User login
Long-acting injectable antipsychotics during COVID-19
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
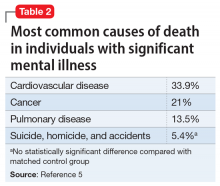
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
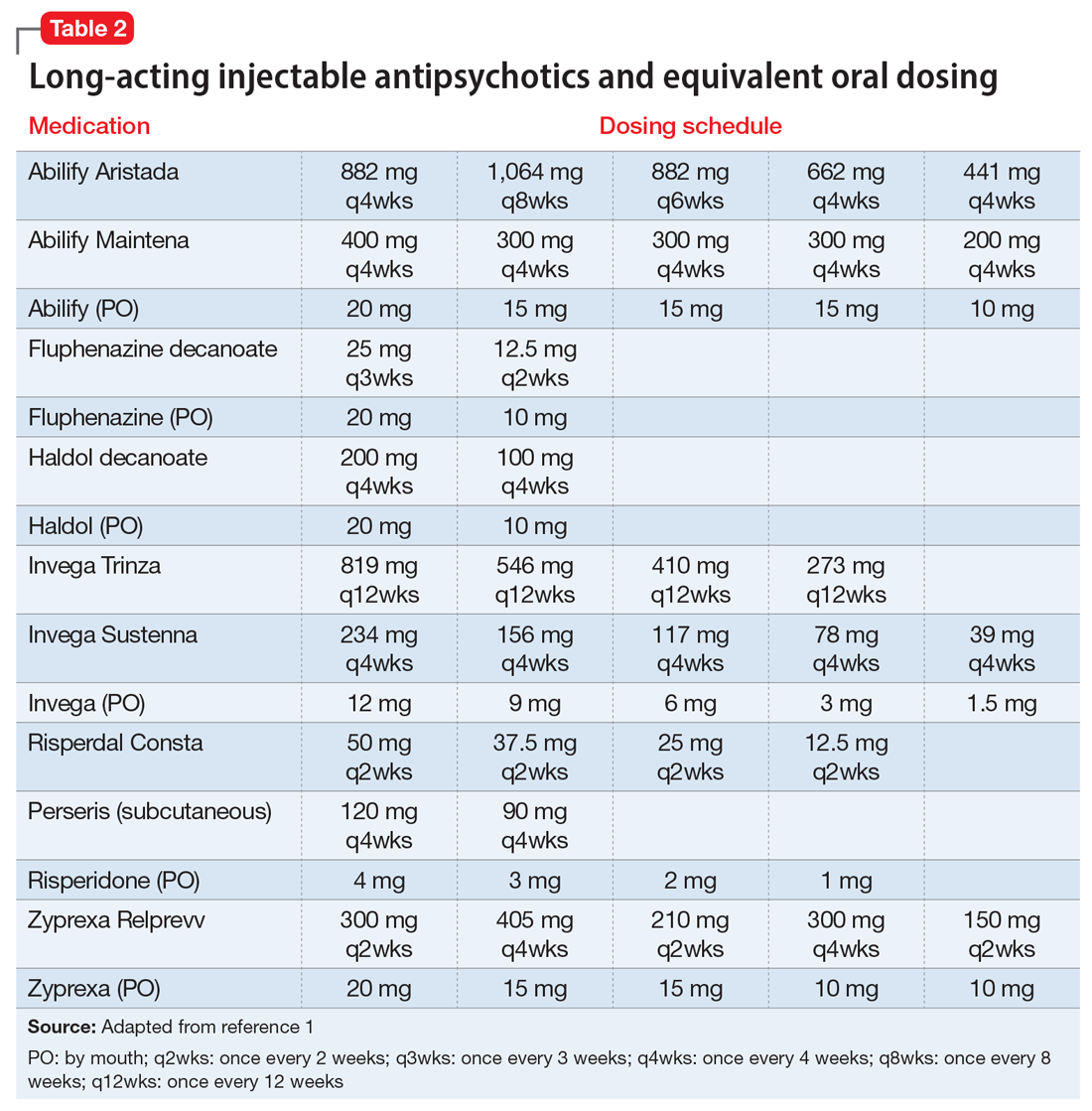
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.
Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
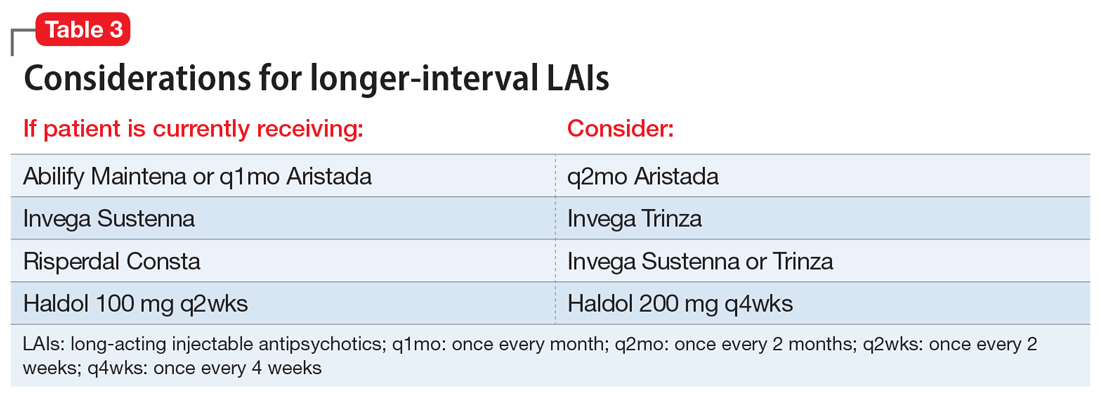
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.
Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.
Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.
Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.
Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.
Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
COVID-19 and the risk of homicide-suicide among older adults
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
Anticonvulsants for alcohol withdrawal: A review of the evidence
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13
Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19
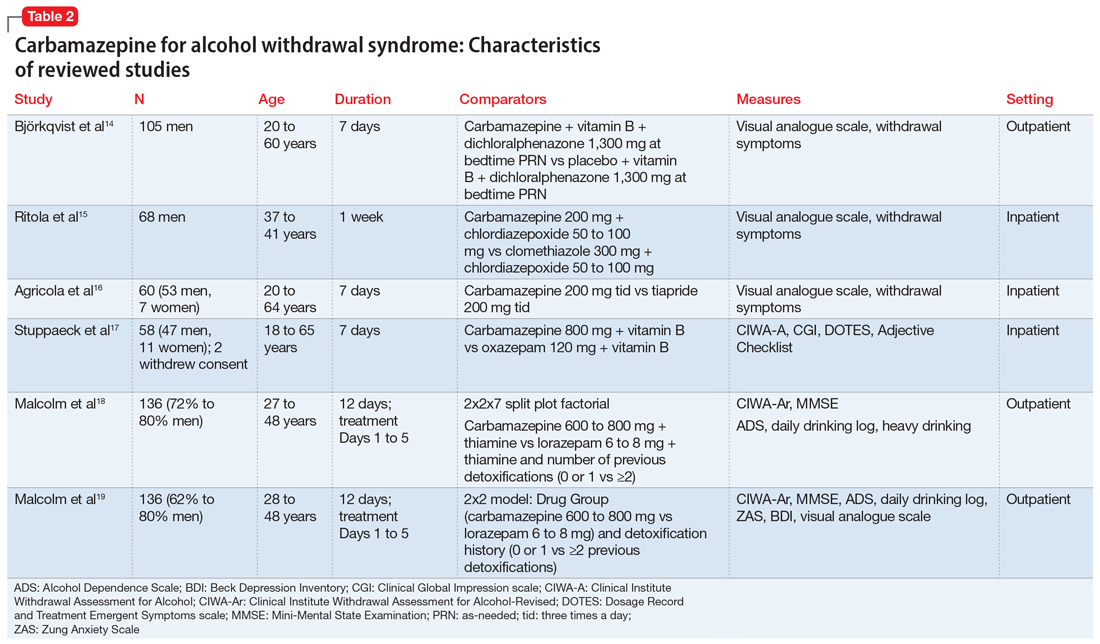
Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21
Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13
Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19

Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21
Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13
Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19

Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21
Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
The rebirth of psychedelic psychiatry
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.
Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.
Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24
Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.
Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.
Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24
Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.
Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.
Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24
Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
Pharmacologic management of autism spectrum disorder: A review of 7 studies
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.
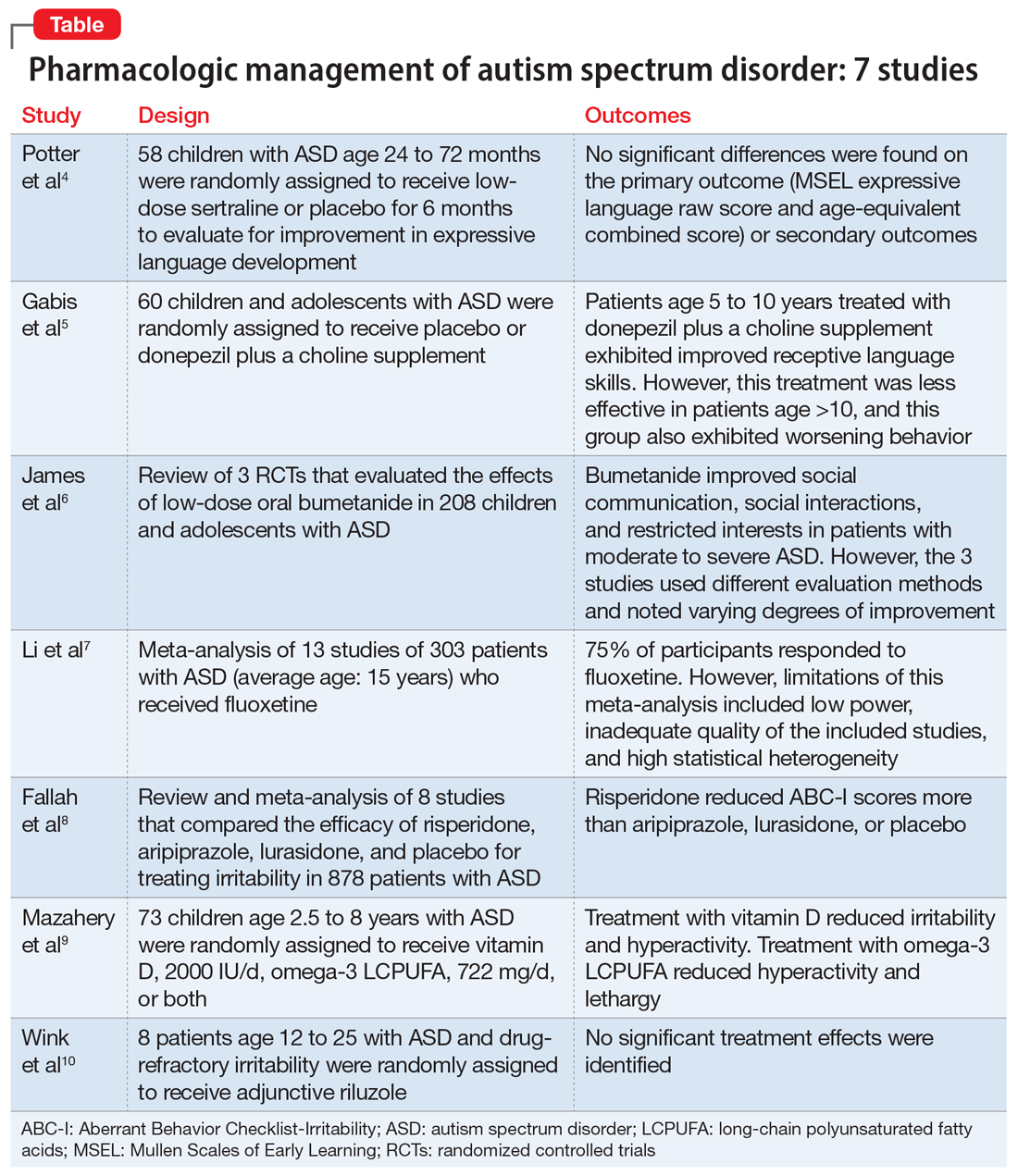
1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.

1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.

1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
2020 Peer Reviewers
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
Caring for outpatients during COVID-19: 4 themes
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.
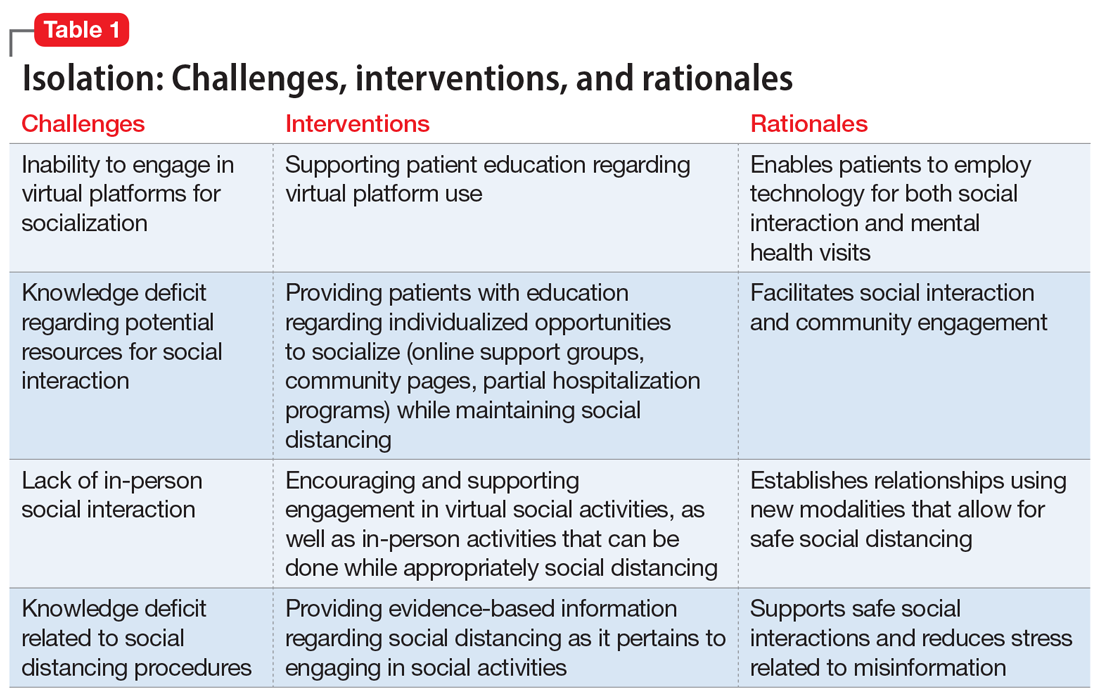
Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11
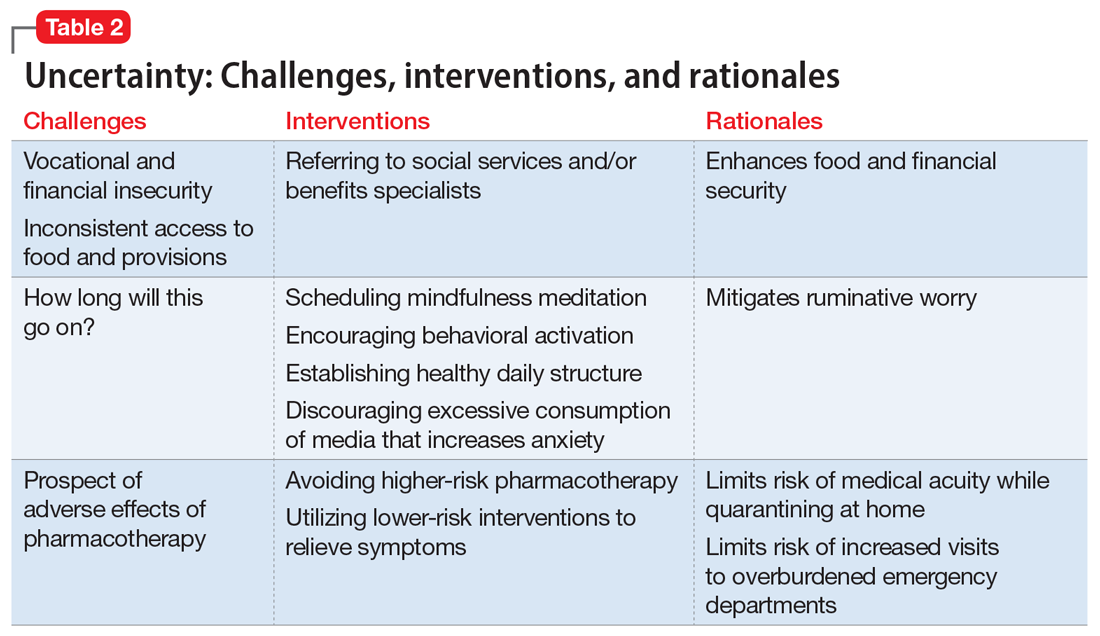
Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16
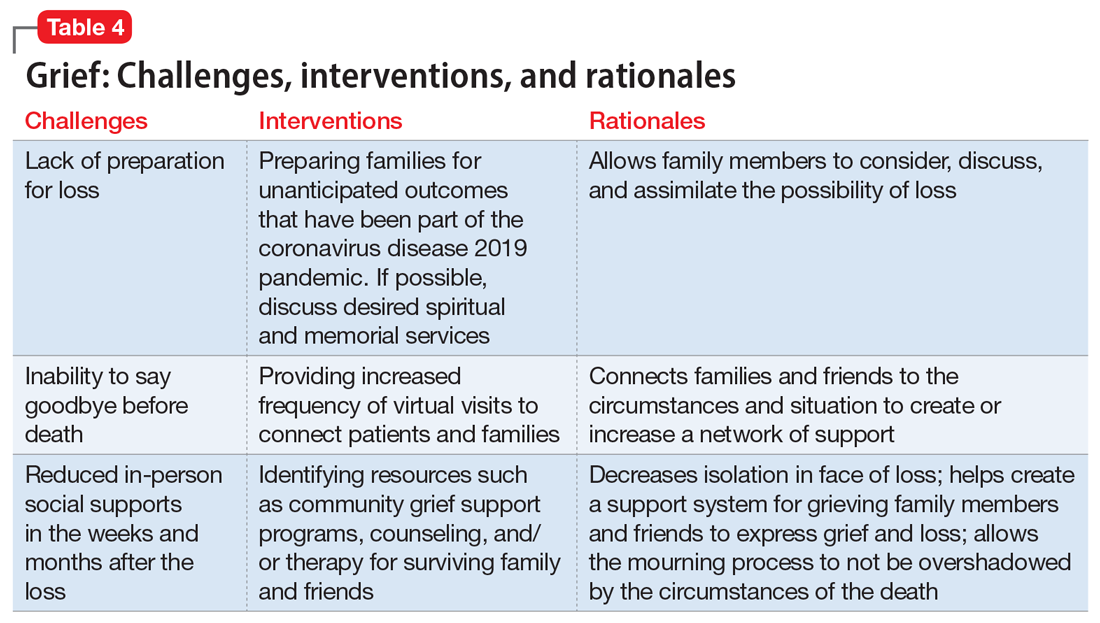
Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.

Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11

Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16

Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.

Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11

Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16

Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
Managing metabolic syndrome in patients with schizophrenia
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.
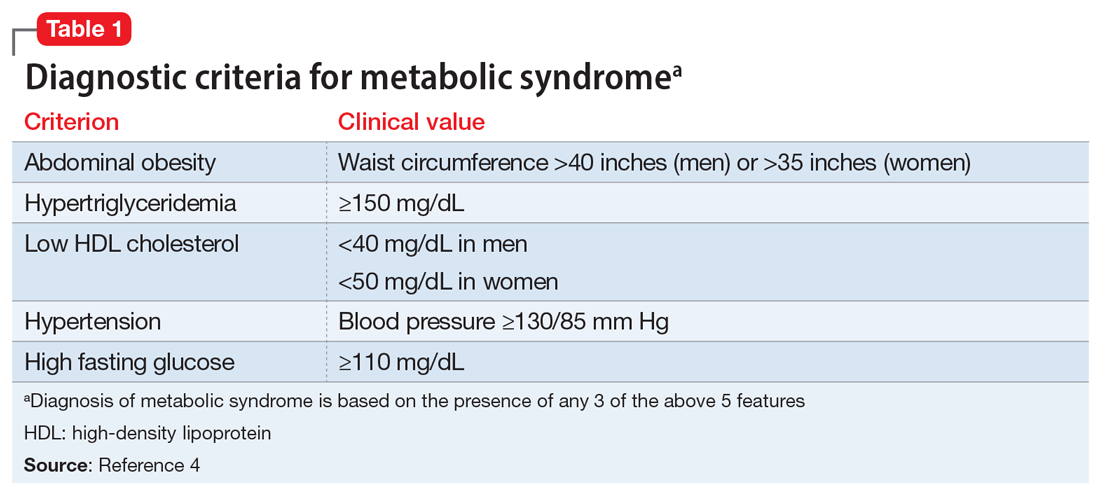
Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.