User login
Health inequities take a societal toll
Arguably one of the most important public health issues in our nation is the gap between high-quality care and the people who need it most. The passage of the Affordable Care Act was meant, in part, to reduce this gap and increase health equity in terms of both eligibility for, and access to, care. However, lower-income residents, especially those from minority groups, are more likely to be hospitalized for asthma, hypertension, heart disease, and diabetes, and to experience infertility, preterm birth, and fetal death.
Health disparities, or inequities, translate not only into greater suffering for certain segments of the population, but also to significantly greater health care costs for everyone. Racial health disparities are associated with an estimated $35 billion annually in excess expenditures, $10 billion in lost productivity, and nearly $200 billion in premature deaths, according to an article in the Harvard Business Review. A 2013 study estimated that reducing racial disparities in adverse pregnancy outcomes – preeclampsia, preterm birth, gestational diabetes mellitus, and fetal death/stillbirth – could generate health care cost savings of up to $214 million per year (Matern Child Health J. 2013 Oct;17[8]:1518-25).
Several years ago, the State of Maryland took a unique approach to reducing health disparities by passing the Maryland Health Improvement and Disparities Reduction Act. One of the major components of this legislation was the creation of Health Enterprise Zones (HEZs), distinct geographical areas across the state dedicated to addressing health disparities and improving access to high-quality care. This incentive-based program provides state-funded resources to primary care providers and community-based health organizations specifically to help the neighborhoods they serve. I was deeply honored to serve as chairman of the task force that recommended the establishment of the HEZs.
For this Master Class, I have invited Melissa A. Simon, MD, the George H. Gardner, MD, Professor of Clinical Gynecology and professor of obstetrics and gynecology at Northwestern University, Chicago, to provide some practical advice on how to create greater health equity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Arguably one of the most important public health issues in our nation is the gap between high-quality care and the people who need it most. The passage of the Affordable Care Act was meant, in part, to reduce this gap and increase health equity in terms of both eligibility for, and access to, care. However, lower-income residents, especially those from minority groups, are more likely to be hospitalized for asthma, hypertension, heart disease, and diabetes, and to experience infertility, preterm birth, and fetal death.
Health disparities, or inequities, translate not only into greater suffering for certain segments of the population, but also to significantly greater health care costs for everyone. Racial health disparities are associated with an estimated $35 billion annually in excess expenditures, $10 billion in lost productivity, and nearly $200 billion in premature deaths, according to an article in the Harvard Business Review. A 2013 study estimated that reducing racial disparities in adverse pregnancy outcomes – preeclampsia, preterm birth, gestational diabetes mellitus, and fetal death/stillbirth – could generate health care cost savings of up to $214 million per year (Matern Child Health J. 2013 Oct;17[8]:1518-25).
Several years ago, the State of Maryland took a unique approach to reducing health disparities by passing the Maryland Health Improvement and Disparities Reduction Act. One of the major components of this legislation was the creation of Health Enterprise Zones (HEZs), distinct geographical areas across the state dedicated to addressing health disparities and improving access to high-quality care. This incentive-based program provides state-funded resources to primary care providers and community-based health organizations specifically to help the neighborhoods they serve. I was deeply honored to serve as chairman of the task force that recommended the establishment of the HEZs.
For this Master Class, I have invited Melissa A. Simon, MD, the George H. Gardner, MD, Professor of Clinical Gynecology and professor of obstetrics and gynecology at Northwestern University, Chicago, to provide some practical advice on how to create greater health equity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Arguably one of the most important public health issues in our nation is the gap between high-quality care and the people who need it most. The passage of the Affordable Care Act was meant, in part, to reduce this gap and increase health equity in terms of both eligibility for, and access to, care. However, lower-income residents, especially those from minority groups, are more likely to be hospitalized for asthma, hypertension, heart disease, and diabetes, and to experience infertility, preterm birth, and fetal death.
Health disparities, or inequities, translate not only into greater suffering for certain segments of the population, but also to significantly greater health care costs for everyone. Racial health disparities are associated with an estimated $35 billion annually in excess expenditures, $10 billion in lost productivity, and nearly $200 billion in premature deaths, according to an article in the Harvard Business Review. A 2013 study estimated that reducing racial disparities in adverse pregnancy outcomes – preeclampsia, preterm birth, gestational diabetes mellitus, and fetal death/stillbirth – could generate health care cost savings of up to $214 million per year (Matern Child Health J. 2013 Oct;17[8]:1518-25).
Several years ago, the State of Maryland took a unique approach to reducing health disparities by passing the Maryland Health Improvement and Disparities Reduction Act. One of the major components of this legislation was the creation of Health Enterprise Zones (HEZs), distinct geographical areas across the state dedicated to addressing health disparities and improving access to high-quality care. This incentive-based program provides state-funded resources to primary care providers and community-based health organizations specifically to help the neighborhoods they serve. I was deeply honored to serve as chairman of the task force that recommended the establishment of the HEZs.
For this Master Class, I have invited Melissa A. Simon, MD, the George H. Gardner, MD, Professor of Clinical Gynecology and professor of obstetrics and gynecology at Northwestern University, Chicago, to provide some practical advice on how to create greater health equity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Moving toward health equity in practice
Of all the medical professions, obstetrics and gynecology should be the strongest champion for equity in women’s health in this country and globally. The question is, what does this mean in the reality of 2017 and moving forward in the 21st century? What does it mean in the context of our own practices and in the landscape of current policy and politics?
Finding answers to these questions requires both a deep understanding of the meaning of health equity and a willingness to rethink the architecture and engineering of how we currently provide care.
The terms equity and equality are sometimes used interchangeably, but they actually have quite different meanings. Imagine three women of different heights standing underneath the lowest branch of a tall apple tree. None of the three women are tall enough to pick an apple from the branch.
If we think about equality, we would assist each woman by giving her a box to stand on, and all three boxes would be the same size. This means that while the tallest woman will now be able to pick an apple, the medium-height woman may be able to touch but not pick the apple, and the shortest woman still may not be able to reach the apple at all.
However, if we think about equity, we’d acknowledge that each woman needs her own personalized box to be able to pick the apple. For instance, the shortest woman may need a box that is three times the height of the box used by the tallest woman.
Achieving true population health for all women requires that we similarly eliminate inequities by providing each patient with her own personalized care plan to help her reach and maintain her health.
Women from minority groups have higher rates of low birth weight, preterm birth, stillbirth, gestational diabetes and its complications, HIV, breast cancer mortality and cervical cancer incidence and mortality, infertility and response to fertility treatment, and maternal mortality.
Yet inequity runs deeper than racial/ethnic labels; disparities also are created by a host of other factors, from cognitive or physical disabilities to gender or sexual identity or orientation, one’s ZIP code, working environment, language, and health literacy.
More than ever, the art of medicine involves understanding how to meet every patient where she is – given her own context and beliefs and levels of support – so that every woman has the opportunity to stand on the right-sized box and pick the apple and thrive.
Our practices
Provider bias and stereotyping can impact health care and health outcomes, and it is important that we work to prevent this in ourselves and in our staff. This means not making assumptions. It means really listening to our patients in ways that we may not have before.
Women who have experienced health inequity may have unique barriers to success. Therefore, we must listen for cues and inquire about our patients’ environment and circumstances, as well as their partnerships and support – or lack thereof. We should then acknowledge and communicate that certain social and environmental factors may impact our ability to achieve a desired outcome.
How can we impact the diet of a patient with gestational diabetes, for instance, if we have not adequately communicated what medical nutritional therapy means in the context of her own culture and ability to access food? If she lives in a food desert or has food insecurity or lives in a violence-ridden neighborhood that keeps her from going to a grocery store regularly, we must think outside the box. Ob.gyns. and their clinical care teams can work with women who have less access to nutritious foods, or who have certain cultural food staples, to suggest recipes and grocery lists that make sense with respect to the types of stores they shop in or their cultural preferences.
When it comes to cancer prevention and treatment, how can we expect a woman to be compliant with screening if we cannot help her understand that she can get screening services for free with her health insurance? How can we help a woman who has coverage for, or access to, free screening but then no funding or coverage for a diagnostic test or cancer treatment? How can we support a patient with abnormal cancer screening results who hasn’t followed up for months because she is afraid to leave home without her partner’s permission?
Such questions and circumstances often involve what we call “social determinants of health,” and they force us to rethink how we can better deliver and optimize care. Re-engineering our practices for health equity may involve employing a more diverse practice staff, linking patients with community resources, modifying our practice hours to align better with working women’s schedules, or finding creative ways to discern patients’ motivating factors and then piggyback on these factors.
We may also need to modify how we approach the number of return visits that we request of women so that follow-up care aligns better with their ability to leave work or find childcare. Simply put, we should strive to set up our patients for success, not failure.
We can pointedly ask patients about the kinds of information and support they want and need. We might ask, for instance: What do you need, and how can I work with you, so that you can effectively monitor and control your glucose levels? How can I work with you to help you get onto a trajectory to stop smoking? How can I help you better understand what tests and procedures are covered under your insurance plan, or whether you qualify for free services?
Patients with lower health literacy may need teach-back methods to validate understanding, or messaging that is more focused and limited at any one time. Self-efficacy through patient-centered education and support should be our goal.
Practices and clinics may also be able to adapt elements of the National Cancer Institute’s multicenter Patient Navigation Research Program, in which community health workers or other “patient navigators” address women’s personal barriers to the timely follow-up of abnormal breast and cervical cancer screening results. Patient navigation through this program and similar projects, including programs that we’ve adapted for different racial and ethnic communities in and around Chicago, has reduced or eliminated delays in diagnostic resolution of gynecologic cancer (Cancer. 2015 Nov 15;121[22]:4025-34, Breast Cancer Res Treat. 2016 Aug;158[3]:523-34, Am J Public Health. 2015 May;105[5]:e87-94).
The patient navigation model is increasingly being adapted and used in a variety of contexts outside of cancer care as well. In a postpartum patient navigation program that we tested at Northwestern University’s Medicaid-based outpatient clinic, a navigator was hired to communicate with patients and support them between delivery and completion of their postpartum care. Patients were reminded through calls and/or texts of their postpartum visits and of the benefits of breastfeeding, effective contraception, and other postpartum practices.
The demonstration project was impactful: Women who were enrolled in the program were more likely to return for postpartum care, to receive World Health Organization Tier 1 or 2 contraception, and to have postpartum screening and vaccinations, compared with women who received care before the program began (Obstet Gynecol. 2017 May;129[5]:925-33).
Connections to our patients will help us to achieve health equity. This includes connections between the primary care we provide and the specialty care our patients sometimes require, both inside and outside of our field. We may refer a patient to an oncology team, for instance, and in the process, unwittingly transfer her care such that other conditions that we’ve been managing – hypertension, depression, or diabetes – fall by the wayside.
Instead, we have to re-engineer our processes so that we maintain personalized connections back to these patients. For example, the referring ob.gyn. could develop and send to the oncologist or gynecologic-oncologist a care plan that includes the patient’s comorbid conditions and how they could be managed. This would allow for clearer communication.
Our communities
As ob.gyns., we have a common goal of championing health equity and true population health for every woman, regardless of whether she lives in rural, urban, or suburban America and regardless of whether she has conservative or liberal values. To do so, we must extend ourselves beyond our own practices.
In a committee opinion on Racial and Ethnic Disparities in Obstetrics and Gynecology, the American College of Obstetricians and Gynecologists advises that ob.gyns. take a number of actions to increase health equity. These include raising awareness about inequity and its effects on health outcomes, promoting quality improvement projects that target disparities, working with public health leadership, and helping recruit ob.gyns. and other health care providers from racial and ethnic minority groups (Obstet Gynecol 2015;126:e130-4).
In Chicago, where 1 out of 5 people lives in poverty and 1 out of 10 lives in deep poverty, we are still in our infancy in combating health inequities. However, with partnerships between academic institutions, departments of health, and other organizations across various sectors, we are beginning to move the needle on these entrenched health inequities.
For example, in 2007, there was a 60% difference in breast cancer mortality between black and white women in Chicago. This disparity sparked the development of the Metropolitan Chicago Breast Cancer Task Force and a series of on-the-ground patient navigator programs, along with several key policy changes and new state laws.
State actions included requiring quality reporting on mammography and increasing the Medicaid reimbursement rate for mammography to the Medicare rate. Nationally, beneficial changes were made to Medicare’s quality metrics and to the National Breast and Cervical Cancer Early Detection Program. All told, through a combination of studies and initiatives focused on improving knowledge, trust, access to care, and quality of care, we have been able to decrease the breast cancer mortality gap by 20%.
We also have a role to play in nurturing and developing a workforce that better aligns with our evolving demographics. This involves redesigning how we plant seeds of opportunity among high school students, undergraduates, and young medical students, and how we seek job applicants. Moreover, when we help people get to the next step in their careers, we need to make sure there is continuous support to retain them and help propel them to the next level.
We should think creatively to establish programs or launch initiatives that can help level the playing field for all women. For example, I created a Massive Open Online Course called “Career 911: Your Future Job in Medicine and Healthcare” as a free workforce development pipeline program. It is accessible on a global platform (https://www.coursera.org/learn/healthcarejobs) and is one example of how we as ob.gyns. can leverage our skills and resources.
Along the way, we also need to train our students and residents – and ourselves – to be more familiar with, and articulate about, health care policy. We need to understand how policy is made and modified and how we can be good communicators and thought leaders.
Right now, our ability to articulate our patients’ stories to policy makers and to the public seems underdeveloped and undertapped. The onus is on us to write and speak about how all women must have the opportunity to not only access care but to access high-quality care and preventive services that are important for full health. Providing health equity isn’t about giving someone a handout, but about giving her a helping hand to take control of her health.
Achieving health equity will involve changing our approach to research. If medical research on women’s health continues to be dominated by studies in which participants are homogeneous and from mainly white or well-resourced populations, we will never have output that is generalizable. As practicing ob.gyns., we can look for opportunities to advocate for diversity in research. We can also acknowledge that, for some women, there is historically-rooted distrust of the health care system that serves as a barrier both to obtaining care and enrolling in trials.
By meeting women where they are, and by tailoring their individual boxes as best we can – in research, in workforce development, and in clinical care delivery – we can work toward solutions.
Strategies for achieving women’s health equity
• Modify office hours/dates to allow flexibility for women who have challenges scheduling childcare and time off from work.
• Ensure handouts, educational materials, and all communications are at appropriate health literacy levels.
• Acknowledge and understand an individual woman’s barriers to care, including social determinants of health, and create a care plan that is achievable for her.
• Learn about and refer women to local community resources needed to overcome barriers to care, such as childcare, social services support, support services for intimate partner violence, and substance abuse counseling.
• Examine office processes to optimize the number of visits women have to attend for a particular health issue. Are there ways to explain results and next steps in a care plan without having to make her come back for an office visit?
Dr. Simon is the George H. Gardner Professor of Clinical Gynecology at Northwestern University, Chicago, and director of the Chicago Cancer Health Equity Collaborative. She is a member of the U.S. Preventive Services Task Force, but the views expressed in this piece are her own.
Of all the medical professions, obstetrics and gynecology should be the strongest champion for equity in women’s health in this country and globally. The question is, what does this mean in the reality of 2017 and moving forward in the 21st century? What does it mean in the context of our own practices and in the landscape of current policy and politics?
Finding answers to these questions requires both a deep understanding of the meaning of health equity and a willingness to rethink the architecture and engineering of how we currently provide care.
The terms equity and equality are sometimes used interchangeably, but they actually have quite different meanings. Imagine three women of different heights standing underneath the lowest branch of a tall apple tree. None of the three women are tall enough to pick an apple from the branch.
If we think about equality, we would assist each woman by giving her a box to stand on, and all three boxes would be the same size. This means that while the tallest woman will now be able to pick an apple, the medium-height woman may be able to touch but not pick the apple, and the shortest woman still may not be able to reach the apple at all.
However, if we think about equity, we’d acknowledge that each woman needs her own personalized box to be able to pick the apple. For instance, the shortest woman may need a box that is three times the height of the box used by the tallest woman.
Achieving true population health for all women requires that we similarly eliminate inequities by providing each patient with her own personalized care plan to help her reach and maintain her health.
Women from minority groups have higher rates of low birth weight, preterm birth, stillbirth, gestational diabetes and its complications, HIV, breast cancer mortality and cervical cancer incidence and mortality, infertility and response to fertility treatment, and maternal mortality.
Yet inequity runs deeper than racial/ethnic labels; disparities also are created by a host of other factors, from cognitive or physical disabilities to gender or sexual identity or orientation, one’s ZIP code, working environment, language, and health literacy.
More than ever, the art of medicine involves understanding how to meet every patient where she is – given her own context and beliefs and levels of support – so that every woman has the opportunity to stand on the right-sized box and pick the apple and thrive.
Our practices
Provider bias and stereotyping can impact health care and health outcomes, and it is important that we work to prevent this in ourselves and in our staff. This means not making assumptions. It means really listening to our patients in ways that we may not have before.
Women who have experienced health inequity may have unique barriers to success. Therefore, we must listen for cues and inquire about our patients’ environment and circumstances, as well as their partnerships and support – or lack thereof. We should then acknowledge and communicate that certain social and environmental factors may impact our ability to achieve a desired outcome.
How can we impact the diet of a patient with gestational diabetes, for instance, if we have not adequately communicated what medical nutritional therapy means in the context of her own culture and ability to access food? If she lives in a food desert or has food insecurity or lives in a violence-ridden neighborhood that keeps her from going to a grocery store regularly, we must think outside the box. Ob.gyns. and their clinical care teams can work with women who have less access to nutritious foods, or who have certain cultural food staples, to suggest recipes and grocery lists that make sense with respect to the types of stores they shop in or their cultural preferences.
When it comes to cancer prevention and treatment, how can we expect a woman to be compliant with screening if we cannot help her understand that she can get screening services for free with her health insurance? How can we help a woman who has coverage for, or access to, free screening but then no funding or coverage for a diagnostic test or cancer treatment? How can we support a patient with abnormal cancer screening results who hasn’t followed up for months because she is afraid to leave home without her partner’s permission?
Such questions and circumstances often involve what we call “social determinants of health,” and they force us to rethink how we can better deliver and optimize care. Re-engineering our practices for health equity may involve employing a more diverse practice staff, linking patients with community resources, modifying our practice hours to align better with working women’s schedules, or finding creative ways to discern patients’ motivating factors and then piggyback on these factors.
We may also need to modify how we approach the number of return visits that we request of women so that follow-up care aligns better with their ability to leave work or find childcare. Simply put, we should strive to set up our patients for success, not failure.
We can pointedly ask patients about the kinds of information and support they want and need. We might ask, for instance: What do you need, and how can I work with you, so that you can effectively monitor and control your glucose levels? How can I work with you to help you get onto a trajectory to stop smoking? How can I help you better understand what tests and procedures are covered under your insurance plan, or whether you qualify for free services?
Patients with lower health literacy may need teach-back methods to validate understanding, or messaging that is more focused and limited at any one time. Self-efficacy through patient-centered education and support should be our goal.
Practices and clinics may also be able to adapt elements of the National Cancer Institute’s multicenter Patient Navigation Research Program, in which community health workers or other “patient navigators” address women’s personal barriers to the timely follow-up of abnormal breast and cervical cancer screening results. Patient navigation through this program and similar projects, including programs that we’ve adapted for different racial and ethnic communities in and around Chicago, has reduced or eliminated delays in diagnostic resolution of gynecologic cancer (Cancer. 2015 Nov 15;121[22]:4025-34, Breast Cancer Res Treat. 2016 Aug;158[3]:523-34, Am J Public Health. 2015 May;105[5]:e87-94).
The patient navigation model is increasingly being adapted and used in a variety of contexts outside of cancer care as well. In a postpartum patient navigation program that we tested at Northwestern University’s Medicaid-based outpatient clinic, a navigator was hired to communicate with patients and support them between delivery and completion of their postpartum care. Patients were reminded through calls and/or texts of their postpartum visits and of the benefits of breastfeeding, effective contraception, and other postpartum practices.
The demonstration project was impactful: Women who were enrolled in the program were more likely to return for postpartum care, to receive World Health Organization Tier 1 or 2 contraception, and to have postpartum screening and vaccinations, compared with women who received care before the program began (Obstet Gynecol. 2017 May;129[5]:925-33).
Connections to our patients will help us to achieve health equity. This includes connections between the primary care we provide and the specialty care our patients sometimes require, both inside and outside of our field. We may refer a patient to an oncology team, for instance, and in the process, unwittingly transfer her care such that other conditions that we’ve been managing – hypertension, depression, or diabetes – fall by the wayside.
Instead, we have to re-engineer our processes so that we maintain personalized connections back to these patients. For example, the referring ob.gyn. could develop and send to the oncologist or gynecologic-oncologist a care plan that includes the patient’s comorbid conditions and how they could be managed. This would allow for clearer communication.
Our communities
As ob.gyns., we have a common goal of championing health equity and true population health for every woman, regardless of whether she lives in rural, urban, or suburban America and regardless of whether she has conservative or liberal values. To do so, we must extend ourselves beyond our own practices.
In a committee opinion on Racial and Ethnic Disparities in Obstetrics and Gynecology, the American College of Obstetricians and Gynecologists advises that ob.gyns. take a number of actions to increase health equity. These include raising awareness about inequity and its effects on health outcomes, promoting quality improvement projects that target disparities, working with public health leadership, and helping recruit ob.gyns. and other health care providers from racial and ethnic minority groups (Obstet Gynecol 2015;126:e130-4).
In Chicago, where 1 out of 5 people lives in poverty and 1 out of 10 lives in deep poverty, we are still in our infancy in combating health inequities. However, with partnerships between academic institutions, departments of health, and other organizations across various sectors, we are beginning to move the needle on these entrenched health inequities.
For example, in 2007, there was a 60% difference in breast cancer mortality between black and white women in Chicago. This disparity sparked the development of the Metropolitan Chicago Breast Cancer Task Force and a series of on-the-ground patient navigator programs, along with several key policy changes and new state laws.
State actions included requiring quality reporting on mammography and increasing the Medicaid reimbursement rate for mammography to the Medicare rate. Nationally, beneficial changes were made to Medicare’s quality metrics and to the National Breast and Cervical Cancer Early Detection Program. All told, through a combination of studies and initiatives focused on improving knowledge, trust, access to care, and quality of care, we have been able to decrease the breast cancer mortality gap by 20%.
We also have a role to play in nurturing and developing a workforce that better aligns with our evolving demographics. This involves redesigning how we plant seeds of opportunity among high school students, undergraduates, and young medical students, and how we seek job applicants. Moreover, when we help people get to the next step in their careers, we need to make sure there is continuous support to retain them and help propel them to the next level.
We should think creatively to establish programs or launch initiatives that can help level the playing field for all women. For example, I created a Massive Open Online Course called “Career 911: Your Future Job in Medicine and Healthcare” as a free workforce development pipeline program. It is accessible on a global platform (https://www.coursera.org/learn/healthcarejobs) and is one example of how we as ob.gyns. can leverage our skills and resources.
Along the way, we also need to train our students and residents – and ourselves – to be more familiar with, and articulate about, health care policy. We need to understand how policy is made and modified and how we can be good communicators and thought leaders.
Right now, our ability to articulate our patients’ stories to policy makers and to the public seems underdeveloped and undertapped. The onus is on us to write and speak about how all women must have the opportunity to not only access care but to access high-quality care and preventive services that are important for full health. Providing health equity isn’t about giving someone a handout, but about giving her a helping hand to take control of her health.
Achieving health equity will involve changing our approach to research. If medical research on women’s health continues to be dominated by studies in which participants are homogeneous and from mainly white or well-resourced populations, we will never have output that is generalizable. As practicing ob.gyns., we can look for opportunities to advocate for diversity in research. We can also acknowledge that, for some women, there is historically-rooted distrust of the health care system that serves as a barrier both to obtaining care and enrolling in trials.
By meeting women where they are, and by tailoring their individual boxes as best we can – in research, in workforce development, and in clinical care delivery – we can work toward solutions.
Strategies for achieving women’s health equity
• Modify office hours/dates to allow flexibility for women who have challenges scheduling childcare and time off from work.
• Ensure handouts, educational materials, and all communications are at appropriate health literacy levels.
• Acknowledge and understand an individual woman’s barriers to care, including social determinants of health, and create a care plan that is achievable for her.
• Learn about and refer women to local community resources needed to overcome barriers to care, such as childcare, social services support, support services for intimate partner violence, and substance abuse counseling.
• Examine office processes to optimize the number of visits women have to attend for a particular health issue. Are there ways to explain results and next steps in a care plan without having to make her come back for an office visit?
Dr. Simon is the George H. Gardner Professor of Clinical Gynecology at Northwestern University, Chicago, and director of the Chicago Cancer Health Equity Collaborative. She is a member of the U.S. Preventive Services Task Force, but the views expressed in this piece are her own.
Of all the medical professions, obstetrics and gynecology should be the strongest champion for equity in women’s health in this country and globally. The question is, what does this mean in the reality of 2017 and moving forward in the 21st century? What does it mean in the context of our own practices and in the landscape of current policy and politics?
Finding answers to these questions requires both a deep understanding of the meaning of health equity and a willingness to rethink the architecture and engineering of how we currently provide care.
The terms equity and equality are sometimes used interchangeably, but they actually have quite different meanings. Imagine three women of different heights standing underneath the lowest branch of a tall apple tree. None of the three women are tall enough to pick an apple from the branch.
If we think about equality, we would assist each woman by giving her a box to stand on, and all three boxes would be the same size. This means that while the tallest woman will now be able to pick an apple, the medium-height woman may be able to touch but not pick the apple, and the shortest woman still may not be able to reach the apple at all.
However, if we think about equity, we’d acknowledge that each woman needs her own personalized box to be able to pick the apple. For instance, the shortest woman may need a box that is three times the height of the box used by the tallest woman.
Achieving true population health for all women requires that we similarly eliminate inequities by providing each patient with her own personalized care plan to help her reach and maintain her health.
Women from minority groups have higher rates of low birth weight, preterm birth, stillbirth, gestational diabetes and its complications, HIV, breast cancer mortality and cervical cancer incidence and mortality, infertility and response to fertility treatment, and maternal mortality.
Yet inequity runs deeper than racial/ethnic labels; disparities also are created by a host of other factors, from cognitive or physical disabilities to gender or sexual identity or orientation, one’s ZIP code, working environment, language, and health literacy.
More than ever, the art of medicine involves understanding how to meet every patient where she is – given her own context and beliefs and levels of support – so that every woman has the opportunity to stand on the right-sized box and pick the apple and thrive.
Our practices
Provider bias and stereotyping can impact health care and health outcomes, and it is important that we work to prevent this in ourselves and in our staff. This means not making assumptions. It means really listening to our patients in ways that we may not have before.
Women who have experienced health inequity may have unique barriers to success. Therefore, we must listen for cues and inquire about our patients’ environment and circumstances, as well as their partnerships and support – or lack thereof. We should then acknowledge and communicate that certain social and environmental factors may impact our ability to achieve a desired outcome.
How can we impact the diet of a patient with gestational diabetes, for instance, if we have not adequately communicated what medical nutritional therapy means in the context of her own culture and ability to access food? If she lives in a food desert or has food insecurity or lives in a violence-ridden neighborhood that keeps her from going to a grocery store regularly, we must think outside the box. Ob.gyns. and their clinical care teams can work with women who have less access to nutritious foods, or who have certain cultural food staples, to suggest recipes and grocery lists that make sense with respect to the types of stores they shop in or their cultural preferences.
When it comes to cancer prevention and treatment, how can we expect a woman to be compliant with screening if we cannot help her understand that she can get screening services for free with her health insurance? How can we help a woman who has coverage for, or access to, free screening but then no funding or coverage for a diagnostic test or cancer treatment? How can we support a patient with abnormal cancer screening results who hasn’t followed up for months because she is afraid to leave home without her partner’s permission?
Such questions and circumstances often involve what we call “social determinants of health,” and they force us to rethink how we can better deliver and optimize care. Re-engineering our practices for health equity may involve employing a more diverse practice staff, linking patients with community resources, modifying our practice hours to align better with working women’s schedules, or finding creative ways to discern patients’ motivating factors and then piggyback on these factors.
We may also need to modify how we approach the number of return visits that we request of women so that follow-up care aligns better with their ability to leave work or find childcare. Simply put, we should strive to set up our patients for success, not failure.
We can pointedly ask patients about the kinds of information and support they want and need. We might ask, for instance: What do you need, and how can I work with you, so that you can effectively monitor and control your glucose levels? How can I work with you to help you get onto a trajectory to stop smoking? How can I help you better understand what tests and procedures are covered under your insurance plan, or whether you qualify for free services?
Patients with lower health literacy may need teach-back methods to validate understanding, or messaging that is more focused and limited at any one time. Self-efficacy through patient-centered education and support should be our goal.
Practices and clinics may also be able to adapt elements of the National Cancer Institute’s multicenter Patient Navigation Research Program, in which community health workers or other “patient navigators” address women’s personal barriers to the timely follow-up of abnormal breast and cervical cancer screening results. Patient navigation through this program and similar projects, including programs that we’ve adapted for different racial and ethnic communities in and around Chicago, has reduced or eliminated delays in diagnostic resolution of gynecologic cancer (Cancer. 2015 Nov 15;121[22]:4025-34, Breast Cancer Res Treat. 2016 Aug;158[3]:523-34, Am J Public Health. 2015 May;105[5]:e87-94).
The patient navigation model is increasingly being adapted and used in a variety of contexts outside of cancer care as well. In a postpartum patient navigation program that we tested at Northwestern University’s Medicaid-based outpatient clinic, a navigator was hired to communicate with patients and support them between delivery and completion of their postpartum care. Patients were reminded through calls and/or texts of their postpartum visits and of the benefits of breastfeeding, effective contraception, and other postpartum practices.
The demonstration project was impactful: Women who were enrolled in the program were more likely to return for postpartum care, to receive World Health Organization Tier 1 or 2 contraception, and to have postpartum screening and vaccinations, compared with women who received care before the program began (Obstet Gynecol. 2017 May;129[5]:925-33).
Connections to our patients will help us to achieve health equity. This includes connections between the primary care we provide and the specialty care our patients sometimes require, both inside and outside of our field. We may refer a patient to an oncology team, for instance, and in the process, unwittingly transfer her care such that other conditions that we’ve been managing – hypertension, depression, or diabetes – fall by the wayside.
Instead, we have to re-engineer our processes so that we maintain personalized connections back to these patients. For example, the referring ob.gyn. could develop and send to the oncologist or gynecologic-oncologist a care plan that includes the patient’s comorbid conditions and how they could be managed. This would allow for clearer communication.
Our communities
As ob.gyns., we have a common goal of championing health equity and true population health for every woman, regardless of whether she lives in rural, urban, or suburban America and regardless of whether she has conservative or liberal values. To do so, we must extend ourselves beyond our own practices.
In a committee opinion on Racial and Ethnic Disparities in Obstetrics and Gynecology, the American College of Obstetricians and Gynecologists advises that ob.gyns. take a number of actions to increase health equity. These include raising awareness about inequity and its effects on health outcomes, promoting quality improvement projects that target disparities, working with public health leadership, and helping recruit ob.gyns. and other health care providers from racial and ethnic minority groups (Obstet Gynecol 2015;126:e130-4).
In Chicago, where 1 out of 5 people lives in poverty and 1 out of 10 lives in deep poverty, we are still in our infancy in combating health inequities. However, with partnerships between academic institutions, departments of health, and other organizations across various sectors, we are beginning to move the needle on these entrenched health inequities.
For example, in 2007, there was a 60% difference in breast cancer mortality between black and white women in Chicago. This disparity sparked the development of the Metropolitan Chicago Breast Cancer Task Force and a series of on-the-ground patient navigator programs, along with several key policy changes and new state laws.
State actions included requiring quality reporting on mammography and increasing the Medicaid reimbursement rate for mammography to the Medicare rate. Nationally, beneficial changes were made to Medicare’s quality metrics and to the National Breast and Cervical Cancer Early Detection Program. All told, through a combination of studies and initiatives focused on improving knowledge, trust, access to care, and quality of care, we have been able to decrease the breast cancer mortality gap by 20%.
We also have a role to play in nurturing and developing a workforce that better aligns with our evolving demographics. This involves redesigning how we plant seeds of opportunity among high school students, undergraduates, and young medical students, and how we seek job applicants. Moreover, when we help people get to the next step in their careers, we need to make sure there is continuous support to retain them and help propel them to the next level.
We should think creatively to establish programs or launch initiatives that can help level the playing field for all women. For example, I created a Massive Open Online Course called “Career 911: Your Future Job in Medicine and Healthcare” as a free workforce development pipeline program. It is accessible on a global platform (https://www.coursera.org/learn/healthcarejobs) and is one example of how we as ob.gyns. can leverage our skills and resources.
Along the way, we also need to train our students and residents – and ourselves – to be more familiar with, and articulate about, health care policy. We need to understand how policy is made and modified and how we can be good communicators and thought leaders.
Right now, our ability to articulate our patients’ stories to policy makers and to the public seems underdeveloped and undertapped. The onus is on us to write and speak about how all women must have the opportunity to not only access care but to access high-quality care and preventive services that are important for full health. Providing health equity isn’t about giving someone a handout, but about giving her a helping hand to take control of her health.
Achieving health equity will involve changing our approach to research. If medical research on women’s health continues to be dominated by studies in which participants are homogeneous and from mainly white or well-resourced populations, we will never have output that is generalizable. As practicing ob.gyns., we can look for opportunities to advocate for diversity in research. We can also acknowledge that, for some women, there is historically-rooted distrust of the health care system that serves as a barrier both to obtaining care and enrolling in trials.
By meeting women where they are, and by tailoring their individual boxes as best we can – in research, in workforce development, and in clinical care delivery – we can work toward solutions.
Strategies for achieving women’s health equity
• Modify office hours/dates to allow flexibility for women who have challenges scheduling childcare and time off from work.
• Ensure handouts, educational materials, and all communications are at appropriate health literacy levels.
• Acknowledge and understand an individual woman’s barriers to care, including social determinants of health, and create a care plan that is achievable for her.
• Learn about and refer women to local community resources needed to overcome barriers to care, such as childcare, social services support, support services for intimate partner violence, and substance abuse counseling.
• Examine office processes to optimize the number of visits women have to attend for a particular health issue. Are there ways to explain results and next steps in a care plan without having to make her come back for an office visit?
Dr. Simon is the George H. Gardner Professor of Clinical Gynecology at Northwestern University, Chicago, and director of the Chicago Cancer Health Equity Collaborative. She is a member of the U.S. Preventive Services Task Force, but the views expressed in this piece are her own.
Demystifying interstitial cystitis
Chronic pelvic pain continues not only to burden the individual, but society as well.
One in seven women between the ages of 18 and 50 endure chronic pelvic pain; with a lifetime incidence of as high as 33%, according to one Gallup poll. Interstitial cystitis/bladder pain syndrome (IC/BPS) has been estimated to have a prevalence of 850 in 100,000 women and 60 in 100,000 men in self-report studies. The RAND Interstitial Cystitis Epidemiology (RICE) study, a symptoms survey, showed that between 2.7% and 6.5% of women (3.3 to 7.9 million women) in the United States have symptoms consistent with a diagnosis of IC/BPS.
Unfortunately, there is little known about the etiology and pathogenesis of IC/PBS. Moreover, oftentimes, the diagnosis is one of exclusion.
To demystify interstitial cystitis/bladder pain syndrome, I have elicited the assistance of Dr. Kenneth Peters, a urologist on staff at William Beaumont Hospital, Royal Oak, Mich. Dr. Peters is the professor and chairman of urology at Oakland University, William Beaumont School of Medicine, and the chairman of urology at Beaumont Health, Royal Oak, Mich.
In his discussion, Dr. Peters will point out that interstitial cystitis actually consists of two different entities: a classic presentation featuring the pathognomonic Hunner’s lesion on cystoscopy and interstitial cystitis/painful bladder syndrome.
It must be acknowledged that Dr. Peters is a practicing urologist. Therefore, some of his recommendations, such as cauterizing Hunner’s lesions via a resectoscope, are beyond the scope of practicing gynecologists. However, it is important for us to realize what our potential referrals possess in their armamentarium. Moreover, it is obvious there is much that can be learned from this excellent diagnostician who professes the importance of physical examination.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is an investigator on an interstitial cystitis study sponsored by Allergan.
Chronic pelvic pain continues not only to burden the individual, but society as well.
One in seven women between the ages of 18 and 50 endure chronic pelvic pain; with a lifetime incidence of as high as 33%, according to one Gallup poll. Interstitial cystitis/bladder pain syndrome (IC/BPS) has been estimated to have a prevalence of 850 in 100,000 women and 60 in 100,000 men in self-report studies. The RAND Interstitial Cystitis Epidemiology (RICE) study, a symptoms survey, showed that between 2.7% and 6.5% of women (3.3 to 7.9 million women) in the United States have symptoms consistent with a diagnosis of IC/BPS.
Unfortunately, there is little known about the etiology and pathogenesis of IC/PBS. Moreover, oftentimes, the diagnosis is one of exclusion.
To demystify interstitial cystitis/bladder pain syndrome, I have elicited the assistance of Dr. Kenneth Peters, a urologist on staff at William Beaumont Hospital, Royal Oak, Mich. Dr. Peters is the professor and chairman of urology at Oakland University, William Beaumont School of Medicine, and the chairman of urology at Beaumont Health, Royal Oak, Mich.
In his discussion, Dr. Peters will point out that interstitial cystitis actually consists of two different entities: a classic presentation featuring the pathognomonic Hunner’s lesion on cystoscopy and interstitial cystitis/painful bladder syndrome.
It must be acknowledged that Dr. Peters is a practicing urologist. Therefore, some of his recommendations, such as cauterizing Hunner’s lesions via a resectoscope, are beyond the scope of practicing gynecologists. However, it is important for us to realize what our potential referrals possess in their armamentarium. Moreover, it is obvious there is much that can be learned from this excellent diagnostician who professes the importance of physical examination.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is an investigator on an interstitial cystitis study sponsored by Allergan.
Chronic pelvic pain continues not only to burden the individual, but society as well.
One in seven women between the ages of 18 and 50 endure chronic pelvic pain; with a lifetime incidence of as high as 33%, according to one Gallup poll. Interstitial cystitis/bladder pain syndrome (IC/BPS) has been estimated to have a prevalence of 850 in 100,000 women and 60 in 100,000 men in self-report studies. The RAND Interstitial Cystitis Epidemiology (RICE) study, a symptoms survey, showed that between 2.7% and 6.5% of women (3.3 to 7.9 million women) in the United States have symptoms consistent with a diagnosis of IC/BPS.
Unfortunately, there is little known about the etiology and pathogenesis of IC/PBS. Moreover, oftentimes, the diagnosis is one of exclusion.
To demystify interstitial cystitis/bladder pain syndrome, I have elicited the assistance of Dr. Kenneth Peters, a urologist on staff at William Beaumont Hospital, Royal Oak, Mich. Dr. Peters is the professor and chairman of urology at Oakland University, William Beaumont School of Medicine, and the chairman of urology at Beaumont Health, Royal Oak, Mich.
In his discussion, Dr. Peters will point out that interstitial cystitis actually consists of two different entities: a classic presentation featuring the pathognomonic Hunner’s lesion on cystoscopy and interstitial cystitis/painful bladder syndrome.
It must be acknowledged that Dr. Peters is a practicing urologist. Therefore, some of his recommendations, such as cauterizing Hunner’s lesions via a resectoscope, are beyond the scope of practicing gynecologists. However, it is important for us to realize what our potential referrals possess in their armamentarium. Moreover, it is obvious there is much that can be learned from this excellent diagnostician who professes the importance of physical examination.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is an investigator on an interstitial cystitis study sponsored by Allergan.
The broad picture of interstitial cystitis
Interstitial cystitis (IC) is a controversial diagnosis that has become muddied and oversimplified. It was originally described as a distinct ulcer (Hunner’s lesion) seen in the bladder on cystoscopy, the treatment of which often led to symptomatic relief. Hunner’s lesion IC is the “classic” form of IC and should be considered a separate disease; it is not a progression of nonulcerative interstitial cystitis/painful bladder syndrome (IC/BPS).
Only a fraction of patients with the key symptoms of IC/BPS – urinary frequency, urgency, and pelvic pain – have ulcers within the bladder. And many of the patients who are diagnosed with IC/BPS are found not to have bladder pathology as the name implies, but rather pelvic floor dysfunction. That the bladder is often an innocent bystander to a larger process means that, as clinicians, we must be thoughtful and astute about our diagnostic process.
Hunner’s lesions
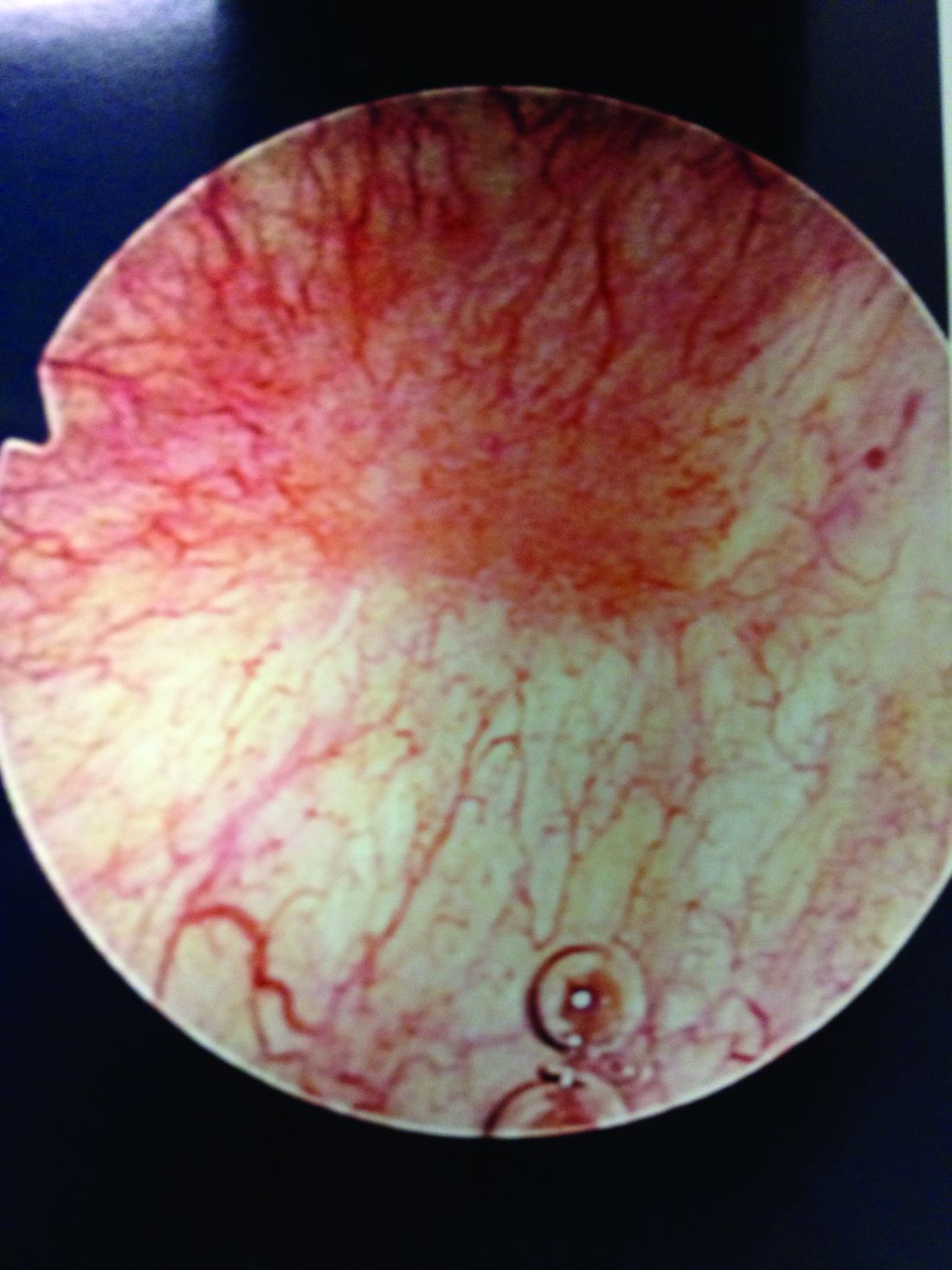
Patients with Hunner’s lesions have a rapid onset of symptoms, typically are older, and have a visible lesion in their bladder that almost always is on the dome or lateral walls. The lesion is often erythematous with central vascularity and mucosal sloughing.
The bladder is a storage organ and urine is toxic. The exposed ulcer results in severe pain with bladder filling and also pain at the end of voiding as the bladder collapses, causing ulcerated tissue to come into contact with other sections of the bladder wall and sending a “jolt” of pain through the pelvis.
If the initial cystoscopy demonstrates inflammatory-appearing lesions or ulcerations suggestive of Hunner’s lesions, I will still do a hydrodistension. By stretching the bladder, the lesions typically expand, crack, and bleed. This helps define the entire diseased area and shows what areas of the bladder need to be cauterized to seal the ulcers and destroy the exposed nerve endings. If this is a new diagnosis, the lesion should be biopsied after the hydrodistension to rule out carcinoma.
Hunner’s lesions can lead to rapid disease progression due to chronic inflammation and subsequent collagen deposition and scarring. Even on initial diagnosis of Hunner’s lesions, a capacity of 350 cc or less (compared with 1,100 cc in a normal bladder) on hydrodistension under anesthesia is not uncommon. This markedly reduced bladder capacity may lead to end-stage bladder impacting the kidneys and requiring a urinary diversion.
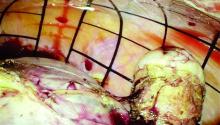
Eradicating the ulcers with resection or cautery often results in marked and immediate improvement in bladder pain, albeit not long-lasting. I will typically place a resectoscope and use a roller ball at 25 watts of current. The entire ulcerated areas are cauterized by rapidly rolling the ball over the area of inflammation and avoiding a deep thermal burn. The goal is to seal the ulcer and destroy the exposed nerve endings so that urine can no longer act as an irritant. Recurrence in 6 months to 1 year is common and retreatment is almost always necessary. We have demonstrated, however, that recurrent cautery of ulcers does not lead to smaller anesthetic bladder capacities (Urology. 2015 Jan;85[1]:74-8).
Low-dose cyclosporine can be very effective at reducing Hunner’s lesion recurrence and improving storage symptoms (Exp Ther Med. 2016 Jul;12[1]:445-50). I use 100 mg twice a day for a month and then 1 pill a day thereafter. This is a relatively low dose, but hypertension can be a side effect and blood pressure should be monitored along with routine labs.
The broader picture
Hunner’s lesion IC is pretty straightforward and clearly a bladder disease. However, in recent years the term IC/BPS has been broadly used to describe women who have symptoms of pelvic pain, urinary urgency, and frequency, but no true bladder pathology to explain their symptoms. One problem: There is no definitive diagnostic test or evidence-based diagnostic process for IC/BPS. In fact, the diagnosis section of the American Urological Association guideline on diagnosis and treatment of IC/BPS, last updated in 2015, is almost entirely consensus-based (J Urol. 2015 May;193[5]:1545-53). It largely remains a diagnosis of exclusion.
As the AUA guidelines state, a careful history, physical examination, and laboratory assessment are all important for documenting symptoms and signs and ruling out significant causes of the symptoms. I frequently see patients who have been diagnosed with IC who have frequency and urgency but no pain (in which case overactive bladder should be considered) or who have pelvic pain but no bladder symptoms, again likely not IC. Pain that worsens with bladder filling and improves after bladder emptying is typical of IC/BPS. This finding in the absence of other confusing symptoms supports the diagnosis of IC/BPS.
It has become too easy for the average clinician to apply a label of IC/BPS to a patient complaining of pelvic pain; this often results in the patient undergoing invasive and nonhelpful therapies such as cystoscopy, hydrodistension, urodynamics, bladder instillations, and other bladder-directed therapies.
More than 20 years of research supported by the National Institutes of Health and industry have failed to show that bladder-directed therapy is superior to placebo. This fact suggests that the bladder may be an innocent bystander in a larger pelvic process. As clinicians, we must be willing to look beyond the bladder and examine for pelvic floor issues and other causes of patient’s symptoms and not be too quick to begin bladder-focused treatments.
A number of disease processes – such as recurrent urinary tract infection, urethral diverticulum, endometriosis, and pudendal neuropathy – can mimic the symptoms of IC/BPS. The most common missed diagnosis in the IC patient is pelvic floor dysfunction that results in a hypertonic contracted state of the levator muscles – a chronic spasm, in essence – that in turn leads to decreased muscle function, increased myofascial pain, and myofascial trigger points (Curr Urol Rep. 2006 Nov;7[6]:450-5).
We and others have reported that up to 85% of patients labeled with IC/BPS have been found on examination to have pelvic floor dysfunction or a diffuse pelvic floor hypersensitivity. The pelvic floor is important in maintaining healthy bladder, bowel, and sexual function. If the pelvic floor is in spasm, this can result in urinary frequency, hesitancy, and pelvic pain.
Many of these patients with contracted pelvic floor muscles report pain with sexual intercourse – often so severe as to cause abstention. In fact, when patients answer no to the question of whether they have pain with intercourse, I know it is unlikely that they have significant pelvic floor dysfunction. This is a key question for history taking.
Other key questions concern the impact of stress on symptoms and a history of any type of abuse. In a study we conducted about 10 years ago, we found that among 76 women who were diagnosed with IC and subsequently evaluated in our clinic, almost half (49%) reported abuse (emotional, physical, and/or sexual). The vast majority (85%) had levator pain (J Urol. 2007 Sep;178[3 Pt 1]:891-5).
Other types of stress – from past surgeries to traumatic life events – may similarly serve as triggers or precursors to pelvic floor dysfunction in some women. I often tell patients that people put stress in different areas of their bodies. While some get tension headaches or low-back aches, others get pelvic pain from contracting and guarding the levator muscles.
Pelvic floor dysfunction
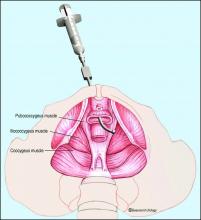
The most important component of the physical exam in patients with the symptoms of frequency, urgency, and pelvic pain – and the most overlooked – is assessment of the levator muscles for tightness and tenderness. Levator pain and trigger points may be identified during the pelvic exam by pressing laterally on the levator complex in each quadrant of the vagina and at the ischial spines. The tension of the muscles and severity of pain should be assessed, and it is helpful to ask the patient if the pain reproduces her normal pelvic pain symptoms.
We’ve found that identifying and treating pelvic floor dysfunction with modalities such as pelvic floor physical therapy with intravaginal myofascial release, intravaginal valium, trigger point injections into the levator complex, pudendal nerve blocks, and neuromodulation can frequently resolve or significantly lessen the patient’s pain and bladder symptoms, suggesting that the diagnosis of IC/BPS was wrong.
Pelvic floor physical therapy works to stretch the contracted anterior pelvic muscles by releasing trigger points and connective tissue restrictions, and by decreasing periurethral tension; it also may decrease neurogenic triggers and central nervous system sensitivity. Kegel exercises will worsen pain in these patients and should be avoided.
When pelvic floor dysfunction is identified, such treatment by a therapist knowledgeable in intravaginal myofascial release is a next reasonable step before any medications or invasive testing, such as bladder hydrodistension, are used.
One of the only National Institutes of Health–funded studies to show benefit of a treatment in an IC population, in fact, was a multicenter randomized controlled trial comparing 10 sessions of myofascial pelvic floor physical therapy with “global therapeutic massage.” Myofascial physical therapy led to significant improvement, compared with the generalized spa-like massage (J Urol. 2012 Jun;187[6]:2113-8).
Our patients with IC/BPS symptoms and pelvic floor dysfunction require 1-2 visits weekly for an average of 12 weeks for tightness and tenderness to be significantly minimized or eliminated. Patients are also prescribed home stretching exercises and advised to use internal vaginal dilators. Most patients will report resolution of their pelvic pain, sexual pain, and bladder symptoms – especially with the combination of physical therapy and trigger point injections. In more severe cases, we may use sacral or pudendal neuromodulation to improve the frequency, urgency and pelvic pain.
Turning to the bladder
When urinary symptoms persist after the completion of pelvic floor therapy, or when pelvic floor dysfunction is not identified in the first place, we proceed with bladder-specific therapies. I will often suggest trials of amitriptyline or hydroxyzine, for instance, and/or changes in hydration and caffeine consumption. I am not a fan of pentosan polysulfate sodium (Elmiron) as it is a very expensive medication that has minimal benefit for the majority of patients.
When conservative therapies do not work, I move to cystoscopy with hydrodistension. The procedure can serve several purposes. It can be diagnostic, enabling us to rule out other potential symptom-causing pathologies, and it can be prognostic, helping us to understand when bladder capacity is severely reduced and to plan treatment. In some patients, it can even be therapeutic. Some of my patients have significant relief of symptoms from a hydrodistension of the bladder once or twice a year.
There is no standard method for performing a hydrodistension. I perform a complete cystoscopy to look for tumors, stones, diverticulum or Hunner’s lesions and, if the bladder is normal in appearance, I proceed with a 2-minute hydrodistension at 80-100 cm of water pressure under anesthesia. The bag is raised above the bladder, allowing the bladder to fill with the force of gravity and the pressures to equalize. The urethra must be compressed so that water doesn’t leak around the cystoscope. After 2 minutes of hydrodistension, the bladder is drained, volume is measured, and the procedure is repeated.
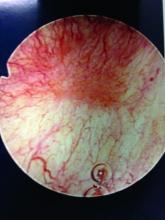
After the hydrodistension, the bladder is reinspected to be certain there is no bladder perforation and to evaluate for diffuse glomerulations (petechial hemorrhages) that are suggestive, but not diagnostic, of IC/BPS.
A holistic approach
Managing patients with voiding dysfunction and chronic pelvic pain can be a challenge, and a multidisciplinary approach is most effective. At Beaumont, we have a Women’s Urology Center that includes urologists, gynecologists, nurse practitioners, pelvic floor physical therapists, pain psychologists, colorectal specialists, sex therapists, and naturopathic and integrative medicine specialists who perform acupuncture, Reiki therapy, medical massage, and guided imagery.
The goal is to break out of our box of specialties and look at the whole patient – mind, body, and soul – while identifying pain triggers and directing therapy toward these triggers using a multidisciplinary, collaborative approach. For us, this approach has been very effective for managing complex pelvic pain issues (Transl Androl Urol. 2015 Dec;4[6]:611-9).
Ongoing studies
A number of research studies are ongoing to help treat the symptoms of IC/BPS. We currently have a Department of Defense grant to prospectively assess bladder-directed therapy (instillations) compared to pelvic floor physical therapy. Patients diagnosed with IC/BPS are being randomized into these two treatment arms and we hope to get a better understanding of the role of these modalities in managing IC/BPS.
Allergan is completing a phase II placebo-controlled trial using a lidocaine delivery device that is placed in the bladder and continuously releases lidocaine over 14 days. The LiNKA trial is designed to assess the impact of lidocaine on not only improving bladder symptoms, but also eradicating Hunner’s lesions through the anti-inflammatory effect of lidocaine. Early open-label data were very promising. In addition, a new medication for IC/BPS that modulates the SHIP1 pathway is being studied by Aquinox Pharmaceuticals. The agent, AQX-1125, is an activator of SHIP1, which controls the phosphoinositide 3-kinase (PI3K) cellular signaling pathway. If the PI3K pathway is overactive, immune cells can produce an abundance of proinflammatory signaling molecules and migrate to and concentrate in tissues, resulting in excessive or chronic inflammation. Early data in IC/BPS patients were supportive of the compound’s potential for reducing the pain associated with this condition.
A note from Charles E. Miller, MD, Master Class Medical Editor:
In a double-blind, placebo-controlled study by J.C. Nickel, et al., pentosan polysulfate sodium was shown to improve pain, urgency, and frequency over the control group (Urology. 2005 Apr;65[4]:654-8). Also, longer duration of treatment with pentosan polysulfate sodium was associated with greater response rates – 50% improved by 26 weeks (J Urol. 2005 Dec;174[6]:2235-8).
Dr. Peters is professor and chairman of urology at Oakland University William Beaumont School of Medicine, Royal Oak, Mich. He reported serving as a consultant for Taris, Medtronic, StimGuard, and Amphora Medical.
Interstitial cystitis (IC) is a controversial diagnosis that has become muddied and oversimplified. It was originally described as a distinct ulcer (Hunner’s lesion) seen in the bladder on cystoscopy, the treatment of which often led to symptomatic relief. Hunner’s lesion IC is the “classic” form of IC and should be considered a separate disease; it is not a progression of nonulcerative interstitial cystitis/painful bladder syndrome (IC/BPS).
Only a fraction of patients with the key symptoms of IC/BPS – urinary frequency, urgency, and pelvic pain – have ulcers within the bladder. And many of the patients who are diagnosed with IC/BPS are found not to have bladder pathology as the name implies, but rather pelvic floor dysfunction. That the bladder is often an innocent bystander to a larger process means that, as clinicians, we must be thoughtful and astute about our diagnostic process.
Hunner’s lesions
Patients with Hunner’s lesions have a rapid onset of symptoms, typically are older, and have a visible lesion in their bladder that almost always is on the dome or lateral walls. The lesion is often erythematous with central vascularity and mucosal sloughing.
The bladder is a storage organ and urine is toxic. The exposed ulcer results in severe pain with bladder filling and also pain at the end of voiding as the bladder collapses, causing ulcerated tissue to come into contact with other sections of the bladder wall and sending a “jolt” of pain through the pelvis.
If the initial cystoscopy demonstrates inflammatory-appearing lesions or ulcerations suggestive of Hunner’s lesions, I will still do a hydrodistension. By stretching the bladder, the lesions typically expand, crack, and bleed. This helps define the entire diseased area and shows what areas of the bladder need to be cauterized to seal the ulcers and destroy the exposed nerve endings. If this is a new diagnosis, the lesion should be biopsied after the hydrodistension to rule out carcinoma.
Hunner’s lesions can lead to rapid disease progression due to chronic inflammation and subsequent collagen deposition and scarring. Even on initial diagnosis of Hunner’s lesions, a capacity of 350 cc or less (compared with 1,100 cc in a normal bladder) on hydrodistension under anesthesia is not uncommon. This markedly reduced bladder capacity may lead to end-stage bladder impacting the kidneys and requiring a urinary diversion.
Eradicating the ulcers with resection or cautery often results in marked and immediate improvement in bladder pain, albeit not long-lasting. I will typically place a resectoscope and use a roller ball at 25 watts of current. The entire ulcerated areas are cauterized by rapidly rolling the ball over the area of inflammation and avoiding a deep thermal burn. The goal is to seal the ulcer and destroy the exposed nerve endings so that urine can no longer act as an irritant. Recurrence in 6 months to 1 year is common and retreatment is almost always necessary. We have demonstrated, however, that recurrent cautery of ulcers does not lead to smaller anesthetic bladder capacities (Urology. 2015 Jan;85[1]:74-8).
Low-dose cyclosporine can be very effective at reducing Hunner’s lesion recurrence and improving storage symptoms (Exp Ther Med. 2016 Jul;12[1]:445-50). I use 100 mg twice a day for a month and then 1 pill a day thereafter. This is a relatively low dose, but hypertension can be a side effect and blood pressure should be monitored along with routine labs.
The broader picture
Hunner’s lesion IC is pretty straightforward and clearly a bladder disease. However, in recent years the term IC/BPS has been broadly used to describe women who have symptoms of pelvic pain, urinary urgency, and frequency, but no true bladder pathology to explain their symptoms. One problem: There is no definitive diagnostic test or evidence-based diagnostic process for IC/BPS. In fact, the diagnosis section of the American Urological Association guideline on diagnosis and treatment of IC/BPS, last updated in 2015, is almost entirely consensus-based (J Urol. 2015 May;193[5]:1545-53). It largely remains a diagnosis of exclusion.
As the AUA guidelines state, a careful history, physical examination, and laboratory assessment are all important for documenting symptoms and signs and ruling out significant causes of the symptoms. I frequently see patients who have been diagnosed with IC who have frequency and urgency but no pain (in which case overactive bladder should be considered) or who have pelvic pain but no bladder symptoms, again likely not IC. Pain that worsens with bladder filling and improves after bladder emptying is typical of IC/BPS. This finding in the absence of other confusing symptoms supports the diagnosis of IC/BPS.
It has become too easy for the average clinician to apply a label of IC/BPS to a patient complaining of pelvic pain; this often results in the patient undergoing invasive and nonhelpful therapies such as cystoscopy, hydrodistension, urodynamics, bladder instillations, and other bladder-directed therapies.
More than 20 years of research supported by the National Institutes of Health and industry have failed to show that bladder-directed therapy is superior to placebo. This fact suggests that the bladder may be an innocent bystander in a larger pelvic process. As clinicians, we must be willing to look beyond the bladder and examine for pelvic floor issues and other causes of patient’s symptoms and not be too quick to begin bladder-focused treatments.
A number of disease processes – such as recurrent urinary tract infection, urethral diverticulum, endometriosis, and pudendal neuropathy – can mimic the symptoms of IC/BPS. The most common missed diagnosis in the IC patient is pelvic floor dysfunction that results in a hypertonic contracted state of the levator muscles – a chronic spasm, in essence – that in turn leads to decreased muscle function, increased myofascial pain, and myofascial trigger points (Curr Urol Rep. 2006 Nov;7[6]:450-5).
We and others have reported that up to 85% of patients labeled with IC/BPS have been found on examination to have pelvic floor dysfunction or a diffuse pelvic floor hypersensitivity. The pelvic floor is important in maintaining healthy bladder, bowel, and sexual function. If the pelvic floor is in spasm, this can result in urinary frequency, hesitancy, and pelvic pain.
Many of these patients with contracted pelvic floor muscles report pain with sexual intercourse – often so severe as to cause abstention. In fact, when patients answer no to the question of whether they have pain with intercourse, I know it is unlikely that they have significant pelvic floor dysfunction. This is a key question for history taking.
Other key questions concern the impact of stress on symptoms and a history of any type of abuse. In a study we conducted about 10 years ago, we found that among 76 women who were diagnosed with IC and subsequently evaluated in our clinic, almost half (49%) reported abuse (emotional, physical, and/or sexual). The vast majority (85%) had levator pain (J Urol. 2007 Sep;178[3 Pt 1]:891-5).
Other types of stress – from past surgeries to traumatic life events – may similarly serve as triggers or precursors to pelvic floor dysfunction in some women. I often tell patients that people put stress in different areas of their bodies. While some get tension headaches or low-back aches, others get pelvic pain from contracting and guarding the levator muscles.
Pelvic floor dysfunction
The most important component of the physical exam in patients with the symptoms of frequency, urgency, and pelvic pain – and the most overlooked – is assessment of the levator muscles for tightness and tenderness. Levator pain and trigger points may be identified during the pelvic exam by pressing laterally on the levator complex in each quadrant of the vagina and at the ischial spines. The tension of the muscles and severity of pain should be assessed, and it is helpful to ask the patient if the pain reproduces her normal pelvic pain symptoms.
We’ve found that identifying and treating pelvic floor dysfunction with modalities such as pelvic floor physical therapy with intravaginal myofascial release, intravaginal valium, trigger point injections into the levator complex, pudendal nerve blocks, and neuromodulation can frequently resolve or significantly lessen the patient’s pain and bladder symptoms, suggesting that the diagnosis of IC/BPS was wrong.
Pelvic floor physical therapy works to stretch the contracted anterior pelvic muscles by releasing trigger points and connective tissue restrictions, and by decreasing periurethral tension; it also may decrease neurogenic triggers and central nervous system sensitivity. Kegel exercises will worsen pain in these patients and should be avoided.
When pelvic floor dysfunction is identified, such treatment by a therapist knowledgeable in intravaginal myofascial release is a next reasonable step before any medications or invasive testing, such as bladder hydrodistension, are used.
One of the only National Institutes of Health–funded studies to show benefit of a treatment in an IC population, in fact, was a multicenter randomized controlled trial comparing 10 sessions of myofascial pelvic floor physical therapy with “global therapeutic massage.” Myofascial physical therapy led to significant improvement, compared with the generalized spa-like massage (J Urol. 2012 Jun;187[6]:2113-8).
Our patients with IC/BPS symptoms and pelvic floor dysfunction require 1-2 visits weekly for an average of 12 weeks for tightness and tenderness to be significantly minimized or eliminated. Patients are also prescribed home stretching exercises and advised to use internal vaginal dilators. Most patients will report resolution of their pelvic pain, sexual pain, and bladder symptoms – especially with the combination of physical therapy and trigger point injections. In more severe cases, we may use sacral or pudendal neuromodulation to improve the frequency, urgency and pelvic pain.
Turning to the bladder
When urinary symptoms persist after the completion of pelvic floor therapy, or when pelvic floor dysfunction is not identified in the first place, we proceed with bladder-specific therapies. I will often suggest trials of amitriptyline or hydroxyzine, for instance, and/or changes in hydration and caffeine consumption. I am not a fan of pentosan polysulfate sodium (Elmiron) as it is a very expensive medication that has minimal benefit for the majority of patients.
When conservative therapies do not work, I move to cystoscopy with hydrodistension. The procedure can serve several purposes. It can be diagnostic, enabling us to rule out other potential symptom-causing pathologies, and it can be prognostic, helping us to understand when bladder capacity is severely reduced and to plan treatment. In some patients, it can even be therapeutic. Some of my patients have significant relief of symptoms from a hydrodistension of the bladder once or twice a year.
There is no standard method for performing a hydrodistension. I perform a complete cystoscopy to look for tumors, stones, diverticulum or Hunner’s lesions and, if the bladder is normal in appearance, I proceed with a 2-minute hydrodistension at 80-100 cm of water pressure under anesthesia. The bag is raised above the bladder, allowing the bladder to fill with the force of gravity and the pressures to equalize. The urethra must be compressed so that water doesn’t leak around the cystoscope. After 2 minutes of hydrodistension, the bladder is drained, volume is measured, and the procedure is repeated.
After the hydrodistension, the bladder is reinspected to be certain there is no bladder perforation and to evaluate for diffuse glomerulations (petechial hemorrhages) that are suggestive, but not diagnostic, of IC/BPS.
A holistic approach
Managing patients with voiding dysfunction and chronic pelvic pain can be a challenge, and a multidisciplinary approach is most effective. At Beaumont, we have a Women’s Urology Center that includes urologists, gynecologists, nurse practitioners, pelvic floor physical therapists, pain psychologists, colorectal specialists, sex therapists, and naturopathic and integrative medicine specialists who perform acupuncture, Reiki therapy, medical massage, and guided imagery.
The goal is to break out of our box of specialties and look at the whole patient – mind, body, and soul – while identifying pain triggers and directing therapy toward these triggers using a multidisciplinary, collaborative approach. For us, this approach has been very effective for managing complex pelvic pain issues (Transl Androl Urol. 2015 Dec;4[6]:611-9).
Ongoing studies
A number of research studies are ongoing to help treat the symptoms of IC/BPS. We currently have a Department of Defense grant to prospectively assess bladder-directed therapy (instillations) compared to pelvic floor physical therapy. Patients diagnosed with IC/BPS are being randomized into these two treatment arms and we hope to get a better understanding of the role of these modalities in managing IC/BPS.
Allergan is completing a phase II placebo-controlled trial using a lidocaine delivery device that is placed in the bladder and continuously releases lidocaine over 14 days. The LiNKA trial is designed to assess the impact of lidocaine on not only improving bladder symptoms, but also eradicating Hunner’s lesions through the anti-inflammatory effect of lidocaine. Early open-label data were very promising. In addition, a new medication for IC/BPS that modulates the SHIP1 pathway is being studied by Aquinox Pharmaceuticals. The agent, AQX-1125, is an activator of SHIP1, which controls the phosphoinositide 3-kinase (PI3K) cellular signaling pathway. If the PI3K pathway is overactive, immune cells can produce an abundance of proinflammatory signaling molecules and migrate to and concentrate in tissues, resulting in excessive or chronic inflammation. Early data in IC/BPS patients were supportive of the compound’s potential for reducing the pain associated with this condition.
A note from Charles E. Miller, MD, Master Class Medical Editor:
In a double-blind, placebo-controlled study by J.C. Nickel, et al., pentosan polysulfate sodium was shown to improve pain, urgency, and frequency over the control group (Urology. 2005 Apr;65[4]:654-8). Also, longer duration of treatment with pentosan polysulfate sodium was associated with greater response rates – 50% improved by 26 weeks (J Urol. 2005 Dec;174[6]:2235-8).
Dr. Peters is professor and chairman of urology at Oakland University William Beaumont School of Medicine, Royal Oak, Mich. He reported serving as a consultant for Taris, Medtronic, StimGuard, and Amphora Medical.
Interstitial cystitis (IC) is a controversial diagnosis that has become muddied and oversimplified. It was originally described as a distinct ulcer (Hunner’s lesion) seen in the bladder on cystoscopy, the treatment of which often led to symptomatic relief. Hunner’s lesion IC is the “classic” form of IC and should be considered a separate disease; it is not a progression of nonulcerative interstitial cystitis/painful bladder syndrome (IC/BPS).
Only a fraction of patients with the key symptoms of IC/BPS – urinary frequency, urgency, and pelvic pain – have ulcers within the bladder. And many of the patients who are diagnosed with IC/BPS are found not to have bladder pathology as the name implies, but rather pelvic floor dysfunction. That the bladder is often an innocent bystander to a larger process means that, as clinicians, we must be thoughtful and astute about our diagnostic process.
Hunner’s lesions
Patients with Hunner’s lesions have a rapid onset of symptoms, typically are older, and have a visible lesion in their bladder that almost always is on the dome or lateral walls. The lesion is often erythematous with central vascularity and mucosal sloughing.
The bladder is a storage organ and urine is toxic. The exposed ulcer results in severe pain with bladder filling and also pain at the end of voiding as the bladder collapses, causing ulcerated tissue to come into contact with other sections of the bladder wall and sending a “jolt” of pain through the pelvis.
If the initial cystoscopy demonstrates inflammatory-appearing lesions or ulcerations suggestive of Hunner’s lesions, I will still do a hydrodistension. By stretching the bladder, the lesions typically expand, crack, and bleed. This helps define the entire diseased area and shows what areas of the bladder need to be cauterized to seal the ulcers and destroy the exposed nerve endings. If this is a new diagnosis, the lesion should be biopsied after the hydrodistension to rule out carcinoma.
Hunner’s lesions can lead to rapid disease progression due to chronic inflammation and subsequent collagen deposition and scarring. Even on initial diagnosis of Hunner’s lesions, a capacity of 350 cc or less (compared with 1,100 cc in a normal bladder) on hydrodistension under anesthesia is not uncommon. This markedly reduced bladder capacity may lead to end-stage bladder impacting the kidneys and requiring a urinary diversion.
Eradicating the ulcers with resection or cautery often results in marked and immediate improvement in bladder pain, albeit not long-lasting. I will typically place a resectoscope and use a roller ball at 25 watts of current. The entire ulcerated areas are cauterized by rapidly rolling the ball over the area of inflammation and avoiding a deep thermal burn. The goal is to seal the ulcer and destroy the exposed nerve endings so that urine can no longer act as an irritant. Recurrence in 6 months to 1 year is common and retreatment is almost always necessary. We have demonstrated, however, that recurrent cautery of ulcers does not lead to smaller anesthetic bladder capacities (Urology. 2015 Jan;85[1]:74-8).
Low-dose cyclosporine can be very effective at reducing Hunner’s lesion recurrence and improving storage symptoms (Exp Ther Med. 2016 Jul;12[1]:445-50). I use 100 mg twice a day for a month and then 1 pill a day thereafter. This is a relatively low dose, but hypertension can be a side effect and blood pressure should be monitored along with routine labs.
The broader picture
Hunner’s lesion IC is pretty straightforward and clearly a bladder disease. However, in recent years the term IC/BPS has been broadly used to describe women who have symptoms of pelvic pain, urinary urgency, and frequency, but no true bladder pathology to explain their symptoms. One problem: There is no definitive diagnostic test or evidence-based diagnostic process for IC/BPS. In fact, the diagnosis section of the American Urological Association guideline on diagnosis and treatment of IC/BPS, last updated in 2015, is almost entirely consensus-based (J Urol. 2015 May;193[5]:1545-53). It largely remains a diagnosis of exclusion.
As the AUA guidelines state, a careful history, physical examination, and laboratory assessment are all important for documenting symptoms and signs and ruling out significant causes of the symptoms. I frequently see patients who have been diagnosed with IC who have frequency and urgency but no pain (in which case overactive bladder should be considered) or who have pelvic pain but no bladder symptoms, again likely not IC. Pain that worsens with bladder filling and improves after bladder emptying is typical of IC/BPS. This finding in the absence of other confusing symptoms supports the diagnosis of IC/BPS.
It has become too easy for the average clinician to apply a label of IC/BPS to a patient complaining of pelvic pain; this often results in the patient undergoing invasive and nonhelpful therapies such as cystoscopy, hydrodistension, urodynamics, bladder instillations, and other bladder-directed therapies.
More than 20 years of research supported by the National Institutes of Health and industry have failed to show that bladder-directed therapy is superior to placebo. This fact suggests that the bladder may be an innocent bystander in a larger pelvic process. As clinicians, we must be willing to look beyond the bladder and examine for pelvic floor issues and other causes of patient’s symptoms and not be too quick to begin bladder-focused treatments.
A number of disease processes – such as recurrent urinary tract infection, urethral diverticulum, endometriosis, and pudendal neuropathy – can mimic the symptoms of IC/BPS. The most common missed diagnosis in the IC patient is pelvic floor dysfunction that results in a hypertonic contracted state of the levator muscles – a chronic spasm, in essence – that in turn leads to decreased muscle function, increased myofascial pain, and myofascial trigger points (Curr Urol Rep. 2006 Nov;7[6]:450-5).
We and others have reported that up to 85% of patients labeled with IC/BPS have been found on examination to have pelvic floor dysfunction or a diffuse pelvic floor hypersensitivity. The pelvic floor is important in maintaining healthy bladder, bowel, and sexual function. If the pelvic floor is in spasm, this can result in urinary frequency, hesitancy, and pelvic pain.
Many of these patients with contracted pelvic floor muscles report pain with sexual intercourse – often so severe as to cause abstention. In fact, when patients answer no to the question of whether they have pain with intercourse, I know it is unlikely that they have significant pelvic floor dysfunction. This is a key question for history taking.
Other key questions concern the impact of stress on symptoms and a history of any type of abuse. In a study we conducted about 10 years ago, we found that among 76 women who were diagnosed with IC and subsequently evaluated in our clinic, almost half (49%) reported abuse (emotional, physical, and/or sexual). The vast majority (85%) had levator pain (J Urol. 2007 Sep;178[3 Pt 1]:891-5).
Other types of stress – from past surgeries to traumatic life events – may similarly serve as triggers or precursors to pelvic floor dysfunction in some women. I often tell patients that people put stress in different areas of their bodies. While some get tension headaches or low-back aches, others get pelvic pain from contracting and guarding the levator muscles.
Pelvic floor dysfunction
The most important component of the physical exam in patients with the symptoms of frequency, urgency, and pelvic pain – and the most overlooked – is assessment of the levator muscles for tightness and tenderness. Levator pain and trigger points may be identified during the pelvic exam by pressing laterally on the levator complex in each quadrant of the vagina and at the ischial spines. The tension of the muscles and severity of pain should be assessed, and it is helpful to ask the patient if the pain reproduces her normal pelvic pain symptoms.
We’ve found that identifying and treating pelvic floor dysfunction with modalities such as pelvic floor physical therapy with intravaginal myofascial release, intravaginal valium, trigger point injections into the levator complex, pudendal nerve blocks, and neuromodulation can frequently resolve or significantly lessen the patient’s pain and bladder symptoms, suggesting that the diagnosis of IC/BPS was wrong.
Pelvic floor physical therapy works to stretch the contracted anterior pelvic muscles by releasing trigger points and connective tissue restrictions, and by decreasing periurethral tension; it also may decrease neurogenic triggers and central nervous system sensitivity. Kegel exercises will worsen pain in these patients and should be avoided.
When pelvic floor dysfunction is identified, such treatment by a therapist knowledgeable in intravaginal myofascial release is a next reasonable step before any medications or invasive testing, such as bladder hydrodistension, are used.
One of the only National Institutes of Health–funded studies to show benefit of a treatment in an IC population, in fact, was a multicenter randomized controlled trial comparing 10 sessions of myofascial pelvic floor physical therapy with “global therapeutic massage.” Myofascial physical therapy led to significant improvement, compared with the generalized spa-like massage (J Urol. 2012 Jun;187[6]:2113-8).
Our patients with IC/BPS symptoms and pelvic floor dysfunction require 1-2 visits weekly for an average of 12 weeks for tightness and tenderness to be significantly minimized or eliminated. Patients are also prescribed home stretching exercises and advised to use internal vaginal dilators. Most patients will report resolution of their pelvic pain, sexual pain, and bladder symptoms – especially with the combination of physical therapy and trigger point injections. In more severe cases, we may use sacral or pudendal neuromodulation to improve the frequency, urgency and pelvic pain.
Turning to the bladder
When urinary symptoms persist after the completion of pelvic floor therapy, or when pelvic floor dysfunction is not identified in the first place, we proceed with bladder-specific therapies. I will often suggest trials of amitriptyline or hydroxyzine, for instance, and/or changes in hydration and caffeine consumption. I am not a fan of pentosan polysulfate sodium (Elmiron) as it is a very expensive medication that has minimal benefit for the majority of patients.
When conservative therapies do not work, I move to cystoscopy with hydrodistension. The procedure can serve several purposes. It can be diagnostic, enabling us to rule out other potential symptom-causing pathologies, and it can be prognostic, helping us to understand when bladder capacity is severely reduced and to plan treatment. In some patients, it can even be therapeutic. Some of my patients have significant relief of symptoms from a hydrodistension of the bladder once or twice a year.
There is no standard method for performing a hydrodistension. I perform a complete cystoscopy to look for tumors, stones, diverticulum or Hunner’s lesions and, if the bladder is normal in appearance, I proceed with a 2-minute hydrodistension at 80-100 cm of water pressure under anesthesia. The bag is raised above the bladder, allowing the bladder to fill with the force of gravity and the pressures to equalize. The urethra must be compressed so that water doesn’t leak around the cystoscope. After 2 minutes of hydrodistension, the bladder is drained, volume is measured, and the procedure is repeated.
After the hydrodistension, the bladder is reinspected to be certain there is no bladder perforation and to evaluate for diffuse glomerulations (petechial hemorrhages) that are suggestive, but not diagnostic, of IC/BPS.
A holistic approach
Managing patients with voiding dysfunction and chronic pelvic pain can be a challenge, and a multidisciplinary approach is most effective. At Beaumont, we have a Women’s Urology Center that includes urologists, gynecologists, nurse practitioners, pelvic floor physical therapists, pain psychologists, colorectal specialists, sex therapists, and naturopathic and integrative medicine specialists who perform acupuncture, Reiki therapy, medical massage, and guided imagery.
The goal is to break out of our box of specialties and look at the whole patient – mind, body, and soul – while identifying pain triggers and directing therapy toward these triggers using a multidisciplinary, collaborative approach. For us, this approach has been very effective for managing complex pelvic pain issues (Transl Androl Urol. 2015 Dec;4[6]:611-9).
Ongoing studies
A number of research studies are ongoing to help treat the symptoms of IC/BPS. We currently have a Department of Defense grant to prospectively assess bladder-directed therapy (instillations) compared to pelvic floor physical therapy. Patients diagnosed with IC/BPS are being randomized into these two treatment arms and we hope to get a better understanding of the role of these modalities in managing IC/BPS.
Allergan is completing a phase II placebo-controlled trial using a lidocaine delivery device that is placed in the bladder and continuously releases lidocaine over 14 days. The LiNKA trial is designed to assess the impact of lidocaine on not only improving bladder symptoms, but also eradicating Hunner’s lesions through the anti-inflammatory effect of lidocaine. Early open-label data were very promising. In addition, a new medication for IC/BPS that modulates the SHIP1 pathway is being studied by Aquinox Pharmaceuticals. The agent, AQX-1125, is an activator of SHIP1, which controls the phosphoinositide 3-kinase (PI3K) cellular signaling pathway. If the PI3K pathway is overactive, immune cells can produce an abundance of proinflammatory signaling molecules and migrate to and concentrate in tissues, resulting in excessive or chronic inflammation. Early data in IC/BPS patients were supportive of the compound’s potential for reducing the pain associated with this condition.
A note from Charles E. Miller, MD, Master Class Medical Editor:
In a double-blind, placebo-controlled study by J.C. Nickel, et al., pentosan polysulfate sodium was shown to improve pain, urgency, and frequency over the control group (Urology. 2005 Apr;65[4]:654-8). Also, longer duration of treatment with pentosan polysulfate sodium was associated with greater response rates – 50% improved by 26 weeks (J Urol. 2005 Dec;174[6]:2235-8).
Dr. Peters is professor and chairman of urology at Oakland University William Beaumont School of Medicine, Royal Oak, Mich. He reported serving as a consultant for Taris, Medtronic, StimGuard, and Amphora Medical.
Refining the use of electronic fetal monitoring
Electronic fetal monitoring (EFM) is the most commonly used instrument in obstetrics and is the perceived standard of care. However, the U.S. Preventive Services Task Force recommended against its use in low-risk women in 1996 (a “D” rating) – signifying the lack of evidence for benefit and the potential for harm – and said there was insufficient evidence to recommend for or against its use in high-risk women (a “C” rating).
Today, available data still suggest that EFM does not reduce overall perinatal mortality or the risk of cerebral palsy. Moreover, its use is associated with increased operative vaginal deliveries and cesarean deliveries.
Given the near-zero positive predictive value of EFM for stillbirth or cerebral palsy, some have called EFM “useless” and a “failure.” However, I see potential in the technology. I believe that we are beginning to see evidence emerge that – if confirmed and expanded – will enable us to quantify and interpret indeterminate EFM patterns in new ways that positively impact clinical outcomes.
Despite EFM’s routine use and our specialty’s well-ingrained clinical habits, we should critically and meaningfully examine new science and new data on category II fetal heart rate tracings as they come to light. In the meantime, there is more we can do to resolve concerning elements of these tracings – without using supplemental oxygen – or to provide reassurance of fetal well-being so that cesarean deliveries are not unnecessarily performed.
Emerging research
An abnormal or indeterminate fetal heart rate tracing is the second most common indication for primary cesarean, after labor arrest, according to a study published in 2011 of more than 32,000 live births. Given the rarity of category III tracings (“abnormal”), it is likely that category II tracings (“indeterminate”) account for most of the cesarean deliveries performed out of concern for fetal acidemia (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Until recently, we’ve known very little about the patterns contained within category II of our current three-tier system for categorizing fetal heart rate patterns. The system was defined by the 2008 consensus workshop sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (Obstet Gynecol. 2008 Sep;112[3]:661-6).
We have reasonable data to know that the vast majority of patients with category I fetal heart rate tracings will have a normal pH. We also have reasonable data showing us that patients with category III tracings have a high risk of acidemia and morbidity. However, the majority of tracings we see during labor at term fall into category II, with no clear indication of risk and characterized most often by the presence of decelerations.
As we’ve delved more deeply into the highly variable and complex category II tracings defined in 2008, we’ve begun to demonstrate that tracings can have different meanings for different patients, and that particular clinical factors can make EFM patterns more informative and predictive. In other words, EFM patterns may require different interpretations based on a priori risk and clinical factors.
One of these factors may be the presence of meconium. In a prospective cohort of more than 3,000 women with category II tracings, the presence of meconium – especially thick meconium – was associated with a higher risk of acidemia and neonatal morbidity than when meconium was absent. Interestingly, the negative predictive value was higher than the overall predictive ability in this cohort, which suggests that the absence of meconium in the setting of a category II tracing can be considered a reassuring feature (Am J Obstet Gynecol. 2014;211:644.e1-8).
We have also found through retrospective cohort studies that magnesium sulfate can impact fetal heart rate tracings, causing a transient decrease in variability (Obstet Gynecol. 2012 Jun;119[6]:1129-36 and Am J Perinatol. 2014 Nov;31[10]:869-74). In addition, intrauterine growth restricted fetuses have a higher risk of decelerations without a commensurate higher risk of morbidity (Am J Perinatol. 2015 Jul;32[9]:873-8).
Such findings need to be reproduced, expanded, and further analyzed to show us how the risk of acidemia can be better predicted. For now, just as we increasingly appreciate that tracings have a transient nature and should be considered with two lenses – one looking back in time and one looking forward – we have a growing sense that EFM should not be interpreted without consideration of clinical factors.
Research at our institution and others has shown that acidemia is more significantly associated with non-NICHD measures of fetal heart rate deceleration than with each of the main deceleration types defined by the 2008 NICHD system (i.e., repetitive variable, repetitive late, and repetitive prolonged).
For instance, Emily Hamilton, MD, and her colleagues at PeriGen, a perinatal software company, found that only prolonged decelerations, in addition to the variability within the deceleration and a depth below 60 beats per minute for more than 60 seconds, could discriminate between cases of metabolic acidosis and those with normal umbilical artery gases (J Matern Fetal Neonatal Med. 2012 Jun;25[6]:648-53).
In a 4-year retrospective cohort study of nearly 5,340 consecutive singleton, term, nonanomalous gestations, we found acidemia was most significantly associated with a calculation of the “total deceleration area” – the sum of the estimates of area within all the decelerations. This measure accounted for the frequency, depth, and duration of all decelerations in the final 30 minutes of EFM.
Each of the NICHD deceleration types was associated in our study with acidemia after adjustment for fever, obesity, prolonged first stage, and nulliparity. However, total deceleration area had superior predictive ability. After the same adjustments were made, an abnormal total deceleration area (greater than the 95th percentile) was significantly associated with an increased risk for acidemia (odds ratio, 3.79) (Am J Obstet Gynecol. 2012 Sep;207[3]:206.e1-8).
Pathophysiologically, it seems logical that the total area is most predictive, as it captures both the temporal and dose effect of decelerations. At this point, however, we can only apply this concept crudely at the bedside. There is more work to do to translate such findings into software-driven bedside tools.
Gaining reassurance
Although efforts to manage intrapartum fetal heart rate tracings focus largely on attempting to better predict who is at greatest risk for acidemia, it is important and worthwhile that we also attempt to determine whether a fetus with a category II tracing is not acidotic.
Research has consistently shown that the presence of accelerations, whether spontaneous or stimulated, is a highly reliable indicator of normal neonatal umbilical cord pH. It is therefore reasonable, when faced with indeterminate tracings (e.g., minimal variability), to consider scalp stimulation to elicit fetal heart rate acceleration. Scalp stimulation is the easiest noninvasive tool to employ to quickly secure clinical reassurance – within a couple of minutes – that the fetus is not acidotic.
For guidance on managing repetitive variable decelerations, amnioinfusion with normal saline is similarly worthy of consideration. It has been demonstrated (Level A evidence) to resolve variable fetal heart rate decelerations and reduce the incidence of cesarean delivery for nonreassuring fetal heart rate patterns. Both amnioinfusion and scalp stimulation are recommended in the 2014 ACOG/SMFM consensus statement on “Safe Prevention of the Primary Cesarean Delivery” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
Oxygen administration, on the other hand, is ingrained in practice and is included in the American College of Obstetricians and Gynecologists’ practice bulletin on managing intrapartum fetal rate tracings. It is listed as a possible resuscitative measure for category II or III tracings, despite the fact that there are extremely limited data for its effectiveness or safety in labor.
Maureen S. Hamel, MD, and her colleagues at the Warren Albert Medical School at Brown University reviewed the literature and concluded that the only two randomized trials investigating the use of maternal oxygen supplementation in laboring women do not support the idea that supplementation may benefit the fetus. Moreover, they contended that oxygen supplementation may even be harmful (Am J Obstet Gynecol. 2014 Aug;211[2]:124-7).
If supplemental oxygen were a medication, we would want to know the dose, as well as the length and duration of administration before fetal heart rate tracing improved. We don’t know the answers to these questions.
There is research ongoing, both observational studies and at least one registered randomized clinical trial, that should provide more information and guidance on the impact of supplemental oxygen in the setting of category II fetal heart rate patterns. I do not expect these findings to resolve all the questions. We’re going to need a thorough body of work to provide us with definitive answers.
Dr. Cahill is the chief of the division of maternal-fetal medicine at Washington University in St. Louis. She reported having no financial disclosures relevant to this Master Class.
Electronic fetal monitoring (EFM) is the most commonly used instrument in obstetrics and is the perceived standard of care. However, the U.S. Preventive Services Task Force recommended against its use in low-risk women in 1996 (a “D” rating) – signifying the lack of evidence for benefit and the potential for harm – and said there was insufficient evidence to recommend for or against its use in high-risk women (a “C” rating).
Today, available data still suggest that EFM does not reduce overall perinatal mortality or the risk of cerebral palsy. Moreover, its use is associated with increased operative vaginal deliveries and cesarean deliveries.
Given the near-zero positive predictive value of EFM for stillbirth or cerebral palsy, some have called EFM “useless” and a “failure.” However, I see potential in the technology. I believe that we are beginning to see evidence emerge that – if confirmed and expanded – will enable us to quantify and interpret indeterminate EFM patterns in new ways that positively impact clinical outcomes.
Despite EFM’s routine use and our specialty’s well-ingrained clinical habits, we should critically and meaningfully examine new science and new data on category II fetal heart rate tracings as they come to light. In the meantime, there is more we can do to resolve concerning elements of these tracings – without using supplemental oxygen – or to provide reassurance of fetal well-being so that cesarean deliveries are not unnecessarily performed.
Emerging research
An abnormal or indeterminate fetal heart rate tracing is the second most common indication for primary cesarean, after labor arrest, according to a study published in 2011 of more than 32,000 live births. Given the rarity of category III tracings (“abnormal”), it is likely that category II tracings (“indeterminate”) account for most of the cesarean deliveries performed out of concern for fetal acidemia (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Until recently, we’ve known very little about the patterns contained within category II of our current three-tier system for categorizing fetal heart rate patterns. The system was defined by the 2008 consensus workshop sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (Obstet Gynecol. 2008 Sep;112[3]:661-6).
We have reasonable data to know that the vast majority of patients with category I fetal heart rate tracings will have a normal pH. We also have reasonable data showing us that patients with category III tracings have a high risk of acidemia and morbidity. However, the majority of tracings we see during labor at term fall into category II, with no clear indication of risk and characterized most often by the presence of decelerations.
As we’ve delved more deeply into the highly variable and complex category II tracings defined in 2008, we’ve begun to demonstrate that tracings can have different meanings for different patients, and that particular clinical factors can make EFM patterns more informative and predictive. In other words, EFM patterns may require different interpretations based on a priori risk and clinical factors.
One of these factors may be the presence of meconium. In a prospective cohort of more than 3,000 women with category II tracings, the presence of meconium – especially thick meconium – was associated with a higher risk of acidemia and neonatal morbidity than when meconium was absent. Interestingly, the negative predictive value was higher than the overall predictive ability in this cohort, which suggests that the absence of meconium in the setting of a category II tracing can be considered a reassuring feature (Am J Obstet Gynecol. 2014;211:644.e1-8).
We have also found through retrospective cohort studies that magnesium sulfate can impact fetal heart rate tracings, causing a transient decrease in variability (Obstet Gynecol. 2012 Jun;119[6]:1129-36 and Am J Perinatol. 2014 Nov;31[10]:869-74). In addition, intrauterine growth restricted fetuses have a higher risk of decelerations without a commensurate higher risk of morbidity (Am J Perinatol. 2015 Jul;32[9]:873-8).
Such findings need to be reproduced, expanded, and further analyzed to show us how the risk of acidemia can be better predicted. For now, just as we increasingly appreciate that tracings have a transient nature and should be considered with two lenses – one looking back in time and one looking forward – we have a growing sense that EFM should not be interpreted without consideration of clinical factors.
Research at our institution and others has shown that acidemia is more significantly associated with non-NICHD measures of fetal heart rate deceleration than with each of the main deceleration types defined by the 2008 NICHD system (i.e., repetitive variable, repetitive late, and repetitive prolonged).
For instance, Emily Hamilton, MD, and her colleagues at PeriGen, a perinatal software company, found that only prolonged decelerations, in addition to the variability within the deceleration and a depth below 60 beats per minute for more than 60 seconds, could discriminate between cases of metabolic acidosis and those with normal umbilical artery gases (J Matern Fetal Neonatal Med. 2012 Jun;25[6]:648-53).
In a 4-year retrospective cohort study of nearly 5,340 consecutive singleton, term, nonanomalous gestations, we found acidemia was most significantly associated with a calculation of the “total deceleration area” – the sum of the estimates of area within all the decelerations. This measure accounted for the frequency, depth, and duration of all decelerations in the final 30 minutes of EFM.
Each of the NICHD deceleration types was associated in our study with acidemia after adjustment for fever, obesity, prolonged first stage, and nulliparity. However, total deceleration area had superior predictive ability. After the same adjustments were made, an abnormal total deceleration area (greater than the 95th percentile) was significantly associated with an increased risk for acidemia (odds ratio, 3.79) (Am J Obstet Gynecol. 2012 Sep;207[3]:206.e1-8).
Pathophysiologically, it seems logical that the total area is most predictive, as it captures both the temporal and dose effect of decelerations. At this point, however, we can only apply this concept crudely at the bedside. There is more work to do to translate such findings into software-driven bedside tools.
Gaining reassurance
Although efforts to manage intrapartum fetal heart rate tracings focus largely on attempting to better predict who is at greatest risk for acidemia, it is important and worthwhile that we also attempt to determine whether a fetus with a category II tracing is not acidotic.
Research has consistently shown that the presence of accelerations, whether spontaneous or stimulated, is a highly reliable indicator of normal neonatal umbilical cord pH. It is therefore reasonable, when faced with indeterminate tracings (e.g., minimal variability), to consider scalp stimulation to elicit fetal heart rate acceleration. Scalp stimulation is the easiest noninvasive tool to employ to quickly secure clinical reassurance – within a couple of minutes – that the fetus is not acidotic.
For guidance on managing repetitive variable decelerations, amnioinfusion with normal saline is similarly worthy of consideration. It has been demonstrated (Level A evidence) to resolve variable fetal heart rate decelerations and reduce the incidence of cesarean delivery for nonreassuring fetal heart rate patterns. Both amnioinfusion and scalp stimulation are recommended in the 2014 ACOG/SMFM consensus statement on “Safe Prevention of the Primary Cesarean Delivery” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
Oxygen administration, on the other hand, is ingrained in practice and is included in the American College of Obstetricians and Gynecologists’ practice bulletin on managing intrapartum fetal rate tracings. It is listed as a possible resuscitative measure for category II or III tracings, despite the fact that there are extremely limited data for its effectiveness or safety in labor.
Maureen S. Hamel, MD, and her colleagues at the Warren Albert Medical School at Brown University reviewed the literature and concluded that the only two randomized trials investigating the use of maternal oxygen supplementation in laboring women do not support the idea that supplementation may benefit the fetus. Moreover, they contended that oxygen supplementation may even be harmful (Am J Obstet Gynecol. 2014 Aug;211[2]:124-7).
If supplemental oxygen were a medication, we would want to know the dose, as well as the length and duration of administration before fetal heart rate tracing improved. We don’t know the answers to these questions.
There is research ongoing, both observational studies and at least one registered randomized clinical trial, that should provide more information and guidance on the impact of supplemental oxygen in the setting of category II fetal heart rate patterns. I do not expect these findings to resolve all the questions. We’re going to need a thorough body of work to provide us with definitive answers.
Dr. Cahill is the chief of the division of maternal-fetal medicine at Washington University in St. Louis. She reported having no financial disclosures relevant to this Master Class.
Electronic fetal monitoring (EFM) is the most commonly used instrument in obstetrics and is the perceived standard of care. However, the U.S. Preventive Services Task Force recommended against its use in low-risk women in 1996 (a “D” rating) – signifying the lack of evidence for benefit and the potential for harm – and said there was insufficient evidence to recommend for or against its use in high-risk women (a “C” rating).
Today, available data still suggest that EFM does not reduce overall perinatal mortality or the risk of cerebral palsy. Moreover, its use is associated with increased operative vaginal deliveries and cesarean deliveries.
Given the near-zero positive predictive value of EFM for stillbirth or cerebral palsy, some have called EFM “useless” and a “failure.” However, I see potential in the technology. I believe that we are beginning to see evidence emerge that – if confirmed and expanded – will enable us to quantify and interpret indeterminate EFM patterns in new ways that positively impact clinical outcomes.
Despite EFM’s routine use and our specialty’s well-ingrained clinical habits, we should critically and meaningfully examine new science and new data on category II fetal heart rate tracings as they come to light. In the meantime, there is more we can do to resolve concerning elements of these tracings – without using supplemental oxygen – or to provide reassurance of fetal well-being so that cesarean deliveries are not unnecessarily performed.
Emerging research
An abnormal or indeterminate fetal heart rate tracing is the second most common indication for primary cesarean, after labor arrest, according to a study published in 2011 of more than 32,000 live births. Given the rarity of category III tracings (“abnormal”), it is likely that category II tracings (“indeterminate”) account for most of the cesarean deliveries performed out of concern for fetal acidemia (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Until recently, we’ve known very little about the patterns contained within category II of our current three-tier system for categorizing fetal heart rate patterns. The system was defined by the 2008 consensus workshop sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (Obstet Gynecol. 2008 Sep;112[3]:661-6).
We have reasonable data to know that the vast majority of patients with category I fetal heart rate tracings will have a normal pH. We also have reasonable data showing us that patients with category III tracings have a high risk of acidemia and morbidity. However, the majority of tracings we see during labor at term fall into category II, with no clear indication of risk and characterized most often by the presence of decelerations.
As we’ve delved more deeply into the highly variable and complex category II tracings defined in 2008, we’ve begun to demonstrate that tracings can have different meanings for different patients, and that particular clinical factors can make EFM patterns more informative and predictive. In other words, EFM patterns may require different interpretations based on a priori risk and clinical factors.
One of these factors may be the presence of meconium. In a prospective cohort of more than 3,000 women with category II tracings, the presence of meconium – especially thick meconium – was associated with a higher risk of acidemia and neonatal morbidity than when meconium was absent. Interestingly, the negative predictive value was higher than the overall predictive ability in this cohort, which suggests that the absence of meconium in the setting of a category II tracing can be considered a reassuring feature (Am J Obstet Gynecol. 2014;211:644.e1-8).
We have also found through retrospective cohort studies that magnesium sulfate can impact fetal heart rate tracings, causing a transient decrease in variability (Obstet Gynecol. 2012 Jun;119[6]:1129-36 and Am J Perinatol. 2014 Nov;31[10]:869-74). In addition, intrauterine growth restricted fetuses have a higher risk of decelerations without a commensurate higher risk of morbidity (Am J Perinatol. 2015 Jul;32[9]:873-8).
Such findings need to be reproduced, expanded, and further analyzed to show us how the risk of acidemia can be better predicted. For now, just as we increasingly appreciate that tracings have a transient nature and should be considered with two lenses – one looking back in time and one looking forward – we have a growing sense that EFM should not be interpreted without consideration of clinical factors.
Research at our institution and others has shown that acidemia is more significantly associated with non-NICHD measures of fetal heart rate deceleration than with each of the main deceleration types defined by the 2008 NICHD system (i.e., repetitive variable, repetitive late, and repetitive prolonged).
For instance, Emily Hamilton, MD, and her colleagues at PeriGen, a perinatal software company, found that only prolonged decelerations, in addition to the variability within the deceleration and a depth below 60 beats per minute for more than 60 seconds, could discriminate between cases of metabolic acidosis and those with normal umbilical artery gases (J Matern Fetal Neonatal Med. 2012 Jun;25[6]:648-53).
In a 4-year retrospective cohort study of nearly 5,340 consecutive singleton, term, nonanomalous gestations, we found acidemia was most significantly associated with a calculation of the “total deceleration area” – the sum of the estimates of area within all the decelerations. This measure accounted for the frequency, depth, and duration of all decelerations in the final 30 minutes of EFM.
Each of the NICHD deceleration types was associated in our study with acidemia after adjustment for fever, obesity, prolonged first stage, and nulliparity. However, total deceleration area had superior predictive ability. After the same adjustments were made, an abnormal total deceleration area (greater than the 95th percentile) was significantly associated with an increased risk for acidemia (odds ratio, 3.79) (Am J Obstet Gynecol. 2012 Sep;207[3]:206.e1-8).
Pathophysiologically, it seems logical that the total area is most predictive, as it captures both the temporal and dose effect of decelerations. At this point, however, we can only apply this concept crudely at the bedside. There is more work to do to translate such findings into software-driven bedside tools.
Gaining reassurance
Although efforts to manage intrapartum fetal heart rate tracings focus largely on attempting to better predict who is at greatest risk for acidemia, it is important and worthwhile that we also attempt to determine whether a fetus with a category II tracing is not acidotic.
Research has consistently shown that the presence of accelerations, whether spontaneous or stimulated, is a highly reliable indicator of normal neonatal umbilical cord pH. It is therefore reasonable, when faced with indeterminate tracings (e.g., minimal variability), to consider scalp stimulation to elicit fetal heart rate acceleration. Scalp stimulation is the easiest noninvasive tool to employ to quickly secure clinical reassurance – within a couple of minutes – that the fetus is not acidotic.
For guidance on managing repetitive variable decelerations, amnioinfusion with normal saline is similarly worthy of consideration. It has been demonstrated (Level A evidence) to resolve variable fetal heart rate decelerations and reduce the incidence of cesarean delivery for nonreassuring fetal heart rate patterns. Both amnioinfusion and scalp stimulation are recommended in the 2014 ACOG/SMFM consensus statement on “Safe Prevention of the Primary Cesarean Delivery” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
Oxygen administration, on the other hand, is ingrained in practice and is included in the American College of Obstetricians and Gynecologists’ practice bulletin on managing intrapartum fetal rate tracings. It is listed as a possible resuscitative measure for category II or III tracings, despite the fact that there are extremely limited data for its effectiveness or safety in labor.
Maureen S. Hamel, MD, and her colleagues at the Warren Albert Medical School at Brown University reviewed the literature and concluded that the only two randomized trials investigating the use of maternal oxygen supplementation in laboring women do not support the idea that supplementation may benefit the fetus. Moreover, they contended that oxygen supplementation may even be harmful (Am J Obstet Gynecol. 2014 Aug;211[2]:124-7).
If supplemental oxygen were a medication, we would want to know the dose, as well as the length and duration of administration before fetal heart rate tracing improved. We don’t know the answers to these questions.
There is research ongoing, both observational studies and at least one registered randomized clinical trial, that should provide more information and guidance on the impact of supplemental oxygen in the setting of category II fetal heart rate patterns. I do not expect these findings to resolve all the questions. We’re going to need a thorough body of work to provide us with definitive answers.
Dr. Cahill is the chief of the division of maternal-fetal medicine at Washington University in St. Louis. She reported having no financial disclosures relevant to this Master Class.
Electronic fetal monitoring: Is it information overload?
There is no question that we are living in the information age where “big data” isn’t just reserved for scientists but is accessible to everyone. Wearable devices have revolutionized when and how often we exercise. Smartphones have changed the way in which we consume news, watch television, take photographs, and record home movies. Online video chatting has allowed people who live miles – or even countries – away to connect on a whole new level. Today’s 7-year-olds have never lived in a world without iPhones and don’t know what life is like without iPads. Technology has improved our daily lives in countless ways. However, is “too much of a good thing” ever just too much?
Last year, we looked back over the preceding 5 decades of ob.gyn. practice. This retrospective analysis demonstrated that today’s practitioners have infinitely more tools at their disposal than many of their mentors did to ensure the best pregnancy outcomes. From prenatal diagnostic approaches, such as ultrasonography and genetic screening, to in utero surgical interventions, our discipline has advanced in leaps and bounds, all over the course of one person’s lifetime.
As technology continues to change and, in many ways, enhance the patient experience, the question we should continually ask is, “just because we can do something, should we do it?” Just because we can perform a chorionic villus sampling, should we perform one? Perhaps not. Just because we can schedule a planned cesarean section, should we? Probably not. The same line of questioning applies to the tools we employ to assist us in labor and delivery, including one of the most ubiquitous ones – the electronic fetal monitor.
The electronic fetal heart rate monitor was developed in the late 1950s to continuously record the fetal heart rate during delivery and to help ob.gyns. identify patterns that might indicate fetal distress. Although the monitors have improved over time, the interpretation of the data obtained, and what measures to employ based on these data, can be unclear. Just because the electronic fetal monitor can detect an abnormal heart rate pattern, should we intervene, and what approaches should we employ?
To help answer these questions, I have invited Dr. Alison G. Cahill, associate professor in the department of obstetrics and gynecology at Washington University, St. Louis, and chief of the division of maternal-fetal medicine, to explore the use, utility, and interpretation of data obtained by electronic fetal monitors.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
There is no question that we are living in the information age where “big data” isn’t just reserved for scientists but is accessible to everyone. Wearable devices have revolutionized when and how often we exercise. Smartphones have changed the way in which we consume news, watch television, take photographs, and record home movies. Online video chatting has allowed people who live miles – or even countries – away to connect on a whole new level. Today’s 7-year-olds have never lived in a world without iPhones and don’t know what life is like without iPads. Technology has improved our daily lives in countless ways. However, is “too much of a good thing” ever just too much?
Last year, we looked back over the preceding 5 decades of ob.gyn. practice. This retrospective analysis demonstrated that today’s practitioners have infinitely more tools at their disposal than many of their mentors did to ensure the best pregnancy outcomes. From prenatal diagnostic approaches, such as ultrasonography and genetic screening, to in utero surgical interventions, our discipline has advanced in leaps and bounds, all over the course of one person’s lifetime.
As technology continues to change and, in many ways, enhance the patient experience, the question we should continually ask is, “just because we can do something, should we do it?” Just because we can perform a chorionic villus sampling, should we perform one? Perhaps not. Just because we can schedule a planned cesarean section, should we? Probably not. The same line of questioning applies to the tools we employ to assist us in labor and delivery, including one of the most ubiquitous ones – the electronic fetal monitor.
The electronic fetal heart rate monitor was developed in the late 1950s to continuously record the fetal heart rate during delivery and to help ob.gyns. identify patterns that might indicate fetal distress. Although the monitors have improved over time, the interpretation of the data obtained, and what measures to employ based on these data, can be unclear. Just because the electronic fetal monitor can detect an abnormal heart rate pattern, should we intervene, and what approaches should we employ?
To help answer these questions, I have invited Dr. Alison G. Cahill, associate professor in the department of obstetrics and gynecology at Washington University, St. Louis, and chief of the division of maternal-fetal medicine, to explore the use, utility, and interpretation of data obtained by electronic fetal monitors.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
There is no question that we are living in the information age where “big data” isn’t just reserved for scientists but is accessible to everyone. Wearable devices have revolutionized when and how often we exercise. Smartphones have changed the way in which we consume news, watch television, take photographs, and record home movies. Online video chatting has allowed people who live miles – or even countries – away to connect on a whole new level. Today’s 7-year-olds have never lived in a world without iPhones and don’t know what life is like without iPads. Technology has improved our daily lives in countless ways. However, is “too much of a good thing” ever just too much?
Last year, we looked back over the preceding 5 decades of ob.gyn. practice. This retrospective analysis demonstrated that today’s practitioners have infinitely more tools at their disposal than many of their mentors did to ensure the best pregnancy outcomes. From prenatal diagnostic approaches, such as ultrasonography and genetic screening, to in utero surgical interventions, our discipline has advanced in leaps and bounds, all over the course of one person’s lifetime.
As technology continues to change and, in many ways, enhance the patient experience, the question we should continually ask is, “just because we can do something, should we do it?” Just because we can perform a chorionic villus sampling, should we perform one? Perhaps not. Just because we can schedule a planned cesarean section, should we? Probably not. The same line of questioning applies to the tools we employ to assist us in labor and delivery, including one of the most ubiquitous ones – the electronic fetal monitor.
The electronic fetal heart rate monitor was developed in the late 1950s to continuously record the fetal heart rate during delivery and to help ob.gyns. identify patterns that might indicate fetal distress. Although the monitors have improved over time, the interpretation of the data obtained, and what measures to employ based on these data, can be unclear. Just because the electronic fetal monitor can detect an abnormal heart rate pattern, should we intervene, and what approaches should we employ?
To help answer these questions, I have invited Dr. Alison G. Cahill, associate professor in the department of obstetrics and gynecology at Washington University, St. Louis, and chief of the division of maternal-fetal medicine, to explore the use, utility, and interpretation of data obtained by electronic fetal monitors.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Moving toward safer morcellation techniques
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
VIDEO: Tips for performing contained power morcellation
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Prenatal surveillance vital in monochorionic twin pregnancies
Prenatal care is important for every pregnancy. It ensures the health and safety of the mother and baby throughout gestation, and alerts the ob.gyn. to any possible complications that may arise. Today, more than ever before, we have a wide array of powerful tools to augment the care we provide for our patients – from imaging technologies, to genomic screens, to advances in fetal surgery. However, every pregnancy can present its own set of challenges, and successful delivery of a healthy newborn cannot be taken for granted.
The importance of proper prenatal care is most essential to women with higher-risk pregnancies, which includes those involving multiple fetuses. From the babies’ perspective, complications of multiple fetuses can include intrauterine growth restriction, cerebral palsy, and stillbirth; from the mother’s perspective, complications of multiple fetuses can include preterm labor, gestational diabetes mellitus, preeclampsia, and placental abruption.
This month, we focus on the range of tools and approaches used to treat complications that can occur in monochorionic twin pregnancies, including twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion. Despite the challenges these conditions pose, increased ultrasonographic and echocardiographic surveillance allow for earlier detection and possible intervention to slow progression or, in some cases, correct defects that would have terminated the pregnancy not too long ago. Additionally, fetal therapy programs utilizing in-utero surgical techniques, such as fetoscopic laser coagulation, have significantly broadened the management and treatment options we can now offer these patients.
Dr. M. Ozhan Turan, an associate professor and director of fetal therapy and complex obstetric surgery in the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, provides an overview of these techniques and technologies which, when applied appropriately, can significantly improve pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Prenatal care is important for every pregnancy. It ensures the health and safety of the mother and baby throughout gestation, and alerts the ob.gyn. to any possible complications that may arise. Today, more than ever before, we have a wide array of powerful tools to augment the care we provide for our patients – from imaging technologies, to genomic screens, to advances in fetal surgery. However, every pregnancy can present its own set of challenges, and successful delivery of a healthy newborn cannot be taken for granted.
The importance of proper prenatal care is most essential to women with higher-risk pregnancies, which includes those involving multiple fetuses. From the babies’ perspective, complications of multiple fetuses can include intrauterine growth restriction, cerebral palsy, and stillbirth; from the mother’s perspective, complications of multiple fetuses can include preterm labor, gestational diabetes mellitus, preeclampsia, and placental abruption.
This month, we focus on the range of tools and approaches used to treat complications that can occur in monochorionic twin pregnancies, including twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion. Despite the challenges these conditions pose, increased ultrasonographic and echocardiographic surveillance allow for earlier detection and possible intervention to slow progression or, in some cases, correct defects that would have terminated the pregnancy not too long ago. Additionally, fetal therapy programs utilizing in-utero surgical techniques, such as fetoscopic laser coagulation, have significantly broadened the management and treatment options we can now offer these patients.
Dr. M. Ozhan Turan, an associate professor and director of fetal therapy and complex obstetric surgery in the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, provides an overview of these techniques and technologies which, when applied appropriately, can significantly improve pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Prenatal care is important for every pregnancy. It ensures the health and safety of the mother and baby throughout gestation, and alerts the ob.gyn. to any possible complications that may arise. Today, more than ever before, we have a wide array of powerful tools to augment the care we provide for our patients – from imaging technologies, to genomic screens, to advances in fetal surgery. However, every pregnancy can present its own set of challenges, and successful delivery of a healthy newborn cannot be taken for granted.
The importance of proper prenatal care is most essential to women with higher-risk pregnancies, which includes those involving multiple fetuses. From the babies’ perspective, complications of multiple fetuses can include intrauterine growth restriction, cerebral palsy, and stillbirth; from the mother’s perspective, complications of multiple fetuses can include preterm labor, gestational diabetes mellitus, preeclampsia, and placental abruption.
This month, we focus on the range of tools and approaches used to treat complications that can occur in monochorionic twin pregnancies, including twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion. Despite the challenges these conditions pose, increased ultrasonographic and echocardiographic surveillance allow for earlier detection and possible intervention to slow progression or, in some cases, correct defects that would have terminated the pregnancy not too long ago. Additionally, fetal therapy programs utilizing in-utero surgical techniques, such as fetoscopic laser coagulation, have significantly broadened the management and treatment options we can now offer these patients.
Dr. M. Ozhan Turan, an associate professor and director of fetal therapy and complex obstetric surgery in the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, provides an overview of these techniques and technologies which, when applied appropriately, can significantly improve pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Detecting and managing monochorionic twin complications
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.