User login
For which patients is maternal oxygen supplementation of value?
Raghuraman N, Temming LA, Doering MM, et al. Maternal oxygen supplementation compared with room air for intrauterine resuscitation: a systematic review and meta-analysis. JAMA Pediatr. January 4, 2021. doi:10.1001/jamapediatrics.2020.5351.
EXPERT COMMENTARY
Maternal oxygen supplementation is widely used in labor for nonreassuring fetal heart rate (FHR) tracings, although its efficacy is uncertain for preventing fetal acidosis, operative intervention, or sequelae of neonatal encephalopathy. Recently, Raghuraman and colleagues reported the results of a systematic review and meta-analysis that included 16 randomized controlled trials. A total of 1,078 women were included in the oxygen group and 974 in the room air group. The primary outcome was umbilical artery pH; 14 trials reported on this outcome.
After analyzing the pooled and stratified results of the effect of maternal oxygen supplementation versus room air on umbilical artery gas measures, the authors concluded that peripartum oxygen supplementation is not associated with clinically relevant improvement in umbilical artery pH. They acknowledged, however, that the published studies were heterogeneous, lacked data on the association of oxygen supplementation with neonatal outcome, and did not assess oxygen use for abnormal FHR tracings, except for one trial with category II FHR tracings.
Effects of O2 supplementation
As maternal arterial hemoglobin is nearly saturated under normal conditions, maternal hyperoxia produces only modest increases in umbilical vein P
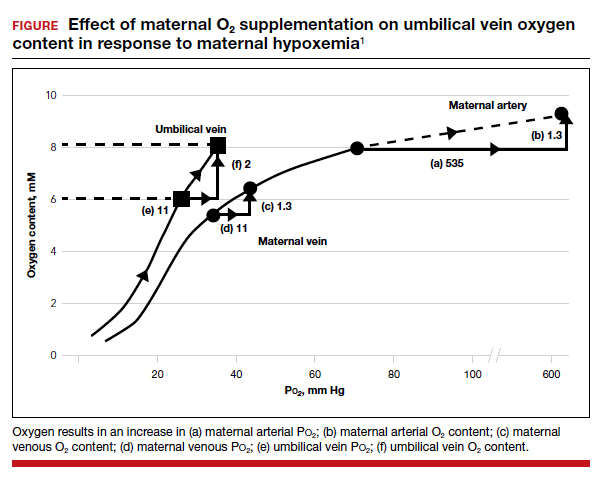
Fetal hypoxemia and acidosis can result from an interruption or an impairment of the mother-to-placenta-to-fetus oxygen pathway. With some interruptions of the oxygen pathway, such as placental abruption and complete cord occlusion–induced bradycardia, there would be less impact of maternal hyperoxia. By contrast, with other oxygen pathway impairments, such as reduced oxygen transfer with placental insufficiency, maternal hyperoxia can be of greater value by increasing maternal uterine artery and vein P
Continue to: Circumstances that may benefit from O2 supplementation...
Circumstances that may benefit from O2 supplementation
Late FHR decelerations reflect impairment of oxygen transfer and thus represent the heart rate pattern that is most likely to benefit from maternal hyperoxia. However, recurrent late decelerations occur in less than 2% of low-risk patients in labor,3 and severe levels of acidosis (umbilical artery pH <7.0 or base deficit [BD] ≥12 mmol/L) occur in only 1% to 2% of near-term or term deliveries.4,5
Variable decelerations also reflect fetal hypoxia and are much more common than late decelerations, so they also may benefit from O2 supplementation. Regardless, O2 supplementation should be seen only as a temporizing strategy while other resuscitative actions are initiated, including preparation for operative delivery, if indicated.
In a prior study by Raghuraman and colleagues (1 of only 4 studies that met selection criteria of oxygen supplementation for patients in labor), newborns of patients not receiving oxygen demonstrated 95% confidence limits of umbilical artery pH (7.24–7.28) and BD (2.9–4.3) well within the normal range.6 Thus, the low prevalence of cases in which a benefit might be anticipated and the low incidence of severe acidosis challenges the design of prospective studies to detect statistically and clinically significant changes in blood gas measures and newborn outcomes.
The normal mild fetal acidosis that develops during labor is likely a result of recurrent interruption of uterine placental blood flow during uterine contractions7 and is unlikely to benefit from maternal hyperoxia. Similarly, as placental oxygen transfer is predominantly flow rather than diffusion limited,8 oxygen supplementation is unlikely to improve severe variable FHR decelerations. Thus, a randomized study of hyperoxia in unselected laboring patients is unlikely to have a measurable effect on clinically significant acidosis.
Oxygen transport pathway guides treatment
For the present, an understanding of oxygen transport can guide clinical oxygen use. Thus, mothers with relative hypoxemia will unquestionably benefit with supplemental oxygen administration. Similarly, fetuses at risk for placental dysfunction (for example, growth restriction, postterm) and particularly those manifesting evidence of impaired oxygen transport (that is, late decelerations) may be most likely to benefit from the increased O2 gradient. For patients with reduced maternal uterine perfusion (such as hypotension or hypovolemia), pressors and/or fluid volume are likely to be more effective, while amnioinfusion is of greater value for umbilical cord compression patterns. A reduction in uterine activity may be of benefit to all fetuses exhibiting compromise. Due to the modest impact on fetal oxygen content, maternal hyperoxia does not produce significant fetal oxidative stress as measured by fetal malondialdehyde levels.
In view of the lack of demonstrated adverse effects of maternal supplemental oxygen, clinicians should not hesitate to use it. However, clinicians should recognize that supplemental oxygen is likely to be of value only in patients with significant impairment in the oxygen pathway, and they should choose additional intrauterine resuscitative measures focused on the etiology.
MICHAEL G. ROSS, MD, MPH,
AND BRYAN S. RICHARDSON, MD
- McNanley T, Woods J. Placental physiology. Glob Libr Women’s Med. (ISSN: 1756-2228). 2008. doi: 10.3843 /GLOWM.10195.
- Richardson BS. Fetal adaptive responses to asphyxia. Clin Perinatol. 1989;16:595-611.
- Sameshima H, Ikenoue T. Predictive value of late decelerations of fetal acidemia in unselective low-risk pregnancies. Am J Perinatol. 2005;22:19-23.
- Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119:824-831.
- Kelly R, Ramaiah SM, Sheridan H, et al. Dose-dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2018;103:F567-F572.
- Raghuraman N, Wan L, Temming LA, et al. Effect of oxygen vs room air on intrauterine fetal resuscitation: a randomized noninferiority clinical trial. JAMA Pediatr. 2018;172:818-823.
- Ramsey EM, Corner JW Jr, Donner MW. Serial and cineradioangiographic visualization of maternal circulation in the primate (hemochorial) placenta. Am J Obstet Gynecol. 1963;86:213-225.
- Nye GA, Ingram E, Johnstone ED, et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol. 2018;596:5523-5534.
Raghuraman N, Temming LA, Doering MM, et al. Maternal oxygen supplementation compared with room air for intrauterine resuscitation: a systematic review and meta-analysis. JAMA Pediatr. January 4, 2021. doi:10.1001/jamapediatrics.2020.5351.
EXPERT COMMENTARY
Maternal oxygen supplementation is widely used in labor for nonreassuring fetal heart rate (FHR) tracings, although its efficacy is uncertain for preventing fetal acidosis, operative intervention, or sequelae of neonatal encephalopathy. Recently, Raghuraman and colleagues reported the results of a systematic review and meta-analysis that included 16 randomized controlled trials. A total of 1,078 women were included in the oxygen group and 974 in the room air group. The primary outcome was umbilical artery pH; 14 trials reported on this outcome.
After analyzing the pooled and stratified results of the effect of maternal oxygen supplementation versus room air on umbilical artery gas measures, the authors concluded that peripartum oxygen supplementation is not associated with clinically relevant improvement in umbilical artery pH. They acknowledged, however, that the published studies were heterogeneous, lacked data on the association of oxygen supplementation with neonatal outcome, and did not assess oxygen use for abnormal FHR tracings, except for one trial with category II FHR tracings.
Effects of O2 supplementation
As maternal arterial hemoglobin is nearly saturated under normal conditions, maternal hyperoxia produces only modest increases in umbilical vein P

Fetal hypoxemia and acidosis can result from an interruption or an impairment of the mother-to-placenta-to-fetus oxygen pathway. With some interruptions of the oxygen pathway, such as placental abruption and complete cord occlusion–induced bradycardia, there would be less impact of maternal hyperoxia. By contrast, with other oxygen pathway impairments, such as reduced oxygen transfer with placental insufficiency, maternal hyperoxia can be of greater value by increasing maternal uterine artery and vein P
Continue to: Circumstances that may benefit from O2 supplementation...
Circumstances that may benefit from O2 supplementation
Late FHR decelerations reflect impairment of oxygen transfer and thus represent the heart rate pattern that is most likely to benefit from maternal hyperoxia. However, recurrent late decelerations occur in less than 2% of low-risk patients in labor,3 and severe levels of acidosis (umbilical artery pH <7.0 or base deficit [BD] ≥12 mmol/L) occur in only 1% to 2% of near-term or term deliveries.4,5
Variable decelerations also reflect fetal hypoxia and are much more common than late decelerations, so they also may benefit from O2 supplementation. Regardless, O2 supplementation should be seen only as a temporizing strategy while other resuscitative actions are initiated, including preparation for operative delivery, if indicated.
In a prior study by Raghuraman and colleagues (1 of only 4 studies that met selection criteria of oxygen supplementation for patients in labor), newborns of patients not receiving oxygen demonstrated 95% confidence limits of umbilical artery pH (7.24–7.28) and BD (2.9–4.3) well within the normal range.6 Thus, the low prevalence of cases in which a benefit might be anticipated and the low incidence of severe acidosis challenges the design of prospective studies to detect statistically and clinically significant changes in blood gas measures and newborn outcomes.
The normal mild fetal acidosis that develops during labor is likely a result of recurrent interruption of uterine placental blood flow during uterine contractions7 and is unlikely to benefit from maternal hyperoxia. Similarly, as placental oxygen transfer is predominantly flow rather than diffusion limited,8 oxygen supplementation is unlikely to improve severe variable FHR decelerations. Thus, a randomized study of hyperoxia in unselected laboring patients is unlikely to have a measurable effect on clinically significant acidosis.
Oxygen transport pathway guides treatment
For the present, an understanding of oxygen transport can guide clinical oxygen use. Thus, mothers with relative hypoxemia will unquestionably benefit with supplemental oxygen administration. Similarly, fetuses at risk for placental dysfunction (for example, growth restriction, postterm) and particularly those manifesting evidence of impaired oxygen transport (that is, late decelerations) may be most likely to benefit from the increased O2 gradient. For patients with reduced maternal uterine perfusion (such as hypotension or hypovolemia), pressors and/or fluid volume are likely to be more effective, while amnioinfusion is of greater value for umbilical cord compression patterns. A reduction in uterine activity may be of benefit to all fetuses exhibiting compromise. Due to the modest impact on fetal oxygen content, maternal hyperoxia does not produce significant fetal oxidative stress as measured by fetal malondialdehyde levels.
In view of the lack of demonstrated adverse effects of maternal supplemental oxygen, clinicians should not hesitate to use it. However, clinicians should recognize that supplemental oxygen is likely to be of value only in patients with significant impairment in the oxygen pathway, and they should choose additional intrauterine resuscitative measures focused on the etiology.
MICHAEL G. ROSS, MD, MPH,
AND BRYAN S. RICHARDSON, MD
Raghuraman N, Temming LA, Doering MM, et al. Maternal oxygen supplementation compared with room air for intrauterine resuscitation: a systematic review and meta-analysis. JAMA Pediatr. January 4, 2021. doi:10.1001/jamapediatrics.2020.5351.
EXPERT COMMENTARY
Maternal oxygen supplementation is widely used in labor for nonreassuring fetal heart rate (FHR) tracings, although its efficacy is uncertain for preventing fetal acidosis, operative intervention, or sequelae of neonatal encephalopathy. Recently, Raghuraman and colleagues reported the results of a systematic review and meta-analysis that included 16 randomized controlled trials. A total of 1,078 women were included in the oxygen group and 974 in the room air group. The primary outcome was umbilical artery pH; 14 trials reported on this outcome.
After analyzing the pooled and stratified results of the effect of maternal oxygen supplementation versus room air on umbilical artery gas measures, the authors concluded that peripartum oxygen supplementation is not associated with clinically relevant improvement in umbilical artery pH. They acknowledged, however, that the published studies were heterogeneous, lacked data on the association of oxygen supplementation with neonatal outcome, and did not assess oxygen use for abnormal FHR tracings, except for one trial with category II FHR tracings.
Effects of O2 supplementation
As maternal arterial hemoglobin is nearly saturated under normal conditions, maternal hyperoxia produces only modest increases in umbilical vein P

Fetal hypoxemia and acidosis can result from an interruption or an impairment of the mother-to-placenta-to-fetus oxygen pathway. With some interruptions of the oxygen pathway, such as placental abruption and complete cord occlusion–induced bradycardia, there would be less impact of maternal hyperoxia. By contrast, with other oxygen pathway impairments, such as reduced oxygen transfer with placental insufficiency, maternal hyperoxia can be of greater value by increasing maternal uterine artery and vein P
Continue to: Circumstances that may benefit from O2 supplementation...
Circumstances that may benefit from O2 supplementation
Late FHR decelerations reflect impairment of oxygen transfer and thus represent the heart rate pattern that is most likely to benefit from maternal hyperoxia. However, recurrent late decelerations occur in less than 2% of low-risk patients in labor,3 and severe levels of acidosis (umbilical artery pH <7.0 or base deficit [BD] ≥12 mmol/L) occur in only 1% to 2% of near-term or term deliveries.4,5
Variable decelerations also reflect fetal hypoxia and are much more common than late decelerations, so they also may benefit from O2 supplementation. Regardless, O2 supplementation should be seen only as a temporizing strategy while other resuscitative actions are initiated, including preparation for operative delivery, if indicated.
In a prior study by Raghuraman and colleagues (1 of only 4 studies that met selection criteria of oxygen supplementation for patients in labor), newborns of patients not receiving oxygen demonstrated 95% confidence limits of umbilical artery pH (7.24–7.28) and BD (2.9–4.3) well within the normal range.6 Thus, the low prevalence of cases in which a benefit might be anticipated and the low incidence of severe acidosis challenges the design of prospective studies to detect statistically and clinically significant changes in blood gas measures and newborn outcomes.
The normal mild fetal acidosis that develops during labor is likely a result of recurrent interruption of uterine placental blood flow during uterine contractions7 and is unlikely to benefit from maternal hyperoxia. Similarly, as placental oxygen transfer is predominantly flow rather than diffusion limited,8 oxygen supplementation is unlikely to improve severe variable FHR decelerations. Thus, a randomized study of hyperoxia in unselected laboring patients is unlikely to have a measurable effect on clinically significant acidosis.
Oxygen transport pathway guides treatment
For the present, an understanding of oxygen transport can guide clinical oxygen use. Thus, mothers with relative hypoxemia will unquestionably benefit with supplemental oxygen administration. Similarly, fetuses at risk for placental dysfunction (for example, growth restriction, postterm) and particularly those manifesting evidence of impaired oxygen transport (that is, late decelerations) may be most likely to benefit from the increased O2 gradient. For patients with reduced maternal uterine perfusion (such as hypotension or hypovolemia), pressors and/or fluid volume are likely to be more effective, while amnioinfusion is of greater value for umbilical cord compression patterns. A reduction in uterine activity may be of benefit to all fetuses exhibiting compromise. Due to the modest impact on fetal oxygen content, maternal hyperoxia does not produce significant fetal oxidative stress as measured by fetal malondialdehyde levels.
In view of the lack of demonstrated adverse effects of maternal supplemental oxygen, clinicians should not hesitate to use it. However, clinicians should recognize that supplemental oxygen is likely to be of value only in patients with significant impairment in the oxygen pathway, and they should choose additional intrauterine resuscitative measures focused on the etiology.
MICHAEL G. ROSS, MD, MPH,
AND BRYAN S. RICHARDSON, MD
- McNanley T, Woods J. Placental physiology. Glob Libr Women’s Med. (ISSN: 1756-2228). 2008. doi: 10.3843 /GLOWM.10195.
- Richardson BS. Fetal adaptive responses to asphyxia. Clin Perinatol. 1989;16:595-611.
- Sameshima H, Ikenoue T. Predictive value of late decelerations of fetal acidemia in unselective low-risk pregnancies. Am J Perinatol. 2005;22:19-23.
- Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119:824-831.
- Kelly R, Ramaiah SM, Sheridan H, et al. Dose-dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2018;103:F567-F572.
- Raghuraman N, Wan L, Temming LA, et al. Effect of oxygen vs room air on intrauterine fetal resuscitation: a randomized noninferiority clinical trial. JAMA Pediatr. 2018;172:818-823.
- Ramsey EM, Corner JW Jr, Donner MW. Serial and cineradioangiographic visualization of maternal circulation in the primate (hemochorial) placenta. Am J Obstet Gynecol. 1963;86:213-225.
- Nye GA, Ingram E, Johnstone ED, et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol. 2018;596:5523-5534.
- McNanley T, Woods J. Placental physiology. Glob Libr Women’s Med. (ISSN: 1756-2228). 2008. doi: 10.3843 /GLOWM.10195.
- Richardson BS. Fetal adaptive responses to asphyxia. Clin Perinatol. 1989;16:595-611.
- Sameshima H, Ikenoue T. Predictive value of late decelerations of fetal acidemia in unselective low-risk pregnancies. Am J Perinatol. 2005;22:19-23.
- Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119:824-831.
- Kelly R, Ramaiah SM, Sheridan H, et al. Dose-dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2018;103:F567-F572.
- Raghuraman N, Wan L, Temming LA, et al. Effect of oxygen vs room air on intrauterine fetal resuscitation: a randomized noninferiority clinical trial. JAMA Pediatr. 2018;172:818-823.
- Ramsey EM, Corner JW Jr, Donner MW. Serial and cineradioangiographic visualization of maternal circulation in the primate (hemochorial) placenta. Am J Obstet Gynecol. 1963;86:213-225.
- Nye GA, Ingram E, Johnstone ED, et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol. 2018;596:5523-5534.
For heavy menstrual bleeding, are long-term outcomes similar for treatment with the LNG-IUS and radiofrequency endometrial ablation?
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Office-based ambulatory cervical ripening prior to inpatient induction of labor
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
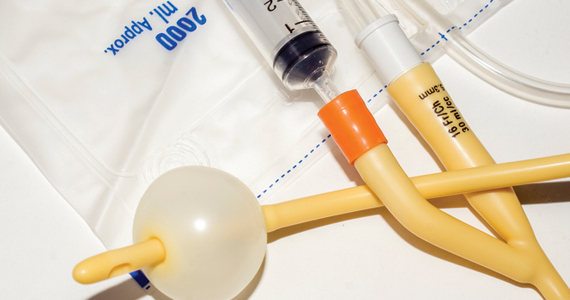
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
Cesarean myomectomy: Safe operation or surgical folly?

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
How does long-term OC use affect breast, ovarian, and endometrial cancer risk?
Karlsson T, Johansson T, Hoguland J, et al. Time-dependent effects of oral contraceptive use on breast, ovarian and endometrial cancers. Cancer Research. 2020;canres.2476.2020. doi:10.1158/0008-5472.CAN-20-2476.
EXPERT COMMENTARY
The long-term effects of OC use on gynecologic and breast cancers has been uncertain, with different reports yielding conflicting findings. To assess the time-dependent and long-term associations between OC use and the risk of breast, ovarian, and endometrial cancer in women born between 1939 and 1970, Karlsson and colleagues used data from the UK Biobank (which includes a large cross-sectional cohort of individuals recruited between 2006 and 2010) and national databases.
Details of the study
A total of 256,661 women were included in this study. Of these, 82% (210,443) had used or were currently using OC (ever-users) and 18% (46,218) had never used OC (never-users). There were 17,739; 1,966; and 2,462 cases of breast, ovarian, and endometrial cancer, respectively, identified.
In analyses adjusted for 10 parameters, the ORs for ovarian (OR, 0.72) and endometrial cancer (OR, 0.68) were lower among ever-users of OC compared with never-users (P<.05). However, the OR for breast cancer (OR, 1.02) was similar among ever-users and never-users of OC (P>.05).
Among women followed to age 55, results were similar for the 2 gynecologic cancers but were significantly higher for breast cancer (OR, 1.10; P<.05). With 20 or more years of OC use, greater prevention of ovarian (OR, 0.60) and, particularly, endometrial cancer (OR, 0.36) was observed (P<.05). However, the risk of breast cancer was similar in never-users and long-term users of OC.
Study strengths and limitations
A strength of this study is that, compared with most previous studies, it had a much longer follow-up period.
The authors noted, however, that among the potential limitations in the study design was the fact that only 6% of participants invited to the UK Biobank volunteered to participate in the study. This may have resulted in participation bias within the cohort, reflecting a healthier cohort that is not representative of the overall population. ●
These study findings from a large cross-sectional cohort by Karlsson and colleagues suggest that controversy regarding the association of breast cancer with OC use may reflect different study methodologies with respect to timing. The authors note that while the lifetime risk of breast cancer may not differ between OC ever-users and never-users, there appears to be a transient elevated risk associated with OC use. By contrast, OC use, particularly when used long-term, appears to “dramatically” reduce the risk of ovarian and endometrial cancer, according to the study authors.
ANDREW M. KAUNITZ, MD
Karlsson T, Johansson T, Hoguland J, et al. Time-dependent effects of oral contraceptive use on breast, ovarian and endometrial cancers. Cancer Research. 2020;canres.2476.2020. doi:10.1158/0008-5472.CAN-20-2476.
EXPERT COMMENTARY
The long-term effects of OC use on gynecologic and breast cancers has been uncertain, with different reports yielding conflicting findings. To assess the time-dependent and long-term associations between OC use and the risk of breast, ovarian, and endometrial cancer in women born between 1939 and 1970, Karlsson and colleagues used data from the UK Biobank (which includes a large cross-sectional cohort of individuals recruited between 2006 and 2010) and national databases.
Details of the study
A total of 256,661 women were included in this study. Of these, 82% (210,443) had used or were currently using OC (ever-users) and 18% (46,218) had never used OC (never-users). There were 17,739; 1,966; and 2,462 cases of breast, ovarian, and endometrial cancer, respectively, identified.
In analyses adjusted for 10 parameters, the ORs for ovarian (OR, 0.72) and endometrial cancer (OR, 0.68) were lower among ever-users of OC compared with never-users (P<.05). However, the OR for breast cancer (OR, 1.02) was similar among ever-users and never-users of OC (P>.05).
Among women followed to age 55, results were similar for the 2 gynecologic cancers but were significantly higher for breast cancer (OR, 1.10; P<.05). With 20 or more years of OC use, greater prevention of ovarian (OR, 0.60) and, particularly, endometrial cancer (OR, 0.36) was observed (P<.05). However, the risk of breast cancer was similar in never-users and long-term users of OC.
Study strengths and limitations
A strength of this study is that, compared with most previous studies, it had a much longer follow-up period.
The authors noted, however, that among the potential limitations in the study design was the fact that only 6% of participants invited to the UK Biobank volunteered to participate in the study. This may have resulted in participation bias within the cohort, reflecting a healthier cohort that is not representative of the overall population. ●
These study findings from a large cross-sectional cohort by Karlsson and colleagues suggest that controversy regarding the association of breast cancer with OC use may reflect different study methodologies with respect to timing. The authors note that while the lifetime risk of breast cancer may not differ between OC ever-users and never-users, there appears to be a transient elevated risk associated with OC use. By contrast, OC use, particularly when used long-term, appears to “dramatically” reduce the risk of ovarian and endometrial cancer, according to the study authors.
ANDREW M. KAUNITZ, MD
Karlsson T, Johansson T, Hoguland J, et al. Time-dependent effects of oral contraceptive use on breast, ovarian and endometrial cancers. Cancer Research. 2020;canres.2476.2020. doi:10.1158/0008-5472.CAN-20-2476.
EXPERT COMMENTARY
The long-term effects of OC use on gynecologic and breast cancers has been uncertain, with different reports yielding conflicting findings. To assess the time-dependent and long-term associations between OC use and the risk of breast, ovarian, and endometrial cancer in women born between 1939 and 1970, Karlsson and colleagues used data from the UK Biobank (which includes a large cross-sectional cohort of individuals recruited between 2006 and 2010) and national databases.
Details of the study
A total of 256,661 women were included in this study. Of these, 82% (210,443) had used or were currently using OC (ever-users) and 18% (46,218) had never used OC (never-users). There were 17,739; 1,966; and 2,462 cases of breast, ovarian, and endometrial cancer, respectively, identified.
In analyses adjusted for 10 parameters, the ORs for ovarian (OR, 0.72) and endometrial cancer (OR, 0.68) were lower among ever-users of OC compared with never-users (P<.05). However, the OR for breast cancer (OR, 1.02) was similar among ever-users and never-users of OC (P>.05).
Among women followed to age 55, results were similar for the 2 gynecologic cancers but were significantly higher for breast cancer (OR, 1.10; P<.05). With 20 or more years of OC use, greater prevention of ovarian (OR, 0.60) and, particularly, endometrial cancer (OR, 0.36) was observed (P<.05). However, the risk of breast cancer was similar in never-users and long-term users of OC.
Study strengths and limitations
A strength of this study is that, compared with most previous studies, it had a much longer follow-up period.
The authors noted, however, that among the potential limitations in the study design was the fact that only 6% of participants invited to the UK Biobank volunteered to participate in the study. This may have resulted in participation bias within the cohort, reflecting a healthier cohort that is not representative of the overall population. ●
These study findings from a large cross-sectional cohort by Karlsson and colleagues suggest that controversy regarding the association of breast cancer with OC use may reflect different study methodologies with respect to timing. The authors note that while the lifetime risk of breast cancer may not differ between OC ever-users and never-users, there appears to be a transient elevated risk associated with OC use. By contrast, OC use, particularly when used long-term, appears to “dramatically” reduce the risk of ovarian and endometrial cancer, according to the study authors.
ANDREW M. KAUNITZ, MD
Treating PPH: A novel vacuum-induced hemorrhage control device
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5
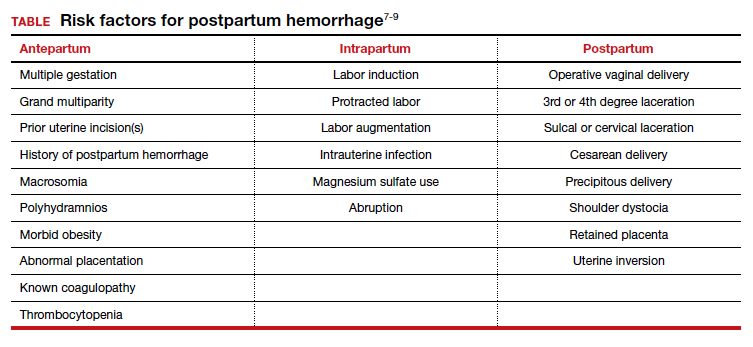
Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).
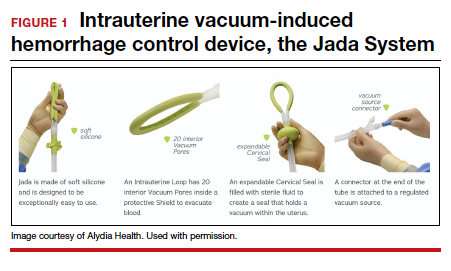
Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.
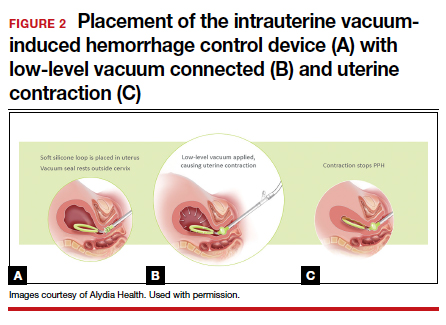
CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5

Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).

Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.

CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5

Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).

Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.

CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
Racism and gynecologic surgery: A time to act
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).
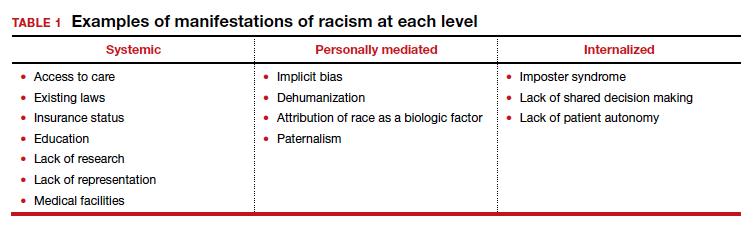
Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).

Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).

Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
Practical obstetrics in pandemic times: Teamwork, flexibility, and creativity promote safety for patients and the care team
Practicing evidence-based medicine, as obstetricians know, is not always possible when one does not have evidence due to lack of data or long-term experience in pregnancy. During the COVID-19 pandemic, the evidence changed so rapidly that we were compelled to alter our strategy frequently as we learned more about the impact of this disease on our vulnerable patient population. The COVID-19 pandemic taught us that, in unprecedented times, centering the safety of the patient, her child, and the health care team requires quick thinking, flexibility, and above all effective communication between team members.
Here, I share our institutional experience in providing practical obstetric care through various stages of the still-evolving COVID-19 pandemic. We based our strategy on guidance from the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG),1,2 and the Society for Maternal-Fetal Medicine (SMFM).3-5 We were reminded yet again that the only constant is change and that timely but thoughtful adjustments were needed to keep up with the coronavirus.
Changes to prenatal care
Like many others, our institution has provided continued in-person outpatient prenatal care to both our low- and high-risk patients throughout each stage of the pandemic. While continuing to provide the necessary obstetric care, we made alterations to limit exposure and practice social distancing when possible.
Limiting patient support persons. One significant change was to restrict or limit support persons in the outpatient clinics based on guidelines reflecting community infection rates. Recognizing that this was not optimal for our patients’ emotional well-being, we needed to become more flexible in using technology to include family or support persons in prenatal visits and ultrasonography exams.
Altering test frequency. Using the guidance from SMFM,1 we changed the frequency of our antenatal testing and ultrasonography exams in the following ways: We increased the duration between indicated growth ultrasonography to every 4 weeks and decreased fetal antenatal testing to weekly, with twice-weekly testing continued for the highest-risk patients. Early first-trimester ultrasonography exams were limited and, when possible, delayed until after 10 to 12 weeks’ gestation or combined with other indications (nuchal translucency). Prenatal visits for low-risk patients were spaced out using existing models if the patient was amenable, especially in early pregnancy.
Adjusting staff assignments and using telehealth. In the early part of the pandemic, we divided into 2 groups to limit the number of clinicians at any one site: a dedicated group of outpatient clinicians who saw patients in the clinic only and a dedicated group of inpatient clinicians who staffed labor and delivery and the inpatient antepartum service. Additionally, our consultative maternal-fetal medicine service transitioned to a telehealth platform and performed the majority of consults remotely. Ultrasonography exams at various sites were read remotely and pertinent findings were communicated directly to patients via phone or the telehealth platform. Amniocentesis continued to be offered.
Responding to lower COVID-19 case numbers. When the number of COVID-19 cases decreased in the summer and fall of 2020, we returned to our prepandemic in-person practices, but we continued to offer telehealth visits as an option for patients who desired it. Patients were limited to one support person.
Shifting gears again. During the second surge of COVID-19 in our region, we used our experiences from the first to transition our practices to reduce in-person contact. Appointment frequency was decreased if appropriate, and we developed a tiered system of antenatal testing frequency based on risk factors. Visitors were again restricted, with exceptions made for extenuating circumstances. Consults were transitioned to telemedicine as appropriate and ultrasonography exams were read remotely when possible to limit exposures. Given the varied experiences with telemedicine and patient preferences, patients who desired in-person consult were (and are still) offered this option.
Some patients who were interested in telehealth but unable to access the technology were offered appointments via telehealth with the use of our clinic devices. Telemedicine increased our flexibility in offering consults as one provider could see patients at different office sites in one session. We continued our routine inpatient and outpatient coverage during this time as this kept our coverage options more flexible and expanded our obstetric backup plan in response to increased rates of community infection that affected both clinicians and patients.
Coordinating care for infected patients. One vital part of our prenatal care during the COVID-19 pandemic was to coordinate with our colleagues in medical specialties to provide outpatient care for patients with confirmed or suspected COVID-19 during their period of isolation or quarantine. Patients could be seen as outpatients in a dedicated space that used appropriate personal protective equipment (PPE) for not only prenatal care but also any needed in-person evaluation for COVID-19. Our obstetric clinicians and sonographers performed exams, antenatal testing (in the form of biophysical profiles), and indicated ultrasonography exams (such as umbilical artery Doppler studies and fetal growth assessments). This required a concerted effort and excellent communication between teams to provide the necessary care in the safest manner possible.
Continue to: Universal testing on labor and delivery...
Universal testing on labor and delivery
Not surprisingly, obstetric delivery volumes in our institution were not affected in the same way as elective surgery volumes. Our inpatient team continued to bring babies into the world at the same if not a higher rate than in prepandemic times. We continued elective inductions when space allowed. Our first COVID-19–positive patient was already at 40 weeks’ gestation when the result of her test, done due to exposure, was received. Creative effort among multiple specialties quickly developed her delivery plan, and she and her infant did well.
As data started coming out of the New York City obstetric experience, concern for preservation of the PPE supply and the potential for asymptomatic/presymptomatic patients led us, in consultation with our infectious disease colleagues, to institute universal testing for all antepartum and laboring patients. At first, all patients were tested on admission with our rapid in-house test. Eventually, we moved toward preoperative testing 3 to 5 days prior to scheduled cesarean deliveries in alignment with the surgical services when elective cases were reinstituted. Finally, we instituted preprocedure testing for all scheduled labor and delivery procedures, including inductions, cerclages, and fetal blood transfusions, while we still used rapid testing for patients who presented urgently or in labor.
We needed to address several considerations almost immediately after instituting universal testing, including:
- what to do in case of patient refusal to be tested
- which precautions to institute while awaiting test results
- potential postponement of elective delivery if a patient tested positive, and
- where best to deliver patients.
What we did at the beginning of the pandemic was not necessarily the same as we do in our current practice, and we expect that our procedures may need to change in the future. Derived from what we learned from others’ experience, we tailored our protocols to our own physical space, staffing capabilities, and testing limitations. We adjusted them often, with input from multiple services, based on updated policy, recommendation for isolation and quarantine durations, rates of community infection, and changes in the unit spaces. As with many things, one protocol did not fit every patient, necessitating case-by-case flexibility.
Delivery considerations
To answer some of the above questions, all patients who declined testing, were awaiting test results while in labor, or were in triage were placed in droplet and contact isolation on our unit, a practice we continue currently. Given the concern of potential aerosolization during the second stage of labor or during intubation, for any patients in those categories who required delivery, we limited the number of staff in their rooms as possible. Additional pediatric staff waited in close proximity of the room and were ready to come in if needed depending on fetal complications and gestational age. For delivery, all team members used full special pathogens precautions (N95 masks, face shields, gowns, and gloves).
Patients who were asymptomatic and tested negative for COVID-19 had and continue to have routine care from a PPE (standard gowns, gloves, face mask, and eye protection) and health care team perspective. We have allowed visitation of one support person per hospital stay for these patients throughout the pandemic.
For the majority of our experience during the pandemic, adult patients who tested positive for COVID-19 were cohorted within dedicated negative pressure units of varying levels of care. As these units included the same intensive care unit (ICU) we utilized in non-COVID times for critical obstetric patients, we had already operationalized their use and they were wired for our electronic fetal monitoring system. These rooms are adjacent to the main operating room (OR) complex, which allows for transition to a dedicated COVID-19 OR for cesarean delivery. We worked with the primary COVID-19 team, ICU team, anesthesia, and neonatal ICU team to develop a written protocol that detailed the care for our COVID-19–positive laboring and postpartum patients in this critical care COVID-19 unit.
For a time, admitted COVID-19–positive patients were not permitted to have support persons. The health care team therefore stepped in to be the patients’ support during the delivery of their child. Care of these patients required a great deal of coordination and communication between teams as well as the addition of a dedicated obstetric physician—separate from the regular labor and delivery team—assigned to care for these patients.
For pregnant patients in the emergency room or in the intermediate or floor COVID-19 units, portable fetal monitors and ultrasonography equipment were used for obstetric consults, fetal testing, and obstetrical ultrasonography as appropriate based on gestational age and medical conditions. Again, communication between teams was essential to provide seamless and timely patient care. Patients usually were admitted to the COVID-19 teams with maternal-fetal medicine or obstetric consult teams following daily; they were admitted and transferred to the ICU COVID-19 unit if delivery was necessary. To limit exposures whenever possible, coordinated care (such as exams and telephone evaluation) was performed outside of the room with the nursing and primary teams.
Continue to: Staying flexible to the changing COVID-19 environment...
Staying flexible to the changing
COVID-19 environment
Postponed in-person visits. Whenever possible, deliveries that were not medically indicated and in-person outpatient care visits were postponed until isolation/quarantine precautions could be lifted to avoid the need for special pathogens precautions, separation of mother and infant, and visitor restrictions. We did not postpone any medically indicated deliveries or appropriate care due to COVID-19 alone. As the CDC guidelines changed regarding the timing of infectivity, we had to continually re-evaluate when a patient could return to regular outpatient care instead of the COVID-19 clinic and/or be delivered.
Mother-infant separation. As outlined in an article we wrote with our pediatric colleagues, originally all infants were immediately separated from their COVID-19–positive mothers, and delayed cord clamping was not performed.6 We adjusted our protocols as experience and data grew regarding the risk of transmission to the newborn from asymptomatic mothers and as updated recommendations were made by ACOG and the CDC. Currently, if desired, asymptomatic mothers are not separated from their well term infants. We practice our standard delayed cord clamping technique for all patients. Masking, hand hygiene, and physical distancing are used to reduce the risk of infection transmission. Breastfeeding is encouraged if the patient desires it, either directly using precautions or supported via pumping.
Reduced workplace exposure. Along with many others, we are even more cognizant of reducing the risk of workplace exposure; thus, we conduct our daily multidisciplinary huddle and physician transition of care sign-outs. We use multiple rooms for our larger group with secure video chats, and we limit huddles to a single representative from each specialty.
Medication protocols. Early in the pandemic in our area, we limited antenatal corticosteroids for fetal lung maturity to patients who were at less than 34 weeks’ gestation, per ACOG recommendations, carefully considering necessity in the critically ill. Now, we continue to administer antenatal steroids according to our usual protocols up to 36 6/7 weeks, per ACOG and SMFM recommendations, regardless of illness severity.7 Nonsteroidal anti-inflammatory drug use, once limited in COVID-19–positive patients, are now used again. Additionally, we had a comprehensive venous thromboembolism (VTE) prophylaxis protocol for our obstetric patients, and we have added special consideration for prophylaxis for patients with moderate to severe illness or other VTE risk factors. While we do not perform routine circumcisions on infants of COVID-19–positive mothers, we have a process in place to provide that service after discharge when isolation precautions are lifted.
Labor accommodations. As COVID-19 cases increased in our hospital during recent months, we made one more significant change in our care protocols. To open up space in the ICU, we moved our care for asymptomatic COVID-19–positive laboring patients to our new labor and delivery unit with implemented special pathogens precautions. This is not revolutionary; many other hospitals did not have the same capability we did with our existing collaboration with the ICU for critical obstetric care. However, this change again required communication and collaboration among multiple care teams, agreement on the qualifications for delivery on labor and delivery versus in the ICU, and physical alteration of our unit to accommodate additional isolation precautions.
Visitor policy. Another change is that we have opened up the visitor policy to welcome an asymptomatic support person for the COVID-19–positive labor patient, giving special attention to adherence to isolation precautions. Our staff members have embraced this change as they have everything else, with cautious optimism and focus on keeping both the patients and the health care team safe. Our moderate to severely ill patients continue to be cared for in the COVID-19 unit in close collaboration with our infectious disease and ICU colleagues.
It’s all about teamwork
I hope I have given a clear example of our approach to providing obstetric care in the ever-changing landscape of the COVID-19 pandemic. We embraced this period of necessary change as practically and safely as possible for both our patients and our health care workers. We learned multiple times along the way that what seemed to be a good idea was not feasible, or not the ideal option, or that COVID-19 had changed the rules of the game again. Our team met daily if not more frequently, as we found we had to constantly adapt and change to each new challenge or new clinical scenario. When we struggled, it generally related to a gap in communication.
I am privileged to work with a dedicated, selfless, multidisciplinary team that rose to the occasion. They had the focused goal to provide the highest quality and safety in obstetric care while offering compassion and empathy for the experience of having a baby during a pandemic. ●
The author would like to acknowledge Danielle Prentice, DO, and Jaimie Maines, MD, for their manuscript review.
- The requirement for reduced in-person contact due to the COVID-19 pandemic challenged our traditional obstetric care models. This led us to comprehensively incorporate technology for communication with patients and their families and to significantly alter how, where, and when we delivered prenatal care.
- Both patients and clinicians needed to adjust to the impact of these changes, especially concerning visitor policies.
- Early incorporation of universal COVID-19 testing for labor and antepartum patients was initially instituted to improve patient and staff safety and to preserve PPE. However, it quickly led to the need for various protocols for both anticipated and unanticipated clinical scenarios.
- As new data emerged and the number of cases fluctuated throughout the pandemic, our approach and protocols necessitated flexibility: Our strategy for maternal and neonatal care early in the pandemic was not the same as our current approach, and it will likely change several more times before we are done.
- One of the biggest challenges to our care team was maintaining standards of excellence and safety in obstetric care while also adhering to the physical barriers of isolation precautions and maintaining vigilance to reduce exposure risk during our routine workflow.
- The physical and operational specifics of our institution determined our approach to obstetric care during COVID-19, in part because halfway through the pandemic we moved our maternity unit from the adult hospital to a new center within our children’s hospital.
- The frequent changes in the knowledge of and recommendations for COVID-19 highlighted the importance of maintaining multidisciplinary communication on a daily, if not more frequent, basis.
- American College of Obstetricians and Gynecologists. Practice advisory: novel coronavirus 2019 (COVID-19): summary of key updates (December 14, 2020). https://www.acog.org/clinical /clinical-guidance/practice-advisory/articles/2020/03/novel -coronavirus-2019. Accessed January 28, 2021.
- American College of Obstetricians and Gynecologists. COVID19 FAQs for obstetrician-gynecologists, obstetrics. Washington, DC: ACOG; 2020. https://www.acog.org/clinical-information /physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Updated November 23, 2020. https: //s3.amazonaws.com/cdn.smfm.org/media/2589/COVID19 -What_MFMs_need_to_know_revision_11-23-20_final.pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Management considerations for pregnant patients with COVID-19. Updated January 7, 2021. https://s3.amazonaws.com/cdn.smfm.org /media/2668/SMFM_COVID_Management_of_COVID_pos _preg_patients_1-7-21_(final).pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. COVID-19 ultrasound clinical practice suggestions. Updated October 20, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2550 /Ultrasound_Covid19_Suggestions_10-20-20_(final).pdf. Accessed January 28, 2020.
- Amatya S, Corr TE, Gandhi CK, et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J Perinatol. 2020;40:987-996.
- American College of Obstetricians and Gynecologists. Committee opinion no 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130:e102-e109.
Practicing evidence-based medicine, as obstetricians know, is not always possible when one does not have evidence due to lack of data or long-term experience in pregnancy. During the COVID-19 pandemic, the evidence changed so rapidly that we were compelled to alter our strategy frequently as we learned more about the impact of this disease on our vulnerable patient population. The COVID-19 pandemic taught us that, in unprecedented times, centering the safety of the patient, her child, and the health care team requires quick thinking, flexibility, and above all effective communication between team members.
Here, I share our institutional experience in providing practical obstetric care through various stages of the still-evolving COVID-19 pandemic. We based our strategy on guidance from the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG),1,2 and the Society for Maternal-Fetal Medicine (SMFM).3-5 We were reminded yet again that the only constant is change and that timely but thoughtful adjustments were needed to keep up with the coronavirus.
Changes to prenatal care
Like many others, our institution has provided continued in-person outpatient prenatal care to both our low- and high-risk patients throughout each stage of the pandemic. While continuing to provide the necessary obstetric care, we made alterations to limit exposure and practice social distancing when possible.
Limiting patient support persons. One significant change was to restrict or limit support persons in the outpatient clinics based on guidelines reflecting community infection rates. Recognizing that this was not optimal for our patients’ emotional well-being, we needed to become more flexible in using technology to include family or support persons in prenatal visits and ultrasonography exams.
Altering test frequency. Using the guidance from SMFM,1 we changed the frequency of our antenatal testing and ultrasonography exams in the following ways: We increased the duration between indicated growth ultrasonography to every 4 weeks and decreased fetal antenatal testing to weekly, with twice-weekly testing continued for the highest-risk patients. Early first-trimester ultrasonography exams were limited and, when possible, delayed until after 10 to 12 weeks’ gestation or combined with other indications (nuchal translucency). Prenatal visits for low-risk patients were spaced out using existing models if the patient was amenable, especially in early pregnancy.
Adjusting staff assignments and using telehealth. In the early part of the pandemic, we divided into 2 groups to limit the number of clinicians at any one site: a dedicated group of outpatient clinicians who saw patients in the clinic only and a dedicated group of inpatient clinicians who staffed labor and delivery and the inpatient antepartum service. Additionally, our consultative maternal-fetal medicine service transitioned to a telehealth platform and performed the majority of consults remotely. Ultrasonography exams at various sites were read remotely and pertinent findings were communicated directly to patients via phone or the telehealth platform. Amniocentesis continued to be offered.
Responding to lower COVID-19 case numbers. When the number of COVID-19 cases decreased in the summer and fall of 2020, we returned to our prepandemic in-person practices, but we continued to offer telehealth visits as an option for patients who desired it. Patients were limited to one support person.
Shifting gears again. During the second surge of COVID-19 in our region, we used our experiences from the first to transition our practices to reduce in-person contact. Appointment frequency was decreased if appropriate, and we developed a tiered system of antenatal testing frequency based on risk factors. Visitors were again restricted, with exceptions made for extenuating circumstances. Consults were transitioned to telemedicine as appropriate and ultrasonography exams were read remotely when possible to limit exposures. Given the varied experiences with telemedicine and patient preferences, patients who desired in-person consult were (and are still) offered this option.
Some patients who were interested in telehealth but unable to access the technology were offered appointments via telehealth with the use of our clinic devices. Telemedicine increased our flexibility in offering consults as one provider could see patients at different office sites in one session. We continued our routine inpatient and outpatient coverage during this time as this kept our coverage options more flexible and expanded our obstetric backup plan in response to increased rates of community infection that affected both clinicians and patients.
Coordinating care for infected patients. One vital part of our prenatal care during the COVID-19 pandemic was to coordinate with our colleagues in medical specialties to provide outpatient care for patients with confirmed or suspected COVID-19 during their period of isolation or quarantine. Patients could be seen as outpatients in a dedicated space that used appropriate personal protective equipment (PPE) for not only prenatal care but also any needed in-person evaluation for COVID-19. Our obstetric clinicians and sonographers performed exams, antenatal testing (in the form of biophysical profiles), and indicated ultrasonography exams (such as umbilical artery Doppler studies and fetal growth assessments). This required a concerted effort and excellent communication between teams to provide the necessary care in the safest manner possible.
Continue to: Universal testing on labor and delivery...
Universal testing on labor and delivery
Not surprisingly, obstetric delivery volumes in our institution were not affected in the same way as elective surgery volumes. Our inpatient team continued to bring babies into the world at the same if not a higher rate than in prepandemic times. We continued elective inductions when space allowed. Our first COVID-19–positive patient was already at 40 weeks’ gestation when the result of her test, done due to exposure, was received. Creative effort among multiple specialties quickly developed her delivery plan, and she and her infant did well.
As data started coming out of the New York City obstetric experience, concern for preservation of the PPE supply and the potential for asymptomatic/presymptomatic patients led us, in consultation with our infectious disease colleagues, to institute universal testing for all antepartum and laboring patients. At first, all patients were tested on admission with our rapid in-house test. Eventually, we moved toward preoperative testing 3 to 5 days prior to scheduled cesarean deliveries in alignment with the surgical services when elective cases were reinstituted. Finally, we instituted preprocedure testing for all scheduled labor and delivery procedures, including inductions, cerclages, and fetal blood transfusions, while we still used rapid testing for patients who presented urgently or in labor.
We needed to address several considerations almost immediately after instituting universal testing, including:
- what to do in case of patient refusal to be tested
- which precautions to institute while awaiting test results
- potential postponement of elective delivery if a patient tested positive, and
- where best to deliver patients.
What we did at the beginning of the pandemic was not necessarily the same as we do in our current practice, and we expect that our procedures may need to change in the future. Derived from what we learned from others’ experience, we tailored our protocols to our own physical space, staffing capabilities, and testing limitations. We adjusted them often, with input from multiple services, based on updated policy, recommendation for isolation and quarantine durations, rates of community infection, and changes in the unit spaces. As with many things, one protocol did not fit every patient, necessitating case-by-case flexibility.
Delivery considerations
To answer some of the above questions, all patients who declined testing, were awaiting test results while in labor, or were in triage were placed in droplet and contact isolation on our unit, a practice we continue currently. Given the concern of potential aerosolization during the second stage of labor or during intubation, for any patients in those categories who required delivery, we limited the number of staff in their rooms as possible. Additional pediatric staff waited in close proximity of the room and were ready to come in if needed depending on fetal complications and gestational age. For delivery, all team members used full special pathogens precautions (N95 masks, face shields, gowns, and gloves).
Patients who were asymptomatic and tested negative for COVID-19 had and continue to have routine care from a PPE (standard gowns, gloves, face mask, and eye protection) and health care team perspective. We have allowed visitation of one support person per hospital stay for these patients throughout the pandemic.
For the majority of our experience during the pandemic, adult patients who tested positive for COVID-19 were cohorted within dedicated negative pressure units of varying levels of care. As these units included the same intensive care unit (ICU) we utilized in non-COVID times for critical obstetric patients, we had already operationalized their use and they were wired for our electronic fetal monitoring system. These rooms are adjacent to the main operating room (OR) complex, which allows for transition to a dedicated COVID-19 OR for cesarean delivery. We worked with the primary COVID-19 team, ICU team, anesthesia, and neonatal ICU team to develop a written protocol that detailed the care for our COVID-19–positive laboring and postpartum patients in this critical care COVID-19 unit.
For a time, admitted COVID-19–positive patients were not permitted to have support persons. The health care team therefore stepped in to be the patients’ support during the delivery of their child. Care of these patients required a great deal of coordination and communication between teams as well as the addition of a dedicated obstetric physician—separate from the regular labor and delivery team—assigned to care for these patients.
For pregnant patients in the emergency room or in the intermediate or floor COVID-19 units, portable fetal monitors and ultrasonography equipment were used for obstetric consults, fetal testing, and obstetrical ultrasonography as appropriate based on gestational age and medical conditions. Again, communication between teams was essential to provide seamless and timely patient care. Patients usually were admitted to the COVID-19 teams with maternal-fetal medicine or obstetric consult teams following daily; they were admitted and transferred to the ICU COVID-19 unit if delivery was necessary. To limit exposures whenever possible, coordinated care (such as exams and telephone evaluation) was performed outside of the room with the nursing and primary teams.
Continue to: Staying flexible to the changing COVID-19 environment...
Staying flexible to the changing
COVID-19 environment
Postponed in-person visits. Whenever possible, deliveries that were not medically indicated and in-person outpatient care visits were postponed until isolation/quarantine precautions could be lifted to avoid the need for special pathogens precautions, separation of mother and infant, and visitor restrictions. We did not postpone any medically indicated deliveries or appropriate care due to COVID-19 alone. As the CDC guidelines changed regarding the timing of infectivity, we had to continually re-evaluate when a patient could return to regular outpatient care instead of the COVID-19 clinic and/or be delivered.
Mother-infant separation. As outlined in an article we wrote with our pediatric colleagues, originally all infants were immediately separated from their COVID-19–positive mothers, and delayed cord clamping was not performed.6 We adjusted our protocols as experience and data grew regarding the risk of transmission to the newborn from asymptomatic mothers and as updated recommendations were made by ACOG and the CDC. Currently, if desired, asymptomatic mothers are not separated from their well term infants. We practice our standard delayed cord clamping technique for all patients. Masking, hand hygiene, and physical distancing are used to reduce the risk of infection transmission. Breastfeeding is encouraged if the patient desires it, either directly using precautions or supported via pumping.
Reduced workplace exposure. Along with many others, we are even more cognizant of reducing the risk of workplace exposure; thus, we conduct our daily multidisciplinary huddle and physician transition of care sign-outs. We use multiple rooms for our larger group with secure video chats, and we limit huddles to a single representative from each specialty.
Medication protocols. Early in the pandemic in our area, we limited antenatal corticosteroids for fetal lung maturity to patients who were at less than 34 weeks’ gestation, per ACOG recommendations, carefully considering necessity in the critically ill. Now, we continue to administer antenatal steroids according to our usual protocols up to 36 6/7 weeks, per ACOG and SMFM recommendations, regardless of illness severity.7 Nonsteroidal anti-inflammatory drug use, once limited in COVID-19–positive patients, are now used again. Additionally, we had a comprehensive venous thromboembolism (VTE) prophylaxis protocol for our obstetric patients, and we have added special consideration for prophylaxis for patients with moderate to severe illness or other VTE risk factors. While we do not perform routine circumcisions on infants of COVID-19–positive mothers, we have a process in place to provide that service after discharge when isolation precautions are lifted.
Labor accommodations. As COVID-19 cases increased in our hospital during recent months, we made one more significant change in our care protocols. To open up space in the ICU, we moved our care for asymptomatic COVID-19–positive laboring patients to our new labor and delivery unit with implemented special pathogens precautions. This is not revolutionary; many other hospitals did not have the same capability we did with our existing collaboration with the ICU for critical obstetric care. However, this change again required communication and collaboration among multiple care teams, agreement on the qualifications for delivery on labor and delivery versus in the ICU, and physical alteration of our unit to accommodate additional isolation precautions.
Visitor policy. Another change is that we have opened up the visitor policy to welcome an asymptomatic support person for the COVID-19–positive labor patient, giving special attention to adherence to isolation precautions. Our staff members have embraced this change as they have everything else, with cautious optimism and focus on keeping both the patients and the health care team safe. Our moderate to severely ill patients continue to be cared for in the COVID-19 unit in close collaboration with our infectious disease and ICU colleagues.
It’s all about teamwork
I hope I have given a clear example of our approach to providing obstetric care in the ever-changing landscape of the COVID-19 pandemic. We embraced this period of necessary change as practically and safely as possible for both our patients and our health care workers. We learned multiple times along the way that what seemed to be a good idea was not feasible, or not the ideal option, or that COVID-19 had changed the rules of the game again. Our team met daily if not more frequently, as we found we had to constantly adapt and change to each new challenge or new clinical scenario. When we struggled, it generally related to a gap in communication.
I am privileged to work with a dedicated, selfless, multidisciplinary team that rose to the occasion. They had the focused goal to provide the highest quality and safety in obstetric care while offering compassion and empathy for the experience of having a baby during a pandemic. ●
The author would like to acknowledge Danielle Prentice, DO, and Jaimie Maines, MD, for their manuscript review.
- The requirement for reduced in-person contact due to the COVID-19 pandemic challenged our traditional obstetric care models. This led us to comprehensively incorporate technology for communication with patients and their families and to significantly alter how, where, and when we delivered prenatal care.
- Both patients and clinicians needed to adjust to the impact of these changes, especially concerning visitor policies.
- Early incorporation of universal COVID-19 testing for labor and antepartum patients was initially instituted to improve patient and staff safety and to preserve PPE. However, it quickly led to the need for various protocols for both anticipated and unanticipated clinical scenarios.
- As new data emerged and the number of cases fluctuated throughout the pandemic, our approach and protocols necessitated flexibility: Our strategy for maternal and neonatal care early in the pandemic was not the same as our current approach, and it will likely change several more times before we are done.
- One of the biggest challenges to our care team was maintaining standards of excellence and safety in obstetric care while also adhering to the physical barriers of isolation precautions and maintaining vigilance to reduce exposure risk during our routine workflow.
- The physical and operational specifics of our institution determined our approach to obstetric care during COVID-19, in part because halfway through the pandemic we moved our maternity unit from the adult hospital to a new center within our children’s hospital.
- The frequent changes in the knowledge of and recommendations for COVID-19 highlighted the importance of maintaining multidisciplinary communication on a daily, if not more frequent, basis.
Practicing evidence-based medicine, as obstetricians know, is not always possible when one does not have evidence due to lack of data or long-term experience in pregnancy. During the COVID-19 pandemic, the evidence changed so rapidly that we were compelled to alter our strategy frequently as we learned more about the impact of this disease on our vulnerable patient population. The COVID-19 pandemic taught us that, in unprecedented times, centering the safety of the patient, her child, and the health care team requires quick thinking, flexibility, and above all effective communication between team members.
Here, I share our institutional experience in providing practical obstetric care through various stages of the still-evolving COVID-19 pandemic. We based our strategy on guidance from the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG),1,2 and the Society for Maternal-Fetal Medicine (SMFM).3-5 We were reminded yet again that the only constant is change and that timely but thoughtful adjustments were needed to keep up with the coronavirus.
Changes to prenatal care
Like many others, our institution has provided continued in-person outpatient prenatal care to both our low- and high-risk patients throughout each stage of the pandemic. While continuing to provide the necessary obstetric care, we made alterations to limit exposure and practice social distancing when possible.
Limiting patient support persons. One significant change was to restrict or limit support persons in the outpatient clinics based on guidelines reflecting community infection rates. Recognizing that this was not optimal for our patients’ emotional well-being, we needed to become more flexible in using technology to include family or support persons in prenatal visits and ultrasonography exams.
Altering test frequency. Using the guidance from SMFM,1 we changed the frequency of our antenatal testing and ultrasonography exams in the following ways: We increased the duration between indicated growth ultrasonography to every 4 weeks and decreased fetal antenatal testing to weekly, with twice-weekly testing continued for the highest-risk patients. Early first-trimester ultrasonography exams were limited and, when possible, delayed until after 10 to 12 weeks’ gestation or combined with other indications (nuchal translucency). Prenatal visits for low-risk patients were spaced out using existing models if the patient was amenable, especially in early pregnancy.
Adjusting staff assignments and using telehealth. In the early part of the pandemic, we divided into 2 groups to limit the number of clinicians at any one site: a dedicated group of outpatient clinicians who saw patients in the clinic only and a dedicated group of inpatient clinicians who staffed labor and delivery and the inpatient antepartum service. Additionally, our consultative maternal-fetal medicine service transitioned to a telehealth platform and performed the majority of consults remotely. Ultrasonography exams at various sites were read remotely and pertinent findings were communicated directly to patients via phone or the telehealth platform. Amniocentesis continued to be offered.
Responding to lower COVID-19 case numbers. When the number of COVID-19 cases decreased in the summer and fall of 2020, we returned to our prepandemic in-person practices, but we continued to offer telehealth visits as an option for patients who desired it. Patients were limited to one support person.
Shifting gears again. During the second surge of COVID-19 in our region, we used our experiences from the first to transition our practices to reduce in-person contact. Appointment frequency was decreased if appropriate, and we developed a tiered system of antenatal testing frequency based on risk factors. Visitors were again restricted, with exceptions made for extenuating circumstances. Consults were transitioned to telemedicine as appropriate and ultrasonography exams were read remotely when possible to limit exposures. Given the varied experiences with telemedicine and patient preferences, patients who desired in-person consult were (and are still) offered this option.
Some patients who were interested in telehealth but unable to access the technology were offered appointments via telehealth with the use of our clinic devices. Telemedicine increased our flexibility in offering consults as one provider could see patients at different office sites in one session. We continued our routine inpatient and outpatient coverage during this time as this kept our coverage options more flexible and expanded our obstetric backup plan in response to increased rates of community infection that affected both clinicians and patients.
Coordinating care for infected patients. One vital part of our prenatal care during the COVID-19 pandemic was to coordinate with our colleagues in medical specialties to provide outpatient care for patients with confirmed or suspected COVID-19 during their period of isolation or quarantine. Patients could be seen as outpatients in a dedicated space that used appropriate personal protective equipment (PPE) for not only prenatal care but also any needed in-person evaluation for COVID-19. Our obstetric clinicians and sonographers performed exams, antenatal testing (in the form of biophysical profiles), and indicated ultrasonography exams (such as umbilical artery Doppler studies and fetal growth assessments). This required a concerted effort and excellent communication between teams to provide the necessary care in the safest manner possible.
Continue to: Universal testing on labor and delivery...
Universal testing on labor and delivery
Not surprisingly, obstetric delivery volumes in our institution were not affected in the same way as elective surgery volumes. Our inpatient team continued to bring babies into the world at the same if not a higher rate than in prepandemic times. We continued elective inductions when space allowed. Our first COVID-19–positive patient was already at 40 weeks’ gestation when the result of her test, done due to exposure, was received. Creative effort among multiple specialties quickly developed her delivery plan, and she and her infant did well.
As data started coming out of the New York City obstetric experience, concern for preservation of the PPE supply and the potential for asymptomatic/presymptomatic patients led us, in consultation with our infectious disease colleagues, to institute universal testing for all antepartum and laboring patients. At first, all patients were tested on admission with our rapid in-house test. Eventually, we moved toward preoperative testing 3 to 5 days prior to scheduled cesarean deliveries in alignment with the surgical services when elective cases were reinstituted. Finally, we instituted preprocedure testing for all scheduled labor and delivery procedures, including inductions, cerclages, and fetal blood transfusions, while we still used rapid testing for patients who presented urgently or in labor.
We needed to address several considerations almost immediately after instituting universal testing, including:
- what to do in case of patient refusal to be tested
- which precautions to institute while awaiting test results
- potential postponement of elective delivery if a patient tested positive, and
- where best to deliver patients.
What we did at the beginning of the pandemic was not necessarily the same as we do in our current practice, and we expect that our procedures may need to change in the future. Derived from what we learned from others’ experience, we tailored our protocols to our own physical space, staffing capabilities, and testing limitations. We adjusted them often, with input from multiple services, based on updated policy, recommendation for isolation and quarantine durations, rates of community infection, and changes in the unit spaces. As with many things, one protocol did not fit every patient, necessitating case-by-case flexibility.
Delivery considerations
To answer some of the above questions, all patients who declined testing, were awaiting test results while in labor, or were in triage were placed in droplet and contact isolation on our unit, a practice we continue currently. Given the concern of potential aerosolization during the second stage of labor or during intubation, for any patients in those categories who required delivery, we limited the number of staff in their rooms as possible. Additional pediatric staff waited in close proximity of the room and were ready to come in if needed depending on fetal complications and gestational age. For delivery, all team members used full special pathogens precautions (N95 masks, face shields, gowns, and gloves).
Patients who were asymptomatic and tested negative for COVID-19 had and continue to have routine care from a PPE (standard gowns, gloves, face mask, and eye protection) and health care team perspective. We have allowed visitation of one support person per hospital stay for these patients throughout the pandemic.
For the majority of our experience during the pandemic, adult patients who tested positive for COVID-19 were cohorted within dedicated negative pressure units of varying levels of care. As these units included the same intensive care unit (ICU) we utilized in non-COVID times for critical obstetric patients, we had already operationalized their use and they were wired for our electronic fetal monitoring system. These rooms are adjacent to the main operating room (OR) complex, which allows for transition to a dedicated COVID-19 OR for cesarean delivery. We worked with the primary COVID-19 team, ICU team, anesthesia, and neonatal ICU team to develop a written protocol that detailed the care for our COVID-19–positive laboring and postpartum patients in this critical care COVID-19 unit.
For a time, admitted COVID-19–positive patients were not permitted to have support persons. The health care team therefore stepped in to be the patients’ support during the delivery of their child. Care of these patients required a great deal of coordination and communication between teams as well as the addition of a dedicated obstetric physician—separate from the regular labor and delivery team—assigned to care for these patients.
For pregnant patients in the emergency room or in the intermediate or floor COVID-19 units, portable fetal monitors and ultrasonography equipment were used for obstetric consults, fetal testing, and obstetrical ultrasonography as appropriate based on gestational age and medical conditions. Again, communication between teams was essential to provide seamless and timely patient care. Patients usually were admitted to the COVID-19 teams with maternal-fetal medicine or obstetric consult teams following daily; they were admitted and transferred to the ICU COVID-19 unit if delivery was necessary. To limit exposures whenever possible, coordinated care (such as exams and telephone evaluation) was performed outside of the room with the nursing and primary teams.
Continue to: Staying flexible to the changing COVID-19 environment...
Staying flexible to the changing
COVID-19 environment
Postponed in-person visits. Whenever possible, deliveries that were not medically indicated and in-person outpatient care visits were postponed until isolation/quarantine precautions could be lifted to avoid the need for special pathogens precautions, separation of mother and infant, and visitor restrictions. We did not postpone any medically indicated deliveries or appropriate care due to COVID-19 alone. As the CDC guidelines changed regarding the timing of infectivity, we had to continually re-evaluate when a patient could return to regular outpatient care instead of the COVID-19 clinic and/or be delivered.
Mother-infant separation. As outlined in an article we wrote with our pediatric colleagues, originally all infants were immediately separated from their COVID-19–positive mothers, and delayed cord clamping was not performed.6 We adjusted our protocols as experience and data grew regarding the risk of transmission to the newborn from asymptomatic mothers and as updated recommendations were made by ACOG and the CDC. Currently, if desired, asymptomatic mothers are not separated from their well term infants. We practice our standard delayed cord clamping technique for all patients. Masking, hand hygiene, and physical distancing are used to reduce the risk of infection transmission. Breastfeeding is encouraged if the patient desires it, either directly using precautions or supported via pumping.
Reduced workplace exposure. Along with many others, we are even more cognizant of reducing the risk of workplace exposure; thus, we conduct our daily multidisciplinary huddle and physician transition of care sign-outs. We use multiple rooms for our larger group with secure video chats, and we limit huddles to a single representative from each specialty.
Medication protocols. Early in the pandemic in our area, we limited antenatal corticosteroids for fetal lung maturity to patients who were at less than 34 weeks’ gestation, per ACOG recommendations, carefully considering necessity in the critically ill. Now, we continue to administer antenatal steroids according to our usual protocols up to 36 6/7 weeks, per ACOG and SMFM recommendations, regardless of illness severity.7 Nonsteroidal anti-inflammatory drug use, once limited in COVID-19–positive patients, are now used again. Additionally, we had a comprehensive venous thromboembolism (VTE) prophylaxis protocol for our obstetric patients, and we have added special consideration for prophylaxis for patients with moderate to severe illness or other VTE risk factors. While we do not perform routine circumcisions on infants of COVID-19–positive mothers, we have a process in place to provide that service after discharge when isolation precautions are lifted.
Labor accommodations. As COVID-19 cases increased in our hospital during recent months, we made one more significant change in our care protocols. To open up space in the ICU, we moved our care for asymptomatic COVID-19–positive laboring patients to our new labor and delivery unit with implemented special pathogens precautions. This is not revolutionary; many other hospitals did not have the same capability we did with our existing collaboration with the ICU for critical obstetric care. However, this change again required communication and collaboration among multiple care teams, agreement on the qualifications for delivery on labor and delivery versus in the ICU, and physical alteration of our unit to accommodate additional isolation precautions.
Visitor policy. Another change is that we have opened up the visitor policy to welcome an asymptomatic support person for the COVID-19–positive labor patient, giving special attention to adherence to isolation precautions. Our staff members have embraced this change as they have everything else, with cautious optimism and focus on keeping both the patients and the health care team safe. Our moderate to severely ill patients continue to be cared for in the COVID-19 unit in close collaboration with our infectious disease and ICU colleagues.
It’s all about teamwork
I hope I have given a clear example of our approach to providing obstetric care in the ever-changing landscape of the COVID-19 pandemic. We embraced this period of necessary change as practically and safely as possible for both our patients and our health care workers. We learned multiple times along the way that what seemed to be a good idea was not feasible, or not the ideal option, or that COVID-19 had changed the rules of the game again. Our team met daily if not more frequently, as we found we had to constantly adapt and change to each new challenge or new clinical scenario. When we struggled, it generally related to a gap in communication.
I am privileged to work with a dedicated, selfless, multidisciplinary team that rose to the occasion. They had the focused goal to provide the highest quality and safety in obstetric care while offering compassion and empathy for the experience of having a baby during a pandemic. ●
The author would like to acknowledge Danielle Prentice, DO, and Jaimie Maines, MD, for their manuscript review.
- The requirement for reduced in-person contact due to the COVID-19 pandemic challenged our traditional obstetric care models. This led us to comprehensively incorporate technology for communication with patients and their families and to significantly alter how, where, and when we delivered prenatal care.
- Both patients and clinicians needed to adjust to the impact of these changes, especially concerning visitor policies.
- Early incorporation of universal COVID-19 testing for labor and antepartum patients was initially instituted to improve patient and staff safety and to preserve PPE. However, it quickly led to the need for various protocols for both anticipated and unanticipated clinical scenarios.
- As new data emerged and the number of cases fluctuated throughout the pandemic, our approach and protocols necessitated flexibility: Our strategy for maternal and neonatal care early in the pandemic was not the same as our current approach, and it will likely change several more times before we are done.
- One of the biggest challenges to our care team was maintaining standards of excellence and safety in obstetric care while also adhering to the physical barriers of isolation precautions and maintaining vigilance to reduce exposure risk during our routine workflow.
- The physical and operational specifics of our institution determined our approach to obstetric care during COVID-19, in part because halfway through the pandemic we moved our maternity unit from the adult hospital to a new center within our children’s hospital.
- The frequent changes in the knowledge of and recommendations for COVID-19 highlighted the importance of maintaining multidisciplinary communication on a daily, if not more frequent, basis.
- American College of Obstetricians and Gynecologists. Practice advisory: novel coronavirus 2019 (COVID-19): summary of key updates (December 14, 2020). https://www.acog.org/clinical /clinical-guidance/practice-advisory/articles/2020/03/novel -coronavirus-2019. Accessed January 28, 2021.
- American College of Obstetricians and Gynecologists. COVID19 FAQs for obstetrician-gynecologists, obstetrics. Washington, DC: ACOG; 2020. https://www.acog.org/clinical-information /physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Updated November 23, 2020. https: //s3.amazonaws.com/cdn.smfm.org/media/2589/COVID19 -What_MFMs_need_to_know_revision_11-23-20_final.pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Management considerations for pregnant patients with COVID-19. Updated January 7, 2021. https://s3.amazonaws.com/cdn.smfm.org /media/2668/SMFM_COVID_Management_of_COVID_pos _preg_patients_1-7-21_(final).pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. COVID-19 ultrasound clinical practice suggestions. Updated October 20, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2550 /Ultrasound_Covid19_Suggestions_10-20-20_(final).pdf. Accessed January 28, 2020.
- Amatya S, Corr TE, Gandhi CK, et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J Perinatol. 2020;40:987-996.
- American College of Obstetricians and Gynecologists. Committee opinion no 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130:e102-e109.
- American College of Obstetricians and Gynecologists. Practice advisory: novel coronavirus 2019 (COVID-19): summary of key updates (December 14, 2020). https://www.acog.org/clinical /clinical-guidance/practice-advisory/articles/2020/03/novel -coronavirus-2019. Accessed January 28, 2021.
- American College of Obstetricians and Gynecologists. COVID19 FAQs for obstetrician-gynecologists, obstetrics. Washington, DC: ACOG; 2020. https://www.acog.org/clinical-information /physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Updated November 23, 2020. https: //s3.amazonaws.com/cdn.smfm.org/media/2589/COVID19 -What_MFMs_need_to_know_revision_11-23-20_final.pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Management considerations for pregnant patients with COVID-19. Updated January 7, 2021. https://s3.amazonaws.com/cdn.smfm.org /media/2668/SMFM_COVID_Management_of_COVID_pos _preg_patients_1-7-21_(final).pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. COVID-19 ultrasound clinical practice suggestions. Updated October 20, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2550 /Ultrasound_Covid19_Suggestions_10-20-20_(final).pdf. Accessed January 28, 2020.
- Amatya S, Corr TE, Gandhi CK, et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J Perinatol. 2020;40:987-996.
- American College of Obstetricians and Gynecologists. Committee opinion no 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130:e102-e109.