User login
A step away from immediate umbilical cord clamping
The common practice of immediate cord clamping, which generally means clamping within 15-20 seconds after birth, was fueled by efforts to reduce the risk of postpartum hemorrhage, a leading cause of maternal death worldwide. Immediate clamping was part of a full active management intervention recommended in 2007 by the World Health Organization, along with the use of uterotonics (generally oxytocin) immediately after birth and controlled cord traction to quickly deliver the placenta.
Adoption of the WHO-recommended “active management during the third stage of labor” (AMTSL) worked, leading to a 70% reduction in postpartum hemorrhage and a 60% reduction in blood transfusion over passive management. However, it appears that immediate cord clamping has not played an important role in these reductions. Several randomized controlled trials have shown that early clamping does not impact the risk of postpartum hemorrhage (> 1000 cc or > 500 cc), nor does it impact the need for manual removal of the placenta or the need for blood transfusion.
Instead, the critical component of the AMTSL package appears to be administration of a uterotonic, as reported in a large WHO-directed multicenter clinical trial published in 2012. The study also found that women who received controlled cord traction bled an average of 11 cc less – an insignificant difference – than did women who delivered their placentas by their own effort. Moreover, they had a third stage of labor that was an average of 6 minutes shorter (Lancet 2012;379:1721-7).

With assurance that the timing of umbilical cord clamping does not impact maternal outcomes, investigators have begun to look more at the impact of immediate versus delayed cord clamping on the health of the baby.
Thus far, the issues in this arena are a bit more complicated than on the maternal side. There are indications, however, that slight delays in umbilical cord clamping may be beneficial for the newborn – particularly for preterm infants, who appear in systemic reviews to have a nearly 50% reduction in intraventricular hemorrhage when clamping is delayed.
Timing in term infants
The theoretical benefits of delayed cord clamping include increased neonatal blood volume (improved perfusion and decreased organ injury), more time for spontaneous breathing (reduced risks of resuscitation and a smoother transition of cardiopulmonary and cerebral circulation), and increased stem cells for the infant (anti-inflammatory, neurotropic, and neuroprotective effects).
Theoretically, delayed clamping will increase the infant’s iron stores and lower the incidence of iron deficiency anemia during infancy. This is particularly relevant in developing countries, where up to 50% of infants have anemia by 1 year of age. Anemia is consistently associated with abnormal neurodevelopment, and treatment may not always reverse developmental issues.
On the negative side, delayed clamping is associated with theoretical concerns about hyperbilirubinemia and jaundice, hypothermia, polycythemia, and delays in the bonding of infants and mothers.
For term infants, our best reading on the benefits and risks of delayed umbilical cord clamping comes from a 2013 Cochrane systematic review that assessed results from 15 randomized controlled trials involving 3,911 women and infant pairs. Early cord clamping was generally carried out within 60 seconds of birth, whereas delayed cord clamping involved clamping the umbilical cord more than 1 minute after birth or when cord pulsation has ceased.
The review found that delayed clamping was associated with a significantly higher neonatal hemoglobin concentration at 24-48 hours postpartum (a weighted mean difference of 2 g/dL) and increased iron reserves up to 6 months after birth. Infants in the early clamping group were more than twice as likely to be iron deficient at 3-6 months compared with infants whose cord clamping was delayed (Cochrane Database Syst. Rev. 2013;7:CD004074)
There were no significant differences between early and late clamping in neonatal mortality or for most other neonatal morbidity outcomes. Delayed clamping also did not increase the risk of severe postpartum hemorrhage, blood loss, or reduced hemoglobin levels in mothers.
The downside to delayed cord clamping was an increased risk of jaundice requiring phototherapy. Infants in the later cord clamping group were 40% more likely to need phototherapy – a difference that equates to 3% of infants in the early clamping group and 5% of infants in the late clamping group.
Data were insufficient in the Cochrane review to draw reliable conclusions about the comparative effects on other short-term outcomes such as symptomatic polycythemia, respiratory problems, hypothermia, and infection, as data were limited on long-term outcomes.
In practice, this means that the risk of jaundice must be weighed against the risk of iron deficiency. In developed countries we have the resources both to increase iron stores of infants and to provide phototherapy. While the WHO recommends umbilical cord clamping after 1-3 minutes to improve an infant’s iron status, I do not believe the evidence is strong enough to universally adopt such delayed cord clamping in the United States.
Considering the risks of jaundice and the relative infrequency of iron deficiency in the United States, we should not routinely delay clamping for term infants at this point.
A recent committee opinion developed by the American College of Obstetricians and Gynecologists and endorsed by the American Academy of Pediatrics (No. 543, December 2012) captures this view by concluding that “insufficient evidence exists to support or to refute the benefits from delayed umbilical cord clamping for term infants that are born in settings with rich resources.” Although the ACOG opinion preceded the Cochrane review, the committee, of which I was a member, reviewed much of the same literature.
Timing in preterm infants
Preterm neonates are at increased risk of temperature dysregulation, hypotension, and the need for rapid initial pediatric care and blood transfusion. The increased risk of intraventricular hemorrhage and necrotizing enterocolitis in preterm infants is possibly related to the increased risk of hypotension.
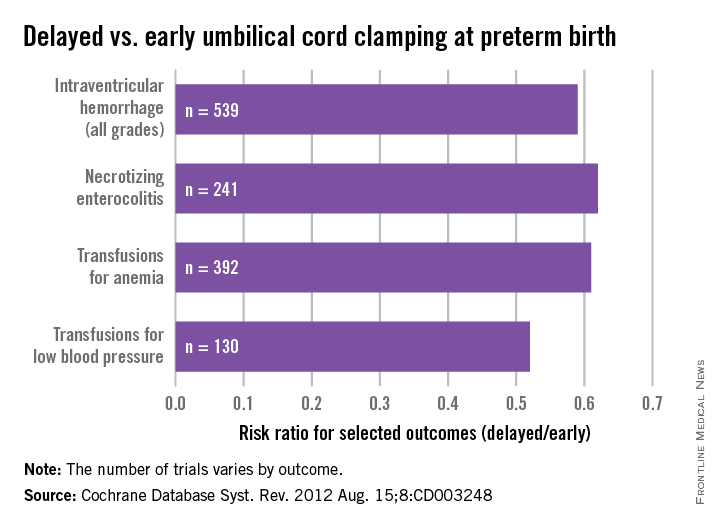
As with term infants, a 2012 Cochrane systematic review offers good insight on our current knowledge. This review of umbilical cord clamping at preterm birth covers 15 studies that included 738 infants delivered between 24 and 36 weeks of gestation. The timing of umbilical cord clamping ranged from 25 seconds to a maximum of 180 seconds (Cochrane Database Syst. Rev. 2012;8:CD003248).
Delayed cord clamping was associated with fewer transfusions for anemia or low blood pressure, less intraventricular hemorrhage of all grades (relative risk 0.59), and a lower risk for necrotizing enterocolitis (relative risk 0.62), compared with immediate clamping.
While there were no clear differences with respect to severe intraventricular hemorrhage (grades 3-4), the nearly 50% reduction in intraventricular hemorrhage overall among deliveries with delayed clamping was significant enough to prompt ACOG to conclude that delayed cord clamping should be considered for preterm infants. This reduction in intraventricular hemorrhage appears to be the single most important benefit, based on current findings.
The data on cord clamping in preterm infants are suggestive of benefit, but are not robust. The studies published thus far have been small, and many of them, as the 2012 Cochrane review points out, involved incomplete reporting and wide confidence intervals. Moreover, just as with the studies on term infants, there has been a lack of long-term follow-up in most of the published trials.
When considering delayed cord clamping in preterm infants, as the ACOG Committee Opinion recommends, I urge focusing on earlier gestational ages. Allowing more placental transfusion at births that occur at or after 36 weeks of gestation may not make much sense because by that point the risk of intraventricular hemorrhage is almost nonexistent.
Our practice and the future
At our institution, births that occur at less than 32 weeks of gestation are eligible for delayed umbilical cord clamping, usually at 30-45 seconds after birth. The main contraindications are placental abruption and multiples.
We do not perform any milking or stripping of the umbilical cord, as the risks are unknown and it is not yet clear whether such practices are equivalent to delayed cord clamping. Compared with delayed cord clamping, which is a natural passive transfusion of placental blood to the infant, milking and stripping are not physiologic.
Additional data from an ongoing large international multicenter study, the Australian Placental Transfusion Study, may resolve some of the current controversy. This study is evaluating the cord clamping in neonates < 30 weeks’ gestation. Another study ongoing in Europe should also provide more information.
These studies – and other trials that are larger and longer than the trials published thus far – are necessary to evaluate long-term outcomes and to establish the ideal timing for umbilical cord clamping. Research is also needed to evaluate the management of the third stage of labor relative to umbilical cord clamping as well as the timing in relation to the initiation of voluntary or assisted ventilation.
Dr. Macones said he had no relevant financial disclosures.
Dr. Macones is the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis.
The common practice of immediate cord clamping, which generally means clamping within 15-20 seconds after birth, was fueled by efforts to reduce the risk of postpartum hemorrhage, a leading cause of maternal death worldwide. Immediate clamping was part of a full active management intervention recommended in 2007 by the World Health Organization, along with the use of uterotonics (generally oxytocin) immediately after birth and controlled cord traction to quickly deliver the placenta.
Adoption of the WHO-recommended “active management during the third stage of labor” (AMTSL) worked, leading to a 70% reduction in postpartum hemorrhage and a 60% reduction in blood transfusion over passive management. However, it appears that immediate cord clamping has not played an important role in these reductions. Several randomized controlled trials have shown that early clamping does not impact the risk of postpartum hemorrhage (> 1000 cc or > 500 cc), nor does it impact the need for manual removal of the placenta or the need for blood transfusion.
Instead, the critical component of the AMTSL package appears to be administration of a uterotonic, as reported in a large WHO-directed multicenter clinical trial published in 2012. The study also found that women who received controlled cord traction bled an average of 11 cc less – an insignificant difference – than did women who delivered their placentas by their own effort. Moreover, they had a third stage of labor that was an average of 6 minutes shorter (Lancet 2012;379:1721-7).

With assurance that the timing of umbilical cord clamping does not impact maternal outcomes, investigators have begun to look more at the impact of immediate versus delayed cord clamping on the health of the baby.
Thus far, the issues in this arena are a bit more complicated than on the maternal side. There are indications, however, that slight delays in umbilical cord clamping may be beneficial for the newborn – particularly for preterm infants, who appear in systemic reviews to have a nearly 50% reduction in intraventricular hemorrhage when clamping is delayed.
Timing in term infants
The theoretical benefits of delayed cord clamping include increased neonatal blood volume (improved perfusion and decreased organ injury), more time for spontaneous breathing (reduced risks of resuscitation and a smoother transition of cardiopulmonary and cerebral circulation), and increased stem cells for the infant (anti-inflammatory, neurotropic, and neuroprotective effects).
Theoretically, delayed clamping will increase the infant’s iron stores and lower the incidence of iron deficiency anemia during infancy. This is particularly relevant in developing countries, where up to 50% of infants have anemia by 1 year of age. Anemia is consistently associated with abnormal neurodevelopment, and treatment may not always reverse developmental issues.
On the negative side, delayed clamping is associated with theoretical concerns about hyperbilirubinemia and jaundice, hypothermia, polycythemia, and delays in the bonding of infants and mothers.
For term infants, our best reading on the benefits and risks of delayed umbilical cord clamping comes from a 2013 Cochrane systematic review that assessed results from 15 randomized controlled trials involving 3,911 women and infant pairs. Early cord clamping was generally carried out within 60 seconds of birth, whereas delayed cord clamping involved clamping the umbilical cord more than 1 minute after birth or when cord pulsation has ceased.
The review found that delayed clamping was associated with a significantly higher neonatal hemoglobin concentration at 24-48 hours postpartum (a weighted mean difference of 2 g/dL) and increased iron reserves up to 6 months after birth. Infants in the early clamping group were more than twice as likely to be iron deficient at 3-6 months compared with infants whose cord clamping was delayed (Cochrane Database Syst. Rev. 2013;7:CD004074)
There were no significant differences between early and late clamping in neonatal mortality or for most other neonatal morbidity outcomes. Delayed clamping also did not increase the risk of severe postpartum hemorrhage, blood loss, or reduced hemoglobin levels in mothers.
The downside to delayed cord clamping was an increased risk of jaundice requiring phototherapy. Infants in the later cord clamping group were 40% more likely to need phototherapy – a difference that equates to 3% of infants in the early clamping group and 5% of infants in the late clamping group.
Data were insufficient in the Cochrane review to draw reliable conclusions about the comparative effects on other short-term outcomes such as symptomatic polycythemia, respiratory problems, hypothermia, and infection, as data were limited on long-term outcomes.
In practice, this means that the risk of jaundice must be weighed against the risk of iron deficiency. In developed countries we have the resources both to increase iron stores of infants and to provide phototherapy. While the WHO recommends umbilical cord clamping after 1-3 minutes to improve an infant’s iron status, I do not believe the evidence is strong enough to universally adopt such delayed cord clamping in the United States.
Considering the risks of jaundice and the relative infrequency of iron deficiency in the United States, we should not routinely delay clamping for term infants at this point.
A recent committee opinion developed by the American College of Obstetricians and Gynecologists and endorsed by the American Academy of Pediatrics (No. 543, December 2012) captures this view by concluding that “insufficient evidence exists to support or to refute the benefits from delayed umbilical cord clamping for term infants that are born in settings with rich resources.” Although the ACOG opinion preceded the Cochrane review, the committee, of which I was a member, reviewed much of the same literature.
Timing in preterm infants
Preterm neonates are at increased risk of temperature dysregulation, hypotension, and the need for rapid initial pediatric care and blood transfusion. The increased risk of intraventricular hemorrhage and necrotizing enterocolitis in preterm infants is possibly related to the increased risk of hypotension.
As with term infants, a 2012 Cochrane systematic review offers good insight on our current knowledge. This review of umbilical cord clamping at preterm birth covers 15 studies that included 738 infants delivered between 24 and 36 weeks of gestation. The timing of umbilical cord clamping ranged from 25 seconds to a maximum of 180 seconds (Cochrane Database Syst. Rev. 2012;8:CD003248).
Delayed cord clamping was associated with fewer transfusions for anemia or low blood pressure, less intraventricular hemorrhage of all grades (relative risk 0.59), and a lower risk for necrotizing enterocolitis (relative risk 0.62), compared with immediate clamping.
While there were no clear differences with respect to severe intraventricular hemorrhage (grades 3-4), the nearly 50% reduction in intraventricular hemorrhage overall among deliveries with delayed clamping was significant enough to prompt ACOG to conclude that delayed cord clamping should be considered for preterm infants. This reduction in intraventricular hemorrhage appears to be the single most important benefit, based on current findings.
The data on cord clamping in preterm infants are suggestive of benefit, but are not robust. The studies published thus far have been small, and many of them, as the 2012 Cochrane review points out, involved incomplete reporting and wide confidence intervals. Moreover, just as with the studies on term infants, there has been a lack of long-term follow-up in most of the published trials.
When considering delayed cord clamping in preterm infants, as the ACOG Committee Opinion recommends, I urge focusing on earlier gestational ages. Allowing more placental transfusion at births that occur at or after 36 weeks of gestation may not make much sense because by that point the risk of intraventricular hemorrhage is almost nonexistent.
Our practice and the future
At our institution, births that occur at less than 32 weeks of gestation are eligible for delayed umbilical cord clamping, usually at 30-45 seconds after birth. The main contraindications are placental abruption and multiples.
We do not perform any milking or stripping of the umbilical cord, as the risks are unknown and it is not yet clear whether such practices are equivalent to delayed cord clamping. Compared with delayed cord clamping, which is a natural passive transfusion of placental blood to the infant, milking and stripping are not physiologic.
Additional data from an ongoing large international multicenter study, the Australian Placental Transfusion Study, may resolve some of the current controversy. This study is evaluating the cord clamping in neonates < 30 weeks’ gestation. Another study ongoing in Europe should also provide more information.
These studies – and other trials that are larger and longer than the trials published thus far – are necessary to evaluate long-term outcomes and to establish the ideal timing for umbilical cord clamping. Research is also needed to evaluate the management of the third stage of labor relative to umbilical cord clamping as well as the timing in relation to the initiation of voluntary or assisted ventilation.
Dr. Macones said he had no relevant financial disclosures.
Dr. Macones is the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis.
The common practice of immediate cord clamping, which generally means clamping within 15-20 seconds after birth, was fueled by efforts to reduce the risk of postpartum hemorrhage, a leading cause of maternal death worldwide. Immediate clamping was part of a full active management intervention recommended in 2007 by the World Health Organization, along with the use of uterotonics (generally oxytocin) immediately after birth and controlled cord traction to quickly deliver the placenta.
Adoption of the WHO-recommended “active management during the third stage of labor” (AMTSL) worked, leading to a 70% reduction in postpartum hemorrhage and a 60% reduction in blood transfusion over passive management. However, it appears that immediate cord clamping has not played an important role in these reductions. Several randomized controlled trials have shown that early clamping does not impact the risk of postpartum hemorrhage (> 1000 cc or > 500 cc), nor does it impact the need for manual removal of the placenta or the need for blood transfusion.
Instead, the critical component of the AMTSL package appears to be administration of a uterotonic, as reported in a large WHO-directed multicenter clinical trial published in 2012. The study also found that women who received controlled cord traction bled an average of 11 cc less – an insignificant difference – than did women who delivered their placentas by their own effort. Moreover, they had a third stage of labor that was an average of 6 minutes shorter (Lancet 2012;379:1721-7).

With assurance that the timing of umbilical cord clamping does not impact maternal outcomes, investigators have begun to look more at the impact of immediate versus delayed cord clamping on the health of the baby.
Thus far, the issues in this arena are a bit more complicated than on the maternal side. There are indications, however, that slight delays in umbilical cord clamping may be beneficial for the newborn – particularly for preterm infants, who appear in systemic reviews to have a nearly 50% reduction in intraventricular hemorrhage when clamping is delayed.
Timing in term infants
The theoretical benefits of delayed cord clamping include increased neonatal blood volume (improved perfusion and decreased organ injury), more time for spontaneous breathing (reduced risks of resuscitation and a smoother transition of cardiopulmonary and cerebral circulation), and increased stem cells for the infant (anti-inflammatory, neurotropic, and neuroprotective effects).
Theoretically, delayed clamping will increase the infant’s iron stores and lower the incidence of iron deficiency anemia during infancy. This is particularly relevant in developing countries, where up to 50% of infants have anemia by 1 year of age. Anemia is consistently associated with abnormal neurodevelopment, and treatment may not always reverse developmental issues.
On the negative side, delayed clamping is associated with theoretical concerns about hyperbilirubinemia and jaundice, hypothermia, polycythemia, and delays in the bonding of infants and mothers.
For term infants, our best reading on the benefits and risks of delayed umbilical cord clamping comes from a 2013 Cochrane systematic review that assessed results from 15 randomized controlled trials involving 3,911 women and infant pairs. Early cord clamping was generally carried out within 60 seconds of birth, whereas delayed cord clamping involved clamping the umbilical cord more than 1 minute after birth or when cord pulsation has ceased.
The review found that delayed clamping was associated with a significantly higher neonatal hemoglobin concentration at 24-48 hours postpartum (a weighted mean difference of 2 g/dL) and increased iron reserves up to 6 months after birth. Infants in the early clamping group were more than twice as likely to be iron deficient at 3-6 months compared with infants whose cord clamping was delayed (Cochrane Database Syst. Rev. 2013;7:CD004074)
There were no significant differences between early and late clamping in neonatal mortality or for most other neonatal morbidity outcomes. Delayed clamping also did not increase the risk of severe postpartum hemorrhage, blood loss, or reduced hemoglobin levels in mothers.
The downside to delayed cord clamping was an increased risk of jaundice requiring phototherapy. Infants in the later cord clamping group were 40% more likely to need phototherapy – a difference that equates to 3% of infants in the early clamping group and 5% of infants in the late clamping group.
Data were insufficient in the Cochrane review to draw reliable conclusions about the comparative effects on other short-term outcomes such as symptomatic polycythemia, respiratory problems, hypothermia, and infection, as data were limited on long-term outcomes.
In practice, this means that the risk of jaundice must be weighed against the risk of iron deficiency. In developed countries we have the resources both to increase iron stores of infants and to provide phototherapy. While the WHO recommends umbilical cord clamping after 1-3 minutes to improve an infant’s iron status, I do not believe the evidence is strong enough to universally adopt such delayed cord clamping in the United States.
Considering the risks of jaundice and the relative infrequency of iron deficiency in the United States, we should not routinely delay clamping for term infants at this point.
A recent committee opinion developed by the American College of Obstetricians and Gynecologists and endorsed by the American Academy of Pediatrics (No. 543, December 2012) captures this view by concluding that “insufficient evidence exists to support or to refute the benefits from delayed umbilical cord clamping for term infants that are born in settings with rich resources.” Although the ACOG opinion preceded the Cochrane review, the committee, of which I was a member, reviewed much of the same literature.
Timing in preterm infants
Preterm neonates are at increased risk of temperature dysregulation, hypotension, and the need for rapid initial pediatric care and blood transfusion. The increased risk of intraventricular hemorrhage and necrotizing enterocolitis in preterm infants is possibly related to the increased risk of hypotension.
As with term infants, a 2012 Cochrane systematic review offers good insight on our current knowledge. This review of umbilical cord clamping at preterm birth covers 15 studies that included 738 infants delivered between 24 and 36 weeks of gestation. The timing of umbilical cord clamping ranged from 25 seconds to a maximum of 180 seconds (Cochrane Database Syst. Rev. 2012;8:CD003248).
Delayed cord clamping was associated with fewer transfusions for anemia or low blood pressure, less intraventricular hemorrhage of all grades (relative risk 0.59), and a lower risk for necrotizing enterocolitis (relative risk 0.62), compared with immediate clamping.
While there were no clear differences with respect to severe intraventricular hemorrhage (grades 3-4), the nearly 50% reduction in intraventricular hemorrhage overall among deliveries with delayed clamping was significant enough to prompt ACOG to conclude that delayed cord clamping should be considered for preterm infants. This reduction in intraventricular hemorrhage appears to be the single most important benefit, based on current findings.
The data on cord clamping in preterm infants are suggestive of benefit, but are not robust. The studies published thus far have been small, and many of them, as the 2012 Cochrane review points out, involved incomplete reporting and wide confidence intervals. Moreover, just as with the studies on term infants, there has been a lack of long-term follow-up in most of the published trials.
When considering delayed cord clamping in preterm infants, as the ACOG Committee Opinion recommends, I urge focusing on earlier gestational ages. Allowing more placental transfusion at births that occur at or after 36 weeks of gestation may not make much sense because by that point the risk of intraventricular hemorrhage is almost nonexistent.
Our practice and the future
At our institution, births that occur at less than 32 weeks of gestation are eligible for delayed umbilical cord clamping, usually at 30-45 seconds after birth. The main contraindications are placental abruption and multiples.
We do not perform any milking or stripping of the umbilical cord, as the risks are unknown and it is not yet clear whether such practices are equivalent to delayed cord clamping. Compared with delayed cord clamping, which is a natural passive transfusion of placental blood to the infant, milking and stripping are not physiologic.
Additional data from an ongoing large international multicenter study, the Australian Placental Transfusion Study, may resolve some of the current controversy. This study is evaluating the cord clamping in neonates < 30 weeks’ gestation. Another study ongoing in Europe should also provide more information.
These studies – and other trials that are larger and longer than the trials published thus far – are necessary to evaluate long-term outcomes and to establish the ideal timing for umbilical cord clamping. Research is also needed to evaluate the management of the third stage of labor relative to umbilical cord clamping as well as the timing in relation to the initiation of voluntary or assisted ventilation.
Dr. Macones said he had no relevant financial disclosures.
Dr. Macones is the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis.
Expert panel endorses continued use of morcellation
VANCOUVER – Morcellation is an effective, lifesaving tool in gynecologic surgery when used appropriately and should not be abandoned despite recent concerns about the dissemination of occult cancers, according to an expert panel that weighed in on this issue at a meeting sponsored by AAGL.
Panelists presented new data to inform the intense debate over this procedure, which has culminated in the Food and Drug Administration (FDA) recommending against the use of power morcellators during fibroid removal by hysterectomy or myomectomy for most women.
Earlier this year, AAGL convened the Tissue Extraction Task Force to study this issue and respond to the controversy. The association presented a statement to the FDA on power morcellation and published the task force findings that morcellation can be done safely and effectively when performed by trained and experienced surgeons in informed, carefully screened premenopausal women (J. Minim. Invasive Gynecol. 2014;21:517-30).
Abandoning it may raise mortality
“The priority of this entire discussion needs to focus on the patient’s welfare,” contended panelist Dr. Jubilee Brown, an associate professor in the department of gynecologic oncology and reproductive medicine, University of Texas M.D. Anderson Cancer Center, Houston. “For every piece of data that we look at, we need to keep that in the back of our minds as we analyze this.”
In a new study, she and her colleagues retrospectively studied outcomes in 808 consecutive patients with planned laparoscopic supracervical hysterectomy with morcellation who had at least 5 years of follow-up. The leading indications for surgery were menorrhagia and leiomyomata.
Only a single woman had a leiomyosarcoma; she was converted to an open procedure without morcellation but nonetheless died from the disease. “What hasn’t shown up in much of the literature is the wisdom of the operating surgeon, who identified that this uterus looked abnormal and called our group in,” commented Dr. Brown, who is also AAGL’s designated spokesperson on tissue extraction. “Unfortunately, what’s also missed in much of the literature is that leiomyosarcoma is an aggressive and often deadly disease. … In her case, as in so many cases, the problem was not the surgery, the problem was the cancer.”
Among the 778 women who underwent the planned laparoscopic hysterectomy with morcellation, 16 were found to have endometrial hyperplasia, two had adenocarcinoma, and one had an endometrial stromal sarcoma – but reassuringly, none had evidence of disease at follow-up.
“I think that what this tells us is that we need to be absolutely meticulous in our preoperative evaluation of patients in whom we are considering morcellation,” Dr. Brown said. The findings “speak to our obligation to educate our membership and everybody performing preoperative sampling on these patients.”
A decision analysis study also reported at the meeting by first author Dr. R. Wendel Naumann, Carolinas Medical Center in Charlotte, N.C., showed that mortality from laparoscopic hysterectomy with power morcellation – even accounting for possible dissemination of undiagnosed leiomyosarcomas – was 0.077%, still less than the 0.085% mortality from abdominal hysterectomy. “Though it is a small difference, it is an absolute difference in favor of laparoscopic hysterectomy with power morcellation. In fact, if all women were converted to an open hysterectomy, 17 more women each year would die of open hysterectomy than of power morcellation,” Dr. Brown commented.
“Power morcellation is an important tool,” she concluded, reiterating AAGL’s position that its use should be improved, not abandoned.
Low risk of leiomyosarcomas
Panelist Dr. Marit Lieng, an associate professor and consultant in the gynecology department of Oslo University Hospital, and her coinvestigators retrospectively studied 4,765 women who underwent surgery at the hospital for uterine fibroids between 2000 and 2013.
There were 26 cases of leiomyosarcoma (the majority in postmenopausal women), for an incidence of 0.54%, or 1 in 183 women.
However, only a single patient with leiomyosarcoma had laparoscopic supracervical hysterectomy with morcellation, because the tumor was identified or suspected preoperatively or intraoperatively in the rest, reported Dr. Lieng, who is also with the Institute of Clinical Medicine at the University of Oslo.
Therefore, the risk of unintended morcellation of an undiagnosed leiomyosarcoma was just 1 in 4,765 women, or 0.02%.
“I think the findings of our study support the conclusions of the AAGL expert group. … You can do power morcellation in selected patients,” Dr. Lieng commented. “Given a thorough preoperative evaluation, including a cervical cytology, endometrial biopsy, and evaluation of the myometrium by ultrasound or MRI, the risk of unintended morcellation of a uterine leiomyosarcoma in premenopausal women appears to be very low.”
Leiomyosarcomas best removed en bloc
“When you are creating public health care policy, decision analysis must begin with scientifically valid evidence,” asserted panelist Dr. Elizabeth Pritts, medical director of the Wisconsin Fertility Institute, Middleton.
She and her colleagues undertook a comprehensive new meta-analysis assessing the prevalence of occult leiomyosarcomas at hysterectomy or myomectomy for presumed uterine fibroids, including 133 original articles describing 30,193 women having explicit pathology.
Analysis of all prospective data showed that the predicted prevalence rate of occult leiomyosarcoma was 0.12 per 1,000 operations for presumed benign fibroids.
The corresponding 1 in 8,300 operations needed to find a leiomyosarcoma in this new meta-analysis differs greatly from the 1 in 498 found in an FDA meta-analysis, mainly because of the differing evidence base, Dr. Pritts maintained. “It really has to do with initial search criteria,” she said, noting, for example, that the FDA’s search strategy missed studies in which no cancer was found and studies in languages other than English.
Dr. Pritts and her colleagues also conducted a new systematic review looking at outcomes after morcellation of an unsuspected leiomyosarcoma, which was recently published (J. Minim. Invasive Gynecol. 2014 Sept. 2 [doi: 10.1016/j.jmig.2014.08.781]).
Main analyses here were based on six papers that compared morcellation with en bloc removal of leiomyosarcomas, most of which found worse survival for women whose tumors were morcellated.
“Now this is not great evidence, but remember, in evidence-based medicine, you’ve got to look at the very best available evidence. This is it,” Dr. Pritts maintained. “En bloc removal confers benefit—don’t cut into these.”
On closer inspection, only 3 of the 81 cases of morcellation reported were confirmed to be power morcellation. Comparisons of outcome with power versus hand morcellation, albeit limited by small numbers, suggested no difference in survival or upstaging.
“There are no data to suggest that any type of morcellation is better or worse than another type, even when including simple tumor biopsies,” Dr. Pritts concluded.
Dr. Brown, Dr. Lieng, and Dr. Pritts disclosed that they had no relevant conflicts of interest.
VANCOUVER – Morcellation is an effective, lifesaving tool in gynecologic surgery when used appropriately and should not be abandoned despite recent concerns about the dissemination of occult cancers, according to an expert panel that weighed in on this issue at a meeting sponsored by AAGL.
Panelists presented new data to inform the intense debate over this procedure, which has culminated in the Food and Drug Administration (FDA) recommending against the use of power morcellators during fibroid removal by hysterectomy or myomectomy for most women.
Earlier this year, AAGL convened the Tissue Extraction Task Force to study this issue and respond to the controversy. The association presented a statement to the FDA on power morcellation and published the task force findings that morcellation can be done safely and effectively when performed by trained and experienced surgeons in informed, carefully screened premenopausal women (J. Minim. Invasive Gynecol. 2014;21:517-30).
Abandoning it may raise mortality
“The priority of this entire discussion needs to focus on the patient’s welfare,” contended panelist Dr. Jubilee Brown, an associate professor in the department of gynecologic oncology and reproductive medicine, University of Texas M.D. Anderson Cancer Center, Houston. “For every piece of data that we look at, we need to keep that in the back of our minds as we analyze this.”
In a new study, she and her colleagues retrospectively studied outcomes in 808 consecutive patients with planned laparoscopic supracervical hysterectomy with morcellation who had at least 5 years of follow-up. The leading indications for surgery were menorrhagia and leiomyomata.
Only a single woman had a leiomyosarcoma; she was converted to an open procedure without morcellation but nonetheless died from the disease. “What hasn’t shown up in much of the literature is the wisdom of the operating surgeon, who identified that this uterus looked abnormal and called our group in,” commented Dr. Brown, who is also AAGL’s designated spokesperson on tissue extraction. “Unfortunately, what’s also missed in much of the literature is that leiomyosarcoma is an aggressive and often deadly disease. … In her case, as in so many cases, the problem was not the surgery, the problem was the cancer.”
Among the 778 women who underwent the planned laparoscopic hysterectomy with morcellation, 16 were found to have endometrial hyperplasia, two had adenocarcinoma, and one had an endometrial stromal sarcoma – but reassuringly, none had evidence of disease at follow-up.
“I think that what this tells us is that we need to be absolutely meticulous in our preoperative evaluation of patients in whom we are considering morcellation,” Dr. Brown said. The findings “speak to our obligation to educate our membership and everybody performing preoperative sampling on these patients.”
A decision analysis study also reported at the meeting by first author Dr. R. Wendel Naumann, Carolinas Medical Center in Charlotte, N.C., showed that mortality from laparoscopic hysterectomy with power morcellation – even accounting for possible dissemination of undiagnosed leiomyosarcomas – was 0.077%, still less than the 0.085% mortality from abdominal hysterectomy. “Though it is a small difference, it is an absolute difference in favor of laparoscopic hysterectomy with power morcellation. In fact, if all women were converted to an open hysterectomy, 17 more women each year would die of open hysterectomy than of power morcellation,” Dr. Brown commented.
“Power morcellation is an important tool,” she concluded, reiterating AAGL’s position that its use should be improved, not abandoned.
Low risk of leiomyosarcomas
Panelist Dr. Marit Lieng, an associate professor and consultant in the gynecology department of Oslo University Hospital, and her coinvestigators retrospectively studied 4,765 women who underwent surgery at the hospital for uterine fibroids between 2000 and 2013.
There were 26 cases of leiomyosarcoma (the majority in postmenopausal women), for an incidence of 0.54%, or 1 in 183 women.
However, only a single patient with leiomyosarcoma had laparoscopic supracervical hysterectomy with morcellation, because the tumor was identified or suspected preoperatively or intraoperatively in the rest, reported Dr. Lieng, who is also with the Institute of Clinical Medicine at the University of Oslo.
Therefore, the risk of unintended morcellation of an undiagnosed leiomyosarcoma was just 1 in 4,765 women, or 0.02%.
“I think the findings of our study support the conclusions of the AAGL expert group. … You can do power morcellation in selected patients,” Dr. Lieng commented. “Given a thorough preoperative evaluation, including a cervical cytology, endometrial biopsy, and evaluation of the myometrium by ultrasound or MRI, the risk of unintended morcellation of a uterine leiomyosarcoma in premenopausal women appears to be very low.”
Leiomyosarcomas best removed en bloc
“When you are creating public health care policy, decision analysis must begin with scientifically valid evidence,” asserted panelist Dr. Elizabeth Pritts, medical director of the Wisconsin Fertility Institute, Middleton.
She and her colleagues undertook a comprehensive new meta-analysis assessing the prevalence of occult leiomyosarcomas at hysterectomy or myomectomy for presumed uterine fibroids, including 133 original articles describing 30,193 women having explicit pathology.
Analysis of all prospective data showed that the predicted prevalence rate of occult leiomyosarcoma was 0.12 per 1,000 operations for presumed benign fibroids.
The corresponding 1 in 8,300 operations needed to find a leiomyosarcoma in this new meta-analysis differs greatly from the 1 in 498 found in an FDA meta-analysis, mainly because of the differing evidence base, Dr. Pritts maintained. “It really has to do with initial search criteria,” she said, noting, for example, that the FDA’s search strategy missed studies in which no cancer was found and studies in languages other than English.
Dr. Pritts and her colleagues also conducted a new systematic review looking at outcomes after morcellation of an unsuspected leiomyosarcoma, which was recently published (J. Minim. Invasive Gynecol. 2014 Sept. 2 [doi: 10.1016/j.jmig.2014.08.781]).
Main analyses here were based on six papers that compared morcellation with en bloc removal of leiomyosarcomas, most of which found worse survival for women whose tumors were morcellated.
“Now this is not great evidence, but remember, in evidence-based medicine, you’ve got to look at the very best available evidence. This is it,” Dr. Pritts maintained. “En bloc removal confers benefit—don’t cut into these.”
On closer inspection, only 3 of the 81 cases of morcellation reported were confirmed to be power morcellation. Comparisons of outcome with power versus hand morcellation, albeit limited by small numbers, suggested no difference in survival or upstaging.
“There are no data to suggest that any type of morcellation is better or worse than another type, even when including simple tumor biopsies,” Dr. Pritts concluded.
Dr. Brown, Dr. Lieng, and Dr. Pritts disclosed that they had no relevant conflicts of interest.
VANCOUVER – Morcellation is an effective, lifesaving tool in gynecologic surgery when used appropriately and should not be abandoned despite recent concerns about the dissemination of occult cancers, according to an expert panel that weighed in on this issue at a meeting sponsored by AAGL.
Panelists presented new data to inform the intense debate over this procedure, which has culminated in the Food and Drug Administration (FDA) recommending against the use of power morcellators during fibroid removal by hysterectomy or myomectomy for most women.
Earlier this year, AAGL convened the Tissue Extraction Task Force to study this issue and respond to the controversy. The association presented a statement to the FDA on power morcellation and published the task force findings that morcellation can be done safely and effectively when performed by trained and experienced surgeons in informed, carefully screened premenopausal women (J. Minim. Invasive Gynecol. 2014;21:517-30).
Abandoning it may raise mortality
“The priority of this entire discussion needs to focus on the patient’s welfare,” contended panelist Dr. Jubilee Brown, an associate professor in the department of gynecologic oncology and reproductive medicine, University of Texas M.D. Anderson Cancer Center, Houston. “For every piece of data that we look at, we need to keep that in the back of our minds as we analyze this.”
In a new study, she and her colleagues retrospectively studied outcomes in 808 consecutive patients with planned laparoscopic supracervical hysterectomy with morcellation who had at least 5 years of follow-up. The leading indications for surgery were menorrhagia and leiomyomata.
Only a single woman had a leiomyosarcoma; she was converted to an open procedure without morcellation but nonetheless died from the disease. “What hasn’t shown up in much of the literature is the wisdom of the operating surgeon, who identified that this uterus looked abnormal and called our group in,” commented Dr. Brown, who is also AAGL’s designated spokesperson on tissue extraction. “Unfortunately, what’s also missed in much of the literature is that leiomyosarcoma is an aggressive and often deadly disease. … In her case, as in so many cases, the problem was not the surgery, the problem was the cancer.”
Among the 778 women who underwent the planned laparoscopic hysterectomy with morcellation, 16 were found to have endometrial hyperplasia, two had adenocarcinoma, and one had an endometrial stromal sarcoma – but reassuringly, none had evidence of disease at follow-up.
“I think that what this tells us is that we need to be absolutely meticulous in our preoperative evaluation of patients in whom we are considering morcellation,” Dr. Brown said. The findings “speak to our obligation to educate our membership and everybody performing preoperative sampling on these patients.”
A decision analysis study also reported at the meeting by first author Dr. R. Wendel Naumann, Carolinas Medical Center in Charlotte, N.C., showed that mortality from laparoscopic hysterectomy with power morcellation – even accounting for possible dissemination of undiagnosed leiomyosarcomas – was 0.077%, still less than the 0.085% mortality from abdominal hysterectomy. “Though it is a small difference, it is an absolute difference in favor of laparoscopic hysterectomy with power morcellation. In fact, if all women were converted to an open hysterectomy, 17 more women each year would die of open hysterectomy than of power morcellation,” Dr. Brown commented.
“Power morcellation is an important tool,” she concluded, reiterating AAGL’s position that its use should be improved, not abandoned.
Low risk of leiomyosarcomas
Panelist Dr. Marit Lieng, an associate professor and consultant in the gynecology department of Oslo University Hospital, and her coinvestigators retrospectively studied 4,765 women who underwent surgery at the hospital for uterine fibroids between 2000 and 2013.
There were 26 cases of leiomyosarcoma (the majority in postmenopausal women), for an incidence of 0.54%, or 1 in 183 women.
However, only a single patient with leiomyosarcoma had laparoscopic supracervical hysterectomy with morcellation, because the tumor was identified or suspected preoperatively or intraoperatively in the rest, reported Dr. Lieng, who is also with the Institute of Clinical Medicine at the University of Oslo.
Therefore, the risk of unintended morcellation of an undiagnosed leiomyosarcoma was just 1 in 4,765 women, or 0.02%.
“I think the findings of our study support the conclusions of the AAGL expert group. … You can do power morcellation in selected patients,” Dr. Lieng commented. “Given a thorough preoperative evaluation, including a cervical cytology, endometrial biopsy, and evaluation of the myometrium by ultrasound or MRI, the risk of unintended morcellation of a uterine leiomyosarcoma in premenopausal women appears to be very low.”
Leiomyosarcomas best removed en bloc
“When you are creating public health care policy, decision analysis must begin with scientifically valid evidence,” asserted panelist Dr. Elizabeth Pritts, medical director of the Wisconsin Fertility Institute, Middleton.
She and her colleagues undertook a comprehensive new meta-analysis assessing the prevalence of occult leiomyosarcomas at hysterectomy or myomectomy for presumed uterine fibroids, including 133 original articles describing 30,193 women having explicit pathology.
Analysis of all prospective data showed that the predicted prevalence rate of occult leiomyosarcoma was 0.12 per 1,000 operations for presumed benign fibroids.
The corresponding 1 in 8,300 operations needed to find a leiomyosarcoma in this new meta-analysis differs greatly from the 1 in 498 found in an FDA meta-analysis, mainly because of the differing evidence base, Dr. Pritts maintained. “It really has to do with initial search criteria,” she said, noting, for example, that the FDA’s search strategy missed studies in which no cancer was found and studies in languages other than English.
Dr. Pritts and her colleagues also conducted a new systematic review looking at outcomes after morcellation of an unsuspected leiomyosarcoma, which was recently published (J. Minim. Invasive Gynecol. 2014 Sept. 2 [doi: 10.1016/j.jmig.2014.08.781]).
Main analyses here were based on six papers that compared morcellation with en bloc removal of leiomyosarcomas, most of which found worse survival for women whose tumors were morcellated.
“Now this is not great evidence, but remember, in evidence-based medicine, you’ve got to look at the very best available evidence. This is it,” Dr. Pritts maintained. “En bloc removal confers benefit—don’t cut into these.”
On closer inspection, only 3 of the 81 cases of morcellation reported were confirmed to be power morcellation. Comparisons of outcome with power versus hand morcellation, albeit limited by small numbers, suggested no difference in survival or upstaging.
“There are no data to suggest that any type of morcellation is better or worse than another type, even when including simple tumor biopsies,” Dr. Pritts concluded.
Dr. Brown, Dr. Lieng, and Dr. Pritts disclosed that they had no relevant conflicts of interest.
AT THE AAGL GLOBAL CONGRESS
Midurethral slings
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.
Treatment of stress urinary incontinence
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
According to a 2004 article by Dr. Eric S. Rovner and Dr. Alan J. Wein, 200 different surgical procedures have been described to treat stress urinary incontinence (Rev. Urol. 2004;6(Suppl 3):S29-47). Two goals exist in such surgical procedures:
1. Urethra repositioning or stabilization of the urethra and bladder neck through creation of retropubic support that is impervious to intraabdominal pressure changes.
2. Augmentation of the ureteral resistance provided by the intrinsic sphincter unit, with or without impacting urethra and bladder neck support (sling vs. periurethral injectables, or a combination of the two).
Sling procedures were initially introduced almost a century ago and have recently become increasingly popular – in part, secondary to a decrease in associated morbidity. Unlike transabdominal or transvaginal urethropexy, a sling not only provides support to the vesicourethral junction, but also may create some aspect of urethral coaptation or compression.
Midurethral slings were introduced nearly 20 years ago. These procedures can be performed with a local anesthetic or with minimal regional anesthesia – thus, in an outpatient setting. In addition, midurethral slings are associated with decreased pain and postoperative convalescence.
I have asked Dr. Eric Russell Sokol to lead this state-of-the-art discussion on midurethral slings. Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals. It is a pleasure and an honor to welcome Dr. Sokol to this edition of Master Class in Gynecologic Surgery, the second installment on urinary incontinence.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Master Class: Obesity
Obesity not only increases a patient’s lifetime risk of numerous chronic conditions, such as diabetes, heart disease and kidney disease, but it also is a major health issue during pregnancy. Women who are obese in pregnancy have a significantly higher chance of developing adverse perinatal outcomes and experiencing various complications that affect both their health and that of their babies.
With an ever-increasing population of overweight and obese women of reproductive age, as key caregivers for women, we must reexamine our approaches and do much more than microfocusing on a woman’s pre- and postdelivery health. We must play a more active role in helping our patients establish and maintain a healthy lifestyle – one that will help ward off and reduce the incidence of this concerning condition.
Over the previous two Master Class installments on obstetrics, we discussed the extent of the obesity epidemic and its link to diabetes, the alarming number of infants, children, and adolescents who are obese, and the implications of these societal and medical trends for ob.gyns.
In the July Master Class, we discussed the importance of appropriately counseling patients on healthy weight gain and physical activity in pregnancy. Because ob.gyns. may be the only health care professionals that many women may see, it is becoming more important that we help our patients and their children attain and maintain positive health and well-being.
In our September installment, Dr. Thomas R. Moore looked at obesity trends through the lens of the Barker Hypothesis, which got us thinking more than 3 decades ago about the role of intrauterine environment in short- and long-term health of offspring. Dr. Moore discussed how obesity in pregnancy appears to program offspring for downstream cardiovascular risk in adulthood.
He told us that we must not only liberally treat gestational diabetes and optimize glucose control during pregnancy, but, most importantly, we also must emphasize to women the importance of having healthy weights at the time of conception.
This month’s Master Class examines this latter concept in more depth. Dr. Patrick Catalano, professor in the department of obstetrics and gynecology and director of the Center for Reproductive Health at MetroHealth Medical Center, Case Western Reserve University, Cleveland, has been at the forefront of research on the physiologic impact of obesity on the placenta and the fetus, and on approaches for addressing maternal obesity and improving perinatal outcomes.
Dr. Catalano explains here why weight loss before pregnancy appears to be important for preventing adverse perinatal outcomes and breaking the intergenerational transfer of obesity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Obesity not only increases a patient’s lifetime risk of numerous chronic conditions, such as diabetes, heart disease and kidney disease, but it also is a major health issue during pregnancy. Women who are obese in pregnancy have a significantly higher chance of developing adverse perinatal outcomes and experiencing various complications that affect both their health and that of their babies.
With an ever-increasing population of overweight and obese women of reproductive age, as key caregivers for women, we must reexamine our approaches and do much more than microfocusing on a woman’s pre- and postdelivery health. We must play a more active role in helping our patients establish and maintain a healthy lifestyle – one that will help ward off and reduce the incidence of this concerning condition.
Over the previous two Master Class installments on obstetrics, we discussed the extent of the obesity epidemic and its link to diabetes, the alarming number of infants, children, and adolescents who are obese, and the implications of these societal and medical trends for ob.gyns.
In the July Master Class, we discussed the importance of appropriately counseling patients on healthy weight gain and physical activity in pregnancy. Because ob.gyns. may be the only health care professionals that many women may see, it is becoming more important that we help our patients and their children attain and maintain positive health and well-being.
In our September installment, Dr. Thomas R. Moore looked at obesity trends through the lens of the Barker Hypothesis, which got us thinking more than 3 decades ago about the role of intrauterine environment in short- and long-term health of offspring. Dr. Moore discussed how obesity in pregnancy appears to program offspring for downstream cardiovascular risk in adulthood.
He told us that we must not only liberally treat gestational diabetes and optimize glucose control during pregnancy, but, most importantly, we also must emphasize to women the importance of having healthy weights at the time of conception.
This month’s Master Class examines this latter concept in more depth. Dr. Patrick Catalano, professor in the department of obstetrics and gynecology and director of the Center for Reproductive Health at MetroHealth Medical Center, Case Western Reserve University, Cleveland, has been at the forefront of research on the physiologic impact of obesity on the placenta and the fetus, and on approaches for addressing maternal obesity and improving perinatal outcomes.
Dr. Catalano explains here why weight loss before pregnancy appears to be important for preventing adverse perinatal outcomes and breaking the intergenerational transfer of obesity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Obesity not only increases a patient’s lifetime risk of numerous chronic conditions, such as diabetes, heart disease and kidney disease, but it also is a major health issue during pregnancy. Women who are obese in pregnancy have a significantly higher chance of developing adverse perinatal outcomes and experiencing various complications that affect both their health and that of their babies.
With an ever-increasing population of overweight and obese women of reproductive age, as key caregivers for women, we must reexamine our approaches and do much more than microfocusing on a woman’s pre- and postdelivery health. We must play a more active role in helping our patients establish and maintain a healthy lifestyle – one that will help ward off and reduce the incidence of this concerning condition.
Over the previous two Master Class installments on obstetrics, we discussed the extent of the obesity epidemic and its link to diabetes, the alarming number of infants, children, and adolescents who are obese, and the implications of these societal and medical trends for ob.gyns.
In the July Master Class, we discussed the importance of appropriately counseling patients on healthy weight gain and physical activity in pregnancy. Because ob.gyns. may be the only health care professionals that many women may see, it is becoming more important that we help our patients and their children attain and maintain positive health and well-being.
In our September installment, Dr. Thomas R. Moore looked at obesity trends through the lens of the Barker Hypothesis, which got us thinking more than 3 decades ago about the role of intrauterine environment in short- and long-term health of offspring. Dr. Moore discussed how obesity in pregnancy appears to program offspring for downstream cardiovascular risk in adulthood.
He told us that we must not only liberally treat gestational diabetes and optimize glucose control during pregnancy, but, most importantly, we also must emphasize to women the importance of having healthy weights at the time of conception.
This month’s Master Class examines this latter concept in more depth. Dr. Patrick Catalano, professor in the department of obstetrics and gynecology and director of the Center for Reproductive Health at MetroHealth Medical Center, Case Western Reserve University, Cleveland, has been at the forefront of research on the physiologic impact of obesity on the placenta and the fetus, and on approaches for addressing maternal obesity and improving perinatal outcomes.
Dr. Catalano explains here why weight loss before pregnancy appears to be important for preventing adverse perinatal outcomes and breaking the intergenerational transfer of obesity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Timing of lifestyle interventions for obesity
Obesity has become so pervasive that it is now considered a major health concern during pregnancy. Almost 56% of women aged 20-39 years in the United States are overweight or obese, based on the World Health Organization’s criteria for body mass index (BMI) and data from the 2009-2010 National Health and Nutrition Examination Survey (NHANES). Moreover, 7.5% of women in this age group are morbidly obese, with a body mass index (BMI) greater than 40 kg/m2 (JAMA 2012;307:491-7).
Obesity in pregnancy not only increases the risk of spontaneous abortions and congenital anomalies, it also increases the risk of gestational diabetes (GDM), hypertensive disorders, and other metabolic complications that affect both the mother and fetus.
Of much concern is the increased risk of fetal overgrowth and long-term health consequences for children of obese mothers. Obesity in early pregnancy has been shown to more than double the risk of obesity in the offspring, which in turn puts these children at risk for developing the metabolic syndrome – and, as Dr. Thomas Moore pointed out in September’s Master Class – appears to program these offspring for downstream cardiovascular risk in adulthood.
Mean term birth weights have risen in the United States during the past several decades. In Cleveland, we have seen a significant 116 g increase in mean term birth weight since 1975; this increase encompasses weights from the 5th to the 95th percentiles. Even more concerning is our finding that the ponderal index in our neonatal population has increased because of decreased fetal length over the last decade.
Some recent studies have suggested that the increase in birth weight in the United States has reached a plateau, but our analyses of national trends suggest that such change is secondary to factors such as earlier gestational age of delivery. Concurrently, an alarming number of children and adolescents – 17% of those aged 2-19 years, according to the 2009-2010 NHANES data – are overweight or obese (JAMA 2012;307:483-90).
How to best treat obesity for improved maternal and fetal health has thus become a focus of research. Studies on lifestyle interventions for obese women during pregnancy have aimed to prevent excessive gestational weight gain and decrease adverse perinatal outcomes – mainly macrosomia, GDM, and hypertensive disorders.
However, the results of this recent body of research have been disappointing. Lifestyle interventions initiated during pregnancy have had only limited success in improving perinatal outcomes. The research tells us that while we may be able to reduce excessive gestational weight gain, it is unlikely that we will be successful in reducing fetal overgrowth, GDM, or preeclampsia in obese women.
Moreover, other studies show that it is a high pregravid BMI – not excessive gestational weight gain or the development of GDM – that plays the biggest role in fetal overgrowth and fetal adiposity.
A paradigm shift is in order. We must think about lifestyle intervention and weight loss before pregnancy, when the woman’s metabolic condition can be improved in time to minimize adverse perinatal metabolic outcomes and to maximize metabolic benefits relating to fetal body composition and metabolism.
Role of prepregnancy BMI
In 2008, the Institute of Medicine (IOM) and National Research Council reexamined 1990 guidelines for gestational weight gain. They concluded that excessive weight gain in pregnancy was a primary contributor to the development of obesity in women. In fact, according to the 2009 IOM report, “Weight Gain During Pregnancy: Reexamining the Guidelines” (Washington: National Academy Press, 2009), 38% of normal weight, 63% of overweight, and 46% of obese women had gained weight in excess of the earlier guidelines.
Helping our patients to gain within the guidelines is important. Excessive gestational weight gain is a primary risk factor for maternal postpartum weight retention, which increases the risk for maternal obesity in a subsequent pregnancy. It also has been associated with a modest increased risk of preterm birth and development of type 2 diabetes.
Interestingly, however, high gestational weight gain has not been related to an increased risk of fetal overgrowth or macrosomia in many obese women. Increased gestational weight gain is a greater risk for fetal overgrowth in women who are of normal weight prior to pregnancy (J. Clin. Endocrinol. Metab. 2012;97:3648-54).
Our research has found that in overweight and obese women, it is maternal pregravid BMI – and not gestational weight gain – that presents the greatest risk for fetal macrosomia, and more specifically, the greatest risk for fetal obesity. Even when glucose tolerance levels are normal, overweight and obese women have neonates who are heavier and who have significant increases in the percentage of body fat and fat mass (Am. J. Obstet. Gynecol. 2006;195:1100-3).
In an 8-year prospective study of the perinatal risk factors associated with childhood obesity, we similarly found that maternal pregravid BMI – independent of maternal glucose status or gestational weight gain – was the strongest predictor of childhood obesity and metabolic dysfunction (Am. J. Clin. Nutr. 2009;90:1303-13).
Other studies have teased apart the roles of maternal obesity and GDM in long-term health of offspring. This work has found that maternal obesity during pregnancy is associated with metabolic syndrome in the offspring and an increased risk of type 2 diabetes in youth, independent of maternal diabetes during pregnancy. A recent meta-analysis also reported that, although maternal diabetes is associated with an increased BMI z score, this was no longer significant after adjustments were made for prepregnancy BMI (Diabetologia 2011;54:1957-66).
Maternal pregravid obesity, therefore, is not only a risk factor for neonatal adiposity at birth, but also for the longer-term risk of obesity and metabolic dysfunction in the offspring – independent of maternal GDM or excessive gestational weight gain.
Interventions in Pregnancy
Numerous prospective trials have examined lifestyle interventions for obese women during pregnancy. One randomized controlled study of a low glycemic index diet in pregnancy (coined the ROLO study) involved 800 women in Ireland who had previously delivered an infant weighting greater than 4,000 g. Women were randomized to receive the restricted diet or no intervention at 13 weeks. Despite a decrease in gestational weight gain in the intervention group, there were no differences in birth weight, birth weight percentile, ponderal index, or macrosomia between the two groups (BMJ 2012;345:e5605).
Another randomized controlled trial reported by a Danish group involved an intervention that consisted of dietary guidance, free membership in a fitness center, and personal coaching initiated between 10 and 14 weeks of gestation. There was a decrease in gestational weight gain in the intervention group, but paradoxically, the infants in the intervention group also had significantly higher birth weight, compared with controls (Diabetes Care 2011;34:2502-7).
Additionally, there have been at least five meta-analyses published in the past 2 years looking at lifestyle interventions during pregnancy. All have concluded that interventions initiated during pregnancy have limited success in reducing excessive gestational weight gain but not necessarily to within the IOM guidelines. The literature contains scant evidence to support further benefits for infant or maternal health (in other words, fetal overgrowth, GDM, or hypertensive disorders).
A recent Cochrane review also concluded that the results of several randomized controlled trials suggest no significant difference in GDM incidence between women receiving exercise intervention versus routine care.
Just this year, three additional randomized controlled trials of lifestyle interventions during pregnancy were published. Only one, the Treatment of Obese Pregnant Women (TOP) study, showed a modest effect in decreasing gestational weight gain. None found a reduction in GDM or fetal overgrowth.
Focus on prepregnancy
Obesity is an inflammatory condition that increases the risk of insulin resistance, impaired beta-cell function, and abnormal adiponectin concentrations. In pregnancy, maternal obesity and hyperinsulinemia can affect placental growth and gene expression.
We have studied lean and obese women recruited prior to a planned pregnancy, as well as lean and obese women scheduled for elective pregnancy termination in the first trimester. Our research, some of which we reported recently in the American Journal of Physiology , has shown increased expression of lipogenic and inflammatory genes in maternal adipose tissue and in the placenta of obese women in the early first trimester, before any phenotypic change becomes apparent (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
Specifically, hyperinsulinemia and/or defective insulin action in obese women appears to affect the placental programming of genes relating to adipokine expression and lipid metabolism, as well as mitrochondrial function. Altered inflammatory and lipid pathways affect the availability of nutrients for the fetus and, consequently, the size and body composition of the fetus. Fetal overgrowth and neonatal adiposity can result.
In addition, our research has shown that obese women have decreased insulin suppression of lipolysis in white adipose tissue, which during pregnancy results in improved lipid availability for fetal fat accretion and lipotoxicity.
When interventions aimed at weight loss and improved insulin sensitivity are undertaken before pregnancy or in the period between pregnancies, we have the opportunity to increase fat oxidation and reduce oxidative stress in early pregnancy. We also may be able to limit placental inflammation and favorably affect placental growth and gene expression. By the second trimester, our research suggests, gene expression in the placenta and early molecular changes in the white adipose tissue have already been programmed and cannot be reversed (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
In studies by our group and others of interpregnancy weight loss or gain, interpregnancy weight loss has been associated with a lower risk of large-for-gestational-age (LGA) infants, whereas interpregnancy weight gain has been associated with an increased risk of LGA. Preliminary work from our group shows that the decrease in birth weight involves primarily fat and not lean mass.
The 2009 IOM guidelines support weight loss before pregnancy and state that overweight women should receive individual preconceptional counseling to improve diet quality, increase physical activity, and normalize weight. Multifaceted interventions do work: In obese nonpregnant individuals, lifestyle interventions, which include an exercise program, diet, and behavioral modification have been shown to be successful in improving insulin sensitivity, inflammation, and overall metabolic function.
According to the IOM report, preconceptional services aimed at achieving a healthy weight before conceiving will represent “a radical change to the care provided to obese women of childbearing age.” With continuing research and accumulating data, however, the concept is gaining traction as a viable paradigm for improving perinatal outcomes, with long-term benefits for both the mother and her baby.
Dr. Catalano reports that he has no disclosures relevant to this Master Class.
Obesity has become so pervasive that it is now considered a major health concern during pregnancy. Almost 56% of women aged 20-39 years in the United States are overweight or obese, based on the World Health Organization’s criteria for body mass index (BMI) and data from the 2009-2010 National Health and Nutrition Examination Survey (NHANES). Moreover, 7.5% of women in this age group are morbidly obese, with a body mass index (BMI) greater than 40 kg/m2 (JAMA 2012;307:491-7).
Obesity in pregnancy not only increases the risk of spontaneous abortions and congenital anomalies, it also increases the risk of gestational diabetes (GDM), hypertensive disorders, and other metabolic complications that affect both the mother and fetus.
Of much concern is the increased risk of fetal overgrowth and long-term health consequences for children of obese mothers. Obesity in early pregnancy has been shown to more than double the risk of obesity in the offspring, which in turn puts these children at risk for developing the metabolic syndrome – and, as Dr. Thomas Moore pointed out in September’s Master Class – appears to program these offspring for downstream cardiovascular risk in adulthood.
Mean term birth weights have risen in the United States during the past several decades. In Cleveland, we have seen a significant 116 g increase in mean term birth weight since 1975; this increase encompasses weights from the 5th to the 95th percentiles. Even more concerning is our finding that the ponderal index in our neonatal population has increased because of decreased fetal length over the last decade.
Some recent studies have suggested that the increase in birth weight in the United States has reached a plateau, but our analyses of national trends suggest that such change is secondary to factors such as earlier gestational age of delivery. Concurrently, an alarming number of children and adolescents – 17% of those aged 2-19 years, according to the 2009-2010 NHANES data – are overweight or obese (JAMA 2012;307:483-90).
How to best treat obesity for improved maternal and fetal health has thus become a focus of research. Studies on lifestyle interventions for obese women during pregnancy have aimed to prevent excessive gestational weight gain and decrease adverse perinatal outcomes – mainly macrosomia, GDM, and hypertensive disorders.
However, the results of this recent body of research have been disappointing. Lifestyle interventions initiated during pregnancy have had only limited success in improving perinatal outcomes. The research tells us that while we may be able to reduce excessive gestational weight gain, it is unlikely that we will be successful in reducing fetal overgrowth, GDM, or preeclampsia in obese women.
Moreover, other studies show that it is a high pregravid BMI – not excessive gestational weight gain or the development of GDM – that plays the biggest role in fetal overgrowth and fetal adiposity.
A paradigm shift is in order. We must think about lifestyle intervention and weight loss before pregnancy, when the woman’s metabolic condition can be improved in time to minimize adverse perinatal metabolic outcomes and to maximize metabolic benefits relating to fetal body composition and metabolism.
Role of prepregnancy BMI
In 2008, the Institute of Medicine (IOM) and National Research Council reexamined 1990 guidelines for gestational weight gain. They concluded that excessive weight gain in pregnancy was a primary contributor to the development of obesity in women. In fact, according to the 2009 IOM report, “Weight Gain During Pregnancy: Reexamining the Guidelines” (Washington: National Academy Press, 2009), 38% of normal weight, 63% of overweight, and 46% of obese women had gained weight in excess of the earlier guidelines.
Helping our patients to gain within the guidelines is important. Excessive gestational weight gain is a primary risk factor for maternal postpartum weight retention, which increases the risk for maternal obesity in a subsequent pregnancy. It also has been associated with a modest increased risk of preterm birth and development of type 2 diabetes.
Interestingly, however, high gestational weight gain has not been related to an increased risk of fetal overgrowth or macrosomia in many obese women. Increased gestational weight gain is a greater risk for fetal overgrowth in women who are of normal weight prior to pregnancy (J. Clin. Endocrinol. Metab. 2012;97:3648-54).
Our research has found that in overweight and obese women, it is maternal pregravid BMI – and not gestational weight gain – that presents the greatest risk for fetal macrosomia, and more specifically, the greatest risk for fetal obesity. Even when glucose tolerance levels are normal, overweight and obese women have neonates who are heavier and who have significant increases in the percentage of body fat and fat mass (Am. J. Obstet. Gynecol. 2006;195:1100-3).
In an 8-year prospective study of the perinatal risk factors associated with childhood obesity, we similarly found that maternal pregravid BMI – independent of maternal glucose status or gestational weight gain – was the strongest predictor of childhood obesity and metabolic dysfunction (Am. J. Clin. Nutr. 2009;90:1303-13).
Other studies have teased apart the roles of maternal obesity and GDM in long-term health of offspring. This work has found that maternal obesity during pregnancy is associated with metabolic syndrome in the offspring and an increased risk of type 2 diabetes in youth, independent of maternal diabetes during pregnancy. A recent meta-analysis also reported that, although maternal diabetes is associated with an increased BMI z score, this was no longer significant after adjustments were made for prepregnancy BMI (Diabetologia 2011;54:1957-66).
Maternal pregravid obesity, therefore, is not only a risk factor for neonatal adiposity at birth, but also for the longer-term risk of obesity and metabolic dysfunction in the offspring – independent of maternal GDM or excessive gestational weight gain.
Interventions in Pregnancy
Numerous prospective trials have examined lifestyle interventions for obese women during pregnancy. One randomized controlled study of a low glycemic index diet in pregnancy (coined the ROLO study) involved 800 women in Ireland who had previously delivered an infant weighting greater than 4,000 g. Women were randomized to receive the restricted diet or no intervention at 13 weeks. Despite a decrease in gestational weight gain in the intervention group, there were no differences in birth weight, birth weight percentile, ponderal index, or macrosomia between the two groups (BMJ 2012;345:e5605).
Another randomized controlled trial reported by a Danish group involved an intervention that consisted of dietary guidance, free membership in a fitness center, and personal coaching initiated between 10 and 14 weeks of gestation. There was a decrease in gestational weight gain in the intervention group, but paradoxically, the infants in the intervention group also had significantly higher birth weight, compared with controls (Diabetes Care 2011;34:2502-7).
Additionally, there have been at least five meta-analyses published in the past 2 years looking at lifestyle interventions during pregnancy. All have concluded that interventions initiated during pregnancy have limited success in reducing excessive gestational weight gain but not necessarily to within the IOM guidelines. The literature contains scant evidence to support further benefits for infant or maternal health (in other words, fetal overgrowth, GDM, or hypertensive disorders).
A recent Cochrane review also concluded that the results of several randomized controlled trials suggest no significant difference in GDM incidence between women receiving exercise intervention versus routine care.
Just this year, three additional randomized controlled trials of lifestyle interventions during pregnancy were published. Only one, the Treatment of Obese Pregnant Women (TOP) study, showed a modest effect in decreasing gestational weight gain. None found a reduction in GDM or fetal overgrowth.
Focus on prepregnancy
Obesity is an inflammatory condition that increases the risk of insulin resistance, impaired beta-cell function, and abnormal adiponectin concentrations. In pregnancy, maternal obesity and hyperinsulinemia can affect placental growth and gene expression.
We have studied lean and obese women recruited prior to a planned pregnancy, as well as lean and obese women scheduled for elective pregnancy termination in the first trimester. Our research, some of which we reported recently in the American Journal of Physiology , has shown increased expression of lipogenic and inflammatory genes in maternal adipose tissue and in the placenta of obese women in the early first trimester, before any phenotypic change becomes apparent (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
Specifically, hyperinsulinemia and/or defective insulin action in obese women appears to affect the placental programming of genes relating to adipokine expression and lipid metabolism, as well as mitrochondrial function. Altered inflammatory and lipid pathways affect the availability of nutrients for the fetus and, consequently, the size and body composition of the fetus. Fetal overgrowth and neonatal adiposity can result.
In addition, our research has shown that obese women have decreased insulin suppression of lipolysis in white adipose tissue, which during pregnancy results in improved lipid availability for fetal fat accretion and lipotoxicity.
When interventions aimed at weight loss and improved insulin sensitivity are undertaken before pregnancy or in the period between pregnancies, we have the opportunity to increase fat oxidation and reduce oxidative stress in early pregnancy. We also may be able to limit placental inflammation and favorably affect placental growth and gene expression. By the second trimester, our research suggests, gene expression in the placenta and early molecular changes in the white adipose tissue have already been programmed and cannot be reversed (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
In studies by our group and others of interpregnancy weight loss or gain, interpregnancy weight loss has been associated with a lower risk of large-for-gestational-age (LGA) infants, whereas interpregnancy weight gain has been associated with an increased risk of LGA. Preliminary work from our group shows that the decrease in birth weight involves primarily fat and not lean mass.
The 2009 IOM guidelines support weight loss before pregnancy and state that overweight women should receive individual preconceptional counseling to improve diet quality, increase physical activity, and normalize weight. Multifaceted interventions do work: In obese nonpregnant individuals, lifestyle interventions, which include an exercise program, diet, and behavioral modification have been shown to be successful in improving insulin sensitivity, inflammation, and overall metabolic function.
According to the IOM report, preconceptional services aimed at achieving a healthy weight before conceiving will represent “a radical change to the care provided to obese women of childbearing age.” With continuing research and accumulating data, however, the concept is gaining traction as a viable paradigm for improving perinatal outcomes, with long-term benefits for both the mother and her baby.
Dr. Catalano reports that he has no disclosures relevant to this Master Class.
Obesity has become so pervasive that it is now considered a major health concern during pregnancy. Almost 56% of women aged 20-39 years in the United States are overweight or obese, based on the World Health Organization’s criteria for body mass index (BMI) and data from the 2009-2010 National Health and Nutrition Examination Survey (NHANES). Moreover, 7.5% of women in this age group are morbidly obese, with a body mass index (BMI) greater than 40 kg/m2 (JAMA 2012;307:491-7).
Obesity in pregnancy not only increases the risk of spontaneous abortions and congenital anomalies, it also increases the risk of gestational diabetes (GDM), hypertensive disorders, and other metabolic complications that affect both the mother and fetus.
Of much concern is the increased risk of fetal overgrowth and long-term health consequences for children of obese mothers. Obesity in early pregnancy has been shown to more than double the risk of obesity in the offspring, which in turn puts these children at risk for developing the metabolic syndrome – and, as Dr. Thomas Moore pointed out in September’s Master Class – appears to program these offspring for downstream cardiovascular risk in adulthood.
Mean term birth weights have risen in the United States during the past several decades. In Cleveland, we have seen a significant 116 g increase in mean term birth weight since 1975; this increase encompasses weights from the 5th to the 95th percentiles. Even more concerning is our finding that the ponderal index in our neonatal population has increased because of decreased fetal length over the last decade.
Some recent studies have suggested that the increase in birth weight in the United States has reached a plateau, but our analyses of national trends suggest that such change is secondary to factors such as earlier gestational age of delivery. Concurrently, an alarming number of children and adolescents – 17% of those aged 2-19 years, according to the 2009-2010 NHANES data – are overweight or obese (JAMA 2012;307:483-90).
How to best treat obesity for improved maternal and fetal health has thus become a focus of research. Studies on lifestyle interventions for obese women during pregnancy have aimed to prevent excessive gestational weight gain and decrease adverse perinatal outcomes – mainly macrosomia, GDM, and hypertensive disorders.
However, the results of this recent body of research have been disappointing. Lifestyle interventions initiated during pregnancy have had only limited success in improving perinatal outcomes. The research tells us that while we may be able to reduce excessive gestational weight gain, it is unlikely that we will be successful in reducing fetal overgrowth, GDM, or preeclampsia in obese women.
Moreover, other studies show that it is a high pregravid BMI – not excessive gestational weight gain or the development of GDM – that plays the biggest role in fetal overgrowth and fetal adiposity.
A paradigm shift is in order. We must think about lifestyle intervention and weight loss before pregnancy, when the woman’s metabolic condition can be improved in time to minimize adverse perinatal metabolic outcomes and to maximize metabolic benefits relating to fetal body composition and metabolism.
Role of prepregnancy BMI
In 2008, the Institute of Medicine (IOM) and National Research Council reexamined 1990 guidelines for gestational weight gain. They concluded that excessive weight gain in pregnancy was a primary contributor to the development of obesity in women. In fact, according to the 2009 IOM report, “Weight Gain During Pregnancy: Reexamining the Guidelines” (Washington: National Academy Press, 2009), 38% of normal weight, 63% of overweight, and 46% of obese women had gained weight in excess of the earlier guidelines.
Helping our patients to gain within the guidelines is important. Excessive gestational weight gain is a primary risk factor for maternal postpartum weight retention, which increases the risk for maternal obesity in a subsequent pregnancy. It also has been associated with a modest increased risk of preterm birth and development of type 2 diabetes.
Interestingly, however, high gestational weight gain has not been related to an increased risk of fetal overgrowth or macrosomia in many obese women. Increased gestational weight gain is a greater risk for fetal overgrowth in women who are of normal weight prior to pregnancy (J. Clin. Endocrinol. Metab. 2012;97:3648-54).
Our research has found that in overweight and obese women, it is maternal pregravid BMI – and not gestational weight gain – that presents the greatest risk for fetal macrosomia, and more specifically, the greatest risk for fetal obesity. Even when glucose tolerance levels are normal, overweight and obese women have neonates who are heavier and who have significant increases in the percentage of body fat and fat mass (Am. J. Obstet. Gynecol. 2006;195:1100-3).
In an 8-year prospective study of the perinatal risk factors associated with childhood obesity, we similarly found that maternal pregravid BMI – independent of maternal glucose status or gestational weight gain – was the strongest predictor of childhood obesity and metabolic dysfunction (Am. J. Clin. Nutr. 2009;90:1303-13).
Other studies have teased apart the roles of maternal obesity and GDM in long-term health of offspring. This work has found that maternal obesity during pregnancy is associated with metabolic syndrome in the offspring and an increased risk of type 2 diabetes in youth, independent of maternal diabetes during pregnancy. A recent meta-analysis also reported that, although maternal diabetes is associated with an increased BMI z score, this was no longer significant after adjustments were made for prepregnancy BMI (Diabetologia 2011;54:1957-66).
Maternal pregravid obesity, therefore, is not only a risk factor for neonatal adiposity at birth, but also for the longer-term risk of obesity and metabolic dysfunction in the offspring – independent of maternal GDM or excessive gestational weight gain.
Interventions in Pregnancy
Numerous prospective trials have examined lifestyle interventions for obese women during pregnancy. One randomized controlled study of a low glycemic index diet in pregnancy (coined the ROLO study) involved 800 women in Ireland who had previously delivered an infant weighting greater than 4,000 g. Women were randomized to receive the restricted diet or no intervention at 13 weeks. Despite a decrease in gestational weight gain in the intervention group, there were no differences in birth weight, birth weight percentile, ponderal index, or macrosomia between the two groups (BMJ 2012;345:e5605).
Another randomized controlled trial reported by a Danish group involved an intervention that consisted of dietary guidance, free membership in a fitness center, and personal coaching initiated between 10 and 14 weeks of gestation. There was a decrease in gestational weight gain in the intervention group, but paradoxically, the infants in the intervention group also had significantly higher birth weight, compared with controls (Diabetes Care 2011;34:2502-7).
Additionally, there have been at least five meta-analyses published in the past 2 years looking at lifestyle interventions during pregnancy. All have concluded that interventions initiated during pregnancy have limited success in reducing excessive gestational weight gain but not necessarily to within the IOM guidelines. The literature contains scant evidence to support further benefits for infant or maternal health (in other words, fetal overgrowth, GDM, or hypertensive disorders).
A recent Cochrane review also concluded that the results of several randomized controlled trials suggest no significant difference in GDM incidence between women receiving exercise intervention versus routine care.
Just this year, three additional randomized controlled trials of lifestyle interventions during pregnancy were published. Only one, the Treatment of Obese Pregnant Women (TOP) study, showed a modest effect in decreasing gestational weight gain. None found a reduction in GDM or fetal overgrowth.
Focus on prepregnancy
Obesity is an inflammatory condition that increases the risk of insulin resistance, impaired beta-cell function, and abnormal adiponectin concentrations. In pregnancy, maternal obesity and hyperinsulinemia can affect placental growth and gene expression.
We have studied lean and obese women recruited prior to a planned pregnancy, as well as lean and obese women scheduled for elective pregnancy termination in the first trimester. Our research, some of which we reported recently in the American Journal of Physiology , has shown increased expression of lipogenic and inflammatory genes in maternal adipose tissue and in the placenta of obese women in the early first trimester, before any phenotypic change becomes apparent (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
Specifically, hyperinsulinemia and/or defective insulin action in obese women appears to affect the placental programming of genes relating to adipokine expression and lipid metabolism, as well as mitrochondrial function. Altered inflammatory and lipid pathways affect the availability of nutrients for the fetus and, consequently, the size and body composition of the fetus. Fetal overgrowth and neonatal adiposity can result.
In addition, our research has shown that obese women have decreased insulin suppression of lipolysis in white adipose tissue, which during pregnancy results in improved lipid availability for fetal fat accretion and lipotoxicity.
When interventions aimed at weight loss and improved insulin sensitivity are undertaken before pregnancy or in the period between pregnancies, we have the opportunity to increase fat oxidation and reduce oxidative stress in early pregnancy. We also may be able to limit placental inflammation and favorably affect placental growth and gene expression. By the second trimester, our research suggests, gene expression in the placenta and early molecular changes in the white adipose tissue have already been programmed and cannot be reversed (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
In studies by our group and others of interpregnancy weight loss or gain, interpregnancy weight loss has been associated with a lower risk of large-for-gestational-age (LGA) infants, whereas interpregnancy weight gain has been associated with an increased risk of LGA. Preliminary work from our group shows that the decrease in birth weight involves primarily fat and not lean mass.
The 2009 IOM guidelines support weight loss before pregnancy and state that overweight women should receive individual preconceptional counseling to improve diet quality, increase physical activity, and normalize weight. Multifaceted interventions do work: In obese nonpregnant individuals, lifestyle interventions, which include an exercise program, diet, and behavioral modification have been shown to be successful in improving insulin sensitivity, inflammation, and overall metabolic function.
According to the IOM report, preconceptional services aimed at achieving a healthy weight before conceiving will represent “a radical change to the care provided to obese women of childbearing age.” With continuing research and accumulating data, however, the concept is gaining traction as a viable paradigm for improving perinatal outcomes, with long-term benefits for both the mother and her baby.
Dr. Catalano reports that he has no disclosures relevant to this Master Class.
Urinary incontinence – An individual and societal ill
Urinary incontinence is a major health care concern, both in terms of the numbers of women who are suffering and with respect to societal costs and the impact on health care spending. Approximately 15 years ago, an international group reported in the Journal of the American Medical Association that 200 million people worldwide – 75%-80% of them women – were suffering from urinary incontinence (JAMA 1998;280:951-3).
Since then, a high prevalence of urinary incontinence has been documented in various studies and reports. Experts have estimated, for instance, that between 13 million and 25 million adult Americans experience transient or chronic symptoms, and that approximately half of these patients suffer from severe or bothersome symptoms. Again, the majority of these individuals are women.
Consumer-based research suggests that 25% of women over the age of 18 years experience episodes of urinary incontinence, according to prevalence data collected by the National Association for Continence. In 2001, 10% of women under the age of 65 years and 35% of women over 65 had symptoms of involuntary leakage, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Despite this, nearly two-thirds of patients never discussed bladder health with their health care provider and on average, women wait over 6 years from symptom onset before a diagnosis is established. Moreover, the costs are significant; in 2001, the cost for urinary incontinence in the United States was $16.3 billion (Obstet. Gynecol. 2001;98:398-406).
There are four types of urinary incontinence – urge, stress, mixed, and overflow. Urge incontinence typically is accompanied by urgency. Stress incontinence occurs with the increased abdominal pressure that accompanies effort, exertion, laughing, coughing, and sneezing. Overflow incontinence generally involves continuous urinary loss and incomplete bladder emptying.
Over the next four installments of Master Class in Gynecologic Surgery, I have chosen to feature the workup and treatment of urinary incontinence. For our first installment, I have asked my former resident Dr. Sandra Culbertson, who is now a professor in the department of obstetrics and gynecology at the University of Chicago, to share her knowledge of the optimal approach for evaluating urinary incontinence in the office. As she explains, it is critical to discern the uncomplicated cases of stress urinary incontinence from possibly complicated cases that require more assessment.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller had no relevant financial disclosures.
Urinary incontinence is a major health care concern, both in terms of the numbers of women who are suffering and with respect to societal costs and the impact on health care spending. Approximately 15 years ago, an international group reported in the Journal of the American Medical Association that 200 million people worldwide – 75%-80% of them women – were suffering from urinary incontinence (JAMA 1998;280:951-3).
Since then, a high prevalence of urinary incontinence has been documented in various studies and reports. Experts have estimated, for instance, that between 13 million and 25 million adult Americans experience transient or chronic symptoms, and that approximately half of these patients suffer from severe or bothersome symptoms. Again, the majority of these individuals are women.
Consumer-based research suggests that 25% of women over the age of 18 years experience episodes of urinary incontinence, according to prevalence data collected by the National Association for Continence. In 2001, 10% of women under the age of 65 years and 35% of women over 65 had symptoms of involuntary leakage, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Despite this, nearly two-thirds of patients never discussed bladder health with their health care provider and on average, women wait over 6 years from symptom onset before a diagnosis is established. Moreover, the costs are significant; in 2001, the cost for urinary incontinence in the United States was $16.3 billion (Obstet. Gynecol. 2001;98:398-406).
There are four types of urinary incontinence – urge, stress, mixed, and overflow. Urge incontinence typically is accompanied by urgency. Stress incontinence occurs with the increased abdominal pressure that accompanies effort, exertion, laughing, coughing, and sneezing. Overflow incontinence generally involves continuous urinary loss and incomplete bladder emptying.
Over the next four installments of Master Class in Gynecologic Surgery, I have chosen to feature the workup and treatment of urinary incontinence. For our first installment, I have asked my former resident Dr. Sandra Culbertson, who is now a professor in the department of obstetrics and gynecology at the University of Chicago, to share her knowledge of the optimal approach for evaluating urinary incontinence in the office. As she explains, it is critical to discern the uncomplicated cases of stress urinary incontinence from possibly complicated cases that require more assessment.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller had no relevant financial disclosures.
Urinary incontinence is a major health care concern, both in terms of the numbers of women who are suffering and with respect to societal costs and the impact on health care spending. Approximately 15 years ago, an international group reported in the Journal of the American Medical Association that 200 million people worldwide – 75%-80% of them women – were suffering from urinary incontinence (JAMA 1998;280:951-3).
Since then, a high prevalence of urinary incontinence has been documented in various studies and reports. Experts have estimated, for instance, that between 13 million and 25 million adult Americans experience transient or chronic symptoms, and that approximately half of these patients suffer from severe or bothersome symptoms. Again, the majority of these individuals are women.
Consumer-based research suggests that 25% of women over the age of 18 years experience episodes of urinary incontinence, according to prevalence data collected by the National Association for Continence. In 2001, 10% of women under the age of 65 years and 35% of women over 65 had symptoms of involuntary leakage, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Despite this, nearly two-thirds of patients never discussed bladder health with their health care provider and on average, women wait over 6 years from symptom onset before a diagnosis is established. Moreover, the costs are significant; in 2001, the cost for urinary incontinence in the United States was $16.3 billion (Obstet. Gynecol. 2001;98:398-406).
There are four types of urinary incontinence – urge, stress, mixed, and overflow. Urge incontinence typically is accompanied by urgency. Stress incontinence occurs with the increased abdominal pressure that accompanies effort, exertion, laughing, coughing, and sneezing. Overflow incontinence generally involves continuous urinary loss and incomplete bladder emptying.
Over the next four installments of Master Class in Gynecologic Surgery, I have chosen to feature the workup and treatment of urinary incontinence. For our first installment, I have asked my former resident Dr. Sandra Culbertson, who is now a professor in the department of obstetrics and gynecology at the University of Chicago, to share her knowledge of the optimal approach for evaluating urinary incontinence in the office. As she explains, it is critical to discern the uncomplicated cases of stress urinary incontinence from possibly complicated cases that require more assessment.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller had no relevant financial disclosures.
Master Class: Office evaluation for incontinence
Ten years ago, urodynamics were widely viewed as the gold standard for evaluating urinary incontinence. We often turned to such testing to confirm or reject the findings of our basic evaluation before determining the best type of treatment – especially before proceeding with primary anti-incontinence surgery.
What has emerged in recent years is a body of evidence that tells us otherwise. We now know that urodynamics do not give us all the answers, and that we can be much more judicious with its use.

A good history followed by a thorough physical examination and some office tests often enables us to make sound treatment recommendations without costly and potentially uncomfortable urodynamic testing. The key lies in discerning complicated and uncomplicated cases. For patients deemed to have uncomplicated stress urinary incontinence (SUI) – especially those who have failed conservative management – we can comfortably recommend surgical repair without urodynamic testing.
Identifying uncomplicated SUI
The history is the most important part of the evaluation for incontinence. Every patient who answers “yes” to a basic opening question about whether she has any concerns about bladder control should be asked a series of questions that will enable the physician to fully understand her symptoms, their severity, and their impact on her life and daily activities.
It is critical to determine whether you are dealing with pure SUI, pure urge incontinence (UI), or SUI with a component of UI. Mixed incontinence is quite prevalent. An analysis of recent National Health and Nutrition Examination Survey (NHANES) data showed that of those women reporting incontinence symptoms, almost 50% reported pure SUI, and 34% reported mixed incontinence (J. Urol. 2008;179: 656-61). Other studies similarly have shown prevalence rates of mixed incontinence above 30%.
The International Urogynecological Association (IUGA) recommends the use of validated questionnaires to assess incontinence and the relative contribution of UI and SUI symptoms. Some physicians do find the organized and structured format of questionnaires helpful in their practices. Others have incorporated questions from various tools into history-taking templates on their electronic medical records. Still others have made them part of a mental checklist for history taking.
The short-form version of the Urogenital Distress Inventory (UDI-6), for instance, asks the patient whether she experiences – and how much she is bothered by – the following: frequent urination; leakage related to a feeling of urgency; leakage related to activity, coughing, or sneezing; small amount of leakage; difficulty emptying the bladder; and pain or discomfort in the lower abdominal or genital area.
The Incontinence Impact Questionnaire can be used to further assess the impact of symptoms. The short-form IIQ (the IIQ-7) asks, for instance, about the extent to which urine leakage has affected household chores, physical recreation, social activities, or emotional health.
Since the UDI and IIQ were developed about 20 years ago, at least several other urinary incontinence questionnaires have been developed and validated. Whether or not questionnaires are utilized as official tools, history taking should capture their essence and provide you with enough information to ascertain the type of incontinence, frequency of occurrence, severity, and effect on daily life.
The history also must assess the possibility of voiding dysfunction. Positive responses to questions about nocturia, hesitancy, and the need to immediately revoid, for instance, point toward complicated SUI and the need for further assessment before embarking on surgical treatment for SUI.
Patients who have uncomplicated SUI, on the other hand, will answer negatively to questions about symptoms of predominant urgency, functional impairment, continuous leakage, and/or incomplete emptying. They also will not have had recurrent urinary tract infections or medical conditions that can affect lower urinary tract function (such as neurologic disease and poorly controlled diabetes).
The physical exam
Along with the history, the physical exam is important for identifying complicated SUI and confirming which cases of SUI are truly uncomplicated. Evaluation should include a cough stress test to confirm leakage from the urethra under stress, an assessment of urethral mobility, and an assessment for pelvic organ prolapse.
The cough stress test is usually done with the patient in the supine or semirecumbent lithotomy position. If you strongly suspect stress incontinence but have a negative result, consider the following:
• Make sure the patient has a comfortably full bladder.
• Many women will contract their pelvic floor muscles when coughing to try to avoid leaking. You can apply pressure against the posterior vaginal wall either digitally or with half of the bivalve speculum to keep the patient from activating her muscles.
• The cough test can be performed in the standing position.
Assessing urethral mobility similarly involves simple observation while the patient is in a supine lithotomy position and straining. A Q-tip test or the Pelvic Organ Prolapse Quantification (POP-Q) system may be used, but visualization and palpation also are completely acceptable.
Just as the absence of urethral mobility is a red flag, so is prolapse beyond the hymen. This patient could potentially have urethral kinking, which can mask the severity of SUI or be a source of retention. Either finding the absence of urethral mobility or the presence of POP beyond the hymen moves the case from the uncomplicated to complicated category and signals the need for further evaluation with urodynamics or other tests.
These and other findings for uncomplicated versus complicated SUI are outlined in a committee opinion issued recently by the American College of Obstetricians and Gynecologists and the American Urogynecologic Society (Committee Opinion No. 603, Obstet .Gynecol. 2014;123:1403-7).
As the ACOG-AUGS recommendations point out, urinalysis is part of the minimum work-up for stress incontinence. Measurement of postvoid residual urine volume also becomes important when midurethral sling surgery is being contemplated for uncomplicated SUI. A normal volume rules out potential bladder-emptying abnormalities and provides final assurance that the patient is a good candidate for surgical repair.
Recent research on urodynamics
Evidence that a simple office-based incontinence evaluation without preoperative urodynamic testing is appropriate for uncomplicated predominant SUI comes largely from two recent randomized noninferiority trials.
One of these trials – a study from the Urinary Incontinence Treatment Network in the United States, known as the VALUE trial – randomized 630 women with uncomplicated SUI to pretreatment work-up with or without urodynamics. Treatment success at 12 months was similar for the two groups (approximately 77%).
This finding, the authors wrote, suggests that for women with uncomplicated SUI, a “basic office evaluation” (i.e., a positive provocative stress test, a normal postvoiding residual volume, an assessment or urethral mobility, and a negative urinalysis) is a “sufficient preoperative work-up” (N. Engl. J. Med. 2012;366:1987-97).
The diagnosis of SUI as made by office evaluation was confirmed in 97% of women who underwent urodynamic testing, and while there were some adjustments in diagnosis after urodynamics, there were no major changes in treatment decision making after the testing. Approximately 93% of women in both groups underwent midurethral sling surgery.
The second trial, a Dutch study, focused on women who had already undergone urodynamic testing and been shown to have discordant findings on urodynamics and their history and clinical exam. The women – all of whom had uncomplicated predominant SUI – were randomized to undergo immediate midurethral sling surgery or receive individually tailored treatment (including sling surgery, behavioral and physical therapy, pessary, and anticholinergics).
At 1 year, there was no clinically significant difference between the two groups in patients’ assessment of their symptoms as measured by the UDI. The authors concluded that “an immediate midurethral sling operation is not inferior to individually tailored treatment based on urodynamic findings” and that “urodynamics should no longer be advised routinely before primary surgery in these patients” (Obstet. Gynecol. 2013;121:999-1008).
When urge incontinence is involved
Urodynamic testing was never believed to be perfect, but these and other studies have highlighted its imperfections. Urodynamics creates an artificial condition in the bladder, in effect, and some of the findings will involve artifact. A systematic review of studies that compared diagnoses based on symptoms with diagnoses after urodynamic investigation was interesting in this regard; while the review did not assess impact on treatment, it showed that there is poor agreement between clinical symptoms and urodynamic-based diagnoses (Neurourol. Urodyn. 2011;30:495-502).
Certainly, women with complicated SUI – as well as women who have recurrent SUI after a prior surgical intervention – require further assessment, which likely includes multichannel urodynamic testing.
Urodynamics also can play a useful role in decision making and counseling for some patients whose incontinence is predominately SUI, but is believed to involve some degree of urinary urgency. Patients with mixed urinary incontinence fare worse after midurethral sling procedures compared with patients who have SUI alone, and I counsel my patients accordingly, emphasizing that the sling will not address aspects of their incontinence related to urgency. When I sense that a patient may have unreasonably high expectations for surgery, urodynamic testing can provide some perspective on possible postoperative outcomes.
Treatment for UI or overactive bladder often may be initiated after simple office-based evaluation, just as with SUI. The goal, similarly, is to discern relatively uncomplicated or straightforward cases from complicated ones. Urologic, medical, and neurologic histories should be obtained, for instance, and retention issues (which can aggravate UI) should be ruled out through the measurement of postvoid residual urine volume.
Just as with SUI, evaluation of suspected UI more often than not involves careful history taking and clinical probing. A voiding diary can sometimes be helpful; I send patients home with such a tool when the history is inconclusive or I suspect behavioral (excessive fluid intake) or functional issues as significant factors in bladder control.
It is important to keep in mind that patients with severe SUI may have urinary frequency as a learned response. Such patients appear to have overactive bladder in addition to SUI, but may actually be urinating frequently because they’ve learned that doing so results in less leakage. In our practice we’ve observed that patients with a learned response tend not to have nocturia, while those with overactive bladder do report nocturia.
Dr. Culbertson had no relevant financial disclosures.
Dr. Culbertson is a professor in the department of obstetrics and gynecology at the University of Chicago.
Ten years ago, urodynamics were widely viewed as the gold standard for evaluating urinary incontinence. We often turned to such testing to confirm or reject the findings of our basic evaluation before determining the best type of treatment – especially before proceeding with primary anti-incontinence surgery.
What has emerged in recent years is a body of evidence that tells us otherwise. We now know that urodynamics do not give us all the answers, and that we can be much more judicious with its use.

A good history followed by a thorough physical examination and some office tests often enables us to make sound treatment recommendations without costly and potentially uncomfortable urodynamic testing. The key lies in discerning complicated and uncomplicated cases. For patients deemed to have uncomplicated stress urinary incontinence (SUI) – especially those who have failed conservative management – we can comfortably recommend surgical repair without urodynamic testing.
Identifying uncomplicated SUI
The history is the most important part of the evaluation for incontinence. Every patient who answers “yes” to a basic opening question about whether she has any concerns about bladder control should be asked a series of questions that will enable the physician to fully understand her symptoms, their severity, and their impact on her life and daily activities.
It is critical to determine whether you are dealing with pure SUI, pure urge incontinence (UI), or SUI with a component of UI. Mixed incontinence is quite prevalent. An analysis of recent National Health and Nutrition Examination Survey (NHANES) data showed that of those women reporting incontinence symptoms, almost 50% reported pure SUI, and 34% reported mixed incontinence (J. Urol. 2008;179: 656-61). Other studies similarly have shown prevalence rates of mixed incontinence above 30%.
The International Urogynecological Association (IUGA) recommends the use of validated questionnaires to assess incontinence and the relative contribution of UI and SUI symptoms. Some physicians do find the organized and structured format of questionnaires helpful in their practices. Others have incorporated questions from various tools into history-taking templates on their electronic medical records. Still others have made them part of a mental checklist for history taking.
The short-form version of the Urogenital Distress Inventory (UDI-6), for instance, asks the patient whether she experiences – and how much she is bothered by – the following: frequent urination; leakage related to a feeling of urgency; leakage related to activity, coughing, or sneezing; small amount of leakage; difficulty emptying the bladder; and pain or discomfort in the lower abdominal or genital area.
The Incontinence Impact Questionnaire can be used to further assess the impact of symptoms. The short-form IIQ (the IIQ-7) asks, for instance, about the extent to which urine leakage has affected household chores, physical recreation, social activities, or emotional health.
Since the UDI and IIQ were developed about 20 years ago, at least several other urinary incontinence questionnaires have been developed and validated. Whether or not questionnaires are utilized as official tools, history taking should capture their essence and provide you with enough information to ascertain the type of incontinence, frequency of occurrence, severity, and effect on daily life.
The history also must assess the possibility of voiding dysfunction. Positive responses to questions about nocturia, hesitancy, and the need to immediately revoid, for instance, point toward complicated SUI and the need for further assessment before embarking on surgical treatment for SUI.
Patients who have uncomplicated SUI, on the other hand, will answer negatively to questions about symptoms of predominant urgency, functional impairment, continuous leakage, and/or incomplete emptying. They also will not have had recurrent urinary tract infections or medical conditions that can affect lower urinary tract function (such as neurologic disease and poorly controlled diabetes).
The physical exam
Along with the history, the physical exam is important for identifying complicated SUI and confirming which cases of SUI are truly uncomplicated. Evaluation should include a cough stress test to confirm leakage from the urethra under stress, an assessment of urethral mobility, and an assessment for pelvic organ prolapse.
The cough stress test is usually done with the patient in the supine or semirecumbent lithotomy position. If you strongly suspect stress incontinence but have a negative result, consider the following:
• Make sure the patient has a comfortably full bladder.
• Many women will contract their pelvic floor muscles when coughing to try to avoid leaking. You can apply pressure against the posterior vaginal wall either digitally or with half of the bivalve speculum to keep the patient from activating her muscles.
• The cough test can be performed in the standing position.
Assessing urethral mobility similarly involves simple observation while the patient is in a supine lithotomy position and straining. A Q-tip test or the Pelvic Organ Prolapse Quantification (POP-Q) system may be used, but visualization and palpation also are completely acceptable.
Just as the absence of urethral mobility is a red flag, so is prolapse beyond the hymen. This patient could potentially have urethral kinking, which can mask the severity of SUI or be a source of retention. Either finding the absence of urethral mobility or the presence of POP beyond the hymen moves the case from the uncomplicated to complicated category and signals the need for further evaluation with urodynamics or other tests.
These and other findings for uncomplicated versus complicated SUI are outlined in a committee opinion issued recently by the American College of Obstetricians and Gynecologists and the American Urogynecologic Society (Committee Opinion No. 603, Obstet .Gynecol. 2014;123:1403-7).
As the ACOG-AUGS recommendations point out, urinalysis is part of the minimum work-up for stress incontinence. Measurement of postvoid residual urine volume also becomes important when midurethral sling surgery is being contemplated for uncomplicated SUI. A normal volume rules out potential bladder-emptying abnormalities and provides final assurance that the patient is a good candidate for surgical repair.
Recent research on urodynamics
Evidence that a simple office-based incontinence evaluation without preoperative urodynamic testing is appropriate for uncomplicated predominant SUI comes largely from two recent randomized noninferiority trials.
One of these trials – a study from the Urinary Incontinence Treatment Network in the United States, known as the VALUE trial – randomized 630 women with uncomplicated SUI to pretreatment work-up with or without urodynamics. Treatment success at 12 months was similar for the two groups (approximately 77%).
This finding, the authors wrote, suggests that for women with uncomplicated SUI, a “basic office evaluation” (i.e., a positive provocative stress test, a normal postvoiding residual volume, an assessment or urethral mobility, and a negative urinalysis) is a “sufficient preoperative work-up” (N. Engl. J. Med. 2012;366:1987-97).
The diagnosis of SUI as made by office evaluation was confirmed in 97% of women who underwent urodynamic testing, and while there were some adjustments in diagnosis after urodynamics, there were no major changes in treatment decision making after the testing. Approximately 93% of women in both groups underwent midurethral sling surgery.
The second trial, a Dutch study, focused on women who had already undergone urodynamic testing and been shown to have discordant findings on urodynamics and their history and clinical exam. The women – all of whom had uncomplicated predominant SUI – were randomized to undergo immediate midurethral sling surgery or receive individually tailored treatment (including sling surgery, behavioral and physical therapy, pessary, and anticholinergics).
At 1 year, there was no clinically significant difference between the two groups in patients’ assessment of their symptoms as measured by the UDI. The authors concluded that “an immediate midurethral sling operation is not inferior to individually tailored treatment based on urodynamic findings” and that “urodynamics should no longer be advised routinely before primary surgery in these patients” (Obstet. Gynecol. 2013;121:999-1008).
When urge incontinence is involved
Urodynamic testing was never believed to be perfect, but these and other studies have highlighted its imperfections. Urodynamics creates an artificial condition in the bladder, in effect, and some of the findings will involve artifact. A systematic review of studies that compared diagnoses based on symptoms with diagnoses after urodynamic investigation was interesting in this regard; while the review did not assess impact on treatment, it showed that there is poor agreement between clinical symptoms and urodynamic-based diagnoses (Neurourol. Urodyn. 2011;30:495-502).
Certainly, women with complicated SUI – as well as women who have recurrent SUI after a prior surgical intervention – require further assessment, which likely includes multichannel urodynamic testing.
Urodynamics also can play a useful role in decision making and counseling for some patients whose incontinence is predominately SUI, but is believed to involve some degree of urinary urgency. Patients with mixed urinary incontinence fare worse after midurethral sling procedures compared with patients who have SUI alone, and I counsel my patients accordingly, emphasizing that the sling will not address aspects of their incontinence related to urgency. When I sense that a patient may have unreasonably high expectations for surgery, urodynamic testing can provide some perspective on possible postoperative outcomes.
Treatment for UI or overactive bladder often may be initiated after simple office-based evaluation, just as with SUI. The goal, similarly, is to discern relatively uncomplicated or straightforward cases from complicated ones. Urologic, medical, and neurologic histories should be obtained, for instance, and retention issues (which can aggravate UI) should be ruled out through the measurement of postvoid residual urine volume.
Just as with SUI, evaluation of suspected UI more often than not involves careful history taking and clinical probing. A voiding diary can sometimes be helpful; I send patients home with such a tool when the history is inconclusive or I suspect behavioral (excessive fluid intake) or functional issues as significant factors in bladder control.
It is important to keep in mind that patients with severe SUI may have urinary frequency as a learned response. Such patients appear to have overactive bladder in addition to SUI, but may actually be urinating frequently because they’ve learned that doing so results in less leakage. In our practice we’ve observed that patients with a learned response tend not to have nocturia, while those with overactive bladder do report nocturia.
Dr. Culbertson had no relevant financial disclosures.
Dr. Culbertson is a professor in the department of obstetrics and gynecology at the University of Chicago.
Ten years ago, urodynamics were widely viewed as the gold standard for evaluating urinary incontinence. We often turned to such testing to confirm or reject the findings of our basic evaluation before determining the best type of treatment – especially before proceeding with primary anti-incontinence surgery.
What has emerged in recent years is a body of evidence that tells us otherwise. We now know that urodynamics do not give us all the answers, and that we can be much more judicious with its use.

A good history followed by a thorough physical examination and some office tests often enables us to make sound treatment recommendations without costly and potentially uncomfortable urodynamic testing. The key lies in discerning complicated and uncomplicated cases. For patients deemed to have uncomplicated stress urinary incontinence (SUI) – especially those who have failed conservative management – we can comfortably recommend surgical repair without urodynamic testing.
Identifying uncomplicated SUI
The history is the most important part of the evaluation for incontinence. Every patient who answers “yes” to a basic opening question about whether she has any concerns about bladder control should be asked a series of questions that will enable the physician to fully understand her symptoms, their severity, and their impact on her life and daily activities.
It is critical to determine whether you are dealing with pure SUI, pure urge incontinence (UI), or SUI with a component of UI. Mixed incontinence is quite prevalent. An analysis of recent National Health and Nutrition Examination Survey (NHANES) data showed that of those women reporting incontinence symptoms, almost 50% reported pure SUI, and 34% reported mixed incontinence (J. Urol. 2008;179: 656-61). Other studies similarly have shown prevalence rates of mixed incontinence above 30%.
The International Urogynecological Association (IUGA) recommends the use of validated questionnaires to assess incontinence and the relative contribution of UI and SUI symptoms. Some physicians do find the organized and structured format of questionnaires helpful in their practices. Others have incorporated questions from various tools into history-taking templates on their electronic medical records. Still others have made them part of a mental checklist for history taking.
The short-form version of the Urogenital Distress Inventory (UDI-6), for instance, asks the patient whether she experiences – and how much she is bothered by – the following: frequent urination; leakage related to a feeling of urgency; leakage related to activity, coughing, or sneezing; small amount of leakage; difficulty emptying the bladder; and pain or discomfort in the lower abdominal or genital area.
The Incontinence Impact Questionnaire can be used to further assess the impact of symptoms. The short-form IIQ (the IIQ-7) asks, for instance, about the extent to which urine leakage has affected household chores, physical recreation, social activities, or emotional health.
Since the UDI and IIQ were developed about 20 years ago, at least several other urinary incontinence questionnaires have been developed and validated. Whether or not questionnaires are utilized as official tools, history taking should capture their essence and provide you with enough information to ascertain the type of incontinence, frequency of occurrence, severity, and effect on daily life.
The history also must assess the possibility of voiding dysfunction. Positive responses to questions about nocturia, hesitancy, and the need to immediately revoid, for instance, point toward complicated SUI and the need for further assessment before embarking on surgical treatment for SUI.
Patients who have uncomplicated SUI, on the other hand, will answer negatively to questions about symptoms of predominant urgency, functional impairment, continuous leakage, and/or incomplete emptying. They also will not have had recurrent urinary tract infections or medical conditions that can affect lower urinary tract function (such as neurologic disease and poorly controlled diabetes).
The physical exam
Along with the history, the physical exam is important for identifying complicated SUI and confirming which cases of SUI are truly uncomplicated. Evaluation should include a cough stress test to confirm leakage from the urethra under stress, an assessment of urethral mobility, and an assessment for pelvic organ prolapse.
The cough stress test is usually done with the patient in the supine or semirecumbent lithotomy position. If you strongly suspect stress incontinence but have a negative result, consider the following:
• Make sure the patient has a comfortably full bladder.
• Many women will contract their pelvic floor muscles when coughing to try to avoid leaking. You can apply pressure against the posterior vaginal wall either digitally or with half of the bivalve speculum to keep the patient from activating her muscles.
• The cough test can be performed in the standing position.
Assessing urethral mobility similarly involves simple observation while the patient is in a supine lithotomy position and straining. A Q-tip test or the Pelvic Organ Prolapse Quantification (POP-Q) system may be used, but visualization and palpation also are completely acceptable.
Just as the absence of urethral mobility is a red flag, so is prolapse beyond the hymen. This patient could potentially have urethral kinking, which can mask the severity of SUI or be a source of retention. Either finding the absence of urethral mobility or the presence of POP beyond the hymen moves the case from the uncomplicated to complicated category and signals the need for further evaluation with urodynamics or other tests.
These and other findings for uncomplicated versus complicated SUI are outlined in a committee opinion issued recently by the American College of Obstetricians and Gynecologists and the American Urogynecologic Society (Committee Opinion No. 603, Obstet .Gynecol. 2014;123:1403-7).
As the ACOG-AUGS recommendations point out, urinalysis is part of the minimum work-up for stress incontinence. Measurement of postvoid residual urine volume also becomes important when midurethral sling surgery is being contemplated for uncomplicated SUI. A normal volume rules out potential bladder-emptying abnormalities and provides final assurance that the patient is a good candidate for surgical repair.
Recent research on urodynamics
Evidence that a simple office-based incontinence evaluation without preoperative urodynamic testing is appropriate for uncomplicated predominant SUI comes largely from two recent randomized noninferiority trials.
One of these trials – a study from the Urinary Incontinence Treatment Network in the United States, known as the VALUE trial – randomized 630 women with uncomplicated SUI to pretreatment work-up with or without urodynamics. Treatment success at 12 months was similar for the two groups (approximately 77%).
This finding, the authors wrote, suggests that for women with uncomplicated SUI, a “basic office evaluation” (i.e., a positive provocative stress test, a normal postvoiding residual volume, an assessment or urethral mobility, and a negative urinalysis) is a “sufficient preoperative work-up” (N. Engl. J. Med. 2012;366:1987-97).
The diagnosis of SUI as made by office evaluation was confirmed in 97% of women who underwent urodynamic testing, and while there were some adjustments in diagnosis after urodynamics, there were no major changes in treatment decision making after the testing. Approximately 93% of women in both groups underwent midurethral sling surgery.
The second trial, a Dutch study, focused on women who had already undergone urodynamic testing and been shown to have discordant findings on urodynamics and their history and clinical exam. The women – all of whom had uncomplicated predominant SUI – were randomized to undergo immediate midurethral sling surgery or receive individually tailored treatment (including sling surgery, behavioral and physical therapy, pessary, and anticholinergics).
At 1 year, there was no clinically significant difference between the two groups in patients’ assessment of their symptoms as measured by the UDI. The authors concluded that “an immediate midurethral sling operation is not inferior to individually tailored treatment based on urodynamic findings” and that “urodynamics should no longer be advised routinely before primary surgery in these patients” (Obstet. Gynecol. 2013;121:999-1008).
When urge incontinence is involved
Urodynamic testing was never believed to be perfect, but these and other studies have highlighted its imperfections. Urodynamics creates an artificial condition in the bladder, in effect, and some of the findings will involve artifact. A systematic review of studies that compared diagnoses based on symptoms with diagnoses after urodynamic investigation was interesting in this regard; while the review did not assess impact on treatment, it showed that there is poor agreement between clinical symptoms and urodynamic-based diagnoses (Neurourol. Urodyn. 2011;30:495-502).
Certainly, women with complicated SUI – as well as women who have recurrent SUI after a prior surgical intervention – require further assessment, which likely includes multichannel urodynamic testing.
Urodynamics also can play a useful role in decision making and counseling for some patients whose incontinence is predominately SUI, but is believed to involve some degree of urinary urgency. Patients with mixed urinary incontinence fare worse after midurethral sling procedures compared with patients who have SUI alone, and I counsel my patients accordingly, emphasizing that the sling will not address aspects of their incontinence related to urgency. When I sense that a patient may have unreasonably high expectations for surgery, urodynamic testing can provide some perspective on possible postoperative outcomes.
Treatment for UI or overactive bladder often may be initiated after simple office-based evaluation, just as with SUI. The goal, similarly, is to discern relatively uncomplicated or straightforward cases from complicated ones. Urologic, medical, and neurologic histories should be obtained, for instance, and retention issues (which can aggravate UI) should be ruled out through the measurement of postvoid residual urine volume.
Just as with SUI, evaluation of suspected UI more often than not involves careful history taking and clinical probing. A voiding diary can sometimes be helpful; I send patients home with such a tool when the history is inconclusive or I suspect behavioral (excessive fluid intake) or functional issues as significant factors in bladder control.
It is important to keep in mind that patients with severe SUI may have urinary frequency as a learned response. Such patients appear to have overactive bladder in addition to SUI, but may actually be urinating frequently because they’ve learned that doing so results in less leakage. In our practice we’ve observed that patients with a learned response tend not to have nocturia, while those with overactive bladder do report nocturia.
Dr. Culbertson had no relevant financial disclosures.
Dr. Culbertson is a professor in the department of obstetrics and gynecology at the University of Chicago.
Gestational diabetes and the Barker Hypothesis
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).
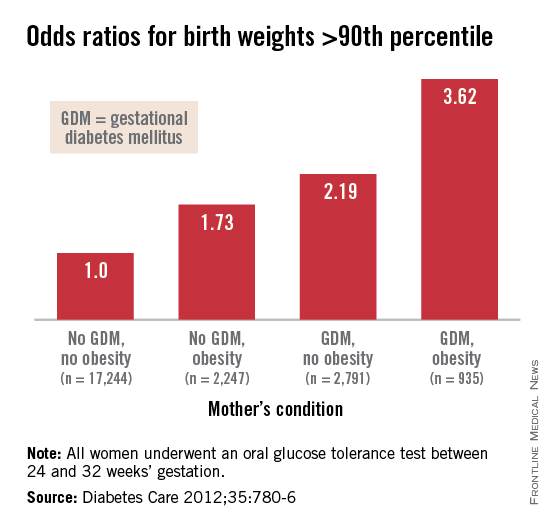
While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.
Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.
Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
The fetal origins hypothesis
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at obnews@frontlinemedcom.com.
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at obnews@frontlinemedcom.com.
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at obnews@frontlinemedcom.com.