excellence in Research
Ron Keren, MD, MPH, associate professor of pediatrics and epidemiology, The Children’s Hospital of Philadelphia, and the University of Pennsylvania School of Medicine.

—Joseph Ming-Wah Li, MD, SFHM, SHM president
Hospitalists across the country are taking the lead—leading their HM groups, leading care teams, and leading quality-improvement (QI) efforts.
With this year’s annual SHM Awards of Excellence, thousands of hospitalists at HM12 saluted the leaders in the specialty. Five individuals and one team were recognized at the San Diego Convention Center for their efforts to transform healthcare and revolutionize patient care.
In addition to being recognized at HM12, the honorees were the subjects of a video presented at HM12 and posted on SHM’s YouTube channel.
Brendon Shank is associate vice president of communications at SHM
The 2012 SHM Award of Excellence winners are:
Excellence in Teamwork and Quality Improvement
The In-Hospital Stroke QI Team at the University of Colorado Hospital, led by Ethan Cumbler, MD, FACP, associate professor of medicine and hospitalist at University of Colorado Hospital, was recognized for exemplary quality improvement initiatives in hospital medicine that engage the full patient care team.
Excellence in Hospital Medicine for Non-Physicians
Jina Saltzman, physician assistant, University of Chicago.
Outstanding Service in Hospital Medicine
William D. Atchley Jr., MD, FACP, SFHM, regional senior medical director, Eagle Hospital Physicians.
Excellence in Teaching
Jeff Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern University Feinberg School of Medicine, Chicago.
Clinical Excellence
Douglas W. Carlson, MD, SFHM, professor of pediatrics and director of the Division of Pediatric Hospital Medicine at Washington University and St. Louis Children’s Hospital.
Excellence in Research
Ron Keren, MD, MPH, associate professor of pediatrics and epidemiology, The Children’s Hospital of Philadelphia, and the University of Pennsylvania School of Medicine.

—Joseph Ming-Wah Li, MD, SFHM, SHM president
Hospitalists across the country are taking the lead—leading their HM groups, leading care teams, and leading quality-improvement (QI) efforts.
With this year’s annual SHM Awards of Excellence, thousands of hospitalists at HM12 saluted the leaders in the specialty. Five individuals and one team were recognized at the San Diego Convention Center for their efforts to transform healthcare and revolutionize patient care.
In addition to being recognized at HM12, the honorees were the subjects of a video presented at HM12 and posted on SHM’s YouTube channel.
Brendon Shank is associate vice president of communications at SHM
The 2012 SHM Award of Excellence winners are:
Excellence in Teamwork and Quality Improvement
The In-Hospital Stroke QI Team at the University of Colorado Hospital, led by Ethan Cumbler, MD, FACP, associate professor of medicine and hospitalist at University of Colorado Hospital, was recognized for exemplary quality improvement initiatives in hospital medicine that engage the full patient care team.
Excellence in Hospital Medicine for Non-Physicians
Jina Saltzman, physician assistant, University of Chicago.
Outstanding Service in Hospital Medicine
William D. Atchley Jr., MD, FACP, SFHM, regional senior medical director, Eagle Hospital Physicians.
Excellence in Teaching
Jeff Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern University Feinberg School of Medicine, Chicago.
Clinical Excellence
Douglas W. Carlson, MD, SFHM, professor of pediatrics and director of the Division of Pediatric Hospital Medicine at Washington University and St. Louis Children’s Hospital.
Excellence in Research
Ron Keren, MD, MPH, associate professor of pediatrics and epidemiology, The Children’s Hospital of Philadelphia, and the University of Pennsylvania School of Medicine.
Complex Medically Ill Patients: A Challenge for PCPs and Hospitalists
Every year, primary-care physicians (PCPs) and hospitalists struggle to manage care for complex medically ill patients, according to a new survey conducted by SHM and QuantiaMD.
Complex medically ill patients have two or more concurrent chronic conditions that require ongoing medical attention or limit activities of daily living. These 4 million patients, which make up 10% of the Medicare population, consume a disproportionate amount of acute healthcare resources, accounting for up to 20% of ED visits, and have significantly higher likelihood of hospital admission and readmission, according to the survey.1 This presents a challenge to both inpatient and outpatient providers charged with coordinating their care.
SHM, in partnership with QuantiaMD, conducted a survey of nearly 4,000 physicians about this topic, which has resulted in a white paper and online expert series to outline opportunities and feature innovative examples to improve patient care of the complex medically ill.
“Communication greatly impacts quality of care and the ability to prevent excess hospital admissions and readmissions,” says Michael Radzienda, MD, SFHM, principal investigator for the Medically Complex Ill Project. “Findings from the study identified barriers to timely and effective communication on the health team who care for the medically complex ill and opportunities to implement the latest innovations to improve patient care and safety.”
A new expert practice series in the reducing readmissions special-interest group from SHM and QuantiaMD, “Innovations in Care Coordination for the Complex Medically Ill,” grew from the study and provides best practices, and also features actual case studies of existing care-coordination efforts. This online interactive forum is the first of its kind, providing resources to address care-coordination challenges that have plagued patient care over the past decade. The series includes nine presentations on topics including patient-centered medical homes, telemedicine, and post-discharge clinics.
Reference
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
Every year, primary-care physicians (PCPs) and hospitalists struggle to manage care for complex medically ill patients, according to a new survey conducted by SHM and QuantiaMD.
Complex medically ill patients have two or more concurrent chronic conditions that require ongoing medical attention or limit activities of daily living. These 4 million patients, which make up 10% of the Medicare population, consume a disproportionate amount of acute healthcare resources, accounting for up to 20% of ED visits, and have significantly higher likelihood of hospital admission and readmission, according to the survey.1 This presents a challenge to both inpatient and outpatient providers charged with coordinating their care.
SHM, in partnership with QuantiaMD, conducted a survey of nearly 4,000 physicians about this topic, which has resulted in a white paper and online expert series to outline opportunities and feature innovative examples to improve patient care of the complex medically ill.
“Communication greatly impacts quality of care and the ability to prevent excess hospital admissions and readmissions,” says Michael Radzienda, MD, SFHM, principal investigator for the Medically Complex Ill Project. “Findings from the study identified barriers to timely and effective communication on the health team who care for the medically complex ill and opportunities to implement the latest innovations to improve patient care and safety.”
A new expert practice series in the reducing readmissions special-interest group from SHM and QuantiaMD, “Innovations in Care Coordination for the Complex Medically Ill,” grew from the study and provides best practices, and also features actual case studies of existing care-coordination efforts. This online interactive forum is the first of its kind, providing resources to address care-coordination challenges that have plagued patient care over the past decade. The series includes nine presentations on topics including patient-centered medical homes, telemedicine, and post-discharge clinics.
Reference
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
Every year, primary-care physicians (PCPs) and hospitalists struggle to manage care for complex medically ill patients, according to a new survey conducted by SHM and QuantiaMD.
Complex medically ill patients have two or more concurrent chronic conditions that require ongoing medical attention or limit activities of daily living. These 4 million patients, which make up 10% of the Medicare population, consume a disproportionate amount of acute healthcare resources, accounting for up to 20% of ED visits, and have significantly higher likelihood of hospital admission and readmission, according to the survey.1 This presents a challenge to both inpatient and outpatient providers charged with coordinating their care.
SHM, in partnership with QuantiaMD, conducted a survey of nearly 4,000 physicians about this topic, which has resulted in a white paper and online expert series to outline opportunities and feature innovative examples to improve patient care of the complex medically ill.
“Communication greatly impacts quality of care and the ability to prevent excess hospital admissions and readmissions,” says Michael Radzienda, MD, SFHM, principal investigator for the Medically Complex Ill Project. “Findings from the study identified barriers to timely and effective communication on the health team who care for the medically complex ill and opportunities to implement the latest innovations to improve patient care and safety.”
A new expert practice series in the reducing readmissions special-interest group from SHM and QuantiaMD, “Innovations in Care Coordination for the Complex Medically Ill,” grew from the study and provides best practices, and also features actual case studies of existing care-coordination efforts. This online interactive forum is the first of its kind, providing resources to address care-coordination challenges that have plagued patient care over the past decade. The series includes nine presentations on topics including patient-centered medical homes, telemedicine, and post-discharge clinics.
Reference
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
Ethics, Advocacy, and Disclosure: The Sunshine Rule
SHM recently joined more than 30 organizations in the Council of Medical Specialty Societies (CMSS) in signing a letter to the Centers for Medicare & Medicaid Services (CMS) to affirm the importance of—and to voice concerns about—some provisions in the proposed Sunshine Rule for doctor-industry relationships.
Specifically, the letter highlights some critical distinctions in compensation for teaching Continuing Medical Education (CME) courses.
The Sunshine Rule was proposed in response to the adoption of the Physician Payments Sunshine Act in Section 6002 of the Affordable Care Act of 2010. The act requires gifts or payments to physicians from pharmaceutical and medical device manufacturers worth more than $10 to be reported publicly by manufacturers. CMS created the proposed rule to frame which situations and exchanges of value fall within and outside the reporting requirements. As part of the rulemaking process, CMS welcomed comments to help refine and develop the final rule.
The proposed rule asserts that the category of “Direct Compensation for Serving as a Faculty or as a Speaker for a Medical Education Program” be broadly understood to encompass any situation in which a manufacturer compensates physicians for speaking engagements. This includes certain indirect payments through a third-party like a CME provider. So if you serve as a faculty member sharing your expertise through accredited or certified CME, your service could be reportable if the provider received industry funding. This could happen even if you have no specific knowledge of the industry funder.
The CMSS letter identifies a distinction between promotional education programs and accredited or certified CME programs, noting that only the former implies a relationship between a physician and manufacturer. Accredited and certified CME programs, on the other hand, already are governed by the Standards for Commercial Support: Standards to Ensure the Independence of CME Activities, which includes guidance to ensure the independence of CME activities from industry funders. Industry grants do not pay accredited or certified CME faculty directly, but rather go to CME providers who organize and develop the programs. This clarification expressly acknowledges the established self-regulation and ethical guidelines of the CME programs. Importantly, the letter does support disclosure of compensation from promotional education programs directly sponsored by industry.
By signing on to this letter, SHM has expressed its support for greater transparency in relationships between physicians and industries, while illuminating areas of concern in the rule.
The Sunshine Rule illustrates some of the richness and complexity of policy initiatives, and highlights potential topics to broaden our conversation and involvement. These types of issues generate robust discussions about ethics and professionalism within the medical establishment.
Hospitalists can, and should, engage these debates both within SHM and from their unique vantage point within the hospital.
As a membership organization, SHM details our efforts at transparency in our relationship with industry partners at www.hospitalmedicine.org/industry.
SHM recently joined more than 30 organizations in the Council of Medical Specialty Societies (CMSS) in signing a letter to the Centers for Medicare & Medicaid Services (CMS) to affirm the importance of—and to voice concerns about—some provisions in the proposed Sunshine Rule for doctor-industry relationships.
Specifically, the letter highlights some critical distinctions in compensation for teaching Continuing Medical Education (CME) courses.
The Sunshine Rule was proposed in response to the adoption of the Physician Payments Sunshine Act in Section 6002 of the Affordable Care Act of 2010. The act requires gifts or payments to physicians from pharmaceutical and medical device manufacturers worth more than $10 to be reported publicly by manufacturers. CMS created the proposed rule to frame which situations and exchanges of value fall within and outside the reporting requirements. As part of the rulemaking process, CMS welcomed comments to help refine and develop the final rule.
The proposed rule asserts that the category of “Direct Compensation for Serving as a Faculty or as a Speaker for a Medical Education Program” be broadly understood to encompass any situation in which a manufacturer compensates physicians for speaking engagements. This includes certain indirect payments through a third-party like a CME provider. So if you serve as a faculty member sharing your expertise through accredited or certified CME, your service could be reportable if the provider received industry funding. This could happen even if you have no specific knowledge of the industry funder.
The CMSS letter identifies a distinction between promotional education programs and accredited or certified CME programs, noting that only the former implies a relationship between a physician and manufacturer. Accredited and certified CME programs, on the other hand, already are governed by the Standards for Commercial Support: Standards to Ensure the Independence of CME Activities, which includes guidance to ensure the independence of CME activities from industry funders. Industry grants do not pay accredited or certified CME faculty directly, but rather go to CME providers who organize and develop the programs. This clarification expressly acknowledges the established self-regulation and ethical guidelines of the CME programs. Importantly, the letter does support disclosure of compensation from promotional education programs directly sponsored by industry.
By signing on to this letter, SHM has expressed its support for greater transparency in relationships between physicians and industries, while illuminating areas of concern in the rule.
The Sunshine Rule illustrates some of the richness and complexity of policy initiatives, and highlights potential topics to broaden our conversation and involvement. These types of issues generate robust discussions about ethics and professionalism within the medical establishment.
Hospitalists can, and should, engage these debates both within SHM and from their unique vantage point within the hospital.
As a membership organization, SHM details our efforts at transparency in our relationship with industry partners at www.hospitalmedicine.org/industry.
SHM recently joined more than 30 organizations in the Council of Medical Specialty Societies (CMSS) in signing a letter to the Centers for Medicare & Medicaid Services (CMS) to affirm the importance of—and to voice concerns about—some provisions in the proposed Sunshine Rule for doctor-industry relationships.
Specifically, the letter highlights some critical distinctions in compensation for teaching Continuing Medical Education (CME) courses.
The Sunshine Rule was proposed in response to the adoption of the Physician Payments Sunshine Act in Section 6002 of the Affordable Care Act of 2010. The act requires gifts or payments to physicians from pharmaceutical and medical device manufacturers worth more than $10 to be reported publicly by manufacturers. CMS created the proposed rule to frame which situations and exchanges of value fall within and outside the reporting requirements. As part of the rulemaking process, CMS welcomed comments to help refine and develop the final rule.
The proposed rule asserts that the category of “Direct Compensation for Serving as a Faculty or as a Speaker for a Medical Education Program” be broadly understood to encompass any situation in which a manufacturer compensates physicians for speaking engagements. This includes certain indirect payments through a third-party like a CME provider. So if you serve as a faculty member sharing your expertise through accredited or certified CME, your service could be reportable if the provider received industry funding. This could happen even if you have no specific knowledge of the industry funder.
The CMSS letter identifies a distinction between promotional education programs and accredited or certified CME programs, noting that only the former implies a relationship between a physician and manufacturer. Accredited and certified CME programs, on the other hand, already are governed by the Standards for Commercial Support: Standards to Ensure the Independence of CME Activities, which includes guidance to ensure the independence of CME activities from industry funders. Industry grants do not pay accredited or certified CME faculty directly, but rather go to CME providers who organize and develop the programs. This clarification expressly acknowledges the established self-regulation and ethical guidelines of the CME programs. Importantly, the letter does support disclosure of compensation from promotional education programs directly sponsored by industry.
By signing on to this letter, SHM has expressed its support for greater transparency in relationships between physicians and industries, while illuminating areas of concern in the rule.
The Sunshine Rule illustrates some of the richness and complexity of policy initiatives, and highlights potential topics to broaden our conversation and involvement. These types of issues generate robust discussions about ethics and professionalism within the medical establishment.
Hospitalists can, and should, engage these debates both within SHM and from their unique vantage point within the hospital.
As a membership organization, SHM details our efforts at transparency in our relationship with industry partners at www.hospitalmedicine.org/industry.
Partnership for Patients: CMS’ Ambitious Program for Patient Safety Improvement

—Katharine Luther, RN, MPM, vice president of hospital portfolio planning and administration, Institute for Healthcare Improvement
Last April, the Centers for Medicare & Medicaid Services (CMS) unveiled “Partnership for Patients,” a landmark event in the patient safety movement that put a national spotlight on the continuing need to improve healthcare safety and quality. A year later, the initiative is getting off the ground, attempting to tackle ambitious goals and overcome methodological hurdles in a very tight timeframe.
Partnership for Patients is a $1 billion, nationwide educational collaborative in which participants pledge that they will try to achieve two things by the end of 2013: Reduce the incidence of preventable hospital-acquired conditions by 40% compared to 2010, and decrease preventable complications during transitions of care to reduce hospital readmissions by 20% compared with 2010.
More than 3,000 hospitals and 2,000 physician and nursing organizations have signed the pledge, and CMS recently awarded contracts to 26 Hospital Engagement Networks (HENs)—state, regional, and national hospitals and health systems that will serve as mobile classrooms that mentor as they implement new intervention strategies, track progress on quality improvement (QI), and develop learning collaboratives to spread effective interventions. CMS also has contracted with outside firms to create patient safety training materials, engage with patients and families to foster more patient-centered care, and evaluate the impact and effectiveness of the initiative.
SHM was one of the first physician groups to sign on to the initiative’s pledge of support, and both its Project BOOST (to reduce preventable readmissions) and its VTE Resource Room (to prevent hospital-acquired venous thromboembolism) are among the resources it is making available to the initiative’s HENs, says Wendy Nickel, MPH, associate vice president of SHM’s Center for Hospital Innovation and Improvement.
Worthy Goals
Despite success stories at some institutions in recent years, patient safety improvement still has far to go at U.S. hospitals. Currently, about 1 in every 20 patients acquires an infection in the hospital, 1 in 7 Medicare patients is harmed in the course of their hospital care, and nearly 1 in 5 is readmitted within 30 days of discharge. CMS estimates that meeting the goals of Partnership for Patients would mean more than 60,000 lives saved over the next three years and over 1.6 million patients spared a preventable complication requiring re-hospitalization within 30 days of discharge—all of which could save Medicare $50 billion over the next 10 years.1
Formidable Obstacles
While the initiative’s goals are certainly worthy, it remains to be seen how prepared hospitals are to achieve them, and whether available metrics are up to the task.
“The initiative is a positive step to improve collaboration among government, communities, and hospital sites in service of better patient care and safety—and so it deserves our endorsement,” says Greg Maynard, MD, MSc, SFHM, health sciences professor of medicine at the University of California at San Diego, director of the UC San Diego Center for Innovation and Improvement Science, and senior vice president of SHM’s Center for Hospital Innovation and Improvement. “But it’s an open question how successful it will be, since it offers no monetary piece of the pie to participating hospitals, and no financial penalties for failing to achieve its goals. The whole project feels rushed, for a major initiative like this, with such ambitious goals.”
The primary “carrot” the initiative offers hospitals, Dr. Maynard notes, is access to patient safety improvement expertise and resources that they would otherwise have to purchase on their own, including training materials, implementation guides, webinars, and site visits by HEN representatives.
As an indirect inducement to participate, CMS’ Hospital Readmissions Reduction Program begins this October and will penalize hospitals by as much as 1% of their total Medicare billings (increasing to 3% in 2015) for high rates of readmissions related to heart attack, heart failure, and pneumonia. CMS’ Value-Based Purchasing program also continues to reward and punish hospitals for their performance on core measures and patient satisfaction, with more metrics forthcoming.
Metric Morass
A major challenge to the success of Partnership for Patients will be the ability to formulate and share reliable, uniform patient safety metrics across institutions. The initiative gives each of the 26 HENs the flexibility to tailor their activities to the sites they are mentoring, and there is no clear way of making standardized comparisons of hospital performance across the HENs, Dr. Maynard says.
Metric validity is a crucial component of any QI initiative. And yet, the ability to reliably measure patient harm/adverse event rates at hospitals—and therefore achieving a solid “denominator” baseline with which to track progress—remains elusive. In a recent report, the U.S. Department of Health and Human Services’ Office of the Inspector General noted that hospital incident reporting systems capture only an estimated 14% of the patient harm events experienced by Medicare patients, reporting requirements remain unclear, and hospital staff continue to harbor misperceptions about what constitutes patient harm.2
In what almost sounds like “back to the drawing board,” the report recommended that CMS and the Agency for Healthcare Research and Quality (AHRQ) collaborate to create and promote a list of potentially reportable events for hospitals to use, and that CMS provide guidance to accreditors regarding their assessments of hospital efforts to track and analyze events.
The problem is that voluntary incident reporting systems are only one tool for identifying patient harm. Typically, however, they miss many things that can harm patients and are grossly under-reported. As a result, they need to be used in conjunction with other data sources, such as hospital infection rates, daily safety rounding on hospital units, and patient chart sampling, says Katharine Luther, RN, MPM, the Institute for Healthcare Improvement’s vice president of hospital portfolio planning and administration.
Another excellent surveillance instrument for capturing a count of possible harm events is a Global Trigger Tool, which samples patient charts to identify aberrant lab values, drug dosages, and other untoward events that might indicate harm, even though they might not easily be recognized as harmful by hospital staff, Luther says.
Warranted Optimism
Despite its aggressive timeline and inherent methodological challenges, Luther says the Partnership for Patients will galvanize and focus hospitals’ patient safety improvement efforts and provide a much-needed framework for implementation.
“We know of organizations that have greatly reduced the incidence of pressure ulcers, and have gone for a year or more with no cases of ventilator-associated pneumonia or central-line-associated bloodstream infection (CLABSI),” Luther says. “Exemplars like these are out there, so it can be done. Partnership for Patients brings a spotlight and an energy to the issue that will last long beyond the 24 months of this program.”
Chris Guadagnino is a freelance medical writer in Philadelphia.
References
- CMS Fact Sheet. Hospital Engagement Networks: Connecting Hospitals to Improve Care. Centers for Medicare & Medicaid website. Available at: http://www.cms.gov/apps/media/press/factsheet.asp?Counter=4219&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=6&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date. Accessed Feb. 12, 2012.
- HHS Office of Inspector General. Hospital Incident Reporting Systems Do Not Capture Most Patient Harm. Office of Inspector General website. Available at: http://oig.hhs.gov/oei/reports/oei-06-09-00091.pdf. Accessed Feb. 12, 2012.

—Katharine Luther, RN, MPM, vice president of hospital portfolio planning and administration, Institute for Healthcare Improvement
Last April, the Centers for Medicare & Medicaid Services (CMS) unveiled “Partnership for Patients,” a landmark event in the patient safety movement that put a national spotlight on the continuing need to improve healthcare safety and quality. A year later, the initiative is getting off the ground, attempting to tackle ambitious goals and overcome methodological hurdles in a very tight timeframe.
Partnership for Patients is a $1 billion, nationwide educational collaborative in which participants pledge that they will try to achieve two things by the end of 2013: Reduce the incidence of preventable hospital-acquired conditions by 40% compared to 2010, and decrease preventable complications during transitions of care to reduce hospital readmissions by 20% compared with 2010.
More than 3,000 hospitals and 2,000 physician and nursing organizations have signed the pledge, and CMS recently awarded contracts to 26 Hospital Engagement Networks (HENs)—state, regional, and national hospitals and health systems that will serve as mobile classrooms that mentor as they implement new intervention strategies, track progress on quality improvement (QI), and develop learning collaboratives to spread effective interventions. CMS also has contracted with outside firms to create patient safety training materials, engage with patients and families to foster more patient-centered care, and evaluate the impact and effectiveness of the initiative.
SHM was one of the first physician groups to sign on to the initiative’s pledge of support, and both its Project BOOST (to reduce preventable readmissions) and its VTE Resource Room (to prevent hospital-acquired venous thromboembolism) are among the resources it is making available to the initiative’s HENs, says Wendy Nickel, MPH, associate vice president of SHM’s Center for Hospital Innovation and Improvement.
Worthy Goals
Despite success stories at some institutions in recent years, patient safety improvement still has far to go at U.S. hospitals. Currently, about 1 in every 20 patients acquires an infection in the hospital, 1 in 7 Medicare patients is harmed in the course of their hospital care, and nearly 1 in 5 is readmitted within 30 days of discharge. CMS estimates that meeting the goals of Partnership for Patients would mean more than 60,000 lives saved over the next three years and over 1.6 million patients spared a preventable complication requiring re-hospitalization within 30 days of discharge—all of which could save Medicare $50 billion over the next 10 years.1
Formidable Obstacles
While the initiative’s goals are certainly worthy, it remains to be seen how prepared hospitals are to achieve them, and whether available metrics are up to the task.
“The initiative is a positive step to improve collaboration among government, communities, and hospital sites in service of better patient care and safety—and so it deserves our endorsement,” says Greg Maynard, MD, MSc, SFHM, health sciences professor of medicine at the University of California at San Diego, director of the UC San Diego Center for Innovation and Improvement Science, and senior vice president of SHM’s Center for Hospital Innovation and Improvement. “But it’s an open question how successful it will be, since it offers no monetary piece of the pie to participating hospitals, and no financial penalties for failing to achieve its goals. The whole project feels rushed, for a major initiative like this, with such ambitious goals.”
The primary “carrot” the initiative offers hospitals, Dr. Maynard notes, is access to patient safety improvement expertise and resources that they would otherwise have to purchase on their own, including training materials, implementation guides, webinars, and site visits by HEN representatives.
As an indirect inducement to participate, CMS’ Hospital Readmissions Reduction Program begins this October and will penalize hospitals by as much as 1% of their total Medicare billings (increasing to 3% in 2015) for high rates of readmissions related to heart attack, heart failure, and pneumonia. CMS’ Value-Based Purchasing program also continues to reward and punish hospitals for their performance on core measures and patient satisfaction, with more metrics forthcoming.
Metric Morass
A major challenge to the success of Partnership for Patients will be the ability to formulate and share reliable, uniform patient safety metrics across institutions. The initiative gives each of the 26 HENs the flexibility to tailor their activities to the sites they are mentoring, and there is no clear way of making standardized comparisons of hospital performance across the HENs, Dr. Maynard says.
Metric validity is a crucial component of any QI initiative. And yet, the ability to reliably measure patient harm/adverse event rates at hospitals—and therefore achieving a solid “denominator” baseline with which to track progress—remains elusive. In a recent report, the U.S. Department of Health and Human Services’ Office of the Inspector General noted that hospital incident reporting systems capture only an estimated 14% of the patient harm events experienced by Medicare patients, reporting requirements remain unclear, and hospital staff continue to harbor misperceptions about what constitutes patient harm.2
In what almost sounds like “back to the drawing board,” the report recommended that CMS and the Agency for Healthcare Research and Quality (AHRQ) collaborate to create and promote a list of potentially reportable events for hospitals to use, and that CMS provide guidance to accreditors regarding their assessments of hospital efforts to track and analyze events.
The problem is that voluntary incident reporting systems are only one tool for identifying patient harm. Typically, however, they miss many things that can harm patients and are grossly under-reported. As a result, they need to be used in conjunction with other data sources, such as hospital infection rates, daily safety rounding on hospital units, and patient chart sampling, says Katharine Luther, RN, MPM, the Institute for Healthcare Improvement’s vice president of hospital portfolio planning and administration.
Another excellent surveillance instrument for capturing a count of possible harm events is a Global Trigger Tool, which samples patient charts to identify aberrant lab values, drug dosages, and other untoward events that might indicate harm, even though they might not easily be recognized as harmful by hospital staff, Luther says.
Warranted Optimism
Despite its aggressive timeline and inherent methodological challenges, Luther says the Partnership for Patients will galvanize and focus hospitals’ patient safety improvement efforts and provide a much-needed framework for implementation.
“We know of organizations that have greatly reduced the incidence of pressure ulcers, and have gone for a year or more with no cases of ventilator-associated pneumonia or central-line-associated bloodstream infection (CLABSI),” Luther says. “Exemplars like these are out there, so it can be done. Partnership for Patients brings a spotlight and an energy to the issue that will last long beyond the 24 months of this program.”
Chris Guadagnino is a freelance medical writer in Philadelphia.
References
- CMS Fact Sheet. Hospital Engagement Networks: Connecting Hospitals to Improve Care. Centers for Medicare & Medicaid website. Available at: http://www.cms.gov/apps/media/press/factsheet.asp?Counter=4219&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=6&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date. Accessed Feb. 12, 2012.
- HHS Office of Inspector General. Hospital Incident Reporting Systems Do Not Capture Most Patient Harm. Office of Inspector General website. Available at: http://oig.hhs.gov/oei/reports/oei-06-09-00091.pdf. Accessed Feb. 12, 2012.

—Katharine Luther, RN, MPM, vice president of hospital portfolio planning and administration, Institute for Healthcare Improvement
Last April, the Centers for Medicare & Medicaid Services (CMS) unveiled “Partnership for Patients,” a landmark event in the patient safety movement that put a national spotlight on the continuing need to improve healthcare safety and quality. A year later, the initiative is getting off the ground, attempting to tackle ambitious goals and overcome methodological hurdles in a very tight timeframe.
Partnership for Patients is a $1 billion, nationwide educational collaborative in which participants pledge that they will try to achieve two things by the end of 2013: Reduce the incidence of preventable hospital-acquired conditions by 40% compared to 2010, and decrease preventable complications during transitions of care to reduce hospital readmissions by 20% compared with 2010.
More than 3,000 hospitals and 2,000 physician and nursing organizations have signed the pledge, and CMS recently awarded contracts to 26 Hospital Engagement Networks (HENs)—state, regional, and national hospitals and health systems that will serve as mobile classrooms that mentor as they implement new intervention strategies, track progress on quality improvement (QI), and develop learning collaboratives to spread effective interventions. CMS also has contracted with outside firms to create patient safety training materials, engage with patients and families to foster more patient-centered care, and evaluate the impact and effectiveness of the initiative.
SHM was one of the first physician groups to sign on to the initiative’s pledge of support, and both its Project BOOST (to reduce preventable readmissions) and its VTE Resource Room (to prevent hospital-acquired venous thromboembolism) are among the resources it is making available to the initiative’s HENs, says Wendy Nickel, MPH, associate vice president of SHM’s Center for Hospital Innovation and Improvement.
Worthy Goals
Despite success stories at some institutions in recent years, patient safety improvement still has far to go at U.S. hospitals. Currently, about 1 in every 20 patients acquires an infection in the hospital, 1 in 7 Medicare patients is harmed in the course of their hospital care, and nearly 1 in 5 is readmitted within 30 days of discharge. CMS estimates that meeting the goals of Partnership for Patients would mean more than 60,000 lives saved over the next three years and over 1.6 million patients spared a preventable complication requiring re-hospitalization within 30 days of discharge—all of which could save Medicare $50 billion over the next 10 years.1
Formidable Obstacles
While the initiative’s goals are certainly worthy, it remains to be seen how prepared hospitals are to achieve them, and whether available metrics are up to the task.
“The initiative is a positive step to improve collaboration among government, communities, and hospital sites in service of better patient care and safety—and so it deserves our endorsement,” says Greg Maynard, MD, MSc, SFHM, health sciences professor of medicine at the University of California at San Diego, director of the UC San Diego Center for Innovation and Improvement Science, and senior vice president of SHM’s Center for Hospital Innovation and Improvement. “But it’s an open question how successful it will be, since it offers no monetary piece of the pie to participating hospitals, and no financial penalties for failing to achieve its goals. The whole project feels rushed, for a major initiative like this, with such ambitious goals.”
The primary “carrot” the initiative offers hospitals, Dr. Maynard notes, is access to patient safety improvement expertise and resources that they would otherwise have to purchase on their own, including training materials, implementation guides, webinars, and site visits by HEN representatives.
As an indirect inducement to participate, CMS’ Hospital Readmissions Reduction Program begins this October and will penalize hospitals by as much as 1% of their total Medicare billings (increasing to 3% in 2015) for high rates of readmissions related to heart attack, heart failure, and pneumonia. CMS’ Value-Based Purchasing program also continues to reward and punish hospitals for their performance on core measures and patient satisfaction, with more metrics forthcoming.
Metric Morass
A major challenge to the success of Partnership for Patients will be the ability to formulate and share reliable, uniform patient safety metrics across institutions. The initiative gives each of the 26 HENs the flexibility to tailor their activities to the sites they are mentoring, and there is no clear way of making standardized comparisons of hospital performance across the HENs, Dr. Maynard says.
Metric validity is a crucial component of any QI initiative. And yet, the ability to reliably measure patient harm/adverse event rates at hospitals—and therefore achieving a solid “denominator” baseline with which to track progress—remains elusive. In a recent report, the U.S. Department of Health and Human Services’ Office of the Inspector General noted that hospital incident reporting systems capture only an estimated 14% of the patient harm events experienced by Medicare patients, reporting requirements remain unclear, and hospital staff continue to harbor misperceptions about what constitutes patient harm.2
In what almost sounds like “back to the drawing board,” the report recommended that CMS and the Agency for Healthcare Research and Quality (AHRQ) collaborate to create and promote a list of potentially reportable events for hospitals to use, and that CMS provide guidance to accreditors regarding their assessments of hospital efforts to track and analyze events.
The problem is that voluntary incident reporting systems are only one tool for identifying patient harm. Typically, however, they miss many things that can harm patients and are grossly under-reported. As a result, they need to be used in conjunction with other data sources, such as hospital infection rates, daily safety rounding on hospital units, and patient chart sampling, says Katharine Luther, RN, MPM, the Institute for Healthcare Improvement’s vice president of hospital portfolio planning and administration.
Another excellent surveillance instrument for capturing a count of possible harm events is a Global Trigger Tool, which samples patient charts to identify aberrant lab values, drug dosages, and other untoward events that might indicate harm, even though they might not easily be recognized as harmful by hospital staff, Luther says.
Warranted Optimism
Despite its aggressive timeline and inherent methodological challenges, Luther says the Partnership for Patients will galvanize and focus hospitals’ patient safety improvement efforts and provide a much-needed framework for implementation.
“We know of organizations that have greatly reduced the incidence of pressure ulcers, and have gone for a year or more with no cases of ventilator-associated pneumonia or central-line-associated bloodstream infection (CLABSI),” Luther says. “Exemplars like these are out there, so it can be done. Partnership for Patients brings a spotlight and an energy to the issue that will last long beyond the 24 months of this program.”
Chris Guadagnino is a freelance medical writer in Philadelphia.
References
- CMS Fact Sheet. Hospital Engagement Networks: Connecting Hospitals to Improve Care. Centers for Medicare & Medicaid website. Available at: http://www.cms.gov/apps/media/press/factsheet.asp?Counter=4219&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=6&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date. Accessed Feb. 12, 2012.
- HHS Office of Inspector General. Hospital Incident Reporting Systems Do Not Capture Most Patient Harm. Office of Inspector General website. Available at: http://oig.hhs.gov/oei/reports/oei-06-09-00091.pdf. Accessed Feb. 12, 2012.
Verify Your Liability Coverage before Taking that New Job
Does your employer provide your medical malpractice insurance coverage? Are you looking for new employment? Are you in the market to purchase a professional malpractice insurance policy? Are you planning to retire soon?
If you answered “yes” to any of these questions, you likely will confront the concept of “tail” insurance at some point in your medical career.
Now is the time to dust off your employment agreement and professional liability insurance policy and review what happens in the event a lawsuit is filed against you after you leave your current employer. This means paying special attention to whether your professional liability insurance policy provides for claims-made or occurrence-based coverage, and, if it’s the former, who is responsible for purchasing tail coverage.
When Do I Need Tail Coverage?
Tail insurance issues frequently arise when a physician leaves his or her place of employment, whether due to switching jobs, retirement, or a buyout of a physician’s ownership interest. If the physician is leaving an employer that has claims-made professional liability insurance, the physician’s insurance coverage might not be seamless. Instead, tail or similar coverage is required.
Claims-made coverage protects a physician for professional negligence, as long as a two-part test is met: First, the physician must have the claims-made coverage in place when the negligent act occurs (with employer No. 1); second, the physician must be covered by the same carrier when he or she is notified of the claim while employed by employer No. 2. If either test is not satisfied, the current claims-made insurance policy will not provide coverage to the physician in the event a lawsuit is filed for an act of negligence that took place while employed by employer No. 1. Alternatively, some employers offer “nose” coverage from its insurance carrier, which will cover negligent acts that might have occurred during your current job. The vast majority of professional liability insurance policies written for medical practice groups are for claims-made coverage.
If, however, an employer has occurrence-based professional liability insurance, the departing physician’s insurance coverage is seamless and no tail insurance is required.
Example A
Here is a common example of what happens when a physician leaves an employer with claims-made professional liability coverage:
An employer maintains claims-made professional liability insurance coverage for its physicians with ABC Insurance Co. A physician decides to leave his or her current employer and accepts employment by a new employer, which maintains claims-made coverage with XYZ Insurance Co.
Within a few months of the physician’s new employment, a medical malpractice lawsuit is filed by a patient for medical treatment the patient received when the physician was employed by the former employer. By leaving the former employer, the departing physician automatically fails the two-part test for claims-made coverage, as the second prong is not satisfied. Therefore, even though the physician has liability coverage through the new employer, this insurance policy will not cover the lawsuit described above.
Unless the physician has tail insurance (or nose coverage) to cover lawsuits related to the former employment, a gap in liability coverage will exist. If claims-made insurance is the benefit you have received in your employment agreement, it is imperative that you understand that tail coverage is necessary when you leave.
However, if a physician leaves and a) is subsequently employed within the same state and b) stays insured by the same insurance carrier, then the insurance carrier will provide continuous coverage and no tail insurance policy is needed.
Who Pays the Premium?
If the physician will need tail coverage, the next critical question is, Who pays for such coverage? Even though tail coverage comes into effect when a physician leaves an employer, tail coverage should be addressed before the physician informs the employer of their departure; an even better approach would be while the employment agreement is negotiated. Payment of tail coverage should be defined in the physician’s employment agreement.
In terms of payment for the coverage, there are several options. First, the cost of tail coverage can be attributed 100% to either physician or employer. In specialties for which recruitment of new physicians is challenging (i.e. HM), employers are more likely to pay a substantial portion, if not all, of the cost as a benefit or inducement.
Second, the physician can connect the payment of tail coverage to the manner in which employment is terminated. For example, if the physician terminates the agreement for cause or if the employer terminates the physician’s employment without cause, the employer could be required to pay for the tail insurance. Alternatively, if the physician terminates the agreement without cause or if the employer terminates the physician’s employment with cause, the physician could be required to pay for the tail coverage. Frequently, physician employment agreements require physicians to pay for tail coverage if the physician violates a restrictive covenant (e.g. non-competition).
A third option is to split the cost of tail insurance between the former employer and the physician based on a percentage, or to include a vesting schedule, for example, such that the former employer pays one-third of the coverage if employment ends in the second year, two-thirds of the coverage if employment ends in the third year, and 100% of the coverage if employment ends in the fourth year or later.
Whatever arrangement the parties agree upon should be included in the physician’s employment agreement in order to prevent an expensive surprise.
Review Your Policy
Now that you have an understanding of claims-made coverage, occurrence-based coverage and tail insurance, it’s time to review your insurance policy. When reviewing your current policy, look for answers to the following important questions:
- Is your policy claims-made or occurrence-based?
- Does your insurance policy only cover professional negligence claims? Does your policy also cover claims of unprofessional conduct reported to state medical licensing boards? Does your policy also cover medical staff bylaw disputes and state licensing matters?
- How is loss defined? “Pure loss” is coverage for the amount awarded to the plaintiff; “ultimate net loss” covers what pure loss covers, plus attorneys’ fees and costs.
- What procedures do you need to follow in order to properly notify the insurance carrier of a claim? Are you precluded from full coverage if you fail to properly report?
- What does the “duty to defend” provision cover? Will you be reimbursed for lost wages for your time in court? What services will be provided as part of your defense?
- What does the “consent to settle” provision say? If a settlement is negotiated between the plaintiff (patient) and the insurance company and the physician does not consent to the settlement, is the physician responsible for the ongoing defense costs and the amount of any verdict in excess of the recommended settlement amount?
It is important to both understand your insurance policy and what your employment agreement says about the policy. If you will be responsible for purchasing a tail policy at the end of your current employment, you should be well aware—and financially prepared—for this post-employment responsibility. Make sure your tail is not left exposed.
Steven M. Harris, Esq., is a nationally recognized healthcare attorney and a member of the law firm McDonald Hopkins LLC in Chicago. Write to him at sharris@mcdonaldhopkins.com.
Does your employer provide your medical malpractice insurance coverage? Are you looking for new employment? Are you in the market to purchase a professional malpractice insurance policy? Are you planning to retire soon?
If you answered “yes” to any of these questions, you likely will confront the concept of “tail” insurance at some point in your medical career.
Now is the time to dust off your employment agreement and professional liability insurance policy and review what happens in the event a lawsuit is filed against you after you leave your current employer. This means paying special attention to whether your professional liability insurance policy provides for claims-made or occurrence-based coverage, and, if it’s the former, who is responsible for purchasing tail coverage.
When Do I Need Tail Coverage?
Tail insurance issues frequently arise when a physician leaves his or her place of employment, whether due to switching jobs, retirement, or a buyout of a physician’s ownership interest. If the physician is leaving an employer that has claims-made professional liability insurance, the physician’s insurance coverage might not be seamless. Instead, tail or similar coverage is required.
Claims-made coverage protects a physician for professional negligence, as long as a two-part test is met: First, the physician must have the claims-made coverage in place when the negligent act occurs (with employer No. 1); second, the physician must be covered by the same carrier when he or she is notified of the claim while employed by employer No. 2. If either test is not satisfied, the current claims-made insurance policy will not provide coverage to the physician in the event a lawsuit is filed for an act of negligence that took place while employed by employer No. 1. Alternatively, some employers offer “nose” coverage from its insurance carrier, which will cover negligent acts that might have occurred during your current job. The vast majority of professional liability insurance policies written for medical practice groups are for claims-made coverage.
If, however, an employer has occurrence-based professional liability insurance, the departing physician’s insurance coverage is seamless and no tail insurance is required.
Example A
Here is a common example of what happens when a physician leaves an employer with claims-made professional liability coverage:
An employer maintains claims-made professional liability insurance coverage for its physicians with ABC Insurance Co. A physician decides to leave his or her current employer and accepts employment by a new employer, which maintains claims-made coverage with XYZ Insurance Co.
Within a few months of the physician’s new employment, a medical malpractice lawsuit is filed by a patient for medical treatment the patient received when the physician was employed by the former employer. By leaving the former employer, the departing physician automatically fails the two-part test for claims-made coverage, as the second prong is not satisfied. Therefore, even though the physician has liability coverage through the new employer, this insurance policy will not cover the lawsuit described above.
Unless the physician has tail insurance (or nose coverage) to cover lawsuits related to the former employment, a gap in liability coverage will exist. If claims-made insurance is the benefit you have received in your employment agreement, it is imperative that you understand that tail coverage is necessary when you leave.
However, if a physician leaves and a) is subsequently employed within the same state and b) stays insured by the same insurance carrier, then the insurance carrier will provide continuous coverage and no tail insurance policy is needed.
Who Pays the Premium?
If the physician will need tail coverage, the next critical question is, Who pays for such coverage? Even though tail coverage comes into effect when a physician leaves an employer, tail coverage should be addressed before the physician informs the employer of their departure; an even better approach would be while the employment agreement is negotiated. Payment of tail coverage should be defined in the physician’s employment agreement.
In terms of payment for the coverage, there are several options. First, the cost of tail coverage can be attributed 100% to either physician or employer. In specialties for which recruitment of new physicians is challenging (i.e. HM), employers are more likely to pay a substantial portion, if not all, of the cost as a benefit or inducement.
Second, the physician can connect the payment of tail coverage to the manner in which employment is terminated. For example, if the physician terminates the agreement for cause or if the employer terminates the physician’s employment without cause, the employer could be required to pay for the tail insurance. Alternatively, if the physician terminates the agreement without cause or if the employer terminates the physician’s employment with cause, the physician could be required to pay for the tail coverage. Frequently, physician employment agreements require physicians to pay for tail coverage if the physician violates a restrictive covenant (e.g. non-competition).
A third option is to split the cost of tail insurance between the former employer and the physician based on a percentage, or to include a vesting schedule, for example, such that the former employer pays one-third of the coverage if employment ends in the second year, two-thirds of the coverage if employment ends in the third year, and 100% of the coverage if employment ends in the fourth year or later.
Whatever arrangement the parties agree upon should be included in the physician’s employment agreement in order to prevent an expensive surprise.
Review Your Policy
Now that you have an understanding of claims-made coverage, occurrence-based coverage and tail insurance, it’s time to review your insurance policy. When reviewing your current policy, look for answers to the following important questions:
- Is your policy claims-made or occurrence-based?
- Does your insurance policy only cover professional negligence claims? Does your policy also cover claims of unprofessional conduct reported to state medical licensing boards? Does your policy also cover medical staff bylaw disputes and state licensing matters?
- How is loss defined? “Pure loss” is coverage for the amount awarded to the plaintiff; “ultimate net loss” covers what pure loss covers, plus attorneys’ fees and costs.
- What procedures do you need to follow in order to properly notify the insurance carrier of a claim? Are you precluded from full coverage if you fail to properly report?
- What does the “duty to defend” provision cover? Will you be reimbursed for lost wages for your time in court? What services will be provided as part of your defense?
- What does the “consent to settle” provision say? If a settlement is negotiated between the plaintiff (patient) and the insurance company and the physician does not consent to the settlement, is the physician responsible for the ongoing defense costs and the amount of any verdict in excess of the recommended settlement amount?
It is important to both understand your insurance policy and what your employment agreement says about the policy. If you will be responsible for purchasing a tail policy at the end of your current employment, you should be well aware—and financially prepared—for this post-employment responsibility. Make sure your tail is not left exposed.
Steven M. Harris, Esq., is a nationally recognized healthcare attorney and a member of the law firm McDonald Hopkins LLC in Chicago. Write to him at sharris@mcdonaldhopkins.com.
Does your employer provide your medical malpractice insurance coverage? Are you looking for new employment? Are you in the market to purchase a professional malpractice insurance policy? Are you planning to retire soon?
If you answered “yes” to any of these questions, you likely will confront the concept of “tail” insurance at some point in your medical career.
Now is the time to dust off your employment agreement and professional liability insurance policy and review what happens in the event a lawsuit is filed against you after you leave your current employer. This means paying special attention to whether your professional liability insurance policy provides for claims-made or occurrence-based coverage, and, if it’s the former, who is responsible for purchasing tail coverage.
When Do I Need Tail Coverage?
Tail insurance issues frequently arise when a physician leaves his or her place of employment, whether due to switching jobs, retirement, or a buyout of a physician’s ownership interest. If the physician is leaving an employer that has claims-made professional liability insurance, the physician’s insurance coverage might not be seamless. Instead, tail or similar coverage is required.
Claims-made coverage protects a physician for professional negligence, as long as a two-part test is met: First, the physician must have the claims-made coverage in place when the negligent act occurs (with employer No. 1); second, the physician must be covered by the same carrier when he or she is notified of the claim while employed by employer No. 2. If either test is not satisfied, the current claims-made insurance policy will not provide coverage to the physician in the event a lawsuit is filed for an act of negligence that took place while employed by employer No. 1. Alternatively, some employers offer “nose” coverage from its insurance carrier, which will cover negligent acts that might have occurred during your current job. The vast majority of professional liability insurance policies written for medical practice groups are for claims-made coverage.
If, however, an employer has occurrence-based professional liability insurance, the departing physician’s insurance coverage is seamless and no tail insurance is required.
Example A
Here is a common example of what happens when a physician leaves an employer with claims-made professional liability coverage:
An employer maintains claims-made professional liability insurance coverage for its physicians with ABC Insurance Co. A physician decides to leave his or her current employer and accepts employment by a new employer, which maintains claims-made coverage with XYZ Insurance Co.
Within a few months of the physician’s new employment, a medical malpractice lawsuit is filed by a patient for medical treatment the patient received when the physician was employed by the former employer. By leaving the former employer, the departing physician automatically fails the two-part test for claims-made coverage, as the second prong is not satisfied. Therefore, even though the physician has liability coverage through the new employer, this insurance policy will not cover the lawsuit described above.
Unless the physician has tail insurance (or nose coverage) to cover lawsuits related to the former employment, a gap in liability coverage will exist. If claims-made insurance is the benefit you have received in your employment agreement, it is imperative that you understand that tail coverage is necessary when you leave.
However, if a physician leaves and a) is subsequently employed within the same state and b) stays insured by the same insurance carrier, then the insurance carrier will provide continuous coverage and no tail insurance policy is needed.
Who Pays the Premium?
If the physician will need tail coverage, the next critical question is, Who pays for such coverage? Even though tail coverage comes into effect when a physician leaves an employer, tail coverage should be addressed before the physician informs the employer of their departure; an even better approach would be while the employment agreement is negotiated. Payment of tail coverage should be defined in the physician’s employment agreement.
In terms of payment for the coverage, there are several options. First, the cost of tail coverage can be attributed 100% to either physician or employer. In specialties for which recruitment of new physicians is challenging (i.e. HM), employers are more likely to pay a substantial portion, if not all, of the cost as a benefit or inducement.
Second, the physician can connect the payment of tail coverage to the manner in which employment is terminated. For example, if the physician terminates the agreement for cause or if the employer terminates the physician’s employment without cause, the employer could be required to pay for the tail insurance. Alternatively, if the physician terminates the agreement without cause or if the employer terminates the physician’s employment with cause, the physician could be required to pay for the tail coverage. Frequently, physician employment agreements require physicians to pay for tail coverage if the physician violates a restrictive covenant (e.g. non-competition).
A third option is to split the cost of tail insurance between the former employer and the physician based on a percentage, or to include a vesting schedule, for example, such that the former employer pays one-third of the coverage if employment ends in the second year, two-thirds of the coverage if employment ends in the third year, and 100% of the coverage if employment ends in the fourth year or later.
Whatever arrangement the parties agree upon should be included in the physician’s employment agreement in order to prevent an expensive surprise.
Review Your Policy
Now that you have an understanding of claims-made coverage, occurrence-based coverage and tail insurance, it’s time to review your insurance policy. When reviewing your current policy, look for answers to the following important questions:
- Is your policy claims-made or occurrence-based?
- Does your insurance policy only cover professional negligence claims? Does your policy also cover claims of unprofessional conduct reported to state medical licensing boards? Does your policy also cover medical staff bylaw disputes and state licensing matters?
- How is loss defined? “Pure loss” is coverage for the amount awarded to the plaintiff; “ultimate net loss” covers what pure loss covers, plus attorneys’ fees and costs.
- What procedures do you need to follow in order to properly notify the insurance carrier of a claim? Are you precluded from full coverage if you fail to properly report?
- What does the “duty to defend” provision cover? Will you be reimbursed for lost wages for your time in court? What services will be provided as part of your defense?
- What does the “consent to settle” provision say? If a settlement is negotiated between the plaintiff (patient) and the insurance company and the physician does not consent to the settlement, is the physician responsible for the ongoing defense costs and the amount of any verdict in excess of the recommended settlement amount?
It is important to both understand your insurance policy and what your employment agreement says about the policy. If you will be responsible for purchasing a tail policy at the end of your current employment, you should be well aware—and financially prepared—for this post-employment responsibility. Make sure your tail is not left exposed.
Steven M. Harris, Esq., is a nationally recognized healthcare attorney and a member of the law firm McDonald Hopkins LLC in Chicago. Write to him at sharris@mcdonaldhopkins.com.