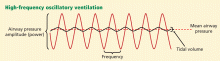
User login
Uniquely Positioned
How can a family physician with a demanding clinical schedule juggle patient care with the grueling administrative and travel duties required of the president-elect of the American Academy of Family Physicians (AAFP)? Lori Heim, MD, FAAFP, found the perfect compromise: Give up the family practice and become a hospitalist.
She did so last November, soon after AAFP members voted her the next president of one of the nation’s largest medical organizations. In fact, she is the only hospitalist at 104-bed Scotland Memorial Hospital in Laurinburg, N.C.
“I was looking for an opportunity while I was working as president-elect and then president of the academy,” says Dr. Heim, who takes over as president of the 94,000-member AAFP in October. “Because of the heavy travel demands, it was not possible to keep my old practice.”
Although the career swap is borne of professional necessity, Dr. Heim says her transition to HM practice has been relatively easy. “I love it. [The new job] utilizes my prior training and skills,” she says. “In private practice, I was doing rounds on my own patients, then I would have to run to the office to see my other patients. I could see the advantages of using the hospitalist services. … Now, here I am on this side.”
An active AAFP member for nearly 25 years, Dr. Heim brings a unique confluence of medical training and experience to her new role. She has firsthand knowledge of the key issues intersecting primary care and hospital-based practice—care coordination, physician reimbursement, and quality improvement. She also acknowledges that walls need to be broken down when it comes to family physicians (FP) transitioning to HM careers. More complete training and improvement in hospital administrations’ understanding of an FP’s clinical capabilities will advance their entrance into hospitalist careers.
“I think it could become a large trend because of the financial constraints on family care,” Dr. Heim says, also noting the lifestyle benefits of an HM career. “FPs often cannot do both inpatient and outpatient care. Your productivity, if you are in the clinic, must make a tradeoff between rounds and office hours—and how late at night do I want to be doing rounds?”
Bumpy Road to HM
The transition from family practice to HM is working out well for Heim, but it wasn’t as smooth as one might expect for a decorated career physician.
The daughter of a military pilot, Dr. Heim earned her bachelor’s degree with honors from Portland State University and her medical degree at the Uniformed Services University of Health Sciences in Bethesda, Md. Following her residency at Andrews Air Force Base in Maryland and a fellowship in faculty development and research at the University of North Carolina at Chapel Hill, her military medical career resembled a spiral staircase. She went from staff physician to clinic chief to residency director to chief of medical staff, with a few stops in between. After 25 years of military service, she retired as an Air Force colonel and opened a private practice.
Even with decades of training and patient care under her belt, Dr. Heim wasn’t rubber-stamped into a hospitalist position. It’s an issue she hopes to address as part of her AAFP tenure. “I know of hospitals where family physicians can admit and treat their patients but not be considered for a hospitalist position. It happened to me,” she explains. “I went and found a hospital that would use my skills.”
Hospitalist Robert Harrington, MD, FHM, knows the feeling. He had a more traditional primary-care practice before entering a HM career, and he understands the intense financial and workload pressures of family practice. Now the vice president of medical affairs for Alpharetta, Ga.-based IN Compass Health Inc. and chair of SHM’s Family Medicine Task Force, he says “there are barriers to hiring because of the wide variability in family physician training.” The root of the problem is that residents in some programs get less hospital time and experience with HM-patient encounters. “In opposed programs, they compete with other specialties and get less time,” Dr. Harrington says. “In unopposed programs, they tend to get more hospital experience and more rotations in inpatient services. Those folks can transition with little to no difficulty to hospital medicine.”
SHM President Scott Flanders, MD, FHM, associate professor and director of the hospitalist program at the University of Michigan in Ann Arbor, sees great value in what FPs can bring to HM. He wants HM to be open to those interested in a career change; however, he agrees physician training and experience can be an obstacle in the recruitment process. “The training in internal medicine is more geared to hospital medicine than it is in family practice [training],” Dr. Flanders says. “FPs must make sure they have hospital training, including the ICU. Many FP programs may not have this.” He also says FPs looking at an HM career—and internists as well—need to be “up to speed” in systems-based practice.

—Lori Heim, MD, FAAFP, Scotland Memorial Hospital, Laurinburg, N.C., AAFP president-elect
Although they represent a small part of SHM’s membership, Dr. Harrington and his task force want FPs to have “representation and a voice” in the society. “They are a small but growing minority,” he says. “Four or five percent of hospitalists are family-medicine-trained by our membership rolls, but we believe it is a bigger number, as some may not be members of SHM. … We run into more problems with hospital administrators. Some of them insist on IM-trained physicians, and there just aren’t enough IMs out there.”
It’s a C-suite roadblock Dr. Heim is familiar with. “Sometimes there is a parochial view in using internists above family physicians. Why should family doctors be second? It’s a misunderstanding of the experience and what family physicians bring to the table,” she says. “They haven’t done it, so people don’t think they can. Some hospitals are underutilizing family physicians in a hospitalist role. If a physician has hospital privileges, there is no reason they should not be considered to be hired as a hospitalist.”
Tenure of Change
As AAFP president, Dr. Heim says she’ll work to improve working conditions for FPs and fight for her constituents’ rights as Washington debates national healthcare reform. The AAFP and SHM share a number of policy interests, including reimbursement reform, new technologies, and patient-safety initiatives.
“I would like to do more with the various societies to increase opportunities for family medicine,” Dr. Heim says. “In some ways, primary care is being squeezed all around. How are you going to increase the numbers of FPs when they earn less? You are not going to unless you reform the system.”
Another top priority is advancing the idea of a national network of electronic health records (EHRs). Dr. Heim says a linked EHR system would improve communication and handoffs, and help physicians limit medical errors. “That’s the key,” she says. “Until then, it is going to be a hodgepodge of solutions. As long as it requires multiple steps, there will always be slipups.
“When I was in the military, records were kept electronically, and I learned the value of having continuity of electronic records.”
Dr. Heim says she would like to partner with hospital-based physicians on QI projects. “Any doctor, it doesn’t matter who you are, has a role to play in quality improvement, either in procedures to improve outcomes in the operating room or ICU or ED. We all have a role in that,” she says. “That is one of the reasons we have supported comparative effectiveness research. Guidelines are valuable.”
As AAFP president, Dr. Heim says she won’t forget her family physician roots. Her platform is ambitious: Reform both the practice and the payment of healthcare in this country. “I think the country recognizes that the current way of paying for healthcare—built on volume and procedures rather than patient outcome—has resulted in a fragmented and disjointed process,” she says. “It’s not an even a system. … We have to look at patient experience and outcome, not ‘what procedure did we do to that patient?’
“Right now, we are paying more for lower-quality healthcare, and we are not getting the bang for our bucks. We cannot afford to continue to spend money and not get value; we really have to change this time.” TH
Carol Berczuk is a freelance writer based in New York City.
How can a family physician with a demanding clinical schedule juggle patient care with the grueling administrative and travel duties required of the president-elect of the American Academy of Family Physicians (AAFP)? Lori Heim, MD, FAAFP, found the perfect compromise: Give up the family practice and become a hospitalist.
She did so last November, soon after AAFP members voted her the next president of one of the nation’s largest medical organizations. In fact, she is the only hospitalist at 104-bed Scotland Memorial Hospital in Laurinburg, N.C.
“I was looking for an opportunity while I was working as president-elect and then president of the academy,” says Dr. Heim, who takes over as president of the 94,000-member AAFP in October. “Because of the heavy travel demands, it was not possible to keep my old practice.”
Although the career swap is borne of professional necessity, Dr. Heim says her transition to HM practice has been relatively easy. “I love it. [The new job] utilizes my prior training and skills,” she says. “In private practice, I was doing rounds on my own patients, then I would have to run to the office to see my other patients. I could see the advantages of using the hospitalist services. … Now, here I am on this side.”
An active AAFP member for nearly 25 years, Dr. Heim brings a unique confluence of medical training and experience to her new role. She has firsthand knowledge of the key issues intersecting primary care and hospital-based practice—care coordination, physician reimbursement, and quality improvement. She also acknowledges that walls need to be broken down when it comes to family physicians (FP) transitioning to HM careers. More complete training and improvement in hospital administrations’ understanding of an FP’s clinical capabilities will advance their entrance into hospitalist careers.
“I think it could become a large trend because of the financial constraints on family care,” Dr. Heim says, also noting the lifestyle benefits of an HM career. “FPs often cannot do both inpatient and outpatient care. Your productivity, if you are in the clinic, must make a tradeoff between rounds and office hours—and how late at night do I want to be doing rounds?”
Bumpy Road to HM
The transition from family practice to HM is working out well for Heim, but it wasn’t as smooth as one might expect for a decorated career physician.
The daughter of a military pilot, Dr. Heim earned her bachelor’s degree with honors from Portland State University and her medical degree at the Uniformed Services University of Health Sciences in Bethesda, Md. Following her residency at Andrews Air Force Base in Maryland and a fellowship in faculty development and research at the University of North Carolina at Chapel Hill, her military medical career resembled a spiral staircase. She went from staff physician to clinic chief to residency director to chief of medical staff, with a few stops in between. After 25 years of military service, she retired as an Air Force colonel and opened a private practice.
Even with decades of training and patient care under her belt, Dr. Heim wasn’t rubber-stamped into a hospitalist position. It’s an issue she hopes to address as part of her AAFP tenure. “I know of hospitals where family physicians can admit and treat their patients but not be considered for a hospitalist position. It happened to me,” she explains. “I went and found a hospital that would use my skills.”
Hospitalist Robert Harrington, MD, FHM, knows the feeling. He had a more traditional primary-care practice before entering a HM career, and he understands the intense financial and workload pressures of family practice. Now the vice president of medical affairs for Alpharetta, Ga.-based IN Compass Health Inc. and chair of SHM’s Family Medicine Task Force, he says “there are barriers to hiring because of the wide variability in family physician training.” The root of the problem is that residents in some programs get less hospital time and experience with HM-patient encounters. “In opposed programs, they compete with other specialties and get less time,” Dr. Harrington says. “In unopposed programs, they tend to get more hospital experience and more rotations in inpatient services. Those folks can transition with little to no difficulty to hospital medicine.”
SHM President Scott Flanders, MD, FHM, associate professor and director of the hospitalist program at the University of Michigan in Ann Arbor, sees great value in what FPs can bring to HM. He wants HM to be open to those interested in a career change; however, he agrees physician training and experience can be an obstacle in the recruitment process. “The training in internal medicine is more geared to hospital medicine than it is in family practice [training],” Dr. Flanders says. “FPs must make sure they have hospital training, including the ICU. Many FP programs may not have this.” He also says FPs looking at an HM career—and internists as well—need to be “up to speed” in systems-based practice.

—Lori Heim, MD, FAAFP, Scotland Memorial Hospital, Laurinburg, N.C., AAFP president-elect
Although they represent a small part of SHM’s membership, Dr. Harrington and his task force want FPs to have “representation and a voice” in the society. “They are a small but growing minority,” he says. “Four or five percent of hospitalists are family-medicine-trained by our membership rolls, but we believe it is a bigger number, as some may not be members of SHM. … We run into more problems with hospital administrators. Some of them insist on IM-trained physicians, and there just aren’t enough IMs out there.”
It’s a C-suite roadblock Dr. Heim is familiar with. “Sometimes there is a parochial view in using internists above family physicians. Why should family doctors be second? It’s a misunderstanding of the experience and what family physicians bring to the table,” she says. “They haven’t done it, so people don’t think they can. Some hospitals are underutilizing family physicians in a hospitalist role. If a physician has hospital privileges, there is no reason they should not be considered to be hired as a hospitalist.”
Tenure of Change
As AAFP president, Dr. Heim says she’ll work to improve working conditions for FPs and fight for her constituents’ rights as Washington debates national healthcare reform. The AAFP and SHM share a number of policy interests, including reimbursement reform, new technologies, and patient-safety initiatives.
“I would like to do more with the various societies to increase opportunities for family medicine,” Dr. Heim says. “In some ways, primary care is being squeezed all around. How are you going to increase the numbers of FPs when they earn less? You are not going to unless you reform the system.”
Another top priority is advancing the idea of a national network of electronic health records (EHRs). Dr. Heim says a linked EHR system would improve communication and handoffs, and help physicians limit medical errors. “That’s the key,” she says. “Until then, it is going to be a hodgepodge of solutions. As long as it requires multiple steps, there will always be slipups.
“When I was in the military, records were kept electronically, and I learned the value of having continuity of electronic records.”
Dr. Heim says she would like to partner with hospital-based physicians on QI projects. “Any doctor, it doesn’t matter who you are, has a role to play in quality improvement, either in procedures to improve outcomes in the operating room or ICU or ED. We all have a role in that,” she says. “That is one of the reasons we have supported comparative effectiveness research. Guidelines are valuable.”
As AAFP president, Dr. Heim says she won’t forget her family physician roots. Her platform is ambitious: Reform both the practice and the payment of healthcare in this country. “I think the country recognizes that the current way of paying for healthcare—built on volume and procedures rather than patient outcome—has resulted in a fragmented and disjointed process,” she says. “It’s not an even a system. … We have to look at patient experience and outcome, not ‘what procedure did we do to that patient?’
“Right now, we are paying more for lower-quality healthcare, and we are not getting the bang for our bucks. We cannot afford to continue to spend money and not get value; we really have to change this time.” TH
Carol Berczuk is a freelance writer based in New York City.
How can a family physician with a demanding clinical schedule juggle patient care with the grueling administrative and travel duties required of the president-elect of the American Academy of Family Physicians (AAFP)? Lori Heim, MD, FAAFP, found the perfect compromise: Give up the family practice and become a hospitalist.
She did so last November, soon after AAFP members voted her the next president of one of the nation’s largest medical organizations. In fact, she is the only hospitalist at 104-bed Scotland Memorial Hospital in Laurinburg, N.C.
“I was looking for an opportunity while I was working as president-elect and then president of the academy,” says Dr. Heim, who takes over as president of the 94,000-member AAFP in October. “Because of the heavy travel demands, it was not possible to keep my old practice.”
Although the career swap is borne of professional necessity, Dr. Heim says her transition to HM practice has been relatively easy. “I love it. [The new job] utilizes my prior training and skills,” she says. “In private practice, I was doing rounds on my own patients, then I would have to run to the office to see my other patients. I could see the advantages of using the hospitalist services. … Now, here I am on this side.”
An active AAFP member for nearly 25 years, Dr. Heim brings a unique confluence of medical training and experience to her new role. She has firsthand knowledge of the key issues intersecting primary care and hospital-based practice—care coordination, physician reimbursement, and quality improvement. She also acknowledges that walls need to be broken down when it comes to family physicians (FP) transitioning to HM careers. More complete training and improvement in hospital administrations’ understanding of an FP’s clinical capabilities will advance their entrance into hospitalist careers.
“I think it could become a large trend because of the financial constraints on family care,” Dr. Heim says, also noting the lifestyle benefits of an HM career. “FPs often cannot do both inpatient and outpatient care. Your productivity, if you are in the clinic, must make a tradeoff between rounds and office hours—and how late at night do I want to be doing rounds?”
Bumpy Road to HM
The transition from family practice to HM is working out well for Heim, but it wasn’t as smooth as one might expect for a decorated career physician.
The daughter of a military pilot, Dr. Heim earned her bachelor’s degree with honors from Portland State University and her medical degree at the Uniformed Services University of Health Sciences in Bethesda, Md. Following her residency at Andrews Air Force Base in Maryland and a fellowship in faculty development and research at the University of North Carolina at Chapel Hill, her military medical career resembled a spiral staircase. She went from staff physician to clinic chief to residency director to chief of medical staff, with a few stops in between. After 25 years of military service, she retired as an Air Force colonel and opened a private practice.
Even with decades of training and patient care under her belt, Dr. Heim wasn’t rubber-stamped into a hospitalist position. It’s an issue she hopes to address as part of her AAFP tenure. “I know of hospitals where family physicians can admit and treat their patients but not be considered for a hospitalist position. It happened to me,” she explains. “I went and found a hospital that would use my skills.”
Hospitalist Robert Harrington, MD, FHM, knows the feeling. He had a more traditional primary-care practice before entering a HM career, and he understands the intense financial and workload pressures of family practice. Now the vice president of medical affairs for Alpharetta, Ga.-based IN Compass Health Inc. and chair of SHM’s Family Medicine Task Force, he says “there are barriers to hiring because of the wide variability in family physician training.” The root of the problem is that residents in some programs get less hospital time and experience with HM-patient encounters. “In opposed programs, they compete with other specialties and get less time,” Dr. Harrington says. “In unopposed programs, they tend to get more hospital experience and more rotations in inpatient services. Those folks can transition with little to no difficulty to hospital medicine.”
SHM President Scott Flanders, MD, FHM, associate professor and director of the hospitalist program at the University of Michigan in Ann Arbor, sees great value in what FPs can bring to HM. He wants HM to be open to those interested in a career change; however, he agrees physician training and experience can be an obstacle in the recruitment process. “The training in internal medicine is more geared to hospital medicine than it is in family practice [training],” Dr. Flanders says. “FPs must make sure they have hospital training, including the ICU. Many FP programs may not have this.” He also says FPs looking at an HM career—and internists as well—need to be “up to speed” in systems-based practice.

—Lori Heim, MD, FAAFP, Scotland Memorial Hospital, Laurinburg, N.C., AAFP president-elect
Although they represent a small part of SHM’s membership, Dr. Harrington and his task force want FPs to have “representation and a voice” in the society. “They are a small but growing minority,” he says. “Four or five percent of hospitalists are family-medicine-trained by our membership rolls, but we believe it is a bigger number, as some may not be members of SHM. … We run into more problems with hospital administrators. Some of them insist on IM-trained physicians, and there just aren’t enough IMs out there.”
It’s a C-suite roadblock Dr. Heim is familiar with. “Sometimes there is a parochial view in using internists above family physicians. Why should family doctors be second? It’s a misunderstanding of the experience and what family physicians bring to the table,” she says. “They haven’t done it, so people don’t think they can. Some hospitals are underutilizing family physicians in a hospitalist role. If a physician has hospital privileges, there is no reason they should not be considered to be hired as a hospitalist.”
Tenure of Change
As AAFP president, Dr. Heim says she’ll work to improve working conditions for FPs and fight for her constituents’ rights as Washington debates national healthcare reform. The AAFP and SHM share a number of policy interests, including reimbursement reform, new technologies, and patient-safety initiatives.
“I would like to do more with the various societies to increase opportunities for family medicine,” Dr. Heim says. “In some ways, primary care is being squeezed all around. How are you going to increase the numbers of FPs when they earn less? You are not going to unless you reform the system.”
Another top priority is advancing the idea of a national network of electronic health records (EHRs). Dr. Heim says a linked EHR system would improve communication and handoffs, and help physicians limit medical errors. “That’s the key,” she says. “Until then, it is going to be a hodgepodge of solutions. As long as it requires multiple steps, there will always be slipups.
“When I was in the military, records were kept electronically, and I learned the value of having continuity of electronic records.”
Dr. Heim says she would like to partner with hospital-based physicians on QI projects. “Any doctor, it doesn’t matter who you are, has a role to play in quality improvement, either in procedures to improve outcomes in the operating room or ICU or ED. We all have a role in that,” she says. “That is one of the reasons we have supported comparative effectiveness research. Guidelines are valuable.”
As AAFP president, Dr. Heim says she won’t forget her family physician roots. Her platform is ambitious: Reform both the practice and the payment of healthcare in this country. “I think the country recognizes that the current way of paying for healthcare—built on volume and procedures rather than patient outcome—has resulted in a fragmented and disjointed process,” she says. “It’s not an even a system. … We have to look at patient experience and outcome, not ‘what procedure did we do to that patient?’
“Right now, we are paying more for lower-quality healthcare, and we are not getting the bang for our bucks. We cannot afford to continue to spend money and not get value; we really have to change this time.” TH
Carol Berczuk is a freelance writer based in New York City.
Financial Fallout
Not once has Vanessa Yasmin Calderón regretted her decision to go into primary care, but she admits she’s disquieted by the amount of debt she’s accumulated while attending the University of California at Los Angeles for medical school and Harvard University’s Kennedy School of Government in pursuit of a master’s degree in public policy.
“I will be 30 years old when I graduate,” says Calderón, who plans to receive her medical degree in 2010. “Right now, I have no retirement account, and I’m staring at loads of debt in a bad economy. There’s a lot to think about.”
Calderón estimates she will have more than $146,000 in loans when she graduates—a daunting sum for someone who used scholarship money and a part-time job to put herself through college. Although Calderón is committed to a career in emergency or general internal medicine (IM), she has watched many of her peers forgo primary care in favor of anesthesiology, dermatology, and surgical specialties—partly because they are worried about how they are going to pay back their education debt.
“I guarantee you that primary care is being the most affected by rising debt,” says Calderón, vice president of finances for the American Medical Student Association (AMSA).
Her personal observations correlate with more than 15 years’ worth of published medical studies that have found compensation plays a role in dissuading medical students who are facing mountains of debt from choosing primary care. That includes careers in IM and, by extension, careers in HM, as more than 82% of hospitalists consider themselves IM specialists, according to SHM’s 2007-2008 “Bi-Annual Survey on the State of the Hospital Medicine Movement.” This doesn’t bode well for the nation’s future, experts say, because primary care and IM comprise the foundation of our nation’s healthcare system.
While the steep decline in IM recruits has leveled off in recent years, the number of medical students choosing IM residency (2,632 seniors entered three-year IM residency programs in 2009) is nowhere near the high point (3,884) of the mid-1980s, says Steven E. Weinberger, MD, FACP, senior vice president for medical education and publishing for the American College of Physicians (ACP).
“If there is not a change in how we support students going through medical school, how can we be surprised when they choose a higher-paying specialty?” says Michael Rosenthal, MD, professor and vice chairman of academic programs and research in the Department of Family and Community Medicine at Thomas Jefferson University in Philadelphia.
Loan Obligations
In 2006, more than 84% of medical school graduates had educational debt, with a median debt of $120,000 for graduates of public medical schools and $160,000 for graduates of private medical schools, according to a 2007 report by the Association of American Medical Colleges (AAMC). In comparison, the same report shows that, in 2001, the median debt for public and private medical school graduates was $86,000 and $120,000, respectively.
Just as the rising cost of healthcare leads to skyrocketing health insurance premiums, so, too, does it result in higher tuition and fees for medical students, says Brian Hurley, MD, MBA, president of AMSA. Public medical schools in particular are affected as state governments, which are obliged to annually balance their budgets, often pay for burgeoning healthcare expenses by cutting subsidies to higher education, he says.
“In a way, universities are balancing their squeezed budgets on the backs of their students,” says Dr. Hurley, who recently graduated from the University of Southern California’s Keck School of Medicine with $300,000 in educational debt.
There is no regulatory body in place that can moderate medical school tuition increases, he laments. But medical students are partly to blame for the spiraling tuition costs, Dr. Hurley says, because students rarely base their school selections on tuition costs. As a result, medical schools aren’t forced to decelerate tuition hikes, because students aren’t taking them to task.
“When pre-med students decide to go to medical school, they have this idea that they will have more opportunities if they can go to Harvard or some other top medical school,” Dr. Hurley says. “Students want to go to the best school they can, and they trust that everything will work itself out in the end.”
Meanwhile, escalating tuition costs and debt loads deter prospective medical students from low-income backgrounds from going to medical school, which hampers efforts to diversify the nation’s medical workforce and provide quality healthcare in poorer communities. “People tend to practice medicine where they came from,” Dr. Hurley says. “It’s not a perfect correlation, but it does match up.”
For its part, AMSA is educating pre-med students on how to select more affordable medical schools that provide a quality education. The association also focuses on teaching medical students how to manage educational debt. “The public perception is that physicians are rich, and it’s a perception we haven’t successfully been able to combat,” Dr. Hurley says. “Right now, medical student debt is not seen as a healthcare issue. We can try to work within the Higher Education Act to better subsidize medical students’ education, but lawmakers tend to focus on undergraduate education.”
Nonprocedurals at Risk
But medical students’ rising debt is a healthcare issue, experts say. “Many students are now leaving medical school with over $200,000 in debt,” says Daniel Dressler, MD, FHM, SHM board member and education director for the HM section and associate program director for the IM residency program at Emory University’s School of Medicine in Atlanta. “As the cost of education increases each year and significantly outpaces the rate of increase in physician salaries, students may look toward specialties where they can pay that off within a more reasonable time frame while they begin their families and build their lives.”
Aside from primary care and IM, the medical fields that have been at the losing end of the bloated-educational-debt trend are nonprocedural-based IM specialties such as geriatrics, endocrinology, pulmonary/critical care, rheumatology, and infectious disease, says Jeffrey Wiese, MD, FACP, FHM, SHM president-elect and associate dean of graduate medical education and director of the IM residency program at Tulane University Hospital in New Orleans.
Doctors in nonprocedural-based IM specialties generally receive lower compensation than those in procedural-based IM specialties like cardiology, gastroenterology, and nephrology. For example, the median annual compensation for private-practice physicians in cardiology and gastroenterology is nearly $385,000; the median salary of endocrinologists and rheumatologists is $184,000; and the median salary for general internists is $166,000, according to a 2007 compensation survey by the Medical Group Management Association.
IM physician salaries always have been significantly less than the salaries of procedure-based specialists, Dr. Wiese says. “But now the workload of general internists has grown, and it hasn’t grown proportional to compensation, as compared to other specialties,” he says. “That’s compelling to students.”
Dr. Weinberger agrees the compensation disparity is disconcerting to medical students who consider IM because “they are choosing a harder lifestyle. It doesn’t help that the doctors who are practicing internal medicine complain about the hassles and the problems with reimbursement. The role models medical students look up to are not as happy as they used to be.”
—Daniel Dressler, MD, FHM, Emory University School of Medicine, Atlanta
HM Holds Its Own
Hospitalists seem to be surviving relatively well in these difficult times, according to data compiled by the American College of Physicians. In 2002, 4% of third-year IM residents surveyed said they were choosing HM. That number has risen steadily, to 10% in 2007 and 2008, Dr. Weinberger notes.
HM compensation varies widely, Dr. Wiese says; however, the mean salary for HM physicians was $196,700 in 2007, according to SHM survey data. That puts hospitalist salaries at the mid- to lower end of the scale when compared with all medical specialties but smack in the middle of IM specialties.
A 2008 study published in the Annals of Internal Medicine suggests that U.S. categorical IM residents with educational debt of $50,000 or more are more likely than those with no debt to choose a HM career, possibly because they can enter the work force right after residency training, as opposed to continuing with fellowship training for a subspecialty at substantially less compensation.1
For HM to continue gaining ground, many say the specialty has to go on the offensive and not wait for medical students and residents to decide to become hospitalists. “It will be more difficult to recruit from residency programs if there are fewer people going into internal medicine,” Dr. Dressler says. “Hospital medicine will simply be competing for a smaller pool of residents.”
Dr. Wiese says academia can contribute by providing a solid foundation in medicine and a clear path to HM careers as next-generation physicians and leaders. “Hospitalists assuming more of a teaching role are good not only for hospital medicine, but internal medicine education,” Dr. Wiese says. “The stronger the mentors, the more internal medicine students you’re going to recruit.”
The same can be said of medical practice settings, Dr. Weinberger explains. Many ambulatory settings in which medical students and residents work are among the most poorly supported and operated, even though they have the sickest patients, he says. That can be a huge turnoff for medical students. To counter that negative, students must be exposed to higher-quality ambulatory settings, Dr. Weinberger says.
Medical schools can help the cause by admitting students who show an inclination to go into primary care and IM, says Dr. Rosenthal, of Thomas Jefferson University. Those students are more likely to leave medical school in pursuit of a generalist career—especially if they’re matched with good IM mentors.
Federal and state governments should consider paying the educational loans of medical students who promise to practice primary care or IM for a certain period of time, especially in high-need communities, Dr. Rosenthal says. Fifteen years ago, he was a lead author in a study published in the Journal of the American Medical Association that found a significant number of fourth-year medical students would go into primary care, including general IM, if positive changes were made to income, hours worked, and loan repayment.2 Dr. Rosenthal says he’s not surprised physicians and researchers are writing about the same topic today.
“The article was written in the Clinton era, at a time when there was a sense the nation’s healthcare system might be reformed. But there was backlash to the plan,” Dr. Rosenthal says. “Today, we are again considering healthcare reform, except this time people are more willing to accept it because the high cost of healthcare is now affecting businesses and the economy.”
Change in Outlook
President Obama’s stated goal of extending health insurance to more Americans makes increasing the ranks of primary-care physicians, general internists, and hospitalists even more urgent, experts say. In Massachusetts, a state that is experimenting with universal health coverage for all of its residents, a shortfall in the primary-care work force is evident, Dr. Weinberger says. It is troubling news, because research consistently shows that when a primary-care physician coordinates a patient’s care, the result is fewer visits to the ED and medical specialists, he says.
“What this means is, we need more internists in the outpatient side to care for these patients longitudinally,” Dr. Dressler says. “We need more hospitalists, as the burden of inpatient care is very likely to grow as well.”
Dr. Rosenthal says more students will be attracted to medicine in part because the recession is making solid, good-paying jobs that play a vital role in communities very attractive. If better support were available for students interested in primary care, he says, he would have reason to hope more students would choose generalist careers.
“There was this expectation among people in their 20s that, if they were bright and able, they would have a nice lifestyle without having to work too hard. But the recession is having an effect on this generation’s outlook,” Dr. Rosenthal says. “I think there is a changing landscape out there.” TH
Lisa Ryan is a freelance writer based in New Jersey.
References
- McDonald FS, West CP, Popkave C, Kolars JC. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149(6): 416-420.
- Rosenthal MP, Diamond JJ, Rabinowitz HK, et al. Influence of income, hours worked, and loan repayment on medical students’ decision to pursue a primary care career. JAMA. 1994;271(12):914-917.
Not once has Vanessa Yasmin Calderón regretted her decision to go into primary care, but she admits she’s disquieted by the amount of debt she’s accumulated while attending the University of California at Los Angeles for medical school and Harvard University’s Kennedy School of Government in pursuit of a master’s degree in public policy.
“I will be 30 years old when I graduate,” says Calderón, who plans to receive her medical degree in 2010. “Right now, I have no retirement account, and I’m staring at loads of debt in a bad economy. There’s a lot to think about.”
Calderón estimates she will have more than $146,000 in loans when she graduates—a daunting sum for someone who used scholarship money and a part-time job to put herself through college. Although Calderón is committed to a career in emergency or general internal medicine (IM), she has watched many of her peers forgo primary care in favor of anesthesiology, dermatology, and surgical specialties—partly because they are worried about how they are going to pay back their education debt.
“I guarantee you that primary care is being the most affected by rising debt,” says Calderón, vice president of finances for the American Medical Student Association (AMSA).
Her personal observations correlate with more than 15 years’ worth of published medical studies that have found compensation plays a role in dissuading medical students who are facing mountains of debt from choosing primary care. That includes careers in IM and, by extension, careers in HM, as more than 82% of hospitalists consider themselves IM specialists, according to SHM’s 2007-2008 “Bi-Annual Survey on the State of the Hospital Medicine Movement.” This doesn’t bode well for the nation’s future, experts say, because primary care and IM comprise the foundation of our nation’s healthcare system.
While the steep decline in IM recruits has leveled off in recent years, the number of medical students choosing IM residency (2,632 seniors entered three-year IM residency programs in 2009) is nowhere near the high point (3,884) of the mid-1980s, says Steven E. Weinberger, MD, FACP, senior vice president for medical education and publishing for the American College of Physicians (ACP).
“If there is not a change in how we support students going through medical school, how can we be surprised when they choose a higher-paying specialty?” says Michael Rosenthal, MD, professor and vice chairman of academic programs and research in the Department of Family and Community Medicine at Thomas Jefferson University in Philadelphia.
Loan Obligations
In 2006, more than 84% of medical school graduates had educational debt, with a median debt of $120,000 for graduates of public medical schools and $160,000 for graduates of private medical schools, according to a 2007 report by the Association of American Medical Colleges (AAMC). In comparison, the same report shows that, in 2001, the median debt for public and private medical school graduates was $86,000 and $120,000, respectively.
Just as the rising cost of healthcare leads to skyrocketing health insurance premiums, so, too, does it result in higher tuition and fees for medical students, says Brian Hurley, MD, MBA, president of AMSA. Public medical schools in particular are affected as state governments, which are obliged to annually balance their budgets, often pay for burgeoning healthcare expenses by cutting subsidies to higher education, he says.
“In a way, universities are balancing their squeezed budgets on the backs of their students,” says Dr. Hurley, who recently graduated from the University of Southern California’s Keck School of Medicine with $300,000 in educational debt.
There is no regulatory body in place that can moderate medical school tuition increases, he laments. But medical students are partly to blame for the spiraling tuition costs, Dr. Hurley says, because students rarely base their school selections on tuition costs. As a result, medical schools aren’t forced to decelerate tuition hikes, because students aren’t taking them to task.
“When pre-med students decide to go to medical school, they have this idea that they will have more opportunities if they can go to Harvard or some other top medical school,” Dr. Hurley says. “Students want to go to the best school they can, and they trust that everything will work itself out in the end.”
Meanwhile, escalating tuition costs and debt loads deter prospective medical students from low-income backgrounds from going to medical school, which hampers efforts to diversify the nation’s medical workforce and provide quality healthcare in poorer communities. “People tend to practice medicine where they came from,” Dr. Hurley says. “It’s not a perfect correlation, but it does match up.”
For its part, AMSA is educating pre-med students on how to select more affordable medical schools that provide a quality education. The association also focuses on teaching medical students how to manage educational debt. “The public perception is that physicians are rich, and it’s a perception we haven’t successfully been able to combat,” Dr. Hurley says. “Right now, medical student debt is not seen as a healthcare issue. We can try to work within the Higher Education Act to better subsidize medical students’ education, but lawmakers tend to focus on undergraduate education.”
Nonprocedurals at Risk
But medical students’ rising debt is a healthcare issue, experts say. “Many students are now leaving medical school with over $200,000 in debt,” says Daniel Dressler, MD, FHM, SHM board member and education director for the HM section and associate program director for the IM residency program at Emory University’s School of Medicine in Atlanta. “As the cost of education increases each year and significantly outpaces the rate of increase in physician salaries, students may look toward specialties where they can pay that off within a more reasonable time frame while they begin their families and build their lives.”
Aside from primary care and IM, the medical fields that have been at the losing end of the bloated-educational-debt trend are nonprocedural-based IM specialties such as geriatrics, endocrinology, pulmonary/critical care, rheumatology, and infectious disease, says Jeffrey Wiese, MD, FACP, FHM, SHM president-elect and associate dean of graduate medical education and director of the IM residency program at Tulane University Hospital in New Orleans.
Doctors in nonprocedural-based IM specialties generally receive lower compensation than those in procedural-based IM specialties like cardiology, gastroenterology, and nephrology. For example, the median annual compensation for private-practice physicians in cardiology and gastroenterology is nearly $385,000; the median salary of endocrinologists and rheumatologists is $184,000; and the median salary for general internists is $166,000, according to a 2007 compensation survey by the Medical Group Management Association.
IM physician salaries always have been significantly less than the salaries of procedure-based specialists, Dr. Wiese says. “But now the workload of general internists has grown, and it hasn’t grown proportional to compensation, as compared to other specialties,” he says. “That’s compelling to students.”
Dr. Weinberger agrees the compensation disparity is disconcerting to medical students who consider IM because “they are choosing a harder lifestyle. It doesn’t help that the doctors who are practicing internal medicine complain about the hassles and the problems with reimbursement. The role models medical students look up to are not as happy as they used to be.”
—Daniel Dressler, MD, FHM, Emory University School of Medicine, Atlanta
HM Holds Its Own
Hospitalists seem to be surviving relatively well in these difficult times, according to data compiled by the American College of Physicians. In 2002, 4% of third-year IM residents surveyed said they were choosing HM. That number has risen steadily, to 10% in 2007 and 2008, Dr. Weinberger notes.
HM compensation varies widely, Dr. Wiese says; however, the mean salary for HM physicians was $196,700 in 2007, according to SHM survey data. That puts hospitalist salaries at the mid- to lower end of the scale when compared with all medical specialties but smack in the middle of IM specialties.
A 2008 study published in the Annals of Internal Medicine suggests that U.S. categorical IM residents with educational debt of $50,000 or more are more likely than those with no debt to choose a HM career, possibly because they can enter the work force right after residency training, as opposed to continuing with fellowship training for a subspecialty at substantially less compensation.1
For HM to continue gaining ground, many say the specialty has to go on the offensive and not wait for medical students and residents to decide to become hospitalists. “It will be more difficult to recruit from residency programs if there are fewer people going into internal medicine,” Dr. Dressler says. “Hospital medicine will simply be competing for a smaller pool of residents.”
Dr. Wiese says academia can contribute by providing a solid foundation in medicine and a clear path to HM careers as next-generation physicians and leaders. “Hospitalists assuming more of a teaching role are good not only for hospital medicine, but internal medicine education,” Dr. Wiese says. “The stronger the mentors, the more internal medicine students you’re going to recruit.”
The same can be said of medical practice settings, Dr. Weinberger explains. Many ambulatory settings in which medical students and residents work are among the most poorly supported and operated, even though they have the sickest patients, he says. That can be a huge turnoff for medical students. To counter that negative, students must be exposed to higher-quality ambulatory settings, Dr. Weinberger says.
Medical schools can help the cause by admitting students who show an inclination to go into primary care and IM, says Dr. Rosenthal, of Thomas Jefferson University. Those students are more likely to leave medical school in pursuit of a generalist career—especially if they’re matched with good IM mentors.
Federal and state governments should consider paying the educational loans of medical students who promise to practice primary care or IM for a certain period of time, especially in high-need communities, Dr. Rosenthal says. Fifteen years ago, he was a lead author in a study published in the Journal of the American Medical Association that found a significant number of fourth-year medical students would go into primary care, including general IM, if positive changes were made to income, hours worked, and loan repayment.2 Dr. Rosenthal says he’s not surprised physicians and researchers are writing about the same topic today.
“The article was written in the Clinton era, at a time when there was a sense the nation’s healthcare system might be reformed. But there was backlash to the plan,” Dr. Rosenthal says. “Today, we are again considering healthcare reform, except this time people are more willing to accept it because the high cost of healthcare is now affecting businesses and the economy.”
Change in Outlook
President Obama’s stated goal of extending health insurance to more Americans makes increasing the ranks of primary-care physicians, general internists, and hospitalists even more urgent, experts say. In Massachusetts, a state that is experimenting with universal health coverage for all of its residents, a shortfall in the primary-care work force is evident, Dr. Weinberger says. It is troubling news, because research consistently shows that when a primary-care physician coordinates a patient’s care, the result is fewer visits to the ED and medical specialists, he says.
“What this means is, we need more internists in the outpatient side to care for these patients longitudinally,” Dr. Dressler says. “We need more hospitalists, as the burden of inpatient care is very likely to grow as well.”
Dr. Rosenthal says more students will be attracted to medicine in part because the recession is making solid, good-paying jobs that play a vital role in communities very attractive. If better support were available for students interested in primary care, he says, he would have reason to hope more students would choose generalist careers.
“There was this expectation among people in their 20s that, if they were bright and able, they would have a nice lifestyle without having to work too hard. But the recession is having an effect on this generation’s outlook,” Dr. Rosenthal says. “I think there is a changing landscape out there.” TH
Lisa Ryan is a freelance writer based in New Jersey.
References
- McDonald FS, West CP, Popkave C, Kolars JC. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149(6): 416-420.
- Rosenthal MP, Diamond JJ, Rabinowitz HK, et al. Influence of income, hours worked, and loan repayment on medical students’ decision to pursue a primary care career. JAMA. 1994;271(12):914-917.
Not once has Vanessa Yasmin Calderón regretted her decision to go into primary care, but she admits she’s disquieted by the amount of debt she’s accumulated while attending the University of California at Los Angeles for medical school and Harvard University’s Kennedy School of Government in pursuit of a master’s degree in public policy.
“I will be 30 years old when I graduate,” says Calderón, who plans to receive her medical degree in 2010. “Right now, I have no retirement account, and I’m staring at loads of debt in a bad economy. There’s a lot to think about.”
Calderón estimates she will have more than $146,000 in loans when she graduates—a daunting sum for someone who used scholarship money and a part-time job to put herself through college. Although Calderón is committed to a career in emergency or general internal medicine (IM), she has watched many of her peers forgo primary care in favor of anesthesiology, dermatology, and surgical specialties—partly because they are worried about how they are going to pay back their education debt.
“I guarantee you that primary care is being the most affected by rising debt,” says Calderón, vice president of finances for the American Medical Student Association (AMSA).
Her personal observations correlate with more than 15 years’ worth of published medical studies that have found compensation plays a role in dissuading medical students who are facing mountains of debt from choosing primary care. That includes careers in IM and, by extension, careers in HM, as more than 82% of hospitalists consider themselves IM specialists, according to SHM’s 2007-2008 “Bi-Annual Survey on the State of the Hospital Medicine Movement.” This doesn’t bode well for the nation’s future, experts say, because primary care and IM comprise the foundation of our nation’s healthcare system.
While the steep decline in IM recruits has leveled off in recent years, the number of medical students choosing IM residency (2,632 seniors entered three-year IM residency programs in 2009) is nowhere near the high point (3,884) of the mid-1980s, says Steven E. Weinberger, MD, FACP, senior vice president for medical education and publishing for the American College of Physicians (ACP).
“If there is not a change in how we support students going through medical school, how can we be surprised when they choose a higher-paying specialty?” says Michael Rosenthal, MD, professor and vice chairman of academic programs and research in the Department of Family and Community Medicine at Thomas Jefferson University in Philadelphia.
Loan Obligations
In 2006, more than 84% of medical school graduates had educational debt, with a median debt of $120,000 for graduates of public medical schools and $160,000 for graduates of private medical schools, according to a 2007 report by the Association of American Medical Colleges (AAMC). In comparison, the same report shows that, in 2001, the median debt for public and private medical school graduates was $86,000 and $120,000, respectively.
Just as the rising cost of healthcare leads to skyrocketing health insurance premiums, so, too, does it result in higher tuition and fees for medical students, says Brian Hurley, MD, MBA, president of AMSA. Public medical schools in particular are affected as state governments, which are obliged to annually balance their budgets, often pay for burgeoning healthcare expenses by cutting subsidies to higher education, he says.
“In a way, universities are balancing their squeezed budgets on the backs of their students,” says Dr. Hurley, who recently graduated from the University of Southern California’s Keck School of Medicine with $300,000 in educational debt.
There is no regulatory body in place that can moderate medical school tuition increases, he laments. But medical students are partly to blame for the spiraling tuition costs, Dr. Hurley says, because students rarely base their school selections on tuition costs. As a result, medical schools aren’t forced to decelerate tuition hikes, because students aren’t taking them to task.
“When pre-med students decide to go to medical school, they have this idea that they will have more opportunities if they can go to Harvard or some other top medical school,” Dr. Hurley says. “Students want to go to the best school they can, and they trust that everything will work itself out in the end.”
Meanwhile, escalating tuition costs and debt loads deter prospective medical students from low-income backgrounds from going to medical school, which hampers efforts to diversify the nation’s medical workforce and provide quality healthcare in poorer communities. “People tend to practice medicine where they came from,” Dr. Hurley says. “It’s not a perfect correlation, but it does match up.”
For its part, AMSA is educating pre-med students on how to select more affordable medical schools that provide a quality education. The association also focuses on teaching medical students how to manage educational debt. “The public perception is that physicians are rich, and it’s a perception we haven’t successfully been able to combat,” Dr. Hurley says. “Right now, medical student debt is not seen as a healthcare issue. We can try to work within the Higher Education Act to better subsidize medical students’ education, but lawmakers tend to focus on undergraduate education.”
Nonprocedurals at Risk
But medical students’ rising debt is a healthcare issue, experts say. “Many students are now leaving medical school with over $200,000 in debt,” says Daniel Dressler, MD, FHM, SHM board member and education director for the HM section and associate program director for the IM residency program at Emory University’s School of Medicine in Atlanta. “As the cost of education increases each year and significantly outpaces the rate of increase in physician salaries, students may look toward specialties where they can pay that off within a more reasonable time frame while they begin their families and build their lives.”
Aside from primary care and IM, the medical fields that have been at the losing end of the bloated-educational-debt trend are nonprocedural-based IM specialties such as geriatrics, endocrinology, pulmonary/critical care, rheumatology, and infectious disease, says Jeffrey Wiese, MD, FACP, FHM, SHM president-elect and associate dean of graduate medical education and director of the IM residency program at Tulane University Hospital in New Orleans.
Doctors in nonprocedural-based IM specialties generally receive lower compensation than those in procedural-based IM specialties like cardiology, gastroenterology, and nephrology. For example, the median annual compensation for private-practice physicians in cardiology and gastroenterology is nearly $385,000; the median salary of endocrinologists and rheumatologists is $184,000; and the median salary for general internists is $166,000, according to a 2007 compensation survey by the Medical Group Management Association.
IM physician salaries always have been significantly less than the salaries of procedure-based specialists, Dr. Wiese says. “But now the workload of general internists has grown, and it hasn’t grown proportional to compensation, as compared to other specialties,” he says. “That’s compelling to students.”
Dr. Weinberger agrees the compensation disparity is disconcerting to medical students who consider IM because “they are choosing a harder lifestyle. It doesn’t help that the doctors who are practicing internal medicine complain about the hassles and the problems with reimbursement. The role models medical students look up to are not as happy as they used to be.”
—Daniel Dressler, MD, FHM, Emory University School of Medicine, Atlanta
HM Holds Its Own
Hospitalists seem to be surviving relatively well in these difficult times, according to data compiled by the American College of Physicians. In 2002, 4% of third-year IM residents surveyed said they were choosing HM. That number has risen steadily, to 10% in 2007 and 2008, Dr. Weinberger notes.
HM compensation varies widely, Dr. Wiese says; however, the mean salary for HM physicians was $196,700 in 2007, according to SHM survey data. That puts hospitalist salaries at the mid- to lower end of the scale when compared with all medical specialties but smack in the middle of IM specialties.
A 2008 study published in the Annals of Internal Medicine suggests that U.S. categorical IM residents with educational debt of $50,000 or more are more likely than those with no debt to choose a HM career, possibly because they can enter the work force right after residency training, as opposed to continuing with fellowship training for a subspecialty at substantially less compensation.1
For HM to continue gaining ground, many say the specialty has to go on the offensive and not wait for medical students and residents to decide to become hospitalists. “It will be more difficult to recruit from residency programs if there are fewer people going into internal medicine,” Dr. Dressler says. “Hospital medicine will simply be competing for a smaller pool of residents.”
Dr. Wiese says academia can contribute by providing a solid foundation in medicine and a clear path to HM careers as next-generation physicians and leaders. “Hospitalists assuming more of a teaching role are good not only for hospital medicine, but internal medicine education,” Dr. Wiese says. “The stronger the mentors, the more internal medicine students you’re going to recruit.”
The same can be said of medical practice settings, Dr. Weinberger explains. Many ambulatory settings in which medical students and residents work are among the most poorly supported and operated, even though they have the sickest patients, he says. That can be a huge turnoff for medical students. To counter that negative, students must be exposed to higher-quality ambulatory settings, Dr. Weinberger says.
Medical schools can help the cause by admitting students who show an inclination to go into primary care and IM, says Dr. Rosenthal, of Thomas Jefferson University. Those students are more likely to leave medical school in pursuit of a generalist career—especially if they’re matched with good IM mentors.
Federal and state governments should consider paying the educational loans of medical students who promise to practice primary care or IM for a certain period of time, especially in high-need communities, Dr. Rosenthal says. Fifteen years ago, he was a lead author in a study published in the Journal of the American Medical Association that found a significant number of fourth-year medical students would go into primary care, including general IM, if positive changes were made to income, hours worked, and loan repayment.2 Dr. Rosenthal says he’s not surprised physicians and researchers are writing about the same topic today.
“The article was written in the Clinton era, at a time when there was a sense the nation’s healthcare system might be reformed. But there was backlash to the plan,” Dr. Rosenthal says. “Today, we are again considering healthcare reform, except this time people are more willing to accept it because the high cost of healthcare is now affecting businesses and the economy.”
Change in Outlook
President Obama’s stated goal of extending health insurance to more Americans makes increasing the ranks of primary-care physicians, general internists, and hospitalists even more urgent, experts say. In Massachusetts, a state that is experimenting with universal health coverage for all of its residents, a shortfall in the primary-care work force is evident, Dr. Weinberger says. It is troubling news, because research consistently shows that when a primary-care physician coordinates a patient’s care, the result is fewer visits to the ED and medical specialists, he says.
“What this means is, we need more internists in the outpatient side to care for these patients longitudinally,” Dr. Dressler says. “We need more hospitalists, as the burden of inpatient care is very likely to grow as well.”
Dr. Rosenthal says more students will be attracted to medicine in part because the recession is making solid, good-paying jobs that play a vital role in communities very attractive. If better support were available for students interested in primary care, he says, he would have reason to hope more students would choose generalist careers.
“There was this expectation among people in their 20s that, if they were bright and able, they would have a nice lifestyle without having to work too hard. But the recession is having an effect on this generation’s outlook,” Dr. Rosenthal says. “I think there is a changing landscape out there.” TH
Lisa Ryan is a freelance writer based in New Jersey.
References
- McDonald FS, West CP, Popkave C, Kolars JC. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149(6): 416-420.
- Rosenthal MP, Diamond JJ, Rabinowitz HK, et al. Influence of income, hours worked, and loan repayment on medical students’ decision to pursue a primary care career. JAMA. 1994;271(12):914-917.
The Big One
In March 2005 the Agency for Healthcare Research and Quality and the HHS Office of Public Health Emergency Preparedness published a report of guidelines for officials on how to plan for delivering health and medical care in a mass casualty event.1
After federal, state, and local authorities’ failure to supply desperately needed assistance following Hurricane Katrina, that report of recommendations from a 39-member panel of experts in bioethics, emergency medicine, emergency management, health administration, health law, and policy is more crucial than ever. This report offers a framework for providing optimal medical care during a potential bioterrorist attack or other public health emergency.
How well do you know your institutions’ plans and protocols for these types of events? How personally prepared are you and your families? Overall, what should your highest concerns be in order to prepare yourself now and in the future?
Definitions
The term disaster is defined many ways, but typically all definitions involve some sort of impact on the community and interruption of services from business as usual beyond the point where outside assistance is needed. Defining what is meant by a mass casualty incident (MCI), on the other hand, is more relative to the location in which it is being declared.
“Typically a mass casualty event is thought of as one in which the number of patients exceeds the amount of resources that are routinely available,” says Andrew Garrett, MD, FAAP, the director of disaster response and pediatric preparedness programs at the National Center for Disaster Preparedness at Columbia University’s Joseph L. Mailman School of Public Health, New York. “But that is a dynamic definition because in Chicago a bus accident with 15 patients might not be a mass casualty incident, but in rural Cody, Wyoming, a car accident with four people might be. It’s where you exceed the resources that are available locally that is important.”
The difference between an emergency, a disaster, or an MCI revolves more around semantics, the environment in which you will work, and the short-term goals of patient care. “We’re not asking people to reinvent the way in which they practice medicine,” says Dr. Garrett “but a disaster or MCI changes the paradigm in which they do it—to do the most good for the most people.”
Who’s in Charge?
The Hospital Emergency Incident Command System (HEICS) was adapted from a plan to coordinate and improve the safety of the wildland firefighting system in California. It was transitioned to serve as a model in hospitals to meet the same goals of staff accountability and safety during a disaster response. HEICS places one “incident commander” at the top of the pyramid in charge of all the separate areas of responsibility, such as logistics, finance, operations, medical care, safety, and so on.
“The way the system works,” says Dr. Garrett, “is that everyone working in a hospital response is supervised by only one person who answers to the command staff. The goal is that there’s one incident commander who knows everything that’s going on at the incident to avoid the trap of multiple people making command decisions at the same time.”
Redundant command structure is a common problem in a large-scale response to disaster. That was certainly the case in Hurricane Katrina, he says, where multiple agencies—federal, state, and local—did not follow this model of disaster response.
“It’s a simple concept,” says Dr. Garrett, “but unless responders practice it, it is difficult to utilize in a real emergency.”
Every hospital should have a HEICS or similar structure set up and the key emergency response roles pre-identified by job title, he says. And while knowledge of weapons of mass destruction (WMD) and incident command is improving, says Stephen V. Cantrill, MD, FACEP, associate director, Department of Emergency Medicine at Denver Health Medical Center, “Some hospitals have taken it seriously; others wish the whole thing would go away.”
More than likely, in the event of a disaster, the HEICS organizational tree is outlined all the way to the top commander in your hospital’s plan. Your role, in general, may have already been determined in this plan, but the conventional wisdom in your hospital (as in most) may be: You’ll learn your roles and responsibilities when the time comes. In fact, depending on your setting, the hospitalist may hold the most senior position in-house overnight or on the weekend—especially if there is not an emergency department at the hospital.
“The thing is, at first people are going to look to the most senior clinician to be in charge during a crisis,” says Dr. Garrett. Perhaps the smaller the hospital, the more you need to know what to do and what is expected of you to fit into the larger picture in the community. “And even if it is a smaller hospital the system and the needs are the same.”
What Types of Care?
Although many types of events can be handled the same way, some involve additional concerns. “With WMD or a contagious disease outbreak, there is the added issue of ‘What’s the risk to me as a provider in the hospital?’” says Dr. Garrett. “And if it’s a community or statewide or national event, ‘What’s the risk to my family?’ Then you’re dealing with issues that aren’t business as usual.”
The hospitalist and the administration will then have to think about other complex issues such as how many people are not going to come to work. Added to that, with a smaller staff, you may need to ask, “What will the scope of my practice be if I’m called to the front of the hospital to help do triage? Roles and responsibilities can change very quickly,” he says.
“Hospitalists are invaluable resources in an institution and in fact [in disaster events] they will be pressed into service because of their location,” says Dr. Cantrill, who with colleagues has trained 15,000 healthcare providers throughout Colorado as one of 17 centers to receive a three-year grant from the Health Resources Services Administration (HRSA) to conduct WMD training. “Especially in the private sector when it hits the fan, the hospitalist is going to be one of the first people to be called.”
In most disasters, the hospitalist’s medical practice will be a departure from the details of daily practice. “Because the majority of hospitalists have internal medicine as their background … they tend to be very detail oriented, which is really their strength,” says Dr. Cantrill. “But in a case like this, they may not have that luxury.”
Another major consideration and “probably the stickiest one,” is altering your standards of care in terms of providing efficiency care or austere care as opposed to what you normally consider appropriate medical care.
What hospitalists will do in any disaster depends on the event—natural, biological, chemical, or use of weaponry—and how your metropolitan or rural area is set up. If it is a biological or bioterrorist event, the pathogen involved may make a difference. Although anthrax is not contagious, for instance, in the event of a large-scale airborne anthrax attack, the need for ventilators will quickly overwhelm resources.2 “That’s one of our largest areas of vulnerability,” says Dr. Cantrill, “whether we’re talking influenza or pneumonic plague, it still is an important factor: How many people can I support?”
The issue of limited ventilators may not be completely soluble, he explains. In ordinary circumstances hospitals can get, say, ventilators from a strategic national stockpile from which equipment can be flown out within 12 hours. Yet if an influenza pandemic breaks out, then the entire country may be involved, rendering that plan inoperable. And even if you have extra ventilators, do you have extra respiratory techs to administer them?
Dr. Cantrill’s institution, with a grant received from HRSA, offers a two-hour course to train people with some medical knowledge to be respiratory assistants who can manage ventilated patients in an emergency.
Injuries may increase exponentially in the case of a disaster. Other needs include vaccinations, treatment for dehydration, serious heat- and cold-related illness, or threats from floodwater (i.e., water laced with toxic chemicals, human waste, fire ants, rats, and snakes).3
Kate Rathbun, MD, MPH, family physician in Baton Rouge, La., is certified in disaster management and knows well the problems that can arise in providing medical care in such an event.4 When Hurricane Katrina hit in 2005, everyone in range of the winds, rain, and destruction, “hunkered down to weather the storm.” The day after the storm, Dr. Rathbun joined other providers and administrators, opened their clinic, and readied themselves to treat trauma and lacerations. It soon became obvious that their biggest health issue was the inability of the displaced to manage their chronic diseases. (Baton Rouge’s normal population of 600,000 exceeded a million within days.)
In cases of diabetes, cardiac disease, HIV infection, or tuberculosis, for example, being without medications might mean lethal disease exacerbations.3 In many cases, patients have no prior history documentation on presentation, and with computers often shut down the provider is faced with prescribing for or actually putting a stock of medications into patients’ hands.
Additional concerns pertain to those who cannot receive hemodialysis or seizure prophylaxis; or disrupted care for those with special needs such as hospice patients, the mentally and physically disabled, the elderly, and individuals in detox programs.
When Dr. Rathbun and her coworkers put a couple of nurses on the phones to handle incoming requests for drugs, she gave them some standards: If it’s for chronic disease medications, prescribe a 30-day supply and three refills (to ensure that 30 days later they would not once again be inundated with calls). When patients requested narcotics or scheduled drugs, they were told they would have to be seen by a provider.
Branching Points and Skill Sets
What will your community expect your institution to respond to and provide in the event of disaster? Here is where hospitalists can delineate what they can do when the time comes, says Erin Stucky, MD, a pediatric hospitalist at Children’s Hospital, San Diego.
“Most disaster preparedness algorithms have roles based on ‘hospital-based providers,’” she says, “but when it comes down to medical administration, many of them stop at the emergency department.”
From that point on they are likely to say “I don’t know”—that is, the rest of that decision tree is left in the hands of whoever is in the lead positions of physician, administrator, and nurse.
“That’s where the hospitalist can say, ‘Let me tell you my skill set,’” says Dr. Stucky, such as “I can triage patients; I can help to coordinate and disseminate information or help to outside providers who are calling; I can help to coordinate provider groups to go to different areas within our hospital to coordinate staffing … because I know operating rooms or I know this subset of patient types.”
At some institutions where hospitalists have been around for a longer time the disaster plan’s algorithm has branching points that don’t end in the emergency department. “Each [branch] has separate blocks that are horizontally equivalent,” says Dr. Stucky, “and the bleed-down [recognizes] the hospitalist as the major ward medical officer responsible for ensuring that floor 6, that’s neuro, and floor 5, hem-onc, and so on, have the correct staffing and are responsible for people reporting to them as well as dividing them as a labor pool into who’s available to go where.”
In general, however, regardless of setting, she says, a “hospitalist knows intimately the structure of the hospital, the flow between units, and can help other patients to get to different parts of the institution where care is still safe, such as observation areas.”
Communications: Up and Down, Out and In
Part of the global-facility thought process must include what communications will be for everything from the county medical system and EMS response to, within an institution, the communication between floors and between people on horizontal lines of authority. In addition, information in and out of the hospital from workers to their families is crucial so that workers can concentrate on the tasks at hand.
Questions must be considered ahead of time: How do I communicate to those people outside whom I need to have come in? How do I get response to the appropriate people who are calling in to find out how many patients we’re caring for? There may be other calls from someone who says, for example, that the ventilator has stopped working for her elderly mother.
And hospitalists must also be ready to support the urgent care or primary care satellite clinics and communicate what’s going on at the hospital, says Dr. Rathbun, “so that someone like me, who is a primary care practitioner in the community, can know that if I call this number or this person, I’m going to be able to say, ‘I’m down here at the [clinic] and here’s what I’ve got,’ or “I know things are terrible, but I have a diabetic you had in the hospital three weeks ago who’s crashed again, and you’ve got to find him a bed.’”
Communication plans might include the provision of satellite phones or two-way radios, says Dr. Stucky, and this will affect concrete issues, such as staffing and allowances for who can come and leave.
“In our institution we make this [communication] a unit-specific responsibility of the nurse team leader,” she says. “The nurses each have a phone and those nurse phones are freed up for any person available on that unit to be used to communicate with the outside world.”
Personal Disaster Plans
“I think another vitally important—and I mean vital importance in the same manner as vital signs—is for each hospitalist to have a personal disaster plan for their family/personal life,” says Mitchell Wilson, MD, medical director, FirstHealth of the Carolinas Hospitalist Services and section chief of Hospital Medicine in the Department of Medicine at the University of North Carolina at Chapel Hill. “As the front line ‘foot soldier,’ the potential to harm our families during a pandemic is enormous.”
Dr. Garrett agrees. “One of the things that we’re not so good at in this country is coming up with emergency plans for our own family—even those of us who are in the medical business and take care of others,” he says. “Taking this step just makes good sense—and serves to be able to maximize your own availability and also be confident that you have the ways and means to know that your family is safe and secure and given the best opportunity to survive in a disaster.”
According to Dr. Wilson, families with vulnerable members, such as the young, elderly, and infirm, must have a plan in place to minimize the risk to them. “The hospitalist who comes home sick [or] infected is a danger to the very safe place [to] which [hospitalists and their families] seek refuge,” he says.
Preparedness includes delineating in your family what your points of contact will be. “Part of the stress that’s involved in being a physician and being expected to report to work [may involve] worrying where your family is or whether they have a safe meeting place; who’s picking up the children from school; does the school for my children have a plan, etc.,” says Dr. Garrett.
If you know that your children’s school has an emergency plan, your spouse’s workplace has a plan, and any relative in a long-term care facility has a plan, you’ll be much more likely to stay on the job and care for patients.
“And if my child is on a school bus that needs to be evacuated somewhere out of town,” he says, “I want to know there’s a phone number that my whole family knows to reconnect somehow.”
No Assumptions
Losing utility power is always a concern in emergencies and disasters. “After 9/11 in New York City, lots of people flooded into emergency departments,” says Ann Waller, ScD, an associate professor in the Department of Emergency Medicine at the University of North Carolina at Chapel Hill and the UNC director for NC DETECT. (See “Disease Surveillance,” p. 20.)
“The emergency departments abandoned their electronic systems and went back to paper and pencil because it was faster to just do the bare minimum … and get them into each team than to enter all the information required,” she explains. “That was a real eye-opener for those of us who rely on electronic data.”
Preparing for crisis involves imagining the inaccessibility of all electronic communications and records, including data collection and surveillance, pharmacy, e-mail, and historical documentation and other medical records.
The general rule in disaster preparedness is to plan for 72-hour capacity: How and what do I need to exist for 72 hours? “And the standard is that you should try to do that for your average daily census plus 100 patients,” says Dr. Stucky.
Scheduling and staffing is another issue. “Be prepared to provide flex staffing and scheduling to provide surge capacity,” says Dr. Wilson.
Think on Your Feet: Training
If they are so inclined, hospitalists can become involved in disaster response, through disaster medical assistance teams, community emergency response teams, or through the Red Cross—to name a few. And there are plenty of ways to take advantage of free training, some of which provide CME.
Another important question to ask of your institution, says Dr. Stucky (who co-presented on the topic of disaster preparedness at this year’s SHM Annual Meeting) is whether they have run any mock disasters.
“You have to do that,” she says. “Half of disaster response is preparedness, but the other half is thinking on your feet. And there’s no way to do that without mocking a drill.”
While there can be value in computer-run mock-ups, “there’s nothing like doing it,” she says. “We learn at least 25 things every time we do it.” And though one drill does not a totally prepared institution make, “it does mean at least you have the right people in those strategic positions [and they] are people who can think on their feet.”
A valuable training resource from AHRQ is listed in the resources at the end of this article.5
Be Prepared
With the vast amount of information on disaster preparedness available, one clear goal is to narrow it to avoid feeling overwhelmed.
“I think that is a real challenge,” says Dr. Cantrill, “but the first step is the motivation to at least look.”
Take for example the motivation of a flu pandemic. “It’s going to happen sooner or later, one of these days, but we know it this time,” says Waller. “We have the ability to be more prepared. … This is a huge opportunity to see it coming and to do as much as we can [correctly]. Which is not to say we can avoid everything, but at least we can be as prepared as we’ve ever been able to be.”
Conclusion
For hospitalists, there are several key techniques for individuals to be able to increase their readiness for disaster in the workplace. The first is to avoid relying initially or entirely on external help to supply a response, says Dr. Garrett: “You are the medical response, and there may be a delay until outside assistance is available.”
A second key is to visualize—as well as possible—any circumstances you might face personally and professionally and to formulate questions, seek answers, and talk to colleagues and supervisors about what your role will be. A third factor is to participate in training in the form of drills and tabletop exercises for your hospital. An unpracticed disaster plan may be more dangerous than no plan at all. TH
Andrea Sattinger also writes the “Alliances” department in this issue.
References
- AHRQ. Altered Standards of Care in Mass Casualty Events. Rockville, MD: Agency for Healthcare Research and Quality; April 2005:Health Systems Research Inc. under Contract No. 290-04-0010.
- Hick JL, Hanfling D, Burstein JL, et al. Health care facility and community strategies for patient care surge capacity. Ann Emerg Med. 2004 Sep;44(3):253-261.
- Greenough PG, Kirsch TD. Hurricane Katrina. Public health response—assessing needs. N Engl J Med. 2005 Oct 13;353(15):1544-1546.
- Rathbun KC, Cranmer H. Hurricane Katrina and disaster medical care. N Engl J Med. 2006 Feb 16;354:772-773.
- Hsu EB, Jenckes MW, Catlett CL, et al. Training of hospital staff to respond to a mass casualty incident. Evidence Report/Technology Assessment No. 95. Rockville, MD: Agency for Healthcare Research and Quality. Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. 290-02-0018; June 2004: AHRQ Publication No. 04-E015-2. Accessible at: www.ahrq.gov/downloads/pub/evidence/pdf/hospmci/hospmci.pdf. Last accessed June 1, 2006.
Resources
National Links
- Centers for Disease Control, Emergency Preparedness and Response: www.bt.cdc.gov/
- The Hospital Emergency Incident Command System (HEICS) is an emergency management system that employs a logical management structure, defined responsibilities, clear reporting channels, and a common nomenclature to help unify hospitals with other emergency responders: www.emsa.cahwnet.gov/dms2/heics3.htm
- State, local, and tribal public health departments have their own public health preparedness and response plans.
- The National Center for Environmental Health (NCEH): www.cdc.gov/nceh/emergency.htm.
- Two other CDC resources contain materials to address public health preparedness needs: the Division of Emergency and Environmental Health Services (EEHS) and the Environmental Public Health Readiness Branch (EPHRB). See all-hazards public health emergency response guide): www.cdc.gov/nceh/eehs/
- U.S. Department of Homeland Security, for family preparedness: www.ready.gov/
- AHRQ bioterrorism link: www.ahrq.gov/news/pubcat/c_biot.htm#biot002
- George Washington University Institute for Crisis, Disaster, and Risk Management offers programs, including training, in the area of crisis, emergency and risk management: www.gwu.edu/~icdrm/
- North Carolina Links—North Carolina Office of Public Health Preparedness and Response (NC PHPR): www.epi.state.nc.us/epi/phpr/provides information and resources regarding the threat of bioterrorism and other emerging infectious diseases within the state and around the nation.
- The Health Alert Network (HAN) system is designed to immediately alert key health officials and care providers in North Carolina to acts of bioterrorism as well as other types of emerging disease threats: www.nchan.org/
- DPH Immunization branch: www.immunizenc.com/
- State Web sites, such as San Diego Country Office of Emergency Systems: www.co.sandiego.ca.us/oes/ or the County of San Diego Health and Human Services Terrorism Preparedness: www.co.san-diego.ca.us/terrorism/links.html
In March 2005 the Agency for Healthcare Research and Quality and the HHS Office of Public Health Emergency Preparedness published a report of guidelines for officials on how to plan for delivering health and medical care in a mass casualty event.1
After federal, state, and local authorities’ failure to supply desperately needed assistance following Hurricane Katrina, that report of recommendations from a 39-member panel of experts in bioethics, emergency medicine, emergency management, health administration, health law, and policy is more crucial than ever. This report offers a framework for providing optimal medical care during a potential bioterrorist attack or other public health emergency.
How well do you know your institutions’ plans and protocols for these types of events? How personally prepared are you and your families? Overall, what should your highest concerns be in order to prepare yourself now and in the future?
Definitions
The term disaster is defined many ways, but typically all definitions involve some sort of impact on the community and interruption of services from business as usual beyond the point where outside assistance is needed. Defining what is meant by a mass casualty incident (MCI), on the other hand, is more relative to the location in which it is being declared.
“Typically a mass casualty event is thought of as one in which the number of patients exceeds the amount of resources that are routinely available,” says Andrew Garrett, MD, FAAP, the director of disaster response and pediatric preparedness programs at the National Center for Disaster Preparedness at Columbia University’s Joseph L. Mailman School of Public Health, New York. “But that is a dynamic definition because in Chicago a bus accident with 15 patients might not be a mass casualty incident, but in rural Cody, Wyoming, a car accident with four people might be. It’s where you exceed the resources that are available locally that is important.”
The difference between an emergency, a disaster, or an MCI revolves more around semantics, the environment in which you will work, and the short-term goals of patient care. “We’re not asking people to reinvent the way in which they practice medicine,” says Dr. Garrett “but a disaster or MCI changes the paradigm in which they do it—to do the most good for the most people.”
Who’s in Charge?
The Hospital Emergency Incident Command System (HEICS) was adapted from a plan to coordinate and improve the safety of the wildland firefighting system in California. It was transitioned to serve as a model in hospitals to meet the same goals of staff accountability and safety during a disaster response. HEICS places one “incident commander” at the top of the pyramid in charge of all the separate areas of responsibility, such as logistics, finance, operations, medical care, safety, and so on.
“The way the system works,” says Dr. Garrett, “is that everyone working in a hospital response is supervised by only one person who answers to the command staff. The goal is that there’s one incident commander who knows everything that’s going on at the incident to avoid the trap of multiple people making command decisions at the same time.”
Redundant command structure is a common problem in a large-scale response to disaster. That was certainly the case in Hurricane Katrina, he says, where multiple agencies—federal, state, and local—did not follow this model of disaster response.
“It’s a simple concept,” says Dr. Garrett, “but unless responders practice it, it is difficult to utilize in a real emergency.”
Every hospital should have a HEICS or similar structure set up and the key emergency response roles pre-identified by job title, he says. And while knowledge of weapons of mass destruction (WMD) and incident command is improving, says Stephen V. Cantrill, MD, FACEP, associate director, Department of Emergency Medicine at Denver Health Medical Center, “Some hospitals have taken it seriously; others wish the whole thing would go away.”
More than likely, in the event of a disaster, the HEICS organizational tree is outlined all the way to the top commander in your hospital’s plan. Your role, in general, may have already been determined in this plan, but the conventional wisdom in your hospital (as in most) may be: You’ll learn your roles and responsibilities when the time comes. In fact, depending on your setting, the hospitalist may hold the most senior position in-house overnight or on the weekend—especially if there is not an emergency department at the hospital.
“The thing is, at first people are going to look to the most senior clinician to be in charge during a crisis,” says Dr. Garrett. Perhaps the smaller the hospital, the more you need to know what to do and what is expected of you to fit into the larger picture in the community. “And even if it is a smaller hospital the system and the needs are the same.”
What Types of Care?
Although many types of events can be handled the same way, some involve additional concerns. “With WMD or a contagious disease outbreak, there is the added issue of ‘What’s the risk to me as a provider in the hospital?’” says Dr. Garrett. “And if it’s a community or statewide or national event, ‘What’s the risk to my family?’ Then you’re dealing with issues that aren’t business as usual.”
The hospitalist and the administration will then have to think about other complex issues such as how many people are not going to come to work. Added to that, with a smaller staff, you may need to ask, “What will the scope of my practice be if I’m called to the front of the hospital to help do triage? Roles and responsibilities can change very quickly,” he says.
“Hospitalists are invaluable resources in an institution and in fact [in disaster events] they will be pressed into service because of their location,” says Dr. Cantrill, who with colleagues has trained 15,000 healthcare providers throughout Colorado as one of 17 centers to receive a three-year grant from the Health Resources Services Administration (HRSA) to conduct WMD training. “Especially in the private sector when it hits the fan, the hospitalist is going to be one of the first people to be called.”
In most disasters, the hospitalist’s medical practice will be a departure from the details of daily practice. “Because the majority of hospitalists have internal medicine as their background … they tend to be very detail oriented, which is really their strength,” says Dr. Cantrill. “But in a case like this, they may not have that luxury.”
Another major consideration and “probably the stickiest one,” is altering your standards of care in terms of providing efficiency care or austere care as opposed to what you normally consider appropriate medical care.
What hospitalists will do in any disaster depends on the event—natural, biological, chemical, or use of weaponry—and how your metropolitan or rural area is set up. If it is a biological or bioterrorist event, the pathogen involved may make a difference. Although anthrax is not contagious, for instance, in the event of a large-scale airborne anthrax attack, the need for ventilators will quickly overwhelm resources.2 “That’s one of our largest areas of vulnerability,” says Dr. Cantrill, “whether we’re talking influenza or pneumonic plague, it still is an important factor: How many people can I support?”
The issue of limited ventilators may not be completely soluble, he explains. In ordinary circumstances hospitals can get, say, ventilators from a strategic national stockpile from which equipment can be flown out within 12 hours. Yet if an influenza pandemic breaks out, then the entire country may be involved, rendering that plan inoperable. And even if you have extra ventilators, do you have extra respiratory techs to administer them?
Dr. Cantrill’s institution, with a grant received from HRSA, offers a two-hour course to train people with some medical knowledge to be respiratory assistants who can manage ventilated patients in an emergency.
Injuries may increase exponentially in the case of a disaster. Other needs include vaccinations, treatment for dehydration, serious heat- and cold-related illness, or threats from floodwater (i.e., water laced with toxic chemicals, human waste, fire ants, rats, and snakes).3
Kate Rathbun, MD, MPH, family physician in Baton Rouge, La., is certified in disaster management and knows well the problems that can arise in providing medical care in such an event.4 When Hurricane Katrina hit in 2005, everyone in range of the winds, rain, and destruction, “hunkered down to weather the storm.” The day after the storm, Dr. Rathbun joined other providers and administrators, opened their clinic, and readied themselves to treat trauma and lacerations. It soon became obvious that their biggest health issue was the inability of the displaced to manage their chronic diseases. (Baton Rouge’s normal population of 600,000 exceeded a million within days.)
In cases of diabetes, cardiac disease, HIV infection, or tuberculosis, for example, being without medications might mean lethal disease exacerbations.3 In many cases, patients have no prior history documentation on presentation, and with computers often shut down the provider is faced with prescribing for or actually putting a stock of medications into patients’ hands.
Additional concerns pertain to those who cannot receive hemodialysis or seizure prophylaxis; or disrupted care for those with special needs such as hospice patients, the mentally and physically disabled, the elderly, and individuals in detox programs.
When Dr. Rathbun and her coworkers put a couple of nurses on the phones to handle incoming requests for drugs, she gave them some standards: If it’s for chronic disease medications, prescribe a 30-day supply and three refills (to ensure that 30 days later they would not once again be inundated with calls). When patients requested narcotics or scheduled drugs, they were told they would have to be seen by a provider.
Branching Points and Skill Sets
What will your community expect your institution to respond to and provide in the event of disaster? Here is where hospitalists can delineate what they can do when the time comes, says Erin Stucky, MD, a pediatric hospitalist at Children’s Hospital, San Diego.
“Most disaster preparedness algorithms have roles based on ‘hospital-based providers,’” she says, “but when it comes down to medical administration, many of them stop at the emergency department.”
From that point on they are likely to say “I don’t know”—that is, the rest of that decision tree is left in the hands of whoever is in the lead positions of physician, administrator, and nurse.
“That’s where the hospitalist can say, ‘Let me tell you my skill set,’” says Dr. Stucky, such as “I can triage patients; I can help to coordinate and disseminate information or help to outside providers who are calling; I can help to coordinate provider groups to go to different areas within our hospital to coordinate staffing … because I know operating rooms or I know this subset of patient types.”
At some institutions where hospitalists have been around for a longer time the disaster plan’s algorithm has branching points that don’t end in the emergency department. “Each [branch] has separate blocks that are horizontally equivalent,” says Dr. Stucky, “and the bleed-down [recognizes] the hospitalist as the major ward medical officer responsible for ensuring that floor 6, that’s neuro, and floor 5, hem-onc, and so on, have the correct staffing and are responsible for people reporting to them as well as dividing them as a labor pool into who’s available to go where.”
In general, however, regardless of setting, she says, a “hospitalist knows intimately the structure of the hospital, the flow between units, and can help other patients to get to different parts of the institution where care is still safe, such as observation areas.”
Communications: Up and Down, Out and In
Part of the global-facility thought process must include what communications will be for everything from the county medical system and EMS response to, within an institution, the communication between floors and between people on horizontal lines of authority. In addition, information in and out of the hospital from workers to their families is crucial so that workers can concentrate on the tasks at hand.
Questions must be considered ahead of time: How do I communicate to those people outside whom I need to have come in? How do I get response to the appropriate people who are calling in to find out how many patients we’re caring for? There may be other calls from someone who says, for example, that the ventilator has stopped working for her elderly mother.
And hospitalists must also be ready to support the urgent care or primary care satellite clinics and communicate what’s going on at the hospital, says Dr. Rathbun, “so that someone like me, who is a primary care practitioner in the community, can know that if I call this number or this person, I’m going to be able to say, ‘I’m down here at the [clinic] and here’s what I’ve got,’ or “I know things are terrible, but I have a diabetic you had in the hospital three weeks ago who’s crashed again, and you’ve got to find him a bed.’”
Communication plans might include the provision of satellite phones or two-way radios, says Dr. Stucky, and this will affect concrete issues, such as staffing and allowances for who can come and leave.
“In our institution we make this [communication] a unit-specific responsibility of the nurse team leader,” she says. “The nurses each have a phone and those nurse phones are freed up for any person available on that unit to be used to communicate with the outside world.”
Personal Disaster Plans
“I think another vitally important—and I mean vital importance in the same manner as vital signs—is for each hospitalist to have a personal disaster plan for their family/personal life,” says Mitchell Wilson, MD, medical director, FirstHealth of the Carolinas Hospitalist Services and section chief of Hospital Medicine in the Department of Medicine at the University of North Carolina at Chapel Hill. “As the front line ‘foot soldier,’ the potential to harm our families during a pandemic is enormous.”
Dr. Garrett agrees. “One of the things that we’re not so good at in this country is coming up with emergency plans for our own family—even those of us who are in the medical business and take care of others,” he says. “Taking this step just makes good sense—and serves to be able to maximize your own availability and also be confident that you have the ways and means to know that your family is safe and secure and given the best opportunity to survive in a disaster.”
According to Dr. Wilson, families with vulnerable members, such as the young, elderly, and infirm, must have a plan in place to minimize the risk to them. “The hospitalist who comes home sick [or] infected is a danger to the very safe place [to] which [hospitalists and their families] seek refuge,” he says.
Preparedness includes delineating in your family what your points of contact will be. “Part of the stress that’s involved in being a physician and being expected to report to work [may involve] worrying where your family is or whether they have a safe meeting place; who’s picking up the children from school; does the school for my children have a plan, etc.,” says Dr. Garrett.
If you know that your children’s school has an emergency plan, your spouse’s workplace has a plan, and any relative in a long-term care facility has a plan, you’ll be much more likely to stay on the job and care for patients.
“And if my child is on a school bus that needs to be evacuated somewhere out of town,” he says, “I want to know there’s a phone number that my whole family knows to reconnect somehow.”
No Assumptions
Losing utility power is always a concern in emergencies and disasters. “After 9/11 in New York City, lots of people flooded into emergency departments,” says Ann Waller, ScD, an associate professor in the Department of Emergency Medicine at the University of North Carolina at Chapel Hill and the UNC director for NC DETECT. (See “Disease Surveillance,” p. 20.)
“The emergency departments abandoned their electronic systems and went back to paper and pencil because it was faster to just do the bare minimum … and get them into each team than to enter all the information required,” she explains. “That was a real eye-opener for those of us who rely on electronic data.”
Preparing for crisis involves imagining the inaccessibility of all electronic communications and records, including data collection and surveillance, pharmacy, e-mail, and historical documentation and other medical records.
The general rule in disaster preparedness is to plan for 72-hour capacity: How and what do I need to exist for 72 hours? “And the standard is that you should try to do that for your average daily census plus 100 patients,” says Dr. Stucky.
Scheduling and staffing is another issue. “Be prepared to provide flex staffing and scheduling to provide surge capacity,” says Dr. Wilson.
Think on Your Feet: Training
If they are so inclined, hospitalists can become involved in disaster response, through disaster medical assistance teams, community emergency response teams, or through the Red Cross—to name a few. And there are plenty of ways to take advantage of free training, some of which provide CME.
Another important question to ask of your institution, says Dr. Stucky (who co-presented on the topic of disaster preparedness at this year’s SHM Annual Meeting) is whether they have run any mock disasters.
“You have to do that,” she says. “Half of disaster response is preparedness, but the other half is thinking on your feet. And there’s no way to do that without mocking a drill.”
While there can be value in computer-run mock-ups, “there’s nothing like doing it,” she says. “We learn at least 25 things every time we do it.” And though one drill does not a totally prepared institution make, “it does mean at least you have the right people in those strategic positions [and they] are people who can think on their feet.”
A valuable training resource from AHRQ is listed in the resources at the end of this article.5
Be Prepared
With the vast amount of information on disaster preparedness available, one clear goal is to narrow it to avoid feeling overwhelmed.
“I think that is a real challenge,” says Dr. Cantrill, “but the first step is the motivation to at least look.”
Take for example the motivation of a flu pandemic. “It’s going to happen sooner or later, one of these days, but we know it this time,” says Waller. “We have the ability to be more prepared. … This is a huge opportunity to see it coming and to do as much as we can [correctly]. Which is not to say we can avoid everything, but at least we can be as prepared as we’ve ever been able to be.”
Conclusion
For hospitalists, there are several key techniques for individuals to be able to increase their readiness for disaster in the workplace. The first is to avoid relying initially or entirely on external help to supply a response, says Dr. Garrett: “You are the medical response, and there may be a delay until outside assistance is available.”
A second key is to visualize—as well as possible—any circumstances you might face personally and professionally and to formulate questions, seek answers, and talk to colleagues and supervisors about what your role will be. A third factor is to participate in training in the form of drills and tabletop exercises for your hospital. An unpracticed disaster plan may be more dangerous than no plan at all. TH
Andrea Sattinger also writes the “Alliances” department in this issue.
References
- AHRQ. Altered Standards of Care in Mass Casualty Events. Rockville, MD: Agency for Healthcare Research and Quality; April 2005:Health Systems Research Inc. under Contract No. 290-04-0010.
- Hick JL, Hanfling D, Burstein JL, et al. Health care facility and community strategies for patient care surge capacity. Ann Emerg Med. 2004 Sep;44(3):253-261.
- Greenough PG, Kirsch TD. Hurricane Katrina. Public health response—assessing needs. N Engl J Med. 2005 Oct 13;353(15):1544-1546.
- Rathbun KC, Cranmer H. Hurricane Katrina and disaster medical care. N Engl J Med. 2006 Feb 16;354:772-773.
- Hsu EB, Jenckes MW, Catlett CL, et al. Training of hospital staff to respond to a mass casualty incident. Evidence Report/Technology Assessment No. 95. Rockville, MD: Agency for Healthcare Research and Quality. Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. 290-02-0018; June 2004: AHRQ Publication No. 04-E015-2. Accessible at: www.ahrq.gov/downloads/pub/evidence/pdf/hospmci/hospmci.pdf. Last accessed June 1, 2006.
Resources
National Links
- Centers for Disease Control, Emergency Preparedness and Response: www.bt.cdc.gov/
- The Hospital Emergency Incident Command System (HEICS) is an emergency management system that employs a logical management structure, defined responsibilities, clear reporting channels, and a common nomenclature to help unify hospitals with other emergency responders: www.emsa.cahwnet.gov/dms2/heics3.htm
- State, local, and tribal public health departments have their own public health preparedness and response plans.
- The National Center for Environmental Health (NCEH): www.cdc.gov/nceh/emergency.htm.
- Two other CDC resources contain materials to address public health preparedness needs: the Division of Emergency and Environmental Health Services (EEHS) and the Environmental Public Health Readiness Branch (EPHRB). See all-hazards public health emergency response guide): www.cdc.gov/nceh/eehs/
- U.S. Department of Homeland Security, for family preparedness: www.ready.gov/
- AHRQ bioterrorism link: www.ahrq.gov/news/pubcat/c_biot.htm#biot002
- George Washington University Institute for Crisis, Disaster, and Risk Management offers programs, including training, in the area of crisis, emergency and risk management: www.gwu.edu/~icdrm/
- North Carolina Links—North Carolina Office of Public Health Preparedness and Response (NC PHPR): www.epi.state.nc.us/epi/phpr/provides information and resources regarding the threat of bioterrorism and other emerging infectious diseases within the state and around the nation.
- The Health Alert Network (HAN) system is designed to immediately alert key health officials and care providers in North Carolina to acts of bioterrorism as well as other types of emerging disease threats: www.nchan.org/
- DPH Immunization branch: www.immunizenc.com/
- State Web sites, such as San Diego Country Office of Emergency Systems: www.co.sandiego.ca.us/oes/ or the County of San Diego Health and Human Services Terrorism Preparedness: www.co.san-diego.ca.us/terrorism/links.html
In March 2005 the Agency for Healthcare Research and Quality and the HHS Office of Public Health Emergency Preparedness published a report of guidelines for officials on how to plan for delivering health and medical care in a mass casualty event.1
After federal, state, and local authorities’ failure to supply desperately needed assistance following Hurricane Katrina, that report of recommendations from a 39-member panel of experts in bioethics, emergency medicine, emergency management, health administration, health law, and policy is more crucial than ever. This report offers a framework for providing optimal medical care during a potential bioterrorist attack or other public health emergency.
How well do you know your institutions’ plans and protocols for these types of events? How personally prepared are you and your families? Overall, what should your highest concerns be in order to prepare yourself now and in the future?
Definitions
The term disaster is defined many ways, but typically all definitions involve some sort of impact on the community and interruption of services from business as usual beyond the point where outside assistance is needed. Defining what is meant by a mass casualty incident (MCI), on the other hand, is more relative to the location in which it is being declared.
“Typically a mass casualty event is thought of as one in which the number of patients exceeds the amount of resources that are routinely available,” says Andrew Garrett, MD, FAAP, the director of disaster response and pediatric preparedness programs at the National Center for Disaster Preparedness at Columbia University’s Joseph L. Mailman School of Public Health, New York. “But that is a dynamic definition because in Chicago a bus accident with 15 patients might not be a mass casualty incident, but in rural Cody, Wyoming, a car accident with four people might be. It’s where you exceed the resources that are available locally that is important.”
The difference between an emergency, a disaster, or an MCI revolves more around semantics, the environment in which you will work, and the short-term goals of patient care. “We’re not asking people to reinvent the way in which they practice medicine,” says Dr. Garrett “but a disaster or MCI changes the paradigm in which they do it—to do the most good for the most people.”
Who’s in Charge?
The Hospital Emergency Incident Command System (HEICS) was adapted from a plan to coordinate and improve the safety of the wildland firefighting system in California. It was transitioned to serve as a model in hospitals to meet the same goals of staff accountability and safety during a disaster response. HEICS places one “incident commander” at the top of the pyramid in charge of all the separate areas of responsibility, such as logistics, finance, operations, medical care, safety, and so on.
“The way the system works,” says Dr. Garrett, “is that everyone working in a hospital response is supervised by only one person who answers to the command staff. The goal is that there’s one incident commander who knows everything that’s going on at the incident to avoid the trap of multiple people making command decisions at the same time.”
Redundant command structure is a common problem in a large-scale response to disaster. That was certainly the case in Hurricane Katrina, he says, where multiple agencies—federal, state, and local—did not follow this model of disaster response.
“It’s a simple concept,” says Dr. Garrett, “but unless responders practice it, it is difficult to utilize in a real emergency.”
Every hospital should have a HEICS or similar structure set up and the key emergency response roles pre-identified by job title, he says. And while knowledge of weapons of mass destruction (WMD) and incident command is improving, says Stephen V. Cantrill, MD, FACEP, associate director, Department of Emergency Medicine at Denver Health Medical Center, “Some hospitals have taken it seriously; others wish the whole thing would go away.”
More than likely, in the event of a disaster, the HEICS organizational tree is outlined all the way to the top commander in your hospital’s plan. Your role, in general, may have already been determined in this plan, but the conventional wisdom in your hospital (as in most) may be: You’ll learn your roles and responsibilities when the time comes. In fact, depending on your setting, the hospitalist may hold the most senior position in-house overnight or on the weekend—especially if there is not an emergency department at the hospital.
“The thing is, at first people are going to look to the most senior clinician to be in charge during a crisis,” says Dr. Garrett. Perhaps the smaller the hospital, the more you need to know what to do and what is expected of you to fit into the larger picture in the community. “And even if it is a smaller hospital the system and the needs are the same.”
What Types of Care?
Although many types of events can be handled the same way, some involve additional concerns. “With WMD or a contagious disease outbreak, there is the added issue of ‘What’s the risk to me as a provider in the hospital?’” says Dr. Garrett. “And if it’s a community or statewide or national event, ‘What’s the risk to my family?’ Then you’re dealing with issues that aren’t business as usual.”
The hospitalist and the administration will then have to think about other complex issues such as how many people are not going to come to work. Added to that, with a smaller staff, you may need to ask, “What will the scope of my practice be if I’m called to the front of the hospital to help do triage? Roles and responsibilities can change very quickly,” he says.
“Hospitalists are invaluable resources in an institution and in fact [in disaster events] they will be pressed into service because of their location,” says Dr. Cantrill, who with colleagues has trained 15,000 healthcare providers throughout Colorado as one of 17 centers to receive a three-year grant from the Health Resources Services Administration (HRSA) to conduct WMD training. “Especially in the private sector when it hits the fan, the hospitalist is going to be one of the first people to be called.”
In most disasters, the hospitalist’s medical practice will be a departure from the details of daily practice. “Because the majority of hospitalists have internal medicine as their background … they tend to be very detail oriented, which is really their strength,” says Dr. Cantrill. “But in a case like this, they may not have that luxury.”
Another major consideration and “probably the stickiest one,” is altering your standards of care in terms of providing efficiency care or austere care as opposed to what you normally consider appropriate medical care.
What hospitalists will do in any disaster depends on the event—natural, biological, chemical, or use of weaponry—and how your metropolitan or rural area is set up. If it is a biological or bioterrorist event, the pathogen involved may make a difference. Although anthrax is not contagious, for instance, in the event of a large-scale airborne anthrax attack, the need for ventilators will quickly overwhelm resources.2 “That’s one of our largest areas of vulnerability,” says Dr. Cantrill, “whether we’re talking influenza or pneumonic plague, it still is an important factor: How many people can I support?”
The issue of limited ventilators may not be completely soluble, he explains. In ordinary circumstances hospitals can get, say, ventilators from a strategic national stockpile from which equipment can be flown out within 12 hours. Yet if an influenza pandemic breaks out, then the entire country may be involved, rendering that plan inoperable. And even if you have extra ventilators, do you have extra respiratory techs to administer them?
Dr. Cantrill’s institution, with a grant received from HRSA, offers a two-hour course to train people with some medical knowledge to be respiratory assistants who can manage ventilated patients in an emergency.
Injuries may increase exponentially in the case of a disaster. Other needs include vaccinations, treatment for dehydration, serious heat- and cold-related illness, or threats from floodwater (i.e., water laced with toxic chemicals, human waste, fire ants, rats, and snakes).3
Kate Rathbun, MD, MPH, family physician in Baton Rouge, La., is certified in disaster management and knows well the problems that can arise in providing medical care in such an event.4 When Hurricane Katrina hit in 2005, everyone in range of the winds, rain, and destruction, “hunkered down to weather the storm.” The day after the storm, Dr. Rathbun joined other providers and administrators, opened their clinic, and readied themselves to treat trauma and lacerations. It soon became obvious that their biggest health issue was the inability of the displaced to manage their chronic diseases. (Baton Rouge’s normal population of 600,000 exceeded a million within days.)
In cases of diabetes, cardiac disease, HIV infection, or tuberculosis, for example, being without medications might mean lethal disease exacerbations.3 In many cases, patients have no prior history documentation on presentation, and with computers often shut down the provider is faced with prescribing for or actually putting a stock of medications into patients’ hands.
Additional concerns pertain to those who cannot receive hemodialysis or seizure prophylaxis; or disrupted care for those with special needs such as hospice patients, the mentally and physically disabled, the elderly, and individuals in detox programs.
When Dr. Rathbun and her coworkers put a couple of nurses on the phones to handle incoming requests for drugs, she gave them some standards: If it’s for chronic disease medications, prescribe a 30-day supply and three refills (to ensure that 30 days later they would not once again be inundated with calls). When patients requested narcotics or scheduled drugs, they were told they would have to be seen by a provider.
Branching Points and Skill Sets
What will your community expect your institution to respond to and provide in the event of disaster? Here is where hospitalists can delineate what they can do when the time comes, says Erin Stucky, MD, a pediatric hospitalist at Children’s Hospital, San Diego.
“Most disaster preparedness algorithms have roles based on ‘hospital-based providers,’” she says, “but when it comes down to medical administration, many of them stop at the emergency department.”
From that point on they are likely to say “I don’t know”—that is, the rest of that decision tree is left in the hands of whoever is in the lead positions of physician, administrator, and nurse.
“That’s where the hospitalist can say, ‘Let me tell you my skill set,’” says Dr. Stucky, such as “I can triage patients; I can help to coordinate and disseminate information or help to outside providers who are calling; I can help to coordinate provider groups to go to different areas within our hospital to coordinate staffing … because I know operating rooms or I know this subset of patient types.”
At some institutions where hospitalists have been around for a longer time the disaster plan’s algorithm has branching points that don’t end in the emergency department. “Each [branch] has separate blocks that are horizontally equivalent,” says Dr. Stucky, “and the bleed-down [recognizes] the hospitalist as the major ward medical officer responsible for ensuring that floor 6, that’s neuro, and floor 5, hem-onc, and so on, have the correct staffing and are responsible for people reporting to them as well as dividing them as a labor pool into who’s available to go where.”
In general, however, regardless of setting, she says, a “hospitalist knows intimately the structure of the hospital, the flow between units, and can help other patients to get to different parts of the institution where care is still safe, such as observation areas.”
Communications: Up and Down, Out and In
Part of the global-facility thought process must include what communications will be for everything from the county medical system and EMS response to, within an institution, the communication between floors and between people on horizontal lines of authority. In addition, information in and out of the hospital from workers to their families is crucial so that workers can concentrate on the tasks at hand.
Questions must be considered ahead of time: How do I communicate to those people outside whom I need to have come in? How do I get response to the appropriate people who are calling in to find out how many patients we’re caring for? There may be other calls from someone who says, for example, that the ventilator has stopped working for her elderly mother.
And hospitalists must also be ready to support the urgent care or primary care satellite clinics and communicate what’s going on at the hospital, says Dr. Rathbun, “so that someone like me, who is a primary care practitioner in the community, can know that if I call this number or this person, I’m going to be able to say, ‘I’m down here at the [clinic] and here’s what I’ve got,’ or “I know things are terrible, but I have a diabetic you had in the hospital three weeks ago who’s crashed again, and you’ve got to find him a bed.’”
Communication plans might include the provision of satellite phones or two-way radios, says Dr. Stucky, and this will affect concrete issues, such as staffing and allowances for who can come and leave.
“In our institution we make this [communication] a unit-specific responsibility of the nurse team leader,” she says. “The nurses each have a phone and those nurse phones are freed up for any person available on that unit to be used to communicate with the outside world.”
Personal Disaster Plans
“I think another vitally important—and I mean vital importance in the same manner as vital signs—is for each hospitalist to have a personal disaster plan for their family/personal life,” says Mitchell Wilson, MD, medical director, FirstHealth of the Carolinas Hospitalist Services and section chief of Hospital Medicine in the Department of Medicine at the University of North Carolina at Chapel Hill. “As the front line ‘foot soldier,’ the potential to harm our families during a pandemic is enormous.”
Dr. Garrett agrees. “One of the things that we’re not so good at in this country is coming up with emergency plans for our own family—even those of us who are in the medical business and take care of others,” he says. “Taking this step just makes good sense—and serves to be able to maximize your own availability and also be confident that you have the ways and means to know that your family is safe and secure and given the best opportunity to survive in a disaster.”
According to Dr. Wilson, families with vulnerable members, such as the young, elderly, and infirm, must have a plan in place to minimize the risk to them. “The hospitalist who comes home sick [or] infected is a danger to the very safe place [to] which [hospitalists and their families] seek refuge,” he says.
Preparedness includes delineating in your family what your points of contact will be. “Part of the stress that’s involved in being a physician and being expected to report to work [may involve] worrying where your family is or whether they have a safe meeting place; who’s picking up the children from school; does the school for my children have a plan, etc.,” says Dr. Garrett.
If you know that your children’s school has an emergency plan, your spouse’s workplace has a plan, and any relative in a long-term care facility has a plan, you’ll be much more likely to stay on the job and care for patients.
“And if my child is on a school bus that needs to be evacuated somewhere out of town,” he says, “I want to know there’s a phone number that my whole family knows to reconnect somehow.”
No Assumptions
Losing utility power is always a concern in emergencies and disasters. “After 9/11 in New York City, lots of people flooded into emergency departments,” says Ann Waller, ScD, an associate professor in the Department of Emergency Medicine at the University of North Carolina at Chapel Hill and the UNC director for NC DETECT. (See “Disease Surveillance,” p. 20.)
“The emergency departments abandoned their electronic systems and went back to paper and pencil because it was faster to just do the bare minimum … and get them into each team than to enter all the information required,” she explains. “That was a real eye-opener for those of us who rely on electronic data.”
Preparing for crisis involves imagining the inaccessibility of all electronic communications and records, including data collection and surveillance, pharmacy, e-mail, and historical documentation and other medical records.
The general rule in disaster preparedness is to plan for 72-hour capacity: How and what do I need to exist for 72 hours? “And the standard is that you should try to do that for your average daily census plus 100 patients,” says Dr. Stucky.
Scheduling and staffing is another issue. “Be prepared to provide flex staffing and scheduling to provide surge capacity,” says Dr. Wilson.
Think on Your Feet: Training
If they are so inclined, hospitalists can become involved in disaster response, through disaster medical assistance teams, community emergency response teams, or through the Red Cross—to name a few. And there are plenty of ways to take advantage of free training, some of which provide CME.
Another important question to ask of your institution, says Dr. Stucky (who co-presented on the topic of disaster preparedness at this year’s SHM Annual Meeting) is whether they have run any mock disasters.
“You have to do that,” she says. “Half of disaster response is preparedness, but the other half is thinking on your feet. And there’s no way to do that without mocking a drill.”
While there can be value in computer-run mock-ups, “there’s nothing like doing it,” she says. “We learn at least 25 things every time we do it.” And though one drill does not a totally prepared institution make, “it does mean at least you have the right people in those strategic positions [and they] are people who can think on their feet.”
A valuable training resource from AHRQ is listed in the resources at the end of this article.5
Be Prepared
With the vast amount of information on disaster preparedness available, one clear goal is to narrow it to avoid feeling overwhelmed.
“I think that is a real challenge,” says Dr. Cantrill, “but the first step is the motivation to at least look.”
Take for example the motivation of a flu pandemic. “It’s going to happen sooner or later, one of these days, but we know it this time,” says Waller. “We have the ability to be more prepared. … This is a huge opportunity to see it coming and to do as much as we can [correctly]. Which is not to say we can avoid everything, but at least we can be as prepared as we’ve ever been able to be.”
Conclusion
For hospitalists, there are several key techniques for individuals to be able to increase their readiness for disaster in the workplace. The first is to avoid relying initially or entirely on external help to supply a response, says Dr. Garrett: “You are the medical response, and there may be a delay until outside assistance is available.”
A second key is to visualize—as well as possible—any circumstances you might face personally and professionally and to formulate questions, seek answers, and talk to colleagues and supervisors about what your role will be. A third factor is to participate in training in the form of drills and tabletop exercises for your hospital. An unpracticed disaster plan may be more dangerous than no plan at all. TH
Andrea Sattinger also writes the “Alliances” department in this issue.
References
- AHRQ. Altered Standards of Care in Mass Casualty Events. Rockville, MD: Agency for Healthcare Research and Quality; April 2005:Health Systems Research Inc. under Contract No. 290-04-0010.
- Hick JL, Hanfling D, Burstein JL, et al. Health care facility and community strategies for patient care surge capacity. Ann Emerg Med. 2004 Sep;44(3):253-261.
- Greenough PG, Kirsch TD. Hurricane Katrina. Public health response—assessing needs. N Engl J Med. 2005 Oct 13;353(15):1544-1546.
- Rathbun KC, Cranmer H. Hurricane Katrina and disaster medical care. N Engl J Med. 2006 Feb 16;354:772-773.
- Hsu EB, Jenckes MW, Catlett CL, et al. Training of hospital staff to respond to a mass casualty incident. Evidence Report/Technology Assessment No. 95. Rockville, MD: Agency for Healthcare Research and Quality. Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. 290-02-0018; June 2004: AHRQ Publication No. 04-E015-2. Accessible at: www.ahrq.gov/downloads/pub/evidence/pdf/hospmci/hospmci.pdf. Last accessed June 1, 2006.
Resources
National Links
- Centers for Disease Control, Emergency Preparedness and Response: www.bt.cdc.gov/
- The Hospital Emergency Incident Command System (HEICS) is an emergency management system that employs a logical management structure, defined responsibilities, clear reporting channels, and a common nomenclature to help unify hospitals with other emergency responders: www.emsa.cahwnet.gov/dms2/heics3.htm
- State, local, and tribal public health departments have their own public health preparedness and response plans.
- The National Center for Environmental Health (NCEH): www.cdc.gov/nceh/emergency.htm.
- Two other CDC resources contain materials to address public health preparedness needs: the Division of Emergency and Environmental Health Services (EEHS) and the Environmental Public Health Readiness Branch (EPHRB). See all-hazards public health emergency response guide): www.cdc.gov/nceh/eehs/
- U.S. Department of Homeland Security, for family preparedness: www.ready.gov/
- AHRQ bioterrorism link: www.ahrq.gov/news/pubcat/c_biot.htm#biot002
- George Washington University Institute for Crisis, Disaster, and Risk Management offers programs, including training, in the area of crisis, emergency and risk management: www.gwu.edu/~icdrm/
- North Carolina Links—North Carolina Office of Public Health Preparedness and Response (NC PHPR): www.epi.state.nc.us/epi/phpr/provides information and resources regarding the threat of bioterrorism and other emerging infectious diseases within the state and around the nation.
- The Health Alert Network (HAN) system is designed to immediately alert key health officials and care providers in North Carolina to acts of bioterrorism as well as other types of emerging disease threats: www.nchan.org/
- DPH Immunization branch: www.immunizenc.com/
- State Web sites, such as San Diego Country Office of Emergency Systems: www.co.sandiego.ca.us/oes/ or the County of San Diego Health and Human Services Terrorism Preparedness: www.co.san-diego.ca.us/terrorism/links.html
Tips to differentiate bipolar II disorder and borderline personality disorder
Alternative modes of mechanical ventilation: A review for the hospitalist
Technologic advances and computerized control of mechanical ventilators have made it possible to deliver ventilatory assistance in new modes. Driving these innovations is the desire to prevent ventilator-induced lung injury, improve patient comfort, and liberate the patient from mechanical ventilation as soon as possible.
We call these innovations “alternative” modes to differentiate them from the plain volume-control and pressure-control modes. Some clinicians rarely use these new modes, but in some medical centers they have become the most common ones used, or are being used unknowingly (the operator misunderstands the mode name). The information we provide on these modes of ventilation is by no means an endorsement of their use, but rather a tool to help the clinician understand their physiologic, theoretical, and clinical effects.
We focused on two goals:
- Explain what the mode does
- Briefly review the theoretical benefits and the actual evidence supporting these alternative modes of ventilation.
STANDARD NOMENCLATURE NEEDED
Since its invention, mechanical ventilation has been plagued by multiple names being used to describe the same things. For example, volume-control ventilation is also called volume-cycled ventilation, assist-control ventilation, volume-limited ventilation, and controlled mechanical ventilation. Similarly, multiple abbreviations are used, each depending on the brand of ventilator, and new acronyms have been added in recent years as new modes have been developed. The vast number of names and modes can confuse even the most seasoned critical care physician.
Efforts to establish a common nomenclature are under way.1
WHAT IS A MODE?
A mode of mechanical ventilation has three essential components:
- The control variable
- The breath sequence
- The targeting scheme.
Similar modes may require more detailed descriptions to distinguish them, but the basic function can be explained by these three components.
The control variable
In general, inspiration is an active process, driven by the patient’s effort, the ventilator, or both, while expiration is passive. For simplicity, in this article a mechanical breath means the inspiratory phase of the breath.
The machine can only control the volume (and flow) or the pressure given. The breaths can be further described on the basis of what triggers the breath, what limits it (the maximum value of a control variable), and what ends (cycles) it.
The breath sequence
There are three possible breath sequences:
- Intermittent mandatory ventilation, in which the patient can take spontaneous breaths between mandatory breaths
- Continuous spontaneous ventilation, in which all breaths are spontaneous (Table 1).
The targeting scheme
The targeting or feedback scheme refers to the ventilator settings and programming that dictate its response to the patient’s lung compliance, lung resistance, and respiratory effort. The regulation can be as simple as controlling the pressure in pressure-control mode, or it can be based on a complicated algorithm.
ADAPTIVE PRESSURE CONTROL
Other names for adaptive pressure control
- Pressure Regulated Volume Control (Maquet Servo-i, Rastatt, Germany)
- AutoFlow (Dräger Medical AG, Lübeck, Germany)
- Adaptive Pressure Ventilation (Hamilton Galileo, Hamilton Medical AG, Bonaduz, Switzerland)
- Volume Control+ (Puritan Bennett, Tyco Healthcare; Mansfield, MA)
- Volume Targeted Pressure Control, Pressure Controlled Volume Guaranteed (Engström, General Electric, Madison, WI).
What does adaptive pressure control do?
The APC mode delivers pressure-controlled breaths with an adaptive targeting scheme (Table 2).
In pressure-control ventilation, tidal volumes depend on the lung’s physiologic mechanics (compliance and resistance) and patient effort (Figure 1). Therefore, the tidal volume varies with changes in lung physiology (ie, larger or smaller tidal volumes than targeted).
To overcome this effect, a machine in APC mode adjusts the inspiratory pressure to deliver the set minimal target tidal volume. If tidal volume increases, the machine decreases the inspiratory pressure, and if tidal volume decreases, the machine increases the inspiratory pressure. However, if the patient effort is large enough, the tidal volume will increase in spite of decreasing the inspiratory pressure (Figure 2). The adjustments to the inspiratory pressure occur after the tidal volume is off-target in a number of breaths.
Common sources of confusion with adaptive pressure control
First, APC is not a volume-control mode. In volume control, the tidal volume does not change; in APC the tidal volume can increase or decrease, and the ventilator will adjust the inflation pressure to achieve the target volume. Thus, APC guarantees an average minimum tidal volume but not a maximum tidal volume.
Second, a characteristic of pressure control (and hence, APC) is that the flow of gas varies to maintain constant airway pressure (ie, maintain the set inspiratory pressure). This characteristic allows a patient who generates an inspiratory effort to receive flow as demanded, which is likely more comfortable. This is essentially different from volume control, in which flow is set by the operator and hence is fixed. Thus, if the patient effort is strong enough (Figure 1), this leads to what is called flow asynchrony, in which the patient does not get the flow asked for in a breath.
Ventilator settings in adaptive pressure control
Ventilator settings in APC are:
- Tidal volume
- Time spent in inspiration (inspiratory time)
- Frequency
- Fraction of inspired oxygen (Fio2)
- Positive end-expiratory pressure (PEEP).
Some ventilators also require setting the speed to reach the peak pressure (also known as slope percent or inspiratory rise time).
Clinical applications of adaptive pressure control
This mode is designed to maintain a consistent tidal volume during pressure-control ventilation and to promote inspiratory flow synchrony. It is a means of automatically reducing ventilatory support (ie, weaning) as the patient’s inspiratory effort becomes stronger, as in awakening from anesthesia.
APC may not be ideal for patients who have an inappropriately increased respiratory drive (eg, in severe metabolic acidosis), since the inspiratory pressure will decrease to maintain the targeted average tidal volume, inappropriately shifting the work of breathing onto the patient.
Theoretical benefits of adaptive pressure control
APC guarantees a minimum average tidal volume (unless the pressure alarm threshold is set too low, so that the target tidal volume is not delivered). Other theoretical benefits are flow synchrony, less ventilator manipulation by the operator, and automatic weaning of ventilator support.
Evidence of benefit of adaptive pressure control
Physiologic benefits. This mode has lower peak inspiratory pressures than does volume-control ventilation,3,4 which is often reported as a positive finding. However, in volume-control mode (the usual comparator), the peak inspiratory pressure is a manifestation of both resistance and compliance. Hence, peak inspiratory pressure is expected to be higher but does not reflect actual lung-distending pressures. It is the plateau pressure, a manifestation of lung compliance, that is related to lung injury.
Patient comfort. APC may increase the work of breathing when using low tidal volume ventilation and when there is increased respiratory effort (drive).5 Interestingly, APC was less comfortable than pressure support ventilation in a small trial.6
Outcomes have not been studied.7
Adaptive pressure control: Bottom line
APC is widely available and widely used, sometimes unknowingly (eg, if the operator thinks it is volume control). It is relatively easy to use and to set; however, evidence of its benefit is scant.
ADAPTIVE SUPPORT VENTILATION
Adaptive support ventilation (ASV) evolved as a form of mandatory minute ventilation implemented with adaptive pressure control. Mandatory minute ventilation is a mode that allows the operator to preset a target minute ventilation, and the ventilator then supplies mandatory breaths, either volume- or pressure-controlled, if the patient’s spontaneous breaths generate a lower minute ventilation.
ASV automatically selects the appropriate tidal volume and frequency for mandatory breaths and the appropriate tidal volume for spontaneous breaths on the basis of the respiratory system mechanics and target minute alveolar ventilation.
Described in 1994 by Laubscher et al,8,9 ASV became commercially available in 1998 in Europe and in 2007 in the United States (Hamilton Galileo ventilator, Hamilton Medical AG). This is the first commercially available ventilator that uses an “optimal” targeting scheme (see below).
What does adaptive support ventilation do?
ASV delivers pressure-controlled breaths using an adaptive (optimal) scheme (Table 2). “Optimal,” in this context, means minimizing the mechanical work of breathing: the machine selects a tidal volume and frequency that the patient’s brain would presumably select if the patient were not connected to a ventilator. This pattern is assumed to encourage the patient to generate spontaneous breaths.
The ventilator calculates the normal required minute ventilation based on the patient’s ideal weight and estimated dead space volume (ie, 2.2 mL/kg). This calculation represents 100% of minute ventilation. The clinician at the bedside sets a target percent of minute ventilation that the ventilator will support—higher than 100% if the patient has increased requirements due, eg, to sepsis or increased dead space, or less than 100% during weaning.
The ventilator initially delivers test breaths, in which it measures the expiratory time constant for the respiratory system and then uses this along with the estimated dead space and normal minute ventilation to calculate an optimal breathing frequency in terms of mechanical work.
The optimal or target tidal volume is calculated as the normal minute ventilation divided by the optimal frequency. The target tidal volume is achieved by the use of APC (see above) (Figure 2). This means that the pressure limit is automatically adjusted to achieve an average delivered tidal volume equal to the target. The ventilator continuously monitors the respiratory system mechanics and adjusts its settings accordingly.
The ventilator adjusts its breaths to avoid air trapping by allowing enough time to exhale, to avoid hypoventilation by delivering tidal volume greater than the dead space, and to avoid volutrauma by avoiding large tidal volumes.
Ventilator settings in adaptive support ventilation
Ventilator settings in ASV are:
- Patient height (to calculate the ideal body weight)
- Sex
- Percent of normal predicted minute ventilation goal
- Fio2
- PEEP.
Clinical applications of adaptive support ventilation
ASV is intended as a sole mode of ventilation, from initial support to weaning.
Theoretical benefits of adaptive support ventilation
In theory, ASV offers automatic selection of ventilator settings, automatic adaptation to changing patient lung mechanics, less need for human manipulation of the machine, improved synchrony, and automatic weaning.
Evidence of benefit of adaptive support ventilation
Physiologic benefits. Ventilator settings are adjusted automatically. ASV selects different tidal volume-respiratory rate combinations based on respiratory mechanics in passive and paralyzed patients.10–12 In actively breathing patients, there was no difference in the ventilator settings chosen by ASV for different clinical scenarios (and lung physiology).10 Compared with pressure-controlled intermittent mandatory ventilation, with ASV, the inspiratory load is less and patient-ventilator interaction is better.13
Patient-ventilator synchrony and comfort have not been studied.
Outcomes. Two trials suggest that ASV may decrease time on mechanical ventilation.14,15 However, in another trial,16 compared with a standard protocol, ASV led to fewer ventilator adjustments but achieved similar postsurgical weaning outcomes. The effect of this mode on the death rate has not been examined.17,18
Adaptive support ventilation: Bottom line
ASV is the first commercially available mode that automatically selects all the ventilator settings except PEEP and Fio2. These seem appropriate for different clinical scenarios in patients with poor respiratory effort or in paralyzed patients. Evidence of the effect in actively breathing patients and on outcomes such as length of stay or death is still lacking.
PROPORTIONAL ASSIST VENTILATION
Patients who have normal respiratory drive but who have difficulty sustaining adequate spontaneous ventilation are often subjected to pressure support ventilation (PSV), in which the ventilator generates a constant pressure throughout inspiration regardless of the intensity of the patient’s effort.
In 1992, Younes and colleagues19,20 developed proportional assist ventilation (PAV) as an alternative in which the ventilator generates pressure in proportion to the patient’s effort. PAV became commercially available in Europe in 1999 and was approved in the United States in 2006, available on the Puritan Bennett 840 ventilator (Puritan Bennett Co, Boulder, CO). PAV has also been used for noninvasive ventilation, but this is not available in the United States.
Other names for proportional assist ventilation
Proportional Pressure Support (Dräger Medical; not yet available in the United States).
What does proportional assist ventilation do?
This mode delivers pressure-controlled breaths with a servo control scheme (Table 2).
To better understand PAV, we can compare it with PSV. With PSV, the pressure applied by the ventilator rises to a preset level that is held constant (a set-point scheme) until a cycling criterion (a percent of the maximum inspiratory flow value) is reached. The inspiratory flow and tidal volume are the result of the patient’s inspiratory effort, the level of pressure applied, and the respiratory system mechanics.
In PAV, as in PSV, all breaths are spontaneous (Table 1). The patient controls the timing and size of the breath. There are no preset pressure, flow, or volume goals, but safety limits on the volume and pressure delivered can be set.
Ventilator settings in proportional assist ventilation
Ventilator settings in PAV are:
- Airway type (endotracheal tube, tracheostomy)
- Airway size (inner diameter)
- Percentage of work supported (assist range 5%–95%)
- Tidal volume limit
- Pressure limit
- Expiratory sensitivity (normally, as inspiration ends, flow should stop; this parameter tells the ventilator at what flow to end inspiration).
Caution when assessing the literature. Earlier ventilator versions, ie, Dräger and Manitoba (University of Manitoba, Winnipeg, MB, Canada), which are not available in the United States, required the repeated calculation of the respiratory system mechanics and the manual setting of flow and volume assists (amplification factors) independently. To overcome this limitation, new software automatically adjusts the flow and volume amplification to support the loads imposed by the automatically measured values of resistance and elastance (inverse of compliance) of the respiratory system.21 This software is included in the model (Puritan Bennett) available in the United States.
Clinical applications of proportional assist ventilation
The PAV mode is indicated for maximizing ventilator patient synchrony for assisted spontaneous ventilation.
PAV is contraindicated in patients with respiratory depression (bradypnea) or large air leaks (eg, bronchopleural fistulas). It should be used with caution in patients with severe hyperinflation, in which the patient may still be exhaling but the ventilator doesn’t recognize it. Another group in which PAV should be used with caution is those with high ventilatory drives, in which the ventilator overestimates respiratory system mechanics. This situation can lead to overassistance due to the “runaway phenomenon,” in which the ventilator continues to provide support even if the patient has stopped inspiration.22
Theoretical benefits of proportional assist ventilation
In theory, PAV should reduce the work of breathing, improve synchrony, automatically adapt to changing patient lung mechanics and effort, decrease the need for ventilator intervention and manipulation, decrease the need for sedation, and improve sleep.
Evidence of benefit of proportional assist ventilation
Physiologic benefits. PAV reduces the work of breathing better than PSV,21 even in the face of changing respiratory mechanics or increased respiratory demand (hypercapnia).23–25 The hemodynamic profile is similar to that in PSV. Tidal volumes are variable; however, in recent reports the tidal volumes were within the lung-protective range (6–8 mL/kg, plateau pressure < 30 cm H20).26,27
Comfort. PAV entails less patient effort and discomfort that PSV does.23,25 PAV significantly reduces asynchrony,27 which in turn may favorably affect sleep in critically ill patients. 28
Outcomes. The probability of spontaneous breathing without assistance was significantly better in critically ill patients ventilated with PAV than with PSV. No trial has reported the effect of PAV on deaths.27,29
Proportional assist ventilation: Bottom line
Extensive basic research has been done with PAV in different forms of respiratory failure, such as obstructive lung disease, acute respiratory distress syndrome (ARDS), and chronic respiratory failure. It fulfills its main goal, which is to improve patient-ventilator synchrony. Clinical experience with PAV in the United States is limited, as it was only recently approved.
AIRWAY PRESSURE-RELEASE VENTILATION AND BIPHASIC POSITIVE AIRWAY PRESSURE
Airway pressure-release ventilation (APRV) was described in 1987 by Stock et al30 as a mode for delivering ventilation in acute lung injury while avoiding high airway pressures. APRV combines high constant positive airway pressure (improving oxygenation and promoting alveolar recruitment) with intermittent releases (causing exhalation).
Other names for biphasic positive airway pressure
Other names for biphasic positive airway pressure are:
- BiLevel (Puritan Bennett)
- BIPAP (Dräger Europe)
- Bi Vent (Siemens)
- BiPhasic (Avea, Cardinal Health, Inc, Dublin, OH)
- PCV+ (Dräger Medical)
- DuoPAP (Hamilton).
Caution—name confusion. In North America, BiPAP (Respironics, Murrysville, PA) and BiLevel are used to refer to noninvasive modes of ventilation.
APRV has no other name.
What do these modes do?
These modes deliver pressure-controlled, time-triggered, and time-cycled breaths using a set-point targeting scheme (Table 2). This means that the ventilator maintains a constant pressure (set point) even in the face of spontaneous breaths.
Caution—source of confusion. The term continuous positive airway pressure (CPAP) is often used to describe this mode. However, CPAP is pressure that is applied continuously at the same level; the patient generates all the work to maintain ventilation (“pressure-controlled continuous spontaneous ventilation” in the current nomenclature). In APRV, the airway pressure is intermittently released and reapplied, generating a tidal volume that supports ventilation. In other words, this is a pressure-controlled breath with a very prolonged inspiratory time and a short expiratory time in which spontaneous ventilation is possible at any point (“pressure-controlled intermittent mandatory ventilation” in the current nomenclature).
How these modes are set in the ventilator may also be a source of confusion. To describe the time spent in high and low airway pressures, we use the terms Thigh and Tlow, respectively. By convention, the difference between APRV and biphasic mode is the duration of Tlow (< 1.5 sec for APRV).
Similarly, Phigh and Plow are used to describe the high and low airway pressure. To better understand this concept, you can create the same mode in conventional pressure-control ventilation by thinking of the Thigh as the inspiratory time, the Tlow as the expiratory time, the Phigh as inspiratory pressure, and the Plow as PEEP.
Hence, APRV is an extreme form of inverse ratio ventilation, with an inspiration-to-expiration ratio of 4:1. This means a patient spends most of the time in Phigh and Thigh, and exhalations are short (Tlow and Plow). In contrast, the biphasic mode uses conventional inspiration-expiration ratios (Figure 4).
As with any form of pressure control, the tidal volume is generated by airway pressure rising above baseline (ie, the end-expiratory value). Hence, to ensure an increase in minute ventilation, the mandatory breath rate must be increased (ie, decreasing Thigh, Tlow, or both) or the tidal volume must be increased (ie, increasing the difference between Phigh and Plow). This means that in APRV the Tlow has to happen more often (by increasing the number of breaths) or be more prolonged (allowing more air to exhale). Because unrestricted spontaneous breaths are permitted at any point of the cycle, the patient contributes to the total minute ventilation (usually 10%–40%).
In APRV and biphasic mode, the operator’s set time and pressure in inspiration and expiration will be delivered regardless of the patient’s breathing efforts—the patient’s spontaneous breath does not trigger a mechanical breath. Some ventilators have automatic adjustments to improve the trigger synchrony.
Ventilator settings in APRV and biphasic mode
These modes require the setting of two pressure levels (Phigh and Plow) and two time durations (Thigh and Tlow). One can add pressure support or automatic tube compensation to assist spontaneous breaths. The difference in Tlow generates differences in the Thigh:Tlow ratio: APRV has a short Tlow (an inspiration-expiration ratio of 4:1). Biphasic mode has a conventional inspiration-expiration ratio of 1:1 to 1:4.
Clinical applications
APRV is used in acute lung injury and ARDS. This mode should be used with caution or not at all in patients with obstructive lung disease or inappropriately increased respiratory drive.32–35
Biphasic mode is intended for both ventilation and weaning. In a patient who has poor respiratory effort or who is paralyzed, biphasic is identical to pressure-control/continuous mandatory ventilation.
Theoretical benefits of APRV and biphasic mode
Multiple benefits have been ascribed to these modes. In theory, APRV will maximize and maintain alveolar recruitment, improve oxygenation, lower inflation pressures, and decrease overinflation. Both APRV and biphasic, by preserving spontaneous breathing, will improve ventilation-perfusion matching and gas diffusion, improve the hemodynamic profile (less need for vasopressors, higher cardiac output, reduced ventricular workload, improved organ perfusion), and improve synchrony (decrease the work of breathing and the need for sedation).
Evidence of benefit of APRV and biphasic mode
APRV and biphasic are different modes. However studies evaluating their effects are combined. This is in part the result of the nomenclature confusion and different practice in different countries.36
Physiologic benefits. In studies, spontaneous breaths contributed to 10% to 40% of minute ventilation,37,38 improved ventilation of dependent areas of the lung, improved ventilation-perfusion match and recruitment,39 and improved hemodynamic profile.40
Patient comfort. These modes are thought to decrease the need for analgesia and sedation,38 but a recent trial showed no difference with pressure-controlled intermittent mandatory ventilation.41 Patient ventilator synchrony and comfort have not been studied.32,42
Outcomes. In small trials, these modes made no difference in terms of deaths, but they may decrease the length of mechanical ventilation.38,41,43,44
APRV and biphasic mode: Bottom line
Maintaining spontaneous breathing while on mechanical ventilation has hemodynamic and ventilatory benefits.
APRV and biphasic mode are not the same thing. APRV’s main goal is to maximize mean airway pressure and, hence, lung recruitment, whereas the main goal of the biphasic mode is synchrony.
There is a plethora of ventilator settings and questions related to physiologic effects.33,34,36
Although these modes are widely used in some centers, there is no evidence yet that they are superior to conventional volume- or pressure-control ventilation with low tidal volume for ARDS and acute lung injury. There is no conclusive evidence that these modes improve synchrony, time to weaning, or patient comfort.
HIGH-FREQUENCY OSCILLATORY VENTILATION
High-frequency oscillatory ventilation (HFOV) was first described and patented in 1952 by Emerson and was clinically developed in the early 1970s by Lunkenheimer.45
The goal of HFOV is to minimize lung injury; its characteristics (discussed below) make it useful in patients with severe ARDS. The US Food and Drug Administration approved it for infants in 1991 and for children in 1995. The adult model has been available since 1993, but it was not approved until 2001 (SensorMedics 3100B, Cardinal Health, Inc).
Other names for high-frequency oscillatory ventilation
While HFOV has no alternative names, the following acronyms describe similar modes:
- HFPPV (high-frequency positive pressure ventilation)
- HFJV (high-frequency jet ventilation)
- HFFI (high-frequency flow interruption)
- HFPV (high-frequency percussive ventilation)
- HFCWO (high-frequency chest wall oscillation).
All of these modes require different specialized ventilators.
What does high-frequency oscillatory ventilation do?
Conceptually, HFOV is a form of pressure-controlled intermittent mandatory ventilation with a set-point control scheme. In contrast to conventional pressure-controlled intermittent mandatory ventilation, in which relatively small spontaneous breaths may be superimposed on relatively large mandatory breaths, HFOV superimposes very small mandatory breaths (oscillations) on top of spontaneous breaths.
Adult patients are usually paralyzed or deeply sedated, since deep spontaneous breathing will trigger alarms and affect ventilator performance.
To manage ventilation (CO2 clearance), one or several of the following maneuvers can be done: decrease the oscillation frequency, increase the amplitude of the oscillations, increase the inspiratory time, or increase bias flow (while allowing an endotracheal tube cuff leak). Oxygenation adjustments are controlled by manipulating the mean airway pressure and the Fio2.
Ventilator settings in high-frequency oscillatory ventilation
Ventilator settings in HFOV are46:
- Airway pressure amplitude (delta P or power)
- Mean airway pressure
- Percent inspiration
- Inspiratory bias flow
- Fio2.
Clinical applications of high-frequency oscillatory ventilation
This mode is usually reserved for ARDS patients for whom conventional ventilation is failing. A recently published protocol46 suggests considering HFOV when there is oxygenation failure (Fio2 ≥ 0.7 and PEEP ≥ 14 cm H2O) or ventilation failure (pH < 7.25 with tidal volume ≥ 6 mL/kg predicted body weight and plateau airway pressure ≥ 30 cm H2O).
This mode is contraindicated when there is known severe airflow obstruction or intracranial hypertension.
Theoretical benefits of high-frequency oscillatory ventilation
Conceptually, HFOV can provide the highest mean airway pressure paired with the lowest tidal volume of any mode. These benefits might make HFOV the ideal lung-protective ventilation strategy.
Evidence of benefit of high-frequency oscillatory ventilation
Physiologic benefits. Animal models have shown less histologic damage and lung inflammation with HFOV than with high-tidal-volume conventional ventilation47,48 and low-tidal-volume conventional ventilation.49
Patient comfort has not been studied. However, current technology does impose undue work of breathing in spontaneously breathing patients.50
Outcomes. Several retrospective case series have described better oxygenation with HFOV as rescue therapy for severe ARDS than with conventional mechanical ventilation. Two randomized controlled trials have studied HFOV vs high-tidal-volume conventional mechanical ventilation for early severe ARDS; HFOV was safe but made no difference in terms of deaths.42,51–54
High-frequency oscillatory ventilation: Bottom line
In theory, HFOV provides all the benefits of an ideal lung-protective strategy, at least for paralyzed or deeply sedated patients. Animal studies support these concepts. In human adults, HFOV has been shown to be safe and to provide better oxygenation but no improvement in death rates compared with conventional mechanical ventilation. Currently, HFOV is better reserved for patients with severe ARDS for whom conventional mechanical ventilation is failing.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Chatburn RL. Computer control of mechanical ventilation. Respir Care 2004; 49:507–517.
- Alvarez A, Subirana M, Benito S. Decelerating flow ventilation effects in acute respiratory failure. J Crit Care 1998; 13:21–25.
- Guldager H, Nielsen SL, Carl P, Soerensen MB. A comparison of volume control and pressure regulated volume control ventilation in acute respiratory failure. Crit Care 1997; 1:75–77.
- Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Work of breathing during lung protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure regulated breathing modes. Respir Care 2005; 50:1623–1631.
- Betensley AD, Khalid I, Crawford J, Pensler RA, DiGiovine B. Patient comfort during pressure support and volume controlled continuous mandatory ventilation. Respir Care 2008; 53:897–902.
- Branson RD, Chatburn RL. Controversies in the critical care setting. Should adaptive pressure control modes be utilized for virtually all patients receiving mechanical ventilation? Respir Care 2007; 52:478–485.
- Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start up procedure for closed-loop controlled ventilation. Int J Clin Monit Comput 1994; 11:19–30.
- Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX. An adaptive lung ventilation controller. IEEE Trans Biomed Eng 1994; 41:51–59.
- Arnal JM, Wysocki M, Nafati C, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med 2008; 34:75–81.
- Campbell RS, Sinamban RP, Johannigman JA, et al. Clinical evaluation of a new closed loop ventilation mode: adaptive supportive ventilation (ASV). Crit Care 1999; 3( suppl 1):083.
- Belliato M, Palo A, Pasero D, Iotti GA, Mojoli F, Braschi A. Evaluation of adaptive support ventilation in paralysed patients and in a physical lung model. Int J Artif Organs 2004; 27:709–716.
- Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient ventilator interactions during partial ventilatory support: a preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med 2002; 30:801–807.
- Gruber PC, Gomersall CD, Leung P, et al. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology 2008; 109:81–87.
- Sulzer CF, Chiolero R, Chassot PG, et al. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology 2001; 95:1339–1345.
- Petter AH, Chiolèro RL, Cassina T, Chassot PG, Müller XM, Revelly JP. Automatic “respirator/weaning” with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analg 2003; 97:1743–1750.
- Brunner JX, Iotti GA. Adaptive support ventilation (ASV). Minerva Anestesiol 2002; 68:365–368.
- Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin North Am 2001; 7:425–440.
- Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis 1992; 145:114–120.
- Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis 1992; 145:121–129.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilatio—proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Med 2006; 32:692–699.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Effect of different levels of pressure support and proportional assist ventilation on breathing pattern, work of breathing and gas exchange in mechanically ventilated hypercapnic COPD patients with acute respiratory failure. Respiration 2003; 70:355–361.
- Grasso S, Puntillo F, Mascia L, et al. Compensation for increase in respiratory workload during mechanical ventilation. Pressure support versus proportional assist ventilation. Am J Respir Crit Care Med 2000; 161:819–826.
- Wrigge H, Golisch W, Zinserling J, Sydow M, Almeling G, Burchardi H. Proportional assist versus pressure support ventilation: effects on breathing pattern and respiratory work of patients with chronic obstructive pulmonary disease. Intensive Care Med 1999; 25:790–798.
- Ranieri VM, Giuliani R, Mascia L, et al. Patient ventilator interaction during acute hypercapnia: pressure support vs. proportional assist ventilation. J Appl Physiol 1996; 81:426–436.
- Kondili E, Xirouchaki N, Vaporidi K, Klimathianaki M, Georgopoulos D. Short-term cardiorespiratory effects of proportional assist and pressure support ventilation in patients with acute lung injury/acute respiratory distress syndrome. Anesthesiology 2006; 105:703–708.
- Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med 2008; 34:2026–2034.
- Bosma K, Ferreyra G, Ambrogio C, et al. Patient ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med 2007; 35:1048–1054.
- Sinderby C, Beck J. Proportional assist ventilation and neurally adjusted ventilatory assist—better approaches to patient ventilator synchrony? Clin Chest Med 2008; 29:329–342.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Baum M, Benzer H, Putensen C, Koller W, Putz G. [Biphasic positive airway pressure (BIPAP)—a new form of augmented ventilation]. Anaesthesist 1989; 38:452–458.
- Seymour CW, Frazer M, Reilly PM, Fuchs BD. Airway pressure release and biphasic intermittent positive airway pressure ventilation: are they ready for prime time? J Trauma 2007; 62:1298–1308.
- Myers TR, MacIntyre NR. Respiratory controversies in the critical care setting. Does airway pressure release ventilation offer important new advantages in mechanical ventilator support? Respir Care 2007; 52:452–458.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Calzia E, Lindner KH, Witt S, et al. Pressure-time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med 1994; 150:904–910.
- Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 2008; 34:1766–1773.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Longterm effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 2001; 5:221–226.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis in 596 patients following adult cardiac surgery. Eur J Anaesthesiol 1997; 14:576–582.
- Habashi NM. Other approaches to open lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33 suppl 3:S228–S240.
- Hess D, Mason S, Branson R. High-frequency ventilation design and equipment issues. Respir Care Clin North Am 2001; 7:577–598.
- Fessler HE, Derdak S, Ferguson ND, et al. A protocol for high frequency oscillatory ventilation in adults: results from a roundtable discussion. Crit Care Med 2007; 35:1649–1654.
- Hamilton PP, Onayemi A, Smyth JA, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol 1983; 55:131–138.
- Sedeek KA, Takeuchi M, Suchodolski K, et al. Open-lung protective ventilation with pressure control ventilation, high-frequency oscillation, and intratracheal pulmonary ventilation results in similar gas exchange, hemodynamics, and lung mechanics. Anesthesiology 2003; 99:1102–1111.
- Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K. Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 2001; 91:1836–1844.
- van Heerde M, Roubik K, Kopelent V, Plötz FB, Markhorst DG. Unloading work of breathing during high-frequency oscillatory ventilation: a bench study. Crit Care 2006; 10:R103.
- Derdak S, Mehta S, Stewart TE, et al., Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial (MOAT) Study Investigators. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002; 166:801–808.
- Bollen CW, van Well GT, Sherry T, et al. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care 2005; 9:R430–R439.
- Mehta S, Granton J, MacDonald RJ, et al. High frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
Technologic advances and computerized control of mechanical ventilators have made it possible to deliver ventilatory assistance in new modes. Driving these innovations is the desire to prevent ventilator-induced lung injury, improve patient comfort, and liberate the patient from mechanical ventilation as soon as possible.
We call these innovations “alternative” modes to differentiate them from the plain volume-control and pressure-control modes. Some clinicians rarely use these new modes, but in some medical centers they have become the most common ones used, or are being used unknowingly (the operator misunderstands the mode name). The information we provide on these modes of ventilation is by no means an endorsement of their use, but rather a tool to help the clinician understand their physiologic, theoretical, and clinical effects.
We focused on two goals:
- Explain what the mode does
- Briefly review the theoretical benefits and the actual evidence supporting these alternative modes of ventilation.

STANDARD NOMENCLATURE NEEDED
Since its invention, mechanical ventilation has been plagued by multiple names being used to describe the same things. For example, volume-control ventilation is also called volume-cycled ventilation, assist-control ventilation, volume-limited ventilation, and controlled mechanical ventilation. Similarly, multiple abbreviations are used, each depending on the brand of ventilator, and new acronyms have been added in recent years as new modes have been developed. The vast number of names and modes can confuse even the most seasoned critical care physician.
Efforts to establish a common nomenclature are under way.1
WHAT IS A MODE?
A mode of mechanical ventilation has three essential components:
- The control variable
- The breath sequence
- The targeting scheme.
Similar modes may require more detailed descriptions to distinguish them, but the basic function can be explained by these three components.
The control variable
In general, inspiration is an active process, driven by the patient’s effort, the ventilator, or both, while expiration is passive. For simplicity, in this article a mechanical breath means the inspiratory phase of the breath.
The machine can only control the volume (and flow) or the pressure given. The breaths can be further described on the basis of what triggers the breath, what limits it (the maximum value of a control variable), and what ends (cycles) it.
The breath sequence
There are three possible breath sequences:
- Intermittent mandatory ventilation, in which the patient can take spontaneous breaths between mandatory breaths
- Continuous spontaneous ventilation, in which all breaths are spontaneous (Table 1).
The targeting scheme
The targeting or feedback scheme refers to the ventilator settings and programming that dictate its response to the patient’s lung compliance, lung resistance, and respiratory effort. The regulation can be as simple as controlling the pressure in pressure-control mode, or it can be based on a complicated algorithm.
ADAPTIVE PRESSURE CONTROL
Other names for adaptive pressure control
- Pressure Regulated Volume Control (Maquet Servo-i, Rastatt, Germany)
- AutoFlow (Dräger Medical AG, Lübeck, Germany)
- Adaptive Pressure Ventilation (Hamilton Galileo, Hamilton Medical AG, Bonaduz, Switzerland)
- Volume Control+ (Puritan Bennett, Tyco Healthcare; Mansfield, MA)
- Volume Targeted Pressure Control, Pressure Controlled Volume Guaranteed (Engström, General Electric, Madison, WI).
What does adaptive pressure control do?
The APC mode delivers pressure-controlled breaths with an adaptive targeting scheme (Table 2).
In pressure-control ventilation, tidal volumes depend on the lung’s physiologic mechanics (compliance and resistance) and patient effort (Figure 1). Therefore, the tidal volume varies with changes in lung physiology (ie, larger or smaller tidal volumes than targeted).
To overcome this effect, a machine in APC mode adjusts the inspiratory pressure to deliver the set minimal target tidal volume. If tidal volume increases, the machine decreases the inspiratory pressure, and if tidal volume decreases, the machine increases the inspiratory pressure. However, if the patient effort is large enough, the tidal volume will increase in spite of decreasing the inspiratory pressure (Figure 2). The adjustments to the inspiratory pressure occur after the tidal volume is off-target in a number of breaths.
Common sources of confusion with adaptive pressure control
First, APC is not a volume-control mode. In volume control, the tidal volume does not change; in APC the tidal volume can increase or decrease, and the ventilator will adjust the inflation pressure to achieve the target volume. Thus, APC guarantees an average minimum tidal volume but not a maximum tidal volume.
Second, a characteristic of pressure control (and hence, APC) is that the flow of gas varies to maintain constant airway pressure (ie, maintain the set inspiratory pressure). This characteristic allows a patient who generates an inspiratory effort to receive flow as demanded, which is likely more comfortable. This is essentially different from volume control, in which flow is set by the operator and hence is fixed. Thus, if the patient effort is strong enough (Figure 1), this leads to what is called flow asynchrony, in which the patient does not get the flow asked for in a breath.
Ventilator settings in adaptive pressure control
Ventilator settings in APC are:
- Tidal volume
- Time spent in inspiration (inspiratory time)
- Frequency
- Fraction of inspired oxygen (Fio2)
- Positive end-expiratory pressure (PEEP).
Some ventilators also require setting the speed to reach the peak pressure (also known as slope percent or inspiratory rise time).
Clinical applications of adaptive pressure control
This mode is designed to maintain a consistent tidal volume during pressure-control ventilation and to promote inspiratory flow synchrony. It is a means of automatically reducing ventilatory support (ie, weaning) as the patient’s inspiratory effort becomes stronger, as in awakening from anesthesia.
APC may not be ideal for patients who have an inappropriately increased respiratory drive (eg, in severe metabolic acidosis), since the inspiratory pressure will decrease to maintain the targeted average tidal volume, inappropriately shifting the work of breathing onto the patient.
Theoretical benefits of adaptive pressure control
APC guarantees a minimum average tidal volume (unless the pressure alarm threshold is set too low, so that the target tidal volume is not delivered). Other theoretical benefits are flow synchrony, less ventilator manipulation by the operator, and automatic weaning of ventilator support.
Evidence of benefit of adaptive pressure control
Physiologic benefits. This mode has lower peak inspiratory pressures than does volume-control ventilation,3,4 which is often reported as a positive finding. However, in volume-control mode (the usual comparator), the peak inspiratory pressure is a manifestation of both resistance and compliance. Hence, peak inspiratory pressure is expected to be higher but does not reflect actual lung-distending pressures. It is the plateau pressure, a manifestation of lung compliance, that is related to lung injury.
Patient comfort. APC may increase the work of breathing when using low tidal volume ventilation and when there is increased respiratory effort (drive).5 Interestingly, APC was less comfortable than pressure support ventilation in a small trial.6
Outcomes have not been studied.7
Adaptive pressure control: Bottom line
APC is widely available and widely used, sometimes unknowingly (eg, if the operator thinks it is volume control). It is relatively easy to use and to set; however, evidence of its benefit is scant.
ADAPTIVE SUPPORT VENTILATION
Adaptive support ventilation (ASV) evolved as a form of mandatory minute ventilation implemented with adaptive pressure control. Mandatory minute ventilation is a mode that allows the operator to preset a target minute ventilation, and the ventilator then supplies mandatory breaths, either volume- or pressure-controlled, if the patient’s spontaneous breaths generate a lower minute ventilation.
ASV automatically selects the appropriate tidal volume and frequency for mandatory breaths and the appropriate tidal volume for spontaneous breaths on the basis of the respiratory system mechanics and target minute alveolar ventilation.
Described in 1994 by Laubscher et al,8,9 ASV became commercially available in 1998 in Europe and in 2007 in the United States (Hamilton Galileo ventilator, Hamilton Medical AG). This is the first commercially available ventilator that uses an “optimal” targeting scheme (see below).
What does adaptive support ventilation do?
ASV delivers pressure-controlled breaths using an adaptive (optimal) scheme (Table 2). “Optimal,” in this context, means minimizing the mechanical work of breathing: the machine selects a tidal volume and frequency that the patient’s brain would presumably select if the patient were not connected to a ventilator. This pattern is assumed to encourage the patient to generate spontaneous breaths.
The ventilator calculates the normal required minute ventilation based on the patient’s ideal weight and estimated dead space volume (ie, 2.2 mL/kg). This calculation represents 100% of minute ventilation. The clinician at the bedside sets a target percent of minute ventilation that the ventilator will support—higher than 100% if the patient has increased requirements due, eg, to sepsis or increased dead space, or less than 100% during weaning.
The ventilator initially delivers test breaths, in which it measures the expiratory time constant for the respiratory system and then uses this along with the estimated dead space and normal minute ventilation to calculate an optimal breathing frequency in terms of mechanical work.
The optimal or target tidal volume is calculated as the normal minute ventilation divided by the optimal frequency. The target tidal volume is achieved by the use of APC (see above) (Figure 2). This means that the pressure limit is automatically adjusted to achieve an average delivered tidal volume equal to the target. The ventilator continuously monitors the respiratory system mechanics and adjusts its settings accordingly.
The ventilator adjusts its breaths to avoid air trapping by allowing enough time to exhale, to avoid hypoventilation by delivering tidal volume greater than the dead space, and to avoid volutrauma by avoiding large tidal volumes.
Ventilator settings in adaptive support ventilation
Ventilator settings in ASV are:
- Patient height (to calculate the ideal body weight)
- Sex
- Percent of normal predicted minute ventilation goal
- Fio2
- PEEP.
Clinical applications of adaptive support ventilation
ASV is intended as a sole mode of ventilation, from initial support to weaning.
Theoretical benefits of adaptive support ventilation
In theory, ASV offers automatic selection of ventilator settings, automatic adaptation to changing patient lung mechanics, less need for human manipulation of the machine, improved synchrony, and automatic weaning.
Evidence of benefit of adaptive support ventilation
Physiologic benefits. Ventilator settings are adjusted automatically. ASV selects different tidal volume-respiratory rate combinations based on respiratory mechanics in passive and paralyzed patients.10–12 In actively breathing patients, there was no difference in the ventilator settings chosen by ASV for different clinical scenarios (and lung physiology).10 Compared with pressure-controlled intermittent mandatory ventilation, with ASV, the inspiratory load is less and patient-ventilator interaction is better.13
Patient-ventilator synchrony and comfort have not been studied.
Outcomes. Two trials suggest that ASV may decrease time on mechanical ventilation.14,15 However, in another trial,16 compared with a standard protocol, ASV led to fewer ventilator adjustments but achieved similar postsurgical weaning outcomes. The effect of this mode on the death rate has not been examined.17,18
Adaptive support ventilation: Bottom line
ASV is the first commercially available mode that automatically selects all the ventilator settings except PEEP and Fio2. These seem appropriate for different clinical scenarios in patients with poor respiratory effort or in paralyzed patients. Evidence of the effect in actively breathing patients and on outcomes such as length of stay or death is still lacking.
PROPORTIONAL ASSIST VENTILATION
Patients who have normal respiratory drive but who have difficulty sustaining adequate spontaneous ventilation are often subjected to pressure support ventilation (PSV), in which the ventilator generates a constant pressure throughout inspiration regardless of the intensity of the patient’s effort.
In 1992, Younes and colleagues19,20 developed proportional assist ventilation (PAV) as an alternative in which the ventilator generates pressure in proportion to the patient’s effort. PAV became commercially available in Europe in 1999 and was approved in the United States in 2006, available on the Puritan Bennett 840 ventilator (Puritan Bennett Co, Boulder, CO). PAV has also been used for noninvasive ventilation, but this is not available in the United States.
Other names for proportional assist ventilation
Proportional Pressure Support (Dräger Medical; not yet available in the United States).
What does proportional assist ventilation do?
This mode delivers pressure-controlled breaths with a servo control scheme (Table 2).
To better understand PAV, we can compare it with PSV. With PSV, the pressure applied by the ventilator rises to a preset level that is held constant (a set-point scheme) until a cycling criterion (a percent of the maximum inspiratory flow value) is reached. The inspiratory flow and tidal volume are the result of the patient’s inspiratory effort, the level of pressure applied, and the respiratory system mechanics.
In PAV, as in PSV, all breaths are spontaneous (Table 1). The patient controls the timing and size of the breath. There are no preset pressure, flow, or volume goals, but safety limits on the volume and pressure delivered can be set.
Ventilator settings in proportional assist ventilation
Ventilator settings in PAV are:
- Airway type (endotracheal tube, tracheostomy)
- Airway size (inner diameter)
- Percentage of work supported (assist range 5%–95%)
- Tidal volume limit
- Pressure limit
- Expiratory sensitivity (normally, as inspiration ends, flow should stop; this parameter tells the ventilator at what flow to end inspiration).
Caution when assessing the literature. Earlier ventilator versions, ie, Dräger and Manitoba (University of Manitoba, Winnipeg, MB, Canada), which are not available in the United States, required the repeated calculation of the respiratory system mechanics and the manual setting of flow and volume assists (amplification factors) independently. To overcome this limitation, new software automatically adjusts the flow and volume amplification to support the loads imposed by the automatically measured values of resistance and elastance (inverse of compliance) of the respiratory system.21 This software is included in the model (Puritan Bennett) available in the United States.
Clinical applications of proportional assist ventilation
The PAV mode is indicated for maximizing ventilator patient synchrony for assisted spontaneous ventilation.
PAV is contraindicated in patients with respiratory depression (bradypnea) or large air leaks (eg, bronchopleural fistulas). It should be used with caution in patients with severe hyperinflation, in which the patient may still be exhaling but the ventilator doesn’t recognize it. Another group in which PAV should be used with caution is those with high ventilatory drives, in which the ventilator overestimates respiratory system mechanics. This situation can lead to overassistance due to the “runaway phenomenon,” in which the ventilator continues to provide support even if the patient has stopped inspiration.22
Theoretical benefits of proportional assist ventilation
In theory, PAV should reduce the work of breathing, improve synchrony, automatically adapt to changing patient lung mechanics and effort, decrease the need for ventilator intervention and manipulation, decrease the need for sedation, and improve sleep.
Evidence of benefit of proportional assist ventilation
Physiologic benefits. PAV reduces the work of breathing better than PSV,21 even in the face of changing respiratory mechanics or increased respiratory demand (hypercapnia).23–25 The hemodynamic profile is similar to that in PSV. Tidal volumes are variable; however, in recent reports the tidal volumes were within the lung-protective range (6–8 mL/kg, plateau pressure < 30 cm H20).26,27
Comfort. PAV entails less patient effort and discomfort that PSV does.23,25 PAV significantly reduces asynchrony,27 which in turn may favorably affect sleep in critically ill patients. 28
Outcomes. The probability of spontaneous breathing without assistance was significantly better in critically ill patients ventilated with PAV than with PSV. No trial has reported the effect of PAV on deaths.27,29
Proportional assist ventilation: Bottom line
Extensive basic research has been done with PAV in different forms of respiratory failure, such as obstructive lung disease, acute respiratory distress syndrome (ARDS), and chronic respiratory failure. It fulfills its main goal, which is to improve patient-ventilator synchrony. Clinical experience with PAV in the United States is limited, as it was only recently approved.
AIRWAY PRESSURE-RELEASE VENTILATION AND BIPHASIC POSITIVE AIRWAY PRESSURE
Airway pressure-release ventilation (APRV) was described in 1987 by Stock et al30 as a mode for delivering ventilation in acute lung injury while avoiding high airway pressures. APRV combines high constant positive airway pressure (improving oxygenation and promoting alveolar recruitment) with intermittent releases (causing exhalation).
Other names for biphasic positive airway pressure
Other names for biphasic positive airway pressure are:
- BiLevel (Puritan Bennett)
- BIPAP (Dräger Europe)
- Bi Vent (Siemens)
- BiPhasic (Avea, Cardinal Health, Inc, Dublin, OH)
- PCV+ (Dräger Medical)
- DuoPAP (Hamilton).
Caution—name confusion. In North America, BiPAP (Respironics, Murrysville, PA) and BiLevel are used to refer to noninvasive modes of ventilation.
APRV has no other name.
What do these modes do?
These modes deliver pressure-controlled, time-triggered, and time-cycled breaths using a set-point targeting scheme (Table 2). This means that the ventilator maintains a constant pressure (set point) even in the face of spontaneous breaths.
Caution—source of confusion. The term continuous positive airway pressure (CPAP) is often used to describe this mode. However, CPAP is pressure that is applied continuously at the same level; the patient generates all the work to maintain ventilation (“pressure-controlled continuous spontaneous ventilation” in the current nomenclature). In APRV, the airway pressure is intermittently released and reapplied, generating a tidal volume that supports ventilation. In other words, this is a pressure-controlled breath with a very prolonged inspiratory time and a short expiratory time in which spontaneous ventilation is possible at any point (“pressure-controlled intermittent mandatory ventilation” in the current nomenclature).
How these modes are set in the ventilator may also be a source of confusion. To describe the time spent in high and low airway pressures, we use the terms Thigh and Tlow, respectively. By convention, the difference between APRV and biphasic mode is the duration of Tlow (< 1.5 sec for APRV).
Similarly, Phigh and Plow are used to describe the high and low airway pressure. To better understand this concept, you can create the same mode in conventional pressure-control ventilation by thinking of the Thigh as the inspiratory time, the Tlow as the expiratory time, the Phigh as inspiratory pressure, and the Plow as PEEP.
Hence, APRV is an extreme form of inverse ratio ventilation, with an inspiration-to-expiration ratio of 4:1. This means a patient spends most of the time in Phigh and Thigh, and exhalations are short (Tlow and Plow). In contrast, the biphasic mode uses conventional inspiration-expiration ratios (Figure 4).
As with any form of pressure control, the tidal volume is generated by airway pressure rising above baseline (ie, the end-expiratory value). Hence, to ensure an increase in minute ventilation, the mandatory breath rate must be increased (ie, decreasing Thigh, Tlow, or both) or the tidal volume must be increased (ie, increasing the difference between Phigh and Plow). This means that in APRV the Tlow has to happen more often (by increasing the number of breaths) or be more prolonged (allowing more air to exhale). Because unrestricted spontaneous breaths are permitted at any point of the cycle, the patient contributes to the total minute ventilation (usually 10%–40%).
In APRV and biphasic mode, the operator’s set time and pressure in inspiration and expiration will be delivered regardless of the patient’s breathing efforts—the patient’s spontaneous breath does not trigger a mechanical breath. Some ventilators have automatic adjustments to improve the trigger synchrony.
Ventilator settings in APRV and biphasic mode
These modes require the setting of two pressure levels (Phigh and Plow) and two time durations (Thigh and Tlow). One can add pressure support or automatic tube compensation to assist spontaneous breaths. The difference in Tlow generates differences in the Thigh:Tlow ratio: APRV has a short Tlow (an inspiration-expiration ratio of 4:1). Biphasic mode has a conventional inspiration-expiration ratio of 1:1 to 1:4.
Clinical applications
APRV is used in acute lung injury and ARDS. This mode should be used with caution or not at all in patients with obstructive lung disease or inappropriately increased respiratory drive.32–35
Biphasic mode is intended for both ventilation and weaning. In a patient who has poor respiratory effort or who is paralyzed, biphasic is identical to pressure-control/continuous mandatory ventilation.
Theoretical benefits of APRV and biphasic mode
Multiple benefits have been ascribed to these modes. In theory, APRV will maximize and maintain alveolar recruitment, improve oxygenation, lower inflation pressures, and decrease overinflation. Both APRV and biphasic, by preserving spontaneous breathing, will improve ventilation-perfusion matching and gas diffusion, improve the hemodynamic profile (less need for vasopressors, higher cardiac output, reduced ventricular workload, improved organ perfusion), and improve synchrony (decrease the work of breathing and the need for sedation).
Evidence of benefit of APRV and biphasic mode
APRV and biphasic are different modes. However studies evaluating their effects are combined. This is in part the result of the nomenclature confusion and different practice in different countries.36
Physiologic benefits. In studies, spontaneous breaths contributed to 10% to 40% of minute ventilation,37,38 improved ventilation of dependent areas of the lung, improved ventilation-perfusion match and recruitment,39 and improved hemodynamic profile.40
Patient comfort. These modes are thought to decrease the need for analgesia and sedation,38 but a recent trial showed no difference with pressure-controlled intermittent mandatory ventilation.41 Patient ventilator synchrony and comfort have not been studied.32,42
Outcomes. In small trials, these modes made no difference in terms of deaths, but they may decrease the length of mechanical ventilation.38,41,43,44
APRV and biphasic mode: Bottom line
Maintaining spontaneous breathing while on mechanical ventilation has hemodynamic and ventilatory benefits.
APRV and biphasic mode are not the same thing. APRV’s main goal is to maximize mean airway pressure and, hence, lung recruitment, whereas the main goal of the biphasic mode is synchrony.
There is a plethora of ventilator settings and questions related to physiologic effects.33,34,36
Although these modes are widely used in some centers, there is no evidence yet that they are superior to conventional volume- or pressure-control ventilation with low tidal volume for ARDS and acute lung injury. There is no conclusive evidence that these modes improve synchrony, time to weaning, or patient comfort.
HIGH-FREQUENCY OSCILLATORY VENTILATION
High-frequency oscillatory ventilation (HFOV) was first described and patented in 1952 by Emerson and was clinically developed in the early 1970s by Lunkenheimer.45
The goal of HFOV is to minimize lung injury; its characteristics (discussed below) make it useful in patients with severe ARDS. The US Food and Drug Administration approved it for infants in 1991 and for children in 1995. The adult model has been available since 1993, but it was not approved until 2001 (SensorMedics 3100B, Cardinal Health, Inc).
Other names for high-frequency oscillatory ventilation
While HFOV has no alternative names, the following acronyms describe similar modes:
- HFPPV (high-frequency positive pressure ventilation)
- HFJV (high-frequency jet ventilation)
- HFFI (high-frequency flow interruption)
- HFPV (high-frequency percussive ventilation)
- HFCWO (high-frequency chest wall oscillation).
All of these modes require different specialized ventilators.
What does high-frequency oscillatory ventilation do?
Conceptually, HFOV is a form of pressure-controlled intermittent mandatory ventilation with a set-point control scheme. In contrast to conventional pressure-controlled intermittent mandatory ventilation, in which relatively small spontaneous breaths may be superimposed on relatively large mandatory breaths, HFOV superimposes very small mandatory breaths (oscillations) on top of spontaneous breaths.
Adult patients are usually paralyzed or deeply sedated, since deep spontaneous breathing will trigger alarms and affect ventilator performance.
To manage ventilation (CO2 clearance), one or several of the following maneuvers can be done: decrease the oscillation frequency, increase the amplitude of the oscillations, increase the inspiratory time, or increase bias flow (while allowing an endotracheal tube cuff leak). Oxygenation adjustments are controlled by manipulating the mean airway pressure and the Fio2.
Ventilator settings in high-frequency oscillatory ventilation
Ventilator settings in HFOV are46:
- Airway pressure amplitude (delta P or power)
- Mean airway pressure
- Percent inspiration
- Inspiratory bias flow
- Fio2.
Clinical applications of high-frequency oscillatory ventilation
This mode is usually reserved for ARDS patients for whom conventional ventilation is failing. A recently published protocol46 suggests considering HFOV when there is oxygenation failure (Fio2 ≥ 0.7 and PEEP ≥ 14 cm H2O) or ventilation failure (pH < 7.25 with tidal volume ≥ 6 mL/kg predicted body weight and plateau airway pressure ≥ 30 cm H2O).
This mode is contraindicated when there is known severe airflow obstruction or intracranial hypertension.
Theoretical benefits of high-frequency oscillatory ventilation
Conceptually, HFOV can provide the highest mean airway pressure paired with the lowest tidal volume of any mode. These benefits might make HFOV the ideal lung-protective ventilation strategy.
Evidence of benefit of high-frequency oscillatory ventilation
Physiologic benefits. Animal models have shown less histologic damage and lung inflammation with HFOV than with high-tidal-volume conventional ventilation47,48 and low-tidal-volume conventional ventilation.49
Patient comfort has not been studied. However, current technology does impose undue work of breathing in spontaneously breathing patients.50
Outcomes. Several retrospective case series have described better oxygenation with HFOV as rescue therapy for severe ARDS than with conventional mechanical ventilation. Two randomized controlled trials have studied HFOV vs high-tidal-volume conventional mechanical ventilation for early severe ARDS; HFOV was safe but made no difference in terms of deaths.42,51–54
High-frequency oscillatory ventilation: Bottom line
In theory, HFOV provides all the benefits of an ideal lung-protective strategy, at least for paralyzed or deeply sedated patients. Animal studies support these concepts. In human adults, HFOV has been shown to be safe and to provide better oxygenation but no improvement in death rates compared with conventional mechanical ventilation. Currently, HFOV is better reserved for patients with severe ARDS for whom conventional mechanical ventilation is failing.
Technologic advances and computerized control of mechanical ventilators have made it possible to deliver ventilatory assistance in new modes. Driving these innovations is the desire to prevent ventilator-induced lung injury, improve patient comfort, and liberate the patient from mechanical ventilation as soon as possible.
We call these innovations “alternative” modes to differentiate them from the plain volume-control and pressure-control modes. Some clinicians rarely use these new modes, but in some medical centers they have become the most common ones used, or are being used unknowingly (the operator misunderstands the mode name). The information we provide on these modes of ventilation is by no means an endorsement of their use, but rather a tool to help the clinician understand their physiologic, theoretical, and clinical effects.
We focused on two goals:
- Explain what the mode does
- Briefly review the theoretical benefits and the actual evidence supporting these alternative modes of ventilation.

STANDARD NOMENCLATURE NEEDED
Since its invention, mechanical ventilation has been plagued by multiple names being used to describe the same things. For example, volume-control ventilation is also called volume-cycled ventilation, assist-control ventilation, volume-limited ventilation, and controlled mechanical ventilation. Similarly, multiple abbreviations are used, each depending on the brand of ventilator, and new acronyms have been added in recent years as new modes have been developed. The vast number of names and modes can confuse even the most seasoned critical care physician.
Efforts to establish a common nomenclature are under way.1
WHAT IS A MODE?
A mode of mechanical ventilation has three essential components:
- The control variable
- The breath sequence
- The targeting scheme.
Similar modes may require more detailed descriptions to distinguish them, but the basic function can be explained by these three components.
The control variable
In general, inspiration is an active process, driven by the patient’s effort, the ventilator, or both, while expiration is passive. For simplicity, in this article a mechanical breath means the inspiratory phase of the breath.
The machine can only control the volume (and flow) or the pressure given. The breaths can be further described on the basis of what triggers the breath, what limits it (the maximum value of a control variable), and what ends (cycles) it.
The breath sequence
There are three possible breath sequences:
- Intermittent mandatory ventilation, in which the patient can take spontaneous breaths between mandatory breaths
- Continuous spontaneous ventilation, in which all breaths are spontaneous (Table 1).
The targeting scheme
The targeting or feedback scheme refers to the ventilator settings and programming that dictate its response to the patient’s lung compliance, lung resistance, and respiratory effort. The regulation can be as simple as controlling the pressure in pressure-control mode, or it can be based on a complicated algorithm.
ADAPTIVE PRESSURE CONTROL
Other names for adaptive pressure control
- Pressure Regulated Volume Control (Maquet Servo-i, Rastatt, Germany)
- AutoFlow (Dräger Medical AG, Lübeck, Germany)
- Adaptive Pressure Ventilation (Hamilton Galileo, Hamilton Medical AG, Bonaduz, Switzerland)
- Volume Control+ (Puritan Bennett, Tyco Healthcare; Mansfield, MA)
- Volume Targeted Pressure Control, Pressure Controlled Volume Guaranteed (Engström, General Electric, Madison, WI).
What does adaptive pressure control do?
The APC mode delivers pressure-controlled breaths with an adaptive targeting scheme (Table 2).
In pressure-control ventilation, tidal volumes depend on the lung’s physiologic mechanics (compliance and resistance) and patient effort (Figure 1). Therefore, the tidal volume varies with changes in lung physiology (ie, larger or smaller tidal volumes than targeted).
To overcome this effect, a machine in APC mode adjusts the inspiratory pressure to deliver the set minimal target tidal volume. If tidal volume increases, the machine decreases the inspiratory pressure, and if tidal volume decreases, the machine increases the inspiratory pressure. However, if the patient effort is large enough, the tidal volume will increase in spite of decreasing the inspiratory pressure (Figure 2). The adjustments to the inspiratory pressure occur after the tidal volume is off-target in a number of breaths.
Common sources of confusion with adaptive pressure control
First, APC is not a volume-control mode. In volume control, the tidal volume does not change; in APC the tidal volume can increase or decrease, and the ventilator will adjust the inflation pressure to achieve the target volume. Thus, APC guarantees an average minimum tidal volume but not a maximum tidal volume.
Second, a characteristic of pressure control (and hence, APC) is that the flow of gas varies to maintain constant airway pressure (ie, maintain the set inspiratory pressure). This characteristic allows a patient who generates an inspiratory effort to receive flow as demanded, which is likely more comfortable. This is essentially different from volume control, in which flow is set by the operator and hence is fixed. Thus, if the patient effort is strong enough (Figure 1), this leads to what is called flow asynchrony, in which the patient does not get the flow asked for in a breath.
Ventilator settings in adaptive pressure control
Ventilator settings in APC are:
- Tidal volume
- Time spent in inspiration (inspiratory time)
- Frequency
- Fraction of inspired oxygen (Fio2)
- Positive end-expiratory pressure (PEEP).
Some ventilators also require setting the speed to reach the peak pressure (also known as slope percent or inspiratory rise time).
Clinical applications of adaptive pressure control
This mode is designed to maintain a consistent tidal volume during pressure-control ventilation and to promote inspiratory flow synchrony. It is a means of automatically reducing ventilatory support (ie, weaning) as the patient’s inspiratory effort becomes stronger, as in awakening from anesthesia.
APC may not be ideal for patients who have an inappropriately increased respiratory drive (eg, in severe metabolic acidosis), since the inspiratory pressure will decrease to maintain the targeted average tidal volume, inappropriately shifting the work of breathing onto the patient.
Theoretical benefits of adaptive pressure control
APC guarantees a minimum average tidal volume (unless the pressure alarm threshold is set too low, so that the target tidal volume is not delivered). Other theoretical benefits are flow synchrony, less ventilator manipulation by the operator, and automatic weaning of ventilator support.
Evidence of benefit of adaptive pressure control
Physiologic benefits. This mode has lower peak inspiratory pressures than does volume-control ventilation,3,4 which is often reported as a positive finding. However, in volume-control mode (the usual comparator), the peak inspiratory pressure is a manifestation of both resistance and compliance. Hence, peak inspiratory pressure is expected to be higher but does not reflect actual lung-distending pressures. It is the plateau pressure, a manifestation of lung compliance, that is related to lung injury.
Patient comfort. APC may increase the work of breathing when using low tidal volume ventilation and when there is increased respiratory effort (drive).5 Interestingly, APC was less comfortable than pressure support ventilation in a small trial.6
Outcomes have not been studied.7
Adaptive pressure control: Bottom line
APC is widely available and widely used, sometimes unknowingly (eg, if the operator thinks it is volume control). It is relatively easy to use and to set; however, evidence of its benefit is scant.
ADAPTIVE SUPPORT VENTILATION
Adaptive support ventilation (ASV) evolved as a form of mandatory minute ventilation implemented with adaptive pressure control. Mandatory minute ventilation is a mode that allows the operator to preset a target minute ventilation, and the ventilator then supplies mandatory breaths, either volume- or pressure-controlled, if the patient’s spontaneous breaths generate a lower minute ventilation.
ASV automatically selects the appropriate tidal volume and frequency for mandatory breaths and the appropriate tidal volume for spontaneous breaths on the basis of the respiratory system mechanics and target minute alveolar ventilation.
Described in 1994 by Laubscher et al,8,9 ASV became commercially available in 1998 in Europe and in 2007 in the United States (Hamilton Galileo ventilator, Hamilton Medical AG). This is the first commercially available ventilator that uses an “optimal” targeting scheme (see below).
What does adaptive support ventilation do?
ASV delivers pressure-controlled breaths using an adaptive (optimal) scheme (Table 2). “Optimal,” in this context, means minimizing the mechanical work of breathing: the machine selects a tidal volume and frequency that the patient’s brain would presumably select if the patient were not connected to a ventilator. This pattern is assumed to encourage the patient to generate spontaneous breaths.
The ventilator calculates the normal required minute ventilation based on the patient’s ideal weight and estimated dead space volume (ie, 2.2 mL/kg). This calculation represents 100% of minute ventilation. The clinician at the bedside sets a target percent of minute ventilation that the ventilator will support—higher than 100% if the patient has increased requirements due, eg, to sepsis or increased dead space, or less than 100% during weaning.
The ventilator initially delivers test breaths, in which it measures the expiratory time constant for the respiratory system and then uses this along with the estimated dead space and normal minute ventilation to calculate an optimal breathing frequency in terms of mechanical work.
The optimal or target tidal volume is calculated as the normal minute ventilation divided by the optimal frequency. The target tidal volume is achieved by the use of APC (see above) (Figure 2). This means that the pressure limit is automatically adjusted to achieve an average delivered tidal volume equal to the target. The ventilator continuously monitors the respiratory system mechanics and adjusts its settings accordingly.
The ventilator adjusts its breaths to avoid air trapping by allowing enough time to exhale, to avoid hypoventilation by delivering tidal volume greater than the dead space, and to avoid volutrauma by avoiding large tidal volumes.
Ventilator settings in adaptive support ventilation
Ventilator settings in ASV are:
- Patient height (to calculate the ideal body weight)
- Sex
- Percent of normal predicted minute ventilation goal
- Fio2
- PEEP.
Clinical applications of adaptive support ventilation
ASV is intended as a sole mode of ventilation, from initial support to weaning.
Theoretical benefits of adaptive support ventilation
In theory, ASV offers automatic selection of ventilator settings, automatic adaptation to changing patient lung mechanics, less need for human manipulation of the machine, improved synchrony, and automatic weaning.
Evidence of benefit of adaptive support ventilation
Physiologic benefits. Ventilator settings are adjusted automatically. ASV selects different tidal volume-respiratory rate combinations based on respiratory mechanics in passive and paralyzed patients.10–12 In actively breathing patients, there was no difference in the ventilator settings chosen by ASV for different clinical scenarios (and lung physiology).10 Compared with pressure-controlled intermittent mandatory ventilation, with ASV, the inspiratory load is less and patient-ventilator interaction is better.13
Patient-ventilator synchrony and comfort have not been studied.
Outcomes. Two trials suggest that ASV may decrease time on mechanical ventilation.14,15 However, in another trial,16 compared with a standard protocol, ASV led to fewer ventilator adjustments but achieved similar postsurgical weaning outcomes. The effect of this mode on the death rate has not been examined.17,18
Adaptive support ventilation: Bottom line
ASV is the first commercially available mode that automatically selects all the ventilator settings except PEEP and Fio2. These seem appropriate for different clinical scenarios in patients with poor respiratory effort or in paralyzed patients. Evidence of the effect in actively breathing patients and on outcomes such as length of stay or death is still lacking.
PROPORTIONAL ASSIST VENTILATION
Patients who have normal respiratory drive but who have difficulty sustaining adequate spontaneous ventilation are often subjected to pressure support ventilation (PSV), in which the ventilator generates a constant pressure throughout inspiration regardless of the intensity of the patient’s effort.
In 1992, Younes and colleagues19,20 developed proportional assist ventilation (PAV) as an alternative in which the ventilator generates pressure in proportion to the patient’s effort. PAV became commercially available in Europe in 1999 and was approved in the United States in 2006, available on the Puritan Bennett 840 ventilator (Puritan Bennett Co, Boulder, CO). PAV has also been used for noninvasive ventilation, but this is not available in the United States.
Other names for proportional assist ventilation
Proportional Pressure Support (Dräger Medical; not yet available in the United States).
What does proportional assist ventilation do?
This mode delivers pressure-controlled breaths with a servo control scheme (Table 2).
To better understand PAV, we can compare it with PSV. With PSV, the pressure applied by the ventilator rises to a preset level that is held constant (a set-point scheme) until a cycling criterion (a percent of the maximum inspiratory flow value) is reached. The inspiratory flow and tidal volume are the result of the patient’s inspiratory effort, the level of pressure applied, and the respiratory system mechanics.
In PAV, as in PSV, all breaths are spontaneous (Table 1). The patient controls the timing and size of the breath. There are no preset pressure, flow, or volume goals, but safety limits on the volume and pressure delivered can be set.
Ventilator settings in proportional assist ventilation
Ventilator settings in PAV are:
- Airway type (endotracheal tube, tracheostomy)
- Airway size (inner diameter)
- Percentage of work supported (assist range 5%–95%)
- Tidal volume limit
- Pressure limit
- Expiratory sensitivity (normally, as inspiration ends, flow should stop; this parameter tells the ventilator at what flow to end inspiration).
Caution when assessing the literature. Earlier ventilator versions, ie, Dräger and Manitoba (University of Manitoba, Winnipeg, MB, Canada), which are not available in the United States, required the repeated calculation of the respiratory system mechanics and the manual setting of flow and volume assists (amplification factors) independently. To overcome this limitation, new software automatically adjusts the flow and volume amplification to support the loads imposed by the automatically measured values of resistance and elastance (inverse of compliance) of the respiratory system.21 This software is included in the model (Puritan Bennett) available in the United States.
Clinical applications of proportional assist ventilation
The PAV mode is indicated for maximizing ventilator patient synchrony for assisted spontaneous ventilation.
PAV is contraindicated in patients with respiratory depression (bradypnea) or large air leaks (eg, bronchopleural fistulas). It should be used with caution in patients with severe hyperinflation, in which the patient may still be exhaling but the ventilator doesn’t recognize it. Another group in which PAV should be used with caution is those with high ventilatory drives, in which the ventilator overestimates respiratory system mechanics. This situation can lead to overassistance due to the “runaway phenomenon,” in which the ventilator continues to provide support even if the patient has stopped inspiration.22
Theoretical benefits of proportional assist ventilation
In theory, PAV should reduce the work of breathing, improve synchrony, automatically adapt to changing patient lung mechanics and effort, decrease the need for ventilator intervention and manipulation, decrease the need for sedation, and improve sleep.
Evidence of benefit of proportional assist ventilation
Physiologic benefits. PAV reduces the work of breathing better than PSV,21 even in the face of changing respiratory mechanics or increased respiratory demand (hypercapnia).23–25 The hemodynamic profile is similar to that in PSV. Tidal volumes are variable; however, in recent reports the tidal volumes were within the lung-protective range (6–8 mL/kg, plateau pressure < 30 cm H20).26,27
Comfort. PAV entails less patient effort and discomfort that PSV does.23,25 PAV significantly reduces asynchrony,27 which in turn may favorably affect sleep in critically ill patients. 28
Outcomes. The probability of spontaneous breathing without assistance was significantly better in critically ill patients ventilated with PAV than with PSV. No trial has reported the effect of PAV on deaths.27,29
Proportional assist ventilation: Bottom line
Extensive basic research has been done with PAV in different forms of respiratory failure, such as obstructive lung disease, acute respiratory distress syndrome (ARDS), and chronic respiratory failure. It fulfills its main goal, which is to improve patient-ventilator synchrony. Clinical experience with PAV in the United States is limited, as it was only recently approved.
AIRWAY PRESSURE-RELEASE VENTILATION AND BIPHASIC POSITIVE AIRWAY PRESSURE
Airway pressure-release ventilation (APRV) was described in 1987 by Stock et al30 as a mode for delivering ventilation in acute lung injury while avoiding high airway pressures. APRV combines high constant positive airway pressure (improving oxygenation and promoting alveolar recruitment) with intermittent releases (causing exhalation).
Other names for biphasic positive airway pressure
Other names for biphasic positive airway pressure are:
- BiLevel (Puritan Bennett)
- BIPAP (Dräger Europe)
- Bi Vent (Siemens)
- BiPhasic (Avea, Cardinal Health, Inc, Dublin, OH)
- PCV+ (Dräger Medical)
- DuoPAP (Hamilton).
Caution—name confusion. In North America, BiPAP (Respironics, Murrysville, PA) and BiLevel are used to refer to noninvasive modes of ventilation.
APRV has no other name.
What do these modes do?
These modes deliver pressure-controlled, time-triggered, and time-cycled breaths using a set-point targeting scheme (Table 2). This means that the ventilator maintains a constant pressure (set point) even in the face of spontaneous breaths.
Caution—source of confusion. The term continuous positive airway pressure (CPAP) is often used to describe this mode. However, CPAP is pressure that is applied continuously at the same level; the patient generates all the work to maintain ventilation (“pressure-controlled continuous spontaneous ventilation” in the current nomenclature). In APRV, the airway pressure is intermittently released and reapplied, generating a tidal volume that supports ventilation. In other words, this is a pressure-controlled breath with a very prolonged inspiratory time and a short expiratory time in which spontaneous ventilation is possible at any point (“pressure-controlled intermittent mandatory ventilation” in the current nomenclature).
How these modes are set in the ventilator may also be a source of confusion. To describe the time spent in high and low airway pressures, we use the terms Thigh and Tlow, respectively. By convention, the difference between APRV and biphasic mode is the duration of Tlow (< 1.5 sec for APRV).
Similarly, Phigh and Plow are used to describe the high and low airway pressure. To better understand this concept, you can create the same mode in conventional pressure-control ventilation by thinking of the Thigh as the inspiratory time, the Tlow as the expiratory time, the Phigh as inspiratory pressure, and the Plow as PEEP.
Hence, APRV is an extreme form of inverse ratio ventilation, with an inspiration-to-expiration ratio of 4:1. This means a patient spends most of the time in Phigh and Thigh, and exhalations are short (Tlow and Plow). In contrast, the biphasic mode uses conventional inspiration-expiration ratios (Figure 4).
As with any form of pressure control, the tidal volume is generated by airway pressure rising above baseline (ie, the end-expiratory value). Hence, to ensure an increase in minute ventilation, the mandatory breath rate must be increased (ie, decreasing Thigh, Tlow, or both) or the tidal volume must be increased (ie, increasing the difference between Phigh and Plow). This means that in APRV the Tlow has to happen more often (by increasing the number of breaths) or be more prolonged (allowing more air to exhale). Because unrestricted spontaneous breaths are permitted at any point of the cycle, the patient contributes to the total minute ventilation (usually 10%–40%).
In APRV and biphasic mode, the operator’s set time and pressure in inspiration and expiration will be delivered regardless of the patient’s breathing efforts—the patient’s spontaneous breath does not trigger a mechanical breath. Some ventilators have automatic adjustments to improve the trigger synchrony.
Ventilator settings in APRV and biphasic mode
These modes require the setting of two pressure levels (Phigh and Plow) and two time durations (Thigh and Tlow). One can add pressure support or automatic tube compensation to assist spontaneous breaths. The difference in Tlow generates differences in the Thigh:Tlow ratio: APRV has a short Tlow (an inspiration-expiration ratio of 4:1). Biphasic mode has a conventional inspiration-expiration ratio of 1:1 to 1:4.
Clinical applications
APRV is used in acute lung injury and ARDS. This mode should be used with caution or not at all in patients with obstructive lung disease or inappropriately increased respiratory drive.32–35
Biphasic mode is intended for both ventilation and weaning. In a patient who has poor respiratory effort or who is paralyzed, biphasic is identical to pressure-control/continuous mandatory ventilation.
Theoretical benefits of APRV and biphasic mode
Multiple benefits have been ascribed to these modes. In theory, APRV will maximize and maintain alveolar recruitment, improve oxygenation, lower inflation pressures, and decrease overinflation. Both APRV and biphasic, by preserving spontaneous breathing, will improve ventilation-perfusion matching and gas diffusion, improve the hemodynamic profile (less need for vasopressors, higher cardiac output, reduced ventricular workload, improved organ perfusion), and improve synchrony (decrease the work of breathing and the need for sedation).
Evidence of benefit of APRV and biphasic mode
APRV and biphasic are different modes. However studies evaluating their effects are combined. This is in part the result of the nomenclature confusion and different practice in different countries.36
Physiologic benefits. In studies, spontaneous breaths contributed to 10% to 40% of minute ventilation,37,38 improved ventilation of dependent areas of the lung, improved ventilation-perfusion match and recruitment,39 and improved hemodynamic profile.40
Patient comfort. These modes are thought to decrease the need for analgesia and sedation,38 but a recent trial showed no difference with pressure-controlled intermittent mandatory ventilation.41 Patient ventilator synchrony and comfort have not been studied.32,42
Outcomes. In small trials, these modes made no difference in terms of deaths, but they may decrease the length of mechanical ventilation.38,41,43,44
APRV and biphasic mode: Bottom line
Maintaining spontaneous breathing while on mechanical ventilation has hemodynamic and ventilatory benefits.
APRV and biphasic mode are not the same thing. APRV’s main goal is to maximize mean airway pressure and, hence, lung recruitment, whereas the main goal of the biphasic mode is synchrony.
There is a plethora of ventilator settings and questions related to physiologic effects.33,34,36
Although these modes are widely used in some centers, there is no evidence yet that they are superior to conventional volume- or pressure-control ventilation with low tidal volume for ARDS and acute lung injury. There is no conclusive evidence that these modes improve synchrony, time to weaning, or patient comfort.
HIGH-FREQUENCY OSCILLATORY VENTILATION
High-frequency oscillatory ventilation (HFOV) was first described and patented in 1952 by Emerson and was clinically developed in the early 1970s by Lunkenheimer.45
The goal of HFOV is to minimize lung injury; its characteristics (discussed below) make it useful in patients with severe ARDS. The US Food and Drug Administration approved it for infants in 1991 and for children in 1995. The adult model has been available since 1993, but it was not approved until 2001 (SensorMedics 3100B, Cardinal Health, Inc).
Other names for high-frequency oscillatory ventilation
While HFOV has no alternative names, the following acronyms describe similar modes:
- HFPPV (high-frequency positive pressure ventilation)
- HFJV (high-frequency jet ventilation)
- HFFI (high-frequency flow interruption)
- HFPV (high-frequency percussive ventilation)
- HFCWO (high-frequency chest wall oscillation).
All of these modes require different specialized ventilators.
What does high-frequency oscillatory ventilation do?
Conceptually, HFOV is a form of pressure-controlled intermittent mandatory ventilation with a set-point control scheme. In contrast to conventional pressure-controlled intermittent mandatory ventilation, in which relatively small spontaneous breaths may be superimposed on relatively large mandatory breaths, HFOV superimposes very small mandatory breaths (oscillations) on top of spontaneous breaths.
Adult patients are usually paralyzed or deeply sedated, since deep spontaneous breathing will trigger alarms and affect ventilator performance.
To manage ventilation (CO2 clearance), one or several of the following maneuvers can be done: decrease the oscillation frequency, increase the amplitude of the oscillations, increase the inspiratory time, or increase bias flow (while allowing an endotracheal tube cuff leak). Oxygenation adjustments are controlled by manipulating the mean airway pressure and the Fio2.
Ventilator settings in high-frequency oscillatory ventilation
Ventilator settings in HFOV are46:
- Airway pressure amplitude (delta P or power)
- Mean airway pressure
- Percent inspiration
- Inspiratory bias flow
- Fio2.
Clinical applications of high-frequency oscillatory ventilation
This mode is usually reserved for ARDS patients for whom conventional ventilation is failing. A recently published protocol46 suggests considering HFOV when there is oxygenation failure (Fio2 ≥ 0.7 and PEEP ≥ 14 cm H2O) or ventilation failure (pH < 7.25 with tidal volume ≥ 6 mL/kg predicted body weight and plateau airway pressure ≥ 30 cm H2O).
This mode is contraindicated when there is known severe airflow obstruction or intracranial hypertension.
Theoretical benefits of high-frequency oscillatory ventilation
Conceptually, HFOV can provide the highest mean airway pressure paired with the lowest tidal volume of any mode. These benefits might make HFOV the ideal lung-protective ventilation strategy.
Evidence of benefit of high-frequency oscillatory ventilation
Physiologic benefits. Animal models have shown less histologic damage and lung inflammation with HFOV than with high-tidal-volume conventional ventilation47,48 and low-tidal-volume conventional ventilation.49
Patient comfort has not been studied. However, current technology does impose undue work of breathing in spontaneously breathing patients.50
Outcomes. Several retrospective case series have described better oxygenation with HFOV as rescue therapy for severe ARDS than with conventional mechanical ventilation. Two randomized controlled trials have studied HFOV vs high-tidal-volume conventional mechanical ventilation for early severe ARDS; HFOV was safe but made no difference in terms of deaths.42,51–54
High-frequency oscillatory ventilation: Bottom line
In theory, HFOV provides all the benefits of an ideal lung-protective strategy, at least for paralyzed or deeply sedated patients. Animal studies support these concepts. In human adults, HFOV has been shown to be safe and to provide better oxygenation but no improvement in death rates compared with conventional mechanical ventilation. Currently, HFOV is better reserved for patients with severe ARDS for whom conventional mechanical ventilation is failing.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Chatburn RL. Computer control of mechanical ventilation. Respir Care 2004; 49:507–517.
- Alvarez A, Subirana M, Benito S. Decelerating flow ventilation effects in acute respiratory failure. J Crit Care 1998; 13:21–25.
- Guldager H, Nielsen SL, Carl P, Soerensen MB. A comparison of volume control and pressure regulated volume control ventilation in acute respiratory failure. Crit Care 1997; 1:75–77.
- Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Work of breathing during lung protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure regulated breathing modes. Respir Care 2005; 50:1623–1631.
- Betensley AD, Khalid I, Crawford J, Pensler RA, DiGiovine B. Patient comfort during pressure support and volume controlled continuous mandatory ventilation. Respir Care 2008; 53:897–902.
- Branson RD, Chatburn RL. Controversies in the critical care setting. Should adaptive pressure control modes be utilized for virtually all patients receiving mechanical ventilation? Respir Care 2007; 52:478–485.
- Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start up procedure for closed-loop controlled ventilation. Int J Clin Monit Comput 1994; 11:19–30.
- Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX. An adaptive lung ventilation controller. IEEE Trans Biomed Eng 1994; 41:51–59.
- Arnal JM, Wysocki M, Nafati C, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med 2008; 34:75–81.
- Campbell RS, Sinamban RP, Johannigman JA, et al. Clinical evaluation of a new closed loop ventilation mode: adaptive supportive ventilation (ASV). Crit Care 1999; 3( suppl 1):083.
- Belliato M, Palo A, Pasero D, Iotti GA, Mojoli F, Braschi A. Evaluation of adaptive support ventilation in paralysed patients and in a physical lung model. Int J Artif Organs 2004; 27:709–716.
- Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient ventilator interactions during partial ventilatory support: a preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med 2002; 30:801–807.
- Gruber PC, Gomersall CD, Leung P, et al. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology 2008; 109:81–87.
- Sulzer CF, Chiolero R, Chassot PG, et al. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology 2001; 95:1339–1345.
- Petter AH, Chiolèro RL, Cassina T, Chassot PG, Müller XM, Revelly JP. Automatic “respirator/weaning” with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analg 2003; 97:1743–1750.
- Brunner JX, Iotti GA. Adaptive support ventilation (ASV). Minerva Anestesiol 2002; 68:365–368.
- Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin North Am 2001; 7:425–440.
- Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis 1992; 145:114–120.
- Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis 1992; 145:121–129.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilatio—proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Med 2006; 32:692–699.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Effect of different levels of pressure support and proportional assist ventilation on breathing pattern, work of breathing and gas exchange in mechanically ventilated hypercapnic COPD patients with acute respiratory failure. Respiration 2003; 70:355–361.
- Grasso S, Puntillo F, Mascia L, et al. Compensation for increase in respiratory workload during mechanical ventilation. Pressure support versus proportional assist ventilation. Am J Respir Crit Care Med 2000; 161:819–826.
- Wrigge H, Golisch W, Zinserling J, Sydow M, Almeling G, Burchardi H. Proportional assist versus pressure support ventilation: effects on breathing pattern and respiratory work of patients with chronic obstructive pulmonary disease. Intensive Care Med 1999; 25:790–798.
- Ranieri VM, Giuliani R, Mascia L, et al. Patient ventilator interaction during acute hypercapnia: pressure support vs. proportional assist ventilation. J Appl Physiol 1996; 81:426–436.
- Kondili E, Xirouchaki N, Vaporidi K, Klimathianaki M, Georgopoulos D. Short-term cardiorespiratory effects of proportional assist and pressure support ventilation in patients with acute lung injury/acute respiratory distress syndrome. Anesthesiology 2006; 105:703–708.
- Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med 2008; 34:2026–2034.
- Bosma K, Ferreyra G, Ambrogio C, et al. Patient ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med 2007; 35:1048–1054.
- Sinderby C, Beck J. Proportional assist ventilation and neurally adjusted ventilatory assist—better approaches to patient ventilator synchrony? Clin Chest Med 2008; 29:329–342.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Baum M, Benzer H, Putensen C, Koller W, Putz G. [Biphasic positive airway pressure (BIPAP)—a new form of augmented ventilation]. Anaesthesist 1989; 38:452–458.
- Seymour CW, Frazer M, Reilly PM, Fuchs BD. Airway pressure release and biphasic intermittent positive airway pressure ventilation: are they ready for prime time? J Trauma 2007; 62:1298–1308.
- Myers TR, MacIntyre NR. Respiratory controversies in the critical care setting. Does airway pressure release ventilation offer important new advantages in mechanical ventilator support? Respir Care 2007; 52:452–458.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Calzia E, Lindner KH, Witt S, et al. Pressure-time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med 1994; 150:904–910.
- Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 2008; 34:1766–1773.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Longterm effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 2001; 5:221–226.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis in 596 patients following adult cardiac surgery. Eur J Anaesthesiol 1997; 14:576–582.
- Habashi NM. Other approaches to open lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33 suppl 3:S228–S240.
- Hess D, Mason S, Branson R. High-frequency ventilation design and equipment issues. Respir Care Clin North Am 2001; 7:577–598.
- Fessler HE, Derdak S, Ferguson ND, et al. A protocol for high frequency oscillatory ventilation in adults: results from a roundtable discussion. Crit Care Med 2007; 35:1649–1654.
- Hamilton PP, Onayemi A, Smyth JA, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol 1983; 55:131–138.
- Sedeek KA, Takeuchi M, Suchodolski K, et al. Open-lung protective ventilation with pressure control ventilation, high-frequency oscillation, and intratracheal pulmonary ventilation results in similar gas exchange, hemodynamics, and lung mechanics. Anesthesiology 2003; 99:1102–1111.
- Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K. Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 2001; 91:1836–1844.
- van Heerde M, Roubik K, Kopelent V, Plötz FB, Markhorst DG. Unloading work of breathing during high-frequency oscillatory ventilation: a bench study. Crit Care 2006; 10:R103.
- Derdak S, Mehta S, Stewart TE, et al., Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial (MOAT) Study Investigators. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002; 166:801–808.
- Bollen CW, van Well GT, Sherry T, et al. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care 2005; 9:R430–R439.
- Mehta S, Granton J, MacDonald RJ, et al. High frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Chatburn RL. Computer control of mechanical ventilation. Respir Care 2004; 49:507–517.
- Alvarez A, Subirana M, Benito S. Decelerating flow ventilation effects in acute respiratory failure. J Crit Care 1998; 13:21–25.
- Guldager H, Nielsen SL, Carl P, Soerensen MB. A comparison of volume control and pressure regulated volume control ventilation in acute respiratory failure. Crit Care 1997; 1:75–77.
- Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Work of breathing during lung protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure regulated breathing modes. Respir Care 2005; 50:1623–1631.
- Betensley AD, Khalid I, Crawford J, Pensler RA, DiGiovine B. Patient comfort during pressure support and volume controlled continuous mandatory ventilation. Respir Care 2008; 53:897–902.
- Branson RD, Chatburn RL. Controversies in the critical care setting. Should adaptive pressure control modes be utilized for virtually all patients receiving mechanical ventilation? Respir Care 2007; 52:478–485.
- Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start up procedure for closed-loop controlled ventilation. Int J Clin Monit Comput 1994; 11:19–30.
- Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX. An adaptive lung ventilation controller. IEEE Trans Biomed Eng 1994; 41:51–59.
- Arnal JM, Wysocki M, Nafati C, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med 2008; 34:75–81.
- Campbell RS, Sinamban RP, Johannigman JA, et al. Clinical evaluation of a new closed loop ventilation mode: adaptive supportive ventilation (ASV). Crit Care 1999; 3( suppl 1):083.
- Belliato M, Palo A, Pasero D, Iotti GA, Mojoli F, Braschi A. Evaluation of adaptive support ventilation in paralysed patients and in a physical lung model. Int J Artif Organs 2004; 27:709–716.
- Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient ventilator interactions during partial ventilatory support: a preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med 2002; 30:801–807.
- Gruber PC, Gomersall CD, Leung P, et al. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology 2008; 109:81–87.
- Sulzer CF, Chiolero R, Chassot PG, et al. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology 2001; 95:1339–1345.
- Petter AH, Chiolèro RL, Cassina T, Chassot PG, Müller XM, Revelly JP. Automatic “respirator/weaning” with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analg 2003; 97:1743–1750.
- Brunner JX, Iotti GA. Adaptive support ventilation (ASV). Minerva Anestesiol 2002; 68:365–368.
- Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin North Am 2001; 7:425–440.
- Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis 1992; 145:114–120.
- Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis 1992; 145:121–129.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilatio—proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Med 2006; 32:692–699.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Effect of different levels of pressure support and proportional assist ventilation on breathing pattern, work of breathing and gas exchange in mechanically ventilated hypercapnic COPD patients with acute respiratory failure. Respiration 2003; 70:355–361.
- Grasso S, Puntillo F, Mascia L, et al. Compensation for increase in respiratory workload during mechanical ventilation. Pressure support versus proportional assist ventilation. Am J Respir Crit Care Med 2000; 161:819–826.
- Wrigge H, Golisch W, Zinserling J, Sydow M, Almeling G, Burchardi H. Proportional assist versus pressure support ventilation: effects on breathing pattern and respiratory work of patients with chronic obstructive pulmonary disease. Intensive Care Med 1999; 25:790–798.
- Ranieri VM, Giuliani R, Mascia L, et al. Patient ventilator interaction during acute hypercapnia: pressure support vs. proportional assist ventilation. J Appl Physiol 1996; 81:426–436.
- Kondili E, Xirouchaki N, Vaporidi K, Klimathianaki M, Georgopoulos D. Short-term cardiorespiratory effects of proportional assist and pressure support ventilation in patients with acute lung injury/acute respiratory distress syndrome. Anesthesiology 2006; 105:703–708.
- Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med 2008; 34:2026–2034.
- Bosma K, Ferreyra G, Ambrogio C, et al. Patient ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med 2007; 35:1048–1054.
- Sinderby C, Beck J. Proportional assist ventilation and neurally adjusted ventilatory assist—better approaches to patient ventilator synchrony? Clin Chest Med 2008; 29:329–342.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Baum M, Benzer H, Putensen C, Koller W, Putz G. [Biphasic positive airway pressure (BIPAP)—a new form of augmented ventilation]. Anaesthesist 1989; 38:452–458.
- Seymour CW, Frazer M, Reilly PM, Fuchs BD. Airway pressure release and biphasic intermittent positive airway pressure ventilation: are they ready for prime time? J Trauma 2007; 62:1298–1308.
- Myers TR, MacIntyre NR. Respiratory controversies in the critical care setting. Does airway pressure release ventilation offer important new advantages in mechanical ventilator support? Respir Care 2007; 52:452–458.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Calzia E, Lindner KH, Witt S, et al. Pressure-time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med 1994; 150:904–910.
- Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 2008; 34:1766–1773.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Longterm effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 2001; 5:221–226.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis in 596 patients following adult cardiac surgery. Eur J Anaesthesiol 1997; 14:576–582.
- Habashi NM. Other approaches to open lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33 suppl 3:S228–S240.
- Hess D, Mason S, Branson R. High-frequency ventilation design and equipment issues. Respir Care Clin North Am 2001; 7:577–598.
- Fessler HE, Derdak S, Ferguson ND, et al. A protocol for high frequency oscillatory ventilation in adults: results from a roundtable discussion. Crit Care Med 2007; 35:1649–1654.
- Hamilton PP, Onayemi A, Smyth JA, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol 1983; 55:131–138.
- Sedeek KA, Takeuchi M, Suchodolski K, et al. Open-lung protective ventilation with pressure control ventilation, high-frequency oscillation, and intratracheal pulmonary ventilation results in similar gas exchange, hemodynamics, and lung mechanics. Anesthesiology 2003; 99:1102–1111.
- Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K. Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 2001; 91:1836–1844.
- van Heerde M, Roubik K, Kopelent V, Plötz FB, Markhorst DG. Unloading work of breathing during high-frequency oscillatory ventilation: a bench study. Crit Care 2006; 10:R103.
- Derdak S, Mehta S, Stewart TE, et al., Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial (MOAT) Study Investigators. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002; 166:801–808.
- Bollen CW, van Well GT, Sherry T, et al. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care 2005; 9:R430–R439.
- Mehta S, Granton J, MacDonald RJ, et al. High frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
KEY POINTS
- The alternative modes of ventilation were developed to prevent lung injury and asynchrony, promote better oxygenation and faster weaning, and be easier to use. However, evidence of their benefit is scant.
- Until now, we have lacked a standard nomenclature for mechanical ventilation, leading to confusion.
- Regardless of the mode used, the goals are to avoid lung injury, keep the patient comfortable, and wean the patient from mechanical ventilation as soon as possible.
A 48-year-old man with uncontrolled diabetes
A 48-year-old white man who has had diabetes mellitus for 6 years presents to the outpatient clinic because his blood sugar levels have been rising for the past week.
Both his parents had diabetes, and at the time of his diagnosis he weighed 278 pounds, all of which supported a diagnosis of type 2 diabetes mellitus. His disease was initially managed with diet, exercise, and metformin (Glucophage). Four months later, with weight loss and exercise, his blood sugar levels were consistently under 100 mg/dL, and metformin was discontinued.
He did well until 1 week ago, when he noted polyuria, polydipsia, and rising fingerstick glucose values, higher than 200 mg/dL. He has been eating well, with no nausea, vomiting, or symptoms of dehydration. He denies having any fever, chills, cough, nasal congestion, chest pain, abdominal pain, or dysuria.
In addition to his type 2 diabetes, he has hypertension, for which he takes losartan (Cozaar); hyperlipidemia, for which he takes atorvastatin (Lipitor); and gout, for which he takes allopurinol (Zyloprim).
His blood pressure is 148/70 mm Hg, pulse 100, and weight 273 pounds, and he is afebrile. On examination, his skin, head, eyes, ears, nose, throat, lungs, heart, and abdomen are normal. Urinalysis in the clinic shows large amounts of glucose and ketones.
WHAT IS THE LEAST LIKELY CAUSE OF HIS POOR CONTROL?
1. Which of the following is the least likely cause of his poorly controlled diabetes?
- Occult infection
- Poor adherence to diet and exercise
- Diabetic ketoacidosis
- Pancreatitis
Until 1 week ago, this patient’s diabetes had been well controlled for several years. Pancreatitis is the least likely cause of his uncontrolled diabetes, because he has no history of pancreatitis and has none of the symptoms of acute pancreatitis (fever, vomiting, or severe midepigastric pain radiating into the back).
Poor adherence to medication and lifestyle issues are very common in patients with poorly controlled diabetes and should always be included in the differential diagnosis.
Occult infection should also be considered in a patient with uncontrolled diabetes. Although this patient had no symptoms or signs of infection, urinalysis was done to look for an occult urinary tract infection and, surprisingly, it showed a large amount of ketones.
Case continued: He is treated for diabetic ketoacidosis
Diabetic ketoacidosis in ‘atypical diabetes’
Diabetic ketoacidosis is one of the most serious complications of diabetes. Many patients present with nausea, vomiting, and abdominal pain. Dehydration is often present because hyperglycemia leads to glucosuria and volume depletion. Interestingly, our patient showed none of these symptoms or signs.
Diabetic ketoacidosis is increasingly being recognized as a complication in patients with type 2 diabetes mellitus.1–4 Since the mid-1990s, clinicians have become increasingly aware of a condition variably termed “atypical diabetes,” “Flatbush diabetes,” “diabetes type 1B,” and “ketosis-prone type 2 diabetes mellitus,” in which patients, usually obese, present with diabetic ketoacidosis as their first manifestation, but are subsequently found to have type 2 diabetes mellitus. These patients typically are African American or of African, Hispanic, or Caribbean descent.
Ketoacidosis results from transient suppression of beta-cell function, the cause of which is unknown. A recent study comparing patients who have type 2 diabetes mellitus with and without diabetic ketoacidosis presenting with decompensated diabetes suggested insulinopenia was the predominant mechanism.5 For many of these patients, insulinopenia is transient: as the ketoacidosis resolves, betacell function improves and, with adequate insulin, lipolysis is reduced.
WHAT CAUSES DIABETIC KETOACIDOSIS?
2. Which of the following hormonal changes underlies the development of diabetic ketoacidosis?
- Insulin resistance
- Insulin deficiency
- Glucagon excess
- Glucagon deficiency
- Insulin deficiency and glucagon excess
- Insulin deficiency and glucagon deficiency
Diabetic ketoacidosis can occur when there is too much glucagon and not enough insulin. Insulin lowers the serum glucose level by promoting glucose uptake in peripheral tissues and by inhibiting gluconeogenesis and glycogenolysis in the liver. Insulin is also anabolic: it inhibits lipolysis in adipocytes and thus decreases the amount of substrate for ketogenesis.
Glucagon is the primary counterregulatory hormone responsible for ketogenesis.6 In the presence of glucagon excess, malonyl CoA production decreases, causing unblocking of carnitine acyltransferase I (CAT I) and allowing beta-oxidation to occur.6
Therefore, the sequence initiating ketogenesis begins with a shift in the ratio of glucagon to insulin, so that there is a relative or absolute excess of glucagon and a deficiency of insulin. A deficiency of insulin accelerates lipolysis, providing more substrate for ketogenesis, while excess glucagon turns on the oxidative sequence for fatty acids in the liver.
Three ketone bodies are produced in diabetic ketoacidosis: two ketoacids (beta-hydroxybutyric acid and acetoacetic acid), and one neutral ketone (acetone). The concentration of insulin required to suppress lipolysis is only one-tenth of that required to promote glucose utilization.7 Diabetic ketoacidosis is uncommon in patients with type 2 diabetes because they typically have enough insulin to inhibit lipolysis (and therefore ketoacid formation) but not enough to promote glucose utilization.
RISK FACTORS FOR DIABETIC KETOACIDOSIS
3. Which of the following is not a risk factor for diabetic ketoacidosis in type 2 diabetes mellitus?
- Acute illness
- Age > 65
- Inadequate insulin doses
- Antipsychotic drugs
- Ethnicity
Diabetic ketoacidosis is often precipitated by an acute illness such as an infection, cerebrovascular accident, myocardial infarction, or acute pancreatitis.8–12 These acute illnesses induce stress in the body and elevate counterregulatory hormones.
Inadequate insulin doses can also lead to diabetic ketoacidosis.
Drugs that affect carbohydrate metabolism are also risk factors. These include glucocorticoids, thiazide diuretics in high doses (> 50 mg daily), sympathomimetic agents, and second-generation antipsychotic agents (also called “atypical” antipsychotics) such as clozapine (Clozaril) and olanzapine (Zyprexa), although some are worse than others.13,14
Ketosis-prone type 2 diabetes mellitus is more prevalent in African Americans and Hispanics.8,15,16
Age is not a risk factor for developing diabetic ketoacidosis. In fact, diabetic ketoacidosis is the leading cause of morbidity and death in children with type 1 diabetes and can also occur in children with type 2 diabetes, particularly in obese African American adolescents.2
DISTINGUISHING TYPE 1 FROM TYPE 2
4. Which of the following is most specific in distinguishing type 1 from type 2 diabetes mellitus?
- C-peptide levels
- Islet cell antibodies
- Body mass index
- Family history
- Hemoglobin A1c level
Type 1 diabetes is characterized by destruction of pancreatic beta cells, leading to absolute insulin deficiency. The process is usually mediated by autoimmunity; therefore, testing for antibodies to islet cells, glutamic acid decarboxylase, insulin, and tyrosine phosphatase is the most specific way to distinguish type 1 from type 2 diabetes mellitus.
The hemoglobin A1c level correlates with the mean blood glucose level over the previous 8 to 12 weeks. The hemoglobin A1c is typically elevated in both type 1 and type 2 diabetes mellitus and therefore is not a useful distinguishing feature.
C-peptide is made when proinsulin is cleaved into insulin and C-peptide. It is released from endocytic vesicles with insulin in a one-to-one molar ratio. Thus, the level of C-peptide in the blood can show how much insulin is being made by the pancreas. C-peptide levels can help distinguish between type 1 and type 2 diabetes mellitus later in the course of the disease (levels are usually lower in a patient with type 1 diabetes), but they are not as useful early on because they can be normal early in the course of type 1 diabetes.17
A family history of diabetes is more common in type 2 diabetes, but patients with either type 1 or type 2 can have an affected close relative.
Patients with type 2 diabetes are generally overweight, with a body mass index greater than the 85th percentile for their age and sex. In contrast, patients with type 1 diabetes are usually not overweight and often have a recent history of weight loss. There are exceptions, however, and some patients with type 1 diabetes have an elevated body mass index, while some patients with type 2 diabetes are thin.
Although individually, C-peptide, family history, and body mass index are not very specific in distinguishing type 1 from type 2 diabetes mellitus, together they often give the clinician a good idea of the type of diabetes the patient has. In our case, although islet cell antibodies were not drawn, the normal C-peptide level, high body mass index, and family history all support a diagnosis of type 2 diabetes mellitus.
THE PATIENT CONTINUES TO DO WELL
The patient is discharged from the hospital on an insulin regimen. His blood sugar levels are closely monitored and remain near normal. Six months after the episode of diabetic ketoacidosis, his insulin is discontinued.
TAKE-HOME POINTS
Diabetic ketoacidosis is not unique to type 1 diabetes mellitus. It can occur in type 2, more commonly in patients who are nonwhite and who have precipitating factors such as acute illness, inadequate insulin treatment, or newly diagnosed diabetes. Clinicians should be aware of the possibility of diabetic ketoacidosis even in patients with type 2 diabetes who may not have these risk factors.
One approach to recognizing diabetic ketoacidosis better in patients with type 2 diabetes mellitus would include checking urine for ketones and serum electrolytes for high anion gap acidosis when patients with type 2 diabetes present with uncontrolled blood sugar levels. If ketonuria or acidosis is present, serum ketone and beta-hydroxybutyrate levels should be obtained to evaluate for diabetic ketoacidosis.
Patients should take insulin for an indeterminate period of time after initial treatment of diabetic ketoacidosis. As our case illustrates, in many cases, beta-cell function will return sufficiently to allow insulin to be discontinued. There are no clear guidelines for how long to continue insulin, but most practitioners continue it for weeks to months and discontinue it when glucose levels are stable and remain so with tapering doses. Sometimes oral agents need to be added as insulin is tapered.
Insulin therapy is tailored to the individual patient on the basis of blood glucose values. There are no data on which type of insulin is the most effective, and there are no data on whether these patients are at greater risk of hypoglycemia than other patients taking insulin. In general, there is no evidence that “prophylactic” insulin (ie, giving insulin to prevent diabetic ketoacidosis during times of illness or stress) is required. However, blood glucose monitoring is appropriate during infection or stress, and if hyperglycemia occurs in these situations, insulin use is prudent to reduce the risks of recurrent diabetic ketoacidosis.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes 1995; 44:790–795.
- Valabhji J, Watson M, Cox J, Poulter C, Elwig C, Elkeles RS. Type 2 diabetes presenting as diabetic ketacidosis in adolescence. Diabet Med 2003; 20:416–417.
- Westphal SA. The occurrence of diabetic ketoacidosis in non-insulin-dependent diabetes and newly diagnosed diabetic adults. Am J Med 1996; 101:19–24.
- Welch B, Zib I. Case study: diabetic ketoacidosis in type 2 diabetes: “look under the sheets.” Clin Diabetes 2004; 22:198–200.
- Linfoot P, Bergstrom C, Ipp E. Pathophysiology of ketoacidosis in type 2 diabetes mellitus. Diabet Med 2005; 22:1414–1419.
- Foster DW, McGarry JD. The regulation of ketogenesis. Ciba Found Symp 1982; 87:120–131.
- Zierler KL, Rabinowitz D. Effect of very small concentrations of insulin on forearm metabolism: persistence of its action on potassium and free fatty acids without its effect on glucose. J Clin Invest 1964; 43:950–962.
- Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med 2004; 164:1925–1931.
- Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med 1997; 157:669–675.
- Jabbour SA, Miller JL. Uncontrolled diabetes mellitus. Clin Lab Med 2001; 21:99–110.
- Ennis ED, Kreisberg RA. Diabetic ketoacidosis and the hyperglycemic hyperosmolar syndrome. In: Leroith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus. Lippincott-Raven Publishers; Philadelphia, 1996:276–286.
- Case CC, Maldonado M. Diabetic ketoacidosis associated with Metabolife: a report of two cases. Diabetes Obes Metab 2002; 4:402–406.
- Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. In:DeFronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International Textbook of Diabetes Mellitus, 3rd ed. John Wiley and Sons, Ltd: Chichester, UK, 2004:1101–1119.
- Newcomer JW. Second generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19( suppl 1):1–93.
- Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis: type 1 vs. type 2 diabetes and the effect of ethnicity. Arch Intern Med 1999; 159:2317–2322.
- Davis SN, Umpierrez GE. Diabetic ketoacidosis in type 2 diabetes mellitus—pathophsyiology and clinical presentation. Nat Clin Pract Endocrinol Metab 2007; 3:730–731.
- Hoogwerf B, Rich S, Barbosa J. Meal-stimulated Cpeptide and insulin antibodies in type I diabetic subjects and their nondiabetic siblings characterized by HLA-DR antigens. Diabetes 1985; 34:440–445.
A 48-year-old white man who has had diabetes mellitus for 6 years presents to the outpatient clinic because his blood sugar levels have been rising for the past week.
Both his parents had diabetes, and at the time of his diagnosis he weighed 278 pounds, all of which supported a diagnosis of type 2 diabetes mellitus. His disease was initially managed with diet, exercise, and metformin (Glucophage). Four months later, with weight loss and exercise, his blood sugar levels were consistently under 100 mg/dL, and metformin was discontinued.
He did well until 1 week ago, when he noted polyuria, polydipsia, and rising fingerstick glucose values, higher than 200 mg/dL. He has been eating well, with no nausea, vomiting, or symptoms of dehydration. He denies having any fever, chills, cough, nasal congestion, chest pain, abdominal pain, or dysuria.
In addition to his type 2 diabetes, he has hypertension, for which he takes losartan (Cozaar); hyperlipidemia, for which he takes atorvastatin (Lipitor); and gout, for which he takes allopurinol (Zyloprim).
His blood pressure is 148/70 mm Hg, pulse 100, and weight 273 pounds, and he is afebrile. On examination, his skin, head, eyes, ears, nose, throat, lungs, heart, and abdomen are normal. Urinalysis in the clinic shows large amounts of glucose and ketones.
WHAT IS THE LEAST LIKELY CAUSE OF HIS POOR CONTROL?
1. Which of the following is the least likely cause of his poorly controlled diabetes?
- Occult infection
- Poor adherence to diet and exercise
- Diabetic ketoacidosis
- Pancreatitis
Until 1 week ago, this patient’s diabetes had been well controlled for several years. Pancreatitis is the least likely cause of his uncontrolled diabetes, because he has no history of pancreatitis and has none of the symptoms of acute pancreatitis (fever, vomiting, or severe midepigastric pain radiating into the back).
Poor adherence to medication and lifestyle issues are very common in patients with poorly controlled diabetes and should always be included in the differential diagnosis.
Occult infection should also be considered in a patient with uncontrolled diabetes. Although this patient had no symptoms or signs of infection, urinalysis was done to look for an occult urinary tract infection and, surprisingly, it showed a large amount of ketones.
Case continued: He is treated for diabetic ketoacidosis
Diabetic ketoacidosis in ‘atypical diabetes’
Diabetic ketoacidosis is one of the most serious complications of diabetes. Many patients present with nausea, vomiting, and abdominal pain. Dehydration is often present because hyperglycemia leads to glucosuria and volume depletion. Interestingly, our patient showed none of these symptoms or signs.
Diabetic ketoacidosis is increasingly being recognized as a complication in patients with type 2 diabetes mellitus.1–4 Since the mid-1990s, clinicians have become increasingly aware of a condition variably termed “atypical diabetes,” “Flatbush diabetes,” “diabetes type 1B,” and “ketosis-prone type 2 diabetes mellitus,” in which patients, usually obese, present with diabetic ketoacidosis as their first manifestation, but are subsequently found to have type 2 diabetes mellitus. These patients typically are African American or of African, Hispanic, or Caribbean descent.
Ketoacidosis results from transient suppression of beta-cell function, the cause of which is unknown. A recent study comparing patients who have type 2 diabetes mellitus with and without diabetic ketoacidosis presenting with decompensated diabetes suggested insulinopenia was the predominant mechanism.5 For many of these patients, insulinopenia is transient: as the ketoacidosis resolves, betacell function improves and, with adequate insulin, lipolysis is reduced.
WHAT CAUSES DIABETIC KETOACIDOSIS?
2. Which of the following hormonal changes underlies the development of diabetic ketoacidosis?
- Insulin resistance
- Insulin deficiency
- Glucagon excess
- Glucagon deficiency
- Insulin deficiency and glucagon excess
- Insulin deficiency and glucagon deficiency
Diabetic ketoacidosis can occur when there is too much glucagon and not enough insulin. Insulin lowers the serum glucose level by promoting glucose uptake in peripheral tissues and by inhibiting gluconeogenesis and glycogenolysis in the liver. Insulin is also anabolic: it inhibits lipolysis in adipocytes and thus decreases the amount of substrate for ketogenesis.
Glucagon is the primary counterregulatory hormone responsible for ketogenesis.6 In the presence of glucagon excess, malonyl CoA production decreases, causing unblocking of carnitine acyltransferase I (CAT I) and allowing beta-oxidation to occur.6
Therefore, the sequence initiating ketogenesis begins with a shift in the ratio of glucagon to insulin, so that there is a relative or absolute excess of glucagon and a deficiency of insulin. A deficiency of insulin accelerates lipolysis, providing more substrate for ketogenesis, while excess glucagon turns on the oxidative sequence for fatty acids in the liver.
Three ketone bodies are produced in diabetic ketoacidosis: two ketoacids (beta-hydroxybutyric acid and acetoacetic acid), and one neutral ketone (acetone). The concentration of insulin required to suppress lipolysis is only one-tenth of that required to promote glucose utilization.7 Diabetic ketoacidosis is uncommon in patients with type 2 diabetes because they typically have enough insulin to inhibit lipolysis (and therefore ketoacid formation) but not enough to promote glucose utilization.
RISK FACTORS FOR DIABETIC KETOACIDOSIS
3. Which of the following is not a risk factor for diabetic ketoacidosis in type 2 diabetes mellitus?
- Acute illness
- Age > 65
- Inadequate insulin doses
- Antipsychotic drugs
- Ethnicity
Diabetic ketoacidosis is often precipitated by an acute illness such as an infection, cerebrovascular accident, myocardial infarction, or acute pancreatitis.8–12 These acute illnesses induce stress in the body and elevate counterregulatory hormones.
Inadequate insulin doses can also lead to diabetic ketoacidosis.
Drugs that affect carbohydrate metabolism are also risk factors. These include glucocorticoids, thiazide diuretics in high doses (> 50 mg daily), sympathomimetic agents, and second-generation antipsychotic agents (also called “atypical” antipsychotics) such as clozapine (Clozaril) and olanzapine (Zyprexa), although some are worse than others.13,14
Ketosis-prone type 2 diabetes mellitus is more prevalent in African Americans and Hispanics.8,15,16
Age is not a risk factor for developing diabetic ketoacidosis. In fact, diabetic ketoacidosis is the leading cause of morbidity and death in children with type 1 diabetes and can also occur in children with type 2 diabetes, particularly in obese African American adolescents.2
DISTINGUISHING TYPE 1 FROM TYPE 2
4. Which of the following is most specific in distinguishing type 1 from type 2 diabetes mellitus?
- C-peptide levels
- Islet cell antibodies
- Body mass index
- Family history
- Hemoglobin A1c level
Type 1 diabetes is characterized by destruction of pancreatic beta cells, leading to absolute insulin deficiency. The process is usually mediated by autoimmunity; therefore, testing for antibodies to islet cells, glutamic acid decarboxylase, insulin, and tyrosine phosphatase is the most specific way to distinguish type 1 from type 2 diabetes mellitus.
The hemoglobin A1c level correlates with the mean blood glucose level over the previous 8 to 12 weeks. The hemoglobin A1c is typically elevated in both type 1 and type 2 diabetes mellitus and therefore is not a useful distinguishing feature.
C-peptide is made when proinsulin is cleaved into insulin and C-peptide. It is released from endocytic vesicles with insulin in a one-to-one molar ratio. Thus, the level of C-peptide in the blood can show how much insulin is being made by the pancreas. C-peptide levels can help distinguish between type 1 and type 2 diabetes mellitus later in the course of the disease (levels are usually lower in a patient with type 1 diabetes), but they are not as useful early on because they can be normal early in the course of type 1 diabetes.17
A family history of diabetes is more common in type 2 diabetes, but patients with either type 1 or type 2 can have an affected close relative.
Patients with type 2 diabetes are generally overweight, with a body mass index greater than the 85th percentile for their age and sex. In contrast, patients with type 1 diabetes are usually not overweight and often have a recent history of weight loss. There are exceptions, however, and some patients with type 1 diabetes have an elevated body mass index, while some patients with type 2 diabetes are thin.
Although individually, C-peptide, family history, and body mass index are not very specific in distinguishing type 1 from type 2 diabetes mellitus, together they often give the clinician a good idea of the type of diabetes the patient has. In our case, although islet cell antibodies were not drawn, the normal C-peptide level, high body mass index, and family history all support a diagnosis of type 2 diabetes mellitus.
THE PATIENT CONTINUES TO DO WELL
The patient is discharged from the hospital on an insulin regimen. His blood sugar levels are closely monitored and remain near normal. Six months after the episode of diabetic ketoacidosis, his insulin is discontinued.
TAKE-HOME POINTS
Diabetic ketoacidosis is not unique to type 1 diabetes mellitus. It can occur in type 2, more commonly in patients who are nonwhite and who have precipitating factors such as acute illness, inadequate insulin treatment, or newly diagnosed diabetes. Clinicians should be aware of the possibility of diabetic ketoacidosis even in patients with type 2 diabetes who may not have these risk factors.
One approach to recognizing diabetic ketoacidosis better in patients with type 2 diabetes mellitus would include checking urine for ketones and serum electrolytes for high anion gap acidosis when patients with type 2 diabetes present with uncontrolled blood sugar levels. If ketonuria or acidosis is present, serum ketone and beta-hydroxybutyrate levels should be obtained to evaluate for diabetic ketoacidosis.
Patients should take insulin for an indeterminate period of time after initial treatment of diabetic ketoacidosis. As our case illustrates, in many cases, beta-cell function will return sufficiently to allow insulin to be discontinued. There are no clear guidelines for how long to continue insulin, but most practitioners continue it for weeks to months and discontinue it when glucose levels are stable and remain so with tapering doses. Sometimes oral agents need to be added as insulin is tapered.
Insulin therapy is tailored to the individual patient on the basis of blood glucose values. There are no data on which type of insulin is the most effective, and there are no data on whether these patients are at greater risk of hypoglycemia than other patients taking insulin. In general, there is no evidence that “prophylactic” insulin (ie, giving insulin to prevent diabetic ketoacidosis during times of illness or stress) is required. However, blood glucose monitoring is appropriate during infection or stress, and if hyperglycemia occurs in these situations, insulin use is prudent to reduce the risks of recurrent diabetic ketoacidosis.
A 48-year-old white man who has had diabetes mellitus for 6 years presents to the outpatient clinic because his blood sugar levels have been rising for the past week.
Both his parents had diabetes, and at the time of his diagnosis he weighed 278 pounds, all of which supported a diagnosis of type 2 diabetes mellitus. His disease was initially managed with diet, exercise, and metformin (Glucophage). Four months later, with weight loss and exercise, his blood sugar levels were consistently under 100 mg/dL, and metformin was discontinued.
He did well until 1 week ago, when he noted polyuria, polydipsia, and rising fingerstick glucose values, higher than 200 mg/dL. He has been eating well, with no nausea, vomiting, or symptoms of dehydration. He denies having any fever, chills, cough, nasal congestion, chest pain, abdominal pain, or dysuria.
In addition to his type 2 diabetes, he has hypertension, for which he takes losartan (Cozaar); hyperlipidemia, for which he takes atorvastatin (Lipitor); and gout, for which he takes allopurinol (Zyloprim).
His blood pressure is 148/70 mm Hg, pulse 100, and weight 273 pounds, and he is afebrile. On examination, his skin, head, eyes, ears, nose, throat, lungs, heart, and abdomen are normal. Urinalysis in the clinic shows large amounts of glucose and ketones.
WHAT IS THE LEAST LIKELY CAUSE OF HIS POOR CONTROL?
1. Which of the following is the least likely cause of his poorly controlled diabetes?
- Occult infection
- Poor adherence to diet and exercise
- Diabetic ketoacidosis
- Pancreatitis
Until 1 week ago, this patient’s diabetes had been well controlled for several years. Pancreatitis is the least likely cause of his uncontrolled diabetes, because he has no history of pancreatitis and has none of the symptoms of acute pancreatitis (fever, vomiting, or severe midepigastric pain radiating into the back).
Poor adherence to medication and lifestyle issues are very common in patients with poorly controlled diabetes and should always be included in the differential diagnosis.
Occult infection should also be considered in a patient with uncontrolled diabetes. Although this patient had no symptoms or signs of infection, urinalysis was done to look for an occult urinary tract infection and, surprisingly, it showed a large amount of ketones.
Case continued: He is treated for diabetic ketoacidosis
Diabetic ketoacidosis in ‘atypical diabetes’
Diabetic ketoacidosis is one of the most serious complications of diabetes. Many patients present with nausea, vomiting, and abdominal pain. Dehydration is often present because hyperglycemia leads to glucosuria and volume depletion. Interestingly, our patient showed none of these symptoms or signs.
Diabetic ketoacidosis is increasingly being recognized as a complication in patients with type 2 diabetes mellitus.1–4 Since the mid-1990s, clinicians have become increasingly aware of a condition variably termed “atypical diabetes,” “Flatbush diabetes,” “diabetes type 1B,” and “ketosis-prone type 2 diabetes mellitus,” in which patients, usually obese, present with diabetic ketoacidosis as their first manifestation, but are subsequently found to have type 2 diabetes mellitus. These patients typically are African American or of African, Hispanic, or Caribbean descent.
Ketoacidosis results from transient suppression of beta-cell function, the cause of which is unknown. A recent study comparing patients who have type 2 diabetes mellitus with and without diabetic ketoacidosis presenting with decompensated diabetes suggested insulinopenia was the predominant mechanism.5 For many of these patients, insulinopenia is transient: as the ketoacidosis resolves, betacell function improves and, with adequate insulin, lipolysis is reduced.
WHAT CAUSES DIABETIC KETOACIDOSIS?
2. Which of the following hormonal changes underlies the development of diabetic ketoacidosis?
- Insulin resistance
- Insulin deficiency
- Glucagon excess
- Glucagon deficiency
- Insulin deficiency and glucagon excess
- Insulin deficiency and glucagon deficiency
Diabetic ketoacidosis can occur when there is too much glucagon and not enough insulin. Insulin lowers the serum glucose level by promoting glucose uptake in peripheral tissues and by inhibiting gluconeogenesis and glycogenolysis in the liver. Insulin is also anabolic: it inhibits lipolysis in adipocytes and thus decreases the amount of substrate for ketogenesis.
Glucagon is the primary counterregulatory hormone responsible for ketogenesis.6 In the presence of glucagon excess, malonyl CoA production decreases, causing unblocking of carnitine acyltransferase I (CAT I) and allowing beta-oxidation to occur.6
Therefore, the sequence initiating ketogenesis begins with a shift in the ratio of glucagon to insulin, so that there is a relative or absolute excess of glucagon and a deficiency of insulin. A deficiency of insulin accelerates lipolysis, providing more substrate for ketogenesis, while excess glucagon turns on the oxidative sequence for fatty acids in the liver.
Three ketone bodies are produced in diabetic ketoacidosis: two ketoacids (beta-hydroxybutyric acid and acetoacetic acid), and one neutral ketone (acetone). The concentration of insulin required to suppress lipolysis is only one-tenth of that required to promote glucose utilization.7 Diabetic ketoacidosis is uncommon in patients with type 2 diabetes because they typically have enough insulin to inhibit lipolysis (and therefore ketoacid formation) but not enough to promote glucose utilization.
RISK FACTORS FOR DIABETIC KETOACIDOSIS
3. Which of the following is not a risk factor for diabetic ketoacidosis in type 2 diabetes mellitus?
- Acute illness
- Age > 65
- Inadequate insulin doses
- Antipsychotic drugs
- Ethnicity
Diabetic ketoacidosis is often precipitated by an acute illness such as an infection, cerebrovascular accident, myocardial infarction, or acute pancreatitis.8–12 These acute illnesses induce stress in the body and elevate counterregulatory hormones.
Inadequate insulin doses can also lead to diabetic ketoacidosis.
Drugs that affect carbohydrate metabolism are also risk factors. These include glucocorticoids, thiazide diuretics in high doses (> 50 mg daily), sympathomimetic agents, and second-generation antipsychotic agents (also called “atypical” antipsychotics) such as clozapine (Clozaril) and olanzapine (Zyprexa), although some are worse than others.13,14
Ketosis-prone type 2 diabetes mellitus is more prevalent in African Americans and Hispanics.8,15,16
Age is not a risk factor for developing diabetic ketoacidosis. In fact, diabetic ketoacidosis is the leading cause of morbidity and death in children with type 1 diabetes and can also occur in children with type 2 diabetes, particularly in obese African American adolescents.2
DISTINGUISHING TYPE 1 FROM TYPE 2
4. Which of the following is most specific in distinguishing type 1 from type 2 diabetes mellitus?
- C-peptide levels
- Islet cell antibodies
- Body mass index
- Family history
- Hemoglobin A1c level
Type 1 diabetes is characterized by destruction of pancreatic beta cells, leading to absolute insulin deficiency. The process is usually mediated by autoimmunity; therefore, testing for antibodies to islet cells, glutamic acid decarboxylase, insulin, and tyrosine phosphatase is the most specific way to distinguish type 1 from type 2 diabetes mellitus.
The hemoglobin A1c level correlates with the mean blood glucose level over the previous 8 to 12 weeks. The hemoglobin A1c is typically elevated in both type 1 and type 2 diabetes mellitus and therefore is not a useful distinguishing feature.
C-peptide is made when proinsulin is cleaved into insulin and C-peptide. It is released from endocytic vesicles with insulin in a one-to-one molar ratio. Thus, the level of C-peptide in the blood can show how much insulin is being made by the pancreas. C-peptide levels can help distinguish between type 1 and type 2 diabetes mellitus later in the course of the disease (levels are usually lower in a patient with type 1 diabetes), but they are not as useful early on because they can be normal early in the course of type 1 diabetes.17
A family history of diabetes is more common in type 2 diabetes, but patients with either type 1 or type 2 can have an affected close relative.
Patients with type 2 diabetes are generally overweight, with a body mass index greater than the 85th percentile for their age and sex. In contrast, patients with type 1 diabetes are usually not overweight and often have a recent history of weight loss. There are exceptions, however, and some patients with type 1 diabetes have an elevated body mass index, while some patients with type 2 diabetes are thin.
Although individually, C-peptide, family history, and body mass index are not very specific in distinguishing type 1 from type 2 diabetes mellitus, together they often give the clinician a good idea of the type of diabetes the patient has. In our case, although islet cell antibodies were not drawn, the normal C-peptide level, high body mass index, and family history all support a diagnosis of type 2 diabetes mellitus.
THE PATIENT CONTINUES TO DO WELL
The patient is discharged from the hospital on an insulin regimen. His blood sugar levels are closely monitored and remain near normal. Six months after the episode of diabetic ketoacidosis, his insulin is discontinued.
TAKE-HOME POINTS
Diabetic ketoacidosis is not unique to type 1 diabetes mellitus. It can occur in type 2, more commonly in patients who are nonwhite and who have precipitating factors such as acute illness, inadequate insulin treatment, or newly diagnosed diabetes. Clinicians should be aware of the possibility of diabetic ketoacidosis even in patients with type 2 diabetes who may not have these risk factors.
One approach to recognizing diabetic ketoacidosis better in patients with type 2 diabetes mellitus would include checking urine for ketones and serum electrolytes for high anion gap acidosis when patients with type 2 diabetes present with uncontrolled blood sugar levels. If ketonuria or acidosis is present, serum ketone and beta-hydroxybutyrate levels should be obtained to evaluate for diabetic ketoacidosis.
Patients should take insulin for an indeterminate period of time after initial treatment of diabetic ketoacidosis. As our case illustrates, in many cases, beta-cell function will return sufficiently to allow insulin to be discontinued. There are no clear guidelines for how long to continue insulin, but most practitioners continue it for weeks to months and discontinue it when glucose levels are stable and remain so with tapering doses. Sometimes oral agents need to be added as insulin is tapered.
Insulin therapy is tailored to the individual patient on the basis of blood glucose values. There are no data on which type of insulin is the most effective, and there are no data on whether these patients are at greater risk of hypoglycemia than other patients taking insulin. In general, there is no evidence that “prophylactic” insulin (ie, giving insulin to prevent diabetic ketoacidosis during times of illness or stress) is required. However, blood glucose monitoring is appropriate during infection or stress, and if hyperglycemia occurs in these situations, insulin use is prudent to reduce the risks of recurrent diabetic ketoacidosis.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes 1995; 44:790–795.
- Valabhji J, Watson M, Cox J, Poulter C, Elwig C, Elkeles RS. Type 2 diabetes presenting as diabetic ketacidosis in adolescence. Diabet Med 2003; 20:416–417.
- Westphal SA. The occurrence of diabetic ketoacidosis in non-insulin-dependent diabetes and newly diagnosed diabetic adults. Am J Med 1996; 101:19–24.
- Welch B, Zib I. Case study: diabetic ketoacidosis in type 2 diabetes: “look under the sheets.” Clin Diabetes 2004; 22:198–200.
- Linfoot P, Bergstrom C, Ipp E. Pathophysiology of ketoacidosis in type 2 diabetes mellitus. Diabet Med 2005; 22:1414–1419.
- Foster DW, McGarry JD. The regulation of ketogenesis. Ciba Found Symp 1982; 87:120–131.
- Zierler KL, Rabinowitz D. Effect of very small concentrations of insulin on forearm metabolism: persistence of its action on potassium and free fatty acids without its effect on glucose. J Clin Invest 1964; 43:950–962.
- Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med 2004; 164:1925–1931.
- Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med 1997; 157:669–675.
- Jabbour SA, Miller JL. Uncontrolled diabetes mellitus. Clin Lab Med 2001; 21:99–110.
- Ennis ED, Kreisberg RA. Diabetic ketoacidosis and the hyperglycemic hyperosmolar syndrome. In: Leroith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus. Lippincott-Raven Publishers; Philadelphia, 1996:276–286.
- Case CC, Maldonado M. Diabetic ketoacidosis associated with Metabolife: a report of two cases. Diabetes Obes Metab 2002; 4:402–406.
- Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. In:DeFronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International Textbook of Diabetes Mellitus, 3rd ed. John Wiley and Sons, Ltd: Chichester, UK, 2004:1101–1119.
- Newcomer JW. Second generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19( suppl 1):1–93.
- Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis: type 1 vs. type 2 diabetes and the effect of ethnicity. Arch Intern Med 1999; 159:2317–2322.
- Davis SN, Umpierrez GE. Diabetic ketoacidosis in type 2 diabetes mellitus—pathophsyiology and clinical presentation. Nat Clin Pract Endocrinol Metab 2007; 3:730–731.
- Hoogwerf B, Rich S, Barbosa J. Meal-stimulated Cpeptide and insulin antibodies in type I diabetic subjects and their nondiabetic siblings characterized by HLA-DR antigens. Diabetes 1985; 34:440–445.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes 1995; 44:790–795.
- Valabhji J, Watson M, Cox J, Poulter C, Elwig C, Elkeles RS. Type 2 diabetes presenting as diabetic ketacidosis in adolescence. Diabet Med 2003; 20:416–417.
- Westphal SA. The occurrence of diabetic ketoacidosis in non-insulin-dependent diabetes and newly diagnosed diabetic adults. Am J Med 1996; 101:19–24.
- Welch B, Zib I. Case study: diabetic ketoacidosis in type 2 diabetes: “look under the sheets.” Clin Diabetes 2004; 22:198–200.
- Linfoot P, Bergstrom C, Ipp E. Pathophysiology of ketoacidosis in type 2 diabetes mellitus. Diabet Med 2005; 22:1414–1419.
- Foster DW, McGarry JD. The regulation of ketogenesis. Ciba Found Symp 1982; 87:120–131.
- Zierler KL, Rabinowitz D. Effect of very small concentrations of insulin on forearm metabolism: persistence of its action on potassium and free fatty acids without its effect on glucose. J Clin Invest 1964; 43:950–962.
- Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med 2004; 164:1925–1931.
- Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med 1997; 157:669–675.
- Jabbour SA, Miller JL. Uncontrolled diabetes mellitus. Clin Lab Med 2001; 21:99–110.
- Ennis ED, Kreisberg RA. Diabetic ketoacidosis and the hyperglycemic hyperosmolar syndrome. In: Leroith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus. Lippincott-Raven Publishers; Philadelphia, 1996:276–286.
- Case CC, Maldonado M. Diabetic ketoacidosis associated with Metabolife: a report of two cases. Diabetes Obes Metab 2002; 4:402–406.
- Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. In:DeFronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International Textbook of Diabetes Mellitus, 3rd ed. John Wiley and Sons, Ltd: Chichester, UK, 2004:1101–1119.
- Newcomer JW. Second generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19( suppl 1):1–93.
- Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis: type 1 vs. type 2 diabetes and the effect of ethnicity. Arch Intern Med 1999; 159:2317–2322.
- Davis SN, Umpierrez GE. Diabetic ketoacidosis in type 2 diabetes mellitus—pathophsyiology and clinical presentation. Nat Clin Pract Endocrinol Metab 2007; 3:730–731.
- Hoogwerf B, Rich S, Barbosa J. Meal-stimulated Cpeptide and insulin antibodies in type I diabetic subjects and their nondiabetic siblings characterized by HLA-DR antigens. Diabetes 1985; 34:440–445.
Painful eye with a facial rash
A 75-year-old man presents 4 days after painful cutaneous lesions appeared on the left side of his face, associated with severe ocular pain. Two days before the eruption, he had had an intense headache, which was diagnosed as a tension headache and was treated with oral acetaminophen (Tylenol), but with no improvement.
The remainder of his physical examination is normal. Laboratory tests, including red and white blood cell counts, hemoglobin, and basic metabolic and coagulation tests reveal no abnormalities.
Q: What is your diagnosis?
- Allergic contact dermatitis
- Herpes simplex
- Varicella
- Ramsay-Hunt syndrome
- Herpes zoster ophthalmicus and herpetic keratitis
A: Herpes zoster ophthalmicus is the correct diagnosis. It represents a reactivation of the varicella zoster virus.1
Varicella zoster virus, like others of the herpes family, has developed a complex control of virus-host interactions to ensure its survival in humans. It lies dormant in the sensory ganglia and, when reactivated, moves down the neurons and satellite cells along the sensory axons to the skin.1 The reactivation is related to diminished cell-mediated immunity, which occurs as a physiologic part of aging, which is why the elderly tend to be the most often affected. 2 The incidence of herpes zoster varies from 2.2 to 3.4 per 1,000 people per year.3 Its incidence in people over age 80 is about 10 per 1,000 people per year.3
CLINICAL PRESENTATION
Herpes zoster typically presents as a dermatome-grouped vesicular eruption over an erythematous base, accompanied or preceded by local pain. It has two main complications, postherpetic neuralgia and ocular involvement. Postherpetic neuralgia is neuropathic pain that persists or develops after the dermatomal rash has healed.4 Independent predictors of postherpetic neuralgia are older age, severe acute pain, severe rash, a shorter duration of rash before consultation, and ocular involvement.5 It occurs in 36.6% of patients over age 60, and in 47.5% over 70.6 Persistent postherpetic neuralgia has been linked to suicide in patients over 70.7
Ocular infection occurs with involvement of the ophthalmic division of the fifth cranial nerve. Before the antiviral era, it was seen in as many as 50% of patients.8 Hutchinson’s sign is skin lesions on the tip, side, or root of the nose and is an important predictor of ocular involvement.1 Lesions may include folliculopapillar conjunctivitis, episcleritis, scleritis, keratitis (dendritic, pseudodendritic, and interstitial), uveitis, and necrotizing retinitis.
DIAGNOSIS
The diagnosis of herpes zoster is usually based on clinical observation of the characteristic rash, although viral culture and molecular techniques are available when definitive diagnosis is required. When ophthalmic division is affected and Hutchinson’s sign, unexplained ocular redness with pain, or complaints of visual problems are present, the patient should be referred promptly to an ophthalmologist, because serious visual impairment can occur. The fluorescein dye may show no staining or the typical dendritic keratitis (Figure 2).
TREATMENT
Oral antiviral drugs have made the treatment of zoster possible when, effectively, no treatment existed before. Ideally, an antiviral should be given within 72 hours of symptom onset. Starting treatment as early as possible—especially within 72 hours of onset—has been shown to be effective in alleviating acute pain and in preventing or limiting the duration and severity of postherpetic neuralgia.3
Acyclovir (Zovirax) 800 mg five times a day for 7 days or one of its derivatives—eg, famciclovir (Famvir), penciclovir (Denavir), or valacyclovir (Valtrex)—has been shown to be safe and effective in the treatment of active disease, as well as in preventing or shortening the duration of postherpetic neuralgia.3 It has also been shown to reduce the rate of eye involvement from 50% to 20% or 30%.9 This is why all patients with this dermatomal involvement must be treated.
Second-generation antivirals
Valacyclovir 1,000 mg three times a day and famciclovir 500 mg three times a day seem to be as effective as acyclovir in reducing zoster-associated pain, but their efficacy in reducing eye involvement has not been studied. In clinical practice, however, these second-generation antivirals may be more effective than acyclovir because patients are more likely to comply with the treatment regimen of three rather than five daily doses.
Other considerations
In patients with kidney failure, the non-nephrotoxic antiviral brivudine is preferred, but it is not available in the United States. Therefore, one must use acyclovir or one of the other drugs, carefully adjusting the dose according to the creatinine clearance and making sure the patient is well hydrated.
The efficacy of antiviral treatment that is started more than 72 hours after the onset of skin rash has never been confirmed.
Although the additional effectiveness of acyclovir eye ointment has never been established, topical acyclovir can be considered in cases of dendritic or pseudodendritic keratitis.
- Liesegang TJ. Herpes zoster ophthalmicus: natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008; 115( suppl 2):S3–S12.
- Opstelten W, Eekhof J, Neven AK, Verheij T. Treatment of herpes zoster. Can Fam Physician 2008; 54:373–377.
- Opstelten W, Zaal MJ. Managing ophthalmic herpes zoster in primary care. BMJ 2005; 331:147–151.
- Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med 1995; 155:1605–1609.
- Opstelten W, Zuithoff NP, van Essen GA, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain 2007; 132( suppl 1):S52–S59.
- De Morgas JM, Kierland RR. The outcome of patients with herpes zoster. AMA Arch Derm 1957; 75:193–196.
- Hess TM, Lutz LJ, Nauss LA, Lamer TJ. Treatment of acute herpetic neuralgia. A case report and review of the literature. Minn Med 1990; 73:37–40.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol 1987; 71:353–358.
- Cobo LM, Foulks GN, Liesegang T, et al. Oral acyclovir in the treatment of acute herpes zoster ophthalmicus. Ophthalmology 1986; 93:763–770.
A 75-year-old man presents 4 days after painful cutaneous lesions appeared on the left side of his face, associated with severe ocular pain. Two days before the eruption, he had had an intense headache, which was diagnosed as a tension headache and was treated with oral acetaminophen (Tylenol), but with no improvement.
The remainder of his physical examination is normal. Laboratory tests, including red and white blood cell counts, hemoglobin, and basic metabolic and coagulation tests reveal no abnormalities.
Q: What is your diagnosis?
- Allergic contact dermatitis
- Herpes simplex
- Varicella
- Ramsay-Hunt syndrome
- Herpes zoster ophthalmicus and herpetic keratitis
A: Herpes zoster ophthalmicus is the correct diagnosis. It represents a reactivation of the varicella zoster virus.1
Varicella zoster virus, like others of the herpes family, has developed a complex control of virus-host interactions to ensure its survival in humans. It lies dormant in the sensory ganglia and, when reactivated, moves down the neurons and satellite cells along the sensory axons to the skin.1 The reactivation is related to diminished cell-mediated immunity, which occurs as a physiologic part of aging, which is why the elderly tend to be the most often affected. 2 The incidence of herpes zoster varies from 2.2 to 3.4 per 1,000 people per year.3 Its incidence in people over age 80 is about 10 per 1,000 people per year.3
CLINICAL PRESENTATION
Herpes zoster typically presents as a dermatome-grouped vesicular eruption over an erythematous base, accompanied or preceded by local pain. It has two main complications, postherpetic neuralgia and ocular involvement. Postherpetic neuralgia is neuropathic pain that persists or develops after the dermatomal rash has healed.4 Independent predictors of postherpetic neuralgia are older age, severe acute pain, severe rash, a shorter duration of rash before consultation, and ocular involvement.5 It occurs in 36.6% of patients over age 60, and in 47.5% over 70.6 Persistent postherpetic neuralgia has been linked to suicide in patients over 70.7
Ocular infection occurs with involvement of the ophthalmic division of the fifth cranial nerve. Before the antiviral era, it was seen in as many as 50% of patients.8 Hutchinson’s sign is skin lesions on the tip, side, or root of the nose and is an important predictor of ocular involvement.1 Lesions may include folliculopapillar conjunctivitis, episcleritis, scleritis, keratitis (dendritic, pseudodendritic, and interstitial), uveitis, and necrotizing retinitis.
DIAGNOSIS
The diagnosis of herpes zoster is usually based on clinical observation of the characteristic rash, although viral culture and molecular techniques are available when definitive diagnosis is required. When ophthalmic division is affected and Hutchinson’s sign, unexplained ocular redness with pain, or complaints of visual problems are present, the patient should be referred promptly to an ophthalmologist, because serious visual impairment can occur. The fluorescein dye may show no staining or the typical dendritic keratitis (Figure 2).
TREATMENT
Oral antiviral drugs have made the treatment of zoster possible when, effectively, no treatment existed before. Ideally, an antiviral should be given within 72 hours of symptom onset. Starting treatment as early as possible—especially within 72 hours of onset—has been shown to be effective in alleviating acute pain and in preventing or limiting the duration and severity of postherpetic neuralgia.3
Acyclovir (Zovirax) 800 mg five times a day for 7 days or one of its derivatives—eg, famciclovir (Famvir), penciclovir (Denavir), or valacyclovir (Valtrex)—has been shown to be safe and effective in the treatment of active disease, as well as in preventing or shortening the duration of postherpetic neuralgia.3 It has also been shown to reduce the rate of eye involvement from 50% to 20% or 30%.9 This is why all patients with this dermatomal involvement must be treated.
Second-generation antivirals
Valacyclovir 1,000 mg three times a day and famciclovir 500 mg three times a day seem to be as effective as acyclovir in reducing zoster-associated pain, but their efficacy in reducing eye involvement has not been studied. In clinical practice, however, these second-generation antivirals may be more effective than acyclovir because patients are more likely to comply with the treatment regimen of three rather than five daily doses.
Other considerations
In patients with kidney failure, the non-nephrotoxic antiviral brivudine is preferred, but it is not available in the United States. Therefore, one must use acyclovir or one of the other drugs, carefully adjusting the dose according to the creatinine clearance and making sure the patient is well hydrated.
The efficacy of antiviral treatment that is started more than 72 hours after the onset of skin rash has never been confirmed.
Although the additional effectiveness of acyclovir eye ointment has never been established, topical acyclovir can be considered in cases of dendritic or pseudodendritic keratitis.
A 75-year-old man presents 4 days after painful cutaneous lesions appeared on the left side of his face, associated with severe ocular pain. Two days before the eruption, he had had an intense headache, which was diagnosed as a tension headache and was treated with oral acetaminophen (Tylenol), but with no improvement.
The remainder of his physical examination is normal. Laboratory tests, including red and white blood cell counts, hemoglobin, and basic metabolic and coagulation tests reveal no abnormalities.
Q: What is your diagnosis?
- Allergic contact dermatitis
- Herpes simplex
- Varicella
- Ramsay-Hunt syndrome
- Herpes zoster ophthalmicus and herpetic keratitis
A: Herpes zoster ophthalmicus is the correct diagnosis. It represents a reactivation of the varicella zoster virus.1
Varicella zoster virus, like others of the herpes family, has developed a complex control of virus-host interactions to ensure its survival in humans. It lies dormant in the sensory ganglia and, when reactivated, moves down the neurons and satellite cells along the sensory axons to the skin.1 The reactivation is related to diminished cell-mediated immunity, which occurs as a physiologic part of aging, which is why the elderly tend to be the most often affected. 2 The incidence of herpes zoster varies from 2.2 to 3.4 per 1,000 people per year.3 Its incidence in people over age 80 is about 10 per 1,000 people per year.3
CLINICAL PRESENTATION
Herpes zoster typically presents as a dermatome-grouped vesicular eruption over an erythematous base, accompanied or preceded by local pain. It has two main complications, postherpetic neuralgia and ocular involvement. Postherpetic neuralgia is neuropathic pain that persists or develops after the dermatomal rash has healed.4 Independent predictors of postherpetic neuralgia are older age, severe acute pain, severe rash, a shorter duration of rash before consultation, and ocular involvement.5 It occurs in 36.6% of patients over age 60, and in 47.5% over 70.6 Persistent postherpetic neuralgia has been linked to suicide in patients over 70.7
Ocular infection occurs with involvement of the ophthalmic division of the fifth cranial nerve. Before the antiviral era, it was seen in as many as 50% of patients.8 Hutchinson’s sign is skin lesions on the tip, side, or root of the nose and is an important predictor of ocular involvement.1 Lesions may include folliculopapillar conjunctivitis, episcleritis, scleritis, keratitis (dendritic, pseudodendritic, and interstitial), uveitis, and necrotizing retinitis.
DIAGNOSIS
The diagnosis of herpes zoster is usually based on clinical observation of the characteristic rash, although viral culture and molecular techniques are available when definitive diagnosis is required. When ophthalmic division is affected and Hutchinson’s sign, unexplained ocular redness with pain, or complaints of visual problems are present, the patient should be referred promptly to an ophthalmologist, because serious visual impairment can occur. The fluorescein dye may show no staining or the typical dendritic keratitis (Figure 2).
TREATMENT
Oral antiviral drugs have made the treatment of zoster possible when, effectively, no treatment existed before. Ideally, an antiviral should be given within 72 hours of symptom onset. Starting treatment as early as possible—especially within 72 hours of onset—has been shown to be effective in alleviating acute pain and in preventing or limiting the duration and severity of postherpetic neuralgia.3
Acyclovir (Zovirax) 800 mg five times a day for 7 days or one of its derivatives—eg, famciclovir (Famvir), penciclovir (Denavir), or valacyclovir (Valtrex)—has been shown to be safe and effective in the treatment of active disease, as well as in preventing or shortening the duration of postherpetic neuralgia.3 It has also been shown to reduce the rate of eye involvement from 50% to 20% or 30%.9 This is why all patients with this dermatomal involvement must be treated.
Second-generation antivirals
Valacyclovir 1,000 mg three times a day and famciclovir 500 mg three times a day seem to be as effective as acyclovir in reducing zoster-associated pain, but their efficacy in reducing eye involvement has not been studied. In clinical practice, however, these second-generation antivirals may be more effective than acyclovir because patients are more likely to comply with the treatment regimen of three rather than five daily doses.
Other considerations
In patients with kidney failure, the non-nephrotoxic antiviral brivudine is preferred, but it is not available in the United States. Therefore, one must use acyclovir or one of the other drugs, carefully adjusting the dose according to the creatinine clearance and making sure the patient is well hydrated.
The efficacy of antiviral treatment that is started more than 72 hours after the onset of skin rash has never been confirmed.
Although the additional effectiveness of acyclovir eye ointment has never been established, topical acyclovir can be considered in cases of dendritic or pseudodendritic keratitis.
- Liesegang TJ. Herpes zoster ophthalmicus: natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008; 115( suppl 2):S3–S12.
- Opstelten W, Eekhof J, Neven AK, Verheij T. Treatment of herpes zoster. Can Fam Physician 2008; 54:373–377.
- Opstelten W, Zaal MJ. Managing ophthalmic herpes zoster in primary care. BMJ 2005; 331:147–151.
- Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med 1995; 155:1605–1609.
- Opstelten W, Zuithoff NP, van Essen GA, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain 2007; 132( suppl 1):S52–S59.
- De Morgas JM, Kierland RR. The outcome of patients with herpes zoster. AMA Arch Derm 1957; 75:193–196.
- Hess TM, Lutz LJ, Nauss LA, Lamer TJ. Treatment of acute herpetic neuralgia. A case report and review of the literature. Minn Med 1990; 73:37–40.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol 1987; 71:353–358.
- Cobo LM, Foulks GN, Liesegang T, et al. Oral acyclovir in the treatment of acute herpes zoster ophthalmicus. Ophthalmology 1986; 93:763–770.
- Liesegang TJ. Herpes zoster ophthalmicus: natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008; 115( suppl 2):S3–S12.
- Opstelten W, Eekhof J, Neven AK, Verheij T. Treatment of herpes zoster. Can Fam Physician 2008; 54:373–377.
- Opstelten W, Zaal MJ. Managing ophthalmic herpes zoster in primary care. BMJ 2005; 331:147–151.
- Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med 1995; 155:1605–1609.
- Opstelten W, Zuithoff NP, van Essen GA, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain 2007; 132( suppl 1):S52–S59.
- De Morgas JM, Kierland RR. The outcome of patients with herpes zoster. AMA Arch Derm 1957; 75:193–196.
- Hess TM, Lutz LJ, Nauss LA, Lamer TJ. Treatment of acute herpetic neuralgia. A case report and review of the literature. Minn Med 1990; 73:37–40.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol 1987; 71:353–358.
- Cobo LM, Foulks GN, Liesegang T, et al. Oral acyclovir in the treatment of acute herpes zoster ophthalmicus. Ophthalmology 1986; 93:763–770.
Back pain made simple: An approach based on principles and evidence
Low back pain should be understood as a remittent, intermittent predicament of life. Its cause is indeterminate, but its course is predictable. Its link to work-related injury is tenuous and confounded by psychosocial issues, including workers’ compensation. It challenges function, compromises performance, and calls for empathy and understanding.1
In this brief paper, we offer a simple approach to one of the most common human afflictions, based on principles and evidence.
WHY IS BACK PAIN IMPORTANT?
Low back pain is common and affects people of all ages. It is second only to the common cold as the most common affliction of mankind, and it is among the leading complaints bringing patients to physicians’ offices. Its lifetime prevalence exceeds 70% in most industrialized countries, with an annual incidence of 15% to 20% in the United States.
Its social and economic impact is substantial. It is the most frequent cause of disability for people under age 45. In 2005, the mean age- and sex-adjusted medical expenditure among respondents with spine problems was $6,096 vs $3,516 in those without spine problems, and it had increased by 65% (adjusted for inflation) from 1997 to 2005.2
WHAT ARE THE GOALS AND PRINCIPLES OF MANAGING LOW BACK PAIN?
The goals of management for patients with low back pain are to:
- Decrease the pain
- Restore mobility
- Hasten recovery so the patient can resume normal daily activities as soon as possible
- Prevent development of a chronic recurrent condition: low back pain is considered acute when it persists for less than 6 weeks, subacute between 6 weeks and 3 months, and chronic when it lasts longer than 3 months
- Restore and preserve physical and financial independence and comfort.
Principles of management
- Most back pain has no recognizable cause and is therefore termed “mechanical” or “musculoskeletal.”
- Underlying systemic disease is rare.
- Most episodes of back pain are unpreventable.
- Confounding psychosocial issues are often contributory, important, and relevant.
- A careful, informed history and physical examination are invaluable; diagnostic studies, however technologically sophisticated, are never a substitute.
- Defer diagnostic studies for specific indications.
- Refer patients only if they have underlying disease or progressive neurologic dysfunction, or if they do not respond to conservative management.
- Encouragement of activity is benign and perhaps salutary for back pain and is desirable for general physical and mental health; there is only scant evidence to support bed rest.3
- Few if any treatments have been proven effective for low back pain.
- Talking to the patient and explaining the issues involved are critical to successful management.4
INITIAL CONSIDERATIONS WHEN EVALUATING A PATIENT
When encountering a patient with back pain, the initial consideration is whether the symptoms are regional—ie, local, mechanical, and musculoskeletal—or if they reflect a systemic disease. It is also important to look for evidence of social or psychological distress that may amplify, prolong, or confound the pain or the patient’s perception of it.
What are the clues to a systemic process?
Does the patient have a regional low back syndrome?
Regional low back syndromes account for 90% of the causes of low back pain. They are usually mechanical in origin.
Regional back pain is due to overuse of a normal mechanical structure (muscle strain, “lumbago”) or is secondary to trauma, deformity, or degeneration of an anatomical structure (herniated nucleus pulposus, fracture, and spondyloarthropathy, including facet joint arthritis). Chronic regional back syndromes include osteoarthritis of the spine (ie, spondylosis), spinal stenosis, and facet joint arthropathy.
Characteristically, mechanical disorders are exacerbated by certain physical activities, such as lifting, and are relieved by others, such as assuming a supine position.
Does the patient have sciatica or another nerve root compression syndrome?
The obvious manifestation of nerve root irritation is usually sciatica, a sharp or burning pain radiating down the posterior or lateral aspect of the leg usually to the foot or the ankle and often associated with numbness or paresthesias. The pain is sometimes aggravated by coughing, sneezing, or the Valsalva maneuver. It is most commonly seen in lumbar disk herniation, cauda equina syndrome, and spinal stenosis.
Might the patient have spinal stenosis?
More than 20% of people over age 60 have radiographic evidence of lumbar spinal canal stenosis, even if they have no symptoms.5 For this reason, the diagnosis of spinal stenosis as a cause of low back pain must be based on the history and physical examination.
The classic history of spinal stenosis is that of neurogenic claudication (“pseudoclaudication”), which is pain that occurs in the legs after walking or prolonged standing and is relieved with sitting. It may sometimes be associated with a varying and transient neurologic deficit. Lumbar flexion increases and lumbar extension decreases the cross-sectional area of the spinal canal—hence, the relief of symptoms of spinal stenosis on stooping or bending forward. Pain is commonly perceived in the back, buttock, or thigh and is elicited by prolonged lumbar extension.
On neurologic examination, about 50% of patients with spinal stenosis have a deficit in vibratory sensibility, temperature sensitivity, or muscle strength. The nerve root involved is most commonly L5, followed by S1 and L4.
Many patients have balance disturbance (wide-based gait or Romberg sign), particularly later in the course of the disorder, with normal cerebellar signs (“pseudocerebellar” presentation).
Patients with bilateral hip osteoarthritis may present with similar symptoms of buttock or thigh pain, which can be distinguished with the above clinical examination. Rotation of the hip is painful in osteoarthritis but not in spinal stenosis. If both conditions overlap, injection of a steroid or lidocaine in the painful hip should decrease the pain associated with hip osteoarthritis.
Does the patient have evidence of neurologic compromise?
Muscle strength is tested by examining the:
- L2 nerve root (which supplies the iliopsoas muscle and is tested by hip flexion)
- L3 nerve root (quadriceps, tested by knee extension)
- L4 nerve root (tibialis anterior, assessed by evaluating ankle dorsiflexion and inversion at the subtalar joint)
- L5 nerve root (extensor hallucis longus and extensor digitorum longus, tested by asking the patient to dorsiflex the great toe, then the other toes)
- S1 nerve root (flexor hallucis longus, flexor digitorum longus, and tendoachilles, tested by asking the patient to plantar-flex the great toe, then the other toes, and then the ankle).
The patient is also asked to walk a few steps on the toes and then on the heels. Inability to toe-walk indicates S1 nerve root involvement; inability to heel-walk may indicate L4 or L5 involvement. If the patient cannot heelwalk, ask him or her to squat; inability to do so indicates L4 problems.6
Radiculopathy. Detecting and locating the cause of radiculopathy may be helpful. In L3–L4 disk herniation, there is pain and paresthesia with numbness and hypalgesia in the anteromedial thigh and the knee. In L4–L5 disk herniation, there is usually involvement of the exiting L5 nerve root, which presents as numbness or paresthesias in the anterolateral calf, great toe, first web space, and medial foot. In L5-S1 disk herniation, the S1 nerve root is involved, presenting as numbness and hypalgesia in the fifth toe, lateral aspect of the foot, sole, and posterolateral calf and thigh.
Reflexes. Exaggerated or decreased reflexes do not always indicate a neurologic abnormality, but reflex asymmetry is significant. The knee-jerk reflex is diminished in L3–L4 nerve root involvement, and the ankle-jerk reflex is diminished with S1 nerve root involvement. The Babinski sign indicates pyramidal tract involvement.
Gait. Observe the patient’s gait as he or she rises and moves to the examining table, to determine whether it is shortened, asymmetrical, or antalgic.7 Also note any foot drop, which may indicate a potentially serious problem (L5 radiculopathy).
What is an adequate examination of the back?
A good back examination can elicit important information about the cause and the extent of back pain. It includes inspection, palpation, and range of movement of the spine along with a detailed neurologic examination.
Inspect it for any deformities, scoliosis, asymmetry, paraspinal muscle spasm, unusual hair growth, listing to one side, decrease or increase in lumbar lordosis, or muscle atrophy or fasciculation.
Palpate it for paraspinal muscle spasm, warmth, and localized bone pain.
Move it. The normal ranges of motion of the lumbar spine are 15 degrees of extension, 40 degrees of flexion, 30 degrees of lateral bending, and 40 degrees of lateral rotation to each side.
Assess it. This includes estimating the tone and nutrition of the muscles, testing their strength (Table 2), examining vibratory or proprioception and pinprick sensation in each dermatome (see below), testing the Achilles and patellar reflexes, and looking for the Babinski sign and clonus. In addition, perform the straight-leg-raising and the cross-straightleg-raising tests, which are positive in most patients with lower lumbar disk herniations.
The femoral stretch test is usually positive in upper lumbar disk herniations (L2–L3, L3– L4). It is performed with the patient in the prone position, with the knee being gradually flexed from full extension. Pain radiating along the anterior aspect of the thigh indicates a positive test.
The examination of the spine must be supplemented with examination of the hip and sacroiliac joints, since back pain may be a referred symptom from any pathology affecting these joints.
When should patients be referred to a specialist?
Patients should be referred to a neurologist, neurosurgeon, orthopedist, or other specialist if they have cauda equina syndrome; severe or progressive neurologic deficits; infections, tumors, or fractures compressing the spinal cord; or, perhaps, no response to conservative therapy for 4 to 6 weeks for patients with a herniated lumbar disk or 8 to 12 weeks for those with spinal stenosis.
If there is profound motor involvement at the time of the initial evaluation, patients must be promptly given systemic corticosteroids such as methylprednisolone (Medrol) or dexamethasone (Decadron) to decrease spinal cord edema.
Are there signs of psychological distress?
Psychosocial factors can significantly affect pain and functional disability in patients who have low back pain.8,9 These are known as “yellow flags” and are better predictors of treatment outcome than physical factors. 10 Anatomically inappropriate signs may be helpful in identifying psychological distress as a result of or as an amplifier of low back symptoms.
Waddell et al11 proposed five categories of these nonorganic signs. These are:
- Inappropriate tenderness that is superficial or widespread
- Pain on simulated axial loading by pressing on the top of the head or simulated spine rotation
- Distraction signs such as inconsistent performance between straight-leg-raising in the seated position vs the supine position
- Regional disturbances in strength and sensation that do not correspond with nerve root innervation patterns
- Overreaction during the physical examination.
The occurrence of any one of the signs is of limited value, but positive findings in three of the five categories suggest psychological distress.11
Which diagnostic studies are useful, cost-effective, and supported by evidence?
Since most abnormalities found on imaging studies are nonspecific, such studies are not necessary during the initial evaluation of acute low back pain unless there are red flags that suggest a more ominous source of pain.
Routine plain lumbosacral spine radiographs with anteroposterior and lateral views may be appropriate initially if the patient has risk factors for vertebral fractures (Table 1), or if the patient does not improve after a course of conservative treatment (usually 4–6 weeks).
Magnetic resonance imaging (MRI) is the preferred test if one suspects a tumor, infection, disk pathology, or spinal stenosis.
Computed tomography (CT) shows bony details better than MRI does. Hence, it is preferred when one needs to evaluate bony details (fractures, scoliosis) and when there are contraindications to MRI, as in patients with metal implant devices and those who are claustrophobic (although now there are “open system” MRI machines, in which the feeling of claustrophobia is much less).
MRI and CT should not be ordered routinely, but only for specific indications to answer specific questions, when specific findings would indicate specific treatment.
In most cases, contrast is not needed for CT or MRI to rule out common causes of low back pain, except in cases of suspected intraspinal tumor. Patients with compromised renal function who need contrast for CT need to be hydrated before the scan to lower the risk of contrast-induced nephropathy. These patients are also at higher risk of nephrogenic fibrosing dermopathy when they receive gadolinium contrast for MRI.
Bone scans can be used to look for infections or fractures not noted on plain radiography. However, MRI provides similar or better diagnostic accuracy without radiation.
Electrodiagnostic studies may be used in patients with radiculopathy when clinical examination suggests multilevel root lesions, when symptoms do not match imaging studies, and when patients have breakaway weakness (fluctuating levels of strength in one or more muscle groups).
Other useful diagnostic and laboratory studies may include the erythrocyte sedimentation rate to screen for malignancy and infection when these are suspected, blood culture for osteomyelitis, and bone aspiration and biopsy for histopathologic diagnosis of infection, malignancy, or other lesions.
WHICH TREATMENTS ARE SUPPORTED BY ROBUST EVIDENCE?
The primary treatment of low back pain should be conservative care, reassurance, and education, allowing patients to improve on their own and helping them cope with their predicament.
Limited bed rest. While 2 or 3 days of limited bed rest may help improve symptoms in patients who have acute radiculopathy, several studies have shown that long periods of bed rest are not beneficial for acute or subacute low back pain.12 Encouraging activity modification allows patients with nonspecific back pain or radicular symptoms to remain active while avoiding activities that may aggravate pain and is shown to lead to a more rapid recovery than bed rest.13,14 The most common situations to avoid are prolonged sitting or standing.15 Low-stress aerobic activities, especially walking, are the best early activities.15
Exercise is one of the only evidence-based, effective treatments for chronic low back pain.16 The most commonly prescribed exercises are aimed at retraining the multifidus (a back muscle) and transversus abdominis (a deep abdominal muscle), supplemented with exercises for the pelvic floor and breathing control.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen (Tylenol) are the drugs of choice for pain control in acute back pain17,18 and are as effective as muscle relaxants or opioids.
Muscle relaxants and opioids offer few advantages over NSAIDs and acetaminophen, except when there is severe muscle spasm associated with the back pain or if acetaminophen or NSAIDs do not relieve the pain. Muscle relaxants and opioids are both associated with more severe adverse effects. If prescribed, they should be used for a short, clearly defined period (1 to 2 weeks).19
Epidural corticosteroids, when used for sciatica, give mild to moderate short-term improvement in leg pain and sensory deficit but no significant long-term functional benefit or reduction in the need for surgery.20
Surgery may be considered in cases of cauda equina syndrome, which is a surgical emergency; severe or progressive neurologic deficit; infections, tumors, and fractures compressing the spinal cord; mechanical instability of the back; and, perhaps, intractable pain (leg pain equal to or greater than back pain) with a positive straight-leg-raising test and no response to conservative therapy.
The term “instability” implies an abnormal motion under physiologic loads. Lumbar instability is defined as translation of more than 4 mm or 10 degrees of angular motion between flexion and extension on an upright lateral radiograph.
Although Weinstein et al21 showed that patients with spinal stenosis who underwent surgery showed significantly more improvement in all primary outcomes than did patients treated nonsurgically, many patients can be effectively treated without surgery.
WHAT SHOULD BE REMEMBERED ABOUT LOW BACK PAIN?
Low back pain is a common and costly medical condition with only a weak correlation between symptoms and pathologic changes, resulting in a lack of objective clinical findings on which a definitive diagnosis can be based.22 Most back pain has no recognizable cause and is usually regional and musculoskeletal. Back pain as a result of an underlying systemic disease is rare and needs to be excluded by a good history and physical examination. Diagnostic studies are best reserved for specific indications.
Referral to a specialist is warranted when the patient is not responding to conservative treatment, when a progressive neurologic deficit or cauda equina syndrome is noted or suspected, or when the patient has an underlying malignancy, infection, fracture, or spinal instability.
Bed rest is best avoided, and activity within the limits of pain is encouraged. NSAIDs and acetaminophen are usually the drugs of choice for controlling acute low back pain.
Ultimately, the goal for clinicians is to identify serious conditions and to prevent the back pain from becoming chronic pain by promptly identifying the various risk factors.
- Hadler NM. Low back pain. In:Koopman WJ, editor. Arthritis and Allied Conditions. 14th ed. Philadelphia, PA: Lipincott Williams and Wilkins; 2001:2026–2041.
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008; 299:656–664.
- NASS Task Force on clinical guidelines. Phase III clinical guidelines for multidisciplinary spine care specialists. Unremitting low back pain. 1st ed. Burr Ridge, IL: North American Spine Society; 2000.
- Cailliet R. Low back pain. In: Soft Tissue Pain and Disability. 3rd ed. Philadelphia, PA: FA Davis; 1996:101–170.
- Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994; 331:69–73.
- A gency for Health Care Policy and Research. Acute low back pain problems in adults: assessment and treatment. http://www.chirobase.org/07Strategy/AHCPR/ahcprclinician.html. Accessed March 2009.
- Cohen R, Chopra P, Upshur C. Primary care work-up of acute and chronic symptoms. Geriatrics 2001; 56:26–37.
- Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine 2002; 27:E109–E120.
- Carragee EJ. Clinical practice: persistent low back pain. N Engl J Med 2005; 352:1891–1898.
- Pincus T, Vlaeyen JW, Kendall NA, Von Korff MR, Kalauokalani DA, Reis S. Cognitive-behavioral therapy and psychosocial factors in low back pain: directions for the future. Spine 2002; 27:E133–E138.
- Waddell G, McCulloch JA, Kummel E, Venner RM. Nonorganic physical signs in low back pain. Spine 1980; 5:117–125.
- Hagen KB, Hilde G, Jamtvedt G, Winnem M. Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev 2004; 4:CD001254.
- Patel AT, Ogle AA. Diagnosis and management of acute low back pain. Am Fam Physician 2000; 61:1779–1790.
- Grotle M, Brox JI, Glomsrød B, Lønn JH, Vøllestad NK. Prognostic factors in first-time care seekers due to acute low back pain. Eur J Pain 2007; 11:290–298.
- Atlas SJ, Deyo RA. Evaluating and managing acute low back pain in the primary care setting. J Gen Intern Med 2001; 16:120–131.
- Maher CG. Effective physical treatment for low back pain. Orthop Clin North Am 2004; 35:57–64.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147:478–491.
- Chou R, Huffman LHAmerican Pain Society. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007; 147:505–514.
- Cherkin DC, Wheeler KJ, Barlow W, Deyo RA. Medication use for low back pain in primary care. Spine 1998; 23:607–614.
- Carette S, Leclaire R, Marcoux S, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med 1997; 336:1634–1640.
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus non-surgical therapy for lumbar spinal stenosis. N Engl J Med 2008; 358:794–810.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA 1992; 268:760–765.
Low back pain should be understood as a remittent, intermittent predicament of life. Its cause is indeterminate, but its course is predictable. Its link to work-related injury is tenuous and confounded by psychosocial issues, including workers’ compensation. It challenges function, compromises performance, and calls for empathy and understanding.1
In this brief paper, we offer a simple approach to one of the most common human afflictions, based on principles and evidence.
WHY IS BACK PAIN IMPORTANT?
Low back pain is common and affects people of all ages. It is second only to the common cold as the most common affliction of mankind, and it is among the leading complaints bringing patients to physicians’ offices. Its lifetime prevalence exceeds 70% in most industrialized countries, with an annual incidence of 15% to 20% in the United States.
Its social and economic impact is substantial. It is the most frequent cause of disability for people under age 45. In 2005, the mean age- and sex-adjusted medical expenditure among respondents with spine problems was $6,096 vs $3,516 in those without spine problems, and it had increased by 65% (adjusted for inflation) from 1997 to 2005.2
WHAT ARE THE GOALS AND PRINCIPLES OF MANAGING LOW BACK PAIN?
The goals of management for patients with low back pain are to:
- Decrease the pain
- Restore mobility
- Hasten recovery so the patient can resume normal daily activities as soon as possible
- Prevent development of a chronic recurrent condition: low back pain is considered acute when it persists for less than 6 weeks, subacute between 6 weeks and 3 months, and chronic when it lasts longer than 3 months
- Restore and preserve physical and financial independence and comfort.
Principles of management
- Most back pain has no recognizable cause and is therefore termed “mechanical” or “musculoskeletal.”
- Underlying systemic disease is rare.
- Most episodes of back pain are unpreventable.
- Confounding psychosocial issues are often contributory, important, and relevant.
- A careful, informed history and physical examination are invaluable; diagnostic studies, however technologically sophisticated, are never a substitute.
- Defer diagnostic studies for specific indications.
- Refer patients only if they have underlying disease or progressive neurologic dysfunction, or if they do not respond to conservative management.
- Encouragement of activity is benign and perhaps salutary for back pain and is desirable for general physical and mental health; there is only scant evidence to support bed rest.3
- Few if any treatments have been proven effective for low back pain.
- Talking to the patient and explaining the issues involved are critical to successful management.4
INITIAL CONSIDERATIONS WHEN EVALUATING A PATIENT
When encountering a patient with back pain, the initial consideration is whether the symptoms are regional—ie, local, mechanical, and musculoskeletal—or if they reflect a systemic disease. It is also important to look for evidence of social or psychological distress that may amplify, prolong, or confound the pain or the patient’s perception of it.
What are the clues to a systemic process?
Does the patient have a regional low back syndrome?
Regional low back syndromes account for 90% of the causes of low back pain. They are usually mechanical in origin.
Regional back pain is due to overuse of a normal mechanical structure (muscle strain, “lumbago”) or is secondary to trauma, deformity, or degeneration of an anatomical structure (herniated nucleus pulposus, fracture, and spondyloarthropathy, including facet joint arthritis). Chronic regional back syndromes include osteoarthritis of the spine (ie, spondylosis), spinal stenosis, and facet joint arthropathy.
Characteristically, mechanical disorders are exacerbated by certain physical activities, such as lifting, and are relieved by others, such as assuming a supine position.
Does the patient have sciatica or another nerve root compression syndrome?
The obvious manifestation of nerve root irritation is usually sciatica, a sharp or burning pain radiating down the posterior or lateral aspect of the leg usually to the foot or the ankle and often associated with numbness or paresthesias. The pain is sometimes aggravated by coughing, sneezing, or the Valsalva maneuver. It is most commonly seen in lumbar disk herniation, cauda equina syndrome, and spinal stenosis.
Might the patient have spinal stenosis?
More than 20% of people over age 60 have radiographic evidence of lumbar spinal canal stenosis, even if they have no symptoms.5 For this reason, the diagnosis of spinal stenosis as a cause of low back pain must be based on the history and physical examination.
The classic history of spinal stenosis is that of neurogenic claudication (“pseudoclaudication”), which is pain that occurs in the legs after walking or prolonged standing and is relieved with sitting. It may sometimes be associated with a varying and transient neurologic deficit. Lumbar flexion increases and lumbar extension decreases the cross-sectional area of the spinal canal—hence, the relief of symptoms of spinal stenosis on stooping or bending forward. Pain is commonly perceived in the back, buttock, or thigh and is elicited by prolonged lumbar extension.
On neurologic examination, about 50% of patients with spinal stenosis have a deficit in vibratory sensibility, temperature sensitivity, or muscle strength. The nerve root involved is most commonly L5, followed by S1 and L4.
Many patients have balance disturbance (wide-based gait or Romberg sign), particularly later in the course of the disorder, with normal cerebellar signs (“pseudocerebellar” presentation).
Patients with bilateral hip osteoarthritis may present with similar symptoms of buttock or thigh pain, which can be distinguished with the above clinical examination. Rotation of the hip is painful in osteoarthritis but not in spinal stenosis. If both conditions overlap, injection of a steroid or lidocaine in the painful hip should decrease the pain associated with hip osteoarthritis.
Does the patient have evidence of neurologic compromise?
Muscle strength is tested by examining the:
- L2 nerve root (which supplies the iliopsoas muscle and is tested by hip flexion)
- L3 nerve root (quadriceps, tested by knee extension)
- L4 nerve root (tibialis anterior, assessed by evaluating ankle dorsiflexion and inversion at the subtalar joint)
- L5 nerve root (extensor hallucis longus and extensor digitorum longus, tested by asking the patient to dorsiflex the great toe, then the other toes)
- S1 nerve root (flexor hallucis longus, flexor digitorum longus, and tendoachilles, tested by asking the patient to plantar-flex the great toe, then the other toes, and then the ankle).
The patient is also asked to walk a few steps on the toes and then on the heels. Inability to toe-walk indicates S1 nerve root involvement; inability to heel-walk may indicate L4 or L5 involvement. If the patient cannot heelwalk, ask him or her to squat; inability to do so indicates L4 problems.6
Radiculopathy. Detecting and locating the cause of radiculopathy may be helpful. In L3–L4 disk herniation, there is pain and paresthesia with numbness and hypalgesia in the anteromedial thigh and the knee. In L4–L5 disk herniation, there is usually involvement of the exiting L5 nerve root, which presents as numbness or paresthesias in the anterolateral calf, great toe, first web space, and medial foot. In L5-S1 disk herniation, the S1 nerve root is involved, presenting as numbness and hypalgesia in the fifth toe, lateral aspect of the foot, sole, and posterolateral calf and thigh.
Reflexes. Exaggerated or decreased reflexes do not always indicate a neurologic abnormality, but reflex asymmetry is significant. The knee-jerk reflex is diminished in L3–L4 nerve root involvement, and the ankle-jerk reflex is diminished with S1 nerve root involvement. The Babinski sign indicates pyramidal tract involvement.
Gait. Observe the patient’s gait as he or she rises and moves to the examining table, to determine whether it is shortened, asymmetrical, or antalgic.7 Also note any foot drop, which may indicate a potentially serious problem (L5 radiculopathy).
What is an adequate examination of the back?
A good back examination can elicit important information about the cause and the extent of back pain. It includes inspection, palpation, and range of movement of the spine along with a detailed neurologic examination.
Inspect it for any deformities, scoliosis, asymmetry, paraspinal muscle spasm, unusual hair growth, listing to one side, decrease or increase in lumbar lordosis, or muscle atrophy or fasciculation.
Palpate it for paraspinal muscle spasm, warmth, and localized bone pain.
Move it. The normal ranges of motion of the lumbar spine are 15 degrees of extension, 40 degrees of flexion, 30 degrees of lateral bending, and 40 degrees of lateral rotation to each side.
Assess it. This includes estimating the tone and nutrition of the muscles, testing their strength (Table 2), examining vibratory or proprioception and pinprick sensation in each dermatome (see below), testing the Achilles and patellar reflexes, and looking for the Babinski sign and clonus. In addition, perform the straight-leg-raising and the cross-straightleg-raising tests, which are positive in most patients with lower lumbar disk herniations.
The femoral stretch test is usually positive in upper lumbar disk herniations (L2–L3, L3– L4). It is performed with the patient in the prone position, with the knee being gradually flexed from full extension. Pain radiating along the anterior aspect of the thigh indicates a positive test.
The examination of the spine must be supplemented with examination of the hip and sacroiliac joints, since back pain may be a referred symptom from any pathology affecting these joints.
When should patients be referred to a specialist?
Patients should be referred to a neurologist, neurosurgeon, orthopedist, or other specialist if they have cauda equina syndrome; severe or progressive neurologic deficits; infections, tumors, or fractures compressing the spinal cord; or, perhaps, no response to conservative therapy for 4 to 6 weeks for patients with a herniated lumbar disk or 8 to 12 weeks for those with spinal stenosis.
If there is profound motor involvement at the time of the initial evaluation, patients must be promptly given systemic corticosteroids such as methylprednisolone (Medrol) or dexamethasone (Decadron) to decrease spinal cord edema.
Are there signs of psychological distress?
Psychosocial factors can significantly affect pain and functional disability in patients who have low back pain.8,9 These are known as “yellow flags” and are better predictors of treatment outcome than physical factors. 10 Anatomically inappropriate signs may be helpful in identifying psychological distress as a result of or as an amplifier of low back symptoms.
Waddell et al11 proposed five categories of these nonorganic signs. These are:
- Inappropriate tenderness that is superficial or widespread
- Pain on simulated axial loading by pressing on the top of the head or simulated spine rotation
- Distraction signs such as inconsistent performance between straight-leg-raising in the seated position vs the supine position
- Regional disturbances in strength and sensation that do not correspond with nerve root innervation patterns
- Overreaction during the physical examination.
The occurrence of any one of the signs is of limited value, but positive findings in three of the five categories suggest psychological distress.11
Which diagnostic studies are useful, cost-effective, and supported by evidence?
Since most abnormalities found on imaging studies are nonspecific, such studies are not necessary during the initial evaluation of acute low back pain unless there are red flags that suggest a more ominous source of pain.
Routine plain lumbosacral spine radiographs with anteroposterior and lateral views may be appropriate initially if the patient has risk factors for vertebral fractures (Table 1), or if the patient does not improve after a course of conservative treatment (usually 4–6 weeks).
Magnetic resonance imaging (MRI) is the preferred test if one suspects a tumor, infection, disk pathology, or spinal stenosis.
Computed tomography (CT) shows bony details better than MRI does. Hence, it is preferred when one needs to evaluate bony details (fractures, scoliosis) and when there are contraindications to MRI, as in patients with metal implant devices and those who are claustrophobic (although now there are “open system” MRI machines, in which the feeling of claustrophobia is much less).
MRI and CT should not be ordered routinely, but only for specific indications to answer specific questions, when specific findings would indicate specific treatment.
In most cases, contrast is not needed for CT or MRI to rule out common causes of low back pain, except in cases of suspected intraspinal tumor. Patients with compromised renal function who need contrast for CT need to be hydrated before the scan to lower the risk of contrast-induced nephropathy. These patients are also at higher risk of nephrogenic fibrosing dermopathy when they receive gadolinium contrast for MRI.
Bone scans can be used to look for infections or fractures not noted on plain radiography. However, MRI provides similar or better diagnostic accuracy without radiation.
Electrodiagnostic studies may be used in patients with radiculopathy when clinical examination suggests multilevel root lesions, when symptoms do not match imaging studies, and when patients have breakaway weakness (fluctuating levels of strength in one or more muscle groups).
Other useful diagnostic and laboratory studies may include the erythrocyte sedimentation rate to screen for malignancy and infection when these are suspected, blood culture for osteomyelitis, and bone aspiration and biopsy for histopathologic diagnosis of infection, malignancy, or other lesions.
WHICH TREATMENTS ARE SUPPORTED BY ROBUST EVIDENCE?
The primary treatment of low back pain should be conservative care, reassurance, and education, allowing patients to improve on their own and helping them cope with their predicament.
Limited bed rest. While 2 or 3 days of limited bed rest may help improve symptoms in patients who have acute radiculopathy, several studies have shown that long periods of bed rest are not beneficial for acute or subacute low back pain.12 Encouraging activity modification allows patients with nonspecific back pain or radicular symptoms to remain active while avoiding activities that may aggravate pain and is shown to lead to a more rapid recovery than bed rest.13,14 The most common situations to avoid are prolonged sitting or standing.15 Low-stress aerobic activities, especially walking, are the best early activities.15
Exercise is one of the only evidence-based, effective treatments for chronic low back pain.16 The most commonly prescribed exercises are aimed at retraining the multifidus (a back muscle) and transversus abdominis (a deep abdominal muscle), supplemented with exercises for the pelvic floor and breathing control.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen (Tylenol) are the drugs of choice for pain control in acute back pain17,18 and are as effective as muscle relaxants or opioids.
Muscle relaxants and opioids offer few advantages over NSAIDs and acetaminophen, except when there is severe muscle spasm associated with the back pain or if acetaminophen or NSAIDs do not relieve the pain. Muscle relaxants and opioids are both associated with more severe adverse effects. If prescribed, they should be used for a short, clearly defined period (1 to 2 weeks).19
Epidural corticosteroids, when used for sciatica, give mild to moderate short-term improvement in leg pain and sensory deficit but no significant long-term functional benefit or reduction in the need for surgery.20
Surgery may be considered in cases of cauda equina syndrome, which is a surgical emergency; severe or progressive neurologic deficit; infections, tumors, and fractures compressing the spinal cord; mechanical instability of the back; and, perhaps, intractable pain (leg pain equal to or greater than back pain) with a positive straight-leg-raising test and no response to conservative therapy.
The term “instability” implies an abnormal motion under physiologic loads. Lumbar instability is defined as translation of more than 4 mm or 10 degrees of angular motion between flexion and extension on an upright lateral radiograph.
Although Weinstein et al21 showed that patients with spinal stenosis who underwent surgery showed significantly more improvement in all primary outcomes than did patients treated nonsurgically, many patients can be effectively treated without surgery.
WHAT SHOULD BE REMEMBERED ABOUT LOW BACK PAIN?
Low back pain is a common and costly medical condition with only a weak correlation between symptoms and pathologic changes, resulting in a lack of objective clinical findings on which a definitive diagnosis can be based.22 Most back pain has no recognizable cause and is usually regional and musculoskeletal. Back pain as a result of an underlying systemic disease is rare and needs to be excluded by a good history and physical examination. Diagnostic studies are best reserved for specific indications.
Referral to a specialist is warranted when the patient is not responding to conservative treatment, when a progressive neurologic deficit or cauda equina syndrome is noted or suspected, or when the patient has an underlying malignancy, infection, fracture, or spinal instability.
Bed rest is best avoided, and activity within the limits of pain is encouraged. NSAIDs and acetaminophen are usually the drugs of choice for controlling acute low back pain.
Ultimately, the goal for clinicians is to identify serious conditions and to prevent the back pain from becoming chronic pain by promptly identifying the various risk factors.
Low back pain should be understood as a remittent, intermittent predicament of life. Its cause is indeterminate, but its course is predictable. Its link to work-related injury is tenuous and confounded by psychosocial issues, including workers’ compensation. It challenges function, compromises performance, and calls for empathy and understanding.1
In this brief paper, we offer a simple approach to one of the most common human afflictions, based on principles and evidence.
WHY IS BACK PAIN IMPORTANT?
Low back pain is common and affects people of all ages. It is second only to the common cold as the most common affliction of mankind, and it is among the leading complaints bringing patients to physicians’ offices. Its lifetime prevalence exceeds 70% in most industrialized countries, with an annual incidence of 15% to 20% in the United States.
Its social and economic impact is substantial. It is the most frequent cause of disability for people under age 45. In 2005, the mean age- and sex-adjusted medical expenditure among respondents with spine problems was $6,096 vs $3,516 in those without spine problems, and it had increased by 65% (adjusted for inflation) from 1997 to 2005.2
WHAT ARE THE GOALS AND PRINCIPLES OF MANAGING LOW BACK PAIN?
The goals of management for patients with low back pain are to:
- Decrease the pain
- Restore mobility
- Hasten recovery so the patient can resume normal daily activities as soon as possible
- Prevent development of a chronic recurrent condition: low back pain is considered acute when it persists for less than 6 weeks, subacute between 6 weeks and 3 months, and chronic when it lasts longer than 3 months
- Restore and preserve physical and financial independence and comfort.
Principles of management
- Most back pain has no recognizable cause and is therefore termed “mechanical” or “musculoskeletal.”
- Underlying systemic disease is rare.
- Most episodes of back pain are unpreventable.
- Confounding psychosocial issues are often contributory, important, and relevant.
- A careful, informed history and physical examination are invaluable; diagnostic studies, however technologically sophisticated, are never a substitute.
- Defer diagnostic studies for specific indications.
- Refer patients only if they have underlying disease or progressive neurologic dysfunction, or if they do not respond to conservative management.
- Encouragement of activity is benign and perhaps salutary for back pain and is desirable for general physical and mental health; there is only scant evidence to support bed rest.3
- Few if any treatments have been proven effective for low back pain.
- Talking to the patient and explaining the issues involved are critical to successful management.4
INITIAL CONSIDERATIONS WHEN EVALUATING A PATIENT
When encountering a patient with back pain, the initial consideration is whether the symptoms are regional—ie, local, mechanical, and musculoskeletal—or if they reflect a systemic disease. It is also important to look for evidence of social or psychological distress that may amplify, prolong, or confound the pain or the patient’s perception of it.
What are the clues to a systemic process?
Does the patient have a regional low back syndrome?
Regional low back syndromes account for 90% of the causes of low back pain. They are usually mechanical in origin.
Regional back pain is due to overuse of a normal mechanical structure (muscle strain, “lumbago”) or is secondary to trauma, deformity, or degeneration of an anatomical structure (herniated nucleus pulposus, fracture, and spondyloarthropathy, including facet joint arthritis). Chronic regional back syndromes include osteoarthritis of the spine (ie, spondylosis), spinal stenosis, and facet joint arthropathy.
Characteristically, mechanical disorders are exacerbated by certain physical activities, such as lifting, and are relieved by others, such as assuming a supine position.
Does the patient have sciatica or another nerve root compression syndrome?
The obvious manifestation of nerve root irritation is usually sciatica, a sharp or burning pain radiating down the posterior or lateral aspect of the leg usually to the foot or the ankle and often associated with numbness or paresthesias. The pain is sometimes aggravated by coughing, sneezing, or the Valsalva maneuver. It is most commonly seen in lumbar disk herniation, cauda equina syndrome, and spinal stenosis.
Might the patient have spinal stenosis?
More than 20% of people over age 60 have radiographic evidence of lumbar spinal canal stenosis, even if they have no symptoms.5 For this reason, the diagnosis of spinal stenosis as a cause of low back pain must be based on the history and physical examination.
The classic history of spinal stenosis is that of neurogenic claudication (“pseudoclaudication”), which is pain that occurs in the legs after walking or prolonged standing and is relieved with sitting. It may sometimes be associated with a varying and transient neurologic deficit. Lumbar flexion increases and lumbar extension decreases the cross-sectional area of the spinal canal—hence, the relief of symptoms of spinal stenosis on stooping or bending forward. Pain is commonly perceived in the back, buttock, or thigh and is elicited by prolonged lumbar extension.
On neurologic examination, about 50% of patients with spinal stenosis have a deficit in vibratory sensibility, temperature sensitivity, or muscle strength. The nerve root involved is most commonly L5, followed by S1 and L4.
Many patients have balance disturbance (wide-based gait or Romberg sign), particularly later in the course of the disorder, with normal cerebellar signs (“pseudocerebellar” presentation).
Patients with bilateral hip osteoarthritis may present with similar symptoms of buttock or thigh pain, which can be distinguished with the above clinical examination. Rotation of the hip is painful in osteoarthritis but not in spinal stenosis. If both conditions overlap, injection of a steroid or lidocaine in the painful hip should decrease the pain associated with hip osteoarthritis.
Does the patient have evidence of neurologic compromise?
Muscle strength is tested by examining the:
- L2 nerve root (which supplies the iliopsoas muscle and is tested by hip flexion)
- L3 nerve root (quadriceps, tested by knee extension)
- L4 nerve root (tibialis anterior, assessed by evaluating ankle dorsiflexion and inversion at the subtalar joint)
- L5 nerve root (extensor hallucis longus and extensor digitorum longus, tested by asking the patient to dorsiflex the great toe, then the other toes)
- S1 nerve root (flexor hallucis longus, flexor digitorum longus, and tendoachilles, tested by asking the patient to plantar-flex the great toe, then the other toes, and then the ankle).
The patient is also asked to walk a few steps on the toes and then on the heels. Inability to toe-walk indicates S1 nerve root involvement; inability to heel-walk may indicate L4 or L5 involvement. If the patient cannot heelwalk, ask him or her to squat; inability to do so indicates L4 problems.6
Radiculopathy. Detecting and locating the cause of radiculopathy may be helpful. In L3–L4 disk herniation, there is pain and paresthesia with numbness and hypalgesia in the anteromedial thigh and the knee. In L4–L5 disk herniation, there is usually involvement of the exiting L5 nerve root, which presents as numbness or paresthesias in the anterolateral calf, great toe, first web space, and medial foot. In L5-S1 disk herniation, the S1 nerve root is involved, presenting as numbness and hypalgesia in the fifth toe, lateral aspect of the foot, sole, and posterolateral calf and thigh.
Reflexes. Exaggerated or decreased reflexes do not always indicate a neurologic abnormality, but reflex asymmetry is significant. The knee-jerk reflex is diminished in L3–L4 nerve root involvement, and the ankle-jerk reflex is diminished with S1 nerve root involvement. The Babinski sign indicates pyramidal tract involvement.
Gait. Observe the patient’s gait as he or she rises and moves to the examining table, to determine whether it is shortened, asymmetrical, or antalgic.7 Also note any foot drop, which may indicate a potentially serious problem (L5 radiculopathy).
What is an adequate examination of the back?
A good back examination can elicit important information about the cause and the extent of back pain. It includes inspection, palpation, and range of movement of the spine along with a detailed neurologic examination.
Inspect it for any deformities, scoliosis, asymmetry, paraspinal muscle spasm, unusual hair growth, listing to one side, decrease or increase in lumbar lordosis, or muscle atrophy or fasciculation.
Palpate it for paraspinal muscle spasm, warmth, and localized bone pain.
Move it. The normal ranges of motion of the lumbar spine are 15 degrees of extension, 40 degrees of flexion, 30 degrees of lateral bending, and 40 degrees of lateral rotation to each side.
Assess it. This includes estimating the tone and nutrition of the muscles, testing their strength (Table 2), examining vibratory or proprioception and pinprick sensation in each dermatome (see below), testing the Achilles and patellar reflexes, and looking for the Babinski sign and clonus. In addition, perform the straight-leg-raising and the cross-straightleg-raising tests, which are positive in most patients with lower lumbar disk herniations.
The femoral stretch test is usually positive in upper lumbar disk herniations (L2–L3, L3– L4). It is performed with the patient in the prone position, with the knee being gradually flexed from full extension. Pain radiating along the anterior aspect of the thigh indicates a positive test.
The examination of the spine must be supplemented with examination of the hip and sacroiliac joints, since back pain may be a referred symptom from any pathology affecting these joints.
When should patients be referred to a specialist?
Patients should be referred to a neurologist, neurosurgeon, orthopedist, or other specialist if they have cauda equina syndrome; severe or progressive neurologic deficits; infections, tumors, or fractures compressing the spinal cord; or, perhaps, no response to conservative therapy for 4 to 6 weeks for patients with a herniated lumbar disk or 8 to 12 weeks for those with spinal stenosis.
If there is profound motor involvement at the time of the initial evaluation, patients must be promptly given systemic corticosteroids such as methylprednisolone (Medrol) or dexamethasone (Decadron) to decrease spinal cord edema.
Are there signs of psychological distress?
Psychosocial factors can significantly affect pain and functional disability in patients who have low back pain.8,9 These are known as “yellow flags” and are better predictors of treatment outcome than physical factors. 10 Anatomically inappropriate signs may be helpful in identifying psychological distress as a result of or as an amplifier of low back symptoms.
Waddell et al11 proposed five categories of these nonorganic signs. These are:
- Inappropriate tenderness that is superficial or widespread
- Pain on simulated axial loading by pressing on the top of the head or simulated spine rotation
- Distraction signs such as inconsistent performance between straight-leg-raising in the seated position vs the supine position
- Regional disturbances in strength and sensation that do not correspond with nerve root innervation patterns
- Overreaction during the physical examination.
The occurrence of any one of the signs is of limited value, but positive findings in three of the five categories suggest psychological distress.11
Which diagnostic studies are useful, cost-effective, and supported by evidence?
Since most abnormalities found on imaging studies are nonspecific, such studies are not necessary during the initial evaluation of acute low back pain unless there are red flags that suggest a more ominous source of pain.
Routine plain lumbosacral spine radiographs with anteroposterior and lateral views may be appropriate initially if the patient has risk factors for vertebral fractures (Table 1), or if the patient does not improve after a course of conservative treatment (usually 4–6 weeks).
Magnetic resonance imaging (MRI) is the preferred test if one suspects a tumor, infection, disk pathology, or spinal stenosis.
Computed tomography (CT) shows bony details better than MRI does. Hence, it is preferred when one needs to evaluate bony details (fractures, scoliosis) and when there are contraindications to MRI, as in patients with metal implant devices and those who are claustrophobic (although now there are “open system” MRI machines, in which the feeling of claustrophobia is much less).
MRI and CT should not be ordered routinely, but only for specific indications to answer specific questions, when specific findings would indicate specific treatment.
In most cases, contrast is not needed for CT or MRI to rule out common causes of low back pain, except in cases of suspected intraspinal tumor. Patients with compromised renal function who need contrast for CT need to be hydrated before the scan to lower the risk of contrast-induced nephropathy. These patients are also at higher risk of nephrogenic fibrosing dermopathy when they receive gadolinium contrast for MRI.
Bone scans can be used to look for infections or fractures not noted on plain radiography. However, MRI provides similar or better diagnostic accuracy without radiation.
Electrodiagnostic studies may be used in patients with radiculopathy when clinical examination suggests multilevel root lesions, when symptoms do not match imaging studies, and when patients have breakaway weakness (fluctuating levels of strength in one or more muscle groups).
Other useful diagnostic and laboratory studies may include the erythrocyte sedimentation rate to screen for malignancy and infection when these are suspected, blood culture for osteomyelitis, and bone aspiration and biopsy for histopathologic diagnosis of infection, malignancy, or other lesions.
WHICH TREATMENTS ARE SUPPORTED BY ROBUST EVIDENCE?
The primary treatment of low back pain should be conservative care, reassurance, and education, allowing patients to improve on their own and helping them cope with their predicament.
Limited bed rest. While 2 or 3 days of limited bed rest may help improve symptoms in patients who have acute radiculopathy, several studies have shown that long periods of bed rest are not beneficial for acute or subacute low back pain.12 Encouraging activity modification allows patients with nonspecific back pain or radicular symptoms to remain active while avoiding activities that may aggravate pain and is shown to lead to a more rapid recovery than bed rest.13,14 The most common situations to avoid are prolonged sitting or standing.15 Low-stress aerobic activities, especially walking, are the best early activities.15
Exercise is one of the only evidence-based, effective treatments for chronic low back pain.16 The most commonly prescribed exercises are aimed at retraining the multifidus (a back muscle) and transversus abdominis (a deep abdominal muscle), supplemented with exercises for the pelvic floor and breathing control.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen (Tylenol) are the drugs of choice for pain control in acute back pain17,18 and are as effective as muscle relaxants or opioids.
Muscle relaxants and opioids offer few advantages over NSAIDs and acetaminophen, except when there is severe muscle spasm associated with the back pain or if acetaminophen or NSAIDs do not relieve the pain. Muscle relaxants and opioids are both associated with more severe adverse effects. If prescribed, they should be used for a short, clearly defined period (1 to 2 weeks).19
Epidural corticosteroids, when used for sciatica, give mild to moderate short-term improvement in leg pain and sensory deficit but no significant long-term functional benefit or reduction in the need for surgery.20
Surgery may be considered in cases of cauda equina syndrome, which is a surgical emergency; severe or progressive neurologic deficit; infections, tumors, and fractures compressing the spinal cord; mechanical instability of the back; and, perhaps, intractable pain (leg pain equal to or greater than back pain) with a positive straight-leg-raising test and no response to conservative therapy.
The term “instability” implies an abnormal motion under physiologic loads. Lumbar instability is defined as translation of more than 4 mm or 10 degrees of angular motion between flexion and extension on an upright lateral radiograph.
Although Weinstein et al21 showed that patients with spinal stenosis who underwent surgery showed significantly more improvement in all primary outcomes than did patients treated nonsurgically, many patients can be effectively treated without surgery.
WHAT SHOULD BE REMEMBERED ABOUT LOW BACK PAIN?
Low back pain is a common and costly medical condition with only a weak correlation between symptoms and pathologic changes, resulting in a lack of objective clinical findings on which a definitive diagnosis can be based.22 Most back pain has no recognizable cause and is usually regional and musculoskeletal. Back pain as a result of an underlying systemic disease is rare and needs to be excluded by a good history and physical examination. Diagnostic studies are best reserved for specific indications.
Referral to a specialist is warranted when the patient is not responding to conservative treatment, when a progressive neurologic deficit or cauda equina syndrome is noted or suspected, or when the patient has an underlying malignancy, infection, fracture, or spinal instability.
Bed rest is best avoided, and activity within the limits of pain is encouraged. NSAIDs and acetaminophen are usually the drugs of choice for controlling acute low back pain.
Ultimately, the goal for clinicians is to identify serious conditions and to prevent the back pain from becoming chronic pain by promptly identifying the various risk factors.
- Hadler NM. Low back pain. In:Koopman WJ, editor. Arthritis and Allied Conditions. 14th ed. Philadelphia, PA: Lipincott Williams and Wilkins; 2001:2026–2041.
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008; 299:656–664.
- NASS Task Force on clinical guidelines. Phase III clinical guidelines for multidisciplinary spine care specialists. Unremitting low back pain. 1st ed. Burr Ridge, IL: North American Spine Society; 2000.
- Cailliet R. Low back pain. In: Soft Tissue Pain and Disability. 3rd ed. Philadelphia, PA: FA Davis; 1996:101–170.
- Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994; 331:69–73.
- A gency for Health Care Policy and Research. Acute low back pain problems in adults: assessment and treatment. http://www.chirobase.org/07Strategy/AHCPR/ahcprclinician.html. Accessed March 2009.
- Cohen R, Chopra P, Upshur C. Primary care work-up of acute and chronic symptoms. Geriatrics 2001; 56:26–37.
- Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine 2002; 27:E109–E120.
- Carragee EJ. Clinical practice: persistent low back pain. N Engl J Med 2005; 352:1891–1898.
- Pincus T, Vlaeyen JW, Kendall NA, Von Korff MR, Kalauokalani DA, Reis S. Cognitive-behavioral therapy and psychosocial factors in low back pain: directions for the future. Spine 2002; 27:E133–E138.
- Waddell G, McCulloch JA, Kummel E, Venner RM. Nonorganic physical signs in low back pain. Spine 1980; 5:117–125.
- Hagen KB, Hilde G, Jamtvedt G, Winnem M. Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev 2004; 4:CD001254.
- Patel AT, Ogle AA. Diagnosis and management of acute low back pain. Am Fam Physician 2000; 61:1779–1790.
- Grotle M, Brox JI, Glomsrød B, Lønn JH, Vøllestad NK. Prognostic factors in first-time care seekers due to acute low back pain. Eur J Pain 2007; 11:290–298.
- Atlas SJ, Deyo RA. Evaluating and managing acute low back pain in the primary care setting. J Gen Intern Med 2001; 16:120–131.
- Maher CG. Effective physical treatment for low back pain. Orthop Clin North Am 2004; 35:57–64.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147:478–491.
- Chou R, Huffman LHAmerican Pain Society. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007; 147:505–514.
- Cherkin DC, Wheeler KJ, Barlow W, Deyo RA. Medication use for low back pain in primary care. Spine 1998; 23:607–614.
- Carette S, Leclaire R, Marcoux S, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med 1997; 336:1634–1640.
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus non-surgical therapy for lumbar spinal stenosis. N Engl J Med 2008; 358:794–810.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA 1992; 268:760–765.
- Hadler NM. Low back pain. In:Koopman WJ, editor. Arthritis and Allied Conditions. 14th ed. Philadelphia, PA: Lipincott Williams and Wilkins; 2001:2026–2041.
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008; 299:656–664.
- NASS Task Force on clinical guidelines. Phase III clinical guidelines for multidisciplinary spine care specialists. Unremitting low back pain. 1st ed. Burr Ridge, IL: North American Spine Society; 2000.
- Cailliet R. Low back pain. In: Soft Tissue Pain and Disability. 3rd ed. Philadelphia, PA: FA Davis; 1996:101–170.
- Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994; 331:69–73.
- A gency for Health Care Policy and Research. Acute low back pain problems in adults: assessment and treatment. http://www.chirobase.org/07Strategy/AHCPR/ahcprclinician.html. Accessed March 2009.
- Cohen R, Chopra P, Upshur C. Primary care work-up of acute and chronic symptoms. Geriatrics 2001; 56:26–37.
- Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine 2002; 27:E109–E120.
- Carragee EJ. Clinical practice: persistent low back pain. N Engl J Med 2005; 352:1891–1898.
- Pincus T, Vlaeyen JW, Kendall NA, Von Korff MR, Kalauokalani DA, Reis S. Cognitive-behavioral therapy and psychosocial factors in low back pain: directions for the future. Spine 2002; 27:E133–E138.
- Waddell G, McCulloch JA, Kummel E, Venner RM. Nonorganic physical signs in low back pain. Spine 1980; 5:117–125.
- Hagen KB, Hilde G, Jamtvedt G, Winnem M. Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev 2004; 4:CD001254.
- Patel AT, Ogle AA. Diagnosis and management of acute low back pain. Am Fam Physician 2000; 61:1779–1790.
- Grotle M, Brox JI, Glomsrød B, Lønn JH, Vøllestad NK. Prognostic factors in first-time care seekers due to acute low back pain. Eur J Pain 2007; 11:290–298.
- Atlas SJ, Deyo RA. Evaluating and managing acute low back pain in the primary care setting. J Gen Intern Med 2001; 16:120–131.
- Maher CG. Effective physical treatment for low back pain. Orthop Clin North Am 2004; 35:57–64.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147:478–491.
- Chou R, Huffman LHAmerican Pain Society. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007; 147:505–514.
- Cherkin DC, Wheeler KJ, Barlow W, Deyo RA. Medication use for low back pain in primary care. Spine 1998; 23:607–614.
- Carette S, Leclaire R, Marcoux S, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med 1997; 336:1634–1640.
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus non-surgical therapy for lumbar spinal stenosis. N Engl J Med 2008; 358:794–810.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA 1992; 268:760–765.
KEY POINTS
- Most back pain has no recognizable cause and is therefore termed “mechanical” or “musculoskeletal.” Underlying systemic disease is rare.
- Most episodes of back pain are not preventable.
- Confounding psychosocial issues are common.
- A careful, informed history and physical examination are invaluable; diagnostic studies, however sophisticated, are never a substitute. Defer them for specific indications.
- Refer patients only if they have underlying disease or progressive neurologic dysfunction or do not respond to conservative management.
- Encouragement of activity is benign and perhaps salutary for back pain and is desirable for general physical and mental health. Evidence to support bed rest is scant.
- Few if any treatments have been proven effective for low back pain.
The blade, the flea, and the colon
As Elder et al point out in this issue of the Journal, the management of ischemic colitis presents an interesting clinical paradox: the internist makes the diagnosis of potentially life-threatening impending tissue necrosis, while the surgeon, consulted to act, tends to be a cheerleader for temperate observation.
Ischemic colitis may account for 1 in 1,000 hospitalizations. Many patients present with a combination of focal lower abdominal pain and some bloody diarrhea. The examiner often localizes the tender colon either by anterior palpation or by rectal examination, unlike the scenario of life-threatening small bowel ischemia, in which severe pain may be accompanied by a fairly “benign” examination.
Some cases of ischemic colitis require resection of a gangrenous colon or become chronic and lead to the development of a stricture. But far more often the ischemic episode resolves after several days of watchful waiting. The typical but not specific endoscopic findings and the thumb-printing and thickening seen on radiographic imaging resolve.
Whatever the assumed cause (a specific one is often not found), ischemic colitis gives the internist and the surgeon a chance to commiserate on the power of informed watchful waiting.
As Elder et al point out in this issue of the Journal, the management of ischemic colitis presents an interesting clinical paradox: the internist makes the diagnosis of potentially life-threatening impending tissue necrosis, while the surgeon, consulted to act, tends to be a cheerleader for temperate observation.
Ischemic colitis may account for 1 in 1,000 hospitalizations. Many patients present with a combination of focal lower abdominal pain and some bloody diarrhea. The examiner often localizes the tender colon either by anterior palpation or by rectal examination, unlike the scenario of life-threatening small bowel ischemia, in which severe pain may be accompanied by a fairly “benign” examination.
Some cases of ischemic colitis require resection of a gangrenous colon or become chronic and lead to the development of a stricture. But far more often the ischemic episode resolves after several days of watchful waiting. The typical but not specific endoscopic findings and the thumb-printing and thickening seen on radiographic imaging resolve.
Whatever the assumed cause (a specific one is often not found), ischemic colitis gives the internist and the surgeon a chance to commiserate on the power of informed watchful waiting.
As Elder et al point out in this issue of the Journal, the management of ischemic colitis presents an interesting clinical paradox: the internist makes the diagnosis of potentially life-threatening impending tissue necrosis, while the surgeon, consulted to act, tends to be a cheerleader for temperate observation.
Ischemic colitis may account for 1 in 1,000 hospitalizations. Many patients present with a combination of focal lower abdominal pain and some bloody diarrhea. The examiner often localizes the tender colon either by anterior palpation or by rectal examination, unlike the scenario of life-threatening small bowel ischemia, in which severe pain may be accompanied by a fairly “benign” examination.
Some cases of ischemic colitis require resection of a gangrenous colon or become chronic and lead to the development of a stricture. But far more often the ischemic episode resolves after several days of watchful waiting. The typical but not specific endoscopic findings and the thumb-printing and thickening seen on radiographic imaging resolve.
Whatever the assumed cause (a specific one is often not found), ischemic colitis gives the internist and the surgeon a chance to commiserate on the power of informed watchful waiting.
Clinical approach to colonic ischemia
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
KEY POINTS
- The incidence of colonic ischemia is difficult to ascertain, as most cases are transient and either not reported or misdiagnosed.
- Most cases are in the elderly.
- The clinical presentation is not specific, as other conditions also present with abdominal pain and hematochezia.
- The most common mechanisms are hypotension and hypovolemia caused by dehydration or bleeding that results in systemic hypoperfusion.
- Endoscopy has become the diagnostic procedure of choice.
- Although most patients can be treated conservatively with intravenous fluids, bowel rest, and antibiotics, some develop peritonitis or clinically deteriorate and require surgery.