User login
Conflicting Measures of Hospital Quality / Halasyamani and Davis
National concerns about the quality of health care in the United States have prompted calls for transparent efforts to measure and report hospital performance to the public. Consumer groups, payers, and credentialing organizations now rate the quality of hospitals and health care through a variety of mechanisms, yielding a kaleidoscope of quality measurement scorecards. However, health care consumers have minimal information about how hospital quality rating systems compare with each other or which rating system might best address their information needs.
The Hospital Compare Web site was launched in April 2005 by the Hospital Quality Alliance (HQA), a public‐private collaboration among organizations, including the Centers for Medicare and Medicaid Services (CMS). The CMS describes Hospital Compare as information [that] measures how well hospitals care for their patients.1 A limited set of Hospital Compare data from 2004 were posted online in 2005 for more than 4200 hospitals, permitting community‐specific comparisons of hospitals' self‐reported standardized core measures that reflect quality of care for acute myocardial infarction (AMI), congestive heart failure (CHF), and community‐acquired pneumonia (CAP) in adult patients.
Other current hospital quality evaluation tools target payers and purchasers of health care. However, many of these evaluations require that institutions pay a fee for submitting their data to be benchmarked against other participating institutions or require that the requesting individual or organization pay a fee to examine a hospital's performance on a specific condition or procedure.
We examined Hospital Compare data alongside that of another hospital rating system that has existed for a longer period of time and is likely better known to the lay publicthe Best Hospitals lists published annually by U.S. News and World Report.2, 3 Together, Hospital Compare and Best Hospitals are hospital quality scorecards that offer consumers assessments of hospital performance on a national scale. However, their measures of hospital quality differ, and we investigated whether they would provide consumers with concordant assessments of hospital quality.
METHODS
Data Sources
Hospital Compare
Core measure performance data were obtained by the investigators from the Hospital Compare Web site.3 Information in the database was provided by hospitals for the period January‐June 2004. Hospitals self‐reported their performance on the core measures using standardized medical record abstraction programs. The measures reported are cumulative averages based on monthly performance summaries.
Fourteen core measures were used in the study to form 3 core measure sets (Table 1): the AMI set comprised 6 measures, the CHF set comprised 4 measures, and the CAP site comprised 4 measures. Of the 17 core measures available on the Hospital Compare Web site, core measures of timing of thrombolytic agents or percutaneous transluminal coronary angioplasty for patients with AMI were excluded from the analysis because fewer than 10% of institutions reported such measures. Data on the core measure about oxygenation measurement for CAP were also excluded because of minimal variation between hospitals (national mean = 98%; the national mean for all other measures was less than 92%).3
| Condition | Core Measures |
|---|---|
| |
| Acute myocardial infarction (AMI) |
|
| Congestive heart failure (CHF) |
|
| Community‐acquired pneumonia (CAP) |
|
Core measures that CMS defined as having too few cases (< 25) to reliably ascertain an estimate of hospital performance, or for which hospitals were not reporting data, were not eligible for analysis. To generate a composite score for each of the disease‐specific core measure sets, scores for all eligible core measures within each set were summed and then divided by the number of eligible measures available. This permitted standardization of the scores in the majority of instances when institutions did not report all eligible measures within a given set.
Best Hospitals
Ratings of hospitals were drawn from the 2004 and 2005 editions of the Best Hospitals listings of the U.S. News and World Report, the editions that most closely reflect performance data and physician survey data concurrent with Hospital Compare data analyzed for this study.4 In each year, ratings were developed for more than 2000 hospitals that met specific criteria related to teaching hospital status, medical school affiliation, or availability of specific technology‐related services.5 The Best Hospitals rating system is based on 3 central elements of evaluation: (a) reputation, judged by responses to a national mail survey of physicians asked to list the 5 hospitals best in their specialty for difficult cases, without economic or geographic considerations; (b) in‐hospital mortality rates for Medicare patients, adjusted for severity of illness; and (c) a combination of other factors, such as the nurse‐to‐patient ratio and the number of a set of predetermined key technologies available, as determined from institutions' responses to the American Hospital Association's annual survey.5
The 50 Best Hospitals for heart and heart surgery, 50 Best Hospitals for respiratory disorders, and all Honor Roll hospitals (as determined by breadth of institutional excellence, with top performance in 6 or more of 17 specialties) named in 2004 and 2005 were included in this study, except that National Jewish Medical and Research Center was listed as a Best Hospital for respiratory disorders in both years but did not report sufficient numbers of cases to have eligible core measures in Hospital Compare. Of note, there were 11 institutions newly listed as Best Hospitals for heart and heart surgery and 10 institutions newly listed as Best Hospitals for respiratory disorders in 2005 versus 2004; 14 hospitals made the Best Hospitals Honor Roll in 2004, and 2 others were added for 2005.
Data Analysis
To examine the internal validity of the Hospital Compare measures, we calculated pairwise correlation coefficients among the 14 core‐measure components, using all eligible data points. We then calculated Cronbach's , a measure of the internal consistency of scales of measures, to characterize each of the sets of Hospital Compare core measures separately (AMI, CHF, CAP). We also generated Cronbach's for a measure we called the combined core‐measures score, which we intended to be analogous to the Best Hospitals Honor Roll, defined as the AMI, CHF, and CAP measure sets scored together.
To compare Hospital Compare data with the Best Hospitals rankings (for heart and heart surgery, respiratory disorders, and the Honor Roll), we first established national quartile score cut points for each of the 3 Hospital Compare core measure sets and for the combined core measures, using all U.S. hospitals eligible for our analysis. We used quartiles to avoid the misclassification that would be more likely to occur with deciles (based on confidence intervals for the core measures provided by CMS).6
We calculated Hospital Compare scores for each institution listed as a Best Hospital in 2004 and 2005 and classified the Best Hospitals into scoring quartiles based on national score cut points (eg, if the national cutoff for AMI core measures for the top quartile was 95.2%, then a Best Hospital with an AMI score for the core‐measures set 95.2% was classified in the first [top] quartile). AMI and CHF core measure sets were used for comparison with the Best Hospitals for heart and heart surgery, the CAP core‐measure set was used for comparison with the Best Hospitals for respiratory disorders, and the combined core‐measure set was used for comparison with the Honor Roll hospitals.
Sensitivity Analyses
To investigate the effect of missing Hospital Compare data on our study findings, we conducted sensitivity analyses. We used only those institutions with complete data for the AMI, CHF, and CAP core measure sets to establish new quartile cut points and then reexamined the quartile distribution for institutions in the corresponding Best Hospitals lists. We also compared the Best Hospitals' Hospital Compare data completeness with that of all Hospital Compare institutions.
RESULTS
Core Performance Measures in Hospital Compare
Of 4203 hospitals that submitted core measures as part of Hospital Compare, 4126 had at least 1 core measure eligible for analysis (> 25 observations). Of these 4126 hospitals, 2165 (52.5%) had at least 1 eligible AMI core measure, and 398 (9.7%) had all 6 measures eligible for analysis; 3130 had at least 1 eligible CHF core measure (75.9%), and 289 (7.0%) had all 4 measures eligible for analysis; and 3462 (83.9%) had at least one eligible CAP core measure and 302 (7.3%) had all 4 measures eligible for analysis. For the combined core‐measure score, 2119 (51.4%) had at least 4 eligible measures, and 120 (2.9%) had all 14 measures eligible for analysis.
Pairwise correlation coefficients within each of the disease‐specific core measure sets was highest for the AMI measures, and was generally higher for measures that reflected similar clinical activities (eg, aspirin and ‐blocker at discharge for AMI care; tobacco cessation counseling for AMI, CHF, and CAP; Table 2). In general, the AMI and CHF performance measures correlated more strongly with each other than did the AMI or CHF measures with the CAP measures.
Internal consistency within each of the disease‐specific measures was moderate to strong, with Cronbach's = .83 for AMI, Cronbach's = .58 for CHF, and Cronbach's = .49 for CAP. For the combined performance measure set (all 14 core measures together), Cronbach's = .74.
Hospital Compare Scores for Institutions Listed as Best Hospitals
Best Hospitals for heart and heart surgery and for respiratory disorders in U.S. News and World Report in 2004 and 2005 exhibited a broad distribution of Hospital Compare core measure scores (Table 3). For none of the core measure sets did a majority of Best Hospitals score in the top quartile in either year.
| Hospital Compare Scores | Best Hospitals for Heart Disease: AMI Core Measures (n = 50 hospitals)* | Best Hospitals for Heart Disease: CHF Core Measures (n = 50 hospitals)* | Best Hospitals for Respiratory Disorders: CAP Core Measures (n = 49 hospitals)* | |||
|---|---|---|---|---|---|---|
| ||||||
| 2004 | 2005 | 2004 | 2005 | 2004 | 2005 | |
| First quartile | 20 (40%) | 15 (30%) | 19 (38%) | 19 (38%) | 5 (10%) | 7 (14%) |
| Second quartile | 16 (32%) | 21 (42%) | 14 (28%) | 15 (30%) | 8 (16%) | 6 (12%) |
| Third quartile | 11 (22%) | 10 (20%) | 11 (22%) | 12 (24%) | 13 (27%) | 15 (31%) |
| Fourth quartile | 3 (6%) | 4 (8%) | 6 (12%) | 4 (8%) | 23 (47%) | 21 (43%) |
Among the 50 hospitals identified as best for cardiac care, only 20 (40%) in the 2004 list and 15 (30%) in the 2005 list had AMI core‐measure scores in the top quartile nationally, and 14 (28%) scored below the national median in both years. Among those same 50 hospitals, only 19 (38%) had CHF core‐measure scores in the top quartile nationally in both years, whereas 17 (34%) scored below the national median in 2004 and 16 in 2005. On the CAP core measures, Best Hospitals for respiratory disorders generally scored poorly, with only 5 (10%) from the 2004 list and 7 (14%) from the 2005 list in the top quartile nationally and nearly half the institutions scoring in the bottom national quartile (Table 3).
For the 14 hospitals named to the 2004 Honor Roll of Best Hospitals, the comparison with the combined core‐measure score (AMI, CHF, and CAP together) revealed a similarly broad distribution of core measure performance. Only five hospitals scored in the top quartile, 2 in the second quartile, 5 in the third quartile, and 2 in the bottom quartile. The distribution for hospitals in the 2005 Honor Roll was similar (5‐3‐6‐2 by quartile).
Sensitivity Analyses
National quartile Hospital Compare core‐measure cut points were slightly lower (1%‐2% in absolute terms) for those institutions with complete data than for institutions overall; in other words, institutions reporting on all 17 measures were generally more likely to have somewhat lower scores. These differences were substantive enough to shift the distribution of Best Hospitals in 2004 and 2005 up to higher quartiles for the AMI and CHF Hospital Compare measures but not for the CAP measures. For example, using the complete data AMI cut points, 23 of the 50 Best Hospitals for cardiac care in 2005 scored in the top quartile, 16 in the second quartile, 6 in the third quartile, and 5 in the bottom quartile (compared with 15‐21‐10‐4; Table 3). With complete data CHF cut points, the distribution was 26, 11, 9, and 4 for the 2005 Best Hospitals for cardiac care from the top through bottom quartiles, respectively (compared with 19‐15‐12‐4; Table 3). Results for 2004 sensitivity analyses were similar.
Institutions named as Best Hospitals appeared more likely than institutions overall to have complete Hospital Compare data. Whereas fewer than 10% of institutions in Hospital Compare had complete data for the AMI, CHF, and CAP core measures, 60% of Best Hospitals for cardiac care in 2005 had complete data for AMI measures and 44% for CHF measures, whereas 32% of Best Hospitals for respiratory care had complete CAP data.
DISCUSSION
With the public release of Hospital Compare data for more than 4200 hospitals in April 2005, national efforts to report hospital quality to the public passed a major milestone. Our findings indicate that the separate Hospital Compare measures for AMI, CHF, and CAP care have moderate to strong internal consistency, which suggests they are capturing similar hospital‐level care behaviors across institutions for these 3 common conditions.
However, Hospital Compare scores are largely discordant with the Best Hospital rank lists for cardiac and respiratory disorders care. Several institutions listed as Best Hospitals nationally scored below the national median on disease‐specific Hospital Compare core measures, perhaps leaving data‐conscious consumers to wonder how to synthesize rating systems that employ different indicators and measure different aspects of health care delivery.
Lack of Agreement in Hospital Quality Measurement
Discordance between the Hospital Compare and Best Hospitals rating systems is not all that surprising, given that their methods of institutional assessment differ markedly. Although both approaches share the goal of allowing consumers a comparative look at institutional performance nationally, they clearly measure different aspects of hospital care.
Hospital Compare measures focus on the delivery of disease‐specific, evidence‐based practices for 3 acute medical conditions from the emergency department to discharge. In comparison, the Best Hospitals rankings emphasize the reputation and mortality data of hospitals and health systems across a variety of general and subspecialty care settings (including several in which core quality measures have not yet been developed), combined with factors related to nursing and technology availability that may also influence consumers' choices. Of note, the Best Hospitals rating approach has been criticized in the past for its strong reliance on physicians' ratings of institutional reputation, which may have little to do with functional measures of quality.7
In essence, the Hospital Compare measures indicate how hospitals perform for an average case, while Best Hospitals relies on reputation and focus on mortality to indicate how institutions perform on the toughest cases. The question at hand is: are these institutional quality measures complementary or contradictory? Our findings suggest that Hospital Compare and Best Hospitals measures offer consumers a mix of complementary and contradictory information, depending on the institution.
The ratings systems differ in other respects as well. In Hospital Compare, performance data are available for more than 4000 hospitals, which permits consumers to examine their local institutions, whereas the Best Hospitals lists offer information only on the top performers. On the other hand, the more established Best Hospitals listings have been published annually for the last 15 years,5 permitting some longitudinal evaluation of hospitals' quality consistency. Importantly, neither rating system includes measures of patient satisfaction with hospital care.
One dimension that both rating systems share is the migration of quality measurement from the local and institutional level to the national stage. Historically, health care quality measurement has been a local phenomenon, as institutions work to gain larger shares of their local markets. A few hospitals have marketed their care and services regionally or even nationally and internationally, but these institutionswhich previously primarily used their reputation rather than specific outcome metrics to reach beyond their local communitiesare a minority of U.S. hospitals.
Although Hospital Compare and Best Hospitals are both national in scope, only Hospital Compare allows consumers to understand the quality of care in most of their community hospitals and health systems. Other investigators analyzing the same data set have highlighted significant differences in hospital performance according to for‐profit status, academic status, and size (number of beds).8
However, it is not yet clear if and how hospital ratings influence consumers' health care decisions. In fact, some studies suggest that only a minority of patients are inclined to use performance reports in their decisions about health care.9, 10 Moreover, if illness is acute, the factors driving choice of hospital may be geographic proximity, bed availability, and payer contracts rather than performance measures.
These constraints on the utility of hospital quality metrics from the consumer perspective are reminders that such metrics may have other benefits. Specifically, ratings such as Hospital Compare and Best Hospitals, as well as others such as those of the Leapfrog Group11 and the Joint Commission on Accreditation of Healthcare Organizations,12 offer differing arrays of performance measures that may induce hospitals to improve their quality of care.1, 13 Institutions that score well or improve their scores over time can use such scores not only to benchmark their processes and outcomes but also to signal the comparative value of their care to the public. In the past, hospitals named to the Best Hospitals Honor Roll have trumpeted their achievements through plaques on their walls and in advertisements for their services. Whether institutions will do the same regarding their Hospital Compare scores remains to be seen.
Study Limitations
The chief limitation of this analysis is that not all hospitals reported data for the Hospital Compare core measures. We standardized the core‐measure sets for AMI, CHF, and CAP care for the number of measures reported in each set in order to include as many hospitals as possible in our analyses. Participation in Hospital Compare is voluntary (although strongly encouraged because of better Medicare reimbursement for institutions that participate), so it is possible that there was a systematic scoring bias in hospitals' incomplete reporting across all measures, that is, hospitals might not report specific core measure scores if they were particularly poor.13 That scale score medians were slightly lower for hospitals with complete data than for hospitals overall may indicate some reporting bias in the Hospital Compare data. Nevertheless, in the sensitivity analyses we performed using only those hospitals with complete data on the Hospital Compare core measures, comparisons with the Best Hospitals lists still predominantly indicated discordance between the rating systems.
Another limitation of this work is that we examined only 2 of several currently available hospital‐rating schemes. We chose to examine Hospital Compare because it is the first governmental effort to report specific hospital quality measures to the public, and we elected to look at Hospital Compare alongside the Best Hospitals lists because the latter are arguably the hospital ratings best known to the lay public.
A third potential limitation is that the Best Hospitals lists for 2004 were based in part on mortality figures and hospital survey data from 2002, which were the most recent data available at the time of the rankings; for the 2005 Best Hospitals lists, the most recent mortality and hospital survey data were collected in 2003.4 Hospital Compare scores were calculated on the basis of patients discharged in 2004, and therefore the ratings systems reflect somewhat different time frames. Nonetheless, we do not believe that this mismatch explains the extent of discordance between the 2 rating scales, particularly because there was such stability in the Best Hospital lists over the 2 years.
CONCLUSIONS
The Best Hospitals lists and Hospital Compare core measure scores agree only a minority of the time on the best institutions for the care of cardiac and respiratory conditions in the United States. Prominent, publicly reported hospital quality scorecards that paint discordant pictures of institutional performance potentially present a conundrum for physicians, patients, and payers with growing incentives to compare institutional quality.
If the movement to improve health care quality is to succeed, the challenge will be to harness the growing professional and lay interest in quality measurement to create rating scales that reflect the best aspects of Hospital Compare and the Best Hospitals lists, with the broadest inclusion of institutions and scope of conditions. For example, it would be more helpful to the public if the Best Hospitals lists included available Hospital Compare measures. It would also benefit consumers if Hospital Compare included more metrics about preventive and elective procedures, domains in which consumers can maximally exercise their choice of health care institutions. Moreover, voluntary reporting may constrain the quality effort. Only with mandatory reporting on quality measures will consistent and sufficient institutional accountability be achieved.
- .Public performance reports and the will for change.JAMA.2002;288:1523–1524.
- .Improving the quality of care—can we practice what we preach?N Engl J Med.2003;348:2681–2683.
- U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov. Accessed May 12,2005.
- U.S. News and World Report. Best hospitals 2005. Available at: http://www.usnews.com/usnews/health/best‐hospitals/tophosp.htm. Accessed July 10,2005.
- . Best hospitals 2005: methodology behind the rankings. U.S. News and World Report. Available at: http://www.usnews.com/usnews/health/best‐hospitals/methodology.htm. Accessed July 10,2005.
- U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Hospital Compare: information for professionals. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Static/Data‐Professionals.asp?dest=NAV|Home|DataDetails|ProfessionalInfo#TabTop. Accessed May 12,2005.
- ,,,.In search of America's best hospitals: the promise and reality of quality assessment.JAMA.1997;277:1152–1155.
- ,,,.Care in US hospitals—the Hospital Quality Alliance program.N Engl Jour Med.2005;353:265–274.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279:1638–1642.
- Kaiser Family Foundation and Agency for Health Care Research and Quality.National Survey on Consumers' Experiences with Patient Safety and Quality Information.Washington, DC:Kaiser Family Foundation;2004.
- Leapfrog Group for Patient Safety. Available at: http://www.leapfroggroup.org. Accessed May 12,2005.
- Joint Commission on Accreditation of Healthcare Organizations. Quality check. Available at: http://www.jcaho.org/quality+check/index.htm. Accessed May 12,2005.
- ,.The unintended consequences of publicly reporting quality information.JAMA.2005;293:1239–1244.
National concerns about the quality of health care in the United States have prompted calls for transparent efforts to measure and report hospital performance to the public. Consumer groups, payers, and credentialing organizations now rate the quality of hospitals and health care through a variety of mechanisms, yielding a kaleidoscope of quality measurement scorecards. However, health care consumers have minimal information about how hospital quality rating systems compare with each other or which rating system might best address their information needs.
The Hospital Compare Web site was launched in April 2005 by the Hospital Quality Alliance (HQA), a public‐private collaboration among organizations, including the Centers for Medicare and Medicaid Services (CMS). The CMS describes Hospital Compare as information [that] measures how well hospitals care for their patients.1 A limited set of Hospital Compare data from 2004 were posted online in 2005 for more than 4200 hospitals, permitting community‐specific comparisons of hospitals' self‐reported standardized core measures that reflect quality of care for acute myocardial infarction (AMI), congestive heart failure (CHF), and community‐acquired pneumonia (CAP) in adult patients.
Other current hospital quality evaluation tools target payers and purchasers of health care. However, many of these evaluations require that institutions pay a fee for submitting their data to be benchmarked against other participating institutions or require that the requesting individual or organization pay a fee to examine a hospital's performance on a specific condition or procedure.
We examined Hospital Compare data alongside that of another hospital rating system that has existed for a longer period of time and is likely better known to the lay publicthe Best Hospitals lists published annually by U.S. News and World Report.2, 3 Together, Hospital Compare and Best Hospitals are hospital quality scorecards that offer consumers assessments of hospital performance on a national scale. However, their measures of hospital quality differ, and we investigated whether they would provide consumers with concordant assessments of hospital quality.
METHODS
Data Sources
Hospital Compare
Core measure performance data were obtained by the investigators from the Hospital Compare Web site.3 Information in the database was provided by hospitals for the period January‐June 2004. Hospitals self‐reported their performance on the core measures using standardized medical record abstraction programs. The measures reported are cumulative averages based on monthly performance summaries.
Fourteen core measures were used in the study to form 3 core measure sets (Table 1): the AMI set comprised 6 measures, the CHF set comprised 4 measures, and the CAP site comprised 4 measures. Of the 17 core measures available on the Hospital Compare Web site, core measures of timing of thrombolytic agents or percutaneous transluminal coronary angioplasty for patients with AMI were excluded from the analysis because fewer than 10% of institutions reported such measures. Data on the core measure about oxygenation measurement for CAP were also excluded because of minimal variation between hospitals (national mean = 98%; the national mean for all other measures was less than 92%).3
| Condition | Core Measures |
|---|---|
| |
| Acute myocardial infarction (AMI) |
|
| Congestive heart failure (CHF) |
|
| Community‐acquired pneumonia (CAP) |
|
Core measures that CMS defined as having too few cases (< 25) to reliably ascertain an estimate of hospital performance, or for which hospitals were not reporting data, were not eligible for analysis. To generate a composite score for each of the disease‐specific core measure sets, scores for all eligible core measures within each set were summed and then divided by the number of eligible measures available. This permitted standardization of the scores in the majority of instances when institutions did not report all eligible measures within a given set.
Best Hospitals
Ratings of hospitals were drawn from the 2004 and 2005 editions of the Best Hospitals listings of the U.S. News and World Report, the editions that most closely reflect performance data and physician survey data concurrent with Hospital Compare data analyzed for this study.4 In each year, ratings were developed for more than 2000 hospitals that met specific criteria related to teaching hospital status, medical school affiliation, or availability of specific technology‐related services.5 The Best Hospitals rating system is based on 3 central elements of evaluation: (a) reputation, judged by responses to a national mail survey of physicians asked to list the 5 hospitals best in their specialty for difficult cases, without economic or geographic considerations; (b) in‐hospital mortality rates for Medicare patients, adjusted for severity of illness; and (c) a combination of other factors, such as the nurse‐to‐patient ratio and the number of a set of predetermined key technologies available, as determined from institutions' responses to the American Hospital Association's annual survey.5
The 50 Best Hospitals for heart and heart surgery, 50 Best Hospitals for respiratory disorders, and all Honor Roll hospitals (as determined by breadth of institutional excellence, with top performance in 6 or more of 17 specialties) named in 2004 and 2005 were included in this study, except that National Jewish Medical and Research Center was listed as a Best Hospital for respiratory disorders in both years but did not report sufficient numbers of cases to have eligible core measures in Hospital Compare. Of note, there were 11 institutions newly listed as Best Hospitals for heart and heart surgery and 10 institutions newly listed as Best Hospitals for respiratory disorders in 2005 versus 2004; 14 hospitals made the Best Hospitals Honor Roll in 2004, and 2 others were added for 2005.
Data Analysis
To examine the internal validity of the Hospital Compare measures, we calculated pairwise correlation coefficients among the 14 core‐measure components, using all eligible data points. We then calculated Cronbach's , a measure of the internal consistency of scales of measures, to characterize each of the sets of Hospital Compare core measures separately (AMI, CHF, CAP). We also generated Cronbach's for a measure we called the combined core‐measures score, which we intended to be analogous to the Best Hospitals Honor Roll, defined as the AMI, CHF, and CAP measure sets scored together.
To compare Hospital Compare data with the Best Hospitals rankings (for heart and heart surgery, respiratory disorders, and the Honor Roll), we first established national quartile score cut points for each of the 3 Hospital Compare core measure sets and for the combined core measures, using all U.S. hospitals eligible for our analysis. We used quartiles to avoid the misclassification that would be more likely to occur with deciles (based on confidence intervals for the core measures provided by CMS).6
We calculated Hospital Compare scores for each institution listed as a Best Hospital in 2004 and 2005 and classified the Best Hospitals into scoring quartiles based on national score cut points (eg, if the national cutoff for AMI core measures for the top quartile was 95.2%, then a Best Hospital with an AMI score for the core‐measures set 95.2% was classified in the first [top] quartile). AMI and CHF core measure sets were used for comparison with the Best Hospitals for heart and heart surgery, the CAP core‐measure set was used for comparison with the Best Hospitals for respiratory disorders, and the combined core‐measure set was used for comparison with the Honor Roll hospitals.
Sensitivity Analyses
To investigate the effect of missing Hospital Compare data on our study findings, we conducted sensitivity analyses. We used only those institutions with complete data for the AMI, CHF, and CAP core measure sets to establish new quartile cut points and then reexamined the quartile distribution for institutions in the corresponding Best Hospitals lists. We also compared the Best Hospitals' Hospital Compare data completeness with that of all Hospital Compare institutions.
RESULTS
Core Performance Measures in Hospital Compare
Of 4203 hospitals that submitted core measures as part of Hospital Compare, 4126 had at least 1 core measure eligible for analysis (> 25 observations). Of these 4126 hospitals, 2165 (52.5%) had at least 1 eligible AMI core measure, and 398 (9.7%) had all 6 measures eligible for analysis; 3130 had at least 1 eligible CHF core measure (75.9%), and 289 (7.0%) had all 4 measures eligible for analysis; and 3462 (83.9%) had at least one eligible CAP core measure and 302 (7.3%) had all 4 measures eligible for analysis. For the combined core‐measure score, 2119 (51.4%) had at least 4 eligible measures, and 120 (2.9%) had all 14 measures eligible for analysis.
Pairwise correlation coefficients within each of the disease‐specific core measure sets was highest for the AMI measures, and was generally higher for measures that reflected similar clinical activities (eg, aspirin and ‐blocker at discharge for AMI care; tobacco cessation counseling for AMI, CHF, and CAP; Table 2). In general, the AMI and CHF performance measures correlated more strongly with each other than did the AMI or CHF measures with the CAP measures.
Internal consistency within each of the disease‐specific measures was moderate to strong, with Cronbach's = .83 for AMI, Cronbach's = .58 for CHF, and Cronbach's = .49 for CAP. For the combined performance measure set (all 14 core measures together), Cronbach's = .74.
Hospital Compare Scores for Institutions Listed as Best Hospitals
Best Hospitals for heart and heart surgery and for respiratory disorders in U.S. News and World Report in 2004 and 2005 exhibited a broad distribution of Hospital Compare core measure scores (Table 3). For none of the core measure sets did a majority of Best Hospitals score in the top quartile in either year.
| Hospital Compare Scores | Best Hospitals for Heart Disease: AMI Core Measures (n = 50 hospitals)* | Best Hospitals for Heart Disease: CHF Core Measures (n = 50 hospitals)* | Best Hospitals for Respiratory Disorders: CAP Core Measures (n = 49 hospitals)* | |||
|---|---|---|---|---|---|---|
| ||||||
| 2004 | 2005 | 2004 | 2005 | 2004 | 2005 | |
| First quartile | 20 (40%) | 15 (30%) | 19 (38%) | 19 (38%) | 5 (10%) | 7 (14%) |
| Second quartile | 16 (32%) | 21 (42%) | 14 (28%) | 15 (30%) | 8 (16%) | 6 (12%) |
| Third quartile | 11 (22%) | 10 (20%) | 11 (22%) | 12 (24%) | 13 (27%) | 15 (31%) |
| Fourth quartile | 3 (6%) | 4 (8%) | 6 (12%) | 4 (8%) | 23 (47%) | 21 (43%) |
Among the 50 hospitals identified as best for cardiac care, only 20 (40%) in the 2004 list and 15 (30%) in the 2005 list had AMI core‐measure scores in the top quartile nationally, and 14 (28%) scored below the national median in both years. Among those same 50 hospitals, only 19 (38%) had CHF core‐measure scores in the top quartile nationally in both years, whereas 17 (34%) scored below the national median in 2004 and 16 in 2005. On the CAP core measures, Best Hospitals for respiratory disorders generally scored poorly, with only 5 (10%) from the 2004 list and 7 (14%) from the 2005 list in the top quartile nationally and nearly half the institutions scoring in the bottom national quartile (Table 3).
For the 14 hospitals named to the 2004 Honor Roll of Best Hospitals, the comparison with the combined core‐measure score (AMI, CHF, and CAP together) revealed a similarly broad distribution of core measure performance. Only five hospitals scored in the top quartile, 2 in the second quartile, 5 in the third quartile, and 2 in the bottom quartile. The distribution for hospitals in the 2005 Honor Roll was similar (5‐3‐6‐2 by quartile).
Sensitivity Analyses
National quartile Hospital Compare core‐measure cut points were slightly lower (1%‐2% in absolute terms) for those institutions with complete data than for institutions overall; in other words, institutions reporting on all 17 measures were generally more likely to have somewhat lower scores. These differences were substantive enough to shift the distribution of Best Hospitals in 2004 and 2005 up to higher quartiles for the AMI and CHF Hospital Compare measures but not for the CAP measures. For example, using the complete data AMI cut points, 23 of the 50 Best Hospitals for cardiac care in 2005 scored in the top quartile, 16 in the second quartile, 6 in the third quartile, and 5 in the bottom quartile (compared with 15‐21‐10‐4; Table 3). With complete data CHF cut points, the distribution was 26, 11, 9, and 4 for the 2005 Best Hospitals for cardiac care from the top through bottom quartiles, respectively (compared with 19‐15‐12‐4; Table 3). Results for 2004 sensitivity analyses were similar.
Institutions named as Best Hospitals appeared more likely than institutions overall to have complete Hospital Compare data. Whereas fewer than 10% of institutions in Hospital Compare had complete data for the AMI, CHF, and CAP core measures, 60% of Best Hospitals for cardiac care in 2005 had complete data for AMI measures and 44% for CHF measures, whereas 32% of Best Hospitals for respiratory care had complete CAP data.
DISCUSSION
With the public release of Hospital Compare data for more than 4200 hospitals in April 2005, national efforts to report hospital quality to the public passed a major milestone. Our findings indicate that the separate Hospital Compare measures for AMI, CHF, and CAP care have moderate to strong internal consistency, which suggests they are capturing similar hospital‐level care behaviors across institutions for these 3 common conditions.
However, Hospital Compare scores are largely discordant with the Best Hospital rank lists for cardiac and respiratory disorders care. Several institutions listed as Best Hospitals nationally scored below the national median on disease‐specific Hospital Compare core measures, perhaps leaving data‐conscious consumers to wonder how to synthesize rating systems that employ different indicators and measure different aspects of health care delivery.
Lack of Agreement in Hospital Quality Measurement
Discordance between the Hospital Compare and Best Hospitals rating systems is not all that surprising, given that their methods of institutional assessment differ markedly. Although both approaches share the goal of allowing consumers a comparative look at institutional performance nationally, they clearly measure different aspects of hospital care.
Hospital Compare measures focus on the delivery of disease‐specific, evidence‐based practices for 3 acute medical conditions from the emergency department to discharge. In comparison, the Best Hospitals rankings emphasize the reputation and mortality data of hospitals and health systems across a variety of general and subspecialty care settings (including several in which core quality measures have not yet been developed), combined with factors related to nursing and technology availability that may also influence consumers' choices. Of note, the Best Hospitals rating approach has been criticized in the past for its strong reliance on physicians' ratings of institutional reputation, which may have little to do with functional measures of quality.7
In essence, the Hospital Compare measures indicate how hospitals perform for an average case, while Best Hospitals relies on reputation and focus on mortality to indicate how institutions perform on the toughest cases. The question at hand is: are these institutional quality measures complementary or contradictory? Our findings suggest that Hospital Compare and Best Hospitals measures offer consumers a mix of complementary and contradictory information, depending on the institution.
The ratings systems differ in other respects as well. In Hospital Compare, performance data are available for more than 4000 hospitals, which permits consumers to examine their local institutions, whereas the Best Hospitals lists offer information only on the top performers. On the other hand, the more established Best Hospitals listings have been published annually for the last 15 years,5 permitting some longitudinal evaluation of hospitals' quality consistency. Importantly, neither rating system includes measures of patient satisfaction with hospital care.
One dimension that both rating systems share is the migration of quality measurement from the local and institutional level to the national stage. Historically, health care quality measurement has been a local phenomenon, as institutions work to gain larger shares of their local markets. A few hospitals have marketed their care and services regionally or even nationally and internationally, but these institutionswhich previously primarily used their reputation rather than specific outcome metrics to reach beyond their local communitiesare a minority of U.S. hospitals.
Although Hospital Compare and Best Hospitals are both national in scope, only Hospital Compare allows consumers to understand the quality of care in most of their community hospitals and health systems. Other investigators analyzing the same data set have highlighted significant differences in hospital performance according to for‐profit status, academic status, and size (number of beds).8
However, it is not yet clear if and how hospital ratings influence consumers' health care decisions. In fact, some studies suggest that only a minority of patients are inclined to use performance reports in their decisions about health care.9, 10 Moreover, if illness is acute, the factors driving choice of hospital may be geographic proximity, bed availability, and payer contracts rather than performance measures.
These constraints on the utility of hospital quality metrics from the consumer perspective are reminders that such metrics may have other benefits. Specifically, ratings such as Hospital Compare and Best Hospitals, as well as others such as those of the Leapfrog Group11 and the Joint Commission on Accreditation of Healthcare Organizations,12 offer differing arrays of performance measures that may induce hospitals to improve their quality of care.1, 13 Institutions that score well or improve their scores over time can use such scores not only to benchmark their processes and outcomes but also to signal the comparative value of their care to the public. In the past, hospitals named to the Best Hospitals Honor Roll have trumpeted their achievements through plaques on their walls and in advertisements for their services. Whether institutions will do the same regarding their Hospital Compare scores remains to be seen.
Study Limitations
The chief limitation of this analysis is that not all hospitals reported data for the Hospital Compare core measures. We standardized the core‐measure sets for AMI, CHF, and CAP care for the number of measures reported in each set in order to include as many hospitals as possible in our analyses. Participation in Hospital Compare is voluntary (although strongly encouraged because of better Medicare reimbursement for institutions that participate), so it is possible that there was a systematic scoring bias in hospitals' incomplete reporting across all measures, that is, hospitals might not report specific core measure scores if they were particularly poor.13 That scale score medians were slightly lower for hospitals with complete data than for hospitals overall may indicate some reporting bias in the Hospital Compare data. Nevertheless, in the sensitivity analyses we performed using only those hospitals with complete data on the Hospital Compare core measures, comparisons with the Best Hospitals lists still predominantly indicated discordance between the rating systems.
Another limitation of this work is that we examined only 2 of several currently available hospital‐rating schemes. We chose to examine Hospital Compare because it is the first governmental effort to report specific hospital quality measures to the public, and we elected to look at Hospital Compare alongside the Best Hospitals lists because the latter are arguably the hospital ratings best known to the lay public.
A third potential limitation is that the Best Hospitals lists for 2004 were based in part on mortality figures and hospital survey data from 2002, which were the most recent data available at the time of the rankings; for the 2005 Best Hospitals lists, the most recent mortality and hospital survey data were collected in 2003.4 Hospital Compare scores were calculated on the basis of patients discharged in 2004, and therefore the ratings systems reflect somewhat different time frames. Nonetheless, we do not believe that this mismatch explains the extent of discordance between the 2 rating scales, particularly because there was such stability in the Best Hospital lists over the 2 years.
CONCLUSIONS
The Best Hospitals lists and Hospital Compare core measure scores agree only a minority of the time on the best institutions for the care of cardiac and respiratory conditions in the United States. Prominent, publicly reported hospital quality scorecards that paint discordant pictures of institutional performance potentially present a conundrum for physicians, patients, and payers with growing incentives to compare institutional quality.
If the movement to improve health care quality is to succeed, the challenge will be to harness the growing professional and lay interest in quality measurement to create rating scales that reflect the best aspects of Hospital Compare and the Best Hospitals lists, with the broadest inclusion of institutions and scope of conditions. For example, it would be more helpful to the public if the Best Hospitals lists included available Hospital Compare measures. It would also benefit consumers if Hospital Compare included more metrics about preventive and elective procedures, domains in which consumers can maximally exercise their choice of health care institutions. Moreover, voluntary reporting may constrain the quality effort. Only with mandatory reporting on quality measures will consistent and sufficient institutional accountability be achieved.
National concerns about the quality of health care in the United States have prompted calls for transparent efforts to measure and report hospital performance to the public. Consumer groups, payers, and credentialing organizations now rate the quality of hospitals and health care through a variety of mechanisms, yielding a kaleidoscope of quality measurement scorecards. However, health care consumers have minimal information about how hospital quality rating systems compare with each other or which rating system might best address their information needs.
The Hospital Compare Web site was launched in April 2005 by the Hospital Quality Alliance (HQA), a public‐private collaboration among organizations, including the Centers for Medicare and Medicaid Services (CMS). The CMS describes Hospital Compare as information [that] measures how well hospitals care for their patients.1 A limited set of Hospital Compare data from 2004 were posted online in 2005 for more than 4200 hospitals, permitting community‐specific comparisons of hospitals' self‐reported standardized core measures that reflect quality of care for acute myocardial infarction (AMI), congestive heart failure (CHF), and community‐acquired pneumonia (CAP) in adult patients.
Other current hospital quality evaluation tools target payers and purchasers of health care. However, many of these evaluations require that institutions pay a fee for submitting their data to be benchmarked against other participating institutions or require that the requesting individual or organization pay a fee to examine a hospital's performance on a specific condition or procedure.
We examined Hospital Compare data alongside that of another hospital rating system that has existed for a longer period of time and is likely better known to the lay publicthe Best Hospitals lists published annually by U.S. News and World Report.2, 3 Together, Hospital Compare and Best Hospitals are hospital quality scorecards that offer consumers assessments of hospital performance on a national scale. However, their measures of hospital quality differ, and we investigated whether they would provide consumers with concordant assessments of hospital quality.
METHODS
Data Sources
Hospital Compare
Core measure performance data were obtained by the investigators from the Hospital Compare Web site.3 Information in the database was provided by hospitals for the period January‐June 2004. Hospitals self‐reported their performance on the core measures using standardized medical record abstraction programs. The measures reported are cumulative averages based on monthly performance summaries.
Fourteen core measures were used in the study to form 3 core measure sets (Table 1): the AMI set comprised 6 measures, the CHF set comprised 4 measures, and the CAP site comprised 4 measures. Of the 17 core measures available on the Hospital Compare Web site, core measures of timing of thrombolytic agents or percutaneous transluminal coronary angioplasty for patients with AMI were excluded from the analysis because fewer than 10% of institutions reported such measures. Data on the core measure about oxygenation measurement for CAP were also excluded because of minimal variation between hospitals (national mean = 98%; the national mean for all other measures was less than 92%).3
| Condition | Core Measures |
|---|---|
| |
| Acute myocardial infarction (AMI) |
|
| Congestive heart failure (CHF) |
|
| Community‐acquired pneumonia (CAP) |
|
Core measures that CMS defined as having too few cases (< 25) to reliably ascertain an estimate of hospital performance, or for which hospitals were not reporting data, were not eligible for analysis. To generate a composite score for each of the disease‐specific core measure sets, scores for all eligible core measures within each set were summed and then divided by the number of eligible measures available. This permitted standardization of the scores in the majority of instances when institutions did not report all eligible measures within a given set.
Best Hospitals
Ratings of hospitals were drawn from the 2004 and 2005 editions of the Best Hospitals listings of the U.S. News and World Report, the editions that most closely reflect performance data and physician survey data concurrent with Hospital Compare data analyzed for this study.4 In each year, ratings were developed for more than 2000 hospitals that met specific criteria related to teaching hospital status, medical school affiliation, or availability of specific technology‐related services.5 The Best Hospitals rating system is based on 3 central elements of evaluation: (a) reputation, judged by responses to a national mail survey of physicians asked to list the 5 hospitals best in their specialty for difficult cases, without economic or geographic considerations; (b) in‐hospital mortality rates for Medicare patients, adjusted for severity of illness; and (c) a combination of other factors, such as the nurse‐to‐patient ratio and the number of a set of predetermined key technologies available, as determined from institutions' responses to the American Hospital Association's annual survey.5
The 50 Best Hospitals for heart and heart surgery, 50 Best Hospitals for respiratory disorders, and all Honor Roll hospitals (as determined by breadth of institutional excellence, with top performance in 6 or more of 17 specialties) named in 2004 and 2005 were included in this study, except that National Jewish Medical and Research Center was listed as a Best Hospital for respiratory disorders in both years but did not report sufficient numbers of cases to have eligible core measures in Hospital Compare. Of note, there were 11 institutions newly listed as Best Hospitals for heart and heart surgery and 10 institutions newly listed as Best Hospitals for respiratory disorders in 2005 versus 2004; 14 hospitals made the Best Hospitals Honor Roll in 2004, and 2 others were added for 2005.
Data Analysis
To examine the internal validity of the Hospital Compare measures, we calculated pairwise correlation coefficients among the 14 core‐measure components, using all eligible data points. We then calculated Cronbach's , a measure of the internal consistency of scales of measures, to characterize each of the sets of Hospital Compare core measures separately (AMI, CHF, CAP). We also generated Cronbach's for a measure we called the combined core‐measures score, which we intended to be analogous to the Best Hospitals Honor Roll, defined as the AMI, CHF, and CAP measure sets scored together.
To compare Hospital Compare data with the Best Hospitals rankings (for heart and heart surgery, respiratory disorders, and the Honor Roll), we first established national quartile score cut points for each of the 3 Hospital Compare core measure sets and for the combined core measures, using all U.S. hospitals eligible for our analysis. We used quartiles to avoid the misclassification that would be more likely to occur with deciles (based on confidence intervals for the core measures provided by CMS).6
We calculated Hospital Compare scores for each institution listed as a Best Hospital in 2004 and 2005 and classified the Best Hospitals into scoring quartiles based on national score cut points (eg, if the national cutoff for AMI core measures for the top quartile was 95.2%, then a Best Hospital with an AMI score for the core‐measures set 95.2% was classified in the first [top] quartile). AMI and CHF core measure sets were used for comparison with the Best Hospitals for heart and heart surgery, the CAP core‐measure set was used for comparison with the Best Hospitals for respiratory disorders, and the combined core‐measure set was used for comparison with the Honor Roll hospitals.
Sensitivity Analyses
To investigate the effect of missing Hospital Compare data on our study findings, we conducted sensitivity analyses. We used only those institutions with complete data for the AMI, CHF, and CAP core measure sets to establish new quartile cut points and then reexamined the quartile distribution for institutions in the corresponding Best Hospitals lists. We also compared the Best Hospitals' Hospital Compare data completeness with that of all Hospital Compare institutions.
RESULTS
Core Performance Measures in Hospital Compare
Of 4203 hospitals that submitted core measures as part of Hospital Compare, 4126 had at least 1 core measure eligible for analysis (> 25 observations). Of these 4126 hospitals, 2165 (52.5%) had at least 1 eligible AMI core measure, and 398 (9.7%) had all 6 measures eligible for analysis; 3130 had at least 1 eligible CHF core measure (75.9%), and 289 (7.0%) had all 4 measures eligible for analysis; and 3462 (83.9%) had at least one eligible CAP core measure and 302 (7.3%) had all 4 measures eligible for analysis. For the combined core‐measure score, 2119 (51.4%) had at least 4 eligible measures, and 120 (2.9%) had all 14 measures eligible for analysis.
Pairwise correlation coefficients within each of the disease‐specific core measure sets was highest for the AMI measures, and was generally higher for measures that reflected similar clinical activities (eg, aspirin and ‐blocker at discharge for AMI care; tobacco cessation counseling for AMI, CHF, and CAP; Table 2). In general, the AMI and CHF performance measures correlated more strongly with each other than did the AMI or CHF measures with the CAP measures.
Internal consistency within each of the disease‐specific measures was moderate to strong, with Cronbach's = .83 for AMI, Cronbach's = .58 for CHF, and Cronbach's = .49 for CAP. For the combined performance measure set (all 14 core measures together), Cronbach's = .74.
Hospital Compare Scores for Institutions Listed as Best Hospitals
Best Hospitals for heart and heart surgery and for respiratory disorders in U.S. News and World Report in 2004 and 2005 exhibited a broad distribution of Hospital Compare core measure scores (Table 3). For none of the core measure sets did a majority of Best Hospitals score in the top quartile in either year.
| Hospital Compare Scores | Best Hospitals for Heart Disease: AMI Core Measures (n = 50 hospitals)* | Best Hospitals for Heart Disease: CHF Core Measures (n = 50 hospitals)* | Best Hospitals for Respiratory Disorders: CAP Core Measures (n = 49 hospitals)* | |||
|---|---|---|---|---|---|---|
| ||||||
| 2004 | 2005 | 2004 | 2005 | 2004 | 2005 | |
| First quartile | 20 (40%) | 15 (30%) | 19 (38%) | 19 (38%) | 5 (10%) | 7 (14%) |
| Second quartile | 16 (32%) | 21 (42%) | 14 (28%) | 15 (30%) | 8 (16%) | 6 (12%) |
| Third quartile | 11 (22%) | 10 (20%) | 11 (22%) | 12 (24%) | 13 (27%) | 15 (31%) |
| Fourth quartile | 3 (6%) | 4 (8%) | 6 (12%) | 4 (8%) | 23 (47%) | 21 (43%) |
Among the 50 hospitals identified as best for cardiac care, only 20 (40%) in the 2004 list and 15 (30%) in the 2005 list had AMI core‐measure scores in the top quartile nationally, and 14 (28%) scored below the national median in both years. Among those same 50 hospitals, only 19 (38%) had CHF core‐measure scores in the top quartile nationally in both years, whereas 17 (34%) scored below the national median in 2004 and 16 in 2005. On the CAP core measures, Best Hospitals for respiratory disorders generally scored poorly, with only 5 (10%) from the 2004 list and 7 (14%) from the 2005 list in the top quartile nationally and nearly half the institutions scoring in the bottom national quartile (Table 3).
For the 14 hospitals named to the 2004 Honor Roll of Best Hospitals, the comparison with the combined core‐measure score (AMI, CHF, and CAP together) revealed a similarly broad distribution of core measure performance. Only five hospitals scored in the top quartile, 2 in the second quartile, 5 in the third quartile, and 2 in the bottom quartile. The distribution for hospitals in the 2005 Honor Roll was similar (5‐3‐6‐2 by quartile).
Sensitivity Analyses
National quartile Hospital Compare core‐measure cut points were slightly lower (1%‐2% in absolute terms) for those institutions with complete data than for institutions overall; in other words, institutions reporting on all 17 measures were generally more likely to have somewhat lower scores. These differences were substantive enough to shift the distribution of Best Hospitals in 2004 and 2005 up to higher quartiles for the AMI and CHF Hospital Compare measures but not for the CAP measures. For example, using the complete data AMI cut points, 23 of the 50 Best Hospitals for cardiac care in 2005 scored in the top quartile, 16 in the second quartile, 6 in the third quartile, and 5 in the bottom quartile (compared with 15‐21‐10‐4; Table 3). With complete data CHF cut points, the distribution was 26, 11, 9, and 4 for the 2005 Best Hospitals for cardiac care from the top through bottom quartiles, respectively (compared with 19‐15‐12‐4; Table 3). Results for 2004 sensitivity analyses were similar.
Institutions named as Best Hospitals appeared more likely than institutions overall to have complete Hospital Compare data. Whereas fewer than 10% of institutions in Hospital Compare had complete data for the AMI, CHF, and CAP core measures, 60% of Best Hospitals for cardiac care in 2005 had complete data for AMI measures and 44% for CHF measures, whereas 32% of Best Hospitals for respiratory care had complete CAP data.
DISCUSSION
With the public release of Hospital Compare data for more than 4200 hospitals in April 2005, national efforts to report hospital quality to the public passed a major milestone. Our findings indicate that the separate Hospital Compare measures for AMI, CHF, and CAP care have moderate to strong internal consistency, which suggests they are capturing similar hospital‐level care behaviors across institutions for these 3 common conditions.
However, Hospital Compare scores are largely discordant with the Best Hospital rank lists for cardiac and respiratory disorders care. Several institutions listed as Best Hospitals nationally scored below the national median on disease‐specific Hospital Compare core measures, perhaps leaving data‐conscious consumers to wonder how to synthesize rating systems that employ different indicators and measure different aspects of health care delivery.
Lack of Agreement in Hospital Quality Measurement
Discordance between the Hospital Compare and Best Hospitals rating systems is not all that surprising, given that their methods of institutional assessment differ markedly. Although both approaches share the goal of allowing consumers a comparative look at institutional performance nationally, they clearly measure different aspects of hospital care.
Hospital Compare measures focus on the delivery of disease‐specific, evidence‐based practices for 3 acute medical conditions from the emergency department to discharge. In comparison, the Best Hospitals rankings emphasize the reputation and mortality data of hospitals and health systems across a variety of general and subspecialty care settings (including several in which core quality measures have not yet been developed), combined with factors related to nursing and technology availability that may also influence consumers' choices. Of note, the Best Hospitals rating approach has been criticized in the past for its strong reliance on physicians' ratings of institutional reputation, which may have little to do with functional measures of quality.7
In essence, the Hospital Compare measures indicate how hospitals perform for an average case, while Best Hospitals relies on reputation and focus on mortality to indicate how institutions perform on the toughest cases. The question at hand is: are these institutional quality measures complementary or contradictory? Our findings suggest that Hospital Compare and Best Hospitals measures offer consumers a mix of complementary and contradictory information, depending on the institution.
The ratings systems differ in other respects as well. In Hospital Compare, performance data are available for more than 4000 hospitals, which permits consumers to examine their local institutions, whereas the Best Hospitals lists offer information only on the top performers. On the other hand, the more established Best Hospitals listings have been published annually for the last 15 years,5 permitting some longitudinal evaluation of hospitals' quality consistency. Importantly, neither rating system includes measures of patient satisfaction with hospital care.
One dimension that both rating systems share is the migration of quality measurement from the local and institutional level to the national stage. Historically, health care quality measurement has been a local phenomenon, as institutions work to gain larger shares of their local markets. A few hospitals have marketed their care and services regionally or even nationally and internationally, but these institutionswhich previously primarily used their reputation rather than specific outcome metrics to reach beyond their local communitiesare a minority of U.S. hospitals.
Although Hospital Compare and Best Hospitals are both national in scope, only Hospital Compare allows consumers to understand the quality of care in most of their community hospitals and health systems. Other investigators analyzing the same data set have highlighted significant differences in hospital performance according to for‐profit status, academic status, and size (number of beds).8
However, it is not yet clear if and how hospital ratings influence consumers' health care decisions. In fact, some studies suggest that only a minority of patients are inclined to use performance reports in their decisions about health care.9, 10 Moreover, if illness is acute, the factors driving choice of hospital may be geographic proximity, bed availability, and payer contracts rather than performance measures.
These constraints on the utility of hospital quality metrics from the consumer perspective are reminders that such metrics may have other benefits. Specifically, ratings such as Hospital Compare and Best Hospitals, as well as others such as those of the Leapfrog Group11 and the Joint Commission on Accreditation of Healthcare Organizations,12 offer differing arrays of performance measures that may induce hospitals to improve their quality of care.1, 13 Institutions that score well or improve their scores over time can use such scores not only to benchmark their processes and outcomes but also to signal the comparative value of their care to the public. In the past, hospitals named to the Best Hospitals Honor Roll have trumpeted their achievements through plaques on their walls and in advertisements for their services. Whether institutions will do the same regarding their Hospital Compare scores remains to be seen.
Study Limitations
The chief limitation of this analysis is that not all hospitals reported data for the Hospital Compare core measures. We standardized the core‐measure sets for AMI, CHF, and CAP care for the number of measures reported in each set in order to include as many hospitals as possible in our analyses. Participation in Hospital Compare is voluntary (although strongly encouraged because of better Medicare reimbursement for institutions that participate), so it is possible that there was a systematic scoring bias in hospitals' incomplete reporting across all measures, that is, hospitals might not report specific core measure scores if they were particularly poor.13 That scale score medians were slightly lower for hospitals with complete data than for hospitals overall may indicate some reporting bias in the Hospital Compare data. Nevertheless, in the sensitivity analyses we performed using only those hospitals with complete data on the Hospital Compare core measures, comparisons with the Best Hospitals lists still predominantly indicated discordance between the rating systems.
Another limitation of this work is that we examined only 2 of several currently available hospital‐rating schemes. We chose to examine Hospital Compare because it is the first governmental effort to report specific hospital quality measures to the public, and we elected to look at Hospital Compare alongside the Best Hospitals lists because the latter are arguably the hospital ratings best known to the lay public.
A third potential limitation is that the Best Hospitals lists for 2004 were based in part on mortality figures and hospital survey data from 2002, which were the most recent data available at the time of the rankings; for the 2005 Best Hospitals lists, the most recent mortality and hospital survey data were collected in 2003.4 Hospital Compare scores were calculated on the basis of patients discharged in 2004, and therefore the ratings systems reflect somewhat different time frames. Nonetheless, we do not believe that this mismatch explains the extent of discordance between the 2 rating scales, particularly because there was such stability in the Best Hospital lists over the 2 years.
CONCLUSIONS
The Best Hospitals lists and Hospital Compare core measure scores agree only a minority of the time on the best institutions for the care of cardiac and respiratory conditions in the United States. Prominent, publicly reported hospital quality scorecards that paint discordant pictures of institutional performance potentially present a conundrum for physicians, patients, and payers with growing incentives to compare institutional quality.
If the movement to improve health care quality is to succeed, the challenge will be to harness the growing professional and lay interest in quality measurement to create rating scales that reflect the best aspects of Hospital Compare and the Best Hospitals lists, with the broadest inclusion of institutions and scope of conditions. For example, it would be more helpful to the public if the Best Hospitals lists included available Hospital Compare measures. It would also benefit consumers if Hospital Compare included more metrics about preventive and elective procedures, domains in which consumers can maximally exercise their choice of health care institutions. Moreover, voluntary reporting may constrain the quality effort. Only with mandatory reporting on quality measures will consistent and sufficient institutional accountability be achieved.
- .Public performance reports and the will for change.JAMA.2002;288:1523–1524.
- .Improving the quality of care—can we practice what we preach?N Engl J Med.2003;348:2681–2683.
- U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov. Accessed May 12,2005.
- U.S. News and World Report. Best hospitals 2005. Available at: http://www.usnews.com/usnews/health/best‐hospitals/tophosp.htm. Accessed July 10,2005.
- . Best hospitals 2005: methodology behind the rankings. U.S. News and World Report. Available at: http://www.usnews.com/usnews/health/best‐hospitals/methodology.htm. Accessed July 10,2005.
- U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Hospital Compare: information for professionals. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Static/Data‐Professionals.asp?dest=NAV|Home|DataDetails|ProfessionalInfo#TabTop. Accessed May 12,2005.
- ,,,.In search of America's best hospitals: the promise and reality of quality assessment.JAMA.1997;277:1152–1155.
- ,,,.Care in US hospitals—the Hospital Quality Alliance program.N Engl Jour Med.2005;353:265–274.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279:1638–1642.
- Kaiser Family Foundation and Agency for Health Care Research and Quality.National Survey on Consumers' Experiences with Patient Safety and Quality Information.Washington, DC:Kaiser Family Foundation;2004.
- Leapfrog Group for Patient Safety. Available at: http://www.leapfroggroup.org. Accessed May 12,2005.
- Joint Commission on Accreditation of Healthcare Organizations. Quality check. Available at: http://www.jcaho.org/quality+check/index.htm. Accessed May 12,2005.
- ,.The unintended consequences of publicly reporting quality information.JAMA.2005;293:1239–1244.
- .Public performance reports and the will for change.JAMA.2002;288:1523–1524.
- .Improving the quality of care—can we practice what we preach?N Engl J Med.2003;348:2681–2683.
- U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov. Accessed May 12,2005.
- U.S. News and World Report. Best hospitals 2005. Available at: http://www.usnews.com/usnews/health/best‐hospitals/tophosp.htm. Accessed July 10,2005.
- . Best hospitals 2005: methodology behind the rankings. U.S. News and World Report. Available at: http://www.usnews.com/usnews/health/best‐hospitals/methodology.htm. Accessed July 10,2005.
- U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Hospital Compare: information for professionals. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Static/Data‐Professionals.asp?dest=NAV|Home|DataDetails|ProfessionalInfo#TabTop. Accessed May 12,2005.
- ,,,.In search of America's best hospitals: the promise and reality of quality assessment.JAMA.1997;277:1152–1155.
- ,,,.Care in US hospitals—the Hospital Quality Alliance program.N Engl Jour Med.2005;353:265–274.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279:1638–1642.
- Kaiser Family Foundation and Agency for Health Care Research and Quality.National Survey on Consumers' Experiences with Patient Safety and Quality Information.Washington, DC:Kaiser Family Foundation;2004.
- Leapfrog Group for Patient Safety. Available at: http://www.leapfroggroup.org. Accessed May 12,2005.
- Joint Commission on Accreditation of Healthcare Organizations. Quality check. Available at: http://www.jcaho.org/quality+check/index.htm. Accessed May 12,2005.
- ,.The unintended consequences of publicly reporting quality information.JAMA.2005;293:1239–1244.
Copyright © 2007 Society of Hospital Medicine
Impact of a Bedside Procedure Service
Inpatient bedside procedures are a major source of preventable adverse events in hospitals.1, 2 Unfortunately, many future inpatient physicians may lack the training3 and confidence4 to correct this problem. One proposed model for improving the teaching, performance, and evaluation of bedside procedures is a procedure service that is staffed by faculty who are experts at inpatient procedures.5 In a recent survey of internal medicine residents from our hospital, 86% (30 of 35) believed that expert supervision would improve central venous catheterization technique (Trick WE, personal communication).
Primary considerations in the development of a procedure service are the baseline demand for bedside procedures and whether a procedure service may affect this demand. Though variations in population‐based rates of some hospital procedures have been described,6, 7 there is little written on the demand for procedures performed at the bedsides of inpatients. Concomitant increases in demand and availability of other technologies810 suggest that improving the availability of bedside procedures may lead to an increase in their demand, regardless of whether such an increase benefits patients.11
Therefore, we sought to determine the impact of a bedside procedure service on the baseline number of paracenteses, thoracenteses, lumbar punctures (LPs), and central venous catheterizations (CVCs) performed on general medicine inpatients at our teaching hospital. In addition, we examined whether this service leads to more successful and safe procedure attempts.
METHODS
Design and Setting
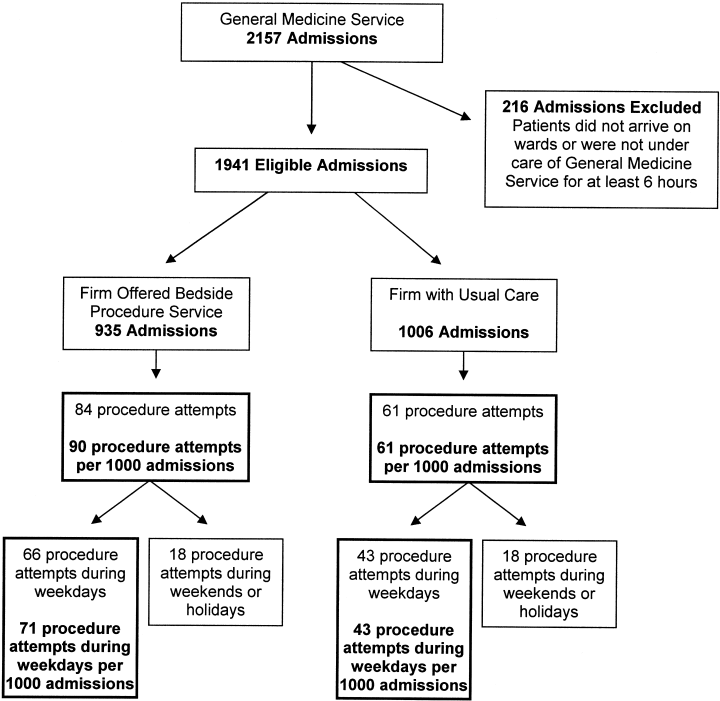
In this prospective cohort study, the cohort was all patients admitted to the general medicine service at Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, in January and February of 2006. The general medicine inpatient service is divided into 3 firms (A, B, and C), each with 4 separate teams of physicians and students. Admissions from the emergency department or other services in the hospital, such as intensive care units (which are closed and therefore staffed by separate teams of physicians), are distributed in sequence to on‐call teams from each firm. During the study period, the availability of a bedside procedure service varied by firm. Throughout the first 4 weeks, the service was available to only 1 of 3 firms (firm A). Then, during weeks 5 through 8, the service crossed over to the other 2 firms (firms B and C) and was unavailable to the original firm. Firm assignments for residents assigned to the inpatient service for all 8 weeks did not change. Of the 16 residents assigned to firm A during the first 4 weeks, when the procedure service was available, 3 remained on the wards during the second 4 weeks, when the procedure service was not available.
We chose to collect data on 4 bedside procedures: paracentesis, thoracentesis, LP, and CVC. Similar to those at other teaching hospitals, our residents informally acquire the skills to perform these procedures while assisting and being assisted by more experienced senior residents in a see one, do one, teach one apprenticeship model of learning.4 To improve the training and performance of these bedside procedures, the Department of Medicine piloted a bedside procedure service to teach procedural skills and assist residents during these procedures. Use of the service, though voluntary, was actively encouraged at residents' monthly orientation meetings and regular conferences.
One attending inpatient physician (J.A.) staffed the bedside procedure service, which was available during normal work hours on weekdays. Requests for procedures were made by general medicine residents through an online database and, after approval by the procedure service attending physician, were performed under his direct supervision. A hand‐carried ultrasound (MicroMaxx, Sonosite, Inc., Bothell, WA) that generates a 2‐dimensional gray‐scale image was used to both confirm the presence and location of fluid prior to paracentesis and thoracentesis and provide real‐time guidance during central venous catheterization. When the bedside procedure service was unavailable, residents performed bedside procedures in the usual fashion, typically without direct attending physician supervision. But if requested, an on‐call chief medical resident with access to a hand‐carried ultrasound device used by the intensive care unit was available for assistance at any time.
Subjects
The study subjects were all patients admitted to the general medical service during the 8‐week pilot period. Patients were excluded if they had been discharged before arrival on the medical wards or if they were under the care of the general medicine service for less than 6 hours before discharge or transfer to another service. We chose 6 hours because we reasoned that such brief admissions were not potential candidates for invasive bedside procedures.
Data Collection
Each morning an investigator contacted the senior residents who had admitted patients during the previous 24‐hour shift and confirmed that newly admitted patients were under the care of the general medicine service for more than 6 hours. To examine how the number of attempts may have been affected by procedures done in the emergency room or intensive care units before admission to the general medicine service, investigators also asked admitting residents whether a bedside procedure had been attempted in the 72 hours before admission. Every general medicine service resident was asked to fill out a brief data collection form after an attempt to perform any procedure on the general medical wards. In addition, chief residents asked each member of the general medicine service at mandatory sign‐out rounds at the end of each weekday whether any procedures had been attempted, and on weekend days investigators contacted senior residents from each general medicine service team.
We report on this quality assurance study, which was conducted during a pilot phase. This report has been reviewed and judged exempt by our institutional review board.
Primary OutcomeNumber of Procedure Attempts
For all bedside procedures attempted by residents on the general medical wards, investigators determined whether the residents were members of firms that were offered the bedside procedure service and, if so, whether the procedure service attending directly supervised the procedure attempt. Multiple procedure attempts of the same type were counted for an individual patient if (1) the procedure attempts did not occur during the same admissions and (2) neither the physicians attempting the procedure nor the primary indications for it were the same. Therefore, neither attempts performed after initially unsuccessful ones nor repeated procedures, such as large‐volume therapeutic paracentesis and thoracentesis, were counted twice. We reasoned that when these criteria were met, procedure attempts could be considered independently.
Secondary Outcomes
Investigators asked residents who attempted procedures to indicate whether (1) the indication for the procedure was solely diagnostic or was, at least in part, therapeutic; (2) the procedure was successful; and (3) there were any immediate major periprocedural complications. A procedure was considered to have been successfully performed if it fulfilled 2 criteria: it had to be completed during a single continuous attempt, even if multiple sites or procedure kits were used; and it had to fulfill the indication for it being done. For example, if the indication for thoracentesis was therapeutic, this procedure would be considered successful if it yielded a large enough volume of fluid to alleviate the patient's symptoms, but if the indication was diagnostic, then thoracentesis would be considered successful if it yielded enough fluid for laboratory processing. Residents were asked to report any periprocedural complications that they considered major; 2 illustrative examples were provided: a pneumothorax and severe bleeding.
Data Analyses
On the basis of earlier pilot data, we estimated that 8%10% of all admissions to the general medicine service underwent at least 1 procedure (paracentesis, thoracentesis, lumbar puncture, or central vein catheterization). We planned for a sample size of 1900 admissions, which would have 80% power to detect a clinically meaningful 50% relative increase in the mean number of bedside procedures with a double‐sided alpha error of 0.05. We used permutation tests to compare the mean number of procedures attempted between firms and bootstrap simulation to construct 95% confidence intervals for those means and the differences between and ratios of them. Fisher's exact test was used to compare proportions of successfully performed procedures and preadmission procedure attempts. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, LP, College Station, TX).
RESULTS
Subjects
During this 8‐week pilot study, there were 2157 admissions to the general medicine service. Among these admissions, 216 were excluded from our study because the patients did not arrive on the medical wards or were not under the care of the general medicine service for at least 6 hours before discharge or before being transferred to another service. Of the remaining 1941 admissions, 935 were to firms with the bedside procedure service available, and 1006 were to firms without the service available (Fig. 1)
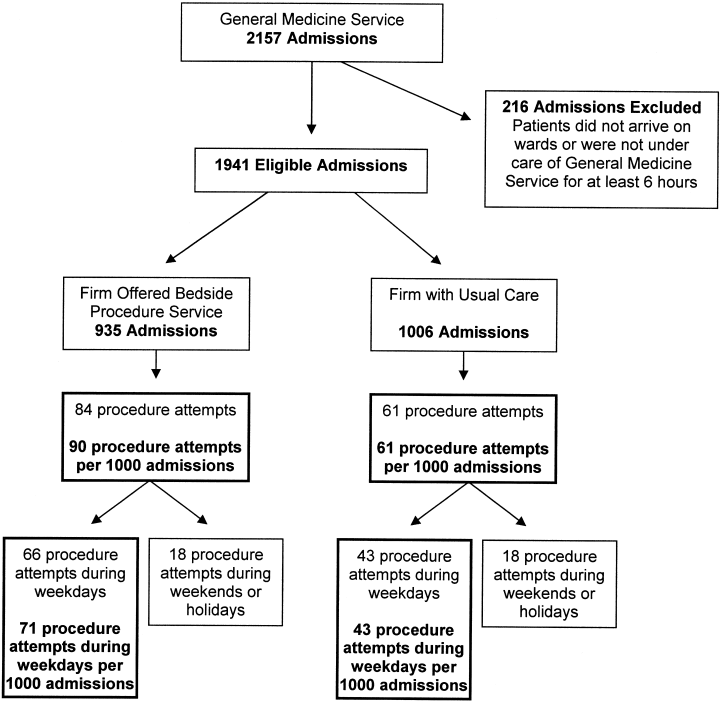
Primary OutcomeNumber of Procedure Attempts
Overall, 122 patients underwent 145 procedure attempts that met our criteria for independence. The mean number of procedure attempts in firms offered the bedside procedure service was 48% higher (90 versus 61 per 1000 admissions; RR 1.48, 95% CI 1.062.10; P = .030; Fig. 1). When procedures attempted on weekends and holidays were excluded, the relative increase in procedure attempts in firms offered the bedside procedure service was even higher (70 versus 43 per 1000 admissions; RR 1.63, 95% CI 1.092.49; P = .023; Fig. 1). When grouped according to whether procedure attempts occurred before or after crossover of the procedure service, the mean number of procedure attempts in firms was higher when the service was offered: firm A dropped from 84 to 70 per 1000 admissions (P = .58) after losing the service, whereas firms B and C increased from 57 to 94 per 1000 admissions (P = .025) on gaining the service. There were 40 procedure attempts performed on patients within 72 hours before admission, but there was no difference between firms in the proportions of these preadmission procedures (P = .43).
Secondary Outcomes
Table 1 shows how of each type of procedure contributed to the overall difference. Attempts of CVC and therapeutic paracentesis and thoracentesis accounted for 86% of the overall increase in procedure attempts for admissions to firms offered the bedside procedure service, whereas only 14% of this increase was a result of diagnostic procedures. There were no differences in the proportions of successfully performed procedures, whether grouped by firm (P = 1.0) or by direct supervision from the procedure service attending (P = .64; Table 2). There were 3 self‐reported major periprocedural complications; all were related to excessive bleeding from CVC attempts. Two occurred without direct supervision from the bedside procedure service attending, one hemomediastinum from an internal jugular CVC attempt and one groin hematoma from a femoral CVC attempt. The third, a groin hematoma from a femoral CVC attempt, occurred during direct supervision from the bedside procedure service attending.
| Bedside procedure and indication | Firms with bedside procedure service 935 admissions | Firms with usual care 1006 admissions | Absolute rate difference (proportion of overall difference)* |
|---|---|---|---|
| Total for entire study (total for weekend days and holidays) | |||
| |||
| Total | 90 (19) | 61 (18) | 29 (100%) |
| Thoracentesis | 30 (10) | 18 (7) | 12 (41%) |
| Diagnosis | 9 (5) | 6 (2) | 3 (9%) |
| Treatment | 21 (4) | 12 (5) | 9 (32%) |
| Paracentesis | 32 (5) | 25 (6) | 7 (25%) |
| Diagnosis | 9 (1) | 11 (3) | 2 (8%) |
| Treatment | 24 (4) | 14 (3) | 10 (33%) |
| Central venous catheterization | 17 (3) | 11 (4) | 6 (21%) |
| Lumbar puncture | 11 (1) | 7 (1) | 4 (13%) |
| Diagnosis | 10 (1) | 6 (1) | 4 (13%) |
| Treatment | 1 (0) | 1 (0) | 0 (0%) |
| Admission to firm with | P value of difference in proportions | ||||||
|---|---|---|---|---|---|---|---|
| Procedure service available | Usual care | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| |||||||
| Central venous catheterization | 16 | 13 | 81 | 11 | 9 | 82 | 1.00 |
| Paracentesis, thoracentesis, or lumbar puncture | 68 | 54 | 79 | 50 | 40 | 80 | 1.00 |
| Total | 84 | 67 | 80 | 61 | 49 | 80 | 1.00 |
| Procedure service attending | Pvalue of difference in proportions | ||||||
| Directly supervised | Did not directly supervise | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| Central venous catheterization | 10 | 10 | 100 | 17 | 12 | 71 | 0.28 |
| Paracentesis, thoracentesis, or lumbar puncture | 40 | 33 | 83 | 78 | 61 | 78 | 0.12 |
| Total | 50 | 43 | 86 | 95 | 73 | 77 | 0.64 |
DISCUSSION
We found that the mean number of bedside procedures increased by 48% (95% CI, 6% to 110%) from 61 to 90 per 1000 general medicine admissions when firms were offered a bedside procedure service. This suggests that a procedure service may lead to an increase in the number of procedures performed. For example, in our hospital, where 12,500 patients are admitted annually to the general medical service, 365 additional procedures per year (95% CI, 45840) may be performed if a procedure service is available. Despite this potential increase in demand, we were unable to demonstrate a parallel increase in bedside procedure success, even when the procedure service attending was directly supervising residents (Table 2). Though our conclusions may not be applicable to other settings, this study is, to our knowledge, the first to describe the demand for bedside procedures performed on general medicine inpatients at an urban teaching hospital and the first to demonstrate that this demand increases with the availability of a procedure service.
Because 86% of the observed increase in procedure attempts was due to therapeutic indications (Table 1), most of the observed difference may be due to undertreatment in the usual care cohort, overtreatment in the bedside procedure service cohort, or a combination of both. However, our study was not designed to determine if patients were undertreated because we did not review the appropriateness of physicians' decisions to not attempt procedures. And even though the bedside procedure service attending physician prospectively confirmed the appropriateness of each procedure attempt in that cohort, we did not examine what physicians' baseline treatment thresholds were or if they were lowered by the availability of the bedside procedure service.11 In other words, we cannot claim that the observed increase in procedure attempts was indicated based on patients' clinical factors. Nevertheless, the observed increase supports the important idea that discrete physician‐level decisions, in this case, whether to perform a bedside procedure, may be affected by broader system‐wide adoptions of new technologies like our bedside procedure service.12 Other nonclinical factors not observed in our study, such as fee‐for‐service compensation and variable physician‐level diagnostic and therapeutic thresholds, may also affect the rate of bedside procedures.
Our study had several limitations. We studied only one group of patients at one hospital: admissions to physicians in different settings may have different rates of bedside procedures. Our study design was observational. However, the predetermined sequential allocation of admissions and the varied assignments of the bedside procedure service during the study period should have limited selection bias. Our identification of procedure attempts, particularly in the usual care group, relied on resident physicians' self‐reports, and we cannot exclude a reporting bias. However, we believe that the daily interactions between investigators and residents from each team on the general medicine service limited the number of procedure attempts that went unrecorded. Finally, though sufficiently powered to determine our primary outcome, our study was underpowered to confirm statistical differences between firms in proportions of successfully performed procedures. For example, approximately 400 additional procedures (or more than 5000 additional admissions) would have been needed to sufficiently power the observed 9% increase in successful attempts that we observed with direct supervision by the procedure service attending (77% versus 86%; P = .64; Table 2). Our current sample size may be adequate in future research if success rates diverge as the experience of the procedure service attending increases. Though expert in performing bedside procedures, he had limited experience teaching them, particularly with the use of a hand‐carried ultrasound device. Just as there is a learning curve to gain the experience to successfully perform procedures,13 so may there be a learning curve to successfully teach procedures.14
Future research could address these limitations by more closely observing the decision‐making processes of physicians who order bedside procedures for general medicine inpatients in various settings. Our findings suggest that although patients' clinical circumstances are likely the most important consideration, nonclinical factors may also affect physicians' decisions.12 Like other multifaceted decision‐making processes of physicians,15 the complexity of this decision is important to examine because, as our pilot data suggest, a procedure service may not lead to more successful procedure attempts or reductions in the number of major complications. Although the cumulative expertise of our service or the innovative methods of training of other institutions may improve the performance of bedside procedures,5, 13 physicians' decisions about whether to order them will remain paramount, because any improvement in procedural competence will do little to reduce the relative danger of unnecessary procedures16 or the missed benefit of procedures left undone. Physicians of inpatients17, 18 should refine the indications for and anticipated benefits from these commonly performed invasive procedures.
- ,,, et al.The nature of adverse events in hospitalized patients: Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,, et al.Cost of medical injuries in Utah and Colorado.Inquiry.36;255–264.
- ,,,.Procedural Skills Training in Internal Medicine Residencies: A Survey of Program Directors.Ann Intern Med1989;111:932–38.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures.Am J Med.2006;119:71.e17–.e24.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Variation in the use of cardiac procedures after acute myocardial infarction.N Engl J Med.1995;333:573–578.
- ,,.Frequency and morbidity of inpatient procedures: report of a pilot study from two teaching hospitals.Arch Intern Med.1978;138:1809–1811.
- ,.The impact of diagnostic testing on therapeutic interventions.JAMA.1996;275:1189–1191.
- ,,, et al.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Coronary artery bypass graft surgery in Ontario and New York State: which rate is right?Ann Intern Med.1997;126:13–19.
- ,.Avoiding the unintended consequences of growth in medical care. How might more be worse?JAMA.1999;281:446–453.
- ,,.Professional uncertainty and the problem of supplier‐induced demand.Soc Sci Med.1982;811–824.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,, et al.Confidence of Academic General Internists and Family Physicians to Teach Ambulatory Procedures.J Gen Intern Med.2000;15:353–360.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19:402–409.
- .Medical care—is more always better?N Engl J Med.2003;349:1665–1667.
- ,.Point/counterpoint: should hospital medicine become a distinct specialty?Hospitalist.2005;9(1):15–19.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hospital Med.2006;1:S1–S95.
Inpatient bedside procedures are a major source of preventable adverse events in hospitals.1, 2 Unfortunately, many future inpatient physicians may lack the training3 and confidence4 to correct this problem. One proposed model for improving the teaching, performance, and evaluation of bedside procedures is a procedure service that is staffed by faculty who are experts at inpatient procedures.5 In a recent survey of internal medicine residents from our hospital, 86% (30 of 35) believed that expert supervision would improve central venous catheterization technique (Trick WE, personal communication).
Primary considerations in the development of a procedure service are the baseline demand for bedside procedures and whether a procedure service may affect this demand. Though variations in population‐based rates of some hospital procedures have been described,6, 7 there is little written on the demand for procedures performed at the bedsides of inpatients. Concomitant increases in demand and availability of other technologies810 suggest that improving the availability of bedside procedures may lead to an increase in their demand, regardless of whether such an increase benefits patients.11
Therefore, we sought to determine the impact of a bedside procedure service on the baseline number of paracenteses, thoracenteses, lumbar punctures (LPs), and central venous catheterizations (CVCs) performed on general medicine inpatients at our teaching hospital. In addition, we examined whether this service leads to more successful and safe procedure attempts.
METHODS
Design and Setting
In this prospective cohort study, the cohort was all patients admitted to the general medicine service at Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, in January and February of 2006. The general medicine inpatient service is divided into 3 firms (A, B, and C), each with 4 separate teams of physicians and students. Admissions from the emergency department or other services in the hospital, such as intensive care units (which are closed and therefore staffed by separate teams of physicians), are distributed in sequence to on‐call teams from each firm. During the study period, the availability of a bedside procedure service varied by firm. Throughout the first 4 weeks, the service was available to only 1 of 3 firms (firm A). Then, during weeks 5 through 8, the service crossed over to the other 2 firms (firms B and C) and was unavailable to the original firm. Firm assignments for residents assigned to the inpatient service for all 8 weeks did not change. Of the 16 residents assigned to firm A during the first 4 weeks, when the procedure service was available, 3 remained on the wards during the second 4 weeks, when the procedure service was not available.
We chose to collect data on 4 bedside procedures: paracentesis, thoracentesis, LP, and CVC. Similar to those at other teaching hospitals, our residents informally acquire the skills to perform these procedures while assisting and being assisted by more experienced senior residents in a see one, do one, teach one apprenticeship model of learning.4 To improve the training and performance of these bedside procedures, the Department of Medicine piloted a bedside procedure service to teach procedural skills and assist residents during these procedures. Use of the service, though voluntary, was actively encouraged at residents' monthly orientation meetings and regular conferences.
One attending inpatient physician (J.A.) staffed the bedside procedure service, which was available during normal work hours on weekdays. Requests for procedures were made by general medicine residents through an online database and, after approval by the procedure service attending physician, were performed under his direct supervision. A hand‐carried ultrasound (MicroMaxx, Sonosite, Inc., Bothell, WA) that generates a 2‐dimensional gray‐scale image was used to both confirm the presence and location of fluid prior to paracentesis and thoracentesis and provide real‐time guidance during central venous catheterization. When the bedside procedure service was unavailable, residents performed bedside procedures in the usual fashion, typically without direct attending physician supervision. But if requested, an on‐call chief medical resident with access to a hand‐carried ultrasound device used by the intensive care unit was available for assistance at any time.
Subjects
The study subjects were all patients admitted to the general medical service during the 8‐week pilot period. Patients were excluded if they had been discharged before arrival on the medical wards or if they were under the care of the general medicine service for less than 6 hours before discharge or transfer to another service. We chose 6 hours because we reasoned that such brief admissions were not potential candidates for invasive bedside procedures.
Data Collection
Each morning an investigator contacted the senior residents who had admitted patients during the previous 24‐hour shift and confirmed that newly admitted patients were under the care of the general medicine service for more than 6 hours. To examine how the number of attempts may have been affected by procedures done in the emergency room or intensive care units before admission to the general medicine service, investigators also asked admitting residents whether a bedside procedure had been attempted in the 72 hours before admission. Every general medicine service resident was asked to fill out a brief data collection form after an attempt to perform any procedure on the general medical wards. In addition, chief residents asked each member of the general medicine service at mandatory sign‐out rounds at the end of each weekday whether any procedures had been attempted, and on weekend days investigators contacted senior residents from each general medicine service team.
We report on this quality assurance study, which was conducted during a pilot phase. This report has been reviewed and judged exempt by our institutional review board.
Primary OutcomeNumber of Procedure Attempts
For all bedside procedures attempted by residents on the general medical wards, investigators determined whether the residents were members of firms that were offered the bedside procedure service and, if so, whether the procedure service attending directly supervised the procedure attempt. Multiple procedure attempts of the same type were counted for an individual patient if (1) the procedure attempts did not occur during the same admissions and (2) neither the physicians attempting the procedure nor the primary indications for it were the same. Therefore, neither attempts performed after initially unsuccessful ones nor repeated procedures, such as large‐volume therapeutic paracentesis and thoracentesis, were counted twice. We reasoned that when these criteria were met, procedure attempts could be considered independently.
Secondary Outcomes
Investigators asked residents who attempted procedures to indicate whether (1) the indication for the procedure was solely diagnostic or was, at least in part, therapeutic; (2) the procedure was successful; and (3) there were any immediate major periprocedural complications. A procedure was considered to have been successfully performed if it fulfilled 2 criteria: it had to be completed during a single continuous attempt, even if multiple sites or procedure kits were used; and it had to fulfill the indication for it being done. For example, if the indication for thoracentesis was therapeutic, this procedure would be considered successful if it yielded a large enough volume of fluid to alleviate the patient's symptoms, but if the indication was diagnostic, then thoracentesis would be considered successful if it yielded enough fluid for laboratory processing. Residents were asked to report any periprocedural complications that they considered major; 2 illustrative examples were provided: a pneumothorax and severe bleeding.
Data Analyses
On the basis of earlier pilot data, we estimated that 8%10% of all admissions to the general medicine service underwent at least 1 procedure (paracentesis, thoracentesis, lumbar puncture, or central vein catheterization). We planned for a sample size of 1900 admissions, which would have 80% power to detect a clinically meaningful 50% relative increase in the mean number of bedside procedures with a double‐sided alpha error of 0.05. We used permutation tests to compare the mean number of procedures attempted between firms and bootstrap simulation to construct 95% confidence intervals for those means and the differences between and ratios of them. Fisher's exact test was used to compare proportions of successfully performed procedures and preadmission procedure attempts. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, LP, College Station, TX).
RESULTS
Subjects
During this 8‐week pilot study, there were 2157 admissions to the general medicine service. Among these admissions, 216 were excluded from our study because the patients did not arrive on the medical wards or were not under the care of the general medicine service for at least 6 hours before discharge or before being transferred to another service. Of the remaining 1941 admissions, 935 were to firms with the bedside procedure service available, and 1006 were to firms without the service available (Fig. 1)

Primary OutcomeNumber of Procedure Attempts
Overall, 122 patients underwent 145 procedure attempts that met our criteria for independence. The mean number of procedure attempts in firms offered the bedside procedure service was 48% higher (90 versus 61 per 1000 admissions; RR 1.48, 95% CI 1.062.10; P = .030; Fig. 1). When procedures attempted on weekends and holidays were excluded, the relative increase in procedure attempts in firms offered the bedside procedure service was even higher (70 versus 43 per 1000 admissions; RR 1.63, 95% CI 1.092.49; P = .023; Fig. 1). When grouped according to whether procedure attempts occurred before or after crossover of the procedure service, the mean number of procedure attempts in firms was higher when the service was offered: firm A dropped from 84 to 70 per 1000 admissions (P = .58) after losing the service, whereas firms B and C increased from 57 to 94 per 1000 admissions (P = .025) on gaining the service. There were 40 procedure attempts performed on patients within 72 hours before admission, but there was no difference between firms in the proportions of these preadmission procedures (P = .43).
Secondary Outcomes
Table 1 shows how of each type of procedure contributed to the overall difference. Attempts of CVC and therapeutic paracentesis and thoracentesis accounted for 86% of the overall increase in procedure attempts for admissions to firms offered the bedside procedure service, whereas only 14% of this increase was a result of diagnostic procedures. There were no differences in the proportions of successfully performed procedures, whether grouped by firm (P = 1.0) or by direct supervision from the procedure service attending (P = .64; Table 2). There were 3 self‐reported major periprocedural complications; all were related to excessive bleeding from CVC attempts. Two occurred without direct supervision from the bedside procedure service attending, one hemomediastinum from an internal jugular CVC attempt and one groin hematoma from a femoral CVC attempt. The third, a groin hematoma from a femoral CVC attempt, occurred during direct supervision from the bedside procedure service attending.
| Bedside procedure and indication | Firms with bedside procedure service 935 admissions | Firms with usual care 1006 admissions | Absolute rate difference (proportion of overall difference)* |
|---|---|---|---|
| Total for entire study (total for weekend days and holidays) | |||
| |||
| Total | 90 (19) | 61 (18) | 29 (100%) |
| Thoracentesis | 30 (10) | 18 (7) | 12 (41%) |
| Diagnosis | 9 (5) | 6 (2) | 3 (9%) |
| Treatment | 21 (4) | 12 (5) | 9 (32%) |
| Paracentesis | 32 (5) | 25 (6) | 7 (25%) |
| Diagnosis | 9 (1) | 11 (3) | 2 (8%) |
| Treatment | 24 (4) | 14 (3) | 10 (33%) |
| Central venous catheterization | 17 (3) | 11 (4) | 6 (21%) |
| Lumbar puncture | 11 (1) | 7 (1) | 4 (13%) |
| Diagnosis | 10 (1) | 6 (1) | 4 (13%) |
| Treatment | 1 (0) | 1 (0) | 0 (0%) |
| Admission to firm with | P value of difference in proportions | ||||||
|---|---|---|---|---|---|---|---|
| Procedure service available | Usual care | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| |||||||
| Central venous catheterization | 16 | 13 | 81 | 11 | 9 | 82 | 1.00 |
| Paracentesis, thoracentesis, or lumbar puncture | 68 | 54 | 79 | 50 | 40 | 80 | 1.00 |
| Total | 84 | 67 | 80 | 61 | 49 | 80 | 1.00 |
| Procedure service attending | Pvalue of difference in proportions | ||||||
| Directly supervised | Did not directly supervise | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| Central venous catheterization | 10 | 10 | 100 | 17 | 12 | 71 | 0.28 |
| Paracentesis, thoracentesis, or lumbar puncture | 40 | 33 | 83 | 78 | 61 | 78 | 0.12 |
| Total | 50 | 43 | 86 | 95 | 73 | 77 | 0.64 |
DISCUSSION
We found that the mean number of bedside procedures increased by 48% (95% CI, 6% to 110%) from 61 to 90 per 1000 general medicine admissions when firms were offered a bedside procedure service. This suggests that a procedure service may lead to an increase in the number of procedures performed. For example, in our hospital, where 12,500 patients are admitted annually to the general medical service, 365 additional procedures per year (95% CI, 45840) may be performed if a procedure service is available. Despite this potential increase in demand, we were unable to demonstrate a parallel increase in bedside procedure success, even when the procedure service attending was directly supervising residents (Table 2). Though our conclusions may not be applicable to other settings, this study is, to our knowledge, the first to describe the demand for bedside procedures performed on general medicine inpatients at an urban teaching hospital and the first to demonstrate that this demand increases with the availability of a procedure service.
Because 86% of the observed increase in procedure attempts was due to therapeutic indications (Table 1), most of the observed difference may be due to undertreatment in the usual care cohort, overtreatment in the bedside procedure service cohort, or a combination of both. However, our study was not designed to determine if patients were undertreated because we did not review the appropriateness of physicians' decisions to not attempt procedures. And even though the bedside procedure service attending physician prospectively confirmed the appropriateness of each procedure attempt in that cohort, we did not examine what physicians' baseline treatment thresholds were or if they were lowered by the availability of the bedside procedure service.11 In other words, we cannot claim that the observed increase in procedure attempts was indicated based on patients' clinical factors. Nevertheless, the observed increase supports the important idea that discrete physician‐level decisions, in this case, whether to perform a bedside procedure, may be affected by broader system‐wide adoptions of new technologies like our bedside procedure service.12 Other nonclinical factors not observed in our study, such as fee‐for‐service compensation and variable physician‐level diagnostic and therapeutic thresholds, may also affect the rate of bedside procedures.
Our study had several limitations. We studied only one group of patients at one hospital: admissions to physicians in different settings may have different rates of bedside procedures. Our study design was observational. However, the predetermined sequential allocation of admissions and the varied assignments of the bedside procedure service during the study period should have limited selection bias. Our identification of procedure attempts, particularly in the usual care group, relied on resident physicians' self‐reports, and we cannot exclude a reporting bias. However, we believe that the daily interactions between investigators and residents from each team on the general medicine service limited the number of procedure attempts that went unrecorded. Finally, though sufficiently powered to determine our primary outcome, our study was underpowered to confirm statistical differences between firms in proportions of successfully performed procedures. For example, approximately 400 additional procedures (or more than 5000 additional admissions) would have been needed to sufficiently power the observed 9% increase in successful attempts that we observed with direct supervision by the procedure service attending (77% versus 86%; P = .64; Table 2). Our current sample size may be adequate in future research if success rates diverge as the experience of the procedure service attending increases. Though expert in performing bedside procedures, he had limited experience teaching them, particularly with the use of a hand‐carried ultrasound device. Just as there is a learning curve to gain the experience to successfully perform procedures,13 so may there be a learning curve to successfully teach procedures.14
Future research could address these limitations by more closely observing the decision‐making processes of physicians who order bedside procedures for general medicine inpatients in various settings. Our findings suggest that although patients' clinical circumstances are likely the most important consideration, nonclinical factors may also affect physicians' decisions.12 Like other multifaceted decision‐making processes of physicians,15 the complexity of this decision is important to examine because, as our pilot data suggest, a procedure service may not lead to more successful procedure attempts or reductions in the number of major complications. Although the cumulative expertise of our service or the innovative methods of training of other institutions may improve the performance of bedside procedures,5, 13 physicians' decisions about whether to order them will remain paramount, because any improvement in procedural competence will do little to reduce the relative danger of unnecessary procedures16 or the missed benefit of procedures left undone. Physicians of inpatients17, 18 should refine the indications for and anticipated benefits from these commonly performed invasive procedures.
Inpatient bedside procedures are a major source of preventable adverse events in hospitals.1, 2 Unfortunately, many future inpatient physicians may lack the training3 and confidence4 to correct this problem. One proposed model for improving the teaching, performance, and evaluation of bedside procedures is a procedure service that is staffed by faculty who are experts at inpatient procedures.5 In a recent survey of internal medicine residents from our hospital, 86% (30 of 35) believed that expert supervision would improve central venous catheterization technique (Trick WE, personal communication).
Primary considerations in the development of a procedure service are the baseline demand for bedside procedures and whether a procedure service may affect this demand. Though variations in population‐based rates of some hospital procedures have been described,6, 7 there is little written on the demand for procedures performed at the bedsides of inpatients. Concomitant increases in demand and availability of other technologies810 suggest that improving the availability of bedside procedures may lead to an increase in their demand, regardless of whether such an increase benefits patients.11
Therefore, we sought to determine the impact of a bedside procedure service on the baseline number of paracenteses, thoracenteses, lumbar punctures (LPs), and central venous catheterizations (CVCs) performed on general medicine inpatients at our teaching hospital. In addition, we examined whether this service leads to more successful and safe procedure attempts.
METHODS
Design and Setting
In this prospective cohort study, the cohort was all patients admitted to the general medicine service at Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, in January and February of 2006. The general medicine inpatient service is divided into 3 firms (A, B, and C), each with 4 separate teams of physicians and students. Admissions from the emergency department or other services in the hospital, such as intensive care units (which are closed and therefore staffed by separate teams of physicians), are distributed in sequence to on‐call teams from each firm. During the study period, the availability of a bedside procedure service varied by firm. Throughout the first 4 weeks, the service was available to only 1 of 3 firms (firm A). Then, during weeks 5 through 8, the service crossed over to the other 2 firms (firms B and C) and was unavailable to the original firm. Firm assignments for residents assigned to the inpatient service for all 8 weeks did not change. Of the 16 residents assigned to firm A during the first 4 weeks, when the procedure service was available, 3 remained on the wards during the second 4 weeks, when the procedure service was not available.
We chose to collect data on 4 bedside procedures: paracentesis, thoracentesis, LP, and CVC. Similar to those at other teaching hospitals, our residents informally acquire the skills to perform these procedures while assisting and being assisted by more experienced senior residents in a see one, do one, teach one apprenticeship model of learning.4 To improve the training and performance of these bedside procedures, the Department of Medicine piloted a bedside procedure service to teach procedural skills and assist residents during these procedures. Use of the service, though voluntary, was actively encouraged at residents' monthly orientation meetings and regular conferences.
One attending inpatient physician (J.A.) staffed the bedside procedure service, which was available during normal work hours on weekdays. Requests for procedures were made by general medicine residents through an online database and, after approval by the procedure service attending physician, were performed under his direct supervision. A hand‐carried ultrasound (MicroMaxx, Sonosite, Inc., Bothell, WA) that generates a 2‐dimensional gray‐scale image was used to both confirm the presence and location of fluid prior to paracentesis and thoracentesis and provide real‐time guidance during central venous catheterization. When the bedside procedure service was unavailable, residents performed bedside procedures in the usual fashion, typically without direct attending physician supervision. But if requested, an on‐call chief medical resident with access to a hand‐carried ultrasound device used by the intensive care unit was available for assistance at any time.
Subjects
The study subjects were all patients admitted to the general medical service during the 8‐week pilot period. Patients were excluded if they had been discharged before arrival on the medical wards or if they were under the care of the general medicine service for less than 6 hours before discharge or transfer to another service. We chose 6 hours because we reasoned that such brief admissions were not potential candidates for invasive bedside procedures.
Data Collection
Each morning an investigator contacted the senior residents who had admitted patients during the previous 24‐hour shift and confirmed that newly admitted patients were under the care of the general medicine service for more than 6 hours. To examine how the number of attempts may have been affected by procedures done in the emergency room or intensive care units before admission to the general medicine service, investigators also asked admitting residents whether a bedside procedure had been attempted in the 72 hours before admission. Every general medicine service resident was asked to fill out a brief data collection form after an attempt to perform any procedure on the general medical wards. In addition, chief residents asked each member of the general medicine service at mandatory sign‐out rounds at the end of each weekday whether any procedures had been attempted, and on weekend days investigators contacted senior residents from each general medicine service team.
We report on this quality assurance study, which was conducted during a pilot phase. This report has been reviewed and judged exempt by our institutional review board.
Primary OutcomeNumber of Procedure Attempts
For all bedside procedures attempted by residents on the general medical wards, investigators determined whether the residents were members of firms that were offered the bedside procedure service and, if so, whether the procedure service attending directly supervised the procedure attempt. Multiple procedure attempts of the same type were counted for an individual patient if (1) the procedure attempts did not occur during the same admissions and (2) neither the physicians attempting the procedure nor the primary indications for it were the same. Therefore, neither attempts performed after initially unsuccessful ones nor repeated procedures, such as large‐volume therapeutic paracentesis and thoracentesis, were counted twice. We reasoned that when these criteria were met, procedure attempts could be considered independently.
Secondary Outcomes
Investigators asked residents who attempted procedures to indicate whether (1) the indication for the procedure was solely diagnostic or was, at least in part, therapeutic; (2) the procedure was successful; and (3) there were any immediate major periprocedural complications. A procedure was considered to have been successfully performed if it fulfilled 2 criteria: it had to be completed during a single continuous attempt, even if multiple sites or procedure kits were used; and it had to fulfill the indication for it being done. For example, if the indication for thoracentesis was therapeutic, this procedure would be considered successful if it yielded a large enough volume of fluid to alleviate the patient's symptoms, but if the indication was diagnostic, then thoracentesis would be considered successful if it yielded enough fluid for laboratory processing. Residents were asked to report any periprocedural complications that they considered major; 2 illustrative examples were provided: a pneumothorax and severe bleeding.
Data Analyses
On the basis of earlier pilot data, we estimated that 8%10% of all admissions to the general medicine service underwent at least 1 procedure (paracentesis, thoracentesis, lumbar puncture, or central vein catheterization). We planned for a sample size of 1900 admissions, which would have 80% power to detect a clinically meaningful 50% relative increase in the mean number of bedside procedures with a double‐sided alpha error of 0.05. We used permutation tests to compare the mean number of procedures attempted between firms and bootstrap simulation to construct 95% confidence intervals for those means and the differences between and ratios of them. Fisher's exact test was used to compare proportions of successfully performed procedures and preadmission procedure attempts. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, LP, College Station, TX).
RESULTS
Subjects
During this 8‐week pilot study, there were 2157 admissions to the general medicine service. Among these admissions, 216 were excluded from our study because the patients did not arrive on the medical wards or were not under the care of the general medicine service for at least 6 hours before discharge or before being transferred to another service. Of the remaining 1941 admissions, 935 were to firms with the bedside procedure service available, and 1006 were to firms without the service available (Fig. 1)

Primary OutcomeNumber of Procedure Attempts
Overall, 122 patients underwent 145 procedure attempts that met our criteria for independence. The mean number of procedure attempts in firms offered the bedside procedure service was 48% higher (90 versus 61 per 1000 admissions; RR 1.48, 95% CI 1.062.10; P = .030; Fig. 1). When procedures attempted on weekends and holidays were excluded, the relative increase in procedure attempts in firms offered the bedside procedure service was even higher (70 versus 43 per 1000 admissions; RR 1.63, 95% CI 1.092.49; P = .023; Fig. 1). When grouped according to whether procedure attempts occurred before or after crossover of the procedure service, the mean number of procedure attempts in firms was higher when the service was offered: firm A dropped from 84 to 70 per 1000 admissions (P = .58) after losing the service, whereas firms B and C increased from 57 to 94 per 1000 admissions (P = .025) on gaining the service. There were 40 procedure attempts performed on patients within 72 hours before admission, but there was no difference between firms in the proportions of these preadmission procedures (P = .43).
Secondary Outcomes
Table 1 shows how of each type of procedure contributed to the overall difference. Attempts of CVC and therapeutic paracentesis and thoracentesis accounted for 86% of the overall increase in procedure attempts for admissions to firms offered the bedside procedure service, whereas only 14% of this increase was a result of diagnostic procedures. There were no differences in the proportions of successfully performed procedures, whether grouped by firm (P = 1.0) or by direct supervision from the procedure service attending (P = .64; Table 2). There were 3 self‐reported major periprocedural complications; all were related to excessive bleeding from CVC attempts. Two occurred without direct supervision from the bedside procedure service attending, one hemomediastinum from an internal jugular CVC attempt and one groin hematoma from a femoral CVC attempt. The third, a groin hematoma from a femoral CVC attempt, occurred during direct supervision from the bedside procedure service attending.
| Bedside procedure and indication | Firms with bedside procedure service 935 admissions | Firms with usual care 1006 admissions | Absolute rate difference (proportion of overall difference)* |
|---|---|---|---|
| Total for entire study (total for weekend days and holidays) | |||
| |||
| Total | 90 (19) | 61 (18) | 29 (100%) |
| Thoracentesis | 30 (10) | 18 (7) | 12 (41%) |
| Diagnosis | 9 (5) | 6 (2) | 3 (9%) |
| Treatment | 21 (4) | 12 (5) | 9 (32%) |
| Paracentesis | 32 (5) | 25 (6) | 7 (25%) |
| Diagnosis | 9 (1) | 11 (3) | 2 (8%) |
| Treatment | 24 (4) | 14 (3) | 10 (33%) |
| Central venous catheterization | 17 (3) | 11 (4) | 6 (21%) |
| Lumbar puncture | 11 (1) | 7 (1) | 4 (13%) |
| Diagnosis | 10 (1) | 6 (1) | 4 (13%) |
| Treatment | 1 (0) | 1 (0) | 0 (0%) |
| Admission to firm with | P value of difference in proportions | ||||||
|---|---|---|---|---|---|---|---|
| Procedure service available | Usual care | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| |||||||
| Central venous catheterization | 16 | 13 | 81 | 11 | 9 | 82 | 1.00 |
| Paracentesis, thoracentesis, or lumbar puncture | 68 | 54 | 79 | 50 | 40 | 80 | 1.00 |
| Total | 84 | 67 | 80 | 61 | 49 | 80 | 1.00 |
| Procedure service attending | Pvalue of difference in proportions | ||||||
| Directly supervised | Did not directly supervise | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| Central venous catheterization | 10 | 10 | 100 | 17 | 12 | 71 | 0.28 |
| Paracentesis, thoracentesis, or lumbar puncture | 40 | 33 | 83 | 78 | 61 | 78 | 0.12 |
| Total | 50 | 43 | 86 | 95 | 73 | 77 | 0.64 |
DISCUSSION
We found that the mean number of bedside procedures increased by 48% (95% CI, 6% to 110%) from 61 to 90 per 1000 general medicine admissions when firms were offered a bedside procedure service. This suggests that a procedure service may lead to an increase in the number of procedures performed. For example, in our hospital, where 12,500 patients are admitted annually to the general medical service, 365 additional procedures per year (95% CI, 45840) may be performed if a procedure service is available. Despite this potential increase in demand, we were unable to demonstrate a parallel increase in bedside procedure success, even when the procedure service attending was directly supervising residents (Table 2). Though our conclusions may not be applicable to other settings, this study is, to our knowledge, the first to describe the demand for bedside procedures performed on general medicine inpatients at an urban teaching hospital and the first to demonstrate that this demand increases with the availability of a procedure service.
Because 86% of the observed increase in procedure attempts was due to therapeutic indications (Table 1), most of the observed difference may be due to undertreatment in the usual care cohort, overtreatment in the bedside procedure service cohort, or a combination of both. However, our study was not designed to determine if patients were undertreated because we did not review the appropriateness of physicians' decisions to not attempt procedures. And even though the bedside procedure service attending physician prospectively confirmed the appropriateness of each procedure attempt in that cohort, we did not examine what physicians' baseline treatment thresholds were or if they were lowered by the availability of the bedside procedure service.11 In other words, we cannot claim that the observed increase in procedure attempts was indicated based on patients' clinical factors. Nevertheless, the observed increase supports the important idea that discrete physician‐level decisions, in this case, whether to perform a bedside procedure, may be affected by broader system‐wide adoptions of new technologies like our bedside procedure service.12 Other nonclinical factors not observed in our study, such as fee‐for‐service compensation and variable physician‐level diagnostic and therapeutic thresholds, may also affect the rate of bedside procedures.
Our study had several limitations. We studied only one group of patients at one hospital: admissions to physicians in different settings may have different rates of bedside procedures. Our study design was observational. However, the predetermined sequential allocation of admissions and the varied assignments of the bedside procedure service during the study period should have limited selection bias. Our identification of procedure attempts, particularly in the usual care group, relied on resident physicians' self‐reports, and we cannot exclude a reporting bias. However, we believe that the daily interactions between investigators and residents from each team on the general medicine service limited the number of procedure attempts that went unrecorded. Finally, though sufficiently powered to determine our primary outcome, our study was underpowered to confirm statistical differences between firms in proportions of successfully performed procedures. For example, approximately 400 additional procedures (or more than 5000 additional admissions) would have been needed to sufficiently power the observed 9% increase in successful attempts that we observed with direct supervision by the procedure service attending (77% versus 86%; P = .64; Table 2). Our current sample size may be adequate in future research if success rates diverge as the experience of the procedure service attending increases. Though expert in performing bedside procedures, he had limited experience teaching them, particularly with the use of a hand‐carried ultrasound device. Just as there is a learning curve to gain the experience to successfully perform procedures,13 so may there be a learning curve to successfully teach procedures.14
Future research could address these limitations by more closely observing the decision‐making processes of physicians who order bedside procedures for general medicine inpatients in various settings. Our findings suggest that although patients' clinical circumstances are likely the most important consideration, nonclinical factors may also affect physicians' decisions.12 Like other multifaceted decision‐making processes of physicians,15 the complexity of this decision is important to examine because, as our pilot data suggest, a procedure service may not lead to more successful procedure attempts or reductions in the number of major complications. Although the cumulative expertise of our service or the innovative methods of training of other institutions may improve the performance of bedside procedures,5, 13 physicians' decisions about whether to order them will remain paramount, because any improvement in procedural competence will do little to reduce the relative danger of unnecessary procedures16 or the missed benefit of procedures left undone. Physicians of inpatients17, 18 should refine the indications for and anticipated benefits from these commonly performed invasive procedures.
- ,,, et al.The nature of adverse events in hospitalized patients: Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,, et al.Cost of medical injuries in Utah and Colorado.Inquiry.36;255–264.
- ,,,.Procedural Skills Training in Internal Medicine Residencies: A Survey of Program Directors.Ann Intern Med1989;111:932–38.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures.Am J Med.2006;119:71.e17–.e24.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Variation in the use of cardiac procedures after acute myocardial infarction.N Engl J Med.1995;333:573–578.
- ,,.Frequency and morbidity of inpatient procedures: report of a pilot study from two teaching hospitals.Arch Intern Med.1978;138:1809–1811.
- ,.The impact of diagnostic testing on therapeutic interventions.JAMA.1996;275:1189–1191.
- ,,, et al.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Coronary artery bypass graft surgery in Ontario and New York State: which rate is right?Ann Intern Med.1997;126:13–19.
- ,.Avoiding the unintended consequences of growth in medical care. How might more be worse?JAMA.1999;281:446–453.
- ,,.Professional uncertainty and the problem of supplier‐induced demand.Soc Sci Med.1982;811–824.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,, et al.Confidence of Academic General Internists and Family Physicians to Teach Ambulatory Procedures.J Gen Intern Med.2000;15:353–360.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19:402–409.
- .Medical care—is more always better?N Engl J Med.2003;349:1665–1667.
- ,.Point/counterpoint: should hospital medicine become a distinct specialty?Hospitalist.2005;9(1):15–19.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hospital Med.2006;1:S1–S95.
- ,,, et al.The nature of adverse events in hospitalized patients: Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,, et al.Cost of medical injuries in Utah and Colorado.Inquiry.36;255–264.
- ,,,.Procedural Skills Training in Internal Medicine Residencies: A Survey of Program Directors.Ann Intern Med1989;111:932–38.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures.Am J Med.2006;119:71.e17–.e24.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Variation in the use of cardiac procedures after acute myocardial infarction.N Engl J Med.1995;333:573–578.
- ,,.Frequency and morbidity of inpatient procedures: report of a pilot study from two teaching hospitals.Arch Intern Med.1978;138:1809–1811.
- ,.The impact of diagnostic testing on therapeutic interventions.JAMA.1996;275:1189–1191.
- ,,, et al.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Coronary artery bypass graft surgery in Ontario and New York State: which rate is right?Ann Intern Med.1997;126:13–19.
- ,.Avoiding the unintended consequences of growth in medical care. How might more be worse?JAMA.1999;281:446–453.
- ,,.Professional uncertainty and the problem of supplier‐induced demand.Soc Sci Med.1982;811–824.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,, et al.Confidence of Academic General Internists and Family Physicians to Teach Ambulatory Procedures.J Gen Intern Med.2000;15:353–360.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19:402–409.
- .Medical care—is more always better?N Engl J Med.2003;349:1665–1667.
- ,.Point/counterpoint: should hospital medicine become a distinct specialty?Hospitalist.2005;9(1):15–19.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hospital Med.2006;1:S1–S95.
Copyright © 2007 Society of Hospital Medicine
NICU‐Based Influenza Vaccine
Influenza is a common infectious agent in the pediatric population, infecting 15% to 42% of preschool children, with a fatality rate of 3.8 per 100,000.13 Those with underlying respiratory and cardiac disease are more likely to require hospitalization and more susceptible to morbidity from the disease.47 Trivalent inactivated influenza vaccine is a safe, cost‐effective method of preventing influenza in children, with a seroconversion rate of up to 89%.810 Both the American Academy of Pediatrics and the Advisory Committee on Immunization Practices recommend that the influenza vaccine be administered to household contacts and out‐of‐home caretakers of infants up to 6 months of age.11 Also included in this high‐risk category are children with chronic respiratory and cardiac disease.8
The immunization rate in the indicated pediatric population ranges from 9% to 22%.12 Because most adults who meet eligible criteria are not vaccinated, it has been proposed that the NICU begin to administer the influenza vaccine to parents of high‐risk infants, eliminating commonly encountered obstacles to vaccine administration and preventing infection in these close contacts of infants, who likely serve as infectious agents of disease in the infants.13, 14
Yet the cost of instituting such a program remains a concern, especially given the recent shortages of the inactivated influenza vaccine, which have increased cost.15 The economic implications of instituting an inactivated influenza vaccination program for parents of patients in the NICU have not been fully evaluated. Given that upwards of 40,000 premature infants are admitted to intensive care units each year, an examination of cost savings is critical prior to implementing such a program.16
METHODS
Data and Assumptions
A 3‐ and 4‐tiered computer model (with the tiers reflecting the variables presence of lung disease, having siblings, and sibling immunization status and the fourth tier reflecting parental immunization in the NICU as a function of the immunization program) assessing influenza vaccination status of parents of a cohort of 2632 patients admitted to the New York Regional Perinatal Center NICU during the influenza season of 2003‐2004 was constructed using the viewpoint of a large multinetwork medical center predominantly serving a lower socioeconomic status population. The likelihood of influenza infection of an infant, the need for infant hospitalization, subsequent length of stay, and the need for the patient to have outpatient physician visits were based on the following clinical variables: lung disease in the infant (defined as a 28‐day‐old patient whose birth weight was less than 1500 g being oxygen dependent); having school‐age siblings, sibling vaccination status; parental vaccination status; and parental compliance. Variables of the model were based on published results when possible. For the purposes of this model, we assumed a 10% reduction in influenza infectivity for parents of children who were immunized in the absence of other confounders based on the risk of needing medical attention of children less than 6 months old for documented influenza with parental vaccination in the 9 states that make up the Emerging Infections Program Network of the Centers for Disease Control.1719 Infected patients younger than 6 months of age were also programmed to have a 10% chance of an outpatient hospitalization visit. No deaths were introduced in the cohort. An outline of the different groups into which patients were classified (before and after the influenza vaccination campaign) is outlined in Figure 1.
Direct Costs
Medical Costs
The average wholesale cost of a dose of influenza vaccine including administration was $15.20 Each parent received 1 dose of influenza vaccine administered during the influenza season (the 5 months from October thru February). In our model, the vaccine is administered by nurses, physicians, or physician‐extenders in a neonatal intensive care unit and thus does not require increased personnel to support the program. Hence, no increased costs were included for administration of the vaccine.
Siblings were not offered immunization in the NICU program. Most NICUs do not allow children younger than 13 years to visit during influenza and respiratory syncytial virus season to prevent infection of newborns. Immunization of younger siblings requires prior knowledge of their vaccination status, as those previously immunized require 1 dose of vaccine, whereas those less than 9 years old not immunized require 2 doses scheduled 1 month apart. As this was considered logistically difficult for a high‐acuity NICU, sibling immunization was deferred to that sibling's primary medical doctor, a policy consistent with that of the American Academy of Pediatrics Medical Home Initiative.
Infant Hospitalization for Influenza
Cost estimates were obtained from published data on the length of stay of infants with respiratory disease.21 In this series the average length of stay of former NICU patients with low socioeconomic status hospitalized for influenza was 4.5 4 days. Average hospital costs were estimated as $1508/day.22, 23 No intensive care unit days were factored into the current cost model. Hospitalization costs for each group were estimated by (number of infants in each group from the New York Regional Perinatal Center 2004 Database hospitalization rate of each group number of days hospitalized $1508/day). This estimation technique was previously validated and used.21
Intensive care unit cost was estimated as 2.5 times the cost of nonintensive care ($3770/day). Intensive care hospitalization for influenza is difficult to measure, as it correlates with bacterial superinfection, which has an incidence of 0.5/10,000 patients with documented influenza.5 However, in another study, ICU hospitalization of infected patients was 0.5%, which would translate to 13 patients in the studied group.18 The length of ICU stay was 1 day, and by univariate analysis, bacterial coinfection was again the highest predictor of ICU admission. These patients were admitted because positive results of outpatient blood cultures, signs of shock, and influenza were noted until several days into the hospitalization and may have been nosocomial in origin.18 Thus, costs are reported in the tables without intensive care unit stays for the 13 patients who may have required them in the model. But to acknowledge the role ICU admission plays in deferring costs, 2 cost‐estimate graphs were generated, 1 including ICU admission.
Outpatient Costs
For patients in each cohort who were unprotected from influenza because of parental or sibling immunization, a 10% increase in the number of outpatient medical visits was considered. Outpatient costs were tallied on the basis of average general pediatrician's salary of $68/hour.23 Duration of outpatient visits was estimated as 20 minutes with no accounting for extra nursing time. Hence, tallies were made by (number of unprotected infants 10% 20 minutes/visit $68/hour 1 hour/60 minutes). As 3% of actual cases of influenza in the group of those less than 6 months old can be misdiagnosed as clinical bacterial pneumonia, prescription costs were estimated as $3.20 for a 7‐day course of generic amoxicillin, which was the only prescribed antibiotic considered.
Indirect Costs
For each outpatient office visit, we used the cost‐estimation scheme outlined by Yount et al.20. We assumed that 1 parent accompanied the infant and 3 hours of lost work should be accounted for. Using the U.S. Bureau of Labor and Statistics 2002 average wage of $17/hour, lost wages for each extra outpatient visits were tabulated by (number of extra MD visits per group 3 hours $17/hour).24 No travel or transportation costs were considered.
Hospitalization
For each hospitalization, we assumed 1 parent stayed with an infant at bedside during the infant's inpatient stay. We calculated the average length of stay for patients with lung disease as 8 days and for those without lung disease as 4.5 days. Calculations were obtained using the following formula: (number of infants in each group from the New York Regional Perinatal Center 2004 Database hospitalization rate of each group number of days hospitalized $17/hour 8 work hours/day 5/7 workdays/week).
Sensitivity Analysis
We evaluated the sensitivity of the model to variations in the assumptions made. We varied the sibling immunization rate from 12% to 17% and the reduction in hospitalization for parents who received influenza vaccine from 10% to 20%. A summary of variables used in the analysis is included in Table 1.
| Compliance of parents offered influenza vaccine | 89% (17) |
| Seroconversion rate of vaccine recipients | 89% (17) |
| Percentage of siblings vaccinated | 12% (12) |
| Excess PMD visits of infected patients | 10% (7) |
| Hospitalization rate of lung disease patients without siblings | 10% (7) |
| Hospitalization rate of lung disease patients with siblings | 15% (7) |
| Length of hospitalization of Lung Disease patients | 8 days (7) |
| Hospitalization rate of nonlung disease patients without siblings | 7/1000 (19) |
| Hospitalization rate of nonlung disease patients with siblings | 19/1000 (19) |
| Length of hospitalization of nonlung disease patients | 4.5 days (19) |
RESULTS
Influenza Costs Prior to Implementation of NICU‐Based Parental Vaccination
Direct and indirect costs of influenza hospitalization of the NICU graduates are summarized in Table 2. The total per‐patient cost of influenza vaccination obtained in the NICU for the 2632 patients in the source data 1 one season was $181.20. NICU patients with lung disease and siblings who were not protected from or immunized for influenza demonstrated the greatest per capita inpatient cost, $1925/patient. Vaccination of patients without lung disease who had no siblings cost $51, the same amount that it cost to vaccinate patients without lung disease who had vaccinated siblings.
| Subgroup type | Cost per patient ($) | Direct costs ($) | Indirect dosts ($) |
|---|---|---|---|
| |||
| Patients with lung disease whose siblings were protected | 1284 | 10,857.60 | 699.42 |
| Patients with lung disease whose siblings were unprotected | 1925 | 142,958.40 | 9,170.29 |
| Patients with lung disease without siblings | 1284 | 63,939.20 | 4,118.85 |
| Patients without lung disease whose siblings were protected | 51 | 7,885.33 | 507.96 |
| Patients without lung disease whose siblings were unprotected | 137 | 179,347.19 | 11,553.25 |
| Patients without lung disease without siblings | 51 | 44,366.86 | 2,858.04 |
Outpatient costs of influenza hospitalization based on source data revealed summarized costs for 1 season of $6.80/patient. This reflected 245 excess primary care visits at a total cost of $5569.20. The cost of excess prescriptions of the antibiotic amoxicillin because of misdiagnoses. Indirect costs secondary to parent lost work hours while attending to their infants in the hospital totaled $12,530.70. Thus, the total cost of influenza in the source population for 1 season including inpatient, outpatient, direct, and indirect costs was $188/patient.
Influenza Costs after Implementation of an NICU‐Based Parental Vaccination Program
Direct and indirect costs of influenza hospitalization for neonates with lung disease are summarized in Table 3. The introduction of parental vaccination decreased the per‐patient cost in the cohort of patients with lung disease and unprotected siblings to $1732 from $1925. This group showed the largest cost savings compared with the costs for this group prior to introduction of the campaign.
| Subgroup type | Cost/patient ($) | Direct costs ($) | Indirect costs ($) |
|---|---|---|---|
| |||
| Patients with lung disease with protected siblings/unprotected parents | 1283 | 2412.80 | 154.49 |
| Patients with lung disease with protected siblings/protected parents | 1155 | 7600.32 | 486.66 |
| Patients with lung disease with unprotected siblings/protected parents | 1732 (Pre‐1925) | 102,604.32 | 6569.94 |
| Patients with lung disease with unprotected siblings/unprotected parents | 1925 | 28,953.60 | 1853.95 |
| Patients with lung disease without siblings/with protected parents | 1155 | 45,601.92 | 2919.97 |
| Patients with lung disease without siblings/with unprotected parents | 1283 | 13,270.40 | 849.73 |
Direct and indirect costs of influenza hospitalization for infants without lung disease are summarized in Table 4. The introduction of parental vaccination to disrupt the cycle of infectious transmission to infant decreased per‐patient costs in patients whose parents and siblings received vaccinations to $45. This reduction of $6/patient was the greatest savings among all the groups in the cohort without lung disease.
| Subgroup type | Cost/Patient ($) | Direct Costs ($) | Indirect Costs ($) |
|---|---|---|---|
| |||
| Patients without lung disease with protected siblings/unprotected parents | 51 | 1662.57 | 106.45 |
| Patients without lung disease with protected siblings/protected parents | 45 (pre‐51) | 5600.48 | 256.30 |
| Patients without lung disease with unprotected siblings/unprotected parents | 137 | 37,261.92 | 2385.51 |
| Patients without lung disease with unprotected siblings/protected parents | 123 | 127,876.74 | 8168.97 |
| Patients without lung disease without siblings/with protected parents | 45 | 31,215.60 | 1998.79 |
| Patients without lung disease without siblings/with unprotected parents | 51 | 9215.38 | 586.60 |
Outpatient costs were reduced after the introduction of the campaign to $1.40/patient, reflecting the decrease in the number of outpatient visits from 245 to 51. Thus, the total cost of influenza in the source population after the introduction of an NICU‐based parental vaccination campaign was $200/patient. The $193/patient savings in the lung disease cohort with unprotected siblings ($1925 vs. $1732) was not sufficient to cover the increased cost of the vaccine. For this population of 2632 NICU patients, administration of NICU‐based parental influenza cost $12 extra/patient.
Financial Modeling Based on Source Data
Using the financial model, cost per patient was determined using the same estimates of incidence of the variables (ie, lung disease, siblings); only the number of enrollees in the program was varied. The relationship of cost per patient with number of NICU patients is shown in Figure 2. Cost per patient was zero at 4000 patients. Beyond that point, cost savings occurred, increasing with number of NICU admissions.
Estimating a 1‐day ICU admission rate of 0.5% at $3770/day reduces the required patient population for costs/patient to zero. This occurs at 3700 patients. Initially there is no added benefit with ICU admission, as the overall patient population is not large enough to support a significant ICU burden. As the population increases to 3000 patients, cost savings begin.
The relationship of variable immunization rates in siblings of the 2632 NICU patients in the source data is presented in Figure 3. Cost savings were not achieved until 37% of siblings had been immunized. A steep reduction in cost was seen as the immunization rate of siblings increased in the cohort. Marginal cost effectiveness was also increased in sibling immunization, meaning greater cost savings is achieved by immunizing a sibling of a high‐risk infant than by immunizing the parents, reflecting that siblings are more likely than parents to be vectors of disease in multichild households.
DISCUSSION
This is the first computer‐based model of the cost effectiveness of offering inactivated influenza vaccine to parents of patients in the NICU for the purpose of preventing illness in their offspring. Based on the source data, the study has demonstrated that offering immunization to parents in the NICU is not cost effective until the NICU population covered is at least 4000 patients. Cost effectiveness can also be reached in smaller populations by increasing the level of sibling immunization. These factors should be considered by public health specialists when mandating administration of influenza vaccine to parents in the NICU setting.
Cost‐effectiveness studies are limited by the variables chosen, by hospitalization rates, and by estimates made. Although we attempted to obtain hospitalization rates based on previously validated, published data, any variation in these rates will alter the cost‐savings model we constructed. For variables affecting the infectivity of and hospitalization for influenza, we chose lung disease, siblings with immunization rate, and parental immunization rate. Other variables, notably day care attendance, were not believed to highly influence infections due to respiratory pathogens.18
Another potential source of error in construction of the model is calculation of indirect costs. Although estimates of lost wages from work hours spent while a patient is hospitalized were calculated as an indirect cost, Leader et al. points out that there are also indirect costs after hospitalization secondary to increased outpatient physician surveillance.25 Furthermore, our model based lost wages on parents of patients earning an average salary of $17/hour. However, our source data represented the Regional Perinatal Center, a consortium of NICUs in New York City serving a primarily uninsured, indigent population. Hence, these estimates of lost wages may be overestimated.
Most cost‐utility analysis studies are performed to help compare public health policy policies across medical disciplines. Most data on adults and on children calculate the cost of quality‐of‐life‐adjusted year. In our study no such calculations were made because influenza was not thought to affect life long term. In other words, quality of life was not thought to be more likely to be affected by the variables NICU admission and birth weight than by the variable influenza infection, and these factors were considered in estimating hospitalization rates. Furthermore, because mortality from influenza is roughly 1 of every 100,000 for children less than 6 months old, no patients in the source data would have died, making quality‐of‐life‐adjusted year difficult to factor.26
Given a limited amount of medical resources, it is imperative to critically evaluate the economic implications of any widespread public health strategy. This cost analysis has demonstrated that the benefits of sponsoring NICU‐based immunization programs for parents will remain low unless the issue of sibling immunization is addressed or the number of patients in the cohort increases to a scale larger than any single traditional NICU may provide.
- ,.Interpandemic influenza in the Houston area, 1974‐1976.N Engl J Med.1978;298:587–592.
- ,,, et al.Burden of interpandemic influenza in children younger than 5 years: a 25‐year prospective study.J Infect Dis.2002;185:147–152.
- .Serious morbidity and mortality associated with influenza epidemics.Epidemiol Rev.1982;4:25–44.
- ,,, et al.Impact of respiratory virus infections on persons with chronic underlying conditions.JAMA.2000;283:499–505.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,.Impact of influenza on morbidity in children with cystic fibrosis.J Paediatr Child Health.1991;27:308–311.
- ,,, et al.The burden of influenza illness in children with asthma and other chronic medical conditions.J Pediatr.2000;137:856–864.
- ,;Committee on Infectious Diseases.Technical report. Reduction of the influenza burden in children.Pediatrics.2002;110:e80. Available at: http://www.pediatrics.org/cgi/content/full/110/6/e80.
- ,,, et al.Clinical reactions and serologic responses after vaccination with while‐virus or split‐virus influenza vaccines in children aged 6 to 36 months.Pediatrics.1982;69:404–408.
- ,.Economic impact of influenza vaccination in preschool children.Pediatrics.2000;106:973–976.
- Committee on Infectious Disease Policy Statement.Reduction of the influenza burden in children.Pediatrics.2002;110:1246–1252.
- ,.Change in recommendation affects influenza vaccinations among children 6 to 59 months of age.Pediatrics.2004;114;948–952.
- ,,.Factors associated with influenza vaccination coverage among the elderly: role of health care personnel.Public Health.1996;110:163–168.
- ,.Optimizing long‐term care by administration of influenza vaccine to parents of NICU patients.J Perinatol.2004;24:273–274.
- ,.Reduction of the influenza burden in children: policy statement of the Committee on Infectious Diseases: American Academy of Pediatrics.Pediatrics.2002;110:1246–1252.
- National Center for Health Statistics. Incidence of prematurity data. Available at: http://www.marchofdimes.com/peristats. Accessed April 16,2006.
- Centers for Disease Control and Prevention (CDC).Estimated influenza vaccination coverage among adults and children—United States, September 1, 2004‐January 31, 2005.MMWR Morb Mortal Wkly Rep.2005;54:304–307.
- ,,, et al.Multistate surveillance for laboratory‐confirmed, influenza‐associated hospitalizations in children: 2003‐2004.Pediatr Infect Dis J.2006;25:395–400.
- .., et al.Population‐based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children.Pediatrics.2004;113:1758–1764.
- New York University Outpatient Pharmacy, personal communication.
- ,.Economic analysis of palivizumab in infants with congenital heart disease.Pediatrics.2004;114:1606–1611.
- Equitable Life Assurance Society of the United States.Hospital Daily Service Charges.New York, NY:Equitable Life Assurance Company,1982.
- ,,Hospital costs of pediatric intensive care.Crit Care Med.1999;27:2079–2085.
- U.S. Bureau of Labor Statistics. Employment and earnings2004. Available at: www.bls.gov/bls/wages.html.
- ,,,,,.Time and out‐of‐pocket costs associated with respiratory syncytial virus hospitalization of infants.Value Health.2003;6:100–106.
- ,,, et al.Influenza‐associated deaths among children in the United States.N Engl J Med.2005;353:2559–2569.
Influenza is a common infectious agent in the pediatric population, infecting 15% to 42% of preschool children, with a fatality rate of 3.8 per 100,000.13 Those with underlying respiratory and cardiac disease are more likely to require hospitalization and more susceptible to morbidity from the disease.47 Trivalent inactivated influenza vaccine is a safe, cost‐effective method of preventing influenza in children, with a seroconversion rate of up to 89%.810 Both the American Academy of Pediatrics and the Advisory Committee on Immunization Practices recommend that the influenza vaccine be administered to household contacts and out‐of‐home caretakers of infants up to 6 months of age.11 Also included in this high‐risk category are children with chronic respiratory and cardiac disease.8
The immunization rate in the indicated pediatric population ranges from 9% to 22%.12 Because most adults who meet eligible criteria are not vaccinated, it has been proposed that the NICU begin to administer the influenza vaccine to parents of high‐risk infants, eliminating commonly encountered obstacles to vaccine administration and preventing infection in these close contacts of infants, who likely serve as infectious agents of disease in the infants.13, 14
Yet the cost of instituting such a program remains a concern, especially given the recent shortages of the inactivated influenza vaccine, which have increased cost.15 The economic implications of instituting an inactivated influenza vaccination program for parents of patients in the NICU have not been fully evaluated. Given that upwards of 40,000 premature infants are admitted to intensive care units each year, an examination of cost savings is critical prior to implementing such a program.16
METHODS
Data and Assumptions
A 3‐ and 4‐tiered computer model (with the tiers reflecting the variables presence of lung disease, having siblings, and sibling immunization status and the fourth tier reflecting parental immunization in the NICU as a function of the immunization program) assessing influenza vaccination status of parents of a cohort of 2632 patients admitted to the New York Regional Perinatal Center NICU during the influenza season of 2003‐2004 was constructed using the viewpoint of a large multinetwork medical center predominantly serving a lower socioeconomic status population. The likelihood of influenza infection of an infant, the need for infant hospitalization, subsequent length of stay, and the need for the patient to have outpatient physician visits were based on the following clinical variables: lung disease in the infant (defined as a 28‐day‐old patient whose birth weight was less than 1500 g being oxygen dependent); having school‐age siblings, sibling vaccination status; parental vaccination status; and parental compliance. Variables of the model were based on published results when possible. For the purposes of this model, we assumed a 10% reduction in influenza infectivity for parents of children who were immunized in the absence of other confounders based on the risk of needing medical attention of children less than 6 months old for documented influenza with parental vaccination in the 9 states that make up the Emerging Infections Program Network of the Centers for Disease Control.1719 Infected patients younger than 6 months of age were also programmed to have a 10% chance of an outpatient hospitalization visit. No deaths were introduced in the cohort. An outline of the different groups into which patients were classified (before and after the influenza vaccination campaign) is outlined in Figure 1.

Direct Costs
Medical Costs
The average wholesale cost of a dose of influenza vaccine including administration was $15.20 Each parent received 1 dose of influenza vaccine administered during the influenza season (the 5 months from October thru February). In our model, the vaccine is administered by nurses, physicians, or physician‐extenders in a neonatal intensive care unit and thus does not require increased personnel to support the program. Hence, no increased costs were included for administration of the vaccine.
Siblings were not offered immunization in the NICU program. Most NICUs do not allow children younger than 13 years to visit during influenza and respiratory syncytial virus season to prevent infection of newborns. Immunization of younger siblings requires prior knowledge of their vaccination status, as those previously immunized require 1 dose of vaccine, whereas those less than 9 years old not immunized require 2 doses scheduled 1 month apart. As this was considered logistically difficult for a high‐acuity NICU, sibling immunization was deferred to that sibling's primary medical doctor, a policy consistent with that of the American Academy of Pediatrics Medical Home Initiative.
Infant Hospitalization for Influenza
Cost estimates were obtained from published data on the length of stay of infants with respiratory disease.21 In this series the average length of stay of former NICU patients with low socioeconomic status hospitalized for influenza was 4.5 4 days. Average hospital costs were estimated as $1508/day.22, 23 No intensive care unit days were factored into the current cost model. Hospitalization costs for each group were estimated by (number of infants in each group from the New York Regional Perinatal Center 2004 Database hospitalization rate of each group number of days hospitalized $1508/day). This estimation technique was previously validated and used.21
Intensive care unit cost was estimated as 2.5 times the cost of nonintensive care ($3770/day). Intensive care hospitalization for influenza is difficult to measure, as it correlates with bacterial superinfection, which has an incidence of 0.5/10,000 patients with documented influenza.5 However, in another study, ICU hospitalization of infected patients was 0.5%, which would translate to 13 patients in the studied group.18 The length of ICU stay was 1 day, and by univariate analysis, bacterial coinfection was again the highest predictor of ICU admission. These patients were admitted because positive results of outpatient blood cultures, signs of shock, and influenza were noted until several days into the hospitalization and may have been nosocomial in origin.18 Thus, costs are reported in the tables without intensive care unit stays for the 13 patients who may have required them in the model. But to acknowledge the role ICU admission plays in deferring costs, 2 cost‐estimate graphs were generated, 1 including ICU admission.
Outpatient Costs
For patients in each cohort who were unprotected from influenza because of parental or sibling immunization, a 10% increase in the number of outpatient medical visits was considered. Outpatient costs were tallied on the basis of average general pediatrician's salary of $68/hour.23 Duration of outpatient visits was estimated as 20 minutes with no accounting for extra nursing time. Hence, tallies were made by (number of unprotected infants 10% 20 minutes/visit $68/hour 1 hour/60 minutes). As 3% of actual cases of influenza in the group of those less than 6 months old can be misdiagnosed as clinical bacterial pneumonia, prescription costs were estimated as $3.20 for a 7‐day course of generic amoxicillin, which was the only prescribed antibiotic considered.
Indirect Costs
For each outpatient office visit, we used the cost‐estimation scheme outlined by Yount et al.20. We assumed that 1 parent accompanied the infant and 3 hours of lost work should be accounted for. Using the U.S. Bureau of Labor and Statistics 2002 average wage of $17/hour, lost wages for each extra outpatient visits were tabulated by (number of extra MD visits per group 3 hours $17/hour).24 No travel or transportation costs were considered.
Hospitalization
For each hospitalization, we assumed 1 parent stayed with an infant at bedside during the infant's inpatient stay. We calculated the average length of stay for patients with lung disease as 8 days and for those without lung disease as 4.5 days. Calculations were obtained using the following formula: (number of infants in each group from the New York Regional Perinatal Center 2004 Database hospitalization rate of each group number of days hospitalized $17/hour 8 work hours/day 5/7 workdays/week).
Sensitivity Analysis
We evaluated the sensitivity of the model to variations in the assumptions made. We varied the sibling immunization rate from 12% to 17% and the reduction in hospitalization for parents who received influenza vaccine from 10% to 20%. A summary of variables used in the analysis is included in Table 1.
| Compliance of parents offered influenza vaccine | 89% (17) |
| Seroconversion rate of vaccine recipients | 89% (17) |
| Percentage of siblings vaccinated | 12% (12) |
| Excess PMD visits of infected patients | 10% (7) |
| Hospitalization rate of lung disease patients without siblings | 10% (7) |
| Hospitalization rate of lung disease patients with siblings | 15% (7) |
| Length of hospitalization of Lung Disease patients | 8 days (7) |
| Hospitalization rate of nonlung disease patients without siblings | 7/1000 (19) |
| Hospitalization rate of nonlung disease patients with siblings | 19/1000 (19) |
| Length of hospitalization of nonlung disease patients | 4.5 days (19) |
RESULTS
Influenza Costs Prior to Implementation of NICU‐Based Parental Vaccination
Direct and indirect costs of influenza hospitalization of the NICU graduates are summarized in Table 2. The total per‐patient cost of influenza vaccination obtained in the NICU for the 2632 patients in the source data 1 one season was $181.20. NICU patients with lung disease and siblings who were not protected from or immunized for influenza demonstrated the greatest per capita inpatient cost, $1925/patient. Vaccination of patients without lung disease who had no siblings cost $51, the same amount that it cost to vaccinate patients without lung disease who had vaccinated siblings.
| Subgroup type | Cost per patient ($) | Direct costs ($) | Indirect dosts ($) |
|---|---|---|---|
| |||
| Patients with lung disease whose siblings were protected | 1284 | 10,857.60 | 699.42 |
| Patients with lung disease whose siblings were unprotected | 1925 | 142,958.40 | 9,170.29 |
| Patients with lung disease without siblings | 1284 | 63,939.20 | 4,118.85 |
| Patients without lung disease whose siblings were protected | 51 | 7,885.33 | 507.96 |
| Patients without lung disease whose siblings were unprotected | 137 | 179,347.19 | 11,553.25 |
| Patients without lung disease without siblings | 51 | 44,366.86 | 2,858.04 |
Outpatient costs of influenza hospitalization based on source data revealed summarized costs for 1 season of $6.80/patient. This reflected 245 excess primary care visits at a total cost of $5569.20. The cost of excess prescriptions of the antibiotic amoxicillin because of misdiagnoses. Indirect costs secondary to parent lost work hours while attending to their infants in the hospital totaled $12,530.70. Thus, the total cost of influenza in the source population for 1 season including inpatient, outpatient, direct, and indirect costs was $188/patient.
Influenza Costs after Implementation of an NICU‐Based Parental Vaccination Program
Direct and indirect costs of influenza hospitalization for neonates with lung disease are summarized in Table 3. The introduction of parental vaccination decreased the per‐patient cost in the cohort of patients with lung disease and unprotected siblings to $1732 from $1925. This group showed the largest cost savings compared with the costs for this group prior to introduction of the campaign.
| Subgroup type | Cost/patient ($) | Direct costs ($) | Indirect costs ($) |
|---|---|---|---|
| |||
| Patients with lung disease with protected siblings/unprotected parents | 1283 | 2412.80 | 154.49 |
| Patients with lung disease with protected siblings/protected parents | 1155 | 7600.32 | 486.66 |
| Patients with lung disease with unprotected siblings/protected parents | 1732 (Pre‐1925) | 102,604.32 | 6569.94 |
| Patients with lung disease with unprotected siblings/unprotected parents | 1925 | 28,953.60 | 1853.95 |
| Patients with lung disease without siblings/with protected parents | 1155 | 45,601.92 | 2919.97 |
| Patients with lung disease without siblings/with unprotected parents | 1283 | 13,270.40 | 849.73 |
Direct and indirect costs of influenza hospitalization for infants without lung disease are summarized in Table 4. The introduction of parental vaccination to disrupt the cycle of infectious transmission to infant decreased per‐patient costs in patients whose parents and siblings received vaccinations to $45. This reduction of $6/patient was the greatest savings among all the groups in the cohort without lung disease.
| Subgroup type | Cost/Patient ($) | Direct Costs ($) | Indirect Costs ($) |
|---|---|---|---|
| |||
| Patients without lung disease with protected siblings/unprotected parents | 51 | 1662.57 | 106.45 |
| Patients without lung disease with protected siblings/protected parents | 45 (pre‐51) | 5600.48 | 256.30 |
| Patients without lung disease with unprotected siblings/unprotected parents | 137 | 37,261.92 | 2385.51 |
| Patients without lung disease with unprotected siblings/protected parents | 123 | 127,876.74 | 8168.97 |
| Patients without lung disease without siblings/with protected parents | 45 | 31,215.60 | 1998.79 |
| Patients without lung disease without siblings/with unprotected parents | 51 | 9215.38 | 586.60 |
Outpatient costs were reduced after the introduction of the campaign to $1.40/patient, reflecting the decrease in the number of outpatient visits from 245 to 51. Thus, the total cost of influenza in the source population after the introduction of an NICU‐based parental vaccination campaign was $200/patient. The $193/patient savings in the lung disease cohort with unprotected siblings ($1925 vs. $1732) was not sufficient to cover the increased cost of the vaccine. For this population of 2632 NICU patients, administration of NICU‐based parental influenza cost $12 extra/patient.
Financial Modeling Based on Source Data
Using the financial model, cost per patient was determined using the same estimates of incidence of the variables (ie, lung disease, siblings); only the number of enrollees in the program was varied. The relationship of cost per patient with number of NICU patients is shown in Figure 2. Cost per patient was zero at 4000 patients. Beyond that point, cost savings occurred, increasing with number of NICU admissions.

Estimating a 1‐day ICU admission rate of 0.5% at $3770/day reduces the required patient population for costs/patient to zero. This occurs at 3700 patients. Initially there is no added benefit with ICU admission, as the overall patient population is not large enough to support a significant ICU burden. As the population increases to 3000 patients, cost savings begin.
The relationship of variable immunization rates in siblings of the 2632 NICU patients in the source data is presented in Figure 3. Cost savings were not achieved until 37% of siblings had been immunized. A steep reduction in cost was seen as the immunization rate of siblings increased in the cohort. Marginal cost effectiveness was also increased in sibling immunization, meaning greater cost savings is achieved by immunizing a sibling of a high‐risk infant than by immunizing the parents, reflecting that siblings are more likely than parents to be vectors of disease in multichild households.

DISCUSSION
This is the first computer‐based model of the cost effectiveness of offering inactivated influenza vaccine to parents of patients in the NICU for the purpose of preventing illness in their offspring. Based on the source data, the study has demonstrated that offering immunization to parents in the NICU is not cost effective until the NICU population covered is at least 4000 patients. Cost effectiveness can also be reached in smaller populations by increasing the level of sibling immunization. These factors should be considered by public health specialists when mandating administration of influenza vaccine to parents in the NICU setting.
Cost‐effectiveness studies are limited by the variables chosen, by hospitalization rates, and by estimates made. Although we attempted to obtain hospitalization rates based on previously validated, published data, any variation in these rates will alter the cost‐savings model we constructed. For variables affecting the infectivity of and hospitalization for influenza, we chose lung disease, siblings with immunization rate, and parental immunization rate. Other variables, notably day care attendance, were not believed to highly influence infections due to respiratory pathogens.18
Another potential source of error in construction of the model is calculation of indirect costs. Although estimates of lost wages from work hours spent while a patient is hospitalized were calculated as an indirect cost, Leader et al. points out that there are also indirect costs after hospitalization secondary to increased outpatient physician surveillance.25 Furthermore, our model based lost wages on parents of patients earning an average salary of $17/hour. However, our source data represented the Regional Perinatal Center, a consortium of NICUs in New York City serving a primarily uninsured, indigent population. Hence, these estimates of lost wages may be overestimated.
Most cost‐utility analysis studies are performed to help compare public health policy policies across medical disciplines. Most data on adults and on children calculate the cost of quality‐of‐life‐adjusted year. In our study no such calculations were made because influenza was not thought to affect life long term. In other words, quality of life was not thought to be more likely to be affected by the variables NICU admission and birth weight than by the variable influenza infection, and these factors were considered in estimating hospitalization rates. Furthermore, because mortality from influenza is roughly 1 of every 100,000 for children less than 6 months old, no patients in the source data would have died, making quality‐of‐life‐adjusted year difficult to factor.26
Given a limited amount of medical resources, it is imperative to critically evaluate the economic implications of any widespread public health strategy. This cost analysis has demonstrated that the benefits of sponsoring NICU‐based immunization programs for parents will remain low unless the issue of sibling immunization is addressed or the number of patients in the cohort increases to a scale larger than any single traditional NICU may provide.
Influenza is a common infectious agent in the pediatric population, infecting 15% to 42% of preschool children, with a fatality rate of 3.8 per 100,000.13 Those with underlying respiratory and cardiac disease are more likely to require hospitalization and more susceptible to morbidity from the disease.47 Trivalent inactivated influenza vaccine is a safe, cost‐effective method of preventing influenza in children, with a seroconversion rate of up to 89%.810 Both the American Academy of Pediatrics and the Advisory Committee on Immunization Practices recommend that the influenza vaccine be administered to household contacts and out‐of‐home caretakers of infants up to 6 months of age.11 Also included in this high‐risk category are children with chronic respiratory and cardiac disease.8
The immunization rate in the indicated pediatric population ranges from 9% to 22%.12 Because most adults who meet eligible criteria are not vaccinated, it has been proposed that the NICU begin to administer the influenza vaccine to parents of high‐risk infants, eliminating commonly encountered obstacles to vaccine administration and preventing infection in these close contacts of infants, who likely serve as infectious agents of disease in the infants.13, 14
Yet the cost of instituting such a program remains a concern, especially given the recent shortages of the inactivated influenza vaccine, which have increased cost.15 The economic implications of instituting an inactivated influenza vaccination program for parents of patients in the NICU have not been fully evaluated. Given that upwards of 40,000 premature infants are admitted to intensive care units each year, an examination of cost savings is critical prior to implementing such a program.16
METHODS
Data and Assumptions
A 3‐ and 4‐tiered computer model (with the tiers reflecting the variables presence of lung disease, having siblings, and sibling immunization status and the fourth tier reflecting parental immunization in the NICU as a function of the immunization program) assessing influenza vaccination status of parents of a cohort of 2632 patients admitted to the New York Regional Perinatal Center NICU during the influenza season of 2003‐2004 was constructed using the viewpoint of a large multinetwork medical center predominantly serving a lower socioeconomic status population. The likelihood of influenza infection of an infant, the need for infant hospitalization, subsequent length of stay, and the need for the patient to have outpatient physician visits were based on the following clinical variables: lung disease in the infant (defined as a 28‐day‐old patient whose birth weight was less than 1500 g being oxygen dependent); having school‐age siblings, sibling vaccination status; parental vaccination status; and parental compliance. Variables of the model were based on published results when possible. For the purposes of this model, we assumed a 10% reduction in influenza infectivity for parents of children who were immunized in the absence of other confounders based on the risk of needing medical attention of children less than 6 months old for documented influenza with parental vaccination in the 9 states that make up the Emerging Infections Program Network of the Centers for Disease Control.1719 Infected patients younger than 6 months of age were also programmed to have a 10% chance of an outpatient hospitalization visit. No deaths were introduced in the cohort. An outline of the different groups into which patients were classified (before and after the influenza vaccination campaign) is outlined in Figure 1.

Direct Costs
Medical Costs
The average wholesale cost of a dose of influenza vaccine including administration was $15.20 Each parent received 1 dose of influenza vaccine administered during the influenza season (the 5 months from October thru February). In our model, the vaccine is administered by nurses, physicians, or physician‐extenders in a neonatal intensive care unit and thus does not require increased personnel to support the program. Hence, no increased costs were included for administration of the vaccine.
Siblings were not offered immunization in the NICU program. Most NICUs do not allow children younger than 13 years to visit during influenza and respiratory syncytial virus season to prevent infection of newborns. Immunization of younger siblings requires prior knowledge of their vaccination status, as those previously immunized require 1 dose of vaccine, whereas those less than 9 years old not immunized require 2 doses scheduled 1 month apart. As this was considered logistically difficult for a high‐acuity NICU, sibling immunization was deferred to that sibling's primary medical doctor, a policy consistent with that of the American Academy of Pediatrics Medical Home Initiative.
Infant Hospitalization for Influenza
Cost estimates were obtained from published data on the length of stay of infants with respiratory disease.21 In this series the average length of stay of former NICU patients with low socioeconomic status hospitalized for influenza was 4.5 4 days. Average hospital costs were estimated as $1508/day.22, 23 No intensive care unit days were factored into the current cost model. Hospitalization costs for each group were estimated by (number of infants in each group from the New York Regional Perinatal Center 2004 Database hospitalization rate of each group number of days hospitalized $1508/day). This estimation technique was previously validated and used.21
Intensive care unit cost was estimated as 2.5 times the cost of nonintensive care ($3770/day). Intensive care hospitalization for influenza is difficult to measure, as it correlates with bacterial superinfection, which has an incidence of 0.5/10,000 patients with documented influenza.5 However, in another study, ICU hospitalization of infected patients was 0.5%, which would translate to 13 patients in the studied group.18 The length of ICU stay was 1 day, and by univariate analysis, bacterial coinfection was again the highest predictor of ICU admission. These patients were admitted because positive results of outpatient blood cultures, signs of shock, and influenza were noted until several days into the hospitalization and may have been nosocomial in origin.18 Thus, costs are reported in the tables without intensive care unit stays for the 13 patients who may have required them in the model. But to acknowledge the role ICU admission plays in deferring costs, 2 cost‐estimate graphs were generated, 1 including ICU admission.
Outpatient Costs
For patients in each cohort who were unprotected from influenza because of parental or sibling immunization, a 10% increase in the number of outpatient medical visits was considered. Outpatient costs were tallied on the basis of average general pediatrician's salary of $68/hour.23 Duration of outpatient visits was estimated as 20 minutes with no accounting for extra nursing time. Hence, tallies were made by (number of unprotected infants 10% 20 minutes/visit $68/hour 1 hour/60 minutes). As 3% of actual cases of influenza in the group of those less than 6 months old can be misdiagnosed as clinical bacterial pneumonia, prescription costs were estimated as $3.20 for a 7‐day course of generic amoxicillin, which was the only prescribed antibiotic considered.
Indirect Costs
For each outpatient office visit, we used the cost‐estimation scheme outlined by Yount et al.20. We assumed that 1 parent accompanied the infant and 3 hours of lost work should be accounted for. Using the U.S. Bureau of Labor and Statistics 2002 average wage of $17/hour, lost wages for each extra outpatient visits were tabulated by (number of extra MD visits per group 3 hours $17/hour).24 No travel or transportation costs were considered.
Hospitalization
For each hospitalization, we assumed 1 parent stayed with an infant at bedside during the infant's inpatient stay. We calculated the average length of stay for patients with lung disease as 8 days and for those without lung disease as 4.5 days. Calculations were obtained using the following formula: (number of infants in each group from the New York Regional Perinatal Center 2004 Database hospitalization rate of each group number of days hospitalized $17/hour 8 work hours/day 5/7 workdays/week).
Sensitivity Analysis
We evaluated the sensitivity of the model to variations in the assumptions made. We varied the sibling immunization rate from 12% to 17% and the reduction in hospitalization for parents who received influenza vaccine from 10% to 20%. A summary of variables used in the analysis is included in Table 1.
| Compliance of parents offered influenza vaccine | 89% (17) |
| Seroconversion rate of vaccine recipients | 89% (17) |
| Percentage of siblings vaccinated | 12% (12) |
| Excess PMD visits of infected patients | 10% (7) |
| Hospitalization rate of lung disease patients without siblings | 10% (7) |
| Hospitalization rate of lung disease patients with siblings | 15% (7) |
| Length of hospitalization of Lung Disease patients | 8 days (7) |
| Hospitalization rate of nonlung disease patients without siblings | 7/1000 (19) |
| Hospitalization rate of nonlung disease patients with siblings | 19/1000 (19) |
| Length of hospitalization of nonlung disease patients | 4.5 days (19) |
RESULTS
Influenza Costs Prior to Implementation of NICU‐Based Parental Vaccination
Direct and indirect costs of influenza hospitalization of the NICU graduates are summarized in Table 2. The total per‐patient cost of influenza vaccination obtained in the NICU for the 2632 patients in the source data 1 one season was $181.20. NICU patients with lung disease and siblings who were not protected from or immunized for influenza demonstrated the greatest per capita inpatient cost, $1925/patient. Vaccination of patients without lung disease who had no siblings cost $51, the same amount that it cost to vaccinate patients without lung disease who had vaccinated siblings.
| Subgroup type | Cost per patient ($) | Direct costs ($) | Indirect dosts ($) |
|---|---|---|---|
| |||
| Patients with lung disease whose siblings were protected | 1284 | 10,857.60 | 699.42 |
| Patients with lung disease whose siblings were unprotected | 1925 | 142,958.40 | 9,170.29 |
| Patients with lung disease without siblings | 1284 | 63,939.20 | 4,118.85 |
| Patients without lung disease whose siblings were protected | 51 | 7,885.33 | 507.96 |
| Patients without lung disease whose siblings were unprotected | 137 | 179,347.19 | 11,553.25 |
| Patients without lung disease without siblings | 51 | 44,366.86 | 2,858.04 |
Outpatient costs of influenza hospitalization based on source data revealed summarized costs for 1 season of $6.80/patient. This reflected 245 excess primary care visits at a total cost of $5569.20. The cost of excess prescriptions of the antibiotic amoxicillin because of misdiagnoses. Indirect costs secondary to parent lost work hours while attending to their infants in the hospital totaled $12,530.70. Thus, the total cost of influenza in the source population for 1 season including inpatient, outpatient, direct, and indirect costs was $188/patient.
Influenza Costs after Implementation of an NICU‐Based Parental Vaccination Program
Direct and indirect costs of influenza hospitalization for neonates with lung disease are summarized in Table 3. The introduction of parental vaccination decreased the per‐patient cost in the cohort of patients with lung disease and unprotected siblings to $1732 from $1925. This group showed the largest cost savings compared with the costs for this group prior to introduction of the campaign.
| Subgroup type | Cost/patient ($) | Direct costs ($) | Indirect costs ($) |
|---|---|---|---|
| |||
| Patients with lung disease with protected siblings/unprotected parents | 1283 | 2412.80 | 154.49 |
| Patients with lung disease with protected siblings/protected parents | 1155 | 7600.32 | 486.66 |
| Patients with lung disease with unprotected siblings/protected parents | 1732 (Pre‐1925) | 102,604.32 | 6569.94 |
| Patients with lung disease with unprotected siblings/unprotected parents | 1925 | 28,953.60 | 1853.95 |
| Patients with lung disease without siblings/with protected parents | 1155 | 45,601.92 | 2919.97 |
| Patients with lung disease without siblings/with unprotected parents | 1283 | 13,270.40 | 849.73 |
Direct and indirect costs of influenza hospitalization for infants without lung disease are summarized in Table 4. The introduction of parental vaccination to disrupt the cycle of infectious transmission to infant decreased per‐patient costs in patients whose parents and siblings received vaccinations to $45. This reduction of $6/patient was the greatest savings among all the groups in the cohort without lung disease.
| Subgroup type | Cost/Patient ($) | Direct Costs ($) | Indirect Costs ($) |
|---|---|---|---|
| |||
| Patients without lung disease with protected siblings/unprotected parents | 51 | 1662.57 | 106.45 |
| Patients without lung disease with protected siblings/protected parents | 45 (pre‐51) | 5600.48 | 256.30 |
| Patients without lung disease with unprotected siblings/unprotected parents | 137 | 37,261.92 | 2385.51 |
| Patients without lung disease with unprotected siblings/protected parents | 123 | 127,876.74 | 8168.97 |
| Patients without lung disease without siblings/with protected parents | 45 | 31,215.60 | 1998.79 |
| Patients without lung disease without siblings/with unprotected parents | 51 | 9215.38 | 586.60 |
Outpatient costs were reduced after the introduction of the campaign to $1.40/patient, reflecting the decrease in the number of outpatient visits from 245 to 51. Thus, the total cost of influenza in the source population after the introduction of an NICU‐based parental vaccination campaign was $200/patient. The $193/patient savings in the lung disease cohort with unprotected siblings ($1925 vs. $1732) was not sufficient to cover the increased cost of the vaccine. For this population of 2632 NICU patients, administration of NICU‐based parental influenza cost $12 extra/patient.
Financial Modeling Based on Source Data
Using the financial model, cost per patient was determined using the same estimates of incidence of the variables (ie, lung disease, siblings); only the number of enrollees in the program was varied. The relationship of cost per patient with number of NICU patients is shown in Figure 2. Cost per patient was zero at 4000 patients. Beyond that point, cost savings occurred, increasing with number of NICU admissions.

Estimating a 1‐day ICU admission rate of 0.5% at $3770/day reduces the required patient population for costs/patient to zero. This occurs at 3700 patients. Initially there is no added benefit with ICU admission, as the overall patient population is not large enough to support a significant ICU burden. As the population increases to 3000 patients, cost savings begin.
The relationship of variable immunization rates in siblings of the 2632 NICU patients in the source data is presented in Figure 3. Cost savings were not achieved until 37% of siblings had been immunized. A steep reduction in cost was seen as the immunization rate of siblings increased in the cohort. Marginal cost effectiveness was also increased in sibling immunization, meaning greater cost savings is achieved by immunizing a sibling of a high‐risk infant than by immunizing the parents, reflecting that siblings are more likely than parents to be vectors of disease in multichild households.

DISCUSSION
This is the first computer‐based model of the cost effectiveness of offering inactivated influenza vaccine to parents of patients in the NICU for the purpose of preventing illness in their offspring. Based on the source data, the study has demonstrated that offering immunization to parents in the NICU is not cost effective until the NICU population covered is at least 4000 patients. Cost effectiveness can also be reached in smaller populations by increasing the level of sibling immunization. These factors should be considered by public health specialists when mandating administration of influenza vaccine to parents in the NICU setting.
Cost‐effectiveness studies are limited by the variables chosen, by hospitalization rates, and by estimates made. Although we attempted to obtain hospitalization rates based on previously validated, published data, any variation in these rates will alter the cost‐savings model we constructed. For variables affecting the infectivity of and hospitalization for influenza, we chose lung disease, siblings with immunization rate, and parental immunization rate. Other variables, notably day care attendance, were not believed to highly influence infections due to respiratory pathogens.18
Another potential source of error in construction of the model is calculation of indirect costs. Although estimates of lost wages from work hours spent while a patient is hospitalized were calculated as an indirect cost, Leader et al. points out that there are also indirect costs after hospitalization secondary to increased outpatient physician surveillance.25 Furthermore, our model based lost wages on parents of patients earning an average salary of $17/hour. However, our source data represented the Regional Perinatal Center, a consortium of NICUs in New York City serving a primarily uninsured, indigent population. Hence, these estimates of lost wages may be overestimated.
Most cost‐utility analysis studies are performed to help compare public health policy policies across medical disciplines. Most data on adults and on children calculate the cost of quality‐of‐life‐adjusted year. In our study no such calculations were made because influenza was not thought to affect life long term. In other words, quality of life was not thought to be more likely to be affected by the variables NICU admission and birth weight than by the variable influenza infection, and these factors were considered in estimating hospitalization rates. Furthermore, because mortality from influenza is roughly 1 of every 100,000 for children less than 6 months old, no patients in the source data would have died, making quality‐of‐life‐adjusted year difficult to factor.26
Given a limited amount of medical resources, it is imperative to critically evaluate the economic implications of any widespread public health strategy. This cost analysis has demonstrated that the benefits of sponsoring NICU‐based immunization programs for parents will remain low unless the issue of sibling immunization is addressed or the number of patients in the cohort increases to a scale larger than any single traditional NICU may provide.
- ,.Interpandemic influenza in the Houston area, 1974‐1976.N Engl J Med.1978;298:587–592.
- ,,, et al.Burden of interpandemic influenza in children younger than 5 years: a 25‐year prospective study.J Infect Dis.2002;185:147–152.
- .Serious morbidity and mortality associated with influenza epidemics.Epidemiol Rev.1982;4:25–44.
- ,,, et al.Impact of respiratory virus infections on persons with chronic underlying conditions.JAMA.2000;283:499–505.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,.Impact of influenza on morbidity in children with cystic fibrosis.J Paediatr Child Health.1991;27:308–311.
- ,,, et al.The burden of influenza illness in children with asthma and other chronic medical conditions.J Pediatr.2000;137:856–864.
- ,;Committee on Infectious Diseases.Technical report. Reduction of the influenza burden in children.Pediatrics.2002;110:e80. Available at: http://www.pediatrics.org/cgi/content/full/110/6/e80.
- ,,, et al.Clinical reactions and serologic responses after vaccination with while‐virus or split‐virus influenza vaccines in children aged 6 to 36 months.Pediatrics.1982;69:404–408.
- ,.Economic impact of influenza vaccination in preschool children.Pediatrics.2000;106:973–976.
- Committee on Infectious Disease Policy Statement.Reduction of the influenza burden in children.Pediatrics.2002;110:1246–1252.
- ,.Change in recommendation affects influenza vaccinations among children 6 to 59 months of age.Pediatrics.2004;114;948–952.
- ,,.Factors associated with influenza vaccination coverage among the elderly: role of health care personnel.Public Health.1996;110:163–168.
- ,.Optimizing long‐term care by administration of influenza vaccine to parents of NICU patients.J Perinatol.2004;24:273–274.
- ,.Reduction of the influenza burden in children: policy statement of the Committee on Infectious Diseases: American Academy of Pediatrics.Pediatrics.2002;110:1246–1252.
- National Center for Health Statistics. Incidence of prematurity data. Available at: http://www.marchofdimes.com/peristats. Accessed April 16,2006.
- Centers for Disease Control and Prevention (CDC).Estimated influenza vaccination coverage among adults and children—United States, September 1, 2004‐January 31, 2005.MMWR Morb Mortal Wkly Rep.2005;54:304–307.
- ,,, et al.Multistate surveillance for laboratory‐confirmed, influenza‐associated hospitalizations in children: 2003‐2004.Pediatr Infect Dis J.2006;25:395–400.
- .., et al.Population‐based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children.Pediatrics.2004;113:1758–1764.
- New York University Outpatient Pharmacy, personal communication.
- ,.Economic analysis of palivizumab in infants with congenital heart disease.Pediatrics.2004;114:1606–1611.
- Equitable Life Assurance Society of the United States.Hospital Daily Service Charges.New York, NY:Equitable Life Assurance Company,1982.
- ,,Hospital costs of pediatric intensive care.Crit Care Med.1999;27:2079–2085.
- U.S. Bureau of Labor Statistics. Employment and earnings2004. Available at: www.bls.gov/bls/wages.html.
- ,,,,,.Time and out‐of‐pocket costs associated with respiratory syncytial virus hospitalization of infants.Value Health.2003;6:100–106.
- ,,, et al.Influenza‐associated deaths among children in the United States.N Engl J Med.2005;353:2559–2569.
- ,.Interpandemic influenza in the Houston area, 1974‐1976.N Engl J Med.1978;298:587–592.
- ,,, et al.Burden of interpandemic influenza in children younger than 5 years: a 25‐year prospective study.J Infect Dis.2002;185:147–152.
- .Serious morbidity and mortality associated with influenza epidemics.Epidemiol Rev.1982;4:25–44.
- ,,, et al.Impact of respiratory virus infections on persons with chronic underlying conditions.JAMA.2000;283:499–505.
- ,,, et al.Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med.2000;342:232–239.
- ,,.Impact of influenza on morbidity in children with cystic fibrosis.J Paediatr Child Health.1991;27:308–311.
- ,,, et al.The burden of influenza illness in children with asthma and other chronic medical conditions.J Pediatr.2000;137:856–864.
- ,;Committee on Infectious Diseases.Technical report. Reduction of the influenza burden in children.Pediatrics.2002;110:e80. Available at: http://www.pediatrics.org/cgi/content/full/110/6/e80.
- ,,, et al.Clinical reactions and serologic responses after vaccination with while‐virus or split‐virus influenza vaccines in children aged 6 to 36 months.Pediatrics.1982;69:404–408.
- ,.Economic impact of influenza vaccination in preschool children.Pediatrics.2000;106:973–976.
- Committee on Infectious Disease Policy Statement.Reduction of the influenza burden in children.Pediatrics.2002;110:1246–1252.
- ,.Change in recommendation affects influenza vaccinations among children 6 to 59 months of age.Pediatrics.2004;114;948–952.
- ,,.Factors associated with influenza vaccination coverage among the elderly: role of health care personnel.Public Health.1996;110:163–168.
- ,.Optimizing long‐term care by administration of influenza vaccine to parents of NICU patients.J Perinatol.2004;24:273–274.
- ,.Reduction of the influenza burden in children: policy statement of the Committee on Infectious Diseases: American Academy of Pediatrics.Pediatrics.2002;110:1246–1252.
- National Center for Health Statistics. Incidence of prematurity data. Available at: http://www.marchofdimes.com/peristats. Accessed April 16,2006.
- Centers for Disease Control and Prevention (CDC).Estimated influenza vaccination coverage among adults and children—United States, September 1, 2004‐January 31, 2005.MMWR Morb Mortal Wkly Rep.2005;54:304–307.
- ,,, et al.Multistate surveillance for laboratory‐confirmed, influenza‐associated hospitalizations in children: 2003‐2004.Pediatr Infect Dis J.2006;25:395–400.
- .., et al.Population‐based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children.Pediatrics.2004;113:1758–1764.
- New York University Outpatient Pharmacy, personal communication.
- ,.Economic analysis of palivizumab in infants with congenital heart disease.Pediatrics.2004;114:1606–1611.
- Equitable Life Assurance Society of the United States.Hospital Daily Service Charges.New York, NY:Equitable Life Assurance Company,1982.
- ,,Hospital costs of pediatric intensive care.Crit Care Med.1999;27:2079–2085.
- U.S. Bureau of Labor Statistics. Employment and earnings2004. Available at: www.bls.gov/bls/wages.html.
- ,,,,,.Time and out‐of‐pocket costs associated with respiratory syncytial virus hospitalization of infants.Value Health.2003;6:100–106.
- ,,, et al.Influenza‐associated deaths among children in the United States.N Engl J Med.2005;353:2559–2569.
Copyright © 2007 Society of Hospital Medicine
Quality of Life of Children with NI after Fundoplication for GERD
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).
Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).

Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).

Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
Once Upon a Tenens
Having finished my family practice residency, and not being tied down by friends or family, I thought a round of working locum tenens would be a good way to see some different styles of practice.
I contacted a company called Locum-motion, filled out my paper work and was on my way. I had graduated from the University of Hamlin a few weeks earlier, armed with stethoscope and hammer. Dr. Claudio Prince (that’s me) was ready to stamp out disease and save lives.
There were several practice options available, but I chose a small hospital in Bremen. The three doctors who ran the hospital were going on a musical cruise for a week, and I would cover the entire facility for that time—ED and floor. I met with Drs. Baker, Butcher, and Maker briefly before they left for a trip. There were only a few inpatients, and one patient in the ICU. Then they were gone, and I was on my own.
My First Patient: I thought I’d start with the inpatients. The first was a Mr. B.B. Wolfe. He had been admitted for myalgia.
I was shocked when I walked into the room. He had severe hypertrichosis, prominent dentition, and proptosis. I briefly considered porphyria. I noted his history of muscle aches, high fever, facial swelling, and visual disturbance. His admit lab had showed a high CPK and LDH.
It sounded like an infestation of Trichinella to me. I questioned him about the ingestion of raw pork. He looked me in the eye and asked if his answer would be part of his medical record. I told him he needed to tell the truth for me to help him. Finally, he admitted he had eaten several portions of uncooked pig. I began to explain to him about the workup, the need for a muscle biopsy, and treatment options like mebendazole or steroids. I described the intestinal stage, which occurs between two and seven days after ingestion, when encysted larvae are liberated from the meat by gastric juices. I told him how the larvae mature into adult worms that burrow into the intestinal mucosa. I described the muscle stage, which develops after the first week and represents the period when adult-derived larvae in the intestines enter the bloodstream and disseminate hematogenously, then enter skeletal muscle causing pain.
He jumped out of bed and said he was leaving AMA; he had an appointment with a red-hooded girl. Whatever. I let him go; not much I could do to prevent his departure.
In the ED: I got a call from the ED about an old lady who had come in, having nearly choked on a bug. She looked fine to me, and I let her go. While down there, another older woman came in—in active labor. She admitted to having taken her friend’s Clomid, and had had little antepartum care secondary to a dearth of health insurance. Before I knew it, we were in the labor suite. First came one boy, then another, then another. I thought I was done, then two girls, another boy, then another girl. Seven babies—incredible! She moaned, not knowing what she was going to do with all these children.
I headed back to the floor to see more patients. The second one for the day was simple: a scrotal burn on a Mr. J.B. Nimble, who had been injured jumping over a flame. He was ready for discharge. The third patient was interesting, a Mrs. Spratt. I had been called by the lab with word that her serum looked like mayonnaise. She had abdominal pain, hepatosplenomegaly, memory loss, dyspnea and eruptive xanthomas. It sounded like type V hyperlipoproteinemia with chylomicronemia syndrome. What an interesting case, probably worse secondary to her very high-fat diet.
I walked down the hall to see my last inpatient, billed as a young man with psychotic depression pending psychiatric placement. I heard him yelling about burning witches and eating the walls. It sounded pretty psychotic. (I learned that his sister had been arraigned on manslaughter charges.) I entered the room and was struck by the smell of his breath, like cherries. A fruity smell, could he be ketotic? I instantly thought about ethylene glycol toxicity, maybe he had been sipping antifreeze, but there were no oxalate crystals in his urine. Was it delirium? I checked an O2 saturation; 95%, that wasn’t it. His blood pressure was 120/60. How about checking his finger-stick glucose? It read >400. This boy was in DKA! I started an insulin drip and hydration. It was only later I learned he had been on a starvation diet for weeks, then had binged on candy. His story about burning an old woman in a stove was unfortunately true. I called child protective services.
The ED pager went off again. That old lady who had swallowed a bug had ingested another, possibly a spider. I was worried about brown recluse or black widow envenomation, but it seemed to have been a simple barn spider, as she was now feeling OK. Again I discharged her with stern warnings to limit her invertebrate consumption. I stopped by the multiparous patient. She was happy to have the children and that they were all healthy, but what was she going to do? Where would she go? I called a social work consult.
ICU Time: I headed to the ICU. A sad story: a 24-year-old woman was unresponsive. She had a living will and did not want prolonged life support. She had choked on a piece of fruit. I walked into a room crowded with her family; it looked like seven very short uncles—one of whom was a doctor. They watched sadly as I pulled the endotracheal tube and the IVs. I told them I’d give them some time with her alone and would be back in an hour. It was a somber and tearful affair. She was so young and so beautiful. What a tragic end.
Another ED call; this bug-swallowing lady was driving them nuts. Now she was claiming she had swallowed a thrush, or maybe had thrush. Either way, she was gone before I got down there to see her.
I met with the social worker. She was dressed in a fancy gown and said she had just been at a party. She heard the story of the lady who had had so many children. She thought for a moment then said she had a few leads to check and would get back to me later that day.
We had a rush in the ED: a boy I thought might have rhinophyma and stiff-man syndrome, a girl with warts on her lips she attributed to kissing a frog, another with a glass splinter in her foot. There was a Mr. W.W. Winkee with hypothermia, and a young girl named Mary who thought she had contracted anthrax from a sheep. There was a boy named Jackie Horner with a tenosynovitis of the thumb.
The old lady came back in again. Now she was complaining of abdominal distention. When I finally laid eyes on her, I noted she certainly had a large abdomen. I grabbed a quick X-ray. Apparently she had taken my warnings against consuming avian and invertebrate entities, as now she had radiographic evidence of a feline skeleton. I planned to send her to a tertiary-care facility; perhaps they could do an endoscopic cat removal. Whatever they did, I was afraid if she kept this up she was bound to die.
I headed back to the ICU. They had dressed the girl, Miss White, in her street clothes and done her hair and makeup, but nothing could hide her severe pallor. She looked so peaceful.
Her “uncles” expectantly greeted me. They said in unison, “Welcome, Dr. Prince.” They were all inappropriately smiling. What was going on here? I went to declare her dead. I held a mirror up to her face, no signs of breathing, no lung sounds with auscultation. I laid my fingers gently across her throat. No pulse, but her skin was strangely pliant and warm.
I stared at her lovely face and the rest of the world suddenly shut itself off from me. I felt like singing. I could not stop myself … how unprofessional. But I bent over and gently kissed her goodbye. Suddenly there was music playing and—even more strangely—woodland animals frolicking at my feet. The uncles, who turned out to be roommates not relatives (had I known that I would never have stopped life support!), danced merrily. I looked back at her, and her eyes were open. She was smiling and gazing at her future husband, me.
Epilogue: The masticatory old lady eventually died from eating tainted horse meat. Mrs. Sprat improved with a low-fat diet. Mr. Wolfe died in a tragic logging accident, killed by the swing of an ax. The housing issues of the old lady with so many kids she did not know what to do was settled by the social worker, who became the septuplets’ godmother and found them housing in a refurbished, oversize shoe. As for Snow and me, we plan on living happily ever after. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
Having finished my family practice residency, and not being tied down by friends or family, I thought a round of working locum tenens would be a good way to see some different styles of practice.
I contacted a company called Locum-motion, filled out my paper work and was on my way. I had graduated from the University of Hamlin a few weeks earlier, armed with stethoscope and hammer. Dr. Claudio Prince (that’s me) was ready to stamp out disease and save lives.
There were several practice options available, but I chose a small hospital in Bremen. The three doctors who ran the hospital were going on a musical cruise for a week, and I would cover the entire facility for that time—ED and floor. I met with Drs. Baker, Butcher, and Maker briefly before they left for a trip. There were only a few inpatients, and one patient in the ICU. Then they were gone, and I was on my own.
My First Patient: I thought I’d start with the inpatients. The first was a Mr. B.B. Wolfe. He had been admitted for myalgia.
I was shocked when I walked into the room. He had severe hypertrichosis, prominent dentition, and proptosis. I briefly considered porphyria. I noted his history of muscle aches, high fever, facial swelling, and visual disturbance. His admit lab had showed a high CPK and LDH.
It sounded like an infestation of Trichinella to me. I questioned him about the ingestion of raw pork. He looked me in the eye and asked if his answer would be part of his medical record. I told him he needed to tell the truth for me to help him. Finally, he admitted he had eaten several portions of uncooked pig. I began to explain to him about the workup, the need for a muscle biopsy, and treatment options like mebendazole or steroids. I described the intestinal stage, which occurs between two and seven days after ingestion, when encysted larvae are liberated from the meat by gastric juices. I told him how the larvae mature into adult worms that burrow into the intestinal mucosa. I described the muscle stage, which develops after the first week and represents the period when adult-derived larvae in the intestines enter the bloodstream and disseminate hematogenously, then enter skeletal muscle causing pain.
He jumped out of bed and said he was leaving AMA; he had an appointment with a red-hooded girl. Whatever. I let him go; not much I could do to prevent his departure.
In the ED: I got a call from the ED about an old lady who had come in, having nearly choked on a bug. She looked fine to me, and I let her go. While down there, another older woman came in—in active labor. She admitted to having taken her friend’s Clomid, and had had little antepartum care secondary to a dearth of health insurance. Before I knew it, we were in the labor suite. First came one boy, then another, then another. I thought I was done, then two girls, another boy, then another girl. Seven babies—incredible! She moaned, not knowing what she was going to do with all these children.
I headed back to the floor to see more patients. The second one for the day was simple: a scrotal burn on a Mr. J.B. Nimble, who had been injured jumping over a flame. He was ready for discharge. The third patient was interesting, a Mrs. Spratt. I had been called by the lab with word that her serum looked like mayonnaise. She had abdominal pain, hepatosplenomegaly, memory loss, dyspnea and eruptive xanthomas. It sounded like type V hyperlipoproteinemia with chylomicronemia syndrome. What an interesting case, probably worse secondary to her very high-fat diet.
I walked down the hall to see my last inpatient, billed as a young man with psychotic depression pending psychiatric placement. I heard him yelling about burning witches and eating the walls. It sounded pretty psychotic. (I learned that his sister had been arraigned on manslaughter charges.) I entered the room and was struck by the smell of his breath, like cherries. A fruity smell, could he be ketotic? I instantly thought about ethylene glycol toxicity, maybe he had been sipping antifreeze, but there were no oxalate crystals in his urine. Was it delirium? I checked an O2 saturation; 95%, that wasn’t it. His blood pressure was 120/60. How about checking his finger-stick glucose? It read >400. This boy was in DKA! I started an insulin drip and hydration. It was only later I learned he had been on a starvation diet for weeks, then had binged on candy. His story about burning an old woman in a stove was unfortunately true. I called child protective services.
The ED pager went off again. That old lady who had swallowed a bug had ingested another, possibly a spider. I was worried about brown recluse or black widow envenomation, but it seemed to have been a simple barn spider, as she was now feeling OK. Again I discharged her with stern warnings to limit her invertebrate consumption. I stopped by the multiparous patient. She was happy to have the children and that they were all healthy, but what was she going to do? Where would she go? I called a social work consult.
ICU Time: I headed to the ICU. A sad story: a 24-year-old woman was unresponsive. She had a living will and did not want prolonged life support. She had choked on a piece of fruit. I walked into a room crowded with her family; it looked like seven very short uncles—one of whom was a doctor. They watched sadly as I pulled the endotracheal tube and the IVs. I told them I’d give them some time with her alone and would be back in an hour. It was a somber and tearful affair. She was so young and so beautiful. What a tragic end.
Another ED call; this bug-swallowing lady was driving them nuts. Now she was claiming she had swallowed a thrush, or maybe had thrush. Either way, she was gone before I got down there to see her.
I met with the social worker. She was dressed in a fancy gown and said she had just been at a party. She heard the story of the lady who had had so many children. She thought for a moment then said she had a few leads to check and would get back to me later that day.
We had a rush in the ED: a boy I thought might have rhinophyma and stiff-man syndrome, a girl with warts on her lips she attributed to kissing a frog, another with a glass splinter in her foot. There was a Mr. W.W. Winkee with hypothermia, and a young girl named Mary who thought she had contracted anthrax from a sheep. There was a boy named Jackie Horner with a tenosynovitis of the thumb.
The old lady came back in again. Now she was complaining of abdominal distention. When I finally laid eyes on her, I noted she certainly had a large abdomen. I grabbed a quick X-ray. Apparently she had taken my warnings against consuming avian and invertebrate entities, as now she had radiographic evidence of a feline skeleton. I planned to send her to a tertiary-care facility; perhaps they could do an endoscopic cat removal. Whatever they did, I was afraid if she kept this up she was bound to die.
I headed back to the ICU. They had dressed the girl, Miss White, in her street clothes and done her hair and makeup, but nothing could hide her severe pallor. She looked so peaceful.
Her “uncles” expectantly greeted me. They said in unison, “Welcome, Dr. Prince.” They were all inappropriately smiling. What was going on here? I went to declare her dead. I held a mirror up to her face, no signs of breathing, no lung sounds with auscultation. I laid my fingers gently across her throat. No pulse, but her skin was strangely pliant and warm.
I stared at her lovely face and the rest of the world suddenly shut itself off from me. I felt like singing. I could not stop myself … how unprofessional. But I bent over and gently kissed her goodbye. Suddenly there was music playing and—even more strangely—woodland animals frolicking at my feet. The uncles, who turned out to be roommates not relatives (had I known that I would never have stopped life support!), danced merrily. I looked back at her, and her eyes were open. She was smiling and gazing at her future husband, me.
Epilogue: The masticatory old lady eventually died from eating tainted horse meat. Mrs. Sprat improved with a low-fat diet. Mr. Wolfe died in a tragic logging accident, killed by the swing of an ax. The housing issues of the old lady with so many kids she did not know what to do was settled by the social worker, who became the septuplets’ godmother and found them housing in a refurbished, oversize shoe. As for Snow and me, we plan on living happily ever after. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
Having finished my family practice residency, and not being tied down by friends or family, I thought a round of working locum tenens would be a good way to see some different styles of practice.
I contacted a company called Locum-motion, filled out my paper work and was on my way. I had graduated from the University of Hamlin a few weeks earlier, armed with stethoscope and hammer. Dr. Claudio Prince (that’s me) was ready to stamp out disease and save lives.
There were several practice options available, but I chose a small hospital in Bremen. The three doctors who ran the hospital were going on a musical cruise for a week, and I would cover the entire facility for that time—ED and floor. I met with Drs. Baker, Butcher, and Maker briefly before they left for a trip. There were only a few inpatients, and one patient in the ICU. Then they were gone, and I was on my own.
My First Patient: I thought I’d start with the inpatients. The first was a Mr. B.B. Wolfe. He had been admitted for myalgia.
I was shocked when I walked into the room. He had severe hypertrichosis, prominent dentition, and proptosis. I briefly considered porphyria. I noted his history of muscle aches, high fever, facial swelling, and visual disturbance. His admit lab had showed a high CPK and LDH.
It sounded like an infestation of Trichinella to me. I questioned him about the ingestion of raw pork. He looked me in the eye and asked if his answer would be part of his medical record. I told him he needed to tell the truth for me to help him. Finally, he admitted he had eaten several portions of uncooked pig. I began to explain to him about the workup, the need for a muscle biopsy, and treatment options like mebendazole or steroids. I described the intestinal stage, which occurs between two and seven days after ingestion, when encysted larvae are liberated from the meat by gastric juices. I told him how the larvae mature into adult worms that burrow into the intestinal mucosa. I described the muscle stage, which develops after the first week and represents the period when adult-derived larvae in the intestines enter the bloodstream and disseminate hematogenously, then enter skeletal muscle causing pain.
He jumped out of bed and said he was leaving AMA; he had an appointment with a red-hooded girl. Whatever. I let him go; not much I could do to prevent his departure.
In the ED: I got a call from the ED about an old lady who had come in, having nearly choked on a bug. She looked fine to me, and I let her go. While down there, another older woman came in—in active labor. She admitted to having taken her friend’s Clomid, and had had little antepartum care secondary to a dearth of health insurance. Before I knew it, we were in the labor suite. First came one boy, then another, then another. I thought I was done, then two girls, another boy, then another girl. Seven babies—incredible! She moaned, not knowing what she was going to do with all these children.
I headed back to the floor to see more patients. The second one for the day was simple: a scrotal burn on a Mr. J.B. Nimble, who had been injured jumping over a flame. He was ready for discharge. The third patient was interesting, a Mrs. Spratt. I had been called by the lab with word that her serum looked like mayonnaise. She had abdominal pain, hepatosplenomegaly, memory loss, dyspnea and eruptive xanthomas. It sounded like type V hyperlipoproteinemia with chylomicronemia syndrome. What an interesting case, probably worse secondary to her very high-fat diet.
I walked down the hall to see my last inpatient, billed as a young man with psychotic depression pending psychiatric placement. I heard him yelling about burning witches and eating the walls. It sounded pretty psychotic. (I learned that his sister had been arraigned on manslaughter charges.) I entered the room and was struck by the smell of his breath, like cherries. A fruity smell, could he be ketotic? I instantly thought about ethylene glycol toxicity, maybe he had been sipping antifreeze, but there were no oxalate crystals in his urine. Was it delirium? I checked an O2 saturation; 95%, that wasn’t it. His blood pressure was 120/60. How about checking his finger-stick glucose? It read >400. This boy was in DKA! I started an insulin drip and hydration. It was only later I learned he had been on a starvation diet for weeks, then had binged on candy. His story about burning an old woman in a stove was unfortunately true. I called child protective services.
The ED pager went off again. That old lady who had swallowed a bug had ingested another, possibly a spider. I was worried about brown recluse or black widow envenomation, but it seemed to have been a simple barn spider, as she was now feeling OK. Again I discharged her with stern warnings to limit her invertebrate consumption. I stopped by the multiparous patient. She was happy to have the children and that they were all healthy, but what was she going to do? Where would she go? I called a social work consult.
ICU Time: I headed to the ICU. A sad story: a 24-year-old woman was unresponsive. She had a living will and did not want prolonged life support. She had choked on a piece of fruit. I walked into a room crowded with her family; it looked like seven very short uncles—one of whom was a doctor. They watched sadly as I pulled the endotracheal tube and the IVs. I told them I’d give them some time with her alone and would be back in an hour. It was a somber and tearful affair. She was so young and so beautiful. What a tragic end.
Another ED call; this bug-swallowing lady was driving them nuts. Now she was claiming she had swallowed a thrush, or maybe had thrush. Either way, she was gone before I got down there to see her.
I met with the social worker. She was dressed in a fancy gown and said she had just been at a party. She heard the story of the lady who had had so many children. She thought for a moment then said she had a few leads to check and would get back to me later that day.
We had a rush in the ED: a boy I thought might have rhinophyma and stiff-man syndrome, a girl with warts on her lips she attributed to kissing a frog, another with a glass splinter in her foot. There was a Mr. W.W. Winkee with hypothermia, and a young girl named Mary who thought she had contracted anthrax from a sheep. There was a boy named Jackie Horner with a tenosynovitis of the thumb.
The old lady came back in again. Now she was complaining of abdominal distention. When I finally laid eyes on her, I noted she certainly had a large abdomen. I grabbed a quick X-ray. Apparently she had taken my warnings against consuming avian and invertebrate entities, as now she had radiographic evidence of a feline skeleton. I planned to send her to a tertiary-care facility; perhaps they could do an endoscopic cat removal. Whatever they did, I was afraid if she kept this up she was bound to die.
I headed back to the ICU. They had dressed the girl, Miss White, in her street clothes and done her hair and makeup, but nothing could hide her severe pallor. She looked so peaceful.
Her “uncles” expectantly greeted me. They said in unison, “Welcome, Dr. Prince.” They were all inappropriately smiling. What was going on here? I went to declare her dead. I held a mirror up to her face, no signs of breathing, no lung sounds with auscultation. I laid my fingers gently across her throat. No pulse, but her skin was strangely pliant and warm.
I stared at her lovely face and the rest of the world suddenly shut itself off from me. I felt like singing. I could not stop myself … how unprofessional. But I bent over and gently kissed her goodbye. Suddenly there was music playing and—even more strangely—woodland animals frolicking at my feet. The uncles, who turned out to be roommates not relatives (had I known that I would never have stopped life support!), danced merrily. I looked back at her, and her eyes were open. She was smiling and gazing at her future husband, me.
Epilogue: The masticatory old lady eventually died from eating tainted horse meat. Mrs. Sprat improved with a low-fat diet. Mr. Wolfe died in a tragic logging accident, killed by the swing of an ax. The housing issues of the old lady with so many kids she did not know what to do was settled by the social worker, who became the septuplets’ godmother and found them housing in a refurbished, oversize shoe. As for Snow and me, we plan on living happily ever after. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
How to Hire and Use Clerical Staff
For the first few years of my career I was my own secretary. The hospitalist group I was part of ranged in size from two to nine doctors, and each of us handled all our own telephone correspondence and paperwork without clerical help. If you looked up our “office” phone number in the hospital’s physician directory you would find each individual’s pager number.
As a result, each of us got many pages every day regarding routine administrative issues such as hospital medical records, death certificates, and billing questions. Sometimes I felt as though I were answering nearly as many calls via pager as the hospital operator. And the pages about important clinical issues were mixed with all these routine inquiries.
While doing without clerical support in a hospitalist practice can help keep your overhead really low (ours was always well under 10%), it is not an efficient way to operate. A nonclinical support person is nearly always worthwhile. But, while the group I was part of made the mistake of trying to do without such a person (a problem we eventually fixed), a number of groups make the opposite mistake and hire too much clerical help, making it difficult or impossible to justify the cost.
Think carefully about clerical support positions. Unfortunately, in many practices in which the hospitalists are employees of the hospital, the doctors may not be engaged in deciding the optimal role and staffing (number of fulltime employees, or FTEs) for this position. To the doctors, it feels as though this person doesn’t cost them anything (in many cases the doctors aren’t paying for it directly, the hospital is), so they might not spend a lot of time thinking about whether they’re really getting good value for the money. But the doctors are in a much better position than other hospital administrators to know whether that position optimally supports the practice.
The amount of staffing and precise job descriptions will vary tremendously from one practice to another. I want to offer some general guidelines worth consideration by nearly all practices. This discussion is not about support personnel, such as case managers dedicated to the hospitalist practice, midlevel providers, or other clinical support staff. This discussion is really about the front-office support staff for your practice.
How Many to Hire?
My experience suggests a hospitalist practice should have about one FTE of clerical support for every five to 15 FTE hospitalists. The optimal staffing for a particular practice will vary depending on the person’s precise responsibilities. A practice that operates at more than one site (e.g., one hospitalist group covers two hospitals) will usually need more support than one that operates in one hospital.
Practices smaller than five or six FTE hospitalists often need less than full-time support. They might work well using part-time clerical support from an existing member of the hospital’s staff, such as someone in administration or the medical staff office. In many cases this might mean the person has one incoming phone line dedicated to hospitalist calls and another dedicated to the other activity. Depending on which line rings, he/she answers by saying, “hospitalist office,” or “medical staff office.” Usually it is best for the person to be responsible for both activities all day long and not divide his/her time into working for the hospitalists only until noon, then spending the rest of each day supporting the other activity. Until the group I am currently part of grew to eight FTE hospitalists, our clerical support person had a full-time job—half of which was devoted to supporting our practice and the other half to supporting the hospital’s Institutional Review Board (IRB).
Define the Job
There are a number of common ways for a support person to contribute to the practice, which I have grouped into several broad categories:
Handle telephone correspondence. This person should answer all calls to the practice’s main office number. Most practices will have a separate number for billing inquiries, and clinical calls from the hospital’s nursing staff are usually paged directly to the doctor by a nurse. But that still leaves a lot of calls that will go to the support person, such as administrative questions about the practice, calls from former patients (who have been discharged) and families, pharmacies (e.g., asking about refills), funeral homes, and others.
Some practices use a “triage pager” system in which all calls about new referrals to the practice (e.g., from ED doctors, referring PCPs, surgeons requesting consults) always go to the triage pager—day or night. Usually the individual doctors take turns carrying and responding to the triage pager, and after hearing about a referral to the practice they will call the doctor who is up next for new patients and pass the information along. In a large practice, that pager can generate a huge number of daytime calls, making it difficult or impossible for the person holding the triage pager to also care for patients.
Some practices have found that the practice clerical support person can take all those calls during the daytime Monday through Friday and pass them along to the appropriate hospitalist. The clerical person would typically get only the patient name and location and the referring doctor’s name and contact information, then page it to the hospitalist next in line for a new referral. That hospitalist would then call back the referring physician to get more clinical information. That relieves a member of the practice from taking all the calls. And, it puts the referring physician directly in contact with the hospitalist who will see the patient, rather than a triage doctor who won’t be caring for the patient. This should mean a better handoff.
Handle paper correspondence. This person can sort all the faxes, mail, and medical records that come to the practice, and put them in each doctor’s mail box in the office. He/she might initiate work on some forms. For example, upon arrival of a form to certify medical necessity for a piece of equipment (e.g., home oxygen ordered on a patient recently discharged) he might open the envelope, complete as much of the form as possible, attach the relevant records from the hospital stay, and leave all this for the doctor to sign.
Another potentially critical function is to request and pursue outside clinical records requested by one of the hospitalists. For example, a hospitalist admits Ms. Smith at 1 a.m. and realizes it will be helpful to get previous creatinine values from the PCP’s office and the report of a prior cardiac cath from an outside hospital. The hospitalist could simply record a voice mail (at 1 a.m., while seeing the patient) requesting that the practice assistant track down these things the next morning. That might include ensuring an appropriate release-of-information form is signed by the patient and faxed to the outside facility. When the records arrive, the assistant would place them on the patient’s chart (and, if necessary, page the hospitalist to report that the records have arrived).
Support billing functions. Practices use many strategies to ensure good documentation, coding, charge capture, and billing. The assistant might play an important role in this process. For example, the doctors might first report all charge data to the practice assistant who reviews it to make sure there are no conflicting charges (e.g., two doctors bill the same service to a patient on the same day) and no missing charges (e.g., a doctor forgot to submit a charge for one day of a patient’s stay). The assistant can be the principle connection between the doctors and the billing service and might be the first person to troubleshoot problems encountered by the billing service (e.g., getting additional documentation, figuring out which doctor can best address an ICD-9 code that lacks a fifth digit).
Perform general practice administrative functions. The assistant can keep track of when each doctor needs to renew his or her state license, DEA certificate, ACLS certificate, as well as keep track of total hours of CME (e.g., know how many more CME hours each doctor needs this year for state licensing requirements). He/she could also assist in various human resource functions such as ensuring each doctor responds during the open-enrollment period for benefits each year.
In some practices it is appropriate for the assistant to create the physician work schedule for the next month, quarter, or year, and serve as the main point of contact for any schedule change the doctor’s need to make. However, for groups that use a complicated scheduling system, the doctors will often need to take an active role in its creation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
For the first few years of my career I was my own secretary. The hospitalist group I was part of ranged in size from two to nine doctors, and each of us handled all our own telephone correspondence and paperwork without clerical help. If you looked up our “office” phone number in the hospital’s physician directory you would find each individual’s pager number.
As a result, each of us got many pages every day regarding routine administrative issues such as hospital medical records, death certificates, and billing questions. Sometimes I felt as though I were answering nearly as many calls via pager as the hospital operator. And the pages about important clinical issues were mixed with all these routine inquiries.
While doing without clerical support in a hospitalist practice can help keep your overhead really low (ours was always well under 10%), it is not an efficient way to operate. A nonclinical support person is nearly always worthwhile. But, while the group I was part of made the mistake of trying to do without such a person (a problem we eventually fixed), a number of groups make the opposite mistake and hire too much clerical help, making it difficult or impossible to justify the cost.
Think carefully about clerical support positions. Unfortunately, in many practices in which the hospitalists are employees of the hospital, the doctors may not be engaged in deciding the optimal role and staffing (number of fulltime employees, or FTEs) for this position. To the doctors, it feels as though this person doesn’t cost them anything (in many cases the doctors aren’t paying for it directly, the hospital is), so they might not spend a lot of time thinking about whether they’re really getting good value for the money. But the doctors are in a much better position than other hospital administrators to know whether that position optimally supports the practice.
The amount of staffing and precise job descriptions will vary tremendously from one practice to another. I want to offer some general guidelines worth consideration by nearly all practices. This discussion is not about support personnel, such as case managers dedicated to the hospitalist practice, midlevel providers, or other clinical support staff. This discussion is really about the front-office support staff for your practice.
How Many to Hire?
My experience suggests a hospitalist practice should have about one FTE of clerical support for every five to 15 FTE hospitalists. The optimal staffing for a particular practice will vary depending on the person’s precise responsibilities. A practice that operates at more than one site (e.g., one hospitalist group covers two hospitals) will usually need more support than one that operates in one hospital.
Practices smaller than five or six FTE hospitalists often need less than full-time support. They might work well using part-time clerical support from an existing member of the hospital’s staff, such as someone in administration or the medical staff office. In many cases this might mean the person has one incoming phone line dedicated to hospitalist calls and another dedicated to the other activity. Depending on which line rings, he/she answers by saying, “hospitalist office,” or “medical staff office.” Usually it is best for the person to be responsible for both activities all day long and not divide his/her time into working for the hospitalists only until noon, then spending the rest of each day supporting the other activity. Until the group I am currently part of grew to eight FTE hospitalists, our clerical support person had a full-time job—half of which was devoted to supporting our practice and the other half to supporting the hospital’s Institutional Review Board (IRB).
Define the Job
There are a number of common ways for a support person to contribute to the practice, which I have grouped into several broad categories:
Handle telephone correspondence. This person should answer all calls to the practice’s main office number. Most practices will have a separate number for billing inquiries, and clinical calls from the hospital’s nursing staff are usually paged directly to the doctor by a nurse. But that still leaves a lot of calls that will go to the support person, such as administrative questions about the practice, calls from former patients (who have been discharged) and families, pharmacies (e.g., asking about refills), funeral homes, and others.
Some practices use a “triage pager” system in which all calls about new referrals to the practice (e.g., from ED doctors, referring PCPs, surgeons requesting consults) always go to the triage pager—day or night. Usually the individual doctors take turns carrying and responding to the triage pager, and after hearing about a referral to the practice they will call the doctor who is up next for new patients and pass the information along. In a large practice, that pager can generate a huge number of daytime calls, making it difficult or impossible for the person holding the triage pager to also care for patients.
Some practices have found that the practice clerical support person can take all those calls during the daytime Monday through Friday and pass them along to the appropriate hospitalist. The clerical person would typically get only the patient name and location and the referring doctor’s name and contact information, then page it to the hospitalist next in line for a new referral. That hospitalist would then call back the referring physician to get more clinical information. That relieves a member of the practice from taking all the calls. And, it puts the referring physician directly in contact with the hospitalist who will see the patient, rather than a triage doctor who won’t be caring for the patient. This should mean a better handoff.
Handle paper correspondence. This person can sort all the faxes, mail, and medical records that come to the practice, and put them in each doctor’s mail box in the office. He/she might initiate work on some forms. For example, upon arrival of a form to certify medical necessity for a piece of equipment (e.g., home oxygen ordered on a patient recently discharged) he might open the envelope, complete as much of the form as possible, attach the relevant records from the hospital stay, and leave all this for the doctor to sign.
Another potentially critical function is to request and pursue outside clinical records requested by one of the hospitalists. For example, a hospitalist admits Ms. Smith at 1 a.m. and realizes it will be helpful to get previous creatinine values from the PCP’s office and the report of a prior cardiac cath from an outside hospital. The hospitalist could simply record a voice mail (at 1 a.m., while seeing the patient) requesting that the practice assistant track down these things the next morning. That might include ensuring an appropriate release-of-information form is signed by the patient and faxed to the outside facility. When the records arrive, the assistant would place them on the patient’s chart (and, if necessary, page the hospitalist to report that the records have arrived).
Support billing functions. Practices use many strategies to ensure good documentation, coding, charge capture, and billing. The assistant might play an important role in this process. For example, the doctors might first report all charge data to the practice assistant who reviews it to make sure there are no conflicting charges (e.g., two doctors bill the same service to a patient on the same day) and no missing charges (e.g., a doctor forgot to submit a charge for one day of a patient’s stay). The assistant can be the principle connection between the doctors and the billing service and might be the first person to troubleshoot problems encountered by the billing service (e.g., getting additional documentation, figuring out which doctor can best address an ICD-9 code that lacks a fifth digit).
Perform general practice administrative functions. The assistant can keep track of when each doctor needs to renew his or her state license, DEA certificate, ACLS certificate, as well as keep track of total hours of CME (e.g., know how many more CME hours each doctor needs this year for state licensing requirements). He/she could also assist in various human resource functions such as ensuring each doctor responds during the open-enrollment period for benefits each year.
In some practices it is appropriate for the assistant to create the physician work schedule for the next month, quarter, or year, and serve as the main point of contact for any schedule change the doctor’s need to make. However, for groups that use a complicated scheduling system, the doctors will often need to take an active role in its creation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
For the first few years of my career I was my own secretary. The hospitalist group I was part of ranged in size from two to nine doctors, and each of us handled all our own telephone correspondence and paperwork without clerical help. If you looked up our “office” phone number in the hospital’s physician directory you would find each individual’s pager number.
As a result, each of us got many pages every day regarding routine administrative issues such as hospital medical records, death certificates, and billing questions. Sometimes I felt as though I were answering nearly as many calls via pager as the hospital operator. And the pages about important clinical issues were mixed with all these routine inquiries.
While doing without clerical support in a hospitalist practice can help keep your overhead really low (ours was always well under 10%), it is not an efficient way to operate. A nonclinical support person is nearly always worthwhile. But, while the group I was part of made the mistake of trying to do without such a person (a problem we eventually fixed), a number of groups make the opposite mistake and hire too much clerical help, making it difficult or impossible to justify the cost.
Think carefully about clerical support positions. Unfortunately, in many practices in which the hospitalists are employees of the hospital, the doctors may not be engaged in deciding the optimal role and staffing (number of fulltime employees, or FTEs) for this position. To the doctors, it feels as though this person doesn’t cost them anything (in many cases the doctors aren’t paying for it directly, the hospital is), so they might not spend a lot of time thinking about whether they’re really getting good value for the money. But the doctors are in a much better position than other hospital administrators to know whether that position optimally supports the practice.
The amount of staffing and precise job descriptions will vary tremendously from one practice to another. I want to offer some general guidelines worth consideration by nearly all practices. This discussion is not about support personnel, such as case managers dedicated to the hospitalist practice, midlevel providers, or other clinical support staff. This discussion is really about the front-office support staff for your practice.
How Many to Hire?
My experience suggests a hospitalist practice should have about one FTE of clerical support for every five to 15 FTE hospitalists. The optimal staffing for a particular practice will vary depending on the person’s precise responsibilities. A practice that operates at more than one site (e.g., one hospitalist group covers two hospitals) will usually need more support than one that operates in one hospital.
Practices smaller than five or six FTE hospitalists often need less than full-time support. They might work well using part-time clerical support from an existing member of the hospital’s staff, such as someone in administration or the medical staff office. In many cases this might mean the person has one incoming phone line dedicated to hospitalist calls and another dedicated to the other activity. Depending on which line rings, he/she answers by saying, “hospitalist office,” or “medical staff office.” Usually it is best for the person to be responsible for both activities all day long and not divide his/her time into working for the hospitalists only until noon, then spending the rest of each day supporting the other activity. Until the group I am currently part of grew to eight FTE hospitalists, our clerical support person had a full-time job—half of which was devoted to supporting our practice and the other half to supporting the hospital’s Institutional Review Board (IRB).
Define the Job
There are a number of common ways for a support person to contribute to the practice, which I have grouped into several broad categories:
Handle telephone correspondence. This person should answer all calls to the practice’s main office number. Most practices will have a separate number for billing inquiries, and clinical calls from the hospital’s nursing staff are usually paged directly to the doctor by a nurse. But that still leaves a lot of calls that will go to the support person, such as administrative questions about the practice, calls from former patients (who have been discharged) and families, pharmacies (e.g., asking about refills), funeral homes, and others.
Some practices use a “triage pager” system in which all calls about new referrals to the practice (e.g., from ED doctors, referring PCPs, surgeons requesting consults) always go to the triage pager—day or night. Usually the individual doctors take turns carrying and responding to the triage pager, and after hearing about a referral to the practice they will call the doctor who is up next for new patients and pass the information along. In a large practice, that pager can generate a huge number of daytime calls, making it difficult or impossible for the person holding the triage pager to also care for patients.
Some practices have found that the practice clerical support person can take all those calls during the daytime Monday through Friday and pass them along to the appropriate hospitalist. The clerical person would typically get only the patient name and location and the referring doctor’s name and contact information, then page it to the hospitalist next in line for a new referral. That hospitalist would then call back the referring physician to get more clinical information. That relieves a member of the practice from taking all the calls. And, it puts the referring physician directly in contact with the hospitalist who will see the patient, rather than a triage doctor who won’t be caring for the patient. This should mean a better handoff.
Handle paper correspondence. This person can sort all the faxes, mail, and medical records that come to the practice, and put them in each doctor’s mail box in the office. He/she might initiate work on some forms. For example, upon arrival of a form to certify medical necessity for a piece of equipment (e.g., home oxygen ordered on a patient recently discharged) he might open the envelope, complete as much of the form as possible, attach the relevant records from the hospital stay, and leave all this for the doctor to sign.
Another potentially critical function is to request and pursue outside clinical records requested by one of the hospitalists. For example, a hospitalist admits Ms. Smith at 1 a.m. and realizes it will be helpful to get previous creatinine values from the PCP’s office and the report of a prior cardiac cath from an outside hospital. The hospitalist could simply record a voice mail (at 1 a.m., while seeing the patient) requesting that the practice assistant track down these things the next morning. That might include ensuring an appropriate release-of-information form is signed by the patient and faxed to the outside facility. When the records arrive, the assistant would place them on the patient’s chart (and, if necessary, page the hospitalist to report that the records have arrived).
Support billing functions. Practices use many strategies to ensure good documentation, coding, charge capture, and billing. The assistant might play an important role in this process. For example, the doctors might first report all charge data to the practice assistant who reviews it to make sure there are no conflicting charges (e.g., two doctors bill the same service to a patient on the same day) and no missing charges (e.g., a doctor forgot to submit a charge for one day of a patient’s stay). The assistant can be the principle connection between the doctors and the billing service and might be the first person to troubleshoot problems encountered by the billing service (e.g., getting additional documentation, figuring out which doctor can best address an ICD-9 code that lacks a fifth digit).
Perform general practice administrative functions. The assistant can keep track of when each doctor needs to renew his or her state license, DEA certificate, ACLS certificate, as well as keep track of total hours of CME (e.g., know how many more CME hours each doctor needs this year for state licensing requirements). He/she could also assist in various human resource functions such as ensuring each doctor responds during the open-enrollment period for benefits each year.
In some practices it is appropriate for the assistant to create the physician work schedule for the next month, quarter, or year, and serve as the main point of contact for any schedule change the doctor’s need to make. However, for groups that use a complicated scheduling system, the doctors will often need to take an active role in its creation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
In the Literature
Thrombocytopenia Reaction to Vancomycin
Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007 Mar 1;356(9):904-910
The use of vancomycin has grown exponentially in the past 20 years.1 Physicians have become increasingly aware of its major side effects, such as red man syndrome, hypersensitivity, neutropenia, and nephrotoxicity. But there have been only a few case reports of thrombocytopenia associated with this drug. This article looked at cases of thrombocytopenia in patients referred for clinical suspicion of vancomycin-induced thrombocytopenia.
From 2001-2005, serum samples were sent to the Platelet and Neutrophil Immunology Laboratory at the BloodCenter of Wisconsin in Milwaukee for testing for vancomycin-dependent antibodies from several sites. Clinical information regarding these patients was obtained from their referring physicians and one of the authors. Platelet reactive antibodies were detected by flow cytometry.
IgG and IgM vancomycin-dependent antibodies were detected in 34 patients. It was found that platelets dropped an average of 93% from pretreatment levels, and the average nadir occurred on day eight. The mean platelet count was 13,600. After vancomycin was discontinued, the platelet count returned to normal in all patients except for the three who died. The average time for resolution of thrombocytopenia was 7.5 days.
Unlike other drug-induced thrombocytopenia, these cases of thrombocytopenia associated with vancomycin appear to be more prone to significant hemorrhage. In this group 34% were found to have had severe hemorrhage defined in this study as florid petechial hemorrhages, ecchymoses, and oozing form the buccal mucosa. Three patients who had renal insufficiency were found to be profoundly thrombocytopenic for a longer duration, presumably due to delayed clearance of vancomycin in this setting.
Based on this study, it appears thrombocytopenia is a significant adverse reaction that can be attributed to vancomycin. Unlike other drug-induced thrombocytopenias, it appears to be associated with a higher likelihood of significant hemorrhage, as well.
Thrombocytopenia is a common occurrence in the acutely ill hospitalized patient and has been linked to increased hospital mortality and increased length of stay.2 Many drugs and diseases that hospitalists treat are associated with thrombocytopenia. The indications for usage of vancomycin continues to grow with the increasing number of patients with prosthetic devices and intravascular access, and the increasing prevalence of MRSA. This study raises awareness of a significant side effect that can be associated with vancomycin.
References
- Ena J, Dick RW, Jones RN, et al. The epidemiology of intravenous vancomycin usage in a university hospital: a 10-year study. JAMA. 1993 Feb 3;269(5):598-602. Comment in JAMA. 1993 Sep 22-29;270(12):1426.
- Crowther MA, Cook DJ, Meade M, et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005 Dec;20(4):248-253.
Can the mBRS Stratify Pts Admitted for Nonvariceal Upper GI Bleeds?
Romagnuolo J, Barkun AN, Enns R, et al. Simple clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch Intern Med. 2007 Feb 12;167(3):265-270.
Nonvariceal upper gastrointestinal bleeding is one of the top 10 admission diagnoses based on reviews of diagnosis-related groups. Patients with low-risk lesions on endoscopy, such as ulcers with a clean base, esophagitis, gastritis, duodenitis, or Mallory-Weiss tears, are felt to have less than a 5% chance of recurrent bleeding. In some instances, these patients can be treated successfully and discharged to home.1
Unfortunately, endoscopy is not always available—especially late at night and on weekends. It would be helpful to have a clinical prediction rule to identify patients at low risk for bleeding who could be safely discharged to get endoscopy within a few days.
In the study, 1,869 patients who had undergone upper endoscopy for upper gastrointestinal bleeding were entered into a Canadian national Registry for Upper GI Bleeding and Endoscopy (RUGBE). A modified Blatchford risk score (mBRS) was calculated to see if it could predict the presence of high-risk stigmata of bleeding, rebleeding rates, and mortality.
This mBRS was also compared with another scoring system—the Rockall score. The mBRS uses clinical and laboratory data to risk assess nonvariceal bleeding. The variables included in the scoring system include hemoglobin, systolic blood pressure, heart rate, melena, liver disease, and heart failure. High-risk endoscopic stigmata were defined as adherent clot after irrigation, a bleeding, oozing or spurting vessel, or a nonbleeding visible vessel. Rebleeding was defined as hematemesis, melena, or a bloody nasogastric aspirate in the presence of shock or a decrease in hemoglobin of 2 g/dL or more.
Patients who had a modified Blatchford risk score of <1 were found to have a lower likelihood of high-risk stigmata on endoscopy and were at a low risk for rebleeding (5%). Patients who had high-risk stigmata on endoscopy but an mBRS score of <1 were also found to have low rebleeding rates. The mBRS seemed to a better predictor than the Rockall score for high-risk stigmata and for rebleeding rates.
Patients with nonvariceal upper gastrointestinal tract bleeding may be identified as low risk for re-bleeding if they are normotensive, not tachycardic, not anemic, and do not have active melena, liver disease, or heart failure. It is conceivable that if endoscopy were not available, these patients could be sent home on high-dose proton pump inhibitor and asked to return for outpatient upper endoscopy within a few days.
The study certainly raises interesting questions. Whether it is acceptable practice to discharge a “low-risk” patient with an upper gastrointestinal hemorrhage on a high-dose proton pump inhibitor with good social support and close outpatient follow-up, but without diagnostic endoscopy is still unclear.
The study is limited by the fact that it is a retrospective analysis; however, it does examine a large cohort of patients. The authors acknowledge this, and this work could lead to a prospective randomized trial that would help answer this question. In the meantime, the mBRS may be a helpful tool to help risk stratify patients admitted for nonvariceal upper gastrointestinal bleeding.
References
- Cipolletta L, Bianco M, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002;55(1):1-5.
Lumbar Puncture to Reduce Adverse Events
Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006 Oct 25;296(16):2012-2022.
Lumbar punctures (LPs) remain a common diagnostic test performed by physicians to rule out meningitis. This procedure may be associated with adverse events, with headache and backache the most commonly reported. This systematic review and meta-analysis sought to review the evidence regarding diagnostic lumbar puncture techniques that might reduce the risk of adverse events, and to examine the accuracy of cerebrospinal fluid (CSF) analysis in the diagnosis of bacterial meningitis.
Studies were identified through searches of the Cochrane Library (www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/AboutCochrane.html), MEDLINE from 1966 to January 2006, and EMBASE from 1980 to January 2006 (without language restrictions) to identify relevant studies. Bibliographies of retrieved articles were also used as data sources.
Randomized controlled trials of patients 18 or older undergoing lumbar puncture testing interventions to facilitate a successful diagnostic procedure or reduce adverse events were identified and selected. As a secondary outcome, trials that assessed the accuracy of CSF biochemical analysis for the diagnosis of bacterial meningitis were also identified and included. Trials that studied spinal anesthesia or myelography were excluded.
Study appraisals for quality (randomization, blinding, and outcome assessment) and data extraction were performed by two investigators independently. Fifteen randomized trials of interventions to reduce adverse events met criteria for inclusion, and four studies of the diagnostic test characteristics of CSF analysis met criteria and were included.
Meta-analysis with a random effects model of five studies (total of 587 patients) comparing atraumatic needles with standard needles yielded a nonsignificant decrease in the odds of headache with an atraumatic needle (absolute risk reduction [ARR], 12.3%; 95% confidence interval [CI], –1.72% to 26.2%). A single study of reinsertion of the stylet before needle removal (600 patients) showed a decreased risk of headache (ARR, 11.3%; 95% CI, 6.50%-16.2%). Meta-analysis of four studies (717 patients) revealed a nonsignificant decrease in headache in patients mobilized after LP (ARR 2.9%; 95% CI, –3.4 to 9.3%).
Data from the diagnostic test studies yielded the following likelihood ratios for diagnosing bacterial meningitis: A CSF–blood glucose ratio of 0.4 or less with a likelihood ratio of 18 (95% CI, 12-27); CSF white blood cell count of 500/µL or higher with a likelihood ratio of 15 (95% CI, 10-22); and CSF lactate level of >31.53 mg/dL with a likelihood ration of 21 (95% CI, 14-32) in accurately diagnosed bacterial meningitis.
These data support the reinsertion of the stylet before needle removal to reduce the risk of headache after lumbar puncture and that patients do not require bed rest after diagnostic lumbar puncture. Biochemical analyses, including CSF-blood glucose ratio, CSF leukocyte count and lactate level are useful in diagnosing bacterial meningitis.
This Rational Clinical Examination systematic review and meta-analysis provides a nice review of the available data on optimizing diagnostic lumbar puncture technique to reduce adverse events. It is somewhat remarkable so little has changed in our knowledge about this long-standing diagnostic procedure. Post-lumbar puncture headaches remain a challenge that may affect patient satisfaction as well as hospital (or observation unit) course particularly for patients who do not have evidence of bacterial meningitis once the analysis is complete.
This review seems to provide some useful answers for physicians performing lumbar puncture, who should consider selecting a small gauge needle and reinserting the stylet prior to removal. Future studies of other maneuvers to reduce post-procedure adverse events should be considered for the question of atraumatic needles, which may be technically more difficult to use. The review confirms and helps quantify the utility of CSF biochemical analysis in the diagnosis of bacterial meningitis.
Who’s Performing Procedures?
Wigton RS, Alguire P. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007 Mar 6;146(5):355-360. Comment in Ann Intern Med. 2007 Mar 6; 146(5):392-393.
Prior surveys of physicians documented that general internists performed a variety and significant number of procedures in their practice. Much has changed since those prior assessments, including physician training, practice settings, availability of subspecialists, and regulatory requirements that have altered physician’s practice with regard to procedures. This study sought to reassess the volume and variety of procedures performed by general internists compared with the prior survey of 1986. The final sample included 990 completed surveys from general internists from 1,389 returned questionnaires for a successful completion rate of 39.6%.
The median number of different procedures performed in practice decreased from 16 in 1986 to seven in 2004. Internists who practiced in smaller hospitals or smaller towns reported performing almost twice as many procedures as physicians in the largest hospitals and cities. Hours spent in the care of hospitalized patients were also associated with an increased number of different procedures—in particular mechanical ventilation, central venous catheter placement, and thoracentesis. For all but one of the 34 procedures common to both surveys, fewer general internists performed them in 2004 compared with 1986. Remarkably, for 22 of the 34 procedures, a greater than 50% reduction in the proportion of respondents who performed the procedure was noted.
In the 1986 survey, the majority of internists performed all but one of the six procedures required by the American Board of Internal Medicine (ABIM) for certification (abdominal paracentesis, arterial puncture for blood gases, central venous catheter placement, joint aspiration, lumbar puncture, and thoracentesis). Except for joint aspiration, in 2004 these required procedures were performed by 25% or fewer of the respondents.
The 2004 survey demonstrated a striking reduction in the number of different procedures performed by general internists, and a decrease in the proportion of internists who do most procedures. These reductions may stem from a variety of changes in physician practices, including the emergence of hospitalists, availability of subspecialty physicians and proceduralists, and changes in technology and regulatory environments.
Regardless of the forces behind these changes, internal medicine residents’ training in procedures should be re-examined.
Many of those in academic hospital medicine have noted a decline in procedures performed by general internists at large academic centers. This study affirms this trend overall and in particular for physicians in large urban settings or in the largest hospitals. The emergence of hospital medicine may have played a role in reducing the procedures performed by primary care (outpatient) physicians who now spend less time caring for medically ill hospitalized patients.
Residency programs now must consider how to incorporate procedure skills and training to align with the needs of internists. The rising interest in careers in hospital medicine (as opposed to outpatient primary care) necessitates a new approach and individualized plans for gaining procedural skills to match career goals and practice settings. The new ABIM policy acknowledges this greater variability in the procedures performed by internists in practice, and takes steps to more closely align procedure requirements and core manual skills with physician practice.
These changes and new flexibility in requirements provide another opportunity for academic hospital medicine programs to provide leadership, and help shape the training of inpatient physicians. TH
Thrombocytopenia Reaction to Vancomycin
Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007 Mar 1;356(9):904-910
The use of vancomycin has grown exponentially in the past 20 years.1 Physicians have become increasingly aware of its major side effects, such as red man syndrome, hypersensitivity, neutropenia, and nephrotoxicity. But there have been only a few case reports of thrombocytopenia associated with this drug. This article looked at cases of thrombocytopenia in patients referred for clinical suspicion of vancomycin-induced thrombocytopenia.
From 2001-2005, serum samples were sent to the Platelet and Neutrophil Immunology Laboratory at the BloodCenter of Wisconsin in Milwaukee for testing for vancomycin-dependent antibodies from several sites. Clinical information regarding these patients was obtained from their referring physicians and one of the authors. Platelet reactive antibodies were detected by flow cytometry.
IgG and IgM vancomycin-dependent antibodies were detected in 34 patients. It was found that platelets dropped an average of 93% from pretreatment levels, and the average nadir occurred on day eight. The mean platelet count was 13,600. After vancomycin was discontinued, the platelet count returned to normal in all patients except for the three who died. The average time for resolution of thrombocytopenia was 7.5 days.
Unlike other drug-induced thrombocytopenia, these cases of thrombocytopenia associated with vancomycin appear to be more prone to significant hemorrhage. In this group 34% were found to have had severe hemorrhage defined in this study as florid petechial hemorrhages, ecchymoses, and oozing form the buccal mucosa. Three patients who had renal insufficiency were found to be profoundly thrombocytopenic for a longer duration, presumably due to delayed clearance of vancomycin in this setting.
Based on this study, it appears thrombocytopenia is a significant adverse reaction that can be attributed to vancomycin. Unlike other drug-induced thrombocytopenias, it appears to be associated with a higher likelihood of significant hemorrhage, as well.
Thrombocytopenia is a common occurrence in the acutely ill hospitalized patient and has been linked to increased hospital mortality and increased length of stay.2 Many drugs and diseases that hospitalists treat are associated with thrombocytopenia. The indications for usage of vancomycin continues to grow with the increasing number of patients with prosthetic devices and intravascular access, and the increasing prevalence of MRSA. This study raises awareness of a significant side effect that can be associated with vancomycin.
References
- Ena J, Dick RW, Jones RN, et al. The epidemiology of intravenous vancomycin usage in a university hospital: a 10-year study. JAMA. 1993 Feb 3;269(5):598-602. Comment in JAMA. 1993 Sep 22-29;270(12):1426.
- Crowther MA, Cook DJ, Meade M, et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005 Dec;20(4):248-253.
Can the mBRS Stratify Pts Admitted for Nonvariceal Upper GI Bleeds?
Romagnuolo J, Barkun AN, Enns R, et al. Simple clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch Intern Med. 2007 Feb 12;167(3):265-270.
Nonvariceal upper gastrointestinal bleeding is one of the top 10 admission diagnoses based on reviews of diagnosis-related groups. Patients with low-risk lesions on endoscopy, such as ulcers with a clean base, esophagitis, gastritis, duodenitis, or Mallory-Weiss tears, are felt to have less than a 5% chance of recurrent bleeding. In some instances, these patients can be treated successfully and discharged to home.1
Unfortunately, endoscopy is not always available—especially late at night and on weekends. It would be helpful to have a clinical prediction rule to identify patients at low risk for bleeding who could be safely discharged to get endoscopy within a few days.
In the study, 1,869 patients who had undergone upper endoscopy for upper gastrointestinal bleeding were entered into a Canadian national Registry for Upper GI Bleeding and Endoscopy (RUGBE). A modified Blatchford risk score (mBRS) was calculated to see if it could predict the presence of high-risk stigmata of bleeding, rebleeding rates, and mortality.
This mBRS was also compared with another scoring system—the Rockall score. The mBRS uses clinical and laboratory data to risk assess nonvariceal bleeding. The variables included in the scoring system include hemoglobin, systolic blood pressure, heart rate, melena, liver disease, and heart failure. High-risk endoscopic stigmata were defined as adherent clot after irrigation, a bleeding, oozing or spurting vessel, or a nonbleeding visible vessel. Rebleeding was defined as hematemesis, melena, or a bloody nasogastric aspirate in the presence of shock or a decrease in hemoglobin of 2 g/dL or more.
Patients who had a modified Blatchford risk score of <1 were found to have a lower likelihood of high-risk stigmata on endoscopy and were at a low risk for rebleeding (5%). Patients who had high-risk stigmata on endoscopy but an mBRS score of <1 were also found to have low rebleeding rates. The mBRS seemed to a better predictor than the Rockall score for high-risk stigmata and for rebleeding rates.
Patients with nonvariceal upper gastrointestinal tract bleeding may be identified as low risk for re-bleeding if they are normotensive, not tachycardic, not anemic, and do not have active melena, liver disease, or heart failure. It is conceivable that if endoscopy were not available, these patients could be sent home on high-dose proton pump inhibitor and asked to return for outpatient upper endoscopy within a few days.
The study certainly raises interesting questions. Whether it is acceptable practice to discharge a “low-risk” patient with an upper gastrointestinal hemorrhage on a high-dose proton pump inhibitor with good social support and close outpatient follow-up, but without diagnostic endoscopy is still unclear.
The study is limited by the fact that it is a retrospective analysis; however, it does examine a large cohort of patients. The authors acknowledge this, and this work could lead to a prospective randomized trial that would help answer this question. In the meantime, the mBRS may be a helpful tool to help risk stratify patients admitted for nonvariceal upper gastrointestinal bleeding.
References
- Cipolletta L, Bianco M, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002;55(1):1-5.
Lumbar Puncture to Reduce Adverse Events
Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006 Oct 25;296(16):2012-2022.
Lumbar punctures (LPs) remain a common diagnostic test performed by physicians to rule out meningitis. This procedure may be associated with adverse events, with headache and backache the most commonly reported. This systematic review and meta-analysis sought to review the evidence regarding diagnostic lumbar puncture techniques that might reduce the risk of adverse events, and to examine the accuracy of cerebrospinal fluid (CSF) analysis in the diagnosis of bacterial meningitis.
Studies were identified through searches of the Cochrane Library (www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/AboutCochrane.html), MEDLINE from 1966 to January 2006, and EMBASE from 1980 to January 2006 (without language restrictions) to identify relevant studies. Bibliographies of retrieved articles were also used as data sources.
Randomized controlled trials of patients 18 or older undergoing lumbar puncture testing interventions to facilitate a successful diagnostic procedure or reduce adverse events were identified and selected. As a secondary outcome, trials that assessed the accuracy of CSF biochemical analysis for the diagnosis of bacterial meningitis were also identified and included. Trials that studied spinal anesthesia or myelography were excluded.
Study appraisals for quality (randomization, blinding, and outcome assessment) and data extraction were performed by two investigators independently. Fifteen randomized trials of interventions to reduce adverse events met criteria for inclusion, and four studies of the diagnostic test characteristics of CSF analysis met criteria and were included.
Meta-analysis with a random effects model of five studies (total of 587 patients) comparing atraumatic needles with standard needles yielded a nonsignificant decrease in the odds of headache with an atraumatic needle (absolute risk reduction [ARR], 12.3%; 95% confidence interval [CI], –1.72% to 26.2%). A single study of reinsertion of the stylet before needle removal (600 patients) showed a decreased risk of headache (ARR, 11.3%; 95% CI, 6.50%-16.2%). Meta-analysis of four studies (717 patients) revealed a nonsignificant decrease in headache in patients mobilized after LP (ARR 2.9%; 95% CI, –3.4 to 9.3%).
Data from the diagnostic test studies yielded the following likelihood ratios for diagnosing bacterial meningitis: A CSF–blood glucose ratio of 0.4 or less with a likelihood ratio of 18 (95% CI, 12-27); CSF white blood cell count of 500/µL or higher with a likelihood ratio of 15 (95% CI, 10-22); and CSF lactate level of >31.53 mg/dL with a likelihood ration of 21 (95% CI, 14-32) in accurately diagnosed bacterial meningitis.
These data support the reinsertion of the stylet before needle removal to reduce the risk of headache after lumbar puncture and that patients do not require bed rest after diagnostic lumbar puncture. Biochemical analyses, including CSF-blood glucose ratio, CSF leukocyte count and lactate level are useful in diagnosing bacterial meningitis.
This Rational Clinical Examination systematic review and meta-analysis provides a nice review of the available data on optimizing diagnostic lumbar puncture technique to reduce adverse events. It is somewhat remarkable so little has changed in our knowledge about this long-standing diagnostic procedure. Post-lumbar puncture headaches remain a challenge that may affect patient satisfaction as well as hospital (or observation unit) course particularly for patients who do not have evidence of bacterial meningitis once the analysis is complete.
This review seems to provide some useful answers for physicians performing lumbar puncture, who should consider selecting a small gauge needle and reinserting the stylet prior to removal. Future studies of other maneuvers to reduce post-procedure adverse events should be considered for the question of atraumatic needles, which may be technically more difficult to use. The review confirms and helps quantify the utility of CSF biochemical analysis in the diagnosis of bacterial meningitis.
Who’s Performing Procedures?
Wigton RS, Alguire P. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007 Mar 6;146(5):355-360. Comment in Ann Intern Med. 2007 Mar 6; 146(5):392-393.
Prior surveys of physicians documented that general internists performed a variety and significant number of procedures in their practice. Much has changed since those prior assessments, including physician training, practice settings, availability of subspecialists, and regulatory requirements that have altered physician’s practice with regard to procedures. This study sought to reassess the volume and variety of procedures performed by general internists compared with the prior survey of 1986. The final sample included 990 completed surveys from general internists from 1,389 returned questionnaires for a successful completion rate of 39.6%.
The median number of different procedures performed in practice decreased from 16 in 1986 to seven in 2004. Internists who practiced in smaller hospitals or smaller towns reported performing almost twice as many procedures as physicians in the largest hospitals and cities. Hours spent in the care of hospitalized patients were also associated with an increased number of different procedures—in particular mechanical ventilation, central venous catheter placement, and thoracentesis. For all but one of the 34 procedures common to both surveys, fewer general internists performed them in 2004 compared with 1986. Remarkably, for 22 of the 34 procedures, a greater than 50% reduction in the proportion of respondents who performed the procedure was noted.
In the 1986 survey, the majority of internists performed all but one of the six procedures required by the American Board of Internal Medicine (ABIM) for certification (abdominal paracentesis, arterial puncture for blood gases, central venous catheter placement, joint aspiration, lumbar puncture, and thoracentesis). Except for joint aspiration, in 2004 these required procedures were performed by 25% or fewer of the respondents.
The 2004 survey demonstrated a striking reduction in the number of different procedures performed by general internists, and a decrease in the proportion of internists who do most procedures. These reductions may stem from a variety of changes in physician practices, including the emergence of hospitalists, availability of subspecialty physicians and proceduralists, and changes in technology and regulatory environments.
Regardless of the forces behind these changes, internal medicine residents’ training in procedures should be re-examined.
Many of those in academic hospital medicine have noted a decline in procedures performed by general internists at large academic centers. This study affirms this trend overall and in particular for physicians in large urban settings or in the largest hospitals. The emergence of hospital medicine may have played a role in reducing the procedures performed by primary care (outpatient) physicians who now spend less time caring for medically ill hospitalized patients.
Residency programs now must consider how to incorporate procedure skills and training to align with the needs of internists. The rising interest in careers in hospital medicine (as opposed to outpatient primary care) necessitates a new approach and individualized plans for gaining procedural skills to match career goals and practice settings. The new ABIM policy acknowledges this greater variability in the procedures performed by internists in practice, and takes steps to more closely align procedure requirements and core manual skills with physician practice.
These changes and new flexibility in requirements provide another opportunity for academic hospital medicine programs to provide leadership, and help shape the training of inpatient physicians. TH
Thrombocytopenia Reaction to Vancomycin
Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007 Mar 1;356(9):904-910
The use of vancomycin has grown exponentially in the past 20 years.1 Physicians have become increasingly aware of its major side effects, such as red man syndrome, hypersensitivity, neutropenia, and nephrotoxicity. But there have been only a few case reports of thrombocytopenia associated with this drug. This article looked at cases of thrombocytopenia in patients referred for clinical suspicion of vancomycin-induced thrombocytopenia.
From 2001-2005, serum samples were sent to the Platelet and Neutrophil Immunology Laboratory at the BloodCenter of Wisconsin in Milwaukee for testing for vancomycin-dependent antibodies from several sites. Clinical information regarding these patients was obtained from their referring physicians and one of the authors. Platelet reactive antibodies were detected by flow cytometry.
IgG and IgM vancomycin-dependent antibodies were detected in 34 patients. It was found that platelets dropped an average of 93% from pretreatment levels, and the average nadir occurred on day eight. The mean platelet count was 13,600. After vancomycin was discontinued, the platelet count returned to normal in all patients except for the three who died. The average time for resolution of thrombocytopenia was 7.5 days.
Unlike other drug-induced thrombocytopenia, these cases of thrombocytopenia associated with vancomycin appear to be more prone to significant hemorrhage. In this group 34% were found to have had severe hemorrhage defined in this study as florid petechial hemorrhages, ecchymoses, and oozing form the buccal mucosa. Three patients who had renal insufficiency were found to be profoundly thrombocytopenic for a longer duration, presumably due to delayed clearance of vancomycin in this setting.
Based on this study, it appears thrombocytopenia is a significant adverse reaction that can be attributed to vancomycin. Unlike other drug-induced thrombocytopenias, it appears to be associated with a higher likelihood of significant hemorrhage, as well.
Thrombocytopenia is a common occurrence in the acutely ill hospitalized patient and has been linked to increased hospital mortality and increased length of stay.2 Many drugs and diseases that hospitalists treat are associated with thrombocytopenia. The indications for usage of vancomycin continues to grow with the increasing number of patients with prosthetic devices and intravascular access, and the increasing prevalence of MRSA. This study raises awareness of a significant side effect that can be associated with vancomycin.
References
- Ena J, Dick RW, Jones RN, et al. The epidemiology of intravenous vancomycin usage in a university hospital: a 10-year study. JAMA. 1993 Feb 3;269(5):598-602. Comment in JAMA. 1993 Sep 22-29;270(12):1426.
- Crowther MA, Cook DJ, Meade M, et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005 Dec;20(4):248-253.
Can the mBRS Stratify Pts Admitted for Nonvariceal Upper GI Bleeds?
Romagnuolo J, Barkun AN, Enns R, et al. Simple clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch Intern Med. 2007 Feb 12;167(3):265-270.
Nonvariceal upper gastrointestinal bleeding is one of the top 10 admission diagnoses based on reviews of diagnosis-related groups. Patients with low-risk lesions on endoscopy, such as ulcers with a clean base, esophagitis, gastritis, duodenitis, or Mallory-Weiss tears, are felt to have less than a 5% chance of recurrent bleeding. In some instances, these patients can be treated successfully and discharged to home.1
Unfortunately, endoscopy is not always available—especially late at night and on weekends. It would be helpful to have a clinical prediction rule to identify patients at low risk for bleeding who could be safely discharged to get endoscopy within a few days.
In the study, 1,869 patients who had undergone upper endoscopy for upper gastrointestinal bleeding were entered into a Canadian national Registry for Upper GI Bleeding and Endoscopy (RUGBE). A modified Blatchford risk score (mBRS) was calculated to see if it could predict the presence of high-risk stigmata of bleeding, rebleeding rates, and mortality.
This mBRS was also compared with another scoring system—the Rockall score. The mBRS uses clinical and laboratory data to risk assess nonvariceal bleeding. The variables included in the scoring system include hemoglobin, systolic blood pressure, heart rate, melena, liver disease, and heart failure. High-risk endoscopic stigmata were defined as adherent clot after irrigation, a bleeding, oozing or spurting vessel, or a nonbleeding visible vessel. Rebleeding was defined as hematemesis, melena, or a bloody nasogastric aspirate in the presence of shock or a decrease in hemoglobin of 2 g/dL or more.
Patients who had a modified Blatchford risk score of <1 were found to have a lower likelihood of high-risk stigmata on endoscopy and were at a low risk for rebleeding (5%). Patients who had high-risk stigmata on endoscopy but an mBRS score of <1 were also found to have low rebleeding rates. The mBRS seemed to a better predictor than the Rockall score for high-risk stigmata and for rebleeding rates.
Patients with nonvariceal upper gastrointestinal tract bleeding may be identified as low risk for re-bleeding if they are normotensive, not tachycardic, not anemic, and do not have active melena, liver disease, or heart failure. It is conceivable that if endoscopy were not available, these patients could be sent home on high-dose proton pump inhibitor and asked to return for outpatient upper endoscopy within a few days.
The study certainly raises interesting questions. Whether it is acceptable practice to discharge a “low-risk” patient with an upper gastrointestinal hemorrhage on a high-dose proton pump inhibitor with good social support and close outpatient follow-up, but without diagnostic endoscopy is still unclear.
The study is limited by the fact that it is a retrospective analysis; however, it does examine a large cohort of patients. The authors acknowledge this, and this work could lead to a prospective randomized trial that would help answer this question. In the meantime, the mBRS may be a helpful tool to help risk stratify patients admitted for nonvariceal upper gastrointestinal bleeding.
References
- Cipolletta L, Bianco M, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002;55(1):1-5.
Lumbar Puncture to Reduce Adverse Events
Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006 Oct 25;296(16):2012-2022.
Lumbar punctures (LPs) remain a common diagnostic test performed by physicians to rule out meningitis. This procedure may be associated with adverse events, with headache and backache the most commonly reported. This systematic review and meta-analysis sought to review the evidence regarding diagnostic lumbar puncture techniques that might reduce the risk of adverse events, and to examine the accuracy of cerebrospinal fluid (CSF) analysis in the diagnosis of bacterial meningitis.
Studies were identified through searches of the Cochrane Library (www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/AboutCochrane.html), MEDLINE from 1966 to January 2006, and EMBASE from 1980 to January 2006 (without language restrictions) to identify relevant studies. Bibliographies of retrieved articles were also used as data sources.
Randomized controlled trials of patients 18 or older undergoing lumbar puncture testing interventions to facilitate a successful diagnostic procedure or reduce adverse events were identified and selected. As a secondary outcome, trials that assessed the accuracy of CSF biochemical analysis for the diagnosis of bacterial meningitis were also identified and included. Trials that studied spinal anesthesia or myelography were excluded.
Study appraisals for quality (randomization, blinding, and outcome assessment) and data extraction were performed by two investigators independently. Fifteen randomized trials of interventions to reduce adverse events met criteria for inclusion, and four studies of the diagnostic test characteristics of CSF analysis met criteria and were included.
Meta-analysis with a random effects model of five studies (total of 587 patients) comparing atraumatic needles with standard needles yielded a nonsignificant decrease in the odds of headache with an atraumatic needle (absolute risk reduction [ARR], 12.3%; 95% confidence interval [CI], –1.72% to 26.2%). A single study of reinsertion of the stylet before needle removal (600 patients) showed a decreased risk of headache (ARR, 11.3%; 95% CI, 6.50%-16.2%). Meta-analysis of four studies (717 patients) revealed a nonsignificant decrease in headache in patients mobilized after LP (ARR 2.9%; 95% CI, –3.4 to 9.3%).
Data from the diagnostic test studies yielded the following likelihood ratios for diagnosing bacterial meningitis: A CSF–blood glucose ratio of 0.4 or less with a likelihood ratio of 18 (95% CI, 12-27); CSF white blood cell count of 500/µL or higher with a likelihood ratio of 15 (95% CI, 10-22); and CSF lactate level of >31.53 mg/dL with a likelihood ration of 21 (95% CI, 14-32) in accurately diagnosed bacterial meningitis.
These data support the reinsertion of the stylet before needle removal to reduce the risk of headache after lumbar puncture and that patients do not require bed rest after diagnostic lumbar puncture. Biochemical analyses, including CSF-blood glucose ratio, CSF leukocyte count and lactate level are useful in diagnosing bacterial meningitis.
This Rational Clinical Examination systematic review and meta-analysis provides a nice review of the available data on optimizing diagnostic lumbar puncture technique to reduce adverse events. It is somewhat remarkable so little has changed in our knowledge about this long-standing diagnostic procedure. Post-lumbar puncture headaches remain a challenge that may affect patient satisfaction as well as hospital (or observation unit) course particularly for patients who do not have evidence of bacterial meningitis once the analysis is complete.
This review seems to provide some useful answers for physicians performing lumbar puncture, who should consider selecting a small gauge needle and reinserting the stylet prior to removal. Future studies of other maneuvers to reduce post-procedure adverse events should be considered for the question of atraumatic needles, which may be technically more difficult to use. The review confirms and helps quantify the utility of CSF biochemical analysis in the diagnosis of bacterial meningitis.
Who’s Performing Procedures?
Wigton RS, Alguire P. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007 Mar 6;146(5):355-360. Comment in Ann Intern Med. 2007 Mar 6; 146(5):392-393.
Prior surveys of physicians documented that general internists performed a variety and significant number of procedures in their practice. Much has changed since those prior assessments, including physician training, practice settings, availability of subspecialists, and regulatory requirements that have altered physician’s practice with regard to procedures. This study sought to reassess the volume and variety of procedures performed by general internists compared with the prior survey of 1986. The final sample included 990 completed surveys from general internists from 1,389 returned questionnaires for a successful completion rate of 39.6%.
The median number of different procedures performed in practice decreased from 16 in 1986 to seven in 2004. Internists who practiced in smaller hospitals or smaller towns reported performing almost twice as many procedures as physicians in the largest hospitals and cities. Hours spent in the care of hospitalized patients were also associated with an increased number of different procedures—in particular mechanical ventilation, central venous catheter placement, and thoracentesis. For all but one of the 34 procedures common to both surveys, fewer general internists performed them in 2004 compared with 1986. Remarkably, for 22 of the 34 procedures, a greater than 50% reduction in the proportion of respondents who performed the procedure was noted.
In the 1986 survey, the majority of internists performed all but one of the six procedures required by the American Board of Internal Medicine (ABIM) for certification (abdominal paracentesis, arterial puncture for blood gases, central venous catheter placement, joint aspiration, lumbar puncture, and thoracentesis). Except for joint aspiration, in 2004 these required procedures were performed by 25% or fewer of the respondents.
The 2004 survey demonstrated a striking reduction in the number of different procedures performed by general internists, and a decrease in the proportion of internists who do most procedures. These reductions may stem from a variety of changes in physician practices, including the emergence of hospitalists, availability of subspecialty physicians and proceduralists, and changes in technology and regulatory environments.
Regardless of the forces behind these changes, internal medicine residents’ training in procedures should be re-examined.
Many of those in academic hospital medicine have noted a decline in procedures performed by general internists at large academic centers. This study affirms this trend overall and in particular for physicians in large urban settings or in the largest hospitals. The emergence of hospital medicine may have played a role in reducing the procedures performed by primary care (outpatient) physicians who now spend less time caring for medically ill hospitalized patients.
Residency programs now must consider how to incorporate procedure skills and training to align with the needs of internists. The rising interest in careers in hospital medicine (as opposed to outpatient primary care) necessitates a new approach and individualized plans for gaining procedural skills to match career goals and practice settings. The new ABIM policy acknowledges this greater variability in the procedures performed by internists in practice, and takes steps to more closely align procedure requirements and core manual skills with physician practice.
These changes and new flexibility in requirements provide another opportunity for academic hospital medicine programs to provide leadership, and help shape the training of inpatient physicians. TH
Vigilant Awareness
In hospitals, clinicians constantly encounter conflicting and ambiguous information,” says Ronald M. Epstein, MD, professor of family medicine, psychiatry, and oncology at the University of Rochester Medical Center (URMC) N.Y. “This information often gets processed tacitly, outside of awareness, and often results in various undesired consequences. For example, premature closure of diagnostic thinking or ordering a test rather than inquiring further of the patient.” In the average hospital, distractions and sensory inputs, including smells, sights, sounds, and tactile sensations, as well as multiple tasks to complete, can all seem pretty overwhelming. Faced with so much data, says Dr. Epstein, the tendency of the mind is to simplify and reduce it in some way. And that’s when error can rear its ugly head.
“Simplification is often arbitrary and unconscious,” he says, and thus “the trick of working in hospital is to develop a vigilant awareness of the ambient stimuli that are all around you, making choices as to what you attend to, relegating other stimuli to the background, and in that way avoiding becoming overwhelmed or controlled by them. In that way, you have the capacity for making better judgments.”
Some clinical decisions can be made fairly easily and routinely (low-level decisions), he says, whereas other patient situations require a fair bit of deliberation (high-level decisions). (See Tables 1 and 2, right.) The human mind tends to avoid the unpleasant and to give more attention to what is compelling. Also, the ambiguity of role and responsibility—especially in large hospitals—may further confound a hospitalist’s mental capacity. Keen attention to each moment also boosts physician well being.
“Hospitalists are often working in crowded, stressful, high-paced, windowless environments in which there is no natural form of respite,” says Dr. Epstein. Therefore, all physicians need ways of keeping themselves from being overwhelmed by the challenges of sensory input and intense emotions caused by exposure to suffering, conflicts, imperatives for critical thinking, and so on.
“If practitioners were able to be more mindful,” he says, “they might experience greater well-being, because they would be able to make more choices about what they attend to and how they react to them.”
Dr. Epstein and his colleagues at the University of Rochester Medical Center—Timothy Quill, MD, Michael Krasner, MD, and Howard Beckman, MD—have studied the qualities of mind required to exercise that awareness extensively, especially as they relate to clinical practice and education.2 They were recently awarded three complementary grants to teach mindfulness to physicians: one from the Arthur Vining Davis Foundations, another from the Physicians’ Foundation for Health Systems Excellence, and the Mannix Award for Excellence in Medical Education.
But just what does mindfulness in medicine entail?
Defining Mindful Practice
“Mindful practice is recognizing where you are at every moment. If you’re distressed, if you’re content or unhappy, if you’re comfortable or in pain, if you’re experiencing some kind of positive or negative effect, if you’re feeling in tune or disconnected from yourself. It’s that monitoring function to be able to say, I’m angry or I’m uncomfortable, or, possibly, I’m in the flow,” says Dr. Epstein.
For physicians to be able to exercise those qualities of mind, to watch and deconstruct their own behavior (what Dr. Epstein describes as “the ability to observe the observer observing the observed”) is something that goes back a long way for him.3 “There’s nothing really mystical about it,” he says. “People do this all the time. It’s part of being an excellent professional in lots of fields. It’s just that no one has organized the science of doing so in the context of medical training.”
In the late 1990s, Dr. Epstein and his coworkers implemented a curriculum reform process at URMC, and his particular charge was to assess the competence of medical students. To accomplish this, he did two things. First, he reviewed the literature on the assessment and definitions of special competence. Second, he turned the magnifying glass on himself. “I thought that it might be a useful exercise to try to understand what made me practice at my best and what barriers there were to doing so.”
The resulting article from this self-monitoring and evaluation was published in JAMA in 1999, before the review article on defining and assessing professional competence appeared in that same journal.3,4 Exploring the nature of his own mindful practice reacquainted him with two areas in which he had participated as a teenager: music and the study of mind—particularly the use of meditation to enhance mental capacities. Those inquiries led him to explore the psychology of a number of qualities of mind: attentiveness, curiosity, decision-making, and the use of cognitive knowledge. The literature was convergent in a number of ways, he says, and “seemed to point to the fact that a lot of competence is not a matter of book knowledge or the kind of knowledge we can explain but tacit knowledge, things we do semi-automatically that really take some effort to deconstruct.”
He realized that “what distinguished an excellent clinician from someone who wasn’t quite so excellent had to do with some of those same qualities that one sees in accomplished musicians, athletes, and meditators, which is the ability to make fine distinctions, lower one’s own level of reactivity, respond in a more conscious way, and pay attention to the unexpected—the surprises that are part of everyday work but that we often ignore.”
All of this rather radicalized his view of what medical education should be doing. He came to believe that—on top of a foundation of knowledge and skills—physicians need to be attentive to their own mental processes and alert to the effects of bias or prior experience.
Writing about excellent clinical practice in this way drew a crescendo of response from readers of the JAMA. The JAMA editors had thoroughly engaged in helping him refine and present the ideas in a way that would really speak to clinical practitioners and educators.3 After publication, he was amazed to receive hundreds of letters from all over the world from physicians in different specialties expressing their appreciation “for having articulated something that was really at the heart of medicine,” he says. “For me, that was incredibly gratifying.”
Hospitalist Qualities of Mind
What qualities of mind are important for a hospitalist to have?
“You have to be enthusiastic, fast-paced individuals,” says Yousaf Ali, MD, hospitalist at URMC and assistant professor of medicine in the Hospital Medicine Division. You also have to be able to immediately connect with patients and families and to have the knowledge and passion that makes that possible. Further, he says, you need to quickly access knowledge pertaining to caring for patients with multiple problems.
Traci Ferguson, MD, is a hospitalist at Boca Raton Community Hospital in Florida, which, by affiliating with Florida Atlantic University (the regional campus for the University of Miami School of Medicine), is moving from community hospital to teaching hospital. Dr. Ferguson believes the qualities of mind necessary to be a good hospitalist are the capacity to be aware of reactions and biases toward patients in order to avoid being judgmental.
“I think the major thing is being present and being attentive when you are caring for patients,” she says, “and that occurs when you’re writing a chart, when you’re talking to family members, [and] when you’re talking to nurses, just as it does when you’re at the bedside.”
Other qualities of mind, in Dr. Ferguson’s view, include the whole spectrum of empathy and compassion, being personable in the sense of being open to what patients and families have to say, and being patient. She also believes the quality of mind necessary to express a human touch is sometimes missing.
Valerie Lang, MD, is also a hospitalist at URMC and has studied mindfulness with Dr. Epstein. She is enrolled in Dr. Krasner’s class for healthcare providers on being mindful. What qualities of mind does she think are important for a hospitalist to have?
“I want to say an open mind, but that’s such a broad term,” she says. “Dr. Epstein uses the term ‘beginner’s mind’ [to refer to] when you’re willing to consider many alternatives, where you don’t necessarily jump to a conclusion and then just stick with it. As a hospitalist, you start making those conclusions as soon as you hear what the patient’s chief complaint is. I think that having [a] beginner’s mind … is so important because we don’t know these patients, and it’s easy to jump to conclusions because we have to make decisions very quickly and … repeatedly.” She also believes that “being able to reflect on how you are communicating with another person is incredibly important to their care.”
—Valerie Lang, MD, hospitalist, University of Rochester Medical Center
Operationalizing Mindfulness
In 2004, after the publication of two of Dr. Epstein’s articles on mindful practice in action, the Arthur Vining Davis Foundation approached him and requested a proposal.5,6 At that time, he was in the process of writing an article on reflection and mindfulness in the context of preventing errors.1 (See Table 3, left.)
“This [proposal] was an intriguing possibility,” says Dr. Epstein, “and galvanized my putting together a curriculum that would not just be elective experiences for preclinical students, which is what the offerings related to mindfulness currently are, but something that was really going to influence clinical training.”
In Dr. Epstein’s view, placing educational reform in the first two years of medical school is teaching it when it matters the least. “Where it matters the most is when students are interacting with patients and using the knowledge and skills and doing work that they’ll ultimately end up doing for the next 30 or 40 years,” says Dr. Epstein.
One project plan is to train practicing primary care physicians to communicate more mindfully with their patients. Outcomes of the intervention will be measured by how it has affected the physicians as well as the patients’ ratings of their physicians and their practice styles.
The second project is a series of annual workshops for 100 third-year medical students and about 250 residents in the nine largest programs at the medical center. All participants will take five seminars that include mindfulness techniques to improve the capacity for paying attention and observing, and narrative exercises, whose themes will include, for instance, suffering, meaningful experience, professionalism, physician self-care, and avoiding burnout. The coursework, which will include both cognitive and experiential content, will also involve training a cadre of about 20 faculty members to teach these sessions, and educational outcomes will ultimately be measured for all participants.
Focus on Metacognition
Dr. Epstein, director of The Rochester Center to Improve Communication in Health Care, says metacognition builds on other approaches, such as the Healer’s Art, a course designed by Rachel Remen, MD, and colleagues, which a number of medical centers are incorporating into their curricula.7
“We are building on Dr. Remen’s wonderful work,” he says. Both curricula include self-awareness, humanism, caring, compassion, meaningful experiences, and physician well being. Both address the “informal curriculum”—a term used to refer to the social environment in which medical trainees adopt values, expectations, and clinical habits. In addition, Dr. Epstein and his colleagues focus on quality of clinical care, including medical decision-making and preventing errors.
“Importantly, our initiative is part of the required curriculum,” says Dr. Epstein. “It targets students and residents working in clinical settings at an advanced level, and it also has a faculty component. … We are trying to transform and heal the informal curriculum, not just immunize students against its toxicity.”
In the Thick of It
All this sounds as if it might benefit hospital practice, according to the hospitalists interviewed for this story. All three believe that mindfulness can be cultivated. Dr. Ali believes the aforementioned forces acting on hospitalists require that hospitalists work at their top capacities, but prioritizing remains essential. He believes one way a hospitalist can cultivate mindfulness in the patient-physician relationship is to avoid burnout in any way that works. Having been a hospitalist for almost 10 years, he discusses this with his medical students and residents. In addition to his hospitalist practice and teaching, Dr. Ali does patient-related quality work, which refreshes his energy.
Dr. Ferguson also thinks mindful practice can be cultivated. “I took cues from the nursing profession in realizing that you do have to care for all aspects of the patient,” she says. “But you can learn this from mentors and people who are successful: people you can emulate, shadow, and follow.”
For her, such a person is Lisa Cooper, MD, MPH, an associate professor in the department of medicine at Johns Hopkins University School of Medicine. Dr. Cooper, both a practicing internist and a researcher, studies and teaches about communication between physicians and minorities—that is, how physicians interact with people of the same or different races and ethnicities. Dr. Ferguson says she feels fortunate to have adopted a mindful awareness in that regard.
As director of the medicine clerkship, Dr. Lang came into contact with Dr. Epstein’s project through her Dean’s Teaching Fellowship, a competitive program at the URSM for faculty members who have a special interest in education.
“The discussions with other educators and clinicians really got me thinking about how my own feelings, whether they had to do with a patient or anything else in life, affect my decision-making,” says Dr. Lang. “You see the phenomenon in residency where you’re in morning report when the residents present a patient and everyone is sitting around a table—not involved with the patient—making judgments about what they should have done. It’s so much easier when you’re not involved [in the situation].”
Though Dr. Lang thinks there are a lot of reasons for that, “part of it is that you are not in the excitement of the moment. And the other factor is that when you’re presenting a patient to a group, you wouldn’t convey your own emotions, what else was going on, what were the competing pressures. Even if you have a wonderful intellect and clinical reasoning skills, you might make the wrong decision when you’re in the thick of the situation.”
Mindful Hospital Practice
Dr. Lang has seen a number of outcomes from her study of mindful practice. It has made her aware of her biases and has taught her to say, in certain cases, “OK, I need to think through the problem again to make sure I’m not changing my judgment about what we should be doing clinically based on how I’m feeling about a patient.”
Dr. Lang sometimes asks herself, “How am I feeling about this? Did that wear me down?” Or, sometimes the opposite can occur. A patient can make you feel “puffed up, where they are so complimentary and make you feel so good that you think that every decision you make is perfect,” she explains.
What Dr. Lang has learned about herself has helped her recognize when she might have prematurely closed a differential diagnosis or come to a conclusion too quickly simply because the patient appeared to agree with her clinical assessment.
Dr. Lang also thinks being a mindful physician has made her a better physician and that she is providing better care that results in better outcomes. “I definitely communicate better with my patients. … I think my relationships with my patients have significantly improved.”
What is her recommendation for how her hospitalist colleagues can learn to practice mindfully? “It’s a practice, and it’s a matter of practice,” says Dr. Lang. “It’s not something you get overnight. It’s a matter of every day, every encounter, taking the time before entering the patient’s room to pause, put things aside, and be present with the patient. And then, at the end of the day, take some time to reflect.”
How does education for mindfulness differ from her original medical training? “I don’t think you’re really ever taught how to manage your emotions when you’ve just made a medical error and you are distraught,” says Dr. Lang, “or how to manage doing that when your pager is going off like crazy and yet you need to sit down and be present with your patient. And that’s the kind of thing that ends up being in your way of being the best physician you can be.” TH
References
- Borrell-Carrió F, Epstein RM. Preventing errors in clinical practice: a call for self-awareness. Ann Fam Med. 2004;2:310-316.
- Epstein RM. Assessment in medical education. N Engl J Med. 2007;356(4):387-396.
- Epstein RM. Mindful practice. JAMA. 1999 Sep;282(9):833-839.
- Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002 Jan 9;287(2):226-235.
- Epstein RM. Mindful practice in action (I): technical competence, evidence-based medicine and relationship-centered care. Fam Syst Health. 2003;21:1-9.
- Epstein RM. Mindful practice in action (II): cultivating habits of mind. Fam Syst Health. 2003;21:11-17.
- O’Donnell JF, Rabow MW, Remen RN. The healer’s art: awakening the heart of medicine. Medical Encounter: Newsletter of the American Academy on Communication in Healthcare. 2007;21, No 1.
In hospitals, clinicians constantly encounter conflicting and ambiguous information,” says Ronald M. Epstein, MD, professor of family medicine, psychiatry, and oncology at the University of Rochester Medical Center (URMC) N.Y. “This information often gets processed tacitly, outside of awareness, and often results in various undesired consequences. For example, premature closure of diagnostic thinking or ordering a test rather than inquiring further of the patient.” In the average hospital, distractions and sensory inputs, including smells, sights, sounds, and tactile sensations, as well as multiple tasks to complete, can all seem pretty overwhelming. Faced with so much data, says Dr. Epstein, the tendency of the mind is to simplify and reduce it in some way. And that’s when error can rear its ugly head.
“Simplification is often arbitrary and unconscious,” he says, and thus “the trick of working in hospital is to develop a vigilant awareness of the ambient stimuli that are all around you, making choices as to what you attend to, relegating other stimuli to the background, and in that way avoiding becoming overwhelmed or controlled by them. In that way, you have the capacity for making better judgments.”
Some clinical decisions can be made fairly easily and routinely (low-level decisions), he says, whereas other patient situations require a fair bit of deliberation (high-level decisions). (See Tables 1 and 2, right.) The human mind tends to avoid the unpleasant and to give more attention to what is compelling. Also, the ambiguity of role and responsibility—especially in large hospitals—may further confound a hospitalist’s mental capacity. Keen attention to each moment also boosts physician well being.
“Hospitalists are often working in crowded, stressful, high-paced, windowless environments in which there is no natural form of respite,” says Dr. Epstein. Therefore, all physicians need ways of keeping themselves from being overwhelmed by the challenges of sensory input and intense emotions caused by exposure to suffering, conflicts, imperatives for critical thinking, and so on.
“If practitioners were able to be more mindful,” he says, “they might experience greater well-being, because they would be able to make more choices about what they attend to and how they react to them.”
Dr. Epstein and his colleagues at the University of Rochester Medical Center—Timothy Quill, MD, Michael Krasner, MD, and Howard Beckman, MD—have studied the qualities of mind required to exercise that awareness extensively, especially as they relate to clinical practice and education.2 They were recently awarded three complementary grants to teach mindfulness to physicians: one from the Arthur Vining Davis Foundations, another from the Physicians’ Foundation for Health Systems Excellence, and the Mannix Award for Excellence in Medical Education.
But just what does mindfulness in medicine entail?
Defining Mindful Practice
“Mindful practice is recognizing where you are at every moment. If you’re distressed, if you’re content or unhappy, if you’re comfortable or in pain, if you’re experiencing some kind of positive or negative effect, if you’re feeling in tune or disconnected from yourself. It’s that monitoring function to be able to say, I’m angry or I’m uncomfortable, or, possibly, I’m in the flow,” says Dr. Epstein.
For physicians to be able to exercise those qualities of mind, to watch and deconstruct their own behavior (what Dr. Epstein describes as “the ability to observe the observer observing the observed”) is something that goes back a long way for him.3 “There’s nothing really mystical about it,” he says. “People do this all the time. It’s part of being an excellent professional in lots of fields. It’s just that no one has organized the science of doing so in the context of medical training.”
In the late 1990s, Dr. Epstein and his coworkers implemented a curriculum reform process at URMC, and his particular charge was to assess the competence of medical students. To accomplish this, he did two things. First, he reviewed the literature on the assessment and definitions of special competence. Second, he turned the magnifying glass on himself. “I thought that it might be a useful exercise to try to understand what made me practice at my best and what barriers there were to doing so.”
The resulting article from this self-monitoring and evaluation was published in JAMA in 1999, before the review article on defining and assessing professional competence appeared in that same journal.3,4 Exploring the nature of his own mindful practice reacquainted him with two areas in which he had participated as a teenager: music and the study of mind—particularly the use of meditation to enhance mental capacities. Those inquiries led him to explore the psychology of a number of qualities of mind: attentiveness, curiosity, decision-making, and the use of cognitive knowledge. The literature was convergent in a number of ways, he says, and “seemed to point to the fact that a lot of competence is not a matter of book knowledge or the kind of knowledge we can explain but tacit knowledge, things we do semi-automatically that really take some effort to deconstruct.”
He realized that “what distinguished an excellent clinician from someone who wasn’t quite so excellent had to do with some of those same qualities that one sees in accomplished musicians, athletes, and meditators, which is the ability to make fine distinctions, lower one’s own level of reactivity, respond in a more conscious way, and pay attention to the unexpected—the surprises that are part of everyday work but that we often ignore.”
All of this rather radicalized his view of what medical education should be doing. He came to believe that—on top of a foundation of knowledge and skills—physicians need to be attentive to their own mental processes and alert to the effects of bias or prior experience.
Writing about excellent clinical practice in this way drew a crescendo of response from readers of the JAMA. The JAMA editors had thoroughly engaged in helping him refine and present the ideas in a way that would really speak to clinical practitioners and educators.3 After publication, he was amazed to receive hundreds of letters from all over the world from physicians in different specialties expressing their appreciation “for having articulated something that was really at the heart of medicine,” he says. “For me, that was incredibly gratifying.”
Hospitalist Qualities of Mind
What qualities of mind are important for a hospitalist to have?
“You have to be enthusiastic, fast-paced individuals,” says Yousaf Ali, MD, hospitalist at URMC and assistant professor of medicine in the Hospital Medicine Division. You also have to be able to immediately connect with patients and families and to have the knowledge and passion that makes that possible. Further, he says, you need to quickly access knowledge pertaining to caring for patients with multiple problems.
Traci Ferguson, MD, is a hospitalist at Boca Raton Community Hospital in Florida, which, by affiliating with Florida Atlantic University (the regional campus for the University of Miami School of Medicine), is moving from community hospital to teaching hospital. Dr. Ferguson believes the qualities of mind necessary to be a good hospitalist are the capacity to be aware of reactions and biases toward patients in order to avoid being judgmental.
“I think the major thing is being present and being attentive when you are caring for patients,” she says, “and that occurs when you’re writing a chart, when you’re talking to family members, [and] when you’re talking to nurses, just as it does when you’re at the bedside.”
Other qualities of mind, in Dr. Ferguson’s view, include the whole spectrum of empathy and compassion, being personable in the sense of being open to what patients and families have to say, and being patient. She also believes the quality of mind necessary to express a human touch is sometimes missing.
Valerie Lang, MD, is also a hospitalist at URMC and has studied mindfulness with Dr. Epstein. She is enrolled in Dr. Krasner’s class for healthcare providers on being mindful. What qualities of mind does she think are important for a hospitalist to have?
“I want to say an open mind, but that’s such a broad term,” she says. “Dr. Epstein uses the term ‘beginner’s mind’ [to refer to] when you’re willing to consider many alternatives, where you don’t necessarily jump to a conclusion and then just stick with it. As a hospitalist, you start making those conclusions as soon as you hear what the patient’s chief complaint is. I think that having [a] beginner’s mind … is so important because we don’t know these patients, and it’s easy to jump to conclusions because we have to make decisions very quickly and … repeatedly.” She also believes that “being able to reflect on how you are communicating with another person is incredibly important to their care.”
—Valerie Lang, MD, hospitalist, University of Rochester Medical Center
Operationalizing Mindfulness
In 2004, after the publication of two of Dr. Epstein’s articles on mindful practice in action, the Arthur Vining Davis Foundation approached him and requested a proposal.5,6 At that time, he was in the process of writing an article on reflection and mindfulness in the context of preventing errors.1 (See Table 3, left.)
“This [proposal] was an intriguing possibility,” says Dr. Epstein, “and galvanized my putting together a curriculum that would not just be elective experiences for preclinical students, which is what the offerings related to mindfulness currently are, but something that was really going to influence clinical training.”
In Dr. Epstein’s view, placing educational reform in the first two years of medical school is teaching it when it matters the least. “Where it matters the most is when students are interacting with patients and using the knowledge and skills and doing work that they’ll ultimately end up doing for the next 30 or 40 years,” says Dr. Epstein.
One project plan is to train practicing primary care physicians to communicate more mindfully with their patients. Outcomes of the intervention will be measured by how it has affected the physicians as well as the patients’ ratings of their physicians and their practice styles.
The second project is a series of annual workshops for 100 third-year medical students and about 250 residents in the nine largest programs at the medical center. All participants will take five seminars that include mindfulness techniques to improve the capacity for paying attention and observing, and narrative exercises, whose themes will include, for instance, suffering, meaningful experience, professionalism, physician self-care, and avoiding burnout. The coursework, which will include both cognitive and experiential content, will also involve training a cadre of about 20 faculty members to teach these sessions, and educational outcomes will ultimately be measured for all participants.
Focus on Metacognition
Dr. Epstein, director of The Rochester Center to Improve Communication in Health Care, says metacognition builds on other approaches, such as the Healer’s Art, a course designed by Rachel Remen, MD, and colleagues, which a number of medical centers are incorporating into their curricula.7
“We are building on Dr. Remen’s wonderful work,” he says. Both curricula include self-awareness, humanism, caring, compassion, meaningful experiences, and physician well being. Both address the “informal curriculum”—a term used to refer to the social environment in which medical trainees adopt values, expectations, and clinical habits. In addition, Dr. Epstein and his colleagues focus on quality of clinical care, including medical decision-making and preventing errors.
“Importantly, our initiative is part of the required curriculum,” says Dr. Epstein. “It targets students and residents working in clinical settings at an advanced level, and it also has a faculty component. … We are trying to transform and heal the informal curriculum, not just immunize students against its toxicity.”
In the Thick of It
All this sounds as if it might benefit hospital practice, according to the hospitalists interviewed for this story. All three believe that mindfulness can be cultivated. Dr. Ali believes the aforementioned forces acting on hospitalists require that hospitalists work at their top capacities, but prioritizing remains essential. He believes one way a hospitalist can cultivate mindfulness in the patient-physician relationship is to avoid burnout in any way that works. Having been a hospitalist for almost 10 years, he discusses this with his medical students and residents. In addition to his hospitalist practice and teaching, Dr. Ali does patient-related quality work, which refreshes his energy.
Dr. Ferguson also thinks mindful practice can be cultivated. “I took cues from the nursing profession in realizing that you do have to care for all aspects of the patient,” she says. “But you can learn this from mentors and people who are successful: people you can emulate, shadow, and follow.”
For her, such a person is Lisa Cooper, MD, MPH, an associate professor in the department of medicine at Johns Hopkins University School of Medicine. Dr. Cooper, both a practicing internist and a researcher, studies and teaches about communication between physicians and minorities—that is, how physicians interact with people of the same or different races and ethnicities. Dr. Ferguson says she feels fortunate to have adopted a mindful awareness in that regard.
As director of the medicine clerkship, Dr. Lang came into contact with Dr. Epstein’s project through her Dean’s Teaching Fellowship, a competitive program at the URSM for faculty members who have a special interest in education.
“The discussions with other educators and clinicians really got me thinking about how my own feelings, whether they had to do with a patient or anything else in life, affect my decision-making,” says Dr. Lang. “You see the phenomenon in residency where you’re in morning report when the residents present a patient and everyone is sitting around a table—not involved with the patient—making judgments about what they should have done. It’s so much easier when you’re not involved [in the situation].”
Though Dr. Lang thinks there are a lot of reasons for that, “part of it is that you are not in the excitement of the moment. And the other factor is that when you’re presenting a patient to a group, you wouldn’t convey your own emotions, what else was going on, what were the competing pressures. Even if you have a wonderful intellect and clinical reasoning skills, you might make the wrong decision when you’re in the thick of the situation.”
Mindful Hospital Practice
Dr. Lang has seen a number of outcomes from her study of mindful practice. It has made her aware of her biases and has taught her to say, in certain cases, “OK, I need to think through the problem again to make sure I’m not changing my judgment about what we should be doing clinically based on how I’m feeling about a patient.”
Dr. Lang sometimes asks herself, “How am I feeling about this? Did that wear me down?” Or, sometimes the opposite can occur. A patient can make you feel “puffed up, where they are so complimentary and make you feel so good that you think that every decision you make is perfect,” she explains.
What Dr. Lang has learned about herself has helped her recognize when she might have prematurely closed a differential diagnosis or come to a conclusion too quickly simply because the patient appeared to agree with her clinical assessment.
Dr. Lang also thinks being a mindful physician has made her a better physician and that she is providing better care that results in better outcomes. “I definitely communicate better with my patients. … I think my relationships with my patients have significantly improved.”
What is her recommendation for how her hospitalist colleagues can learn to practice mindfully? “It’s a practice, and it’s a matter of practice,” says Dr. Lang. “It’s not something you get overnight. It’s a matter of every day, every encounter, taking the time before entering the patient’s room to pause, put things aside, and be present with the patient. And then, at the end of the day, take some time to reflect.”
How does education for mindfulness differ from her original medical training? “I don’t think you’re really ever taught how to manage your emotions when you’ve just made a medical error and you are distraught,” says Dr. Lang, “or how to manage doing that when your pager is going off like crazy and yet you need to sit down and be present with your patient. And that’s the kind of thing that ends up being in your way of being the best physician you can be.” TH
References
- Borrell-Carrió F, Epstein RM. Preventing errors in clinical practice: a call for self-awareness. Ann Fam Med. 2004;2:310-316.
- Epstein RM. Assessment in medical education. N Engl J Med. 2007;356(4):387-396.
- Epstein RM. Mindful practice. JAMA. 1999 Sep;282(9):833-839.
- Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002 Jan 9;287(2):226-235.
- Epstein RM. Mindful practice in action (I): technical competence, evidence-based medicine and relationship-centered care. Fam Syst Health. 2003;21:1-9.
- Epstein RM. Mindful practice in action (II): cultivating habits of mind. Fam Syst Health. 2003;21:11-17.
- O’Donnell JF, Rabow MW, Remen RN. The healer’s art: awakening the heart of medicine. Medical Encounter: Newsletter of the American Academy on Communication in Healthcare. 2007;21, No 1.
In hospitals, clinicians constantly encounter conflicting and ambiguous information,” says Ronald M. Epstein, MD, professor of family medicine, psychiatry, and oncology at the University of Rochester Medical Center (URMC) N.Y. “This information often gets processed tacitly, outside of awareness, and often results in various undesired consequences. For example, premature closure of diagnostic thinking or ordering a test rather than inquiring further of the patient.” In the average hospital, distractions and sensory inputs, including smells, sights, sounds, and tactile sensations, as well as multiple tasks to complete, can all seem pretty overwhelming. Faced with so much data, says Dr. Epstein, the tendency of the mind is to simplify and reduce it in some way. And that’s when error can rear its ugly head.
“Simplification is often arbitrary and unconscious,” he says, and thus “the trick of working in hospital is to develop a vigilant awareness of the ambient stimuli that are all around you, making choices as to what you attend to, relegating other stimuli to the background, and in that way avoiding becoming overwhelmed or controlled by them. In that way, you have the capacity for making better judgments.”
Some clinical decisions can be made fairly easily and routinely (low-level decisions), he says, whereas other patient situations require a fair bit of deliberation (high-level decisions). (See Tables 1 and 2, right.) The human mind tends to avoid the unpleasant and to give more attention to what is compelling. Also, the ambiguity of role and responsibility—especially in large hospitals—may further confound a hospitalist’s mental capacity. Keen attention to each moment also boosts physician well being.
“Hospitalists are often working in crowded, stressful, high-paced, windowless environments in which there is no natural form of respite,” says Dr. Epstein. Therefore, all physicians need ways of keeping themselves from being overwhelmed by the challenges of sensory input and intense emotions caused by exposure to suffering, conflicts, imperatives for critical thinking, and so on.
“If practitioners were able to be more mindful,” he says, “they might experience greater well-being, because they would be able to make more choices about what they attend to and how they react to them.”
Dr. Epstein and his colleagues at the University of Rochester Medical Center—Timothy Quill, MD, Michael Krasner, MD, and Howard Beckman, MD—have studied the qualities of mind required to exercise that awareness extensively, especially as they relate to clinical practice and education.2 They were recently awarded three complementary grants to teach mindfulness to physicians: one from the Arthur Vining Davis Foundations, another from the Physicians’ Foundation for Health Systems Excellence, and the Mannix Award for Excellence in Medical Education.
But just what does mindfulness in medicine entail?
Defining Mindful Practice
“Mindful practice is recognizing where you are at every moment. If you’re distressed, if you’re content or unhappy, if you’re comfortable or in pain, if you’re experiencing some kind of positive or negative effect, if you’re feeling in tune or disconnected from yourself. It’s that monitoring function to be able to say, I’m angry or I’m uncomfortable, or, possibly, I’m in the flow,” says Dr. Epstein.
For physicians to be able to exercise those qualities of mind, to watch and deconstruct their own behavior (what Dr. Epstein describes as “the ability to observe the observer observing the observed”) is something that goes back a long way for him.3 “There’s nothing really mystical about it,” he says. “People do this all the time. It’s part of being an excellent professional in lots of fields. It’s just that no one has organized the science of doing so in the context of medical training.”
In the late 1990s, Dr. Epstein and his coworkers implemented a curriculum reform process at URMC, and his particular charge was to assess the competence of medical students. To accomplish this, he did two things. First, he reviewed the literature on the assessment and definitions of special competence. Second, he turned the magnifying glass on himself. “I thought that it might be a useful exercise to try to understand what made me practice at my best and what barriers there were to doing so.”
The resulting article from this self-monitoring and evaluation was published in JAMA in 1999, before the review article on defining and assessing professional competence appeared in that same journal.3,4 Exploring the nature of his own mindful practice reacquainted him with two areas in which he had participated as a teenager: music and the study of mind—particularly the use of meditation to enhance mental capacities. Those inquiries led him to explore the psychology of a number of qualities of mind: attentiveness, curiosity, decision-making, and the use of cognitive knowledge. The literature was convergent in a number of ways, he says, and “seemed to point to the fact that a lot of competence is not a matter of book knowledge or the kind of knowledge we can explain but tacit knowledge, things we do semi-automatically that really take some effort to deconstruct.”
He realized that “what distinguished an excellent clinician from someone who wasn’t quite so excellent had to do with some of those same qualities that one sees in accomplished musicians, athletes, and meditators, which is the ability to make fine distinctions, lower one’s own level of reactivity, respond in a more conscious way, and pay attention to the unexpected—the surprises that are part of everyday work but that we often ignore.”
All of this rather radicalized his view of what medical education should be doing. He came to believe that—on top of a foundation of knowledge and skills—physicians need to be attentive to their own mental processes and alert to the effects of bias or prior experience.
Writing about excellent clinical practice in this way drew a crescendo of response from readers of the JAMA. The JAMA editors had thoroughly engaged in helping him refine and present the ideas in a way that would really speak to clinical practitioners and educators.3 After publication, he was amazed to receive hundreds of letters from all over the world from physicians in different specialties expressing their appreciation “for having articulated something that was really at the heart of medicine,” he says. “For me, that was incredibly gratifying.”
Hospitalist Qualities of Mind
What qualities of mind are important for a hospitalist to have?
“You have to be enthusiastic, fast-paced individuals,” says Yousaf Ali, MD, hospitalist at URMC and assistant professor of medicine in the Hospital Medicine Division. You also have to be able to immediately connect with patients and families and to have the knowledge and passion that makes that possible. Further, he says, you need to quickly access knowledge pertaining to caring for patients with multiple problems.
Traci Ferguson, MD, is a hospitalist at Boca Raton Community Hospital in Florida, which, by affiliating with Florida Atlantic University (the regional campus for the University of Miami School of Medicine), is moving from community hospital to teaching hospital. Dr. Ferguson believes the qualities of mind necessary to be a good hospitalist are the capacity to be aware of reactions and biases toward patients in order to avoid being judgmental.
“I think the major thing is being present and being attentive when you are caring for patients,” she says, “and that occurs when you’re writing a chart, when you’re talking to family members, [and] when you’re talking to nurses, just as it does when you’re at the bedside.”
Other qualities of mind, in Dr. Ferguson’s view, include the whole spectrum of empathy and compassion, being personable in the sense of being open to what patients and families have to say, and being patient. She also believes the quality of mind necessary to express a human touch is sometimes missing.
Valerie Lang, MD, is also a hospitalist at URMC and has studied mindfulness with Dr. Epstein. She is enrolled in Dr. Krasner’s class for healthcare providers on being mindful. What qualities of mind does she think are important for a hospitalist to have?
“I want to say an open mind, but that’s such a broad term,” she says. “Dr. Epstein uses the term ‘beginner’s mind’ [to refer to] when you’re willing to consider many alternatives, where you don’t necessarily jump to a conclusion and then just stick with it. As a hospitalist, you start making those conclusions as soon as you hear what the patient’s chief complaint is. I think that having [a] beginner’s mind … is so important because we don’t know these patients, and it’s easy to jump to conclusions because we have to make decisions very quickly and … repeatedly.” She also believes that “being able to reflect on how you are communicating with another person is incredibly important to their care.”
—Valerie Lang, MD, hospitalist, University of Rochester Medical Center
Operationalizing Mindfulness
In 2004, after the publication of two of Dr. Epstein’s articles on mindful practice in action, the Arthur Vining Davis Foundation approached him and requested a proposal.5,6 At that time, he was in the process of writing an article on reflection and mindfulness in the context of preventing errors.1 (See Table 3, left.)
“This [proposal] was an intriguing possibility,” says Dr. Epstein, “and galvanized my putting together a curriculum that would not just be elective experiences for preclinical students, which is what the offerings related to mindfulness currently are, but something that was really going to influence clinical training.”
In Dr. Epstein’s view, placing educational reform in the first two years of medical school is teaching it when it matters the least. “Where it matters the most is when students are interacting with patients and using the knowledge and skills and doing work that they’ll ultimately end up doing for the next 30 or 40 years,” says Dr. Epstein.
One project plan is to train practicing primary care physicians to communicate more mindfully with their patients. Outcomes of the intervention will be measured by how it has affected the physicians as well as the patients’ ratings of their physicians and their practice styles.
The second project is a series of annual workshops for 100 third-year medical students and about 250 residents in the nine largest programs at the medical center. All participants will take five seminars that include mindfulness techniques to improve the capacity for paying attention and observing, and narrative exercises, whose themes will include, for instance, suffering, meaningful experience, professionalism, physician self-care, and avoiding burnout. The coursework, which will include both cognitive and experiential content, will also involve training a cadre of about 20 faculty members to teach these sessions, and educational outcomes will ultimately be measured for all participants.
Focus on Metacognition
Dr. Epstein, director of The Rochester Center to Improve Communication in Health Care, says metacognition builds on other approaches, such as the Healer’s Art, a course designed by Rachel Remen, MD, and colleagues, which a number of medical centers are incorporating into their curricula.7
“We are building on Dr. Remen’s wonderful work,” he says. Both curricula include self-awareness, humanism, caring, compassion, meaningful experiences, and physician well being. Both address the “informal curriculum”—a term used to refer to the social environment in which medical trainees adopt values, expectations, and clinical habits. In addition, Dr. Epstein and his colleagues focus on quality of clinical care, including medical decision-making and preventing errors.
“Importantly, our initiative is part of the required curriculum,” says Dr. Epstein. “It targets students and residents working in clinical settings at an advanced level, and it also has a faculty component. … We are trying to transform and heal the informal curriculum, not just immunize students against its toxicity.”
In the Thick of It
All this sounds as if it might benefit hospital practice, according to the hospitalists interviewed for this story. All three believe that mindfulness can be cultivated. Dr. Ali believes the aforementioned forces acting on hospitalists require that hospitalists work at their top capacities, but prioritizing remains essential. He believes one way a hospitalist can cultivate mindfulness in the patient-physician relationship is to avoid burnout in any way that works. Having been a hospitalist for almost 10 years, he discusses this with his medical students and residents. In addition to his hospitalist practice and teaching, Dr. Ali does patient-related quality work, which refreshes his energy.
Dr. Ferguson also thinks mindful practice can be cultivated. “I took cues from the nursing profession in realizing that you do have to care for all aspects of the patient,” she says. “But you can learn this from mentors and people who are successful: people you can emulate, shadow, and follow.”
For her, such a person is Lisa Cooper, MD, MPH, an associate professor in the department of medicine at Johns Hopkins University School of Medicine. Dr. Cooper, both a practicing internist and a researcher, studies and teaches about communication between physicians and minorities—that is, how physicians interact with people of the same or different races and ethnicities. Dr. Ferguson says she feels fortunate to have adopted a mindful awareness in that regard.
As director of the medicine clerkship, Dr. Lang came into contact with Dr. Epstein’s project through her Dean’s Teaching Fellowship, a competitive program at the URSM for faculty members who have a special interest in education.
“The discussions with other educators and clinicians really got me thinking about how my own feelings, whether they had to do with a patient or anything else in life, affect my decision-making,” says Dr. Lang. “You see the phenomenon in residency where you’re in morning report when the residents present a patient and everyone is sitting around a table—not involved with the patient—making judgments about what they should have done. It’s so much easier when you’re not involved [in the situation].”
Though Dr. Lang thinks there are a lot of reasons for that, “part of it is that you are not in the excitement of the moment. And the other factor is that when you’re presenting a patient to a group, you wouldn’t convey your own emotions, what else was going on, what were the competing pressures. Even if you have a wonderful intellect and clinical reasoning skills, you might make the wrong decision when you’re in the thick of the situation.”
Mindful Hospital Practice
Dr. Lang has seen a number of outcomes from her study of mindful practice. It has made her aware of her biases and has taught her to say, in certain cases, “OK, I need to think through the problem again to make sure I’m not changing my judgment about what we should be doing clinically based on how I’m feeling about a patient.”
Dr. Lang sometimes asks herself, “How am I feeling about this? Did that wear me down?” Or, sometimes the opposite can occur. A patient can make you feel “puffed up, where they are so complimentary and make you feel so good that you think that every decision you make is perfect,” she explains.
What Dr. Lang has learned about herself has helped her recognize when she might have prematurely closed a differential diagnosis or come to a conclusion too quickly simply because the patient appeared to agree with her clinical assessment.
Dr. Lang also thinks being a mindful physician has made her a better physician and that she is providing better care that results in better outcomes. “I definitely communicate better with my patients. … I think my relationships with my patients have significantly improved.”
What is her recommendation for how her hospitalist colleagues can learn to practice mindfully? “It’s a practice, and it’s a matter of practice,” says Dr. Lang. “It’s not something you get overnight. It’s a matter of every day, every encounter, taking the time before entering the patient’s room to pause, put things aside, and be present with the patient. And then, at the end of the day, take some time to reflect.”
How does education for mindfulness differ from her original medical training? “I don’t think you’re really ever taught how to manage your emotions when you’ve just made a medical error and you are distraught,” says Dr. Lang, “or how to manage doing that when your pager is going off like crazy and yet you need to sit down and be present with your patient. And that’s the kind of thing that ends up being in your way of being the best physician you can be.” TH
References
- Borrell-Carrió F, Epstein RM. Preventing errors in clinical practice: a call for self-awareness. Ann Fam Med. 2004;2:310-316.
- Epstein RM. Assessment in medical education. N Engl J Med. 2007;356(4):387-396.
- Epstein RM. Mindful practice. JAMA. 1999 Sep;282(9):833-839.
- Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002 Jan 9;287(2):226-235.
- Epstein RM. Mindful practice in action (I): technical competence, evidence-based medicine and relationship-centered care. Fam Syst Health. 2003;21:1-9.
- Epstein RM. Mindful practice in action (II): cultivating habits of mind. Fam Syst Health. 2003;21:11-17.
- O’Donnell JF, Rabow MW, Remen RN. The healer’s art: awakening the heart of medicine. Medical Encounter: Newsletter of the American Academy on Communication in Healthcare. 2007;21, No 1.
Pitfalls in Pain Treatment
Note: This is Part 2 of The Hospitalist’s series on pain and hospital medicine. Part 1 appeared on p. 45 of the April issue.
Welcome to Part 2 of our three-part series on managing the pain of hospitalized patients. Last month’s article presented the context for pain management in the hospital—a core competency identified by SHM. It emphasized techniques for assessing patients’ pain, ranging from a zero-to-10 pain score to more complex pain histories addressing type, source, duration, and intensity as well as psychosocial and spiritual factors.
Part 2 delves into some difficult cases and dilemmas of pain management—situations that can take hospitalists out of their comfort zone and challenge their confidence in managing their patients’ pain.
Some of these dilemmas arise from misconceptions about pain and pain treatments and from the fact that, historically, physicians have not been well trained in optimal pain management. General barriers to pain management in the U.S. healthcare system, as identified by the National Association of Attorneys General, include patients’ beliefs, physician and institutional practices, restrictive state polices, and racial and socioeconomic disparities.1
Many of these issues relate specifically to the most common treatments for severe pain, opioid analgesics, which have all sorts of negative associations based on misconceptions about abuse, addiction, and overdose. In other cases, physicians face real challenges in balancing analgesic benefits with side effects and in determining the right medication, dose, and schedule to meet the patient’s need for pain relief.
Hospitalists confronting difficult pain cases work under the added pressure of trying to bring their patients’ acute illnesses under control so they can discharge them to a lower level of care as soon as prudently possible. This time pressure, along with demands arising from the rest of the hospitalist’s caseload, may impose limits on what can be accomplished in difficult situations or with medications that require time to stabilize.
Challenges also arise when the customary approach to pain management—the drug and dosing schedule the hospitalist is most comfortable using for most patients—fails to bring the pain under control. This is often a red flag for the need to try something new, says Stephen Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and a consultant on the medical center’s palliative care service. In some cases, that means calling in a specialist in pain treatment, palliative medicine, psychiatry, or substance abuse.
“You need to work into the equation that there are pitfalls and caveats to everything we say about pain,” Dr. Bekanich observes. “Plus, the common pain treatments are controlled substances, with obvious legal implications and a professional duty for physicians to handle them safely and appropriately.”
When Dr. Bekanich finds himself confronting a difficult pain situation that has caused a conflict with a patient, he often involves one of the hospital’s customer service patient advocates. They are trained to mediate disagreements between patients and the treatment team.
Is This Patient’s Pain Real?
Physicians sometimes wonder if their patients’ reports of pain are accurate. Is the pain really as bad as the patient says it is? “Residents, frequently, are more skeptical of patients’ claims of pain, doubting whether they are truly experiencing that level of pain,” reports Jean Youngwerth, MD, a hospitalist, palliative care consultant, and fellowship associate program director at the University of Colorado Health Sciences Center in Aurora.
“I tell my residents that malingering is rare, and those few cases where it happens really tend to stand out,” Dr. Youngwerth says. “I also tell them that our default position is always to trust the patient, unless given a good reason not to. I have been burned more often when I questioned my patients’ reports of pain than when I didn’t.”
Pain experts emphasize that the patient’s self-report is the most reliable source of information on pain—based on an understanding of pain as a complex, subjective phenomenon associated with actual or potential tissue damage and the patient’s perception of and emotional reaction to that sensation. The phenomenon of pain also includes emotional, social, psychological, even spiritual components and can be mediated by a host of other factors. But that doesn’t mean it isn’t real to the patient.
“Often, younger physicians take the attitude that if the pain is real, then administration of morphine will make it go away,” says Porter Storey, MD, FACP, FAAHPM. “In reality, pain doesn’t always respond to opioids, for all sorts of reasons. Hospitalists value clarity, and they use pain as a screen for all sorts of other problems. Their goal, often, is not so much the comfort of the patient as it is diagnosing, treating, and then discharging the patient from the hospital.” Dr. Storey is a palliative care physician in Boulder, Colo., and executive vice president for Medical Affairs at the American Academy of Hospice and Palliative Medicine (AAHPM).
Physicians need to be reminded, however, that unresolved pain in hospitalized patients has many negative consequences. These range from resistance to rehabilitation to depression to delayed hospital discharge, as well as reduced job satisfaction for the healthcare professionals who care for them.
Will Prescribing Analgesics Cause Addiction?
Fears about causing addiction haunt many pain management discussions. Requests for more medications, obsessing over the next scheduled analgesic dose, and even manipulative or drug-seeking behaviors can be misunderstood by physicians who lack training in the real nature of drug addiction. Actual cases of drug addiction created by appropriate, sufficient, and well-monitored opioid analgesic treatment are rare, pain experts say. There is an important caveat: the patient who brings a prior history of drug abuse to the current acute medical episode.
“There are no good data about iatrogenic addiction,” says Robert Brody, MD, chief of the pain consultation clinic at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. “People who do pain management, certainly including hospice and palliative care physicians, don’t really believe in it. In my own clinical experience, most patients don’t like pain medications and stop them as soon as they can.”
Addiction is more accurately understood as the inappropriate use of a drug for non-medical purposes. It refers to disruptive, drug-seeking behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.2 Addiction experts also describe addiction as a disease syndrome in its own right. Although that concept can sometimes be hard to accept by those who don’t have a lot of experience working with it, it is a useful paradigm to treat addiction as if it were a disease, says Ronald Crossno, MD, Rockdale, Texas-based area medical director for the VistaCare hospice chain.
Pain experts use the term pseudoaddiction for behaviors that are reminiscent of addiction but in fact reflect the pursuit of pain relief. Examples might include hoarding drugs, clock-watching, and exaggerated complaints of pain, such as moaning or crying. If it is pseudoaddiction, once the pain is brought under control, these behaviors cease. The term was coined in 1989 to describe an iatrogenic syndrome resulting from poorly treated pain.3-5
“Pseudoaddiction is a term you need to know,” Dr. Crossno asserted during a presentation on addiction pain at the recent annual conference of AAHPM in Salt Lake City in February. “It is at least as prevalent as addiction—and an indictment of how our healthcare system deals with pain.”
Dr. Youngwerth offers some advice.“We often see pseudoaddiction in response to undertreatment and inadequately managed pain,” she says. “If you treat the pain appropriately, these behaviors go away.” She tries to teach this concept to residents and hospital staff, who sometimes find it hard to put themselves in the shoes of patients experiencing severe pain.
“If you have a 68-year-old patient with no history of addiction or substance abuse who is in the hospital [with the] status post-hip replacement and is now clock-watching and routinely pressing the call button before her next dose of opioids is due, staff may feel that she is displaying addictive behaviors,” Dr. Youngwerth says. “Why would they think that this situation evolved into addiction during her brief hospital stay? It’s more likely that she’s just afraid of having pain.”
The solution to pseudoaddiction is to prescribe opioids at pharmacologically appropriate doses and schedules. Then, titrate up until analgesia is achieved or toxicities necessitate alternative approaches. Use all the techniques described in the first article of this series. It is also important to restore trust and the patient’s confidence in the medical system’s ability to manage his or her pain. Opioid pain regimens in the hospital should also be coordinated with plans for post-discharge medications and with the patient’s primary-care physician.
Two other concepts that often come up in discussions of opioid treatments are tolerance, which is a diminution of the drug’s effects over time, resulting in a need to increase doses of the medication to achieve the same analgesic effect, and physical dependence, in which the abrupt discontinuation of an analgesic after a period of continuous use causes physical symptoms of withdrawal from the drug. Both of these issues can be addressed with proper assessment and management, and neither is diagnostic of addiction.
Pain experts say tolerance, though a real phenomenon of opioids, is not often a serious problem with pain management in the hospital. Instead, the need for escalating analgesic doses may reflect changes in the underlying disease process. Tolerance can also include positive benefits such as its emergence for opioid side effects like nausea or sedation. Physical dependence on opioids is predictable but can be managed if the original cause of the pain is resolved and the analgesic is no longer needed. Most opioids can be gradually reduced, with each day’s dose at 75% of the previous day’s dose, until the drug is tapered off.6
What if the Patient Is an Addict?
Although pain experts believe that drug addiction caused by appropriate and adequate prescribing of opioids for analgesia is rare, this does not mean that hospitalists won’t face the problem of patients who are addicted to pain medications. “You are already treating patients with addiction,” said Dr. Crossno in his presentation at the AAHPM meeting in Salt Lake City.
Given that pre-existing addictions are relatively common in American society (estimates range from 5% to 17% of the population, depending on whether alcohol abuse is included), it is reasonable to expect this segment of the population will be represented among acutely ill, hospitalized patients.7 Sometimes, the substance abuse problem of a friend or family member affects the patient’s care, such as when pain medications are stolen from the patient.
“Some hospitalized patients do abuse opioids,” says Dr. Bekanich. “We catch people with drug paraphernalia or actually shooting up in their rooms.” Providers can exercise some control over what patients do in the hospital, but it is probably not realistic to expect that a hospitalist will be able to resolve long-standing substance abuse problems during the patient’s brief stay in the hospital.
As part of a comprehensive pain assessment, it is appropriate to ask if the patient has a history of drug use. Many patients will freely admit to such a history, may be actively in recovery or on a methadone maintenance program, or may even resist opioid analgesics despite severe pain because of their commitment to recovery. Without the benefit of such candor, however, it will be difficult to reach a conclusive diagnosis of drug addiction during the patient’s acute inpatient stay, because that ordinarily requires observations over time.
“It is not our job as hospitalists to get patients off opioids; there are other institutions and services for that,” Dr. Bekanich adds. “For us to try to do it in a few days in the hospital seems like a hopeless task. That is not to say we shouldn’t be mindful of the issues involved, talking to the patient or even offering a referral to a drug rehabilitation program. But we should not be trying to do drug rehab.”
The basic principles of believing patients’ reports of pain and providing analgesic doses sufficient to relieve the pain still apply—unless side effects or the patient’s problematic behavior demand a modification in this approach. Pain physicians often cite the maxim “trust but verify.” There are various screening tools that can be used for indicating the possibility of substance abuse, and it is imperative the use of controlled substances always be closely monitored.
Urine drug screening tests are easy to order in the hospital and may encourage compliance for patients who have a drug history when presented up front as a routine aspect of pain management. The urine test can detect prescribed medicines that are being taken by the patient as well as non-prescribed opioids, but it is important to be aware of false positives and negatives and opportunities for gaming the system by those who are determined to do so.
“Just as it is a myth that treating pain appropriately leads to addiction, it is also a myth that people with drug histories can’t have their pain treated effectively,” says Scott Irwin, MD, PhD, medical director of palliative care psychiatry at San Diego Hospice and Palliative Care. “The first thing to ask these patients is what are their goals for pain management. Get as much objective information as you can about the pain and the patient’s history. Fully inform the patient about options. Treat the pain just as you would for anyone else.”
Then, if things don’t add up, Dr. Irwin says, it may be necessary to go back and reassess the patient’s pain and history. Is there psychological distress? Perhaps the analgesic dose isn’t adequate. Maybe financial pressures or complicated social relationships are leading to drug diversion.
If the patient is participating in a methadone maintenance program or similar protocol, it is advisable for the hospitalist to speak to the medical director of that program. But effective pain control also supports maintenance. Emphasize long-acting analgesics, add non-opioid adjuvants and, when possible, find alternatives to intravenous administration. But if the patient is addicted, trying to minimize adverse effects from analgesic treatments might be the best the hospitalist can do.
Another approach to managing the patient with a history of drug abuse is the use of a contract or opioid agreement, in which the patient promises to do certain things with a clear understanding of the consequences for not doing so. Establish the rules early and be prepared to enforce them. Explain expectations for the patient and the physician’s role, designate a single pharmacy and a single physician responsible for pain prescribing, and get consent for treatment and drug testing. If a repeat offender breaks the agreement, it may be time to call in an addiction specialist. Such agreements should be negotiated in person by the physician, not delegated to nurses or other professionals, but then make sure other team members are in the loop. For an example of such an agreement, see http://tinyurl.com/y2bbh6.
Will Pain Medications Cause Respiratory Suppression?
Another common fear related to opioid use is that prescribing sufficient analgesic doses for patients with advanced illnesses could lead to toxicities, suppress their breathing, cause an overdose, or even prematurely end their lives. This scenario is often luridly presented as turning up the morphine drip. Pain management experts question the truth of this scenario, arguing that morphine often is falsely credited with deaths that result from advanced disease processes. Morphine is a common treatment for the sensation of dyspnea, while morphine-related toxicity likely will present with drowsiness, confusion, and loss of consciousness before respiratory compromise.8
A main concern of hospitalists is appreciating the need to balance pain relief with the side effects of analgesics, including opioid toxicities, which can be addressed through careful titration and frequent assessments. Respiratory suppression can be a side effect of opioids, and there are special groups of patients for whom any sedation is a major concern. An example is a lung transplant patient, for whom somnolence may suppress the important cough reflex.
Respiratory suppression from morphine is an area without a large evidence base. But a recent study of 725 patients nearing death in 13 hospice programs analyzed those who were receiving opioids and had at least one change in opioid dose prior to death to see if escalating opioid doses was associated with premature death.9 The authors conclude that “final opioid dose, but not percentage change in dose, was one of several factors associated with survival, but the association is very weak … (and explains) only a very small percentage in variation in survival.” They also found support for their conclusion that opioid use is not a major contributor to premature death in the few other published studies on the subject.
“I tell residents that the fear of respiratory suppression is overrated,” Dr. Youngwerth says. “As long as you follow World Health Organization and other recognized guidelines for dosing and titrating opioids, you can safely prescribe pain medications and control the patient’s pain. They get this fear ingrained during residency. In reality, it is not very common. I remind them that there is much more evidence of under-dosing.”
Dr. Bekanich describes a recent patient, a young woman suffering from severe abdominal pain following the birth of her baby. The pain was so difficult to manage that her hospital in rural Idaho transferred her to his medical center in Salt Lake City. She had also experienced respiratory arrest twice secondary to the application of fentanyl analgesic patches. “But she was relatively easy to manage once we tried a different drug, appropriately titrated,” he relates.
Dr. Bekanich spent two hours in the patient’s room adjusting the intravenous analgesic dose and monitoring the patient’s pulse oxygen level and neurological status. “These medicines don’t have to cause respiratory suppression, although it will happen occasionally, especially when there are multiple co-morbidities,” he says. “Hospitalists don’t realize that most of these problems can be avoided if you are meticulous in prescribing.”
Does Regulatory Scrutiny Chill Pain Treatment?
The ubiquitous fear of opioids and their potential side effects, including some unfounded or unrealistic fears, is also reflected in the regulation of controlled substances and physicians’ fears that they will be subjected to oppressive regulatory scrutiny.
Widely publicized cases of physicians being disciplined or prosecuted for over-prescribing opioids have only added to these fears, while the rare case of a physician being sued or sanctioned for under-prescribing pain medications does little to allay them.10
Growing attention to the inadequacies of and barriers to pain management—and the role of controlled substances regulation in those barriers—led to the 1998 promulgation of “Model Guidelines for the Use of Controlled Substances for the Treatment of Pain” by the Federation of State Medical Boards.11 These guidelines, promoting the legitimate role of opioids in relieving pain and acknowledging providers’ concerns about being disciplined, were revised in 2004 and have been adopted by 21 states.12
The effect remains, however. “For decades, physicians have reported being reluctant to prescribe opioids because of the fear of the stress, expense, and consequences of being investigated by licensing agencies or law enforcement,” states a 2006 state report card issued by the Pain & Policy Studies Group at the University of Wisconsin in Madison.13 “Some states—but far from all—have adopted policies which recognize that controlled substances are necessary for public health. … But in some states, pain treatment using opioids is unduly restricted by policies reflecting medical opinions that were discarded decades ago.”
The Pain & Policy Studies Group’s report card, which advocates for a balanced approach to the regulation and prescribing of controlled substances, has given every state a grade for how well it meets this goal. According to the 2006 report card, Michigan and Virginia get top grades for achieving balance in pain policy, while Georgia gets the lowest grade.
“Regulation is a real concern,” says Daniel Burkhardt, MD, associate professor and director of the Acute Pain Service at the University of California-San Francisco. “Every time a prosecutor arrests someone for prescribing too much pain medication, these things travel, adding to the extra regulatory burden on physicians.”
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., says the burden has lessened somewhat in California because that state eliminated its requirements for triplicate paper prescribing forms for controlled substances.
A related concern involves the potential diversion of controlled substances by impaired healthcare professionals for personal use and abuse. This is another of the fears that have driven archaic pain regulation in many states. In fact, current estimates suggest that a substance abuse-related impairment will affect between 8% and 18% percent of physicians sometime in their lives, and that 2% of physicians are dealing with an active substance abuse problem.14
A recent medical journal letter to the editor from the Wisconsin Pain & Policy Studies Group suggests public policies on opioid diversion should focus more on sources of diversion such as “thefts, including armed robberies, night break-ins, and employee and customer pilferage,” rather than just the doctor-patient prescribing relationship.15
Physician diversion data don’t break out hospital medicine as a category, but some hospitalists say they have not heard of diversion problems involving hospitalist colleagues. That may reflect the fact that hospitalists, unlike some other health professionals, generally don’t administer controlled substances directly to the patient or have ready access to hospital drug storage facilities. TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Joranson D, Payne R. Will my pain be managed? In Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- American Pain Society. Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, American Pain Society, and American Society of Addiction Medicine. Available at www.ampainsoc.org/advocacy/opioids2.htm. Last accessed April 13, 2007.
- Weissman DE, Haddox JD. Opioid pseudoaddiction. Pain. 1989 Mar;36(3):363-366.
- Weissman DE. Fast Fact and Concept #68: Is it pain or addiction? [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_68.htm. Last accessed April 13, 2007.
- Weissman DE. Fast Fact and Concept #69: Pseudoaddiction. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_69.htm. Last accessed April 13, 2007.
- Doyle D, Hanks G, Cherny N, et al, eds. The Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, England: Oxford University Press;2005:336.
- Passik SD, Kirsh KL. Chapter 56: Pain in patients with alcohol and drug dependence. In Bruera E, Higginson I, von Gunten C, et al. Textbook of Palliative Medicine. London, England: Hodder Arnold;2006:517-524.
- Von Gunten CF. Fast Fact and Concept #8: Morphine and hastened death. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_008.htm. Last accessed April 13, 2007.
- Portenoy RK, Siberceva U, Smout R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32:532-540.
- Warm EJ, Weissman DE. Fast Fact and Concept #63: The legal liability of under-treatment of pain. [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_63.htm. Last accessed April 13, 2007.
- Federation of the State Medical Boards of the United States. Dallas, Texas. Available at www.fsmb.org. Accessed April 13, 2007.
- National Association of Attorneys General. Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- Pain & Policy Studies Group. University of Wisconsin Paul P. Carbone Comprehensive Cancer Center. Available at: www.painpolicy.wisc.edu. Accessed April 13, 2007.
- Blondell RD. Taking a proactive approach to physician impairment. Postgrad Med. 2005 Jul;118(1):16-18.
- Joranson DE, Gilson AM. Drug crime is a source of abused pain medications in the United States. J Pain Symptom Manage. 2005 Oct;30(4):299-301.
Note: This is Part 2 of The Hospitalist’s series on pain and hospital medicine. Part 1 appeared on p. 45 of the April issue.
Welcome to Part 2 of our three-part series on managing the pain of hospitalized patients. Last month’s article presented the context for pain management in the hospital—a core competency identified by SHM. It emphasized techniques for assessing patients’ pain, ranging from a zero-to-10 pain score to more complex pain histories addressing type, source, duration, and intensity as well as psychosocial and spiritual factors.
Part 2 delves into some difficult cases and dilemmas of pain management—situations that can take hospitalists out of their comfort zone and challenge their confidence in managing their patients’ pain.
Some of these dilemmas arise from misconceptions about pain and pain treatments and from the fact that, historically, physicians have not been well trained in optimal pain management. General barriers to pain management in the U.S. healthcare system, as identified by the National Association of Attorneys General, include patients’ beliefs, physician and institutional practices, restrictive state polices, and racial and socioeconomic disparities.1
Many of these issues relate specifically to the most common treatments for severe pain, opioid analgesics, which have all sorts of negative associations based on misconceptions about abuse, addiction, and overdose. In other cases, physicians face real challenges in balancing analgesic benefits with side effects and in determining the right medication, dose, and schedule to meet the patient’s need for pain relief.
Hospitalists confronting difficult pain cases work under the added pressure of trying to bring their patients’ acute illnesses under control so they can discharge them to a lower level of care as soon as prudently possible. This time pressure, along with demands arising from the rest of the hospitalist’s caseload, may impose limits on what can be accomplished in difficult situations or with medications that require time to stabilize.
Challenges also arise when the customary approach to pain management—the drug and dosing schedule the hospitalist is most comfortable using for most patients—fails to bring the pain under control. This is often a red flag for the need to try something new, says Stephen Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and a consultant on the medical center’s palliative care service. In some cases, that means calling in a specialist in pain treatment, palliative medicine, psychiatry, or substance abuse.
“You need to work into the equation that there are pitfalls and caveats to everything we say about pain,” Dr. Bekanich observes. “Plus, the common pain treatments are controlled substances, with obvious legal implications and a professional duty for physicians to handle them safely and appropriately.”
When Dr. Bekanich finds himself confronting a difficult pain situation that has caused a conflict with a patient, he often involves one of the hospital’s customer service patient advocates. They are trained to mediate disagreements between patients and the treatment team.
Is This Patient’s Pain Real?
Physicians sometimes wonder if their patients’ reports of pain are accurate. Is the pain really as bad as the patient says it is? “Residents, frequently, are more skeptical of patients’ claims of pain, doubting whether they are truly experiencing that level of pain,” reports Jean Youngwerth, MD, a hospitalist, palliative care consultant, and fellowship associate program director at the University of Colorado Health Sciences Center in Aurora.
“I tell my residents that malingering is rare, and those few cases where it happens really tend to stand out,” Dr. Youngwerth says. “I also tell them that our default position is always to trust the patient, unless given a good reason not to. I have been burned more often when I questioned my patients’ reports of pain than when I didn’t.”
Pain experts emphasize that the patient’s self-report is the most reliable source of information on pain—based on an understanding of pain as a complex, subjective phenomenon associated with actual or potential tissue damage and the patient’s perception of and emotional reaction to that sensation. The phenomenon of pain also includes emotional, social, psychological, even spiritual components and can be mediated by a host of other factors. But that doesn’t mean it isn’t real to the patient.
“Often, younger physicians take the attitude that if the pain is real, then administration of morphine will make it go away,” says Porter Storey, MD, FACP, FAAHPM. “In reality, pain doesn’t always respond to opioids, for all sorts of reasons. Hospitalists value clarity, and they use pain as a screen for all sorts of other problems. Their goal, often, is not so much the comfort of the patient as it is diagnosing, treating, and then discharging the patient from the hospital.” Dr. Storey is a palliative care physician in Boulder, Colo., and executive vice president for Medical Affairs at the American Academy of Hospice and Palliative Medicine (AAHPM).
Physicians need to be reminded, however, that unresolved pain in hospitalized patients has many negative consequences. These range from resistance to rehabilitation to depression to delayed hospital discharge, as well as reduced job satisfaction for the healthcare professionals who care for them.
Will Prescribing Analgesics Cause Addiction?
Fears about causing addiction haunt many pain management discussions. Requests for more medications, obsessing over the next scheduled analgesic dose, and even manipulative or drug-seeking behaviors can be misunderstood by physicians who lack training in the real nature of drug addiction. Actual cases of drug addiction created by appropriate, sufficient, and well-monitored opioid analgesic treatment are rare, pain experts say. There is an important caveat: the patient who brings a prior history of drug abuse to the current acute medical episode.
“There are no good data about iatrogenic addiction,” says Robert Brody, MD, chief of the pain consultation clinic at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. “People who do pain management, certainly including hospice and palliative care physicians, don’t really believe in it. In my own clinical experience, most patients don’t like pain medications and stop them as soon as they can.”
Addiction is more accurately understood as the inappropriate use of a drug for non-medical purposes. It refers to disruptive, drug-seeking behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.2 Addiction experts also describe addiction as a disease syndrome in its own right. Although that concept can sometimes be hard to accept by those who don’t have a lot of experience working with it, it is a useful paradigm to treat addiction as if it were a disease, says Ronald Crossno, MD, Rockdale, Texas-based area medical director for the VistaCare hospice chain.
Pain experts use the term pseudoaddiction for behaviors that are reminiscent of addiction but in fact reflect the pursuit of pain relief. Examples might include hoarding drugs, clock-watching, and exaggerated complaints of pain, such as moaning or crying. If it is pseudoaddiction, once the pain is brought under control, these behaviors cease. The term was coined in 1989 to describe an iatrogenic syndrome resulting from poorly treated pain.3-5
“Pseudoaddiction is a term you need to know,” Dr. Crossno asserted during a presentation on addiction pain at the recent annual conference of AAHPM in Salt Lake City in February. “It is at least as prevalent as addiction—and an indictment of how our healthcare system deals with pain.”
Dr. Youngwerth offers some advice.“We often see pseudoaddiction in response to undertreatment and inadequately managed pain,” she says. “If you treat the pain appropriately, these behaviors go away.” She tries to teach this concept to residents and hospital staff, who sometimes find it hard to put themselves in the shoes of patients experiencing severe pain.
“If you have a 68-year-old patient with no history of addiction or substance abuse who is in the hospital [with the] status post-hip replacement and is now clock-watching and routinely pressing the call button before her next dose of opioids is due, staff may feel that she is displaying addictive behaviors,” Dr. Youngwerth says. “Why would they think that this situation evolved into addiction during her brief hospital stay? It’s more likely that she’s just afraid of having pain.”
The solution to pseudoaddiction is to prescribe opioids at pharmacologically appropriate doses and schedules. Then, titrate up until analgesia is achieved or toxicities necessitate alternative approaches. Use all the techniques described in the first article of this series. It is also important to restore trust and the patient’s confidence in the medical system’s ability to manage his or her pain. Opioid pain regimens in the hospital should also be coordinated with plans for post-discharge medications and with the patient’s primary-care physician.
Two other concepts that often come up in discussions of opioid treatments are tolerance, which is a diminution of the drug’s effects over time, resulting in a need to increase doses of the medication to achieve the same analgesic effect, and physical dependence, in which the abrupt discontinuation of an analgesic after a period of continuous use causes physical symptoms of withdrawal from the drug. Both of these issues can be addressed with proper assessment and management, and neither is diagnostic of addiction.
Pain experts say tolerance, though a real phenomenon of opioids, is not often a serious problem with pain management in the hospital. Instead, the need for escalating analgesic doses may reflect changes in the underlying disease process. Tolerance can also include positive benefits such as its emergence for opioid side effects like nausea or sedation. Physical dependence on opioids is predictable but can be managed if the original cause of the pain is resolved and the analgesic is no longer needed. Most opioids can be gradually reduced, with each day’s dose at 75% of the previous day’s dose, until the drug is tapered off.6
What if the Patient Is an Addict?
Although pain experts believe that drug addiction caused by appropriate and adequate prescribing of opioids for analgesia is rare, this does not mean that hospitalists won’t face the problem of patients who are addicted to pain medications. “You are already treating patients with addiction,” said Dr. Crossno in his presentation at the AAHPM meeting in Salt Lake City.
Given that pre-existing addictions are relatively common in American society (estimates range from 5% to 17% of the population, depending on whether alcohol abuse is included), it is reasonable to expect this segment of the population will be represented among acutely ill, hospitalized patients.7 Sometimes, the substance abuse problem of a friend or family member affects the patient’s care, such as when pain medications are stolen from the patient.
“Some hospitalized patients do abuse opioids,” says Dr. Bekanich. “We catch people with drug paraphernalia or actually shooting up in their rooms.” Providers can exercise some control over what patients do in the hospital, but it is probably not realistic to expect that a hospitalist will be able to resolve long-standing substance abuse problems during the patient’s brief stay in the hospital.
As part of a comprehensive pain assessment, it is appropriate to ask if the patient has a history of drug use. Many patients will freely admit to such a history, may be actively in recovery or on a methadone maintenance program, or may even resist opioid analgesics despite severe pain because of their commitment to recovery. Without the benefit of such candor, however, it will be difficult to reach a conclusive diagnosis of drug addiction during the patient’s acute inpatient stay, because that ordinarily requires observations over time.
“It is not our job as hospitalists to get patients off opioids; there are other institutions and services for that,” Dr. Bekanich adds. “For us to try to do it in a few days in the hospital seems like a hopeless task. That is not to say we shouldn’t be mindful of the issues involved, talking to the patient or even offering a referral to a drug rehabilitation program. But we should not be trying to do drug rehab.”
The basic principles of believing patients’ reports of pain and providing analgesic doses sufficient to relieve the pain still apply—unless side effects or the patient’s problematic behavior demand a modification in this approach. Pain physicians often cite the maxim “trust but verify.” There are various screening tools that can be used for indicating the possibility of substance abuse, and it is imperative the use of controlled substances always be closely monitored.
Urine drug screening tests are easy to order in the hospital and may encourage compliance for patients who have a drug history when presented up front as a routine aspect of pain management. The urine test can detect prescribed medicines that are being taken by the patient as well as non-prescribed opioids, but it is important to be aware of false positives and negatives and opportunities for gaming the system by those who are determined to do so.
“Just as it is a myth that treating pain appropriately leads to addiction, it is also a myth that people with drug histories can’t have their pain treated effectively,” says Scott Irwin, MD, PhD, medical director of palliative care psychiatry at San Diego Hospice and Palliative Care. “The first thing to ask these patients is what are their goals for pain management. Get as much objective information as you can about the pain and the patient’s history. Fully inform the patient about options. Treat the pain just as you would for anyone else.”
Then, if things don’t add up, Dr. Irwin says, it may be necessary to go back and reassess the patient’s pain and history. Is there psychological distress? Perhaps the analgesic dose isn’t adequate. Maybe financial pressures or complicated social relationships are leading to drug diversion.
If the patient is participating in a methadone maintenance program or similar protocol, it is advisable for the hospitalist to speak to the medical director of that program. But effective pain control also supports maintenance. Emphasize long-acting analgesics, add non-opioid adjuvants and, when possible, find alternatives to intravenous administration. But if the patient is addicted, trying to minimize adverse effects from analgesic treatments might be the best the hospitalist can do.
Another approach to managing the patient with a history of drug abuse is the use of a contract or opioid agreement, in which the patient promises to do certain things with a clear understanding of the consequences for not doing so. Establish the rules early and be prepared to enforce them. Explain expectations for the patient and the physician’s role, designate a single pharmacy and a single physician responsible for pain prescribing, and get consent for treatment and drug testing. If a repeat offender breaks the agreement, it may be time to call in an addiction specialist. Such agreements should be negotiated in person by the physician, not delegated to nurses or other professionals, but then make sure other team members are in the loop. For an example of such an agreement, see http://tinyurl.com/y2bbh6.
Will Pain Medications Cause Respiratory Suppression?
Another common fear related to opioid use is that prescribing sufficient analgesic doses for patients with advanced illnesses could lead to toxicities, suppress their breathing, cause an overdose, or even prematurely end their lives. This scenario is often luridly presented as turning up the morphine drip. Pain management experts question the truth of this scenario, arguing that morphine often is falsely credited with deaths that result from advanced disease processes. Morphine is a common treatment for the sensation of dyspnea, while morphine-related toxicity likely will present with drowsiness, confusion, and loss of consciousness before respiratory compromise.8
A main concern of hospitalists is appreciating the need to balance pain relief with the side effects of analgesics, including opioid toxicities, which can be addressed through careful titration and frequent assessments. Respiratory suppression can be a side effect of opioids, and there are special groups of patients for whom any sedation is a major concern. An example is a lung transplant patient, for whom somnolence may suppress the important cough reflex.
Respiratory suppression from morphine is an area without a large evidence base. But a recent study of 725 patients nearing death in 13 hospice programs analyzed those who were receiving opioids and had at least one change in opioid dose prior to death to see if escalating opioid doses was associated with premature death.9 The authors conclude that “final opioid dose, but not percentage change in dose, was one of several factors associated with survival, but the association is very weak … (and explains) only a very small percentage in variation in survival.” They also found support for their conclusion that opioid use is not a major contributor to premature death in the few other published studies on the subject.
“I tell residents that the fear of respiratory suppression is overrated,” Dr. Youngwerth says. “As long as you follow World Health Organization and other recognized guidelines for dosing and titrating opioids, you can safely prescribe pain medications and control the patient’s pain. They get this fear ingrained during residency. In reality, it is not very common. I remind them that there is much more evidence of under-dosing.”
Dr. Bekanich describes a recent patient, a young woman suffering from severe abdominal pain following the birth of her baby. The pain was so difficult to manage that her hospital in rural Idaho transferred her to his medical center in Salt Lake City. She had also experienced respiratory arrest twice secondary to the application of fentanyl analgesic patches. “But she was relatively easy to manage once we tried a different drug, appropriately titrated,” he relates.
Dr. Bekanich spent two hours in the patient’s room adjusting the intravenous analgesic dose and monitoring the patient’s pulse oxygen level and neurological status. “These medicines don’t have to cause respiratory suppression, although it will happen occasionally, especially when there are multiple co-morbidities,” he says. “Hospitalists don’t realize that most of these problems can be avoided if you are meticulous in prescribing.”
Does Regulatory Scrutiny Chill Pain Treatment?
The ubiquitous fear of opioids and their potential side effects, including some unfounded or unrealistic fears, is also reflected in the regulation of controlled substances and physicians’ fears that they will be subjected to oppressive regulatory scrutiny.
Widely publicized cases of physicians being disciplined or prosecuted for over-prescribing opioids have only added to these fears, while the rare case of a physician being sued or sanctioned for under-prescribing pain medications does little to allay them.10
Growing attention to the inadequacies of and barriers to pain management—and the role of controlled substances regulation in those barriers—led to the 1998 promulgation of “Model Guidelines for the Use of Controlled Substances for the Treatment of Pain” by the Federation of State Medical Boards.11 These guidelines, promoting the legitimate role of opioids in relieving pain and acknowledging providers’ concerns about being disciplined, were revised in 2004 and have been adopted by 21 states.12
The effect remains, however. “For decades, physicians have reported being reluctant to prescribe opioids because of the fear of the stress, expense, and consequences of being investigated by licensing agencies or law enforcement,” states a 2006 state report card issued by the Pain & Policy Studies Group at the University of Wisconsin in Madison.13 “Some states—but far from all—have adopted policies which recognize that controlled substances are necessary for public health. … But in some states, pain treatment using opioids is unduly restricted by policies reflecting medical opinions that were discarded decades ago.”
The Pain & Policy Studies Group’s report card, which advocates for a balanced approach to the regulation and prescribing of controlled substances, has given every state a grade for how well it meets this goal. According to the 2006 report card, Michigan and Virginia get top grades for achieving balance in pain policy, while Georgia gets the lowest grade.
“Regulation is a real concern,” says Daniel Burkhardt, MD, associate professor and director of the Acute Pain Service at the University of California-San Francisco. “Every time a prosecutor arrests someone for prescribing too much pain medication, these things travel, adding to the extra regulatory burden on physicians.”
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., says the burden has lessened somewhat in California because that state eliminated its requirements for triplicate paper prescribing forms for controlled substances.
A related concern involves the potential diversion of controlled substances by impaired healthcare professionals for personal use and abuse. This is another of the fears that have driven archaic pain regulation in many states. In fact, current estimates suggest that a substance abuse-related impairment will affect between 8% and 18% percent of physicians sometime in their lives, and that 2% of physicians are dealing with an active substance abuse problem.14
A recent medical journal letter to the editor from the Wisconsin Pain & Policy Studies Group suggests public policies on opioid diversion should focus more on sources of diversion such as “thefts, including armed robberies, night break-ins, and employee and customer pilferage,” rather than just the doctor-patient prescribing relationship.15
Physician diversion data don’t break out hospital medicine as a category, but some hospitalists say they have not heard of diversion problems involving hospitalist colleagues. That may reflect the fact that hospitalists, unlike some other health professionals, generally don’t administer controlled substances directly to the patient or have ready access to hospital drug storage facilities. TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Joranson D, Payne R. Will my pain be managed? In Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- American Pain Society. Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, American Pain Society, and American Society of Addiction Medicine. Available at www.ampainsoc.org/advocacy/opioids2.htm. Last accessed April 13, 2007.
- Weissman DE, Haddox JD. Opioid pseudoaddiction. Pain. 1989 Mar;36(3):363-366.
- Weissman DE. Fast Fact and Concept #68: Is it pain or addiction? [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_68.htm. Last accessed April 13, 2007.
- Weissman DE. Fast Fact and Concept #69: Pseudoaddiction. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_69.htm. Last accessed April 13, 2007.
- Doyle D, Hanks G, Cherny N, et al, eds. The Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, England: Oxford University Press;2005:336.
- Passik SD, Kirsh KL. Chapter 56: Pain in patients with alcohol and drug dependence. In Bruera E, Higginson I, von Gunten C, et al. Textbook of Palliative Medicine. London, England: Hodder Arnold;2006:517-524.
- Von Gunten CF. Fast Fact and Concept #8: Morphine and hastened death. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_008.htm. Last accessed April 13, 2007.
- Portenoy RK, Siberceva U, Smout R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32:532-540.
- Warm EJ, Weissman DE. Fast Fact and Concept #63: The legal liability of under-treatment of pain. [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_63.htm. Last accessed April 13, 2007.
- Federation of the State Medical Boards of the United States. Dallas, Texas. Available at www.fsmb.org. Accessed April 13, 2007.
- National Association of Attorneys General. Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- Pain & Policy Studies Group. University of Wisconsin Paul P. Carbone Comprehensive Cancer Center. Available at: www.painpolicy.wisc.edu. Accessed April 13, 2007.
- Blondell RD. Taking a proactive approach to physician impairment. Postgrad Med. 2005 Jul;118(1):16-18.
- Joranson DE, Gilson AM. Drug crime is a source of abused pain medications in the United States. J Pain Symptom Manage. 2005 Oct;30(4):299-301.
Note: This is Part 2 of The Hospitalist’s series on pain and hospital medicine. Part 1 appeared on p. 45 of the April issue.
Welcome to Part 2 of our three-part series on managing the pain of hospitalized patients. Last month’s article presented the context for pain management in the hospital—a core competency identified by SHM. It emphasized techniques for assessing patients’ pain, ranging from a zero-to-10 pain score to more complex pain histories addressing type, source, duration, and intensity as well as psychosocial and spiritual factors.
Part 2 delves into some difficult cases and dilemmas of pain management—situations that can take hospitalists out of their comfort zone and challenge their confidence in managing their patients’ pain.
Some of these dilemmas arise from misconceptions about pain and pain treatments and from the fact that, historically, physicians have not been well trained in optimal pain management. General barriers to pain management in the U.S. healthcare system, as identified by the National Association of Attorneys General, include patients’ beliefs, physician and institutional practices, restrictive state polices, and racial and socioeconomic disparities.1
Many of these issues relate specifically to the most common treatments for severe pain, opioid analgesics, which have all sorts of negative associations based on misconceptions about abuse, addiction, and overdose. In other cases, physicians face real challenges in balancing analgesic benefits with side effects and in determining the right medication, dose, and schedule to meet the patient’s need for pain relief.
Hospitalists confronting difficult pain cases work under the added pressure of trying to bring their patients’ acute illnesses under control so they can discharge them to a lower level of care as soon as prudently possible. This time pressure, along with demands arising from the rest of the hospitalist’s caseload, may impose limits on what can be accomplished in difficult situations or with medications that require time to stabilize.
Challenges also arise when the customary approach to pain management—the drug and dosing schedule the hospitalist is most comfortable using for most patients—fails to bring the pain under control. This is often a red flag for the need to try something new, says Stephen Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and a consultant on the medical center’s palliative care service. In some cases, that means calling in a specialist in pain treatment, palliative medicine, psychiatry, or substance abuse.
“You need to work into the equation that there are pitfalls and caveats to everything we say about pain,” Dr. Bekanich observes. “Plus, the common pain treatments are controlled substances, with obvious legal implications and a professional duty for physicians to handle them safely and appropriately.”
When Dr. Bekanich finds himself confronting a difficult pain situation that has caused a conflict with a patient, he often involves one of the hospital’s customer service patient advocates. They are trained to mediate disagreements between patients and the treatment team.
Is This Patient’s Pain Real?
Physicians sometimes wonder if their patients’ reports of pain are accurate. Is the pain really as bad as the patient says it is? “Residents, frequently, are more skeptical of patients’ claims of pain, doubting whether they are truly experiencing that level of pain,” reports Jean Youngwerth, MD, a hospitalist, palliative care consultant, and fellowship associate program director at the University of Colorado Health Sciences Center in Aurora.
“I tell my residents that malingering is rare, and those few cases where it happens really tend to stand out,” Dr. Youngwerth says. “I also tell them that our default position is always to trust the patient, unless given a good reason not to. I have been burned more often when I questioned my patients’ reports of pain than when I didn’t.”
Pain experts emphasize that the patient’s self-report is the most reliable source of information on pain—based on an understanding of pain as a complex, subjective phenomenon associated with actual or potential tissue damage and the patient’s perception of and emotional reaction to that sensation. The phenomenon of pain also includes emotional, social, psychological, even spiritual components and can be mediated by a host of other factors. But that doesn’t mean it isn’t real to the patient.
“Often, younger physicians take the attitude that if the pain is real, then administration of morphine will make it go away,” says Porter Storey, MD, FACP, FAAHPM. “In reality, pain doesn’t always respond to opioids, for all sorts of reasons. Hospitalists value clarity, and they use pain as a screen for all sorts of other problems. Their goal, often, is not so much the comfort of the patient as it is diagnosing, treating, and then discharging the patient from the hospital.” Dr. Storey is a palliative care physician in Boulder, Colo., and executive vice president for Medical Affairs at the American Academy of Hospice and Palliative Medicine (AAHPM).
Physicians need to be reminded, however, that unresolved pain in hospitalized patients has many negative consequences. These range from resistance to rehabilitation to depression to delayed hospital discharge, as well as reduced job satisfaction for the healthcare professionals who care for them.
Will Prescribing Analgesics Cause Addiction?
Fears about causing addiction haunt many pain management discussions. Requests for more medications, obsessing over the next scheduled analgesic dose, and even manipulative or drug-seeking behaviors can be misunderstood by physicians who lack training in the real nature of drug addiction. Actual cases of drug addiction created by appropriate, sufficient, and well-monitored opioid analgesic treatment are rare, pain experts say. There is an important caveat: the patient who brings a prior history of drug abuse to the current acute medical episode.
“There are no good data about iatrogenic addiction,” says Robert Brody, MD, chief of the pain consultation clinic at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. “People who do pain management, certainly including hospice and palliative care physicians, don’t really believe in it. In my own clinical experience, most patients don’t like pain medications and stop them as soon as they can.”
Addiction is more accurately understood as the inappropriate use of a drug for non-medical purposes. It refers to disruptive, drug-seeking behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.2 Addiction experts also describe addiction as a disease syndrome in its own right. Although that concept can sometimes be hard to accept by those who don’t have a lot of experience working with it, it is a useful paradigm to treat addiction as if it were a disease, says Ronald Crossno, MD, Rockdale, Texas-based area medical director for the VistaCare hospice chain.
Pain experts use the term pseudoaddiction for behaviors that are reminiscent of addiction but in fact reflect the pursuit of pain relief. Examples might include hoarding drugs, clock-watching, and exaggerated complaints of pain, such as moaning or crying. If it is pseudoaddiction, once the pain is brought under control, these behaviors cease. The term was coined in 1989 to describe an iatrogenic syndrome resulting from poorly treated pain.3-5
“Pseudoaddiction is a term you need to know,” Dr. Crossno asserted during a presentation on addiction pain at the recent annual conference of AAHPM in Salt Lake City in February. “It is at least as prevalent as addiction—and an indictment of how our healthcare system deals with pain.”
Dr. Youngwerth offers some advice.“We often see pseudoaddiction in response to undertreatment and inadequately managed pain,” she says. “If you treat the pain appropriately, these behaviors go away.” She tries to teach this concept to residents and hospital staff, who sometimes find it hard to put themselves in the shoes of patients experiencing severe pain.
“If you have a 68-year-old patient with no history of addiction or substance abuse who is in the hospital [with the] status post-hip replacement and is now clock-watching and routinely pressing the call button before her next dose of opioids is due, staff may feel that she is displaying addictive behaviors,” Dr. Youngwerth says. “Why would they think that this situation evolved into addiction during her brief hospital stay? It’s more likely that she’s just afraid of having pain.”
The solution to pseudoaddiction is to prescribe opioids at pharmacologically appropriate doses and schedules. Then, titrate up until analgesia is achieved or toxicities necessitate alternative approaches. Use all the techniques described in the first article of this series. It is also important to restore trust and the patient’s confidence in the medical system’s ability to manage his or her pain. Opioid pain regimens in the hospital should also be coordinated with plans for post-discharge medications and with the patient’s primary-care physician.
Two other concepts that often come up in discussions of opioid treatments are tolerance, which is a diminution of the drug’s effects over time, resulting in a need to increase doses of the medication to achieve the same analgesic effect, and physical dependence, in which the abrupt discontinuation of an analgesic after a period of continuous use causes physical symptoms of withdrawal from the drug. Both of these issues can be addressed with proper assessment and management, and neither is diagnostic of addiction.
Pain experts say tolerance, though a real phenomenon of opioids, is not often a serious problem with pain management in the hospital. Instead, the need for escalating analgesic doses may reflect changes in the underlying disease process. Tolerance can also include positive benefits such as its emergence for opioid side effects like nausea or sedation. Physical dependence on opioids is predictable but can be managed if the original cause of the pain is resolved and the analgesic is no longer needed. Most opioids can be gradually reduced, with each day’s dose at 75% of the previous day’s dose, until the drug is tapered off.6
What if the Patient Is an Addict?
Although pain experts believe that drug addiction caused by appropriate and adequate prescribing of opioids for analgesia is rare, this does not mean that hospitalists won’t face the problem of patients who are addicted to pain medications. “You are already treating patients with addiction,” said Dr. Crossno in his presentation at the AAHPM meeting in Salt Lake City.
Given that pre-existing addictions are relatively common in American society (estimates range from 5% to 17% of the population, depending on whether alcohol abuse is included), it is reasonable to expect this segment of the population will be represented among acutely ill, hospitalized patients.7 Sometimes, the substance abuse problem of a friend or family member affects the patient’s care, such as when pain medications are stolen from the patient.
“Some hospitalized patients do abuse opioids,” says Dr. Bekanich. “We catch people with drug paraphernalia or actually shooting up in their rooms.” Providers can exercise some control over what patients do in the hospital, but it is probably not realistic to expect that a hospitalist will be able to resolve long-standing substance abuse problems during the patient’s brief stay in the hospital.
As part of a comprehensive pain assessment, it is appropriate to ask if the patient has a history of drug use. Many patients will freely admit to such a history, may be actively in recovery or on a methadone maintenance program, or may even resist opioid analgesics despite severe pain because of their commitment to recovery. Without the benefit of such candor, however, it will be difficult to reach a conclusive diagnosis of drug addiction during the patient’s acute inpatient stay, because that ordinarily requires observations over time.
“It is not our job as hospitalists to get patients off opioids; there are other institutions and services for that,” Dr. Bekanich adds. “For us to try to do it in a few days in the hospital seems like a hopeless task. That is not to say we shouldn’t be mindful of the issues involved, talking to the patient or even offering a referral to a drug rehabilitation program. But we should not be trying to do drug rehab.”
The basic principles of believing patients’ reports of pain and providing analgesic doses sufficient to relieve the pain still apply—unless side effects or the patient’s problematic behavior demand a modification in this approach. Pain physicians often cite the maxim “trust but verify.” There are various screening tools that can be used for indicating the possibility of substance abuse, and it is imperative the use of controlled substances always be closely monitored.
Urine drug screening tests are easy to order in the hospital and may encourage compliance for patients who have a drug history when presented up front as a routine aspect of pain management. The urine test can detect prescribed medicines that are being taken by the patient as well as non-prescribed opioids, but it is important to be aware of false positives and negatives and opportunities for gaming the system by those who are determined to do so.
“Just as it is a myth that treating pain appropriately leads to addiction, it is also a myth that people with drug histories can’t have their pain treated effectively,” says Scott Irwin, MD, PhD, medical director of palliative care psychiatry at San Diego Hospice and Palliative Care. “The first thing to ask these patients is what are their goals for pain management. Get as much objective information as you can about the pain and the patient’s history. Fully inform the patient about options. Treat the pain just as you would for anyone else.”
Then, if things don’t add up, Dr. Irwin says, it may be necessary to go back and reassess the patient’s pain and history. Is there psychological distress? Perhaps the analgesic dose isn’t adequate. Maybe financial pressures or complicated social relationships are leading to drug diversion.
If the patient is participating in a methadone maintenance program or similar protocol, it is advisable for the hospitalist to speak to the medical director of that program. But effective pain control also supports maintenance. Emphasize long-acting analgesics, add non-opioid adjuvants and, when possible, find alternatives to intravenous administration. But if the patient is addicted, trying to minimize adverse effects from analgesic treatments might be the best the hospitalist can do.
Another approach to managing the patient with a history of drug abuse is the use of a contract or opioid agreement, in which the patient promises to do certain things with a clear understanding of the consequences for not doing so. Establish the rules early and be prepared to enforce them. Explain expectations for the patient and the physician’s role, designate a single pharmacy and a single physician responsible for pain prescribing, and get consent for treatment and drug testing. If a repeat offender breaks the agreement, it may be time to call in an addiction specialist. Such agreements should be negotiated in person by the physician, not delegated to nurses or other professionals, but then make sure other team members are in the loop. For an example of such an agreement, see http://tinyurl.com/y2bbh6.
Will Pain Medications Cause Respiratory Suppression?
Another common fear related to opioid use is that prescribing sufficient analgesic doses for patients with advanced illnesses could lead to toxicities, suppress their breathing, cause an overdose, or even prematurely end their lives. This scenario is often luridly presented as turning up the morphine drip. Pain management experts question the truth of this scenario, arguing that morphine often is falsely credited with deaths that result from advanced disease processes. Morphine is a common treatment for the sensation of dyspnea, while morphine-related toxicity likely will present with drowsiness, confusion, and loss of consciousness before respiratory compromise.8
A main concern of hospitalists is appreciating the need to balance pain relief with the side effects of analgesics, including opioid toxicities, which can be addressed through careful titration and frequent assessments. Respiratory suppression can be a side effect of opioids, and there are special groups of patients for whom any sedation is a major concern. An example is a lung transplant patient, for whom somnolence may suppress the important cough reflex.
Respiratory suppression from morphine is an area without a large evidence base. But a recent study of 725 patients nearing death in 13 hospice programs analyzed those who were receiving opioids and had at least one change in opioid dose prior to death to see if escalating opioid doses was associated with premature death.9 The authors conclude that “final opioid dose, but not percentage change in dose, was one of several factors associated with survival, but the association is very weak … (and explains) only a very small percentage in variation in survival.” They also found support for their conclusion that opioid use is not a major contributor to premature death in the few other published studies on the subject.
“I tell residents that the fear of respiratory suppression is overrated,” Dr. Youngwerth says. “As long as you follow World Health Organization and other recognized guidelines for dosing and titrating opioids, you can safely prescribe pain medications and control the patient’s pain. They get this fear ingrained during residency. In reality, it is not very common. I remind them that there is much more evidence of under-dosing.”
Dr. Bekanich describes a recent patient, a young woman suffering from severe abdominal pain following the birth of her baby. The pain was so difficult to manage that her hospital in rural Idaho transferred her to his medical center in Salt Lake City. She had also experienced respiratory arrest twice secondary to the application of fentanyl analgesic patches. “But she was relatively easy to manage once we tried a different drug, appropriately titrated,” he relates.
Dr. Bekanich spent two hours in the patient’s room adjusting the intravenous analgesic dose and monitoring the patient’s pulse oxygen level and neurological status. “These medicines don’t have to cause respiratory suppression, although it will happen occasionally, especially when there are multiple co-morbidities,” he says. “Hospitalists don’t realize that most of these problems can be avoided if you are meticulous in prescribing.”
Does Regulatory Scrutiny Chill Pain Treatment?
The ubiquitous fear of opioids and their potential side effects, including some unfounded or unrealistic fears, is also reflected in the regulation of controlled substances and physicians’ fears that they will be subjected to oppressive regulatory scrutiny.
Widely publicized cases of physicians being disciplined or prosecuted for over-prescribing opioids have only added to these fears, while the rare case of a physician being sued or sanctioned for under-prescribing pain medications does little to allay them.10
Growing attention to the inadequacies of and barriers to pain management—and the role of controlled substances regulation in those barriers—led to the 1998 promulgation of “Model Guidelines for the Use of Controlled Substances for the Treatment of Pain” by the Federation of State Medical Boards.11 These guidelines, promoting the legitimate role of opioids in relieving pain and acknowledging providers’ concerns about being disciplined, were revised in 2004 and have been adopted by 21 states.12
The effect remains, however. “For decades, physicians have reported being reluctant to prescribe opioids because of the fear of the stress, expense, and consequences of being investigated by licensing agencies or law enforcement,” states a 2006 state report card issued by the Pain & Policy Studies Group at the University of Wisconsin in Madison.13 “Some states—but far from all—have adopted policies which recognize that controlled substances are necessary for public health. … But in some states, pain treatment using opioids is unduly restricted by policies reflecting medical opinions that were discarded decades ago.”
The Pain & Policy Studies Group’s report card, which advocates for a balanced approach to the regulation and prescribing of controlled substances, has given every state a grade for how well it meets this goal. According to the 2006 report card, Michigan and Virginia get top grades for achieving balance in pain policy, while Georgia gets the lowest grade.
“Regulation is a real concern,” says Daniel Burkhardt, MD, associate professor and director of the Acute Pain Service at the University of California-San Francisco. “Every time a prosecutor arrests someone for prescribing too much pain medication, these things travel, adding to the extra regulatory burden on physicians.”
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., says the burden has lessened somewhat in California because that state eliminated its requirements for triplicate paper prescribing forms for controlled substances.
A related concern involves the potential diversion of controlled substances by impaired healthcare professionals for personal use and abuse. This is another of the fears that have driven archaic pain regulation in many states. In fact, current estimates suggest that a substance abuse-related impairment will affect between 8% and 18% percent of physicians sometime in their lives, and that 2% of physicians are dealing with an active substance abuse problem.14
A recent medical journal letter to the editor from the Wisconsin Pain & Policy Studies Group suggests public policies on opioid diversion should focus more on sources of diversion such as “thefts, including armed robberies, night break-ins, and employee and customer pilferage,” rather than just the doctor-patient prescribing relationship.15
Physician diversion data don’t break out hospital medicine as a category, but some hospitalists say they have not heard of diversion problems involving hospitalist colleagues. That may reflect the fact that hospitalists, unlike some other health professionals, generally don’t administer controlled substances directly to the patient or have ready access to hospital drug storage facilities. TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Joranson D, Payne R. Will my pain be managed? In Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- American Pain Society. Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, American Pain Society, and American Society of Addiction Medicine. Available at www.ampainsoc.org/advocacy/opioids2.htm. Last accessed April 13, 2007.
- Weissman DE, Haddox JD. Opioid pseudoaddiction. Pain. 1989 Mar;36(3):363-366.
- Weissman DE. Fast Fact and Concept #68: Is it pain or addiction? [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_68.htm. Last accessed April 13, 2007.
- Weissman DE. Fast Fact and Concept #69: Pseudoaddiction. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_69.htm. Last accessed April 13, 2007.
- Doyle D, Hanks G, Cherny N, et al, eds. The Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, England: Oxford University Press;2005:336.
- Passik SD, Kirsh KL. Chapter 56: Pain in patients with alcohol and drug dependence. In Bruera E, Higginson I, von Gunten C, et al. Textbook of Palliative Medicine. London, England: Hodder Arnold;2006:517-524.
- Von Gunten CF. Fast Fact and Concept #8: Morphine and hastened death. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_008.htm. Last accessed April 13, 2007.
- Portenoy RK, Siberceva U, Smout R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32:532-540.
- Warm EJ, Weissman DE. Fast Fact and Concept #63: The legal liability of under-treatment of pain. [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_63.htm. Last accessed April 13, 2007.
- Federation of the State Medical Boards of the United States. Dallas, Texas. Available at www.fsmb.org. Accessed April 13, 2007.
- National Association of Attorneys General. Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- Pain & Policy Studies Group. University of Wisconsin Paul P. Carbone Comprehensive Cancer Center. Available at: www.painpolicy.wisc.edu. Accessed April 13, 2007.
- Blondell RD. Taking a proactive approach to physician impairment. Postgrad Med. 2005 Jul;118(1):16-18.
- Joranson DE, Gilson AM. Drug crime is a source of abused pain medications in the United States. J Pain Symptom Manage. 2005 Oct;30(4):299-301.
A Wolf in Sheep's Clothing
Adverse drug reactions are a major clinical problem, accounting for 2%-6% of all hospital admissions. And, 6%-15% of hospitalized patients experience a serious adverse drug reaction that contributes to longer hospital stays and higher costs. It is crucial for clinicians to detect, diagnose, and report adverse drug reactions to ensure safe prescribing and continued drug safety monitoring, as illustrated by this brief case presentation.
The Patient
A 72-year-old male presented to the emergency department in acute respiratory distress due to severe angioedema of the face and tongue; the patient required intubation. He denied prior episodes of angioedema. A careful evaluation of all possible causes of angioedema, including a thorough assessment of the medications used by the patient, led to the conclusion that this life-threatening incident could be attributed only to a reaction to an angiotensin-converting enzyme (ACE) inhibitor. The patient had been on ACE inhibitor therapy for hypertension for more than five years and at the time of admission had been taking a combination of benazepril and amlodipine for more than two years. This medication was immediately discontinued, and he recovered fully after five days in the ICU on mechanical ventilation.
ACE Inhibitor-Associated Angioedema
ACE inhibitors are used by more than 35 million people worldwide to treat hypertension, heart failure, and diabetes mellitus; still, many physicians believe they are underprescribed.1 Angioedema is a serious complication of ACE inhibitor therapy that occurs in 0.1% to 0.68% of patients taking ACE inhibitors.2,3
Angioedema presents with a non-pitting swelling of subcutaneous or submucosal tissue without desquamation. Angioedema associated with ACE inhibitor use is rapid in onset, occurring minutes to hours after ingestion, does not present with urticaria, and usually lasts no more than 48 hours.4 At times, angioedema related to ACE inhibitor therapy occurs in the intestine, causing abdominal pain, diarrhea, and vomiting without mucocutaneous signs.1,4
Certain risk factors for developing ACE inhibitor-related angioedema include age older than 65, seasonal allergies, and black ethnicity. Another risk factor pertinent to our case presentation is the patient’s length of time on ACE inhibitor therapy. One study found that ACE inhibitor-associated angioedema occurred at a rate that was nine times higher during the first month of therapy than during subsequent months of therapy.2 Agostoni and colleagues found that ACE inhibitor-associated angioedema could occur in patients who had been on ACE inhibitor therapy for as long as eight years.5
The Process
ACE inhibitor-induced angioedema is probably a multifactorial process. Angiotensin-converting enzyme (ACE) metabolizes angiotensin I to angiotensin II in vivo and is a major inactivator of bradykinin. ACE and aminopeptidase P are the major pathways of bradykinin metabolism. A minor pathway uses carboxypeptidase N, which metabolizes bradykinin to its active metabolite, des-Arg-bradykinin. Des-Arg-bradykinin can then be inactivated by ACE and aminopeptidase P. In patients who had angioedema caused by ACE inhibitors, higher levels of des-Arg-bradykinin were found due to decreased activity of aminopeptidase P, which normally plays a major role in bradykinin breakdown when an ACE inhibitor is present.6
Bradykinin is a beta2 receptor agonist, but, when it is metabolized by carboxypeptidase N to des-Arg-bradykinin, it becomes a beta1 receptor agonist.6
During ACE inhibitor therapy, bradykinin can be inactivated by aminopeptidase P or metabolized into a beta1 receptor agonist by carboxypeptidase N, which is then broken down by aminopeptidase P. If aminopeptidase P is not active, then bradykinin can be converted to des-Arg-bradykinin, which can then act on upregulated beta1 receptors in the oropharynx and tongue, producing vasodilation, increased capillary permeability, and pain.
Treatment
Treatment of ACE inhibitor-induced angioedema includes discontinuing the ACE inhibitor and providing symptomatic support. Although some ACE inhibitors are more likely than others to cause angioedema, a patient who has had an episode of ACE inhibitor-associated angioedema should never again use any ACE inhibitor.3 Angiotensin receptor blockers (ARBs) do not affect the bradykinin system; however, they can cause angioedema (0.13% in one trial of ARBs), and it is not known if ARBs should be avoided in patients who have had ACE inhibitor-induced angioedema.7 Therapy with a bradykinin receptor antagonist to prevent or resolve ACE inhibitor-associated angioedema has not yet been studied in detail.1
Summary
Adverse drug reactions can present clinically in many different ways, and, indeed, these reactions have deposed syphilis and tuberculosis as the mimic of disease. Many adverse drug reactions are mild, but others can be severe and, occasionally, life-threatening. This variability in manifestations means clinicians always have to consider that the drug may be the cause of the patient’s symptoms. TH
Johnson is a medical student at the Kansas City University of Medicine and Biosciences, Kansas City, Mo. Dr. Egger is a consultant in general internal medicine at the Mayo Clinic, Rochester, Minn.
References
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005 Jul;165(14):1637-1642.
- Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004 Feb;17(2):103-111.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53:373-388.
- Agostoni A, Cicardi M, Cugno M, et al. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology. 1999;44:21-25.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232-237.
- Ward KE, Hume AL. Olmesartan (benicar) for hypertension. Am Fam Physician. 2005;72:673-674.
Adverse drug reactions are a major clinical problem, accounting for 2%-6% of all hospital admissions. And, 6%-15% of hospitalized patients experience a serious adverse drug reaction that contributes to longer hospital stays and higher costs. It is crucial for clinicians to detect, diagnose, and report adverse drug reactions to ensure safe prescribing and continued drug safety monitoring, as illustrated by this brief case presentation.
The Patient
A 72-year-old male presented to the emergency department in acute respiratory distress due to severe angioedema of the face and tongue; the patient required intubation. He denied prior episodes of angioedema. A careful evaluation of all possible causes of angioedema, including a thorough assessment of the medications used by the patient, led to the conclusion that this life-threatening incident could be attributed only to a reaction to an angiotensin-converting enzyme (ACE) inhibitor. The patient had been on ACE inhibitor therapy for hypertension for more than five years and at the time of admission had been taking a combination of benazepril and amlodipine for more than two years. This medication was immediately discontinued, and he recovered fully after five days in the ICU on mechanical ventilation.
ACE Inhibitor-Associated Angioedema
ACE inhibitors are used by more than 35 million people worldwide to treat hypertension, heart failure, and diabetes mellitus; still, many physicians believe they are underprescribed.1 Angioedema is a serious complication of ACE inhibitor therapy that occurs in 0.1% to 0.68% of patients taking ACE inhibitors.2,3
Angioedema presents with a non-pitting swelling of subcutaneous or submucosal tissue without desquamation. Angioedema associated with ACE inhibitor use is rapid in onset, occurring minutes to hours after ingestion, does not present with urticaria, and usually lasts no more than 48 hours.4 At times, angioedema related to ACE inhibitor therapy occurs in the intestine, causing abdominal pain, diarrhea, and vomiting without mucocutaneous signs.1,4
Certain risk factors for developing ACE inhibitor-related angioedema include age older than 65, seasonal allergies, and black ethnicity. Another risk factor pertinent to our case presentation is the patient’s length of time on ACE inhibitor therapy. One study found that ACE inhibitor-associated angioedema occurred at a rate that was nine times higher during the first month of therapy than during subsequent months of therapy.2 Agostoni and colleagues found that ACE inhibitor-associated angioedema could occur in patients who had been on ACE inhibitor therapy for as long as eight years.5
The Process
ACE inhibitor-induced angioedema is probably a multifactorial process. Angiotensin-converting enzyme (ACE) metabolizes angiotensin I to angiotensin II in vivo and is a major inactivator of bradykinin. ACE and aminopeptidase P are the major pathways of bradykinin metabolism. A minor pathway uses carboxypeptidase N, which metabolizes bradykinin to its active metabolite, des-Arg-bradykinin. Des-Arg-bradykinin can then be inactivated by ACE and aminopeptidase P. In patients who had angioedema caused by ACE inhibitors, higher levels of des-Arg-bradykinin were found due to decreased activity of aminopeptidase P, which normally plays a major role in bradykinin breakdown when an ACE inhibitor is present.6
Bradykinin is a beta2 receptor agonist, but, when it is metabolized by carboxypeptidase N to des-Arg-bradykinin, it becomes a beta1 receptor agonist.6
During ACE inhibitor therapy, bradykinin can be inactivated by aminopeptidase P or metabolized into a beta1 receptor agonist by carboxypeptidase N, which is then broken down by aminopeptidase P. If aminopeptidase P is not active, then bradykinin can be converted to des-Arg-bradykinin, which can then act on upregulated beta1 receptors in the oropharynx and tongue, producing vasodilation, increased capillary permeability, and pain.
Treatment
Treatment of ACE inhibitor-induced angioedema includes discontinuing the ACE inhibitor and providing symptomatic support. Although some ACE inhibitors are more likely than others to cause angioedema, a patient who has had an episode of ACE inhibitor-associated angioedema should never again use any ACE inhibitor.3 Angiotensin receptor blockers (ARBs) do not affect the bradykinin system; however, they can cause angioedema (0.13% in one trial of ARBs), and it is not known if ARBs should be avoided in patients who have had ACE inhibitor-induced angioedema.7 Therapy with a bradykinin receptor antagonist to prevent or resolve ACE inhibitor-associated angioedema has not yet been studied in detail.1
Summary
Adverse drug reactions can present clinically in many different ways, and, indeed, these reactions have deposed syphilis and tuberculosis as the mimic of disease. Many adverse drug reactions are mild, but others can be severe and, occasionally, life-threatening. This variability in manifestations means clinicians always have to consider that the drug may be the cause of the patient’s symptoms. TH
Johnson is a medical student at the Kansas City University of Medicine and Biosciences, Kansas City, Mo. Dr. Egger is a consultant in general internal medicine at the Mayo Clinic, Rochester, Minn.
References
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005 Jul;165(14):1637-1642.
- Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004 Feb;17(2):103-111.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53:373-388.
- Agostoni A, Cicardi M, Cugno M, et al. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology. 1999;44:21-25.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232-237.
- Ward KE, Hume AL. Olmesartan (benicar) for hypertension. Am Fam Physician. 2005;72:673-674.
Adverse drug reactions are a major clinical problem, accounting for 2%-6% of all hospital admissions. And, 6%-15% of hospitalized patients experience a serious adverse drug reaction that contributes to longer hospital stays and higher costs. It is crucial for clinicians to detect, diagnose, and report adverse drug reactions to ensure safe prescribing and continued drug safety monitoring, as illustrated by this brief case presentation.
The Patient
A 72-year-old male presented to the emergency department in acute respiratory distress due to severe angioedema of the face and tongue; the patient required intubation. He denied prior episodes of angioedema. A careful evaluation of all possible causes of angioedema, including a thorough assessment of the medications used by the patient, led to the conclusion that this life-threatening incident could be attributed only to a reaction to an angiotensin-converting enzyme (ACE) inhibitor. The patient had been on ACE inhibitor therapy for hypertension for more than five years and at the time of admission had been taking a combination of benazepril and amlodipine for more than two years. This medication was immediately discontinued, and he recovered fully after five days in the ICU on mechanical ventilation.
ACE Inhibitor-Associated Angioedema
ACE inhibitors are used by more than 35 million people worldwide to treat hypertension, heart failure, and diabetes mellitus; still, many physicians believe they are underprescribed.1 Angioedema is a serious complication of ACE inhibitor therapy that occurs in 0.1% to 0.68% of patients taking ACE inhibitors.2,3
Angioedema presents with a non-pitting swelling of subcutaneous or submucosal tissue without desquamation. Angioedema associated with ACE inhibitor use is rapid in onset, occurring minutes to hours after ingestion, does not present with urticaria, and usually lasts no more than 48 hours.4 At times, angioedema related to ACE inhibitor therapy occurs in the intestine, causing abdominal pain, diarrhea, and vomiting without mucocutaneous signs.1,4
Certain risk factors for developing ACE inhibitor-related angioedema include age older than 65, seasonal allergies, and black ethnicity. Another risk factor pertinent to our case presentation is the patient’s length of time on ACE inhibitor therapy. One study found that ACE inhibitor-associated angioedema occurred at a rate that was nine times higher during the first month of therapy than during subsequent months of therapy.2 Agostoni and colleagues found that ACE inhibitor-associated angioedema could occur in patients who had been on ACE inhibitor therapy for as long as eight years.5
The Process
ACE inhibitor-induced angioedema is probably a multifactorial process. Angiotensin-converting enzyme (ACE) metabolizes angiotensin I to angiotensin II in vivo and is a major inactivator of bradykinin. ACE and aminopeptidase P are the major pathways of bradykinin metabolism. A minor pathway uses carboxypeptidase N, which metabolizes bradykinin to its active metabolite, des-Arg-bradykinin. Des-Arg-bradykinin can then be inactivated by ACE and aminopeptidase P. In patients who had angioedema caused by ACE inhibitors, higher levels of des-Arg-bradykinin were found due to decreased activity of aminopeptidase P, which normally plays a major role in bradykinin breakdown when an ACE inhibitor is present.6
Bradykinin is a beta2 receptor agonist, but, when it is metabolized by carboxypeptidase N to des-Arg-bradykinin, it becomes a beta1 receptor agonist.6
During ACE inhibitor therapy, bradykinin can be inactivated by aminopeptidase P or metabolized into a beta1 receptor agonist by carboxypeptidase N, which is then broken down by aminopeptidase P. If aminopeptidase P is not active, then bradykinin can be converted to des-Arg-bradykinin, which can then act on upregulated beta1 receptors in the oropharynx and tongue, producing vasodilation, increased capillary permeability, and pain.
Treatment
Treatment of ACE inhibitor-induced angioedema includes discontinuing the ACE inhibitor and providing symptomatic support. Although some ACE inhibitors are more likely than others to cause angioedema, a patient who has had an episode of ACE inhibitor-associated angioedema should never again use any ACE inhibitor.3 Angiotensin receptor blockers (ARBs) do not affect the bradykinin system; however, they can cause angioedema (0.13% in one trial of ARBs), and it is not known if ARBs should be avoided in patients who have had ACE inhibitor-induced angioedema.7 Therapy with a bradykinin receptor antagonist to prevent or resolve ACE inhibitor-associated angioedema has not yet been studied in detail.1
Summary
Adverse drug reactions can present clinically in many different ways, and, indeed, these reactions have deposed syphilis and tuberculosis as the mimic of disease. Many adverse drug reactions are mild, but others can be severe and, occasionally, life-threatening. This variability in manifestations means clinicians always have to consider that the drug may be the cause of the patient’s symptoms. TH
Johnson is a medical student at the Kansas City University of Medicine and Biosciences, Kansas City, Mo. Dr. Egger is a consultant in general internal medicine at the Mayo Clinic, Rochester, Minn.
References
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005 Jul;165(14):1637-1642.
- Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004 Feb;17(2):103-111.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53:373-388.
- Agostoni A, Cicardi M, Cugno M, et al. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology. 1999;44:21-25.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232-237.
- Ward KE, Hume AL. Olmesartan (benicar) for hypertension. Am Fam Physician. 2005;72:673-674.