User login
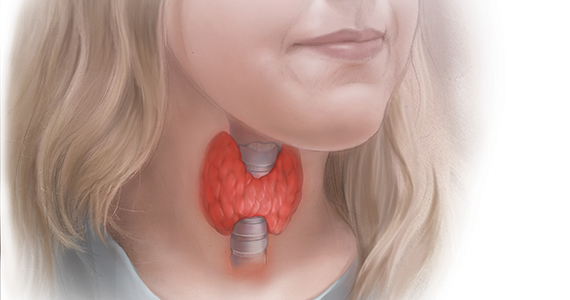
Subclinical hypothyroidism and pregnancy: Public health problem or lab finding with minimal clinical significance?
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
When providing contraceptive counseling to women with migraine headaches, how do you identify migraine with aura?
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
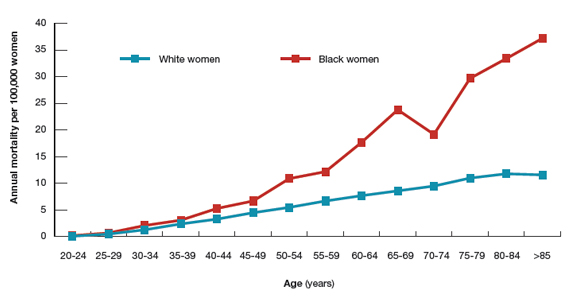
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
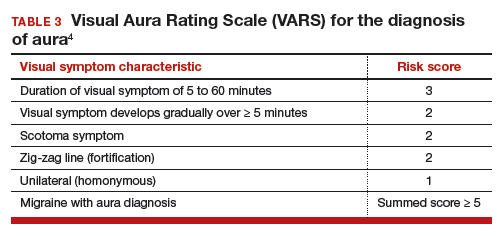
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4
Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Can we discern optimal long-term osteoporosis treatment for women?
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
To AROM or not to AROM: Does early amniotomy during induction of labor increase the risk of cesarean delivery?
De Vivo V, Carbone L, Saccone G, et al. Early amniotomy after cervical ripening for induction of labor: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2019. doi: 10.1016/j.ajog.2019.07.049.
EXPERT COMMENTARY
Induction of labor has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.1 Labor induction at term is associated with perinatal outcomes similar to those with spontaneous labor, without an increase in the CD rate.1-3 Although numerous methods for cervical ripening have been evaluated, the safest and most effective method has yet to be determined.2
Amniotomy—or artificial rupture of membranes (AROM)—has long been used as a technique for labor induction and for augmentation in women in spontaneous labor. Purported benefits include an increased responsiveness to exogenous oxytocin, decreased interval to delivery, and an increased likelihood of spontaneous vaginal delivery. Risks of amniotomy include injury to the fetus or surrounding tissues, bleeding, nonreassuring fetal testing, cord prolapse, and prolonged rupture of membranes (defined as longer than 18 hours), which is a risk factor for intra-amniotic infection.
The optimal timing of amniotomy is not known. The recent study by De Vivo and colleagues was designed to better understand the risk/benefit ratio of early amniotomy after cervical ripening in women undergoing induction of labor.
Details of the study
The authors conducted a systematic review and meta-analysis that included 1,273 women in 4 randomized controlled trials to determine the effectiveness of routine early amniotomy versus late amniotomy/spontaneous rupture of membranes after cervical ripening (with either a Foley catheter or prostaglandins) in women with a singleton vertex fetus undergoing induction of labor in the term or late preterm period.
Early amniotomy was defined as AROM “soon after cervical ripening” (cases); late amniotomy was defined as AROM after the active phase of labor or spontaneous rupture of membranes (controls).
The primary outcome was the incidence of CD. Secondary outcomes included the overall length of labor, latency from induction to delivery, and neonatal morbidity (a composite of birth weight, Apgar scores, meconium-stained amniotic fluid, neonatal sepsis, need for resuscitation, and admission to the neonatal intensive care unit).
Continue to: Findings...
Findings. Women randomly assigned to early amniotomy had a similar risk of CD compared with controls (31.1% vs 30.9% [relative risk (RR), 1.05; 95% confidence interval (CI), 0.71–1.56]) and a shorter interval from induction to delivery of about 5 hours (mean difference, -4.95 hours [95% CI, -8.12 to -1.78]).
There was no difference in any of the secondary outcome measures, although the number of events was small. Specifically, there was no significant difference in rates of chorioamnionitis between the early and late amniotomy cohorts (7.3% vs 4.8% [RR, 1.47; 95% CI, 0.95–2.28]).
Study strengths and limitations
This is the first systematic review to evaluate early versus late amniotomy after cervical ripening for induction of labor. “Systematic review and meta-analysis” is not synonymous with a review of the literature. It has its own methodology and is regarded as original research. A strength of this study is that it was performed by a highly credible team who followed established Cochrane and PRISMA methodological and reporting guidelines.
Study weaknesses include the fact that the meta-analysis contained a relatively small number of trials and study participants. It was significantly underpowered to address issues related to neonatal outcome. The 4 trials included were highly variable in terms of maternal parity and indications for labor induction and CD. The definition of “early amniotomy” was inconsistent, and the overall rate of CD varied greatly among the studies (7.9%–41.1%). Multiple pregnancies were excluded. Taken together, these findings may have limited generalizability.
This is the first systematic review to evaluate early versus late amniotomy/spontaneous rupture of membranes after cervical ripening for induction of labor. The study results suggest that amniotomy soon after cervical ripening does not change the likelihood of CD, but it does shorten the induction-to-delivery interval by around 5 hours. Prior studies have shown that early amniotomy in women in spontaneous labor decreases time to delivery by an average of 3 hours.4 Now we know that this is true also of early amniotomy following cervical ripening for induction of labor.
A number of questions still remain before early amniotomy is introduced into routine practice: Does group B streptococcus colonization status matter? Does this practice increase the risk of chorioamnionitis? At this time, it seems most prudent to individualize amniotomy timing based on a woman's obstetric history, indication for induction, and response to cervical ripening.
ERROL R. NORWITZ, MD, PHD, MBA, AND DIANA KOLETTIS, MD
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Saccone G, Berghella V. Induction of labor at full term in uncomplicated singleton gestations: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015;213:629-636.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Frigoletto FD Jr, Lieberman E, Lang JM, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333:745-750.
De Vivo V, Carbone L, Saccone G, et al. Early amniotomy after cervical ripening for induction of labor: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2019. doi: 10.1016/j.ajog.2019.07.049.
EXPERT COMMENTARY
Induction of labor has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.1 Labor induction at term is associated with perinatal outcomes similar to those with spontaneous labor, without an increase in the CD rate.1-3 Although numerous methods for cervical ripening have been evaluated, the safest and most effective method has yet to be determined.2
Amniotomy—or artificial rupture of membranes (AROM)—has long been used as a technique for labor induction and for augmentation in women in spontaneous labor. Purported benefits include an increased responsiveness to exogenous oxytocin, decreased interval to delivery, and an increased likelihood of spontaneous vaginal delivery. Risks of amniotomy include injury to the fetus or surrounding tissues, bleeding, nonreassuring fetal testing, cord prolapse, and prolonged rupture of membranes (defined as longer than 18 hours), which is a risk factor for intra-amniotic infection.
The optimal timing of amniotomy is not known. The recent study by De Vivo and colleagues was designed to better understand the risk/benefit ratio of early amniotomy after cervical ripening in women undergoing induction of labor.
Details of the study
The authors conducted a systematic review and meta-analysis that included 1,273 women in 4 randomized controlled trials to determine the effectiveness of routine early amniotomy versus late amniotomy/spontaneous rupture of membranes after cervical ripening (with either a Foley catheter or prostaglandins) in women with a singleton vertex fetus undergoing induction of labor in the term or late preterm period.
Early amniotomy was defined as AROM “soon after cervical ripening” (cases); late amniotomy was defined as AROM after the active phase of labor or spontaneous rupture of membranes (controls).
The primary outcome was the incidence of CD. Secondary outcomes included the overall length of labor, latency from induction to delivery, and neonatal morbidity (a composite of birth weight, Apgar scores, meconium-stained amniotic fluid, neonatal sepsis, need for resuscitation, and admission to the neonatal intensive care unit).
Continue to: Findings...
Findings. Women randomly assigned to early amniotomy had a similar risk of CD compared with controls (31.1% vs 30.9% [relative risk (RR), 1.05; 95% confidence interval (CI), 0.71–1.56]) and a shorter interval from induction to delivery of about 5 hours (mean difference, -4.95 hours [95% CI, -8.12 to -1.78]).
There was no difference in any of the secondary outcome measures, although the number of events was small. Specifically, there was no significant difference in rates of chorioamnionitis between the early and late amniotomy cohorts (7.3% vs 4.8% [RR, 1.47; 95% CI, 0.95–2.28]).
Study strengths and limitations
This is the first systematic review to evaluate early versus late amniotomy after cervical ripening for induction of labor. “Systematic review and meta-analysis” is not synonymous with a review of the literature. It has its own methodology and is regarded as original research. A strength of this study is that it was performed by a highly credible team who followed established Cochrane and PRISMA methodological and reporting guidelines.
Study weaknesses include the fact that the meta-analysis contained a relatively small number of trials and study participants. It was significantly underpowered to address issues related to neonatal outcome. The 4 trials included were highly variable in terms of maternal parity and indications for labor induction and CD. The definition of “early amniotomy” was inconsistent, and the overall rate of CD varied greatly among the studies (7.9%–41.1%). Multiple pregnancies were excluded. Taken together, these findings may have limited generalizability.
This is the first systematic review to evaluate early versus late amniotomy/spontaneous rupture of membranes after cervical ripening for induction of labor. The study results suggest that amniotomy soon after cervical ripening does not change the likelihood of CD, but it does shorten the induction-to-delivery interval by around 5 hours. Prior studies have shown that early amniotomy in women in spontaneous labor decreases time to delivery by an average of 3 hours.4 Now we know that this is true also of early amniotomy following cervical ripening for induction of labor.
A number of questions still remain before early amniotomy is introduced into routine practice: Does group B streptococcus colonization status matter? Does this practice increase the risk of chorioamnionitis? At this time, it seems most prudent to individualize amniotomy timing based on a woman's obstetric history, indication for induction, and response to cervical ripening.
ERROL R. NORWITZ, MD, PHD, MBA, AND DIANA KOLETTIS, MD
De Vivo V, Carbone L, Saccone G, et al. Early amniotomy after cervical ripening for induction of labor: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2019. doi: 10.1016/j.ajog.2019.07.049.
EXPERT COMMENTARY
Induction of labor has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.1 Labor induction at term is associated with perinatal outcomes similar to those with spontaneous labor, without an increase in the CD rate.1-3 Although numerous methods for cervical ripening have been evaluated, the safest and most effective method has yet to be determined.2
Amniotomy—or artificial rupture of membranes (AROM)—has long been used as a technique for labor induction and for augmentation in women in spontaneous labor. Purported benefits include an increased responsiveness to exogenous oxytocin, decreased interval to delivery, and an increased likelihood of spontaneous vaginal delivery. Risks of amniotomy include injury to the fetus or surrounding tissues, bleeding, nonreassuring fetal testing, cord prolapse, and prolonged rupture of membranes (defined as longer than 18 hours), which is a risk factor for intra-amniotic infection.
The optimal timing of amniotomy is not known. The recent study by De Vivo and colleagues was designed to better understand the risk/benefit ratio of early amniotomy after cervical ripening in women undergoing induction of labor.
Details of the study
The authors conducted a systematic review and meta-analysis that included 1,273 women in 4 randomized controlled trials to determine the effectiveness of routine early amniotomy versus late amniotomy/spontaneous rupture of membranes after cervical ripening (with either a Foley catheter or prostaglandins) in women with a singleton vertex fetus undergoing induction of labor in the term or late preterm period.
Early amniotomy was defined as AROM “soon after cervical ripening” (cases); late amniotomy was defined as AROM after the active phase of labor or spontaneous rupture of membranes (controls).
The primary outcome was the incidence of CD. Secondary outcomes included the overall length of labor, latency from induction to delivery, and neonatal morbidity (a composite of birth weight, Apgar scores, meconium-stained amniotic fluid, neonatal sepsis, need for resuscitation, and admission to the neonatal intensive care unit).
Continue to: Findings...
Findings. Women randomly assigned to early amniotomy had a similar risk of CD compared with controls (31.1% vs 30.9% [relative risk (RR), 1.05; 95% confidence interval (CI), 0.71–1.56]) and a shorter interval from induction to delivery of about 5 hours (mean difference, -4.95 hours [95% CI, -8.12 to -1.78]).
There was no difference in any of the secondary outcome measures, although the number of events was small. Specifically, there was no significant difference in rates of chorioamnionitis between the early and late amniotomy cohorts (7.3% vs 4.8% [RR, 1.47; 95% CI, 0.95–2.28]).
Study strengths and limitations
This is the first systematic review to evaluate early versus late amniotomy after cervical ripening for induction of labor. “Systematic review and meta-analysis” is not synonymous with a review of the literature. It has its own methodology and is regarded as original research. A strength of this study is that it was performed by a highly credible team who followed established Cochrane and PRISMA methodological and reporting guidelines.
Study weaknesses include the fact that the meta-analysis contained a relatively small number of trials and study participants. It was significantly underpowered to address issues related to neonatal outcome. The 4 trials included were highly variable in terms of maternal parity and indications for labor induction and CD. The definition of “early amniotomy” was inconsistent, and the overall rate of CD varied greatly among the studies (7.9%–41.1%). Multiple pregnancies were excluded. Taken together, these findings may have limited generalizability.
This is the first systematic review to evaluate early versus late amniotomy/spontaneous rupture of membranes after cervical ripening for induction of labor. The study results suggest that amniotomy soon after cervical ripening does not change the likelihood of CD, but it does shorten the induction-to-delivery interval by around 5 hours. Prior studies have shown that early amniotomy in women in spontaneous labor decreases time to delivery by an average of 3 hours.4 Now we know that this is true also of early amniotomy following cervical ripening for induction of labor.
A number of questions still remain before early amniotomy is introduced into routine practice: Does group B streptococcus colonization status matter? Does this practice increase the risk of chorioamnionitis? At this time, it seems most prudent to individualize amniotomy timing based on a woman's obstetric history, indication for induction, and response to cervical ripening.
ERROL R. NORWITZ, MD, PHD, MBA, AND DIANA KOLETTIS, MD
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Saccone G, Berghella V. Induction of labor at full term in uncomplicated singleton gestations: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015;213:629-636.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Frigoletto FD Jr, Lieberman E, Lang JM, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333:745-750.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Saccone G, Berghella V. Induction of labor at full term in uncomplicated singleton gestations: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015;213:629-636.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Frigoletto FD Jr, Lieberman E, Lang JM, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333:745-750.
Supporting our gender-diverse patients
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
Women with epilepsy: 5 clinical pearls for contraception and preconception counseling
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).

For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
Minimally invasive surgery for cervical cancer: Is surgeon volume a factor?
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
Why do so many women aged 65 years and older die of cervical cancer?
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1
A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
How do new BP guidelines affect identifying risk for hypertensive disorders of pregnancy?
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.