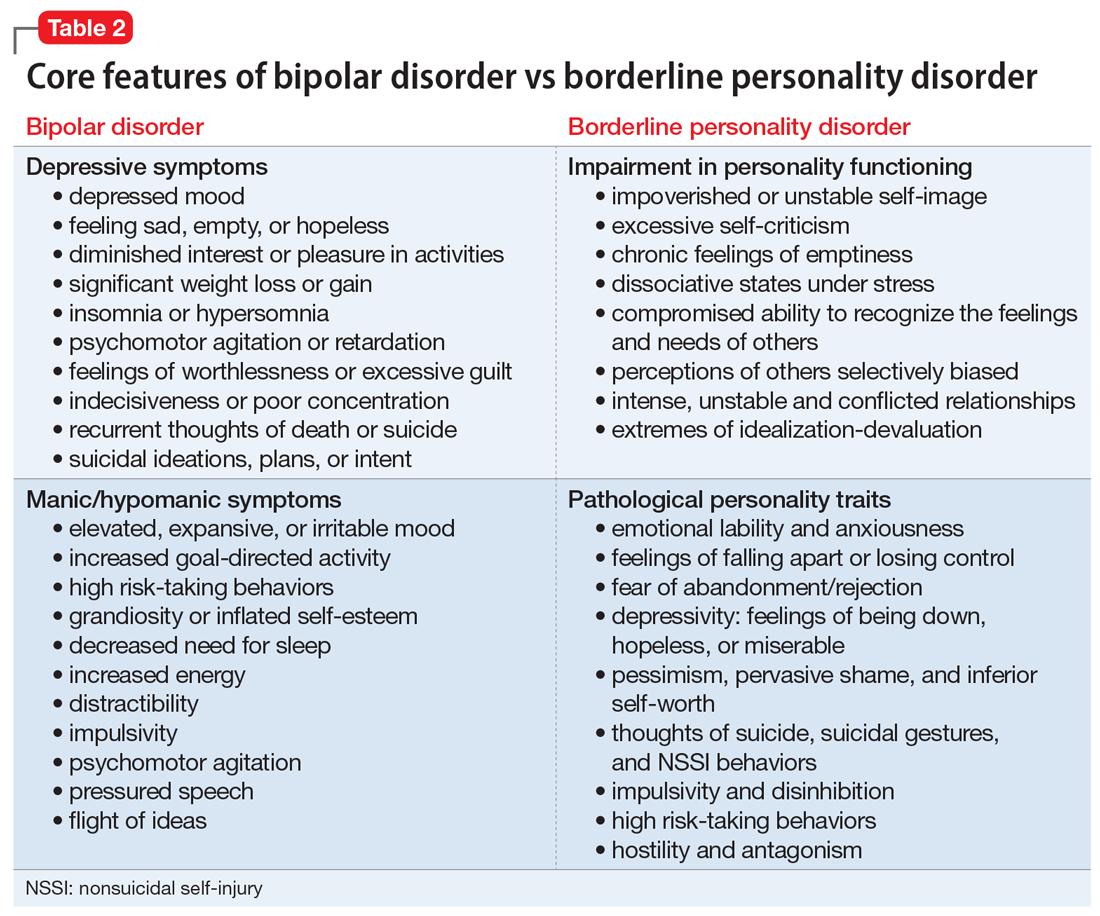
User login
Buspirone: A forgotten friend
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
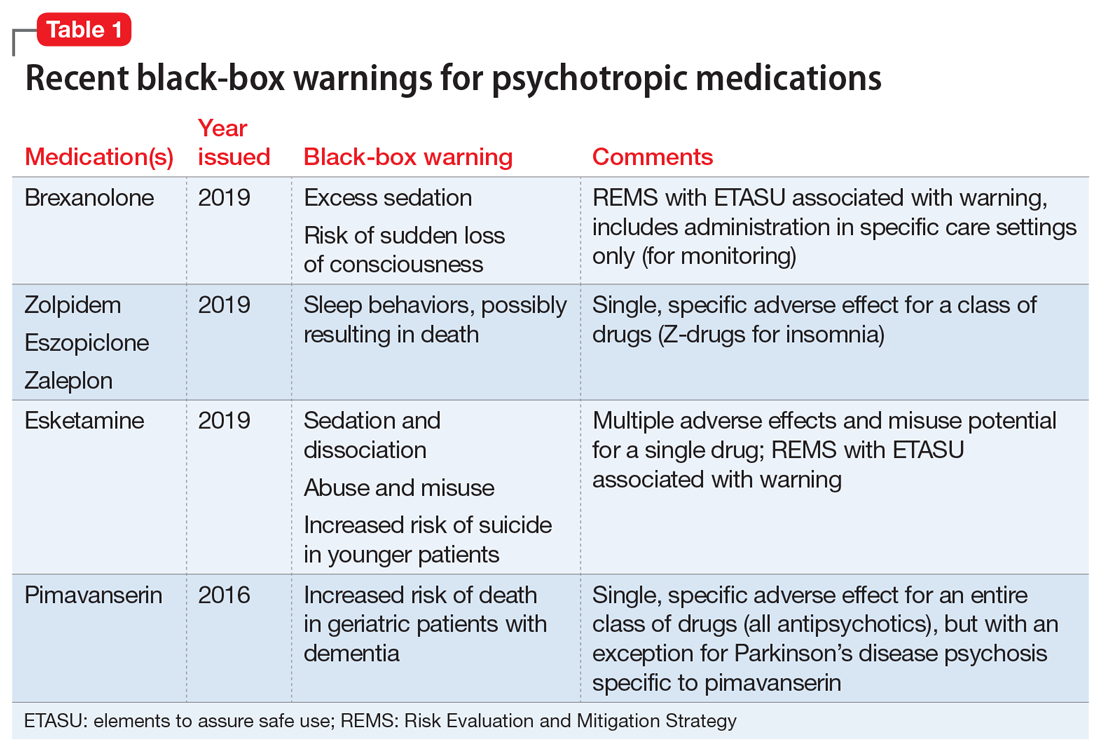
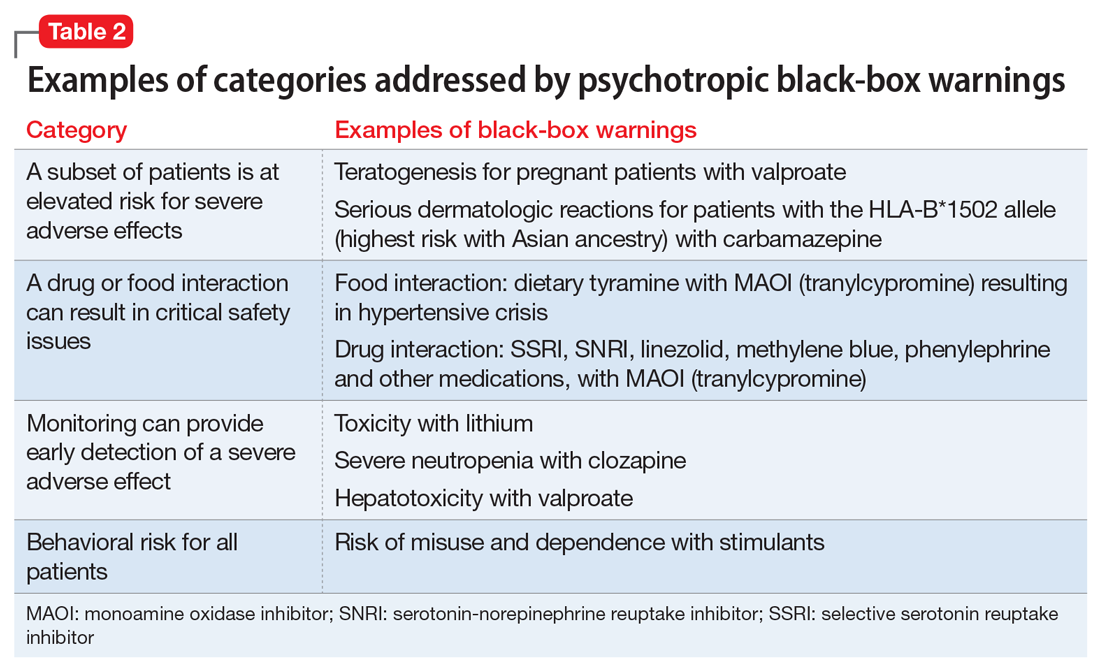
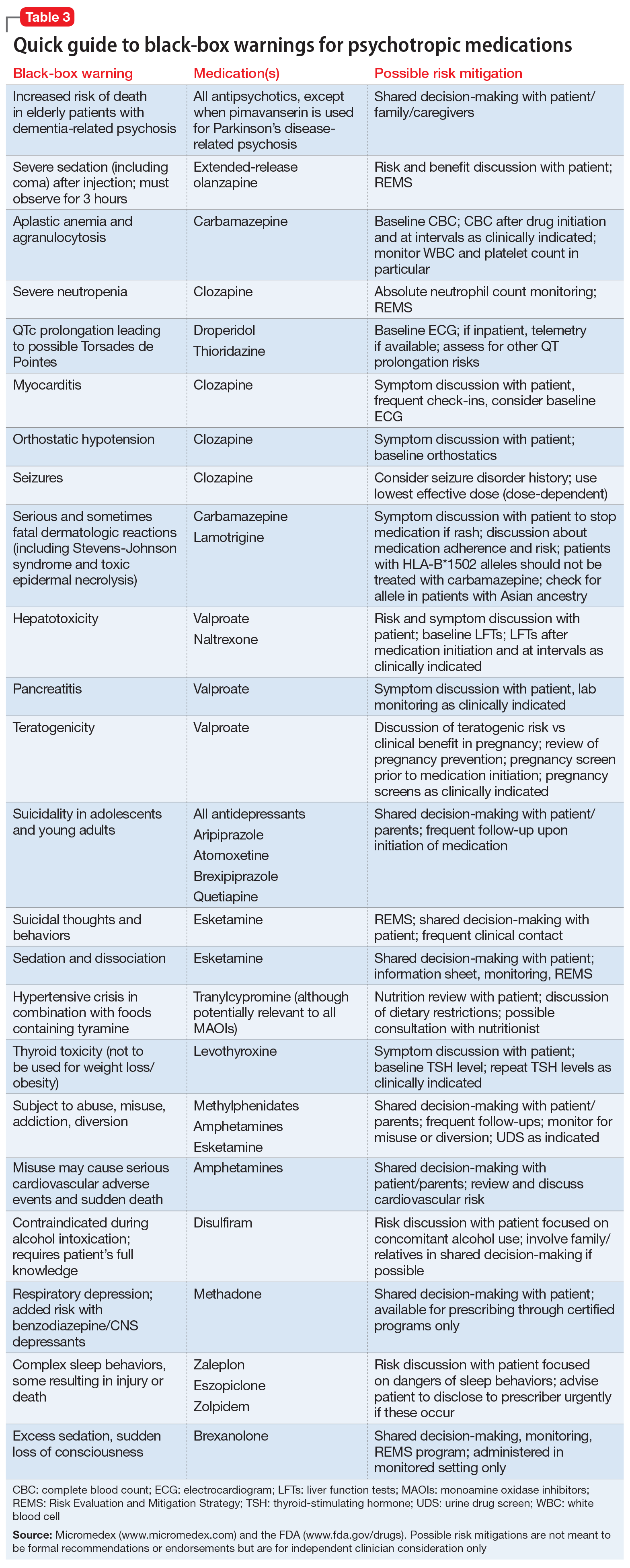
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.
Top research findings of 2018-2019 for clinical practice
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
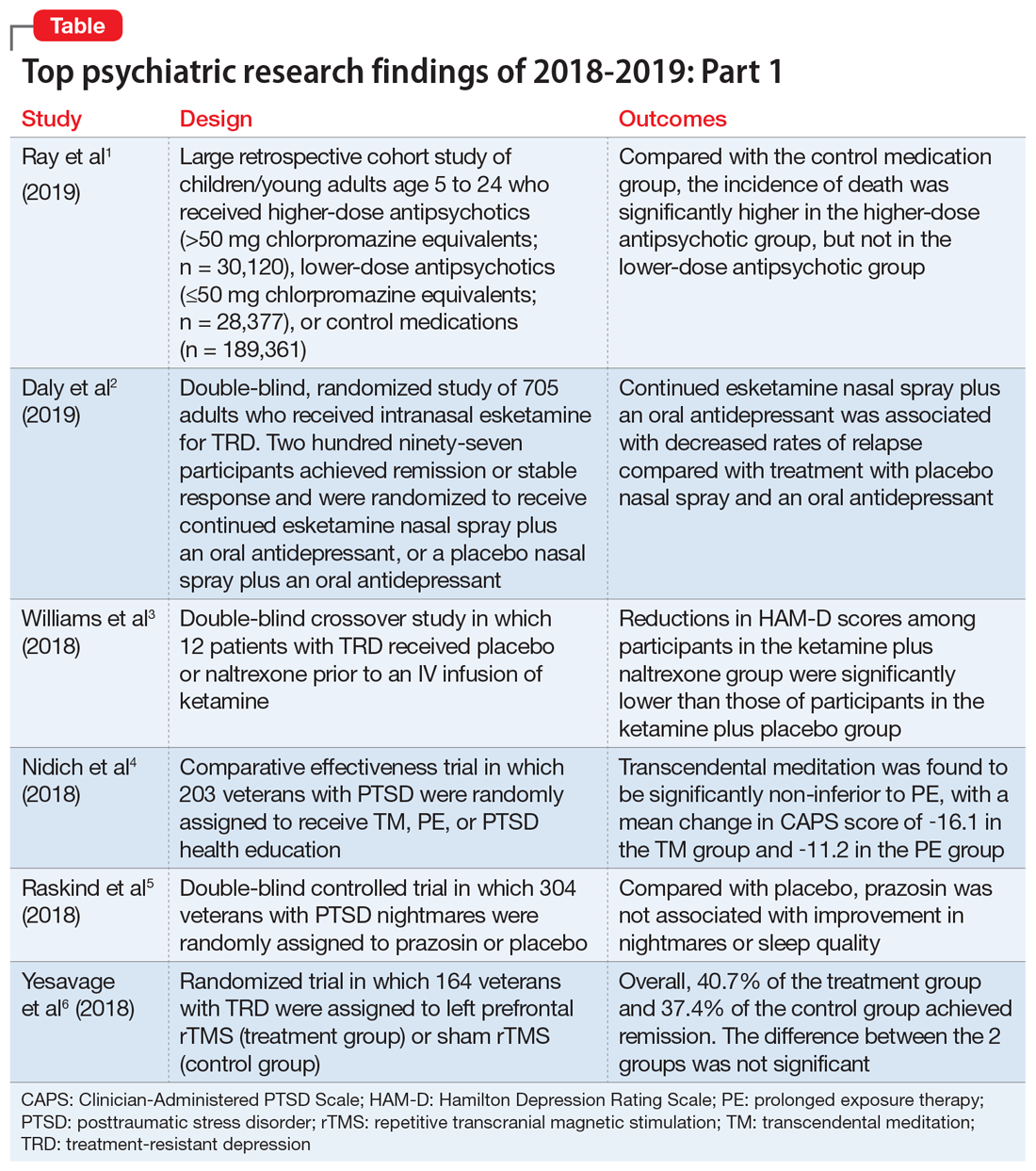
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
Black-box warnings: How they can improve your clinical practice
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).
In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5
Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).
In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5
Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).
In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5
Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
The psychiatrist’s role in liver transplantation
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2
Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9
A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2
Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9
A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
Since the first liver transplant (LT) was performed in 1963 by Starzl et al, there have been considerable advances in the field, with improvements in post-transplant survival.1 There are multiple indications for LT, including acute liver failure and index complications of cirrhosis such as ascites, encephalopathy, and hepatopulmonary syndrome.2 Once a patient develops one of these conditions, he/she is evaluated for LT, even as the complications of liver failure are being managed.
Although the number of LTs has risen, the demand for transplant continues to exceed availability. In 2015, chronic liver disease and cirrhosis was the 12th leading cause of death in the United States.3 In 2016, approximately 50% of waitlisted candidates received a transplant.4 There is also a donor shortage. In part, this shortage may be due to longer life spans and the subsequent increase in the age of the potential donor.5 In light of this shortage and increased demand, the pre-LT workup is comprehensive. The pre-transplant assessment typically consists of cardiology, surgery, hepatology, and psychosocial evaluations, and hence requires a team of experts to determine who is an ideal candidate for transplant.
Psychiatrists play a key role in the pre-transplant psychosocial evaluations. This article describes the elements of these evaluations, and what psychiatrists can do to help patients both before and after they undergo LT.
Elements of the pre-transplant evaluation
The psychosocial evaluation is a critical component of the pre-transplant assessment. As part of the evaluation, patients are screened for psychosocial limitations that may complicate transplantation, such as demonstrated noncompliance, ongoing alcohol or drug use, and lack of social support (Table 12 ). Other goals of the psychosocial evaluation are to identify in the pre-transplant period patients with possible risk factors, such as substance use or psychiatric disorders, and develop treatment plans to optimize transplant outcomes (Table 26). There are relative contraindications to LT (Table 37) but no absolute psychiatric contraindications, according to the 2013 American Association for the Study of Liver Diseases (AASLD) practice guideline for transplantation.2
Adherence. The 2013 AASLD practice guideline states that patients “should be evaluated for and meet reasonable expectations for adherence to medical directives and mental health stability as determined by the psychosocial evaluation.”2 In the transplant setting, adherence is complex. It requires compliance with complicated medication regimens and laboratory testing, frequent follow-up appointments, and close, prompt communication of concerns to the health care team. Patient adherence to medication regimens plays an important role in transplant outcomes.8 In fact, in patients who have undergone renal transplant, nonadherence to therapy is considered the leading cause of avoidable graft failure.9
A retrospective study of adult LT recipients found that pre-transplant chart evidence of nonadherence, such as missed laboratory testing and clinic visits, was a significant predictor of post-transplant nonadherence with immunosuppressant therapy. Pre-transplant unemployment status and a history of substance abuse also were associated with nonadherence.9
Dobbels et al10 found that patients with a self-reported history of pre-transplant non-adherence had a higher risk of being nonadherent with their immunosuppressive therapy after transplant (odd ratio [OR]: 7.9). Their self-report adherence questionnaire included questions that addressed pre-transplant smoking status, alcohol use, and adherence with medication. In this prospective study, researchers also found that patients with a low “conscientiousness” score were at a higher risk for post-transplant medication nonadherence (OR: 0.8).
Continue to: Studies have also found...
Studies have also found that patients with higher education are more at risk for post-transplant medication nonadherence. Higher education may be associated with higher employment status resulting in a busier lifestyle, a known risk factor that may prevent patients from regular medication adherence.11,12 Alternatively, it is possible that higher educated patients are “decisive” nonadherers who prefer independent decision-making regarding their disease and treatment.13
Substance use. The 2013 AASLD practice guideline lists “ongoing alcohol or illicit substance abuse” as one of the contraindications to LT.2 In guidelines from the Austrian Society for Gastroenterology and Hepatology, Graziadei et al14 listed “alcohol addiction without motivation for alcohol abstinence and untreated/ongoing substance abuse” as absolute contraindications and “untreated alcohol abuse and other drug-related addiction” as relative contraindications. Hence, the pre-transplant evaluation should include a thorough substance use history, including duration, amount, previous attempts to quit, and motivation for abstinence.
Substance use history is especially important because alcoholic liver disease is the second most common indication for LT.2 Most LT programs require 6 months of abstinence before a patient can be considered for transplant.15 The 6-month period was based on studies demonstrating that pre-transplant abstinence from alcohol for <6 months is a risk factor for relapse.15 However, this guideline remains controversial because the transplant referral and workup may be delayed as the patient’s liver disease worsens. Other risk factors for substance relapse should also be taken into consideration, such as depression, personality disorders, lack of social support, severity of alcohol use, and family history of alcoholism.16 Lee and Leggio16 developed the Sustained Alcohol Use Post-Liver Transplant (SALT) score to identify patients who were at risk for sustained alcohol use posttransplant. The 4 SALT criteria are:
- >10 drinks per day at initial hospitalization (+4 points)
- multiple prior rehabilitation attempts (+4 points)
- prior alcohol‐related legal issues (+2 points), and
- prior illicit substance abuse (+1 point).
A SALT score can range from 0 to 11. Lee et al17 found a SALT score ≥5 had a 25% positive predictive value (95% confidence interval [CI]: 10% to 47%) and a SALT score of <5 had a 95% negative predictive value (95% CI: 89% to 98%) for sustained alcohol use post‐LT. Thus, the 2013 AASLD guideline cautions against delaying evaluation based on the 6-month abstinence rule, and instead recommends early transplant referral for patients with alcoholic liver disease to encourage such patients to begin addiction treatment.2
As part of the substance use history, it is important to ask about the patient’s smoking history. Approximately 60% of LT candidates have a history of smoking cigarettes.18 Tobacco use history is associated with increased post-transplant vascular complications, such as hepatic artery thrombosis or stenosis, portal vein thrombosis, and deep vein thrombosis.19 The 2013 AASLD guideline recommends that tobacco use should be prohibited in LT candidates.2 Pungpapong et al19 reported that smoking cessation for at least 2 years prior to transplant led to a significantly decreased risk of developing arterial complications, with an absolute risk reduction of approximately 16%.
Continue to: Liver cirrhosis due to...
Liver cirrhosis due to chronic hepatitis C virus (HCV) infection is one of the leading causes for LT. In the United States, HCV is commonly transmitted during injection drug use. According to the 2013 AASLD guideline, ongoing illicit substance use is a relative contraindication to LT.2 It is important to note, however, that methadone maintenance therapy (MMT) is not a contraindication to LT. In fact, the 2013 AASLD guideline recommends that patients receiving MMT should not be required to reduce or stop therapy in order to be listed for transplant.2 Studies have shown that in 80% of patients, tapering MMT leads to illicit opiate relapse.20 Currently, there is no evidence that patients receiving MMT have poorer post-transplant outcomes compared with patients not receiving MMT.21
Whether cannabis use is a relative contraindication to LT remains controversial.22 Possible adverse effects of cannabis use in transplant patients include drug–drug interactions and infections. Hézode et al23 reported that daily cannabis use is significantly associated with an increased fibrosis progression rate in patients with chronic HCV infection. Another recent study found that a history of cannabis use was not associated with worse outcomes among patients on the LT waitlist.24 With the increased legalization of cannabis, more studies are needed to assess ongoing cannabis use in patients on the LT waitlist and post-LT outcomes.
Psychiatric history. When assessing a patient for possible LT, no psychiatric disorder is considered an absolute contraindication. Patients with a serious mental illness, such as schizophrenia, and those with intellectual disability can have successful, long-term outcomes with proper evaluation and preparation, including social support. However, empirical literature regarding transplant outcomes and predictive factors in patients with serious mental illness is scarce.2
Studies examining the predictive value of pre-transplant depression on post-transplant outcomes have had mixed results.25 Depression may predict lower post-transplant quality of life. Pre-LT suicidal thoughts (as noted on the Beck Depression Inventory, for example) are associated with post-LT depression.25 In contrast, available data show no significant effect of pre-transplant anxiety on post-LT outcomes. Similarly, pre-transplant cognitive performance appears not to predict survival or other post-transplant outcomes, but may predict poorer quality of life after transplant.25
A few psychiatric factors are considered relative contraindications for LT. These include severe personality disorders, active substance use with no motivation for treatment or abstinence, active psychosis, severe neurocognitive disorders, suicidality, and factitious disorder.7
Continue to: Social support
Social support. Assessing a pre-LT patient’s level of social support is an essential part of the psychosocial evaluation. According to the 2013 AASLD guideline, patients should have “adequate” social support both during the waitlist and post-operative periods.2 Lack of partnership is a significant predictor of poor post-transplant outcomes, such as late graft loss.10 Satapathy and Sanyal26 reported that among patients who receive an LT for alcoholic liver disease, those with immediate family support were less likely to relapse to using alcohol after transplant. Poor social support was also a predictor of post-transplant medication nonadherence.10 Thus, the patient needs enough social support to engage in the pre-transplant health care requirements and to participate in post-transplant recommendations until he/she is functioning independently post-transplant.
Screening tools
Various screening tools may be useful in a pre-LT evaluation. Three standardized assessment tools available specifically for pre-transplant psychosocial assessments are the Stanford Integrated Psychosocial Assessment for Transplantation (Table 26), the Psychosocial Assessment of Candidates for Transplantation,27 and the Transplant Evaluation Rating Scale.28 Instruments to aid in the assessment of depression, anxiety, and delirium,29-31 a structured personality assessment,32 coping inventories,33 neuropsychological batteries,34 and others also have been used to evaluate patients before LT. The self-rated Beck Depression Inventory and the clinician-rated Hamilton Depression Rating Scale are commonly used.7 Other tools, such as the LEIPAD quality of life instrument and the Brief Symptom Inventory (BSI), have been used to assess for perceived quality of life and psychological distress, respectively.35 These screening tools can be helpful as aids for the pre-LT evaluation; however, diagnoses and treatment plan recommendations require a psychiatric evaluation conducted by a trained clinician.
Treatment after liver transplant
Psychiatric issues. After LT, various psychiatric complications may arise, including (but not limited to) delirium7 and “paradoxical psychiatric syndrome” (PPS).36 Delirium can be managed by administering low-dose antipsychotic medications, limiting the use of benzodiazepines and medications with anticholinergic effects, implementing behavioral interventions (frequent orientation, maintaining sleep/wake cycle, limiting noise, presence of a family member or a sitter at bedside),37 and addressing the underlying etiology. Paradoxical psychiatric syndrome is defined as psychiatric symptoms that occur despite a successful LT. It develops within the first year of transplantation and is characterized by recipients having strong guilt feelings toward their donors.38
Drug interactions. In the post-transplant period, antipsychotics are used for management of delirium and psychosis, antidepressants for anxiety and depression, and benzodiazepines for anxiety and sleep problems.7 Drug–drug interactions between psychotropic medications and the immunosuppressants required after LT must be closely monitored. First-generation antipsychotics should be avoided in post-transplant patients taking tacrolimus due to the increased risk of QTc prolongation. Tacrolimus can also increase the risk of nephrotoxicity when co-administered with lithium. Post-LT patients taking steroids and bupropion have an increased risk of seizure. Carbamazepine may decrease blood levels of cyclosporine due to the induction of hepatic metabolism.39,40 The psychiatrist should review and update the patient’s complete medication list at each visit, checking for possible medication interactions.
Quality of life. In the first 6 months post-transplant, patients typically experience improved quality of life in both physical and psychological domains. However, this improvement vacillates as the patient adjusts to post-transplant life. A reduction in BSI score 1 year after transplant has been reported. The BSI evaluates psychopathological symptoms, which are early indicators of psychological discomfort. One study noted a reduction in the LEIPAD quality of life score, which measures overall quality of life, 2 years after transplant.35 This decline may reflect the difficulties associated with the new challenges after transplant. Patients may endure both physical changes due to medical complications as well as psychological problems as they adjust to their new bodily integrity, their dependence on medications and medical staff, and other changes in function. Three to 5 years after transplant, patients reached a new psychological stability, with reported improvements in quality of life and decreased psychological distress.35
Continue to: Special populations
Special populations
HCV infection. Recurrent HCV infection and liver disease after transplantation are associated with psychological distress. This is particularly evident in patients 6 months after transplantation. Depression and psychological distress have been reported in male patients with recurrent HCV infection within the first year after transplantation.35
Acetaminophen overdose. Patients who receive a transplant for acetaminophen-induced acute liver failure (ALF) had a greater prevalence of psychiatric comorbidity as reflected by predefined diagnoses, medication, and previous suicide attempts.41 Despite this, outcomes for patients transplanted emergently for acetaminophen-induced ALF were comparable to those transplanted for non-acetaminophen-induced ALF and for chronic liver disease. Multidisciplinary approaches with long-term psychiatric follow-up may contribute to low post-transplant suicide rates and low rates of graft loss because of noncompliance.41
CASE REPORT
A complicated presentation
Ms. A, age 45, a married woman with history of chronic back pain and self-reported bipolar disorder, presented to our hospital with acute liver failure secondary to acetaminophen overdose. Her Model for End-Stage Liver Disease (MELD) score on presentation was 38 (range: 0 to 40 with higher scores indicating increased likelihood of mortality). Her urine drug screen was positive for benzodiazepines and opiates. On hospital Day 2, the primary team consulted psychiatry for a pre-transplant evaluation and consideration of suicidality. Hepatology, toxicology, and transplant surgery services also were consulted.
Because Ms. A was intubated for acute respiratory failure, the initial history was gathered from family, a review of the medical record, consultation with her pharmacy, and collateral from her outpatient physician. Ms. A had been taking
Four days before presenting with acute liver failure, Ms. A had visited another hospital for lethargy. Benzodiazepines and opiates were stopped abruptly, and she was discharged with the recommendation to take acetaminophen for her pain. Approximately 24 hours after returning home, Ms. A began having auditory and visual hallucinations, and she did not sleep for days. She continued to complain of pain and was taking acetaminophen as recommended by the outside hospital. Her husband notes that she was intermittently confused. He was unsure how much acetaminophen she was taking.
Continue to: Her family noted...
Her family noted Ms. A had been diagnosed with bipolar disorder “years ago” but was unable to describe any manic episodes, and Ms. A had been treated only with an antidepressant from her primary care physician. She had persistent low mood and increased sleep since developing chronic back pain that severely limited her functioning. Ms. A attempted suicide once years ago by cutting her wrists. She had 2 prior psychiatric hospitalizations for suicidal ideation and the suicide attempt; however, she had not recently voiced suicidal ideation to her husband or family. She was adherent to psychotropic medications and follow-up appointments. Ms. A is a current smoker. She had used marijuana in the past, but her family denies current use, as well as any alcohol use or illicit substance use.
Ms. A’s diagnosis was consistent with tobacco use disorder and major depressive disorder (MDD). She likely developed withdrawal after abrupt cessation of diazepam, which she had been taking as prescribed for years. There was no evidence at the time of her initial psychiatric evaluation that the acetaminophen overdose was a suicide attempt; however, because Ms. A was intubated and sedated at that time, the consultation team recommended direct observation until she could participate in a risk assessment.
For the pre-transplant psychiatric evaluation, our consultation-liaison team noted Ms. A’s history of MDD, with recent active symptoms, chronic pain, and a past suicide attempt. She was a current tobacco smoker, which increases the risk of post-transplant vascular problems. However, she had been adherent to medications and follow-up, had very close family support, and there was no clear evidence that this acetaminophen ingestion was a suicide attempt. We noted that outpatient psychiatric follow-up and better chronic pain management would be helpful post-transplant. We would have to re-evaluate Ms. A when she was medically stable enough to communicate before making any further recommendations. Due to medical complications that developed after our evaluation, the transplant team noted Ms. A was no longer a transplant candidate.
Fortunately, Ms. A recovered with medical management over the next 2 weeks. She denied any suicidal ideation throughout her hospitalization. She was restarted on an antidepressant and received supportive therapy until discharge. Outpatient psychiatry follow-up and pain management was set up before Ms. A was discharged. Inpatient psychiatric hospitalization was not recommended. Per available records, Ms. A followed up with all outpatient appointments, including with her psychiatrist, since discharge.
Avoiding problems, maximizing outcomes
In addition to medical factors, psychosocial factors may affect the success of LT, although empirical data regarding which factors are most predictive of post-transplant outcomes is lacking, especially in patients with serious mental illness. The goals of a psychosocial pre-transplant evaluation are to promote fairness and equal access to care, maximize optimal outcomes, wisely use scarce resources, and ensure that the potential for benefits outweigh surgical risks to the patient. Identifying potential complicating factors (ie, substance abuse, nonadherence, serious psychopathology) can help guide the medical and psychiatric treatment plan and help minimize preventable problems both before and after transplant.42
Continue to: In patients who have...
In patients who have a history of alcohol use and alcohol liver disease, relapse to alcohol is a significant problem. Relapse rates vary from 10% to 30%.7 The duration of abstinence before LT appears to be a poor predictor of abstinence after LT.43 Polysubstance use also adversely affects outcomes in patients with alcohol liver disease. Approximately one-third of patients with polysubstance use who receive a LT relapse to substance use.44 Coffman et al45 showed that the presence of antisocial behavior and eating disorders may increase the risk of relapse after LT.
The psychiatrist’s role in the setting of LT spans from the pre-transplant assessment to post-transplant management and follow-up. Clarifying specific psychiatric diagnoses, psychosocial factors that need to be addressed before transplant, and substance use diagnoses and treatment recommendations can help the transplant team clearly identify modifiable factors that can affect transplant outcomes.
Bottom Line
Psychiatrists can help patients who are candidates for liver transplantation (LT) by performing a pre-transplant psychosocial assessment to identity factors that might complicate transplantation or recovery. After LT, patients require careful monitoring for psychiatric comorbidities, drug interactions, and other factors that can affect quality of life.
Related Resources
- Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018;53(2):135-144.
- Marcangelo MJ, Crone C. Tools, techniques to assess organ transplant candidates. Current Psychiatry. 2007;6(9):56-66.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Hydromorphone • Dilaudid
Lithium • Eskalith, Lithobid
Tacrolimus • Astagraf XL, Envarsus XR
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
1. Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives [Article in English, Portuguese]. Einstein (São Paulo). 2015;13(1):149-152.
2. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144-1165.
3. Centers for Disease Control and Prevention. QuickStats: number of deaths from 10 leading causes,* by sex—National Vital Statistics System, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(15):413.
4. Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31(1):84-89.
5. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59.
6. Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123-132.
7. Grover S, Sarkar S. Liver transplant—psychiatric and psychosocial aspects. J Clin Exp Hepatol. 2012;2(4):382-392.
8. Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760-770.
9. Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824-834.
10. Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497-1504.
11. De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323.
12. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172-183.
13. Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718-1726.
14. Graziadei I, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 2016;128(19):679-690.
15. Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981-987.
16. Lee MR, Leggio L. Management of alcohol use disorder in patients requiring liver transplant. Am J Psychiatry. 2015;172(12):1182-1189.
17. Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the Sustained Alcohol Use Post-Liver Transplant score. Hepatology. 2019;69(4):1477-1487.
18. DiMartini A, Crone C, Dew MA. Alcohol and substance use in liver transplant patients. Clinics in Liver Disease. 2011;15(4):727-751.
19. Pungpapong S, Manzarbeitia C, Ortiz J, et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582-587.
20. Kreek MJ. Pharmacotherapy of opioid dependence: rationale and update. Regulatory Peptides. 1994;53(suppl 1):S255-S256.
21. Jiao M, Greanya ED, Haque M, et al. Methadone maintenance therapy in liver transplantation. Prog Transplant. 2010;20(3):209-214; quiz 215.
22. Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91.
23. Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63-71.
24. Kotwani P, Saxena V, Dodge JL, et al. History of marijuana use does not affect outcomes on the liver transplant waitlist. Transplantation. 2018;102(5):794-802.
25. Fineberg SK, West A, Na PJ, et al. Utility of pretransplant psychological measures to predict posttransplant outcomes in liver transplant patients: a systematic review. Gen Hospl Psychiatry. 2016;40:4-11.
26. Satapathy S, Sanyal A. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
27. Olbrisch ME, Levenson JL, Hamer R. The PACT: a rating scale for the study of clinical decision making in psychosocial screening of organ transplant candidates. Clin Transplant. 1989;3:164-169.
28. Twillman RK, Manetto C, Wellisch DK, et al. Transplant Evaluation Rating Scale: a revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153.
29. Goodier J. Evaluating Stress:97496. In: Zalaquett CP, Wood RJ, eds. Evaluating stress: a book of resources. Lanham, MD: Scarecrow Press; 1997:29-29.
30. Beck AT, Steer RA, Carbin, MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8(1):77-100.
31. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242.
32. Cottle WC. The MMPI: a review. Lawrence, KS: University of Kansas; 1953.
33. Addison CC, Campbell-Jenkins BW, Sarpong DF, et al. Psychometric Evaluation of a Coping Strategies Inventory Short-Form (CSI-SF) in the Jackson Heart Study Cohort. Int J Environ Res Public Health. 2007;4(4):289-295.
34. Mooney S, Hasssanein T, Hilsabeck R, et al. Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175-186.
35. De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33(4):609-615.
36. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42(4):337-343.
37. Landefeld CS, Palme, RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344.
38. Fukunishi I, Sugawara Y, Takayama T, et al. Psychiatric problems in living-related transplantation (II): the association between paradoxical psychiatric syndrome and guilt feelings in adult recipients after living donor liver transplantation. Transplantation Proceedings. 2002;34(7):2632-2633.
39. Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet. 1996;30(2):141-179.
40. Ozkanlar Y, Nishijima Y, Cunha DD, et al. Acute effects of tacrolimus (FK506) on left ventricular mechanics. Pharmacol Res. 2005;52(4):307-312.
41. Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826-833.
42. Maldonado J R. I have been asked to work up a patient who requires a liver transplant how should I proceed? FOCUS. 2009;7(3):332-335.
43. Mccallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol and Alcoholism. 2006;41(4):358-363.
44. Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680-685.
45. Coffman KL, Hoffman A, Sher L, et al. Treatment of the postoperative alcoholic liver transplant recipient with other addictions. Liver Transpl Surg. 1997;3(3):322-327.
2019 Peer Reviewers
Aparna Atluru, MD
Stanford Medicine
Anjan Bhattacharyya, MD
Saint Louis University
Caroline Bonham, MD
The University of New Mexico
Catherine Crone, MD
Inova Health System
Sheila Dowd, PhD
Rush Medical College
Ahmed Z. Elmaadawi, MD
Indiana University School of Medicine
Donald Gilbert, MD, MS
Cincinnati Children’s Hospital Medical Center
Mark Gold, MD
Washington University in St. Louis
Elana Harris, MD, PhD
Cincinnati Children’s Hospital
Susan Hatters-Friedman, MD
Case Western Reserve University
Faisal Islam, MD, MBA
Greenvale, New York
Kaustubh G. Joshi, MD
University of South Carolina School of Medicine
Rita Khoury, MD
Saint George Hospital University Medical Center
Suneeta Kumari, MD, MPH
Howard University Hospital
Michelle Magid, MD
Austin PsychCare PA
Michael Maksimowski, MD
Wayne State University
Jose Maldonado, MD
Stanford University
Thomas W. Meeks, MD
Portland VA Medical Center
John Miller, MD
University of South Florida
Armando Morera-Fumero, MD, PhD
Universidad de La Laguna
Mary K. Morreale, MD
Wayne State University
Philip Muskin, MD
Columbia University College of Physicians and Surgeons
Katharine Nelson, MD
University of Minnesota
Carol North, MD
University of Texas Southwestern Medical Center at Dallas
Douglas Opler, MD
Rutgers University School of Medicine
Joseph Pierre, MD
University of California, Los Angeles
Jerrold Pollak, PhD, ABN, ABPP
Seacoast Mental Health Center
Edwin Raffi, MD, MPH
Massachusetts General Hospital Center for Women’s Mental Health
Y. Pritham Raj, MD
Oregon Health and Science University
Jeffrey Rakofsky, MD
Emory University School of Medicine
Laura Ramsey, PhD
Cincinnati Children’s Hospital Medical Center
Erica Rapp, MD
University of Colorado School of Medicine
Abhishek Reddy, MD
The University of Alabama at Birmingham
Eduardo Rueda Vasquez, MD
Williamsport, Pennsylvania
Stephen Saklad, PharmD, BCPP
The University of Texas at Austin
Lauren Schwarz, PhD, ABPP-CN
Saint Louis University School of Medicine
Andreas Sidiropoulos, MD
University of Michigan
Shirshendu Sinha, MD
Mayo Clinic Health System and Mayo Clinic
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Jeffrey Sung, MD
University of Washington
Thida Thant, MD
University of Colorado at Denver
Adele Viguera, MD
Cleveland Clinic Lerner College of Medicine
Aparna Atluru, MD
Stanford Medicine
Anjan Bhattacharyya, MD
Saint Louis University
Caroline Bonham, MD
The University of New Mexico
Catherine Crone, MD
Inova Health System
Sheila Dowd, PhD
Rush Medical College
Ahmed Z. Elmaadawi, MD
Indiana University School of Medicine
Donald Gilbert, MD, MS
Cincinnati Children’s Hospital Medical Center
Mark Gold, MD
Washington University in St. Louis
Elana Harris, MD, PhD
Cincinnati Children’s Hospital
Susan Hatters-Friedman, MD
Case Western Reserve University
Faisal Islam, MD, MBA
Greenvale, New York
Kaustubh G. Joshi, MD
University of South Carolina School of Medicine
Rita Khoury, MD
Saint George Hospital University Medical Center
Suneeta Kumari, MD, MPH
Howard University Hospital
Michelle Magid, MD
Austin PsychCare PA
Michael Maksimowski, MD
Wayne State University
Jose Maldonado, MD
Stanford University
Thomas W. Meeks, MD
Portland VA Medical Center
John Miller, MD
University of South Florida
Armando Morera-Fumero, MD, PhD
Universidad de La Laguna
Mary K. Morreale, MD
Wayne State University
Philip Muskin, MD
Columbia University College of Physicians and Surgeons
Katharine Nelson, MD
University of Minnesota
Carol North, MD
University of Texas Southwestern Medical Center at Dallas
Douglas Opler, MD
Rutgers University School of Medicine
Joseph Pierre, MD
University of California, Los Angeles
Jerrold Pollak, PhD, ABN, ABPP
Seacoast Mental Health Center
Edwin Raffi, MD, MPH
Massachusetts General Hospital Center for Women’s Mental Health
Y. Pritham Raj, MD
Oregon Health and Science University
Jeffrey Rakofsky, MD
Emory University School of Medicine
Laura Ramsey, PhD
Cincinnati Children’s Hospital Medical Center
Erica Rapp, MD
University of Colorado School of Medicine
Abhishek Reddy, MD
The University of Alabama at Birmingham
Eduardo Rueda Vasquez, MD
Williamsport, Pennsylvania
Stephen Saklad, PharmD, BCPP
The University of Texas at Austin
Lauren Schwarz, PhD, ABPP-CN
Saint Louis University School of Medicine
Andreas Sidiropoulos, MD
University of Michigan
Shirshendu Sinha, MD
Mayo Clinic Health System and Mayo Clinic
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Jeffrey Sung, MD
University of Washington
Thida Thant, MD
University of Colorado at Denver
Adele Viguera, MD
Cleveland Clinic Lerner College of Medicine
Aparna Atluru, MD
Stanford Medicine
Anjan Bhattacharyya, MD
Saint Louis University
Caroline Bonham, MD
The University of New Mexico
Catherine Crone, MD
Inova Health System
Sheila Dowd, PhD
Rush Medical College
Ahmed Z. Elmaadawi, MD
Indiana University School of Medicine
Donald Gilbert, MD, MS
Cincinnati Children’s Hospital Medical Center
Mark Gold, MD
Washington University in St. Louis
Elana Harris, MD, PhD
Cincinnati Children’s Hospital
Susan Hatters-Friedman, MD
Case Western Reserve University
Faisal Islam, MD, MBA
Greenvale, New York
Kaustubh G. Joshi, MD
University of South Carolina School of Medicine
Rita Khoury, MD
Saint George Hospital University Medical Center
Suneeta Kumari, MD, MPH
Howard University Hospital
Michelle Magid, MD
Austin PsychCare PA
Michael Maksimowski, MD
Wayne State University
Jose Maldonado, MD
Stanford University
Thomas W. Meeks, MD
Portland VA Medical Center
John Miller, MD
University of South Florida
Armando Morera-Fumero, MD, PhD
Universidad de La Laguna
Mary K. Morreale, MD
Wayne State University
Philip Muskin, MD
Columbia University College of Physicians and Surgeons
Katharine Nelson, MD
University of Minnesota
Carol North, MD
University of Texas Southwestern Medical Center at Dallas
Douglas Opler, MD
Rutgers University School of Medicine
Joseph Pierre, MD
University of California, Los Angeles
Jerrold Pollak, PhD, ABN, ABPP
Seacoast Mental Health Center
Edwin Raffi, MD, MPH
Massachusetts General Hospital Center for Women’s Mental Health
Y. Pritham Raj, MD
Oregon Health and Science University
Jeffrey Rakofsky, MD
Emory University School of Medicine
Laura Ramsey, PhD
Cincinnati Children’s Hospital Medical Center
Erica Rapp, MD
University of Colorado School of Medicine
Abhishek Reddy, MD
The University of Alabama at Birmingham
Eduardo Rueda Vasquez, MD
Williamsport, Pennsylvania
Stephen Saklad, PharmD, BCPP
The University of Texas at Austin
Lauren Schwarz, PhD, ABPP-CN
Saint Louis University School of Medicine
Andreas Sidiropoulos, MD
University of Michigan
Shirshendu Sinha, MD
Mayo Clinic Health System and Mayo Clinic
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Jeffrey Sung, MD
University of Washington
Thida Thant, MD
University of Colorado at Denver
Adele Viguera, MD
Cleveland Clinic Lerner College of Medicine
Neuropsychological testing: A useful but underutilized resource
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
Bipolar disorder or borderline personality disorder?
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Losing a patient to suicide: Navigating the aftermath
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact education@afsp.org to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.

Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact education@afsp.org to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.

Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact education@afsp.org to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.