User login
Does your patient have the right to refuse medications?
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
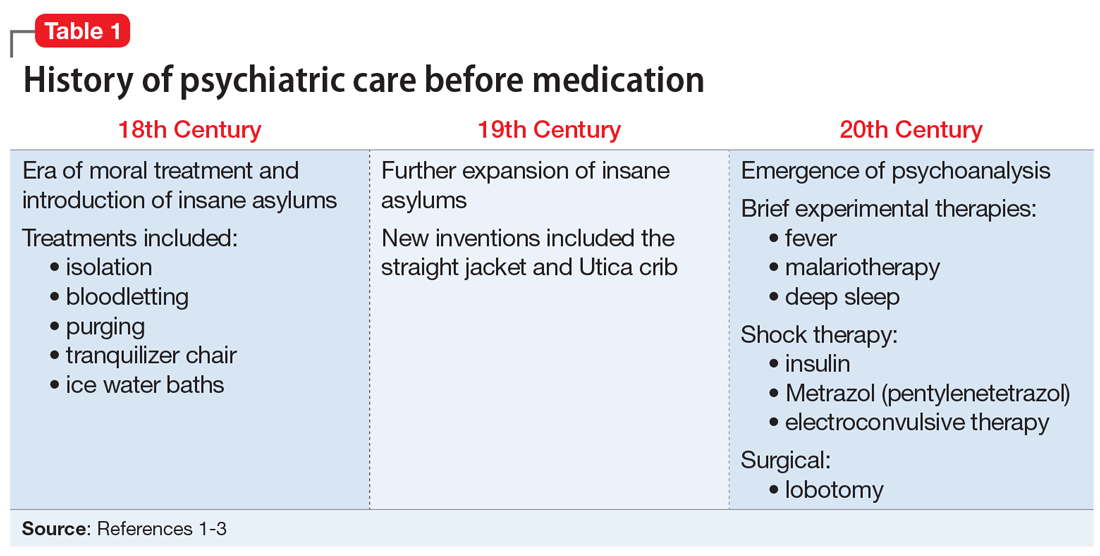
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
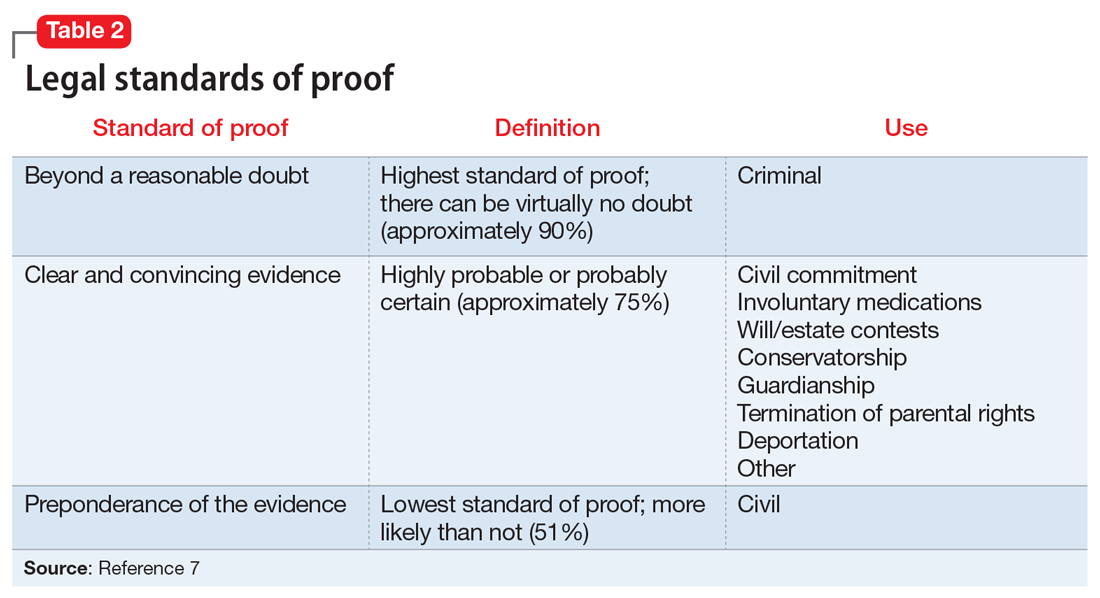
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
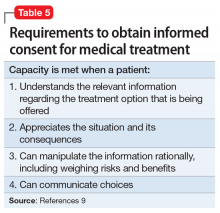
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
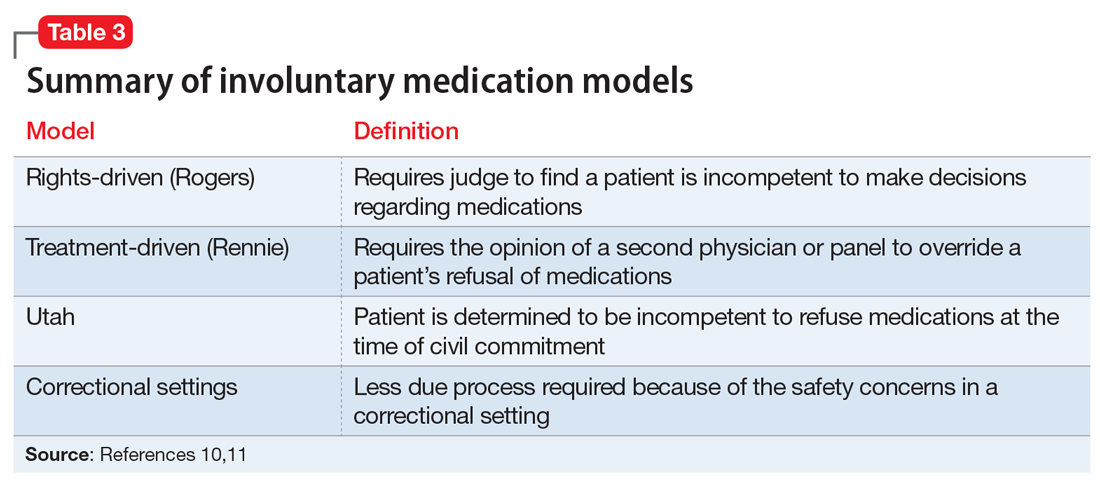
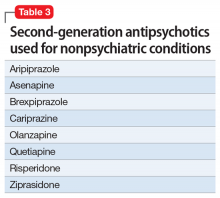
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
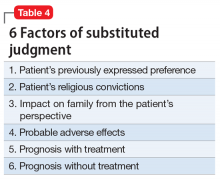
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
Second-generation long-acting injectable antipsychotics: A practical guide
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
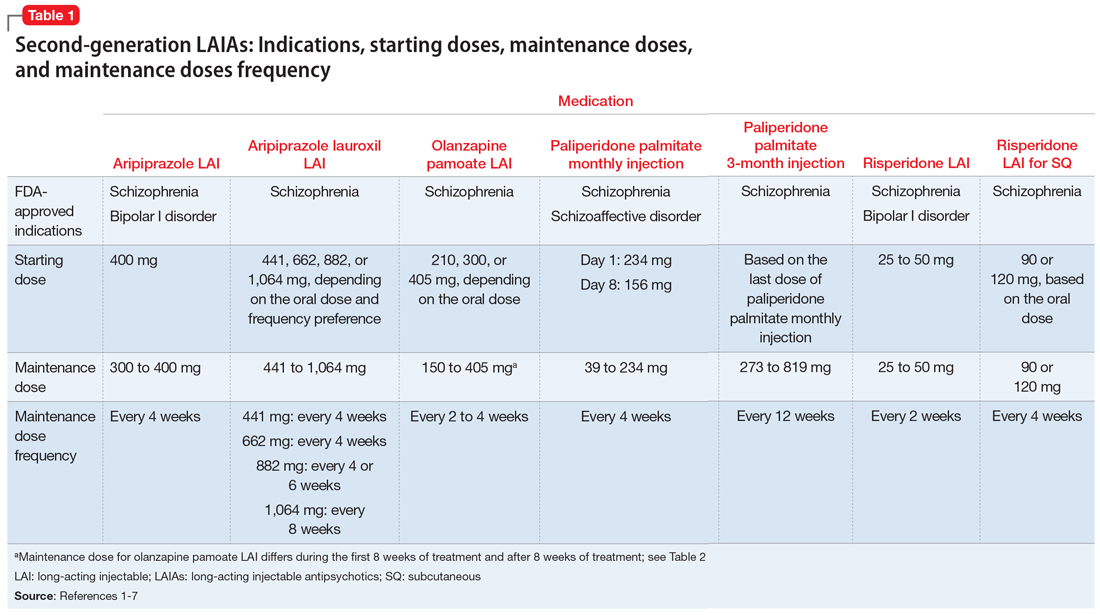
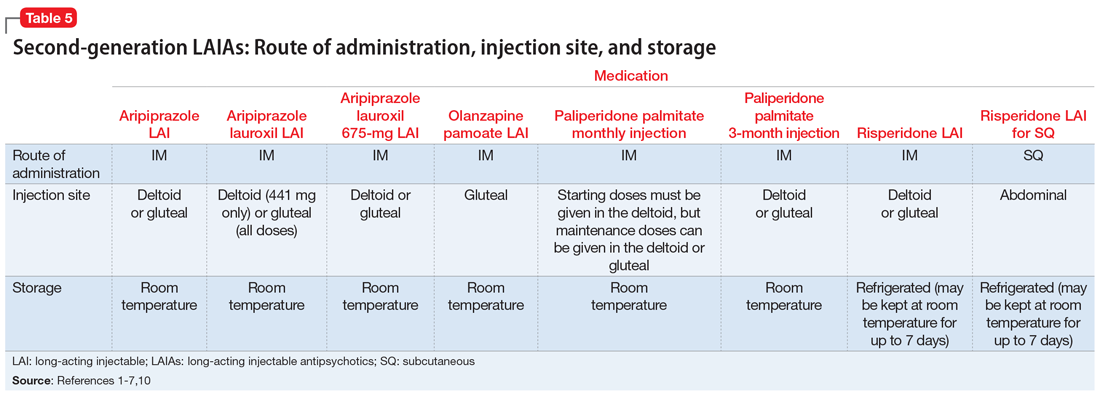
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
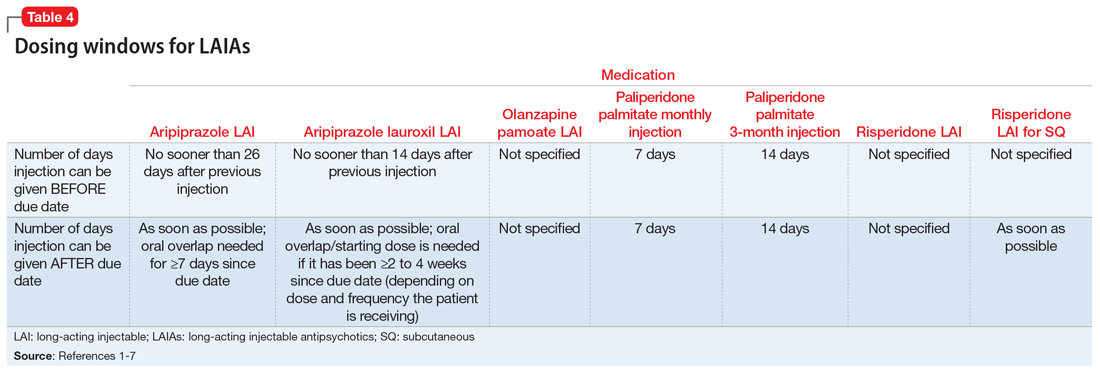
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
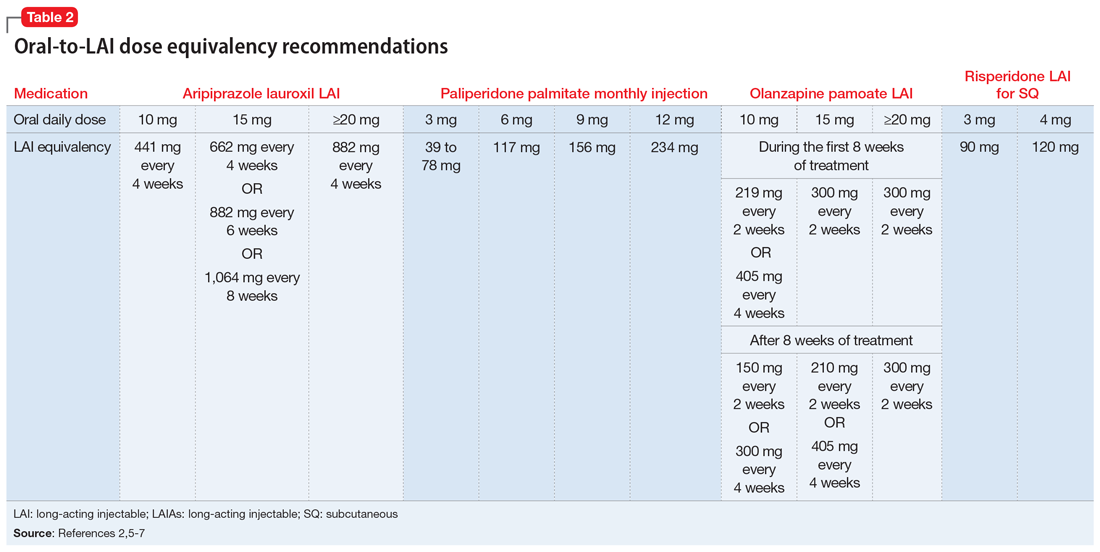
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
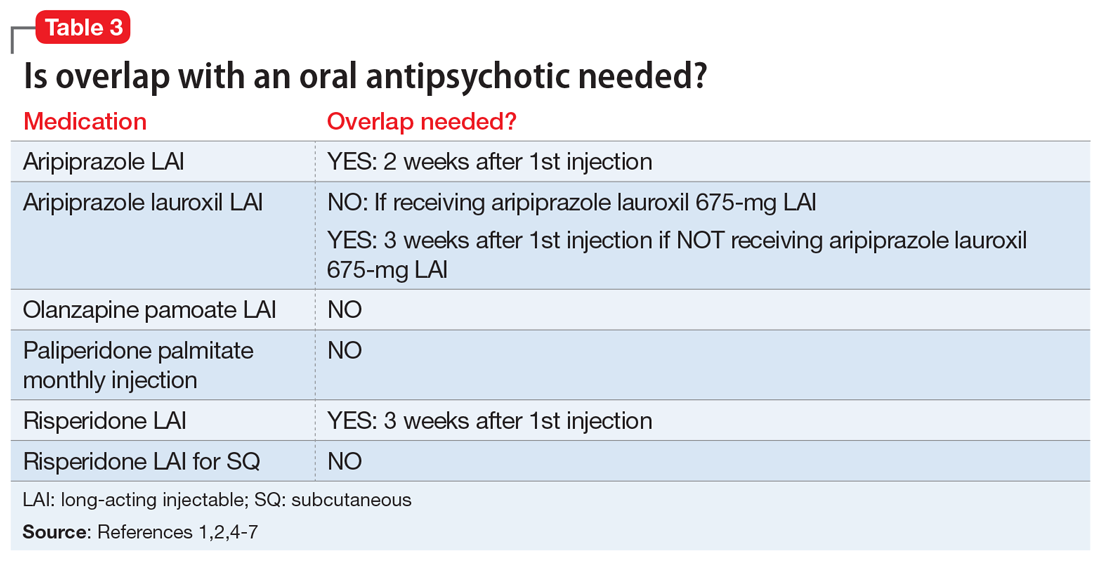
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
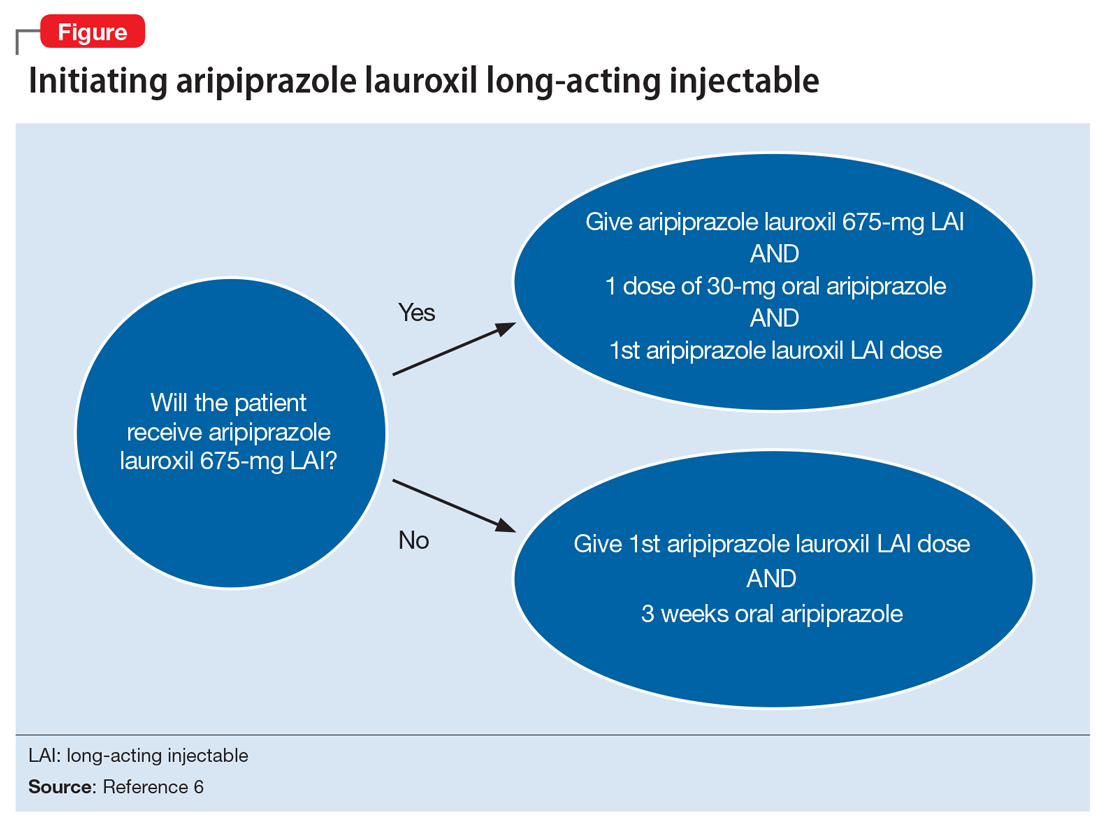
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
Kratom: What we know, what to tell your patients
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
Opioid use disorder in adolescents: An overview
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Top research findings of 2018-2019 for clinical practice
In Part 1 of this article, published in
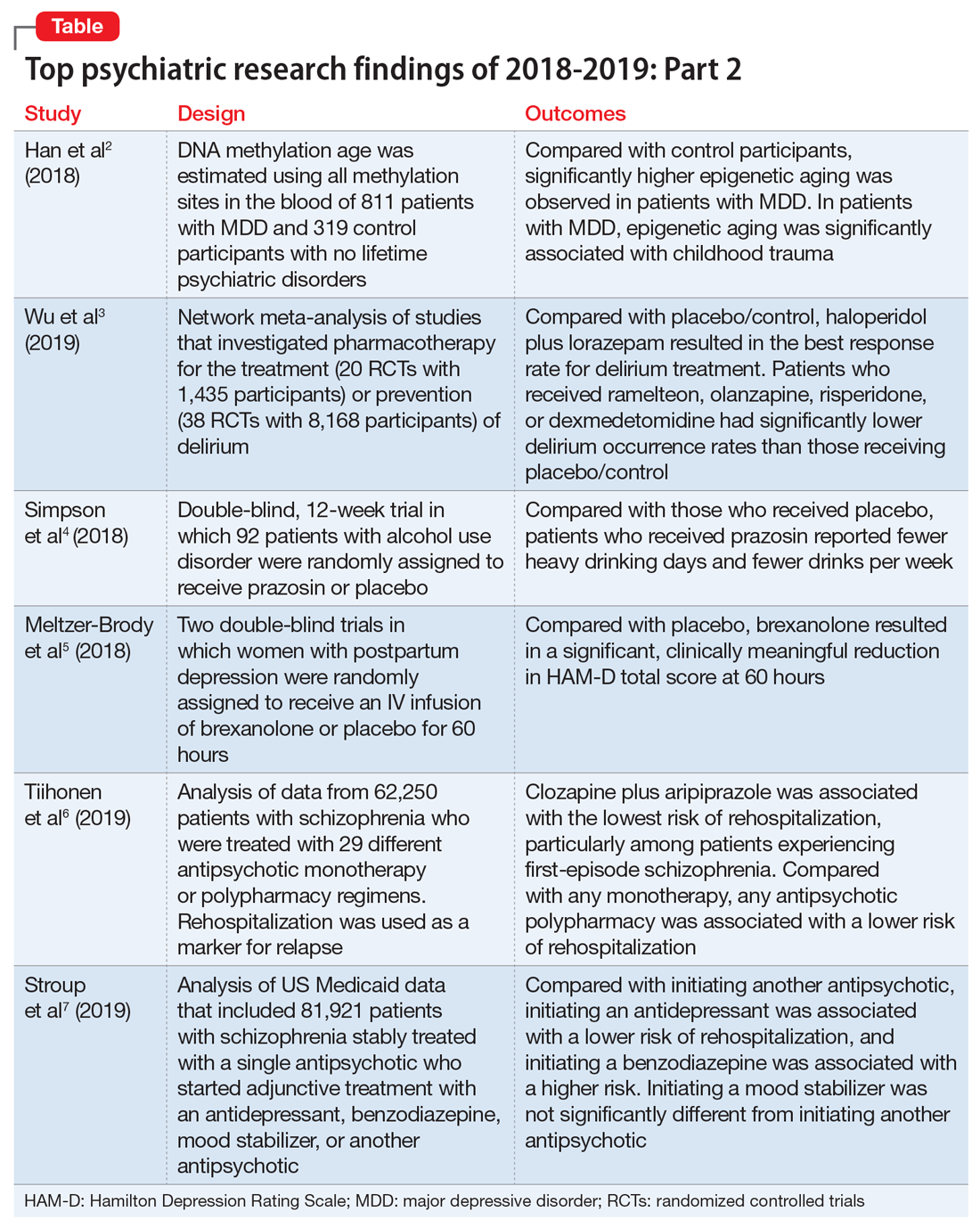
1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
Antipsychotics, dopamine, and pain
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796