User login
Tension Pneumocephalus as Complication of Hematoma Evacuation
A 78‐year‐old man was transferred from an outside hospital where he presented with declining mental status and a history of falls. A computed tomography (CT) scan of the brain revealed a chronic subdural hematoma with superimposed acute hemorrhage. The subdural hematoma was attributed to a fall at home approximately 5 weeks prior to admission. He was taken to the operating room for urgent craniotomy and hemorrhage evacuation and postoperatively comanaged by neurosurgery, hospitalists, and medicine residents. He tolerated the procedure and was noted to have marked improvement in mental status after the procedure. He was monitored overnight in our intensive care unit without intracranial pressure monitoring.
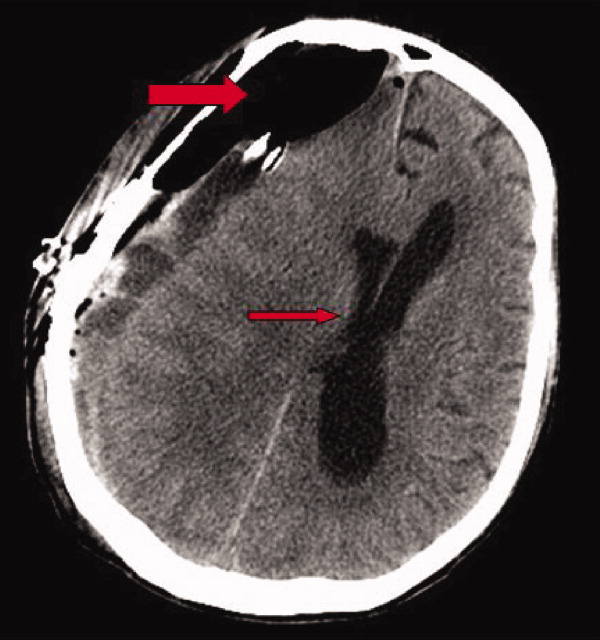
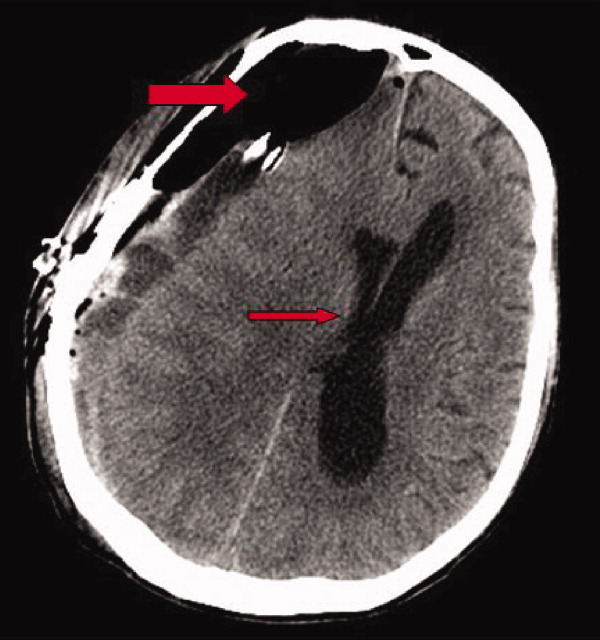
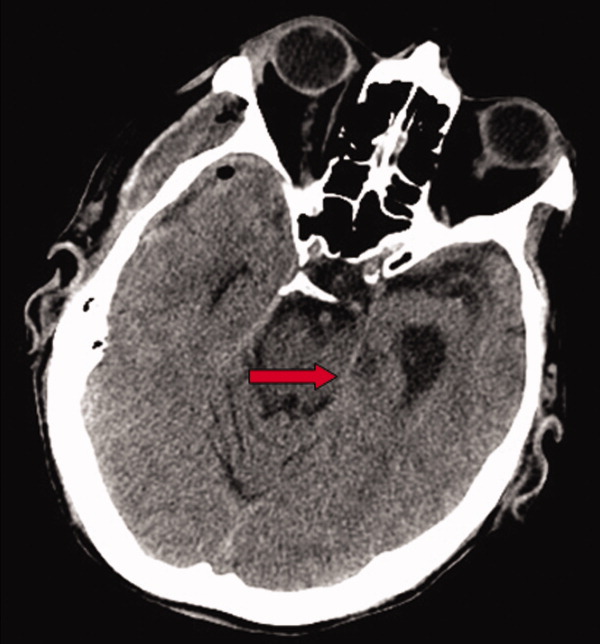
Early on postoperative day 1, he was awake, alert, following commands, and felt to be stable enough to be transferred to our transitional intensive care unit. However, later in the day he became progressively more confused. A follow‐up CT scan of the brain was ordered (Fig. 1) by the medicine team which revealed a large collection of air (wide arrow) and marked midline shift (thin arrow) consistent with tension pneumocephaly and subfalcine herniation (Fig. 2; arrow). Examination revealed that he was grossly obtunded with marked anisocoria, decerebrate posturing, and rigid tone. Neurosurgery was immediately contacted and recommended accessing 2 indwelling catheters left in the cerebrum as part of the normal postoperative course. Approximately 100 mL of serosanguinous fluid and air was aspirated with immediate improvement in his mental status and exam findings. Over the next few days, he remained clinically stable, and repeat CT scan showed slow resolution of the pneumocephalus and a decrease of his mass effect and midline shift. He was ultimately transferred to our skilled nursing facility for physical therapy and has done relatively well.
Pneumocephalus is a relatively common finding in many neurosurgical, intracranial procedures. However, tension pneumocephalus is a rare, life‐threatening form of pneumocephalus in which intracranial air causes mass effect and midline shift. In a review of 295 cases of pneumocephalus, 75% were caused by surgery, mostly intracranial and transsphenoidal, and head trauma. About 9% of cases resulted from infection with gas‐forming bacteria and rare causes include invasion of a nasopharyngeal carcinoma, frequent Valsalva maneuver, and air travel.1 Tension pneumocephalus occurs most commonly after the neurosurgical evacuation of a subdural hematoma. The prevalence of tension pneumocephalus following the evacuation of chronic subdural hematomas has been reported from 2.5% to 16%.2
There are 2 proposed mechanisms for the development of pneumocephalus; 1 proposes that air passes through a dural tear by a ball valve effect in which air can be forced into the intracranial cavity by a rapid increase in intrasinus pressure that occurs during sneezing, coughing, or straining. The air is then trapped intracranially. The second theory proposes that cerebrospinal fluid leakage permits air to enter the intracranial cavity because negative pressure is created as cerebrospinal fluid leaves the space.3 The conversion to tension physiology in either of these theories is less well understood.
- ,,,,.Pneumocephalus secondary to septic thrombosis of the superior sagittal sinus: report of a case.J Formos Med Assoc.2001;100(2):142‐144.
- ,,, et al.Subdural tension pneumocephalus following surgery for chronic subdural hematoma.J Neurosurg.1988;68:58‐61.
- ,,,.Nontraumatic tension pneumocephalus—a differential diagnosis of headache in the ED.Am J Emerg Med.2005, Vol.23, pp235‐236.
A 78‐year‐old man was transferred from an outside hospital where he presented with declining mental status and a history of falls. A computed tomography (CT) scan of the brain revealed a chronic subdural hematoma with superimposed acute hemorrhage. The subdural hematoma was attributed to a fall at home approximately 5 weeks prior to admission. He was taken to the operating room for urgent craniotomy and hemorrhage evacuation and postoperatively comanaged by neurosurgery, hospitalists, and medicine residents. He tolerated the procedure and was noted to have marked improvement in mental status after the procedure. He was monitored overnight in our intensive care unit without intracranial pressure monitoring.
Early on postoperative day 1, he was awake, alert, following commands, and felt to be stable enough to be transferred to our transitional intensive care unit. However, later in the day he became progressively more confused. A follow‐up CT scan of the brain was ordered (Fig. 1) by the medicine team which revealed a large collection of air (wide arrow) and marked midline shift (thin arrow) consistent with tension pneumocephaly and subfalcine herniation (Fig. 2; arrow). Examination revealed that he was grossly obtunded with marked anisocoria, decerebrate posturing, and rigid tone. Neurosurgery was immediately contacted and recommended accessing 2 indwelling catheters left in the cerebrum as part of the normal postoperative course. Approximately 100 mL of serosanguinous fluid and air was aspirated with immediate improvement in his mental status and exam findings. Over the next few days, he remained clinically stable, and repeat CT scan showed slow resolution of the pneumocephalus and a decrease of his mass effect and midline shift. He was ultimately transferred to our skilled nursing facility for physical therapy and has done relatively well.


Pneumocephalus is a relatively common finding in many neurosurgical, intracranial procedures. However, tension pneumocephalus is a rare, life‐threatening form of pneumocephalus in which intracranial air causes mass effect and midline shift. In a review of 295 cases of pneumocephalus, 75% were caused by surgery, mostly intracranial and transsphenoidal, and head trauma. About 9% of cases resulted from infection with gas‐forming bacteria and rare causes include invasion of a nasopharyngeal carcinoma, frequent Valsalva maneuver, and air travel.1 Tension pneumocephalus occurs most commonly after the neurosurgical evacuation of a subdural hematoma. The prevalence of tension pneumocephalus following the evacuation of chronic subdural hematomas has been reported from 2.5% to 16%.2
There are 2 proposed mechanisms for the development of pneumocephalus; 1 proposes that air passes through a dural tear by a ball valve effect in which air can be forced into the intracranial cavity by a rapid increase in intrasinus pressure that occurs during sneezing, coughing, or straining. The air is then trapped intracranially. The second theory proposes that cerebrospinal fluid leakage permits air to enter the intracranial cavity because negative pressure is created as cerebrospinal fluid leaves the space.3 The conversion to tension physiology in either of these theories is less well understood.
A 78‐year‐old man was transferred from an outside hospital where he presented with declining mental status and a history of falls. A computed tomography (CT) scan of the brain revealed a chronic subdural hematoma with superimposed acute hemorrhage. The subdural hematoma was attributed to a fall at home approximately 5 weeks prior to admission. He was taken to the operating room for urgent craniotomy and hemorrhage evacuation and postoperatively comanaged by neurosurgery, hospitalists, and medicine residents. He tolerated the procedure and was noted to have marked improvement in mental status after the procedure. He was monitored overnight in our intensive care unit without intracranial pressure monitoring.
Early on postoperative day 1, he was awake, alert, following commands, and felt to be stable enough to be transferred to our transitional intensive care unit. However, later in the day he became progressively more confused. A follow‐up CT scan of the brain was ordered (Fig. 1) by the medicine team which revealed a large collection of air (wide arrow) and marked midline shift (thin arrow) consistent with tension pneumocephaly and subfalcine herniation (Fig. 2; arrow). Examination revealed that he was grossly obtunded with marked anisocoria, decerebrate posturing, and rigid tone. Neurosurgery was immediately contacted and recommended accessing 2 indwelling catheters left in the cerebrum as part of the normal postoperative course. Approximately 100 mL of serosanguinous fluid and air was aspirated with immediate improvement in his mental status and exam findings. Over the next few days, he remained clinically stable, and repeat CT scan showed slow resolution of the pneumocephalus and a decrease of his mass effect and midline shift. He was ultimately transferred to our skilled nursing facility for physical therapy and has done relatively well.


Pneumocephalus is a relatively common finding in many neurosurgical, intracranial procedures. However, tension pneumocephalus is a rare, life‐threatening form of pneumocephalus in which intracranial air causes mass effect and midline shift. In a review of 295 cases of pneumocephalus, 75% were caused by surgery, mostly intracranial and transsphenoidal, and head trauma. About 9% of cases resulted from infection with gas‐forming bacteria and rare causes include invasion of a nasopharyngeal carcinoma, frequent Valsalva maneuver, and air travel.1 Tension pneumocephalus occurs most commonly after the neurosurgical evacuation of a subdural hematoma. The prevalence of tension pneumocephalus following the evacuation of chronic subdural hematomas has been reported from 2.5% to 16%.2
There are 2 proposed mechanisms for the development of pneumocephalus; 1 proposes that air passes through a dural tear by a ball valve effect in which air can be forced into the intracranial cavity by a rapid increase in intrasinus pressure that occurs during sneezing, coughing, or straining. The air is then trapped intracranially. The second theory proposes that cerebrospinal fluid leakage permits air to enter the intracranial cavity because negative pressure is created as cerebrospinal fluid leaves the space.3 The conversion to tension physiology in either of these theories is less well understood.
- ,,,,.Pneumocephalus secondary to septic thrombosis of the superior sagittal sinus: report of a case.J Formos Med Assoc.2001;100(2):142‐144.
- ,,, et al.Subdural tension pneumocephalus following surgery for chronic subdural hematoma.J Neurosurg.1988;68:58‐61.
- ,,,.Nontraumatic tension pneumocephalus—a differential diagnosis of headache in the ED.Am J Emerg Med.2005, Vol.23, pp235‐236.
- ,,,,.Pneumocephalus secondary to septic thrombosis of the superior sagittal sinus: report of a case.J Formos Med Assoc.2001;100(2):142‐144.
- ,,, et al.Subdural tension pneumocephalus following surgery for chronic subdural hematoma.J Neurosurg.1988;68:58‐61.
- ,,,.Nontraumatic tension pneumocephalus—a differential diagnosis of headache in the ED.Am J Emerg Med.2005, Vol.23, pp235‐236.
Quantification of Bedside Teaching
Bedside teaching, defined as teaching in the presence of a patient, has been an integral, respected part of medical education throughout the history of modern medicine. There is widespread concern among medical educators that bedside teaching is declining, and in particular, physical examination teaching.1‐5 Learning at the bedside accounted for 75% of clinical teaching in the 1960s and only 16% by 1978.2 Current estimates range from 8% to 19%.1
The bedside is the ideal venue for demonstrating, observing, and evaluating medical interviewing skills, physical examination techniques, and interpersonal and communication skills. Role modeling is the primary method by which clinical teachers demonstrate and teach professionalism and humanistic behavior.6 The bedside is also a place to develop clinical reasoning skills, stimulate problem‐based learning,7 and demonstrate teamwork.4 Thus, the decline in bedside teaching is of major concern for more than just the dying of a time‐honored tradition, but for the threat to the development of skills and attitudes essential for the practice of medicine.
With the rapid growth in the number of hospitalists and their presence at most major U.S. teaching hospitals, internal medicine residents and medical students in their medicine clerkships receive much of their inpatient training from attending physicians who are hospitalists.8 Little is known about the teaching practices of hospitalist attending physicians. We investigated the fraction of time hospitalist attending physicians spend at the bedside during attending teaching rounds and the frequency of the demonstration of physical examination skills at 1 academic teaching hospital.
Patients and Methods
The Brigham & Women's Hospitalist Service, a 28‐member academic hospitalist group who serve as both the teaching attendings and patient care attendings on 4 general medicine teams, was studied in a prospective, observational fashion. Internal medicine residents at Brigham & Women's Hospital rotating on the hospitalist service were identified by examining the schedule of inpatient rotations during the 2007‐2008 academic year and were asked to participate in the study via an e‐mail invitation. The Institutional Review Board of Brigham & Women's Hospital approved the study.
Teams were made up of 1 senior resident and 2 interns. Call frequency was every fourth day. Over a period of 23 sequential weekdays, medical residents and interns from each of the 4 hospitalist teams observed and reported the behavior of their attendings on rounds. Their reports captured the fraction of time spent at the bedside during rounds and the frequency of physical examination teaching. Residents and interns were asked to respond to 3 questions in a daily e‐mail. Respondents reported (1) total time spent with their hospitalist attending during attending rounds, (2) time spent inside patient rooms during attending rounds, and (3) whether or not a physical examination finding or skill was demonstrated by their hospitalist attending. When more than 1 team member responded, time reported among team members was averaged and if there was a discrepancy between whether or not a physical examination finding or skill was demonstrated, it was defaulted to the positive response. Hospitalist attendings remained unaware of the daily observations.
Hospitalist attendings were independently invited to complete a baseline needs assessment survey on bedside teaching. Surveys addressed attitudes toward bedside teaching, confidence in ability to lead bedside teaching rounds and teach the physical examination, and adequacy of their own training in these skills. Respondents were asked to comment on obstacles to bedside teaching. Residents were surveyed at the completion of a rotation with a hospitalist attending regarding the value of the time spent at the bedside and their self‐perceived improvement in physical examination skills and bedside teaching skills. The survey solicited the residents' opinion of the most valuable aspect of bedside teaching. The survey questions used a 4‐point Likert scale with response options ranging from 1 = strongly disagree to 4 = strongly agree.
The fraction of time spent at the bedside during attending hospitalist rounds was calculated from the average time spent in patient rooms and the average time of attending rounds. The frequency of physical examination teaching was expressed as a percent of all teaching encounters. Interrater reliability was calculated using the intraclass correlation coefficient with the Spearman‐Brown adjustment. Differences between groups were calculated using the Fisher's exact test for counts and the Wilcoxon rank‐sum test for continuous data. Significance was accepted for P < 0.05.
Results
Thirty‐five residents provided observations on 61 of 92 potentially observed attending rounds (66% response rate) over 23 weekdays, including observations of the rounding behavior of 12 different hospitalists. The interrater reliability was 0.91. The average patient census on each team during this time period was 12 (range 6‐19).
Residents reported that their attendings went to the bedside at least once during 37 of these 61 rounds (61%), and provided physical examination teaching during 23 of these 61 (38%) encounters. Hospitalists spent an average of 101 minutes on rounds and an average of 17 minutes (17%) of their time inside patient rooms.
Rounds that included time spent at the bedside were significantly longer on average than rounds that did not include time spent at the bedside (122 vs. 69 minutes, P < 0.001). During rounds that included bedside teaching, teams spent an average of 29 minutes (24% of the total time) in patient rooms, and rounds were significantly more likely to include teaching on physical diagnosis (23/37 rounds vs. 0/24 rounds, P < 0.001). Physical examination teaching did not significantly prolong those rounds that included bedside teaching (124 vs. 119 minutes, P = 0.56), but did significantly increase the amount of time spent at the bedside (32 vs. 22 minutes, P = 0.046).
Eighteen hospitalists (64% response) with a mean of 5.9 years of experience as attending physicians completed a needs‐assessment survey (Table 1). Fourteen of the 18 hospitalists (78%) reported that they prioritize bedside teaching and 16 (89%) requested more emphasis on bedside teaching in the residency curriculum. Twelve hospitalists (67%) indicated that they were confident in their ability to lead bedside teaching rounds; 9 (50%) were confident in their ability to teach physical examination. Eleven (61%) of the respondents felt poorly prepared to do bedside teaching after completing their residency, and 12 (67%) felt that they had received inadequate training in how to teach the physical examination. Of the obstacles to bedside teaching, time and inadequate training and skills were the most frequently noted, present in 11 and 6 of the reports, respectively. Lack of confidence and lack of role models were also cited in 4 and 2 of the reports, respectively.
| Strongly Disagree (%) | Disagree (%) | Agree (%) | Strongly Agree (%) | |
|---|---|---|---|---|
| ||||
| I make bedside teaching a priority | 0 | 22 | 56 | 22 |
| More emphasis on bedside teaching in the residency curriculum is needed | 0 | 11 | 39 | 50 |
| I feel confident in my ability to lead bedside teaching rounds | 11 | 22 | 50 | 17 |
| I was well‐prepared to do bedside teaching after residency training | 22 | 39 | 28 | 11 |
| I feel confident in my ability to teach the physical exam | 11 | 39 | 33 | 17 |
| I have received adequate training in how to teach the physical exam | 17 | 50 | 22 | 11 |
Seventeen medical residents (49% response) completed a survey regarding their general medical service rotation with a hospitalist upon its completion (Table 2). Sixteen of the respondents (94%) agreed that time spent at the bedside during hospitalist attending teaching rounds that specific rotation was valuable, and 15 (82%) of the residents sought more emphasis on bedside teaching in the residency curriculum. Four of the respondents (24%) reported that their physical examination skills improved over the rotation, 5 (29%) felt better prepared to teach the physical examination, and 9 (53%) felt better prepared to lead bedside teaching rounds. Only 3 (18%) of the respondents reported that they had received helpful feedback on their physical examination skills from their attending. Responding residents noted physical examination teaching, communication and interpersonal skills, focus on patient‐centered care, and integrating the clinical examination with diagnostic and management decisions as the most valuable aspects of bedside teaching.
| Strongly Disagree (%) | Disagree (%) | Agree (%) | Strongly Agree (%) | |
|---|---|---|---|---|
| ||||
| Time spent at the bedside during teaching rounds was valuable | 0 | 6 | 65 | 29 |
| More emphasis on bedside teaching in the residency curriculum is needed | 0 | 18 | 53 | 29 |
| I feel better prepared to lead bedside teaching rounds | 6 | 41 | 53 | 0 |
| My physical exam skills improved over the rotation | 6 | 71 | 24 | 0 |
| I feel better prepared to teach the physical exam | 6 | 65 | 29 | 0 |
| I received helpful feedback on my physical exam skills | 18 | 65 | 18 | 0 |
Discussion
Bedside teaching is highly valued by clinicians and trainees, though there is little evidence supporting its efficacy. Patients also enjoy and are accepting of bedside presentations7, 9, 10 if certain rules are adhered to (eg, avoid medical jargon) and benefit by having a better understanding of their illness.9 This study supports previous views of medical residents, students,1, 5, 7 and faculty11 of the value and need for greater emphasis on bedside teaching in medical education.
This study of rounding behavior found that hospitalists in this academic center go to the bedside most days, but 39% of attending teaching rounds did not include a bedside encounter. Physical examination teaching is infrequent. Though time spent at the bedside was only a small fraction of total teaching time (17%) in this practice, this fraction is at the high end of previous reports. Teaching rounds that did not include bedside teaching most likely occurred in the confines of a conference room.
Many factors appear to contribute to the paucity of time spent at the bedside: time constraints, shorter hospital stays, greater work demands,11 residency duty‐hour regulations,12 declining bedside teaching skills, unrealistic expectations of the encounter, and erosion of the teaching ethic.3 A decline in clinical examination skills among trainees and attending physicians leads to a growing reliance on data and technology, thereby perpetuating the cycle of declining bedside skills.4
The hospitalists in this study identify time as the most dominant obstacle to bedside teaching. On days when hospitalist attending physicians went to the bedside, rounds were on average 53 minutes longer than on those days when they did not go to the bedside. This time increase varied little whether or not physical examination teaching occurred. The difference in rounding time may be partially explained by the admitting cycle and patient census. Teaching attendings are likely to go to the bedside to see new patients on postcall days when the patient census is also the highest.
Many members of this hospitalist group indicated that they felt inadequately prepared to lead bedside teaching rounds. Of those who responded to the survey, 67% did not feel that they received adequate training in how to teach the physical examination. Consequently, only one‐half of responding hospitalists expressed confidence in their ability to teach the physical examination. Not surprisingly, physical examination skills were a component of a minority of teaching sessions and only one‐quarter of the medical residents perceived that their physical examination skills improved during the rotation with a hospitalist attending. The paucity of feedback to the house‐staff likely contributed to this stagnancy. Residents who become hospitalists ill‐prepared to lead bedside teaching and teach the physical examination will perpetuate the decline in bedside teaching.
Though a substantial portion of the hospitalists in this study lacked confidence, an overwhelming majority of medical residents found their time spent at the bedside with a hospitalist to be valuable. More than one‐half of residents reported that they were better prepared to lead bedside teaching after the rotation. Residents recognize that bedside teaching can include communication and clinical reasoning skills. Hospitalists should be made aware that a broad range of skills and content can be taught at the bedside.
Hospitalists have an increasing influence on the education of medical residents and students and are appropriate targets for faculty development programs aimed at improving bedside teaching. As a newer, growing specialty, hospitalists tend to be younger physicians, and are therefore more reliant on the education attained during residency to support their bedside activities. Many residencies have developed resident as educator programs in an attempt to create a future generation of attendings better able to teach.13
Several limitations should be acknowledged when interpreting the results of this study. The study was limited to a hospitalist group at a single academic medical center and relied on resident recall. Though the response rate to the daily e‐mails was relatively low, the interrater reliability was high, and a broad range of residents and attendings were represented. Residents with greater patient censuses may have been too busy to respond, but it is unclear in which direction this would bias the results.
Conclusions
This study provides additional evidence that bedside and physical examination teaching are in decline. Time is an increasingly precious commodity for hospitalists; though many commentators echo the sentiments of the respondents in this study that more time at the bedside is needed, the amount of time that should be optimally spent at the bedside remains unclear. Research to improve the quality of bedside learning and its influence on patient care outcomes is needed.
- ,,,.Improving bedside teaching: findings from a focus group study of learners.Acad Med.2008;83(3):257–264.
- .On bedside teaching.Ann Intern Med.1997;126(3):217–220.
- ,,,.Whither bedside teaching? A focus‐group study of clinical teachers.Acad Med.2003;78(4):384–390.
- .Bedside rounds revisited.N Engl J Med.1997;336(16):1174–1175.
- ,,,.Effect of a physical examination teaching program on the behavior of medical residents.J Gen Intern Med.2005;20(8):710–714.
- ,,,,.Role modeling humanistic behavior: learning bedside manner from the experts.Acad Med.2006;81(7):661–667.
- ,,.Student and patient perspectives on bedside teaching.Med Educ.1997;31(5):341–346.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- ,,,.A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit.Pediatrics.2007;120(2):275–280.
- ,,.Impediments to bed‐side teaching.Med Educ.1998;32(2):159–162.
- ,,, et al.Internal medicine and general surgery residents' attitudes about the ACGME duty hours regulations: a multicenter study.Acad Med.2006;81(12):1052–1058.
- ,,.Resident as teacher: educating the educators.Mt Sinai J Med.2006;73(8):1165–1169.
Bedside teaching, defined as teaching in the presence of a patient, has been an integral, respected part of medical education throughout the history of modern medicine. There is widespread concern among medical educators that bedside teaching is declining, and in particular, physical examination teaching.1‐5 Learning at the bedside accounted for 75% of clinical teaching in the 1960s and only 16% by 1978.2 Current estimates range from 8% to 19%.1
The bedside is the ideal venue for demonstrating, observing, and evaluating medical interviewing skills, physical examination techniques, and interpersonal and communication skills. Role modeling is the primary method by which clinical teachers demonstrate and teach professionalism and humanistic behavior.6 The bedside is also a place to develop clinical reasoning skills, stimulate problem‐based learning,7 and demonstrate teamwork.4 Thus, the decline in bedside teaching is of major concern for more than just the dying of a time‐honored tradition, but for the threat to the development of skills and attitudes essential for the practice of medicine.
With the rapid growth in the number of hospitalists and their presence at most major U.S. teaching hospitals, internal medicine residents and medical students in their medicine clerkships receive much of their inpatient training from attending physicians who are hospitalists.8 Little is known about the teaching practices of hospitalist attending physicians. We investigated the fraction of time hospitalist attending physicians spend at the bedside during attending teaching rounds and the frequency of the demonstration of physical examination skills at 1 academic teaching hospital.
Patients and Methods
The Brigham & Women's Hospitalist Service, a 28‐member academic hospitalist group who serve as both the teaching attendings and patient care attendings on 4 general medicine teams, was studied in a prospective, observational fashion. Internal medicine residents at Brigham & Women's Hospital rotating on the hospitalist service were identified by examining the schedule of inpatient rotations during the 2007‐2008 academic year and were asked to participate in the study via an e‐mail invitation. The Institutional Review Board of Brigham & Women's Hospital approved the study.
Teams were made up of 1 senior resident and 2 interns. Call frequency was every fourth day. Over a period of 23 sequential weekdays, medical residents and interns from each of the 4 hospitalist teams observed and reported the behavior of their attendings on rounds. Their reports captured the fraction of time spent at the bedside during rounds and the frequency of physical examination teaching. Residents and interns were asked to respond to 3 questions in a daily e‐mail. Respondents reported (1) total time spent with their hospitalist attending during attending rounds, (2) time spent inside patient rooms during attending rounds, and (3) whether or not a physical examination finding or skill was demonstrated by their hospitalist attending. When more than 1 team member responded, time reported among team members was averaged and if there was a discrepancy between whether or not a physical examination finding or skill was demonstrated, it was defaulted to the positive response. Hospitalist attendings remained unaware of the daily observations.
Hospitalist attendings were independently invited to complete a baseline needs assessment survey on bedside teaching. Surveys addressed attitudes toward bedside teaching, confidence in ability to lead bedside teaching rounds and teach the physical examination, and adequacy of their own training in these skills. Respondents were asked to comment on obstacles to bedside teaching. Residents were surveyed at the completion of a rotation with a hospitalist attending regarding the value of the time spent at the bedside and their self‐perceived improvement in physical examination skills and bedside teaching skills. The survey solicited the residents' opinion of the most valuable aspect of bedside teaching. The survey questions used a 4‐point Likert scale with response options ranging from 1 = strongly disagree to 4 = strongly agree.
The fraction of time spent at the bedside during attending hospitalist rounds was calculated from the average time spent in patient rooms and the average time of attending rounds. The frequency of physical examination teaching was expressed as a percent of all teaching encounters. Interrater reliability was calculated using the intraclass correlation coefficient with the Spearman‐Brown adjustment. Differences between groups were calculated using the Fisher's exact test for counts and the Wilcoxon rank‐sum test for continuous data. Significance was accepted for P < 0.05.
Results
Thirty‐five residents provided observations on 61 of 92 potentially observed attending rounds (66% response rate) over 23 weekdays, including observations of the rounding behavior of 12 different hospitalists. The interrater reliability was 0.91. The average patient census on each team during this time period was 12 (range 6‐19).
Residents reported that their attendings went to the bedside at least once during 37 of these 61 rounds (61%), and provided physical examination teaching during 23 of these 61 (38%) encounters. Hospitalists spent an average of 101 minutes on rounds and an average of 17 minutes (17%) of their time inside patient rooms.
Rounds that included time spent at the bedside were significantly longer on average than rounds that did not include time spent at the bedside (122 vs. 69 minutes, P < 0.001). During rounds that included bedside teaching, teams spent an average of 29 minutes (24% of the total time) in patient rooms, and rounds were significantly more likely to include teaching on physical diagnosis (23/37 rounds vs. 0/24 rounds, P < 0.001). Physical examination teaching did not significantly prolong those rounds that included bedside teaching (124 vs. 119 minutes, P = 0.56), but did significantly increase the amount of time spent at the bedside (32 vs. 22 minutes, P = 0.046).
Eighteen hospitalists (64% response) with a mean of 5.9 years of experience as attending physicians completed a needs‐assessment survey (Table 1). Fourteen of the 18 hospitalists (78%) reported that they prioritize bedside teaching and 16 (89%) requested more emphasis on bedside teaching in the residency curriculum. Twelve hospitalists (67%) indicated that they were confident in their ability to lead bedside teaching rounds; 9 (50%) were confident in their ability to teach physical examination. Eleven (61%) of the respondents felt poorly prepared to do bedside teaching after completing their residency, and 12 (67%) felt that they had received inadequate training in how to teach the physical examination. Of the obstacles to bedside teaching, time and inadequate training and skills were the most frequently noted, present in 11 and 6 of the reports, respectively. Lack of confidence and lack of role models were also cited in 4 and 2 of the reports, respectively.
| Strongly Disagree (%) | Disagree (%) | Agree (%) | Strongly Agree (%) | |
|---|---|---|---|---|
| ||||
| I make bedside teaching a priority | 0 | 22 | 56 | 22 |
| More emphasis on bedside teaching in the residency curriculum is needed | 0 | 11 | 39 | 50 |
| I feel confident in my ability to lead bedside teaching rounds | 11 | 22 | 50 | 17 |
| I was well‐prepared to do bedside teaching after residency training | 22 | 39 | 28 | 11 |
| I feel confident in my ability to teach the physical exam | 11 | 39 | 33 | 17 |
| I have received adequate training in how to teach the physical exam | 17 | 50 | 22 | 11 |
Seventeen medical residents (49% response) completed a survey regarding their general medical service rotation with a hospitalist upon its completion (Table 2). Sixteen of the respondents (94%) agreed that time spent at the bedside during hospitalist attending teaching rounds that specific rotation was valuable, and 15 (82%) of the residents sought more emphasis on bedside teaching in the residency curriculum. Four of the respondents (24%) reported that their physical examination skills improved over the rotation, 5 (29%) felt better prepared to teach the physical examination, and 9 (53%) felt better prepared to lead bedside teaching rounds. Only 3 (18%) of the respondents reported that they had received helpful feedback on their physical examination skills from their attending. Responding residents noted physical examination teaching, communication and interpersonal skills, focus on patient‐centered care, and integrating the clinical examination with diagnostic and management decisions as the most valuable aspects of bedside teaching.
| Strongly Disagree (%) | Disagree (%) | Agree (%) | Strongly Agree (%) | |
|---|---|---|---|---|
| ||||
| Time spent at the bedside during teaching rounds was valuable | 0 | 6 | 65 | 29 |
| More emphasis on bedside teaching in the residency curriculum is needed | 0 | 18 | 53 | 29 |
| I feel better prepared to lead bedside teaching rounds | 6 | 41 | 53 | 0 |
| My physical exam skills improved over the rotation | 6 | 71 | 24 | 0 |
| I feel better prepared to teach the physical exam | 6 | 65 | 29 | 0 |
| I received helpful feedback on my physical exam skills | 18 | 65 | 18 | 0 |
Discussion
Bedside teaching is highly valued by clinicians and trainees, though there is little evidence supporting its efficacy. Patients also enjoy and are accepting of bedside presentations7, 9, 10 if certain rules are adhered to (eg, avoid medical jargon) and benefit by having a better understanding of their illness.9 This study supports previous views of medical residents, students,1, 5, 7 and faculty11 of the value and need for greater emphasis on bedside teaching in medical education.
This study of rounding behavior found that hospitalists in this academic center go to the bedside most days, but 39% of attending teaching rounds did not include a bedside encounter. Physical examination teaching is infrequent. Though time spent at the bedside was only a small fraction of total teaching time (17%) in this practice, this fraction is at the high end of previous reports. Teaching rounds that did not include bedside teaching most likely occurred in the confines of a conference room.
Many factors appear to contribute to the paucity of time spent at the bedside: time constraints, shorter hospital stays, greater work demands,11 residency duty‐hour regulations,12 declining bedside teaching skills, unrealistic expectations of the encounter, and erosion of the teaching ethic.3 A decline in clinical examination skills among trainees and attending physicians leads to a growing reliance on data and technology, thereby perpetuating the cycle of declining bedside skills.4
The hospitalists in this study identify time as the most dominant obstacle to bedside teaching. On days when hospitalist attending physicians went to the bedside, rounds were on average 53 minutes longer than on those days when they did not go to the bedside. This time increase varied little whether or not physical examination teaching occurred. The difference in rounding time may be partially explained by the admitting cycle and patient census. Teaching attendings are likely to go to the bedside to see new patients on postcall days when the patient census is also the highest.
Many members of this hospitalist group indicated that they felt inadequately prepared to lead bedside teaching rounds. Of those who responded to the survey, 67% did not feel that they received adequate training in how to teach the physical examination. Consequently, only one‐half of responding hospitalists expressed confidence in their ability to teach the physical examination. Not surprisingly, physical examination skills were a component of a minority of teaching sessions and only one‐quarter of the medical residents perceived that their physical examination skills improved during the rotation with a hospitalist attending. The paucity of feedback to the house‐staff likely contributed to this stagnancy. Residents who become hospitalists ill‐prepared to lead bedside teaching and teach the physical examination will perpetuate the decline in bedside teaching.
Though a substantial portion of the hospitalists in this study lacked confidence, an overwhelming majority of medical residents found their time spent at the bedside with a hospitalist to be valuable. More than one‐half of residents reported that they were better prepared to lead bedside teaching after the rotation. Residents recognize that bedside teaching can include communication and clinical reasoning skills. Hospitalists should be made aware that a broad range of skills and content can be taught at the bedside.
Hospitalists have an increasing influence on the education of medical residents and students and are appropriate targets for faculty development programs aimed at improving bedside teaching. As a newer, growing specialty, hospitalists tend to be younger physicians, and are therefore more reliant on the education attained during residency to support their bedside activities. Many residencies have developed resident as educator programs in an attempt to create a future generation of attendings better able to teach.13
Several limitations should be acknowledged when interpreting the results of this study. The study was limited to a hospitalist group at a single academic medical center and relied on resident recall. Though the response rate to the daily e‐mails was relatively low, the interrater reliability was high, and a broad range of residents and attendings were represented. Residents with greater patient censuses may have been too busy to respond, but it is unclear in which direction this would bias the results.
Conclusions
This study provides additional evidence that bedside and physical examination teaching are in decline. Time is an increasingly precious commodity for hospitalists; though many commentators echo the sentiments of the respondents in this study that more time at the bedside is needed, the amount of time that should be optimally spent at the bedside remains unclear. Research to improve the quality of bedside learning and its influence on patient care outcomes is needed.
Bedside teaching, defined as teaching in the presence of a patient, has been an integral, respected part of medical education throughout the history of modern medicine. There is widespread concern among medical educators that bedside teaching is declining, and in particular, physical examination teaching.1‐5 Learning at the bedside accounted for 75% of clinical teaching in the 1960s and only 16% by 1978.2 Current estimates range from 8% to 19%.1
The bedside is the ideal venue for demonstrating, observing, and evaluating medical interviewing skills, physical examination techniques, and interpersonal and communication skills. Role modeling is the primary method by which clinical teachers demonstrate and teach professionalism and humanistic behavior.6 The bedside is also a place to develop clinical reasoning skills, stimulate problem‐based learning,7 and demonstrate teamwork.4 Thus, the decline in bedside teaching is of major concern for more than just the dying of a time‐honored tradition, but for the threat to the development of skills and attitudes essential for the practice of medicine.
With the rapid growth in the number of hospitalists and their presence at most major U.S. teaching hospitals, internal medicine residents and medical students in their medicine clerkships receive much of their inpatient training from attending physicians who are hospitalists.8 Little is known about the teaching practices of hospitalist attending physicians. We investigated the fraction of time hospitalist attending physicians spend at the bedside during attending teaching rounds and the frequency of the demonstration of physical examination skills at 1 academic teaching hospital.
Patients and Methods
The Brigham & Women's Hospitalist Service, a 28‐member academic hospitalist group who serve as both the teaching attendings and patient care attendings on 4 general medicine teams, was studied in a prospective, observational fashion. Internal medicine residents at Brigham & Women's Hospital rotating on the hospitalist service were identified by examining the schedule of inpatient rotations during the 2007‐2008 academic year and were asked to participate in the study via an e‐mail invitation. The Institutional Review Board of Brigham & Women's Hospital approved the study.
Teams were made up of 1 senior resident and 2 interns. Call frequency was every fourth day. Over a period of 23 sequential weekdays, medical residents and interns from each of the 4 hospitalist teams observed and reported the behavior of their attendings on rounds. Their reports captured the fraction of time spent at the bedside during rounds and the frequency of physical examination teaching. Residents and interns were asked to respond to 3 questions in a daily e‐mail. Respondents reported (1) total time spent with their hospitalist attending during attending rounds, (2) time spent inside patient rooms during attending rounds, and (3) whether or not a physical examination finding or skill was demonstrated by their hospitalist attending. When more than 1 team member responded, time reported among team members was averaged and if there was a discrepancy between whether or not a physical examination finding or skill was demonstrated, it was defaulted to the positive response. Hospitalist attendings remained unaware of the daily observations.
Hospitalist attendings were independently invited to complete a baseline needs assessment survey on bedside teaching. Surveys addressed attitudes toward bedside teaching, confidence in ability to lead bedside teaching rounds and teach the physical examination, and adequacy of their own training in these skills. Respondents were asked to comment on obstacles to bedside teaching. Residents were surveyed at the completion of a rotation with a hospitalist attending regarding the value of the time spent at the bedside and their self‐perceived improvement in physical examination skills and bedside teaching skills. The survey solicited the residents' opinion of the most valuable aspect of bedside teaching. The survey questions used a 4‐point Likert scale with response options ranging from 1 = strongly disagree to 4 = strongly agree.
The fraction of time spent at the bedside during attending hospitalist rounds was calculated from the average time spent in patient rooms and the average time of attending rounds. The frequency of physical examination teaching was expressed as a percent of all teaching encounters. Interrater reliability was calculated using the intraclass correlation coefficient with the Spearman‐Brown adjustment. Differences between groups were calculated using the Fisher's exact test for counts and the Wilcoxon rank‐sum test for continuous data. Significance was accepted for P < 0.05.
Results
Thirty‐five residents provided observations on 61 of 92 potentially observed attending rounds (66% response rate) over 23 weekdays, including observations of the rounding behavior of 12 different hospitalists. The interrater reliability was 0.91. The average patient census on each team during this time period was 12 (range 6‐19).
Residents reported that their attendings went to the bedside at least once during 37 of these 61 rounds (61%), and provided physical examination teaching during 23 of these 61 (38%) encounters. Hospitalists spent an average of 101 minutes on rounds and an average of 17 minutes (17%) of their time inside patient rooms.
Rounds that included time spent at the bedside were significantly longer on average than rounds that did not include time spent at the bedside (122 vs. 69 minutes, P < 0.001). During rounds that included bedside teaching, teams spent an average of 29 minutes (24% of the total time) in patient rooms, and rounds were significantly more likely to include teaching on physical diagnosis (23/37 rounds vs. 0/24 rounds, P < 0.001). Physical examination teaching did not significantly prolong those rounds that included bedside teaching (124 vs. 119 minutes, P = 0.56), but did significantly increase the amount of time spent at the bedside (32 vs. 22 minutes, P = 0.046).
Eighteen hospitalists (64% response) with a mean of 5.9 years of experience as attending physicians completed a needs‐assessment survey (Table 1). Fourteen of the 18 hospitalists (78%) reported that they prioritize bedside teaching and 16 (89%) requested more emphasis on bedside teaching in the residency curriculum. Twelve hospitalists (67%) indicated that they were confident in their ability to lead bedside teaching rounds; 9 (50%) were confident in their ability to teach physical examination. Eleven (61%) of the respondents felt poorly prepared to do bedside teaching after completing their residency, and 12 (67%) felt that they had received inadequate training in how to teach the physical examination. Of the obstacles to bedside teaching, time and inadequate training and skills were the most frequently noted, present in 11 and 6 of the reports, respectively. Lack of confidence and lack of role models were also cited in 4 and 2 of the reports, respectively.
| Strongly Disagree (%) | Disagree (%) | Agree (%) | Strongly Agree (%) | |
|---|---|---|---|---|
| ||||
| I make bedside teaching a priority | 0 | 22 | 56 | 22 |
| More emphasis on bedside teaching in the residency curriculum is needed | 0 | 11 | 39 | 50 |
| I feel confident in my ability to lead bedside teaching rounds | 11 | 22 | 50 | 17 |
| I was well‐prepared to do bedside teaching after residency training | 22 | 39 | 28 | 11 |
| I feel confident in my ability to teach the physical exam | 11 | 39 | 33 | 17 |
| I have received adequate training in how to teach the physical exam | 17 | 50 | 22 | 11 |
Seventeen medical residents (49% response) completed a survey regarding their general medical service rotation with a hospitalist upon its completion (Table 2). Sixteen of the respondents (94%) agreed that time spent at the bedside during hospitalist attending teaching rounds that specific rotation was valuable, and 15 (82%) of the residents sought more emphasis on bedside teaching in the residency curriculum. Four of the respondents (24%) reported that their physical examination skills improved over the rotation, 5 (29%) felt better prepared to teach the physical examination, and 9 (53%) felt better prepared to lead bedside teaching rounds. Only 3 (18%) of the respondents reported that they had received helpful feedback on their physical examination skills from their attending. Responding residents noted physical examination teaching, communication and interpersonal skills, focus on patient‐centered care, and integrating the clinical examination with diagnostic and management decisions as the most valuable aspects of bedside teaching.
| Strongly Disagree (%) | Disagree (%) | Agree (%) | Strongly Agree (%) | |
|---|---|---|---|---|
| ||||
| Time spent at the bedside during teaching rounds was valuable | 0 | 6 | 65 | 29 |
| More emphasis on bedside teaching in the residency curriculum is needed | 0 | 18 | 53 | 29 |
| I feel better prepared to lead bedside teaching rounds | 6 | 41 | 53 | 0 |
| My physical exam skills improved over the rotation | 6 | 71 | 24 | 0 |
| I feel better prepared to teach the physical exam | 6 | 65 | 29 | 0 |
| I received helpful feedback on my physical exam skills | 18 | 65 | 18 | 0 |
Discussion
Bedside teaching is highly valued by clinicians and trainees, though there is little evidence supporting its efficacy. Patients also enjoy and are accepting of bedside presentations7, 9, 10 if certain rules are adhered to (eg, avoid medical jargon) and benefit by having a better understanding of their illness.9 This study supports previous views of medical residents, students,1, 5, 7 and faculty11 of the value and need for greater emphasis on bedside teaching in medical education.
This study of rounding behavior found that hospitalists in this academic center go to the bedside most days, but 39% of attending teaching rounds did not include a bedside encounter. Physical examination teaching is infrequent. Though time spent at the bedside was only a small fraction of total teaching time (17%) in this practice, this fraction is at the high end of previous reports. Teaching rounds that did not include bedside teaching most likely occurred in the confines of a conference room.
Many factors appear to contribute to the paucity of time spent at the bedside: time constraints, shorter hospital stays, greater work demands,11 residency duty‐hour regulations,12 declining bedside teaching skills, unrealistic expectations of the encounter, and erosion of the teaching ethic.3 A decline in clinical examination skills among trainees and attending physicians leads to a growing reliance on data and technology, thereby perpetuating the cycle of declining bedside skills.4
The hospitalists in this study identify time as the most dominant obstacle to bedside teaching. On days when hospitalist attending physicians went to the bedside, rounds were on average 53 minutes longer than on those days when they did not go to the bedside. This time increase varied little whether or not physical examination teaching occurred. The difference in rounding time may be partially explained by the admitting cycle and patient census. Teaching attendings are likely to go to the bedside to see new patients on postcall days when the patient census is also the highest.
Many members of this hospitalist group indicated that they felt inadequately prepared to lead bedside teaching rounds. Of those who responded to the survey, 67% did not feel that they received adequate training in how to teach the physical examination. Consequently, only one‐half of responding hospitalists expressed confidence in their ability to teach the physical examination. Not surprisingly, physical examination skills were a component of a minority of teaching sessions and only one‐quarter of the medical residents perceived that their physical examination skills improved during the rotation with a hospitalist attending. The paucity of feedback to the house‐staff likely contributed to this stagnancy. Residents who become hospitalists ill‐prepared to lead bedside teaching and teach the physical examination will perpetuate the decline in bedside teaching.
Though a substantial portion of the hospitalists in this study lacked confidence, an overwhelming majority of medical residents found their time spent at the bedside with a hospitalist to be valuable. More than one‐half of residents reported that they were better prepared to lead bedside teaching after the rotation. Residents recognize that bedside teaching can include communication and clinical reasoning skills. Hospitalists should be made aware that a broad range of skills and content can be taught at the bedside.
Hospitalists have an increasing influence on the education of medical residents and students and are appropriate targets for faculty development programs aimed at improving bedside teaching. As a newer, growing specialty, hospitalists tend to be younger physicians, and are therefore more reliant on the education attained during residency to support their bedside activities. Many residencies have developed resident as educator programs in an attempt to create a future generation of attendings better able to teach.13
Several limitations should be acknowledged when interpreting the results of this study. The study was limited to a hospitalist group at a single academic medical center and relied on resident recall. Though the response rate to the daily e‐mails was relatively low, the interrater reliability was high, and a broad range of residents and attendings were represented. Residents with greater patient censuses may have been too busy to respond, but it is unclear in which direction this would bias the results.
Conclusions
This study provides additional evidence that bedside and physical examination teaching are in decline. Time is an increasingly precious commodity for hospitalists; though many commentators echo the sentiments of the respondents in this study that more time at the bedside is needed, the amount of time that should be optimally spent at the bedside remains unclear. Research to improve the quality of bedside learning and its influence on patient care outcomes is needed.
- ,,,.Improving bedside teaching: findings from a focus group study of learners.Acad Med.2008;83(3):257–264.
- .On bedside teaching.Ann Intern Med.1997;126(3):217–220.
- ,,,.Whither bedside teaching? A focus‐group study of clinical teachers.Acad Med.2003;78(4):384–390.
- .Bedside rounds revisited.N Engl J Med.1997;336(16):1174–1175.
- ,,,.Effect of a physical examination teaching program on the behavior of medical residents.J Gen Intern Med.2005;20(8):710–714.
- ,,,,.Role modeling humanistic behavior: learning bedside manner from the experts.Acad Med.2006;81(7):661–667.
- ,,.Student and patient perspectives on bedside teaching.Med Educ.1997;31(5):341–346.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- ,,,.A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit.Pediatrics.2007;120(2):275–280.
- ,,.Impediments to bed‐side teaching.Med Educ.1998;32(2):159–162.
- ,,, et al.Internal medicine and general surgery residents' attitudes about the ACGME duty hours regulations: a multicenter study.Acad Med.2006;81(12):1052–1058.
- ,,.Resident as teacher: educating the educators.Mt Sinai J Med.2006;73(8):1165–1169.
- ,,,.Improving bedside teaching: findings from a focus group study of learners.Acad Med.2008;83(3):257–264.
- .On bedside teaching.Ann Intern Med.1997;126(3):217–220.
- ,,,.Whither bedside teaching? A focus‐group study of clinical teachers.Acad Med.2003;78(4):384–390.
- .Bedside rounds revisited.N Engl J Med.1997;336(16):1174–1175.
- ,,,.Effect of a physical examination teaching program on the behavior of medical residents.J Gen Intern Med.2005;20(8):710–714.
- ,,,,.Role modeling humanistic behavior: learning bedside manner from the experts.Acad Med.2006;81(7):661–667.
- ,,.Student and patient perspectives on bedside teaching.Med Educ.1997;31(5):341–346.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- ,,,,.The effect of bedside case presentations on patients' perceptions of their medical care.N Engl J Med.1997;336(16):1150–1155.
- ,,,.A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit.Pediatrics.2007;120(2):275–280.
- ,,.Impediments to bed‐side teaching.Med Educ.1998;32(2):159–162.
- ,,, et al.Internal medicine and general surgery residents' attitudes about the ACGME duty hours regulations: a multicenter study.Acad Med.2006;81(12):1052–1058.
- ,,.Resident as teacher: educating the educators.Mt Sinai J Med.2006;73(8):1165–1169.
Copyright © 2009 Society of Hospital Medicine
A numbered day in the life
It seemed like years had passed since he was told he had cancer. While he basked in the cool white ambiance of the examination room, Jim mentally traced his many steps up and down the nearby hospital hallways. From this room to that, he had shuffled through most of the rooms on this hospital ward. Jim had read every outdated Time and National Geographic magazine, and all of the kids' books. From sitting in waiting rooms, he had even developed a deep appreciation for Thomas the Tank Engine. As he sat there, he realized that he had only spent 8 days in this hospital ward. But in here, 8 days might as well be 11 years. Time doesn't so much pass in hospital wards as it stands perfectly still on your chest. The total isolation for those who must stay is startling. Jim had begun judging time by the movements of those lucky enough to go home. Instead of Mondays, Tuesdays, and Wednesdays, he was also measuring time by food. Days of the week had become known as Styrofoam meatloaf, highly suspect lasagna, and inedible beef Wellington; all had become units of time measurement.
When he was told about his cancerthe doctor told him it was a type of hematological neo‐something‐or‐otherJim felt strangely aroused by the news. He felt energy racing through his body as he geared for battle. His immediate response was to think about how he would volunteer for the harshest, meanest, nastiest treatment he could get the doctor to agree with. Poke him full of holes, pour poison straight in his veins, run him on treadmills while doing all of thatit didn't matter. There was nothing he would not do to beat this thing. The doctor had finished telling Jim about all those options at the very minute Jim was ready to hear them. Whatever, whatever, Doc, let's get going with this, was his response.
Then, he had gradually noticed that the pace of medicine was something a little less urgent than he had thought. On TV the doctors run everywhere, but here they walked with a brisk but awkward gait, as if afraid of falling on the floor by going too fast. While waiting for his appointments, Jim noticed that everything was about waitingwaiting for everything. Nothing happens in the hospital. There are people walking everywhere with some projected sense of purpose but it all seems so meaningless when there's a hundred people in hospital uniforms walking past a whole room of patients.
Finally, the door to the examination room burst open and in walked Dr. Day. Standing slightly less than 6 feet tall, Docas Jim called himwas one of those well‐preserved 50‐year‐olds who could be found wind‐surfing his way back to his convertible sports car during his off‐hours. Jim imagined that Doc had been the star student, the handsome rover, the jock. Age had started to claw at his youthful looks, but vanity had led the charge against age for Doc. His behavior and choices worked against his clock, and he was not going quietly into that dark night. Doc's athletic stride made it seem like his feet never touched the floor, and he wafted deep into the room before the doors had even fully opened. Doc never looked forward, but always studied the charts in front of him. He was intense; it was as if he had to truly concentrate sometimes to keep pace with his own mind. Doc was talking, but it was unclear to whom. Finally, he looked up with an expectant pause, and Jim, battered with indifference, nodded in affirmationto what, he didn't care. Doc then gave Jim the thumbs‐up, turned on his heel and headed toward the door; he spun around on the spot and looked back, Yeah, you'll need to change into a robe with nothing on underneath it. He gestured to the one wall where a shelf held neatly folded paper‐thin gowns. Jim put one on and could barely believe how sheer it was. It had the density of a paper napkin, he thought. Then again, this was hardly a cause for modesty. The cancer, he had learned, was actually lymphoma, and it had settled in his groin. At first, Jim was ashamed to have doctors and nurses poke and prod his nether regions, but after a while he became quite causal about it with the usual array of doctors and nurses who populated his monotonous life in this shiny new white palace. After the requisite 15 minutes of unexplained absence, Dr. Day returned through the doors. There was something different about him now, and in a world so dominated by sameness, predictability, and routine, change was a dark storm cloud and sudden wind in Jim's mind.
Lie down on that table please, Doc said in his usual my hand may be making a tactile map of your groin, but my mind is in Bermuda manner. As Jim hopped up on the table and shifted his diseased area closer to the end, the Doc seemed to brighten up, Stay right there, he said as he moved quickly from the room once again. Jim pondered the instruction. As opposed to going where? Jim groaned. He would go somewhere, but his treatment, eventually, would happen here. If he left, he didn't know if would ever come back.
In through the door, one more time, came Dr. Day, but this time he seemed to be leading some kind of tour. Trailing behind him in different states of interest and alertness was a team of young people, all in the little training smocks they give them that look just like the big‐boy coat that Dr. Day wore. Their smocks were more wrinkled and more ivory colored, but they still looked official. Dr. Day hardly looked toward the patient as he smoothly rolled into the side of the table nearest Jim's now‐exposed groin.
Jim looked up between his upstretched knees to see them, all of them, standing around trying to decide if they should be looking at the Doc or looking at the affected area. Jim was embarrassed. Jim was mad. Jim was embarrassed again. He tried to make eye contact with every single visitor in the room, and all he could see were eyes looking straight down under the flap of his hospital gown. Doc had broken into his whole song and dance when he stopped short and looked to Jim, almost apologetically, You're alright with this, right? These are first‐year residents and I wanted them to see this kind of tumor up close. Doc hardly took a full breath and he had turned and was back into his blather about mito, crypto, this, that, and some other bullshit. Jim felt like if he rested his head back maybe no one would ever know that he was the real fleshy cadaver that they stared at that day. He might never see any of these students again, and even if he did, none of them could bring themselves to look him anywhere near the eye anyway. Not much danger that any of them could pick him out of a police lineup, even if he did it without any pants on.
It's important to palpate the region, each of you need to feel what this is like, starting with you. Jim heard this particular instruction and snapped his head up to see exactly where the students were now headed to see and feel the thing they just had to touch. Imagine his dismay when he saw that all of them were still there, still transfixed on whatever they had found to look at studiously during this whole period of time. Doc had motioned to the smallest, frailest, most out‐of‐her‐element young woman he had ever seen. She visibly swallowed hard at the news that she would be first. Her eyes, previously fixed without distraction on some point on Jim's leg, now began to frantically search the eyes of others, possibly looking for some permission to run away. Her eyes met Jim's quite by accident, and she shared with him a look of total and complete shame. He took out his annoyance on her by fixing her with a murderous stare, while he watched as her hand inched ever closer to his leg. In a continuous, but painfully slow motion, she reached under the robe and Jim felt the slightest touch of what he assumed was her finger come to rest on the lowest part of his abdomen. She held her finger there, motionless, and then drew it away quickly while nodding to the Doc. No, no, no, you have to really feel it! Doc chided. He reached down and poked the area firmly, but with a certainty and comfort that comes only from unspoken familiarity. Doc then grabbed the poor girl's hand and guided it and proceeded, with his hand holding her wrist, to make the poking motion with her hand. She was clearly horrified and would have rather been poking through the exposed abdomen of a cadaver at that point. Jim's mood became even more annoyed at her response. It was okay to be embarrassed, but she was now acting like his groin was Elephant‐Man‐esque in its hideousness. He wondered if Doc would set up a barker stand and call to the passers‐by to see the bulbous freak, 50 cents for a viewing! Don't forget about our snack tents! Nobody should go home without candy, everybody loves candy!
After the young resident had endured all that the Doc thought she should, he motioned to the next one and repeated the same process; one after another, after another. The students instinctively formed a line that snaked around the table and spilled out through the room. Jim became callous at this point and began chatting up the students while they stood, quietly waiting their turn for the guided poking, to make them feel even more uncomfortable and intimidated. Jim spied one extremely uncomfortable‐looking male student. You know, if you like this, it doesn't make you gay, Jim shared in an almost caring tone. As the target of his comments shuffled forward, eyes never leaving the floor, Jim targeted another victim with his comments, and then another, and then another. Jim became a sniper of sarcasm, picking off helpless young residents as they stood helplessly in his aim. Doc finally reacted to Jim and shot him a scolding look. Doc leaned into Jim's ear, Fun is fun, but let's just take it easy now, ok? Jim grunted in disagreement, but complied. There was no anger left to vent, and really no need to vent it. Residents weren't the problem, but it was easy to treat them that way. Besides, Jim figured there was many more days for him to make it up to them by being a nicer patient. Today was today, but there were probably 20 more tomorrows for him here.
Finally, the last student had his moment. Jim noticed that the region was now sore from the guiding probing, and Doc had his back to him while addressing the students about what they had seen there today. Jim hopped off the table and proceeded to change back into his clothes while Doc carried on talking. Then, Doc was gone; he sped from the room with his entourage in close pursuit. Jim finished dressing and shuffled down the hallway to his room. Jim sighed under the weight of monotony. Every day was the same, and only the torturous delight he enjoyed at the expense of those residents made the day unique. It was, for the most part, emotion that broke the routine. Emotion was the only thing that Jim controlled at this point, and occasionally, selfishly, he would let it loose on the unsuspecting, simply to bookmark his day. Cancer was not killing Jim, but boredom quite possibly could. As Jim passed the drink machine around the corner from his ward, he saw nurse Janet coming in to work with her neon pink lunch kit slung over her shoulder. She smiled at Jim and asked him how he was feeling. Jim smiled, told her all was getting better, and then made his way back to his room. Janet's arrival meant it was almost supper time, and today was lasagna.
It seemed like years had passed since he was told he had cancer. While he basked in the cool white ambiance of the examination room, Jim mentally traced his many steps up and down the nearby hospital hallways. From this room to that, he had shuffled through most of the rooms on this hospital ward. Jim had read every outdated Time and National Geographic magazine, and all of the kids' books. From sitting in waiting rooms, he had even developed a deep appreciation for Thomas the Tank Engine. As he sat there, he realized that he had only spent 8 days in this hospital ward. But in here, 8 days might as well be 11 years. Time doesn't so much pass in hospital wards as it stands perfectly still on your chest. The total isolation for those who must stay is startling. Jim had begun judging time by the movements of those lucky enough to go home. Instead of Mondays, Tuesdays, and Wednesdays, he was also measuring time by food. Days of the week had become known as Styrofoam meatloaf, highly suspect lasagna, and inedible beef Wellington; all had become units of time measurement.
When he was told about his cancerthe doctor told him it was a type of hematological neo‐something‐or‐otherJim felt strangely aroused by the news. He felt energy racing through his body as he geared for battle. His immediate response was to think about how he would volunteer for the harshest, meanest, nastiest treatment he could get the doctor to agree with. Poke him full of holes, pour poison straight in his veins, run him on treadmills while doing all of thatit didn't matter. There was nothing he would not do to beat this thing. The doctor had finished telling Jim about all those options at the very minute Jim was ready to hear them. Whatever, whatever, Doc, let's get going with this, was his response.
Then, he had gradually noticed that the pace of medicine was something a little less urgent than he had thought. On TV the doctors run everywhere, but here they walked with a brisk but awkward gait, as if afraid of falling on the floor by going too fast. While waiting for his appointments, Jim noticed that everything was about waitingwaiting for everything. Nothing happens in the hospital. There are people walking everywhere with some projected sense of purpose but it all seems so meaningless when there's a hundred people in hospital uniforms walking past a whole room of patients.
Finally, the door to the examination room burst open and in walked Dr. Day. Standing slightly less than 6 feet tall, Docas Jim called himwas one of those well‐preserved 50‐year‐olds who could be found wind‐surfing his way back to his convertible sports car during his off‐hours. Jim imagined that Doc had been the star student, the handsome rover, the jock. Age had started to claw at his youthful looks, but vanity had led the charge against age for Doc. His behavior and choices worked against his clock, and he was not going quietly into that dark night. Doc's athletic stride made it seem like his feet never touched the floor, and he wafted deep into the room before the doors had even fully opened. Doc never looked forward, but always studied the charts in front of him. He was intense; it was as if he had to truly concentrate sometimes to keep pace with his own mind. Doc was talking, but it was unclear to whom. Finally, he looked up with an expectant pause, and Jim, battered with indifference, nodded in affirmationto what, he didn't care. Doc then gave Jim the thumbs‐up, turned on his heel and headed toward the door; he spun around on the spot and looked back, Yeah, you'll need to change into a robe with nothing on underneath it. He gestured to the one wall where a shelf held neatly folded paper‐thin gowns. Jim put one on and could barely believe how sheer it was. It had the density of a paper napkin, he thought. Then again, this was hardly a cause for modesty. The cancer, he had learned, was actually lymphoma, and it had settled in his groin. At first, Jim was ashamed to have doctors and nurses poke and prod his nether regions, but after a while he became quite causal about it with the usual array of doctors and nurses who populated his monotonous life in this shiny new white palace. After the requisite 15 minutes of unexplained absence, Dr. Day returned through the doors. There was something different about him now, and in a world so dominated by sameness, predictability, and routine, change was a dark storm cloud and sudden wind in Jim's mind.
Lie down on that table please, Doc said in his usual my hand may be making a tactile map of your groin, but my mind is in Bermuda manner. As Jim hopped up on the table and shifted his diseased area closer to the end, the Doc seemed to brighten up, Stay right there, he said as he moved quickly from the room once again. Jim pondered the instruction. As opposed to going where? Jim groaned. He would go somewhere, but his treatment, eventually, would happen here. If he left, he didn't know if would ever come back.
In through the door, one more time, came Dr. Day, but this time he seemed to be leading some kind of tour. Trailing behind him in different states of interest and alertness was a team of young people, all in the little training smocks they give them that look just like the big‐boy coat that Dr. Day wore. Their smocks were more wrinkled and more ivory colored, but they still looked official. Dr. Day hardly looked toward the patient as he smoothly rolled into the side of the table nearest Jim's now‐exposed groin.
Jim looked up between his upstretched knees to see them, all of them, standing around trying to decide if they should be looking at the Doc or looking at the affected area. Jim was embarrassed. Jim was mad. Jim was embarrassed again. He tried to make eye contact with every single visitor in the room, and all he could see were eyes looking straight down under the flap of his hospital gown. Doc had broken into his whole song and dance when he stopped short and looked to Jim, almost apologetically, You're alright with this, right? These are first‐year residents and I wanted them to see this kind of tumor up close. Doc hardly took a full breath and he had turned and was back into his blather about mito, crypto, this, that, and some other bullshit. Jim felt like if he rested his head back maybe no one would ever know that he was the real fleshy cadaver that they stared at that day. He might never see any of these students again, and even if he did, none of them could bring themselves to look him anywhere near the eye anyway. Not much danger that any of them could pick him out of a police lineup, even if he did it without any pants on.
It's important to palpate the region, each of you need to feel what this is like, starting with you. Jim heard this particular instruction and snapped his head up to see exactly where the students were now headed to see and feel the thing they just had to touch. Imagine his dismay when he saw that all of them were still there, still transfixed on whatever they had found to look at studiously during this whole period of time. Doc had motioned to the smallest, frailest, most out‐of‐her‐element young woman he had ever seen. She visibly swallowed hard at the news that she would be first. Her eyes, previously fixed without distraction on some point on Jim's leg, now began to frantically search the eyes of others, possibly looking for some permission to run away. Her eyes met Jim's quite by accident, and she shared with him a look of total and complete shame. He took out his annoyance on her by fixing her with a murderous stare, while he watched as her hand inched ever closer to his leg. In a continuous, but painfully slow motion, she reached under the robe and Jim felt the slightest touch of what he assumed was her finger come to rest on the lowest part of his abdomen. She held her finger there, motionless, and then drew it away quickly while nodding to the Doc. No, no, no, you have to really feel it! Doc chided. He reached down and poked the area firmly, but with a certainty and comfort that comes only from unspoken familiarity. Doc then grabbed the poor girl's hand and guided it and proceeded, with his hand holding her wrist, to make the poking motion with her hand. She was clearly horrified and would have rather been poking through the exposed abdomen of a cadaver at that point. Jim's mood became even more annoyed at her response. It was okay to be embarrassed, but she was now acting like his groin was Elephant‐Man‐esque in its hideousness. He wondered if Doc would set up a barker stand and call to the passers‐by to see the bulbous freak, 50 cents for a viewing! Don't forget about our snack tents! Nobody should go home without candy, everybody loves candy!
After the young resident had endured all that the Doc thought she should, he motioned to the next one and repeated the same process; one after another, after another. The students instinctively formed a line that snaked around the table and spilled out through the room. Jim became callous at this point and began chatting up the students while they stood, quietly waiting their turn for the guided poking, to make them feel even more uncomfortable and intimidated. Jim spied one extremely uncomfortable‐looking male student. You know, if you like this, it doesn't make you gay, Jim shared in an almost caring tone. As the target of his comments shuffled forward, eyes never leaving the floor, Jim targeted another victim with his comments, and then another, and then another. Jim became a sniper of sarcasm, picking off helpless young residents as they stood helplessly in his aim. Doc finally reacted to Jim and shot him a scolding look. Doc leaned into Jim's ear, Fun is fun, but let's just take it easy now, ok? Jim grunted in disagreement, but complied. There was no anger left to vent, and really no need to vent it. Residents weren't the problem, but it was easy to treat them that way. Besides, Jim figured there was many more days for him to make it up to them by being a nicer patient. Today was today, but there were probably 20 more tomorrows for him here.
Finally, the last student had his moment. Jim noticed that the region was now sore from the guiding probing, and Doc had his back to him while addressing the students about what they had seen there today. Jim hopped off the table and proceeded to change back into his clothes while Doc carried on talking. Then, Doc was gone; he sped from the room with his entourage in close pursuit. Jim finished dressing and shuffled down the hallway to his room. Jim sighed under the weight of monotony. Every day was the same, and only the torturous delight he enjoyed at the expense of those residents made the day unique. It was, for the most part, emotion that broke the routine. Emotion was the only thing that Jim controlled at this point, and occasionally, selfishly, he would let it loose on the unsuspecting, simply to bookmark his day. Cancer was not killing Jim, but boredom quite possibly could. As Jim passed the drink machine around the corner from his ward, he saw nurse Janet coming in to work with her neon pink lunch kit slung over her shoulder. She smiled at Jim and asked him how he was feeling. Jim smiled, told her all was getting better, and then made his way back to his room. Janet's arrival meant it was almost supper time, and today was lasagna.
It seemed like years had passed since he was told he had cancer. While he basked in the cool white ambiance of the examination room, Jim mentally traced his many steps up and down the nearby hospital hallways. From this room to that, he had shuffled through most of the rooms on this hospital ward. Jim had read every outdated Time and National Geographic magazine, and all of the kids' books. From sitting in waiting rooms, he had even developed a deep appreciation for Thomas the Tank Engine. As he sat there, he realized that he had only spent 8 days in this hospital ward. But in here, 8 days might as well be 11 years. Time doesn't so much pass in hospital wards as it stands perfectly still on your chest. The total isolation for those who must stay is startling. Jim had begun judging time by the movements of those lucky enough to go home. Instead of Mondays, Tuesdays, and Wednesdays, he was also measuring time by food. Days of the week had become known as Styrofoam meatloaf, highly suspect lasagna, and inedible beef Wellington; all had become units of time measurement.
When he was told about his cancerthe doctor told him it was a type of hematological neo‐something‐or‐otherJim felt strangely aroused by the news. He felt energy racing through his body as he geared for battle. His immediate response was to think about how he would volunteer for the harshest, meanest, nastiest treatment he could get the doctor to agree with. Poke him full of holes, pour poison straight in his veins, run him on treadmills while doing all of thatit didn't matter. There was nothing he would not do to beat this thing. The doctor had finished telling Jim about all those options at the very minute Jim was ready to hear them. Whatever, whatever, Doc, let's get going with this, was his response.
Then, he had gradually noticed that the pace of medicine was something a little less urgent than he had thought. On TV the doctors run everywhere, but here they walked with a brisk but awkward gait, as if afraid of falling on the floor by going too fast. While waiting for his appointments, Jim noticed that everything was about waitingwaiting for everything. Nothing happens in the hospital. There are people walking everywhere with some projected sense of purpose but it all seems so meaningless when there's a hundred people in hospital uniforms walking past a whole room of patients.
Finally, the door to the examination room burst open and in walked Dr. Day. Standing slightly less than 6 feet tall, Docas Jim called himwas one of those well‐preserved 50‐year‐olds who could be found wind‐surfing his way back to his convertible sports car during his off‐hours. Jim imagined that Doc had been the star student, the handsome rover, the jock. Age had started to claw at his youthful looks, but vanity had led the charge against age for Doc. His behavior and choices worked against his clock, and he was not going quietly into that dark night. Doc's athletic stride made it seem like his feet never touched the floor, and he wafted deep into the room before the doors had even fully opened. Doc never looked forward, but always studied the charts in front of him. He was intense; it was as if he had to truly concentrate sometimes to keep pace with his own mind. Doc was talking, but it was unclear to whom. Finally, he looked up with an expectant pause, and Jim, battered with indifference, nodded in affirmationto what, he didn't care. Doc then gave Jim the thumbs‐up, turned on his heel and headed toward the door; he spun around on the spot and looked back, Yeah, you'll need to change into a robe with nothing on underneath it. He gestured to the one wall where a shelf held neatly folded paper‐thin gowns. Jim put one on and could barely believe how sheer it was. It had the density of a paper napkin, he thought. Then again, this was hardly a cause for modesty. The cancer, he had learned, was actually lymphoma, and it had settled in his groin. At first, Jim was ashamed to have doctors and nurses poke and prod his nether regions, but after a while he became quite causal about it with the usual array of doctors and nurses who populated his monotonous life in this shiny new white palace. After the requisite 15 minutes of unexplained absence, Dr. Day returned through the doors. There was something different about him now, and in a world so dominated by sameness, predictability, and routine, change was a dark storm cloud and sudden wind in Jim's mind.
Lie down on that table please, Doc said in his usual my hand may be making a tactile map of your groin, but my mind is in Bermuda manner. As Jim hopped up on the table and shifted his diseased area closer to the end, the Doc seemed to brighten up, Stay right there, he said as he moved quickly from the room once again. Jim pondered the instruction. As opposed to going where? Jim groaned. He would go somewhere, but his treatment, eventually, would happen here. If he left, he didn't know if would ever come back.
In through the door, one more time, came Dr. Day, but this time he seemed to be leading some kind of tour. Trailing behind him in different states of interest and alertness was a team of young people, all in the little training smocks they give them that look just like the big‐boy coat that Dr. Day wore. Their smocks were more wrinkled and more ivory colored, but they still looked official. Dr. Day hardly looked toward the patient as he smoothly rolled into the side of the table nearest Jim's now‐exposed groin.
Jim looked up between his upstretched knees to see them, all of them, standing around trying to decide if they should be looking at the Doc or looking at the affected area. Jim was embarrassed. Jim was mad. Jim was embarrassed again. He tried to make eye contact with every single visitor in the room, and all he could see were eyes looking straight down under the flap of his hospital gown. Doc had broken into his whole song and dance when he stopped short and looked to Jim, almost apologetically, You're alright with this, right? These are first‐year residents and I wanted them to see this kind of tumor up close. Doc hardly took a full breath and he had turned and was back into his blather about mito, crypto, this, that, and some other bullshit. Jim felt like if he rested his head back maybe no one would ever know that he was the real fleshy cadaver that they stared at that day. He might never see any of these students again, and even if he did, none of them could bring themselves to look him anywhere near the eye anyway. Not much danger that any of them could pick him out of a police lineup, even if he did it without any pants on.
It's important to palpate the region, each of you need to feel what this is like, starting with you. Jim heard this particular instruction and snapped his head up to see exactly where the students were now headed to see and feel the thing they just had to touch. Imagine his dismay when he saw that all of them were still there, still transfixed on whatever they had found to look at studiously during this whole period of time. Doc had motioned to the smallest, frailest, most out‐of‐her‐element young woman he had ever seen. She visibly swallowed hard at the news that she would be first. Her eyes, previously fixed without distraction on some point on Jim's leg, now began to frantically search the eyes of others, possibly looking for some permission to run away. Her eyes met Jim's quite by accident, and she shared with him a look of total and complete shame. He took out his annoyance on her by fixing her with a murderous stare, while he watched as her hand inched ever closer to his leg. In a continuous, but painfully slow motion, she reached under the robe and Jim felt the slightest touch of what he assumed was her finger come to rest on the lowest part of his abdomen. She held her finger there, motionless, and then drew it away quickly while nodding to the Doc. No, no, no, you have to really feel it! Doc chided. He reached down and poked the area firmly, but with a certainty and comfort that comes only from unspoken familiarity. Doc then grabbed the poor girl's hand and guided it and proceeded, with his hand holding her wrist, to make the poking motion with her hand. She was clearly horrified and would have rather been poking through the exposed abdomen of a cadaver at that point. Jim's mood became even more annoyed at her response. It was okay to be embarrassed, but she was now acting like his groin was Elephant‐Man‐esque in its hideousness. He wondered if Doc would set up a barker stand and call to the passers‐by to see the bulbous freak, 50 cents for a viewing! Don't forget about our snack tents! Nobody should go home without candy, everybody loves candy!
After the young resident had endured all that the Doc thought she should, he motioned to the next one and repeated the same process; one after another, after another. The students instinctively formed a line that snaked around the table and spilled out through the room. Jim became callous at this point and began chatting up the students while they stood, quietly waiting their turn for the guided poking, to make them feel even more uncomfortable and intimidated. Jim spied one extremely uncomfortable‐looking male student. You know, if you like this, it doesn't make you gay, Jim shared in an almost caring tone. As the target of his comments shuffled forward, eyes never leaving the floor, Jim targeted another victim with his comments, and then another, and then another. Jim became a sniper of sarcasm, picking off helpless young residents as they stood helplessly in his aim. Doc finally reacted to Jim and shot him a scolding look. Doc leaned into Jim's ear, Fun is fun, but let's just take it easy now, ok? Jim grunted in disagreement, but complied. There was no anger left to vent, and really no need to vent it. Residents weren't the problem, but it was easy to treat them that way. Besides, Jim figured there was many more days for him to make it up to them by being a nicer patient. Today was today, but there were probably 20 more tomorrows for him here.
Finally, the last student had his moment. Jim noticed that the region was now sore from the guiding probing, and Doc had his back to him while addressing the students about what they had seen there today. Jim hopped off the table and proceeded to change back into his clothes while Doc carried on talking. Then, Doc was gone; he sped from the room with his entourage in close pursuit. Jim finished dressing and shuffled down the hallway to his room. Jim sighed under the weight of monotony. Every day was the same, and only the torturous delight he enjoyed at the expense of those residents made the day unique. It was, for the most part, emotion that broke the routine. Emotion was the only thing that Jim controlled at this point, and occasionally, selfishly, he would let it loose on the unsuspecting, simply to bookmark his day. Cancer was not killing Jim, but boredom quite possibly could. As Jim passed the drink machine around the corner from his ward, he saw nurse Janet coming in to work with her neon pink lunch kit slung over her shoulder. She smiled at Jim and asked him how he was feeling. Jim smiled, told her all was getting better, and then made his way back to his room. Janet's arrival meant it was almost supper time, and today was lasagna.
PRESsed for time
A 36‐year‐old woman was admitted after new‐onset Hseizures. She had been diagnosed with breast cancer 5 years prior to admission. At that time, she underwent left radical mastectomy and lymph node dissection. Lymph nodes were positive for metastatic disease with negative HER‐2‐Neu and positive estrogen and progesterone receptors. She was treated with docetaxel and tamoxifen but subsequently developed metastatic left hip lesions and was treated with letrozole and anastrozole. Three years later, scans revealed further metastatic disease to the liver, lung, and vertebral column. She was subsequently treated with capecitabine, until further disease progression led to the use of carboplatin and paclitaxel. Seven months prior to admission, her cancer was progressing and she was switched to doxorubicin, gemcitabine, and bevacizumab. Six weeks prior to admission, both positron emission tomography (PET) and computed tomography (CT) scan of her whole body and magnetic resonance imaging (MRI) of the brain illustrated significant improvement. Her last dose of bevacizumab was given 3 weeks prior to her admission.
Two weeks prior to admission, patient reported new‐onset daily headache. These were often localized in the occipital region. She reported some associated nausea and occasional emesis. Subsequently, she developed photophobia and phonophobia. On seeking outpatient treatment for her headache, it was noted that her systolic blood pressure had increased from a baseline of 100 mm Hg to 170 mm Hg. On the day prior to admission, she reported severe headache and several episodes of emesis and later that evening had a witnessed tonic‐clonic seizure.
The patient presented to an outside hospital and had an unremarkable noncontrast CT scan of her brain. An examination of her cerebrospinal fluid revealed negative gram stain, and a normal white blood cell count and protein level. She was treated with lorazepam, phenytoin, and decadron. On becoming more alert, she insisted on going home, where she later developed recurrent headache and presented to our emergency room.
On admission to our service, she was noted to be confused and irritable, and unable to provide any history. Her exam revealed a blood pressure of 143/102 mmHg. No localizing neurologic signs were noted and her laboratory values were normal. After sedation, MRI of the brain was obtained (Figure 1). This revealed diffuse and patchy gyriform hyperintensity of the white matter, most consistent with posterior reversible encephalopathy syndrome (PRES).
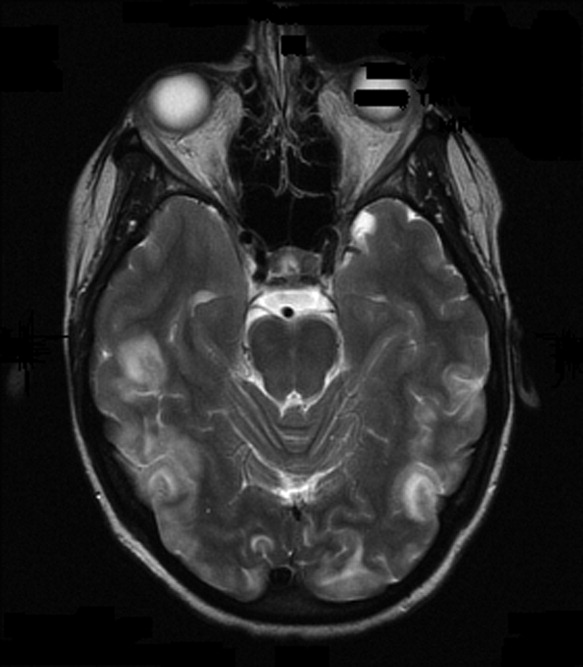
Upon reflection, the patient had new onset hypertension that coincided with the initiation and dosing of bevacizumab. Bevacizumab, an antineoplastic agent, is a recombinant humanized monoclonal antibody that binds to and neutralizes vascular endothelial growth factor, thereby preventing angiogenesis.1 It is known to cause grade 3 hypertension in a minority of patients. Therefore, it was postulated that the patient's persistent blood pressure elevation resulted in vasogenic brain edema, precipitating her seizure. Subsequent to the diagnosis, her blood pressure was aggressively controlled with oral enalapril, metoprolol, triamterene/hydrochlorothiazide, and hydralazine. By hospital day 7, her headache had subsided and her altered mental status had resolved. She had no further episodes of seizures and bevacizumab was discontinued.
PRES has a distinct constellation of clinical symptoms and radiologic findings. The name PRES is a misnomer, as this syndrome is not always reversible, nor is it restricted to the white matter or to the posterior areas of the brain.2 It is hypothesized that a sudden rise in blood pressure leads to elevations in intracranial pressure, which exceeds the brain's autoregulatory mechanisms. This subsequently leads to transudation of fluid into the brain parenchyma. Interestingly, it appears that it is not the absolute level of systolic blood pressure that is critical in the development of PRES, but the rate of change in blood pressure. Hence, patients with chronic hypertension have developed adaptive vascular changes that protect them from this type of parenchymal damage.
PRES has gained increasing recognition due to the use of immunosuppressive and chemotherapeutic medications in organ transplant and oncology patients. Drugs such as cyclosporine, tacrolimus, fludarabine, vincristine, cisplatin, cytarabine, interferon‐alpha, interleukin, antiretroviral therapy, erythropoietin, granulocyte stimulating factor, and intravenous immunoglobulin have all been implicated.3 In addition to increasing blood pressure, these agents likely cause direct toxic injury to the brain, disrupting the blood‐brain barrier and resulting in subsequent edema. Other conditions associated with PRES include renal disease, vasculitis, endocrine disorders, porphyria, cocaine or amphetamine abuse, and stimulant abuse.
Clinically, PRES can present as headache, altered mental status, confusion, drowsiness progressing to stupor, emesis, abnormal visual perceptions, visual neglect, cortical blindness, difficulty with memory and concentration, brisk deep tendon reflexes, weakness, ataxia, and seizure activity. PRES has a characteristic appearance on neuroimaging that differentiates it from other forms of hypertensive encephalopathy. Edema of the white or gray matter in the posterior cerebral hemispheres, particularly the bilateral parietooccipital regions, is seen. PRES can also diffusely involve the brain stem, cerebellum, basal ganglia, and the frontal lobes. Abnormalities on neuroimaging are often symmetric but clinical manifestations can be asymmetric. MRI and CT scans can both be utilized for characterization of PRES.4
There are currently no published guidelines for the management of PRES. Expert opinion suggests removing the underlying cause and aggressively treating the hypertension.5 Furthermore, initiation and duration of antiepileptics remains controversial. After aggressive blood pressure control, resolution of findings on neuroimaging studies are expected anywhere from 8 days to 17 months.
Timely recognition of PRES is critical for prevention of further neurologic compromise. Immediate discontinuation of offending agents, as well as aggressive treatment of blood pressure, is the cornerstone treatment for PRES. In the future, a better understanding of the pathophysiology of PRES can lead to improved diagnostic and management options.
- ,,.Reversible posterior leukoencephalopathy syndrome and bevacizumab.N Engl J Med.2006;354(9):980–982.
- ,,, et al.A reversible posterior leukoencephalopathy syndrome.N Engl J Med.1996;334(8):494–500.
- ,,,,,.Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies.Am J Hematol.2004;77(1):72–76.
- ,,, et al.Posterior leukoencephalopathy without severe hypertension: utility of diffusion‐weighted MRI.Neurology.1998;51(5):1369–1376.
- .Posterior leukoencephalopathy syndrome.Postgrad Med J.2001;77(903):24–28.
A 36‐year‐old woman was admitted after new‐onset Hseizures. She had been diagnosed with breast cancer 5 years prior to admission. At that time, she underwent left radical mastectomy and lymph node dissection. Lymph nodes were positive for metastatic disease with negative HER‐2‐Neu and positive estrogen and progesterone receptors. She was treated with docetaxel and tamoxifen but subsequently developed metastatic left hip lesions and was treated with letrozole and anastrozole. Three years later, scans revealed further metastatic disease to the liver, lung, and vertebral column. She was subsequently treated with capecitabine, until further disease progression led to the use of carboplatin and paclitaxel. Seven months prior to admission, her cancer was progressing and she was switched to doxorubicin, gemcitabine, and bevacizumab. Six weeks prior to admission, both positron emission tomography (PET) and computed tomography (CT) scan of her whole body and magnetic resonance imaging (MRI) of the brain illustrated significant improvement. Her last dose of bevacizumab was given 3 weeks prior to her admission.
Two weeks prior to admission, patient reported new‐onset daily headache. These were often localized in the occipital region. She reported some associated nausea and occasional emesis. Subsequently, she developed photophobia and phonophobia. On seeking outpatient treatment for her headache, it was noted that her systolic blood pressure had increased from a baseline of 100 mm Hg to 170 mm Hg. On the day prior to admission, she reported severe headache and several episodes of emesis and later that evening had a witnessed tonic‐clonic seizure.
The patient presented to an outside hospital and had an unremarkable noncontrast CT scan of her brain. An examination of her cerebrospinal fluid revealed negative gram stain, and a normal white blood cell count and protein level. She was treated with lorazepam, phenytoin, and decadron. On becoming more alert, she insisted on going home, where she later developed recurrent headache and presented to our emergency room.
On admission to our service, she was noted to be confused and irritable, and unable to provide any history. Her exam revealed a blood pressure of 143/102 mmHg. No localizing neurologic signs were noted and her laboratory values were normal. After sedation, MRI of the brain was obtained (Figure 1). This revealed diffuse and patchy gyriform hyperintensity of the white matter, most consistent with posterior reversible encephalopathy syndrome (PRES).

Upon reflection, the patient had new onset hypertension that coincided with the initiation and dosing of bevacizumab. Bevacizumab, an antineoplastic agent, is a recombinant humanized monoclonal antibody that binds to and neutralizes vascular endothelial growth factor, thereby preventing angiogenesis.1 It is known to cause grade 3 hypertension in a minority of patients. Therefore, it was postulated that the patient's persistent blood pressure elevation resulted in vasogenic brain edema, precipitating her seizure. Subsequent to the diagnosis, her blood pressure was aggressively controlled with oral enalapril, metoprolol, triamterene/hydrochlorothiazide, and hydralazine. By hospital day 7, her headache had subsided and her altered mental status had resolved. She had no further episodes of seizures and bevacizumab was discontinued.
PRES has a distinct constellation of clinical symptoms and radiologic findings. The name PRES is a misnomer, as this syndrome is not always reversible, nor is it restricted to the white matter or to the posterior areas of the brain.2 It is hypothesized that a sudden rise in blood pressure leads to elevations in intracranial pressure, which exceeds the brain's autoregulatory mechanisms. This subsequently leads to transudation of fluid into the brain parenchyma. Interestingly, it appears that it is not the absolute level of systolic blood pressure that is critical in the development of PRES, but the rate of change in blood pressure. Hence, patients with chronic hypertension have developed adaptive vascular changes that protect them from this type of parenchymal damage.
PRES has gained increasing recognition due to the use of immunosuppressive and chemotherapeutic medications in organ transplant and oncology patients. Drugs such as cyclosporine, tacrolimus, fludarabine, vincristine, cisplatin, cytarabine, interferon‐alpha, interleukin, antiretroviral therapy, erythropoietin, granulocyte stimulating factor, and intravenous immunoglobulin have all been implicated.3 In addition to increasing blood pressure, these agents likely cause direct toxic injury to the brain, disrupting the blood‐brain barrier and resulting in subsequent edema. Other conditions associated with PRES include renal disease, vasculitis, endocrine disorders, porphyria, cocaine or amphetamine abuse, and stimulant abuse.
Clinically, PRES can present as headache, altered mental status, confusion, drowsiness progressing to stupor, emesis, abnormal visual perceptions, visual neglect, cortical blindness, difficulty with memory and concentration, brisk deep tendon reflexes, weakness, ataxia, and seizure activity. PRES has a characteristic appearance on neuroimaging that differentiates it from other forms of hypertensive encephalopathy. Edema of the white or gray matter in the posterior cerebral hemispheres, particularly the bilateral parietooccipital regions, is seen. PRES can also diffusely involve the brain stem, cerebellum, basal ganglia, and the frontal lobes. Abnormalities on neuroimaging are often symmetric but clinical manifestations can be asymmetric. MRI and CT scans can both be utilized for characterization of PRES.4
There are currently no published guidelines for the management of PRES. Expert opinion suggests removing the underlying cause and aggressively treating the hypertension.5 Furthermore, initiation and duration of antiepileptics remains controversial. After aggressive blood pressure control, resolution of findings on neuroimaging studies are expected anywhere from 8 days to 17 months.
Timely recognition of PRES is critical for prevention of further neurologic compromise. Immediate discontinuation of offending agents, as well as aggressive treatment of blood pressure, is the cornerstone treatment for PRES. In the future, a better understanding of the pathophysiology of PRES can lead to improved diagnostic and management options.
A 36‐year‐old woman was admitted after new‐onset Hseizures. She had been diagnosed with breast cancer 5 years prior to admission. At that time, she underwent left radical mastectomy and lymph node dissection. Lymph nodes were positive for metastatic disease with negative HER‐2‐Neu and positive estrogen and progesterone receptors. She was treated with docetaxel and tamoxifen but subsequently developed metastatic left hip lesions and was treated with letrozole and anastrozole. Three years later, scans revealed further metastatic disease to the liver, lung, and vertebral column. She was subsequently treated with capecitabine, until further disease progression led to the use of carboplatin and paclitaxel. Seven months prior to admission, her cancer was progressing and she was switched to doxorubicin, gemcitabine, and bevacizumab. Six weeks prior to admission, both positron emission tomography (PET) and computed tomography (CT) scan of her whole body and magnetic resonance imaging (MRI) of the brain illustrated significant improvement. Her last dose of bevacizumab was given 3 weeks prior to her admission.
Two weeks prior to admission, patient reported new‐onset daily headache. These were often localized in the occipital region. She reported some associated nausea and occasional emesis. Subsequently, she developed photophobia and phonophobia. On seeking outpatient treatment for her headache, it was noted that her systolic blood pressure had increased from a baseline of 100 mm Hg to 170 mm Hg. On the day prior to admission, she reported severe headache and several episodes of emesis and later that evening had a witnessed tonic‐clonic seizure.
The patient presented to an outside hospital and had an unremarkable noncontrast CT scan of her brain. An examination of her cerebrospinal fluid revealed negative gram stain, and a normal white blood cell count and protein level. She was treated with lorazepam, phenytoin, and decadron. On becoming more alert, she insisted on going home, where she later developed recurrent headache and presented to our emergency room.
On admission to our service, she was noted to be confused and irritable, and unable to provide any history. Her exam revealed a blood pressure of 143/102 mmHg. No localizing neurologic signs were noted and her laboratory values were normal. After sedation, MRI of the brain was obtained (Figure 1). This revealed diffuse and patchy gyriform hyperintensity of the white matter, most consistent with posterior reversible encephalopathy syndrome (PRES).

Upon reflection, the patient had new onset hypertension that coincided with the initiation and dosing of bevacizumab. Bevacizumab, an antineoplastic agent, is a recombinant humanized monoclonal antibody that binds to and neutralizes vascular endothelial growth factor, thereby preventing angiogenesis.1 It is known to cause grade 3 hypertension in a minority of patients. Therefore, it was postulated that the patient's persistent blood pressure elevation resulted in vasogenic brain edema, precipitating her seizure. Subsequent to the diagnosis, her blood pressure was aggressively controlled with oral enalapril, metoprolol, triamterene/hydrochlorothiazide, and hydralazine. By hospital day 7, her headache had subsided and her altered mental status had resolved. She had no further episodes of seizures and bevacizumab was discontinued.
PRES has a distinct constellation of clinical symptoms and radiologic findings. The name PRES is a misnomer, as this syndrome is not always reversible, nor is it restricted to the white matter or to the posterior areas of the brain.2 It is hypothesized that a sudden rise in blood pressure leads to elevations in intracranial pressure, which exceeds the brain's autoregulatory mechanisms. This subsequently leads to transudation of fluid into the brain parenchyma. Interestingly, it appears that it is not the absolute level of systolic blood pressure that is critical in the development of PRES, but the rate of change in blood pressure. Hence, patients with chronic hypertension have developed adaptive vascular changes that protect them from this type of parenchymal damage.
PRES has gained increasing recognition due to the use of immunosuppressive and chemotherapeutic medications in organ transplant and oncology patients. Drugs such as cyclosporine, tacrolimus, fludarabine, vincristine, cisplatin, cytarabine, interferon‐alpha, interleukin, antiretroviral therapy, erythropoietin, granulocyte stimulating factor, and intravenous immunoglobulin have all been implicated.3 In addition to increasing blood pressure, these agents likely cause direct toxic injury to the brain, disrupting the blood‐brain barrier and resulting in subsequent edema. Other conditions associated with PRES include renal disease, vasculitis, endocrine disorders, porphyria, cocaine or amphetamine abuse, and stimulant abuse.
Clinically, PRES can present as headache, altered mental status, confusion, drowsiness progressing to stupor, emesis, abnormal visual perceptions, visual neglect, cortical blindness, difficulty with memory and concentration, brisk deep tendon reflexes, weakness, ataxia, and seizure activity. PRES has a characteristic appearance on neuroimaging that differentiates it from other forms of hypertensive encephalopathy. Edema of the white or gray matter in the posterior cerebral hemispheres, particularly the bilateral parietooccipital regions, is seen. PRES can also diffusely involve the brain stem, cerebellum, basal ganglia, and the frontal lobes. Abnormalities on neuroimaging are often symmetric but clinical manifestations can be asymmetric. MRI and CT scans can both be utilized for characterization of PRES.4
There are currently no published guidelines for the management of PRES. Expert opinion suggests removing the underlying cause and aggressively treating the hypertension.5 Furthermore, initiation and duration of antiepileptics remains controversial. After aggressive blood pressure control, resolution of findings on neuroimaging studies are expected anywhere from 8 days to 17 months.
Timely recognition of PRES is critical for prevention of further neurologic compromise. Immediate discontinuation of offending agents, as well as aggressive treatment of blood pressure, is the cornerstone treatment for PRES. In the future, a better understanding of the pathophysiology of PRES can lead to improved diagnostic and management options.
- ,,.Reversible posterior leukoencephalopathy syndrome and bevacizumab.N Engl J Med.2006;354(9):980–982.
- ,,, et al.A reversible posterior leukoencephalopathy syndrome.N Engl J Med.1996;334(8):494–500.
- ,,,,,.Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies.Am J Hematol.2004;77(1):72–76.
- ,,, et al.Posterior leukoencephalopathy without severe hypertension: utility of diffusion‐weighted MRI.Neurology.1998;51(5):1369–1376.
- .Posterior leukoencephalopathy syndrome.Postgrad Med J.2001;77(903):24–28.
- ,,.Reversible posterior leukoencephalopathy syndrome and bevacizumab.N Engl J Med.2006;354(9):980–982.
- ,,, et al.A reversible posterior leukoencephalopathy syndrome.N Engl J Med.1996;334(8):494–500.
- ,,,,,.Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies.Am J Hematol.2004;77(1):72–76.
- ,,, et al.Posterior leukoencephalopathy without severe hypertension: utility of diffusion‐weighted MRI.Neurology.1998;51(5):1369–1376.
- .Posterior leukoencephalopathy syndrome.Postgrad Med J.2001;77(903):24–28.
Pemetrexed
Dr. Chandra P. Belani reports that maintenance therapy with pemetrexed significantly improves overall survival in advanced non-small cell lung cancer. Damian McNamara of the Global Medical News Network (GMNN) reports from the annual meeting of the American Society of Clinical Oncology in Orlando.
Dr. Chandra P. Belani reports that maintenance therapy with pemetrexed significantly improves overall survival in advanced non-small cell lung cancer. Damian McNamara of the Global Medical News Network (GMNN) reports from the annual meeting of the American Society of Clinical Oncology in Orlando.
Dr. Chandra P. Belani reports that maintenance therapy with pemetrexed significantly improves overall survival in advanced non-small cell lung cancer. Damian McNamara of the Global Medical News Network (GMNN) reports from the annual meeting of the American Society of Clinical Oncology in Orlando.
Elderly Patients & Pneumonia, Metoprolol after Vascular Surgery, and More
Elderly Pneumonia Patients after Antibiotic Switch
Nathan RV, Rhew DC, Bratzler DW, et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006 Jun;119(6):512.e1-7.
Community-acquired pneumonia (CAP) continues to be a common reason for hospital admission—especially among the elderly. As with many infectious diseases, the duration and route of antibiotic therapy is often based on expert recommendations rather than prospective randomized trials. The Patient Outcome Research Team (PORT) trials address the decision to admit a patient, but not other aspects of care. For hospitalists, the decision of when to discharge any patient with reasonable safety is often fraught with uncertainty. This study addresses the necessity of observing a patient for one day following the switch from IV to oral therapy. Two previous smaller retrospective studies have suggested this was unnecessary.
The current study is also retrospective but involves a large database derived from the U.S. Medicare National Pneumonia Project database. Ultimately 5,248 patients over 65 (mean age=80) were selected for analysis; 2,536 were not observed; and 2,712 were observed for one day.) Patients were excluded if their length of stay was greater than seven days or less than two days, suggesting complicated cases in the former instance and mild illness in the latter (i.e., perhaps not even requiring admission). Immunosupressed patients were also excluded. There was no significant difference in the observed 30-day mortality (5.1% in the “not observed” versus 4.4% in the “observed” cohort, respectively).
The obvious limitation of this study is that it was retrospective/observational and thus potentially subject to the bias inherent in this study design. It is possible that the sicker patients were logically watched longer. Propensity analysis was not a component of this study. The authors do present reasons why certain structural weaknesses would have favored the “observed “group.
Certainly there may be other reasons to observe a patient after the switch to oral therapy. A patient with associated gastrointestinal disturbance or a questionable history of GI or other intolerance to a class of antibiotics is an obvious example. Nevertheless, this study should convey a certain confidence to hospitalists when they assess the suitability for discharge for the type of patient covered in this analysis. Interestingly the recently published guidelines for treatment of community acquired pneumonia are concordant with this study.1
Reference
- Mandell L, Wundrelink A, Bartlett J. Guideline for the treatment of community acquired pneumonia. Clin Infect Dis. 2007;44: S27-72.
The Revised Geneva Score for PE
Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department. Ann Intern Med. 2006 Feb 7:144(3):165-171. Comment in: ACP J Club. 2006 Jul-Aug;145(1):25 & Ann Intern Med. 2006 Feb 7;144(3):210-212.
Pulmonary embolism is a diagnosis frequently considered by the hospitalist—both as an explanation for the admitting clinical picture, as well as a complication arising during the course of a hospitalization for another condition.
My institutions’ ability to identify patients with this potentially lethal condition has greatly improved with the advent of multidetector CT angiography and various diagnostic schemata that include d-dimer testing and estimations of pre-test probability. It is a classic consideration whenever there is a onset of pleurisy, dyspnea, or aggravation thereof. Nevertheless multiple other situations arise in the hospital setting, such as unexplained tachycardia, hemoptysis, or vaguely possible but not clear-cut pleuritic chest pain, in which one feels obligated to at least consider the diagnosis. Further, to have to incorporate d-dimer testing into the diagnostic strategy is problematic as up to 80% of hospitalized patients are likely to be positive. Hospitalists need a reasonable strategy to avoid going down that proverbial pathway in certain low risk situations.
The Geneva scoring system and the Wells system are two methodologies that have been used in lieu of or as an adjunct to “clinical judgment.” The former requires arterial blood gases and the latter has as criteria “other diagnosis more likely than pulmonary embolus” that can be problematic and difficult to standardize.
This article presents a revised Geneva scoring system based solely on elements of the history and physical examination. The elements were derived retrospectively from a prior different study on diagnostic strategies for pulmonary thromboembolism (PTE). A different prospective study on PTE was utilized for the validation arm of this study. By logistical regression analysis the following eight elements were incorporated into the revised Geneva score: Age greater than 65 (1 point), previous deep venous thrombosis or pulmonary embolism (3 points), surgery or fracture within one month (2 points), active malignant condition (2 points), unilateral lower limb pain (3 points), hemoptysis (2 points), heart rate 75 to 94 beats/min (3 points) or heart rate 95 beats /minute or more (5 points), and pain on lower limb palpation and unilateral edema (4 points). The prevalence for pulmonary embolism was as follows: low probability or 8% (0 to 3 points), intermediate probability or 28% (4 to 10 points), and high probability or 74% (equal or greater than 11 points).
Significance for hospitalists: This scoring system is not validated a management system per se. However in the imperfect world of clinical reasoning it can help reinforce a thoughtful decision not to embark on the diagnostic path for pulmonary embolism, with its own inherent risks.
Metoprolol after Vascular Surgery
Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of metoprolol after vascular surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006 Nov;152(5):983-990. Comment in Am Heart J. 2006 Nov;152(5):815-818. McCullough PA. Failure of beta-blockers in the reduction of perioperative events: where did we go wrong? Am Heart J. 2006 Nov;152(5):815-818. Comment in: Am Heart J. 2006 Nov;152(5):983-990.
Hospitalists are frequently consulted regarding perioperative risk assessment and reduction for patients undergoing non-cardiac surgery. Over the last decade and supported by a few studies, the perioperative use of beta-blocker therapy has resolved the uncertainty frequently encountered. The McFalls study in 2004 showed no benefit to routine coronary revascularization for patients undergoing vascular surgery deemed at risk for myocardial ischemia.1 This provided further confidence for those of us supplying these preoperative assessments. However, the Lindenauer study in 2005 (a retrospective cohort analysis) was the first indication that perioperative beta blockade could be harmful.2 Lower-risk patients based on the revised cardiovascular index (RCRI) score actually did worse when treated. Still the ACC guidelines published in 2006 suggested perioperative beta blockers be considered for lower risk patients undergoing vascular surgery.3
This study is a randomized placebo-controlled trial of perioperative beta-blocker therapy in 500 treatment-naïve patients undergoing vascular surgery. Metoprolol was started two hours before surgery and continued for one week. Cardiovascular endpoints included cardiac death, arrhythmia requiring treatment, acute myocardial infarction or acute coronary syndrome, and congestive heart failure. No benefit was found for treatment with metoprolol regardless of the number of Revised Cardiac Risk Index (RCRI) factors present. No excess adverse outcomes were noted for therapy although intraoperative bradycardia and hypotension were significantly increased in the active treatment group.
In the accompanying editorial McCullough discusses possible reasons and implications of these findings. In fact, two other trials have reported similar findings. In contrast to the older trials suggesting a benefit to perioperative beta blockade these newer trials are larger and have a stronger design. He also notes that the patients in the more recent trials are more likely to have prior revascularization and hence are less prone to demand-type events, reflective of the type of insult beta blockade would most likely be helpful in preventing. These events may be more closely allied with plaque destabilization of subcritical lesions, with factors such as perioperative hypercoagulability and perhaps inflammation being more important. In this regard it is notable that recent trials on the perioperative use of statins have demonstrated favorable results, with these agents presumably acting to promote plaque stability, the so-called “pleiotropic” function of statins.
Significance for hospitalists: It is reasonable to be more circumspect in the recommendation of perioperative beta blockade. This practice is not likely the magic bullet, which is a common misconcpetion. An indicative situation is an 80-year-old patient undergoing total hip replacement. He has diabetes, COPD, and hypertension, a pulse of 65, a blood pressure of 110/50. There may also be concerns about bradycardia, hypotension, and bronchospasm. Given this analysis a clinician can be confident in withholding perioperative treatment.
The use of beta-blocker therapy in a patient with a single RCRI factor, which is not coronary artery disease, does not seem justified. On the other hand the use of perioperative statins should be more actively entertained. Emerging recommendations from various specialty organizations and other relevant professional entities should be anticipated and sought.
Bibliography
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Eng J Med. 2004Dec 30;351(27):2795-2804.
- Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Eng J Med. 2005 Jul 28;353(4):349-361.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery. J Am Coll Cardiol. 2006;47: 2343-2355
The New C. Diff Epidemic
Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006 Nov 21;145(10):758-764.
C. difficile infection is emerging as one of the most important illnesses for hospitalists to be facile with. It not only occurs frequently, but is also often severe or life threatening, and most importantly iatrogenic and preventable. This review by Bartlett, who elucidated the cause of this disease in 1978, reviews important up-to-date information on C. difficile, focusing on the recent emergence of a more virulent form of the disease.
Infectious diarrhea that develops in the hospital is almost always due to C. difficile. The tissue culture cytotoxic assay (first described in 1978) remains the most sensitive and specific diagnostic tool. The toxin immunoassay used most routinely is only 75% sensitive.
An epidemic of unusually severe C. difficile was first described in Quebec in 2001. Important features include a higher tendency for toxic megacolon and a need for colectomy, protein-losing enteropathy, leukemoid reactions, refractoriness to treatment, a high rate of relapse and an astonishing 16.5% attributable mortality. Fluoroquinolones are the leading associated antibiotic causal factor, although extended spectrum cephalosporins remain important as well in this regard. The new strain is characterized by high levels of toxin production due to the deletion of a toxin production regulatory gene. The strain is also fluoroquinolone resistant, explaining the role of that antibiotic in its genesis.
Treatment of C. difficile colitis (especially the emergent strain) remains problematic. In particular the role of metronidazole versus vancomycin as initial therapy is often contentious. Bartlett cites some evidence suggesting vancomycin may be more effective and is especially recommended for severe disease, characteristics of which are often manifested by this new strain.
This review cites important considerations that hospitalist ought to vigilant and proactive in. Given the high risk of fluoroquinolone treatment we must be sure that these drugs are used appropriately. Nonchalantly stacking on levofloxacin therapy for the COPD flair without evidence for pneumonia should be discouraged. When possible antibiotics with a lower risk for C. difficile (sulfonamides, macrolides, tetracyclines) should be used for any infection. When disease is suspected, a negative toxin immunoassay should not discourage empiric treatment especially in a very ill patient. Isolation and barrier precautions are important in preventing the spread of this potentially lethal infection. C. difficile spores are not killed by alcohol-based detergents, and either soap and water or gloves are necessary to care for these patients. When your hospital experiences a clustering of severe C. difficile infection, alert appropriate infection control personnel. Administrative control of antibiotic use may be indicated.
Baclofen Versus Diazepam to Treat Alcohol Withdrawal
Addolorato G, Leggio L, Abenavoli L, et al. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006 Mar;119 (3):276.e13-18.
Alcohol withdrawal syndrome (AWS) is a frequent problem encountered in hospitalized patients; its management is considered one of SHM’s core competencies for hospitalists. Benzodiazepines are the gold standard of therapy for this problem given their established record for safety and efficacy; however, their use can be problematic in certain instances.
This study involved 37 outpatients, as inpatients may often be sicker and at higher risk of severe withdrawal.
There is a definite risk of oversedation—especially in patients with COPD or chronic liver disease. Some patients require inordinately high doses of benzodiazepines, thus setting the stage for a prolonged hospitalization. Occasional paradoxical or disinhibition reactions to benzodiazepines can also be problematic. Addiction and or diversion are also a concern in patients prone to substance abuse. An otherwise stable patient, ready for discharge, may still be on a relatively high dose of lorazepam, but it is generally not prudent to send the patient out with a supply of medication to finish the course given concerns over resumption of drinking while on the sedative. Conversely, the solution can be cold comfort for the attending physician if the patient resumes drinking, thus eliminating the need for additional medication.
Baclofen, a stereoselective gamma-aminobutyric acid agonist, has a long history of safety in the treatment of spasticity. As such it can counter balance the activation of the glutamate excitatory pathway that characterizes AWS. It has been proposed as an alternative treatment for AWS that would not share the above concerns cited for benzodiazepines.
This study is a randomized controlled trial of baclofen versus valium in the treatment for AWS. Thirty-seven subjects with a history of heavy alcohol use were randomized to either baclofen 30 mg per day or valium 0.5 to 0.75 mg/kg. All were outpatients treated for 10 days. Clinical Institute Withdrawal Assessment-Alcohol (CIWA) scores were assessed daily. Both regimens continuously decreased the baseline elevation of CIWA scores daily over the course of the study, without a significant difference in treatment efficacy. No adverse events or side effects were reported in either group.
Other than baseline CIWA and daily alcohol consumption, it is not clear that the two groups were at equal risk for severe withdrawal reactions. Relevant baseline characteristics such as history of seizures or delirium tremens, factors that raise this risk were not noted.
Significance for hospitalists: With a long history of safety and efficacy, benzodiazepines remain the drugs of choice for hospitalists treating patients with AWS. In certain instances it may be desirable to limit or even avoid their use. How effective and safe baclofen would be in filling this role remains to be fully established. In particular the relative risk for sedation and respiratory depression has not been defined. Nevertheless at least in my institutions, as guided by expert consultation, its use has been carefully considered and proven helpful in some of the situations noted above. TH
Elderly Pneumonia Patients after Antibiotic Switch
Nathan RV, Rhew DC, Bratzler DW, et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006 Jun;119(6):512.e1-7.
Community-acquired pneumonia (CAP) continues to be a common reason for hospital admission—especially among the elderly. As with many infectious diseases, the duration and route of antibiotic therapy is often based on expert recommendations rather than prospective randomized trials. The Patient Outcome Research Team (PORT) trials address the decision to admit a patient, but not other aspects of care. For hospitalists, the decision of when to discharge any patient with reasonable safety is often fraught with uncertainty. This study addresses the necessity of observing a patient for one day following the switch from IV to oral therapy. Two previous smaller retrospective studies have suggested this was unnecessary.
The current study is also retrospective but involves a large database derived from the U.S. Medicare National Pneumonia Project database. Ultimately 5,248 patients over 65 (mean age=80) were selected for analysis; 2,536 were not observed; and 2,712 were observed for one day.) Patients were excluded if their length of stay was greater than seven days or less than two days, suggesting complicated cases in the former instance and mild illness in the latter (i.e., perhaps not even requiring admission). Immunosupressed patients were also excluded. There was no significant difference in the observed 30-day mortality (5.1% in the “not observed” versus 4.4% in the “observed” cohort, respectively).
The obvious limitation of this study is that it was retrospective/observational and thus potentially subject to the bias inherent in this study design. It is possible that the sicker patients were logically watched longer. Propensity analysis was not a component of this study. The authors do present reasons why certain structural weaknesses would have favored the “observed “group.
Certainly there may be other reasons to observe a patient after the switch to oral therapy. A patient with associated gastrointestinal disturbance or a questionable history of GI or other intolerance to a class of antibiotics is an obvious example. Nevertheless, this study should convey a certain confidence to hospitalists when they assess the suitability for discharge for the type of patient covered in this analysis. Interestingly the recently published guidelines for treatment of community acquired pneumonia are concordant with this study.1
Reference
- Mandell L, Wundrelink A, Bartlett J. Guideline for the treatment of community acquired pneumonia. Clin Infect Dis. 2007;44: S27-72.
The Revised Geneva Score for PE
Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department. Ann Intern Med. 2006 Feb 7:144(3):165-171. Comment in: ACP J Club. 2006 Jul-Aug;145(1):25 & Ann Intern Med. 2006 Feb 7;144(3):210-212.
Pulmonary embolism is a diagnosis frequently considered by the hospitalist—both as an explanation for the admitting clinical picture, as well as a complication arising during the course of a hospitalization for another condition.
My institutions’ ability to identify patients with this potentially lethal condition has greatly improved with the advent of multidetector CT angiography and various diagnostic schemata that include d-dimer testing and estimations of pre-test probability. It is a classic consideration whenever there is a onset of pleurisy, dyspnea, or aggravation thereof. Nevertheless multiple other situations arise in the hospital setting, such as unexplained tachycardia, hemoptysis, or vaguely possible but not clear-cut pleuritic chest pain, in which one feels obligated to at least consider the diagnosis. Further, to have to incorporate d-dimer testing into the diagnostic strategy is problematic as up to 80% of hospitalized patients are likely to be positive. Hospitalists need a reasonable strategy to avoid going down that proverbial pathway in certain low risk situations.
The Geneva scoring system and the Wells system are two methodologies that have been used in lieu of or as an adjunct to “clinical judgment.” The former requires arterial blood gases and the latter has as criteria “other diagnosis more likely than pulmonary embolus” that can be problematic and difficult to standardize.
This article presents a revised Geneva scoring system based solely on elements of the history and physical examination. The elements were derived retrospectively from a prior different study on diagnostic strategies for pulmonary thromboembolism (PTE). A different prospective study on PTE was utilized for the validation arm of this study. By logistical regression analysis the following eight elements were incorporated into the revised Geneva score: Age greater than 65 (1 point), previous deep venous thrombosis or pulmonary embolism (3 points), surgery or fracture within one month (2 points), active malignant condition (2 points), unilateral lower limb pain (3 points), hemoptysis (2 points), heart rate 75 to 94 beats/min (3 points) or heart rate 95 beats /minute or more (5 points), and pain on lower limb palpation and unilateral edema (4 points). The prevalence for pulmonary embolism was as follows: low probability or 8% (0 to 3 points), intermediate probability or 28% (4 to 10 points), and high probability or 74% (equal or greater than 11 points).
Significance for hospitalists: This scoring system is not validated a management system per se. However in the imperfect world of clinical reasoning it can help reinforce a thoughtful decision not to embark on the diagnostic path for pulmonary embolism, with its own inherent risks.
Metoprolol after Vascular Surgery
Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of metoprolol after vascular surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006 Nov;152(5):983-990. Comment in Am Heart J. 2006 Nov;152(5):815-818. McCullough PA. Failure of beta-blockers in the reduction of perioperative events: where did we go wrong? Am Heart J. 2006 Nov;152(5):815-818. Comment in: Am Heart J. 2006 Nov;152(5):983-990.
Hospitalists are frequently consulted regarding perioperative risk assessment and reduction for patients undergoing non-cardiac surgery. Over the last decade and supported by a few studies, the perioperative use of beta-blocker therapy has resolved the uncertainty frequently encountered. The McFalls study in 2004 showed no benefit to routine coronary revascularization for patients undergoing vascular surgery deemed at risk for myocardial ischemia.1 This provided further confidence for those of us supplying these preoperative assessments. However, the Lindenauer study in 2005 (a retrospective cohort analysis) was the first indication that perioperative beta blockade could be harmful.2 Lower-risk patients based on the revised cardiovascular index (RCRI) score actually did worse when treated. Still the ACC guidelines published in 2006 suggested perioperative beta blockers be considered for lower risk patients undergoing vascular surgery.3
This study is a randomized placebo-controlled trial of perioperative beta-blocker therapy in 500 treatment-naïve patients undergoing vascular surgery. Metoprolol was started two hours before surgery and continued for one week. Cardiovascular endpoints included cardiac death, arrhythmia requiring treatment, acute myocardial infarction or acute coronary syndrome, and congestive heart failure. No benefit was found for treatment with metoprolol regardless of the number of Revised Cardiac Risk Index (RCRI) factors present. No excess adverse outcomes were noted for therapy although intraoperative bradycardia and hypotension were significantly increased in the active treatment group.
In the accompanying editorial McCullough discusses possible reasons and implications of these findings. In fact, two other trials have reported similar findings. In contrast to the older trials suggesting a benefit to perioperative beta blockade these newer trials are larger and have a stronger design. He also notes that the patients in the more recent trials are more likely to have prior revascularization and hence are less prone to demand-type events, reflective of the type of insult beta blockade would most likely be helpful in preventing. These events may be more closely allied with plaque destabilization of subcritical lesions, with factors such as perioperative hypercoagulability and perhaps inflammation being more important. In this regard it is notable that recent trials on the perioperative use of statins have demonstrated favorable results, with these agents presumably acting to promote plaque stability, the so-called “pleiotropic” function of statins.
Significance for hospitalists: It is reasonable to be more circumspect in the recommendation of perioperative beta blockade. This practice is not likely the magic bullet, which is a common misconcpetion. An indicative situation is an 80-year-old patient undergoing total hip replacement. He has diabetes, COPD, and hypertension, a pulse of 65, a blood pressure of 110/50. There may also be concerns about bradycardia, hypotension, and bronchospasm. Given this analysis a clinician can be confident in withholding perioperative treatment.
The use of beta-blocker therapy in a patient with a single RCRI factor, which is not coronary artery disease, does not seem justified. On the other hand the use of perioperative statins should be more actively entertained. Emerging recommendations from various specialty organizations and other relevant professional entities should be anticipated and sought.
Bibliography
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Eng J Med. 2004Dec 30;351(27):2795-2804.
- Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Eng J Med. 2005 Jul 28;353(4):349-361.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery. J Am Coll Cardiol. 2006;47: 2343-2355
The New C. Diff Epidemic
Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006 Nov 21;145(10):758-764.
C. difficile infection is emerging as one of the most important illnesses for hospitalists to be facile with. It not only occurs frequently, but is also often severe or life threatening, and most importantly iatrogenic and preventable. This review by Bartlett, who elucidated the cause of this disease in 1978, reviews important up-to-date information on C. difficile, focusing on the recent emergence of a more virulent form of the disease.
Infectious diarrhea that develops in the hospital is almost always due to C. difficile. The tissue culture cytotoxic assay (first described in 1978) remains the most sensitive and specific diagnostic tool. The toxin immunoassay used most routinely is only 75% sensitive.
An epidemic of unusually severe C. difficile was first described in Quebec in 2001. Important features include a higher tendency for toxic megacolon and a need for colectomy, protein-losing enteropathy, leukemoid reactions, refractoriness to treatment, a high rate of relapse and an astonishing 16.5% attributable mortality. Fluoroquinolones are the leading associated antibiotic causal factor, although extended spectrum cephalosporins remain important as well in this regard. The new strain is characterized by high levels of toxin production due to the deletion of a toxin production regulatory gene. The strain is also fluoroquinolone resistant, explaining the role of that antibiotic in its genesis.
Treatment of C. difficile colitis (especially the emergent strain) remains problematic. In particular the role of metronidazole versus vancomycin as initial therapy is often contentious. Bartlett cites some evidence suggesting vancomycin may be more effective and is especially recommended for severe disease, characteristics of which are often manifested by this new strain.
This review cites important considerations that hospitalist ought to vigilant and proactive in. Given the high risk of fluoroquinolone treatment we must be sure that these drugs are used appropriately. Nonchalantly stacking on levofloxacin therapy for the COPD flair without evidence for pneumonia should be discouraged. When possible antibiotics with a lower risk for C. difficile (sulfonamides, macrolides, tetracyclines) should be used for any infection. When disease is suspected, a negative toxin immunoassay should not discourage empiric treatment especially in a very ill patient. Isolation and barrier precautions are important in preventing the spread of this potentially lethal infection. C. difficile spores are not killed by alcohol-based detergents, and either soap and water or gloves are necessary to care for these patients. When your hospital experiences a clustering of severe C. difficile infection, alert appropriate infection control personnel. Administrative control of antibiotic use may be indicated.
Baclofen Versus Diazepam to Treat Alcohol Withdrawal
Addolorato G, Leggio L, Abenavoli L, et al. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006 Mar;119 (3):276.e13-18.
Alcohol withdrawal syndrome (AWS) is a frequent problem encountered in hospitalized patients; its management is considered one of SHM’s core competencies for hospitalists. Benzodiazepines are the gold standard of therapy for this problem given their established record for safety and efficacy; however, their use can be problematic in certain instances.
This study involved 37 outpatients, as inpatients may often be sicker and at higher risk of severe withdrawal.
There is a definite risk of oversedation—especially in patients with COPD or chronic liver disease. Some patients require inordinately high doses of benzodiazepines, thus setting the stage for a prolonged hospitalization. Occasional paradoxical or disinhibition reactions to benzodiazepines can also be problematic. Addiction and or diversion are also a concern in patients prone to substance abuse. An otherwise stable patient, ready for discharge, may still be on a relatively high dose of lorazepam, but it is generally not prudent to send the patient out with a supply of medication to finish the course given concerns over resumption of drinking while on the sedative. Conversely, the solution can be cold comfort for the attending physician if the patient resumes drinking, thus eliminating the need for additional medication.
Baclofen, a stereoselective gamma-aminobutyric acid agonist, has a long history of safety in the treatment of spasticity. As such it can counter balance the activation of the glutamate excitatory pathway that characterizes AWS. It has been proposed as an alternative treatment for AWS that would not share the above concerns cited for benzodiazepines.
This study is a randomized controlled trial of baclofen versus valium in the treatment for AWS. Thirty-seven subjects with a history of heavy alcohol use were randomized to either baclofen 30 mg per day or valium 0.5 to 0.75 mg/kg. All were outpatients treated for 10 days. Clinical Institute Withdrawal Assessment-Alcohol (CIWA) scores were assessed daily. Both regimens continuously decreased the baseline elevation of CIWA scores daily over the course of the study, without a significant difference in treatment efficacy. No adverse events or side effects were reported in either group.
Other than baseline CIWA and daily alcohol consumption, it is not clear that the two groups were at equal risk for severe withdrawal reactions. Relevant baseline characteristics such as history of seizures or delirium tremens, factors that raise this risk were not noted.
Significance for hospitalists: With a long history of safety and efficacy, benzodiazepines remain the drugs of choice for hospitalists treating patients with AWS. In certain instances it may be desirable to limit or even avoid their use. How effective and safe baclofen would be in filling this role remains to be fully established. In particular the relative risk for sedation and respiratory depression has not been defined. Nevertheless at least in my institutions, as guided by expert consultation, its use has been carefully considered and proven helpful in some of the situations noted above. TH
Elderly Pneumonia Patients after Antibiotic Switch
Nathan RV, Rhew DC, Bratzler DW, et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006 Jun;119(6):512.e1-7.
Community-acquired pneumonia (CAP) continues to be a common reason for hospital admission—especially among the elderly. As with many infectious diseases, the duration and route of antibiotic therapy is often based on expert recommendations rather than prospective randomized trials. The Patient Outcome Research Team (PORT) trials address the decision to admit a patient, but not other aspects of care. For hospitalists, the decision of when to discharge any patient with reasonable safety is often fraught with uncertainty. This study addresses the necessity of observing a patient for one day following the switch from IV to oral therapy. Two previous smaller retrospective studies have suggested this was unnecessary.
The current study is also retrospective but involves a large database derived from the U.S. Medicare National Pneumonia Project database. Ultimately 5,248 patients over 65 (mean age=80) were selected for analysis; 2,536 were not observed; and 2,712 were observed for one day.) Patients were excluded if their length of stay was greater than seven days or less than two days, suggesting complicated cases in the former instance and mild illness in the latter (i.e., perhaps not even requiring admission). Immunosupressed patients were also excluded. There was no significant difference in the observed 30-day mortality (5.1% in the “not observed” versus 4.4% in the “observed” cohort, respectively).
The obvious limitation of this study is that it was retrospective/observational and thus potentially subject to the bias inherent in this study design. It is possible that the sicker patients were logically watched longer. Propensity analysis was not a component of this study. The authors do present reasons why certain structural weaknesses would have favored the “observed “group.
Certainly there may be other reasons to observe a patient after the switch to oral therapy. A patient with associated gastrointestinal disturbance or a questionable history of GI or other intolerance to a class of antibiotics is an obvious example. Nevertheless, this study should convey a certain confidence to hospitalists when they assess the suitability for discharge for the type of patient covered in this analysis. Interestingly the recently published guidelines for treatment of community acquired pneumonia are concordant with this study.1
Reference
- Mandell L, Wundrelink A, Bartlett J. Guideline for the treatment of community acquired pneumonia. Clin Infect Dis. 2007;44: S27-72.
The Revised Geneva Score for PE
Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department. Ann Intern Med. 2006 Feb 7:144(3):165-171. Comment in: ACP J Club. 2006 Jul-Aug;145(1):25 & Ann Intern Med. 2006 Feb 7;144(3):210-212.
Pulmonary embolism is a diagnosis frequently considered by the hospitalist—both as an explanation for the admitting clinical picture, as well as a complication arising during the course of a hospitalization for another condition.
My institutions’ ability to identify patients with this potentially lethal condition has greatly improved with the advent of multidetector CT angiography and various diagnostic schemata that include d-dimer testing and estimations of pre-test probability. It is a classic consideration whenever there is a onset of pleurisy, dyspnea, or aggravation thereof. Nevertheless multiple other situations arise in the hospital setting, such as unexplained tachycardia, hemoptysis, or vaguely possible but not clear-cut pleuritic chest pain, in which one feels obligated to at least consider the diagnosis. Further, to have to incorporate d-dimer testing into the diagnostic strategy is problematic as up to 80% of hospitalized patients are likely to be positive. Hospitalists need a reasonable strategy to avoid going down that proverbial pathway in certain low risk situations.
The Geneva scoring system and the Wells system are two methodologies that have been used in lieu of or as an adjunct to “clinical judgment.” The former requires arterial blood gases and the latter has as criteria “other diagnosis more likely than pulmonary embolus” that can be problematic and difficult to standardize.
This article presents a revised Geneva scoring system based solely on elements of the history and physical examination. The elements were derived retrospectively from a prior different study on diagnostic strategies for pulmonary thromboembolism (PTE). A different prospective study on PTE was utilized for the validation arm of this study. By logistical regression analysis the following eight elements were incorporated into the revised Geneva score: Age greater than 65 (1 point), previous deep venous thrombosis or pulmonary embolism (3 points), surgery or fracture within one month (2 points), active malignant condition (2 points), unilateral lower limb pain (3 points), hemoptysis (2 points), heart rate 75 to 94 beats/min (3 points) or heart rate 95 beats /minute or more (5 points), and pain on lower limb palpation and unilateral edema (4 points). The prevalence for pulmonary embolism was as follows: low probability or 8% (0 to 3 points), intermediate probability or 28% (4 to 10 points), and high probability or 74% (equal or greater than 11 points).
Significance for hospitalists: This scoring system is not validated a management system per se. However in the imperfect world of clinical reasoning it can help reinforce a thoughtful decision not to embark on the diagnostic path for pulmonary embolism, with its own inherent risks.
Metoprolol after Vascular Surgery
Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of metoprolol after vascular surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006 Nov;152(5):983-990. Comment in Am Heart J. 2006 Nov;152(5):815-818. McCullough PA. Failure of beta-blockers in the reduction of perioperative events: where did we go wrong? Am Heart J. 2006 Nov;152(5):815-818. Comment in: Am Heart J. 2006 Nov;152(5):983-990.
Hospitalists are frequently consulted regarding perioperative risk assessment and reduction for patients undergoing non-cardiac surgery. Over the last decade and supported by a few studies, the perioperative use of beta-blocker therapy has resolved the uncertainty frequently encountered. The McFalls study in 2004 showed no benefit to routine coronary revascularization for patients undergoing vascular surgery deemed at risk for myocardial ischemia.1 This provided further confidence for those of us supplying these preoperative assessments. However, the Lindenauer study in 2005 (a retrospective cohort analysis) was the first indication that perioperative beta blockade could be harmful.2 Lower-risk patients based on the revised cardiovascular index (RCRI) score actually did worse when treated. Still the ACC guidelines published in 2006 suggested perioperative beta blockers be considered for lower risk patients undergoing vascular surgery.3
This study is a randomized placebo-controlled trial of perioperative beta-blocker therapy in 500 treatment-naïve patients undergoing vascular surgery. Metoprolol was started two hours before surgery and continued for one week. Cardiovascular endpoints included cardiac death, arrhythmia requiring treatment, acute myocardial infarction or acute coronary syndrome, and congestive heart failure. No benefit was found for treatment with metoprolol regardless of the number of Revised Cardiac Risk Index (RCRI) factors present. No excess adverse outcomes were noted for therapy although intraoperative bradycardia and hypotension were significantly increased in the active treatment group.
In the accompanying editorial McCullough discusses possible reasons and implications of these findings. In fact, two other trials have reported similar findings. In contrast to the older trials suggesting a benefit to perioperative beta blockade these newer trials are larger and have a stronger design. He also notes that the patients in the more recent trials are more likely to have prior revascularization and hence are less prone to demand-type events, reflective of the type of insult beta blockade would most likely be helpful in preventing. These events may be more closely allied with plaque destabilization of subcritical lesions, with factors such as perioperative hypercoagulability and perhaps inflammation being more important. In this regard it is notable that recent trials on the perioperative use of statins have demonstrated favorable results, with these agents presumably acting to promote plaque stability, the so-called “pleiotropic” function of statins.
Significance for hospitalists: It is reasonable to be more circumspect in the recommendation of perioperative beta blockade. This practice is not likely the magic bullet, which is a common misconcpetion. An indicative situation is an 80-year-old patient undergoing total hip replacement. He has diabetes, COPD, and hypertension, a pulse of 65, a blood pressure of 110/50. There may also be concerns about bradycardia, hypotension, and bronchospasm. Given this analysis a clinician can be confident in withholding perioperative treatment.
The use of beta-blocker therapy in a patient with a single RCRI factor, which is not coronary artery disease, does not seem justified. On the other hand the use of perioperative statins should be more actively entertained. Emerging recommendations from various specialty organizations and other relevant professional entities should be anticipated and sought.
Bibliography
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Eng J Med. 2004Dec 30;351(27):2795-2804.
- Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Eng J Med. 2005 Jul 28;353(4):349-361.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery. J Am Coll Cardiol. 2006;47: 2343-2355
The New C. Diff Epidemic
Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006 Nov 21;145(10):758-764.
C. difficile infection is emerging as one of the most important illnesses for hospitalists to be facile with. It not only occurs frequently, but is also often severe or life threatening, and most importantly iatrogenic and preventable. This review by Bartlett, who elucidated the cause of this disease in 1978, reviews important up-to-date information on C. difficile, focusing on the recent emergence of a more virulent form of the disease.
Infectious diarrhea that develops in the hospital is almost always due to C. difficile. The tissue culture cytotoxic assay (first described in 1978) remains the most sensitive and specific diagnostic tool. The toxin immunoassay used most routinely is only 75% sensitive.
An epidemic of unusually severe C. difficile was first described in Quebec in 2001. Important features include a higher tendency for toxic megacolon and a need for colectomy, protein-losing enteropathy, leukemoid reactions, refractoriness to treatment, a high rate of relapse and an astonishing 16.5% attributable mortality. Fluoroquinolones are the leading associated antibiotic causal factor, although extended spectrum cephalosporins remain important as well in this regard. The new strain is characterized by high levels of toxin production due to the deletion of a toxin production regulatory gene. The strain is also fluoroquinolone resistant, explaining the role of that antibiotic in its genesis.
Treatment of C. difficile colitis (especially the emergent strain) remains problematic. In particular the role of metronidazole versus vancomycin as initial therapy is often contentious. Bartlett cites some evidence suggesting vancomycin may be more effective and is especially recommended for severe disease, characteristics of which are often manifested by this new strain.
This review cites important considerations that hospitalist ought to vigilant and proactive in. Given the high risk of fluoroquinolone treatment we must be sure that these drugs are used appropriately. Nonchalantly stacking on levofloxacin therapy for the COPD flair without evidence for pneumonia should be discouraged. When possible antibiotics with a lower risk for C. difficile (sulfonamides, macrolides, tetracyclines) should be used for any infection. When disease is suspected, a negative toxin immunoassay should not discourage empiric treatment especially in a very ill patient. Isolation and barrier precautions are important in preventing the spread of this potentially lethal infection. C. difficile spores are not killed by alcohol-based detergents, and either soap and water or gloves are necessary to care for these patients. When your hospital experiences a clustering of severe C. difficile infection, alert appropriate infection control personnel. Administrative control of antibiotic use may be indicated.
Baclofen Versus Diazepam to Treat Alcohol Withdrawal
Addolorato G, Leggio L, Abenavoli L, et al. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006 Mar;119 (3):276.e13-18.
Alcohol withdrawal syndrome (AWS) is a frequent problem encountered in hospitalized patients; its management is considered one of SHM’s core competencies for hospitalists. Benzodiazepines are the gold standard of therapy for this problem given their established record for safety and efficacy; however, their use can be problematic in certain instances.
This study involved 37 outpatients, as inpatients may often be sicker and at higher risk of severe withdrawal.
There is a definite risk of oversedation—especially in patients with COPD or chronic liver disease. Some patients require inordinately high doses of benzodiazepines, thus setting the stage for a prolonged hospitalization. Occasional paradoxical or disinhibition reactions to benzodiazepines can also be problematic. Addiction and or diversion are also a concern in patients prone to substance abuse. An otherwise stable patient, ready for discharge, may still be on a relatively high dose of lorazepam, but it is generally not prudent to send the patient out with a supply of medication to finish the course given concerns over resumption of drinking while on the sedative. Conversely, the solution can be cold comfort for the attending physician if the patient resumes drinking, thus eliminating the need for additional medication.
Baclofen, a stereoselective gamma-aminobutyric acid agonist, has a long history of safety in the treatment of spasticity. As such it can counter balance the activation of the glutamate excitatory pathway that characterizes AWS. It has been proposed as an alternative treatment for AWS that would not share the above concerns cited for benzodiazepines.
This study is a randomized controlled trial of baclofen versus valium in the treatment for AWS. Thirty-seven subjects with a history of heavy alcohol use were randomized to either baclofen 30 mg per day or valium 0.5 to 0.75 mg/kg. All were outpatients treated for 10 days. Clinical Institute Withdrawal Assessment-Alcohol (CIWA) scores were assessed daily. Both regimens continuously decreased the baseline elevation of CIWA scores daily over the course of the study, without a significant difference in treatment efficacy. No adverse events or side effects were reported in either group.
Other than baseline CIWA and daily alcohol consumption, it is not clear that the two groups were at equal risk for severe withdrawal reactions. Relevant baseline characteristics such as history of seizures or delirium tremens, factors that raise this risk were not noted.
Significance for hospitalists: With a long history of safety and efficacy, benzodiazepines remain the drugs of choice for hospitalists treating patients with AWS. In certain instances it may be desirable to limit or even avoid their use. How effective and safe baclofen would be in filling this role remains to be fully established. In particular the relative risk for sedation and respiratory depression has not been defined. Nevertheless at least in my institutions, as guided by expert consultation, its use has been carefully considered and proven helpful in some of the situations noted above. TH
New Path to Primary Care?
A new bill that could steer more medical school students toward primary care is winding its way through Congress and will receive a careful look from SHM.
The Preserving Patient Access to Primary Care Act of 2009 (HR 2350) would provide financial assistance to medical students choosing a primary-care career, proposes changes to Medicare reimbursement, and suggests the development of measures to support and expand the patient-centered medical home (PCMH) model of care.
The bill, sponsored by U.S. Rep. Allyson Schwartz, D-Pa., has been endorsed by the American College of Physicians (ACP). In a press release, the ACP referred to the proposal as "the best medicine for curing the growing crisis in primary care."
SHM's Public Policy Committee will discuss HR 2350 during its June meeting. "SHM has been supportive of improving and expanding primary care because a strong primary-care base creates opportunities for a partnership with hospitalists," says Larry Wellikson, MD, FHM, CEO of SHM. "The committee also will look at HR 2350 from the hospitalist perspective and how it fits in with many of the other proposals that are part of healthcare reform."
A new bill that could steer more medical school students toward primary care is winding its way through Congress and will receive a careful look from SHM.
The Preserving Patient Access to Primary Care Act of 2009 (HR 2350) would provide financial assistance to medical students choosing a primary-care career, proposes changes to Medicare reimbursement, and suggests the development of measures to support and expand the patient-centered medical home (PCMH) model of care.
The bill, sponsored by U.S. Rep. Allyson Schwartz, D-Pa., has been endorsed by the American College of Physicians (ACP). In a press release, the ACP referred to the proposal as "the best medicine for curing the growing crisis in primary care."
SHM's Public Policy Committee will discuss HR 2350 during its June meeting. "SHM has been supportive of improving and expanding primary care because a strong primary-care base creates opportunities for a partnership with hospitalists," says Larry Wellikson, MD, FHM, CEO of SHM. "The committee also will look at HR 2350 from the hospitalist perspective and how it fits in with many of the other proposals that are part of healthcare reform."
A new bill that could steer more medical school students toward primary care is winding its way through Congress and will receive a careful look from SHM.
The Preserving Patient Access to Primary Care Act of 2009 (HR 2350) would provide financial assistance to medical students choosing a primary-care career, proposes changes to Medicare reimbursement, and suggests the development of measures to support and expand the patient-centered medical home (PCMH) model of care.
The bill, sponsored by U.S. Rep. Allyson Schwartz, D-Pa., has been endorsed by the American College of Physicians (ACP). In a press release, the ACP referred to the proposal as "the best medicine for curing the growing crisis in primary care."
SHM's Public Policy Committee will discuss HR 2350 during its June meeting. "SHM has been supportive of improving and expanding primary care because a strong primary-care base creates opportunities for a partnership with hospitalists," says Larry Wellikson, MD, FHM, CEO of SHM. "The committee also will look at HR 2350 from the hospitalist perspective and how it fits in with many of the other proposals that are part of healthcare reform."
In the Literature: The Latest Research You Need to Know
Clinical question: Are elevated fasting blood glucose levels independently associated with poor outcomes in all types of acute coronary syndromes (ACS)?
Background: Elevated admission blood glucose levels have been associated with poor outcomes in ACS patients; however, the role of fasting blood glucose levels in these settings, especially non-ST elevation myocardial infarction (NSTEMI), is unclear. It also is uncertain if one is a better predictor of outcomes than the other.
Study design: Prospective cohort of 57,406 patients in the Global Registry of Acute Coronary Events (GRACE).
Setting: 106 hospitals located in 14 countries in North and South America, Europe, Australia, and New Zealand.
Synopsis: 22,001 admission and 13,526 fasting blood glucose levels were extracted from GRACE and categorized into groups ranging from <100 mg/dL to =300 mg/dL. Multivariate logistic regression analysis of the association between these and the primary outcomes of in-hospital and six-month post-discharge all-cause mortality was carried out.
Fasting glucose levels higher than 100 mg/dL were associated with a linear increase in inpatient mortality (irrespective of diagnosis of diabetes) with an eightfold increase at levels =300mg/dL (17.22% vs. 1.71%). Increased six-month mortality, especially in patients with NSTEMI and STEMI, was also noted. However, this was a nonlinear relationship due to a lower mortality at 200-299 mg/dL, possibly reflecting the higher number of diabetics undergoing treatment in this group.
Admission glucose levels showed a linear increase in inpatient mortality at levels higher than 126 mg/dL. In contrast to fasting levels, admission levels were not associated with poor long-term outcome.
Study limitations included the use of registry data from a subgroup analysis and the possibility that fasting glucose levels in severely ill patients might not be representative of actual metabolic state.
Bottom line: Elevated fasting blood glucose in patients with acute coronary syndromes may portend a worse outcome and may be a better predictor than admission glucose levels.
Citation: Sinnaeve PR, Steg PG, Fox KA, et al. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med. 2009;169(4):401-409.
— Reviewed for "TH eWire" by Mital Patel, MD, Alraies Chadi, MD, Saurabh Kandpal, MD, Iqbal Masood, MD, Anuradha Ramaswamy, MD, Department of Hospital Medicine, Cleveland Clinic
Clinical question: Are elevated fasting blood glucose levels independently associated with poor outcomes in all types of acute coronary syndromes (ACS)?
Background: Elevated admission blood glucose levels have been associated with poor outcomes in ACS patients; however, the role of fasting blood glucose levels in these settings, especially non-ST elevation myocardial infarction (NSTEMI), is unclear. It also is uncertain if one is a better predictor of outcomes than the other.
Study design: Prospective cohort of 57,406 patients in the Global Registry of Acute Coronary Events (GRACE).
Setting: 106 hospitals located in 14 countries in North and South America, Europe, Australia, and New Zealand.
Synopsis: 22,001 admission and 13,526 fasting blood glucose levels were extracted from GRACE and categorized into groups ranging from <100 mg/dL to =300 mg/dL. Multivariate logistic regression analysis of the association between these and the primary outcomes of in-hospital and six-month post-discharge all-cause mortality was carried out.
Fasting glucose levels higher than 100 mg/dL were associated with a linear increase in inpatient mortality (irrespective of diagnosis of diabetes) with an eightfold increase at levels =300mg/dL (17.22% vs. 1.71%). Increased six-month mortality, especially in patients with NSTEMI and STEMI, was also noted. However, this was a nonlinear relationship due to a lower mortality at 200-299 mg/dL, possibly reflecting the higher number of diabetics undergoing treatment in this group.
Admission glucose levels showed a linear increase in inpatient mortality at levels higher than 126 mg/dL. In contrast to fasting levels, admission levels were not associated with poor long-term outcome.
Study limitations included the use of registry data from a subgroup analysis and the possibility that fasting glucose levels in severely ill patients might not be representative of actual metabolic state.
Bottom line: Elevated fasting blood glucose in patients with acute coronary syndromes may portend a worse outcome and may be a better predictor than admission glucose levels.
Citation: Sinnaeve PR, Steg PG, Fox KA, et al. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med. 2009;169(4):401-409.
— Reviewed for "TH eWire" by Mital Patel, MD, Alraies Chadi, MD, Saurabh Kandpal, MD, Iqbal Masood, MD, Anuradha Ramaswamy, MD, Department of Hospital Medicine, Cleveland Clinic
Clinical question: Are elevated fasting blood glucose levels independently associated with poor outcomes in all types of acute coronary syndromes (ACS)?
Background: Elevated admission blood glucose levels have been associated with poor outcomes in ACS patients; however, the role of fasting blood glucose levels in these settings, especially non-ST elevation myocardial infarction (NSTEMI), is unclear. It also is uncertain if one is a better predictor of outcomes than the other.
Study design: Prospective cohort of 57,406 patients in the Global Registry of Acute Coronary Events (GRACE).
Setting: 106 hospitals located in 14 countries in North and South America, Europe, Australia, and New Zealand.
Synopsis: 22,001 admission and 13,526 fasting blood glucose levels were extracted from GRACE and categorized into groups ranging from <100 mg/dL to =300 mg/dL. Multivariate logistic regression analysis of the association between these and the primary outcomes of in-hospital and six-month post-discharge all-cause mortality was carried out.
Fasting glucose levels higher than 100 mg/dL were associated with a linear increase in inpatient mortality (irrespective of diagnosis of diabetes) with an eightfold increase at levels =300mg/dL (17.22% vs. 1.71%). Increased six-month mortality, especially in patients with NSTEMI and STEMI, was also noted. However, this was a nonlinear relationship due to a lower mortality at 200-299 mg/dL, possibly reflecting the higher number of diabetics undergoing treatment in this group.
Admission glucose levels showed a linear increase in inpatient mortality at levels higher than 126 mg/dL. In contrast to fasting levels, admission levels were not associated with poor long-term outcome.
Study limitations included the use of registry data from a subgroup analysis and the possibility that fasting glucose levels in severely ill patients might not be representative of actual metabolic state.
Bottom line: Elevated fasting blood glucose in patients with acute coronary syndromes may portend a worse outcome and may be a better predictor than admission glucose levels.
Citation: Sinnaeve PR, Steg PG, Fox KA, et al. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med. 2009;169(4):401-409.
— Reviewed for "TH eWire" by Mital Patel, MD, Alraies Chadi, MD, Saurabh Kandpal, MD, Iqbal Masood, MD, Anuradha Ramaswamy, MD, Department of Hospital Medicine, Cleveland Clinic
Back in the Saddle
When Tait Shanafelt, MD, and his colleagues at the Mayo Clinic in Rochester, Minn., investigated whether medical residents who rated high on burnout also delivered lower quality care, they found an interesting correlation: Physicians who reported errors experienced more burnout, and burned-out physicians made more errors.1
“While the patient safety issue is paramount, there is also a pretty substantial personal cost to physicians when they perceive that they have made an error,” says Dr. Shanafelt, an assistant professor of medicine.
Whether or not a bad outcome stems from “errors,” hospitalists may experience a psychoemotional aftermath. They often suffer in silence, imagining that few other doctors make errors. This common experience has historically been covered by a veil of silence.2
Silent Struggle
Caregivers are largely hesitant to discuss their involvement in adverse events.
“Their reluctance to discuss this with their colleagues is a common barrier to work through this in constructive ways,” Dr. Shanafelt says.
Though clinical decisions have systems and individual components, when mistakes happen it’s the latter with which hospitalists struggle silently—and often dysfunctionally.
“Every serious adverse event has at least two victims: the patient and family; and the caregiver,” says Albert W. Wu, MD, a professor of health policy and management at Johns Hopkins University in Baltimore.3-5
Although there is merit in analyzing the mistake and learning from it, doing so without facing the personal consequences is insufficient.
“Even if there was a system factor that can be identified, physicians still feel personally responsible for their patients and they often carry a sense of guilt around with them,” Dr. Shanafelt says.
That feeling of responsibility is not necessarily a bad thing.
“It is important to recognize that everything is not a systems error,” says Lenny Feldman, MD, an assistant professor and hospitalist with the Division of General Internal Medicine at Johns Hopkins Hospital. “Individuals want to be able to take responsibility for the bad things that have happened, and there is a value to that.”
The medical profession has just begun to fully acknowledge the inevitability of errors and the need for clinicians to be trained to manage them. Emotional responses to bad outcomes or medical errors include fear, guilt, anger, embarrassment, humiliation, and depression, which can last days—or years.
“The cognoscenti of coping know that there are adaptive and maladaptive ways of coping,” says Dr. Wu. “Adaptive would be reframing and growing and learning from the incident, channeling the energy into trying to do better next time. Maladaptive strategies include denial, turning to alcohol, and becoming angry.”
—Albert W. Wu, MD, professor of health policy and management, Johns Hopkins University, Baltimore
What Hospitalists Can Do
Healthcare is changing its culture so reporting adverse events is easier, without an emphasis on assigning blame.
Support is available by phone, disclosure protocols have been created, and practitioners work with risk management personnel to involve patients and families in discussions and apologies. These processes can give physicians a healthy way to be transparent about what happened and also to know that their institutions support them.
Mixed emotions are common after a bad choice or outcome, but the stakes are particularly high in hospital-based specialties.
“Physicians who practice in hospitals are at particular risk for being involved in instances where patients are harmed, partly because of the acuity of patients and partly because they are repeatedly in this environment,” Dr. Wu says. “In a way, for some physicians and nurses, it is like working in a war zone. It is necessary for you to return to that zone to work, even after something bad happens. That, in itself, can be traumatic.”
Experts accentuate the importance of providing hospitalists, especially young ones, the tools to help them recognize and manage post-event emotional baggage. These tools can serve as a roadmap on how to work through their experience.
Periodic debriefing with peers helps hospitalists discuss what’s going on in their practice. But if a hospitalist has not established this trust and support beforehand, it may be difficult to locate the right support after an event occurs.
“There is a healing that goes on when you are able to share with your colleagues who can tell you about their own experiences with this,” says Dr. Shanafelt.
Dr. Feldman agrees. After a couple of bad patient outcomes this year, he needed to talk as much as possible in a few forums, including with his hospitalist group leader and his peers. As the associate program director at his institution, he also checked in with his residents.
“I know how I’m feeling, which is horrible,” Dr. Feldman says. “You know if you’re doing it, your residents are probably doing the same thing.”
Teaching hospitalists can help change the culture by making them willing to share their own experiences with trainees.
“For supervising physicians to say, in effect, to medical students and residents, ‘In my own career, these are things I have experienced and how I’ve worked through them,’ can help young physicians recognize that identifying and working though the consequences of errors on both a professional and personal level is an important part of being a mindful physician,” says Dr. Shanafelt.
Open and humble sharing means trainees can act similarly.
“When we were first presenting our data to the residents in one of our early studies, I often felt like a priest with physicians coming me to confess their mistakes,” Dr. Shanafelt says. “After having this experience repeat itself over and over, I recognized that sharing this was a cathartic event for them.”
The most helpful thing hospitalists can do for each other is listen without judgment.
“We need to realize it is going to happen to each and every one of us, and be prepared to offer a shoulder to cry on to help your colleague work through it,” says Dr. Feldman.
Dr. Wu believes clinicians need to examine their capacity to offer such support.
“We are not as reassuring as we could be,” Dr. Wu says. “I think we tend to hold back, maybe because we are so fearful of the whole idea. Really admitting that this is a normal, common event frightens us too much.”
What Hospitalist Groups Can Do
Cleo Hardin, MD, section chief of pediatric hospital medicine at the University of Arizona in Tucson, says her department deals with post-event distress several ways. Her doctors talk with her, share in their departmental morbidity and mortality conference, and don’t ignore errors and bad outcomes.
“Anytime there is a bad outcome,” Dr. Hardin says, “it is very important for the leader of the group to meet with the individual to ask, ‘What do we need to do to reduce the likelihood that you’re going to develop post-traumatic stress disorder and that you’re going to question everything you do in the future? You are a good doctor. How do we keep you being a good doctor?’ ”
A show of support by the whole team is of utmost importance.
“There is a tremendous amount of camaraderie in the group, and we all understand [that clinical practice] is not an exact science,” Dr. Hardin says. “There’s a lot of art in medicine.”
An expression of that art showed up several years ago in her hospital’s pediatrics unit when a child died on Christmas Day.
“On the day an event happens, I think the person is reeling and in shock and can’t hear anything,” Dr. Hardin says. “I called the hospitalist to ask whether he needed coverage. He declined, saying he was afraid if he got off the horse, he would never get back on.”
The following month they discussed the event at their mortality and morbidity conference; the entire hospitalist section was there.
“We sat together, and it was very clear to the hospitalist involved in the event that he was not there alone,” Dr. Hardin says. “We told him how much we appreciated him and respected him, and we were there to be his support.”
What Leaders Can Do
As the director of the hospitalist program at Johns Hopkins Hospital, Daniel J. Brotman, MD, FACP, understands that the level of upset after a bad outcome can be dramatic.
“Whether or not it was your fault, and independent of any medical-legal ramifications, although those certainly exist,” he says, “there’s a personal sense of just being a human being; of wanting to go back; questioning whether you made a right decision, a wrong decision; and facing whether you would change anything if you could do it again.”
Dr. Brotman believes in leading by example. “What you want is an open-door policy where faculty members can say, ‘Something bad happened, I need to get it off my chest, and I need to do it now,’” he says.
The group leader also needs to know what happened in case there’s a need for damage control beyond the hospitalist group. The leader should ultimately be the one who confers forgiveness.
“If somebody feels they are keeping a secret, it’s going to make matters worse,” Dr. Brotman says.
Research confirms the needs for “confession, restitution, and absolution,” and hospitalists need their competence validated.6
Even though I [have been] honest and said, ‘I don’t think I would have made that decision,’ I also have said, ‘I know that in terms of your thought process, you were acting in the best interest of the patient … and you’re one of the most compassionate physicians I know,” Dr. Brotman says.
As leaders gather information on an adverse event, they may also be in a better position to advocate for the hospitalist in subsequent conversations. In so doing, they can save that individual further embarrassment and humiliation.
“I hope that I’m able to process what I was experiencing in a way that doesn’t paralyze me,” Dr. Feldman says. “But I hope it informs me to the gravity of the situation.” TH
Andrea Sattinger is a medical writer based in North Carolina.
References
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071-1078.
- Goldberg RM, Kuhn G, Andrew LB, et al. Coping with medical mistakes and errors in judgment. Ann Emerg Med. 2002;39(3):287-292.
- Wu AW. Handling hospital errors: is disclosure the best defense? Ann Intern Med. 1999;131(12):970-972.
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726-727.
- Wu AW, Folkman S, McPhee SJ, Lo B. Do house officers learn from their mistakes? JAMA. 1991;265(16):2089-2094.
- Wears RL, Wu AW. Dealing with failure: the aftermath of errors and adverse events. Ann Emerg Med. 2002;39(3):344-346.
When Tait Shanafelt, MD, and his colleagues at the Mayo Clinic in Rochester, Minn., investigated whether medical residents who rated high on burnout also delivered lower quality care, they found an interesting correlation: Physicians who reported errors experienced more burnout, and burned-out physicians made more errors.1
“While the patient safety issue is paramount, there is also a pretty substantial personal cost to physicians when they perceive that they have made an error,” says Dr. Shanafelt, an assistant professor of medicine.
Whether or not a bad outcome stems from “errors,” hospitalists may experience a psychoemotional aftermath. They often suffer in silence, imagining that few other doctors make errors. This common experience has historically been covered by a veil of silence.2
Silent Struggle
Caregivers are largely hesitant to discuss their involvement in adverse events.
“Their reluctance to discuss this with their colleagues is a common barrier to work through this in constructive ways,” Dr. Shanafelt says.
Though clinical decisions have systems and individual components, when mistakes happen it’s the latter with which hospitalists struggle silently—and often dysfunctionally.
“Every serious adverse event has at least two victims: the patient and family; and the caregiver,” says Albert W. Wu, MD, a professor of health policy and management at Johns Hopkins University in Baltimore.3-5
Although there is merit in analyzing the mistake and learning from it, doing so without facing the personal consequences is insufficient.
“Even if there was a system factor that can be identified, physicians still feel personally responsible for their patients and they often carry a sense of guilt around with them,” Dr. Shanafelt says.
That feeling of responsibility is not necessarily a bad thing.
“It is important to recognize that everything is not a systems error,” says Lenny Feldman, MD, an assistant professor and hospitalist with the Division of General Internal Medicine at Johns Hopkins Hospital. “Individuals want to be able to take responsibility for the bad things that have happened, and there is a value to that.”
The medical profession has just begun to fully acknowledge the inevitability of errors and the need for clinicians to be trained to manage them. Emotional responses to bad outcomes or medical errors include fear, guilt, anger, embarrassment, humiliation, and depression, which can last days—or years.
“The cognoscenti of coping know that there are adaptive and maladaptive ways of coping,” says Dr. Wu. “Adaptive would be reframing and growing and learning from the incident, channeling the energy into trying to do better next time. Maladaptive strategies include denial, turning to alcohol, and becoming angry.”
—Albert W. Wu, MD, professor of health policy and management, Johns Hopkins University, Baltimore
What Hospitalists Can Do
Healthcare is changing its culture so reporting adverse events is easier, without an emphasis on assigning blame.
Support is available by phone, disclosure protocols have been created, and practitioners work with risk management personnel to involve patients and families in discussions and apologies. These processes can give physicians a healthy way to be transparent about what happened and also to know that their institutions support them.
Mixed emotions are common after a bad choice or outcome, but the stakes are particularly high in hospital-based specialties.
“Physicians who practice in hospitals are at particular risk for being involved in instances where patients are harmed, partly because of the acuity of patients and partly because they are repeatedly in this environment,” Dr. Wu says. “In a way, for some physicians and nurses, it is like working in a war zone. It is necessary for you to return to that zone to work, even after something bad happens. That, in itself, can be traumatic.”
Experts accentuate the importance of providing hospitalists, especially young ones, the tools to help them recognize and manage post-event emotional baggage. These tools can serve as a roadmap on how to work through their experience.
Periodic debriefing with peers helps hospitalists discuss what’s going on in their practice. But if a hospitalist has not established this trust and support beforehand, it may be difficult to locate the right support after an event occurs.
“There is a healing that goes on when you are able to share with your colleagues who can tell you about their own experiences with this,” says Dr. Shanafelt.
Dr. Feldman agrees. After a couple of bad patient outcomes this year, he needed to talk as much as possible in a few forums, including with his hospitalist group leader and his peers. As the associate program director at his institution, he also checked in with his residents.
“I know how I’m feeling, which is horrible,” Dr. Feldman says. “You know if you’re doing it, your residents are probably doing the same thing.”
Teaching hospitalists can help change the culture by making them willing to share their own experiences with trainees.
“For supervising physicians to say, in effect, to medical students and residents, ‘In my own career, these are things I have experienced and how I’ve worked through them,’ can help young physicians recognize that identifying and working though the consequences of errors on both a professional and personal level is an important part of being a mindful physician,” says Dr. Shanafelt.
Open and humble sharing means trainees can act similarly.
“When we were first presenting our data to the residents in one of our early studies, I often felt like a priest with physicians coming me to confess their mistakes,” Dr. Shanafelt says. “After having this experience repeat itself over and over, I recognized that sharing this was a cathartic event for them.”
The most helpful thing hospitalists can do for each other is listen without judgment.
“We need to realize it is going to happen to each and every one of us, and be prepared to offer a shoulder to cry on to help your colleague work through it,” says Dr. Feldman.
Dr. Wu believes clinicians need to examine their capacity to offer such support.
“We are not as reassuring as we could be,” Dr. Wu says. “I think we tend to hold back, maybe because we are so fearful of the whole idea. Really admitting that this is a normal, common event frightens us too much.”
What Hospitalist Groups Can Do
Cleo Hardin, MD, section chief of pediatric hospital medicine at the University of Arizona in Tucson, says her department deals with post-event distress several ways. Her doctors talk with her, share in their departmental morbidity and mortality conference, and don’t ignore errors and bad outcomes.
“Anytime there is a bad outcome,” Dr. Hardin says, “it is very important for the leader of the group to meet with the individual to ask, ‘What do we need to do to reduce the likelihood that you’re going to develop post-traumatic stress disorder and that you’re going to question everything you do in the future? You are a good doctor. How do we keep you being a good doctor?’ ”
A show of support by the whole team is of utmost importance.
“There is a tremendous amount of camaraderie in the group, and we all understand [that clinical practice] is not an exact science,” Dr. Hardin says. “There’s a lot of art in medicine.”
An expression of that art showed up several years ago in her hospital’s pediatrics unit when a child died on Christmas Day.
“On the day an event happens, I think the person is reeling and in shock and can’t hear anything,” Dr. Hardin says. “I called the hospitalist to ask whether he needed coverage. He declined, saying he was afraid if he got off the horse, he would never get back on.”
The following month they discussed the event at their mortality and morbidity conference; the entire hospitalist section was there.
“We sat together, and it was very clear to the hospitalist involved in the event that he was not there alone,” Dr. Hardin says. “We told him how much we appreciated him and respected him, and we were there to be his support.”
What Leaders Can Do
As the director of the hospitalist program at Johns Hopkins Hospital, Daniel J. Brotman, MD, FACP, understands that the level of upset after a bad outcome can be dramatic.
“Whether or not it was your fault, and independent of any medical-legal ramifications, although those certainly exist,” he says, “there’s a personal sense of just being a human being; of wanting to go back; questioning whether you made a right decision, a wrong decision; and facing whether you would change anything if you could do it again.”
Dr. Brotman believes in leading by example. “What you want is an open-door policy where faculty members can say, ‘Something bad happened, I need to get it off my chest, and I need to do it now,’” he says.
The group leader also needs to know what happened in case there’s a need for damage control beyond the hospitalist group. The leader should ultimately be the one who confers forgiveness.
“If somebody feels they are keeping a secret, it’s going to make matters worse,” Dr. Brotman says.
Research confirms the needs for “confession, restitution, and absolution,” and hospitalists need their competence validated.6
Even though I [have been] honest and said, ‘I don’t think I would have made that decision,’ I also have said, ‘I know that in terms of your thought process, you were acting in the best interest of the patient … and you’re one of the most compassionate physicians I know,” Dr. Brotman says.
As leaders gather information on an adverse event, they may also be in a better position to advocate for the hospitalist in subsequent conversations. In so doing, they can save that individual further embarrassment and humiliation.
“I hope that I’m able to process what I was experiencing in a way that doesn’t paralyze me,” Dr. Feldman says. “But I hope it informs me to the gravity of the situation.” TH
Andrea Sattinger is a medical writer based in North Carolina.
References
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071-1078.
- Goldberg RM, Kuhn G, Andrew LB, et al. Coping with medical mistakes and errors in judgment. Ann Emerg Med. 2002;39(3):287-292.
- Wu AW. Handling hospital errors: is disclosure the best defense? Ann Intern Med. 1999;131(12):970-972.
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726-727.
- Wu AW, Folkman S, McPhee SJ, Lo B. Do house officers learn from their mistakes? JAMA. 1991;265(16):2089-2094.
- Wears RL, Wu AW. Dealing with failure: the aftermath of errors and adverse events. Ann Emerg Med. 2002;39(3):344-346.
When Tait Shanafelt, MD, and his colleagues at the Mayo Clinic in Rochester, Minn., investigated whether medical residents who rated high on burnout also delivered lower quality care, they found an interesting correlation: Physicians who reported errors experienced more burnout, and burned-out physicians made more errors.1
“While the patient safety issue is paramount, there is also a pretty substantial personal cost to physicians when they perceive that they have made an error,” says Dr. Shanafelt, an assistant professor of medicine.
Whether or not a bad outcome stems from “errors,” hospitalists may experience a psychoemotional aftermath. They often suffer in silence, imagining that few other doctors make errors. This common experience has historically been covered by a veil of silence.2
Silent Struggle
Caregivers are largely hesitant to discuss their involvement in adverse events.
“Their reluctance to discuss this with their colleagues is a common barrier to work through this in constructive ways,” Dr. Shanafelt says.
Though clinical decisions have systems and individual components, when mistakes happen it’s the latter with which hospitalists struggle silently—and often dysfunctionally.
“Every serious adverse event has at least two victims: the patient and family; and the caregiver,” says Albert W. Wu, MD, a professor of health policy and management at Johns Hopkins University in Baltimore.3-5
Although there is merit in analyzing the mistake and learning from it, doing so without facing the personal consequences is insufficient.
“Even if there was a system factor that can be identified, physicians still feel personally responsible for their patients and they often carry a sense of guilt around with them,” Dr. Shanafelt says.
That feeling of responsibility is not necessarily a bad thing.
“It is important to recognize that everything is not a systems error,” says Lenny Feldman, MD, an assistant professor and hospitalist with the Division of General Internal Medicine at Johns Hopkins Hospital. “Individuals want to be able to take responsibility for the bad things that have happened, and there is a value to that.”
The medical profession has just begun to fully acknowledge the inevitability of errors and the need for clinicians to be trained to manage them. Emotional responses to bad outcomes or medical errors include fear, guilt, anger, embarrassment, humiliation, and depression, which can last days—or years.
“The cognoscenti of coping know that there are adaptive and maladaptive ways of coping,” says Dr. Wu. “Adaptive would be reframing and growing and learning from the incident, channeling the energy into trying to do better next time. Maladaptive strategies include denial, turning to alcohol, and becoming angry.”
—Albert W. Wu, MD, professor of health policy and management, Johns Hopkins University, Baltimore
What Hospitalists Can Do
Healthcare is changing its culture so reporting adverse events is easier, without an emphasis on assigning blame.
Support is available by phone, disclosure protocols have been created, and practitioners work with risk management personnel to involve patients and families in discussions and apologies. These processes can give physicians a healthy way to be transparent about what happened and also to know that their institutions support them.
Mixed emotions are common after a bad choice or outcome, but the stakes are particularly high in hospital-based specialties.
“Physicians who practice in hospitals are at particular risk for being involved in instances where patients are harmed, partly because of the acuity of patients and partly because they are repeatedly in this environment,” Dr. Wu says. “In a way, for some physicians and nurses, it is like working in a war zone. It is necessary for you to return to that zone to work, even after something bad happens. That, in itself, can be traumatic.”
Experts accentuate the importance of providing hospitalists, especially young ones, the tools to help them recognize and manage post-event emotional baggage. These tools can serve as a roadmap on how to work through their experience.
Periodic debriefing with peers helps hospitalists discuss what’s going on in their practice. But if a hospitalist has not established this trust and support beforehand, it may be difficult to locate the right support after an event occurs.
“There is a healing that goes on when you are able to share with your colleagues who can tell you about their own experiences with this,” says Dr. Shanafelt.
Dr. Feldman agrees. After a couple of bad patient outcomes this year, he needed to talk as much as possible in a few forums, including with his hospitalist group leader and his peers. As the associate program director at his institution, he also checked in with his residents.
“I know how I’m feeling, which is horrible,” Dr. Feldman says. “You know if you’re doing it, your residents are probably doing the same thing.”
Teaching hospitalists can help change the culture by making them willing to share their own experiences with trainees.
“For supervising physicians to say, in effect, to medical students and residents, ‘In my own career, these are things I have experienced and how I’ve worked through them,’ can help young physicians recognize that identifying and working though the consequences of errors on both a professional and personal level is an important part of being a mindful physician,” says Dr. Shanafelt.
Open and humble sharing means trainees can act similarly.
“When we were first presenting our data to the residents in one of our early studies, I often felt like a priest with physicians coming me to confess their mistakes,” Dr. Shanafelt says. “After having this experience repeat itself over and over, I recognized that sharing this was a cathartic event for them.”
The most helpful thing hospitalists can do for each other is listen without judgment.
“We need to realize it is going to happen to each and every one of us, and be prepared to offer a shoulder to cry on to help your colleague work through it,” says Dr. Feldman.
Dr. Wu believes clinicians need to examine their capacity to offer such support.
“We are not as reassuring as we could be,” Dr. Wu says. “I think we tend to hold back, maybe because we are so fearful of the whole idea. Really admitting that this is a normal, common event frightens us too much.”
What Hospitalist Groups Can Do
Cleo Hardin, MD, section chief of pediatric hospital medicine at the University of Arizona in Tucson, says her department deals with post-event distress several ways. Her doctors talk with her, share in their departmental morbidity and mortality conference, and don’t ignore errors and bad outcomes.
“Anytime there is a bad outcome,” Dr. Hardin says, “it is very important for the leader of the group to meet with the individual to ask, ‘What do we need to do to reduce the likelihood that you’re going to develop post-traumatic stress disorder and that you’re going to question everything you do in the future? You are a good doctor. How do we keep you being a good doctor?’ ”
A show of support by the whole team is of utmost importance.
“There is a tremendous amount of camaraderie in the group, and we all understand [that clinical practice] is not an exact science,” Dr. Hardin says. “There’s a lot of art in medicine.”
An expression of that art showed up several years ago in her hospital’s pediatrics unit when a child died on Christmas Day.
“On the day an event happens, I think the person is reeling and in shock and can’t hear anything,” Dr. Hardin says. “I called the hospitalist to ask whether he needed coverage. He declined, saying he was afraid if he got off the horse, he would never get back on.”
The following month they discussed the event at their mortality and morbidity conference; the entire hospitalist section was there.
“We sat together, and it was very clear to the hospitalist involved in the event that he was not there alone,” Dr. Hardin says. “We told him how much we appreciated him and respected him, and we were there to be his support.”
What Leaders Can Do
As the director of the hospitalist program at Johns Hopkins Hospital, Daniel J. Brotman, MD, FACP, understands that the level of upset after a bad outcome can be dramatic.
“Whether or not it was your fault, and independent of any medical-legal ramifications, although those certainly exist,” he says, “there’s a personal sense of just being a human being; of wanting to go back; questioning whether you made a right decision, a wrong decision; and facing whether you would change anything if you could do it again.”
Dr. Brotman believes in leading by example. “What you want is an open-door policy where faculty members can say, ‘Something bad happened, I need to get it off my chest, and I need to do it now,’” he says.
The group leader also needs to know what happened in case there’s a need for damage control beyond the hospitalist group. The leader should ultimately be the one who confers forgiveness.
“If somebody feels they are keeping a secret, it’s going to make matters worse,” Dr. Brotman says.
Research confirms the needs for “confession, restitution, and absolution,” and hospitalists need their competence validated.6
Even though I [have been] honest and said, ‘I don’t think I would have made that decision,’ I also have said, ‘I know that in terms of your thought process, you were acting in the best interest of the patient … and you’re one of the most compassionate physicians I know,” Dr. Brotman says.
As leaders gather information on an adverse event, they may also be in a better position to advocate for the hospitalist in subsequent conversations. In so doing, they can save that individual further embarrassment and humiliation.
“I hope that I’m able to process what I was experiencing in a way that doesn’t paralyze me,” Dr. Feldman says. “But I hope it informs me to the gravity of the situation.” TH
Andrea Sattinger is a medical writer based in North Carolina.
References
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071-1078.
- Goldberg RM, Kuhn G, Andrew LB, et al. Coping with medical mistakes and errors in judgment. Ann Emerg Med. 2002;39(3):287-292.
- Wu AW. Handling hospital errors: is disclosure the best defense? Ann Intern Med. 1999;131(12):970-972.
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726-727.
- Wu AW, Folkman S, McPhee SJ, Lo B. Do house officers learn from their mistakes? JAMA. 1991;265(16):2089-2094.
- Wears RL, Wu AW. Dealing with failure: the aftermath of errors and adverse events. Ann Emerg Med. 2002;39(3):344-346.
Erlotinib in Lung Cancer
Dr. Vincent A. Miller describes study results that suggest the addition of erlotinib to bevacizumab treatment for advanced non-small cell lung cancer extends progression-free survival compared with bevacizumab alone. Damian McNamara of the Global Medical News Network (GMNN) reports from the annual meeting of the American Society of Clinical Oncology in Orlando.
Dr. Vincent A. Miller describes study results that suggest the addition of erlotinib to bevacizumab treatment for advanced non-small cell lung cancer extends progression-free survival compared with bevacizumab alone. Damian McNamara of the Global Medical News Network (GMNN) reports from the annual meeting of the American Society of Clinical Oncology in Orlando.
Dr. Vincent A. Miller describes study results that suggest the addition of erlotinib to bevacizumab treatment for advanced non-small cell lung cancer extends progression-free survival compared with bevacizumab alone. Damian McNamara of the Global Medical News Network (GMNN) reports from the annual meeting of the American Society of Clinical Oncology in Orlando.