User login
COVID-19 vaccination and pregnancy: Benefits outweigh the risks, for now
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.1 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers—and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.2
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the US Food and Drug Administration are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention’s (CDC) V-safe Vaccine Pregnancy Registry.3 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.4 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices in that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered.
As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-data-tracker/#pregnant-population. Accessed April 19, 2021.
Centers for Disease Control and Prevention. V-safe COVID-19 Vaccine Pregnancy Registry. https://www.cdc.gov/coronavirus/2019- ncov/vaccines/safety/vsafepregnancyregistry.html. Updated May 3, 2021. Accessed April 19, 2021.
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.1 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers—and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.2
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the US Food and Drug Administration are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention’s (CDC) V-safe Vaccine Pregnancy Registry.3 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.4 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices in that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered.
As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.1 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers—and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.2
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the US Food and Drug Administration are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention’s (CDC) V-safe Vaccine Pregnancy Registry.3 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.4 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices in that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered.
As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-data-tracker/#pregnant-population. Accessed April 19, 2021.
Centers for Disease Control and Prevention. V-safe COVID-19 Vaccine Pregnancy Registry. https://www.cdc.gov/coronavirus/2019- ncov/vaccines/safety/vsafepregnancyregistry.html. Updated May 3, 2021. Accessed April 19, 2021.
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-data-tracker/#pregnant-population. Accessed April 19, 2021.
Centers for Disease Control and Prevention. V-safe COVID-19 Vaccine Pregnancy Registry. https://www.cdc.gov/coronavirus/2019- ncov/vaccines/safety/vsafepregnancyregistry.html. Updated May 3, 2021. Accessed April 19, 2021.
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
Addressing an uncharted front in the war on COVID-19: Vaccination during pregnancy
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
Stop checking routine lipid panels every year
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
Are pregnant and lactating women and their infants protected with the COVID-19 mRNA vaccines?
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
EXPERT COMMENTARY
Pregnant women are among those at highest risk for severe disease and death from SARS-CoV-2 infection. However, exclusion of pregnant and lactating women from the initial COVID-19 vaccine trials has made counseling these patients challenging due to both the novelty of the vaccines themselves and the general lack of data in this vulnerable population. Data for the efficacy and risks of vaccination are needed to inform shared decision making between clinician and patient.
Details of the study
Gray and colleagues conducted a prospective cohort study of 84 pregnant, 31 lactating, and 16 nonpregnant women who received 1 of the 2 COVID-19 mRNA vaccines approved by the US Food and Drug Administration for emergency use authorization (BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna). The study’s primary objective was to evaluate the humoral immune response (antibody quantification) and adverse effects of these vaccines in the pregnant and lactating women compared with both nonpregnant women and a cohort of 37 women who had natural COVID-19 infection during pregnancy.
Antibody quantification from blood and breast milk was performed at 4 time points: V0, the first vaccine dose; V1, the second vaccine dose; V2, 2 to 6 weeks after the second vaccine dose; and at delivery. Umbilical cord blood also was collected from the subset of delivered patients (n = 13).
Results. The ultimately IgG-dominated antibody response to the vaccine in pregnant and lactating women was comparable to that in nonpregnant women, and all vaccine antibody responses were significantly higher than that in response to natural SARS-CoV-2 infection. IgG antibodies also were found in umbilical cord blood and breast milk, supporting the transfer of immunity to both the fetus and infant. There were no significant differences in adverse effects between pregnant and nonpregnant women.
Study strengths and limitations
This study’s main strength is that it demonstrated a similar increase in humoral immune response to the COVID-19 mRNA vaccines in a previously unstudied population of pregnant and lactating women, supporting the potential efficacy of the vaccines in this group at high risk for complications from SARS-CoV-2. Other data to support this include the increased vaccine antibody response compared with the antibody response after SARS-CoV-2 infection in pregnant women as well as the presence of maternal-infant transfer of antibodies via cord blood and breast milk. All of these are important data to inform patients and practitioners who are trying to make shared, informed decisions about a novel vaccine during a global pandemic.
The main limitation of this study is a limited patient population of mostly White, non-Hispanic, health care workers with few comorbidities and only 13 delivered vaccinated patients within the study period. Long-term immunity, immune responses other than antibody titers, and potential fetal effects also were not explored in this study. ●
The study by Gray and colleagues provides some of the first data supporting the potential efficacy of the novel mRNA vaccines in pregnant and lactating women, as the antibody-mediated response is similar in this population to that in the nonpregnant population. Moreover, it provides reassurance that the antibodies are getting to the fetus and the infant via the umbilical cord blood and breast milk and that the adverse effect profile is similar. Clinicians can use these data to help their patients make more informed decisions about COVID-19 vaccination. Future studies still are needed for long-term data on immunity and safety for the fetus.
JAIMEY M. PAULI, MD
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
EXPERT COMMENTARY
Pregnant women are among those at highest risk for severe disease and death from SARS-CoV-2 infection. However, exclusion of pregnant and lactating women from the initial COVID-19 vaccine trials has made counseling these patients challenging due to both the novelty of the vaccines themselves and the general lack of data in this vulnerable population. Data for the efficacy and risks of vaccination are needed to inform shared decision making between clinician and patient.
Details of the study
Gray and colleagues conducted a prospective cohort study of 84 pregnant, 31 lactating, and 16 nonpregnant women who received 1 of the 2 COVID-19 mRNA vaccines approved by the US Food and Drug Administration for emergency use authorization (BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna). The study’s primary objective was to evaluate the humoral immune response (antibody quantification) and adverse effects of these vaccines in the pregnant and lactating women compared with both nonpregnant women and a cohort of 37 women who had natural COVID-19 infection during pregnancy.
Antibody quantification from blood and breast milk was performed at 4 time points: V0, the first vaccine dose; V1, the second vaccine dose; V2, 2 to 6 weeks after the second vaccine dose; and at delivery. Umbilical cord blood also was collected from the subset of delivered patients (n = 13).
Results. The ultimately IgG-dominated antibody response to the vaccine in pregnant and lactating women was comparable to that in nonpregnant women, and all vaccine antibody responses were significantly higher than that in response to natural SARS-CoV-2 infection. IgG antibodies also were found in umbilical cord blood and breast milk, supporting the transfer of immunity to both the fetus and infant. There were no significant differences in adverse effects between pregnant and nonpregnant women.
Study strengths and limitations
This study’s main strength is that it demonstrated a similar increase in humoral immune response to the COVID-19 mRNA vaccines in a previously unstudied population of pregnant and lactating women, supporting the potential efficacy of the vaccines in this group at high risk for complications from SARS-CoV-2. Other data to support this include the increased vaccine antibody response compared with the antibody response after SARS-CoV-2 infection in pregnant women as well as the presence of maternal-infant transfer of antibodies via cord blood and breast milk. All of these are important data to inform patients and practitioners who are trying to make shared, informed decisions about a novel vaccine during a global pandemic.
The main limitation of this study is a limited patient population of mostly White, non-Hispanic, health care workers with few comorbidities and only 13 delivered vaccinated patients within the study period. Long-term immunity, immune responses other than antibody titers, and potential fetal effects also were not explored in this study. ●
The study by Gray and colleagues provides some of the first data supporting the potential efficacy of the novel mRNA vaccines in pregnant and lactating women, as the antibody-mediated response is similar in this population to that in the nonpregnant population. Moreover, it provides reassurance that the antibodies are getting to the fetus and the infant via the umbilical cord blood and breast milk and that the adverse effect profile is similar. Clinicians can use these data to help their patients make more informed decisions about COVID-19 vaccination. Future studies still are needed for long-term data on immunity and safety for the fetus.
JAIMEY M. PAULI, MD
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
EXPERT COMMENTARY
Pregnant women are among those at highest risk for severe disease and death from SARS-CoV-2 infection. However, exclusion of pregnant and lactating women from the initial COVID-19 vaccine trials has made counseling these patients challenging due to both the novelty of the vaccines themselves and the general lack of data in this vulnerable population. Data for the efficacy and risks of vaccination are needed to inform shared decision making between clinician and patient.
Details of the study
Gray and colleagues conducted a prospective cohort study of 84 pregnant, 31 lactating, and 16 nonpregnant women who received 1 of the 2 COVID-19 mRNA vaccines approved by the US Food and Drug Administration for emergency use authorization (BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna). The study’s primary objective was to evaluate the humoral immune response (antibody quantification) and adverse effects of these vaccines in the pregnant and lactating women compared with both nonpregnant women and a cohort of 37 women who had natural COVID-19 infection during pregnancy.
Antibody quantification from blood and breast milk was performed at 4 time points: V0, the first vaccine dose; V1, the second vaccine dose; V2, 2 to 6 weeks after the second vaccine dose; and at delivery. Umbilical cord blood also was collected from the subset of delivered patients (n = 13).
Results. The ultimately IgG-dominated antibody response to the vaccine in pregnant and lactating women was comparable to that in nonpregnant women, and all vaccine antibody responses were significantly higher than that in response to natural SARS-CoV-2 infection. IgG antibodies also were found in umbilical cord blood and breast milk, supporting the transfer of immunity to both the fetus and infant. There were no significant differences in adverse effects between pregnant and nonpregnant women.
Study strengths and limitations
This study’s main strength is that it demonstrated a similar increase in humoral immune response to the COVID-19 mRNA vaccines in a previously unstudied population of pregnant and lactating women, supporting the potential efficacy of the vaccines in this group at high risk for complications from SARS-CoV-2. Other data to support this include the increased vaccine antibody response compared with the antibody response after SARS-CoV-2 infection in pregnant women as well as the presence of maternal-infant transfer of antibodies via cord blood and breast milk. All of these are important data to inform patients and practitioners who are trying to make shared, informed decisions about a novel vaccine during a global pandemic.
The main limitation of this study is a limited patient population of mostly White, non-Hispanic, health care workers with few comorbidities and only 13 delivered vaccinated patients within the study period. Long-term immunity, immune responses other than antibody titers, and potential fetal effects also were not explored in this study. ●
The study by Gray and colleagues provides some of the first data supporting the potential efficacy of the novel mRNA vaccines in pregnant and lactating women, as the antibody-mediated response is similar in this population to that in the nonpregnant population. Moreover, it provides reassurance that the antibodies are getting to the fetus and the infant via the umbilical cord blood and breast milk and that the adverse effect profile is similar. Clinicians can use these data to help their patients make more informed decisions about COVID-19 vaccination. Future studies still are needed for long-term data on immunity and safety for the fetus.
JAIMEY M. PAULI, MD
Managing herpes simplex virus genital infection in pregnancy
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
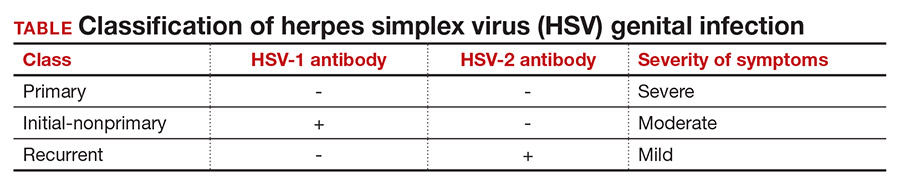
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.
Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1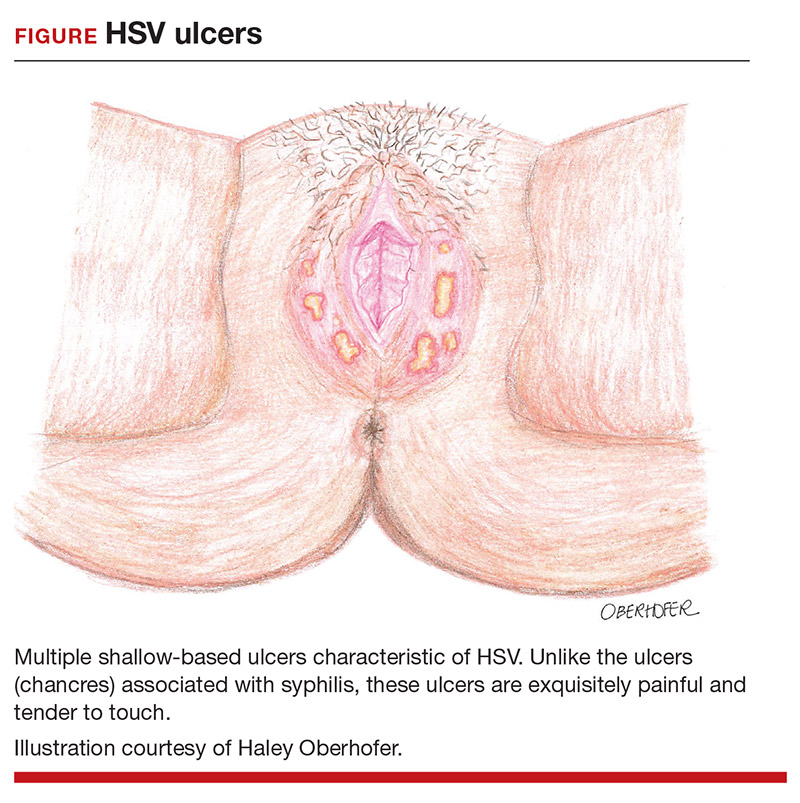
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
Can a once-daily oral formulation treat symptoms of uterine fibroids without causing hot flashes or bone loss?
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Optimize your treatment of endometriosis by using an FDA-approved hormonal medication
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.




