User login
Reimbursement Readiness
Doctors shouldn’t have to worry about financial issues. The welfare of our patients should be our only concern.
We should be able to devote our full attention to studying how best to serve the needs of the people we care for. We shouldn’t need to spend time learning about healthcare reform or things like ICD-9 (or ICD-10!)—things that don’t help us provide better care to patients.
But these are pie-in-the-sky dreams. As far as I can tell, all healthcare systems require caregivers to attend to economics and data management that aren’t directly tied to clinical care. Our system depends on all caregivers devoting some time to learn how the system is organized, and keeping up with how it evolves. And the crisis in runaway costs in U.S. healthcare only increases the need for all who work in healthcare to devote significant time (too much) to the operational (nonclinical side) of healthcare.
Hospitalist practice is a much simpler business to manage and operate than most forms of clinical practice. There usually is no building to rent, few nonclinical employees to manage, and a comparatively simple financial model. And if employed by a hospital or other large entity, nonclinicians handle most of the “business management.” So when it comes to the number of brain cells diverted to business rather than clinical concerns, hospitalists start with an advantage over most other specialties.
Still, we have a lot of nonclinical stuff to keep up with. Consider the concept of “managing to Medicare reimbursement.” This means managing a practice or hospital in a way that minimizes the failure to capture all appropriate Medicare reimbursement dollars. Even if you’ve never heard of this concept before, there are probably a lot of people at your hospital who have this as their main responsibility, and clinicians should know something about it.
So in an effort to distract the fewest brain cells away from clinical matters, here is a very simple overview of some components of managing to Medicare reimbursement relevant to hospitalists. This isn’t a comprehensive list, only some hospitalist-relevant highlights.
Medicare Reimbursement Today
Accurate determination of inpatient vs. observation status. Wow, this can get complicated. Most hospitals have people who devote significant time to doing this for patients every day, and even those experts sometimes disagree on the appropriate status. But all hospitalists should have a basic understanding of how this works and a willingness to answer questions from the hospital’s experts, and, when appropriate, write additional information in the chart to clarify the appropriate status.
Optimal resource utilization, including length of stay. Because Medicare pays an essentially fixed amount based on the diagnoses for each inpatient admission, managing costs is critical to a hospital’s financial well-being. Hospitalists have a huge role in this. And regardless of how Medicare reimburses for services, there is clinical rationale for being careful about resources used and how long someone stays in a hospital. In many cases, more is not better—and it even could be worse—for the patient.
Optimal clinical documentation and accurate DRG assignment. Good documentation is important for clinical care, but beyond that, the precise way things are documented can have significant influence on Medicare reimbursement. Low potassium might in some cases lead to higher reimbursement, but a doctor must write “hypokalemia”; simply writing K+ means the hospital can’t include hypokalemia as a diagnosis. (A doctor, nurse practitioner, or physician assistant must write out “hypokalemia” only once for Medicare purposes; it would then be fine to use K+ in the chart every other time.)
Say you have a patient with a UTI and sepsis. Write only “urosepsis,” and the hospital must bill for cystitis—low reimbursement. Write “urinary tract infection with sepsis,” and the hospital can bill for higher reimbursement.
There should be people at your hospital who are experts at this, and all hospitalists should work with them to learn appropriate documentation language to describe illnesses correctly for billing purposes. Many hospitals use a system of “DRG queries,” which hospitalists should always respond to (though they should agree with the issue raised, such as “was the pneumonia likely due to aspiration?” only when clinically appropriate).
Change Is Coming
Don’t make the mistake of thinking Medicare reimbursement is a static phenomenon. It is undergoing rapid and significant evolution. For example, the Affordable Care Act, aka healthcare reform legislation, provides for a number of changes hospitalists need to understand.
I suggest that you make sure to understand your hospital’s or medical group’s position on accountable-care organizations (ACOs). It is a pretty complicated program that, in the first few years, has modest impact on reimbursement. If the ACO performs well, the additional reimbursement to an organization might pay for little more than the staff salaries of the staff that managed the considerable complexity of enrolling in and reporting for the program. And there is a risk the organization could lose money if it doesn’t perform well. So many organizations have decided not to pursue participation as an ACO, but they may decide to put in place most of the elements of an ACO without enrolling in the program. Some refer to this as an “aco” rather than an “ACO.”
Value-based purchasing (VBP) is set to influence hospital reimbursement rates starting in 2013 based on a hospital’s performance in 2012. SHM has a terrific VBP toolkit available online.
Bundled payments and financial penalties for readmissions also take effect in 2013. Now is the time ensure that you understand the implications of these programs; they are designed so that the financial impact to most organizations will be modest.
Reimbursement penalties for a specified list of hospital-acquired conditions (HACs) will begin in 2015. Conditions most relevant for hospitalists include vascular catheter-related bloodstream infections, catheter-related urinary infection, or manifestations of poor glycemic control (HONK, DKA, hypo-/hyperglycemia).
I plan to address some of these programs in greater detail in future practice management columns.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Doctors shouldn’t have to worry about financial issues. The welfare of our patients should be our only concern.
We should be able to devote our full attention to studying how best to serve the needs of the people we care for. We shouldn’t need to spend time learning about healthcare reform or things like ICD-9 (or ICD-10!)—things that don’t help us provide better care to patients.
But these are pie-in-the-sky dreams. As far as I can tell, all healthcare systems require caregivers to attend to economics and data management that aren’t directly tied to clinical care. Our system depends on all caregivers devoting some time to learn how the system is organized, and keeping up with how it evolves. And the crisis in runaway costs in U.S. healthcare only increases the need for all who work in healthcare to devote significant time (too much) to the operational (nonclinical side) of healthcare.
Hospitalist practice is a much simpler business to manage and operate than most forms of clinical practice. There usually is no building to rent, few nonclinical employees to manage, and a comparatively simple financial model. And if employed by a hospital or other large entity, nonclinicians handle most of the “business management.” So when it comes to the number of brain cells diverted to business rather than clinical concerns, hospitalists start with an advantage over most other specialties.
Still, we have a lot of nonclinical stuff to keep up with. Consider the concept of “managing to Medicare reimbursement.” This means managing a practice or hospital in a way that minimizes the failure to capture all appropriate Medicare reimbursement dollars. Even if you’ve never heard of this concept before, there are probably a lot of people at your hospital who have this as their main responsibility, and clinicians should know something about it.
So in an effort to distract the fewest brain cells away from clinical matters, here is a very simple overview of some components of managing to Medicare reimbursement relevant to hospitalists. This isn’t a comprehensive list, only some hospitalist-relevant highlights.
Medicare Reimbursement Today
Accurate determination of inpatient vs. observation status. Wow, this can get complicated. Most hospitals have people who devote significant time to doing this for patients every day, and even those experts sometimes disagree on the appropriate status. But all hospitalists should have a basic understanding of how this works and a willingness to answer questions from the hospital’s experts, and, when appropriate, write additional information in the chart to clarify the appropriate status.
Optimal resource utilization, including length of stay. Because Medicare pays an essentially fixed amount based on the diagnoses for each inpatient admission, managing costs is critical to a hospital’s financial well-being. Hospitalists have a huge role in this. And regardless of how Medicare reimburses for services, there is clinical rationale for being careful about resources used and how long someone stays in a hospital. In many cases, more is not better—and it even could be worse—for the patient.
Optimal clinical documentation and accurate DRG assignment. Good documentation is important for clinical care, but beyond that, the precise way things are documented can have significant influence on Medicare reimbursement. Low potassium might in some cases lead to higher reimbursement, but a doctor must write “hypokalemia”; simply writing K+ means the hospital can’t include hypokalemia as a diagnosis. (A doctor, nurse practitioner, or physician assistant must write out “hypokalemia” only once for Medicare purposes; it would then be fine to use K+ in the chart every other time.)
Say you have a patient with a UTI and sepsis. Write only “urosepsis,” and the hospital must bill for cystitis—low reimbursement. Write “urinary tract infection with sepsis,” and the hospital can bill for higher reimbursement.
There should be people at your hospital who are experts at this, and all hospitalists should work with them to learn appropriate documentation language to describe illnesses correctly for billing purposes. Many hospitals use a system of “DRG queries,” which hospitalists should always respond to (though they should agree with the issue raised, such as “was the pneumonia likely due to aspiration?” only when clinically appropriate).
Change Is Coming
Don’t make the mistake of thinking Medicare reimbursement is a static phenomenon. It is undergoing rapid and significant evolution. For example, the Affordable Care Act, aka healthcare reform legislation, provides for a number of changes hospitalists need to understand.
I suggest that you make sure to understand your hospital’s or medical group’s position on accountable-care organizations (ACOs). It is a pretty complicated program that, in the first few years, has modest impact on reimbursement. If the ACO performs well, the additional reimbursement to an organization might pay for little more than the staff salaries of the staff that managed the considerable complexity of enrolling in and reporting for the program. And there is a risk the organization could lose money if it doesn’t perform well. So many organizations have decided not to pursue participation as an ACO, but they may decide to put in place most of the elements of an ACO without enrolling in the program. Some refer to this as an “aco” rather than an “ACO.”
Value-based purchasing (VBP) is set to influence hospital reimbursement rates starting in 2013 based on a hospital’s performance in 2012. SHM has a terrific VBP toolkit available online.
Bundled payments and financial penalties for readmissions also take effect in 2013. Now is the time ensure that you understand the implications of these programs; they are designed so that the financial impact to most organizations will be modest.
Reimbursement penalties for a specified list of hospital-acquired conditions (HACs) will begin in 2015. Conditions most relevant for hospitalists include vascular catheter-related bloodstream infections, catheter-related urinary infection, or manifestations of poor glycemic control (HONK, DKA, hypo-/hyperglycemia).
I plan to address some of these programs in greater detail in future practice management columns.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Doctors shouldn’t have to worry about financial issues. The welfare of our patients should be our only concern.
We should be able to devote our full attention to studying how best to serve the needs of the people we care for. We shouldn’t need to spend time learning about healthcare reform or things like ICD-9 (or ICD-10!)—things that don’t help us provide better care to patients.
But these are pie-in-the-sky dreams. As far as I can tell, all healthcare systems require caregivers to attend to economics and data management that aren’t directly tied to clinical care. Our system depends on all caregivers devoting some time to learn how the system is organized, and keeping up with how it evolves. And the crisis in runaway costs in U.S. healthcare only increases the need for all who work in healthcare to devote significant time (too much) to the operational (nonclinical side) of healthcare.
Hospitalist practice is a much simpler business to manage and operate than most forms of clinical practice. There usually is no building to rent, few nonclinical employees to manage, and a comparatively simple financial model. And if employed by a hospital or other large entity, nonclinicians handle most of the “business management.” So when it comes to the number of brain cells diverted to business rather than clinical concerns, hospitalists start with an advantage over most other specialties.
Still, we have a lot of nonclinical stuff to keep up with. Consider the concept of “managing to Medicare reimbursement.” This means managing a practice or hospital in a way that minimizes the failure to capture all appropriate Medicare reimbursement dollars. Even if you’ve never heard of this concept before, there are probably a lot of people at your hospital who have this as their main responsibility, and clinicians should know something about it.
So in an effort to distract the fewest brain cells away from clinical matters, here is a very simple overview of some components of managing to Medicare reimbursement relevant to hospitalists. This isn’t a comprehensive list, only some hospitalist-relevant highlights.
Medicare Reimbursement Today
Accurate determination of inpatient vs. observation status. Wow, this can get complicated. Most hospitals have people who devote significant time to doing this for patients every day, and even those experts sometimes disagree on the appropriate status. But all hospitalists should have a basic understanding of how this works and a willingness to answer questions from the hospital’s experts, and, when appropriate, write additional information in the chart to clarify the appropriate status.
Optimal resource utilization, including length of stay. Because Medicare pays an essentially fixed amount based on the diagnoses for each inpatient admission, managing costs is critical to a hospital’s financial well-being. Hospitalists have a huge role in this. And regardless of how Medicare reimburses for services, there is clinical rationale for being careful about resources used and how long someone stays in a hospital. In many cases, more is not better—and it even could be worse—for the patient.
Optimal clinical documentation and accurate DRG assignment. Good documentation is important for clinical care, but beyond that, the precise way things are documented can have significant influence on Medicare reimbursement. Low potassium might in some cases lead to higher reimbursement, but a doctor must write “hypokalemia”; simply writing K+ means the hospital can’t include hypokalemia as a diagnosis. (A doctor, nurse practitioner, or physician assistant must write out “hypokalemia” only once for Medicare purposes; it would then be fine to use K+ in the chart every other time.)
Say you have a patient with a UTI and sepsis. Write only “urosepsis,” and the hospital must bill for cystitis—low reimbursement. Write “urinary tract infection with sepsis,” and the hospital can bill for higher reimbursement.
There should be people at your hospital who are experts at this, and all hospitalists should work with them to learn appropriate documentation language to describe illnesses correctly for billing purposes. Many hospitals use a system of “DRG queries,” which hospitalists should always respond to (though they should agree with the issue raised, such as “was the pneumonia likely due to aspiration?” only when clinically appropriate).
Change Is Coming
Don’t make the mistake of thinking Medicare reimbursement is a static phenomenon. It is undergoing rapid and significant evolution. For example, the Affordable Care Act, aka healthcare reform legislation, provides for a number of changes hospitalists need to understand.
I suggest that you make sure to understand your hospital’s or medical group’s position on accountable-care organizations (ACOs). It is a pretty complicated program that, in the first few years, has modest impact on reimbursement. If the ACO performs well, the additional reimbursement to an organization might pay for little more than the staff salaries of the staff that managed the considerable complexity of enrolling in and reporting for the program. And there is a risk the organization could lose money if it doesn’t perform well. So many organizations have decided not to pursue participation as an ACO, but they may decide to put in place most of the elements of an ACO without enrolling in the program. Some refer to this as an “aco” rather than an “ACO.”
Value-based purchasing (VBP) is set to influence hospital reimbursement rates starting in 2013 based on a hospital’s performance in 2012. SHM has a terrific VBP toolkit available online.
Bundled payments and financial penalties for readmissions also take effect in 2013. Now is the time ensure that you understand the implications of these programs; they are designed so that the financial impact to most organizations will be modest.
Reimbursement penalties for a specified list of hospital-acquired conditions (HACs) will begin in 2015. Conditions most relevant for hospitalists include vascular catheter-related bloodstream infections, catheter-related urinary infection, or manifestations of poor glycemic control (HONK, DKA, hypo-/hyperglycemia).
I plan to address some of these programs in greater detail in future practice management columns.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
What is the recommended approach to a breast mass in a woman younger than 25 years?
Scant research has focused on breast cancer in very young women. This retrospective case series by investigators at the Mayo Clinic assessed girls and women younger than 25 years who were given a diagnosis of primary breast cancer between 1935 and 2005 and who received care at that institution.
The investigators highlighted many of the challenges clinicians face when a young patient presents with a lump or other signs associated with breast cancer. For example, they note that, in its early stages, breast carcinoma in young women can be similar in appearance to fibroadenoma. When a patient postpones care or a clinician dismisses the lump because of a low index of suspicion, diagnosis can be delayed. That is problematic because invasive breast carcinoma in girls and young women is more aggressive and associated with a poorer prognosis overall.
- When the patient has a medical history that arouses concern, such as a history of malignancy, a family history of breast or ovarian cancer at a young age, a history of BRCA mutation, a rapidly growing mass, or constitutional symptoms of malignancy.
- When the physical examination reveals fever, weight loss, anemia, systemic lymphadenopathy, other masses, or hepatosplenomegaly. Other findings that should arouse concern (and warrant biopsy) are hard masses with an irregular edge, skin tethering, axillary lymphadenopathy, or any combination of these; distorted architecture or asymmetry of the breasts; bloody uniductal nipple discharge; or a mass size of 5 cm or larger.
- When it persists with no sign of regression for 3 to 4 months.
- When there are multiple and bilateral breast masses.
- When imaging detects reason for concern.
* Surgical excisional biopsy or core needle biopsy is recommended.
Source: Simmons PS, et al.
Details of the trial
Eleven women 20 to 24 years old and one 18-year-old teen were found to have breast cancer. Of these, eight of the women detected the mass themselves, one observed bloody nipple discharge associated with constitutional symptoms, and another experienced severe constitutional symptoms associated with disseminated malignancy. In one case, the physician detected a breast mass in an asymptomatic woman. Details on the remaining woman were unavailable.
Palpable masses were noted in most of the women at the time of clinical evaluation, and the median greatest diameter was 4 cm. After the original history and exam, breast cancer was suspected in only 2 of the 11 women.
Among the 11 young women who had breast cancer, one had received mantle and abdominal radiotherapy for previously diagnosed Hodgkin’s disease. Two women had a family history suggesting hereditary breast cancer. None of the women were tested for a BRCA mutation. Regional or local recurrence was identified in three women, and contralateral breast cancer was found in two women (one of whom was subsequently also found to have ovarian cancer). At the time of the last follow-up (a median of 25.5 months), four women had died as a result of breast cancer, one had died from advanced ovarian cancer, two were alive with disease, and five were alive with no evidence of disease.
As the investigators point out, the rarity of malignancy in very young women should not prevent clinicians from evaluating breast masses in women younger than 25 years. At a minimum, evaluation should include palpation and ultrasonographic imaging performed by an expert. Imaging other than ultrasound may best be considered and ordered by a breast surgeon.
The authors propose tissue diagnosis that is based on specific criteria (see the box on page 16). They also note the high prevalence of a hereditary component of breast cancer in young women. Other reports indicate that approximately 10% of women younger than 40 years who have breast cancer harbor a BRCA1 or BRCA2 mutation.
ANDREW M. KAUNITZ, MD
We want to hear from you! Tell us what you think.
Scant research has focused on breast cancer in very young women. This retrospective case series by investigators at the Mayo Clinic assessed girls and women younger than 25 years who were given a diagnosis of primary breast cancer between 1935 and 2005 and who received care at that institution.
The investigators highlighted many of the challenges clinicians face when a young patient presents with a lump or other signs associated with breast cancer. For example, they note that, in its early stages, breast carcinoma in young women can be similar in appearance to fibroadenoma. When a patient postpones care or a clinician dismisses the lump because of a low index of suspicion, diagnosis can be delayed. That is problematic because invasive breast carcinoma in girls and young women is more aggressive and associated with a poorer prognosis overall.
- When the patient has a medical history that arouses concern, such as a history of malignancy, a family history of breast or ovarian cancer at a young age, a history of BRCA mutation, a rapidly growing mass, or constitutional symptoms of malignancy.
- When the physical examination reveals fever, weight loss, anemia, systemic lymphadenopathy, other masses, or hepatosplenomegaly. Other findings that should arouse concern (and warrant biopsy) are hard masses with an irregular edge, skin tethering, axillary lymphadenopathy, or any combination of these; distorted architecture or asymmetry of the breasts; bloody uniductal nipple discharge; or a mass size of 5 cm or larger.
- When it persists with no sign of regression for 3 to 4 months.
- When there are multiple and bilateral breast masses.
- When imaging detects reason for concern.
* Surgical excisional biopsy or core needle biopsy is recommended.
Source: Simmons PS, et al.
Details of the trial
Eleven women 20 to 24 years old and one 18-year-old teen were found to have breast cancer. Of these, eight of the women detected the mass themselves, one observed bloody nipple discharge associated with constitutional symptoms, and another experienced severe constitutional symptoms associated with disseminated malignancy. In one case, the physician detected a breast mass in an asymptomatic woman. Details on the remaining woman were unavailable.
Palpable masses were noted in most of the women at the time of clinical evaluation, and the median greatest diameter was 4 cm. After the original history and exam, breast cancer was suspected in only 2 of the 11 women.
Among the 11 young women who had breast cancer, one had received mantle and abdominal radiotherapy for previously diagnosed Hodgkin’s disease. Two women had a family history suggesting hereditary breast cancer. None of the women were tested for a BRCA mutation. Regional or local recurrence was identified in three women, and contralateral breast cancer was found in two women (one of whom was subsequently also found to have ovarian cancer). At the time of the last follow-up (a median of 25.5 months), four women had died as a result of breast cancer, one had died from advanced ovarian cancer, two were alive with disease, and five were alive with no evidence of disease.
As the investigators point out, the rarity of malignancy in very young women should not prevent clinicians from evaluating breast masses in women younger than 25 years. At a minimum, evaluation should include palpation and ultrasonographic imaging performed by an expert. Imaging other than ultrasound may best be considered and ordered by a breast surgeon.
The authors propose tissue diagnosis that is based on specific criteria (see the box on page 16). They also note the high prevalence of a hereditary component of breast cancer in young women. Other reports indicate that approximately 10% of women younger than 40 years who have breast cancer harbor a BRCA1 or BRCA2 mutation.
ANDREW M. KAUNITZ, MD
We want to hear from you! Tell us what you think.
Scant research has focused on breast cancer in very young women. This retrospective case series by investigators at the Mayo Clinic assessed girls and women younger than 25 years who were given a diagnosis of primary breast cancer between 1935 and 2005 and who received care at that institution.
The investigators highlighted many of the challenges clinicians face when a young patient presents with a lump or other signs associated with breast cancer. For example, they note that, in its early stages, breast carcinoma in young women can be similar in appearance to fibroadenoma. When a patient postpones care or a clinician dismisses the lump because of a low index of suspicion, diagnosis can be delayed. That is problematic because invasive breast carcinoma in girls and young women is more aggressive and associated with a poorer prognosis overall.
- When the patient has a medical history that arouses concern, such as a history of malignancy, a family history of breast or ovarian cancer at a young age, a history of BRCA mutation, a rapidly growing mass, or constitutional symptoms of malignancy.
- When the physical examination reveals fever, weight loss, anemia, systemic lymphadenopathy, other masses, or hepatosplenomegaly. Other findings that should arouse concern (and warrant biopsy) are hard masses with an irregular edge, skin tethering, axillary lymphadenopathy, or any combination of these; distorted architecture or asymmetry of the breasts; bloody uniductal nipple discharge; or a mass size of 5 cm or larger.
- When it persists with no sign of regression for 3 to 4 months.
- When there are multiple and bilateral breast masses.
- When imaging detects reason for concern.
* Surgical excisional biopsy or core needle biopsy is recommended.
Source: Simmons PS, et al.
Details of the trial
Eleven women 20 to 24 years old and one 18-year-old teen were found to have breast cancer. Of these, eight of the women detected the mass themselves, one observed bloody nipple discharge associated with constitutional symptoms, and another experienced severe constitutional symptoms associated with disseminated malignancy. In one case, the physician detected a breast mass in an asymptomatic woman. Details on the remaining woman were unavailable.
Palpable masses were noted in most of the women at the time of clinical evaluation, and the median greatest diameter was 4 cm. After the original history and exam, breast cancer was suspected in only 2 of the 11 women.
Among the 11 young women who had breast cancer, one had received mantle and abdominal radiotherapy for previously diagnosed Hodgkin’s disease. Two women had a family history suggesting hereditary breast cancer. None of the women were tested for a BRCA mutation. Regional or local recurrence was identified in three women, and contralateral breast cancer was found in two women (one of whom was subsequently also found to have ovarian cancer). At the time of the last follow-up (a median of 25.5 months), four women had died as a result of breast cancer, one had died from advanced ovarian cancer, two were alive with disease, and five were alive with no evidence of disease.
As the investigators point out, the rarity of malignancy in very young women should not prevent clinicians from evaluating breast masses in women younger than 25 years. At a minimum, evaluation should include palpation and ultrasonographic imaging performed by an expert. Imaging other than ultrasound may best be considered and ordered by a breast surgeon.
The authors propose tissue diagnosis that is based on specific criteria (see the box on page 16). They also note the high prevalence of a hereditary component of breast cancer in young women. Other reports indicate that approximately 10% of women younger than 40 years who have breast cancer harbor a BRCA1 or BRCA2 mutation.
ANDREW M. KAUNITZ, MD
We want to hear from you! Tell us what you think.
What is the significance of the head-to-body delivery interval in shoulder dystocia?
- Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
Shoulder dystocia is a well-described obstetric complication that occurs in approximately 1% of deliveries.1 It has been associated with adverse maternal outcomes as well as adverse perinatal outcomes, including fracture, nerve palsy, and hypoxic ischemic encephalopathy.
Although multiple risk factors for shoulder dystocia have been described, experts have not yet been able to combine them into an accurate, discriminating, clinically useful shoulder dystocia prediction model; therefore, shoulder dystocia remains an unpredictable event.2 We also lack a strategy to prevent shoulder dystocia. Because we cannot predict or prevent it, a provider’s response to shoulder dystocia, once it occurs, is seminal, in terms of management.
Details of the study
As Lerner and colleagues concisely state, when shoulder dystocia occurs, there is a need for caution in the application of force during maneuvers and a “countervailing need to achieve delivery.” It is in a provider’s interest, then, to have knowledge of whether there is a time at which that countervailing need to achieve delivery takes on greater relative significance.
In an effort to address this issue, the authors examined the relationship between the duration of shoulder dystocia and neonatal depression (defined as the need for cardiopulmonary resuscitation or intubation; a pH level below 7.0; an Apgar score below 6 at 5 minutes; or death).
In their study, 127 births involving uncomplicated shoulder dystocia (i.e., no evidence of neonatal trauma or depression) from a single institution were compared with 55 births involving complicated shoulder dystocia (i.e., the occurrence of brachial plexus palsy with or without neonatal depression).
Lerner and colleagues found a correlation between the duration of shoulder dystocia and the extent of neonatal complications. For example, the median interval from head-to-body delivery for uncomplicated births was 1.0 minute; for births complicated by brachial plexus palsy alone, it was 2.0 minutes; and for births complicated by brachial plexus palsy and neonatal depression, the interval was 5.3 minutes (P <.001). There was no single cutoff, however, that was completely discriminating with regard to whether neonatal depression would occur.
Strengths and weaknesses of the trial
As the authors note, one limitation of their study is a lack of precision in the recorded duration of shoulder dystocia cases, given that “it appears that clinicians often rounded” the stated times.
Other types of bias that may have affected the findings include:
- Selection bias. In an observational study such as this, it is typically ideal to draw the cases and controls from the same underlying population in an effort to limit the occurrence of other potentially confounding factors, both known and unknown. In this study, however, the uncomplicated cases came from one institution over 10 years, whereas the complicated cases came from a medicolegal database one author had accumulated over 15 years. Because these clearly are very different populations, the reported association between head-to-body delivery interval and brachial plexus palsy or neonatal depression may be related to characteristics other than, or in addition to, duration of the dystocia. For example, there may have been complicated cases that did not result in legal action. If the duration of the dystocia is in any way related to the chance that medicolegal action occurs, the relationship between duration and the presence of complication will be affected.
- Ascertainment bias. Because this study lacked a standard approach to the recording of duration, ascertainment bias may have affected the results. It is possible, for example, that the knowledge that a complication did or did not occur could have affected whether the duration was recorded or how much time was documented.
Complications of shoulder dystocia are rare
Ultimately, the primary question posed in this article is difficult to answer. Although shoulder dystocia occurs in approximately 1% of births, major adverse perinatal outcomes occur in only a fraction of these cases. That fact means that an event such as permanent brachial plexus palsy or neonatal depression, let alone actual hypoxic ischemic encephalopathy, occurs only in the context of thousands of births.
The data published to date,3,4 including this study, should offer some reassurance to obstetric care providers. Long-term adverse outcomes are uncommon in shoulder dystocia. Even intermediate outcomes such as neonatal depression, when they do occur, appear to be uncommon when the shoulder dystocia is of relatively short duration.
When shoulder dystocia does occur, however, providers should maintain situational awareness, being cognizant of the time that elapses, so that the continuation of appropriate and coordinated maneuvers can be ensured.
WILLIAM A. GROBMAN, MD, MBA
We want to hear from you! Tell us what you think.
1. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetric nightmare. Clin Obstet Gynecol. 2002;45(2):345-362.
2. Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol. 2006;194(3):888-894.
3. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839-842.
4. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474-479.
- Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
Shoulder dystocia is a well-described obstetric complication that occurs in approximately 1% of deliveries.1 It has been associated with adverse maternal outcomes as well as adverse perinatal outcomes, including fracture, nerve palsy, and hypoxic ischemic encephalopathy.
Although multiple risk factors for shoulder dystocia have been described, experts have not yet been able to combine them into an accurate, discriminating, clinically useful shoulder dystocia prediction model; therefore, shoulder dystocia remains an unpredictable event.2 We also lack a strategy to prevent shoulder dystocia. Because we cannot predict or prevent it, a provider’s response to shoulder dystocia, once it occurs, is seminal, in terms of management.
Details of the study
As Lerner and colleagues concisely state, when shoulder dystocia occurs, there is a need for caution in the application of force during maneuvers and a “countervailing need to achieve delivery.” It is in a provider’s interest, then, to have knowledge of whether there is a time at which that countervailing need to achieve delivery takes on greater relative significance.
In an effort to address this issue, the authors examined the relationship between the duration of shoulder dystocia and neonatal depression (defined as the need for cardiopulmonary resuscitation or intubation; a pH level below 7.0; an Apgar score below 6 at 5 minutes; or death).
In their study, 127 births involving uncomplicated shoulder dystocia (i.e., no evidence of neonatal trauma or depression) from a single institution were compared with 55 births involving complicated shoulder dystocia (i.e., the occurrence of brachial plexus palsy with or without neonatal depression).
Lerner and colleagues found a correlation between the duration of shoulder dystocia and the extent of neonatal complications. For example, the median interval from head-to-body delivery for uncomplicated births was 1.0 minute; for births complicated by brachial plexus palsy alone, it was 2.0 minutes; and for births complicated by brachial plexus palsy and neonatal depression, the interval was 5.3 minutes (P <.001). There was no single cutoff, however, that was completely discriminating with regard to whether neonatal depression would occur.
Strengths and weaknesses of the trial
As the authors note, one limitation of their study is a lack of precision in the recorded duration of shoulder dystocia cases, given that “it appears that clinicians often rounded” the stated times.
Other types of bias that may have affected the findings include:
- Selection bias. In an observational study such as this, it is typically ideal to draw the cases and controls from the same underlying population in an effort to limit the occurrence of other potentially confounding factors, both known and unknown. In this study, however, the uncomplicated cases came from one institution over 10 years, whereas the complicated cases came from a medicolegal database one author had accumulated over 15 years. Because these clearly are very different populations, the reported association between head-to-body delivery interval and brachial plexus palsy or neonatal depression may be related to characteristics other than, or in addition to, duration of the dystocia. For example, there may have been complicated cases that did not result in legal action. If the duration of the dystocia is in any way related to the chance that medicolegal action occurs, the relationship between duration and the presence of complication will be affected.
- Ascertainment bias. Because this study lacked a standard approach to the recording of duration, ascertainment bias may have affected the results. It is possible, for example, that the knowledge that a complication did or did not occur could have affected whether the duration was recorded or how much time was documented.
Complications of shoulder dystocia are rare
Ultimately, the primary question posed in this article is difficult to answer. Although shoulder dystocia occurs in approximately 1% of births, major adverse perinatal outcomes occur in only a fraction of these cases. That fact means that an event such as permanent brachial plexus palsy or neonatal depression, let alone actual hypoxic ischemic encephalopathy, occurs only in the context of thousands of births.
The data published to date,3,4 including this study, should offer some reassurance to obstetric care providers. Long-term adverse outcomes are uncommon in shoulder dystocia. Even intermediate outcomes such as neonatal depression, when they do occur, appear to be uncommon when the shoulder dystocia is of relatively short duration.
When shoulder dystocia does occur, however, providers should maintain situational awareness, being cognizant of the time that elapses, so that the continuation of appropriate and coordinated maneuvers can be ensured.
WILLIAM A. GROBMAN, MD, MBA
We want to hear from you! Tell us what you think.
- Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
Shoulder dystocia is a well-described obstetric complication that occurs in approximately 1% of deliveries.1 It has been associated with adverse maternal outcomes as well as adverse perinatal outcomes, including fracture, nerve palsy, and hypoxic ischemic encephalopathy.
Although multiple risk factors for shoulder dystocia have been described, experts have not yet been able to combine them into an accurate, discriminating, clinically useful shoulder dystocia prediction model; therefore, shoulder dystocia remains an unpredictable event.2 We also lack a strategy to prevent shoulder dystocia. Because we cannot predict or prevent it, a provider’s response to shoulder dystocia, once it occurs, is seminal, in terms of management.
Details of the study
As Lerner and colleagues concisely state, when shoulder dystocia occurs, there is a need for caution in the application of force during maneuvers and a “countervailing need to achieve delivery.” It is in a provider’s interest, then, to have knowledge of whether there is a time at which that countervailing need to achieve delivery takes on greater relative significance.
In an effort to address this issue, the authors examined the relationship between the duration of shoulder dystocia and neonatal depression (defined as the need for cardiopulmonary resuscitation or intubation; a pH level below 7.0; an Apgar score below 6 at 5 minutes; or death).
In their study, 127 births involving uncomplicated shoulder dystocia (i.e., no evidence of neonatal trauma or depression) from a single institution were compared with 55 births involving complicated shoulder dystocia (i.e., the occurrence of brachial plexus palsy with or without neonatal depression).
Lerner and colleagues found a correlation between the duration of shoulder dystocia and the extent of neonatal complications. For example, the median interval from head-to-body delivery for uncomplicated births was 1.0 minute; for births complicated by brachial plexus palsy alone, it was 2.0 minutes; and for births complicated by brachial plexus palsy and neonatal depression, the interval was 5.3 minutes (P <.001). There was no single cutoff, however, that was completely discriminating with regard to whether neonatal depression would occur.
Strengths and weaknesses of the trial
As the authors note, one limitation of their study is a lack of precision in the recorded duration of shoulder dystocia cases, given that “it appears that clinicians often rounded” the stated times.
Other types of bias that may have affected the findings include:
- Selection bias. In an observational study such as this, it is typically ideal to draw the cases and controls from the same underlying population in an effort to limit the occurrence of other potentially confounding factors, both known and unknown. In this study, however, the uncomplicated cases came from one institution over 10 years, whereas the complicated cases came from a medicolegal database one author had accumulated over 15 years. Because these clearly are very different populations, the reported association between head-to-body delivery interval and brachial plexus palsy or neonatal depression may be related to characteristics other than, or in addition to, duration of the dystocia. For example, there may have been complicated cases that did not result in legal action. If the duration of the dystocia is in any way related to the chance that medicolegal action occurs, the relationship between duration and the presence of complication will be affected.
- Ascertainment bias. Because this study lacked a standard approach to the recording of duration, ascertainment bias may have affected the results. It is possible, for example, that the knowledge that a complication did or did not occur could have affected whether the duration was recorded or how much time was documented.
Complications of shoulder dystocia are rare
Ultimately, the primary question posed in this article is difficult to answer. Although shoulder dystocia occurs in approximately 1% of births, major adverse perinatal outcomes occur in only a fraction of these cases. That fact means that an event such as permanent brachial plexus palsy or neonatal depression, let alone actual hypoxic ischemic encephalopathy, occurs only in the context of thousands of births.
The data published to date,3,4 including this study, should offer some reassurance to obstetric care providers. Long-term adverse outcomes are uncommon in shoulder dystocia. Even intermediate outcomes such as neonatal depression, when they do occur, appear to be uncommon when the shoulder dystocia is of relatively short duration.
When shoulder dystocia does occur, however, providers should maintain situational awareness, being cognizant of the time that elapses, so that the continuation of appropriate and coordinated maneuvers can be ensured.
WILLIAM A. GROBMAN, MD, MBA
We want to hear from you! Tell us what you think.
1. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetric nightmare. Clin Obstet Gynecol. 2002;45(2):345-362.
2. Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol. 2006;194(3):888-894.
3. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839-842.
4. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474-479.
1. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetric nightmare. Clin Obstet Gynecol. 2002;45(2):345-362.
2. Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol. 2006;194(3):888-894.
3. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839-842.
4. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474-479.
Holdout Hospitals
I think 70% to 80% of U.S. hospitals now have a hospitalist practice. (Some have more than one hospitalist group operating within their walls.) I arrived at this estimate by relying on both my anecdotal experience and on the annual American Hospital Association survey, which in 2009 showed 58% of hospitals have hospitalists, with an ongoing rapid rate of adoption.
No regular reader of The Hospitalist should be surprised that most U.S. hospitals now have hospitalists, but some might be surprised that 20% to 30% don’t. There are about 5,800 hospitals in the U.S. (a ballpark figure), so that means about 1,100 to 1,800 don’t have hospitalists. What is unique about them?
For some hospitals, the answer is easy. For example, the U.S. has something like 450 psychiatric hospitals. They vary a lot, but many simply don’t accept patients with active medical problems, so these facilities would have little need for medical hospitalists.
Variations in how the term “hospitalist” is used probably account for some facilities reporting no hospitalists. For example, long-term acute-care hospitals (LTACs) might have dedicated inpatient providers but simply don’t call them hospitalists.
Even accounting for these things, there are still a lot of “med-surg” hospitals that say they don’t have hospitalists.
The Holdouts
My experience suggests the two most important reasons some hospitals have not yet developed a hospitalist practice are an oversupply of primary-care physicians (PCPs) and an attractive payor mix in the unassigned patient population. In fact, it is hard for me to imagine a hospital that enjoys both of these attributes ever being able to support hospitalists.
Although it isn’t a common problem, an excess of PCPs (or dearth of patients) removes the most universal and powerful stimulus to develop a hospitalist practice: the desire of PCPs to be relieved of hospital work. And in most cases, those PCPs can offset the loss of hospital work and its associated revenue, with more work in the office. This can mean a better lifestyle (e.g. no trips to the hospital on nights and weekends) and the same or higher income. But if there are too many PCPs in the community, they may be unwilling to give up the hospital work, as there might be no way to replace it in the office. End result: no hospitalists.
For the rare hospital that has an attractive ED-unassigned payor mix, PCPs are more likely to want to continue taking ED call and not support a proposal to develop a hospitalist practice. And access to the ED call roster can be important to new PCPs building a community practice. I have seen situations in which a hospital has addressed the poor reimbursement of unattached ED admissions by paying PCPs to provide that care. Even though that same hospital might want a hospitalist practice, the ED call payment it is providing to PCPs may create a barrier that can’t be overcome. Such a hospital will face the very difficult decision of terminating the payments for ED call and redirecting that money to a hospitalist practice—something that is likely to lead to a lot of frustration on the part of PCPs who depend on the pay-for-call arrangement. A common outcome: no hospitalists.
An occasional reason hospitals are late to the hospitalist party is one or two (rarely more than that) of its private PCPs have simply chosen to work heroic amounts, and in addition to office and hospital care of their private patients, they accept referrals from other PCPs. I have met a number of doctors like this. Some are terrific doctors who actively participate in hospital initiatives; many appear chronically tired and harried, and hospital staff express frustration that they do things like make rounds at 3 a.m., take hours to respond to urgent calls, refuse to use protocols, etc. But because they’ve responded to the PCPs’ desire to be relieved of hospital work, other doctors may rally to their support and prevent the hospital from moving forward with a hospitalist program.
Will Every Hospital Have Hospitalists Eventually?
It is really interesting to think about whether every hospital, outside narrow specialty hospitals, will have hospitalists in the future. I wonder what informed people in the 1970s and early 1980s were predicting for emergency medicine’s future. At that point it probably wasn’t clear that, in the future, dedicated ED doctors essentially would staff every ED in the country, but I think that is exactly what has happened. (I once worked with an approximately 100-bed rural hospital that didn’t have ED physicians until 1999. I wonder if they were the last adopter.)
I think hospitalists are critically important for nearly all med-surg hospitals; however, maybe there will always be a small number that either have PCPs continue to practice in the traditional model, working both outpatient and inpatient, or some other effective configuration that makes hospitalists less necessary. We’ll have to wait and see. But I’m pretty confident
that almost no institutions that have hospitalists will ever return to the pre-hospitalist model of care. It seems there is no going back.
For those hospitals without hospitalists currently who will at some future time have hospitalists, the right time for this to happen is dependent on a combination of local factors. It could be something like the departure (i.e. relocation or retirement) of some of the current doctors, or simply the arrival of someone who has a vision and energy to successfully navigate the obstacles to build one. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.</p>
I think 70% to 80% of U.S. hospitals now have a hospitalist practice. (Some have more than one hospitalist group operating within their walls.) I arrived at this estimate by relying on both my anecdotal experience and on the annual American Hospital Association survey, which in 2009 showed 58% of hospitals have hospitalists, with an ongoing rapid rate of adoption.
No regular reader of The Hospitalist should be surprised that most U.S. hospitals now have hospitalists, but some might be surprised that 20% to 30% don’t. There are about 5,800 hospitals in the U.S. (a ballpark figure), so that means about 1,100 to 1,800 don’t have hospitalists. What is unique about them?
For some hospitals, the answer is easy. For example, the U.S. has something like 450 psychiatric hospitals. They vary a lot, but many simply don’t accept patients with active medical problems, so these facilities would have little need for medical hospitalists.
Variations in how the term “hospitalist” is used probably account for some facilities reporting no hospitalists. For example, long-term acute-care hospitals (LTACs) might have dedicated inpatient providers but simply don’t call them hospitalists.
Even accounting for these things, there are still a lot of “med-surg” hospitals that say they don’t have hospitalists.
The Holdouts
My experience suggests the two most important reasons some hospitals have not yet developed a hospitalist practice are an oversupply of primary-care physicians (PCPs) and an attractive payor mix in the unassigned patient population. In fact, it is hard for me to imagine a hospital that enjoys both of these attributes ever being able to support hospitalists.
Although it isn’t a common problem, an excess of PCPs (or dearth of patients) removes the most universal and powerful stimulus to develop a hospitalist practice: the desire of PCPs to be relieved of hospital work. And in most cases, those PCPs can offset the loss of hospital work and its associated revenue, with more work in the office. This can mean a better lifestyle (e.g. no trips to the hospital on nights and weekends) and the same or higher income. But if there are too many PCPs in the community, they may be unwilling to give up the hospital work, as there might be no way to replace it in the office. End result: no hospitalists.
For the rare hospital that has an attractive ED-unassigned payor mix, PCPs are more likely to want to continue taking ED call and not support a proposal to develop a hospitalist practice. And access to the ED call roster can be important to new PCPs building a community practice. I have seen situations in which a hospital has addressed the poor reimbursement of unattached ED admissions by paying PCPs to provide that care. Even though that same hospital might want a hospitalist practice, the ED call payment it is providing to PCPs may create a barrier that can’t be overcome. Such a hospital will face the very difficult decision of terminating the payments for ED call and redirecting that money to a hospitalist practice—something that is likely to lead to a lot of frustration on the part of PCPs who depend on the pay-for-call arrangement. A common outcome: no hospitalists.
An occasional reason hospitals are late to the hospitalist party is one or two (rarely more than that) of its private PCPs have simply chosen to work heroic amounts, and in addition to office and hospital care of their private patients, they accept referrals from other PCPs. I have met a number of doctors like this. Some are terrific doctors who actively participate in hospital initiatives; many appear chronically tired and harried, and hospital staff express frustration that they do things like make rounds at 3 a.m., take hours to respond to urgent calls, refuse to use protocols, etc. But because they’ve responded to the PCPs’ desire to be relieved of hospital work, other doctors may rally to their support and prevent the hospital from moving forward with a hospitalist program.
Will Every Hospital Have Hospitalists Eventually?
It is really interesting to think about whether every hospital, outside narrow specialty hospitals, will have hospitalists in the future. I wonder what informed people in the 1970s and early 1980s were predicting for emergency medicine’s future. At that point it probably wasn’t clear that, in the future, dedicated ED doctors essentially would staff every ED in the country, but I think that is exactly what has happened. (I once worked with an approximately 100-bed rural hospital that didn’t have ED physicians until 1999. I wonder if they were the last adopter.)
I think hospitalists are critically important for nearly all med-surg hospitals; however, maybe there will always be a small number that either have PCPs continue to practice in the traditional model, working both outpatient and inpatient, or some other effective configuration that makes hospitalists less necessary. We’ll have to wait and see. But I’m pretty confident
that almost no institutions that have hospitalists will ever return to the pre-hospitalist model of care. It seems there is no going back.
For those hospitals without hospitalists currently who will at some future time have hospitalists, the right time for this to happen is dependent on a combination of local factors. It could be something like the departure (i.e. relocation or retirement) of some of the current doctors, or simply the arrival of someone who has a vision and energy to successfully navigate the obstacles to build one. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.</p>
I think 70% to 80% of U.S. hospitals now have a hospitalist practice. (Some have more than one hospitalist group operating within their walls.) I arrived at this estimate by relying on both my anecdotal experience and on the annual American Hospital Association survey, which in 2009 showed 58% of hospitals have hospitalists, with an ongoing rapid rate of adoption.
No regular reader of The Hospitalist should be surprised that most U.S. hospitals now have hospitalists, but some might be surprised that 20% to 30% don’t. There are about 5,800 hospitals in the U.S. (a ballpark figure), so that means about 1,100 to 1,800 don’t have hospitalists. What is unique about them?
For some hospitals, the answer is easy. For example, the U.S. has something like 450 psychiatric hospitals. They vary a lot, but many simply don’t accept patients with active medical problems, so these facilities would have little need for medical hospitalists.
Variations in how the term “hospitalist” is used probably account for some facilities reporting no hospitalists. For example, long-term acute-care hospitals (LTACs) might have dedicated inpatient providers but simply don’t call them hospitalists.
Even accounting for these things, there are still a lot of “med-surg” hospitals that say they don’t have hospitalists.
The Holdouts
My experience suggests the two most important reasons some hospitals have not yet developed a hospitalist practice are an oversupply of primary-care physicians (PCPs) and an attractive payor mix in the unassigned patient population. In fact, it is hard for me to imagine a hospital that enjoys both of these attributes ever being able to support hospitalists.
Although it isn’t a common problem, an excess of PCPs (or dearth of patients) removes the most universal and powerful stimulus to develop a hospitalist practice: the desire of PCPs to be relieved of hospital work. And in most cases, those PCPs can offset the loss of hospital work and its associated revenue, with more work in the office. This can mean a better lifestyle (e.g. no trips to the hospital on nights and weekends) and the same or higher income. But if there are too many PCPs in the community, they may be unwilling to give up the hospital work, as there might be no way to replace it in the office. End result: no hospitalists.
For the rare hospital that has an attractive ED-unassigned payor mix, PCPs are more likely to want to continue taking ED call and not support a proposal to develop a hospitalist practice. And access to the ED call roster can be important to new PCPs building a community practice. I have seen situations in which a hospital has addressed the poor reimbursement of unattached ED admissions by paying PCPs to provide that care. Even though that same hospital might want a hospitalist practice, the ED call payment it is providing to PCPs may create a barrier that can’t be overcome. Such a hospital will face the very difficult decision of terminating the payments for ED call and redirecting that money to a hospitalist practice—something that is likely to lead to a lot of frustration on the part of PCPs who depend on the pay-for-call arrangement. A common outcome: no hospitalists.
An occasional reason hospitals are late to the hospitalist party is one or two (rarely more than that) of its private PCPs have simply chosen to work heroic amounts, and in addition to office and hospital care of their private patients, they accept referrals from other PCPs. I have met a number of doctors like this. Some are terrific doctors who actively participate in hospital initiatives; many appear chronically tired and harried, and hospital staff express frustration that they do things like make rounds at 3 a.m., take hours to respond to urgent calls, refuse to use protocols, etc. But because they’ve responded to the PCPs’ desire to be relieved of hospital work, other doctors may rally to their support and prevent the hospital from moving forward with a hospitalist program.
Will Every Hospital Have Hospitalists Eventually?
It is really interesting to think about whether every hospital, outside narrow specialty hospitals, will have hospitalists in the future. I wonder what informed people in the 1970s and early 1980s were predicting for emergency medicine’s future. At that point it probably wasn’t clear that, in the future, dedicated ED doctors essentially would staff every ED in the country, but I think that is exactly what has happened. (I once worked with an approximately 100-bed rural hospital that didn’t have ED physicians until 1999. I wonder if they were the last adopter.)
I think hospitalists are critically important for nearly all med-surg hospitals; however, maybe there will always be a small number that either have PCPs continue to practice in the traditional model, working both outpatient and inpatient, or some other effective configuration that makes hospitalists less necessary. We’ll have to wait and see. But I’m pretty confident
that almost no institutions that have hospitalists will ever return to the pre-hospitalist model of care. It seems there is no going back.
For those hospitals without hospitalists currently who will at some future time have hospitalists, the right time for this to happen is dependent on a combination of local factors. It could be something like the departure (i.e. relocation or retirement) of some of the current doctors, or simply the arrival of someone who has a vision and energy to successfully navigate the obstacles to build one. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.</p>
Good Citizenship
Hospital medicine is fortunate to have many very dedicated and professionally centered doctors who work enthusiastically to both provide excellent care to their patients and work to make their own practice and their hospital a better place. I am lucky to practice with many of them in our practice in Bellevue, Wash.
Yet a significant portion of hospitalists have chosen this work because they’re looking for relatively-low-commitment work. In essence, they see themselves as dating their practice rather than marrying it. Some of them might even say, “I thought I wanted a career. It turns out all I wanted was a paycheck.”
Most are skilled clinicians who find the energy to do a good job for the patients under their care but don’t have a mindset of owning their practice and investing time in making it perform better.
This gives rise to a dilemma: How can a practice turn these perfectly capable physicians into meaningfully engaged participants in the hospitalist practice itself and the hospital as a whole? What about a salary bonus based on good citizenship? Would that cause them to become more engaged and committed?
There is voluminous research and a whole row of books at your local Barnes & Noble that address these questions more completely that I can, so I’ll just share some real-world experience and insights from one book.
What Might a Citizenship Bonus Look Like?
There are a number of ways to consider designing a citizenship bonus. At a previous SHM practice-management course, Win Whitcomb, MD, MHM, presented one example from Mercy Medical Center in Springfield, Mass. (see Figure 1).
The following kinds of activities might be appropriate for a hospitalist to earn a citizenship bonus:
- Active participation on approved hospital committees (e.g. the pharmacy and therapeutic committees) and regular input from and feedback to the hospitalist group (e.g. via e-mail) about relevant activities of the committee;
- A project to improve clinical care (e.g. improved glycemic control, fall prevention, med reconciliation, discharge processes, readmission rates, ensuring follow-up of tests resulted after discharge, etc.);
- A project to improve business operations—for example, improve our billing/coding accuracy. Such a project could be to develop a new progress note template and collect data regarding its use and effectiveness;
- Work to improve communication and interaction with other hospital staff—for example, joint rounding with nurses, improve throughput, etc.; and
- Project(s) to increase the group’s social cohesion and engagement with hospital initiatives and goals.
Does a Citizenship Bonus Help or Hinder a Practice?
From the experience Mercy Hospital had with the citizenship bonus, Win concluded that many, but not all, hospitalists who don’t seem interested in quality improvement (QI) will become engaged if there is a reward/recognition structure. A relatively small dollar bonus is OK, as long as non-monetary rewards exist (e.g. improvement demonstrable, sense of teamwork, recognition). And hospitalists who were engaged prior to establishing the salary incentive are not likely to change their behavior, but their effort is now recognized—allowing for sustained engagement.
I’m sure many institutions would find a similar desirable outcome from putting into place a citizenship bonus. But it isn’t a guarantee. All performance bonus programs, whether based on “hard” outcomes like patient satisfaction scores or “soft” things like citizenship, are tricky to set up and operate effectively.
I have seen well-intentioned efforts to create a citizenship bonus lead to an increase in hospitalists working on projects outside of direct patient care, but at a cost of leading them to focus more intently on just how much they’re being paid for any work outside of direct patient care. It seems that the bonus might have ignited more frustration and concern about compensation, and any benefit to the practice might have been offset by harm to group culture. And if the bonus goes away, some doctors might be even less engaged than they were before it was turned on.
In “Drive: The Surprising Truth About What Motivates Us,” Daniel Pink makes a pretty convincing case that “the more prominent salary, perks, and benefits are in someone’s work life, the more they can inhibit creativity and unravel performance.” He makes the case that organizations are most demotivating “when they use rewards like money to motivate staff.”
“Effective organizations compensate people in amounts and ways that allow individuals to mostly forget about compensation and instead focus on the work itself,” Pink writes.
How do you allow individuals to forget about compensation? He says ensure internal and external fairness in compensation; pay more than average; and if you use performance metrics, make them wide-ranging, relevant, and hard to game.
So maybe financial compensation for citizenship, whether paid through a bonus, hourly, or some other separate salary element, isn’t such a good idea for a hospitalist practice (or any physician practice?). I don’t have a definitive answer, so you’ll have to decide this for yourself. But my hunch is that groups with a thriving culture might in some cases benefit from a well-designed citizenship bonus. That said, those groups also could be the ones less in need of it.
Groups that already have a weak or unhealthy culture, or are frustrated by what they see is inadequate compensation for clinical work, might find such a bonus leads to problems that offset its benefit.
Training in leadership, quality improvement, and other non-clinical areas that are critical for the success of a hospitalist practice is always worthwhile and might capture many of the benefits of a citizenship bonus without its drawbacks.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Hospital medicine is fortunate to have many very dedicated and professionally centered doctors who work enthusiastically to both provide excellent care to their patients and work to make their own practice and their hospital a better place. I am lucky to practice with many of them in our practice in Bellevue, Wash.
Yet a significant portion of hospitalists have chosen this work because they’re looking for relatively-low-commitment work. In essence, they see themselves as dating their practice rather than marrying it. Some of them might even say, “I thought I wanted a career. It turns out all I wanted was a paycheck.”
Most are skilled clinicians who find the energy to do a good job for the patients under their care but don’t have a mindset of owning their practice and investing time in making it perform better.
This gives rise to a dilemma: How can a practice turn these perfectly capable physicians into meaningfully engaged participants in the hospitalist practice itself and the hospital as a whole? What about a salary bonus based on good citizenship? Would that cause them to become more engaged and committed?
There is voluminous research and a whole row of books at your local Barnes & Noble that address these questions more completely that I can, so I’ll just share some real-world experience and insights from one book.
What Might a Citizenship Bonus Look Like?
There are a number of ways to consider designing a citizenship bonus. At a previous SHM practice-management course, Win Whitcomb, MD, MHM, presented one example from Mercy Medical Center in Springfield, Mass. (see Figure 1).
The following kinds of activities might be appropriate for a hospitalist to earn a citizenship bonus:
- Active participation on approved hospital committees (e.g. the pharmacy and therapeutic committees) and regular input from and feedback to the hospitalist group (e.g. via e-mail) about relevant activities of the committee;
- A project to improve clinical care (e.g. improved glycemic control, fall prevention, med reconciliation, discharge processes, readmission rates, ensuring follow-up of tests resulted after discharge, etc.);
- A project to improve business operations—for example, improve our billing/coding accuracy. Such a project could be to develop a new progress note template and collect data regarding its use and effectiveness;
- Work to improve communication and interaction with other hospital staff—for example, joint rounding with nurses, improve throughput, etc.; and
- Project(s) to increase the group’s social cohesion and engagement with hospital initiatives and goals.
Does a Citizenship Bonus Help or Hinder a Practice?
From the experience Mercy Hospital had with the citizenship bonus, Win concluded that many, but not all, hospitalists who don’t seem interested in quality improvement (QI) will become engaged if there is a reward/recognition structure. A relatively small dollar bonus is OK, as long as non-monetary rewards exist (e.g. improvement demonstrable, sense of teamwork, recognition). And hospitalists who were engaged prior to establishing the salary incentive are not likely to change their behavior, but their effort is now recognized—allowing for sustained engagement.
I’m sure many institutions would find a similar desirable outcome from putting into place a citizenship bonus. But it isn’t a guarantee. All performance bonus programs, whether based on “hard” outcomes like patient satisfaction scores or “soft” things like citizenship, are tricky to set up and operate effectively.
I have seen well-intentioned efforts to create a citizenship bonus lead to an increase in hospitalists working on projects outside of direct patient care, but at a cost of leading them to focus more intently on just how much they’re being paid for any work outside of direct patient care. It seems that the bonus might have ignited more frustration and concern about compensation, and any benefit to the practice might have been offset by harm to group culture. And if the bonus goes away, some doctors might be even less engaged than they were before it was turned on.
In “Drive: The Surprising Truth About What Motivates Us,” Daniel Pink makes a pretty convincing case that “the more prominent salary, perks, and benefits are in someone’s work life, the more they can inhibit creativity and unravel performance.” He makes the case that organizations are most demotivating “when they use rewards like money to motivate staff.”
“Effective organizations compensate people in amounts and ways that allow individuals to mostly forget about compensation and instead focus on the work itself,” Pink writes.
How do you allow individuals to forget about compensation? He says ensure internal and external fairness in compensation; pay more than average; and if you use performance metrics, make them wide-ranging, relevant, and hard to game.
So maybe financial compensation for citizenship, whether paid through a bonus, hourly, or some other separate salary element, isn’t such a good idea for a hospitalist practice (or any physician practice?). I don’t have a definitive answer, so you’ll have to decide this for yourself. But my hunch is that groups with a thriving culture might in some cases benefit from a well-designed citizenship bonus. That said, those groups also could be the ones less in need of it.
Groups that already have a weak or unhealthy culture, or are frustrated by what they see is inadequate compensation for clinical work, might find such a bonus leads to problems that offset its benefit.
Training in leadership, quality improvement, and other non-clinical areas that are critical for the success of a hospitalist practice is always worthwhile and might capture many of the benefits of a citizenship bonus without its drawbacks.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Hospital medicine is fortunate to have many very dedicated and professionally centered doctors who work enthusiastically to both provide excellent care to their patients and work to make their own practice and their hospital a better place. I am lucky to practice with many of them in our practice in Bellevue, Wash.
Yet a significant portion of hospitalists have chosen this work because they’re looking for relatively-low-commitment work. In essence, they see themselves as dating their practice rather than marrying it. Some of them might even say, “I thought I wanted a career. It turns out all I wanted was a paycheck.”
Most are skilled clinicians who find the energy to do a good job for the patients under their care but don’t have a mindset of owning their practice and investing time in making it perform better.
This gives rise to a dilemma: How can a practice turn these perfectly capable physicians into meaningfully engaged participants in the hospitalist practice itself and the hospital as a whole? What about a salary bonus based on good citizenship? Would that cause them to become more engaged and committed?
There is voluminous research and a whole row of books at your local Barnes & Noble that address these questions more completely that I can, so I’ll just share some real-world experience and insights from one book.
What Might a Citizenship Bonus Look Like?
There are a number of ways to consider designing a citizenship bonus. At a previous SHM practice-management course, Win Whitcomb, MD, MHM, presented one example from Mercy Medical Center in Springfield, Mass. (see Figure 1).
The following kinds of activities might be appropriate for a hospitalist to earn a citizenship bonus:
- Active participation on approved hospital committees (e.g. the pharmacy and therapeutic committees) and regular input from and feedback to the hospitalist group (e.g. via e-mail) about relevant activities of the committee;
- A project to improve clinical care (e.g. improved glycemic control, fall prevention, med reconciliation, discharge processes, readmission rates, ensuring follow-up of tests resulted after discharge, etc.);
- A project to improve business operations—for example, improve our billing/coding accuracy. Such a project could be to develop a new progress note template and collect data regarding its use and effectiveness;
- Work to improve communication and interaction with other hospital staff—for example, joint rounding with nurses, improve throughput, etc.; and
- Project(s) to increase the group’s social cohesion and engagement with hospital initiatives and goals.
Does a Citizenship Bonus Help or Hinder a Practice?
From the experience Mercy Hospital had with the citizenship bonus, Win concluded that many, but not all, hospitalists who don’t seem interested in quality improvement (QI) will become engaged if there is a reward/recognition structure. A relatively small dollar bonus is OK, as long as non-monetary rewards exist (e.g. improvement demonstrable, sense of teamwork, recognition). And hospitalists who were engaged prior to establishing the salary incentive are not likely to change their behavior, but their effort is now recognized—allowing for sustained engagement.
I’m sure many institutions would find a similar desirable outcome from putting into place a citizenship bonus. But it isn’t a guarantee. All performance bonus programs, whether based on “hard” outcomes like patient satisfaction scores or “soft” things like citizenship, are tricky to set up and operate effectively.
I have seen well-intentioned efforts to create a citizenship bonus lead to an increase in hospitalists working on projects outside of direct patient care, but at a cost of leading them to focus more intently on just how much they’re being paid for any work outside of direct patient care. It seems that the bonus might have ignited more frustration and concern about compensation, and any benefit to the practice might have been offset by harm to group culture. And if the bonus goes away, some doctors might be even less engaged than they were before it was turned on.
In “Drive: The Surprising Truth About What Motivates Us,” Daniel Pink makes a pretty convincing case that “the more prominent salary, perks, and benefits are in someone’s work life, the more they can inhibit creativity and unravel performance.” He makes the case that organizations are most demotivating “when they use rewards like money to motivate staff.”
“Effective organizations compensate people in amounts and ways that allow individuals to mostly forget about compensation and instead focus on the work itself,” Pink writes.
How do you allow individuals to forget about compensation? He says ensure internal and external fairness in compensation; pay more than average; and if you use performance metrics, make them wide-ranging, relevant, and hard to game.
So maybe financial compensation for citizenship, whether paid through a bonus, hourly, or some other separate salary element, isn’t such a good idea for a hospitalist practice (or any physician practice?). I don’t have a definitive answer, so you’ll have to decide this for yourself. But my hunch is that groups with a thriving culture might in some cases benefit from a well-designed citizenship bonus. That said, those groups also could be the ones less in need of it.
Groups that already have a weak or unhealthy culture, or are frustrated by what they see is inadequate compensation for clinical work, might find such a bonus leads to problems that offset its benefit.
Training in leadership, quality improvement, and other non-clinical areas that are critical for the success of a hospitalist practice is always worthwhile and might capture many of the benefits of a citizenship bonus without its drawbacks.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
What is the optimal interval between administration of antenatal corticosteroids and preterm delivery?
In the early 1970s, as a direct by-product of basic science research on preterm birth using a sheep model, Liggins and Howie discovered that the administration of corticosteroids to women facing impending preterm birth could lower the risk not only of respiratory morbidity but also of intraventricular hemorrhage, necrotizing colitis, and death for their newborns. This discovery was confirmed in other clinical trials, and the novel strategy of a secondary therapy to reduce the sequelae of preterm birth became part of standard perinatal care. Despite this advance, numerous questions remain, including the proper dosing interval and number of doses of corticosteroids necessary to prevent the sequelae of preterm birth.
Authors explored births before 34 weeks’ gestation
To elucidate the proper dosing interval, Wilms and colleagues conducted a retrospective cohort study among women who received antenatal corticosteroids and delivered before 34 weeks’ gestation. Of the 254 infants who were delivered prematurely, those delivered within 7 days of the administration of corticosteroids had a reduced risk of requiring intervention in the NICU. The authors concluded that the efficacy of antenatal corticosteroids diminishes when the treatment-to-delivery interval exceeds 7 days.
Limitations of the study
The investigators admonish readers to carefully consider the timing of the first dose of corticosteroid therapy. Their conclusions are limited by 1) the retrospective nature of their research and 2) the fact that clinicians who cared for newborns in this study were aware of the timing of maternal corticosteroid administration.
Nevertheless, clinicians can draw pertinent points for day-to-day practice:
- The window of benefit seems to be time-limited, with a break-point and diminution at 7 days after administration of corticosteroids
- A second dose of antenatal corticosteroids may be of benefit in selected situations.
Repetitive dosing beyond two rounds 1 week apart appears to have no benefit, according to clinical trials of weekly versus one-time dosing. Moreover, repetitive dosing causes small but significant decrements in birth weight and head circumference. Whether these changes are associated with long-term harm remains unknown.
Other authors have drawn similar conclusions—but we need prospective randomized, controlled trials to clarify the issue of whether to repeat corticosteroid administration and, if so, how many doses are optimal and how often they should be given.
In women who are given antenatal corticosteroids for impending preterm birth, pay attention to the interval between administration and delivery. In this study, an interval of 7 days or less was associated with a reduced need for intervention in the NICU.
John M. Thorp, Jr, MD
We want to hear from you! Tell us what you think.
In the early 1970s, as a direct by-product of basic science research on preterm birth using a sheep model, Liggins and Howie discovered that the administration of corticosteroids to women facing impending preterm birth could lower the risk not only of respiratory morbidity but also of intraventricular hemorrhage, necrotizing colitis, and death for their newborns. This discovery was confirmed in other clinical trials, and the novel strategy of a secondary therapy to reduce the sequelae of preterm birth became part of standard perinatal care. Despite this advance, numerous questions remain, including the proper dosing interval and number of doses of corticosteroids necessary to prevent the sequelae of preterm birth.
Authors explored births before 34 weeks’ gestation
To elucidate the proper dosing interval, Wilms and colleagues conducted a retrospective cohort study among women who received antenatal corticosteroids and delivered before 34 weeks’ gestation. Of the 254 infants who were delivered prematurely, those delivered within 7 days of the administration of corticosteroids had a reduced risk of requiring intervention in the NICU. The authors concluded that the efficacy of antenatal corticosteroids diminishes when the treatment-to-delivery interval exceeds 7 days.
Limitations of the study
The investigators admonish readers to carefully consider the timing of the first dose of corticosteroid therapy. Their conclusions are limited by 1) the retrospective nature of their research and 2) the fact that clinicians who cared for newborns in this study were aware of the timing of maternal corticosteroid administration.
Nevertheless, clinicians can draw pertinent points for day-to-day practice:
- The window of benefit seems to be time-limited, with a break-point and diminution at 7 days after administration of corticosteroids
- A second dose of antenatal corticosteroids may be of benefit in selected situations.
Repetitive dosing beyond two rounds 1 week apart appears to have no benefit, according to clinical trials of weekly versus one-time dosing. Moreover, repetitive dosing causes small but significant decrements in birth weight and head circumference. Whether these changes are associated with long-term harm remains unknown.
Other authors have drawn similar conclusions—but we need prospective randomized, controlled trials to clarify the issue of whether to repeat corticosteroid administration and, if so, how many doses are optimal and how often they should be given.
In women who are given antenatal corticosteroids for impending preterm birth, pay attention to the interval between administration and delivery. In this study, an interval of 7 days or less was associated with a reduced need for intervention in the NICU.
John M. Thorp, Jr, MD
We want to hear from you! Tell us what you think.
In the early 1970s, as a direct by-product of basic science research on preterm birth using a sheep model, Liggins and Howie discovered that the administration of corticosteroids to women facing impending preterm birth could lower the risk not only of respiratory morbidity but also of intraventricular hemorrhage, necrotizing colitis, and death for their newborns. This discovery was confirmed in other clinical trials, and the novel strategy of a secondary therapy to reduce the sequelae of preterm birth became part of standard perinatal care. Despite this advance, numerous questions remain, including the proper dosing interval and number of doses of corticosteroids necessary to prevent the sequelae of preterm birth.
Authors explored births before 34 weeks’ gestation
To elucidate the proper dosing interval, Wilms and colleagues conducted a retrospective cohort study among women who received antenatal corticosteroids and delivered before 34 weeks’ gestation. Of the 254 infants who were delivered prematurely, those delivered within 7 days of the administration of corticosteroids had a reduced risk of requiring intervention in the NICU. The authors concluded that the efficacy of antenatal corticosteroids diminishes when the treatment-to-delivery interval exceeds 7 days.
Limitations of the study
The investigators admonish readers to carefully consider the timing of the first dose of corticosteroid therapy. Their conclusions are limited by 1) the retrospective nature of their research and 2) the fact that clinicians who cared for newborns in this study were aware of the timing of maternal corticosteroid administration.
Nevertheless, clinicians can draw pertinent points for day-to-day practice:
- The window of benefit seems to be time-limited, with a break-point and diminution at 7 days after administration of corticosteroids
- A second dose of antenatal corticosteroids may be of benefit in selected situations.
Repetitive dosing beyond two rounds 1 week apart appears to have no benefit, according to clinical trials of weekly versus one-time dosing. Moreover, repetitive dosing causes small but significant decrements in birth weight and head circumference. Whether these changes are associated with long-term harm remains unknown.
Other authors have drawn similar conclusions—but we need prospective randomized, controlled trials to clarify the issue of whether to repeat corticosteroid administration and, if so, how many doses are optimal and how often they should be given.
In women who are given antenatal corticosteroids for impending preterm birth, pay attention to the interval between administration and delivery. In this study, an interval of 7 days or less was associated with a reduced need for intervention in the NICU.
John M. Thorp, Jr, MD
We want to hear from you! Tell us what you think.
What is the prevalence of cervical cytologic abnormalities and high-risk HPV in the screened population?
The Pap test wrought a sea change in the medical profession’s approach to cervical cancer screening, dramatically lowering the rate of invasive cervical cancer among women who underwent the test on a regular basis. That said, the sensitivity of a single Pap test in the detection of cervical dysplasia or cancer is less than 60%.1
It is well established that oncogenic HPV strains, otherwise known as high-risk HPV infection, are responsible for the development of severe preinvasive dysplasia and cervical cancer. Munoz and colleagues identified subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and 73 as having the greatest oncogenic potential.2 They also noted that HPV 26, 53, and 66 are probably carcinogenic.2
HPV DNA diagnostic tests are available to identify 14 high-risk HPV types. Current guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) recommend both cytology and HPV testing to determine the optimal interval between screening tests and for triage to colposcopy.3
HPV DNA diagnostic tests have evolved one step further and can now detect HPV 16 and HPV 18 individually; these two types of HPV account for 70% of all cervical cancer cases.4 Research is needed to determine what combination of tests will further improve outcomes in the screened population.
Details of the ATHENA study
The study was designed to evaluate the cobas HPV test (Roche), a new polymerase chain reaction–based DNA test that yields a pooled result for 12 high-risk HPV types as well as individual results for HPV 16 and 18.
ATHENA evaluates the test in three scenarios:
- as a triage test for women who have a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- as an adjunctive test to guide clinical management in women who have cytologic findings that are negative for intraepithelial lesions or malignancy (NILM)
- as a potential first-line test in the screening of women 25 years and older, regardless of the cytology result.
The primary endpoint in all three scenarios was to detect CIN 2 or greater.
The baseline results of this study are outlined above. Data from a 3-year follow-up of the women in the ATHENA study will be published at a future date.
Other screening studies are under way
Now that we have identified HPV as the cause of cervical cancer, researchers can investigate the best way to detect high-grade CIN. Published studies have determined that HPV testing is more sensitive and less specific than the Pap test in the detection of CIN 3 and cancer.5
The HPV FOCAL trial is under way to establish the efficiency of testing for high-risk HPV DNA as primary screening and as triage in three arms. In all three arms, CIN 3 is the outcome.1
ATHENA adds a second tier to similar studies by genotyping for HPV 16 and 18.
Unanswered questions
There is no doubt that the Pap test will be replaced as a stand-alone screening test for cervical cancer. Existing ASCCP guidelines already recommend HPV testing in patients who have normal cytology; it remains to be determined how testing specifically for HPV 16 and 18 will be incorporated into the algorithm. The ATHENA trial, and others like it, will be the basis of future cervical cancer screening guidelines.
Among the issues that need to be resolved are:
- the age at which testing for HPV 16 and 18 is appropriate
- the follow-up protocol for women who test positive for HPV 16 and 18, as well as for those who test negative
- the cost of adding testing for HPV 16 and 18 to screening
- the number of women who need to be screened to find those who are positive for HPV 16 and 18 among women infected with high-risk HPV
- the number of extra cases of CIN 3+ that will be identified when women who test positive for high-risk HPV are genotyped for HPV 16 and 18
- the number of women who will undergo unnecessary colposcopy by this approach
- the triage protocol that best balances sensitivity and specificity.
As guidelines become more complex and difficult to remember, compliance will no doubt be mixed. A centralized cervical cancer screening program and database are needed to reduce confusion and improve adherence to guidelines.
The findings of the ATHENA study do not alter current cervical cancer screening guidelines—yet. Until the most effective strategy of incorporating HPV 16 and 18 genotyping into screening is determined, you should follow current ASCCP guidelines. Algorithms for different abnormal cytologic findings are available at http://www.asccp.org/Portals/9/docs/pdfs/Consensus%20Guidelines/algorithms_cyto_07.pdf
RACHEL KUPETS, MD
We want to hear from you! Tell us what you think.
1. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.-
2. Munoz N, Bosch FX, de Sanjose S, et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518-527.doi: 10.1056/NEJMoa021641.
3. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103(2):304-309.
4. De Sanjose S, Quint WG, Alemany L, et al. Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048-1056.
5. Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579-1588.
The Pap test wrought a sea change in the medical profession’s approach to cervical cancer screening, dramatically lowering the rate of invasive cervical cancer among women who underwent the test on a regular basis. That said, the sensitivity of a single Pap test in the detection of cervical dysplasia or cancer is less than 60%.1
It is well established that oncogenic HPV strains, otherwise known as high-risk HPV infection, are responsible for the development of severe preinvasive dysplasia and cervical cancer. Munoz and colleagues identified subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and 73 as having the greatest oncogenic potential.2 They also noted that HPV 26, 53, and 66 are probably carcinogenic.2
HPV DNA diagnostic tests are available to identify 14 high-risk HPV types. Current guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) recommend both cytology and HPV testing to determine the optimal interval between screening tests and for triage to colposcopy.3
HPV DNA diagnostic tests have evolved one step further and can now detect HPV 16 and HPV 18 individually; these two types of HPV account for 70% of all cervical cancer cases.4 Research is needed to determine what combination of tests will further improve outcomes in the screened population.
Details of the ATHENA study
The study was designed to evaluate the cobas HPV test (Roche), a new polymerase chain reaction–based DNA test that yields a pooled result for 12 high-risk HPV types as well as individual results for HPV 16 and 18.
ATHENA evaluates the test in three scenarios:
- as a triage test for women who have a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- as an adjunctive test to guide clinical management in women who have cytologic findings that are negative for intraepithelial lesions or malignancy (NILM)
- as a potential first-line test in the screening of women 25 years and older, regardless of the cytology result.
The primary endpoint in all three scenarios was to detect CIN 2 or greater.
The baseline results of this study are outlined above. Data from a 3-year follow-up of the women in the ATHENA study will be published at a future date.
Other screening studies are under way
Now that we have identified HPV as the cause of cervical cancer, researchers can investigate the best way to detect high-grade CIN. Published studies have determined that HPV testing is more sensitive and less specific than the Pap test in the detection of CIN 3 and cancer.5
The HPV FOCAL trial is under way to establish the efficiency of testing for high-risk HPV DNA as primary screening and as triage in three arms. In all three arms, CIN 3 is the outcome.1
ATHENA adds a second tier to similar studies by genotyping for HPV 16 and 18.
Unanswered questions
There is no doubt that the Pap test will be replaced as a stand-alone screening test for cervical cancer. Existing ASCCP guidelines already recommend HPV testing in patients who have normal cytology; it remains to be determined how testing specifically for HPV 16 and 18 will be incorporated into the algorithm. The ATHENA trial, and others like it, will be the basis of future cervical cancer screening guidelines.
Among the issues that need to be resolved are:
- the age at which testing for HPV 16 and 18 is appropriate
- the follow-up protocol for women who test positive for HPV 16 and 18, as well as for those who test negative
- the cost of adding testing for HPV 16 and 18 to screening
- the number of women who need to be screened to find those who are positive for HPV 16 and 18 among women infected with high-risk HPV
- the number of extra cases of CIN 3+ that will be identified when women who test positive for high-risk HPV are genotyped for HPV 16 and 18
- the number of women who will undergo unnecessary colposcopy by this approach
- the triage protocol that best balances sensitivity and specificity.
As guidelines become more complex and difficult to remember, compliance will no doubt be mixed. A centralized cervical cancer screening program and database are needed to reduce confusion and improve adherence to guidelines.
The findings of the ATHENA study do not alter current cervical cancer screening guidelines—yet. Until the most effective strategy of incorporating HPV 16 and 18 genotyping into screening is determined, you should follow current ASCCP guidelines. Algorithms for different abnormal cytologic findings are available at http://www.asccp.org/Portals/9/docs/pdfs/Consensus%20Guidelines/algorithms_cyto_07.pdf
RACHEL KUPETS, MD
We want to hear from you! Tell us what you think.
The Pap test wrought a sea change in the medical profession’s approach to cervical cancer screening, dramatically lowering the rate of invasive cervical cancer among women who underwent the test on a regular basis. That said, the sensitivity of a single Pap test in the detection of cervical dysplasia or cancer is less than 60%.1
It is well established that oncogenic HPV strains, otherwise known as high-risk HPV infection, are responsible for the development of severe preinvasive dysplasia and cervical cancer. Munoz and colleagues identified subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and 73 as having the greatest oncogenic potential.2 They also noted that HPV 26, 53, and 66 are probably carcinogenic.2
HPV DNA diagnostic tests are available to identify 14 high-risk HPV types. Current guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) recommend both cytology and HPV testing to determine the optimal interval between screening tests and for triage to colposcopy.3
HPV DNA diagnostic tests have evolved one step further and can now detect HPV 16 and HPV 18 individually; these two types of HPV account for 70% of all cervical cancer cases.4 Research is needed to determine what combination of tests will further improve outcomes in the screened population.
Details of the ATHENA study
The study was designed to evaluate the cobas HPV test (Roche), a new polymerase chain reaction–based DNA test that yields a pooled result for 12 high-risk HPV types as well as individual results for HPV 16 and 18.
ATHENA evaluates the test in three scenarios:
- as a triage test for women who have a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- as an adjunctive test to guide clinical management in women who have cytologic findings that are negative for intraepithelial lesions or malignancy (NILM)
- as a potential first-line test in the screening of women 25 years and older, regardless of the cytology result.
The primary endpoint in all three scenarios was to detect CIN 2 or greater.
The baseline results of this study are outlined above. Data from a 3-year follow-up of the women in the ATHENA study will be published at a future date.
Other screening studies are under way
Now that we have identified HPV as the cause of cervical cancer, researchers can investigate the best way to detect high-grade CIN. Published studies have determined that HPV testing is more sensitive and less specific than the Pap test in the detection of CIN 3 and cancer.5
The HPV FOCAL trial is under way to establish the efficiency of testing for high-risk HPV DNA as primary screening and as triage in three arms. In all three arms, CIN 3 is the outcome.1
ATHENA adds a second tier to similar studies by genotyping for HPV 16 and 18.
Unanswered questions
There is no doubt that the Pap test will be replaced as a stand-alone screening test for cervical cancer. Existing ASCCP guidelines already recommend HPV testing in patients who have normal cytology; it remains to be determined how testing specifically for HPV 16 and 18 will be incorporated into the algorithm. The ATHENA trial, and others like it, will be the basis of future cervical cancer screening guidelines.
Among the issues that need to be resolved are:
- the age at which testing for HPV 16 and 18 is appropriate
- the follow-up protocol for women who test positive for HPV 16 and 18, as well as for those who test negative
- the cost of adding testing for HPV 16 and 18 to screening
- the number of women who need to be screened to find those who are positive for HPV 16 and 18 among women infected with high-risk HPV
- the number of extra cases of CIN 3+ that will be identified when women who test positive for high-risk HPV are genotyped for HPV 16 and 18
- the number of women who will undergo unnecessary colposcopy by this approach
- the triage protocol that best balances sensitivity and specificity.
As guidelines become more complex and difficult to remember, compliance will no doubt be mixed. A centralized cervical cancer screening program and database are needed to reduce confusion and improve adherence to guidelines.
The findings of the ATHENA study do not alter current cervical cancer screening guidelines—yet. Until the most effective strategy of incorporating HPV 16 and 18 genotyping into screening is determined, you should follow current ASCCP guidelines. Algorithms for different abnormal cytologic findings are available at http://www.asccp.org/Portals/9/docs/pdfs/Consensus%20Guidelines/algorithms_cyto_07.pdf
RACHEL KUPETS, MD
We want to hear from you! Tell us what you think.
1. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.-
2. Munoz N, Bosch FX, de Sanjose S, et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518-527.doi: 10.1056/NEJMoa021641.
3. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103(2):304-309.
4. De Sanjose S, Quint WG, Alemany L, et al. Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048-1056.
5. Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579-1588.
1. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.-
2. Munoz N, Bosch FX, de Sanjose S, et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518-527.doi: 10.1056/NEJMoa021641.
3. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103(2):304-309.
4. De Sanjose S, Quint WG, Alemany L, et al. Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048-1056.
5. Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579-1588.
Vulvar pain syndromes: Causes and treatment of vestibulodynia
- Part 1: Making the correct diagnosis
(September 2011) - Part 2: A bounty of treatments—but not all of them are proven
(October 2011)
This three-part series concludes with a look at vestibulodynia—pain that is localized to the vulvar vestibule. Much is known about this disorder, compared with our knowledge base in the recent past, but much remains to be discovered. Among the questions explored by the panelists in this article is whether vestibulodynia and generalized vulvodynia are distinct entities—or different manifestations of the same process.
Other questions addressed here:
- Do oral contraceptives (OCs) contribute to vestibulodynia?
- What about herpes and genital warts? Are they causes of vestibular pain?
- Are some women more vulnerable to vestibulodynia than others?
- Is the disorder curable?
- Does vestibulectomy provide definitive treatment?
Part 1 of this series, which appeared in the September 2011 issue, focused on generalized vulvar pain and its causes, features, and diagnosis. Part 2, in the October issue, took as its subject the treatment of vulvar pain. Both are available in the archive at obgmanagement.com.
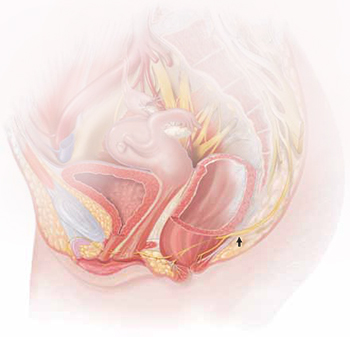
The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
What do we know about the causes of vestibulodynia?
Dr. Lonky: What are the causes of provoked vestibulodynia (PVD), also known as vulvar vestibulitis syndrome? And what are the theories behind those causes?
Dr. Haefner: The specific cause is unknown. Most likely, there isn’t a single cause. Theories that have been proposed include abnormalities of embryologic development, infection, inflammation, genetic and immune factors, and nerve pathways.
Patients who have vestibulodynia may also have interstitial cystitis. It has been noted that tissues from the vestibule and bladder have a common embryologic origin and, therefore, are predisposed to similar pathologic responses when challenged.1,2
Candida albicans infection in patients who experience vestibular pain has also been studied. The exact association is difficult to determine because many patients report Candida infections without verified testing for yeast. Bazin and colleagues found a very weak association between infection and pain on the vestibule.3
Inflammation—the “itis” in vestibulitis—has been excluded from the recent International Society for the Study of Vulvovaginal Disease (ISSVD) terminology because studies found no association between excised tissue and inflammation. Bohm-Starke and colleagues found low expression of the inflammatory markers cyclooxygenase 2 and inducible nitric oxide synthase in the vestibular mucosa of women who had localized vestibular pain, as well as in healthy women in the control group.4
Goetsch was one of the first researchers to explore a genetic association with localized vulvar pain.5 Fifteen percent of patients questioned over a 6-month period were found to have localized vestibular pain. Thirty-two percent had a female relative who had dyspareunia or tampon intolerance, raising the issue of a genetic predisposition. Another genetic connection was found in a study evaluating gene coding for interleukin 1-receptor antagonist.6–8
Krantz examined the nerve characteristics of the vulva and vagina.9 The region of the hymeneal ring was richly supplied with free nerve endings. No corpuscular endings of any form were observed. Only free nerve endings were observed in the fossa navicularis. A sparsity of nerve endings occurred in the vagina, as compared with the region of the fourchette, fossa navicularis, and hymeneal ring. More recent studies have analyzed the nerve factors, thermoreceptors, and nociceptors in women with vulvar pain.10,11
Dr. Edwards: I feel strongly that vestibulodynia and generalized vulvodynia are the same process. For example, tension headaches are supposed to be occipital, but some people experience tension headaches that are periorbital. Both are tension headaches despite the different locations. And almost all patients who experience any subset of vulvodynia have provoked vestibular pain. So the only thing that separates vestibulodynia from other patterns of vulvodynia is the option of vestibulectomy for therapy.
I don’t think that vulvodynia and vestibulodynia are “wastebasket” names for undiagnosable vulvar pain; rather, they are specific disease processes produced by pelvic floor dysfunction that predisposes a woman to neuropathic pain with a trigger or to a systemic pain syndrome that includes an abnormal pelvic floor.
Dr. Gunter: There are probably many causes of PVD, as Dr. Haefner suggested. There may be an ignition hypothesis, whereby some outside inflammatory trigger or trauma produces local neurogenic inflammation. However, given the prevalence of other pain disorders, there is probably also a need to have a lowered threshold for these changes to occur—basically, a vulnerable neurologic platform.
For some women, local neural hyperplasia is probably a factor. It is possible that there are different causes for primary and secondary vestibulodynia.
Vulvodynia and depression often travel together. They are such common comorbidities, in fact, that some physicians theorize that vulvodynia may be a symptom of an underlying mood disorder, such as depression, or that depression may be one manifestation of chronic vulvar pain. Suffice it to say that chronic pain and depression are often associated, and it is frequently difficult to determine whether the relationship is one of cause and effect.
Comprehensive care of the patient who has vulvar pain, therefore, should include a thorough history, looking specifically for depression (including sleep disorders) and eliciting information on any suicidal thoughts or intentions.
Although many patients who have vulvodynia are treated with an antidepressant, the dose that relieves pain may not be high enough to attenuate an accompanying mood disorder. My approach is to team up with a psychiatrist or psychologist who is familiar with vulvar pain syndromes. Together, we monitor the patient and fine-tune the therapeutic response.
—Neal M. Lonky, MD, MPH
Do oral contraceptives contribute to vestibulodynia?
Dr. Lonky: Is PVD more prevalent among OC users?
Dr. Haefner: Controversy surrounds the question of whether vestibulodynia and OC use are linked. Some studies suggest no association12–14 and others suggest a possible effect of OCs on vulvodynia.15–17 A study by Reed and colleagues found no association between taking OCs or hormone therapy at enrollment and incident vulvodynia only in the univariable analysis, but not when controlling for age at enrollment.18 This reflects the finding that younger age was associated with incident disease; younger age and use of OCs are similarly associated.
Dr. Gunter: I am not a believer in a cause-and-effect relationship between OC use and vestibulodynia. I do not find the studies demonstrating an association convincing. Given the supraphysiologic levels of hormones during pregnancy, if high hormone levels played a role, we should also see a greater incidence of vestibulodynia among women who have several pregnancies at an early age.
Dr. Edwards: In my practice, stopping, starting, and changing OCs has made no difference for patients. Topical estrogen supplementation in the occasional OC user who has signs of low estrogen has been useful at times.
Do herpes or genital warts contribute to PVD?
Dr. Lonky: Does a history of vulvar herpes or genital warts have any impact on the incidence of PVD?
Dr. Edwards: No.
Dr. Gunter: I agree that it has no impact.
Dr. Haefner: Herpes is sometimes associated with vulvar pain. The lesions resolve, but pain may continue as post-herpetic neuralgia. As with shingles, a low threshold for starting a patient on gabapentin to control pain after herpes may be beneficial.
Genital warts rarely cause vulvar pain—but the treatment may. Patients sometimes feel pain following topical treatment, as well as pain from surgical wart treatment.
Effect of demographic variables
Dr. Lonky: Does race, skin type, or hair or eye color make a difference in the prevalence, manifestation of symptoms, or treatment of PVD?
Dr. Gunter: I am not aware of any studies that confirm an association between vulvodynia and those factors.
Dr. Edwards: I don’t know whether any of these variables make a difference. My own impression—confirmed by informal study in my office—is that vulvodynia patients weigh less than my general dermatology patients and are better educated. I sometimes get the sense that my vulvodynia patients are more likely to be fair.
Dr. Lonky: What age group is most commonly affected by PVD?
Dr. Edwards: In my experience the most common age group is women 25 to 45 years old, probably because they are the most sexually active group old enough and tough enough to pursue this issue.
Dr. Gunter: I believe it affects all women equally, although women in their reproductive years are more likely to visit a gynecologist and, therefore, probably more likely to be given this diagnosis.
Dr. Lonky: Do you believe that PVD and generalized vulvar dysesthesia are curable—or just treatable?
Dr. Gunter: That depends on many variables. It is far more challenging to cure a patient who has multiple pain syndromes (for example, fibromyalgia, migraines, and irritable bowel syndrome) than the woman who simply has vestibulodynia or generalized vulvar pain. In addition, stress, anxiety, coping skills, and depression all play a role. In my opinion, a woman without comorbidity has a good chance of having her symptoms well-controlled. Some will be cured (that is, able to discontinue medications), and others will need ongoing treatment but will not be bothered by their symptoms.
Dr. Haefner: The response to treatment in many pain patients depends on the amount of time that the pain has been present. Someone who has had pain for 30 years will probably not be cured 3 months after starting treatment. However, someone with a short duration of pain often gets good improvement. One hundred percent improvement is rare, however. Many patients are able to approach the 80% improvement mark.
Dr. Edwards: I would say that these conditions are manageable more than curable, although pure vestibulodynia—which is uncommon—is curable with surgery.
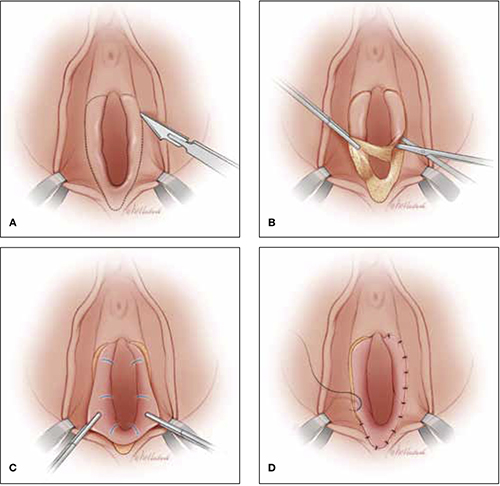
Vestibulectomy technique
(A) Incision. In many cases, the incision needs to extend up to the opening of the Skene’s ducts on the vestibule before it is carried down laterally along Hart’s line to the perianal skin, with the mucosa undermined above the hymeneal ring. (B) Excision. Remove the tissue superior to the hymeneal ring. (C) Advancement of vaginal mucosa. Further undermine the mucosa and advance it to close the defect. (D) Suturing. Close the defect in two layers using absorbable suture.
Is vestibulectomy definitive treatment?
Dr. Lonky: Does vestibulectomy work as a definitive treatment for PVD?
Dr. Gunter: Vestibulectomy—that is, resection of the vestibule and advancement of vaginal mucosa—is well described for women who have localized vestibulodynia. The complication rate is low, with only 3% of women reporting worsening of symptoms after the procedure.19,20 Success rates range from 17% to 89%, although many studies are retrospective reviews or include nonhomogenous populations, or both.19–21 A prospective study (Level II) indicates a 52% reduction in pain scores for women 6 months after vestibulectomy—and the scores continued to improve at evaluation at 2.5 years.22,23
Prospective studies indicate that vestibulectomy improves pain scores more than cognitive behavioral therapy, biofeedback, oral despiramine, and topical lidocaine.22,23 However, even surgery carries a robust placebo response rate, and vulvar biopsy alone has been associated with an improvement in pain scores in 67% of women.24,25
I offer surgery for vestibulodynia after the patient has failed at least two therapies (two topical treatments or one topical and one oral treatment). I offer injections before proceeding, and almost all patients opt to try them. I do not offer vestibulectomy to patients who have unprovoked pain or pain outside of resection margins.
Dr. Haefner: Surgical excision of the vulvar vestibule has met with success, in some studies, in more than 80% of cases, but it should be reserved for women who have longstanding and localized vestibular pain in whom other management options have failed.
The patient should undergo cotton-swab testing to outline areas of pain before anesthesia is administered in the operating room. Often, the incision will need to extend up to the opening of the Skene’s ducts on the vestibule (FIGURE). The incision is carried down laterally along Hart’s line to the perianal skin, and the mucosa is undermined above the hymeneal ring. The specimen is excised superior to the hymeneal ring. The vaginal tissue is further undermined and brought down to close the defect in two layers using absorbable suture. A review of this technique with illustrations has been published.26
Dr. Lonky: Thank you all again for sharing your considerable expertise, experience, and insight.
Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
In this population-based study, 16% of women reported a history of chronic unexplained vulvar pain, and nearly 7% reported current symptoms.
Khandker M, Brady SS, Vitonis AF, Maclehose RF, Stewart EG, Harlow BL. The influence of depression and anxiety on risk of adult onset vulvodynia [published online ahead of print August 8, 2011].
J Womens Health (Larchmt). doi:10.1089/jwh.2010.2661.
Vulvodynia was four times more likely among women who had antecedent mood or anxiety disorders than in women who didn’t. Vulvodynia was also associated with new or recurrent onset of mood or anxiety disorders.
Masheb RM, Wang E, Lozano C, Kerns RD. Prevalence and correlates of depression in treatment-seeking women with vulvodynia. J Obstet Gynaecol. 2005;25(8):786–791.
Comorbid major depressive disorders in women who have vulvodynia are related to greater pain severity and worse functioning.
Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
Women who have vulvodynia are psychologically similar to women who don’t. A primary psychological cause of vulvodynia is not supported.
Tribo MJ, Andion O, Ros S, et al. Clinical characteristics and psychopathological profile of patients with vulvodynia: an observational and descriptive study. Dermatology. 2008;216(1):24–30.
Psychiatric treatment may be a useful option to improve symptoms of vulvodynia.
We want to hear from you! Tell us what you think about this series on Vulvar Pain Syndromes.
1. McCormack WM. Two urogenital sinus syndromes. Interstitial cystitis and focal vulvitis. J Reprod Med. 1990;35(9):873-876.
2. Fitzpatrick CC, DeLancey JP, Elkins TE, McGuire EJ. Vulvar vestibulitis and interstitial cystitis: a disorder of urogenital sinusderived epithelium. Obstet Gynecol. 1993;81(5 Pt 2):860-862.
3. Bazin S, Bouchard C, Brisson J, Morin C, Meisels A, Fortier M. Vulvar vestibulitis syndrome: an exploratory case-control study. Obstet Gynecol. 1994;83(1):47-50.
4. Bohm-Starke N, Falconer C, Rylander E, Hilliges M. The expression of cyclooxygenase 2 and inducible nitric oxide synthase indicates no active inflammation in vulvar vestibulitis. Acta Obstet Gynecol Scand. 2001;80(7):638-644.
5. Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164(6 Pt 1):1609-1616.
6. Jeremias J, Ledger WJ, Witkin SS. Interleukin 1 receptor antagonist gene polymorphism in women with vulvar vestibulitis. Am J Obstet Gynecol. 2000;182(2):283-285.
7. Witkin SS, Gerber S, Ledger WJ. Differential characterization of women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2002;187(3):589-594.
8. Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am J Obstet Gynecol. 2007;196(4):346. e1-8.
9. Krantz KE. Innervation of the human vulva and vagina: a microscopic study. Obstet Gynecol. 1958;12:382-396.
10. Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94(2):177-183.
11. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
12. Bachman GA, Rosen R, Pinn VW, et al. Vulvodynia: a state-of-the-art consensus on definitions, diagnosis and management. J Reprod Med. 2006;51(6):447-456.
13. Harlow BL, Vitonis AF, Stewart EG. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med. 2008;53(2):102-110.
14. Danielsson I, Sjoberg I, Stenlund H, Wikman M. Prevalence and incidence of prolonged and severe dyspareunia in women: results from a population study. Scand J Public Health. 2003;31(2):113-118.
15. Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49(11):888-892.
16. Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J Sexual Med. 2007;4(6):1679-1683.
17. Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol. 2002;156(3):254-261.
18. Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: a 2-year follow-up study. Obstet Gynecol. 2008;112(2 Pt 1):231-237.
19. Goldstein AT, Klingman D, Christopher K, Johnson C, Marinoff SC. Surgical treatment of vulvar vestibulitis syndrome: outcome assessment derived from a postoperative questionnaire. J Sex Med. 2006;3(5):923-931.
20. Eva LJ, Narain S, Orakwue CO, Luesley DM. Is modified vestibulectomy for localized provoked vulvodynia an effective long-term treatment? A follow-up study. J Reprod Med. 2008;53(6):435-440.
21. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genit Tract Dis. 2005;9(1):40-51.
22. Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91(3):297-306.
23. Bergeron S, Khalife S, Glazer HI, Binik Y. Surgical and behavioral treatments for vestibulodynia: two-and-one-half-year follow-up and predictors of outcome. Obstet Gynecol. 2008;111(1):159-166.
24. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
25. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulvar vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
26. Haefner HK. Critique of new gynecologic surgical procedures: surgery for vulvar vestibulitis. Clin Obstet Gynecol. 2000;43(3):689-700.
- Part 1: Making the correct diagnosis
(September 2011) - Part 2: A bounty of treatments—but not all of them are proven
(October 2011)
This three-part series concludes with a look at vestibulodynia—pain that is localized to the vulvar vestibule. Much is known about this disorder, compared with our knowledge base in the recent past, but much remains to be discovered. Among the questions explored by the panelists in this article is whether vestibulodynia and generalized vulvodynia are distinct entities—or different manifestations of the same process.
Other questions addressed here:
- Do oral contraceptives (OCs) contribute to vestibulodynia?
- What about herpes and genital warts? Are they causes of vestibular pain?
- Are some women more vulnerable to vestibulodynia than others?
- Is the disorder curable?
- Does vestibulectomy provide definitive treatment?
Part 1 of this series, which appeared in the September 2011 issue, focused on generalized vulvar pain and its causes, features, and diagnosis. Part 2, in the October issue, took as its subject the treatment of vulvar pain. Both are available in the archive at obgmanagement.com.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
What do we know about the causes of vestibulodynia?
Dr. Lonky: What are the causes of provoked vestibulodynia (PVD), also known as vulvar vestibulitis syndrome? And what are the theories behind those causes?
Dr. Haefner: The specific cause is unknown. Most likely, there isn’t a single cause. Theories that have been proposed include abnormalities of embryologic development, infection, inflammation, genetic and immune factors, and nerve pathways.
Patients who have vestibulodynia may also have interstitial cystitis. It has been noted that tissues from the vestibule and bladder have a common embryologic origin and, therefore, are predisposed to similar pathologic responses when challenged.1,2
Candida albicans infection in patients who experience vestibular pain has also been studied. The exact association is difficult to determine because many patients report Candida infections without verified testing for yeast. Bazin and colleagues found a very weak association between infection and pain on the vestibule.3
Inflammation—the “itis” in vestibulitis—has been excluded from the recent International Society for the Study of Vulvovaginal Disease (ISSVD) terminology because studies found no association between excised tissue and inflammation. Bohm-Starke and colleagues found low expression of the inflammatory markers cyclooxygenase 2 and inducible nitric oxide synthase in the vestibular mucosa of women who had localized vestibular pain, as well as in healthy women in the control group.4
Goetsch was one of the first researchers to explore a genetic association with localized vulvar pain.5 Fifteen percent of patients questioned over a 6-month period were found to have localized vestibular pain. Thirty-two percent had a female relative who had dyspareunia or tampon intolerance, raising the issue of a genetic predisposition. Another genetic connection was found in a study evaluating gene coding for interleukin 1-receptor antagonist.6–8
Krantz examined the nerve characteristics of the vulva and vagina.9 The region of the hymeneal ring was richly supplied with free nerve endings. No corpuscular endings of any form were observed. Only free nerve endings were observed in the fossa navicularis. A sparsity of nerve endings occurred in the vagina, as compared with the region of the fourchette, fossa navicularis, and hymeneal ring. More recent studies have analyzed the nerve factors, thermoreceptors, and nociceptors in women with vulvar pain.10,11
Dr. Edwards: I feel strongly that vestibulodynia and generalized vulvodynia are the same process. For example, tension headaches are supposed to be occipital, but some people experience tension headaches that are periorbital. Both are tension headaches despite the different locations. And almost all patients who experience any subset of vulvodynia have provoked vestibular pain. So the only thing that separates vestibulodynia from other patterns of vulvodynia is the option of vestibulectomy for therapy.
I don’t think that vulvodynia and vestibulodynia are “wastebasket” names for undiagnosable vulvar pain; rather, they are specific disease processes produced by pelvic floor dysfunction that predisposes a woman to neuropathic pain with a trigger or to a systemic pain syndrome that includes an abnormal pelvic floor.
Dr. Gunter: There are probably many causes of PVD, as Dr. Haefner suggested. There may be an ignition hypothesis, whereby some outside inflammatory trigger or trauma produces local neurogenic inflammation. However, given the prevalence of other pain disorders, there is probably also a need to have a lowered threshold for these changes to occur—basically, a vulnerable neurologic platform.
For some women, local neural hyperplasia is probably a factor. It is possible that there are different causes for primary and secondary vestibulodynia.
Vulvodynia and depression often travel together. They are such common comorbidities, in fact, that some physicians theorize that vulvodynia may be a symptom of an underlying mood disorder, such as depression, or that depression may be one manifestation of chronic vulvar pain. Suffice it to say that chronic pain and depression are often associated, and it is frequently difficult to determine whether the relationship is one of cause and effect.
Comprehensive care of the patient who has vulvar pain, therefore, should include a thorough history, looking specifically for depression (including sleep disorders) and eliciting information on any suicidal thoughts or intentions.
Although many patients who have vulvodynia are treated with an antidepressant, the dose that relieves pain may not be high enough to attenuate an accompanying mood disorder. My approach is to team up with a psychiatrist or psychologist who is familiar with vulvar pain syndromes. Together, we monitor the patient and fine-tune the therapeutic response.
—Neal M. Lonky, MD, MPH
Do oral contraceptives contribute to vestibulodynia?
Dr. Lonky: Is PVD more prevalent among OC users?
Dr. Haefner: Controversy surrounds the question of whether vestibulodynia and OC use are linked. Some studies suggest no association12–14 and others suggest a possible effect of OCs on vulvodynia.15–17 A study by Reed and colleagues found no association between taking OCs or hormone therapy at enrollment and incident vulvodynia only in the univariable analysis, but not when controlling for age at enrollment.18 This reflects the finding that younger age was associated with incident disease; younger age and use of OCs are similarly associated.
Dr. Gunter: I am not a believer in a cause-and-effect relationship between OC use and vestibulodynia. I do not find the studies demonstrating an association convincing. Given the supraphysiologic levels of hormones during pregnancy, if high hormone levels played a role, we should also see a greater incidence of vestibulodynia among women who have several pregnancies at an early age.
Dr. Edwards: In my practice, stopping, starting, and changing OCs has made no difference for patients. Topical estrogen supplementation in the occasional OC user who has signs of low estrogen has been useful at times.
Do herpes or genital warts contribute to PVD?
Dr. Lonky: Does a history of vulvar herpes or genital warts have any impact on the incidence of PVD?
Dr. Edwards: No.
Dr. Gunter: I agree that it has no impact.
Dr. Haefner: Herpes is sometimes associated with vulvar pain. The lesions resolve, but pain may continue as post-herpetic neuralgia. As with shingles, a low threshold for starting a patient on gabapentin to control pain after herpes may be beneficial.
Genital warts rarely cause vulvar pain—but the treatment may. Patients sometimes feel pain following topical treatment, as well as pain from surgical wart treatment.
Effect of demographic variables
Dr. Lonky: Does race, skin type, or hair or eye color make a difference in the prevalence, manifestation of symptoms, or treatment of PVD?
Dr. Gunter: I am not aware of any studies that confirm an association between vulvodynia and those factors.
Dr. Edwards: I don’t know whether any of these variables make a difference. My own impression—confirmed by informal study in my office—is that vulvodynia patients weigh less than my general dermatology patients and are better educated. I sometimes get the sense that my vulvodynia patients are more likely to be fair.
Dr. Lonky: What age group is most commonly affected by PVD?
Dr. Edwards: In my experience the most common age group is women 25 to 45 years old, probably because they are the most sexually active group old enough and tough enough to pursue this issue.
Dr. Gunter: I believe it affects all women equally, although women in their reproductive years are more likely to visit a gynecologist and, therefore, probably more likely to be given this diagnosis.
Dr. Lonky: Do you believe that PVD and generalized vulvar dysesthesia are curable—or just treatable?
Dr. Gunter: That depends on many variables. It is far more challenging to cure a patient who has multiple pain syndromes (for example, fibromyalgia, migraines, and irritable bowel syndrome) than the woman who simply has vestibulodynia or generalized vulvar pain. In addition, stress, anxiety, coping skills, and depression all play a role. In my opinion, a woman without comorbidity has a good chance of having her symptoms well-controlled. Some will be cured (that is, able to discontinue medications), and others will need ongoing treatment but will not be bothered by their symptoms.
Dr. Haefner: The response to treatment in many pain patients depends on the amount of time that the pain has been present. Someone who has had pain for 30 years will probably not be cured 3 months after starting treatment. However, someone with a short duration of pain often gets good improvement. One hundred percent improvement is rare, however. Many patients are able to approach the 80% improvement mark.
Dr. Edwards: I would say that these conditions are manageable more than curable, although pure vestibulodynia—which is uncommon—is curable with surgery.

Vestibulectomy technique
(A) Incision. In many cases, the incision needs to extend up to the opening of the Skene’s ducts on the vestibule before it is carried down laterally along Hart’s line to the perianal skin, with the mucosa undermined above the hymeneal ring. (B) Excision. Remove the tissue superior to the hymeneal ring. (C) Advancement of vaginal mucosa. Further undermine the mucosa and advance it to close the defect. (D) Suturing. Close the defect in two layers using absorbable suture.
Is vestibulectomy definitive treatment?
Dr. Lonky: Does vestibulectomy work as a definitive treatment for PVD?
Dr. Gunter: Vestibulectomy—that is, resection of the vestibule and advancement of vaginal mucosa—is well described for women who have localized vestibulodynia. The complication rate is low, with only 3% of women reporting worsening of symptoms after the procedure.19,20 Success rates range from 17% to 89%, although many studies are retrospective reviews or include nonhomogenous populations, or both.19–21 A prospective study (Level II) indicates a 52% reduction in pain scores for women 6 months after vestibulectomy—and the scores continued to improve at evaluation at 2.5 years.22,23
Prospective studies indicate that vestibulectomy improves pain scores more than cognitive behavioral therapy, biofeedback, oral despiramine, and topical lidocaine.22,23 However, even surgery carries a robust placebo response rate, and vulvar biopsy alone has been associated with an improvement in pain scores in 67% of women.24,25
I offer surgery for vestibulodynia after the patient has failed at least two therapies (two topical treatments or one topical and one oral treatment). I offer injections before proceeding, and almost all patients opt to try them. I do not offer vestibulectomy to patients who have unprovoked pain or pain outside of resection margins.
Dr. Haefner: Surgical excision of the vulvar vestibule has met with success, in some studies, in more than 80% of cases, but it should be reserved for women who have longstanding and localized vestibular pain in whom other management options have failed.
The patient should undergo cotton-swab testing to outline areas of pain before anesthesia is administered in the operating room. Often, the incision will need to extend up to the opening of the Skene’s ducts on the vestibule (FIGURE). The incision is carried down laterally along Hart’s line to the perianal skin, and the mucosa is undermined above the hymeneal ring. The specimen is excised superior to the hymeneal ring. The vaginal tissue is further undermined and brought down to close the defect in two layers using absorbable suture. A review of this technique with illustrations has been published.26
Dr. Lonky: Thank you all again for sharing your considerable expertise, experience, and insight.
Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
In this population-based study, 16% of women reported a history of chronic unexplained vulvar pain, and nearly 7% reported current symptoms.
Khandker M, Brady SS, Vitonis AF, Maclehose RF, Stewart EG, Harlow BL. The influence of depression and anxiety on risk of adult onset vulvodynia [published online ahead of print August 8, 2011].
J Womens Health (Larchmt). doi:10.1089/jwh.2010.2661.
Vulvodynia was four times more likely among women who had antecedent mood or anxiety disorders than in women who didn’t. Vulvodynia was also associated with new or recurrent onset of mood or anxiety disorders.
Masheb RM, Wang E, Lozano C, Kerns RD. Prevalence and correlates of depression in treatment-seeking women with vulvodynia. J Obstet Gynaecol. 2005;25(8):786–791.
Comorbid major depressive disorders in women who have vulvodynia are related to greater pain severity and worse functioning.
Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
Women who have vulvodynia are psychologically similar to women who don’t. A primary psychological cause of vulvodynia is not supported.
Tribo MJ, Andion O, Ros S, et al. Clinical characteristics and psychopathological profile of patients with vulvodynia: an observational and descriptive study. Dermatology. 2008;216(1):24–30.
Psychiatric treatment may be a useful option to improve symptoms of vulvodynia.
We want to hear from you! Tell us what you think about this series on Vulvar Pain Syndromes.
- Part 1: Making the correct diagnosis
(September 2011) - Part 2: A bounty of treatments—but not all of them are proven
(October 2011)
This three-part series concludes with a look at vestibulodynia—pain that is localized to the vulvar vestibule. Much is known about this disorder, compared with our knowledge base in the recent past, but much remains to be discovered. Among the questions explored by the panelists in this article is whether vestibulodynia and generalized vulvodynia are distinct entities—or different manifestations of the same process.
Other questions addressed here:
- Do oral contraceptives (OCs) contribute to vestibulodynia?
- What about herpes and genital warts? Are they causes of vestibular pain?
- Are some women more vulnerable to vestibulodynia than others?
- Is the disorder curable?
- Does vestibulectomy provide definitive treatment?
Part 1 of this series, which appeared in the September 2011 issue, focused on generalized vulvar pain and its causes, features, and diagnosis. Part 2, in the October issue, took as its subject the treatment of vulvar pain. Both are available in the archive at obgmanagement.com.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
What do we know about the causes of vestibulodynia?
Dr. Lonky: What are the causes of provoked vestibulodynia (PVD), also known as vulvar vestibulitis syndrome? And what are the theories behind those causes?
Dr. Haefner: The specific cause is unknown. Most likely, there isn’t a single cause. Theories that have been proposed include abnormalities of embryologic development, infection, inflammation, genetic and immune factors, and nerve pathways.
Patients who have vestibulodynia may also have interstitial cystitis. It has been noted that tissues from the vestibule and bladder have a common embryologic origin and, therefore, are predisposed to similar pathologic responses when challenged.1,2
Candida albicans infection in patients who experience vestibular pain has also been studied. The exact association is difficult to determine because many patients report Candida infections without verified testing for yeast. Bazin and colleagues found a very weak association between infection and pain on the vestibule.3
Inflammation—the “itis” in vestibulitis—has been excluded from the recent International Society for the Study of Vulvovaginal Disease (ISSVD) terminology because studies found no association between excised tissue and inflammation. Bohm-Starke and colleagues found low expression of the inflammatory markers cyclooxygenase 2 and inducible nitric oxide synthase in the vestibular mucosa of women who had localized vestibular pain, as well as in healthy women in the control group.4
Goetsch was one of the first researchers to explore a genetic association with localized vulvar pain.5 Fifteen percent of patients questioned over a 6-month period were found to have localized vestibular pain. Thirty-two percent had a female relative who had dyspareunia or tampon intolerance, raising the issue of a genetic predisposition. Another genetic connection was found in a study evaluating gene coding for interleukin 1-receptor antagonist.6–8
Krantz examined the nerve characteristics of the vulva and vagina.9 The region of the hymeneal ring was richly supplied with free nerve endings. No corpuscular endings of any form were observed. Only free nerve endings were observed in the fossa navicularis. A sparsity of nerve endings occurred in the vagina, as compared with the region of the fourchette, fossa navicularis, and hymeneal ring. More recent studies have analyzed the nerve factors, thermoreceptors, and nociceptors in women with vulvar pain.10,11
Dr. Edwards: I feel strongly that vestibulodynia and generalized vulvodynia are the same process. For example, tension headaches are supposed to be occipital, but some people experience tension headaches that are periorbital. Both are tension headaches despite the different locations. And almost all patients who experience any subset of vulvodynia have provoked vestibular pain. So the only thing that separates vestibulodynia from other patterns of vulvodynia is the option of vestibulectomy for therapy.
I don’t think that vulvodynia and vestibulodynia are “wastebasket” names for undiagnosable vulvar pain; rather, they are specific disease processes produced by pelvic floor dysfunction that predisposes a woman to neuropathic pain with a trigger or to a systemic pain syndrome that includes an abnormal pelvic floor.
Dr. Gunter: There are probably many causes of PVD, as Dr. Haefner suggested. There may be an ignition hypothesis, whereby some outside inflammatory trigger or trauma produces local neurogenic inflammation. However, given the prevalence of other pain disorders, there is probably also a need to have a lowered threshold for these changes to occur—basically, a vulnerable neurologic platform.
For some women, local neural hyperplasia is probably a factor. It is possible that there are different causes for primary and secondary vestibulodynia.
Vulvodynia and depression often travel together. They are such common comorbidities, in fact, that some physicians theorize that vulvodynia may be a symptom of an underlying mood disorder, such as depression, or that depression may be one manifestation of chronic vulvar pain. Suffice it to say that chronic pain and depression are often associated, and it is frequently difficult to determine whether the relationship is one of cause and effect.
Comprehensive care of the patient who has vulvar pain, therefore, should include a thorough history, looking specifically for depression (including sleep disorders) and eliciting information on any suicidal thoughts or intentions.
Although many patients who have vulvodynia are treated with an antidepressant, the dose that relieves pain may not be high enough to attenuate an accompanying mood disorder. My approach is to team up with a psychiatrist or psychologist who is familiar with vulvar pain syndromes. Together, we monitor the patient and fine-tune the therapeutic response.
—Neal M. Lonky, MD, MPH
Do oral contraceptives contribute to vestibulodynia?
Dr. Lonky: Is PVD more prevalent among OC users?
Dr. Haefner: Controversy surrounds the question of whether vestibulodynia and OC use are linked. Some studies suggest no association12–14 and others suggest a possible effect of OCs on vulvodynia.15–17 A study by Reed and colleagues found no association between taking OCs or hormone therapy at enrollment and incident vulvodynia only in the univariable analysis, but not when controlling for age at enrollment.18 This reflects the finding that younger age was associated with incident disease; younger age and use of OCs are similarly associated.
Dr. Gunter: I am not a believer in a cause-and-effect relationship between OC use and vestibulodynia. I do not find the studies demonstrating an association convincing. Given the supraphysiologic levels of hormones during pregnancy, if high hormone levels played a role, we should also see a greater incidence of vestibulodynia among women who have several pregnancies at an early age.
Dr. Edwards: In my practice, stopping, starting, and changing OCs has made no difference for patients. Topical estrogen supplementation in the occasional OC user who has signs of low estrogen has been useful at times.
Do herpes or genital warts contribute to PVD?
Dr. Lonky: Does a history of vulvar herpes or genital warts have any impact on the incidence of PVD?
Dr. Edwards: No.
Dr. Gunter: I agree that it has no impact.
Dr. Haefner: Herpes is sometimes associated with vulvar pain. The lesions resolve, but pain may continue as post-herpetic neuralgia. As with shingles, a low threshold for starting a patient on gabapentin to control pain after herpes may be beneficial.
Genital warts rarely cause vulvar pain—but the treatment may. Patients sometimes feel pain following topical treatment, as well as pain from surgical wart treatment.
Effect of demographic variables
Dr. Lonky: Does race, skin type, or hair or eye color make a difference in the prevalence, manifestation of symptoms, or treatment of PVD?
Dr. Gunter: I am not aware of any studies that confirm an association between vulvodynia and those factors.
Dr. Edwards: I don’t know whether any of these variables make a difference. My own impression—confirmed by informal study in my office—is that vulvodynia patients weigh less than my general dermatology patients and are better educated. I sometimes get the sense that my vulvodynia patients are more likely to be fair.
Dr. Lonky: What age group is most commonly affected by PVD?
Dr. Edwards: In my experience the most common age group is women 25 to 45 years old, probably because they are the most sexually active group old enough and tough enough to pursue this issue.
Dr. Gunter: I believe it affects all women equally, although women in their reproductive years are more likely to visit a gynecologist and, therefore, probably more likely to be given this diagnosis.
Dr. Lonky: Do you believe that PVD and generalized vulvar dysesthesia are curable—or just treatable?
Dr. Gunter: That depends on many variables. It is far more challenging to cure a patient who has multiple pain syndromes (for example, fibromyalgia, migraines, and irritable bowel syndrome) than the woman who simply has vestibulodynia or generalized vulvar pain. In addition, stress, anxiety, coping skills, and depression all play a role. In my opinion, a woman without comorbidity has a good chance of having her symptoms well-controlled. Some will be cured (that is, able to discontinue medications), and others will need ongoing treatment but will not be bothered by their symptoms.
Dr. Haefner: The response to treatment in many pain patients depends on the amount of time that the pain has been present. Someone who has had pain for 30 years will probably not be cured 3 months after starting treatment. However, someone with a short duration of pain often gets good improvement. One hundred percent improvement is rare, however. Many patients are able to approach the 80% improvement mark.
Dr. Edwards: I would say that these conditions are manageable more than curable, although pure vestibulodynia—which is uncommon—is curable with surgery.

Vestibulectomy technique
(A) Incision. In many cases, the incision needs to extend up to the opening of the Skene’s ducts on the vestibule before it is carried down laterally along Hart’s line to the perianal skin, with the mucosa undermined above the hymeneal ring. (B) Excision. Remove the tissue superior to the hymeneal ring. (C) Advancement of vaginal mucosa. Further undermine the mucosa and advance it to close the defect. (D) Suturing. Close the defect in two layers using absorbable suture.
Is vestibulectomy definitive treatment?
Dr. Lonky: Does vestibulectomy work as a definitive treatment for PVD?
Dr. Gunter: Vestibulectomy—that is, resection of the vestibule and advancement of vaginal mucosa—is well described for women who have localized vestibulodynia. The complication rate is low, with only 3% of women reporting worsening of symptoms after the procedure.19,20 Success rates range from 17% to 89%, although many studies are retrospective reviews or include nonhomogenous populations, or both.19–21 A prospective study (Level II) indicates a 52% reduction in pain scores for women 6 months after vestibulectomy—and the scores continued to improve at evaluation at 2.5 years.22,23
Prospective studies indicate that vestibulectomy improves pain scores more than cognitive behavioral therapy, biofeedback, oral despiramine, and topical lidocaine.22,23 However, even surgery carries a robust placebo response rate, and vulvar biopsy alone has been associated with an improvement in pain scores in 67% of women.24,25
I offer surgery for vestibulodynia after the patient has failed at least two therapies (two topical treatments or one topical and one oral treatment). I offer injections before proceeding, and almost all patients opt to try them. I do not offer vestibulectomy to patients who have unprovoked pain or pain outside of resection margins.
Dr. Haefner: Surgical excision of the vulvar vestibule has met with success, in some studies, in more than 80% of cases, but it should be reserved for women who have longstanding and localized vestibular pain in whom other management options have failed.
The patient should undergo cotton-swab testing to outline areas of pain before anesthesia is administered in the operating room. Often, the incision will need to extend up to the opening of the Skene’s ducts on the vestibule (FIGURE). The incision is carried down laterally along Hart’s line to the perianal skin, and the mucosa is undermined above the hymeneal ring. The specimen is excised superior to the hymeneal ring. The vaginal tissue is further undermined and brought down to close the defect in two layers using absorbable suture. A review of this technique with illustrations has been published.26
Dr. Lonky: Thank you all again for sharing your considerable expertise, experience, and insight.
Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
In this population-based study, 16% of women reported a history of chronic unexplained vulvar pain, and nearly 7% reported current symptoms.
Khandker M, Brady SS, Vitonis AF, Maclehose RF, Stewart EG, Harlow BL. The influence of depression and anxiety on risk of adult onset vulvodynia [published online ahead of print August 8, 2011].
J Womens Health (Larchmt). doi:10.1089/jwh.2010.2661.
Vulvodynia was four times more likely among women who had antecedent mood or anxiety disorders than in women who didn’t. Vulvodynia was also associated with new or recurrent onset of mood or anxiety disorders.
Masheb RM, Wang E, Lozano C, Kerns RD. Prevalence and correlates of depression in treatment-seeking women with vulvodynia. J Obstet Gynaecol. 2005;25(8):786–791.
Comorbid major depressive disorders in women who have vulvodynia are related to greater pain severity and worse functioning.
Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
Women who have vulvodynia are psychologically similar to women who don’t. A primary psychological cause of vulvodynia is not supported.
Tribo MJ, Andion O, Ros S, et al. Clinical characteristics and psychopathological profile of patients with vulvodynia: an observational and descriptive study. Dermatology. 2008;216(1):24–30.
Psychiatric treatment may be a useful option to improve symptoms of vulvodynia.
We want to hear from you! Tell us what you think about this series on Vulvar Pain Syndromes.
1. McCormack WM. Two urogenital sinus syndromes. Interstitial cystitis and focal vulvitis. J Reprod Med. 1990;35(9):873-876.
2. Fitzpatrick CC, DeLancey JP, Elkins TE, McGuire EJ. Vulvar vestibulitis and interstitial cystitis: a disorder of urogenital sinusderived epithelium. Obstet Gynecol. 1993;81(5 Pt 2):860-862.
3. Bazin S, Bouchard C, Brisson J, Morin C, Meisels A, Fortier M. Vulvar vestibulitis syndrome: an exploratory case-control study. Obstet Gynecol. 1994;83(1):47-50.
4. Bohm-Starke N, Falconer C, Rylander E, Hilliges M. The expression of cyclooxygenase 2 and inducible nitric oxide synthase indicates no active inflammation in vulvar vestibulitis. Acta Obstet Gynecol Scand. 2001;80(7):638-644.
5. Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164(6 Pt 1):1609-1616.
6. Jeremias J, Ledger WJ, Witkin SS. Interleukin 1 receptor antagonist gene polymorphism in women with vulvar vestibulitis. Am J Obstet Gynecol. 2000;182(2):283-285.
7. Witkin SS, Gerber S, Ledger WJ. Differential characterization of women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2002;187(3):589-594.
8. Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am J Obstet Gynecol. 2007;196(4):346. e1-8.
9. Krantz KE. Innervation of the human vulva and vagina: a microscopic study. Obstet Gynecol. 1958;12:382-396.
10. Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94(2):177-183.
11. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
12. Bachman GA, Rosen R, Pinn VW, et al. Vulvodynia: a state-of-the-art consensus on definitions, diagnosis and management. J Reprod Med. 2006;51(6):447-456.
13. Harlow BL, Vitonis AF, Stewart EG. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med. 2008;53(2):102-110.
14. Danielsson I, Sjoberg I, Stenlund H, Wikman M. Prevalence and incidence of prolonged and severe dyspareunia in women: results from a population study. Scand J Public Health. 2003;31(2):113-118.
15. Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49(11):888-892.
16. Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J Sexual Med. 2007;4(6):1679-1683.
17. Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol. 2002;156(3):254-261.
18. Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: a 2-year follow-up study. Obstet Gynecol. 2008;112(2 Pt 1):231-237.
19. Goldstein AT, Klingman D, Christopher K, Johnson C, Marinoff SC. Surgical treatment of vulvar vestibulitis syndrome: outcome assessment derived from a postoperative questionnaire. J Sex Med. 2006;3(5):923-931.
20. Eva LJ, Narain S, Orakwue CO, Luesley DM. Is modified vestibulectomy for localized provoked vulvodynia an effective long-term treatment? A follow-up study. J Reprod Med. 2008;53(6):435-440.
21. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genit Tract Dis. 2005;9(1):40-51.
22. Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91(3):297-306.
23. Bergeron S, Khalife S, Glazer HI, Binik Y. Surgical and behavioral treatments for vestibulodynia: two-and-one-half-year follow-up and predictors of outcome. Obstet Gynecol. 2008;111(1):159-166.
24. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
25. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulvar vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
26. Haefner HK. Critique of new gynecologic surgical procedures: surgery for vulvar vestibulitis. Clin Obstet Gynecol. 2000;43(3):689-700.
1. McCormack WM. Two urogenital sinus syndromes. Interstitial cystitis and focal vulvitis. J Reprod Med. 1990;35(9):873-876.
2. Fitzpatrick CC, DeLancey JP, Elkins TE, McGuire EJ. Vulvar vestibulitis and interstitial cystitis: a disorder of urogenital sinusderived epithelium. Obstet Gynecol. 1993;81(5 Pt 2):860-862.
3. Bazin S, Bouchard C, Brisson J, Morin C, Meisels A, Fortier M. Vulvar vestibulitis syndrome: an exploratory case-control study. Obstet Gynecol. 1994;83(1):47-50.
4. Bohm-Starke N, Falconer C, Rylander E, Hilliges M. The expression of cyclooxygenase 2 and inducible nitric oxide synthase indicates no active inflammation in vulvar vestibulitis. Acta Obstet Gynecol Scand. 2001;80(7):638-644.
5. Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164(6 Pt 1):1609-1616.
6. Jeremias J, Ledger WJ, Witkin SS. Interleukin 1 receptor antagonist gene polymorphism in women with vulvar vestibulitis. Am J Obstet Gynecol. 2000;182(2):283-285.
7. Witkin SS, Gerber S, Ledger WJ. Differential characterization of women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2002;187(3):589-594.
8. Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am J Obstet Gynecol. 2007;196(4):346. e1-8.
9. Krantz KE. Innervation of the human vulva and vagina: a microscopic study. Obstet Gynecol. 1958;12:382-396.
10. Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94(2):177-183.
11. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
12. Bachman GA, Rosen R, Pinn VW, et al. Vulvodynia: a state-of-the-art consensus on definitions, diagnosis and management. J Reprod Med. 2006;51(6):447-456.
13. Harlow BL, Vitonis AF, Stewart EG. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med. 2008;53(2):102-110.
14. Danielsson I, Sjoberg I, Stenlund H, Wikman M. Prevalence and incidence of prolonged and severe dyspareunia in women: results from a population study. Scand J Public Health. 2003;31(2):113-118.
15. Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49(11):888-892.
16. Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J Sexual Med. 2007;4(6):1679-1683.
17. Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol. 2002;156(3):254-261.
18. Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: a 2-year follow-up study. Obstet Gynecol. 2008;112(2 Pt 1):231-237.
19. Goldstein AT, Klingman D, Christopher K, Johnson C, Marinoff SC. Surgical treatment of vulvar vestibulitis syndrome: outcome assessment derived from a postoperative questionnaire. J Sex Med. 2006;3(5):923-931.
20. Eva LJ, Narain S, Orakwue CO, Luesley DM. Is modified vestibulectomy for localized provoked vulvodynia an effective long-term treatment? A follow-up study. J Reprod Med. 2008;53(6):435-440.
21. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genit Tract Dis. 2005;9(1):40-51.
22. Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91(3):297-306.
23. Bergeron S, Khalife S, Glazer HI, Binik Y. Surgical and behavioral treatments for vestibulodynia: two-and-one-half-year follow-up and predictors of outcome. Obstet Gynecol. 2008;111(1):159-166.
24. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
25. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulvar vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
26. Haefner HK. Critique of new gynecologic surgical procedures: surgery for vulvar vestibulitis. Clin Obstet Gynecol. 2000;43(3):689-700.
Laborists, Defined
Last month (see “Hospital-Focused Practice,” Septem-ber 2011, p. 61), I discussed the adoption of the hospitalist model of practice by many specialties, some of the common issues they face, and highlighted a national meeting to examine this phenomenon (for more information on the meeting, visit www.hospitalmedicine/hfpm). This month, relying mostly on my own experience with this practice model, I’ll drill deeper into OB hospitalists (also known as laborists). While there are a lot of ways in which hospitalist practice in many specialties are the same, laborists differ from those in other fields in important and interesting ways.
Prevalence
One of the most informative sources about the “laborist movement” is ObGynHospitalist.com, a website started and managed by Dr. Rob Olson, an enterprising laborist in Bellingham, Wash. As of July, the site listed 132 laborist programs nationwide (and that figure likely underestimates the actual number in operation). A survey of registered users of the website in April yielded 106 responses, representing a 24% response rate. Seventy-five of the respondents indicated they were full-time laborists.
Unique Drivers
Because obstetric malpractice costs are so high, and many lawsuits are related to delayed response to obstetric emergencies, there is hope (not much hard proof yet) that outcomes will be better, and lawsuits less common or less costly.1 So the hope of reduced malpractice costs figures more prominently into the cost-benefit analysis of the OB hospitalist model than most other types of HM practice.
Financial Model
It appears that all hospitalist models require financial support over and above professional fee revenue. Hospitals usually are willing (happy?) to provide this money because they can make back even more as a result of increased patient volume/market share or lower costs. And, as is the case for hospitalists in other specialties, laborist presence can be an asset in recruitment and retention of other OBGYNs.
I think the most interesting feature of laborist practice is that in many settings, it has the potential to open new sources of revenue—both hospital “facility fee” and professional fee revenue. A common practice in many hospitals is for obstetricians to send patients, or for them to self-present, to labor and delivery to be checked for a cold, vomiting, or whether labor has started. Many times, a nurse performs these checks, communicates with a doctor, then discharges the patient—and no bill is generated. An on-site laborist can see the same patients (presumably making for a higher-quality visit for the patient) and, assuming the visit is medically necessary, both a facility and professional charge can be submitted. Revenue from such visits can go a long way toward making up the difference between the total cost of the laborist program and fee collections. This adds to patient safety, as each patient is evaluated in person by a physician rather than only a nurse.
In most settings, the laborist submits a charge for delivery only for unassigned patients. For those patients who “belong to” another OB who provided prenatal care, it is often most practical for that doctor to submit the global fee for prenatal care and delivery, and to pay the laborist program an agreed-upon rate for each service provided.
Compensation
Laborists often are paid an hourly rate, and they typically don’t have a salary component tied to work relative-value unit (wRVU) production or other productivity metrics. Total annual compensation is typically lower than private-practice OBGYN physicians. It also varies widely, depending on local market forces, job description, and workload. Most programs are trying to implement meaningful quality bonuses for laborists.
Scope of Practice
Laborists typically provide care to all unassigned patients who present to labor and delivery, and perform deliveries, C-sections, and other services on patients when requested by OBs in traditional practice. Requests arise when an OB simply needs to be relieved of being on call for their private patients, or when an emergency arises. (These “as-needed” referrals are different from the most common arrangement for “medical hospitalist” practices that ask other doctors to refer all or none of their patients, not just when they are otherwise occupied.)
Lastly, the laborist might serve as surgical assistant to other OBGYNs. In nearly all settings, there is no need to require that any physicians refer to the laborist, and the other OBs are free to decide when to refer.
A reasonably common scenario is that, to avoid disruption of scheduled office hours, an OB in traditional practice might ask that the laborist manage a patient who presents in labor. But if still undelivered at the close of office hours, the traditional OB might assume care from that point on or have the laborist remain responsible through delivery. The traditional OB usually will make post-partum “rounding” visits on all of their patients but could rely on the laborist for these visits.
In most cases, the laborist does not have any scheduled gynecologic procedures, though he or she may see GYN consults throughout the hospital as time permits. Laborists typically have no outpatient responsibilities, but some OBGYN hospitalists cover GYN in the ED.
Operational Structure
Although models vary significantly, the single most common arrangement is for laborists to work 24-hour, in-house shifts. Rarely is there a need or justification to have more than one laborist on at a time. For a single physician, seven or eight 24-hour shifts per month is considered full-time. My experience is that most laborists are employed by the hospital in which they work.
As is the case in every specialty, some large OBGYN groups adopt a rotating laborist model, in which one member of their group becomes the laborist for 24 hours at a time, during which they are relieved of all other responsibilities.
Recruitment
ObGynHospitalist.com shows that, as of July, 40 of the 132 laborist programs that had identified themselves on the site were recruiting. My experience is that unlike “medical hospitalist” practices, which tend to successfully recruit those very early in their career, or “surgical hospitalist” programs, which target mid- to late-career general surgeons, laborist candidates come from any point in their careers. Most programs prefer that a laborist has several years of post-residency experience, but they generally have no other preference.
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Reference
Last month (see “Hospital-Focused Practice,” Septem-ber 2011, p. 61), I discussed the adoption of the hospitalist model of practice by many specialties, some of the common issues they face, and highlighted a national meeting to examine this phenomenon (for more information on the meeting, visit www.hospitalmedicine/hfpm). This month, relying mostly on my own experience with this practice model, I’ll drill deeper into OB hospitalists (also known as laborists). While there are a lot of ways in which hospitalist practice in many specialties are the same, laborists differ from those in other fields in important and interesting ways.
Prevalence
One of the most informative sources about the “laborist movement” is ObGynHospitalist.com, a website started and managed by Dr. Rob Olson, an enterprising laborist in Bellingham, Wash. As of July, the site listed 132 laborist programs nationwide (and that figure likely underestimates the actual number in operation). A survey of registered users of the website in April yielded 106 responses, representing a 24% response rate. Seventy-five of the respondents indicated they were full-time laborists.
Unique Drivers
Because obstetric malpractice costs are so high, and many lawsuits are related to delayed response to obstetric emergencies, there is hope (not much hard proof yet) that outcomes will be better, and lawsuits less common or less costly.1 So the hope of reduced malpractice costs figures more prominently into the cost-benefit analysis of the OB hospitalist model than most other types of HM practice.
Financial Model
It appears that all hospitalist models require financial support over and above professional fee revenue. Hospitals usually are willing (happy?) to provide this money because they can make back even more as a result of increased patient volume/market share or lower costs. And, as is the case for hospitalists in other specialties, laborist presence can be an asset in recruitment and retention of other OBGYNs.
I think the most interesting feature of laborist practice is that in many settings, it has the potential to open new sources of revenue—both hospital “facility fee” and professional fee revenue. A common practice in many hospitals is for obstetricians to send patients, or for them to self-present, to labor and delivery to be checked for a cold, vomiting, or whether labor has started. Many times, a nurse performs these checks, communicates with a doctor, then discharges the patient—and no bill is generated. An on-site laborist can see the same patients (presumably making for a higher-quality visit for the patient) and, assuming the visit is medically necessary, both a facility and professional charge can be submitted. Revenue from such visits can go a long way toward making up the difference between the total cost of the laborist program and fee collections. This adds to patient safety, as each patient is evaluated in person by a physician rather than only a nurse.
In most settings, the laborist submits a charge for delivery only for unassigned patients. For those patients who “belong to” another OB who provided prenatal care, it is often most practical for that doctor to submit the global fee for prenatal care and delivery, and to pay the laborist program an agreed-upon rate for each service provided.
Compensation
Laborists often are paid an hourly rate, and they typically don’t have a salary component tied to work relative-value unit (wRVU) production or other productivity metrics. Total annual compensation is typically lower than private-practice OBGYN physicians. It also varies widely, depending on local market forces, job description, and workload. Most programs are trying to implement meaningful quality bonuses for laborists.
Scope of Practice
Laborists typically provide care to all unassigned patients who present to labor and delivery, and perform deliveries, C-sections, and other services on patients when requested by OBs in traditional practice. Requests arise when an OB simply needs to be relieved of being on call for their private patients, or when an emergency arises. (These “as-needed” referrals are different from the most common arrangement for “medical hospitalist” practices that ask other doctors to refer all or none of their patients, not just when they are otherwise occupied.)
Lastly, the laborist might serve as surgical assistant to other OBGYNs. In nearly all settings, there is no need to require that any physicians refer to the laborist, and the other OBs are free to decide when to refer.
A reasonably common scenario is that, to avoid disruption of scheduled office hours, an OB in traditional practice might ask that the laborist manage a patient who presents in labor. But if still undelivered at the close of office hours, the traditional OB might assume care from that point on or have the laborist remain responsible through delivery. The traditional OB usually will make post-partum “rounding” visits on all of their patients but could rely on the laborist for these visits.
In most cases, the laborist does not have any scheduled gynecologic procedures, though he or she may see GYN consults throughout the hospital as time permits. Laborists typically have no outpatient responsibilities, but some OBGYN hospitalists cover GYN in the ED.
Operational Structure
Although models vary significantly, the single most common arrangement is for laborists to work 24-hour, in-house shifts. Rarely is there a need or justification to have more than one laborist on at a time. For a single physician, seven or eight 24-hour shifts per month is considered full-time. My experience is that most laborists are employed by the hospital in which they work.
As is the case in every specialty, some large OBGYN groups adopt a rotating laborist model, in which one member of their group becomes the laborist for 24 hours at a time, during which they are relieved of all other responsibilities.
Recruitment
ObGynHospitalist.com shows that, as of July, 40 of the 132 laborist programs that had identified themselves on the site were recruiting. My experience is that unlike “medical hospitalist” practices, which tend to successfully recruit those very early in their career, or “surgical hospitalist” programs, which target mid- to late-career general surgeons, laborist candidates come from any point in their careers. Most programs prefer that a laborist has several years of post-residency experience, but they generally have no other preference.
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Reference
Last month (see “Hospital-Focused Practice,” Septem-ber 2011, p. 61), I discussed the adoption of the hospitalist model of practice by many specialties, some of the common issues they face, and highlighted a national meeting to examine this phenomenon (for more information on the meeting, visit www.hospitalmedicine/hfpm). This month, relying mostly on my own experience with this practice model, I’ll drill deeper into OB hospitalists (also known as laborists). While there are a lot of ways in which hospitalist practice in many specialties are the same, laborists differ from those in other fields in important and interesting ways.
Prevalence
One of the most informative sources about the “laborist movement” is ObGynHospitalist.com, a website started and managed by Dr. Rob Olson, an enterprising laborist in Bellingham, Wash. As of July, the site listed 132 laborist programs nationwide (and that figure likely underestimates the actual number in operation). A survey of registered users of the website in April yielded 106 responses, representing a 24% response rate. Seventy-five of the respondents indicated they were full-time laborists.
Unique Drivers
Because obstetric malpractice costs are so high, and many lawsuits are related to delayed response to obstetric emergencies, there is hope (not much hard proof yet) that outcomes will be better, and lawsuits less common or less costly.1 So the hope of reduced malpractice costs figures more prominently into the cost-benefit analysis of the OB hospitalist model than most other types of HM practice.
Financial Model
It appears that all hospitalist models require financial support over and above professional fee revenue. Hospitals usually are willing (happy?) to provide this money because they can make back even more as a result of increased patient volume/market share or lower costs. And, as is the case for hospitalists in other specialties, laborist presence can be an asset in recruitment and retention of other OBGYNs.
I think the most interesting feature of laborist practice is that in many settings, it has the potential to open new sources of revenue—both hospital “facility fee” and professional fee revenue. A common practice in many hospitals is for obstetricians to send patients, or for them to self-present, to labor and delivery to be checked for a cold, vomiting, or whether labor has started. Many times, a nurse performs these checks, communicates with a doctor, then discharges the patient—and no bill is generated. An on-site laborist can see the same patients (presumably making for a higher-quality visit for the patient) and, assuming the visit is medically necessary, both a facility and professional charge can be submitted. Revenue from such visits can go a long way toward making up the difference between the total cost of the laborist program and fee collections. This adds to patient safety, as each patient is evaluated in person by a physician rather than only a nurse.
In most settings, the laborist submits a charge for delivery only for unassigned patients. For those patients who “belong to” another OB who provided prenatal care, it is often most practical for that doctor to submit the global fee for prenatal care and delivery, and to pay the laborist program an agreed-upon rate for each service provided.
Compensation
Laborists often are paid an hourly rate, and they typically don’t have a salary component tied to work relative-value unit (wRVU) production or other productivity metrics. Total annual compensation is typically lower than private-practice OBGYN physicians. It also varies widely, depending on local market forces, job description, and workload. Most programs are trying to implement meaningful quality bonuses for laborists.
Scope of Practice
Laborists typically provide care to all unassigned patients who present to labor and delivery, and perform deliveries, C-sections, and other services on patients when requested by OBs in traditional practice. Requests arise when an OB simply needs to be relieved of being on call for their private patients, or when an emergency arises. (These “as-needed” referrals are different from the most common arrangement for “medical hospitalist” practices that ask other doctors to refer all or none of their patients, not just when they are otherwise occupied.)
Lastly, the laborist might serve as surgical assistant to other OBGYNs. In nearly all settings, there is no need to require that any physicians refer to the laborist, and the other OBs are free to decide when to refer.
A reasonably common scenario is that, to avoid disruption of scheduled office hours, an OB in traditional practice might ask that the laborist manage a patient who presents in labor. But if still undelivered at the close of office hours, the traditional OB might assume care from that point on or have the laborist remain responsible through delivery. The traditional OB usually will make post-partum “rounding” visits on all of their patients but could rely on the laborist for these visits.
In most cases, the laborist does not have any scheduled gynecologic procedures, though he or she may see GYN consults throughout the hospital as time permits. Laborists typically have no outpatient responsibilities, but some OBGYN hospitalists cover GYN in the ED.
Operational Structure
Although models vary significantly, the single most common arrangement is for laborists to work 24-hour, in-house shifts. Rarely is there a need or justification to have more than one laborist on at a time. For a single physician, seven or eight 24-hour shifts per month is considered full-time. My experience is that most laborists are employed by the hospital in which they work.
As is the case in every specialty, some large OBGYN groups adopt a rotating laborist model, in which one member of their group becomes the laborist for 24 hours at a time, during which they are relieved of all other responsibilities.
Recruitment
ObGynHospitalist.com shows that, as of July, 40 of the 132 laborist programs that had identified themselves on the site were recruiting. My experience is that unlike “medical hospitalist” practices, which tend to successfully recruit those very early in their career, or “surgical hospitalist” programs, which target mid- to late-career general surgeons, laborist candidates come from any point in their careers. Most programs prefer that a laborist has several years of post-residency experience, but they generally have no other preference.
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Reference
Are new tools for correcting prolapse and incontinence better just because they’re new?
The author reports no financial relationships relevant to this article.
From my vantage point, it appears that economic factors are playing an increasingly important role in how pelvic organ prolapse (POP) and urinary incontinence (UI) are managed—particularly, in regard to the use of surgical devices. As such, the topic of treating POP and UI deserves our attention to ensure that we make the best decisions for our patients.
Now, I’m a staunch supporter of innovation in treatment; certainly, there is room for improvement in current approaches—particularly in surgery—for treating POP and UI. At the same time, I strongly believe that innovation must be demonstrated to be an improvement before it is incorporated into practice. Although innovation is commonly taken on faith, we should know better than to equate “new” with “better” until evidence, gathered through clinical research, has demonstrated this conclusively. A look at the US Food and Drug Administration’s (FDA’s) process for clearing medical devices for clinical use reveals that such a standard often doesn’t apply—and this should matter to us.
The meaning of 510(k)
Most medical devices are evaluated through an FDA clearance mechanism known as the 510(k) process. This is wholly distinct from the agency’s drug approval process with which most of us are familiar. It’s beyond the scope of this commentary for me to go into detail about 510(k); if you are interested, see two recent commentaries1,2 and visit http://www.fda.gov/cdrh/devadvice/314.html.
In a nutshell, the 510(k) process requires only that an applicant demonstrate that a new medical device has “substantial equivalence” to an already legally marketed device, known as the predicate, which may also have been cleared only through the 510(k) process. That means it’s possible to have generations of products cleared on the basis of one predicate device that was itself never studied adequately.
Indeed, this is the case with most medical devices that have been sold for the surgical treatment of POP and UI—from before the ProteGen Sling (Boston Scientific), through Tension-Free Vaginal Tape (TVT) (Gynecare), and continuing with the newest devices.
The story of the ProteGen Sling (FIGURE) offers a cautionary tale about what can go wrong when new devices are cleared by the FDA through 510(k), rather than evaluated through rigorous clinical trials, as drugs are. More recently, experience with the ObTape (Mentor Corporation) followed virtually the same trajectory of events; the product was pulled from the market in 2006 and is now the focus of lawsuits nationwide.
Fortunately, for our patients, experience with TVT (Gynecare) has been favorable. Although TVT was also cleared by the FDA through 510(k), clinical research performed after TVT was introduced has demonstrated its effectiveness and relative safety. Indeed, TVT has revolutionized the treatment of stress UI in women—and, even, our understanding of its etiology.
Several companies are capitalizing on the success of TVT by introducing competing products that are designed to be 1) similar enough to ride on the coattails of TVT yet 2) different enough to capture their own share of market—without evidence of safety or effectiveness required. Even Gynecare (part of Ethicon Women’s Health and Urology, a subsidiary of Johnson & Johnson) has introduced TVT SECUR to compete with its own TVT—again, without independent evidence of safety or effectiveness.
The current market in devices for stress UI is a moving target that makes it nearly impossible—even for research organizations, such as the federally funded Pelvic Floor Disorders Network, that are independent of industry—to develop and implement sound clinical trials of those devices. Why do I say “moving target”? First, there are no assurances that any device chosen for study will remain the same for the duration of a trial. Second, there is no way to foresee which products will be abandoned over the time required for a large clinical trial.
FIGURE The saga of the ProteGen Sling
Transparency over what might be considered “experimental”
Until the FDA changes its process—to one in which 1) medical devices are adequately assessed before they reach market and 2) postmarketing surveillance is required—it’s our duty to insist on evidence of safety and effectiveness before adopting the latest and greatest products that companies have to offer.
Of all the questions that a patient might ask before treatment, three of the most important, surely, are:
- “Will this help me?”
- “If it helps me, how long will it help?”
- “Whether or not this treatment helps me, what risks—in the short-term and over the long-term—does it present?”
Until we can provide our patients with answers that are supported by evidence, products that lack such evidence should be considered experimental, and patients should be counseled accordingly.
Some patients may accept what they’ve been advised are new and unproven treatments—in the way that some physicians are early adopters. Nevertheless, I am concerned that some clinicians do not appear to appreciate the true lack of evidence that accompanies most marketed devices for prolapse and incontinence. They may mistake the FDA 510(k) process of clearance for something similar to the agency’s extended and complex drug approval process. They may accept claims made in industry-produced white papers that are often largely promotional materials, and fail to look further into those claims.
Now, more than ever and above all else, we must stand between marketing and our patients’ safety. We are familiar with the toll that prolapse and incontinence, as chronic conditions, take on our patients; yet it’s that very chronic nature that should lead us to adopt patience and caution in accepting new treatments before they have been adequately studied.
If we cannot always rely on industry to provide clear information about the risks and benefits of new devices, neither can we routinely look to our professional organizations for unbiased information. Often, professional organizations accept cash contributions from industry, raising the question of conflict of interest that may undermine their actions when the priorities of industry do not align with the goal of safeguarding patients’ well-being.
In an unprecedented example of how a professional association can interfere with its own, expert-authored clinical practice guidelines, the American College of Obstetricians and Gynecologists (ACOG) more than a year ago rescinded one of its published guidelines on POP (Issue 79, February 20073) and replaced it with a new guideline (Issue 85, September 20074). The new guideline is nearly identical to the prior one—save for one sentence, in which “experimental” is deleted in a discussion of kits of trocar-based synthetic materials sold for the surgical treatment of pelvic organ prolapse (see the EXCERPT).
The deletion is crucial because offering informed consent for surgery requires a patient to accept risks in balance with an expectation of benefit. A patient cannot be appropriately informed when no evidence of benefit exists and evidence of postoperative risk is extremely limited.
Now, I am not declaring that ACOG acted with bias because of a financial conflict of interest with industry in this instance; the fact that a financial conflict of interest exists for ACOG, however, cannot be disputed if one examines the College’s Annual Report, where contributors are listed. (For a comprehensive, if disillusioning, treatise on the many effects of financial conflict of interest within medicine, I recommend the book On The Take.5)
organ prolapse
Differences between the two bulletins are marked in boldface
Bulletin #79 (original wording): “Given the limited data and frequent changes in the marketed products (particularly with regard to type of mesh material itself, which is most closely associated with several of the postoperative risks especially mesh erosion), if clinicians recommend these procedures before evidence of their risk-benefit is fully understood, the procedures should be considered experimental and patients consented for surgery with that understanding.”
Bulletin #84 (revised wording): “Given the limited data and frequent changes in the marketed products for vaginal surgery for prolapse repair (particularly with regard to type of mesh material itself, which is most closely associated with several of the postoperative risks especially mesh erosion), patients should consent to surgery with an understanding of the post-operative risks and lack of long-term outcomes data.”
Case: Radiofrequency therapy. Even when clinical experience demonstrates lack of effectiveness or an unacceptable rate of complications associated with certain techniques or devices, unequivocal evidence of such problems does not always appear in the literature. One example of this is how a technique was translated into a treatment for incontinence by way of its use in other fields.
Radiofrequency has, among other uses, been used to ablate nerves in intractable chronic pain and to address joint instability in orthopedic surgery. Radiofrequency energy was then, by extension, applied transvaginally to tissue (known as “endopelvic” fascia, of a distinctly different nature than parietal fascia involved in orthopedic procedures) surrounding the urethra. The goal was to coagulate “supporting” tissues and “correct” urethral hypermobility that purportedly causes stress incontinence.
Marketing of the SURx Transvaginal System (CooperSurgical, Inc.) began in 2002, followed by reports of success. One industry-sponsored study, for example, reported a 73% rate of either continence or improvement after 12 months.6
Despite such favorable early results, however, in 2006 CooperSurgical decided to abandon this system, citing “technique-dependent” results of the procedure. Since then, independent research has shown a very low initial success rate that declines rapidly—within weeks or months—of treatment.7,8
A different radiofrequency technique continues to be marketed—the Renessa System™ (NovaSys Medical), which uses a urethral catheter-mounted system to deliver radiofrequency energy through the urethral mucosa to the submucosa and adjacent tissues. Once again, initial reports of industry-sponsored research showed promising results; one study of 110 patients reported 74% achieved continence or improvement after 1 year.9 In a follow-up report of 21 of the original 110 patients, “improvement” was reported in 74% after 3 years.10 Independent research has yet to be reported in the literature.
Of particular concern, no data exist on the long-term effect of denaturing collagen in the urethra and adjacent tissues in relation to UI, other aspects of bladder function, or sexual function. An apparent lack of immediate complications cannot be equated with safety; we need long-term studies to determine whether urethral function is affected adversely compared with that in untreated women and women treated with surgery.
Bring on innovation—in context!
For those who consider my argument anti-innovation, let me repeat: I believe strongly in innovation to improve care for our patients. Am I anti-industry? Only when there is an unbridled race to profit from marketing products without safeguards to ensure, first and foremost, the safety of our patients and, second, their long-term effectiveness. Knowingly or unknowingly, patients then become the guinea pigs on whom these products are tested—just not in the appropriate context of clinical research and informed consent for participation.
Instead (as happened in the US Public Health Service’s Tuskegee syphilis experiments), patients serve as research subjects without their consent when they receive untested products and undergo unproven treatments. And because clinicians are the conduit through which patients receive untested and unproven treatments, who is ultimately responsible for the outcome?
Industry brings innovation to clinical practice. But it is incumbent on clinicians to recognize, with unflinching honesty, the bottom line on which industry operates. Prolapse and incontinence are deeply distressing for our patients, but these chronic conditions are not life-threatening; virtually all women who suffer these conditions have been symptomatic for years before they come for care. I see no need, except to increase that bottom line, to rush products to market before they have been evaluated sufficiently to determine whether “new” is actually “better.”
For clinicians who style themselves as early adopters, remember: It’s not you, but your patient, who is “adopting” a foreign material and having it placed deep in the most intimate area of her body—a foreign material intended to stay for life (except for those unfortunate patients who must have it removed). Above all, we must do no harm—an elusive goal when some of us try to attract patients by being the first to use a product before evidence of its risk and utility have been established in practice.
Does this kind of talk encourage litigation?
Does a commentary like this one provide fodder for plaintiff attorneys who are seeking grounds for product liability lawsuits against manufacturers and malpractice claims against clinicians? Please! Spend a moment on the Web, and you will see that the lawyers are already busy—especially since the FDA’s October 2008 alert about complications with surgical mesh for prolapse and incontinence. [See “FDA alert: Transvaginal placement of surgical mesh carries serious risks,” in the December 2008 issue of OBG Management.] It’s worth noting how these lawyers see themselves: They would likely tell you that they “provide an important service in protecting patients from unscrupulous manufacturers who profit from the vulnerability of people seeking treatment for distressing conditions.” As clinicians, are we absolutely sure that we can say the same of ourselves?
Is it wrong to harp on what happened in the past?
Why revisit events surrounding, for example, the ProteGen Sling? My reply is another question: Where is the evidence that such sequences of events are in the past? Among clinicians, who knows which is best in a collection of kits that changes from one month to the next, without their promoters skipping a beat in proclaiming theirs as the “best”? It isn’t shameful to admit that one doesn’t know which one is best; but it is a shame to act as if one does know, especially when the risk falls to another. The names change; the events are the same.
What is the solution to this problem?
Businesses succeed only when their products are purchased. If clinicians refused to be participants whenever the device industry introduces unproven treatments into the market, industry would be compelled to test their products beforehand. Patients would benefit—by being able to make truly informed choices, with adequate information about risk and benefit. Clinicians would benefit—by being able to provide the most effective treatment without sacrificing their integrity in the process. Ultimately, industry would benefit, by profiting appropriately from products that truly help our patients. Is that an impossible wish?
We want to hear from you! Tell us what you think.
1. Goldman HB. Is new always better? Curr Urol Rep. 2007;8(4):253-254.
2. Ostergard DR. Lessons from the past: directions for the future. Do new marketed surgical procedures and grafts produce ethical, personal liability, and legal concerns for physicians? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:591-598.
3. ACOG Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 79: Pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):461-473.
4. ACOG Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110:717-729.
5. Kassirer JP. On the Take: How Medicine’s Complicity with Big Business Can Endanger Your Health. New York: Oxford University Press; 2005.
6. Dmochowski RR, Avon M, Ross J, et al. Transvaginal radiofrequency treatment of the endopelvic fascia: a prospective evaluation for the treatment of genuine stress urinary incontinence. J Urol. 2003;169:1028-1032.
7. Buchsbaum GM, McConville J, Korni R, Duecy EE. Outcome of transvaginal radiofrequency for treatment of women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(3):263-265.
8. Ismail SI. Radiofrequency remodelling of the endopelvic fascia is not an effective procedure for urodynamic stress incontinence in women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1205-1209.
9. Appell RA, Juma S, Wells WG, et al. Transurethral radio-frequency energy collagen micro-remodeling for the treatment of female stress urinary incontinence. Neurourol Urodyn. 2006;25(4):331-336.
10. Appell RA, Singh G, Klimberg IW, et al. Nonsurgical radiofrequency collagen denaturation for stress urinary incontinence: retrospective 3-year evaluation. Expert Rev Med Devices. 2007;4:455-461.
just because they’re new;Anne M. Weber MD;Commentary;economic factors;urinary incontinence;pelvic organ prolapse;POP;transvaginal mesh;urethral sling;UI;innovation in treatment;FDA;medical device;510(k);ProteGen Sling;ObTape;TVT;Gynecare;TVT Secur;early adopters;ACOG;Clinical Practice Bulletin;informed consent;financial conflict of interest;radiofrequency therapy;SURx Transvaginal system;Renessa;do no harm;FDA Alert;
The author reports no financial relationships relevant to this article.
From my vantage point, it appears that economic factors are playing an increasingly important role in how pelvic organ prolapse (POP) and urinary incontinence (UI) are managed—particularly, in regard to the use of surgical devices. As such, the topic of treating POP and UI deserves our attention to ensure that we make the best decisions for our patients.
Now, I’m a staunch supporter of innovation in treatment; certainly, there is room for improvement in current approaches—particularly in surgery—for treating POP and UI. At the same time, I strongly believe that innovation must be demonstrated to be an improvement before it is incorporated into practice. Although innovation is commonly taken on faith, we should know better than to equate “new” with “better” until evidence, gathered through clinical research, has demonstrated this conclusively. A look at the US Food and Drug Administration’s (FDA’s) process for clearing medical devices for clinical use reveals that such a standard often doesn’t apply—and this should matter to us.
The meaning of 510(k)
Most medical devices are evaluated through an FDA clearance mechanism known as the 510(k) process. This is wholly distinct from the agency’s drug approval process with which most of us are familiar. It’s beyond the scope of this commentary for me to go into detail about 510(k); if you are interested, see two recent commentaries1,2 and visit http://www.fda.gov/cdrh/devadvice/314.html.
In a nutshell, the 510(k) process requires only that an applicant demonstrate that a new medical device has “substantial equivalence” to an already legally marketed device, known as the predicate, which may also have been cleared only through the 510(k) process. That means it’s possible to have generations of products cleared on the basis of one predicate device that was itself never studied adequately.
Indeed, this is the case with most medical devices that have been sold for the surgical treatment of POP and UI—from before the ProteGen Sling (Boston Scientific), through Tension-Free Vaginal Tape (TVT) (Gynecare), and continuing with the newest devices.
The story of the ProteGen Sling (FIGURE) offers a cautionary tale about what can go wrong when new devices are cleared by the FDA through 510(k), rather than evaluated through rigorous clinical trials, as drugs are. More recently, experience with the ObTape (Mentor Corporation) followed virtually the same trajectory of events; the product was pulled from the market in 2006 and is now the focus of lawsuits nationwide.
Fortunately, for our patients, experience with TVT (Gynecare) has been favorable. Although TVT was also cleared by the FDA through 510(k), clinical research performed after TVT was introduced has demonstrated its effectiveness and relative safety. Indeed, TVT has revolutionized the treatment of stress UI in women—and, even, our understanding of its etiology.
Several companies are capitalizing on the success of TVT by introducing competing products that are designed to be 1) similar enough to ride on the coattails of TVT yet 2) different enough to capture their own share of market—without evidence of safety or effectiveness required. Even Gynecare (part of Ethicon Women’s Health and Urology, a subsidiary of Johnson & Johnson) has introduced TVT SECUR to compete with its own TVT—again, without independent evidence of safety or effectiveness.
The current market in devices for stress UI is a moving target that makes it nearly impossible—even for research organizations, such as the federally funded Pelvic Floor Disorders Network, that are independent of industry—to develop and implement sound clinical trials of those devices. Why do I say “moving target”? First, there are no assurances that any device chosen for study will remain the same for the duration of a trial. Second, there is no way to foresee which products will be abandoned over the time required for a large clinical trial.
FIGURE The saga of the ProteGen Sling
Transparency over what might be considered “experimental”
Until the FDA changes its process—to one in which 1) medical devices are adequately assessed before they reach market and 2) postmarketing surveillance is required—it’s our duty to insist on evidence of safety and effectiveness before adopting the latest and greatest products that companies have to offer.
Of all the questions that a patient might ask before treatment, three of the most important, surely, are:
- “Will this help me?”
- “If it helps me, how long will it help?”
- “Whether or not this treatment helps me, what risks—in the short-term and over the long-term—does it present?”
Until we can provide our patients with answers that are supported by evidence, products that lack such evidence should be considered experimental, and patients should be counseled accordingly.
Some patients may accept what they’ve been advised are new and unproven treatments—in the way that some physicians are early adopters. Nevertheless, I am concerned that some clinicians do not appear to appreciate the true lack of evidence that accompanies most marketed devices for prolapse and incontinence. They may mistake the FDA 510(k) process of clearance for something similar to the agency’s extended and complex drug approval process. They may accept claims made in industry-produced white papers that are often largely promotional materials, and fail to look further into those claims.
Now, more than ever and above all else, we must stand between marketing and our patients’ safety. We are familiar with the toll that prolapse and incontinence, as chronic conditions, take on our patients; yet it’s that very chronic nature that should lead us to adopt patience and caution in accepting new treatments before they have been adequately studied.
If we cannot always rely on industry to provide clear information about the risks and benefits of new devices, neither can we routinely look to our professional organizations for unbiased information. Often, professional organizations accept cash contributions from industry, raising the question of conflict of interest that may undermine their actions when the priorities of industry do not align with the goal of safeguarding patients’ well-being.
In an unprecedented example of how a professional association can interfere with its own, expert-authored clinical practice guidelines, the American College of Obstetricians and Gynecologists (ACOG) more than a year ago rescinded one of its published guidelines on POP (Issue 79, February 20073) and replaced it with a new guideline (Issue 85, September 20074). The new guideline is nearly identical to the prior one—save for one sentence, in which “experimental” is deleted in a discussion of kits of trocar-based synthetic materials sold for the surgical treatment of pelvic organ prolapse (see the EXCERPT).
The deletion is crucial because offering informed consent for surgery requires a patient to accept risks in balance with an expectation of benefit. A patient cannot be appropriately informed when no evidence of benefit exists and evidence of postoperative risk is extremely limited.
Now, I am not declaring that ACOG acted with bias because of a financial conflict of interest with industry in this instance; the fact that a financial conflict of interest exists for ACOG, however, cannot be disputed if one examines the College’s Annual Report, where contributors are listed. (For a comprehensive, if disillusioning, treatise on the many effects of financial conflict of interest within medicine, I recommend the book On The Take.5)
organ prolapse
Differences between the two bulletins are marked in boldface
Bulletin #79 (original wording): “Given the limited data and frequent changes in the marketed products (particularly with regard to type of mesh material itself, which is most closely associated with several of the postoperative risks especially mesh erosion), if clinicians recommend these procedures before evidence of their risk-benefit is fully understood, the procedures should be considered experimental and patients consented for surgery with that understanding.”
Bulletin #84 (revised wording): “Given the limited data and frequent changes in the marketed products for vaginal surgery for prolapse repair (particularly with regard to type of mesh material itself, which is most closely associated with several of the postoperative risks especially mesh erosion), patients should consent to surgery with an understanding of the post-operative risks and lack of long-term outcomes data.”
Case: Radiofrequency therapy. Even when clinical experience demonstrates lack of effectiveness or an unacceptable rate of complications associated with certain techniques or devices, unequivocal evidence of such problems does not always appear in the literature. One example of this is how a technique was translated into a treatment for incontinence by way of its use in other fields.
Radiofrequency has, among other uses, been used to ablate nerves in intractable chronic pain and to address joint instability in orthopedic surgery. Radiofrequency energy was then, by extension, applied transvaginally to tissue (known as “endopelvic” fascia, of a distinctly different nature than parietal fascia involved in orthopedic procedures) surrounding the urethra. The goal was to coagulate “supporting” tissues and “correct” urethral hypermobility that purportedly causes stress incontinence.
Marketing of the SURx Transvaginal System (CooperSurgical, Inc.) began in 2002, followed by reports of success. One industry-sponsored study, for example, reported a 73% rate of either continence or improvement after 12 months.6
Despite such favorable early results, however, in 2006 CooperSurgical decided to abandon this system, citing “technique-dependent” results of the procedure. Since then, independent research has shown a very low initial success rate that declines rapidly—within weeks or months—of treatment.7,8
A different radiofrequency technique continues to be marketed—the Renessa System™ (NovaSys Medical), which uses a urethral catheter-mounted system to deliver radiofrequency energy through the urethral mucosa to the submucosa and adjacent tissues. Once again, initial reports of industry-sponsored research showed promising results; one study of 110 patients reported 74% achieved continence or improvement after 1 year.9 In a follow-up report of 21 of the original 110 patients, “improvement” was reported in 74% after 3 years.10 Independent research has yet to be reported in the literature.
Of particular concern, no data exist on the long-term effect of denaturing collagen in the urethra and adjacent tissues in relation to UI, other aspects of bladder function, or sexual function. An apparent lack of immediate complications cannot be equated with safety; we need long-term studies to determine whether urethral function is affected adversely compared with that in untreated women and women treated with surgery.
Bring on innovation—in context!
For those who consider my argument anti-innovation, let me repeat: I believe strongly in innovation to improve care for our patients. Am I anti-industry? Only when there is an unbridled race to profit from marketing products without safeguards to ensure, first and foremost, the safety of our patients and, second, their long-term effectiveness. Knowingly or unknowingly, patients then become the guinea pigs on whom these products are tested—just not in the appropriate context of clinical research and informed consent for participation.
Instead (as happened in the US Public Health Service’s Tuskegee syphilis experiments), patients serve as research subjects without their consent when they receive untested products and undergo unproven treatments. And because clinicians are the conduit through which patients receive untested and unproven treatments, who is ultimately responsible for the outcome?
Industry brings innovation to clinical practice. But it is incumbent on clinicians to recognize, with unflinching honesty, the bottom line on which industry operates. Prolapse and incontinence are deeply distressing for our patients, but these chronic conditions are not life-threatening; virtually all women who suffer these conditions have been symptomatic for years before they come for care. I see no need, except to increase that bottom line, to rush products to market before they have been evaluated sufficiently to determine whether “new” is actually “better.”
For clinicians who style themselves as early adopters, remember: It’s not you, but your patient, who is “adopting” a foreign material and having it placed deep in the most intimate area of her body—a foreign material intended to stay for life (except for those unfortunate patients who must have it removed). Above all, we must do no harm—an elusive goal when some of us try to attract patients by being the first to use a product before evidence of its risk and utility have been established in practice.
Does this kind of talk encourage litigation?
Does a commentary like this one provide fodder for plaintiff attorneys who are seeking grounds for product liability lawsuits against manufacturers and malpractice claims against clinicians? Please! Spend a moment on the Web, and you will see that the lawyers are already busy—especially since the FDA’s October 2008 alert about complications with surgical mesh for prolapse and incontinence. [See “FDA alert: Transvaginal placement of surgical mesh carries serious risks,” in the December 2008 issue of OBG Management.] It’s worth noting how these lawyers see themselves: They would likely tell you that they “provide an important service in protecting patients from unscrupulous manufacturers who profit from the vulnerability of people seeking treatment for distressing conditions.” As clinicians, are we absolutely sure that we can say the same of ourselves?
Is it wrong to harp on what happened in the past?
Why revisit events surrounding, for example, the ProteGen Sling? My reply is another question: Where is the evidence that such sequences of events are in the past? Among clinicians, who knows which is best in a collection of kits that changes from one month to the next, without their promoters skipping a beat in proclaiming theirs as the “best”? It isn’t shameful to admit that one doesn’t know which one is best; but it is a shame to act as if one does know, especially when the risk falls to another. The names change; the events are the same.
What is the solution to this problem?
Businesses succeed only when their products are purchased. If clinicians refused to be participants whenever the device industry introduces unproven treatments into the market, industry would be compelled to test their products beforehand. Patients would benefit—by being able to make truly informed choices, with adequate information about risk and benefit. Clinicians would benefit—by being able to provide the most effective treatment without sacrificing their integrity in the process. Ultimately, industry would benefit, by profiting appropriately from products that truly help our patients. Is that an impossible wish?
We want to hear from you! Tell us what you think.
The author reports no financial relationships relevant to this article.
From my vantage point, it appears that economic factors are playing an increasingly important role in how pelvic organ prolapse (POP) and urinary incontinence (UI) are managed—particularly, in regard to the use of surgical devices. As such, the topic of treating POP and UI deserves our attention to ensure that we make the best decisions for our patients.
Now, I’m a staunch supporter of innovation in treatment; certainly, there is room for improvement in current approaches—particularly in surgery—for treating POP and UI. At the same time, I strongly believe that innovation must be demonstrated to be an improvement before it is incorporated into practice. Although innovation is commonly taken on faith, we should know better than to equate “new” with “better” until evidence, gathered through clinical research, has demonstrated this conclusively. A look at the US Food and Drug Administration’s (FDA’s) process for clearing medical devices for clinical use reveals that such a standard often doesn’t apply—and this should matter to us.
The meaning of 510(k)
Most medical devices are evaluated through an FDA clearance mechanism known as the 510(k) process. This is wholly distinct from the agency’s drug approval process with which most of us are familiar. It’s beyond the scope of this commentary for me to go into detail about 510(k); if you are interested, see two recent commentaries1,2 and visit http://www.fda.gov/cdrh/devadvice/314.html.
In a nutshell, the 510(k) process requires only that an applicant demonstrate that a new medical device has “substantial equivalence” to an already legally marketed device, known as the predicate, which may also have been cleared only through the 510(k) process. That means it’s possible to have generations of products cleared on the basis of one predicate device that was itself never studied adequately.
Indeed, this is the case with most medical devices that have been sold for the surgical treatment of POP and UI—from before the ProteGen Sling (Boston Scientific), through Tension-Free Vaginal Tape (TVT) (Gynecare), and continuing with the newest devices.
The story of the ProteGen Sling (FIGURE) offers a cautionary tale about what can go wrong when new devices are cleared by the FDA through 510(k), rather than evaluated through rigorous clinical trials, as drugs are. More recently, experience with the ObTape (Mentor Corporation) followed virtually the same trajectory of events; the product was pulled from the market in 2006 and is now the focus of lawsuits nationwide.
Fortunately, for our patients, experience with TVT (Gynecare) has been favorable. Although TVT was also cleared by the FDA through 510(k), clinical research performed after TVT was introduced has demonstrated its effectiveness and relative safety. Indeed, TVT has revolutionized the treatment of stress UI in women—and, even, our understanding of its etiology.
Several companies are capitalizing on the success of TVT by introducing competing products that are designed to be 1) similar enough to ride on the coattails of TVT yet 2) different enough to capture their own share of market—without evidence of safety or effectiveness required. Even Gynecare (part of Ethicon Women’s Health and Urology, a subsidiary of Johnson & Johnson) has introduced TVT SECUR to compete with its own TVT—again, without independent evidence of safety or effectiveness.
The current market in devices for stress UI is a moving target that makes it nearly impossible—even for research organizations, such as the federally funded Pelvic Floor Disorders Network, that are independent of industry—to develop and implement sound clinical trials of those devices. Why do I say “moving target”? First, there are no assurances that any device chosen for study will remain the same for the duration of a trial. Second, there is no way to foresee which products will be abandoned over the time required for a large clinical trial.
FIGURE The saga of the ProteGen Sling
Transparency over what might be considered “experimental”
Until the FDA changes its process—to one in which 1) medical devices are adequately assessed before they reach market and 2) postmarketing surveillance is required—it’s our duty to insist on evidence of safety and effectiveness before adopting the latest and greatest products that companies have to offer.
Of all the questions that a patient might ask before treatment, three of the most important, surely, are:
- “Will this help me?”
- “If it helps me, how long will it help?”
- “Whether or not this treatment helps me, what risks—in the short-term and over the long-term—does it present?”
Until we can provide our patients with answers that are supported by evidence, products that lack such evidence should be considered experimental, and patients should be counseled accordingly.
Some patients may accept what they’ve been advised are new and unproven treatments—in the way that some physicians are early adopters. Nevertheless, I am concerned that some clinicians do not appear to appreciate the true lack of evidence that accompanies most marketed devices for prolapse and incontinence. They may mistake the FDA 510(k) process of clearance for something similar to the agency’s extended and complex drug approval process. They may accept claims made in industry-produced white papers that are often largely promotional materials, and fail to look further into those claims.
Now, more than ever and above all else, we must stand between marketing and our patients’ safety. We are familiar with the toll that prolapse and incontinence, as chronic conditions, take on our patients; yet it’s that very chronic nature that should lead us to adopt patience and caution in accepting new treatments before they have been adequately studied.
If we cannot always rely on industry to provide clear information about the risks and benefits of new devices, neither can we routinely look to our professional organizations for unbiased information. Often, professional organizations accept cash contributions from industry, raising the question of conflict of interest that may undermine their actions when the priorities of industry do not align with the goal of safeguarding patients’ well-being.
In an unprecedented example of how a professional association can interfere with its own, expert-authored clinical practice guidelines, the American College of Obstetricians and Gynecologists (ACOG) more than a year ago rescinded one of its published guidelines on POP (Issue 79, February 20073) and replaced it with a new guideline (Issue 85, September 20074). The new guideline is nearly identical to the prior one—save for one sentence, in which “experimental” is deleted in a discussion of kits of trocar-based synthetic materials sold for the surgical treatment of pelvic organ prolapse (see the EXCERPT).
The deletion is crucial because offering informed consent for surgery requires a patient to accept risks in balance with an expectation of benefit. A patient cannot be appropriately informed when no evidence of benefit exists and evidence of postoperative risk is extremely limited.
Now, I am not declaring that ACOG acted with bias because of a financial conflict of interest with industry in this instance; the fact that a financial conflict of interest exists for ACOG, however, cannot be disputed if one examines the College’s Annual Report, where contributors are listed. (For a comprehensive, if disillusioning, treatise on the many effects of financial conflict of interest within medicine, I recommend the book On The Take.5)
organ prolapse
Differences between the two bulletins are marked in boldface
Bulletin #79 (original wording): “Given the limited data and frequent changes in the marketed products (particularly with regard to type of mesh material itself, which is most closely associated with several of the postoperative risks especially mesh erosion), if clinicians recommend these procedures before evidence of their risk-benefit is fully understood, the procedures should be considered experimental and patients consented for surgery with that understanding.”
Bulletin #84 (revised wording): “Given the limited data and frequent changes in the marketed products for vaginal surgery for prolapse repair (particularly with regard to type of mesh material itself, which is most closely associated with several of the postoperative risks especially mesh erosion), patients should consent to surgery with an understanding of the post-operative risks and lack of long-term outcomes data.”
Case: Radiofrequency therapy. Even when clinical experience demonstrates lack of effectiveness or an unacceptable rate of complications associated with certain techniques or devices, unequivocal evidence of such problems does not always appear in the literature. One example of this is how a technique was translated into a treatment for incontinence by way of its use in other fields.
Radiofrequency has, among other uses, been used to ablate nerves in intractable chronic pain and to address joint instability in orthopedic surgery. Radiofrequency energy was then, by extension, applied transvaginally to tissue (known as “endopelvic” fascia, of a distinctly different nature than parietal fascia involved in orthopedic procedures) surrounding the urethra. The goal was to coagulate “supporting” tissues and “correct” urethral hypermobility that purportedly causes stress incontinence.
Marketing of the SURx Transvaginal System (CooperSurgical, Inc.) began in 2002, followed by reports of success. One industry-sponsored study, for example, reported a 73% rate of either continence or improvement after 12 months.6
Despite such favorable early results, however, in 2006 CooperSurgical decided to abandon this system, citing “technique-dependent” results of the procedure. Since then, independent research has shown a very low initial success rate that declines rapidly—within weeks or months—of treatment.7,8
A different radiofrequency technique continues to be marketed—the Renessa System™ (NovaSys Medical), which uses a urethral catheter-mounted system to deliver radiofrequency energy through the urethral mucosa to the submucosa and adjacent tissues. Once again, initial reports of industry-sponsored research showed promising results; one study of 110 patients reported 74% achieved continence or improvement after 1 year.9 In a follow-up report of 21 of the original 110 patients, “improvement” was reported in 74% after 3 years.10 Independent research has yet to be reported in the literature.
Of particular concern, no data exist on the long-term effect of denaturing collagen in the urethra and adjacent tissues in relation to UI, other aspects of bladder function, or sexual function. An apparent lack of immediate complications cannot be equated with safety; we need long-term studies to determine whether urethral function is affected adversely compared with that in untreated women and women treated with surgery.
Bring on innovation—in context!
For those who consider my argument anti-innovation, let me repeat: I believe strongly in innovation to improve care for our patients. Am I anti-industry? Only when there is an unbridled race to profit from marketing products without safeguards to ensure, first and foremost, the safety of our patients and, second, their long-term effectiveness. Knowingly or unknowingly, patients then become the guinea pigs on whom these products are tested—just not in the appropriate context of clinical research and informed consent for participation.
Instead (as happened in the US Public Health Service’s Tuskegee syphilis experiments), patients serve as research subjects without their consent when they receive untested products and undergo unproven treatments. And because clinicians are the conduit through which patients receive untested and unproven treatments, who is ultimately responsible for the outcome?
Industry brings innovation to clinical practice. But it is incumbent on clinicians to recognize, with unflinching honesty, the bottom line on which industry operates. Prolapse and incontinence are deeply distressing for our patients, but these chronic conditions are not life-threatening; virtually all women who suffer these conditions have been symptomatic for years before they come for care. I see no need, except to increase that bottom line, to rush products to market before they have been evaluated sufficiently to determine whether “new” is actually “better.”
For clinicians who style themselves as early adopters, remember: It’s not you, but your patient, who is “adopting” a foreign material and having it placed deep in the most intimate area of her body—a foreign material intended to stay for life (except for those unfortunate patients who must have it removed). Above all, we must do no harm—an elusive goal when some of us try to attract patients by being the first to use a product before evidence of its risk and utility have been established in practice.
Does this kind of talk encourage litigation?
Does a commentary like this one provide fodder for plaintiff attorneys who are seeking grounds for product liability lawsuits against manufacturers and malpractice claims against clinicians? Please! Spend a moment on the Web, and you will see that the lawyers are already busy—especially since the FDA’s October 2008 alert about complications with surgical mesh for prolapse and incontinence. [See “FDA alert: Transvaginal placement of surgical mesh carries serious risks,” in the December 2008 issue of OBG Management.] It’s worth noting how these lawyers see themselves: They would likely tell you that they “provide an important service in protecting patients from unscrupulous manufacturers who profit from the vulnerability of people seeking treatment for distressing conditions.” As clinicians, are we absolutely sure that we can say the same of ourselves?
Is it wrong to harp on what happened in the past?
Why revisit events surrounding, for example, the ProteGen Sling? My reply is another question: Where is the evidence that such sequences of events are in the past? Among clinicians, who knows which is best in a collection of kits that changes from one month to the next, without their promoters skipping a beat in proclaiming theirs as the “best”? It isn’t shameful to admit that one doesn’t know which one is best; but it is a shame to act as if one does know, especially when the risk falls to another. The names change; the events are the same.
What is the solution to this problem?
Businesses succeed only when their products are purchased. If clinicians refused to be participants whenever the device industry introduces unproven treatments into the market, industry would be compelled to test their products beforehand. Patients would benefit—by being able to make truly informed choices, with adequate information about risk and benefit. Clinicians would benefit—by being able to provide the most effective treatment without sacrificing their integrity in the process. Ultimately, industry would benefit, by profiting appropriately from products that truly help our patients. Is that an impossible wish?
We want to hear from you! Tell us what you think.
1. Goldman HB. Is new always better? Curr Urol Rep. 2007;8(4):253-254.
2. Ostergard DR. Lessons from the past: directions for the future. Do new marketed surgical procedures and grafts produce ethical, personal liability, and legal concerns for physicians? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:591-598.
3. ACOG Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 79: Pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):461-473.
4. ACOG Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110:717-729.
5. Kassirer JP. On the Take: How Medicine’s Complicity with Big Business Can Endanger Your Health. New York: Oxford University Press; 2005.
6. Dmochowski RR, Avon M, Ross J, et al. Transvaginal radiofrequency treatment of the endopelvic fascia: a prospective evaluation for the treatment of genuine stress urinary incontinence. J Urol. 2003;169:1028-1032.
7. Buchsbaum GM, McConville J, Korni R, Duecy EE. Outcome of transvaginal radiofrequency for treatment of women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(3):263-265.
8. Ismail SI. Radiofrequency remodelling of the endopelvic fascia is not an effective procedure for urodynamic stress incontinence in women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1205-1209.
9. Appell RA, Juma S, Wells WG, et al. Transurethral radio-frequency energy collagen micro-remodeling for the treatment of female stress urinary incontinence. Neurourol Urodyn. 2006;25(4):331-336.
10. Appell RA, Singh G, Klimberg IW, et al. Nonsurgical radiofrequency collagen denaturation for stress urinary incontinence: retrospective 3-year evaluation. Expert Rev Med Devices. 2007;4:455-461.
1. Goldman HB. Is new always better? Curr Urol Rep. 2007;8(4):253-254.
2. Ostergard DR. Lessons from the past: directions for the future. Do new marketed surgical procedures and grafts produce ethical, personal liability, and legal concerns for physicians? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:591-598.
3. ACOG Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 79: Pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):461-473.
4. ACOG Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110:717-729.
5. Kassirer JP. On the Take: How Medicine’s Complicity with Big Business Can Endanger Your Health. New York: Oxford University Press; 2005.
6. Dmochowski RR, Avon M, Ross J, et al. Transvaginal radiofrequency treatment of the endopelvic fascia: a prospective evaluation for the treatment of genuine stress urinary incontinence. J Urol. 2003;169:1028-1032.
7. Buchsbaum GM, McConville J, Korni R, Duecy EE. Outcome of transvaginal radiofrequency for treatment of women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(3):263-265.
8. Ismail SI. Radiofrequency remodelling of the endopelvic fascia is not an effective procedure for urodynamic stress incontinence in women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1205-1209.
9. Appell RA, Juma S, Wells WG, et al. Transurethral radio-frequency energy collagen micro-remodeling for the treatment of female stress urinary incontinence. Neurourol Urodyn. 2006;25(4):331-336.
10. Appell RA, Singh G, Klimberg IW, et al. Nonsurgical radiofrequency collagen denaturation for stress urinary incontinence: retrospective 3-year evaluation. Expert Rev Med Devices. 2007;4:455-461.
just because they’re new;Anne M. Weber MD;Commentary;economic factors;urinary incontinence;pelvic organ prolapse;POP;transvaginal mesh;urethral sling;UI;innovation in treatment;FDA;medical device;510(k);ProteGen Sling;ObTape;TVT;Gynecare;TVT Secur;early adopters;ACOG;Clinical Practice Bulletin;informed consent;financial conflict of interest;radiofrequency therapy;SURx Transvaginal system;Renessa;do no harm;FDA Alert;
just because they’re new;Anne M. Weber MD;Commentary;economic factors;urinary incontinence;pelvic organ prolapse;POP;transvaginal mesh;urethral sling;UI;innovation in treatment;FDA;medical device;510(k);ProteGen Sling;ObTape;TVT;Gynecare;TVT Secur;early adopters;ACOG;Clinical Practice Bulletin;informed consent;financial conflict of interest;radiofrequency therapy;SURx Transvaginal system;Renessa;do no harm;FDA Alert;








