User login
Payment changes drive hysteroscopy to the office
The benefits of integrating hysteroscopy into office practice have been compelling for some time. An in-office approach is patient centered, more efficient, and clinically valuable. It also has had the potential to be economically valuable for practices that are able to perform a mix of diagnostic and therapeutic/operative hysteroscopies.
Dramatic shifts within the Centers for Medicare & Medicaid Services fee schedule in 2017 – and commensurate changes in the private insurance market – have now ramped up this value, making it all the more important that physicians consider investing in equipment and adopting an in-office approach.
Central to this increase, in turn, is a significant increase in practice expense reimbursement. CMS has included the costs of equipment, including the costs of the hysteroscopic fluid management system and the hysteroscopic tissue resection system, in recalibrating the practice expense relative value unit. Clearly, physicians are being encouraged to move hysteroscopic procedures into the office.
Weighing an investment
In the Medicare resource-based relative value scale payment system, relative value units (RVUs) are calculated based on three elements: physician work, practice expenses, and malpractice cost. Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
This isn’t the first year that the payment system – a standard for many other payers in determining compensation – allows for higher reimbursement for some hysteroscopic procedures performed in the office. The practice expense relative units have been higher for some time for certain hysteroscopic procedures – such as diagnostic hysteroscopy (code 58555), removal of a foreign body (58562), endometrial ablation (58353), and biopsy/polypectomy (58558) – when these procedures are performed in the office, compared with the hospital or an ambulatory surgical center.
However, the new increase in physician office payment for 58558 changes the equation significantly and ensures a better return on investment. In 2017, CMS offered a 12% increase in the facility fee paid to hospitals and a 2% increase in the facility fee paid to outpatient surgery centers when a hysteroscopic biopsy/polypectomy is performed in these settings, but the physician reimbursement in these cases declined 11%-19%.
On the flip side, an in-office approach to hysteroscopic biopsy/polypectomy has been rewarded in 2017 through a significantly higher practice expense RVU and a “non-facility” total RVU of 38.51 – a 237% increase over the 2016 practice expense RVU of 11.4. Such dramatic differences between the practice RVUs – and total RVUs – for in-office and out-of-office hysteroscopic procedures will continue for 2018.
Private insurers are following suit, and some are increasing their reimbursement even more. As of June 2017 in metropolitan Chicago, Blue Cross Blue Shield has been reimbursing in-office hysteroscopic biopsy/polypectomy at approximately $2,424.00; prior to June, the allowable charge was $742.81.
Equipment costs for in-office hysteroscopy can range from $15,000 to $35,000, based on whether equipment is new or used, the number of trays, and the style of camera and monitor system. Ancillary equipment/disposables cost $10 or less, and $40-$50 or less for diagnostic and many operative procedures, respectively. The prices for handpiece mechanical resection disposables or tissue removal devices vary based on company and blade type, so these costs will need to be accounted for if such equipment is incorporated. Again, the CMS increase in reimbursement for offices accommodates for the inclusion of these disposables as well as fluid management disposable costs.
If diagnostic hysteroscopy (as a separate procedure) is the procedure that you perform most often, the investment will look less favorable. However, if you anticipate performing hysteroscopic biopsies and/or polypectomies as well, the investment will look significantly more favorable now than it has in past years.
Once you have established your in-office system, even those procedures that are weighted equally for the practice setting (non-facility) and hospital/surgery center setting, such as hysteroscopic lysis of adhesions (58559), can be easily incorporated from a financial point of view.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. The setup is relatively simple and requires a dedicated exam room, not a surgical suite. You can perform one or two annual exams while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Hysteroscopy at the hospital, or even at an ambulatory surgical center, involves time driving, changing, and waiting for anesthesia.
For our patients, most importantly, an in-office approach offers less out-of-pocket expense (deductibles), less time away from family/work, avoidance of general anesthesia/intubation, and greater patient comfort from being within a familiar environment. For diagnostic procedures, the patient can be in and out in less than 30 minutes, and for operative procedures, she can be in and out in 1-2 hours, compared with more than 4 hours at the hospital.
Preparing the office
Physicians in Europe have been performing in-office hysteroscopy for years. But in the United States, it is a newer concept, with most gynecologic surgeons having been taught to perform surgical procedures in the operating room. Undoubtedly, our unfamiliarity with in-office surgery has played a role in the slow uptake of hysteroscopy in our practices.
Open communication about everything the patient will see hear and feel before, during and after the procedure is important. Focusing on these details can improve your patient’s experience and your professional relationship with her.
In an earlier edition of Master Class, I addressed instrumentation and technique, elements of pain control and anesthesia, and the value of a vaginoscopic approach to hysteroscopy. Vaginoscopy avoids the use of a vaginal speculum or cervical tenaculum, and is so tolerable to many patients that I use minimal premedication and only rarely use any local anesthetic and/or sedation, even for biopsies and polypectomies.
Preparing your practice for hysteroscopy is a multifaceted process involving not only the purchase and/or rental of equipment but also compliance with guidelines, regulatory considerations, patient rights, hospital transfer arrangements, and other issues. ACOG’s Report of the Presidential Task Force on Patient Safety in the Office Setting is a valuable resource for getting started. The report discusses anesthesia levels and the benefits and risks of a contract anesthesiologist, for instance, as well as the role of and processes for credentialing, privileging, and accreditation.
Checklists and drills are important for ensuring a safe practice, and the report discusses each of these elements and provides templates and examples. A sample “Office Surgical Safety Checklist” to be used for each procedure, for instance, has sections with preoperative steps (before anesthesia/analgesia, and before incision), intraoperative steps, postoperative steps, and discharge steps. Similar in format to checklists used in the aviation industry, each step has a box to be checked off to verify completion.
Mock drills help ensure that staff are knowledgeable about their roles and coordinated in their response to potential complications, such as vasovagal episodes, respiratory arrest caused by laryngospasm, and local anesthetic toxicity reactions. And, while not the focus of drills, we also must be prepared to manage cervical strictures and stenosis, cervical laceration, uterine perforation, and other complications.
Outpatient surgery guidelines from organizations such as the American College of Surgeons, the Joint Commission, state regulatory agencies, and professional liability insurers, can also be useful resources. With the use of ACOG’s report and other such resources, the set-up and the transition to in-office hysteroscopy need not be daunting. For most gynecologic surgeons, it will all feel comfortable after only a few procedures.
Dr. Cholkeri-Singh is with the University of Illinois at Chicago, and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital in Park Ridge, Ill. She is in private practice in Chicago. She is a consultant for Hologic, Bayer HealthCare, Olympus, Caldera Medical, Karl Storz, Medtronic, DYSIS Medical, and Channel Medsystems.
The benefits of integrating hysteroscopy into office practice have been compelling for some time. An in-office approach is patient centered, more efficient, and clinically valuable. It also has had the potential to be economically valuable for practices that are able to perform a mix of diagnostic and therapeutic/operative hysteroscopies.
Dramatic shifts within the Centers for Medicare & Medicaid Services fee schedule in 2017 – and commensurate changes in the private insurance market – have now ramped up this value, making it all the more important that physicians consider investing in equipment and adopting an in-office approach.
Central to this increase, in turn, is a significant increase in practice expense reimbursement. CMS has included the costs of equipment, including the costs of the hysteroscopic fluid management system and the hysteroscopic tissue resection system, in recalibrating the practice expense relative value unit. Clearly, physicians are being encouraged to move hysteroscopic procedures into the office.
Weighing an investment
In the Medicare resource-based relative value scale payment system, relative value units (RVUs) are calculated based on three elements: physician work, practice expenses, and malpractice cost. Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
This isn’t the first year that the payment system – a standard for many other payers in determining compensation – allows for higher reimbursement for some hysteroscopic procedures performed in the office. The practice expense relative units have been higher for some time for certain hysteroscopic procedures – such as diagnostic hysteroscopy (code 58555), removal of a foreign body (58562), endometrial ablation (58353), and biopsy/polypectomy (58558) – when these procedures are performed in the office, compared with the hospital or an ambulatory surgical center.
However, the new increase in physician office payment for 58558 changes the equation significantly and ensures a better return on investment. In 2017, CMS offered a 12% increase in the facility fee paid to hospitals and a 2% increase in the facility fee paid to outpatient surgery centers when a hysteroscopic biopsy/polypectomy is performed in these settings, but the physician reimbursement in these cases declined 11%-19%.
On the flip side, an in-office approach to hysteroscopic biopsy/polypectomy has been rewarded in 2017 through a significantly higher practice expense RVU and a “non-facility” total RVU of 38.51 – a 237% increase over the 2016 practice expense RVU of 11.4. Such dramatic differences between the practice RVUs – and total RVUs – for in-office and out-of-office hysteroscopic procedures will continue for 2018.
Private insurers are following suit, and some are increasing their reimbursement even more. As of June 2017 in metropolitan Chicago, Blue Cross Blue Shield has been reimbursing in-office hysteroscopic biopsy/polypectomy at approximately $2,424.00; prior to June, the allowable charge was $742.81.
Equipment costs for in-office hysteroscopy can range from $15,000 to $35,000, based on whether equipment is new or used, the number of trays, and the style of camera and monitor system. Ancillary equipment/disposables cost $10 or less, and $40-$50 or less for diagnostic and many operative procedures, respectively. The prices for handpiece mechanical resection disposables or tissue removal devices vary based on company and blade type, so these costs will need to be accounted for if such equipment is incorporated. Again, the CMS increase in reimbursement for offices accommodates for the inclusion of these disposables as well as fluid management disposable costs.
If diagnostic hysteroscopy (as a separate procedure) is the procedure that you perform most often, the investment will look less favorable. However, if you anticipate performing hysteroscopic biopsies and/or polypectomies as well, the investment will look significantly more favorable now than it has in past years.
Once you have established your in-office system, even those procedures that are weighted equally for the practice setting (non-facility) and hospital/surgery center setting, such as hysteroscopic lysis of adhesions (58559), can be easily incorporated from a financial point of view.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. The setup is relatively simple and requires a dedicated exam room, not a surgical suite. You can perform one or two annual exams while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Hysteroscopy at the hospital, or even at an ambulatory surgical center, involves time driving, changing, and waiting for anesthesia.
For our patients, most importantly, an in-office approach offers less out-of-pocket expense (deductibles), less time away from family/work, avoidance of general anesthesia/intubation, and greater patient comfort from being within a familiar environment. For diagnostic procedures, the patient can be in and out in less than 30 minutes, and for operative procedures, she can be in and out in 1-2 hours, compared with more than 4 hours at the hospital.
Preparing the office
Physicians in Europe have been performing in-office hysteroscopy for years. But in the United States, it is a newer concept, with most gynecologic surgeons having been taught to perform surgical procedures in the operating room. Undoubtedly, our unfamiliarity with in-office surgery has played a role in the slow uptake of hysteroscopy in our practices.
Open communication about everything the patient will see hear and feel before, during and after the procedure is important. Focusing on these details can improve your patient’s experience and your professional relationship with her.
In an earlier edition of Master Class, I addressed instrumentation and technique, elements of pain control and anesthesia, and the value of a vaginoscopic approach to hysteroscopy. Vaginoscopy avoids the use of a vaginal speculum or cervical tenaculum, and is so tolerable to many patients that I use minimal premedication and only rarely use any local anesthetic and/or sedation, even for biopsies and polypectomies.
Preparing your practice for hysteroscopy is a multifaceted process involving not only the purchase and/or rental of equipment but also compliance with guidelines, regulatory considerations, patient rights, hospital transfer arrangements, and other issues. ACOG’s Report of the Presidential Task Force on Patient Safety in the Office Setting is a valuable resource for getting started. The report discusses anesthesia levels and the benefits and risks of a contract anesthesiologist, for instance, as well as the role of and processes for credentialing, privileging, and accreditation.
Checklists and drills are important for ensuring a safe practice, and the report discusses each of these elements and provides templates and examples. A sample “Office Surgical Safety Checklist” to be used for each procedure, for instance, has sections with preoperative steps (before anesthesia/analgesia, and before incision), intraoperative steps, postoperative steps, and discharge steps. Similar in format to checklists used in the aviation industry, each step has a box to be checked off to verify completion.
Mock drills help ensure that staff are knowledgeable about their roles and coordinated in their response to potential complications, such as vasovagal episodes, respiratory arrest caused by laryngospasm, and local anesthetic toxicity reactions. And, while not the focus of drills, we also must be prepared to manage cervical strictures and stenosis, cervical laceration, uterine perforation, and other complications.
Outpatient surgery guidelines from organizations such as the American College of Surgeons, the Joint Commission, state regulatory agencies, and professional liability insurers, can also be useful resources. With the use of ACOG’s report and other such resources, the set-up and the transition to in-office hysteroscopy need not be daunting. For most gynecologic surgeons, it will all feel comfortable after only a few procedures.
Dr. Cholkeri-Singh is with the University of Illinois at Chicago, and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital in Park Ridge, Ill. She is in private practice in Chicago. She is a consultant for Hologic, Bayer HealthCare, Olympus, Caldera Medical, Karl Storz, Medtronic, DYSIS Medical, and Channel Medsystems.
The benefits of integrating hysteroscopy into office practice have been compelling for some time. An in-office approach is patient centered, more efficient, and clinically valuable. It also has had the potential to be economically valuable for practices that are able to perform a mix of diagnostic and therapeutic/operative hysteroscopies.
Dramatic shifts within the Centers for Medicare & Medicaid Services fee schedule in 2017 – and commensurate changes in the private insurance market – have now ramped up this value, making it all the more important that physicians consider investing in equipment and adopting an in-office approach.
Central to this increase, in turn, is a significant increase in practice expense reimbursement. CMS has included the costs of equipment, including the costs of the hysteroscopic fluid management system and the hysteroscopic tissue resection system, in recalibrating the practice expense relative value unit. Clearly, physicians are being encouraged to move hysteroscopic procedures into the office.
Weighing an investment
In the Medicare resource-based relative value scale payment system, relative value units (RVUs) are calculated based on three elements: physician work, practice expenses, and malpractice cost. Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
This isn’t the first year that the payment system – a standard for many other payers in determining compensation – allows for higher reimbursement for some hysteroscopic procedures performed in the office. The practice expense relative units have been higher for some time for certain hysteroscopic procedures – such as diagnostic hysteroscopy (code 58555), removal of a foreign body (58562), endometrial ablation (58353), and biopsy/polypectomy (58558) – when these procedures are performed in the office, compared with the hospital or an ambulatory surgical center.
However, the new increase in physician office payment for 58558 changes the equation significantly and ensures a better return on investment. In 2017, CMS offered a 12% increase in the facility fee paid to hospitals and a 2% increase in the facility fee paid to outpatient surgery centers when a hysteroscopic biopsy/polypectomy is performed in these settings, but the physician reimbursement in these cases declined 11%-19%.
On the flip side, an in-office approach to hysteroscopic biopsy/polypectomy has been rewarded in 2017 through a significantly higher practice expense RVU and a “non-facility” total RVU of 38.51 – a 237% increase over the 2016 practice expense RVU of 11.4. Such dramatic differences between the practice RVUs – and total RVUs – for in-office and out-of-office hysteroscopic procedures will continue for 2018.
Private insurers are following suit, and some are increasing their reimbursement even more. As of June 2017 in metropolitan Chicago, Blue Cross Blue Shield has been reimbursing in-office hysteroscopic biopsy/polypectomy at approximately $2,424.00; prior to June, the allowable charge was $742.81.
Equipment costs for in-office hysteroscopy can range from $15,000 to $35,000, based on whether equipment is new or used, the number of trays, and the style of camera and monitor system. Ancillary equipment/disposables cost $10 or less, and $40-$50 or less for diagnostic and many operative procedures, respectively. The prices for handpiece mechanical resection disposables or tissue removal devices vary based on company and blade type, so these costs will need to be accounted for if such equipment is incorporated. Again, the CMS increase in reimbursement for offices accommodates for the inclusion of these disposables as well as fluid management disposable costs.
If diagnostic hysteroscopy (as a separate procedure) is the procedure that you perform most often, the investment will look less favorable. However, if you anticipate performing hysteroscopic biopsies and/or polypectomies as well, the investment will look significantly more favorable now than it has in past years.
Once you have established your in-office system, even those procedures that are weighted equally for the practice setting (non-facility) and hospital/surgery center setting, such as hysteroscopic lysis of adhesions (58559), can be easily incorporated from a financial point of view.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. The setup is relatively simple and requires a dedicated exam room, not a surgical suite. You can perform one or two annual exams while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Hysteroscopy at the hospital, or even at an ambulatory surgical center, involves time driving, changing, and waiting for anesthesia.
For our patients, most importantly, an in-office approach offers less out-of-pocket expense (deductibles), less time away from family/work, avoidance of general anesthesia/intubation, and greater patient comfort from being within a familiar environment. For diagnostic procedures, the patient can be in and out in less than 30 minutes, and for operative procedures, she can be in and out in 1-2 hours, compared with more than 4 hours at the hospital.
Preparing the office
Physicians in Europe have been performing in-office hysteroscopy for years. But in the United States, it is a newer concept, with most gynecologic surgeons having been taught to perform surgical procedures in the operating room. Undoubtedly, our unfamiliarity with in-office surgery has played a role in the slow uptake of hysteroscopy in our practices.
Open communication about everything the patient will see hear and feel before, during and after the procedure is important. Focusing on these details can improve your patient’s experience and your professional relationship with her.
In an earlier edition of Master Class, I addressed instrumentation and technique, elements of pain control and anesthesia, and the value of a vaginoscopic approach to hysteroscopy. Vaginoscopy avoids the use of a vaginal speculum or cervical tenaculum, and is so tolerable to many patients that I use minimal premedication and only rarely use any local anesthetic and/or sedation, even for biopsies and polypectomies.
Preparing your practice for hysteroscopy is a multifaceted process involving not only the purchase and/or rental of equipment but also compliance with guidelines, regulatory considerations, patient rights, hospital transfer arrangements, and other issues. ACOG’s Report of the Presidential Task Force on Patient Safety in the Office Setting is a valuable resource for getting started. The report discusses anesthesia levels and the benefits and risks of a contract anesthesiologist, for instance, as well as the role of and processes for credentialing, privileging, and accreditation.
Checklists and drills are important for ensuring a safe practice, and the report discusses each of these elements and provides templates and examples. A sample “Office Surgical Safety Checklist” to be used for each procedure, for instance, has sections with preoperative steps (before anesthesia/analgesia, and before incision), intraoperative steps, postoperative steps, and discharge steps. Similar in format to checklists used in the aviation industry, each step has a box to be checked off to verify completion.
Mock drills help ensure that staff are knowledgeable about their roles and coordinated in their response to potential complications, such as vasovagal episodes, respiratory arrest caused by laryngospasm, and local anesthetic toxicity reactions. And, while not the focus of drills, we also must be prepared to manage cervical strictures and stenosis, cervical laceration, uterine perforation, and other complications.
Outpatient surgery guidelines from organizations such as the American College of Surgeons, the Joint Commission, state regulatory agencies, and professional liability insurers, can also be useful resources. With the use of ACOG’s report and other such resources, the set-up and the transition to in-office hysteroscopy need not be daunting. For most gynecologic surgeons, it will all feel comfortable after only a few procedures.
Dr. Cholkeri-Singh is with the University of Illinois at Chicago, and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital in Park Ridge, Ill. She is in private practice in Chicago. She is a consultant for Hologic, Bayer HealthCare, Olympus, Caldera Medical, Karl Storz, Medtronic, DYSIS Medical, and Channel Medsystems.
Understanding the new economic benefits of in-office hysteroscopy
As a practicing reproductive endocrinologist and minimally invasive gynecologic surgeon, falling reimbursement has become routine. Furthermore, it was disadvantageous to perform in-office procedures while physician reimbursement was similar whether cases were performed in office, the hospital, or a surgery center. Higher procedural costs in the office, including reusable and disposable instrumentation and staffing, actually discouraged the physician who wanted to perform cases in the office, as it led to an overall reduction in reimbursement. Of course, certain outlying procedures have been reimbursed at a far greater rate in office and, as a result, global endometrial ablation and the Essure procedure now are generally performed in office.
In order to help us all understand the “nuts and bolts” behind the changes in physician compensation for in-office hysteroscopic procedures, I have once again called upon internationally recognized expert in hysteroscopic surgery, Aarathi Cholkeri-Singh, MD. At the AAGL 45th Global Congress on Minimally Invasive Gynecologic Surgery in 2016, Dr. Cholkeri-Singh was the chair and faculty of the postgraduate course, “Hysteroscopy 360° Beyond the Basics: Maximize Treatment, Minimize Failures.” At this year’s Global Congress, Dr. Cholkeri-Singh is a cochair of the postgraduate course “Advanced Operative Hysteroscopy: Expect the Unexpected.”
I am sure after reading Dr. Cholkeri-Singh’s comments, many of our readers of the Master Class in Gynecologic Surgery will add hysteroscopic surgery to their surgical repertoire.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is a consultant for Medtronic.
As a practicing reproductive endocrinologist and minimally invasive gynecologic surgeon, falling reimbursement has become routine. Furthermore, it was disadvantageous to perform in-office procedures while physician reimbursement was similar whether cases were performed in office, the hospital, or a surgery center. Higher procedural costs in the office, including reusable and disposable instrumentation and staffing, actually discouraged the physician who wanted to perform cases in the office, as it led to an overall reduction in reimbursement. Of course, certain outlying procedures have been reimbursed at a far greater rate in office and, as a result, global endometrial ablation and the Essure procedure now are generally performed in office.
In order to help us all understand the “nuts and bolts” behind the changes in physician compensation for in-office hysteroscopic procedures, I have once again called upon internationally recognized expert in hysteroscopic surgery, Aarathi Cholkeri-Singh, MD. At the AAGL 45th Global Congress on Minimally Invasive Gynecologic Surgery in 2016, Dr. Cholkeri-Singh was the chair and faculty of the postgraduate course, “Hysteroscopy 360° Beyond the Basics: Maximize Treatment, Minimize Failures.” At this year’s Global Congress, Dr. Cholkeri-Singh is a cochair of the postgraduate course “Advanced Operative Hysteroscopy: Expect the Unexpected.”
I am sure after reading Dr. Cholkeri-Singh’s comments, many of our readers of the Master Class in Gynecologic Surgery will add hysteroscopic surgery to their surgical repertoire.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is a consultant for Medtronic.
As a practicing reproductive endocrinologist and minimally invasive gynecologic surgeon, falling reimbursement has become routine. Furthermore, it was disadvantageous to perform in-office procedures while physician reimbursement was similar whether cases were performed in office, the hospital, or a surgery center. Higher procedural costs in the office, including reusable and disposable instrumentation and staffing, actually discouraged the physician who wanted to perform cases in the office, as it led to an overall reduction in reimbursement. Of course, certain outlying procedures have been reimbursed at a far greater rate in office and, as a result, global endometrial ablation and the Essure procedure now are generally performed in office.
In order to help us all understand the “nuts and bolts” behind the changes in physician compensation for in-office hysteroscopic procedures, I have once again called upon internationally recognized expert in hysteroscopic surgery, Aarathi Cholkeri-Singh, MD. At the AAGL 45th Global Congress on Minimally Invasive Gynecologic Surgery in 2016, Dr. Cholkeri-Singh was the chair and faculty of the postgraduate course, “Hysteroscopy 360° Beyond the Basics: Maximize Treatment, Minimize Failures.” At this year’s Global Congress, Dr. Cholkeri-Singh is a cochair of the postgraduate course “Advanced Operative Hysteroscopy: Expect the Unexpected.”
I am sure after reading Dr. Cholkeri-Singh’s comments, many of our readers of the Master Class in Gynecologic Surgery will add hysteroscopic surgery to their surgical repertoire.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is a consultant for Medtronic.
Morbidly adherent placenta: A multidisciplinary approach
The rate of placenta accreta has been rising, almost certainly as a consequence of the increasing cesarean delivery rate. It is estimated that morbidly adherent placenta (placenta accreta, increta, and percreta) occurs today in approximately 1 in 500 pregnancies. Women who have had prior cesarean deliveries or other uterine surgery, such as myomectomy, are at higher risk.
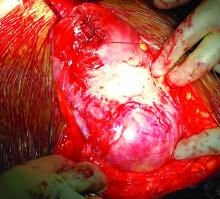
Morbidly adherent placenta (MAP) is associated with significant hemorrhage and morbidity – not only in cases of attempted placental removal, which is usually not advisable, but also in cases of cesarean hysterectomy. Cesarean hysterectomy is technically complex and completely different from other hysterectomies. The abnormal vasculature of MAP requires intricate, stepwise, vessel-by-vessel dissection and not only the uterine artery ligation that is the focus in hysterectomies performed for other indications.
In the last several years, we have demonstrated improved outcomes with such an approach at the University of Maryland, Baltimore. In 2014, we instituted a multidisciplinary complex obstetric surgery program for patients with MAP and others at high risk of intrapartum and postpartum complications. The program brings together obstetric anesthesiologists, the blood bank staff, the neonatal and surgical intensive care unit staff, vascular surgeons, perinatologists, interventional radiologists, urologists, and others.
Since the program was implemented, we have reduced our transfusion rate in patients with MAP by more than 60% while caring for increasing numbers of patients with the condition. We also have reduced the intensive care unit admission rate and improved overall surgical morbidity, including bladder complications. Moreover, our multidisciplinary approach is allowing us to develop more algorithms for management and to selectively take conservative approaches while also allowing us to lay the groundwork for future research.
The patients at risk
Anticipation is important: Identifying patient populations at high risk – and then evaluating individual risks – is essential for the prevention of delivery complications and the reduction of maternal morbidity.
Having had multiple cesarean deliveries – especially in pregnancies involving placenta previa – is one of the most important risk factors for developing MAP. One prospective cohort study of more than 30,000 women in 19 academic centers who had had cesarean deliveries found that, in cases of placenta previa, the risk of placenta accreta went from 3% after one cesarean delivery to 67% after five or more cesarean deliveries (Obstet Gynecol. 2006 Jun;107[6]:1226-32). Placenta accreta was defined in this study as the placenta’s being adherent to the uterine wall without easy separation. This definition included all forms of MAP.
Even without a history of placenta previa, patients who have had multiple cesarean deliveries – and developed consequent myometrial damage and scarring – should be evaluated for placental location during future pregnancies, as should patients who have had a myomectomy. A placenta that is anteriorly located in a patient who had a prior classical cesarean incision should also be thoroughly investigated. Overall, there is a risk of MAP whenever the placenta attaches to an area of uterine scarring.
Diagnosis of MAP can be made – as best as is currently possible – by ultrasonography or by MRI, the latter of which is performed in high-risk or ambiguous cases to look more closely at the depth of placental growth.
Our outcomes and process
In our complex obstetric surgery program, we identify and evaluate patients at risk for developing MAP and also prepare comprehensive surgical plans. Each individual’s plan addresses the optimal timing of and conditions for delivery, how the patient and the team should prepare for high-quality perioperative care, and how possible complications and emergency surgery should be handled, such as who should be called in the case of emergency preterm delivery.
Indeed, research has shown that the value of a multidisciplinary approach is greatest when MAP is identified or suspected before delivery. For instance, investigators who analyzed the pregnancies complicated by placenta accreta in Utah over a 12-year period found that cases managed by a multidisciplinary care team had a 50% risk reduction for early morbidities, compared with cases managed with standard obstetric care. The benefits were even greater when placenta accreta (defined in the study to include the spectrum of MAP) was suspected before delivery; this group had a nearly 80% risk reduction with multidisciplinary care (Obstet Gynecol. 2011 Feb;117[2 Pt 1]:331-7).
We recently compared our outcomes before and after the multidisciplinary complex obstetric surgery program was established. For patients with MAP, estimated blood loss has decreased by 40%, and the use of blood products has fallen by 60%-70%, with a corresponding reduction in intensive care unit admission. Moreover, our bladder complication rate fell to 6% after program implementation. This and our reoperation rate, among other outcomes, are lower than published rates from other similar medical centers that use a multidisciplinary approach.
We strive to have two surgeons in the operating room – either two senior surgeons or one senior surgeon and one junior surgeon – as well as a separate “operation supervisor” who monitors blood loss (volume and sources), vital signs, and other clinical points and who is continually thinking about next steps. The operation supervisor is not necessarily a third surgeon but could be an experienced surgical nurse or an obstetric anesthesiologist.
Obstetric anesthesiologists and the blood bank staff have proven to be especially important parts of our multidisciplinary team. At 28-30 weeks’ gestation, each patient has an anesthesia consult and also is tested for blood type and screened for antibodies. Patients also are tested for anemia at this time so that it may be corrected if necessary before surgery.
As determined by our multidisciplinary team, all deliveries are performed under general anesthesia, with early placement of both a central venous catheter and a peripheral arterial line to enable rapid transfusions of blood or fluid. Patients are routinely placed in the dorsal lithotomy position, which enables direct access to the vagina and better assessment of vaginal bleeding. And, when significant blood loss is anticipated, the intensive care unit team prepares a bed, and our surgical colleagues are alerted.
Conservative management
Interest in conservative management – in avoiding hysterectomy when it is deemed to carry much higher risks of hemorrhage or injury to adjacent tissue than leaving the placenta in situ – has resurged in Europe. However, research is still in its infancy regarding the benefits and safety of conservative management, and clear guidance about eligibility and contraindications is still needed (Am J Obstet Gynecol. 2015 Dec;213[6]:755-60).
One patient with the placenta left in situ had an urgent hysterectomy within 2 hours of delivery because of vaginal bleeding, with the total blood loss within an acceptable range and without complications. Another required an urgent hysterectomy 6 weeks after delivery because of severe hemorrhaging. The remaining two had nonurgent hysterectomies at least 6 weeks later, with the total blood loss minimized by the period of recovery and by some spontaneous regression of the placental bulk.
As we have gained more experience with conservative management and spent more time shaping multidisciplinary protocols, it has become clear to us that programs must have in place excellent protocols and strict rules for monitoring and follow-up given the risks of life-threatening hemorrhage and other significant complications when the placenta is left in situ.
A conservative approach also may be preferred by women who desire fertility preservation. Currently, in such cases, we have performed segmental or local resection with uterine repair. We do not yet have any data on subsequent pregnancies.
Research conducted within the growing sphere of complex obstetric surgery should help us to improve decision making and management of MAP. For instance, we need better imaging techniques to more accurately predict MAP and show us the degree of placental invasion. A study published several years ago that blinded sonographers from information about patients’ clinical history and risk factors found significant interobserver variability for the diagnosis of placenta accreta and sensitivity (53.5%) that was significantly lower than previously described (J Ultrasound Med. 2014 Dec;33[12]:2153-8).
Dr. Turan’s stepwise dissection
In addition to a multidisciplinary approach, a meticulous dissection technique can help drive improved outcomes. The morbidly adherent placenta is a hypervascular organ; it recruits a host of blood vessels, largely from the vaginal arteries, superior vesical arteries, and vaginal venous plexus.
Moreover, in most cases, this vascular remodeling exacerbates vascular patterns that are distorted to begin with as a result of the scarring process following previous uterine surgery. Scarred tissue is already hypervascular.
I have found that most of the blood loss during hysterectomy occurs during dissection of the poorly defined interface between the lower uterine segment and the bladder and not during dissection of the uterine artery. Identification of the cleavage plane and ligation of each individual vessel using a bipolar or small hand-held desiccation device are key in reducing blood loss. This can take a significant amount of time but is well worth it.
Managing super morbid obesity
The number of pregnant women who require challenging obstetric surgeries is increasing, and this includes women with super morbid obesity (BMI greater than 50 kg/m2 or weight greater than 350 lb). Cesarean deliveries for these patients have proven to be much more complicated, involving special anesthesia needs, for instance.
In addition to women with placental implantation abnormalities (MAP and placenta previa, for instance) and those with extreme morbid obesity, the complex obstetric surgery program also aims to manage patients with increased risk for surgical morbidities based on previous surgery, patients whose fetuses require ex utero intrapartum treatment, and women who require abdominal cerclage.
Dr. Turan is director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, as well as an associate professor of obstetrics, gynecology, and reproductive sciences. He reported having no relevant financial disclosures.
The rate of placenta accreta has been rising, almost certainly as a consequence of the increasing cesarean delivery rate. It is estimated that morbidly adherent placenta (placenta accreta, increta, and percreta) occurs today in approximately 1 in 500 pregnancies. Women who have had prior cesarean deliveries or other uterine surgery, such as myomectomy, are at higher risk.
Morbidly adherent placenta (MAP) is associated with significant hemorrhage and morbidity – not only in cases of attempted placental removal, which is usually not advisable, but also in cases of cesarean hysterectomy. Cesarean hysterectomy is technically complex and completely different from other hysterectomies. The abnormal vasculature of MAP requires intricate, stepwise, vessel-by-vessel dissection and not only the uterine artery ligation that is the focus in hysterectomies performed for other indications.
In the last several years, we have demonstrated improved outcomes with such an approach at the University of Maryland, Baltimore. In 2014, we instituted a multidisciplinary complex obstetric surgery program for patients with MAP and others at high risk of intrapartum and postpartum complications. The program brings together obstetric anesthesiologists, the blood bank staff, the neonatal and surgical intensive care unit staff, vascular surgeons, perinatologists, interventional radiologists, urologists, and others.
Since the program was implemented, we have reduced our transfusion rate in patients with MAP by more than 60% while caring for increasing numbers of patients with the condition. We also have reduced the intensive care unit admission rate and improved overall surgical morbidity, including bladder complications. Moreover, our multidisciplinary approach is allowing us to develop more algorithms for management and to selectively take conservative approaches while also allowing us to lay the groundwork for future research.
The patients at risk
Anticipation is important: Identifying patient populations at high risk – and then evaluating individual risks – is essential for the prevention of delivery complications and the reduction of maternal morbidity.
Having had multiple cesarean deliveries – especially in pregnancies involving placenta previa – is one of the most important risk factors for developing MAP. One prospective cohort study of more than 30,000 women in 19 academic centers who had had cesarean deliveries found that, in cases of placenta previa, the risk of placenta accreta went from 3% after one cesarean delivery to 67% after five or more cesarean deliveries (Obstet Gynecol. 2006 Jun;107[6]:1226-32). Placenta accreta was defined in this study as the placenta’s being adherent to the uterine wall without easy separation. This definition included all forms of MAP.
Even without a history of placenta previa, patients who have had multiple cesarean deliveries – and developed consequent myometrial damage and scarring – should be evaluated for placental location during future pregnancies, as should patients who have had a myomectomy. A placenta that is anteriorly located in a patient who had a prior classical cesarean incision should also be thoroughly investigated. Overall, there is a risk of MAP whenever the placenta attaches to an area of uterine scarring.
Diagnosis of MAP can be made – as best as is currently possible – by ultrasonography or by MRI, the latter of which is performed in high-risk or ambiguous cases to look more closely at the depth of placental growth.
Our outcomes and process
In our complex obstetric surgery program, we identify and evaluate patients at risk for developing MAP and also prepare comprehensive surgical plans. Each individual’s plan addresses the optimal timing of and conditions for delivery, how the patient and the team should prepare for high-quality perioperative care, and how possible complications and emergency surgery should be handled, such as who should be called in the case of emergency preterm delivery.
Indeed, research has shown that the value of a multidisciplinary approach is greatest when MAP is identified or suspected before delivery. For instance, investigators who analyzed the pregnancies complicated by placenta accreta in Utah over a 12-year period found that cases managed by a multidisciplinary care team had a 50% risk reduction for early morbidities, compared with cases managed with standard obstetric care. The benefits were even greater when placenta accreta (defined in the study to include the spectrum of MAP) was suspected before delivery; this group had a nearly 80% risk reduction with multidisciplinary care (Obstet Gynecol. 2011 Feb;117[2 Pt 1]:331-7).
We recently compared our outcomes before and after the multidisciplinary complex obstetric surgery program was established. For patients with MAP, estimated blood loss has decreased by 40%, and the use of blood products has fallen by 60%-70%, with a corresponding reduction in intensive care unit admission. Moreover, our bladder complication rate fell to 6% after program implementation. This and our reoperation rate, among other outcomes, are lower than published rates from other similar medical centers that use a multidisciplinary approach.
We strive to have two surgeons in the operating room – either two senior surgeons or one senior surgeon and one junior surgeon – as well as a separate “operation supervisor” who monitors blood loss (volume and sources), vital signs, and other clinical points and who is continually thinking about next steps. The operation supervisor is not necessarily a third surgeon but could be an experienced surgical nurse or an obstetric anesthesiologist.
Obstetric anesthesiologists and the blood bank staff have proven to be especially important parts of our multidisciplinary team. At 28-30 weeks’ gestation, each patient has an anesthesia consult and also is tested for blood type and screened for antibodies. Patients also are tested for anemia at this time so that it may be corrected if necessary before surgery.
As determined by our multidisciplinary team, all deliveries are performed under general anesthesia, with early placement of both a central venous catheter and a peripheral arterial line to enable rapid transfusions of blood or fluid. Patients are routinely placed in the dorsal lithotomy position, which enables direct access to the vagina and better assessment of vaginal bleeding. And, when significant blood loss is anticipated, the intensive care unit team prepares a bed, and our surgical colleagues are alerted.
Conservative management
Interest in conservative management – in avoiding hysterectomy when it is deemed to carry much higher risks of hemorrhage or injury to adjacent tissue than leaving the placenta in situ – has resurged in Europe. However, research is still in its infancy regarding the benefits and safety of conservative management, and clear guidance about eligibility and contraindications is still needed (Am J Obstet Gynecol. 2015 Dec;213[6]:755-60).
One patient with the placenta left in situ had an urgent hysterectomy within 2 hours of delivery because of vaginal bleeding, with the total blood loss within an acceptable range and without complications. Another required an urgent hysterectomy 6 weeks after delivery because of severe hemorrhaging. The remaining two had nonurgent hysterectomies at least 6 weeks later, with the total blood loss minimized by the period of recovery and by some spontaneous regression of the placental bulk.
As we have gained more experience with conservative management and spent more time shaping multidisciplinary protocols, it has become clear to us that programs must have in place excellent protocols and strict rules for monitoring and follow-up given the risks of life-threatening hemorrhage and other significant complications when the placenta is left in situ.
A conservative approach also may be preferred by women who desire fertility preservation. Currently, in such cases, we have performed segmental or local resection with uterine repair. We do not yet have any data on subsequent pregnancies.
Research conducted within the growing sphere of complex obstetric surgery should help us to improve decision making and management of MAP. For instance, we need better imaging techniques to more accurately predict MAP and show us the degree of placental invasion. A study published several years ago that blinded sonographers from information about patients’ clinical history and risk factors found significant interobserver variability for the diagnosis of placenta accreta and sensitivity (53.5%) that was significantly lower than previously described (J Ultrasound Med. 2014 Dec;33[12]:2153-8).
Dr. Turan’s stepwise dissection
In addition to a multidisciplinary approach, a meticulous dissection technique can help drive improved outcomes. The morbidly adherent placenta is a hypervascular organ; it recruits a host of blood vessels, largely from the vaginal arteries, superior vesical arteries, and vaginal venous plexus.
Moreover, in most cases, this vascular remodeling exacerbates vascular patterns that are distorted to begin with as a result of the scarring process following previous uterine surgery. Scarred tissue is already hypervascular.
I have found that most of the blood loss during hysterectomy occurs during dissection of the poorly defined interface between the lower uterine segment and the bladder and not during dissection of the uterine artery. Identification of the cleavage plane and ligation of each individual vessel using a bipolar or small hand-held desiccation device are key in reducing blood loss. This can take a significant amount of time but is well worth it.
Managing super morbid obesity
The number of pregnant women who require challenging obstetric surgeries is increasing, and this includes women with super morbid obesity (BMI greater than 50 kg/m2 or weight greater than 350 lb). Cesarean deliveries for these patients have proven to be much more complicated, involving special anesthesia needs, for instance.
In addition to women with placental implantation abnormalities (MAP and placenta previa, for instance) and those with extreme morbid obesity, the complex obstetric surgery program also aims to manage patients with increased risk for surgical morbidities based on previous surgery, patients whose fetuses require ex utero intrapartum treatment, and women who require abdominal cerclage.
Dr. Turan is director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, as well as an associate professor of obstetrics, gynecology, and reproductive sciences. He reported having no relevant financial disclosures.
The rate of placenta accreta has been rising, almost certainly as a consequence of the increasing cesarean delivery rate. It is estimated that morbidly adherent placenta (placenta accreta, increta, and percreta) occurs today in approximately 1 in 500 pregnancies. Women who have had prior cesarean deliveries or other uterine surgery, such as myomectomy, are at higher risk.
Morbidly adherent placenta (MAP) is associated with significant hemorrhage and morbidity – not only in cases of attempted placental removal, which is usually not advisable, but also in cases of cesarean hysterectomy. Cesarean hysterectomy is technically complex and completely different from other hysterectomies. The abnormal vasculature of MAP requires intricate, stepwise, vessel-by-vessel dissection and not only the uterine artery ligation that is the focus in hysterectomies performed for other indications.
In the last several years, we have demonstrated improved outcomes with such an approach at the University of Maryland, Baltimore. In 2014, we instituted a multidisciplinary complex obstetric surgery program for patients with MAP and others at high risk of intrapartum and postpartum complications. The program brings together obstetric anesthesiologists, the blood bank staff, the neonatal and surgical intensive care unit staff, vascular surgeons, perinatologists, interventional radiologists, urologists, and others.
Since the program was implemented, we have reduced our transfusion rate in patients with MAP by more than 60% while caring for increasing numbers of patients with the condition. We also have reduced the intensive care unit admission rate and improved overall surgical morbidity, including bladder complications. Moreover, our multidisciplinary approach is allowing us to develop more algorithms for management and to selectively take conservative approaches while also allowing us to lay the groundwork for future research.
The patients at risk
Anticipation is important: Identifying patient populations at high risk – and then evaluating individual risks – is essential for the prevention of delivery complications and the reduction of maternal morbidity.
Having had multiple cesarean deliveries – especially in pregnancies involving placenta previa – is one of the most important risk factors for developing MAP. One prospective cohort study of more than 30,000 women in 19 academic centers who had had cesarean deliveries found that, in cases of placenta previa, the risk of placenta accreta went from 3% after one cesarean delivery to 67% after five or more cesarean deliveries (Obstet Gynecol. 2006 Jun;107[6]:1226-32). Placenta accreta was defined in this study as the placenta’s being adherent to the uterine wall without easy separation. This definition included all forms of MAP.
Even without a history of placenta previa, patients who have had multiple cesarean deliveries – and developed consequent myometrial damage and scarring – should be evaluated for placental location during future pregnancies, as should patients who have had a myomectomy. A placenta that is anteriorly located in a patient who had a prior classical cesarean incision should also be thoroughly investigated. Overall, there is a risk of MAP whenever the placenta attaches to an area of uterine scarring.
Diagnosis of MAP can be made – as best as is currently possible – by ultrasonography or by MRI, the latter of which is performed in high-risk or ambiguous cases to look more closely at the depth of placental growth.
Our outcomes and process
In our complex obstetric surgery program, we identify and evaluate patients at risk for developing MAP and also prepare comprehensive surgical plans. Each individual’s plan addresses the optimal timing of and conditions for delivery, how the patient and the team should prepare for high-quality perioperative care, and how possible complications and emergency surgery should be handled, such as who should be called in the case of emergency preterm delivery.
Indeed, research has shown that the value of a multidisciplinary approach is greatest when MAP is identified or suspected before delivery. For instance, investigators who analyzed the pregnancies complicated by placenta accreta in Utah over a 12-year period found that cases managed by a multidisciplinary care team had a 50% risk reduction for early morbidities, compared with cases managed with standard obstetric care. The benefits were even greater when placenta accreta (defined in the study to include the spectrum of MAP) was suspected before delivery; this group had a nearly 80% risk reduction with multidisciplinary care (Obstet Gynecol. 2011 Feb;117[2 Pt 1]:331-7).
We recently compared our outcomes before and after the multidisciplinary complex obstetric surgery program was established. For patients with MAP, estimated blood loss has decreased by 40%, and the use of blood products has fallen by 60%-70%, with a corresponding reduction in intensive care unit admission. Moreover, our bladder complication rate fell to 6% after program implementation. This and our reoperation rate, among other outcomes, are lower than published rates from other similar medical centers that use a multidisciplinary approach.
We strive to have two surgeons in the operating room – either two senior surgeons or one senior surgeon and one junior surgeon – as well as a separate “operation supervisor” who monitors blood loss (volume and sources), vital signs, and other clinical points and who is continually thinking about next steps. The operation supervisor is not necessarily a third surgeon but could be an experienced surgical nurse or an obstetric anesthesiologist.
Obstetric anesthesiologists and the blood bank staff have proven to be especially important parts of our multidisciplinary team. At 28-30 weeks’ gestation, each patient has an anesthesia consult and also is tested for blood type and screened for antibodies. Patients also are tested for anemia at this time so that it may be corrected if necessary before surgery.
As determined by our multidisciplinary team, all deliveries are performed under general anesthesia, with early placement of both a central venous catheter and a peripheral arterial line to enable rapid transfusions of blood or fluid. Patients are routinely placed in the dorsal lithotomy position, which enables direct access to the vagina and better assessment of vaginal bleeding. And, when significant blood loss is anticipated, the intensive care unit team prepares a bed, and our surgical colleagues are alerted.
Conservative management
Interest in conservative management – in avoiding hysterectomy when it is deemed to carry much higher risks of hemorrhage or injury to adjacent tissue than leaving the placenta in situ – has resurged in Europe. However, research is still in its infancy regarding the benefits and safety of conservative management, and clear guidance about eligibility and contraindications is still needed (Am J Obstet Gynecol. 2015 Dec;213[6]:755-60).
One patient with the placenta left in situ had an urgent hysterectomy within 2 hours of delivery because of vaginal bleeding, with the total blood loss within an acceptable range and without complications. Another required an urgent hysterectomy 6 weeks after delivery because of severe hemorrhaging. The remaining two had nonurgent hysterectomies at least 6 weeks later, with the total blood loss minimized by the period of recovery and by some spontaneous regression of the placental bulk.
As we have gained more experience with conservative management and spent more time shaping multidisciplinary protocols, it has become clear to us that programs must have in place excellent protocols and strict rules for monitoring and follow-up given the risks of life-threatening hemorrhage and other significant complications when the placenta is left in situ.
A conservative approach also may be preferred by women who desire fertility preservation. Currently, in such cases, we have performed segmental or local resection with uterine repair. We do not yet have any data on subsequent pregnancies.
Research conducted within the growing sphere of complex obstetric surgery should help us to improve decision making and management of MAP. For instance, we need better imaging techniques to more accurately predict MAP and show us the degree of placental invasion. A study published several years ago that blinded sonographers from information about patients’ clinical history and risk factors found significant interobserver variability for the diagnosis of placenta accreta and sensitivity (53.5%) that was significantly lower than previously described (J Ultrasound Med. 2014 Dec;33[12]:2153-8).
Dr. Turan’s stepwise dissection
In addition to a multidisciplinary approach, a meticulous dissection technique can help drive improved outcomes. The morbidly adherent placenta is a hypervascular organ; it recruits a host of blood vessels, largely from the vaginal arteries, superior vesical arteries, and vaginal venous plexus.
Moreover, in most cases, this vascular remodeling exacerbates vascular patterns that are distorted to begin with as a result of the scarring process following previous uterine surgery. Scarred tissue is already hypervascular.
I have found that most of the blood loss during hysterectomy occurs during dissection of the poorly defined interface between the lower uterine segment and the bladder and not during dissection of the uterine artery. Identification of the cleavage plane and ligation of each individual vessel using a bipolar or small hand-held desiccation device are key in reducing blood loss. This can take a significant amount of time but is well worth it.
Managing super morbid obesity
The number of pregnant women who require challenging obstetric surgeries is increasing, and this includes women with super morbid obesity (BMI greater than 50 kg/m2 or weight greater than 350 lb). Cesarean deliveries for these patients have proven to be much more complicated, involving special anesthesia needs, for instance.
In addition to women with placental implantation abnormalities (MAP and placenta previa, for instance) and those with extreme morbid obesity, the complex obstetric surgery program also aims to manage patients with increased risk for surgical morbidities based on previous surgery, patients whose fetuses require ex utero intrapartum treatment, and women who require abdominal cerclage.
Dr. Turan is director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, as well as an associate professor of obstetrics, gynecology, and reproductive sciences. He reported having no relevant financial disclosures.
Multidisciplinary teams offer key to complex deliveries
Medical practice has evolved, and will continue to do so, as we begin pushing for more personalized and better precision health care. Gone are the days of the general practitioner who attempted to treat all conditions in all patients. Health care is now so complex that not only specialists but also so-called superspecialists are needed to manage complicated cases successfully.
One of the biggest challenges, and greatest opportunities, in ob.gyn. is the need to establish a multidisciplinary health team to the address the needs of today’s patients. More than ever, we are working with patients with advanced maternal age having their first pregnancies. More than ever, we are managing patients who have preexisting diabetes and are concurrently overweight or obese. More than ever, our patients are having multiple cesarean deliveries. More than ever, our patients are hoping – perhaps even expecting – to retain their fertility after a complicated delivery. More than ever, a single patient may need the guidance and care of not just an ob.gyn. or maternal-fetal medicine subspecialist but also an endocrinologist, cardiologist, diabetologist, genetic counselor, nutritionist – the list could go on.
The emergence and continued growth of personalized and preventive medicine in the very near future will catalyze fundamental changes at many different levels in health care and health systems. The need to establish multidisciplinary care teams is already apparent in ob.gyn. but is especially necessary in helping patients who experience complicated deliveries that could jeopardize their immediate and long-term health and fertility.
This month, we have invited M. Ozhan Turan, MD, PhD, the director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, to discuss the use of a multidisciplinary team in the management of patients with placenta accreta and other forms of morbidly adherent placenta.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column.
Medical practice has evolved, and will continue to do so, as we begin pushing for more personalized and better precision health care. Gone are the days of the general practitioner who attempted to treat all conditions in all patients. Health care is now so complex that not only specialists but also so-called superspecialists are needed to manage complicated cases successfully.
One of the biggest challenges, and greatest opportunities, in ob.gyn. is the need to establish a multidisciplinary health team to the address the needs of today’s patients. More than ever, we are working with patients with advanced maternal age having their first pregnancies. More than ever, we are managing patients who have preexisting diabetes and are concurrently overweight or obese. More than ever, our patients are having multiple cesarean deliveries. More than ever, our patients are hoping – perhaps even expecting – to retain their fertility after a complicated delivery. More than ever, a single patient may need the guidance and care of not just an ob.gyn. or maternal-fetal medicine subspecialist but also an endocrinologist, cardiologist, diabetologist, genetic counselor, nutritionist – the list could go on.
The emergence and continued growth of personalized and preventive medicine in the very near future will catalyze fundamental changes at many different levels in health care and health systems. The need to establish multidisciplinary care teams is already apparent in ob.gyn. but is especially necessary in helping patients who experience complicated deliveries that could jeopardize their immediate and long-term health and fertility.
This month, we have invited M. Ozhan Turan, MD, PhD, the director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, to discuss the use of a multidisciplinary team in the management of patients with placenta accreta and other forms of morbidly adherent placenta.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column.
Medical practice has evolved, and will continue to do so, as we begin pushing for more personalized and better precision health care. Gone are the days of the general practitioner who attempted to treat all conditions in all patients. Health care is now so complex that not only specialists but also so-called superspecialists are needed to manage complicated cases successfully.
One of the biggest challenges, and greatest opportunities, in ob.gyn. is the need to establish a multidisciplinary health team to the address the needs of today’s patients. More than ever, we are working with patients with advanced maternal age having their first pregnancies. More than ever, we are managing patients who have preexisting diabetes and are concurrently overweight or obese. More than ever, our patients are having multiple cesarean deliveries. More than ever, our patients are hoping – perhaps even expecting – to retain their fertility after a complicated delivery. More than ever, a single patient may need the guidance and care of not just an ob.gyn. or maternal-fetal medicine subspecialist but also an endocrinologist, cardiologist, diabetologist, genetic counselor, nutritionist – the list could go on.
The emergence and continued growth of personalized and preventive medicine in the very near future will catalyze fundamental changes at many different levels in health care and health systems. The need to establish multidisciplinary care teams is already apparent in ob.gyn. but is especially necessary in helping patients who experience complicated deliveries that could jeopardize their immediate and long-term health and fertility.
This month, we have invited M. Ozhan Turan, MD, PhD, the director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, to discuss the use of a multidisciplinary team in the management of patients with placenta accreta and other forms of morbidly adherent placenta.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column.
A multidisciplinary approach to diaphragmatic endometriosis
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.
Diaphragmatic and thoracic endometriosis
The first case of diaphragmatic endometriosis was reported by Alan Brews in 19541. Unfortunately, no guidelines exist to enhance the recognition and treatment.
Diaphragmatic and thoracic endometriosis often is overlooked by the gynecologist, not only because of lack of appreciation of the symptoms but also because of the failure to properly work-up the patient and evaluate the diaphragm at time of surgery. In a retrospective review of 3,008 patients with pelvic endometriosis published in Surgical Endoscopy in 2013, Marcello Ceccaroni, MD, PhD, and his colleagues found 46 cases (1.53%) with the intraoperative diagnosis of diaphragmatic endometriosis, six with liver involvement. Multiple diaphragmatic endometriosis lesions were seen in 70% of patients and, the vast majority being right-sided lesions (87%), with 11% of cases having bilateral lesions.2 While in the study, superficial lesions were generally vaporized using the argon beam coagulator, deep lesions were removed by sharp dissection, highlighting the need to have adequately trained minimally invasive surgeons treating diaphragmatic lesions via incision. If a pneumothorax occurred, and reabsorbable suture was placed after adequate expansion of the lung via positive pressure ventilation and progressive air suctioning with complete evacuation of the pneumothorax prior to the final closure (i.e., a purse string around the suction device), then the integrity of the closure could be proven using a bubble test with 500cc of saline placed at the diaphragm.
As the gynecologic surgeon studies Dr. Nezhat’s thorough discourse, it is obvious that, at times, a multidisciplinary team must be involved. Although possible, it would appear that risk of diaphragm paralysis secondary to injury of the phrenic nerve is indeed rare. This likely is because of the greater incidence of right-sided disease, rather than involving the central tendon, and lower likelihood that the lesion penetrates deeply. Nevertheless, a prudent multidisciplinary approach and knowledge of the anatomy will inevitably further reduce this rare complication.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.
References
1. Proc R Soc Med. 1954 Jun; 47(6):461-8.
2. Surg Endosc. 2013 Feb;27(2):625-32.
The first case of diaphragmatic endometriosis was reported by Alan Brews in 19541. Unfortunately, no guidelines exist to enhance the recognition and treatment.
Diaphragmatic and thoracic endometriosis often is overlooked by the gynecologist, not only because of lack of appreciation of the symptoms but also because of the failure to properly work-up the patient and evaluate the diaphragm at time of surgery. In a retrospective review of 3,008 patients with pelvic endometriosis published in Surgical Endoscopy in 2013, Marcello Ceccaroni, MD, PhD, and his colleagues found 46 cases (1.53%) with the intraoperative diagnosis of diaphragmatic endometriosis, six with liver involvement. Multiple diaphragmatic endometriosis lesions were seen in 70% of patients and, the vast majority being right-sided lesions (87%), with 11% of cases having bilateral lesions.2 While in the study, superficial lesions were generally vaporized using the argon beam coagulator, deep lesions were removed by sharp dissection, highlighting the need to have adequately trained minimally invasive surgeons treating diaphragmatic lesions via incision. If a pneumothorax occurred, and reabsorbable suture was placed after adequate expansion of the lung via positive pressure ventilation and progressive air suctioning with complete evacuation of the pneumothorax prior to the final closure (i.e., a purse string around the suction device), then the integrity of the closure could be proven using a bubble test with 500cc of saline placed at the diaphragm.
As the gynecologic surgeon studies Dr. Nezhat’s thorough discourse, it is obvious that, at times, a multidisciplinary team must be involved. Although possible, it would appear that risk of diaphragm paralysis secondary to injury of the phrenic nerve is indeed rare. This likely is because of the greater incidence of right-sided disease, rather than involving the central tendon, and lower likelihood that the lesion penetrates deeply. Nevertheless, a prudent multidisciplinary approach and knowledge of the anatomy will inevitably further reduce this rare complication.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.
References
1. Proc R Soc Med. 1954 Jun; 47(6):461-8.
2. Surg Endosc. 2013 Feb;27(2):625-32.
The first case of diaphragmatic endometriosis was reported by Alan Brews in 19541. Unfortunately, no guidelines exist to enhance the recognition and treatment.
Diaphragmatic and thoracic endometriosis often is overlooked by the gynecologist, not only because of lack of appreciation of the symptoms but also because of the failure to properly work-up the patient and evaluate the diaphragm at time of surgery. In a retrospective review of 3,008 patients with pelvic endometriosis published in Surgical Endoscopy in 2013, Marcello Ceccaroni, MD, PhD, and his colleagues found 46 cases (1.53%) with the intraoperative diagnosis of diaphragmatic endometriosis, six with liver involvement. Multiple diaphragmatic endometriosis lesions were seen in 70% of patients and, the vast majority being right-sided lesions (87%), with 11% of cases having bilateral lesions.2 While in the study, superficial lesions were generally vaporized using the argon beam coagulator, deep lesions were removed by sharp dissection, highlighting the need to have adequately trained minimally invasive surgeons treating diaphragmatic lesions via incision. If a pneumothorax occurred, and reabsorbable suture was placed after adequate expansion of the lung via positive pressure ventilation and progressive air suctioning with complete evacuation of the pneumothorax prior to the final closure (i.e., a purse string around the suction device), then the integrity of the closure could be proven using a bubble test with 500cc of saline placed at the diaphragm.
As the gynecologic surgeon studies Dr. Nezhat’s thorough discourse, it is obvious that, at times, a multidisciplinary team must be involved. Although possible, it would appear that risk of diaphragm paralysis secondary to injury of the phrenic nerve is indeed rare. This likely is because of the greater incidence of right-sided disease, rather than involving the central tendon, and lower likelihood that the lesion penetrates deeply. Nevertheless, a prudent multidisciplinary approach and knowledge of the anatomy will inevitably further reduce this rare complication.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.
References
1. Proc R Soc Med. 1954 Jun; 47(6):461-8.
2. Surg Endosc. 2013 Feb;27(2):625-32.
Diabetes’ social determinants: What they mean in our practices
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.
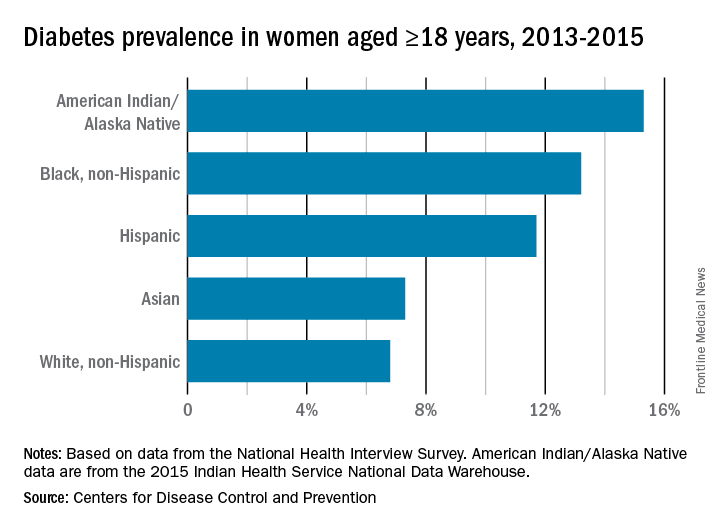
In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.

In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.

In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
The moving target of gestational diabetes care
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Tips and advice for Essure removal
Essure tubal microinserts were not designed to be removed. However, a small minority of women are requesting removal because of regret, complications, or the development of pelvic pain and other symptoms that may or may not be caused by the device.
Minimally invasive gynecologic surgeons have developed a variety of removal procedures and techniques for these women. There is general agreement that hysteroscopic removal is feasible only in the first 3 months following insertion of the device. After that, laparoscopic removal has become the norm. Small published reports and case series have documented the use of laparoscopic bilateral salpingectomy (BS) with and without hysterectomy, laparoscopic BS with cornuectomy, and laparoscopic salpingostomy, often followed by salpingectomy. There is not yet enough data to demonstrate that one method is superior to another, and we each have our own preferred approaches for preoperative imaging and removal.
Here is some of our advice on working with patients to assess the need for, and possible outcomes of, removal, as well as how to approach the surgery.
Counseling, assessment, and consent
Dr. Cohen: We have to be frank with our patients that symptoms may or may not improve following Essure removal. In a recently published case series of 52 women who underwent Essure removal at our institution, three-quarters of the patients reported near or total improvement in the quality of life. However, a relatively high number – roughly 30% – reported some ongoing symptoms (J Minim Invasive Gynecol. 2017 Jun 6. doi: 10:1016/j.jmig.2017.05.015).
The most common indication for Essure removal in this series was pelvic pain (96%), followed by abnormal uterine bleeding (35%) and patient-reported allergic reaction (21%). The indications were not mutually exclusive.
Importantly, could endometriosis or another underlying condition have developed since placement or worsened over time? Or, could her pelvic pain be worsened because of the cessation of hormonal contraception that coincided with Essure placement, rather than the device itself? For some women, Essure removal alone will not cure their symptoms.
In our cohort of 52 women, interestingly, 44% of those with pelvic pain had one or more concomitant or alternate causes of pain, including endometriosis, adenomyosis, and adhesions.
Dr. Levie: We can’t assume that Essure coils are at fault when patients present with pain and other symptoms, nor can we minimize complaints and concerns. We have to explore them.
It’s important that we inform women that pain may not be related to Essure microinserts. However, if, after thorough evaluation, the patient believes that the coils are the etiology of her pain and I cannot find another reason – or if she has regrets or is concerned about potential problems in the future – I am happy to remove them.
Dr. Yunker: In our case series of 29 women who underwent removal for the primary indication of pelvic pain, 88.5% reported significant relief at their postoperative visit (Contraception. 2016 Aug;94[2]:190-2). This, and other unpublished data, show that patients with gynecologic complaints specifically are the most likely to have resolution of symptoms, compared with those with more systemic or nongynecologic complaints.
Some patients have systemic symptoms that they feel are related and new since the device was placed. My counseling in these cases is that, while I do not have any physiologic evidence that the Essure coil is causing their symptoms, I’m hopeful that symptoms will improve with removal. If they do not, these patients must follow up with their primary care doctor for further work-up.
Device structure and use of imaging
Essure is a 4-cm long device (0.8 mm in diameter) with two parts: an inner coil made of stainless steel and PET fibers, the latter of which induces the fibrosis responsible for tubal occlusion, and an outer coil made of nitinol, a nickel titanium alloy.
Dr. Cohen: While the exact mechanism is unclear, it’s possible that the PET fibers may be drivers of the systemic inflammatory-type symptoms that some women report. Nickel allergies are also possible albeit uncommon. They appear to manifest as rash, urticaria, and other symptoms characteristic of contact allergic reactions.
The brittle nature of the outer coil makes a grasp-and-pull approach disadvantageous, unless you’re removing coils early on hysteroscopically. In general, one must avoid fracturing the outer coil, or parts of the device will be left behind. Pulling too hard may also cause the outer coil to unravel and expand to be quite long, which further increases the risk of fracture.
Hysterosalpingogram (HSG) and ultrasound are typically first-line options for looking at coil position. A diagnostic hysteroscopy may also help identify coils, and intraoperative fluoroscopy may be useful for either the hysteroscopic or laparoscopic approach, if there’s any question about portions of the device not being recovered.
Dr. Levie: Ultrasound is often sufficient for operative planning, but, if it does not detect devices in the cornual region, then further imaging may be warranted.
It’s important to be aware that some devices that appear to have correct placement on ultrasound or HSG may actually be partially tracking subserosally. In these cases, the distal portion of the device may have tracked through the mucosal layer and along the muscularis but below the serosa in the fallopian tube, causing pain. Imaging won’t be helpful in making this diagnosis. It will be identified laparoscopically.
Dr. Yunker: When patients have completed the 3-month HSG (to confirm occlusion of the Fallopian tubes post placement), I will review the images myself rather than relying on the report. Without an HSG – and, in many cases, even when I have it in hand – I will order a plain film x-ray of the abdomen and pelvis to look for coils. In almost all cases, I also order an ultrasound, which is helpful in assessing for ovarian and uterine conditions.
I’ve found plain film imaging to be valuable for identifying additional or misplaced Essure inserts. I have found up to four in one tube. In interpreting x-rays, one must appreciate that the outer coil is not radio-opaque (other than the tiny marker at the end) and will not show up. Occasionally we’ll add hysteroscopy to see how much of a coil is trailing into the uterus, but the ultrasound and x-ray are usually enough.
Some patients ask about postremoval imaging. I do not routinely do this, but I’m not opposed to it.
Surgical techniques
Dr. Cohen: I advise dissecting around each coil without cutting the outer portion and removing the coil intact, resecting all the way down to the interstitial portion of the tube, then proceeding with bilateral salpingectomy to ensure contraception.
If the patient’s symptoms are systemic and possibly reflective of PET fiber reactions, a wedge resection of the cornua may provide more peace of mind that PET fibers will not be left in situ. This procedure can be approached similarly to myomectomy, with the use of hemostatic agents such as misoprostol or vasopressin and suture closure in multiple layers.
If there are multiple coils present in the cavity, one option, to avoid having to pull them all out from the abdominal side, is to transect and remove the intracavity portion of the device hysteroscopically then dissect and remove the tubal/interstitial potion laparoscopically. As a general rule, I send all the removed tissue to pathology.
Dr. Levie: In general, I do a linear salpingostomy after using a uterine manipulator and a grasper to first identify the site of the distal portion of the device. One can usually feel where the tubes bend onto the device.
A bit proximal to where I visually and mentally mark the distal end of the device, I make a 2-3 cm incision over the device. With a fine-tip grasper, I can usually release the distal portion of the inner coil. Using two graspers over the inner and outer coils together and a hand-over-hand motion, I pull without excess traction in the access of the tubes, and the proximal portion will usually follow and deliver fairly simply. If the proximal portion breaks, I advise looking for it hysteroscopically and delivering it through the uterus.
Some surgeons have recommended hysteroscopy at the beginning of the procedure with cutting (using scissors) at the proximal end of the outer coil to avoid its getting caught in the cornua.
Most patients continue to want permanent sterilization, so we proceed with salpingectomy. Sometimes, given underlying pathologies, we’ll decide on laparoscopic or vaginal hysterectomy as well or bilateral salpingectomy without doing the salpingostomy. When hysterectomy is part of the surgery, we don’t need to worry at all about broken devices.
When the device is removed separately from the fallopian tube, one should inspect it afterward to ensure that all four markers of the device – the markers that are recommended by the manufacturer for radiologic confirmation of proper placement – have been delivered.
Dr. Yunker: When everything looks normal on the ultrasound – and when the coils on either HSG and/or plain film x-ray appear to be in the appropriate position in the tubes – then removal of the coils and tubes only is an option.
The closer the coil is to the fimbriae, the easier it is to come straight across the tube as you would in a regular salpingectomy without concern of breaking or cutting the coil. However, the closer the coil is the uterine side, the deeper you’ll need to dissect into the cornual region of the uterus. A cornual wedge resection may be necessary in order to remove the coil intact.
Our procedure has evolved over the years and we have moved away from salpingectomy as a means to dissect out the coils. With the theoretical risk of retained coil fragments and PET fibers, we prefer to remove the coils and tubes en bloc.
Dr. Cohen is director of research and the fellowship program director of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston. She reported that she has no financial disclosures. Dr. Levie is professor and associate chairman of the department of obstetrics and gynecology and women’s health and director of the minimally invasive surgery fellowship at Montefiore Medical Center, New York. Dr. Levie reported that he is an investigator in two studies involving Essure and sat on the Essure medical advisory board for Bayer but did not receive personal renumeration. Dr. Yunker is an assistant professor of obstetrics and gynecology at Vanderbilt University, Nashville. She reported that she is a consultant for Olympus.
Essure tubal microinserts were not designed to be removed. However, a small minority of women are requesting removal because of regret, complications, or the development of pelvic pain and other symptoms that may or may not be caused by the device.
Minimally invasive gynecologic surgeons have developed a variety of removal procedures and techniques for these women. There is general agreement that hysteroscopic removal is feasible only in the first 3 months following insertion of the device. After that, laparoscopic removal has become the norm. Small published reports and case series have documented the use of laparoscopic bilateral salpingectomy (BS) with and without hysterectomy, laparoscopic BS with cornuectomy, and laparoscopic salpingostomy, often followed by salpingectomy. There is not yet enough data to demonstrate that one method is superior to another, and we each have our own preferred approaches for preoperative imaging and removal.
Here is some of our advice on working with patients to assess the need for, and possible outcomes of, removal, as well as how to approach the surgery.
Counseling, assessment, and consent
Dr. Cohen: We have to be frank with our patients that symptoms may or may not improve following Essure removal. In a recently published case series of 52 women who underwent Essure removal at our institution, three-quarters of the patients reported near or total improvement in the quality of life. However, a relatively high number – roughly 30% – reported some ongoing symptoms (J Minim Invasive Gynecol. 2017 Jun 6. doi: 10:1016/j.jmig.2017.05.015).
The most common indication for Essure removal in this series was pelvic pain (96%), followed by abnormal uterine bleeding (35%) and patient-reported allergic reaction (21%). The indications were not mutually exclusive.
Importantly, could endometriosis or another underlying condition have developed since placement or worsened over time? Or, could her pelvic pain be worsened because of the cessation of hormonal contraception that coincided with Essure placement, rather than the device itself? For some women, Essure removal alone will not cure their symptoms.
In our cohort of 52 women, interestingly, 44% of those with pelvic pain had one or more concomitant or alternate causes of pain, including endometriosis, adenomyosis, and adhesions.
Dr. Levie: We can’t assume that Essure coils are at fault when patients present with pain and other symptoms, nor can we minimize complaints and concerns. We have to explore them.
It’s important that we inform women that pain may not be related to Essure microinserts. However, if, after thorough evaluation, the patient believes that the coils are the etiology of her pain and I cannot find another reason – or if she has regrets or is concerned about potential problems in the future – I am happy to remove them.
Dr. Yunker: In our case series of 29 women who underwent removal for the primary indication of pelvic pain, 88.5% reported significant relief at their postoperative visit (Contraception. 2016 Aug;94[2]:190-2). This, and other unpublished data, show that patients with gynecologic complaints specifically are the most likely to have resolution of symptoms, compared with those with more systemic or nongynecologic complaints.
Some patients have systemic symptoms that they feel are related and new since the device was placed. My counseling in these cases is that, while I do not have any physiologic evidence that the Essure coil is causing their symptoms, I’m hopeful that symptoms will improve with removal. If they do not, these patients must follow up with their primary care doctor for further work-up.
Device structure and use of imaging
Essure is a 4-cm long device (0.8 mm in diameter) with two parts: an inner coil made of stainless steel and PET fibers, the latter of which induces the fibrosis responsible for tubal occlusion, and an outer coil made of nitinol, a nickel titanium alloy.
Dr. Cohen: While the exact mechanism is unclear, it’s possible that the PET fibers may be drivers of the systemic inflammatory-type symptoms that some women report. Nickel allergies are also possible albeit uncommon. They appear to manifest as rash, urticaria, and other symptoms characteristic of contact allergic reactions.
The brittle nature of the outer coil makes a grasp-and-pull approach disadvantageous, unless you’re removing coils early on hysteroscopically. In general, one must avoid fracturing the outer coil, or parts of the device will be left behind. Pulling too hard may also cause the outer coil to unravel and expand to be quite long, which further increases the risk of fracture.
Hysterosalpingogram (HSG) and ultrasound are typically first-line options for looking at coil position. A diagnostic hysteroscopy may also help identify coils, and intraoperative fluoroscopy may be useful for either the hysteroscopic or laparoscopic approach, if there’s any question about portions of the device not being recovered.
Dr. Levie: Ultrasound is often sufficient for operative planning, but, if it does not detect devices in the cornual region, then further imaging may be warranted.
It’s important to be aware that some devices that appear to have correct placement on ultrasound or HSG may actually be partially tracking subserosally. In these cases, the distal portion of the device may have tracked through the mucosal layer and along the muscularis but below the serosa in the fallopian tube, causing pain. Imaging won’t be helpful in making this diagnosis. It will be identified laparoscopically.
Dr. Yunker: When patients have completed the 3-month HSG (to confirm occlusion of the Fallopian tubes post placement), I will review the images myself rather than relying on the report. Without an HSG – and, in many cases, even when I have it in hand – I will order a plain film x-ray of the abdomen and pelvis to look for coils. In almost all cases, I also order an ultrasound, which is helpful in assessing for ovarian and uterine conditions.
I’ve found plain film imaging to be valuable for identifying additional or misplaced Essure inserts. I have found up to four in one tube. In interpreting x-rays, one must appreciate that the outer coil is not radio-opaque (other than the tiny marker at the end) and will not show up. Occasionally we’ll add hysteroscopy to see how much of a coil is trailing into the uterus, but the ultrasound and x-ray are usually enough.
Some patients ask about postremoval imaging. I do not routinely do this, but I’m not opposed to it.
Surgical techniques
Dr. Cohen: I advise dissecting around each coil without cutting the outer portion and removing the coil intact, resecting all the way down to the interstitial portion of the tube, then proceeding with bilateral salpingectomy to ensure contraception.
If the patient’s symptoms are systemic and possibly reflective of PET fiber reactions, a wedge resection of the cornua may provide more peace of mind that PET fibers will not be left in situ. This procedure can be approached similarly to myomectomy, with the use of hemostatic agents such as misoprostol or vasopressin and suture closure in multiple layers.
If there are multiple coils present in the cavity, one option, to avoid having to pull them all out from the abdominal side, is to transect and remove the intracavity portion of the device hysteroscopically then dissect and remove the tubal/interstitial potion laparoscopically. As a general rule, I send all the removed tissue to pathology.
Dr. Levie: In general, I do a linear salpingostomy after using a uterine manipulator and a grasper to first identify the site of the distal portion of the device. One can usually feel where the tubes bend onto the device.
A bit proximal to where I visually and mentally mark the distal end of the device, I make a 2-3 cm incision over the device. With a fine-tip grasper, I can usually release the distal portion of the inner coil. Using two graspers over the inner and outer coils together and a hand-over-hand motion, I pull without excess traction in the access of the tubes, and the proximal portion will usually follow and deliver fairly simply. If the proximal portion breaks, I advise looking for it hysteroscopically and delivering it through the uterus.
Some surgeons have recommended hysteroscopy at the beginning of the procedure with cutting (using scissors) at the proximal end of the outer coil to avoid its getting caught in the cornua.
Most patients continue to want permanent sterilization, so we proceed with salpingectomy. Sometimes, given underlying pathologies, we’ll decide on laparoscopic or vaginal hysterectomy as well or bilateral salpingectomy without doing the salpingostomy. When hysterectomy is part of the surgery, we don’t need to worry at all about broken devices.
When the device is removed separately from the fallopian tube, one should inspect it afterward to ensure that all four markers of the device – the markers that are recommended by the manufacturer for radiologic confirmation of proper placement – have been delivered.
Dr. Yunker: When everything looks normal on the ultrasound – and when the coils on either HSG and/or plain film x-ray appear to be in the appropriate position in the tubes – then removal of the coils and tubes only is an option.
The closer the coil is to the fimbriae, the easier it is to come straight across the tube as you would in a regular salpingectomy without concern of breaking or cutting the coil. However, the closer the coil is the uterine side, the deeper you’ll need to dissect into the cornual region of the uterus. A cornual wedge resection may be necessary in order to remove the coil intact.
Our procedure has evolved over the years and we have moved away from salpingectomy as a means to dissect out the coils. With the theoretical risk of retained coil fragments and PET fibers, we prefer to remove the coils and tubes en bloc.
Dr. Cohen is director of research and the fellowship program director of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston. She reported that she has no financial disclosures. Dr. Levie is professor and associate chairman of the department of obstetrics and gynecology and women’s health and director of the minimally invasive surgery fellowship at Montefiore Medical Center, New York. Dr. Levie reported that he is an investigator in two studies involving Essure and sat on the Essure medical advisory board for Bayer but did not receive personal renumeration. Dr. Yunker is an assistant professor of obstetrics and gynecology at Vanderbilt University, Nashville. She reported that she is a consultant for Olympus.
Essure tubal microinserts were not designed to be removed. However, a small minority of women are requesting removal because of regret, complications, or the development of pelvic pain and other symptoms that may or may not be caused by the device.
Minimally invasive gynecologic surgeons have developed a variety of removal procedures and techniques for these women. There is general agreement that hysteroscopic removal is feasible only in the first 3 months following insertion of the device. After that, laparoscopic removal has become the norm. Small published reports and case series have documented the use of laparoscopic bilateral salpingectomy (BS) with and without hysterectomy, laparoscopic BS with cornuectomy, and laparoscopic salpingostomy, often followed by salpingectomy. There is not yet enough data to demonstrate that one method is superior to another, and we each have our own preferred approaches for preoperative imaging and removal.
Here is some of our advice on working with patients to assess the need for, and possible outcomes of, removal, as well as how to approach the surgery.
Counseling, assessment, and consent
Dr. Cohen: We have to be frank with our patients that symptoms may or may not improve following Essure removal. In a recently published case series of 52 women who underwent Essure removal at our institution, three-quarters of the patients reported near or total improvement in the quality of life. However, a relatively high number – roughly 30% – reported some ongoing symptoms (J Minim Invasive Gynecol. 2017 Jun 6. doi: 10:1016/j.jmig.2017.05.015).
The most common indication for Essure removal in this series was pelvic pain (96%), followed by abnormal uterine bleeding (35%) and patient-reported allergic reaction (21%). The indications were not mutually exclusive.
Importantly, could endometriosis or another underlying condition have developed since placement or worsened over time? Or, could her pelvic pain be worsened because of the cessation of hormonal contraception that coincided with Essure placement, rather than the device itself? For some women, Essure removal alone will not cure their symptoms.
In our cohort of 52 women, interestingly, 44% of those with pelvic pain had one or more concomitant or alternate causes of pain, including endometriosis, adenomyosis, and adhesions.
Dr. Levie: We can’t assume that Essure coils are at fault when patients present with pain and other symptoms, nor can we minimize complaints and concerns. We have to explore them.
It’s important that we inform women that pain may not be related to Essure microinserts. However, if, after thorough evaluation, the patient believes that the coils are the etiology of her pain and I cannot find another reason – or if she has regrets or is concerned about potential problems in the future – I am happy to remove them.
Dr. Yunker: In our case series of 29 women who underwent removal for the primary indication of pelvic pain, 88.5% reported significant relief at their postoperative visit (Contraception. 2016 Aug;94[2]:190-2). This, and other unpublished data, show that patients with gynecologic complaints specifically are the most likely to have resolution of symptoms, compared with those with more systemic or nongynecologic complaints.
Some patients have systemic symptoms that they feel are related and new since the device was placed. My counseling in these cases is that, while I do not have any physiologic evidence that the Essure coil is causing their symptoms, I’m hopeful that symptoms will improve with removal. If they do not, these patients must follow up with their primary care doctor for further work-up.
Device structure and use of imaging
Essure is a 4-cm long device (0.8 mm in diameter) with two parts: an inner coil made of stainless steel and PET fibers, the latter of which induces the fibrosis responsible for tubal occlusion, and an outer coil made of nitinol, a nickel titanium alloy.
Dr. Cohen: While the exact mechanism is unclear, it’s possible that the PET fibers may be drivers of the systemic inflammatory-type symptoms that some women report. Nickel allergies are also possible albeit uncommon. They appear to manifest as rash, urticaria, and other symptoms characteristic of contact allergic reactions.
The brittle nature of the outer coil makes a grasp-and-pull approach disadvantageous, unless you’re removing coils early on hysteroscopically. In general, one must avoid fracturing the outer coil, or parts of the device will be left behind. Pulling too hard may also cause the outer coil to unravel and expand to be quite long, which further increases the risk of fracture.
Hysterosalpingogram (HSG) and ultrasound are typically first-line options for looking at coil position. A diagnostic hysteroscopy may also help identify coils, and intraoperative fluoroscopy may be useful for either the hysteroscopic or laparoscopic approach, if there’s any question about portions of the device not being recovered.
Dr. Levie: Ultrasound is often sufficient for operative planning, but, if it does not detect devices in the cornual region, then further imaging may be warranted.
It’s important to be aware that some devices that appear to have correct placement on ultrasound or HSG may actually be partially tracking subserosally. In these cases, the distal portion of the device may have tracked through the mucosal layer and along the muscularis but below the serosa in the fallopian tube, causing pain. Imaging won’t be helpful in making this diagnosis. It will be identified laparoscopically.
Dr. Yunker: When patients have completed the 3-month HSG (to confirm occlusion of the Fallopian tubes post placement), I will review the images myself rather than relying on the report. Without an HSG – and, in many cases, even when I have it in hand – I will order a plain film x-ray of the abdomen and pelvis to look for coils. In almost all cases, I also order an ultrasound, which is helpful in assessing for ovarian and uterine conditions.
I’ve found plain film imaging to be valuable for identifying additional or misplaced Essure inserts. I have found up to four in one tube. In interpreting x-rays, one must appreciate that the outer coil is not radio-opaque (other than the tiny marker at the end) and will not show up. Occasionally we’ll add hysteroscopy to see how much of a coil is trailing into the uterus, but the ultrasound and x-ray are usually enough.
Some patients ask about postremoval imaging. I do not routinely do this, but I’m not opposed to it.
Surgical techniques
Dr. Cohen: I advise dissecting around each coil without cutting the outer portion and removing the coil intact, resecting all the way down to the interstitial portion of the tube, then proceeding with bilateral salpingectomy to ensure contraception.
If the patient’s symptoms are systemic and possibly reflective of PET fiber reactions, a wedge resection of the cornua may provide more peace of mind that PET fibers will not be left in situ. This procedure can be approached similarly to myomectomy, with the use of hemostatic agents such as misoprostol or vasopressin and suture closure in multiple layers.
If there are multiple coils present in the cavity, one option, to avoid having to pull them all out from the abdominal side, is to transect and remove the intracavity portion of the device hysteroscopically then dissect and remove the tubal/interstitial potion laparoscopically. As a general rule, I send all the removed tissue to pathology.
Dr. Levie: In general, I do a linear salpingostomy after using a uterine manipulator and a grasper to first identify the site of the distal portion of the device. One can usually feel where the tubes bend onto the device.
A bit proximal to where I visually and mentally mark the distal end of the device, I make a 2-3 cm incision over the device. With a fine-tip grasper, I can usually release the distal portion of the inner coil. Using two graspers over the inner and outer coils together and a hand-over-hand motion, I pull without excess traction in the access of the tubes, and the proximal portion will usually follow and deliver fairly simply. If the proximal portion breaks, I advise looking for it hysteroscopically and delivering it through the uterus.
Some surgeons have recommended hysteroscopy at the beginning of the procedure with cutting (using scissors) at the proximal end of the outer coil to avoid its getting caught in the cornua.
Most patients continue to want permanent sterilization, so we proceed with salpingectomy. Sometimes, given underlying pathologies, we’ll decide on laparoscopic or vaginal hysterectomy as well or bilateral salpingectomy without doing the salpingostomy. When hysterectomy is part of the surgery, we don’t need to worry at all about broken devices.
When the device is removed separately from the fallopian tube, one should inspect it afterward to ensure that all four markers of the device – the markers that are recommended by the manufacturer for radiologic confirmation of proper placement – have been delivered.
Dr. Yunker: When everything looks normal on the ultrasound – and when the coils on either HSG and/or plain film x-ray appear to be in the appropriate position in the tubes – then removal of the coils and tubes only is an option.
The closer the coil is to the fimbriae, the easier it is to come straight across the tube as you would in a regular salpingectomy without concern of breaking or cutting the coil. However, the closer the coil is the uterine side, the deeper you’ll need to dissect into the cornual region of the uterus. A cornual wedge resection may be necessary in order to remove the coil intact.
Our procedure has evolved over the years and we have moved away from salpingectomy as a means to dissect out the coils. With the theoretical risk of retained coil fragments and PET fibers, we prefer to remove the coils and tubes en bloc.
Dr. Cohen is director of research and the fellowship program director of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston. She reported that she has no financial disclosures. Dr. Levie is professor and associate chairman of the department of obstetrics and gynecology and women’s health and director of the minimally invasive surgery fellowship at Montefiore Medical Center, New York. Dr. Levie reported that he is an investigator in two studies involving Essure and sat on the Essure medical advisory board for Bayer but did not receive personal renumeration. Dr. Yunker is an assistant professor of obstetrics and gynecology at Vanderbilt University, Nashville. She reported that she is a consultant for Olympus.
So you want your Essure device removed ...
Despite recent negative lay press and a boxed safety warning from the Food and Drug Administration, Essure tubal microinserts continue to be a popular method of permanent contraception. It is imperative for patients to understand that this method of contraception cannot be reversed, and thereafter, the only method to achieve pregnancy would be via in vitro fertilization. Furthermore, preoperatively, patients must be counseled that placement of the Essure tubal microinserts may be associated with pelvic pain, abnormal bleeding, and even allergic reaction.
Even with our best effort to properly inform our patients as to the risks and benefits of permanent sterilization via Essure tubal microinserts, secondary to undesired side effects, patients desire their removal. This can be a challenging endeavor for the practitioner, especially if the women is not interested in hysterectomy.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.
Despite recent negative lay press and a boxed safety warning from the Food and Drug Administration, Essure tubal microinserts continue to be a popular method of permanent contraception. It is imperative for patients to understand that this method of contraception cannot be reversed, and thereafter, the only method to achieve pregnancy would be via in vitro fertilization. Furthermore, preoperatively, patients must be counseled that placement of the Essure tubal microinserts may be associated with pelvic pain, abnormal bleeding, and even allergic reaction.
Even with our best effort to properly inform our patients as to the risks and benefits of permanent sterilization via Essure tubal microinserts, secondary to undesired side effects, patients desire their removal. This can be a challenging endeavor for the practitioner, especially if the women is not interested in hysterectomy.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.
Despite recent negative lay press and a boxed safety warning from the Food and Drug Administration, Essure tubal microinserts continue to be a popular method of permanent contraception. It is imperative for patients to understand that this method of contraception cannot be reversed, and thereafter, the only method to achieve pregnancy would be via in vitro fertilization. Furthermore, preoperatively, patients must be counseled that placement of the Essure tubal microinserts may be associated with pelvic pain, abnormal bleeding, and even allergic reaction.
Even with our best effort to properly inform our patients as to the risks and benefits of permanent sterilization via Essure tubal microinserts, secondary to undesired side effects, patients desire their removal. This can be a challenging endeavor for the practitioner, especially if the women is not interested in hysterectomy.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.