User login
Making Breastfeeding Easier for Soldiers
The Army has revised its breastfeeding and lactation support policy by making it easier for soldiers to meet the demands of both baby and job. Commanders now will provide soldiers with a location to pump, even during field exercises, as well as a means of storage and time to transport or discard.
Other revisions include a requirement that a location to pump is not a restroom. It must be a private secure place with a seat, a shelf or other surface for the pump, an electrical outlet, and access to a reasonably close water source. The policy now also requires providing soldier with adequate time to express milk and recognizing that lactation breaks may be necessary for at least 1 year after the child is born.
“Extensive medical research has documented that breastfeeding has significant health, nutritional, immunologic, developmental, emotional, social, and economic benefits for both mother and child,” the directive says. “In light of those benefits, commanders are responsible for notifying all Soldiers of this [policy] during initial pregnancy counseling.”
The Army has revised its breastfeeding and lactation support policy by making it easier for soldiers to meet the demands of both baby and job. Commanders now will provide soldiers with a location to pump, even during field exercises, as well as a means of storage and time to transport or discard.
Other revisions include a requirement that a location to pump is not a restroom. It must be a private secure place with a seat, a shelf or other surface for the pump, an electrical outlet, and access to a reasonably close water source. The policy now also requires providing soldier with adequate time to express milk and recognizing that lactation breaks may be necessary for at least 1 year after the child is born.
“Extensive medical research has documented that breastfeeding has significant health, nutritional, immunologic, developmental, emotional, social, and economic benefits for both mother and child,” the directive says. “In light of those benefits, commanders are responsible for notifying all Soldiers of this [policy] during initial pregnancy counseling.”
The Army has revised its breastfeeding and lactation support policy by making it easier for soldiers to meet the demands of both baby and job. Commanders now will provide soldiers with a location to pump, even during field exercises, as well as a means of storage and time to transport or discard.
Other revisions include a requirement that a location to pump is not a restroom. It must be a private secure place with a seat, a shelf or other surface for the pump, an electrical outlet, and access to a reasonably close water source. The policy now also requires providing soldier with adequate time to express milk and recognizing that lactation breaks may be necessary for at least 1 year after the child is born.
“Extensive medical research has documented that breastfeeding has significant health, nutritional, immunologic, developmental, emotional, social, and economic benefits for both mother and child,” the directive says. “In light of those benefits, commanders are responsible for notifying all Soldiers of this [policy] during initial pregnancy counseling.”
VA Pledges to Strengthen Veterans Crisis Line Operations in New York
The VA Office of Inspector General (OIG) released a report of its investigation of quality assurance concerns regarding the Veterans Crisis Line (VCL) in Canandaigua, New York. The investigation began in 2014. Allegations of calls going unanswered or answered by voicemail and improperly trained responders sparked the investigation.
“It is important for veterans and our key stakeholders to know that VA undertook actions to strengthen Veterans Crisis Line operations long before publication of the inspector general report,” Deputy Secretary Sloan Gibson responded in a blog post. Gibson insisted that the VA will “continue that work until the Veterans Crisis Line is the world-class crisis response center veterans deserve.”
The OIG report stated that it “substantiated allegations that some calls routed to backup crisis centers were answered by voicemail, and callers did not always receive immediate assistance from VCL and/or backup center staff.” The investigation also found that the VCL management team did not provide adequate orientation and ongoing training for social service assistants.
Upon the investigation’s completion in fiscal year 2015, the OIG made 7 recommendations to improve the quality of the VCL service for veterans and their families. The executive director of the Office of Mental Health Operations (OMHO) concurred with all 7 and implemented steps to ensure compliance.
For example, OMHO and VCL staff now must submit daily, weekly, and monthly reports that track system issues—including response hold times. The VCL also hired 68 additional crisis line responders, and VCL policy now states that backup centers cannot place callers on hold. Routing “rollover” calls also ensures that no caller waits longer than 2 minutes.
The VCL service has seen its usage grow by 467,000 annual calls from 2008 to 2015. Spurred by this increase in call volume, the VCL also made technology investments last year that upgraded its phone systems and allows for a higher volume of calls.
A New Employee Orientation program, updated employee handbook, and a formal quality assurance program combine to ensure that all VCL employees receive proper training. A VCL Talent Management System also will track and record all employees’ performance data for frequent analysis.
All 7 recommendations are scheduled to be fulfilled by September 30, 2016.
The VA Office of Inspector General (OIG) released a report of its investigation of quality assurance concerns regarding the Veterans Crisis Line (VCL) in Canandaigua, New York. The investigation began in 2014. Allegations of calls going unanswered or answered by voicemail and improperly trained responders sparked the investigation.
“It is important for veterans and our key stakeholders to know that VA undertook actions to strengthen Veterans Crisis Line operations long before publication of the inspector general report,” Deputy Secretary Sloan Gibson responded in a blog post. Gibson insisted that the VA will “continue that work until the Veterans Crisis Line is the world-class crisis response center veterans deserve.”
The OIG report stated that it “substantiated allegations that some calls routed to backup crisis centers were answered by voicemail, and callers did not always receive immediate assistance from VCL and/or backup center staff.” The investigation also found that the VCL management team did not provide adequate orientation and ongoing training for social service assistants.
Upon the investigation’s completion in fiscal year 2015, the OIG made 7 recommendations to improve the quality of the VCL service for veterans and their families. The executive director of the Office of Mental Health Operations (OMHO) concurred with all 7 and implemented steps to ensure compliance.
For example, OMHO and VCL staff now must submit daily, weekly, and monthly reports that track system issues—including response hold times. The VCL also hired 68 additional crisis line responders, and VCL policy now states that backup centers cannot place callers on hold. Routing “rollover” calls also ensures that no caller waits longer than 2 minutes.
The VCL service has seen its usage grow by 467,000 annual calls from 2008 to 2015. Spurred by this increase in call volume, the VCL also made technology investments last year that upgraded its phone systems and allows for a higher volume of calls.
A New Employee Orientation program, updated employee handbook, and a formal quality assurance program combine to ensure that all VCL employees receive proper training. A VCL Talent Management System also will track and record all employees’ performance data for frequent analysis.
All 7 recommendations are scheduled to be fulfilled by September 30, 2016.
The VA Office of Inspector General (OIG) released a report of its investigation of quality assurance concerns regarding the Veterans Crisis Line (VCL) in Canandaigua, New York. The investigation began in 2014. Allegations of calls going unanswered or answered by voicemail and improperly trained responders sparked the investigation.
“It is important for veterans and our key stakeholders to know that VA undertook actions to strengthen Veterans Crisis Line operations long before publication of the inspector general report,” Deputy Secretary Sloan Gibson responded in a blog post. Gibson insisted that the VA will “continue that work until the Veterans Crisis Line is the world-class crisis response center veterans deserve.”
The OIG report stated that it “substantiated allegations that some calls routed to backup crisis centers were answered by voicemail, and callers did not always receive immediate assistance from VCL and/or backup center staff.” The investigation also found that the VCL management team did not provide adequate orientation and ongoing training for social service assistants.
Upon the investigation’s completion in fiscal year 2015, the OIG made 7 recommendations to improve the quality of the VCL service for veterans and their families. The executive director of the Office of Mental Health Operations (OMHO) concurred with all 7 and implemented steps to ensure compliance.
For example, OMHO and VCL staff now must submit daily, weekly, and monthly reports that track system issues—including response hold times. The VCL also hired 68 additional crisis line responders, and VCL policy now states that backup centers cannot place callers on hold. Routing “rollover” calls also ensures that no caller waits longer than 2 minutes.
The VCL service has seen its usage grow by 467,000 annual calls from 2008 to 2015. Spurred by this increase in call volume, the VCL also made technology investments last year that upgraded its phone systems and allows for a higher volume of calls.
A New Employee Orientation program, updated employee handbook, and a formal quality assurance program combine to ensure that all VCL employees receive proper training. A VCL Talent Management System also will track and record all employees’ performance data for frequent analysis.
All 7 recommendations are scheduled to be fulfilled by September 30, 2016.
HIV Antibody Infusion Safely Reduces Viral Load
According to a small study reported by the NIH, antibody infusions dramatically suppressed the level of HIV virus in patients not taking antiretroviral therapy (ART).
The phase 1 clinical trial at the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases (NIAID) involved 23 HIV-infected people, of whom 15 were taking ART and 8 were not. Patients on ART were given 2 infusions of VRC01 28 days apart, those not on ART received 1 antibody infusion. The researchers say infusing the antibodies into a vein or under the skin was safe and well tolerated.
Related: Initiatives Aim at Improving HIV and Mental Health Services
The antibody infusions did not reduce the amount of HIV in the blood cells, but these infusions reduced plasma viral load by 10-fold in 6 patients not on ART. The antibody also did not appear to have any effect in people taking ART whose virus was already suppressed.
Related:Anthrax Antitoxin Drugs Added to the Stockpile
In 2 patients who began with the lowest viral loads, the antibody suppressed HIV to extremely low levels for approximately 3 weeks or as long as VRC01 was present at therapeutic concentrations. In 4 other people whose HIV levels declined, viral load fell “substantially” although not to undetectable levels. In 2 people not on ART, viral loads remained steady. The researchers subsequently found that the predominant HIV strain in these patients’ bodies had been resistant to VRC01 at the outset.
According to a small study reported by the NIH, antibody infusions dramatically suppressed the level of HIV virus in patients not taking antiretroviral therapy (ART).
The phase 1 clinical trial at the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases (NIAID) involved 23 HIV-infected people, of whom 15 were taking ART and 8 were not. Patients on ART were given 2 infusions of VRC01 28 days apart, those not on ART received 1 antibody infusion. The researchers say infusing the antibodies into a vein or under the skin was safe and well tolerated.
Related: Initiatives Aim at Improving HIV and Mental Health Services
The antibody infusions did not reduce the amount of HIV in the blood cells, but these infusions reduced plasma viral load by 10-fold in 6 patients not on ART. The antibody also did not appear to have any effect in people taking ART whose virus was already suppressed.
Related:Anthrax Antitoxin Drugs Added to the Stockpile
In 2 patients who began with the lowest viral loads, the antibody suppressed HIV to extremely low levels for approximately 3 weeks or as long as VRC01 was present at therapeutic concentrations. In 4 other people whose HIV levels declined, viral load fell “substantially” although not to undetectable levels. In 2 people not on ART, viral loads remained steady. The researchers subsequently found that the predominant HIV strain in these patients’ bodies had been resistant to VRC01 at the outset.
According to a small study reported by the NIH, antibody infusions dramatically suppressed the level of HIV virus in patients not taking antiretroviral therapy (ART).
The phase 1 clinical trial at the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases (NIAID) involved 23 HIV-infected people, of whom 15 were taking ART and 8 were not. Patients on ART were given 2 infusions of VRC01 28 days apart, those not on ART received 1 antibody infusion. The researchers say infusing the antibodies into a vein or under the skin was safe and well tolerated.
Related: Initiatives Aim at Improving HIV and Mental Health Services
The antibody infusions did not reduce the amount of HIV in the blood cells, but these infusions reduced plasma viral load by 10-fold in 6 patients not on ART. The antibody also did not appear to have any effect in people taking ART whose virus was already suppressed.
Related:Anthrax Antitoxin Drugs Added to the Stockpile
In 2 patients who began with the lowest viral loads, the antibody suppressed HIV to extremely low levels for approximately 3 weeks or as long as VRC01 was present at therapeutic concentrations. In 4 other people whose HIV levels declined, viral load fell “substantially” although not to undetectable levels. In 2 people not on ART, viral loads remained steady. The researchers subsequently found that the predominant HIV strain in these patients’ bodies had been resistant to VRC01 at the outset.
White House Places All Hands On Deck to Combat Emerging Zika Threat
As the Zika virus continues to emerge as a potential health risk within the U.S., the Obama administration announced its intention to ask Congress for $1.8 billion to respond to the Zika virus threat. The White House summary stressed the need to accelerate research efforts, increase the availability of diagnostic tests, develop vaccines and treatment plans, and bolster education and prevention efforts.
Related:Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Pending Congressional approval, the Department of Health and Human Services (HHS) will receive the lion’s share of these funds—nearly $1.5 billion. Given its prior experience in treating the virus, HHS has already asked experts within the DoD to support its research.
“This is an area where the DoD has done some research in the past, and I think some of that expertise will be brought to this effort," Pentagon Press Secretary Peter Cook said in a briefing. "We'll be supporting HHS in whatever way we can."
Related:The Role of Procalcitonin in the Management of Infectious Diseases
The announcement comes after a meeting attended by President Obama and leaders of both the DoD and HHS. Topics of discussion included how the Zika virus is transmitted and how widespread that transmission could be. A summary of the meeting shows that attendees discussed how to protect the American public effectively. Attendees also discussed the need to accelerate treatment research to provide guidance for health care providers who care for pregnant women—particularly given the virus’ potential links to birth defects and Guillain-Barré syndrome.
Related: White House Budget Invests in Cancer, VA Hiring, and TRICARE
The Centers for Disease Control and Prevention has issued a travel alert for people traveling to regions and countries where Zika virus transmission is ongoing: Brazil, Colombia, El Salvador, French Guiana, Guatemala, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Suriname, Venezuela, and Puerto Rico.
As the Zika virus continues to emerge as a potential health risk within the U.S., the Obama administration announced its intention to ask Congress for $1.8 billion to respond to the Zika virus threat. The White House summary stressed the need to accelerate research efforts, increase the availability of diagnostic tests, develop vaccines and treatment plans, and bolster education and prevention efforts.
Related:Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Pending Congressional approval, the Department of Health and Human Services (HHS) will receive the lion’s share of these funds—nearly $1.5 billion. Given its prior experience in treating the virus, HHS has already asked experts within the DoD to support its research.
“This is an area where the DoD has done some research in the past, and I think some of that expertise will be brought to this effort," Pentagon Press Secretary Peter Cook said in a briefing. "We'll be supporting HHS in whatever way we can."
Related:The Role of Procalcitonin in the Management of Infectious Diseases
The announcement comes after a meeting attended by President Obama and leaders of both the DoD and HHS. Topics of discussion included how the Zika virus is transmitted and how widespread that transmission could be. A summary of the meeting shows that attendees discussed how to protect the American public effectively. Attendees also discussed the need to accelerate treatment research to provide guidance for health care providers who care for pregnant women—particularly given the virus’ potential links to birth defects and Guillain-Barré syndrome.
Related: White House Budget Invests in Cancer, VA Hiring, and TRICARE
The Centers for Disease Control and Prevention has issued a travel alert for people traveling to regions and countries where Zika virus transmission is ongoing: Brazil, Colombia, El Salvador, French Guiana, Guatemala, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Suriname, Venezuela, and Puerto Rico.
As the Zika virus continues to emerge as a potential health risk within the U.S., the Obama administration announced its intention to ask Congress for $1.8 billion to respond to the Zika virus threat. The White House summary stressed the need to accelerate research efforts, increase the availability of diagnostic tests, develop vaccines and treatment plans, and bolster education and prevention efforts.
Related:Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Pending Congressional approval, the Department of Health and Human Services (HHS) will receive the lion’s share of these funds—nearly $1.5 billion. Given its prior experience in treating the virus, HHS has already asked experts within the DoD to support its research.
“This is an area where the DoD has done some research in the past, and I think some of that expertise will be brought to this effort," Pentagon Press Secretary Peter Cook said in a briefing. "We'll be supporting HHS in whatever way we can."
Related:The Role of Procalcitonin in the Management of Infectious Diseases
The announcement comes after a meeting attended by President Obama and leaders of both the DoD and HHS. Topics of discussion included how the Zika virus is transmitted and how widespread that transmission could be. A summary of the meeting shows that attendees discussed how to protect the American public effectively. Attendees also discussed the need to accelerate treatment research to provide guidance for health care providers who care for pregnant women—particularly given the virus’ potential links to birth defects and Guillain-Barré syndrome.
Related: White House Budget Invests in Cancer, VA Hiring, and TRICARE
The Centers for Disease Control and Prevention has issued a travel alert for people traveling to regions and countries where Zika virus transmission is ongoing: Brazil, Colombia, El Salvador, French Guiana, Guatemala, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Suriname, Venezuela, and Puerto Rico.
VA Facilities Compare Favorably in Large-Scale Comparative Study
A study that compared mortality and readmission rates at VA and non-VA hospitals found little differences in older male patients with acute myocardial infarction (AMI), heart failure (HF), or pneumonia. The JAMA study examined records for 104 VA and 1,513 non-VA hospitals between 2010 and 2013 and compared patient populations in the same metropolitan statistical areas (MSAs).
The finding are “reassuring” according to Ashish Jha, MD, MPH, in an accompanying editorial, noting that “even though the VA has much work to do, it is starting off from a substantially better place than it was 2 decades ago.”
Among men aged ≥ 65 years with AMI, HF, or pneumonia, hospitalization at VA hospitals was associated with “lower 30-day risk-standardized all-cause mortality rates for AMI and HF.” However, as the authors report, the 30-day, risk-standardized all-cause readmission rate for patients with all 3 conditions was higher. These trends were seen both nationally and within similar geographic areas. The authors cautioned that the “absolute differences between these outcomes at VA and non-VA hospitals were small.”
Mortality rates were lower at VA hospitals for patients with AMI and HF (13.5% and 11.4%, respectively) than those for non-VA hospitals (13.7% and 11.9%, respectively). Readmission rates were reversed for pneumonia with the VA facilities lagging behind (12.6% vs 12.2%). However, the VA also lagged behind in readmission rates for AMI (17.8% vs 17.2%), HF (24.7% vs 23.5%), and pneumonia (19.4% vs 18.7%).
Success is significant with respect to mortality rates for the VA. As Jha suggests, readmission rates largely reflect “how sick and poor the patient population is, not how good or how integrated the care is.” Still the research examined only a small cross-section of VA health care, which suggests the need for more research comparing VA and non-VA facilities
A study that compared mortality and readmission rates at VA and non-VA hospitals found little differences in older male patients with acute myocardial infarction (AMI), heart failure (HF), or pneumonia. The JAMA study examined records for 104 VA and 1,513 non-VA hospitals between 2010 and 2013 and compared patient populations in the same metropolitan statistical areas (MSAs).
The finding are “reassuring” according to Ashish Jha, MD, MPH, in an accompanying editorial, noting that “even though the VA has much work to do, it is starting off from a substantially better place than it was 2 decades ago.”
Among men aged ≥ 65 years with AMI, HF, or pneumonia, hospitalization at VA hospitals was associated with “lower 30-day risk-standardized all-cause mortality rates for AMI and HF.” However, as the authors report, the 30-day, risk-standardized all-cause readmission rate for patients with all 3 conditions was higher. These trends were seen both nationally and within similar geographic areas. The authors cautioned that the “absolute differences between these outcomes at VA and non-VA hospitals were small.”
Mortality rates were lower at VA hospitals for patients with AMI and HF (13.5% and 11.4%, respectively) than those for non-VA hospitals (13.7% and 11.9%, respectively). Readmission rates were reversed for pneumonia with the VA facilities lagging behind (12.6% vs 12.2%). However, the VA also lagged behind in readmission rates for AMI (17.8% vs 17.2%), HF (24.7% vs 23.5%), and pneumonia (19.4% vs 18.7%).
Success is significant with respect to mortality rates for the VA. As Jha suggests, readmission rates largely reflect “how sick and poor the patient population is, not how good or how integrated the care is.” Still the research examined only a small cross-section of VA health care, which suggests the need for more research comparing VA and non-VA facilities
A study that compared mortality and readmission rates at VA and non-VA hospitals found little differences in older male patients with acute myocardial infarction (AMI), heart failure (HF), or pneumonia. The JAMA study examined records for 104 VA and 1,513 non-VA hospitals between 2010 and 2013 and compared patient populations in the same metropolitan statistical areas (MSAs).
The finding are “reassuring” according to Ashish Jha, MD, MPH, in an accompanying editorial, noting that “even though the VA has much work to do, it is starting off from a substantially better place than it was 2 decades ago.”
Among men aged ≥ 65 years with AMI, HF, or pneumonia, hospitalization at VA hospitals was associated with “lower 30-day risk-standardized all-cause mortality rates for AMI and HF.” However, as the authors report, the 30-day, risk-standardized all-cause readmission rate for patients with all 3 conditions was higher. These trends were seen both nationally and within similar geographic areas. The authors cautioned that the “absolute differences between these outcomes at VA and non-VA hospitals were small.”
Mortality rates were lower at VA hospitals for patients with AMI and HF (13.5% and 11.4%, respectively) than those for non-VA hospitals (13.7% and 11.9%, respectively). Readmission rates were reversed for pneumonia with the VA facilities lagging behind (12.6% vs 12.2%). However, the VA also lagged behind in readmission rates for AMI (17.8% vs 17.2%), HF (24.7% vs 23.5%), and pneumonia (19.4% vs 18.7%).
Success is significant with respect to mortality rates for the VA. As Jha suggests, readmission rates largely reflect “how sick and poor the patient population is, not how good or how integrated the care is.” Still the research examined only a small cross-section of VA health care, which suggests the need for more research comparing VA and non-VA facilities
White House Budget Invests in Cancer, VA Hiring, and TRICARE
President Barak Obama released a $4 trillion national budget proposal yesterday, which included a request for $78.7 billion in discretionary funding for VA health care—a 5% rise over the 2016 budget. Nearly half the budget’s discretionary spending would go to a beefed-up DoD, although health care spending is a small fraction of that total. The budget is not expected to pass the Republican-led House of Representatives, but it offers a road map for the President’s priorities during his final year in office.
One of these priorities is precision medicine and fighting cancer. The National Institutes of Health (NIH) would receive $195 million in new cancer initiatives for the cancer “moonshot;” another $33.1 billion would support biomedical research through NIH, providing “about 10,000 new and competing NIH grants that will help us better understand the fundamental causes and mechanisms of disease, like the BRAIN Initiative and Precision Medicine.”
Hiring more health care providers remains a top priority for the VA, which expects to spend $1.4 billion in 2016 and $853 million in 2017 on 9,700 new medical care staff hired through the Veterans Choice Act. The VA also targets more than $1 billion for facility improvements over the course of 2016 and 2017.
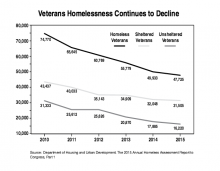
The proposed budget also dedicated $1.6 billion for fully eliminating veteran homelessness. While the President’s time line has slipped from 2015 to 2020, the White House is still focused on the topic. Most of that funding will go toward housing vouchers and rapid rehousing and includes $300 million for Supportive Services for Veteran Families, $496 million for VA case management services for at-risk veterans, and $247 million in grant and per diem payments that support temporary housing provided by community-based organizations.
The budget proposal also noted the recent efforts of the DoD to modernize and streamline its health care operations under the Defense Health Agency. Further goals outlined within the budget proposal call for the DoD to “simplify TRICARE” and add choices for beneficiaries—including TRICARE for life—to better use existing military treatment resources.
President Barak Obama released a $4 trillion national budget proposal yesterday, which included a request for $78.7 billion in discretionary funding for VA health care—a 5% rise over the 2016 budget. Nearly half the budget’s discretionary spending would go to a beefed-up DoD, although health care spending is a small fraction of that total. The budget is not expected to pass the Republican-led House of Representatives, but it offers a road map for the President’s priorities during his final year in office.
One of these priorities is precision medicine and fighting cancer. The National Institutes of Health (NIH) would receive $195 million in new cancer initiatives for the cancer “moonshot;” another $33.1 billion would support biomedical research through NIH, providing “about 10,000 new and competing NIH grants that will help us better understand the fundamental causes and mechanisms of disease, like the BRAIN Initiative and Precision Medicine.”
Hiring more health care providers remains a top priority for the VA, which expects to spend $1.4 billion in 2016 and $853 million in 2017 on 9,700 new medical care staff hired through the Veterans Choice Act. The VA also targets more than $1 billion for facility improvements over the course of 2016 and 2017.
The proposed budget also dedicated $1.6 billion for fully eliminating veteran homelessness. While the President’s time line has slipped from 2015 to 2020, the White House is still focused on the topic. Most of that funding will go toward housing vouchers and rapid rehousing and includes $300 million for Supportive Services for Veteran Families, $496 million for VA case management services for at-risk veterans, and $247 million in grant and per diem payments that support temporary housing provided by community-based organizations.
The budget proposal also noted the recent efforts of the DoD to modernize and streamline its health care operations under the Defense Health Agency. Further goals outlined within the budget proposal call for the DoD to “simplify TRICARE” and add choices for beneficiaries—including TRICARE for life—to better use existing military treatment resources.
President Barak Obama released a $4 trillion national budget proposal yesterday, which included a request for $78.7 billion in discretionary funding for VA health care—a 5% rise over the 2016 budget. Nearly half the budget’s discretionary spending would go to a beefed-up DoD, although health care spending is a small fraction of that total. The budget is not expected to pass the Republican-led House of Representatives, but it offers a road map for the President’s priorities during his final year in office.
One of these priorities is precision medicine and fighting cancer. The National Institutes of Health (NIH) would receive $195 million in new cancer initiatives for the cancer “moonshot;” another $33.1 billion would support biomedical research through NIH, providing “about 10,000 new and competing NIH grants that will help us better understand the fundamental causes and mechanisms of disease, like the BRAIN Initiative and Precision Medicine.”
Hiring more health care providers remains a top priority for the VA, which expects to spend $1.4 billion in 2016 and $853 million in 2017 on 9,700 new medical care staff hired through the Veterans Choice Act. The VA also targets more than $1 billion for facility improvements over the course of 2016 and 2017.
The proposed budget also dedicated $1.6 billion for fully eliminating veteran homelessness. While the President’s time line has slipped from 2015 to 2020, the White House is still focused on the topic. Most of that funding will go toward housing vouchers and rapid rehousing and includes $300 million for Supportive Services for Veteran Families, $496 million for VA case management services for at-risk veterans, and $247 million in grant and per diem payments that support temporary housing provided by community-based organizations.
The budget proposal also noted the recent efforts of the DoD to modernize and streamline its health care operations under the Defense Health Agency. Further goals outlined within the budget proposal call for the DoD to “simplify TRICARE” and add choices for beneficiaries—including TRICARE for life—to better use existing military treatment resources.
Home-Visiting Program to Support Young Native American Families
The Denan Project, a Connecticut-based nonprofit, has begun training more than 20 Native American medical community health nurses, paraprofessionals, and health technicians to support young parents and their families. Home-based lessons will be given to expectant and young mothers from pregnancy to 3 years postpartum. By the end of the year the program will support 150 at-risk families.
The Denan Project is an all-volunteer community-based organization that provides health and development assistance to underserved people in remote areas. It is committing financial resources for more than 2 years to support the Family Spirit program of the Johns Hopkins Center for American Indian Health. Family Spirit is an evidence-based and culturally tailored home-visiting program delivered by Native American paraprofessionals. It operates in 59 reservation and urban Native American communities in 14 states. After 12 years of operation in Africa, Asia, and Latin America, the Denan Project launches its first US-based program with training in the Navajo communities of Chinle, Pinon, and Tsaile, in Arizona.
With a mission to provide help to people living in the most remote and poorest places in the world, Dick Young, president and founder said, “we felt it was right to work closer to home and identified the Family Spirit initiative as an excellent partner.”
The Denan Project, a Connecticut-based nonprofit, has begun training more than 20 Native American medical community health nurses, paraprofessionals, and health technicians to support young parents and their families. Home-based lessons will be given to expectant and young mothers from pregnancy to 3 years postpartum. By the end of the year the program will support 150 at-risk families.
The Denan Project is an all-volunteer community-based organization that provides health and development assistance to underserved people in remote areas. It is committing financial resources for more than 2 years to support the Family Spirit program of the Johns Hopkins Center for American Indian Health. Family Spirit is an evidence-based and culturally tailored home-visiting program delivered by Native American paraprofessionals. It operates in 59 reservation and urban Native American communities in 14 states. After 12 years of operation in Africa, Asia, and Latin America, the Denan Project launches its first US-based program with training in the Navajo communities of Chinle, Pinon, and Tsaile, in Arizona.
With a mission to provide help to people living in the most remote and poorest places in the world, Dick Young, president and founder said, “we felt it was right to work closer to home and identified the Family Spirit initiative as an excellent partner.”
The Denan Project, a Connecticut-based nonprofit, has begun training more than 20 Native American medical community health nurses, paraprofessionals, and health technicians to support young parents and their families. Home-based lessons will be given to expectant and young mothers from pregnancy to 3 years postpartum. By the end of the year the program will support 150 at-risk families.
The Denan Project is an all-volunteer community-based organization that provides health and development assistance to underserved people in remote areas. It is committing financial resources for more than 2 years to support the Family Spirit program of the Johns Hopkins Center for American Indian Health. Family Spirit is an evidence-based and culturally tailored home-visiting program delivered by Native American paraprofessionals. It operates in 59 reservation and urban Native American communities in 14 states. After 12 years of operation in Africa, Asia, and Latin America, the Denan Project launches its first US-based program with training in the Navajo communities of Chinle, Pinon, and Tsaile, in Arizona.
With a mission to provide help to people living in the most remote and poorest places in the world, Dick Young, president and founder said, “we felt it was right to work closer to home and identified the Family Spirit initiative as an excellent partner.”
VA/DoD to Help Lead New Cancer Initiative
President Barak Obama plans to ask Congress for an additional $1 billion for a sweeping effort to cure cancer. The White House Cancer Moonshot Task Force aims to “bring about a decade’s worth of advances in five years.” The task force will include representatives from the VA, DoD, FDA, HHS, National Cancer Institute, National Institutes of Health (NIH), among other agencies. The White House has already outlined the roles for the VA, DoD, and HHS.
The White House specifically referenced 3 VA programs that will play important roles in the initiative. For example, the Million Veteran Program (MVP) includes genetic information from more than 445,000 veterans, and nearly one-third of participants have a cancer diagnosis. According to the White House, MVP may provide a potential rich clinical database for genetic exploration and analyses. Moreover, the Precision Oncology Program (POP), which is part of the Massachusetts Veterans Epidemiology Research and Information Center, has already developed a mechanism to capture genetic information and match patients with appropriate clinical trials. In addition, the VA National Radiation Oncology Program conducts multiple initiatives in cancer research. In all, the VA has nearly 250 research projects related to cancer, which include 170 clinical studies.
With 3 Cancer Centers of Excellence and the Murtha Cancer Treatment Center at the Walter Reed National Military Medical Center, the DoD also will play an important role in the moonshot, according to the White House. The DoD already receives Congressional Special Initiative funding for research, much of which is focused on treatment of highly prevalent forms of cancer or others associated with exposure to hazardous materials that some service members may encounter while on duty.
According to the White House, The Moonshot initiative will begin with a request for $195 million in new cancer activities at the NIH in fiscal year 2016 and another $755 million in mandatory funds for new cancer-related research activities at both NIH and the FDA.
“The goal of this initiative — this ‘Moonshot’ — is to seize this moment,” Biden explained in a blog post announcing the initiative. “To accelerate our efforts to progress towards a cure, and to unleash new discoveries and breakthroughs for other deadly diseases.”
Biden pledged that the federal government “will do everything it possibly can— through funding, targeted incentives, and increased private-sector coordination — to support research and enable progress.” Later this month Biden will meet with cabinet secretaries and heads of relevant agencies to discuss ways to improve federal investment and support of cancer research and treatment.
President Barak Obama plans to ask Congress for an additional $1 billion for a sweeping effort to cure cancer. The White House Cancer Moonshot Task Force aims to “bring about a decade’s worth of advances in five years.” The task force will include representatives from the VA, DoD, FDA, HHS, National Cancer Institute, National Institutes of Health (NIH), among other agencies. The White House has already outlined the roles for the VA, DoD, and HHS.
The White House specifically referenced 3 VA programs that will play important roles in the initiative. For example, the Million Veteran Program (MVP) includes genetic information from more than 445,000 veterans, and nearly one-third of participants have a cancer diagnosis. According to the White House, MVP may provide a potential rich clinical database for genetic exploration and analyses. Moreover, the Precision Oncology Program (POP), which is part of the Massachusetts Veterans Epidemiology Research and Information Center, has already developed a mechanism to capture genetic information and match patients with appropriate clinical trials. In addition, the VA National Radiation Oncology Program conducts multiple initiatives in cancer research. In all, the VA has nearly 250 research projects related to cancer, which include 170 clinical studies.
With 3 Cancer Centers of Excellence and the Murtha Cancer Treatment Center at the Walter Reed National Military Medical Center, the DoD also will play an important role in the moonshot, according to the White House. The DoD already receives Congressional Special Initiative funding for research, much of which is focused on treatment of highly prevalent forms of cancer or others associated with exposure to hazardous materials that some service members may encounter while on duty.
According to the White House, The Moonshot initiative will begin with a request for $195 million in new cancer activities at the NIH in fiscal year 2016 and another $755 million in mandatory funds for new cancer-related research activities at both NIH and the FDA.
“The goal of this initiative — this ‘Moonshot’ — is to seize this moment,” Biden explained in a blog post announcing the initiative. “To accelerate our efforts to progress towards a cure, and to unleash new discoveries and breakthroughs for other deadly diseases.”
Biden pledged that the federal government “will do everything it possibly can— through funding, targeted incentives, and increased private-sector coordination — to support research and enable progress.” Later this month Biden will meet with cabinet secretaries and heads of relevant agencies to discuss ways to improve federal investment and support of cancer research and treatment.
President Barak Obama plans to ask Congress for an additional $1 billion for a sweeping effort to cure cancer. The White House Cancer Moonshot Task Force aims to “bring about a decade’s worth of advances in five years.” The task force will include representatives from the VA, DoD, FDA, HHS, National Cancer Institute, National Institutes of Health (NIH), among other agencies. The White House has already outlined the roles for the VA, DoD, and HHS.
The White House specifically referenced 3 VA programs that will play important roles in the initiative. For example, the Million Veteran Program (MVP) includes genetic information from more than 445,000 veterans, and nearly one-third of participants have a cancer diagnosis. According to the White House, MVP may provide a potential rich clinical database for genetic exploration and analyses. Moreover, the Precision Oncology Program (POP), which is part of the Massachusetts Veterans Epidemiology Research and Information Center, has already developed a mechanism to capture genetic information and match patients with appropriate clinical trials. In addition, the VA National Radiation Oncology Program conducts multiple initiatives in cancer research. In all, the VA has nearly 250 research projects related to cancer, which include 170 clinical studies.
With 3 Cancer Centers of Excellence and the Murtha Cancer Treatment Center at the Walter Reed National Military Medical Center, the DoD also will play an important role in the moonshot, according to the White House. The DoD already receives Congressional Special Initiative funding for research, much of which is focused on treatment of highly prevalent forms of cancer or others associated with exposure to hazardous materials that some service members may encounter while on duty.
According to the White House, The Moonshot initiative will begin with a request for $195 million in new cancer activities at the NIH in fiscal year 2016 and another $755 million in mandatory funds for new cancer-related research activities at both NIH and the FDA.
“The goal of this initiative — this ‘Moonshot’ — is to seize this moment,” Biden explained in a blog post announcing the initiative. “To accelerate our efforts to progress towards a cure, and to unleash new discoveries and breakthroughs for other deadly diseases.”
Biden pledged that the federal government “will do everything it possibly can— through funding, targeted incentives, and increased private-sector coordination — to support research and enable progress.” Later this month Biden will meet with cabinet secretaries and heads of relevant agencies to discuss ways to improve federal investment and support of cancer research and treatment.
CDC Reviews a Year of Health ‘Nightmares’
The CDC recently released an article titled 2015: What Kept Us Up at Night and What Will Keep Us Busy in 2016, which lists antibiotic resistance as a top concern. According to the CDC, in 2015 more than 23,000 Americans died from these “largely preventable” infections; but it “learned that when health care facilities coordinate their efforts, they can prevent the spread of nightmare bacteria resistant to most antibiotics.” Newly published guidelines intended to support better communication and prevent the spread of bacteria provide instructions on how state and local health departments can alert local facilities when antibiotic-resistant bacteria are reported in their area.
In 2016, the CDC will aim to reverse the number of deaths from infections resistant to antibiotics. The next steps include the debut of the AR Patient Safety Atlas, an interactive web platform with open access to antibiotic resistance data on healthcare-associated infections reported to the National Healthcare Safety Network.
This year the CDC also will release the first antibiotic stewardship report on progress in prescribing practices. “We must preserve these miracle medications,” the year-end review says, “so we can avoid returning to the pre-antibiotic era when minor infections often led to death.”
The CDC recently released an article titled 2015: What Kept Us Up at Night and What Will Keep Us Busy in 2016, which lists antibiotic resistance as a top concern. According to the CDC, in 2015 more than 23,000 Americans died from these “largely preventable” infections; but it “learned that when health care facilities coordinate their efforts, they can prevent the spread of nightmare bacteria resistant to most antibiotics.” Newly published guidelines intended to support better communication and prevent the spread of bacteria provide instructions on how state and local health departments can alert local facilities when antibiotic-resistant bacteria are reported in their area.
In 2016, the CDC will aim to reverse the number of deaths from infections resistant to antibiotics. The next steps include the debut of the AR Patient Safety Atlas, an interactive web platform with open access to antibiotic resistance data on healthcare-associated infections reported to the National Healthcare Safety Network.
This year the CDC also will release the first antibiotic stewardship report on progress in prescribing practices. “We must preserve these miracle medications,” the year-end review says, “so we can avoid returning to the pre-antibiotic era when minor infections often led to death.”
The CDC recently released an article titled 2015: What Kept Us Up at Night and What Will Keep Us Busy in 2016, which lists antibiotic resistance as a top concern. According to the CDC, in 2015 more than 23,000 Americans died from these “largely preventable” infections; but it “learned that when health care facilities coordinate their efforts, they can prevent the spread of nightmare bacteria resistant to most antibiotics.” Newly published guidelines intended to support better communication and prevent the spread of bacteria provide instructions on how state and local health departments can alert local facilities when antibiotic-resistant bacteria are reported in their area.
In 2016, the CDC will aim to reverse the number of deaths from infections resistant to antibiotics. The next steps include the debut of the AR Patient Safety Atlas, an interactive web platform with open access to antibiotic resistance data on healthcare-associated infections reported to the National Healthcare Safety Network.
This year the CDC also will release the first antibiotic stewardship report on progress in prescribing practices. “We must preserve these miracle medications,” the year-end review says, “so we can avoid returning to the pre-antibiotic era when minor infections often led to death.”
DoD Increases Support Provided to Military Families
The DoD highlighted reforms to its Force of the Future program that are designed to improve the quality of life for military personnel with or planning families. Female members of the military will now receive up to 12 weeks paid maternity leave in 2016, regardless of the military branch. Previously each of the services had established different policies, ranging from 18 weeks in the Navy and Marines to just 6 weeks in the Army.
During a briefing at the Pentagon on January 28, Defense Secretary Ash Carter noted that the goal of this action was increase the support provided to military families while also improving retention.
"Our calculation is quite simple, we want our people to be able to balance two of the most solemn commitments they can ever make: a commitment to serve their country and a commitment to start and support a family," Carter said. "This puts DoD in the top tier of institutions nationwide and will have significant influence on decision-making for our military family members."
Some of the other reforms highlighted by Carter during his briefing include:
- Increased hours of military child care to 14 hours of the day across the force;
- The option for military personnel to remain at their current location in exchange for additional service obligations; and
- Coverage for family planning benefits, such as the cost of freezing sperm or eggs, as well as reproductive technologies that include in vitro fertilization.
"By providing our troops with child care they can rely on from before reveille until after taps, we provide one more reason for them to stay on board," he said. "We show them that supporting a family and serving our country are by no means incompatible goals."
The DoD highlighted reforms to its Force of the Future program that are designed to improve the quality of life for military personnel with or planning families. Female members of the military will now receive up to 12 weeks paid maternity leave in 2016, regardless of the military branch. Previously each of the services had established different policies, ranging from 18 weeks in the Navy and Marines to just 6 weeks in the Army.
During a briefing at the Pentagon on January 28, Defense Secretary Ash Carter noted that the goal of this action was increase the support provided to military families while also improving retention.
"Our calculation is quite simple, we want our people to be able to balance two of the most solemn commitments they can ever make: a commitment to serve their country and a commitment to start and support a family," Carter said. "This puts DoD in the top tier of institutions nationwide and will have significant influence on decision-making for our military family members."
Some of the other reforms highlighted by Carter during his briefing include:
- Increased hours of military child care to 14 hours of the day across the force;
- The option for military personnel to remain at their current location in exchange for additional service obligations; and
- Coverage for family planning benefits, such as the cost of freezing sperm or eggs, as well as reproductive technologies that include in vitro fertilization.
"By providing our troops with child care they can rely on from before reveille until after taps, we provide one more reason for them to stay on board," he said. "We show them that supporting a family and serving our country are by no means incompatible goals."
The DoD highlighted reforms to its Force of the Future program that are designed to improve the quality of life for military personnel with or planning families. Female members of the military will now receive up to 12 weeks paid maternity leave in 2016, regardless of the military branch. Previously each of the services had established different policies, ranging from 18 weeks in the Navy and Marines to just 6 weeks in the Army.
During a briefing at the Pentagon on January 28, Defense Secretary Ash Carter noted that the goal of this action was increase the support provided to military families while also improving retention.
"Our calculation is quite simple, we want our people to be able to balance two of the most solemn commitments they can ever make: a commitment to serve their country and a commitment to start and support a family," Carter said. "This puts DoD in the top tier of institutions nationwide and will have significant influence on decision-making for our military family members."
Some of the other reforms highlighted by Carter during his briefing include:
- Increased hours of military child care to 14 hours of the day across the force;
- The option for military personnel to remain at their current location in exchange for additional service obligations; and
- Coverage for family planning benefits, such as the cost of freezing sperm or eggs, as well as reproductive technologies that include in vitro fertilization.
"By providing our troops with child care they can rely on from before reveille until after taps, we provide one more reason for them to stay on board," he said. "We show them that supporting a family and serving our country are by no means incompatible goals."