User login
How do new BP guidelines affect identifying risk for hypertensive disorders of pregnancy?
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol. 2019. pii: S0002-9378(19)30807-5. doi: 10.1016/j.ajog.2019.06.031.
EXPERT COMMENTARY
Hauspurg and colleagues set out to determine whether redefined BP category (normal, < 120/80 mm Hg) and trajectory (a difference of ≥ 5 mm Hg systolic, diastolic, or mean arterial pressure between the first and second prenatal visit) helps to identify women at increased risk for developing hypertensive disorders of pregnancy or preeclampsia.
With respect to the former variable, such an association was demonstrated in the first National Institutes of Health–funded preeclampsia prevention trial published in 1993, which used low-dose aspirin.1 In that trial, low-dose aspirin was not found to be effective in preventing preeclampsia in young, healthy nulliparous women. Interestingly, the 2 factors most associated with developing preeclampsia were an initial systolic BP of 120 to 134 mm Hg and an initial weight of >60 kg. For most clinicians, these findings would not be helpful in trying to better identify a high-risk group.
Details of the study
The idea of BP “trajectory” is interesting in the Hauspurg and colleagues’ study. The authors analyzed data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort study, and included a very large population of almost 9,000 women in the analysis. Participants were classified according to their BP measurement at the first study visit, with BP categories based on updated American College of Cardiology/American Heart Association guidelines. The primary outcome was the risk of hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia.
The data analysis found that elevated BP was associated with an adjusted risk ratio (aRR) of 1.54 (95% confidence interval [CI], 1.18–2.02). Stage 1 hypertension was associated with an aRR of 2.16 (95% CI, 1.31–3.57). Compared with women whose BP had a downward systolic trajectory, women with normal BP and an upward systolic trajectory had a 41% increased risk of any hypertensive disorder of pregnancy (aRR, 1.41; 95% CI, 1.20–1.65).
Study strengths and limitations
While the large study population is a strength of this study, there are a number of limitations, such as the use of BP measurements during pregnancy only, without having pre-pregnancy measurements available. Further, a single BP measurement during each visit is also a drawback, although the standardized measurement by study staff is a strength.
Anticlimactic conclusions. The conclusions of the study, however, are either not surprising, not clinically meaningful, or of little value to clinicians at present, at least with respect to patient management.
Continue to: Conclusions that were not surprising included...
Conclusions that were not surprising included a statistically lower chance of indicated preterm delivery in the normal BP group than in the elevated BP or stage 1 hypertension groups. Conclusions that were not meaningful included a statistically significant lower birthweight in the elevated BP group (3,269 g) and in the stage 1 hypertension group (3,258 g) compared with the normal BP group (3,279 g), but the clinical significance of these differences is arguable.
Lastly is the issue of what these data mean for clinical practice. The idea of identifying high-risk groups is attractive, provided that there are effective intervention strategies available. If one follows the United States Preventive Services Task Force (USPSTF) recommendations for preeclampsia prevention,2 then virtually every nulliparous woman is a candidate for low-dose aspirin for preeclampsia prophylaxis. Beyond that, the current data do not support any change in the standard clinical practice of managing these “now identified” high-risk women. Increasing prenatal visits, using biomarkers to further delineate risk, and using uterine artery Doppler studies are all strategies that have been or are being investigated, but as yet they are not supported by conclusive data documenting improved outcomes—a sentiment supported by both the USPSTF3 and the authors of the study.
Until further data are available, my advice to clinicians is to pay close attention to all risk factors for any of the hypertensive disorders of pregnancy. Initial BP and BP trajectory are important but probably something that sound clinical judgment would identify anyway. My recommendation is to continue to use those methods of prophylaxis, fetal surveillance, and indications for delivery that are supported by current data and await the additional investigations that Hauspurg and colleagues suggest need to be done before altering your management of women at increased risk for any of the hypertensive disorders of pregnancy.
JOHN T. REPKE, MD
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.
- Sibai BM, Caritis SN, Thom E, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine. Prevention of preeclampsia with low-dose aspirin in healthy nulliparous pregnant women. N Engl J Med. 1993;329:1213-1218.
- United States Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. September 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed July 30, 2019.
- United States Preventive Service Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for preeclampsia: US Preventive Services Task Force recommendation statement. JAMA. 2017;387:1661-1667.
Tips to improve immunization rates in your office
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.
Expert advice for immediate postpartum LARC insertion
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
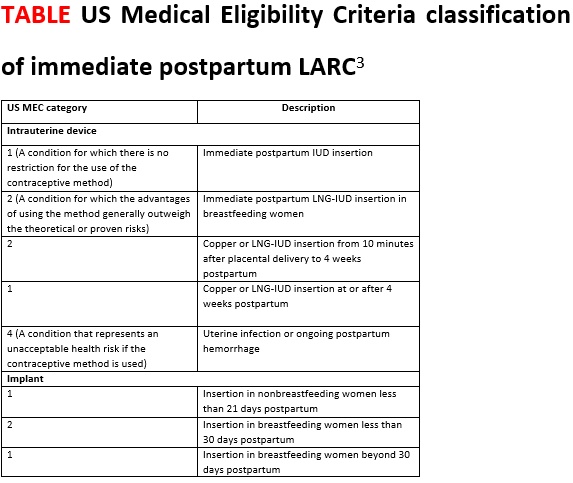
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.
OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.

OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.

OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.
Is the vaginal or buccal route more effective when administering prostaglandins for cervical ripening at term?
Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
EXPERT COMMENTARY
Cervical ripening is routine practice in women undergoing induction of labor who have an unfavorable cervical examination.1 This is because generating contractions against a long thick cervix is more likely to lead to failed induction and cesarean delivery. Cervical ripening can be achieved using mechanical or pharmacologic methods.
Misoprostol (a prostaglandin E1 [PGE1] analog) is approved by the US Food and Drug Administration for the treatment of peptic ulcer disease, but it also is widely used off-label for cervical ripening, partly due to its low cost. Misoprostol’s optimal dosing regimen and route of administration are not known. The IMPROVE trial was designed to address this knowledge gap, specifically to compare the efficacy and safety of VM versus BM in women undergoing labor induction at term.
Details of the study
The IMPROVE trial was a prospective, randomized, noninferiority, triple-masked, placebo-controlled trial of 300 women with a singleton vertex fetus requiring cervical ripening for induction of labor at term.2 Enrolled women were randomly assigned to VM or BM (same dosing regimen) and to a matching placebo administered via the opposite route.
Primary outcomes included time-to-vaginal-delivery from first dose, which was reduced in VM vs BM (20.1 vs 28.1 hours; P = .006), and urgent cesarean delivery for nonreassuring fetal testing, which was similarly reduced in VM (3.3% vs 9.5%; P = .33). These differences persisted after controlling for covariates. There was also a greater difference seen in multiparous versus nulliparous women.
Secondary outcomes also favored VM over BM, including more vaginal deliverieswithin 24 hours, fewer doses to achieve active labor, and a lower maximum dose of oxytocin.
Overall cesarean delivery rates were similar in the 2 groups (VM, 15.8%; BM, 22.3%; P = .15). There were no significant differences in other delivery characteristics or in maternal or fetal adverse events.
While a number of studies have evaluated the risk of cesarean delivery (CD) with the use of cervical ripening agents by different routes of administration, Handal-Orefice and colleagues studied this outcome specifically in a predominantly overweight population at a tertiary care center.1
The retrospective study included 276 women, of whom 91% had a body mass index (BMI) of 25 kg/m2 or more and 61% had a BMI of 30 kg/m2 or more at the time of delivery.
For cervical ripening, 138 women received vaginal misoprostol (25 µg) and 138 received oral misoprostol (50 µg). The frequency of CD (the primary study outcome) was significantly higher with oral compared with vaginal misoprostol use (32% vs 21%; P = .04). When the analysis was adjusted for age, BMI, parity, indication for induction, and Foley catheter use, the risk of CD remained significantly higher for the oral misoprostol group (adjusted odds ratio [aOR], 2.01; 95% confidence interval [CI], 1.07-3.76).
Other key findings:
- frequency of CD among nulliparous women: 41% in the oral misoprostol group, 28% in the vaginal misoprostol group (aOR, 2.79; 95% CI, 1.26-6.19)
- time to vaginal delivery: 41 hours for the oral misoprostol group, 31 hours for the vaginal misoprostol group (P = .01)
- uterine tachysystole: 11% in the oral misoprostol group, 20% in the vaginal misoprostol group (P = .04).
The authors noted that the strengths of the study, including the racial and ethnic diversity of the population (72% of women were of either black or Hispanic race or ethnicity), the commonly used doses of misoprostol, and the performance of inductions outside a research protocol, add to the generalizability of the results.
Reference
1. Handal-Orefice R, Friedman AM, Chouinard SM, et al. Oral or vaginal misoprostol for labor induction and cesarean delivery risk. Obstet Gynecol. 2019. doi:10.1097/AOG.0000000000003274.
Continue to: Study strengths and limitations...
Study strengths and limitations
The IMPROVE trial had a triple-blinded study design with an intention-to-treat paradigm and good follow-up. There was also standardization of PGE1 administration criteria, which was consistent with the American College of Obstetricians and Gynecologists standards of care. Results were similar to those of prior studies regarding rates of tachysystole, urgent cesarean delivery, and vaginal delivery.
The study has good generalizability as it included both elective and medically indicated inductions; however, patients with ruptured membranes were excluded. Although there was no difference in the overall cesarean delivery rates, the study was underpowered to look at this outcome. The authors included a patient satisfaction survey, but this is hard to interpret since study participants all received tablets orally and vaginally. The study did not address efficacy of VM versus BM administration at different doses or time intervals.
Labor induction has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.3 This number is likely to increase given recent data suggesting that routine induction at 39 weeks may significantly decrease cesarean delivery rates.4 It is critical, therefore, that we identify the optimal technique for cervical ripening, including the ideal dosing regimen and route of administration. Results of the IMPROVE trial suggest that vaginal administration of misoprostol (25 μg initial dose, 50 μg subsequent doses) may be superior to the buccal route, with more rapid vaginal delivery, more vaginal deliveries within 24 hours, and fewer urgent cesareans for nonreassuring fetal testing (although the overall cesarean delivery rate was not significantly different).
ERROL R. NORWITZ, MD, PHD, MBA; JULIE M. STONE, MD
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 107. Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
- Martin JA, Hamilton BE, Osterman M, et al. Births: final data for 2016. Nat Vital Stat Rep. 2018;67:1-55.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
EXPERT COMMENTARY
Cervical ripening is routine practice in women undergoing induction of labor who have an unfavorable cervical examination.1 This is because generating contractions against a long thick cervix is more likely to lead to failed induction and cesarean delivery. Cervical ripening can be achieved using mechanical or pharmacologic methods.
Misoprostol (a prostaglandin E1 [PGE1] analog) is approved by the US Food and Drug Administration for the treatment of peptic ulcer disease, but it also is widely used off-label for cervical ripening, partly due to its low cost. Misoprostol’s optimal dosing regimen and route of administration are not known. The IMPROVE trial was designed to address this knowledge gap, specifically to compare the efficacy and safety of VM versus BM in women undergoing labor induction at term.
Details of the study
The IMPROVE trial was a prospective, randomized, noninferiority, triple-masked, placebo-controlled trial of 300 women with a singleton vertex fetus requiring cervical ripening for induction of labor at term.2 Enrolled women were randomly assigned to VM or BM (same dosing regimen) and to a matching placebo administered via the opposite route.
Primary outcomes included time-to-vaginal-delivery from first dose, which was reduced in VM vs BM (20.1 vs 28.1 hours; P = .006), and urgent cesarean delivery for nonreassuring fetal testing, which was similarly reduced in VM (3.3% vs 9.5%; P = .33). These differences persisted after controlling for covariates. There was also a greater difference seen in multiparous versus nulliparous women.
Secondary outcomes also favored VM over BM, including more vaginal deliverieswithin 24 hours, fewer doses to achieve active labor, and a lower maximum dose of oxytocin.
Overall cesarean delivery rates were similar in the 2 groups (VM, 15.8%; BM, 22.3%; P = .15). There were no significant differences in other delivery characteristics or in maternal or fetal adverse events.
While a number of studies have evaluated the risk of cesarean delivery (CD) with the use of cervical ripening agents by different routes of administration, Handal-Orefice and colleagues studied this outcome specifically in a predominantly overweight population at a tertiary care center.1
The retrospective study included 276 women, of whom 91% had a body mass index (BMI) of 25 kg/m2 or more and 61% had a BMI of 30 kg/m2 or more at the time of delivery.
For cervical ripening, 138 women received vaginal misoprostol (25 µg) and 138 received oral misoprostol (50 µg). The frequency of CD (the primary study outcome) was significantly higher with oral compared with vaginal misoprostol use (32% vs 21%; P = .04). When the analysis was adjusted for age, BMI, parity, indication for induction, and Foley catheter use, the risk of CD remained significantly higher for the oral misoprostol group (adjusted odds ratio [aOR], 2.01; 95% confidence interval [CI], 1.07-3.76).
Other key findings:
- frequency of CD among nulliparous women: 41% in the oral misoprostol group, 28% in the vaginal misoprostol group (aOR, 2.79; 95% CI, 1.26-6.19)
- time to vaginal delivery: 41 hours for the oral misoprostol group, 31 hours for the vaginal misoprostol group (P = .01)
- uterine tachysystole: 11% in the oral misoprostol group, 20% in the vaginal misoprostol group (P = .04).
The authors noted that the strengths of the study, including the racial and ethnic diversity of the population (72% of women were of either black or Hispanic race or ethnicity), the commonly used doses of misoprostol, and the performance of inductions outside a research protocol, add to the generalizability of the results.
Reference
1. Handal-Orefice R, Friedman AM, Chouinard SM, et al. Oral or vaginal misoprostol for labor induction and cesarean delivery risk. Obstet Gynecol. 2019. doi:10.1097/AOG.0000000000003274.
Continue to: Study strengths and limitations...
Study strengths and limitations
The IMPROVE trial had a triple-blinded study design with an intention-to-treat paradigm and good follow-up. There was also standardization of PGE1 administration criteria, which was consistent with the American College of Obstetricians and Gynecologists standards of care. Results were similar to those of prior studies regarding rates of tachysystole, urgent cesarean delivery, and vaginal delivery.
The study has good generalizability as it included both elective and medically indicated inductions; however, patients with ruptured membranes were excluded. Although there was no difference in the overall cesarean delivery rates, the study was underpowered to look at this outcome. The authors included a patient satisfaction survey, but this is hard to interpret since study participants all received tablets orally and vaginally. The study did not address efficacy of VM versus BM administration at different doses or time intervals.
Labor induction has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.3 This number is likely to increase given recent data suggesting that routine induction at 39 weeks may significantly decrease cesarean delivery rates.4 It is critical, therefore, that we identify the optimal technique for cervical ripening, including the ideal dosing regimen and route of administration. Results of the IMPROVE trial suggest that vaginal administration of misoprostol (25 μg initial dose, 50 μg subsequent doses) may be superior to the buccal route, with more rapid vaginal delivery, more vaginal deliveries within 24 hours, and fewer urgent cesareans for nonreassuring fetal testing (although the overall cesarean delivery rate was not significantly different).
ERROL R. NORWITZ, MD, PHD, MBA; JULIE M. STONE, MD
Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
EXPERT COMMENTARY
Cervical ripening is routine practice in women undergoing induction of labor who have an unfavorable cervical examination.1 This is because generating contractions against a long thick cervix is more likely to lead to failed induction and cesarean delivery. Cervical ripening can be achieved using mechanical or pharmacologic methods.
Misoprostol (a prostaglandin E1 [PGE1] analog) is approved by the US Food and Drug Administration for the treatment of peptic ulcer disease, but it also is widely used off-label for cervical ripening, partly due to its low cost. Misoprostol’s optimal dosing regimen and route of administration are not known. The IMPROVE trial was designed to address this knowledge gap, specifically to compare the efficacy and safety of VM versus BM in women undergoing labor induction at term.
Details of the study
The IMPROVE trial was a prospective, randomized, noninferiority, triple-masked, placebo-controlled trial of 300 women with a singleton vertex fetus requiring cervical ripening for induction of labor at term.2 Enrolled women were randomly assigned to VM or BM (same dosing regimen) and to a matching placebo administered via the opposite route.
Primary outcomes included time-to-vaginal-delivery from first dose, which was reduced in VM vs BM (20.1 vs 28.1 hours; P = .006), and urgent cesarean delivery for nonreassuring fetal testing, which was similarly reduced in VM (3.3% vs 9.5%; P = .33). These differences persisted after controlling for covariates. There was also a greater difference seen in multiparous versus nulliparous women.
Secondary outcomes also favored VM over BM, including more vaginal deliverieswithin 24 hours, fewer doses to achieve active labor, and a lower maximum dose of oxytocin.
Overall cesarean delivery rates were similar in the 2 groups (VM, 15.8%; BM, 22.3%; P = .15). There were no significant differences in other delivery characteristics or in maternal or fetal adverse events.
While a number of studies have evaluated the risk of cesarean delivery (CD) with the use of cervical ripening agents by different routes of administration, Handal-Orefice and colleagues studied this outcome specifically in a predominantly overweight population at a tertiary care center.1
The retrospective study included 276 women, of whom 91% had a body mass index (BMI) of 25 kg/m2 or more and 61% had a BMI of 30 kg/m2 or more at the time of delivery.
For cervical ripening, 138 women received vaginal misoprostol (25 µg) and 138 received oral misoprostol (50 µg). The frequency of CD (the primary study outcome) was significantly higher with oral compared with vaginal misoprostol use (32% vs 21%; P = .04). When the analysis was adjusted for age, BMI, parity, indication for induction, and Foley catheter use, the risk of CD remained significantly higher for the oral misoprostol group (adjusted odds ratio [aOR], 2.01; 95% confidence interval [CI], 1.07-3.76).
Other key findings:
- frequency of CD among nulliparous women: 41% in the oral misoprostol group, 28% in the vaginal misoprostol group (aOR, 2.79; 95% CI, 1.26-6.19)
- time to vaginal delivery: 41 hours for the oral misoprostol group, 31 hours for the vaginal misoprostol group (P = .01)
- uterine tachysystole: 11% in the oral misoprostol group, 20% in the vaginal misoprostol group (P = .04).
The authors noted that the strengths of the study, including the racial and ethnic diversity of the population (72% of women were of either black or Hispanic race or ethnicity), the commonly used doses of misoprostol, and the performance of inductions outside a research protocol, add to the generalizability of the results.
Reference
1. Handal-Orefice R, Friedman AM, Chouinard SM, et al. Oral or vaginal misoprostol for labor induction and cesarean delivery risk. Obstet Gynecol. 2019. doi:10.1097/AOG.0000000000003274.
Continue to: Study strengths and limitations...
Study strengths and limitations
The IMPROVE trial had a triple-blinded study design with an intention-to-treat paradigm and good follow-up. There was also standardization of PGE1 administration criteria, which was consistent with the American College of Obstetricians and Gynecologists standards of care. Results were similar to those of prior studies regarding rates of tachysystole, urgent cesarean delivery, and vaginal delivery.
The study has good generalizability as it included both elective and medically indicated inductions; however, patients with ruptured membranes were excluded. Although there was no difference in the overall cesarean delivery rates, the study was underpowered to look at this outcome. The authors included a patient satisfaction survey, but this is hard to interpret since study participants all received tablets orally and vaginally. The study did not address efficacy of VM versus BM administration at different doses or time intervals.
Labor induction has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.3 This number is likely to increase given recent data suggesting that routine induction at 39 weeks may significantly decrease cesarean delivery rates.4 It is critical, therefore, that we identify the optimal technique for cervical ripening, including the ideal dosing regimen and route of administration. Results of the IMPROVE trial suggest that vaginal administration of misoprostol (25 μg initial dose, 50 μg subsequent doses) may be superior to the buccal route, with more rapid vaginal delivery, more vaginal deliveries within 24 hours, and fewer urgent cesareans for nonreassuring fetal testing (although the overall cesarean delivery rate was not significantly different).
ERROL R. NORWITZ, MD, PHD, MBA; JULIE M. STONE, MD
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 107. Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
- Martin JA, Hamilton BE, Osterman M, et al. Births: final data for 2016. Nat Vital Stat Rep. 2018;67:1-55.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 107. Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
- Martin JA, Hamilton BE, Osterman M, et al. Births: final data for 2016. Nat Vital Stat Rep. 2018;67:1-55.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
Uterus-sparing interventions to treat postpartum hemorrhage during cesarean delivery surgery
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.
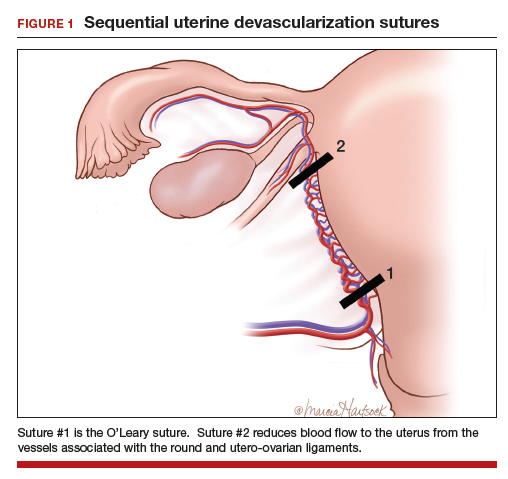
In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.
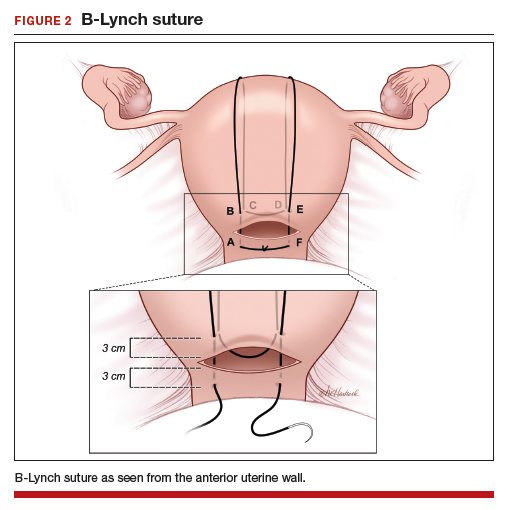
The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4
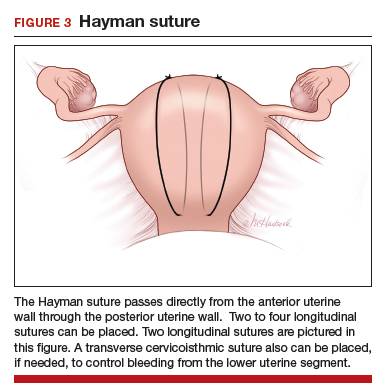
The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.
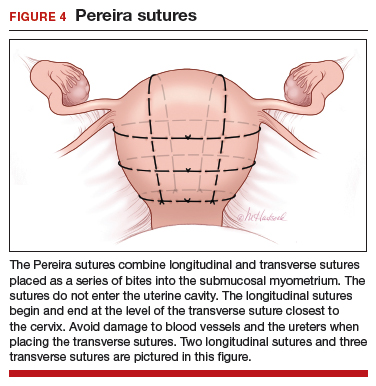
Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
What’s in store for ObGyn reimbursement in the EHR age and beyond
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In women with late preterm mild hypertensive disorders, does immediate delivery versus expectant management differ in terms of neonatal neurodevelopmental outcomes?
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
Maternal mortality: Critical next steps in addressing the crisis
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1
A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1

A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1

A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.