User login
The psychiatric consequences of COVID-19: 8 Studies
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
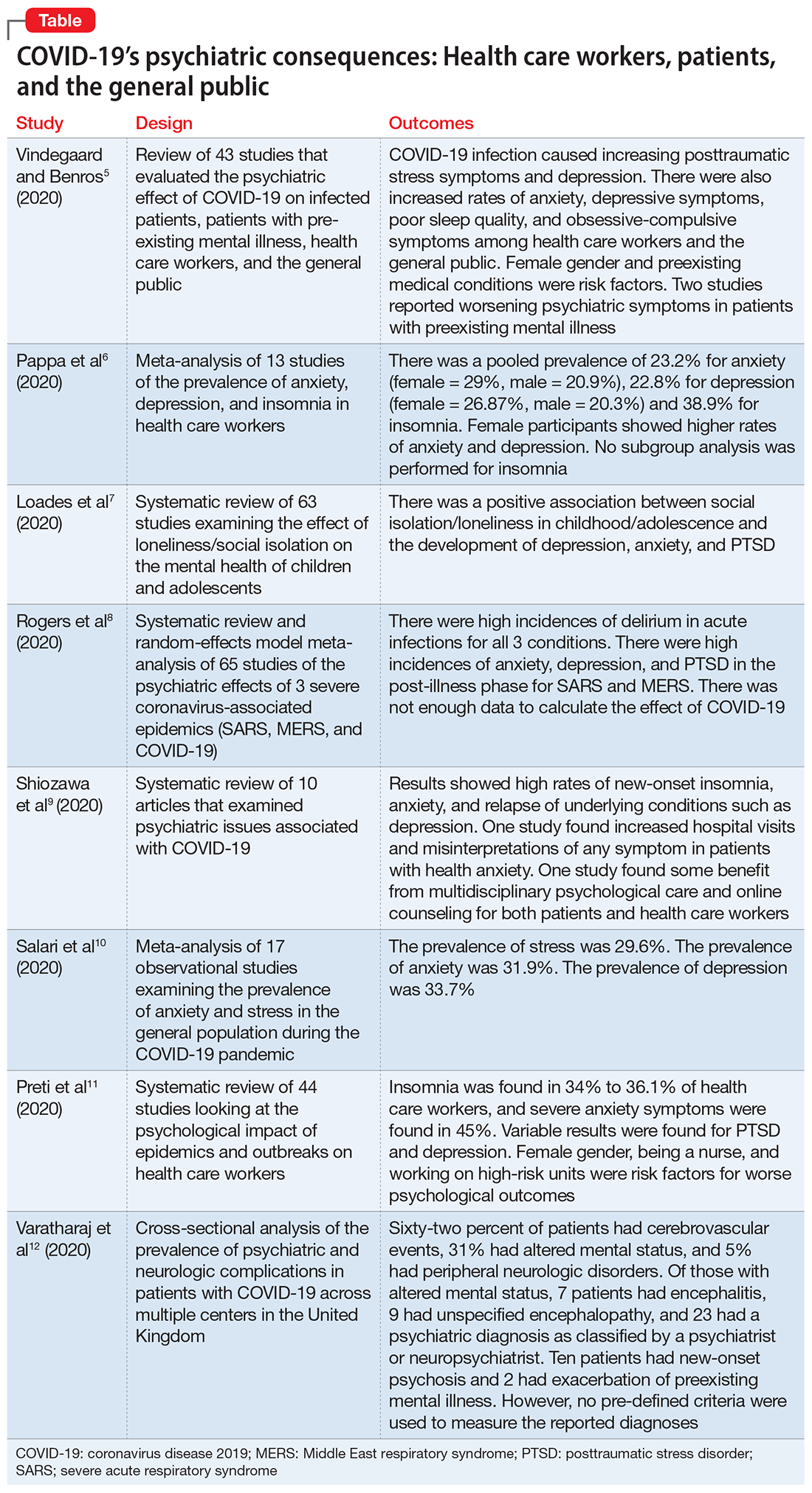
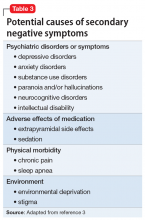
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Using seclusion to prevent COVID-19 transmission on inpatient psychiatry units
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.
Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.
Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.
Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
Negative symptoms of schizophrenia: An update
The negative symptoms of schizophrenia have been recognized for 100 years. Characterized by a loss of a function that should be present, negative symptoms include anhedonia, asociality, amotivation, and affective blunting. Individuals with schizophrenia who have a preponderance of negative symptoms (“deficit syndrome”) may comprise a special subset of patients. Compared with positive symptoms, negative symptoms are associated with worse global functioning and worse response to antipsychotic medication. Treatment of negative symptoms is challenging. Secondary negative symptoms—those that simulate or resemble primary negative symptoms but are attributable to another cause, such as major depressive disorder or the adverse effects of antipsychotic medication—need to be ruled out. Emerging evidence suggests that newer antipsychotics with novel mechanisms might be effective in treating negative symptoms. Antidepressants might also play a role.
This article describes types of negative symptoms, their clinical relevance, neuroanatomical and neurotransmission factors associated with negative symptoms, and current and future treatment options.
Modest improvements with antipsychotics
Schizophrenia affects an estimated 1% of the population.1 Antipsychotic medication has been the mainstay of schizophrenia treatment since
All antipsychotics are believed to exert their therapeutic effects by blocking dopamine (D2) receptors and are effective in ameliorating the positive symptoms of schizophrenia, including hallucinations, delusions, bizarre behavior, disordered thinking, and agitation.1 Early research had suggested that SGAs might also reduce the negative symptoms of schizophrenia, perhaps because they also block serotonin 2A receptors, a property thought to broaden their therapeutic profile. Over time, it became clear that neither FGAs nor SGAs conferred an advantage in treating negative symptoms, and that the observed improvements were modest.2-5 However, recent research suggests that several newer antipsychotics might be effective in targeting negative symptoms.2,6,7
History of negative symptoms
In the early 20th century, Swiss psychiatrist Eugen Bleuler coined the term schizophrenia to emphasize the cognitive impairment that occurs in patients with this illness, and which he conceptualized as a fragmenting of the psychic process.8 He believed that certain symptoms were fundamental to the illness, and described affective blunting, disturbance of association (ie, distorted thinking) autism (ie, impaired relationships), and ambivalence (ie, fragmented emotional responses). He viewed hallucinations and delusions as accessory symptoms because they were not unique to schizophrenia but were also found in other disorders (eg, mood disorders). Bleuler’s ideas took root, and generations of psychiatrists were taught his fundamental symptoms (“the 4 A’s”), the forerunner of today’s negative symptoms. Later, other experts chose to emphasize psychotic symptoms as most characteristic of schizophrenia, including Schneider’s “first-rank symptoms,” such as voices conversing or delusions of passivity.9
Negative symptoms were rediscovered in the 1970s and 1980s by psychiatric researchers interested in descriptive phenomenology.10,11 Research confirmed the presence of a positive dimension in schizophrenia characterized by the loss of boundaries between the patient and the real world (eg, hallucinations, delusions), and a negative dimension characterized by the loss of a function that should be present, such as alogia and asociality. These experts carefully described negative symptoms and created scales to measure them, including the Scale for the Assessment of Negative Symptoms (SANS),12 the Positive and Negative Syndrome Scale (PANSS),13 the Brief Negative Symptom Scale (BNSS),14 and the 16-item Negative Symptom Assessment (NSA-16).15 Contemporaneous to this work, a “deficit syndrome” was identified among patients with schizophrenia with prominent negative symptoms. The deficit syndrome is found in 25% to 30% of chronic cases.16 Negative symptoms are very common in patients with schizophrenia (Table 19).8,17
Early editions of the DSM defined schizophrenia mainly on the basis of disturbance of cognition, mood, and behavior, and a retreat from reality. With the publication of DSM-III in 1980, and in subsequent editions, schizophrenia was redefined as a relatively severe psychotic illness in which positive and negative symptoms were present, thereby acknowledging the importance of Bleuler’s fundamental symptoms. In DSM-5, negative symptoms are described as accounting for “a substantial portion of the morbidity associated with schizophrenia but are less prominent in other psychotic disorders.”18
Continue to: Types of negative symptoms
Types of negative symptoms
The following symptoms fall within the negative dimension19:
Alogia refers to the impoverished thinking and cognition that often occur in patients with schizophrenia. The patient’s thinking processes seem empty, turgid, or slow, as inferred from the patient’s speech. The 2 major manifestations of alogia are poverty of speech (nonfluent empty speech) and poverty of content of speech (fluent but empty speech). Examples of each appear in Table 2.19
Affective flattening or blunting manifests as a general impoverishment of emotional expression, reactivity, and feeling. Affective flattening can be assessed through observing a patient’s behavior and responsiveness during the interview.
Avolition-apathy manifests itself as a lack of energy and drive. Patients become inert and are unable to mobilize themselves to initiate or persist in completing many kinds of tasks.
Anhedonia-asociality encompasses the patient’s difficulties in experiencing interest or pleasure. It may express itself as a loss of interest in pleasurable activities, an inability to experience pleasure when participating in activities normally considered pleasurable, or a lack of involvement in social relationships.
Continue to: Attention
Attention is often poor in patients with severe mental illnesses. The patient may have trouble focusing his/her attention or may be able to focus only sporadically and erratically. He/she may ignore attempts to converse with him/her, wander away during an activity or a task, or appear to be inattentive when engaged in formal testing or interviewing.
Clinical relevance of negative symptoms
According to DSM-5, “Negative symptoms are more closely related to prognosis than are positive symptoms and tend to be the most persistent.”18 Research has shown that, compared with positive symptoms, negative symptoms are associated with greater impairment in overall functioning, social interaction, interpersonal relationships, economic functioning, and recreational activities.1,3,5 Negative symptoms also are associated with poorer response to medication and a positive family history of schizophrenia. Research shows that negative symptoms are persistent over time, and, in fact, become more prominent as the patient ages, whereas positive symptoms become less prominent.20
Secondary negative symptoms
Potential secondary causes of negative symptoms should be ruled out before concluding that the negative symptoms are due to schizophrenia.3 What might appear to be a negative symptom of schizophrenia, such as poor motivation or flattened affect, could be due to the presence of major depressive disorder. Such symptoms might resolve with treatment. Alternatively, a patient could have developed pseudoparkinsonism from antipsychotic medication and display unchanging facial expression and decreased spontaneous movements. These symptoms could resolve by adding
The neuroanatomy of negative symptoms
Although the neuroanatomical basis of negative symptoms has not been determined, neuroimaging studies have provided important clues.3 Structural brain imaging has consistently shown that negative symptoms in patients with schizophrenia correlate with decreased prefrontal white matter volume, anterior cingulate volume, insular cortex volume, left temporal cortex volume, and ventricular enlargement. Interestingly, volume loss starts before the appearance of negative symptoms.21,22 Functional imaging has shown that negative symptoms correlate with reduced cerebral blood perfusion in frontal, prefrontal, posterior cingulate, thalamus, parietal, and striatal regions.21,22 These findings may help explain the apathy, failure to initiate activities, and impaired social relatedness in patients with schizophrenia.
Neurotransmission and negative symptoms
Some experts have hypothesized that lowered cortical dopamine transmission in mesocortical pathways could give rise to negative symptoms, whereas excess transmission in subcortical structures leads to positive symptoms.23 There is also evidence for a noradrenalin deficiency based on the finding that low levels of cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol (MHPG), a noradrenaline metabolite, correlates with greater negative symptom severity.24 The presence of a serotonin deficiency has been proposed based on evidence that negative symptoms might be mitigated by serotonergic agents.25 More recently, some experts have posited that the dopamine D3 receptor might be involved in the etiology of negative symptoms. The dopamine D3 receptor activity is expressed in brain regions thought to control reward, emotions, and motivation.2 Newer medications with novel mechanisms suggest that other neurotransmitter pathways could be involved.6,7
Continue to: Treatment options
Treatment options
Treating negative symptoms remains challenging and there are no clear answers. When they were introduced in the 1990s, SGAs were initially thought to be superior to FGAs in targeting negative symptoms. Subsequent research, including recent reviews and meta-analyses, has shown that SGAs are not superior to FGAs in treating negative symptoms, and the effect of either medication class on negative symptoms is modest.2-5 One exception is amisulpride (not available in the United States), which is known to antagonize D2 and D3 receptors. A meta-analysis of the efficacy of antipsychotics in schizophrenia showed that amisulpride was significantly more effective than placebo in treating negative symptoms in 590 patients who received the medication.26 The authors suggested that amisulpride was effective due to its binding to presynaptic receptors in the frontal cortex, thereby enhancing dopamine transmission in this region.
Cariprazine, which acts as a partial agonist at the D2 and D3 receptors, with a 10-fold affinity for the D3 receptor, also has shown promise in treating negative symptoms.2 In a clinical trial of 460 patients with predominant negative symptoms, treatment with cariprazine led to a greater reduction in negative symptoms than
Other promising agentsinclude
Antidepressants also could be effective in reducing negative symptoms.3 A meta-analysis of randomized controlled trials evaluating the use of antidepressants as adjuncts to antipsychotic medications showed that adding an antidepressant was effective in reducing negative symptoms.29 The mechanism by which an antidepressant might cause a reduction in negative symptoms is uncertain, and it is possible that the antidepressant might treat depressive symptoms that are causing or contributing to the negative symptoms.
Bottom Line
Negative symptoms in patients with schizophrenia are associated with a worse functional outcome and poorer response to antipsychotic medication than positive symptoms. First- and second-generation antipsychotics are largely ineffective in consistently treating negative symptoms. Antipsychotic medications that target the D3 receptor might be more effective. Roluperidone, which targets serotonin 2A and sigma receptors, and SEP-363856, which targets TAAR1 and serotonin 1A receptors, are being studied for their effects on negative symptoms.
Continue to: Related Resources
Related Resources
- Galderisi S, Färden A, Kaiser S. Dissecting negative symptoms of schizophrenia: History, assessment, pathophysiological mechanisms and treatment. Schizophr Res. 2017;186:1-2.
- Rabinowitz J. Treating negative symptoms of schizophrenia. Current Psychiatry. 2018;17(12):19-23.
Drug Brand Names
Benztropine • Cogentin
Cariprazine • Vraylar
Chlorpromazine • Promapar, Thorazine
Risperidone • Risperdal
1. Owen MJ, Sawa A, Mortensen PD. Schizophrenia. Lancet. 2016;388(10039):86-97.
2. Cerviri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat. 2019;15:1525-1535.
3. Mitra S, Mahintamani T, Kavoor AR, et al. Negative symptoms in schizophrenia. Ind Psychiatr J. 2016;25(2):135-144.
4. Fusa-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892-899.
5. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms: an update. Curr Treat Options Psych. 2016;3:133-150.
6. Harvey PD, Saoud JB, Luthringer R, et al. Effects of roluperidone (MIN-101) on two dimensions of negative symptoms factor score: reduced emotional experience and reduced emotional expression. Schizophr Res. 2020;215:352-356.
7. Dedic N, Jones PG, Hopkins SC, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Psychopharmacol Exp Ther. 2019;371(1):1-14.
8. Bleuler E. Dementia praecox or the group of schizophrenia. New York, New York: International Universities Press; 1950.
9. Andreasen NC. The diagnosis of schizophrenia. Schizophr Bull. 1987;13(1):9-22.
10. Andreasen NC. Thought, language, and communication disorders I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36(12):1315-1321.
11. Crow TJ. Molecular pathology of schizophrenia: more than one disease process? Br Med J. 1980;280(6207):66-68.
12. Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39(7):789-794.
13. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
14. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305.
15. Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptoms Assessment. J Psychiatr Res. 1993;27(3):253-258.
16. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578-583.
17. Bobes J, Arango C, Garcia-Garcia M, et al. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS Study. J Clin Psychiatry. 2010;71(3):280-286.
18. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
19. Black DW, Andreasen NC. Interviewing and assessment. In: Introductory textbook of psychiatry, 7th ed. Black DW, Andreasen NC, eds. Washington, DC: American Psychiatric Publishing; 2020:15-53.
20. Pfohl B, Winokur G. The micropsychopathology of hebephrenic/catatonic schizophrenia. J Nerv Ment Dis. 1983;171(5):296-300.
21. Hovington CL, Lepage M. Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother. 2012;12(1):53-69.
22. Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, et al. A review of schizophrenia, melancholic depression and Parkinson’s disease. Brain Res Bull. 2006;70(4-6):312-321.
23. Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329-336.
24. Yoshimura R, Hori H, Katsuki A, et al. Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF, and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Ann Gen Psychiatry. 2016;15:1.
25. Moller HJ. Management of negative symptoms of schizophrenia: new treatment options. CNS Drugs. 2003;17(11):793-823.
26. Leucht S. Amisulpride: a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2004;7(suppl 1):S15-S20. doi: 10.1017/S1461145704004109.
27. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomized, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
28. Neill JC, Grayson, Kiss B, et al. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26(1):3-14.
29. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9):876-886.
The negative symptoms of schizophrenia have been recognized for 100 years. Characterized by a loss of a function that should be present, negative symptoms include anhedonia, asociality, amotivation, and affective blunting. Individuals with schizophrenia who have a preponderance of negative symptoms (“deficit syndrome”) may comprise a special subset of patients. Compared with positive symptoms, negative symptoms are associated with worse global functioning and worse response to antipsychotic medication. Treatment of negative symptoms is challenging. Secondary negative symptoms—those that simulate or resemble primary negative symptoms but are attributable to another cause, such as major depressive disorder or the adverse effects of antipsychotic medication—need to be ruled out. Emerging evidence suggests that newer antipsychotics with novel mechanisms might be effective in treating negative symptoms. Antidepressants might also play a role.
This article describes types of negative symptoms, their clinical relevance, neuroanatomical and neurotransmission factors associated with negative symptoms, and current and future treatment options.
Modest improvements with antipsychotics
Schizophrenia affects an estimated 1% of the population.1 Antipsychotic medication has been the mainstay of schizophrenia treatment since
All antipsychotics are believed to exert their therapeutic effects by blocking dopamine (D2) receptors and are effective in ameliorating the positive symptoms of schizophrenia, including hallucinations, delusions, bizarre behavior, disordered thinking, and agitation.1 Early research had suggested that SGAs might also reduce the negative symptoms of schizophrenia, perhaps because they also block serotonin 2A receptors, a property thought to broaden their therapeutic profile. Over time, it became clear that neither FGAs nor SGAs conferred an advantage in treating negative symptoms, and that the observed improvements were modest.2-5 However, recent research suggests that several newer antipsychotics might be effective in targeting negative symptoms.2,6,7
History of negative symptoms
In the early 20th century, Swiss psychiatrist Eugen Bleuler coined the term schizophrenia to emphasize the cognitive impairment that occurs in patients with this illness, and which he conceptualized as a fragmenting of the psychic process.8 He believed that certain symptoms were fundamental to the illness, and described affective blunting, disturbance of association (ie, distorted thinking) autism (ie, impaired relationships), and ambivalence (ie, fragmented emotional responses). He viewed hallucinations and delusions as accessory symptoms because they were not unique to schizophrenia but were also found in other disorders (eg, mood disorders). Bleuler’s ideas took root, and generations of psychiatrists were taught his fundamental symptoms (“the 4 A’s”), the forerunner of today’s negative symptoms. Later, other experts chose to emphasize psychotic symptoms as most characteristic of schizophrenia, including Schneider’s “first-rank symptoms,” such as voices conversing or delusions of passivity.9
Negative symptoms were rediscovered in the 1970s and 1980s by psychiatric researchers interested in descriptive phenomenology.10,11 Research confirmed the presence of a positive dimension in schizophrenia characterized by the loss of boundaries between the patient and the real world (eg, hallucinations, delusions), and a negative dimension characterized by the loss of a function that should be present, such as alogia and asociality. These experts carefully described negative symptoms and created scales to measure them, including the Scale for the Assessment of Negative Symptoms (SANS),12 the Positive and Negative Syndrome Scale (PANSS),13 the Brief Negative Symptom Scale (BNSS),14 and the 16-item Negative Symptom Assessment (NSA-16).15 Contemporaneous to this work, a “deficit syndrome” was identified among patients with schizophrenia with prominent negative symptoms. The deficit syndrome is found in 25% to 30% of chronic cases.16 Negative symptoms are very common in patients with schizophrenia (Table 19).8,17
Early editions of the DSM defined schizophrenia mainly on the basis of disturbance of cognition, mood, and behavior, and a retreat from reality. With the publication of DSM-III in 1980, and in subsequent editions, schizophrenia was redefined as a relatively severe psychotic illness in which positive and negative symptoms were present, thereby acknowledging the importance of Bleuler’s fundamental symptoms. In DSM-5, negative symptoms are described as accounting for “a substantial portion of the morbidity associated with schizophrenia but are less prominent in other psychotic disorders.”18
Continue to: Types of negative symptoms
Types of negative symptoms
The following symptoms fall within the negative dimension19:
Alogia refers to the impoverished thinking and cognition that often occur in patients with schizophrenia. The patient’s thinking processes seem empty, turgid, or slow, as inferred from the patient’s speech. The 2 major manifestations of alogia are poverty of speech (nonfluent empty speech) and poverty of content of speech (fluent but empty speech). Examples of each appear in Table 2.19
Affective flattening or blunting manifests as a general impoverishment of emotional expression, reactivity, and feeling. Affective flattening can be assessed through observing a patient’s behavior and responsiveness during the interview.
Avolition-apathy manifests itself as a lack of energy and drive. Patients become inert and are unable to mobilize themselves to initiate or persist in completing many kinds of tasks.
Anhedonia-asociality encompasses the patient’s difficulties in experiencing interest or pleasure. It may express itself as a loss of interest in pleasurable activities, an inability to experience pleasure when participating in activities normally considered pleasurable, or a lack of involvement in social relationships.
Continue to: Attention
Attention is often poor in patients with severe mental illnesses. The patient may have trouble focusing his/her attention or may be able to focus only sporadically and erratically. He/she may ignore attempts to converse with him/her, wander away during an activity or a task, or appear to be inattentive when engaged in formal testing or interviewing.
Clinical relevance of negative symptoms
According to DSM-5, “Negative symptoms are more closely related to prognosis than are positive symptoms and tend to be the most persistent.”18 Research has shown that, compared with positive symptoms, negative symptoms are associated with greater impairment in overall functioning, social interaction, interpersonal relationships, economic functioning, and recreational activities.1,3,5 Negative symptoms also are associated with poorer response to medication and a positive family history of schizophrenia. Research shows that negative symptoms are persistent over time, and, in fact, become more prominent as the patient ages, whereas positive symptoms become less prominent.20
Secondary negative symptoms
Potential secondary causes of negative symptoms should be ruled out before concluding that the negative symptoms are due to schizophrenia.3 What might appear to be a negative symptom of schizophrenia, such as poor motivation or flattened affect, could be due to the presence of major depressive disorder. Such symptoms might resolve with treatment. Alternatively, a patient could have developed pseudoparkinsonism from antipsychotic medication and display unchanging facial expression and decreased spontaneous movements. These symptoms could resolve by adding
The neuroanatomy of negative symptoms
Although the neuroanatomical basis of negative symptoms has not been determined, neuroimaging studies have provided important clues.3 Structural brain imaging has consistently shown that negative symptoms in patients with schizophrenia correlate with decreased prefrontal white matter volume, anterior cingulate volume, insular cortex volume, left temporal cortex volume, and ventricular enlargement. Interestingly, volume loss starts before the appearance of negative symptoms.21,22 Functional imaging has shown that negative symptoms correlate with reduced cerebral blood perfusion in frontal, prefrontal, posterior cingulate, thalamus, parietal, and striatal regions.21,22 These findings may help explain the apathy, failure to initiate activities, and impaired social relatedness in patients with schizophrenia.
Neurotransmission and negative symptoms
Some experts have hypothesized that lowered cortical dopamine transmission in mesocortical pathways could give rise to negative symptoms, whereas excess transmission in subcortical structures leads to positive symptoms.23 There is also evidence for a noradrenalin deficiency based on the finding that low levels of cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol (MHPG), a noradrenaline metabolite, correlates with greater negative symptom severity.24 The presence of a serotonin deficiency has been proposed based on evidence that negative symptoms might be mitigated by serotonergic agents.25 More recently, some experts have posited that the dopamine D3 receptor might be involved in the etiology of negative symptoms. The dopamine D3 receptor activity is expressed in brain regions thought to control reward, emotions, and motivation.2 Newer medications with novel mechanisms suggest that other neurotransmitter pathways could be involved.6,7
Continue to: Treatment options
Treatment options
Treating negative symptoms remains challenging and there are no clear answers. When they were introduced in the 1990s, SGAs were initially thought to be superior to FGAs in targeting negative symptoms. Subsequent research, including recent reviews and meta-analyses, has shown that SGAs are not superior to FGAs in treating negative symptoms, and the effect of either medication class on negative symptoms is modest.2-5 One exception is amisulpride (not available in the United States), which is known to antagonize D2 and D3 receptors. A meta-analysis of the efficacy of antipsychotics in schizophrenia showed that amisulpride was significantly more effective than placebo in treating negative symptoms in 590 patients who received the medication.26 The authors suggested that amisulpride was effective due to its binding to presynaptic receptors in the frontal cortex, thereby enhancing dopamine transmission in this region.
Cariprazine, which acts as a partial agonist at the D2 and D3 receptors, with a 10-fold affinity for the D3 receptor, also has shown promise in treating negative symptoms.2 In a clinical trial of 460 patients with predominant negative symptoms, treatment with cariprazine led to a greater reduction in negative symptoms than
Other promising agentsinclude
Antidepressants also could be effective in reducing negative symptoms.3 A meta-analysis of randomized controlled trials evaluating the use of antidepressants as adjuncts to antipsychotic medications showed that adding an antidepressant was effective in reducing negative symptoms.29 The mechanism by which an antidepressant might cause a reduction in negative symptoms is uncertain, and it is possible that the antidepressant might treat depressive symptoms that are causing or contributing to the negative symptoms.
Bottom Line
Negative symptoms in patients with schizophrenia are associated with a worse functional outcome and poorer response to antipsychotic medication than positive symptoms. First- and second-generation antipsychotics are largely ineffective in consistently treating negative symptoms. Antipsychotic medications that target the D3 receptor might be more effective. Roluperidone, which targets serotonin 2A and sigma receptors, and SEP-363856, which targets TAAR1 and serotonin 1A receptors, are being studied for their effects on negative symptoms.
Continue to: Related Resources
Related Resources
- Galderisi S, Färden A, Kaiser S. Dissecting negative symptoms of schizophrenia: History, assessment, pathophysiological mechanisms and treatment. Schizophr Res. 2017;186:1-2.
- Rabinowitz J. Treating negative symptoms of schizophrenia. Current Psychiatry. 2018;17(12):19-23.
Drug Brand Names
Benztropine • Cogentin
Cariprazine • Vraylar
Chlorpromazine • Promapar, Thorazine
Risperidone • Risperdal
The negative symptoms of schizophrenia have been recognized for 100 years. Characterized by a loss of a function that should be present, negative symptoms include anhedonia, asociality, amotivation, and affective blunting. Individuals with schizophrenia who have a preponderance of negative symptoms (“deficit syndrome”) may comprise a special subset of patients. Compared with positive symptoms, negative symptoms are associated with worse global functioning and worse response to antipsychotic medication. Treatment of negative symptoms is challenging. Secondary negative symptoms—those that simulate or resemble primary negative symptoms but are attributable to another cause, such as major depressive disorder or the adverse effects of antipsychotic medication—need to be ruled out. Emerging evidence suggests that newer antipsychotics with novel mechanisms might be effective in treating negative symptoms. Antidepressants might also play a role.
This article describes types of negative symptoms, their clinical relevance, neuroanatomical and neurotransmission factors associated with negative symptoms, and current and future treatment options.
Modest improvements with antipsychotics
Schizophrenia affects an estimated 1% of the population.1 Antipsychotic medication has been the mainstay of schizophrenia treatment since
All antipsychotics are believed to exert their therapeutic effects by blocking dopamine (D2) receptors and are effective in ameliorating the positive symptoms of schizophrenia, including hallucinations, delusions, bizarre behavior, disordered thinking, and agitation.1 Early research had suggested that SGAs might also reduce the negative symptoms of schizophrenia, perhaps because they also block serotonin 2A receptors, a property thought to broaden their therapeutic profile. Over time, it became clear that neither FGAs nor SGAs conferred an advantage in treating negative symptoms, and that the observed improvements were modest.2-5 However, recent research suggests that several newer antipsychotics might be effective in targeting negative symptoms.2,6,7
History of negative symptoms
In the early 20th century, Swiss psychiatrist Eugen Bleuler coined the term schizophrenia to emphasize the cognitive impairment that occurs in patients with this illness, and which he conceptualized as a fragmenting of the psychic process.8 He believed that certain symptoms were fundamental to the illness, and described affective blunting, disturbance of association (ie, distorted thinking) autism (ie, impaired relationships), and ambivalence (ie, fragmented emotional responses). He viewed hallucinations and delusions as accessory symptoms because they were not unique to schizophrenia but were also found in other disorders (eg, mood disorders). Bleuler’s ideas took root, and generations of psychiatrists were taught his fundamental symptoms (“the 4 A’s”), the forerunner of today’s negative symptoms. Later, other experts chose to emphasize psychotic symptoms as most characteristic of schizophrenia, including Schneider’s “first-rank symptoms,” such as voices conversing or delusions of passivity.9
Negative symptoms were rediscovered in the 1970s and 1980s by psychiatric researchers interested in descriptive phenomenology.10,11 Research confirmed the presence of a positive dimension in schizophrenia characterized by the loss of boundaries between the patient and the real world (eg, hallucinations, delusions), and a negative dimension characterized by the loss of a function that should be present, such as alogia and asociality. These experts carefully described negative symptoms and created scales to measure them, including the Scale for the Assessment of Negative Symptoms (SANS),12 the Positive and Negative Syndrome Scale (PANSS),13 the Brief Negative Symptom Scale (BNSS),14 and the 16-item Negative Symptom Assessment (NSA-16).15 Contemporaneous to this work, a “deficit syndrome” was identified among patients with schizophrenia with prominent negative symptoms. The deficit syndrome is found in 25% to 30% of chronic cases.16 Negative symptoms are very common in patients with schizophrenia (Table 19).8,17
Early editions of the DSM defined schizophrenia mainly on the basis of disturbance of cognition, mood, and behavior, and a retreat from reality. With the publication of DSM-III in 1980, and in subsequent editions, schizophrenia was redefined as a relatively severe psychotic illness in which positive and negative symptoms were present, thereby acknowledging the importance of Bleuler’s fundamental symptoms. In DSM-5, negative symptoms are described as accounting for “a substantial portion of the morbidity associated with schizophrenia but are less prominent in other psychotic disorders.”18
Continue to: Types of negative symptoms
Types of negative symptoms
The following symptoms fall within the negative dimension19:
Alogia refers to the impoverished thinking and cognition that often occur in patients with schizophrenia. The patient’s thinking processes seem empty, turgid, or slow, as inferred from the patient’s speech. The 2 major manifestations of alogia are poverty of speech (nonfluent empty speech) and poverty of content of speech (fluent but empty speech). Examples of each appear in Table 2.19
Affective flattening or blunting manifests as a general impoverishment of emotional expression, reactivity, and feeling. Affective flattening can be assessed through observing a patient’s behavior and responsiveness during the interview.
Avolition-apathy manifests itself as a lack of energy and drive. Patients become inert and are unable to mobilize themselves to initiate or persist in completing many kinds of tasks.
Anhedonia-asociality encompasses the patient’s difficulties in experiencing interest or pleasure. It may express itself as a loss of interest in pleasurable activities, an inability to experience pleasure when participating in activities normally considered pleasurable, or a lack of involvement in social relationships.
Continue to: Attention
Attention is often poor in patients with severe mental illnesses. The patient may have trouble focusing his/her attention or may be able to focus only sporadically and erratically. He/she may ignore attempts to converse with him/her, wander away during an activity or a task, or appear to be inattentive when engaged in formal testing or interviewing.
Clinical relevance of negative symptoms
According to DSM-5, “Negative symptoms are more closely related to prognosis than are positive symptoms and tend to be the most persistent.”18 Research has shown that, compared with positive symptoms, negative symptoms are associated with greater impairment in overall functioning, social interaction, interpersonal relationships, economic functioning, and recreational activities.1,3,5 Negative symptoms also are associated with poorer response to medication and a positive family history of schizophrenia. Research shows that negative symptoms are persistent over time, and, in fact, become more prominent as the patient ages, whereas positive symptoms become less prominent.20
Secondary negative symptoms
Potential secondary causes of negative symptoms should be ruled out before concluding that the negative symptoms are due to schizophrenia.3 What might appear to be a negative symptom of schizophrenia, such as poor motivation or flattened affect, could be due to the presence of major depressive disorder. Such symptoms might resolve with treatment. Alternatively, a patient could have developed pseudoparkinsonism from antipsychotic medication and display unchanging facial expression and decreased spontaneous movements. These symptoms could resolve by adding
The neuroanatomy of negative symptoms
Although the neuroanatomical basis of negative symptoms has not been determined, neuroimaging studies have provided important clues.3 Structural brain imaging has consistently shown that negative symptoms in patients with schizophrenia correlate with decreased prefrontal white matter volume, anterior cingulate volume, insular cortex volume, left temporal cortex volume, and ventricular enlargement. Interestingly, volume loss starts before the appearance of negative symptoms.21,22 Functional imaging has shown that negative symptoms correlate with reduced cerebral blood perfusion in frontal, prefrontal, posterior cingulate, thalamus, parietal, and striatal regions.21,22 These findings may help explain the apathy, failure to initiate activities, and impaired social relatedness in patients with schizophrenia.
Neurotransmission and negative symptoms
Some experts have hypothesized that lowered cortical dopamine transmission in mesocortical pathways could give rise to negative symptoms, whereas excess transmission in subcortical structures leads to positive symptoms.23 There is also evidence for a noradrenalin deficiency based on the finding that low levels of cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol (MHPG), a noradrenaline metabolite, correlates with greater negative symptom severity.24 The presence of a serotonin deficiency has been proposed based on evidence that negative symptoms might be mitigated by serotonergic agents.25 More recently, some experts have posited that the dopamine D3 receptor might be involved in the etiology of negative symptoms. The dopamine D3 receptor activity is expressed in brain regions thought to control reward, emotions, and motivation.2 Newer medications with novel mechanisms suggest that other neurotransmitter pathways could be involved.6,7
Continue to: Treatment options
Treatment options
Treating negative symptoms remains challenging and there are no clear answers. When they were introduced in the 1990s, SGAs were initially thought to be superior to FGAs in targeting negative symptoms. Subsequent research, including recent reviews and meta-analyses, has shown that SGAs are not superior to FGAs in treating negative symptoms, and the effect of either medication class on negative symptoms is modest.2-5 One exception is amisulpride (not available in the United States), which is known to antagonize D2 and D3 receptors. A meta-analysis of the efficacy of antipsychotics in schizophrenia showed that amisulpride was significantly more effective than placebo in treating negative symptoms in 590 patients who received the medication.26 The authors suggested that amisulpride was effective due to its binding to presynaptic receptors in the frontal cortex, thereby enhancing dopamine transmission in this region.
Cariprazine, which acts as a partial agonist at the D2 and D3 receptors, with a 10-fold affinity for the D3 receptor, also has shown promise in treating negative symptoms.2 In a clinical trial of 460 patients with predominant negative symptoms, treatment with cariprazine led to a greater reduction in negative symptoms than
Other promising agentsinclude
Antidepressants also could be effective in reducing negative symptoms.3 A meta-analysis of randomized controlled trials evaluating the use of antidepressants as adjuncts to antipsychotic medications showed that adding an antidepressant was effective in reducing negative symptoms.29 The mechanism by which an antidepressant might cause a reduction in negative symptoms is uncertain, and it is possible that the antidepressant might treat depressive symptoms that are causing or contributing to the negative symptoms.
Bottom Line
Negative symptoms in patients with schizophrenia are associated with a worse functional outcome and poorer response to antipsychotic medication than positive symptoms. First- and second-generation antipsychotics are largely ineffective in consistently treating negative symptoms. Antipsychotic medications that target the D3 receptor might be more effective. Roluperidone, which targets serotonin 2A and sigma receptors, and SEP-363856, which targets TAAR1 and serotonin 1A receptors, are being studied for their effects on negative symptoms.
Continue to: Related Resources
Related Resources
- Galderisi S, Färden A, Kaiser S. Dissecting negative symptoms of schizophrenia: History, assessment, pathophysiological mechanisms and treatment. Schizophr Res. 2017;186:1-2.
- Rabinowitz J. Treating negative symptoms of schizophrenia. Current Psychiatry. 2018;17(12):19-23.
Drug Brand Names
Benztropine • Cogentin
Cariprazine • Vraylar
Chlorpromazine • Promapar, Thorazine
Risperidone • Risperdal
1. Owen MJ, Sawa A, Mortensen PD. Schizophrenia. Lancet. 2016;388(10039):86-97.
2. Cerviri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat. 2019;15:1525-1535.
3. Mitra S, Mahintamani T, Kavoor AR, et al. Negative symptoms in schizophrenia. Ind Psychiatr J. 2016;25(2):135-144.
4. Fusa-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892-899.
5. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms: an update. Curr Treat Options Psych. 2016;3:133-150.
6. Harvey PD, Saoud JB, Luthringer R, et al. Effects of roluperidone (MIN-101) on two dimensions of negative symptoms factor score: reduced emotional experience and reduced emotional expression. Schizophr Res. 2020;215:352-356.
7. Dedic N, Jones PG, Hopkins SC, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Psychopharmacol Exp Ther. 2019;371(1):1-14.
8. Bleuler E. Dementia praecox or the group of schizophrenia. New York, New York: International Universities Press; 1950.
9. Andreasen NC. The diagnosis of schizophrenia. Schizophr Bull. 1987;13(1):9-22.
10. Andreasen NC. Thought, language, and communication disorders I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36(12):1315-1321.
11. Crow TJ. Molecular pathology of schizophrenia: more than one disease process? Br Med J. 1980;280(6207):66-68.
12. Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39(7):789-794.
13. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
14. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305.
15. Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptoms Assessment. J Psychiatr Res. 1993;27(3):253-258.
16. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578-583.
17. Bobes J, Arango C, Garcia-Garcia M, et al. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS Study. J Clin Psychiatry. 2010;71(3):280-286.
18. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
19. Black DW, Andreasen NC. Interviewing and assessment. In: Introductory textbook of psychiatry, 7th ed. Black DW, Andreasen NC, eds. Washington, DC: American Psychiatric Publishing; 2020:15-53.
20. Pfohl B, Winokur G. The micropsychopathology of hebephrenic/catatonic schizophrenia. J Nerv Ment Dis. 1983;171(5):296-300.
21. Hovington CL, Lepage M. Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother. 2012;12(1):53-69.
22. Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, et al. A review of schizophrenia, melancholic depression and Parkinson’s disease. Brain Res Bull. 2006;70(4-6):312-321.
23. Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329-336.
24. Yoshimura R, Hori H, Katsuki A, et al. Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF, and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Ann Gen Psychiatry. 2016;15:1.
25. Moller HJ. Management of negative symptoms of schizophrenia: new treatment options. CNS Drugs. 2003;17(11):793-823.
26. Leucht S. Amisulpride: a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2004;7(suppl 1):S15-S20. doi: 10.1017/S1461145704004109.
27. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomized, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
28. Neill JC, Grayson, Kiss B, et al. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26(1):3-14.
29. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9):876-886.
1. Owen MJ, Sawa A, Mortensen PD. Schizophrenia. Lancet. 2016;388(10039):86-97.
2. Cerviri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat. 2019;15:1525-1535.
3. Mitra S, Mahintamani T, Kavoor AR, et al. Negative symptoms in schizophrenia. Ind Psychiatr J. 2016;25(2):135-144.
4. Fusa-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892-899.
5. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms: an update. Curr Treat Options Psych. 2016;3:133-150.
6. Harvey PD, Saoud JB, Luthringer R, et al. Effects of roluperidone (MIN-101) on two dimensions of negative symptoms factor score: reduced emotional experience and reduced emotional expression. Schizophr Res. 2020;215:352-356.
7. Dedic N, Jones PG, Hopkins SC, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Psychopharmacol Exp Ther. 2019;371(1):1-14.
8. Bleuler E. Dementia praecox or the group of schizophrenia. New York, New York: International Universities Press; 1950.
9. Andreasen NC. The diagnosis of schizophrenia. Schizophr Bull. 1987;13(1):9-22.
10. Andreasen NC. Thought, language, and communication disorders I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36(12):1315-1321.
11. Crow TJ. Molecular pathology of schizophrenia: more than one disease process? Br Med J. 1980;280(6207):66-68.
12. Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39(7):789-794.
13. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
14. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305.
15. Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptoms Assessment. J Psychiatr Res. 1993;27(3):253-258.
16. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578-583.
17. Bobes J, Arango C, Garcia-Garcia M, et al. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS Study. J Clin Psychiatry. 2010;71(3):280-286.
18. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
19. Black DW, Andreasen NC. Interviewing and assessment. In: Introductory textbook of psychiatry, 7th ed. Black DW, Andreasen NC, eds. Washington, DC: American Psychiatric Publishing; 2020:15-53.
20. Pfohl B, Winokur G. The micropsychopathology of hebephrenic/catatonic schizophrenia. J Nerv Ment Dis. 1983;171(5):296-300.
21. Hovington CL, Lepage M. Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother. 2012;12(1):53-69.
22. Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, et al. A review of schizophrenia, melancholic depression and Parkinson’s disease. Brain Res Bull. 2006;70(4-6):312-321.
23. Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329-336.
24. Yoshimura R, Hori H, Katsuki A, et al. Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF, and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Ann Gen Psychiatry. 2016;15:1.
25. Moller HJ. Management of negative symptoms of schizophrenia: new treatment options. CNS Drugs. 2003;17(11):793-823.
26. Leucht S. Amisulpride: a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2004;7(suppl 1):S15-S20. doi: 10.1017/S1461145704004109.
27. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomized, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
28. Neill JC, Grayson, Kiss B, et al. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26(1):3-14.
29. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9):876-886.
Evaluating patients’ decision-making capacity during COVID-19
The coronavirus disease 2019 (COVID-19) pandemic has introduced many new clinical challenges. Consider the patient with fever and dyspnea who tests positive for COVID-19 but does not believe in COVID-19 and wants to leave the hospital against medical advice (AMA). Or the patient with numerous cardiovascular risk factors and crushing substernal chest pain who is too afraid of contracting COVID-19 to come to the emergency department. These challenging clinical scenarios can be addressed in the context of decision-making capacity (DMC), for which our medical colleagues often call upon psychiatrists to assist. This article reviews the framework for DMC assessment, describes how COVID-19 affects DMC assessment, and discusses approaches to these scenarios using the DMC framework.
Review of decision-making capacity
Assessment of DMC is a fundamental clinical skill. It allows a physician to balance autonomy with beneficence and non-maleficence. An autonomous decision is a decision that is made intentionally, with understanding, and without controlling influences (these are the elements of informed consent).1 However, if a patient cannot make a decision with intention and understanding, then beneficence and non-maleficence must prevail in order to protect the patient. Capacity assessments evaluate a patient’s ability to make an intentional and understood choice.
In order to prove capacity, a patient must demonstrate 4 functional abilities:
- choice refers to the ability to communicate a relatively stable choice2,3
- understanding refers to the ability to convey information about the illness, risks/benefits of the chosen intervention, and risks/benefits of alternative options.2,3 Understanding measures objective information about the medical situation
- appreciation refers to the patient’s ability to apply that information to his/her own life.2,3 Appreciation requires insight into having the illness and the ability to anticipate how one’s life would be impacted by one’s condition and choice. This is where life experiences and values come into play
- reasoning is intimately tied to appreciation. It refers to the ability to explain how the decision was made and which factors were most important.2,3
Most clinicians and ethicists endorse a “threshold” approach to decisional capacity, which specifies that the level of evidence required to prove capacity depends on the gravity of the medical situation (Figure 1A).1,4,5 The gravity of the situation is based on the risk/benefit analysis. Consider two treatments with equal benefit: one has minimal adverse effects (gastrointestinal upset) and the second has significant adverse effects (myelosuppression). Accepting the first treatment requires less intentionality and understanding than accepting the second because the risk is much lower and thus has a lower capacity threshold (Figure 1B). The capacity to refuse these treatments results in the opposite ranking (Figure 1C).
Establishing a threshold helps guide the physician in determining how robust the patient’s responses must be to have decisional capacity. For a high-threshold decision, the patient must have a well-developed and highly detailed level of understanding, appreciation, and reasoning.
How COVID-19 affects assessment of decision-making capacity
Three characteristics of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 illness impact decision-making assessment:
- high level of contagiousness
- high health-care utilization
- the uncertainty about its clinical course and outcomes.
The high level of contagiousness stems from this virus’s estimated basic reproduction number (R0) of 2.2 to 5.7 (which indicates the expected number of cases from any single case), its long incubation period, and the potential for asymptomatic and pre-symptomatic shedding.6-9 Decision-making capacity assessments must therefore consider community-level effects in the risk/benefit analysis. Because SARS-CoV-2 is a new virus affecting humans, it can easily overwhelm existing hospital systems. This happened in Wuhan, China; Lombardy, Italy; and New York. In a stressed system, physicians will have to factor health-care utilization into the risk/benefit analysis. Finally, because this is a novel virus, there is still considerable uncertainty about the epidemiology, clinical course, and outcomes.10 The minimal dose of virus needed to cause illness is unknown. Patients can deteriorate quickly and unpredictably into needing ventilator support.11 Treatment options are limited, and many candidates are being investigated.12 This uncertainty hinders physicians’ ability to accurately estimate risks and benefits for an individual patient when discussing various medical decisions. As our understanding of SARS-CoV-2 improves, this uncertainty will lessen.
Continue to: Effects of the sociopolitical climate
Effects of the sociopolitical climate
In the United States, the COVID-19 pandemic emerged during a time of deep sociopolitical divide. Accordingly, beliefs about viral infectivity, severity of illness, and precautionary measures have varied. Some politicians, media outlets, and physicians have shared information that contradicts guidelines and recommendations from mainstream national and international medical and scientific organizations. Patients who subscribe to these reports and beliefs may not meet the threshold for understanding, appreciation, or reasoning. For example, if a patient’s beliefs about the virus depart from well-established medical evidence, they would technically lack understanding. The usual remedy for addressing misunderstanding is education and time. However, because of the divisiveness of the sociopolitical climate, the limited time physicians have with patients, and the fact that many DMC assessments will occur in acute-care settings, it may be difficult or near impossible to correct the misunderstanding.
The sociopolitical climate and its accompanying potentially erroneous or imbalanced narrative may thus directly impact patients’ understanding, appreciation, and reasoning. However, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. Additionally, some clinicians and ethicists might object to declaring incapacity in a patient with no underlying mental or neurologic dysfunction. The United States has a functional approach to capacity, based solely on meeting criteria for the 4 functional abilities.3,13 Mental or neurologic dysfunction is not legally required in the United States, but in practice, the consideration of incapacity is often closely linked to some form of cognitive impairment.14 Other countries do make dysfunction a specific criterion; for example, the United Kingdom dictates that mental incapacity can only occur in someone with “impairment of, or a disturbance in the functioning of, the mind or brain.”15
Leaving against medical advice
In the case of a patient who is COVID-19–positive, symptomatic, and wants to leave AMA, the threshold is automatically elevated because of societal-level risks (the risk of potential exposure or infection of others if a patient who is COVID-19–positive is not properly isolated). Furthermore, the individual risk of the patient leaving AMA depends on his/her age, comorbidities, and current clinical status; because of the uncertainty and rapid deterioration seen with COVID-19 illness, the calculated risk may actually be higher than for a non-COVID-19–related illness. Thus, in order to leave AMA, the patient’s responses must be fairly robust (Figure 2). Table 1 describes the information needed for robust understanding, appreciation, and reasoning.
For patients who do not meet this threshold, it is important to determine why. If a patient has a psychiatric condition that not only impacts DMC but also meets criteria for a psychiatric hold (ie, an imminent risk of harm to self or others), a psychiatric hold should be placed. If the patient does not meet the threshold because of altered mental status or some other neurologic or cognitive comorbidity, a medical hold should be placed. Most states do not have an explicit legal basis for a medical hold, although it does fall under the incapacity laws in the United States; in the absence of a surrogate, declaration of medical emergency can also be used if applicable.16,17 As a caveat, it can be difficult to detain someone on a medical hold because security officers may be afraid to physically detain someone without explicit legal paperwork.17
If a patient does not meet the capacity threshold but there does not seem to be a psychiatric, neurologic, or cognitive explanation, several options are possible. The first step would be to assess whether the patient is amenable to further discussion and compromise. A nonjudgmental and nonconfrontational approach that aims to further clarify the patient’s perspective and identify shared goals is key. Any plan that lowers the risks sufficiently would allow the patient to leave by lowering the capacity threshold. Enlisting the support of family and friends can be helpful. If this does not work, theoretically the patient should be detained in the hospital. Practically speaking, this may be difficult or unadvised. First, as described above, security officers may refuse to physically detain the patient.17 Second, the patient’s legally mandated surrogate may espouse similar COVID-related views as the patient; thus, this approach may not help keep the patient in the hospital. If the physician has serious concern about the risk of the patient leaving, he/she would have to consult the facility’s Ethics and Legal staff to determine capacity of the surrogate. Third, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. In the current sociopolitical climate, involuntary detention may elicit a political backlash. Using medical detention for impending deterioration of clinical status would be more acceptable than using medical detention for isolation. Presently, there are no such laws for patients with COVID-19 (although this is not without precedent, as with active tuberculosis or Ebola18,19), but individual jurisdictions may have isolation or quarantine orders; the local health department could be contacted and may evaluate on a case-by-case basis.
Continue to: Refusing to seek medical care
Refusing to seek medical care
Anecdotally, many physicians have reported an increase in patients who are refusing clinic- or hospital-based treatment for a medical condition because they fear they may catch the virus. Although this is not strictly a capacity case—there is little recourse for action if a patient is refusing treatment from home (unless the patient requires a psychiatric hold or already has a guardian for medical decisions)—the same elements of thresholds apply and can be helpful in guiding conversations with the patient.
For the patient, the benefits of staying at home are to avoid potentially exposing themselves and the members of their household to the virus and COVID-19 illness. The risks of staying home include progression of the patient’s primary illness, which could lead to increased morbidity and mortality. Staying home has an ancillary benefit to the community of reducing health-care utilization, but at the risk of increasing utilization in the future.
The risk/benefit profile is shown on the thresholds graph in Figure 3. There is considerable variability. It is helpful to stratify the risk of progression of the primary condition as low (can be postponed indefinitely with minimal risk), medium (can be postponed for a short amount of time; risk of increased morbidity with ongoing delay and possibly increased mortality), or high (cannot be postponed; will have greater morbidity and/or higher risk of mortality). Because of the uncertainty about COVID-19, it is harder to quantify the benefits of refusing care and staying at home, although older patients and patients with underlying health issues are at higher risk of severe illness and death.20 However, by taking appropriate precautions when seeking care, viral exposure and risk of infection can be mitigated.
This risk/benefit analysis will help set the threshold for whether staying at home is reasonable or whether it would incur more risk of harm. If the latter, then the physician must elicit the patient’s understanding, appreciation, and reasoning related to their current medical condition and COVID-19. It is likely they are undervaluing the former and overvaluing the latter. Table 2 lists important points to cover during these discussions.
Although there is no legal recourse to force patients at home to come to the clinic or hospital for medical treatment, there are several possible strategies to motivate them to do so. One is to ask patients how likely (on a scale of 0 to 100) they think they are to contract COVID-19 if they came for evaluation/treatment, and how likely they feel they are to experience a bad outcome from their primary condition. Then, after providing psychoeducation about their primary medical condition and COVID-19–related precautions and risk, repeat this question. Another strategy is to empathize with the patient’s fears while also expressing concern about the primary medical condition and connecting with the patient on the shared desire to protect his/her health. A third is to draw a risk/benefit diagram (similar to Figure 3) or reassure the patient by describing the ways in which the clinic or hospital is minimizing exposure and infection risk. A final strategy is to enlist the help of the patient’s family or friends.
Continue to: Bottom Line
Bottom Line
In order to have decision-making capacity, a patient must demonstrate choice, understanding, appreciation, and reasoning. The degree of understanding, appreciation, and reasoning required depends on the capacity threshold, which is determined by a risk/benefit analysis. Conducting a risk/benefit analysis during the coronavirus disease 2019 (COVID-19) pandemic requires consideration of societallevel factors (such as contagiousness to others and health-care utilization) and is complicated by a wide range of uncertainties and divisive sociopolitical views regarding COVID-19.
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
- Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
Acknowledgments
The author thanks Drs. Awais Aftab, Zackary D. Berger, and R. Brett Lloyd for their helpful discussions on the topic.
1. Beauchamp TL, Childress JF. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013.
2. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
3. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
4. Magid M, Dodd ML, Bostwick MJ, et al. Is your patient making the ‘wrong’ treatment choice? Current Psychiatry. 2006;5(3):13-20.
5. Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. Association of Directors of Medical Student Education in Psychiatry. 2020. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
6. Sanche S, Lin YT, Xu C, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470-1477.
7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
8. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469.
9. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180.
10. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382(13):1194-1196.
11. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singapore. 2020;49(3):108-118.
12. Asai A, Konno M, Ozaki M, et al. COVID-19 drug discovery using intensive approaches. Int J Mol Sci. 2020;21(8):2839.
13. Siegel AM, Barnwell AS, Sisti DA. Assessing decision-making capacity: a primer for the development of hospital practice guidelines. HEC Forum. 2014;26(2):159-168.
14. Karlawish J. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated February 24, 2020. Accessed May 27, 2020.
15. Mental Capacity Act 2005. Chapter 9. http://www.legislation.gov.uk/ukpga/2005/9/part/1. Accessed May 27, 2020.
16. Kersten C. The doctor as jailer: medical detention of non-psychiatric patients. J Law Biosci. 2019;6(1):310-316.
17. Cheung EH, Heldt J, Strouse T, et al. The medical incapacity hold: a policy on the involuntary medical hospitalization of patients who lack decisional capacity. Psychosomatics. 2018;59(2):169-176.
18. Parmet WE, Sinha MS. Covid-19 - the law and limits of quarantine. N Engl J Med. 2020;382(15):e28.
19. Coker R, Thomas M, Lock K, et al. Detention and the evolving threat of tuberculosis: evidence, ethics, and law. J Law Med Ethics. 2007;35(4):609-615.
20. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
The coronavirus disease 2019 (COVID-19) pandemic has introduced many new clinical challenges. Consider the patient with fever and dyspnea who tests positive for COVID-19 but does not believe in COVID-19 and wants to leave the hospital against medical advice (AMA). Or the patient with numerous cardiovascular risk factors and crushing substernal chest pain who is too afraid of contracting COVID-19 to come to the emergency department. These challenging clinical scenarios can be addressed in the context of decision-making capacity (DMC), for which our medical colleagues often call upon psychiatrists to assist. This article reviews the framework for DMC assessment, describes how COVID-19 affects DMC assessment, and discusses approaches to these scenarios using the DMC framework.
Review of decision-making capacity
Assessment of DMC is a fundamental clinical skill. It allows a physician to balance autonomy with beneficence and non-maleficence. An autonomous decision is a decision that is made intentionally, with understanding, and without controlling influences (these are the elements of informed consent).1 However, if a patient cannot make a decision with intention and understanding, then beneficence and non-maleficence must prevail in order to protect the patient. Capacity assessments evaluate a patient’s ability to make an intentional and understood choice.
In order to prove capacity, a patient must demonstrate 4 functional abilities:
- choice refers to the ability to communicate a relatively stable choice2,3
- understanding refers to the ability to convey information about the illness, risks/benefits of the chosen intervention, and risks/benefits of alternative options.2,3 Understanding measures objective information about the medical situation
- appreciation refers to the patient’s ability to apply that information to his/her own life.2,3 Appreciation requires insight into having the illness and the ability to anticipate how one’s life would be impacted by one’s condition and choice. This is where life experiences and values come into play
- reasoning is intimately tied to appreciation. It refers to the ability to explain how the decision was made and which factors were most important.2,3
Most clinicians and ethicists endorse a “threshold” approach to decisional capacity, which specifies that the level of evidence required to prove capacity depends on the gravity of the medical situation (Figure 1A).1,4,5 The gravity of the situation is based on the risk/benefit analysis. Consider two treatments with equal benefit: one has minimal adverse effects (gastrointestinal upset) and the second has significant adverse effects (myelosuppression). Accepting the first treatment requires less intentionality and understanding than accepting the second because the risk is much lower and thus has a lower capacity threshold (Figure 1B). The capacity to refuse these treatments results in the opposite ranking (Figure 1C).
Establishing a threshold helps guide the physician in determining how robust the patient’s responses must be to have decisional capacity. For a high-threshold decision, the patient must have a well-developed and highly detailed level of understanding, appreciation, and reasoning.
How COVID-19 affects assessment of decision-making capacity
Three characteristics of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 illness impact decision-making assessment:
- high level of contagiousness
- high health-care utilization
- the uncertainty about its clinical course and outcomes.
The high level of contagiousness stems from this virus’s estimated basic reproduction number (R0) of 2.2 to 5.7 (which indicates the expected number of cases from any single case), its long incubation period, and the potential for asymptomatic and pre-symptomatic shedding.6-9 Decision-making capacity assessments must therefore consider community-level effects in the risk/benefit analysis. Because SARS-CoV-2 is a new virus affecting humans, it can easily overwhelm existing hospital systems. This happened in Wuhan, China; Lombardy, Italy; and New York. In a stressed system, physicians will have to factor health-care utilization into the risk/benefit analysis. Finally, because this is a novel virus, there is still considerable uncertainty about the epidemiology, clinical course, and outcomes.10 The minimal dose of virus needed to cause illness is unknown. Patients can deteriorate quickly and unpredictably into needing ventilator support.11 Treatment options are limited, and many candidates are being investigated.12 This uncertainty hinders physicians’ ability to accurately estimate risks and benefits for an individual patient when discussing various medical decisions. As our understanding of SARS-CoV-2 improves, this uncertainty will lessen.
Continue to: Effects of the sociopolitical climate
Effects of the sociopolitical climate
In the United States, the COVID-19 pandemic emerged during a time of deep sociopolitical divide. Accordingly, beliefs about viral infectivity, severity of illness, and precautionary measures have varied. Some politicians, media outlets, and physicians have shared information that contradicts guidelines and recommendations from mainstream national and international medical and scientific organizations. Patients who subscribe to these reports and beliefs may not meet the threshold for understanding, appreciation, or reasoning. For example, if a patient’s beliefs about the virus depart from well-established medical evidence, they would technically lack understanding. The usual remedy for addressing misunderstanding is education and time. However, because of the divisiveness of the sociopolitical climate, the limited time physicians have with patients, and the fact that many DMC assessments will occur in acute-care settings, it may be difficult or near impossible to correct the misunderstanding.
The sociopolitical climate and its accompanying potentially erroneous or imbalanced narrative may thus directly impact patients’ understanding, appreciation, and reasoning. However, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. Additionally, some clinicians and ethicists might object to declaring incapacity in a patient with no underlying mental or neurologic dysfunction. The United States has a functional approach to capacity, based solely on meeting criteria for the 4 functional abilities.3,13 Mental or neurologic dysfunction is not legally required in the United States, but in practice, the consideration of incapacity is often closely linked to some form of cognitive impairment.14 Other countries do make dysfunction a specific criterion; for example, the United Kingdom dictates that mental incapacity can only occur in someone with “impairment of, or a disturbance in the functioning of, the mind or brain.”15
Leaving against medical advice
In the case of a patient who is COVID-19–positive, symptomatic, and wants to leave AMA, the threshold is automatically elevated because of societal-level risks (the risk of potential exposure or infection of others if a patient who is COVID-19–positive is not properly isolated). Furthermore, the individual risk of the patient leaving AMA depends on his/her age, comorbidities, and current clinical status; because of the uncertainty and rapid deterioration seen with COVID-19 illness, the calculated risk may actually be higher than for a non-COVID-19–related illness. Thus, in order to leave AMA, the patient’s responses must be fairly robust (Figure 2). Table 1 describes the information needed for robust understanding, appreciation, and reasoning.
For patients who do not meet this threshold, it is important to determine why. If a patient has a psychiatric condition that not only impacts DMC but also meets criteria for a psychiatric hold (ie, an imminent risk of harm to self or others), a psychiatric hold should be placed. If the patient does not meet the threshold because of altered mental status or some other neurologic or cognitive comorbidity, a medical hold should be placed. Most states do not have an explicit legal basis for a medical hold, although it does fall under the incapacity laws in the United States; in the absence of a surrogate, declaration of medical emergency can also be used if applicable.16,17 As a caveat, it can be difficult to detain someone on a medical hold because security officers may be afraid to physically detain someone without explicit legal paperwork.17
If a patient does not meet the capacity threshold but there does not seem to be a psychiatric, neurologic, or cognitive explanation, several options are possible. The first step would be to assess whether the patient is amenable to further discussion and compromise. A nonjudgmental and nonconfrontational approach that aims to further clarify the patient’s perspective and identify shared goals is key. Any plan that lowers the risks sufficiently would allow the patient to leave by lowering the capacity threshold. Enlisting the support of family and friends can be helpful. If this does not work, theoretically the patient should be detained in the hospital. Practically speaking, this may be difficult or unadvised. First, as described above, security officers may refuse to physically detain the patient.17 Second, the patient’s legally mandated surrogate may espouse similar COVID-related views as the patient; thus, this approach may not help keep the patient in the hospital. If the physician has serious concern about the risk of the patient leaving, he/she would have to consult the facility’s Ethics and Legal staff to determine capacity of the surrogate. Third, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. In the current sociopolitical climate, involuntary detention may elicit a political backlash. Using medical detention for impending deterioration of clinical status would be more acceptable than using medical detention for isolation. Presently, there are no such laws for patients with COVID-19 (although this is not without precedent, as with active tuberculosis or Ebola18,19), but individual jurisdictions may have isolation or quarantine orders; the local health department could be contacted and may evaluate on a case-by-case basis.
Continue to: Refusing to seek medical care
Refusing to seek medical care
Anecdotally, many physicians have reported an increase in patients who are refusing clinic- or hospital-based treatment for a medical condition because they fear they may catch the virus. Although this is not strictly a capacity case—there is little recourse for action if a patient is refusing treatment from home (unless the patient requires a psychiatric hold or already has a guardian for medical decisions)—the same elements of thresholds apply and can be helpful in guiding conversations with the patient.
For the patient, the benefits of staying at home are to avoid potentially exposing themselves and the members of their household to the virus and COVID-19 illness. The risks of staying home include progression of the patient’s primary illness, which could lead to increased morbidity and mortality. Staying home has an ancillary benefit to the community of reducing health-care utilization, but at the risk of increasing utilization in the future.
The risk/benefit profile is shown on the thresholds graph in Figure 3. There is considerable variability. It is helpful to stratify the risk of progression of the primary condition as low (can be postponed indefinitely with minimal risk), medium (can be postponed for a short amount of time; risk of increased morbidity with ongoing delay and possibly increased mortality), or high (cannot be postponed; will have greater morbidity and/or higher risk of mortality). Because of the uncertainty about COVID-19, it is harder to quantify the benefits of refusing care and staying at home, although older patients and patients with underlying health issues are at higher risk of severe illness and death.20 However, by taking appropriate precautions when seeking care, viral exposure and risk of infection can be mitigated.
This risk/benefit analysis will help set the threshold for whether staying at home is reasonable or whether it would incur more risk of harm. If the latter, then the physician must elicit the patient’s understanding, appreciation, and reasoning related to their current medical condition and COVID-19. It is likely they are undervaluing the former and overvaluing the latter. Table 2 lists important points to cover during these discussions.
Although there is no legal recourse to force patients at home to come to the clinic or hospital for medical treatment, there are several possible strategies to motivate them to do so. One is to ask patients how likely (on a scale of 0 to 100) they think they are to contract COVID-19 if they came for evaluation/treatment, and how likely they feel they are to experience a bad outcome from their primary condition. Then, after providing psychoeducation about their primary medical condition and COVID-19–related precautions and risk, repeat this question. Another strategy is to empathize with the patient’s fears while also expressing concern about the primary medical condition and connecting with the patient on the shared desire to protect his/her health. A third is to draw a risk/benefit diagram (similar to Figure 3) or reassure the patient by describing the ways in which the clinic or hospital is minimizing exposure and infection risk. A final strategy is to enlist the help of the patient’s family or friends.
Continue to: Bottom Line
Bottom Line
In order to have decision-making capacity, a patient must demonstrate choice, understanding, appreciation, and reasoning. The degree of understanding, appreciation, and reasoning required depends on the capacity threshold, which is determined by a risk/benefit analysis. Conducting a risk/benefit analysis during the coronavirus disease 2019 (COVID-19) pandemic requires consideration of societallevel factors (such as contagiousness to others and health-care utilization) and is complicated by a wide range of uncertainties and divisive sociopolitical views regarding COVID-19.
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
- Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
Acknowledgments
The author thanks Drs. Awais Aftab, Zackary D. Berger, and R. Brett Lloyd for their helpful discussions on the topic.
The coronavirus disease 2019 (COVID-19) pandemic has introduced many new clinical challenges. Consider the patient with fever and dyspnea who tests positive for COVID-19 but does not believe in COVID-19 and wants to leave the hospital against medical advice (AMA). Or the patient with numerous cardiovascular risk factors and crushing substernal chest pain who is too afraid of contracting COVID-19 to come to the emergency department. These challenging clinical scenarios can be addressed in the context of decision-making capacity (DMC), for which our medical colleagues often call upon psychiatrists to assist. This article reviews the framework for DMC assessment, describes how COVID-19 affects DMC assessment, and discusses approaches to these scenarios using the DMC framework.
Review of decision-making capacity
Assessment of DMC is a fundamental clinical skill. It allows a physician to balance autonomy with beneficence and non-maleficence. An autonomous decision is a decision that is made intentionally, with understanding, and without controlling influences (these are the elements of informed consent).1 However, if a patient cannot make a decision with intention and understanding, then beneficence and non-maleficence must prevail in order to protect the patient. Capacity assessments evaluate a patient’s ability to make an intentional and understood choice.
In order to prove capacity, a patient must demonstrate 4 functional abilities:
- choice refers to the ability to communicate a relatively stable choice2,3
- understanding refers to the ability to convey information about the illness, risks/benefits of the chosen intervention, and risks/benefits of alternative options.2,3 Understanding measures objective information about the medical situation
- appreciation refers to the patient’s ability to apply that information to his/her own life.2,3 Appreciation requires insight into having the illness and the ability to anticipate how one’s life would be impacted by one’s condition and choice. This is where life experiences and values come into play
- reasoning is intimately tied to appreciation. It refers to the ability to explain how the decision was made and which factors were most important.2,3
Most clinicians and ethicists endorse a “threshold” approach to decisional capacity, which specifies that the level of evidence required to prove capacity depends on the gravity of the medical situation (Figure 1A).1,4,5 The gravity of the situation is based on the risk/benefit analysis. Consider two treatments with equal benefit: one has minimal adverse effects (gastrointestinal upset) and the second has significant adverse effects (myelosuppression). Accepting the first treatment requires less intentionality and understanding than accepting the second because the risk is much lower and thus has a lower capacity threshold (Figure 1B). The capacity to refuse these treatments results in the opposite ranking (Figure 1C).
Establishing a threshold helps guide the physician in determining how robust the patient’s responses must be to have decisional capacity. For a high-threshold decision, the patient must have a well-developed and highly detailed level of understanding, appreciation, and reasoning.
How COVID-19 affects assessment of decision-making capacity
Three characteristics of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 illness impact decision-making assessment:
- high level of contagiousness
- high health-care utilization
- the uncertainty about its clinical course and outcomes.
The high level of contagiousness stems from this virus’s estimated basic reproduction number (R0) of 2.2 to 5.7 (which indicates the expected number of cases from any single case), its long incubation period, and the potential for asymptomatic and pre-symptomatic shedding.6-9 Decision-making capacity assessments must therefore consider community-level effects in the risk/benefit analysis. Because SARS-CoV-2 is a new virus affecting humans, it can easily overwhelm existing hospital systems. This happened in Wuhan, China; Lombardy, Italy; and New York. In a stressed system, physicians will have to factor health-care utilization into the risk/benefit analysis. Finally, because this is a novel virus, there is still considerable uncertainty about the epidemiology, clinical course, and outcomes.10 The minimal dose of virus needed to cause illness is unknown. Patients can deteriorate quickly and unpredictably into needing ventilator support.11 Treatment options are limited, and many candidates are being investigated.12 This uncertainty hinders physicians’ ability to accurately estimate risks and benefits for an individual patient when discussing various medical decisions. As our understanding of SARS-CoV-2 improves, this uncertainty will lessen.
Continue to: Effects of the sociopolitical climate
Effects of the sociopolitical climate
In the United States, the COVID-19 pandemic emerged during a time of deep sociopolitical divide. Accordingly, beliefs about viral infectivity, severity of illness, and precautionary measures have varied. Some politicians, media outlets, and physicians have shared information that contradicts guidelines and recommendations from mainstream national and international medical and scientific organizations. Patients who subscribe to these reports and beliefs may not meet the threshold for understanding, appreciation, or reasoning. For example, if a patient’s beliefs about the virus depart from well-established medical evidence, they would technically lack understanding. The usual remedy for addressing misunderstanding is education and time. However, because of the divisiveness of the sociopolitical climate, the limited time physicians have with patients, and the fact that many DMC assessments will occur in acute-care settings, it may be difficult or near impossible to correct the misunderstanding.
The sociopolitical climate and its accompanying potentially erroneous or imbalanced narrative may thus directly impact patients’ understanding, appreciation, and reasoning. However, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. Additionally, some clinicians and ethicists might object to declaring incapacity in a patient with no underlying mental or neurologic dysfunction. The United States has a functional approach to capacity, based solely on meeting criteria for the 4 functional abilities.3,13 Mental or neurologic dysfunction is not legally required in the United States, but in practice, the consideration of incapacity is often closely linked to some form of cognitive impairment.14 Other countries do make dysfunction a specific criterion; for example, the United Kingdom dictates that mental incapacity can only occur in someone with “impairment of, or a disturbance in the functioning of, the mind or brain.”15
Leaving against medical advice
In the case of a patient who is COVID-19–positive, symptomatic, and wants to leave AMA, the threshold is automatically elevated because of societal-level risks (the risk of potential exposure or infection of others if a patient who is COVID-19–positive is not properly isolated). Furthermore, the individual risk of the patient leaving AMA depends on his/her age, comorbidities, and current clinical status; because of the uncertainty and rapid deterioration seen with COVID-19 illness, the calculated risk may actually be higher than for a non-COVID-19–related illness. Thus, in order to leave AMA, the patient’s responses must be fairly robust (Figure 2). Table 1 describes the information needed for robust understanding, appreciation, and reasoning.
For patients who do not meet this threshold, it is important to determine why. If a patient has a psychiatric condition that not only impacts DMC but also meets criteria for a psychiatric hold (ie, an imminent risk of harm to self or others), a psychiatric hold should be placed. If the patient does not meet the threshold because of altered mental status or some other neurologic or cognitive comorbidity, a medical hold should be placed. Most states do not have an explicit legal basis for a medical hold, although it does fall under the incapacity laws in the United States; in the absence of a surrogate, declaration of medical emergency can also be used if applicable.16,17 As a caveat, it can be difficult to detain someone on a medical hold because security officers may be afraid to physically detain someone without explicit legal paperwork.17
If a patient does not meet the capacity threshold but there does not seem to be a psychiatric, neurologic, or cognitive explanation, several options are possible. The first step would be to assess whether the patient is amenable to further discussion and compromise. A nonjudgmental and nonconfrontational approach that aims to further clarify the patient’s perspective and identify shared goals is key. Any plan that lowers the risks sufficiently would allow the patient to leave by lowering the capacity threshold. Enlisting the support of family and friends can be helpful. If this does not work, theoretically the patient should be detained in the hospital. Practically speaking, this may be difficult or unadvised. First, as described above, security officers may refuse to physically detain the patient.17 Second, the patient’s legally mandated surrogate may espouse similar COVID-related views as the patient; thus, this approach may not help keep the patient in the hospital. If the physician has serious concern about the risk of the patient leaving, he/she would have to consult the facility’s Ethics and Legal staff to determine capacity of the surrogate. Third, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. In the current sociopolitical climate, involuntary detention may elicit a political backlash. Using medical detention for impending deterioration of clinical status would be more acceptable than using medical detention for isolation. Presently, there are no such laws for patients with COVID-19 (although this is not without precedent, as with active tuberculosis or Ebola18,19), but individual jurisdictions may have isolation or quarantine orders; the local health department could be contacted and may evaluate on a case-by-case basis.
Continue to: Refusing to seek medical care
Refusing to seek medical care
Anecdotally, many physicians have reported an increase in patients who are refusing clinic- or hospital-based treatment for a medical condition because they fear they may catch the virus. Although this is not strictly a capacity case—there is little recourse for action if a patient is refusing treatment from home (unless the patient requires a psychiatric hold or already has a guardian for medical decisions)—the same elements of thresholds apply and can be helpful in guiding conversations with the patient.
For the patient, the benefits of staying at home are to avoid potentially exposing themselves and the members of their household to the virus and COVID-19 illness. The risks of staying home include progression of the patient’s primary illness, which could lead to increased morbidity and mortality. Staying home has an ancillary benefit to the community of reducing health-care utilization, but at the risk of increasing utilization in the future.
The risk/benefit profile is shown on the thresholds graph in Figure 3. There is considerable variability. It is helpful to stratify the risk of progression of the primary condition as low (can be postponed indefinitely with minimal risk), medium (can be postponed for a short amount of time; risk of increased morbidity with ongoing delay and possibly increased mortality), or high (cannot be postponed; will have greater morbidity and/or higher risk of mortality). Because of the uncertainty about COVID-19, it is harder to quantify the benefits of refusing care and staying at home, although older patients and patients with underlying health issues are at higher risk of severe illness and death.20 However, by taking appropriate precautions when seeking care, viral exposure and risk of infection can be mitigated.
This risk/benefit analysis will help set the threshold for whether staying at home is reasonable or whether it would incur more risk of harm. If the latter, then the physician must elicit the patient’s understanding, appreciation, and reasoning related to their current medical condition and COVID-19. It is likely they are undervaluing the former and overvaluing the latter. Table 2 lists important points to cover during these discussions.
Although there is no legal recourse to force patients at home to come to the clinic or hospital for medical treatment, there are several possible strategies to motivate them to do so. One is to ask patients how likely (on a scale of 0 to 100) they think they are to contract COVID-19 if they came for evaluation/treatment, and how likely they feel they are to experience a bad outcome from their primary condition. Then, after providing psychoeducation about their primary medical condition and COVID-19–related precautions and risk, repeat this question. Another strategy is to empathize with the patient’s fears while also expressing concern about the primary medical condition and connecting with the patient on the shared desire to protect his/her health. A third is to draw a risk/benefit diagram (similar to Figure 3) or reassure the patient by describing the ways in which the clinic or hospital is minimizing exposure and infection risk. A final strategy is to enlist the help of the patient’s family or friends.
Continue to: Bottom Line
Bottom Line
In order to have decision-making capacity, a patient must demonstrate choice, understanding, appreciation, and reasoning. The degree of understanding, appreciation, and reasoning required depends on the capacity threshold, which is determined by a risk/benefit analysis. Conducting a risk/benefit analysis during the coronavirus disease 2019 (COVID-19) pandemic requires consideration of societallevel factors (such as contagiousness to others and health-care utilization) and is complicated by a wide range of uncertainties and divisive sociopolitical views regarding COVID-19.
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
- Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
Acknowledgments
The author thanks Drs. Awais Aftab, Zackary D. Berger, and R. Brett Lloyd for their helpful discussions on the topic.
1. Beauchamp TL, Childress JF. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013.
2. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
3. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
4. Magid M, Dodd ML, Bostwick MJ, et al. Is your patient making the ‘wrong’ treatment choice? Current Psychiatry. 2006;5(3):13-20.
5. Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. Association of Directors of Medical Student Education in Psychiatry. 2020. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
6. Sanche S, Lin YT, Xu C, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470-1477.
7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
8. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469.
9. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180.
10. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382(13):1194-1196.
11. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singapore. 2020;49(3):108-118.
12. Asai A, Konno M, Ozaki M, et al. COVID-19 drug discovery using intensive approaches. Int J Mol Sci. 2020;21(8):2839.
13. Siegel AM, Barnwell AS, Sisti DA. Assessing decision-making capacity: a primer for the development of hospital practice guidelines. HEC Forum. 2014;26(2):159-168.
14. Karlawish J. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated February 24, 2020. Accessed May 27, 2020.
15. Mental Capacity Act 2005. Chapter 9. http://www.legislation.gov.uk/ukpga/2005/9/part/1. Accessed May 27, 2020.
16. Kersten C. The doctor as jailer: medical detention of non-psychiatric patients. J Law Biosci. 2019;6(1):310-316.
17. Cheung EH, Heldt J, Strouse T, et al. The medical incapacity hold: a policy on the involuntary medical hospitalization of patients who lack decisional capacity. Psychosomatics. 2018;59(2):169-176.
18. Parmet WE, Sinha MS. Covid-19 - the law and limits of quarantine. N Engl J Med. 2020;382(15):e28.
19. Coker R, Thomas M, Lock K, et al. Detention and the evolving threat of tuberculosis: evidence, ethics, and law. J Law Med Ethics. 2007;35(4):609-615.
20. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
1. Beauchamp TL, Childress JF. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013.
2. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
3. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
4. Magid M, Dodd ML, Bostwick MJ, et al. Is your patient making the ‘wrong’ treatment choice? Current Psychiatry. 2006;5(3):13-20.
5. Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. Association of Directors of Medical Student Education in Psychiatry. 2020. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
6. Sanche S, Lin YT, Xu C, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470-1477.
7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
8. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469.
9. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180.
10. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382(13):1194-1196.
11. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singapore. 2020;49(3):108-118.
12. Asai A, Konno M, Ozaki M, et al. COVID-19 drug discovery using intensive approaches. Int J Mol Sci. 2020;21(8):2839.
13. Siegel AM, Barnwell AS, Sisti DA. Assessing decision-making capacity: a primer for the development of hospital practice guidelines. HEC Forum. 2014;26(2):159-168.
14. Karlawish J. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated February 24, 2020. Accessed May 27, 2020.
15. Mental Capacity Act 2005. Chapter 9. http://www.legislation.gov.uk/ukpga/2005/9/part/1. Accessed May 27, 2020.
16. Kersten C. The doctor as jailer: medical detention of non-psychiatric patients. J Law Biosci. 2019;6(1):310-316.
17. Cheung EH, Heldt J, Strouse T, et al. The medical incapacity hold: a policy on the involuntary medical hospitalization of patients who lack decisional capacity. Psychosomatics. 2018;59(2):169-176.
18. Parmet WE, Sinha MS. Covid-19 - the law and limits of quarantine. N Engl J Med. 2020;382(15):e28.
19. Coker R, Thomas M, Lock K, et al. Detention and the evolving threat of tuberculosis: evidence, ethics, and law. J Law Med Ethics. 2007;35(4):609-615.
20. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
Helping older adults overcome the challenges of technology
Technology is pervasive, and for many people, it is central to their daily activities. Younger people who have been exposed to technology for their entire lives take this for granted, but older individuals often have had much less experience with it. Many technological developments that are now a part of most people’s daily life, such as personal computers, cell phones, and automated teller machines (ATMs), have occurred in the past 4 decades, with the pace accelerating in the last 15 to 20 years.
Such changes have had a substantial impact on older adults who were never exposed to these technologies during their working life. For example, an 85-year-old person who retired at age 65 would probably have not been exposed to wireless internet prior to retirement. Therefore, all of the tasks that they are now required to complete online would have been performed in other ways. Banking, accessing instruction manuals for new devices, and even scheduling and confirming health care appointments and accessing medical records all now require individuals to have a level of technological skills that many older individuals find challenging. At times, this can limit their ability to complete routine daily activities, and also can have clinical implications (Table).
Fortunately, there are strategies clinicians can use to help their older patients face these challenges. In this article, we describe the cognitive domains associated with learning technological skills, how aging affects these domains, and what can be done to help older adults improve their technological skills.
Limited training on how to use new technology
Technological skills are similar to any other skills in one critical way: they need to be learned. At the same time, technological skills also differ from many other skills, such as playing a musical instrument, because of the constant updating of devices, programs, and applications. When smartphones or computers update their operating systems, the visual appearance of the screen and the way that tasks are performed also can change. Buttons can move and sequences of commands can be altered. Updates often happen with little or no notice, and users may need to navigate a completely different device landscape in order to perform tasks that they had previously mastered.
In addition, the creators/distributors of technology typically provide little training or documentation. Further, institutions such as banks or health care systems frequently do not provide any specific training for using their systems. For example, when patients are required to use technology to refill prescriptions, typically there is no training available on how the system operates.
Cognitive domains associated with technological skills
Because there are minimal opportunities to receive training in how to use most aspects of technology, users have to be able to learn by exposure and experience. This requires several different cognitive abilities to work together. In a recent review, Harvey1 described cognition and cognitive assessment in the general population, with a focus on cognitive domains. Here we discuss several of these domains in terms of the relationship to real-world functional tasks and discuss their importance for mastering technology.
Reasoning and problem solving. Because most technological devices and applications are designed to be “intuitive,” the user needs to be able to adopt a sequential approach to learning the task. For example, using the internet to refill a prescription requires several steps:
- accessing the internet
- finding the pharmacy web site
- establishing a user ID and password
- navigating the web site to the prescriptions section
- identifying the correct prescription
- requesting the refill
- selecting the pickup date and time.
Continue to: After navigating these steps...
After navigating these steps, an individual still needs other cognitive abilities to refill other prescriptions later. However, executive functioning is also critical for maintaining organization across different technological demands. For example, web sites have different password rules and require frequent changes without re-using old passwords, so it becomes critical to maintain an organized list of web site addresses and their passwords.
Refilling a prescription with a telephone voice menu also requires a series of steps. Typically, this process is simpler than an internet refill, because no log-in information is necessary. However, it still requires a structured series of tasks.
Working memory refers to the ability to hold information in consciousness long enough to operate on it. At each step of the navigation process, the user needs to remember which steps he/she has already completed, because repeating steps can slow down the process or lead to error messages. Thus, remembering which steps have been completed is as critical for performing tasks as is correctly understanding the anticipated sequence of steps. Further, when a password is forgotten, the user needs to remember the newly provided password.
Working memory can be spatial as well. For example, most web sites do not display a password while it is being entered, which eliminates spatial working memory from the equation. Thus, the ability to remember which characters have been entered and which still need to be entered is necessary.
Episodic memory is the process of learning and retaining newly presented verbal or spatial information as well as recalling it later for adaptive use. After successfully using a new technology, it is critical to be able to remember what to do the next time it is used. This includes both recalling how to access the technology (including the web address, user ID, and password), recalling the steps needed to be performed and their sequence, and recognizing the buttons and instructions presented onscreen.
Continue to: Procedural memory
Procedural memory is memory for motor acts and sequences. For instance, remembering how to ride a bicycle is a procedural memory, as is the ability to perform motor acts in sequence, such as peeling, cutting, and cooking vegetables. Interestingly, procedural memory can be spared in individuals with major challenges in episodic memory, such as those with amnestic conditions or cortical dementia. Thus, it may be possible for people to continue to perform technology-based skills despite declines in episodic memory. Many current technological functional tasks have fixed sequences of events that, if remembered, can lead to increased efficiency and higher chances of success in performance of functional tasks.
Prospective memory is the ability to remember to perform tasks in the future. This can include event-related tasks (eg, enter your password before trying to make a hotel reservation on a web site) or time-related tasks (eg, refill your prescriptions next Friday). Technology can actually facilitate prospective memory by providing reminders to individuals, such as alarms for appointments. However, prospective memory is required to initially set up such alarms, and setting up confusing or incorrect alarms can impede task performance.
Processing speed is the ability to perform cognitively demanding tasks under time constraints. Traditional processing speed tasks include coding and sorting tasks, which require processing new information and effort for relatively short periods of time. In our research, we discovered that processing speed measured with traditional tests was strongly correlated with the time required to perform functional tasks such as an ATM banking task.2,3 This correlation makes sense in terms of the fact that many real-world functional tasks with technology often have a series of sequential demands that must be accomplished before progression to the next task.
Manual dexterity is also important for using technology. Many electronic devices have small, touch screen-based keyboards. Being able to touch the correct key requires dexterity and can be made more difficult by age-related vision changes, a tremor, or reduced sensation in extremities.
Cognitive changes and aging
It is normal for certain cognitive abilities to change with aging. There are a set of cognitive skills that are generally stable from early adulthood until the early “senescent” period. Some of these skills decline normatively after age 60 to 65, or earlier in some individuals. These include processing new information, solving new problems, and learning and remembering information. Referred to as “fluid intelligence,” these abilities show age-related decline during healthy aging, and even greater decline in individuals with age-related cognitive conditions.
Continue to: On the other hand...
On the other hand, some cognitive abilities do not decline with aging. These include previously acquired knowledge, such as vocabulary and mathematics skills, as well as factual information, such as academic information and the faces of familiar people. These are referred to as “crystallized intelligence,” and there is limited evidence that they decline with age. In fact, these abilities do not decline until the moderately severe stage of cortical dementias, and are commonly used to index premorbid cognitive functioning and cognitive reserve.
Why is this distinction between fluid intelligence and crystallized intelligence important? As noted above, many older people do not have early-life experience with technology. Thus, their crystallized intelligence, which is not as vulnerable to decline with aging, does not include information about how to perform many technological tasks. In contrast to today’s adolescents and young adults, older adults’ academic history typically does not include using smartphones, doing homework via Google Docs, or having homework and classwork assigned via the internet.
Learning how to use new technology requires fluid intelligence, and these abilities are less efficient in older adults. So for many older people, technological tasks can be complex and unfamiliar, and the skills needed to learn how to perform them are also more limited, even in comparison to older adults’ own ability when younger. Because many technology-based activities require concurrent performance of multiple tasks, older adults are at a disadvantage.4 It is not surprising, therefore, that a subset of older adults rate their technology skills as weak, and technology-based tasks as challenging or anxiety-provoking.
However, studies show most older adults’ attitudes toward technology remain largely positive, and that they are capable of attaining the necessary skills to use information and communication technology.4,5 An individual’s perception of his/her age, age-related beliefs, and self-efficacy are associated not only with attitudes toward technology, but possibly with cognition itself.6
Education level and socioeconomic factors also influence a person’s ability to become proficient in using technology.7-9 In fact, socioeconomic factors are more strongly related to access to the internet than age. Many older adults have internet access, but this access does not always translate into full use of its services.
Continue to: The Box...
The Box10-22 describes some of the effects of aging on the brain, and how these changes are reflected in cognitive abilities.
Box
The global baseline intensity of human brain activity, determined by indirectly measuring blood oxygenation, decreases with age.10 Multiple domains of fluid cognition decline with age; these cognitive abilities include processing speed,11,12 working memory,11 episodic memory,11 and executive function.11 Expected neuroanatomic changes of aging include a decrease in cerebral grey matter volume as well as decreased white matter integrity, which is associated with diminished executive function and impaired working memory.13 Processing speed is associated with increased white matter microstructure during neurodevelopment.14 Diminished processing speed in older adults also may predict increased mortality risk.15 Individuals with advanced age may have augmented difficulty with episodic memory, especially when they are required to integrate information from more than one source.11 Diminished hippocampal volume13 and reduced activity of the middle frontal gyrus are associated with age-related decline in episodic memory retrieval.10 Working memory16 is known to share a neurocircuitry overlap with attention processes.17 Working memory capacity also is closely associated with other cognitive functions, such as shifting and inhibition.10 Enhanced cerebellar activity is related to working memory; increased cerebellar activity is likely due to compensatory recruitment of neurons due to reduced activity in the superior frontal gyrus.10 The superior frontal gyrus contributes to both working memory as well as executive processing.10
Although the cognitive decline associated with aging is inevitable, individuals who experience cognitive decline at an increased rate are predisposed to worse outcomes. One longitudinal cohort study found that adults in their 8th and 9th decades of life with preserved cognitive function had a lower risk of disability and death.18
On the other hand, crystallized cognitive functions such as semantic memory,13 shortterm memory,13 and emotion regulation16 remain largely intact throughout the aging process. Semantic memory, a subtype of episodic memory, is related to associated facts or interpretations of previous occurrences.19 This type of memory is detached from an individual’s personal experience.20 Semantic memory loss classically presents with anomia and detectable lesions in the anterior and temporal lobes.20 Emotion regulation deficits are not a part of normal aging; in fact, emotional well-being is known to either improve or remain consistent with age.21 Emotional experiences in patients of advanced age may be more complex and unique in comparison to other cognitive abilities.22
The role of cognitive training
Existing interventions for helping older adults improve their technology proficiency generally focus on improving cognition, and not necessarily on addressing skills learning. Skills learning and cognition are related; however, the brain depends on neural plasticity for skills learning, whereas cognitive declines are a result of gradual and functional worsening of memory, processing speed, executive functioning, and attention.23 Interventions such as cognitive strategy training are capable of altering brain neurocircuitry to improve attention and memory.10,11 Other interventions known to improve cognition include exercise10 and processing speed training.24 On the other hand, skills learning is more effectively targeted by interventions that focus on stimulating realistic environments to mimic activities of daily living that involve technology.
Studies have consistently demonstrated cognitive improvements associated with computerized cognitive training (CCT). The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study was designed to evaluate the efficacy of cognitive training in 2,832 healthy adults age >65 across 6 recruitment sites in the United States.25 Participants were randomized to a control group (no treatment) or to 1 of 3 treatment groups:
- memory strategy training (instructor-led, not computerized)
- reasoning training (instructor-led, not computerized)
- speed training (no instructor, adaptive computerized training).
Each treatment group received 10 sessions of classroom-based training (1 hour each, twice per week for 5 weeks). Following the intervention, participants who had completed ≥8 sessions were randomized to receive 4 booster sessions at 11 and 35 months after the initial training, or no booster sessions.
Each cognitive training program significantly improved performance on within-domain cognitive tests relative to the control group. Effect sizes were large immediately following training; they declined over time, but were still significant at 10-year follow-up. As hypothesized, training effects did not generalize to neuropsychological tests in other training domains. The booster subgroup of speed training showed improved performance on a separate functional speed measure at 2-year26 and 5-year follow-up.27 Each condition showed slower decline in instrumental activities of daily living relative to the control group.
Continue to: The Figure...
The Figure shows the type of stimuli presented in the speed training, a procedure where individuals are taught high-speed multitasking by having to identify and locate visual information quickly in a divided-attention format. A stimulus appears in the center of the screen—either a car or a truck—and at the same time, a “Route 66” sign appears in the periphery. For every successful response, the next stimulus is presented at a shorter duration after every successful response, and more slowly after errors.
Secondary outcome analyses demonstrated that for older adults, speed training reduced rates of driving cessation,27 improved driving habits, and lowered the incidence of at-fault crashes28 (based on motor vehicle records). Speed training also resulted in improvements in health-related quality of life,29,30 depression,31 locus of control,32 and medical expenditures.33 An analysis of 10-year outcomes34 found that speed training was associated with a 29% reduction in risk of developing of dementia, while the other 2 interventions were not. However, despite these multiple areas of benefit, there was no evidence that new functional skills were acquired as a result of the training.26-34
Functional skills training
While there is a long history of using functional skills training to help patients with schizophrenia, for healthy older people, there are considerably more challenges. First, aging is not a disease. Consequently, functional skills training is typically not covered by health insurance. Second, functional skills training delivered by a human trainer can be expensive and is not readily available. Finally, there are no real curricula for training functional skills, particularly those that are device-based (phone, tablet, or computer).
Recently, researchers have developed a functional skills assessment and training program that was originally piloted as a fixed difficulty simulation as described in 2 studies by Czaja et al.2,3 The original assessment was used to compare healthy control individuals with people with mild cognitive impairment (MCI) or schizophrenia. Most recently, training modules for 6 different technology-based functional tasks have been developed and piloted in samples of healthy controls and patients with MCI in a randomized trial.35 Half of the participants in each of the 2 groups were randomized to receive speed training similar to the ACTIVE study, and the other half received skills training alone. All participants were trained for 24 sessions over 12 weeks or until they mastered all 6 simulations.
Both patients with MCI and healthy controls improved in all 6 simulations. Although patients with MCI were considerably less efficient at baseline, their training gains per session were equivalent to that of healthy controls. Finally, concurrent cognitive training increased the efficiency of skills training. At the end of the study, functional gains were the same for people in both groups randomized to either condition, even though individuals in the combined cognitive and skills training interventions received only half as much skills training time.
Continue to: What to tell patients
What to tell patients
Older patients might ask their clinicians what they can do to “exercise their brain.” Let them know that CCT has been shown to improve cognitive performance in healthy older people, and that there are several evidence-based, commercially available products for this purpose. Two such self-administrable systems with supportive data are BrainHQ (www.brainhq.com) and Happy Neuron (www.happy-neuron.com). Explain that it is likely that the best strategy is a combination of cognitive and functional skills training. One commercially available functional skills training program with supportive data is i-Function (www.i-Function.com). (Editor’s note: One of the authors, PDH, is an employee of i-Function, Inc.)
Bottom Line
Clinicians should ensure older patients that they have the cognitive capacity to learn new technology-related functional skills, and that such patients have the opportunity to learn these skills. Clinicians need to be able to identify people who are at high risk of not being able to adhere to instructions and suggestions that require interactions with technology. Treatment options include computerized cognitive training and functional skills training.
Related Resources
- Hill NT, Mowszowski L, Naismith SL, et al. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and metaanalysis. Am J Psychiatry. 2017;174(4):329-340.
- Harvey PD, McGurk SR, Mahncke H, et al. Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(11):907-915.
1. Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neuro. 2019;21(3):227-237.
2. Czaja SJ, Loewenstein DA, Sabbag SA, et al. A novel method for direct assessment of everyday competence among older adults. J Alzheimers Dis. 2017;57(4):1229-1238.
3. Czaja SJ, Loewenstein DA, Lee CC, et al. Assessing functional performance using computer-based simulations of everyday activities. Schizophr Res. 2017;183:130-136.
4. Tsai HS, Shillair R, Cotten SR. Social support and “playing around”: an examination of how older adults acquire digital literacy with tablet computers. J Appl Gerontol. 2017;36(1):29-55.
5. Cabrita M, Tabak M, Vollenbroek-Hutten MM. Older adults’ attitudes toward ambulatory technology to support monitoring and coaching of healthy behaviors: qualitative study. JMIR Aging. 2019;2(1):e10476. doi: 10.2196/10476.
6. Lim KY, Chang KJ, Kim HJ, et al. P.5.a.010 association between memory age identity and cognition in the elderly. Eur Neuropsychopharmacol. 2010;20(suppl 3):S555.
7. Moraes C, Pinto JA Jr, Lopes MA, et al. Impact of sociodemographic and health variables on mini-mental state examination in a community-based sample of older people. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):535-542.
8. Freitas S, Simões MR, Alves L, et al. The relevance of sociodemographic and health variables on MMSE normative data. Appl Neuropsychol Adult. 2015;22(4):311-319.
9. Han C, Jo SA, Jo I, et al. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008;47(3):302-310.
10. Yin S, Zhu X, Li R, et al. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci Rep. 2014;4:7309.
11. Bender AR, Prindle JJ, Brandmaier AM, et al. White matter and memory in healthy adults: coupled changes over two years. Neuroimage. 2016;131:193-204.
12. Guye S, von Bastian CC. Working memory training in older adults: Bayesian evidence supporting the absence of transfer. Psychol Aging. 2017;32(8):732-746.
13. Taki Y, Kinomura S, Sato K, et al. Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn. 2011;75(2):170-176.
14. Cassidy AR, White MT, DeMaso DR, et al. Processing speed, executive function, and academic achievement in children with dextro-transposition of the great arteries: Testing a longitudinal developmental cascade model. Neuropsychology. 2016;30(7):874-885.
15. Aichele S, Rabbitt P, Ghisletta P. Life span decrements in fluid intelligence and processing speed predict mortality risk. Psychol Aging. 2015;30(3):598-612.
16. Eich TS, Castel AD. The cognitive control of emotional versus value-based information in younger and older adults. Psychol Aging. 2016;31(5):503-512.
17. Rolle CE, Anguera JA, Skinner SN, et al. Enhancing spatial attention and working memory in younger and older adults. J Cogn Neurosci. 2017;29(9):1483-1497.
18. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58(5):889-894.
19. Madore KP, Schacter DL. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychol Aging. 2014;29(4):913-924.
20. Matthews BR. Memory dysfunction. Continuum (Minneap Minn). 2015;21(3 Behavioral Neurology and Neuropsychiatry):613-626.
21. Mather M. The emotion paradox in the aging brain. Ann N Y Acad Sci. 2012;1251(1):33-49.
22. Gurera JW, Isaacowitz DM. Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. Prog Brain Res. 2019;247:329-351.
23. Cai L, Chan JS, Yan JH, et al. Brain plasticity and motor practice in cognitive aging. Front Aging Neurosci. 2014;6:31.
24. Cassetta BD, Tomfohr-Madsen LM, Goghari VM. A randomized controlled trial of working memory and processing speed training in schizophrenia. Psychol Med. 2019;49(12):2009-2019.
25. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271-2281.
26. Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16-24.
27. Edwards JD, Delahunt PB, Mahncke HW. Cognitive speed of processing training delays driving cessation. J Gerontol A Biol Sci Med Sci. 2009;64(12):1262-1267.
28. Ball K, Edwards JD, Ross LA, et al. Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107-2113.
29. Wolinsky FD, Unverzagt FW, Smith DM, et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):S281-S287.
30. Wolinsky FD, Unverzagt FW, Smith DM, et al. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61(12):1324-1329.
31. Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64(4):468-472.
32. Wolinsky FD, Vander Weg MW, Martin R, et al. Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci. 2010;65(5):591-598.
33. Wolinsky FD, Mahncke HW, Kosinski M, et al. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv Res. 2009;9:109.
34. Edwards JD, Xu H, Clark DO, et al. Speed of processing training results in lower risk of dementia. Alzheimers Dement (N Y). 2017;3(4):603-611.
35. Harvey PD, Tibiriçá L, Kallestrup P, et al. A computerized functional skills assessment and training program targeting technology based everyday functional skills. J Vis Exp. 2020;156:e60330. doi: 10.3791/60330.
Technology is pervasive, and for many people, it is central to their daily activities. Younger people who have been exposed to technology for their entire lives take this for granted, but older individuals often have had much less experience with it. Many technological developments that are now a part of most people’s daily life, such as personal computers, cell phones, and automated teller machines (ATMs), have occurred in the past 4 decades, with the pace accelerating in the last 15 to 20 years.
Such changes have had a substantial impact on older adults who were never exposed to these technologies during their working life. For example, an 85-year-old person who retired at age 65 would probably have not been exposed to wireless internet prior to retirement. Therefore, all of the tasks that they are now required to complete online would have been performed in other ways. Banking, accessing instruction manuals for new devices, and even scheduling and confirming health care appointments and accessing medical records all now require individuals to have a level of technological skills that many older individuals find challenging. At times, this can limit their ability to complete routine daily activities, and also can have clinical implications (Table).
Fortunately, there are strategies clinicians can use to help their older patients face these challenges. In this article, we describe the cognitive domains associated with learning technological skills, how aging affects these domains, and what can be done to help older adults improve their technological skills.
Limited training on how to use new technology
Technological skills are similar to any other skills in one critical way: they need to be learned. At the same time, technological skills also differ from many other skills, such as playing a musical instrument, because of the constant updating of devices, programs, and applications. When smartphones or computers update their operating systems, the visual appearance of the screen and the way that tasks are performed also can change. Buttons can move and sequences of commands can be altered. Updates often happen with little or no notice, and users may need to navigate a completely different device landscape in order to perform tasks that they had previously mastered.
In addition, the creators/distributors of technology typically provide little training or documentation. Further, institutions such as banks or health care systems frequently do not provide any specific training for using their systems. For example, when patients are required to use technology to refill prescriptions, typically there is no training available on how the system operates.
Cognitive domains associated with technological skills
Because there are minimal opportunities to receive training in how to use most aspects of technology, users have to be able to learn by exposure and experience. This requires several different cognitive abilities to work together. In a recent review, Harvey1 described cognition and cognitive assessment in the general population, with a focus on cognitive domains. Here we discuss several of these domains in terms of the relationship to real-world functional tasks and discuss their importance for mastering technology.
Reasoning and problem solving. Because most technological devices and applications are designed to be “intuitive,” the user needs to be able to adopt a sequential approach to learning the task. For example, using the internet to refill a prescription requires several steps:
- accessing the internet
- finding the pharmacy web site
- establishing a user ID and password
- navigating the web site to the prescriptions section
- identifying the correct prescription
- requesting the refill
- selecting the pickup date and time.
Continue to: After navigating these steps...
After navigating these steps, an individual still needs other cognitive abilities to refill other prescriptions later. However, executive functioning is also critical for maintaining organization across different technological demands. For example, web sites have different password rules and require frequent changes without re-using old passwords, so it becomes critical to maintain an organized list of web site addresses and their passwords.
Refilling a prescription with a telephone voice menu also requires a series of steps. Typically, this process is simpler than an internet refill, because no log-in information is necessary. However, it still requires a structured series of tasks.
Working memory refers to the ability to hold information in consciousness long enough to operate on it. At each step of the navigation process, the user needs to remember which steps he/she has already completed, because repeating steps can slow down the process or lead to error messages. Thus, remembering which steps have been completed is as critical for performing tasks as is correctly understanding the anticipated sequence of steps. Further, when a password is forgotten, the user needs to remember the newly provided password.
Working memory can be spatial as well. For example, most web sites do not display a password while it is being entered, which eliminates spatial working memory from the equation. Thus, the ability to remember which characters have been entered and which still need to be entered is necessary.
Episodic memory is the process of learning and retaining newly presented verbal or spatial information as well as recalling it later for adaptive use. After successfully using a new technology, it is critical to be able to remember what to do the next time it is used. This includes both recalling how to access the technology (including the web address, user ID, and password), recalling the steps needed to be performed and their sequence, and recognizing the buttons and instructions presented onscreen.
Continue to: Procedural memory
Procedural memory is memory for motor acts and sequences. For instance, remembering how to ride a bicycle is a procedural memory, as is the ability to perform motor acts in sequence, such as peeling, cutting, and cooking vegetables. Interestingly, procedural memory can be spared in individuals with major challenges in episodic memory, such as those with amnestic conditions or cortical dementia. Thus, it may be possible for people to continue to perform technology-based skills despite declines in episodic memory. Many current technological functional tasks have fixed sequences of events that, if remembered, can lead to increased efficiency and higher chances of success in performance of functional tasks.
Prospective memory is the ability to remember to perform tasks in the future. This can include event-related tasks (eg, enter your password before trying to make a hotel reservation on a web site) or time-related tasks (eg, refill your prescriptions next Friday). Technology can actually facilitate prospective memory by providing reminders to individuals, such as alarms for appointments. However, prospective memory is required to initially set up such alarms, and setting up confusing or incorrect alarms can impede task performance.
Processing speed is the ability to perform cognitively demanding tasks under time constraints. Traditional processing speed tasks include coding and sorting tasks, which require processing new information and effort for relatively short periods of time. In our research, we discovered that processing speed measured with traditional tests was strongly correlated with the time required to perform functional tasks such as an ATM banking task.2,3 This correlation makes sense in terms of the fact that many real-world functional tasks with technology often have a series of sequential demands that must be accomplished before progression to the next task.
Manual dexterity is also important for using technology. Many electronic devices have small, touch screen-based keyboards. Being able to touch the correct key requires dexterity and can be made more difficult by age-related vision changes, a tremor, or reduced sensation in extremities.
Cognitive changes and aging
It is normal for certain cognitive abilities to change with aging. There are a set of cognitive skills that are generally stable from early adulthood until the early “senescent” period. Some of these skills decline normatively after age 60 to 65, or earlier in some individuals. These include processing new information, solving new problems, and learning and remembering information. Referred to as “fluid intelligence,” these abilities show age-related decline during healthy aging, and even greater decline in individuals with age-related cognitive conditions.
Continue to: On the other hand...
On the other hand, some cognitive abilities do not decline with aging. These include previously acquired knowledge, such as vocabulary and mathematics skills, as well as factual information, such as academic information and the faces of familiar people. These are referred to as “crystallized intelligence,” and there is limited evidence that they decline with age. In fact, these abilities do not decline until the moderately severe stage of cortical dementias, and are commonly used to index premorbid cognitive functioning and cognitive reserve.
Why is this distinction between fluid intelligence and crystallized intelligence important? As noted above, many older people do not have early-life experience with technology. Thus, their crystallized intelligence, which is not as vulnerable to decline with aging, does not include information about how to perform many technological tasks. In contrast to today’s adolescents and young adults, older adults’ academic history typically does not include using smartphones, doing homework via Google Docs, or having homework and classwork assigned via the internet.
Learning how to use new technology requires fluid intelligence, and these abilities are less efficient in older adults. So for many older people, technological tasks can be complex and unfamiliar, and the skills needed to learn how to perform them are also more limited, even in comparison to older adults’ own ability when younger. Because many technology-based activities require concurrent performance of multiple tasks, older adults are at a disadvantage.4 It is not surprising, therefore, that a subset of older adults rate their technology skills as weak, and technology-based tasks as challenging or anxiety-provoking.
However, studies show most older adults’ attitudes toward technology remain largely positive, and that they are capable of attaining the necessary skills to use information and communication technology.4,5 An individual’s perception of his/her age, age-related beliefs, and self-efficacy are associated not only with attitudes toward technology, but possibly with cognition itself.6
Education level and socioeconomic factors also influence a person’s ability to become proficient in using technology.7-9 In fact, socioeconomic factors are more strongly related to access to the internet than age. Many older adults have internet access, but this access does not always translate into full use of its services.
Continue to: The Box...
The Box10-22 describes some of the effects of aging on the brain, and how these changes are reflected in cognitive abilities.
Box
The global baseline intensity of human brain activity, determined by indirectly measuring blood oxygenation, decreases with age.10 Multiple domains of fluid cognition decline with age; these cognitive abilities include processing speed,11,12 working memory,11 episodic memory,11 and executive function.11 Expected neuroanatomic changes of aging include a decrease in cerebral grey matter volume as well as decreased white matter integrity, which is associated with diminished executive function and impaired working memory.13 Processing speed is associated with increased white matter microstructure during neurodevelopment.14 Diminished processing speed in older adults also may predict increased mortality risk.15 Individuals with advanced age may have augmented difficulty with episodic memory, especially when they are required to integrate information from more than one source.11 Diminished hippocampal volume13 and reduced activity of the middle frontal gyrus are associated with age-related decline in episodic memory retrieval.10 Working memory16 is known to share a neurocircuitry overlap with attention processes.17 Working memory capacity also is closely associated with other cognitive functions, such as shifting and inhibition.10 Enhanced cerebellar activity is related to working memory; increased cerebellar activity is likely due to compensatory recruitment of neurons due to reduced activity in the superior frontal gyrus.10 The superior frontal gyrus contributes to both working memory as well as executive processing.10
Although the cognitive decline associated with aging is inevitable, individuals who experience cognitive decline at an increased rate are predisposed to worse outcomes. One longitudinal cohort study found that adults in their 8th and 9th decades of life with preserved cognitive function had a lower risk of disability and death.18
On the other hand, crystallized cognitive functions such as semantic memory,13 shortterm memory,13 and emotion regulation16 remain largely intact throughout the aging process. Semantic memory, a subtype of episodic memory, is related to associated facts or interpretations of previous occurrences.19 This type of memory is detached from an individual’s personal experience.20 Semantic memory loss classically presents with anomia and detectable lesions in the anterior and temporal lobes.20 Emotion regulation deficits are not a part of normal aging; in fact, emotional well-being is known to either improve or remain consistent with age.21 Emotional experiences in patients of advanced age may be more complex and unique in comparison to other cognitive abilities.22
The role of cognitive training
Existing interventions for helping older adults improve their technology proficiency generally focus on improving cognition, and not necessarily on addressing skills learning. Skills learning and cognition are related; however, the brain depends on neural plasticity for skills learning, whereas cognitive declines are a result of gradual and functional worsening of memory, processing speed, executive functioning, and attention.23 Interventions such as cognitive strategy training are capable of altering brain neurocircuitry to improve attention and memory.10,11 Other interventions known to improve cognition include exercise10 and processing speed training.24 On the other hand, skills learning is more effectively targeted by interventions that focus on stimulating realistic environments to mimic activities of daily living that involve technology.
Studies have consistently demonstrated cognitive improvements associated with computerized cognitive training (CCT). The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study was designed to evaluate the efficacy of cognitive training in 2,832 healthy adults age >65 across 6 recruitment sites in the United States.25 Participants were randomized to a control group (no treatment) or to 1 of 3 treatment groups:
- memory strategy training (instructor-led, not computerized)
- reasoning training (instructor-led, not computerized)
- speed training (no instructor, adaptive computerized training).
Each treatment group received 10 sessions of classroom-based training (1 hour each, twice per week for 5 weeks). Following the intervention, participants who had completed ≥8 sessions were randomized to receive 4 booster sessions at 11 and 35 months after the initial training, or no booster sessions.
Each cognitive training program significantly improved performance on within-domain cognitive tests relative to the control group. Effect sizes were large immediately following training; they declined over time, but were still significant at 10-year follow-up. As hypothesized, training effects did not generalize to neuropsychological tests in other training domains. The booster subgroup of speed training showed improved performance on a separate functional speed measure at 2-year26 and 5-year follow-up.27 Each condition showed slower decline in instrumental activities of daily living relative to the control group.
Continue to: The Figure...
The Figure shows the type of stimuli presented in the speed training, a procedure where individuals are taught high-speed multitasking by having to identify and locate visual information quickly in a divided-attention format. A stimulus appears in the center of the screen—either a car or a truck—and at the same time, a “Route 66” sign appears in the periphery. For every successful response, the next stimulus is presented at a shorter duration after every successful response, and more slowly after errors.
Secondary outcome analyses demonstrated that for older adults, speed training reduced rates of driving cessation,27 improved driving habits, and lowered the incidence of at-fault crashes28 (based on motor vehicle records). Speed training also resulted in improvements in health-related quality of life,29,30 depression,31 locus of control,32 and medical expenditures.33 An analysis of 10-year outcomes34 found that speed training was associated with a 29% reduction in risk of developing of dementia, while the other 2 interventions were not. However, despite these multiple areas of benefit, there was no evidence that new functional skills were acquired as a result of the training.26-34
Functional skills training
While there is a long history of using functional skills training to help patients with schizophrenia, for healthy older people, there are considerably more challenges. First, aging is not a disease. Consequently, functional skills training is typically not covered by health insurance. Second, functional skills training delivered by a human trainer can be expensive and is not readily available. Finally, there are no real curricula for training functional skills, particularly those that are device-based (phone, tablet, or computer).
Recently, researchers have developed a functional skills assessment and training program that was originally piloted as a fixed difficulty simulation as described in 2 studies by Czaja et al.2,3 The original assessment was used to compare healthy control individuals with people with mild cognitive impairment (MCI) or schizophrenia. Most recently, training modules for 6 different technology-based functional tasks have been developed and piloted in samples of healthy controls and patients with MCI in a randomized trial.35 Half of the participants in each of the 2 groups were randomized to receive speed training similar to the ACTIVE study, and the other half received skills training alone. All participants were trained for 24 sessions over 12 weeks or until they mastered all 6 simulations.
Both patients with MCI and healthy controls improved in all 6 simulations. Although patients with MCI were considerably less efficient at baseline, their training gains per session were equivalent to that of healthy controls. Finally, concurrent cognitive training increased the efficiency of skills training. At the end of the study, functional gains were the same for people in both groups randomized to either condition, even though individuals in the combined cognitive and skills training interventions received only half as much skills training time.
Continue to: What to tell patients
What to tell patients
Older patients might ask their clinicians what they can do to “exercise their brain.” Let them know that CCT has been shown to improve cognitive performance in healthy older people, and that there are several evidence-based, commercially available products for this purpose. Two such self-administrable systems with supportive data are BrainHQ (www.brainhq.com) and Happy Neuron (www.happy-neuron.com). Explain that it is likely that the best strategy is a combination of cognitive and functional skills training. One commercially available functional skills training program with supportive data is i-Function (www.i-Function.com). (Editor’s note: One of the authors, PDH, is an employee of i-Function, Inc.)
Bottom Line
Clinicians should ensure older patients that they have the cognitive capacity to learn new technology-related functional skills, and that such patients have the opportunity to learn these skills. Clinicians need to be able to identify people who are at high risk of not being able to adhere to instructions and suggestions that require interactions with technology. Treatment options include computerized cognitive training and functional skills training.
Related Resources
- Hill NT, Mowszowski L, Naismith SL, et al. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and metaanalysis. Am J Psychiatry. 2017;174(4):329-340.
- Harvey PD, McGurk SR, Mahncke H, et al. Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(11):907-915.
Technology is pervasive, and for many people, it is central to their daily activities. Younger people who have been exposed to technology for their entire lives take this for granted, but older individuals often have had much less experience with it. Many technological developments that are now a part of most people’s daily life, such as personal computers, cell phones, and automated teller machines (ATMs), have occurred in the past 4 decades, with the pace accelerating in the last 15 to 20 years.
Such changes have had a substantial impact on older adults who were never exposed to these technologies during their working life. For example, an 85-year-old person who retired at age 65 would probably have not been exposed to wireless internet prior to retirement. Therefore, all of the tasks that they are now required to complete online would have been performed in other ways. Banking, accessing instruction manuals for new devices, and even scheduling and confirming health care appointments and accessing medical records all now require individuals to have a level of technological skills that many older individuals find challenging. At times, this can limit their ability to complete routine daily activities, and also can have clinical implications (Table).
Fortunately, there are strategies clinicians can use to help their older patients face these challenges. In this article, we describe the cognitive domains associated with learning technological skills, how aging affects these domains, and what can be done to help older adults improve their technological skills.
Limited training on how to use new technology
Technological skills are similar to any other skills in one critical way: they need to be learned. At the same time, technological skills also differ from many other skills, such as playing a musical instrument, because of the constant updating of devices, programs, and applications. When smartphones or computers update their operating systems, the visual appearance of the screen and the way that tasks are performed also can change. Buttons can move and sequences of commands can be altered. Updates often happen with little or no notice, and users may need to navigate a completely different device landscape in order to perform tasks that they had previously mastered.
In addition, the creators/distributors of technology typically provide little training or documentation. Further, institutions such as banks or health care systems frequently do not provide any specific training for using their systems. For example, when patients are required to use technology to refill prescriptions, typically there is no training available on how the system operates.
Cognitive domains associated with technological skills
Because there are minimal opportunities to receive training in how to use most aspects of technology, users have to be able to learn by exposure and experience. This requires several different cognitive abilities to work together. In a recent review, Harvey1 described cognition and cognitive assessment in the general population, with a focus on cognitive domains. Here we discuss several of these domains in terms of the relationship to real-world functional tasks and discuss their importance for mastering technology.
Reasoning and problem solving. Because most technological devices and applications are designed to be “intuitive,” the user needs to be able to adopt a sequential approach to learning the task. For example, using the internet to refill a prescription requires several steps:
- accessing the internet
- finding the pharmacy web site
- establishing a user ID and password
- navigating the web site to the prescriptions section
- identifying the correct prescription
- requesting the refill
- selecting the pickup date and time.
Continue to: After navigating these steps...
After navigating these steps, an individual still needs other cognitive abilities to refill other prescriptions later. However, executive functioning is also critical for maintaining organization across different technological demands. For example, web sites have different password rules and require frequent changes without re-using old passwords, so it becomes critical to maintain an organized list of web site addresses and their passwords.
Refilling a prescription with a telephone voice menu also requires a series of steps. Typically, this process is simpler than an internet refill, because no log-in information is necessary. However, it still requires a structured series of tasks.
Working memory refers to the ability to hold information in consciousness long enough to operate on it. At each step of the navigation process, the user needs to remember which steps he/she has already completed, because repeating steps can slow down the process or lead to error messages. Thus, remembering which steps have been completed is as critical for performing tasks as is correctly understanding the anticipated sequence of steps. Further, when a password is forgotten, the user needs to remember the newly provided password.
Working memory can be spatial as well. For example, most web sites do not display a password while it is being entered, which eliminates spatial working memory from the equation. Thus, the ability to remember which characters have been entered and which still need to be entered is necessary.
Episodic memory is the process of learning and retaining newly presented verbal or spatial information as well as recalling it later for adaptive use. After successfully using a new technology, it is critical to be able to remember what to do the next time it is used. This includes both recalling how to access the technology (including the web address, user ID, and password), recalling the steps needed to be performed and their sequence, and recognizing the buttons and instructions presented onscreen.
Continue to: Procedural memory
Procedural memory is memory for motor acts and sequences. For instance, remembering how to ride a bicycle is a procedural memory, as is the ability to perform motor acts in sequence, such as peeling, cutting, and cooking vegetables. Interestingly, procedural memory can be spared in individuals with major challenges in episodic memory, such as those with amnestic conditions or cortical dementia. Thus, it may be possible for people to continue to perform technology-based skills despite declines in episodic memory. Many current technological functional tasks have fixed sequences of events that, if remembered, can lead to increased efficiency and higher chances of success in performance of functional tasks.
Prospective memory is the ability to remember to perform tasks in the future. This can include event-related tasks (eg, enter your password before trying to make a hotel reservation on a web site) or time-related tasks (eg, refill your prescriptions next Friday). Technology can actually facilitate prospective memory by providing reminders to individuals, such as alarms for appointments. However, prospective memory is required to initially set up such alarms, and setting up confusing or incorrect alarms can impede task performance.
Processing speed is the ability to perform cognitively demanding tasks under time constraints. Traditional processing speed tasks include coding and sorting tasks, which require processing new information and effort for relatively short periods of time. In our research, we discovered that processing speed measured with traditional tests was strongly correlated with the time required to perform functional tasks such as an ATM banking task.2,3 This correlation makes sense in terms of the fact that many real-world functional tasks with technology often have a series of sequential demands that must be accomplished before progression to the next task.
Manual dexterity is also important for using technology. Many electronic devices have small, touch screen-based keyboards. Being able to touch the correct key requires dexterity and can be made more difficult by age-related vision changes, a tremor, or reduced sensation in extremities.
Cognitive changes and aging
It is normal for certain cognitive abilities to change with aging. There are a set of cognitive skills that are generally stable from early adulthood until the early “senescent” period. Some of these skills decline normatively after age 60 to 65, or earlier in some individuals. These include processing new information, solving new problems, and learning and remembering information. Referred to as “fluid intelligence,” these abilities show age-related decline during healthy aging, and even greater decline in individuals with age-related cognitive conditions.
Continue to: On the other hand...
On the other hand, some cognitive abilities do not decline with aging. These include previously acquired knowledge, such as vocabulary and mathematics skills, as well as factual information, such as academic information and the faces of familiar people. These are referred to as “crystallized intelligence,” and there is limited evidence that they decline with age. In fact, these abilities do not decline until the moderately severe stage of cortical dementias, and are commonly used to index premorbid cognitive functioning and cognitive reserve.
Why is this distinction between fluid intelligence and crystallized intelligence important? As noted above, many older people do not have early-life experience with technology. Thus, their crystallized intelligence, which is not as vulnerable to decline with aging, does not include information about how to perform many technological tasks. In contrast to today’s adolescents and young adults, older adults’ academic history typically does not include using smartphones, doing homework via Google Docs, or having homework and classwork assigned via the internet.
Learning how to use new technology requires fluid intelligence, and these abilities are less efficient in older adults. So for many older people, technological tasks can be complex and unfamiliar, and the skills needed to learn how to perform them are also more limited, even in comparison to older adults’ own ability when younger. Because many technology-based activities require concurrent performance of multiple tasks, older adults are at a disadvantage.4 It is not surprising, therefore, that a subset of older adults rate their technology skills as weak, and technology-based tasks as challenging or anxiety-provoking.
However, studies show most older adults’ attitudes toward technology remain largely positive, and that they are capable of attaining the necessary skills to use information and communication technology.4,5 An individual’s perception of his/her age, age-related beliefs, and self-efficacy are associated not only with attitudes toward technology, but possibly with cognition itself.6
Education level and socioeconomic factors also influence a person’s ability to become proficient in using technology.7-9 In fact, socioeconomic factors are more strongly related to access to the internet than age. Many older adults have internet access, but this access does not always translate into full use of its services.
Continue to: The Box...
The Box10-22 describes some of the effects of aging on the brain, and how these changes are reflected in cognitive abilities.
Box
The global baseline intensity of human brain activity, determined by indirectly measuring blood oxygenation, decreases with age.10 Multiple domains of fluid cognition decline with age; these cognitive abilities include processing speed,11,12 working memory,11 episodic memory,11 and executive function.11 Expected neuroanatomic changes of aging include a decrease in cerebral grey matter volume as well as decreased white matter integrity, which is associated with diminished executive function and impaired working memory.13 Processing speed is associated with increased white matter microstructure during neurodevelopment.14 Diminished processing speed in older adults also may predict increased mortality risk.15 Individuals with advanced age may have augmented difficulty with episodic memory, especially when they are required to integrate information from more than one source.11 Diminished hippocampal volume13 and reduced activity of the middle frontal gyrus are associated with age-related decline in episodic memory retrieval.10 Working memory16 is known to share a neurocircuitry overlap with attention processes.17 Working memory capacity also is closely associated with other cognitive functions, such as shifting and inhibition.10 Enhanced cerebellar activity is related to working memory; increased cerebellar activity is likely due to compensatory recruitment of neurons due to reduced activity in the superior frontal gyrus.10 The superior frontal gyrus contributes to both working memory as well as executive processing.10
Although the cognitive decline associated with aging is inevitable, individuals who experience cognitive decline at an increased rate are predisposed to worse outcomes. One longitudinal cohort study found that adults in their 8th and 9th decades of life with preserved cognitive function had a lower risk of disability and death.18
On the other hand, crystallized cognitive functions such as semantic memory,13 shortterm memory,13 and emotion regulation16 remain largely intact throughout the aging process. Semantic memory, a subtype of episodic memory, is related to associated facts or interpretations of previous occurrences.19 This type of memory is detached from an individual’s personal experience.20 Semantic memory loss classically presents with anomia and detectable lesions in the anterior and temporal lobes.20 Emotion regulation deficits are not a part of normal aging; in fact, emotional well-being is known to either improve or remain consistent with age.21 Emotional experiences in patients of advanced age may be more complex and unique in comparison to other cognitive abilities.22
The role of cognitive training
Existing interventions for helping older adults improve their technology proficiency generally focus on improving cognition, and not necessarily on addressing skills learning. Skills learning and cognition are related; however, the brain depends on neural plasticity for skills learning, whereas cognitive declines are a result of gradual and functional worsening of memory, processing speed, executive functioning, and attention.23 Interventions such as cognitive strategy training are capable of altering brain neurocircuitry to improve attention and memory.10,11 Other interventions known to improve cognition include exercise10 and processing speed training.24 On the other hand, skills learning is more effectively targeted by interventions that focus on stimulating realistic environments to mimic activities of daily living that involve technology.
Studies have consistently demonstrated cognitive improvements associated with computerized cognitive training (CCT). The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study was designed to evaluate the efficacy of cognitive training in 2,832 healthy adults age >65 across 6 recruitment sites in the United States.25 Participants were randomized to a control group (no treatment) or to 1 of 3 treatment groups:
- memory strategy training (instructor-led, not computerized)
- reasoning training (instructor-led, not computerized)
- speed training (no instructor, adaptive computerized training).
Each treatment group received 10 sessions of classroom-based training (1 hour each, twice per week for 5 weeks). Following the intervention, participants who had completed ≥8 sessions were randomized to receive 4 booster sessions at 11 and 35 months after the initial training, or no booster sessions.
Each cognitive training program significantly improved performance on within-domain cognitive tests relative to the control group. Effect sizes were large immediately following training; they declined over time, but were still significant at 10-year follow-up. As hypothesized, training effects did not generalize to neuropsychological tests in other training domains. The booster subgroup of speed training showed improved performance on a separate functional speed measure at 2-year26 and 5-year follow-up.27 Each condition showed slower decline in instrumental activities of daily living relative to the control group.
Continue to: The Figure...
The Figure shows the type of stimuli presented in the speed training, a procedure where individuals are taught high-speed multitasking by having to identify and locate visual information quickly in a divided-attention format. A stimulus appears in the center of the screen—either a car or a truck—and at the same time, a “Route 66” sign appears in the periphery. For every successful response, the next stimulus is presented at a shorter duration after every successful response, and more slowly after errors.
Secondary outcome analyses demonstrated that for older adults, speed training reduced rates of driving cessation,27 improved driving habits, and lowered the incidence of at-fault crashes28 (based on motor vehicle records). Speed training also resulted in improvements in health-related quality of life,29,30 depression,31 locus of control,32 and medical expenditures.33 An analysis of 10-year outcomes34 found that speed training was associated with a 29% reduction in risk of developing of dementia, while the other 2 interventions were not. However, despite these multiple areas of benefit, there was no evidence that new functional skills were acquired as a result of the training.26-34
Functional skills training
While there is a long history of using functional skills training to help patients with schizophrenia, for healthy older people, there are considerably more challenges. First, aging is not a disease. Consequently, functional skills training is typically not covered by health insurance. Second, functional skills training delivered by a human trainer can be expensive and is not readily available. Finally, there are no real curricula for training functional skills, particularly those that are device-based (phone, tablet, or computer).
Recently, researchers have developed a functional skills assessment and training program that was originally piloted as a fixed difficulty simulation as described in 2 studies by Czaja et al.2,3 The original assessment was used to compare healthy control individuals with people with mild cognitive impairment (MCI) or schizophrenia. Most recently, training modules for 6 different technology-based functional tasks have been developed and piloted in samples of healthy controls and patients with MCI in a randomized trial.35 Half of the participants in each of the 2 groups were randomized to receive speed training similar to the ACTIVE study, and the other half received skills training alone. All participants were trained for 24 sessions over 12 weeks or until they mastered all 6 simulations.
Both patients with MCI and healthy controls improved in all 6 simulations. Although patients with MCI were considerably less efficient at baseline, their training gains per session were equivalent to that of healthy controls. Finally, concurrent cognitive training increased the efficiency of skills training. At the end of the study, functional gains were the same for people in both groups randomized to either condition, even though individuals in the combined cognitive and skills training interventions received only half as much skills training time.
Continue to: What to tell patients
What to tell patients
Older patients might ask their clinicians what they can do to “exercise their brain.” Let them know that CCT has been shown to improve cognitive performance in healthy older people, and that there are several evidence-based, commercially available products for this purpose. Two such self-administrable systems with supportive data are BrainHQ (www.brainhq.com) and Happy Neuron (www.happy-neuron.com). Explain that it is likely that the best strategy is a combination of cognitive and functional skills training. One commercially available functional skills training program with supportive data is i-Function (www.i-Function.com). (Editor’s note: One of the authors, PDH, is an employee of i-Function, Inc.)
Bottom Line
Clinicians should ensure older patients that they have the cognitive capacity to learn new technology-related functional skills, and that such patients have the opportunity to learn these skills. Clinicians need to be able to identify people who are at high risk of not being able to adhere to instructions and suggestions that require interactions with technology. Treatment options include computerized cognitive training and functional skills training.
Related Resources
- Hill NT, Mowszowski L, Naismith SL, et al. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and metaanalysis. Am J Psychiatry. 2017;174(4):329-340.
- Harvey PD, McGurk SR, Mahncke H, et al. Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(11):907-915.
1. Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neuro. 2019;21(3):227-237.
2. Czaja SJ, Loewenstein DA, Sabbag SA, et al. A novel method for direct assessment of everyday competence among older adults. J Alzheimers Dis. 2017;57(4):1229-1238.
3. Czaja SJ, Loewenstein DA, Lee CC, et al. Assessing functional performance using computer-based simulations of everyday activities. Schizophr Res. 2017;183:130-136.
4. Tsai HS, Shillair R, Cotten SR. Social support and “playing around”: an examination of how older adults acquire digital literacy with tablet computers. J Appl Gerontol. 2017;36(1):29-55.
5. Cabrita M, Tabak M, Vollenbroek-Hutten MM. Older adults’ attitudes toward ambulatory technology to support monitoring and coaching of healthy behaviors: qualitative study. JMIR Aging. 2019;2(1):e10476. doi: 10.2196/10476.
6. Lim KY, Chang KJ, Kim HJ, et al. P.5.a.010 association between memory age identity and cognition in the elderly. Eur Neuropsychopharmacol. 2010;20(suppl 3):S555.
7. Moraes C, Pinto JA Jr, Lopes MA, et al. Impact of sociodemographic and health variables on mini-mental state examination in a community-based sample of older people. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):535-542.
8. Freitas S, Simões MR, Alves L, et al. The relevance of sociodemographic and health variables on MMSE normative data. Appl Neuropsychol Adult. 2015;22(4):311-319.
9. Han C, Jo SA, Jo I, et al. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008;47(3):302-310.
10. Yin S, Zhu X, Li R, et al. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci Rep. 2014;4:7309.
11. Bender AR, Prindle JJ, Brandmaier AM, et al. White matter and memory in healthy adults: coupled changes over two years. Neuroimage. 2016;131:193-204.
12. Guye S, von Bastian CC. Working memory training in older adults: Bayesian evidence supporting the absence of transfer. Psychol Aging. 2017;32(8):732-746.
13. Taki Y, Kinomura S, Sato K, et al. Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn. 2011;75(2):170-176.
14. Cassidy AR, White MT, DeMaso DR, et al. Processing speed, executive function, and academic achievement in children with dextro-transposition of the great arteries: Testing a longitudinal developmental cascade model. Neuropsychology. 2016;30(7):874-885.
15. Aichele S, Rabbitt P, Ghisletta P. Life span decrements in fluid intelligence and processing speed predict mortality risk. Psychol Aging. 2015;30(3):598-612.
16. Eich TS, Castel AD. The cognitive control of emotional versus value-based information in younger and older adults. Psychol Aging. 2016;31(5):503-512.
17. Rolle CE, Anguera JA, Skinner SN, et al. Enhancing spatial attention and working memory in younger and older adults. J Cogn Neurosci. 2017;29(9):1483-1497.
18. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58(5):889-894.
19. Madore KP, Schacter DL. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychol Aging. 2014;29(4):913-924.
20. Matthews BR. Memory dysfunction. Continuum (Minneap Minn). 2015;21(3 Behavioral Neurology and Neuropsychiatry):613-626.
21. Mather M. The emotion paradox in the aging brain. Ann N Y Acad Sci. 2012;1251(1):33-49.
22. Gurera JW, Isaacowitz DM. Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. Prog Brain Res. 2019;247:329-351.
23. Cai L, Chan JS, Yan JH, et al. Brain plasticity and motor practice in cognitive aging. Front Aging Neurosci. 2014;6:31.
24. Cassetta BD, Tomfohr-Madsen LM, Goghari VM. A randomized controlled trial of working memory and processing speed training in schizophrenia. Psychol Med. 2019;49(12):2009-2019.
25. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271-2281.
26. Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16-24.
27. Edwards JD, Delahunt PB, Mahncke HW. Cognitive speed of processing training delays driving cessation. J Gerontol A Biol Sci Med Sci. 2009;64(12):1262-1267.
28. Ball K, Edwards JD, Ross LA, et al. Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107-2113.
29. Wolinsky FD, Unverzagt FW, Smith DM, et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):S281-S287.
30. Wolinsky FD, Unverzagt FW, Smith DM, et al. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61(12):1324-1329.
31. Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64(4):468-472.
32. Wolinsky FD, Vander Weg MW, Martin R, et al. Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci. 2010;65(5):591-598.
33. Wolinsky FD, Mahncke HW, Kosinski M, et al. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv Res. 2009;9:109.
34. Edwards JD, Xu H, Clark DO, et al. Speed of processing training results in lower risk of dementia. Alzheimers Dement (N Y). 2017;3(4):603-611.
35. Harvey PD, Tibiriçá L, Kallestrup P, et al. A computerized functional skills assessment and training program targeting technology based everyday functional skills. J Vis Exp. 2020;156:e60330. doi: 10.3791/60330.
1. Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neuro. 2019;21(3):227-237.
2. Czaja SJ, Loewenstein DA, Sabbag SA, et al. A novel method for direct assessment of everyday competence among older adults. J Alzheimers Dis. 2017;57(4):1229-1238.
3. Czaja SJ, Loewenstein DA, Lee CC, et al. Assessing functional performance using computer-based simulations of everyday activities. Schizophr Res. 2017;183:130-136.
4. Tsai HS, Shillair R, Cotten SR. Social support and “playing around”: an examination of how older adults acquire digital literacy with tablet computers. J Appl Gerontol. 2017;36(1):29-55.
5. Cabrita M, Tabak M, Vollenbroek-Hutten MM. Older adults’ attitudes toward ambulatory technology to support monitoring and coaching of healthy behaviors: qualitative study. JMIR Aging. 2019;2(1):e10476. doi: 10.2196/10476.
6. Lim KY, Chang KJ, Kim HJ, et al. P.5.a.010 association between memory age identity and cognition in the elderly. Eur Neuropsychopharmacol. 2010;20(suppl 3):S555.
7. Moraes C, Pinto JA Jr, Lopes MA, et al. Impact of sociodemographic and health variables on mini-mental state examination in a community-based sample of older people. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):535-542.
8. Freitas S, Simões MR, Alves L, et al. The relevance of sociodemographic and health variables on MMSE normative data. Appl Neuropsychol Adult. 2015;22(4):311-319.
9. Han C, Jo SA, Jo I, et al. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008;47(3):302-310.
10. Yin S, Zhu X, Li R, et al. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci Rep. 2014;4:7309.
11. Bender AR, Prindle JJ, Brandmaier AM, et al. White matter and memory in healthy adults: coupled changes over two years. Neuroimage. 2016;131:193-204.
12. Guye S, von Bastian CC. Working memory training in older adults: Bayesian evidence supporting the absence of transfer. Psychol Aging. 2017;32(8):732-746.
13. Taki Y, Kinomura S, Sato K, et al. Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn. 2011;75(2):170-176.
14. Cassidy AR, White MT, DeMaso DR, et al. Processing speed, executive function, and academic achievement in children with dextro-transposition of the great arteries: Testing a longitudinal developmental cascade model. Neuropsychology. 2016;30(7):874-885.
15. Aichele S, Rabbitt P, Ghisletta P. Life span decrements in fluid intelligence and processing speed predict mortality risk. Psychol Aging. 2015;30(3):598-612.
16. Eich TS, Castel AD. The cognitive control of emotional versus value-based information in younger and older adults. Psychol Aging. 2016;31(5):503-512.
17. Rolle CE, Anguera JA, Skinner SN, et al. Enhancing spatial attention and working memory in younger and older adults. J Cogn Neurosci. 2017;29(9):1483-1497.
18. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58(5):889-894.
19. Madore KP, Schacter DL. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychol Aging. 2014;29(4):913-924.
20. Matthews BR. Memory dysfunction. Continuum (Minneap Minn). 2015;21(3 Behavioral Neurology and Neuropsychiatry):613-626.
21. Mather M. The emotion paradox in the aging brain. Ann N Y Acad Sci. 2012;1251(1):33-49.
22. Gurera JW, Isaacowitz DM. Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. Prog Brain Res. 2019;247:329-351.
23. Cai L, Chan JS, Yan JH, et al. Brain plasticity and motor practice in cognitive aging. Front Aging Neurosci. 2014;6:31.
24. Cassetta BD, Tomfohr-Madsen LM, Goghari VM. A randomized controlled trial of working memory and processing speed training in schizophrenia. Psychol Med. 2019;49(12):2009-2019.
25. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271-2281.
26. Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16-24.
27. Edwards JD, Delahunt PB, Mahncke HW. Cognitive speed of processing training delays driving cessation. J Gerontol A Biol Sci Med Sci. 2009;64(12):1262-1267.
28. Ball K, Edwards JD, Ross LA, et al. Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107-2113.
29. Wolinsky FD, Unverzagt FW, Smith DM, et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):S281-S287.
30. Wolinsky FD, Unverzagt FW, Smith DM, et al. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61(12):1324-1329.
31. Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64(4):468-472.
32. Wolinsky FD, Vander Weg MW, Martin R, et al. Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci. 2010;65(5):591-598.
33. Wolinsky FD, Mahncke HW, Kosinski M, et al. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv Res. 2009;9:109.
34. Edwards JD, Xu H, Clark DO, et al. Speed of processing training results in lower risk of dementia. Alzheimers Dement (N Y). 2017;3(4):603-611.
35. Harvey PD, Tibiriçá L, Kallestrup P, et al. A computerized functional skills assessment and training program targeting technology based everyday functional skills. J Vis Exp. 2020;156:e60330. doi: 10.3791/60330.
COVID-19 and patients with serious mental illness
“This whole thing is not about heroism. It’s about decency. It may seem a ridiculous idea, but the only way to fight the plague is with decency . ”
– Albert Camus, La Peste (1947)1
Severe acute respiratory syndrome (SARS), H1N1 swine flu, Ebola, Zika, and Middle East respiratory syndrome (MERS): the 21st century has already been witness to several serious infectious outbreaks and pandemics,2 but none has been as deadly and consequential as the current one. The ongoing SARS-coronavirus-2 (SARS-CoV-2) pandemic is shaping not only current psychiatric care but the future of psychiatry. Now that we are beyond the initial stages of the coronavirus disease 2019 (COVID-19) pandemic, when psychiatrists had a crash course in disaster psychiatry, our attention must shift to rebuilding and managing disillusionment and other psychological fallout of the intense early days.3
In this article, we offer guidance to psychiatrists caring for patients with serious mental illness (SMI) during the SARS-CoV-2 pandemic. Patients with SMI are easily forgotten as other issues (eg, preserving ICU capacity) overshadow the already historically neglected needs of this impoverished group.4 From both human and public-health perspectives, this inattention is a mistake. Assuring psychiatric stability is critically important to prevent the spread of COVID-19 in marginalized communities comprised of individuals who are poor, members of racial minorities, and others who already experience health disparities.5 Without controlling transmission in these groups, the pandemic will not be sufficiently contained.
We begin by highlighting general principles of pandemic management because caring for patients with SMI does not occur in a vacuum. Infectious outbreaks require not only helping those who need direct medical care because they are infected, but also managing populations that are at risk of getting infected, including health care and other essential workers.
Principles of pandemic management
Delivery of medical care during a pandemic differs from routine care. An effective disaster response requires collaboration and coordination among public-health, treatment, and emergency systems. Many institutions shift to an incident management system and crisis leadership, with clear lines of authority to coordinate responders and build medical surge capacity. Such a top-down leadership approach must plan and allow for the emergence of other credible leaders and for the restoration of people’s agency.
Unfortunately, adaptive capacity may be limited, especially in the public sector and psychiatric care system, where resources are already poor. Particularly early in a pandemic, services considered non-essential—which includes most psychiatric outpatient care—can become unavailable. A major effort is needed to prevent the psychiatric care system from contracting further, as happened during 9/11.6 Additionally, “essential” cannot be conflated with “emergent,” as can easily occur in extreme circumstances. Early and sustained efforts are required to ensure that patients with SMI who may be teetering on the edge of emergency status do not slip off that edge, especially when the emergency medical system is operating over capacity.
A comprehensive outbreak response must consider that a pandemic is not only a medical crisis but a mental health crisis and a communication emergency.7 Mental health clinicians need to provide accurate information and help patients cope with their fears.
Continue to: Psychological aspects of pandemics
Psychological aspects of pandemics. Previous infectious outbreaks have reaffirmed that mental health plays an outsized role during epidemics. Chaos, uncertainty, fear of death, and loss of income and housing cause prolonged stress and exact a psychological toll.
Adverse psychological impacts include expectable, normal reactions such as stress-induced anxiety or insomnia. In addition, new-onset psychiatric illnesses or exacerbations of existing ones may emerge.8 As disillusionment and demoralization appear in the wake of the acute phase, with persistently high unemployment, suicide prevention becomes an important goal.9
Pandemics lead to expectable behavioral responses (eg, increases in substance use and interpersonal conflict). Fear-based decisions may result in unhelpful behavior, such as hoarding medications (which may result in shortages) or dangerous, unsupervised use of unproven medications (eg, hydroxychloroquine). Trust is needed to accept public-health measures, and recommendations (eg, wearing masks) must be culturally informed to be credible and effective.
Because people are affected differently, at individual, cultural, and socioeconomic levels, they will view the situation differently. For many people, secondary stressors (eg, job loss) may be more disastrous than the primary medical event (ie, the pandemic). This distinction is critical because concrete financial help, not psychiatric care, is needed. Sometimes, even when a psychiatric disorder such as SMI or major neurocognitive disorder is present, the illusion of an acute decompensation can be created by the loss of social and structural supports that previously scaffolded a person’s life.
Mental illness prevention. Community mental-health surveillance is important to monitor for distress, psychiatric symptoms, health-risk behaviors, risk and safety perception, and preparedness. Clinicians must be ready to normalize expectable and temporary distress, while recognizing when that distress becomes pathological. This may be difficult in patients with SMI who often already have reduced stress tolerance or problem-based coping skills.10
Continue to: Psychological first aid...
Psychological first aid (PFA) is a standard intervention recommended by the World Health Organization for most individuals following a disaster; it is evidence-informed and has face validity.11 Intended to relieve distress by creating an environment that is safe, calm, and connected, PFA fosters self-efficacy and hope. While PFA is a form of universal prevention, it is not designed for patients with SMI, is not a psychiatric intervention, and is not provided by clinicians. Its principles, however, can easily be applied to patients with SMI to prevent distressing symptoms from becoming a relapse.
Communication. Good risk and crisis communication are critical because individual and population behavior will be governed by the perception of risk and fear, and not by facts. Failure to manage the “infodemic”7—with its misinformation, contradictory messages, and rumors—jeopardizes infection control if patients become paralyzed by uncertainty and fear. Scapegoating occurs easily during times of threat, and society must contain the parallel epidemic of xenophobia based on stigma and misinformation.12
Decision-making under uncertainty is not perfect and subject to revision as better information becomes available. Pointing this out to the public is delicate but essential to curtail skepticism and mistrust when policies are adjusted in response to new circumstances and knowledge.
Mistrust of an authority’s legitimacy and fear-based decisions lead to lack of cooperation with public-health measures, which can undermine an effective response to the pandemic. Travel restrictions or quarantine measures will not be followed if individuals question their importance. Like the general public, patients need education and clear communication to address their fear of contagion, dangers posed to family (and pets), and mistrust of authority and government. A lack of appreciation of the seriousness of the pandemic and individual responsibility may need to be addressed. Two important measures to accomplish this are steering patients to reputable sources of information and advising that they limit media exposure.
Resilience-building. Community and workplace resilience are important aspects of making it through a disaster as best as possible. Resilience is not innate and fixed; it must be deliberately built.13 Choosing an attitude of post-traumatic growth over the victim narrative is a helpful stance. Practicing self-care (rest, nutrition, exercise) and self-compassion (self-kindness, common humanity, mindfulness) is good advice for patients and caregivers alike.
Continue to: Workforce protection
Workforce protection. Compared to other disasters, infectious outbreaks disproportionally affect the medical community, and care delivery is at stake. While psychological and psychiatric needs may increase during a pandemic, services often contract, day programs and clinics close, teams are reduced to skeleton crews, and only emergency psychiatric care is available. Workforce protection is critical to avoid illness or simple absenteeism due to mistrust of protective measures.
Only a well-briefed, well-led, well-supported, and adequately resourced workforce is going to be effective in managing this public-health emergency. Burnout and moral injury are feared long-term consequences for health care workers that need to be proactively addressed.14 As opposed to other forms of disasters, managing your own fears about safety is important. Clinicians and their patients sit in the proverbial same boat.
Ethics. The anticipated need to ration life-saving care (eg, ventilators) has been at the forefront of ethical concerns.15 In psychiatry, the question of involuntary public-health interventions for uncooperative psychiatric patients sits uncomfortably between public-health ethics and human rights, and is an opportunity for collaboration with public-health and infectious-disease colleagues.
Redeployed clinicians and those working under substandard conditions may be concerned about civil liability due to a modified standard of care during a crisis. Some clinicians may ask if their duty to care must override their natural instinct to protect themselves. There is a lot of room for resentment in these circumstances. Redeployed or otherwise “conscripted” clinicians may resent administrators, especially those administering from the safety of their homes. Those “left behind” to work in potentially precarious circumstances may resent their absent colleagues. Moreover, these front-line clinicians may have been forced to make ethical decisions for which they were not prepared.16 Maintaining morale is far from trivial, not just during the pandemic, but afterward, when (and if) the entire workforce is reunited. All parties need to be mindful of how their actions and decisions impact and are perceived by others, both in the hospital and at home.
Managing patients with SMI during COVID-19
Patients with SMI are potentially hard hit by COVID-19 due to a “tragic” epidemiologic triad of agent-host-environment: SARS-CoV-2 is a highly infectious agent affecting patients with SMI who are vulnerable hosts in permissive environments (Figure).
Continue to: While not as infectious as measles...
While not as infectious as measles, COVID-19 is more infectious than the seasonal flu virus.17 It can lead to uncontrolled infection within a short period of time, particularly in enclosed settings. Outbreaks have occurred readily on cruise ships and aircraft carriers as well as in nursing homes, homeless shelters, prisons, and group homes.
Patients with SMI are vulnerable hosts because they have many of the medical risk factors18 that portend a poor prognosis if they become infected, including pre-existing lung conditions and heart disease19 as well as diabetes and obesity.20 Obesity likely creates a hyperinflammatory state and a decrease in vital capacity. Patient-related behavioral factors include poor early-symptom reporting and ineffective infection control.
Unfavorable social determinants of health include not only poverty but crowded housing that is a perfect incubator for COVID-19.
Priority treatment goals. The overarching goal during a pandemic is to keep patients with SMI in psychiatric treatment and prevent them from disengaging from care in the service of infection control. Urgent tasks include infection control, relapse prevention, and preventing treatment disengagement and loneliness.
Infection control. As trusted sources of information, psychiatrists can play an important role in infection control in several important ways:
- educating patients about infection-control measures and public-health recommendations
- helping patients understand what testing can accomplish and when to pursue it
- encouraging protective health behaviors (eg, hand washing, mask wearing, physical distancing)
- assessing patients’ risk appreciation
- assessing for and addressing obstacles to implementing and complying with infection-control measures
- explaining contact tracing
- providing reassurance.
Continue to: Materials and explanations...
Materials and explanations must be adapted for patient understanding.
Patients with disorganization or cognitive disturbances may have difficulties cooperating or problem-solving. Patients with negative symptoms may be inappropriately unconcerned and also inaccurately report symptoms that suggest COVID-19. Acute psychosis or mania can prevent patients from complying with public-health efforts. Some measures may be difficult to implement if the means are simply not there (eg, physical distancing in a crowded apartment). Previously open settings (eg, group homes) have had to develop new mechanisms under the primacy of infection control. Inpatient units—traditionally places where community, shared healing, and group therapy are prized—have had to decrease maximum occupancy, limit the number of patients attending groups, and discourage or outrightly prohibit social interaction (eg, dining together).
Relapse prevention. Patients who take maintenance medications need to be supported. A manic or psychotic relapse during a pandemic puts patients at risk of acquiring and spreading COVID-19. “Treatment as prevention” is a slogan from human immunodeficiency virus (HIV) care that captures the importance of antiretroviral treatment to prevent medical complications from HIV, and also to reduce infecting other people. By analogy, psychiatric treatment for patients with SMI can prevent psychiatric instability and thereby control viral transmission. Avoiding sending psychiatric patients to a potentially stressed acute-care system is important.
Psychosocial support. Clinics need to ensure that patients continue to engage in care beyond medication-taking to proactively prevent psychiatric exacerbations. Healthful, resilience-building behaviors should be encouraged while monitoring and counseling against maladaptive ones (eg, increased substance use). Supporting patients emotionally and helping them solve problems are critical, particularly for those who are subjected to quarantine or isolation. Obviously, in these latter situations, outreach will be necessary and may require creative delivery systems and dedicated clinicians for patients who lack access to the technology necessary for virtual visits. Havens and Ghaemi21 have suggested that a good therapeutic alliance can be viewed as a mood stabilizer. Helping patients grieve losses (loved ones, jobs, sense of safety) may be an important part of support.
Even before COVID-19, loneliness was a major factor for patients with schizophrenia.22 A psychiatric clinic is one aspect of a person with SMI’s social network; during the initial phase of the pandemic, many clinics and treatment programs closed. Patients for whom clinics structure and anchor their activities are at high risk of disconnecting from treatment, staying at home, and becoming lonely.
Continue to: Caregivers are always important...
Caregivers are always important to SMI patients, but they may assume an even bigger role during this pandemic. Some patients may have moved in with a relative, after years of living on their own. In other cases, stable caregiver relationships may be disrupted due to COVID-19–related sickness in the caregiver; if not addressed, this can result in a patient’s clinical decompensation. Clinicians should take the opportunity to understand who a patient’s caregivers are (group home staff, families) and rekindle clinical contact with them. Relationships with caregivers that may have been on “autopilot” during normal times are opportunities for welcome support and guidance, to the benefit of both patients and caregivers.
Table 1 summarizes clinical tasks that need to be kept in mind when conducting clinic visits during COVID-19 in order to achieve the high-priority treatment goals of infection control, relapse prevention, and psychosocial support.
Differential diagnosis. Neuropsychiatric syndromes have long been observed in influenza pandemics,23 due both to direct viral effects and to the effects of critical illness on the brain. Two core symptoms of COVID-19—anosmia and ageusia—suggest that COVID-19 can directly affect the brain. While neurologic manifestations are common,24 it remains unclear to what extent COVID-19 can directly “cause” psychiatric symptoms, or if such symptoms are the result of cytokines25 or other medical processes (eg, thromboembolism).26 Psychosis due to COVID-19 may, in some cases, represent a stress-related brief psychotic disorder.27
Hospitalized patients who have recovered from COVID-19 may have experienced prolonged sedation and severe delirium in an ICU.28 Complications such as posttraumatic stress disorder,29 hypoperfusion-related brain injuries, or other long-term cognitive difficulties may result. In previous flu epidemics, patients developed serious neurologic complications such as post-encephalitic Parkinson’s disease.30
Any person subjected to isolation or quarantine is at risk for psychiatric complications.31 Patients with SMI who live in group homes may be particularly susceptible to new rules, including no-visitor policies.
Continue to: Outpatients whose primary disorder...
Outpatients whose primary disorder is well controlled may, like anyone else, struggle with the effects of the pandemic. It is necessary to carefully differentiate non-specific symptoms associated with stress from the emergence of a new disorder resulting from stress.32 For some patients, grief or adjustment disorders should be considered. Prolonged stress and uncertainty may eventually lead to an exacerbation of a primary disorder, particularly if the situation (eg, financial loss) does not improve or worsens. Demoralization and suicidal thinking need to be monitored. Relapse or increased use of alcohol or other substances as a response to stress may also complicate the clinical picture.33 Last, smoking cessation as a major treatment goal in general should be re-emphasized and not ignored during the ongoing pandemic.34
Table 2 summarizes psychiatric symptoms that need to be considered when managing a patient with SMI during this pandemic.
Treatment tools
Psychopharmacology. Even though crisis-mode prescribing may be necessary, the safe use of psychotropics remains the goal of psychiatric prescribing. Access to medications becomes a larger consideration; for many patients, a 90-day supply may be indicated. Review of polypharmacy, including for pneumonia risk, should be undertaken. Preventing drooling (eg, from sedation, clozapine, extrapyramidal symptoms [EPS]) will decrease aspiration risk.
In general, treatment of psychiatric symptoms in a patient with COVID-19 follows usual guidelines. The best treatment for COVID-19 patients with delirium, however, remains to be established, particularly how to manage severe agitation.28 Pharmacodynamic and pharmacokinetic drug–drug interactions between psychotropics and antiviral treatments for COVID-19 (eg, QTc prolongation) can be expected and need to be reviewed.35 For stress-related anxiety, judicious pharmacotherapy can be helpful. Diazepam given at the earliest signs of a psychotic relapse may stave off a relapse for patients with schizophrenia.36 Even if permitted under relaxed prescribing rules during a public-health emergency, prescribing controlled substances without seeing patients in person requires additional thought. In some cases, adjusting the primary medication to buffer against stress may be preferred (eg, adjusting an antipsychotic in a patient on maintenance treatment for schizophrenia, particularly if a low-dose strategy is pursued).
Clozapine requires registry-based prescribing and bloodwork (“no blood, no drug”). The use of clozapine during this public-health emergency has been made easier because of FDA guidance that allows clozapine to be dispensed without blood work if obtaining blood work is not possible (eg, a patient is quarantined) or can be accomplished only at substantial risk to patients and the population at large. Under certain conditions, clozapine can be dispensed safely and in a way that is consistent with infection prevention. Clozapine-treated patients admitted with COVID-19 should be monitored for clozapine toxicity and the clozapine dose adjusted.37 A consensus statement consistent with the FDA and clinical considerations for using clozapine during COVID-19 is summarized in Table 3.38
Continue to: Long-acting injectable antipsychotics...
Long-acting injectable antipsychotics (LAIs) pose a problem because they require in-person visits. Ideally, during a pandemic, patients should be seen in person as frequently as medically necessary but as infrequently as possible to limit exposure of both patients and staff. Table 4 provides some clinical recommendations on how to use LAIs during the pandemic.39
Supportive psychotherapy may be the most important tool we have in helping patients with loss and uncertainty during these challenging months.40 Simply staying in contact with patients plays a major role in preventing care discontinuity. Even routine interactions have become stressful, with everyone wearing a mask that partially obscures the face. People with impaired hearing may find it even more difficult to understand you.
Education, problem-solving, and a directive, encouraging style are major tools of supportive psychotherapy to reduce symptoms and increase adaptive skills. Clarify that social distancing refers to physical, not emotional, distancing. The judicious and temporary use of anxiolytics is appropriate to reduce anxiety. Concrete help and problem-solving (eg, filling out forms) are examples of proactive crisis intervention.
Telepsychiatry emerged in the pandemic’s early days as the default mode of practice in order to limit in-person contacts.41 Like all new technology, telepsychiatry brings progress and peril.42 While it has gone surprisingly well for most, the “digital divide” does not afford all patients access to the needed technology. The long-term effectiveness and acceptance of telehealth remain to be seen. (Editor’s Note: For more about this topic, see “Telepsychiatry: What you need to know.”
Lessons learned and outlook
Infectious outbreaks have historically inflicted long-term disruptions on societies and altered the course of history. However, each disaster is unique, and lessons from previous disasters may only partially apply.43 We do not yet know how this one will end, including how long it will take for the world’s economies to recover. If nothing else, the current public-health emergency has brought to the forefront what psychiatrists have always known: health disparities are partially responsible for different disease risks (in this case, the risk of getting infected with SARS-CoV-2).5 It may not be a coincidence that the Black Lives Matter movement is becoming a major impetus for social change at a time when the pandemic is exposing health-care inequalities.
Continue to: Some areas of the country...
Some areas of the country succeeded in reducing infections and limiting community spread, which ushered in an uneasy sense of normalcy even while the pandemic continues. At least for now, these locales can focus on rebuilding and preparing for expectable fluctuations in disease activity, including the arrival of the annual flu season on top of COVID-19.44 Recovery is not a return to the status quo ante but building stronger communities—“building back better.”45 Unless there is a continuum of care, shortcomings in one sector will have ripple effects through the entire system, particularly for psychiatric care for patients with SMI, which was inadequate before the pandemic.
Ensuring access to critical care was a priority during the pandemic’s early phase but came at the price of deferring other types of care, such as routine primary care; the coming months will see the downstream consequences of this approach,46 including for patients with SMI.
In the meantime, doing our job as clinicians, as Camus’s fictitious Dr. Bernard Rieux from the epigraph responds when asked how to define decency, may be the best we can do in these times. This includes contributing to and molding our field’s future and fostering a sense of agency in our patients and in ourselves. Major goals will be to preserve lessons learned, maintain flexibility, and avoid a return to unhelpful overregulation and payment models that do not reflect the flexible, person-centered care so important for patients with SMI.47
Bottom Line
During a pandemic, patients with serious mental illness may be easily forgotten as other issues overshadow the needs of this impoverished group. During a pandemic, the priority treatment goals for these patients are infection control, relapse prevention, and preventing treatment disengagement and loneliness. A pandemic requires changes in how patients with serious mental illness will receive psychopharmacology and psychotherapy.
Related Resources
- Huremović D (ed). Psychiatry of pandemics: a mental health response to infection outbreak. Cham, Switzerland: Springer Nature Switzerland AG; 2019.
- Ursano RJ, Fullerton CS, Weisaeth L, et al (eds). Textbook of disaster psychiatry. 2nd ed. Cambridge, UK: Cambridge University Press; 2017.
- Centers for Disease Control and Prevention. Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- American Psychiatric Association. Coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus.
- SMI Adviser. Make informed decisions related to COVID-19 and mental health. https://smiadviser.org/about/covid.
Drug Brand Names
Clozapine • Clozaril
Diazepam • Valium
Hydroxychloroquine • Plaquenil
1. Camus A. La peste. Paris, France: Éditions Gallimard; 1947.
2. Huremovic
3. Substance Abuse and Mental Health Services Administration. Phases of disaster. https://www.samhsa.gov/dtac/recovering-disasters/phases-disaster. Updated June 17, 2020. Accessed August 7, 2020.
4. Geller J. COVID-19 and advocacy—the good and the unacceptable. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5b13. Published May 7, 2020. Accessed August 7, 2020.
5. Webb Hooper M, Nápoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467.
6. Sederer LI, Lanzara CB, Essock SM, et al. Lessons learned from the New York State mental health response to the September 11, 2001, attacks. Psychiatr Serv. 2011;62(9):1085-1089.
7. World Health Organization. Infodemic management – infodemiology. https://www.who.int/teams/risk-communication/infodemic-management. Accessed August 7, 2020.
8. Zhou J, Liu L, Xue P, et al. Mental health response to the COVID-19 outbreak in China. Am J Psychiatry. 2020;117(7):574-575.
9. Kawohl W, Nordt C. COVID-19, unemployment, and suicide. Lancet Psychiatry. 2020;7(5):389-390.
10. Yao H, Chen JH, Xu YF. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4):e21. doi: 10.1016/S2215-0366(20)30090-0.
11. Minihan E, Gavin B, Kelly BD, et al. Covid-19, mental health and psychological first aid. Ir J Psychol Med. 2020:1-12.
12. Adja KYC, Golinelli D, Lenzi J, et al. Pandemics and social stigma: who’s next? Italy’s experience with COVID-19. Public Health. 2020;185:39-41.
13. Rosenberg AR. Cultivating deliberate resilience during the coronavirus disease 2019 pandemic [published online April 14, 2020]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2020.1436.
14. Dean W, Talbot SG, Caplan A. Clarifying the language of clinician distress [published online January 31, 2020]. JAMA. doi: 10.1001/jama.2019.21576.
15. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055.
16. Rosenbaum L. Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875.
17. Viceconte G, Petrosillo N. COVID-19 R0: magic number or conundrum? Infect Dis Rep. 2020;12(1):8516.
18. de Hert M, Schreurs V, Vancampfort D, van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
19. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97-105.
20. Finer N, Garnett SP, Bruun JM. COVID-19 and obesity. Clin Obes. 2020;10(3):e12365. doi: 10.1111/cob.12365.
21. Havens LL, Ghaemi SN. Existential despair and bipolar disorder: the therapeutic alliance as a mood stabilizer. Am J Psychother. 2005;59(2):137-147.
22. Trémeau F, Antonius D, Malaspina D, et al. Loneliness in schizophrenia and its possible correlates. An exploratory study. Psychiatry Res. 2016;246:211-217.
23. Menninger KA. Psychoses associated with influenza: I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
24. Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832.
25. Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.012.
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
27. Martin Jr. EB. Brief psychotic disorder triggered by fear of coronavirus? Psychiatric Times. https://www.psychiatrictimes.com/view/brief-psychotic-disorder-triggered-fear-coronavirus-small-case-series. Published May 8, 2020. Accessed August 7, 2020.
28. Sher Y, Rabkin B, Maldonado JR, et al. COVID-19-associated hyperactive intensive care unit delirium with proposed pathophysiology and treatment: a case report [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.007.
29. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813.
30. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6(3):114-124.
31. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
32. Maercker A, Brewin CR, Bryant RA, et al. Diagnosis and classification of disorders specifically associated with stress: proposals for ICD-11. World Psychiatry. 2013;12(3):198-206.
33. Ornell F, Moura HF, Scherer JN, et al. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi: 10.1016/j.psychres.2020.113096.
34. Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking [published online April 3, 2020]. Nicotine Tob Res. https://doi.org/10.1093/ntr/ntaa059.
35. Back D, Marzolini C, Hodge C, et al. COVID-19 treatment in patients with comorbidities: awareness of drug-drug interactions [published online May 8, 2020]. Br J Clin Pharmacol. doi: 10.1111/bcp.14358.
36. Carpenter WT Jr., Buchanan RW, Kirkpatrick B, et al. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156(2):299-303.
37. Dotson S, Hartvigsen N, Wesner T, et al. Clozapine toxicity in the setting of COVID-19 [published online May 30, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.025.
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223.
39. Schnitzer K, MacLaurin S, Freudenreich O. Long-acting injectable antipsychotics during the COVID-19 pandemic. Current Psychiatry. In press.
40. Winston A, Rosenthal RN, Pinsker H. Learning supportive psychotherapy: an illustrated guide. Washington, DC: American Psychiatric Publishing; 2012.
41. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
42. Jordan A, Dixon LB. Considerations for telepsychiatry service implementation in the era of COVID-19. Psychiatr Serv. 2020;71(6):643-644.
43. DePierro J, Lowe S, Katz C. Lessons learned from 9/11: mental health perspectives on the COVID-19 pandemic. Psychiatry Res. 2020;288:113024.
44. Hussain S. Immunization and vaccination. In: Huremovic
45. Epping-Jordan JE, van Ommeren M, Ashour HN, et al. Beyond the crisis: building back better mental health care in 10 emergency-affected areas using a longer-term perspective. Int J Ment Health Syst. 2015;9:15.
46. Rosenbaum L. The untold toll - the pandemic’s effects on patients without Covid-19. N Engl J Med. 2020;382(24):2368-2371.
47. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness [published online June 3, 2020]. Psychiatr Serv. doi: 10.1176/appi.ps.202000244.
“This whole thing is not about heroism. It’s about decency. It may seem a ridiculous idea, but the only way to fight the plague is with decency . ”
– Albert Camus, La Peste (1947)1
Severe acute respiratory syndrome (SARS), H1N1 swine flu, Ebola, Zika, and Middle East respiratory syndrome (MERS): the 21st century has already been witness to several serious infectious outbreaks and pandemics,2 but none has been as deadly and consequential as the current one. The ongoing SARS-coronavirus-2 (SARS-CoV-2) pandemic is shaping not only current psychiatric care but the future of psychiatry. Now that we are beyond the initial stages of the coronavirus disease 2019 (COVID-19) pandemic, when psychiatrists had a crash course in disaster psychiatry, our attention must shift to rebuilding and managing disillusionment and other psychological fallout of the intense early days.3
In this article, we offer guidance to psychiatrists caring for patients with serious mental illness (SMI) during the SARS-CoV-2 pandemic. Patients with SMI are easily forgotten as other issues (eg, preserving ICU capacity) overshadow the already historically neglected needs of this impoverished group.4 From both human and public-health perspectives, this inattention is a mistake. Assuring psychiatric stability is critically important to prevent the spread of COVID-19 in marginalized communities comprised of individuals who are poor, members of racial minorities, and others who already experience health disparities.5 Without controlling transmission in these groups, the pandemic will not be sufficiently contained.
We begin by highlighting general principles of pandemic management because caring for patients with SMI does not occur in a vacuum. Infectious outbreaks require not only helping those who need direct medical care because they are infected, but also managing populations that are at risk of getting infected, including health care and other essential workers.
Principles of pandemic management
Delivery of medical care during a pandemic differs from routine care. An effective disaster response requires collaboration and coordination among public-health, treatment, and emergency systems. Many institutions shift to an incident management system and crisis leadership, with clear lines of authority to coordinate responders and build medical surge capacity. Such a top-down leadership approach must plan and allow for the emergence of other credible leaders and for the restoration of people’s agency.
Unfortunately, adaptive capacity may be limited, especially in the public sector and psychiatric care system, where resources are already poor. Particularly early in a pandemic, services considered non-essential—which includes most psychiatric outpatient care—can become unavailable. A major effort is needed to prevent the psychiatric care system from contracting further, as happened during 9/11.6 Additionally, “essential” cannot be conflated with “emergent,” as can easily occur in extreme circumstances. Early and sustained efforts are required to ensure that patients with SMI who may be teetering on the edge of emergency status do not slip off that edge, especially when the emergency medical system is operating over capacity.
A comprehensive outbreak response must consider that a pandemic is not only a medical crisis but a mental health crisis and a communication emergency.7 Mental health clinicians need to provide accurate information and help patients cope with their fears.
Continue to: Psychological aspects of pandemics
Psychological aspects of pandemics. Previous infectious outbreaks have reaffirmed that mental health plays an outsized role during epidemics. Chaos, uncertainty, fear of death, and loss of income and housing cause prolonged stress and exact a psychological toll.
Adverse psychological impacts include expectable, normal reactions such as stress-induced anxiety or insomnia. In addition, new-onset psychiatric illnesses or exacerbations of existing ones may emerge.8 As disillusionment and demoralization appear in the wake of the acute phase, with persistently high unemployment, suicide prevention becomes an important goal.9
Pandemics lead to expectable behavioral responses (eg, increases in substance use and interpersonal conflict). Fear-based decisions may result in unhelpful behavior, such as hoarding medications (which may result in shortages) or dangerous, unsupervised use of unproven medications (eg, hydroxychloroquine). Trust is needed to accept public-health measures, and recommendations (eg, wearing masks) must be culturally informed to be credible and effective.
Because people are affected differently, at individual, cultural, and socioeconomic levels, they will view the situation differently. For many people, secondary stressors (eg, job loss) may be more disastrous than the primary medical event (ie, the pandemic). This distinction is critical because concrete financial help, not psychiatric care, is needed. Sometimes, even when a psychiatric disorder such as SMI or major neurocognitive disorder is present, the illusion of an acute decompensation can be created by the loss of social and structural supports that previously scaffolded a person’s life.
Mental illness prevention. Community mental-health surveillance is important to monitor for distress, psychiatric symptoms, health-risk behaviors, risk and safety perception, and preparedness. Clinicians must be ready to normalize expectable and temporary distress, while recognizing when that distress becomes pathological. This may be difficult in patients with SMI who often already have reduced stress tolerance or problem-based coping skills.10
Continue to: Psychological first aid...
Psychological first aid (PFA) is a standard intervention recommended by the World Health Organization for most individuals following a disaster; it is evidence-informed and has face validity.11 Intended to relieve distress by creating an environment that is safe, calm, and connected, PFA fosters self-efficacy and hope. While PFA is a form of universal prevention, it is not designed for patients with SMI, is not a psychiatric intervention, and is not provided by clinicians. Its principles, however, can easily be applied to patients with SMI to prevent distressing symptoms from becoming a relapse.
Communication. Good risk and crisis communication are critical because individual and population behavior will be governed by the perception of risk and fear, and not by facts. Failure to manage the “infodemic”7—with its misinformation, contradictory messages, and rumors—jeopardizes infection control if patients become paralyzed by uncertainty and fear. Scapegoating occurs easily during times of threat, and society must contain the parallel epidemic of xenophobia based on stigma and misinformation.12
Decision-making under uncertainty is not perfect and subject to revision as better information becomes available. Pointing this out to the public is delicate but essential to curtail skepticism and mistrust when policies are adjusted in response to new circumstances and knowledge.
Mistrust of an authority’s legitimacy and fear-based decisions lead to lack of cooperation with public-health measures, which can undermine an effective response to the pandemic. Travel restrictions or quarantine measures will not be followed if individuals question their importance. Like the general public, patients need education and clear communication to address their fear of contagion, dangers posed to family (and pets), and mistrust of authority and government. A lack of appreciation of the seriousness of the pandemic and individual responsibility may need to be addressed. Two important measures to accomplish this are steering patients to reputable sources of information and advising that they limit media exposure.
Resilience-building. Community and workplace resilience are important aspects of making it through a disaster as best as possible. Resilience is not innate and fixed; it must be deliberately built.13 Choosing an attitude of post-traumatic growth over the victim narrative is a helpful stance. Practicing self-care (rest, nutrition, exercise) and self-compassion (self-kindness, common humanity, mindfulness) is good advice for patients and caregivers alike.
Continue to: Workforce protection
Workforce protection. Compared to other disasters, infectious outbreaks disproportionally affect the medical community, and care delivery is at stake. While psychological and psychiatric needs may increase during a pandemic, services often contract, day programs and clinics close, teams are reduced to skeleton crews, and only emergency psychiatric care is available. Workforce protection is critical to avoid illness or simple absenteeism due to mistrust of protective measures.
Only a well-briefed, well-led, well-supported, and adequately resourced workforce is going to be effective in managing this public-health emergency. Burnout and moral injury are feared long-term consequences for health care workers that need to be proactively addressed.14 As opposed to other forms of disasters, managing your own fears about safety is important. Clinicians and their patients sit in the proverbial same boat.
Ethics. The anticipated need to ration life-saving care (eg, ventilators) has been at the forefront of ethical concerns.15 In psychiatry, the question of involuntary public-health interventions for uncooperative psychiatric patients sits uncomfortably between public-health ethics and human rights, and is an opportunity for collaboration with public-health and infectious-disease colleagues.
Redeployed clinicians and those working under substandard conditions may be concerned about civil liability due to a modified standard of care during a crisis. Some clinicians may ask if their duty to care must override their natural instinct to protect themselves. There is a lot of room for resentment in these circumstances. Redeployed or otherwise “conscripted” clinicians may resent administrators, especially those administering from the safety of their homes. Those “left behind” to work in potentially precarious circumstances may resent their absent colleagues. Moreover, these front-line clinicians may have been forced to make ethical decisions for which they were not prepared.16 Maintaining morale is far from trivial, not just during the pandemic, but afterward, when (and if) the entire workforce is reunited. All parties need to be mindful of how their actions and decisions impact and are perceived by others, both in the hospital and at home.
Managing patients with SMI during COVID-19
Patients with SMI are potentially hard hit by COVID-19 due to a “tragic” epidemiologic triad of agent-host-environment: SARS-CoV-2 is a highly infectious agent affecting patients with SMI who are vulnerable hosts in permissive environments (Figure).
Continue to: While not as infectious as measles...
While not as infectious as measles, COVID-19 is more infectious than the seasonal flu virus.17 It can lead to uncontrolled infection within a short period of time, particularly in enclosed settings. Outbreaks have occurred readily on cruise ships and aircraft carriers as well as in nursing homes, homeless shelters, prisons, and group homes.
Patients with SMI are vulnerable hosts because they have many of the medical risk factors18 that portend a poor prognosis if they become infected, including pre-existing lung conditions and heart disease19 as well as diabetes and obesity.20 Obesity likely creates a hyperinflammatory state and a decrease in vital capacity. Patient-related behavioral factors include poor early-symptom reporting and ineffective infection control.
Unfavorable social determinants of health include not only poverty but crowded housing that is a perfect incubator for COVID-19.
Priority treatment goals. The overarching goal during a pandemic is to keep patients with SMI in psychiatric treatment and prevent them from disengaging from care in the service of infection control. Urgent tasks include infection control, relapse prevention, and preventing treatment disengagement and loneliness.
Infection control. As trusted sources of information, psychiatrists can play an important role in infection control in several important ways:
- educating patients about infection-control measures and public-health recommendations
- helping patients understand what testing can accomplish and when to pursue it
- encouraging protective health behaviors (eg, hand washing, mask wearing, physical distancing)
- assessing patients’ risk appreciation
- assessing for and addressing obstacles to implementing and complying with infection-control measures
- explaining contact tracing
- providing reassurance.
Continue to: Materials and explanations...
Materials and explanations must be adapted for patient understanding.
Patients with disorganization or cognitive disturbances may have difficulties cooperating or problem-solving. Patients with negative symptoms may be inappropriately unconcerned and also inaccurately report symptoms that suggest COVID-19. Acute psychosis or mania can prevent patients from complying with public-health efforts. Some measures may be difficult to implement if the means are simply not there (eg, physical distancing in a crowded apartment). Previously open settings (eg, group homes) have had to develop new mechanisms under the primacy of infection control. Inpatient units—traditionally places where community, shared healing, and group therapy are prized—have had to decrease maximum occupancy, limit the number of patients attending groups, and discourage or outrightly prohibit social interaction (eg, dining together).
Relapse prevention. Patients who take maintenance medications need to be supported. A manic or psychotic relapse during a pandemic puts patients at risk of acquiring and spreading COVID-19. “Treatment as prevention” is a slogan from human immunodeficiency virus (HIV) care that captures the importance of antiretroviral treatment to prevent medical complications from HIV, and also to reduce infecting other people. By analogy, psychiatric treatment for patients with SMI can prevent psychiatric instability and thereby control viral transmission. Avoiding sending psychiatric patients to a potentially stressed acute-care system is important.
Psychosocial support. Clinics need to ensure that patients continue to engage in care beyond medication-taking to proactively prevent psychiatric exacerbations. Healthful, resilience-building behaviors should be encouraged while monitoring and counseling against maladaptive ones (eg, increased substance use). Supporting patients emotionally and helping them solve problems are critical, particularly for those who are subjected to quarantine or isolation. Obviously, in these latter situations, outreach will be necessary and may require creative delivery systems and dedicated clinicians for patients who lack access to the technology necessary for virtual visits. Havens and Ghaemi21 have suggested that a good therapeutic alliance can be viewed as a mood stabilizer. Helping patients grieve losses (loved ones, jobs, sense of safety) may be an important part of support.
Even before COVID-19, loneliness was a major factor for patients with schizophrenia.22 A psychiatric clinic is one aspect of a person with SMI’s social network; during the initial phase of the pandemic, many clinics and treatment programs closed. Patients for whom clinics structure and anchor their activities are at high risk of disconnecting from treatment, staying at home, and becoming lonely.
Continue to: Caregivers are always important...
Caregivers are always important to SMI patients, but they may assume an even bigger role during this pandemic. Some patients may have moved in with a relative, after years of living on their own. In other cases, stable caregiver relationships may be disrupted due to COVID-19–related sickness in the caregiver; if not addressed, this can result in a patient’s clinical decompensation. Clinicians should take the opportunity to understand who a patient’s caregivers are (group home staff, families) and rekindle clinical contact with them. Relationships with caregivers that may have been on “autopilot” during normal times are opportunities for welcome support and guidance, to the benefit of both patients and caregivers.
Table 1 summarizes clinical tasks that need to be kept in mind when conducting clinic visits during COVID-19 in order to achieve the high-priority treatment goals of infection control, relapse prevention, and psychosocial support.
Differential diagnosis. Neuropsychiatric syndromes have long been observed in influenza pandemics,23 due both to direct viral effects and to the effects of critical illness on the brain. Two core symptoms of COVID-19—anosmia and ageusia—suggest that COVID-19 can directly affect the brain. While neurologic manifestations are common,24 it remains unclear to what extent COVID-19 can directly “cause” psychiatric symptoms, or if such symptoms are the result of cytokines25 or other medical processes (eg, thromboembolism).26 Psychosis due to COVID-19 may, in some cases, represent a stress-related brief psychotic disorder.27
Hospitalized patients who have recovered from COVID-19 may have experienced prolonged sedation and severe delirium in an ICU.28 Complications such as posttraumatic stress disorder,29 hypoperfusion-related brain injuries, or other long-term cognitive difficulties may result. In previous flu epidemics, patients developed serious neurologic complications such as post-encephalitic Parkinson’s disease.30
Any person subjected to isolation or quarantine is at risk for psychiatric complications.31 Patients with SMI who live in group homes may be particularly susceptible to new rules, including no-visitor policies.
Continue to: Outpatients whose primary disorder...
Outpatients whose primary disorder is well controlled may, like anyone else, struggle with the effects of the pandemic. It is necessary to carefully differentiate non-specific symptoms associated with stress from the emergence of a new disorder resulting from stress.32 For some patients, grief or adjustment disorders should be considered. Prolonged stress and uncertainty may eventually lead to an exacerbation of a primary disorder, particularly if the situation (eg, financial loss) does not improve or worsens. Demoralization and suicidal thinking need to be monitored. Relapse or increased use of alcohol or other substances as a response to stress may also complicate the clinical picture.33 Last, smoking cessation as a major treatment goal in general should be re-emphasized and not ignored during the ongoing pandemic.34
Table 2 summarizes psychiatric symptoms that need to be considered when managing a patient with SMI during this pandemic.
Treatment tools
Psychopharmacology. Even though crisis-mode prescribing may be necessary, the safe use of psychotropics remains the goal of psychiatric prescribing. Access to medications becomes a larger consideration; for many patients, a 90-day supply may be indicated. Review of polypharmacy, including for pneumonia risk, should be undertaken. Preventing drooling (eg, from sedation, clozapine, extrapyramidal symptoms [EPS]) will decrease aspiration risk.
In general, treatment of psychiatric symptoms in a patient with COVID-19 follows usual guidelines. The best treatment for COVID-19 patients with delirium, however, remains to be established, particularly how to manage severe agitation.28 Pharmacodynamic and pharmacokinetic drug–drug interactions between psychotropics and antiviral treatments for COVID-19 (eg, QTc prolongation) can be expected and need to be reviewed.35 For stress-related anxiety, judicious pharmacotherapy can be helpful. Diazepam given at the earliest signs of a psychotic relapse may stave off a relapse for patients with schizophrenia.36 Even if permitted under relaxed prescribing rules during a public-health emergency, prescribing controlled substances without seeing patients in person requires additional thought. In some cases, adjusting the primary medication to buffer against stress may be preferred (eg, adjusting an antipsychotic in a patient on maintenance treatment for schizophrenia, particularly if a low-dose strategy is pursued).
Clozapine requires registry-based prescribing and bloodwork (“no blood, no drug”). The use of clozapine during this public-health emergency has been made easier because of FDA guidance that allows clozapine to be dispensed without blood work if obtaining blood work is not possible (eg, a patient is quarantined) or can be accomplished only at substantial risk to patients and the population at large. Under certain conditions, clozapine can be dispensed safely and in a way that is consistent with infection prevention. Clozapine-treated patients admitted with COVID-19 should be monitored for clozapine toxicity and the clozapine dose adjusted.37 A consensus statement consistent with the FDA and clinical considerations for using clozapine during COVID-19 is summarized in Table 3.38
Continue to: Long-acting injectable antipsychotics...
Long-acting injectable antipsychotics (LAIs) pose a problem because they require in-person visits. Ideally, during a pandemic, patients should be seen in person as frequently as medically necessary but as infrequently as possible to limit exposure of both patients and staff. Table 4 provides some clinical recommendations on how to use LAIs during the pandemic.39
Supportive psychotherapy may be the most important tool we have in helping patients with loss and uncertainty during these challenging months.40 Simply staying in contact with patients plays a major role in preventing care discontinuity. Even routine interactions have become stressful, with everyone wearing a mask that partially obscures the face. People with impaired hearing may find it even more difficult to understand you.
Education, problem-solving, and a directive, encouraging style are major tools of supportive psychotherapy to reduce symptoms and increase adaptive skills. Clarify that social distancing refers to physical, not emotional, distancing. The judicious and temporary use of anxiolytics is appropriate to reduce anxiety. Concrete help and problem-solving (eg, filling out forms) are examples of proactive crisis intervention.
Telepsychiatry emerged in the pandemic’s early days as the default mode of practice in order to limit in-person contacts.41 Like all new technology, telepsychiatry brings progress and peril.42 While it has gone surprisingly well for most, the “digital divide” does not afford all patients access to the needed technology. The long-term effectiveness and acceptance of telehealth remain to be seen. (Editor’s Note: For more about this topic, see “Telepsychiatry: What you need to know.”
Lessons learned and outlook
Infectious outbreaks have historically inflicted long-term disruptions on societies and altered the course of history. However, each disaster is unique, and lessons from previous disasters may only partially apply.43 We do not yet know how this one will end, including how long it will take for the world’s economies to recover. If nothing else, the current public-health emergency has brought to the forefront what psychiatrists have always known: health disparities are partially responsible for different disease risks (in this case, the risk of getting infected with SARS-CoV-2).5 It may not be a coincidence that the Black Lives Matter movement is becoming a major impetus for social change at a time when the pandemic is exposing health-care inequalities.
Continue to: Some areas of the country...
Some areas of the country succeeded in reducing infections and limiting community spread, which ushered in an uneasy sense of normalcy even while the pandemic continues. At least for now, these locales can focus on rebuilding and preparing for expectable fluctuations in disease activity, including the arrival of the annual flu season on top of COVID-19.44 Recovery is not a return to the status quo ante but building stronger communities—“building back better.”45 Unless there is a continuum of care, shortcomings in one sector will have ripple effects through the entire system, particularly for psychiatric care for patients with SMI, which was inadequate before the pandemic.
Ensuring access to critical care was a priority during the pandemic’s early phase but came at the price of deferring other types of care, such as routine primary care; the coming months will see the downstream consequences of this approach,46 including for patients with SMI.
In the meantime, doing our job as clinicians, as Camus’s fictitious Dr. Bernard Rieux from the epigraph responds when asked how to define decency, may be the best we can do in these times. This includes contributing to and molding our field’s future and fostering a sense of agency in our patients and in ourselves. Major goals will be to preserve lessons learned, maintain flexibility, and avoid a return to unhelpful overregulation and payment models that do not reflect the flexible, person-centered care so important for patients with SMI.47
Bottom Line
During a pandemic, patients with serious mental illness may be easily forgotten as other issues overshadow the needs of this impoverished group. During a pandemic, the priority treatment goals for these patients are infection control, relapse prevention, and preventing treatment disengagement and loneliness. A pandemic requires changes in how patients with serious mental illness will receive psychopharmacology and psychotherapy.
Related Resources
- Huremović D (ed). Psychiatry of pandemics: a mental health response to infection outbreak. Cham, Switzerland: Springer Nature Switzerland AG; 2019.
- Ursano RJ, Fullerton CS, Weisaeth L, et al (eds). Textbook of disaster psychiatry. 2nd ed. Cambridge, UK: Cambridge University Press; 2017.
- Centers for Disease Control and Prevention. Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- American Psychiatric Association. Coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus.
- SMI Adviser. Make informed decisions related to COVID-19 and mental health. https://smiadviser.org/about/covid.
Drug Brand Names
Clozapine • Clozaril
Diazepam • Valium
Hydroxychloroquine • Plaquenil
“This whole thing is not about heroism. It’s about decency. It may seem a ridiculous idea, but the only way to fight the plague is with decency . ”
– Albert Camus, La Peste (1947)1
Severe acute respiratory syndrome (SARS), H1N1 swine flu, Ebola, Zika, and Middle East respiratory syndrome (MERS): the 21st century has already been witness to several serious infectious outbreaks and pandemics,2 but none has been as deadly and consequential as the current one. The ongoing SARS-coronavirus-2 (SARS-CoV-2) pandemic is shaping not only current psychiatric care but the future of psychiatry. Now that we are beyond the initial stages of the coronavirus disease 2019 (COVID-19) pandemic, when psychiatrists had a crash course in disaster psychiatry, our attention must shift to rebuilding and managing disillusionment and other psychological fallout of the intense early days.3
In this article, we offer guidance to psychiatrists caring for patients with serious mental illness (SMI) during the SARS-CoV-2 pandemic. Patients with SMI are easily forgotten as other issues (eg, preserving ICU capacity) overshadow the already historically neglected needs of this impoverished group.4 From both human and public-health perspectives, this inattention is a mistake. Assuring psychiatric stability is critically important to prevent the spread of COVID-19 in marginalized communities comprised of individuals who are poor, members of racial minorities, and others who already experience health disparities.5 Without controlling transmission in these groups, the pandemic will not be sufficiently contained.
We begin by highlighting general principles of pandemic management because caring for patients with SMI does not occur in a vacuum. Infectious outbreaks require not only helping those who need direct medical care because they are infected, but also managing populations that are at risk of getting infected, including health care and other essential workers.
Principles of pandemic management
Delivery of medical care during a pandemic differs from routine care. An effective disaster response requires collaboration and coordination among public-health, treatment, and emergency systems. Many institutions shift to an incident management system and crisis leadership, with clear lines of authority to coordinate responders and build medical surge capacity. Such a top-down leadership approach must plan and allow for the emergence of other credible leaders and for the restoration of people’s agency.
Unfortunately, adaptive capacity may be limited, especially in the public sector and psychiatric care system, where resources are already poor. Particularly early in a pandemic, services considered non-essential—which includes most psychiatric outpatient care—can become unavailable. A major effort is needed to prevent the psychiatric care system from contracting further, as happened during 9/11.6 Additionally, “essential” cannot be conflated with “emergent,” as can easily occur in extreme circumstances. Early and sustained efforts are required to ensure that patients with SMI who may be teetering on the edge of emergency status do not slip off that edge, especially when the emergency medical system is operating over capacity.
A comprehensive outbreak response must consider that a pandemic is not only a medical crisis but a mental health crisis and a communication emergency.7 Mental health clinicians need to provide accurate information and help patients cope with their fears.
Continue to: Psychological aspects of pandemics
Psychological aspects of pandemics. Previous infectious outbreaks have reaffirmed that mental health plays an outsized role during epidemics. Chaos, uncertainty, fear of death, and loss of income and housing cause prolonged stress and exact a psychological toll.
Adverse psychological impacts include expectable, normal reactions such as stress-induced anxiety or insomnia. In addition, new-onset psychiatric illnesses or exacerbations of existing ones may emerge.8 As disillusionment and demoralization appear in the wake of the acute phase, with persistently high unemployment, suicide prevention becomes an important goal.9
Pandemics lead to expectable behavioral responses (eg, increases in substance use and interpersonal conflict). Fear-based decisions may result in unhelpful behavior, such as hoarding medications (which may result in shortages) or dangerous, unsupervised use of unproven medications (eg, hydroxychloroquine). Trust is needed to accept public-health measures, and recommendations (eg, wearing masks) must be culturally informed to be credible and effective.
Because people are affected differently, at individual, cultural, and socioeconomic levels, they will view the situation differently. For many people, secondary stressors (eg, job loss) may be more disastrous than the primary medical event (ie, the pandemic). This distinction is critical because concrete financial help, not psychiatric care, is needed. Sometimes, even when a psychiatric disorder such as SMI or major neurocognitive disorder is present, the illusion of an acute decompensation can be created by the loss of social and structural supports that previously scaffolded a person’s life.
Mental illness prevention. Community mental-health surveillance is important to monitor for distress, psychiatric symptoms, health-risk behaviors, risk and safety perception, and preparedness. Clinicians must be ready to normalize expectable and temporary distress, while recognizing when that distress becomes pathological. This may be difficult in patients with SMI who often already have reduced stress tolerance or problem-based coping skills.10
Continue to: Psychological first aid...
Psychological first aid (PFA) is a standard intervention recommended by the World Health Organization for most individuals following a disaster; it is evidence-informed and has face validity.11 Intended to relieve distress by creating an environment that is safe, calm, and connected, PFA fosters self-efficacy and hope. While PFA is a form of universal prevention, it is not designed for patients with SMI, is not a psychiatric intervention, and is not provided by clinicians. Its principles, however, can easily be applied to patients with SMI to prevent distressing symptoms from becoming a relapse.
Communication. Good risk and crisis communication are critical because individual and population behavior will be governed by the perception of risk and fear, and not by facts. Failure to manage the “infodemic”7—with its misinformation, contradictory messages, and rumors—jeopardizes infection control if patients become paralyzed by uncertainty and fear. Scapegoating occurs easily during times of threat, and society must contain the parallel epidemic of xenophobia based on stigma and misinformation.12
Decision-making under uncertainty is not perfect and subject to revision as better information becomes available. Pointing this out to the public is delicate but essential to curtail skepticism and mistrust when policies are adjusted in response to new circumstances and knowledge.
Mistrust of an authority’s legitimacy and fear-based decisions lead to lack of cooperation with public-health measures, which can undermine an effective response to the pandemic. Travel restrictions or quarantine measures will not be followed if individuals question their importance. Like the general public, patients need education and clear communication to address their fear of contagion, dangers posed to family (and pets), and mistrust of authority and government. A lack of appreciation of the seriousness of the pandemic and individual responsibility may need to be addressed. Two important measures to accomplish this are steering patients to reputable sources of information and advising that they limit media exposure.
Resilience-building. Community and workplace resilience are important aspects of making it through a disaster as best as possible. Resilience is not innate and fixed; it must be deliberately built.13 Choosing an attitude of post-traumatic growth over the victim narrative is a helpful stance. Practicing self-care (rest, nutrition, exercise) and self-compassion (self-kindness, common humanity, mindfulness) is good advice for patients and caregivers alike.
Continue to: Workforce protection
Workforce protection. Compared to other disasters, infectious outbreaks disproportionally affect the medical community, and care delivery is at stake. While psychological and psychiatric needs may increase during a pandemic, services often contract, day programs and clinics close, teams are reduced to skeleton crews, and only emergency psychiatric care is available. Workforce protection is critical to avoid illness or simple absenteeism due to mistrust of protective measures.
Only a well-briefed, well-led, well-supported, and adequately resourced workforce is going to be effective in managing this public-health emergency. Burnout and moral injury are feared long-term consequences for health care workers that need to be proactively addressed.14 As opposed to other forms of disasters, managing your own fears about safety is important. Clinicians and their patients sit in the proverbial same boat.
Ethics. The anticipated need to ration life-saving care (eg, ventilators) has been at the forefront of ethical concerns.15 In psychiatry, the question of involuntary public-health interventions for uncooperative psychiatric patients sits uncomfortably between public-health ethics and human rights, and is an opportunity for collaboration with public-health and infectious-disease colleagues.
Redeployed clinicians and those working under substandard conditions may be concerned about civil liability due to a modified standard of care during a crisis. Some clinicians may ask if their duty to care must override their natural instinct to protect themselves. There is a lot of room for resentment in these circumstances. Redeployed or otherwise “conscripted” clinicians may resent administrators, especially those administering from the safety of their homes. Those “left behind” to work in potentially precarious circumstances may resent their absent colleagues. Moreover, these front-line clinicians may have been forced to make ethical decisions for which they were not prepared.16 Maintaining morale is far from trivial, not just during the pandemic, but afterward, when (and if) the entire workforce is reunited. All parties need to be mindful of how their actions and decisions impact and are perceived by others, both in the hospital and at home.
Managing patients with SMI during COVID-19
Patients with SMI are potentially hard hit by COVID-19 due to a “tragic” epidemiologic triad of agent-host-environment: SARS-CoV-2 is a highly infectious agent affecting patients with SMI who are vulnerable hosts in permissive environments (Figure).
Continue to: While not as infectious as measles...
While not as infectious as measles, COVID-19 is more infectious than the seasonal flu virus.17 It can lead to uncontrolled infection within a short period of time, particularly in enclosed settings. Outbreaks have occurred readily on cruise ships and aircraft carriers as well as in nursing homes, homeless shelters, prisons, and group homes.
Patients with SMI are vulnerable hosts because they have many of the medical risk factors18 that portend a poor prognosis if they become infected, including pre-existing lung conditions and heart disease19 as well as diabetes and obesity.20 Obesity likely creates a hyperinflammatory state and a decrease in vital capacity. Patient-related behavioral factors include poor early-symptom reporting and ineffective infection control.
Unfavorable social determinants of health include not only poverty but crowded housing that is a perfect incubator for COVID-19.
Priority treatment goals. The overarching goal during a pandemic is to keep patients with SMI in psychiatric treatment and prevent them from disengaging from care in the service of infection control. Urgent tasks include infection control, relapse prevention, and preventing treatment disengagement and loneliness.
Infection control. As trusted sources of information, psychiatrists can play an important role in infection control in several important ways:
- educating patients about infection-control measures and public-health recommendations
- helping patients understand what testing can accomplish and when to pursue it
- encouraging protective health behaviors (eg, hand washing, mask wearing, physical distancing)
- assessing patients’ risk appreciation
- assessing for and addressing obstacles to implementing and complying with infection-control measures
- explaining contact tracing
- providing reassurance.
Continue to: Materials and explanations...
Materials and explanations must be adapted for patient understanding.
Patients with disorganization or cognitive disturbances may have difficulties cooperating or problem-solving. Patients with negative symptoms may be inappropriately unconcerned and also inaccurately report symptoms that suggest COVID-19. Acute psychosis or mania can prevent patients from complying with public-health efforts. Some measures may be difficult to implement if the means are simply not there (eg, physical distancing in a crowded apartment). Previously open settings (eg, group homes) have had to develop new mechanisms under the primacy of infection control. Inpatient units—traditionally places where community, shared healing, and group therapy are prized—have had to decrease maximum occupancy, limit the number of patients attending groups, and discourage or outrightly prohibit social interaction (eg, dining together).
Relapse prevention. Patients who take maintenance medications need to be supported. A manic or psychotic relapse during a pandemic puts patients at risk of acquiring and spreading COVID-19. “Treatment as prevention” is a slogan from human immunodeficiency virus (HIV) care that captures the importance of antiretroviral treatment to prevent medical complications from HIV, and also to reduce infecting other people. By analogy, psychiatric treatment for patients with SMI can prevent psychiatric instability and thereby control viral transmission. Avoiding sending psychiatric patients to a potentially stressed acute-care system is important.
Psychosocial support. Clinics need to ensure that patients continue to engage in care beyond medication-taking to proactively prevent psychiatric exacerbations. Healthful, resilience-building behaviors should be encouraged while monitoring and counseling against maladaptive ones (eg, increased substance use). Supporting patients emotionally and helping them solve problems are critical, particularly for those who are subjected to quarantine or isolation. Obviously, in these latter situations, outreach will be necessary and may require creative delivery systems and dedicated clinicians for patients who lack access to the technology necessary for virtual visits. Havens and Ghaemi21 have suggested that a good therapeutic alliance can be viewed as a mood stabilizer. Helping patients grieve losses (loved ones, jobs, sense of safety) may be an important part of support.
Even before COVID-19, loneliness was a major factor for patients with schizophrenia.22 A psychiatric clinic is one aspect of a person with SMI’s social network; during the initial phase of the pandemic, many clinics and treatment programs closed. Patients for whom clinics structure and anchor their activities are at high risk of disconnecting from treatment, staying at home, and becoming lonely.
Continue to: Caregivers are always important...
Caregivers are always important to SMI patients, but they may assume an even bigger role during this pandemic. Some patients may have moved in with a relative, after years of living on their own. In other cases, stable caregiver relationships may be disrupted due to COVID-19–related sickness in the caregiver; if not addressed, this can result in a patient’s clinical decompensation. Clinicians should take the opportunity to understand who a patient’s caregivers are (group home staff, families) and rekindle clinical contact with them. Relationships with caregivers that may have been on “autopilot” during normal times are opportunities for welcome support and guidance, to the benefit of both patients and caregivers.
Table 1 summarizes clinical tasks that need to be kept in mind when conducting clinic visits during COVID-19 in order to achieve the high-priority treatment goals of infection control, relapse prevention, and psychosocial support.
Differential diagnosis. Neuropsychiatric syndromes have long been observed in influenza pandemics,23 due both to direct viral effects and to the effects of critical illness on the brain. Two core symptoms of COVID-19—anosmia and ageusia—suggest that COVID-19 can directly affect the brain. While neurologic manifestations are common,24 it remains unclear to what extent COVID-19 can directly “cause” psychiatric symptoms, or if such symptoms are the result of cytokines25 or other medical processes (eg, thromboembolism).26 Psychosis due to COVID-19 may, in some cases, represent a stress-related brief psychotic disorder.27
Hospitalized patients who have recovered from COVID-19 may have experienced prolonged sedation and severe delirium in an ICU.28 Complications such as posttraumatic stress disorder,29 hypoperfusion-related brain injuries, or other long-term cognitive difficulties may result. In previous flu epidemics, patients developed serious neurologic complications such as post-encephalitic Parkinson’s disease.30
Any person subjected to isolation or quarantine is at risk for psychiatric complications.31 Patients with SMI who live in group homes may be particularly susceptible to new rules, including no-visitor policies.
Continue to: Outpatients whose primary disorder...
Outpatients whose primary disorder is well controlled may, like anyone else, struggle with the effects of the pandemic. It is necessary to carefully differentiate non-specific symptoms associated with stress from the emergence of a new disorder resulting from stress.32 For some patients, grief or adjustment disorders should be considered. Prolonged stress and uncertainty may eventually lead to an exacerbation of a primary disorder, particularly if the situation (eg, financial loss) does not improve or worsens. Demoralization and suicidal thinking need to be monitored. Relapse or increased use of alcohol or other substances as a response to stress may also complicate the clinical picture.33 Last, smoking cessation as a major treatment goal in general should be re-emphasized and not ignored during the ongoing pandemic.34
Table 2 summarizes psychiatric symptoms that need to be considered when managing a patient with SMI during this pandemic.
Treatment tools
Psychopharmacology. Even though crisis-mode prescribing may be necessary, the safe use of psychotropics remains the goal of psychiatric prescribing. Access to medications becomes a larger consideration; for many patients, a 90-day supply may be indicated. Review of polypharmacy, including for pneumonia risk, should be undertaken. Preventing drooling (eg, from sedation, clozapine, extrapyramidal symptoms [EPS]) will decrease aspiration risk.
In general, treatment of psychiatric symptoms in a patient with COVID-19 follows usual guidelines. The best treatment for COVID-19 patients with delirium, however, remains to be established, particularly how to manage severe agitation.28 Pharmacodynamic and pharmacokinetic drug–drug interactions between psychotropics and antiviral treatments for COVID-19 (eg, QTc prolongation) can be expected and need to be reviewed.35 For stress-related anxiety, judicious pharmacotherapy can be helpful. Diazepam given at the earliest signs of a psychotic relapse may stave off a relapse for patients with schizophrenia.36 Even if permitted under relaxed prescribing rules during a public-health emergency, prescribing controlled substances without seeing patients in person requires additional thought. In some cases, adjusting the primary medication to buffer against stress may be preferred (eg, adjusting an antipsychotic in a patient on maintenance treatment for schizophrenia, particularly if a low-dose strategy is pursued).
Clozapine requires registry-based prescribing and bloodwork (“no blood, no drug”). The use of clozapine during this public-health emergency has been made easier because of FDA guidance that allows clozapine to be dispensed without blood work if obtaining blood work is not possible (eg, a patient is quarantined) or can be accomplished only at substantial risk to patients and the population at large. Under certain conditions, clozapine can be dispensed safely and in a way that is consistent with infection prevention. Clozapine-treated patients admitted with COVID-19 should be monitored for clozapine toxicity and the clozapine dose adjusted.37 A consensus statement consistent with the FDA and clinical considerations for using clozapine during COVID-19 is summarized in Table 3.38
Continue to: Long-acting injectable antipsychotics...
Long-acting injectable antipsychotics (LAIs) pose a problem because they require in-person visits. Ideally, during a pandemic, patients should be seen in person as frequently as medically necessary but as infrequently as possible to limit exposure of both patients and staff. Table 4 provides some clinical recommendations on how to use LAIs during the pandemic.39
Supportive psychotherapy may be the most important tool we have in helping patients with loss and uncertainty during these challenging months.40 Simply staying in contact with patients plays a major role in preventing care discontinuity. Even routine interactions have become stressful, with everyone wearing a mask that partially obscures the face. People with impaired hearing may find it even more difficult to understand you.
Education, problem-solving, and a directive, encouraging style are major tools of supportive psychotherapy to reduce symptoms and increase adaptive skills. Clarify that social distancing refers to physical, not emotional, distancing. The judicious and temporary use of anxiolytics is appropriate to reduce anxiety. Concrete help and problem-solving (eg, filling out forms) are examples of proactive crisis intervention.
Telepsychiatry emerged in the pandemic’s early days as the default mode of practice in order to limit in-person contacts.41 Like all new technology, telepsychiatry brings progress and peril.42 While it has gone surprisingly well for most, the “digital divide” does not afford all patients access to the needed technology. The long-term effectiveness and acceptance of telehealth remain to be seen. (Editor’s Note: For more about this topic, see “Telepsychiatry: What you need to know.”
Lessons learned and outlook
Infectious outbreaks have historically inflicted long-term disruptions on societies and altered the course of history. However, each disaster is unique, and lessons from previous disasters may only partially apply.43 We do not yet know how this one will end, including how long it will take for the world’s economies to recover. If nothing else, the current public-health emergency has brought to the forefront what psychiatrists have always known: health disparities are partially responsible for different disease risks (in this case, the risk of getting infected with SARS-CoV-2).5 It may not be a coincidence that the Black Lives Matter movement is becoming a major impetus for social change at a time when the pandemic is exposing health-care inequalities.
Continue to: Some areas of the country...
Some areas of the country succeeded in reducing infections and limiting community spread, which ushered in an uneasy sense of normalcy even while the pandemic continues. At least for now, these locales can focus on rebuilding and preparing for expectable fluctuations in disease activity, including the arrival of the annual flu season on top of COVID-19.44 Recovery is not a return to the status quo ante but building stronger communities—“building back better.”45 Unless there is a continuum of care, shortcomings in one sector will have ripple effects through the entire system, particularly for psychiatric care for patients with SMI, which was inadequate before the pandemic.
Ensuring access to critical care was a priority during the pandemic’s early phase but came at the price of deferring other types of care, such as routine primary care; the coming months will see the downstream consequences of this approach,46 including for patients with SMI.
In the meantime, doing our job as clinicians, as Camus’s fictitious Dr. Bernard Rieux from the epigraph responds when asked how to define decency, may be the best we can do in these times. This includes contributing to and molding our field’s future and fostering a sense of agency in our patients and in ourselves. Major goals will be to preserve lessons learned, maintain flexibility, and avoid a return to unhelpful overregulation and payment models that do not reflect the flexible, person-centered care so important for patients with SMI.47
Bottom Line
During a pandemic, patients with serious mental illness may be easily forgotten as other issues overshadow the needs of this impoverished group. During a pandemic, the priority treatment goals for these patients are infection control, relapse prevention, and preventing treatment disengagement and loneliness. A pandemic requires changes in how patients with serious mental illness will receive psychopharmacology and psychotherapy.
Related Resources
- Huremović D (ed). Psychiatry of pandemics: a mental health response to infection outbreak. Cham, Switzerland: Springer Nature Switzerland AG; 2019.
- Ursano RJ, Fullerton CS, Weisaeth L, et al (eds). Textbook of disaster psychiatry. 2nd ed. Cambridge, UK: Cambridge University Press; 2017.
- Centers for Disease Control and Prevention. Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- American Psychiatric Association. Coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus.
- SMI Adviser. Make informed decisions related to COVID-19 and mental health. https://smiadviser.org/about/covid.
Drug Brand Names
Clozapine • Clozaril
Diazepam • Valium
Hydroxychloroquine • Plaquenil
1. Camus A. La peste. Paris, France: Éditions Gallimard; 1947.
2. Huremovic
3. Substance Abuse and Mental Health Services Administration. Phases of disaster. https://www.samhsa.gov/dtac/recovering-disasters/phases-disaster. Updated June 17, 2020. Accessed August 7, 2020.
4. Geller J. COVID-19 and advocacy—the good and the unacceptable. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5b13. Published May 7, 2020. Accessed August 7, 2020.
5. Webb Hooper M, Nápoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467.
6. Sederer LI, Lanzara CB, Essock SM, et al. Lessons learned from the New York State mental health response to the September 11, 2001, attacks. Psychiatr Serv. 2011;62(9):1085-1089.
7. World Health Organization. Infodemic management – infodemiology. https://www.who.int/teams/risk-communication/infodemic-management. Accessed August 7, 2020.
8. Zhou J, Liu L, Xue P, et al. Mental health response to the COVID-19 outbreak in China. Am J Psychiatry. 2020;117(7):574-575.
9. Kawohl W, Nordt C. COVID-19, unemployment, and suicide. Lancet Psychiatry. 2020;7(5):389-390.
10. Yao H, Chen JH, Xu YF. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4):e21. doi: 10.1016/S2215-0366(20)30090-0.
11. Minihan E, Gavin B, Kelly BD, et al. Covid-19, mental health and psychological first aid. Ir J Psychol Med. 2020:1-12.
12. Adja KYC, Golinelli D, Lenzi J, et al. Pandemics and social stigma: who’s next? Italy’s experience with COVID-19. Public Health. 2020;185:39-41.
13. Rosenberg AR. Cultivating deliberate resilience during the coronavirus disease 2019 pandemic [published online April 14, 2020]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2020.1436.
14. Dean W, Talbot SG, Caplan A. Clarifying the language of clinician distress [published online January 31, 2020]. JAMA. doi: 10.1001/jama.2019.21576.
15. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055.
16. Rosenbaum L. Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875.
17. Viceconte G, Petrosillo N. COVID-19 R0: magic number or conundrum? Infect Dis Rep. 2020;12(1):8516.
18. de Hert M, Schreurs V, Vancampfort D, van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
19. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97-105.
20. Finer N, Garnett SP, Bruun JM. COVID-19 and obesity. Clin Obes. 2020;10(3):e12365. doi: 10.1111/cob.12365.
21. Havens LL, Ghaemi SN. Existential despair and bipolar disorder: the therapeutic alliance as a mood stabilizer. Am J Psychother. 2005;59(2):137-147.
22. Trémeau F, Antonius D, Malaspina D, et al. Loneliness in schizophrenia and its possible correlates. An exploratory study. Psychiatry Res. 2016;246:211-217.
23. Menninger KA. Psychoses associated with influenza: I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
24. Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832.
25. Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.012.
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
27. Martin Jr. EB. Brief psychotic disorder triggered by fear of coronavirus? Psychiatric Times. https://www.psychiatrictimes.com/view/brief-psychotic-disorder-triggered-fear-coronavirus-small-case-series. Published May 8, 2020. Accessed August 7, 2020.
28. Sher Y, Rabkin B, Maldonado JR, et al. COVID-19-associated hyperactive intensive care unit delirium with proposed pathophysiology and treatment: a case report [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.007.
29. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813.
30. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6(3):114-124.
31. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
32. Maercker A, Brewin CR, Bryant RA, et al. Diagnosis and classification of disorders specifically associated with stress: proposals for ICD-11. World Psychiatry. 2013;12(3):198-206.
33. Ornell F, Moura HF, Scherer JN, et al. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi: 10.1016/j.psychres.2020.113096.
34. Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking [published online April 3, 2020]. Nicotine Tob Res. https://doi.org/10.1093/ntr/ntaa059.
35. Back D, Marzolini C, Hodge C, et al. COVID-19 treatment in patients with comorbidities: awareness of drug-drug interactions [published online May 8, 2020]. Br J Clin Pharmacol. doi: 10.1111/bcp.14358.
36. Carpenter WT Jr., Buchanan RW, Kirkpatrick B, et al. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156(2):299-303.
37. Dotson S, Hartvigsen N, Wesner T, et al. Clozapine toxicity in the setting of COVID-19 [published online May 30, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.025.
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223.
39. Schnitzer K, MacLaurin S, Freudenreich O. Long-acting injectable antipsychotics during the COVID-19 pandemic. Current Psychiatry. In press.
40. Winston A, Rosenthal RN, Pinsker H. Learning supportive psychotherapy: an illustrated guide. Washington, DC: American Psychiatric Publishing; 2012.
41. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
42. Jordan A, Dixon LB. Considerations for telepsychiatry service implementation in the era of COVID-19. Psychiatr Serv. 2020;71(6):643-644.
43. DePierro J, Lowe S, Katz C. Lessons learned from 9/11: mental health perspectives on the COVID-19 pandemic. Psychiatry Res. 2020;288:113024.
44. Hussain S. Immunization and vaccination. In: Huremovic
45. Epping-Jordan JE, van Ommeren M, Ashour HN, et al. Beyond the crisis: building back better mental health care in 10 emergency-affected areas using a longer-term perspective. Int J Ment Health Syst. 2015;9:15.
46. Rosenbaum L. The untold toll - the pandemic’s effects on patients without Covid-19. N Engl J Med. 2020;382(24):2368-2371.
47. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness [published online June 3, 2020]. Psychiatr Serv. doi: 10.1176/appi.ps.202000244.
1. Camus A. La peste. Paris, France: Éditions Gallimard; 1947.
2. Huremovic
3. Substance Abuse and Mental Health Services Administration. Phases of disaster. https://www.samhsa.gov/dtac/recovering-disasters/phases-disaster. Updated June 17, 2020. Accessed August 7, 2020.
4. Geller J. COVID-19 and advocacy—the good and the unacceptable. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5b13. Published May 7, 2020. Accessed August 7, 2020.
5. Webb Hooper M, Nápoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467.
6. Sederer LI, Lanzara CB, Essock SM, et al. Lessons learned from the New York State mental health response to the September 11, 2001, attacks. Psychiatr Serv. 2011;62(9):1085-1089.
7. World Health Organization. Infodemic management – infodemiology. https://www.who.int/teams/risk-communication/infodemic-management. Accessed August 7, 2020.
8. Zhou J, Liu L, Xue P, et al. Mental health response to the COVID-19 outbreak in China. Am J Psychiatry. 2020;117(7):574-575.
9. Kawohl W, Nordt C. COVID-19, unemployment, and suicide. Lancet Psychiatry. 2020;7(5):389-390.
10. Yao H, Chen JH, Xu YF. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4):e21. doi: 10.1016/S2215-0366(20)30090-0.
11. Minihan E, Gavin B, Kelly BD, et al. Covid-19, mental health and psychological first aid. Ir J Psychol Med. 2020:1-12.
12. Adja KYC, Golinelli D, Lenzi J, et al. Pandemics and social stigma: who’s next? Italy’s experience with COVID-19. Public Health. 2020;185:39-41.
13. Rosenberg AR. Cultivating deliberate resilience during the coronavirus disease 2019 pandemic [published online April 14, 2020]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2020.1436.
14. Dean W, Talbot SG, Caplan A. Clarifying the language of clinician distress [published online January 31, 2020]. JAMA. doi: 10.1001/jama.2019.21576.
15. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055.
16. Rosenbaum L. Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875.
17. Viceconte G, Petrosillo N. COVID-19 R0: magic number or conundrum? Infect Dis Rep. 2020;12(1):8516.
18. de Hert M, Schreurs V, Vancampfort D, van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
19. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97-105.
20. Finer N, Garnett SP, Bruun JM. COVID-19 and obesity. Clin Obes. 2020;10(3):e12365. doi: 10.1111/cob.12365.
21. Havens LL, Ghaemi SN. Existential despair and bipolar disorder: the therapeutic alliance as a mood stabilizer. Am J Psychother. 2005;59(2):137-147.
22. Trémeau F, Antonius D, Malaspina D, et al. Loneliness in schizophrenia and its possible correlates. An exploratory study. Psychiatry Res. 2016;246:211-217.
23. Menninger KA. Psychoses associated with influenza: I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
24. Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832.
25. Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.012.
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
27. Martin Jr. EB. Brief psychotic disorder triggered by fear of coronavirus? Psychiatric Times. https://www.psychiatrictimes.com/view/brief-psychotic-disorder-triggered-fear-coronavirus-small-case-series. Published May 8, 2020. Accessed August 7, 2020.
28. Sher Y, Rabkin B, Maldonado JR, et al. COVID-19-associated hyperactive intensive care unit delirium with proposed pathophysiology and treatment: a case report [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.007.
29. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813.
30. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6(3):114-124.
31. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
32. Maercker A, Brewin CR, Bryant RA, et al. Diagnosis and classification of disorders specifically associated with stress: proposals for ICD-11. World Psychiatry. 2013;12(3):198-206.
33. Ornell F, Moura HF, Scherer JN, et al. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi: 10.1016/j.psychres.2020.113096.
34. Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking [published online April 3, 2020]. Nicotine Tob Res. https://doi.org/10.1093/ntr/ntaa059.
35. Back D, Marzolini C, Hodge C, et al. COVID-19 treatment in patients with comorbidities: awareness of drug-drug interactions [published online May 8, 2020]. Br J Clin Pharmacol. doi: 10.1111/bcp.14358.
36. Carpenter WT Jr., Buchanan RW, Kirkpatrick B, et al. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156(2):299-303.
37. Dotson S, Hartvigsen N, Wesner T, et al. Clozapine toxicity in the setting of COVID-19 [published online May 30, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.025.
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223.
39. Schnitzer K, MacLaurin S, Freudenreich O. Long-acting injectable antipsychotics during the COVID-19 pandemic. Current Psychiatry. In press.
40. Winston A, Rosenthal RN, Pinsker H. Learning supportive psychotherapy: an illustrated guide. Washington, DC: American Psychiatric Publishing; 2012.
41. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
42. Jordan A, Dixon LB. Considerations for telepsychiatry service implementation in the era of COVID-19. Psychiatr Serv. 2020;71(6):643-644.
43. DePierro J, Lowe S, Katz C. Lessons learned from 9/11: mental health perspectives on the COVID-19 pandemic. Psychiatry Res. 2020;288:113024.
44. Hussain S. Immunization and vaccination. In: Huremovic
45. Epping-Jordan JE, van Ommeren M, Ashour HN, et al. Beyond the crisis: building back better mental health care in 10 emergency-affected areas using a longer-term perspective. Int J Ment Health Syst. 2015;9:15.
46. Rosenbaum L. The untold toll - the pandemic’s effects on patients without Covid-19. N Engl J Med. 2020;382(24):2368-2371.
47. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness [published online June 3, 2020]. Psychiatr Serv. doi: 10.1176/appi.ps.202000244.
Cognitive-behavioral therapy for insomnia: A review of 8 studies
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16
1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16
1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16
1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.