User login
Lupus in Hispanics: A matter of serious concern
Some diseases are either more serious or more frequent in US Hispanics, and systemic lupus erythematosus is one of them. This fact has not yet diffused to all providers, many of whom will be the ones dealing with these individuals when the disease first emerges.
In order to raise physicians’ awareness of this situation, we will briefly review here the salient features of lupus in US Hispanics and its short-term and long-term impact.
HISPANICS ARE THE LARGEST MINORITY IN THE UNITED STATES
Over the last 30 years, the Hispanic population in the United States has increased to the point that it is now the largest US minority group, and the fastest-growing. In the 2010 US census, Hispanics surpassed the 50 million mark.1 Physicians and health care providers are becoming familiar with this growing population and its ailments, but more needs to be done to familiarize them with specific conditions that are more frequent and more serious in US Hispanics.
No population-based study has yet defined the prevalence and incidence of lupus in US Hispanics. However, on the basis of hospital and outpatient visits in regions in which Hispanics make up a large part of the population, it has been inferred that this group has a higher frequency of lupus, probably as high as in African Americans.
Likewise, clinicians taking care of these patients have suspected that lupus is more severe in US Hispanics than in non-Hispanic Caucasians, but this was documented and brought to general attention only with the publication of reports from the Lupus in Minorities: Nature versus Nurture (LUMINA) study.2
LUMINA, a longitudinal study
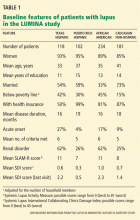
LUMINA is a longitudinal study of 640 patients with lupus from four populations: Hispanic from Texas, Hispanic from Puerto Rico, African American, and Caucasian non-Hispanic (Table 1). At the time of recruitment, patients were at least 16 years old and had had lupus for 5 years or less. They come in for periodic visits to the University of Alabama at Birmingham, the University of Texas Health Science Center at Houston, and the University of Puerto Rico Medical Sciences Campus. Recruitment began in 1994 and finished in 2007. Follow-up ranges from 1 to 14 years, with a mean of 4.5 years.
LUMINA is supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes of Health General Clinical Research Centers program, the National Center for Research Resources Clinical Research Infrastructure Initiative, the Mary Kirkland Center for Lupus Research Scholars Program, and Rheuminations Inc (New York, NY).
The purpose of the study is to shed light on the interplay of genetics and environment in this disease and, in the process, to raise awareness about the problem of lupus in Hispanics. In fact, much of the information in the following sections is from the LUMINA study.
HISPANICS ARE NOT A HOMOGENEOUS GROUP
In the United States, the term Hispanic describes anyone whose origin goes back to a Spanish-speaking country. However, US Hispanics are not a homogeneous racial group: they differ in genetics, culture, and problems.
The largest US Hispanic subgroup and the one more likely to be seen by US physicians is Hispanics of Mexican origin, who account for 66% of all US Hispanics. This group has a higher percentage of Amerindian genes than those of Puerto Rican ancestry.3 LUMINA researchers analyzed the DNA of 492 patients and found the following mixtures of genes3:
- Hispanics in Texas (mostly of Mexican origin): 48% Amerindian, 18% African, 34% European
- Hispanics from Puerto Rico: 20% Amerindian, 45% African, 35% European
- African Americans: 0% Amerindian, 79% African, 21% European
- Non-Hispanic Caucasians: 10% Amerindian, 18% African, 72% European.
Latin Americans of mixed European and Amerindian ancestry (which includes Aztec, Mayan, Quechuan, Aymaran, and other Central and South American groups) are called mestizos. Not all people in Latin America are mestizos: some are of European, African, or Asian ancestry, but in the United States they are all called Hispanics.
LUPUS DIFFERS AMONG SUBGROUPS
LUMINA research has revealed that lupus is heterogeneous also among US Hispanic subgroups. When people from Puerto Rico get lupus, it is generally less serious and devastating than in those from Mexico or Central America. Since US Hispanics of Mexican or Central American origin possess more Amerindian genes, this observation supports the notion that these genes are important contributors to the occurrence and expression of the disease.
Amerindian genes contribute to a greater susceptibility to lupus,4,5 although there is an interplay between genetic and nongenetic factors in the etiology and expression.6 Lupus starts at a younger age in Hispanics of predominantly Amerindian ancestry than in non-Hispanic Caucasians, and the onset is more likely to be acute.7
Renal involvement in these patients8 and mestizos from Latin America is rather common, probably as common as it is in US African Americans, and it tends to develop earlier than in non-Hispanic Caucasians.9 Amerindian ancestral genes, like African genes, contribute to the occurrence of renal disease in lupus patients.4 Furthermore, once nephritis ensues, end-stage renal disease occurs more often in US Hispanic and African American than in non-Hispanic Caucasian children, as demonstrated by Hiraki et al10 using national databases, and the same is true in adults, as shown in the LUMINA cohort.11
Other potentially serious manifestations of the disease are also more common, including hematologic and central nervous system manifestations. Not surprisingly, then, these patients show a higher degree of disease activity, both early in the course of the disease12,13 and over time.14
Table 1 compares the demographic and clinical features of LUMINA patients according to ethnicity. By and large, Hispanics from Texas have lower levels of education and income (comparable with levels in African Americans), and this can adversely affect the disease course by limiting these patients’ access to adequate care.15
DISEASE ACTIVITY AND ORGAN DAMAGE ARE GREATER IN HISPANICS
Disease activity in lupus reflects the ongoing immune-mediated inflammatory process. In LUMINA patients, regardless of the time at which disease activity was ascertained, it was higher in Hispanics from Texas and in African Americans than in non-Hispanic Caucasians and in Hispanics from Puerto Rico.7,12,16–18 Similar findings were seen in the Grupo Latinoamericano de Estudio de Lupus (GLADEL) cohort,13 in which mestizos and Hispanics of mixed African and European ancestry had higher maximum disease activity scores than non-Hispanic Caucasians.13
In addition, organ damage in lupus—the irreversible changes that occur in organ systems as a consequence of the disease or its treatments (eg, glucocorticoids, immunosuppressive drugs)—is more severe and develops sooner in Hispanics from Texas than in other groups.6,18,19 Using multivariate analysis, LUMINA investigators19 estimated the hazard ratio for the time until organ damage appeared for various risk factors, with values of 1 or greater indicating a shorter time and lower values indicating a longer time. Being a Hispanic from Texas carried a hazard ratio of 2.11 (95% confidence interval 1.15–3.88).
Because organ damage is an important and independent predictor of further damage20 and death,21 physicians need to take this disease quite seriously and try to prevent damage early in people at risk. To achieve that, the need to control disease activity must be balanced against the risk of overtreatment, as the important contribution of glucocorticoids to organ damage is well recognized.22
HISPANICS HAVE MORE COMORBIDITIES
Obesity, hypertension, diabetes, and metabolic syndrome are more common in US Hispanics, particularly those of Amerindian ancestry, than in the majority population of non-Hispanic Caucasians.23,24 The potential deleterious effects of glucocorticoids in patients already predisposed to these conditions need to be considered, balancing adequate disease control against the potential adverse effects.22
QUALITY OF LIFE IS WORSE WITH LUPUS
Whether it is measured with a generic instrument such as the Short Form 36 (SF-36), as it was in LUMINA,25 or with a disease-specific tool such as the Lupus-Pro, quality of life is significantly worsened by lupus. Furthermore, Fernandez et al26 found that a low level of health-related quality of life, as measured by the SF-6D version of the SF-36, was predictive of poor outcomes in LUMINA patients.
POVERTY, NOT ETHNICITY, ACCOUNTS FOR HIGHER MORTALITY RATE
As yet, we have no population-based data comparing survival in US Hispanic patients with lupus vs that of other population groups.
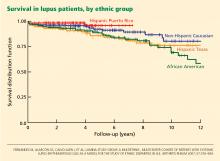
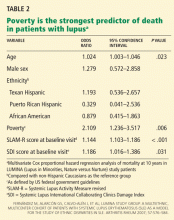
At first inspection, data from LUMINA indicate that Hispanics of primarily Amerindian ancestry have a lower survival rate than patients in other ethnic groups (Figure 1).6 However, when all other factors are taken into consideration, poverty, not ethnicity, is the major contributing factor (Table 2).6,27
This finding illustrates the important interplay between genetic and nongenetic factors in the course and final outcome of lupus, as already alluded to, although the exact relationship between them is not clear. It remains to be determined whether poverty is only a proxy for other population characteristics such as illiteracy, limited access to specialized care, limited access to medications, or cultural beliefs that may interfere with proper care.
ANTIMALARIAL DRUGS INCREASE SURVIVAL
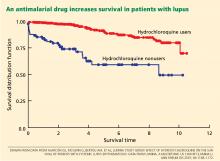
Using statistical analysis that adjusts for confounding by indication, we and others28–30 have shown that antimalarial drugs exert an independent and important protective effect on survival in lupus (Figure 2).
Important also is the protective effect of antimalarials on organ damage and the possibility of using them from disease outset in Hispanic patients at risk of early and rapid damage accrual,11 renal damage, and even lupus nephritis.31,32 This has very practical implications for the adequate and prompt management of these Hispanic patients.
PRACTICAL IMPLICATIONS
Lupus in US Hispanics is a serious disease with devastating consequences. Prompt diagnosis is paramount to prevent early organ damage and to prolong survival.
The disease may present in many different and unexpected ways, but joint pain, sun-sensitive rashes, renal involvement, cytopenias, and other manifestations should prompt the clinician to consider lupus in the differential diagnosis. Patients are often dismissed as having “arthritis” without being asked about other manifestations that may suggest a systemic connective tissue disease such as lupus. The same goes for skin rashes or unusual central nervous system manifestations.
The diagnosis of lupus is clinical, but some laboratory studies are essential to rule in or rule out renal or hematologic abnormalities and determine the level of disease activity. Tests usually ordered in patients suspected of having lupus include antinuclear antibody, complement levels, a complete blood cell count and differential, and a urinalysis. The need for additional tests depends on the results of the tests listed.
Once the disease is diagnosed, treatment should be tailored to the severity and type of clinical manifestations present. In general, glucocorticoids should be used at the smallest possible dose, antimalarials should be prescribed from the outset to all patients (following current guidelines in order to avoid ocular toxicity),33 and immunosuppressants and other treatments should be considered in certain instances. In parallel, consideration should be given to sun protection, adequate exercise, tobacco avoidance, osteoporosis and atherosclerosis prevention, planned conception, and compliance.
The goal in these people at risk is to control their lupus manifestations without causing undue damage, to preserve their quality of life, and to prevent an early demise.
- Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. 2010 Census briefs; 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed October 20, 2012.
- Reveille JD, Moulds JM, Ahn C, et al; for the LUMINA study Group. Systemic lupus erythematosus in three ethnic groups. I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity and disease onset. Arthritis Rheum 1998; 41:1161–1172.
- Alarcón GS, Beasley TM, Roseman JM, et al; LUMINA Study Group. Ethnic disparities in health and disease: the need to account for ancestral admixture when estimating the genetic contribution to both (LUMINA XXVI) (Letter). Lupus 2005; 14:867–868.
- Alarcón GS, Bastian HM, Beasley TM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA) XXXII: [corrected] contributions of admixture and socioeconomic status to renal involvement. Lupus 2006; 15:26–31.
- Sanchez E, Webb RD, Rasmussen A, et al. Genetically determined Amerindian ancestry correlates with increased frequency of risk alleles for systemic lupus erythematosus. Arthritis Rheum 2010; 62:3722–3729.
- Fernández M, Alarcón GS, Calvo-Alén J, et al; LUMINA Study Group. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 2007; 57:576–584.
- Alarcón GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs Nurture. Lupus 1999; 8:197–209.
- Bastian HM, Alarcón GS, Roseman JM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XL II: factors predictive of new or worsening proteinuria. Rheumatology (Oxford) 2007; 46:683–689.
- Burgos PI, McGwin G, Pons-Estel GJ, Reveille JD, Alarcón GS, Vilá LM. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann Rheum Dis 2011; 70:393–394.
- Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum 2011; 63:1988–1997.
- Pons-Estel GJ, Alarcón GS, McGwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 2009; 61:830–839.
- Alarcón GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998; 41:1173–1180.
- Pons-Estel BA, Catoggio LJ, Cardiel MH, et al; Grupo Latinoamericano de Estudio del Lupus. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics.” Medicine (Baltimore) 2004; 83:1–17.
- Alarcón GS, Calvo-Alén J, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006; 65:1168–1174.
- Vilá LM, Alarcón GS, McGwin G, Bastian HM, Fessler BJ, Reveille JD; Lumina Study Group. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006; 55:799–806.
- Zhang J, González LA, Roseman JM, Vilá LM, Reveille JD, Alárcon GS. Predictors of the rate of change in disease activity over time in LUMINA, a multiethnic US cohort of patients with systemic lupus erythematosus: LUMINA LXX. Lupus 2010; 19:727–733.
- Vilá LM, Alarcón GS, McGwin G, et al; LUMINA Study Group. Early clinical manifestations, disease activity and damage of systemic lupus erythematosus among two distinct US Hispanic subpopulations. Rheumatology (Oxford) 2004; 43:358–363.
- Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39:363–369.
- Toloza SM, Roseman JM, Alarcón GS, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXII. Predictors of time to the occurrence of initial damage. Arthritis Rheum 2004; 50:3177–3186.
- Alarcón GS, Roseman JM, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004; 43:202–205.
- Alarcón GS, McGwin G, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum 2001; 45:191–202.
- Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012 E-pub ahead of print.
- Jordan HT, Tabaei BP, Nash D, Angell SY, Chamany S, Kerker B. Metabolic syndrome among adults in New York City, 2004 New York City Health and Nutrition Examination Survey. Prev Chronic Dis 2012; 9:E04.
- Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). Am Heart J 2005; 149:1066–1073.
- Alarcón GS, McGwin G, Uribe A, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of selfreported health-related quality of life early in the disease course. Arthritis Rheum 2004; 51:465–474.
- Fernández M, Alarcón GS, McGwin G, et al; LUMINA Study Group. Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US cohort. Arthritis Rheum 2007; 57:986–992.
- Durán S, Apte M, Alarcón GSLUMINA Study Group. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007; 99:1196–1198.
- Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006; 15:577–583.
- Alarcón GS, McGwin G, Bertoli AM, et al; LUMINA Study Group. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007; 66:1168–1172.
- Shinjo SK, Bonfá E, Wojdyla D, et al; Grupo Latino Americano de Estudio del Lupus Eritematoso (Gladel). Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum 2010; 62:855–862.
- Fessler BJ, Alarcón GS, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005; 52:1473–1480.
- Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Antimalarials have a protective effect against the development of renal disease in Latin American SLE patients. The 9th International Congress on SLE June 24–27, 2010, Vancouver, Canada. Lupus 2010; 19(suppl 1):31–32.
- Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69:20–28.
Some diseases are either more serious or more frequent in US Hispanics, and systemic lupus erythematosus is one of them. This fact has not yet diffused to all providers, many of whom will be the ones dealing with these individuals when the disease first emerges.
In order to raise physicians’ awareness of this situation, we will briefly review here the salient features of lupus in US Hispanics and its short-term and long-term impact.
HISPANICS ARE THE LARGEST MINORITY IN THE UNITED STATES
Over the last 30 years, the Hispanic population in the United States has increased to the point that it is now the largest US minority group, and the fastest-growing. In the 2010 US census, Hispanics surpassed the 50 million mark.1 Physicians and health care providers are becoming familiar with this growing population and its ailments, but more needs to be done to familiarize them with specific conditions that are more frequent and more serious in US Hispanics.
No population-based study has yet defined the prevalence and incidence of lupus in US Hispanics. However, on the basis of hospital and outpatient visits in regions in which Hispanics make up a large part of the population, it has been inferred that this group has a higher frequency of lupus, probably as high as in African Americans.
Likewise, clinicians taking care of these patients have suspected that lupus is more severe in US Hispanics than in non-Hispanic Caucasians, but this was documented and brought to general attention only with the publication of reports from the Lupus in Minorities: Nature versus Nurture (LUMINA) study.2
LUMINA, a longitudinal study
LUMINA is a longitudinal study of 640 patients with lupus from four populations: Hispanic from Texas, Hispanic from Puerto Rico, African American, and Caucasian non-Hispanic (Table 1). At the time of recruitment, patients were at least 16 years old and had had lupus for 5 years or less. They come in for periodic visits to the University of Alabama at Birmingham, the University of Texas Health Science Center at Houston, and the University of Puerto Rico Medical Sciences Campus. Recruitment began in 1994 and finished in 2007. Follow-up ranges from 1 to 14 years, with a mean of 4.5 years.
LUMINA is supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes of Health General Clinical Research Centers program, the National Center for Research Resources Clinical Research Infrastructure Initiative, the Mary Kirkland Center for Lupus Research Scholars Program, and Rheuminations Inc (New York, NY).
The purpose of the study is to shed light on the interplay of genetics and environment in this disease and, in the process, to raise awareness about the problem of lupus in Hispanics. In fact, much of the information in the following sections is from the LUMINA study.
HISPANICS ARE NOT A HOMOGENEOUS GROUP
In the United States, the term Hispanic describes anyone whose origin goes back to a Spanish-speaking country. However, US Hispanics are not a homogeneous racial group: they differ in genetics, culture, and problems.
The largest US Hispanic subgroup and the one more likely to be seen by US physicians is Hispanics of Mexican origin, who account for 66% of all US Hispanics. This group has a higher percentage of Amerindian genes than those of Puerto Rican ancestry.3 LUMINA researchers analyzed the DNA of 492 patients and found the following mixtures of genes3:
- Hispanics in Texas (mostly of Mexican origin): 48% Amerindian, 18% African, 34% European
- Hispanics from Puerto Rico: 20% Amerindian, 45% African, 35% European
- African Americans: 0% Amerindian, 79% African, 21% European
- Non-Hispanic Caucasians: 10% Amerindian, 18% African, 72% European.
Latin Americans of mixed European and Amerindian ancestry (which includes Aztec, Mayan, Quechuan, Aymaran, and other Central and South American groups) are called mestizos. Not all people in Latin America are mestizos: some are of European, African, or Asian ancestry, but in the United States they are all called Hispanics.
LUPUS DIFFERS AMONG SUBGROUPS
LUMINA research has revealed that lupus is heterogeneous also among US Hispanic subgroups. When people from Puerto Rico get lupus, it is generally less serious and devastating than in those from Mexico or Central America. Since US Hispanics of Mexican or Central American origin possess more Amerindian genes, this observation supports the notion that these genes are important contributors to the occurrence and expression of the disease.
Amerindian genes contribute to a greater susceptibility to lupus,4,5 although there is an interplay between genetic and nongenetic factors in the etiology and expression.6 Lupus starts at a younger age in Hispanics of predominantly Amerindian ancestry than in non-Hispanic Caucasians, and the onset is more likely to be acute.7
Renal involvement in these patients8 and mestizos from Latin America is rather common, probably as common as it is in US African Americans, and it tends to develop earlier than in non-Hispanic Caucasians.9 Amerindian ancestral genes, like African genes, contribute to the occurrence of renal disease in lupus patients.4 Furthermore, once nephritis ensues, end-stage renal disease occurs more often in US Hispanic and African American than in non-Hispanic Caucasian children, as demonstrated by Hiraki et al10 using national databases, and the same is true in adults, as shown in the LUMINA cohort.11
Other potentially serious manifestations of the disease are also more common, including hematologic and central nervous system manifestations. Not surprisingly, then, these patients show a higher degree of disease activity, both early in the course of the disease12,13 and over time.14
Table 1 compares the demographic and clinical features of LUMINA patients according to ethnicity. By and large, Hispanics from Texas have lower levels of education and income (comparable with levels in African Americans), and this can adversely affect the disease course by limiting these patients’ access to adequate care.15
DISEASE ACTIVITY AND ORGAN DAMAGE ARE GREATER IN HISPANICS
Disease activity in lupus reflects the ongoing immune-mediated inflammatory process. In LUMINA patients, regardless of the time at which disease activity was ascertained, it was higher in Hispanics from Texas and in African Americans than in non-Hispanic Caucasians and in Hispanics from Puerto Rico.7,12,16–18 Similar findings were seen in the Grupo Latinoamericano de Estudio de Lupus (GLADEL) cohort,13 in which mestizos and Hispanics of mixed African and European ancestry had higher maximum disease activity scores than non-Hispanic Caucasians.13
In addition, organ damage in lupus—the irreversible changes that occur in organ systems as a consequence of the disease or its treatments (eg, glucocorticoids, immunosuppressive drugs)—is more severe and develops sooner in Hispanics from Texas than in other groups.6,18,19 Using multivariate analysis, LUMINA investigators19 estimated the hazard ratio for the time until organ damage appeared for various risk factors, with values of 1 or greater indicating a shorter time and lower values indicating a longer time. Being a Hispanic from Texas carried a hazard ratio of 2.11 (95% confidence interval 1.15–3.88).
Because organ damage is an important and independent predictor of further damage20 and death,21 physicians need to take this disease quite seriously and try to prevent damage early in people at risk. To achieve that, the need to control disease activity must be balanced against the risk of overtreatment, as the important contribution of glucocorticoids to organ damage is well recognized.22
HISPANICS HAVE MORE COMORBIDITIES
Obesity, hypertension, diabetes, and metabolic syndrome are more common in US Hispanics, particularly those of Amerindian ancestry, than in the majority population of non-Hispanic Caucasians.23,24 The potential deleterious effects of glucocorticoids in patients already predisposed to these conditions need to be considered, balancing adequate disease control against the potential adverse effects.22
QUALITY OF LIFE IS WORSE WITH LUPUS
Whether it is measured with a generic instrument such as the Short Form 36 (SF-36), as it was in LUMINA,25 or with a disease-specific tool such as the Lupus-Pro, quality of life is significantly worsened by lupus. Furthermore, Fernandez et al26 found that a low level of health-related quality of life, as measured by the SF-6D version of the SF-36, was predictive of poor outcomes in LUMINA patients.
POVERTY, NOT ETHNICITY, ACCOUNTS FOR HIGHER MORTALITY RATE
As yet, we have no population-based data comparing survival in US Hispanic patients with lupus vs that of other population groups.
At first inspection, data from LUMINA indicate that Hispanics of primarily Amerindian ancestry have a lower survival rate than patients in other ethnic groups (Figure 1).6 However, when all other factors are taken into consideration, poverty, not ethnicity, is the major contributing factor (Table 2).6,27
This finding illustrates the important interplay between genetic and nongenetic factors in the course and final outcome of lupus, as already alluded to, although the exact relationship between them is not clear. It remains to be determined whether poverty is only a proxy for other population characteristics such as illiteracy, limited access to specialized care, limited access to medications, or cultural beliefs that may interfere with proper care.
ANTIMALARIAL DRUGS INCREASE SURVIVAL
Using statistical analysis that adjusts for confounding by indication, we and others28–30 have shown that antimalarial drugs exert an independent and important protective effect on survival in lupus (Figure 2).
Important also is the protective effect of antimalarials on organ damage and the possibility of using them from disease outset in Hispanic patients at risk of early and rapid damage accrual,11 renal damage, and even lupus nephritis.31,32 This has very practical implications for the adequate and prompt management of these Hispanic patients.
PRACTICAL IMPLICATIONS
Lupus in US Hispanics is a serious disease with devastating consequences. Prompt diagnosis is paramount to prevent early organ damage and to prolong survival.
The disease may present in many different and unexpected ways, but joint pain, sun-sensitive rashes, renal involvement, cytopenias, and other manifestations should prompt the clinician to consider lupus in the differential diagnosis. Patients are often dismissed as having “arthritis” without being asked about other manifestations that may suggest a systemic connective tissue disease such as lupus. The same goes for skin rashes or unusual central nervous system manifestations.
The diagnosis of lupus is clinical, but some laboratory studies are essential to rule in or rule out renal or hematologic abnormalities and determine the level of disease activity. Tests usually ordered in patients suspected of having lupus include antinuclear antibody, complement levels, a complete blood cell count and differential, and a urinalysis. The need for additional tests depends on the results of the tests listed.
Once the disease is diagnosed, treatment should be tailored to the severity and type of clinical manifestations present. In general, glucocorticoids should be used at the smallest possible dose, antimalarials should be prescribed from the outset to all patients (following current guidelines in order to avoid ocular toxicity),33 and immunosuppressants and other treatments should be considered in certain instances. In parallel, consideration should be given to sun protection, adequate exercise, tobacco avoidance, osteoporosis and atherosclerosis prevention, planned conception, and compliance.
The goal in these people at risk is to control their lupus manifestations without causing undue damage, to preserve their quality of life, and to prevent an early demise.
Some diseases are either more serious or more frequent in US Hispanics, and systemic lupus erythematosus is one of them. This fact has not yet diffused to all providers, many of whom will be the ones dealing with these individuals when the disease first emerges.
In order to raise physicians’ awareness of this situation, we will briefly review here the salient features of lupus in US Hispanics and its short-term and long-term impact.
HISPANICS ARE THE LARGEST MINORITY IN THE UNITED STATES
Over the last 30 years, the Hispanic population in the United States has increased to the point that it is now the largest US minority group, and the fastest-growing. In the 2010 US census, Hispanics surpassed the 50 million mark.1 Physicians and health care providers are becoming familiar with this growing population and its ailments, but more needs to be done to familiarize them with specific conditions that are more frequent and more serious in US Hispanics.
No population-based study has yet defined the prevalence and incidence of lupus in US Hispanics. However, on the basis of hospital and outpatient visits in regions in which Hispanics make up a large part of the population, it has been inferred that this group has a higher frequency of lupus, probably as high as in African Americans.
Likewise, clinicians taking care of these patients have suspected that lupus is more severe in US Hispanics than in non-Hispanic Caucasians, but this was documented and brought to general attention only with the publication of reports from the Lupus in Minorities: Nature versus Nurture (LUMINA) study.2
LUMINA, a longitudinal study
LUMINA is a longitudinal study of 640 patients with lupus from four populations: Hispanic from Texas, Hispanic from Puerto Rico, African American, and Caucasian non-Hispanic (Table 1). At the time of recruitment, patients were at least 16 years old and had had lupus for 5 years or less. They come in for periodic visits to the University of Alabama at Birmingham, the University of Texas Health Science Center at Houston, and the University of Puerto Rico Medical Sciences Campus. Recruitment began in 1994 and finished in 2007. Follow-up ranges from 1 to 14 years, with a mean of 4.5 years.
LUMINA is supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes of Health General Clinical Research Centers program, the National Center for Research Resources Clinical Research Infrastructure Initiative, the Mary Kirkland Center for Lupus Research Scholars Program, and Rheuminations Inc (New York, NY).
The purpose of the study is to shed light on the interplay of genetics and environment in this disease and, in the process, to raise awareness about the problem of lupus in Hispanics. In fact, much of the information in the following sections is from the LUMINA study.
HISPANICS ARE NOT A HOMOGENEOUS GROUP
In the United States, the term Hispanic describes anyone whose origin goes back to a Spanish-speaking country. However, US Hispanics are not a homogeneous racial group: they differ in genetics, culture, and problems.
The largest US Hispanic subgroup and the one more likely to be seen by US physicians is Hispanics of Mexican origin, who account for 66% of all US Hispanics. This group has a higher percentage of Amerindian genes than those of Puerto Rican ancestry.3 LUMINA researchers analyzed the DNA of 492 patients and found the following mixtures of genes3:
- Hispanics in Texas (mostly of Mexican origin): 48% Amerindian, 18% African, 34% European
- Hispanics from Puerto Rico: 20% Amerindian, 45% African, 35% European
- African Americans: 0% Amerindian, 79% African, 21% European
- Non-Hispanic Caucasians: 10% Amerindian, 18% African, 72% European.
Latin Americans of mixed European and Amerindian ancestry (which includes Aztec, Mayan, Quechuan, Aymaran, and other Central and South American groups) are called mestizos. Not all people in Latin America are mestizos: some are of European, African, or Asian ancestry, but in the United States they are all called Hispanics.
LUPUS DIFFERS AMONG SUBGROUPS
LUMINA research has revealed that lupus is heterogeneous also among US Hispanic subgroups. When people from Puerto Rico get lupus, it is generally less serious and devastating than in those from Mexico or Central America. Since US Hispanics of Mexican or Central American origin possess more Amerindian genes, this observation supports the notion that these genes are important contributors to the occurrence and expression of the disease.
Amerindian genes contribute to a greater susceptibility to lupus,4,5 although there is an interplay between genetic and nongenetic factors in the etiology and expression.6 Lupus starts at a younger age in Hispanics of predominantly Amerindian ancestry than in non-Hispanic Caucasians, and the onset is more likely to be acute.7
Renal involvement in these patients8 and mestizos from Latin America is rather common, probably as common as it is in US African Americans, and it tends to develop earlier than in non-Hispanic Caucasians.9 Amerindian ancestral genes, like African genes, contribute to the occurrence of renal disease in lupus patients.4 Furthermore, once nephritis ensues, end-stage renal disease occurs more often in US Hispanic and African American than in non-Hispanic Caucasian children, as demonstrated by Hiraki et al10 using national databases, and the same is true in adults, as shown in the LUMINA cohort.11
Other potentially serious manifestations of the disease are also more common, including hematologic and central nervous system manifestations. Not surprisingly, then, these patients show a higher degree of disease activity, both early in the course of the disease12,13 and over time.14
Table 1 compares the demographic and clinical features of LUMINA patients according to ethnicity. By and large, Hispanics from Texas have lower levels of education and income (comparable with levels in African Americans), and this can adversely affect the disease course by limiting these patients’ access to adequate care.15
DISEASE ACTIVITY AND ORGAN DAMAGE ARE GREATER IN HISPANICS
Disease activity in lupus reflects the ongoing immune-mediated inflammatory process. In LUMINA patients, regardless of the time at which disease activity was ascertained, it was higher in Hispanics from Texas and in African Americans than in non-Hispanic Caucasians and in Hispanics from Puerto Rico.7,12,16–18 Similar findings were seen in the Grupo Latinoamericano de Estudio de Lupus (GLADEL) cohort,13 in which mestizos and Hispanics of mixed African and European ancestry had higher maximum disease activity scores than non-Hispanic Caucasians.13
In addition, organ damage in lupus—the irreversible changes that occur in organ systems as a consequence of the disease or its treatments (eg, glucocorticoids, immunosuppressive drugs)—is more severe and develops sooner in Hispanics from Texas than in other groups.6,18,19 Using multivariate analysis, LUMINA investigators19 estimated the hazard ratio for the time until organ damage appeared for various risk factors, with values of 1 or greater indicating a shorter time and lower values indicating a longer time. Being a Hispanic from Texas carried a hazard ratio of 2.11 (95% confidence interval 1.15–3.88).
Because organ damage is an important and independent predictor of further damage20 and death,21 physicians need to take this disease quite seriously and try to prevent damage early in people at risk. To achieve that, the need to control disease activity must be balanced against the risk of overtreatment, as the important contribution of glucocorticoids to organ damage is well recognized.22
HISPANICS HAVE MORE COMORBIDITIES
Obesity, hypertension, diabetes, and metabolic syndrome are more common in US Hispanics, particularly those of Amerindian ancestry, than in the majority population of non-Hispanic Caucasians.23,24 The potential deleterious effects of glucocorticoids in patients already predisposed to these conditions need to be considered, balancing adequate disease control against the potential adverse effects.22
QUALITY OF LIFE IS WORSE WITH LUPUS
Whether it is measured with a generic instrument such as the Short Form 36 (SF-36), as it was in LUMINA,25 or with a disease-specific tool such as the Lupus-Pro, quality of life is significantly worsened by lupus. Furthermore, Fernandez et al26 found that a low level of health-related quality of life, as measured by the SF-6D version of the SF-36, was predictive of poor outcomes in LUMINA patients.
POVERTY, NOT ETHNICITY, ACCOUNTS FOR HIGHER MORTALITY RATE
As yet, we have no population-based data comparing survival in US Hispanic patients with lupus vs that of other population groups.
At first inspection, data from LUMINA indicate that Hispanics of primarily Amerindian ancestry have a lower survival rate than patients in other ethnic groups (Figure 1).6 However, when all other factors are taken into consideration, poverty, not ethnicity, is the major contributing factor (Table 2).6,27
This finding illustrates the important interplay between genetic and nongenetic factors in the course and final outcome of lupus, as already alluded to, although the exact relationship between them is not clear. It remains to be determined whether poverty is only a proxy for other population characteristics such as illiteracy, limited access to specialized care, limited access to medications, or cultural beliefs that may interfere with proper care.
ANTIMALARIAL DRUGS INCREASE SURVIVAL
Using statistical analysis that adjusts for confounding by indication, we and others28–30 have shown that antimalarial drugs exert an independent and important protective effect on survival in lupus (Figure 2).
Important also is the protective effect of antimalarials on organ damage and the possibility of using them from disease outset in Hispanic patients at risk of early and rapid damage accrual,11 renal damage, and even lupus nephritis.31,32 This has very practical implications for the adequate and prompt management of these Hispanic patients.
PRACTICAL IMPLICATIONS
Lupus in US Hispanics is a serious disease with devastating consequences. Prompt diagnosis is paramount to prevent early organ damage and to prolong survival.
The disease may present in many different and unexpected ways, but joint pain, sun-sensitive rashes, renal involvement, cytopenias, and other manifestations should prompt the clinician to consider lupus in the differential diagnosis. Patients are often dismissed as having “arthritis” without being asked about other manifestations that may suggest a systemic connective tissue disease such as lupus. The same goes for skin rashes or unusual central nervous system manifestations.
The diagnosis of lupus is clinical, but some laboratory studies are essential to rule in or rule out renal or hematologic abnormalities and determine the level of disease activity. Tests usually ordered in patients suspected of having lupus include antinuclear antibody, complement levels, a complete blood cell count and differential, and a urinalysis. The need for additional tests depends on the results of the tests listed.
Once the disease is diagnosed, treatment should be tailored to the severity and type of clinical manifestations present. In general, glucocorticoids should be used at the smallest possible dose, antimalarials should be prescribed from the outset to all patients (following current guidelines in order to avoid ocular toxicity),33 and immunosuppressants and other treatments should be considered in certain instances. In parallel, consideration should be given to sun protection, adequate exercise, tobacco avoidance, osteoporosis and atherosclerosis prevention, planned conception, and compliance.
The goal in these people at risk is to control their lupus manifestations without causing undue damage, to preserve their quality of life, and to prevent an early demise.
- Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. 2010 Census briefs; 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed October 20, 2012.
- Reveille JD, Moulds JM, Ahn C, et al; for the LUMINA study Group. Systemic lupus erythematosus in three ethnic groups. I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity and disease onset. Arthritis Rheum 1998; 41:1161–1172.
- Alarcón GS, Beasley TM, Roseman JM, et al; LUMINA Study Group. Ethnic disparities in health and disease: the need to account for ancestral admixture when estimating the genetic contribution to both (LUMINA XXVI) (Letter). Lupus 2005; 14:867–868.
- Alarcón GS, Bastian HM, Beasley TM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA) XXXII: [corrected] contributions of admixture and socioeconomic status to renal involvement. Lupus 2006; 15:26–31.
- Sanchez E, Webb RD, Rasmussen A, et al. Genetically determined Amerindian ancestry correlates with increased frequency of risk alleles for systemic lupus erythematosus. Arthritis Rheum 2010; 62:3722–3729.
- Fernández M, Alarcón GS, Calvo-Alén J, et al; LUMINA Study Group. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 2007; 57:576–584.
- Alarcón GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs Nurture. Lupus 1999; 8:197–209.
- Bastian HM, Alarcón GS, Roseman JM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XL II: factors predictive of new or worsening proteinuria. Rheumatology (Oxford) 2007; 46:683–689.
- Burgos PI, McGwin G, Pons-Estel GJ, Reveille JD, Alarcón GS, Vilá LM. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann Rheum Dis 2011; 70:393–394.
- Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum 2011; 63:1988–1997.
- Pons-Estel GJ, Alarcón GS, McGwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 2009; 61:830–839.
- Alarcón GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998; 41:1173–1180.
- Pons-Estel BA, Catoggio LJ, Cardiel MH, et al; Grupo Latinoamericano de Estudio del Lupus. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics.” Medicine (Baltimore) 2004; 83:1–17.
- Alarcón GS, Calvo-Alén J, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006; 65:1168–1174.
- Vilá LM, Alarcón GS, McGwin G, Bastian HM, Fessler BJ, Reveille JD; Lumina Study Group. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006; 55:799–806.
- Zhang J, González LA, Roseman JM, Vilá LM, Reveille JD, Alárcon GS. Predictors of the rate of change in disease activity over time in LUMINA, a multiethnic US cohort of patients with systemic lupus erythematosus: LUMINA LXX. Lupus 2010; 19:727–733.
- Vilá LM, Alarcón GS, McGwin G, et al; LUMINA Study Group. Early clinical manifestations, disease activity and damage of systemic lupus erythematosus among two distinct US Hispanic subpopulations. Rheumatology (Oxford) 2004; 43:358–363.
- Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39:363–369.
- Toloza SM, Roseman JM, Alarcón GS, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXII. Predictors of time to the occurrence of initial damage. Arthritis Rheum 2004; 50:3177–3186.
- Alarcón GS, Roseman JM, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004; 43:202–205.
- Alarcón GS, McGwin G, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum 2001; 45:191–202.
- Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012 E-pub ahead of print.
- Jordan HT, Tabaei BP, Nash D, Angell SY, Chamany S, Kerker B. Metabolic syndrome among adults in New York City, 2004 New York City Health and Nutrition Examination Survey. Prev Chronic Dis 2012; 9:E04.
- Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). Am Heart J 2005; 149:1066–1073.
- Alarcón GS, McGwin G, Uribe A, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of selfreported health-related quality of life early in the disease course. Arthritis Rheum 2004; 51:465–474.
- Fernández M, Alarcón GS, McGwin G, et al; LUMINA Study Group. Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US cohort. Arthritis Rheum 2007; 57:986–992.
- Durán S, Apte M, Alarcón GSLUMINA Study Group. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007; 99:1196–1198.
- Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006; 15:577–583.
- Alarcón GS, McGwin G, Bertoli AM, et al; LUMINA Study Group. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007; 66:1168–1172.
- Shinjo SK, Bonfá E, Wojdyla D, et al; Grupo Latino Americano de Estudio del Lupus Eritematoso (Gladel). Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum 2010; 62:855–862.
- Fessler BJ, Alarcón GS, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005; 52:1473–1480.
- Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Antimalarials have a protective effect against the development of renal disease in Latin American SLE patients. The 9th International Congress on SLE June 24–27, 2010, Vancouver, Canada. Lupus 2010; 19(suppl 1):31–32.
- Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69:20–28.
- Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. 2010 Census briefs; 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed October 20, 2012.
- Reveille JD, Moulds JM, Ahn C, et al; for the LUMINA study Group. Systemic lupus erythematosus in three ethnic groups. I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity and disease onset. Arthritis Rheum 1998; 41:1161–1172.
- Alarcón GS, Beasley TM, Roseman JM, et al; LUMINA Study Group. Ethnic disparities in health and disease: the need to account for ancestral admixture when estimating the genetic contribution to both (LUMINA XXVI) (Letter). Lupus 2005; 14:867–868.
- Alarcón GS, Bastian HM, Beasley TM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA) XXXII: [corrected] contributions of admixture and socioeconomic status to renal involvement. Lupus 2006; 15:26–31.
- Sanchez E, Webb RD, Rasmussen A, et al. Genetically determined Amerindian ancestry correlates with increased frequency of risk alleles for systemic lupus erythematosus. Arthritis Rheum 2010; 62:3722–3729.
- Fernández M, Alarcón GS, Calvo-Alén J, et al; LUMINA Study Group. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 2007; 57:576–584.
- Alarcón GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs Nurture. Lupus 1999; 8:197–209.
- Bastian HM, Alarcón GS, Roseman JM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XL II: factors predictive of new or worsening proteinuria. Rheumatology (Oxford) 2007; 46:683–689.
- Burgos PI, McGwin G, Pons-Estel GJ, Reveille JD, Alarcón GS, Vilá LM. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann Rheum Dis 2011; 70:393–394.
- Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum 2011; 63:1988–1997.
- Pons-Estel GJ, Alarcón GS, McGwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 2009; 61:830–839.
- Alarcón GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998; 41:1173–1180.
- Pons-Estel BA, Catoggio LJ, Cardiel MH, et al; Grupo Latinoamericano de Estudio del Lupus. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics.” Medicine (Baltimore) 2004; 83:1–17.
- Alarcón GS, Calvo-Alén J, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006; 65:1168–1174.
- Vilá LM, Alarcón GS, McGwin G, Bastian HM, Fessler BJ, Reveille JD; Lumina Study Group. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006; 55:799–806.
- Zhang J, González LA, Roseman JM, Vilá LM, Reveille JD, Alárcon GS. Predictors of the rate of change in disease activity over time in LUMINA, a multiethnic US cohort of patients with systemic lupus erythematosus: LUMINA LXX. Lupus 2010; 19:727–733.
- Vilá LM, Alarcón GS, McGwin G, et al; LUMINA Study Group. Early clinical manifestations, disease activity and damage of systemic lupus erythematosus among two distinct US Hispanic subpopulations. Rheumatology (Oxford) 2004; 43:358–363.
- Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39:363–369.
- Toloza SM, Roseman JM, Alarcón GS, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXII. Predictors of time to the occurrence of initial damage. Arthritis Rheum 2004; 50:3177–3186.
- Alarcón GS, Roseman JM, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004; 43:202–205.
- Alarcón GS, McGwin G, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum 2001; 45:191–202.
- Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012 E-pub ahead of print.
- Jordan HT, Tabaei BP, Nash D, Angell SY, Chamany S, Kerker B. Metabolic syndrome among adults in New York City, 2004 New York City Health and Nutrition Examination Survey. Prev Chronic Dis 2012; 9:E04.
- Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). Am Heart J 2005; 149:1066–1073.
- Alarcón GS, McGwin G, Uribe A, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of selfreported health-related quality of life early in the disease course. Arthritis Rheum 2004; 51:465–474.
- Fernández M, Alarcón GS, McGwin G, et al; LUMINA Study Group. Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US cohort. Arthritis Rheum 2007; 57:986–992.
- Durán S, Apte M, Alarcón GSLUMINA Study Group. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007; 99:1196–1198.
- Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006; 15:577–583.
- Alarcón GS, McGwin G, Bertoli AM, et al; LUMINA Study Group. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007; 66:1168–1172.
- Shinjo SK, Bonfá E, Wojdyla D, et al; Grupo Latino Americano de Estudio del Lupus Eritematoso (Gladel). Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum 2010; 62:855–862.
- Fessler BJ, Alarcón GS, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005; 52:1473–1480.
- Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Antimalarials have a protective effect against the development of renal disease in Latin American SLE patients. The 9th International Congress on SLE June 24–27, 2010, Vancouver, Canada. Lupus 2010; 19(suppl 1):31–32.
- Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69:20–28.
KEY POINTS
- Amerindian genes contribute to a greater susceptibility to lupus, although there is an interplay between genetic and nongenetic factors in its etiology and expression.
- In large studies, disease activity and organ damage were greater in African Americans and in Hispanics from Texas than in Caucasians and Hispanics from Puerto Rico.
- Hispanics of primarily Amerindian ancestry (which includes Aztec, Mayan, Quechuan, Aymaran, and other Central and South American groups) have a lower survival rate than patients in other ethnic groups, but poverty is the responsible factor.
- The need to control disease activity with corticosteroids must be balanced against the risk of overtreatment and organ damage.
- Antimalarial drugs such as chloroquine and hydroxychloroquine should be prescribed from the outset to all patients with lupus, according to current guidelines designed to avoid ocular toxicity.
Hypertensive chronic kidney disease in African Americans: Strategies for improving care
“Healthy citizens are the greatest asset any country can have.”
—Winston Churchill
Diabetes and high blood pressure take a toll on the kidneys, especially in African Americans. To prevent chronic kidney disease (CKD) and to slow or stop its progression, the same principles apply in African Americans as in other patients—ie, vigilance for the onset of proteinuria, aggressive control of blood pressure, drug treatment to block the renin-angiotensin system, and attention to lifestyle factors (Table 1). However, we need to try to do better in the care of African Americans.
The purpose of this article is to review recent evidence- and consensus-based recommendations and to present a practical approach for the evaluation and treatment of CKD in African Americans.
CKD DEFINED
In 2002, the National Kidney Foundation1 defined CKD as either:
- Kidney damage for 3 or more months, as defined by structural or functional abnormalities of the kidney, with or without a decreased glomerular filtration rate (GFR), manifested either by pathologic abnormalities or by markers of kidney damage, including abnormalities in the composition of the blood or urine (eg, proteinuria), or abnormalities in imaging tests; or
- A GFR less than 60 mL/min/1.73 m2 for 3 or more months, with or without kidney damage.
The definition divides CKD into five progressive stages according to the GFR:
- Stage 1 (kidney damage with normal or increased GFR): GFR ≥ 90 mL/min/1.73m2
- Stage 2 (kidney damage with mildly decreased GFR): GFR 60–89
- Stage 3 (moderately decreased GFR): GFR 30–59
- Stage 4 (severely decreased GFR): GFR 15–29
- Stage 5 (kidney failure): GFR < 15 or dialysis.
Because the definition includes markers of kidney damage such as albuminuria, it allows CKD to be detected in its earliest stages, when the estimated GFR might still be well within normal limits.
CKD APPEARS EARLIER, PROGRESSES FASTER IN AFRICAN AMERICANS
“Not everything that counts can be counted, and not everything that can be counted counts.”
—Albert Einstein
CKD with or without a sustained reduction in the estimated GFR affects about one in every nine American adults.2 Its course varies depending on the cause and also from patient to patient, even in those with the same cause of CKD.
In general, the prevalence of early CKD is comparable across racial and ethnic groups in the United States, but CKD progresses to end-stage renal disease far more rapidly in minority populations, with rates nearly four times higher in black Americans than in white Americans.3 Also, the onset of CKD is earlier in African Americans.
HYPERTENSION AND DIABETES AS REASONS FOR THE DISPARITIES
Part of the reason for these differences is that minority populations have higher rates of diabetes and hypertension, and these diseases tend to be more severe in these groups. Poverty, less access to health care, exposure to environmental toxins, and genetic variation may also contribute.4–7
Compared with whites, blacks have higher rates of diabetes and hypertension and earlier onset of these diseases, poorer control, and higher rates of complications such as CKD, stroke, and heart disease.8,9 The higher rate of hypertension and the lower rate of blood pressure control in African Americans with CKD may contribute to the more rapid progression of CKD to end-stage renal disease.
In the Chronic Renal Insufficiency Cohort, 10 a racially and ethnically diverse group of 3,612 adults with a broad spectrum of renal disease severity, 93% of African Americans had hypertension at baseline compared with 80% of whites. In addition, African Americans were 18% less likely to have their blood pressure controlled to 140/90 mm Hg (the rates of control were 76% vs 60%), and 28% were less likely to have it controlled to 130/80 mm Hg (56% vs 38%).10 These factors may partially explain the faster progression to end-stage renal disease in African Americans with CKD.
Despite the potential efficacy of strict control of serum glucose levels and blood pressure,11 the high rate of poor blood pressure control has contributed to the epidemic of diabetic nephropathy, especially among African Americans. Fortunately, hypertension control in the general population, while still not ideal, has improved from 27% in 1988–1994 to 50% in 2007–2008 and is now similar across racial and ethnic groups.12 This, hopefully, is a preface for improved hypertension-related outcomes for all Americans over the next decade.
OTHER REASONS FOR THE DISPARITIES
“There are no unnatural or supernatural phenomena, only a very large gap in our knowledge of what is natural.”
—Edgar Mitchell, Apollo 14 astronaut
Proteinuria
Proteinuria is another key cardiorenal risk factor prevalent in African Americans.
Knight et al,13 analyzing data from the Third National Health and Nutrition Examination Survey, found that people with high-normal blood pressure (systolic pressure 130–139 mm Hg or diastolic pressure 85–89 mm Hg) were twice as likely to have microalbuminuria (odds ratio 2.13, 95% confidence interval [CI] 1.51–3.01) compared with people with optimal blood pressure (systolic pressure < 120 mm Hg and diastolic pressure < 80 mm Hg). Compared with whites as the reference group, Mexican Americans had slightly but not statistically significantly higher odds of microalbuminuria (odds ratio 1.16; 95% CI 0.90–1.51), and African Americans had significantly higher odds (odds ratio 1.30; 95% CI 1.04–1.64).
The incidence of hypertension-related end-stage renal disease is nearly five times higher in African Americans than in whites, and the rate of hypertension-related end-stage renal disease is 15 times higher in African American men ages 24 to 44 than in whites of the same ages.3 The greater risk of proteinuria in African Americans at any given level of higher blood pressure is thought to contribute in part to these disparate rates.
The renin-angiotensin system
The renin-angiotensin system plays a role in modulating hypertension and mediating hypertension-related complications. Hypertensive African Americans are more likely than hypertensive whites to have low-renin, salt-sensitive hypertension. Therein lies a paradox.
Since the renin-angiotensin system promotes the progression of CKD, we would expect patients with low-renin hypertension to have a lower risk of hypertension-related endorgan damage than patients with high-renin hypertension. However, many African Americans (who as a group have high rates of sodium sensitivity and low plasma renin levels) experience more severe hypertension-related end-organ complications such as proteinuria and cardiorenal disease.14
A reason for this paradox may be that the circulating renin-angiotensin system is separate from the intrarenal one. Supporting this theory is the observation that up-regulation of the intrarenal renin-angiotensin system accompanies renal interstitial inflammation and oxidative stress in the kidneys and cardiovascular tissues of salt-sensitive rats fed a high-salt diet.15 In other experiments in salt-sensitive rats, renin-angiotensin system blockade reversed endothelial dysfunction, attenuated proteinuria, and reduced renal injury independent of blood pressure changes even though the animals had low circulating renin levels.16
These findings imply that drugs that block the renin-angiotensin system, ie, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, could still be a rational therapy for CKD patients with low-renin hypertension, particularly African Americans, in whom local up-regulation of the renin-angiotensin system in the kidney could exacerbate both diabetic and hypertensive CKD.17 Although these drugs may not lower blood pressure as much in low-renin hypertension as in high-renin hypertension, they may still afford the same cardiorenal protection.
Genetic factors
Variations in the MYH9 and APOL1 genes on chromosome 22 have recently been found in genome-wide admixture mapping studies and may explain as much as 70% of the differences in the rates of nondiabetic end-stage renal disease between white and black Americans.7,18,19 In addition, genetic variations may modulate differences in blood-pressure response to antihypertensive medications across racial and ethnic groups,20 complicating treatment recommendations and clinical outcomes in our increasingly diverse nation.
Comment. The pathophysiologic basis for the variability in the course of CKD is probably multifactorial and is still poorly understood. Nevertheless, we may be able to delay the progression of CKD and prevent its complications with specific therapeutic and life-style interventions.
Race and ethnicity are associated with sociocultural and biologic variations that influence the risk and progression of CKD. Understanding these factors for minority populations can help in targeting interventions to attenuate the disproportionately high rates of CKD progression and complications.
The pathophysiologic reason African Americans have a greater prevalence of end-stage renal disease and a more rapid progression of CKD is complex and probably involves the interplay of biological, behavioral, and environmental factors such as salt intake, stress levels, and exposure to heavy metals.21
TRIALS OF ANTIHYPERTENSIVE THERAPY IN AFRICAN AMERICANS WITH CKD
“If we knew what we were doing, it wouldn’t be called research.”
—Albert Einstein
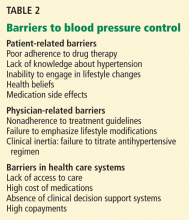
Until recently, trials of antihypertensive therapy in patients with CKD did not include adequate numbers of African American participants, but the following clinical trials have added to our knowledge (Table 2).22–26
African American Study of Kidney Disease and Hypertension (AASK)
The African American Study of Kidney Disease and Hypertension (AASK),22,23 with 1,094 patients, was the largest prospective study of CKD to date designed to focus on African Americans.
AASK examined the effects of two levels of blood-pressure control:
- Standard, with a goal blood pressure of 135–140/85–90 mm Hg (mean arterial pressure 102–107 mm Hg)
- Intensive, with a goal of 120/80 mm Hg or less (mean arterial pressure ≤ 92 mm Hg).
In a two-by two factorial design, patients were also randomized to receive one of three antihypertensive drugs as initial therapy:
- The ACE inhibitor ramipril (Altace)
- The sustained-release beta-blocker metoprolol succinate (Toprol XL)
- The calcium channel blocker amlodipine (Norvasc).
To enter the study, patients had to be African American, have at least one diastolic pressure reading of 95 mm Hg or greater during the screening period, and have a measured GFR between 20 and 65 mL/min/1.83 m2. They could not have diabetes, substantial proteinuria (> 2.5 g/day), or other causes of CKD.22
AASK was distinct from many of the larger hypertension trials in which secondary analyses of outcomes in patients with CKD were performed in that it was implicit in the design that most, if not all, study participants had substantial GFR reduction and would need diuretic therapy.
At baseline, after blood pressure medications had been tapered to define eligibility and then reintroduced before randomization, 20.0% of the patients in the intensive blood pressure goal group had pressure lower than 140/90 mm Hg, and this increased to 78.9% by 14 months after randomization. In the standard goal group, the numbers were 21.5% at baseline but only 41.8% at 14 months.23 In spite of this difference, the rate of decline in GFR (the main clinical outcome measure) was the same in both groups.
However, the class of drug did make a difference. Secondary clinical outcomes, including the composite end point of development of end-stage renal disease, doubling of serum creatinine, or death, were less frequent in the ACE inhibitor group than in the beta-blocker and calcium channel blocker groups. As anticipated and consistent with real world practice, nearly 90% of all participants received concomitant diuretic therapy to achieve target blood pressure levels.
Comments. AASK showed that blood pressure can be controlled in African Americans who have CKD and that clinical cardiorenal outcomes can be improved by using an ACE inhibitor as initial therapy rather than a beta-blocker or calcium channel blocker, with diuretics and other agents added as needed.
AASK cohort phase
After completing the trial phase, patients were invited to enroll in a cohort phase in which the blood pressure target was less than 130/80 mm Hg. The combined follow-up period was 8.8 to 12.2 years.24
During the trial phase, the mean blood pressure was 130/78 mm Hg in the intensive group and 141/86 mm Hg in the standard group. During the cohort phase, the mean blood pressures were 131/78 mm Hg and 134/78 mm Hg, respectively, in these groups.
In both phases, there was no significant difference between groups in clinical outcomes (hazard ratio in the intensive-control group 0.91, P = .27). However, the groups differed when stratified by baseline level of proteinuria (P = .02 for the interaction), with a potential benefit of a blood pressure target lower than 130/80 mm Hg in patients with a protein-to-creatinine ratio of more than 0.22 (hazard ratio 0.73, P = .01).24
Comment. Given that many African Americans with hypertension and CKD have a protein-to-creatinine ratio of more than 0.22, these findings support a practical approach in clinical practice for a target blood pressure less than 130/80 mm Hg, using a first-line combination of a renin-angiotensin system inhibitor and a diuretic.
RENAAL study
The Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan (RENAAL) study25 included 1,513 patients, of whom 15% were African American and 18% were Hispanic; all had type 2 diabetes mellitus and nephropathy. They were randomized to receive the angiotensin II receptor antagonist losartan (Cozaar) or placebo in addition to other antihypertensive drugs.
At 3.4 years, the blood pressure was about 141/74 mm Hg in both groups. A post hoc analysis found lower rates of albuminuria and end-stage renal disease in the group treated with losartan,25 with no racial or ethnic differences in its renoprotective effect.
Comments. While these findings support the recommendation of inhibiting the renin-angiotensin system for improving clinical outcomes in diabetic nephropathy in racial and ethnic minorities, the AASK study also proved a second important point. These patients required intense blood pressure management for several years in a clinical trial environment, which may be difficult to do in many clinical practice models.
To be cost-effective in today’s health care environment, such care will likely be limited to larger group practices or health care plans with large comprehensive covered populations. Payers and providers need to be willing to invest in intense early care in such high-risk subgroups with the understanding that they could recognize downstream gains from long-term improved outcomes. However, even in these settings, the ability to provide effective care to high-risk subgroups without generating significant financial losses remains a concern.
ALLHAT
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)26 enrolled more than 33,000 hypertensive patients at high risk, of whom 32% were black, 16% were Hispanic, and 36% had diabetes. Their mean serum creatinine level was 1 mg/dL. Follow-up was for up to 8 years. At year 5, the mean blood pressure was 135/75 mm Hg.
In a secondary analysis, patients were stratified by GFR:
- Normal (> 90 mL/min/1.73 m2; n = 8,126)
- Mild reduction (60–89 mL/min/1.73 m2; n = 18,109)
- Moderate-severe reduction (< 60 mL/min/1.73 m2; n = 5,662).
In all three groups, amlodipine, lisinopril (Zestril), and chlorthalidone were equivalent as initial monotherapy in reducing the rate of the composite end point of end-stage renal disease or 50% or greater decrement in GFR.
Comments. The combined AASK, RENAAL, and ALLHAT findings are consistent with the practical recommendation of a diuretic, renin-angiotensin system inhibitor, or both, as initial therapy for blood pressure control in African American patients who have CKD, with a target blood pressure of less than 130/80 mm Hg.
A COMPREHENSIVE APPROACH TO CHRONIC KIDNEY DISEASE CARE
“It is much more important to know what sort of a patient has a disease, than what sort of disease a patient has.”
—William Osler
Many of the risk factors for cardiovascular disease in African Americans are behavioral and modifiable. These include too much salt and fat in the diet, too little physical activity, excessive alcohol intake, and smoking.
Education is key, to identify and communicate the risk attributable to health beliefs and behaviors, particularly in patients with known cardiovascular disease, and to encourage the patient to be proactive in risk-reduction strategies (Table 1). However, effective communication depends on compassion and concern by the health care provider to engender a sense of trust.27 Other health care professionals such as dietitians, pharmacists, and social workers as well as family members can reinforce messages and improve communication with the patient to optimize outcomes.
The International Society on Hypertension in Blacks recommends a blood pressure target of less than 130/80 mm Hg in blacks with elevated blood pressure and target-organ damage. The authors suggest monotherapy with a diuretic or calcium channel blocker if the blood pressure is 10 mm Hg or less above target levels. When blood pressure is more than 15/10 mm Hg above target, two-drug therapy is recommended, either with a calcium channel blocker plus a renin-angiotensin system blocker or, alternatively, in edematous or volume-overload states, with a thiazide diuretic plus a renin-angiotensin system blocker.28,29
The Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease of the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative recommend starting anti-hypertensive therapy with an ACE inhibitor or an angiotensin receptor blocker for most patients with CKD, regardless of ethnicity, recognizing that many will require combination therapy.30 Evaluation of the response to therapy should include not only checking that the blood pressure is at or below the recommended target of 130/80 mm Hg, but also assessing for complications and monitoring the change in the level of proteinuria, which is a powerful predictor of progression of hypertensive kidney disease in all patients at any given GFR.31
OUR RECOMMENDATIONS
African Americans with hypertension and kidney disease require an aggressive and comprehensive approach to slow the progression of kidney disease and its complications, often necessitating aggressive care of the primary cause and the use of two or more antihypertensive agents to control blood pressure, proteinuria, or both (Figure 1).32
We recommend that the initial evaluation of patients with hypertension include a screening for albuminuria and that the initial therapy for hypertension or proteinuria in all patients with CKD include renin-angiotensin system inhibition with a diuretic, because this combination appears most effective to achieve blood pressure control and to confer additional cardiorenal protection beyond that offered by blood-pressure control alone. Although some studies have reported that African Americans have lower blood-pressure response rates than whites to renin-angiotensin system inhibition, 18 it is nevertheless beneficial for clinical outcomes in this group, especially in the presence of proteinuria, a hallmark of hypertension-related CKD in African Americans. Thus, until more data are available, ethnicity should not be the primary criterion for selecting a given class of antihypertensive therapy, especially in patients with hypertensive nephropathy.
The overall treatment decision should be guided by individual response, coexisting risk factors, and potential cultural and socioeconomic considerations such as cost of medications and insurance coverage, which affect adherence to both pharmacologic and nonpharmacologic interventions.33
Future studies should strive for adequate representation of racial and ethnic minority populations in order to enhance the evidence base for CKD treatment as we move toward using personalized medicine approaches in an increasingly diverse society.34
Acknowledgment: Support for this paper was provided in part by NIH grants RR026138 and MD000182.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010.
- Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 2008; 19:1261–1270.
- Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int 2005; 68:914–924.
- Powe NR. To have and have not: health and health care disparities in chronic kidney disease. Kidney Int 2003; 64:763–772.
- Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329:841–845.
- Rosamond W, Flegal K, Furie K, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117:e25–e146.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Muntner P, Anderson A, Charleston J, et al; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2010; 55:441–451.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Knight EL, Kramer HM, Curhan GC. High-normal blood pressure and microalbuminuria. Am J Kidney Dis 2003; 41:588–595.
- Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation 1979; 59:643–650.
- Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol 2008; 28:158–167.
- Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation 1997; 96:2407–2413.
- Norris KC, Tareen N, Martins D, Vaziri ND. Implications of ethnicity for the treatment of hypertensive kidney disease, with an emphasis on African Americans. Nat Clin Pract Nephrol 2008; 4:538–549.
- Kao WH, Klag MJ, Meoni LA, et al; Family Investigation of Nephropathy and Diabetes Research Group. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 2008; 40:1185–1192.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993; 328:914–921. Erratum in N Engl J Med 1994; 330:1689.
- Norris KC, Francis CK. Gender and ethnic differences and considerations in cardiovascular risk assessment and prevention in African Americans. In:Wong N, Gardin JM, Black HR, editors. Practical Strategies in Preventing Heart Disease. New York, NY: McGraw-Hill; 2004:415–440.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431. Erratum in JAMA 2006; 295:2726.
- Wright JT, Agodoa L, Contreras G, et al; African American Study of Kidney Disease and Hypertension Study Group. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med 2002; 162:1636–1643.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- de Zeeuw D, Ramjit D, Zhang Z, et al. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int 2006; 69:1675–1682.
- Rahman M, Pressel S, Davis BR, et al. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 2005; 165:936–946.
- Barrier PA, Li JT, Jensen NM. Two words to improve physician-patient communication: what else? Mayo Clin Proc 2003; 78:211–214.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Wright JT, Agodoa LY, Appel L, et al. New recommendations for treating hypertension in black patients: evidence and/or consensus? Hypertension 2010; 56:801–803.
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease: executive summary. Am J Kid Dis 2004; 43(suppl 1):S16–S33.
- Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med 2005; 165:947–953.
- Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000; 36:646–661.
- Martins D, Norris K. Hypertension treatment in African Americans: physiology is less important than sociology. Cleve Clin J Med 2004; 71:735–743.
- Cooper RS, Psaty BM. Should ethnicity serve as the basis for clinical trial design? Diversity and inclusiveness should remain the guiding principles for clinical trials. Circulation 2005; 112:3660–3665.
“Healthy citizens are the greatest asset any country can have.”
—Winston Churchill
Diabetes and high blood pressure take a toll on the kidneys, especially in African Americans. To prevent chronic kidney disease (CKD) and to slow or stop its progression, the same principles apply in African Americans as in other patients—ie, vigilance for the onset of proteinuria, aggressive control of blood pressure, drug treatment to block the renin-angiotensin system, and attention to lifestyle factors (Table 1). However, we need to try to do better in the care of African Americans.
The purpose of this article is to review recent evidence- and consensus-based recommendations and to present a practical approach for the evaluation and treatment of CKD in African Americans.
CKD DEFINED
In 2002, the National Kidney Foundation1 defined CKD as either:
- Kidney damage for 3 or more months, as defined by structural or functional abnormalities of the kidney, with or without a decreased glomerular filtration rate (GFR), manifested either by pathologic abnormalities or by markers of kidney damage, including abnormalities in the composition of the blood or urine (eg, proteinuria), or abnormalities in imaging tests; or
- A GFR less than 60 mL/min/1.73 m2 for 3 or more months, with or without kidney damage.
The definition divides CKD into five progressive stages according to the GFR:
- Stage 1 (kidney damage with normal or increased GFR): GFR ≥ 90 mL/min/1.73m2
- Stage 2 (kidney damage with mildly decreased GFR): GFR 60–89
- Stage 3 (moderately decreased GFR): GFR 30–59
- Stage 4 (severely decreased GFR): GFR 15–29
- Stage 5 (kidney failure): GFR < 15 or dialysis.
Because the definition includes markers of kidney damage such as albuminuria, it allows CKD to be detected in its earliest stages, when the estimated GFR might still be well within normal limits.
CKD APPEARS EARLIER, PROGRESSES FASTER IN AFRICAN AMERICANS
“Not everything that counts can be counted, and not everything that can be counted counts.”
—Albert Einstein
CKD with or without a sustained reduction in the estimated GFR affects about one in every nine American adults.2 Its course varies depending on the cause and also from patient to patient, even in those with the same cause of CKD.
In general, the prevalence of early CKD is comparable across racial and ethnic groups in the United States, but CKD progresses to end-stage renal disease far more rapidly in minority populations, with rates nearly four times higher in black Americans than in white Americans.3 Also, the onset of CKD is earlier in African Americans.
HYPERTENSION AND DIABETES AS REASONS FOR THE DISPARITIES
Part of the reason for these differences is that minority populations have higher rates of diabetes and hypertension, and these diseases tend to be more severe in these groups. Poverty, less access to health care, exposure to environmental toxins, and genetic variation may also contribute.4–7
Compared with whites, blacks have higher rates of diabetes and hypertension and earlier onset of these diseases, poorer control, and higher rates of complications such as CKD, stroke, and heart disease.8,9 The higher rate of hypertension and the lower rate of blood pressure control in African Americans with CKD may contribute to the more rapid progression of CKD to end-stage renal disease.
In the Chronic Renal Insufficiency Cohort, 10 a racially and ethnically diverse group of 3,612 adults with a broad spectrum of renal disease severity, 93% of African Americans had hypertension at baseline compared with 80% of whites. In addition, African Americans were 18% less likely to have their blood pressure controlled to 140/90 mm Hg (the rates of control were 76% vs 60%), and 28% were less likely to have it controlled to 130/80 mm Hg (56% vs 38%).10 These factors may partially explain the faster progression to end-stage renal disease in African Americans with CKD.
Despite the potential efficacy of strict control of serum glucose levels and blood pressure,11 the high rate of poor blood pressure control has contributed to the epidemic of diabetic nephropathy, especially among African Americans. Fortunately, hypertension control in the general population, while still not ideal, has improved from 27% in 1988–1994 to 50% in 2007–2008 and is now similar across racial and ethnic groups.12 This, hopefully, is a preface for improved hypertension-related outcomes for all Americans over the next decade.
OTHER REASONS FOR THE DISPARITIES
“There are no unnatural or supernatural phenomena, only a very large gap in our knowledge of what is natural.”
—Edgar Mitchell, Apollo 14 astronaut
Proteinuria
Proteinuria is another key cardiorenal risk factor prevalent in African Americans.
Knight et al,13 analyzing data from the Third National Health and Nutrition Examination Survey, found that people with high-normal blood pressure (systolic pressure 130–139 mm Hg or diastolic pressure 85–89 mm Hg) were twice as likely to have microalbuminuria (odds ratio 2.13, 95% confidence interval [CI] 1.51–3.01) compared with people with optimal blood pressure (systolic pressure < 120 mm Hg and diastolic pressure < 80 mm Hg). Compared with whites as the reference group, Mexican Americans had slightly but not statistically significantly higher odds of microalbuminuria (odds ratio 1.16; 95% CI 0.90–1.51), and African Americans had significantly higher odds (odds ratio 1.30; 95% CI 1.04–1.64).
The incidence of hypertension-related end-stage renal disease is nearly five times higher in African Americans than in whites, and the rate of hypertension-related end-stage renal disease is 15 times higher in African American men ages 24 to 44 than in whites of the same ages.3 The greater risk of proteinuria in African Americans at any given level of higher blood pressure is thought to contribute in part to these disparate rates.
The renin-angiotensin system
The renin-angiotensin system plays a role in modulating hypertension and mediating hypertension-related complications. Hypertensive African Americans are more likely than hypertensive whites to have low-renin, salt-sensitive hypertension. Therein lies a paradox.
Since the renin-angiotensin system promotes the progression of CKD, we would expect patients with low-renin hypertension to have a lower risk of hypertension-related endorgan damage than patients with high-renin hypertension. However, many African Americans (who as a group have high rates of sodium sensitivity and low plasma renin levels) experience more severe hypertension-related end-organ complications such as proteinuria and cardiorenal disease.14
A reason for this paradox may be that the circulating renin-angiotensin system is separate from the intrarenal one. Supporting this theory is the observation that up-regulation of the intrarenal renin-angiotensin system accompanies renal interstitial inflammation and oxidative stress in the kidneys and cardiovascular tissues of salt-sensitive rats fed a high-salt diet.15 In other experiments in salt-sensitive rats, renin-angiotensin system blockade reversed endothelial dysfunction, attenuated proteinuria, and reduced renal injury independent of blood pressure changes even though the animals had low circulating renin levels.16
These findings imply that drugs that block the renin-angiotensin system, ie, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, could still be a rational therapy for CKD patients with low-renin hypertension, particularly African Americans, in whom local up-regulation of the renin-angiotensin system in the kidney could exacerbate both diabetic and hypertensive CKD.17 Although these drugs may not lower blood pressure as much in low-renin hypertension as in high-renin hypertension, they may still afford the same cardiorenal protection.
Genetic factors
Variations in the MYH9 and APOL1 genes on chromosome 22 have recently been found in genome-wide admixture mapping studies and may explain as much as 70% of the differences in the rates of nondiabetic end-stage renal disease between white and black Americans.7,18,19 In addition, genetic variations may modulate differences in blood-pressure response to antihypertensive medications across racial and ethnic groups,20 complicating treatment recommendations and clinical outcomes in our increasingly diverse nation.
Comment. The pathophysiologic basis for the variability in the course of CKD is probably multifactorial and is still poorly understood. Nevertheless, we may be able to delay the progression of CKD and prevent its complications with specific therapeutic and life-style interventions.
Race and ethnicity are associated with sociocultural and biologic variations that influence the risk and progression of CKD. Understanding these factors for minority populations can help in targeting interventions to attenuate the disproportionately high rates of CKD progression and complications.
The pathophysiologic reason African Americans have a greater prevalence of end-stage renal disease and a more rapid progression of CKD is complex and probably involves the interplay of biological, behavioral, and environmental factors such as salt intake, stress levels, and exposure to heavy metals.21
TRIALS OF ANTIHYPERTENSIVE THERAPY IN AFRICAN AMERICANS WITH CKD
“If we knew what we were doing, it wouldn’t be called research.”
—Albert Einstein
Until recently, trials of antihypertensive therapy in patients with CKD did not include adequate numbers of African American participants, but the following clinical trials have added to our knowledge (Table 2).22–26
African American Study of Kidney Disease and Hypertension (AASK)
The African American Study of Kidney Disease and Hypertension (AASK),22,23 with 1,094 patients, was the largest prospective study of CKD to date designed to focus on African Americans.
AASK examined the effects of two levels of blood-pressure control:
- Standard, with a goal blood pressure of 135–140/85–90 mm Hg (mean arterial pressure 102–107 mm Hg)
- Intensive, with a goal of 120/80 mm Hg or less (mean arterial pressure ≤ 92 mm Hg).
In a two-by two factorial design, patients were also randomized to receive one of three antihypertensive drugs as initial therapy:
- The ACE inhibitor ramipril (Altace)
- The sustained-release beta-blocker metoprolol succinate (Toprol XL)
- The calcium channel blocker amlodipine (Norvasc).
To enter the study, patients had to be African American, have at least one diastolic pressure reading of 95 mm Hg or greater during the screening period, and have a measured GFR between 20 and 65 mL/min/1.83 m2. They could not have diabetes, substantial proteinuria (> 2.5 g/day), or other causes of CKD.22
AASK was distinct from many of the larger hypertension trials in which secondary analyses of outcomes in patients with CKD were performed in that it was implicit in the design that most, if not all, study participants had substantial GFR reduction and would need diuretic therapy.
At baseline, after blood pressure medications had been tapered to define eligibility and then reintroduced before randomization, 20.0% of the patients in the intensive blood pressure goal group had pressure lower than 140/90 mm Hg, and this increased to 78.9% by 14 months after randomization. In the standard goal group, the numbers were 21.5% at baseline but only 41.8% at 14 months.23 In spite of this difference, the rate of decline in GFR (the main clinical outcome measure) was the same in both groups.
However, the class of drug did make a difference. Secondary clinical outcomes, including the composite end point of development of end-stage renal disease, doubling of serum creatinine, or death, were less frequent in the ACE inhibitor group than in the beta-blocker and calcium channel blocker groups. As anticipated and consistent with real world practice, nearly 90% of all participants received concomitant diuretic therapy to achieve target blood pressure levels.
Comments. AASK showed that blood pressure can be controlled in African Americans who have CKD and that clinical cardiorenal outcomes can be improved by using an ACE inhibitor as initial therapy rather than a beta-blocker or calcium channel blocker, with diuretics and other agents added as needed.
AASK cohort phase
After completing the trial phase, patients were invited to enroll in a cohort phase in which the blood pressure target was less than 130/80 mm Hg. The combined follow-up period was 8.8 to 12.2 years.24
During the trial phase, the mean blood pressure was 130/78 mm Hg in the intensive group and 141/86 mm Hg in the standard group. During the cohort phase, the mean blood pressures were 131/78 mm Hg and 134/78 mm Hg, respectively, in these groups.
In both phases, there was no significant difference between groups in clinical outcomes (hazard ratio in the intensive-control group 0.91, P = .27). However, the groups differed when stratified by baseline level of proteinuria (P = .02 for the interaction), with a potential benefit of a blood pressure target lower than 130/80 mm Hg in patients with a protein-to-creatinine ratio of more than 0.22 (hazard ratio 0.73, P = .01).24
Comment. Given that many African Americans with hypertension and CKD have a protein-to-creatinine ratio of more than 0.22, these findings support a practical approach in clinical practice for a target blood pressure less than 130/80 mm Hg, using a first-line combination of a renin-angiotensin system inhibitor and a diuretic.
RENAAL study
The Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan (RENAAL) study25 included 1,513 patients, of whom 15% were African American and 18% were Hispanic; all had type 2 diabetes mellitus and nephropathy. They were randomized to receive the angiotensin II receptor antagonist losartan (Cozaar) or placebo in addition to other antihypertensive drugs.
At 3.4 years, the blood pressure was about 141/74 mm Hg in both groups. A post hoc analysis found lower rates of albuminuria and end-stage renal disease in the group treated with losartan,25 with no racial or ethnic differences in its renoprotective effect.
Comments. While these findings support the recommendation of inhibiting the renin-angiotensin system for improving clinical outcomes in diabetic nephropathy in racial and ethnic minorities, the AASK study also proved a second important point. These patients required intense blood pressure management for several years in a clinical trial environment, which may be difficult to do in many clinical practice models.
To be cost-effective in today’s health care environment, such care will likely be limited to larger group practices or health care plans with large comprehensive covered populations. Payers and providers need to be willing to invest in intense early care in such high-risk subgroups with the understanding that they could recognize downstream gains from long-term improved outcomes. However, even in these settings, the ability to provide effective care to high-risk subgroups without generating significant financial losses remains a concern.
ALLHAT
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)26 enrolled more than 33,000 hypertensive patients at high risk, of whom 32% were black, 16% were Hispanic, and 36% had diabetes. Their mean serum creatinine level was 1 mg/dL. Follow-up was for up to 8 years. At year 5, the mean blood pressure was 135/75 mm Hg.
In a secondary analysis, patients were stratified by GFR:
- Normal (> 90 mL/min/1.73 m2; n = 8,126)
- Mild reduction (60–89 mL/min/1.73 m2; n = 18,109)
- Moderate-severe reduction (< 60 mL/min/1.73 m2; n = 5,662).
In all three groups, amlodipine, lisinopril (Zestril), and chlorthalidone were equivalent as initial monotherapy in reducing the rate of the composite end point of end-stage renal disease or 50% or greater decrement in GFR.
Comments. The combined AASK, RENAAL, and ALLHAT findings are consistent with the practical recommendation of a diuretic, renin-angiotensin system inhibitor, or both, as initial therapy for blood pressure control in African American patients who have CKD, with a target blood pressure of less than 130/80 mm Hg.
A COMPREHENSIVE APPROACH TO CHRONIC KIDNEY DISEASE CARE
“It is much more important to know what sort of a patient has a disease, than what sort of disease a patient has.”
—William Osler
Many of the risk factors for cardiovascular disease in African Americans are behavioral and modifiable. These include too much salt and fat in the diet, too little physical activity, excessive alcohol intake, and smoking.
Education is key, to identify and communicate the risk attributable to health beliefs and behaviors, particularly in patients with known cardiovascular disease, and to encourage the patient to be proactive in risk-reduction strategies (Table 1). However, effective communication depends on compassion and concern by the health care provider to engender a sense of trust.27 Other health care professionals such as dietitians, pharmacists, and social workers as well as family members can reinforce messages and improve communication with the patient to optimize outcomes.
The International Society on Hypertension in Blacks recommends a blood pressure target of less than 130/80 mm Hg in blacks with elevated blood pressure and target-organ damage. The authors suggest monotherapy with a diuretic or calcium channel blocker if the blood pressure is 10 mm Hg or less above target levels. When blood pressure is more than 15/10 mm Hg above target, two-drug therapy is recommended, either with a calcium channel blocker plus a renin-angiotensin system blocker or, alternatively, in edematous or volume-overload states, with a thiazide diuretic plus a renin-angiotensin system blocker.28,29
The Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease of the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative recommend starting anti-hypertensive therapy with an ACE inhibitor or an angiotensin receptor blocker for most patients with CKD, regardless of ethnicity, recognizing that many will require combination therapy.30 Evaluation of the response to therapy should include not only checking that the blood pressure is at or below the recommended target of 130/80 mm Hg, but also assessing for complications and monitoring the change in the level of proteinuria, which is a powerful predictor of progression of hypertensive kidney disease in all patients at any given GFR.31
OUR RECOMMENDATIONS
African Americans with hypertension and kidney disease require an aggressive and comprehensive approach to slow the progression of kidney disease and its complications, often necessitating aggressive care of the primary cause and the use of two or more antihypertensive agents to control blood pressure, proteinuria, or both (Figure 1).32
We recommend that the initial evaluation of patients with hypertension include a screening for albuminuria and that the initial therapy for hypertension or proteinuria in all patients with CKD include renin-angiotensin system inhibition with a diuretic, because this combination appears most effective to achieve blood pressure control and to confer additional cardiorenal protection beyond that offered by blood-pressure control alone. Although some studies have reported that African Americans have lower blood-pressure response rates than whites to renin-angiotensin system inhibition, 18 it is nevertheless beneficial for clinical outcomes in this group, especially in the presence of proteinuria, a hallmark of hypertension-related CKD in African Americans. Thus, until more data are available, ethnicity should not be the primary criterion for selecting a given class of antihypertensive therapy, especially in patients with hypertensive nephropathy.
The overall treatment decision should be guided by individual response, coexisting risk factors, and potential cultural and socioeconomic considerations such as cost of medications and insurance coverage, which affect adherence to both pharmacologic and nonpharmacologic interventions.33
Future studies should strive for adequate representation of racial and ethnic minority populations in order to enhance the evidence base for CKD treatment as we move toward using personalized medicine approaches in an increasingly diverse society.34
Acknowledgment: Support for this paper was provided in part by NIH grants RR026138 and MD000182.
“Healthy citizens are the greatest asset any country can have.”
—Winston Churchill
Diabetes and high blood pressure take a toll on the kidneys, especially in African Americans. To prevent chronic kidney disease (CKD) and to slow or stop its progression, the same principles apply in African Americans as in other patients—ie, vigilance for the onset of proteinuria, aggressive control of blood pressure, drug treatment to block the renin-angiotensin system, and attention to lifestyle factors (Table 1). However, we need to try to do better in the care of African Americans.
The purpose of this article is to review recent evidence- and consensus-based recommendations and to present a practical approach for the evaluation and treatment of CKD in African Americans.
CKD DEFINED
In 2002, the National Kidney Foundation1 defined CKD as either:
- Kidney damage for 3 or more months, as defined by structural or functional abnormalities of the kidney, with or without a decreased glomerular filtration rate (GFR), manifested either by pathologic abnormalities or by markers of kidney damage, including abnormalities in the composition of the blood or urine (eg, proteinuria), or abnormalities in imaging tests; or
- A GFR less than 60 mL/min/1.73 m2 for 3 or more months, with or without kidney damage.
The definition divides CKD into five progressive stages according to the GFR:
- Stage 1 (kidney damage with normal or increased GFR): GFR ≥ 90 mL/min/1.73m2
- Stage 2 (kidney damage with mildly decreased GFR): GFR 60–89
- Stage 3 (moderately decreased GFR): GFR 30–59
- Stage 4 (severely decreased GFR): GFR 15–29
- Stage 5 (kidney failure): GFR < 15 or dialysis.
Because the definition includes markers of kidney damage such as albuminuria, it allows CKD to be detected in its earliest stages, when the estimated GFR might still be well within normal limits.
CKD APPEARS EARLIER, PROGRESSES FASTER IN AFRICAN AMERICANS
“Not everything that counts can be counted, and not everything that can be counted counts.”
—Albert Einstein
CKD with or without a sustained reduction in the estimated GFR affects about one in every nine American adults.2 Its course varies depending on the cause and also from patient to patient, even in those with the same cause of CKD.
In general, the prevalence of early CKD is comparable across racial and ethnic groups in the United States, but CKD progresses to end-stage renal disease far more rapidly in minority populations, with rates nearly four times higher in black Americans than in white Americans.3 Also, the onset of CKD is earlier in African Americans.
HYPERTENSION AND DIABETES AS REASONS FOR THE DISPARITIES
Part of the reason for these differences is that minority populations have higher rates of diabetes and hypertension, and these diseases tend to be more severe in these groups. Poverty, less access to health care, exposure to environmental toxins, and genetic variation may also contribute.4–7
Compared with whites, blacks have higher rates of diabetes and hypertension and earlier onset of these diseases, poorer control, and higher rates of complications such as CKD, stroke, and heart disease.8,9 The higher rate of hypertension and the lower rate of blood pressure control in African Americans with CKD may contribute to the more rapid progression of CKD to end-stage renal disease.
In the Chronic Renal Insufficiency Cohort, 10 a racially and ethnically diverse group of 3,612 adults with a broad spectrum of renal disease severity, 93% of African Americans had hypertension at baseline compared with 80% of whites. In addition, African Americans were 18% less likely to have their blood pressure controlled to 140/90 mm Hg (the rates of control were 76% vs 60%), and 28% were less likely to have it controlled to 130/80 mm Hg (56% vs 38%).10 These factors may partially explain the faster progression to end-stage renal disease in African Americans with CKD.
Despite the potential efficacy of strict control of serum glucose levels and blood pressure,11 the high rate of poor blood pressure control has contributed to the epidemic of diabetic nephropathy, especially among African Americans. Fortunately, hypertension control in the general population, while still not ideal, has improved from 27% in 1988–1994 to 50% in 2007–2008 and is now similar across racial and ethnic groups.12 This, hopefully, is a preface for improved hypertension-related outcomes for all Americans over the next decade.
OTHER REASONS FOR THE DISPARITIES
“There are no unnatural or supernatural phenomena, only a very large gap in our knowledge of what is natural.”
—Edgar Mitchell, Apollo 14 astronaut
Proteinuria
Proteinuria is another key cardiorenal risk factor prevalent in African Americans.
Knight et al,13 analyzing data from the Third National Health and Nutrition Examination Survey, found that people with high-normal blood pressure (systolic pressure 130–139 mm Hg or diastolic pressure 85–89 mm Hg) were twice as likely to have microalbuminuria (odds ratio 2.13, 95% confidence interval [CI] 1.51–3.01) compared with people with optimal blood pressure (systolic pressure < 120 mm Hg and diastolic pressure < 80 mm Hg). Compared with whites as the reference group, Mexican Americans had slightly but not statistically significantly higher odds of microalbuminuria (odds ratio 1.16; 95% CI 0.90–1.51), and African Americans had significantly higher odds (odds ratio 1.30; 95% CI 1.04–1.64).
The incidence of hypertension-related end-stage renal disease is nearly five times higher in African Americans than in whites, and the rate of hypertension-related end-stage renal disease is 15 times higher in African American men ages 24 to 44 than in whites of the same ages.3 The greater risk of proteinuria in African Americans at any given level of higher blood pressure is thought to contribute in part to these disparate rates.
The renin-angiotensin system
The renin-angiotensin system plays a role in modulating hypertension and mediating hypertension-related complications. Hypertensive African Americans are more likely than hypertensive whites to have low-renin, salt-sensitive hypertension. Therein lies a paradox.
Since the renin-angiotensin system promotes the progression of CKD, we would expect patients with low-renin hypertension to have a lower risk of hypertension-related endorgan damage than patients with high-renin hypertension. However, many African Americans (who as a group have high rates of sodium sensitivity and low plasma renin levels) experience more severe hypertension-related end-organ complications such as proteinuria and cardiorenal disease.14
A reason for this paradox may be that the circulating renin-angiotensin system is separate from the intrarenal one. Supporting this theory is the observation that up-regulation of the intrarenal renin-angiotensin system accompanies renal interstitial inflammation and oxidative stress in the kidneys and cardiovascular tissues of salt-sensitive rats fed a high-salt diet.15 In other experiments in salt-sensitive rats, renin-angiotensin system blockade reversed endothelial dysfunction, attenuated proteinuria, and reduced renal injury independent of blood pressure changes even though the animals had low circulating renin levels.16
These findings imply that drugs that block the renin-angiotensin system, ie, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, could still be a rational therapy for CKD patients with low-renin hypertension, particularly African Americans, in whom local up-regulation of the renin-angiotensin system in the kidney could exacerbate both diabetic and hypertensive CKD.17 Although these drugs may not lower blood pressure as much in low-renin hypertension as in high-renin hypertension, they may still afford the same cardiorenal protection.
Genetic factors
Variations in the MYH9 and APOL1 genes on chromosome 22 have recently been found in genome-wide admixture mapping studies and may explain as much as 70% of the differences in the rates of nondiabetic end-stage renal disease between white and black Americans.7,18,19 In addition, genetic variations may modulate differences in blood-pressure response to antihypertensive medications across racial and ethnic groups,20 complicating treatment recommendations and clinical outcomes in our increasingly diverse nation.
Comment. The pathophysiologic basis for the variability in the course of CKD is probably multifactorial and is still poorly understood. Nevertheless, we may be able to delay the progression of CKD and prevent its complications with specific therapeutic and life-style interventions.
Race and ethnicity are associated with sociocultural and biologic variations that influence the risk and progression of CKD. Understanding these factors for minority populations can help in targeting interventions to attenuate the disproportionately high rates of CKD progression and complications.
The pathophysiologic reason African Americans have a greater prevalence of end-stage renal disease and a more rapid progression of CKD is complex and probably involves the interplay of biological, behavioral, and environmental factors such as salt intake, stress levels, and exposure to heavy metals.21
TRIALS OF ANTIHYPERTENSIVE THERAPY IN AFRICAN AMERICANS WITH CKD
“If we knew what we were doing, it wouldn’t be called research.”
—Albert Einstein
Until recently, trials of antihypertensive therapy in patients with CKD did not include adequate numbers of African American participants, but the following clinical trials have added to our knowledge (Table 2).22–26
African American Study of Kidney Disease and Hypertension (AASK)
The African American Study of Kidney Disease and Hypertension (AASK),22,23 with 1,094 patients, was the largest prospective study of CKD to date designed to focus on African Americans.
AASK examined the effects of two levels of blood-pressure control:
- Standard, with a goal blood pressure of 135–140/85–90 mm Hg (mean arterial pressure 102–107 mm Hg)
- Intensive, with a goal of 120/80 mm Hg or less (mean arterial pressure ≤ 92 mm Hg).
In a two-by two factorial design, patients were also randomized to receive one of three antihypertensive drugs as initial therapy:
- The ACE inhibitor ramipril (Altace)
- The sustained-release beta-blocker metoprolol succinate (Toprol XL)
- The calcium channel blocker amlodipine (Norvasc).
To enter the study, patients had to be African American, have at least one diastolic pressure reading of 95 mm Hg or greater during the screening period, and have a measured GFR between 20 and 65 mL/min/1.83 m2. They could not have diabetes, substantial proteinuria (> 2.5 g/day), or other causes of CKD.22
AASK was distinct from many of the larger hypertension trials in which secondary analyses of outcomes in patients with CKD were performed in that it was implicit in the design that most, if not all, study participants had substantial GFR reduction and would need diuretic therapy.
At baseline, after blood pressure medications had been tapered to define eligibility and then reintroduced before randomization, 20.0% of the patients in the intensive blood pressure goal group had pressure lower than 140/90 mm Hg, and this increased to 78.9% by 14 months after randomization. In the standard goal group, the numbers were 21.5% at baseline but only 41.8% at 14 months.23 In spite of this difference, the rate of decline in GFR (the main clinical outcome measure) was the same in both groups.
However, the class of drug did make a difference. Secondary clinical outcomes, including the composite end point of development of end-stage renal disease, doubling of serum creatinine, or death, were less frequent in the ACE inhibitor group than in the beta-blocker and calcium channel blocker groups. As anticipated and consistent with real world practice, nearly 90% of all participants received concomitant diuretic therapy to achieve target blood pressure levels.
Comments. AASK showed that blood pressure can be controlled in African Americans who have CKD and that clinical cardiorenal outcomes can be improved by using an ACE inhibitor as initial therapy rather than a beta-blocker or calcium channel blocker, with diuretics and other agents added as needed.
AASK cohort phase
After completing the trial phase, patients were invited to enroll in a cohort phase in which the blood pressure target was less than 130/80 mm Hg. The combined follow-up period was 8.8 to 12.2 years.24
During the trial phase, the mean blood pressure was 130/78 mm Hg in the intensive group and 141/86 mm Hg in the standard group. During the cohort phase, the mean blood pressures were 131/78 mm Hg and 134/78 mm Hg, respectively, in these groups.
In both phases, there was no significant difference between groups in clinical outcomes (hazard ratio in the intensive-control group 0.91, P = .27). However, the groups differed when stratified by baseline level of proteinuria (P = .02 for the interaction), with a potential benefit of a blood pressure target lower than 130/80 mm Hg in patients with a protein-to-creatinine ratio of more than 0.22 (hazard ratio 0.73, P = .01).24
Comment. Given that many African Americans with hypertension and CKD have a protein-to-creatinine ratio of more than 0.22, these findings support a practical approach in clinical practice for a target blood pressure less than 130/80 mm Hg, using a first-line combination of a renin-angiotensin system inhibitor and a diuretic.
RENAAL study
The Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan (RENAAL) study25 included 1,513 patients, of whom 15% were African American and 18% were Hispanic; all had type 2 diabetes mellitus and nephropathy. They were randomized to receive the angiotensin II receptor antagonist losartan (Cozaar) or placebo in addition to other antihypertensive drugs.
At 3.4 years, the blood pressure was about 141/74 mm Hg in both groups. A post hoc analysis found lower rates of albuminuria and end-stage renal disease in the group treated with losartan,25 with no racial or ethnic differences in its renoprotective effect.
Comments. While these findings support the recommendation of inhibiting the renin-angiotensin system for improving clinical outcomes in diabetic nephropathy in racial and ethnic minorities, the AASK study also proved a second important point. These patients required intense blood pressure management for several years in a clinical trial environment, which may be difficult to do in many clinical practice models.
To be cost-effective in today’s health care environment, such care will likely be limited to larger group practices or health care plans with large comprehensive covered populations. Payers and providers need to be willing to invest in intense early care in such high-risk subgroups with the understanding that they could recognize downstream gains from long-term improved outcomes. However, even in these settings, the ability to provide effective care to high-risk subgroups without generating significant financial losses remains a concern.
ALLHAT
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)26 enrolled more than 33,000 hypertensive patients at high risk, of whom 32% were black, 16% were Hispanic, and 36% had diabetes. Their mean serum creatinine level was 1 mg/dL. Follow-up was for up to 8 years. At year 5, the mean blood pressure was 135/75 mm Hg.
In a secondary analysis, patients were stratified by GFR:
- Normal (> 90 mL/min/1.73 m2; n = 8,126)
- Mild reduction (60–89 mL/min/1.73 m2; n = 18,109)
- Moderate-severe reduction (< 60 mL/min/1.73 m2; n = 5,662).
In all three groups, amlodipine, lisinopril (Zestril), and chlorthalidone were equivalent as initial monotherapy in reducing the rate of the composite end point of end-stage renal disease or 50% or greater decrement in GFR.
Comments. The combined AASK, RENAAL, and ALLHAT findings are consistent with the practical recommendation of a diuretic, renin-angiotensin system inhibitor, or both, as initial therapy for blood pressure control in African American patients who have CKD, with a target blood pressure of less than 130/80 mm Hg.
A COMPREHENSIVE APPROACH TO CHRONIC KIDNEY DISEASE CARE
“It is much more important to know what sort of a patient has a disease, than what sort of disease a patient has.”
—William Osler
Many of the risk factors for cardiovascular disease in African Americans are behavioral and modifiable. These include too much salt and fat in the diet, too little physical activity, excessive alcohol intake, and smoking.
Education is key, to identify and communicate the risk attributable to health beliefs and behaviors, particularly in patients with known cardiovascular disease, and to encourage the patient to be proactive in risk-reduction strategies (Table 1). However, effective communication depends on compassion and concern by the health care provider to engender a sense of trust.27 Other health care professionals such as dietitians, pharmacists, and social workers as well as family members can reinforce messages and improve communication with the patient to optimize outcomes.
The International Society on Hypertension in Blacks recommends a blood pressure target of less than 130/80 mm Hg in blacks with elevated blood pressure and target-organ damage. The authors suggest monotherapy with a diuretic or calcium channel blocker if the blood pressure is 10 mm Hg or less above target levels. When blood pressure is more than 15/10 mm Hg above target, two-drug therapy is recommended, either with a calcium channel blocker plus a renin-angiotensin system blocker or, alternatively, in edematous or volume-overload states, with a thiazide diuretic plus a renin-angiotensin system blocker.28,29
The Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease of the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative recommend starting anti-hypertensive therapy with an ACE inhibitor or an angiotensin receptor blocker for most patients with CKD, regardless of ethnicity, recognizing that many will require combination therapy.30 Evaluation of the response to therapy should include not only checking that the blood pressure is at or below the recommended target of 130/80 mm Hg, but also assessing for complications and monitoring the change in the level of proteinuria, which is a powerful predictor of progression of hypertensive kidney disease in all patients at any given GFR.31
OUR RECOMMENDATIONS
African Americans with hypertension and kidney disease require an aggressive and comprehensive approach to slow the progression of kidney disease and its complications, often necessitating aggressive care of the primary cause and the use of two or more antihypertensive agents to control blood pressure, proteinuria, or both (Figure 1).32
We recommend that the initial evaluation of patients with hypertension include a screening for albuminuria and that the initial therapy for hypertension or proteinuria in all patients with CKD include renin-angiotensin system inhibition with a diuretic, because this combination appears most effective to achieve blood pressure control and to confer additional cardiorenal protection beyond that offered by blood-pressure control alone. Although some studies have reported that African Americans have lower blood-pressure response rates than whites to renin-angiotensin system inhibition, 18 it is nevertheless beneficial for clinical outcomes in this group, especially in the presence of proteinuria, a hallmark of hypertension-related CKD in African Americans. Thus, until more data are available, ethnicity should not be the primary criterion for selecting a given class of antihypertensive therapy, especially in patients with hypertensive nephropathy.
The overall treatment decision should be guided by individual response, coexisting risk factors, and potential cultural and socioeconomic considerations such as cost of medications and insurance coverage, which affect adherence to both pharmacologic and nonpharmacologic interventions.33
Future studies should strive for adequate representation of racial and ethnic minority populations in order to enhance the evidence base for CKD treatment as we move toward using personalized medicine approaches in an increasingly diverse society.34
Acknowledgment: Support for this paper was provided in part by NIH grants RR026138 and MD000182.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010.
- Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 2008; 19:1261–1270.
- Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int 2005; 68:914–924.
- Powe NR. To have and have not: health and health care disparities in chronic kidney disease. Kidney Int 2003; 64:763–772.
- Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329:841–845.
- Rosamond W, Flegal K, Furie K, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117:e25–e146.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Muntner P, Anderson A, Charleston J, et al; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2010; 55:441–451.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Knight EL, Kramer HM, Curhan GC. High-normal blood pressure and microalbuminuria. Am J Kidney Dis 2003; 41:588–595.
- Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation 1979; 59:643–650.
- Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol 2008; 28:158–167.
- Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation 1997; 96:2407–2413.
- Norris KC, Tareen N, Martins D, Vaziri ND. Implications of ethnicity for the treatment of hypertensive kidney disease, with an emphasis on African Americans. Nat Clin Pract Nephrol 2008; 4:538–549.
- Kao WH, Klag MJ, Meoni LA, et al; Family Investigation of Nephropathy and Diabetes Research Group. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 2008; 40:1185–1192.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993; 328:914–921. Erratum in N Engl J Med 1994; 330:1689.
- Norris KC, Francis CK. Gender and ethnic differences and considerations in cardiovascular risk assessment and prevention in African Americans. In:Wong N, Gardin JM, Black HR, editors. Practical Strategies in Preventing Heart Disease. New York, NY: McGraw-Hill; 2004:415–440.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431. Erratum in JAMA 2006; 295:2726.
- Wright JT, Agodoa L, Contreras G, et al; African American Study of Kidney Disease and Hypertension Study Group. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med 2002; 162:1636–1643.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- de Zeeuw D, Ramjit D, Zhang Z, et al. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int 2006; 69:1675–1682.
- Rahman M, Pressel S, Davis BR, et al. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 2005; 165:936–946.
- Barrier PA, Li JT, Jensen NM. Two words to improve physician-patient communication: what else? Mayo Clin Proc 2003; 78:211–214.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Wright JT, Agodoa LY, Appel L, et al. New recommendations for treating hypertension in black patients: evidence and/or consensus? Hypertension 2010; 56:801–803.
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease: executive summary. Am J Kid Dis 2004; 43(suppl 1):S16–S33.
- Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med 2005; 165:947–953.
- Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000; 36:646–661.
- Martins D, Norris K. Hypertension treatment in African Americans: physiology is less important than sociology. Cleve Clin J Med 2004; 71:735–743.
- Cooper RS, Psaty BM. Should ethnicity serve as the basis for clinical trial design? Diversity and inclusiveness should remain the guiding principles for clinical trials. Circulation 2005; 112:3660–3665.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010.
- Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 2008; 19:1261–1270.
- Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int 2005; 68:914–924.
- Powe NR. To have and have not: health and health care disparities in chronic kidney disease. Kidney Int 2003; 64:763–772.
- Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329:841–845.
- Rosamond W, Flegal K, Furie K, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117:e25–e146.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Muntner P, Anderson A, Charleston J, et al; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2010; 55:441–451.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Knight EL, Kramer HM, Curhan GC. High-normal blood pressure and microalbuminuria. Am J Kidney Dis 2003; 41:588–595.
- Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation 1979; 59:643–650.
- Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol 2008; 28:158–167.
- Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation 1997; 96:2407–2413.
- Norris KC, Tareen N, Martins D, Vaziri ND. Implications of ethnicity for the treatment of hypertensive kidney disease, with an emphasis on African Americans. Nat Clin Pract Nephrol 2008; 4:538–549.
- Kao WH, Klag MJ, Meoni LA, et al; Family Investigation of Nephropathy and Diabetes Research Group. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 2008; 40:1185–1192.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993; 328:914–921. Erratum in N Engl J Med 1994; 330:1689.
- Norris KC, Francis CK. Gender and ethnic differences and considerations in cardiovascular risk assessment and prevention in African Americans. In:Wong N, Gardin JM, Black HR, editors. Practical Strategies in Preventing Heart Disease. New York, NY: McGraw-Hill; 2004:415–440.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431. Erratum in JAMA 2006; 295:2726.
- Wright JT, Agodoa L, Contreras G, et al; African American Study of Kidney Disease and Hypertension Study Group. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med 2002; 162:1636–1643.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- de Zeeuw D, Ramjit D, Zhang Z, et al. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int 2006; 69:1675–1682.
- Rahman M, Pressel S, Davis BR, et al. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 2005; 165:936–946.
- Barrier PA, Li JT, Jensen NM. Two words to improve physician-patient communication: what else? Mayo Clin Proc 2003; 78:211–214.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Wright JT, Agodoa LY, Appel L, et al. New recommendations for treating hypertension in black patients: evidence and/or consensus? Hypertension 2010; 56:801–803.
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease: executive summary. Am J Kid Dis 2004; 43(suppl 1):S16–S33.
- Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med 2005; 165:947–953.
- Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000; 36:646–661.
- Martins D, Norris K. Hypertension treatment in African Americans: physiology is less important than sociology. Cleve Clin J Med 2004; 71:735–743.
- Cooper RS, Psaty BM. Should ethnicity serve as the basis for clinical trial design? Diversity and inclusiveness should remain the guiding principles for clinical trials. Circulation 2005; 112:3660–3665.
KEY POINTS
- To provide optimal care for African Americans, we need to be sensitive to factors that may pose barriers to care, such as poverty, unemployment, lack of insurance, low education level, lack of family support, inaccurate health beliefs, and unhealthy behaviors.
- If we detect CKD earlier, we can better implement strategies to prevent its progression, refer the patient to specialists, and possibly arrange for preemptive kidney transplantation if needed.
- Progression of CKD can be prevented or slowed by controlling blood pressure, proteinuria, and blood glucose. However, CKD progresses in a subset of patients despite evidence-based therapy to target goals.
- African Americans with hypertensive CKD and proteinuria should receive a diuretic, a renin-angiotensin system inhibitor, or both as initial therapy, with a target blood pressure of less than 130/80 mm Hg.
Disparities in prostate cancer in African American men: What primary care physicians can do
Prostate cancer is the most common cancer affecting American men. In 2010, an estimated 217,730 men were diagnosed with it and 32,050 died of it.1 African American men are disproportionately affected, with a prostate cancer incidence two-thirds higher than whites and a mortality rate twice as high.1 Owing to such disparities, the life expectancy of African Americans is several years shorter than that of non-Hispanic whites.2
For the primary care provider, who is often the first access point for health care in the United States, it is important to understand what mechanisms may underlie these differences and what can be done to narrow the gap.3
WHAT IS THE CAUSE OF THESE DIFFERENCES?
Many studies have looked into the causes of the higher incidence of prostate cancer in African American men and their higher mortality rate from it. The disparity may be due to a variety of factors, some socioeconomic and some biologic.
Poorer access to care, or lower-quality care?
A study of US servicemen who had equal access to care showed that African American men had a higher rate of prostate cancer regardless of access to care and socioeconomic status.4
However, the 2002 Institute of Medicine report, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, found evidence that racial and ethnic minorities tend to receive lower-quality health care than whites, “even when access-related factors, such as patients’ insurance status and income, are controlled.”5
Genetic predisposition?
Some have proposed that the disparity may be a function of genetic predisposition.
Evidence of a genetic component to the high incidence and mortality rate in African American men comes from epidemiologic studies of men with similar genetic backgrounds. For example, men in Nigeria and Ghana also have a high incidence of prostate cancer, as do men of African descent in the Caribbean islands and in the United Kingdom.6
Chromosome 8q24 variants have been shown in several studies to be associated with prostate cancer risk and are more common in African American men.7–10 Some studies have also shown a higher rate of variations in cell apoptosis genes such as BCL211 and tumor-suppression genes such as EphB2 in African American men.12
These findings suggest that genetic differences may contribute to the higher prostate cancer incidence and mortality rate seen in African American men.
More-aggressive cancer, or later detection?
Not only do African American men tend to have a higher incidence of prostate cancer, they also tend to have more-aggressive disease (ie, a higher pathologic grade) at the time of diagnosis, which may contribute to the disparity in mortality rates.13–19
Initially, there was some controversy as to whether this observation is a result of genetic and biologic factors that may predispose African American men to more-aggressive disease, or if it is due to inadequate screening and delayed presentation. However, a body of evidence supports the contention that prostate cancer is more aggressive in African American men.
For example, a study of autopsy data from men who died of prostate cancer at ages 20 to 49 showed that the age of onset of prostate cancer was similar between African American and white men.20 The Surveillance Epidemiology and End Results (SEER) database showed that African American men had a higher incidence of metastatic disease across all age groups.20 A similar study conducted 10 years later confirmed that rates of subclinical prostate cancer in African American and white men do not differ by race at the early ages, but that advanced or metastatic disease occurred nearly four times as frequently in African American men.21
Another study examined prostate biopsies from African American men and found that their tumors expressed higher levels of biomarkers, suggesting they had more-aggressive disease.22
SCREENING FOR PROSTATE CANCER
Serum prostate-specific antigen (PSA) testing has become the method of choice for prostate cancer screening. However, PSA screening in asymptomatic men is under debate, because it can lead to overdetection and subsequent overtreatment of indolent disease.23
Several recent studies showed differing results from prostate cancer screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial found that the mortality rate was no lower with combined PSA screening and digital rectal examination during a median follow-up of 11 years than in a control group that had a lower rate of screening.24 However, further analysis of these data, with stratifying by comorbidities, showed that PSA screening in young and healthy men reduces the risk of death from prostate cancer, with minimal overtreatment.25
The European Randomized Study of Screening for Prostate Cancer found a statistically significant 20% reduction in deaths from prostate cancer with PSA screening, but that it was necessary to treat 48 men in order to save one life.26
Another study, published in 2010, showed that regular PSA screening reduced the rate of prostate cancer mortality by half over 14 years.27
African American men generally present with disease that is more advanced than in white men.28 This historically has been attributed to the fact that African Americans have been less likely to be screened for prostate cancer, though recent data indicate the gap is lessening.29–31 A cross-sectional study from the Texas Medical Center showed that 54.4% of African American men had received PSA screening, compared with 63.2% of white men.32
Another study showed that African Americans were more likely to have had a longer interval between PSA screenings before diagnosis, and that a longer PSA screening interval was associated with greater odds of having advanced disease at diagnosis.33 However, when the researchers controlled for the PSA screening interval, they found that African Americans had the same odds of being diagnosed with advanced prostate cancer as white patients did. They concluded that more frequent or systematic PSA screening may reduce the racial differences in cancer stage at diagnosis and in deaths.
Reasons for the disparities in screening
Many reasons have been proposed to explain why African Americans receive less screening, including poor communication between physicians and minority patients due to lack of cultural competency among physicians, lack of health insurance (and poor access to quality care as a result), and deficiency of knowledge about screening. Though awareness is rising, many African Americans are unaware of early detection methods for prostate cancer (eg, PSA testing), and other barriers such as cost and transportation exist that may prevent African American men from being screened.34,35
As gatekeepers, primary care physicians are in a position to address these shortcomings in patient education and to enhance the physician-patient relationship.36
Black men have higher PSA levels, with or without cancer
Physicians must also be aware of racial differences in PSA levels and realize that the predictive value of PSA in the diagnosis of prostate cancer may differ between African Americans and whites.
Black men, with or without prostate cancer, have been found to have higher PSA levels. Kyle and colleagues37 found that African American men without prostate cancer had significantly higher mean PSA levels than white men across all age groups. Furthermore, Vijayakumar et al38 found that African Americans with newly diagnosed localized prostate cancer had higher serum PSA levels than whites at diagnosis.
Although PSA cutoff levels have not been officially modified according to race, primary care physicians should have a lower threshold for referring African American men who have a suspiciously high PSA level for further urologic evaluation. Close partnership between the internist, family practitioner, and urologist will aid in the optimal use of PSA testing for the early detection of prostate cancer.
When to start PSA screening? How often to screen?
The age at which African American men should begin to have their PSA levels checked (with or without a digital rectal examination) continues to debated. However, the American Cancer Society39 recommends that African American men who have a father or brother who had prostate cancer before age 65 should begin having discussions with their physician on this topic and, with their informed consent, screening at age 45.
The frequency of PSA screening depends on the individual’s PSA level. The National Comprehensive Cancer Network40 recommends that men at high risk be offered a baseline PSA measurement and digital rectal examination at age 40 and, if the PSA level is higher than 1 ng/mL, that they be offered annual follow-ups. If the PSA level is less than 1 ng/mL, they recommend screening again at age 45. Risk factors for prostate cancer include family history as well as African American race.41
How should PSA levels be interpreted?
Interpreting PSA results is important in detecting prostate cancer at early stages.
At first, we believed the normal range of PSA for all men was 4.0 ng/mL or less. However, the American Urological Association now recognizes that the normal PSA range, in addition to varying along racial lines, also is age-dependent.42 The Cleveland Clinic Minority Men's Health Center's suggested normal ranges of PSA in African American men are:
- Age 40–49: ≤ 2.5 ng/mL
- Age 50–59: ≤ 3.0 ng/mL
- Age 60–69: ≤ 3.5 ng/mL
- Age 70–79: ≤ 4.5 ng/mL
- Age > 80: ≤ 5.0 ng/mL.
Remember that an elevated PSA does not necessarily signify prostate cancer, and that these are reference ranges only and may vary in individual men.
SURVIVAL AFTER DIAGNOSIS
African American men with prostate cancer have significantly higher mortality rates than white men. The possible causes of worse outcomes are many, and there have been many studies that attempted to address this disparity. The question of a more biologically aggressive cancer was previously discussed, but additional factors such as socioeconomic factors, comorbidities, and treatment received have also been studied, and data are mixed.43–45
In a large SEER database review, once confounding variables of socioeconomic status, cancer stage, and treatment received were eliminated, African Americans had similar stage-for-stage survival from prostate cancer.46 Another study found, in 2,046 men, that differences in socioeconomic status explained the difference in mortality rates between white and black patients.47
However, other studies that adjusted for socioeconomic status as well as patient and tumor characteristics found that African American and Hispanic men were more likely to die of prostate cancer than white men.48
Do African American men receive less-aggressive care?
Studies have also determined that there may be differences in treatments offered to patients, which in turn negatively affect survival.28,49–53 Potentially curative local therapies (including radical surgery or radiation) may be recommended less often to black men because of major comorbidities or socioeconomic considerations.49–52
Additionally, potential metastatic disease may be identified in a less timely and accurate manner, as African American men are less likely to undergo pelvic lymph node dissection. This was associated with worse survival in men with poorly differentiated prostate cancer.53
However, returning to the possibility that prostate cancer is biologically more aggressive in African American men, some studies have shown that even after adjusting for treatment, African Americans continue to have worse survival rates.54,55 One study in men with stage T1 to T3 prostate cancer who chose brachytherapy for treatment reported that after adjusting for PSA, clinical stage, socioeconomic status, and comorbidities, African American and Hispanic race were associated with higher all-cause mortality rates.55
Equal care, equal outcomes?
In total, these results suggest that factors unrelated to tumor biology may be additional reasons for the poorer survival rates in African American men with prostate cancer. More favorable survival outcomes for African Americans with localized disease may be achieved with uniform assignment of treatment.
Fowler and Terrell56 reviewed the outcomes of 148 black and 209 white men with localized prostate cancer treated with surgery or radiation therapy over an 11-year period at a Veterans Administration hospital. Not surprisingly, the black men presented more often with advanced disease. However, survival outcomes were equivalent between whites and blacks when treatment was assigned in a uniform manner without regard to race. After a median follow-up of 96 months, there were no significant differences in all-cause, cause-specific, metastasis-free, clinical disease-free, or PSA recurrence-free survival rates in 109 black and 167 white men with low-stage cancer treated with surgery or radiation therapy or in 39 black and 42 white men with high-stage disease treated with radiotherapy.56
Similarly, Tewari et al57 studied a cohort of 402 African American and 642 white men, all of whom underwent radical prostatectomy for clinically localized prostate cancer. They were followed for PSA recurrence to determine if race-specific differences in PSA doubling time or histopathologic variables might account for the higher mortality rate in black men. While there were race-specific differences in baseline serum PSA and incidence of high-grade prostatic intraepithelial neoplasia, race was not an independent risk factor for biochemical recurrence. Instead, other variables such as the Gleason pathology score, bilateral cancers, and margin positivity were independently associated with biochemical recurrence.
Furthermore, researchers at Louisiana State University58 retrospectively analyzed data from 205 men of different races with early-stage prostate cancer. The African American men had a higher serum PSA level, suggesting more advanced disease or greater tumor burden at presentation, but no statistically significant differences were found among the pretreatment biopsy variables, including prostate volume (measured by ultrasonography), Gleason score, millimeters of cancer within the biopsy specimen, and percentage of cancer within the biopsy specimen. After treatment, there were no significant differences in survival outcomes along racial lines, leading the authors to conclude that early detection and treatment of prostate cancer in African Americans would be the best approach to lowering mortality rates.
Taken together, these data suggest that if localized prostate cancer is treated adequately and appropriately, African American patients may have improved survival rates.
DIETARY AND LIFESTYLE FACTORS
The incidence of prostate cancer is increasing in other countries where Western diets and lifestyles have been adopted,59,60 suggesting that nutritional factors may also contribute partly to prostate carcinogenesis. Culture- and race-specific differences in diet may play an important role in prostate cancer risk in certain racial minorities. Many aspects of diet and nutrition have been studied for their impact on prostate cancer.
Dietary risk factors
Too much red meat and processed meat? Although some have suggested that diets high in red and processed meats may lead to a higher risk of prostate cancer, a meta-analysis showed no association.61,62
Too much calcium? The European Prospective Investigation Into Cancer and Nutrition study found that high dietary intake of dairy protein and calcium from dairy products was associated with a higher risk of prostate cancer.63 A cohort study in the United States had similar findings with regard to calcium.64 However, the higher risk of prostate cancer was associated with consumption of 2,000 mg or more of calcium per day, which was consumed by only 2% of the study’s cohort and, as the study’s authors reported, fewer than 1% of US men. As such, only a small population of American men seem to be exposing themselves to a higher risk of prostate cancer by high calcium consumption.
High fat intake? Certain fatty acids have been implicated in general tumor genesis, and that risk has been extrapolated to prostate cancer.65 For example, high fat intake and obesity are associated with increased levels of insulin-like growth factor 1, which in turn has been shown to correlate with a significantly elevated risk of prostate cancer.63,65
Obesity has been shown to increase the risk of more-aggressive prostate cancer, but not of less-aggressive tumors.66 Moreover, men who lost weight had a lower risk of prostate cancer than those who maintained their weight over 10 years.66 Obesity may be particularly risky for African American men, in whom it was found to be associated with shorter biochemical relapse-free survival, whereas it was not an independent risk factor in white men.67
Preventive dietary agents have been elusive
Unfortunately, despite attempts to identify preventive dietary agents, none has yet been confirmed.
No benefit from selenium or vitamin E. The Selenium and Vitamin E Cancer Prevention Trial was discontinued, as there was no evidence that either agent prevented prostate cancer in relatively healthy men.68
Vitamin D? It has been suggested that lower levels of vitamin D could contribute to the higher rates of prostate cancer in African Americans, as vitamin D deficiency is more common in African Americans.69 However, several meta-analyses have shown no association between vitamin D and prostate cancer.70–72
Soy? Attempts at correlating the relatively low incidence of prostate cancer in Asians have revealed that high soy intake may be protective. Asians consume more soy than Americans do (100 vs 3 mg/day), and soy isoflavones such as genistein, glycitein, and daidzein lower the incidence of prostate cancer in laboratory mice.73
Other lifestyle factors
Other lifestyle factors have also been analyzed to see if they contribute to prostate cancer.
Pollution. Some studies have suggested that the etiology of prostate cancer may lie in environmental exposures to pesticides,74 metal industrial facilities,75 and urban living.76
Smoking. Watters et al77 found that current and former cigarette smokers were actually at a lower risk of being diagnosed with non-advanced prostate cancer, but current smokers were at higher risk of dying from prostate cancer.
Physical activity. A prospective study of lifetime physical activity of more than 45,000 men found that men who were not sedentary during work and who walked or bicycled more than 30 minutes per day during adult life had an approximately 20% lower incidence of prostate cancer.78
In sum, primary care providers who are generally promoting healthy lifestyles can point to a reduction in risk for prostate cancer as yet another benefit to a low-fat diet, a healthy body mass index, and daily exercise.
HOW PRIMARY CARE PHYSICIANS CAN HELP CLOSE THE GAP
Primary care physicians serve as the first point of health access for many in the United States today.
The diagnosis of prostate cancer is made more frequently in African American men than in other American men, often at a higher pathological grade, and with a worse mortality rate. Primary care physicians can help improve these statistics. Interventions targeting overall health, such as promotion of a healthy diet, could be established at primary care visits and could also reduce the incidence of prostate cancer in African American men. Patient education regarding prostate cancer screening, the impact of family history, and the rate of PSA screening could be improved.
Primary care physicians serve a vital role in health education and prostate cancer screening, and therefore they begin the process in potentially reducing the impact of prostate cancer in African American men. The racial disparity seen in prostate cancer may begin to be minimized with primary care physicians and specialists working together to ensure that all men receive appropriate treatment.
- Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. Accessed April 2, 2011.
- Arias E. United States life tables, 2007. National vital statistics reports; vol 59 no 9. Hyattsville, MD: National Center for Health Statistics. 2011.
- Klein JB, Nguyen CT, Saffore L, Modlin C, Modlin CS. Racial disparities in urologic health care. J Natl Med Assoc 2010; 102:108–117.
- Wells TS, Bukowinski AT, Smith TC, et al. Racial differences in prostate cancer risk remain among US servicemen with equal access to care. Prostate 2010; 70:727–734.
- Smedley BD, Stith AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine. National Academy Press; 2002.
- Odedina FT, Akinremi TO, Chinegwundoh F, et al. Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer 2009; 4(suppl 1):S2.
- Okobia MN, Zmuda JM, Ferrell RE, Patrick AL, Bunker CH. Chromosome 8q24 variants are associated with prostate cancer risk in a high risk population of African ancestry. Prostate 2011; 71:1054–1063.
- Haiman CA, Chen GK, Blot WJ, et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet 2011; 7:e1001387.
- Freedman ML, Haiman CA, Patterson N, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A 2006; 103:14068–14073.
- Chang BL, Isaacs SD, Wiley KE, et al. Genome-wide screen for prostate cancer susceptibility genes in men with clinically significant disease. Prostate 2005; 64:356–361.
- Hatcher D, Daniels G, Osman I, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Transl Res 2009; 1:235–248.
- Robbins CM, Hooker S, Kittles RA, Carpten JD. EphB2 SNPs and sporadic prostate cancer risk in African American men. PLoS One 2011; 6:e19494.
- American Cancer Society. Cancer Facts & Figures for African Americans 2009–2010. http://www.cancer.org/acs/groups/content/@nho/documents/document/cffaa20092010pdf.pdf. Accessed April 2, 2012.
- Ayanian JZ, Udvarhelyi IS, Gatsonis CA, Pashos CL, Epstein AM. Racial differences in the use of revascularization procedures after coronary angiography. JAMA 1993; 269:2642–2646.
- Fine MJ, Ibrahim SA, Thomas SB. The role of race and genetics in health disparities research. Am J Public Health 2005; 95:2125–2128.
- Horner RD, Oddone EZ, Matchar DB. Theories explaining racial differences in the utilization of diagnostic and therapeutic procedures for cerebrovascular disease. Milbank Q 1995; 73:443–462.
- Juckett G. Cross-cultural medicine. Am Fam Physician 2005; 72:2267–2274.
- Ndubuisi SC, Kofie VY, Andoh JY, Schwartz EM. Black-white differences in the stage at presentation of prostate cancer in the District of Columbia. Urology 1995; 46:71–77.
- Misra-Hebert AD. Physician cultural competence: cross-cultural communication improves care. Cleve Clin J Med 2003; 70:289,293,296–298.
- Powell I, Sakr W, Weiss L, et al. Prostate cancer is biologically more aggressive among African Americans than Caucasian men under age 70: hypothesis supported by autopsy and SEER data. Program and abstracts from the American Urological Association 95th Annual Meeting; April 29–May 4, 2000: Atlanta, GA.
- Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol 2010; 183:1792–1796.
- Kim HS, Moreira DM, Jayachandran J, et al. Prostate biopsies from black men express higher levels of aggressive disease biomarkers than prostate biopsies from white men. Prostate Cancer Prostatic Dis 2011; 14:262–265.
- Duffy MJ. Prostate-specific antigen: does the current evidence support its use in prostate cancer screening? Ann Clin Biochem 2011; 48:310–316.
- Andriole GL, Crawford ED, Grubb RL, et al; PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360:1310–1319.
- Crawford ED, Grubb R, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol 2011; 29:355–361.
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360:1320–1328.
- Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010; 11:725–732.
- Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate 2011; 71:985–997.
- Boyd MD, Weinrich SP, Weinrich M, Norton A. Obstacles to prostate cancer screening in African-American men. J Natl Black Nurses Assoc 2001; 12:1–5.
- Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate 2005; 62:243–252.
- Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 2008; 17:636–644.
- Hosain GM, Sanderson M, Du XL, Chan W, Strom SS. Racial/ethnic differences in predictors of PSA screening in a tri-ethnic population. Cent Eur J Public Health 2011; 19:30–34.
- Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control 2010; 21:1071–1080.
- Betancourt JR, Maina AW. The Institute of Medicine report “Unequal Treatment”: implications for academic health centers. Mt Sinai J Med 2004; 71:314–321.
- Patel K, Kenerson D, Wang H, et al. Factors influencing prostate cancer screening in low-income African Americans in Tennessee. J Health Care Poor Underserved 2010; 21(suppl 1):114–126.
- Modlin CS. Culture, race, and disparities in health care. Cleve Clin J Med 2003; 70:283–288.
- Kyle C, Ewing T, Wu XC, et al. Statewide analysis of serum prostate specific antigen levels in Louisiana men without prostate cancer. J La State Med Soc 2004; 156:319–323.
- Vijayakumar S, Winter K, Sause W, et al. Prostate-specific antigen levels are higher in African-American than in white patients in a multicenter registration study: results of RTOG 94-12. Int J Radiat Oncol Biol Phys 1998; 40:17–25.
- Chang BL, Spangler E, Gallagher S, et al. Validation of genome-wide prostate cancer associations in men of African descent. Cancer Epidemiol Biomarkers Prev 2011; 20:23–32.
- National Comprehensive Cancer Network (NCCN). NCCN Stresses Importance of PSA Testing in High-Risk Men. http://www.nccn.org/about/news/newsinfo.asp?NewsID=218. Accessed April 2, 2012.
- National Cancer Institute. Prostate-Specific Antigen (PSA) Test. http://www.cancer.gov/cancertopics/factsheet/detection/PSA. Accessed April 2, 2012.
- Duggan D, Zheng SL, Knowlton M, et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. Natl Cancer Inst 2007; 99:1836–1844.
- Grossfeld GD, Latini DM, Downs T, Lubeck DP, Mehta SS, Carroll PR. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol 2002; 168:2510–2515.
- Hoffman RM, Harlan LC, Klabunde CN, et al. Racial differences in initial treatment for clinically localized prostate cancer. Results from the prostate cancer outcomes study. J Gen Intern Med 2003; 18:845–853.
- Polednak AP. Prostate cancer treatment in black and white men: the need to consider both stage at diagnosis and socioeconomic status. J Natl Med Assoc 1998; 90:101–104.
- Merrill RM, Lyon JL. Explaining the difference in prostate cancer mortality rates between white and black men in the United States. Urology 2000; 55:730–735.
- Tewari AK, Gold HT, Demers RY, et al. Effect of socioeconomic factors on long-term mortality in men with clinically localized prostate cancer. Urology 2009; 73:624–630.
- White A, Coker AL, Du XL, Eggleston KS, Williams M. Racial/ethnic disparities in survival among men diagnosed with prostate cancer in Texas. Cancer 2011; 117:1080–1088.
- Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol 2010; 28:1069–1074.
- Demers RY, Tiwari A, Wei J, Weiss LK, Severson RK, Montie J. Trends in the utilization of androgen-deprivation therapy for patients with prostate carcinoma suggest an effect on mortality. Cancer 2001; 92:2309–2317.
- Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci 2006; 11:1388–1413.
- Schwartz K, Powell IJ, Underwood W, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 2009; 74:1296–1302.
- Hayn MH, Orom H, Shavers VL, et al. Racial/ethnic differences in receipt of pelvic lymph node dissection among men with localized/regional prostate cancer. Cancer 2011. [Epub ahead of print]
- Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979–2003. Cancer 2011; 117:3242–3251.
- Winkfield KM, Chen MH, Dosoretz DE, et al. Race and survival following brachytherapy-based treatment for men with localized or locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 20115; 81:e345–e350.
- Fowler JE, Terrell F. Survival in blacks and whites after treatment for localized prostate cancer. J Urol 1996; 156:133–136.
- Tewari A, Horninger W, Badani KK, et al. Racial differences in serum prostate-specific antigen (PSA) doubling time, histopathological variables and long-term PSA recurrence between African-American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int 2005; 96:29–33.
- Bozeman C, Williams BJ, Whatley T, Crow A, Eastham J. Clinical and biopsy specimen features in black and white men with clinically localized prostate cancer. South Med J 2000; 93:400–402.
- Delongchamps NB, Singh A, Haas GP. Epidemiology of prostate cancer in Africa: another step in the understanding of the disease? Curr Probl Cancer 2007; 31:226–236.
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int 2002; 90:162–173.
- Muller DC, Severi G, Baglietto L, et al. Dietary patterns and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2009; 18:3126–3129.
- Alexander DD, Mink PJ, Cushing CA, Sceurman B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr J 2010; 9:50.
- Gonzalez CA, Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer 2010; 46:2555–2562.
- Rodriguez C, McCullough ML, Mondul AM, et al. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol Biomarkers Prev 2003; 12:597–603.
- McCarty MF. Mortality from Western cancers rose dramatically among African-Americans during the 20th century: are dietary animal products to blame? Med Hypotheses 2001; 57:169–174.
- Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2007; 16:63–69.
- Spangler E, Zeigler-Johnson CM, Coomes M, Malkowicz SB, Wein A, Rebbeck TR. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol 2007; 178:1939–1944.
- Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009; 301:39–51.
- Oakley-Girvan I, Feldman D, Eccleshall TR, et al. Risk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptor. Cancer Epidemiol Biomarkers Prev 2004; 13:1325–1330.
- Gilbert R, Martin RM, Beynon R, et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control 2011; 22:319–340.
- Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 2011; 128:1414–1424.
- Yin L, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer Epidemiol 2009; 33:435–445.
- McCormick DL, Johnson WD, Bosland MC, Lubet RA, Steele VE. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr Cancer 2007; 57:184–193.
- Belpomme D, Irigaray P, Ossondo M, Vacque D, Martin M. Prostate cancer as an environmental disease: an ecological study in the French Caribbean islands, Martinique and Guadeloupe. Int J Oncol 2009; 34:1037–1044.
- Ramis R, Diggle P, Cambra K, López-Abente G. Prostate cancer and industrial pollution. Risk around putative focus in a multi-source scenario. Environ Int 2011; 37:577–585.
- Dey S, Zhang Z, Hablas A, et al. Geographic patterns of cancer in the population-based registry of Egypt: possible links to environmental exposures. Cancer Epidemiol 2011; 35:254–264.
- Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol Biomarkers Prev 2009; 18:2427–2435.
- Orsini N, Bellocco R, Bottai M, et al. A prospective study of lifetime physical activity and prostate cancer incidence and mortality. Br J Cancer 2009; 101:1932–1938.
Prostate cancer is the most common cancer affecting American men. In 2010, an estimated 217,730 men were diagnosed with it and 32,050 died of it.1 African American men are disproportionately affected, with a prostate cancer incidence two-thirds higher than whites and a mortality rate twice as high.1 Owing to such disparities, the life expectancy of African Americans is several years shorter than that of non-Hispanic whites.2
For the primary care provider, who is often the first access point for health care in the United States, it is important to understand what mechanisms may underlie these differences and what can be done to narrow the gap.3
WHAT IS THE CAUSE OF THESE DIFFERENCES?
Many studies have looked into the causes of the higher incidence of prostate cancer in African American men and their higher mortality rate from it. The disparity may be due to a variety of factors, some socioeconomic and some biologic.
Poorer access to care, or lower-quality care?
A study of US servicemen who had equal access to care showed that African American men had a higher rate of prostate cancer regardless of access to care and socioeconomic status.4
However, the 2002 Institute of Medicine report, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, found evidence that racial and ethnic minorities tend to receive lower-quality health care than whites, “even when access-related factors, such as patients’ insurance status and income, are controlled.”5
Genetic predisposition?
Some have proposed that the disparity may be a function of genetic predisposition.
Evidence of a genetic component to the high incidence and mortality rate in African American men comes from epidemiologic studies of men with similar genetic backgrounds. For example, men in Nigeria and Ghana also have a high incidence of prostate cancer, as do men of African descent in the Caribbean islands and in the United Kingdom.6
Chromosome 8q24 variants have been shown in several studies to be associated with prostate cancer risk and are more common in African American men.7–10 Some studies have also shown a higher rate of variations in cell apoptosis genes such as BCL211 and tumor-suppression genes such as EphB2 in African American men.12
These findings suggest that genetic differences may contribute to the higher prostate cancer incidence and mortality rate seen in African American men.
More-aggressive cancer, or later detection?
Not only do African American men tend to have a higher incidence of prostate cancer, they also tend to have more-aggressive disease (ie, a higher pathologic grade) at the time of diagnosis, which may contribute to the disparity in mortality rates.13–19
Initially, there was some controversy as to whether this observation is a result of genetic and biologic factors that may predispose African American men to more-aggressive disease, or if it is due to inadequate screening and delayed presentation. However, a body of evidence supports the contention that prostate cancer is more aggressive in African American men.
For example, a study of autopsy data from men who died of prostate cancer at ages 20 to 49 showed that the age of onset of prostate cancer was similar between African American and white men.20 The Surveillance Epidemiology and End Results (SEER) database showed that African American men had a higher incidence of metastatic disease across all age groups.20 A similar study conducted 10 years later confirmed that rates of subclinical prostate cancer in African American and white men do not differ by race at the early ages, but that advanced or metastatic disease occurred nearly four times as frequently in African American men.21
Another study examined prostate biopsies from African American men and found that their tumors expressed higher levels of biomarkers, suggesting they had more-aggressive disease.22
SCREENING FOR PROSTATE CANCER
Serum prostate-specific antigen (PSA) testing has become the method of choice for prostate cancer screening. However, PSA screening in asymptomatic men is under debate, because it can lead to overdetection and subsequent overtreatment of indolent disease.23
Several recent studies showed differing results from prostate cancer screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial found that the mortality rate was no lower with combined PSA screening and digital rectal examination during a median follow-up of 11 years than in a control group that had a lower rate of screening.24 However, further analysis of these data, with stratifying by comorbidities, showed that PSA screening in young and healthy men reduces the risk of death from prostate cancer, with minimal overtreatment.25
The European Randomized Study of Screening for Prostate Cancer found a statistically significant 20% reduction in deaths from prostate cancer with PSA screening, but that it was necessary to treat 48 men in order to save one life.26
Another study, published in 2010, showed that regular PSA screening reduced the rate of prostate cancer mortality by half over 14 years.27
African American men generally present with disease that is more advanced than in white men.28 This historically has been attributed to the fact that African Americans have been less likely to be screened for prostate cancer, though recent data indicate the gap is lessening.29–31 A cross-sectional study from the Texas Medical Center showed that 54.4% of African American men had received PSA screening, compared with 63.2% of white men.32
Another study showed that African Americans were more likely to have had a longer interval between PSA screenings before diagnosis, and that a longer PSA screening interval was associated with greater odds of having advanced disease at diagnosis.33 However, when the researchers controlled for the PSA screening interval, they found that African Americans had the same odds of being diagnosed with advanced prostate cancer as white patients did. They concluded that more frequent or systematic PSA screening may reduce the racial differences in cancer stage at diagnosis and in deaths.
Reasons for the disparities in screening
Many reasons have been proposed to explain why African Americans receive less screening, including poor communication between physicians and minority patients due to lack of cultural competency among physicians, lack of health insurance (and poor access to quality care as a result), and deficiency of knowledge about screening. Though awareness is rising, many African Americans are unaware of early detection methods for prostate cancer (eg, PSA testing), and other barriers such as cost and transportation exist that may prevent African American men from being screened.34,35
As gatekeepers, primary care physicians are in a position to address these shortcomings in patient education and to enhance the physician-patient relationship.36
Black men have higher PSA levels, with or without cancer
Physicians must also be aware of racial differences in PSA levels and realize that the predictive value of PSA in the diagnosis of prostate cancer may differ between African Americans and whites.
Black men, with or without prostate cancer, have been found to have higher PSA levels. Kyle and colleagues37 found that African American men without prostate cancer had significantly higher mean PSA levels than white men across all age groups. Furthermore, Vijayakumar et al38 found that African Americans with newly diagnosed localized prostate cancer had higher serum PSA levels than whites at diagnosis.
Although PSA cutoff levels have not been officially modified according to race, primary care physicians should have a lower threshold for referring African American men who have a suspiciously high PSA level for further urologic evaluation. Close partnership between the internist, family practitioner, and urologist will aid in the optimal use of PSA testing for the early detection of prostate cancer.
When to start PSA screening? How often to screen?
The age at which African American men should begin to have their PSA levels checked (with or without a digital rectal examination) continues to debated. However, the American Cancer Society39 recommends that African American men who have a father or brother who had prostate cancer before age 65 should begin having discussions with their physician on this topic and, with their informed consent, screening at age 45.
The frequency of PSA screening depends on the individual’s PSA level. The National Comprehensive Cancer Network40 recommends that men at high risk be offered a baseline PSA measurement and digital rectal examination at age 40 and, if the PSA level is higher than 1 ng/mL, that they be offered annual follow-ups. If the PSA level is less than 1 ng/mL, they recommend screening again at age 45. Risk factors for prostate cancer include family history as well as African American race.41
How should PSA levels be interpreted?
Interpreting PSA results is important in detecting prostate cancer at early stages.
At first, we believed the normal range of PSA for all men was 4.0 ng/mL or less. However, the American Urological Association now recognizes that the normal PSA range, in addition to varying along racial lines, also is age-dependent.42 The Cleveland Clinic Minority Men's Health Center's suggested normal ranges of PSA in African American men are:
- Age 40–49: ≤ 2.5 ng/mL
- Age 50–59: ≤ 3.0 ng/mL
- Age 60–69: ≤ 3.5 ng/mL
- Age 70–79: ≤ 4.5 ng/mL
- Age > 80: ≤ 5.0 ng/mL.
Remember that an elevated PSA does not necessarily signify prostate cancer, and that these are reference ranges only and may vary in individual men.
SURVIVAL AFTER DIAGNOSIS
African American men with prostate cancer have significantly higher mortality rates than white men. The possible causes of worse outcomes are many, and there have been many studies that attempted to address this disparity. The question of a more biologically aggressive cancer was previously discussed, but additional factors such as socioeconomic factors, comorbidities, and treatment received have also been studied, and data are mixed.43–45
In a large SEER database review, once confounding variables of socioeconomic status, cancer stage, and treatment received were eliminated, African Americans had similar stage-for-stage survival from prostate cancer.46 Another study found, in 2,046 men, that differences in socioeconomic status explained the difference in mortality rates between white and black patients.47
However, other studies that adjusted for socioeconomic status as well as patient and tumor characteristics found that African American and Hispanic men were more likely to die of prostate cancer than white men.48
Do African American men receive less-aggressive care?
Studies have also determined that there may be differences in treatments offered to patients, which in turn negatively affect survival.28,49–53 Potentially curative local therapies (including radical surgery or radiation) may be recommended less often to black men because of major comorbidities or socioeconomic considerations.49–52
Additionally, potential metastatic disease may be identified in a less timely and accurate manner, as African American men are less likely to undergo pelvic lymph node dissection. This was associated with worse survival in men with poorly differentiated prostate cancer.53
However, returning to the possibility that prostate cancer is biologically more aggressive in African American men, some studies have shown that even after adjusting for treatment, African Americans continue to have worse survival rates.54,55 One study in men with stage T1 to T3 prostate cancer who chose brachytherapy for treatment reported that after adjusting for PSA, clinical stage, socioeconomic status, and comorbidities, African American and Hispanic race were associated with higher all-cause mortality rates.55
Equal care, equal outcomes?
In total, these results suggest that factors unrelated to tumor biology may be additional reasons for the poorer survival rates in African American men with prostate cancer. More favorable survival outcomes for African Americans with localized disease may be achieved with uniform assignment of treatment.
Fowler and Terrell56 reviewed the outcomes of 148 black and 209 white men with localized prostate cancer treated with surgery or radiation therapy over an 11-year period at a Veterans Administration hospital. Not surprisingly, the black men presented more often with advanced disease. However, survival outcomes were equivalent between whites and blacks when treatment was assigned in a uniform manner without regard to race. After a median follow-up of 96 months, there were no significant differences in all-cause, cause-specific, metastasis-free, clinical disease-free, or PSA recurrence-free survival rates in 109 black and 167 white men with low-stage cancer treated with surgery or radiation therapy or in 39 black and 42 white men with high-stage disease treated with radiotherapy.56
Similarly, Tewari et al57 studied a cohort of 402 African American and 642 white men, all of whom underwent radical prostatectomy for clinically localized prostate cancer. They were followed for PSA recurrence to determine if race-specific differences in PSA doubling time or histopathologic variables might account for the higher mortality rate in black men. While there were race-specific differences in baseline serum PSA and incidence of high-grade prostatic intraepithelial neoplasia, race was not an independent risk factor for biochemical recurrence. Instead, other variables such as the Gleason pathology score, bilateral cancers, and margin positivity were independently associated with biochemical recurrence.
Furthermore, researchers at Louisiana State University58 retrospectively analyzed data from 205 men of different races with early-stage prostate cancer. The African American men had a higher serum PSA level, suggesting more advanced disease or greater tumor burden at presentation, but no statistically significant differences were found among the pretreatment biopsy variables, including prostate volume (measured by ultrasonography), Gleason score, millimeters of cancer within the biopsy specimen, and percentage of cancer within the biopsy specimen. After treatment, there were no significant differences in survival outcomes along racial lines, leading the authors to conclude that early detection and treatment of prostate cancer in African Americans would be the best approach to lowering mortality rates.
Taken together, these data suggest that if localized prostate cancer is treated adequately and appropriately, African American patients may have improved survival rates.
DIETARY AND LIFESTYLE FACTORS
The incidence of prostate cancer is increasing in other countries where Western diets and lifestyles have been adopted,59,60 suggesting that nutritional factors may also contribute partly to prostate carcinogenesis. Culture- and race-specific differences in diet may play an important role in prostate cancer risk in certain racial minorities. Many aspects of diet and nutrition have been studied for their impact on prostate cancer.
Dietary risk factors
Too much red meat and processed meat? Although some have suggested that diets high in red and processed meats may lead to a higher risk of prostate cancer, a meta-analysis showed no association.61,62
Too much calcium? The European Prospective Investigation Into Cancer and Nutrition study found that high dietary intake of dairy protein and calcium from dairy products was associated with a higher risk of prostate cancer.63 A cohort study in the United States had similar findings with regard to calcium.64 However, the higher risk of prostate cancer was associated with consumption of 2,000 mg or more of calcium per day, which was consumed by only 2% of the study’s cohort and, as the study’s authors reported, fewer than 1% of US men. As such, only a small population of American men seem to be exposing themselves to a higher risk of prostate cancer by high calcium consumption.
High fat intake? Certain fatty acids have been implicated in general tumor genesis, and that risk has been extrapolated to prostate cancer.65 For example, high fat intake and obesity are associated with increased levels of insulin-like growth factor 1, which in turn has been shown to correlate with a significantly elevated risk of prostate cancer.63,65
Obesity has been shown to increase the risk of more-aggressive prostate cancer, but not of less-aggressive tumors.66 Moreover, men who lost weight had a lower risk of prostate cancer than those who maintained their weight over 10 years.66 Obesity may be particularly risky for African American men, in whom it was found to be associated with shorter biochemical relapse-free survival, whereas it was not an independent risk factor in white men.67
Preventive dietary agents have been elusive
Unfortunately, despite attempts to identify preventive dietary agents, none has yet been confirmed.
No benefit from selenium or vitamin E. The Selenium and Vitamin E Cancer Prevention Trial was discontinued, as there was no evidence that either agent prevented prostate cancer in relatively healthy men.68
Vitamin D? It has been suggested that lower levels of vitamin D could contribute to the higher rates of prostate cancer in African Americans, as vitamin D deficiency is more common in African Americans.69 However, several meta-analyses have shown no association between vitamin D and prostate cancer.70–72
Soy? Attempts at correlating the relatively low incidence of prostate cancer in Asians have revealed that high soy intake may be protective. Asians consume more soy than Americans do (100 vs 3 mg/day), and soy isoflavones such as genistein, glycitein, and daidzein lower the incidence of prostate cancer in laboratory mice.73
Other lifestyle factors
Other lifestyle factors have also been analyzed to see if they contribute to prostate cancer.
Pollution. Some studies have suggested that the etiology of prostate cancer may lie in environmental exposures to pesticides,74 metal industrial facilities,75 and urban living.76
Smoking. Watters et al77 found that current and former cigarette smokers were actually at a lower risk of being diagnosed with non-advanced prostate cancer, but current smokers were at higher risk of dying from prostate cancer.
Physical activity. A prospective study of lifetime physical activity of more than 45,000 men found that men who were not sedentary during work and who walked or bicycled more than 30 minutes per day during adult life had an approximately 20% lower incidence of prostate cancer.78
In sum, primary care providers who are generally promoting healthy lifestyles can point to a reduction in risk for prostate cancer as yet another benefit to a low-fat diet, a healthy body mass index, and daily exercise.
HOW PRIMARY CARE PHYSICIANS CAN HELP CLOSE THE GAP
Primary care physicians serve as the first point of health access for many in the United States today.
The diagnosis of prostate cancer is made more frequently in African American men than in other American men, often at a higher pathological grade, and with a worse mortality rate. Primary care physicians can help improve these statistics. Interventions targeting overall health, such as promotion of a healthy diet, could be established at primary care visits and could also reduce the incidence of prostate cancer in African American men. Patient education regarding prostate cancer screening, the impact of family history, and the rate of PSA screening could be improved.
Primary care physicians serve a vital role in health education and prostate cancer screening, and therefore they begin the process in potentially reducing the impact of prostate cancer in African American men. The racial disparity seen in prostate cancer may begin to be minimized with primary care physicians and specialists working together to ensure that all men receive appropriate treatment.
Prostate cancer is the most common cancer affecting American men. In 2010, an estimated 217,730 men were diagnosed with it and 32,050 died of it.1 African American men are disproportionately affected, with a prostate cancer incidence two-thirds higher than whites and a mortality rate twice as high.1 Owing to such disparities, the life expectancy of African Americans is several years shorter than that of non-Hispanic whites.2
For the primary care provider, who is often the first access point for health care in the United States, it is important to understand what mechanisms may underlie these differences and what can be done to narrow the gap.3
WHAT IS THE CAUSE OF THESE DIFFERENCES?
Many studies have looked into the causes of the higher incidence of prostate cancer in African American men and their higher mortality rate from it. The disparity may be due to a variety of factors, some socioeconomic and some biologic.
Poorer access to care, or lower-quality care?
A study of US servicemen who had equal access to care showed that African American men had a higher rate of prostate cancer regardless of access to care and socioeconomic status.4
However, the 2002 Institute of Medicine report, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, found evidence that racial and ethnic minorities tend to receive lower-quality health care than whites, “even when access-related factors, such as patients’ insurance status and income, are controlled.”5
Genetic predisposition?
Some have proposed that the disparity may be a function of genetic predisposition.
Evidence of a genetic component to the high incidence and mortality rate in African American men comes from epidemiologic studies of men with similar genetic backgrounds. For example, men in Nigeria and Ghana also have a high incidence of prostate cancer, as do men of African descent in the Caribbean islands and in the United Kingdom.6
Chromosome 8q24 variants have been shown in several studies to be associated with prostate cancer risk and are more common in African American men.7–10 Some studies have also shown a higher rate of variations in cell apoptosis genes such as BCL211 and tumor-suppression genes such as EphB2 in African American men.12
These findings suggest that genetic differences may contribute to the higher prostate cancer incidence and mortality rate seen in African American men.
More-aggressive cancer, or later detection?
Not only do African American men tend to have a higher incidence of prostate cancer, they also tend to have more-aggressive disease (ie, a higher pathologic grade) at the time of diagnosis, which may contribute to the disparity in mortality rates.13–19
Initially, there was some controversy as to whether this observation is a result of genetic and biologic factors that may predispose African American men to more-aggressive disease, or if it is due to inadequate screening and delayed presentation. However, a body of evidence supports the contention that prostate cancer is more aggressive in African American men.
For example, a study of autopsy data from men who died of prostate cancer at ages 20 to 49 showed that the age of onset of prostate cancer was similar between African American and white men.20 The Surveillance Epidemiology and End Results (SEER) database showed that African American men had a higher incidence of metastatic disease across all age groups.20 A similar study conducted 10 years later confirmed that rates of subclinical prostate cancer in African American and white men do not differ by race at the early ages, but that advanced or metastatic disease occurred nearly four times as frequently in African American men.21
Another study examined prostate biopsies from African American men and found that their tumors expressed higher levels of biomarkers, suggesting they had more-aggressive disease.22
SCREENING FOR PROSTATE CANCER
Serum prostate-specific antigen (PSA) testing has become the method of choice for prostate cancer screening. However, PSA screening in asymptomatic men is under debate, because it can lead to overdetection and subsequent overtreatment of indolent disease.23
Several recent studies showed differing results from prostate cancer screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial found that the mortality rate was no lower with combined PSA screening and digital rectal examination during a median follow-up of 11 years than in a control group that had a lower rate of screening.24 However, further analysis of these data, with stratifying by comorbidities, showed that PSA screening in young and healthy men reduces the risk of death from prostate cancer, with minimal overtreatment.25
The European Randomized Study of Screening for Prostate Cancer found a statistically significant 20% reduction in deaths from prostate cancer with PSA screening, but that it was necessary to treat 48 men in order to save one life.26
Another study, published in 2010, showed that regular PSA screening reduced the rate of prostate cancer mortality by half over 14 years.27
African American men generally present with disease that is more advanced than in white men.28 This historically has been attributed to the fact that African Americans have been less likely to be screened for prostate cancer, though recent data indicate the gap is lessening.29–31 A cross-sectional study from the Texas Medical Center showed that 54.4% of African American men had received PSA screening, compared with 63.2% of white men.32
Another study showed that African Americans were more likely to have had a longer interval between PSA screenings before diagnosis, and that a longer PSA screening interval was associated with greater odds of having advanced disease at diagnosis.33 However, when the researchers controlled for the PSA screening interval, they found that African Americans had the same odds of being diagnosed with advanced prostate cancer as white patients did. They concluded that more frequent or systematic PSA screening may reduce the racial differences in cancer stage at diagnosis and in deaths.
Reasons for the disparities in screening
Many reasons have been proposed to explain why African Americans receive less screening, including poor communication between physicians and minority patients due to lack of cultural competency among physicians, lack of health insurance (and poor access to quality care as a result), and deficiency of knowledge about screening. Though awareness is rising, many African Americans are unaware of early detection methods for prostate cancer (eg, PSA testing), and other barriers such as cost and transportation exist that may prevent African American men from being screened.34,35
As gatekeepers, primary care physicians are in a position to address these shortcomings in patient education and to enhance the physician-patient relationship.36
Black men have higher PSA levels, with or without cancer
Physicians must also be aware of racial differences in PSA levels and realize that the predictive value of PSA in the diagnosis of prostate cancer may differ between African Americans and whites.
Black men, with or without prostate cancer, have been found to have higher PSA levels. Kyle and colleagues37 found that African American men without prostate cancer had significantly higher mean PSA levels than white men across all age groups. Furthermore, Vijayakumar et al38 found that African Americans with newly diagnosed localized prostate cancer had higher serum PSA levels than whites at diagnosis.
Although PSA cutoff levels have not been officially modified according to race, primary care physicians should have a lower threshold for referring African American men who have a suspiciously high PSA level for further urologic evaluation. Close partnership between the internist, family practitioner, and urologist will aid in the optimal use of PSA testing for the early detection of prostate cancer.
When to start PSA screening? How often to screen?
The age at which African American men should begin to have their PSA levels checked (with or without a digital rectal examination) continues to debated. However, the American Cancer Society39 recommends that African American men who have a father or brother who had prostate cancer before age 65 should begin having discussions with their physician on this topic and, with their informed consent, screening at age 45.
The frequency of PSA screening depends on the individual’s PSA level. The National Comprehensive Cancer Network40 recommends that men at high risk be offered a baseline PSA measurement and digital rectal examination at age 40 and, if the PSA level is higher than 1 ng/mL, that they be offered annual follow-ups. If the PSA level is less than 1 ng/mL, they recommend screening again at age 45. Risk factors for prostate cancer include family history as well as African American race.41
How should PSA levels be interpreted?
Interpreting PSA results is important in detecting prostate cancer at early stages.
At first, we believed the normal range of PSA for all men was 4.0 ng/mL or less. However, the American Urological Association now recognizes that the normal PSA range, in addition to varying along racial lines, also is age-dependent.42 The Cleveland Clinic Minority Men's Health Center's suggested normal ranges of PSA in African American men are:
- Age 40–49: ≤ 2.5 ng/mL
- Age 50–59: ≤ 3.0 ng/mL
- Age 60–69: ≤ 3.5 ng/mL
- Age 70–79: ≤ 4.5 ng/mL
- Age > 80: ≤ 5.0 ng/mL.
Remember that an elevated PSA does not necessarily signify prostate cancer, and that these are reference ranges only and may vary in individual men.
SURVIVAL AFTER DIAGNOSIS
African American men with prostate cancer have significantly higher mortality rates than white men. The possible causes of worse outcomes are many, and there have been many studies that attempted to address this disparity. The question of a more biologically aggressive cancer was previously discussed, but additional factors such as socioeconomic factors, comorbidities, and treatment received have also been studied, and data are mixed.43–45
In a large SEER database review, once confounding variables of socioeconomic status, cancer stage, and treatment received were eliminated, African Americans had similar stage-for-stage survival from prostate cancer.46 Another study found, in 2,046 men, that differences in socioeconomic status explained the difference in mortality rates between white and black patients.47
However, other studies that adjusted for socioeconomic status as well as patient and tumor characteristics found that African American and Hispanic men were more likely to die of prostate cancer than white men.48
Do African American men receive less-aggressive care?
Studies have also determined that there may be differences in treatments offered to patients, which in turn negatively affect survival.28,49–53 Potentially curative local therapies (including radical surgery or radiation) may be recommended less often to black men because of major comorbidities or socioeconomic considerations.49–52
Additionally, potential metastatic disease may be identified in a less timely and accurate manner, as African American men are less likely to undergo pelvic lymph node dissection. This was associated with worse survival in men with poorly differentiated prostate cancer.53
However, returning to the possibility that prostate cancer is biologically more aggressive in African American men, some studies have shown that even after adjusting for treatment, African Americans continue to have worse survival rates.54,55 One study in men with stage T1 to T3 prostate cancer who chose brachytherapy for treatment reported that after adjusting for PSA, clinical stage, socioeconomic status, and comorbidities, African American and Hispanic race were associated with higher all-cause mortality rates.55
Equal care, equal outcomes?
In total, these results suggest that factors unrelated to tumor biology may be additional reasons for the poorer survival rates in African American men with prostate cancer. More favorable survival outcomes for African Americans with localized disease may be achieved with uniform assignment of treatment.
Fowler and Terrell56 reviewed the outcomes of 148 black and 209 white men with localized prostate cancer treated with surgery or radiation therapy over an 11-year period at a Veterans Administration hospital. Not surprisingly, the black men presented more often with advanced disease. However, survival outcomes were equivalent between whites and blacks when treatment was assigned in a uniform manner without regard to race. After a median follow-up of 96 months, there were no significant differences in all-cause, cause-specific, metastasis-free, clinical disease-free, or PSA recurrence-free survival rates in 109 black and 167 white men with low-stage cancer treated with surgery or radiation therapy or in 39 black and 42 white men with high-stage disease treated with radiotherapy.56
Similarly, Tewari et al57 studied a cohort of 402 African American and 642 white men, all of whom underwent radical prostatectomy for clinically localized prostate cancer. They were followed for PSA recurrence to determine if race-specific differences in PSA doubling time or histopathologic variables might account for the higher mortality rate in black men. While there were race-specific differences in baseline serum PSA and incidence of high-grade prostatic intraepithelial neoplasia, race was not an independent risk factor for biochemical recurrence. Instead, other variables such as the Gleason pathology score, bilateral cancers, and margin positivity were independently associated with biochemical recurrence.
Furthermore, researchers at Louisiana State University58 retrospectively analyzed data from 205 men of different races with early-stage prostate cancer. The African American men had a higher serum PSA level, suggesting more advanced disease or greater tumor burden at presentation, but no statistically significant differences were found among the pretreatment biopsy variables, including prostate volume (measured by ultrasonography), Gleason score, millimeters of cancer within the biopsy specimen, and percentage of cancer within the biopsy specimen. After treatment, there were no significant differences in survival outcomes along racial lines, leading the authors to conclude that early detection and treatment of prostate cancer in African Americans would be the best approach to lowering mortality rates.
Taken together, these data suggest that if localized prostate cancer is treated adequately and appropriately, African American patients may have improved survival rates.
DIETARY AND LIFESTYLE FACTORS
The incidence of prostate cancer is increasing in other countries where Western diets and lifestyles have been adopted,59,60 suggesting that nutritional factors may also contribute partly to prostate carcinogenesis. Culture- and race-specific differences in diet may play an important role in prostate cancer risk in certain racial minorities. Many aspects of diet and nutrition have been studied for their impact on prostate cancer.
Dietary risk factors
Too much red meat and processed meat? Although some have suggested that diets high in red and processed meats may lead to a higher risk of prostate cancer, a meta-analysis showed no association.61,62
Too much calcium? The European Prospective Investigation Into Cancer and Nutrition study found that high dietary intake of dairy protein and calcium from dairy products was associated with a higher risk of prostate cancer.63 A cohort study in the United States had similar findings with regard to calcium.64 However, the higher risk of prostate cancer was associated with consumption of 2,000 mg or more of calcium per day, which was consumed by only 2% of the study’s cohort and, as the study’s authors reported, fewer than 1% of US men. As such, only a small population of American men seem to be exposing themselves to a higher risk of prostate cancer by high calcium consumption.
High fat intake? Certain fatty acids have been implicated in general tumor genesis, and that risk has been extrapolated to prostate cancer.65 For example, high fat intake and obesity are associated with increased levels of insulin-like growth factor 1, which in turn has been shown to correlate with a significantly elevated risk of prostate cancer.63,65
Obesity has been shown to increase the risk of more-aggressive prostate cancer, but not of less-aggressive tumors.66 Moreover, men who lost weight had a lower risk of prostate cancer than those who maintained their weight over 10 years.66 Obesity may be particularly risky for African American men, in whom it was found to be associated with shorter biochemical relapse-free survival, whereas it was not an independent risk factor in white men.67
Preventive dietary agents have been elusive
Unfortunately, despite attempts to identify preventive dietary agents, none has yet been confirmed.
No benefit from selenium or vitamin E. The Selenium and Vitamin E Cancer Prevention Trial was discontinued, as there was no evidence that either agent prevented prostate cancer in relatively healthy men.68
Vitamin D? It has been suggested that lower levels of vitamin D could contribute to the higher rates of prostate cancer in African Americans, as vitamin D deficiency is more common in African Americans.69 However, several meta-analyses have shown no association between vitamin D and prostate cancer.70–72
Soy? Attempts at correlating the relatively low incidence of prostate cancer in Asians have revealed that high soy intake may be protective. Asians consume more soy than Americans do (100 vs 3 mg/day), and soy isoflavones such as genistein, glycitein, and daidzein lower the incidence of prostate cancer in laboratory mice.73
Other lifestyle factors
Other lifestyle factors have also been analyzed to see if they contribute to prostate cancer.
Pollution. Some studies have suggested that the etiology of prostate cancer may lie in environmental exposures to pesticides,74 metal industrial facilities,75 and urban living.76
Smoking. Watters et al77 found that current and former cigarette smokers were actually at a lower risk of being diagnosed with non-advanced prostate cancer, but current smokers were at higher risk of dying from prostate cancer.
Physical activity. A prospective study of lifetime physical activity of more than 45,000 men found that men who were not sedentary during work and who walked or bicycled more than 30 minutes per day during adult life had an approximately 20% lower incidence of prostate cancer.78
In sum, primary care providers who are generally promoting healthy lifestyles can point to a reduction in risk for prostate cancer as yet another benefit to a low-fat diet, a healthy body mass index, and daily exercise.
HOW PRIMARY CARE PHYSICIANS CAN HELP CLOSE THE GAP
Primary care physicians serve as the first point of health access for many in the United States today.
The diagnosis of prostate cancer is made more frequently in African American men than in other American men, often at a higher pathological grade, and with a worse mortality rate. Primary care physicians can help improve these statistics. Interventions targeting overall health, such as promotion of a healthy diet, could be established at primary care visits and could also reduce the incidence of prostate cancer in African American men. Patient education regarding prostate cancer screening, the impact of family history, and the rate of PSA screening could be improved.
Primary care physicians serve a vital role in health education and prostate cancer screening, and therefore they begin the process in potentially reducing the impact of prostate cancer in African American men. The racial disparity seen in prostate cancer may begin to be minimized with primary care physicians and specialists working together to ensure that all men receive appropriate treatment.
- Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. Accessed April 2, 2011.
- Arias E. United States life tables, 2007. National vital statistics reports; vol 59 no 9. Hyattsville, MD: National Center for Health Statistics. 2011.
- Klein JB, Nguyen CT, Saffore L, Modlin C, Modlin CS. Racial disparities in urologic health care. J Natl Med Assoc 2010; 102:108–117.
- Wells TS, Bukowinski AT, Smith TC, et al. Racial differences in prostate cancer risk remain among US servicemen with equal access to care. Prostate 2010; 70:727–734.
- Smedley BD, Stith AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine. National Academy Press; 2002.
- Odedina FT, Akinremi TO, Chinegwundoh F, et al. Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer 2009; 4(suppl 1):S2.
- Okobia MN, Zmuda JM, Ferrell RE, Patrick AL, Bunker CH. Chromosome 8q24 variants are associated with prostate cancer risk in a high risk population of African ancestry. Prostate 2011; 71:1054–1063.
- Haiman CA, Chen GK, Blot WJ, et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet 2011; 7:e1001387.
- Freedman ML, Haiman CA, Patterson N, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A 2006; 103:14068–14073.
- Chang BL, Isaacs SD, Wiley KE, et al. Genome-wide screen for prostate cancer susceptibility genes in men with clinically significant disease. Prostate 2005; 64:356–361.
- Hatcher D, Daniels G, Osman I, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Transl Res 2009; 1:235–248.
- Robbins CM, Hooker S, Kittles RA, Carpten JD. EphB2 SNPs and sporadic prostate cancer risk in African American men. PLoS One 2011; 6:e19494.
- American Cancer Society. Cancer Facts & Figures for African Americans 2009–2010. http://www.cancer.org/acs/groups/content/@nho/documents/document/cffaa20092010pdf.pdf. Accessed April 2, 2012.
- Ayanian JZ, Udvarhelyi IS, Gatsonis CA, Pashos CL, Epstein AM. Racial differences in the use of revascularization procedures after coronary angiography. JAMA 1993; 269:2642–2646.
- Fine MJ, Ibrahim SA, Thomas SB. The role of race and genetics in health disparities research. Am J Public Health 2005; 95:2125–2128.
- Horner RD, Oddone EZ, Matchar DB. Theories explaining racial differences in the utilization of diagnostic and therapeutic procedures for cerebrovascular disease. Milbank Q 1995; 73:443–462.
- Juckett G. Cross-cultural medicine. Am Fam Physician 2005; 72:2267–2274.
- Ndubuisi SC, Kofie VY, Andoh JY, Schwartz EM. Black-white differences in the stage at presentation of prostate cancer in the District of Columbia. Urology 1995; 46:71–77.
- Misra-Hebert AD. Physician cultural competence: cross-cultural communication improves care. Cleve Clin J Med 2003; 70:289,293,296–298.
- Powell I, Sakr W, Weiss L, et al. Prostate cancer is biologically more aggressive among African Americans than Caucasian men under age 70: hypothesis supported by autopsy and SEER data. Program and abstracts from the American Urological Association 95th Annual Meeting; April 29–May 4, 2000: Atlanta, GA.
- Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol 2010; 183:1792–1796.
- Kim HS, Moreira DM, Jayachandran J, et al. Prostate biopsies from black men express higher levels of aggressive disease biomarkers than prostate biopsies from white men. Prostate Cancer Prostatic Dis 2011; 14:262–265.
- Duffy MJ. Prostate-specific antigen: does the current evidence support its use in prostate cancer screening? Ann Clin Biochem 2011; 48:310–316.
- Andriole GL, Crawford ED, Grubb RL, et al; PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360:1310–1319.
- Crawford ED, Grubb R, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol 2011; 29:355–361.
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360:1320–1328.
- Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010; 11:725–732.
- Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate 2011; 71:985–997.
- Boyd MD, Weinrich SP, Weinrich M, Norton A. Obstacles to prostate cancer screening in African-American men. J Natl Black Nurses Assoc 2001; 12:1–5.
- Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate 2005; 62:243–252.
- Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 2008; 17:636–644.
- Hosain GM, Sanderson M, Du XL, Chan W, Strom SS. Racial/ethnic differences in predictors of PSA screening in a tri-ethnic population. Cent Eur J Public Health 2011; 19:30–34.
- Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control 2010; 21:1071–1080.
- Betancourt JR, Maina AW. The Institute of Medicine report “Unequal Treatment”: implications for academic health centers. Mt Sinai J Med 2004; 71:314–321.
- Patel K, Kenerson D, Wang H, et al. Factors influencing prostate cancer screening in low-income African Americans in Tennessee. J Health Care Poor Underserved 2010; 21(suppl 1):114–126.
- Modlin CS. Culture, race, and disparities in health care. Cleve Clin J Med 2003; 70:283–288.
- Kyle C, Ewing T, Wu XC, et al. Statewide analysis of serum prostate specific antigen levels in Louisiana men without prostate cancer. J La State Med Soc 2004; 156:319–323.
- Vijayakumar S, Winter K, Sause W, et al. Prostate-specific antigen levels are higher in African-American than in white patients in a multicenter registration study: results of RTOG 94-12. Int J Radiat Oncol Biol Phys 1998; 40:17–25.
- Chang BL, Spangler E, Gallagher S, et al. Validation of genome-wide prostate cancer associations in men of African descent. Cancer Epidemiol Biomarkers Prev 2011; 20:23–32.
- National Comprehensive Cancer Network (NCCN). NCCN Stresses Importance of PSA Testing in High-Risk Men. http://www.nccn.org/about/news/newsinfo.asp?NewsID=218. Accessed April 2, 2012.
- National Cancer Institute. Prostate-Specific Antigen (PSA) Test. http://www.cancer.gov/cancertopics/factsheet/detection/PSA. Accessed April 2, 2012.
- Duggan D, Zheng SL, Knowlton M, et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. Natl Cancer Inst 2007; 99:1836–1844.
- Grossfeld GD, Latini DM, Downs T, Lubeck DP, Mehta SS, Carroll PR. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol 2002; 168:2510–2515.
- Hoffman RM, Harlan LC, Klabunde CN, et al. Racial differences in initial treatment for clinically localized prostate cancer. Results from the prostate cancer outcomes study. J Gen Intern Med 2003; 18:845–853.
- Polednak AP. Prostate cancer treatment in black and white men: the need to consider both stage at diagnosis and socioeconomic status. J Natl Med Assoc 1998; 90:101–104.
- Merrill RM, Lyon JL. Explaining the difference in prostate cancer mortality rates between white and black men in the United States. Urology 2000; 55:730–735.
- Tewari AK, Gold HT, Demers RY, et al. Effect of socioeconomic factors on long-term mortality in men with clinically localized prostate cancer. Urology 2009; 73:624–630.
- White A, Coker AL, Du XL, Eggleston KS, Williams M. Racial/ethnic disparities in survival among men diagnosed with prostate cancer in Texas. Cancer 2011; 117:1080–1088.
- Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol 2010; 28:1069–1074.
- Demers RY, Tiwari A, Wei J, Weiss LK, Severson RK, Montie J. Trends in the utilization of androgen-deprivation therapy for patients with prostate carcinoma suggest an effect on mortality. Cancer 2001; 92:2309–2317.
- Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci 2006; 11:1388–1413.
- Schwartz K, Powell IJ, Underwood W, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 2009; 74:1296–1302.
- Hayn MH, Orom H, Shavers VL, et al. Racial/ethnic differences in receipt of pelvic lymph node dissection among men with localized/regional prostate cancer. Cancer 2011. [Epub ahead of print]
- Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979–2003. Cancer 2011; 117:3242–3251.
- Winkfield KM, Chen MH, Dosoretz DE, et al. Race and survival following brachytherapy-based treatment for men with localized or locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 20115; 81:e345–e350.
- Fowler JE, Terrell F. Survival in blacks and whites after treatment for localized prostate cancer. J Urol 1996; 156:133–136.
- Tewari A, Horninger W, Badani KK, et al. Racial differences in serum prostate-specific antigen (PSA) doubling time, histopathological variables and long-term PSA recurrence between African-American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int 2005; 96:29–33.
- Bozeman C, Williams BJ, Whatley T, Crow A, Eastham J. Clinical and biopsy specimen features in black and white men with clinically localized prostate cancer. South Med J 2000; 93:400–402.
- Delongchamps NB, Singh A, Haas GP. Epidemiology of prostate cancer in Africa: another step in the understanding of the disease? Curr Probl Cancer 2007; 31:226–236.
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int 2002; 90:162–173.
- Muller DC, Severi G, Baglietto L, et al. Dietary patterns and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2009; 18:3126–3129.
- Alexander DD, Mink PJ, Cushing CA, Sceurman B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr J 2010; 9:50.
- Gonzalez CA, Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer 2010; 46:2555–2562.
- Rodriguez C, McCullough ML, Mondul AM, et al. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol Biomarkers Prev 2003; 12:597–603.
- McCarty MF. Mortality from Western cancers rose dramatically among African-Americans during the 20th century: are dietary animal products to blame? Med Hypotheses 2001; 57:169–174.
- Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2007; 16:63–69.
- Spangler E, Zeigler-Johnson CM, Coomes M, Malkowicz SB, Wein A, Rebbeck TR. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol 2007; 178:1939–1944.
- Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009; 301:39–51.
- Oakley-Girvan I, Feldman D, Eccleshall TR, et al. Risk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptor. Cancer Epidemiol Biomarkers Prev 2004; 13:1325–1330.
- Gilbert R, Martin RM, Beynon R, et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control 2011; 22:319–340.
- Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 2011; 128:1414–1424.
- Yin L, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer Epidemiol 2009; 33:435–445.
- McCormick DL, Johnson WD, Bosland MC, Lubet RA, Steele VE. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr Cancer 2007; 57:184–193.
- Belpomme D, Irigaray P, Ossondo M, Vacque D, Martin M. Prostate cancer as an environmental disease: an ecological study in the French Caribbean islands, Martinique and Guadeloupe. Int J Oncol 2009; 34:1037–1044.
- Ramis R, Diggle P, Cambra K, López-Abente G. Prostate cancer and industrial pollution. Risk around putative focus in a multi-source scenario. Environ Int 2011; 37:577–585.
- Dey S, Zhang Z, Hablas A, et al. Geographic patterns of cancer in the population-based registry of Egypt: possible links to environmental exposures. Cancer Epidemiol 2011; 35:254–264.
- Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol Biomarkers Prev 2009; 18:2427–2435.
- Orsini N, Bellocco R, Bottai M, et al. A prospective study of lifetime physical activity and prostate cancer incidence and mortality. Br J Cancer 2009; 101:1932–1938.
- Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. Accessed April 2, 2011.
- Arias E. United States life tables, 2007. National vital statistics reports; vol 59 no 9. Hyattsville, MD: National Center for Health Statistics. 2011.
- Klein JB, Nguyen CT, Saffore L, Modlin C, Modlin CS. Racial disparities in urologic health care. J Natl Med Assoc 2010; 102:108–117.
- Wells TS, Bukowinski AT, Smith TC, et al. Racial differences in prostate cancer risk remain among US servicemen with equal access to care. Prostate 2010; 70:727–734.
- Smedley BD, Stith AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine. National Academy Press; 2002.
- Odedina FT, Akinremi TO, Chinegwundoh F, et al. Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer 2009; 4(suppl 1):S2.
- Okobia MN, Zmuda JM, Ferrell RE, Patrick AL, Bunker CH. Chromosome 8q24 variants are associated with prostate cancer risk in a high risk population of African ancestry. Prostate 2011; 71:1054–1063.
- Haiman CA, Chen GK, Blot WJ, et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet 2011; 7:e1001387.
- Freedman ML, Haiman CA, Patterson N, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A 2006; 103:14068–14073.
- Chang BL, Isaacs SD, Wiley KE, et al. Genome-wide screen for prostate cancer susceptibility genes in men with clinically significant disease. Prostate 2005; 64:356–361.
- Hatcher D, Daniels G, Osman I, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Transl Res 2009; 1:235–248.
- Robbins CM, Hooker S, Kittles RA, Carpten JD. EphB2 SNPs and sporadic prostate cancer risk in African American men. PLoS One 2011; 6:e19494.
- American Cancer Society. Cancer Facts & Figures for African Americans 2009–2010. http://www.cancer.org/acs/groups/content/@nho/documents/document/cffaa20092010pdf.pdf. Accessed April 2, 2012.
- Ayanian JZ, Udvarhelyi IS, Gatsonis CA, Pashos CL, Epstein AM. Racial differences in the use of revascularization procedures after coronary angiography. JAMA 1993; 269:2642–2646.
- Fine MJ, Ibrahim SA, Thomas SB. The role of race and genetics in health disparities research. Am J Public Health 2005; 95:2125–2128.
- Horner RD, Oddone EZ, Matchar DB. Theories explaining racial differences in the utilization of diagnostic and therapeutic procedures for cerebrovascular disease. Milbank Q 1995; 73:443–462.
- Juckett G. Cross-cultural medicine. Am Fam Physician 2005; 72:2267–2274.
- Ndubuisi SC, Kofie VY, Andoh JY, Schwartz EM. Black-white differences in the stage at presentation of prostate cancer in the District of Columbia. Urology 1995; 46:71–77.
- Misra-Hebert AD. Physician cultural competence: cross-cultural communication improves care. Cleve Clin J Med 2003; 70:289,293,296–298.
- Powell I, Sakr W, Weiss L, et al. Prostate cancer is biologically more aggressive among African Americans than Caucasian men under age 70: hypothesis supported by autopsy and SEER data. Program and abstracts from the American Urological Association 95th Annual Meeting; April 29–May 4, 2000: Atlanta, GA.
- Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol 2010; 183:1792–1796.
- Kim HS, Moreira DM, Jayachandran J, et al. Prostate biopsies from black men express higher levels of aggressive disease biomarkers than prostate biopsies from white men. Prostate Cancer Prostatic Dis 2011; 14:262–265.
- Duffy MJ. Prostate-specific antigen: does the current evidence support its use in prostate cancer screening? Ann Clin Biochem 2011; 48:310–316.
- Andriole GL, Crawford ED, Grubb RL, et al; PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360:1310–1319.
- Crawford ED, Grubb R, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol 2011; 29:355–361.
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360:1320–1328.
- Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010; 11:725–732.
- Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate 2011; 71:985–997.
- Boyd MD, Weinrich SP, Weinrich M, Norton A. Obstacles to prostate cancer screening in African-American men. J Natl Black Nurses Assoc 2001; 12:1–5.
- Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate 2005; 62:243–252.
- Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 2008; 17:636–644.
- Hosain GM, Sanderson M, Du XL, Chan W, Strom SS. Racial/ethnic differences in predictors of PSA screening in a tri-ethnic population. Cent Eur J Public Health 2011; 19:30–34.
- Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control 2010; 21:1071–1080.
- Betancourt JR, Maina AW. The Institute of Medicine report “Unequal Treatment”: implications for academic health centers. Mt Sinai J Med 2004; 71:314–321.
- Patel K, Kenerson D, Wang H, et al. Factors influencing prostate cancer screening in low-income African Americans in Tennessee. J Health Care Poor Underserved 2010; 21(suppl 1):114–126.
- Modlin CS. Culture, race, and disparities in health care. Cleve Clin J Med 2003; 70:283–288.
- Kyle C, Ewing T, Wu XC, et al. Statewide analysis of serum prostate specific antigen levels in Louisiana men without prostate cancer. J La State Med Soc 2004; 156:319–323.
- Vijayakumar S, Winter K, Sause W, et al. Prostate-specific antigen levels are higher in African-American than in white patients in a multicenter registration study: results of RTOG 94-12. Int J Radiat Oncol Biol Phys 1998; 40:17–25.
- Chang BL, Spangler E, Gallagher S, et al. Validation of genome-wide prostate cancer associations in men of African descent. Cancer Epidemiol Biomarkers Prev 2011; 20:23–32.
- National Comprehensive Cancer Network (NCCN). NCCN Stresses Importance of PSA Testing in High-Risk Men. http://www.nccn.org/about/news/newsinfo.asp?NewsID=218. Accessed April 2, 2012.
- National Cancer Institute. Prostate-Specific Antigen (PSA) Test. http://www.cancer.gov/cancertopics/factsheet/detection/PSA. Accessed April 2, 2012.
- Duggan D, Zheng SL, Knowlton M, et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. Natl Cancer Inst 2007; 99:1836–1844.
- Grossfeld GD, Latini DM, Downs T, Lubeck DP, Mehta SS, Carroll PR. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol 2002; 168:2510–2515.
- Hoffman RM, Harlan LC, Klabunde CN, et al. Racial differences in initial treatment for clinically localized prostate cancer. Results from the prostate cancer outcomes study. J Gen Intern Med 2003; 18:845–853.
- Polednak AP. Prostate cancer treatment in black and white men: the need to consider both stage at diagnosis and socioeconomic status. J Natl Med Assoc 1998; 90:101–104.
- Merrill RM, Lyon JL. Explaining the difference in prostate cancer mortality rates between white and black men in the United States. Urology 2000; 55:730–735.
- Tewari AK, Gold HT, Demers RY, et al. Effect of socioeconomic factors on long-term mortality in men with clinically localized prostate cancer. Urology 2009; 73:624–630.
- White A, Coker AL, Du XL, Eggleston KS, Williams M. Racial/ethnic disparities in survival among men diagnosed with prostate cancer in Texas. Cancer 2011; 117:1080–1088.
- Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol 2010; 28:1069–1074.
- Demers RY, Tiwari A, Wei J, Weiss LK, Severson RK, Montie J. Trends in the utilization of androgen-deprivation therapy for patients with prostate carcinoma suggest an effect on mortality. Cancer 2001; 92:2309–2317.
- Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci 2006; 11:1388–1413.
- Schwartz K, Powell IJ, Underwood W, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 2009; 74:1296–1302.
- Hayn MH, Orom H, Shavers VL, et al. Racial/ethnic differences in receipt of pelvic lymph node dissection among men with localized/regional prostate cancer. Cancer 2011. [Epub ahead of print]
- Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979–2003. Cancer 2011; 117:3242–3251.
- Winkfield KM, Chen MH, Dosoretz DE, et al. Race and survival following brachytherapy-based treatment for men with localized or locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 20115; 81:e345–e350.
- Fowler JE, Terrell F. Survival in blacks and whites after treatment for localized prostate cancer. J Urol 1996; 156:133–136.
- Tewari A, Horninger W, Badani KK, et al. Racial differences in serum prostate-specific antigen (PSA) doubling time, histopathological variables and long-term PSA recurrence between African-American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int 2005; 96:29–33.
- Bozeman C, Williams BJ, Whatley T, Crow A, Eastham J. Clinical and biopsy specimen features in black and white men with clinically localized prostate cancer. South Med J 2000; 93:400–402.
- Delongchamps NB, Singh A, Haas GP. Epidemiology of prostate cancer in Africa: another step in the understanding of the disease? Curr Probl Cancer 2007; 31:226–236.
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int 2002; 90:162–173.
- Muller DC, Severi G, Baglietto L, et al. Dietary patterns and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2009; 18:3126–3129.
- Alexander DD, Mink PJ, Cushing CA, Sceurman B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr J 2010; 9:50.
- Gonzalez CA, Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer 2010; 46:2555–2562.
- Rodriguez C, McCullough ML, Mondul AM, et al. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol Biomarkers Prev 2003; 12:597–603.
- McCarty MF. Mortality from Western cancers rose dramatically among African-Americans during the 20th century: are dietary animal products to blame? Med Hypotheses 2001; 57:169–174.
- Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2007; 16:63–69.
- Spangler E, Zeigler-Johnson CM, Coomes M, Malkowicz SB, Wein A, Rebbeck TR. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol 2007; 178:1939–1944.
- Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009; 301:39–51.
- Oakley-Girvan I, Feldman D, Eccleshall TR, et al. Risk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptor. Cancer Epidemiol Biomarkers Prev 2004; 13:1325–1330.
- Gilbert R, Martin RM, Beynon R, et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control 2011; 22:319–340.
- Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 2011; 128:1414–1424.
- Yin L, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer Epidemiol 2009; 33:435–445.
- McCormick DL, Johnson WD, Bosland MC, Lubet RA, Steele VE. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr Cancer 2007; 57:184–193.
- Belpomme D, Irigaray P, Ossondo M, Vacque D, Martin M. Prostate cancer as an environmental disease: an ecological study in the French Caribbean islands, Martinique and Guadeloupe. Int J Oncol 2009; 34:1037–1044.
- Ramis R, Diggle P, Cambra K, López-Abente G. Prostate cancer and industrial pollution. Risk around putative focus in a multi-source scenario. Environ Int 2011; 37:577–585.
- Dey S, Zhang Z, Hablas A, et al. Geographic patterns of cancer in the population-based registry of Egypt: possible links to environmental exposures. Cancer Epidemiol 2011; 35:254–264.
- Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol Biomarkers Prev 2009; 18:2427–2435.
- Orsini N, Bellocco R, Bottai M, et al. A prospective study of lifetime physical activity and prostate cancer incidence and mortality. Br J Cancer 2009; 101:1932–1938.
KEY POINTS
- African American men have the dual disadvantages of being less likely to receive adequate care and also, possibly, of having biological differences that make them more prone to prostate cancer and more-aggressive cancer.
- Prostate-specific antigen (PSA) cutoff levels have not been officially modified according to race, but we believe primary care physicians should have a lower threshold for referring African American men who have a suspiciously high PSA level for further urologic evaluation.
- A healthy lifestyle, with a low-fat diet, healthy body mass index, and daily exercise, may decrease the risk of prostate cancer, among other benefits.
- Primary care physicians, who are often the gatekeepers to care, play a key role in educating and screening their patients.
Asthma in African Americans: What can we do about the higher rates of disease?
The last several decades have seen a dramatic surge in the prevalence of asthma. In 2009, there were an estimated 17.5 million adults and almost 7.1 million children with asthma in the United States,1 up from 9.5 million adults and slightly more than 5 million children in 1995.2
Multiple factors contribute to these disparities, including genetics, socioeconomic factors, cultural factors, health maintenance behaviors, provider-patient communication, air quality, and obesity.
GENETICS: 70% OF DESTINY?
The trend towards personalized medicine has spurred extensive research into the genetics of asthma. Studies in twins and familial aggregation studies suggest genetics plays a significant role, with estimates of the heritability of asthma as high as 70%.4,5 More than 100 candidate genes have been shown to be associated with asthma and atopy, 30 of them in five or more independent studies.6
Researchers face many challenges when investigating the genetics involved in asthma for a particular race. Race is both a biologic and a social construct and, as such, is a poor substitute for genetics. Race constitutes not only genetic differences in individuals, but also the behaviors, beliefs, and experiences that vary among races.
The clinical disease—the phenotype—is the product of the interaction of genes and these differing behaviors and exposures. Genetics can affect how environmental factors found in association with socioeconomic factors relate to asthma morbidity and mortality.
For example, as we will discuss below, African Americans are more likely than whites to be sensitized to cockroach allergen, even after controlling for socioeconomic variables that may be associated with greater exposure.7 High-level exposure to cockroach allergen in sensitized children has been associated with poor asthma outcomes.8 This suggests that a genetic difference may exist between African Americans and whites with respect to the potential to develop cockroach sensitization, and this difference may be of particular importance for those African Americans living in areas with higher levels of cockroach exposure.
Two polymorphisms
Two polymorphisms have garnered attention for their influence on African Americans with asthma:
TheADRB2gene. This gene codes for the beta-2 adrenergic receptor and resides at chromosome 5q13.9 The receptor is found on several types of cells in the lung, including airway smooth muscle and epithelial cells, and is responsible for the salutary effects of inhaled beta-2 agonists such as albuterol (eg, Proventil).
Allelic polymorphisms of this gene are clinically relevant. The substitution of arginine (Arg) for glycine (Gly) at codon 16 of this gene is responsible for differences in response to short-acting beta-2 agonists. The allelic frequency of Arg16 is lower in white Americans (39.3%) than in African Americans (49.2%), and thus African Americans are more likely to be homozygous for Arg16 (ie, to have the Arg/Arg genotype).10
People who are homozygous for Arg16 who use albuterol on a regular basis are at higher risk of untoward asthma outcomes.11 This is important, for several reasons. In general, adherence to inhaled corticosteroids is poor (not only in African Americans),12 and patients who do not take their inhaled corticosteroids as they should may rely on short-acting beta-2 agonists more frequently. Furthermore, African Americans may have a poorer response to the repeated doses of albuterol that are typically given in the emergency department and in the hospital for severe asthma exacerbations.13 Additionally, data suggest that Arg/Arg individuals have more frequent exacerbations independent of beta-agonist use,14 although curiously, patients who are homozygous for Arg16 have a greater benefit from single doses of short-acting beta-2 agonists than those who are Gly16 homozygous.15
TheCD14gene. An interesting relationship between innate immunity and asthma has recently been described. Polymorphisms of CD14, which codes for a receptor for endotoxin, have been uncovered. The single-nucleotide polymorphism variant thymine (T) at position −260 has been found in greater frequency in whites than in African Americans, who are more likely to have the cytosine (C) allele.16 An association between the CC genotype and atopy has been reported,16 although this has not been consistent.17
A possible explanation for these inconsistencies may lie in complex gene-environment interactions. The amount of endotoxin exposure may play a role in phenotypic expression. Individuals with the CC genotype were at lower risk of developing atopy when exposed to high levels of endotoxin; however, when exposed to lower levels of endotoxin, the CC genotype was associated with a higher risk of atopy.18 Nonfarm homes in westernized countries tend to have lower levels of endotoxin than farm homes, even in low-income urban areas.19 This implies that individuals with the CC allele, who are more likely to be African American, would be at greater risk for atopy in the United States. Greater knowledge of these types of gene-environment interactions may lead to improved understanding of the observations that have generated controversy concerning the “hygiene hypothesis.”
The details of how microbial exposure can influence the human immune response to antigen exposure are still being elucidated.20
These examples highlight not only the importance of genetics in the development of asthma, but also the role genes play in variation of treatment response and subsequent risk of morbidity and death. An understanding of these genetic differences among patients is clearly important for moving towards personalized treatment strategies for asthma.
Ancestry-informative markers
A developing strategy to assess the differences in asthma prevalence, severity, and response to treatment between racial groups is the use of ancestry-informative markers (AIMs).
AIMs are single-nucleotide polymorphisms that occur in varying allelic frequencies between ancestral groups, eg, continental Africans or European whites.21 AIMs provide an estimate of an individual’s proportion of ancestry—ie, of how “African” an African American is genetically.
African ancestry, determined using AIMs, was found to be associated with asthma in people living on the Caribbean coast of Colombia.22 However, one study found that AIMs could not predict an individual’s response to inhaled corticosteroids.23
Further research is necessary to find a technique to determine how groups of individuals can be characterized more precisely and managed more appropriately.
SOCIOECONOMIC FACTORS
African Americans living in low-income urban areas have an even greater prevalence of asthma and a greater risk of asthma-related morbidity and death than African Americans overall.3,24,25 Urban areas typically have a high proportion of residents living at or below the poverty level, and minorities often constitute a substantial proportion of the population in these areas. Evidence suggests that both African American race and lower socioeconomic status are independent risk factors for asthma prevalence, morbidity, and death.3,25
To provide better care for African Americans living in low-income urban areas, it is important to understand the factors that may be contributing to the higher morbidity and mortality rates in low-income urban areas.
Inadequate follow-up
Proper and routine follow-up for evaluation of asthma symptoms is essential for appropriate management. The Expert Panel Report 3 (EPR-3) of the National Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma,26 published in 2007, recommends that patients be seen at least every 6 months if they have been experiencing good control. While gaining control, patients should be seen every 2 to 6 weeks.26
Despite these recommendations, numerous studies have suggested that African Americans do not receive adequate follow-up. Children who are poor, are nonwhite or Hispanic, or are underinsured are more likely to lack routine health care27 and, more specifically, routine asthma care.28 Low-income patients are also more likely to receive care in a large hospital-run clinic or neighborhood clinic,27,28 where continuity of care may be less than ideal.29 Even among patients enrolled in Medicaid or Medicare, African American children with a primary care provider have fewer asthma visits compared with white Medicaid-insured children.30
Insufficient follow-up care contributes to greater asthma morbidity, resulting in, for example, more emergency department visits for asthma in African Americans.27,31,32
Suboptimal care
Data also suggest that the quality of care that residents of low-income urban areas receive is often suboptimal. Many people living in low-income urban areas are not provided with the knowledge and tools to treat asthma exacerbations at home.33 African Americans are also less likely to be seen by an asthma specialist31,34 as recommended for those with moderate or severe asthma.26
The EPR-3 guidelines also stress the importance of inhaled corticosteroids as the preferred therapy for all patients with persistent asthma. Even after controlling for the number of primary care visits, insurance status, and disease severity, African Americans are less likely to receive a prescription for inhaled corticosteroids, or they receive the same dosage of inhaled corticosteroids in the face of more severe disease.31,33,35,36
The reasons for these differences in treatment are not fully understood but are likely multiple. Lack of access to an asthma specialist and financial or formulary constraints are some of the potential barriers to optimal asthma care outcomes.
Misdiagnosis in the acute setting may also be a source of less-than-ideal care, as patients seen in emergency departments may be misdiagnosed with viral infection or bronchitis.
African Americans may report different symptoms than whites
Intriguing studies suggest that African Americans report different symptoms while describing asthma exacerbations.
In one study, compared with whites, African Americans were less likely to report nocturnal symptoms, dyspnea, or chest pain during exacerbations.37 In another study, when given a methacholine challenge that induced a significant drop in forced expiratory volume in 1 second (FEV1), African Americans with asthma were more likely to complain of upper airway symptoms as opposed to lower airway symptoms, compared with white patients.38
The symptoms that African Americans describe, such as having a tight throat or voice, are not typically regarded as related to asthma; for this reason, such descriptions may be an obstacle to correct diagnosis, management, and follow-up.
Asthma care providers should be aware of these observations to ensure that their patients are managed appropriately.
Lack of social support
Living in a low-income urban area presents many challenges that can interfere with proper asthma control.
Asthma diagnosis, management, and morbidity are affected by family support.39 Patients with asthma who lack sufficient financial support for treatment, who lack adequate psychological support, and who have more major life stressors are at higher risk of untoward outcomes. Disruption and dysfunction of the family and the supports available have been associated with greater asthma morbidity.40–42 Unfortunately, these types of stressors are all too common in families living in low-income urban areas.43–45
Multiple stressors that can occur more often in low-income urban areas, including exposure to violent crime, have also been linked to greater asthma morbidity.45–47
POOR PHYSICIAN-PATIENT COMMUNICATION
A consistent theme in focus groups of African Americans living in inner-city areas is the perception that health care providers are not effectively communicating and taking the time to listen to their concerns.48,49 Respondents believed they had better insight into their illness than their providers, and for this reason were better able to manage their disease.
The importance of an optimal provider-patient relationship was highlighted by a prospective cohort study in which Medicaid children receiving care at physician’s offices with the highest cultural competency scores were more adherent with their asthma controller medications.50
MEDICATION ADHERENCE RATES ARE DISTURBINGLY LOW
Rates of medication adherence for chronic diseases is disturbingly low, and may be even worse for pulmonary diseases.51 Reported rates of adherence to asthma medications among all patients range from 50% to 60%.52,53 Several studies showed that African Americans have a lower rate of adherence than do whites,53–55 even after adjusting for multiple socioeconomic variables.56
Many explanations have been proposed for this discrepancy, and all likely play a role in particular environments. For example, the incidence of crime in the surrounding area was inversely related to medication adherence after adjusting for socioeconomic factors.57 African Americans may have more concern about side effects associated with inhaled corticosteroid use and may be less likely to understand how these drugs work.52,53 A poor provider-patient relationship has also been cited as a barrier to adherence.55,57 Finally, physicians are more likely to underestimate asthma severity in an African American patient than in a white patient.58
Taking the time to ensure that patients truly understand all aspects of their disease and establishing a health care environment that is culturally appropriate may have a significant impact in patients with asthma.
ENVIRONMENTAL EXPOSURES
Air quality contributes to the greater asthma morbidity observed in urban residents, including African Americans. While poor outdoor air quality has not been clearly linked to a higher incidence of asthma, it has been associated with greater asthma morbidity. Poor air quality may affect individuals of all races, but with respect to ambient pollutants such as particulate matter and diesel exhaust, outdoor air quality is worse in urban environments where greater proportions of people of low socioeconomic status reside.59,60
The most extensively studied components of air pollution are ozone, sulfur dioxide, and particulate matter. These pollutants have been associated with a higher rate of emergency department visits,61,62 worse asthma symptoms,63,64 and higher exhaled nitric oxide levels.65
Tobacco smoke
Despite the substantial success of smoking cessation efforts nationwide, exposure to tobacco smoke continues to be common and is a significant risk factor for poor asthma control. Recent data suggest that African Americans and whites have a similar prevalence of smoking,66 but a study found a very high prevalence in low-income African Americans.67
Active smoking has been associated with worse asthma control and a higher risk of death.68 People with asthma who smoke are less likely to improve in their lung function and symptom scores when treated with short courses of oral glucocorticoids compared with both nonsmokers and former smokers.69
Secondhand smoke hurts too. Many children living in low-income urban areas are exposed to secondhand smoke or environmental tobacco smoke.70,71 Passive exposure in children has been associated with worse asthma outcomes, and data suggest such exposure may be a cause of asthma.68,72–74
Environmental tobacco smoke has also been implicated in gene-environment interactions. Patients who are either homozygous or heterozygous for the Arg allele at codon 16 of the ADRB2 gene (discussed above) had significantly lower FEV1 and forced vital capacity (FVC) values when exposed to passive tobacco smoke. This difference was not seen in people who were not exposed.75
Cockroach allergen
The type and condition of a person’s housing also plays a role in asthma-related morbidity and death. Across several socioeconomic levels, it has been suggested that African Americans have poorer-quality housing compared with whites.76 Some of the conditions found in low-quality houses, such as interruptions in heat, plumbing leaks, and the presence of rodents, have been associated with a higher prevalence of asthma in the household.77
Cockroach allergen exposure and sensitization is a major contributor to asthma morbidity in African Americans, particularly those living in poorer urban areas where cockroach allergen may be the most common indoor allergen.8 Living in older housing in urban areas is associated with higher exposure to cockroach allergen, and with subsequent sensitization.78,79 Exposure to high levels of the major cockroach allergen, Bla g 1, in sensitized individuals has been linked to a greater risk of hospitalization and unscheduled medical visits for asthma. This was not found to be the case for other common indoor allergens, such as dust mite and cat dander.8
However, it is not only exposure to high cockroach allergen levels that puts African Americans at risk. African Americans living in low-income urban areas may also be more likely than whites living in low-income urban areas to become sensitized to cockroach allergen.7,80 This suggests a gene-environment interaction that may be unique to African Americans. Moreover, cockroach sensitization may occur early in life.81,82
While successful cockroach avoidance measures and environmental control may be challenging, such measures have been shown to decrease rates of asthma morbidity.83
OBESITY
Obesity has been linked to an ever-growing list of diseases, one of which is asthma. Obesity is not a unique challenge for African Americans, but recent data from the US Centers for Disease Control and Prevention show that African Americans have a 51% higher prevalence of obesity compared with whites.84
Obesity is a risk factor for greater asthma morbidity and is a significant challenge in the African American community. The rise in obesity rates has paralleled the rise in asthma in recent decades. The higher one’s body mass index, the higher one’s risk of asthma.85 This association appears to be stronger in people without concurrent atopic disease.86 Obesity has also been associated with a poorer response to inhaled corticosteroids and a higher risk of asthma exacerbations.87 Interestingly, significant weight loss has been associated with improvements in both asthma control and lung function.88,89
What is the mechanism?
The underlying pathogenic mechanisms have not been completely elucidated, and they are likely multiple.
Adipokines (cytokines secreted by adipocytes) have been implicated. Two of the most extensively studied adipokines are leptin and adiponectin. Leptin production is increased in obesity, and it has inflammatory effects on both the innate and adaptive immune systems.90 The opposite is true for adiponectin, which may have anti-inflammatory properties and which decreases as the body mass index increases.90 The precise role these molecules may have in lung disease is undergoing further investigation.
Mechanical alterations in lung function may also contribute. Obese people have a lower functional residual capacity and expiratory reserve volume. Breathing with a lower-volume functional residual capacity results in decreased airway diameter and contributes to increased airway resistance.90 The decreased airway diameter may alter the contractile properties of airway smooth muscle and lead to increased airway responsiveness.90 These differences are in addition to the lower mean values of common spirometry indices such as the FEV1 and FVC, found in nonasthmatic African Americans compared with whites.91
Data suggest these differences are primarily due to anthropometric factors, with nutritional and environmental factors playing a less significant role.92 On this basis, the American Thoracic Society recommends applying race-specific reference standards for use with spirometry in order to accurately gauge lung function in African Americans.
APPROPRIATE CARE AND EDUCATION
The cause of greater asthma prevalence and severity among African Americans is multifactorial. It is likely that a number of factors work together, rather than separately, in influencing the development of asthma and its course.
Some risk factors are avoidable, and it is important to identify and ameliorate them. Others are not preventable, but knowledge of them may provide more specific management strategies and may lead to new therapies in the future.
While more work is needed to further unravel the complex risk factors associated with asthma, ensuring that higher-risk patients are provided the appropriate care and the knowledge to help control their disease is a necessary step in improving the disparities in asthma care outcomes.
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 2011; 32:1–14.
- National Institutes of Health. National Heart, Lung, and Blood Institute. Data Fact Sheet. Asthma statistics. January 1999. http://www.nhlbi.nih.gov/health/prof/lung/asthma/asthstat.pdf. Accessed February 1, 2012.
- Lang DM, Polansky M, Sherman MS. Hospitalizations for asthma in an urban population: 1995–1999. Ann Allergy Asthma Immunol 2009; 103:128–133.
- Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis 1990; 142:1351–1358.
- Koeppen-Schomerus G, Stevenson J, Plomin R. Genes and environment in asthma: a study of 4 year old twins. Arch Dis Child 2001; 85:398–400.
- Meng JF, Rosenwasser LJ. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol Res 2010; 2:215–227.
- Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol 2001; 108:747–752.
- Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997; 336:1356–1363.
- Kobilka BK, Dixon RA, Frielle T, et al. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A 1987; 84:46–50.
- Maxwell TJ, Ameyaw MM, Pritchard S, et al. Beta-2 adrenergic receptor genotypes and haplotypes in different ethnic groups. Int J Mol Med 2005; 16:573–580.
- Israel E, Chinchilli VM, Ford JG, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004; 364:1505–1512.
- Wells K, Pladevall M, Peterson EL, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med 2008; 178:1194–1201.
- Carroll CL, Stoltz P, Schramm CM, Zucker AR. Beta2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations. Chest 2009; 135:1186–1192.
- Bleecker ER, Nelson HS, Kraft M, et al. Beta2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionate. Am J Respir Crit Care Med 2010; 181:676–687.
- Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta-agonists in children with asthma: a meta-analysis. J Asthma 2009; 46:900–905.
- Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A polymorphism in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol 1999; 20:976–983.
- Kedda MA, Lose F, Duffy D, Bell E, Thompson PJ, Upham J. The CD14 C-159T polymorphism is not associated with asthma or asthma severity in an Australian adult population. Thorax 2005; 60:211–214.
- Zambelli-Weiner A, Ehrlich E, Stockton ML, et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol 2005; 115:1203–1209.
- Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006; 117:1082–1089.
- Williams LK, Oliver J, Peterson EL, et al. Gene-environment interactions between CD14 C-260T and endotoxin exposure on Foxp3+ and Foxp3− CD4+ lymphocyte numbers and total serum IgE levels in early childhood. Ann Allergy Asthma Immunol 2008; 100:128–136.
- Barnes KC. Ancestry, ancestry-informative markers, asthma, and the quest for personalized medicine. J Allergy Clin Immunol 2010; 126:1139–1140.
- Vergara C, Caraballo L, Mercado D, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet 2009; 125:565–579.
- Gould W, Peterson EL, Karungi G, et al. Factors predicting inhaled corticosteroid responsiveness in African American patients with asthma. J Allergy Clin Immunol 2010; 126:1131–1138.
- Lang DM, Polansky M. Patterns of asthma mortality in Philadelphia from 1969 to 1991. N Engl J Med 1994; 331:1542–1546.
- Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis 1990; 142:555–562.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Newacheck PW, Hughes DC, Stoddard JJ. Children’s access to primary care: differences by race, income, and insurance status. Pediatrics 1996; 97:26–32.
- Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics 1993; 91:56–61.
- Burt CW, Knapp DE. Ambulatory care visits for asthma: United States, 1993–94. Adv Data 1996; 277:1.
- Lieu TA, Lozano P, Finkelstein JA, et al. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002; 109:857–865.
- Zoratti EM, Havstad S, Rodriguez J, Robens-Paradise Y, Lafata JE, McCarthy B. Health service use by African Americans and Caucasians with asthma in a managed care setting. Am J Respir Crit Care Med 1998; 158:371–377.
- Wisnivesky JP, Leventhal H, Halm EA. Predictors of asthma-related health care utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol 2005; 116:636–642.
- Halm EA, Wisnivesky JP, Leventhal H. Quality and access to care among a cohort of inner-city adults with asthma: who gets guideline concordant care? Chest 2005; 128:1943–1950.
- Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, Wu AW. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med 2001; 161:1660–1668.
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94:666–668.
- Boudreaux ED, Emond SD, Clark S, Camargo CA; Multicenter Airway Research Collaboration Investigators. Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics 2003; 111:e615–e621.
- Trochtenberg DS, BeLue R, Piphus S, Washington N. Differing reports of asthma symptoms in African Americans and Caucasians. J Asthma 2008; 45:165–170.
- Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest 2000; 117:935–943.
- Clark NM, Levison MJ, Evans D, Wasilewski Y, Feldman CH, Mellins RB. Communication within low income families and the management of asthma. Patient Educ Couns 1990; 15:191–210.
- Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes in adolescents: barriers to adherence as a mediator. J Adolesc Health 2010; 47:472–478.
- Quinn K, Kaufman JS, Siddiqi A, Yeatts KB. Parent perceptions of neighborhood stressors are associated with general health and child respiratory health among low-income, urban families. J Asthma 2010; 47:281–289.
- Loerbroks A, Apfelbacher CJ, Bosch JA, Stürmer T. Depressive symptoms, social support, and risk of adult asthma in a population-based cohort study. Psychosom Med 2010; 72:309–315.
- Wood DL, Valdez RB, Hayashi T, Shen A. Health of homeless children and housed, poor children. Pediatrics 1990; 86:858–866.
- O’Donnell L, Stueve A, Myint-U A. Parenting and violence toward self, partners, and others among inner-city young adults. Am J Public Health 2009; 99:2255–2260.
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 2000; 126:309–337.
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A 2009; 106:12406–12411.
- Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health 2004; 94:625–632.
- George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research-enhanced understanding of patients’ beliefs: results of focus groups with low-income, urban, African American adults with asthma. J Allergy Clin Immunol 2003; 111:967–973.
- Laster N, Holsey CN, Shendell DG, Mccarty FA, Celano M. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma 2009; 46:731–739.
- Lieu TA, Finkelstein JA, Lozano P, et al. Cultural competence policies and other predictors of asthma care quality for Medicaid-insured children. Pediatrics 2004; 114:e102–e110.
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004; 42:200–209.
- Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol 2000; 85:416–421.
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol 2004; 114:1288–1293.
- Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol 2003; 111:1219–1226.
- Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med 1998; 157:1810–1817.
- Williams LK, Joseph CL, Peterson EL, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol 2007; 119:168–175.
- O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med 2004; 38:777–785.
- Okelo SO, Wu AW, Merriman B, Krishnan JA, Diette GB. Are physician estimates of asthma severity less accurate in black than in white patients? J Gen Intern Med 2007; 22:976–981.
- Kinney PL, Aggarwal M, Northridge ME, Janssen NA, Shepard P. Airborne concentrations of PM(2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect 2000; 108:213–218.
- O’Neill MS, Jerrett M, Kawachi I, et al; Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect 2003; 111:1861–1870.
- Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 1993; 147:826–831.
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 1999; 107:489–493.
- Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ Health Perspect 2000; 108:1209–1214.
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann Allergy Asthma Immunol 2003; 91:346–353.
- Koenig JQ, Jansen K, Mar TF, et al. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ Health Perspect 2003; 111:1625–1629.
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1227–1232.
- Delva J, Tellez M, Finlayson TL, et al. Cigarette smoking among low-income African Americans: a serious public health problem. Am J Prev Med 2005; 29:218–220.
- McLeish AC, Zvolensky MJ. Asthma and cigarette smoking: a review of the empirical literature. J Asthma 2010; 47:345–361.
- Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med 2003; 168:1308–1311.
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect 2005; 113:362–367.
- Huss K, Rand CS, Butz AM, et al. Home environmental risk factors in urban minority asthmatic children. Ann Allergy 1994; 72:173–177.
- Samir S, Colin Y, Thomas S. Impact of environmental tobacco smoke on children admitted with status asthmaticus in the pediatric intensive care unit. Pediatr Pulmonol 2010. [Epub ahead of print]
- Lannerö E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res 2006; 7:3.
- Hedman L, Bjerg A, Sundberg S, Forsberg B, Rönmark E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax 2011; 66:20–25.
- Zhang G, Hayden CM, Khoo SK, et al. Beta2-adrenoceptor polymorphisms and asthma phenotypes: interactions with passive smoking. Eur Respir J 2007; 30:48–55.
- Rosenbaum E, Friedman S. The Housing Divide: How Generations of Immigrants Fare in New York’s Housing Market. New York, NY: New York University Press; 2007.
- Rosenbaum E. Racial/ethnic differences in asthma prevalence: the role of housing and neighborhood environments. J Health Soc Behav 2008; 49:131–145.
- Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect 2002; 110( suppl 2):323–327.
- Eggleston PA, Rosenstreich D, Lynn H, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol 1998; 102:563–570.
- Togias A, Horowitz E, Joyner D, Guydon L, Malveaux F. Evaluating the factors that relate to asthma severity in adolescents. Int Arch Allergy Immunol 1997; 113:87–95.
- Alp H, Yu BH, Grant EN, Rao V, Moy JN. Cockroach allergy appears early in life in inner-city children with recurrent wheezing. Ann Allergy Asthma Immunol 2001; 86:51–54.
- Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med 2001; 164:995–1001.
- Morgan WJ, Crain EF, Gruchalla RS, et al; Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004; 351:1068–1080.
- Centers for Disease Control and Prevention (CDC). Overweight and Obesity. US Obesity Trends. http://templatelab.com/us-obesity-trends/. Accessed February 1, 2012.
- Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175:661–666.
- Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010; 47:822–829.
- Camargo CA, Sutherland ER, Bailey W, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin 2010; 26:1629–1635.
- Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000; 118:1315–1321.
- Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000; 320:827–832.
- Dixon AE, Holguin F, Sood A, et al; American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010; 7:325–335.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999; 159:179–187.
- Harik-Khan RI, Muller DC, Wise RA. Racial difference in lung function in African-American and white children: effect of anthropometric, socioeconomic, nutritional, and environmental factors. Am J Epidemiol 2004; 160:893–900.
The last several decades have seen a dramatic surge in the prevalence of asthma. In 2009, there were an estimated 17.5 million adults and almost 7.1 million children with asthma in the United States,1 up from 9.5 million adults and slightly more than 5 million children in 1995.2
Multiple factors contribute to these disparities, including genetics, socioeconomic factors, cultural factors, health maintenance behaviors, provider-patient communication, air quality, and obesity.
GENETICS: 70% OF DESTINY?
The trend towards personalized medicine has spurred extensive research into the genetics of asthma. Studies in twins and familial aggregation studies suggest genetics plays a significant role, with estimates of the heritability of asthma as high as 70%.4,5 More than 100 candidate genes have been shown to be associated with asthma and atopy, 30 of them in five or more independent studies.6
Researchers face many challenges when investigating the genetics involved in asthma for a particular race. Race is both a biologic and a social construct and, as such, is a poor substitute for genetics. Race constitutes not only genetic differences in individuals, but also the behaviors, beliefs, and experiences that vary among races.
The clinical disease—the phenotype—is the product of the interaction of genes and these differing behaviors and exposures. Genetics can affect how environmental factors found in association with socioeconomic factors relate to asthma morbidity and mortality.
For example, as we will discuss below, African Americans are more likely than whites to be sensitized to cockroach allergen, even after controlling for socioeconomic variables that may be associated with greater exposure.7 High-level exposure to cockroach allergen in sensitized children has been associated with poor asthma outcomes.8 This suggests that a genetic difference may exist between African Americans and whites with respect to the potential to develop cockroach sensitization, and this difference may be of particular importance for those African Americans living in areas with higher levels of cockroach exposure.
Two polymorphisms
Two polymorphisms have garnered attention for their influence on African Americans with asthma:
TheADRB2gene. This gene codes for the beta-2 adrenergic receptor and resides at chromosome 5q13.9 The receptor is found on several types of cells in the lung, including airway smooth muscle and epithelial cells, and is responsible for the salutary effects of inhaled beta-2 agonists such as albuterol (eg, Proventil).
Allelic polymorphisms of this gene are clinically relevant. The substitution of arginine (Arg) for glycine (Gly) at codon 16 of this gene is responsible for differences in response to short-acting beta-2 agonists. The allelic frequency of Arg16 is lower in white Americans (39.3%) than in African Americans (49.2%), and thus African Americans are more likely to be homozygous for Arg16 (ie, to have the Arg/Arg genotype).10
People who are homozygous for Arg16 who use albuterol on a regular basis are at higher risk of untoward asthma outcomes.11 This is important, for several reasons. In general, adherence to inhaled corticosteroids is poor (not only in African Americans),12 and patients who do not take their inhaled corticosteroids as they should may rely on short-acting beta-2 agonists more frequently. Furthermore, African Americans may have a poorer response to the repeated doses of albuterol that are typically given in the emergency department and in the hospital for severe asthma exacerbations.13 Additionally, data suggest that Arg/Arg individuals have more frequent exacerbations independent of beta-agonist use,14 although curiously, patients who are homozygous for Arg16 have a greater benefit from single doses of short-acting beta-2 agonists than those who are Gly16 homozygous.15
TheCD14gene. An interesting relationship between innate immunity and asthma has recently been described. Polymorphisms of CD14, which codes for a receptor for endotoxin, have been uncovered. The single-nucleotide polymorphism variant thymine (T) at position −260 has been found in greater frequency in whites than in African Americans, who are more likely to have the cytosine (C) allele.16 An association between the CC genotype and atopy has been reported,16 although this has not been consistent.17
A possible explanation for these inconsistencies may lie in complex gene-environment interactions. The amount of endotoxin exposure may play a role in phenotypic expression. Individuals with the CC genotype were at lower risk of developing atopy when exposed to high levels of endotoxin; however, when exposed to lower levels of endotoxin, the CC genotype was associated with a higher risk of atopy.18 Nonfarm homes in westernized countries tend to have lower levels of endotoxin than farm homes, even in low-income urban areas.19 This implies that individuals with the CC allele, who are more likely to be African American, would be at greater risk for atopy in the United States. Greater knowledge of these types of gene-environment interactions may lead to improved understanding of the observations that have generated controversy concerning the “hygiene hypothesis.”
The details of how microbial exposure can influence the human immune response to antigen exposure are still being elucidated.20
These examples highlight not only the importance of genetics in the development of asthma, but also the role genes play in variation of treatment response and subsequent risk of morbidity and death. An understanding of these genetic differences among patients is clearly important for moving towards personalized treatment strategies for asthma.
Ancestry-informative markers
A developing strategy to assess the differences in asthma prevalence, severity, and response to treatment between racial groups is the use of ancestry-informative markers (AIMs).
AIMs are single-nucleotide polymorphisms that occur in varying allelic frequencies between ancestral groups, eg, continental Africans or European whites.21 AIMs provide an estimate of an individual’s proportion of ancestry—ie, of how “African” an African American is genetically.
African ancestry, determined using AIMs, was found to be associated with asthma in people living on the Caribbean coast of Colombia.22 However, one study found that AIMs could not predict an individual’s response to inhaled corticosteroids.23
Further research is necessary to find a technique to determine how groups of individuals can be characterized more precisely and managed more appropriately.
SOCIOECONOMIC FACTORS
African Americans living in low-income urban areas have an even greater prevalence of asthma and a greater risk of asthma-related morbidity and death than African Americans overall.3,24,25 Urban areas typically have a high proportion of residents living at or below the poverty level, and minorities often constitute a substantial proportion of the population in these areas. Evidence suggests that both African American race and lower socioeconomic status are independent risk factors for asthma prevalence, morbidity, and death.3,25
To provide better care for African Americans living in low-income urban areas, it is important to understand the factors that may be contributing to the higher morbidity and mortality rates in low-income urban areas.
Inadequate follow-up
Proper and routine follow-up for evaluation of asthma symptoms is essential for appropriate management. The Expert Panel Report 3 (EPR-3) of the National Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma,26 published in 2007, recommends that patients be seen at least every 6 months if they have been experiencing good control. While gaining control, patients should be seen every 2 to 6 weeks.26
Despite these recommendations, numerous studies have suggested that African Americans do not receive adequate follow-up. Children who are poor, are nonwhite or Hispanic, or are underinsured are more likely to lack routine health care27 and, more specifically, routine asthma care.28 Low-income patients are also more likely to receive care in a large hospital-run clinic or neighborhood clinic,27,28 where continuity of care may be less than ideal.29 Even among patients enrolled in Medicaid or Medicare, African American children with a primary care provider have fewer asthma visits compared with white Medicaid-insured children.30
Insufficient follow-up care contributes to greater asthma morbidity, resulting in, for example, more emergency department visits for asthma in African Americans.27,31,32
Suboptimal care
Data also suggest that the quality of care that residents of low-income urban areas receive is often suboptimal. Many people living in low-income urban areas are not provided with the knowledge and tools to treat asthma exacerbations at home.33 African Americans are also less likely to be seen by an asthma specialist31,34 as recommended for those with moderate or severe asthma.26
The EPR-3 guidelines also stress the importance of inhaled corticosteroids as the preferred therapy for all patients with persistent asthma. Even after controlling for the number of primary care visits, insurance status, and disease severity, African Americans are less likely to receive a prescription for inhaled corticosteroids, or they receive the same dosage of inhaled corticosteroids in the face of more severe disease.31,33,35,36
The reasons for these differences in treatment are not fully understood but are likely multiple. Lack of access to an asthma specialist and financial or formulary constraints are some of the potential barriers to optimal asthma care outcomes.
Misdiagnosis in the acute setting may also be a source of less-than-ideal care, as patients seen in emergency departments may be misdiagnosed with viral infection or bronchitis.
African Americans may report different symptoms than whites
Intriguing studies suggest that African Americans report different symptoms while describing asthma exacerbations.
In one study, compared with whites, African Americans were less likely to report nocturnal symptoms, dyspnea, or chest pain during exacerbations.37 In another study, when given a methacholine challenge that induced a significant drop in forced expiratory volume in 1 second (FEV1), African Americans with asthma were more likely to complain of upper airway symptoms as opposed to lower airway symptoms, compared with white patients.38
The symptoms that African Americans describe, such as having a tight throat or voice, are not typically regarded as related to asthma; for this reason, such descriptions may be an obstacle to correct diagnosis, management, and follow-up.
Asthma care providers should be aware of these observations to ensure that their patients are managed appropriately.
Lack of social support
Living in a low-income urban area presents many challenges that can interfere with proper asthma control.
Asthma diagnosis, management, and morbidity are affected by family support.39 Patients with asthma who lack sufficient financial support for treatment, who lack adequate psychological support, and who have more major life stressors are at higher risk of untoward outcomes. Disruption and dysfunction of the family and the supports available have been associated with greater asthma morbidity.40–42 Unfortunately, these types of stressors are all too common in families living in low-income urban areas.43–45
Multiple stressors that can occur more often in low-income urban areas, including exposure to violent crime, have also been linked to greater asthma morbidity.45–47
POOR PHYSICIAN-PATIENT COMMUNICATION
A consistent theme in focus groups of African Americans living in inner-city areas is the perception that health care providers are not effectively communicating and taking the time to listen to their concerns.48,49 Respondents believed they had better insight into their illness than their providers, and for this reason were better able to manage their disease.
The importance of an optimal provider-patient relationship was highlighted by a prospective cohort study in which Medicaid children receiving care at physician’s offices with the highest cultural competency scores were more adherent with their asthma controller medications.50
MEDICATION ADHERENCE RATES ARE DISTURBINGLY LOW
Rates of medication adherence for chronic diseases is disturbingly low, and may be even worse for pulmonary diseases.51 Reported rates of adherence to asthma medications among all patients range from 50% to 60%.52,53 Several studies showed that African Americans have a lower rate of adherence than do whites,53–55 even after adjusting for multiple socioeconomic variables.56
Many explanations have been proposed for this discrepancy, and all likely play a role in particular environments. For example, the incidence of crime in the surrounding area was inversely related to medication adherence after adjusting for socioeconomic factors.57 African Americans may have more concern about side effects associated with inhaled corticosteroid use and may be less likely to understand how these drugs work.52,53 A poor provider-patient relationship has also been cited as a barrier to adherence.55,57 Finally, physicians are more likely to underestimate asthma severity in an African American patient than in a white patient.58
Taking the time to ensure that patients truly understand all aspects of their disease and establishing a health care environment that is culturally appropriate may have a significant impact in patients with asthma.
ENVIRONMENTAL EXPOSURES
Air quality contributes to the greater asthma morbidity observed in urban residents, including African Americans. While poor outdoor air quality has not been clearly linked to a higher incidence of asthma, it has been associated with greater asthma morbidity. Poor air quality may affect individuals of all races, but with respect to ambient pollutants such as particulate matter and diesel exhaust, outdoor air quality is worse in urban environments where greater proportions of people of low socioeconomic status reside.59,60
The most extensively studied components of air pollution are ozone, sulfur dioxide, and particulate matter. These pollutants have been associated with a higher rate of emergency department visits,61,62 worse asthma symptoms,63,64 and higher exhaled nitric oxide levels.65
Tobacco smoke
Despite the substantial success of smoking cessation efforts nationwide, exposure to tobacco smoke continues to be common and is a significant risk factor for poor asthma control. Recent data suggest that African Americans and whites have a similar prevalence of smoking,66 but a study found a very high prevalence in low-income African Americans.67
Active smoking has been associated with worse asthma control and a higher risk of death.68 People with asthma who smoke are less likely to improve in their lung function and symptom scores when treated with short courses of oral glucocorticoids compared with both nonsmokers and former smokers.69
Secondhand smoke hurts too. Many children living in low-income urban areas are exposed to secondhand smoke or environmental tobacco smoke.70,71 Passive exposure in children has been associated with worse asthma outcomes, and data suggest such exposure may be a cause of asthma.68,72–74
Environmental tobacco smoke has also been implicated in gene-environment interactions. Patients who are either homozygous or heterozygous for the Arg allele at codon 16 of the ADRB2 gene (discussed above) had significantly lower FEV1 and forced vital capacity (FVC) values when exposed to passive tobacco smoke. This difference was not seen in people who were not exposed.75
Cockroach allergen
The type and condition of a person’s housing also plays a role in asthma-related morbidity and death. Across several socioeconomic levels, it has been suggested that African Americans have poorer-quality housing compared with whites.76 Some of the conditions found in low-quality houses, such as interruptions in heat, plumbing leaks, and the presence of rodents, have been associated with a higher prevalence of asthma in the household.77
Cockroach allergen exposure and sensitization is a major contributor to asthma morbidity in African Americans, particularly those living in poorer urban areas where cockroach allergen may be the most common indoor allergen.8 Living in older housing in urban areas is associated with higher exposure to cockroach allergen, and with subsequent sensitization.78,79 Exposure to high levels of the major cockroach allergen, Bla g 1, in sensitized individuals has been linked to a greater risk of hospitalization and unscheduled medical visits for asthma. This was not found to be the case for other common indoor allergens, such as dust mite and cat dander.8
However, it is not only exposure to high cockroach allergen levels that puts African Americans at risk. African Americans living in low-income urban areas may also be more likely than whites living in low-income urban areas to become sensitized to cockroach allergen.7,80 This suggests a gene-environment interaction that may be unique to African Americans. Moreover, cockroach sensitization may occur early in life.81,82
While successful cockroach avoidance measures and environmental control may be challenging, such measures have been shown to decrease rates of asthma morbidity.83
OBESITY
Obesity has been linked to an ever-growing list of diseases, one of which is asthma. Obesity is not a unique challenge for African Americans, but recent data from the US Centers for Disease Control and Prevention show that African Americans have a 51% higher prevalence of obesity compared with whites.84
Obesity is a risk factor for greater asthma morbidity and is a significant challenge in the African American community. The rise in obesity rates has paralleled the rise in asthma in recent decades. The higher one’s body mass index, the higher one’s risk of asthma.85 This association appears to be stronger in people without concurrent atopic disease.86 Obesity has also been associated with a poorer response to inhaled corticosteroids and a higher risk of asthma exacerbations.87 Interestingly, significant weight loss has been associated with improvements in both asthma control and lung function.88,89
What is the mechanism?
The underlying pathogenic mechanisms have not been completely elucidated, and they are likely multiple.
Adipokines (cytokines secreted by adipocytes) have been implicated. Two of the most extensively studied adipokines are leptin and adiponectin. Leptin production is increased in obesity, and it has inflammatory effects on both the innate and adaptive immune systems.90 The opposite is true for adiponectin, which may have anti-inflammatory properties and which decreases as the body mass index increases.90 The precise role these molecules may have in lung disease is undergoing further investigation.
Mechanical alterations in lung function may also contribute. Obese people have a lower functional residual capacity and expiratory reserve volume. Breathing with a lower-volume functional residual capacity results in decreased airway diameter and contributes to increased airway resistance.90 The decreased airway diameter may alter the contractile properties of airway smooth muscle and lead to increased airway responsiveness.90 These differences are in addition to the lower mean values of common spirometry indices such as the FEV1 and FVC, found in nonasthmatic African Americans compared with whites.91
Data suggest these differences are primarily due to anthropometric factors, with nutritional and environmental factors playing a less significant role.92 On this basis, the American Thoracic Society recommends applying race-specific reference standards for use with spirometry in order to accurately gauge lung function in African Americans.
APPROPRIATE CARE AND EDUCATION
The cause of greater asthma prevalence and severity among African Americans is multifactorial. It is likely that a number of factors work together, rather than separately, in influencing the development of asthma and its course.
Some risk factors are avoidable, and it is important to identify and ameliorate them. Others are not preventable, but knowledge of them may provide more specific management strategies and may lead to new therapies in the future.
While more work is needed to further unravel the complex risk factors associated with asthma, ensuring that higher-risk patients are provided the appropriate care and the knowledge to help control their disease is a necessary step in improving the disparities in asthma care outcomes.
The last several decades have seen a dramatic surge in the prevalence of asthma. In 2009, there were an estimated 17.5 million adults and almost 7.1 million children with asthma in the United States,1 up from 9.5 million adults and slightly more than 5 million children in 1995.2
Multiple factors contribute to these disparities, including genetics, socioeconomic factors, cultural factors, health maintenance behaviors, provider-patient communication, air quality, and obesity.
GENETICS: 70% OF DESTINY?
The trend towards personalized medicine has spurred extensive research into the genetics of asthma. Studies in twins and familial aggregation studies suggest genetics plays a significant role, with estimates of the heritability of asthma as high as 70%.4,5 More than 100 candidate genes have been shown to be associated with asthma and atopy, 30 of them in five or more independent studies.6
Researchers face many challenges when investigating the genetics involved in asthma for a particular race. Race is both a biologic and a social construct and, as such, is a poor substitute for genetics. Race constitutes not only genetic differences in individuals, but also the behaviors, beliefs, and experiences that vary among races.
The clinical disease—the phenotype—is the product of the interaction of genes and these differing behaviors and exposures. Genetics can affect how environmental factors found in association with socioeconomic factors relate to asthma morbidity and mortality.
For example, as we will discuss below, African Americans are more likely than whites to be sensitized to cockroach allergen, even after controlling for socioeconomic variables that may be associated with greater exposure.7 High-level exposure to cockroach allergen in sensitized children has been associated with poor asthma outcomes.8 This suggests that a genetic difference may exist between African Americans and whites with respect to the potential to develop cockroach sensitization, and this difference may be of particular importance for those African Americans living in areas with higher levels of cockroach exposure.
Two polymorphisms
Two polymorphisms have garnered attention for their influence on African Americans with asthma:
TheADRB2gene. This gene codes for the beta-2 adrenergic receptor and resides at chromosome 5q13.9 The receptor is found on several types of cells in the lung, including airway smooth muscle and epithelial cells, and is responsible for the salutary effects of inhaled beta-2 agonists such as albuterol (eg, Proventil).
Allelic polymorphisms of this gene are clinically relevant. The substitution of arginine (Arg) for glycine (Gly) at codon 16 of this gene is responsible for differences in response to short-acting beta-2 agonists. The allelic frequency of Arg16 is lower in white Americans (39.3%) than in African Americans (49.2%), and thus African Americans are more likely to be homozygous for Arg16 (ie, to have the Arg/Arg genotype).10
People who are homozygous for Arg16 who use albuterol on a regular basis are at higher risk of untoward asthma outcomes.11 This is important, for several reasons. In general, adherence to inhaled corticosteroids is poor (not only in African Americans),12 and patients who do not take their inhaled corticosteroids as they should may rely on short-acting beta-2 agonists more frequently. Furthermore, African Americans may have a poorer response to the repeated doses of albuterol that are typically given in the emergency department and in the hospital for severe asthma exacerbations.13 Additionally, data suggest that Arg/Arg individuals have more frequent exacerbations independent of beta-agonist use,14 although curiously, patients who are homozygous for Arg16 have a greater benefit from single doses of short-acting beta-2 agonists than those who are Gly16 homozygous.15
TheCD14gene. An interesting relationship between innate immunity and asthma has recently been described. Polymorphisms of CD14, which codes for a receptor for endotoxin, have been uncovered. The single-nucleotide polymorphism variant thymine (T) at position −260 has been found in greater frequency in whites than in African Americans, who are more likely to have the cytosine (C) allele.16 An association between the CC genotype and atopy has been reported,16 although this has not been consistent.17
A possible explanation for these inconsistencies may lie in complex gene-environment interactions. The amount of endotoxin exposure may play a role in phenotypic expression. Individuals with the CC genotype were at lower risk of developing atopy when exposed to high levels of endotoxin; however, when exposed to lower levels of endotoxin, the CC genotype was associated with a higher risk of atopy.18 Nonfarm homes in westernized countries tend to have lower levels of endotoxin than farm homes, even in low-income urban areas.19 This implies that individuals with the CC allele, who are more likely to be African American, would be at greater risk for atopy in the United States. Greater knowledge of these types of gene-environment interactions may lead to improved understanding of the observations that have generated controversy concerning the “hygiene hypothesis.”
The details of how microbial exposure can influence the human immune response to antigen exposure are still being elucidated.20
These examples highlight not only the importance of genetics in the development of asthma, but also the role genes play in variation of treatment response and subsequent risk of morbidity and death. An understanding of these genetic differences among patients is clearly important for moving towards personalized treatment strategies for asthma.
Ancestry-informative markers
A developing strategy to assess the differences in asthma prevalence, severity, and response to treatment between racial groups is the use of ancestry-informative markers (AIMs).
AIMs are single-nucleotide polymorphisms that occur in varying allelic frequencies between ancestral groups, eg, continental Africans or European whites.21 AIMs provide an estimate of an individual’s proportion of ancestry—ie, of how “African” an African American is genetically.
African ancestry, determined using AIMs, was found to be associated with asthma in people living on the Caribbean coast of Colombia.22 However, one study found that AIMs could not predict an individual’s response to inhaled corticosteroids.23
Further research is necessary to find a technique to determine how groups of individuals can be characterized more precisely and managed more appropriately.
SOCIOECONOMIC FACTORS
African Americans living in low-income urban areas have an even greater prevalence of asthma and a greater risk of asthma-related morbidity and death than African Americans overall.3,24,25 Urban areas typically have a high proportion of residents living at or below the poverty level, and minorities often constitute a substantial proportion of the population in these areas. Evidence suggests that both African American race and lower socioeconomic status are independent risk factors for asthma prevalence, morbidity, and death.3,25
To provide better care for African Americans living in low-income urban areas, it is important to understand the factors that may be contributing to the higher morbidity and mortality rates in low-income urban areas.
Inadequate follow-up
Proper and routine follow-up for evaluation of asthma symptoms is essential for appropriate management. The Expert Panel Report 3 (EPR-3) of the National Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma,26 published in 2007, recommends that patients be seen at least every 6 months if they have been experiencing good control. While gaining control, patients should be seen every 2 to 6 weeks.26
Despite these recommendations, numerous studies have suggested that African Americans do not receive adequate follow-up. Children who are poor, are nonwhite or Hispanic, or are underinsured are more likely to lack routine health care27 and, more specifically, routine asthma care.28 Low-income patients are also more likely to receive care in a large hospital-run clinic or neighborhood clinic,27,28 where continuity of care may be less than ideal.29 Even among patients enrolled in Medicaid or Medicare, African American children with a primary care provider have fewer asthma visits compared with white Medicaid-insured children.30
Insufficient follow-up care contributes to greater asthma morbidity, resulting in, for example, more emergency department visits for asthma in African Americans.27,31,32
Suboptimal care
Data also suggest that the quality of care that residents of low-income urban areas receive is often suboptimal. Many people living in low-income urban areas are not provided with the knowledge and tools to treat asthma exacerbations at home.33 African Americans are also less likely to be seen by an asthma specialist31,34 as recommended for those with moderate or severe asthma.26
The EPR-3 guidelines also stress the importance of inhaled corticosteroids as the preferred therapy for all patients with persistent asthma. Even after controlling for the number of primary care visits, insurance status, and disease severity, African Americans are less likely to receive a prescription for inhaled corticosteroids, or they receive the same dosage of inhaled corticosteroids in the face of more severe disease.31,33,35,36
The reasons for these differences in treatment are not fully understood but are likely multiple. Lack of access to an asthma specialist and financial or formulary constraints are some of the potential barriers to optimal asthma care outcomes.
Misdiagnosis in the acute setting may also be a source of less-than-ideal care, as patients seen in emergency departments may be misdiagnosed with viral infection or bronchitis.
African Americans may report different symptoms than whites
Intriguing studies suggest that African Americans report different symptoms while describing asthma exacerbations.
In one study, compared with whites, African Americans were less likely to report nocturnal symptoms, dyspnea, or chest pain during exacerbations.37 In another study, when given a methacholine challenge that induced a significant drop in forced expiratory volume in 1 second (FEV1), African Americans with asthma were more likely to complain of upper airway symptoms as opposed to lower airway symptoms, compared with white patients.38
The symptoms that African Americans describe, such as having a tight throat or voice, are not typically regarded as related to asthma; for this reason, such descriptions may be an obstacle to correct diagnosis, management, and follow-up.
Asthma care providers should be aware of these observations to ensure that their patients are managed appropriately.
Lack of social support
Living in a low-income urban area presents many challenges that can interfere with proper asthma control.
Asthma diagnosis, management, and morbidity are affected by family support.39 Patients with asthma who lack sufficient financial support for treatment, who lack adequate psychological support, and who have more major life stressors are at higher risk of untoward outcomes. Disruption and dysfunction of the family and the supports available have been associated with greater asthma morbidity.40–42 Unfortunately, these types of stressors are all too common in families living in low-income urban areas.43–45
Multiple stressors that can occur more often in low-income urban areas, including exposure to violent crime, have also been linked to greater asthma morbidity.45–47
POOR PHYSICIAN-PATIENT COMMUNICATION
A consistent theme in focus groups of African Americans living in inner-city areas is the perception that health care providers are not effectively communicating and taking the time to listen to their concerns.48,49 Respondents believed they had better insight into their illness than their providers, and for this reason were better able to manage their disease.
The importance of an optimal provider-patient relationship was highlighted by a prospective cohort study in which Medicaid children receiving care at physician’s offices with the highest cultural competency scores were more adherent with their asthma controller medications.50
MEDICATION ADHERENCE RATES ARE DISTURBINGLY LOW
Rates of medication adherence for chronic diseases is disturbingly low, and may be even worse for pulmonary diseases.51 Reported rates of adherence to asthma medications among all patients range from 50% to 60%.52,53 Several studies showed that African Americans have a lower rate of adherence than do whites,53–55 even after adjusting for multiple socioeconomic variables.56
Many explanations have been proposed for this discrepancy, and all likely play a role in particular environments. For example, the incidence of crime in the surrounding area was inversely related to medication adherence after adjusting for socioeconomic factors.57 African Americans may have more concern about side effects associated with inhaled corticosteroid use and may be less likely to understand how these drugs work.52,53 A poor provider-patient relationship has also been cited as a barrier to adherence.55,57 Finally, physicians are more likely to underestimate asthma severity in an African American patient than in a white patient.58
Taking the time to ensure that patients truly understand all aspects of their disease and establishing a health care environment that is culturally appropriate may have a significant impact in patients with asthma.
ENVIRONMENTAL EXPOSURES
Air quality contributes to the greater asthma morbidity observed in urban residents, including African Americans. While poor outdoor air quality has not been clearly linked to a higher incidence of asthma, it has been associated with greater asthma morbidity. Poor air quality may affect individuals of all races, but with respect to ambient pollutants such as particulate matter and diesel exhaust, outdoor air quality is worse in urban environments where greater proportions of people of low socioeconomic status reside.59,60
The most extensively studied components of air pollution are ozone, sulfur dioxide, and particulate matter. These pollutants have been associated with a higher rate of emergency department visits,61,62 worse asthma symptoms,63,64 and higher exhaled nitric oxide levels.65
Tobacco smoke
Despite the substantial success of smoking cessation efforts nationwide, exposure to tobacco smoke continues to be common and is a significant risk factor for poor asthma control. Recent data suggest that African Americans and whites have a similar prevalence of smoking,66 but a study found a very high prevalence in low-income African Americans.67
Active smoking has been associated with worse asthma control and a higher risk of death.68 People with asthma who smoke are less likely to improve in their lung function and symptom scores when treated with short courses of oral glucocorticoids compared with both nonsmokers and former smokers.69
Secondhand smoke hurts too. Many children living in low-income urban areas are exposed to secondhand smoke or environmental tobacco smoke.70,71 Passive exposure in children has been associated with worse asthma outcomes, and data suggest such exposure may be a cause of asthma.68,72–74
Environmental tobacco smoke has also been implicated in gene-environment interactions. Patients who are either homozygous or heterozygous for the Arg allele at codon 16 of the ADRB2 gene (discussed above) had significantly lower FEV1 and forced vital capacity (FVC) values when exposed to passive tobacco smoke. This difference was not seen in people who were not exposed.75
Cockroach allergen
The type and condition of a person’s housing also plays a role in asthma-related morbidity and death. Across several socioeconomic levels, it has been suggested that African Americans have poorer-quality housing compared with whites.76 Some of the conditions found in low-quality houses, such as interruptions in heat, plumbing leaks, and the presence of rodents, have been associated with a higher prevalence of asthma in the household.77
Cockroach allergen exposure and sensitization is a major contributor to asthma morbidity in African Americans, particularly those living in poorer urban areas where cockroach allergen may be the most common indoor allergen.8 Living in older housing in urban areas is associated with higher exposure to cockroach allergen, and with subsequent sensitization.78,79 Exposure to high levels of the major cockroach allergen, Bla g 1, in sensitized individuals has been linked to a greater risk of hospitalization and unscheduled medical visits for asthma. This was not found to be the case for other common indoor allergens, such as dust mite and cat dander.8
However, it is not only exposure to high cockroach allergen levels that puts African Americans at risk. African Americans living in low-income urban areas may also be more likely than whites living in low-income urban areas to become sensitized to cockroach allergen.7,80 This suggests a gene-environment interaction that may be unique to African Americans. Moreover, cockroach sensitization may occur early in life.81,82
While successful cockroach avoidance measures and environmental control may be challenging, such measures have been shown to decrease rates of asthma morbidity.83
OBESITY
Obesity has been linked to an ever-growing list of diseases, one of which is asthma. Obesity is not a unique challenge for African Americans, but recent data from the US Centers for Disease Control and Prevention show that African Americans have a 51% higher prevalence of obesity compared with whites.84
Obesity is a risk factor for greater asthma morbidity and is a significant challenge in the African American community. The rise in obesity rates has paralleled the rise in asthma in recent decades. The higher one’s body mass index, the higher one’s risk of asthma.85 This association appears to be stronger in people without concurrent atopic disease.86 Obesity has also been associated with a poorer response to inhaled corticosteroids and a higher risk of asthma exacerbations.87 Interestingly, significant weight loss has been associated with improvements in both asthma control and lung function.88,89
What is the mechanism?
The underlying pathogenic mechanisms have not been completely elucidated, and they are likely multiple.
Adipokines (cytokines secreted by adipocytes) have been implicated. Two of the most extensively studied adipokines are leptin and adiponectin. Leptin production is increased in obesity, and it has inflammatory effects on both the innate and adaptive immune systems.90 The opposite is true for adiponectin, which may have anti-inflammatory properties and which decreases as the body mass index increases.90 The precise role these molecules may have in lung disease is undergoing further investigation.
Mechanical alterations in lung function may also contribute. Obese people have a lower functional residual capacity and expiratory reserve volume. Breathing with a lower-volume functional residual capacity results in decreased airway diameter and contributes to increased airway resistance.90 The decreased airway diameter may alter the contractile properties of airway smooth muscle and lead to increased airway responsiveness.90 These differences are in addition to the lower mean values of common spirometry indices such as the FEV1 and FVC, found in nonasthmatic African Americans compared with whites.91
Data suggest these differences are primarily due to anthropometric factors, with nutritional and environmental factors playing a less significant role.92 On this basis, the American Thoracic Society recommends applying race-specific reference standards for use with spirometry in order to accurately gauge lung function in African Americans.
APPROPRIATE CARE AND EDUCATION
The cause of greater asthma prevalence and severity among African Americans is multifactorial. It is likely that a number of factors work together, rather than separately, in influencing the development of asthma and its course.
Some risk factors are avoidable, and it is important to identify and ameliorate them. Others are not preventable, but knowledge of them may provide more specific management strategies and may lead to new therapies in the future.
While more work is needed to further unravel the complex risk factors associated with asthma, ensuring that higher-risk patients are provided the appropriate care and the knowledge to help control their disease is a necessary step in improving the disparities in asthma care outcomes.
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 2011; 32:1–14.
- National Institutes of Health. National Heart, Lung, and Blood Institute. Data Fact Sheet. Asthma statistics. January 1999. http://www.nhlbi.nih.gov/health/prof/lung/asthma/asthstat.pdf. Accessed February 1, 2012.
- Lang DM, Polansky M, Sherman MS. Hospitalizations for asthma in an urban population: 1995–1999. Ann Allergy Asthma Immunol 2009; 103:128–133.
- Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis 1990; 142:1351–1358.
- Koeppen-Schomerus G, Stevenson J, Plomin R. Genes and environment in asthma: a study of 4 year old twins. Arch Dis Child 2001; 85:398–400.
- Meng JF, Rosenwasser LJ. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol Res 2010; 2:215–227.
- Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol 2001; 108:747–752.
- Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997; 336:1356–1363.
- Kobilka BK, Dixon RA, Frielle T, et al. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A 1987; 84:46–50.
- Maxwell TJ, Ameyaw MM, Pritchard S, et al. Beta-2 adrenergic receptor genotypes and haplotypes in different ethnic groups. Int J Mol Med 2005; 16:573–580.
- Israel E, Chinchilli VM, Ford JG, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004; 364:1505–1512.
- Wells K, Pladevall M, Peterson EL, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med 2008; 178:1194–1201.
- Carroll CL, Stoltz P, Schramm CM, Zucker AR. Beta2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations. Chest 2009; 135:1186–1192.
- Bleecker ER, Nelson HS, Kraft M, et al. Beta2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionate. Am J Respir Crit Care Med 2010; 181:676–687.
- Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta-agonists in children with asthma: a meta-analysis. J Asthma 2009; 46:900–905.
- Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A polymorphism in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol 1999; 20:976–983.
- Kedda MA, Lose F, Duffy D, Bell E, Thompson PJ, Upham J. The CD14 C-159T polymorphism is not associated with asthma or asthma severity in an Australian adult population. Thorax 2005; 60:211–214.
- Zambelli-Weiner A, Ehrlich E, Stockton ML, et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol 2005; 115:1203–1209.
- Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006; 117:1082–1089.
- Williams LK, Oliver J, Peterson EL, et al. Gene-environment interactions between CD14 C-260T and endotoxin exposure on Foxp3+ and Foxp3− CD4+ lymphocyte numbers and total serum IgE levels in early childhood. Ann Allergy Asthma Immunol 2008; 100:128–136.
- Barnes KC. Ancestry, ancestry-informative markers, asthma, and the quest for personalized medicine. J Allergy Clin Immunol 2010; 126:1139–1140.
- Vergara C, Caraballo L, Mercado D, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet 2009; 125:565–579.
- Gould W, Peterson EL, Karungi G, et al. Factors predicting inhaled corticosteroid responsiveness in African American patients with asthma. J Allergy Clin Immunol 2010; 126:1131–1138.
- Lang DM, Polansky M. Patterns of asthma mortality in Philadelphia from 1969 to 1991. N Engl J Med 1994; 331:1542–1546.
- Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis 1990; 142:555–562.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Newacheck PW, Hughes DC, Stoddard JJ. Children’s access to primary care: differences by race, income, and insurance status. Pediatrics 1996; 97:26–32.
- Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics 1993; 91:56–61.
- Burt CW, Knapp DE. Ambulatory care visits for asthma: United States, 1993–94. Adv Data 1996; 277:1.
- Lieu TA, Lozano P, Finkelstein JA, et al. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002; 109:857–865.
- Zoratti EM, Havstad S, Rodriguez J, Robens-Paradise Y, Lafata JE, McCarthy B. Health service use by African Americans and Caucasians with asthma in a managed care setting. Am J Respir Crit Care Med 1998; 158:371–377.
- Wisnivesky JP, Leventhal H, Halm EA. Predictors of asthma-related health care utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol 2005; 116:636–642.
- Halm EA, Wisnivesky JP, Leventhal H. Quality and access to care among a cohort of inner-city adults with asthma: who gets guideline concordant care? Chest 2005; 128:1943–1950.
- Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, Wu AW. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med 2001; 161:1660–1668.
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94:666–668.
- Boudreaux ED, Emond SD, Clark S, Camargo CA; Multicenter Airway Research Collaboration Investigators. Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics 2003; 111:e615–e621.
- Trochtenberg DS, BeLue R, Piphus S, Washington N. Differing reports of asthma symptoms in African Americans and Caucasians. J Asthma 2008; 45:165–170.
- Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest 2000; 117:935–943.
- Clark NM, Levison MJ, Evans D, Wasilewski Y, Feldman CH, Mellins RB. Communication within low income families and the management of asthma. Patient Educ Couns 1990; 15:191–210.
- Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes in adolescents: barriers to adherence as a mediator. J Adolesc Health 2010; 47:472–478.
- Quinn K, Kaufman JS, Siddiqi A, Yeatts KB. Parent perceptions of neighborhood stressors are associated with general health and child respiratory health among low-income, urban families. J Asthma 2010; 47:281–289.
- Loerbroks A, Apfelbacher CJ, Bosch JA, Stürmer T. Depressive symptoms, social support, and risk of adult asthma in a population-based cohort study. Psychosom Med 2010; 72:309–315.
- Wood DL, Valdez RB, Hayashi T, Shen A. Health of homeless children and housed, poor children. Pediatrics 1990; 86:858–866.
- O’Donnell L, Stueve A, Myint-U A. Parenting and violence toward self, partners, and others among inner-city young adults. Am J Public Health 2009; 99:2255–2260.
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 2000; 126:309–337.
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A 2009; 106:12406–12411.
- Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health 2004; 94:625–632.
- George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research-enhanced understanding of patients’ beliefs: results of focus groups with low-income, urban, African American adults with asthma. J Allergy Clin Immunol 2003; 111:967–973.
- Laster N, Holsey CN, Shendell DG, Mccarty FA, Celano M. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma 2009; 46:731–739.
- Lieu TA, Finkelstein JA, Lozano P, et al. Cultural competence policies and other predictors of asthma care quality for Medicaid-insured children. Pediatrics 2004; 114:e102–e110.
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004; 42:200–209.
- Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol 2000; 85:416–421.
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol 2004; 114:1288–1293.
- Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol 2003; 111:1219–1226.
- Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med 1998; 157:1810–1817.
- Williams LK, Joseph CL, Peterson EL, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol 2007; 119:168–175.
- O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med 2004; 38:777–785.
- Okelo SO, Wu AW, Merriman B, Krishnan JA, Diette GB. Are physician estimates of asthma severity less accurate in black than in white patients? J Gen Intern Med 2007; 22:976–981.
- Kinney PL, Aggarwal M, Northridge ME, Janssen NA, Shepard P. Airborne concentrations of PM(2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect 2000; 108:213–218.
- O’Neill MS, Jerrett M, Kawachi I, et al; Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect 2003; 111:1861–1870.
- Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 1993; 147:826–831.
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 1999; 107:489–493.
- Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ Health Perspect 2000; 108:1209–1214.
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann Allergy Asthma Immunol 2003; 91:346–353.
- Koenig JQ, Jansen K, Mar TF, et al. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ Health Perspect 2003; 111:1625–1629.
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1227–1232.
- Delva J, Tellez M, Finlayson TL, et al. Cigarette smoking among low-income African Americans: a serious public health problem. Am J Prev Med 2005; 29:218–220.
- McLeish AC, Zvolensky MJ. Asthma and cigarette smoking: a review of the empirical literature. J Asthma 2010; 47:345–361.
- Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med 2003; 168:1308–1311.
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect 2005; 113:362–367.
- Huss K, Rand CS, Butz AM, et al. Home environmental risk factors in urban minority asthmatic children. Ann Allergy 1994; 72:173–177.
- Samir S, Colin Y, Thomas S. Impact of environmental tobacco smoke on children admitted with status asthmaticus in the pediatric intensive care unit. Pediatr Pulmonol 2010. [Epub ahead of print]
- Lannerö E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res 2006; 7:3.
- Hedman L, Bjerg A, Sundberg S, Forsberg B, Rönmark E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax 2011; 66:20–25.
- Zhang G, Hayden CM, Khoo SK, et al. Beta2-adrenoceptor polymorphisms and asthma phenotypes: interactions with passive smoking. Eur Respir J 2007; 30:48–55.
- Rosenbaum E, Friedman S. The Housing Divide: How Generations of Immigrants Fare in New York’s Housing Market. New York, NY: New York University Press; 2007.
- Rosenbaum E. Racial/ethnic differences in asthma prevalence: the role of housing and neighborhood environments. J Health Soc Behav 2008; 49:131–145.
- Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect 2002; 110( suppl 2):323–327.
- Eggleston PA, Rosenstreich D, Lynn H, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol 1998; 102:563–570.
- Togias A, Horowitz E, Joyner D, Guydon L, Malveaux F. Evaluating the factors that relate to asthma severity in adolescents. Int Arch Allergy Immunol 1997; 113:87–95.
- Alp H, Yu BH, Grant EN, Rao V, Moy JN. Cockroach allergy appears early in life in inner-city children with recurrent wheezing. Ann Allergy Asthma Immunol 2001; 86:51–54.
- Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med 2001; 164:995–1001.
- Morgan WJ, Crain EF, Gruchalla RS, et al; Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004; 351:1068–1080.
- Centers for Disease Control and Prevention (CDC). Overweight and Obesity. US Obesity Trends. http://templatelab.com/us-obesity-trends/. Accessed February 1, 2012.
- Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175:661–666.
- Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010; 47:822–829.
- Camargo CA, Sutherland ER, Bailey W, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin 2010; 26:1629–1635.
- Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000; 118:1315–1321.
- Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000; 320:827–832.
- Dixon AE, Holguin F, Sood A, et al; American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010; 7:325–335.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999; 159:179–187.
- Harik-Khan RI, Muller DC, Wise RA. Racial difference in lung function in African-American and white children: effect of anthropometric, socioeconomic, nutritional, and environmental factors. Am J Epidemiol 2004; 160:893–900.
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 2011; 32:1–14.
- National Institutes of Health. National Heart, Lung, and Blood Institute. Data Fact Sheet. Asthma statistics. January 1999. http://www.nhlbi.nih.gov/health/prof/lung/asthma/asthstat.pdf. Accessed February 1, 2012.
- Lang DM, Polansky M, Sherman MS. Hospitalizations for asthma in an urban population: 1995–1999. Ann Allergy Asthma Immunol 2009; 103:128–133.
- Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis 1990; 142:1351–1358.
- Koeppen-Schomerus G, Stevenson J, Plomin R. Genes and environment in asthma: a study of 4 year old twins. Arch Dis Child 2001; 85:398–400.
- Meng JF, Rosenwasser LJ. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol Res 2010; 2:215–227.
- Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol 2001; 108:747–752.
- Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997; 336:1356–1363.
- Kobilka BK, Dixon RA, Frielle T, et al. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A 1987; 84:46–50.
- Maxwell TJ, Ameyaw MM, Pritchard S, et al. Beta-2 adrenergic receptor genotypes and haplotypes in different ethnic groups. Int J Mol Med 2005; 16:573–580.
- Israel E, Chinchilli VM, Ford JG, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004; 364:1505–1512.
- Wells K, Pladevall M, Peterson EL, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med 2008; 178:1194–1201.
- Carroll CL, Stoltz P, Schramm CM, Zucker AR. Beta2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations. Chest 2009; 135:1186–1192.
- Bleecker ER, Nelson HS, Kraft M, et al. Beta2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionate. Am J Respir Crit Care Med 2010; 181:676–687.
- Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta-agonists in children with asthma: a meta-analysis. J Asthma 2009; 46:900–905.
- Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A polymorphism in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol 1999; 20:976–983.
- Kedda MA, Lose F, Duffy D, Bell E, Thompson PJ, Upham J. The CD14 C-159T polymorphism is not associated with asthma or asthma severity in an Australian adult population. Thorax 2005; 60:211–214.
- Zambelli-Weiner A, Ehrlich E, Stockton ML, et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol 2005; 115:1203–1209.
- Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006; 117:1082–1089.
- Williams LK, Oliver J, Peterson EL, et al. Gene-environment interactions between CD14 C-260T and endotoxin exposure on Foxp3+ and Foxp3− CD4+ lymphocyte numbers and total serum IgE levels in early childhood. Ann Allergy Asthma Immunol 2008; 100:128–136.
- Barnes KC. Ancestry, ancestry-informative markers, asthma, and the quest for personalized medicine. J Allergy Clin Immunol 2010; 126:1139–1140.
- Vergara C, Caraballo L, Mercado D, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet 2009; 125:565–579.
- Gould W, Peterson EL, Karungi G, et al. Factors predicting inhaled corticosteroid responsiveness in African American patients with asthma. J Allergy Clin Immunol 2010; 126:1131–1138.
- Lang DM, Polansky M. Patterns of asthma mortality in Philadelphia from 1969 to 1991. N Engl J Med 1994; 331:1542–1546.
- Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis 1990; 142:555–562.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Newacheck PW, Hughes DC, Stoddard JJ. Children’s access to primary care: differences by race, income, and insurance status. Pediatrics 1996; 97:26–32.
- Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics 1993; 91:56–61.
- Burt CW, Knapp DE. Ambulatory care visits for asthma: United States, 1993–94. Adv Data 1996; 277:1.
- Lieu TA, Lozano P, Finkelstein JA, et al. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002; 109:857–865.
- Zoratti EM, Havstad S, Rodriguez J, Robens-Paradise Y, Lafata JE, McCarthy B. Health service use by African Americans and Caucasians with asthma in a managed care setting. Am J Respir Crit Care Med 1998; 158:371–377.
- Wisnivesky JP, Leventhal H, Halm EA. Predictors of asthma-related health care utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol 2005; 116:636–642.
- Halm EA, Wisnivesky JP, Leventhal H. Quality and access to care among a cohort of inner-city adults with asthma: who gets guideline concordant care? Chest 2005; 128:1943–1950.
- Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, Wu AW. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med 2001; 161:1660–1668.
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94:666–668.
- Boudreaux ED, Emond SD, Clark S, Camargo CA; Multicenter Airway Research Collaboration Investigators. Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics 2003; 111:e615–e621.
- Trochtenberg DS, BeLue R, Piphus S, Washington N. Differing reports of asthma symptoms in African Americans and Caucasians. J Asthma 2008; 45:165–170.
- Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest 2000; 117:935–943.
- Clark NM, Levison MJ, Evans D, Wasilewski Y, Feldman CH, Mellins RB. Communication within low income families and the management of asthma. Patient Educ Couns 1990; 15:191–210.
- Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes in adolescents: barriers to adherence as a mediator. J Adolesc Health 2010; 47:472–478.
- Quinn K, Kaufman JS, Siddiqi A, Yeatts KB. Parent perceptions of neighborhood stressors are associated with general health and child respiratory health among low-income, urban families. J Asthma 2010; 47:281–289.
- Loerbroks A, Apfelbacher CJ, Bosch JA, Stürmer T. Depressive symptoms, social support, and risk of adult asthma in a population-based cohort study. Psychosom Med 2010; 72:309–315.
- Wood DL, Valdez RB, Hayashi T, Shen A. Health of homeless children and housed, poor children. Pediatrics 1990; 86:858–866.
- O’Donnell L, Stueve A, Myint-U A. Parenting and violence toward self, partners, and others among inner-city young adults. Am J Public Health 2009; 99:2255–2260.
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 2000; 126:309–337.
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A 2009; 106:12406–12411.
- Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health 2004; 94:625–632.
- George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research-enhanced understanding of patients’ beliefs: results of focus groups with low-income, urban, African American adults with asthma. J Allergy Clin Immunol 2003; 111:967–973.
- Laster N, Holsey CN, Shendell DG, Mccarty FA, Celano M. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma 2009; 46:731–739.
- Lieu TA, Finkelstein JA, Lozano P, et al. Cultural competence policies and other predictors of asthma care quality for Medicaid-insured children. Pediatrics 2004; 114:e102–e110.
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004; 42:200–209.
- Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol 2000; 85:416–421.
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol 2004; 114:1288–1293.
- Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol 2003; 111:1219–1226.
- Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med 1998; 157:1810–1817.
- Williams LK, Joseph CL, Peterson EL, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol 2007; 119:168–175.
- O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med 2004; 38:777–785.
- Okelo SO, Wu AW, Merriman B, Krishnan JA, Diette GB. Are physician estimates of asthma severity less accurate in black than in white patients? J Gen Intern Med 2007; 22:976–981.
- Kinney PL, Aggarwal M, Northridge ME, Janssen NA, Shepard P. Airborne concentrations of PM(2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect 2000; 108:213–218.
- O’Neill MS, Jerrett M, Kawachi I, et al; Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect 2003; 111:1861–1870.
- Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 1993; 147:826–831.
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 1999; 107:489–493.
- Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ Health Perspect 2000; 108:1209–1214.
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann Allergy Asthma Immunol 2003; 91:346–353.
- Koenig JQ, Jansen K, Mar TF, et al. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ Health Perspect 2003; 111:1625–1629.
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1227–1232.
- Delva J, Tellez M, Finlayson TL, et al. Cigarette smoking among low-income African Americans: a serious public health problem. Am J Prev Med 2005; 29:218–220.
- McLeish AC, Zvolensky MJ. Asthma and cigarette smoking: a review of the empirical literature. J Asthma 2010; 47:345–361.
- Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med 2003; 168:1308–1311.
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect 2005; 113:362–367.
- Huss K, Rand CS, Butz AM, et al. Home environmental risk factors in urban minority asthmatic children. Ann Allergy 1994; 72:173–177.
- Samir S, Colin Y, Thomas S. Impact of environmental tobacco smoke on children admitted with status asthmaticus in the pediatric intensive care unit. Pediatr Pulmonol 2010. [Epub ahead of print]
- Lannerö E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res 2006; 7:3.
- Hedman L, Bjerg A, Sundberg S, Forsberg B, Rönmark E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax 2011; 66:20–25.
- Zhang G, Hayden CM, Khoo SK, et al. Beta2-adrenoceptor polymorphisms and asthma phenotypes: interactions with passive smoking. Eur Respir J 2007; 30:48–55.
- Rosenbaum E, Friedman S. The Housing Divide: How Generations of Immigrants Fare in New York’s Housing Market. New York, NY: New York University Press; 2007.
- Rosenbaum E. Racial/ethnic differences in asthma prevalence: the role of housing and neighborhood environments. J Health Soc Behav 2008; 49:131–145.
- Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect 2002; 110( suppl 2):323–327.
- Eggleston PA, Rosenstreich D, Lynn H, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol 1998; 102:563–570.
- Togias A, Horowitz E, Joyner D, Guydon L, Malveaux F. Evaluating the factors that relate to asthma severity in adolescents. Int Arch Allergy Immunol 1997; 113:87–95.
- Alp H, Yu BH, Grant EN, Rao V, Moy JN. Cockroach allergy appears early in life in inner-city children with recurrent wheezing. Ann Allergy Asthma Immunol 2001; 86:51–54.
- Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med 2001; 164:995–1001.
- Morgan WJ, Crain EF, Gruchalla RS, et al; Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004; 351:1068–1080.
- Centers for Disease Control and Prevention (CDC). Overweight and Obesity. US Obesity Trends. http://templatelab.com/us-obesity-trends/. Accessed February 1, 2012.
- Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175:661–666.
- Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010; 47:822–829.
- Camargo CA, Sutherland ER, Bailey W, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin 2010; 26:1629–1635.
- Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000; 118:1315–1321.
- Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000; 320:827–832.
- Dixon AE, Holguin F, Sood A, et al; American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010; 7:325–335.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999; 159:179–187.
- Harik-Khan RI, Muller DC, Wise RA. Racial difference in lung function in African-American and white children: effect of anthropometric, socioeconomic, nutritional, and environmental factors. Am J Epidemiol 2004; 160:893–900.
KEY POINTS
- To better identify those at risk, researchers are looking at genetic markers such as polymorphisms in ADRB2 and CD14.
- Exposure to tobacco smoke and to cockroach allergen contribute to higher rates of asthma prevalence and morbidity.
- African Americans are more likely to receive suboptimal care, in particular to be misdiagnosed with other conditions, to not receive inhaled corticosteroids, and to not receive proper follow-up.
- Better physician-patient communication is one of the keys to improving this problem.
Overcoming health care disparities via better cross-cultural communication and health literacy
An english-speaking middle-aged woman from an ethnic minority group presents to her internist for follow-up of her chronic medical problems, which include diabetes, high blood pressure, asthma, and high cholesterol. Although she sees her physician regularly, her medical conditions are not optimally controlled.
At one of the visits, her physician gives her a list of her medications and, while reviewing it, explains—not for the first time—the importance of taking all of them as prescribed. The patient looks at the paper for a while, and then cautiously tells the physician, “But I can’t read.”
This patient presented to our practice several years ago. The scenario may be familiar to many primary physicians, except for the ending— ie, the patient telling her physician that she cannot read.
Her case raises several questions:
- Why did the physician not realize at the first encounter that she could not read the names of her prescribed medications?
- Why did the patient wait to tell her physician that important fact?
- And to what extent did her inability to read contribute to the poor control of her chronic medical problems?
Patients like this one are the human faces behind the statistics about health disparities—the worse outcomes noted in minority populations. Here, we discuss the issues of cross-cultural communication and health literacy as they relate to health care disparities.
DISPARITY IS NOT ONLY DUE TO LACK OF ACCESS
Health care disparity has been an important topic of discussion in medicine in the past decade.
In a 2003 publication,1 the Institute of Medicine identified lower quality of health care in minority populations as a serious problem. Further, it disputed the long-held belief that the differences in health care between minority and nonminority populations could be explained by lack of access to medical services in minority groups. Instead, it cited factors at the level of the health care system, the level of the patient, and the “care-process level” (ie, the physician-patient encounter) as contributing in distinct ways to the problem.1
A CALL FOR CULTURAL COMPETENCE
In a policy paper published in 2010, the American College of Physicians2 reviewed the progress made in addressing health care disparities. In addition, noting that an individual’s environment, income, level of education, and other factors all affect health, it called for a concerted effort to improve insurance coverage, health literacy, and the health care delivery system; to address stressors both within and outside the health care system; and to recruit more minority health care workers.
None of these things seems like anything a busy practicing clinician could do much about. However, we can try to improve our cultural competence in our interactions with patients on an individual level.
The report recommends that physicians and other health care professionals be sensitive to cultural diversity among patients. It also says we should recognize our preconceived perceptions of minority patients that may affect their treatment and contribute to disparities in health care in minorities. To those ends, it calls for cultural competence training in medical school to improve cultural awareness and sensitivity.2
The Office of Minority Health broadly defines cultural and linguistic competence in health as “a set of congruent behaviors, attitudes, and policies that come together in a system, agency, or among professionals that enables effective work in cross-cultural situations.”3 Cultural competence training should focus on being aware of one’s personal bias, as well as on education about culture-specific norms or knowledge of possible causes of mistrust in minority groups.
For example, many African Americans may mistrust the medical system, given the awareness of previous inequities such as the notorious Tuskegee syphilis study (in which informed consent was not used and treatment that was needed was withheld). Further, beliefs about health in minority populations may be discordant with the Western medical model.4
RECOGNIZING OUR OWN BIASES
Preconceived perceptions on the part of the physician may be shaped by previous experiences with patients from a specific minority group or by personal bias. Unfortunately, even a well-meaning physician who has tried to learn about cultural norms of specific minority groups can be at risk of stereotyping by assuming that all members of that group hold the same beliefs. From the patient’s viewpoint, they can also be molded by previous experiences of health care inequities or unfavorable interactions with physicians.
For example, in the case we described above, perhaps the physician had assumed that the patient was noncompliant and therefore did not look for reasons for the poor control of her medical problems, or maybe the patient did not trust the physician enough to explain the reason for her difficulty with understanding how to take her medications.
Being aware of our own unconscious stereotyping of minority groups is an important step in effectively communicating with patients from different cultural backgrounds or with low health literacy. We also need to reflect about our own health belief system and try to incorporate the patient’s viewpoint into decision-making.
If, on reflection, we recognize that we do harbor biases, we ought to think about ways to better accommodate patients from different backgrounds and literacy levels, including trying to learn more about their culture or mastering techniques to effectively explain treatment plans to low-literacy patients.
ALL ENCOUNTERS WITH PATIENTS ARE ‘CROSS-CULTURAL’
In health care, “cross-cultural communication” does not refer only to interactions between persons from different ethnic backgrounds or with different beliefs about health. Health care has a culture of its own, creating a cross-cultural encounter the moment a person enters your office or clinic in the role of “patient.”
Carillo et al5 categorized issues that may pose difficulties in a cross-cultural encounter as those of authority, physical contact, communication styles, gender, sexuality, and family.
Physician-patient communication is a complicated issue. Many patients will not question a physician if their own cultural norms view it as disrespectful—even if they have very specific fears about the diagnosis or treatment plan. They may also defer any important decision to a family member who has the authority to make decisions for the family.
Frequently, miscommunication is unintentional. In a recent study of hospitalized patients,6 77% of the physicians believed that their patients understood their diagnoses, while only 57% of patients could correctly state this information.
WHAT DOES THE PATIENT THINK?
A key issue in cross-cultural communication, and one that is often neglected, is to address a patient’s fears about his or her illness. In the study mentioned above, more than half of the patients who reported having anxieties or fears in the hospital stated that their physicians did not discuss their fears.6 But if we fail to do so, patients may be less satisfied with the treatment plan and may not accept our recommendations.
A patient’s understanding of his or her illness may be very different from the biomedical explanation. For example, we once saw an elderly man who was admitted to the hospital with back pain due to metastatic prostate cancer, but who was convinced that his symptoms were caused by a voodoo “hex” placed on him by his ex-wife.
For example, for the man who thought that his ex-wife put a hex on him, asking him “What do you think has caused your problem?” during the initial history-taking would allow him to express his concern about the hex and give the physician an opportunity to learn of this fear and then to offer the biomedical explanation for the problem and for the recommended treatment.
What happens more often in practice is that the specific fear is not addressed at the start of the encounter. Consequently, the patient is less likely to follow through with the treatment plan, as he or she does not feel the prescribed treatment is fixing the real problem. This process of exploring the explanatory model of illness may be viewed on a practical level as a way of managing expectations in the clinical care of culturally diverse populations.
HEALTH LITERACY: MORE THAN THE ABILITY TO READ
The better you know how to read, the healthier you probably are. In fact, a study found that a person’s literacy level correlated more strongly with health than did race or formal education level.9 (Apparently, attending school does not necessarily mean that people know how to read, and not attending school doesn’t mean that they don’t.)
Even more important than literacy may be health literacy, defined by Ratzan and Parker as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”8 It includes basic math and critical-thinking skills that allow patients to use medications properly and participate in treatment decisions. Thus, health literacy is much more than the ability to read.
Even people who read and write very well may have trouble when confronted with the complexities of navigating our health care system, such as appointment scheduling, specialty referrals, and follow-up testing and procedures: their health literacy may be lower than their general literacy. We had a patient, a highly trained professional, who was confused by instructions for preparing for colonoscopy on a patient handout. Another similar patient could not understand the dosing of eye drops after cataract surgery because the instructions on the discharge paperwork were unclear.
However, limited health literacy disproportionately affects minority groups and is linked to poorer health care outcomes. Thus, addressing limited health literacy is important in addressing health care disparities. Effective physician-patient communication about treatment plans is fundamental to providing equitable care to patients from minority groups, some of whom may be at high risk for low health literacy.
Below, we will review some of the data on health literacy and offer suggestions for screening and interventions for those whose health literacy is limited.
36% have basic or below-basic reading skills
Every 10 years, the US Department of Education completes its National Assessment of Adult Literacy. Its 2003 survey—the most recent—included 19,000 adults in the community and in prison, interviewed at their place of residence.10 Each participant completed a set of tasks to measure his or her ability to read, understand, and interpret text and to use and interpret numbers.
Participants were divided into four categories based on the results: proficient (12%), intermediate (53%), basic (22%), and below basic (14%). Additionally, 5% of potential participants could not be tested because they had insufficient skills to participate in the survey.
Low literacy puts patients at risk
Although literacy is not the same as health literacy, functionally, those who have basic or below-basic literacy skills (36% of the US population) are at high risk for encountering problems in the US health care system. For example, they would have difficulty with most patient education handouts and health insurance forms.
Limited health literacy exacts both personal and financial costs. Patients with low health literacy are less likely to understand how to take their medications, what prescription warning labels mean, how to schedule follow-up appointments, and how to fill out health insurance forms.11–14
Medicare managed-care enrollees are more likely to be hospitalized if they have limited health literacy,15 and diabetic Medicaid patients who have limited health literacy are less likely to have good glycemic control.16 One study showed annual health care costs of $10,688 for Medicaid enrollees with limited health literacy compared with $2,891 for all enrollees.17 The total cost of limited health literacy to the US health care system is estimated to be between $50 and $73 billion per year.18
Screening for limited health literacy: You can’t tell just by looking
Given the high costs of low health literacy, identifying patients who have it is of paramount importance.
Groups who are more likely to have limited health literacy include the elderly, the poor, the unemployed, high school dropouts, members of minority groups, recent immigrants, and people for whom English is a second language.
However, these demographic factors are not sufficient as a screen for low health literacy—you can't tell just by looking. Red flags for low health literacy include difficulty filling out forms in the office, missed appointments, nonadherence to medication regimens, failure to follow up with scheduled testing, and difficulty reading written materials, often masked with a statement such as “I forgot my glasses and will read this at home.”
A number of screening tests have been developed, including the Rapid Estimate of Adult Literacy in Medicine (REALM)19 and the Test for Functional Health Literacy in Adults (TOFHLA).20 These tests are long, making them difficult to incorporate into a patient visit in a busy primary care practice, but they are useful for research. A newer screening test asks the patient to review a nutrition label and answer six questions.21
The most useful screening test for clinical use may consist of a single question. Questions that have been validated:
- “How often do you need to have someone help you when you read instructions, pamphlets, or other written material from your doctor or pharmacy?” Positive answers are “sometimes,” “often,” or “always.”
- “How confident are you filling out medical forms by yourself?” Positive answers are “somewhat,” “a little bit,” or “not at all.”22–24
These questions can be included either in the initial screening by a nurse or medical assistant or as part of the social history portion of the interview with the physician.
A “brown bag review” can also be helpful. Patients are asked to bring in their medications (often in a brown bag—hence the name). Asking the patient to identify each medication by name and the indication for it can uncover knowledge gaps that indicate low health literacy.
The point to remember is that patients with low health literacy will probably not tell you that they do not understand. However, they would appreciate being asked in a nonthreatening manner.
Make your office a shame-free environment
Many experts advocate a “universal precautions approach,” in which interventions to address low health literacy are incorporated into routine office practice for all patients. Practice sites should adopt a culture of a “shame-free environment,” in which support staff encourage patients to ask questions and are trained to offer assistance to those having difficulty reading or filling out forms.
On a broader level, medical offices and hospitals can partner with adult-learning specialists to help patients gain skills to navigate the health care system. All signage should be clear and should use plain language as opposed to medical terms. Medical forms and questionnaires should be designed to collect only essential information and should be written at a sixth-grade reading level or below. Patient instructions and educational materials should also be clear and free of jargon.
The ‘teach-back’ technique
The “teach-back” technique is a simple method to confirm patient understanding at the end of the visit. This involves asking patients in a nonthreatening way to explain or demonstrate what they have been told. Examples:
- “I want to make sure I have explained things correctly. Can you tell me how you plan to take your medication when you go home?”
- “I want to make sure I have done a good job explaining things to you. When you go home and tell your spouse about your visit today, what will you say?”
These questions should be asked in a nonthreatening way. Put the burden of explanation on yourself as the first step, and let the patient know you are willing to explain again more thoroughly any instructions that may have not been clearly understood.
Other measures
Pictures and computer-based education may be useful for some patients who have difficulty reading.
Weiss25 advocates six steps to improve communication with patients in all encounters: slow down; use plain, nonmedical language; show or draw pictures; limit the amount of information provided; use the teach-back technique; and create a shame-free environment, encouraging questions.
Improving health literacy, as it relates to cross-cultural communication of treatment plans, must encompass understanding of health beliefs often based on cultural norms, in order to come to agreement on a mutually acceptable plan of care. Physicians should be aware of preferences for nontraditional or complementary treatments that may reflect specific cultural beliefs.
IF THE PATIENT DOES NOT SPEAK ENGLISH
Verbal communication across language barriers poses another layer of challenge. A trained interpreter should be used whenever possible when treating a patient who speaks a different language than that of the practitioner. When family members are used as interpreters, there are risks that the patient may not fully disclose facts about the history of illness or specific symptoms, and also that family members may place their own “twist” on the story when translating.
The physician should speak directly to the patient in a normal tone of voice. In this setting, also remember that nonverbal communication can be misinterpreted. Gestures should be avoided. Finally, be aware that personal space is viewed differently depending on cultural background, as is eye contact.
It is helpful to have a pre-interview meeting with the interpreter to explain the format of the interview, as well as a post-interview meeting to ensure all parties felt they effectively communicated during the encounter.
TOWARD EQUITABLE CARE
Health care disparities are the result of multiple determinants. In December 2008, a National Institutes of Health summit conference cited not only barriers to access, but also the interaction of biological, behavioral, social, environmental, economic, cultural, and political factors, and noted that the causes and effects of health disparities transcend health care.26
Clearly, an individual physician’s efforts will not be all that is needed to eliminate health disparities. A team-based approach is essential, using skills of nonphysician members of the health care team such as nurses, medical assistants, social workers, and case managers. Continued opportunity for professional training and development in provider-patient communication skills should be offered.
However, the impact of effective cross-cultural communication and managing low health literacy populations on the physician-patient level should not be understated. As practitioners treating patients from diverse backgrounds, improving self-awareness, eliciting the patient’s explanatory model, and assuring understanding of treatment plans for patients with low health literacy or with language barriers, we can do our part in working toward equitable care for all patients.
- Institute of Medicine of the National Academies. Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare; 2003. http://www.nap.edu/openbook.php?record_id=12875&page=R1. Accessed January 5, 2012.
- American College of Physicians. Racial and Ethnic Disparities in Health Care, Updated 2010. Philadelphia: American College of Physicians; 2010: Policy Paper.
- US Department of Health and Human Services. The Office of Minority Health. What Is Cultural Competency? http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlid=11. Accessed January 5, 2012.
- Eiser AR, Ellis G. Viewpoint: cultural competence and the African American experience with health care: the case for specific content in cross-cultural education. Acad Med 2007; 82:176–183.
- Carrillo JE, Green AR, Betancourt JR. Cross-cultural primary care: a patient-based approach. Ann Intern Med 1999; 130:829–834.
- Olson DP, Windish DM. Communication discrepancies between physicians and hospitalized patients. Arch Intern Med 2010; 170:1302–1307.
- Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med 1978; 88:251–258.
- National Library of Medicine. Current bibliographies in medicine 2000–1. Health Literacy. www.nlm.nih.gov/archive//20061214/pubs/cbm/hliteracy.html. Accessed January 5, 2012.
- Sentell TL, Halpin HA. Importance of adult literacy in understanding health disparities. J Gen Intern Med 2006; 21:862–866.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). US Department of Education. Washington, DC: National Center for Education Statistics; 2006. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed January 5, 2012.
- Williams MV, Parker RM, Baker DW, et al. Inadequate functional health literacy among patients at two public hospitals. JAMA 1995; 274:1677–1682.
- Baker DW, Parker RM, Williams MV, et al. The health care experience of patients with low literacy. Arch Fam Med 1996; 5:329–334.
- Fact Sheet: health literacy and understanding medical information. Lawrenceville, NJ: Center for Health Care Strategies; 2002.
- Wolf MS, Davis TC, Tilson HH, Bass PF, Parker RM. Misunderstanding of prescription drug warning labels among patients with low literacy. Am J Health Syst Pharm 2006; 63:1048–1055.
- Baker DW, Gazmararian JA, Williams MV, et al. Functional health literacy and the risk of hospital admission among Medicare managed care enrollees. Am J Public Health 2002; 92:1278–1283.
- Schillinger D, Barton LR, Karter AJ, Wang F, Adler N. Does literacy mediate the relationship between education and health outcomes? A study of a low-income population with diabetes. Public Health Rep 2006; 121:245–254.
- Weiss BD, Palmer R. Relationship between health care costs and very low literacy skills in a medically needy and indigent Medicaid population. J Am Board Fam Pract 2004; 17:44–47.
- Friedland RB. Understanding health literacy: new estimates of the costs of inadequate health literacy. Washington, DC: National Academy on an Aging Society; 1998.
- Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med 1993; 25:391–395.
- Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns 1999; 38:33–42.
- Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005; 3:514–522.
- Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004; 36:588–594.
- Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract 2006; 7:21.
- Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med 2006; 21:874–877.
- Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. 2nd ed. American Medical Association Foundation and American Medical Association. www.ama-assn.org/ama1/pub/upload/mm/367/healthlitclinicians.pdf. Accessed January 5, 2012.
- Dankwa-Mullan I, Rhee KB, Williams K, et al. The science of eliminating health disparities: summary and analysis of the NIH summit recommendations. Am J Public Health 2010; 100(suppl 1):S12–S18.
An english-speaking middle-aged woman from an ethnic minority group presents to her internist for follow-up of her chronic medical problems, which include diabetes, high blood pressure, asthma, and high cholesterol. Although she sees her physician regularly, her medical conditions are not optimally controlled.
At one of the visits, her physician gives her a list of her medications and, while reviewing it, explains—not for the first time—the importance of taking all of them as prescribed. The patient looks at the paper for a while, and then cautiously tells the physician, “But I can’t read.”
This patient presented to our practice several years ago. The scenario may be familiar to many primary physicians, except for the ending— ie, the patient telling her physician that she cannot read.
Her case raises several questions:
- Why did the physician not realize at the first encounter that she could not read the names of her prescribed medications?
- Why did the patient wait to tell her physician that important fact?
- And to what extent did her inability to read contribute to the poor control of her chronic medical problems?
Patients like this one are the human faces behind the statistics about health disparities—the worse outcomes noted in minority populations. Here, we discuss the issues of cross-cultural communication and health literacy as they relate to health care disparities.
DISPARITY IS NOT ONLY DUE TO LACK OF ACCESS
Health care disparity has been an important topic of discussion in medicine in the past decade.
In a 2003 publication,1 the Institute of Medicine identified lower quality of health care in minority populations as a serious problem. Further, it disputed the long-held belief that the differences in health care between minority and nonminority populations could be explained by lack of access to medical services in minority groups. Instead, it cited factors at the level of the health care system, the level of the patient, and the “care-process level” (ie, the physician-patient encounter) as contributing in distinct ways to the problem.1
A CALL FOR CULTURAL COMPETENCE
In a policy paper published in 2010, the American College of Physicians2 reviewed the progress made in addressing health care disparities. In addition, noting that an individual’s environment, income, level of education, and other factors all affect health, it called for a concerted effort to improve insurance coverage, health literacy, and the health care delivery system; to address stressors both within and outside the health care system; and to recruit more minority health care workers.
None of these things seems like anything a busy practicing clinician could do much about. However, we can try to improve our cultural competence in our interactions with patients on an individual level.
The report recommends that physicians and other health care professionals be sensitive to cultural diversity among patients. It also says we should recognize our preconceived perceptions of minority patients that may affect their treatment and contribute to disparities in health care in minorities. To those ends, it calls for cultural competence training in medical school to improve cultural awareness and sensitivity.2
The Office of Minority Health broadly defines cultural and linguistic competence in health as “a set of congruent behaviors, attitudes, and policies that come together in a system, agency, or among professionals that enables effective work in cross-cultural situations.”3 Cultural competence training should focus on being aware of one’s personal bias, as well as on education about culture-specific norms or knowledge of possible causes of mistrust in minority groups.
For example, many African Americans may mistrust the medical system, given the awareness of previous inequities such as the notorious Tuskegee syphilis study (in which informed consent was not used and treatment that was needed was withheld). Further, beliefs about health in minority populations may be discordant with the Western medical model.4
RECOGNIZING OUR OWN BIASES
Preconceived perceptions on the part of the physician may be shaped by previous experiences with patients from a specific minority group or by personal bias. Unfortunately, even a well-meaning physician who has tried to learn about cultural norms of specific minority groups can be at risk of stereotyping by assuming that all members of that group hold the same beliefs. From the patient’s viewpoint, they can also be molded by previous experiences of health care inequities or unfavorable interactions with physicians.
For example, in the case we described above, perhaps the physician had assumed that the patient was noncompliant and therefore did not look for reasons for the poor control of her medical problems, or maybe the patient did not trust the physician enough to explain the reason for her difficulty with understanding how to take her medications.
Being aware of our own unconscious stereotyping of minority groups is an important step in effectively communicating with patients from different cultural backgrounds or with low health literacy. We also need to reflect about our own health belief system and try to incorporate the patient’s viewpoint into decision-making.
If, on reflection, we recognize that we do harbor biases, we ought to think about ways to better accommodate patients from different backgrounds and literacy levels, including trying to learn more about their culture or mastering techniques to effectively explain treatment plans to low-literacy patients.
ALL ENCOUNTERS WITH PATIENTS ARE ‘CROSS-CULTURAL’
In health care, “cross-cultural communication” does not refer only to interactions between persons from different ethnic backgrounds or with different beliefs about health. Health care has a culture of its own, creating a cross-cultural encounter the moment a person enters your office or clinic in the role of “patient.”
Carillo et al5 categorized issues that may pose difficulties in a cross-cultural encounter as those of authority, physical contact, communication styles, gender, sexuality, and family.
Physician-patient communication is a complicated issue. Many patients will not question a physician if their own cultural norms view it as disrespectful—even if they have very specific fears about the diagnosis or treatment plan. They may also defer any important decision to a family member who has the authority to make decisions for the family.
Frequently, miscommunication is unintentional. In a recent study of hospitalized patients,6 77% of the physicians believed that their patients understood their diagnoses, while only 57% of patients could correctly state this information.
WHAT DOES THE PATIENT THINK?
A key issue in cross-cultural communication, and one that is often neglected, is to address a patient’s fears about his or her illness. In the study mentioned above, more than half of the patients who reported having anxieties or fears in the hospital stated that their physicians did not discuss their fears.6 But if we fail to do so, patients may be less satisfied with the treatment plan and may not accept our recommendations.
A patient’s understanding of his or her illness may be very different from the biomedical explanation. For example, we once saw an elderly man who was admitted to the hospital with back pain due to metastatic prostate cancer, but who was convinced that his symptoms were caused by a voodoo “hex” placed on him by his ex-wife.
For example, for the man who thought that his ex-wife put a hex on him, asking him “What do you think has caused your problem?” during the initial history-taking would allow him to express his concern about the hex and give the physician an opportunity to learn of this fear and then to offer the biomedical explanation for the problem and for the recommended treatment.
What happens more often in practice is that the specific fear is not addressed at the start of the encounter. Consequently, the patient is less likely to follow through with the treatment plan, as he or she does not feel the prescribed treatment is fixing the real problem. This process of exploring the explanatory model of illness may be viewed on a practical level as a way of managing expectations in the clinical care of culturally diverse populations.
HEALTH LITERACY: MORE THAN THE ABILITY TO READ
The better you know how to read, the healthier you probably are. In fact, a study found that a person’s literacy level correlated more strongly with health than did race or formal education level.9 (Apparently, attending school does not necessarily mean that people know how to read, and not attending school doesn’t mean that they don’t.)
Even more important than literacy may be health literacy, defined by Ratzan and Parker as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”8 It includes basic math and critical-thinking skills that allow patients to use medications properly and participate in treatment decisions. Thus, health literacy is much more than the ability to read.
Even people who read and write very well may have trouble when confronted with the complexities of navigating our health care system, such as appointment scheduling, specialty referrals, and follow-up testing and procedures: their health literacy may be lower than their general literacy. We had a patient, a highly trained professional, who was confused by instructions for preparing for colonoscopy on a patient handout. Another similar patient could not understand the dosing of eye drops after cataract surgery because the instructions on the discharge paperwork were unclear.
However, limited health literacy disproportionately affects minority groups and is linked to poorer health care outcomes. Thus, addressing limited health literacy is important in addressing health care disparities. Effective physician-patient communication about treatment plans is fundamental to providing equitable care to patients from minority groups, some of whom may be at high risk for low health literacy.
Below, we will review some of the data on health literacy and offer suggestions for screening and interventions for those whose health literacy is limited.
36% have basic or below-basic reading skills
Every 10 years, the US Department of Education completes its National Assessment of Adult Literacy. Its 2003 survey—the most recent—included 19,000 adults in the community and in prison, interviewed at their place of residence.10 Each participant completed a set of tasks to measure his or her ability to read, understand, and interpret text and to use and interpret numbers.
Participants were divided into four categories based on the results: proficient (12%), intermediate (53%), basic (22%), and below basic (14%). Additionally, 5% of potential participants could not be tested because they had insufficient skills to participate in the survey.
Low literacy puts patients at risk
Although literacy is not the same as health literacy, functionally, those who have basic or below-basic literacy skills (36% of the US population) are at high risk for encountering problems in the US health care system. For example, they would have difficulty with most patient education handouts and health insurance forms.
Limited health literacy exacts both personal and financial costs. Patients with low health literacy are less likely to understand how to take their medications, what prescription warning labels mean, how to schedule follow-up appointments, and how to fill out health insurance forms.11–14
Medicare managed-care enrollees are more likely to be hospitalized if they have limited health literacy,15 and diabetic Medicaid patients who have limited health literacy are less likely to have good glycemic control.16 One study showed annual health care costs of $10,688 for Medicaid enrollees with limited health literacy compared with $2,891 for all enrollees.17 The total cost of limited health literacy to the US health care system is estimated to be between $50 and $73 billion per year.18
Screening for limited health literacy: You can’t tell just by looking
Given the high costs of low health literacy, identifying patients who have it is of paramount importance.
Groups who are more likely to have limited health literacy include the elderly, the poor, the unemployed, high school dropouts, members of minority groups, recent immigrants, and people for whom English is a second language.
However, these demographic factors are not sufficient as a screen for low health literacy—you can't tell just by looking. Red flags for low health literacy include difficulty filling out forms in the office, missed appointments, nonadherence to medication regimens, failure to follow up with scheduled testing, and difficulty reading written materials, often masked with a statement such as “I forgot my glasses and will read this at home.”
A number of screening tests have been developed, including the Rapid Estimate of Adult Literacy in Medicine (REALM)19 and the Test for Functional Health Literacy in Adults (TOFHLA).20 These tests are long, making them difficult to incorporate into a patient visit in a busy primary care practice, but they are useful for research. A newer screening test asks the patient to review a nutrition label and answer six questions.21
The most useful screening test for clinical use may consist of a single question. Questions that have been validated:
- “How often do you need to have someone help you when you read instructions, pamphlets, or other written material from your doctor or pharmacy?” Positive answers are “sometimes,” “often,” or “always.”
- “How confident are you filling out medical forms by yourself?” Positive answers are “somewhat,” “a little bit,” or “not at all.”22–24
These questions can be included either in the initial screening by a nurse or medical assistant or as part of the social history portion of the interview with the physician.
A “brown bag review” can also be helpful. Patients are asked to bring in their medications (often in a brown bag—hence the name). Asking the patient to identify each medication by name and the indication for it can uncover knowledge gaps that indicate low health literacy.
The point to remember is that patients with low health literacy will probably not tell you that they do not understand. However, they would appreciate being asked in a nonthreatening manner.
Make your office a shame-free environment
Many experts advocate a “universal precautions approach,” in which interventions to address low health literacy are incorporated into routine office practice for all patients. Practice sites should adopt a culture of a “shame-free environment,” in which support staff encourage patients to ask questions and are trained to offer assistance to those having difficulty reading or filling out forms.
On a broader level, medical offices and hospitals can partner with adult-learning specialists to help patients gain skills to navigate the health care system. All signage should be clear and should use plain language as opposed to medical terms. Medical forms and questionnaires should be designed to collect only essential information and should be written at a sixth-grade reading level or below. Patient instructions and educational materials should also be clear and free of jargon.
The ‘teach-back’ technique
The “teach-back” technique is a simple method to confirm patient understanding at the end of the visit. This involves asking patients in a nonthreatening way to explain or demonstrate what they have been told. Examples:
- “I want to make sure I have explained things correctly. Can you tell me how you plan to take your medication when you go home?”
- “I want to make sure I have done a good job explaining things to you. When you go home and tell your spouse about your visit today, what will you say?”
These questions should be asked in a nonthreatening way. Put the burden of explanation on yourself as the first step, and let the patient know you are willing to explain again more thoroughly any instructions that may have not been clearly understood.
Other measures
Pictures and computer-based education may be useful for some patients who have difficulty reading.
Weiss25 advocates six steps to improve communication with patients in all encounters: slow down; use plain, nonmedical language; show or draw pictures; limit the amount of information provided; use the teach-back technique; and create a shame-free environment, encouraging questions.
Improving health literacy, as it relates to cross-cultural communication of treatment plans, must encompass understanding of health beliefs often based on cultural norms, in order to come to agreement on a mutually acceptable plan of care. Physicians should be aware of preferences for nontraditional or complementary treatments that may reflect specific cultural beliefs.
IF THE PATIENT DOES NOT SPEAK ENGLISH
Verbal communication across language barriers poses another layer of challenge. A trained interpreter should be used whenever possible when treating a patient who speaks a different language than that of the practitioner. When family members are used as interpreters, there are risks that the patient may not fully disclose facts about the history of illness or specific symptoms, and also that family members may place their own “twist” on the story when translating.
The physician should speak directly to the patient in a normal tone of voice. In this setting, also remember that nonverbal communication can be misinterpreted. Gestures should be avoided. Finally, be aware that personal space is viewed differently depending on cultural background, as is eye contact.
It is helpful to have a pre-interview meeting with the interpreter to explain the format of the interview, as well as a post-interview meeting to ensure all parties felt they effectively communicated during the encounter.
TOWARD EQUITABLE CARE
Health care disparities are the result of multiple determinants. In December 2008, a National Institutes of Health summit conference cited not only barriers to access, but also the interaction of biological, behavioral, social, environmental, economic, cultural, and political factors, and noted that the causes and effects of health disparities transcend health care.26
Clearly, an individual physician’s efforts will not be all that is needed to eliminate health disparities. A team-based approach is essential, using skills of nonphysician members of the health care team such as nurses, medical assistants, social workers, and case managers. Continued opportunity for professional training and development in provider-patient communication skills should be offered.
However, the impact of effective cross-cultural communication and managing low health literacy populations on the physician-patient level should not be understated. As practitioners treating patients from diverse backgrounds, improving self-awareness, eliciting the patient’s explanatory model, and assuring understanding of treatment plans for patients with low health literacy or with language barriers, we can do our part in working toward equitable care for all patients.
An english-speaking middle-aged woman from an ethnic minority group presents to her internist for follow-up of her chronic medical problems, which include diabetes, high blood pressure, asthma, and high cholesterol. Although she sees her physician regularly, her medical conditions are not optimally controlled.
At one of the visits, her physician gives her a list of her medications and, while reviewing it, explains—not for the first time—the importance of taking all of them as prescribed. The patient looks at the paper for a while, and then cautiously tells the physician, “But I can’t read.”
This patient presented to our practice several years ago. The scenario may be familiar to many primary physicians, except for the ending— ie, the patient telling her physician that she cannot read.
Her case raises several questions:
- Why did the physician not realize at the first encounter that she could not read the names of her prescribed medications?
- Why did the patient wait to tell her physician that important fact?
- And to what extent did her inability to read contribute to the poor control of her chronic medical problems?
Patients like this one are the human faces behind the statistics about health disparities—the worse outcomes noted in minority populations. Here, we discuss the issues of cross-cultural communication and health literacy as they relate to health care disparities.
DISPARITY IS NOT ONLY DUE TO LACK OF ACCESS
Health care disparity has been an important topic of discussion in medicine in the past decade.
In a 2003 publication,1 the Institute of Medicine identified lower quality of health care in minority populations as a serious problem. Further, it disputed the long-held belief that the differences in health care between minority and nonminority populations could be explained by lack of access to medical services in minority groups. Instead, it cited factors at the level of the health care system, the level of the patient, and the “care-process level” (ie, the physician-patient encounter) as contributing in distinct ways to the problem.1
A CALL FOR CULTURAL COMPETENCE
In a policy paper published in 2010, the American College of Physicians2 reviewed the progress made in addressing health care disparities. In addition, noting that an individual’s environment, income, level of education, and other factors all affect health, it called for a concerted effort to improve insurance coverage, health literacy, and the health care delivery system; to address stressors both within and outside the health care system; and to recruit more minority health care workers.
None of these things seems like anything a busy practicing clinician could do much about. However, we can try to improve our cultural competence in our interactions with patients on an individual level.
The report recommends that physicians and other health care professionals be sensitive to cultural diversity among patients. It also says we should recognize our preconceived perceptions of minority patients that may affect their treatment and contribute to disparities in health care in minorities. To those ends, it calls for cultural competence training in medical school to improve cultural awareness and sensitivity.2
The Office of Minority Health broadly defines cultural and linguistic competence in health as “a set of congruent behaviors, attitudes, and policies that come together in a system, agency, or among professionals that enables effective work in cross-cultural situations.”3 Cultural competence training should focus on being aware of one’s personal bias, as well as on education about culture-specific norms or knowledge of possible causes of mistrust in minority groups.
For example, many African Americans may mistrust the medical system, given the awareness of previous inequities such as the notorious Tuskegee syphilis study (in which informed consent was not used and treatment that was needed was withheld). Further, beliefs about health in minority populations may be discordant with the Western medical model.4
RECOGNIZING OUR OWN BIASES
Preconceived perceptions on the part of the physician may be shaped by previous experiences with patients from a specific minority group or by personal bias. Unfortunately, even a well-meaning physician who has tried to learn about cultural norms of specific minority groups can be at risk of stereotyping by assuming that all members of that group hold the same beliefs. From the patient’s viewpoint, they can also be molded by previous experiences of health care inequities or unfavorable interactions with physicians.
For example, in the case we described above, perhaps the physician had assumed that the patient was noncompliant and therefore did not look for reasons for the poor control of her medical problems, or maybe the patient did not trust the physician enough to explain the reason for her difficulty with understanding how to take her medications.
Being aware of our own unconscious stereotyping of minority groups is an important step in effectively communicating with patients from different cultural backgrounds or with low health literacy. We also need to reflect about our own health belief system and try to incorporate the patient’s viewpoint into decision-making.
If, on reflection, we recognize that we do harbor biases, we ought to think about ways to better accommodate patients from different backgrounds and literacy levels, including trying to learn more about their culture or mastering techniques to effectively explain treatment plans to low-literacy patients.
ALL ENCOUNTERS WITH PATIENTS ARE ‘CROSS-CULTURAL’
In health care, “cross-cultural communication” does not refer only to interactions between persons from different ethnic backgrounds or with different beliefs about health. Health care has a culture of its own, creating a cross-cultural encounter the moment a person enters your office or clinic in the role of “patient.”
Carillo et al5 categorized issues that may pose difficulties in a cross-cultural encounter as those of authority, physical contact, communication styles, gender, sexuality, and family.
Physician-patient communication is a complicated issue. Many patients will not question a physician if their own cultural norms view it as disrespectful—even if they have very specific fears about the diagnosis or treatment plan. They may also defer any important decision to a family member who has the authority to make decisions for the family.
Frequently, miscommunication is unintentional. In a recent study of hospitalized patients,6 77% of the physicians believed that their patients understood their diagnoses, while only 57% of patients could correctly state this information.
WHAT DOES THE PATIENT THINK?
A key issue in cross-cultural communication, and one that is often neglected, is to address a patient’s fears about his or her illness. In the study mentioned above, more than half of the patients who reported having anxieties or fears in the hospital stated that their physicians did not discuss their fears.6 But if we fail to do so, patients may be less satisfied with the treatment plan and may not accept our recommendations.
A patient’s understanding of his or her illness may be very different from the biomedical explanation. For example, we once saw an elderly man who was admitted to the hospital with back pain due to metastatic prostate cancer, but who was convinced that his symptoms were caused by a voodoo “hex” placed on him by his ex-wife.
For example, for the man who thought that his ex-wife put a hex on him, asking him “What do you think has caused your problem?” during the initial history-taking would allow him to express his concern about the hex and give the physician an opportunity to learn of this fear and then to offer the biomedical explanation for the problem and for the recommended treatment.
What happens more often in practice is that the specific fear is not addressed at the start of the encounter. Consequently, the patient is less likely to follow through with the treatment plan, as he or she does not feel the prescribed treatment is fixing the real problem. This process of exploring the explanatory model of illness may be viewed on a practical level as a way of managing expectations in the clinical care of culturally diverse populations.
HEALTH LITERACY: MORE THAN THE ABILITY TO READ
The better you know how to read, the healthier you probably are. In fact, a study found that a person’s literacy level correlated more strongly with health than did race or formal education level.9 (Apparently, attending school does not necessarily mean that people know how to read, and not attending school doesn’t mean that they don’t.)
Even more important than literacy may be health literacy, defined by Ratzan and Parker as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”8 It includes basic math and critical-thinking skills that allow patients to use medications properly and participate in treatment decisions. Thus, health literacy is much more than the ability to read.
Even people who read and write very well may have trouble when confronted with the complexities of navigating our health care system, such as appointment scheduling, specialty referrals, and follow-up testing and procedures: their health literacy may be lower than their general literacy. We had a patient, a highly trained professional, who was confused by instructions for preparing for colonoscopy on a patient handout. Another similar patient could not understand the dosing of eye drops after cataract surgery because the instructions on the discharge paperwork were unclear.
However, limited health literacy disproportionately affects minority groups and is linked to poorer health care outcomes. Thus, addressing limited health literacy is important in addressing health care disparities. Effective physician-patient communication about treatment plans is fundamental to providing equitable care to patients from minority groups, some of whom may be at high risk for low health literacy.
Below, we will review some of the data on health literacy and offer suggestions for screening and interventions for those whose health literacy is limited.
36% have basic or below-basic reading skills
Every 10 years, the US Department of Education completes its National Assessment of Adult Literacy. Its 2003 survey—the most recent—included 19,000 adults in the community and in prison, interviewed at their place of residence.10 Each participant completed a set of tasks to measure his or her ability to read, understand, and interpret text and to use and interpret numbers.
Participants were divided into four categories based on the results: proficient (12%), intermediate (53%), basic (22%), and below basic (14%). Additionally, 5% of potential participants could not be tested because they had insufficient skills to participate in the survey.
Low literacy puts patients at risk
Although literacy is not the same as health literacy, functionally, those who have basic or below-basic literacy skills (36% of the US population) are at high risk for encountering problems in the US health care system. For example, they would have difficulty with most patient education handouts and health insurance forms.
Limited health literacy exacts both personal and financial costs. Patients with low health literacy are less likely to understand how to take their medications, what prescription warning labels mean, how to schedule follow-up appointments, and how to fill out health insurance forms.11–14
Medicare managed-care enrollees are more likely to be hospitalized if they have limited health literacy,15 and diabetic Medicaid patients who have limited health literacy are less likely to have good glycemic control.16 One study showed annual health care costs of $10,688 for Medicaid enrollees with limited health literacy compared with $2,891 for all enrollees.17 The total cost of limited health literacy to the US health care system is estimated to be between $50 and $73 billion per year.18
Screening for limited health literacy: You can’t tell just by looking
Given the high costs of low health literacy, identifying patients who have it is of paramount importance.
Groups who are more likely to have limited health literacy include the elderly, the poor, the unemployed, high school dropouts, members of minority groups, recent immigrants, and people for whom English is a second language.
However, these demographic factors are not sufficient as a screen for low health literacy—you can't tell just by looking. Red flags for low health literacy include difficulty filling out forms in the office, missed appointments, nonadherence to medication regimens, failure to follow up with scheduled testing, and difficulty reading written materials, often masked with a statement such as “I forgot my glasses and will read this at home.”
A number of screening tests have been developed, including the Rapid Estimate of Adult Literacy in Medicine (REALM)19 and the Test for Functional Health Literacy in Adults (TOFHLA).20 These tests are long, making them difficult to incorporate into a patient visit in a busy primary care practice, but they are useful for research. A newer screening test asks the patient to review a nutrition label and answer six questions.21
The most useful screening test for clinical use may consist of a single question. Questions that have been validated:
- “How often do you need to have someone help you when you read instructions, pamphlets, or other written material from your doctor or pharmacy?” Positive answers are “sometimes,” “often,” or “always.”
- “How confident are you filling out medical forms by yourself?” Positive answers are “somewhat,” “a little bit,” or “not at all.”22–24
These questions can be included either in the initial screening by a nurse or medical assistant or as part of the social history portion of the interview with the physician.
A “brown bag review” can also be helpful. Patients are asked to bring in their medications (often in a brown bag—hence the name). Asking the patient to identify each medication by name and the indication for it can uncover knowledge gaps that indicate low health literacy.
The point to remember is that patients with low health literacy will probably not tell you that they do not understand. However, they would appreciate being asked in a nonthreatening manner.
Make your office a shame-free environment
Many experts advocate a “universal precautions approach,” in which interventions to address low health literacy are incorporated into routine office practice for all patients. Practice sites should adopt a culture of a “shame-free environment,” in which support staff encourage patients to ask questions and are trained to offer assistance to those having difficulty reading or filling out forms.
On a broader level, medical offices and hospitals can partner with adult-learning specialists to help patients gain skills to navigate the health care system. All signage should be clear and should use plain language as opposed to medical terms. Medical forms and questionnaires should be designed to collect only essential information and should be written at a sixth-grade reading level or below. Patient instructions and educational materials should also be clear and free of jargon.
The ‘teach-back’ technique
The “teach-back” technique is a simple method to confirm patient understanding at the end of the visit. This involves asking patients in a nonthreatening way to explain or demonstrate what they have been told. Examples:
- “I want to make sure I have explained things correctly. Can you tell me how you plan to take your medication when you go home?”
- “I want to make sure I have done a good job explaining things to you. When you go home and tell your spouse about your visit today, what will you say?”
These questions should be asked in a nonthreatening way. Put the burden of explanation on yourself as the first step, and let the patient know you are willing to explain again more thoroughly any instructions that may have not been clearly understood.
Other measures
Pictures and computer-based education may be useful for some patients who have difficulty reading.
Weiss25 advocates six steps to improve communication with patients in all encounters: slow down; use plain, nonmedical language; show or draw pictures; limit the amount of information provided; use the teach-back technique; and create a shame-free environment, encouraging questions.
Improving health literacy, as it relates to cross-cultural communication of treatment plans, must encompass understanding of health beliefs often based on cultural norms, in order to come to agreement on a mutually acceptable plan of care. Physicians should be aware of preferences for nontraditional or complementary treatments that may reflect specific cultural beliefs.
IF THE PATIENT DOES NOT SPEAK ENGLISH
Verbal communication across language barriers poses another layer of challenge. A trained interpreter should be used whenever possible when treating a patient who speaks a different language than that of the practitioner. When family members are used as interpreters, there are risks that the patient may not fully disclose facts about the history of illness or specific symptoms, and also that family members may place their own “twist” on the story when translating.
The physician should speak directly to the patient in a normal tone of voice. In this setting, also remember that nonverbal communication can be misinterpreted. Gestures should be avoided. Finally, be aware that personal space is viewed differently depending on cultural background, as is eye contact.
It is helpful to have a pre-interview meeting with the interpreter to explain the format of the interview, as well as a post-interview meeting to ensure all parties felt they effectively communicated during the encounter.
TOWARD EQUITABLE CARE
Health care disparities are the result of multiple determinants. In December 2008, a National Institutes of Health summit conference cited not only barriers to access, but also the interaction of biological, behavioral, social, environmental, economic, cultural, and political factors, and noted that the causes and effects of health disparities transcend health care.26
Clearly, an individual physician’s efforts will not be all that is needed to eliminate health disparities. A team-based approach is essential, using skills of nonphysician members of the health care team such as nurses, medical assistants, social workers, and case managers. Continued opportunity for professional training and development in provider-patient communication skills should be offered.
However, the impact of effective cross-cultural communication and managing low health literacy populations on the physician-patient level should not be understated. As practitioners treating patients from diverse backgrounds, improving self-awareness, eliciting the patient’s explanatory model, and assuring understanding of treatment plans for patients with low health literacy or with language barriers, we can do our part in working toward equitable care for all patients.
- Institute of Medicine of the National Academies. Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare; 2003. http://www.nap.edu/openbook.php?record_id=12875&page=R1. Accessed January 5, 2012.
- American College of Physicians. Racial and Ethnic Disparities in Health Care, Updated 2010. Philadelphia: American College of Physicians; 2010: Policy Paper.
- US Department of Health and Human Services. The Office of Minority Health. What Is Cultural Competency? http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlid=11. Accessed January 5, 2012.
- Eiser AR, Ellis G. Viewpoint: cultural competence and the African American experience with health care: the case for specific content in cross-cultural education. Acad Med 2007; 82:176–183.
- Carrillo JE, Green AR, Betancourt JR. Cross-cultural primary care: a patient-based approach. Ann Intern Med 1999; 130:829–834.
- Olson DP, Windish DM. Communication discrepancies between physicians and hospitalized patients. Arch Intern Med 2010; 170:1302–1307.
- Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med 1978; 88:251–258.
- National Library of Medicine. Current bibliographies in medicine 2000–1. Health Literacy. www.nlm.nih.gov/archive//20061214/pubs/cbm/hliteracy.html. Accessed January 5, 2012.
- Sentell TL, Halpin HA. Importance of adult literacy in understanding health disparities. J Gen Intern Med 2006; 21:862–866.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). US Department of Education. Washington, DC: National Center for Education Statistics; 2006. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed January 5, 2012.
- Williams MV, Parker RM, Baker DW, et al. Inadequate functional health literacy among patients at two public hospitals. JAMA 1995; 274:1677–1682.
- Baker DW, Parker RM, Williams MV, et al. The health care experience of patients with low literacy. Arch Fam Med 1996; 5:329–334.
- Fact Sheet: health literacy and understanding medical information. Lawrenceville, NJ: Center for Health Care Strategies; 2002.
- Wolf MS, Davis TC, Tilson HH, Bass PF, Parker RM. Misunderstanding of prescription drug warning labels among patients with low literacy. Am J Health Syst Pharm 2006; 63:1048–1055.
- Baker DW, Gazmararian JA, Williams MV, et al. Functional health literacy and the risk of hospital admission among Medicare managed care enrollees. Am J Public Health 2002; 92:1278–1283.
- Schillinger D, Barton LR, Karter AJ, Wang F, Adler N. Does literacy mediate the relationship between education and health outcomes? A study of a low-income population with diabetes. Public Health Rep 2006; 121:245–254.
- Weiss BD, Palmer R. Relationship between health care costs and very low literacy skills in a medically needy and indigent Medicaid population. J Am Board Fam Pract 2004; 17:44–47.
- Friedland RB. Understanding health literacy: new estimates of the costs of inadequate health literacy. Washington, DC: National Academy on an Aging Society; 1998.
- Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med 1993; 25:391–395.
- Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns 1999; 38:33–42.
- Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005; 3:514–522.
- Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004; 36:588–594.
- Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract 2006; 7:21.
- Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med 2006; 21:874–877.
- Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. 2nd ed. American Medical Association Foundation and American Medical Association. www.ama-assn.org/ama1/pub/upload/mm/367/healthlitclinicians.pdf. Accessed January 5, 2012.
- Dankwa-Mullan I, Rhee KB, Williams K, et al. The science of eliminating health disparities: summary and analysis of the NIH summit recommendations. Am J Public Health 2010; 100(suppl 1):S12–S18.
- Institute of Medicine of the National Academies. Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare; 2003. http://www.nap.edu/openbook.php?record_id=12875&page=R1. Accessed January 5, 2012.
- American College of Physicians. Racial and Ethnic Disparities in Health Care, Updated 2010. Philadelphia: American College of Physicians; 2010: Policy Paper.
- US Department of Health and Human Services. The Office of Minority Health. What Is Cultural Competency? http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlid=11. Accessed January 5, 2012.
- Eiser AR, Ellis G. Viewpoint: cultural competence and the African American experience with health care: the case for specific content in cross-cultural education. Acad Med 2007; 82:176–183.
- Carrillo JE, Green AR, Betancourt JR. Cross-cultural primary care: a patient-based approach. Ann Intern Med 1999; 130:829–834.
- Olson DP, Windish DM. Communication discrepancies between physicians and hospitalized patients. Arch Intern Med 2010; 170:1302–1307.
- Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med 1978; 88:251–258.
- National Library of Medicine. Current bibliographies in medicine 2000–1. Health Literacy. www.nlm.nih.gov/archive//20061214/pubs/cbm/hliteracy.html. Accessed January 5, 2012.
- Sentell TL, Halpin HA. Importance of adult literacy in understanding health disparities. J Gen Intern Med 2006; 21:862–866.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). US Department of Education. Washington, DC: National Center for Education Statistics; 2006. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed January 5, 2012.
- Williams MV, Parker RM, Baker DW, et al. Inadequate functional health literacy among patients at two public hospitals. JAMA 1995; 274:1677–1682.
- Baker DW, Parker RM, Williams MV, et al. The health care experience of patients with low literacy. Arch Fam Med 1996; 5:329–334.
- Fact Sheet: health literacy and understanding medical information. Lawrenceville, NJ: Center for Health Care Strategies; 2002.
- Wolf MS, Davis TC, Tilson HH, Bass PF, Parker RM. Misunderstanding of prescription drug warning labels among patients with low literacy. Am J Health Syst Pharm 2006; 63:1048–1055.
- Baker DW, Gazmararian JA, Williams MV, et al. Functional health literacy and the risk of hospital admission among Medicare managed care enrollees. Am J Public Health 2002; 92:1278–1283.
- Schillinger D, Barton LR, Karter AJ, Wang F, Adler N. Does literacy mediate the relationship between education and health outcomes? A study of a low-income population with diabetes. Public Health Rep 2006; 121:245–254.
- Weiss BD, Palmer R. Relationship between health care costs and very low literacy skills in a medically needy and indigent Medicaid population. J Am Board Fam Pract 2004; 17:44–47.
- Friedland RB. Understanding health literacy: new estimates of the costs of inadequate health literacy. Washington, DC: National Academy on an Aging Society; 1998.
- Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med 1993; 25:391–395.
- Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns 1999; 38:33–42.
- Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005; 3:514–522.
- Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004; 36:588–594.
- Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract 2006; 7:21.
- Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med 2006; 21:874–877.
- Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. 2nd ed. American Medical Association Foundation and American Medical Association. www.ama-assn.org/ama1/pub/upload/mm/367/healthlitclinicians.pdf. Accessed January 5, 2012.
- Dankwa-Mullan I, Rhee KB, Williams K, et al. The science of eliminating health disparities: summary and analysis of the NIH summit recommendations. Am J Public Health 2010; 100(suppl 1):S12–S18.
KEY POINTS
- To provide optimal care, physicians and staff need to think about ways to accommodate patients of other cultures and backgrounds, in particular by learning more about the patient’s culture and by examining themselves for possible bias.
- Even people who read and write very well may have limited health literacy. We should not assume that patients understand what we are talking about.
- Weiss (2011) advocates six steps to improve communication with patients in all encounters: slow down; use plain, nonmedical language; show or draw pictures; limit the amount of information provided; use the “teach-back” technique; and create a shame-free environment, encouraging questions.
- The “teach-back” technique is a simple way to confirm a patient’s understanding at the end of the visit. This involves asking the patient in a nonthreatening way to explain or show what he or she has been told.
Overcoming barriers to hypertension control in African Americans
High blood pressure takes a devastating toll on African Americans. Better control can go a long way to closing the “mortality gap” between African Americans and white Americans. But which strategies are best to address this complex problem?
In this report, we review the evidence on practice-based approaches to improving blood pressure control, from new styles of patient education to home blood pressure monitoring, focusing on studies in African Americans (Table 1).1–11
BETTER CONTROL IS NEEDED
PATIENT-RELATED BARRIERS
Patient-related barriers24–40 include:
- Poor knowledge about hypertension and its consequences31,32
- Poor adherence to drug therapy (a major factor,24–26 as African Americans have poorer adherence rates than whites,27–29 which may explain some of the racial disparity in blood pressure control30)
- False health beliefs34–37
- Inability to change one’s lifestyle
- Side effects of antihypertensive drugs32
- Unrealistic expectations of treatment (eg, a cure33)
- Demographic factors (eg, socioeconomic status, educational level, age, sex).24,38–40
Perhaps the most salient and easily modifiable of these factors are patients’ reluctance to modify their lifestyle and their misconceptions about the causes, treatment, and prevention of hypertension. Patients whose beliefs are discordant with traditional biomedical concepts of hypertension have poorer blood pressure control than those whose beliefs are concordant.41 This may be more relevant to African Americans, since they are known to have cultural health beliefs that differ from those of Western culture (eg, that hypertension is a curable rather than a chronic illness, and that hypertension is a disease of nerves that often affects the blood and clogs the arteries).42
PHYSICIAN-RELATED BARRIERS
Barriers to effective blood pressure control at the physician level43–48 include:
- Nonadherence to treatment guidelines44
- Failure to intensify the regimen if goals are not met45
- Failure to emphasize therapeutic lifestyle changes.43,46–48
When primary care physicians do not follow evidence-based guidelines, the reason may be that they are not aware of them or that they do not understand them. In a national survey of 1,029 physicians that was designed to explore how well physicians know the indications for specific antihypertensive drugs and how closely their opinions and practice agreed with national guidelines, only 37.3% correctly answered all of the knowledge-related questions.49
Other reasons for nonadherence are that physicians may disagree with the guidelines, may not be able to follow the guidelines, may not believe that following them will achieve the desired effect, or may have no motivation to change their practice.50
Whatever the reason, Hyman et al51 reported that as many as 30% of physicians did not recommend treatment for patients with diastolic blood pressures of 90 to 100 mm Hg, and a higher proportion did not treat patients with systolic blood pressures of 140 to 160 mm Hg.
BARRIERS IN HEALTH CARE SYSTEMS
Although health care systems present barriers to optimal blood pressure control,20,27,31,52 there is evidence that most cases of uncontrolled hypertension occur in patients with good access to care.32,53,54 For example, an NHANES study53 suggested that most patients with uncontrolled hypertension had in fact seen a physician on average at least three times in the previous year. And this may be more pervasive in African Americans: one survey found hypertension was uncontrolled in 75% of hypertensive African American patients despite free access to care, free medications, and regular follow-up visits.41
Thus, the most significant barriers to blood pressure control appear to be patient-related and physician-related.
INTERVENTIONS AIMED AT PATIENTS
The most common approaches to improving blood pressure control at the patient level, regardless of race, are patient education,55–61 home blood pressure monitoring,62–67 and behavioral counseling to address misconceptions about hypertension,68 to improve adherence to drug therapy,69–73 and to encourage lifestyle modifications.74–78
Patient education
Patient education can improve blood pressure control.58,79–82 Its aims are to increase patients’ understanding of the disease83 and to encourage them to be more active in their own care.80,84,85
Patient education has a moderate effect on blood pressure control. The average proportion of patients whose hypertension was under control in community-based trials of various interventions ranged from 60% to 70%, compared with 38% to 46% with usual care.56,80,81
However, these strategies largely did not address misconceptions patients have about hypertension. This issue is especially critical in African Americans, who may have different perceptions of hypertension and different expectations for care41: beliefs that hypertension is “curable,” not chronic, and that medication is needed only for hypertension-related symptoms may translate to poorer rates of medication adherence.
Levine et al1 evaluated the efficacy of home visits by trained community health advisory board workers in a neighborhood in Baltimore, MD, with a high prevalence of hypertension. Participants were randomized to receive either one visit or five visits during the 40-month study period. Both groups had a statistically significant reduction in blood pressure, and in both groups the proportion of patients with adequate blood pressure control increased significantly. The results support the use of a practice- and community-based partnership to improve blood pressure control in African American patients.
Ogedegbe et al2 randomized 190 hypertensive African American patients to receive usual care or quarterly counseling sessions that used motivational interviewing focused on medication adherence. The counseled patients stayed adherent to their medications, whereas adherence declined significantly in those receiving usual care. This effect was associated with a modest, nonsignificant trend toward a net reduction in systolic blood pressure with motivational interviewing.
A novel method of health education is the use of narrative communication—ie, storytelling. It has a good amount of evidence to support it, as culturally appropriate storytelling may allow patients to identify with a story as it relates to their own lives.86–89 Examples of educational storytelling include:
- A woman with hypertension discussing what it means to have high blood pressure, and the benefits of controlling it, such as living long enough to see her grandchildren grow up
- A man discussing the importance of involving family and friends to help control blood pressure, and how dietary modifications can be made to ensure that salt alternatives are used when the family does the cooking.
Storytelling should be done in a culturally appropriate context. For example, storytellers should have the same background as the patient (ie, similar socioeconomic status and ethnic background): patients are more likely to be influenced if they identify with the storyteller and imagine themselves in a similar situation.
Houston et al3 randomized 299 hypertensive African Americans to view either three DVDs that featured patients with hypertension or three “attention-control DVDs” on topics not related to hypertension. The intervention group’s DVDs focused on storytelling and “learning more.” In the storytelling section, patients told personal stories about what it meant to have hypertension and gave advice on how to best interact with health care providers and methods to improve medication adherence. A “learning more” section focused on what high blood pressure is, addressed therapeutic lifestyle changes, and encouraged patients to communicate with their health care providers. The patients who viewed the patient narratives had significantly lower blood pressure at 3 months than those assigned to usual care. Although blood pressure subsequently increased in both groups, the benefits of the intervention still existed at the end of follow-up.
Important to note about two of the above three studies1,3 is that the interventions were done by people other than physicians, thus emphasizing the importance of a team approach to blood pressure control.
Behavioral counseling
The effectiveness of lifestyle modifications such as diet, weight loss, and physical activity in preventing and treating hypertension is well established.74–78 For example:
- In the Dietary Approaches to Stop Hypertension (DASH) trial,76 a healthy diet lowered blood pressure about as much as single drugs do, particularly in African Americans.
- The Trial of Nonpharmacologic Interventions in the Elderly (TONE)74 showed that exercise can lower blood pressure in obese hypertensive patients.
- The PREMIER trial (Lifestyle Interventions for Blood Pressure Control)75 showed that a single brief counseling session could produce substantial decreases in blood pressure in patients with stage 1 hypertension or high-normal blood pressure.
Unfortunately, these results have been hard to translate into primary care practice, especially for African American patients. Several studies have evaluated the impact of lifestyle interventions on blood pressure control in primary care practices with a large population of African American patients.
Bosworth et al,4 in a study of a practice in which almost half the patients were African American, randomized patients to receive usual care, nurse-administered tailored behavioral telephone counseling, home blood pressure monitoring, or home monitoring plus tailored behavioral telephone counseling. The combination of home monitoring and tailored behavioral telephone counseling led to a statistically significant improvement at 24 months compared with baseline.
Home blood pressure monitoring
The effectiveness of self-monitoring in improving blood pressure control is also well documented.62,63,65–67,90–95
Pickering et al62 studied patients with poorly controlled hypertension in a managed-care setting and found a reduction of 7 mm Hg systolic and 5 mm Hg diastolic pressure after 3 to 6 months of home monitoring compared with usual care.
Mengden et al,94 in a similar study, found average blood pressure reductions at 6 months of 19.3/11.9 mm Hg in the home-monitoring group vs 10.6/8.8 mm Hg in the usual-care group.
The effect of home blood pressure monitoring may be greater in African Americans.
Rogers et al93 found it to be more effective at lowering blood pressure than usual care in a group of 121 patients with poorly controlled hypertension followed in primary care practices, and these reductions were twice as large in African American patients than in white patients.93
Bondmass,92 in a study of 33 African American patients with poorly controlled hypertension, reported a 53% control rate within 4 weeks of home monitoring. All patients in the study had uncontrolled blood pressure at baseline (> 140/90 mm Hg).
Artinian et al5 evaluated the effect of nurse-managed telemonitoring on blood pressure control vs enhanced usual care. All participants were African American. The monitored group had a significantly greater reduction in systolic pressure at 12 months compared with those who received enhanced usual care.
PHYSICIAN-LEVEL INTERVENTIONS
Most interventions to improve how physicians manage patients with hypertension are designed to improve adherence to treatment guidelines. In most cases, these interventions are based on continuous quality improvement and disease management concepts such as physician education and academic detailing, reminders, feedback on performance measures, and risk-assessment tools.96,97
Physician education
Interest is increasing in physician educational interventions for blood pressure control.24,98
Inui et al,99 in an early study in a primary care practice, found that patients of physicians who received tutorials on hypertension management were more compliant with their drug regimens and had better blood pressure control than patients of physicians in the control group.
Jennett et al,100 in a similar randomized clinical trial, found that physicians who participated in an education activity were more adherent to treatment guidelines at 6 and 12 months compared with those who did not participate.
Maue et al101 showed that rates of blood pressure control improved from 41% to 52% after a 6-month educational intervention for physicians in a managed-care setting.
Tu et al102 reviewed 12 studies in which seven different physician educational interventions were used either alone or in combination and concluded that physician education improves compliance with guidelines for managing hypertension.
Unfortunately, these studies did not report outcomes separately for African American and white patients.
Hicks et al6 found that disease management approaches that target physicians whose patients with hypertension are mostly African American did not yield clinically relevant improvement in these patients, and that minority patients were significantly less likely to have their blood pressure controlled at the end of the study compared with their non-Hispanic white counterparts.
Feedback to providers
Several studies have shown that, given reminders and feedback systems, physicians will change their practice.103–106
Mashru and Lant104 combined chart audits and physician education in primary care practices and found they improved physician performance measures such as accuracy of diagnosis, number of patients who received cardiovascular risk assessment, and number of patients whose treatment was based on clinical laboratory assessments.
Feedback takes many forms but consists mostly of computerized information107 or peer-to-peer academic detailing with opinion leaders.108–110
Dickinson et al,106 for instance, showed that computer-generated listings of patients’ blood pressures combined with a physician education program on clinical management of hypertension led to increased knowledge and better follow-up on their patients.
Again, however, these studies did not distinguish between African American and white patients, which makes it difficult to judge whether or not these approaches work differently for physicians with a large proportion of African American patients.
Computerized decision-support systems
Computerized decision-support systems have proliferated in primary care practices.111
McAlister et al103 found that general practitioners randomized to manage hypertension with the assistance of a computer obtained better outcomes than with usual care.
Montgomery and Fahey,107 in a systematic review, found improved blood pressure control in two of the three trials that compared computer-generated feedback reports and reminders to usual care. Specifically, 51% of patients whose physicians received reminders either had controlled blood pressure or were at least receiving treatment vs 33% in the control group at 12 months. This difference was even higher at 24 months.
Montgomery et al7 later randomized primary care practices to use a computer-based decision-support system and a cardiovascular risk chart, the risk chart alone, or to continue as usual. Results indicated no reduction in cardiovascular risk in the computer-system or the chart-only group, whereas patients in the chart-only group had a significant reduction in systolic pressure and were prescribed more cardiovascular drugs. This study indicates that use of a computerized decision-support system is not superior to chart review and audit feedback alone.
Evidence that computerized decision systems improve blood pressure control in African Americans is scant. However, when one looks at the evidence from studies of African Americans, the outcomes do not seem to differ between African American and white patients.
Hicks et al6 examined the effectiveness of computerized decision support in improving hypertension care in a racially diverse population. Physicians were randomized to receive computerized decision support or to provide usual care without computerized support. Both groups improved significantly in prescribing appropriate drugs but not in overall blood pressure control. Furthermore, the study showed no reduction in racial disparities of care and blood pressure control.
A potential explanation for the lack of improvement in blood pressure was that the intervention dealt with making sure the appropriate drugs were prescribed rather than making sure physicians also appropriately intensified antihypertensive management when necessary.
INTERVENTIONS TARGETING PATIENTS AND PHYSICIANS
Several studies have targeted both patient and physician-level barriers to blood pressure control in practice-based settings.
Roumie et al8 randomized physicians to one of three intervention groups:
- “Provider education” consisting of an email message with a Web-based link to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7)
- Provider education plus a computer alert with information about their patient’s blood pressure
- Provider education, a computer alert, and patient education (ie, patients received a letter encouraging adherence to drug therapy, changing their lifestyle, and talking with their doctor about their blood pressure).
Patients whose providers were randomized to the third group had better blood pressure control. The report did not differentiate African American vs white patients. The data, however, did show the effectiveness of adding patient education to provider education to improve blood pressure control.
Bosworth et al,112 in a study in which 40% of patients were African American, randomized patients to usual care or to bimonthly nurse-delivered behavioral telephone counseling. They also randomized providers either to receive computer-generated decision support designed to improve adherence to guidelines or to receive no support.
There were no significant differences in rates of blood pressure control in the intervention groups compared with a control group. Although differences in blood pressure control between groups were not significant, patients randomized to behavioral intervention had significantly better blood pressure control at the 24-month follow-up than at baseline.
Svetkey et al9 evaluated the effects of physician intervention, patient intervention, and physician intervention plus patient intervention compared with control on systolic blood pressure at 6 months. They found that an intensive behavioral lifestyle intervention led to a significant reduction in systolic pressure at 6 months. By itself, the physician intervention did not have a meaningful effect, but patients in the combined physician-and-patient-intervention group experienced the greatest reduction (9.7 ± 12.7 mm Hg).
It takes a team
Physicians should not be the only focus in helping patients achieve blood pressure control. Although physician and patient factors need to be addressed to improve blood pressure control in African Americans, emphasis should also be placed on interdisciplinary, team-based care utilizing health care providers such as nurses, physician assistants, and pharmacists. Team-based care has been shown to have the greatest impact of all the strategies for improving blood pressure control.113 There is a good amount of evidence involving interventions with a focus on health care providers other than physicians, although the data lack a sufficient focus on African Americans.
Carter et al,10 in a randomized controlled trial in which 26.3% of the patients were African American, found that an intervention consisting of clinical pharmacists giving physicians drug therapy recommendations based on national guidelines resulted in a significantly lower blood pressure compared with a control group: the mean reduction was 20.7/9.7 mm Hg in the intervention group vs 6.8/4.5 mm Hg in the control group.
Carter et al114 performed a meta-analysis of 37 studies and found that two strategies led to a significant reduction in blood pressure: a pharmacist-led intervention with treatment recommendations to physicians resulted in a systolic pressure reduction of 9.30 mm Hg; and nurse-led interventions resulted in a systolic pressure reduction of 4.80 mm Hg. Again, many of the studies cited in this meta-analysis lacked a focus on African Americans.
Hunt et al11 conducted a randomized controlled trial in which pharmacists actively participated in the management of blood pressure. They were involved with every aspect of care, including reviewing medications and adverse drug reactions, assessing lifestyle behaviors and barriers to adherence, making dosing adjustments, and adding medications. Patients randomized to the intervention group achieved significantly lower systolic and diastolic pressures (137/75 vs 143/78 mm Hg in the control group). However, information about race was not included.
The above studies are just a few out of a large body of evidence demonstrating the value of team-based care to improve blood pressure control. It has yet to be determined whether these models can improve blood pressure control specifically in African Americans, since so many of these trials lacked a focus on this group. Promising is an ongoing randomized prospective trial by Carter et al115 evaluating a model of collaboration between physicians and pharmacists, with a focus on patients in underrepresented minorities.
SO WHAT WORKS?
Although there is a growing body of literature on interventions to try to reduce disparities in hypertension and blood pressure control between African Americans and whites, only a few randomized controlled trials have focused on African Americans, and several have not reported their results.116 So the question remains: How should we interpret the available data, which are aggregated across racial groups, and put it into practice when caring for hypertensive African American patients?
Patient education. In trying to overcome patient-related barriers, emphasis should be on patient education, in particular addressing misconceptions about hypertension and promoting adherence to antihypertensive therapy. This is evident from the narrative storytelling intervention by Houston et al.3 Although this is the first study of its kind, this strategy may be something to consider if future studies replicate these findings. Culturally appropriate storytelling may allow patients to identify with the stories as they relate to their own personal lives. It can be an effective way to address patient education and change behaviors.
Self-monitoring with a home blood pressure monitor has also proven effective in the management of hypertension in African Americans. Indeed, the few studies that reported findings in African Americans showed impressive reductions in blood pressure. The benefits of home monitoring are well documented, and the effect on physician-related barriers such as clinical inertia are also quite impressive.117 However, most of these studies did not assess the long-term impact or cost-effectiveness of home monitoring on blood pressure control.
Behavioral counseling. Although we have good evidence of the effectiveness of behavioral counseling, whether this is sustained long-term has been less studied in African Americans. Thus, while interventions that targeted African Americans have reported impressive reductions in blood pressure, the effect tends to be greatest during the first few months of implementation, with the benefits disappearing over time.
Physician-related interventions. With regard to physician-level interventions, research has focused on physician education, utilizing alerts and computerized clinical decision-support systems. Evidence is scant on whether the use of computerized systems results in improves hypertension care in African Americans. However, a closer look at the data from studies that report outcomes in African American and white patients shows that the results do not seem to differ between these groups. Still, there is insufficient information about the impact on hypertensive African Americans.6
Strategies that address both patient- and physician-related barriers can improve overall blood pressure control; however, there is a lack of data comparing outcomes in hypertensive African Americans with those of whites, making it difficult to know if this would be an effective strategy in African American patients alone.
More studies needed that focus on African Americans
Developing interventions to improve blood pressure control in African Americans should be an ongoing priority for research if we intend to address racial disparities in cardiovascular disease. Although it is reassuring that there is a growing body of evidence and research with this focus,118–121 more research is needed to determine effective strategies that address barriers related to physician practice and to the health care system overall as they relate to blood pressure control in African Americans. More importantly, these strategies should also emphasize a team-based approach that includes nurses, pharmacists, and physician assistants. Developing targeted interventions for hypertensive African Americans will help reduce disparities in the rates of cardiovascular illness and death in this patient population.
- Levine DM, Bone LR, Hill MN, et al. The effectiveness of a community/academic health center partnership in decreasing the level of blood pressure in an urban African-American population. Ethn Dis 2003; 13:354–361.
- Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens 2008; 21:1137–1143.
- Houston TK, Allison JJ, Sussman M, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med 2011; 154:77–84.
- Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009; 151:687–695.
- Artinian NT, Flack JM, Nordstrom CK, et al. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res 2007; 56:312–322.
- Hicks LS, Sequist TD, Ayanian JZ, et al. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med 2008; 23:429–441.
- Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ 2000; 320:686–690.
- Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006; 145:165–175.
- Svetkey LP, Pollak KI, Yancy WS, et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension 2009; 54:1226–1233.
- Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med 2009; 169:1996–2002.
- Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med 2008; 23:1966–1972.
- US Department of Health and Human Services: Office of Disease Prevention and Health Promotion—Healthy People 2010. Nasnewsletter 2000; 15:3.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- US Centers for Disease Control and Prevention. Age-specific excess deaths associated with stroke among racial/ethnic minority populations–United States, 1997. JAMA 2000; 283:2382–2383.
- Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black-white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med 1995; 155:1319–1324.
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 1997; 277:1293–1298.
- Pavlik VN, Hyman DJ, Vallbona C, Toronjo C, Louis K. Hypertension awareness and control in an inner-city African-American sample. J Hum Hypertens 1997; 11:277–283.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation Feb 1; 123( 4):e18–e209.
- Chobanian AV, Bakris GL, Black HR, et al., Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bone LR, Hill MN, Stallings R, et al. Community health survey in an urban African-American neighborhood: distribution and correlates of elevated blood pressure. Ethn Dis 2000; 10:87–95.
- Weber MA. Strategies for improving blood pressure control. Am J Hypertens 1998; 11:897–899.
- Hill MN, Sutton BS. Barriers to hypertension care and control. Curr Hypertens Rep 2000; 2:445–450.
- Alderman MH. Barriers to blood pressure control. Am J Hypertens 1999; 12:1268–1269.
- Chobanian AV. Control of hypertension—an important national priority. N Engl J Med 2001; 345:534–535.
- Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation 1997; 95:1085–1090.
- Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In:Haynes RB, Taylor DW, Sackett DL, eds. Compliance in Health Care. Baltimore, MD: John Hopkins University Press; 1979:11–22.
- Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Correlates of nonadherence to hypertension treatment in an inner-city minority population. Am J Public Health 1992; 82:1607–1612.
- Kirscht JP, Rosenstock IM. Patient adherence to antihypertensive medical regimens. J Community Health. 1977; 3:115–124.
- Hershey JC, Morton BG, Davis JB, Reichgott MJ. Patient compliance with antihypertensive medication. Am J Public Health 1980; 70:1081–1089.
- Bosworth HB, Powers B, Grubber JM, et al. Racial differences in blood pressure control: potential explanatory factors. J Gen Intern Med 2008; 23:692–698.
- Douglas JG, Ferdinand KC, Bakris GL, Sowers JR. Barriers to blood pressure control in African Americans. Overcoming obstacles is challenging, but target goals can be attained. Postgrad Med 2002; 112:51–52,55,59–62passim.
- Knight EL, Bohn RL, Wang PS, Glynn RJ, Mogun H, Avorn J. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension 2001; 38:809–814.
- Ogedegbe G, Mancuso CA, Allegrante JP. Expectations of blood pressure management in hypertensive African-American patients: a qualitative study. J Natl Med Assoc 2004; 96:442–449.
- Blumhagen D. Hypertension: a folk illness with a medical name. Cult Med Psychiatry 1980; 4:197–224.
- Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: the example of hypertension. Health Psychol 1985; 4:115–135.
- Nelson EC, Stason WB, Neutra RR, Solomon HS, McArdle PJ. Impact of patient perceptions on compliance with treatment for hypertension. Med Care 1978; 16:893–906.
- Heurtin-Roberts S. ‘High-pertension’—the uses of a chronic folk illness for personal adaptation. Soc Sci Med 1993; 37:285–294.
- Lang T. Social and economic factors as obstacles to blood pressure control. Am J Hypertens 1998; 11:900–902.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Stamler R, Shipley M, Elliott P, Dyer A, Sans S, Stamler J. Higher blood pressure in adults with less education. Some explanations from INTERSALT. Hypertension 1992; 19:237–241.
- Heurtin-Roberts S, Reisin E. The relation of culturally influenced lay models of hypertension to compliance with treatment. Am J Hypertens 1992; 5:787–792.
- Snow LF. Folk medical beliefs and their implications for care of patients. A review bases on studies among black Americans. Ann Intern Med 1974; 81:82–96.
- Hicks LS, Shaykevich S, Bates DW, Ayanian JZ. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC Cardiovasc Disord 2005; 5:16.
- Mehta SS, Wilcox CS, Schulman KA. Treatment of hypertension in patients with comorbidities: results from the study of hypertensive prescribing practices (SHyPP). Am J Hypertens 1999; 12:333–340.
- Ballard DJ, Strogatz DS, Wagner EH, et al. Hypertension control in a rural southern community: medical care process and dropping out. Am J Prev Med 1988; 4:133–139.
- Hajjar I, Miller K, Hirth V. Age-related bias in the management of hypertension: a national survey of physicians’ opinions on hypertension in elderly adults. J Gerontol A Biol Sci Med Sci 2002; 57:M487–M491.
- McAlister FA, Laupacis A, Teo KK, Hamilton PG, Montague TJ. A survey of clinician attitudes and management practices in hypertension. J Hum Hypertens 1997; 11:413–419.
- Trilling JS, Froom J. The urgent need to improve hypertension care. Arch Fam Med 2000; 9:794–801.
- Huse DM, Roht LH, Alpert JS, Hartz SC. Physicians’ knowledge, attitudes, and practice of pharmacologic treatment of hypertension. Ann Pharmacother 2001; 35:1173–1179.
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282:1458–1465.
- Hyman DJ, Pavlik VN, Vallbona C. Physician role in lack of awareness and control of hypertension. J Clin Hypertens (Greenwich) 2000; 2:324–330.
- Morley Kotchen J, Walker WE, Kotchen TA. Rationale for a community approach to hypertension control among inner city minority populations. Heart Dis Stroke 1994; 3:61–62.
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med 2001; 345:479–486.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Pierce JP, Watson DS, Knights S, Gliddon T, Williams S, Watson R. A controlled trial of health education in the physician’s office. Prev Med 1984; 13:185–194.
- Morisky DE, DeMuth NM, Field-Fass M, Green LW, Levine DM. Evaluation of family health education to build social support for long-term control of high blood pressure. Health Educ Q 1985; 12:35–50.
- Lorgelly P, Siatis I, Brooks A, et al. Is ambulatory blood pressure monitoring cost-effective in the routine surveillance of treated hypertensive patients in primary care? Br J Gen Pract 2003; 53:794–796.
- Green LW, Levine DM, Wolle J, Deeds S. Development of randomized patient education experiments with urban poor hypertensives. Patient Couns Health Educ 1979; 1:106–111.
- Gruesser M, Hartmann P, Schlottmann N, Lohmann FW, Sawicki PT, Joergens V. Structured patient education for out-patients with hypertension in general practice: a model project in Germany. J Hum Hypertens 1997; 11:501–506.
- Mühlhauser I, Sawicki PT, Didjurgeit U, Jörgens V, Trampisch HJ, Berger M. Evaluation of a structured treatment and teaching programme on hypertension in general practice. Clin Exp Hypertens 1993; 15:125–142.
- Roca B, Nadal E, Rovira RE, Valls S, Lapuebla C, Lloría N. Usefulness of a hypertension education program. South Med J 2003; 96:1133–1137.
- Pickering TG, Gerin W, Holland JK. Home blood pressure teletransmission for better diagnosis and treatment. Curr Hypertens Rep 1999; 1:489–494.
- Yarows SA, Julius S, Pickering TG. Home blood pressure monitoring. Arch Intern Med 2000; 160:1251–1257.
- Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet 1976; 1:1265–1268.
- Johnson AL, Taylor DW, Sackett DL, Dunnett CW, Shimizu AG. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J 1978; 119:1034–1039.
- Carnahan JE, Nugent CA. The effects of self-monitoring by patients on the control of hypertension. Am J Med Sci 1975; 269:69–73.
- Stahl SM, Kelley CR, Neill PJ, Grim CE, Mamlin J. Effects of home blood pressure measurement on long-term BP control. Am J Public Health 1984; 74:704–709.
- Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med 2001; 21:221–232.
- Haynes RB, Mattson ME, Engebretson TO. Patient compliance to prescribed antihypertensive medication regimens: a report to the National Heart, Lung, and Blood institute. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, 1980. NIH publication 81-2102.
- Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med 1997; 19:239–263.
- Dunbar-Jacob J, Dwyer K, Dunning EJ. Compliance with antihypertensive regimen: a review of the research in the 1980s. Ann Behav Med 1991; 13:31–39.
- Haynes RB, Montague P, Oliver T, McKibbon KA, Brouwers MC, Kanani R. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2000; ( 2):CD000011.
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care 1998; 36:1138–1161.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Appel LJ, Champagne CM, Harsha DW, et al; Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093.
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336:1117–1124.
- Moore TJ, Conlin PR, Ard J, Svetkey LP. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension 2001; 38:155–158.
- Stevens VJ, Obarzanek E, Cook NR, et al; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134:1–11.
- Sawicki PT, Mühlhauser I, Didjurgeit U, Berger M. Improvement of hypertension care by a structured treatment and teaching programme. J Hum Hypertens 1993; 7:571–573.
- Morisky DE, Bowler MH, Finlay JS. An educational and behavioral approach toward increasing patient activation in hypertension management. J Community Health 1982; 7:171–182.
- Levine DM, Green LW, Deeds SG, Chwalow J, Russell RP, Finlay J. Health education for hypertensive patients. JAMA 1979; 241:1700–1703.
- Iso H, Shimamoto T, Yokota K, Sankai T, Jacobs DR, Komachi Y. Community-based education classes for hypertension control. A 1.5-year randomized controlled trial. Hypertension 1996; 27:968–974.
- Cuspidi C, Sampieri L, Macca G, et al. Improvement of patients’ knowledge by a single educational meeting on hypertension. J Hum Hypertens 2001; 15:57–61.
- Nessman DG, Carnahan JE, Nugent CA. Increasing compliance. Patient-operated hypertension groups. Arch Intern Med 1980; 140:1427–1430.
- Casasanta L, Patel S. Outcomes of an educational component of a disease management program for hypertension. Manag Care Interface 1999; 12:70–73.
- McAdams DP. The Stories We Live By: Personal Myths and the Making of the Self. New York NY: The Guilford Press; 1993.
- Bruner J. Acts of Meaning. Cambridge, MA: Harvard Univ Pr; 1990.
- Slater MD, Rouner D. Entertainment—education and elaboration likelihood: Understanding the processing of narrative persuasion. Commun Theory 2002; 12:173–191.
- Dal CS, Zanna MP, Fong GT. Narrative persuasion and overcoming resistance. In:Knowles ES, Linn J, eds. Resistance and Persuasion. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004:175–191.
- Artinian NT, Washington OG, Templin TN. Effects of home telemonitoring and community-based monitoring on blood pressure control in urban African Americans: a pilot study. Heart Lung 2001; 30:191–199.
- Bailey B, Carney SL, Gillies AA, Smith AJ. Antihypertensive drug treatment: a comparison of usual care with self blood pressure measurement. J Hum Hypertens 1999; 13:147–150.
- Bondmass M. The effect of home monitoring and telemanagement on blood pressure control among African Americans. Telemed J 2000; 6:15–23.
- Rogers MA, Small D, Buchan DA, et al. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med 2001; 134:1024–1032.
- Mengden T, Uen S, Baulmann J, Vetter H. Significance of blood pressure self-measurement as compared with office blood pressure measurement and ambulatory 24-hour blood pressure measurement in pharmacological studies. Blood Press Monit 2003; 8:169–172.
- Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens 1996; 9:285–292.
- Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995; 153:1423–1431.
- Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract 1998; 48:991–997.
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995; 274:700–705.
- Inui TS, Yourtee EL, Williamson JW. Improved outcomes in hypertension after physician tutorials. A controlled trial. Ann Intern Med 1976; 84:646–651.
- Jennett PA, Wilson TW, Hayton RC, Mainprize GW, Laxdal OE. Desirable behaviours in the office management of hypertension addressed through continuing medical education. Can J Public Health 1989; 80:359–362.
- Maue SK, Rivo ML, Weiss B, Farrelly EW, Brower-Stenger S. Effect of a primary care physician-focused, population-based approach to blood pressure control. Fam Med 2002; 34:508–513.
- Tu K, Davis D. Can we alter physician behavior by educational methods? Lessons learned from studies of the management and follow-up of hypertension. J Contin Educ Health Prof 2002; 22:11–22.
- McAlister NH, Covvey HD, Tong C, Lee A, Wigle ED. Randomised controlled trial of computer assisted management of hypertension in primary care. Br Med J (Clin Res Ed) 1986; 293:670–674.
- Mashru M, Lant A. Interpractice audit of diagnosis and management of hypertension in primary care: educational intervention and review of medical records. BMJ 1997; 314:942–946.
- Degoulet P, Menard J, Berger C, Plouin PF, Devries C, Hirel JC. Hypertension management: the computer as a participant. Am J Med 1980; 68:559–567.
- Dickinson JC, Warshaw GA, Gehlbach SH, Bobula JA, Muhlbaier LH, Parkerson GR. Improving hypertension control: impact of computer feedback and physician education. Med Care 1981; 19:843–854.
- Montgomery AA, Fahey T. A systematic review of the use of computers in the management of hypertension. J Epidemiol Community Health 1998; 52:520–525.
- Coleman MT, Lott JA, Sharma S. Use of continuous quality improvement to identify barriers in the management of hypertension. Am J Med Qual 2000; 15:72–77.
- Goldberg HI, Wagner EH, Fihn SD, et al. A randomized controlled trial of CQI teams and academic detailing: can they alter compliance with guidelines? Jt Comm J Qual Improv 1998; 24:130–142.
- Horowitz CR, Goldberg HI, Martin DP, et al. Conducting a randomized controlled trial of CQI and academic detailing to implement clinical guidelines. Jt Comm J Qual Improv 1996; 22:734–750.
- Johnson B, McNair D, Kailasam K, et al. Discern—an integrated prospective decision support system. Proc Annu Symp Comput Appl Med Care 1994; 969.
- Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J 2009; 157:450–456.
- Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care 2006; 44:646–657.
- Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med 2009; 169:1748–1755.
- Carter BL, Clarke W, Ardery G, et al; Collaboration Among Pharmacists Physicians To Improve Outcomes Now (CAPTION) Trial Investigators. A cluster-randomized effectiveness trial of a physician-pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:418–423.
- Einhorn PT. National heart, lung, and blood institute-initiated program “interventions to improve hypertension control rates in African Americans”: background and implementation. Circ Cardiovasc Qual Outcomes 2009; 2:236–240.
- Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011; 57:29–38.
- Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns 2008; 70:338–347.
- Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans’ study to improve the control of hypertension (V-STITCH): design and methodology. Contemp Clin Trials 2005; 26:155–168.
- Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension (CAATCH) trial: a multi-level intervention to improve blood pressure control in hypertensive blacks. Circ Cardiovasc Qual Outcomes 2009; 2:249–256.
- Bosworth HB, Almirall D, Weiner BJ, et al. The implementation of a translational study involving a primary care based behavioral program to improve blood pressure control: The HTN-IMPROVE study protocol (01295). Implement Sci 2010; 5:54.
High blood pressure takes a devastating toll on African Americans. Better control can go a long way to closing the “mortality gap” between African Americans and white Americans. But which strategies are best to address this complex problem?
In this report, we review the evidence on practice-based approaches to improving blood pressure control, from new styles of patient education to home blood pressure monitoring, focusing on studies in African Americans (Table 1).1–11
BETTER CONTROL IS NEEDED
PATIENT-RELATED BARRIERS
Patient-related barriers24–40 include:
- Poor knowledge about hypertension and its consequences31,32
- Poor adherence to drug therapy (a major factor,24–26 as African Americans have poorer adherence rates than whites,27–29 which may explain some of the racial disparity in blood pressure control30)
- False health beliefs34–37
- Inability to change one’s lifestyle
- Side effects of antihypertensive drugs32
- Unrealistic expectations of treatment (eg, a cure33)
- Demographic factors (eg, socioeconomic status, educational level, age, sex).24,38–40
Perhaps the most salient and easily modifiable of these factors are patients’ reluctance to modify their lifestyle and their misconceptions about the causes, treatment, and prevention of hypertension. Patients whose beliefs are discordant with traditional biomedical concepts of hypertension have poorer blood pressure control than those whose beliefs are concordant.41 This may be more relevant to African Americans, since they are known to have cultural health beliefs that differ from those of Western culture (eg, that hypertension is a curable rather than a chronic illness, and that hypertension is a disease of nerves that often affects the blood and clogs the arteries).42
PHYSICIAN-RELATED BARRIERS
Barriers to effective blood pressure control at the physician level43–48 include:
- Nonadherence to treatment guidelines44
- Failure to intensify the regimen if goals are not met45
- Failure to emphasize therapeutic lifestyle changes.43,46–48
When primary care physicians do not follow evidence-based guidelines, the reason may be that they are not aware of them or that they do not understand them. In a national survey of 1,029 physicians that was designed to explore how well physicians know the indications for specific antihypertensive drugs and how closely their opinions and practice agreed with national guidelines, only 37.3% correctly answered all of the knowledge-related questions.49
Other reasons for nonadherence are that physicians may disagree with the guidelines, may not be able to follow the guidelines, may not believe that following them will achieve the desired effect, or may have no motivation to change their practice.50
Whatever the reason, Hyman et al51 reported that as many as 30% of physicians did not recommend treatment for patients with diastolic blood pressures of 90 to 100 mm Hg, and a higher proportion did not treat patients with systolic blood pressures of 140 to 160 mm Hg.
BARRIERS IN HEALTH CARE SYSTEMS
Although health care systems present barriers to optimal blood pressure control,20,27,31,52 there is evidence that most cases of uncontrolled hypertension occur in patients with good access to care.32,53,54 For example, an NHANES study53 suggested that most patients with uncontrolled hypertension had in fact seen a physician on average at least three times in the previous year. And this may be more pervasive in African Americans: one survey found hypertension was uncontrolled in 75% of hypertensive African American patients despite free access to care, free medications, and regular follow-up visits.41
Thus, the most significant barriers to blood pressure control appear to be patient-related and physician-related.
INTERVENTIONS AIMED AT PATIENTS
The most common approaches to improving blood pressure control at the patient level, regardless of race, are patient education,55–61 home blood pressure monitoring,62–67 and behavioral counseling to address misconceptions about hypertension,68 to improve adherence to drug therapy,69–73 and to encourage lifestyle modifications.74–78
Patient education
Patient education can improve blood pressure control.58,79–82 Its aims are to increase patients’ understanding of the disease83 and to encourage them to be more active in their own care.80,84,85
Patient education has a moderate effect on blood pressure control. The average proportion of patients whose hypertension was under control in community-based trials of various interventions ranged from 60% to 70%, compared with 38% to 46% with usual care.56,80,81
However, these strategies largely did not address misconceptions patients have about hypertension. This issue is especially critical in African Americans, who may have different perceptions of hypertension and different expectations for care41: beliefs that hypertension is “curable,” not chronic, and that medication is needed only for hypertension-related symptoms may translate to poorer rates of medication adherence.
Levine et al1 evaluated the efficacy of home visits by trained community health advisory board workers in a neighborhood in Baltimore, MD, with a high prevalence of hypertension. Participants were randomized to receive either one visit or five visits during the 40-month study period. Both groups had a statistically significant reduction in blood pressure, and in both groups the proportion of patients with adequate blood pressure control increased significantly. The results support the use of a practice- and community-based partnership to improve blood pressure control in African American patients.
Ogedegbe et al2 randomized 190 hypertensive African American patients to receive usual care or quarterly counseling sessions that used motivational interviewing focused on medication adherence. The counseled patients stayed adherent to their medications, whereas adherence declined significantly in those receiving usual care. This effect was associated with a modest, nonsignificant trend toward a net reduction in systolic blood pressure with motivational interviewing.
A novel method of health education is the use of narrative communication—ie, storytelling. It has a good amount of evidence to support it, as culturally appropriate storytelling may allow patients to identify with a story as it relates to their own lives.86–89 Examples of educational storytelling include:
- A woman with hypertension discussing what it means to have high blood pressure, and the benefits of controlling it, such as living long enough to see her grandchildren grow up
- A man discussing the importance of involving family and friends to help control blood pressure, and how dietary modifications can be made to ensure that salt alternatives are used when the family does the cooking.
Storytelling should be done in a culturally appropriate context. For example, storytellers should have the same background as the patient (ie, similar socioeconomic status and ethnic background): patients are more likely to be influenced if they identify with the storyteller and imagine themselves in a similar situation.
Houston et al3 randomized 299 hypertensive African Americans to view either three DVDs that featured patients with hypertension or three “attention-control DVDs” on topics not related to hypertension. The intervention group’s DVDs focused on storytelling and “learning more.” In the storytelling section, patients told personal stories about what it meant to have hypertension and gave advice on how to best interact with health care providers and methods to improve medication adherence. A “learning more” section focused on what high blood pressure is, addressed therapeutic lifestyle changes, and encouraged patients to communicate with their health care providers. The patients who viewed the patient narratives had significantly lower blood pressure at 3 months than those assigned to usual care. Although blood pressure subsequently increased in both groups, the benefits of the intervention still existed at the end of follow-up.
Important to note about two of the above three studies1,3 is that the interventions were done by people other than physicians, thus emphasizing the importance of a team approach to blood pressure control.
Behavioral counseling
The effectiveness of lifestyle modifications such as diet, weight loss, and physical activity in preventing and treating hypertension is well established.74–78 For example:
- In the Dietary Approaches to Stop Hypertension (DASH) trial,76 a healthy diet lowered blood pressure about as much as single drugs do, particularly in African Americans.
- The Trial of Nonpharmacologic Interventions in the Elderly (TONE)74 showed that exercise can lower blood pressure in obese hypertensive patients.
- The PREMIER trial (Lifestyle Interventions for Blood Pressure Control)75 showed that a single brief counseling session could produce substantial decreases in blood pressure in patients with stage 1 hypertension or high-normal blood pressure.
Unfortunately, these results have been hard to translate into primary care practice, especially for African American patients. Several studies have evaluated the impact of lifestyle interventions on blood pressure control in primary care practices with a large population of African American patients.
Bosworth et al,4 in a study of a practice in which almost half the patients were African American, randomized patients to receive usual care, nurse-administered tailored behavioral telephone counseling, home blood pressure monitoring, or home monitoring plus tailored behavioral telephone counseling. The combination of home monitoring and tailored behavioral telephone counseling led to a statistically significant improvement at 24 months compared with baseline.
Home blood pressure monitoring
The effectiveness of self-monitoring in improving blood pressure control is also well documented.62,63,65–67,90–95
Pickering et al62 studied patients with poorly controlled hypertension in a managed-care setting and found a reduction of 7 mm Hg systolic and 5 mm Hg diastolic pressure after 3 to 6 months of home monitoring compared with usual care.
Mengden et al,94 in a similar study, found average blood pressure reductions at 6 months of 19.3/11.9 mm Hg in the home-monitoring group vs 10.6/8.8 mm Hg in the usual-care group.
The effect of home blood pressure monitoring may be greater in African Americans.
Rogers et al93 found it to be more effective at lowering blood pressure than usual care in a group of 121 patients with poorly controlled hypertension followed in primary care practices, and these reductions were twice as large in African American patients than in white patients.93
Bondmass,92 in a study of 33 African American patients with poorly controlled hypertension, reported a 53% control rate within 4 weeks of home monitoring. All patients in the study had uncontrolled blood pressure at baseline (> 140/90 mm Hg).
Artinian et al5 evaluated the effect of nurse-managed telemonitoring on blood pressure control vs enhanced usual care. All participants were African American. The monitored group had a significantly greater reduction in systolic pressure at 12 months compared with those who received enhanced usual care.
PHYSICIAN-LEVEL INTERVENTIONS
Most interventions to improve how physicians manage patients with hypertension are designed to improve adherence to treatment guidelines. In most cases, these interventions are based on continuous quality improvement and disease management concepts such as physician education and academic detailing, reminders, feedback on performance measures, and risk-assessment tools.96,97
Physician education
Interest is increasing in physician educational interventions for blood pressure control.24,98
Inui et al,99 in an early study in a primary care practice, found that patients of physicians who received tutorials on hypertension management were more compliant with their drug regimens and had better blood pressure control than patients of physicians in the control group.
Jennett et al,100 in a similar randomized clinical trial, found that physicians who participated in an education activity were more adherent to treatment guidelines at 6 and 12 months compared with those who did not participate.
Maue et al101 showed that rates of blood pressure control improved from 41% to 52% after a 6-month educational intervention for physicians in a managed-care setting.
Tu et al102 reviewed 12 studies in which seven different physician educational interventions were used either alone or in combination and concluded that physician education improves compliance with guidelines for managing hypertension.
Unfortunately, these studies did not report outcomes separately for African American and white patients.
Hicks et al6 found that disease management approaches that target physicians whose patients with hypertension are mostly African American did not yield clinically relevant improvement in these patients, and that minority patients were significantly less likely to have their blood pressure controlled at the end of the study compared with their non-Hispanic white counterparts.
Feedback to providers
Several studies have shown that, given reminders and feedback systems, physicians will change their practice.103–106
Mashru and Lant104 combined chart audits and physician education in primary care practices and found they improved physician performance measures such as accuracy of diagnosis, number of patients who received cardiovascular risk assessment, and number of patients whose treatment was based on clinical laboratory assessments.
Feedback takes many forms but consists mostly of computerized information107 or peer-to-peer academic detailing with opinion leaders.108–110
Dickinson et al,106 for instance, showed that computer-generated listings of patients’ blood pressures combined with a physician education program on clinical management of hypertension led to increased knowledge and better follow-up on their patients.
Again, however, these studies did not distinguish between African American and white patients, which makes it difficult to judge whether or not these approaches work differently for physicians with a large proportion of African American patients.
Computerized decision-support systems
Computerized decision-support systems have proliferated in primary care practices.111
McAlister et al103 found that general practitioners randomized to manage hypertension with the assistance of a computer obtained better outcomes than with usual care.
Montgomery and Fahey,107 in a systematic review, found improved blood pressure control in two of the three trials that compared computer-generated feedback reports and reminders to usual care. Specifically, 51% of patients whose physicians received reminders either had controlled blood pressure or were at least receiving treatment vs 33% in the control group at 12 months. This difference was even higher at 24 months.
Montgomery et al7 later randomized primary care practices to use a computer-based decision-support system and a cardiovascular risk chart, the risk chart alone, or to continue as usual. Results indicated no reduction in cardiovascular risk in the computer-system or the chart-only group, whereas patients in the chart-only group had a significant reduction in systolic pressure and were prescribed more cardiovascular drugs. This study indicates that use of a computerized decision-support system is not superior to chart review and audit feedback alone.
Evidence that computerized decision systems improve blood pressure control in African Americans is scant. However, when one looks at the evidence from studies of African Americans, the outcomes do not seem to differ between African American and white patients.
Hicks et al6 examined the effectiveness of computerized decision support in improving hypertension care in a racially diverse population. Physicians were randomized to receive computerized decision support or to provide usual care without computerized support. Both groups improved significantly in prescribing appropriate drugs but not in overall blood pressure control. Furthermore, the study showed no reduction in racial disparities of care and blood pressure control.
A potential explanation for the lack of improvement in blood pressure was that the intervention dealt with making sure the appropriate drugs were prescribed rather than making sure physicians also appropriately intensified antihypertensive management when necessary.
INTERVENTIONS TARGETING PATIENTS AND PHYSICIANS
Several studies have targeted both patient and physician-level barriers to blood pressure control in practice-based settings.
Roumie et al8 randomized physicians to one of three intervention groups:
- “Provider education” consisting of an email message with a Web-based link to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7)
- Provider education plus a computer alert with information about their patient’s blood pressure
- Provider education, a computer alert, and patient education (ie, patients received a letter encouraging adherence to drug therapy, changing their lifestyle, and talking with their doctor about their blood pressure).
Patients whose providers were randomized to the third group had better blood pressure control. The report did not differentiate African American vs white patients. The data, however, did show the effectiveness of adding patient education to provider education to improve blood pressure control.
Bosworth et al,112 in a study in which 40% of patients were African American, randomized patients to usual care or to bimonthly nurse-delivered behavioral telephone counseling. They also randomized providers either to receive computer-generated decision support designed to improve adherence to guidelines or to receive no support.
There were no significant differences in rates of blood pressure control in the intervention groups compared with a control group. Although differences in blood pressure control between groups were not significant, patients randomized to behavioral intervention had significantly better blood pressure control at the 24-month follow-up than at baseline.
Svetkey et al9 evaluated the effects of physician intervention, patient intervention, and physician intervention plus patient intervention compared with control on systolic blood pressure at 6 months. They found that an intensive behavioral lifestyle intervention led to a significant reduction in systolic pressure at 6 months. By itself, the physician intervention did not have a meaningful effect, but patients in the combined physician-and-patient-intervention group experienced the greatest reduction (9.7 ± 12.7 mm Hg).
It takes a team
Physicians should not be the only focus in helping patients achieve blood pressure control. Although physician and patient factors need to be addressed to improve blood pressure control in African Americans, emphasis should also be placed on interdisciplinary, team-based care utilizing health care providers such as nurses, physician assistants, and pharmacists. Team-based care has been shown to have the greatest impact of all the strategies for improving blood pressure control.113 There is a good amount of evidence involving interventions with a focus on health care providers other than physicians, although the data lack a sufficient focus on African Americans.
Carter et al,10 in a randomized controlled trial in which 26.3% of the patients were African American, found that an intervention consisting of clinical pharmacists giving physicians drug therapy recommendations based on national guidelines resulted in a significantly lower blood pressure compared with a control group: the mean reduction was 20.7/9.7 mm Hg in the intervention group vs 6.8/4.5 mm Hg in the control group.
Carter et al114 performed a meta-analysis of 37 studies and found that two strategies led to a significant reduction in blood pressure: a pharmacist-led intervention with treatment recommendations to physicians resulted in a systolic pressure reduction of 9.30 mm Hg; and nurse-led interventions resulted in a systolic pressure reduction of 4.80 mm Hg. Again, many of the studies cited in this meta-analysis lacked a focus on African Americans.
Hunt et al11 conducted a randomized controlled trial in which pharmacists actively participated in the management of blood pressure. They were involved with every aspect of care, including reviewing medications and adverse drug reactions, assessing lifestyle behaviors and barriers to adherence, making dosing adjustments, and adding medications. Patients randomized to the intervention group achieved significantly lower systolic and diastolic pressures (137/75 vs 143/78 mm Hg in the control group). However, information about race was not included.
The above studies are just a few out of a large body of evidence demonstrating the value of team-based care to improve blood pressure control. It has yet to be determined whether these models can improve blood pressure control specifically in African Americans, since so many of these trials lacked a focus on this group. Promising is an ongoing randomized prospective trial by Carter et al115 evaluating a model of collaboration between physicians and pharmacists, with a focus on patients in underrepresented minorities.
SO WHAT WORKS?
Although there is a growing body of literature on interventions to try to reduce disparities in hypertension and blood pressure control between African Americans and whites, only a few randomized controlled trials have focused on African Americans, and several have not reported their results.116 So the question remains: How should we interpret the available data, which are aggregated across racial groups, and put it into practice when caring for hypertensive African American patients?
Patient education. In trying to overcome patient-related barriers, emphasis should be on patient education, in particular addressing misconceptions about hypertension and promoting adherence to antihypertensive therapy. This is evident from the narrative storytelling intervention by Houston et al.3 Although this is the first study of its kind, this strategy may be something to consider if future studies replicate these findings. Culturally appropriate storytelling may allow patients to identify with the stories as they relate to their own personal lives. It can be an effective way to address patient education and change behaviors.
Self-monitoring with a home blood pressure monitor has also proven effective in the management of hypertension in African Americans. Indeed, the few studies that reported findings in African Americans showed impressive reductions in blood pressure. The benefits of home monitoring are well documented, and the effect on physician-related barriers such as clinical inertia are also quite impressive.117 However, most of these studies did not assess the long-term impact or cost-effectiveness of home monitoring on blood pressure control.
Behavioral counseling. Although we have good evidence of the effectiveness of behavioral counseling, whether this is sustained long-term has been less studied in African Americans. Thus, while interventions that targeted African Americans have reported impressive reductions in blood pressure, the effect tends to be greatest during the first few months of implementation, with the benefits disappearing over time.
Physician-related interventions. With regard to physician-level interventions, research has focused on physician education, utilizing alerts and computerized clinical decision-support systems. Evidence is scant on whether the use of computerized systems results in improves hypertension care in African Americans. However, a closer look at the data from studies that report outcomes in African American and white patients shows that the results do not seem to differ between these groups. Still, there is insufficient information about the impact on hypertensive African Americans.6
Strategies that address both patient- and physician-related barriers can improve overall blood pressure control; however, there is a lack of data comparing outcomes in hypertensive African Americans with those of whites, making it difficult to know if this would be an effective strategy in African American patients alone.
More studies needed that focus on African Americans
Developing interventions to improve blood pressure control in African Americans should be an ongoing priority for research if we intend to address racial disparities in cardiovascular disease. Although it is reassuring that there is a growing body of evidence and research with this focus,118–121 more research is needed to determine effective strategies that address barriers related to physician practice and to the health care system overall as they relate to blood pressure control in African Americans. More importantly, these strategies should also emphasize a team-based approach that includes nurses, pharmacists, and physician assistants. Developing targeted interventions for hypertensive African Americans will help reduce disparities in the rates of cardiovascular illness and death in this patient population.
High blood pressure takes a devastating toll on African Americans. Better control can go a long way to closing the “mortality gap” between African Americans and white Americans. But which strategies are best to address this complex problem?
In this report, we review the evidence on practice-based approaches to improving blood pressure control, from new styles of patient education to home blood pressure monitoring, focusing on studies in African Americans (Table 1).1–11
BETTER CONTROL IS NEEDED
PATIENT-RELATED BARRIERS
Patient-related barriers24–40 include:
- Poor knowledge about hypertension and its consequences31,32
- Poor adherence to drug therapy (a major factor,24–26 as African Americans have poorer adherence rates than whites,27–29 which may explain some of the racial disparity in blood pressure control30)
- False health beliefs34–37
- Inability to change one’s lifestyle
- Side effects of antihypertensive drugs32
- Unrealistic expectations of treatment (eg, a cure33)
- Demographic factors (eg, socioeconomic status, educational level, age, sex).24,38–40
Perhaps the most salient and easily modifiable of these factors are patients’ reluctance to modify their lifestyle and their misconceptions about the causes, treatment, and prevention of hypertension. Patients whose beliefs are discordant with traditional biomedical concepts of hypertension have poorer blood pressure control than those whose beliefs are concordant.41 This may be more relevant to African Americans, since they are known to have cultural health beliefs that differ from those of Western culture (eg, that hypertension is a curable rather than a chronic illness, and that hypertension is a disease of nerves that often affects the blood and clogs the arteries).42
PHYSICIAN-RELATED BARRIERS
Barriers to effective blood pressure control at the physician level43–48 include:
- Nonadherence to treatment guidelines44
- Failure to intensify the regimen if goals are not met45
- Failure to emphasize therapeutic lifestyle changes.43,46–48
When primary care physicians do not follow evidence-based guidelines, the reason may be that they are not aware of them or that they do not understand them. In a national survey of 1,029 physicians that was designed to explore how well physicians know the indications for specific antihypertensive drugs and how closely their opinions and practice agreed with national guidelines, only 37.3% correctly answered all of the knowledge-related questions.49
Other reasons for nonadherence are that physicians may disagree with the guidelines, may not be able to follow the guidelines, may not believe that following them will achieve the desired effect, or may have no motivation to change their practice.50
Whatever the reason, Hyman et al51 reported that as many as 30% of physicians did not recommend treatment for patients with diastolic blood pressures of 90 to 100 mm Hg, and a higher proportion did not treat patients with systolic blood pressures of 140 to 160 mm Hg.
BARRIERS IN HEALTH CARE SYSTEMS
Although health care systems present barriers to optimal blood pressure control,20,27,31,52 there is evidence that most cases of uncontrolled hypertension occur in patients with good access to care.32,53,54 For example, an NHANES study53 suggested that most patients with uncontrolled hypertension had in fact seen a physician on average at least three times in the previous year. And this may be more pervasive in African Americans: one survey found hypertension was uncontrolled in 75% of hypertensive African American patients despite free access to care, free medications, and regular follow-up visits.41
Thus, the most significant barriers to blood pressure control appear to be patient-related and physician-related.
INTERVENTIONS AIMED AT PATIENTS
The most common approaches to improving blood pressure control at the patient level, regardless of race, are patient education,55–61 home blood pressure monitoring,62–67 and behavioral counseling to address misconceptions about hypertension,68 to improve adherence to drug therapy,69–73 and to encourage lifestyle modifications.74–78
Patient education
Patient education can improve blood pressure control.58,79–82 Its aims are to increase patients’ understanding of the disease83 and to encourage them to be more active in their own care.80,84,85
Patient education has a moderate effect on blood pressure control. The average proportion of patients whose hypertension was under control in community-based trials of various interventions ranged from 60% to 70%, compared with 38% to 46% with usual care.56,80,81
However, these strategies largely did not address misconceptions patients have about hypertension. This issue is especially critical in African Americans, who may have different perceptions of hypertension and different expectations for care41: beliefs that hypertension is “curable,” not chronic, and that medication is needed only for hypertension-related symptoms may translate to poorer rates of medication adherence.
Levine et al1 evaluated the efficacy of home visits by trained community health advisory board workers in a neighborhood in Baltimore, MD, with a high prevalence of hypertension. Participants were randomized to receive either one visit or five visits during the 40-month study period. Both groups had a statistically significant reduction in blood pressure, and in both groups the proportion of patients with adequate blood pressure control increased significantly. The results support the use of a practice- and community-based partnership to improve blood pressure control in African American patients.
Ogedegbe et al2 randomized 190 hypertensive African American patients to receive usual care or quarterly counseling sessions that used motivational interviewing focused on medication adherence. The counseled patients stayed adherent to their medications, whereas adherence declined significantly in those receiving usual care. This effect was associated with a modest, nonsignificant trend toward a net reduction in systolic blood pressure with motivational interviewing.
A novel method of health education is the use of narrative communication—ie, storytelling. It has a good amount of evidence to support it, as culturally appropriate storytelling may allow patients to identify with a story as it relates to their own lives.86–89 Examples of educational storytelling include:
- A woman with hypertension discussing what it means to have high blood pressure, and the benefits of controlling it, such as living long enough to see her grandchildren grow up
- A man discussing the importance of involving family and friends to help control blood pressure, and how dietary modifications can be made to ensure that salt alternatives are used when the family does the cooking.
Storytelling should be done in a culturally appropriate context. For example, storytellers should have the same background as the patient (ie, similar socioeconomic status and ethnic background): patients are more likely to be influenced if they identify with the storyteller and imagine themselves in a similar situation.
Houston et al3 randomized 299 hypertensive African Americans to view either three DVDs that featured patients with hypertension or three “attention-control DVDs” on topics not related to hypertension. The intervention group’s DVDs focused on storytelling and “learning more.” In the storytelling section, patients told personal stories about what it meant to have hypertension and gave advice on how to best interact with health care providers and methods to improve medication adherence. A “learning more” section focused on what high blood pressure is, addressed therapeutic lifestyle changes, and encouraged patients to communicate with their health care providers. The patients who viewed the patient narratives had significantly lower blood pressure at 3 months than those assigned to usual care. Although blood pressure subsequently increased in both groups, the benefits of the intervention still existed at the end of follow-up.
Important to note about two of the above three studies1,3 is that the interventions were done by people other than physicians, thus emphasizing the importance of a team approach to blood pressure control.
Behavioral counseling
The effectiveness of lifestyle modifications such as diet, weight loss, and physical activity in preventing and treating hypertension is well established.74–78 For example:
- In the Dietary Approaches to Stop Hypertension (DASH) trial,76 a healthy diet lowered blood pressure about as much as single drugs do, particularly in African Americans.
- The Trial of Nonpharmacologic Interventions in the Elderly (TONE)74 showed that exercise can lower blood pressure in obese hypertensive patients.
- The PREMIER trial (Lifestyle Interventions for Blood Pressure Control)75 showed that a single brief counseling session could produce substantial decreases in blood pressure in patients with stage 1 hypertension or high-normal blood pressure.
Unfortunately, these results have been hard to translate into primary care practice, especially for African American patients. Several studies have evaluated the impact of lifestyle interventions on blood pressure control in primary care practices with a large population of African American patients.
Bosworth et al,4 in a study of a practice in which almost half the patients were African American, randomized patients to receive usual care, nurse-administered tailored behavioral telephone counseling, home blood pressure monitoring, or home monitoring plus tailored behavioral telephone counseling. The combination of home monitoring and tailored behavioral telephone counseling led to a statistically significant improvement at 24 months compared with baseline.
Home blood pressure monitoring
The effectiveness of self-monitoring in improving blood pressure control is also well documented.62,63,65–67,90–95
Pickering et al62 studied patients with poorly controlled hypertension in a managed-care setting and found a reduction of 7 mm Hg systolic and 5 mm Hg diastolic pressure after 3 to 6 months of home monitoring compared with usual care.
Mengden et al,94 in a similar study, found average blood pressure reductions at 6 months of 19.3/11.9 mm Hg in the home-monitoring group vs 10.6/8.8 mm Hg in the usual-care group.
The effect of home blood pressure monitoring may be greater in African Americans.
Rogers et al93 found it to be more effective at lowering blood pressure than usual care in a group of 121 patients with poorly controlled hypertension followed in primary care practices, and these reductions were twice as large in African American patients than in white patients.93
Bondmass,92 in a study of 33 African American patients with poorly controlled hypertension, reported a 53% control rate within 4 weeks of home monitoring. All patients in the study had uncontrolled blood pressure at baseline (> 140/90 mm Hg).
Artinian et al5 evaluated the effect of nurse-managed telemonitoring on blood pressure control vs enhanced usual care. All participants were African American. The monitored group had a significantly greater reduction in systolic pressure at 12 months compared with those who received enhanced usual care.
PHYSICIAN-LEVEL INTERVENTIONS
Most interventions to improve how physicians manage patients with hypertension are designed to improve adherence to treatment guidelines. In most cases, these interventions are based on continuous quality improvement and disease management concepts such as physician education and academic detailing, reminders, feedback on performance measures, and risk-assessment tools.96,97
Physician education
Interest is increasing in physician educational interventions for blood pressure control.24,98
Inui et al,99 in an early study in a primary care practice, found that patients of physicians who received tutorials on hypertension management were more compliant with their drug regimens and had better blood pressure control than patients of physicians in the control group.
Jennett et al,100 in a similar randomized clinical trial, found that physicians who participated in an education activity were more adherent to treatment guidelines at 6 and 12 months compared with those who did not participate.
Maue et al101 showed that rates of blood pressure control improved from 41% to 52% after a 6-month educational intervention for physicians in a managed-care setting.
Tu et al102 reviewed 12 studies in which seven different physician educational interventions were used either alone or in combination and concluded that physician education improves compliance with guidelines for managing hypertension.
Unfortunately, these studies did not report outcomes separately for African American and white patients.
Hicks et al6 found that disease management approaches that target physicians whose patients with hypertension are mostly African American did not yield clinically relevant improvement in these patients, and that minority patients were significantly less likely to have their blood pressure controlled at the end of the study compared with their non-Hispanic white counterparts.
Feedback to providers
Several studies have shown that, given reminders and feedback systems, physicians will change their practice.103–106
Mashru and Lant104 combined chart audits and physician education in primary care practices and found they improved physician performance measures such as accuracy of diagnosis, number of patients who received cardiovascular risk assessment, and number of patients whose treatment was based on clinical laboratory assessments.
Feedback takes many forms but consists mostly of computerized information107 or peer-to-peer academic detailing with opinion leaders.108–110
Dickinson et al,106 for instance, showed that computer-generated listings of patients’ blood pressures combined with a physician education program on clinical management of hypertension led to increased knowledge and better follow-up on their patients.
Again, however, these studies did not distinguish between African American and white patients, which makes it difficult to judge whether or not these approaches work differently for physicians with a large proportion of African American patients.
Computerized decision-support systems
Computerized decision-support systems have proliferated in primary care practices.111
McAlister et al103 found that general practitioners randomized to manage hypertension with the assistance of a computer obtained better outcomes than with usual care.
Montgomery and Fahey,107 in a systematic review, found improved blood pressure control in two of the three trials that compared computer-generated feedback reports and reminders to usual care. Specifically, 51% of patients whose physicians received reminders either had controlled blood pressure or were at least receiving treatment vs 33% in the control group at 12 months. This difference was even higher at 24 months.
Montgomery et al7 later randomized primary care practices to use a computer-based decision-support system and a cardiovascular risk chart, the risk chart alone, or to continue as usual. Results indicated no reduction in cardiovascular risk in the computer-system or the chart-only group, whereas patients in the chart-only group had a significant reduction in systolic pressure and were prescribed more cardiovascular drugs. This study indicates that use of a computerized decision-support system is not superior to chart review and audit feedback alone.
Evidence that computerized decision systems improve blood pressure control in African Americans is scant. However, when one looks at the evidence from studies of African Americans, the outcomes do not seem to differ between African American and white patients.
Hicks et al6 examined the effectiveness of computerized decision support in improving hypertension care in a racially diverse population. Physicians were randomized to receive computerized decision support or to provide usual care without computerized support. Both groups improved significantly in prescribing appropriate drugs but not in overall blood pressure control. Furthermore, the study showed no reduction in racial disparities of care and blood pressure control.
A potential explanation for the lack of improvement in blood pressure was that the intervention dealt with making sure the appropriate drugs were prescribed rather than making sure physicians also appropriately intensified antihypertensive management when necessary.
INTERVENTIONS TARGETING PATIENTS AND PHYSICIANS
Several studies have targeted both patient and physician-level barriers to blood pressure control in practice-based settings.
Roumie et al8 randomized physicians to one of three intervention groups:
- “Provider education” consisting of an email message with a Web-based link to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7)
- Provider education plus a computer alert with information about their patient’s blood pressure
- Provider education, a computer alert, and patient education (ie, patients received a letter encouraging adherence to drug therapy, changing their lifestyle, and talking with their doctor about their blood pressure).
Patients whose providers were randomized to the third group had better blood pressure control. The report did not differentiate African American vs white patients. The data, however, did show the effectiveness of adding patient education to provider education to improve blood pressure control.
Bosworth et al,112 in a study in which 40% of patients were African American, randomized patients to usual care or to bimonthly nurse-delivered behavioral telephone counseling. They also randomized providers either to receive computer-generated decision support designed to improve adherence to guidelines or to receive no support.
There were no significant differences in rates of blood pressure control in the intervention groups compared with a control group. Although differences in blood pressure control between groups were not significant, patients randomized to behavioral intervention had significantly better blood pressure control at the 24-month follow-up than at baseline.
Svetkey et al9 evaluated the effects of physician intervention, patient intervention, and physician intervention plus patient intervention compared with control on systolic blood pressure at 6 months. They found that an intensive behavioral lifestyle intervention led to a significant reduction in systolic pressure at 6 months. By itself, the physician intervention did not have a meaningful effect, but patients in the combined physician-and-patient-intervention group experienced the greatest reduction (9.7 ± 12.7 mm Hg).
It takes a team
Physicians should not be the only focus in helping patients achieve blood pressure control. Although physician and patient factors need to be addressed to improve blood pressure control in African Americans, emphasis should also be placed on interdisciplinary, team-based care utilizing health care providers such as nurses, physician assistants, and pharmacists. Team-based care has been shown to have the greatest impact of all the strategies for improving blood pressure control.113 There is a good amount of evidence involving interventions with a focus on health care providers other than physicians, although the data lack a sufficient focus on African Americans.
Carter et al,10 in a randomized controlled trial in which 26.3% of the patients were African American, found that an intervention consisting of clinical pharmacists giving physicians drug therapy recommendations based on national guidelines resulted in a significantly lower blood pressure compared with a control group: the mean reduction was 20.7/9.7 mm Hg in the intervention group vs 6.8/4.5 mm Hg in the control group.
Carter et al114 performed a meta-analysis of 37 studies and found that two strategies led to a significant reduction in blood pressure: a pharmacist-led intervention with treatment recommendations to physicians resulted in a systolic pressure reduction of 9.30 mm Hg; and nurse-led interventions resulted in a systolic pressure reduction of 4.80 mm Hg. Again, many of the studies cited in this meta-analysis lacked a focus on African Americans.
Hunt et al11 conducted a randomized controlled trial in which pharmacists actively participated in the management of blood pressure. They were involved with every aspect of care, including reviewing medications and adverse drug reactions, assessing lifestyle behaviors and barriers to adherence, making dosing adjustments, and adding medications. Patients randomized to the intervention group achieved significantly lower systolic and diastolic pressures (137/75 vs 143/78 mm Hg in the control group). However, information about race was not included.
The above studies are just a few out of a large body of evidence demonstrating the value of team-based care to improve blood pressure control. It has yet to be determined whether these models can improve blood pressure control specifically in African Americans, since so many of these trials lacked a focus on this group. Promising is an ongoing randomized prospective trial by Carter et al115 evaluating a model of collaboration between physicians and pharmacists, with a focus on patients in underrepresented minorities.
SO WHAT WORKS?
Although there is a growing body of literature on interventions to try to reduce disparities in hypertension and blood pressure control between African Americans and whites, only a few randomized controlled trials have focused on African Americans, and several have not reported their results.116 So the question remains: How should we interpret the available data, which are aggregated across racial groups, and put it into practice when caring for hypertensive African American patients?
Patient education. In trying to overcome patient-related barriers, emphasis should be on patient education, in particular addressing misconceptions about hypertension and promoting adherence to antihypertensive therapy. This is evident from the narrative storytelling intervention by Houston et al.3 Although this is the first study of its kind, this strategy may be something to consider if future studies replicate these findings. Culturally appropriate storytelling may allow patients to identify with the stories as they relate to their own personal lives. It can be an effective way to address patient education and change behaviors.
Self-monitoring with a home blood pressure monitor has also proven effective in the management of hypertension in African Americans. Indeed, the few studies that reported findings in African Americans showed impressive reductions in blood pressure. The benefits of home monitoring are well documented, and the effect on physician-related barriers such as clinical inertia are also quite impressive.117 However, most of these studies did not assess the long-term impact or cost-effectiveness of home monitoring on blood pressure control.
Behavioral counseling. Although we have good evidence of the effectiveness of behavioral counseling, whether this is sustained long-term has been less studied in African Americans. Thus, while interventions that targeted African Americans have reported impressive reductions in blood pressure, the effect tends to be greatest during the first few months of implementation, with the benefits disappearing over time.
Physician-related interventions. With regard to physician-level interventions, research has focused on physician education, utilizing alerts and computerized clinical decision-support systems. Evidence is scant on whether the use of computerized systems results in improves hypertension care in African Americans. However, a closer look at the data from studies that report outcomes in African American and white patients shows that the results do not seem to differ between these groups. Still, there is insufficient information about the impact on hypertensive African Americans.6
Strategies that address both patient- and physician-related barriers can improve overall blood pressure control; however, there is a lack of data comparing outcomes in hypertensive African Americans with those of whites, making it difficult to know if this would be an effective strategy in African American patients alone.
More studies needed that focus on African Americans
Developing interventions to improve blood pressure control in African Americans should be an ongoing priority for research if we intend to address racial disparities in cardiovascular disease. Although it is reassuring that there is a growing body of evidence and research with this focus,118–121 more research is needed to determine effective strategies that address barriers related to physician practice and to the health care system overall as they relate to blood pressure control in African Americans. More importantly, these strategies should also emphasize a team-based approach that includes nurses, pharmacists, and physician assistants. Developing targeted interventions for hypertensive African Americans will help reduce disparities in the rates of cardiovascular illness and death in this patient population.
- Levine DM, Bone LR, Hill MN, et al. The effectiveness of a community/academic health center partnership in decreasing the level of blood pressure in an urban African-American population. Ethn Dis 2003; 13:354–361.
- Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens 2008; 21:1137–1143.
- Houston TK, Allison JJ, Sussman M, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med 2011; 154:77–84.
- Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009; 151:687–695.
- Artinian NT, Flack JM, Nordstrom CK, et al. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res 2007; 56:312–322.
- Hicks LS, Sequist TD, Ayanian JZ, et al. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med 2008; 23:429–441.
- Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ 2000; 320:686–690.
- Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006; 145:165–175.
- Svetkey LP, Pollak KI, Yancy WS, et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension 2009; 54:1226–1233.
- Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med 2009; 169:1996–2002.
- Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med 2008; 23:1966–1972.
- US Department of Health and Human Services: Office of Disease Prevention and Health Promotion—Healthy People 2010. Nasnewsletter 2000; 15:3.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- US Centers for Disease Control and Prevention. Age-specific excess deaths associated with stroke among racial/ethnic minority populations–United States, 1997. JAMA 2000; 283:2382–2383.
- Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black-white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med 1995; 155:1319–1324.
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 1997; 277:1293–1298.
- Pavlik VN, Hyman DJ, Vallbona C, Toronjo C, Louis K. Hypertension awareness and control in an inner-city African-American sample. J Hum Hypertens 1997; 11:277–283.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation Feb 1; 123( 4):e18–e209.
- Chobanian AV, Bakris GL, Black HR, et al., Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bone LR, Hill MN, Stallings R, et al. Community health survey in an urban African-American neighborhood: distribution and correlates of elevated blood pressure. Ethn Dis 2000; 10:87–95.
- Weber MA. Strategies for improving blood pressure control. Am J Hypertens 1998; 11:897–899.
- Hill MN, Sutton BS. Barriers to hypertension care and control. Curr Hypertens Rep 2000; 2:445–450.
- Alderman MH. Barriers to blood pressure control. Am J Hypertens 1999; 12:1268–1269.
- Chobanian AV. Control of hypertension—an important national priority. N Engl J Med 2001; 345:534–535.
- Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation 1997; 95:1085–1090.
- Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In:Haynes RB, Taylor DW, Sackett DL, eds. Compliance in Health Care. Baltimore, MD: John Hopkins University Press; 1979:11–22.
- Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Correlates of nonadherence to hypertension treatment in an inner-city minority population. Am J Public Health 1992; 82:1607–1612.
- Kirscht JP, Rosenstock IM. Patient adherence to antihypertensive medical regimens. J Community Health. 1977; 3:115–124.
- Hershey JC, Morton BG, Davis JB, Reichgott MJ. Patient compliance with antihypertensive medication. Am J Public Health 1980; 70:1081–1089.
- Bosworth HB, Powers B, Grubber JM, et al. Racial differences in blood pressure control: potential explanatory factors. J Gen Intern Med 2008; 23:692–698.
- Douglas JG, Ferdinand KC, Bakris GL, Sowers JR. Barriers to blood pressure control in African Americans. Overcoming obstacles is challenging, but target goals can be attained. Postgrad Med 2002; 112:51–52,55,59–62passim.
- Knight EL, Bohn RL, Wang PS, Glynn RJ, Mogun H, Avorn J. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension 2001; 38:809–814.
- Ogedegbe G, Mancuso CA, Allegrante JP. Expectations of blood pressure management in hypertensive African-American patients: a qualitative study. J Natl Med Assoc 2004; 96:442–449.
- Blumhagen D. Hypertension: a folk illness with a medical name. Cult Med Psychiatry 1980; 4:197–224.
- Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: the example of hypertension. Health Psychol 1985; 4:115–135.
- Nelson EC, Stason WB, Neutra RR, Solomon HS, McArdle PJ. Impact of patient perceptions on compliance with treatment for hypertension. Med Care 1978; 16:893–906.
- Heurtin-Roberts S. ‘High-pertension’—the uses of a chronic folk illness for personal adaptation. Soc Sci Med 1993; 37:285–294.
- Lang T. Social and economic factors as obstacles to blood pressure control. Am J Hypertens 1998; 11:900–902.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Stamler R, Shipley M, Elliott P, Dyer A, Sans S, Stamler J. Higher blood pressure in adults with less education. Some explanations from INTERSALT. Hypertension 1992; 19:237–241.
- Heurtin-Roberts S, Reisin E. The relation of culturally influenced lay models of hypertension to compliance with treatment. Am J Hypertens 1992; 5:787–792.
- Snow LF. Folk medical beliefs and their implications for care of patients. A review bases on studies among black Americans. Ann Intern Med 1974; 81:82–96.
- Hicks LS, Shaykevich S, Bates DW, Ayanian JZ. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC Cardiovasc Disord 2005; 5:16.
- Mehta SS, Wilcox CS, Schulman KA. Treatment of hypertension in patients with comorbidities: results from the study of hypertensive prescribing practices (SHyPP). Am J Hypertens 1999; 12:333–340.
- Ballard DJ, Strogatz DS, Wagner EH, et al. Hypertension control in a rural southern community: medical care process and dropping out. Am J Prev Med 1988; 4:133–139.
- Hajjar I, Miller K, Hirth V. Age-related bias in the management of hypertension: a national survey of physicians’ opinions on hypertension in elderly adults. J Gerontol A Biol Sci Med Sci 2002; 57:M487–M491.
- McAlister FA, Laupacis A, Teo KK, Hamilton PG, Montague TJ. A survey of clinician attitudes and management practices in hypertension. J Hum Hypertens 1997; 11:413–419.
- Trilling JS, Froom J. The urgent need to improve hypertension care. Arch Fam Med 2000; 9:794–801.
- Huse DM, Roht LH, Alpert JS, Hartz SC. Physicians’ knowledge, attitudes, and practice of pharmacologic treatment of hypertension. Ann Pharmacother 2001; 35:1173–1179.
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282:1458–1465.
- Hyman DJ, Pavlik VN, Vallbona C. Physician role in lack of awareness and control of hypertension. J Clin Hypertens (Greenwich) 2000; 2:324–330.
- Morley Kotchen J, Walker WE, Kotchen TA. Rationale for a community approach to hypertension control among inner city minority populations. Heart Dis Stroke 1994; 3:61–62.
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med 2001; 345:479–486.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Pierce JP, Watson DS, Knights S, Gliddon T, Williams S, Watson R. A controlled trial of health education in the physician’s office. Prev Med 1984; 13:185–194.
- Morisky DE, DeMuth NM, Field-Fass M, Green LW, Levine DM. Evaluation of family health education to build social support for long-term control of high blood pressure. Health Educ Q 1985; 12:35–50.
- Lorgelly P, Siatis I, Brooks A, et al. Is ambulatory blood pressure monitoring cost-effective in the routine surveillance of treated hypertensive patients in primary care? Br J Gen Pract 2003; 53:794–796.
- Green LW, Levine DM, Wolle J, Deeds S. Development of randomized patient education experiments with urban poor hypertensives. Patient Couns Health Educ 1979; 1:106–111.
- Gruesser M, Hartmann P, Schlottmann N, Lohmann FW, Sawicki PT, Joergens V. Structured patient education for out-patients with hypertension in general practice: a model project in Germany. J Hum Hypertens 1997; 11:501–506.
- Mühlhauser I, Sawicki PT, Didjurgeit U, Jörgens V, Trampisch HJ, Berger M. Evaluation of a structured treatment and teaching programme on hypertension in general practice. Clin Exp Hypertens 1993; 15:125–142.
- Roca B, Nadal E, Rovira RE, Valls S, Lapuebla C, Lloría N. Usefulness of a hypertension education program. South Med J 2003; 96:1133–1137.
- Pickering TG, Gerin W, Holland JK. Home blood pressure teletransmission for better diagnosis and treatment. Curr Hypertens Rep 1999; 1:489–494.
- Yarows SA, Julius S, Pickering TG. Home blood pressure monitoring. Arch Intern Med 2000; 160:1251–1257.
- Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet 1976; 1:1265–1268.
- Johnson AL, Taylor DW, Sackett DL, Dunnett CW, Shimizu AG. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J 1978; 119:1034–1039.
- Carnahan JE, Nugent CA. The effects of self-monitoring by patients on the control of hypertension. Am J Med Sci 1975; 269:69–73.
- Stahl SM, Kelley CR, Neill PJ, Grim CE, Mamlin J. Effects of home blood pressure measurement on long-term BP control. Am J Public Health 1984; 74:704–709.
- Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med 2001; 21:221–232.
- Haynes RB, Mattson ME, Engebretson TO. Patient compliance to prescribed antihypertensive medication regimens: a report to the National Heart, Lung, and Blood institute. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, 1980. NIH publication 81-2102.
- Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med 1997; 19:239–263.
- Dunbar-Jacob J, Dwyer K, Dunning EJ. Compliance with antihypertensive regimen: a review of the research in the 1980s. Ann Behav Med 1991; 13:31–39.
- Haynes RB, Montague P, Oliver T, McKibbon KA, Brouwers MC, Kanani R. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2000; ( 2):CD000011.
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care 1998; 36:1138–1161.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Appel LJ, Champagne CM, Harsha DW, et al; Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093.
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336:1117–1124.
- Moore TJ, Conlin PR, Ard J, Svetkey LP. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension 2001; 38:155–158.
- Stevens VJ, Obarzanek E, Cook NR, et al; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134:1–11.
- Sawicki PT, Mühlhauser I, Didjurgeit U, Berger M. Improvement of hypertension care by a structured treatment and teaching programme. J Hum Hypertens 1993; 7:571–573.
- Morisky DE, Bowler MH, Finlay JS. An educational and behavioral approach toward increasing patient activation in hypertension management. J Community Health 1982; 7:171–182.
- Levine DM, Green LW, Deeds SG, Chwalow J, Russell RP, Finlay J. Health education for hypertensive patients. JAMA 1979; 241:1700–1703.
- Iso H, Shimamoto T, Yokota K, Sankai T, Jacobs DR, Komachi Y. Community-based education classes for hypertension control. A 1.5-year randomized controlled trial. Hypertension 1996; 27:968–974.
- Cuspidi C, Sampieri L, Macca G, et al. Improvement of patients’ knowledge by a single educational meeting on hypertension. J Hum Hypertens 2001; 15:57–61.
- Nessman DG, Carnahan JE, Nugent CA. Increasing compliance. Patient-operated hypertension groups. Arch Intern Med 1980; 140:1427–1430.
- Casasanta L, Patel S. Outcomes of an educational component of a disease management program for hypertension. Manag Care Interface 1999; 12:70–73.
- McAdams DP. The Stories We Live By: Personal Myths and the Making of the Self. New York NY: The Guilford Press; 1993.
- Bruner J. Acts of Meaning. Cambridge, MA: Harvard Univ Pr; 1990.
- Slater MD, Rouner D. Entertainment—education and elaboration likelihood: Understanding the processing of narrative persuasion. Commun Theory 2002; 12:173–191.
- Dal CS, Zanna MP, Fong GT. Narrative persuasion and overcoming resistance. In:Knowles ES, Linn J, eds. Resistance and Persuasion. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004:175–191.
- Artinian NT, Washington OG, Templin TN. Effects of home telemonitoring and community-based monitoring on blood pressure control in urban African Americans: a pilot study. Heart Lung 2001; 30:191–199.
- Bailey B, Carney SL, Gillies AA, Smith AJ. Antihypertensive drug treatment: a comparison of usual care with self blood pressure measurement. J Hum Hypertens 1999; 13:147–150.
- Bondmass M. The effect of home monitoring and telemanagement on blood pressure control among African Americans. Telemed J 2000; 6:15–23.
- Rogers MA, Small D, Buchan DA, et al. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med 2001; 134:1024–1032.
- Mengden T, Uen S, Baulmann J, Vetter H. Significance of blood pressure self-measurement as compared with office blood pressure measurement and ambulatory 24-hour blood pressure measurement in pharmacological studies. Blood Press Monit 2003; 8:169–172.
- Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens 1996; 9:285–292.
- Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995; 153:1423–1431.
- Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract 1998; 48:991–997.
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995; 274:700–705.
- Inui TS, Yourtee EL, Williamson JW. Improved outcomes in hypertension after physician tutorials. A controlled trial. Ann Intern Med 1976; 84:646–651.
- Jennett PA, Wilson TW, Hayton RC, Mainprize GW, Laxdal OE. Desirable behaviours in the office management of hypertension addressed through continuing medical education. Can J Public Health 1989; 80:359–362.
- Maue SK, Rivo ML, Weiss B, Farrelly EW, Brower-Stenger S. Effect of a primary care physician-focused, population-based approach to blood pressure control. Fam Med 2002; 34:508–513.
- Tu K, Davis D. Can we alter physician behavior by educational methods? Lessons learned from studies of the management and follow-up of hypertension. J Contin Educ Health Prof 2002; 22:11–22.
- McAlister NH, Covvey HD, Tong C, Lee A, Wigle ED. Randomised controlled trial of computer assisted management of hypertension in primary care. Br Med J (Clin Res Ed) 1986; 293:670–674.
- Mashru M, Lant A. Interpractice audit of diagnosis and management of hypertension in primary care: educational intervention and review of medical records. BMJ 1997; 314:942–946.
- Degoulet P, Menard J, Berger C, Plouin PF, Devries C, Hirel JC. Hypertension management: the computer as a participant. Am J Med 1980; 68:559–567.
- Dickinson JC, Warshaw GA, Gehlbach SH, Bobula JA, Muhlbaier LH, Parkerson GR. Improving hypertension control: impact of computer feedback and physician education. Med Care 1981; 19:843–854.
- Montgomery AA, Fahey T. A systematic review of the use of computers in the management of hypertension. J Epidemiol Community Health 1998; 52:520–525.
- Coleman MT, Lott JA, Sharma S. Use of continuous quality improvement to identify barriers in the management of hypertension. Am J Med Qual 2000; 15:72–77.
- Goldberg HI, Wagner EH, Fihn SD, et al. A randomized controlled trial of CQI teams and academic detailing: can they alter compliance with guidelines? Jt Comm J Qual Improv 1998; 24:130–142.
- Horowitz CR, Goldberg HI, Martin DP, et al. Conducting a randomized controlled trial of CQI and academic detailing to implement clinical guidelines. Jt Comm J Qual Improv 1996; 22:734–750.
- Johnson B, McNair D, Kailasam K, et al. Discern—an integrated prospective decision support system. Proc Annu Symp Comput Appl Med Care 1994; 969.
- Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J 2009; 157:450–456.
- Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care 2006; 44:646–657.
- Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med 2009; 169:1748–1755.
- Carter BL, Clarke W, Ardery G, et al; Collaboration Among Pharmacists Physicians To Improve Outcomes Now (CAPTION) Trial Investigators. A cluster-randomized effectiveness trial of a physician-pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:418–423.
- Einhorn PT. National heart, lung, and blood institute-initiated program “interventions to improve hypertension control rates in African Americans”: background and implementation. Circ Cardiovasc Qual Outcomes 2009; 2:236–240.
- Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011; 57:29–38.
- Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns 2008; 70:338–347.
- Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans’ study to improve the control of hypertension (V-STITCH): design and methodology. Contemp Clin Trials 2005; 26:155–168.
- Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension (CAATCH) trial: a multi-level intervention to improve blood pressure control in hypertensive blacks. Circ Cardiovasc Qual Outcomes 2009; 2:249–256.
- Bosworth HB, Almirall D, Weiner BJ, et al. The implementation of a translational study involving a primary care based behavioral program to improve blood pressure control: The HTN-IMPROVE study protocol (01295). Implement Sci 2010; 5:54.
- Levine DM, Bone LR, Hill MN, et al. The effectiveness of a community/academic health center partnership in decreasing the level of blood pressure in an urban African-American population. Ethn Dis 2003; 13:354–361.
- Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens 2008; 21:1137–1143.
- Houston TK, Allison JJ, Sussman M, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med 2011; 154:77–84.
- Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009; 151:687–695.
- Artinian NT, Flack JM, Nordstrom CK, et al. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res 2007; 56:312–322.
- Hicks LS, Sequist TD, Ayanian JZ, et al. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med 2008; 23:429–441.
- Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ 2000; 320:686–690.
- Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006; 145:165–175.
- Svetkey LP, Pollak KI, Yancy WS, et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension 2009; 54:1226–1233.
- Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med 2009; 169:1996–2002.
- Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med 2008; 23:1966–1972.
- US Department of Health and Human Services: Office of Disease Prevention and Health Promotion—Healthy People 2010. Nasnewsletter 2000; 15:3.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- US Centers for Disease Control and Prevention. Age-specific excess deaths associated with stroke among racial/ethnic minority populations–United States, 1997. JAMA 2000; 283:2382–2383.
- Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black-white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med 1995; 155:1319–1324.
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 1997; 277:1293–1298.
- Pavlik VN, Hyman DJ, Vallbona C, Toronjo C, Louis K. Hypertension awareness and control in an inner-city African-American sample. J Hum Hypertens 1997; 11:277–283.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation Feb 1; 123( 4):e18–e209.
- Chobanian AV, Bakris GL, Black HR, et al., Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bone LR, Hill MN, Stallings R, et al. Community health survey in an urban African-American neighborhood: distribution and correlates of elevated blood pressure. Ethn Dis 2000; 10:87–95.
- Weber MA. Strategies for improving blood pressure control. Am J Hypertens 1998; 11:897–899.
- Hill MN, Sutton BS. Barriers to hypertension care and control. Curr Hypertens Rep 2000; 2:445–450.
- Alderman MH. Barriers to blood pressure control. Am J Hypertens 1999; 12:1268–1269.
- Chobanian AV. Control of hypertension—an important national priority. N Engl J Med 2001; 345:534–535.
- Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation 1997; 95:1085–1090.
- Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In:Haynes RB, Taylor DW, Sackett DL, eds. Compliance in Health Care. Baltimore, MD: John Hopkins University Press; 1979:11–22.
- Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Correlates of nonadherence to hypertension treatment in an inner-city minority population. Am J Public Health 1992; 82:1607–1612.
- Kirscht JP, Rosenstock IM. Patient adherence to antihypertensive medical regimens. J Community Health. 1977; 3:115–124.
- Hershey JC, Morton BG, Davis JB, Reichgott MJ. Patient compliance with antihypertensive medication. Am J Public Health 1980; 70:1081–1089.
- Bosworth HB, Powers B, Grubber JM, et al. Racial differences in blood pressure control: potential explanatory factors. J Gen Intern Med 2008; 23:692–698.
- Douglas JG, Ferdinand KC, Bakris GL, Sowers JR. Barriers to blood pressure control in African Americans. Overcoming obstacles is challenging, but target goals can be attained. Postgrad Med 2002; 112:51–52,55,59–62passim.
- Knight EL, Bohn RL, Wang PS, Glynn RJ, Mogun H, Avorn J. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension 2001; 38:809–814.
- Ogedegbe G, Mancuso CA, Allegrante JP. Expectations of blood pressure management in hypertensive African-American patients: a qualitative study. J Natl Med Assoc 2004; 96:442–449.
- Blumhagen D. Hypertension: a folk illness with a medical name. Cult Med Psychiatry 1980; 4:197–224.
- Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: the example of hypertension. Health Psychol 1985; 4:115–135.
- Nelson EC, Stason WB, Neutra RR, Solomon HS, McArdle PJ. Impact of patient perceptions on compliance with treatment for hypertension. Med Care 1978; 16:893–906.
- Heurtin-Roberts S. ‘High-pertension’—the uses of a chronic folk illness for personal adaptation. Soc Sci Med 1993; 37:285–294.
- Lang T. Social and economic factors as obstacles to blood pressure control. Am J Hypertens 1998; 11:900–902.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Stamler R, Shipley M, Elliott P, Dyer A, Sans S, Stamler J. Higher blood pressure in adults with less education. Some explanations from INTERSALT. Hypertension 1992; 19:237–241.
- Heurtin-Roberts S, Reisin E. The relation of culturally influenced lay models of hypertension to compliance with treatment. Am J Hypertens 1992; 5:787–792.
- Snow LF. Folk medical beliefs and their implications for care of patients. A review bases on studies among black Americans. Ann Intern Med 1974; 81:82–96.
- Hicks LS, Shaykevich S, Bates DW, Ayanian JZ. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC Cardiovasc Disord 2005; 5:16.
- Mehta SS, Wilcox CS, Schulman KA. Treatment of hypertension in patients with comorbidities: results from the study of hypertensive prescribing practices (SHyPP). Am J Hypertens 1999; 12:333–340.
- Ballard DJ, Strogatz DS, Wagner EH, et al. Hypertension control in a rural southern community: medical care process and dropping out. Am J Prev Med 1988; 4:133–139.
- Hajjar I, Miller K, Hirth V. Age-related bias in the management of hypertension: a national survey of physicians’ opinions on hypertension in elderly adults. J Gerontol A Biol Sci Med Sci 2002; 57:M487–M491.
- McAlister FA, Laupacis A, Teo KK, Hamilton PG, Montague TJ. A survey of clinician attitudes and management practices in hypertension. J Hum Hypertens 1997; 11:413–419.
- Trilling JS, Froom J. The urgent need to improve hypertension care. Arch Fam Med 2000; 9:794–801.
- Huse DM, Roht LH, Alpert JS, Hartz SC. Physicians’ knowledge, attitudes, and practice of pharmacologic treatment of hypertension. Ann Pharmacother 2001; 35:1173–1179.
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282:1458–1465.
- Hyman DJ, Pavlik VN, Vallbona C. Physician role in lack of awareness and control of hypertension. J Clin Hypertens (Greenwich) 2000; 2:324–330.
- Morley Kotchen J, Walker WE, Kotchen TA. Rationale for a community approach to hypertension control among inner city minority populations. Heart Dis Stroke 1994; 3:61–62.
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med 2001; 345:479–486.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Pierce JP, Watson DS, Knights S, Gliddon T, Williams S, Watson R. A controlled trial of health education in the physician’s office. Prev Med 1984; 13:185–194.
- Morisky DE, DeMuth NM, Field-Fass M, Green LW, Levine DM. Evaluation of family health education to build social support for long-term control of high blood pressure. Health Educ Q 1985; 12:35–50.
- Lorgelly P, Siatis I, Brooks A, et al. Is ambulatory blood pressure monitoring cost-effective in the routine surveillance of treated hypertensive patients in primary care? Br J Gen Pract 2003; 53:794–796.
- Green LW, Levine DM, Wolle J, Deeds S. Development of randomized patient education experiments with urban poor hypertensives. Patient Couns Health Educ 1979; 1:106–111.
- Gruesser M, Hartmann P, Schlottmann N, Lohmann FW, Sawicki PT, Joergens V. Structured patient education for out-patients with hypertension in general practice: a model project in Germany. J Hum Hypertens 1997; 11:501–506.
- Mühlhauser I, Sawicki PT, Didjurgeit U, Jörgens V, Trampisch HJ, Berger M. Evaluation of a structured treatment and teaching programme on hypertension in general practice. Clin Exp Hypertens 1993; 15:125–142.
- Roca B, Nadal E, Rovira RE, Valls S, Lapuebla C, Lloría N. Usefulness of a hypertension education program. South Med J 2003; 96:1133–1137.
- Pickering TG, Gerin W, Holland JK. Home blood pressure teletransmission for better diagnosis and treatment. Curr Hypertens Rep 1999; 1:489–494.
- Yarows SA, Julius S, Pickering TG. Home blood pressure monitoring. Arch Intern Med 2000; 160:1251–1257.
- Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet 1976; 1:1265–1268.
- Johnson AL, Taylor DW, Sackett DL, Dunnett CW, Shimizu AG. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J 1978; 119:1034–1039.
- Carnahan JE, Nugent CA. The effects of self-monitoring by patients on the control of hypertension. Am J Med Sci 1975; 269:69–73.
- Stahl SM, Kelley CR, Neill PJ, Grim CE, Mamlin J. Effects of home blood pressure measurement on long-term BP control. Am J Public Health 1984; 74:704–709.
- Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med 2001; 21:221–232.
- Haynes RB, Mattson ME, Engebretson TO. Patient compliance to prescribed antihypertensive medication regimens: a report to the National Heart, Lung, and Blood institute. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, 1980. NIH publication 81-2102.
- Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med 1997; 19:239–263.
- Dunbar-Jacob J, Dwyer K, Dunning EJ. Compliance with antihypertensive regimen: a review of the research in the 1980s. Ann Behav Med 1991; 13:31–39.
- Haynes RB, Montague P, Oliver T, McKibbon KA, Brouwers MC, Kanani R. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2000; ( 2):CD000011.
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care 1998; 36:1138–1161.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Appel LJ, Champagne CM, Harsha DW, et al; Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093.
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336:1117–1124.
- Moore TJ, Conlin PR, Ard J, Svetkey LP. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension 2001; 38:155–158.
- Stevens VJ, Obarzanek E, Cook NR, et al; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134:1–11.
- Sawicki PT, Mühlhauser I, Didjurgeit U, Berger M. Improvement of hypertension care by a structured treatment and teaching programme. J Hum Hypertens 1993; 7:571–573.
- Morisky DE, Bowler MH, Finlay JS. An educational and behavioral approach toward increasing patient activation in hypertension management. J Community Health 1982; 7:171–182.
- Levine DM, Green LW, Deeds SG, Chwalow J, Russell RP, Finlay J. Health education for hypertensive patients. JAMA 1979; 241:1700–1703.
- Iso H, Shimamoto T, Yokota K, Sankai T, Jacobs DR, Komachi Y. Community-based education classes for hypertension control. A 1.5-year randomized controlled trial. Hypertension 1996; 27:968–974.
- Cuspidi C, Sampieri L, Macca G, et al. Improvement of patients’ knowledge by a single educational meeting on hypertension. J Hum Hypertens 2001; 15:57–61.
- Nessman DG, Carnahan JE, Nugent CA. Increasing compliance. Patient-operated hypertension groups. Arch Intern Med 1980; 140:1427–1430.
- Casasanta L, Patel S. Outcomes of an educational component of a disease management program for hypertension. Manag Care Interface 1999; 12:70–73.
- McAdams DP. The Stories We Live By: Personal Myths and the Making of the Self. New York NY: The Guilford Press; 1993.
- Bruner J. Acts of Meaning. Cambridge, MA: Harvard Univ Pr; 1990.
- Slater MD, Rouner D. Entertainment—education and elaboration likelihood: Understanding the processing of narrative persuasion. Commun Theory 2002; 12:173–191.
- Dal CS, Zanna MP, Fong GT. Narrative persuasion and overcoming resistance. In:Knowles ES, Linn J, eds. Resistance and Persuasion. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004:175–191.
- Artinian NT, Washington OG, Templin TN. Effects of home telemonitoring and community-based monitoring on blood pressure control in urban African Americans: a pilot study. Heart Lung 2001; 30:191–199.
- Bailey B, Carney SL, Gillies AA, Smith AJ. Antihypertensive drug treatment: a comparison of usual care with self blood pressure measurement. J Hum Hypertens 1999; 13:147–150.
- Bondmass M. The effect of home monitoring and telemanagement on blood pressure control among African Americans. Telemed J 2000; 6:15–23.
- Rogers MA, Small D, Buchan DA, et al. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med 2001; 134:1024–1032.
- Mengden T, Uen S, Baulmann J, Vetter H. Significance of blood pressure self-measurement as compared with office blood pressure measurement and ambulatory 24-hour blood pressure measurement in pharmacological studies. Blood Press Monit 2003; 8:169–172.
- Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens 1996; 9:285–292.
- Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995; 153:1423–1431.
- Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract 1998; 48:991–997.
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995; 274:700–705.
- Inui TS, Yourtee EL, Williamson JW. Improved outcomes in hypertension after physician tutorials. A controlled trial. Ann Intern Med 1976; 84:646–651.
- Jennett PA, Wilson TW, Hayton RC, Mainprize GW, Laxdal OE. Desirable behaviours in the office management of hypertension addressed through continuing medical education. Can J Public Health 1989; 80:359–362.
- Maue SK, Rivo ML, Weiss B, Farrelly EW, Brower-Stenger S. Effect of a primary care physician-focused, population-based approach to blood pressure control. Fam Med 2002; 34:508–513.
- Tu K, Davis D. Can we alter physician behavior by educational methods? Lessons learned from studies of the management and follow-up of hypertension. J Contin Educ Health Prof 2002; 22:11–22.
- McAlister NH, Covvey HD, Tong C, Lee A, Wigle ED. Randomised controlled trial of computer assisted management of hypertension in primary care. Br Med J (Clin Res Ed) 1986; 293:670–674.
- Mashru M, Lant A. Interpractice audit of diagnosis and management of hypertension in primary care: educational intervention and review of medical records. BMJ 1997; 314:942–946.
- Degoulet P, Menard J, Berger C, Plouin PF, Devries C, Hirel JC. Hypertension management: the computer as a participant. Am J Med 1980; 68:559–567.
- Dickinson JC, Warshaw GA, Gehlbach SH, Bobula JA, Muhlbaier LH, Parkerson GR. Improving hypertension control: impact of computer feedback and physician education. Med Care 1981; 19:843–854.
- Montgomery AA, Fahey T. A systematic review of the use of computers in the management of hypertension. J Epidemiol Community Health 1998; 52:520–525.
- Coleman MT, Lott JA, Sharma S. Use of continuous quality improvement to identify barriers in the management of hypertension. Am J Med Qual 2000; 15:72–77.
- Goldberg HI, Wagner EH, Fihn SD, et al. A randomized controlled trial of CQI teams and academic detailing: can they alter compliance with guidelines? Jt Comm J Qual Improv 1998; 24:130–142.
- Horowitz CR, Goldberg HI, Martin DP, et al. Conducting a randomized controlled trial of CQI and academic detailing to implement clinical guidelines. Jt Comm J Qual Improv 1996; 22:734–750.
- Johnson B, McNair D, Kailasam K, et al. Discern—an integrated prospective decision support system. Proc Annu Symp Comput Appl Med Care 1994; 969.
- Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J 2009; 157:450–456.
- Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care 2006; 44:646–657.
- Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med 2009; 169:1748–1755.
- Carter BL, Clarke W, Ardery G, et al; Collaboration Among Pharmacists Physicians To Improve Outcomes Now (CAPTION) Trial Investigators. A cluster-randomized effectiveness trial of a physician-pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:418–423.
- Einhorn PT. National heart, lung, and blood institute-initiated program “interventions to improve hypertension control rates in African Americans”: background and implementation. Circ Cardiovasc Qual Outcomes 2009; 2:236–240.
- Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011; 57:29–38.
- Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns 2008; 70:338–347.
- Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans’ study to improve the control of hypertension (V-STITCH): design and methodology. Contemp Clin Trials 2005; 26:155–168.
- Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension (CAATCH) trial: a multi-level intervention to improve blood pressure control in hypertensive blacks. Circ Cardiovasc Qual Outcomes 2009; 2:249–256.
- Bosworth HB, Almirall D, Weiner BJ, et al. The implementation of a translational study involving a primary care based behavioral program to improve blood pressure control: The HTN-IMPROVE study protocol (01295). Implement Sci 2010; 5:54.
KEY POINTS
- Rates of cardiovascular disease and related death are disparately high in African Americans.
- Ways to improve how physicians manage blood pressure in this patient population may include chart audit with feedback, a computerized clinical decision-support system, and keeping up-to-date with treatment guidelines. However, more data are needed to determine the effectiveness of these interventions.
- A novel method of health education is the use of narrative communication—ie, storytelling. Culturally appropriate storytelling may allow patients to identify with a story as it relates to their own lives.
- A team-based approach to blood pressure control that involves nurses, pharmacists, and physician assistants should be emphasized, even though studies that have shown positive results did not focus specifically on African Americans.
Addressing disparities in health care
In the united states, minority populations are rapidly increasing. In 1970, minorities—ie, African American, Hispanic, Asian, and Native American—accounted for 12.3% of the US population, but they now account for 25%. And this growth is expected to continue, so that by 2050 one of every two Americans will be African American, Hispanic, Asian, Pacific Islander, or Native American.1
Also, while advances in medicine over the past several decades have reduced death rates from cancer and coronary artery disease and have contributed to a longer life expectancy for Americans, minority populations have not benefited equally from these improvements.2 In fact, the growing minority populations suffer from disparities in health care compared with white patients: minority patients have a higher incidence and burden of disease, and poorer health outcomes, contributing to shorter life expectancy.
Clearly, there is an urgent need for physicians, other health care providers, health systems, and medical researchers to increase their awareness of disparities in health care and their impact on patients, as well as on the US health system and the US economy. Now more than ever, we need to equip ourselves to more effectively engage minorities and to deliver culturally competent health care that improves outcomes in our minority patients.
A MULTIFACTORIAL PROBLEM
Disparities in health care are often thought to be the result of poverty and a related lack of access to quality health care. But clinical experience and research show that this is overly simplistic. In fact, disparities result from a variety of factors. Patient-related factors can include culturally related beliefs,1 dietary preferences, and health-seeking behaviors (perhaps influenced by a distrust of doctors, researchers, and the health care system), in addition to poor health literacy. Physician-related factors include poor cultural competency, which leads to poor communication with the patient. Other factors are a continuing lack of representation of minority patients in clinical research trials, as well as biologic factors.3
TAKING ACTION
In view of the disparities in health care that affect racial and ethnic minorities, and the many factors underlying the problem, the US Department of Health and Human Services launched the initiative Healthy People 2020, a continuation of the previous 10-year Healthy People initiatives. Healthy People 2020 calls for health providers and health systems to devise effective ways to eliminate health disparities.4 It outlines high-priority health issues, sets 10-year goals for improving the health of all Americans, and suggests specific actions to take to address health disparities.4
On another front, in 2010 the National Institutes of Health formally established its National Institute of Health and Health Disparities, which funds research into the pathogenesis of health disparities in racial and ethnic minorities.5 Clearly, racial, ethnic, and cultural factors need to be considered for health care to result in better outcomes in minority populations.
OUR NEW SERIES
In this issue of the Cleveland Clinic Journal of Medicine, we launch a series we hope will provide practical tools for physicians to address the disparities in our health care system. The first installment, by Odesosu et al (page 46), addresses barriers to optimal hypertension control in African Americans by outlining potential tactics for both patients and physicians. Future articles will address the challenge of health literacy and cultural issues in medicine, slowing the progression of renal disease in African Americans (especially the complex issue of which antihypertensive agents to use), and the challenges of diabetes in Hispanics. We also plan articles on kidney transplantation in African Americans and on prostate cancer, heart failure, lupus, and diabetes.
We look forward to your comments on this series as well as suggestions for future topics. We believe that as physicians, other health providers, health systems, health insurers and policy-makers become more aware of the disparities in health care, they will embrace ways in which to deliver or promote personalized, culturally competent health care. We hope this series will provide practical tools for physicians to address these complex issues.
- Modlin CS. Culture, race, and disparities in health care. Cleve Clin J Med 2003; 70:283–288.
- Centers for Disease Control and Prevention life expectancy data. www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed December 5, 2011.
- Klein JB, Nguyen CT, Saffore L, Modlin C, Modlin CS. Racial disparities in urologic health care. J Natl Med Assoc 2010: 102:108–117.
- Healthy People 2020. www.healthypeople.gov/2020/. Accessed December 5, 2011.
- National Institutes of Health. NIH announces Institute on minority health and health disparities. www.nih.gov/news/health/sep2010/nimhd-27.htm. Accessed December 5, 2011.
In the united states, minority populations are rapidly increasing. In 1970, minorities—ie, African American, Hispanic, Asian, and Native American—accounted for 12.3% of the US population, but they now account for 25%. And this growth is expected to continue, so that by 2050 one of every two Americans will be African American, Hispanic, Asian, Pacific Islander, or Native American.1
Also, while advances in medicine over the past several decades have reduced death rates from cancer and coronary artery disease and have contributed to a longer life expectancy for Americans, minority populations have not benefited equally from these improvements.2 In fact, the growing minority populations suffer from disparities in health care compared with white patients: minority patients have a higher incidence and burden of disease, and poorer health outcomes, contributing to shorter life expectancy.
Clearly, there is an urgent need for physicians, other health care providers, health systems, and medical researchers to increase their awareness of disparities in health care and their impact on patients, as well as on the US health system and the US economy. Now more than ever, we need to equip ourselves to more effectively engage minorities and to deliver culturally competent health care that improves outcomes in our minority patients.
A MULTIFACTORIAL PROBLEM
Disparities in health care are often thought to be the result of poverty and a related lack of access to quality health care. But clinical experience and research show that this is overly simplistic. In fact, disparities result from a variety of factors. Patient-related factors can include culturally related beliefs,1 dietary preferences, and health-seeking behaviors (perhaps influenced by a distrust of doctors, researchers, and the health care system), in addition to poor health literacy. Physician-related factors include poor cultural competency, which leads to poor communication with the patient. Other factors are a continuing lack of representation of minority patients in clinical research trials, as well as biologic factors.3
TAKING ACTION
In view of the disparities in health care that affect racial and ethnic minorities, and the many factors underlying the problem, the US Department of Health and Human Services launched the initiative Healthy People 2020, a continuation of the previous 10-year Healthy People initiatives. Healthy People 2020 calls for health providers and health systems to devise effective ways to eliminate health disparities.4 It outlines high-priority health issues, sets 10-year goals for improving the health of all Americans, and suggests specific actions to take to address health disparities.4
On another front, in 2010 the National Institutes of Health formally established its National Institute of Health and Health Disparities, which funds research into the pathogenesis of health disparities in racial and ethnic minorities.5 Clearly, racial, ethnic, and cultural factors need to be considered for health care to result in better outcomes in minority populations.
OUR NEW SERIES
In this issue of the Cleveland Clinic Journal of Medicine, we launch a series we hope will provide practical tools for physicians to address the disparities in our health care system. The first installment, by Odesosu et al (page 46), addresses barriers to optimal hypertension control in African Americans by outlining potential tactics for both patients and physicians. Future articles will address the challenge of health literacy and cultural issues in medicine, slowing the progression of renal disease in African Americans (especially the complex issue of which antihypertensive agents to use), and the challenges of diabetes in Hispanics. We also plan articles on kidney transplantation in African Americans and on prostate cancer, heart failure, lupus, and diabetes.
We look forward to your comments on this series as well as suggestions for future topics. We believe that as physicians, other health providers, health systems, health insurers and policy-makers become more aware of the disparities in health care, they will embrace ways in which to deliver or promote personalized, culturally competent health care. We hope this series will provide practical tools for physicians to address these complex issues.
In the united states, minority populations are rapidly increasing. In 1970, minorities—ie, African American, Hispanic, Asian, and Native American—accounted for 12.3% of the US population, but they now account for 25%. And this growth is expected to continue, so that by 2050 one of every two Americans will be African American, Hispanic, Asian, Pacific Islander, or Native American.1
Also, while advances in medicine over the past several decades have reduced death rates from cancer and coronary artery disease and have contributed to a longer life expectancy for Americans, minority populations have not benefited equally from these improvements.2 In fact, the growing minority populations suffer from disparities in health care compared with white patients: minority patients have a higher incidence and burden of disease, and poorer health outcomes, contributing to shorter life expectancy.
Clearly, there is an urgent need for physicians, other health care providers, health systems, and medical researchers to increase their awareness of disparities in health care and their impact on patients, as well as on the US health system and the US economy. Now more than ever, we need to equip ourselves to more effectively engage minorities and to deliver culturally competent health care that improves outcomes in our minority patients.
A MULTIFACTORIAL PROBLEM
Disparities in health care are often thought to be the result of poverty and a related lack of access to quality health care. But clinical experience and research show that this is overly simplistic. In fact, disparities result from a variety of factors. Patient-related factors can include culturally related beliefs,1 dietary preferences, and health-seeking behaviors (perhaps influenced by a distrust of doctors, researchers, and the health care system), in addition to poor health literacy. Physician-related factors include poor cultural competency, which leads to poor communication with the patient. Other factors are a continuing lack of representation of minority patients in clinical research trials, as well as biologic factors.3
TAKING ACTION
In view of the disparities in health care that affect racial and ethnic minorities, and the many factors underlying the problem, the US Department of Health and Human Services launched the initiative Healthy People 2020, a continuation of the previous 10-year Healthy People initiatives. Healthy People 2020 calls for health providers and health systems to devise effective ways to eliminate health disparities.4 It outlines high-priority health issues, sets 10-year goals for improving the health of all Americans, and suggests specific actions to take to address health disparities.4
On another front, in 2010 the National Institutes of Health formally established its National Institute of Health and Health Disparities, which funds research into the pathogenesis of health disparities in racial and ethnic minorities.5 Clearly, racial, ethnic, and cultural factors need to be considered for health care to result in better outcomes in minority populations.
OUR NEW SERIES
In this issue of the Cleveland Clinic Journal of Medicine, we launch a series we hope will provide practical tools for physicians to address the disparities in our health care system. The first installment, by Odesosu et al (page 46), addresses barriers to optimal hypertension control in African Americans by outlining potential tactics for both patients and physicians. Future articles will address the challenge of health literacy and cultural issues in medicine, slowing the progression of renal disease in African Americans (especially the complex issue of which antihypertensive agents to use), and the challenges of diabetes in Hispanics. We also plan articles on kidney transplantation in African Americans and on prostate cancer, heart failure, lupus, and diabetes.
We look forward to your comments on this series as well as suggestions for future topics. We believe that as physicians, other health providers, health systems, health insurers and policy-makers become more aware of the disparities in health care, they will embrace ways in which to deliver or promote personalized, culturally competent health care. We hope this series will provide practical tools for physicians to address these complex issues.
- Modlin CS. Culture, race, and disparities in health care. Cleve Clin J Med 2003; 70:283–288.
- Centers for Disease Control and Prevention life expectancy data. www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed December 5, 2011.
- Klein JB, Nguyen CT, Saffore L, Modlin C, Modlin CS. Racial disparities in urologic health care. J Natl Med Assoc 2010: 102:108–117.
- Healthy People 2020. www.healthypeople.gov/2020/. Accessed December 5, 2011.
- National Institutes of Health. NIH announces Institute on minority health and health disparities. www.nih.gov/news/health/sep2010/nimhd-27.htm. Accessed December 5, 2011.
- Modlin CS. Culture, race, and disparities in health care. Cleve Clin J Med 2003; 70:283–288.
- Centers for Disease Control and Prevention life expectancy data. www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed December 5, 2011.
- Klein JB, Nguyen CT, Saffore L, Modlin C, Modlin CS. Racial disparities in urologic health care. J Natl Med Assoc 2010: 102:108–117.
- Healthy People 2020. www.healthypeople.gov/2020/. Accessed December 5, 2011.
- National Institutes of Health. NIH announces Institute on minority health and health disparities. www.nih.gov/news/health/sep2010/nimhd-27.htm. Accessed December 5, 2011.