User login
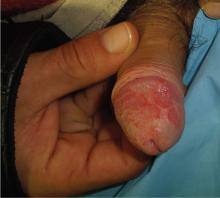
Erythematous patches with keratotic annular borders on the glans penis
A 31-year-old man presented to the emergency department with meatal inflammation, dysuria, and mucopurulent penile discharge, diagnosed as urethritis and treated empirically with levofloxacin. He was referred to the genitourinary medicine clinic for a full screening for sexually transmitted disease. The results were negative.
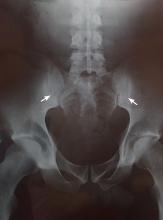
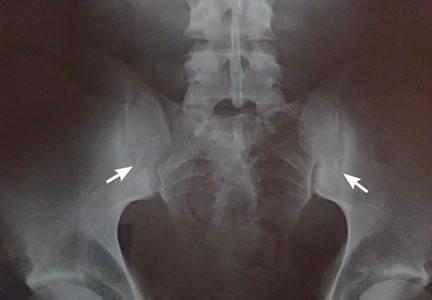
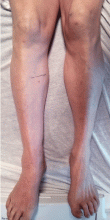
Two months later, he returned with pain and redness in his left eye and inflammatory lumbar pain. The glans penis had small pustules that ruptured, leaving painless superficial erosions that coalesced to form a serpiginous pattern (Figure 1). Radiography and magnetic resonance imaging revealed features of grade 3 bilateral sacroiliitis (Figure 2): subchondral sclerosis of both sacral and iliac articular margins (predominantly on the iliac side), erosions, reduced articular space, widening of the joint space, and incipient ankylosis. A diagnosis of reactive arthritis was made based on the presence of urethritis, ocular symptoms, circinate balanitis, and radiologic evidence of sacroiliitis. In addition, the chronic inflammatory back pain and bilateral sacroiliitis indicated developing ankylosing spondylitis according to the modified New York criteria.
Our patient’s circinate balanitis resolved with hydrocortisone cream, and treatment with a nonsteroidal anti-inflammatory drug brought improvement of the joint symptoms.
THE MANY FEATURES OF REACTIVE ARTHRITIS
The American Rheumatology Association diagnostic criteria for reactive arthritis include asymmetric arthritis that lasts at least 1 month and at least one of the following symptoms: urethritis, inflammatory eye disease, mouth ulcers, circinate balanitis, and radiographic evidence of sarcoiliitis, periostitis, or heel spurs.1,2
Keratoderma blennorrhagicum is another extra-articular manifestation. These symptoms typically start within 1 to 6 weeks after urogenital infection with Chlamydia trachomatis or gastrointestinal infection with Salmonella, Shigella, Yersinia, or Campylobacter species.1–3
There is great variation in the severity, number, and timing of clinical features in reactive arthritis. The diagnosis can be difficult because only about one-third of patients show the complete classic triad (conjunctivitis, urethritis, arthritis). HLA-B27 positivity is associated with more frequent skin lesions and axial involvement.4,5
- Wu IB, Schwartz RA. Reiter’s syndrome: the classic triad and more. J Am Acad Dermatol 2008; 59:113–121.
- Koga T, Miyashita T, Watanabe T, et al. Reactive arthritis which occurred one year after acute chlamydial urethritis. Intern Med 2008; 47:663–666.
- Carter JD. Treating reactive arthritis: insights for the clinician. Ther Adv Musculoskelet Dis 2010; 2:45–54.
- Willkens RF, Arnett FC, Bitter T, et al. Reiter’s syndrome: evaluation of preliminary criteria for definite disease. Arthritis Rheum 1981; 24:844–849.
- Bakkour W, Chularojanamontri L, Motta L, Chalmers RJ. Successful use of dapsone for the management of circinate balanitis. Clin Exp Dermatol 2014; 39:333–335.
A 31-year-old man presented to the emergency department with meatal inflammation, dysuria, and mucopurulent penile discharge, diagnosed as urethritis and treated empirically with levofloxacin. He was referred to the genitourinary medicine clinic for a full screening for sexually transmitted disease. The results were negative.
Two months later, he returned with pain and redness in his left eye and inflammatory lumbar pain. The glans penis had small pustules that ruptured, leaving painless superficial erosions that coalesced to form a serpiginous pattern (Figure 1). Radiography and magnetic resonance imaging revealed features of grade 3 bilateral sacroiliitis (Figure 2): subchondral sclerosis of both sacral and iliac articular margins (predominantly on the iliac side), erosions, reduced articular space, widening of the joint space, and incipient ankylosis. A diagnosis of reactive arthritis was made based on the presence of urethritis, ocular symptoms, circinate balanitis, and radiologic evidence of sacroiliitis. In addition, the chronic inflammatory back pain and bilateral sacroiliitis indicated developing ankylosing spondylitis according to the modified New York criteria.
Our patient’s circinate balanitis resolved with hydrocortisone cream, and treatment with a nonsteroidal anti-inflammatory drug brought improvement of the joint symptoms.
THE MANY FEATURES OF REACTIVE ARTHRITIS
The American Rheumatology Association diagnostic criteria for reactive arthritis include asymmetric arthritis that lasts at least 1 month and at least one of the following symptoms: urethritis, inflammatory eye disease, mouth ulcers, circinate balanitis, and radiographic evidence of sarcoiliitis, periostitis, or heel spurs.1,2
Keratoderma blennorrhagicum is another extra-articular manifestation. These symptoms typically start within 1 to 6 weeks after urogenital infection with Chlamydia trachomatis or gastrointestinal infection with Salmonella, Shigella, Yersinia, or Campylobacter species.1–3
There is great variation in the severity, number, and timing of clinical features in reactive arthritis. The diagnosis can be difficult because only about one-third of patients show the complete classic triad (conjunctivitis, urethritis, arthritis). HLA-B27 positivity is associated with more frequent skin lesions and axial involvement.4,5
A 31-year-old man presented to the emergency department with meatal inflammation, dysuria, and mucopurulent penile discharge, diagnosed as urethritis and treated empirically with levofloxacin. He was referred to the genitourinary medicine clinic for a full screening for sexually transmitted disease. The results were negative.
Two months later, he returned with pain and redness in his left eye and inflammatory lumbar pain. The glans penis had small pustules that ruptured, leaving painless superficial erosions that coalesced to form a serpiginous pattern (Figure 1). Radiography and magnetic resonance imaging revealed features of grade 3 bilateral sacroiliitis (Figure 2): subchondral sclerosis of both sacral and iliac articular margins (predominantly on the iliac side), erosions, reduced articular space, widening of the joint space, and incipient ankylosis. A diagnosis of reactive arthritis was made based on the presence of urethritis, ocular symptoms, circinate balanitis, and radiologic evidence of sacroiliitis. In addition, the chronic inflammatory back pain and bilateral sacroiliitis indicated developing ankylosing spondylitis according to the modified New York criteria.
Our patient’s circinate balanitis resolved with hydrocortisone cream, and treatment with a nonsteroidal anti-inflammatory drug brought improvement of the joint symptoms.
THE MANY FEATURES OF REACTIVE ARTHRITIS
The American Rheumatology Association diagnostic criteria for reactive arthritis include asymmetric arthritis that lasts at least 1 month and at least one of the following symptoms: urethritis, inflammatory eye disease, mouth ulcers, circinate balanitis, and radiographic evidence of sarcoiliitis, periostitis, or heel spurs.1,2
Keratoderma blennorrhagicum is another extra-articular manifestation. These symptoms typically start within 1 to 6 weeks after urogenital infection with Chlamydia trachomatis or gastrointestinal infection with Salmonella, Shigella, Yersinia, or Campylobacter species.1–3
There is great variation in the severity, number, and timing of clinical features in reactive arthritis. The diagnosis can be difficult because only about one-third of patients show the complete classic triad (conjunctivitis, urethritis, arthritis). HLA-B27 positivity is associated with more frequent skin lesions and axial involvement.4,5
- Wu IB, Schwartz RA. Reiter’s syndrome: the classic triad and more. J Am Acad Dermatol 2008; 59:113–121.
- Koga T, Miyashita T, Watanabe T, et al. Reactive arthritis which occurred one year after acute chlamydial urethritis. Intern Med 2008; 47:663–666.
- Carter JD. Treating reactive arthritis: insights for the clinician. Ther Adv Musculoskelet Dis 2010; 2:45–54.
- Willkens RF, Arnett FC, Bitter T, et al. Reiter’s syndrome: evaluation of preliminary criteria for definite disease. Arthritis Rheum 1981; 24:844–849.
- Bakkour W, Chularojanamontri L, Motta L, Chalmers RJ. Successful use of dapsone for the management of circinate balanitis. Clin Exp Dermatol 2014; 39:333–335.
- Wu IB, Schwartz RA. Reiter’s syndrome: the classic triad and more. J Am Acad Dermatol 2008; 59:113–121.
- Koga T, Miyashita T, Watanabe T, et al. Reactive arthritis which occurred one year after acute chlamydial urethritis. Intern Med 2008; 47:663–666.
- Carter JD. Treating reactive arthritis: insights for the clinician. Ther Adv Musculoskelet Dis 2010; 2:45–54.
- Willkens RF, Arnett FC, Bitter T, et al. Reiter’s syndrome: evaluation of preliminary criteria for definite disease. Arthritis Rheum 1981; 24:844–849.
- Bakkour W, Chularojanamontri L, Motta L, Chalmers RJ. Successful use of dapsone for the management of circinate balanitis. Clin Exp Dermatol 2014; 39:333–335.
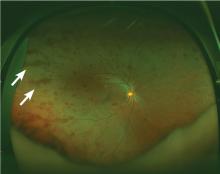
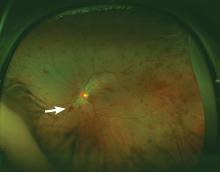
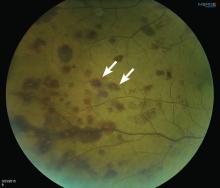
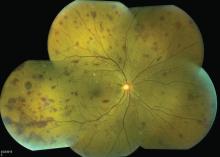
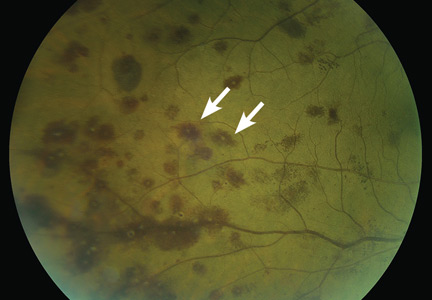
Lymphoplasmacytic lymphoma presenting as retinal hemorrhage
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.
Imaging suggestive, but symptoms atypical
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
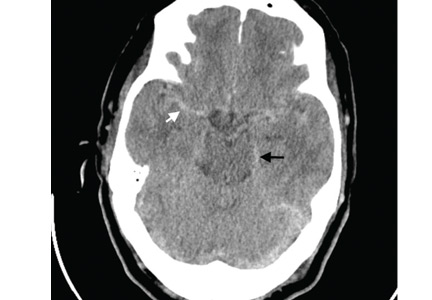
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
Minocycline-induced hyperpigmentation
A 64-year-old woman had a remote history of generalized fatigue, tightness of the hands, tingling and numbness of the face, joint stiffness, and bluish discoloration of the fingers that worsened with cold weather. Laboratory testing at that time had revealed an antinuclear antibody titer over 1:320 (reference range < 1:10), anti-Scl-70 antibody 100 U/mL (< 32 U/mL), and thyroid-stimulating hormone 10.78 mIU/L (0.4–5.5). Pulmonary function testing showed a pattern of restrictive lung disease. She was diagnosed with hypothyroidism, Raynaud phenomenon, and scleroderma. She was referred to a rheumatologist, who prescribed levothyroxine and penicillamine.
Despite treatment, she continued to feel fatigued, and she requested the addition of minocycline to the scleroderma treatment after seeing a report on television. Minocycline 100 mg twice daily was prescribed. She reported improvement of her symptoms for the next 2 years but was then lost to follow-up with the rheumatologist. She continued to take penicillamine and minocycline as prescribed by her primary care physician.
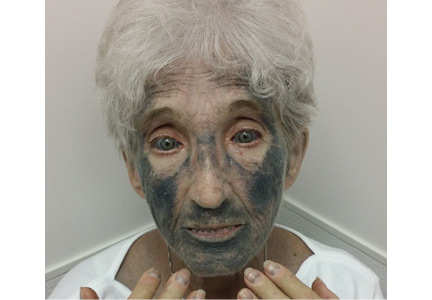
She presented to our clinic with bluish discoloration (Figure 1) that had started 1 year before as a small area but had spread to involve the entire face, fingers, gums, teeth, and sclera, and included a dark discoloration of the neck and upper chest. She had been taking minocycline for nearly 9 years. We referred her to a dermatologist, who diagnosed minocycline-induced hyperpigmentation. Her minocycline was stopped. Skin biopsy was not done, as the dermatologist was confident making the diagnosis without biopsy. At 1 year later, she continued to have the widespread skin pigmentation with no improvement at all.
DIFFERENTIAL DIAGNOSIS
Hyperpigmentation is the darkening in the natural color of the skin, usually from increased deposition of melanin in the epidermis or dermis, or both. It can occur in different degrees of blue, brown, and black (from lightest to darkest). Less frequently, it may be caused by the deposition in the dermis of an endogenous or exogenous pigment, such as hemosiderin, iron, or heavy metal.1 The hyperpigmentation can be circumscribed or more diffuse.
The differential diagnosis of diffuse skin pigmentation includes Addison disease, hyperthyroidism, hemochromatosis, erythema dyschromicum perstans, cutaneous malignancies, sunburn, and drug-induced hyperpigmentation.1,2 Medications commonly cited as causing hyperpigmentation include minocycline, amiodarone, bleomycin, prostaglandins, oral contraceptives, phenothiazine, and antimalarial drugs.1,3 In Addison disease, the pigmentation is typically diffuse, with accentuation in sun-exposed areas, flexures, palmar and plantar creases, and areas of pressure or friction.2 The bronze discoloration of hemochromatosis is from a combination of hemosiderin deposition and increased melanin production.1 Erythema dyschromicum perstans presents with brownish oval-shaped macules and patches. Early lesions may have thin, raised, erythematous borders that typically involve the trunk, but they may spread to the neck, upper extremities, and face.4
The role of minocycline in the treatment of scleroderma is controversial. Early reports involving a small number of patients showed a benefit of minocycline in decreasing symptoms,5,6 but these findings were not achieved in a larger multicenter trial.7
Types of minocycline-induced hyperpigmentation
Three types of minocycline-induced hyperpigmentation occur3,8:
- Type 1—blue-grey coloration on the face in areas of inflammation
- Type 2—blue-grey coloration on normal skin on the skin of the shins and forearms
- Type 3—the least common, characterized by diffuse muddy brown or blue-grey discoloration in sun-exposed areas, as in our patient.
The prevalence of minocycline-induced hyperpigmentation varies between 2.4% and 41% and is highest in patients with rheumatoid arthritis.3,9 Type 1 pigmentation is not correlated with treatment duration or cumulative dose, while type 2 and 3 are associated with long-term therapy.8 In type 3, changes are nonspecific, consisting of increased melanin in basal keratinocytes and melanin-only staining dermal melanophages. Types 1 and 2 may resolve slowly, whereas type 3 can persist indefinitely.3,8,10
TREATMENT
Treatment involves early recognition, discontinuation of the drug, and avoidance of sun exposure. Treatment with pigment-specific lasers has shown promise.8,10
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Thiboutot DM. Clinical review 74: dermatological manifestations of endocrine disorders. J Clin Endocrinol Metab 1995; 80:3082–3087.
- Geria AN, Tajirian AL, Kihiczak G, Schwartz RA. Minocycline-induced skin pigmentation: an update. Acta Dermatovenerol Croat 2009; 17:123–126.
- Schwartz RA. Erythema dyschromicum perstans: the continuing enigma of Cinderella or ashy dermatosis. Int J Dermatol 2004; 43:230–232.
- Le CH, Morales A, Trentham DE. Minocycline in early diffuse scleroderma. Lancet 1998; 352:1755–1756.
- Robertson LP, Marshall RW, Hickling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis 2003; 62:267–269.
- Mayes MD, O’Donnell D, Rothfield NF, Csuka ME. Minocycline is not effective in systemic sclerosis: results of an open-label multicenter trial. Arthritis Rheum 2004; 50:553–557.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. London, UK: Saunders/Elsevier; 2011:125–126.
- Roberts G, Capell HA. The frequency and distribution of minocycline induced hyperpigmentation in a rheumatoid arthritis population. J Rheumatol 2006; 33:1254–1257.
- Vangipuram RK, DeLozier WL, Geddes E, Friedman PM. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med 2016; 48:234–237.
A 64-year-old woman had a remote history of generalized fatigue, tightness of the hands, tingling and numbness of the face, joint stiffness, and bluish discoloration of the fingers that worsened with cold weather. Laboratory testing at that time had revealed an antinuclear antibody titer over 1:320 (reference range < 1:10), anti-Scl-70 antibody 100 U/mL (< 32 U/mL), and thyroid-stimulating hormone 10.78 mIU/L (0.4–5.5). Pulmonary function testing showed a pattern of restrictive lung disease. She was diagnosed with hypothyroidism, Raynaud phenomenon, and scleroderma. She was referred to a rheumatologist, who prescribed levothyroxine and penicillamine.
Despite treatment, she continued to feel fatigued, and she requested the addition of minocycline to the scleroderma treatment after seeing a report on television. Minocycline 100 mg twice daily was prescribed. She reported improvement of her symptoms for the next 2 years but was then lost to follow-up with the rheumatologist. She continued to take penicillamine and minocycline as prescribed by her primary care physician.
She presented to our clinic with bluish discoloration (Figure 1) that had started 1 year before as a small area but had spread to involve the entire face, fingers, gums, teeth, and sclera, and included a dark discoloration of the neck and upper chest. She had been taking minocycline for nearly 9 years. We referred her to a dermatologist, who diagnosed minocycline-induced hyperpigmentation. Her minocycline was stopped. Skin biopsy was not done, as the dermatologist was confident making the diagnosis without biopsy. At 1 year later, she continued to have the widespread skin pigmentation with no improvement at all.
DIFFERENTIAL DIAGNOSIS
Hyperpigmentation is the darkening in the natural color of the skin, usually from increased deposition of melanin in the epidermis or dermis, or both. It can occur in different degrees of blue, brown, and black (from lightest to darkest). Less frequently, it may be caused by the deposition in the dermis of an endogenous or exogenous pigment, such as hemosiderin, iron, or heavy metal.1 The hyperpigmentation can be circumscribed or more diffuse.
The differential diagnosis of diffuse skin pigmentation includes Addison disease, hyperthyroidism, hemochromatosis, erythema dyschromicum perstans, cutaneous malignancies, sunburn, and drug-induced hyperpigmentation.1,2 Medications commonly cited as causing hyperpigmentation include minocycline, amiodarone, bleomycin, prostaglandins, oral contraceptives, phenothiazine, and antimalarial drugs.1,3 In Addison disease, the pigmentation is typically diffuse, with accentuation in sun-exposed areas, flexures, palmar and plantar creases, and areas of pressure or friction.2 The bronze discoloration of hemochromatosis is from a combination of hemosiderin deposition and increased melanin production.1 Erythema dyschromicum perstans presents with brownish oval-shaped macules and patches. Early lesions may have thin, raised, erythematous borders that typically involve the trunk, but they may spread to the neck, upper extremities, and face.4
The role of minocycline in the treatment of scleroderma is controversial. Early reports involving a small number of patients showed a benefit of minocycline in decreasing symptoms,5,6 but these findings were not achieved in a larger multicenter trial.7
Types of minocycline-induced hyperpigmentation
Three types of minocycline-induced hyperpigmentation occur3,8:
- Type 1—blue-grey coloration on the face in areas of inflammation
- Type 2—blue-grey coloration on normal skin on the skin of the shins and forearms
- Type 3—the least common, characterized by diffuse muddy brown or blue-grey discoloration in sun-exposed areas, as in our patient.
The prevalence of minocycline-induced hyperpigmentation varies between 2.4% and 41% and is highest in patients with rheumatoid arthritis.3,9 Type 1 pigmentation is not correlated with treatment duration or cumulative dose, while type 2 and 3 are associated with long-term therapy.8 In type 3, changes are nonspecific, consisting of increased melanin in basal keratinocytes and melanin-only staining dermal melanophages. Types 1 and 2 may resolve slowly, whereas type 3 can persist indefinitely.3,8,10
TREATMENT
Treatment involves early recognition, discontinuation of the drug, and avoidance of sun exposure. Treatment with pigment-specific lasers has shown promise.8,10
A 64-year-old woman had a remote history of generalized fatigue, tightness of the hands, tingling and numbness of the face, joint stiffness, and bluish discoloration of the fingers that worsened with cold weather. Laboratory testing at that time had revealed an antinuclear antibody titer over 1:320 (reference range < 1:10), anti-Scl-70 antibody 100 U/mL (< 32 U/mL), and thyroid-stimulating hormone 10.78 mIU/L (0.4–5.5). Pulmonary function testing showed a pattern of restrictive lung disease. She was diagnosed with hypothyroidism, Raynaud phenomenon, and scleroderma. She was referred to a rheumatologist, who prescribed levothyroxine and penicillamine.
Despite treatment, she continued to feel fatigued, and she requested the addition of minocycline to the scleroderma treatment after seeing a report on television. Minocycline 100 mg twice daily was prescribed. She reported improvement of her symptoms for the next 2 years but was then lost to follow-up with the rheumatologist. She continued to take penicillamine and minocycline as prescribed by her primary care physician.
She presented to our clinic with bluish discoloration (Figure 1) that had started 1 year before as a small area but had spread to involve the entire face, fingers, gums, teeth, and sclera, and included a dark discoloration of the neck and upper chest. She had been taking minocycline for nearly 9 years. We referred her to a dermatologist, who diagnosed minocycline-induced hyperpigmentation. Her minocycline was stopped. Skin biopsy was not done, as the dermatologist was confident making the diagnosis without biopsy. At 1 year later, she continued to have the widespread skin pigmentation with no improvement at all.
DIFFERENTIAL DIAGNOSIS
Hyperpigmentation is the darkening in the natural color of the skin, usually from increased deposition of melanin in the epidermis or dermis, or both. It can occur in different degrees of blue, brown, and black (from lightest to darkest). Less frequently, it may be caused by the deposition in the dermis of an endogenous or exogenous pigment, such as hemosiderin, iron, or heavy metal.1 The hyperpigmentation can be circumscribed or more diffuse.
The differential diagnosis of diffuse skin pigmentation includes Addison disease, hyperthyroidism, hemochromatosis, erythema dyschromicum perstans, cutaneous malignancies, sunburn, and drug-induced hyperpigmentation.1,2 Medications commonly cited as causing hyperpigmentation include minocycline, amiodarone, bleomycin, prostaglandins, oral contraceptives, phenothiazine, and antimalarial drugs.1,3 In Addison disease, the pigmentation is typically diffuse, with accentuation in sun-exposed areas, flexures, palmar and plantar creases, and areas of pressure or friction.2 The bronze discoloration of hemochromatosis is from a combination of hemosiderin deposition and increased melanin production.1 Erythema dyschromicum perstans presents with brownish oval-shaped macules and patches. Early lesions may have thin, raised, erythematous borders that typically involve the trunk, but they may spread to the neck, upper extremities, and face.4
The role of minocycline in the treatment of scleroderma is controversial. Early reports involving a small number of patients showed a benefit of minocycline in decreasing symptoms,5,6 but these findings were not achieved in a larger multicenter trial.7
Types of minocycline-induced hyperpigmentation
Three types of minocycline-induced hyperpigmentation occur3,8:
- Type 1—blue-grey coloration on the face in areas of inflammation
- Type 2—blue-grey coloration on normal skin on the skin of the shins and forearms
- Type 3—the least common, characterized by diffuse muddy brown or blue-grey discoloration in sun-exposed areas, as in our patient.
The prevalence of minocycline-induced hyperpigmentation varies between 2.4% and 41% and is highest in patients with rheumatoid arthritis.3,9 Type 1 pigmentation is not correlated with treatment duration or cumulative dose, while type 2 and 3 are associated with long-term therapy.8 In type 3, changes are nonspecific, consisting of increased melanin in basal keratinocytes and melanin-only staining dermal melanophages. Types 1 and 2 may resolve slowly, whereas type 3 can persist indefinitely.3,8,10
TREATMENT
Treatment involves early recognition, discontinuation of the drug, and avoidance of sun exposure. Treatment with pigment-specific lasers has shown promise.8,10
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Thiboutot DM. Clinical review 74: dermatological manifestations of endocrine disorders. J Clin Endocrinol Metab 1995; 80:3082–3087.
- Geria AN, Tajirian AL, Kihiczak G, Schwartz RA. Minocycline-induced skin pigmentation: an update. Acta Dermatovenerol Croat 2009; 17:123–126.
- Schwartz RA. Erythema dyschromicum perstans: the continuing enigma of Cinderella or ashy dermatosis. Int J Dermatol 2004; 43:230–232.
- Le CH, Morales A, Trentham DE. Minocycline in early diffuse scleroderma. Lancet 1998; 352:1755–1756.
- Robertson LP, Marshall RW, Hickling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis 2003; 62:267–269.
- Mayes MD, O’Donnell D, Rothfield NF, Csuka ME. Minocycline is not effective in systemic sclerosis: results of an open-label multicenter trial. Arthritis Rheum 2004; 50:553–557.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. London, UK: Saunders/Elsevier; 2011:125–126.
- Roberts G, Capell HA. The frequency and distribution of minocycline induced hyperpigmentation in a rheumatoid arthritis population. J Rheumatol 2006; 33:1254–1257.
- Vangipuram RK, DeLozier WL, Geddes E, Friedman PM. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med 2016; 48:234–237.
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Thiboutot DM. Clinical review 74: dermatological manifestations of endocrine disorders. J Clin Endocrinol Metab 1995; 80:3082–3087.
- Geria AN, Tajirian AL, Kihiczak G, Schwartz RA. Minocycline-induced skin pigmentation: an update. Acta Dermatovenerol Croat 2009; 17:123–126.
- Schwartz RA. Erythema dyschromicum perstans: the continuing enigma of Cinderella or ashy dermatosis. Int J Dermatol 2004; 43:230–232.
- Le CH, Morales A, Trentham DE. Minocycline in early diffuse scleroderma. Lancet 1998; 352:1755–1756.
- Robertson LP, Marshall RW, Hickling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis 2003; 62:267–269.
- Mayes MD, O’Donnell D, Rothfield NF, Csuka ME. Minocycline is not effective in systemic sclerosis: results of an open-label multicenter trial. Arthritis Rheum 2004; 50:553–557.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. London, UK: Saunders/Elsevier; 2011:125–126.
- Roberts G, Capell HA. The frequency and distribution of minocycline induced hyperpigmentation in a rheumatoid arthritis population. J Rheumatol 2006; 33:1254–1257.
- Vangipuram RK, DeLozier WL, Geddes E, Friedman PM. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med 2016; 48:234–237.
Phlegmasia cerulea dolens from radiation-induced venous stenosis
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
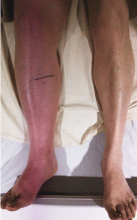
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
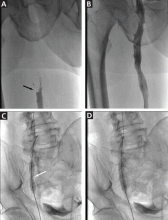
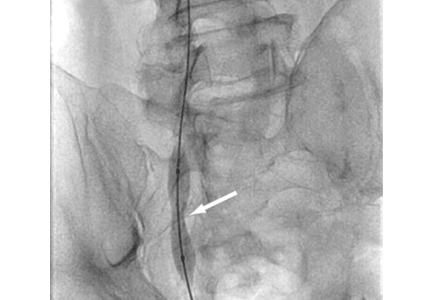
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
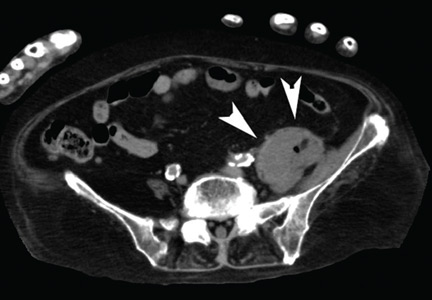
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
Abdominal pain under immunosuppressive conditions
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.
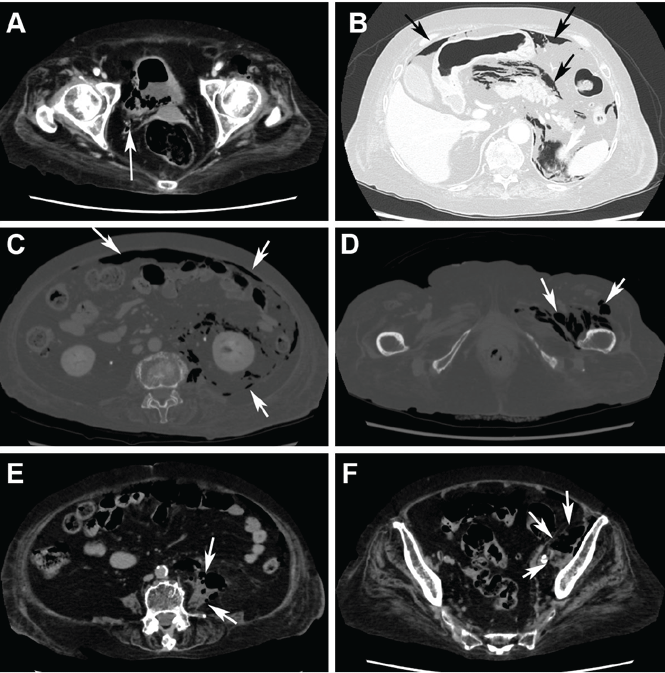
Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.
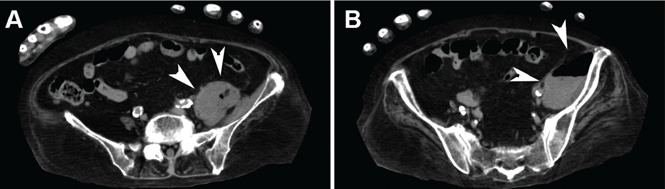
EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.

Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.

EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.

Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.

EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
Bilateral earlobe creases and coronary artery disease
A 70-year-old man with hypertension and hypercholesterolemia presented to the emergency department after the acute onset of substernal, pressure-like chest pain while climbing a flight of stairs. His physical examination was normal, but he was noted to have bilateral diagonal earlobe creases (the Frank sign) (Figure 1), considered by some to indicate risk of coronary artery disease.1–5
Electrocardiography showed atrial fibrillation with a ventricular rate of 149 beats per minute, ST-segment elevation in leads V1 and aVR, and ST-segment depression in leads V3 to V6, II, III, and aVF.
Urgent coronary arteriography showed severe coronary artery disease (Figure 2). Left ventriculography showed an ejection fraction of 50% with mild anterior wall hypokinesis. A drug-eluting stent was placed in the mid-left anterior descending artery. The patient tolerated the procedure well, and his chest pain resolved afterward.
A STILL-UNCLEAR ASSOCIATION
Sanders T. Frank, in 1973, first described a diagonal wrinkle-like line on the earlobe as a sign of coronary artery disease.1 Subsequently, autopsy studies suggested that deep bilateral earlobe creases could be an important sign of coronary atherosclerosis.2 Diagonal earlobe creases have been shown to be independently associated with increased prevalence, extent, and severity of coronary artery disease.3,4 They are also associated with major adverse cardiovascular events4 and ischemic stroke.5 The mechanism linking diagonal earlobe creases and atherosclerotic disease is not yet clear.
This patient’s presentation and evaluation remind us that bilateral earlobe creases may be useful to include in the clinical examination of patients with suspected coronary artery disease and may facilitate early recognition of disease in a patient at high risk.
- Frank ST. Aural sign of coronary-artery disease. N Engl J Med 1973; 289:327–328.
- Patel V, Champ C, Andrews PS, Gostelow BE, Gunasekara NP, Davidson AR. Diagonal earlobe creases and atheromatous disease: a postmortem study. J R Coll Phys Lond 1992; 26:274–277.
- Kaukola S, Manninen V, Valle M, Halonen PI. Ear-lobe crease and coronary atherosclerosis. Lancet 1979; 2:1377.
- Shmilovich H, Cheng VY, Rajani R, et al. Relation of diagonal ear lobe crease to the presence, extent, and severity of coronary artery disease determined by coronary computed tomography angiography. Am J Cardiol 2012; 109:1283–1287.
- Zapata-Wainberg G, Vivancos J. Images in clinical medicine: bilateral earlobe creases. N Engl J Med 2013; 368:e32.
A 70-year-old man with hypertension and hypercholesterolemia presented to the emergency department after the acute onset of substernal, pressure-like chest pain while climbing a flight of stairs. His physical examination was normal, but he was noted to have bilateral diagonal earlobe creases (the Frank sign) (Figure 1), considered by some to indicate risk of coronary artery disease.1–5
Electrocardiography showed atrial fibrillation with a ventricular rate of 149 beats per minute, ST-segment elevation in leads V1 and aVR, and ST-segment depression in leads V3 to V6, II, III, and aVF.
Urgent coronary arteriography showed severe coronary artery disease (Figure 2). Left ventriculography showed an ejection fraction of 50% with mild anterior wall hypokinesis. A drug-eluting stent was placed in the mid-left anterior descending artery. The patient tolerated the procedure well, and his chest pain resolved afterward.
A STILL-UNCLEAR ASSOCIATION
Sanders T. Frank, in 1973, first described a diagonal wrinkle-like line on the earlobe as a sign of coronary artery disease.1 Subsequently, autopsy studies suggested that deep bilateral earlobe creases could be an important sign of coronary atherosclerosis.2 Diagonal earlobe creases have been shown to be independently associated with increased prevalence, extent, and severity of coronary artery disease.3,4 They are also associated with major adverse cardiovascular events4 and ischemic stroke.5 The mechanism linking diagonal earlobe creases and atherosclerotic disease is not yet clear.
This patient’s presentation and evaluation remind us that bilateral earlobe creases may be useful to include in the clinical examination of patients with suspected coronary artery disease and may facilitate early recognition of disease in a patient at high risk.
A 70-year-old man with hypertension and hypercholesterolemia presented to the emergency department after the acute onset of substernal, pressure-like chest pain while climbing a flight of stairs. His physical examination was normal, but he was noted to have bilateral diagonal earlobe creases (the Frank sign) (Figure 1), considered by some to indicate risk of coronary artery disease.1–5
Electrocardiography showed atrial fibrillation with a ventricular rate of 149 beats per minute, ST-segment elevation in leads V1 and aVR, and ST-segment depression in leads V3 to V6, II, III, and aVF.
Urgent coronary arteriography showed severe coronary artery disease (Figure 2). Left ventriculography showed an ejection fraction of 50% with mild anterior wall hypokinesis. A drug-eluting stent was placed in the mid-left anterior descending artery. The patient tolerated the procedure well, and his chest pain resolved afterward.
A STILL-UNCLEAR ASSOCIATION
Sanders T. Frank, in 1973, first described a diagonal wrinkle-like line on the earlobe as a sign of coronary artery disease.1 Subsequently, autopsy studies suggested that deep bilateral earlobe creases could be an important sign of coronary atherosclerosis.2 Diagonal earlobe creases have been shown to be independently associated with increased prevalence, extent, and severity of coronary artery disease.3,4 They are also associated with major adverse cardiovascular events4 and ischemic stroke.5 The mechanism linking diagonal earlobe creases and atherosclerotic disease is not yet clear.
This patient’s presentation and evaluation remind us that bilateral earlobe creases may be useful to include in the clinical examination of patients with suspected coronary artery disease and may facilitate early recognition of disease in a patient at high risk.
- Frank ST. Aural sign of coronary-artery disease. N Engl J Med 1973; 289:327–328.
- Patel V, Champ C, Andrews PS, Gostelow BE, Gunasekara NP, Davidson AR. Diagonal earlobe creases and atheromatous disease: a postmortem study. J R Coll Phys Lond 1992; 26:274–277.
- Kaukola S, Manninen V, Valle M, Halonen PI. Ear-lobe crease and coronary atherosclerosis. Lancet 1979; 2:1377.
- Shmilovich H, Cheng VY, Rajani R, et al. Relation of diagonal ear lobe crease to the presence, extent, and severity of coronary artery disease determined by coronary computed tomography angiography. Am J Cardiol 2012; 109:1283–1287.
- Zapata-Wainberg G, Vivancos J. Images in clinical medicine: bilateral earlobe creases. N Engl J Med 2013; 368:e32.
- Frank ST. Aural sign of coronary-artery disease. N Engl J Med 1973; 289:327–328.
- Patel V, Champ C, Andrews PS, Gostelow BE, Gunasekara NP, Davidson AR. Diagonal earlobe creases and atheromatous disease: a postmortem study. J R Coll Phys Lond 1992; 26:274–277.
- Kaukola S, Manninen V, Valle M, Halonen PI. Ear-lobe crease and coronary atherosclerosis. Lancet 1979; 2:1377.
- Shmilovich H, Cheng VY, Rajani R, et al. Relation of diagonal ear lobe crease to the presence, extent, and severity of coronary artery disease determined by coronary computed tomography angiography. Am J Cardiol 2012; 109:1283–1287.
- Zapata-Wainberg G, Vivancos J. Images in clinical medicine: bilateral earlobe creases. N Engl J Med 2013; 368:e32.